User login
Surgery for Blastomycosis of the Spine
Blastomycosis is a rare fungal infection that primarily produces acute lung infections but may on occasion disseminate to multiple sites, including the skin, bone, central nervous system (CNS), and oropharynx.1-30 In the case of a primary infection of the lung, if there is a high index of suspicion and a thorough diagnostic workup, the diagnosis can be made from sputum or bronchoscopy.24 Patients present with acute pneumonia that either resolves spontaneously or proceeds to chronic pneumonia with extrapulmonary spread to multiple organs, including the spine. Once vertebral involvement occurs, an untreated infection may result in vertebral body destruction and paraspinal and epidural abscess formation followed by neurologic injury and loss of structural integrity of the spine.11,13,17,23,27,29
In this article, we present a case of blastomycosis of the vertebral body and provide a detailed review of the literature concerning this extremely rare infection of the spine. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
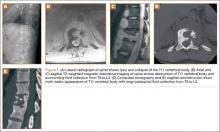
A 30-year-old African American man with known pulmonary blastomycosis, for which he had been treated with oral itraconazole 200 mg twice daily for 6 months, was admitted to the hospital with a 2-month history of mild thoracolumbar back pain. He reported transient numbness and tingling in the lower extremities but no weakness. He denied weight loss, fatigue, appetite loss, and significant night pain. On physical examination, he was alert and oriented, well nourished, and in no acute distress. Percussion revealed limited range of motion and pain. Further examination of the spine demonstrated no spasm, swelling, erythema, or drainage. The lower extremities had intact sensation, motor strength, reflexes, and pulses, and clonus was absent. White blood cell count was 8100 cells/μL (normal), erythrocyte sedimentation rate was 77 mm/h (normal range, 0-20 mm/h), and C-reactive protein level was 57.2 mg/L (normal, ≤ 10 mg/L). The patient was HIV-negative. Chest radiographs were normal except for a small pleural effusion. Radiographs showed a destructive lesion of T11 with an extensive paravertebral and retropleural abscess tracking a spinal level above and below with extension into the spinal canal (Figure 1).
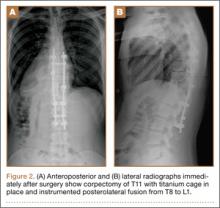
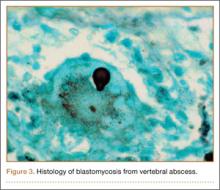
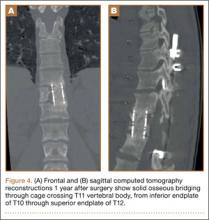
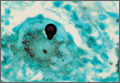
As the patient had signs of spinal cord compression, he was taken to surgery for incision and drainage and culture procurement and corpectomy of T11 with autogenous rib graft. One week later, he was stabilized with posterior fusion and instrumentation (Figure 2). Gram stain of the specimen demonstrated broad-based budding yeast forms 15 to 20 micrometers in size, consistent with blastomycosis. Cultures were positive for Blastomyces dermatitidis. Histopathologic slides (Figure 3) of the surgical pathology specimen showed granulomatous inflammation. Oral itraconazole 200 mg twice daily was continued, as it has been found to be efficacious in treating immunocompetent patients with blastomycosis17 and is considered the medication of choice for non–life-threatening, non-CNS blastomycosis. (Intravenous amphotericin B was ruled out because of its known serious side effects, such as bone marrow suppression and renal function impairment10; itraconazole was the better alternative.) The patient was placed in a thoracolumbar orthosis and discharged. As the effect of presence of instrumentation in the setting of a fungal infection is unknown, it was deemed prudent to maintain the patient on chronic antifungal suppression. One year after surgery, computed tomography (CT) showed solid osseous bridging through the cage crossing the T11 vertebral body, from the inferior endplate of T10 through the superior endplate of T12 (Figure 4). In addition, there had been no recurrence of the spinal infection, and the patient was neurologically intact and doing well.
Discussion
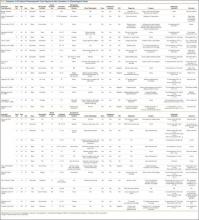
North American blastomycosis (B dermatitidis) is a ubiquitous dimorphic fungus that occurs worldwide and on occasion causes serious infections in humans.9,23,26,29 It was first characterized in 1894 by Gilchrist and Stokes (Gilchrist disease) when they recovered the fungus from the lung tissue of a patient.3 In North America, blastomycosis infections occur from central Canada to the Gulf Coast to east of the Mississippi River.2,5,7,8,13,14,17,21,22,24,27,29 Additional cases of the disease have been reported in Africa,9,16,23,28 Asia,12,19 and South America7,8 (Table [on pages E270-E271]). Recent epidemiologic studies have linked transmission of the disease to bodies of water and have questioned previous reports of male predominance and racial preference for African Americans (Table).
