User login
Progressive Widespread Warty Skin Growths
Epidermodysplasia Verruciformis
Epidermodysplasia verruciformis (EV) is a rare hereditary disorder that predisposes affected individuals to widespread infection with various forms of human papillomavirus (HPV). It is inherited in an autosomal-recessive pattern.1 The first manifestations generally are seen in childhood. The clinical appearance of lesions can vary, at times mimicking other disease processes. Patients can present with flat wartlike papules resembling verruca plana distributed in sun-exposed areas. Another distinct presentation is multiple salmon-colored, hyperpigmented, or hypopigmented macules, papules, or plaques with overlying scale that can resemble tinea versicolor.1,2 A large percentage of patients will go on to develop actinic keratosis and squamous cell carcinoma by 40 years of age.2 The malignancies most commonly develop in sun-exposed areas, suggesting UV radiation as an important contributor to development along with HPV infection. Mutations in the EVER1 and EVER2 genes that code for transmembrane proteins on the endoplasmic reticulum that are involved in zinc transport lead to EV. The mutations lead to decreased zinc movement into the cytoplasm, which is thought to play a role in preventing HPV infection. The decreased protection against HPV leads to infections from both common subtypes and those that immunocompetent individuals would be resistant to, namely the β-genus HPV-5, -8, -9 and -20.1,2 Immunosuppressed individuals, such as those with human immunodeficiency virus/AIDS, also are at an increased risk for infection with these HPV subtypes and generally have similar clinical and histological presentations.1 It is important to promote sunscreen use for preventive care in patients with EV due to the increased risk for squamous cell carcinoma.
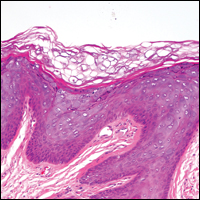
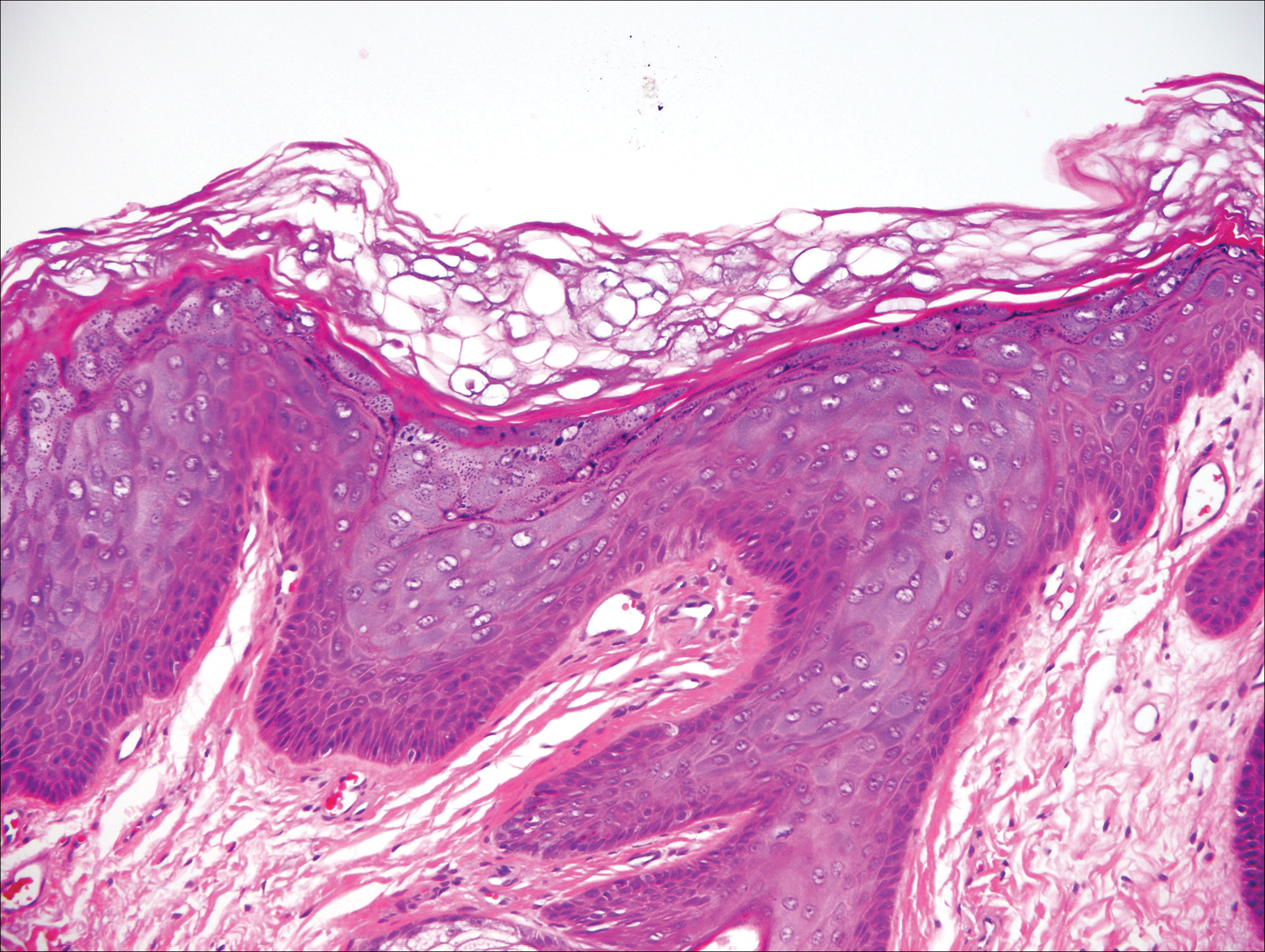
