User login
Peripartum depression: Early recognition improves outcomes
Contrary to common belief, pregnancy does not confer protection against depression.1,2 In fact, pregnant women are just as likely as nonpregnant women to become or remain depressed, and up to 12.7% of pregnant women meet criteria for depression.1
In the postpartum period, women are particularly vulnerable to a major depressive episode, whether a first episode or a recurrence. The estimated prevalence of a depressive episode in the first 3 postpartum months is 19.2%,2 making postpartum depression the most common complication of childbearing.2 At the same time, peripartum depression remains largely underrecognized and undertreated.3
As evidence mounts regarding the deleterious impact of untreated mental illness on the mother, the developing fetus, and the infant, early detection and intervention for peripartum depression are paramount.3
DEPRESSION DURING PREGNANCY: SIGNIFICANT CONSEQUENCES
Although the rates of depression in pregnant and nonpregnant women are similar, depression in pregnancy carries additional significant consequences. Further, many depressed pregnant women believe their depression will lift once their baby is born, though it is well documented that depression during pregnancy is the strongest predictor of postpartum depression and that if left untreated it can be devastating for mother, infant, and family.4
Compared with nondepressed pregnant women, depressed pregnant women have poorer overall health status,5 are more likely to engage in behaviors that pose risk to the developing fetus such as smoking,5 alcohol consumption, and substance use,6 and have poor nutrition and inadequate weight gain.7,8
Pregnant women who are depressed and are also experiencing domestic violence are especially at risk for poor prenatal care as they tend to miss more prenatal appointments.9 Evidence also suggests that depressed pregnant women are less attached to the fetus and more likely to have elective terminations.10,11
Depression in pregnancy is associated with higher rates of adverse pregnancy outcomes such as preterm birth, low birth weight, operative delivery, and longer predelivery hospital stay.3,12 Depression and anxiety during pregnancy have been associated with prenatal hypertension,13 gestational diabetes,14 preeclampsia,15 and HELLP syndrome (ie, hemolysis, elevated liver enzymes, and low platelet count).15 Depression and anxiety during pregnancy are associated with subsequent poorer infant attachment16,17 and an overall unfavorable impact on infant and child development.18
Risk factors for depression during pregnancy include past episodes of depression, current anxiety, poor social support, unintended pregnancy, life stress, being single, domestic violence, and being on Medicaid.19
Undoubtedly the most devastating consequence of severe depression during pregnancy is suicide. Rates of suicide are lower in peripartum women,20 but when suicide does occur, pregnant women tend to use more violent means than nonpregnant women. Pregnant adolescents represent a particularly high-risk group.21
POSTPARTUM DEPRESSION
Postpartum depression is the most common complication of childbearing. Although the precise pathogenesis is undetermined, there is converging evidence of a subset of women particularly sensitive to dramatic fluctuations in levels of estradiol and progesterone that occur during childbirth.22,23 There is also evidence that dysregulation of the hypothalamic-pituitary-adrenal axis contributes to the development of postpartum depression in certain women.24 Further, women who have depression or anxiety during pregnancy are much more likely to experience postpartum depression than those who are not symptomatic during pregnancy.4 A history of peripartum depression or other lifetime depressive episodes, poverty, conflict with a primary partner, poor social support, stressful life events, and low self-esteem are strongly associated with postpartum depression.25
When unrecognized and untreated, postpartum depression can have profound and persistent effects on the mother and the developing infant.18,26 Mothers with postpartum depression are much more likely than mothers without depression to have impaired bonding,27 to be less responsive to their infant’s needs,17 and to be more likely to miss well-baby checkups.28
Postpartum depression’s effects on maternal-infant interactions can include maternal withdrawal, disengagement, intrusion, and hostility and can lead to long-term effects on child development, including poor cognitive functioning, emotional maladjustment, and behavioral inhibition.29,30 Infants and children of mothers with untreated postpartum depression have been shown to exhibit a higher incidence of colic, excessive crying, sleep problems, and irritability.31,32 Women with postpartum depression may be less likely to initiate or maintain breastfeeding, and depressive symptoms have been noted to precede the discontinuation of breastfeeding.33–35
Risk factors for postpartum depression
Characteristics to look for in the prenatal care of pregnant women include the following:
- Depression during pregnancy
- History of postpartum or other depressive episode
- Poverty
- Conflict with primary partner
- Poor social support
- Low self-esteem
- Single status.
DIFFERENTIATING ‘POSTPARTUM BLUES’ FROM MAJOR DEPRESSION
Primary care providers are often the first point of contact for depressed women. The diagnosis of major depression in pregnant and postpartum women is challenging because of changes in sleep, appetite, and energy brought on by pregnancy, complications of delivery, and demands of caring for a newborn.36 Many pregnant and postpartum women are reluctant to disclose their symptoms due to a sense of shame and guilt for being depressed during a time in their life that society commonly regards as joyful, and this contributes to under-detection.
In the first few days postpartum, fatigue, emotionality, irritability, and worry over the infant’s well-being affect up to 75% of women. This period, typically referred to as the “baby blues” or “postpartum blues,” is not considered a disorder and responds well to support, reassurance, and adequate sleep, and it typically resolves within 2 weeks.37,38 Table 1 lists features that help distinguish postpartum blues from major depression.
Signs of major depressive disorder
Major depressive disorder is a serious and disabling condition. To meet criteria for major depressive disorder, women must report depressed mood and loss of interest or pleasure in normally pleasurable activities for at least 2 weeks. Completing the symptom profile, at least 5 of the following must be present: sleep disturbance (insomnia or hypersomnia), lack of energy, feelings of worthlessness or low self-esteem, guilt, difficulty concentrating, indecisiveness, psychomotor retardation or agitation, and thoughts of suicide or death.
The Diagnostic and Statistical Manual of Mental Disorders (5th edition) recognizes that postpartum depression commonly begins during pregnancy, and now uses “peripartum onset” as the specifier for major depressive disorder that occurs during pregnancy, postpartum, or both.39 Other hallmark symptoms with peripartum onset include a lack of interest in or attachment to the pregnancy or infant, and anxiety and worry often accompanied by intrusive, unwanted thoughts of harm befalling the infant.40
Postpartum psychosis
Postpartum psychosis is a far less common presentation, occurring in 1 to 2 per 1,000 births, but it constitutes a psychiatric emergency requiring immediate referral to a psychiatric care setting. Women at highest risk are those with a personal or family history of bipolar disorder.
The clinical presentation is most commonly characterized by confusion, agitation, hallucinations, delusional beliefs, and disorientation. Suicide and infanticide, while rare, are more likely to occur in the context of a psychotic episode.41
SCREENING RECOMMENDATIONS
Screening for depression is routine in primary care settings and is no less important for peripartum women.
In 2016, the US Preventive Services Task Force issued a recommendation that all pregnant and postpartum women be screened for depression,42 highlighting the need for all medical providers to be alert to the potentially serious consequences of unrecognized and untreated maternal psychiatric illness.
The American College of Obstetricians and Gynecologists (ACOG) recommends screening for depression and anxiety at least once during the peripartum period,43 and the American Academy of Pediatrics recommends screening mothers for depression at the 1-, 2-, and 4-month well-baby visits.44
The peripartum period is associated with changes in sleep, appetite, and energy levels, but these are also typical of depression. Taking this into account, the Edinburgh Postnatal Depression Scale (EPDS) was developed to screen for depression specifically in this population.45 The EPDS is a validated and widely used 10-item self-reporting questionnaire with a high degree of sensitivity and specificity; it is easily administered and quickly scored. A cutoff score of 13 (of a maximum of 30) is considered indicative of depressed mood and signals the need for further assessment.
ACOG, the American Academy of Pediatrics, and the US Preventive Services Task Force recommend a standardized validated tool and cite both the EPDS (https://psychology-tools.com/epds/) and the Patient Health Questionnaire-9 (PHQ-9) (Figure 1) as appropriate to screen for peripartum depression.42–44 Primary care providers tend to be most familiar with the PHQ-9, a highly sensitive and specific 9-item depression screen that has been validated in primary care and obstetric clinic patients.46 A score on the PHQ-9 ranging from 5 to 10 indicates mild depression, 10 to 14 moderate depression, 15 to 19 moderate to severe depression, and greater than 19 severe depression.
CLINICAL MANAGEMENT
Many women prefer nondrug therapy
The gold standard treatment for moderate to severe major depressive disorder is psychotherapy plus pharmacotherapy. Yet many peripartum women voice concerns about exposure to pharmacologic treatment, and studies have shown that many women prefer nonpharmacologic intervention.47
Evidence-based psychotherapies that have demonstrated efficacy in peripartum women include cognitive behavioral therapy48 and interpersonal psychotherapy when administered by a psychotherapist trained in these treatments. Pregnant and breastfeeding women often express preference for psychotherapy and complementary and alternative treatments as a means of avoiding fetal and infant exposure to antidepressants.47
For mild to moderate depression, complementary therapies such as exercise, yoga, bright light therapy, and acupuncture have shown efficacy and can be used alone or adjunctively.49 Because a poor marital relationship is consistently associated with peripartum depression,25 primary care physicians who routinely address social support and screen for family conflict are well positioned to detect this significant correlate and to recommend marital or family therapy as a primary or adjunctive treatment.
When to consider drug therapy
The decision to recommend drug therapy must be individualized and based on the severity of symptoms, functional impairment, number and frequency of depressive episodes, history of response to medications, and the preferences of the patient, with the recognition that no decision is risk-free and that antidepressants enter the amniotic fluid, so fetal exposure is unavoidable.
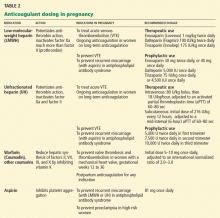
Table 2 lists common antidepressants. The antidepressants most commonly prescribed, especially in the primary care setting, are selective serotonin reuptake inhibitors (SSRIs), which are favored because of their effectiveness, low side-effect profile, and lack of overdose toxicity.
Serotonin syndrome is no more likely to occur in pregnant than in nonpregnant women. Close monitoring for this condition is warranted only when patients are taking very high doses of SSRIs or SSRIs in combination with other serotonergic agonists.
Prescribing antidepressants for pregnant or breastfeeding women requires thoughtful consideration of the patient’s preferences, as well as weighing the risks and benefits of fetal and infant exposure to maternal depression vs exposure to medications. Additional considerations include monotherapy, avoiding medication changes, choosing drugs that have been effective in the past, and avoiding drugs with known drug-drug interactions or teratogenic effects.50
There is increasing consensus that the short- and long-term consequences of undertreatment or nontreatment of maternal depression outweigh the risk of fetal exposure to SSRIs.3,51,52 Cohen et al53 have recommended that if a woman is on an antidepressant and learns she is pregnant, she should not discontinue it because of the likelihood of relapse; they found a 68% relapse rate in women who discontinued their antidepressant in the first trimester of pregnancy.53
In a comprehensive review of studies published between 1996 and 2012 that examined antidepressant use during pregnancy, Byatt et al54 found little or no evidence of increased teratogenic risk with antidepressants with the exception of paroxetine, which is associated with a small but significant increased risk of cardiac malformation during first-trimester exposure.54
These conclusions were underscored in a large cohort study in the United Kingdom.55 In addition, a joint task force of the American Psychiatric Association and ACOG reviewed studies looking at the association between depression, antidepressants, and birth outcomes including miscarriage, preterm birth, cardiac abnormalities (resulting from first trimester exposure), persistent pulmonary hypertension (related to second- and third-trimester exposure), and neonatal adaptation syndrome (associated with third-trimester exposure).8 They concluded that the available data neither support nor refute a link between the use of antidepressants and several of the above outcomes. No increase in risk of congenital malformations (including cardiac abnormalities) was found. An increased risk of persistent pulmonary hypertension was noted, although the absolute risk of this disorder remained low, at 3 to 6 per 1,000 infants exposed to SSRIs in utero.8,56
Neonatal adaptation syndrome
Neonatal adaptation syndrome is characterized by jitteriness, irritability, decreased muscle tone, and feeding difficulty in the neonate. It can occur in 15% to 30% of infants exposed to SSRIs antenatally.57,58 These symptoms, however, are transient and typically resolve within 7 to 10 days after birth. A more recent study suggested that neurobehavioral symptoms for some infants extend beyond 2 weeks and that concomitant exposure to benzodiazepines results in even higher rates of this syndrome.59 There is no evidence that tapering or discontinuing antidepressants near term is necessary, safe, or effective in preventing transient neonatal complications. However, this approach would increase the risk of relapse for the mother.
Autism spectrum disorders
The possible association between antidepressants and autism spectrum disorders in pregnancy has captured much attention in recent years. One study based on healthcare claims60 and one registry-based study61 associated in utero exposure to antidepressants with autism liability in children. However, a large-scale Danish registry-based study did not replicate this association.62 In addition, 2 recent cohort studies, identifying children with autism spectrum disorder or attention-deficit hyperactivity disorder from electronic health records, found that neither disorder was significantly associated with prenatal antidepressant exposure in crude or adjusted models. However, both studies found a significant association with the use of antidepressants before pregnancy, indicating that the risk of autism observed with prenatal antidepressant exposure is likely confounded by the severity of maternal illness.63,64
Concerns about drug therapy during breastfeeding
For infants of breastfeeding women, exposure to antidepressants through breast milk is minimal. Amounts in breast milk depend on the timing of the antidepressant dose, timing of feeding, and genetically influenced metabolic activity in mother and infant. The current literature supports antidepressant use for breastfeeding mothers of healthy full-term infants.65
The 2 most widely studied antidepressants in breastfed infants are paroxetine and sertraline. It has been shown that very little can be detected in the infant’s serum, with relative infant doses ranging from 0.4% to 2.8%.65 While clinicians are cautioned against prescribing paroxetine for pregnant women, the drug remains a suitable alternative for breastfeeding women.
If an antidepressant is started postpartum, the recommendation is to start with a low dose and then slowly titrate upward while monitoring the infant for adverse effects.65,66 Possible adverse effects in breastfeeding infants include irritability, sedation, poor weight gain, and a change in feeding patterns.67 Adverse events are most likely to occur in newborns up to 8 weeks of age, and infants born prematurely or with medical problems may be particularly at risk.65,68
Helping patients weigh risks and benefits of drug therapy
Women may hear about the risks of medications to the fetus and during breastfeeding and so may be reluctant to seek or accept intervention. Often, the information is not from a reliable, scientifically based source. Primary care physicians are well positioned to guide peripartum women in risk-benefit analysis of proper treatment of their depression vs no treatment or undertreatment. In addition, establishing referral sources—ideally with a peripartum mental health specialist—is advisable. Online resources that clinicians can refer patients to for help in managing peripartum depression include the following:
- www.postpartum.net
- www.womensmentalhealth.org
- www.mothertobaby.org (for pharmacologic guidance).
