User login
Dermatology and Vaccines: We Must Do Better
Vaccines work. They are powerful tools that have saved millions of lives worldwide; however, a robust antivaccine movement has taken hold in the United States and worldwide despite overwhelming data in support of vaccination. In fact, vaccine hesitancy—the reluctance or refusal to vaccinate despite the availability of vaccines—was listed by the World Health Organization as one of the top 10 global health threats in 2019.1
Several vaccines have a role in dermatology, including the human papillomavirus (HPV) vaccine (Gardasil 9 [Merck Sharp & Dohme Corp]), the herpes zoster vaccines (Zostavax [Merck Sharp & Dohme Corp] and Shingrix [GlaxoSmithKline Biologicals]), and the measles-mumps-rubella vaccine, among others. These vaccinations are necessary for children and many adults alike, and they play a critical role in protecting both healthy and immunosuppressed patients.
Vaccine hesitancy is a growing threat to individual and public health that requires a response from all physicians. In our experience, dermatologists have been somewhat passive in advocating for vaccinations, possibly due to knowledge barriers or time constraints; however, this stance must change. Dermatologists must join the front lines in advocating for vaccinations, which are a proven and effective modality in promoting public health.
Dermatologists can employ the following practical tips to improve vaccination compliance among patients:
• Familiarize yourself with the Centers for Disease Control and Prevention immunization schedules and vaccination information sheets (https://www.cdc.gov/vaccines/hcp/vis/current-vis.html). Printed copies of informational handouts should be readily available to provide to patients in the office. The Centers for Disease Control and Prevention also offers tip sheets to guide conversations with patients (https://www.cdc.gov/vaccines/hcp/conversations/index.html).
• Prior to starting an immunosuppressive medication, confirm the patient’s immunization status. You should know which vaccines are live (containing an attenuated pathogen) and which are inactivated. Live vaccines typically are not administered to immunosuppressed patients.
• Use electronic medical records to help provide reminders to prompt administration of any necessary vaccines.
• Know the facts, especially regarding purported vaccine controversies, and be able to cite data on vaccine safety and efficacy. For example, when having a conversation with a patient you could state that vaccination against HPV, which can cause genital warts and certain cancers, has decreased the number of HPV infections by more than 70% in young women and 80% in teenaged girls.2 Cervical precancers were reduced by 40% in women vaccinated against HPV. Twelve years of monitoring data validates the safety and efficacy of the HPV vaccine—it is safe and effective, with benefits that outweigh any potential risks.2
• Tailor counseling based on the patient’s age and focus on benefits that directly impact the patient. For example, consider showing young adults photographs of genital warts while educating them that the HPV vaccine can help prevent this kind of infection in the future.
• Emphasize that vaccines are a routine part of comprehensive patient care and support this point by providing data and specific reasons for recommending vaccines.3 Avoid phrases such as, “Do you want the vaccine?” or “You could consider receiving the vaccine today,” which can imply that the vaccine is not necessary.
• Offer vaccines in your office or provide clear printed informational sheets directing patients to nearby primary care clinics, infectious disease clinics, or pharmacies where vaccinations are offered.
• Consider using social media to promote the benefits of vaccination among patients.
The recent coronavirus disease 2019 pandemic has brought the topic of vaccination into the limelight while highlighting that rampant misinformation can lead to distrust of health care workers. Dermatologists, along with all physicians, should be trusted advisors and advocates for public health. In addition to being knowledgeable, dermatologists must remain open-minded in having conversations with skeptical patients. Physicians must take the time and effort to promote vaccinations—the health of patients and the general public depends on it.
- Akbar R. Ten threats to global health in 2019. World Health Organization website. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019. Published March 21, 2019. Accessed November 11, 2020.
- HPV vaccination is safe and effective. Centers for Disease Control and Prevention website. https://www.cdc.gov/hpv/parents/vaccinesafety.html. Updated April 29, 2019. Accessed November 11, 2020.
- How to give a strong recommendation to adult patients who require vaccination. Medscape website. https://www.medscape.com/viewarticle/842874. Published April 16, 2015. Accessed November 11, 2020.
Vaccines work. They are powerful tools that have saved millions of lives worldwide; however, a robust antivaccine movement has taken hold in the United States and worldwide despite overwhelming data in support of vaccination. In fact, vaccine hesitancy—the reluctance or refusal to vaccinate despite the availability of vaccines—was listed by the World Health Organization as one of the top 10 global health threats in 2019.1
Several vaccines have a role in dermatology, including the human papillomavirus (HPV) vaccine (Gardasil 9 [Merck Sharp & Dohme Corp]), the herpes zoster vaccines (Zostavax [Merck Sharp & Dohme Corp] and Shingrix [GlaxoSmithKline Biologicals]), and the measles-mumps-rubella vaccine, among others. These vaccinations are necessary for children and many adults alike, and they play a critical role in protecting both healthy and immunosuppressed patients.
Vaccine hesitancy is a growing threat to individual and public health that requires a response from all physicians. In our experience, dermatologists have been somewhat passive in advocating for vaccinations, possibly due to knowledge barriers or time constraints; however, this stance must change. Dermatologists must join the front lines in advocating for vaccinations, which are a proven and effective modality in promoting public health.
Dermatologists can employ the following practical tips to improve vaccination compliance among patients:
• Familiarize yourself with the Centers for Disease Control and Prevention immunization schedules and vaccination information sheets (https://www.cdc.gov/vaccines/hcp/vis/current-vis.html). Printed copies of informational handouts should be readily available to provide to patients in the office. The Centers for Disease Control and Prevention also offers tip sheets to guide conversations with patients (https://www.cdc.gov/vaccines/hcp/conversations/index.html).
• Prior to starting an immunosuppressive medication, confirm the patient’s immunization status. You should know which vaccines are live (containing an attenuated pathogen) and which are inactivated. Live vaccines typically are not administered to immunosuppressed patients.
• Use electronic medical records to help provide reminders to prompt administration of any necessary vaccines.
• Know the facts, especially regarding purported vaccine controversies, and be able to cite data on vaccine safety and efficacy. For example, when having a conversation with a patient you could state that vaccination against HPV, which can cause genital warts and certain cancers, has decreased the number of HPV infections by more than 70% in young women and 80% in teenaged girls.2 Cervical precancers were reduced by 40% in women vaccinated against HPV. Twelve years of monitoring data validates the safety and efficacy of the HPV vaccine—it is safe and effective, with benefits that outweigh any potential risks.2
• Tailor counseling based on the patient’s age and focus on benefits that directly impact the patient. For example, consider showing young adults photographs of genital warts while educating them that the HPV vaccine can help prevent this kind of infection in the future.
• Emphasize that vaccines are a routine part of comprehensive patient care and support this point by providing data and specific reasons for recommending vaccines.3 Avoid phrases such as, “Do you want the vaccine?” or “You could consider receiving the vaccine today,” which can imply that the vaccine is not necessary.
• Offer vaccines in your office or provide clear printed informational sheets directing patients to nearby primary care clinics, infectious disease clinics, or pharmacies where vaccinations are offered.
• Consider using social media to promote the benefits of vaccination among patients.
The recent coronavirus disease 2019 pandemic has brought the topic of vaccination into the limelight while highlighting that rampant misinformation can lead to distrust of health care workers. Dermatologists, along with all physicians, should be trusted advisors and advocates for public health. In addition to being knowledgeable, dermatologists must remain open-minded in having conversations with skeptical patients. Physicians must take the time and effort to promote vaccinations—the health of patients and the general public depends on it.
Vaccines work. They are powerful tools that have saved millions of lives worldwide; however, a robust antivaccine movement has taken hold in the United States and worldwide despite overwhelming data in support of vaccination. In fact, vaccine hesitancy—the reluctance or refusal to vaccinate despite the availability of vaccines—was listed by the World Health Organization as one of the top 10 global health threats in 2019.1
Several vaccines have a role in dermatology, including the human papillomavirus (HPV) vaccine (Gardasil 9 [Merck Sharp & Dohme Corp]), the herpes zoster vaccines (Zostavax [Merck Sharp & Dohme Corp] and Shingrix [GlaxoSmithKline Biologicals]), and the measles-mumps-rubella vaccine, among others. These vaccinations are necessary for children and many adults alike, and they play a critical role in protecting both healthy and immunosuppressed patients.
Vaccine hesitancy is a growing threat to individual and public health that requires a response from all physicians. In our experience, dermatologists have been somewhat passive in advocating for vaccinations, possibly due to knowledge barriers or time constraints; however, this stance must change. Dermatologists must join the front lines in advocating for vaccinations, which are a proven and effective modality in promoting public health.
Dermatologists can employ the following practical tips to improve vaccination compliance among patients:
• Familiarize yourself with the Centers for Disease Control and Prevention immunization schedules and vaccination information sheets (https://www.cdc.gov/vaccines/hcp/vis/current-vis.html). Printed copies of informational handouts should be readily available to provide to patients in the office. The Centers for Disease Control and Prevention also offers tip sheets to guide conversations with patients (https://www.cdc.gov/vaccines/hcp/conversations/index.html).
• Prior to starting an immunosuppressive medication, confirm the patient’s immunization status. You should know which vaccines are live (containing an attenuated pathogen) and which are inactivated. Live vaccines typically are not administered to immunosuppressed patients.
• Use electronic medical records to help provide reminders to prompt administration of any necessary vaccines.
• Know the facts, especially regarding purported vaccine controversies, and be able to cite data on vaccine safety and efficacy. For example, when having a conversation with a patient you could state that vaccination against HPV, which can cause genital warts and certain cancers, has decreased the number of HPV infections by more than 70% in young women and 80% in teenaged girls.2 Cervical precancers were reduced by 40% in women vaccinated against HPV. Twelve years of monitoring data validates the safety and efficacy of the HPV vaccine—it is safe and effective, with benefits that outweigh any potential risks.2
• Tailor counseling based on the patient’s age and focus on benefits that directly impact the patient. For example, consider showing young adults photographs of genital warts while educating them that the HPV vaccine can help prevent this kind of infection in the future.
• Emphasize that vaccines are a routine part of comprehensive patient care and support this point by providing data and specific reasons for recommending vaccines.3 Avoid phrases such as, “Do you want the vaccine?” or “You could consider receiving the vaccine today,” which can imply that the vaccine is not necessary.
• Offer vaccines in your office or provide clear printed informational sheets directing patients to nearby primary care clinics, infectious disease clinics, or pharmacies where vaccinations are offered.
• Consider using social media to promote the benefits of vaccination among patients.
The recent coronavirus disease 2019 pandemic has brought the topic of vaccination into the limelight while highlighting that rampant misinformation can lead to distrust of health care workers. Dermatologists, along with all physicians, should be trusted advisors and advocates for public health. In addition to being knowledgeable, dermatologists must remain open-minded in having conversations with skeptical patients. Physicians must take the time and effort to promote vaccinations—the health of patients and the general public depends on it.
- Akbar R. Ten threats to global health in 2019. World Health Organization website. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019. Published March 21, 2019. Accessed November 11, 2020.
- HPV vaccination is safe and effective. Centers for Disease Control and Prevention website. https://www.cdc.gov/hpv/parents/vaccinesafety.html. Updated April 29, 2019. Accessed November 11, 2020.
- How to give a strong recommendation to adult patients who require vaccination. Medscape website. https://www.medscape.com/viewarticle/842874. Published April 16, 2015. Accessed November 11, 2020.
- Akbar R. Ten threats to global health in 2019. World Health Organization website. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019. Published March 21, 2019. Accessed November 11, 2020.
- HPV vaccination is safe and effective. Centers for Disease Control and Prevention website. https://www.cdc.gov/hpv/parents/vaccinesafety.html. Updated April 29, 2019. Accessed November 11, 2020.
- How to give a strong recommendation to adult patients who require vaccination. Medscape website. https://www.medscape.com/viewarticle/842874. Published April 16, 2015. Accessed November 11, 2020.
Bedbugs: Helping your patient through an infestation
Bedbugs have been unwelcome bedfellows for humans for thousands of years. An increase in pyrethroid resistance, a ban on the insecticide dichloro-diphenyl-trichloroethane (DDT), increased international travel, and increased population density in large cities have led to an exponential rise in the incidence of bedbug infestations. Physicians are often at the forefront of bedbug infestation diagnosis.
Once the diagnosis is suggested, symptomatic treatment of the patient and extermination of the pests are essential, though time-consuming, costly, and often problematic. Measures to eliminate infestation and to prevent spread include identification of the pest, early detection, patient education, and professional eradication.
BEDBUGS: A BRIEF HISTORY
The term bedbug refers to the obligate parasitic arthropod Cimex lectularius (the common bedbug) and, less commonly, its tropical cousin C hemipterus. Bedbugs have coexisted with humans for centuries, dating back to the ancient Egyptians 3,500 years ago.1 Through the mid-20th century, about 30% of US households were infested with bedbugs.2 The introduction of pesticides during World War II markedly decreased the incidence, but with increased international travel, pesticide resistance, and the banning of certain pesticides in the last decade, bedbugs have reemerged worldwide.3
BIOLOGY
Bedbugs are red-brown, wingless, oval-shaped insects measuring 4 to 5 mm in length (Figure 1). They are hematophagous ectoparasites that preferentially feed on human blood, although they feed on some animals as well.2
Cimex lectularius dwells in temperate climates and C hemipterus in more tropical climates, but overlap and interbreeding are common. The usual life cycle is about 6 months, but some bugs live 12 months or longer. The female bedbug lays 5 to 8 eggs per week, or approximately 500 eggs in her lifetime, and each egg hatches in 5 to 10 days.4
These photophobic parasites do not live on their human hosts but rather simply visit for a meal. They cohabitate in dark locations, attacking human hosts when they are inactive or sleeping for long periods of time. Common living areas include mattress seams, box springs, bed linens and clothes, wallpaper seams, electrical outlets, and furniture seams (Table 1).5 The female bedbug lays her eggs in these secluded crevices, ensuring their safety until hatching. The dense nests of adult bedbugs, their eggs, and accumulated fecal matter allow for easy visual identification of infestation.5
Bedbugs typically feed between 1:00 am and 5:00 am. Though wingless, they successfully navigate towards their human host, attracted by emitted heat and carbon dioxide.2 Once attached to human skin, the bedbug bite releases enzymes and chemicals including nitrophorin and nitric oxide that facilitate bleeding; these substances are responsible for the resultant dermatitis. (Of note, bedbugs with experimentally excised salivary glands do not cause skin disease in humans.6) After feeding for 3 to 20 minutes, the length and weight of the arthropod can increase by 50% to 200%. A fully sated bedbug can survive for a year until its next meal.2,7 Even if an establishment, home, room, or article of clothing infested with bedbugs has been abandoned for several months, without proper eradication the item still represents a possible nidus for recurrent disease if used, inhabited, or worn again.
EPIDEMIOLOGY
From the earliest documented cases of Cimex in ancient Egyptian tombs to the mid-1900s, the cohabitation of humans and bedbugs was seen as inevitable. With the introduction of DDT 60 years ago, the bedbug population significantly decreased.8 Since DDT’s prohibition, coupled with increased travel and heightened resistance to over-the-counter insecticides, the bedbug population has reemerged exponentially.9,10
Infestations have been reported worldwide, on every continent, and in all 50 of the United States. In Australia, infestations have risen 4,500% in the last 10 to 15 years.11 In the United States, infestation occurs exclusively with C lectularius and the incidence is rising. Philadelphia and New York City are among the most bedbug-infested US cities. New York City experienced a 2,000% increase in bedbug complaints between 2004 and 2009.8
Bedbugs can be transmitted either through active migration of colonies from one area to another adjacent living area through wall spaces or ventilation, or through passive transportation in luggage, clothing, furniture, used mattresses, bookbags, and other personal items.1 Although infestation affects people of all socioeconomic classes and backgrounds, the likelihood increases in people who frequently travel and people who live in lower income neighborhoods with tightly packed apartments. Bedbug infestations are also common in refugee camps: 98% of the rooms in a refugee camp in Sierra Leone had bedbugs, and almost 90% of the residents had signs of bites.12 Unlike scabies, direct person-to-person, skin-to-skin transfer is rare.
CLINICAL FINDINGS
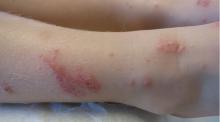
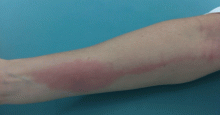
Bedbug bites are analogous, almost identical, to other arthropod bites: bites begin as pink macules that progress to papules (Figure 2), large plaques, or wheals (hives).13 Bites can arise minutes or even days after the initial assault. Some papules and plaques may have a central crust or erosion suggesting a bite.
Bites are typically intensely pruritic, and occasionally, hypersensitive victims can develop bullae, necrotic plaques, or even vasculitis. New papules and plaques form as older ones heal. Some patients may have fever and malaise.13 About 30% of patients may not have skin disease from bedbugs, making diagnosis in those individuals impossible.
The nonspecific nature of this presentation and the subsequent difficulty in prompt diagnosis can lead to a prolonged period of morbidity for the patient, as well as increasing the window of opportunity for the bedbugs to affect other surrounding individuals.
THE DIFFERENTIAL DIAGNOSIS IS BROAD
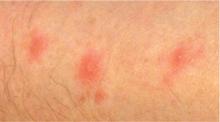
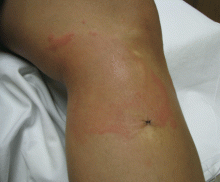
Commonly, bedbug bites have been misdiagnosed as drug eruptions, food allergies, dermatitis herpetiformis, staphylococcal or varicella infection, and scabies, as well as other arthropod bites.11 This broad differential diagnosis can often be narrowed by careful observation of the bite distribution. The clustering of bites in groups of 3, often in a linear pattern, sometimes overlying blood vessels, is known as the “breakfast, lunch, and dinner” sign (Figure 3), and this can help to guide the clinician toward the diagnosis of a bite as opposed to a diffuse urticarial response.2
If the characteristic clusters of bites are not present, distinguishing clinically between the various causes of pruritic urticarial lesions is difficult. Subtle clues that point towards bedbug bites can be that the rash appears to be most edematous in the morning and flattens throughout the day, as the bites occur typically during sleep.14 Likewise, the rash associated with bedbug bites has also been reported to last longer, to blanch less, and to be less responsive to steroid and antihistamine treatment than other urticarial rashes.14 If a skin biopsy specimen is available, histologic assessment can help to rule out similarly presenting conditions such as prodromal bullous pemphigoid, dermatitis herpetiformis, and urticarial dermatosis, even if it cannot provide a definitive answer as to the etiology.15
Bedbug bites vs other arthropod bites
Once a bite is suspected, differentiating between bedbug and other arthropod bites is the next challenge.
Once again, a detailed assessment of the location of the bites can yield valuable information. The waist, axillae, and uncovered parts of the body are the usual sites for bedbug bites.2 Likewise, inflammatory papules along the eyelid (the “eyelid sign”) are highly suggestive of a bedbug bite.16
The scant involvement of covered body areas, the lack of shallow burrows in the skin, and the lack of scabetic elements on skin scrapings exclude scabies as a diagnosis.
Skin biopsy is not helpful in differentiating arthropod bites, as the histologic findings are nonspecific. The key to a definitive diagnosis in these cases is identification of the suspected bug in characteristic locations. Patients should be encouraged to carefully inspect mattresses, floorboards, and other crevices for the small ovaloid bugs or the reddish-brown specks of heme and feces they typically leave behind on bed linens.15 A positive reported sighting of the bugs can lend credence to the diagnosis, whereas capture and laboratory assessment of a specimen is ideal.
BEDBUGS AS DISEASE VECTORS
Extracutaneous manifestations of bedbug assault are rare. Anaphylaxis to proteins in Cimex saliva may occur, as well as significant blood loss, even anemia, from extensive feeding.17 Bedbug infestations can exacerbate asthma, preexisting mental illness, anxiety, and insomnia.18 Since bedbugs extract blood from hosts, they have a putative ability to act as vectors of disease. Some 45 known pathogens have been isolated from the Cimex species including hepatitis B, human immunodeficiency virus (HIV), Trypanosoma cruzi, and methicillin-resistant Staphylococcus aureus. To date, however, there is no evidence to demonstrate transmission of pathogens to humans.5
TREATMENT AND ERADICATION
Treatment is mainly symptomatic—systemic antihistamines and topical corticosteroids to reduce pruritus and alleviate the dermatitis.2 Patients should be instructed to avoid scratching to prevent infection. Secondary bacterial infection can be treated with topical or systemic antibiotics. Rare cases of bite-induced asthma or anaphylaxis necessitate appropriate emergency treatment. Extermination of infestation is critical to therapy.
If bedbug infestation is suggested, mattresses, bedding, sleeping areas, and bed clothing should be inspected for insects, eggs, and fecal spotting. Adhesives or traps that emit heat or carbon dioxide can be used to capture the bedbugs. During widespread infestation, the arthropods release a pungent odor, which allows trained dogs to detect them with 95% to 98% accuracy.19
Eradication techniques
Once infestation is confirmed, patients should contact an exterminator who can confirm the presence of bedbugs. Typical eradication measures often require nonchemical control and chemical pesticides.
Professional exterminators have special equipment that can heat a room to 48 to 50°C (118–122°F). Heat sustained at this temperature for 90 minutes is sufficient to kill bedbugs.20
The infested area should be vacuumed daily, and vacuum bags and unwanted items should be sealed in plastic before discarding. Clothing, linens, and infested fabrics should be washed and dried in heat at 60°C (140°F) or greater.
Mattresses and furniture should be sealed in a special plastic that allows treatment with heat, steaming, or pesticides. Most professional pesticides contain pyrethroids, but resistance to these products is common, necessitating the use of multiple formulations to overcome resistance.8
Over-the-counter pesticides, almost exclusively pyrethroids, are variably effective and potentially hazardous to consumers.8 Patients must be advised to follow label directions to avoid adverse effects and toxicity.
Alternative chemical eradication methods to circumvent the problem of resistance include piperonyl butoxide, S-methoprene, boric acid, silicates (diatomaceous earth dust), and sulfuryl fluoride. Recent research has also posited the use of antiparasitic agents such as ivermectin and moxidectin in cases of resistant bedbug infestation, with promising results.21
All extermination products and techniques have variable risks, efficacies, and costs,8 and repeat inspections and retreatment are often required.
Prevention strategies include visual inspection of possibly infested rooms, with particular attention to mattress seams and crevices, placing luggage on a luggage rack away from the floor and bed, and careful examination of acquired second-hand items.7
Educating patients is the key to success
While all of the above eradication techniques are important curative strategies, the success of any treatment is contingent on appropriate patient education about the nature of the problem.
Resolving a bedbug infestation is notoriously difficult and requires meticulous adherence to hygiene and cleansing instructions throughout the household or institution for a sustained period of time. Information from sources such as the US Environmental Protection Agency (www.epa.gov) can empower patients to perform the necessary eradication protocols, and clinicians should routinely recommended them as part of a holistic treatment strategy.
- Krause-Parello CA, Sciscione P. Bedbugs: an equal opportunist and cosmopolitan creature. J Sch Nurs 2009; 25:126–132.
- Sfeir M, Munoz-Price LS. Scabies and bedbugs in hospital outbreaks. Curr Infect Dis Rep 2014; 16:412.
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 2007; 44:175–178.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis 2011; 52:200–210.
- Doggett SL, Dwyer DE, Penas PF, Russell RC. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 2012; 25:164–192.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol 2013; 149:372–373.
- Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 2009; 301:1358–1366.
- Davies TG, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 2012; 26:241–254.