Histologically, the lesions in EV are composed of acanthosis and hyperkeratosis with keratinocytes arranged in clusters.1,3 There is orthokeratosis and parakeratosis.1 Scattered or clustered keratinocytes in the granular layer or upper stratum spinosum appear swollen with foamy blue-gray cytoplasm (quiz image and Figure 1).1,4 The keratinocytes may become atypical and progress to squamous cell carcinoma, particularly in sun-exposed regions. Cell differentiation becomes disorganized and nuclei become enlarged and hyperchromatic.1

Condyloma acuminatum will have pronounced acanthosis and hyperkeratosis with exophytic growth. Rounded parakeratosis is visible. The characteristic cell is the koilocyte, a keratinocyte that has an enlarged nucleus with areas of surrounding clearing, increased dark color in the nucleus, and wrinkled nuclear membrane (Figure 2).1,3 True koilocytes may be rare in condyloma acuminatum.4 Other distinct features include coarse hypergranulosis and a compact stratum corneum.

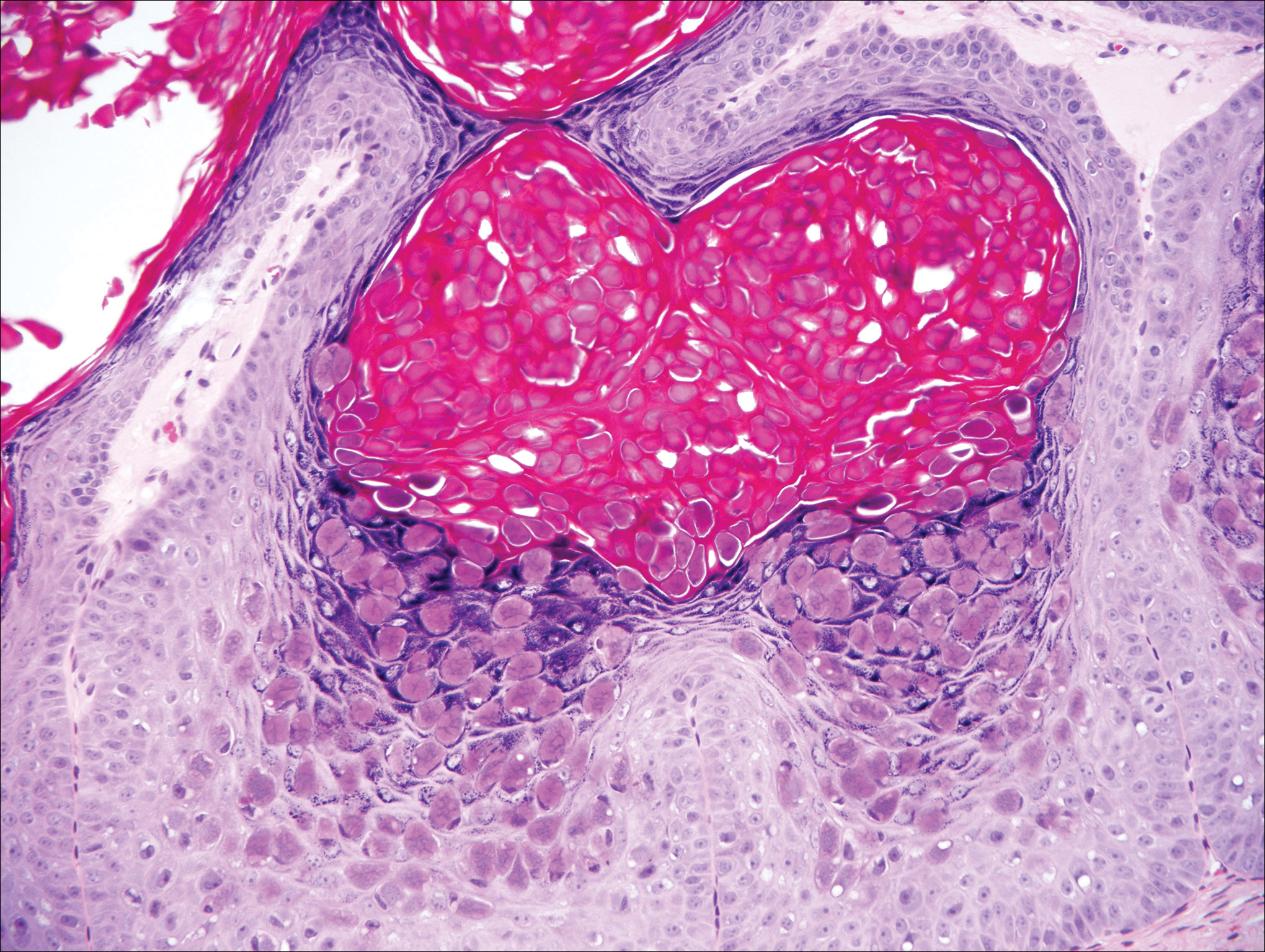
Herpesvirus lesions typically demonstrate ballooning degeneration of keratinocytes.1 They will become pale and fuse to form multinucleated giant cells, a feature not found in verruca. The nuclei will be slate gray with margination of the chromatin, which can be identified due to its increased basophilic appearance (Figure 3).1,4 Inclusion bodies can be found, but unlike molluscum contagiosum (MC), these bodies are intranuclear as opposed to cytoplasmic.1
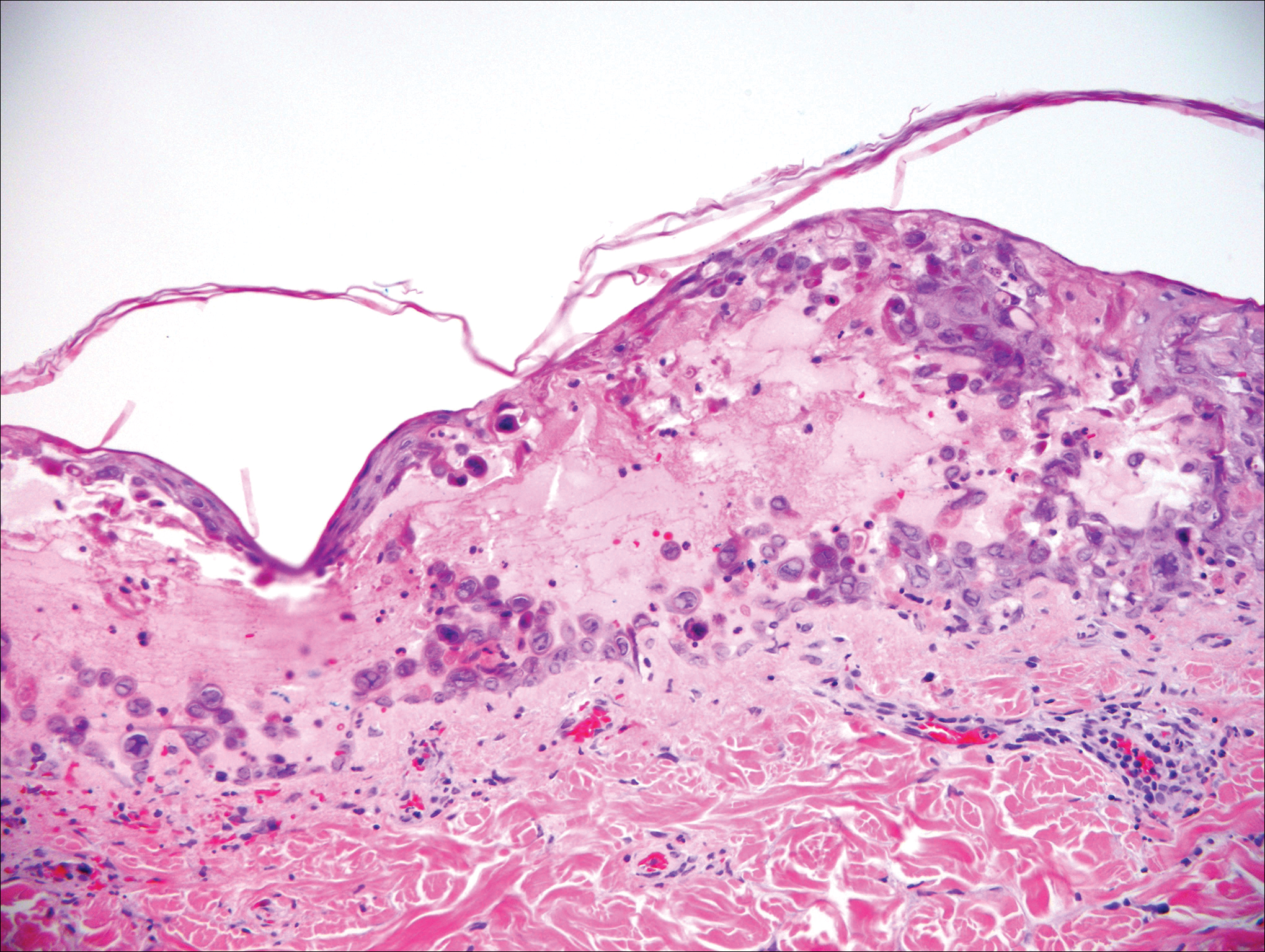
The telltale Henderson-Patterson (molluscum) bodies can identify MC histologically.4 Located within keratinocytes, these cytoplasmic inclusions can vary in both color and size as they mature. As the keratinocytes develop outward, the molluscum bodies grow larger and become more eosinophilic (Figure 4).1,4 Another feature of MC that can be used to differentiate it from EV is the scalloped borders located on lesions.4
