User login
Growing Ears, Noses, and Skin: The New Frontier in Dermatology and Dermatologic Surgery?
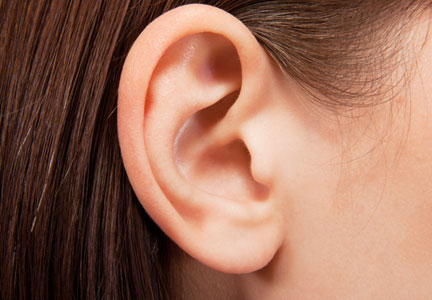
In an article published online in Maclean’s magazine on October 15, 2013, the concept of developing artificial body parts is discussed. The technology now exists for scientists to grow tissue organs, such as ears, noses, and fingers. Several groups of investigators, including Anthony Atala, MD (Wake Forest Institute for Regenerative Medicine, Winston-Salem, North Carolina) and Alexander Seifalian, PhD (University College London, England), are busy making ears by creating a biodegradable scaffold onto which cells—either stem cells from the patient or cells that have been harvested from the patient’s original organ—are layered and permitted to multiply. The cell-layered scaffold is then placed in a bioreactor for a couple of weeks. Once ready, the new ear is transplanted onto the patient; subsequently, the scaffold melts away.
Dr. Atala’s laboratory also is busy growing fingers. Meanwhile, Dr. Seifalian grew a nose (inside the patient’s own arm) in only 3 months after the patient lost his nose to skin cancer. He made a mold based on the patient’s original nose, created a scaffold composed of nanocomposite material, added the patient’s stem cells to the scaffold, and put the nose in a bioreactor to mature. Meanwhile, he placed a tissue expander in the patient’s arm and subsequently inserted the nose into the arm so that it could become vascularized and covered by skin. The nose was transplanted to the patient’s face; additional surgery is planned to open the nostrils, followed by seeding them with stem cells to return his sense of smell.
Marc Jeschke, MD, PhD (Ross Tilley Burn Centre, Sunnybrook Health Sciences Centre, Toronto, Ontario) is developing a bioprinter to create skin. The bioprinter dispenses different types of cells (grown from the patient’s own cells) into a hydrogel matrix in 3 dimensions. Currently, Dr. Jeschke is working with mice; however, in a few years, he anticipates treating human patients. Other investigators, such as Michael C. McAlpine (Princeton University, New Jersey), recently used a commercial 3-dimensional printer to make an ear; the “inks” included calf cells and a silver nanoparticle paste that formed a coiled antenna inside the cartilage that was capable of receiving electromagnetic signals and transmitting them to the brain.
What’s the issue?
The ability to grow tissue organs is going to revolutionize the surgical management of patients who need solid organ replacement; kidneys, lungs, pancreases, spleens, and tracheas have already been successfully grown. Indeed several investigators are making ears and noses. How long will it be before flaps and grafts to repair wound defects following extensive and deforming skin cancer surgery are replaced by ears and noses that are grown from the patient’s own tissue cells or stem cells? Although it seems like science fiction today, how soon will it be before a cutaneous 3-dimensional printer becomes a standard piece of equipment in the dermatologist and dermatologic surgeon’s office for use to create skin to cover postoperative sites that cannot be closed by directly bringing the wound edges together?
In an article published online in Maclean’s magazine on October 15, 2013, the concept of developing artificial body parts is discussed. The technology now exists for scientists to grow tissue organs, such as ears, noses, and fingers. Several groups of investigators, including Anthony Atala, MD (Wake Forest Institute for Regenerative Medicine, Winston-Salem, North Carolina) and Alexander Seifalian, PhD (University College London, England), are busy making ears by creating a biodegradable scaffold onto which cells—either stem cells from the patient or cells that have been harvested from the patient’s original organ—are layered and permitted to multiply. The cell-layered scaffold is then placed in a bioreactor for a couple of weeks. Once ready, the new ear is transplanted onto the patient; subsequently, the scaffold melts away.
