User login
UV protection and sunscreens: What to tell patients
Everyone should avoid overexposure to the sun’s rays. But the desire for the “perfect tan,” the belief that a tan enables one to spend more time in the sun, and a lack of awareness about the dangers of ultraviolet (UV) radiation are factors that contribute to UV-induced skin damage and to an increased risk of skin cancer. Physicians need to be prepared to counsel patients on why and how to avoid damaging UV radiation.
See the patient education handout
Some measures are straightforward, such as wearing protective clothing, limiting sun exposure during the peak daylight hours, and avoiding tanning booths. The issue of which sunscreen to use can be more difficult, given the quantity of sunscreen products and the confusing claims made on product labels.
In this article, we review UV radiation, the consequences of increased exposure to different parts of the UV spectrum, tanning, and the fundamentals of sunscreens. We also briefly review current guidelines from professional organizations and rulings on sunscreen products by the US Food and Drug Administration (FDA).
FACTORS AFFECTING UV EXPOSURE
UV radiation from the sun is strongest between 10:00 am and 4:00 pm at equatorial latitudes and during summer months.1 Certain wavelengths of UV radiation have long been known to contribute to skin cancer in humans: the wavelengths considered most damaging are those from 320 to 400 nm, referred to as UV-A, and from 290 to 320 nm, referred to as UV-B.1,2 The UV spectrum also includes UV-C and other subdivisions, but in this article we are mainly concerned with UV-A and UV-B. From 90% to 95% of UV radiation that reaches the earth’s surface is UV-A, and most of the rest is UV-B.
The different wavelengths of UV-A and UV-B have different effects on the skin. Much of the shorter-wavelength UV-B radiation is scattered by the atmospheric ozone layer, by clouds, by air pollution, and by glass; on the other hand, UV-B rays are the main cause of sunburn in humans. The longer-wavelength UV-A radiation penetrates more deeply into the skin and so may have greater destructive potential.1,3
The daily UV index
The daily UV index of the US National Weather Service and the US Environmental Protection Agency (EPA) (www.epa.gov/sunwise/uvindex.html) offers a direct measurement of the level of UV radiation on a scale of 1 (low) to 11+ (extremely high). The higher the number, the greater the risk of sunburn for a fair-skinned person, even after allowing for cloud cover.
UV EXPOSURE RISKS ARE WELL KNOWN
The American Cancer Society has estimated that the annual incidence of nonmelanoma skin cancer is greater than 2 million, and the incidence of melanoma is from 65,000 to 70,000.4 The incidence of all types of skin cancer has been increasing for the last 30 years.4,5
Exposure to UV radiation is the major environmental risk factor for nonmelanoma skin cancer.6 It is also believed to be a major risk factor for melanoma; although definitive evidence is still lacking, research is beginning to uncover mechanisms linking UV-related gene damage to melanoma.7
UV LIGHT’S EFFECTS ON THE SKIN
The effects of UV light on the skin can be immediate (eg, erythema) and long-term (eg, photoaging, immunosuppression, carcinogenicity).1
Sunburn
Excessive UV damage creates a biochemical milieu that manifests grossly on the skin as a “sunburn.” Excessive UV exposure is damaging regardless of whether a sunburn occurs. Intensive intermittent UV exposure in childhood and teen years leading to blistering sunburn is a risk factor for basal cell carcinoma and malignant melanoma, whereas excessive chronic cumulative exposure is a risk factor for squamous cell carcinoma. In addition, both types of exposure can lead to photoaging.
Sunburn is noticeable 3 to 4 hours after exposure, peaking at around 24 hours.
Photoaging
A long-term effect of UV exposure is photoaging. Although how photoaging occurs is unclear, studies suggest that UV-A contributes more to photoaging, while UV-B contributes to burning, which results in extracellular matrix degradation and dysregulation of collagen metabolism. These changes in matrix and collagen may cause wrinkles and loss of skin turgor; increases in vascular growth factors may induce telangiectasia. All of these effects are characteristic of photoaging.8,9
Immunosuppression, sun exposure, cancer
Profound systemic immunosuppression, such as in organ transplantation patients, can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
But sun exposure itself can also cause both local and systemic immunosuppression depending on the area of exposure and the dosage of UV radiation. The immunosuppressive and carcinogenic effects of UV light on the skin are complex, involving a variety of cell types, including antigen-presenting cells, lymphocytes, and cytokines. UV radiation can cause dysregulation of antigen-presenting cells such as Langerhans cells and dermal dendritic cells, which in turn can activate regulatory T cells to suppress the immune system. UV radiation can also induce keratinocytes to produce immunosuppressive cytokines that inhibit the production of a number of “repair cytokines” that fix UV-induced DNA damage. The repair cytokines can mitigate UV-induced immunosuppression.6,11 These effects can suppress the induction of local, systemic, and memory immunity.
Both UV-A and UV-B interact to enhance UV-induced immunosuppression, and this can occur even at doses that do not cause erythema.12 Profound immunosuppression—whether UV-induced or due to HIV infection or immunosuppressive drugs—can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
Animal studies linking UV-B exposure to skin cancer found that UV-B energy is directly absorbed by DNA, resulting in the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone photoproducts in the DNA, which block replication and transcription.6 The resulting mutations specifically occur in the tumor suppressor gene p53, and these mutations have been linked to squamous cell carcinoma.13,14
UV-A light has also been reported to induce cyclobutane dimers, but via an indirect mechanism, since DNA does not directly absorb UV-A. Dimers induced by UV-A light are apparently cleared at a slower rate than those induced by UV-B, suggesting that UV-A may have a greater potential for carcinogenesis.15 UV-A light can also directly induce carcinogenesis through reactive oxygen species that cause tumorogenic modified bases in the DNA. These modified bases can be misread, leading to decreased DNA integrity.6
WHAT IS TANNING?
UV radiation produces darkening of the skin, or tanning. UV exposure results in both immediate and persistent pigment darkening. Immediate pigment darkening, which is visible and transient, occurs within seconds of UV exposure as a result of the formation of reactive oxygen species and photooxidation of preexisting melanin, and it resolves in a couple of hours. Persistent pigment darkening results from photooxidation and redistribution of preexisting melanin, occurring 2 to 24 hours after sun exposure. Neither type of pigment darkening protects the skin, since no new melanin is produced.16,17
UV-B rays can induce skin erythema, edema, and sunburn, followed by skin desquamation and tanning. Its effects can be seen immediately, but typically the erythema reaches its peak 24 hours later.1
“Delayed tanning” is an adaptive response seen about 3 days after sun exposure and is caused by increased melanocyte activity and new melanin formation in response to UV-B; this effect is considered mildly photoprotective, with a sun protection factor (SPF) of 3. In other words, there is a tiny bit of truth to the common belief that a tan that develops a few days after sun exposure (delayed tanning) can provide a small increase in protection from sunburn. However, the real health concern is not only sunburn, but increased cancer risk and photoaging from UV exposure.
INDOOR TANNING
Every year, nearly 28 million Americans use a sunbed or a sunlamp, and 2.3 million of them are teenagers.18,19 Every day in the United States more than 1 million people use an indoor tanning device.20 Nearly 70% of those who use tanning devices are white women ages 16 to 29.21
Tanning is big business. In 2010, there were 20,000 tanning salons in the United States, and the number of health clubs and spas with tanning beds was between 15,000 and 20,000. In 2010, the tanning industry generated an estimated $4.7 billion in revenue.22
In their search for the perfect tan, people receive very large doses of UV light, and most tanning lamps emit 95% to 99% of their light as UV-A. In fact, the typical sunlamp user can receive an annual dose of UV-A that is 0.3 to 1.2 times the average annual cumulative dose received from sun exposure (7,700 kJ/m2).11 A typical customer of a tanning salon in the course of 20 sessions is exposed to up to 1.2 times the average normal annual exposure from sunlight. Also, for a frequent tanner, the exposure can increase to 4.7 times the average normal annual exposure and up to 12 times the exposure if using high-pressure sunlamps.11 Indoor tanners not only receive large doses of a known carcinogen, but the body’s pigmentary responses to a sunlamp’s UV-A (immediate and persistent pigment darkening) do not protect it from sunburn, cancer-inducing DNA damage, immunosuppression, or photoaging.
Additionally, even though tanning bed lamps only emit 1% to 5% of their light in the UV-B spectrum, one can still receive a very large dose of UV-B radiation with enough exposure.
The American Academy of Dermatology opposes indoor tanning and supports a ban on the nonmedical production and sale of indoor tanning devices. The World Health Organization classifies tanning lamps as carcinogenic and advises minors to avoid indoor tanning.23
SUNSCREEN PROTECTION
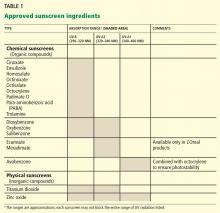
Sunscreen products must contain an active sunscreen ingredient that absorbs radiation in the range of 290 to 400 nm. In “physical” sunscreens, the ingredient is an inorganic compound with particles that physically block out UV radiation; in “chemical” sunscreens, the ingredient is an organic compound that absorbs UV radiation.
Most organic UV filters absorb UV-B radiation, and a few act in the UV-A2 range (320–340 nm). Only one FDA-approved organic sunscreen, avobenzone, protects against UV-A1 (340–400 nm).
Inorganic compounds function by physically reflecting and scattering UV radiation from a film of inert metal particles, ie, in a manner similar to protective clothing.24 Two FDA-approved inorganic sunscreens—titanium dioxide and zinc oxide—provide UV-A and UV-B protection. Zinc oxide and the non-micronized form of titanium dioxide provide UV-A1 and UV-A2 protection.
Inorganic sunscreens have a thick consistency and tend to clump. Advances in nanoparticle technology have improved their consistency,25 but micronized titanium dioxide does not provide UV-A1 protection.
The FDA regulates the active ingredients in sunscreen products, determines the methods of testing them, and dictates labelling requirements.
CATEGORIES OF SUNSCREENS
Sunscreens are categorized according to their SPF,26 UV-A protection,27,28 substantivity, and stability.29
Understanding the ‘sun protection factor’
SPF is a laboratory measure of sunscreen efficacy and is defined as the amount of UV radiation required to produce a sunburn on protected skin relative to that of unprotected skin. Since SPF assessment is based on erythema, it is mainly a measure of UV-B exposure, not UV-A exposure.