INCREASED AWARENESS IS KEY
Primary care physicians must remain alert to the high prevalence of depression in women of childbearing age and embrace routine screening for depression. (See the sidebar, “The primary care management of peripartum depression.”) Since half of pregnancies are unintended, awareness of the risks of undetected and untreated peripartum depression to the mother, developing fetus, and infant is essential. Untreated antepartum depression has been linked to poor pregnancy outcomes, nutritional deficits, and substance abuse. Untreated postpartum depression negatively affects mother-infant attachment, infant, and child development and maternal self care.
Not treating depression is hazardous
Drug treatment during pregnancy and breastfeeding poses challenges for the patient and physician due to the inevitability of fetal and infant exposure, but lack of treatment can be hazardous.
To date, the evidence on the use of antidepressants in pregnant and lactating women is reassuring. Specialized peripartum psychiatric partial hospital programs69 and inpatient programs70 exist for women who need a higher level of care. There is also substantial evidence that psychotherapy, especially cognitive behavioral therapy and interpersonal therapy, is highly effective, and emerging data on complementary and alternative treatments are promising. Coordinated care between primary care and behavioral healthcare providers with expertise in treating peripartum depression is most likely to yield optimal outcomes.
- World Health Organization (WHO). A message from the Director General. www.who.int/whr/2001/dg_message/en/index.html. Accessed March 6, 2017.
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obset Gynecol 2005; 106:1071–1083.
- Davalos DB, Yadon CA, Tregellas HC. Untreated prenatal maternal depression and the potential risks to offspring: a review. Arch Women’s Mental Health 2012; 15:1–14.
- Chaudron LH, Klein MH, Remington P, Palta M, Allen C, Essex MJ. Predictors, prodromes and incidence of postpartum depression. J Psychosom Obstet Gynaecol 2001; 22:103–112.
- Orr ST, Blazer DG, Orr CA. Maternal prenatal depressive symptoms, nicotine addiction, and smoking-related knowledge, attitudes, beliefs, and behaviors. Matern Child Health J 2012; 16:973–978.
- Flynn HA, Chermack ST. Prenatal alcohol use: the role of lifetime problems with alcohol,drugs, depression, and violence. J Stud Alcohol Drugs 2008; 69:500–509.
- Bodnar LM, Wisner KL, Moses-Kolko E, Sit DK, Hanusa BH. Prepregnancy body mass index, gestational weight gain, and the likelihood of major depressive disorder during pregnancy. J Clin Psychiatry 2009; 70:1290–1296.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol 2009; 114:703–713.
- Han A, Stewart DE. Maternal and fetal outcomes of intimate partner violence associated with pregnancy in the Latin American and Caribbean region. Int J Gynecol Obstet 2014; 124:6–11.
- McFarland J, Salisbury AL, Battler CL, Hawes K, Halloran K, Lester BM. Major depressive disorder during pregnancy and emotional attachment to the fetus. Arch Womens Ment Health 2011; 14:425–434.
- Suri R, Althuler LA, Mintz J. Depression and the decision to abort. Am J Psychiatry 2004; 161:1502.
- Kim DR, Sockol LE, Sammel MD, Kelly C, Moseley M, Epperson CN. Elevated risk of adverse obstetric outcomes in pregnant women with depression. Arch Women’s Ment Health 2013; 16:475–482.
- Mautner E, Greimel E, Trutnovsky G, Daghofer F, Egger JW, Lang U. Quality of life outcomes in pregnancy and postpartum complicated by hypertensive disorders, gestational diabetes, and preterm birth. J Psychosom Obstet Gynaecol 2009; 30:231–237.
- Katon JG, Russo J, Gavin AR, Melville JL, Katon WJ. Diabetes and depression in pregnancy: is there an association? J Women’s Health (Larchmt) 2011; 20:983–989.
- Delahaije DH, Dirksen CD, Peeters LL, Smits LJ. Anxiety and depression following preeclampsia or HELLP syndrome: a systematic review. Acta Obstet Gynecol Scand 2013; 92:746–761.
- O’Higgins M, Roberts IS, Glover V, Taylor A. Mother-child bonding at 1 year; associations with symptoms of postnatal depression and bonding in the first few weeks. Arch Women’s Ment Health 2013; 16:381–389.
- Field T, Healy BT, Goldstein S, Guthertz M. Behavior-state matching and synchrony in mother-infant interactions of nondepressed versus depressed dyads. Dev Psychol 1990; 26:7–14.
- Kingston D, Tough S, Whitfield H. Prenatal and postpartum maternal psychological distress and infant development: a systematic review. Child Psychiatry Hum Dev 2012; 43:683–714.
- Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol 2010; 202:5–14.
- Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Women’s Ment Health 2005; 8:77–87.
- Appleby L. Suicide after pregnancy and the first postnatal year. BMJ 1991; 302:137–140.
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000; 157:924–930.
- Workman JL, Barha CK, Galea LAM. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci 2012; 126:54–72.
- Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci 2011; 13:89–100.
- O’Hara MW, McCabe JE. Postpartum depression: current status and future directions. Annu Rev Clin Psychol 2013; 9:379–407.
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev 2011; 14:1–27
- Muzik M, Bocknek EL, Broderick A, et al. Mother-infant bonding impairment across the first 6 months postpartum: the primacy of psychopathology in women with childhood abuse and neglect histories. Arch Women’s Ment Health 2013; 16:29–38.
- Farr SL, Dietz PM, Rizzo JH, et al. Health care utilisation in the first year of life among infants of mothers with perinatal depression or anxiety. Paediatr Perinat Epidemiol 2013; 27:81–88.
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Women’s Ment Health 2003; 6:263–274.
- Murray L, Cooper PJ. Postpartum depression and child development. Psychol Med 1997; 27:253–260.
- Orhon FS, Ulukol B, Soykan A. Postpartum mood disorders and maternal perceptions of infant patterns in well-child follow-up visits. Acta Paediatr 2007; 96:1777–1783.
- Dennis CL, Ross L. Relationships among infant sleep patterns, maternal fatigue, and development of depressive symptomatology. Birth 2005; 32:187–193.
- Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 2007; 153:1–186.
- Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Paediatr 2007; 96:590–594.
- Hatton DC, Harrison-Hohner J, Coste S, Dorato V, Curet LB, McCarron DA. Symptoms of postpartum depression and breastfeeding. J Hum Lact 2005; 21:444–449.
- Klein MH, Essex MJ. Pregnant or depressed? The effect of overlap between symptoms of depression and somatic complaints of pregnancy on rates of major depression in the second trimester. Depression 1994; 2:308–314.
- Seyfried LS, Marcus SM. Postpartum mood disorders. Int Rev Psychiatry 2003; 15:231–242.
- Buttner MM, O’Hara MW, Watson D. The structure of women’s mood in the early postpartum. Assessment 2012; 19:247–256.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA; American Psychiatric Association Publishing: 2013.
- Wisner KL, Peindl KS, Gigliotti T, Hanusa BH. Obsessions and compulsions in women with postpartum depression. J Clin Psychiatry 1999: 60:176-180.
- Di Florio A, Smith S, Jones I. Postpartum psychosis. The Obstetrician & Gynecologist 2013; 15:145–150.
- O’Connor E, Rossom RC, Henniger M, Groom HC, Burda BU. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315:388–406.
- Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol 2015; 125:1268–1271.
- Earls MF; Committee on Psychosocial Aspects of Child and Family Health American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics 2010; 126:1032–1039.
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry 1987; 150:782–786.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–616.
- Battle CL, Salisbury AL, Schofield CA, Ortiz-Hernandez S. Perinatal antidepressant use: understanding women’s preferences and concerns. J Psychiatr Pract 2013; 19:443–453.
- Stuart S, Koleva H. Psychological treatments for perinatal depression. Best Pract Res Clin Obstet Gynaecol 2014; 28:61–70.
- Deligiannidis KM, Freeman MP. Complementary and alternative medicine therapies for perinatal depression. Best Pract Res Clin Obstet Gynaecol 2014; 28:85–95.
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin: clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces Practice Bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008; 111:1001–1020.
- Ornoy A, Koren G. Selective serotonin reuptake inhibitors in human pregnancy: on the way to resolving the controversy. Semin Fetal Neonatal Med 2014; 19:188–194.
- Salisbury AL, Wisner KL, Pearlstein T, Battle CL, Stroud L, Lester BM. Newborn neurobehavioral patterns are differentially related to prenatal maternal major depressive disorder and serotonin reuptake inhibitor treatment. Depress Anxiety 2011; 28:1008–1019.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006; 295:499–507.
- Byatt N, Deligiannidis KM, Freeman MP. Antidepressant use in pregnancy: a critical review focused on risks and controversies. Acta Psychiatr Scand 2013; 127:94–114.
- Ban L, Gibson JE, West J, et al. Maternal depression, antidepressant prescriptions, and congenital anomaly risk in offspring: a population-based cohort study. BJOG 2014; 121:1471–1481.
- Kallen B, Olausson P. Maternal use of selective serotonin re-uptake inhibitors and persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf 2008; 17:801–806.
- Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med 1996; 335:1010–1015.
- Costei AM, Kozer E, Ho T, Ito S, Koren G. Perinatal outcome following third trimester exposure to paroxetine. Arch Pediatr Adolesc Med 2002; 156:1129–1132.
- Salisbury AL, O’Grady KE, Battle CL, et al. The roles of maternal depression, serotonin reuptake inhibitor treatment, and concomitant benzodiazepine use on infant neurobehavioral functioning over the first postnatal month. Am J Psychiatry 2016; 173:147–157.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68:1104–1112.
- Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ 2013; 346:f2059.
- Sorensen MJ, Gronborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol 2013; 5:449–459.
- Clements CC, Castro VM, Blumenthal SR, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20:727–734.
- Castro VM, Kong SW, Clements CC, et al. Absence of evidence for increase in risk for autism or attention-deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study. Transl Psychiatry 2016; 6:e708.
- Hale TW, Rowe HE. Medications and Mothers’ Milk. 16th ed. Amarillo, TX: Hale Publishing, L.P; 2014.
- Abreu AC, Stuart S. Pharmacologic and hormonal treatments for postpartum depression. Psychiatr Ann 2005; 35:568–576.
- Sit DK, Wisner KL. Decision making for postpartum depression treatment. Psychiatr Ann 2005; 35:577–584.
- Wisner KL, Parry BL, Piontek CM. Clinical practice. Postpartum depression. N Engl J Med 2002; 347:194–199.
- Howard M, Battle CL, Pearlstein T, Rosene-Montella K. A psychiatric mother-baby day hospital for pregnant and postpartum women. Arch Women’s Ment Health 2006; 9:213–218.
- Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Women’s Ment Health 2014; 17:107–113.
Contrary to common belief, pregnancy does not confer protection against depression.1,2 In fact, pregnant women are just as likely as nonpregnant women to become or remain depressed, and up to 12.7% of pregnant women meet criteria for depression.1
In the postpartum period, women are particularly vulnerable to a major depressive episode, whether a first episode or a recurrence. The estimated prevalence of a depressive episode in the first 3 postpartum months is 19.2%,2 making postpartum depression the most common complication of childbearing.2 At the same time, peripartum depression remains largely underrecognized and undertreated.3
As evidence mounts regarding the deleterious impact of untreated mental illness on the mother, the developing fetus, and the infant, early detection and intervention for peripartum depression are paramount.3
DEPRESSION DURING PREGNANCY: SIGNIFICANT CONSEQUENCES
Although the rates of depression in pregnant and nonpregnant women are similar, depression in pregnancy carries additional significant consequences. Further, many depressed pregnant women believe their depression will lift once their baby is born, though it is well documented that depression during pregnancy is the strongest predictor of postpartum depression and that if left untreated it can be devastating for mother, infant, and family.4
Compared with nondepressed pregnant women, depressed pregnant women have poorer overall health status,5 are more likely to engage in behaviors that pose risk to the developing fetus such as smoking,5 alcohol consumption, and substance use,6 and have poor nutrition and inadequate weight gain.7,8
Pregnant women who are depressed and are also experiencing domestic violence are especially at risk for poor prenatal care as they tend to miss more prenatal appointments.9 Evidence also suggests that depressed pregnant women are less attached to the fetus and more likely to have elective terminations.10,11
Depression in pregnancy is associated with higher rates of adverse pregnancy outcomes such as preterm birth, low birth weight, operative delivery, and longer predelivery hospital stay.3,12 Depression and anxiety during pregnancy have been associated with prenatal hypertension,13 gestational diabetes,14 preeclampsia,15 and HELLP syndrome (ie, hemolysis, elevated liver enzymes, and low platelet count).15 Depression and anxiety during pregnancy are associated with subsequent poorer infant attachment16,17 and an overall unfavorable impact on infant and child development.18
Risk factors for depression during pregnancy include past episodes of depression, current anxiety, poor social support, unintended pregnancy, life stress, being single, domestic violence, and being on Medicaid.19
Undoubtedly the most devastating consequence of severe depression during pregnancy is suicide. Rates of suicide are lower in peripartum women,20 but when suicide does occur, pregnant women tend to use more violent means than nonpregnant women. Pregnant adolescents represent a particularly high-risk group.21
POSTPARTUM DEPRESSION
Postpartum depression is the most common complication of childbearing. Although the precise pathogenesis is undetermined, there is converging evidence of a subset of women particularly sensitive to dramatic fluctuations in levels of estradiol and progesterone that occur during childbirth.22,23 There is also evidence that dysregulation of the hypothalamic-pituitary-adrenal axis contributes to the development of postpartum depression in certain women.24 Further, women who have depression or anxiety during pregnancy are much more likely to experience postpartum depression than those who are not symptomatic during pregnancy.4 A history of peripartum depression or other lifetime depressive episodes, poverty, conflict with a primary partner, poor social support, stressful life events, and low self-esteem are strongly associated with postpartum depression.25
When unrecognized and untreated, postpartum depression can have profound and persistent effects on the mother and the developing infant.18,26 Mothers with postpartum depression are much more likely than mothers without depression to have impaired bonding,27 to be less responsive to their infant’s needs,17 and to be more likely to miss well-baby checkups.28
Postpartum depression’s effects on maternal-infant interactions can include maternal withdrawal, disengagement, intrusion, and hostility and can lead to long-term effects on child development, including poor cognitive functioning, emotional maladjustment, and behavioral inhibition.29,30 Infants and children of mothers with untreated postpartum depression have been shown to exhibit a higher incidence of colic, excessive crying, sleep problems, and irritability.31,32 Women with postpartum depression may be less likely to initiate or maintain breastfeeding, and depressive symptoms have been noted to precede the discontinuation of breastfeeding.33–35
Risk factors for postpartum depression
Characteristics to look for in the prenatal care of pregnant women include the following:
- Depression during pregnancy
- History of postpartum or other depressive episode
- Poverty
- Conflict with primary partner
- Poor social support
- Low self-esteem
- Single status.