Dr. Atala’s laboratory also is busy growing fingers. Meanwhile, Dr. Seifalian grew a nose (inside the patient’s own arm) in only 3 months after the patient lost his nose to skin cancer. He made a mold based on the patient’s original nose, created a scaffold composed of nanocomposite material, added the patient’s stem cells to the scaffold, and put the nose in a bioreactor to mature. Meanwhile, he placed a tissue expander in the patient’s arm and subsequently inserted the nose into the arm so that it could become vascularized and covered by skin. The nose was transplanted to the patient’s face; additional surgery is planned to open the nostrils, followed by seeding them with stem cells to return his sense of smell.
Marc Jeschke, MD, PhD (Ross Tilley Burn Centre, Sunnybrook Health Sciences Centre, Toronto, Ontario) is developing a bioprinter to create skin. The bioprinter dispenses different types of cells (grown from the patient’s own cells) into a hydrogel matrix in 3 dimensions. Currently, Dr. Jeschke is working with mice; however, in a few years, he anticipates treating human patients. Other investigators, such as Michael C. McAlpine (Princeton University, New Jersey), recently used a commercial 3-dimensional printer to make an ear; the “inks” included calf cells and a silver nanoparticle paste that formed a coiled antenna inside the cartilage that was capable of receiving electromagnetic signals and transmitting them to the brain.
What’s the issue?
The ability to grow tissue organs is going to revolutionize the surgical management of patients who need solid organ replacement; kidneys, lungs, pancreases, spleens, and tracheas have already been successfully grown. Indeed several investigators are making ears and noses. How long will it be before flaps and grafts to repair wound defects following extensive and deforming skin cancer surgery are replaced by ears and noses that are grown from the patient’s own tissue cells or stem cells? Although it seems like science fiction today, how soon will it be before a cutaneous 3-dimensional printer becomes a standard piece of equipment in the dermatologist and dermatologic surgeon’s office for use to create skin to cover postoperative sites that cannot be closed by directly bringing the wound edges together?
In an article published online in Maclean’s magazine on October 15, 2013, the concept of developing artificial body parts is discussed. The technology now exists for scientists to grow tissue organs, such as ears, noses, and fingers. Several groups of investigators, including Anthony Atala, MD (Wake Forest Institute for Regenerative Medicine, Winston-Salem, North Carolina) and Alexander Seifalian, PhD (University College London, England), are busy making ears by creating a biodegradable scaffold onto which cells—either stem cells from the patient or cells that have been harvested from the patient’s original organ—are layered and permitted to multiply. The cell-layered scaffold is then placed in a bioreactor for a couple of weeks. Once ready, the new ear is transplanted onto the patient; subsequently, the scaffold melts away.
Dr. Atala’s laboratory also is busy growing fingers. Meanwhile, Dr. Seifalian grew a nose (inside the patient’s own arm) in only 3 months after the patient lost his nose to skin cancer. He made a mold based on the patient’s original nose, created a scaffold composed of nanocomposite material, added the patient’s stem cells to the scaffold, and put the nose in a bioreactor to mature. Meanwhile, he placed a tissue expander in the patient’s arm and subsequently inserted the nose into the arm so that it could become vascularized and covered by skin. The nose was transplanted to the patient’s face; additional surgery is planned to open the nostrils, followed by seeding them with stem cells to return his sense of smell.
Marc Jeschke, MD, PhD (Ross Tilley Burn Centre, Sunnybrook Health Sciences Centre, Toronto, Ontario) is developing a bioprinter to create skin. The bioprinter dispenses different types of cells (grown from the patient’s own cells) into a hydrogel matrix in 3 dimensions. Currently, Dr. Jeschke is working with mice; however, in a few years, he anticipates treating human patients. Other investigators, such as Michael C. McAlpine (Princeton University, New Jersey), recently used a commercial 3-dimensional printer to make an ear; the “inks” included calf cells and a silver nanoparticle paste that formed a coiled antenna inside the cartilage that was capable of receiving electromagnetic signals and transmitting them to the brain.
What’s the issue?