Contrary to popular belief, the SPF of a product is not related to the duration of UV exposure.30 Also, the relationship between SPF and UV-B protection is not linear: a sunscreen with an SPF of 15 can filter 94% of UV-B radiation, whereas an SPF of 30 provides greater than 97% protection at an equal UV-B dosage. UV radiation dosage depends on both the duration of exposure and the intensity of the UV radiation. Thus, a sunscreen with twice the SPF does not necessarily mean one can stay out in the sun twice as long before developing a sunburn.
Ability to block UV-A radiation
As UV-A causes significant immunosuppression and is the major type of UV radiation reaching Earth, a systematic and repeatable method of measuring a sunscreen’s ability to block UV-A light is necessary.
For each sunscreen, laboratory testing generates a curve of the absorbance within the UV spectrum. The area under this curve is calculated, and a “critical wavelength” is defined as the wavelength where the area under the absorbance curve up to that value is 90% of the total area under the curve. A sunscreen with “broad-spectrum” UV-A protection is one for which the critical wavelength is greater than or equal to 370 nm. The critical wavelength measures the breadth of UV-A absorbance by a sunscreen and must be used in combination with the SPF value to provide a complete assessment of UV protection.27,28,32,33
Substantivity
Substantivity is a sunscreen’s ability to remain effective under adverse conditions such as exposure to water and sweat. A water-resistant product maintains the indicated protection after 40 minutes of water immersion, whereas a very-water-resistant (formerly called “waterproof”) product maintains the indicated protection after 80 minutes of water immersion.27,28,32,33
Stability
The stability of the sunscreen is important for long-lasting protection with continuous exposure to UV light, in particular to prevent photodegradation. The FDA has established maximum levels of each filter allowed in the sunscreen. Several filters can be combined to achieve a high SPF level, to provide broadspectrum UV-A and UV-B protection, and to prevent photodegradation. For example, octocrylene prevents the degradation of the photosensitive compound avobenzone, whereas ecamsule has been combined with avobenzone and octocrylene to provide broad-spectrum UV-A and UV-B protection. Ecamsule is currently patent-protected by L’Oreal and is found only in products produced by it and its subsidiaries.
SUNSCREEN USES AND ABUSES
Sunscreen use generally falls into three categories: daily use, short-term use (eg, for an activity involving increased sun exposure, such as outdoor exercise or work), and use for preventing sunburn during tan acquisition, ie, to increase the time of UV radiation exposure.
Most published studies report on the effects of daily sunscreen protection or on cutaneous immune responses to sunscreen use. However, the use of sunscreens to enhance tan acquisition and to increase sun exposure duration is an abuse of the product and can actually increase the risk of skin cancer. A common misperception is that sunscreens decrease the risk of burning and allow people to increase their exposure to UV radiation. This results in increased exposure to UV-A and thus increases the risk of skin cancers and facilitates photoaging.34
In 2003, Baron et al35 published a randomized trial evaluating the protective effects of UV-B sunscreens (SPF 15) and UV-A/UV-B sunscreens (SPF 15) against UV radiation, using contact hypersensitivity as a model for immunosuppression. The study involved 211 volunteers ages 18 to 59. Measuring skinfold thickness vs total UV dose to calculate an immune protection factor, they reported that the UV-A/UV-B sunscreens had a greater average immune protection factor than the UV-B sunscreen. They concluded that though both types of sunscreen can protect against immunosuppression, the addition of a UV-A filter provides greater protection against immunosuppression.35
A French study36 in 104 volunteers examined the immunoprotective effects of sunscreens with equal SPF but differing levels of UV-A protection after UV exposure, and used delayed-type hypersensitivity as a model for cutaneous immune response. Broader UV-A protection yielded smaller reductions in delayed-type hypersensitivity after UV exposure, leading to the conclusion that UV-A contributes greatly to cutaneous immunosuppression and that UV-A filters can mitigate some of these effects.36
Sunscreens and photoaging
Only a few clinical studies have examined the effects of sunscreen use on photoaging.
In 1995, a randomized, double-blind, placebo-controlled trial involving 53 adults with previously diagnosed with actinic keratosis or skin cancer, or both, showed that those who applied a UV-A/UV-B sunscreen over a 24-month period had less solar elastosis on biopsy compared with controls.37
In 2008, a French study of 12 volunteers showed that broad-spectrum UV protection prevented histologic changes attributed to 6 weeks of chronic UV exposure. The control group exhibited structural and molecular evidence of UV damage (eg, epidermal thickening, decreased procollagen expression, higher lysozyme-to-elastin ratio), whereas chronic use of a broad-spectrum sunscreen either minimized or abrogated these findings.12
Evidence also suggests that broad-spectrum sunscreens can prevent damage from suberythemal doses of UV. A study published in 200738 investigated whether broad-spectrum sunscreen use affects the development of genetic and cellular markers of UV damage after daily suberythemal UV exposure. It reported that unprotected individuals exhibited more thymine dimers, higher p53 expression, and loss of Langerhans cells compared with protected individuals.38
Similarly, a study published in 201012 assessed cellular and molecular markers of photodamage after 19 daily suberythemal UV exposures with or without a broad-spectrum, low-SPF (SPF 8) sunscreen and found that consistent sunscreen use resulted in fewer p53-positive cells, a lower lysozyme-to-elastin ratio, a decreased number and size of melanocytes, and an increased number of Langerhans cells.
Thus, evidence supports the idea that consistent use of a broad-spectrum sunscreen can protect against photodamage, even at doses that do not cause erythema.12
Sunscreens and squamous cell carcinoma
Several large trials provide appreciable evidence that sunscreen is effective in preventing squamous cell carcinoma.
A randomized, controlled, 7-month trial in Australia of a broad-spectrum sunscreen with an SPF of 17 noted a dose-dependent reduction in the development of new actinic keratosis.39 Another randomized, controlled trial from Australia showed a 40% reduction in the development of squamous cell carcinoma over a 4.5-year period in participants who applied a broad-spectrum SPF-16 sunscreen 3 to 4 days per week vs discretionary use.40 Follow-up data at 8 years showed that daily sunscreen users continued to have a 40% lower incidence rate of squamous cell carcinoma than controls.41
Sunscreens and basal cell carcinoma
Although sunscreens appear to be effective in preventing actinic keratosis and squamous cell carcinoma, the evidence that they also prevent basal cell carcinoma and melanoma has been inconclusive.
Sunscreens and melanoma
Using a high number of nevi as a surrogate measure of the risk of developing melanoma, a randomized controlled trial of a broad-spectrum SPF-30 sunscreen in Canadian children over a 3-year period showed a slight decrease in the number of new nevi compared with controls. However, this effect was seen only in children with freckles.42
In a large European study of white school-age children, sunscreen use was associated with an increased number of nevi compared with the use of clothing, which prevented new nevi.43
A large meta-analysis of 18 case-controlled studies failed to show a protective association of sunscreen use with melanoma.44 Postulated confounding factors in earlier studies included older sunscreen formulations with no UV-A protection, low SPF, and limited substantivity. In many cases, sunscreen users exposed themselves to higher doses of UV because of the perceived decreased risk of burning with sunscreen use. This is especially the case when sun exposure was intentional to acquire a tan.34 Individuals who burn easily or may have had a family history of melanoma tended to use more sunscreen, thus creating another confounder. Finally, extrapolation of results from data performed in different geographic latitudes may not be appropriate.
Recently, Green et al45 published a study using the same cohort from a previous study of sunscreens and nonmelanoma skin cancer to examine new primary melanomas as a secondary outcome. They reported that, during the 5-year trial period and during the 10-year follow-up, fewer participants in the intervention group developed primary melanoma compared with the control group (11 vs 21). They concluded that regular applications of a broad-spectrum SPF-16 sunscreen in white adults ages 25 to 75 can decrease the incidence of melanoma.45 The study had serious limitations: the authors admitted that the results were marginally statistically significant; intervention sites of sunscreen application were chosen for nonmelanoma skin cancer and excluded the trunk and lower extremities, where melanomas often occur; and the entire body was analyzed for melanomas, not just the intervention site.46 Thus, despite providing some of the first evidence supporting sunscreen’s ability to prevent melanoma, these results are controversial and are by no means conclusive.
HOW TO USE SUNSCREEN
The American Academy of Dermatology guidelines47 recommend daily, year-round use of a broad-spectrum, water-resistant sunscreen with an SPF of at least 30, regardless of age or skin type. Cloud cover and windows block UV-B but not UV-A. Additionally, 80% of UV light can pass through cloud cover, while 25% is reflected by sand and 80% by snow. Thus, sunscreen should be used daily throughout the year.
Sunscreen should be applied to exposed dry skin 15 to 30 minutes before sun exposure, paying particular attention to common areas of nonmelanoma skin cancer, such as the face, ears, hands, arms, and lips. The standard amount of sunscreen used in SPF testing is 2 mg/cm2, which is difficult to translate into real use; most people apply only 25% to 50% of the recommended amount of sunscreen.48 According to the guidelines, 1 oz of sunscreen—2 tablespoons, or enough to fill a shot glass—is enough to cover sun-exposed parts of the adult body. Sunscreen should be reapplied every 2 hours or after swimming or heavy perspiration; many water-resistant sunscreens lose effectiveness after 40 minutes in the water.
Despite the protective effects of sunscreen, the following are still recommended:
- Seek shade or avoid exposure between 10:00 am and 4:00 pm, ie, when the sun’s rays are strongest
- Take caution around water, sand, and snow, which reflect UV radiation
- Wear protective clothing such as long-sleeved shirts, pants, sunglasses, and wide-brimmed hats
- Do not use tanning beds
- Do not use sunscreens to increase the time of UV exposure.
SPECIAL CONSIDERATIONS: INFANTS
Infants and toddlers are at higher risk of UV damage and skin cancer. Structurally, children’s skin is thinner than that of adults and has lower melanin concentrations. Thus, UV penetrates more deeply into skin that is less able to absorb UV radiation. Animal studies suggest that the skin of children, especially infants, is immunologically immature and less able to respond to UV damage than adult skin. Therefore, extra care must be taken to protect children from UV exposure.49
The American Academy of Pediatrics recommends that infants under 6 months of age should be kept out of direct sunlight whenever possible. A broad-spectrum, water-resistant sunscreen with an SPF of at least 30 should be applied to skin that is not protected by clothing or shade (eg, face, hands, neck).50
NEW FDA GUIDELINES AND OTHER PROPOSED CHANGES
The FDA’s SPF labeling requirements remained unchanged; however, the FDA instituted new regulations regarding UV-A protection. Sunscreens that qualify as broad-spectrum are to be labeled as such, indicating that they protect against radiation in the entire UV spectrum. Products that are “broad-spectrum SPF ≥ 15” can now include the following statement in the “drug facts” part of the label: “If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.”
