User login
Progressively Worsening Scaly Patches and Plaques in an Infant
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
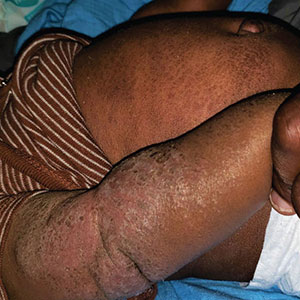
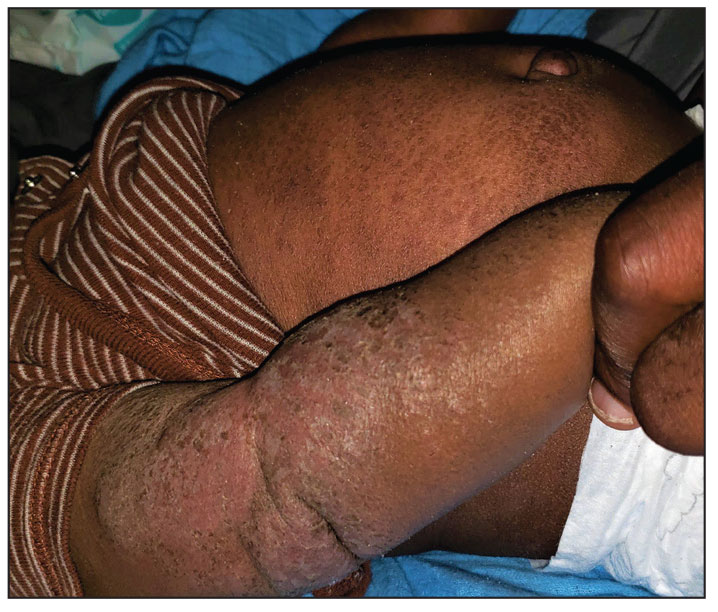
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
A 5-month-old male with moderately brown skin that rarely burns and tans profusely presented to the emergency department with a worsening red rash of more than 4 months’ duration. The patient had diffuse erythroderma and eczematous patches and plaques covering 95% of the total body surface area, including lichenified plaques on the arms and elbows, with no signs of infection. He initially presented for his 1-month appointment at the pediatric clinic with scaly patches and plaques on the face and trunk as well as diffuse xerosis. He was prescribed daily oatmeal baths and topical Minerin (Major Pharmaceuticals)—containing water, petrolatum, mineral oil, mineral wax, lanolin alcohol, methylchloroisothiazolinone, and methylisothiazolinone—to be applied to the whole body twice daily. At the patient’s 2-month well visit, symptoms persisted. The patient’s pediatrician increased application of Minerin to 2 to 3 times daily, and hydrocortisone cream 2.5% application 2 to 3 times daily was added.
Tense Bullae With Widespread Erosions
The Diagnosis: Linear IgA Bullous Dermatosis
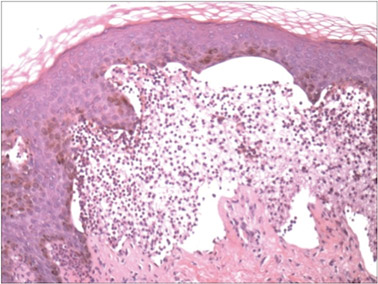
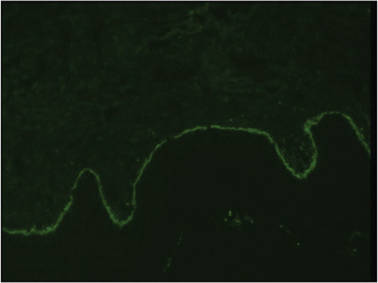
A biopsy specimen from an intact vesicle was obtained. Histologic findings showed a basket weave stratum corneum suggestive of an acute process. There was subepidermal separation with an inflammatory infiltrate of neutrophils (Figure 1). Direct immunofluorescence yielded a pattern of IgA deposition along the dermoepidermal junction (Figure 2). A diagnosis of linear IgA bullous dermatosis (LABD) was made. The patient was started on 100 mg daily of dapsone. The dose was subsequently increased to 175 mg twice daily, resulting in complete clearance. He became dermatologically disease free after 10 months and the dapsone was successfully tapered.