- Saenz VL, Booth W, Schal C, Vargo EL. Genetic analysis of bed bug populations reveals small propagule size within individual infestations but high genetic diversity across infestations from the eastern United States. J Med Entomol 2012; 49:865–875.
- Jones SC, Bryant JL. Ineffectiveness of over-the-counter total-release foggers against the bed bug (Heteroptera: cimicidae). J Econ Entomol 2012; 105:957–963.
- Doggett SL, Russell R. Bed bugs—what the GP needs to know. Aust Fam Physician 2009; 38:880–884.
- Gbakima AA, Terry BC, Kanja F, Kortequee S, Dukuley I, Sahr F. High prevalence of bedbugs Cimex hemipterus and Cimex lectularis in camps for internally displaced persons in Freetown, Sierra Leone: a pilot humanitarian investigation. West Afr J Med 2002; 21:268–271.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Scarupa MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol 2006; 117:1508–1509.
- Thomas I, Kihiczak GG, Schwartz RA. Bedbug bites: a review. Int J Dermatol 2004; 43:430–433.
- Quach KA, Zaenglein AL. The eyelid sign: a clue to bed bug bites. Pediatr Dermatol 2014; 31:353–355.
- Paulke-Korinek M, Szell M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Goddard J, de Shazo R. Psychological effects of bed bug attacks (Cimex lectularius L). Am J Med 2012; 125:101–103.
- Pfiester M, Koehler PG, Pereira RM. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J Econ Entomol 2008; 101:1389–1396.
- Kells SA, Goblirsch MJ. Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011; 2:412–422.
- Sheele JM, Ridge GE. Toxicity and potential utility of ivermectin and moxidectin as xenointoxicants against the common bed bug Cimex lectularius L. Parasitol Res 2016; 115:3071–3081.
Bedbugs have been unwelcome bedfellows for humans for thousands of years. An increase in pyrethroid resistance, a ban on the insecticide dichloro-diphenyl-trichloroethane (DDT), increased international travel, and increased population density in large cities have led to an exponential rise in the incidence of bedbug infestations. Physicians are often at the forefront of bedbug infestation diagnosis.
Once the diagnosis is suggested, symptomatic treatment of the patient and extermination of the pests are essential, though time-consuming, costly, and often problematic. Measures to eliminate infestation and to prevent spread include identification of the pest, early detection, patient education, and professional eradication.
BEDBUGS: A BRIEF HISTORY
The term bedbug refers to the obligate parasitic arthropod Cimex lectularius (the common bedbug) and, less commonly, its tropical cousin C hemipterus. Bedbugs have coexisted with humans for centuries, dating back to the ancient Egyptians 3,500 years ago.1 Through the mid-20th century, about 30% of US households were infested with bedbugs.2 The introduction of pesticides during World War II markedly decreased the incidence, but with increased international travel, pesticide resistance, and the banning of certain pesticides in the last decade, bedbugs have reemerged worldwide.3
BIOLOGY
Bedbugs are red-brown, wingless, oval-shaped insects measuring 4 to 5 mm in length (Figure 1). They are hematophagous ectoparasites that preferentially feed on human blood, although they feed on some animals as well.2
Cimex lectularius dwells in temperate climates and C hemipterus in more tropical climates, but overlap and interbreeding are common. The usual life cycle is about 6 months, but some bugs live 12 months or longer. The female bedbug lays 5 to 8 eggs per week, or approximately 500 eggs in her lifetime, and each egg hatches in 5 to 10 days.4
These photophobic parasites do not live on their human hosts but rather simply visit for a meal. They cohabitate in dark locations, attacking human hosts when they are inactive or sleeping for long periods of time. Common living areas include mattress seams, box springs, bed linens and clothes, wallpaper seams, electrical outlets, and furniture seams (Table 1).5 The female bedbug lays her eggs in these secluded crevices, ensuring their safety until hatching. The dense nests of adult bedbugs, their eggs, and accumulated fecal matter allow for easy visual identification of infestation.5
Bedbugs typically feed between 1:00 am and 5:00 am. Though wingless, they successfully navigate towards their human host, attracted by emitted heat and carbon dioxide.2 Once attached to human skin, the bedbug bite releases enzymes and chemicals including nitrophorin and nitric oxide that facilitate bleeding; these substances are responsible for the resultant dermatitis. (Of note, bedbugs with experimentally excised salivary glands do not cause skin disease in humans.6) After feeding for 3 to 20 minutes, the length and weight of the arthropod can increase by 50% to 200%. A fully sated bedbug can survive for a year until its next meal.2,7 Even if an establishment, home, room, or article of clothing infested with bedbugs has been abandoned for several months, without proper eradication the item still represents a possible nidus for recurrent disease if used, inhabited, or worn again.
EPIDEMIOLOGY
From the earliest documented cases of Cimex in ancient Egyptian tombs to the mid-1900s, the cohabitation of humans and bedbugs was seen as inevitable. With the introduction of DDT 60 years ago, the bedbug population significantly decreased.8 Since DDT’s prohibition, coupled with increased travel and heightened resistance to over-the-counter insecticides, the bedbug population has reemerged exponentially.9,10
Infestations have been reported worldwide, on every continent, and in all 50 of the United States. In Australia, infestations have risen 4,500% in the last 10 to 15 years.11 In the United States, infestation occurs exclusively with C lectularius and the incidence is rising. Philadelphia and New York City are among the most bedbug-infested US cities. New York City experienced a 2,000% increase in bedbug complaints between 2004 and 2009.8
Bedbugs can be transmitted either through active migration of colonies from one area to another adjacent living area through wall spaces or ventilation, or through passive transportation in luggage, clothing, furniture, used mattresses, bookbags, and other personal items.1 Although infestation affects people of all socioeconomic classes and backgrounds, the likelihood increases in people who frequently travel and people who live in lower income neighborhoods with tightly packed apartments. Bedbug infestations are also common in refugee camps: 98% of the rooms in a refugee camp in Sierra Leone had bedbugs, and almost 90% of the residents had signs of bites.12 Unlike scabies, direct person-to-person, skin-to-skin transfer is rare.
CLINICAL FINDINGS
Bedbug bites are analogous, almost identical, to other arthropod bites: bites begin as pink macules that progress to papules (Figure 2), large plaques, or wheals (hives).13 Bites can arise minutes or even days after the initial assault. Some papules and plaques may have a central crust or erosion suggesting a bite.
Bites are typically intensely pruritic, and occasionally, hypersensitive victims can develop bullae, necrotic plaques, or even vasculitis. New papules and plaques form as older ones heal. Some patients may have fever and malaise.13 About 30% of patients may not have skin disease from bedbugs, making diagnosis in those individuals impossible.
The nonspecific nature of this presentation and the subsequent difficulty in prompt diagnosis can lead to a prolonged period of morbidity for the patient, as well as increasing the window of opportunity for the bedbugs to affect other surrounding individuals.
THE DIFFERENTIAL DIAGNOSIS IS BROAD
Commonly, bedbug bites have been misdiagnosed as drug eruptions, food allergies, dermatitis herpetiformis, staphylococcal or varicella infection, and scabies, as well as other arthropod bites.11 This broad differential diagnosis can often be narrowed by careful observation of the bite distribution. The clustering of bites in groups of 3, often in a linear pattern, sometimes overlying blood vessels, is known as the “breakfast, lunch, and dinner” sign (Figure 3), and this can help to guide the clinician toward the diagnosis of a bite as opposed to a diffuse urticarial response.2
If the characteristic clusters of bites are not present, distinguishing clinically between the various causes of pruritic urticarial lesions is difficult. Subtle clues that point towards bedbug bites can be that the rash appears to be most edematous in the morning and flattens throughout the day, as the bites occur typically during sleep.14 Likewise, the rash associated with bedbug bites has also been reported to last longer, to blanch less, and to be less responsive to steroid and antihistamine treatment than other urticarial rashes.14 If a skin biopsy specimen is available, histologic assessment can help to rule out similarly presenting conditions such as prodromal bullous pemphigoid, dermatitis herpetiformis, and urticarial dermatosis, even if it cannot provide a definitive answer as to the etiology.15
Bedbug bites vs other arthropod bites
Once a bite is suspected, differentiating between bedbug and other arthropod bites is the next challenge.
Once again, a detailed assessment of the location of the bites can yield valuable information. The waist, axillae, and uncovered parts of the body are the usual sites for bedbug bites.2 Likewise, inflammatory papules along the eyelid (the “eyelid sign”) are highly suggestive of a bedbug bite.16
The scant involvement of covered body areas, the lack of shallow burrows in the skin, and the lack of scabetic elements on skin scrapings exclude scabies as a diagnosis.
Skin biopsy is not helpful in differentiating arthropod bites, as the histologic findings are nonspecific. The key to a definitive diagnosis in these cases is identification of the suspected bug in characteristic locations. Patients should be encouraged to carefully inspect mattresses, floorboards, and other crevices for the small ovaloid bugs or the reddish-brown specks of heme and feces they typically leave behind on bed linens.15 A positive reported sighting of the bugs can lend credence to the diagnosis, whereas capture and laboratory assessment of a specimen is ideal.
BEDBUGS AS DISEASE VECTORS
Extracutaneous manifestations of bedbug assault are rare. Anaphylaxis to proteins in Cimex saliva may occur, as well as significant blood loss, even anemia, from extensive feeding.17 Bedbug infestations can exacerbate asthma, preexisting mental illness, anxiety, and insomnia.18 Since bedbugs extract blood from hosts, they have a putative ability to act as vectors of disease. Some 45 known pathogens have been isolated from the Cimex species including hepatitis B, human immunodeficiency virus (HIV), Trypanosoma cruzi, and methicillin-resistant Staphylococcus aureus. To date, however, there is no evidence to demonstrate transmission of pathogens to humans.5
TREATMENT AND ERADICATION
Treatment is mainly symptomatic—systemic antihistamines and topical corticosteroids to reduce pruritus and alleviate the dermatitis.2 Patients should be instructed to avoid scratching to prevent infection. Secondary bacterial infection can be treated with topical or systemic antibiotics. Rare cases of bite-induced asthma or anaphylaxis necessitate appropriate emergency treatment. Extermination of infestation is critical to therapy.
If bedbug infestation is suggested, mattresses, bedding, sleeping areas, and bed clothing should be inspected for insects, eggs, and fecal spotting. Adhesives or traps that emit heat or carbon dioxide can be used to capture the bedbugs. During widespread infestation, the arthropods release a pungent odor, which allows trained dogs to detect them with 95% to 98% accuracy.19
Eradication techniques
Once infestation is confirmed, patients should contact an exterminator who can confirm the presence of bedbugs. Typical eradication measures often require nonchemical control and chemical pesticides.
Professional exterminators have special equipment that can heat a room to 48 to 50°C (118–122°F). Heat sustained at this temperature for 90 minutes is sufficient to kill bedbugs.20
The infested area should be vacuumed daily, and vacuum bags and unwanted items should be sealed in plastic before discarding. Clothing, linens, and infested fabrics should be washed and dried in heat at 60°C (140°F) or greater.
Mattresses and furniture should be sealed in a special plastic that allows treatment with heat, steaming, or pesticides. Most professional pesticides contain pyrethroids, but resistance to these products is common, necessitating the use of multiple formulations to overcome resistance.8
Over-the-counter pesticides, almost exclusively pyrethroids, are variably effective and potentially hazardous to consumers.8 Patients must be advised to follow label directions to avoid adverse effects and toxicity.
Alternative chemical eradication methods to circumvent the problem of resistance include piperonyl butoxide, S-methoprene, boric acid, silicates (diatomaceous earth dust), and sulfuryl fluoride. Recent research has also posited the use of antiparasitic agents such as ivermectin and moxidectin in cases of resistant bedbug infestation, with promising results.21
All extermination products and techniques have variable risks, efficacies, and costs,8 and repeat inspections and retreatment are often required.
Prevention strategies include visual inspection of possibly infested rooms, with particular attention to mattress seams and crevices, placing luggage on a luggage rack away from the floor and bed, and careful examination of acquired second-hand items.7
Educating patients is the key to success
While all of the above eradication techniques are important curative strategies, the success of any treatment is contingent on appropriate patient education about the nature of the problem.
Resolving a bedbug infestation is notoriously difficult and requires meticulous adherence to hygiene and cleansing instructions throughout the household or institution for a sustained period of time. Information from sources such as the US Environmental Protection Agency (www.epa.gov) can empower patients to perform the necessary eradication protocols, and clinicians should routinely recommended them as part of a holistic treatment strategy.
Bedbugs have been unwelcome bedfellows for humans for thousands of years. An increase in pyrethroid resistance, a ban on the insecticide dichloro-diphenyl-trichloroethane (DDT), increased international travel, and increased population density in large cities have led to an exponential rise in the incidence of bedbug infestations. Physicians are often at the forefront of bedbug infestation diagnosis.
Once the diagnosis is suggested, symptomatic treatment of the patient and extermination of the pests are essential, though time-consuming, costly, and often problematic. Measures to eliminate infestation and to prevent spread include identification of the pest, early detection, patient education, and professional eradication.
BEDBUGS: A BRIEF HISTORY
The term bedbug refers to the obligate parasitic arthropod Cimex lectularius (the common bedbug) and, less commonly, its tropical cousin C hemipterus. Bedbugs have coexisted with humans for centuries, dating back to the ancient Egyptians 3,500 years ago.1 Through the mid-20th century, about 30% of US households were infested with bedbugs.2 The introduction of pesticides during World War II markedly decreased the incidence, but with increased international travel, pesticide resistance, and the banning of certain pesticides in the last decade, bedbugs have reemerged worldwide.3
BIOLOGY
Bedbugs are red-brown, wingless, oval-shaped insects measuring 4 to 5 mm in length (Figure 1). They are hematophagous ectoparasites that preferentially feed on human blood, although they feed on some animals as well.2
Cimex lectularius dwells in temperate climates and C hemipterus in more tropical climates, but overlap and interbreeding are common. The usual life cycle is about 6 months, but some bugs live 12 months or longer. The female bedbug lays 5 to 8 eggs per week, or approximately 500 eggs in her lifetime, and each egg hatches in 5 to 10 days.4
These photophobic parasites do not live on their human hosts but rather simply visit for a meal. They cohabitate in dark locations, attacking human hosts when they are inactive or sleeping for long periods of time. Common living areas include mattress seams, box springs, bed linens and clothes, wallpaper seams, electrical outlets, and furniture seams (Table 1).5 The female bedbug lays her eggs in these secluded crevices, ensuring their safety until hatching. The dense nests of adult bedbugs, their eggs, and accumulated fecal matter allow for easy visual identification of infestation.5
Bedbugs typically feed between 1:00 am and 5:00 am. Though wingless, they successfully navigate towards their human host, attracted by emitted heat and carbon dioxide.2 Once attached to human skin, the bedbug bite releases enzymes and chemicals including nitrophorin and nitric oxide that facilitate bleeding; these substances are responsible for the resultant dermatitis. (Of note, bedbugs with experimentally excised salivary glands do not cause skin disease in humans.6) After feeding for 3 to 20 minutes, the length and weight of the arthropod can increase by 50% to 200%. A fully sated bedbug can survive for a year until its next meal.2,7 Even if an establishment, home, room, or article of clothing infested with bedbugs has been abandoned for several months, without proper eradication the item still represents a possible nidus for recurrent disease if used, inhabited, or worn again.
EPIDEMIOLOGY
From the earliest documented cases of Cimex in ancient Egyptian tombs to the mid-1900s, the cohabitation of humans and bedbugs was seen as inevitable. With the introduction of DDT 60 years ago, the bedbug population significantly decreased.8 Since DDT’s prohibition, coupled with increased travel and heightened resistance to over-the-counter insecticides, the bedbug population has reemerged exponentially.9,10
Infestations have been reported worldwide, on every continent, and in all 50 of the United States. In Australia, infestations have risen 4,500% in the last 10 to 15 years.11 In the United States, infestation occurs exclusively with C lectularius and the incidence is rising. Philadelphia and New York City are among the most bedbug-infested US cities. New York City experienced a 2,000% increase in bedbug complaints between 2004 and 2009.8
Bedbugs can be transmitted either through active migration of colonies from one area to another adjacent living area through wall spaces or ventilation, or through passive transportation in luggage, clothing, furniture, used mattresses, bookbags, and other personal items.1 Although infestation affects people of all socioeconomic classes and backgrounds, the likelihood increases in people who frequently travel and people who live in lower income neighborhoods with tightly packed apartments. Bedbug infestations are also common in refugee camps: 98% of the rooms in a refugee camp in Sierra Leone had bedbugs, and almost 90% of the residents had signs of bites.12 Unlike scabies, direct person-to-person, skin-to-skin transfer is rare.
CLINICAL FINDINGS
Bedbug bites are analogous, almost identical, to other arthropod bites: bites begin as pink macules that progress to papules (Figure 2), large plaques, or wheals (hives).13 Bites can arise minutes or even days after the initial assault. Some papules and plaques may have a central crust or erosion suggesting a bite.
Bites are typically intensely pruritic, and occasionally, hypersensitive victims can develop bullae, necrotic plaques, or even vasculitis. New papules and plaques form as older ones heal. Some patients may have fever and malaise.13 About 30% of patients may not have skin disease from bedbugs, making diagnosis in those individuals impossible.
The nonspecific nature of this presentation and the subsequent difficulty in prompt diagnosis can lead to a prolonged period of morbidity for the patient, as well as increasing the window of opportunity for the bedbugs to affect other surrounding individuals.
THE DIFFERENTIAL DIAGNOSIS IS BROAD
Commonly, bedbug bites have been misdiagnosed as drug eruptions, food allergies, dermatitis herpetiformis, staphylococcal or varicella infection, and scabies, as well as other arthropod bites.11 This broad differential diagnosis can often be narrowed by careful observation of the bite distribution. The clustering of bites in groups of 3, often in a linear pattern, sometimes overlying blood vessels, is known as the “breakfast, lunch, and dinner” sign (Figure 3), and this can help to guide the clinician toward the diagnosis of a bite as opposed to a diffuse urticarial response.2
If the characteristic clusters of bites are not present, distinguishing clinically between the various causes of pruritic urticarial lesions is difficult. Subtle clues that point towards bedbug bites can be that the rash appears to be most edematous in the morning and flattens throughout the day, as the bites occur typically during sleep.14 Likewise, the rash associated with bedbug bites has also been reported to last longer, to blanch less, and to be less responsive to steroid and antihistamine treatment than other urticarial rashes.14 If a skin biopsy specimen is available, histologic assessment can help to rule out similarly presenting conditions such as prodromal bullous pemphigoid, dermatitis herpetiformis, and urticarial dermatosis, even if it cannot provide a definitive answer as to the etiology.15
Bedbug bites vs other arthropod bites
Once a bite is suspected, differentiating between bedbug and other arthropod bites is the next challenge.
Once again, a detailed assessment of the location of the bites can yield valuable information. The waist, axillae, and uncovered parts of the body are the usual sites for bedbug bites.2 Likewise, inflammatory papules along the eyelid (the “eyelid sign”) are highly suggestive of a bedbug bite.16
The scant involvement of covered body areas, the lack of shallow burrows in the skin, and the lack of scabetic elements on skin scrapings exclude scabies as a diagnosis.
Skin biopsy is not helpful in differentiating arthropod bites, as the histologic findings are nonspecific. The key to a definitive diagnosis in these cases is identification of the suspected bug in characteristic locations. Patients should be encouraged to carefully inspect mattresses, floorboards, and other crevices for the small ovaloid bugs or the reddish-brown specks of heme and feces they typically leave behind on bed linens.15 A positive reported sighting of the bugs can lend credence to the diagnosis, whereas capture and laboratory assessment of a specimen is ideal.
BEDBUGS AS DISEASE VECTORS
Extracutaneous manifestations of bedbug assault are rare. Anaphylaxis to proteins in Cimex saliva may occur, as well as significant blood loss, even anemia, from extensive feeding.17 Bedbug infestations can exacerbate asthma, preexisting mental illness, anxiety, and insomnia.18 Since bedbugs extract blood from hosts, they have a putative ability to act as vectors of disease. Some 45 known pathogens have been isolated from the Cimex species including hepatitis B, human immunodeficiency virus (HIV), Trypanosoma cruzi, and methicillin-resistant Staphylococcus aureus. To date, however, there is no evidence to demonstrate transmission of pathogens to humans.5
TREATMENT AND ERADICATION
Treatment is mainly symptomatic—systemic antihistamines and topical corticosteroids to reduce pruritus and alleviate the dermatitis.2 Patients should be instructed to avoid scratching to prevent infection. Secondary bacterial infection can be treated with topical or systemic antibiotics. Rare cases of bite-induced asthma or anaphylaxis necessitate appropriate emergency treatment. Extermination of infestation is critical to therapy.
If bedbug infestation is suggested, mattresses, bedding, sleeping areas, and bed clothing should be inspected for insects, eggs, and fecal spotting. Adhesives or traps that emit heat or carbon dioxide can be used to capture the bedbugs. During widespread infestation, the arthropods release a pungent odor, which allows trained dogs to detect them with 95% to 98% accuracy.19
Eradication techniques
Once infestation is confirmed, patients should contact an exterminator who can confirm the presence of bedbugs. Typical eradication measures often require nonchemical control and chemical pesticides.
Professional exterminators have special equipment that can heat a room to 48 to 50°C (118–122°F). Heat sustained at this temperature for 90 minutes is sufficient to kill bedbugs.20
The infested area should be vacuumed daily, and vacuum bags and unwanted items should be sealed in plastic before discarding. Clothing, linens, and infested fabrics should be washed and dried in heat at 60°C (140°F) or greater.
Mattresses and furniture should be sealed in a special plastic that allows treatment with heat, steaming, or pesticides. Most professional pesticides contain pyrethroids, but resistance to these products is common, necessitating the use of multiple formulations to overcome resistance.8
Over-the-counter pesticides, almost exclusively pyrethroids, are variably effective and potentially hazardous to consumers.8 Patients must be advised to follow label directions to avoid adverse effects and toxicity.
Alternative chemical eradication methods to circumvent the problem of resistance include piperonyl butoxide, S-methoprene, boric acid, silicates (diatomaceous earth dust), and sulfuryl fluoride. Recent research has also posited the use of antiparasitic agents such as ivermectin and moxidectin in cases of resistant bedbug infestation, with promising results.21
All extermination products and techniques have variable risks, efficacies, and costs,8 and repeat inspections and retreatment are often required.
Prevention strategies include visual inspection of possibly infested rooms, with particular attention to mattress seams and crevices, placing luggage on a luggage rack away from the floor and bed, and careful examination of acquired second-hand items.7
Educating patients is the key to success
While all of the above eradication techniques are important curative strategies, the success of any treatment is contingent on appropriate patient education about the nature of the problem.
Resolving a bedbug infestation is notoriously difficult and requires meticulous adherence to hygiene and cleansing instructions throughout the household or institution for a sustained period of time. Information from sources such as the US Environmental Protection Agency (www.epa.gov) can empower patients to perform the necessary eradication protocols, and clinicians should routinely recommended them as part of a holistic treatment strategy.
- Krause-Parello CA, Sciscione P. Bedbugs: an equal opportunist and cosmopolitan creature. J Sch Nurs 2009; 25:126–132.
- Sfeir M, Munoz-Price LS. Scabies and bedbugs in hospital outbreaks. Curr Infect Dis Rep 2014; 16:412.
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 2007; 44:175–178.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis 2011; 52:200–210.
- Doggett SL, Dwyer DE, Penas PF, Russell RC. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 2012; 25:164–192.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol 2013; 149:372–373.
- Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 2009; 301:1358–1366.
- Davies TG, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 2012; 26:241–254.