The FDA now requires sunscreens that are not broad-spectrum or that have an SPF less than 15 to include the following alert: “Spending time in the sun increases your risk of skin cancer and early skin aging.”33 These products can only claim protection from sunburn with the statement: “This product has been shown only to prevent sunburn, not skin cancer or early skin aging.”27,28,32,33
In terms of water resistance, the FDA now bans the terms “sunblock,” “waterproof,” or “sweatproof,” as these claims cannot be substantiated. Instead, the label on the front of the package can only read either “water resistant (40 minutes)” or “water resistant (80 minutes).” Also, sunscreens may no longer claim to provide “instant protection,” nor can they claim to maintain efficacy for more than 2 hours without reapplication.27,28,32,33
Some sunscreen products have been labeled with SPF values exceeding 100. The FDA decided that because there is insufficient evidence of clinical benefit for such SPFs, sunscreen product labels may claim a maximum SPF value of “50+.”28,52
The FDA now also specifies approved formulations for sunscreen products. Oils, lotions, creams, gels, butters, pastes, and ointments are acceptable, and this applies to all products that contain sunscreens, including cosmetics. Wipes, towelettes, powders, body washes, and shampoos are not acceptable as sunscreen products. The FDA now considers the popular spray form as potentially acceptable; a final decision awaits the results of further testing.28,53
Editor’s note: As this paper was being sent to press, the US Food and Drug Administration announced that sunscreen manufacturers would have an additional 6 months to comply with the new labeling rules for sunscreens. The new deadline is December 2012. Smaller companies have until December 2013 to implement the labeling changes.
- Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol 2005; 52:937–958.
- Sivamani RK, Ghiya M, Maibach HI. Shedding light on sunscreens and their labels: testing policies need to match actual use. Am J Prev Med 2010; 38:679–681.
- Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res 2011; 24:136–147.
- American Cancer Society. What are the key statistics about basal and squamous cell skin cancers? http://www.cancer.org/Cancer/SkinCancer-BasalandSquamousCell/DetailedGuide/skin-cancer-basal-and-squamous-cell-key-statistics. Accessed May 9, 2012.
- American Cancer Society. What are the key statistics about melanoma? http://www.cancer.org/Cancer/SkinCancer-Melanoma/DetailedGuide/melanoma-skin-cancer-key-statistics. Accessed May 9, 2012.
- Jou PC, McCormick TS, Baron ED. UV immunosuppression and cutaneous malignancies. Expert Rev Dermatol 2011; 6:61–74.
- Wang Y, Digiovanna JJ, Stern JB, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A 2009; 106:6279–6284.
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol 2005; 152:115–121.
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol 2006; 55:1–19.
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol 2008; 128:447–454.
- Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochem Photobiol 1998; 68:63–70.
- Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol 2008; 58(5 suppl 2):S160–166.
- Besaratinia A, Synold TW, Chen HH, et al. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci U S A 2005; 102:10058–10063.
- May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 1999; 18:7621–7636.
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA 2006; 103:13765–13770.
- Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res 2008; 21:487–491.
- Miyamura Y, Coelho SG, Wolber R, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res 2007; 20:2–13.
- Kwon HT, Mayer JA, Walker KK, Yu H, Lewis EC, Belch GE. Promotion of frequent tanning sessions by indoor tanning facilities: two studies. J Am Acad Dermatol 2002; 46:700–705.
- Dellavalle RP, Parker ER, Cersonsky N, et al. Youth access laws: in the dark at the tanning parlor? Arch Dermatol 2003; 139:443–448.
- Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol 2001; 44:775–780.
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J Am Acad Dermatol 1998; 38:89–98.
- IBISWorld. Tanning salons in the US: Market research report NAICS 81219c. www.ibisworld.com. Accesssed May 9, 2012.
- American Academy of Dermatology Tanning Website. Stats and facts. Prevention and care. Indoor tanning. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/indoor-tanning. Accessed May 9, 2012.
- Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 2007; 370:528–537.
- Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photodermatol Photoimmunol Photomed 2011 Apr; 27( 2):58–67.
- US Food and Drug Administration (FDA). CFR - Code of Federal Regulations Title 21, Chapter 1, Part 352: Sunscreen drug products for over-the-counter human use. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352. Accessed May 9, 2012.
- Wang SQ, Lim HW. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration’s final rule on labeling and effectiveness testing. J Am Acad Dermatol 2011; 65:863–869.
- Food and Drug Administration (FDA). Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use (final rule). Federal Register 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf. Accessed May 9, 2012.
- Scherschun L, Lim HW. Photoprotection by sunscreens. Am J Clin Dermatol 2001; 2:131–134.
- US Food and Drug Administration (FDA). Sunburn protection factor (SPF). http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106351.htm. Accessed May 9, 2012.
- DeSimone EM. FDA proposes changes in sunscreen regulations. Am Pharm 1994; NS34:26–31.
- US Food and Drug Administration (FDA). Questions and answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the US (updated 6/23/11). http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicine-Safely/UnderstandingOver-the-CounterMedicines/ucm258468.htm. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). FDA Press Release. FDA announces changes to better inform consumers about sunscreen: new rules give consumers more information to help reduce the risk of skin cancer, early aging. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258940.htm. Accessed May 9, 2012.
- Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol 2009; 161(suppl 3):40–45.
- Baron ED, Fourtanier A, Compan D, Medaisko C, Cooper KD, Stevens SR. High ultraviolet A protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans. J Invest Dermatol 2003; 121:869–875.
- Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol 2008; 58(suppl 2):S149–S154.
- Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol 1995; 33:941–946.
- Young AR, Orchard GE, Harrison GI, Klock JL. The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen. J Invest Dermatol 2007; 127:975–978.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993; 329:1147–1151.
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–729.
- van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–2548.
- Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA 2000; 283:2955–2960.
- Autier P, Doré JF, Cattaruzza MS, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst 1998; 90:1873–1880.
- Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med 2003; 139:966–978.
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257–263.
- Goldenhersh MA, Koslowsky M. Increased melanoma after regular sunscreen use? J Clin Oncol 2011; 29:e557–e558.
- American Academey of Dermatology Sunscreen Website. Stats and facts. Prevention and care. Sunscreens. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/sunscreens. Accessed May 9, 2012.
- Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol 2002; 138:1319–1325.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics 2011; 128:92–102.
- American Academy of Pediatrics. HealthyChildren. Safety & prevention: Sun safety. http://www.healthychildren.org/english/safety-prevention/at-play/pages/Sun-Safety.aspx. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). Information for consumers (drugs). Sunscreen. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm239463.htm. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Revised effectiveness determination; Sunscreen drug products for over-the-counter human use (proposed rule.) Federal Register 2011. http://69.175.53.6/register/2011/jun/17/2011-14769.pdf. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Sunscreen drug products for over-the-counter human use: Request for data and information regarding dosage forms (advance notice of proposed rulemaking), Federal Register 2011). http://69.175.53.6/register/2011/jun/17/2011-14768.pdf. Accessed May 9, 2012.
Everyone should avoid overexposure to the sun’s rays. But the desire for the “perfect tan,” the belief that a tan enables one to spend more time in the sun, and a lack of awareness about the dangers of ultraviolet (UV) radiation are factors that contribute to UV-induced skin damage and to an increased risk of skin cancer. Physicians need to be prepared to counsel patients on why and how to avoid damaging UV radiation.
See the patient education handout
Some measures are straightforward, such as wearing protective clothing, limiting sun exposure during the peak daylight hours, and avoiding tanning booths. The issue of which sunscreen to use can be more difficult, given the quantity of sunscreen products and the confusing claims made on product labels.
In this article, we review UV radiation, the consequences of increased exposure to different parts of the UV spectrum, tanning, and the fundamentals of sunscreens. We also briefly review current guidelines from professional organizations and rulings on sunscreen products by the US Food and Drug Administration (FDA).
FACTORS AFFECTING UV EXPOSURE
UV radiation from the sun is strongest between 10:00 am and 4:00 pm at equatorial latitudes and during summer months.1 Certain wavelengths of UV radiation have long been known to contribute to skin cancer in humans: the wavelengths considered most damaging are those from 320 to 400 nm, referred to as UV-A, and from 290 to 320 nm, referred to as UV-B.1,2 The UV spectrum also includes UV-C and other subdivisions, but in this article we are mainly concerned with UV-A and UV-B. From 90% to 95% of UV radiation that reaches the earth’s surface is UV-A, and most of the rest is UV-B.
The different wavelengths of UV-A and UV-B have different effects on the skin. Much of the shorter-wavelength UV-B radiation is scattered by the atmospheric ozone layer, by clouds, by air pollution, and by glass; on the other hand, UV-B rays are the main cause of sunburn in humans. The longer-wavelength UV-A radiation penetrates more deeply into the skin and so may have greater destructive potential.1,3
The daily UV index
The daily UV index of the US National Weather Service and the US Environmental Protection Agency (EPA) (www.epa.gov/sunwise/uvindex.html) offers a direct measurement of the level of UV radiation on a scale of 1 (low) to 11+ (extremely high). The higher the number, the greater the risk of sunburn for a fair-skinned person, even after allowing for cloud cover.
UV EXPOSURE RISKS ARE WELL KNOWN
The American Cancer Society has estimated that the annual incidence of nonmelanoma skin cancer is greater than 2 million, and the incidence of melanoma is from 65,000 to 70,000.4 The incidence of all types of skin cancer has been increasing for the last 30 years.4,5
Exposure to UV radiation is the major environmental risk factor for nonmelanoma skin cancer.6 It is also believed to be a major risk factor for melanoma; although definitive evidence is still lacking, research is beginning to uncover mechanisms linking UV-related gene damage to melanoma.7
UV LIGHT’S EFFECTS ON THE SKIN
The effects of UV light on the skin can be immediate (eg, erythema) and long-term (eg, photoaging, immunosuppression, carcinogenicity).1
Sunburn
Excessive UV damage creates a biochemical milieu that manifests grossly on the skin as a “sunburn.” Excessive UV exposure is damaging regardless of whether a sunburn occurs. Intensive intermittent UV exposure in childhood and teen years leading to blistering sunburn is a risk factor for basal cell carcinoma and malignant melanoma, whereas excessive chronic cumulative exposure is a risk factor for squamous cell carcinoma. In addition, both types of exposure can lead to photoaging.
Sunburn is noticeable 3 to 4 hours after exposure, peaking at around 24 hours.
