User login
Evidence-based tools for premenstrual disorders
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
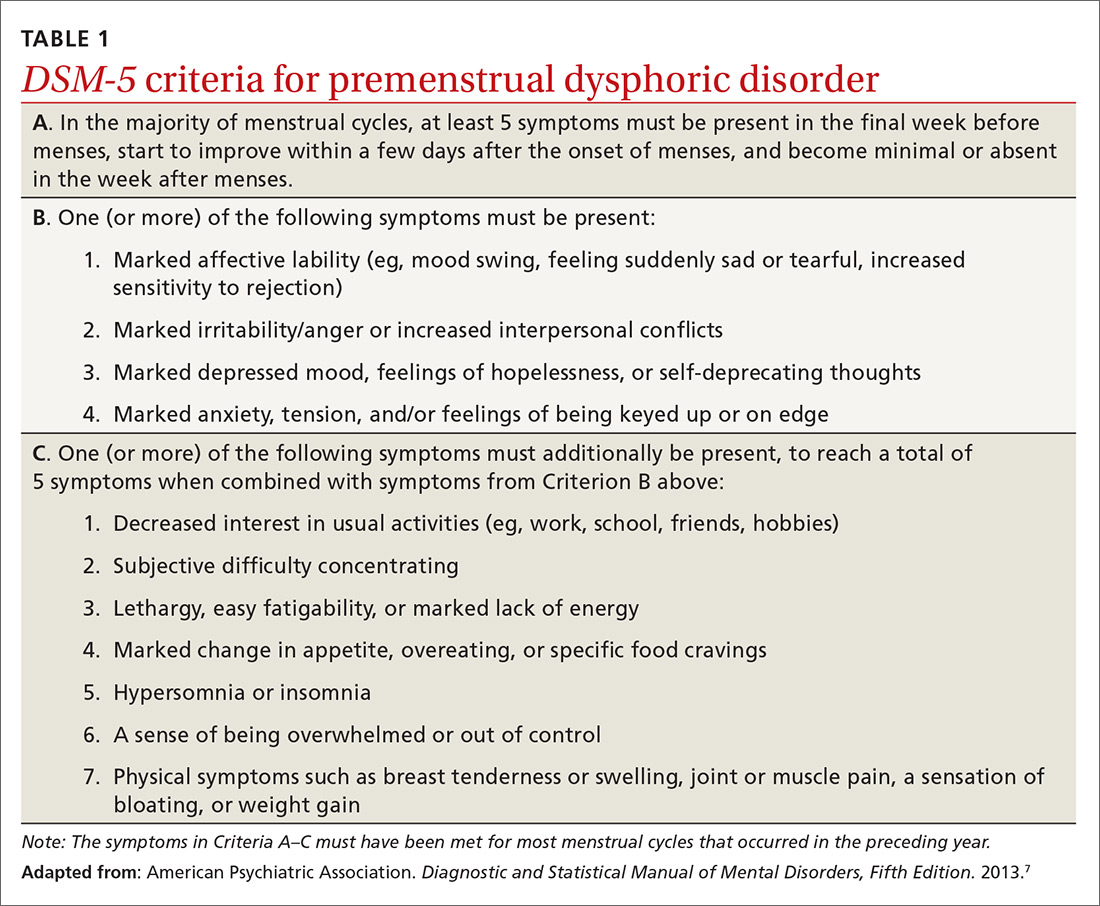
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7
Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
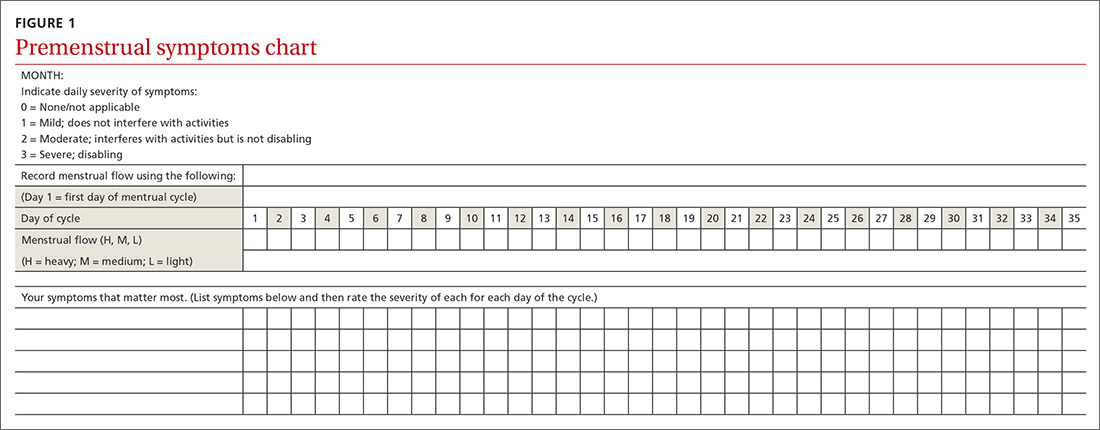
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.
Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
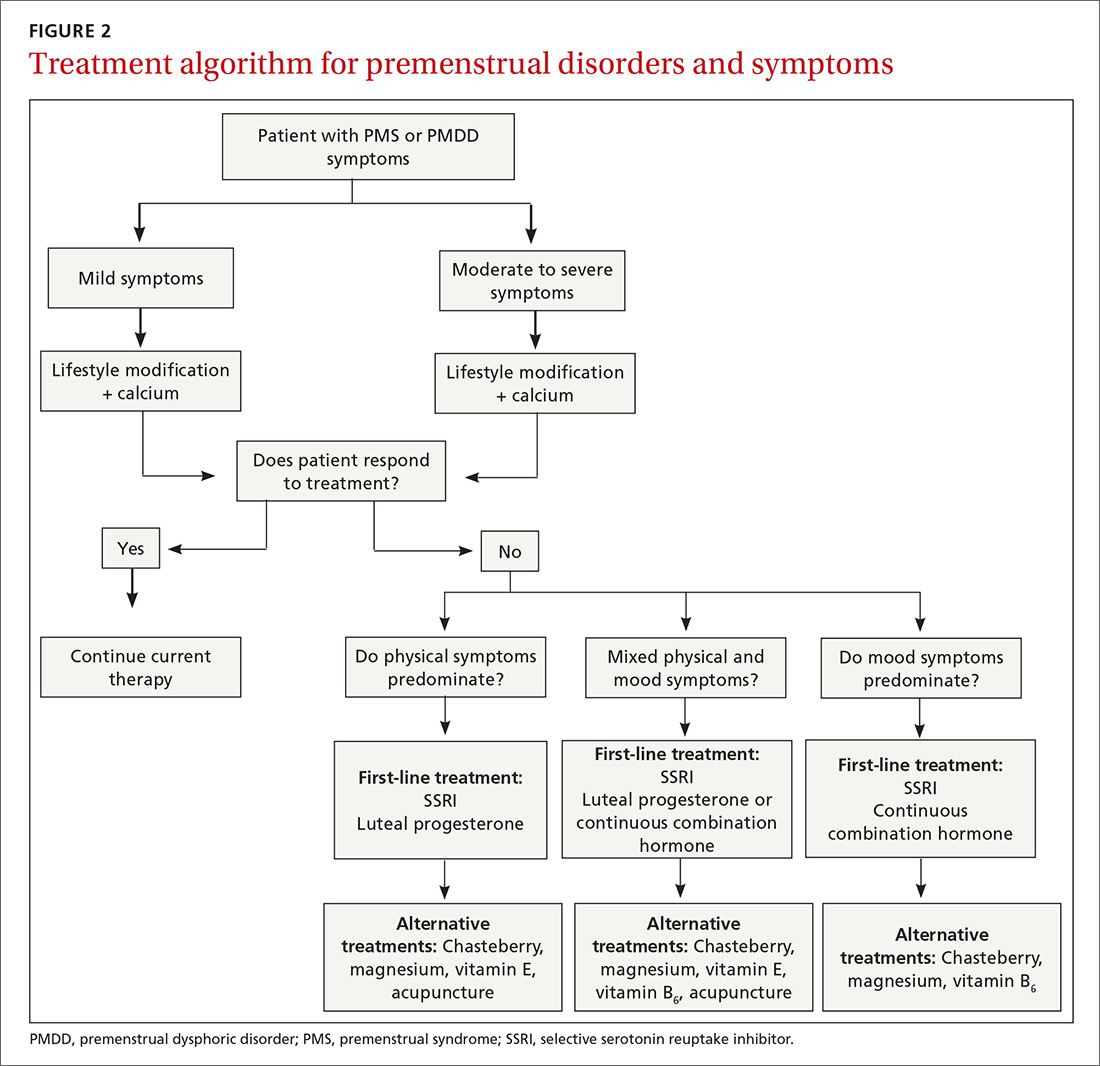
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness
Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
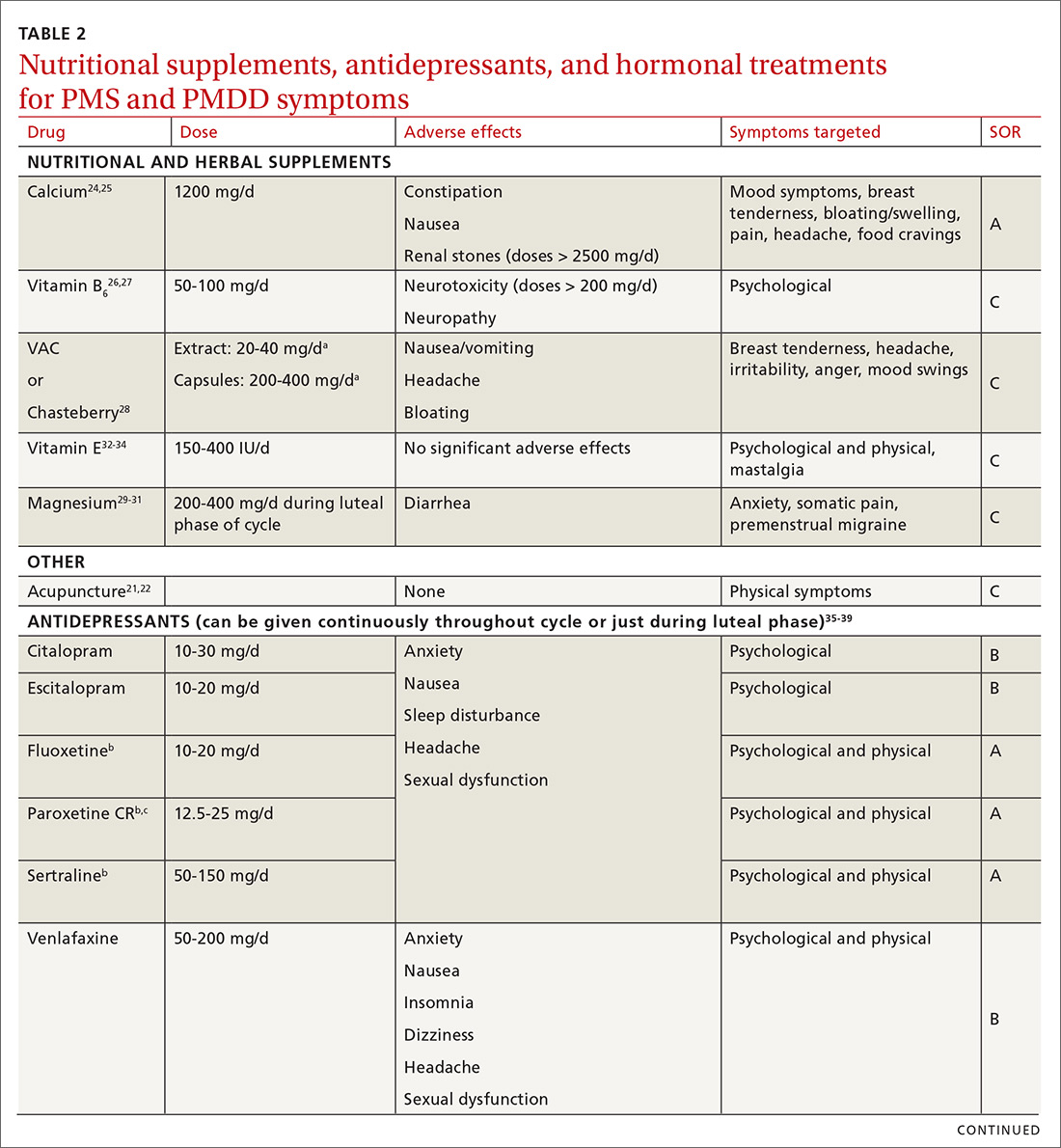
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25
Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27
Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
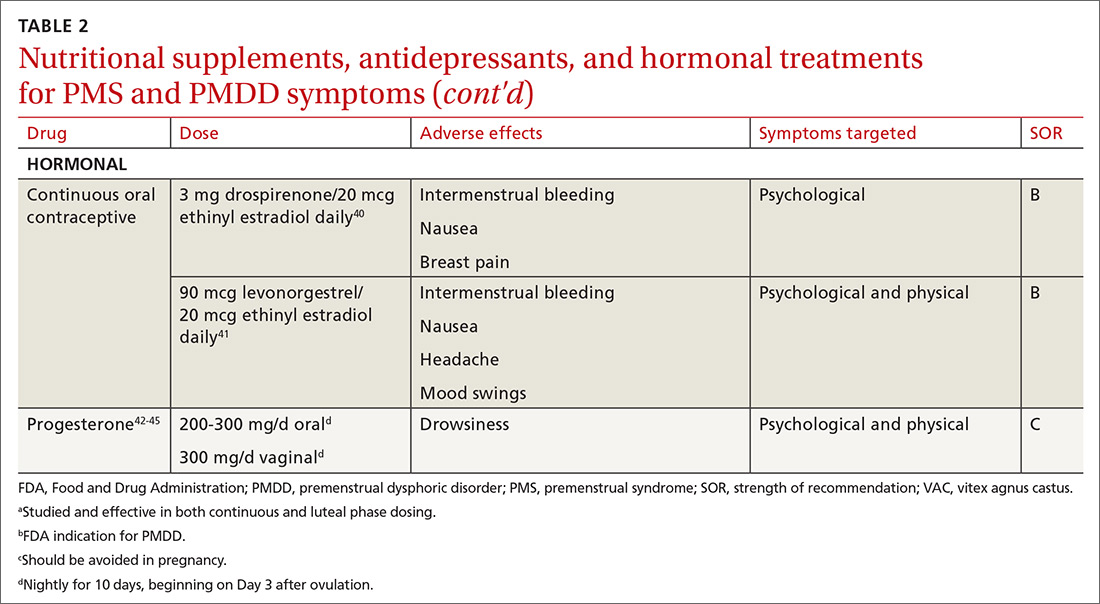
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; Peter.Danis@mercy.net.
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7
Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.
Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness
Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25
Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27
Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; Peter.Danis@mercy.net.
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7
Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.
Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness
Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25
Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27
Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; Peter.Danis@mercy.net.
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
PRACTICE RECOMMENDATIONS
› Start calcium supplementation in all patients who report significant premenstrual symptoms. A
› Add a selective serotonin reuptake inhibitor (SSRI) to calcium supplementationfor patients who have more severe premenstrual psychological symptoms. A
› Consider hormonal treatment options for patients who require treatment beyond calcium and an SSRI. B
› Provide nutrition and exercise information to all patients who report significant premenstrual symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Which patients with gastroesophageal reflux disease (GERD) should have esophagogastroduoudenoscopy (EGD)?
No evidence was identified that provides a basis for determining whether EGD leads to improved outcomes in patients with GERD. However, patients with GERD referred for elective EGD who were found to have Barrett’s esophagus were more likely to have symptoms for more than 1 year than patients who did not have Barrett’s esophagus. Patients with esophageal adenocarcinoma were more likely to have frequent, severe, or longer duration of GERD symptoms. The calculated odds ratios (OR) for esophageal adenocarcinoma increased with increasing frequency, severity, or duration of GERD symptoms, independently or in combination.
