User login
Consequences of Missed Opportunities
A 58‐year‐old man was evaluated for 3 weeks of leg numbness and weakness. His symptoms began with numbness and tingling in the distal left leg that progressed to weakness that impaired his ability to walk. He had no history of trauma or incontinence but endorsed several months of back pain that worsened when lying flat. He had a history of type 2 diabetes mellitus, hepatitis C infection, hypertension, and posttraumatic stress disorder. He had a remote history of intravenous drug use and had quit tobacco 9 years earlier. Medications he was taking included hydrochlorothiazide, rosiglitazone, oxycodone/acetaminophen, baclofen, ibuprofen, and gabapentin.
Internists see this constellation of complaints frequently in an acute care setting. Finding a unifying diagnosis may be difficult initially, so thinking of the symptoms in series is helpful. The complaint of leg weakness and the pattern of numbness should be further elucidated. Is this true weakness, or is it a feeling of instability because of foot numbness? What is the pattern of the numbness? Peripheral neuropathy typically begins in a symmetric stocking pattern (involving the plantar surface of the feet), then progresses to a glove distribution (involving the hands from the fingers distally to the wrist proximally). Such a pattern in a patient with diabetes would be consistent with distal polyneuropathy, a mixed sensory and motor process. Other possible causes of peripheral neuropathy in this patient include HIV, B12 deficiency, and syphilis. These symptoms could be tied to the back pain if this were intervertebral disk disease, a compression fracture, or a lytic lesion in the vertebrae with resulting nerve impingement or if it were epidural spinal cord compression. The lack of bowel or bladder dysfunction speaks against a cauda equina syndrome but does not rule out more cepahalad spinal pathology.
On neurological examination, I would concentrate on differentiating weakness from pain. I would attempt to determine whether the weakness was of central or peripheral nerve etiology. Helpful findings would include increased tone with upper motor lesions and flaccid tone with lower motor lesions, hyperreflexia with upper motor lesions and hyporeflexia with lower motor lesions, a Babinski sign, muscle atrophy or fasciculations, and gait. A rectal examination would also be helpful to assess for deficits in rectal tone, wink reflex, or saddle anesthesia.
All patients with low back pain who have alarm signs of age older than 50, pain duration of more than 1 month, known cancer, lack of relief with conservative measures, or systemic B symptoms should have imaging of the spine. Although plain films may reveal bony abnormalities, computed tomography (CT) is better for evaluating osseous structures and magnetic resonance imaging (MRI) for evaluating pathology in patients suspected of having an infection or a malignancy. I would obtain imaging of the spine in this patient.
The patient was receiving care at an outside clinic for 2 liver lesions discovered on abdominal ultrasound 19 months prior to admission. CT showed that the lesions were 4.0 and 2.3 cm in diameter 17 months prior to admission and 5.0 and 3.0 cm in diameter 5 months prior to admission. No cirrhosis was appreciated on the ultrasound or CT. The patient was referred for CT‐guided biopsy of the larger mass after the second CT, but he became anxious and left before the biopsy was obtained.
This piece of the history is ominous, as it increases the possibility of cancer in our differential. Metastatic disease could provide a unifying diagnosis, explaining the constellation of back pain, leg weakness, and liver lesions. Lung cancer commonly metastasizes to the liver and to bone, so I would obtain a chest x‐ray. Other possible types of cancer in this situation include cancer of the prostate, colon, or thyroid and melanoma. In this patient, who has hepatitis C, hepatocellular carcinoma (HCC) could be the primary etiology, although cirrhosis was not seen on CT and HCC metastasizes to the spine less commonly than do other primary cancers (eg, lung, breast, prostate). Nonetheless, I would obtain an alpha‐fetoprotein level, which would confirm HCC in a patient with liver lesions if it was greater than 200 g/L. Pancreatic cancer has been associated with both type 2 diabetes and liver lesions and could explain his abdominal pain.
There is no comment on the arterial‐phase CT imaging of the liver lesions. Dual‐phase CT scans examine the hepatic arterial and portal vein phases of contrast filling. Triple‐phase CT scans also examine the portal vein influx phase. Both hemangiomas and hypervascular HCCs enhance on the arterial phase, as they derive their blood flow from the hepatic artery. Therefore, arterial‐phase imaging can help to distinguish vascular tumors that flush with contrast, such as hemangiomas, melanoma, and HCC, from less vascular tumors such as pancreatic and colon cancer. Other liver lesions such as focal nodular hyperplasia and adenomas cannot be excluded in this situation as they also may enhance during the arterial phase and can grow over time, as this patient's repeat imaging documented. It seems unlikely that this patient has a liver abscess because he has a paucity of constitutional symptoms and no travel history. The liver lesions seen on initial imaging were larger than 1.0 cm, so I would have favored an earlier biopsy to obtain a tissue diagnosis.
The patient was afebrile, and all other vital signs were normal. He appeared well nourished and anicteric. There was no lymphadenopathy. Cardiac auscultation was regular without murmurs. The lungs were clear. The abdomen was without fluid wave or hepatosplenomegaly and was tender to palpation in the right upper and lower quadrants. There was no midline tenderness to palpation of the spine.
Cranial nerves II‐XII were intact. Lower extremity muscle tone could not be accurately assessed due to splinting from back pain. Strength was 3 of 5 in the left hip extensors and left knee flexors and extensors, and 1 of 5 in the left hip flexors. He had no motor strength in the distal left lower extremity extensors. Bilateral upper extremity and right leg strength were normal. Sensation to light touch, temperature, and pain was decreased circumferentially below the xiphoid. The patient had hyperesthesia in a band around the thorax just above the xiphoid and paresthesia of the perineal area. Left patellar tendon reflexes were brisk, and left ankle jerk was absent, but other reflexes were normal. Toes were down‐going bilaterally. The anal wink was absent, and rectal tone was decreased. Results of the cerebellar exam were normal. Gait could not be assessed.
The results of the exam are notable for not showing the stigmata of end‐stage liver disease. The results of the neurological exam are concerning, with decreased sensation at approximately the T7 level that is almost certainly a result of epidural compression of the spinal cord. Hematogenous metastasis to the vertebrae from one of the tumors mentioned above, with spread into the thecal sac, is the most likely culprit. An epidural abscess is possible because the patient has diabetes and a history of injection drug use.
The thoracic spine is involved in 60% of spinal cord metastases. This patient's left‐sided distal leg weakness is consistent with having corticospinal tract compression and indicates thoracic spine involvement. Flaccid paralysis is classically found in lower motor neuron weakness, but is also seen in the early stages of upper motor neuron pathology. Lesions found above the cauda equina often spare the perineal area, but low thoracic lesions involving the conus medullaris (from T10 to L1) could explain both his loss of anal wink and his decreased rectal tone.
This patient's presentation is unfortunately classic for epidural spinal cord compression. Because the onset of compression is insidious, the diagnosis is often delayed, even in patients with known cancer. Urgent imaging is imperative to evaluate this possibility, as having any meaningful chance of recovery of function depends on rapid relief of the spinal cord compression. I would obtain an emergent MRI of the thoracic and lumbosacral spine.