Photoaging
A long-term effect of UV exposure is photoaging. Although how photoaging occurs is unclear, studies suggest that UV-A contributes more to photoaging, while UV-B contributes to burning, which results in extracellular matrix degradation and dysregulation of collagen metabolism. These changes in matrix and collagen may cause wrinkles and loss of skin turgor; increases in vascular growth factors may induce telangiectasia. All of these effects are characteristic of photoaging.8,9
Immunosuppression, sun exposure, cancer
Profound systemic immunosuppression, such as in organ transplantation patients, can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
But sun exposure itself can also cause both local and systemic immunosuppression depending on the area of exposure and the dosage of UV radiation. The immunosuppressive and carcinogenic effects of UV light on the skin are complex, involving a variety of cell types, including antigen-presenting cells, lymphocytes, and cytokines. UV radiation can cause dysregulation of antigen-presenting cells such as Langerhans cells and dermal dendritic cells, which in turn can activate regulatory T cells to suppress the immune system. UV radiation can also induce keratinocytes to produce immunosuppressive cytokines that inhibit the production of a number of “repair cytokines” that fix UV-induced DNA damage. The repair cytokines can mitigate UV-induced immunosuppression.6,11 These effects can suppress the induction of local, systemic, and memory immunity.
Both UV-A and UV-B interact to enhance UV-induced immunosuppression, and this can occur even at doses that do not cause erythema.12 Profound immunosuppression—whether UV-induced or due to HIV infection or immunosuppressive drugs—can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
Animal studies linking UV-B exposure to skin cancer found that UV-B energy is directly absorbed by DNA, resulting in the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone photoproducts in the DNA, which block replication and transcription.6 The resulting mutations specifically occur in the tumor suppressor gene p53, and these mutations have been linked to squamous cell carcinoma.13,14
UV-A light has also been reported to induce cyclobutane dimers, but via an indirect mechanism, since DNA does not directly absorb UV-A. Dimers induced by UV-A light are apparently cleared at a slower rate than those induced by UV-B, suggesting that UV-A may have a greater potential for carcinogenesis.15 UV-A light can also directly induce carcinogenesis through reactive oxygen species that cause tumorogenic modified bases in the DNA. These modified bases can be misread, leading to decreased DNA integrity.6
WHAT IS TANNING?
UV radiation produces darkening of the skin, or tanning. UV exposure results in both immediate and persistent pigment darkening. Immediate pigment darkening, which is visible and transient, occurs within seconds of UV exposure as a result of the formation of reactive oxygen species and photooxidation of preexisting melanin, and it resolves in a couple of hours. Persistent pigment darkening results from photooxidation and redistribution of preexisting melanin, occurring 2 to 24 hours after sun exposure. Neither type of pigment darkening protects the skin, since no new melanin is produced.16,17
UV-B rays can induce skin erythema, edema, and sunburn, followed by skin desquamation and tanning. Its effects can be seen immediately, but typically the erythema reaches its peak 24 hours later.1
“Delayed tanning” is an adaptive response seen about 3 days after sun exposure and is caused by increased melanocyte activity and new melanin formation in response to UV-B; this effect is considered mildly photoprotective, with a sun protection factor (SPF) of 3. In other words, there is a tiny bit of truth to the common belief that a tan that develops a few days after sun exposure (delayed tanning) can provide a small increase in protection from sunburn. However, the real health concern is not only sunburn, but increased cancer risk and photoaging from UV exposure.
INDOOR TANNING
Every year, nearly 28 million Americans use a sunbed or a sunlamp, and 2.3 million of them are teenagers.18,19 Every day in the United States more than 1 million people use an indoor tanning device.20 Nearly 70% of those who use tanning devices are white women ages 16 to 29.21
Tanning is big business. In 2010, there were 20,000 tanning salons in the United States, and the number of health clubs and spas with tanning beds was between 15,000 and 20,000. In 2010, the tanning industry generated an estimated $4.7 billion in revenue.22
In their search for the perfect tan, people receive very large doses of UV light, and most tanning lamps emit 95% to 99% of their light as UV-A. In fact, the typical sunlamp user can receive an annual dose of UV-A that is 0.3 to 1.2 times the average annual cumulative dose received from sun exposure (7,700 kJ/m2).11 A typical customer of a tanning salon in the course of 20 sessions is exposed to up to 1.2 times the average normal annual exposure from sunlight. Also, for a frequent tanner, the exposure can increase to 4.7 times the average normal annual exposure and up to 12 times the exposure if using high-pressure sunlamps.11 Indoor tanners not only receive large doses of a known carcinogen, but the body’s pigmentary responses to a sunlamp’s UV-A (immediate and persistent pigment darkening) do not protect it from sunburn, cancer-inducing DNA damage, immunosuppression, or photoaging.
Additionally, even though tanning bed lamps only emit 1% to 5% of their light in the UV-B spectrum, one can still receive a very large dose of UV-B radiation with enough exposure.
The American Academy of Dermatology opposes indoor tanning and supports a ban on the nonmedical production and sale of indoor tanning devices. The World Health Organization classifies tanning lamps as carcinogenic and advises minors to avoid indoor tanning.23
SUNSCREEN PROTECTION
Sunscreen products must contain an active sunscreen ingredient that absorbs radiation in the range of 290 to 400 nm. In “physical” sunscreens, the ingredient is an inorganic compound with particles that physically block out UV radiation; in “chemical” sunscreens, the ingredient is an organic compound that absorbs UV radiation.
Most organic UV filters absorb UV-B radiation, and a few act in the UV-A2 range (320–340 nm). Only one FDA-approved organic sunscreen, avobenzone, protects against UV-A1 (340–400 nm).
Inorganic compounds function by physically reflecting and scattering UV radiation from a film of inert metal particles, ie, in a manner similar to protective clothing.24 Two FDA-approved inorganic sunscreens—titanium dioxide and zinc oxide—provide UV-A and UV-B protection. Zinc oxide and the non-micronized form of titanium dioxide provide UV-A1 and UV-A2 protection.
Inorganic sunscreens have a thick consistency and tend to clump. Advances in nanoparticle technology have improved their consistency,25 but micronized titanium dioxide does not provide UV-A1 protection.
The FDA regulates the active ingredients in sunscreen products, determines the methods of testing them, and dictates labelling requirements.
CATEGORIES OF SUNSCREENS
Sunscreens are categorized according to their SPF,26 UV-A protection,27,28 substantivity, and stability.29
Understanding the ‘sun protection factor’
SPF is a laboratory measure of sunscreen efficacy and is defined as the amount of UV radiation required to produce a sunburn on protected skin relative to that of unprotected skin. Since SPF assessment is based on erythema, it is mainly a measure of UV-B exposure, not UV-A exposure.
Contrary to popular belief, the SPF of a product is not related to the duration of UV exposure.30 Also, the relationship between SPF and UV-B protection is not linear: a sunscreen with an SPF of 15 can filter 94% of UV-B radiation, whereas an SPF of 30 provides greater than 97% protection at an equal UV-B dosage. UV radiation dosage depends on both the duration of exposure and the intensity of the UV radiation. Thus, a sunscreen with twice the SPF does not necessarily mean one can stay out in the sun twice as long before developing a sunburn.
Ability to block UV-A radiation
As UV-A causes significant immunosuppression and is the major type of UV radiation reaching Earth, a systematic and repeatable method of measuring a sunscreen’s ability to block UV-A light is necessary.
For each sunscreen, laboratory testing generates a curve of the absorbance within the UV spectrum. The area under this curve is calculated, and a “critical wavelength” is defined as the wavelength where the area under the absorbance curve up to that value is 90% of the total area under the curve. A sunscreen with “broad-spectrum” UV-A protection is one for which the critical wavelength is greater than or equal to 370 nm. The critical wavelength measures the breadth of UV-A absorbance by a sunscreen and must be used in combination with the SPF value to provide a complete assessment of UV protection.27,28,32,33
Substantivity
Substantivity is a sunscreen’s ability to remain effective under adverse conditions such as exposure to water and sweat. A water-resistant product maintains the indicated protection after 40 minutes of water immersion, whereas a very-water-resistant (formerly called “waterproof”) product maintains the indicated protection after 80 minutes of water immersion.27,28,32,33
Stability
The stability of the sunscreen is important for long-lasting protection with continuous exposure to UV light, in particular to prevent photodegradation. The FDA has established maximum levels of each filter allowed in the sunscreen. Several filters can be combined to achieve a high SPF level, to provide broadspectrum UV-A and UV-B protection, and to prevent photodegradation. For example, octocrylene prevents the degradation of the photosensitive compound avobenzone, whereas ecamsule has been combined with avobenzone and octocrylene to provide broad-spectrum UV-A and UV-B protection. Ecamsule is currently patent-protected by L’Oreal and is found only in products produced by it and its subsidiaries.
SUNSCREEN USES AND ABUSES
Sunscreen use generally falls into three categories: daily use, short-term use (eg, for an activity involving increased sun exposure, such as outdoor exercise or work), and use for preventing sunburn during tan acquisition, ie, to increase the time of UV radiation exposure.
Most published studies report on the effects of daily sunscreen protection or on cutaneous immune responses to sunscreen use. However, the use of sunscreens to enhance tan acquisition and to increase sun exposure duration is an abuse of the product and can actually increase the risk of skin cancer. A common misperception is that sunscreens decrease the risk of burning and allow people to increase their exposure to UV radiation. This results in increased exposure to UV-A and thus increases the risk of skin cancers and facilitates photoaging.34
In 2003, Baron et al35 published a randomized trial evaluating the protective effects of UV-B sunscreens (SPF 15) and UV-A/UV-B sunscreens (SPF 15) against UV radiation, using contact hypersensitivity as a model for immunosuppression. The study involved 211 volunteers ages 18 to 59. Measuring skinfold thickness vs total UV dose to calculate an immune protection factor, they reported that the UV-A/UV-B sunscreens had a greater average immune protection factor than the UV-B sunscreen. They concluded that though both types of sunscreen can protect against immunosuppression, the addition of a UV-A filter provides greater protection against immunosuppression.35
A French study36 in 104 volunteers examined the immunoprotective effects of sunscreens with equal SPF but differing levels of UV-A protection after UV exposure, and used delayed-type hypersensitivity as a model for cutaneous immune response. Broader UV-A protection yielded smaller reductions in delayed-type hypersensitivity after UV exposure, leading to the conclusion that UV-A contributes greatly to cutaneous immunosuppression and that UV-A filters can mitigate some of these effects.36
Sunscreens and photoaging
Only a few clinical studies have examined the effects of sunscreen use on photoaging.
