Article

Sustentaculum Lunatum: Appreciation of the Palmar Lunate Facet in Management of Complex Intra-Articular Fractures of the Distal Radius
- Author:
- Paryavi E
- Christian MW
- Eglseder WA
- Pensy RA
Fracture of the distal radius is the most common wrist injury. Treatment of complex intra-articular fractures of the distal radius requires an...
Article
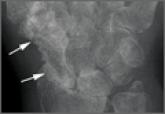
Septic Arthritis and Osteomyelitis Caused by Pasteurella multocida
- Author:
- Vranis N
- Paryavi E
- Christian M
- Joshi M
- Pensy RA
This report presents a case of progressive septic arthritis and osteomyelitis caused by a rare pathogen, Pasteurella multocida, thought to be...