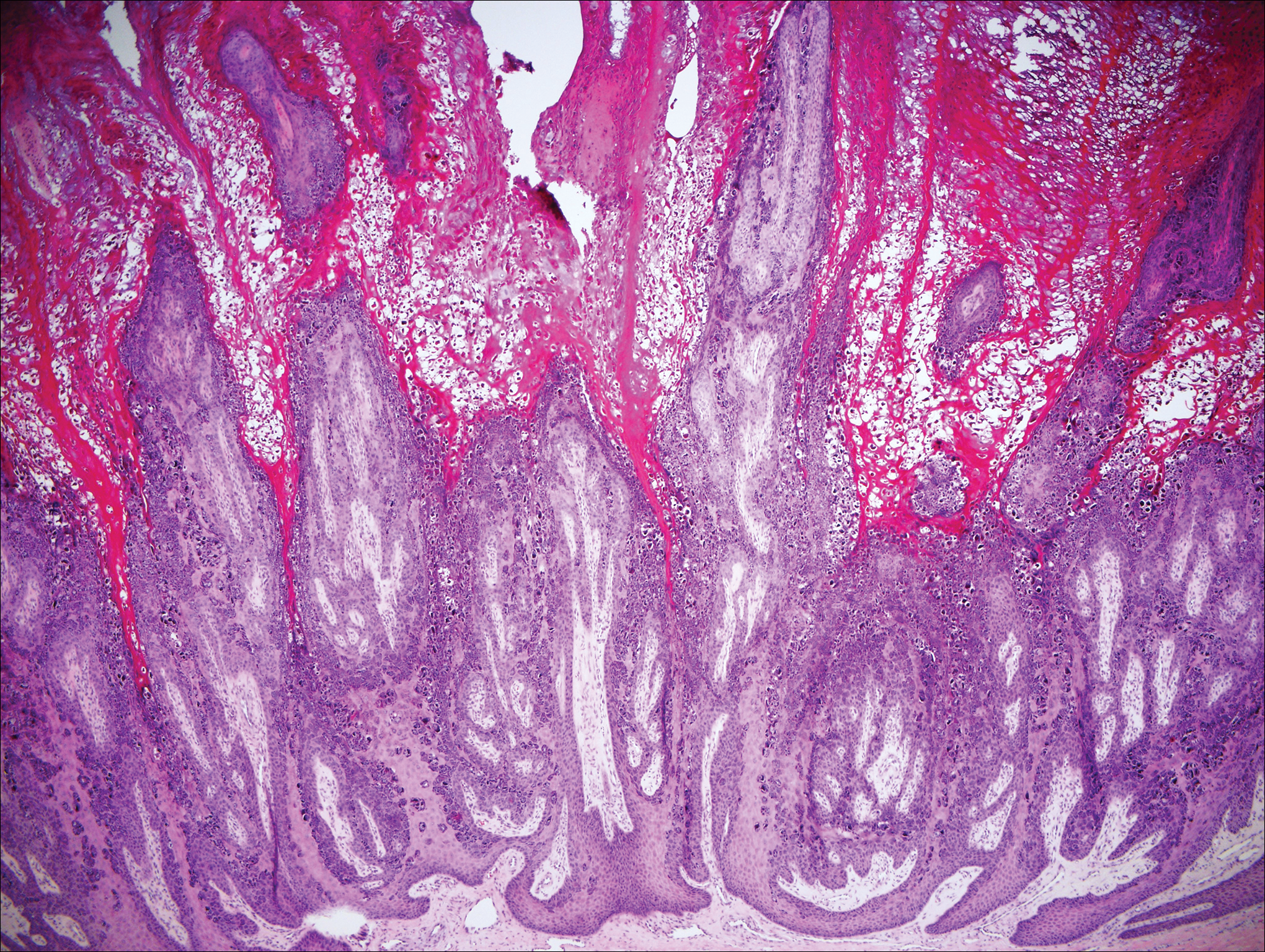
On histology, verruca vulgaris will have pronounced acanthosis with orthokeratosis and vertical tiers of parakeratosis.3,4 Growth is exophytic. The granular layer will have large irregular basophilic granules. Koilocytes may be seen. A distinctive feature is the papillomatosis with inward bending of rete ridges.3,4 It is common to see invasion of tortuous blood vessels into the exophytic projections.3 In myrmecia (palmoplantar warts) it is common to see thrombosis of these vessels and inclusions of red cytoplasmic bodies (Figure 5).1,4
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Hunzeker CM, Soldano AC, Prystowsky S. Epidermodysplasia verruciformis. Dermatology Online J. 2008;14:2.
- Calonje E, McKee PH. McKee's Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012.
- Elston DM, Ko CJ, Ferringer T. Dermatopathology. Edinburgh, Scotland: Saunders/Elsevier; 2009.
