User login
2019 Update in perioperative cardiovascular medicine
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
Perioperative medicine is an evolving field with a rapidly growing body of literature, particularly in cardiology.
In this update, we review 6 articles to answer questions related to preoperative cardiac risk assessment, perioperative medication management, and postoperative cardiac complications. We surveyed perioperative literature from February 2018 through January 2019 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
These summaries are derived from “Updates in Perioperative Medicine” presented at the 14th Annual Perioperative Medicine Summit (Orlando, FL, February 13–16, 2019) and the 2019 Society of Hospital Medicine Annual Meeting (National Harbor, MD, March 24–27, 2019).
PREOPERATIVE CARDIAC EVALUATION
How well do measures of functional capacity predict perioperative complications and mortality in noncardiac surgical patients?
Functional capacity is commonly assessed in preoperative evaluations to estimate patients’ risks of perioperative complications and death. The American College of Cardiology/American Heart Association1 and the European Society of Cardiology2 guidelines both include estimation of cardiopulmonary fitness as a step in preoperative assessment before major noncardiac surgery.
“Subjective assessment” is one way to estimate functional capacity. Simply put, clinicians try to form a rough idea about the fitness of patients by asking questions about routine activities such as walking or climbing stairs. Although commonly used, subjective assessment of functional capacity lacks strong evidence that it predicts adverse perioperative events.
Cardiopulmonary exercise testing is a third option. It measures peak oxygen consumption and anaerobic threshold during exercise. It is probably the best objective measurement of functional capacity, but not necessarily for predicting postoperative cardiac complications, and it is performed relatively infrequently.
[Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0]
In a multicenter, prospective cohort study, Wijeysundera et al4 compared subjective functional capacity assessment, the Duke Activity Status Index, cardiopulmonary exercise testing, and the preoperative N-terminal pro-B-type natriuretic peptide (NT-proBNP) level in their ability to predict complications and death in 1,401 noncardiac surgery patients older than 40 with at least 1 cardiovascular risk factor. After surgery, patients had daily electrocardiograms and troponin measurements until postoperative day 3 or discharge.
The primary outcome was the 30-day incidence of death or myocardial infarction (MI). Additional outcomes included the 30-day incidence of death or myocardial injury after noncardiac surgery (MINS), the 1-year mortality rate, and moderate to severe in-hospital perioperative complications.
Findings. Two percent of patients died or had an MI within 30 days of surgery.4
Subjective assessment had only a 19.2% sensitivity (95% confidence interval [CI] 14.2–25) but a 94.7% specificity (95% CI 93.2–95.9) for predicting inability to attain 4 metabolic equivalents during exercise.4
A lower Duke Activity Status Index predicted the primary outcome of death or MI within 30 days (adjusted odds ratio [OR] 0.96, 95% CI 0.83–0.99, P = .03), and it was the only measure that did so. Additionally, the Duke index and NT-proBNP level predicted the risk of death or MINS within 30 days.4
Only elevated NT-proBNP was associated with death at 1 year.4
On exercise testing, low peak oxygen consumption was significantly associated with perioperative complications.
Limitations. The number of primary outcome events (death and MI) was low, potentially affecting the statistical power of the study.
Conclusions. Subjective assessment of functional capacity misclassifies too many patients as being at low risk of perioperative complications and should not be used for preoperative risk stratification. Other tools, such as the Duke Activity Status Index and NT-proBNP levels, are better predictors of adverse perioperative cardiovascular outcomes and should be considered for use in preoperative cardiac risk assessment.
Although the Duke Activity Status Index is a better predictor of adverse outcomes than subjective functional capacity assessment, a specific perioperative threshold for risk classification has not been established. Its correlate for metabolic equivalents should be considered for use in clinical practice at this point.
PERIOPERATIVE MEDICATION MANAGEMENT
Is perioperative aspirin beneficial in patients undergoing vascular surgery?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial,5 a 2-by-2 factorial randomized controlled trial in which patients received perioperative aspirin, clonidine, both, or neither, demonstrated that perioperative aspirin did not reduce cardiovascular events and increased major bleeding. Patients with recently placed coronary stents and those undergoing carotid endarterectomy were excluded because aspirin is known to have a beneficial effect in these patients.
A subsequent substudy6 found perioperative aspirin to be beneficial in patients with coronary stents placed more than a year before noncardiac surgery. Whether perioperative aspirin is beneficial in other subgroups was unknown.
[Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925]
Biccard et al7 investigated the effect of perioperative aspirin in the subgroup of patients from the POISE-2 trial who underwent vascular surgery. The primary outcome was death or MI within 30 days. Secondary outcomes in this substudy included vascular occlusive complications (amputation and peripheral arterial thrombosis) and major or life-threatening bleeding.
Limitations. There were few adverse events, and this substudy was underpowered for the primary and secondary outcomes.
Conclusion. Starting or continuing aspirin did not improve outcomes, and withdrawing it did not increase cardiovascular or occlusive complications.
Do ACE inhibitors affect risk in noncardiac nonvascular surgery?
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are some of the most commonly used medications for treating hypertension. But whether patients should continue receiving them on the day of surgery or whether they should be held remains unclear.
Although current recommendations are inconsistent, the most recent American College of Cardiology/American Heart Association1 perioperative practice guidelines say that continuing ACE inhibitors or ARBs is reasonable perioperatively. This recommendation, however, acknowledges that published evidence is limited. There is general agreement that preoperative exposure to ACE inhibitors and ARBs is associated with intraoperative hypotension, but whether this increases the risk of adverse clinical outcomes remains unclear. Needed was a study to determine the effect on perioperative morbidity and mortality of continuing vs withholding ACE inhibitors and ARBs before surgery.
[Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036]
Shiffermiller et al8 performed a randomized controlled trial comparing the effect of 2 preoperative ACE inhibitor management protocols in patients undergoing noncardiac nonvascular surgery. Patients were randomized to either receive or not receive their final preoperative ACE inhibitor dose, whether scheduled on the morning of surgery or the night before.
Exclusion criteria included hypotension or hypertension at their preoperative clinic appointment (defined as systolic blood pressure < 90 or ≥ 160 mm Hg, and diastolic blood pressure < 60 or ≥ 95 mm Hg), moderate to severe heart failure, and end-stage renal disease requiring dialysis. Excluded surgery types were cardiac, vascular, organ transplant, oncologic, and all outpatient procedures. Patients taking ARBs were also excluded.
The primary outcome was intraoperative hypotension defined as any systolic blood pressure less than 80 mm Hg from the time of anesthesia induction until transfer to the postanesthesia care unit. Secondary outcomes were measured until hospital discharge and included postoperative acute kidney injury, postoperative hypotension (systolic pressure < 90 mm Hg) and hypertension (systolic pressure > 180 mm Hg), major cardiac events (composite of acute coronary syndrome, acute heart failure, or new-onset arrhythmia), and death.
Findings. A total of 453 patients were screened for eligibility, and of these, 291 were included for randomization. Their average age was 64, 48% were men, and 87% were white. About 50% underwent general anesthesia, 25% spinal, and 25% regional. Over half of the surgeries were orthopedic, and 20% were spine surgeries.
The primary outcome of intraoperative hypotension occurred significantly less often in patients randomized to ACE inhibitor omission than in the continuation group (55% vs 69%, relative risk [RR] 0.81, 95% CI 0.67–0.97, P = .03). This translates to 1 case of intraoperative hypotension for every 7.5 patients continuing an ACE inhibitor perioperatively (number needed to harm 7.5). Intraoperative hypotension associated with vasopressor administration also occurred significantly less frequently in the ACE inhibitor omission group.
Patients in the ACE inhibitor omission group were also less likely to experience postoperative hypotension, but on the other hand, they were more likely to experience severe postoperative hypertension (defined as any systolic blood pressure > 180 mm Hg). The two groups fared the same in terms of rates of acute kidney injury and major adverse cardiac events (MACE) and hospital length of stay, and no patients died in either group.
Limitations. Several factors limit the generalizability of this single-center study, including the many exclusion criteria, the predominance of orthopedic and spine surgeries, and the low-risk patient population (the average Revised Cardiac Risk Index score was 0, range 0–3). Other limitations include not controlling for the specific ACE inhibitor used and not including the precise timing of the final dose in relation to surgery. Lastly, this study lacked power to measure postoperative outcomes.
Conclusions. Continuing ACE inhibitor treatment before noncardiac nonvascular surgery is associated with a greater frequency and duration of intraoperative hypotension, but it did not increase the incidences of acute kidney injury, MACE, or death nor the hospital length of stay.
[Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837]
Hollmann et al9 performed a meta-analysis to determine whether it is better to continue or withhold ACE inhibitors and ARBs before surgery. The patients were adults undergoing noncardiac surgery and receiving an ACE inhibitor or ARB, which was either withheld or continued on the morning of surgery.
Primary outcomes were all-cause mortality and MACE, while secondary outcomes included the incidence of acute kidney injury, heart failure, stroke, intraoperative and postoperative hypotension, and length of hospital stay. Randomized controlled trials and observational studies were included, while case reports and case-control studies were excluded.
Findings. This meta-analysis included 5 randomized controlled trials and 4 cohort studies, with a total of 6,022 patients; 1,816 had their ACE inhibitor or ARB withheld before surgery, while 4,206 continued therapy. It found no difference between the 2 groups in the incidence of death or MACE, and there were not enough data to determine a difference in heart failure, stroke, acute kidney injury, or hospital length of stay.
Seven studies, with 5,414 patients, examined intraoperative hypotension. The overall incidence was 30%, but was significantly lower if the ACE inhibitor or ARB was withheld (OR 0.63, 95% CI 0.47–0.85, P = .002). Findings were similar in an analysis of only the randomized controlled trials. No difference was observed in postoperative hypotension.
Limitations. There was no standard definition of the morbidity outcomes, including hypotension and MACE. The assessment of MACE included data only for MI and not MINS. The specific duration of hypotension was not reported, and this meta-analysis did not take into account different anesthetic techniques. The duration of follow-up varied widely among studies, ranging from the day of hospital discharge to 30 days after surgery. And the randomized controlled trial performed by Shiffermiller et al8 was not included.
Conclusions. While continuing ACE inhibitors or ARBs before noncardiac surgery was associated with intraoperative hypotension, it did not seem to affect other outcomes, including death and MACE. The authors propose that a large randomized controlled trial is needed to determine whether continuing or withholding ACE inhibitor or ARB therapy before surgery is safer.
POSTOPERATIVE CARDIAC COMPLICATIONS
How should we treat MINS?
MINS is associated with an increased risk of cardiovascular events and death in both the short term and long term. MINS is defined as an elevated postoperative troponin level related to an ischemic etiology. However, whether to routinely measure troponin after surgery is unclear, as most patients do not present with ischemic symptoms, and there is no standard of care for treatment of this entity. Limited observational data suggest that starting or intensifying cardiac medications, particularly aspirin and statins, may be beneficial in terms of reducing 30-day mortality rates in patients with MI or cardiac events at 1 year in vascular surgery patients with MINS.
The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial was designed to evaluate the potential of the anticoagulant dabigatran to prevent major vascular complications in patients with MINS.
[Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8]
Devereaux et al10 randomized patients who were at least 45 years old and had developed MINS within the previous 35 days to receive dabigatran 110 mg orally twice daily or placebo for up to 2 years. Patients not already taking a proton pump inhibitor were also randomized to take either omeprazole 20 mg once daily or placebo.
The primary efficacy outcome initially was major vascular complications, which included vascular mortality, nonfatal MI, nonhemorrhagic stroke, and peripheral arterial thrombosis. However, amputation and symptomatic venous thromboembolism were subsequently added during the study.
The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding. Major bleeding required a decrease in hemoglobin of at least 4 g/dL, transfusion of at least 3 units of red blood cells within a 24-hour period, or a procedure to stop the bleeding.
Findings. The original goal was to recruit 3,200 patients, but due to slow enrollment and loss of funding, the sample was reduced to 1,754 patients (877 in each group). Approximately 45% of each group stopped taking the study drug prematurely.
The primary efficacy outcome occurred in significantly fewer patients receiving dabigatran (97, 11%) than placebo (133, 15%, HR 0.72, 95% CI 0.55–0.93, P = .0115). The incidence of the primary safety outcome was similar in both groups: 3% with dabigatran and 4% with placebo (HR 0.92, 95% CI 0.55–1.53, P = .76). The only individual efficacy outcome meeting statistical significance was a lower rate of nonhemorrhagic stroke in the dabigatran group. Subgroup analyses showed a trend benefiting patients randomized within 5 days of MINS or with a diagnosis of MI, although it was not statistically significant.
Limitations. The efficacy outcomes were expanded to include venous thromboembolism and others not directly related to MINS, raising questions about the conclusions. Further, as defined by the protocol, bleeding had to be fairly severe to be deemed major. The high number of patients who discontinued the study drug is another limitation of this study.
Conclusion. Dabigatran lowered the risk of major vascular complications with no significant increase in major bleeding in patients with MINS.
What is the risk of thromboembolism in postoperative atrial fibrillation, and what are the benefits of anticoagulation?
Although nonvalvular atrial fibrillation is associated with increased risks of ischemic stroke and systemic embolic events in nonsurgical patients, the association of new-onset postoperative atrial fibrillation with long-term thromboembolic events in the noncardiac surgical population is not well established.
[Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088]
In this retrospective cohort study using a nationwide registry in Denmark, Butt et al11 assessed the long-term risk of thromboembolic events in noncardiac surgical patients with new postoperative atrial fibrillation. Patients were identified who had no previous history of atrial fibrillation and developed it after noncardiac, nonobstetric surgeries, and were matched in a 1:4 ratio with patients who developed nonvalvular atrial fibrillation during nonsurgical hospitalizations. Matching was based on age, sex, heart failure, hypertension, diabetes, known history of thromboembolic events, ischemic heart disease, and the year patients presented with new atrial fibrillation.
Patients were excluded if they received antiarrhythmic drugs or oral anticoagulants before hospitalization or surgery, had cancer in the year prior, or died in the hospital.
The primary outcome of the study was thromboembolic events—a composite of ischemic stroke, transient cerebral ischemia, and peripheral arterial thrombosis or embolism. Secondary outcomes included rehospitalization for atrial fibrillation and all-cause mortality.
Findings. Overall, 0.4% of patients developed new postoperative atrial fibrillation, of whom 3,380 were matched with 15,320 patients with nonvalvular atrial fibrillation. Over a median follow-up of 3.2 years, the risk of thromboembolic events was similar in both groups (31.7 and 29.9 per 1,000 person-years, HR 0.95, 95% CI 0.85–1.07). The groups did not differ in their CHA2DS2-VASc risk scores, HAS-BLED risk scores, or year in which patients were diagnosed.
Anticoagulation lowered the risk of thromboembolic events to a similar extent in both groups compared with no anticoagulation:
- In postoperative atrial fibrillation—HR 0.57, 95% CI 0.40–0.67
- In nonvalvular atrial fibrillation—HR 0.56, 95% CI 0.51–0.62.
Despite the similar reduction in thromboembolic events, only 24.4% of the postoperative atrial fibrillation patients were started on anticoagulation therapy within 30 days of discharge, compared with 41.5% of those with nonvalvular atrial fibrillation.
Limitations. Although this was a large study with excellent follow-up data, it was observational. It may have underestimated the number of patients who developed postoperative atrial fibrillation because episodes that were judged not to be clinically significant may not have been charted. Many patients are not monitored with continuous telemetry postoperatively, which also may have led to underestimation of the number of atrial fibrillation events.
The study also did not examine the number of atrial fibrillation episodes per patient, the heart rhythm at discharge or long-term, or indication for and duration of anticoagulation. There were no data regarding international normalized ratio levels.
Conclusions. Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases the risks of stroke and death. However, substantially fewer patients with postoperative atrial fibrillation receive anticoagulation. Anticoagulation should be considered in these patients, while noting bleeding risk.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64(22):e77–137. doi:10.1016/j.jacc.2014.07.944
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35(35):2383–2431. doi:10.1093/eurheartj/ehu282
- Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (The Duke Activity Status Index). Am J Cardiol 1989; 64(10):651–654. doi:10.1016/0002-9149(89)90496-7
- Wijeysundera DN, Pearse RM, Sulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018; 391(10140):2631–2640. doi:10.1016/S0140-6736(18)31131-0
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168(4):237–244. pmid:29132159
- Biccard BM, Sigamani A, Chan MTV, et al. Effect of aspirin in vascular surgery in patients from a randomized clinical trial (POISE-2). Br J Surg 2018; 105(12):1591–1597. doi:10.1002/bjs.10925
- Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018; 13(10):661–667. doi:10.12788/jhm.3036
- Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018; 127(3):678–687. doi:10.1213/ANE.0000000000002837
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Butt JH, Olesen JB, Havers-Borgersen E, et al. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol 2018; 72(17):2027–2036. doi:10.1016/j.jacc.2018.07.088
KEY POINTS
- The Duke Activity Status Index is a better tool for assessing cardiopulmonary fitness than subjective assessment, and it should be considered for use in guideline algorithms.
- Aspirin should not be given perioperatively in patients undergoing vascular surgery other than carotid endarterectomy.
- Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are associated with intraoperative hypotension if given before surgery. Further study is needed to determined how best to manage ACE inhibitors and ARBs perioperatively.
- In a study, dabigatran given to patients with myocardial injury after noncardiac surgery lowered the risk of major vascular complications, with no significant increase in major bleeding. But the study had major limitations.
- Postoperative atrial fibrillation is associated with outcomes similar to those of nonsurgical nonvalvular atrial fibrillation. Anticoagulation decreases its stroke and mortality risk.
Perioperative cardiovascular medicine: 5 questions for 2018
A plethora of studies are under way in the field of perioperative medicine. As a result, evidence-based care of surgical patients is evolving at an exponential rate.
We performed a literature search and, using consensus, identified recent articles we believe will have a great impact on perioperative cardiovascular medicine. These articles report studies that were presented at national meetings in 2018, including the Perioperative Medicine Summit, Society of General Internal Medicine, and Society of Hospital Medicine. These articles are grouped under 5 questions that will help guide clinical practice in perioperative cardiovascular medicine.
SHOULD ASPIRIN BE CONTINUED PERIOPERATIVELY IN PATIENTS WITH A CORONARY STENT?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial1 found that giving aspirin before surgery and throughout the early postoperative period had no significant effect on the rate of a composite of death or nonfatal myocardial infarction; moreover, aspirin increased the risk of major bleeding. However, many experts felt uncomfortable stopping aspirin preoperatively in patients taking it for secondary prophylaxis, particularly patients with a coronary stent.
[Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244.]
This post hoc subgroup analysis2 of POISE-2 evaluated the benefit and harm of perioperative aspirin in patients who had previously undergone percutaneous coronary intervention, more than 90% of whom had received a stent. Patients were age 45 or older with atherosclerotic heart disease or risk factors for it who had previously undergone percutaneous coronary intervention and were now undergoing noncardiac surgery.