In 1995, a randomized, double-blind, placebo-controlled trial involving 53 adults with previously diagnosed with actinic keratosis or skin cancer, or both, showed that those who applied a UV-A/UV-B sunscreen over a 24-month period had less solar elastosis on biopsy compared with controls.37
In 2008, a French study of 12 volunteers showed that broad-spectrum UV protection prevented histologic changes attributed to 6 weeks of chronic UV exposure. The control group exhibited structural and molecular evidence of UV damage (eg, epidermal thickening, decreased procollagen expression, higher lysozyme-to-elastin ratio), whereas chronic use of a broad-spectrum sunscreen either minimized or abrogated these findings.12
Evidence also suggests that broad-spectrum sunscreens can prevent damage from suberythemal doses of UV. A study published in 200738 investigated whether broad-spectrum sunscreen use affects the development of genetic and cellular markers of UV damage after daily suberythemal UV exposure. It reported that unprotected individuals exhibited more thymine dimers, higher p53 expression, and loss of Langerhans cells compared with protected individuals.38
Similarly, a study published in 201012 assessed cellular and molecular markers of photodamage after 19 daily suberythemal UV exposures with or without a broad-spectrum, low-SPF (SPF 8) sunscreen and found that consistent sunscreen use resulted in fewer p53-positive cells, a lower lysozyme-to-elastin ratio, a decreased number and size of melanocytes, and an increased number of Langerhans cells.
Thus, evidence supports the idea that consistent use of a broad-spectrum sunscreen can protect against photodamage, even at doses that do not cause erythema.12
Sunscreens and squamous cell carcinoma
Several large trials provide appreciable evidence that sunscreen is effective in preventing squamous cell carcinoma.
A randomized, controlled, 7-month trial in Australia of a broad-spectrum sunscreen with an SPF of 17 noted a dose-dependent reduction in the development of new actinic keratosis.39 Another randomized, controlled trial from Australia showed a 40% reduction in the development of squamous cell carcinoma over a 4.5-year period in participants who applied a broad-spectrum SPF-16 sunscreen 3 to 4 days per week vs discretionary use.40 Follow-up data at 8 years showed that daily sunscreen users continued to have a 40% lower incidence rate of squamous cell carcinoma than controls.41
Sunscreens and basal cell carcinoma
Although sunscreens appear to be effective in preventing actinic keratosis and squamous cell carcinoma, the evidence that they also prevent basal cell carcinoma and melanoma has been inconclusive.
Sunscreens and melanoma
Using a high number of nevi as a surrogate measure of the risk of developing melanoma, a randomized controlled trial of a broad-spectrum SPF-30 sunscreen in Canadian children over a 3-year period showed a slight decrease in the number of new nevi compared with controls. However, this effect was seen only in children with freckles.42
In a large European study of white school-age children, sunscreen use was associated with an increased number of nevi compared with the use of clothing, which prevented new nevi.43
A large meta-analysis of 18 case-controlled studies failed to show a protective association of sunscreen use with melanoma.44 Postulated confounding factors in earlier studies included older sunscreen formulations with no UV-A protection, low SPF, and limited substantivity. In many cases, sunscreen users exposed themselves to higher doses of UV because of the perceived decreased risk of burning with sunscreen use. This is especially the case when sun exposure was intentional to acquire a tan.34 Individuals who burn easily or may have had a family history of melanoma tended to use more sunscreen, thus creating another confounder. Finally, extrapolation of results from data performed in different geographic latitudes may not be appropriate.
Recently, Green et al45 published a study using the same cohort from a previous study of sunscreens and nonmelanoma skin cancer to examine new primary melanomas as a secondary outcome. They reported that, during the 5-year trial period and during the 10-year follow-up, fewer participants in the intervention group developed primary melanoma compared with the control group (11 vs 21). They concluded that regular applications of a broad-spectrum SPF-16 sunscreen in white adults ages 25 to 75 can decrease the incidence of melanoma.45 The study had serious limitations: the authors admitted that the results were marginally statistically significant; intervention sites of sunscreen application were chosen for nonmelanoma skin cancer and excluded the trunk and lower extremities, where melanomas often occur; and the entire body was analyzed for melanomas, not just the intervention site.46 Thus, despite providing some of the first evidence supporting sunscreen’s ability to prevent melanoma, these results are controversial and are by no means conclusive.
HOW TO USE SUNSCREEN
The American Academy of Dermatology guidelines47 recommend daily, year-round use of a broad-spectrum, water-resistant sunscreen with an SPF of at least 30, regardless of age or skin type. Cloud cover and windows block UV-B but not UV-A. Additionally, 80% of UV light can pass through cloud cover, while 25% is reflected by sand and 80% by snow. Thus, sunscreen should be used daily throughout the year.
Sunscreen should be applied to exposed dry skin 15 to 30 minutes before sun exposure, paying particular attention to common areas of nonmelanoma skin cancer, such as the face, ears, hands, arms, and lips. The standard amount of sunscreen used in SPF testing is 2 mg/cm2, which is difficult to translate into real use; most people apply only 25% to 50% of the recommended amount of sunscreen.48 According to the guidelines, 1 oz of sunscreen—2 tablespoons, or enough to fill a shot glass—is enough to cover sun-exposed parts of the adult body. Sunscreen should be reapplied every 2 hours or after swimming or heavy perspiration; many water-resistant sunscreens lose effectiveness after 40 minutes in the water.
Despite the protective effects of sunscreen, the following are still recommended:
- Seek shade or avoid exposure between 10:00 am and 4:00 pm, ie, when the sun’s rays are strongest
- Take caution around water, sand, and snow, which reflect UV radiation
- Wear protective clothing such as long-sleeved shirts, pants, sunglasses, and wide-brimmed hats
- Do not use tanning beds
- Do not use sunscreens to increase the time of UV exposure.
SPECIAL CONSIDERATIONS: INFANTS
Infants and toddlers are at higher risk of UV damage and skin cancer. Structurally, children’s skin is thinner than that of adults and has lower melanin concentrations. Thus, UV penetrates more deeply into skin that is less able to absorb UV radiation. Animal studies suggest that the skin of children, especially infants, is immunologically immature and less able to respond to UV damage than adult skin. Therefore, extra care must be taken to protect children from UV exposure.49
The American Academy of Pediatrics recommends that infants under 6 months of age should be kept out of direct sunlight whenever possible. A broad-spectrum, water-resistant sunscreen with an SPF of at least 30 should be applied to skin that is not protected by clothing or shade (eg, face, hands, neck).50
NEW FDA GUIDELINES AND OTHER PROPOSED CHANGES
The FDA’s SPF labeling requirements remained unchanged; however, the FDA instituted new regulations regarding UV-A protection. Sunscreens that qualify as broad-spectrum are to be labeled as such, indicating that they protect against radiation in the entire UV spectrum. Products that are “broad-spectrum SPF ≥ 15” can now include the following statement in the “drug facts” part of the label: “If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.”
The FDA now requires sunscreens that are not broad-spectrum or that have an SPF less than 15 to include the following alert: “Spending time in the sun increases your risk of skin cancer and early skin aging.”33 These products can only claim protection from sunburn with the statement: “This product has been shown only to prevent sunburn, not skin cancer or early skin aging.”27,28,32,33
In terms of water resistance, the FDA now bans the terms “sunblock,” “waterproof,” or “sweatproof,” as these claims cannot be substantiated. Instead, the label on the front of the package can only read either “water resistant (40 minutes)” or “water resistant (80 minutes).” Also, sunscreens may no longer claim to provide “instant protection,” nor can they claim to maintain efficacy for more than 2 hours without reapplication.27,28,32,33
Some sunscreen products have been labeled with SPF values exceeding 100. The FDA decided that because there is insufficient evidence of clinical benefit for such SPFs, sunscreen product labels may claim a maximum SPF value of “50+.”28,52
The FDA now also specifies approved formulations for sunscreen products. Oils, lotions, creams, gels, butters, pastes, and ointments are acceptable, and this applies to all products that contain sunscreens, including cosmetics. Wipes, towelettes, powders, body washes, and shampoos are not acceptable as sunscreen products. The FDA now considers the popular spray form as potentially acceptable; a final decision awaits the results of further testing.28,53
Editor’s note: As this paper was being sent to press, the US Food and Drug Administration announced that sunscreen manufacturers would have an additional 6 months to comply with the new labeling rules for sunscreens. The new deadline is December 2012. Smaller companies have until December 2013 to implement the labeling changes.
Everyone should avoid overexposure to the sun’s rays. But the desire for the “perfect tan,” the belief that a tan enables one to spend more time in the sun, and a lack of awareness about the dangers of ultraviolet (UV) radiation are factors that contribute to UV-induced skin damage and to an increased risk of skin cancer. Physicians need to be prepared to counsel patients on why and how to avoid damaging UV radiation.
See the patient education handout
Some measures are straightforward, such as wearing protective clothing, limiting sun exposure during the peak daylight hours, and avoiding tanning booths. The issue of which sunscreen to use can be more difficult, given the quantity of sunscreen products and the confusing claims made on product labels.
In this article, we review UV radiation, the consequences of increased exposure to different parts of the UV spectrum, tanning, and the fundamentals of sunscreens. We also briefly review current guidelines from professional organizations and rulings on sunscreen products by the US Food and Drug Administration (FDA).
FACTORS AFFECTING UV EXPOSURE
UV radiation from the sun is strongest between 10:00 am and 4:00 pm at equatorial latitudes and during summer months.1 Certain wavelengths of UV radiation have long been known to contribute to skin cancer in humans: the wavelengths considered most damaging are those from 320 to 400 nm, referred to as UV-A, and from 290 to 320 nm, referred to as UV-B.1,2 The UV spectrum also includes UV-C and other subdivisions, but in this article we are mainly concerned with UV-A and UV-B. From 90% to 95% of UV radiation that reaches the earth’s surface is UV-A, and most of the rest is UV-B.
The different wavelengths of UV-A and UV-B have different effects on the skin. Much of the shorter-wavelength UV-B radiation is scattered by the atmospheric ozone layer, by clouds, by air pollution, and by glass; on the other hand, UV-B rays are the main cause of sunburn in humans. The longer-wavelength UV-A radiation penetrates more deeply into the skin and so may have greater destructive potential.1,3
The daily UV index
The daily UV index of the US National Weather Service and the US Environmental Protection Agency (EPA) (www.epa.gov/sunwise/uvindex.html) offers a direct measurement of the level of UV radiation on a scale of 1 (low) to 11+ (extremely high). The higher the number, the greater the risk of sunburn for a fair-skinned person, even after allowing for cloud cover.