The ability to grow tissue organs is going to revolutionize the surgical management of patients who need solid organ replacement; kidneys, lungs, pancreases, spleens, and tracheas have already been successfully grown. Indeed several investigators are making ears and noses. How long will it be before flaps and grafts to repair wound defects following extensive and deforming skin cancer surgery are replaced by ears and noses that are grown from the patient’s own tissue cells or stem cells? Although it seems like science fiction today, how soon will it be before a cutaneous 3-dimensional printer becomes a standard piece of equipment in the dermatologist and dermatologic surgeon’s office for use to create skin to cover postoperative sites that cannot be closed by directly bringing the wound edges together?
Cutaneous SCC in a Renal Transplant Patient Derived From Donor Kidney Tumor Cells: Should Donor Transplant Organs Undergo Genetic Profiling for Cancer-Associated Mutations?
Squamous cell carcinoma (SCC) on sun-exposed sites following prolonged immunosuppression is one of the main long-term complications of allogeneic transplantations. In an article published in the Journal of Clinical Investigation (2013;123:3797-3801), the investigators demonstrated that the SCC tumor cells from a renal transplant patient had the donor genotype and harbored a TP53 (tumor-suppressing p53) mutation in codon 175; in addition, the same TP53 mutation had previously been documented 7 years earlier in p53+ cells in the renal tubules from a kidney graft biopsy. The observations in this patient provide evidence that the kidney donor can contribute to subsequent SCCs in renal transplant recipients.
What’s the issue?
Donor contribution to the malignant epithelium of cutaneous cancer in organ transplant recipients has several important implications. First, this observation provides additional insight into the initiation and progression of tumor carcinogenesis. Second, because there is longer survival of patients following renal transplant and therefore a prolonged duration of immunosuppression in these individuals, the development of long-term complications such as SCC of sun-exposed skin may be greater. Third, should genetic profiling of kidneys from potential donors to screen for cancer-associated mutations be performed? And fourth, is this phenomenon restricted only to patients receiving kidneys or might it be a possible complication in the recipients of other organs such as hearts, lungs, or even faces?
Squamous cell carcinoma (SCC) on sun-exposed sites following prolonged immunosuppression is one of the main long-term complications of allogeneic transplantations. In an article published in the Journal of Clinical Investigation (2013;123:3797-3801), the investigators demonstrated that the SCC tumor cells from a renal transplant patient had the donor genotype and harbored a TP53 (tumor-suppressing p53) mutation in codon 175; in addition, the same TP53 mutation had previously been documented 7 years earlier in p53+ cells in the renal tubules from a kidney graft biopsy. The observations in this patient provide evidence that the kidney donor can contribute to subsequent SCCs in renal transplant recipients.
What’s the issue?
Donor contribution to the malignant epithelium of cutaneous cancer in organ transplant recipients has several important implications. First, this observation provides additional insight into the initiation and progression of tumor carcinogenesis. Second, because there is longer survival of patients following renal transplant and therefore a prolonged duration of immunosuppression in these individuals, the development of long-term complications such as SCC of sun-exposed skin may be greater. Third, should genetic profiling of kidneys from potential donors to screen for cancer-associated mutations be performed? And fourth, is this phenomenon restricted only to patients receiving kidneys or might it be a possible complication in the recipients of other organs such as hearts, lungs, or even faces?
Squamous cell carcinoma (SCC) on sun-exposed sites following prolonged immunosuppression is one of the main long-term complications of allogeneic transplantations. In an article published in the Journal of Clinical Investigation (2013;123:3797-3801), the investigators demonstrated that the SCC tumor cells from a renal transplant patient had the donor genotype and harbored a TP53 (tumor-suppressing p53) mutation in codon 175; in addition, the same TP53 mutation had previously been documented 7 years earlier in p53+ cells in the renal tubules from a kidney graft biopsy. The observations in this patient provide evidence that the kidney donor can contribute to subsequent SCCs in renal transplant recipients.
What’s the issue?