Patients who had received a bare-metal stent within the previous 6 weeks or a drug-eluting stent within 12 months before surgery were excluded because guidelines at that time said to continue dual antiplatelet therapy for that long. Recommendations have since changed; the optimal duration for dual antiplatelet therapy with drug-eluting stents is now 6 months. Second-generation drug-eluting stents pose a lower risk of stent thrombosis and require a shorter duration of dual antiplatelet therapy than first-generation drug-eluting stents. Approximately 25% of the percutaneous coronary intervention subgroup had a drug-eluting stent, but the authors did not specify the type of drug-eluting stent.
The post hoc analysis2 included a subgroup of 234 of 4,998 patients receiving aspirin and 236 of 5,012 patients receiving placebo initiated within 4 hours before surgery and continued postoperatively. The primary outcome measured was the rate of death or nonfatal myocardial infarction within 30 days after surgery, and bleeding was a secondary outcome.
Findings. Although the overall POISE-2 study found no benefit from aspirin, in the subgroup who had previously undergone percutaneous coronary intervention, aspirin significantly reduced the risk of the primary outcome, which occurred in 6% vs 11.5% of the patients:
- Absolute risk reduction 5.5% (95% confidence interval 0.4%–10.5%)
- Hazard ratio 0.50 (0.26–0.95).
The reduction was primarily due to fewer myocardial infarctions:
- Absolute risk reduction 5.9% (1.0%–10.8%)
- Hazard ratio 0.44 (0.22–0.87).
The type of stent had no effect on the primary outcome, although this subgroup analysis had limited power. In the nonpercutaneous coronary intervention subgroup, there was no significant difference in outcomes between the aspirin and placebo groups. This subgroup analysis was underpowered to evaluate the effect of aspirin on the composite of major and life-threatening bleeding in patients with prior percutaneous coronary intervention, which was reported as “uncertain” due to wide confidence intervals (absolute risk increase 1.3%, 95% confidence interval –2.6% to 5.2%), but the increased risk of major or life-threatening bleeding with aspirin demonstrated in the overall POISE-2 study population likely applies:
- Absolute risk increase 0.8% (0.1%–1.6%)
- Hazard ratio 1.22 (1.01–1.48).
Limitations. This was a nonspecified subgroup analysis that was underpowered and had a relatively small sample size with few events.
Conclusion. In the absence of a very high bleeding risk, continuing aspirin perioperatively in patients with prior percutaneous coronary intervention undergoing noncardiac surgery is more likely to result in benefit than harm. This finding is in agreement with current recommendations from the American College Cardiology and American Heart Association (class I; level of evidence C).3
WHAT IS THE INCIDENCE OF MINS? IS MEASURING TROPONIN USEFUL?
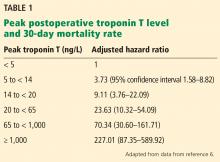
Despite advances in anesthesia and surgical techniques, about 1% of patients over age 45 die within 30 days of noncardiac surgery.4 Studies have demonstrated a high mortality rate in patients who experience myocardial injury after noncardiac surgery (MINS), defined as elevations of troponin T with or without ischemic symptoms or electrocardiographic changes.5 Most of these studies used earlier, “non-high-sensitivity” troponin T assays. Fifth-generation, highly sensitive troponin T assays are now available that can detect troponin T at lower concentrations, but their utility in predicting postoperative outcomes remains uncertain. Two recent studies provide further insight into these issues.
[Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651.]
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study5 was an international, prospective cohort study that initially evaluated the association between MINS and the 30-day mortality rate using a non-high-sensitivity troponin T assay (Roche fourth-generation Elecsys TnT assay) in patients age 45 or older undergoing noncardiac surgery and requiring hospital admission for at least 1 night. After the first 15,000 patients, the study switched to the Roche fifth-generation assay, with measurements at 6 to 12 hours after surgery and on postoperative days 1, 2, and 3.
A 2017 analysis by Devereaux et al6 included only these later-enrolled patients and correlated their high-sensitivity troponin T levels with 30-day mortality rates. Patients with a level 14 ng/L or higher, the upper limit of normal in this study, were also assessed for ischemic symptoms and electrocardiographic changes. Although not required by the study, more than 7,800 patients had their troponin T levels measured before surgery, and the absolute change was also analyzed for an association with the 30-day mortality rate.
Findings. Of the 21,842 patients, about two-thirds underwent some form of major surgery; some of them had more than 1 type. A total of 1.2% of the patients died within 30 days of surgery.
Based on their analysis, the authors proposed that MINS be defined as:
- A postoperative troponin T level of 65 ng/L or higher, or
- A level in the range of 20 ng/L to less than 65 ng/L with an absolute increase from the preoperative level at least 5 ng/L, not attributable to a nonischemic cause.
Seventeen percent of the study patients met these criteria, and of these, 21.7% met the universal definition of myocardial infarction, although only 6.9% had symptoms of it.
Limitations. Only 40.4% of the patients had a preoperative high-sensitivity troponin T measurement for comparison, and in 13.8% of patients who had an elevated perioperative measurement, their preoperative value was the same or higher than their postoperative one. Thus, the incidence of MINS may have been overestimated if patients were otherwise not known to have troponin T elevations before surgery.
[Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232.]
Puelacher et al7 investigated the prevalence of MINS in 2,018 patients at increased cardiovascular risk (age ≥ 65, or age ≥ 45 with a history of coronary artery disease, peripheral vascular disease, or stroke) who underwent major noncardiac surgery (planned overnight stay ≥ 24 hours) at a university hospital in Switzerland. Patients had their troponin T measured with a high-sensitivity assay within 30 days before surgery and on postoperative days 1 and 2.
Instead of MINS, the investigators used the term “perioperative myocardial injury” (PMI), defined as an absolute increase in troponin T of at least 14 ng/L from before surgery to the peak postoperative reading. Similar to MINS, PMI did not require ischemic features, but in this study, noncardiac triggers (sepsis, stroke, or pulmonary embolus) were not excluded.
Findings. PMI occurred in 16% of surgeries, and of the patients with PMI, 6% had typical chest pain and 18% had any ischemic symptoms. Unlike in the POISE-2 study discussed above, PMI triggered an automatic referral to a cardiologist.
The unadjusted 30-day mortality rate was 8.9% among patients with PMI and 1.5% in those without. Multivariable logistic regression analysis showed an adjusted hazard ratio for 30-day mortality of 2.7 (95% CI 1.5–4.8) for those with PMI vs without, and this difference persisted for at least 1 year.
In patients with PMI, the authors compared the 30-day mortality rate of those with no ischemic signs or symptoms (71% of the patients) with those who met the criteria for myocardial infarction and found no difference. Patients with PMI triggered by a noncardiac event had a worse prognosis than those with a presumed cardiac etiology.
Limitations. Despite the multivariate analysis that included adjustment for age, nonelective surgery, and Revised Cardiac Risk Index (RCRI), the increased risk associated with PMI could simply reflect higher risk at baseline. Although PMI resulted in automatic referral to a cardiologist, only 10% of patients eventually underwent coronary angiography; a similar percentage were discharged with additional medical therapy such as aspirin, a statin, or a beta-blocker. The effect of these interventions is not known.
Conclusions. MINS is common and has a strong association with mortality risk proportional to the degree of troponin T elevation using high-sensitivity assays, consistent with data from previous studies of earlier assays. Because the mechanism of MINS may differ from that of myocardial infarction, its prevention and treatment may differ, and it remains unclear how serial measurement in postoperative patients should change clinical practice.
The recently published Dabigatran in Patients With Myocardial Injury After Non-cardiac Surgery (MANAGE) trial8 suggests that dabigatran may reduce arterial and venous complications in patients with MINS, but the study had a number of limitations that may restrict the clinical applicability of this finding.
While awaiting further clinical outcomes data, pre- and postoperative troponin T measurement may be beneficial in higher-risk patients (such as those with cardiovascular disease or multiple RCRI risk factors) if the information will change perioperative management.
WHAT IS THE ROLE OF HYPOTENSION OR BLOOD PRESSURE CONTROL?
Intraoperative hypotension is associated with organ ischemia, which may cause postoperative myocardial infarction, myocardial injury, and acute kidney injury.9 Traditional anesthesia practice is to maintain intraoperative blood pressure within 20% of the preoperative baseline, based on the notion that hypertensive patients require higher perfusion pressures.
[Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357.]
Futier et al10 sought to address uncertainty in intraoperative and immediate postoperative management of systolic blood pressure. In this multicenter, randomized, parallel-group trial, 298 patients at increased risk of postoperative renal complications were randomized to blood pressure management that was either “individualized” (within 10% of resting systolic pressure) or “standard” (≥ 80 mm Hg or ≥ 40% of resting systolic pressure) from induction to 4 hours postoperatively.
Blood pressure was monitored using radial arterial lines and maintained using a combination of intravenous fluids, norepinephrine (the first-line agent for the individualized group), and ephedrine (in the standard treatment group only). The primary outcome was a composite of systemic inflammatory response syndrome (SIRS) and organ dysfunction affecting at least 1 organ system (cardiovascular, respiratory, renal, hematologic, or neurologic).
Findings. Data on the primary outcome were available for 292 of 298 patients enrolled. The mean age was 70 years, 15% were women, and 82% had previously diagnosed hypertension. Despite the requirement for an elevated risk of acute kidney injury, only 13% of the patients had a baseline estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, and the median was 88 mL/min/1.73 m2. Ninety-five percent of patients underwent abdominal surgery, and 50% of the surgeries were elective.
The mean systolic blood pressure was 123 mm Hg in the individualized treatment group compared with 116 mm Hg in the standard treatment group. Despite this small difference, 96% of individualized treatment patients received norepinephrine, compared with 26% in the standard treatment group.
The primary outcome of SIRS with organ dysfunction occurred in 38.1% of patients in the individualized treatment group and 51.7% of those in the standard treatment group. After adjusting for center, surgical urgency, surgical site, and acute kidney injury risk index, the relative risk of developing SIRS in those receiving individualized management was 0.73 (P = .02). Renal dysfunction (based on Acute Dialysis Quality Initiative criteria11) occurred in 32.7% of individualized treatment patients and 49% of standardized treatment patients.
Limitations of this study included differences in pharmacologic approach to maintain blood pressure in the 2 protocols (ephedrine and fluids vs norepinephrine) and a modest sample size.
Conclusions. Despite this, the difference in organ dysfunction was striking, with a number needed to treat of only 7 patients. This intervention extended 4 hours postoperatively, a time when many of these patients have left the postanesthesia care unit and have returned to hospitalist care on inpatient wards.
While optimal management of intraoperative and immediate postoperative blood pressure may not be settled, this study suggests that even mild relative hypotension may justify immediate action. Further studies may be useful to delineate high- and low-risk populations, the timing of greatest risk, and indications for intraarterial blood pressure monitoring.
[Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65.]
This retrospective cohort study12 assessed the association between myocardial or kidney injury and absolute or relative thresholds of intraoperative mean arterial pressure. It included 57,315 adults who underwent inpatient noncardiac surgery, had a preoperative and at least 1 postoperative serum creatinine measurement within 7 days, and had blood pressure recorded in preoperative appointments within 6 months. Patients with chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2) and those on dialysis were excluded. The outcomes were MINS5 and acute kidney injury as defined by the Acute Kidney Injury Network.9
Findings. A mean arterial pressure below an absolute threshold of 65 mm Hg or a relative threshold of 20% lower than baseline value was associated with myocardial and kidney injury. At each threshold, prolonged periods of hypotension were associated with progressively increased risk.
An important conclusion of the study was that relative thresholds of mean arterial pressure were not any more predictive than absolute thresholds. Absolute thresholds are easier to use intraoperatively, especially when baseline values are not available. The authors did not find a clinically significant interaction between baseline blood pressure and the association of hypotension and myocardial and kidney injury.
Limitations included use of cardiac enzymes postoperatively to define MINS. Since these were not routinely collected, clinically silent myocardial injury may have been missed. Baseline blood pressure may have important implications in other forms of organ injury (ie, cerebral ischemia) that were not studied.
Summary. The lowest absolute mean arterial pressure is as predictive of postoperative myocardial and kidney injury as the relative pressure reduction, at least in patients with normal renal function. Limiting exposure to intraoperative hypotension is important. Baseline blood pressure values may have limited utility for intraoperative management.
In combination, these studies confirm that intraoperative hypotension is a predictor of postoperative organ dysfunction, but the definition and management remain unclear. While aggressive intraoperative management is likely beneficial, how to manage the antihypertensive therapy the patient has been taking as an outpatient when he or she comes into the hospital for surgery remains uncertain.
DOES PATENT FORAMEN OVALE INCREASE THE RISK OF STROKE?
Perioperative stroke is an uncommon, severe complication of noncardiac surgery. The pathophysiology has been better defined in cardiac than in noncardiac surgeries. In nonsurgical patients, patent foramen ovale (PFO) is associated with stroke, even in patients considered to be at low risk.13 Perioperative patients have additional risk for venous thromboembolism and may have periprocedural antithrombotic medications altered, increasing their risk of paradoxical embolism through the PFO.
[Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462.]
This retrospective cohort study of noncardiac surgery patients at 3 hospitals14 sought to determine the association of preoperatively diagnosed PFO with the risk of perioperative ischemic stroke identified by International Classification of Diseases diagnoses.
Of 150,198 patients, 1.0% had a preoperative diagnosis of PFO, and at baseline, those with PFO had significantly more comorbidities than those without PFO. Stroke occurred in 3.2% of patients with PFO vs 0.5% of those without. Patients known to have a PFO were much more likely to have cardiovascular and thromboembolic risk factors for stroke. In the adjusted analysis, the absolute risk difference between groups was 0.4% (95% CI 0.2–0.6%), with an estimated perioperative stroke risk of 5.9 per 1,000 in patients with known patent foramen ovale and 2.2 per 1,000 in those without. A diagnosis of PFO was also associated with increased risk of large-vessel-territory stroke and more severe neurologic deficit.
Further attempts to adjust for baseline risk factors and other potential bias, including a propensity score-matched cohort analysis and an analysis limited to patients who had echocardiography performed in the same healthcare system, still showed a higher risk of perioperative stroke among patients with preoperatively detected patent foramen ovale.
Limitations. The study was retrospective and observational, used administrative data, and had a low rate of PFO diagnosis (1%), compared with about 25% in population-based studies.15 Indications for preoperative echocardiography are unknown. In addition, the study specifically examined preoperatively diagnosed PFO, rather than including those diagnosed in the postoperative period.
Discussion. How does this study affect clinical practice? The absolute stroke risk was increased by 0.4% in patients with PFO compared with those without. Although this is a relatively small increase, millions of patients undergo noncardiac surgery annually. The risks of therapeutic anticoagulation or PFO closure are likely too high in this context; however, clinicians may approach the perioperative management of antiplatelet agents and venous thromboembolism prophylaxis in patients with known PFO with additional caution.
HOW DOES TIMING OF EMERGENCY SURGERY AFTER PRIOR STROKE AFFECT OUTCOMES?
A history of stroke or transient ischemic attack is a known risk factor for perioperative vascular complications. A recent large cohort study demonstrated that a history of stroke within 9 months of elective surgery was associated with increased adverse outcomes.16 Little is known, however, of the perioperative risk in patients with a history of stroke who undergo emergency surgery.
[Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19.]
In this study,17 all emergency noncardiac and nonintracranial surgeries from 2005 to 2011 were analyzed using multiple national patient registries in Denmark according to time elapsed between previous stroke and surgery. Primary outcomes were 30-day all-cause mortality and 30-day major adverse cardiac events (MACE), defined as nonfatal ischemic stroke, nonfatal myocardial infarction, and cardiovascular death. Statistical analysis to assess the risk of adverse outcomes included logistic regression models, spline analyses, and propensity-score matching.
Findings. The authors identified 146,694 emergency surgeries, with 7,861 patients (5.4%) having had a previous stroke (transient ischemic attacks and hemorrhagic strokes were not included). Rates of postoperative stroke were as follows:
- 9.9% in patents with a history of ischemic stroke within 3 months of surgery
- 2.8% in patients with a history of stroke 3 to 9 months before surgery
- 0.3% in patients with no previous stroke.
The risk plateaued when the time between stroke and surgery exceeded 4 to 5 months.15
Interestingly, in patients who underwent emergency surgery within 14 days of stroke, the risk of MACE was significantly lower immediately after surgery (1–3 days after stroke) compared with surgery that took place 4 to 14 days after stroke. The authors hypothesized that because cerebral autoregulation does not become compromised until approximately 5 days after a stroke, the risk was lower 1 to 3 days after surgery and increased thereafter.
Limitations of this study included the possibility of residual confounding, given its retrospective design using administrative data, not accounting for preoperative antithrombotic and anticoagulation therapy, and lack of information regarding the etiology of recurrent stroke (eg, thromboembolic, atherothrombotic, hypoperfusion).
Conclusions. Although it would be impractical to postpone emergency surgery in a patient who recently had a stroke, this study shows that the incidence rates of postoperative recurrent stroke and MACE are high. Therefore, it is important that the patient and perioperative team be aware of the risk. Further research is needed to confirm these estimates of postoperative adverse events in more diverse patient populations.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130(24):2215–2245. doi:10.1161/CIR.0000000000000105
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2(2):181–187. doi:10.1001/jamacardio.2016.4792
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651. doi:10.1001/jama.2017.4360
- Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232. doi:10.1161/CIRCULATIONAHA.117.030114
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357. doi:10.1001/jama.2017.14172
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204. doi:10.1186/cc2872
- Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65. doi:10.1097/ALN.0000000000001432
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318(18):1148–1152. doi:10.1056/NEJM198805053181802
- Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462. doi:10.1001/jama.2017.21899
- Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc 1999; 74(9):862–869. pmid:10488786
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277. doi:10.1001/jama.2014.8165
- Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19. doi:10.1097/ALN.0000000000001685
A plethora of studies are under way in the field of perioperative medicine. As a result, evidence-based care of surgical patients is evolving at an exponential rate.
We performed a literature search and, using consensus, identified recent articles we believe will have a great impact on perioperative cardiovascular medicine. These articles report studies that were presented at national meetings in 2018, including the Perioperative Medicine Summit, Society of General Internal Medicine, and Society of Hospital Medicine. These articles are grouped under 5 questions that will help guide clinical practice in perioperative cardiovascular medicine.
SHOULD ASPIRIN BE CONTINUED PERIOPERATIVELY IN PATIENTS WITH A CORONARY STENT?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial1 found that giving aspirin before surgery and throughout the early postoperative period had no significant effect on the rate of a composite of death or nonfatal myocardial infarction; moreover, aspirin increased the risk of major bleeding. However, many experts felt uncomfortable stopping aspirin preoperatively in patients taking it for secondary prophylaxis, particularly patients with a coronary stent.
[Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244.]
This post hoc subgroup analysis2 of POISE-2 evaluated the benefit and harm of perioperative aspirin in patients who had previously undergone percutaneous coronary intervention, more than 90% of whom had received a stent. Patients were age 45 or older with atherosclerotic heart disease or risk factors for it who had previously undergone percutaneous coronary intervention and were now undergoing noncardiac surgery.