DIFFERENTIATING ‘POSTPARTUM BLUES’ FROM MAJOR DEPRESSION
Primary care providers are often the first point of contact for depressed women. The diagnosis of major depression in pregnant and postpartum women is challenging because of changes in sleep, appetite, and energy brought on by pregnancy, complications of delivery, and demands of caring for a newborn.36 Many pregnant and postpartum women are reluctant to disclose their symptoms due to a sense of shame and guilt for being depressed during a time in their life that society commonly regards as joyful, and this contributes to under-detection.
In the first few days postpartum, fatigue, emotionality, irritability, and worry over the infant’s well-being affect up to 75% of women. This period, typically referred to as the “baby blues” or “postpartum blues,” is not considered a disorder and responds well to support, reassurance, and adequate sleep, and it typically resolves within 2 weeks.37,38 Table 1 lists features that help distinguish postpartum blues from major depression.
Signs of major depressive disorder
Major depressive disorder is a serious and disabling condition. To meet criteria for major depressive disorder, women must report depressed mood and loss of interest or pleasure in normally pleasurable activities for at least 2 weeks. Completing the symptom profile, at least 5 of the following must be present: sleep disturbance (insomnia or hypersomnia), lack of energy, feelings of worthlessness or low self-esteem, guilt, difficulty concentrating, indecisiveness, psychomotor retardation or agitation, and thoughts of suicide or death.
The Diagnostic and Statistical Manual of Mental Disorders (5th edition) recognizes that postpartum depression commonly begins during pregnancy, and now uses “peripartum onset” as the specifier for major depressive disorder that occurs during pregnancy, postpartum, or both.39 Other hallmark symptoms with peripartum onset include a lack of interest in or attachment to the pregnancy or infant, and anxiety and worry often accompanied by intrusive, unwanted thoughts of harm befalling the infant.40
Postpartum psychosis
Postpartum psychosis is a far less common presentation, occurring in 1 to 2 per 1,000 births, but it constitutes a psychiatric emergency requiring immediate referral to a psychiatric care setting. Women at highest risk are those with a personal or family history of bipolar disorder.
The clinical presentation is most commonly characterized by confusion, agitation, hallucinations, delusional beliefs, and disorientation. Suicide and infanticide, while rare, are more likely to occur in the context of a psychotic episode.41
SCREENING RECOMMENDATIONS
Screening for depression is routine in primary care settings and is no less important for peripartum women.
In 2016, the US Preventive Services Task Force issued a recommendation that all pregnant and postpartum women be screened for depression,42 highlighting the need for all medical providers to be alert to the potentially serious consequences of unrecognized and untreated maternal psychiatric illness.
The American College of Obstetricians and Gynecologists (ACOG) recommends screening for depression and anxiety at least once during the peripartum period,43 and the American Academy of Pediatrics recommends screening mothers for depression at the 1-, 2-, and 4-month well-baby visits.44
The peripartum period is associated with changes in sleep, appetite, and energy levels, but these are also typical of depression. Taking this into account, the Edinburgh Postnatal Depression Scale (EPDS) was developed to screen for depression specifically in this population.45 The EPDS is a validated and widely used 10-item self-reporting questionnaire with a high degree of sensitivity and specificity; it is easily administered and quickly scored. A cutoff score of 13 (of a maximum of 30) is considered indicative of depressed mood and signals the need for further assessment.
ACOG, the American Academy of Pediatrics, and the US Preventive Services Task Force recommend a standardized validated tool and cite both the EPDS (https://psychology-tools.com/epds/) and the Patient Health Questionnaire-9 (PHQ-9) (Figure 1) as appropriate to screen for peripartum depression.42–44 Primary care providers tend to be most familiar with the PHQ-9, a highly sensitive and specific 9-item depression screen that has been validated in primary care and obstetric clinic patients.46 A score on the PHQ-9 ranging from 5 to 10 indicates mild depression, 10 to 14 moderate depression, 15 to 19 moderate to severe depression, and greater than 19 severe depression.
CLINICAL MANAGEMENT
Many women prefer nondrug therapy
The gold standard treatment for moderate to severe major depressive disorder is psychotherapy plus pharmacotherapy. Yet many peripartum women voice concerns about exposure to pharmacologic treatment, and studies have shown that many women prefer nonpharmacologic intervention.47
Evidence-based psychotherapies that have demonstrated efficacy in peripartum women include cognitive behavioral therapy48 and interpersonal psychotherapy when administered by a psychotherapist trained in these treatments. Pregnant and breastfeeding women often express preference for psychotherapy and complementary and alternative treatments as a means of avoiding fetal and infant exposure to antidepressants.47
For mild to moderate depression, complementary therapies such as exercise, yoga, bright light therapy, and acupuncture have shown efficacy and can be used alone or adjunctively.49 Because a poor marital relationship is consistently associated with peripartum depression,25 primary care physicians who routinely address social support and screen for family conflict are well positioned to detect this significant correlate and to recommend marital or family therapy as a primary or adjunctive treatment.
When to consider drug therapy
The decision to recommend drug therapy must be individualized and based on the severity of symptoms, functional impairment, number and frequency of depressive episodes, history of response to medications, and the preferences of the patient, with the recognition that no decision is risk-free and that antidepressants enter the amniotic fluid, so fetal exposure is unavoidable.
Table 2 lists common antidepressants. The antidepressants most commonly prescribed, especially in the primary care setting, are selective serotonin reuptake inhibitors (SSRIs), which are favored because of their effectiveness, low side-effect profile, and lack of overdose toxicity.
Serotonin syndrome is no more likely to occur in pregnant than in nonpregnant women. Close monitoring for this condition is warranted only when patients are taking very high doses of SSRIs or SSRIs in combination with other serotonergic agonists.
Prescribing antidepressants for pregnant or breastfeeding women requires thoughtful consideration of the patient’s preferences, as well as weighing the risks and benefits of fetal and infant exposure to maternal depression vs exposure to medications. Additional considerations include monotherapy, avoiding medication changes, choosing drugs that have been effective in the past, and avoiding drugs with known drug-drug interactions or teratogenic effects.50
There is increasing consensus that the short- and long-term consequences of undertreatment or nontreatment of maternal depression outweigh the risk of fetal exposure to SSRIs.3,51,52 Cohen et al53 have recommended that if a woman is on an antidepressant and learns she is pregnant, she should not discontinue it because of the likelihood of relapse; they found a 68% relapse rate in women who discontinued their antidepressant in the first trimester of pregnancy.53
In a comprehensive review of studies published between 1996 and 2012 that examined antidepressant use during pregnancy, Byatt et al54 found little or no evidence of increased teratogenic risk with antidepressants with the exception of paroxetine, which is associated with a small but significant increased risk of cardiac malformation during first-trimester exposure.54
These conclusions were underscored in a large cohort study in the United Kingdom.55 In addition, a joint task force of the American Psychiatric Association and ACOG reviewed studies looking at the association between depression, antidepressants, and birth outcomes including miscarriage, preterm birth, cardiac abnormalities (resulting from first trimester exposure), persistent pulmonary hypertension (related to second- and third-trimester exposure), and neonatal adaptation syndrome (associated with third-trimester exposure).8 They concluded that the available data neither support nor refute a link between the use of antidepressants and several of the above outcomes. No increase in risk of congenital malformations (including cardiac abnormalities) was found. An increased risk of persistent pulmonary hypertension was noted, although the absolute risk of this disorder remained low, at 3 to 6 per 1,000 infants exposed to SSRIs in utero.8,56
Neonatal adaptation syndrome
Neonatal adaptation syndrome is characterized by jitteriness, irritability, decreased muscle tone, and feeding difficulty in the neonate. It can occur in 15% to 30% of infants exposed to SSRIs antenatally.57,58 These symptoms, however, are transient and typically resolve within 7 to 10 days after birth. A more recent study suggested that neurobehavioral symptoms for some infants extend beyond 2 weeks and that concomitant exposure to benzodiazepines results in even higher rates of this syndrome.59 There is no evidence that tapering or discontinuing antidepressants near term is necessary, safe, or effective in preventing transient neonatal complications. However, this approach would increase the risk of relapse for the mother.
Autism spectrum disorders
The possible association between antidepressants and autism spectrum disorders in pregnancy has captured much attention in recent years. One study based on healthcare claims60 and one registry-based study61 associated in utero exposure to antidepressants with autism liability in children. However, a large-scale Danish registry-based study did not replicate this association.62 In addition, 2 recent cohort studies, identifying children with autism spectrum disorder or attention-deficit hyperactivity disorder from electronic health records, found that neither disorder was significantly associated with prenatal antidepressant exposure in crude or adjusted models. However, both studies found a significant association with the use of antidepressants before pregnancy, indicating that the risk of autism observed with prenatal antidepressant exposure is likely confounded by the severity of maternal illness.63,64
Concerns about drug therapy during breastfeeding
For infants of breastfeeding women, exposure to antidepressants through breast milk is minimal. Amounts in breast milk depend on the timing of the antidepressant dose, timing of feeding, and genetically influenced metabolic activity in mother and infant. The current literature supports antidepressant use for breastfeeding mothers of healthy full-term infants.65
The 2 most widely studied antidepressants in breastfed infants are paroxetine and sertraline. It has been shown that very little can be detected in the infant’s serum, with relative infant doses ranging from 0.4% to 2.8%.65 While clinicians are cautioned against prescribing paroxetine for pregnant women, the drug remains a suitable alternative for breastfeeding women.
If an antidepressant is started postpartum, the recommendation is to start with a low dose and then slowly titrate upward while monitoring the infant for adverse effects.65,66 Possible adverse effects in breastfeeding infants include irritability, sedation, poor weight gain, and a change in feeding patterns.67 Adverse events are most likely to occur in newborns up to 8 weeks of age, and infants born prematurely or with medical problems may be particularly at risk.65,68
Helping patients weigh risks and benefits of drug therapy
Women may hear about the risks of medications to the fetus and during breastfeeding and so may be reluctant to seek or accept intervention. Often, the information is not from a reliable, scientifically based source. Primary care physicians are well positioned to guide peripartum women in risk-benefit analysis of proper treatment of their depression vs no treatment or undertreatment. In addition, establishing referral sources—ideally with a peripartum mental health specialist—is advisable. Online resources that clinicians can refer patients to for help in managing peripartum depression include the following:
- www.postpartum.net
- www.womensmentalhealth.org
- www.mothertobaby.org (for pharmacologic guidance).
INCREASED AWARENESS IS KEY
Primary care physicians must remain alert to the high prevalence of depression in women of childbearing age and embrace routine screening for depression. (See the sidebar, “The primary care management of peripartum depression.”) Since half of pregnancies are unintended, awareness of the risks of undetected and untreated peripartum depression to the mother, developing fetus, and infant is essential. Untreated antepartum depression has been linked to poor pregnancy outcomes, nutritional deficits, and substance abuse. Untreated postpartum depression negatively affects mother-infant attachment, infant, and child development and maternal self care.
Not treating depression is hazardous
Drug treatment during pregnancy and breastfeeding poses challenges for the patient and physician due to the inevitability of fetal and infant exposure, but lack of treatment can be hazardous.
To date, the evidence on the use of antidepressants in pregnant and lactating women is reassuring. Specialized peripartum psychiatric partial hospital programs69 and inpatient programs70 exist for women who need a higher level of care. There is also substantial evidence that psychotherapy, especially cognitive behavioral therapy and interpersonal therapy, is highly effective, and emerging data on complementary and alternative treatments are promising. Coordinated care between primary care and behavioral healthcare providers with expertise in treating peripartum depression is most likely to yield optimal outcomes.