Laboratory studies showed the following: hemoglobin, 13.1 g/dL; mean corpuscular volume, 80 m3; platelet count, 149,000/L; creatinine, 1.9 mg/dL; aspartate aminotransferase, 66 U/L (5‐35 U/L); alanine aminotransferase, 66 U/L (7‐56 U/L); alkaline phosphatase, 87 U/L (40‐125 U/L); total bilirubin, 1.3 mg/dL; prostate specific antigen (PSA), 1.6 g/dL; and alpha‐fetoprotein (AFP), 10.3 g/L. White cell count, sodium, glucose, calcium, and albumin levels, and prothrombin and partial‐thromboplastin times were within normal ranges.
His liver function tests likely reflect chronic hepatitis C infection. His renal insufficiency could be a result of hypertension, diabetes, or dehydration given that he has been bed‐bound.
Most intriguing are the normal PSA level and only slightly elevated AFP level. PSA is useful for detecting recurrence of prostate cancer or following response of therapy, but the utility of PSA as a screening tool remains controversial in part because of its low specificity. Prostate cancer is the most commonly diagnosed cancer among men and cannot be ruled out by a normal PSA. In a patient with hepatitis C, cirrhosis (which we have not conclusively diagnosed), and a radiologically suspicious liver lesion, an AFP > 200 g/L would be diagnostic of HCC. In this case, however, mildly elevated AFP does not help us to either diagnose or exclude HCC.
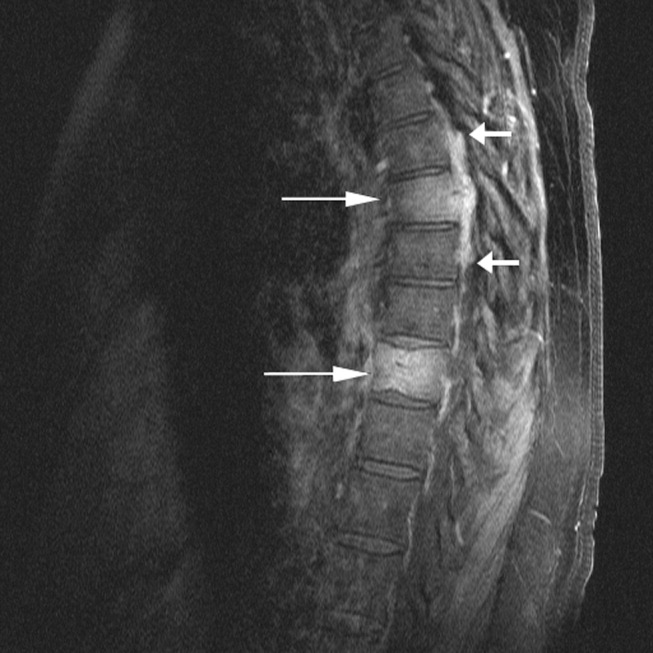
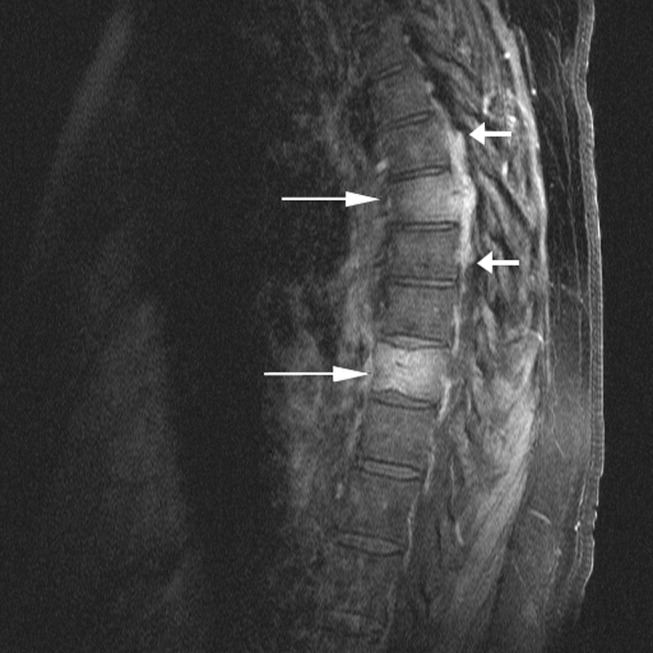
The chest x‐ray showed no abnormalities. MRI of the spine revealed lytic lesions in the T7‐T10 vertebral bodies with spinal cord compression at the T7 level (Fig. 1).
A repeat CT scan of the abdomen showed a coarse, nodular liver with 2 heterogeneous, early‐enhancing masses (4.7 4.2 and 3.4 2.4 cm in diameter) with surrounding satellite lesions (Fig. 2).
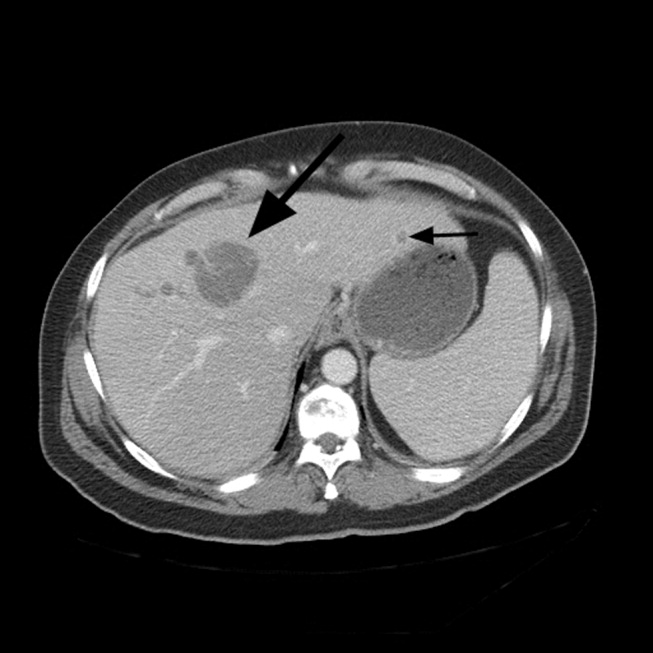
The enhancement pattern on dual‐phase liver protocol CT was not characteristic of HCC. The left portal vein was not visualized. Splenomegaly and esophageal varices were observed. The adrenal glands showed bilateral, heterogeneous enhancing masses. The epiphrenic, retroperitoneal, and periportal lymph nodes were enlarged. Lytic lesions were seen in the sacrum, left iliac wing, and T7‐T10 vertebral bodies.
Intravenous high‐dose steroids were started. The neurosurgery team advised that no surgical interventions were appropriate because of the patient's poor functional status and the extent of his disease.
It is unfortunate that no neurosurgical interventions could help this patient, especially because we are not yet sure of the final diagnosis. Standard indications for neurosurgical decompression include compression from bone fragments, spinal instability requiring fixation, and lack of response to radiation therapy. Patients must also be able to tolerate surgery. Although evidence supports the use of corticosteroids in reducing edema, inflammation, and neurological deficits in malignant spinal cord compression, there is not consensus on what the optimal dose is. Doses of 16‐100 mg of dexamethasone per day appear to be beneficial, as long as higher doses are rapidly tapered to avoid toxic effects. High‐dose steroids minimize the initial edema but are unlikely to change the long‐term outcome of patients who are nonambulatory on arrival.