Patients who had received a bare-metal stent within the previous 6 weeks or a drug-eluting stent within 12 months before surgery were excluded because guidelines at that time said to continue dual antiplatelet therapy for that long. Recommendations have since changed; the optimal duration for dual antiplatelet therapy with drug-eluting stents is now 6 months. Second-generation drug-eluting stents pose a lower risk of stent thrombosis and require a shorter duration of dual antiplatelet therapy than first-generation drug-eluting stents. Approximately 25% of the percutaneous coronary intervention subgroup had a drug-eluting stent, but the authors did not specify the type of drug-eluting stent.
The post hoc analysis2 included a subgroup of 234 of 4,998 patients receiving aspirin and 236 of 5,012 patients receiving placebo initiated within 4 hours before surgery and continued postoperatively. The primary outcome measured was the rate of death or nonfatal myocardial infarction within 30 days after surgery, and bleeding was a secondary outcome.
Findings. Although the overall POISE-2 study found no benefit from aspirin, in the subgroup who had previously undergone percutaneous coronary intervention, aspirin significantly reduced the risk of the primary outcome, which occurred in 6% vs 11.5% of the patients:
- Absolute risk reduction 5.5% (95% confidence interval 0.4%–10.5%)
- Hazard ratio 0.50 (0.26–0.95).
The reduction was primarily due to fewer myocardial infarctions:
- Absolute risk reduction 5.9% (1.0%–10.8%)
- Hazard ratio 0.44 (0.22–0.87).
The type of stent had no effect on the primary outcome, although this subgroup analysis had limited power. In the nonpercutaneous coronary intervention subgroup, there was no significant difference in outcomes between the aspirin and placebo groups. This subgroup analysis was underpowered to evaluate the effect of aspirin on the composite of major and life-threatening bleeding in patients with prior percutaneous coronary intervention, which was reported as “uncertain” due to wide confidence intervals (absolute risk increase 1.3%, 95% confidence interval –2.6% to 5.2%), but the increased risk of major or life-threatening bleeding with aspirin demonstrated in the overall POISE-2 study population likely applies:
- Absolute risk increase 0.8% (0.1%–1.6%)
- Hazard ratio 1.22 (1.01–1.48).
Limitations. This was a nonspecified subgroup analysis that was underpowered and had a relatively small sample size with few events.
Conclusion. In the absence of a very high bleeding risk, continuing aspirin perioperatively in patients with prior percutaneous coronary intervention undergoing noncardiac surgery is more likely to result in benefit than harm. This finding is in agreement with current recommendations from the American College Cardiology and American Heart Association (class I; level of evidence C).3
WHAT IS THE INCIDENCE OF MINS? IS MEASURING TROPONIN USEFUL?
Despite advances in anesthesia and surgical techniques, about 1% of patients over age 45 die within 30 days of noncardiac surgery.4 Studies have demonstrated a high mortality rate in patients who experience myocardial injury after noncardiac surgery (MINS), defined as elevations of troponin T with or without ischemic symptoms or electrocardiographic changes.5 Most of these studies used earlier, “non-high-sensitivity” troponin T assays. Fifth-generation, highly sensitive troponin T assays are now available that can detect troponin T at lower concentrations, but their utility in predicting postoperative outcomes remains uncertain. Two recent studies provide further insight into these issues.
[Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651.]
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study5 was an international, prospective cohort study that initially evaluated the association between MINS and the 30-day mortality rate using a non-high-sensitivity troponin T assay (Roche fourth-generation Elecsys TnT assay) in patients age 45 or older undergoing noncardiac surgery and requiring hospital admission for at least 1 night. After the first 15,000 patients, the study switched to the Roche fifth-generation assay, with measurements at 6 to 12 hours after surgery and on postoperative days 1, 2, and 3.
A 2017 analysis by Devereaux et al6 included only these later-enrolled patients and correlated their high-sensitivity troponin T levels with 30-day mortality rates. Patients with a level 14 ng/L or higher, the upper limit of normal in this study, were also assessed for ischemic symptoms and electrocardiographic changes. Although not required by the study, more than 7,800 patients had their troponin T levels measured before surgery, and the absolute change was also analyzed for an association with the 30-day mortality rate.
Findings. Of the 21,842 patients, about two-thirds underwent some form of major surgery; some of them had more than 1 type. A total of 1.2% of the patients died within 30 days of surgery.
Based on their analysis, the authors proposed that MINS be defined as:
- A postoperative troponin T level of 65 ng/L or higher, or
- A level in the range of 20 ng/L to less than 65 ng/L with an absolute increase from the preoperative level at least 5 ng/L, not attributable to a nonischemic cause.
Seventeen percent of the study patients met these criteria, and of these, 21.7% met the universal definition of myocardial infarction, although only 6.9% had symptoms of it.
Limitations. Only 40.4% of the patients had a preoperative high-sensitivity troponin T measurement for comparison, and in 13.8% of patients who had an elevated perioperative measurement, their preoperative value was the same or higher than their postoperative one. Thus, the incidence of MINS may have been overestimated if patients were otherwise not known to have troponin T elevations before surgery.
[Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232.]
Puelacher et al7 investigated the prevalence of MINS in 2,018 patients at increased cardiovascular risk (age ≥ 65, or age ≥ 45 with a history of coronary artery disease, peripheral vascular disease, or stroke) who underwent major noncardiac surgery (planned overnight stay ≥ 24 hours) at a university hospital in Switzerland. Patients had their troponin T measured with a high-sensitivity assay within 30 days before surgery and on postoperative days 1 and 2.
Instead of MINS, the investigators used the term “perioperative myocardial injury” (PMI), defined as an absolute increase in troponin T of at least 14 ng/L from before surgery to the peak postoperative reading. Similar to MINS, PMI did not require ischemic features, but in this study, noncardiac triggers (sepsis, stroke, or pulmonary embolus) were not excluded.
Findings. PMI occurred in 16% of surgeries, and of the patients with PMI, 6% had typical chest pain and 18% had any ischemic symptoms. Unlike in the POISE-2 study discussed above, PMI triggered an automatic referral to a cardiologist.
The unadjusted 30-day mortality rate was 8.9% among patients with PMI and 1.5% in those without. Multivariable logistic regression analysis showed an adjusted hazard ratio for 30-day mortality of 2.7 (95% CI 1.5–4.8) for those with PMI vs without, and this difference persisted for at least 1 year.
In patients with PMI, the authors compared the 30-day mortality rate of those with no ischemic signs or symptoms (71% of the patients) with those who met the criteria for myocardial infarction and found no difference. Patients with PMI triggered by a noncardiac event had a worse prognosis than those with a presumed cardiac etiology.
Limitations. Despite the multivariate analysis that included adjustment for age, nonelective surgery, and Revised Cardiac Risk Index (RCRI), the increased risk associated with PMI could simply reflect higher risk at baseline. Although PMI resulted in automatic referral to a cardiologist, only 10% of patients eventually underwent coronary angiography; a similar percentage were discharged with additional medical therapy such as aspirin, a statin, or a beta-blocker. The effect of these interventions is not known.
Conclusions. MINS is common and has a strong association with mortality risk proportional to the degree of troponin T elevation using high-sensitivity assays, consistent with data from previous studies of earlier assays. Because the mechanism of MINS may differ from that of myocardial infarction, its prevention and treatment may differ, and it remains unclear how serial measurement in postoperative patients should change clinical practice.
The recently published Dabigatran in Patients With Myocardial Injury After Non-cardiac Surgery (MANAGE) trial8 suggests that dabigatran may reduce arterial and venous complications in patients with MINS, but the study had a number of limitations that may restrict the clinical applicability of this finding.
While awaiting further clinical outcomes data, pre- and postoperative troponin T measurement may be beneficial in higher-risk patients (such as those with cardiovascular disease or multiple RCRI risk factors) if the information will change perioperative management.
WHAT IS THE ROLE OF HYPOTENSION OR BLOOD PRESSURE CONTROL?
Intraoperative hypotension is associated with organ ischemia, which may cause postoperative myocardial infarction, myocardial injury, and acute kidney injury.9 Traditional anesthesia practice is to maintain intraoperative blood pressure within 20% of the preoperative baseline, based on the notion that hypertensive patients require higher perfusion pressures.
[Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357.]
Futier et al10 sought to address uncertainty in intraoperative and immediate postoperative management of systolic blood pressure. In this multicenter, randomized, parallel-group trial, 298 patients at increased risk of postoperative renal complications were randomized to blood pressure management that was either “individualized” (within 10% of resting systolic pressure) or “standard” (≥ 80 mm Hg or ≥ 40% of resting systolic pressure) from induction to 4 hours postoperatively.
Blood pressure was monitored using radial arterial lines and maintained using a combination of intravenous fluids, norepinephrine (the first-line agent for the individualized group), and ephedrine (in the standard treatment group only). The primary outcome was a composite of systemic inflammatory response syndrome (SIRS) and organ dysfunction affecting at least 1 organ system (cardiovascular, respiratory, renal, hematologic, or neurologic).
Findings. Data on the primary outcome were available for 292 of 298 patients enrolled. The mean age was 70 years, 15% were women, and 82% had previously diagnosed hypertension. Despite the requirement for an elevated risk of acute kidney injury, only 13% of the patients had a baseline estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, and the median was 88 mL/min/1.73 m2. Ninety-five percent of patients underwent abdominal surgery, and 50% of the surgeries were elective.
The mean systolic blood pressure was 123 mm Hg in the individualized treatment group compared with 116 mm Hg in the standard treatment group. Despite this small difference, 96% of individualized treatment patients received norepinephrine, compared with 26% in the standard treatment group.
The primary outcome of SIRS with organ dysfunction occurred in 38.1% of patients in the individualized treatment group and 51.7% of those in the standard treatment group. After adjusting for center, surgical urgency, surgical site, and acute kidney injury risk index, the relative risk of developing SIRS in those receiving individualized management was 0.73 (P = .02). Renal dysfunction (based on Acute Dialysis Quality Initiative criteria11) occurred in 32.7% of individualized treatment patients and 49% of standardized treatment patients.
Limitations of this study included differences in pharmacologic approach to maintain blood pressure in the 2 protocols (ephedrine and fluids vs norepinephrine) and a modest sample size.
Conclusions. Despite this, the difference in organ dysfunction was striking, with a number needed to treat of only 7 patients. This intervention extended 4 hours postoperatively, a time when many of these patients have left the postanesthesia care unit and have returned to hospitalist care on inpatient wards.
While optimal management of intraoperative and immediate postoperative blood pressure may not be settled, this study suggests that even mild relative hypotension may justify immediate action. Further studies may be useful to delineate high- and low-risk populations, the timing of greatest risk, and indications for intraarterial blood pressure monitoring.
[Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65.]
This retrospective cohort study12 assessed the association between myocardial or kidney injury and absolute or relative thresholds of intraoperative mean arterial pressure. It included 57,315 adults who underwent inpatient noncardiac surgery, had a preoperative and at least 1 postoperative serum creatinine measurement within 7 days, and had blood pressure recorded in preoperative appointments within 6 months. Patients with chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2) and those on dialysis were excluded. The outcomes were MINS5 and acute kidney injury as defined by the Acute Kidney Injury Network.9
Findings. A mean arterial pressure below an absolute threshold of 65 mm Hg or a relative threshold of 20% lower than baseline value was associated with myocardial and kidney injury. At each threshold, prolonged periods of hypotension were associated with progressively increased risk.
An important conclusion of the study was that relative thresholds of mean arterial pressure were not any more predictive than absolute thresholds. Absolute thresholds are easier to use intraoperatively, especially when baseline values are not available. The authors did not find a clinically significant interaction between baseline blood pressure and the association of hypotension and myocardial and kidney injury.
Limitations included use of cardiac enzymes postoperatively to define MINS. Since these were not routinely collected, clinically silent myocardial injury may have been missed. Baseline blood pressure may have important implications in other forms of organ injury (ie, cerebral ischemia) that were not studied.
Summary. The lowest absolute mean arterial pressure is as predictive of postoperative myocardial and kidney injury as the relative pressure reduction, at least in patients with normal renal function. Limiting exposure to intraoperative hypotension is important. Baseline blood pressure values may have limited utility for intraoperative management.
In combination, these studies confirm that intraoperative hypotension is a predictor of postoperative organ dysfunction, but the definition and management remain unclear. While aggressive intraoperative management is likely beneficial, how to manage the antihypertensive therapy the patient has been taking as an outpatient when he or she comes into the hospital for surgery remains uncertain.
DOES PATENT FORAMEN OVALE INCREASE THE RISK OF STROKE?
Perioperative stroke is an uncommon, severe complication of noncardiac surgery. The pathophysiology has been better defined in cardiac than in noncardiac surgeries. In nonsurgical patients, patent foramen ovale (PFO) is associated with stroke, even in patients considered to be at low risk.13 Perioperative patients have additional risk for venous thromboembolism and may have periprocedural antithrombotic medications altered, increasing their risk of paradoxical embolism through the PFO.
[Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462.]
This retrospective cohort study of noncardiac surgery patients at 3 hospitals14 sought to determine the association of preoperatively diagnosed PFO with the risk of perioperative ischemic stroke identified by International Classification of Diseases diagnoses.
Of 150,198 patients, 1.0% had a preoperative diagnosis of PFO, and at baseline, those with PFO had significantly more comorbidities than those without PFO. Stroke occurred in 3.2% of patients with PFO vs 0.5% of those without. Patients known to have a PFO were much more likely to have cardiovascular and thromboembolic risk factors for stroke. In the adjusted analysis, the absolute risk difference between groups was 0.4% (95% CI 0.2–0.6%), with an estimated perioperative stroke risk of 5.9 per 1,000 in patients with known patent foramen ovale and 2.2 per 1,000 in those without. A diagnosis of PFO was also associated with increased risk of large-vessel-territory stroke and more severe neurologic deficit.
Further attempts to adjust for baseline risk factors and other potential bias, including a propensity score-matched cohort analysis and an analysis limited to patients who had echocardiography performed in the same healthcare system, still showed a higher risk of perioperative stroke among patients with preoperatively detected patent foramen ovale.
Limitations. The study was retrospective and observational, used administrative data, and had a low rate of PFO diagnosis (1%), compared with about 25% in population-based studies.15 Indications for preoperative echocardiography are unknown. In addition, the study specifically examined preoperatively diagnosed PFO, rather than including those diagnosed in the postoperative period.
Discussion. How does this study affect clinical practice? The absolute stroke risk was increased by 0.4% in patients with PFO compared with those without. Although this is a relatively small increase, millions of patients undergo noncardiac surgery annually. The risks of therapeutic anticoagulation or PFO closure are likely too high in this context; however, clinicians may approach the perioperative management of antiplatelet agents and venous thromboembolism prophylaxis in patients with known PFO with additional caution.
HOW DOES TIMING OF EMERGENCY SURGERY AFTER PRIOR STROKE AFFECT OUTCOMES?
A history of stroke or transient ischemic attack is a known risk factor for perioperative vascular complications. A recent large cohort study demonstrated that a history of stroke within 9 months of elective surgery was associated with increased adverse outcomes.16 Little is known, however, of the perioperative risk in patients with a history of stroke who undergo emergency surgery.
[Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19.]
In this study,17 all emergency noncardiac and nonintracranial surgeries from 2005 to 2011 were analyzed using multiple national patient registries in Denmark according to time elapsed between previous stroke and surgery. Primary outcomes were 30-day all-cause mortality and 30-day major adverse cardiac events (MACE), defined as nonfatal ischemic stroke, nonfatal myocardial infarction, and cardiovascular death. Statistical analysis to assess the risk of adverse outcomes included logistic regression models, spline analyses, and propensity-score matching.
Findings. The authors identified 146,694 emergency surgeries, with 7,861 patients (5.4%) having had a previous stroke (transient ischemic attacks and hemorrhagic strokes were not included). Rates of postoperative stroke were as follows:
- 9.9% in patents with a history of ischemic stroke within 3 months of surgery
- 2.8% in patients with a history of stroke 3 to 9 months before surgery
- 0.3% in patients with no previous stroke.
The risk plateaued when the time between stroke and surgery exceeded 4 to 5 months.15
Interestingly, in patients who underwent emergency surgery within 14 days of stroke, the risk of MACE was significantly lower immediately after surgery (1–3 days after stroke) compared with surgery that took place 4 to 14 days after stroke. The authors hypothesized that because cerebral autoregulation does not become compromised until approximately 5 days after a stroke, the risk was lower 1 to 3 days after surgery and increased thereafter.
Limitations of this study included the possibility of residual confounding, given its retrospective design using administrative data, not accounting for preoperative antithrombotic and anticoagulation therapy, and lack of information regarding the etiology of recurrent stroke (eg, thromboembolic, atherothrombotic, hypoperfusion).
Conclusions. Although it would be impractical to postpone emergency surgery in a patient who recently had a stroke, this study shows that the incidence rates of postoperative recurrent stroke and MACE are high. Therefore, it is important that the patient and perioperative team be aware of the risk. Further research is needed to confirm these estimates of postoperative adverse events in more diverse patient populations.
A plethora of studies are under way in the field of perioperative medicine. As a result, evidence-based care of surgical patients is evolving at an exponential rate.
We performed a literature search and, using consensus, identified recent articles we believe will have a great impact on perioperative cardiovascular medicine. These articles report studies that were presented at national meetings in 2018, including the Perioperative Medicine Summit, Society of General Internal Medicine, and Society of Hospital Medicine. These articles are grouped under 5 questions that will help guide clinical practice in perioperative cardiovascular medicine.
SHOULD ASPIRIN BE CONTINUED PERIOPERATIVELY IN PATIENTS WITH A CORONARY STENT?
The Perioperative Ischemic Evaluation 2 (POISE-2) trial1 found that giving aspirin before surgery and throughout the early postoperative period had no significant effect on the rate of a composite of death or nonfatal myocardial infarction; moreover, aspirin increased the risk of major bleeding. However, many experts felt uncomfortable stopping aspirin preoperatively in patients taking it for secondary prophylaxis, particularly patients with a coronary stent.
[Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244.]
This post hoc subgroup analysis2 of POISE-2 evaluated the benefit and harm of perioperative aspirin in patients who had previously undergone percutaneous coronary intervention, more than 90% of whom had received a stent. Patients were age 45 or older with atherosclerotic heart disease or risk factors for it who had previously undergone percutaneous coronary intervention and were now undergoing noncardiac surgery.
Patients who had received a bare-metal stent within the previous 6 weeks or a drug-eluting stent within 12 months before surgery were excluded because guidelines at that time said to continue dual antiplatelet therapy for that long. Recommendations have since changed; the optimal duration for dual antiplatelet therapy with drug-eluting stents is now 6 months. Second-generation drug-eluting stents pose a lower risk of stent thrombosis and require a shorter duration of dual antiplatelet therapy than first-generation drug-eluting stents. Approximately 25% of the percutaneous coronary intervention subgroup had a drug-eluting stent, but the authors did not specify the type of drug-eluting stent.
