User login
Cisplatin-Induced Acute Kidney Injury and Renal Salt Wasting Syndrome
A treatment strategy that incorporates both water restrictions and sodium supplementation may be appropriate when differentiating between diagnoses of renal salt wasting syndrome and syndrome of inappropriate antidiuretic hormone secretion.
Cisplatin is a potent antineoplastic agent derived from platinum and commonly used in the treatment of head and neck, bladder, ovarian, and testicular malignancies.1,2 Approximately 20% of all cancer patients are prescribed platinum-based chemotherapeutics.3 Although considered highly effective, cisplatin is also a dose-dependent nephrotoxin, inducing apoptosis in the proximal tubules of the nephron and reducing glomerular filtration rate. This nephron injury leads to inflammation and reduced medullary blood flow, causing further ischemic damage to the tubular cells.4 Given that the proximal tubule reabsorbs 67% of all sodium, cisplatin-induced nephron injuries can also lead to hyponatremia.5
The primary mechanisms of hyponatremia following cisplatin chemotherapy are syndrome of inappropriate antidiuretic hormone secretion (SIADH) and renal salt wasting syndrome (RSWS). Though these diagnoses have similar presentations, the treatment recommendations are different due to pathophysiologic differences. Fluid restriction is the hallmark of SIADH treatment, while increased sodium intake remains the hallmark of RSWS treatment.6 This patient presented with a combination of cisplatin-induced acute kidney injury (AKI) and hyponatremia secondary to RSWS. While RSWS and AKI are known complications of cisplatin chemotherapy, the combination is underreported in the literature. Therefore, this case report highlights the combination of these cisplatin-induced complications, emphasizes the clinical challenges in differentiating SIADH from RSWS, especially in the presence of a concomitant AKI, and suggests a treatment approach during diagnostic uncertainty.
Case Presentation
A 71-year-old man with a medical history of squamous cell carcinoma (SCC) of the left neck on cycle 1, day 8 of cisplatin-based chemotherapy and ongoing radiation therapy (720 cGy of 6300 cGy), lung adenocarcinoma status postresection, and hyperlipidemia presented to the emergency department (ED) at the request of his oncologist for abnormal laboratory values. In the ED, his metabolic panel showed a 131-mmol/L serum sodium, 3.3 mmol/L potassium, 83 mmol/L chloride, 29 mmol/L bicarbonate, 61 mg/dL blood urea nitrogen (BUN), and 8.8 mg/dL creatinine (baseline, 0.9 mg/dL). The patient reported throbbing headaches, persistent nausea, and multiple episodes of nonbloody emesis for several days that he attributed to his chemotherapy. He noted decreased urination without discomfort or changes in color or odor and no fatigue, fevers, chills, hematuria, flank, abdominal pain, thirst, or polydipsia. He reported no toxic ingestions or IV drug use. The patient had no relevant family history or additional social history. His outpatient medications included 10 mg cetirizine, 8 mg ondansetron, and 81 mg aspirin. On initial examination, his 137/66 mm Hg blood pressure was mildly elevated. The physical examination findings were notable for a 5-cm mass in the left neck that was firm and irregularly-shaped. His physical examination was otherwise unremarkable. He was admitted to the inpatient medicine service for an AKI complicated by symptomatic hyponatremia.
Investigations
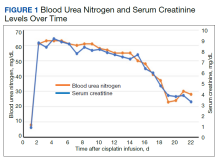
We evaluated the patient’s AKI based on treatment responsiveness, imaging, and laboratory testing. Renal and bladder ultrasound showed no evidence of hydronephrosis or obstruction. He had a benign urinalysis with microscopy absent for protein, blood, ketones, leukocyte esterase, nitrites, and cellular casts. His urine pH was 5.5 (reference range, 5.0-9.0) and specific gravity was 1.011 (reference range, 1.005-1.030). His urine electrolytes revealed 45-mmol/L urine sodium (reference range, 40-220), 33-mmol/L urine chloride (reference range, 110-250), 10-mmol/L urine potassium (reference range, 25-120), 106.7-mg/dL urine creatinine (reference range, 10-400) and a calculated 2.7% fractional excretion of sodium (FENa) and 22.0-mEq/L elevated urine anion gap. As a fluid challenge, he was treated with IV 0.9% sodium chloride at 100-125 mL/h, receiving 3 liters over the first 48 hours of his hospitalization. His creatinine peaked at 9.2 mg/dL and stabilized before improving later in his hospitalization (Figure 1). The patient initially had oliguria (< 0.5 mL/kg/h), which slowly improved over his hospital course. Unfortunately, due to multiple system and clinical factors, accurate inputs and outputs were not adequately maintained during his hospitalization.
We evaluated hyponatremia with a combination of serum and urine laboratory tests. In addition to urine electrolytes, the initial evaluation focused on trending his clinical trajectory. We repeated a basic metabolic panel every 4 to 6 hours. He had 278-mOsm/kg serum osmolality (reference range, 285-295) with an effective 217-mOsm/kg serum tonicity. His urine osmolality was 270.5 mOsm/kg.
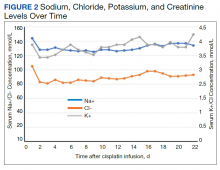
Despite administering 462 mEq sodium via crystalloid, his sodium worsened over the first 48 hours, reaching a nadir at 125 mmol/L on hospital day 3 (Figure 2). While he continued to appear euvolemic on physical examination, his blood pressure became difficult to control with 160- to 180-mm Hg systolic blood pressure readings. His thyroid stimulating hormone (TSH) was normal and aldosterone was low (4 ng/dL). Additional urine studies, including a 24-hour urine sample, were collected for further evaluation. His urine uric acid was 140 mg/d (reference range, 120-820); his serum uric acid level was 8.2 mg/dL (reference range, 3.0-9.0). His 24-hour urine creatinine was 0.57 g/d (reference range, 0.50-2.15) and uric acid to creatinine ratio was 246 mg/g (reference range, 60-580). His serum creatinine collected from the same day as his 24-hour urine sample was 7.3 mg/dL. His fractional excretion of uric acid (FEurate) was 21.9%.
Differential Diagnosis
The patient’s recent administration of cisplatin raised clinical suspicion of cisplatin-induced AKI. To avoid premature diagnostic closure, we used a systematic approach for thinking about our patient’s AKI, considering prerenal, intrarenal, and postrenal etiologies. The unremarkable renal and bladder ultrasound made a postrenal etiology unlikely. The patient’s 2.7% FENa in the absence of a diuretic, limited responsiveness to crystalloid fluid resuscitation, 7.5 serum BUN/creatinine ratio, and 270.5 mOsm/kg urine osmolality suggested an intrarenal etiology, which can be further divided into problems with glomeruli, tubules, small vessels, or interstitial space. The patient’s normal urinary microscopy with no evidence of protein, blood, ketones, leukocyte esterase, nitrites, or cellular casts made a glomerular etiology less likely. The acute onset and lack of additional systemic features, other than hypertension, made a vascular etiology less likely. A tubular etiology, such as acute tubular necrosis (ATN), was highest on the differential and was followed by an interstitial etiology, such as acute interstitial nephritis (AIN).
Patients with drug-induced AIN commonly present with signs and symptoms of an allergic-type reaction, including fever, rash, hematuria, pyuria, and costovertebral angle tenderness. The patient lacked these symptoms. However, cisplatin is known to cause ATN in up to 20-30% of patients.7 Therefore, despite the lack of the classic muddy-brown, granular casts on urine microscopy, cisplatin-induced ATN remained the most likely etiology of his AKI. Moreover, ATN can cause hyponatremia. ATN is characterized by 3 phases: initiation, maintenance, and recovery phases.8 Hyponatremia occurs during the recovery phase, typically starting weeks after renal insult and associated with high urine output and diuresis. This patient presented 1 week after injury and had persistent oliguria, making ATN an unlikely culprit of his hyponatremia.
Our patient presented with hypotonic hyponatremia with a 131 mmol/L initial sodium level and an < 280 mOsm/kg effective serum osmolality, or serum tonicity. The serum tonicity is equivalent to the difference between the measured serum osmolality and the BUN. In the setting of profound AKI, this adjustment is essential for correctly categorizing a patient’s hyponatremia as hyper-, iso-, or hypotonic. The differential diagnosis for this patient’s hypotonic hyponatremia included dilutional effects of hypervolemia, SIADH, hyperthyroidism, adrenal insufficiency, and RSWS. The patient’s volume examination, lack of predisposing comorbidities or suggestive biomarkers, and > 20 mmol/L urinary sodium made hypervolemia unlikely. His urinary osmolality and specific gravity made primary polydipsia unlikely. We worked up his hyponatremia according to a diagnostic algorithm (eAppendix available at doi:10.12788/fp.0198).
The patient had a 217 mOsm/kg serum tonicity and a 270.5 mOsm/kg urine osmolality, consistent with impaired water excretion. His presentation, TSH, and concordant decrease in sodium and potassium made an endocrine etiology of his hyponatremia less likely. In hindsight, a serum cortisol would have been beneficial to more completely exclude adrenal insufficiency. His urine sodium was elevated at 45 mmol/L, raising concern for RSWS or SIADH. The FEurate helped to distinguish between SIADH and RSWS. While FEurate is often elevated in both SIADH and RSWS initially, the FEurate normalizes in SIADH with normalization of the serum sodium. The ideal cutoff for posthyponatremia correction FEurate is debated; however, a FEurate value after sodium correction < 11% suggests SIADH while a value > 11% suggests RSWS.9 Our patient’s FEurate following the sodium correction (serum sodium 134 mmol/L) was 21.9%, most suggestive of RSWS.
Treatment
Upon admission, initial treatment focused on resolving the patient’s AKI. The oncology team discontinued the cisplatin-based chemotherapy. His medication dosages were adjusted for his renal function and additional nephrotoxins avoided. In consultation, the nephrology service recommended 100 mL/h fluid resuscitation. After the patient received 3 L of 0.9% sodium chloride, his creatinine showed limited improvement and his sodium worsened, trending from 131 mmol/L to a nadir of 125 mmol/L. We initiated oral free-water restriction while continuing IV infusion of 0.9% sodium chloride at 125 mL/h.
We further augmented his sodium intake with 1-g sodium chloride tablets with each meal. By hospital day 6, the patient’s serum sodium, BUN, and creatinine improved to 130 mEq/L, 50 mg/dL, and 7.7 mg/dL, respectively. We then discontinued the oral sodium chloride tablets, fluid restriction, and IV fluids in a stepwise fashion prior to discharge. At discharge, the patient’s serum sodium was 136 mEq/L and creatinine, 4.8 mg/dL. The patient’s clinical course was complicated by symptomatic hypertension with systolic blood pressures about 180 mm Hg, requiring intermittent IV hydralazine, which was transitioned to daily nifedipine. Concerned that fluid resuscitation contributed to his hypertension, the patient also received several doses of furosemide. At time of discharge, the patient remained hypertensive and was discharged with nifedipine 90 mg daily.
Outcome and Follow-up
The patient has remained stable clinically since discharge. One week after discharge, his serum sodium and creatinine were 138 mmol/L and 3.8 mg/dL, respectively. More than 1 month after discharge, his sodium remains in the reference range and his creatinine was stable at about 3.5 mg/dL. He continues to follow-up with nephrology, oncology, and radiation oncology. He has restarted chemotherapy with a carboplatin-based regimen without recurrence of hyponatremia or AKI. His blood pressure has gradually improved to the point where he no longer requires nifedipine.
Discussion
The US Food and Drug Administration first approved the use of cisplatin, an alkylating agent that inhibits DNA replication, in 1978 for the treatment of testicular cancer.10 Since its approval, cisplatin has increased in popularity and is now considered one of the most effective antineoplastic agents for the treatment of solid tumors.1 Unfortunately, cisplatin has a well-documented adverse effect profile that includes neurotoxicity, gastrointestinal toxicity, nephrotoxicity, and ototoxicity.4 Despite frequent nephrotoxicity, cisplatin only occasionally causes hyponatremia and rarely causes RSWS, a known but potentially fatal complication. Moreover, the combination of AKI and RSWS is unique. Our patient presented with the unique combination of AKI and hyponatremia, most consistent with RSWS, likely precipitated from cisplatin chemotherapy. Through this case, we review cisplatin-associated electrolyte abnormalities, highlight the challenge of differentiating SIADH and RSWS, and suggest a treatment approach for hyponatremia during the period of diagnostic uncertainty.
Blachley and colleagues first discussed renal and electrolyte disturbances, specifically magnesium wasting, secondary to cisplatin use in 1981. In 1984, Kurtzberg and colleagues noted salt wasting in 2 patients receiving cisplatin therapy. The authors suggested that cisplatin inhibits solute transport in the thick ascending limb, causing clinically significant electrolyte abnormalities, coining the term cisplatin-induced salt wasting.11
The prevalence of cisplatin-induced salt wasting is unclear and likely underreported. In 1988, Hutchinson and colleagues conducted a prospective cohort study and noted 10% of patients (n = 70) developed RSWS at some point over 18 months of cisplatin therapy—a higher rate than previously estimated.12 In 1992, another prospective cohort study evaluated the adverse effects of 47 patients with non-small cell lung cancer treated with cisplatin and reported hyponatremia in 43% of its 93 courses of chemotherapy. The authors did not report the etiology of these hyponatremia cases.13 Given the diagnostic challenge, RSWS may be underrepresented as a confirmed etiology of hyponatremia in cisplatin treatment.