|
Linear IgA bullous dermatosis is an autoimmune subepidermal blistering disease with linear IgA deposits found along the basement membrane of the skin. There are 3 major categories of LABD: drug induced, systemic disorder related, and idiopathic.1 Patients with LABD present with a pruritic vesicobullous eruption that tends to favor the trunk, proximal extremities, and acral regions of the body. Mucous membrane lesions are present in less than 50% of patients.2 Linear IgA bullous dermatosis may resemble bullous pemphigoid, erythema multiforme, dermatitis herpetiformis, or toxic epidermal necrolysis. The gold standard for diagnosis is immunofluorescence staining that shows linear IgA deposition along the skin’s basement membrane.1 Prognosis for LABD is variable; there is risk for persistence and scarring.2 The drug-induced form of LABD is associated with clearance with the removal of the inciting agent.1
There are several autoimmune disorders that have been described in association with human immunodeficiency virus (HIV).3 Autoimmune bullous dermatoses, while described, are very uncommon in the setting of HIV infection. Previously reported cases include bullous pemphigoid, epidermolysis bullosa acquisita, pemphigus herpetiformis, pemphigus vegetans, pemphigus vulgaris, and cicatricial pemphigoid.4-12 The presentation of LABD in an HIV-positive patient is extremely rare.
There are 3 proposed mechanisms by which HIV and autoimmune bullous dermatoses coexist: unregulated B-cell activation, loss of T-suppressor cell regulation, and molecular mimicry. In patients with HIV, infected macrophages increase production of IL-1 and IL-6, causing nonspecific stimulation of B cells. Further production of tumor necrosis factor and other lymphotoxins may kill CD8+ T-suppressor cells, which further reduces B-cell regulation and production of nonspecific antibodies. Unregulated B-cell activation could lead to proliferation of antiself-specific B cells and autoantibodies. Additionally, various autoantibodies may arise due to mimicry between HIV antigens and human proteins. Some of the antibodies produced may be cytotoxic antilymphocyte antibodies that further disrupt B-cell regulation.13,14
Zandman-Goddard and Shoenfeld14 proposed a staging system of autoimmune disease and HIV with respect to CD4 count and viral load. Stage I is clinical latency of HIV, with a high CD4 count (>500 cells/mm3) and high viral load, which correlates with an acute infection of HIV and an intact immune system. Autoimmune disease can be seen in this stage. Stage II is cellular response, a quiescent period without overt manifestations of AIDS. The CD4 count is declining (200–499 cells/mm3), indicating immunosuppression, and the viral count is high. Autoimmune disease can occur and typically includes immune complex–mediated disease and vasculitis. Stage III is immune deficiency. The CD4 count is low (<200 cells/mm3), viral load is high, and AIDS develops. Autoimmune disease is not seen during this stage. Stage IV is the period of immune restoration following the advent of highly active antiretroviral therapy. There is a high CD4 count (>500 cells/mm3) and low viral load. There is a resurgence of autoimmune disease in this stage. Autoimmune disease can occur with an immune system capable of B- and T-cell interactions and a normal CD4 count. Autoimmunity is possible in stages I, II, and IV.14 Our patient developed bullous disease in stage II.
Although uncommon, autoimmune disease is possible in the setting of immune deficiency. The presence of autoimmune disease in a patient with HIV can only be seen during certain stages of infection. Knowledge of the possible scenarios of autoimmune disease can assist the clinician with monitoring status of the HIV infection or immune reconstitution.
1. Bouldin MB, Clowers-Webb HE, Davis JL, et al. Naproxen-associated linear IgA bullous dermatosis: case report and review. Mayo Clin Proc. 2000;75:967-970.
2. Nousari HC, Kimyai-Asadi A, Caeiro JP, et al. Clinical, demographic, and immunohistologic features of vancomycin-induced linear IgA bullous disease of the skin: report of 2 cases and review of the literature. Medicine. 1999;78:1-8.