Evidence summary
A total of 701 patients referred for EGD by gastroenterologists for GERD symptoms yielded 77 cases of Barrett’s esophagus. Compared with patients without Barrett’s esophagus, patients with the condition were more likely to have symptoms greater than 1 year: 1 to 5 years (OR=3.0 [95% confidence interval (CI), 1.2-8.0]), 5 to 10 years [OR=5.1 (95% CI, 1.2-14.7)], more than 10 years [OR=6.4 (95% CI, 2.4-17.10)].1
A case control study conducted at Duke University compared 79 patients with Barrett’s esophagus with 2 control groups: patients undergoing endoscopy for GERD symptoms and patients undergoing endoscopy for other indications. Patients with Barrett’s esophagus developed symptoms at an earlier age (mean age = 35.3 years ± 16 years compared with 43.7 years ± 13 years and 42.7 years ± 13 years, respectively) and had a longer duration of symptoms (mean duration = 16.36 years [range = 1-63 years] compared with 11.81 years [range = 1-55 years] and 13.03 years [range = 1-53 years]) than patients without Barrett’s esophagus.
A Swedish case-controlled study compared 189 cases of esophageal adenocarcinoma and 167 cases of squamous cell carcinoma with 820 control subjects recruited from the general population. Patients with esophageal adenocarcinoma were 7.7 times more likely to report symptoms of GERD at least once weekly 5 years before their diagnosis with adenocarcinoma. Other ORs are as follows: symptoms once per week (5.1), symptoms more than 3 times per week (16.7), nocturnal symptoms at least once per week (10.8), and duration more than 20 years (16.4).3 Also, obesity was a risk factor for esophageal adenocarcinoma in this population. Patients with a body mass index (BMI) greater than 30 had increased risk of adenocarcinoma compared with patients with a BMI less than 25 (OR=16.2; 95% CI, 6.3-41.4).4 These authors calculated numbers of endoscopies needed to detect 1 case of esophageal adenocarcinoma. Among Swedish men aged 50 to 79 years with symptoms at least 1 time per week and BMI greater than 30, 594 endoscopies (95% CI, 385-972) are needed to find 1 case of esophageal adenocarcinoma.5 These authors estimate incidence of adenocarcinoma using population-wide surveillance data.
There is no current evidence that evaluation of GERD patients with EGD improves outcomes, although 2 retrospective studies have shown improved outcomes for patients with Barrett’s esophagus undergoing surveillance endoscopy compared with those without surveillance.6,7
Recommendations from others
The American College of Gastroenterology recommends EGD for GERD patients who do not respond to therapy, experience alarm symptoms, who have chronic symptoms and are at risk for Barrett’s esophagus, and need continuous chronic therapy.8
The Alberta (Canada) clinical practice guideline recommends EGD for GERD patients with alarm symptoms or failure to respond to 4 to 8 weeks of therapy with a proton pump inhibitor.9
Heidi Chumley, MD
University of Texas Health Science Center at San Antonio
Indigestion is a very common gastrointestinal symptom. The majority of the time, it is related to GERD. For young to middle-aged patients who appear to be healthy, lifestyle modifications and antacids or H2-blockers will usually provide relief of their symptoms. A lack of response to these interventions or the development of other gastrointestinal symptoms may imply a more significant disease process or a different diagnosis. In addition to the alarm symptoms provided Table 1, I also become more concerned if patients have tobacco or alcohol problems, or require chronic proton pump inhibitor therapy (>12 weeks) to control their symptoms. Upper endoscopy is a useful tool to confirm a diagnosis in this small subset of patients.
1. Lieberman DA. Risk factors for Barrett’s esophagus in a community based practice: GORGE consortium (Gastroenterology outcomes research group in endoscopy). Am J Gastroenterol 1997;92:1293-97.
2. Eisen GM, Sandler RS, Murray S, Gottfried M. The relationship between gastroesophageal reflux and its complications with Barrett’s esophagus. Am J Gastroenterol 1997;92:1-3.
3. Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. New Engl J Med 1999;340:825-31.
4. Lagergren J, Bergstrom R, Nyren O. Association between body mass index and adenocarcinoma of the esophagus and gastria. Ann Intern Med 1999;130:883-90
5. Lagergren J, Ye W, Bergstrom R, Nyren O. Utility of endoscopic screening for upper gastrointestinal adenocarcinoma. JAMA 2000;284:961-2.
6. Streitz JM, Andrews CW, Ellis FH. Endoscopic surveillance of Barrett’s esophagus: does it help? J Thoracic Cardiac Surg 1993;105:383-8.