Hyponatremia from cisplatin may present as either SIADH or RSWS, complicating treatment decisions. Both conditions lead to hypotonic hyponatremia with urine osmolality > 100 mOSm/kg and urine sodium levels > 40 mmol/L. However, pathophysiology behind SIADH and RSWS is different. In RSWS, proximal tubule damage causes hyponatremia, decreasing sodium reabsorption, and leading to impaired concentration gradient in every segment of the nephron. As a result, RSWS can lead to profound hyponatremia. Treatment typically consists of increasing sodium intake to correct serum sodium with salt tablets and hypertonic sodium chloride while treating the underlying etiology, in our case removing the offending agent, and waiting for proximal tubule function to recover.6 On the other hand, in SIADH, elevated antidiuretic hormone (ADH) increases water reabsorption in the collecting duct, which has no impact on concentration gradients of the other nephron segments.14 Free-water restriction is the hallmark of SIADH treatment. Severe SIADH may require sodium repletion and/or the initiation of vaptans, ADH antagonists that competitively inhibit V2 receptors in the collecting duct to prevent water reabsorption.15
Our patient had an uncertain etiology of his hyponatremia throughout most of his treatment course, complicating our treatment decision-making. Initially, his measured serum osmolality was 278 mOsm/kg; however, his effective tonicity was lower. His AKI elevated his BUN, which in turnrequired us to calculate his serum tonicity (217 mOsm/kg) that was consistent with hypotonic hyponatremia. His elevated urine osmolality and urine sodium levels made SIADH and RSWS the most likely etiologies of his hyponatremia. To confirm the etiology, we waited for correction of his serum sodium. Therefore, we treated him with a combination of sodium repletion with 0.9% sodium chloride (154 mEq/L), hypertonic relative to his serum sodium, sodium chloride tablets, and free-water restriction. In this approach, we attempted to harmonize the treatment strategies for both SIADH and RSWS and effectively corrected his serum sodium. We evaluated his response to our treatment with a basic metabolic panel every 6 to 8 hours. Had his serum sodium decreased < 120 mmol/L, we planned to transfer the patient to the intensive care unit for 3% sodium chloride and/or intensification of his fluid restriction. A significant worsening of his hyponatremia would have strongly suggested hyponatremia secondary to SIADH since isotonic saline can worsen hyponatremia due to increased free-water reabsorption in the collecting duct.16
To differentiate between SIADH and RSWS, we relied on the FEurate after sodium correction. Multiple case reports from Japan have characterized the distinction between the processes through FEurate and serum uric acid. While the optimal cut-off values for FEurate require additional investigation, values < 11% after serum sodium correction suggests SIADH, while a value > 11% suggests RSWS.17 Prior cases have also emphasized serum hypouricemia as a distinguishing characteristic in RSWS. However, our case illustrates that serum hypouricemia is less reliable in the setting of AKI. Due to his severe AKI, our patient could not efficiently clear uric acid, likely contributing to his hyperuricemia.
Ultimately, our patient had an FEurate > 20%, which was suggestive of RSWS. Nevertheless, we recognize limitations and confounders in our diagnosis and have reflected on our diagnostic and management choices. First, the sensitivity and specificity of postsodium correction FEurate is unknown. Tracking the change in FEurate with our interventions would have increased our diagnostic utility, as suggested by Maesaka and colleagues.14 Second, our patient’s serum sodium was still at the lower end of the reference range after treatment, which may decrease the specificity of FEurate. Third, a plasma ADH collected during the initial phase of symptomatic hyponatremia would have helped differentiate between SIADH and RSWS.
Other diagnostic tests that could have excluded alternative diagnoses with even greater certainty include plasma adrenocorticotropic hormone, B-type natriuretic peptide, renin, cortisol, and thyroid function tests. From a practical standpoint, these laboratory results (excluding thyroid function test and brain natriuretic peptide) would have taken several weeks to result at our institution, limiting their clinical utility. Similarly, FEurate also has limited clinical utility, requiring effective treatment as part of the diagnostic test. Therefore, we recommend focusing on optimal treatment for hyponatremia of uncertain etiology, especially where SIADH and RSWS are the leading diagnoses.
Conclusions
We described a rare case of concomitant cisplatin-induced severe AKI and RSWS. We have emphasized the diagnostic challenge of distinguishing between SIADH and RSWS, especially with concomitant AKI, and have acknowledged that optimal treatment relies on accurate differentiation. However, differentiation may not be clinically feasible. Therefore, we suggest a treatment strategy that incorporates both free-water restriction and sodium supplementation via IV and/or oral administration.
1. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364-378. doi:10.1016/j.ejphar.2014.07.025
2. Holditch SJ, Brown CN, Lombardi AM, Nguyen KN, Edelstein CL. Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int J Mol Sci. 2019;20(12):3011. Published 2019 Jun 20. doi:10.3390/ijms20123011
3. National Institutes of Health, National Cancer Institute. The “accidental” cure—platinum-based treatment for cancer: the discovery of cisplatin. Published May 30, 2014. Accessed November 10, 2021. https://www.cancer.gov/research/progress/discovery/cisplatin
4. Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014;2014:967826. doi:10.1155/2014/967826
5. Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol. 2015;10(4):676-687. doi:10.2215/CJN.12391213
6. Bitew S, Imbriano L, Miyawaki N, Fishbane S, Maesaka JK. More on renal salt wasting without cerebral disease: response to saline infusion. Clin J Am Soc Nephrol. 2009;4(2):309-315. doi:10.2215/CJN.02740608
7. Shirali AC, Perazella MA. Tubulointerstitial injury associated with chemotherapeutic agents. Adv Chronic Kidney Dis. 2014;21(1):56-63. doi:10.1053/j.ackd.2013.06.010
8. Agrawal M, Swartz R. Acute renal failure [published correction appears in Am Fam Physician 2001 Feb 1;63(3):445]. Am Fam Physician. 2000;61(7):2077-2088.
9. Milionis HJ, Liamis GL, Elisaf MS. The hyponatremic patient: a systematic approach to laboratory diagnosis. CMAJ. 2002;166(8):1056-1062.
10. Monneret C. Platinum anticancer drugs. From serendipity to rational design. Ann Pharm Fr. 2011;69(6):286-295. doi:10.1016/j.pharma.2011.10.001
11. Kurtzberg J, Dennis VW, Kinney TR. Cisplatinum-induced renal salt wasting. Med Pediatr Oncol. 1984;12(2):150-154. doi:10.1002/mpo.2950120219
12. Hutchison FN, Perez EA, Gandara DR, Lawrence HJ, Kaysen GA. Renal salt wasting in patients treated with cisplatin. Ann Intern Med. 1988;108(1):21-25. doi:10.7326/0003-4819-108-1-21
13. Lee YK, Shin DM. Renal salt wasting in patients treated with high-dose cisplatin, etoposide, and mitomycin in patients with advanced non-small cell lung cancer. Korean J Intern Med. 1992;7(2):118-121. doi:10.3904/kjim.1992.7.2.118
14. Maesaka JK, Imbriano L, Mattana J, Gallagher D, Bade N, Sharif S. Differentiating SIADH from cerebral/renal salt wasting: failure of the volume approach and need for a new approach to hyponatremia. J Clin Med. 2014;3(4):1373-1385. Published 2014 Dec 8. doi:10.3390/jcm3041373
15. Palmer BF. The role of v2 receptor antagonists in the treatment of hyponatremia. Electrolyte Blood Press. 2013;11(1):1-8. doi:10.5049/EBP.2013.11.1.1
16. Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med. 2007;120(11 Suppl 1):S1-S21. doi:10.1016/j.amjmed.2007.09.001
17. Maesaka JK, Imbriano LJ, Miyawaki N. High prevalence of renal salt wasting without cerebral disease as cause of hyponatremia in general medical wards. Am J Med Sci. 2018;356(1):15-22. doi:10.1016/j.amjms.2018.03.02
A treatment strategy that incorporates both water restrictions and sodium supplementation may be appropriate when differentiating between diagnoses of renal salt wasting syndrome and syndrome of inappropriate antidiuretic hormone secretion.
A treatment strategy that incorporates both water restrictions and sodium supplementation may be appropriate when differentiating between diagnoses of renal salt wasting syndrome and syndrome of inappropriate antidiuretic hormone secretion.
Cisplatin is a potent antineoplastic agent derived from platinum and commonly used in the treatment of head and neck, bladder, ovarian, and testicular malignancies.1,2 Approximately 20% of all cancer patients are prescribed platinum-based chemotherapeutics.3 Although considered highly effective, cisplatin is also a dose-dependent nephrotoxin, inducing apoptosis in the proximal tubules of the nephron and reducing glomerular filtration rate. This nephron injury leads to inflammation and reduced medullary blood flow, causing further ischemic damage to the tubular cells.4 Given that the proximal tubule reabsorbs 67% of all sodium, cisplatin-induced nephron injuries can also lead to hyponatremia.5
The primary mechanisms of hyponatremia following cisplatin chemotherapy are syndrome of inappropriate antidiuretic hormone secretion (SIADH) and renal salt wasting syndrome (RSWS). Though these diagnoses have similar presentations, the treatment recommendations are different due to pathophysiologic differences. Fluid restriction is the hallmark of SIADH treatment, while increased sodium intake remains the hallmark of RSWS treatment.6 This patient presented with a combination of cisplatin-induced acute kidney injury (AKI) and hyponatremia secondary to RSWS. While RSWS and AKI are known complications of cisplatin chemotherapy, the combination is underreported in the literature. Therefore, this case report highlights the combination of these cisplatin-induced complications, emphasizes the clinical challenges in differentiating SIADH from RSWS, especially in the presence of a concomitant AKI, and suggests a treatment approach during diagnostic uncertainty.
Case Presentation
A 71-year-old man with a medical history of squamous cell carcinoma (SCC) of the left neck on cycle 1, day 8 of cisplatin-based chemotherapy and ongoing radiation therapy (720 cGy of 6300 cGy), lung adenocarcinoma status postresection, and hyperlipidemia presented to the emergency department (ED) at the request of his oncologist for abnormal laboratory values. In the ED, his metabolic panel showed a 131-mmol/L serum sodium, 3.3 mmol/L potassium, 83 mmol/L chloride, 29 mmol/L bicarbonate, 61 mg/dL blood urea nitrogen (BUN), and 8.8 mg/dL creatinine (baseline, 0.9 mg/dL). The patient reported throbbing headaches, persistent nausea, and multiple episodes of nonbloody emesis for several days that he attributed to his chemotherapy. He noted decreased urination without discomfort or changes in color or odor and no fatigue, fevers, chills, hematuria, flank, abdominal pain, thirst, or polydipsia. He reported no toxic ingestions or IV drug use. The patient had no relevant family history or additional social history. His outpatient medications included 10 mg cetirizine, 8 mg ondansetron, and 81 mg aspirin. On initial examination, his 137/66 mm Hg blood pressure was mildly elevated. The physical examination findings were notable for a 5-cm mass in the left neck that was firm and irregularly-shaped. His physical examination was otherwise unremarkable. He was admitted to the inpatient medicine service for an AKI complicated by symptomatic hyponatremia.
Investigations
We evaluated the patient’s AKI based on treatment responsiveness, imaging, and laboratory testing. Renal and bladder ultrasound showed no evidence of hydronephrosis or obstruction. He had a benign urinalysis with microscopy absent for protein, blood, ketones, leukocyte esterase, nitrites, and cellular casts. His urine pH was 5.5 (reference range, 5.0-9.0) and specific gravity was 1.011 (reference range, 1.005-1.030). His urine electrolytes revealed 45-mmol/L urine sodium (reference range, 40-220), 33-mmol/L urine chloride (reference range, 110-250), 10-mmol/L urine potassium (reference range, 25-120), 106.7-mg/dL urine creatinine (reference range, 10-400) and a calculated 2.7% fractional excretion of sodium (FENa) and 22.0-mEq/L elevated urine anion gap. As a fluid challenge, he was treated with IV 0.9% sodium chloride at 100-125 mL/h, receiving 3 liters over the first 48 hours of his hospitalization. His creatinine peaked at 9.2 mg/dL and stabilized before improving later in his hospitalization (Figure 1). The patient initially had oliguria (< 0.5 mL/kg/h), which slowly improved over his hospital course. Unfortunately, due to multiple system and clinical factors, accurate inputs and outputs were not adequately maintained during his hospitalization.
We evaluated hyponatremia with a combination of serum and urine laboratory tests. In addition to urine electrolytes, the initial evaluation focused on trending his clinical trajectory. We repeated a basic metabolic panel every 4 to 6 hours. He had 278-mOsm/kg serum osmolality (reference range, 285-295) with an effective 217-mOsm/kg serum tonicity. His urine osmolality was 270.5 mOsm/kg.
Despite administering 462 mEq sodium via crystalloid, his sodium worsened over the first 48 hours, reaching a nadir at 125 mmol/L on hospital day 3 (Figure 2). While he continued to appear euvolemic on physical examination, his blood pressure became difficult to control with 160- to 180-mm Hg systolic blood pressure readings. His thyroid stimulating hormone (TSH) was normal and aldosterone was low (4 ng/dL). Additional urine studies, including a 24-hour urine sample, were collected for further evaluation. His urine uric acid was 140 mg/d (reference range, 120-820); his serum uric acid level was 8.2 mg/dL (reference range, 3.0-9.0). His 24-hour urine creatinine was 0.57 g/d (reference range, 0.50-2.15) and uric acid to creatinine ratio was 246 mg/g (reference range, 60-580). His serum creatinine collected from the same day as his 24-hour urine sample was 7.3 mg/dL. His fractional excretion of uric acid (FEurate) was 21.9%.
Differential Diagnosis
The patient’s recent administration of cisplatin raised clinical suspicion of cisplatin-induced AKI. To avoid premature diagnostic closure, we used a systematic approach for thinking about our patient’s AKI, considering prerenal, intrarenal, and postrenal etiologies. The unremarkable renal and bladder ultrasound made a postrenal etiology unlikely. The patient’s 2.7% FENa in the absence of a diuretic, limited responsiveness to crystalloid fluid resuscitation, 7.5 serum BUN/creatinine ratio, and 270.5 mOsm/kg urine osmolality suggested an intrarenal etiology, which can be further divided into problems with glomeruli, tubules, small vessels, or interstitial space. The patient’s normal urinary microscopy with no evidence of protein, blood, ketones, leukocyte esterase, nitrites, or cellular casts made a glomerular etiology less likely. The acute onset and lack of additional systemic features, other than hypertension, made a vascular etiology less likely. A tubular etiology, such as acute tubular necrosis (ATN), was highest on the differential and was followed by an interstitial etiology, such as acute interstitial nephritis (AIN).
Patients with drug-induced AIN commonly present with signs and symptoms of an allergic-type reaction, including fever, rash, hematuria, pyuria, and costovertebral angle tenderness. The patient lacked these symptoms. However, cisplatin is known to cause ATN in up to 20-30% of patients.7 Therefore, despite the lack of the classic muddy-brown, granular casts on urine microscopy, cisplatin-induced ATN remained the most likely etiology of his AKI. Moreover, ATN can cause hyponatremia. ATN is characterized by 3 phases: initiation, maintenance, and recovery phases.8 Hyponatremia occurs during the recovery phase, typically starting weeks after renal insult and associated with high urine output and diuresis. This patient presented 1 week after injury and had persistent oliguria, making ATN an unlikely culprit of his hyponatremia.