Blastomycosis is acquired when inhaled fungus (airborne conidia spores) causes a primary pulmonary infection or, rarely, when there is direct inoculation through the skin. The differential diagnosis includes neoplasm, tuberculosis, actinomycosis, bacterial infections, cryptococcosis, and coccidioidomycosis.3,9,12,20,25,31 Blastomycosis occurs in adults and children.1-30 The rate of mortality is much higher in immunocompromised patients. Initial symptoms include fever, chills, fatigue, malaise, myalgia, arthalgia, weight loss, and stigmata of chronic disease.1-30 Acute pulmonary infection with blastomycosis generally resolves spontaneously but may progress to acute respiratory distress syndrome, which has a mortality rate of 50% to 89%.19 With systemic dissemination, the infection may spread to other organs11—there is a particular predilection for the skin9,20,29—and to the long bones7,16 and the oropharynx.16,26,28
In 50% to 64% of cases, bone involvement may be the first disease manifestation.6,7,16,22 Osseous involvement with blastomycosis most commonly affects the long bones15 but may include the vertebrae,1-29 the ribs,26 and the carpal or tarsal bones.7,16 The most common vertebral involvement occurs in the thoracic or lumbar spine1,2,7-9,11-14,17,19,21-24,26 and typically results in destruction of the body, development of a paraspinal abscess, and potential extension into the spinal canal, causing an epidural abscess and development of chronic draining cutaneous sinuses.2,7,9,11-13,16,17,19,22,23,26,28,29 In the present case, we do not know whether the vertebral body was involved before the patient presented with mid-thoracolumbar back pain. There may have been bony involvement during initial presentation.
Diagnosis is often difficult because of a low index of suspicion, leading to a significant delay in treatment. Primary pulmonary infections are successfully diagnosed 86% of the time from sputum and 92% of the time from bronchoscopy.19 Once the infection involves the spine, plain radiographs, CT, and magnetic resonance imaging (MRI) can be used to identify not only the bony involvement but also any adjacent soft-tissue extension.13 The radiographic findings, typical of tuberculosis or a neoplasm, include disc space narrowing, vertebral body destruction and collapse, late segmental kyphotic deformity, and development of a psoas abscess or a retropleural abscess.7,26 Such abscesses lend themselves well to fine-needle aspiration,7,8,11,13,14,17,19,26 which, when combined with CT and MRI guidance, reliably assists in the diagnosis of blastomycosis.1,13,17 If fine-needle aspiration fails, then open biopsy and surgical débridement specimens may be effective in the diagnosis.2,9,12,21,22,27
The mortality rate for systemic blastomycosis exceeded 90% before the development of antifungal medications, and these medications remain the primary treatment for most initial infections.15 For severe infections in critically ill patients and for patients with CNS involvement, amphotericin B has been effective, with cure rates approaching 97%.17 Itraconazole, which is well tolerated, has replaced ketoconazole as the preferred long-term oral treatment for blastomycosis. Cure rates for itraconazole approach 90% when treatment is instituted over 2 years in a compliant patient.10,19,20 Nonsurgical (antifungal) treatment for blastomycosis of the spine has also proved successful in neurologically intact patients.7,9,11,26,28
A case involving the spine and requiring surgical drainage was first reported in 19085; since then, only a few more cases have been reported.1,2,5,7-9,11-14,16,17,19,21-24,26-29 Thus, the literature includes very little information that can be used to establish indications for surgery for a blastomycotic infection of the spine. However, there is enough evidence to establish that surgery is indicated for patients who have a known blastomycosis infection and are developing neurologic or structural loss of integrity of the spinal column or have an abscess that requires drainage and débridement.
Our patient had been on long-term antifungal treatment but nevertheless developed a destructive spinal lesion with a concurrent epidural and retropleural abscess. Given his risk of pathologic fracture, we performed anterior débridement and stabilization followed by posterior fusion and instrumentation. We are unaware of any other cases in which an anterior titanium cage was combined with rib autograft after anterior débridement and vertebrectomy combined with posterior instrumentation for blastomycosis. This technique proved very useful, as it allowed for immediate stabilization of the spine. Therefore, the treatment goal is similar to that for any destructive infection that fails medical treatment: preservation of neurologic function, stabilization of spinal vertebrae, débridement of abscess cavity, and definitive culture procurement.
Conclusion
Although there is little reported information regarding surgical indications for blastomycotic vertebral osteomyelitis that has failed medical management—in patients with a destructive lesion and compromise of both the spinal canal and the integrity of the vertebral column—anterior débridement and stabilization followed by posterior fusion and instrumentation are useful in preventing vertebral collapse, further canal compromise, and possible cord injury.
1. Akhtar I, Flowers R, Siddiqi A, Heard K, Baliga M. Fine needle aspiration biopsy of vertebral and paravertebral lesions: retrospective study of 124 cases [published correction appears in Acta Cytol. 2006;50(5):600]. Acta Cytol. 2006;50(4):364-371.
2. Arvin MC, Gehring RL, Crecelius JL, Curfman MF. Man with progressive lower back pain. Indiana Med. 1991;84(8):554-556.
3. Baylin GJ, Wear JM. Blastomycosis and actinomycosis of the spine. Am J Roentgenol Radium Ther Nucl Med. 1953;69(3):395-398.
4. Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17(1):21-40.
5. Brewer GE, Wood FC. XII. Blastomycosis of the spine: double lesion: two operations: recovery. Ann Surg. 1908;48(6):889-896.
6. Carman WF, Frean JA, Crewe-Brown HH, Culligan GA, Young CN. Blastomycosis in Africa. A review of known cases diagnosed between 1951 and 1987. Mycopathologica. 1989;107(1):25-32.
7. Challapalli M, Cunningham DG. North American blastomycosis of the vertebrae in an adolescent. Clin Infect Dis. 1996;23(4):853-854.
8. Detrisac DA, Harding WG, Greiner AL, Dunn CR, Mayfield FH. Vertebral North American blastomycosis. Surg Neurol. 1980;13(4):311-312.