Donor contribution to the malignant epithelium of cutaneous cancer in organ transplant recipients has several important implications. First, this observation provides additional insight into the initiation and progression of tumor carcinogenesis. Second, because there is longer survival of patients following renal transplant and therefore a prolonged duration of immunosuppression in these individuals, the development of long-term complications such as SCC of sun-exposed skin may be greater. Third, should genetic profiling of kidneys from potential donors to screen for cancer-associated mutations be performed? And fourth, is this phenomenon restricted only to patients receiving kidneys or might it be a possible complication in the recipients of other organs such as hearts, lungs, or even faces?
Lung Cancer–Associated Scalp Hair Loss: A Rare Cause of Secondary Alopecia Neoplastica
Shared Medical Appointments: A New Practice Model for Dermatologists?
In a recent article published online in Time magazine, “The New Group Medical Checkup,” the potential benefits of shared medical appointments are discussed. The author mentions that “[a]mong those who try shared visits, about 85% don’t go back to individual exams for everything from diabetes to weight loss and skin-related issues.”
A shared medical appointment is a group visit for the patient. For example, patients sign in and have their vital signs taken. If the visit includes a one-on-one examination by the physician, the nonphysician office personnel answers questions for the other patients. Discussion of the patients’ problems and treatments is then conducted in a group setting; additional time (eg, 90 minutes) is allocated to the visit so that the patients can learn from each other’s medical problems and have ample opportunity to ask questions.
Group visits are already being conducted by family medicine practices. It also is anticipated that additional specialties may incorporate shared medical appointments because group visits shall provide an efficient means for lowering costs when major provisions of the Patient Protection and Affordable Care Act are implemented next year.
Group visits can provide advantages for both the physician and the patient. The shared medical appointment enables the physician to streamline the delivery of care without having to repeat the same recommendations to each patient separately. The group visit encourages patients to inquire about their disease and to learn more about their condition by being able to share the experience with other patients.
What’s the issue?
The practice of medicine (and dermatology) continues to evolve, from sole practitioners to group practices to multispecialty groups to corporate-owned and -managed institutions. Some of the established means for interaction between the physician and the patient occur in a setting that is either private, hospital based, or concierge. However, social and financial influences are promoting another practice model: shared medical appointments. Is this approach to medical management appropriate for dermatology? If group visits are considered to be an appropriate approach to dermatology patient care, should dermatologists embrace this new concept and incorporate it into their practices?
In a recent article published online in Time magazine, “The New Group Medical Checkup,” the potential benefits of shared medical appointments are discussed. The author mentions that “[a]mong those who try shared visits, about 85% don’t go back to individual exams for everything from diabetes to weight loss and skin-related issues.”
A shared medical appointment is a group visit for the patient. For example, patients sign in and have their vital signs taken. If the visit includes a one-on-one examination by the physician, the nonphysician office personnel answers questions for the other patients. Discussion of the patients’ problems and treatments is then conducted in a group setting; additional time (eg, 90 minutes) is allocated to the visit so that the patients can learn from each other’s medical problems and have ample opportunity to ask questions.
Group visits are already being conducted by family medicine practices. It also is anticipated that additional specialties may incorporate shared medical appointments because group visits shall provide an efficient means for lowering costs when major provisions of the Patient Protection and Affordable Care Act are implemented next year.
Group visits can provide advantages for both the physician and the patient. The shared medical appointment enables the physician to streamline the delivery of care without having to repeat the same recommendations to each patient separately. The group visit encourages patients to inquire about their disease and to learn more about their condition by being able to share the experience with other patients.
What’s the issue?
The practice of medicine (and dermatology) continues to evolve, from sole practitioners to group practices to multispecialty groups to corporate-owned and -managed institutions. Some of the established means for interaction between the physician and the patient occur in a setting that is either private, hospital based, or concierge. However, social and financial influences are promoting another practice model: shared medical appointments. Is this approach to medical management appropriate for dermatology? If group visits are considered to be an appropriate approach to dermatology patient care, should dermatologists embrace this new concept and incorporate it into their practices?
In a recent article published online in Time magazine, “The New Group Medical Checkup,” the potential benefits of shared medical appointments are discussed. The author mentions that “[a]mong those who try shared visits, about 85% don’t go back to individual exams for everything from diabetes to weight loss and skin-related issues.”