Our patient presented with hypotonic hyponatremia with a 131 mmol/L initial sodium level and an < 280 mOsm/kg effective serum osmolality, or serum tonicity. The serum tonicity is equivalent to the difference between the measured serum osmolality and the BUN. In the setting of profound AKI, this adjustment is essential for correctly categorizing a patient’s hyponatremia as hyper-, iso-, or hypotonic. The differential diagnosis for this patient’s hypotonic hyponatremia included dilutional effects of hypervolemia, SIADH, hyperthyroidism, adrenal insufficiency, and RSWS. The patient’s volume examination, lack of predisposing comorbidities or suggestive biomarkers, and > 20 mmol/L urinary sodium made hypervolemia unlikely. His urinary osmolality and specific gravity made primary polydipsia unlikely. We worked up his hyponatremia according to a diagnostic algorithm (eAppendix available at doi:10.12788/fp.0198).
The patient had a 217 mOsm/kg serum tonicity and a 270.5 mOsm/kg urine osmolality, consistent with impaired water excretion. His presentation, TSH, and concordant decrease in sodium and potassium made an endocrine etiology of his hyponatremia less likely. In hindsight, a serum cortisol would have been beneficial to more completely exclude adrenal insufficiency. His urine sodium was elevated at 45 mmol/L, raising concern for RSWS or SIADH. The FEurate helped to distinguish between SIADH and RSWS. While FEurate is often elevated in both SIADH and RSWS initially, the FEurate normalizes in SIADH with normalization of the serum sodium. The ideal cutoff for posthyponatremia correction FEurate is debated; however, a FEurate value after sodium correction < 11% suggests SIADH while a value > 11% suggests RSWS.9 Our patient’s FEurate following the sodium correction (serum sodium 134 mmol/L) was 21.9%, most suggestive of RSWS.
Treatment
Upon admission, initial treatment focused on resolving the patient’s AKI. The oncology team discontinued the cisplatin-based chemotherapy. His medication dosages were adjusted for his renal function and additional nephrotoxins avoided. In consultation, the nephrology service recommended 100 mL/h fluid resuscitation. After the patient received 3 L of 0.9% sodium chloride, his creatinine showed limited improvement and his sodium worsened, trending from 131 mmol/L to a nadir of 125 mmol/L. We initiated oral free-water restriction while continuing IV infusion of 0.9% sodium chloride at 125 mL/h.
We further augmented his sodium intake with 1-g sodium chloride tablets with each meal. By hospital day 6, the patient’s serum sodium, BUN, and creatinine improved to 130 mEq/L, 50 mg/dL, and 7.7 mg/dL, respectively. We then discontinued the oral sodium chloride tablets, fluid restriction, and IV fluids in a stepwise fashion prior to discharge. At discharge, the patient’s serum sodium was 136 mEq/L and creatinine, 4.8 mg/dL. The patient’s clinical course was complicated by symptomatic hypertension with systolic blood pressures about 180 mm Hg, requiring intermittent IV hydralazine, which was transitioned to daily nifedipine. Concerned that fluid resuscitation contributed to his hypertension, the patient also received several doses of furosemide. At time of discharge, the patient remained hypertensive and was discharged with nifedipine 90 mg daily.
Outcome and Follow-up
The patient has remained stable clinically since discharge. One week after discharge, his serum sodium and creatinine were 138 mmol/L and 3.8 mg/dL, respectively. More than 1 month after discharge, his sodium remains in the reference range and his creatinine was stable at about 3.5 mg/dL. He continues to follow-up with nephrology, oncology, and radiation oncology. He has restarted chemotherapy with a carboplatin-based regimen without recurrence of hyponatremia or AKI. His blood pressure has gradually improved to the point where he no longer requires nifedipine.
Discussion
The US Food and Drug Administration first approved the use of cisplatin, an alkylating agent that inhibits DNA replication, in 1978 for the treatment of testicular cancer.10 Since its approval, cisplatin has increased in popularity and is now considered one of the most effective antineoplastic agents for the treatment of solid tumors.1 Unfortunately, cisplatin has a well-documented adverse effect profile that includes neurotoxicity, gastrointestinal toxicity, nephrotoxicity, and ototoxicity.4 Despite frequent nephrotoxicity, cisplatin only occasionally causes hyponatremia and rarely causes RSWS, a known but potentially fatal complication. Moreover, the combination of AKI and RSWS is unique. Our patient presented with the unique combination of AKI and hyponatremia, most consistent with RSWS, likely precipitated from cisplatin chemotherapy. Through this case, we review cisplatin-associated electrolyte abnormalities, highlight the challenge of differentiating SIADH and RSWS, and suggest a treatment approach for hyponatremia during the period of diagnostic uncertainty.
Blachley and colleagues first discussed renal and electrolyte disturbances, specifically magnesium wasting, secondary to cisplatin use in 1981. In 1984, Kurtzberg and colleagues noted salt wasting in 2 patients receiving cisplatin therapy. The authors suggested that cisplatin inhibits solute transport in the thick ascending limb, causing clinically significant electrolyte abnormalities, coining the term cisplatin-induced salt wasting.11
The prevalence of cisplatin-induced salt wasting is unclear and likely underreported. In 1988, Hutchinson and colleagues conducted a prospective cohort study and noted 10% of patients (n = 70) developed RSWS at some point over 18 months of cisplatin therapy—a higher rate than previously estimated.12 In 1992, another prospective cohort study evaluated the adverse effects of 47 patients with non-small cell lung cancer treated with cisplatin and reported hyponatremia in 43% of its 93 courses of chemotherapy. The authors did not report the etiology of these hyponatremia cases.13 Given the diagnostic challenge, RSWS may be underrepresented as a confirmed etiology of hyponatremia in cisplatin treatment.
Hyponatremia from cisplatin may present as either SIADH or RSWS, complicating treatment decisions. Both conditions lead to hypotonic hyponatremia with urine osmolality > 100 mOSm/kg and urine sodium levels > 40 mmol/L. However, pathophysiology behind SIADH and RSWS is different. In RSWS, proximal tubule damage causes hyponatremia, decreasing sodium reabsorption, and leading to impaired concentration gradient in every segment of the nephron. As a result, RSWS can lead to profound hyponatremia. Treatment typically consists of increasing sodium intake to correct serum sodium with salt tablets and hypertonic sodium chloride while treating the underlying etiology, in our case removing the offending agent, and waiting for proximal tubule function to recover.6 On the other hand, in SIADH, elevated antidiuretic hormone (ADH) increases water reabsorption in the collecting duct, which has no impact on concentration gradients of the other nephron segments.14 Free-water restriction is the hallmark of SIADH treatment. Severe SIADH may require sodium repletion and/or the initiation of vaptans, ADH antagonists that competitively inhibit V2 receptors in the collecting duct to prevent water reabsorption.15
Our patient had an uncertain etiology of his hyponatremia throughout most of his treatment course, complicating our treatment decision-making. Initially, his measured serum osmolality was 278 mOsm/kg; however, his effective tonicity was lower. His AKI elevated his BUN, which in turnrequired us to calculate his serum tonicity (217 mOsm/kg) that was consistent with hypotonic hyponatremia. His elevated urine osmolality and urine sodium levels made SIADH and RSWS the most likely etiologies of his hyponatremia. To confirm the etiology, we waited for correction of his serum sodium. Therefore, we treated him with a combination of sodium repletion with 0.9% sodium chloride (154 mEq/L), hypertonic relative to his serum sodium, sodium chloride tablets, and free-water restriction. In this approach, we attempted to harmonize the treatment strategies for both SIADH and RSWS and effectively corrected his serum sodium. We evaluated his response to our treatment with a basic metabolic panel every 6 to 8 hours. Had his serum sodium decreased < 120 mmol/L, we planned to transfer the patient to the intensive care unit for 3% sodium chloride and/or intensification of his fluid restriction. A significant worsening of his hyponatremia would have strongly suggested hyponatremia secondary to SIADH since isotonic saline can worsen hyponatremia due to increased free-water reabsorption in the collecting duct.16
To differentiate between SIADH and RSWS, we relied on the FEurate after sodium correction. Multiple case reports from Japan have characterized the distinction between the processes through FEurate and serum uric acid. While the optimal cut-off values for FEurate require additional investigation, values < 11% after serum sodium correction suggests SIADH, while a value > 11% suggests RSWS.17 Prior cases have also emphasized serum hypouricemia as a distinguishing characteristic in RSWS. However, our case illustrates that serum hypouricemia is less reliable in the setting of AKI. Due to his severe AKI, our patient could not efficiently clear uric acid, likely contributing to his hyperuricemia.
Ultimately, our patient had an FEurate > 20%, which was suggestive of RSWS. Nevertheless, we recognize limitations and confounders in our diagnosis and have reflected on our diagnostic and management choices. First, the sensitivity and specificity of postsodium correction FEurate is unknown. Tracking the change in FEurate with our interventions would have increased our diagnostic utility, as suggested by Maesaka and colleagues.14 Second, our patient’s serum sodium was still at the lower end of the reference range after treatment, which may decrease the specificity of FEurate. Third, a plasma ADH collected during the initial phase of symptomatic hyponatremia would have helped differentiate between SIADH and RSWS.
Other diagnostic tests that could have excluded alternative diagnoses with even greater certainty include plasma adrenocorticotropic hormone, B-type natriuretic peptide, renin, cortisol, and thyroid function tests. From a practical standpoint, these laboratory results (excluding thyroid function test and brain natriuretic peptide) would have taken several weeks to result at our institution, limiting their clinical utility. Similarly, FEurate also has limited clinical utility, requiring effective treatment as part of the diagnostic test. Therefore, we recommend focusing on optimal treatment for hyponatremia of uncertain etiology, especially where SIADH and RSWS are the leading diagnoses.
Conclusions
We described a rare case of concomitant cisplatin-induced severe AKI and RSWS. We have emphasized the diagnostic challenge of distinguishing between SIADH and RSWS, especially with concomitant AKI, and have acknowledged that optimal treatment relies on accurate differentiation. However, differentiation may not be clinically feasible. Therefore, we suggest a treatment strategy that incorporates both free-water restriction and sodium supplementation via IV and/or oral administration.
Cisplatin is a potent antineoplastic agent derived from platinum and commonly used in the treatment of head and neck, bladder, ovarian, and testicular malignancies.1,2 Approximately 20% of all cancer patients are prescribed platinum-based chemotherapeutics.3 Although considered highly effective, cisplatin is also a dose-dependent nephrotoxin, inducing apoptosis in the proximal tubules of the nephron and reducing glomerular filtration rate. This nephron injury leads to inflammation and reduced medullary blood flow, causing further ischemic damage to the tubular cells.4 Given that the proximal tubule reabsorbs 67% of all sodium, cisplatin-induced nephron injuries can also lead to hyponatremia.5
The primary mechanisms of hyponatremia following cisplatin chemotherapy are syndrome of inappropriate antidiuretic hormone secretion (SIADH) and renal salt wasting syndrome (RSWS). Though these diagnoses have similar presentations, the treatment recommendations are different due to pathophysiologic differences. Fluid restriction is the hallmark of SIADH treatment, while increased sodium intake remains the hallmark of RSWS treatment.6 This patient presented with a combination of cisplatin-induced acute kidney injury (AKI) and hyponatremia secondary to RSWS. While RSWS and AKI are known complications of cisplatin chemotherapy, the combination is underreported in the literature. Therefore, this case report highlights the combination of these cisplatin-induced complications, emphasizes the clinical challenges in differentiating SIADH from RSWS, especially in the presence of a concomitant AKI, and suggests a treatment approach during diagnostic uncertainty.
Case Presentation
A 71-year-old man with a medical history of squamous cell carcinoma (SCC) of the left neck on cycle 1, day 8 of cisplatin-based chemotherapy and ongoing radiation therapy (720 cGy of 6300 cGy), lung adenocarcinoma status postresection, and hyperlipidemia presented to the emergency department (ED) at the request of his oncologist for abnormal laboratory values. In the ED, his metabolic panel showed a 131-mmol/L serum sodium, 3.3 mmol/L potassium, 83 mmol/L chloride, 29 mmol/L bicarbonate, 61 mg/dL blood urea nitrogen (BUN), and 8.8 mg/dL creatinine (baseline, 0.9 mg/dL). The patient reported throbbing headaches, persistent nausea, and multiple episodes of nonbloody emesis for several days that he attributed to his chemotherapy. He noted decreased urination without discomfort or changes in color or odor and no fatigue, fevers, chills, hematuria, flank, abdominal pain, thirst, or polydipsia. He reported no toxic ingestions or IV drug use. The patient had no relevant family history or additional social history. His outpatient medications included 10 mg cetirizine, 8 mg ondansetron, and 81 mg aspirin. On initial examination, his 137/66 mm Hg blood pressure was mildly elevated. The physical examination findings were notable for a 5-cm mass in the left neck that was firm and irregularly-shaped. His physical examination was otherwise unremarkable. He was admitted to the inpatient medicine service for an AKI complicated by symptomatic hyponatremia.
Investigations
We evaluated the patient’s AKI based on treatment responsiveness, imaging, and laboratory testing. Renal and bladder ultrasound showed no evidence of hydronephrosis or obstruction. He had a benign urinalysis with microscopy absent for protein, blood, ketones, leukocyte esterase, nitrites, and cellular casts. His urine pH was 5.5 (reference range, 5.0-9.0) and specific gravity was 1.011 (reference range, 1.005-1.030). His urine electrolytes revealed 45-mmol/L urine sodium (reference range, 40-220), 33-mmol/L urine chloride (reference range, 110-250), 10-mmol/L urine potassium (reference range, 25-120), 106.7-mg/dL urine creatinine (reference range, 10-400) and a calculated 2.7% fractional excretion of sodium (FENa) and 22.0-mEq/L elevated urine anion gap. As a fluid challenge, he was treated with IV 0.9% sodium chloride at 100-125 mL/h, receiving 3 liters over the first 48 hours of his hospitalization. His creatinine peaked at 9.2 mg/dL and stabilized before improving later in his hospitalization (Figure 1). The patient initially had oliguria (< 0.5 mL/kg/h), which slowly improved over his hospital course. Unfortunately, due to multiple system and clinical factors, accurate inputs and outputs were not adequately maintained during his hospitalization.
We evaluated hyponatremia with a combination of serum and urine laboratory tests. In addition to urine electrolytes, the initial evaluation focused on trending his clinical trajectory. We repeated a basic metabolic panel every 4 to 6 hours. He had 278-mOsm/kg serum osmolality (reference range, 285-295) with an effective 217-mOsm/kg serum tonicity. His urine osmolality was 270.5 mOsm/kg.