9. Frean J, Blumberg L, Woolf M. Disseminated blastomycosis masquerading as tuberculosis. J Infect. 1993;26(2):203-206.
10. Goodman LS, Brunton LL, Chabner B, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill Medical; 2011.
11. Gottlieb JR, Eismont FJ. Nonoperative treatment of vertebral blastomycosis osteomyelitis associated with paraspinal abscess and cord compression. A case report. J Bone Joint Surg Am. 2006;88(4):854-856.
12. Güler N, Palanduz A, Ones U, et al. Progressive vertebral blastomycosis mimicking tuberculosis. Pediatr Infect Dis J. 1995;14(9):816-818.
13. Hadjipavlou AG, Mader JT, Nauta HJ, Necessary JT, Chaljub G, Adesokan A. Blastomycosis of the lumbar spine: case report and review of the literature, with emphasis on diagnostic laboratory tools and management. Eur Spine J. 1998;7(5):416-421.
14. Hardjasudarma M, Willis B, Black-Payne C, Edwards R. Pediatric spinal blastomycosis: case report. Neurosurgery. 1995;37(3):534-536.
15. Jahangir AA, Heck RK. Blastomycosis: case report of an isolated lesion in the distal fibula. Am J Orthop. 2010;39(3):E22-E24.
16. Koen AF, Blumberg LH. North American blastomycosis in South Africa simulating tuberculosis. Clin Radiol. 1999;54(4):260-262.
17. Lagging LM, Breland CM, Kennedy DJ, Milligan TW, Sokol-Anderson ML, Westblom TU. Delayed treatment of pulmonary blastomycosis causing vertebral osteomyelitis, paraspinal abscess, and spinal cord compression. Scand J Infect Dis. 1994;26(1):111-115.
18. MacDonald PB, Black GB, MacKenzie R. Orthopaedic manifestations of blastomycosis. J Bone Joint Surg Am. 1990;72(6):860-864.
19. Mahiquez M, Bunton KL, Carney G, Weinstein MA, Small JM. Nonsurgical treatment of lumbosacral blastomycosis involving L2–S1: a case report. Spine. 2008;33(13):E442-E446.
20. McKinnell JA, Pappas PG. Blastomycosis: new insights into diagnosis, prevention, and treatment. Clin Chest Med. 2009;30(2):227-239.
21. Moore RM, Green NE. Blastomycosis of bone. A report of six cases. J Bone Joint Surg Am. 1982;64(7):1097-1101.
22. Muñiz AE, Evans T. Chronic paronychia, osteomyelitis, and paravertebral abscess in a child with blastomycosis. J Emerg Med. 2000;19(3):245-248.
23. Osmond JD, Schweitzer G, Dunbar JM, Villet W. Blastomycosis of the spine with paraplegia. S Afr Med J. 1971;45(16):431-434.
24. Parr AM, Fewer D. Intramedullary blastomycosis in a child: case report. Can J Neurol Sci. 2004;31(2):282-285.
25. Rein MF, Fischetti JL, Sande MA. Osteomyelitis caused by concurrent infection with Mycobacterium tuberculosis and Blastomyces dermatitidis. Am Rev Respir Dis. 1974;109(2):286-289.
26. Saccente M, Abernathy RS, Pappas PG, Shah HR, Bradsher RW. Vertebral blastomycosis with paravertebral abscess: report of eight cases and review of the literature. Clin Infect Dis. 1998;26(2):413-418.
27. Titrud LA. Blastomycosis of the cervical spine. Minn Med. 1975;58(10):729-732.
28. Vandepitte J, Gatti F. A case of North American blastomycosis in Africa. Its existence in Republic of Zaire. Ann Soc Belg Med Trop. 1972;52(4):467-479.
29. Voris HC, Greenwood RC. Blastomycosis of the spine with invasion of the spinal canal. Proc Inst Med Chic. 1947;16(17):463.
30. Witorsch P, Utz JP. North American blastomycosis: a study of 40 patients. Medicine. 1968;47(3):169-200.
31. Lucio E, Adesokan A, Hadjipavlou AG, Crow WN, Adegboyega PA. Pyogenic spondylodiskitis: a radiologic/pathologic and culture correlation study. Arch Pathol Lab Med. 2000;124(5):712-716.
Blastomycosis is a rare fungal infection that primarily produces acute lung infections but may on occasion disseminate to multiple sites, including the skin, bone, central nervous system (CNS), and oropharynx.1-30 In the case of a primary infection of the lung, if there is a high index of suspicion and a thorough diagnostic workup, the diagnosis can be made from sputum or bronchoscopy.24 Patients present with acute pneumonia that either resolves spontaneously or proceeds to chronic pneumonia with extrapulmonary spread to multiple organs, including the spine. Once vertebral involvement occurs, an untreated infection may result in vertebral body destruction and paraspinal and epidural abscess formation followed by neurologic injury and loss of structural integrity of the spine.11,13,17,23,27,29
In this article, we present a case of blastomycosis of the vertebral body and provide a detailed review of the literature concerning this extremely rare infection of the spine. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old African American man with known pulmonary blastomycosis, for which he had been treated with oral itraconazole 200 mg twice daily for 6 months, was admitted to the hospital with a 2-month history of mild thoracolumbar back pain. He reported transient numbness and tingling in the lower extremities but no weakness. He denied weight loss, fatigue, appetite loss, and significant night pain. On physical examination, he was alert and oriented, well nourished, and in no acute distress. Percussion revealed limited range of motion and pain. Further examination of the spine demonstrated no spasm, swelling, erythema, or drainage. The lower extremities had intact sensation, motor strength, reflexes, and pulses, and clonus was absent. White blood cell count was 8100 cells/μL (normal), erythrocyte sedimentation rate was 77 mm/h (normal range, 0-20 mm/h), and C-reactive protein level was 57.2 mg/L (normal, ≤ 10 mg/L). The patient was HIV-negative. Chest radiographs were normal except for a small pleural effusion. Radiographs showed a destructive lesion of T11 with an extensive paravertebral and retropleural abscess tracking a spinal level above and below with extension into the spinal canal (Figure 1).