3. Gala S, Fulcher DA. How HIV leads to autoimmune disorders. Med J Aust. 1996;164:224-226.
4. Lateef A, Packles MR, White SM, et al. Pemphigus vegetans in association with human immunodeficiency virus. Int J Dermatol. 1999;38:778-781.
5. Levy PM, Balavoine JF, Salomon D, et al. Ritodrine-responsive bullous pemphigoing in a patient with AIDS-related complex. Br J Dermatol. 1986;114:635-636.
6. Bull RH, Fallowfield ME, Marsden RA. Autoimmune blistering diseases associated with HIV infection. Clin Exp Dermatol. 1994;19:47-50.
7. Chou K, Kauh YC, Jacoby RA, et al. Autoimmune bullous disease in a patient with HIV infection. J Am Acad Dermatol. 1991;24:1022-1023.
8. Mahé A, Flageul B, Prost C, et al. Pemphigus vegetans in an HIV-1-infected man. Clin Exp Dermatol. 1994;19:447.
9. Capizzi R, Marasca G, De Luca A, et al. Pemphigus vulgaris in a human-immunodeficiency-virus-infected patient. Dermatology. 1998;197:97-98.
10. Splaver A, Silos S, Lowell B, et al. Case report: pemphigus vulgaris in a patient infected with HIV. AIDS Patient Care STDS. 2000;14:295-296.
11. Hodgson TA, Fidler SJ, Speight PM, et al. Oral pemphigus vulgaris associated with HIV infection. J Am Acad Dermatol. 2003;49:313-315.
12. Demathé A, Arede LT, Miyahara GI. Mucous membrane pemphigoid in HIV patient: a case report. Cases J. 2008;1:345.
13. Etzioni A. Immune deficiency and autoimmunity. Autoimmun Rev. 2003;2:364-369.
14. Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329-337.
The Diagnosis: Linear IgA Bullous Dermatosis
A biopsy specimen from an intact vesicle was obtained. Histologic findings showed a basket weave stratum corneum suggestive of an acute process. There was subepidermal separation with an inflammatory infiltrate of neutrophils (Figure 1). Direct immunofluorescence yielded a pattern of IgA deposition along the dermoepidermal junction (Figure 2). A diagnosis of linear IgA bullous dermatosis (LABD) was made. The patient was started on 100 mg daily of dapsone. The dose was subsequently increased to 175 mg twice daily, resulting in complete clearance. He became dermatologically disease free after 10 months and the dapsone was successfully tapered.
|
Linear IgA bullous dermatosis is an autoimmune subepidermal blistering disease with linear IgA deposits found along the basement membrane of the skin. There are 3 major categories of LABD: drug induced, systemic disorder related, and idiopathic.1 Patients with LABD present with a pruritic vesicobullous eruption that tends to favor the trunk, proximal extremities, and acral regions of the body. Mucous membrane lesions are present in less than 50% of patients.2 Linear IgA bullous dermatosis may resemble bullous pemphigoid, erythema multiforme, dermatitis herpetiformis, or toxic epidermal necrolysis. The gold standard for diagnosis is immunofluorescence staining that shows linear IgA deposition along the skin’s basement membrane.1 Prognosis for LABD is variable; there is risk for persistence and scarring.2 The drug-induced form of LABD is associated with clearance with the removal of the inciting agent.1
There are several autoimmune disorders that have been described in association with human immunodeficiency virus (HIV).3 Autoimmune bullous dermatoses, while described, are very uncommon in the setting of HIV infection. Previously reported cases include bullous pemphigoid, epidermolysis bullosa acquisita, pemphigus herpetiformis, pemphigus vegetans, pemphigus vulgaris, and cicatricial pemphigoid.4-12 The presentation of LABD in an HIV-positive patient is extremely rare.