Despite administering 462 mEq sodium via crystalloid, his sodium worsened over the first 48 hours, reaching a nadir at 125 mmol/L on hospital day 3 (Figure 2). While he continued to appear euvolemic on physical examination, his blood pressure became difficult to control with 160- to 180-mm Hg systolic blood pressure readings. His thyroid stimulating hormone (TSH) was normal and aldosterone was low (4 ng/dL). Additional urine studies, including a 24-hour urine sample, were collected for further evaluation. His urine uric acid was 140 mg/d (reference range, 120-820); his serum uric acid level was 8.2 mg/dL (reference range, 3.0-9.0). His 24-hour urine creatinine was 0.57 g/d (reference range, 0.50-2.15) and uric acid to creatinine ratio was 246 mg/g (reference range, 60-580). His serum creatinine collected from the same day as his 24-hour urine sample was 7.3 mg/dL. His fractional excretion of uric acid (FEurate) was 21.9%.
Differential Diagnosis
The patient’s recent administration of cisplatin raised clinical suspicion of cisplatin-induced AKI. To avoid premature diagnostic closure, we used a systematic approach for thinking about our patient’s AKI, considering prerenal, intrarenal, and postrenal etiologies. The unremarkable renal and bladder ultrasound made a postrenal etiology unlikely. The patient’s 2.7% FENa in the absence of a diuretic, limited responsiveness to crystalloid fluid resuscitation, 7.5 serum BUN/creatinine ratio, and 270.5 mOsm/kg urine osmolality suggested an intrarenal etiology, which can be further divided into problems with glomeruli, tubules, small vessels, or interstitial space. The patient’s normal urinary microscopy with no evidence of protein, blood, ketones, leukocyte esterase, nitrites, or cellular casts made a glomerular etiology less likely. The acute onset and lack of additional systemic features, other than hypertension, made a vascular etiology less likely. A tubular etiology, such as acute tubular necrosis (ATN), was highest on the differential and was followed by an interstitial etiology, such as acute interstitial nephritis (AIN).
Patients with drug-induced AIN commonly present with signs and symptoms of an allergic-type reaction, including fever, rash, hematuria, pyuria, and costovertebral angle tenderness. The patient lacked these symptoms. However, cisplatin is known to cause ATN in up to 20-30% of patients.7 Therefore, despite the lack of the classic muddy-brown, granular casts on urine microscopy, cisplatin-induced ATN remained the most likely etiology of his AKI. Moreover, ATN can cause hyponatremia. ATN is characterized by 3 phases: initiation, maintenance, and recovery phases.8 Hyponatremia occurs during the recovery phase, typically starting weeks after renal insult and associated with high urine output and diuresis. This patient presented 1 week after injury and had persistent oliguria, making ATN an unlikely culprit of his hyponatremia.
Our patient presented with hypotonic hyponatremia with a 131 mmol/L initial sodium level and an < 280 mOsm/kg effective serum osmolality, or serum tonicity. The serum tonicity is equivalent to the difference between the measured serum osmolality and the BUN. In the setting of profound AKI, this adjustment is essential for correctly categorizing a patient’s hyponatremia as hyper-, iso-, or hypotonic. The differential diagnosis for this patient’s hypotonic hyponatremia included dilutional effects of hypervolemia, SIADH, hyperthyroidism, adrenal insufficiency, and RSWS. The patient’s volume examination, lack of predisposing comorbidities or suggestive biomarkers, and > 20 mmol/L urinary sodium made hypervolemia unlikely. His urinary osmolality and specific gravity made primary polydipsia unlikely. We worked up his hyponatremia according to a diagnostic algorithm (eAppendix available at doi:10.12788/fp.0198).
The patient had a 217 mOsm/kg serum tonicity and a 270.5 mOsm/kg urine osmolality, consistent with impaired water excretion. His presentation, TSH, and concordant decrease in sodium and potassium made an endocrine etiology of his hyponatremia less likely. In hindsight, a serum cortisol would have been beneficial to more completely exclude adrenal insufficiency. His urine sodium was elevated at 45 mmol/L, raising concern for RSWS or SIADH. The FEurate helped to distinguish between SIADH and RSWS. While FEurate is often elevated in both SIADH and RSWS initially, the FEurate normalizes in SIADH with normalization of the serum sodium. The ideal cutoff for posthyponatremia correction FEurate is debated; however, a FEurate value after sodium correction < 11% suggests SIADH while a value > 11% suggests RSWS.9 Our patient’s FEurate following the sodium correction (serum sodium 134 mmol/L) was 21.9%, most suggestive of RSWS.
Treatment
Upon admission, initial treatment focused on resolving the patient’s AKI. The oncology team discontinued the cisplatin-based chemotherapy. His medication dosages were adjusted for his renal function and additional nephrotoxins avoided. In consultation, the nephrology service recommended 100 mL/h fluid resuscitation. After the patient received 3 L of 0.9% sodium chloride, his creatinine showed limited improvement and his sodium worsened, trending from 131 mmol/L to a nadir of 125 mmol/L. We initiated oral free-water restriction while continuing IV infusion of 0.9% sodium chloride at 125 mL/h.
We further augmented his sodium intake with 1-g sodium chloride tablets with each meal. By hospital day 6, the patient’s serum sodium, BUN, and creatinine improved to 130 mEq/L, 50 mg/dL, and 7.7 mg/dL, respectively. We then discontinued the oral sodium chloride tablets, fluid restriction, and IV fluids in a stepwise fashion prior to discharge. At discharge, the patient’s serum sodium was 136 mEq/L and creatinine, 4.8 mg/dL. The patient’s clinical course was complicated by symptomatic hypertension with systolic blood pressures about 180 mm Hg, requiring intermittent IV hydralazine, which was transitioned to daily nifedipine. Concerned that fluid resuscitation contributed to his hypertension, the patient also received several doses of furosemide. At time of discharge, the patient remained hypertensive and was discharged with nifedipine 90 mg daily.
Outcome and Follow-up
The patient has remained stable clinically since discharge. One week after discharge, his serum sodium and creatinine were 138 mmol/L and 3.8 mg/dL, respectively. More than 1 month after discharge, his sodium remains in the reference range and his creatinine was stable at about 3.5 mg/dL. He continues to follow-up with nephrology, oncology, and radiation oncology. He has restarted chemotherapy with a carboplatin-based regimen without recurrence of hyponatremia or AKI. His blood pressure has gradually improved to the point where he no longer requires nifedipine.
Discussion
The US Food and Drug Administration first approved the use of cisplatin, an alkylating agent that inhibits DNA replication, in 1978 for the treatment of testicular cancer.10 Since its approval, cisplatin has increased in popularity and is now considered one of the most effective antineoplastic agents for the treatment of solid tumors.1 Unfortunately, cisplatin has a well-documented adverse effect profile that includes neurotoxicity, gastrointestinal toxicity, nephrotoxicity, and ototoxicity.4 Despite frequent nephrotoxicity, cisplatin only occasionally causes hyponatremia and rarely causes RSWS, a known but potentially fatal complication. Moreover, the combination of AKI and RSWS is unique. Our patient presented with the unique combination of AKI and hyponatremia, most consistent with RSWS, likely precipitated from cisplatin chemotherapy. Through this case, we review cisplatin-associated electrolyte abnormalities, highlight the challenge of differentiating SIADH and RSWS, and suggest a treatment approach for hyponatremia during the period of diagnostic uncertainty.
Blachley and colleagues first discussed renal and electrolyte disturbances, specifically magnesium wasting, secondary to cisplatin use in 1981. In 1984, Kurtzberg and colleagues noted salt wasting in 2 patients receiving cisplatin therapy. The authors suggested that cisplatin inhibits solute transport in the thick ascending limb, causing clinically significant electrolyte abnormalities, coining the term cisplatin-induced salt wasting.11
The prevalence of cisplatin-induced salt wasting is unclear and likely underreported. In 1988, Hutchinson and colleagues conducted a prospective cohort study and noted 10% of patients (n = 70) developed RSWS at some point over 18 months of cisplatin therapy—a higher rate than previously estimated.12 In 1992, another prospective cohort study evaluated the adverse effects of 47 patients with non-small cell lung cancer treated with cisplatin and reported hyponatremia in 43% of its 93 courses of chemotherapy. The authors did not report the etiology of these hyponatremia cases.13 Given the diagnostic challenge, RSWS may be underrepresented as a confirmed etiology of hyponatremia in cisplatin treatment.
Hyponatremia from cisplatin may present as either SIADH or RSWS, complicating treatment decisions. Both conditions lead to hypotonic hyponatremia with urine osmolality > 100 mOSm/kg and urine sodium levels > 40 mmol/L. However, pathophysiology behind SIADH and RSWS is different. In RSWS, proximal tubule damage causes hyponatremia, decreasing sodium reabsorption, and leading to impaired concentration gradient in every segment of the nephron. As a result, RSWS can lead to profound hyponatremia. Treatment typically consists of increasing sodium intake to correct serum sodium with salt tablets and hypertonic sodium chloride while treating the underlying etiology, in our case removing the offending agent, and waiting for proximal tubule function to recover.6 On the other hand, in SIADH, elevated antidiuretic hormone (ADH) increases water reabsorption in the collecting duct, which has no impact on concentration gradients of the other nephron segments.14 Free-water restriction is the hallmark of SIADH treatment. Severe SIADH may require sodium repletion and/or the initiation of vaptans, ADH antagonists that competitively inhibit V2 receptors in the collecting duct to prevent water reabsorption.15
Our patient had an uncertain etiology of his hyponatremia throughout most of his treatment course, complicating our treatment decision-making. Initially, his measured serum osmolality was 278 mOsm/kg; however, his effective tonicity was lower. His AKI elevated his BUN, which in turnrequired us to calculate his serum tonicity (217 mOsm/kg) that was consistent with hypotonic hyponatremia. His elevated urine osmolality and urine sodium levels made SIADH and RSWS the most likely etiologies of his hyponatremia. To confirm the etiology, we waited for correction of his serum sodium. Therefore, we treated him with a combination of sodium repletion with 0.9% sodium chloride (154 mEq/L), hypertonic relative to his serum sodium, sodium chloride tablets, and free-water restriction. In this approach, we attempted to harmonize the treatment strategies for both SIADH and RSWS and effectively corrected his serum sodium. We evaluated his response to our treatment with a basic metabolic panel every 6 to 8 hours. Had his serum sodium decreased < 120 mmol/L, we planned to transfer the patient to the intensive care unit for 3% sodium chloride and/or intensification of his fluid restriction. A significant worsening of his hyponatremia would have strongly suggested hyponatremia secondary to SIADH since isotonic saline can worsen hyponatremia due to increased free-water reabsorption in the collecting duct.16
To differentiate between SIADH and RSWS, we relied on the FEurate after sodium correction. Multiple case reports from Japan have characterized the distinction between the processes through FEurate and serum uric acid. While the optimal cut-off values for FEurate require additional investigation, values < 11% after serum sodium correction suggests SIADH, while a value > 11% suggests RSWS.17 Prior cases have also emphasized serum hypouricemia as a distinguishing characteristic in RSWS. However, our case illustrates that serum hypouricemia is less reliable in the setting of AKI. Due to his severe AKI, our patient could not efficiently clear uric acid, likely contributing to his hyperuricemia.
Ultimately, our patient had an FEurate > 20%, which was suggestive of RSWS. Nevertheless, we recognize limitations and confounders in our diagnosis and have reflected on our diagnostic and management choices. First, the sensitivity and specificity of postsodium correction FEurate is unknown. Tracking the change in FEurate with our interventions would have increased our diagnostic utility, as suggested by Maesaka and colleagues.14 Second, our patient’s serum sodium was still at the lower end of the reference range after treatment, which may decrease the specificity of FEurate. Third, a plasma ADH collected during the initial phase of symptomatic hyponatremia would have helped differentiate between SIADH and RSWS.
Other diagnostic tests that could have excluded alternative diagnoses with even greater certainty include plasma adrenocorticotropic hormone, B-type natriuretic peptide, renin, cortisol, and thyroid function tests. From a practical standpoint, these laboratory results (excluding thyroid function test and brain natriuretic peptide) would have taken several weeks to result at our institution, limiting their clinical utility. Similarly, FEurate also has limited clinical utility, requiring effective treatment as part of the diagnostic test. Therefore, we recommend focusing on optimal treatment for hyponatremia of uncertain etiology, especially where SIADH and RSWS are the leading diagnoses.
Conclusions
We described a rare case of concomitant cisplatin-induced severe AKI and RSWS. We have emphasized the diagnostic challenge of distinguishing between SIADH and RSWS, especially with concomitant AKI, and have acknowledged that optimal treatment relies on accurate differentiation. However, differentiation may not be clinically feasible. Therefore, we suggest a treatment strategy that incorporates both free-water restriction and sodium supplementation via IV and/or oral administration.
1. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364-378. doi:10.1016/j.ejphar.2014.07.025
2. Holditch SJ, Brown CN, Lombardi AM, Nguyen KN, Edelstein CL. Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int J Mol Sci. 2019;20(12):3011. Published 2019 Jun 20. doi:10.3390/ijms20123011
3. National Institutes of Health, National Cancer Institute. The “accidental” cure—platinum-based treatment for cancer: the discovery of cisplatin. Published May 30, 2014. Accessed November 10, 2021. https://www.cancer.gov/research/progress/discovery/cisplatin
4. Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014;2014:967826. doi:10.1155/2014/967826
5. Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol. 2015;10(4):676-687. doi:10.2215/CJN.12391213
6. Bitew S, Imbriano L, Miyawaki N, Fishbane S, Maesaka JK. More on renal salt wasting without cerebral disease: response to saline infusion. Clin J Am Soc Nephrol. 2009;4(2):309-315. doi:10.2215/CJN.02740608
7. Shirali AC, Perazella MA. Tubulointerstitial injury associated with chemotherapeutic agents. Adv Chronic Kidney Dis. 2014;21(1):56-63. doi:10.1053/j.ackd.2013.06.010
8. Agrawal M, Swartz R. Acute renal failure [published correction appears in Am Fam Physician 2001 Feb 1;63(3):445]. Am Fam Physician. 2000;61(7):2077-2088.
9. Milionis HJ, Liamis GL, Elisaf MS. The hyponatremic patient: a systematic approach to laboratory diagnosis. CMAJ. 2002;166(8):1056-1062.