UV EXPOSURE RISKS ARE WELL KNOWN
The American Cancer Society has estimated that the annual incidence of nonmelanoma skin cancer is greater than 2 million, and the incidence of melanoma is from 65,000 to 70,000.4 The incidence of all types of skin cancer has been increasing for the last 30 years.4,5
Exposure to UV radiation is the major environmental risk factor for nonmelanoma skin cancer.6 It is also believed to be a major risk factor for melanoma; although definitive evidence is still lacking, research is beginning to uncover mechanisms linking UV-related gene damage to melanoma.7
UV LIGHT’S EFFECTS ON THE SKIN
The effects of UV light on the skin can be immediate (eg, erythema) and long-term (eg, photoaging, immunosuppression, carcinogenicity).1
Sunburn
Excessive UV damage creates a biochemical milieu that manifests grossly on the skin as a “sunburn.” Excessive UV exposure is damaging regardless of whether a sunburn occurs. Intensive intermittent UV exposure in childhood and teen years leading to blistering sunburn is a risk factor for basal cell carcinoma and malignant melanoma, whereas excessive chronic cumulative exposure is a risk factor for squamous cell carcinoma. In addition, both types of exposure can lead to photoaging.
Sunburn is noticeable 3 to 4 hours after exposure, peaking at around 24 hours.
Photoaging
A long-term effect of UV exposure is photoaging. Although how photoaging occurs is unclear, studies suggest that UV-A contributes more to photoaging, while UV-B contributes to burning, which results in extracellular matrix degradation and dysregulation of collagen metabolism. These changes in matrix and collagen may cause wrinkles and loss of skin turgor; increases in vascular growth factors may induce telangiectasia. All of these effects are characteristic of photoaging.8,9
Immunosuppression, sun exposure, cancer
Profound systemic immunosuppression, such as in organ transplantation patients, can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
But sun exposure itself can also cause both local and systemic immunosuppression depending on the area of exposure and the dosage of UV radiation. The immunosuppressive and carcinogenic effects of UV light on the skin are complex, involving a variety of cell types, including antigen-presenting cells, lymphocytes, and cytokines. UV radiation can cause dysregulation of antigen-presenting cells such as Langerhans cells and dermal dendritic cells, which in turn can activate regulatory T cells to suppress the immune system. UV radiation can also induce keratinocytes to produce immunosuppressive cytokines that inhibit the production of a number of “repair cytokines” that fix UV-induced DNA damage. The repair cytokines can mitigate UV-induced immunosuppression.6,11 These effects can suppress the induction of local, systemic, and memory immunity.
Both UV-A and UV-B interact to enhance UV-induced immunosuppression, and this can occur even at doses that do not cause erythema.12 Profound immunosuppression—whether UV-induced or due to HIV infection or immunosuppressive drugs—can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
Animal studies linking UV-B exposure to skin cancer found that UV-B energy is directly absorbed by DNA, resulting in the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone photoproducts in the DNA, which block replication and transcription.6 The resulting mutations specifically occur in the tumor suppressor gene p53, and these mutations have been linked to squamous cell carcinoma.13,14
UV-A light has also been reported to induce cyclobutane dimers, but via an indirect mechanism, since DNA does not directly absorb UV-A. Dimers induced by UV-A light are apparently cleared at a slower rate than those induced by UV-B, suggesting that UV-A may have a greater potential for carcinogenesis.15 UV-A light can also directly induce carcinogenesis through reactive oxygen species that cause tumorogenic modified bases in the DNA. These modified bases can be misread, leading to decreased DNA integrity.6
WHAT IS TANNING?
UV radiation produces darkening of the skin, or tanning. UV exposure results in both immediate and persistent pigment darkening. Immediate pigment darkening, which is visible and transient, occurs within seconds of UV exposure as a result of the formation of reactive oxygen species and photooxidation of preexisting melanin, and it resolves in a couple of hours. Persistent pigment darkening results from photooxidation and redistribution of preexisting melanin, occurring 2 to 24 hours after sun exposure. Neither type of pigment darkening protects the skin, since no new melanin is produced.16,17
UV-B rays can induce skin erythema, edema, and sunburn, followed by skin desquamation and tanning. Its effects can be seen immediately, but typically the erythema reaches its peak 24 hours later.1
“Delayed tanning” is an adaptive response seen about 3 days after sun exposure and is caused by increased melanocyte activity and new melanin formation in response to UV-B; this effect is considered mildly photoprotective, with a sun protection factor (SPF) of 3. In other words, there is a tiny bit of truth to the common belief that a tan that develops a few days after sun exposure (delayed tanning) can provide a small increase in protection from sunburn. However, the real health concern is not only sunburn, but increased cancer risk and photoaging from UV exposure.
INDOOR TANNING
Every year, nearly 28 million Americans use a sunbed or a sunlamp, and 2.3 million of them are teenagers.18,19 Every day in the United States more than 1 million people use an indoor tanning device.20 Nearly 70% of those who use tanning devices are white women ages 16 to 29.21
Tanning is big business. In 2010, there were 20,000 tanning salons in the United States, and the number of health clubs and spas with tanning beds was between 15,000 and 20,000. In 2010, the tanning industry generated an estimated $4.7 billion in revenue.22
In their search for the perfect tan, people receive very large doses of UV light, and most tanning lamps emit 95% to 99% of their light as UV-A. In fact, the typical sunlamp user can receive an annual dose of UV-A that is 0.3 to 1.2 times the average annual cumulative dose received from sun exposure (7,700 kJ/m2).11 A typical customer of a tanning salon in the course of 20 sessions is exposed to up to 1.2 times the average normal annual exposure from sunlight. Also, for a frequent tanner, the exposure can increase to 4.7 times the average normal annual exposure and up to 12 times the exposure if using high-pressure sunlamps.11 Indoor tanners not only receive large doses of a known carcinogen, but the body’s pigmentary responses to a sunlamp’s UV-A (immediate and persistent pigment darkening) do not protect it from sunburn, cancer-inducing DNA damage, immunosuppression, or photoaging.
Additionally, even though tanning bed lamps only emit 1% to 5% of their light in the UV-B spectrum, one can still receive a very large dose of UV-B radiation with enough exposure.
The American Academy of Dermatology opposes indoor tanning and supports a ban on the nonmedical production and sale of indoor tanning devices. The World Health Organization classifies tanning lamps as carcinogenic and advises minors to avoid indoor tanning.23
SUNSCREEN PROTECTION
Sunscreen products must contain an active sunscreen ingredient that absorbs radiation in the range of 290 to 400 nm. In “physical” sunscreens, the ingredient is an inorganic compound with particles that physically block out UV radiation; in “chemical” sunscreens, the ingredient is an organic compound that absorbs UV radiation.
Most organic UV filters absorb UV-B radiation, and a few act in the UV-A2 range (320–340 nm). Only one FDA-approved organic sunscreen, avobenzone, protects against UV-A1 (340–400 nm).
Inorganic compounds function by physically reflecting and scattering UV radiation from a film of inert metal particles, ie, in a manner similar to protective clothing.24 Two FDA-approved inorganic sunscreens—titanium dioxide and zinc oxide—provide UV-A and UV-B protection. Zinc oxide and the non-micronized form of titanium dioxide provide UV-A1 and UV-A2 protection.
Inorganic sunscreens have a thick consistency and tend to clump. Advances in nanoparticle technology have improved their consistency,25 but micronized titanium dioxide does not provide UV-A1 protection.
The FDA regulates the active ingredients in sunscreen products, determines the methods of testing them, and dictates labelling requirements.
CATEGORIES OF SUNSCREENS
Sunscreens are categorized according to their SPF,26 UV-A protection,27,28 substantivity, and stability.29
Understanding the ‘sun protection factor’
SPF is a laboratory measure of sunscreen efficacy and is defined as the amount of UV radiation required to produce a sunburn on protected skin relative to that of unprotected skin. Since SPF assessment is based on erythema, it is mainly a measure of UV-B exposure, not UV-A exposure.
Contrary to popular belief, the SPF of a product is not related to the duration of UV exposure.30 Also, the relationship between SPF and UV-B protection is not linear: a sunscreen with an SPF of 15 can filter 94% of UV-B radiation, whereas an SPF of 30 provides greater than 97% protection at an equal UV-B dosage. UV radiation dosage depends on both the duration of exposure and the intensity of the UV radiation. Thus, a sunscreen with twice the SPF does not necessarily mean one can stay out in the sun twice as long before developing a sunburn.
Ability to block UV-A radiation
As UV-A causes significant immunosuppression and is the major type of UV radiation reaching Earth, a systematic and repeatable method of measuring a sunscreen’s ability to block UV-A light is necessary.
For each sunscreen, laboratory testing generates a curve of the absorbance within the UV spectrum. The area under this curve is calculated, and a “critical wavelength” is defined as the wavelength where the area under the absorbance curve up to that value is 90% of the total area under the curve. A sunscreen with “broad-spectrum” UV-A protection is one for which the critical wavelength is greater than or equal to 370 nm. The critical wavelength measures the breadth of UV-A absorbance by a sunscreen and must be used in combination with the SPF value to provide a complete assessment of UV protection.27,28,32,33
Substantivity
Substantivity is a sunscreen’s ability to remain effective under adverse conditions such as exposure to water and sweat. A water-resistant product maintains the indicated protection after 40 minutes of water immersion, whereas a very-water-resistant (formerly called “waterproof”) product maintains the indicated protection after 80 minutes of water immersion.27,28,32,33
Stability
The stability of the sunscreen is important for long-lasting protection with continuous exposure to UV light, in particular to prevent photodegradation. The FDA has established maximum levels of each filter allowed in the sunscreen. Several filters can be combined to achieve a high SPF level, to provide broadspectrum UV-A and UV-B protection, and to prevent photodegradation. For example, octocrylene prevents the degradation of the photosensitive compound avobenzone, whereas ecamsule has been combined with avobenzone and octocrylene to provide broad-spectrum UV-A and UV-B protection. Ecamsule is currently patent-protected by L’Oreal and is found only in products produced by it and its subsidiaries.
SUNSCREEN USES AND ABUSES
Sunscreen use generally falls into three categories: daily use, short-term use (eg, for an activity involving increased sun exposure, such as outdoor exercise or work), and use for preventing sunburn during tan acquisition, ie, to increase the time of UV radiation exposure.