The CT scan does not help us distinguish between metastatic cancer and primary HCC. Adrenal metastases are very uncommon in HCC. Lung cancer, however, metastasizes to the liver, adrenal glands, and spine, even without significant pulmonary symptoms. HCC may be seen on CT as a solitary mass, a dominant mass with surrounding satellite lesions, multifocal lesions, or a diffusely infiltrating tumor. This diagnosis now seems more likely given the finding of cirrhosis, which increases the risk of HCC in individuals with hepatitis C infection.
We need to obtain tissue for diagnosis and prognosis and to guide therapy. I would consult with radiology and gastroenterology colleagues about the best location to biopsy, but a bone biopsy should be avoided because the pathologic yield is lower.
The radiology and gastroenterology consultants recommended adrenal biopsy because there was easier posterior access for tissue. A liver biopsy was avoided because of the risk of bleeding with hypervascular masses. Fine‐needle aspiration of the mass in the right adrenal gland was performed. The pathology demonstrated bile production and hexagonal arrangement of cells with endothelial cuffing consistent with hepatocellular carcinoma. The oncology staff was consulted about palliative chemotherapy options. The patient began radiation therapy directed at the T7 lesion compressing the spinal cord. He regained minimal movement of his foot. After discussing treatment options with the oncology staff, the patient declined chemotherapy and was transitioned to hospice, where he died 3 weeks later.
COMMENTARY
Hepatocellular carcinoma (HCC) is the third‐leading cause of cancer death and the fifth‐leading cause of cancer worldwide. It causes nearly 1 million deaths annually, and unlike many other cancers, its incidence and mortality rate are rising. Most cases of HCC in Africa and Asia are a result of chronic hepatitis B infection, but in the United States HCC is primarily attributable to hepatitis C infection.1 The annual incidence of HCC in the U.S. population, now about 4 cases per 100,000 people,2 is rising because of the increased prevalence of hepatitis C. Other causes of HCC, such as alcoholic liver disease, hepatitis B infection, and hemochromatosis, have remained stable and have not contributed as significantly to the rising incidence of HCC. For the individual patient, hepatitis C infection conveys a 20‐fold increase in the risk for HCC (2%‐8% risk/year).1 Eighty percent of cases of HCC develop in patients with cirrhosis.3 Unlike patients with hepatitis B infection, persons chronically infected with hepatitis C rarely develop HCC unless they have cirrhosis.
The American Association for the Study of Liver Disease recommends that hepatitis Binfected individuals at high risk for HCC (eg, men older than 40 years and persons with cirrhosis or a family history of HCC) and hepatitis Cinfected individuals with cirrhosis4 be periodically screened for HCC with alpha‐fetoprotein (AFP) and ultrasonography (every 6 months to approximate the doubling time of the tumor5). Using the most commonly reported cutoff for a positive test result for hepatocellular carcinoma (AFP level > 20 g/L) resulted in the following test characteristics: sensitivity, 41%‐65%; specificity, 80%‐94%; positive likelihood ratio, 3.1‐6.8; and negative likelihood ratio, 0.4‐0.6.6 AFP alone is therefore a poor screening test for HCC, and as shown in this case, AFP levels can be normal or only minimally elevated in the setting of diffusely metastatic disease. Ultrasonography alone is only 35%‐87% sensitive in detecting HCC,79 but the combination of AFP and ultrasonography identified 100% of the HCC cases in one small case series.10
For the patient in this case, the optimal clinical pathway would have been to transition from screening to diagnostic measures in a timely manner. Consensus guidelines from the European Association for Study of the Liver in 2001 recommend biopsy of all focal liver lesions that are between 1 and 2 cm.11 The American Association for the Study of Liver Diseases (AASLD) recommends that focal liver lesions between 1 and 2 cm found on ultrasound in cirrhotic livers be followed by 2 dynamic studies: CT, MRI, or contrast ultrasound. If 2 separate studies reveal typical characteristics of HCC, then the lesion should be treated as HCC, and if not typical, then the lesion should be biopsied.4 Although no studies were available to support the recommendations, both the EASL and AASLD advise that lesions greater than 2 cm with demonstrated vascularity on both ultrasonography and CT can be diagnosed as HCC without biopsy and that lesions smaller than 1 cm be monitored.4, 11
Hepatocellular carcinoma can metastasize to almost anywhere in the body by hematologic or lymphatic spread or by direct extension. The most common site for metastases of HCC is the lung. Metastases to the lung arise primarily from arterial emboli and therefore are most common in the lower lobes, where there is greater perfusion.12 The second most common site is intraabdominal lymph nodes. The axial skeleton is the third most common site of metastases and, as in this case, primarily involves the spine.13 Other sites of metastases include the peritoneum, the inferior vena cava and right atrium by direct extension, and, less commonly, the gallbladder and spleen. Autopsy studies of patients with HCC found that 8% had metastases to the adrenal glands, as did this patient.13 Metastasis to the central nervous system is rare.
There were several challenging aspects of this case, including atypical radiologic appearance, an unusual metastatic pattern, and minimally elevated AFP level. This case raises 3 key points that we must remember as clinicians:
-
Patients infected with hepatitis C who are found to have suspicious hepatic lesions should be aggressively evaluated for HCC.
-
Using an AFP level < 20 g/L as a screening test is not helpful because this level can be seen even with widely metastatic disease.
-
Knowledge of available screening tests as well as the many possible manifestations of HCC helps clinicians to diagnose HCC earlier, when the disease is potentially curable.
Acknowledgements
The authors thank Gurpreet Dhaliwal, MD, for reviewing an early version of this manuscript.
- .Hepatocellular carcinoma: epidemiology, risk factors, and screening.Semin Liver Dis.2005;25:143–154.
- American Cancer Society. Cancer Facts and Figures 2005. Atlanta, GA: American Cancer Society, 2005. Available at: http://www.cancer.org/docroot/STT/stt_0.asp. Accessed October 17,2005.
- ,,.Hepatocellular carcinoma.Lancet.2003;362:1907–1917.
- ,.Management of hepatocellular carcinoma. AASLD Practice Guideline.Hepatology.2005;42:1208–1236.
- ,,, et al.Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications.Gastroenterology.1985;89:259–266.
- ,,.Test characteristics of alpha‐fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C.Ann Intern Med.2003;139:46–50.
- ,,,.Sonographic screening for hepatocellular carcinoma in patients with chronic hepatitis or cirrhosis: an evaluation.Am J Roentgenol.1998;171:433–435.
- ,,,,.Detection of malignant tumors in end‐stage cirrhotic livers: efficacy of sonography as a screening technique.Am J Roentgenol.1992;159:727–733.
- ,,, et al.The diagnosis of small hepatocellular carcinomas: efficacy of various imaging procedures in 100 patients.Am J Roentgenol.1990;155:49–54
- ,,,,,.Outcome of 67 patients with hepatocellular cancer detected during screening of 1125 patients with chronic hepatitis.Ann Surg.1998;277:513–518.