- Saenz VL, Booth W, Schal C, Vargo EL. Genetic analysis of bed bug populations reveals small propagule size within individual infestations but high genetic diversity across infestations from the eastern United States. J Med Entomol 2012; 49:865–875.
- Jones SC, Bryant JL. Ineffectiveness of over-the-counter total-release foggers against the bed bug (Heteroptera: cimicidae). J Econ Entomol 2012; 105:957–963.
- Doggett SL, Russell R. Bed bugs—what the GP needs to know. Aust Fam Physician 2009; 38:880–884.
- Gbakima AA, Terry BC, Kanja F, Kortequee S, Dukuley I, Sahr F. High prevalence of bedbugs Cimex hemipterus and Cimex lectularis in camps for internally displaced persons in Freetown, Sierra Leone: a pilot humanitarian investigation. West Afr J Med 2002; 21:268–271.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Scarupa MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol 2006; 117:1508–1509.
- Thomas I, Kihiczak GG, Schwartz RA. Bedbug bites: a review. Int J Dermatol 2004; 43:430–433.
- Quach KA, Zaenglein AL. The eyelid sign: a clue to bed bug bites. Pediatr Dermatol 2014; 31:353–355.
- Paulke-Korinek M, Szell M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Goddard J, de Shazo R. Psychological effects of bed bug attacks (Cimex lectularius L). Am J Med 2012; 125:101–103.
- Pfiester M, Koehler PG, Pereira RM. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J Econ Entomol 2008; 101:1389–1396.
- Kells SA, Goblirsch MJ. Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011; 2:412–422.
- Sheele JM, Ridge GE. Toxicity and potential utility of ivermectin and moxidectin as xenointoxicants against the common bed bug Cimex lectularius L. Parasitol Res 2016; 115:3071–3081.
- Krause-Parello CA, Sciscione P. Bedbugs: an equal opportunist and cosmopolitan creature. J Sch Nurs 2009; 25:126–132.
- Sfeir M, Munoz-Price LS. Scabies and bedbugs in hospital outbreaks. Curr Infect Dis Rep 2014; 16:412.
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 2007; 44:175–178.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis 2011; 52:200–210.
- Doggett SL, Dwyer DE, Penas PF, Russell RC. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 2012; 25:164–192.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol 2013; 149:372–373.
- Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 2009; 301:1358–1366.
- Davies TG, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 2012; 26:241–254.
- Saenz VL, Booth W, Schal C, Vargo EL. Genetic analysis of bed bug populations reveals small propagule size within individual infestations but high genetic diversity across infestations from the eastern United States. J Med Entomol 2012; 49:865–875.
- Jones SC, Bryant JL. Ineffectiveness of over-the-counter total-release foggers against the bed bug (Heteroptera: cimicidae). J Econ Entomol 2012; 105:957–963.
- Doggett SL, Russell R. Bed bugs—what the GP needs to know. Aust Fam Physician 2009; 38:880–884.
- Gbakima AA, Terry BC, Kanja F, Kortequee S, Dukuley I, Sahr F. High prevalence of bedbugs Cimex hemipterus and Cimex lectularis in camps for internally displaced persons in Freetown, Sierra Leone: a pilot humanitarian investigation. West Afr J Med 2002; 21:268–271.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Scarupa MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol 2006; 117:1508–1509.
- Thomas I, Kihiczak GG, Schwartz RA. Bedbug bites: a review. Int J Dermatol 2004; 43:430–433.
- Quach KA, Zaenglein AL. The eyelid sign: a clue to bed bug bites. Pediatr Dermatol 2014; 31:353–355.
- Paulke-Korinek M, Szell M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Goddard J, de Shazo R. Psychological effects of bed bug attacks (Cimex lectularius L). Am J Med 2012; 125:101–103.
- Pfiester M, Koehler PG, Pereira RM. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J Econ Entomol 2008; 101:1389–1396.
- Kells SA, Goblirsch MJ. Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011; 2:412–422.
- Sheele JM, Ridge GE. Toxicity and potential utility of ivermectin and moxidectin as xenointoxicants against the common bed bug Cimex lectularius L. Parasitol Res 2016; 115:3071–3081.
KEY POINTS
- The increase in pyrethroid resistance, the ban of DDT, the ease and frequency of travel, and the increased population density in large cities have led to an exponential rise in the incidence of bedbug infection.
- Once the diagnosis is suggested, patients deserve symptomatic treatment, and extermination of the pests becomes essential, though time-consuming, costly, and often problematic.
- Measures to eliminate infestation and prevent spread include early detection, identification of the pest, patient education, and professional eradication.
Parvovirus mimicking acute HIV infection
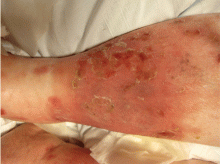
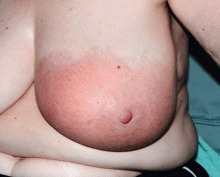
A 25-year-old Jamaican man presented to the emergency department for evaluation of a rash on his face, back, and hands (Figure 1). He recalled a puncture injury after handling garbage at work. He denied recent travel, blood transfusions, or sick contacts. He was not aware of any recent arthropod bites. All standard vaccines including a tetanus booster were up to date.
Examination revealed edema of the hands and uvula. He was discharged with diphenhydramine and a short course of a systemic corticosteroid for presumed contact dermatitis.
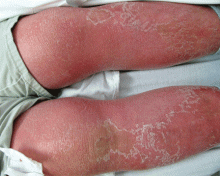
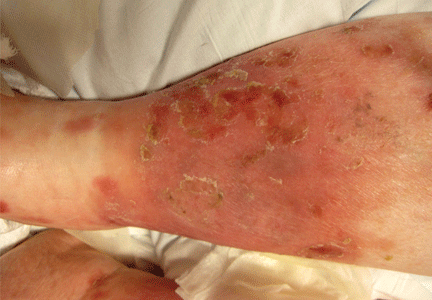
He returned 4 days later with new symptoms, including sore throat, fever with a temperature of 103°F (39.4°C), oropharyngeal pain, dysphagia, dysuria, and purpura on the hands, abdomen, and legs. He was admitted to the hospital.
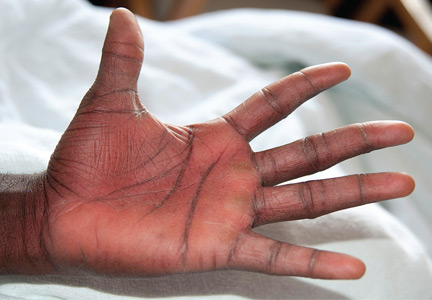
Examination revealed bright red confluent erythema of both legs extending to the lower abdomen, with petechiae on the palms (Figure 2), soles, toes, and fingers. Several small scrotal ulcers with well-defined borders were noted. Oral examination revealed white-yellow adherent plaques on the tongue and similar small ulcers on the lower lip and soft and hard palates.
A complete blood cell count revealed absolute lymphopenia, with a white blood cell count of 0.64 × 109/L (reference range 1.0–4.8) and a neutrophil percentage of 78.9% (39%–68%). Other values were within normal limits, with a red blood cell count of 5.3 × 1012/L (3.9–5.5) and a platelet count of 161 × 109/L (150–350). Serum liver enzymes were also within normal limits.
Treatment with intravenous fluids and intramuscular penicillin G was started empirically, pending a workup for infectious disease. Tests for syphilis immunoglobulin G (IgG), streptococci, anti-streptolysin O, Epstein-Barr virus, and human immunodeficiency virus (HIV) 1 and 2 were negative. The scrotal ulcers were swabbed, and culture and direct fluorescent antibody testing for cytomegalovirus and herpes simplex virus were negative. Urine testing for gonococcal and chlamydial infection was negative.
On the fifth day of hospitalization, the patient’s condition was improving, but there was still no definitive diagnosis. Consultation with the inpatient dermatology team prompted testing for parvovirus B19 infection, based on the gloves-and-socks distribution of the purpura. Testing revealed a slightly elevated parvovirus B19 IgG titer (2.61) and a significantly elevated parvovirus B19 IgM titer (12.74), which confirmed acute parvovirus infection.
The patient’s condition improved over several days with fluid administration, and he was discharged in good condition. He returned 1 week later for a follow-up appointment, at which time only superficial desquamation was noted in the areas previously affected by purpura.
PARVOVIRUS B19: NOT ONLY IN CHILDREN
Parvovirus B19 is responsible for the common childhood viral exanthem known as fifth disease.1 However, although much less common, the virus can also affect young adults, precipitating a dermatosis referred to as gloves-and-socks syndrome characterized by purpura on the hands and feet,2,3 and with a higher incidence in the spring and summer.1
Although papular-purpuric gloves-and- socks syndrome is characterized by purpura on the hands and feet, the cheeks, oral mucosa, inner thighs, buttocks, and genitalia are affected in about 50% of patients.2 In one report, in two-thirds of adult patients the presentation was caused by parvovirus B19 infection,4 but the syndrome has also been associated with Epstein-Barr virus, cytomegalovirus, human herpesvirus types 6 and 7, hepatitis B virus, rubella virus, and varicella zoster virus.4
Parvovirus B19 infection is commonly associated with systemic manifestations such as fever, fatigue, and lymphadenopathy, as well as swelling of the lips, cutaneous and mucosal ulcerations, polyarthritis, and petechiae involving the hard palate, the soft palate, or both.1
The syndrome is self-limited and resolves within 1 to 2 weeks.1
THE DIAGNOSTIC CHALLENGE
The differential diagnosis of the syndrome’s gloves-and-socks presentation includes hand-foot-mouth disease, erythema multiforme, Henoch-Schönlein purpura, and Kawasaki disease,4 in addition to viral exanthems and sexually transmitted diseases. Our patient’s fever, rash, and absolute lymphopenia focused attention on possible HIV infection, which caused the patient significant anxiety while awaiting the results of HIV testing. Heightened awareness of the cutaneous presentation of parvovirus B19 infection can help avoid unnecessary hospitalization and patient anxiety.
- Smith PT, Landry ML, Carey H, Krasnoff J, Cooney E. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis 1998; 27:164–168.
- Harms M, Feldmann R, Saurat JH. Papular-purpuric “gloves and socks” syndrome. J Am Acad Dermatol 1990; 23:850–854.
- Bagot M, Revuz J. Papular-purpuric “gloves and socks” syndrome: primary infection with parvovirus B19? J Am Acad Dermatol 1991; 25:341–342.
- Gutermuth J, Nadas K, Zirbs M, et al. Papular-purpuric gloves and socks syndrome. Lancet 2011; 378:198.
A 25-year-old Jamaican man presented to the emergency department for evaluation of a rash on his face, back, and hands (Figure 1). He recalled a puncture injury after handling garbage at work. He denied recent travel, blood transfusions, or sick contacts. He was not aware of any recent arthropod bites. All standard vaccines including a tetanus booster were up to date.
Examination revealed edema of the hands and uvula. He was discharged with diphenhydramine and a short course of a systemic corticosteroid for presumed contact dermatitis.
He returned 4 days later with new symptoms, including sore throat, fever with a temperature of 103°F (39.4°C), oropharyngeal pain, dysphagia, dysuria, and purpura on the hands, abdomen, and legs. He was admitted to the hospital.
Examination revealed bright red confluent erythema of both legs extending to the lower abdomen, with petechiae on the palms (Figure 2), soles, toes, and fingers. Several small scrotal ulcers with well-defined borders were noted. Oral examination revealed white-yellow adherent plaques on the tongue and similar small ulcers on the lower lip and soft and hard palates.
A complete blood cell count revealed absolute lymphopenia, with a white blood cell count of 0.64 × 109/L (reference range 1.0–4.8) and a neutrophil percentage of 78.9% (39%–68%). Other values were within normal limits, with a red blood cell count of 5.3 × 1012/L (3.9–5.5) and a platelet count of 161 × 109/L (150–350). Serum liver enzymes were also within normal limits.
Treatment with intravenous fluids and intramuscular penicillin G was started empirically, pending a workup for infectious disease. Tests for syphilis immunoglobulin G (IgG), streptococci, anti-streptolysin O, Epstein-Barr virus, and human immunodeficiency virus (HIV) 1 and 2 were negative. The scrotal ulcers were swabbed, and culture and direct fluorescent antibody testing for cytomegalovirus and herpes simplex virus were negative. Urine testing for gonococcal and chlamydial infection was negative.
On the fifth day of hospitalization, the patient’s condition was improving, but there was still no definitive diagnosis. Consultation with the inpatient dermatology team prompted testing for parvovirus B19 infection, based on the gloves-and-socks distribution of the purpura. Testing revealed a slightly elevated parvovirus B19 IgG titer (2.61) and a significantly elevated parvovirus B19 IgM titer (12.74), which confirmed acute parvovirus infection.
The patient’s condition improved over several days with fluid administration, and he was discharged in good condition. He returned 1 week later for a follow-up appointment, at which time only superficial desquamation was noted in the areas previously affected by purpura.
PARVOVIRUS B19: NOT ONLY IN CHILDREN
Parvovirus B19 is responsible for the common childhood viral exanthem known as fifth disease.1 However, although much less common, the virus can also affect young adults, precipitating a dermatosis referred to as gloves-and-socks syndrome characterized by purpura on the hands and feet,2,3 and with a higher incidence in the spring and summer.1
Although papular-purpuric gloves-and- socks syndrome is characterized by purpura on the hands and feet, the cheeks, oral mucosa, inner thighs, buttocks, and genitalia are affected in about 50% of patients.2 In one report, in two-thirds of adult patients the presentation was caused by parvovirus B19 infection,4 but the syndrome has also been associated with Epstein-Barr virus, cytomegalovirus, human herpesvirus types 6 and 7, hepatitis B virus, rubella virus, and varicella zoster virus.4
Parvovirus B19 infection is commonly associated with systemic manifestations such as fever, fatigue, and lymphadenopathy, as well as swelling of the lips, cutaneous and mucosal ulcerations, polyarthritis, and petechiae involving the hard palate, the soft palate, or both.1
The syndrome is self-limited and resolves within 1 to 2 weeks.1
THE DIAGNOSTIC CHALLENGE
The differential diagnosis of the syndrome’s gloves-and-socks presentation includes hand-foot-mouth disease, erythema multiforme, Henoch-Schönlein purpura, and Kawasaki disease,4 in addition to viral exanthems and sexually transmitted diseases. Our patient’s fever, rash, and absolute lymphopenia focused attention on possible HIV infection, which caused the patient significant anxiety while awaiting the results of HIV testing. Heightened awareness of the cutaneous presentation of parvovirus B19 infection can help avoid unnecessary hospitalization and patient anxiety.
A 25-year-old Jamaican man presented to the emergency department for evaluation of a rash on his face, back, and hands (Figure 1). He recalled a puncture injury after handling garbage at work. He denied recent travel, blood transfusions, or sick contacts. He was not aware of any recent arthropod bites. All standard vaccines including a tetanus booster were up to date.
Examination revealed edema of the hands and uvula. He was discharged with diphenhydramine and a short course of a systemic corticosteroid for presumed contact dermatitis.
He returned 4 days later with new symptoms, including sore throat, fever with a temperature of 103°F (39.4°C), oropharyngeal pain, dysphagia, dysuria, and purpura on the hands, abdomen, and legs. He was admitted to the hospital.
Examination revealed bright red confluent erythema of both legs extending to the lower abdomen, with petechiae on the palms (Figure 2), soles, toes, and fingers. Several small scrotal ulcers with well-defined borders were noted. Oral examination revealed white-yellow adherent plaques on the tongue and similar small ulcers on the lower lip and soft and hard palates.
A complete blood cell count revealed absolute lymphopenia, with a white blood cell count of 0.64 × 109/L (reference range 1.0–4.8) and a neutrophil percentage of 78.9% (39%–68%). Other values were within normal limits, with a red blood cell count of 5.3 × 1012/L (3.9–5.5) and a platelet count of 161 × 109/L (150–350). Serum liver enzymes were also within normal limits.
Treatment with intravenous fluids and intramuscular penicillin G was started empirically, pending a workup for infectious disease. Tests for syphilis immunoglobulin G (IgG), streptococci, anti-streptolysin O, Epstein-Barr virus, and human immunodeficiency virus (HIV) 1 and 2 were negative. The scrotal ulcers were swabbed, and culture and direct fluorescent antibody testing for cytomegalovirus and herpes simplex virus were negative. Urine testing for gonococcal and chlamydial infection was negative.
On the fifth day of hospitalization, the patient’s condition was improving, but there was still no definitive diagnosis. Consultation with the inpatient dermatology team prompted testing for parvovirus B19 infection, based on the gloves-and-socks distribution of the purpura. Testing revealed a slightly elevated parvovirus B19 IgG titer (2.61) and a significantly elevated parvovirus B19 IgM titer (12.74), which confirmed acute parvovirus infection.
The patient’s condition improved over several days with fluid administration, and he was discharged in good condition. He returned 1 week later for a follow-up appointment, at which time only superficial desquamation was noted in the areas previously affected by purpura.
PARVOVIRUS B19: NOT ONLY IN CHILDREN
Parvovirus B19 is responsible for the common childhood viral exanthem known as fifth disease.1 However, although much less common, the virus can also affect young adults, precipitating a dermatosis referred to as gloves-and-socks syndrome characterized by purpura on the hands and feet,2,3 and with a higher incidence in the spring and summer.1
Although papular-purpuric gloves-and- socks syndrome is characterized by purpura on the hands and feet, the cheeks, oral mucosa, inner thighs, buttocks, and genitalia are affected in about 50% of patients.2 In one report, in two-thirds of adult patients the presentation was caused by parvovirus B19 infection,4 but the syndrome has also been associated with Epstein-Barr virus, cytomegalovirus, human herpesvirus types 6 and 7, hepatitis B virus, rubella virus, and varicella zoster virus.4
Parvovirus B19 infection is commonly associated with systemic manifestations such as fever, fatigue, and lymphadenopathy, as well as swelling of the lips, cutaneous and mucosal ulcerations, polyarthritis, and petechiae involving the hard palate, the soft palate, or both.1
The syndrome is self-limited and resolves within 1 to 2 weeks.1
THE DIAGNOSTIC CHALLENGE
The differential diagnosis of the syndrome’s gloves-and-socks presentation includes hand-foot-mouth disease, erythema multiforme, Henoch-Schönlein purpura, and Kawasaki disease,4 in addition to viral exanthems and sexually transmitted diseases. Our patient’s fever, rash, and absolute lymphopenia focused attention on possible HIV infection, which caused the patient significant anxiety while awaiting the results of HIV testing. Heightened awareness of the cutaneous presentation of parvovirus B19 infection can help avoid unnecessary hospitalization and patient anxiety.
- Smith PT, Landry ML, Carey H, Krasnoff J, Cooney E. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis 1998; 27:164–168.
- Harms M, Feldmann R, Saurat JH. Papular-purpuric “gloves and socks” syndrome. J Am Acad Dermatol 1990; 23:850–854.
- Bagot M, Revuz J. Papular-purpuric “gloves and socks” syndrome: primary infection with parvovirus B19? J Am Acad Dermatol 1991; 25:341–342.
- Gutermuth J, Nadas K, Zirbs M, et al. Papular-purpuric gloves and socks syndrome. Lancet 2011; 378:198.
- Smith PT, Landry ML, Carey H, Krasnoff J, Cooney E. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis 1998; 27:164–168.
- Harms M, Feldmann R, Saurat JH. Papular-purpuric “gloves and socks” syndrome. J Am Acad Dermatol 1990; 23:850–854.
- Bagot M, Revuz J. Papular-purpuric “gloves and socks” syndrome: primary infection with parvovirus B19? J Am Acad Dermatol 1991; 25:341–342.
- Gutermuth J, Nadas K, Zirbs M, et al. Papular-purpuric gloves and socks syndrome. Lancet 2011; 378:198.
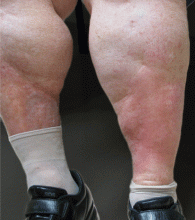
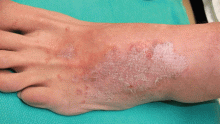
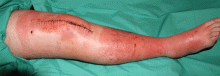
Distinguishing cellulitis from its mimics
More than 10% of patients labeled as having cellulitis do not have cellulitis.1 This is unfortunate, as it leads to excessive and incorrect use of antibiotics and to delays in appropriate therapy.2 However, it is not surprising, given the number of conditions that bear a striking similarity to cellulitis. A familiarity with the features of true cellulitis and with the handful of conditions that can bear a striking similarity to it is the way out of this potential diagnostic quagmire.
WHAT CELLULITIS IS—AND IS NOT
The key characteristics of cellulitis are redness, warmth, tenderness, and swelling of the skin. A history of trauma and pain in the affected area and evidence of leukocytosis3 suggest cellulitis. A symmetric or diffusely scattered pattern indicates a condition other than cellulitis, which is overwhelmingly unilateral, with smooth, indistinct borders4,5 Other factors pointing to cellulitis are underlying immunosuppression, a more rapid progression, previous episodes, systemic symptoms (eg, fever, leukocytosis), new medications, new travel or outdoor exposure, and comorbidities such as diabetes and peripheral vascular disease. A long-standing, slowly progressive course and a history of unsuccessful treatment with antibiotics are strong indicators of a condition other than cellulitis.
Consultation with a dermatologist is recommended to narrow the differential diagnosis. The dermatologist can determine if biopsy is necessary, as many dermatoses that mimic cellulitis can be diagnosed by visual recognition alone.
STASIS DERMATITIS
The most common mimic of cellulitis is stasis dermatitis (Figure 1).2 Patients can present with ill-defined, bilateral, pitting edema of the lower extremities, typically with erythema, hyperpigmentation, serous drainage, and superficial desquamation.3,6,7
The inciting factor is chronic venous insufficiency, leading to interstitial edema, extravasation of red blood cells, and decreased tissue oxygenation. This process causes micro-vascular changes and microthrombi that up-regulate transforming growth factor beta and fibroblastic growth factor.7 If the process is allowed to continue, stasis dermatitis may progress to lipodermatosclerosis.
Tip: Stasis dermatitis is generally bilateral, the process will have been ongoing for years, there is often pitting edema, and the legs should be nontender.
LIPODERMATOSCLEROSIS
Lipodermatosclerosis is a sclerosing panniculitis classically described as an “inverted champagne bottle” or “inverted bowling pin” appearance of the leg, ie, the diameter of the leg is sharply narrowed directly below the calf (Figure 2).
There is an acute and a chronic phase. The acute phase is characterized by inflammation and erythema, and the chronic phase is characterized by fibrosis.8 The acute phase presents with severe lower-extremity pain above the medial malleolus, erythema, edema, and warmth; there is no sharp demarcation between affected and unaffected skin.9,10 This phase can be difficult to distinguish from cellulitis, so the history plays a key role. Known venous insufficiency, cutaneous changes of stasis dermatitis, and the absence of systemic symptoms all point to lipodermatosclerosis.
The chronic phase is characterized by unilateral or bilateral, indurated, sclerotic plaques with a “bound-down” appearance (ie, they appear as if tethered—or bound—to the subcutaneous tissue) affecting the skin from below the knee to the ankle; there is a sharp demarcation between affected and unaffected skin.9–11 The skin is often bronze or brown secondary to hemosiderin deposits. There can be prominent varicosities and scattered ulcerations depending on the course of the disease.
This condition is thought to be the result of long-standing chronic venous insufficiency.7,8,9,11 It is proposed that venous incompetence leads to extravasation of interstitial fluid and red blood cells, decreased diffusion of oxygen to the tissues, and eventual tissue and endothelial damage. As the endothelium is damaged, microthrombi formation and infarction ensue, stimulating fibroblasts to form granulation tissue.