Contrary to common belief, pregnancy does not confer protection against depression.1,2 In fact, pregnant women are just as likely as nonpregnant women to become or remain depressed, and up to 12.7% of pregnant women meet criteria for depression.1
In the postpartum period, women are particularly vulnerable to a major depressive episode, whether a first episode or a recurrence. The estimated prevalence of a depressive episode in the first 3 postpartum months is 19.2%,2 making postpartum depression the most common complication of childbearing.2 At the same time, peripartum depression remains largely underrecognized and undertreated.3
As evidence mounts regarding the deleterious impact of untreated mental illness on the mother, the developing fetus, and the infant, early detection and intervention for peripartum depression are paramount.3
DEPRESSION DURING PREGNANCY: SIGNIFICANT CONSEQUENCES
Although the rates of depression in pregnant and nonpregnant women are similar, depression in pregnancy carries additional significant consequences. Further, many depressed pregnant women believe their depression will lift once their baby is born, though it is well documented that depression during pregnancy is the strongest predictor of postpartum depression and that if left untreated it can be devastating for mother, infant, and family.4
Compared with nondepressed pregnant women, depressed pregnant women have poorer overall health status,5 are more likely to engage in behaviors that pose risk to the developing fetus such as smoking,5 alcohol consumption, and substance use,6 and have poor nutrition and inadequate weight gain.7,8
Pregnant women who are depressed and are also experiencing domestic violence are especially at risk for poor prenatal care as they tend to miss more prenatal appointments.9 Evidence also suggests that depressed pregnant women are less attached to the fetus and more likely to have elective terminations.10,11
Depression in pregnancy is associated with higher rates of adverse pregnancy outcomes such as preterm birth, low birth weight, operative delivery, and longer predelivery hospital stay.3,12 Depression and anxiety during pregnancy have been associated with prenatal hypertension,13 gestational diabetes,14 preeclampsia,15 and HELLP syndrome (ie, hemolysis, elevated liver enzymes, and low platelet count).15 Depression and anxiety during pregnancy are associated with subsequent poorer infant attachment16,17 and an overall unfavorable impact on infant and child development.18
Risk factors for depression during pregnancy include past episodes of depression, current anxiety, poor social support, unintended pregnancy, life stress, being single, domestic violence, and being on Medicaid.19
Undoubtedly the most devastating consequence of severe depression during pregnancy is suicide. Rates of suicide are lower in peripartum women,20 but when suicide does occur, pregnant women tend to use more violent means than nonpregnant women. Pregnant adolescents represent a particularly high-risk group.21
POSTPARTUM DEPRESSION
Postpartum depression is the most common complication of childbearing. Although the precise pathogenesis is undetermined, there is converging evidence of a subset of women particularly sensitive to dramatic fluctuations in levels of estradiol and progesterone that occur during childbirth.22,23 There is also evidence that dysregulation of the hypothalamic-pituitary-adrenal axis contributes to the development of postpartum depression in certain women.24 Further, women who have depression or anxiety during pregnancy are much more likely to experience postpartum depression than those who are not symptomatic during pregnancy.4 A history of peripartum depression or other lifetime depressive episodes, poverty, conflict with a primary partner, poor social support, stressful life events, and low self-esteem are strongly associated with postpartum depression.25
When unrecognized and untreated, postpartum depression can have profound and persistent effects on the mother and the developing infant.18,26 Mothers with postpartum depression are much more likely than mothers without depression to have impaired bonding,27 to be less responsive to their infant’s needs,17 and to be more likely to miss well-baby checkups.28
Postpartum depression’s effects on maternal-infant interactions can include maternal withdrawal, disengagement, intrusion, and hostility and can lead to long-term effects on child development, including poor cognitive functioning, emotional maladjustment, and behavioral inhibition.29,30 Infants and children of mothers with untreated postpartum depression have been shown to exhibit a higher incidence of colic, excessive crying, sleep problems, and irritability.31,32 Women with postpartum depression may be less likely to initiate or maintain breastfeeding, and depressive symptoms have been noted to precede the discontinuation of breastfeeding.33–35
Risk factors for postpartum depression
Characteristics to look for in the prenatal care of pregnant women include the following:
- Depression during pregnancy
- History of postpartum or other depressive episode
- Poverty
- Conflict with primary partner
- Poor social support
- Low self-esteem
- Single status.
DIFFERENTIATING ‘POSTPARTUM BLUES’ FROM MAJOR DEPRESSION
Primary care providers are often the first point of contact for depressed women. The diagnosis of major depression in pregnant and postpartum women is challenging because of changes in sleep, appetite, and energy brought on by pregnancy, complications of delivery, and demands of caring for a newborn.36 Many pregnant and postpartum women are reluctant to disclose their symptoms due to a sense of shame and guilt for being depressed during a time in their life that society commonly regards as joyful, and this contributes to under-detection.
In the first few days postpartum, fatigue, emotionality, irritability, and worry over the infant’s well-being affect up to 75% of women. This period, typically referred to as the “baby blues” or “postpartum blues,” is not considered a disorder and responds well to support, reassurance, and adequate sleep, and it typically resolves within 2 weeks.37,38 Table 1 lists features that help distinguish postpartum blues from major depression.
Signs of major depressive disorder
Major depressive disorder is a serious and disabling condition. To meet criteria for major depressive disorder, women must report depressed mood and loss of interest or pleasure in normally pleasurable activities for at least 2 weeks. Completing the symptom profile, at least 5 of the following must be present: sleep disturbance (insomnia or hypersomnia), lack of energy, feelings of worthlessness or low self-esteem, guilt, difficulty concentrating, indecisiveness, psychomotor retardation or agitation, and thoughts of suicide or death.
The Diagnostic and Statistical Manual of Mental Disorders (5th edition) recognizes that postpartum depression commonly begins during pregnancy, and now uses “peripartum onset” as the specifier for major depressive disorder that occurs during pregnancy, postpartum, or both.39 Other hallmark symptoms with peripartum onset include a lack of interest in or attachment to the pregnancy or infant, and anxiety and worry often accompanied by intrusive, unwanted thoughts of harm befalling the infant.40
Postpartum psychosis
Postpartum psychosis is a far less common presentation, occurring in 1 to 2 per 1,000 births, but it constitutes a psychiatric emergency requiring immediate referral to a psychiatric care setting. Women at highest risk are those with a personal or family history of bipolar disorder.
The clinical presentation is most commonly characterized by confusion, agitation, hallucinations, delusional beliefs, and disorientation. Suicide and infanticide, while rare, are more likely to occur in the context of a psychotic episode.41
SCREENING RECOMMENDATIONS
Screening for depression is routine in primary care settings and is no less important for peripartum women.
In 2016, the US Preventive Services Task Force issued a recommendation that all pregnant and postpartum women be screened for depression,42 highlighting the need for all medical providers to be alert to the potentially serious consequences of unrecognized and untreated maternal psychiatric illness.
The American College of Obstetricians and Gynecologists (ACOG) recommends screening for depression and anxiety at least once during the peripartum period,43 and the American Academy of Pediatrics recommends screening mothers for depression at the 1-, 2-, and 4-month well-baby visits.44
The peripartum period is associated with changes in sleep, appetite, and energy levels, but these are also typical of depression. Taking this into account, the Edinburgh Postnatal Depression Scale (EPDS) was developed to screen for depression specifically in this population.45 The EPDS is a validated and widely used 10-item self-reporting questionnaire with a high degree of sensitivity and specificity; it is easily administered and quickly scored. A cutoff score of 13 (of a maximum of 30) is considered indicative of depressed mood and signals the need for further assessment.
ACOG, the American Academy of Pediatrics, and the US Preventive Services Task Force recommend a standardized validated tool and cite both the EPDS (https://psychology-tools.com/epds/) and the Patient Health Questionnaire-9 (PHQ-9) (Figure 1) as appropriate to screen for peripartum depression.42–44 Primary care providers tend to be most familiar with the PHQ-9, a highly sensitive and specific 9-item depression screen that has been validated in primary care and obstetric clinic patients.46 A score on the PHQ-9 ranging from 5 to 10 indicates mild depression, 10 to 14 moderate depression, 15 to 19 moderate to severe depression, and greater than 19 severe depression.
CLINICAL MANAGEMENT
Many women prefer nondrug therapy
The gold standard treatment for moderate to severe major depressive disorder is psychotherapy plus pharmacotherapy. Yet many peripartum women voice concerns about exposure to pharmacologic treatment, and studies have shown that many women prefer nonpharmacologic intervention.47
Evidence-based psychotherapies that have demonstrated efficacy in peripartum women include cognitive behavioral therapy48 and interpersonal psychotherapy when administered by a psychotherapist trained in these treatments. Pregnant and breastfeeding women often express preference for psychotherapy and complementary and alternative treatments as a means of avoiding fetal and infant exposure to antidepressants.47
For mild to moderate depression, complementary therapies such as exercise, yoga, bright light therapy, and acupuncture have shown efficacy and can be used alone or adjunctively.49 Because a poor marital relationship is consistently associated with peripartum depression,25 primary care physicians who routinely address social support and screen for family conflict are well positioned to detect this significant correlate and to recommend marital or family therapy as a primary or adjunctive treatment.
When to consider drug therapy
The decision to recommend drug therapy must be individualized and based on the severity of symptoms, functional impairment, number and frequency of depressive episodes, history of response to medications, and the preferences of the patient, with the recognition that no decision is risk-free and that antidepressants enter the amniotic fluid, so fetal exposure is unavoidable.
Table 2 lists common antidepressants. The antidepressants most commonly prescribed, especially in the primary care setting, are selective serotonin reuptake inhibitors (SSRIs), which are favored because of their effectiveness, low side-effect profile, and lack of overdose toxicity.
Serotonin syndrome is no more likely to occur in pregnant than in nonpregnant women. Close monitoring for this condition is warranted only when patients are taking very high doses of SSRIs or SSRIs in combination with other serotonergic agonists.
Prescribing antidepressants for pregnant or breastfeeding women requires thoughtful consideration of the patient’s preferences, as well as weighing the risks and benefits of fetal and infant exposure to maternal depression vs exposure to medications. Additional considerations include monotherapy, avoiding medication changes, choosing drugs that have been effective in the past, and avoiding drugs with known drug-drug interactions or teratogenic effects.50
There is increasing consensus that the short- and long-term consequences of undertreatment or nontreatment of maternal depression outweigh the risk of fetal exposure to SSRIs.3,51,52 Cohen et al53 have recommended that if a woman is on an antidepressant and learns she is pregnant, she should not discontinue it because of the likelihood of relapse; they found a 68% relapse rate in women who discontinued their antidepressant in the first trimester of pregnancy.53
In a comprehensive review of studies published between 1996 and 2012 that examined antidepressant use during pregnancy, Byatt et al54 found little or no evidence of increased teratogenic risk with antidepressants with the exception of paroxetine, which is associated with a small but significant increased risk of cardiac malformation during first-trimester exposure.54
These conclusions were underscored in a large cohort study in the United Kingdom.55 In addition, a joint task force of the American Psychiatric Association and ACOG reviewed studies looking at the association between depression, antidepressants, and birth outcomes including miscarriage, preterm birth, cardiac abnormalities (resulting from first trimester exposure), persistent pulmonary hypertension (related to second- and third-trimester exposure), and neonatal adaptation syndrome (associated with third-trimester exposure).8 They concluded that the available data neither support nor refute a link between the use of antidepressants and several of the above outcomes. No increase in risk of congenital malformations (including cardiac abnormalities) was found. An increased risk of persistent pulmonary hypertension was noted, although the absolute risk of this disorder remained low, at 3 to 6 per 1,000 infants exposed to SSRIs in utero.8,56
Neonatal adaptation syndrome
Neonatal adaptation syndrome is characterized by jitteriness, irritability, decreased muscle tone, and feeding difficulty in the neonate. It can occur in 15% to 30% of infants exposed to SSRIs antenatally.57,58 These symptoms, however, are transient and typically resolve within 7 to 10 days after birth. A more recent study suggested that neurobehavioral symptoms for some infants extend beyond 2 weeks and that concomitant exposure to benzodiazepines results in even higher rates of this syndrome.59 There is no evidence that tapering or discontinuing antidepressants near term is necessary, safe, or effective in preventing transient neonatal complications. However, this approach would increase the risk of relapse for the mother.
Autism spectrum disorders
The possible association between antidepressants and autism spectrum disorders in pregnancy has captured much attention in recent years. One study based on healthcare claims60 and one registry-based study61 associated in utero exposure to antidepressants with autism liability in children. However, a large-scale Danish registry-based study did not replicate this association.62 In addition, 2 recent cohort studies, identifying children with autism spectrum disorder or attention-deficit hyperactivity disorder from electronic health records, found that neither disorder was significantly associated with prenatal antidepressant exposure in crude or adjusted models. However, both studies found a significant association with the use of antidepressants before pregnancy, indicating that the risk of autism observed with prenatal antidepressant exposure is likely confounded by the severity of maternal illness.63,64
Concerns about drug therapy during breastfeeding
For infants of breastfeeding women, exposure to antidepressants through breast milk is minimal. Amounts in breast milk depend on the timing of the antidepressant dose, timing of feeding, and genetically influenced metabolic activity in mother and infant. The current literature supports antidepressant use for breastfeeding mothers of healthy full-term infants.65
The 2 most widely studied antidepressants in breastfed infants are paroxetine and sertraline. It has been shown that very little can be detected in the infant’s serum, with relative infant doses ranging from 0.4% to 2.8%.65 While clinicians are cautioned against prescribing paroxetine for pregnant women, the drug remains a suitable alternative for breastfeeding women.
If an antidepressant is started postpartum, the recommendation is to start with a low dose and then slowly titrate upward while monitoring the infant for adverse effects.65,66 Possible adverse effects in breastfeeding infants include irritability, sedation, poor weight gain, and a change in feeding patterns.67 Adverse events are most likely to occur in newborns up to 8 weeks of age, and infants born prematurely or with medical problems may be particularly at risk.65,68
Helping patients weigh risks and benefits of drug therapy
Women may hear about the risks of medications to the fetus and during breastfeeding and so may be reluctant to seek or accept intervention. Often, the information is not from a reliable, scientifically based source. Primary care physicians are well positioned to guide peripartum women in risk-benefit analysis of proper treatment of their depression vs no treatment or undertreatment. In addition, establishing referral sources—ideally with a peripartum mental health specialist—is advisable. Online resources that clinicians can refer patients to for help in managing peripartum depression include the following:
- www.postpartum.net
- www.womensmentalhealth.org
- www.mothertobaby.org (for pharmacologic guidance).
INCREASED AWARENESS IS KEY
Primary care physicians must remain alert to the high prevalence of depression in women of childbearing age and embrace routine screening for depression. (See the sidebar, “The primary care management of peripartum depression.”) Since half of pregnancies are unintended, awareness of the risks of undetected and untreated peripartum depression to the mother, developing fetus, and infant is essential. Untreated antepartum depression has been linked to poor pregnancy outcomes, nutritional deficits, and substance abuse. Untreated postpartum depression negatively affects mother-infant attachment, infant, and child development and maternal self care.
Not treating depression is hazardous
Drug treatment during pregnancy and breastfeeding poses challenges for the patient and physician due to the inevitability of fetal and infant exposure, but lack of treatment can be hazardous.
To date, the evidence on the use of antidepressants in pregnant and lactating women is reassuring. Specialized peripartum psychiatric partial hospital programs69 and inpatient programs70 exist for women who need a higher level of care. There is also substantial evidence that psychotherapy, especially cognitive behavioral therapy and interpersonal therapy, is highly effective, and emerging data on complementary and alternative treatments are promising. Coordinated care between primary care and behavioral healthcare providers with expertise in treating peripartum depression is most likely to yield optimal outcomes.
- World Health Organization (WHO). A message from the Director General. www.who.int/whr/2001/dg_message/en/index.html. Accessed March 6, 2017.
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obset Gynecol 2005; 106:1071–1083.
- Davalos DB, Yadon CA, Tregellas HC. Untreated prenatal maternal depression and the potential risks to offspring: a review. Arch Women’s Mental Health 2012; 15:1–14.
- Chaudron LH, Klein MH, Remington P, Palta M, Allen C, Essex MJ. Predictors, prodromes and incidence of postpartum depression. J Psychosom Obstet Gynaecol 2001; 22:103–112.