- ,,, et al.;EASL Panel of Experts on HCC.Clinical management of hepatocellular carcinoma: conclusions of the Barcelona‐2000 EASL conference: European Association for the Study of the Liver.J Hepatol.2001;35:421–430.
- ,,, et al.Extrahepatic spread of hepatocellular carcinoma: a pictorial review.Eur Radiol.2003;13:874–882.
- ,,,,,.Extrahepatic metastases of hepatocellular carcinoma.Radiology.2000;216:698–703.
A 58‐year‐old man was evaluated for 3 weeks of leg numbness and weakness. His symptoms began with numbness and tingling in the distal left leg that progressed to weakness that impaired his ability to walk. He had no history of trauma or incontinence but endorsed several months of back pain that worsened when lying flat. He had a history of type 2 diabetes mellitus, hepatitis C infection, hypertension, and posttraumatic stress disorder. He had a remote history of intravenous drug use and had quit tobacco 9 years earlier. Medications he was taking included hydrochlorothiazide, rosiglitazone, oxycodone/acetaminophen, baclofen, ibuprofen, and gabapentin.
Internists see this constellation of complaints frequently in an acute care setting. Finding a unifying diagnosis may be difficult initially, so thinking of the symptoms in series is helpful. The complaint of leg weakness and the pattern of numbness should be further elucidated. Is this true weakness, or is it a feeling of instability because of foot numbness? What is the pattern of the numbness? Peripheral neuropathy typically begins in a symmetric stocking pattern (involving the plantar surface of the feet), then progresses to a glove distribution (involving the hands from the fingers distally to the wrist proximally). Such a pattern in a patient with diabetes would be consistent with distal polyneuropathy, a mixed sensory and motor process. Other possible causes of peripheral neuropathy in this patient include HIV, B12 deficiency, and syphilis. These symptoms could be tied to the back pain if this were intervertebral disk disease, a compression fracture, or a lytic lesion in the vertebrae with resulting nerve impingement or if it were epidural spinal cord compression. The lack of bowel or bladder dysfunction speaks against a cauda equina syndrome but does not rule out more cepahalad spinal pathology.
On neurological examination, I would concentrate on differentiating weakness from pain. I would attempt to determine whether the weakness was of central or peripheral nerve etiology. Helpful findings would include increased tone with upper motor lesions and flaccid tone with lower motor lesions, hyperreflexia with upper motor lesions and hyporeflexia with lower motor lesions, a Babinski sign, muscle atrophy or fasciculations, and gait. A rectal examination would also be helpful to assess for deficits in rectal tone, wink reflex, or saddle anesthesia.
All patients with low back pain who have alarm signs of age older than 50, pain duration of more than 1 month, known cancer, lack of relief with conservative measures, or systemic B symptoms should have imaging of the spine. Although plain films may reveal bony abnormalities, computed tomography (CT) is better for evaluating osseous structures and magnetic resonance imaging (MRI) for evaluating pathology in patients suspected of having an infection or a malignancy. I would obtain imaging of the spine in this patient.
The patient was receiving care at an outside clinic for 2 liver lesions discovered on abdominal ultrasound 19 months prior to admission. CT showed that the lesions were 4.0 and 2.3 cm in diameter 17 months prior to admission and 5.0 and 3.0 cm in diameter 5 months prior to admission. No cirrhosis was appreciated on the ultrasound or CT. The patient was referred for CT‐guided biopsy of the larger mass after the second CT, but he became anxious and left before the biopsy was obtained.
This piece of the history is ominous, as it increases the possibility of cancer in our differential. Metastatic disease could provide a unifying diagnosis, explaining the constellation of back pain, leg weakness, and liver lesions. Lung cancer commonly metastasizes to the liver and to bone, so I would obtain a chest x‐ray. Other possible types of cancer in this situation include cancer of the prostate, colon, or thyroid and melanoma. In this patient, who has hepatitis C, hepatocellular carcinoma (HCC) could be the primary etiology, although cirrhosis was not seen on CT and HCC metastasizes to the spine less commonly than do other primary cancers (eg, lung, breast, prostate). Nonetheless, I would obtain an alpha‐fetoprotein level, which would confirm HCC in a patient with liver lesions if it was greater than 200 g/L. Pancreatic cancer has been associated with both type 2 diabetes and liver lesions and could explain his abdominal pain.
There is no comment on the arterial‐phase CT imaging of the liver lesions. Dual‐phase CT scans examine the hepatic arterial and portal vein phases of contrast filling. Triple‐phase CT scans also examine the portal vein influx phase. Both hemangiomas and hypervascular HCCs enhance on the arterial phase, as they derive their blood flow from the hepatic artery. Therefore, arterial‐phase imaging can help to distinguish vascular tumors that flush with contrast, such as hemangiomas, melanoma, and HCC, from less vascular tumors such as pancreatic and colon cancer. Other liver lesions such as focal nodular hyperplasia and adenomas cannot be excluded in this situation as they also may enhance during the arterial phase and can grow over time, as this patient's repeat imaging documented. It seems unlikely that this patient has a liver abscess because he has a paucity of constitutional symptoms and no travel history. The liver lesions seen on initial imaging were larger than 1.0 cm, so I would have favored an earlier biopsy to obtain a tissue diagnosis.
The patient was afebrile, and all other vital signs were normal. He appeared well nourished and anicteric. There was no lymphadenopathy. Cardiac auscultation was regular without murmurs. The lungs were clear. The abdomen was without fluid wave or hepatosplenomegaly and was tender to palpation in the right upper and lower quadrants. There was no midline tenderness to palpation of the spine.
Cranial nerves II‐XII were intact. Lower extremity muscle tone could not be accurately assessed due to splinting from back pain. Strength was 3 of 5 in the left hip extensors and left knee flexors and extensors, and 1 of 5 in the left hip flexors. He had no motor strength in the distal left lower extremity extensors. Bilateral upper extremity and right leg strength were normal. Sensation to light touch, temperature, and pain was decreased circumferentially below the xiphoid. The patient had hyperesthesia in a band around the thorax just above the xiphoid and paresthesia of the perineal area. Left patellar tendon reflexes were brisk, and left ankle jerk was absent, but other reflexes were normal. Toes were down‐going bilaterally. The anal wink was absent, and rectal tone was decreased. Results of the cerebellar exam were normal. Gait could not be assessed.
The results of the exam are notable for not showing the stigmata of end‐stage liver disease. The results of the neurological exam are concerning, with decreased sensation at approximately the T7 level that is almost certainly a result of epidural compression of the spinal cord. Hematogenous metastasis to the vertebrae from one of the tumors mentioned above, with spread into the thecal sac, is the most likely culprit. An epidural abscess is possible because the patient has diabetes and a history of injection drug use.
The thoracic spine is involved in 60% of spinal cord metastases. This patient's left‐sided distal leg weakness is consistent with having corticospinal tract compression and indicates thoracic spine involvement. Flaccid paralysis is classically found in lower motor neuron weakness, but is also seen in the early stages of upper motor neuron pathology. Lesions found above the cauda equina often spare the perineal area, but low thoracic lesions involving the conus medullaris (from T10 to L1) could explain both his loss of anal wink and his decreased rectal tone.