As the patient had signs of spinal cord compression, he was taken to surgery for incision and drainage and culture procurement and corpectomy of T11 with autogenous rib graft. One week later, he was stabilized with posterior fusion and instrumentation (Figure 2). Gram stain of the specimen demonstrated broad-based budding yeast forms 15 to 20 micrometers in size, consistent with blastomycosis. Cultures were positive for Blastomyces dermatitidis. Histopathologic slides (Figure 3) of the surgical pathology specimen showed granulomatous inflammation. Oral itraconazole 200 mg twice daily was continued, as it has been found to be efficacious in treating immunocompetent patients with blastomycosis17 and is considered the medication of choice for non–life-threatening, non-CNS blastomycosis. (Intravenous amphotericin B was ruled out because of its known serious side effects, such as bone marrow suppression and renal function impairment10; itraconazole was the better alternative.) The patient was placed in a thoracolumbar orthosis and discharged. As the effect of presence of instrumentation in the setting of a fungal infection is unknown, it was deemed prudent to maintain the patient on chronic antifungal suppression. One year after surgery, computed tomography (CT) showed solid osseous bridging through the cage crossing the T11 vertebral body, from the inferior endplate of T10 through the superior endplate of T12 (Figure 4). In addition, there had been no recurrence of the spinal infection, and the patient was neurologically intact and doing well.
Discussion
North American blastomycosis (B dermatitidis) is a ubiquitous dimorphic fungus that occurs worldwide and on occasion causes serious infections in humans.9,23,26,29 It was first characterized in 1894 by Gilchrist and Stokes (Gilchrist disease) when they recovered the fungus from the lung tissue of a patient.3 In North America, blastomycosis infections occur from central Canada to the Gulf Coast to east of the Mississippi River.2,5,7,8,13,14,17,21,22,24,27,29 Additional cases of the disease have been reported in Africa,9,16,23,28 Asia,12,19 and South America7,8 (Table [on pages E270-E271]). Recent epidemiologic studies have linked transmission of the disease to bodies of water and have questioned previous reports of male predominance and racial preference for African Americans (Table).
Blastomycosis is acquired when inhaled fungus (airborne conidia spores) causes a primary pulmonary infection or, rarely, when there is direct inoculation through the skin. The differential diagnosis includes neoplasm, tuberculosis, actinomycosis, bacterial infections, cryptococcosis, and coccidioidomycosis.3,9,12,20,25,31 Blastomycosis occurs in adults and children.1-30 The rate of mortality is much higher in immunocompromised patients. Initial symptoms include fever, chills, fatigue, malaise, myalgia, arthalgia, weight loss, and stigmata of chronic disease.1-30 Acute pulmonary infection with blastomycosis generally resolves spontaneously but may progress to acute respiratory distress syndrome, which has a mortality rate of 50% to 89%.19 With systemic dissemination, the infection may spread to other organs11—there is a particular predilection for the skin9,20,29—and to the long bones7,16 and the oropharynx.16,26,28
In 50% to 64% of cases, bone involvement may be the first disease manifestation.6,7,16,22 Osseous involvement with blastomycosis most commonly affects the long bones15 but may include the vertebrae,1-29 the ribs,26 and the carpal or tarsal bones.7,16 The most common vertebral involvement occurs in the thoracic or lumbar spine1,2,7-9,11-14,17,19,21-24,26 and typically results in destruction of the body, development of a paraspinal abscess, and potential extension into the spinal canal, causing an epidural abscess and development of chronic draining cutaneous sinuses.2,7,9,11-13,16,17,19,22,23,26,28,29 In the present case, we do not know whether the vertebral body was involved before the patient presented with mid-thoracolumbar back pain. There may have been bony involvement during initial presentation.
Diagnosis is often difficult because of a low index of suspicion, leading to a significant delay in treatment. Primary pulmonary infections are successfully diagnosed 86% of the time from sputum and 92% of the time from bronchoscopy.19 Once the infection involves the spine, plain radiographs, CT, and magnetic resonance imaging (MRI) can be used to identify not only the bony involvement but also any adjacent soft-tissue extension.13 The radiographic findings, typical of tuberculosis or a neoplasm, include disc space narrowing, vertebral body destruction and collapse, late segmental kyphotic deformity, and development of a psoas abscess or a retropleural abscess.7,26 Such abscesses lend themselves well to fine-needle aspiration,7,8,11,13,14,17,19,26 which, when combined with CT and MRI guidance, reliably assists in the diagnosis of blastomycosis.1,13,17 If fine-needle aspiration fails, then open biopsy and surgical débridement specimens may be effective in the diagnosis.2,9,12,21,22,27
The mortality rate for systemic blastomycosis exceeded 90% before the development of antifungal medications, and these medications remain the primary treatment for most initial infections.15 For severe infections in critically ill patients and for patients with CNS involvement, amphotericin B has been effective, with cure rates approaching 97%.17 Itraconazole, which is well tolerated, has replaced ketoconazole as the preferred long-term oral treatment for blastomycosis. Cure rates for itraconazole approach 90% when treatment is instituted over 2 years in a compliant patient.10,19,20 Nonsurgical (antifungal) treatment for blastomycosis of the spine has also proved successful in neurologically intact patients.7,9,11,26,28
A case involving the spine and requiring surgical drainage was first reported in 19085; since then, only a few more cases have been reported.1,2,5,7-9,11-14,16,17,19,21-24,26-29 Thus, the literature includes very little information that can be used to establish indications for surgery for a blastomycotic infection of the spine. However, there is enough evidence to establish that surgery is indicated for patients who have a known blastomycosis infection and are developing neurologic or structural loss of integrity of the spinal column or have an abscess that requires drainage and débridement.