A shared medical appointment is a group visit for the patient. For example, patients sign in and have their vital signs taken. If the visit includes a one-on-one examination by the physician, the nonphysician office personnel answers questions for the other patients. Discussion of the patients’ problems and treatments is then conducted in a group setting; additional time (eg, 90 minutes) is allocated to the visit so that the patients can learn from each other’s medical problems and have ample opportunity to ask questions.
Group visits are already being conducted by family medicine practices. It also is anticipated that additional specialties may incorporate shared medical appointments because group visits shall provide an efficient means for lowering costs when major provisions of the Patient Protection and Affordable Care Act are implemented next year.
Group visits can provide advantages for both the physician and the patient. The shared medical appointment enables the physician to streamline the delivery of care without having to repeat the same recommendations to each patient separately. The group visit encourages patients to inquire about their disease and to learn more about their condition by being able to share the experience with other patients.
What’s the issue?
The practice of medicine (and dermatology) continues to evolve, from sole practitioners to group practices to multispecialty groups to corporate-owned and -managed institutions. Some of the established means for interaction between the physician and the patient occur in a setting that is either private, hospital based, or concierge. However, social and financial influences are promoting another practice model: shared medical appointments. Is this approach to medical management appropriate for dermatology? If group visits are considered to be an appropriate approach to dermatology patient care, should dermatologists embrace this new concept and incorporate it into their practices?
Telomeres and Telomerase: Can a Topical Telomerase Activator Reverse Cutaneous Aging?
In an article published online in Maclean’s magazine on May 14, 2013, postulated hypotheses regarding the potential cellular pathophysiology of longevity are summarized. Telomeres, telomerase, and telomerase activators each—individually or in concert—may have a critical role in this process.
Telomeres, the tiny bits of DNA that cap the ends of chromosomes, shorten each time a cell divides and also as people age. Telomeres function to prevent chromosomes from unraveling and fusing with each other, yet when they become too short, the cell dies. Shorter telomeres have been observed in patients with chronic stress, such as mothers of children with chronic illnesses and spouses who care for parents with Alzheimer disease; domestic abuse victims; and individuals with untreated depression. Several systemic conditions also are associated with shorter telomeres, including cancer, cardiovascular disease, dementia, diabetes mellitus, and osteoporosis. However, individuals who are older than 100 years have remarkably long telomeres. Hence, telomere length may be a more accurate indicator of a person’s physiologic age than one’s date of birth.
Telomerase, an antiaging enzyme, rebuilds telomeres and protects them from wearing down. Lifestyle changes, such as diet (with increased omega-3 fatty acids found in fish oils), exercise (approximately 30 minutes 4 or 5 times a week), and meditation, can potentially slow down the shortening of telomeres, increase telomerase activity, or both. Experiments performed on genetically engineered mice with a controllable telomerase gene show that when the enzyme is turned off, it becomes prematurely old, mentally impaired, and infertile. However, even after the mice reach this state of severe degeneration, the changes reverse when the gene is turned on again; the mice eventually resemble young active adults, with a healthy sheen restored to their hair coat, improved cognition, and restored fertility.
Telomerase activators, products that can stimulate telomerase, are the next logical progression in this quest to remain young. Indeed, at least one agent is commercially available (and sold as a nutritional supplement); however, the US Food and Drug Administration has not approved the oral agent.
What’s the issue?
The skin is the largest organ of the body. It is reasonable to speculate that the aging of one’s skin may be related to overall senescence. Therefore, the cellular longevity of a person’s keratinocytes also might be related to the length of their telomeres. Increasing the telomerase activity of these keratinocytes should favorably influence the length of the telomeres. To the best of my knowledge, a topical telomerase activator remains to be developed. However, it is very intriguing to consider the potential possibilities of a new topical cutaneous antiaging agent. Will the next antiaging agent for the skin be a topical telomerase activator?