7. Van Sandick JW, van Lanschot JJ, Kuiken BW, Tytgat GN, Offerhaus GJ, Obertop H. Impact of endoscopic biopsy surveillance of Barrett’s esophagus on pathological stage and clinical outcome of Barrett’s carcinoma. Gut 1998;43:216-22.
8. DeVault KR. Updated treatment guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The practice parameters committee of the American College of Gastroenterology. Am J Gastroenter 1999;94:1434-42.
9. Alberta Clinical Practice Guidelines: Guidelines for treatment of gastroesophageal reflux in adults. Canadian Consensus Conference for the management of patients with gastroesophageal reflux disease. www.amda.ab.ca/cpg/catalogue/documents/gastroenterology/gerd/guideline.html
No evidence was identified that provides a basis for determining whether EGD leads to improved outcomes in patients with GERD. However, patients with GERD referred for elective EGD who were found to have Barrett’s esophagus were more likely to have symptoms for more than 1 year than patients who did not have Barrett’s esophagus. Patients with esophageal adenocarcinoma were more likely to have frequent, severe, or longer duration of GERD symptoms. The calculated odds ratios (OR) for esophageal adenocarcinoma increased with increasing frequency, severity, or duration of GERD symptoms, independently or in combination.
Evidence summary
A total of 701 patients referred for EGD by gastroenterologists for GERD symptoms yielded 77 cases of Barrett’s esophagus. Compared with patients without Barrett’s esophagus, patients with the condition were more likely to have symptoms greater than 1 year: 1 to 5 years (OR=3.0 [95% confidence interval (CI), 1.2-8.0]), 5 to 10 years [OR=5.1 (95% CI, 1.2-14.7)], more than 10 years [OR=6.4 (95% CI, 2.4-17.10)].1
A case control study conducted at Duke University compared 79 patients with Barrett’s esophagus with 2 control groups: patients undergoing endoscopy for GERD symptoms and patients undergoing endoscopy for other indications. Patients with Barrett’s esophagus developed symptoms at an earlier age (mean age = 35.3 years ± 16 years compared with 43.7 years ± 13 years and 42.7 years ± 13 years, respectively) and had a longer duration of symptoms (mean duration = 16.36 years [range = 1-63 years] compared with 11.81 years [range = 1-55 years] and 13.03 years [range = 1-53 years]) than patients without Barrett’s esophagus.
A Swedish case-controlled study compared 189 cases of esophageal adenocarcinoma and 167 cases of squamous cell carcinoma with 820 control subjects recruited from the general population. Patients with esophageal adenocarcinoma were 7.7 times more likely to report symptoms of GERD at least once weekly 5 years before their diagnosis with adenocarcinoma. Other ORs are as follows: symptoms once per week (5.1), symptoms more than 3 times per week (16.7), nocturnal symptoms at least once per week (10.8), and duration more than 20 years (16.4).3 Also, obesity was a risk factor for esophageal adenocarcinoma in this population. Patients with a body mass index (BMI) greater than 30 had increased risk of adenocarcinoma compared with patients with a BMI less than 25 (OR=16.2; 95% CI, 6.3-41.4).4 These authors calculated numbers of endoscopies needed to detect 1 case of esophageal adenocarcinoma. Among Swedish men aged 50 to 79 years with symptoms at least 1 time per week and BMI greater than 30, 594 endoscopies (95% CI, 385-972) are needed to find 1 case of esophageal adenocarcinoma.5 These authors estimate incidence of adenocarcinoma using population-wide surveillance data.