There are 3 proposed mechanisms by which HIV and autoimmune bullous dermatoses coexist: unregulated B-cell activation, loss of T-suppressor cell regulation, and molecular mimicry. In patients with HIV, infected macrophages increase production of IL-1 and IL-6, causing nonspecific stimulation of B cells. Further production of tumor necrosis factor and other lymphotoxins may kill CD8+ T-suppressor cells, which further reduces B-cell regulation and production of nonspecific antibodies. Unregulated B-cell activation could lead to proliferation of antiself-specific B cells and autoantibodies. Additionally, various autoantibodies may arise due to mimicry between HIV antigens and human proteins. Some of the antibodies produced may be cytotoxic antilymphocyte antibodies that further disrupt B-cell regulation.13,14
Zandman-Goddard and Shoenfeld14 proposed a staging system of autoimmune disease and HIV with respect to CD4 count and viral load. Stage I is clinical latency of HIV, with a high CD4 count (>500 cells/mm3) and high viral load, which correlates with an acute infection of HIV and an intact immune system. Autoimmune disease can be seen in this stage. Stage II is cellular response, a quiescent period without overt manifestations of AIDS. The CD4 count is declining (200–499 cells/mm3), indicating immunosuppression, and the viral count is high. Autoimmune disease can occur and typically includes immune complex–mediated disease and vasculitis. Stage III is immune deficiency. The CD4 count is low (<200 cells/mm3), viral load is high, and AIDS develops. Autoimmune disease is not seen during this stage. Stage IV is the period of immune restoration following the advent of highly active antiretroviral therapy. There is a high CD4 count (>500 cells/mm3) and low viral load. There is a resurgence of autoimmune disease in this stage. Autoimmune disease can occur with an immune system capable of B- and T-cell interactions and a normal CD4 count. Autoimmunity is possible in stages I, II, and IV.14 Our patient developed bullous disease in stage II.
Although uncommon, autoimmune disease is possible in the setting of immune deficiency. The presence of autoimmune disease in a patient with HIV can only be seen during certain stages of infection. Knowledge of the possible scenarios of autoimmune disease can assist the clinician with monitoring status of the HIV infection or immune reconstitution.
The Diagnosis: Linear IgA Bullous Dermatosis
A biopsy specimen from an intact vesicle was obtained. Histologic findings showed a basket weave stratum corneum suggestive of an acute process. There was subepidermal separation with an inflammatory infiltrate of neutrophils (Figure 1). Direct immunofluorescence yielded a pattern of IgA deposition along the dermoepidermal junction (Figure 2). A diagnosis of linear IgA bullous dermatosis (LABD) was made. The patient was started on 100 mg daily of dapsone. The dose was subsequently increased to 175 mg twice daily, resulting in complete clearance. He became dermatologically disease free after 10 months and the dapsone was successfully tapered.
|
Linear IgA bullous dermatosis is an autoimmune subepidermal blistering disease with linear IgA deposits found along the basement membrane of the skin. There are 3 major categories of LABD: drug induced, systemic disorder related, and idiopathic.1 Patients with LABD present with a pruritic vesicobullous eruption that tends to favor the trunk, proximal extremities, and acral regions of the body. Mucous membrane lesions are present in less than 50% of patients.2 Linear IgA bullous dermatosis may resemble bullous pemphigoid, erythema multiforme, dermatitis herpetiformis, or toxic epidermal necrolysis. The gold standard for diagnosis is immunofluorescence staining that shows linear IgA deposition along the skin’s basement membrane.1 Prognosis for LABD is variable; there is risk for persistence and scarring.2 The drug-induced form of LABD is associated with clearance with the removal of the inciting agent.1
There are several autoimmune disorders that have been described in association with human immunodeficiency virus (HIV).3 Autoimmune bullous dermatoses, while described, are very uncommon in the setting of HIV infection. Previously reported cases include bullous pemphigoid, epidermolysis bullosa acquisita, pemphigus herpetiformis, pemphigus vegetans, pemphigus vulgaris, and cicatricial pemphigoid.4-12 The presentation of LABD in an HIV-positive patient is extremely rare.