- Orr ST, Blazer DG, Orr CA. Maternal prenatal depressive symptoms, nicotine addiction, and smoking-related knowledge, attitudes, beliefs, and behaviors. Matern Child Health J 2012; 16:973–978.
- Flynn HA, Chermack ST. Prenatal alcohol use: the role of lifetime problems with alcohol,drugs, depression, and violence. J Stud Alcohol Drugs 2008; 69:500–509.
- Bodnar LM, Wisner KL, Moses-Kolko E, Sit DK, Hanusa BH. Prepregnancy body mass index, gestational weight gain, and the likelihood of major depressive disorder during pregnancy. J Clin Psychiatry 2009; 70:1290–1296.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol 2009; 114:703–713.
- Han A, Stewart DE. Maternal and fetal outcomes of intimate partner violence associated with pregnancy in the Latin American and Caribbean region. Int J Gynecol Obstet 2014; 124:6–11.
- McFarland J, Salisbury AL, Battler CL, Hawes K, Halloran K, Lester BM. Major depressive disorder during pregnancy and emotional attachment to the fetus. Arch Womens Ment Health 2011; 14:425–434.
- Suri R, Althuler LA, Mintz J. Depression and the decision to abort. Am J Psychiatry 2004; 161:1502.
- Kim DR, Sockol LE, Sammel MD, Kelly C, Moseley M, Epperson CN. Elevated risk of adverse obstetric outcomes in pregnant women with depression. Arch Women’s Ment Health 2013; 16:475–482.
- Mautner E, Greimel E, Trutnovsky G, Daghofer F, Egger JW, Lang U. Quality of life outcomes in pregnancy and postpartum complicated by hypertensive disorders, gestational diabetes, and preterm birth. J Psychosom Obstet Gynaecol 2009; 30:231–237.
- Katon JG, Russo J, Gavin AR, Melville JL, Katon WJ. Diabetes and depression in pregnancy: is there an association? J Women’s Health (Larchmt) 2011; 20:983–989.
- Delahaije DH, Dirksen CD, Peeters LL, Smits LJ. Anxiety and depression following preeclampsia or HELLP syndrome: a systematic review. Acta Obstet Gynecol Scand 2013; 92:746–761.
- O’Higgins M, Roberts IS, Glover V, Taylor A. Mother-child bonding at 1 year; associations with symptoms of postnatal depression and bonding in the first few weeks. Arch Women’s Ment Health 2013; 16:381–389.
- Field T, Healy BT, Goldstein S, Guthertz M. Behavior-state matching and synchrony in mother-infant interactions of nondepressed versus depressed dyads. Dev Psychol 1990; 26:7–14.
- Kingston D, Tough S, Whitfield H. Prenatal and postpartum maternal psychological distress and infant development: a systematic review. Child Psychiatry Hum Dev 2012; 43:683–714.
- Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol 2010; 202:5–14.
- Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Women’s Ment Health 2005; 8:77–87.
- Appleby L. Suicide after pregnancy and the first postnatal year. BMJ 1991; 302:137–140.
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000; 157:924–930.
- Workman JL, Barha CK, Galea LAM. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci 2012; 126:54–72.
- Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci 2011; 13:89–100.
- O’Hara MW, McCabe JE. Postpartum depression: current status and future directions. Annu Rev Clin Psychol 2013; 9:379–407.
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev 2011; 14:1–27
- Muzik M, Bocknek EL, Broderick A, et al. Mother-infant bonding impairment across the first 6 months postpartum: the primacy of psychopathology in women with childhood abuse and neglect histories. Arch Women’s Ment Health 2013; 16:29–38.
- Farr SL, Dietz PM, Rizzo JH, et al. Health care utilisation in the first year of life among infants of mothers with perinatal depression or anxiety. Paediatr Perinat Epidemiol 2013; 27:81–88.
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Women’s Ment Health 2003; 6:263–274.
- Murray L, Cooper PJ. Postpartum depression and child development. Psychol Med 1997; 27:253–260.
- Orhon FS, Ulukol B, Soykan A. Postpartum mood disorders and maternal perceptions of infant patterns in well-child follow-up visits. Acta Paediatr 2007; 96:1777–1783.
- Dennis CL, Ross L. Relationships among infant sleep patterns, maternal fatigue, and development of depressive symptomatology. Birth 2005; 32:187–193.
- Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 2007; 153:1–186.
- Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Paediatr 2007; 96:590–594.
- Hatton DC, Harrison-Hohner J, Coste S, Dorato V, Curet LB, McCarron DA. Symptoms of postpartum depression and breastfeeding. J Hum Lact 2005; 21:444–449.
- Klein MH, Essex MJ. Pregnant or depressed? The effect of overlap between symptoms of depression and somatic complaints of pregnancy on rates of major depression in the second trimester. Depression 1994; 2:308–314.
- Seyfried LS, Marcus SM. Postpartum mood disorders. Int Rev Psychiatry 2003; 15:231–242.
- Buttner MM, O’Hara MW, Watson D. The structure of women’s mood in the early postpartum. Assessment 2012; 19:247–256.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA; American Psychiatric Association Publishing: 2013.
- Wisner KL, Peindl KS, Gigliotti T, Hanusa BH. Obsessions and compulsions in women with postpartum depression. J Clin Psychiatry 1999: 60:176-180.
- Di Florio A, Smith S, Jones I. Postpartum psychosis. The Obstetrician & Gynecologist 2013; 15:145–150.
- O’Connor E, Rossom RC, Henniger M, Groom HC, Burda BU. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315:388–406.
- Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol 2015; 125:1268–1271.
- Earls MF; Committee on Psychosocial Aspects of Child and Family Health American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics 2010; 126:1032–1039.
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry 1987; 150:782–786.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–616.
- Battle CL, Salisbury AL, Schofield CA, Ortiz-Hernandez S. Perinatal antidepressant use: understanding women’s preferences and concerns. J Psychiatr Pract 2013; 19:443–453.
- Stuart S, Koleva H. Psychological treatments for perinatal depression. Best Pract Res Clin Obstet Gynaecol 2014; 28:61–70.
- Deligiannidis KM, Freeman MP. Complementary and alternative medicine therapies for perinatal depression. Best Pract Res Clin Obstet Gynaecol 2014; 28:85–95.
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin: clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces Practice Bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008; 111:1001–1020.
- Ornoy A, Koren G. Selective serotonin reuptake inhibitors in human pregnancy: on the way to resolving the controversy. Semin Fetal Neonatal Med 2014; 19:188–194.
- Salisbury AL, Wisner KL, Pearlstein T, Battle CL, Stroud L, Lester BM. Newborn neurobehavioral patterns are differentially related to prenatal maternal major depressive disorder and serotonin reuptake inhibitor treatment. Depress Anxiety 2011; 28:1008–1019.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006; 295:499–507.
- Byatt N, Deligiannidis KM, Freeman MP. Antidepressant use in pregnancy: a critical review focused on risks and controversies. Acta Psychiatr Scand 2013; 127:94–114.
- Ban L, Gibson JE, West J, et al. Maternal depression, antidepressant prescriptions, and congenital anomaly risk in offspring: a population-based cohort study. BJOG 2014; 121:1471–1481.
- Kallen B, Olausson P. Maternal use of selective serotonin re-uptake inhibitors and persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf 2008; 17:801–806.
- Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med 1996; 335:1010–1015.
- Costei AM, Kozer E, Ho T, Ito S, Koren G. Perinatal outcome following third trimester exposure to paroxetine. Arch Pediatr Adolesc Med 2002; 156:1129–1132.
- Salisbury AL, O’Grady KE, Battle CL, et al. The roles of maternal depression, serotonin reuptake inhibitor treatment, and concomitant benzodiazepine use on infant neurobehavioral functioning over the first postnatal month. Am J Psychiatry 2016; 173:147–157.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68:1104–1112.
- Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ 2013; 346:f2059.
- Sorensen MJ, Gronborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol 2013; 5:449–459.
- Clements CC, Castro VM, Blumenthal SR, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20:727–734.
- Castro VM, Kong SW, Clements CC, et al. Absence of evidence for increase in risk for autism or attention-deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study. Transl Psychiatry 2016; 6:e708.
- Hale TW, Rowe HE. Medications and Mothers’ Milk. 16th ed. Amarillo, TX: Hale Publishing, L.P; 2014.
- Abreu AC, Stuart S. Pharmacologic and hormonal treatments for postpartum depression. Psychiatr Ann 2005; 35:568–576.
- Sit DK, Wisner KL. Decision making for postpartum depression treatment. Psychiatr Ann 2005; 35:577–584.
- Wisner KL, Parry BL, Piontek CM. Clinical practice. Postpartum depression. N Engl J Med 2002; 347:194–199.
- Howard M, Battle CL, Pearlstein T, Rosene-Montella K. A psychiatric mother-baby day hospital for pregnant and postpartum women. Arch Women’s Ment Health 2006; 9:213–218.
- Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Women’s Ment Health 2014; 17:107–113.
- World Health Organization (WHO). A message from the Director General. www.who.int/whr/2001/dg_message/en/index.html. Accessed March 6, 2017.
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obset Gynecol 2005; 106:1071–1083.
- Davalos DB, Yadon CA, Tregellas HC. Untreated prenatal maternal depression and the potential risks to offspring: a review. Arch Women’s Mental Health 2012; 15:1–14.
- Chaudron LH, Klein MH, Remington P, Palta M, Allen C, Essex MJ. Predictors, prodromes and incidence of postpartum depression. J Psychosom Obstet Gynaecol 2001; 22:103–112.
- Orr ST, Blazer DG, Orr CA. Maternal prenatal depressive symptoms, nicotine addiction, and smoking-related knowledge, attitudes, beliefs, and behaviors. Matern Child Health J 2012; 16:973–978.
- Flynn HA, Chermack ST. Prenatal alcohol use: the role of lifetime problems with alcohol,drugs, depression, and violence. J Stud Alcohol Drugs 2008; 69:500–509.
- Bodnar LM, Wisner KL, Moses-Kolko E, Sit DK, Hanusa BH. Prepregnancy body mass index, gestational weight gain, and the likelihood of major depressive disorder during pregnancy. J Clin Psychiatry 2009; 70:1290–1296.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol 2009; 114:703–713.
- Han A, Stewart DE. Maternal and fetal outcomes of intimate partner violence associated with pregnancy in the Latin American and Caribbean region. Int J Gynecol Obstet 2014; 124:6–11.
- McFarland J, Salisbury AL, Battler CL, Hawes K, Halloran K, Lester BM. Major depressive disorder during pregnancy and emotional attachment to the fetus. Arch Womens Ment Health 2011; 14:425–434.
- Suri R, Althuler LA, Mintz J. Depression and the decision to abort. Am J Psychiatry 2004; 161:1502.
- Kim DR, Sockol LE, Sammel MD, Kelly C, Moseley M, Epperson CN. Elevated risk of adverse obstetric outcomes in pregnant women with depression. Arch Women’s Ment Health 2013; 16:475–482.
- Mautner E, Greimel E, Trutnovsky G, Daghofer F, Egger JW, Lang U. Quality of life outcomes in pregnancy and postpartum complicated by hypertensive disorders, gestational diabetes, and preterm birth. J Psychosom Obstet Gynaecol 2009; 30:231–237.
- Katon JG, Russo J, Gavin AR, Melville JL, Katon WJ. Diabetes and depression in pregnancy: is there an association? J Women’s Health (Larchmt) 2011; 20:983–989.
- Delahaije DH, Dirksen CD, Peeters LL, Smits LJ. Anxiety and depression following preeclampsia or HELLP syndrome: a systematic review. Acta Obstet Gynecol Scand 2013; 92:746–761.
- O’Higgins M, Roberts IS, Glover V, Taylor A. Mother-child bonding at 1 year; associations with symptoms of postnatal depression and bonding in the first few weeks. Arch Women’s Ment Health 2013; 16:381–389.
- Field T, Healy BT, Goldstein S, Guthertz M. Behavior-state matching and synchrony in mother-infant interactions of nondepressed versus depressed dyads. Dev Psychol 1990; 26:7–14.
- Kingston D, Tough S, Whitfield H. Prenatal and postpartum maternal psychological distress and infant development: a systematic review. Child Psychiatry Hum Dev 2012; 43:683–714.
- Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstet Gynecol 2010; 202:5–14.
- Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Women’s Ment Health 2005; 8:77–87.
- Appleby L. Suicide after pregnancy and the first postnatal year. BMJ 1991; 302:137–140.
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000; 157:924–930.
- Workman JL, Barha CK, Galea LAM. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci 2012; 126:54–72.
- Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci 2011; 13:89–100.
- O’Hara MW, McCabe JE. Postpartum depression: current status and future directions. Annu Rev Clin Psychol 2013; 9:379–407.
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev 2011; 14:1–27
- Muzik M, Bocknek EL, Broderick A, et al. Mother-infant bonding impairment across the first 6 months postpartum: the primacy of psychopathology in women with childhood abuse and neglect histories. Arch Women’s Ment Health 2013; 16:29–38.
- Farr SL, Dietz PM, Rizzo JH, et al. Health care utilisation in the first year of life among infants of mothers with perinatal depression or anxiety. Paediatr Perinat Epidemiol 2013; 27:81–88.
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Women’s Ment Health 2003; 6:263–274.
- Murray L, Cooper PJ. Postpartum depression and child development. Psychol Med 1997; 27:253–260.
- Orhon FS, Ulukol B, Soykan A. Postpartum mood disorders and maternal perceptions of infant patterns in well-child follow-up visits. Acta Paediatr 2007; 96:1777–1783.
- Dennis CL, Ross L. Relationships among infant sleep patterns, maternal fatigue, and development of depressive symptomatology. Birth 2005; 32:187–193.
- Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 2007; 153:1–186.
- Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Paediatr 2007; 96:590–594.
- Hatton DC, Harrison-Hohner J, Coste S, Dorato V, Curet LB, McCarron DA. Symptoms of postpartum depression and breastfeeding. J Hum Lact 2005; 21:444–449.
- Klein MH, Essex MJ. Pregnant or depressed? The effect of overlap between symptoms of depression and somatic complaints of pregnancy on rates of major depression in the second trimester. Depression 1994; 2:308–314.
- Seyfried LS, Marcus SM. Postpartum mood disorders. Int Rev Psychiatry 2003; 15:231–242.