The post hoc analysis2 included a subgroup of 234 of 4,998 patients receiving aspirin and 236 of 5,012 patients receiving placebo initiated within 4 hours before surgery and continued postoperatively. The primary outcome measured was the rate of death or nonfatal myocardial infarction within 30 days after surgery, and bleeding was a secondary outcome.
Findings. Although the overall POISE-2 study found no benefit from aspirin, in the subgroup who had previously undergone percutaneous coronary intervention, aspirin significantly reduced the risk of the primary outcome, which occurred in 6% vs 11.5% of the patients:
- Absolute risk reduction 5.5% (95% confidence interval 0.4%–10.5%)
- Hazard ratio 0.50 (0.26–0.95).
The reduction was primarily due to fewer myocardial infarctions:
- Absolute risk reduction 5.9% (1.0%–10.8%)
- Hazard ratio 0.44 (0.22–0.87).
The type of stent had no effect on the primary outcome, although this subgroup analysis had limited power. In the nonpercutaneous coronary intervention subgroup, there was no significant difference in outcomes between the aspirin and placebo groups. This subgroup analysis was underpowered to evaluate the effect of aspirin on the composite of major and life-threatening bleeding in patients with prior percutaneous coronary intervention, which was reported as “uncertain” due to wide confidence intervals (absolute risk increase 1.3%, 95% confidence interval –2.6% to 5.2%), but the increased risk of major or life-threatening bleeding with aspirin demonstrated in the overall POISE-2 study population likely applies:
- Absolute risk increase 0.8% (0.1%–1.6%)
- Hazard ratio 1.22 (1.01–1.48).
Limitations. This was a nonspecified subgroup analysis that was underpowered and had a relatively small sample size with few events.
Conclusion. In the absence of a very high bleeding risk, continuing aspirin perioperatively in patients with prior percutaneous coronary intervention undergoing noncardiac surgery is more likely to result in benefit than harm. This finding is in agreement with current recommendations from the American College Cardiology and American Heart Association (class I; level of evidence C).3
WHAT IS THE INCIDENCE OF MINS? IS MEASURING TROPONIN USEFUL?
Despite advances in anesthesia and surgical techniques, about 1% of patients over age 45 die within 30 days of noncardiac surgery.4 Studies have demonstrated a high mortality rate in patients who experience myocardial injury after noncardiac surgery (MINS), defined as elevations of troponin T with or without ischemic symptoms or electrocardiographic changes.5 Most of these studies used earlier, “non-high-sensitivity” troponin T assays. Fifth-generation, highly sensitive troponin T assays are now available that can detect troponin T at lower concentrations, but their utility in predicting postoperative outcomes remains uncertain. Two recent studies provide further insight into these issues.
[Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651.]
The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study5 was an international, prospective cohort study that initially evaluated the association between MINS and the 30-day mortality rate using a non-high-sensitivity troponin T assay (Roche fourth-generation Elecsys TnT assay) in patients age 45 or older undergoing noncardiac surgery and requiring hospital admission for at least 1 night. After the first 15,000 patients, the study switched to the Roche fifth-generation assay, with measurements at 6 to 12 hours after surgery and on postoperative days 1, 2, and 3.
A 2017 analysis by Devereaux et al6 included only these later-enrolled patients and correlated their high-sensitivity troponin T levels with 30-day mortality rates. Patients with a level 14 ng/L or higher, the upper limit of normal in this study, were also assessed for ischemic symptoms and electrocardiographic changes. Although not required by the study, more than 7,800 patients had their troponin T levels measured before surgery, and the absolute change was also analyzed for an association with the 30-day mortality rate.
Findings. Of the 21,842 patients, about two-thirds underwent some form of major surgery; some of them had more than 1 type. A total of 1.2% of the patients died within 30 days of surgery.
Based on their analysis, the authors proposed that MINS be defined as:
- A postoperative troponin T level of 65 ng/L or higher, or
- A level in the range of 20 ng/L to less than 65 ng/L with an absolute increase from the preoperative level at least 5 ng/L, not attributable to a nonischemic cause.
Seventeen percent of the study patients met these criteria, and of these, 21.7% met the universal definition of myocardial infarction, although only 6.9% had symptoms of it.
Limitations. Only 40.4% of the patients had a preoperative high-sensitivity troponin T measurement for comparison, and in 13.8% of patients who had an elevated perioperative measurement, their preoperative value was the same or higher than their postoperative one. Thus, the incidence of MINS may have been overestimated if patients were otherwise not known to have troponin T elevations before surgery.
[Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232.]
Puelacher et al7 investigated the prevalence of MINS in 2,018 patients at increased cardiovascular risk (age ≥ 65, or age ≥ 45 with a history of coronary artery disease, peripheral vascular disease, or stroke) who underwent major noncardiac surgery (planned overnight stay ≥ 24 hours) at a university hospital in Switzerland. Patients had their troponin T measured with a high-sensitivity assay within 30 days before surgery and on postoperative days 1 and 2.
Instead of MINS, the investigators used the term “perioperative myocardial injury” (PMI), defined as an absolute increase in troponin T of at least 14 ng/L from before surgery to the peak postoperative reading. Similar to MINS, PMI did not require ischemic features, but in this study, noncardiac triggers (sepsis, stroke, or pulmonary embolus) were not excluded.
Findings. PMI occurred in 16% of surgeries, and of the patients with PMI, 6% had typical chest pain and 18% had any ischemic symptoms. Unlike in the POISE-2 study discussed above, PMI triggered an automatic referral to a cardiologist.
The unadjusted 30-day mortality rate was 8.9% among patients with PMI and 1.5% in those without. Multivariable logistic regression analysis showed an adjusted hazard ratio for 30-day mortality of 2.7 (95% CI 1.5–4.8) for those with PMI vs without, and this difference persisted for at least 1 year.
In patients with PMI, the authors compared the 30-day mortality rate of those with no ischemic signs or symptoms (71% of the patients) with those who met the criteria for myocardial infarction and found no difference. Patients with PMI triggered by a noncardiac event had a worse prognosis than those with a presumed cardiac etiology.
Limitations. Despite the multivariate analysis that included adjustment for age, nonelective surgery, and Revised Cardiac Risk Index (RCRI), the increased risk associated with PMI could simply reflect higher risk at baseline. Although PMI resulted in automatic referral to a cardiologist, only 10% of patients eventually underwent coronary angiography; a similar percentage were discharged with additional medical therapy such as aspirin, a statin, or a beta-blocker. The effect of these interventions is not known.
Conclusions. MINS is common and has a strong association with mortality risk proportional to the degree of troponin T elevation using high-sensitivity assays, consistent with data from previous studies of earlier assays. Because the mechanism of MINS may differ from that of myocardial infarction, its prevention and treatment may differ, and it remains unclear how serial measurement in postoperative patients should change clinical practice.
The recently published Dabigatran in Patients With Myocardial Injury After Non-cardiac Surgery (MANAGE) trial8 suggests that dabigatran may reduce arterial and venous complications in patients with MINS, but the study had a number of limitations that may restrict the clinical applicability of this finding.
While awaiting further clinical outcomes data, pre- and postoperative troponin T measurement may be beneficial in higher-risk patients (such as those with cardiovascular disease or multiple RCRI risk factors) if the information will change perioperative management.
WHAT IS THE ROLE OF HYPOTENSION OR BLOOD PRESSURE CONTROL?
Intraoperative hypotension is associated with organ ischemia, which may cause postoperative myocardial infarction, myocardial injury, and acute kidney injury.9 Traditional anesthesia practice is to maintain intraoperative blood pressure within 20% of the preoperative baseline, based on the notion that hypertensive patients require higher perfusion pressures.
[Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357.]
Futier et al10 sought to address uncertainty in intraoperative and immediate postoperative management of systolic blood pressure. In this multicenter, randomized, parallel-group trial, 298 patients at increased risk of postoperative renal complications were randomized to blood pressure management that was either “individualized” (within 10% of resting systolic pressure) or “standard” (≥ 80 mm Hg or ≥ 40% of resting systolic pressure) from induction to 4 hours postoperatively.
Blood pressure was monitored using radial arterial lines and maintained using a combination of intravenous fluids, norepinephrine (the first-line agent for the individualized group), and ephedrine (in the standard treatment group only). The primary outcome was a composite of systemic inflammatory response syndrome (SIRS) and organ dysfunction affecting at least 1 organ system (cardiovascular, respiratory, renal, hematologic, or neurologic).
Findings. Data on the primary outcome were available for 292 of 298 patients enrolled. The mean age was 70 years, 15% were women, and 82% had previously diagnosed hypertension. Despite the requirement for an elevated risk of acute kidney injury, only 13% of the patients had a baseline estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, and the median was 88 mL/min/1.73 m2. Ninety-five percent of patients underwent abdominal surgery, and 50% of the surgeries were elective.
The mean systolic blood pressure was 123 mm Hg in the individualized treatment group compared with 116 mm Hg in the standard treatment group. Despite this small difference, 96% of individualized treatment patients received norepinephrine, compared with 26% in the standard treatment group.
The primary outcome of SIRS with organ dysfunction occurred in 38.1% of patients in the individualized treatment group and 51.7% of those in the standard treatment group. After adjusting for center, surgical urgency, surgical site, and acute kidney injury risk index, the relative risk of developing SIRS in those receiving individualized management was 0.73 (P = .02). Renal dysfunction (based on Acute Dialysis Quality Initiative criteria11) occurred in 32.7% of individualized treatment patients and 49% of standardized treatment patients.
Limitations of this study included differences in pharmacologic approach to maintain blood pressure in the 2 protocols (ephedrine and fluids vs norepinephrine) and a modest sample size.
Conclusions. Despite this, the difference in organ dysfunction was striking, with a number needed to treat of only 7 patients. This intervention extended 4 hours postoperatively, a time when many of these patients have left the postanesthesia care unit and have returned to hospitalist care on inpatient wards.
While optimal management of intraoperative and immediate postoperative blood pressure may not be settled, this study suggests that even mild relative hypotension may justify immediate action. Further studies may be useful to delineate high- and low-risk populations, the timing of greatest risk, and indications for intraarterial blood pressure monitoring.
[Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65.]
This retrospective cohort study12 assessed the association between myocardial or kidney injury and absolute or relative thresholds of intraoperative mean arterial pressure. It included 57,315 adults who underwent inpatient noncardiac surgery, had a preoperative and at least 1 postoperative serum creatinine measurement within 7 days, and had blood pressure recorded in preoperative appointments within 6 months. Patients with chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2) and those on dialysis were excluded. The outcomes were MINS5 and acute kidney injury as defined by the Acute Kidney Injury Network.9
Findings. A mean arterial pressure below an absolute threshold of 65 mm Hg or a relative threshold of 20% lower than baseline value was associated with myocardial and kidney injury. At each threshold, prolonged periods of hypotension were associated with progressively increased risk.
An important conclusion of the study was that relative thresholds of mean arterial pressure were not any more predictive than absolute thresholds. Absolute thresholds are easier to use intraoperatively, especially when baseline values are not available. The authors did not find a clinically significant interaction between baseline blood pressure and the association of hypotension and myocardial and kidney injury.
Limitations included use of cardiac enzymes postoperatively to define MINS. Since these were not routinely collected, clinically silent myocardial injury may have been missed. Baseline blood pressure may have important implications in other forms of organ injury (ie, cerebral ischemia) that were not studied.
Summary. The lowest absolute mean arterial pressure is as predictive of postoperative myocardial and kidney injury as the relative pressure reduction, at least in patients with normal renal function. Limiting exposure to intraoperative hypotension is important. Baseline blood pressure values may have limited utility for intraoperative management.
In combination, these studies confirm that intraoperative hypotension is a predictor of postoperative organ dysfunction, but the definition and management remain unclear. While aggressive intraoperative management is likely beneficial, how to manage the antihypertensive therapy the patient has been taking as an outpatient when he or she comes into the hospital for surgery remains uncertain.
DOES PATENT FORAMEN OVALE INCREASE THE RISK OF STROKE?
Perioperative stroke is an uncommon, severe complication of noncardiac surgery. The pathophysiology has been better defined in cardiac than in noncardiac surgeries. In nonsurgical patients, patent foramen ovale (PFO) is associated with stroke, even in patients considered to be at low risk.13 Perioperative patients have additional risk for venous thromboembolism and may have periprocedural antithrombotic medications altered, increasing their risk of paradoxical embolism through the PFO.
[Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462.]
This retrospective cohort study of noncardiac surgery patients at 3 hospitals14 sought to determine the association of preoperatively diagnosed PFO with the risk of perioperative ischemic stroke identified by International Classification of Diseases diagnoses.
Of 150,198 patients, 1.0% had a preoperative diagnosis of PFO, and at baseline, those with PFO had significantly more comorbidities than those without PFO. Stroke occurred in 3.2% of patients with PFO vs 0.5% of those without. Patients known to have a PFO were much more likely to have cardiovascular and thromboembolic risk factors for stroke. In the adjusted analysis, the absolute risk difference between groups was 0.4% (95% CI 0.2–0.6%), with an estimated perioperative stroke risk of 5.9 per 1,000 in patients with known patent foramen ovale and 2.2 per 1,000 in those without. A diagnosis of PFO was also associated with increased risk of large-vessel-territory stroke and more severe neurologic deficit.
Further attempts to adjust for baseline risk factors and other potential bias, including a propensity score-matched cohort analysis and an analysis limited to patients who had echocardiography performed in the same healthcare system, still showed a higher risk of perioperative stroke among patients with preoperatively detected patent foramen ovale.
Limitations. The study was retrospective and observational, used administrative data, and had a low rate of PFO diagnosis (1%), compared with about 25% in population-based studies.15 Indications for preoperative echocardiography are unknown. In addition, the study specifically examined preoperatively diagnosed PFO, rather than including those diagnosed in the postoperative period.
Discussion. How does this study affect clinical practice? The absolute stroke risk was increased by 0.4% in patients with PFO compared with those without. Although this is a relatively small increase, millions of patients undergo noncardiac surgery annually. The risks of therapeutic anticoagulation or PFO closure are likely too high in this context; however, clinicians may approach the perioperative management of antiplatelet agents and venous thromboembolism prophylaxis in patients with known PFO with additional caution.
HOW DOES TIMING OF EMERGENCY SURGERY AFTER PRIOR STROKE AFFECT OUTCOMES?
A history of stroke or transient ischemic attack is a known risk factor for perioperative vascular complications. A recent large cohort study demonstrated that a history of stroke within 9 months of elective surgery was associated with increased adverse outcomes.16 Little is known, however, of the perioperative risk in patients with a history of stroke who undergo emergency surgery.
[Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19.]
In this study,17 all emergency noncardiac and nonintracranial surgeries from 2005 to 2011 were analyzed using multiple national patient registries in Denmark according to time elapsed between previous stroke and surgery. Primary outcomes were 30-day all-cause mortality and 30-day major adverse cardiac events (MACE), defined as nonfatal ischemic stroke, nonfatal myocardial infarction, and cardiovascular death. Statistical analysis to assess the risk of adverse outcomes included logistic regression models, spline analyses, and propensity-score matching.
Findings. The authors identified 146,694 emergency surgeries, with 7,861 patients (5.4%) having had a previous stroke (transient ischemic attacks and hemorrhagic strokes were not included). Rates of postoperative stroke were as follows:
- 9.9% in patents with a history of ischemic stroke within 3 months of surgery
- 2.8% in patients with a history of stroke 3 to 9 months before surgery
- 0.3% in patients with no previous stroke.
The risk plateaued when the time between stroke and surgery exceeded 4 to 5 months.15
Interestingly, in patients who underwent emergency surgery within 14 days of stroke, the risk of MACE was significantly lower immediately after surgery (1–3 days after stroke) compared with surgery that took place 4 to 14 days after stroke. The authors hypothesized that because cerebral autoregulation does not become compromised until approximately 5 days after a stroke, the risk was lower 1 to 3 days after surgery and increased thereafter.
Limitations of this study included the possibility of residual confounding, given its retrospective design using administrative data, not accounting for preoperative antithrombotic and anticoagulation therapy, and lack of information regarding the etiology of recurrent stroke (eg, thromboembolic, atherothrombotic, hypoperfusion).
Conclusions. Although it would be impractical to postpone emergency surgery in a patient who recently had a stroke, this study shows that the incidence rates of postoperative recurrent stroke and MACE are high. Therefore, it is important that the patient and perioperative team be aware of the risk. Further research is needed to confirm these estimates of postoperative adverse events in more diverse patient populations.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130(24):2215–2245. doi:10.1161/CIR.0000000000000105
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2(2):181–187. doi:10.1001/jamacardio.2016.4792
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651. doi:10.1001/jama.2017.4360
- Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232. doi:10.1161/CIRCULATIONAHA.117.030114
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357. doi:10.1001/jama.2017.14172
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204. doi:10.1186/cc2872
- Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65. doi:10.1097/ALN.0000000000001432
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318(18):1148–1152. doi:10.1056/NEJM198805053181802
- Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462. doi:10.1001/jama.2017.21899
- Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc 1999; 74(9):862–869. pmid:10488786
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277. doi:10.1001/jama.2014.8165
- Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19. doi:10.1097/ALN.0000000000001685
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Graham MM, Sessler DI, Parlow JL, et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018; 168(4):237–244. doi:10.7326/M17-2341
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130(24):2215–2245. doi:10.1161/CIR.0000000000000105
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2(2):181–187. doi:10.1001/jamacardio.2016.4792
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317(16):1642–1651. doi:10.1001/jama.2017.4360
- Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137(12):1221–1232. doi:10.1161/CIRCULATIONAHA.117.030114
- Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet 2018; 391(10137):2325–2334. doi:10.1016/S0140-6736(18)30832-8
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013; 119(3):507–515. doi:10.1097/ALN.0b013e3182a10e26
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 2017; 318(14):1346–1357. doi:10.1001/jama.2017.14172
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8:R204. doi:10.1186/cc2872
- Salmasi V, Maheswari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126(1):47–65. doi:10.1097/ALN.0000000000001432
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318(18):1148–1152. doi:10.1056/NEJM198805053181802
- Ng PY, Ng AK, Subramaniam B, et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA 2018; 319(5):452–462. doi:10.1001/jama.2017.21899
- Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc 1999; 74(9):862–869. pmid:10488786
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277. doi:10.1001/jama.2014.8165
- Christiansen MN, Andersson C, Gislason GH, et al. Risks of cardiovascular adverse events and death in patients with previous stroke undergoing emergency noncardiac, nonintracranial surgery: the importance of operative timing. Anesthesiology 2017; 127(1):9–19. doi:10.1097/ALN.0000000000001685
KEY POINTS
- Patients undergoing noncardiac surgery who have a history of percutaneous coronary intervention will benefit from continuing aspirin perioperatively if they are not at very high risk of bleeding.
- Myocardial injury after noncardiac surgery is strongly associated with a risk of death, and the higher the troponin level, the higher the risk. Measuring troponin T before and after surgery may be beneficial in patients at high risk if the information leads to a change in management.
- Perioperative hypotension can lead to end-organ dysfunction postoperatively. There is conflicting evidence whether the absolute or relative reduction in blood pressure is more predictive.