Tip: The history helps to distinguish acute lipodermatosclerosis from cellulitis. Chroniclipodermatoslcerosis will have been ongoing for years, the legs should be nontender, the skin will be bound-down, and the diameter of the leg will sharply decrease from knee to ankle.
CONTACT DERMATITIS
Allergic and irritant forms of contact dermatitis are often mistaken for cellulitis. Irritant contact dermatitis (Figure 3) presents with erythematous patches and plaques with well-defined borders, often in a geometric distribution where the skin was exposed to an irritant.12 Allergic contact dermatitis is a delayed hypersensitivity dermatitis that can be secondary to something ingested, applied to the skin, or airborne (Figure 4). It presents as erythematous macules, papules, and plaques that may have serous drainage or vesiculation. Lesions of allergic contact dermatitis are usually confined to the site of contact with the allergen, but they can infrequently be found at distant sites, in which case it is considered systemic contact dermatitis.3,5 Depending on the severity of the allergy, patients may complain of intense pain and pruritus.3
Additionally, chronic, nonhealing leg ulcers may have a confounding allergic contact dermatitis.7 Although patients may believe they are helping the ulcer heal by applying topical antibiotics or other lubricants, they may in fact be impeding the healing process. Always inquire as to what the patient is applying if he or she has leg ulceration with surrounding edema and erythema that has not resolved with conventional treatments.13,14
Tip: The key to distinguishing contact dermatitis from cellulitis is the history. For example, ask about recent changes in medications, soaps, and laundry detergents, new hobbies, or recent surgeries. The involved site is often confined to the area where the allergen contacted the skin, except in cases of exposure to an airborne allergen.
LYMPHEDEMA
Lymphedema is characterized by localized edema of an affected extremity, with induration, erythema, and secondary cutaneous changes such as hyperkeratosis, dyspigmentation, and wart-like architecture (Figure 5).
Primary lymphedema appears in the setting of congenital abnormalities, whereas secondary lymphedema results from an interruption of a previously functioning lymphatic system (eg, after radical mastectomy).
Patients often present with unilateral nonpitting edema and erythema in the absence of systemic symptoms.12 Many patients presenting with lower-extremity lymphedema are overweight or obese, as the weight they carry causes obstruction of the inguinal lymphatics.6
The pathophysiology is not clearly delineated but is thought to be a consequence of decreased oxygenation of tissue secondary to extravasated lymph. As the oxygen is compromised, macrophages and fibroblasts are recruited, resulting in fibrosis.6
Patients with lymphedema are more susceptible to superficial and deep skin infections, as the natural defense system in the epidermis and papillary dermis is compromised by impaired lymphatic drainage.15
To differentiate uncomplicated lymphedema from a secondary cutaneous infection, the clinician should take into account the presence or absence of warmth, pain, increased erythema, and systemic symptoms (Figure 6).
Tip: Primary lymphedema will most likely present in childhood with no inciting factors and will require a full workup. Obtaining a history should make secondary lymphedema a relatively straightforward diagnosis: Has the patient undergone lymph node dissection? Has the patient had an injury in the affected leg? Lymphedema is overwhelmingly unilateral and nonpitting, and is often seen in overweight people (if no precipitating factor is present).
EOSINOPHILIC CELLULITIS
Eosinophilic cellulitis, or Wells syndrome, was first described in 1971 as a granulomatous dermatitis.16 It is a recurrent hypersensitivity reaction to a drug, to a vaccine, or to an insect bite, or to a viral or fungal infection that presents on the extremities as localized erythema, edema, and induration with sharp borders and a green or gray hue (Figure 7).17–19 The lesions commonly progress to firm, indurated plaques that resemble morphea. The plaques may take weeks or years to resolve, but they do so without scarring.12,17,20,21
As patients tend to have recurrent bouts of eosinophilic cellulitis, they may have lesions in different stages of healing. Patients tend to report itching and burning that precedes the onset of plaques.22 The complete blood count typically shows a transient hypereosinophilia.12,16,17,23–25
Tip: This diagnosis often requires biopsy for confirmation, but helpful clues are a history of recurrent episodes, the color of the lesions, and peripheral eosinophilia.
PAPULAR URTICARIA
Papular urticaria is a dermal hypersensitivity reaction to an insect bite, most commonly from a flea or mosquito.26 Patients are often children, as their immune system may be hypersensitive. But children often develop tolerance before puberty.27
The presentation may vary, from numerous urticarial papules near the site of a bite, to generalized, large, indurated, erythematous plaques reminiscent of cellulitis (Figure 8).5,26 The lesions usually develop within hours of a bite and persist for an average of 1 to 2 weeks.28 The areas typically affected are the head and neck or the upper or lower extremities; the palms, soles, and trunk are usually spared.27
Patients most often complain of intense itching.12 The pathogenesis is proposed to be mediated by the immune complex, and tissue biopsy study shows increased eosinophils. The eosinophils stimulate mast cells, causing release of histamine, leading to increased vascular permeability, edema, and erythema.28,29
Tip: Biopsy may be necessary to confirm the diagnosis, though often the history may be sufficient. The patient may or may not recall a bite, so probe into recent activities such as outdoor sports or contact with a new pet. The papules and plaques are generally very pruritic but not painful.
DERMATOLOGY CONSULT
If the clinical presentation and history do not correlate, or if the skin condition has been treated with antibiotics yet has failed to respond, the possibility of other cutaneous dermatoses should be entertained. A dermatology consult can help determine the diagnosis, the need for further evaluation, and the best treatment course.
- Hepburn MJ, Dooley DP, Ellis MW. Alternative diagnoses that often mimic cellulitis. Am Fam Physician 2003; 67:2471.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J 2011; 17:1.
- Bailey E, Kroshinsky D. Cellulitis: diagnosis and management. Dermatol Ther 2011; 24:229–239.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Lio PA. The many faces of cellulitis. Arch Dis Child Educ Pract Ed 2009; 94:50–54.
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol 2007; 56:901–916.
- Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol 2009; 10:73–86.
- Kirsner RS, Pardes JB, Eaglstein WH, Falanga V. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol 1993; 28:623–627.
- Miteva M, Romanelli P, Kirsner RS. Lipodermatosclerosis. Dermatol Ther 2010; 23:375–388.
- Barron GS, Jacob SE, Kirsner RS. Dermatologic complications of chronic venous disease: medical management and beyond. Ann Vasc Surg 2007; 21:652–662.
- Bruce AJ, Bennett DD, Lohse CM, Rooke TW, Davis MD. Lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol 2002; 46:187–192.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Wilson CL, Cameron J, Powell SM, Cherry G, Ryan TJ. High incidence of contact dermatitis in leg-ulcer patients—implications for management. Clin Exp Dermatol 1991; 16:250–253.
- Wolf R. The lanolin paradox. Dermatology 1996; 192:198–202.
- Keeley VL. Lymphoedema and cellulitis: chicken or egg? Br J Dermatol 2008; 158:1175–1176.
- Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc 1971; 57:46–56.
- Ferreli C, Pinna AL, Atzori L, Aste N. Eosinophilic cellulitis (Well’s syndrome): a new case description. J Eur Acad Dermatol Venereol 1999; 13:41–45.
- Ladoyanni E, Vlachou C, Thushara R, Snead D. A patient with Wells’ syndrome. Clin Exp Dermatol 2010; 35:e3–e4.
- Moon HS, Park K, Lee JH, Son SJ. Eosinophilic cellulitis in an infant. Int J Dermatol 2010; 49:592–593.
- Walker P, Long D, James C, Marshman G. Exaggerated insect bite reaction exacerbated by a pyogenic infection in a patient with chronic lymphocytic leukaemia. Australas J Dermatol 2007; 48:165–169.
- Laliwala NM, Kulshrestha R, Singh R, Balasubramaniam P. A case of eosinophilic cellulitis of the hand mimicking bacterial cellulitis. J Hand Surg Eur Vol 2009; 34:410–411.
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol 2006; 5:908–911.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin 1990; 8:287–293.
- Spigel GT, Winkelmann RK. Wells’ syndrome. Recurrent granulomatous dermatitis with eosinophilia. Arch Dermatol 1979; 115:611–613.
- Clark DP, Anderson PC. Eosinophilic cellulitis caused by arthropod bites. Int J Dermatol 1988; 27:411–412.
- Howard R, Frieden IJ. Papular urticaria in children. Pediatr Dermatol 1996; 13:246–249.
- Hernandez RG, Cohen BA. Insect bite-induced hypersensitivity and the SCRATCH principles: a new approach to papular urticaria. Pediatrics 2006; 118:e189–e196.
- Heng MC, Kloss SG, Haberfelde GC. Pathogenesis of papular urticaria. J Am Acad Dermatol 1984; 10:1030–1034.
- Kossard S, Hamann I, Wilkinson B. Defining urticarial dermatitis: a subset of dermal hypersensitivity reaction pattern. Arch Dermatol 2006; 142:29–34.
More than 10% of patients labeled as having cellulitis do not have cellulitis.1 This is unfortunate, as it leads to excessive and incorrect use of antibiotics and to delays in appropriate therapy.2 However, it is not surprising, given the number of conditions that bear a striking similarity to cellulitis. A familiarity with the features of true cellulitis and with the handful of conditions that can bear a striking similarity to it is the way out of this potential diagnostic quagmire.
WHAT CELLULITIS IS—AND IS NOT
The key characteristics of cellulitis are redness, warmth, tenderness, and swelling of the skin. A history of trauma and pain in the affected area and evidence of leukocytosis3 suggest cellulitis. A symmetric or diffusely scattered pattern indicates a condition other than cellulitis, which is overwhelmingly unilateral, with smooth, indistinct borders4,5 Other factors pointing to cellulitis are underlying immunosuppression, a more rapid progression, previous episodes, systemic symptoms (eg, fever, leukocytosis), new medications, new travel or outdoor exposure, and comorbidities such as diabetes and peripheral vascular disease. A long-standing, slowly progressive course and a history of unsuccessful treatment with antibiotics are strong indicators of a condition other than cellulitis.
Consultation with a dermatologist is recommended to narrow the differential diagnosis. The dermatologist can determine if biopsy is necessary, as many dermatoses that mimic cellulitis can be diagnosed by visual recognition alone.
STASIS DERMATITIS
The most common mimic of cellulitis is stasis dermatitis (Figure 1).2 Patients can present with ill-defined, bilateral, pitting edema of the lower extremities, typically with erythema, hyperpigmentation, serous drainage, and superficial desquamation.3,6,7
The inciting factor is chronic venous insufficiency, leading to interstitial edema, extravasation of red blood cells, and decreased tissue oxygenation. This process causes micro-vascular changes and microthrombi that up-regulate transforming growth factor beta and fibroblastic growth factor.7 If the process is allowed to continue, stasis dermatitis may progress to lipodermatosclerosis.
Tip: Stasis dermatitis is generally bilateral, the process will have been ongoing for years, there is often pitting edema, and the legs should be nontender.
LIPODERMATOSCLEROSIS
Lipodermatosclerosis is a sclerosing panniculitis classically described as an “inverted champagne bottle” or “inverted bowling pin” appearance of the leg, ie, the diameter of the leg is sharply narrowed directly below the calf (Figure 2).
There is an acute and a chronic phase. The acute phase is characterized by inflammation and erythema, and the chronic phase is characterized by fibrosis.8 The acute phase presents with severe lower-extremity pain above the medial malleolus, erythema, edema, and warmth; there is no sharp demarcation between affected and unaffected skin.9,10 This phase can be difficult to distinguish from cellulitis, so the history plays a key role. Known venous insufficiency, cutaneous changes of stasis dermatitis, and the absence of systemic symptoms all point to lipodermatosclerosis.
The chronic phase is characterized by unilateral or bilateral, indurated, sclerotic plaques with a “bound-down” appearance (ie, they appear as if tethered—or bound—to the subcutaneous tissue) affecting the skin from below the knee to the ankle; there is a sharp demarcation between affected and unaffected skin.9–11 The skin is often bronze or brown secondary to hemosiderin deposits. There can be prominent varicosities and scattered ulcerations depending on the course of the disease.
This condition is thought to be the result of long-standing chronic venous insufficiency.7,8,9,11 It is proposed that venous incompetence leads to extravasation of interstitial fluid and red blood cells, decreased diffusion of oxygen to the tissues, and eventual tissue and endothelial damage. As the endothelium is damaged, microthrombi formation and infarction ensue, stimulating fibroblasts to form granulation tissue.
Tip: The history helps to distinguish acute lipodermatosclerosis from cellulitis. Chroniclipodermatoslcerosis will have been ongoing for years, the legs should be nontender, the skin will be bound-down, and the diameter of the leg will sharply decrease from knee to ankle.
CONTACT DERMATITIS
Allergic and irritant forms of contact dermatitis are often mistaken for cellulitis. Irritant contact dermatitis (Figure 3) presents with erythematous patches and plaques with well-defined borders, often in a geometric distribution where the skin was exposed to an irritant.12 Allergic contact dermatitis is a delayed hypersensitivity dermatitis that can be secondary to something ingested, applied to the skin, or airborne (Figure 4). It presents as erythematous macules, papules, and plaques that may have serous drainage or vesiculation. Lesions of allergic contact dermatitis are usually confined to the site of contact with the allergen, but they can infrequently be found at distant sites, in which case it is considered systemic contact dermatitis.3,5 Depending on the severity of the allergy, patients may complain of intense pain and pruritus.3
Additionally, chronic, nonhealing leg ulcers may have a confounding allergic contact dermatitis.7 Although patients may believe they are helping the ulcer heal by applying topical antibiotics or other lubricants, they may in fact be impeding the healing process. Always inquire as to what the patient is applying if he or she has leg ulceration with surrounding edema and erythema that has not resolved with conventional treatments.13,14
Tip: The key to distinguishing contact dermatitis from cellulitis is the history. For example, ask about recent changes in medications, soaps, and laundry detergents, new hobbies, or recent surgeries. The involved site is often confined to the area where the allergen contacted the skin, except in cases of exposure to an airborne allergen.
LYMPHEDEMA
Lymphedema is characterized by localized edema of an affected extremity, with induration, erythema, and secondary cutaneous changes such as hyperkeratosis, dyspigmentation, and wart-like architecture (Figure 5).
Primary lymphedema appears in the setting of congenital abnormalities, whereas secondary lymphedema results from an interruption of a previously functioning lymphatic system (eg, after radical mastectomy).
Patients often present with unilateral nonpitting edema and erythema in the absence of systemic symptoms.12 Many patients presenting with lower-extremity lymphedema are overweight or obese, as the weight they carry causes obstruction of the inguinal lymphatics.6
The pathophysiology is not clearly delineated but is thought to be a consequence of decreased oxygenation of tissue secondary to extravasated lymph. As the oxygen is compromised, macrophages and fibroblasts are recruited, resulting in fibrosis.6
Patients with lymphedema are more susceptible to superficial and deep skin infections, as the natural defense system in the epidermis and papillary dermis is compromised by impaired lymphatic drainage.15
To differentiate uncomplicated lymphedema from a secondary cutaneous infection, the clinician should take into account the presence or absence of warmth, pain, increased erythema, and systemic symptoms (Figure 6).
Tip: Primary lymphedema will most likely present in childhood with no inciting factors and will require a full workup. Obtaining a history should make secondary lymphedema a relatively straightforward diagnosis: Has the patient undergone lymph node dissection? Has the patient had an injury in the affected leg? Lymphedema is overwhelmingly unilateral and nonpitting, and is often seen in overweight people (if no precipitating factor is present).
EOSINOPHILIC CELLULITIS
Eosinophilic cellulitis, or Wells syndrome, was first described in 1971 as a granulomatous dermatitis.16 It is a recurrent hypersensitivity reaction to a drug, to a vaccine, or to an insect bite, or to a viral or fungal infection that presents on the extremities as localized erythema, edema, and induration with sharp borders and a green or gray hue (Figure 7).17–19 The lesions commonly progress to firm, indurated plaques that resemble morphea. The plaques may take weeks or years to resolve, but they do so without scarring.12,17,20,21
As patients tend to have recurrent bouts of eosinophilic cellulitis, they may have lesions in different stages of healing. Patients tend to report itching and burning that precedes the onset of plaques.22 The complete blood count typically shows a transient hypereosinophilia.12,16,17,23–25
Tip: This diagnosis often requires biopsy for confirmation, but helpful clues are a history of recurrent episodes, the color of the lesions, and peripheral eosinophilia.
PAPULAR URTICARIA
Papular urticaria is a dermal hypersensitivity reaction to an insect bite, most commonly from a flea or mosquito.26 Patients are often children, as their immune system may be hypersensitive. But children often develop tolerance before puberty.27
The presentation may vary, from numerous urticarial papules near the site of a bite, to generalized, large, indurated, erythematous plaques reminiscent of cellulitis (Figure 8).5,26 The lesions usually develop within hours of a bite and persist for an average of 1 to 2 weeks.28 The areas typically affected are the head and neck or the upper or lower extremities; the palms, soles, and trunk are usually spared.27
Patients most often complain of intense itching.12 The pathogenesis is proposed to be mediated by the immune complex, and tissue biopsy study shows increased eosinophils. The eosinophils stimulate mast cells, causing release of histamine, leading to increased vascular permeability, edema, and erythema.28,29
Tip: Biopsy may be necessary to confirm the diagnosis, though often the history may be sufficient. The patient may or may not recall a bite, so probe into recent activities such as outdoor sports or contact with a new pet. The papules and plaques are generally very pruritic but not painful.
DERMATOLOGY CONSULT
If the clinical presentation and history do not correlate, or if the skin condition has been treated with antibiotics yet has failed to respond, the possibility of other cutaneous dermatoses should be entertained. A dermatology consult can help determine the diagnosis, the need for further evaluation, and the best treatment course.
More than 10% of patients labeled as having cellulitis do not have cellulitis.1 This is unfortunate, as it leads to excessive and incorrect use of antibiotics and to delays in appropriate therapy.2 However, it is not surprising, given the number of conditions that bear a striking similarity to cellulitis. A familiarity with the features of true cellulitis and with the handful of conditions that can bear a striking similarity to it is the way out of this potential diagnostic quagmire.
WHAT CELLULITIS IS—AND IS NOT
The key characteristics of cellulitis are redness, warmth, tenderness, and swelling of the skin. A history of trauma and pain in the affected area and evidence of leukocytosis3 suggest cellulitis. A symmetric or diffusely scattered pattern indicates a condition other than cellulitis, which is overwhelmingly unilateral, with smooth, indistinct borders4,5 Other factors pointing to cellulitis are underlying immunosuppression, a more rapid progression, previous episodes, systemic symptoms (eg, fever, leukocytosis), new medications, new travel or outdoor exposure, and comorbidities such as diabetes and peripheral vascular disease. A long-standing, slowly progressive course and a history of unsuccessful treatment with antibiotics are strong indicators of a condition other than cellulitis.
Consultation with a dermatologist is recommended to narrow the differential diagnosis. The dermatologist can determine if biopsy is necessary, as many dermatoses that mimic cellulitis can be diagnosed by visual recognition alone.
STASIS DERMATITIS
The most common mimic of cellulitis is stasis dermatitis (Figure 1).2 Patients can present with ill-defined, bilateral, pitting edema of the lower extremities, typically with erythema, hyperpigmentation, serous drainage, and superficial desquamation.3,6,7
The inciting factor is chronic venous insufficiency, leading to interstitial edema, extravasation of red blood cells, and decreased tissue oxygenation. This process causes micro-vascular changes and microthrombi that up-regulate transforming growth factor beta and fibroblastic growth factor.7 If the process is allowed to continue, stasis dermatitis may progress to lipodermatosclerosis.
Tip: Stasis dermatitis is generally bilateral, the process will have been ongoing for years, there is often pitting edema, and the legs should be nontender.
LIPODERMATOSCLEROSIS
Lipodermatosclerosis is a sclerosing panniculitis classically described as an “inverted champagne bottle” or “inverted bowling pin” appearance of the leg, ie, the diameter of the leg is sharply narrowed directly below the calf (Figure 2).
There is an acute and a chronic phase. The acute phase is characterized by inflammation and erythema, and the chronic phase is characterized by fibrosis.8 The acute phase presents with severe lower-extremity pain above the medial malleolus, erythema, edema, and warmth; there is no sharp demarcation between affected and unaffected skin.9,10 This phase can be difficult to distinguish from cellulitis, so the history plays a key role. Known venous insufficiency, cutaneous changes of stasis dermatitis, and the absence of systemic symptoms all point to lipodermatosclerosis.
The chronic phase is characterized by unilateral or bilateral, indurated, sclerotic plaques with a “bound-down” appearance (ie, they appear as if tethered—or bound—to the subcutaneous tissue) affecting the skin from below the knee to the ankle; there is a sharp demarcation between affected and unaffected skin.9–11 The skin is often bronze or brown secondary to hemosiderin deposits. There can be prominent varicosities and scattered ulcerations depending on the course of the disease.
This condition is thought to be the result of long-standing chronic venous insufficiency.7,8,9,11 It is proposed that venous incompetence leads to extravasation of interstitial fluid and red blood cells, decreased diffusion of oxygen to the tissues, and eventual tissue and endothelial damage. As the endothelium is damaged, microthrombi formation and infarction ensue, stimulating fibroblasts to form granulation tissue.
Tip: The history helps to distinguish acute lipodermatosclerosis from cellulitis. Chroniclipodermatoslcerosis will have been ongoing for years, the legs should be nontender, the skin will be bound-down, and the diameter of the leg will sharply decrease from knee to ankle.
CONTACT DERMATITIS
Allergic and irritant forms of contact dermatitis are often mistaken for cellulitis. Irritant contact dermatitis (Figure 3) presents with erythematous patches and plaques with well-defined borders, often in a geometric distribution where the skin was exposed to an irritant.12 Allergic contact dermatitis is a delayed hypersensitivity dermatitis that can be secondary to something ingested, applied to the skin, or airborne (Figure 4). It presents as erythematous macules, papules, and plaques that may have serous drainage or vesiculation. Lesions of allergic contact dermatitis are usually confined to the site of contact with the allergen, but they can infrequently be found at distant sites, in which case it is considered systemic contact dermatitis.3,5 Depending on the severity of the allergy, patients may complain of intense pain and pruritus.3
Additionally, chronic, nonhealing leg ulcers may have a confounding allergic contact dermatitis.7 Although patients may believe they are helping the ulcer heal by applying topical antibiotics or other lubricants, they may in fact be impeding the healing process. Always inquire as to what the patient is applying if he or she has leg ulceration with surrounding edema and erythema that has not resolved with conventional treatments.13,14
Tip: The key to distinguishing contact dermatitis from cellulitis is the history. For example, ask about recent changes in medications, soaps, and laundry detergents, new hobbies, or recent surgeries. The involved site is often confined to the area where the allergen contacted the skin, except in cases of exposure to an airborne allergen.
LYMPHEDEMA
Lymphedema is characterized by localized edema of an affected extremity, with induration, erythema, and secondary cutaneous changes such as hyperkeratosis, dyspigmentation, and wart-like architecture (Figure 5).
Primary lymphedema appears in the setting of congenital abnormalities, whereas secondary lymphedema results from an interruption of a previously functioning lymphatic system (eg, after radical mastectomy).
Patients often present with unilateral nonpitting edema and erythema in the absence of systemic symptoms.12 Many patients presenting with lower-extremity lymphedema are overweight or obese, as the weight they carry causes obstruction of the inguinal lymphatics.6
The pathophysiology is not clearly delineated but is thought to be a consequence of decreased oxygenation of tissue secondary to extravasated lymph. As the oxygen is compromised, macrophages and fibroblasts are recruited, resulting in fibrosis.6
Patients with lymphedema are more susceptible to superficial and deep skin infections, as the natural defense system in the epidermis and papillary dermis is compromised by impaired lymphatic drainage.15
To differentiate uncomplicated lymphedema from a secondary cutaneous infection, the clinician should take into account the presence or absence of warmth, pain, increased erythema, and systemic symptoms (Figure 6).