There is no current evidence that evaluation of GERD patients with EGD improves outcomes, although 2 retrospective studies have shown improved outcomes for patients with Barrett’s esophagus undergoing surveillance endoscopy compared with those without surveillance.6,7
Recommendations from others
The American College of Gastroenterology recommends EGD for GERD patients who do not respond to therapy, experience alarm symptoms, who have chronic symptoms and are at risk for Barrett’s esophagus, and need continuous chronic therapy.8
The Alberta (Canada) clinical practice guideline recommends EGD for GERD patients with alarm symptoms or failure to respond to 4 to 8 weeks of therapy with a proton pump inhibitor.9
Heidi Chumley, MD
University of Texas Health Science Center at San Antonio
Indigestion is a very common gastrointestinal symptom. The majority of the time, it is related to GERD. For young to middle-aged patients who appear to be healthy, lifestyle modifications and antacids or H2-blockers will usually provide relief of their symptoms. A lack of response to these interventions or the development of other gastrointestinal symptoms may imply a more significant disease process or a different diagnosis. In addition to the alarm symptoms provided Table 1, I also become more concerned if patients have tobacco or alcohol problems, or require chronic proton pump inhibitor therapy (>12 weeks) to control their symptoms. Upper endoscopy is a useful tool to confirm a diagnosis in this small subset of patients.
No evidence was identified that provides a basis for determining whether EGD leads to improved outcomes in patients with GERD. However, patients with GERD referred for elective EGD who were found to have Barrett’s esophagus were more likely to have symptoms for more than 1 year than patients who did not have Barrett’s esophagus. Patients with esophageal adenocarcinoma were more likely to have frequent, severe, or longer duration of GERD symptoms. The calculated odds ratios (OR) for esophageal adenocarcinoma increased with increasing frequency, severity, or duration of GERD symptoms, independently or in combination.
Evidence summary
A total of 701 patients referred for EGD by gastroenterologists for GERD symptoms yielded 77 cases of Barrett’s esophagus. Compared with patients without Barrett’s esophagus, patients with the condition were more likely to have symptoms greater than 1 year: 1 to 5 years (OR=3.0 [95% confidence interval (CI), 1.2-8.0]), 5 to 10 years [OR=5.1 (95% CI, 1.2-14.7)], more than 10 years [OR=6.4 (95% CI, 2.4-17.10)].1
A case control study conducted at Duke University compared 79 patients with Barrett’s esophagus with 2 control groups: patients undergoing endoscopy for GERD symptoms and patients undergoing endoscopy for other indications. Patients with Barrett’s esophagus developed symptoms at an earlier age (mean age = 35.3 years ± 16 years compared with 43.7 years ± 13 years and 42.7 years ± 13 years, respectively) and had a longer duration of symptoms (mean duration = 16.36 years [range = 1-63 years] compared with 11.81 years [range = 1-55 years] and 13.03 years [range = 1-53 years]) than patients without Barrett’s esophagus.
A Swedish case-controlled study compared 189 cases of esophageal adenocarcinoma and 167 cases of squamous cell carcinoma with 820 control subjects recruited from the general population. Patients with esophageal adenocarcinoma were 7.7 times more likely to report symptoms of GERD at least once weekly 5 years before their diagnosis with adenocarcinoma. Other ORs are as follows: symptoms once per week (5.1), symptoms more than 3 times per week (16.7), nocturnal symptoms at least once per week (10.8), and duration more than 20 years (16.4).3 Also, obesity was a risk factor for esophageal adenocarcinoma in this population. Patients with a body mass index (BMI) greater than 30 had increased risk of adenocarcinoma compared with patients with a BMI less than 25 (OR=16.2; 95% CI, 6.3-41.4).4 These authors calculated numbers of endoscopies needed to detect 1 case of esophageal adenocarcinoma. Among Swedish men aged 50 to 79 years with symptoms at least 1 time per week and BMI greater than 30, 594 endoscopies (95% CI, 385-972) are needed to find 1 case of esophageal adenocarcinoma.5 These authors estimate incidence of adenocarcinoma using population-wide surveillance data.
There is no current evidence that evaluation of GERD patients with EGD improves outcomes, although 2 retrospective studies have shown improved outcomes for patients with Barrett’s esophagus undergoing surveillance endoscopy compared with those without surveillance.6,7
Recommendations from others
The American College of Gastroenterology recommends EGD for GERD patients who do not respond to therapy, experience alarm symptoms, who have chronic symptoms and are at risk for Barrett’s esophagus, and need continuous chronic therapy.8
The Alberta (Canada) clinical practice guideline recommends EGD for GERD patients with alarm symptoms or failure to respond to 4 to 8 weeks of therapy with a proton pump inhibitor.9
Heidi Chumley, MD
University of Texas Health Science Center at San Antonio
Indigestion is a very common gastrointestinal symptom. The majority of the time, it is related to GERD. For young to middle-aged patients who appear to be healthy, lifestyle modifications and antacids or H2-blockers will usually provide relief of their symptoms. A lack of response to these interventions or the development of other gastrointestinal symptoms may imply a more significant disease process or a different diagnosis. In addition to the alarm symptoms provided Table 1, I also become more concerned if patients have tobacco or alcohol problems, or require chronic proton pump inhibitor therapy (>12 weeks) to control their symptoms. Upper endoscopy is a useful tool to confirm a diagnosis in this small subset of patients.