In an article published online in Maclean’s magazine on May 14, 2013, postulated hypotheses regarding the potential cellular pathophysiology of longevity are summarized. Telomeres, telomerase, and telomerase activators each—individually or in concert—may have a critical role in this process.
Telomeres, the tiny bits of DNA that cap the ends of chromosomes, shorten each time a cell divides and also as people age. Telomeres function to prevent chromosomes from unraveling and fusing with each other, yet when they become too short, the cell dies. Shorter telomeres have been observed in patients with chronic stress, such as mothers of children with chronic illnesses and spouses who care for parents with Alzheimer disease; domestic abuse victims; and individuals with untreated depression. Several systemic conditions also are associated with shorter telomeres, including cancer, cardiovascular disease, dementia, diabetes mellitus, and osteoporosis. However, individuals who are older than 100 years have remarkably long telomeres. Hence, telomere length may be a more accurate indicator of a person’s physiologic age than one’s date of birth.
Telomerase, an antiaging enzyme, rebuilds telomeres and protects them from wearing down. Lifestyle changes, such as diet (with increased omega-3 fatty acids found in fish oils), exercise (approximately 30 minutes 4 or 5 times a week), and meditation, can potentially slow down the shortening of telomeres, increase telomerase activity, or both. Experiments performed on genetically engineered mice with a controllable telomerase gene show that when the enzyme is turned off, it becomes prematurely old, mentally impaired, and infertile. However, even after the mice reach this state of severe degeneration, the changes reverse when the gene is turned on again; the mice eventually resemble young active adults, with a healthy sheen restored to their hair coat, improved cognition, and restored fertility.
Telomerase activators, products that can stimulate telomerase, are the next logical progression in this quest to remain young. Indeed, at least one agent is commercially available (and sold as a nutritional supplement); however, the US Food and Drug Administration has not approved the oral agent.
What’s the issue?
The skin is the largest organ of the body. It is reasonable to speculate that the aging of one’s skin may be related to overall senescence. Therefore, the cellular longevity of a person’s keratinocytes also might be related to the length of their telomeres. Increasing the telomerase activity of these keratinocytes should favorably influence the length of the telomeres. To the best of my knowledge, a topical telomerase activator remains to be developed. However, it is very intriguing to consider the potential possibilities of a new topical cutaneous antiaging agent. Will the next antiaging agent for the skin be a topical telomerase activator?
In an article published online in Maclean’s magazine on May 14, 2013, postulated hypotheses regarding the potential cellular pathophysiology of longevity are summarized. Telomeres, telomerase, and telomerase activators each—individually or in concert—may have a critical role in this process.
Telomeres, the tiny bits of DNA that cap the ends of chromosomes, shorten each time a cell divides and also as people age. Telomeres function to prevent chromosomes from unraveling and fusing with each other, yet when they become too short, the cell dies. Shorter telomeres have been observed in patients with chronic stress, such as mothers of children with chronic illnesses and spouses who care for parents with Alzheimer disease; domestic abuse victims; and individuals with untreated depression. Several systemic conditions also are associated with shorter telomeres, including cancer, cardiovascular disease, dementia, diabetes mellitus, and osteoporosis. However, individuals who are older than 100 years have remarkably long telomeres. Hence, telomere length may be a more accurate indicator of a person’s physiologic age than one’s date of birth.
Telomerase, an antiaging enzyme, rebuilds telomeres and protects them from wearing down. Lifestyle changes, such as diet (with increased omega-3 fatty acids found in fish oils), exercise (approximately 30 minutes 4 or 5 times a week), and meditation, can potentially slow down the shortening of telomeres, increase telomerase activity, or both. Experiments performed on genetically engineered mice with a controllable telomerase gene show that when the enzyme is turned off, it becomes prematurely old, mentally impaired, and infertile. However, even after the mice reach this state of severe degeneration, the changes reverse when the gene is turned on again; the mice eventually resemble young active adults, with a healthy sheen restored to their hair coat, improved cognition, and restored fertility.