Tip: Primary lymphedema will most likely present in childhood with no inciting factors and will require a full workup. Obtaining a history should make secondary lymphedema a relatively straightforward diagnosis: Has the patient undergone lymph node dissection? Has the patient had an injury in the affected leg? Lymphedema is overwhelmingly unilateral and nonpitting, and is often seen in overweight people (if no precipitating factor is present).
EOSINOPHILIC CELLULITIS
Eosinophilic cellulitis, or Wells syndrome, was first described in 1971 as a granulomatous dermatitis.16 It is a recurrent hypersensitivity reaction to a drug, to a vaccine, or to an insect bite, or to a viral or fungal infection that presents on the extremities as localized erythema, edema, and induration with sharp borders and a green or gray hue (Figure 7).17–19 The lesions commonly progress to firm, indurated plaques that resemble morphea. The plaques may take weeks or years to resolve, but they do so without scarring.12,17,20,21
As patients tend to have recurrent bouts of eosinophilic cellulitis, they may have lesions in different stages of healing. Patients tend to report itching and burning that precedes the onset of plaques.22 The complete blood count typically shows a transient hypereosinophilia.12,16,17,23–25
Tip: This diagnosis often requires biopsy for confirmation, but helpful clues are a history of recurrent episodes, the color of the lesions, and peripheral eosinophilia.
PAPULAR URTICARIA
Papular urticaria is a dermal hypersensitivity reaction to an insect bite, most commonly from a flea or mosquito.26 Patients are often children, as their immune system may be hypersensitive. But children often develop tolerance before puberty.27
The presentation may vary, from numerous urticarial papules near the site of a bite, to generalized, large, indurated, erythematous plaques reminiscent of cellulitis (Figure 8).5,26 The lesions usually develop within hours of a bite and persist for an average of 1 to 2 weeks.28 The areas typically affected are the head and neck or the upper or lower extremities; the palms, soles, and trunk are usually spared.27
Patients most often complain of intense itching.12 The pathogenesis is proposed to be mediated by the immune complex, and tissue biopsy study shows increased eosinophils. The eosinophils stimulate mast cells, causing release of histamine, leading to increased vascular permeability, edema, and erythema.28,29
Tip: Biopsy may be necessary to confirm the diagnosis, though often the history may be sufficient. The patient may or may not recall a bite, so probe into recent activities such as outdoor sports or contact with a new pet. The papules and plaques are generally very pruritic but not painful.
DERMATOLOGY CONSULT
If the clinical presentation and history do not correlate, or if the skin condition has been treated with antibiotics yet has failed to respond, the possibility of other cutaneous dermatoses should be entertained. A dermatology consult can help determine the diagnosis, the need for further evaluation, and the best treatment course.
- Hepburn MJ, Dooley DP, Ellis MW. Alternative diagnoses that often mimic cellulitis. Am Fam Physician 2003; 67:2471.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J 2011; 17:1.
- Bailey E, Kroshinsky D. Cellulitis: diagnosis and management. Dermatol Ther 2011; 24:229–239.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Lio PA. The many faces of cellulitis. Arch Dis Child Educ Pract Ed 2009; 94:50–54.
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol 2007; 56:901–916.
- Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol 2009; 10:73–86.
- Kirsner RS, Pardes JB, Eaglstein WH, Falanga V. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol 1993; 28:623–627.
- Miteva M, Romanelli P, Kirsner RS. Lipodermatosclerosis. Dermatol Ther 2010; 23:375–388.
- Barron GS, Jacob SE, Kirsner RS. Dermatologic complications of chronic venous disease: medical management and beyond. Ann Vasc Surg 2007; 21:652–662.
- Bruce AJ, Bennett DD, Lohse CM, Rooke TW, Davis MD. Lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol 2002; 46:187–192.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Wilson CL, Cameron J, Powell SM, Cherry G, Ryan TJ. High incidence of contact dermatitis in leg-ulcer patients—implications for management. Clin Exp Dermatol 1991; 16:250–253.
- Wolf R. The lanolin paradox. Dermatology 1996; 192:198–202.
- Keeley VL. Lymphoedema and cellulitis: chicken or egg? Br J Dermatol 2008; 158:1175–1176.
- Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc 1971; 57:46–56.
- Ferreli C, Pinna AL, Atzori L, Aste N. Eosinophilic cellulitis (Well’s syndrome): a new case description. J Eur Acad Dermatol Venereol 1999; 13:41–45.
- Ladoyanni E, Vlachou C, Thushara R, Snead D. A patient with Wells’ syndrome. Clin Exp Dermatol 2010; 35:e3–e4.
- Moon HS, Park K, Lee JH, Son SJ. Eosinophilic cellulitis in an infant. Int J Dermatol 2010; 49:592–593.
- Walker P, Long D, James C, Marshman G. Exaggerated insect bite reaction exacerbated by a pyogenic infection in a patient with chronic lymphocytic leukaemia. Australas J Dermatol 2007; 48:165–169.
- Laliwala NM, Kulshrestha R, Singh R, Balasubramaniam P. A case of eosinophilic cellulitis of the hand mimicking bacterial cellulitis. J Hand Surg Eur Vol 2009; 34:410–411.
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol 2006; 5:908–911.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin 1990; 8:287–293.
- Spigel GT, Winkelmann RK. Wells’ syndrome. Recurrent granulomatous dermatitis with eosinophilia. Arch Dermatol 1979; 115:611–613.
- Clark DP, Anderson PC. Eosinophilic cellulitis caused by arthropod bites. Int J Dermatol 1988; 27:411–412.
- Howard R, Frieden IJ. Papular urticaria in children. Pediatr Dermatol 1996; 13:246–249.
- Hernandez RG, Cohen BA. Insect bite-induced hypersensitivity and the SCRATCH principles: a new approach to papular urticaria. Pediatrics 2006; 118:e189–e196.
- Heng MC, Kloss SG, Haberfelde GC. Pathogenesis of papular urticaria. J Am Acad Dermatol 1984; 10:1030–1034.
- Kossard S, Hamann I, Wilkinson B. Defining urticarial dermatitis: a subset of dermal hypersensitivity reaction pattern. Arch Dermatol 2006; 142:29–34.
- Hepburn MJ, Dooley DP, Ellis MW. Alternative diagnoses that often mimic cellulitis. Am Fam Physician 2003; 67:2471.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J 2011; 17:1.
- Bailey E, Kroshinsky D. Cellulitis: diagnosis and management. Dermatol Ther 2011; 24:229–239.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Lio PA. The many faces of cellulitis. Arch Dis Child Educ Pract Ed 2009; 94:50–54.
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol 2007; 56:901–916.
- Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol 2009; 10:73–86.
- Kirsner RS, Pardes JB, Eaglstein WH, Falanga V. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol 1993; 28:623–627.
- Miteva M, Romanelli P, Kirsner RS. Lipodermatosclerosis. Dermatol Ther 2010; 23:375–388.
- Barron GS, Jacob SE, Kirsner RS. Dermatologic complications of chronic venous disease: medical management and beyond. Ann Vasc Surg 2007; 21:652–662.
- Bruce AJ, Bennett DD, Lohse CM, Rooke TW, Davis MD. Lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol 2002; 46:187–192.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Wilson CL, Cameron J, Powell SM, Cherry G, Ryan TJ. High incidence of contact dermatitis in leg-ulcer patients—implications for management. Clin Exp Dermatol 1991; 16:250–253.
- Wolf R. The lanolin paradox. Dermatology 1996; 192:198–202.
- Keeley VL. Lymphoedema and cellulitis: chicken or egg? Br J Dermatol 2008; 158:1175–1176.
- Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc 1971; 57:46–56.
- Ferreli C, Pinna AL, Atzori L, Aste N. Eosinophilic cellulitis (Well’s syndrome): a new case description. J Eur Acad Dermatol Venereol 1999; 13:41–45.
- Ladoyanni E, Vlachou C, Thushara R, Snead D. A patient with Wells’ syndrome. Clin Exp Dermatol 2010; 35:e3–e4.
- Moon HS, Park K, Lee JH, Son SJ. Eosinophilic cellulitis in an infant. Int J Dermatol 2010; 49:592–593.
- Walker P, Long D, James C, Marshman G. Exaggerated insect bite reaction exacerbated by a pyogenic infection in a patient with chronic lymphocytic leukaemia. Australas J Dermatol 2007; 48:165–169.
- Laliwala NM, Kulshrestha R, Singh R, Balasubramaniam P. A case of eosinophilic cellulitis of the hand mimicking bacterial cellulitis. J Hand Surg Eur Vol 2009; 34:410–411.
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol 2006; 5:908–911.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin 1990; 8:287–293.
- Spigel GT, Winkelmann RK. Wells’ syndrome. Recurrent granulomatous dermatitis with eosinophilia. Arch Dermatol 1979; 115:611–613.
- Clark DP, Anderson PC. Eosinophilic cellulitis caused by arthropod bites. Int J Dermatol 1988; 27:411–412.
- Howard R, Frieden IJ. Papular urticaria in children. Pediatr Dermatol 1996; 13:246–249.
- Hernandez RG, Cohen BA. Insect bite-induced hypersensitivity and the SCRATCH principles: a new approach to papular urticaria. Pediatrics 2006; 118:e189–e196.
- Heng MC, Kloss SG, Haberfelde GC. Pathogenesis of papular urticaria. J Am Acad Dermatol 1984; 10:1030–1034.
- Kossard S, Hamann I, Wilkinson B. Defining urticarial dermatitis: a subset of dermal hypersensitivity reaction pattern. Arch Dermatol 2006; 142:29–34.
KEY POINTS
- Cellulitis is rarely bilateral.
- Patients with cellulitis often have systemic symptoms, such as fever and leukocytosis.
- A chronic course points to a diagnosis other than cellulitis.
- Plaques with a “bound-down” appearance or dark pigmentation point to a chronic disease rather than cellulitis.
- Stasis dermatitis is the most common mimic of cellulitis.
UV protection and sunscreens: What to tell patients
Everyone should avoid overexposure to the sun’s rays. But the desire for the “perfect tan,” the belief that a tan enables one to spend more time in the sun, and a lack of awareness about the dangers of ultraviolet (UV) radiation are factors that contribute to UV-induced skin damage and to an increased risk of skin cancer. Physicians need to be prepared to counsel patients on why and how to avoid damaging UV radiation.
See the patient education handout
Some measures are straightforward, such as wearing protective clothing, limiting sun exposure during the peak daylight hours, and avoiding tanning booths. The issue of which sunscreen to use can be more difficult, given the quantity of sunscreen products and the confusing claims made on product labels.
In this article, we review UV radiation, the consequences of increased exposure to different parts of the UV spectrum, tanning, and the fundamentals of sunscreens. We also briefly review current guidelines from professional organizations and rulings on sunscreen products by the US Food and Drug Administration (FDA).
FACTORS AFFECTING UV EXPOSURE
UV radiation from the sun is strongest between 10:00 am and 4:00 pm at equatorial latitudes and during summer months.1 Certain wavelengths of UV radiation have long been known to contribute to skin cancer in humans: the wavelengths considered most damaging are those from 320 to 400 nm, referred to as UV-A, and from 290 to 320 nm, referred to as UV-B.1,2 The UV spectrum also includes UV-C and other subdivisions, but in this article we are mainly concerned with UV-A and UV-B. From 90% to 95% of UV radiation that reaches the earth’s surface is UV-A, and most of the rest is UV-B.
The different wavelengths of UV-A and UV-B have different effects on the skin. Much of the shorter-wavelength UV-B radiation is scattered by the atmospheric ozone layer, by clouds, by air pollution, and by glass; on the other hand, UV-B rays are the main cause of sunburn in humans. The longer-wavelength UV-A radiation penetrates more deeply into the skin and so may have greater destructive potential.1,3
The daily UV index
The daily UV index of the US National Weather Service and the US Environmental Protection Agency (EPA) (www.epa.gov/sunwise/uvindex.html) offers a direct measurement of the level of UV radiation on a scale of 1 (low) to 11+ (extremely high). The higher the number, the greater the risk of sunburn for a fair-skinned person, even after allowing for cloud cover.
UV EXPOSURE RISKS ARE WELL KNOWN
The American Cancer Society has estimated that the annual incidence of nonmelanoma skin cancer is greater than 2 million, and the incidence of melanoma is from 65,000 to 70,000.4 The incidence of all types of skin cancer has been increasing for the last 30 years.4,5
Exposure to UV radiation is the major environmental risk factor for nonmelanoma skin cancer.6 It is also believed to be a major risk factor for melanoma; although definitive evidence is still lacking, research is beginning to uncover mechanisms linking UV-related gene damage to melanoma.7
UV LIGHT’S EFFECTS ON THE SKIN
The effects of UV light on the skin can be immediate (eg, erythema) and long-term (eg, photoaging, immunosuppression, carcinogenicity).1
Sunburn
Excessive UV damage creates a biochemical milieu that manifests grossly on the skin as a “sunburn.” Excessive UV exposure is damaging regardless of whether a sunburn occurs. Intensive intermittent UV exposure in childhood and teen years leading to blistering sunburn is a risk factor for basal cell carcinoma and malignant melanoma, whereas excessive chronic cumulative exposure is a risk factor for squamous cell carcinoma. In addition, both types of exposure can lead to photoaging.
Sunburn is noticeable 3 to 4 hours after exposure, peaking at around 24 hours.
Photoaging
A long-term effect of UV exposure is photoaging. Although how photoaging occurs is unclear, studies suggest that UV-A contributes more to photoaging, while UV-B contributes to burning, which results in extracellular matrix degradation and dysregulation of collagen metabolism. These changes in matrix and collagen may cause wrinkles and loss of skin turgor; increases in vascular growth factors may induce telangiectasia. All of these effects are characteristic of photoaging.8,9
Immunosuppression, sun exposure, cancer
Profound systemic immunosuppression, such as in organ transplantation patients, can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
But sun exposure itself can also cause both local and systemic immunosuppression depending on the area of exposure and the dosage of UV radiation. The immunosuppressive and carcinogenic effects of UV light on the skin are complex, involving a variety of cell types, including antigen-presenting cells, lymphocytes, and cytokines. UV radiation can cause dysregulation of antigen-presenting cells such as Langerhans cells and dermal dendritic cells, which in turn can activate regulatory T cells to suppress the immune system. UV radiation can also induce keratinocytes to produce immunosuppressive cytokines that inhibit the production of a number of “repair cytokines” that fix UV-induced DNA damage. The repair cytokines can mitigate UV-induced immunosuppression.6,11 These effects can suppress the induction of local, systemic, and memory immunity.
Both UV-A and UV-B interact to enhance UV-induced immunosuppression, and this can occur even at doses that do not cause erythema.12 Profound immunosuppression—whether UV-induced or due to HIV infection or immunosuppressive drugs—can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
Animal studies linking UV-B exposure to skin cancer found that UV-B energy is directly absorbed by DNA, resulting in the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone photoproducts in the DNA, which block replication and transcription.6 The resulting mutations specifically occur in the tumor suppressor gene p53, and these mutations have been linked to squamous cell carcinoma.13,14
UV-A light has also been reported to induce cyclobutane dimers, but via an indirect mechanism, since DNA does not directly absorb UV-A. Dimers induced by UV-A light are apparently cleared at a slower rate than those induced by UV-B, suggesting that UV-A may have a greater potential for carcinogenesis.15 UV-A light can also directly induce carcinogenesis through reactive oxygen species that cause tumorogenic modified bases in the DNA. These modified bases can be misread, leading to decreased DNA integrity.6
WHAT IS TANNING?
UV radiation produces darkening of the skin, or tanning. UV exposure results in both immediate and persistent pigment darkening. Immediate pigment darkening, which is visible and transient, occurs within seconds of UV exposure as a result of the formation of reactive oxygen species and photooxidation of preexisting melanin, and it resolves in a couple of hours. Persistent pigment darkening results from photooxidation and redistribution of preexisting melanin, occurring 2 to 24 hours after sun exposure. Neither type of pigment darkening protects the skin, since no new melanin is produced.16,17
UV-B rays can induce skin erythema, edema, and sunburn, followed by skin desquamation and tanning. Its effects can be seen immediately, but typically the erythema reaches its peak 24 hours later.1
“Delayed tanning” is an adaptive response seen about 3 days after sun exposure and is caused by increased melanocyte activity and new melanin formation in response to UV-B; this effect is considered mildly photoprotective, with a sun protection factor (SPF) of 3. In other words, there is a tiny bit of truth to the common belief that a tan that develops a few days after sun exposure (delayed tanning) can provide a small increase in protection from sunburn. However, the real health concern is not only sunburn, but increased cancer risk and photoaging from UV exposure.
INDOOR TANNING
Every year, nearly 28 million Americans use a sunbed or a sunlamp, and 2.3 million of them are teenagers.18,19 Every day in the United States more than 1 million people use an indoor tanning device.20 Nearly 70% of those who use tanning devices are white women ages 16 to 29.21
Tanning is big business. In 2010, there were 20,000 tanning salons in the United States, and the number of health clubs and spas with tanning beds was between 15,000 and 20,000. In 2010, the tanning industry generated an estimated $4.7 billion in revenue.22
In their search for the perfect tan, people receive very large doses of UV light, and most tanning lamps emit 95% to 99% of their light as UV-A. In fact, the typical sunlamp user can receive an annual dose of UV-A that is 0.3 to 1.2 times the average annual cumulative dose received from sun exposure (7,700 kJ/m2).11 A typical customer of a tanning salon in the course of 20 sessions is exposed to up to 1.2 times the average normal annual exposure from sunlight. Also, for a frequent tanner, the exposure can increase to 4.7 times the average normal annual exposure and up to 12 times the exposure if using high-pressure sunlamps.11 Indoor tanners not only receive large doses of a known carcinogen, but the body’s pigmentary responses to a sunlamp’s UV-A (immediate and persistent pigment darkening) do not protect it from sunburn, cancer-inducing DNA damage, immunosuppression, or photoaging.
Additionally, even though tanning bed lamps only emit 1% to 5% of their light in the UV-B spectrum, one can still receive a very large dose of UV-B radiation with enough exposure.
The American Academy of Dermatology opposes indoor tanning and supports a ban on the nonmedical production and sale of indoor tanning devices. The World Health Organization classifies tanning lamps as carcinogenic and advises minors to avoid indoor tanning.23
SUNSCREEN PROTECTION
Sunscreen products must contain an active sunscreen ingredient that absorbs radiation in the range of 290 to 400 nm. In “physical” sunscreens, the ingredient is an inorganic compound with particles that physically block out UV radiation; in “chemical” sunscreens, the ingredient is an organic compound that absorbs UV radiation.
Most organic UV filters absorb UV-B radiation, and a few act in the UV-A2 range (320–340 nm). Only one FDA-approved organic sunscreen, avobenzone, protects against UV-A1 (340–400 nm).
Inorganic compounds function by physically reflecting and scattering UV radiation from a film of inert metal particles, ie, in a manner similar to protective clothing.24 Two FDA-approved inorganic sunscreens—titanium dioxide and zinc oxide—provide UV-A and UV-B protection. Zinc oxide and the non-micronized form of titanium dioxide provide UV-A1 and UV-A2 protection.
Inorganic sunscreens have a thick consistency and tend to clump. Advances in nanoparticle technology have improved their consistency,25 but micronized titanium dioxide does not provide UV-A1 protection.
The FDA regulates the active ingredients in sunscreen products, determines the methods of testing them, and dictates labelling requirements.
CATEGORIES OF SUNSCREENS
Sunscreens are categorized according to their SPF,26 UV-A protection,27,28 substantivity, and stability.29
Understanding the ‘sun protection factor’
SPF is a laboratory measure of sunscreen efficacy and is defined as the amount of UV radiation required to produce a sunburn on protected skin relative to that of unprotected skin. Since SPF assessment is based on erythema, it is mainly a measure of UV-B exposure, not UV-A exposure.
Contrary to popular belief, the SPF of a product is not related to the duration of UV exposure.30 Also, the relationship between SPF and UV-B protection is not linear: a sunscreen with an SPF of 15 can filter 94% of UV-B radiation, whereas an SPF of 30 provides greater than 97% protection at an equal UV-B dosage. UV radiation dosage depends on both the duration of exposure and the intensity of the UV radiation. Thus, a sunscreen with twice the SPF does not necessarily mean one can stay out in the sun twice as long before developing a sunburn.
Ability to block UV-A radiation
As UV-A causes significant immunosuppression and is the major type of UV radiation reaching Earth, a systematic and repeatable method of measuring a sunscreen’s ability to block UV-A light is necessary.
For each sunscreen, laboratory testing generates a curve of the absorbance within the UV spectrum. The area under this curve is calculated, and a “critical wavelength” is defined as the wavelength where the area under the absorbance curve up to that value is 90% of the total area under the curve. A sunscreen with “broad-spectrum” UV-A protection is one for which the critical wavelength is greater than or equal to 370 nm. The critical wavelength measures the breadth of UV-A absorbance by a sunscreen and must be used in combination with the SPF value to provide a complete assessment of UV protection.27,28,32,33
Substantivity
Substantivity is a sunscreen’s ability to remain effective under adverse conditions such as exposure to water and sweat. A water-resistant product maintains the indicated protection after 40 minutes of water immersion, whereas a very-water-resistant (formerly called “waterproof”) product maintains the indicated protection after 80 minutes of water immersion.27,28,32,33
Stability
The stability of the sunscreen is important for long-lasting protection with continuous exposure to UV light, in particular to prevent photodegradation. The FDA has established maximum levels of each filter allowed in the sunscreen. Several filters can be combined to achieve a high SPF level, to provide broadspectrum UV-A and UV-B protection, and to prevent photodegradation. For example, octocrylene prevents the degradation of the photosensitive compound avobenzone, whereas ecamsule has been combined with avobenzone and octocrylene to provide broad-spectrum UV-A and UV-B protection. Ecamsule is currently patent-protected by L’Oreal and is found only in products produced by it and its subsidiaries.
SUNSCREEN USES AND ABUSES
Sunscreen use generally falls into three categories: daily use, short-term use (eg, for an activity involving increased sun exposure, such as outdoor exercise or work), and use for preventing sunburn during tan acquisition, ie, to increase the time of UV radiation exposure.
Most published studies report on the effects of daily sunscreen protection or on cutaneous immune responses to sunscreen use. However, the use of sunscreens to enhance tan acquisition and to increase sun exposure duration is an abuse of the product and can actually increase the risk of skin cancer. A common misperception is that sunscreens decrease the risk of burning and allow people to increase their exposure to UV radiation. This results in increased exposure to UV-A and thus increases the risk of skin cancers and facilitates photoaging.34
In 2003, Baron et al35 published a randomized trial evaluating the protective effects of UV-B sunscreens (SPF 15) and UV-A/UV-B sunscreens (SPF 15) against UV radiation, using contact hypersensitivity as a model for immunosuppression. The study involved 211 volunteers ages 18 to 59. Measuring skinfold thickness vs total UV dose to calculate an immune protection factor, they reported that the UV-A/UV-B sunscreens had a greater average immune protection factor than the UV-B sunscreen. They concluded that though both types of sunscreen can protect against immunosuppression, the addition of a UV-A filter provides greater protection against immunosuppression.35
A French study36 in 104 volunteers examined the immunoprotective effects of sunscreens with equal SPF but differing levels of UV-A protection after UV exposure, and used delayed-type hypersensitivity as a model for cutaneous immune response. Broader UV-A protection yielded smaller reductions in delayed-type hypersensitivity after UV exposure, leading to the conclusion that UV-A contributes greatly to cutaneous immunosuppression and that UV-A filters can mitigate some of these effects.36
Sunscreens and photoaging
Only a few clinical studies have examined the effects of sunscreen use on photoaging.