1. Lieberman DA. Risk factors for Barrett’s esophagus in a community based practice: GORGE consortium (Gastroenterology outcomes research group in endoscopy). Am J Gastroenterol 1997;92:1293-97.
2. Eisen GM, Sandler RS, Murray S, Gottfried M. The relationship between gastroesophageal reflux and its complications with Barrett’s esophagus. Am J Gastroenterol 1997;92:1-3.
3. Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. New Engl J Med 1999;340:825-31.
4. Lagergren J, Bergstrom R, Nyren O. Association between body mass index and adenocarcinoma of the esophagus and gastria. Ann Intern Med 1999;130:883-90
5. Lagergren J, Ye W, Bergstrom R, Nyren O. Utility of endoscopic screening for upper gastrointestinal adenocarcinoma. JAMA 2000;284:961-2.
6. Streitz JM, Andrews CW, Ellis FH. Endoscopic surveillance of Barrett’s esophagus: does it help? J Thoracic Cardiac Surg 1993;105:383-8.
7. Van Sandick JW, van Lanschot JJ, Kuiken BW, Tytgat GN, Offerhaus GJ, Obertop H. Impact of endoscopic biopsy surveillance of Barrett’s esophagus on pathological stage and clinical outcome of Barrett’s carcinoma. Gut 1998;43:216-22.
8. DeVault KR. Updated treatment guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The practice parameters committee of the American College of Gastroenterology. Am J Gastroenter 1999;94:1434-42.
9. Alberta Clinical Practice Guidelines: Guidelines for treatment of gastroesophageal reflux in adults. Canadian Consensus Conference for the management of patients with gastroesophageal reflux disease. www.amda.ab.ca/cpg/catalogue/documents/gastroenterology/gerd/guideline.html
1. Lieberman DA. Risk factors for Barrett’s esophagus in a community based practice: GORGE consortium (Gastroenterology outcomes research group in endoscopy). Am J Gastroenterol 1997;92:1293-97.
2. Eisen GM, Sandler RS, Murray S, Gottfried M. The relationship between gastroesophageal reflux and its complications with Barrett’s esophagus. Am J Gastroenterol 1997;92:1-3.
3. Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. New Engl J Med 1999;340:825-31.
4. Lagergren J, Bergstrom R, Nyren O. Association between body mass index and adenocarcinoma of the esophagus and gastria. Ann Intern Med 1999;130:883-90
5. Lagergren J, Ye W, Bergstrom R, Nyren O. Utility of endoscopic screening for upper gastrointestinal adenocarcinoma. JAMA 2000;284:961-2.
6. Streitz JM, Andrews CW, Ellis FH. Endoscopic surveillance of Barrett’s esophagus: does it help? J Thoracic Cardiac Surg 1993;105:383-8.
7. Van Sandick JW, van Lanschot JJ, Kuiken BW, Tytgat GN, Offerhaus GJ, Obertop H. Impact of endoscopic biopsy surveillance of Barrett’s esophagus on pathological stage and clinical outcome of Barrett’s carcinoma. Gut 1998;43:216-22.
8. DeVault KR. Updated treatment guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The practice parameters committee of the American College of Gastroenterology. Am J Gastroenter 1999;94:1434-42.
9. Alberta Clinical Practice Guidelines: Guidelines for treatment of gastroesophageal reflux in adults. Canadian Consensus Conference for the management of patients with gastroesophageal reflux disease. www.amda.ab.ca/cpg/catalogue/documents/gastroenterology/gerd/guideline.html
Evidence-based answers from the Family Physicians Inquiries Network