There are 3 proposed mechanisms by which HIV and autoimmune bullous dermatoses coexist: unregulated B-cell activation, loss of T-suppressor cell regulation, and molecular mimicry. In patients with HIV, infected macrophages increase production of IL-1 and IL-6, causing nonspecific stimulation of B cells. Further production of tumor necrosis factor and other lymphotoxins may kill CD8+ T-suppressor cells, which further reduces B-cell regulation and production of nonspecific antibodies. Unregulated B-cell activation could lead to proliferation of antiself-specific B cells and autoantibodies. Additionally, various autoantibodies may arise due to mimicry between HIV antigens and human proteins. Some of the antibodies produced may be cytotoxic antilymphocyte antibodies that further disrupt B-cell regulation.13,14
Zandman-Goddard and Shoenfeld14 proposed a staging system of autoimmune disease and HIV with respect to CD4 count and viral load. Stage I is clinical latency of HIV, with a high CD4 count (>500 cells/mm3) and high viral load, which correlates with an acute infection of HIV and an intact immune system. Autoimmune disease can be seen in this stage. Stage II is cellular response, a quiescent period without overt manifestations of AIDS. The CD4 count is declining (200–499 cells/mm3), indicating immunosuppression, and the viral count is high. Autoimmune disease can occur and typically includes immune complex–mediated disease and vasculitis. Stage III is immune deficiency. The CD4 count is low (<200 cells/mm3), viral load is high, and AIDS develops. Autoimmune disease is not seen during this stage. Stage IV is the period of immune restoration following the advent of highly active antiretroviral therapy. There is a high CD4 count (>500 cells/mm3) and low viral load. There is a resurgence of autoimmune disease in this stage. Autoimmune disease can occur with an immune system capable of B- and T-cell interactions and a normal CD4 count. Autoimmunity is possible in stages I, II, and IV.14 Our patient developed bullous disease in stage II.
Although uncommon, autoimmune disease is possible in the setting of immune deficiency. The presence of autoimmune disease in a patient with HIV can only be seen during certain stages of infection. Knowledge of the possible scenarios of autoimmune disease can assist the clinician with monitoring status of the HIV infection or immune reconstitution.
1. Bouldin MB, Clowers-Webb HE, Davis JL, et al. Naproxen-associated linear IgA bullous dermatosis: case report and review. Mayo Clin Proc. 2000;75:967-970.
2. Nousari HC, Kimyai-Asadi A, Caeiro JP, et al. Clinical, demographic, and immunohistologic features of vancomycin-induced linear IgA bullous disease of the skin: report of 2 cases and review of the literature. Medicine. 1999;78:1-8.
3. Gala S, Fulcher DA. How HIV leads to autoimmune disorders. Med J Aust. 1996;164:224-226.
4. Lateef A, Packles MR, White SM, et al. Pemphigus vegetans in association with human immunodeficiency virus. Int J Dermatol. 1999;38:778-781.
5. Levy PM, Balavoine JF, Salomon D, et al. Ritodrine-responsive bullous pemphigoing in a patient with AIDS-related complex. Br J Dermatol. 1986;114:635-636.
6. Bull RH, Fallowfield ME, Marsden RA. Autoimmune blistering diseases associated with HIV infection. Clin Exp Dermatol. 1994;19:47-50.
7. Chou K, Kauh YC, Jacoby RA, et al. Autoimmune bullous disease in a patient with HIV infection. J Am Acad Dermatol. 1991;24:1022-1023.
8. Mahé A, Flageul B, Prost C, et al. Pemphigus vegetans in an HIV-1-infected man. Clin Exp Dermatol. 1994;19:447.
9. Capizzi R, Marasca G, De Luca A, et al. Pemphigus vulgaris in a human-immunodeficiency-virus-infected patient. Dermatology. 1998;197:97-98.
10. Splaver A, Silos S, Lowell B, et al. Case report: pemphigus vulgaris in a patient infected with HIV. AIDS Patient Care STDS. 2000;14:295-296.
11. Hodgson TA, Fidler SJ, Speight PM, et al. Oral pemphigus vulgaris associated with HIV infection. J Am Acad Dermatol. 2003;49:313-315.
12. Demathé A, Arede LT, Miyahara GI. Mucous membrane pemphigoid in HIV patient: a case report. Cases J. 2008;1:345.
13. Etzioni A. Immune deficiency and autoimmunity. Autoimmun Rev. 2003;2:364-369.
14. Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329-337.
1. Bouldin MB, Clowers-Webb HE, Davis JL, et al. Naproxen-associated linear IgA bullous dermatosis: case report and review. Mayo Clin Proc. 2000;75:967-970.