10. Monneret C. Platinum anticancer drugs. From serendipity to rational design. Ann Pharm Fr. 2011;69(6):286-295. doi:10.1016/j.pharma.2011.10.001
11. Kurtzberg J, Dennis VW, Kinney TR. Cisplatinum-induced renal salt wasting. Med Pediatr Oncol. 1984;12(2):150-154. doi:10.1002/mpo.2950120219
12. Hutchison FN, Perez EA, Gandara DR, Lawrence HJ, Kaysen GA. Renal salt wasting in patients treated with cisplatin. Ann Intern Med. 1988;108(1):21-25. doi:10.7326/0003-4819-108-1-21
13. Lee YK, Shin DM. Renal salt wasting in patients treated with high-dose cisplatin, etoposide, and mitomycin in patients with advanced non-small cell lung cancer. Korean J Intern Med. 1992;7(2):118-121. doi:10.3904/kjim.1992.7.2.118
14. Maesaka JK, Imbriano L, Mattana J, Gallagher D, Bade N, Sharif S. Differentiating SIADH from cerebral/renal salt wasting: failure of the volume approach and need for a new approach to hyponatremia. J Clin Med. 2014;3(4):1373-1385. Published 2014 Dec 8. doi:10.3390/jcm3041373
15. Palmer BF. The role of v2 receptor antagonists in the treatment of hyponatremia. Electrolyte Blood Press. 2013;11(1):1-8. doi:10.5049/EBP.2013.11.1.1
16. Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med. 2007;120(11 Suppl 1):S1-S21. doi:10.1016/j.amjmed.2007.09.001
17. Maesaka JK, Imbriano LJ, Miyawaki N. High prevalence of renal salt wasting without cerebral disease as cause of hyponatremia in general medical wards. Am J Med Sci. 2018;356(1):15-22. doi:10.1016/j.amjms.2018.03.02
1. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364-378. doi:10.1016/j.ejphar.2014.07.025
2. Holditch SJ, Brown CN, Lombardi AM, Nguyen KN, Edelstein CL. Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int J Mol Sci. 2019;20(12):3011. Published 2019 Jun 20. doi:10.3390/ijms20123011
3. National Institutes of Health, National Cancer Institute. The “accidental” cure—platinum-based treatment for cancer: the discovery of cisplatin. Published May 30, 2014. Accessed November 10, 2021. https://www.cancer.gov/research/progress/discovery/cisplatin
4. Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014;2014:967826. doi:10.1155/2014/967826
5. Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol. 2015;10(4):676-687. doi:10.2215/CJN.12391213
6. Bitew S, Imbriano L, Miyawaki N, Fishbane S, Maesaka JK. More on renal salt wasting without cerebral disease: response to saline infusion. Clin J Am Soc Nephrol. 2009;4(2):309-315. doi:10.2215/CJN.02740608
7. Shirali AC, Perazella MA. Tubulointerstitial injury associated with chemotherapeutic agents. Adv Chronic Kidney Dis. 2014;21(1):56-63. doi:10.1053/j.ackd.2013.06.010
8. Agrawal M, Swartz R. Acute renal failure [published correction appears in Am Fam Physician 2001 Feb 1;63(3):445]. Am Fam Physician. 2000;61(7):2077-2088.
9. Milionis HJ, Liamis GL, Elisaf MS. The hyponatremic patient: a systematic approach to laboratory diagnosis. CMAJ. 2002;166(8):1056-1062.
10. Monneret C. Platinum anticancer drugs. From serendipity to rational design. Ann Pharm Fr. 2011;69(6):286-295. doi:10.1016/j.pharma.2011.10.001
11. Kurtzberg J, Dennis VW, Kinney TR. Cisplatinum-induced renal salt wasting. Med Pediatr Oncol. 1984;12(2):150-154. doi:10.1002/mpo.2950120219
12. Hutchison FN, Perez EA, Gandara DR, Lawrence HJ, Kaysen GA. Renal salt wasting in patients treated with cisplatin. Ann Intern Med. 1988;108(1):21-25. doi:10.7326/0003-4819-108-1-21
13. Lee YK, Shin DM. Renal salt wasting in patients treated with high-dose cisplatin, etoposide, and mitomycin in patients with advanced non-small cell lung cancer. Korean J Intern Med. 1992;7(2):118-121. doi:10.3904/kjim.1992.7.2.118
14. Maesaka JK, Imbriano L, Mattana J, Gallagher D, Bade N, Sharif S. Differentiating SIADH from cerebral/renal salt wasting: failure of the volume approach and need for a new approach to hyponatremia. J Clin Med. 2014;3(4):1373-1385. Published 2014 Dec 8. doi:10.3390/jcm3041373
15. Palmer BF. The role of v2 receptor antagonists in the treatment of hyponatremia. Electrolyte Blood Press. 2013;11(1):1-8. doi:10.5049/EBP.2013.11.1.1
16. Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med. 2007;120(11 Suppl 1):S1-S21. doi:10.1016/j.amjmed.2007.09.001
17. Maesaka JK, Imbriano LJ, Miyawaki N. High prevalence of renal salt wasting without cerebral disease as cause of hyponatremia in general medical wards. Am J Med Sci. 2018;356(1):15-22. doi:10.1016/j.amjms.2018.03.02
Albuterol, Acidosis, and Aneurysms
A patient with a complicated medical history on admission for dyspnea was administered nebulizer therapy but after 72 hours developed asymptomatic acute kidney injury and anion-gap metabolic acidosis.
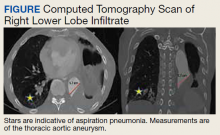
An 88-year-old male veteran with a medical history of chronic obstructive pulmonary disease (COPD) on home oxygen, chronic alcohol use, squamous cell carcinoma of the lung status after left upper lobectomy, and a 5.7 cm thoracic aortic aneurysm was admitted to the inpatient medical service for progressive dyspnea and productive cough. The patient was in his usual state of health until 2 days before presentation. A chest computed tomography scan showed a right lower lobe infiltrate, concerning for pneumonia, and stable thoracic aortic aneurysm (Figure). On admission, the patient was started on IV ceftriaxone 2 g daily for pneumonia and
The patient responded well to therapy, and his cough and dyspnea improved. However, 72 hours after admission, he developed an asymptomatic acute kidney injury (AKI) and anion-gap metabolic acidosis. His serum creatinine increased from baseline 0.6 mg/dL to 1.2 mg/dL. He also had an anion gap of 21 mmol/L and a decrease in bicarbonate from 23 mmol/L to 17 mmol/L. His condition was further complicated by new-onset hypertension (153/111 mm Hg). His calculated fractional excretion of sodium (FENa) was 0.5%, and his lactate level returned elevated at 3.6 mmol/L. On further investigation, he reported alcohol use the night prior; however, his β-hydroxybutyrate was negative, and serum alcohol level was undetectable. Meanwhile, the patient continued to receive antibiotics and scheduled nebulizer treatments. Although his AKI resolved with initial fluid resuscitation, his repeat lactate levels continued to trend upward to a peak of 4.0 mmol/L.
- What is your diagnosis?
- How would you treat this patient?
Although IV fluids resolved his AKI, prerenal in etiology given the calculated FENa at 0.5%, his lactate levels continued to uptrend to a peak of 4.0 mmol/L complicated by elevated blood pressure (BP) > 150/100 mm Hg. Given his thoracic aneurysm, his BP was treated with metoprolol tartrate and amlodipine 10 mg daily. The patient remained asymptomatic with no evidence of ischemia or sepsis.
We suspected the nebulizer treatments to be the etiology of the patient’s hyperlactatemia and subsequent anion-gap metabolic acidosis. His scheduled albuterol and ipratropium nebulizer treatments were discontinued, and the patient experienced rapid resolution of his anion gap and hyperlactatemia to 1.2 mmol/L over 24 hours. On discontinuation of the nebulization therapy, mild wheezing was noted on physical examination. The patient reported no symptoms and was at his baseline. The patient finished his antibiotic course for his community-acquired pneumonia and was discharged in stable condition with instructions to continue his previously established home COPD medication regimen of umeclidinium/vilanterol 62.5/25 mcg daily and albuterol metered-dose inhaler as needed.
Discussion
Short-acting β-agonists, such as albuterol, are widely used in COPD and are a guideline-recommended treatment in maintenance and exacerbation of asthma and COPD.1 Short-acting β-agonist adverse effects (AEs) include nausea, vomiting, tremors, headache, and tachycardia; abnormal laboratory results include hypocalcemia, hypokalemia, hypophosphatemia, hypomagnesemia, and hyperglycemia.2,3 Albuterol-induced hyperlactatemia and lactic acidosis also are known but often overlooked and underreported AEs.
In a randomized control trial, researchers identified a positive correlation between nebulized albuterol use and hyperlactatemia in asthmatics with asthma exacerbation.4 One systematic review identified ≤ 20% of patients on either IV or nebulized high-dose treatments with selective β2-agonists may experience hyperlactatemia.5 However, aerosolized administration of albuterol as opposed to IV administration is less likely to result in AEs and abnormal laboratory results given decreased systemic absorption.3
Hyperlactatemia and lactic acidosis are associated with increased morbidity and mortality.6 Lactic acidosis is classified as either type A or type B. Type A lactic acidosis is characterized by hypoperfusion as subsequent ischemic injuries lead to anaerobic metabolism and elevated lactate. Diseases such as septic, cardiogenic, and hypovolemic shock are often associated with type A lactic acidosis. Type B lactic acidosis, however, encapsulates all nonhypoperfusion-related elevations in lactate, including malignancy, ethanol intoxication, and medication-induced lactic acidosis.7,8
In this case, the diagnosis was elusive as the patient had multiple comorbidities. His history included COPD, which is associated with elevated lactate levels.5 However, his initial laboratory workup did not show an anion gap, confirming a lack of an underlying acidotic process on admission. Because the patient was admitted for pneumonia, a known infectious source, complicated by an acute elevation in lactate, sepsis must be and was effectively ruled out. The patient also reported alcohol use during his admission, which confounded his presentation but was unlikely to impact the etiology of his lactic acidosis, given the unremarkable β-hydroxybutyrate and serum alcohol levels.
Furthermore, the patient harbored an enlarged thoracic aortic aneurysm and remained hypertensive above the goal of BP 130/80 mm Hg for patients with thoracoabdominal aneurysms.9 Lactic acidosis in the context of hemodynamic instability for this patient might have indicated tissue hypoperfusion secondary to a ruptured aneurysm or aortic dissection. Fortunately, the patient did not manifest any signs or symptoms suggestive of a ruptured aortic aneurysm. Last, on discontinuing the nebulizer therapy, the patient’s hyperlactatemia resolved within 24 hours, highly indicative of albuterol-induced lactic acidosis as the proper diagnosis.
As a β-agonist, albuterol stimulates β-adrenergic receptors, which increases lipolysis and glycolysis. The biochemical reactions increase the product pyruvate, which is used in both aerobic and anaerobic metabolisms. With an increase in pyruvate, capacity for aerobic metabolism is maximized with increased shunting toward anaerobic metabolism, leading to elevated lactate levels and lactic acidosis.8,10,11
Regardless, albuterol-induced lactic acidosis is a diagnosis of exclusion.6 It is thus prudent to rule out life-threatening etiologies of hyperlactatemia, given the association with increased morbidity and mortality. This case illustrates the importance of ruling out life-threatening etiologies of hyperlactatemia and lactic acidosis in an older patient with multiple comorbidities. This case also recognizes the acute AEs of hyperlactatemia and lactic acidosis secondary to scheduled albuterol nebulization therapy in acutely ill patients. Of note, patients presenting with an acute medical illness may be more susceptible to hyperlactatemia secondary to scheduled albuterol nebulization therapy.
Conclusions
We encourage heightened clinical suspicion of albuterol-induced lactic acidosis in acutely ill patients with COPD on albuterol therapy on rule out of life-threatening etiologies and
1. Global Initiative for Asthma. Pocket Guide to COPD Diagnosis, Management, and Prevention: A Guide for Health Care Professionals (2020 Report). Global Initiative for Chronic Lung Diseases, Inc; 2020. Accessed April 16, 2021. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. Jat KR, Khairwa A. Levalbuterol versus albuterol for acute asthma: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2013;26(2):239-248. doi:10.1016/j.pupt.2012.11.003
3. Ahrens RC, Smith GD. Albuterol: an adrenergic agent for use in the treatment of asthma pharmacology, pharmacokinetics and clinical use. Pharmacotherapy. 1984;4(3):105- 121. doi:10.1002/j.1875-9114.1984.tb03330.x
4. Lewis LM, Ferguson I, House SL, et al. Albuterol administration is commonly associated with increases in serum lactate in patients with asthma treated for acute exacerbation of asthma. Chest. 2014;145(1):53-59. doi:10.1378/chest.13-0930
5. Liedtke AG, Lava SAG, Milani GP, et al. Selective β2-adrenoceptor agonists and relevant hyperlactatemia: systematic review and meta-analysis. J Clin Med. 2019;9(1):71. doi:10.3390/jcm9010071
6. Smith ZR, Horng M, Rech MA. Medication-induced hyperlactatemia and lactic acidosis: a systematic review of the literature. Pharmacotherapy. 2019;39(9):946-963. doi:10.1002/phar.2316
7. Hockstein M, Diercks D. Significant lactic acidosis from albuterol. Clin Pract Cases Emerg Med. 2018;2(2):128-131. doi:10.5811/cpcem.2018.1.36024
8. Foucher CD, Tubben RE. Lactic acidosis. StatPearls Publishing; 2020. Updated November 21, 2020. Accessed April 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK470202
9. Aronow WS. Treatment of thoracic aortic aneurysm. Ann Transl Med. 2018;6(3):66. doi:10.21037/atm.2018.01.07
10. Lau E, Mazer J, Carino G. Inhaled β-agonist therapy and respiratory muscle fatigue as under-recognised causes of lactic acidosis. BMJ Case Rep. 2013;2013:bcr2013201015. Published October 14, 2013. doi:10.1136/bcr-2013-201015
11. Ramakrishna KN, Virk J, Gambhir HS. Albuterol-induced lactic acidosis. Am J Ther. 2019;26(5):e635-e636. doi:10.1097/MJT.0000000000000843
A patient with a complicated medical history on admission for dyspnea was administered nebulizer therapy but after 72 hours developed asymptomatic acute kidney injury and anion-gap metabolic acidosis.
A patient with a complicated medical history on admission for dyspnea was administered nebulizer therapy but after 72 hours developed asymptomatic acute kidney injury and anion-gap metabolic acidosis.