- Perioperative risk of stroke is higher in patients with patent foramen ovale than in those without.
- Many patients who recently had a stroke suffer recurrent stroke and major adverse cardiac events if they undergo emergency surgery.
Update in perioperative cardiac medicine
Perioperative medicine is an evolving field with a rapidly growing body of literature. Because physicians and patients are often concerned about cardiac risk, we focus this review on perioperative cardiology.
The information we present here is derived from presentations at the Perioperative Medicine Summit and the annual meetings of the Society of Hospital Medicine and Society of General Internal Medicine in 2016. We surveyed perioperative literature from January 2015 through March 2016 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
We have divided this review into four sections:
- Preoperative cardiac risk assessment
- Medical therapy to reduce postoperative cardiac complications (beta-blockers, statins, and angiotensin II receptor blockers [ARBs])
- Perioperative management of patients with a coronary stent on antiplatelet therapy
- Perioperative bridging anticoagulation.
PREOPERATIVE ASSESSMENT OF CARDIAC RISK
Functionally independent patients do better
Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
Functional capacity is an independent predictor of perioperative death and is included in the algorithm of the current joint American College of Cardiology/American Heart Association (ACC/AHA) guidelines,1 but it is not in the Revised Cardiac Risk Index2 or the American Society of Anesthesiologists (ASA) classification.3
The study. Visnjevac et al4 performed a retrospective, observational cohort study of 12,324 patients who underwent noncardiac surgery, stratifying rates of all-cause mortality and 30-day postoperative complications based on ASA class and functional capacity.
The ASA physical status classification is defined as:
- 1—Normal healthy patient
- 2—Patient with mild systemic disease
- 3—Patient with severe systemic disease
- 4—Patient with severe systemic disease that is a constant threat to life
- 5—Moribund patient not expected to survive without surgery.
Functional capacity was defined as the ability to perform all activities of daily living. It was prospectively assessed during the patient interview by pre-anesthesia personnel and entered into the database of the Veterans Affairs Surgical Quality Improvement Program.
Results. Within each ASA class, the mortality rate was significantly lower for functionally independent patients than for partially or fully dependent patients:
- In class 2—odds ratio (OR) 0.14 for functionally independent patients
- In class 3—OR 0.29 for functionally independent patients
- In class 4—OR 0.5 for functionally independent patients.
The mortality rate was higher for dependent patients than for independent patients who were one ASA class higher, despite the higher class having greater rates of comorbidity.
Adding functional capacity to the ASA classification improved the area under the receiver operating curve from 0.811 to 0.848 (a perfect test would have a value of 1.0), suggesting that physicians should incorporate functional capacity into their preoperative evaluation, perhaps by increasing a patient’s ASA class to the next higher class if he or she is functionally dependent.
Angina portends poor outcomes
Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
Coronary artery disease is a risk factor for adverse perioperative outcomes, but the risk varies depending on whether the patient has had a myocardial infarction (and how long ago) and whether he or she has anginal symptoms (and how severe they are).
The study. Pandey et al5 used data from the American College of Surgeons National Surgical Quality Improvement Program to evaluate the impact of stable angina in 1,568 patients who underwent noncardiac surgery after a myocardial infarction.
Results. Postoperative myocardial infarction or cardiac arrest occurred in 5.5% of patients. The incidence was significantly greater in those who had anginal symptoms before surgery than in those without symptoms (8.4% vs 5%, P = .035); reintervention rates and length of stay were also higher in this group. In multivariate analysis, preoperative angina remained a significant predictor of postoperative myocardial infarction (OR 2.49, 95% confidence interval [CI] 1.20–5.81) and reintervention (OR 2.4, 95% CI 1.44–3.82.
The authors cautioned against relying on predictive tools such as the Revised Cardiac Risk Index that do not consider stable angina and previous myocardial infarction as separate independent risk factors.
Implications for clinical practice. While functional capacity is an integral part of the ACC/AHA guideline algorithm,1 the findings of these two studies suggest that other current tools to calculate perioperative risk (ASA class and Revised Cardiac Risk Index) could be improved by including functional capacity and stable angina.
PERIOPERATIVE MEDICAL THERAPY
Beta-blockers help only those at high risk and may harm others
Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
Beta-blockers have been used perioperatively for nearly 2 decades to try to reduce rates of postoperative major adverse cardiovascular events. However, in view of recent trials, fewer patients are likely to benefit from this intervention than has been thought.
The study. Friedell et al6 retrospectively analyzed data from 343,645 patients in Veterans Affairs hospitals to determine the effect of beta-blockers on major adverse cardiac event rates after major noncardiac surgery. Beta-blockers were considered to have been used perioperatively if given any time between 8 hours before and 24 hours after surgery. The outcome studied was the mortality rate at 30 days.
The authors derived a novel risk score and used multivariate analysis to attempt to adjust for confounding factors. The risk score was based on four risk factors identified a priori:
- Serum creatinine level > 2.0 mg/dL
- Coronary artery disease
- Diabetes
- Surgery in a major body cavity (abdomen or chest).
Results. In this cohort, 43.2% of patients had received a beta-blocker. The unadjusted mortality rates by risk category for patients receiving or not receiving a beta-blocker were:
- No risk factors: 1.0% with a beta-blocker vs 0.6% without
- One or two risk factors: 1.7% vs 1.5%
- Three or four risk factors: 2.3% vs 4.5%.
After adjustment for confounding factors, the 30-day mortality rate was higher in low-risk patients and lower in high-risk patients who received beta-blockers. Odds ratios for death in beta-blocker users (entire cohort) by risk category were:
- No risk factors: 1.19
- One or two risk factors 0.97
- Three or four risk factors 0.76.
In the 3.8% of the total cohort who underwent cardiac surgery, beta-blockers had no significant effect—beneficial or harmful—in any risk group.
Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
The study. Jørgensen et al7 investigated the association between chronic beta-blocker use for the treatment of hypertension and 30-day rates of mortality and major adverse cardiac events. Eligible patients (N = 55,320) were at least 20 years old and were undergoing any type of noncardiac surgery. The authors established that hypertension was present through use of an algorithm based on the International Classification of Diseases (10th edition). Patients with existing cardiovascular disease and renal disease were excluded. The authors used multivariate analysis to adjust for confounding factors.
Results. Twenty-six percent of the patients were on chronic beta-blocker therapy for hypertension. The mortality rate at 30 days was 1.93% in patients treated with a beta-blocker alone or in combination with other antihypertensive drugs; the rate was 1.32% for patients receiving any combination of renin-angiotensin system inhibitor, calcium antagonist, or thiazide, but no beta-blocker. Similarly, the 30-day major adverse cardiac event rates were 1.32% with beta-blockers and 0.84% without beta-blockers.
In subgroup analysis, each medication combination that included a beta-blocker was associated with higher rates of death and major adverse cardiac events than the same combination without a beta-blocker. Odds ratios for major adverse cardiac events with beta-blocker combinations ranged from 1.22 to 2.16 compared with regimens with no beta-blocker.
Implications for clinical practice. These two studies added to a growing chorus of concerns about the value and safety of beta-blockers in surgical patients. Friedell et al6 made an observation that was remarkably similar to one reported by Lindenauer et al8 in 2005: when patients were stratified by baseline risk of death, only those with the highest baseline risk benefited from beta-blocker therapy. Those in the lowest risk group actually were harmed by beta-blocker use, ie, the mortality rate was higher.
More interesting is the novel observation by Jørgensen et al7 that even in patients with no known cardiovascular disease who are on chronic beta-blocker therapy—presumably on stable doses and not solely for perioperative risk reduction—rates of mortality and major adverse cardiac events were higher than for patients not on chronic beta-blocker therapy.
The current studies support a cautious, selective approach to the perioperative use of beta-blockers—they should be used only in high-risk patients undergoing high-risk surgery, as has been proposed by the ACC/AHA.1
Statins protect
Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
The study9 was a comprehensive meta-analysis of randomized controlled trials and observational studies of the effects of HMG-CoA reductase inhibitors (statins) on perioperative outcomes in patients undergoing vascular surgery (but not for intracranial or coronary artery disease). Twenty-four studies were included, 4 randomized controlled trials and 20 observational studies (including 16 cohort and 4 case-controlled studies), with a total of 22,536 patients, 8,052 receiving statins and 15,484 not receiving statins.
Results. Although there was no significant difference in cardiovascular mortality rates, patients receiving statins had significantly lower rates of all-cause mortality, myocardial infarction, stroke, and a composite of myocardial infarction, stroke, and death at 30 days postoperatively than patients not receiving statins. Additionally, there was no difference in the incidence of kidney injury between groups. The possibility of publication bias was thought to be low for all of these outcomes.
Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
The study. The Vascular Events in Non-cardiac Surgery Patients Cohort Evaluation (VISION) study10,11 was an international prospective cohort study of more than 40,000 patients age 45 and older undergoing major noncardiac surgery with either general or regional anesthesia. Postoperative troponin measurements were obtained in all patients 6 to 12 hours after surgery and for the first 3 postoperative days. The authors evaluated the effect of preoperative statin use on cardiovascular outcomes at 30 days after surgery using a multivariate logistic model and propensity score analysis to correct for confounding factors. Statin use was defined as exposure within 7 days before surgery or 3 days after.
Results. In the 15,478 patients included in the analysis, statin use conferred a significant reduction in the primary outcome (composite of all-cause mortality, myocardial injury after noncardiac surgery, or stroke); the absolute risk reduction was 2.0%. Statin users also had a significantly lower risk of all-cause mortality, cardiovascular mortality, and myocardial injury after noncardiac surgery, but not of postoperative myocardial infarction or stroke. This analysis did not address the type of statin, dosing, or safety markers such as liver and muscle function.
Implications for clinical practice. With largely observational data and a few small randomized trials, these meta-analyses provide important information with respect to perioperative cardiovascular protection by statins. Starting a statin before surgery and continuing it perioperatively seems appropriate in patients at high risk (as recommended by the ACC/AHA guidelines1). Based on other data, the benefit may be evident in as little as 5 days, as this is when statins appear to reach their plateau with regard to their vascular pleiotropic effects.12 The incidence of adverse effects of statins, including muscle and liver injury, appears to be low in the perioperative setting.13
Given the inconsistent data regarding perioperative beta-blocker therapy, statins may very well be the most important perioperative medication with respect to cardiovascular risk reduction. However, a large randomized trial would help to confirm this belief.
Restart angiotensin II receptor blockers soon after surgery
Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
A concern about perioperative use of ARBs is that they impair the renin-angiotensin-aldosterone system, which maintains blood pressure under general anesthesia. ARB-induced intraoperative hypotension is particularly difficult to control, as it is often refractory to treatment with conventional adrenergic vasopressors.
The study. Lee et al14 conducted a retrospective cohort trial to evaluate the effects of continuing to withhold ARBs postoperatively. Of the 30,173 patients admitted for surgery in the Veterans Affairs system from 1999 through 2011 who were taking an ARB before surgery and who met the inclusion criteria, 10,205 (33.8%) were not restarted on their medication by postoperative day 2.
Results. The mortality rate at 30 days was higher in those whose ARBs were withheld than in those in whom it was resumed, with a multivariable-adjusted hazard ratio of 1.74 (95% CI 1.47–2.06; P < .001). The risk of withholding ARBs was more pronounced in younger patients (hazard ratio 2.52; 95% CI 1.69–3.76 in those under age 60) than in older patients (hazard ratio 1.42, 95% CI 1.09–1.85 in those over age 75).
Implications for clinical practice. While not addressing whether to continue or withhold ARBs preoperatively, this retrospective study presented evidence that delay in resuming chronic ARB therapy after surgery was common and appeared to be associated with a higher 30-day mortality rate. The ACC/AHA guidelines1 state:
- Continuing angiotensin-converting enzyme (ACE) inhibitors or ARBs perioperatively is reasonable (class IIa recommendation, level of evidence B) (Table 1).
- If an ACE inhibitor or ARB is withheld before surgery, it is reasonable to restart it postoperatively as soon as clinically feasible (class IIa recommendation, level of evidence C).
Close attention to medication reconciliation in the postoperative period is necessary to facilitate early resumption of ARBs.
CORONARY STENTS AND ANTIPLATELET THERAPY IN NONCARDIAC SURGERY PATIENTS
Considerations in the management of noncardiac surgery patients with stents include risks of stent thrombosis, bleeding, and potentially delaying procedures to continue uninterrupted dual antiplatelet therapy. Evidence is evolving regarding the risks of perioperative complications in patients with bare-metal stents and drug-eluting stents, as well as the optimal timing before noncardiac surgery.
Bare-metal vs drug-eluting stents
Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
The study. Bangalore et al15 compared the safety of drug-eluting vs bare-metal stents in noncardiac surgery patients and investigated adverse events stratified by time since stent placement. This was a retrospective observational study of 8,415 patients in the Massachusetts claims database who underwent noncardiac surgery 1 year or less after percutaneous coronary intervention.
Results. There was no significant difference in the incidence of the primary outcome (composite of death, myocardial infarction, and bleeding) between the two groups.
With drug-eluting stents, patients had lower 30-day postoperative mortality rates, and their rate of the primary outcome decreased with time from percutaneous coronary intervention to surgery, being lowest beyond 90 days:
- 8.6% in days 1–30
- 7.5% in days 31–90
- 5.2% in days 91–180
- 5.8% in days 181–365 (P = .02).
With bare-metal stents, the event rate remained high over time:
- 8.2% in days 1–30
- 6.6% in days 31–90
- 8.1% in days 91–180
- 8.8% in days 181–365 (P = .60).
This study did not report information about perioperative antiplatelet management and was limited to first-generation drug-eluting stents.
Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
The study. Saia et al16 retrospectively examined predictors of periprocedural ischemic and bleeding events among cardiac and noncardiac surgical patients who had previously undergone percutaneous coronary intervention. They also assessed the risks associated with stent type and time from percutaneous coronary intervention to surgery.
Of 39,362 patients, 13,128 underwent procedures during the 5-year study period. The cumulative incidence of surgery was 3.6% at 30 days, 14% at 1 year, and 40% at 5 years after percutaneous coronary intervention. Almost 30% of the procedures were done urgently.
Results. The 30-day rate of postoperative cardiac death was 2.5%, nonfatal myocardial infarction 1.5%, and serious bleeding events 6.5%. Older drug-eluting stents were associated with higher risks of adverse events than newer drug-eluting stents at any time point (odds ratio 2.1 at 0–180 days, 1.9 at 6–12 months, and 1.45 after 12 months). Surgery performed 6 to 12 months after percutaneous coronary intervention had lower rates of adverse outcomes than surgery performed within 6 months. Beyond 6 months from percutaneous coronary intervention, bare-metal stents and newer drug-eluting stents did not have significantly different adverse event rates; however, newer drug-eluting stents appeared safer than bare-metal stents from 0 to 180 days.
Limitations of this study included lack of information regarding periprocedural antiplatelet management and a relatively small subset of newer drug-eluting stent patients.
Implications for clinical practice. These studies added to earlier work that demonstrated that the risk of perioperative adverse events differs by both the stent type and the time from percutaneous coronary intervention to noncardiac surgery. In patients with a drug-eluting stent, the risk levels off 90 days after percutaneous coronary intervention, suggesting that the previously recommended 12 months of uninterrupted dual antiplatelet therapy (per the 2014 ACC/AHA guidelines1) may not be needed, particularly with newer-generation drug-eluting stents. Based on new evidence, the ACC/AHA guidelines regarding perioperative management of dual antiplatelet therapy in noncardiac surgery patients were updated,17 as noted below.
An update to the ACC/AHA guidelines on dual antiplatelet therapy
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29. DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]
The 2016 update17 provides the following recommendations for patients with coronary stents who undergo noncardiac surgery:
- Delay elective surgery for 30 days after placement of a bare-metal stent (class I recommendation, level of evidence B).
- It is optimal to delay elective surgery 6 months after drug-eluting stent placement (class I recommendation, level of evidence B).
- If dual antiplatelet therapy must be discontinued, then continue aspirin if possible and restart the P2Y12 inhibitor as soon as possible postoperatively (class I recommendation, level of evidence C ).
- A consensus decision among treating clinicians is useful regarding the risks of surgery and discontinuation or continuation of antiplatelet therapy (class IIa recommendation, level of evidence C).
- If dual antiplatelet therapy must be discontinued, then elective surgery should not be performed less than 30 days after bare-metal stent placement, or less than 3 months after drug-eluting stent placement (class III recommendation, level of evidence B).
- Elective surgery after drug-eluting stent placement when the P2Y12 inhibitor must be discontinued may be considered 3 months after drug-eluting stent placement if the risk of surgical delay is greater than the risk of stent thrombosis (class IIb recommendation, level of evidence C).
The basic differences are the new recommendations for a minimum of 6 months of dual antiplatelet therapy as opposed to 12 months after drug-eluting stent placement before elective noncardiac surgery, and to allow surgery after 3 months (as opposed to 6 months) if the risk of delaying surgery outweighs the risk of stent thrombosis or myocardial infarction.
PERIOPERATIVE ANTICOAGULATION
The optimal perioperative management of patients with atrial fibrillation who are on warfarin is uncertain. The American College of Chest Physicians guidelines18 categorized patients with atrial fibrillation into low, moderate, and high thromboembolic risk. Based primarily on observational data, these guidelines recommended perioperative bridging anticoagulation for those at high risk but not for those at low risk. For intermediate-risk patients, there were insufficient data to make any recommendation.
Bridging may not benefit those at intermediate risk
Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
The study. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial19 was the first randomized controlled trial to examine the effects of perioperative bridging anticoagulation in patients with atrial fibrillation without mechanical heart valves.
Results. In 1,884 patients undergoing elective surgery, the incidence of arterial thromboembolism was 0.4% in the no-bridging group and 0.3% in the bridging group (95% CI −0.6 to 0.8; P = .01 for noninferiority). Major bleeding occurred in 1.3% of patients in the no-bridging group and 3.2% in the bridging group (95% CI 0.20–0.78; P = .005 for superiority).
These results suggest that the risks of bridging therapy are greater than the benefits. Of note, the mean CHADS2 score (1 point each for congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus; 2 points for previous stroke or transient ischemic attack; a total score > 2 indicates significant risk of stroke) for patients enrolled in this trial was 2.3, and it may be difficult to extrapolate these results to the limited number of patients at highest risk, ie, who have a CHADS2 score of 5 or 6. Also, this study did not address patients with arterial or venous thromboembolism.
Implications for clinical practice. Despite the limitations noted above, this study does provide guidance for management of the intermediate-risk group with atrial fibrillation as defined by the American College of Chest Physicians: a no-bridging strategy is the best option.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA 1961; 178:261–266.
- Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
- Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
- Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
- Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol 2001; 88:1306–1307.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
- Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
- Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29 DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]. Accessed August 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S. Erratum in Chest 2012; 141:1129.
- Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
Perioperative medicine is an evolving field with a rapidly growing body of literature. Because physicians and patients are often concerned about cardiac risk, we focus this review on perioperative cardiology.