Our patient had been on long-term antifungal treatment but nevertheless developed a destructive spinal lesion with a concurrent epidural and retropleural abscess. Given his risk of pathologic fracture, we performed anterior débridement and stabilization followed by posterior fusion and instrumentation. We are unaware of any other cases in which an anterior titanium cage was combined with rib autograft after anterior débridement and vertebrectomy combined with posterior instrumentation for blastomycosis. This technique proved very useful, as it allowed for immediate stabilization of the spine. Therefore, the treatment goal is similar to that for any destructive infection that fails medical treatment: preservation of neurologic function, stabilization of spinal vertebrae, débridement of abscess cavity, and definitive culture procurement.
Conclusion
Although there is little reported information regarding surgical indications for blastomycotic vertebral osteomyelitis that has failed medical management—in patients with a destructive lesion and compromise of both the spinal canal and the integrity of the vertebral column—anterior débridement and stabilization followed by posterior fusion and instrumentation are useful in preventing vertebral collapse, further canal compromise, and possible cord injury.
Blastomycosis is a rare fungal infection that primarily produces acute lung infections but may on occasion disseminate to multiple sites, including the skin, bone, central nervous system (CNS), and oropharynx.1-30 In the case of a primary infection of the lung, if there is a high index of suspicion and a thorough diagnostic workup, the diagnosis can be made from sputum or bronchoscopy.24 Patients present with acute pneumonia that either resolves spontaneously or proceeds to chronic pneumonia with extrapulmonary spread to multiple organs, including the spine. Once vertebral involvement occurs, an untreated infection may result in vertebral body destruction and paraspinal and epidural abscess formation followed by neurologic injury and loss of structural integrity of the spine.11,13,17,23,27,29
In this article, we present a case of blastomycosis of the vertebral body and provide a detailed review of the literature concerning this extremely rare infection of the spine. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old African American man with known pulmonary blastomycosis, for which he had been treated with oral itraconazole 200 mg twice daily for 6 months, was admitted to the hospital with a 2-month history of mild thoracolumbar back pain. He reported transient numbness and tingling in the lower extremities but no weakness. He denied weight loss, fatigue, appetite loss, and significant night pain. On physical examination, he was alert and oriented, well nourished, and in no acute distress. Percussion revealed limited range of motion and pain. Further examination of the spine demonstrated no spasm, swelling, erythema, or drainage. The lower extremities had intact sensation, motor strength, reflexes, and pulses, and clonus was absent. White blood cell count was 8100 cells/μL (normal), erythrocyte sedimentation rate was 77 mm/h (normal range, 0-20 mm/h), and C-reactive protein level was 57.2 mg/L (normal, ≤ 10 mg/L). The patient was HIV-negative. Chest radiographs were normal except for a small pleural effusion. Radiographs showed a destructive lesion of T11 with an extensive paravertebral and retropleural abscess tracking a spinal level above and below with extension into the spinal canal (Figure 1).
As the patient had signs of spinal cord compression, he was taken to surgery for incision and drainage and culture procurement and corpectomy of T11 with autogenous rib graft. One week later, he was stabilized with posterior fusion and instrumentation (Figure 2). Gram stain of the specimen demonstrated broad-based budding yeast forms 15 to 20 micrometers in size, consistent with blastomycosis. Cultures were positive for Blastomyces dermatitidis. Histopathologic slides (Figure 3) of the surgical pathology specimen showed granulomatous inflammation. Oral itraconazole 200 mg twice daily was continued, as it has been found to be efficacious in treating immunocompetent patients with blastomycosis17 and is considered the medication of choice for non–life-threatening, non-CNS blastomycosis. (Intravenous amphotericin B was ruled out because of its known serious side effects, such as bone marrow suppression and renal function impairment10; itraconazole was the better alternative.) The patient was placed in a thoracolumbar orthosis and discharged. As the effect of presence of instrumentation in the setting of a fungal infection is unknown, it was deemed prudent to maintain the patient on chronic antifungal suppression. One year after surgery, computed tomography (CT) showed solid osseous bridging through the cage crossing the T11 vertebral body, from the inferior endplate of T10 through the superior endplate of T12 (Figure 4). In addition, there had been no recurrence of the spinal infection, and the patient was neurologically intact and doing well.