An 88-year-old male veteran with a medical history of chronic obstructive pulmonary disease (COPD) on home oxygen, chronic alcohol use, squamous cell carcinoma of the lung status after left upper lobectomy, and a 5.7 cm thoracic aortic aneurysm was admitted to the inpatient medical service for progressive dyspnea and productive cough. The patient was in his usual state of health until 2 days before presentation. A chest computed tomography scan showed a right lower lobe infiltrate, concerning for pneumonia, and stable thoracic aortic aneurysm (Figure). On admission, the patient was started on IV ceftriaxone 2 g daily for pneumonia and
The patient responded well to therapy, and his cough and dyspnea improved. However, 72 hours after admission, he developed an asymptomatic acute kidney injury (AKI) and anion-gap metabolic acidosis. His serum creatinine increased from baseline 0.6 mg/dL to 1.2 mg/dL. He also had an anion gap of 21 mmol/L and a decrease in bicarbonate from 23 mmol/L to 17 mmol/L. His condition was further complicated by new-onset hypertension (153/111 mm Hg). His calculated fractional excretion of sodium (FENa) was 0.5%, and his lactate level returned elevated at 3.6 mmol/L. On further investigation, he reported alcohol use the night prior; however, his β-hydroxybutyrate was negative, and serum alcohol level was undetectable. Meanwhile, the patient continued to receive antibiotics and scheduled nebulizer treatments. Although his AKI resolved with initial fluid resuscitation, his repeat lactate levels continued to trend upward to a peak of 4.0 mmol/L.
- What is your diagnosis?
- How would you treat this patient?
Although IV fluids resolved his AKI, prerenal in etiology given the calculated FENa at 0.5%, his lactate levels continued to uptrend to a peak of 4.0 mmol/L complicated by elevated blood pressure (BP) > 150/100 mm Hg. Given his thoracic aneurysm, his BP was treated with metoprolol tartrate and amlodipine 10 mg daily. The patient remained asymptomatic with no evidence of ischemia or sepsis.
We suspected the nebulizer treatments to be the etiology of the patient’s hyperlactatemia and subsequent anion-gap metabolic acidosis. His scheduled albuterol and ipratropium nebulizer treatments were discontinued, and the patient experienced rapid resolution of his anion gap and hyperlactatemia to 1.2 mmol/L over 24 hours. On discontinuation of the nebulization therapy, mild wheezing was noted on physical examination. The patient reported no symptoms and was at his baseline. The patient finished his antibiotic course for his community-acquired pneumonia and was discharged in stable condition with instructions to continue his previously established home COPD medication regimen of umeclidinium/vilanterol 62.5/25 mcg daily and albuterol metered-dose inhaler as needed.
Discussion
Short-acting β-agonists, such as albuterol, are widely used in COPD and are a guideline-recommended treatment in maintenance and exacerbation of asthma and COPD.1 Short-acting β-agonist adverse effects (AEs) include nausea, vomiting, tremors, headache, and tachycardia; abnormal laboratory results include hypocalcemia, hypokalemia, hypophosphatemia, hypomagnesemia, and hyperglycemia.2,3 Albuterol-induced hyperlactatemia and lactic acidosis also are known but often overlooked and underreported AEs.
In a randomized control trial, researchers identified a positive correlation between nebulized albuterol use and hyperlactatemia in asthmatics with asthma exacerbation.4 One systematic review identified ≤ 20% of patients on either IV or nebulized high-dose treatments with selective β2-agonists may experience hyperlactatemia.5 However, aerosolized administration of albuterol as opposed to IV administration is less likely to result in AEs and abnormal laboratory results given decreased systemic absorption.3
Hyperlactatemia and lactic acidosis are associated with increased morbidity and mortality.6 Lactic acidosis is classified as either type A or type B. Type A lactic acidosis is characterized by hypoperfusion as subsequent ischemic injuries lead to anaerobic metabolism and elevated lactate. Diseases such as septic, cardiogenic, and hypovolemic shock are often associated with type A lactic acidosis. Type B lactic acidosis, however, encapsulates all nonhypoperfusion-related elevations in lactate, including malignancy, ethanol intoxication, and medication-induced lactic acidosis.7,8
In this case, the diagnosis was elusive as the patient had multiple comorbidities. His history included COPD, which is associated with elevated lactate levels.5 However, his initial laboratory workup did not show an anion gap, confirming a lack of an underlying acidotic process on admission. Because the patient was admitted for pneumonia, a known infectious source, complicated by an acute elevation in lactate, sepsis must be and was effectively ruled out. The patient also reported alcohol use during his admission, which confounded his presentation but was unlikely to impact the etiology of his lactic acidosis, given the unremarkable β-hydroxybutyrate and serum alcohol levels.
Furthermore, the patient harbored an enlarged thoracic aortic aneurysm and remained hypertensive above the goal of BP 130/80 mm Hg for patients with thoracoabdominal aneurysms.9 Lactic acidosis in the context of hemodynamic instability for this patient might have indicated tissue hypoperfusion secondary to a ruptured aneurysm or aortic dissection. Fortunately, the patient did not manifest any signs or symptoms suggestive of a ruptured aortic aneurysm. Last, on discontinuing the nebulizer therapy, the patient’s hyperlactatemia resolved within 24 hours, highly indicative of albuterol-induced lactic acidosis as the proper diagnosis.
As a β-agonist, albuterol stimulates β-adrenergic receptors, which increases lipolysis and glycolysis. The biochemical reactions increase the product pyruvate, which is used in both aerobic and anaerobic metabolisms. With an increase in pyruvate, capacity for aerobic metabolism is maximized with increased shunting toward anaerobic metabolism, leading to elevated lactate levels and lactic acidosis.8,10,11
Regardless, albuterol-induced lactic acidosis is a diagnosis of exclusion.6 It is thus prudent to rule out life-threatening etiologies of hyperlactatemia, given the association with increased morbidity and mortality. This case illustrates the importance of ruling out life-threatening etiologies of hyperlactatemia and lactic acidosis in an older patient with multiple comorbidities. This case also recognizes the acute AEs of hyperlactatemia and lactic acidosis secondary to scheduled albuterol nebulization therapy in acutely ill patients. Of note, patients presenting with an acute medical illness may be more susceptible to hyperlactatemia secondary to scheduled albuterol nebulization therapy.
Conclusions
We encourage heightened clinical suspicion of albuterol-induced lactic acidosis in acutely ill patients with COPD on albuterol therapy on rule out of life-threatening etiologies and
An 88-year-old male veteran with a medical history of chronic obstructive pulmonary disease (COPD) on home oxygen, chronic alcohol use, squamous cell carcinoma of the lung status after left upper lobectomy, and a 5.7 cm thoracic aortic aneurysm was admitted to the inpatient medical service for progressive dyspnea and productive cough. The patient was in his usual state of health until 2 days before presentation. A chest computed tomography scan showed a right lower lobe infiltrate, concerning for pneumonia, and stable thoracic aortic aneurysm (Figure). On admission, the patient was started on IV ceftriaxone 2 g daily for pneumonia and
The patient responded well to therapy, and his cough and dyspnea improved. However, 72 hours after admission, he developed an asymptomatic acute kidney injury (AKI) and anion-gap metabolic acidosis. His serum creatinine increased from baseline 0.6 mg/dL to 1.2 mg/dL. He also had an anion gap of 21 mmol/L and a decrease in bicarbonate from 23 mmol/L to 17 mmol/L. His condition was further complicated by new-onset hypertension (153/111 mm Hg). His calculated fractional excretion of sodium (FENa) was 0.5%, and his lactate level returned elevated at 3.6 mmol/L. On further investigation, he reported alcohol use the night prior; however, his β-hydroxybutyrate was negative, and serum alcohol level was undetectable. Meanwhile, the patient continued to receive antibiotics and scheduled nebulizer treatments. Although his AKI resolved with initial fluid resuscitation, his repeat lactate levels continued to trend upward to a peak of 4.0 mmol/L.
- What is your diagnosis?
- How would you treat this patient?
Although IV fluids resolved his AKI, prerenal in etiology given the calculated FENa at 0.5%, his lactate levels continued to uptrend to a peak of 4.0 mmol/L complicated by elevated blood pressure (BP) > 150/100 mm Hg. Given his thoracic aneurysm, his BP was treated with metoprolol tartrate and amlodipine 10 mg daily. The patient remained asymptomatic with no evidence of ischemia or sepsis.
We suspected the nebulizer treatments to be the etiology of the patient’s hyperlactatemia and subsequent anion-gap metabolic acidosis. His scheduled albuterol and ipratropium nebulizer treatments were discontinued, and the patient experienced rapid resolution of his anion gap and hyperlactatemia to 1.2 mmol/L over 24 hours. On discontinuation of the nebulization therapy, mild wheezing was noted on physical examination. The patient reported no symptoms and was at his baseline. The patient finished his antibiotic course for his community-acquired pneumonia and was discharged in stable condition with instructions to continue his previously established home COPD medication regimen of umeclidinium/vilanterol 62.5/25 mcg daily and albuterol metered-dose inhaler as needed.
Discussion
Short-acting β-agonists, such as albuterol, are widely used in COPD and are a guideline-recommended treatment in maintenance and exacerbation of asthma and COPD.1 Short-acting β-agonist adverse effects (AEs) include nausea, vomiting, tremors, headache, and tachycardia; abnormal laboratory results include hypocalcemia, hypokalemia, hypophosphatemia, hypomagnesemia, and hyperglycemia.2,3 Albuterol-induced hyperlactatemia and lactic acidosis also are known but often overlooked and underreported AEs.
In a randomized control trial, researchers identified a positive correlation between nebulized albuterol use and hyperlactatemia in asthmatics with asthma exacerbation.4 One systematic review identified ≤ 20% of patients on either IV or nebulized high-dose treatments with selective β2-agonists may experience hyperlactatemia.5 However, aerosolized administration of albuterol as opposed to IV administration is less likely to result in AEs and abnormal laboratory results given decreased systemic absorption.3
Hyperlactatemia and lactic acidosis are associated with increased morbidity and mortality.6 Lactic acidosis is classified as either type A or type B. Type A lactic acidosis is characterized by hypoperfusion as subsequent ischemic injuries lead to anaerobic metabolism and elevated lactate. Diseases such as septic, cardiogenic, and hypovolemic shock are often associated with type A lactic acidosis. Type B lactic acidosis, however, encapsulates all nonhypoperfusion-related elevations in lactate, including malignancy, ethanol intoxication, and medication-induced lactic acidosis.7,8
In this case, the diagnosis was elusive as the patient had multiple comorbidities. His history included COPD, which is associated with elevated lactate levels.5 However, his initial laboratory workup did not show an anion gap, confirming a lack of an underlying acidotic process on admission. Because the patient was admitted for pneumonia, a known infectious source, complicated by an acute elevation in lactate, sepsis must be and was effectively ruled out. The patient also reported alcohol use during his admission, which confounded his presentation but was unlikely to impact the etiology of his lactic acidosis, given the unremarkable β-hydroxybutyrate and serum alcohol levels.
Furthermore, the patient harbored an enlarged thoracic aortic aneurysm and remained hypertensive above the goal of BP 130/80 mm Hg for patients with thoracoabdominal aneurysms.9 Lactic acidosis in the context of hemodynamic instability for this patient might have indicated tissue hypoperfusion secondary to a ruptured aneurysm or aortic dissection. Fortunately, the patient did not manifest any signs or symptoms suggestive of a ruptured aortic aneurysm. Last, on discontinuing the nebulizer therapy, the patient’s hyperlactatemia resolved within 24 hours, highly indicative of albuterol-induced lactic acidosis as the proper diagnosis.
As a β-agonist, albuterol stimulates β-adrenergic receptors, which increases lipolysis and glycolysis. The biochemical reactions increase the product pyruvate, which is used in both aerobic and anaerobic metabolisms. With an increase in pyruvate, capacity for aerobic metabolism is maximized with increased shunting toward anaerobic metabolism, leading to elevated lactate levels and lactic acidosis.8,10,11
Regardless, albuterol-induced lactic acidosis is a diagnosis of exclusion.6 It is thus prudent to rule out life-threatening etiologies of hyperlactatemia, given the association with increased morbidity and mortality. This case illustrates the importance of ruling out life-threatening etiologies of hyperlactatemia and lactic acidosis in an older patient with multiple comorbidities. This case also recognizes the acute AEs of hyperlactatemia and lactic acidosis secondary to scheduled albuterol nebulization therapy in acutely ill patients. Of note, patients presenting with an acute medical illness may be more susceptible to hyperlactatemia secondary to scheduled albuterol nebulization therapy.