The information we present here is derived from presentations at the Perioperative Medicine Summit and the annual meetings of the Society of Hospital Medicine and Society of General Internal Medicine in 2016. We surveyed perioperative literature from January 2015 through March 2016 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
We have divided this review into four sections:
- Preoperative cardiac risk assessment
- Medical therapy to reduce postoperative cardiac complications (beta-blockers, statins, and angiotensin II receptor blockers [ARBs])
- Perioperative management of patients with a coronary stent on antiplatelet therapy
- Perioperative bridging anticoagulation.
PREOPERATIVE ASSESSMENT OF CARDIAC RISK
Functionally independent patients do better
Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
Functional capacity is an independent predictor of perioperative death and is included in the algorithm of the current joint American College of Cardiology/American Heart Association (ACC/AHA) guidelines,1 but it is not in the Revised Cardiac Risk Index2 or the American Society of Anesthesiologists (ASA) classification.3
The study. Visnjevac et al4 performed a retrospective, observational cohort study of 12,324 patients who underwent noncardiac surgery, stratifying rates of all-cause mortality and 30-day postoperative complications based on ASA class and functional capacity.
The ASA physical status classification is defined as:
- 1—Normal healthy patient
- 2—Patient with mild systemic disease
- 3—Patient with severe systemic disease
- 4—Patient with severe systemic disease that is a constant threat to life
- 5—Moribund patient not expected to survive without surgery.
Functional capacity was defined as the ability to perform all activities of daily living. It was prospectively assessed during the patient interview by pre-anesthesia personnel and entered into the database of the Veterans Affairs Surgical Quality Improvement Program.
Results. Within each ASA class, the mortality rate was significantly lower for functionally independent patients than for partially or fully dependent patients:
- In class 2—odds ratio (OR) 0.14 for functionally independent patients
- In class 3—OR 0.29 for functionally independent patients
- In class 4—OR 0.5 for functionally independent patients.
The mortality rate was higher for dependent patients than for independent patients who were one ASA class higher, despite the higher class having greater rates of comorbidity.
Adding functional capacity to the ASA classification improved the area under the receiver operating curve from 0.811 to 0.848 (a perfect test would have a value of 1.0), suggesting that physicians should incorporate functional capacity into their preoperative evaluation, perhaps by increasing a patient’s ASA class to the next higher class if he or she is functionally dependent.
Angina portends poor outcomes
Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
Coronary artery disease is a risk factor for adverse perioperative outcomes, but the risk varies depending on whether the patient has had a myocardial infarction (and how long ago) and whether he or she has anginal symptoms (and how severe they are).
The study. Pandey et al5 used data from the American College of Surgeons National Surgical Quality Improvement Program to evaluate the impact of stable angina in 1,568 patients who underwent noncardiac surgery after a myocardial infarction.
Results. Postoperative myocardial infarction or cardiac arrest occurred in 5.5% of patients. The incidence was significantly greater in those who had anginal symptoms before surgery than in those without symptoms (8.4% vs 5%, P = .035); reintervention rates and length of stay were also higher in this group. In multivariate analysis, preoperative angina remained a significant predictor of postoperative myocardial infarction (OR 2.49, 95% confidence interval [CI] 1.20–5.81) and reintervention (OR 2.4, 95% CI 1.44–3.82.
The authors cautioned against relying on predictive tools such as the Revised Cardiac Risk Index that do not consider stable angina and previous myocardial infarction as separate independent risk factors.
Implications for clinical practice. While functional capacity is an integral part of the ACC/AHA guideline algorithm,1 the findings of these two studies suggest that other current tools to calculate perioperative risk (ASA class and Revised Cardiac Risk Index) could be improved by including functional capacity and stable angina.
PERIOPERATIVE MEDICAL THERAPY
Beta-blockers help only those at high risk and may harm others
Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
Beta-blockers have been used perioperatively for nearly 2 decades to try to reduce rates of postoperative major adverse cardiovascular events. However, in view of recent trials, fewer patients are likely to benefit from this intervention than has been thought.
The study. Friedell et al6 retrospectively analyzed data from 343,645 patients in Veterans Affairs hospitals to determine the effect of beta-blockers on major adverse cardiac event rates after major noncardiac surgery. Beta-blockers were considered to have been used perioperatively if given any time between 8 hours before and 24 hours after surgery. The outcome studied was the mortality rate at 30 days.
The authors derived a novel risk score and used multivariate analysis to attempt to adjust for confounding factors. The risk score was based on four risk factors identified a priori:
- Serum creatinine level > 2.0 mg/dL
- Coronary artery disease
- Diabetes
- Surgery in a major body cavity (abdomen or chest).
Results. In this cohort, 43.2% of patients had received a beta-blocker. The unadjusted mortality rates by risk category for patients receiving or not receiving a beta-blocker were:
- No risk factors: 1.0% with a beta-blocker vs 0.6% without
- One or two risk factors: 1.7% vs 1.5%
- Three or four risk factors: 2.3% vs 4.5%.
After adjustment for confounding factors, the 30-day mortality rate was higher in low-risk patients and lower in high-risk patients who received beta-blockers. Odds ratios for death in beta-blocker users (entire cohort) by risk category were:
- No risk factors: 1.19
- One or two risk factors 0.97
- Three or four risk factors 0.76.
In the 3.8% of the total cohort who underwent cardiac surgery, beta-blockers had no significant effect—beneficial or harmful—in any risk group.
Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
The study. Jørgensen et al7 investigated the association between chronic beta-blocker use for the treatment of hypertension and 30-day rates of mortality and major adverse cardiac events. Eligible patients (N = 55,320) were at least 20 years old and were undergoing any type of noncardiac surgery. The authors established that hypertension was present through use of an algorithm based on the International Classification of Diseases (10th edition). Patients with existing cardiovascular disease and renal disease were excluded. The authors used multivariate analysis to adjust for confounding factors.
Results. Twenty-six percent of the patients were on chronic beta-blocker therapy for hypertension. The mortality rate at 30 days was 1.93% in patients treated with a beta-blocker alone or in combination with other antihypertensive drugs; the rate was 1.32% for patients receiving any combination of renin-angiotensin system inhibitor, calcium antagonist, or thiazide, but no beta-blocker. Similarly, the 30-day major adverse cardiac event rates were 1.32% with beta-blockers and 0.84% without beta-blockers.
In subgroup analysis, each medication combination that included a beta-blocker was associated with higher rates of death and major adverse cardiac events than the same combination without a beta-blocker. Odds ratios for major adverse cardiac events with beta-blocker combinations ranged from 1.22 to 2.16 compared with regimens with no beta-blocker.
Implications for clinical practice. These two studies added to a growing chorus of concerns about the value and safety of beta-blockers in surgical patients. Friedell et al6 made an observation that was remarkably similar to one reported by Lindenauer et al8 in 2005: when patients were stratified by baseline risk of death, only those with the highest baseline risk benefited from beta-blocker therapy. Those in the lowest risk group actually were harmed by beta-blocker use, ie, the mortality rate was higher.
More interesting is the novel observation by Jørgensen et al7 that even in patients with no known cardiovascular disease who are on chronic beta-blocker therapy—presumably on stable doses and not solely for perioperative risk reduction—rates of mortality and major adverse cardiac events were higher than for patients not on chronic beta-blocker therapy.
The current studies support a cautious, selective approach to the perioperative use of beta-blockers—they should be used only in high-risk patients undergoing high-risk surgery, as has been proposed by the ACC/AHA.1
Statins protect
Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
The study9 was a comprehensive meta-analysis of randomized controlled trials and observational studies of the effects of HMG-CoA reductase inhibitors (statins) on perioperative outcomes in patients undergoing vascular surgery (but not for intracranial or coronary artery disease). Twenty-four studies were included, 4 randomized controlled trials and 20 observational studies (including 16 cohort and 4 case-controlled studies), with a total of 22,536 patients, 8,052 receiving statins and 15,484 not receiving statins.
Results. Although there was no significant difference in cardiovascular mortality rates, patients receiving statins had significantly lower rates of all-cause mortality, myocardial infarction, stroke, and a composite of myocardial infarction, stroke, and death at 30 days postoperatively than patients not receiving statins. Additionally, there was no difference in the incidence of kidney injury between groups. The possibility of publication bias was thought to be low for all of these outcomes.
Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
The study. The Vascular Events in Non-cardiac Surgery Patients Cohort Evaluation (VISION) study10,11 was an international prospective cohort study of more than 40,000 patients age 45 and older undergoing major noncardiac surgery with either general or regional anesthesia. Postoperative troponin measurements were obtained in all patients 6 to 12 hours after surgery and for the first 3 postoperative days. The authors evaluated the effect of preoperative statin use on cardiovascular outcomes at 30 days after surgery using a multivariate logistic model and propensity score analysis to correct for confounding factors. Statin use was defined as exposure within 7 days before surgery or 3 days after.
Results. In the 15,478 patients included in the analysis, statin use conferred a significant reduction in the primary outcome (composite of all-cause mortality, myocardial injury after noncardiac surgery, or stroke); the absolute risk reduction was 2.0%. Statin users also had a significantly lower risk of all-cause mortality, cardiovascular mortality, and myocardial injury after noncardiac surgery, but not of postoperative myocardial infarction or stroke. This analysis did not address the type of statin, dosing, or safety markers such as liver and muscle function.
Implications for clinical practice. With largely observational data and a few small randomized trials, these meta-analyses provide important information with respect to perioperative cardiovascular protection by statins. Starting a statin before surgery and continuing it perioperatively seems appropriate in patients at high risk (as recommended by the ACC/AHA guidelines1). Based on other data, the benefit may be evident in as little as 5 days, as this is when statins appear to reach their plateau with regard to their vascular pleiotropic effects.12 The incidence of adverse effects of statins, including muscle and liver injury, appears to be low in the perioperative setting.13
Given the inconsistent data regarding perioperative beta-blocker therapy, statins may very well be the most important perioperative medication with respect to cardiovascular risk reduction. However, a large randomized trial would help to confirm this belief.
Restart angiotensin II receptor blockers soon after surgery
Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
A concern about perioperative use of ARBs is that they impair the renin-angiotensin-aldosterone system, which maintains blood pressure under general anesthesia. ARB-induced intraoperative hypotension is particularly difficult to control, as it is often refractory to treatment with conventional adrenergic vasopressors.
The study. Lee et al14 conducted a retrospective cohort trial to evaluate the effects of continuing to withhold ARBs postoperatively. Of the 30,173 patients admitted for surgery in the Veterans Affairs system from 1999 through 2011 who were taking an ARB before surgery and who met the inclusion criteria, 10,205 (33.8%) were not restarted on their medication by postoperative day 2.
Results. The mortality rate at 30 days was higher in those whose ARBs were withheld than in those in whom it was resumed, with a multivariable-adjusted hazard ratio of 1.74 (95% CI 1.47–2.06; P < .001). The risk of withholding ARBs was more pronounced in younger patients (hazard ratio 2.52; 95% CI 1.69–3.76 in those under age 60) than in older patients (hazard ratio 1.42, 95% CI 1.09–1.85 in those over age 75).
Implications for clinical practice. While not addressing whether to continue or withhold ARBs preoperatively, this retrospective study presented evidence that delay in resuming chronic ARB therapy after surgery was common and appeared to be associated with a higher 30-day mortality rate. The ACC/AHA guidelines1 state:
- Continuing angiotensin-converting enzyme (ACE) inhibitors or ARBs perioperatively is reasonable (class IIa recommendation, level of evidence B) (Table 1).
- If an ACE inhibitor or ARB is withheld before surgery, it is reasonable to restart it postoperatively as soon as clinically feasible (class IIa recommendation, level of evidence C).
Close attention to medication reconciliation in the postoperative period is necessary to facilitate early resumption of ARBs.
CORONARY STENTS AND ANTIPLATELET THERAPY IN NONCARDIAC SURGERY PATIENTS
Considerations in the management of noncardiac surgery patients with stents include risks of stent thrombosis, bleeding, and potentially delaying procedures to continue uninterrupted dual antiplatelet therapy. Evidence is evolving regarding the risks of perioperative complications in patients with bare-metal stents and drug-eluting stents, as well as the optimal timing before noncardiac surgery.
Bare-metal vs drug-eluting stents
Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
The study. Bangalore et al15 compared the safety of drug-eluting vs bare-metal stents in noncardiac surgery patients and investigated adverse events stratified by time since stent placement. This was a retrospective observational study of 8,415 patients in the Massachusetts claims database who underwent noncardiac surgery 1 year or less after percutaneous coronary intervention.
Results. There was no significant difference in the incidence of the primary outcome (composite of death, myocardial infarction, and bleeding) between the two groups.
With drug-eluting stents, patients had lower 30-day postoperative mortality rates, and their rate of the primary outcome decreased with time from percutaneous coronary intervention to surgery, being lowest beyond 90 days:
- 8.6% in days 1–30
- 7.5% in days 31–90
- 5.2% in days 91–180
- 5.8% in days 181–365 (P = .02).
With bare-metal stents, the event rate remained high over time:
- 8.2% in days 1–30
- 6.6% in days 31–90
- 8.1% in days 91–180
- 8.8% in days 181–365 (P = .60).
This study did not report information about perioperative antiplatelet management and was limited to first-generation drug-eluting stents.
Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
The study. Saia et al16 retrospectively examined predictors of periprocedural ischemic and bleeding events among cardiac and noncardiac surgical patients who had previously undergone percutaneous coronary intervention. They also assessed the risks associated with stent type and time from percutaneous coronary intervention to surgery.
Of 39,362 patients, 13,128 underwent procedures during the 5-year study period. The cumulative incidence of surgery was 3.6% at 30 days, 14% at 1 year, and 40% at 5 years after percutaneous coronary intervention. Almost 30% of the procedures were done urgently.
Results. The 30-day rate of postoperative cardiac death was 2.5%, nonfatal myocardial infarction 1.5%, and serious bleeding events 6.5%. Older drug-eluting stents were associated with higher risks of adverse events than newer drug-eluting stents at any time point (odds ratio 2.1 at 0–180 days, 1.9 at 6–12 months, and 1.45 after 12 months). Surgery performed 6 to 12 months after percutaneous coronary intervention had lower rates of adverse outcomes than surgery performed within 6 months. Beyond 6 months from percutaneous coronary intervention, bare-metal stents and newer drug-eluting stents did not have significantly different adverse event rates; however, newer drug-eluting stents appeared safer than bare-metal stents from 0 to 180 days.
Limitations of this study included lack of information regarding periprocedural antiplatelet management and a relatively small subset of newer drug-eluting stent patients.
Implications for clinical practice. These studies added to earlier work that demonstrated that the risk of perioperative adverse events differs by both the stent type and the time from percutaneous coronary intervention to noncardiac surgery. In patients with a drug-eluting stent, the risk levels off 90 days after percutaneous coronary intervention, suggesting that the previously recommended 12 months of uninterrupted dual antiplatelet therapy (per the 2014 ACC/AHA guidelines1) may not be needed, particularly with newer-generation drug-eluting stents. Based on new evidence, the ACC/AHA guidelines regarding perioperative management of dual antiplatelet therapy in noncardiac surgery patients were updated,17 as noted below.
An update to the ACC/AHA guidelines on dual antiplatelet therapy
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29. DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]
The 2016 update17 provides the following recommendations for patients with coronary stents who undergo noncardiac surgery:
- Delay elective surgery for 30 days after placement of a bare-metal stent (class I recommendation, level of evidence B).
- It is optimal to delay elective surgery 6 months after drug-eluting stent placement (class I recommendation, level of evidence B).
- If dual antiplatelet therapy must be discontinued, then continue aspirin if possible and restart the P2Y12 inhibitor as soon as possible postoperatively (class I recommendation, level of evidence C ).
- A consensus decision among treating clinicians is useful regarding the risks of surgery and discontinuation or continuation of antiplatelet therapy (class IIa recommendation, level of evidence C).
- If dual antiplatelet therapy must be discontinued, then elective surgery should not be performed less than 30 days after bare-metal stent placement, or less than 3 months after drug-eluting stent placement (class III recommendation, level of evidence B).
- Elective surgery after drug-eluting stent placement when the P2Y12 inhibitor must be discontinued may be considered 3 months after drug-eluting stent placement if the risk of surgical delay is greater than the risk of stent thrombosis (class IIb recommendation, level of evidence C).
The basic differences are the new recommendations for a minimum of 6 months of dual antiplatelet therapy as opposed to 12 months after drug-eluting stent placement before elective noncardiac surgery, and to allow surgery after 3 months (as opposed to 6 months) if the risk of delaying surgery outweighs the risk of stent thrombosis or myocardial infarction.
PERIOPERATIVE ANTICOAGULATION
The optimal perioperative management of patients with atrial fibrillation who are on warfarin is uncertain. The American College of Chest Physicians guidelines18 categorized patients with atrial fibrillation into low, moderate, and high thromboembolic risk. Based primarily on observational data, these guidelines recommended perioperative bridging anticoagulation for those at high risk but not for those at low risk. For intermediate-risk patients, there were insufficient data to make any recommendation.
Bridging may not benefit those at intermediate risk
Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
The study. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial19 was the first randomized controlled trial to examine the effects of perioperative bridging anticoagulation in patients with atrial fibrillation without mechanical heart valves.
Results. In 1,884 patients undergoing elective surgery, the incidence of arterial thromboembolism was 0.4% in the no-bridging group and 0.3% in the bridging group (95% CI −0.6 to 0.8; P = .01 for noninferiority). Major bleeding occurred in 1.3% of patients in the no-bridging group and 3.2% in the bridging group (95% CI 0.20–0.78; P = .005 for superiority).
These results suggest that the risks of bridging therapy are greater than the benefits. Of note, the mean CHADS2 score (1 point each for congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus; 2 points for previous stroke or transient ischemic attack; a total score > 2 indicates significant risk of stroke) for patients enrolled in this trial was 2.3, and it may be difficult to extrapolate these results to the limited number of patients at highest risk, ie, who have a CHADS2 score of 5 or 6. Also, this study did not address patients with arterial or venous thromboembolism.
Implications for clinical practice. Despite the limitations noted above, this study does provide guidance for management of the intermediate-risk group with atrial fibrillation as defined by the American College of Chest Physicians: a no-bridging strategy is the best option.
Perioperative medicine is an evolving field with a rapidly growing body of literature. Because physicians and patients are often concerned about cardiac risk, we focus this review on perioperative cardiology.
The information we present here is derived from presentations at the Perioperative Medicine Summit and the annual meetings of the Society of Hospital Medicine and Society of General Internal Medicine in 2016. We surveyed perioperative literature from January 2015 through March 2016 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
We have divided this review into four sections:
- Preoperative cardiac risk assessment
- Medical therapy to reduce postoperative cardiac complications (beta-blockers, statins, and angiotensin II receptor blockers [ARBs])
- Perioperative management of patients with a coronary stent on antiplatelet therapy
- Perioperative bridging anticoagulation.