- Buttner MM, O’Hara MW, Watson D. The structure of women’s mood in the early postpartum. Assessment 2012; 19:247–256.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA; American Psychiatric Association Publishing: 2013.
- Wisner KL, Peindl KS, Gigliotti T, Hanusa BH. Obsessions and compulsions in women with postpartum depression. J Clin Psychiatry 1999: 60:176-180.
- Di Florio A, Smith S, Jones I. Postpartum psychosis. The Obstetrician & Gynecologist 2013; 15:145–150.
- O’Connor E, Rossom RC, Henniger M, Groom HC, Burda BU. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315:388–406.
- Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol 2015; 125:1268–1271.
- Earls MF; Committee on Psychosocial Aspects of Child and Family Health American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics 2010; 126:1032–1039.
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry 1987; 150:782–786.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–616.
- Battle CL, Salisbury AL, Schofield CA, Ortiz-Hernandez S. Perinatal antidepressant use: understanding women’s preferences and concerns. J Psychiatr Pract 2013; 19:443–453.
- Stuart S, Koleva H. Psychological treatments for perinatal depression. Best Pract Res Clin Obstet Gynaecol 2014; 28:61–70.
- Deligiannidis KM, Freeman MP. Complementary and alternative medicine therapies for perinatal depression. Best Pract Res Clin Obstet Gynaecol 2014; 28:85–95.
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin: clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces Practice Bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008; 111:1001–1020.
- Ornoy A, Koren G. Selective serotonin reuptake inhibitors in human pregnancy: on the way to resolving the controversy. Semin Fetal Neonatal Med 2014; 19:188–194.
- Salisbury AL, Wisner KL, Pearlstein T, Battle CL, Stroud L, Lester BM. Newborn neurobehavioral patterns are differentially related to prenatal maternal major depressive disorder and serotonin reuptake inhibitor treatment. Depress Anxiety 2011; 28:1008–1019.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006; 295:499–507.
- Byatt N, Deligiannidis KM, Freeman MP. Antidepressant use in pregnancy: a critical review focused on risks and controversies. Acta Psychiatr Scand 2013; 127:94–114.
- Ban L, Gibson JE, West J, et al. Maternal depression, antidepressant prescriptions, and congenital anomaly risk in offspring: a population-based cohort study. BJOG 2014; 121:1471–1481.
- Kallen B, Olausson P. Maternal use of selective serotonin re-uptake inhibitors and persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf 2008; 17:801–806.
- Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med 1996; 335:1010–1015.
- Costei AM, Kozer E, Ho T, Ito S, Koren G. Perinatal outcome following third trimester exposure to paroxetine. Arch Pediatr Adolesc Med 2002; 156:1129–1132.
- Salisbury AL, O’Grady KE, Battle CL, et al. The roles of maternal depression, serotonin reuptake inhibitor treatment, and concomitant benzodiazepine use on infant neurobehavioral functioning over the first postnatal month. Am J Psychiatry 2016; 173:147–157.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68:1104–1112.
- Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ 2013; 346:f2059.
- Sorensen MJ, Gronborg TK, Christensen J, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol 2013; 5:449–459.
- Clements CC, Castro VM, Blumenthal SR, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20:727–734.
- Castro VM, Kong SW, Clements CC, et al. Absence of evidence for increase in risk for autism or attention-deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study. Transl Psychiatry 2016; 6:e708.
- Hale TW, Rowe HE. Medications and Mothers’ Milk. 16th ed. Amarillo, TX: Hale Publishing, L.P; 2014.
- Abreu AC, Stuart S. Pharmacologic and hormonal treatments for postpartum depression. Psychiatr Ann 2005; 35:568–576.
- Sit DK, Wisner KL. Decision making for postpartum depression treatment. Psychiatr Ann 2005; 35:577–584.
- Wisner KL, Parry BL, Piontek CM. Clinical practice. Postpartum depression. N Engl J Med 2002; 347:194–199.
- Howard M, Battle CL, Pearlstein T, Rosene-Montella K. A psychiatric mother-baby day hospital for pregnant and postpartum women. Arch Women’s Ment Health 2006; 9:213–218.
- Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Women’s Ment Health 2014; 17:107–113.
KEY POINTS
- Depression occurs in up to 13% of pregnant women, a prevalence similar to that in nonpregnant women, but the incidence rises postpartum.
- Depressed pregnant women are more likely to engage in behaviors that pose a risk to the fetus.
- Depression in pregnancy is associated with adverse pregnancy outcomes such as preterm birth, low birth weight, gestational diabetes, and hypertensive disorders of pregnancy.
- Risk factors for depression in pregnancy include past episodes of depression, poor social support, unwanted pregnancy, and domestic violence.
Anticoagulants and pregnancy: When are they safe?
Anticoagulation is essential in a wide variety of conditions in women of child-bearing age. Some, such as venous thromboembolism, occur more often during pregnancy. Others, such as recurrent fetal loss in the setting of antiphospholipid antibodies, are specific to pregnancy.
While anticoagulants are useful in many circumstances, their use during pregnancy increases the risk of hemorrhage and other adverse effects on the mother and the fetus. Treatment with anticoagulants during pregnancy must therefore be carefully considered, with judicious selection of the agent, and with reflection on the physiologic changes of pregnancy to ensure appropriate dosing. In this article, we review these issues.
WHY IS THROMBOTIC RISK HIGHER DURING PREGNANCY?
Venous thromboembolism is among the leading causes of maternal death in developed countries.1–3 Modern care has dramatically reduced the risk of maternal death from hemorrhage, infection, and hypertension, but rates of morbidity and death from thrombosis have remained stable or increased in recent years.4
- Much higher levels of fibrinogen and factors VII, VIII, IX, and X
- Lower levels of protein S and increased resistance to activated protein C
- Impaired fibrinolysis, due to inhibitors derived from the placenta.
Acquired antithrombin deficiency may also occur in high-proteinuric states such as nephrotic syndrome or preeclampsia, further increasing thrombotic risk. Pooling of venous blood, caused by progesterone-mediated venous dilation and compounded by compression of the inferior vena cava by the uterus in later pregnancy, also increases thrombotic risk. And endothelial disruption of the pelvic vessels may occur during delivery, particularly during cesarean section.
Additional factors that increase thrombotic risk include immobilization, such as bed rest for pregnancy complications; surgery, including cesarean section; ovarian hyperstimulation during gonadotropin use for in vitro fertilization; trauma; malignancy; and hereditary or acquired hypercoagulable states.6 These hypercoagulable states include deficiencies of antithrombin or the intrinsic anticoagulant proteins C or S; resistance to activated protein C, usually due to the factor V Leiden mutation; the PT20210A mutation of the prothrombin gene; hyperhomocystinemia due to mutation of the methyltetrahydrofolate reductase (MTHFR) gene; and the sustained presence of antiphospholipid antibodies, including lupus anticoagulant antibodies, sometimes also with moderately high titers of anticardiolipin or beta-2-glycoprotein I antibodies.
Other conditions that increase thrombotic risk include hyperemesis gravidarum, obesity, inflammatory bowel disease, infection, smoking, and indwelling intravenous catheters.6 Given the multitude of risk factors, pregnant women have a risk of thrombotic complications three to five times higher than nonpregnant women.7
HEPARIN USE DURING PREGNANCY
Low-molecular-weight heparins (LMWHs)8 and unfractionated heparin bind to anti-thrombin and thus change the shape of the antithrombin molecule, dramatically increasing its interaction with the clotting factors Xa and prothrombin (factor II). The enhanced clearance of these procoagulant proteins leads to the anticoagulant effect. Unfractionated heparin has roughly equivalent interaction with factors Xa and II and prolongs the activated partial thromboplastin time (aPTT), which is therefore used to monitor the intensity of anticoagulation.
LMWHs, on the other hand, interact relatively little with factor II and do not predictably prolong the aPTT. Monitoring their effect is therefore more difficult and requires direct measurement of anti-factor-Xa activity. This test is widely available, but it is time-consuming (it takes several hours and results may not be available within 24 hours if the test is requested “after hours”), and therefore it is of limited use in the acute clinical setting. While weight-based dosing of LMWHs is reliable and safe in nonpregnant patients, it has not yet been validated for pregnant women.
Unfractionated heparin has been used for decades for many indications during pregnancy. It is a large molecule, so it does not cross the placenta and thus, in contrast to the coumarin derivatives, does not cause teratogenesis or toxic fetal effects. Its main limitations in pregnancy are its inconvenient dosing (at least twice daily when given subcutaneously) and its potential maternal adverse effects (mainly osteoporosis and heparin-induced thrombocytopenia).
Over the last 10 years LMWHs have become the preferred anticoagulants for treating and preventing thromboembolism in all patients. They are equivalent or superior to unfractionated heparin in efficacy and safety in the initial treatment of acute deep venous thrombosis9,10 and pulmonary embolism11,12 outside of pregnancy. While comparative data are much less robust in pregnant patients, several series have confirmed the safety and efficacy of LMWHs in pregnancy.13–15 LMWHs do not cross the placenta15–17 and thus have a fetal safety profile equivalent to that of unfractionated heparin.
Pregnancy alters metabolism of LMWHs
The physiologic changes of pregnancy alter the metabolism of LMWH, resulting in lower peak levels and a higher rate of clearance,18,19 and so a pregnant woman may need higher doses or more frequent dosing.
Recent evidence suggests that thromboprophylaxis can be done with lower, fixed, once-daily doses of LMWH throughout pregnancy,20 although some clinicians still prefer twice-daily dosing (particularly during the latter half of pregnancy).
Pending more research on weight-based dosing of LMWH in pregnancy, anti-factor- Xa activity levels should be measured after treatment is started and every 1 to 3 months thereafter during pregnancy.21 Doses should be adjusted to keep the peak anti-Xa level (ie, 4 hours after the dose) at 0.5 to 1.2 U/mL.22
Heparin-induced thrombocytopenia
Type-2 heparin-induced thrombocytopenia is an uncommon but serious adverse effect of unfractionated heparin therapy (and, less commonly of LMWH), caused by heparin-dependent immunoglobulin G (IgG) antibodies that activate platelets via their Fc receptors, potentially precipitating life-threatening arterial or venous thrombosis.
In a trial in nonpregnant orthopedic patients,23 clinical heparin-induced thrombocytopenia occurred in 2.7% of patients receiving unfractionated heparin vs 0% of those receiving LMWH; heparin-dependent IgG was present in 7.8% vs 2.2%, respectively.
Fortunately, heparin-induced thrombocytopenia seems to be very rare in pregnancy: two recent prospective series evaluating prolonged LMWH use in pregnancy13,15 revealed no episodes of this disease. Nonetheless, it is reasonable to measure the platelet count once or twice weekly during the first few weeks of LMWH use and less often thereafter, unless symptoms of heparin-induced thrombocytopenia develop. In pregnant women with heparin-induced thrombocytopenia or heparin-related skin reactions, other anticoagulants must be considered24 (see discussion later).
Heparin-induced osteoporosis
Heparin-induced osteoporosis, a potential effect of prolonged heparin therapy, is of concern, given the prolonged duration and high doses of unfractionated heparin often needed to treat venous thromboembolism during pregnancy. Several studies found significant loss of bone mineral density in the proximal femur25 and lumbar spine26 during extended use of unfractionated heparin in pregnancy.
Fortunately, LMWH appears to be much safer with respect to bone loss. Three recent studies27–30 evaluated the use of LMWH for extended periods during pregnancy, and none found any greater loss of bone mineral density than that seen in normal pregnant controls. Giving supplemental calcium (1,000–1,500 mg/day) and vitamin D (400–1,000 IU/day) concomitantly with unfractionated heparin or LMWH in pregnancy is advisable to further reduce the risk.
Interrupt heparin to permit regional anesthesia
Heparin therapy should be temporarily stopped during the immediate peripartum interval to minimize the risk of hemorrhage and to permit regional anesthesia. Because of the theoretical risk of paraspinal hemorrhage in women receiving heparin who undergo epidural or spinal anesthesia, many anesthetists will not perform neuraxial regional anesthesia in women who have recently received heparin.
Since unfractionated heparin has a relatively short duration of action, the American Society of Regional Anesthesia states that subcutaneous unfractionated heparin prophylaxis is not a contraindication to neuraxial regional anesthesia.31 However, LMWHs should be stopped for at least 12 to 24 hours before regional anesthesia can be considered safe. This issue is discussed in more detail in the section on peripartum and postpartum management of anticoagulation, below.
In summary, LMWH during pregnancy offers a number of advantages over unfractionated heparin: equivalent efficacy, once- or twice-daily dosing, lower risk of heparin-induced thrombocytopenia and osteoporosis, and less-intensive monitoring. Unfractionated heparin can be offered to women who cannot afford LMWH (which costs four to five times more), and it may be used peripartum to reduce hemorrhagic risk and to permit regional anesthesia.
COUMARINS
Coumarins are the mainstay of anticoagulant therapy in most nonpregnant women beyond the immediate thrombotic period.
Warfarin (Coumadin) is the most widely used coumarin because it has a predictable onset and duration of action and excellent bioavailability.32 Others, such as acenocoumarol (Sintrom) and phenprocoumon (Marcoumar), are used more outside the United States but can be ordered or brought into the United States.
Coumarins interfere with vitamin K metabolism, inhibiting the generation of vitamin-K-dependent procoagulant proteins (factors II, VII, IX, and X) and thereby preventing clotting. They also inhibit the formation of the vitamin-K-dependent intrinsic anticoagulant proteins C and S.
Major bleeding is the most significant side effect of coumarin therapy, occurring at a rate of 4% to 6% over 3 months when the prothrombin time is maintained at an international normalized ratio (INR) of 2 to 3,33 and more often if the INR is higher.
Other issues with warfarin are the effect of variations in dietary vitamin K intake on anticoagulation and potential drug interactions that may alter the anticoagulant effect. Thus, the INR needs to be monitored closely.
Risks to the fetus and the mother
Unlike the heparins, coumarins freely cross the placenta and thus pose a risk of teratogenicity. A cluster of fetal malformations including “warfarin embryopathy” (nasal bone hypoplasia and chondrodysplasia punctata) can occur when the drug is used between 6 and 12 weeks of gestation. Warfarin embryopathy may be avoided by stopping warfarin prior to 6 weeks from the onset of the last menstrual period (ie, 6-week “menstrual age” or 4-week gestational age34).