In 1995, a randomized, double-blind, placebo-controlled trial involving 53 adults with previously diagnosed with actinic keratosis or skin cancer, or both, showed that those who applied a UV-A/UV-B sunscreen over a 24-month period had less solar elastosis on biopsy compared with controls.37
In 2008, a French study of 12 volunteers showed that broad-spectrum UV protection prevented histologic changes attributed to 6 weeks of chronic UV exposure. The control group exhibited structural and molecular evidence of UV damage (eg, epidermal thickening, decreased procollagen expression, higher lysozyme-to-elastin ratio), whereas chronic use of a broad-spectrum sunscreen either minimized or abrogated these findings.12
Evidence also suggests that broad-spectrum sunscreens can prevent damage from suberythemal doses of UV. A study published in 200738 investigated whether broad-spectrum sunscreen use affects the development of genetic and cellular markers of UV damage after daily suberythemal UV exposure. It reported that unprotected individuals exhibited more thymine dimers, higher p53 expression, and loss of Langerhans cells compared with protected individuals.38
Similarly, a study published in 201012 assessed cellular and molecular markers of photodamage after 19 daily suberythemal UV exposures with or without a broad-spectrum, low-SPF (SPF 8) sunscreen and found that consistent sunscreen use resulted in fewer p53-positive cells, a lower lysozyme-to-elastin ratio, a decreased number and size of melanocytes, and an increased number of Langerhans cells.
Thus, evidence supports the idea that consistent use of a broad-spectrum sunscreen can protect against photodamage, even at doses that do not cause erythema.12
Sunscreens and squamous cell carcinoma
Several large trials provide appreciable evidence that sunscreen is effective in preventing squamous cell carcinoma.
A randomized, controlled, 7-month trial in Australia of a broad-spectrum sunscreen with an SPF of 17 noted a dose-dependent reduction in the development of new actinic keratosis.39 Another randomized, controlled trial from Australia showed a 40% reduction in the development of squamous cell carcinoma over a 4.5-year period in participants who applied a broad-spectrum SPF-16 sunscreen 3 to 4 days per week vs discretionary use.40 Follow-up data at 8 years showed that daily sunscreen users continued to have a 40% lower incidence rate of squamous cell carcinoma than controls.41
Sunscreens and basal cell carcinoma
Although sunscreens appear to be effective in preventing actinic keratosis and squamous cell carcinoma, the evidence that they also prevent basal cell carcinoma and melanoma has been inconclusive.
Sunscreens and melanoma
Using a high number of nevi as a surrogate measure of the risk of developing melanoma, a randomized controlled trial of a broad-spectrum SPF-30 sunscreen in Canadian children over a 3-year period showed a slight decrease in the number of new nevi compared with controls. However, this effect was seen only in children with freckles.42
In a large European study of white school-age children, sunscreen use was associated with an increased number of nevi compared with the use of clothing, which prevented new nevi.43
A large meta-analysis of 18 case-controlled studies failed to show a protective association of sunscreen use with melanoma.44 Postulated confounding factors in earlier studies included older sunscreen formulations with no UV-A protection, low SPF, and limited substantivity. In many cases, sunscreen users exposed themselves to higher doses of UV because of the perceived decreased risk of burning with sunscreen use. This is especially the case when sun exposure was intentional to acquire a tan.34 Individuals who burn easily or may have had a family history of melanoma tended to use more sunscreen, thus creating another confounder. Finally, extrapolation of results from data performed in different geographic latitudes may not be appropriate.
Recently, Green et al45 published a study using the same cohort from a previous study of sunscreens and nonmelanoma skin cancer to examine new primary melanomas as a secondary outcome. They reported that, during the 5-year trial period and during the 10-year follow-up, fewer participants in the intervention group developed primary melanoma compared with the control group (11 vs 21). They concluded that regular applications of a broad-spectrum SPF-16 sunscreen in white adults ages 25 to 75 can decrease the incidence of melanoma.45 The study had serious limitations: the authors admitted that the results were marginally statistically significant; intervention sites of sunscreen application were chosen for nonmelanoma skin cancer and excluded the trunk and lower extremities, where melanomas often occur; and the entire body was analyzed for melanomas, not just the intervention site.46 Thus, despite providing some of the first evidence supporting sunscreen’s ability to prevent melanoma, these results are controversial and are by no means conclusive.
HOW TO USE SUNSCREEN
The American Academy of Dermatology guidelines47 recommend daily, year-round use of a broad-spectrum, water-resistant sunscreen with an SPF of at least 30, regardless of age or skin type. Cloud cover and windows block UV-B but not UV-A. Additionally, 80% of UV light can pass through cloud cover, while 25% is reflected by sand and 80% by snow. Thus, sunscreen should be used daily throughout the year.
Sunscreen should be applied to exposed dry skin 15 to 30 minutes before sun exposure, paying particular attention to common areas of nonmelanoma skin cancer, such as the face, ears, hands, arms, and lips. The standard amount of sunscreen used in SPF testing is 2 mg/cm2, which is difficult to translate into real use; most people apply only 25% to 50% of the recommended amount of sunscreen.48 According to the guidelines, 1 oz of sunscreen—2 tablespoons, or enough to fill a shot glass—is enough to cover sun-exposed parts of the adult body. Sunscreen should be reapplied every 2 hours or after swimming or heavy perspiration; many water-resistant sunscreens lose effectiveness after 40 minutes in the water.
Despite the protective effects of sunscreen, the following are still recommended:
- Seek shade or avoid exposure between 10:00 am and 4:00 pm, ie, when the sun’s rays are strongest
- Take caution around water, sand, and snow, which reflect UV radiation
- Wear protective clothing such as long-sleeved shirts, pants, sunglasses, and wide-brimmed hats
- Do not use tanning beds
- Do not use sunscreens to increase the time of UV exposure.
SPECIAL CONSIDERATIONS: INFANTS
Infants and toddlers are at higher risk of UV damage and skin cancer. Structurally, children’s skin is thinner than that of adults and has lower melanin concentrations. Thus, UV penetrates more deeply into skin that is less able to absorb UV radiation. Animal studies suggest that the skin of children, especially infants, is immunologically immature and less able to respond to UV damage than adult skin. Therefore, extra care must be taken to protect children from UV exposure.49
The American Academy of Pediatrics recommends that infants under 6 months of age should be kept out of direct sunlight whenever possible. A broad-spectrum, water-resistant sunscreen with an SPF of at least 30 should be applied to skin that is not protected by clothing or shade (eg, face, hands, neck).50
NEW FDA GUIDELINES AND OTHER PROPOSED CHANGES
The FDA’s SPF labeling requirements remained unchanged; however, the FDA instituted new regulations regarding UV-A protection. Sunscreens that qualify as broad-spectrum are to be labeled as such, indicating that they protect against radiation in the entire UV spectrum. Products that are “broad-spectrum SPF ≥ 15” can now include the following statement in the “drug facts” part of the label: “If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.”
The FDA now requires sunscreens that are not broad-spectrum or that have an SPF less than 15 to include the following alert: “Spending time in the sun increases your risk of skin cancer and early skin aging.”33 These products can only claim protection from sunburn with the statement: “This product has been shown only to prevent sunburn, not skin cancer or early skin aging.”27,28,32,33
In terms of water resistance, the FDA now bans the terms “sunblock,” “waterproof,” or “sweatproof,” as these claims cannot be substantiated. Instead, the label on the front of the package can only read either “water resistant (40 minutes)” or “water resistant (80 minutes).” Also, sunscreens may no longer claim to provide “instant protection,” nor can they claim to maintain efficacy for more than 2 hours without reapplication.27,28,32,33
Some sunscreen products have been labeled with SPF values exceeding 100. The FDA decided that because there is insufficient evidence of clinical benefit for such SPFs, sunscreen product labels may claim a maximum SPF value of “50+.”28,52
The FDA now also specifies approved formulations for sunscreen products. Oils, lotions, creams, gels, butters, pastes, and ointments are acceptable, and this applies to all products that contain sunscreens, including cosmetics. Wipes, towelettes, powders, body washes, and shampoos are not acceptable as sunscreen products. The FDA now considers the popular spray form as potentially acceptable; a final decision awaits the results of further testing.28,53
Editor’s note: As this paper was being sent to press, the US Food and Drug Administration announced that sunscreen manufacturers would have an additional 6 months to comply with the new labeling rules for sunscreens. The new deadline is December 2012. Smaller companies have until December 2013 to implement the labeling changes.
- Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol 2005; 52:937–958.
- Sivamani RK, Ghiya M, Maibach HI. Shedding light on sunscreens and their labels: testing policies need to match actual use. Am J Prev Med 2010; 38:679–681.
- Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res 2011; 24:136–147.
- American Cancer Society. What are the key statistics about basal and squamous cell skin cancers? http://www.cancer.org/Cancer/SkinCancer-BasalandSquamousCell/DetailedGuide/skin-cancer-basal-and-squamous-cell-key-statistics. Accessed May 9, 2012.
- American Cancer Society. What are the key statistics about melanoma? http://www.cancer.org/Cancer/SkinCancer-Melanoma/DetailedGuide/melanoma-skin-cancer-key-statistics. Accessed May 9, 2012.
- Jou PC, McCormick TS, Baron ED. UV immunosuppression and cutaneous malignancies. Expert Rev Dermatol 2011; 6:61–74.
- Wang Y, Digiovanna JJ, Stern JB, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A 2009; 106:6279–6284.
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol 2005; 152:115–121.
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol 2006; 55:1–19.
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol 2008; 128:447–454.
- Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochem Photobiol 1998; 68:63–70.
- Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol 2008; 58(5 suppl 2):S160–166.
- Besaratinia A, Synold TW, Chen HH, et al. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci U S A 2005; 102:10058–10063.
- May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 1999; 18:7621–7636.
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA 2006; 103:13765–13770.
- Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res 2008; 21:487–491.
- Miyamura Y, Coelho SG, Wolber R, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res 2007; 20:2–13.
- Kwon HT, Mayer JA, Walker KK, Yu H, Lewis EC, Belch GE. Promotion of frequent tanning sessions by indoor tanning facilities: two studies. J Am Acad Dermatol 2002; 46:700–705.
- Dellavalle RP, Parker ER, Cersonsky N, et al. Youth access laws: in the dark at the tanning parlor? Arch Dermatol 2003; 139:443–448.
- Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol 2001; 44:775–780.
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J Am Acad Dermatol 1998; 38:89–98.
- IBISWorld. Tanning salons in the US: Market research report NAICS 81219c. www.ibisworld.com. Accesssed May 9, 2012.
- American Academy of Dermatology Tanning Website. Stats and facts. Prevention and care. Indoor tanning. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/indoor-tanning. Accessed May 9, 2012.
- Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 2007; 370:528–537.
- Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photodermatol Photoimmunol Photomed 2011 Apr; 27( 2):58–67.
- US Food and Drug Administration (FDA). CFR - Code of Federal Regulations Title 21, Chapter 1, Part 352: Sunscreen drug products for over-the-counter human use. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352. Accessed May 9, 2012.
- Wang SQ, Lim HW. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration’s final rule on labeling and effectiveness testing. J Am Acad Dermatol 2011; 65:863–869.
- Food and Drug Administration (FDA). Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use (final rule). Federal Register 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf. Accessed May 9, 2012.
- Scherschun L, Lim HW. Photoprotection by sunscreens. Am J Clin Dermatol 2001; 2:131–134.
- US Food and Drug Administration (FDA). Sunburn protection factor (SPF). http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106351.htm. Accessed May 9, 2012.
- DeSimone EM. FDA proposes changes in sunscreen regulations. Am Pharm 1994; NS34:26–31.
- US Food and Drug Administration (FDA). Questions and answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the US (updated 6/23/11). http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicine-Safely/UnderstandingOver-the-CounterMedicines/ucm258468.htm. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). FDA Press Release. FDA announces changes to better inform consumers about sunscreen: new rules give consumers more information to help reduce the risk of skin cancer, early aging. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258940.htm. Accessed May 9, 2012.
- Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol 2009; 161(suppl 3):40–45.
- Baron ED, Fourtanier A, Compan D, Medaisko C, Cooper KD, Stevens SR. High ultraviolet A protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans. J Invest Dermatol 2003; 121:869–875.
- Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol 2008; 58(suppl 2):S149–S154.
- Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol 1995; 33:941–946.
- Young AR, Orchard GE, Harrison GI, Klock JL. The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen. J Invest Dermatol 2007; 127:975–978.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993; 329:1147–1151.
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–729.
- van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–2548.
- Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA 2000; 283:2955–2960.
- Autier P, Doré JF, Cattaruzza MS, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst 1998; 90:1873–1880.
- Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med 2003; 139:966–978.
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257–263.
- Goldenhersh MA, Koslowsky M. Increased melanoma after regular sunscreen use? J Clin Oncol 2011; 29:e557–e558.
- American Academey of Dermatology Sunscreen Website. Stats and facts. Prevention and care. Sunscreens. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/sunscreens. Accessed May 9, 2012.
- Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol 2002; 138:1319–1325.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics 2011; 128:92–102.
- American Academy of Pediatrics. HealthyChildren. Safety & prevention: Sun safety. http://www.healthychildren.org/english/safety-prevention/at-play/pages/Sun-Safety.aspx. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). Information for consumers (drugs). Sunscreen. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm239463.htm. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Revised effectiveness determination; Sunscreen drug products for over-the-counter human use (proposed rule.) Federal Register 2011. http://69.175.53.6/register/2011/jun/17/2011-14769.pdf. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Sunscreen drug products for over-the-counter human use: Request for data and information regarding dosage forms (advance notice of proposed rulemaking), Federal Register 2011). http://69.175.53.6/register/2011/jun/17/2011-14768.pdf. Accessed May 9, 2012.
Everyone should avoid overexposure to the sun’s rays. But the desire for the “perfect tan,” the belief that a tan enables one to spend more time in the sun, and a lack of awareness about the dangers of ultraviolet (UV) radiation are factors that contribute to UV-induced skin damage and to an increased risk of skin cancer. Physicians need to be prepared to counsel patients on why and how to avoid damaging UV radiation.
See the patient education handout
Some measures are straightforward, such as wearing protective clothing, limiting sun exposure during the peak daylight hours, and avoiding tanning booths. The issue of which sunscreen to use can be more difficult, given the quantity of sunscreen products and the confusing claims made on product labels.
In this article, we review UV radiation, the consequences of increased exposure to different parts of the UV spectrum, tanning, and the fundamentals of sunscreens. We also briefly review current guidelines from professional organizations and rulings on sunscreen products by the US Food and Drug Administration (FDA).
FACTORS AFFECTING UV EXPOSURE
UV radiation from the sun is strongest between 10:00 am and 4:00 pm at equatorial latitudes and during summer months.1 Certain wavelengths of UV radiation have long been known to contribute to skin cancer in humans: the wavelengths considered most damaging are those from 320 to 400 nm, referred to as UV-A, and from 290 to 320 nm, referred to as UV-B.1,2 The UV spectrum also includes UV-C and other subdivisions, but in this article we are mainly concerned with UV-A and UV-B. From 90% to 95% of UV radiation that reaches the earth’s surface is UV-A, and most of the rest is UV-B.
The different wavelengths of UV-A and UV-B have different effects on the skin. Much of the shorter-wavelength UV-B radiation is scattered by the atmospheric ozone layer, by clouds, by air pollution, and by glass; on the other hand, UV-B rays are the main cause of sunburn in humans. The longer-wavelength UV-A radiation penetrates more deeply into the skin and so may have greater destructive potential.1,3
The daily UV index
The daily UV index of the US National Weather Service and the US Environmental Protection Agency (EPA) (www.epa.gov/sunwise/uvindex.html) offers a direct measurement of the level of UV radiation on a scale of 1 (low) to 11+ (extremely high). The higher the number, the greater the risk of sunburn for a fair-skinned person, even after allowing for cloud cover.
UV EXPOSURE RISKS ARE WELL KNOWN
The American Cancer Society has estimated that the annual incidence of nonmelanoma skin cancer is greater than 2 million, and the incidence of melanoma is from 65,000 to 70,000.4 The incidence of all types of skin cancer has been increasing for the last 30 years.4,5
Exposure to UV radiation is the major environmental risk factor for nonmelanoma skin cancer.6 It is also believed to be a major risk factor for melanoma; although definitive evidence is still lacking, research is beginning to uncover mechanisms linking UV-related gene damage to melanoma.7
UV LIGHT’S EFFECTS ON THE SKIN
The effects of UV light on the skin can be immediate (eg, erythema) and long-term (eg, photoaging, immunosuppression, carcinogenicity).1
Sunburn
Excessive UV damage creates a biochemical milieu that manifests grossly on the skin as a “sunburn.” Excessive UV exposure is damaging regardless of whether a sunburn occurs. Intensive intermittent UV exposure in childhood and teen years leading to blistering sunburn is a risk factor for basal cell carcinoma and malignant melanoma, whereas excessive chronic cumulative exposure is a risk factor for squamous cell carcinoma. In addition, both types of exposure can lead to photoaging.
Sunburn is noticeable 3 to 4 hours after exposure, peaking at around 24 hours.
Photoaging
A long-term effect of UV exposure is photoaging. Although how photoaging occurs is unclear, studies suggest that UV-A contributes more to photoaging, while UV-B contributes to burning, which results in extracellular matrix degradation and dysregulation of collagen metabolism. These changes in matrix and collagen may cause wrinkles and loss of skin turgor; increases in vascular growth factors may induce telangiectasia. All of these effects are characteristic of photoaging.8,9
Immunosuppression, sun exposure, cancer
Profound systemic immunosuppression, such as in organ transplantation patients, can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
But sun exposure itself can also cause both local and systemic immunosuppression depending on the area of exposure and the dosage of UV radiation. The immunosuppressive and carcinogenic effects of UV light on the skin are complex, involving a variety of cell types, including antigen-presenting cells, lymphocytes, and cytokines. UV radiation can cause dysregulation of antigen-presenting cells such as Langerhans cells and dermal dendritic cells, which in turn can activate regulatory T cells to suppress the immune system. UV radiation can also induce keratinocytes to produce immunosuppressive cytokines that inhibit the production of a number of “repair cytokines” that fix UV-induced DNA damage. The repair cytokines can mitigate UV-induced immunosuppression.6,11 These effects can suppress the induction of local, systemic, and memory immunity.
Both UV-A and UV-B interact to enhance UV-induced immunosuppression, and this can occur even at doses that do not cause erythema.12 Profound immunosuppression—whether UV-induced or due to HIV infection or immunosuppressive drugs—can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
Animal studies linking UV-B exposure to skin cancer found that UV-B energy is directly absorbed by DNA, resulting in the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone photoproducts in the DNA, which block replication and transcription.6 The resulting mutations specifically occur in the tumor suppressor gene p53, and these mutations have been linked to squamous cell carcinoma.13,14
UV-A light has also been reported to induce cyclobutane dimers, but via an indirect mechanism, since DNA does not directly absorb UV-A. Dimers induced by UV-A light are apparently cleared at a slower rate than those induced by UV-B, suggesting that UV-A may have a greater potential for carcinogenesis.15 UV-A light can also directly induce carcinogenesis through reactive oxygen species that cause tumorogenic modified bases in the DNA. These modified bases can be misread, leading to decreased DNA integrity.6
WHAT IS TANNING?
UV radiation produces darkening of the skin, or tanning. UV exposure results in both immediate and persistent pigment darkening. Immediate pigment darkening, which is visible and transient, occurs within seconds of UV exposure as a result of the formation of reactive oxygen species and photooxidation of preexisting melanin, and it resolves in a couple of hours. Persistent pigment darkening results from photooxidation and redistribution of preexisting melanin, occurring 2 to 24 hours after sun exposure. Neither type of pigment darkening protects the skin, since no new melanin is produced.16,17
UV-B rays can induce skin erythema, edema, and sunburn, followed by skin desquamation and tanning. Its effects can be seen immediately, but typically the erythema reaches its peak 24 hours later.1
“Delayed tanning” is an adaptive response seen about 3 days after sun exposure and is caused by increased melanocyte activity and new melanin formation in response to UV-B; this effect is considered mildly photoprotective, with a sun protection factor (SPF) of 3. In other words, there is a tiny bit of truth to the common belief that a tan that develops a few days after sun exposure (delayed tanning) can provide a small increase in protection from sunburn. However, the real health concern is not only sunburn, but increased cancer risk and photoaging from UV exposure.
INDOOR TANNING
Every year, nearly 28 million Americans use a sunbed or a sunlamp, and 2.3 million of them are teenagers.18,19 Every day in the United States more than 1 million people use an indoor tanning device.20 Nearly 70% of those who use tanning devices are white women ages 16 to 29.21
Tanning is big business. In 2010, there were 20,000 tanning salons in the United States, and the number of health clubs and spas with tanning beds was between 15,000 and 20,000. In 2010, the tanning industry generated an estimated $4.7 billion in revenue.22
In their search for the perfect tan, people receive very large doses of UV light, and most tanning lamps emit 95% to 99% of their light as UV-A. In fact, the typical sunlamp user can receive an annual dose of UV-A that is 0.3 to 1.2 times the average annual cumulative dose received from sun exposure (7,700 kJ/m2).11 A typical customer of a tanning salon in the course of 20 sessions is exposed to up to 1.2 times the average normal annual exposure from sunlight. Also, for a frequent tanner, the exposure can increase to 4.7 times the average normal annual exposure and up to 12 times the exposure if using high-pressure sunlamps.11 Indoor tanners not only receive large doses of a known carcinogen, but the body’s pigmentary responses to a sunlamp’s UV-A (immediate and persistent pigment darkening) do not protect it from sunburn, cancer-inducing DNA damage, immunosuppression, or photoaging.
Additionally, even though tanning bed lamps only emit 1% to 5% of their light in the UV-B spectrum, one can still receive a very large dose of UV-B radiation with enough exposure.
The American Academy of Dermatology opposes indoor tanning and supports a ban on the nonmedical production and sale of indoor tanning devices. The World Health Organization classifies tanning lamps as carcinogenic and advises minors to avoid indoor tanning.23
SUNSCREEN PROTECTION
Sunscreen products must contain an active sunscreen ingredient that absorbs radiation in the range of 290 to 400 nm. In “physical” sunscreens, the ingredient is an inorganic compound with particles that physically block out UV radiation; in “chemical” sunscreens, the ingredient is an organic compound that absorbs UV radiation.
Most organic UV filters absorb UV-B radiation, and a few act in the UV-A2 range (320–340 nm). Only one FDA-approved organic sunscreen, avobenzone, protects against UV-A1 (340–400 nm).
Inorganic compounds function by physically reflecting and scattering UV radiation from a film of inert metal particles, ie, in a manner similar to protective clothing.24 Two FDA-approved inorganic sunscreens—titanium dioxide and zinc oxide—provide UV-A and UV-B protection. Zinc oxide and the non-micronized form of titanium dioxide provide UV-A1 and UV-A2 protection.
Inorganic sunscreens have a thick consistency and tend to clump. Advances in nanoparticle technology have improved their consistency,25 but micronized titanium dioxide does not provide UV-A1 protection.
The FDA regulates the active ingredients in sunscreen products, determines the methods of testing them, and dictates labelling requirements.