Discussion
North American blastomycosis (B dermatitidis) is a ubiquitous dimorphic fungus that occurs worldwide and on occasion causes serious infections in humans.9,23,26,29 It was first characterized in 1894 by Gilchrist and Stokes (Gilchrist disease) when they recovered the fungus from the lung tissue of a patient.3 In North America, blastomycosis infections occur from central Canada to the Gulf Coast to east of the Mississippi River.2,5,7,8,13,14,17,21,22,24,27,29 Additional cases of the disease have been reported in Africa,9,16,23,28 Asia,12,19 and South America7,8 (Table [on pages E270-E271]). Recent epidemiologic studies have linked transmission of the disease to bodies of water and have questioned previous reports of male predominance and racial preference for African Americans (Table).
Blastomycosis is acquired when inhaled fungus (airborne conidia spores) causes a primary pulmonary infection or, rarely, when there is direct inoculation through the skin. The differential diagnosis includes neoplasm, tuberculosis, actinomycosis, bacterial infections, cryptococcosis, and coccidioidomycosis.3,9,12,20,25,31 Blastomycosis occurs in adults and children.1-30 The rate of mortality is much higher in immunocompromised patients. Initial symptoms include fever, chills, fatigue, malaise, myalgia, arthalgia, weight loss, and stigmata of chronic disease.1-30 Acute pulmonary infection with blastomycosis generally resolves spontaneously but may progress to acute respiratory distress syndrome, which has a mortality rate of 50% to 89%.19 With systemic dissemination, the infection may spread to other organs11—there is a particular predilection for the skin9,20,29—and to the long bones7,16 and the oropharynx.16,26,28
In 50% to 64% of cases, bone involvement may be the first disease manifestation.6,7,16,22 Osseous involvement with blastomycosis most commonly affects the long bones15 but may include the vertebrae,1-29 the ribs,26 and the carpal or tarsal bones.7,16 The most common vertebral involvement occurs in the thoracic or lumbar spine1,2,7-9,11-14,17,19,21-24,26 and typically results in destruction of the body, development of a paraspinal abscess, and potential extension into the spinal canal, causing an epidural abscess and development of chronic draining cutaneous sinuses.2,7,9,11-13,16,17,19,22,23,26,28,29 In the present case, we do not know whether the vertebral body was involved before the patient presented with mid-thoracolumbar back pain. There may have been bony involvement during initial presentation.
Diagnosis is often difficult because of a low index of suspicion, leading to a significant delay in treatment. Primary pulmonary infections are successfully diagnosed 86% of the time from sputum and 92% of the time from bronchoscopy.19 Once the infection involves the spine, plain radiographs, CT, and magnetic resonance imaging (MRI) can be used to identify not only the bony involvement but also any adjacent soft-tissue extension.13 The radiographic findings, typical of tuberculosis or a neoplasm, include disc space narrowing, vertebral body destruction and collapse, late segmental kyphotic deformity, and development of a psoas abscess or a retropleural abscess.7,26 Such abscesses lend themselves well to fine-needle aspiration,7,8,11,13,14,17,19,26 which, when combined with CT and MRI guidance, reliably assists in the diagnosis of blastomycosis.1,13,17 If fine-needle aspiration fails, then open biopsy and surgical débridement specimens may be effective in the diagnosis.2,9,12,21,22,27
The mortality rate for systemic blastomycosis exceeded 90% before the development of antifungal medications, and these medications remain the primary treatment for most initial infections.15 For severe infections in critically ill patients and for patients with CNS involvement, amphotericin B has been effective, with cure rates approaching 97%.17 Itraconazole, which is well tolerated, has replaced ketoconazole as the preferred long-term oral treatment for blastomycosis. Cure rates for itraconazole approach 90% when treatment is instituted over 2 years in a compliant patient.10,19,20 Nonsurgical (antifungal) treatment for blastomycosis of the spine has also proved successful in neurologically intact patients.7,9,11,26,28
A case involving the spine and requiring surgical drainage was first reported in 19085; since then, only a few more cases have been reported.1,2,5,7-9,11-14,16,17,19,21-24,26-29 Thus, the literature includes very little information that can be used to establish indications for surgery for a blastomycotic infection of the spine. However, there is enough evidence to establish that surgery is indicated for patients who have a known blastomycosis infection and are developing neurologic or structural loss of integrity of the spinal column or have an abscess that requires drainage and débridement.
Our patient had been on long-term antifungal treatment but nevertheless developed a destructive spinal lesion with a concurrent epidural and retropleural abscess. Given his risk of pathologic fracture, we performed anterior débridement and stabilization followed by posterior fusion and instrumentation. We are unaware of any other cases in which an anterior titanium cage was combined with rib autograft after anterior débridement and vertebrectomy combined with posterior instrumentation for blastomycosis. This technique proved very useful, as it allowed for immediate stabilization of the spine. Therefore, the treatment goal is similar to that for any destructive infection that fails medical treatment: preservation of neurologic function, stabilization of spinal vertebrae, débridement of abscess cavity, and definitive culture procurement.
Conclusion
Although there is little reported information regarding surgical indications for blastomycotic vertebral osteomyelitis that has failed medical management—in patients with a destructive lesion and compromise of both the spinal canal and the integrity of the vertebral column—anterior débridement and stabilization followed by posterior fusion and instrumentation are useful in preventing vertebral collapse, further canal compromise, and possible cord injury.
1. Akhtar I, Flowers R, Siddiqi A, Heard K, Baliga M. Fine needle aspiration biopsy of vertebral and paravertebral lesions: retrospective study of 124 cases [published correction appears in Acta Cytol. 2006;50(5):600]. Acta Cytol. 2006;50(4):364-371.