Later in pregnancy, warfarin is associated with potential fetal bleeding complications leading to central nervous system abnormalities, increased rates of intrauterine fetal death, and pregnancy loss. In pregnant women with mechanical cardiac valve prostheses who received oral anticoagulants throughout pregnancy, the incidence of congenital anomalies was 6.4% to 10.2%.35 Fetal demise (spontaneous abortion, stillbirth, neonatal death) was also very common (29.7% to 33.6% of pregnancies) in coumarin-treated women.
Severe maternal hemorrhage may also occur in pregnant women on oral anticoagulants, particularly those who remain fully anticoagulated around the time of labor and delivery.
General caveats to warfarin in pregnancy
Because of the many maternal and fetal concerns, oral anticoagulant use in pregnancy is largely restricted to women with older-generation prosthetic heart valves in whom the very high maternal thrombotic risk may outweigh the risk of maternal and fetal side effects.
While there are limited data on warfarin use in pregnant women with antiphospholipid syndrome,36 warfarin use in such patients should be considered only for those at highest risk and with careful informed consent. These issues are discussed further below in the section on mechanical heart valve prostheses.
ANTIPLATELET DRUGS
Aspirin is an antiplatelet agent rather than an anticoagulant. Although considered inadequate for preventing venous thrombosis in high-risk groups when used alone, aspirin can moderately reduce the risk of deep venous thrombosis and pulmonary embolism in nonpregnant patients.37 It also has a well-accepted role in preventing arterial thrombotic events, ie, coronary artery disease and stroke.38
Low-dose aspirin (≤ 100 mg/day) has been extensively evaluated during pregnancy39–41 and has been shown to be safe and effective in reducing the risk of preeclampsia in high-risk women39 and in treating women with antiphospholipid antibodies and recurrent pregnancy loss42 (in conjunction with prophylactic doses of heparin). Although higher doses of aspirin and other nonsteroidal anti-inflammatory drugs can be toxic to the fetus, low doses have been shown to be safe throughout pregnancy.43
Dipyridamole (Persantine) has been studied extensively in pregnancy, and while it appears to be safe, it has not found a well-defined therapeutic role.
Other antiplatelet drugs have been only rarely used, and data on their safety and efficacy during pregnancy are limited to case reports, for example, on ticlopidine44 (Ticlid) and clopidogrel45,46 (Plavix) given during pregnancy in women with cardiac disease. These drugs do not appear to be major teratogens or to cause specific fetal harm. Their use may be reasonable in some high-risk situations, such as recurrent thrombotic stroke despite aspirin therapy. They may be used alone or with other anticoagulants in women with a coronary or other vascular stent if fetal safety is uncertain or if there is an increased risk of maternal bleeding.
NEWER ANTICOAGULANTS
Danaparoid
The heparinoid danaparoid (Orgaran) is an LMWH, a combination of heparan, dermatan, and chondroitin sulfate. Since it is derived from heparin, in theory it can cross-react with antiheparin antibodies, but this is generally not a problem. Danaparoid inhibits factor Xa, and monitoring is via measurement of anti-factor-Xa activity levels. It has been shown to be safe and effective in nonpregnant patients with heparin-induced thrombocytopenia.51
Although no controlled study has been published on danaparoid in pregnancy, at least 51 pregnancies in 49 patients treated with danaparoid have been reported.52 Thirty-two of the patients received danaparoid because of heparin-induced thrombocytopenia and 19 because of heparin-induced skin intolerance. These reports suggest that danaparoid does not cross the placenta53 and that it may be effective and safe during pregnancy.54 For this reason, it is probably the preferred anticoagulant in pregnant patients with heparin-induced thrombocytopenia or other serious reactions to heparin.
Unfortunately, danaparoid has two major disadvantages. First, it has a prolonged half-life and no effective reversing agent, which makes its use problematic close to the time of delivery. Second, and perhaps more relevant to this discussion, it is not readily available in the United States; it was removed from the market by its manufacturer in April 2002 for business reasons rather than because of concerns over toxicity. It is still available in Canada and Europe, and it can be obtained in special circumstances in the United States via the US Food and Drug Administration (FDA); this may be worthwhile in pregnant patients who require a nonurgent alternative to heparin.
Direct thrombin inhibitors
Lepirudin (Refludan), bivalirudin (Angiomax), and argatroban are direct thrombin inhibitors and exert their anticoagulant effect independently of antithrombin. They are given by continuous intravenous infusion, and they have a very short half-life.
Lepirudin and argatroban are typically monitored via the aPTT. Bivalirudin can be monitored with the activated clotting time, partial thromboplastin time, or INR, depending on the circumstances. None of these agents generates or cross-reacts with antibodies generated in heparin-induced thrombocytopenia. None has an antidote, but the short half-life usually obviates the need for one.
Unfortunately, pregnancy data are very sparse for all three of these new agents. Argatroban has a low molecular weight and likely crosses the placenta. Also, because these agents are given intravenously, they are not practical for long-term use in pregnancy.
Fondaparinux
Fondaparinux (Arixtra), a direct factor Xa inhibitor, binds to antithrombin, causing an irreversible conformational change that increases antithrombin’s ability to inactivate factor Xa (as do the heparins). It has no effect on factor IIa (thrombin) and does not predictably affect the aPTT. Its half-life is 17 hours, and no agent is known to reverse its anticoagulant effect, although some experts would recommend a trial of high-dose recombinant factor VIIa (Novo-Seven) in uncontrolled hemorrhage.
While not FDA-approved for treating heparin-induced thrombocytopenia, it has been used for this in some patients.55–58 Animal studies and in vitro human placental perfusion studies suggest that fondaparinux does not cross the placenta in significant amounts.49 Since danaparoid is not available in the United States, fondaparinux would likely be the first choice among the newer anticoagulants when treating heparin-induced thrombocytopenia in pregnancy.
INDICATIONS FOR ANTICOAGULANTS DURING PREGNANCY
Acute deep venous thrombosis and pulmonary embolism
Anticoagulant therapy should begin as full doses of either LMWH or intravenous unfractionated heparin. We prefer starting with LMWH, as it can be started rapidly with less need for nursing care (eg, no need to start and maintain an intravenous line and monitor the aPTT) and has excellent safety. If LMWH is selected, initial dosing should be based on the current weight (Table 2). Subsequent monitoring of the peak anti-factor-Xa activity levels (ie, 4 hours after the dose) is recommended, with the first level drawn in the first few days of treatment, and repeat levels every 1 to 3 months for the rest of treatment. As mentioned earlier, weight-based dosing has not been systematically evaluated in pregnancy.
If unfractionated heparin is the initial agent, it should be given as a bolus followed by a continuous infusion, ideally utilizing a weight-based nomogram to estimate required doses, with adjustment of the infusion rate to maintain the aPTT at 1.5 to 2.5 times the baseline value (obtained during pregnancy). After several days, the heparin may be switched to LMWH in therapeutic doses (Table 2).
Alternatively, in women approaching term or who cannot afford LMWH, anticoagulation may be continued as adjusted-dose subcutaneous unfractionated heparin, ie, two or three large daily doses of subcutaneous heparin to provide therapeutic levels of anticoagulation. The starting dose can be calculated as the total units of heparin required to maintain full anticoagulation intravenously over 24 hours, given as two or three divided doses (Table 2). The aPTT at the mid-dosing interval (eg, 6 hours after the subcutaneous dose during every-12-hour dosing) should be monitored and the dose adjusted to maintain the aPTT at 1.5 to 2.5 times the baseline value.
A therapeutic level of anticoagulation should be maintained for at least 3 months after an acute thrombotic event during pregnancy, though many physicians prefer to continue full anticoagulation for a total of 6 months. Beyond this interval, if the woman is still pregnant, the anticoagulation may be reduced in intensity, perhaps even to a prophylactic level for the duration of the pregnancy (see discussion below on prior venous thromboembolic events) (Table 2). Peripartum and postpartum anticoagulation are discussed further below.
PRIOR VENOUS THROMBOEMBOLIC EVENT
While all pregnant women are at higher risk of venous thrombosis, the overall incidence of thromboembolism is only about one event per 1,000 pregnancies. Routine thromboprophylaxis in all pregnant women is therefore not justified. However, women who have previously had a venous thromboembolic event are at a substantially higher risk of recurrent thrombosis and should be considered for thromboprophylaxis in all subsequent high-risk situations, including pregnancy.
For women on indefinite therapeutic anticoagulation (ie, because of recurrent thrombosis), full therapeutic anticoagulation with LMWH or adjusted-dose unfractionated heparin should be maintained throughout pregnancy, as described above.
Which other women should receive prophylactic anticoagulation is a topic of ongoing debate and controversy.
How great is the risk of recurrent thromboembolism?
A small observational study59 examined the risk of recurrent venous thromboembolism during subsequent pregnancies in women with a prior thrombotic event. Anticoagulation was withheld during the antepartum period and restarted briefly after delivery. Among the 125 women enrolled, recurrent venous thromboembolism occurred in 4.8%, with half of the events occurring during the antepartum period. Among those with underlying thrombophilia, the rate of recurrent venous thromboembolism was 13% (95% confidence interval [CI] 1.7%–40.5%) to 20% (95% CI 2.5%–56.5%), and those with a prior idiopathic clot without thrombophilia had an event rate of 7.7% (95% CI 0.01%–25.1%). The subgroup with a prior reversible risk factor (at the time of their initial venous thromboembolic event) and without detectable thrombophilia had no recurrent events.
This study suggests that women with prior venous thromboembolism and thrombophilia or a prior idiopathic thrombotic event are at a substantial risk of recurrent thrombotic events during pregnancy. And other data confirm the high risk of recurrent venous thromboembolism in thrombophilic pregnant women.60 These women should all be offered active antepartum and postpartum thromboprophylaxis with LMWH or unfractionated heparin (Tables 2 and 4). Women without thrombophilia but with a history of venous thromboembolism related to pregnancy or oral contraceptive use also have a substantial risk of recurrent venous thrombosis and should be offered antepartum and postpartum thromboprophylaxis.61 In contrast, women with a prior “secondary” clot, no thrombophilia, and no additional current risk factors (Table 1) appear to be at low risk of recurrent venous thromboembolism.
The risks should be discussed with these women, with an option for close clinical surveillance during pregnancy (Table 4), but with a low threshold to investigate any worrisome symptoms. Such women may also elect to take LMWH or unfractionated heparin during pregnancy.
Which heparin to use?
Prophylactic anticoagulation during pregnancy can be with either LMWH or unfractionated heparin. For most women this involves “prophylactic” dosing with the goal of maintaining a mid-interval anti-factor-Xa activity level of approximately 0.05 to 0.2 U/mL. Thromboprophylaxis with LMWH can be with lower, fixed, once-daily doses throughout pregnancy20 (Table 2), although some clinicians still prefer twice-daily dosing. The heparin should be started as soon as pregnancy is confirmed, as the pregnancy-associated increase in thrombotic risk begins by the middle of the first trimester.
To maintain effective prophylactic levels, the dose of unfractionated heparin should be increased sequentially over the trimesters62,63: approximately 5,000 units subcutaneously twice daily in the first trimester, then 7,500 units twice daily in the second trimester, and 10,000 units twice daily in the third trimester for a woman of average size.
When to add low-dose aspirin
Women with antiphospholipid antibodies, particularly those with prior recurrent pregnancy loss or fetal demise, should receive aspirin 81 mg/day in addition to heparin.39 The aspirin may be started prior to conception or when pregnancy is confirmed.
Other measures
Women on anticoagulant therapy who are at risk of recurrent venous thromboembolism should be encouraged to wear elastic compression stockings. Intermittent pneumatic compression of the legs via automated devices may be considered for women hospitalized for any reason or on bedrest.
Whichever measures are used, a high index of suspicion and a low threshold for investigating for recurrent thrombosis should be maintained throughout pregnancy and the puerperium.
PERIPARTUM AND POSTPARTUM MANAGEMENT OF ANTICOAGULATION
Heparin therapy must be interrupted temporarily during the immediate peripartum interval to minimize the risk of hemorrhage and to allow for the option of regional anesthesia. As mentioned earlier, because of the theoretical risk of paraspinal hemorrhage in women receiving heparin who undergo epidural or spinal anesthesia, the American Society of Regional Anesthesia guidelines advise waiting to insert the needle at least 10 to 12 hours after the last prophylactic dose of LMWH, and at least 24 hours after the last therapeutic dose.31
The guidelines state that neuraxial anesthesia is not contraindicated in patients on prophylactic unfractionated heparin.31
To facilitate use of regional anesthesia in these women, therefore, options include:
- Electively stopping LMWH 24 hours before planned induction of labor
- Electively stopping prophylactic-dose LMWH or unfractionated heparin at about 38 weeks of gestation, to await spontaneous labor, or
- Switching therapeutic or prophylactic LMWH to unfractionated heparin at about 36 weeks of gestation, with instructions to discontinue the injections in the earliest stages of spontaneous labor. This aims to shorten the heparin-free period required before neuraxial anesthesia while minimizing maternal thrombotic risk.
Additional advantages to using unfractionated heparin peripartum include the option of obtaining a rapid aPTT measurement to confirm the absence of a significant ongoing heparin effect prior to regional anesthesia or delivery, and the ability to completely reverse the heparin effect with protamine sulfate if major bleeding occurs. LMWHs are only partially reversible.64
Interrupting anticoagulation after an initial thrombotic event
If therapeutic anticoagulation must be interrupted for labor within 1 month of the initial thrombotic event, the risk of recurrent thrombotic complications is high65; these women must be observed very carefully and may benefit from intravenous heparin before and after delivery. They may even merit placement of a temporary vena cava filter (particularly if less than 2 weeks have elapsed since the venous thromboembolic event and in women with a large deep venous clot burden), a procedure that has been used safely but little studied in pregnant women.66
Fluoroscopic guidance may be needed for filter placement. This exposes the fetus to radiation, but the low-level exposure at this late gestational age is unlikely to pose a significant risk. The filter may be removed within 1 to 2 weeks postpartum, assuming there are no ongoing contraindications to anticoagulation.
In the rare woman with antithrombin deficiency and a recent or prior thrombotic event, giving antithrombin concentrate during the peripartum (heparin-free) interval has been described and may be considered under the guidance of a hematologist.67
Ongoing anticoagulation is essential postpartum, as the puerperium is the period of highest day-to-day risk of thromboembolic events: about one-third of pregnancy-associated events occur during these 6 to 12 weeks.2 Heparin should be resumed 6 to 12 hours after delivery, once hemostasis is confirmed.