This patient's presentation is unfortunately classic for epidural spinal cord compression. Because the onset of compression is insidious, the diagnosis is often delayed, even in patients with known cancer. Urgent imaging is imperative to evaluate this possibility, as having any meaningful chance of recovery of function depends on rapid relief of the spinal cord compression. I would obtain an emergent MRI of the thoracic and lumbosacral spine.
Laboratory studies showed the following: hemoglobin, 13.1 g/dL; mean corpuscular volume, 80 m3; platelet count, 149,000/L; creatinine, 1.9 mg/dL; aspartate aminotransferase, 66 U/L (5‐35 U/L); alanine aminotransferase, 66 U/L (7‐56 U/L); alkaline phosphatase, 87 U/L (40‐125 U/L); total bilirubin, 1.3 mg/dL; prostate specific antigen (PSA), 1.6 g/dL; and alpha‐fetoprotein (AFP), 10.3 g/L. White cell count, sodium, glucose, calcium, and albumin levels, and prothrombin and partial‐thromboplastin times were within normal ranges.
His liver function tests likely reflect chronic hepatitis C infection. His renal insufficiency could be a result of hypertension, diabetes, or dehydration given that he has been bed‐bound.
Most intriguing are the normal PSA level and only slightly elevated AFP level. PSA is useful for detecting recurrence of prostate cancer or following response of therapy, but the utility of PSA as a screening tool remains controversial in part because of its low specificity. Prostate cancer is the most commonly diagnosed cancer among men and cannot be ruled out by a normal PSA. In a patient with hepatitis C, cirrhosis (which we have not conclusively diagnosed), and a radiologically suspicious liver lesion, an AFP > 200 g/L would be diagnostic of HCC. In this case, however, mildly elevated AFP does not help us to either diagnose or exclude HCC.
The chest x‐ray showed no abnormalities. MRI of the spine revealed lytic lesions in the T7‐T10 vertebral bodies with spinal cord compression at the T7 level (Fig. 1).

A repeat CT scan of the abdomen showed a coarse, nodular liver with 2 heterogeneous, early‐enhancing masses (4.7 4.2 and 3.4 2.4 cm in diameter) with surrounding satellite lesions (Fig. 2).

The enhancement pattern on dual‐phase liver protocol CT was not characteristic of HCC. The left portal vein was not visualized. Splenomegaly and esophageal varices were observed. The adrenal glands showed bilateral, heterogeneous enhancing masses. The epiphrenic, retroperitoneal, and periportal lymph nodes were enlarged. Lytic lesions were seen in the sacrum, left iliac wing, and T7‐T10 vertebral bodies.
Intravenous high‐dose steroids were started. The neurosurgery team advised that no surgical interventions were appropriate because of the patient's poor functional status and the extent of his disease.
It is unfortunate that no neurosurgical interventions could help this patient, especially because we are not yet sure of the final diagnosis. Standard indications for neurosurgical decompression include compression from bone fragments, spinal instability requiring fixation, and lack of response to radiation therapy. Patients must also be able to tolerate surgery. Although evidence supports the use of corticosteroids in reducing edema, inflammation, and neurological deficits in malignant spinal cord compression, there is not consensus on what the optimal dose is. Doses of 16‐100 mg of dexamethasone per day appear to be beneficial, as long as higher doses are rapidly tapered to avoid toxic effects. High‐dose steroids minimize the initial edema but are unlikely to change the long‐term outcome of patients who are nonambulatory on arrival.
The CT scan does not help us distinguish between metastatic cancer and primary HCC. Adrenal metastases are very uncommon in HCC. Lung cancer, however, metastasizes to the liver, adrenal glands, and spine, even without significant pulmonary symptoms. HCC may be seen on CT as a solitary mass, a dominant mass with surrounding satellite lesions, multifocal lesions, or a diffusely infiltrating tumor. This diagnosis now seems more likely given the finding of cirrhosis, which increases the risk of HCC in individuals with hepatitis C infection.
We need to obtain tissue for diagnosis and prognosis and to guide therapy. I would consult with radiology and gastroenterology colleagues about the best location to biopsy, but a bone biopsy should be avoided because the pathologic yield is lower.
The radiology and gastroenterology consultants recommended adrenal biopsy because there was easier posterior access for tissue. A liver biopsy was avoided because of the risk of bleeding with hypervascular masses. Fine‐needle aspiration of the mass in the right adrenal gland was performed. The pathology demonstrated bile production and hexagonal arrangement of cells with endothelial cuffing consistent with hepatocellular carcinoma. The oncology staff was consulted about palliative chemotherapy options. The patient began radiation therapy directed at the T7 lesion compressing the spinal cord. He regained minimal movement of his foot. After discussing treatment options with the oncology staff, the patient declined chemotherapy and was transitioned to hospice, where he died 3 weeks later.
COMMENTARY
Hepatocellular carcinoma (HCC) is the third‐leading cause of cancer death and the fifth‐leading cause of cancer worldwide. It causes nearly 1 million deaths annually, and unlike many other cancers, its incidence and mortality rate are rising. Most cases of HCC in Africa and Asia are a result of chronic hepatitis B infection, but in the United States HCC is primarily attributable to hepatitis C infection.1 The annual incidence of HCC in the U.S. population, now about 4 cases per 100,000 people,2 is rising because of the increased prevalence of hepatitis C. Other causes of HCC, such as alcoholic liver disease, hepatitis B infection, and hemochromatosis, have remained stable and have not contributed as significantly to the rising incidence of HCC. For the individual patient, hepatitis C infection conveys a 20‐fold increase in the risk for HCC (2%‐8% risk/year).1 Eighty percent of cases of HCC develop in patients with cirrhosis.3 Unlike patients with hepatitis B infection, persons chronically infected with hepatitis C rarely develop HCC unless they have cirrhosis.