Most published studies report on the effects of daily sunscreen protection or on cutaneous immune responses to sunscreen use. However, the use of sunscreens to enhance tan acquisition and to increase sun exposure duration is an abuse of the product and can actually increase the risk of skin cancer. A common misperception is that sunscreens decrease the risk of burning and allow people to increase their exposure to UV radiation. This results in increased exposure to UV-A and thus increases the risk of skin cancers and facilitates photoaging.34
In 2003, Baron et al35 published a randomized trial evaluating the protective effects of UV-B sunscreens (SPF 15) and UV-A/UV-B sunscreens (SPF 15) against UV radiation, using contact hypersensitivity as a model for immunosuppression. The study involved 211 volunteers ages 18 to 59. Measuring skinfold thickness vs total UV dose to calculate an immune protection factor, they reported that the UV-A/UV-B sunscreens had a greater average immune protection factor than the UV-B sunscreen. They concluded that though both types of sunscreen can protect against immunosuppression, the addition of a UV-A filter provides greater protection against immunosuppression.35
A French study36 in 104 volunteers examined the immunoprotective effects of sunscreens with equal SPF but differing levels of UV-A protection after UV exposure, and used delayed-type hypersensitivity as a model for cutaneous immune response. Broader UV-A protection yielded smaller reductions in delayed-type hypersensitivity after UV exposure, leading to the conclusion that UV-A contributes greatly to cutaneous immunosuppression and that UV-A filters can mitigate some of these effects.36
Sunscreens and photoaging
Only a few clinical studies have examined the effects of sunscreen use on photoaging.
In 1995, a randomized, double-blind, placebo-controlled trial involving 53 adults with previously diagnosed with actinic keratosis or skin cancer, or both, showed that those who applied a UV-A/UV-B sunscreen over a 24-month period had less solar elastosis on biopsy compared with controls.37
In 2008, a French study of 12 volunteers showed that broad-spectrum UV protection prevented histologic changes attributed to 6 weeks of chronic UV exposure. The control group exhibited structural and molecular evidence of UV damage (eg, epidermal thickening, decreased procollagen expression, higher lysozyme-to-elastin ratio), whereas chronic use of a broad-spectrum sunscreen either minimized or abrogated these findings.12
Evidence also suggests that broad-spectrum sunscreens can prevent damage from suberythemal doses of UV. A study published in 200738 investigated whether broad-spectrum sunscreen use affects the development of genetic and cellular markers of UV damage after daily suberythemal UV exposure. It reported that unprotected individuals exhibited more thymine dimers, higher p53 expression, and loss of Langerhans cells compared with protected individuals.38
Similarly, a study published in 201012 assessed cellular and molecular markers of photodamage after 19 daily suberythemal UV exposures with or without a broad-spectrum, low-SPF (SPF 8) sunscreen and found that consistent sunscreen use resulted in fewer p53-positive cells, a lower lysozyme-to-elastin ratio, a decreased number and size of melanocytes, and an increased number of Langerhans cells.
Thus, evidence supports the idea that consistent use of a broad-spectrum sunscreen can protect against photodamage, even at doses that do not cause erythema.12
Sunscreens and squamous cell carcinoma
Several large trials provide appreciable evidence that sunscreen is effective in preventing squamous cell carcinoma.
A randomized, controlled, 7-month trial in Australia of a broad-spectrum sunscreen with an SPF of 17 noted a dose-dependent reduction in the development of new actinic keratosis.39 Another randomized, controlled trial from Australia showed a 40% reduction in the development of squamous cell carcinoma over a 4.5-year period in participants who applied a broad-spectrum SPF-16 sunscreen 3 to 4 days per week vs discretionary use.40 Follow-up data at 8 years showed that daily sunscreen users continued to have a 40% lower incidence rate of squamous cell carcinoma than controls.41
Sunscreens and basal cell carcinoma
Although sunscreens appear to be effective in preventing actinic keratosis and squamous cell carcinoma, the evidence that they also prevent basal cell carcinoma and melanoma has been inconclusive.
Sunscreens and melanoma
Using a high number of nevi as a surrogate measure of the risk of developing melanoma, a randomized controlled trial of a broad-spectrum SPF-30 sunscreen in Canadian children over a 3-year period showed a slight decrease in the number of new nevi compared with controls. However, this effect was seen only in children with freckles.42
In a large European study of white school-age children, sunscreen use was associated with an increased number of nevi compared with the use of clothing, which prevented new nevi.43
A large meta-analysis of 18 case-controlled studies failed to show a protective association of sunscreen use with melanoma.44 Postulated confounding factors in earlier studies included older sunscreen formulations with no UV-A protection, low SPF, and limited substantivity. In many cases, sunscreen users exposed themselves to higher doses of UV because of the perceived decreased risk of burning with sunscreen use. This is especially the case when sun exposure was intentional to acquire a tan.34 Individuals who burn easily or may have had a family history of melanoma tended to use more sunscreen, thus creating another confounder. Finally, extrapolation of results from data performed in different geographic latitudes may not be appropriate.
Recently, Green et al45 published a study using the same cohort from a previous study of sunscreens and nonmelanoma skin cancer to examine new primary melanomas as a secondary outcome. They reported that, during the 5-year trial period and during the 10-year follow-up, fewer participants in the intervention group developed primary melanoma compared with the control group (11 vs 21). They concluded that regular applications of a broad-spectrum SPF-16 sunscreen in white adults ages 25 to 75 can decrease the incidence of melanoma.45 The study had serious limitations: the authors admitted that the results were marginally statistically significant; intervention sites of sunscreen application were chosen for nonmelanoma skin cancer and excluded the trunk and lower extremities, where melanomas often occur; and the entire body was analyzed for melanomas, not just the intervention site.46 Thus, despite providing some of the first evidence supporting sunscreen’s ability to prevent melanoma, these results are controversial and are by no means conclusive.
HOW TO USE SUNSCREEN
The American Academy of Dermatology guidelines47 recommend daily, year-round use of a broad-spectrum, water-resistant sunscreen with an SPF of at least 30, regardless of age or skin type. Cloud cover and windows block UV-B but not UV-A. Additionally, 80% of UV light can pass through cloud cover, while 25% is reflected by sand and 80% by snow. Thus, sunscreen should be used daily throughout the year.
Sunscreen should be applied to exposed dry skin 15 to 30 minutes before sun exposure, paying particular attention to common areas of nonmelanoma skin cancer, such as the face, ears, hands, arms, and lips. The standard amount of sunscreen used in SPF testing is 2 mg/cm2, which is difficult to translate into real use; most people apply only 25% to 50% of the recommended amount of sunscreen.48 According to the guidelines, 1 oz of sunscreen—2 tablespoons, or enough to fill a shot glass—is enough to cover sun-exposed parts of the adult body. Sunscreen should be reapplied every 2 hours or after swimming or heavy perspiration; many water-resistant sunscreens lose effectiveness after 40 minutes in the water.
Despite the protective effects of sunscreen, the following are still recommended:
- Seek shade or avoid exposure between 10:00 am and 4:00 pm, ie, when the sun’s rays are strongest
- Take caution around water, sand, and snow, which reflect UV radiation
- Wear protective clothing such as long-sleeved shirts, pants, sunglasses, and wide-brimmed hats
- Do not use tanning beds
- Do not use sunscreens to increase the time of UV exposure.
SPECIAL CONSIDERATIONS: INFANTS
Infants and toddlers are at higher risk of UV damage and skin cancer. Structurally, children’s skin is thinner than that of adults and has lower melanin concentrations. Thus, UV penetrates more deeply into skin that is less able to absorb UV radiation. Animal studies suggest that the skin of children, especially infants, is immunologically immature and less able to respond to UV damage than adult skin. Therefore, extra care must be taken to protect children from UV exposure.49
The American Academy of Pediatrics recommends that infants under 6 months of age should be kept out of direct sunlight whenever possible. A broad-spectrum, water-resistant sunscreen with an SPF of at least 30 should be applied to skin that is not protected by clothing or shade (eg, face, hands, neck).50
NEW FDA GUIDELINES AND OTHER PROPOSED CHANGES
The FDA’s SPF labeling requirements remained unchanged; however, the FDA instituted new regulations regarding UV-A protection. Sunscreens that qualify as broad-spectrum are to be labeled as such, indicating that they protect against radiation in the entire UV spectrum. Products that are “broad-spectrum SPF ≥ 15” can now include the following statement in the “drug facts” part of the label: “If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.”
The FDA now requires sunscreens that are not broad-spectrum or that have an SPF less than 15 to include the following alert: “Spending time in the sun increases your risk of skin cancer and early skin aging.”33 These products can only claim protection from sunburn with the statement: “This product has been shown only to prevent sunburn, not skin cancer or early skin aging.”27,28,32,33
In terms of water resistance, the FDA now bans the terms “sunblock,” “waterproof,” or “sweatproof,” as these claims cannot be substantiated. Instead, the label on the front of the package can only read either “water resistant (40 minutes)” or “water resistant (80 minutes).” Also, sunscreens may no longer claim to provide “instant protection,” nor can they claim to maintain efficacy for more than 2 hours without reapplication.27,28,32,33
Some sunscreen products have been labeled with SPF values exceeding 100. The FDA decided that because there is insufficient evidence of clinical benefit for such SPFs, sunscreen product labels may claim a maximum SPF value of “50+.”28,52
The FDA now also specifies approved formulations for sunscreen products. Oils, lotions, creams, gels, butters, pastes, and ointments are acceptable, and this applies to all products that contain sunscreens, including cosmetics. Wipes, towelettes, powders, body washes, and shampoos are not acceptable as sunscreen products. The FDA now considers the popular spray form as potentially acceptable; a final decision awaits the results of further testing.28,53
Editor’s note: As this paper was being sent to press, the US Food and Drug Administration announced that sunscreen manufacturers would have an additional 6 months to comply with the new labeling rules for sunscreens. The new deadline is December 2012. Smaller companies have until December 2013 to implement the labeling changes.
- Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol 2005; 52:937–958.
- Sivamani RK, Ghiya M, Maibach HI. Shedding light on sunscreens and their labels: testing policies need to match actual use. Am J Prev Med 2010; 38:679–681.
- Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res 2011; 24:136–147.
- American Cancer Society. What are the key statistics about basal and squamous cell skin cancers? http://www.cancer.org/Cancer/SkinCancer-BasalandSquamousCell/DetailedGuide/skin-cancer-basal-and-squamous-cell-key-statistics. Accessed May 9, 2012.
- American Cancer Society. What are the key statistics about melanoma? http://www.cancer.org/Cancer/SkinCancer-Melanoma/DetailedGuide/melanoma-skin-cancer-key-statistics. Accessed May 9, 2012.
- Jou PC, McCormick TS, Baron ED. UV immunosuppression and cutaneous malignancies. Expert Rev Dermatol 2011; 6:61–74.
