Article
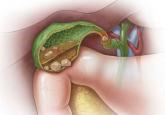
Gallstones: Watch and wait, or intervene?
- Author:
- Mounir Ibrahim, MD
- Shashank Sarvepalli, MD
- Gareth Morris-Stiff, MD, PhD
- Maged Rizk, MD
- Amit Bhatt, MD
- R. Matthew Walsh, MD
- Umar Hayat, MD
- Ari Garber, MD, EDD
- John Vargo, MD
- Carol A. Burke, MD
Consider laparoscopic cholecystectomy for symptomatic cholelithiasis, expectant management for asymptomatic cases.
Article
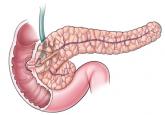
Total pancreatectomy and islet cell autotransplantation: Definitive treatment for chronic pancreatitis
- Author:
- Karla M. Arce, MD
- Yu Kuei Lin, MD
- Tyler Stevens, MD
- R. Matthew Walsh, MD
- Betul A. Hatipoglu, MD
Most patients report less pain after this procedure, and many avoid overt diabetes.