The American Association for the Study of Liver Disease recommends that hepatitis Binfected individuals at high risk for HCC (eg, men older than 40 years and persons with cirrhosis or a family history of HCC) and hepatitis Cinfected individuals with cirrhosis4 be periodically screened for HCC with alpha‐fetoprotein (AFP) and ultrasonography (every 6 months to approximate the doubling time of the tumor5). Using the most commonly reported cutoff for a positive test result for hepatocellular carcinoma (AFP level > 20 g/L) resulted in the following test characteristics: sensitivity, 41%‐65%; specificity, 80%‐94%; positive likelihood ratio, 3.1‐6.8; and negative likelihood ratio, 0.4‐0.6.6 AFP alone is therefore a poor screening test for HCC, and as shown in this case, AFP levels can be normal or only minimally elevated in the setting of diffusely metastatic disease. Ultrasonography alone is only 35%‐87% sensitive in detecting HCC,79 but the combination of AFP and ultrasonography identified 100% of the HCC cases in one small case series.10
For the patient in this case, the optimal clinical pathway would have been to transition from screening to diagnostic measures in a timely manner. Consensus guidelines from the European Association for Study of the Liver in 2001 recommend biopsy of all focal liver lesions that are between 1 and 2 cm.11 The American Association for the Study of Liver Diseases (AASLD) recommends that focal liver lesions between 1 and 2 cm found on ultrasound in cirrhotic livers be followed by 2 dynamic studies: CT, MRI, or contrast ultrasound. If 2 separate studies reveal typical characteristics of HCC, then the lesion should be treated as HCC, and if not typical, then the lesion should be biopsied.4 Although no studies were available to support the recommendations, both the EASL and AASLD advise that lesions greater than 2 cm with demonstrated vascularity on both ultrasonography and CT can be diagnosed as HCC without biopsy and that lesions smaller than 1 cm be monitored.4, 11
Hepatocellular carcinoma can metastasize to almost anywhere in the body by hematologic or lymphatic spread or by direct extension. The most common site for metastases of HCC is the lung. Metastases to the lung arise primarily from arterial emboli and therefore are most common in the lower lobes, where there is greater perfusion.12 The second most common site is intraabdominal lymph nodes. The axial skeleton is the third most common site of metastases and, as in this case, primarily involves the spine.13 Other sites of metastases include the peritoneum, the inferior vena cava and right atrium by direct extension, and, less commonly, the gallbladder and spleen. Autopsy studies of patients with HCC found that 8% had metastases to the adrenal glands, as did this patient.13 Metastasis to the central nervous system is rare.
There were several challenging aspects of this case, including atypical radiologic appearance, an unusual metastatic pattern, and minimally elevated AFP level. This case raises 3 key points that we must remember as clinicians:
-
Patients infected with hepatitis C who are found to have suspicious hepatic lesions should be aggressively evaluated for HCC.
-
Using an AFP level < 20 g/L as a screening test is not helpful because this level can be seen even with widely metastatic disease.
-
Knowledge of available screening tests as well as the many possible manifestations of HCC helps clinicians to diagnose HCC earlier, when the disease is potentially curable.
Acknowledgements
The authors thank Gurpreet Dhaliwal, MD, for reviewing an early version of this manuscript.
A 58‐year‐old man was evaluated for 3 weeks of leg numbness and weakness. His symptoms began with numbness and tingling in the distal left leg that progressed to weakness that impaired his ability to walk. He had no history of trauma or incontinence but endorsed several months of back pain that worsened when lying flat. He had a history of type 2 diabetes mellitus, hepatitis C infection, hypertension, and posttraumatic stress disorder. He had a remote history of intravenous drug use and had quit tobacco 9 years earlier. Medications he was taking included hydrochlorothiazide, rosiglitazone, oxycodone/acetaminophen, baclofen, ibuprofen, and gabapentin.
Internists see this constellation of complaints frequently in an acute care setting. Finding a unifying diagnosis may be difficult initially, so thinking of the symptoms in series is helpful. The complaint of leg weakness and the pattern of numbness should be further elucidated. Is this true weakness, or is it a feeling of instability because of foot numbness? What is the pattern of the numbness? Peripheral neuropathy typically begins in a symmetric stocking pattern (involving the plantar surface of the feet), then progresses to a glove distribution (involving the hands from the fingers distally to the wrist proximally). Such a pattern in a patient with diabetes would be consistent with distal polyneuropathy, a mixed sensory and motor process. Other possible causes of peripheral neuropathy in this patient include HIV, B12 deficiency, and syphilis. These symptoms could be tied to the back pain if this were intervertebral disk disease, a compression fracture, or a lytic lesion in the vertebrae with resulting nerve impingement or if it were epidural spinal cord compression. The lack of bowel or bladder dysfunction speaks against a cauda equina syndrome but does not rule out more cepahalad spinal pathology.
On neurological examination, I would concentrate on differentiating weakness from pain. I would attempt to determine whether the weakness was of central or peripheral nerve etiology. Helpful findings would include increased tone with upper motor lesions and flaccid tone with lower motor lesions, hyperreflexia with upper motor lesions and hyporeflexia with lower motor lesions, a Babinski sign, muscle atrophy or fasciculations, and gait. A rectal examination would also be helpful to assess for deficits in rectal tone, wink reflex, or saddle anesthesia.
All patients with low back pain who have alarm signs of age older than 50, pain duration of more than 1 month, known cancer, lack of relief with conservative measures, or systemic B symptoms should have imaging of the spine. Although plain films may reveal bony abnormalities, computed tomography (CT) is better for evaluating osseous structures and magnetic resonance imaging (MRI) for evaluating pathology in patients suspected of having an infection or a malignancy. I would obtain imaging of the spine in this patient.
The patient was receiving care at an outside clinic for 2 liver lesions discovered on abdominal ultrasound 19 months prior to admission. CT showed that the lesions were 4.0 and 2.3 cm in diameter 17 months prior to admission and 5.0 and 3.0 cm in diameter 5 months prior to admission. No cirrhosis was appreciated on the ultrasound or CT. The patient was referred for CT‐guided biopsy of the larger mass after the second CT, but he became anxious and left before the biopsy was obtained.
This piece of the history is ominous, as it increases the possibility of cancer in our differential. Metastatic disease could provide a unifying diagnosis, explaining the constellation of back pain, leg weakness, and liver lesions. Lung cancer commonly metastasizes to the liver and to bone, so I would obtain a chest x‐ray. Other possible types of cancer in this situation include cancer of the prostate, colon, or thyroid and melanoma. In this patient, who has hepatitis C, hepatocellular carcinoma (HCC) could be the primary etiology, although cirrhosis was not seen on CT and HCC metastasizes to the spine less commonly than do other primary cancers (eg, lung, breast, prostate). Nonetheless, I would obtain an alpha‐fetoprotein level, which would confirm HCC in a patient with liver lesions if it was greater than 200 g/L. Pancreatic cancer has been associated with both type 2 diabetes and liver lesions and could explain his abdominal pain.
There is no comment on the arterial‐phase CT imaging of the liver lesions. Dual‐phase CT scans examine the hepatic arterial and portal vein phases of contrast filling. Triple‐phase CT scans also examine the portal vein influx phase. Both hemangiomas and hypervascular HCCs enhance on the arterial phase, as they derive their blood flow from the hepatic artery. Therefore, arterial‐phase imaging can help to distinguish vascular tumors that flush with contrast, such as hemangiomas, melanoma, and HCC, from less vascular tumors such as pancreatic and colon cancer. Other liver lesions such as focal nodular hyperplasia and adenomas cannot be excluded in this situation as they also may enhance during the arterial phase and can grow over time, as this patient's repeat imaging documented. It seems unlikely that this patient has a liver abscess because he has a paucity of constitutional symptoms and no travel history. The liver lesions seen on initial imaging were larger than 1.0 cm, so I would have favored an earlier biopsy to obtain a tissue diagnosis.
The patient was afebrile, and all other vital signs were normal. He appeared well nourished and anicteric. There was no lymphadenopathy. Cardiac auscultation was regular without murmurs. The lungs were clear. The abdomen was without fluid wave or hepatosplenomegaly and was tender to palpation in the right upper and lower quadrants. There was no midline tenderness to palpation of the spine.