Telomerase activators, products that can stimulate telomerase, are the next logical progression in this quest to remain young. Indeed, at least one agent is commercially available (and sold as a nutritional supplement); however, the US Food and Drug Administration has not approved the oral agent.
What’s the issue?
The skin is the largest organ of the body. It is reasonable to speculate that the aging of one’s skin may be related to overall senescence. Therefore, the cellular longevity of a person’s keratinocytes also might be related to the length of their telomeres. Increasing the telomerase activity of these keratinocytes should favorably influence the length of the telomeres. To the best of my knowledge, a topical telomerase activator remains to be developed. However, it is very intriguing to consider the potential possibilities of a new topical cutaneous antiaging agent. Will the next antiaging agent for the skin be a topical telomerase activator?
The Deplorable Imprisonment of a Doctor: A Warning Signal for Physicians
Professor Cyril Karabus is a 78-year-old pediatric oncologist who has dedicated his life to treating children with malignancy and is widely respected for his expertise and compassion. He was arrested in the United Arab Emirates (UAE) on August 18, 2012, while returning to his home in South Africa with his wife and daughter following his son’s wedding in Canada. The flight had stopped at Abu Dhabi International Airport overnight and he was detained by passport control. He was unaware that more than 10 years earlier he had been tried and convicted in absentia for manslaughter and falsifying documents (medical records) after the death of a 3-year-old girl with acute myeloblastic leukemia in 2002 while he was temporarily working in the UAE (Br Med J. 2012;345:e6815)(The Cancer Letter. 2012;38[46]:11).
The injustice of Professor Karabus’ arrest and detention by the UAE’s judicial system is deplorable. A South African activist organization called the Treatment Action Campaign stated that by no modern principle of jurisprudence is it acceptable to try a foreign citizen in absentia without informing him/her. The South African Medical Association has cautioned members about working in the UAE and the British Medical Association has protested the conditions in which he had been held (Br Med J. 2012;345:e6815). More recently, the World Medical Association (WMA) stated that an advisory notice will be published in the World Medical Journal and on the WMA Web site about the working conditions and legal risks for physicians working in the UAE (http://www.wma.net/en/30publications/10policies/30council/cr_16/).
Professor Karabus recently was released after being held for 9 months (since August 2012) even though the prosecution could not find the disputed medical records. On March 21, 2013, a judge acquitted Professor Karabus of all charges. However, the prosecution elected to appeal the judge’s decision, keeping Professor Karabus in the UAE. He was finally released and was back home in South Africa on May 18, 2013. The WMA continues to emphasize the risks for physicians working in the UAE (http://www.wma.net/en/40news/20archives/2013/2013_15/index.html).
What’s the issue?
First, is it still safe for doctors to accept locum tenens abroad? Perhaps yes, but it may be prudent to carefully assess the judicial system of the country in which one is considering to work before accepting the employment opportunity.
Second, should academic centers and particularly medical institutions be receiving large sums of money from foreign dictatorships, such as the UAE? Currently, there are several major medical institutions—Johns Hopkins University in Baltimore, Maryland; the Children’s National Medical Center in Washington, DC; and The University of Texas MD Anderson Cancer Center in Houston—that accept substantial financial contributions from the Zayed family who govern the UAE (Clin Dermatol. 2013;31:325-326).
Professor Cyril Karabus is a 78-year-old pediatric oncologist who has dedicated his life to treating children with malignancy and is widely respected for his expertise and compassion. He was arrested in the United Arab Emirates (UAE) on August 18, 2012, while returning to his home in South Africa with his wife and daughter following his son’s wedding in Canada. The flight had stopped at Abu Dhabi International Airport overnight and he was detained by passport control. He was unaware that more than 10 years earlier he had been tried and convicted in absentia for manslaughter and falsifying documents (medical records) after the death of a 3-year-old girl with acute myeloblastic leukemia in 2002 while he was temporarily working in the UAE (Br Med J. 2012;345:e6815)(The Cancer Letter. 2012;38[46]:11).