- Wang Y, Digiovanna JJ, Stern JB, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A 2009; 106:6279–6284.
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol 2005; 152:115–121.
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol 2006; 55:1–19.
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol 2008; 128:447–454.
- Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochem Photobiol 1998; 68:63–70.
- Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol 2008; 58(5 suppl 2):S160–166.
- Besaratinia A, Synold TW, Chen HH, et al. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci U S A 2005; 102:10058–10063.
- May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 1999; 18:7621–7636.
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA 2006; 103:13765–13770.
- Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res 2008; 21:487–491.
- Miyamura Y, Coelho SG, Wolber R, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res 2007; 20:2–13.
- Kwon HT, Mayer JA, Walker KK, Yu H, Lewis EC, Belch GE. Promotion of frequent tanning sessions by indoor tanning facilities: two studies. J Am Acad Dermatol 2002; 46:700–705.
- Dellavalle RP, Parker ER, Cersonsky N, et al. Youth access laws: in the dark at the tanning parlor? Arch Dermatol 2003; 139:443–448.
- Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol 2001; 44:775–780.
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J Am Acad Dermatol 1998; 38:89–98.
- IBISWorld. Tanning salons in the US: Market research report NAICS 81219c. www.ibisworld.com. Accesssed May 9, 2012.
- American Academy of Dermatology Tanning Website. Stats and facts. Prevention and care. Indoor tanning. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/indoor-tanning. Accessed May 9, 2012.
- Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 2007; 370:528–537.
- Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photodermatol Photoimmunol Photomed 2011 Apr; 27( 2):58–67.
- US Food and Drug Administration (FDA). CFR - Code of Federal Regulations Title 21, Chapter 1, Part 352: Sunscreen drug products for over-the-counter human use. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352. Accessed May 9, 2012.
- Wang SQ, Lim HW. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration’s final rule on labeling and effectiveness testing. J Am Acad Dermatol 2011; 65:863–869.
- Food and Drug Administration (FDA). Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use (final rule). Federal Register 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf. Accessed May 9, 2012.
- Scherschun L, Lim HW. Photoprotection by sunscreens. Am J Clin Dermatol 2001; 2:131–134.
- US Food and Drug Administration (FDA). Sunburn protection factor (SPF). http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106351.htm. Accessed May 9, 2012.
- DeSimone EM. FDA proposes changes in sunscreen regulations. Am Pharm 1994; NS34:26–31.
- US Food and Drug Administration (FDA). Questions and answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the US (updated 6/23/11). http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicine-Safely/UnderstandingOver-the-CounterMedicines/ucm258468.htm. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). FDA Press Release. FDA announces changes to better inform consumers about sunscreen: new rules give consumers more information to help reduce the risk of skin cancer, early aging. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258940.htm. Accessed May 9, 2012.
- Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol 2009; 161(suppl 3):40–45.
- Baron ED, Fourtanier A, Compan D, Medaisko C, Cooper KD, Stevens SR. High ultraviolet A protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans. J Invest Dermatol 2003; 121:869–875.
- Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol 2008; 58(suppl 2):S149–S154.
- Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol 1995; 33:941–946.
- Young AR, Orchard GE, Harrison GI, Klock JL. The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen. J Invest Dermatol 2007; 127:975–978.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993; 329:1147–1151.
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–729.
- van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–2548.
- Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA 2000; 283:2955–2960.
- Autier P, Doré JF, Cattaruzza MS, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst 1998; 90:1873–1880.
- Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med 2003; 139:966–978.
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257–263.
- Goldenhersh MA, Koslowsky M. Increased melanoma after regular sunscreen use? J Clin Oncol 2011; 29:e557–e558.
- American Academey of Dermatology Sunscreen Website. Stats and facts. Prevention and care. Sunscreens. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/sunscreens. Accessed May 9, 2012.
- Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol 2002; 138:1319–1325.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics 2011; 128:92–102.
- American Academy of Pediatrics. HealthyChildren. Safety & prevention: Sun safety. http://www.healthychildren.org/english/safety-prevention/at-play/pages/Sun-Safety.aspx. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). Information for consumers (drugs). Sunscreen. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm239463.htm. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Revised effectiveness determination; Sunscreen drug products for over-the-counter human use (proposed rule.) Federal Register 2011. http://69.175.53.6/register/2011/jun/17/2011-14769.pdf. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Sunscreen drug products for over-the-counter human use: Request for data and information regarding dosage forms (advance notice of proposed rulemaking), Federal Register 2011). http://69.175.53.6/register/2011/jun/17/2011-14768.pdf. Accessed May 9, 2012.
- Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol 2005; 52:937–958.
- Sivamani RK, Ghiya M, Maibach HI. Shedding light on sunscreens and their labels: testing policies need to match actual use. Am J Prev Med 2010; 38:679–681.
- Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res 2011; 24:136–147.
- American Cancer Society. What are the key statistics about basal and squamous cell skin cancers? http://www.cancer.org/Cancer/SkinCancer-BasalandSquamousCell/DetailedGuide/skin-cancer-basal-and-squamous-cell-key-statistics. Accessed May 9, 2012.
- American Cancer Society. What are the key statistics about melanoma? http://www.cancer.org/Cancer/SkinCancer-Melanoma/DetailedGuide/melanoma-skin-cancer-key-statistics. Accessed May 9, 2012.
- Jou PC, McCormick TS, Baron ED. UV immunosuppression and cutaneous malignancies. Expert Rev Dermatol 2011; 6:61–74.
- Wang Y, Digiovanna JJ, Stern JB, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A 2009; 106:6279–6284.
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol 2005; 152:115–121.
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol 2006; 55:1–19.
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol 2008; 128:447–454.
- Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochem Photobiol 1998; 68:63–70.
- Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol 2008; 58(5 suppl 2):S160–166.
- Besaratinia A, Synold TW, Chen HH, et al. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci U S A 2005; 102:10058–10063.
- May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 1999; 18:7621–7636.
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA 2006; 103:13765–13770.
- Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res 2008; 21:487–491.
- Miyamura Y, Coelho SG, Wolber R, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res 2007; 20:2–13.
- Kwon HT, Mayer JA, Walker KK, Yu H, Lewis EC, Belch GE. Promotion of frequent tanning sessions by indoor tanning facilities: two studies. J Am Acad Dermatol 2002; 46:700–705.
- Dellavalle RP, Parker ER, Cersonsky N, et al. Youth access laws: in the dark at the tanning parlor? Arch Dermatol 2003; 139:443–448.
- Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol 2001; 44:775–780.
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J Am Acad Dermatol 1998; 38:89–98.
- IBISWorld. Tanning salons in the US: Market research report NAICS 81219c. www.ibisworld.com. Accesssed May 9, 2012.
- American Academy of Dermatology Tanning Website. Stats and facts. Prevention and care. Indoor tanning. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/indoor-tanning. Accessed May 9, 2012.
- Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 2007; 370:528–537.
- Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photodermatol Photoimmunol Photomed 2011 Apr; 27( 2):58–67.
- US Food and Drug Administration (FDA). CFR - Code of Federal Regulations Title 21, Chapter 1, Part 352: Sunscreen drug products for over-the-counter human use. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352. Accessed May 9, 2012.
- Wang SQ, Lim HW. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration’s final rule on labeling and effectiveness testing. J Am Acad Dermatol 2011; 65:863–869.
- Food and Drug Administration (FDA). Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use (final rule). Federal Register 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf. Accessed May 9, 2012.
- Scherschun L, Lim HW. Photoprotection by sunscreens. Am J Clin Dermatol 2001; 2:131–134.
- US Food and Drug Administration (FDA). Sunburn protection factor (SPF). http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106351.htm. Accessed May 9, 2012.
- DeSimone EM. FDA proposes changes in sunscreen regulations. Am Pharm 1994; NS34:26–31.
- US Food and Drug Administration (FDA). Questions and answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the US (updated 6/23/11). http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicine-Safely/UnderstandingOver-the-CounterMedicines/ucm258468.htm. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). FDA Press Release. FDA announces changes to better inform consumers about sunscreen: new rules give consumers more information to help reduce the risk of skin cancer, early aging. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258940.htm. Accessed May 9, 2012.
- Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol 2009; 161(suppl 3):40–45.
- Baron ED, Fourtanier A, Compan D, Medaisko C, Cooper KD, Stevens SR. High ultraviolet A protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans. J Invest Dermatol 2003; 121:869–875.
- Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol 2008; 58(suppl 2):S149–S154.
- Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol 1995; 33:941–946.
- Young AR, Orchard GE, Harrison GI, Klock JL. The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen. J Invest Dermatol 2007; 127:975–978.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993; 329:1147–1151.
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–729.
- van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–2548.
- Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA 2000; 283:2955–2960.
- Autier P, Doré JF, Cattaruzza MS, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst 1998; 90:1873–1880.
- Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med 2003; 139:966–978.
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257–263.
- Goldenhersh MA, Koslowsky M. Increased melanoma after regular sunscreen use? J Clin Oncol 2011; 29:e557–e558.
- American Academey of Dermatology Sunscreen Website. Stats and facts. Prevention and care. Sunscreens. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/sunscreens. Accessed May 9, 2012.
- Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol 2002; 138:1319–1325.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics 2011; 128:92–102.
- American Academy of Pediatrics. HealthyChildren. Safety & prevention: Sun safety. http://www.healthychildren.org/english/safety-prevention/at-play/pages/Sun-Safety.aspx. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). Information for consumers (drugs). Sunscreen. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm239463.htm. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Revised effectiveness determination; Sunscreen drug products for over-the-counter human use (proposed rule.) Federal Register 2011. http://69.175.53.6/register/2011/jun/17/2011-14769.pdf. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Sunscreen drug products for over-the-counter human use: Request for data and information regarding dosage forms (advance notice of proposed rulemaking), Federal Register 2011). http://69.175.53.6/register/2011/jun/17/2011-14768.pdf. Accessed May 9, 2012.
KEY POINTS
- Despite the known risks, nearly 28 million Americans use a sunbed or a sunlamp every year, and 70% of those are white women ages 16 to 29.
- Sunscreens have been a source of confusion in their labeling and their sun protection factor ratings. Revised FDA labeling requirements may help clinicians provide useful guidance to patients.
- The American Academy of Dermatology supports a ban on the nonmedical production and sale of indoor tanning devices.
- Recommendations to prevent UV damage include minimizing sun exposure during peak daylight hours, wearing clothing such as long-sleeve shirts, wide-brimmed hats, and sunglasses, and application of a broad-spectrum sunscreen with UV-A protection. Infants less than 6 months of age require additional protective measures.