2. Nousari HC, Kimyai-Asadi A, Caeiro JP, et al. Clinical, demographic, and immunohistologic features of vancomycin-induced linear IgA bullous disease of the skin: report of 2 cases and review of the literature. Medicine. 1999;78:1-8.
3. Gala S, Fulcher DA. How HIV leads to autoimmune disorders. Med J Aust. 1996;164:224-226.
4. Lateef A, Packles MR, White SM, et al. Pemphigus vegetans in association with human immunodeficiency virus. Int J Dermatol. 1999;38:778-781.
5. Levy PM, Balavoine JF, Salomon D, et al. Ritodrine-responsive bullous pemphigoing in a patient with AIDS-related complex. Br J Dermatol. 1986;114:635-636.
6. Bull RH, Fallowfield ME, Marsden RA. Autoimmune blistering diseases associated with HIV infection. Clin Exp Dermatol. 1994;19:47-50.
7. Chou K, Kauh YC, Jacoby RA, et al. Autoimmune bullous disease in a patient with HIV infection. J Am Acad Dermatol. 1991;24:1022-1023.
8. Mahé A, Flageul B, Prost C, et al. Pemphigus vegetans in an HIV-1-infected man. Clin Exp Dermatol. 1994;19:447.
9. Capizzi R, Marasca G, De Luca A, et al. Pemphigus vulgaris in a human-immunodeficiency-virus-infected patient. Dermatology. 1998;197:97-98.
10. Splaver A, Silos S, Lowell B, et al. Case report: pemphigus vulgaris in a patient infected with HIV. AIDS Patient Care STDS. 2000;14:295-296.
11. Hodgson TA, Fidler SJ, Speight PM, et al. Oral pemphigus vulgaris associated with HIV infection. J Am Acad Dermatol. 2003;49:313-315.
12. Demathé A, Arede LT, Miyahara GI. Mucous membrane pemphigoid in HIV patient: a case report. Cases J. 2008;1:345.
13. Etzioni A. Immune deficiency and autoimmunity. Autoimmun Rev. 2003;2:364-369.
14. Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329-337.
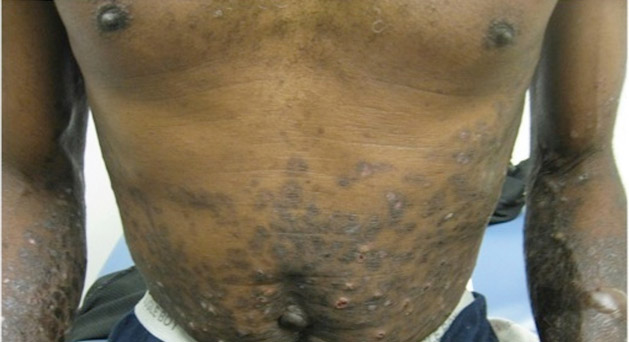
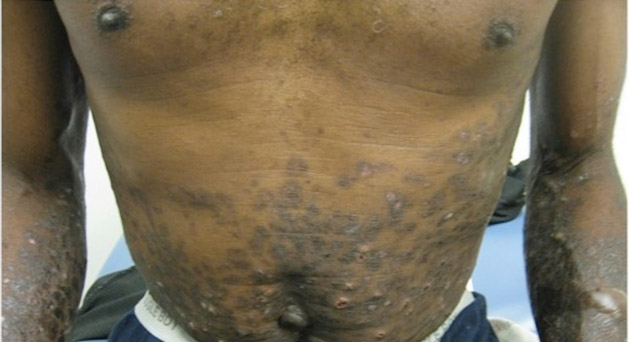
A 50-year-old black man presented with a new-onset widespread pruritic bullous eruption 7 months after being diagnosed with human immunodeficiency virus. The CD4 lymphocyte count was 421 cells/mm3 and viral load was 7818 copies/mL. Results of a viral culture were negative for herpes simplex virus. Dermatologic examination revealed numerous intact tense bullae as well as scattered erosions on the trunk and extremities. Postinflammatory hyperpigmentation was prominent, with some areas of hypopigmentation and depigmentation.