Conclusions
We encourage heightened clinical suspicion of albuterol-induced lactic acidosis in acutely ill patients with COPD on albuterol therapy on rule out of life-threatening etiologies and
1. Global Initiative for Asthma. Pocket Guide to COPD Diagnosis, Management, and Prevention: A Guide for Health Care Professionals (2020 Report). Global Initiative for Chronic Lung Diseases, Inc; 2020. Accessed April 16, 2021. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. Jat KR, Khairwa A. Levalbuterol versus albuterol for acute asthma: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2013;26(2):239-248. doi:10.1016/j.pupt.2012.11.003
3. Ahrens RC, Smith GD. Albuterol: an adrenergic agent for use in the treatment of asthma pharmacology, pharmacokinetics and clinical use. Pharmacotherapy. 1984;4(3):105- 121. doi:10.1002/j.1875-9114.1984.tb03330.x
4. Lewis LM, Ferguson I, House SL, et al. Albuterol administration is commonly associated with increases in serum lactate in patients with asthma treated for acute exacerbation of asthma. Chest. 2014;145(1):53-59. doi:10.1378/chest.13-0930
5. Liedtke AG, Lava SAG, Milani GP, et al. Selective β2-adrenoceptor agonists and relevant hyperlactatemia: systematic review and meta-analysis. J Clin Med. 2019;9(1):71. doi:10.3390/jcm9010071
6. Smith ZR, Horng M, Rech MA. Medication-induced hyperlactatemia and lactic acidosis: a systematic review of the literature. Pharmacotherapy. 2019;39(9):946-963. doi:10.1002/phar.2316
7. Hockstein M, Diercks D. Significant lactic acidosis from albuterol. Clin Pract Cases Emerg Med. 2018;2(2):128-131. doi:10.5811/cpcem.2018.1.36024
8. Foucher CD, Tubben RE. Lactic acidosis. StatPearls Publishing; 2020. Updated November 21, 2020. Accessed April 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK470202
9. Aronow WS. Treatment of thoracic aortic aneurysm. Ann Transl Med. 2018;6(3):66. doi:10.21037/atm.2018.01.07
10. Lau E, Mazer J, Carino G. Inhaled β-agonist therapy and respiratory muscle fatigue as under-recognised causes of lactic acidosis. BMJ Case Rep. 2013;2013:bcr2013201015. Published October 14, 2013. doi:10.1136/bcr-2013-201015
11. Ramakrishna KN, Virk J, Gambhir HS. Albuterol-induced lactic acidosis. Am J Ther. 2019;26(5):e635-e636. doi:10.1097/MJT.0000000000000843
1. Global Initiative for Asthma. Pocket Guide to COPD Diagnosis, Management, and Prevention: A Guide for Health Care Professionals (2020 Report). Global Initiative for Chronic Lung Diseases, Inc; 2020. Accessed April 16, 2021. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
2. Jat KR, Khairwa A. Levalbuterol versus albuterol for acute asthma: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2013;26(2):239-248. doi:10.1016/j.pupt.2012.11.003
3. Ahrens RC, Smith GD. Albuterol: an adrenergic agent for use in the treatment of asthma pharmacology, pharmacokinetics and clinical use. Pharmacotherapy. 1984;4(3):105- 121. doi:10.1002/j.1875-9114.1984.tb03330.x
4. Lewis LM, Ferguson I, House SL, et al. Albuterol administration is commonly associated with increases in serum lactate in patients with asthma treated for acute exacerbation of asthma. Chest. 2014;145(1):53-59. doi:10.1378/chest.13-0930
5. Liedtke AG, Lava SAG, Milani GP, et al. Selective β2-adrenoceptor agonists and relevant hyperlactatemia: systematic review and meta-analysis. J Clin Med. 2019;9(1):71. doi:10.3390/jcm9010071
6. Smith ZR, Horng M, Rech MA. Medication-induced hyperlactatemia and lactic acidosis: a systematic review of the literature. Pharmacotherapy. 2019;39(9):946-963. doi:10.1002/phar.2316
7. Hockstein M, Diercks D. Significant lactic acidosis from albuterol. Clin Pract Cases Emerg Med. 2018;2(2):128-131. doi:10.5811/cpcem.2018.1.36024
8. Foucher CD, Tubben RE. Lactic acidosis. StatPearls Publishing; 2020. Updated November 21, 2020. Accessed April 16, 2021. https://www.ncbi.nlm.nih.gov/books/NBK470202
9. Aronow WS. Treatment of thoracic aortic aneurysm. Ann Transl Med. 2018;6(3):66. doi:10.21037/atm.2018.01.07
10. Lau E, Mazer J, Carino G. Inhaled β-agonist therapy and respiratory muscle fatigue as under-recognised causes of lactic acidosis. BMJ Case Rep. 2013;2013:bcr2013201015. Published October 14, 2013. doi:10.1136/bcr-2013-201015
11. Ramakrishna KN, Virk J, Gambhir HS. Albuterol-induced lactic acidosis. Am J Ther. 2019;26(5):e635-e636. doi:10.1097/MJT.0000000000000843
Gastrointestinal Symptoms and Lactic Acidosis in a Chronic Marijuana User
A 57-year-old woman with a history of traumatic brain injury, posttraumatic stress disorder, depression, migraines, hypothyroidism, and a hiatal hernia repair presented to the emergency department with a 1-day history of nausea, vomiting, and diffuse abdominal pain. She reported that her symptoms were relieved by hot showers. She also reported having similar symptoms and a previous gastric-emptying study that showed a slow-emptying stomach. Her history also consisted of frequent cannabis use for mood and appetite stimulation along with eliminating meat and fish from her diet, an increase in consumption of simple carbohydrates in the past year, and no alcohol use. Her medications included topiramate 100 mg and clonidine 0.3 mg nightly for migraines; levothyroxine 200 mcg daily for hypothyroidism; tizanidine 4 mg twice a day for muscle spasm; famotidine 40 mg twice a day as needed for gastric reflux; and bupropion 50 mg daily, citalopram 20 mg daily, and lamotrigine 25 mg nightly for mood.
The patient’s physical examination was notable for bradycardia (43 beats/min) and epigastric tenderness. Admission laboratory results were notable for an elevated lactic acid level of 4.8 (normal range, 0.50-2.20) mmol/L and a leukocytosis count of 10.8×109 cells/L. Serum alcohol level and blood cultures were negative. Liver function test, hemoglobin A1c, and lipase test were unremarkable. Her electrocardiogram showed an unchanged right bundle branch block. Chest X-ray, computed tomography (CT) of her abdomen/pelvis and echocardiogram were unremarkable.
What is your diagnosis?
How would you treat this patient?
This patient was diagnosed with gastrointestinal beriberi. Because of her dietary changes, lactic acidosis, and bradycardia, thiamine deficiency was suspected after ruling out other possibilities on the differential diagnosis (Table). The patient’s symptoms resolved after administration of high-dose IV thiamine 500 mg 3 times daily for 4 days. Her white blood cell count and lactic acid level normalized. Unfortunately, thiamine levels were not obtained for the patient before treatment was initiated. After administration of IV thiamine, her plasma thiamine level was > 1,200 (normal range, 8-30) nmol/L.
Her differential diagnosis included infectious etiology. Given her leukocytosis and lactic acidosis, vancomycin and piperacillin/tazobactam were started on admission. One day later, her leukocytosis count doubled to 20.7×109 cells/L. However, after 48 hours of negative blood cultures, antibiotics were discontinued.
Small bowel obstruction was suspected due to the patient’s history of abdominal surgery but was ruled out with CT imaging. Similarly, pancreatitis was ruled out based on negative CT imaging and the patient’s normal lipase level. Gastroparesis also was considered because of the patient’s history of hypothyroidism, tobacco use, and her prior gastric-emptying study. The patient was treated for gastroparesis with a course of metoclopramide and erythromycin without improvement in symptoms. Additionally, gastroparesis would not explain the patient’s leukocytosis.
Cannabinoid hyperemesis syndrome (CHS) was suspected because the patient’s symptoms improved with cannabis discontinuation and hot showers.1 In chronic users, however, tetrahydrocannabinol levels have a half-life of 5 to 13 days.2 Although lactic acidosis and leukocytosis have been previously reported with cannabis use, it is unlikely that the patient would have such significant improvement within the first 4 days after discontinuation.1,3,4 Although the patient had many psychiatric comorbidities with previous hospitalizations describing concern for somatization disorder, her leukocytosis and elevated lactic acid levels were suggestive of an organic rather than a psychiatric etiology of her symptoms.
Discussion
Gastrointestinal beriberi has been reported in chronic cannabis users who present with nausea, vomiting, epigastric pain, leukocytosis, and lactic acidosis; all these symptoms rapidly improve after thiamine administration.5,6 The patient’s dietary change also eliminated her intake of vitamin B12, which compounded her condition. Thiamine deficiency produces lactic acidosis by disrupting pyruvate metabolism.7 Bradycardia also can be a sign of thiamine deficiency, although the patient’s use of clonidine for migraines is a confounder.8
Chronically ill patients are prone to nutritional deficiencies, including deficiencies of thiamine.7,9 Many patients with chronic illnesses also use cannabis to ameliorate physical and neuropsychiatric symptoms.2 Recent reports suggest cannabis users are prone to gastrointestinal beriberi and Wernicke encephalopathy.5,10 Treating gastrointestinal symptoms in these patients can be challenging to diagnose because gastrointestinal beriberi and CHS share many clinical manifestations.
The patient’s presentation is likely multifactorial resulting from the combination of gastrointestinal beriberi and CHS. However, thiamine deficiency seems to play the dominant role.
There is no standard treatment regimen for thiamine deficiency with neurologic deficits, and patients only retain about 10 to 15% of intramuscular (IM) injections of cyanocobalamin.11,12 The British Committee for Standards in Haematology recommends IM injections of 1,000 mcg of cyanocobalamin 3 times a week for 2 weeks and then reassess the need for continued treatment.13 The British Columbia guidelines also recommend IM injections of 1,000 mcg daily for 1 to 5 days before transitioning to oral repletion.14 European Neurology guidelines for the treatment of Wernicke encephalopathy recommend IV cyanocobalamin 200 mg 3 times daily.15 Low-level evidence with observational studies informs these decisions and is why there is variation.
The patient’s serum lactate and leukocytosis normalized 1 day after the administration of thiamine. Thiamine deficiency classically causes Wernicke encephalopathy and wet beriberi.16 The patient did not present with Wernicke encephalopathy’s triad: ophthalmoplegia, ataxia, or confusion. She also was euvolemic without signs or symptoms of wet beriberi.
Conclusions
Thiamine deficiency is principally a clinical diagnosis. Thiamine laboratory testing may not be readily available in all medical centers, and confirming a diagnosis of thiamine deficiency should not delay treatment when thiamine deficiency is suspected. This patient’s thiamine levels resulted a week after collection. The administration of thiamine before sampling also can alter the result as it did in this case. Additionally, laboratories may offer whole blood and serum testing. Whole blood testing is more accurate because most bioactive thiamine is found in red blood cells.17
1. Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc. 2011;111(3):166-169. doi:10.7556/jaoa.2011.111.3.166
2. Sharma P, Murthy P, Bharath MM. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 2012;7(4):149-156.
3. Antill T, Jakkoju A, Dieguez J, Laskhmiprasad L. Lactic acidosis: a rare manifestation of synthetic marijuana intoxication. J La State Med Soc. 2015;167(3):155.
4. Sullivan S. Cannabinoid hyperemesis. Can J Gastroenterol. 2010;24(5):284-285. doi:10.1155/2010/481940
5. Duca J, Lum CJ, Lo AM. Elevated lactate secondary to gastrointestinal beriberi. J Gen Intern Med. 2016;31(1):133-136. doi:10.1007/s11606-015-3326-2
6. Prakash S. Gastrointestinal beriberi: a forme fruste of Wernicke’s encephalopathy? BMJ Case Rep. 2018;bcr2018224841. doi:10.1136/bcr-2018-224841
7. Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine (Baltimore). 2007;86(4):225-232. doi:10.1097/MD.0b013e318125759a
8. Liang CC. Bradycardia in thiamin deficiency and the role of glyoxylate. J Nutrition Sci Vitaminology. 1977;23(1):1-6. doi:10.3177/jnsv.23.1
9. Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci. 2018;356(4):382-390. doi:10.1016/j.amjms.2018.06.015
10. Chaudhari A, Li ZY, Long A, Afshinnik A. Heavy cannabis use associated with Wernicke’s encephalopathy. Cureus. 2019;11(7):e5109. doi:10.7759/cureus.5109
11. Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149-160. doi:10.1056/NEJMcp1113996
12. Green R, Allen LH, Bjørke-Monsen A-L, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3(1):17040. doi:10.1038/nrdp.2017.40
13. Devalia V, Hamilton MS, Molloy AM. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513. doi:10.1111/bjh.12959
14. British Columbia Ministry of Health; Guidelines and Protocols and Advisory Committee. Guidelines and protocols cobalamin (vitamin B12) deficiency–investigation & management. Effective January 1, 2012. Revised May 1, 2013. Accessed March 10, 2021. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/vitamin-b12
15. Galvin R, Brathen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408-1418. doi:10.1111/j.1468-1331.2010.03153.x
16. Wiley KD, Gupta M. Vitamin B1 thiamine deficiency (beriberi). In: StatPearls. StatPearls Publishing LLC; 2019.
17. Jenco J, Krcmova LK, Solichova D, Solich P. Recent trends in determination of thiamine and its derivatives in clinical practice. J Chromatogra A. 2017;1510:1-12. doi:10.1016/j.chroma.2017.06.048
A 57-year-old woman with a history of traumatic brain injury, posttraumatic stress disorder, depression, migraines, hypothyroidism, and a hiatal hernia repair presented to the emergency department with a 1-day history of nausea, vomiting, and diffuse abdominal pain. She reported that her symptoms were relieved by hot showers. She also reported having similar symptoms and a previous gastric-emptying study that showed a slow-emptying stomach. Her history also consisted of frequent cannabis use for mood and appetite stimulation along with eliminating meat and fish from her diet, an increase in consumption of simple carbohydrates in the past year, and no alcohol use. Her medications included topiramate 100 mg and clonidine 0.3 mg nightly for migraines; levothyroxine 200 mcg daily for hypothyroidism; tizanidine 4 mg twice a day for muscle spasm; famotidine 40 mg twice a day as needed for gastric reflux; and bupropion 50 mg daily, citalopram 20 mg daily, and lamotrigine 25 mg nightly for mood.
The patient’s physical examination was notable for bradycardia (43 beats/min) and epigastric tenderness. Admission laboratory results were notable for an elevated lactic acid level of 4.8 (normal range, 0.50-2.20) mmol/L and a leukocytosis count of 10.8×109 cells/L. Serum alcohol level and blood cultures were negative. Liver function test, hemoglobin A1c, and lipase test were unremarkable. Her electrocardiogram showed an unchanged right bundle branch block. Chest X-ray, computed tomography (CT) of her abdomen/pelvis and echocardiogram were unremarkable.
What is your diagnosis?
How would you treat this patient?
This patient was diagnosed with gastrointestinal beriberi. Because of her dietary changes, lactic acidosis, and bradycardia, thiamine deficiency was suspected after ruling out other possibilities on the differential diagnosis (Table). The patient’s symptoms resolved after administration of high-dose IV thiamine 500 mg 3 times daily for 4 days. Her white blood cell count and lactic acid level normalized. Unfortunately, thiamine levels were not obtained for the patient before treatment was initiated. After administration of IV thiamine, her plasma thiamine level was > 1,200 (normal range, 8-30) nmol/L.
Her differential diagnosis included infectious etiology. Given her leukocytosis and lactic acidosis, vancomycin and piperacillin/tazobactam were started on admission. One day later, her leukocytosis count doubled to 20.7×109 cells/L. However, after 48 hours of negative blood cultures, antibiotics were discontinued.
Small bowel obstruction was suspected due to the patient’s history of abdominal surgery but was ruled out with CT imaging. Similarly, pancreatitis was ruled out based on negative CT imaging and the patient’s normal lipase level. Gastroparesis also was considered because of the patient’s history of hypothyroidism, tobacco use, and her prior gastric-emptying study. The patient was treated for gastroparesis with a course of metoclopramide and erythromycin without improvement in symptoms. Additionally, gastroparesis would not explain the patient’s leukocytosis.
