Article

Mental Health Services: The Missing Piece or Missing Peace for Patients With Atopic Dermatitis
- Author:
- Peter A. Lio, MD
There is a well-established connection between the mind and the skin, and it is clear that this relationship is bidirectional—not only does skin...
Article
Dietary Triggers for Atopic Dermatitis in Children
- Author:
- Peter A. Lio, MD
It is important not to dismiss food as a factor in atopic dermatitis (AD), as it can play a number of roles in the condition.
Article
Patch Testing on Dupilumab: Reliable or Not?
- Author:
- Alexandra Kuzyk, MD, PhD
- Alim R. Devani, MD
- Vimal H. Prajapati, MD
- Peter A. Lio, MD
Patch testing is important to help determine a possible allergic contactant, but there is confusion about its accuracy in patients taking...
Video

Atopic Dermatitis and Peanut Allergy Prevention: New Guidelines
- Author:
- Peter A. Lio, MD
Delaying introduction of allergenic foods such as peanuts in infants with atopic dermatitis may not be appropriate. Dr. Peter Lio reviews new...
Article

Updated Guidelines on Peanut Allergy Prevention in Infants With Atopic Dermatitis
- Author:
- Peter A. Lio, MD
Article
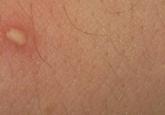
Identification of Cutaneous Warts: Cryotherapy-Induced Acetowhitelike Epithelium
- Author:
- Shivani Nanda, MD
- Peter A. Lio, MD
Cutaneous warts are benign proliferations of the epidermis that occur secondary to human papillomavirus infection. The diagnosis of cutaneous...
Article
In-Office Diagnosis of Cutaneous Mycosis: A Comparison of Potassium Hydroxide, Swartz-Lamkins, and Chlorazol Black E Fungal Stains
- Author:
- Vivian Y. Shi, MD
- Peter A. Lio, MD
Cutaneous mycosis represents the most common type of skin infection and accounts for up to 17% of dermatology office visits.
Article
Derm emergencies— detecting early signs of trouble
- Author:
- Alisa McQueen, MD
- Stephen A. Martin, MD, EdM
- Peter A. Lio, MD
Life-threatening dermatologic conditions do not always present with classic findings. This review—and the accompanying images—will help you...