Epidermodysplasia Verruciformis
Epidermodysplasia verruciformis (EV) is a rare hereditary disorder that predisposes affected individuals to widespread infection with various forms of human papillomavirus (HPV). It is inherited in an autosomal-recessive pattern.1 The first manifestations generally are seen in childhood. The clinical appearance of lesions can vary, at times mimicking other disease processes. Patients can present with flat wartlike papules resembling verruca plana distributed in sun-exposed areas. Another distinct presentation is multiple salmon-colored, hyperpigmented, or hypopigmented macules, papules, or plaques with overlying scale that can resemble tinea versicolor.1,2 A large percentage of patients will go on to develop actinic keratosis and squamous cell carcinoma by 40 years of age.2 The malignancies most commonly develop in sun-exposed areas, suggesting UV radiation as an important contributor to development along with HPV infection. Mutations in the EVER1 and EVER2 genes that code for transmembrane proteins on the endoplasmic reticulum that are involved in zinc transport lead to EV. The mutations lead to decreased zinc movement into the cytoplasm, which is thought to play a role in preventing HPV infection. The decreased protection against HPV leads to infections from both common subtypes and those that immunocompetent individuals would be resistant to, namely the β-genus HPV-5, -8, -9 and -20.1,2 Immunosuppressed individuals, such as those with human immunodeficiency virus/AIDS, also are at an increased risk for infection with these HPV subtypes and generally have similar clinical and histological presentations.1 It is important to promote sunscreen use for preventive care in patients with EV due to the increased risk for squamous cell carcinoma.
Histologically, the lesions in EV are composed of acanthosis and hyperkeratosis with keratinocytes arranged in clusters.1,3 There is orthokeratosis and parakeratosis.1 Scattered or clustered keratinocytes in the granular layer or upper stratum spinosum appear swollen with foamy blue-gray cytoplasm (quiz image and Figure 1).1,4 The keratinocytes may become atypical and progress to squamous cell carcinoma, particularly in sun-exposed regions. Cell differentiation becomes disorganized and nuclei become enlarged and hyperchromatic.1

Condyloma acuminatum will have pronounced acanthosis and hyperkeratosis with exophytic growth. Rounded parakeratosis is visible. The characteristic cell is the koilocyte, a keratinocyte that has an enlarged nucleus with areas of surrounding clearing, increased dark color in the nucleus, and wrinkled nuclear membrane (Figure 2).1,3 True koilocytes may be rare in condyloma acuminatum.4 Other distinct features include coarse hypergranulosis and a compact stratum corneum.

Herpesvirus lesions typically demonstrate ballooning degeneration of keratinocytes.1 They will become pale and fuse to form multinucleated giant cells, a feature not found in verruca. The nuclei will be slate gray with margination of the chromatin, which can be identified due to its increased basophilic appearance (Figure 3).1,4 Inclusion bodies can be found, but unlike molluscum contagiosum (MC), these bodies are intranuclear as opposed to cytoplasmic.1

The telltale Henderson-Patterson (molluscum) bodies can identify MC histologically.4 Located within keratinocytes, these cytoplasmic inclusions can vary in both color and size as they mature. As the keratinocytes develop outward, the molluscum bodies grow larger and become more eosinophilic (Figure 4).1,4 Another feature of MC that can be used to differentiate it from EV is the scalloped borders located on lesions.4

On histology, verruca vulgaris will have pronounced acanthosis with orthokeratosis and vertical tiers of parakeratosis.3,4 Growth is exophytic. The granular layer will have large irregular basophilic granules. Koilocytes may be seen. A distinctive feature is the papillomatosis with inward bending of rete ridges.3,4 It is common to see invasion of tortuous blood vessels into the exophytic projections.3 In myrmecia (palmoplantar warts) it is common to see thrombosis of these vessels and inclusions of red cytoplasmic bodies (Figure 5).1,4

Epidermodysplasia Verruciformis
Epidermodysplasia verruciformis (EV) is a rare hereditary disorder that predisposes affected individuals to widespread infection with various forms of human papillomavirus (HPV). It is inherited in an autosomal-recessive pattern.1 The first manifestations generally are seen in childhood. The clinical appearance of lesions can vary, at times mimicking other disease processes. Patients can present with flat wartlike papules resembling verruca plana distributed in sun-exposed areas. Another distinct presentation is multiple salmon-colored, hyperpigmented, or hypopigmented macules, papules, or plaques with overlying scale that can resemble tinea versicolor.1,2 A large percentage of patients will go on to develop actinic keratosis and squamous cell carcinoma by 40 years of age.2 The malignancies most commonly develop in sun-exposed areas, suggesting UV radiation as an important contributor to development along with HPV infection. Mutations in the EVER1 and EVER2 genes that code for transmembrane proteins on the endoplasmic reticulum that are involved in zinc transport lead to EV. The mutations lead to decreased zinc movement into the cytoplasm, which is thought to play a role in preventing HPV infection. The decreased protection against HPV leads to infections from both common subtypes and those that immunocompetent individuals would be resistant to, namely the β-genus HPV-5, -8, -9 and -20.1,2 Immunosuppressed individuals, such as those with human immunodeficiency virus/AIDS, also are at an increased risk for infection with these HPV subtypes and generally have similar clinical and histological presentations.1 It is important to promote sunscreen use for preventive care in patients with EV due to the increased risk for squamous cell carcinoma.