Cannabinoid hyperemesis syndrome (CHS) was suspected because the patient’s symptoms improved with cannabis discontinuation and hot showers.1 In chronic users, however, tetrahydrocannabinol levels have a half-life of 5 to 13 days.2 Although lactic acidosis and leukocytosis have been previously reported with cannabis use, it is unlikely that the patient would have such significant improvement within the first 4 days after discontinuation.1,3,4 Although the patient had many psychiatric comorbidities with previous hospitalizations describing concern for somatization disorder, her leukocytosis and elevated lactic acid levels were suggestive of an organic rather than a psychiatric etiology of her symptoms.
Discussion
Gastrointestinal beriberi has been reported in chronic cannabis users who present with nausea, vomiting, epigastric pain, leukocytosis, and lactic acidosis; all these symptoms rapidly improve after thiamine administration.5,6 The patient’s dietary change also eliminated her intake of vitamin B12, which compounded her condition. Thiamine deficiency produces lactic acidosis by disrupting pyruvate metabolism.7 Bradycardia also can be a sign of thiamine deficiency, although the patient’s use of clonidine for migraines is a confounder.8
Chronically ill patients are prone to nutritional deficiencies, including deficiencies of thiamine.7,9 Many patients with chronic illnesses also use cannabis to ameliorate physical and neuropsychiatric symptoms.2 Recent reports suggest cannabis users are prone to gastrointestinal beriberi and Wernicke encephalopathy.5,10 Treating gastrointestinal symptoms in these patients can be challenging to diagnose because gastrointestinal beriberi and CHS share many clinical manifestations.
The patient’s presentation is likely multifactorial resulting from the combination of gastrointestinal beriberi and CHS. However, thiamine deficiency seems to play the dominant role.
There is no standard treatment regimen for thiamine deficiency with neurologic deficits, and patients only retain about 10 to 15% of intramuscular (IM) injections of cyanocobalamin.11,12 The British Committee for Standards in Haematology recommends IM injections of 1,000 mcg of cyanocobalamin 3 times a week for 2 weeks and then reassess the need for continued treatment.13 The British Columbia guidelines also recommend IM injections of 1,000 mcg daily for 1 to 5 days before transitioning to oral repletion.14 European Neurology guidelines for the treatment of Wernicke encephalopathy recommend IV cyanocobalamin 200 mg 3 times daily.15 Low-level evidence with observational studies informs these decisions and is why there is variation.
The patient’s serum lactate and leukocytosis normalized 1 day after the administration of thiamine. Thiamine deficiency classically causes Wernicke encephalopathy and wet beriberi.16 The patient did not present with Wernicke encephalopathy’s triad: ophthalmoplegia, ataxia, or confusion. She also was euvolemic without signs or symptoms of wet beriberi.
Conclusions
Thiamine deficiency is principally a clinical diagnosis. Thiamine laboratory testing may not be readily available in all medical centers, and confirming a diagnosis of thiamine deficiency should not delay treatment when thiamine deficiency is suspected. This patient’s thiamine levels resulted a week after collection. The administration of thiamine before sampling also can alter the result as it did in this case. Additionally, laboratories may offer whole blood and serum testing. Whole blood testing is more accurate because most bioactive thiamine is found in red blood cells.17
A 57-year-old woman with a history of traumatic brain injury, posttraumatic stress disorder, depression, migraines, hypothyroidism, and a hiatal hernia repair presented to the emergency department with a 1-day history of nausea, vomiting, and diffuse abdominal pain. She reported that her symptoms were relieved by hot showers. She also reported having similar symptoms and a previous gastric-emptying study that showed a slow-emptying stomach. Her history also consisted of frequent cannabis use for mood and appetite stimulation along with eliminating meat and fish from her diet, an increase in consumption of simple carbohydrates in the past year, and no alcohol use. Her medications included topiramate 100 mg and clonidine 0.3 mg nightly for migraines; levothyroxine 200 mcg daily for hypothyroidism; tizanidine 4 mg twice a day for muscle spasm; famotidine 40 mg twice a day as needed for gastric reflux; and bupropion 50 mg daily, citalopram 20 mg daily, and lamotrigine 25 mg nightly for mood.
The patient’s physical examination was notable for bradycardia (43 beats/min) and epigastric tenderness. Admission laboratory results were notable for an elevated lactic acid level of 4.8 (normal range, 0.50-2.20) mmol/L and a leukocytosis count of 10.8×109 cells/L. Serum alcohol level and blood cultures were negative. Liver function test, hemoglobin A1c, and lipase test were unremarkable. Her electrocardiogram showed an unchanged right bundle branch block. Chest X-ray, computed tomography (CT) of her abdomen/pelvis and echocardiogram were unremarkable.
What is your diagnosis?
How would you treat this patient?
This patient was diagnosed with gastrointestinal beriberi. Because of her dietary changes, lactic acidosis, and bradycardia, thiamine deficiency was suspected after ruling out other possibilities on the differential diagnosis (Table). The patient’s symptoms resolved after administration of high-dose IV thiamine 500 mg 3 times daily for 4 days. Her white blood cell count and lactic acid level normalized. Unfortunately, thiamine levels were not obtained for the patient before treatment was initiated. After administration of IV thiamine, her plasma thiamine level was > 1,200 (normal range, 8-30) nmol/L.
Her differential diagnosis included infectious etiology. Given her leukocytosis and lactic acidosis, vancomycin and piperacillin/tazobactam were started on admission. One day later, her leukocytosis count doubled to 20.7×109 cells/L. However, after 48 hours of negative blood cultures, antibiotics were discontinued.
Small bowel obstruction was suspected due to the patient’s history of abdominal surgery but was ruled out with CT imaging. Similarly, pancreatitis was ruled out based on negative CT imaging and the patient’s normal lipase level. Gastroparesis also was considered because of the patient’s history of hypothyroidism, tobacco use, and her prior gastric-emptying study. The patient was treated for gastroparesis with a course of metoclopramide and erythromycin without improvement in symptoms. Additionally, gastroparesis would not explain the patient’s leukocytosis.
Cannabinoid hyperemesis syndrome (CHS) was suspected because the patient’s symptoms improved with cannabis discontinuation and hot showers.1 In chronic users, however, tetrahydrocannabinol levels have a half-life of 5 to 13 days.2 Although lactic acidosis and leukocytosis have been previously reported with cannabis use, it is unlikely that the patient would have such significant improvement within the first 4 days after discontinuation.1,3,4 Although the patient had many psychiatric comorbidities with previous hospitalizations describing concern for somatization disorder, her leukocytosis and elevated lactic acid levels were suggestive of an organic rather than a psychiatric etiology of her symptoms.
Discussion
Gastrointestinal beriberi has been reported in chronic cannabis users who present with nausea, vomiting, epigastric pain, leukocytosis, and lactic acidosis; all these symptoms rapidly improve after thiamine administration.5,6 The patient’s dietary change also eliminated her intake of vitamin B12, which compounded her condition. Thiamine deficiency produces lactic acidosis by disrupting pyruvate metabolism.7 Bradycardia also can be a sign of thiamine deficiency, although the patient’s use of clonidine for migraines is a confounder.8
Chronically ill patients are prone to nutritional deficiencies, including deficiencies of thiamine.7,9 Many patients with chronic illnesses also use cannabis to ameliorate physical and neuropsychiatric symptoms.2 Recent reports suggest cannabis users are prone to gastrointestinal beriberi and Wernicke encephalopathy.5,10 Treating gastrointestinal symptoms in these patients can be challenging to diagnose because gastrointestinal beriberi and CHS share many clinical manifestations.
The patient’s presentation is likely multifactorial resulting from the combination of gastrointestinal beriberi and CHS. However, thiamine deficiency seems to play the dominant role.
There is no standard treatment regimen for thiamine deficiency with neurologic deficits, and patients only retain about 10 to 15% of intramuscular (IM) injections of cyanocobalamin.11,12 The British Committee for Standards in Haematology recommends IM injections of 1,000 mcg of cyanocobalamin 3 times a week for 2 weeks and then reassess the need for continued treatment.13 The British Columbia guidelines also recommend IM injections of 1,000 mcg daily for 1 to 5 days before transitioning to oral repletion.14 European Neurology guidelines for the treatment of Wernicke encephalopathy recommend IV cyanocobalamin 200 mg 3 times daily.15 Low-level evidence with observational studies informs these decisions and is why there is variation.
The patient’s serum lactate and leukocytosis normalized 1 day after the administration of thiamine. Thiamine deficiency classically causes Wernicke encephalopathy and wet beriberi.16 The patient did not present with Wernicke encephalopathy’s triad: ophthalmoplegia, ataxia, or confusion. She also was euvolemic without signs or symptoms of wet beriberi.
Conclusions
Thiamine deficiency is principally a clinical diagnosis. Thiamine laboratory testing may not be readily available in all medical centers, and confirming a diagnosis of thiamine deficiency should not delay treatment when thiamine deficiency is suspected. This patient’s thiamine levels resulted a week after collection. The administration of thiamine before sampling also can alter the result as it did in this case. Additionally, laboratories may offer whole blood and serum testing. Whole blood testing is more accurate because most bioactive thiamine is found in red blood cells.17
1. Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc. 2011;111(3):166-169. doi:10.7556/jaoa.2011.111.3.166
2. Sharma P, Murthy P, Bharath MM. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 2012;7(4):149-156.
3. Antill T, Jakkoju A, Dieguez J, Laskhmiprasad L. Lactic acidosis: a rare manifestation of synthetic marijuana intoxication. J La State Med Soc. 2015;167(3):155.
4. Sullivan S. Cannabinoid hyperemesis. Can J Gastroenterol. 2010;24(5):284-285. doi:10.1155/2010/481940
5. Duca J, Lum CJ, Lo AM. Elevated lactate secondary to gastrointestinal beriberi. J Gen Intern Med. 2016;31(1):133-136. doi:10.1007/s11606-015-3326-2
6. Prakash S. Gastrointestinal beriberi: a forme fruste of Wernicke’s encephalopathy? BMJ Case Rep. 2018;bcr2018224841. doi:10.1136/bcr-2018-224841
7. Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine (Baltimore). 2007;86(4):225-232. doi:10.1097/MD.0b013e318125759a
8. Liang CC. Bradycardia in thiamin deficiency and the role of glyoxylate. J Nutrition Sci Vitaminology. 1977;23(1):1-6. doi:10.3177/jnsv.23.1
9. Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci. 2018;356(4):382-390. doi:10.1016/j.amjms.2018.06.015
10. Chaudhari A, Li ZY, Long A, Afshinnik A. Heavy cannabis use associated with Wernicke’s encephalopathy. Cureus. 2019;11(7):e5109. doi:10.7759/cureus.5109
11. Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149-160. doi:10.1056/NEJMcp1113996
12. Green R, Allen LH, Bjørke-Monsen A-L, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3(1):17040. doi:10.1038/nrdp.2017.40
13. Devalia V, Hamilton MS, Molloy AM. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513. doi:10.1111/bjh.12959
14. British Columbia Ministry of Health; Guidelines and Protocols and Advisory Committee. Guidelines and protocols cobalamin (vitamin B12) deficiency–investigation & management. Effective January 1, 2012. Revised May 1, 2013. Accessed March 10, 2021. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/vitamin-b12
15. Galvin R, Brathen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408-1418. doi:10.1111/j.1468-1331.2010.03153.x
16. Wiley KD, Gupta M. Vitamin B1 thiamine deficiency (beriberi). In: StatPearls. StatPearls Publishing LLC; 2019.
17. Jenco J, Krcmova LK, Solichova D, Solich P. Recent trends in determination of thiamine and its derivatives in clinical practice. J Chromatogra A. 2017;1510:1-12. doi:10.1016/j.chroma.2017.06.048
1. Price SL, Fisher C, Kumar R, Hilgerson A. Cannabinoid hyperemesis syndrome as the underlying cause of intractable nausea and vomiting. J Am Osteopath Assoc. 2011;111(3):166-169. doi:10.7556/jaoa.2011.111.3.166
2. Sharma P, Murthy P, Bharath MM. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 2012;7(4):149-156.
3. Antill T, Jakkoju A, Dieguez J, Laskhmiprasad L. Lactic acidosis: a rare manifestation of synthetic marijuana intoxication. J La State Med Soc. 2015;167(3):155.
4. Sullivan S. Cannabinoid hyperemesis. Can J Gastroenterol. 2010;24(5):284-285. doi:10.1155/2010/481940
5. Duca J, Lum CJ, Lo AM. Elevated lactate secondary to gastrointestinal beriberi. J Gen Intern Med. 2016;31(1):133-136. doi:10.1007/s11606-015-3326-2
6. Prakash S. Gastrointestinal beriberi: a forme fruste of Wernicke’s encephalopathy? BMJ Case Rep. 2018;bcr2018224841. doi:10.1136/bcr-2018-224841
7. Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine (Baltimore). 2007;86(4):225-232. doi:10.1097/MD.0b013e318125759a
8. Liang CC. Bradycardia in thiamin deficiency and the role of glyoxylate. J Nutrition Sci Vitaminology. 1977;23(1):1-6. doi:10.3177/jnsv.23.1
9. Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci. 2018;356(4):382-390. doi:10.1016/j.amjms.2018.06.015
10. Chaudhari A, Li ZY, Long A, Afshinnik A. Heavy cannabis use associated with Wernicke’s encephalopathy. Cureus. 2019;11(7):e5109. doi:10.7759/cureus.5109
11. Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149-160. doi:10.1056/NEJMcp1113996
12. Green R, Allen LH, Bjørke-Monsen A-L, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3(1):17040. doi:10.1038/nrdp.2017.40
13. Devalia V, Hamilton MS, Molloy AM. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513. doi:10.1111/bjh.12959
14. British Columbia Ministry of Health; Guidelines and Protocols and Advisory Committee. Guidelines and protocols cobalamin (vitamin B12) deficiency–investigation & management. Effective January 1, 2012. Revised May 1, 2013. Accessed March 10, 2021. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/vitamin-b12
15. Galvin R, Brathen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408-1418. doi:10.1111/j.1468-1331.2010.03153.x
16. Wiley KD, Gupta M. Vitamin B1 thiamine deficiency (beriberi). In: StatPearls. StatPearls Publishing LLC; 2019.
17. Jenco J, Krcmova LK, Solichova D, Solich P. Recent trends in determination of thiamine and its derivatives in clinical practice. J Chromatogra A. 2017;1510:1-12. doi:10.1016/j.chroma.2017.06.048