The injustice of Professor Karabus’ arrest and detention by the UAE’s judicial system is deplorable. A South African activist organization called the Treatment Action Campaign stated that by no modern principle of jurisprudence is it acceptable to try a foreign citizen in absentia without informing him/her. The South African Medical Association has cautioned members about working in the UAE and the British Medical Association has protested the conditions in which he had been held (Br Med J. 2012;345:e6815). More recently, the World Medical Association (WMA) stated that an advisory notice will be published in the World Medical Journal and on the WMA Web site about the working conditions and legal risks for physicians working in the UAE (http://www.wma.net/en/30publications/10policies/30council/cr_16/).
Professor Karabus recently was released after being held for 9 months (since August 2012) even though the prosecution could not find the disputed medical records. On March 21, 2013, a judge acquitted Professor Karabus of all charges. However, the prosecution elected to appeal the judge’s decision, keeping Professor Karabus in the UAE. He was finally released and was back home in South Africa on May 18, 2013. The WMA continues to emphasize the risks for physicians working in the UAE (http://www.wma.net/en/40news/20archives/2013/2013_15/index.html).
What’s the issue?
First, is it still safe for doctors to accept locum tenens abroad? Perhaps yes, but it may be prudent to carefully assess the judicial system of the country in which one is considering to work before accepting the employment opportunity.
Second, should academic centers and particularly medical institutions be receiving large sums of money from foreign dictatorships, such as the UAE? Currently, there are several major medical institutions—Johns Hopkins University in Baltimore, Maryland; the Children’s National Medical Center in Washington, DC; and The University of Texas MD Anderson Cancer Center in Houston—that accept substantial financial contributions from the Zayed family who govern the UAE (Clin Dermatol. 2013;31:325-326).
Professor Cyril Karabus is a 78-year-old pediatric oncologist who has dedicated his life to treating children with malignancy and is widely respected for his expertise and compassion. He was arrested in the United Arab Emirates (UAE) on August 18, 2012, while returning to his home in South Africa with his wife and daughter following his son’s wedding in Canada. The flight had stopped at Abu Dhabi International Airport overnight and he was detained by passport control. He was unaware that more than 10 years earlier he had been tried and convicted in absentia for manslaughter and falsifying documents (medical records) after the death of a 3-year-old girl with acute myeloblastic leukemia in 2002 while he was temporarily working in the UAE (Br Med J. 2012;345:e6815)(The Cancer Letter. 2012;38[46]:11).
The injustice of Professor Karabus’ arrest and detention by the UAE’s judicial system is deplorable. A South African activist organization called the Treatment Action Campaign stated that by no modern principle of jurisprudence is it acceptable to try a foreign citizen in absentia without informing him/her. The South African Medical Association has cautioned members about working in the UAE and the British Medical Association has protested the conditions in which he had been held (Br Med J. 2012;345:e6815). More recently, the World Medical Association (WMA) stated that an advisory notice will be published in the World Medical Journal and on the WMA Web site about the working conditions and legal risks for physicians working in the UAE (http://www.wma.net/en/30publications/10policies/30council/cr_16/).
Professor Karabus recently was released after being held for 9 months (since August 2012) even though the prosecution could not find the disputed medical records. On March 21, 2013, a judge acquitted Professor Karabus of all charges. However, the prosecution elected to appeal the judge’s decision, keeping Professor Karabus in the UAE. He was finally released and was back home in South Africa on May 18, 2013. The WMA continues to emphasize the risks for physicians working in the UAE (http://www.wma.net/en/40news/20archives/2013/2013_15/index.html).
What’s the issue?
First, is it still safe for doctors to accept locum tenens abroad? Perhaps yes, but it may be prudent to carefully assess the judicial system of the country in which one is considering to work before accepting the employment opportunity.
Second, should academic centers and particularly medical institutions be receiving large sums of money from foreign dictatorships, such as the UAE? Currently, there are several major medical institutions—Johns Hopkins University in Baltimore, Maryland; the Children’s National Medical Center in Washington, DC; and The University of Texas MD Anderson Cancer Center in Houston—that accept substantial financial contributions from the Zayed family who govern the UAE (Clin Dermatol. 2013;31:325-326).