User login
Unusual Cardiac Rhythm Device Infection
A 35‐year‐old woman with a history of hypertrophic cardiomyopathy survived a ventricular fibrillation cardiac arrest. She subsequently underwent placement of a single, transvenous right ventricular lead implantable cardioverter defibrillator (ICD) system. The lead was an active fixation model, and the generator was placed in a left infraclavicular subcutaneous pocket.
Six months later, she presented with a 5‐week illness consisting of productive cough, fever, anorexia, and myalgias. Physical exam was notable for a rapid heart rate with a variable S1. Labs were notable for a leukocytosis with predominance of neutrophils. An ECG demonstrated atrial fibrillation.
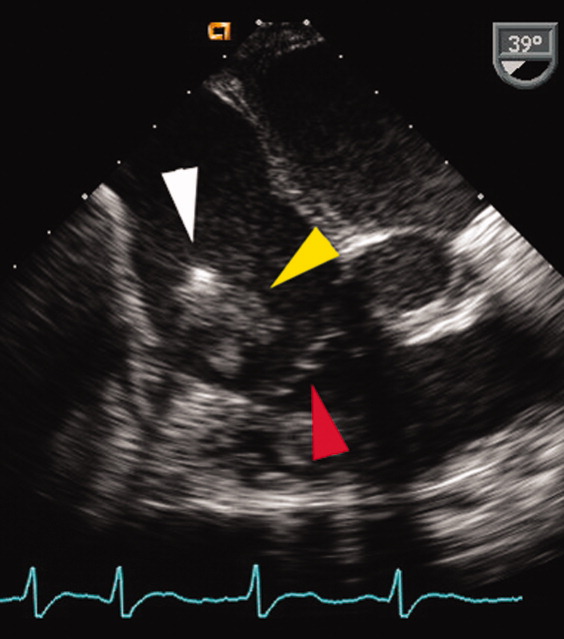
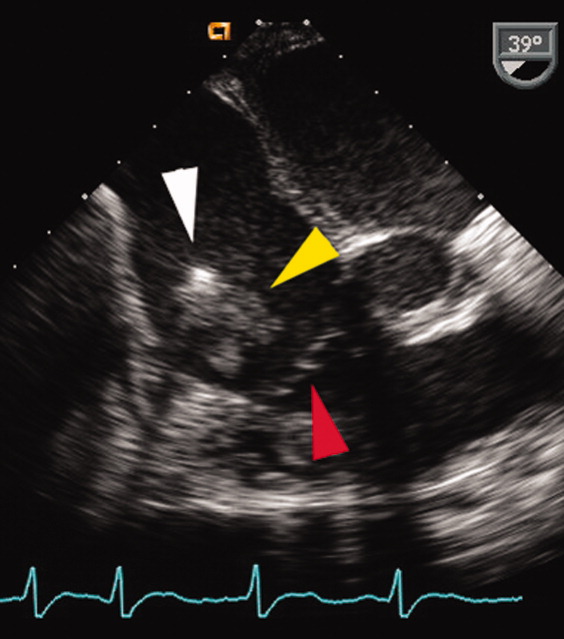
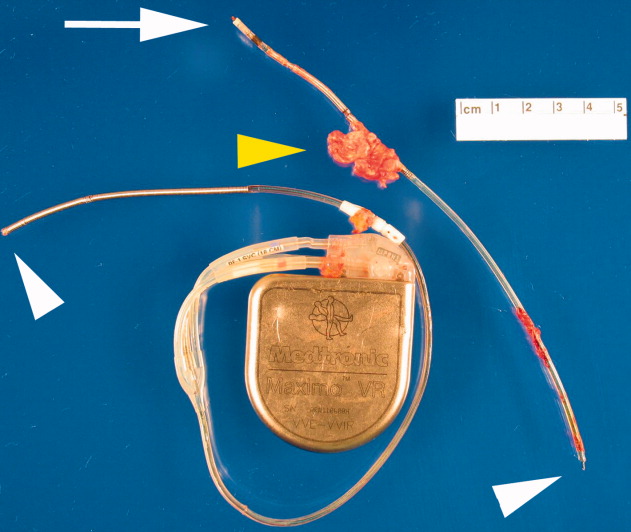
A transesophageal echocardiogram (TEE) was performed in anticipation of a cardioversion for atrial fibrillation. The TEE demonstrated a mass in the right atrium that was attached to the ICD lead, with possible involvement of the tricuspid valve leaflets (Fig. 1). The mass, characterized as multiple confluent bulky segments, was freely mobile and measured about 1.8 cm at its greatest dimension. Therefore, cardioversion was not performed. Within 72 hours, multiple aerobic BACTEC blood cultures identified Haemophilus parainfluenzae, beta lactamase negative. The patient underwent a median sternotomy to remove the ICD lead and generator (Fig. 2). The septal leaflet of the tricuspid valve was debrided. The patient was treated with a prolonged course of ceftriaxone without clinical or microbiologic signs of persistent infection.