2. Arvin MC, Gehring RL, Crecelius JL, Curfman MF. Man with progressive lower back pain. Indiana Med. 1991;84(8):554-556.
3. Baylin GJ, Wear JM. Blastomycosis and actinomycosis of the spine. Am J Roentgenol Radium Ther Nucl Med. 1953;69(3):395-398.
4. Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17(1):21-40.
5. Brewer GE, Wood FC. XII. Blastomycosis of the spine: double lesion: two operations: recovery. Ann Surg. 1908;48(6):889-896.
6. Carman WF, Frean JA, Crewe-Brown HH, Culligan GA, Young CN. Blastomycosis in Africa. A review of known cases diagnosed between 1951 and 1987. Mycopathologica. 1989;107(1):25-32.
7. Challapalli M, Cunningham DG. North American blastomycosis of the vertebrae in an adolescent. Clin Infect Dis. 1996;23(4):853-854.
8. Detrisac DA, Harding WG, Greiner AL, Dunn CR, Mayfield FH. Vertebral North American blastomycosis. Surg Neurol. 1980;13(4):311-312.
9. Frean J, Blumberg L, Woolf M. Disseminated blastomycosis masquerading as tuberculosis. J Infect. 1993;26(2):203-206.
10. Goodman LS, Brunton LL, Chabner B, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill Medical; 2011.
11. Gottlieb JR, Eismont FJ. Nonoperative treatment of vertebral blastomycosis osteomyelitis associated with paraspinal abscess and cord compression. A case report. J Bone Joint Surg Am. 2006;88(4):854-856.
12. Güler N, Palanduz A, Ones U, et al. Progressive vertebral blastomycosis mimicking tuberculosis. Pediatr Infect Dis J. 1995;14(9):816-818.
13. Hadjipavlou AG, Mader JT, Nauta HJ, Necessary JT, Chaljub G, Adesokan A. Blastomycosis of the lumbar spine: case report and review of the literature, with emphasis on diagnostic laboratory tools and management. Eur Spine J. 1998;7(5):416-421.
14. Hardjasudarma M, Willis B, Black-Payne C, Edwards R. Pediatric spinal blastomycosis: case report. Neurosurgery. 1995;37(3):534-536.
15. Jahangir AA, Heck RK. Blastomycosis: case report of an isolated lesion in the distal fibula. Am J Orthop. 2010;39(3):E22-E24.
16. Koen AF, Blumberg LH. North American blastomycosis in South Africa simulating tuberculosis. Clin Radiol. 1999;54(4):260-262.
17. Lagging LM, Breland CM, Kennedy DJ, Milligan TW, Sokol-Anderson ML, Westblom TU. Delayed treatment of pulmonary blastomycosis causing vertebral osteomyelitis, paraspinal abscess, and spinal cord compression. Scand J Infect Dis. 1994;26(1):111-115.
18. MacDonald PB, Black GB, MacKenzie R. Orthopaedic manifestations of blastomycosis. J Bone Joint Surg Am. 1990;72(6):860-864.
19. Mahiquez M, Bunton KL, Carney G, Weinstein MA, Small JM. Nonsurgical treatment of lumbosacral blastomycosis involving L2–S1: a case report. Spine. 2008;33(13):E442-E446.
20. McKinnell JA, Pappas PG. Blastomycosis: new insights into diagnosis, prevention, and treatment. Clin Chest Med. 2009;30(2):227-239.
21. Moore RM, Green NE. Blastomycosis of bone. A report of six cases. J Bone Joint Surg Am. 1982;64(7):1097-1101.
22. Muñiz AE, Evans T. Chronic paronychia, osteomyelitis, and paravertebral abscess in a child with blastomycosis. J Emerg Med. 2000;19(3):245-248.
23. Osmond JD, Schweitzer G, Dunbar JM, Villet W. Blastomycosis of the spine with paraplegia. S Afr Med J. 1971;45(16):431-434.
24. Parr AM, Fewer D. Intramedullary blastomycosis in a child: case report. Can J Neurol Sci. 2004;31(2):282-285.
25. Rein MF, Fischetti JL, Sande MA. Osteomyelitis caused by concurrent infection with Mycobacterium tuberculosis and Blastomyces dermatitidis. Am Rev Respir Dis. 1974;109(2):286-289.
26. Saccente M, Abernathy RS, Pappas PG, Shah HR, Bradsher RW. Vertebral blastomycosis with paravertebral abscess: report of eight cases and review of the literature. Clin Infect Dis. 1998;26(2):413-418.
27. Titrud LA. Blastomycosis of the cervical spine. Minn Med. 1975;58(10):729-732.
28. Vandepitte J, Gatti F. A case of North American blastomycosis in Africa. Its existence in Republic of Zaire. Ann Soc Belg Med Trop. 1972;52(4):467-479.
29. Voris HC, Greenwood RC. Blastomycosis of the spine with invasion of the spinal canal. Proc Inst Med Chic. 1947;16(17):463.
30. Witorsch P, Utz JP. North American blastomycosis: a study of 40 patients. Medicine. 1968;47(3):169-200.
31. Lucio E, Adesokan A, Hadjipavlou AG, Crow WN, Adegboyega PA. Pyogenic spondylodiskitis: a radiologic/pathologic and culture correlation study. Arch Pathol Lab Med. 2000;124(5):712-716.
1. Akhtar I, Flowers R, Siddiqi A, Heard K, Baliga M. Fine needle aspiration biopsy of vertebral and paravertebral lesions: retrospective study of 124 cases [published correction appears in Acta Cytol. 2006;50(5):600]. Acta Cytol. 2006;50(4):364-371.