CATEGORIES OF SUNSCREENS
Sunscreens are categorized according to their SPF,26 UV-A protection,27,28 substantivity, and stability.29
Understanding the ‘sun protection factor’
SPF is a laboratory measure of sunscreen efficacy and is defined as the amount of UV radiation required to produce a sunburn on protected skin relative to that of unprotected skin. Since SPF assessment is based on erythema, it is mainly a measure of UV-B exposure, not UV-A exposure.
Contrary to popular belief, the SPF of a product is not related to the duration of UV exposure.30 Also, the relationship between SPF and UV-B protection is not linear: a sunscreen with an SPF of 15 can filter 94% of UV-B radiation, whereas an SPF of 30 provides greater than 97% protection at an equal UV-B dosage. UV radiation dosage depends on both the duration of exposure and the intensity of the UV radiation. Thus, a sunscreen with twice the SPF does not necessarily mean one can stay out in the sun twice as long before developing a sunburn.
Ability to block UV-A radiation
As UV-A causes significant immunosuppression and is the major type of UV radiation reaching Earth, a systematic and repeatable method of measuring a sunscreen’s ability to block UV-A light is necessary.
For each sunscreen, laboratory testing generates a curve of the absorbance within the UV spectrum. The area under this curve is calculated, and a “critical wavelength” is defined as the wavelength where the area under the absorbance curve up to that value is 90% of the total area under the curve. A sunscreen with “broad-spectrum” UV-A protection is one for which the critical wavelength is greater than or equal to 370 nm. The critical wavelength measures the breadth of UV-A absorbance by a sunscreen and must be used in combination with the SPF value to provide a complete assessment of UV protection.27,28,32,33
Substantivity
Substantivity is a sunscreen’s ability to remain effective under adverse conditions such as exposure to water and sweat. A water-resistant product maintains the indicated protection after 40 minutes of water immersion, whereas a very-water-resistant (formerly called “waterproof”) product maintains the indicated protection after 80 minutes of water immersion.27,28,32,33
Stability
The stability of the sunscreen is important for long-lasting protection with continuous exposure to UV light, in particular to prevent photodegradation. The FDA has established maximum levels of each filter allowed in the sunscreen. Several filters can be combined to achieve a high SPF level, to provide broadspectrum UV-A and UV-B protection, and to prevent photodegradation. For example, octocrylene prevents the degradation of the photosensitive compound avobenzone, whereas ecamsule has been combined with avobenzone and octocrylene to provide broad-spectrum UV-A and UV-B protection. Ecamsule is currently patent-protected by L’Oreal and is found only in products produced by it and its subsidiaries.
SUNSCREEN USES AND ABUSES
Sunscreen use generally falls into three categories: daily use, short-term use (eg, for an activity involving increased sun exposure, such as outdoor exercise or work), and use for preventing sunburn during tan acquisition, ie, to increase the time of UV radiation exposure.
Most published studies report on the effects of daily sunscreen protection or on cutaneous immune responses to sunscreen use. However, the use of sunscreens to enhance tan acquisition and to increase sun exposure duration is an abuse of the product and can actually increase the risk of skin cancer. A common misperception is that sunscreens decrease the risk of burning and allow people to increase their exposure to UV radiation. This results in increased exposure to UV-A and thus increases the risk of skin cancers and facilitates photoaging.34
In 2003, Baron et al35 published a randomized trial evaluating the protective effects of UV-B sunscreens (SPF 15) and UV-A/UV-B sunscreens (SPF 15) against UV radiation, using contact hypersensitivity as a model for immunosuppression. The study involved 211 volunteers ages 18 to 59. Measuring skinfold thickness vs total UV dose to calculate an immune protection factor, they reported that the UV-A/UV-B sunscreens had a greater average immune protection factor than the UV-B sunscreen. They concluded that though both types of sunscreen can protect against immunosuppression, the addition of a UV-A filter provides greater protection against immunosuppression.35
A French study36 in 104 volunteers examined the immunoprotective effects of sunscreens with equal SPF but differing levels of UV-A protection after UV exposure, and used delayed-type hypersensitivity as a model for cutaneous immune response. Broader UV-A protection yielded smaller reductions in delayed-type hypersensitivity after UV exposure, leading to the conclusion that UV-A contributes greatly to cutaneous immunosuppression and that UV-A filters can mitigate some of these effects.36
Sunscreens and photoaging
Only a few clinical studies have examined the effects of sunscreen use on photoaging.
In 1995, a randomized, double-blind, placebo-controlled trial involving 53 adults with previously diagnosed with actinic keratosis or skin cancer, or both, showed that those who applied a UV-A/UV-B sunscreen over a 24-month period had less solar elastosis on biopsy compared with controls.37
In 2008, a French study of 12 volunteers showed that broad-spectrum UV protection prevented histologic changes attributed to 6 weeks of chronic UV exposure. The control group exhibited structural and molecular evidence of UV damage (eg, epidermal thickening, decreased procollagen expression, higher lysozyme-to-elastin ratio), whereas chronic use of a broad-spectrum sunscreen either minimized or abrogated these findings.12
Evidence also suggests that broad-spectrum sunscreens can prevent damage from suberythemal doses of UV. A study published in 200738 investigated whether broad-spectrum sunscreen use affects the development of genetic and cellular markers of UV damage after daily suberythemal UV exposure. It reported that unprotected individuals exhibited more thymine dimers, higher p53 expression, and loss of Langerhans cells compared with protected individuals.38
Similarly, a study published in 201012 assessed cellular and molecular markers of photodamage after 19 daily suberythemal UV exposures with or without a broad-spectrum, low-SPF (SPF 8) sunscreen and found that consistent sunscreen use resulted in fewer p53-positive cells, a lower lysozyme-to-elastin ratio, a decreased number and size of melanocytes, and an increased number of Langerhans cells.
Thus, evidence supports the idea that consistent use of a broad-spectrum sunscreen can protect against photodamage, even at doses that do not cause erythema.12
Sunscreens and squamous cell carcinoma
Several large trials provide appreciable evidence that sunscreen is effective in preventing squamous cell carcinoma.
A randomized, controlled, 7-month trial in Australia of a broad-spectrum sunscreen with an SPF of 17 noted a dose-dependent reduction in the development of new actinic keratosis.39 Another randomized, controlled trial from Australia showed a 40% reduction in the development of squamous cell carcinoma over a 4.5-year period in participants who applied a broad-spectrum SPF-16 sunscreen 3 to 4 days per week vs discretionary use.40 Follow-up data at 8 years showed that daily sunscreen users continued to have a 40% lower incidence rate of squamous cell carcinoma than controls.41
Sunscreens and basal cell carcinoma
Although sunscreens appear to be effective in preventing actinic keratosis and squamous cell carcinoma, the evidence that they also prevent basal cell carcinoma and melanoma has been inconclusive.
Sunscreens and melanoma
Using a high number of nevi as a surrogate measure of the risk of developing melanoma, a randomized controlled trial of a broad-spectrum SPF-30 sunscreen in Canadian children over a 3-year period showed a slight decrease in the number of new nevi compared with controls. However, this effect was seen only in children with freckles.42
In a large European study of white school-age children, sunscreen use was associated with an increased number of nevi compared with the use of clothing, which prevented new nevi.43
A large meta-analysis of 18 case-controlled studies failed to show a protective association of sunscreen use with melanoma.44 Postulated confounding factors in earlier studies included older sunscreen formulations with no UV-A protection, low SPF, and limited substantivity. In many cases, sunscreen users exposed themselves to higher doses of UV because of the perceived decreased risk of burning with sunscreen use. This is especially the case when sun exposure was intentional to acquire a tan.34 Individuals who burn easily or may have had a family history of melanoma tended to use more sunscreen, thus creating another confounder. Finally, extrapolation of results from data performed in different geographic latitudes may not be appropriate.
Recently, Green et al45 published a study using the same cohort from a previous study of sunscreens and nonmelanoma skin cancer to examine new primary melanomas as a secondary outcome. They reported that, during the 5-year trial period and during the 10-year follow-up, fewer participants in the intervention group developed primary melanoma compared with the control group (11 vs 21). They concluded that regular applications of a broad-spectrum SPF-16 sunscreen in white adults ages 25 to 75 can decrease the incidence of melanoma.45 The study had serious limitations: the authors admitted that the results were marginally statistically significant; intervention sites of sunscreen application were chosen for nonmelanoma skin cancer and excluded the trunk and lower extremities, where melanomas often occur; and the entire body was analyzed for melanomas, not just the intervention site.46 Thus, despite providing some of the first evidence supporting sunscreen’s ability to prevent melanoma, these results are controversial and are by no means conclusive.
HOW TO USE SUNSCREEN
The American Academy of Dermatology guidelines47 recommend daily, year-round use of a broad-spectrum, water-resistant sunscreen with an SPF of at least 30, regardless of age or skin type. Cloud cover and windows block UV-B but not UV-A. Additionally, 80% of UV light can pass through cloud cover, while 25% is reflected by sand and 80% by snow. Thus, sunscreen should be used daily throughout the year.
Sunscreen should be applied to exposed dry skin 15 to 30 minutes before sun exposure, paying particular attention to common areas of nonmelanoma skin cancer, such as the face, ears, hands, arms, and lips. The standard amount of sunscreen used in SPF testing is 2 mg/cm2, which is difficult to translate into real use; most people apply only 25% to 50% of the recommended amount of sunscreen.48 According to the guidelines, 1 oz of sunscreen—2 tablespoons, or enough to fill a shot glass—is enough to cover sun-exposed parts of the adult body. Sunscreen should be reapplied every 2 hours or after swimming or heavy perspiration; many water-resistant sunscreens lose effectiveness after 40 minutes in the water.
Despite the protective effects of sunscreen, the following are still recommended:
- Seek shade or avoid exposure between 10:00 am and 4:00 pm, ie, when the sun’s rays are strongest
- Take caution around water, sand, and snow, which reflect UV radiation
- Wear protective clothing such as long-sleeved shirts, pants, sunglasses, and wide-brimmed hats
- Do not use tanning beds
- Do not use sunscreens to increase the time of UV exposure.
SPECIAL CONSIDERATIONS: INFANTS
Infants and toddlers are at higher risk of UV damage and skin cancer. Structurally, children’s skin is thinner than that of adults and has lower melanin concentrations. Thus, UV penetrates more deeply into skin that is less able to absorb UV radiation. Animal studies suggest that the skin of children, especially infants, is immunologically immature and less able to respond to UV damage than adult skin. Therefore, extra care must be taken to protect children from UV exposure.49
The American Academy of Pediatrics recommends that infants under 6 months of age should be kept out of direct sunlight whenever possible. A broad-spectrum, water-resistant sunscreen with an SPF of at least 30 should be applied to skin that is not protected by clothing or shade (eg, face, hands, neck).50
NEW FDA GUIDELINES AND OTHER PROPOSED CHANGES
The FDA’s SPF labeling requirements remained unchanged; however, the FDA instituted new regulations regarding UV-A protection. Sunscreens that qualify as broad-spectrum are to be labeled as such, indicating that they protect against radiation in the entire UV spectrum. Products that are “broad-spectrum SPF ≥ 15” can now include the following statement in the “drug facts” part of the label: “If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.”
The FDA now requires sunscreens that are not broad-spectrum or that have an SPF less than 15 to include the following alert: “Spending time in the sun increases your risk of skin cancer and early skin aging.”33 These products can only claim protection from sunburn with the statement: “This product has been shown only to prevent sunburn, not skin cancer or early skin aging.”27,28,32,33
In terms of water resistance, the FDA now bans the terms “sunblock,” “waterproof,” or “sweatproof,” as these claims cannot be substantiated. Instead, the label on the front of the package can only read either “water resistant (40 minutes)” or “water resistant (80 minutes).” Also, sunscreens may no longer claim to provide “instant protection,” nor can they claim to maintain efficacy for more than 2 hours without reapplication.27,28,32,33
Some sunscreen products have been labeled with SPF values exceeding 100. The FDA decided that because there is insufficient evidence of clinical benefit for such SPFs, sunscreen product labels may claim a maximum SPF value of “50+.”28,52
The FDA now also specifies approved formulations for sunscreen products. Oils, lotions, creams, gels, butters, pastes, and ointments are acceptable, and this applies to all products that contain sunscreens, including cosmetics. Wipes, towelettes, powders, body washes, and shampoos are not acceptable as sunscreen products. The FDA now considers the popular spray form as potentially acceptable; a final decision awaits the results of further testing.28,53
Editor’s note: As this paper was being sent to press, the US Food and Drug Administration announced that sunscreen manufacturers would have an additional 6 months to comply with the new labeling rules for sunscreens. The new deadline is December 2012. Smaller companies have until December 2013 to implement the labeling changes.
Everyone should avoid overexposure to the sun’s rays. But the desire for the “perfect tan,” the belief that a tan enables one to spend more time in the sun, and a lack of awareness about the dangers of ultraviolet (UV) radiation are factors that contribute to UV-induced skin damage and to an increased risk of skin cancer. Physicians need to be prepared to counsel patients on why and how to avoid damaging UV radiation.
See the patient education handout
Some measures are straightforward, such as wearing protective clothing, limiting sun exposure during the peak daylight hours, and avoiding tanning booths. The issue of which sunscreen to use can be more difficult, given the quantity of sunscreen products and the confusing claims made on product labels.
In this article, we review UV radiation, the consequences of increased exposure to different parts of the UV spectrum, tanning, and the fundamentals of sunscreens. We also briefly review current guidelines from professional organizations and rulings on sunscreen products by the US Food and Drug Administration (FDA).
FACTORS AFFECTING UV EXPOSURE
UV radiation from the sun is strongest between 10:00 am and 4:00 pm at equatorial latitudes and during summer months.1 Certain wavelengths of UV radiation have long been known to contribute to skin cancer in humans: the wavelengths considered most damaging are those from 320 to 400 nm, referred to as UV-A, and from 290 to 320 nm, referred to as UV-B.1,2 The UV spectrum also includes UV-C and other subdivisions, but in this article we are mainly concerned with UV-A and UV-B. From 90% to 95% of UV radiation that reaches the earth’s surface is UV-A, and most of the rest is UV-B.
The different wavelengths of UV-A and UV-B have different effects on the skin. Much of the shorter-wavelength UV-B radiation is scattered by the atmospheric ozone layer, by clouds, by air pollution, and by glass; on the other hand, UV-B rays are the main cause of sunburn in humans. The longer-wavelength UV-A radiation penetrates more deeply into the skin and so may have greater destructive potential.1,3
The daily UV index
The daily UV index of the US National Weather Service and the US Environmental Protection Agency (EPA) (www.epa.gov/sunwise/uvindex.html) offers a direct measurement of the level of UV radiation on a scale of 1 (low) to 11+ (extremely high). The higher the number, the greater the risk of sunburn for a fair-skinned person, even after allowing for cloud cover.
UV EXPOSURE RISKS ARE WELL KNOWN
The American Cancer Society has estimated that the annual incidence of nonmelanoma skin cancer is greater than 2 million, and the incidence of melanoma is from 65,000 to 70,000.4 The incidence of all types of skin cancer has been increasing for the last 30 years.4,5
Exposure to UV radiation is the major environmental risk factor for nonmelanoma skin cancer.6 It is also believed to be a major risk factor for melanoma; although definitive evidence is still lacking, research is beginning to uncover mechanisms linking UV-related gene damage to melanoma.7
UV LIGHT’S EFFECTS ON THE SKIN
The effects of UV light on the skin can be immediate (eg, erythema) and long-term (eg, photoaging, immunosuppression, carcinogenicity).1
Sunburn
Excessive UV damage creates a biochemical milieu that manifests grossly on the skin as a “sunburn.” Excessive UV exposure is damaging regardless of whether a sunburn occurs. Intensive intermittent UV exposure in childhood and teen years leading to blistering sunburn is a risk factor for basal cell carcinoma and malignant melanoma, whereas excessive chronic cumulative exposure is a risk factor for squamous cell carcinoma. In addition, both types of exposure can lead to photoaging.
Sunburn is noticeable 3 to 4 hours after exposure, peaking at around 24 hours.
Photoaging
A long-term effect of UV exposure is photoaging. Although how photoaging occurs is unclear, studies suggest that UV-A contributes more to photoaging, while UV-B contributes to burning, which results in extracellular matrix degradation and dysregulation of collagen metabolism. These changes in matrix and collagen may cause wrinkles and loss of skin turgor; increases in vascular growth factors may induce telangiectasia. All of these effects are characteristic of photoaging.8,9
Immunosuppression, sun exposure, cancer
Profound systemic immunosuppression, such as in organ transplantation patients, can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
But sun exposure itself can also cause both local and systemic immunosuppression depending on the area of exposure and the dosage of UV radiation. The immunosuppressive and carcinogenic effects of UV light on the skin are complex, involving a variety of cell types, including antigen-presenting cells, lymphocytes, and cytokines. UV radiation can cause dysregulation of antigen-presenting cells such as Langerhans cells and dermal dendritic cells, which in turn can activate regulatory T cells to suppress the immune system. UV radiation can also induce keratinocytes to produce immunosuppressive cytokines that inhibit the production of a number of “repair cytokines” that fix UV-induced DNA damage. The repair cytokines can mitigate UV-induced immunosuppression.6,11 These effects can suppress the induction of local, systemic, and memory immunity.
Both UV-A and UV-B interact to enhance UV-induced immunosuppression, and this can occur even at doses that do not cause erythema.12 Profound immunosuppression—whether UV-induced or due to HIV infection or immunosuppressive drugs—can lead to an increased risk of skin cancer, as evidenced by the frequent development of nonmelanoma skin cancers in patients who have undergone organ transplantation, with reported incidence rates of 21% to 50%.6,10
Animal studies linking UV-B exposure to skin cancer found that UV-B energy is directly absorbed by DNA, resulting in the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone photoproducts in the DNA, which block replication and transcription.6 The resulting mutations specifically occur in the tumor suppressor gene p53, and these mutations have been linked to squamous cell carcinoma.13,14
UV-A light has also been reported to induce cyclobutane dimers, but via an indirect mechanism, since DNA does not directly absorb UV-A. Dimers induced by UV-A light are apparently cleared at a slower rate than those induced by UV-B, suggesting that UV-A may have a greater potential for carcinogenesis.15 UV-A light can also directly induce carcinogenesis through reactive oxygen species that cause tumorogenic modified bases in the DNA. These modified bases can be misread, leading to decreased DNA integrity.6
WHAT IS TANNING?
UV radiation produces darkening of the skin, or tanning. UV exposure results in both immediate and persistent pigment darkening. Immediate pigment darkening, which is visible and transient, occurs within seconds of UV exposure as a result of the formation of reactive oxygen species and photooxidation of preexisting melanin, and it resolves in a couple of hours. Persistent pigment darkening results from photooxidation and redistribution of preexisting melanin, occurring 2 to 24 hours after sun exposure. Neither type of pigment darkening protects the skin, since no new melanin is produced.16,17
UV-B rays can induce skin erythema, edema, and sunburn, followed by skin desquamation and tanning. Its effects can be seen immediately, but typically the erythema reaches its peak 24 hours later.1
“Delayed tanning” is an adaptive response seen about 3 days after sun exposure and is caused by increased melanocyte activity and new melanin formation in response to UV-B; this effect is considered mildly photoprotective, with a sun protection factor (SPF) of 3. In other words, there is a tiny bit of truth to the common belief that a tan that develops a few days after sun exposure (delayed tanning) can provide a small increase in protection from sunburn. However, the real health concern is not only sunburn, but increased cancer risk and photoaging from UV exposure.
INDOOR TANNING
Every year, nearly 28 million Americans use a sunbed or a sunlamp, and 2.3 million of them are teenagers.18,19 Every day in the United States more than 1 million people use an indoor tanning device.20 Nearly 70% of those who use tanning devices are white women ages 16 to 29.21
Tanning is big business. In 2010, there were 20,000 tanning salons in the United States, and the number of health clubs and spas with tanning beds was between 15,000 and 20,000. In 2010, the tanning industry generated an estimated $4.7 billion in revenue.22
In their search for the perfect tan, people receive very large doses of UV light, and most tanning lamps emit 95% to 99% of their light as UV-A. In fact, the typical sunlamp user can receive an annual dose of UV-A that is 0.3 to 1.2 times the average annual cumulative dose received from sun exposure (7,700 kJ/m2).11 A typical customer of a tanning salon in the course of 20 sessions is exposed to up to 1.2 times the average normal annual exposure from sunlight. Also, for a frequent tanner, the exposure can increase to 4.7 times the average normal annual exposure and up to 12 times the exposure if using high-pressure sunlamps.11 Indoor tanners not only receive large doses of a known carcinogen, but the body’s pigmentary responses to a sunlamp’s UV-A (immediate and persistent pigment darkening) do not protect it from sunburn, cancer-inducing DNA damage, immunosuppression, or photoaging.
Additionally, even though tanning bed lamps only emit 1% to 5% of their light in the UV-B spectrum, one can still receive a very large dose of UV-B radiation with enough exposure.
The American Academy of Dermatology opposes indoor tanning and supports a ban on the nonmedical production and sale of indoor tanning devices. The World Health Organization classifies tanning lamps as carcinogenic and advises minors to avoid indoor tanning.23
SUNSCREEN PROTECTION
Sunscreen products must contain an active sunscreen ingredient that absorbs radiation in the range of 290 to 400 nm. In “physical” sunscreens, the ingredient is an inorganic compound with particles that physically block out UV radiation; in “chemical” sunscreens, the ingredient is an organic compound that absorbs UV radiation.
Most organic UV filters absorb UV-B radiation, and a few act in the UV-A2 range (320–340 nm). Only one FDA-approved organic sunscreen, avobenzone, protects against UV-A1 (340–400 nm).
Inorganic compounds function by physically reflecting and scattering UV radiation from a film of inert metal particles, ie, in a manner similar to protective clothing.24 Two FDA-approved inorganic sunscreens—titanium dioxide and zinc oxide—provide UV-A and UV-B protection. Zinc oxide and the non-micronized form of titanium dioxide provide UV-A1 and UV-A2 protection.
Inorganic sunscreens have a thick consistency and tend to clump. Advances in nanoparticle technology have improved their consistency,25 but micronized titanium dioxide does not provide UV-A1 protection.
The FDA regulates the active ingredients in sunscreen products, determines the methods of testing them, and dictates labelling requirements.
CATEGORIES OF SUNSCREENS
Sunscreens are categorized according to their SPF,26 UV-A protection,27,28 substantivity, and stability.29
Understanding the ‘sun protection factor’
SPF is a laboratory measure of sunscreen efficacy and is defined as the amount of UV radiation required to produce a sunburn on protected skin relative to that of unprotected skin. Since SPF assessment is based on erythema, it is mainly a measure of UV-B exposure, not UV-A exposure.
Contrary to popular belief, the SPF of a product is not related to the duration of UV exposure.30 Also, the relationship between SPF and UV-B protection is not linear: a sunscreen with an SPF of 15 can filter 94% of UV-B radiation, whereas an SPF of 30 provides greater than 97% protection at an equal UV-B dosage. UV radiation dosage depends on both the duration of exposure and the intensity of the UV radiation. Thus, a sunscreen with twice the SPF does not necessarily mean one can stay out in the sun twice as long before developing a sunburn.
Ability to block UV-A radiation
As UV-A causes significant immunosuppression and is the major type of UV radiation reaching Earth, a systematic and repeatable method of measuring a sunscreen’s ability to block UV-A light is necessary.