DISCUSSION
Research has demonstrated that the rise in cardiac device infections is greater than the rise in the rate of implantation of these devices over the same time period.1 Most infections with cardiac rhythm devices are primary infections, which begin at the pocket and frequently present around generator placement or exchange.2, 3 Because the intravascular leads are continuous to the pocket, there remains a risk for lead and systemic infection.
This case illustrates 2 important concepts. Secondary device infections, which usually result from bacteria originating at a site other than the generator pocket, are less common and tend to involve the intravascular lead.2, 4 Seeding of the intravascular lead frequently occurs with either Staphylococcus aureus or coagulase‐negative Staphylococci.2, 4 Therefore, H. parainfluenzae, a gram‐negative bacillus that can be part of the normal flora of the upper respiratory tract, is not a commonly encountered pathogen for secondary lead infections. Given the respiratory tract symptoms, this was likely the source in this patient. When the lead, generator, or both are infected, this necessitates removal of the entire system.
Furthermore, H. parainfluenzae is categorized with the HACEK organisms (Haemophilus species including H. aphrophilus, H. parainfluenzae, and H. paraphrophilus; Actinobacillus actinomycetemcomitans; Cardiobacterium hominis; Eikenella corrodens; Kingella kingae), a group of fastidious gram‐negative bacilli historically thought to be a common cause of culture‐negative endocarditis. Recent retrospective studies suggest that a prolonged incubation for HACEK organisms is generally not necessary because of advances in culture media and automated blood culture systems.5, 6 As shown in this case, the organism was cultured in less than 72 hours. Therefore, HACEK organisms, when used with modern culture media in addition to automated blood culture systems, are unlikely to be causes of true culture‐negative device or valve infection, provided the patient has had no recent exposure to antibiotics and adequate blood cultures have been obtained. If a cardiac device infection is suspected, blood cultures obtained before commencement of antibiotics and adequate sampling of blood for culture are more likely to identify the pathogen than are blood cultures from prolonged incubation.
- ,,, et al.Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999.Am Heart J.2004;147:582–586.
- ,.Infections of intracardiac devices. Cardiol Clin. 2003; 21: 253–271
- ,,,,,.Diagnosis and management of infections involving implantable electrophysiologic cardiac devices.Ann Intern Med.2000;133:604–608.
- ,,, et al.Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter‐defibrillators.Circulation.2001;104:1029–1033.
- ,,, et al.Utility of extended blood culture incubation for isolation of Haemophilus, Actinobacillus, Cardiobacterium, Eikenella and Kingella organisms: a retrospective multicenter evaluation.J Clin Microbiol.2006;44(1):257–259.
- ,,.Prolonged incubation and extensive subculturing do not increase recovery of clinically significant microorganisms from standard automated blood cultures.Clin Infect Dis.2005;41:1677–1680.
A 35‐year‐old woman with a history of hypertrophic cardiomyopathy survived a ventricular fibrillation cardiac arrest. She subsequently underwent placement of a single, transvenous right ventricular lead implantable cardioverter defibrillator (ICD) system. The lead was an active fixation model, and the generator was placed in a left infraclavicular subcutaneous pocket.