2. Arvin MC, Gehring RL, Crecelius JL, Curfman MF. Man with progressive lower back pain. Indiana Med. 1991;84(8):554-556.
3. Baylin GJ, Wear JM. Blastomycosis and actinomycosis of the spine. Am J Roentgenol Radium Ther Nucl Med. 1953;69(3):395-398.
4. Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17(1):21-40.
5. Brewer GE, Wood FC. XII. Blastomycosis of the spine: double lesion: two operations: recovery. Ann Surg. 1908;48(6):889-896.
6. Carman WF, Frean JA, Crewe-Brown HH, Culligan GA, Young CN. Blastomycosis in Africa. A review of known cases diagnosed between 1951 and 1987. Mycopathologica. 1989;107(1):25-32.
7. Challapalli M, Cunningham DG. North American blastomycosis of the vertebrae in an adolescent. Clin Infect Dis. 1996;23(4):853-854.
8. Detrisac DA, Harding WG, Greiner AL, Dunn CR, Mayfield FH. Vertebral North American blastomycosis. Surg Neurol. 1980;13(4):311-312.
9. Frean J, Blumberg L, Woolf M. Disseminated blastomycosis masquerading as tuberculosis. J Infect. 1993;26(2):203-206.
10. Goodman LS, Brunton LL, Chabner B, Knollman BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill Medical; 2011.
11. Gottlieb JR, Eismont FJ. Nonoperative treatment of vertebral blastomycosis osteomyelitis associated with paraspinal abscess and cord compression. A case report. J Bone Joint Surg Am. 2006;88(4):854-856.
12. Güler N, Palanduz A, Ones U, et al. Progressive vertebral blastomycosis mimicking tuberculosis. Pediatr Infect Dis J. 1995;14(9):816-818.
13. Hadjipavlou AG, Mader JT, Nauta HJ, Necessary JT, Chaljub G, Adesokan A. Blastomycosis of the lumbar spine: case report and review of the literature, with emphasis on diagnostic laboratory tools and management. Eur Spine J. 1998;7(5):416-421.
14. Hardjasudarma M, Willis B, Black-Payne C, Edwards R. Pediatric spinal blastomycosis: case report. Neurosurgery. 1995;37(3):534-536.
15. Jahangir AA, Heck RK. Blastomycosis: case report of an isolated lesion in the distal fibula. Am J Orthop. 2010;39(3):E22-E24.
16. Koen AF, Blumberg LH. North American blastomycosis in South Africa simulating tuberculosis. Clin Radiol. 1999;54(4):260-262.
17. Lagging LM, Breland CM, Kennedy DJ, Milligan TW, Sokol-Anderson ML, Westblom TU. Delayed treatment of pulmonary blastomycosis causing vertebral osteomyelitis, paraspinal abscess, and spinal cord compression. Scand J Infect Dis. 1994;26(1):111-115.
18. MacDonald PB, Black GB, MacKenzie R. Orthopaedic manifestations of blastomycosis. J Bone Joint Surg Am. 1990;72(6):860-864.
19. Mahiquez M, Bunton KL, Carney G, Weinstein MA, Small JM. Nonsurgical treatment of lumbosacral blastomycosis involving L2–S1: a case report. Spine. 2008;33(13):E442-E446.
20. McKinnell JA, Pappas PG. Blastomycosis: new insights into diagnosis, prevention, and treatment. Clin Chest Med. 2009;30(2):227-239.
21. Moore RM, Green NE. Blastomycosis of bone. A report of six cases. J Bone Joint Surg Am. 1982;64(7):1097-1101.
22. Muñiz AE, Evans T. Chronic paronychia, osteomyelitis, and paravertebral abscess in a child with blastomycosis. J Emerg Med. 2000;19(3):245-248.
23. Osmond JD, Schweitzer G, Dunbar JM, Villet W. Blastomycosis of the spine with paraplegia. S Afr Med J. 1971;45(16):431-434.
24. Parr AM, Fewer D. Intramedullary blastomycosis in a child: case report. Can J Neurol Sci. 2004;31(2):282-285.
25. Rein MF, Fischetti JL, Sande MA. Osteomyelitis caused by concurrent infection with Mycobacterium tuberculosis and Blastomyces dermatitidis. Am Rev Respir Dis. 1974;109(2):286-289.
26. Saccente M, Abernathy RS, Pappas PG, Shah HR, Bradsher RW. Vertebral blastomycosis with paravertebral abscess: report of eight cases and review of the literature. Clin Infect Dis. 1998;26(2):413-418.
27. Titrud LA. Blastomycosis of the cervical spine. Minn Med. 1975;58(10):729-732.
28. Vandepitte J, Gatti F. A case of North American blastomycosis in Africa. Its existence in Republic of Zaire. Ann Soc Belg Med Trop. 1972;52(4):467-479.
29. Voris HC, Greenwood RC. Blastomycosis of the spine with invasion of the spinal canal. Proc Inst Med Chic. 1947;16(17):463.
30. Witorsch P, Utz JP. North American blastomycosis: a study of 40 patients. Medicine. 1968;47(3):169-200.
31. Lucio E, Adesokan A, Hadjipavlou AG, Crow WN, Adegboyega PA. Pyogenic spondylodiskitis: a radiologic/pathologic and culture correlation study. Arch Pathol Lab Med. 2000;124(5):712-716.