PREOPERATIVE ASSESSMENT OF CARDIAC RISK
Functionally independent patients do better
Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
Functional capacity is an independent predictor of perioperative death and is included in the algorithm of the current joint American College of Cardiology/American Heart Association (ACC/AHA) guidelines,1 but it is not in the Revised Cardiac Risk Index2 or the American Society of Anesthesiologists (ASA) classification.3
The study. Visnjevac et al4 performed a retrospective, observational cohort study of 12,324 patients who underwent noncardiac surgery, stratifying rates of all-cause mortality and 30-day postoperative complications based on ASA class and functional capacity.
The ASA physical status classification is defined as:
- 1—Normal healthy patient
- 2—Patient with mild systemic disease
- 3—Patient with severe systemic disease
- 4—Patient with severe systemic disease that is a constant threat to life
- 5—Moribund patient not expected to survive without surgery.
Functional capacity was defined as the ability to perform all activities of daily living. It was prospectively assessed during the patient interview by pre-anesthesia personnel and entered into the database of the Veterans Affairs Surgical Quality Improvement Program.
Results. Within each ASA class, the mortality rate was significantly lower for functionally independent patients than for partially or fully dependent patients:
- In class 2—odds ratio (OR) 0.14 for functionally independent patients
- In class 3—OR 0.29 for functionally independent patients
- In class 4—OR 0.5 for functionally independent patients.
The mortality rate was higher for dependent patients than for independent patients who were one ASA class higher, despite the higher class having greater rates of comorbidity.
Adding functional capacity to the ASA classification improved the area under the receiver operating curve from 0.811 to 0.848 (a perfect test would have a value of 1.0), suggesting that physicians should incorporate functional capacity into their preoperative evaluation, perhaps by increasing a patient’s ASA class to the next higher class if he or she is functionally dependent.
Angina portends poor outcomes
Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
Coronary artery disease is a risk factor for adverse perioperative outcomes, but the risk varies depending on whether the patient has had a myocardial infarction (and how long ago) and whether he or she has anginal symptoms (and how severe they are).
The study. Pandey et al5 used data from the American College of Surgeons National Surgical Quality Improvement Program to evaluate the impact of stable angina in 1,568 patients who underwent noncardiac surgery after a myocardial infarction.
Results. Postoperative myocardial infarction or cardiac arrest occurred in 5.5% of patients. The incidence was significantly greater in those who had anginal symptoms before surgery than in those without symptoms (8.4% vs 5%, P = .035); reintervention rates and length of stay were also higher in this group. In multivariate analysis, preoperative angina remained a significant predictor of postoperative myocardial infarction (OR 2.49, 95% confidence interval [CI] 1.20–5.81) and reintervention (OR 2.4, 95% CI 1.44–3.82.
The authors cautioned against relying on predictive tools such as the Revised Cardiac Risk Index that do not consider stable angina and previous myocardial infarction as separate independent risk factors.
Implications for clinical practice. While functional capacity is an integral part of the ACC/AHA guideline algorithm,1 the findings of these two studies suggest that other current tools to calculate perioperative risk (ASA class and Revised Cardiac Risk Index) could be improved by including functional capacity and stable angina.
PERIOPERATIVE MEDICAL THERAPY
Beta-blockers help only those at high risk and may harm others
Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
Beta-blockers have been used perioperatively for nearly 2 decades to try to reduce rates of postoperative major adverse cardiovascular events. However, in view of recent trials, fewer patients are likely to benefit from this intervention than has been thought.
The study. Friedell et al6 retrospectively analyzed data from 343,645 patients in Veterans Affairs hospitals to determine the effect of beta-blockers on major adverse cardiac event rates after major noncardiac surgery. Beta-blockers were considered to have been used perioperatively if given any time between 8 hours before and 24 hours after surgery. The outcome studied was the mortality rate at 30 days.
The authors derived a novel risk score and used multivariate analysis to attempt to adjust for confounding factors. The risk score was based on four risk factors identified a priori:
- Serum creatinine level > 2.0 mg/dL
- Coronary artery disease
- Diabetes
- Surgery in a major body cavity (abdomen or chest).
Results. In this cohort, 43.2% of patients had received a beta-blocker. The unadjusted mortality rates by risk category for patients receiving or not receiving a beta-blocker were:
- No risk factors: 1.0% with a beta-blocker vs 0.6% without
- One or two risk factors: 1.7% vs 1.5%
- Three or four risk factors: 2.3% vs 4.5%.
After adjustment for confounding factors, the 30-day mortality rate was higher in low-risk patients and lower in high-risk patients who received beta-blockers. Odds ratios for death in beta-blocker users (entire cohort) by risk category were:
- No risk factors: 1.19
- One or two risk factors 0.97
- Three or four risk factors 0.76.
In the 3.8% of the total cohort who underwent cardiac surgery, beta-blockers had no significant effect—beneficial or harmful—in any risk group.
Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
The study. Jørgensen et al7 investigated the association between chronic beta-blocker use for the treatment of hypertension and 30-day rates of mortality and major adverse cardiac events. Eligible patients (N = 55,320) were at least 20 years old and were undergoing any type of noncardiac surgery. The authors established that hypertension was present through use of an algorithm based on the International Classification of Diseases (10th edition). Patients with existing cardiovascular disease and renal disease were excluded. The authors used multivariate analysis to adjust for confounding factors.
Results. Twenty-six percent of the patients were on chronic beta-blocker therapy for hypertension. The mortality rate at 30 days was 1.93% in patients treated with a beta-blocker alone or in combination with other antihypertensive drugs; the rate was 1.32% for patients receiving any combination of renin-angiotensin system inhibitor, calcium antagonist, or thiazide, but no beta-blocker. Similarly, the 30-day major adverse cardiac event rates were 1.32% with beta-blockers and 0.84% without beta-blockers.
In subgroup analysis, each medication combination that included a beta-blocker was associated with higher rates of death and major adverse cardiac events than the same combination without a beta-blocker. Odds ratios for major adverse cardiac events with beta-blocker combinations ranged from 1.22 to 2.16 compared with regimens with no beta-blocker.
Implications for clinical practice. These two studies added to a growing chorus of concerns about the value and safety of beta-blockers in surgical patients. Friedell et al6 made an observation that was remarkably similar to one reported by Lindenauer et al8 in 2005: when patients were stratified by baseline risk of death, only those with the highest baseline risk benefited from beta-blocker therapy. Those in the lowest risk group actually were harmed by beta-blocker use, ie, the mortality rate was higher.
More interesting is the novel observation by Jørgensen et al7 that even in patients with no known cardiovascular disease who are on chronic beta-blocker therapy—presumably on stable doses and not solely for perioperative risk reduction—rates of mortality and major adverse cardiac events were higher than for patients not on chronic beta-blocker therapy.
The current studies support a cautious, selective approach to the perioperative use of beta-blockers—they should be used only in high-risk patients undergoing high-risk surgery, as has been proposed by the ACC/AHA.1
Statins protect
Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
The study9 was a comprehensive meta-analysis of randomized controlled trials and observational studies of the effects of HMG-CoA reductase inhibitors (statins) on perioperative outcomes in patients undergoing vascular surgery (but not for intracranial or coronary artery disease). Twenty-four studies were included, 4 randomized controlled trials and 20 observational studies (including 16 cohort and 4 case-controlled studies), with a total of 22,536 patients, 8,052 receiving statins and 15,484 not receiving statins.
Results. Although there was no significant difference in cardiovascular mortality rates, patients receiving statins had significantly lower rates of all-cause mortality, myocardial infarction, stroke, and a composite of myocardial infarction, stroke, and death at 30 days postoperatively than patients not receiving statins. Additionally, there was no difference in the incidence of kidney injury between groups. The possibility of publication bias was thought to be low for all of these outcomes.
Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
The study. The Vascular Events in Non-cardiac Surgery Patients Cohort Evaluation (VISION) study10,11 was an international prospective cohort study of more than 40,000 patients age 45 and older undergoing major noncardiac surgery with either general or regional anesthesia. Postoperative troponin measurements were obtained in all patients 6 to 12 hours after surgery and for the first 3 postoperative days. The authors evaluated the effect of preoperative statin use on cardiovascular outcomes at 30 days after surgery using a multivariate logistic model and propensity score analysis to correct for confounding factors. Statin use was defined as exposure within 7 days before surgery or 3 days after.
Results. In the 15,478 patients included in the analysis, statin use conferred a significant reduction in the primary outcome (composite of all-cause mortality, myocardial injury after noncardiac surgery, or stroke); the absolute risk reduction was 2.0%. Statin users also had a significantly lower risk of all-cause mortality, cardiovascular mortality, and myocardial injury after noncardiac surgery, but not of postoperative myocardial infarction or stroke. This analysis did not address the type of statin, dosing, or safety markers such as liver and muscle function.
Implications for clinical practice. With largely observational data and a few small randomized trials, these meta-analyses provide important information with respect to perioperative cardiovascular protection by statins. Starting a statin before surgery and continuing it perioperatively seems appropriate in patients at high risk (as recommended by the ACC/AHA guidelines1). Based on other data, the benefit may be evident in as little as 5 days, as this is when statins appear to reach their plateau with regard to their vascular pleiotropic effects.12 The incidence of adverse effects of statins, including muscle and liver injury, appears to be low in the perioperative setting.13
Given the inconsistent data regarding perioperative beta-blocker therapy, statins may very well be the most important perioperative medication with respect to cardiovascular risk reduction. However, a large randomized trial would help to confirm this belief.
Restart angiotensin II receptor blockers soon after surgery
Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
A concern about perioperative use of ARBs is that they impair the renin-angiotensin-aldosterone system, which maintains blood pressure under general anesthesia. ARB-induced intraoperative hypotension is particularly difficult to control, as it is often refractory to treatment with conventional adrenergic vasopressors.
The study. Lee et al14 conducted a retrospective cohort trial to evaluate the effects of continuing to withhold ARBs postoperatively. Of the 30,173 patients admitted for surgery in the Veterans Affairs system from 1999 through 2011 who were taking an ARB before surgery and who met the inclusion criteria, 10,205 (33.8%) were not restarted on their medication by postoperative day 2.
Results. The mortality rate at 30 days was higher in those whose ARBs were withheld than in those in whom it was resumed, with a multivariable-adjusted hazard ratio of 1.74 (95% CI 1.47–2.06; P < .001). The risk of withholding ARBs was more pronounced in younger patients (hazard ratio 2.52; 95% CI 1.69–3.76 in those under age 60) than in older patients (hazard ratio 1.42, 95% CI 1.09–1.85 in those over age 75).
Implications for clinical practice. While not addressing whether to continue or withhold ARBs preoperatively, this retrospective study presented evidence that delay in resuming chronic ARB therapy after surgery was common and appeared to be associated with a higher 30-day mortality rate. The ACC/AHA guidelines1 state:
- Continuing angiotensin-converting enzyme (ACE) inhibitors or ARBs perioperatively is reasonable (class IIa recommendation, level of evidence B) (Table 1).
- If an ACE inhibitor or ARB is withheld before surgery, it is reasonable to restart it postoperatively as soon as clinically feasible (class IIa recommendation, level of evidence C).
Close attention to medication reconciliation in the postoperative period is necessary to facilitate early resumption of ARBs.
CORONARY STENTS AND ANTIPLATELET THERAPY IN NONCARDIAC SURGERY PATIENTS
Considerations in the management of noncardiac surgery patients with stents include risks of stent thrombosis, bleeding, and potentially delaying procedures to continue uninterrupted dual antiplatelet therapy. Evidence is evolving regarding the risks of perioperative complications in patients with bare-metal stents and drug-eluting stents, as well as the optimal timing before noncardiac surgery.
Bare-metal vs drug-eluting stents
Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
The study. Bangalore et al15 compared the safety of drug-eluting vs bare-metal stents in noncardiac surgery patients and investigated adverse events stratified by time since stent placement. This was a retrospective observational study of 8,415 patients in the Massachusetts claims database who underwent noncardiac surgery 1 year or less after percutaneous coronary intervention.
Results. There was no significant difference in the incidence of the primary outcome (composite of death, myocardial infarction, and bleeding) between the two groups.
With drug-eluting stents, patients had lower 30-day postoperative mortality rates, and their rate of the primary outcome decreased with time from percutaneous coronary intervention to surgery, being lowest beyond 90 days:
- 8.6% in days 1–30
- 7.5% in days 31–90
- 5.2% in days 91–180
- 5.8% in days 181–365 (P = .02).
With bare-metal stents, the event rate remained high over time:
- 8.2% in days 1–30
- 6.6% in days 31–90
- 8.1% in days 91–180
- 8.8% in days 181–365 (P = .60).
This study did not report information about perioperative antiplatelet management and was limited to first-generation drug-eluting stents.
Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
The study. Saia et al16 retrospectively examined predictors of periprocedural ischemic and bleeding events among cardiac and noncardiac surgical patients who had previously undergone percutaneous coronary intervention. They also assessed the risks associated with stent type and time from percutaneous coronary intervention to surgery.
Of 39,362 patients, 13,128 underwent procedures during the 5-year study period. The cumulative incidence of surgery was 3.6% at 30 days, 14% at 1 year, and 40% at 5 years after percutaneous coronary intervention. Almost 30% of the procedures were done urgently.
Results. The 30-day rate of postoperative cardiac death was 2.5%, nonfatal myocardial infarction 1.5%, and serious bleeding events 6.5%. Older drug-eluting stents were associated with higher risks of adverse events than newer drug-eluting stents at any time point (odds ratio 2.1 at 0–180 days, 1.9 at 6–12 months, and 1.45 after 12 months). Surgery performed 6 to 12 months after percutaneous coronary intervention had lower rates of adverse outcomes than surgery performed within 6 months. Beyond 6 months from percutaneous coronary intervention, bare-metal stents and newer drug-eluting stents did not have significantly different adverse event rates; however, newer drug-eluting stents appeared safer than bare-metal stents from 0 to 180 days.
Limitations of this study included lack of information regarding periprocedural antiplatelet management and a relatively small subset of newer drug-eluting stent patients.
Implications for clinical practice. These studies added to earlier work that demonstrated that the risk of perioperative adverse events differs by both the stent type and the time from percutaneous coronary intervention to noncardiac surgery. In patients with a drug-eluting stent, the risk levels off 90 days after percutaneous coronary intervention, suggesting that the previously recommended 12 months of uninterrupted dual antiplatelet therapy (per the 2014 ACC/AHA guidelines1) may not be needed, particularly with newer-generation drug-eluting stents. Based on new evidence, the ACC/AHA guidelines regarding perioperative management of dual antiplatelet therapy in noncardiac surgery patients were updated,17 as noted below.
An update to the ACC/AHA guidelines on dual antiplatelet therapy
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29. DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]
The 2016 update17 provides the following recommendations for patients with coronary stents who undergo noncardiac surgery:
- Delay elective surgery for 30 days after placement of a bare-metal stent (class I recommendation, level of evidence B).
- It is optimal to delay elective surgery 6 months after drug-eluting stent placement (class I recommendation, level of evidence B).
- If dual antiplatelet therapy must be discontinued, then continue aspirin if possible and restart the P2Y12 inhibitor as soon as possible postoperatively (class I recommendation, level of evidence C ).
- A consensus decision among treating clinicians is useful regarding the risks of surgery and discontinuation or continuation of antiplatelet therapy (class IIa recommendation, level of evidence C).
- If dual antiplatelet therapy must be discontinued, then elective surgery should not be performed less than 30 days after bare-metal stent placement, or less than 3 months after drug-eluting stent placement (class III recommendation, level of evidence B).
- Elective surgery after drug-eluting stent placement when the P2Y12 inhibitor must be discontinued may be considered 3 months after drug-eluting stent placement if the risk of surgical delay is greater than the risk of stent thrombosis (class IIb recommendation, level of evidence C).
The basic differences are the new recommendations for a minimum of 6 months of dual antiplatelet therapy as opposed to 12 months after drug-eluting stent placement before elective noncardiac surgery, and to allow surgery after 3 months (as opposed to 6 months) if the risk of delaying surgery outweighs the risk of stent thrombosis or myocardial infarction.
PERIOPERATIVE ANTICOAGULATION
The optimal perioperative management of patients with atrial fibrillation who are on warfarin is uncertain. The American College of Chest Physicians guidelines18 categorized patients with atrial fibrillation into low, moderate, and high thromboembolic risk. Based primarily on observational data, these guidelines recommended perioperative bridging anticoagulation for those at high risk but not for those at low risk. For intermediate-risk patients, there were insufficient data to make any recommendation.
Bridging may not benefit those at intermediate risk
Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
The study. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial19 was the first randomized controlled trial to examine the effects of perioperative bridging anticoagulation in patients with atrial fibrillation without mechanical heart valves.
Results. In 1,884 patients undergoing elective surgery, the incidence of arterial thromboembolism was 0.4% in the no-bridging group and 0.3% in the bridging group (95% CI −0.6 to 0.8; P = .01 for noninferiority). Major bleeding occurred in 1.3% of patients in the no-bridging group and 3.2% in the bridging group (95% CI 0.20–0.78; P = .005 for superiority).
These results suggest that the risks of bridging therapy are greater than the benefits. Of note, the mean CHADS2 score (1 point each for congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus; 2 points for previous stroke or transient ischemic attack; a total score > 2 indicates significant risk of stroke) for patients enrolled in this trial was 2.3, and it may be difficult to extrapolate these results to the limited number of patients at highest risk, ie, who have a CHADS2 score of 5 or 6. Also, this study did not address patients with arterial or venous thromboembolism.
Implications for clinical practice. Despite the limitations noted above, this study does provide guidance for management of the intermediate-risk group with atrial fibrillation as defined by the American College of Chest Physicians: a no-bridging strategy is the best option.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA 1961; 178:261–266.
- Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
- Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
- Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
- Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol 2001; 88:1306–1307.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
- Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
- Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29 DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]. Accessed August 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S. Erratum in Chest 2012; 141:1129.
- Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA 1961; 178:261–266.
- Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
- Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
- Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
- Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol 2001; 88:1306–1307.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
- Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
- Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29 DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]. Accessed August 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S. Erratum in Chest 2012; 141:1129.
- Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
KEY POINTS
- Outcomes are worse in patients with poor functional capacity or stable angina, and these factors should be considered in preoperative risk assessment.
- Perioperative use of beta-blockers may benefit only patients at highest risk and may harm other patients.
- Statins seem to provide perioperative protection.
- If an ARB is withheld for surgery, it should be restarted soon after.
- For patients with a coronary stent, the type of stent and duration of dual antiplatelet therapy need to be considered before noncardiac surgery.
- Bridging anticoagulant therapy should not be used in patients at intermediate or low risk of thromboembolism.
Perioperative medicine: Combining the science and the art
In this issue of the Cleveland Clinic Journal of Medicine,1 Dr. Steven L. Cohn provides a succinct review of the recently published guidelines by the American College of Cardiology and American Heart Association (ACC/AHA) on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery.2 Although no drastic changes have been made in these guidelines, several significant modifications have been implemented and are highlighted in his review.
A BREACH OF SCIENTIFIC INTEGRITY
First, I am pleased Dr. Cohn described how the writing committee of the new guidelines handled the well-publicized breaches of scientific integrity by Dr. Don Poldermans, a prolific perioperative-medicine researcher at Erasmus University in the Netherlands who has contributed an abundance of literature that influenced clinical practice. Although some of his key publications were excluded by the ACC/AHA committee in its overall analysis, it remains unclear to me if simply ignoring some of his work is truly possible. For better or for worse, his publications have significantly shaped clinical practice in addition to guiding subsequent research in this field.
