Article
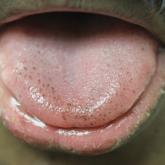
Pigmented Fungiform Papillae of the Tongue in an Indian Male
- Author:
- Jan M. Smogorzewski, MD
- Patrick Armstrong, MD
- Lorraine Young, MD
Pigmented fungiform papillae of the tongue are common lingual hyperpigmented macules in patients with skin of color. It is important to be aware...
Article
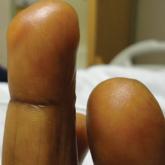
Acral Cutaneous Metastasis From a Primary Breast Carcinoma Following Chemotherapy With Bevacizumab and Paclitaxel
- Author:
- Patrick Armstrong, MD
- Meghan M. Woody, MD, MPH
- Jason S. Reichenberg, MD
- Alde Carlo P. Gavino, MD
Acral cutaneous metastasis from internal malignancy is rare, and the pathogenesis is not completely understood. We present the case of a 54-year-...