Six months later, she presented with a 5‐week illness consisting of productive cough, fever, anorexia, and myalgias. Physical exam was notable for a rapid heart rate with a variable S1. Labs were notable for a leukocytosis with predominance of neutrophils. An ECG demonstrated atrial fibrillation.
A transesophageal echocardiogram (TEE) was performed in anticipation of a cardioversion for atrial fibrillation. The TEE demonstrated a mass in the right atrium that was attached to the ICD lead, with possible involvement of the tricuspid valve leaflets (Fig. 1). The mass, characterized as multiple confluent bulky segments, was freely mobile and measured about 1.8 cm at its greatest dimension. Therefore, cardioversion was not performed. Within 72 hours, multiple aerobic BACTEC blood cultures identified Haemophilus parainfluenzae, beta lactamase negative. The patient underwent a median sternotomy to remove the ICD lead and generator (Fig. 2). The septal leaflet of the tricuspid valve was debrided. The patient was treated with a prolonged course of ceftriaxone without clinical or microbiologic signs of persistent infection.


DISCUSSION
Research has demonstrated that the rise in cardiac device infections is greater than the rise in the rate of implantation of these devices over the same time period.1 Most infections with cardiac rhythm devices are primary infections, which begin at the pocket and frequently present around generator placement or exchange.2, 3 Because the intravascular leads are continuous to the pocket, there remains a risk for lead and systemic infection.
This case illustrates 2 important concepts. Secondary device infections, which usually result from bacteria originating at a site other than the generator pocket, are less common and tend to involve the intravascular lead.2, 4 Seeding of the intravascular lead frequently occurs with either Staphylococcus aureus or coagulase‐negative Staphylococci.2, 4 Therefore, H. parainfluenzae, a gram‐negative bacillus that can be part of the normal flora of the upper respiratory tract, is not a commonly encountered pathogen for secondary lead infections. Given the respiratory tract symptoms, this was likely the source in this patient. When the lead, generator, or both are infected, this necessitates removal of the entire system.
Furthermore, H. parainfluenzae is categorized with the HACEK organisms (Haemophilus species including H. aphrophilus, H. parainfluenzae, and H. paraphrophilus; Actinobacillus actinomycetemcomitans; Cardiobacterium hominis; Eikenella corrodens; Kingella kingae), a group of fastidious gram‐negative bacilli historically thought to be a common cause of culture‐negative endocarditis. Recent retrospective studies suggest that a prolonged incubation for HACEK organisms is generally not necessary because of advances in culture media and automated blood culture systems.5, 6 As shown in this case, the organism was cultured in less than 72 hours. Therefore, HACEK organisms, when used with modern culture media in addition to automated blood culture systems, are unlikely to be causes of true culture‐negative device or valve infection, provided the patient has had no recent exposure to antibiotics and adequate blood cultures have been obtained. If a cardiac device infection is suspected, blood cultures obtained before commencement of antibiotics and adequate sampling of blood for culture are more likely to identify the pathogen than are blood cultures from prolonged incubation.
A 35‐year‐old woman with a history of hypertrophic cardiomyopathy survived a ventricular fibrillation cardiac arrest. She subsequently underwent placement of a single, transvenous right ventricular lead implantable cardioverter defibrillator (ICD) system. The lead was an active fixation model, and the generator was placed in a left infraclavicular subcutaneous pocket.
Six months later, she presented with a 5‐week illness consisting of productive cough, fever, anorexia, and myalgias. Physical exam was notable for a rapid heart rate with a variable S1. Labs were notable for a leukocytosis with predominance of neutrophils. An ECG demonstrated atrial fibrillation.