For each sunscreen, laboratory testing generates a curve of the absorbance within the UV spectrum. The area under this curve is calculated, and a “critical wavelength” is defined as the wavelength where the area under the absorbance curve up to that value is 90% of the total area under the curve. A sunscreen with “broad-spectrum” UV-A protection is one for which the critical wavelength is greater than or equal to 370 nm. The critical wavelength measures the breadth of UV-A absorbance by a sunscreen and must be used in combination with the SPF value to provide a complete assessment of UV protection.27,28,32,33
Substantivity
Substantivity is a sunscreen’s ability to remain effective under adverse conditions such as exposure to water and sweat. A water-resistant product maintains the indicated protection after 40 minutes of water immersion, whereas a very-water-resistant (formerly called “waterproof”) product maintains the indicated protection after 80 minutes of water immersion.27,28,32,33
Stability
The stability of the sunscreen is important for long-lasting protection with continuous exposure to UV light, in particular to prevent photodegradation. The FDA has established maximum levels of each filter allowed in the sunscreen. Several filters can be combined to achieve a high SPF level, to provide broadspectrum UV-A and UV-B protection, and to prevent photodegradation. For example, octocrylene prevents the degradation of the photosensitive compound avobenzone, whereas ecamsule has been combined with avobenzone and octocrylene to provide broad-spectrum UV-A and UV-B protection. Ecamsule is currently patent-protected by L’Oreal and is found only in products produced by it and its subsidiaries.
SUNSCREEN USES AND ABUSES
Sunscreen use generally falls into three categories: daily use, short-term use (eg, for an activity involving increased sun exposure, such as outdoor exercise or work), and use for preventing sunburn during tan acquisition, ie, to increase the time of UV radiation exposure.
Most published studies report on the effects of daily sunscreen protection or on cutaneous immune responses to sunscreen use. However, the use of sunscreens to enhance tan acquisition and to increase sun exposure duration is an abuse of the product and can actually increase the risk of skin cancer. A common misperception is that sunscreens decrease the risk of burning and allow people to increase their exposure to UV radiation. This results in increased exposure to UV-A and thus increases the risk of skin cancers and facilitates photoaging.34
In 2003, Baron et al35 published a randomized trial evaluating the protective effects of UV-B sunscreens (SPF 15) and UV-A/UV-B sunscreens (SPF 15) against UV radiation, using contact hypersensitivity as a model for immunosuppression. The study involved 211 volunteers ages 18 to 59. Measuring skinfold thickness vs total UV dose to calculate an immune protection factor, they reported that the UV-A/UV-B sunscreens had a greater average immune protection factor than the UV-B sunscreen. They concluded that though both types of sunscreen can protect against immunosuppression, the addition of a UV-A filter provides greater protection against immunosuppression.35
A French study36 in 104 volunteers examined the immunoprotective effects of sunscreens with equal SPF but differing levels of UV-A protection after UV exposure, and used delayed-type hypersensitivity as a model for cutaneous immune response. Broader UV-A protection yielded smaller reductions in delayed-type hypersensitivity after UV exposure, leading to the conclusion that UV-A contributes greatly to cutaneous immunosuppression and that UV-A filters can mitigate some of these effects.36
Sunscreens and photoaging
Only a few clinical studies have examined the effects of sunscreen use on photoaging.
In 1995, a randomized, double-blind, placebo-controlled trial involving 53 adults with previously diagnosed with actinic keratosis or skin cancer, or both, showed that those who applied a UV-A/UV-B sunscreen over a 24-month period had less solar elastosis on biopsy compared with controls.37
In 2008, a French study of 12 volunteers showed that broad-spectrum UV protection prevented histologic changes attributed to 6 weeks of chronic UV exposure. The control group exhibited structural and molecular evidence of UV damage (eg, epidermal thickening, decreased procollagen expression, higher lysozyme-to-elastin ratio), whereas chronic use of a broad-spectrum sunscreen either minimized or abrogated these findings.12
Evidence also suggests that broad-spectrum sunscreens can prevent damage from suberythemal doses of UV. A study published in 200738 investigated whether broad-spectrum sunscreen use affects the development of genetic and cellular markers of UV damage after daily suberythemal UV exposure. It reported that unprotected individuals exhibited more thymine dimers, higher p53 expression, and loss of Langerhans cells compared with protected individuals.38
Similarly, a study published in 201012 assessed cellular and molecular markers of photodamage after 19 daily suberythemal UV exposures with or without a broad-spectrum, low-SPF (SPF 8) sunscreen and found that consistent sunscreen use resulted in fewer p53-positive cells, a lower lysozyme-to-elastin ratio, a decreased number and size of melanocytes, and an increased number of Langerhans cells.
Thus, evidence supports the idea that consistent use of a broad-spectrum sunscreen can protect against photodamage, even at doses that do not cause erythema.12
Sunscreens and squamous cell carcinoma
Several large trials provide appreciable evidence that sunscreen is effective in preventing squamous cell carcinoma.
A randomized, controlled, 7-month trial in Australia of a broad-spectrum sunscreen with an SPF of 17 noted a dose-dependent reduction in the development of new actinic keratosis.39 Another randomized, controlled trial from Australia showed a 40% reduction in the development of squamous cell carcinoma over a 4.5-year period in participants who applied a broad-spectrum SPF-16 sunscreen 3 to 4 days per week vs discretionary use.40 Follow-up data at 8 years showed that daily sunscreen users continued to have a 40% lower incidence rate of squamous cell carcinoma than controls.41
Sunscreens and basal cell carcinoma
Although sunscreens appear to be effective in preventing actinic keratosis and squamous cell carcinoma, the evidence that they also prevent basal cell carcinoma and melanoma has been inconclusive.
Sunscreens and melanoma
Using a high number of nevi as a surrogate measure of the risk of developing melanoma, a randomized controlled trial of a broad-spectrum SPF-30 sunscreen in Canadian children over a 3-year period showed a slight decrease in the number of new nevi compared with controls. However, this effect was seen only in children with freckles.42
In a large European study of white school-age children, sunscreen use was associated with an increased number of nevi compared with the use of clothing, which prevented new nevi.43
A large meta-analysis of 18 case-controlled studies failed to show a protective association of sunscreen use with melanoma.44 Postulated confounding factors in earlier studies included older sunscreen formulations with no UV-A protection, low SPF, and limited substantivity. In many cases, sunscreen users exposed themselves to higher doses of UV because of the perceived decreased risk of burning with sunscreen use. This is especially the case when sun exposure was intentional to acquire a tan.34 Individuals who burn easily or may have had a family history of melanoma tended to use more sunscreen, thus creating another confounder. Finally, extrapolation of results from data performed in different geographic latitudes may not be appropriate.
Recently, Green et al45 published a study using the same cohort from a previous study of sunscreens and nonmelanoma skin cancer to examine new primary melanomas as a secondary outcome. They reported that, during the 5-year trial period and during the 10-year follow-up, fewer participants in the intervention group developed primary melanoma compared with the control group (11 vs 21). They concluded that regular applications of a broad-spectrum SPF-16 sunscreen in white adults ages 25 to 75 can decrease the incidence of melanoma.45 The study had serious limitations: the authors admitted that the results were marginally statistically significant; intervention sites of sunscreen application were chosen for nonmelanoma skin cancer and excluded the trunk and lower extremities, where melanomas often occur; and the entire body was analyzed for melanomas, not just the intervention site.46 Thus, despite providing some of the first evidence supporting sunscreen’s ability to prevent melanoma, these results are controversial and are by no means conclusive.
HOW TO USE SUNSCREEN
The American Academy of Dermatology guidelines47 recommend daily, year-round use of a broad-spectrum, water-resistant sunscreen with an SPF of at least 30, regardless of age or skin type. Cloud cover and windows block UV-B but not UV-A. Additionally, 80% of UV light can pass through cloud cover, while 25% is reflected by sand and 80% by snow. Thus, sunscreen should be used daily throughout the year.
Sunscreen should be applied to exposed dry skin 15 to 30 minutes before sun exposure, paying particular attention to common areas of nonmelanoma skin cancer, such as the face, ears, hands, arms, and lips. The standard amount of sunscreen used in SPF testing is 2 mg/cm2, which is difficult to translate into real use; most people apply only 25% to 50% of the recommended amount of sunscreen.48 According to the guidelines, 1 oz of sunscreen—2 tablespoons, or enough to fill a shot glass—is enough to cover sun-exposed parts of the adult body. Sunscreen should be reapplied every 2 hours or after swimming or heavy perspiration; many water-resistant sunscreens lose effectiveness after 40 minutes in the water.
Despite the protective effects of sunscreen, the following are still recommended:
- Seek shade or avoid exposure between 10:00 am and 4:00 pm, ie, when the sun’s rays are strongest
- Take caution around water, sand, and snow, which reflect UV radiation
- Wear protective clothing such as long-sleeved shirts, pants, sunglasses, and wide-brimmed hats
- Do not use tanning beds
- Do not use sunscreens to increase the time of UV exposure.
SPECIAL CONSIDERATIONS: INFANTS
Infants and toddlers are at higher risk of UV damage and skin cancer. Structurally, children’s skin is thinner than that of adults and has lower melanin concentrations. Thus, UV penetrates more deeply into skin that is less able to absorb UV radiation. Animal studies suggest that the skin of children, especially infants, is immunologically immature and less able to respond to UV damage than adult skin. Therefore, extra care must be taken to protect children from UV exposure.49
The American Academy of Pediatrics recommends that infants under 6 months of age should be kept out of direct sunlight whenever possible. A broad-spectrum, water-resistant sunscreen with an SPF of at least 30 should be applied to skin that is not protected by clothing or shade (eg, face, hands, neck).50
NEW FDA GUIDELINES AND OTHER PROPOSED CHANGES
The FDA’s SPF labeling requirements remained unchanged; however, the FDA instituted new regulations regarding UV-A protection. Sunscreens that qualify as broad-spectrum are to be labeled as such, indicating that they protect against radiation in the entire UV spectrum. Products that are “broad-spectrum SPF ≥ 15” can now include the following statement in the “drug facts” part of the label: “If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.”
The FDA now requires sunscreens that are not broad-spectrum or that have an SPF less than 15 to include the following alert: “Spending time in the sun increases your risk of skin cancer and early skin aging.”33 These products can only claim protection from sunburn with the statement: “This product has been shown only to prevent sunburn, not skin cancer or early skin aging.”27,28,32,33
In terms of water resistance, the FDA now bans the terms “sunblock,” “waterproof,” or “sweatproof,” as these claims cannot be substantiated. Instead, the label on the front of the package can only read either “water resistant (40 minutes)” or “water resistant (80 minutes).” Also, sunscreens may no longer claim to provide “instant protection,” nor can they claim to maintain efficacy for more than 2 hours without reapplication.27,28,32,33
Some sunscreen products have been labeled with SPF values exceeding 100. The FDA decided that because there is insufficient evidence of clinical benefit for such SPFs, sunscreen product labels may claim a maximum SPF value of “50+.”28,52
The FDA now also specifies approved formulations for sunscreen products. Oils, lotions, creams, gels, butters, pastes, and ointments are acceptable, and this applies to all products that contain sunscreens, including cosmetics. Wipes, towelettes, powders, body washes, and shampoos are not acceptable as sunscreen products. The FDA now considers the popular spray form as potentially acceptable; a final decision awaits the results of further testing.28,53
Editor’s note: As this paper was being sent to press, the US Food and Drug Administration announced that sunscreen manufacturers would have an additional 6 months to comply with the new labeling rules for sunscreens. The new deadline is December 2012. Smaller companies have until December 2013 to implement the labeling changes.
- Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol 2005; 52:937–958.
- Sivamani RK, Ghiya M, Maibach HI. Shedding light on sunscreens and their labels: testing policies need to match actual use. Am J Prev Med 2010; 38:679–681.
- Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res 2011; 24:136–147.
- American Cancer Society. What are the key statistics about basal and squamous cell skin cancers? http://www.cancer.org/Cancer/SkinCancer-BasalandSquamousCell/DetailedGuide/skin-cancer-basal-and-squamous-cell-key-statistics. Accessed May 9, 2012.
- American Cancer Society. What are the key statistics about melanoma? http://www.cancer.org/Cancer/SkinCancer-Melanoma/DetailedGuide/melanoma-skin-cancer-key-statistics. Accessed May 9, 2012.
- Jou PC, McCormick TS, Baron ED. UV immunosuppression and cutaneous malignancies. Expert Rev Dermatol 2011; 6:61–74.
- Wang Y, Digiovanna JJ, Stern JB, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A 2009; 106:6279–6284.
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol 2005; 152:115–121.
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol 2006; 55:1–19.
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol 2008; 128:447–454.
- Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochem Photobiol 1998; 68:63–70.
- Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol 2008; 58(5 suppl 2):S160–166.
- Besaratinia A, Synold TW, Chen HH, et al. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci U S A 2005; 102:10058–10063.
- May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 1999; 18:7621–7636.
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA 2006; 103:13765–13770.
- Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res 2008; 21:487–491.
- Miyamura Y, Coelho SG, Wolber R, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res 2007; 20:2–13.
- Kwon HT, Mayer JA, Walker KK, Yu H, Lewis EC, Belch GE. Promotion of frequent tanning sessions by indoor tanning facilities: two studies. J Am Acad Dermatol 2002; 46:700–705.
- Dellavalle RP, Parker ER, Cersonsky N, et al. Youth access laws: in the dark at the tanning parlor? Arch Dermatol 2003; 139:443–448.
- Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol 2001; 44:775–780.
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J Am Acad Dermatol 1998; 38:89–98.
- IBISWorld. Tanning salons in the US: Market research report NAICS 81219c. www.ibisworld.com. Accesssed May 9, 2012.
- American Academy of Dermatology Tanning Website. Stats and facts. Prevention and care. Indoor tanning. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/indoor-tanning. Accessed May 9, 2012.
- Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 2007; 370:528–537.
- Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photodermatol Photoimmunol Photomed 2011 Apr; 27( 2):58–67.
- US Food and Drug Administration (FDA). CFR - Code of Federal Regulations Title 21, Chapter 1, Part 352: Sunscreen drug products for over-the-counter human use. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352. Accessed May 9, 2012.
- Wang SQ, Lim HW. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration’s final rule on labeling and effectiveness testing. J Am Acad Dermatol 2011; 65:863–869.
- Food and Drug Administration (FDA). Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use (final rule). Federal Register 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf. Accessed May 9, 2012.
- Scherschun L, Lim HW. Photoprotection by sunscreens. Am J Clin Dermatol 2001; 2:131–134.
- US Food and Drug Administration (FDA). Sunburn protection factor (SPF). http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106351.htm. Accessed May 9, 2012.
- DeSimone EM. FDA proposes changes in sunscreen regulations. Am Pharm 1994; NS34:26–31.
- US Food and Drug Administration (FDA). Questions and answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the US (updated 6/23/11). http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicine-Safely/UnderstandingOver-the-CounterMedicines/ucm258468.htm. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). FDA Press Release. FDA announces changes to better inform consumers about sunscreen: new rules give consumers more information to help reduce the risk of skin cancer, early aging. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258940.htm. Accessed May 9, 2012.
- Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol 2009; 161(suppl 3):40–45.
- Baron ED, Fourtanier A, Compan D, Medaisko C, Cooper KD, Stevens SR. High ultraviolet A protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans. J Invest Dermatol 2003; 121:869–875.
- Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol 2008; 58(suppl 2):S149–S154.
- Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol 1995; 33:941–946.
- Young AR, Orchard GE, Harrison GI, Klock JL. The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen. J Invest Dermatol 2007; 127:975–978.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993; 329:1147–1151.
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–729.
- van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–2548.
- Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA 2000; 283:2955–2960.
- Autier P, Doré JF, Cattaruzza MS, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst 1998; 90:1873–1880.
- Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med 2003; 139:966–978.
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257–263.
- Goldenhersh MA, Koslowsky M. Increased melanoma after regular sunscreen use? J Clin Oncol 2011; 29:e557–e558.
- American Academey of Dermatology Sunscreen Website. Stats and facts. Prevention and care. Sunscreens. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/sunscreens. Accessed May 9, 2012.
- Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol 2002; 138:1319–1325.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics 2011; 128:92–102.
- American Academy of Pediatrics. HealthyChildren. Safety & prevention: Sun safety. http://www.healthychildren.org/english/safety-prevention/at-play/pages/Sun-Safety.aspx. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). Information for consumers (drugs). Sunscreen. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm239463.htm. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Revised effectiveness determination; Sunscreen drug products for over-the-counter human use (proposed rule.) Federal Register 2011. http://69.175.53.6/register/2011/jun/17/2011-14769.pdf. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Sunscreen drug products for over-the-counter human use: Request for data and information regarding dosage forms (advance notice of proposed rulemaking), Federal Register 2011). http://69.175.53.6/register/2011/jun/17/2011-14768.pdf. Accessed May 9, 2012.
- Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol 2005; 52:937–958.
- Sivamani RK, Ghiya M, Maibach HI. Shedding light on sunscreens and their labels: testing policies need to match actual use. Am J Prev Med 2010; 38:679–681.
- Miyamura Y, Coelho SG, Schlenz K, et al. The deceptive nature of UVA tanning versus the modest protective effects of UVB tanning on human skin. Pigment Cell Melanoma Res 2011; 24:136–147.
- American Cancer Society. What are the key statistics about basal and squamous cell skin cancers? http://www.cancer.org/Cancer/SkinCancer-BasalandSquamousCell/DetailedGuide/skin-cancer-basal-and-squamous-cell-key-statistics. Accessed May 9, 2012.
- American Cancer Society. What are the key statistics about melanoma? http://www.cancer.org/Cancer/SkinCancer-Melanoma/DetailedGuide/melanoma-skin-cancer-key-statistics. Accessed May 9, 2012.
- Jou PC, McCormick TS, Baron ED. UV immunosuppression and cutaneous malignancies. Expert Rev Dermatol 2011; 6:61–74.
- Wang Y, Digiovanna JJ, Stern JB, et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A 2009; 106:6279–6284.
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol 2005; 152:115–121.
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol 2006; 55:1–19.
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol 2008; 128:447–454.
- Miller SA, Hamilton SL, Wester UG, Cyr WH. An analysis of UVA emissions from sunlamps and the potential importance for melanoma. Photochem Photobiol 1998; 68:63–70.
- Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol 2008; 58(5 suppl 2):S160–166.
- Besaratinia A, Synold TW, Chen HH, et al. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci U S A 2005; 102:10058–10063.
- May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 1999; 18:7621–7636.
- Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci USA 2006; 103:13765–13770.
- Wolber R, Schlenz K, Wakamatsu K, et al. Pigmentation effects of solar-simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res 2008; 21:487–491.
- Miyamura Y, Coelho SG, Wolber R, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res 2007; 20:2–13.
- Kwon HT, Mayer JA, Walker KK, Yu H, Lewis EC, Belch GE. Promotion of frequent tanning sessions by indoor tanning facilities: two studies. J Am Acad Dermatol 2002; 46:700–705.
- Dellavalle RP, Parker ER, Cersonsky N, et al. Youth access laws: in the dark at the tanning parlor? Arch Dermatol 2003; 139:443–448.
- Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol 2001; 44:775–780.
- Swerdlow AJ, Weinstock MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J Am Acad Dermatol 1998; 38:89–98.
- IBISWorld. Tanning salons in the US: Market research report NAICS 81219c. www.ibisworld.com. Accesssed May 9, 2012.
- American Academy of Dermatology Tanning Website. Stats and facts. Prevention and care. Indoor tanning. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/indoor-tanning. Accessed May 9, 2012.
- Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet 2007; 370:528–537.
- Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photodermatol Photoimmunol Photomed 2011 Apr; 27( 2):58–67.
- US Food and Drug Administration (FDA). CFR - Code of Federal Regulations Title 21, Chapter 1, Part 352: Sunscreen drug products for over-the-counter human use. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352. Accessed May 9, 2012.
- Wang SQ, Lim HW. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration’s final rule on labeling and effectiveness testing. J Am Acad Dermatol 2011; 65:863–869.
- Food and Drug Administration (FDA). Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use (final rule). Federal Register 2011. http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf. Accessed May 9, 2012.
- Scherschun L, Lim HW. Photoprotection by sunscreens. Am J Clin Dermatol 2001; 2:131–134.
- US Food and Drug Administration (FDA). Sunburn protection factor (SPF). http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm106351.htm. Accessed May 9, 2012.
- DeSimone EM. FDA proposes changes in sunscreen regulations. Am Pharm 1994; NS34:26–31.
- US Food and Drug Administration (FDA). Questions and answers: FDA announces new requirements for over-the-counter (OTC) sunscreen products marketed in the US (updated 6/23/11). http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicine-Safely/UnderstandingOver-the-CounterMedicines/ucm258468.htm. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). FDA Press Release. FDA announces changes to better inform consumers about sunscreen: new rules give consumers more information to help reduce the risk of skin cancer, early aging. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258940.htm. Accessed May 9, 2012.
- Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol 2009; 161(suppl 3):40–45.
- Baron ED, Fourtanier A, Compan D, Medaisko C, Cooper KD, Stevens SR. High ultraviolet A protection affords greater immune protection confirming that ultraviolet A contributes to photoimmunosuppression in humans. J Invest Dermatol 2003; 121:869–875.
- Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol 2008; 58(suppl 2):S149–S154.
- Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol 1995; 33:941–946.
- Young AR, Orchard GE, Harrison GI, Klock JL. The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen. J Invest Dermatol 2007; 127:975–978.
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med 1993; 329:1147–1151.
- Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–729.
- van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–2548.
- Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: a randomized controlled trial. JAMA 2000; 283:2955–2960.
- Autier P, Doré JF, Cattaruzza MS, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst 1998; 90:1873–1880.
- Dennis LK, Beane Freeman LE, VanBeek MJ. Sunscreen use and the risk for melanoma: a quantitative review. Ann Intern Med 2003; 139:966–978.
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257–263.
- Goldenhersh MA, Koslowsky M. Increased melanoma after regular sunscreen use? J Clin Oncol 2011; 29:e557–e558.
- American Academey of Dermatology Sunscreen Website. Stats and facts. Prevention and care. Sunscreens. http://www.aad.org/media-resources/stats-and-facts/prevention-and-care/sunscreens. Accessed May 9, 2012.
- Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol 2002; 138:1319–1325.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics 2011; 128:92–102.
- American Academy of Pediatrics. HealthyChildren. Safety & prevention: Sun safety. http://www.healthychildren.org/english/safety-prevention/at-play/pages/Sun-Safety.aspx. Accessed May 9, 2012.
- US Food and Drug Administration (FDA). Information for consumers (drugs). Sunscreen. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingOver-the-CounterMedicines/ucm239463.htm. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Revised effectiveness determination; Sunscreen drug products for over-the-counter human use (proposed rule.) Federal Register 2011. http://69.175.53.6/register/2011/jun/17/2011-14769.pdf. Accessed May 9, 2012.
- Food and Drug Administration (FDA). Sunscreen drug products for over-the-counter human use: Request for data and information regarding dosage forms (advance notice of proposed rulemaking), Federal Register 2011). http://69.175.53.6/register/2011/jun/17/2011-14768.pdf. Accessed May 9, 2012.
KEY POINTS
- Despite the known risks, nearly 28 million Americans use a sunbed or a sunlamp every year, and 70% of those are white women ages 16 to 29.
- Sunscreens have been a source of confusion in their labeling and their sun protection factor ratings. Revised FDA labeling requirements may help clinicians provide useful guidance to patients.
- The American Academy of Dermatology supports a ban on the nonmedical production and sale of indoor tanning devices.
- Recommendations to prevent UV damage include minimizing sun exposure during peak daylight hours, wearing clothing such as long-sleeve shirts, wide-brimmed hats, and sunglasses, and application of a broad-spectrum sunscreen with UV-A protection. Infants less than 6 months of age require additional protective measures.