Cranial nerves II‐XII were intact. Lower extremity muscle tone could not be accurately assessed due to splinting from back pain. Strength was 3 of 5 in the left hip extensors and left knee flexors and extensors, and 1 of 5 in the left hip flexors. He had no motor strength in the distal left lower extremity extensors. Bilateral upper extremity and right leg strength were normal. Sensation to light touch, temperature, and pain was decreased circumferentially below the xiphoid. The patient had hyperesthesia in a band around the thorax just above the xiphoid and paresthesia of the perineal area. Left patellar tendon reflexes were brisk, and left ankle jerk was absent, but other reflexes were normal. Toes were down‐going bilaterally. The anal wink was absent, and rectal tone was decreased. Results of the cerebellar exam were normal. Gait could not be assessed.
The results of the exam are notable for not showing the stigmata of end‐stage liver disease. The results of the neurological exam are concerning, with decreased sensation at approximately the T7 level that is almost certainly a result of epidural compression of the spinal cord. Hematogenous metastasis to the vertebrae from one of the tumors mentioned above, with spread into the thecal sac, is the most likely culprit. An epidural abscess is possible because the patient has diabetes and a history of injection drug use.
The thoracic spine is involved in 60% of spinal cord metastases. This patient's left‐sided distal leg weakness is consistent with having corticospinal tract compression and indicates thoracic spine involvement. Flaccid paralysis is classically found in lower motor neuron weakness, but is also seen in the early stages of upper motor neuron pathology. Lesions found above the cauda equina often spare the perineal area, but low thoracic lesions involving the conus medullaris (from T10 to L1) could explain both his loss of anal wink and his decreased rectal tone.
This patient's presentation is unfortunately classic for epidural spinal cord compression. Because the onset of compression is insidious, the diagnosis is often delayed, even in patients with known cancer. Urgent imaging is imperative to evaluate this possibility, as having any meaningful chance of recovery of function depends on rapid relief of the spinal cord compression. I would obtain an emergent MRI of the thoracic and lumbosacral spine.
Laboratory studies showed the following: hemoglobin, 13.1 g/dL; mean corpuscular volume, 80 m3; platelet count, 149,000/L; creatinine, 1.9 mg/dL; aspartate aminotransferase, 66 U/L (5‐35 U/L); alanine aminotransferase, 66 U/L (7‐56 U/L); alkaline phosphatase, 87 U/L (40‐125 U/L); total bilirubin, 1.3 mg/dL; prostate specific antigen (PSA), 1.6 g/dL; and alpha‐fetoprotein (AFP), 10.3 g/L. White cell count, sodium, glucose, calcium, and albumin levels, and prothrombin and partial‐thromboplastin times were within normal ranges.
His liver function tests likely reflect chronic hepatitis C infection. His renal insufficiency could be a result of hypertension, diabetes, or dehydration given that he has been bed‐bound.
Most intriguing are the normal PSA level and only slightly elevated AFP level. PSA is useful for detecting recurrence of prostate cancer or following response of therapy, but the utility of PSA as a screening tool remains controversial in part because of its low specificity. Prostate cancer is the most commonly diagnosed cancer among men and cannot be ruled out by a normal PSA. In a patient with hepatitis C, cirrhosis (which we have not conclusively diagnosed), and a radiologically suspicious liver lesion, an AFP > 200 g/L would be diagnostic of HCC. In this case, however, mildly elevated AFP does not help us to either diagnose or exclude HCC.
The chest x‐ray showed no abnormalities. MRI of the spine revealed lytic lesions in the T7‐T10 vertebral bodies with spinal cord compression at the T7 level (Fig. 1).

A repeat CT scan of the abdomen showed a coarse, nodular liver with 2 heterogeneous, early‐enhancing masses (4.7 4.2 and 3.4 2.4 cm in diameter) with surrounding satellite lesions (Fig. 2).

The enhancement pattern on dual‐phase liver protocol CT was not characteristic of HCC. The left portal vein was not visualized. Splenomegaly and esophageal varices were observed. The adrenal glands showed bilateral, heterogeneous enhancing masses. The epiphrenic, retroperitoneal, and periportal lymph nodes were enlarged. Lytic lesions were seen in the sacrum, left iliac wing, and T7‐T10 vertebral bodies.
Intravenous high‐dose steroids were started. The neurosurgery team advised that no surgical interventions were appropriate because of the patient's poor functional status and the extent of his disease.
It is unfortunate that no neurosurgical interventions could help this patient, especially because we are not yet sure of the final diagnosis. Standard indications for neurosurgical decompression include compression from bone fragments, spinal instability requiring fixation, and lack of response to radiation therapy. Patients must also be able to tolerate surgery. Although evidence supports the use of corticosteroids in reducing edema, inflammation, and neurological deficits in malignant spinal cord compression, there is not consensus on what the optimal dose is. Doses of 16‐100 mg of dexamethasone per day appear to be beneficial, as long as higher doses are rapidly tapered to avoid toxic effects. High‐dose steroids minimize the initial edema but are unlikely to change the long‐term outcome of patients who are nonambulatory on arrival.
The CT scan does not help us distinguish between metastatic cancer and primary HCC. Adrenal metastases are very uncommon in HCC. Lung cancer, however, metastasizes to the liver, adrenal glands, and spine, even without significant pulmonary symptoms. HCC may be seen on CT as a solitary mass, a dominant mass with surrounding satellite lesions, multifocal lesions, or a diffusely infiltrating tumor. This diagnosis now seems more likely given the finding of cirrhosis, which increases the risk of HCC in individuals with hepatitis C infection.
We need to obtain tissue for diagnosis and prognosis and to guide therapy. I would consult with radiology and gastroenterology colleagues about the best location to biopsy, but a bone biopsy should be avoided because the pathologic yield is lower.
The radiology and gastroenterology consultants recommended adrenal biopsy because there was easier posterior access for tissue. A liver biopsy was avoided because of the risk of bleeding with hypervascular masses. Fine‐needle aspiration of the mass in the right adrenal gland was performed. The pathology demonstrated bile production and hexagonal arrangement of cells with endothelial cuffing consistent with hepatocellular carcinoma. The oncology staff was consulted about palliative chemotherapy options. The patient began radiation therapy directed at the T7 lesion compressing the spinal cord. He regained minimal movement of his foot. After discussing treatment options with the oncology staff, the patient declined chemotherapy and was transitioned to hospice, where he died 3 weeks later.
COMMENTARY
Hepatocellular carcinoma (HCC) is the third‐leading cause of cancer death and the fifth‐leading cause of cancer worldwide. It causes nearly 1 million deaths annually, and unlike many other cancers, its incidence and mortality rate are rising. Most cases of HCC in Africa and Asia are a result of chronic hepatitis B infection, but in the United States HCC is primarily attributable to hepatitis C infection.1 The annual incidence of HCC in the U.S. population, now about 4 cases per 100,000 people,2 is rising because of the increased prevalence of hepatitis C. Other causes of HCC, such as alcoholic liver disease, hepatitis B infection, and hemochromatosis, have remained stable and have not contributed as significantly to the rising incidence of HCC. For the individual patient, hepatitis C infection conveys a 20‐fold increase in the risk for HCC (2%‐8% risk/year).1 Eighty percent of cases of HCC develop in patients with cirrhosis.3 Unlike patients with hepatitis B infection, persons chronically infected with hepatitis C rarely develop HCC unless they have cirrhosis.