ASSESSING RISK
Along with continuing to endorse the Revised Cardiac Risk Index (RCRI),3 the guidelines now include another option for objective preoperative cardiovascular risk assessment. Dr. Cohn nicely outlines the pros and cons of the surgical risk calculator (often referred to as the “Gupta calculator”) derived from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database.4
Although the RCRI is not perfect, I agree with Dr. Cohn that the ACS NSQIP tool has limitations, including a cumbersome calculation (requiring a smartphone application or online calculator), lack of external validation, and use of the American Society of Anesthesiologists Physical Status Classification System, which has been notoriously confusing for generalists and has demonstrated poor inter-rater reliability among anesthesiologists.5,6
Of note, a patient may have very different risk-prediction scores depending on which tool is used. For example, a 66-year-old man with a history of ischemic heart disease, diabetes on insulin therapy, hypertension, and chronic kidney disease with a serum creatinine level greater than 2.0 mg/dL who is scheduled to undergo total hip arthroplasty would have a risk of a perioperative cardiovascular event of about 10% according to the RCRI, but only 1.1% according to the ACS NSQIP calculator. How widely this newer risk-stratification tool will be adopted in clinical practice will be interesting to observe.
In what appears to be an effort to simplify the guidelines, the ACC/AHA now recommends combining the patient’s clinical and surgical risks into estimating an overall perioperative risk for developing major adverse cardiac events. This estimate is now whittled down to only two categories: “low risk” and “elevated risk.” I am concerned that the new guidelines may have become too streamlined and lack the direction to assist providers in making important clinical decisions. Most notably, and as Dr. Cohn appropriately suggests, many patients will be in a gray zone with respect to whether cardiac stress testing should be obtained before surgery.
STRESS TESTING
Significant background knowledge is required to answer the important question in the ACC/AHA algorithm, ie, whether further testing will have an impact on decision-making or perioperative care.2 Dr. Cohn provides some of this information by noting the abysmal positive predictive value of preoperative noninvasive cardiac testing (with studies ranging from 0% to 37%) and by correctly stating that no benefit has been observed with preoperative cardiac revascularization.
If this is not widely known, I share Dr. Cohn’s fear that the new guidelines may stimulate increased ordering of preoperative stress tests. We observed this trend with the highly scripted 2002 ACC/AHA perioperative guidelines7 and subsequently learned that stress testing before surgery very seldom changes patient management.
A preoperative stress test should be reserved for patients with symptoms suggestive of ischemic heart disease. As a diagnostic study, the value of stress testing is excellent. This is not true when it is used as a screening test for asymptomatic patients, where its ability to predict perioperative cardiovascular events is extremely poor. The only other indication for preoperative stress testing is the rare occasion when further risk stratification is desired for exceptionally high-risk patients. In this scenario, test results may influence the decision to proceed with surgery vs seeking nonoperative approaches or palliative care.
MANAGING MEDICATIONS
Dr. Cohn discusses pertinent issues in the perioperative management of patients’ medications, an important component of the preoperative evaluation.
Despite the inconsistent clinical trial results on perioperative beta-blockers, his assessment of their risks and benefits is clinically accurate and practical. Furthermore, I fully agree with Dr. Cohn’s thoughtful approach regarding perioperative statins, despite the limited data available from randomized controlled trials.
With respect to perioperative aspirin use, I have concerns with Dr. Cohn’s statement that it may be reasonable to continue aspirin perioperatively if the risk of potential cardiac events outweighs the risk of bleeding. Given the result of the recently published second Perioperative Ischemic Evaluation (POISE-2) trial8 that showed a significantly higher risk of major perioperative bleeding in patients randomized to low-dose aspirin, it is difficult to advocate continuing aspirin when no cardiovascular protection was found in this very large trial. I agree with Dr. Cohn that this applies only to patients with no history of coronary artery stent placement, as patients with a stent should remain on low-dose aspirin throughout the entire perioperative period.
Controversy also surrounds angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Dr. Cohn agrees with the ACC/AHA guidelines to continue these agents before surgery; however, I favor holding them on the day of surgery. Although the risk of hypotension-induced cardiac events has not been clearly demonstrated, a recent retrospective study involving more than 1,100 patients showed significantly more acute kidney injury (even after adjusting for hypotension) as well as an increased length of hospital stay in the patients exposed to these agents before surgery.9 Given these findings, in addition to the postinduction hypotension (which can be profound) commonly observed by our anesthesiology colleagues, I recommend holding angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the day of surgery, with very few exceptions.
THE SCIENCE AND ART OF MEDICINE
Dr. Cohn acknowledges that we lack scientific data to answer many questions that arise when caring for the perioperative patient and thus we rely on the ACC/AHA guidelines to provide a framework. These scientific knowledge gaps emphasize the importance of the art of medicine in the perioperative arena. Although we may desire “cookbook” guidelines, the significant gaps in the perioperative medicine evidence base reinforce the necessity to provide individual patient-level care in a multidisciplinary environment with our surgery and anesthesiology colleagues. Without the proper balance of science and art in perioperative medicine, we sacrifice our ability to deliver optimal care for this high-risk patient population.
- Cohn SL. Updated guidelines on cardiovascular evaluation before noncardiac surgery: a view from the trenches. Cleve Clin J Med 2014; 81:742–751.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Aronson WL, McAuliffe MS, Miller K. Variability in the American Society of Anesthesiologists Physical Status Classification Scale. AANA J 2003; 71:265–274.
- Mak PH, Campbell RC, Irwin MG; American Society of Anesthesiologists. The ASA Physical Status Classification: inter-observer consistency. American Society of Anesthesiologists. Anaesth Intensive Care 2002; 30:633–640.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2002; 39:542–553.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9:283–288.
In this issue of the Cleveland Clinic Journal of Medicine,1 Dr. Steven L. Cohn provides a succinct review of the recently published guidelines by the American College of Cardiology and American Heart Association (ACC/AHA) on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery.2 Although no drastic changes have been made in these guidelines, several significant modifications have been implemented and are highlighted in his review.
A BREACH OF SCIENTIFIC INTEGRITY
First, I am pleased Dr. Cohn described how the writing committee of the new guidelines handled the well-publicized breaches of scientific integrity by Dr. Don Poldermans, a prolific perioperative-medicine researcher at Erasmus University in the Netherlands who has contributed an abundance of literature that influenced clinical practice. Although some of his key publications were excluded by the ACC/AHA committee in its overall analysis, it remains unclear to me if simply ignoring some of his work is truly possible. For better or for worse, his publications have significantly shaped clinical practice in addition to guiding subsequent research in this field.
ASSESSING RISK
Along with continuing to endorse the Revised Cardiac Risk Index (RCRI),3 the guidelines now include another option for objective preoperative cardiovascular risk assessment. Dr. Cohn nicely outlines the pros and cons of the surgical risk calculator (often referred to as the “Gupta calculator”) derived from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database.4
Although the RCRI is not perfect, I agree with Dr. Cohn that the ACS NSQIP tool has limitations, including a cumbersome calculation (requiring a smartphone application or online calculator), lack of external validation, and use of the American Society of Anesthesiologists Physical Status Classification System, which has been notoriously confusing for generalists and has demonstrated poor inter-rater reliability among anesthesiologists.5,6
Of note, a patient may have very different risk-prediction scores depending on which tool is used. For example, a 66-year-old man with a history of ischemic heart disease, diabetes on insulin therapy, hypertension, and chronic kidney disease with a serum creatinine level greater than 2.0 mg/dL who is scheduled to undergo total hip arthroplasty would have a risk of a perioperative cardiovascular event of about 10% according to the RCRI, but only 1.1% according to the ACS NSQIP calculator. How widely this newer risk-stratification tool will be adopted in clinical practice will be interesting to observe.
In what appears to be an effort to simplify the guidelines, the ACC/AHA now recommends combining the patient’s clinical and surgical risks into estimating an overall perioperative risk for developing major adverse cardiac events. This estimate is now whittled down to only two categories: “low risk” and “elevated risk.” I am concerned that the new guidelines may have become too streamlined and lack the direction to assist providers in making important clinical decisions. Most notably, and as Dr. Cohn appropriately suggests, many patients will be in a gray zone with respect to whether cardiac stress testing should be obtained before surgery.
STRESS TESTING
Significant background knowledge is required to answer the important question in the ACC/AHA algorithm, ie, whether further testing will have an impact on decision-making or perioperative care.2 Dr. Cohn provides some of this information by noting the abysmal positive predictive value of preoperative noninvasive cardiac testing (with studies ranging from 0% to 37%) and by correctly stating that no benefit has been observed with preoperative cardiac revascularization.
If this is not widely known, I share Dr. Cohn’s fear that the new guidelines may stimulate increased ordering of preoperative stress tests. We observed this trend with the highly scripted 2002 ACC/AHA perioperative guidelines7 and subsequently learned that stress testing before surgery very seldom changes patient management.
A preoperative stress test should be reserved for patients with symptoms suggestive of ischemic heart disease. As a diagnostic study, the value of stress testing is excellent. This is not true when it is used as a screening test for asymptomatic patients, where its ability to predict perioperative cardiovascular events is extremely poor. The only other indication for preoperative stress testing is the rare occasion when further risk stratification is desired for exceptionally high-risk patients. In this scenario, test results may influence the decision to proceed with surgery vs seeking nonoperative approaches or palliative care.
MANAGING MEDICATIONS
Dr. Cohn discusses pertinent issues in the perioperative management of patients’ medications, an important component of the preoperative evaluation.
Despite the inconsistent clinical trial results on perioperative beta-blockers, his assessment of their risks and benefits is clinically accurate and practical. Furthermore, I fully agree with Dr. Cohn’s thoughtful approach regarding perioperative statins, despite the limited data available from randomized controlled trials.
With respect to perioperative aspirin use, I have concerns with Dr. Cohn’s statement that it may be reasonable to continue aspirin perioperatively if the risk of potential cardiac events outweighs the risk of bleeding. Given the result of the recently published second Perioperative Ischemic Evaluation (POISE-2) trial8 that showed a significantly higher risk of major perioperative bleeding in patients randomized to low-dose aspirin, it is difficult to advocate continuing aspirin when no cardiovascular protection was found in this very large trial. I agree with Dr. Cohn that this applies only to patients with no history of coronary artery stent placement, as patients with a stent should remain on low-dose aspirin throughout the entire perioperative period.
Controversy also surrounds angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Dr. Cohn agrees with the ACC/AHA guidelines to continue these agents before surgery; however, I favor holding them on the day of surgery. Although the risk of hypotension-induced cardiac events has not been clearly demonstrated, a recent retrospective study involving more than 1,100 patients showed significantly more acute kidney injury (even after adjusting for hypotension) as well as an increased length of hospital stay in the patients exposed to these agents before surgery.9 Given these findings, in addition to the postinduction hypotension (which can be profound) commonly observed by our anesthesiology colleagues, I recommend holding angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the day of surgery, with very few exceptions.
THE SCIENCE AND ART OF MEDICINE
Dr. Cohn acknowledges that we lack scientific data to answer many questions that arise when caring for the perioperative patient and thus we rely on the ACC/AHA guidelines to provide a framework. These scientific knowledge gaps emphasize the importance of the art of medicine in the perioperative arena. Although we may desire “cookbook” guidelines, the significant gaps in the perioperative medicine evidence base reinforce the necessity to provide individual patient-level care in a multidisciplinary environment with our surgery and anesthesiology colleagues. Without the proper balance of science and art in perioperative medicine, we sacrifice our ability to deliver optimal care for this high-risk patient population.
In this issue of the Cleveland Clinic Journal of Medicine,1 Dr. Steven L. Cohn provides a succinct review of the recently published guidelines by the American College of Cardiology and American Heart Association (ACC/AHA) on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery.2 Although no drastic changes have been made in these guidelines, several significant modifications have been implemented and are highlighted in his review.
A BREACH OF SCIENTIFIC INTEGRITY
First, I am pleased Dr. Cohn described how the writing committee of the new guidelines handled the well-publicized breaches of scientific integrity by Dr. Don Poldermans, a prolific perioperative-medicine researcher at Erasmus University in the Netherlands who has contributed an abundance of literature that influenced clinical practice. Although some of his key publications were excluded by the ACC/AHA committee in its overall analysis, it remains unclear to me if simply ignoring some of his work is truly possible. For better or for worse, his publications have significantly shaped clinical practice in addition to guiding subsequent research in this field.
ASSESSING RISK
Along with continuing to endorse the Revised Cardiac Risk Index (RCRI),3 the guidelines now include another option for objective preoperative cardiovascular risk assessment. Dr. Cohn nicely outlines the pros and cons of the surgical risk calculator (often referred to as the “Gupta calculator”) derived from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database.4
Although the RCRI is not perfect, I agree with Dr. Cohn that the ACS NSQIP tool has limitations, including a cumbersome calculation (requiring a smartphone application or online calculator), lack of external validation, and use of the American Society of Anesthesiologists Physical Status Classification System, which has been notoriously confusing for generalists and has demonstrated poor inter-rater reliability among anesthesiologists.5,6
Of note, a patient may have very different risk-prediction scores depending on which tool is used. For example, a 66-year-old man with a history of ischemic heart disease, diabetes on insulin therapy, hypertension, and chronic kidney disease with a serum creatinine level greater than 2.0 mg/dL who is scheduled to undergo total hip arthroplasty would have a risk of a perioperative cardiovascular event of about 10% according to the RCRI, but only 1.1% according to the ACS NSQIP calculator. How widely this newer risk-stratification tool will be adopted in clinical practice will be interesting to observe.
In what appears to be an effort to simplify the guidelines, the ACC/AHA now recommends combining the patient’s clinical and surgical risks into estimating an overall perioperative risk for developing major adverse cardiac events. This estimate is now whittled down to only two categories: “low risk” and “elevated risk.” I am concerned that the new guidelines may have become too streamlined and lack the direction to assist providers in making important clinical decisions. Most notably, and as Dr. Cohn appropriately suggests, many patients will be in a gray zone with respect to whether cardiac stress testing should be obtained before surgery.
STRESS TESTING
Significant background knowledge is required to answer the important question in the ACC/AHA algorithm, ie, whether further testing will have an impact on decision-making or perioperative care.2 Dr. Cohn provides some of this information by noting the abysmal positive predictive value of preoperative noninvasive cardiac testing (with studies ranging from 0% to 37%) and by correctly stating that no benefit has been observed with preoperative cardiac revascularization.
If this is not widely known, I share Dr. Cohn’s fear that the new guidelines may stimulate increased ordering of preoperative stress tests. We observed this trend with the highly scripted 2002 ACC/AHA perioperative guidelines7 and subsequently learned that stress testing before surgery very seldom changes patient management.
A preoperative stress test should be reserved for patients with symptoms suggestive of ischemic heart disease. As a diagnostic study, the value of stress testing is excellent. This is not true when it is used as a screening test for asymptomatic patients, where its ability to predict perioperative cardiovascular events is extremely poor. The only other indication for preoperative stress testing is the rare occasion when further risk stratification is desired for exceptionally high-risk patients. In this scenario, test results may influence the decision to proceed with surgery vs seeking nonoperative approaches or palliative care.
MANAGING MEDICATIONS
Dr. Cohn discusses pertinent issues in the perioperative management of patients’ medications, an important component of the preoperative evaluation.
Despite the inconsistent clinical trial results on perioperative beta-blockers, his assessment of their risks and benefits is clinically accurate and practical. Furthermore, I fully agree with Dr. Cohn’s thoughtful approach regarding perioperative statins, despite the limited data available from randomized controlled trials.
With respect to perioperative aspirin use, I have concerns with Dr. Cohn’s statement that it may be reasonable to continue aspirin perioperatively if the risk of potential cardiac events outweighs the risk of bleeding. Given the result of the recently published second Perioperative Ischemic Evaluation (POISE-2) trial8 that showed a significantly higher risk of major perioperative bleeding in patients randomized to low-dose aspirin, it is difficult to advocate continuing aspirin when no cardiovascular protection was found in this very large trial. I agree with Dr. Cohn that this applies only to patients with no history of coronary artery stent placement, as patients with a stent should remain on low-dose aspirin throughout the entire perioperative period.
Controversy also surrounds angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Dr. Cohn agrees with the ACC/AHA guidelines to continue these agents before surgery; however, I favor holding them on the day of surgery. Although the risk of hypotension-induced cardiac events has not been clearly demonstrated, a recent retrospective study involving more than 1,100 patients showed significantly more acute kidney injury (even after adjusting for hypotension) as well as an increased length of hospital stay in the patients exposed to these agents before surgery.9 Given these findings, in addition to the postinduction hypotension (which can be profound) commonly observed by our anesthesiology colleagues, I recommend holding angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the day of surgery, with very few exceptions.
THE SCIENCE AND ART OF MEDICINE
Dr. Cohn acknowledges that we lack scientific data to answer many questions that arise when caring for the perioperative patient and thus we rely on the ACC/AHA guidelines to provide a framework. These scientific knowledge gaps emphasize the importance of the art of medicine in the perioperative arena. Although we may desire “cookbook” guidelines, the significant gaps in the perioperative medicine evidence base reinforce the necessity to provide individual patient-level care in a multidisciplinary environment with our surgery and anesthesiology colleagues. Without the proper balance of science and art in perioperative medicine, we sacrifice our ability to deliver optimal care for this high-risk patient population.
- Cohn SL. Updated guidelines on cardiovascular evaluation before noncardiac surgery: a view from the trenches. Cleve Clin J Med 2014; 81:742–751.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Aronson WL, McAuliffe MS, Miller K. Variability in the American Society of Anesthesiologists Physical Status Classification Scale. AANA J 2003; 71:265–274.
- Mak PH, Campbell RC, Irwin MG; American Society of Anesthesiologists. The ASA Physical Status Classification: inter-observer consistency. American Society of Anesthesiologists. Anaesth Intensive Care 2002; 30:633–640.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2002; 39:542–553.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9:283–288.
- Cohn SL. Updated guidelines on cardiovascular evaluation before noncardiac surgery: a view from the trenches. Cleve Clin J Med 2014; 81:742–751.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; Jul 29. pii: S0735-1097(14)05536-3. doi: 10.1016/j.jacc.2014.07.944. [Epub ahead of print].
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Aronson WL, McAuliffe MS, Miller K. Variability in the American Society of Anesthesiologists Physical Status Classification Scale. AANA J 2003; 71:265–274.
- Mak PH, Campbell RC, Irwin MG; American Society of Anesthesiologists. The ASA Physical Status Classification: inter-observer consistency. American Society of Anesthesiologists. Anaesth Intensive Care 2002; 30:633–640.
- Eagle KA, Berger PB, Calkins H, et al; American College of Cardiology; American Heart Association. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2002; 39:542–553.
- Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370:1494–1503.
- Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med 2014; 9:283–288.