Histologically, the lesions in EV are composed of acanthosis and hyperkeratosis with keratinocytes arranged in clusters.1,3 There is orthokeratosis and parakeratosis.1 Scattered or clustered keratinocytes in the granular layer or upper stratum spinosum appear swollen with foamy blue-gray cytoplasm (quiz image and Figure 1).1,4 The keratinocytes may become atypical and progress to squamous cell carcinoma, particularly in sun-exposed regions. Cell differentiation becomes disorganized and nuclei become enlarged and hyperchromatic.1

Condyloma acuminatum will have pronounced acanthosis and hyperkeratosis with exophytic growth. Rounded parakeratosis is visible. The characteristic cell is the koilocyte, a keratinocyte that has an enlarged nucleus with areas of surrounding clearing, increased dark color in the nucleus, and wrinkled nuclear membrane (Figure 2).1,3 True koilocytes may be rare in condyloma acuminatum.4 Other distinct features include coarse hypergranulosis and a compact stratum corneum.

Herpesvirus lesions typically demonstrate ballooning degeneration of keratinocytes.1 They will become pale and fuse to form multinucleated giant cells, a feature not found in verruca. The nuclei will be slate gray with margination of the chromatin, which can be identified due to its increased basophilic appearance (Figure 3).1,4 Inclusion bodies can be found, but unlike molluscum contagiosum (MC), these bodies are intranuclear as opposed to cytoplasmic.1

The telltale Henderson-Patterson (molluscum) bodies can identify MC histologically.4 Located within keratinocytes, these cytoplasmic inclusions can vary in both color and size as they mature. As the keratinocytes develop outward, the molluscum bodies grow larger and become more eosinophilic (Figure 4).1,4 Another feature of MC that can be used to differentiate it from EV is the scalloped borders located on lesions.4

On histology, verruca vulgaris will have pronounced acanthosis with orthokeratosis and vertical tiers of parakeratosis.3,4 Growth is exophytic. The granular layer will have large irregular basophilic granules. Koilocytes may be seen. A distinctive feature is the papillomatosis with inward bending of rete ridges.3,4 It is common to see invasion of tortuous blood vessels into the exophytic projections.3 In myrmecia (palmoplantar warts) it is common to see thrombosis of these vessels and inclusions of red cytoplasmic bodies (Figure 5).1,4

- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Hunzeker CM, Soldano AC, Prystowsky S. Epidermodysplasia verruciformis. Dermatology Online J. 2008;14:2.
- Calonje E, McKee PH. McKee's Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012.
- Elston DM, Ko CJ, Ferringer T. Dermatopathology. Edinburgh, Scotland: Saunders/Elsevier; 2009.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
- Hunzeker CM, Soldano AC, Prystowsky S. Epidermodysplasia verruciformis. Dermatology Online J. 2008;14:2.
- Calonje E, McKee PH. McKee's Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier/Saunders; 2012.
- Elston DM, Ko CJ, Ferringer T. Dermatopathology. Edinburgh, Scotland: Saunders/Elsevier; 2009.
A 33-year-old man presented with progressive widespread warty skin growths that had been present since 6 years of age. Physical examination revealed numerous verrucous papules on the face and neck along with verrucous, tan-pink papules and plaques diffusely scattered on the trunk, arms, and legs. A biopsy of a lesion on the neck was performed.