The American Association for the Study of Liver Disease recommends that hepatitis Binfected individuals at high risk for HCC (eg, men older than 40 years and persons with cirrhosis or a family history of HCC) and hepatitis Cinfected individuals with cirrhosis4 be periodically screened for HCC with alpha‐fetoprotein (AFP) and ultrasonography (every 6 months to approximate the doubling time of the tumor5). Using the most commonly reported cutoff for a positive test result for hepatocellular carcinoma (AFP level > 20 g/L) resulted in the following test characteristics: sensitivity, 41%‐65%; specificity, 80%‐94%; positive likelihood ratio, 3.1‐6.8; and negative likelihood ratio, 0.4‐0.6.6 AFP alone is therefore a poor screening test for HCC, and as shown in this case, AFP levels can be normal or only minimally elevated in the setting of diffusely metastatic disease. Ultrasonography alone is only 35%‐87% sensitive in detecting HCC,79 but the combination of AFP and ultrasonography identified 100% of the HCC cases in one small case series.10
For the patient in this case, the optimal clinical pathway would have been to transition from screening to diagnostic measures in a timely manner. Consensus guidelines from the European Association for Study of the Liver in 2001 recommend biopsy of all focal liver lesions that are between 1 and 2 cm.11 The American Association for the Study of Liver Diseases (AASLD) recommends that focal liver lesions between 1 and 2 cm found on ultrasound in cirrhotic livers be followed by 2 dynamic studies: CT, MRI, or contrast ultrasound. If 2 separate studies reveal typical characteristics of HCC, then the lesion should be treated as HCC, and if not typical, then the lesion should be biopsied.4 Although no studies were available to support the recommendations, both the EASL and AASLD advise that lesions greater than 2 cm with demonstrated vascularity on both ultrasonography and CT can be diagnosed as HCC without biopsy and that lesions smaller than 1 cm be monitored.4, 11
Hepatocellular carcinoma can metastasize to almost anywhere in the body by hematologic or lymphatic spread or by direct extension. The most common site for metastases of HCC is the lung. Metastases to the lung arise primarily from arterial emboli and therefore are most common in the lower lobes, where there is greater perfusion.12 The second most common site is intraabdominal lymph nodes. The axial skeleton is the third most common site of metastases and, as in this case, primarily involves the spine.13 Other sites of metastases include the peritoneum, the inferior vena cava and right atrium by direct extension, and, less commonly, the gallbladder and spleen. Autopsy studies of patients with HCC found that 8% had metastases to the adrenal glands, as did this patient.13 Metastasis to the central nervous system is rare.
There were several challenging aspects of this case, including atypical radiologic appearance, an unusual metastatic pattern, and minimally elevated AFP level. This case raises 3 key points that we must remember as clinicians:
-
Patients infected with hepatitis C who are found to have suspicious hepatic lesions should be aggressively evaluated for HCC.
-
Using an AFP level < 20 g/L as a screening test is not helpful because this level can be seen even with widely metastatic disease.
-
Knowledge of available screening tests as well as the many possible manifestations of HCC helps clinicians to diagnose HCC earlier, when the disease is potentially curable.
Acknowledgements
The authors thank Gurpreet Dhaliwal, MD, for reviewing an early version of this manuscript.
- .Hepatocellular carcinoma: epidemiology, risk factors, and screening.Semin Liver Dis.2005;25:143–154.
- American Cancer Society. Cancer Facts and Figures 2005. Atlanta, GA: American Cancer Society, 2005. Available at: http://www.cancer.org/docroot/STT/stt_0.asp. Accessed October 17,2005.
- ,,.Hepatocellular carcinoma.Lancet.2003;362:1907–1917.
- ,.Management of hepatocellular carcinoma. AASLD Practice Guideline.Hepatology.2005;42:1208–1236.
- ,,, et al.Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications.Gastroenterology.1985;89:259–266.
- ,,.Test characteristics of alpha‐fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C.Ann Intern Med.2003;139:46–50.
- ,,,.Sonographic screening for hepatocellular carcinoma in patients with chronic hepatitis or cirrhosis: an evaluation.Am J Roentgenol.1998;171:433–435.
- ,,,,.Detection of malignant tumors in end‐stage cirrhotic livers: efficacy of sonography as a screening technique.Am J Roentgenol.1992;159:727–733.
- ,,, et al.The diagnosis of small hepatocellular carcinomas: efficacy of various imaging procedures in 100 patients.Am J Roentgenol.1990;155:49–54
- ,,,,,.Outcome of 67 patients with hepatocellular cancer detected during screening of 1125 patients with chronic hepatitis.Ann Surg.1998;277:513–518.
- ,,, et al.;EASL Panel of Experts on HCC.Clinical management of hepatocellular carcinoma: conclusions of the Barcelona‐2000 EASL conference: European Association for the Study of the Liver.J Hepatol.2001;35:421–430.
- ,,, et al.Extrahepatic spread of hepatocellular carcinoma: a pictorial review.Eur Radiol.2003;13:874–882.
- ,,,,,.Extrahepatic metastases of hepatocellular carcinoma.Radiology.2000;216:698–703.
- .Hepatocellular carcinoma: epidemiology, risk factors, and screening.Semin Liver Dis.2005;25:143–154.
- American Cancer Society. Cancer Facts and Figures 2005. Atlanta, GA: American Cancer Society, 2005. Available at: http://www.cancer.org/docroot/STT/stt_0.asp. Accessed October 17,2005.
- ,,.Hepatocellular carcinoma.Lancet.2003;362:1907–1917.
- ,.Management of hepatocellular carcinoma. AASLD Practice Guideline.Hepatology.2005;42:1208–1236.
- ,,, et al.Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications.Gastroenterology.1985;89:259–266.
- ,,.Test characteristics of alpha‐fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C.Ann Intern Med.2003;139:46–50.
- ,,,.Sonographic screening for hepatocellular carcinoma in patients with chronic hepatitis or cirrhosis: an evaluation.Am J Roentgenol.1998;171:433–435.
- ,,,,.Detection of malignant tumors in end‐stage cirrhotic livers: efficacy of sonography as a screening technique.Am J Roentgenol.1992;159:727–733.
- ,,, et al.The diagnosis of small hepatocellular carcinomas: efficacy of various imaging procedures in 100 patients.Am J Roentgenol.1990;155:49–54
- ,,,,,.Outcome of 67 patients with hepatocellular cancer detected during screening of 1125 patients with chronic hepatitis.Ann Surg.1998;277:513–518.
- ,,, et al.;EASL Panel of Experts on HCC.Clinical management of hepatocellular carcinoma: conclusions of the Barcelona‐2000 EASL conference: European Association for the Study of the Liver.J Hepatol.2001;35:421–430.
- ,,, et al.Extrahepatic spread of hepatocellular carcinoma: a pictorial review.Eur Radiol.2003;13:874–882.
- ,,,,,.Extrahepatic metastases of hepatocellular carcinoma.Radiology.2000;216:698–703.