A transesophageal echocardiogram (TEE) was performed in anticipation of a cardioversion for atrial fibrillation. The TEE demonstrated a mass in the right atrium that was attached to the ICD lead, with possible involvement of the tricuspid valve leaflets (Fig. 1). The mass, characterized as multiple confluent bulky segments, was freely mobile and measured about 1.8 cm at its greatest dimension. Therefore, cardioversion was not performed. Within 72 hours, multiple aerobic BACTEC blood cultures identified Haemophilus parainfluenzae, beta lactamase negative. The patient underwent a median sternotomy to remove the ICD lead and generator (Fig. 2). The septal leaflet of the tricuspid valve was debrided. The patient was treated with a prolonged course of ceftriaxone without clinical or microbiologic signs of persistent infection.


DISCUSSION
Research has demonstrated that the rise in cardiac device infections is greater than the rise in the rate of implantation of these devices over the same time period.1 Most infections with cardiac rhythm devices are primary infections, which begin at the pocket and frequently present around generator placement or exchange.2, 3 Because the intravascular leads are continuous to the pocket, there remains a risk for lead and systemic infection.
This case illustrates 2 important concepts. Secondary device infections, which usually result from bacteria originating at a site other than the generator pocket, are less common and tend to involve the intravascular lead.2, 4 Seeding of the intravascular lead frequently occurs with either Staphylococcus aureus or coagulase‐negative Staphylococci.2, 4 Therefore, H. parainfluenzae, a gram‐negative bacillus that can be part of the normal flora of the upper respiratory tract, is not a commonly encountered pathogen for secondary lead infections. Given the respiratory tract symptoms, this was likely the source in this patient. When the lead, generator, or both are infected, this necessitates removal of the entire system.
Furthermore, H. parainfluenzae is categorized with the HACEK organisms (Haemophilus species including H. aphrophilus, H. parainfluenzae, and H. paraphrophilus; Actinobacillus actinomycetemcomitans; Cardiobacterium hominis; Eikenella corrodens; Kingella kingae), a group of fastidious gram‐negative bacilli historically thought to be a common cause of culture‐negative endocarditis. Recent retrospective studies suggest that a prolonged incubation for HACEK organisms is generally not necessary because of advances in culture media and automated blood culture systems.5, 6 As shown in this case, the organism was cultured in less than 72 hours. Therefore, HACEK organisms, when used with modern culture media in addition to automated blood culture systems, are unlikely to be causes of true culture‐negative device or valve infection, provided the patient has had no recent exposure to antibiotics and adequate blood cultures have been obtained. If a cardiac device infection is suspected, blood cultures obtained before commencement of antibiotics and adequate sampling of blood for culture are more likely to identify the pathogen than are blood cultures from prolonged incubation.
- ,,, et al.Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999.Am Heart J.2004;147:582–586.
- ,.Infections of intracardiac devices. Cardiol Clin. 2003; 21: 253–271
- ,,,,,.Diagnosis and management of infections involving implantable electrophysiologic cardiac devices.Ann Intern Med.2000;133:604–608.
- ,,, et al.Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter‐defibrillators.Circulation.2001;104:1029–1033.
- ,,, et al.Utility of extended blood culture incubation for isolation of Haemophilus, Actinobacillus, Cardiobacterium, Eikenella and Kingella organisms: a retrospective multicenter evaluation.J Clin Microbiol.2006;44(1):257–259.
- ,,.Prolonged incubation and extensive subculturing do not increase recovery of clinically significant microorganisms from standard automated blood cultures.Clin Infect Dis.2005;41:1677–1680.
- ,,, et al.Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999.Am Heart J.2004;147:582–586.
- ,.Infections of intracardiac devices. Cardiol Clin. 2003; 21: 253–271
- ,,,,,.Diagnosis and management of infections involving implantable electrophysiologic cardiac devices.Ann Intern Med.2000;133:604–608.
- ,,, et al.Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter‐defibrillators.Circulation.2001;104:1029–1033.
- ,,, et al.Utility of extended blood culture incubation for isolation of Haemophilus, Actinobacillus, Cardiobacterium, Eikenella and Kingella organisms: a retrospective multicenter evaluation.J Clin Microbiol.2006;44(1):257–259.
- ,,.Prolonged incubation and extensive subculturing do not increase recovery of clinically significant microorganisms from standard automated blood cultures.Clin Infect Dis.2005;41:1677–1680.