Options for women requiring ongoing therapeutic anticoagulation include intravenous heparin started without a bolus, to minimize bleeding risk, with aPTT measured 12 hours later, or an initial prophylactic dose of LMWH 6 to 12 hours postpartum, with therapeutic dosing resumed on postpartum day 1. If prophylactic dosing is desired, unfractionated heparin or LMWH may be given subcutaneously starting at about 6 hours postpartum.
Warfarin in the puerperium
Women may subsequently be maintained on either LMWH or unfractionated heparin, or switched to an oral anticoagulant such as warfarin. Although warfarin may appear in minute amounts in breast milk, it has not been associated with adverse events in newborns and is considered compatible with breastfeeding.68 Heparin should be continued during the initial days of warfarin therapy, until the INR is at a therapeutic level for 24 hours. Some physicians prefer to delay warfarin for several days, giving LMWH alone in the immediate postpartum period, to allow wound-healing and to reduce bleeding risk.
Postpartum, anticoagulation should be continued for at least 6 to 12 weeks, at which point the physiologic changes in the coagulation system related to pregnancy will have returned to normal.
THROMBOPHILIA WITHOUT A PREVIOUS THROMBOEMBOLIC EVENT
Over the last 5 to 10 years, practitioners have been seeing many more young women with genetic or acquired thrombophilias who have never had a venous thromboembolic event. Physicians must advise these women about their risk of thromboembolic events during pregnancy and about the appropriateness of anticoagulant use.
Thrombophilias are often detected in women who develop venous thrombosis during pregnancy,69–71 but they are also very common in the general population (around 15%). While women with thrombophilia are at above-average risk of venous thromboembolism during pregnancy, the magnitude of risk in an individual patient is often difficult to estimate.
Data suggest that some types of thrombophilia confer greater thrombotic risk than others. McColl et al72 derived risk estimates for a primary event in women with several of the disorders: 0.23% in women heterozygous for the factor V Leiden mutation, 0.88% in women with protein C deficiency, and 2.4% to 35.7% in women with antithrombin deficiency. A case-control study70 found that all thrombophilic states were more common in women with pregnancy-associated venous thromboembolism than in healthy pregnant controls, except those with the MTHFR mutation and protein S deficiency. The estimated risk during pregnancy was 0.03% in women with no defect, 0.1% in women with protein C deficiency, 0.25% in women with the factor V Leiden mutation, 0.4% in those with antithrombin deficiency, 0.5% in those with the prothrombin gene mutation, and 4.6% in those with both factor V Leiden and prothrombin gene mutations.
Routine anticoagulation not advised in pregnant thrombophilic women
Because the risk of a primary venous thromboembolic event is less than 1% for most thrombophilic women, routine anticoagulant therapy does not seem prudent for this indication. Given the low absolute risk of venous thromboembolism, the cost and potential side effects of anticoagulant use are difficult to justify.
The women who seem at higher risk and in whom anticoagulation should be considered include those with antithrombin deficiency; those with high-titer anticardiolipin antibodies or a lupus anticoagulant antibody (treat with heparin and low-dose aspirin); those with combined thrombophilic defects or who are homozygotes for the factor V Leiden or prothrombin gene mutations; and those with multiple other current risk factors for venous thromboembolism (Table 1).
Since anticoagulants for primary prevention of adverse pregnancy outcomes in thrombophilic women have not yet been shown to have a definitive benefit, they are not recommended for this purpose.
ADVERSE PREGNANCY OUTCOMES IN WOMEN WITH THROMBOPHILIAS
Women with antiphospholipid antibodies and a previous poor obstetric outcome are clearly at increased risk of recurrent adverse pregnancy outcomes such as recurrent spontaneous abortion, unexplained fetal death, placental insufficiency, and early or severe preeclampsia. In such women who have both antiphospholipid antibodies and a history of venous thromboembolism or adverse pregnancy outcome, treatment during subsequent pregnancy with low-dose aspirin and prophylactic-dose LMWH or unfractionated heparin improves pregnancy outcomes.36–42 Women with antiphospholipid antibodies without previous thrombosis or pregnancy complications may also be at increased risk, but it is unclear whether thromboprophylaxis improves their outcomes.
Recent epidemiologic data reveal that women with other thrombophilic conditions also are at increased risk of early, severe preeclampsia73 as well as other pregnancy complications, including recurrent pregnancy loss, placental abruption, fetal growth restriction, and stillbirth.74 A recent meta-analysis75 looked at individual thrombophilias and found that factor V Leiden and prothrombin gene mutations were associated with recurrent fetal loss, stillbirth, and preeclampsia; that protein S deficiency was associated with recurrent fetal loss and stillbirth; that antiphospholipid antibodies were associated with recurrent pregnancy loss, preeclampsia, and intrauterine growth restriction; that the MTHFR mutation (homozygous) was associated with preeclampsia; and that protein C and antithrombin deficiencies were not significantly associated with adverse pregnancy outcomes. Data were scant for some of the rarer thrombophilias.75
Several recent small studies76–78 suggest that anticoagulants may improve pregnancy outcomes in women with genetic thrombophilias and recurrent pregnancy loss. These findings have not yet been confirmed in high-quality clinical trials, but such trials are under way. It is still unclear whether anticoagulants also reduce the risk of other adverse pregnancy outcomes associated with thrombophilias.
The current American College of Chest Physicians guidelines recommend testing of women with adverse pregnancy outcomes (recurrent pregnancy loss, prior severe or recurrent preeclampsia, abruptions, or otherwise unexplained intrauterine death) for congenital thrombophilias and antiphospholipid antibodies, and offering treatment to such women, if thrombophilic, with low-dose aspirin plus prophylactic heparin (unfractionated or LMWH).22 The authors of the guidelines admit that the evidence for this recommendation is weak, but they argue that the heparin will also serve as thromboprophylaxis in this high-risk group. Hopefully, the randomized clinical trials currently under way will provide clearer guidance regarding the most appropriate therapy in this difficult clinical situation.
MECHANICAL HEART VALVES
Internists may occasionally encounter a woman with a mechanical heart valve prosthesis who is either pregnant or is planning a pregnancy and therefore needs advice regarding optimal anticoagulant management. This should generally be undertaken in a multi-disciplinary fashion, with input from cardiology, hematology, and maternal-fetal medicine. The substantial maternal and fetal risks and the lack of definitive data on which to base treatment decisions make it a treacherous and stressful undertaking. Nonetheless, all internists should have a basic understanding of the complex issues regarding this management.
Outside of pregnancy, oral anticoagulants are the mainstay of therapy for patients with mechanical heart valves. Unfortunately, as discussed above, the use of these agents during pregnancy carries a risk of teratogenicity and toxic fetal effects and increases the risk of pregnancy loss and maternal hemorrhage. Heparins have been used in this setting for many years, but data on their efficacy and safety are very limited, and there are numerous reports of catastrophic maternal thrombotic complications.79,80
A systematic review of anticoagulation in pregnant women with prosthetic heart valves34 found very limited data on heparin use throughout pregnancy. Women maintained on warfarin vs heparin between pregnancy weeks 6 and 12 had higher rates of congenital anomalies (6.4% with warfarin vs 3.4% with heparin) and total fetal wastage (33.6% vs 26.5%). The warfarin group had fewer maternal thromboembolic complications (3.9% vs 9.2%), however, and a slightly lower rate of maternal death (1.8% vs 4.2%). Most of the women had higher-risk older-generation valves in the mitral position.
Recent data on LMWH consist mainly of case reports and case series,81 with a likely bias to publication of worse outcomes. Controlled trials in this area will be difficult to conduct. Still, aggressive anticoagulation with LMWH or unfractionated heparin, with close monitoring of the intensity of anticoagulation, may be safe and effective for pregnant women with newer-generation mechanical heart valves.82 A recent consensus statement22 suggested several regimens for pregnant women with mechanical heart valves:
- Twice-daily LMWH throughout pregnancy, with the dose adjusted either by weight, or to keep the 4-hour postinjection anti-factor-Xa activity level around 1.0 to 1.2 U/mL
- Aggressive adjusted-dose unfractionated heparin throughout pregnancy, given subcutaneously every 12 hours and adjusted to keep the mid-interval aPTT at least twice the control value or to attain a mid-interval anti-factor-Xa activity level of 0.35 to 0.70 U/mL
- Unfractionated heparin or LMWH (as above) until gestation week 13, then warfarin until the middle of the third trimester, and then heparin again.22
The authors also recommended adding low-dose aspirin (75–162 mg/day) in high-risk women.22
These options all seem reasonable, given our current knowledge, though warfarin use during pregnancy should be restricted to very-high-risk situations, such as women with older-generation mitral prostheses. LM-WHs may become the preferred therapy for this indication once further controlled data regarding their efficacy and safety become available.
- Chang J, Elam-Evans LD, Berg CJ, et al. Pregnancy-related mortality surveillance-United States, 1991–1999. MMWR Surveill Summ 2003; 52:1–8.
- Lewis G, Drife JO, Clutton-Brock T, et al. Why Mothers Die, 2000–2002. The Sixth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG Press, 2004.
- Health Canada. Special Report on Maternal Mortality and Severe Morbidity in Canada—Enhanced Surveillance: The Path to Prevention. Ottawa: Minister of Public Works and Government Services Canada, 2004. www.phac-aspc.gc.ca/rhs-ssg/srmm-rsmm/page1-eng.php. Accessed 11/26/2008.
- Stein PD, Hull RD, Kayali F, et al. Venous thromboembolism in pregnancy: 21-year trends. Am J Med 2004; 117:121–125.
- Greer IA. Thrombosis in pregnancy: maternal and fetal issues. Lancet 1999; 353:1258–1265.
- Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet 1999; 353:1167–1173.
- Gherman RB, Goodwin TM, Leung B, et al. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol 1999; 94:730–734.
- Weitz JI. Low-molecular-weight heparins. N Engl J Med 1997; 337:688–698.
- Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996; 334:677–681.
- Koopman MM, Prandoni P, Piovella F, et al. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. The Tasman Study Group. N Engl J Med 1996; 334:682–687.
- Simonneau G, Sors H, Charbonnier B, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. The THESEE Study Group. Tinzaparine ou Heparine Standard: Evaluations dans l’Embolie Pulmonaire. N Engl J Med 1997; 337:663–669.
- Hull RD, Raskob GE, Brant RF, et al. Low-molecular-weight heparin vs heparin in the treatment of patients with pulmonary embolism. American-Canadian Thrombosis Study Group. Arch Intern Med 2000; 160:229–236.
- Sanson BJ, Lensing AW, Prins MH, et al. Safety of low-molecular-weight heparin in pregnancy: a systematic review. Thromb Haemost 1999; 81:668–672.
- Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood 2005; 106:401–407.
- Melissari E, Parker CJ, Wilson NV, et al. Use of low molecular weight heparin in pregnancy. Thromb Haemost 1992; 68:652–656.
- Forestier F, Daffos F, Capella-Pavlovsky M. Low molecular weight heparin (PK 10169) does not cross the placenta during the second trimester of pregnancy study by direct fetal blood sampling under ultrasound. Thromb Res 1984; 34:557–560.
- Forestier F, Daffos F, Rainaut M, Toulemonde F. Low molecular weight heparin (CY 216) does not cross the placenta during the third trimester of pregnancy. Thromb Haemost 1987; 57:234.
- Barbour LA, Oja JL, Schultz LK. A prospective trial that demonstrates that dalteparin requirements increase in pregnancy to maintain therapeutic levels of anticoagulation. Am J Obstet Gynecol 2004; 191:1024–1029.
- Smith MP, Norris LA, Steer PJ, Savidge GF, Bonnar J. Tinzaparin sodium for thrombosis treatment and prevention during pregnancy. Am J Obstet Gynecol 2004; 190:495–501.
- Ellison J, Walker ID, Greer IA. Antenatal use of enoxaparin for prevention and treatment of thromboembolism in pregnancy. BJOG 2000; 107:1116–1121.
- Sarig G, Brenner B. Monitoring of low molecular weight heparin (LMWH) in pregnancy. Thromb Res 2005; 115 suppl 1:84–86.
- Bates SM, Greer IA, Hirsh J, Ginsberg JS. Use of antithrombotic agents during pregnancy: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004; 126 suppl 3:627S–644S.
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995; 332:1330–1335.
- Hassell K. The management of patients with heparin-induced thrombocytopenia who require anticoagulant therapy. Chest 2005; 127 suppl 2:1S–8S.
- Barbour LA, Kick SD, Steiner JF, et al. A prospective study of heparin-induced osteoporosis in pregnancy using bone densitometry. Am J Obst Gynecol 1994; 170:862–869.
- Douketis JD, Ginsberg JS, Burrows RF, Duku EK, Webber CE, Brill-Edwards P. The effects of long-term heparin therapy during pregnancy on bone density. A prospective matched cohort study. Thromb Haemost 1996; 75:254–257.
- Pettila V, Leinonen P, Markkola A, Hiilesmaa V, Kaaja R. Postpartum bone mineral density in women treated for thromboprophylaxis with unfractionated heparin or LMW heparin. Thromb Haemost 2002; 87:182–186.
- Carlin AJ, Farquharson RG, Quenby SM, Topping J, Fraser WD. Prospective observational study of bone mineral density during pregnancy: low molecular weight heparin versus control. Hum Reprod 2004; 19:1211–1214.
- Casele HL, Laifer SA. Prospective evaluation of bone density in pregnant women receiving the low molecular weight heparin enoxaparin sodium. J Matern Fetal Med 2000; 9:122–125.
- Casele H, Haney EI, James A, Rosene-Montella K, Carson M. Bone density changes in women who receive thromboprophylaxis in pregnancy. Am J Obstet Gynecol 2006; 195:1109–1113.
- Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med 2003; 28:172–197.
- Hirsh J, Dalen JE, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 2001; 119 suppl 1:8S–21S.
- Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004; 126 suppl 3:287S–310S.
- Holmes LB. Teratogen-induced limb defects. Am J Med Genet 2002; 112:297–303.
- Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med 2000; 160:191–196.
- Pauzner R, Dulitzki M, Langevitz P, Livneh A, Kenett R, Many A. Low molecular weight heparin and warfarin in the treatment of patients with antiphospholipid syndrome during pregnancy. Thromb Haemost 2001; 86:1379–1384.
- Pulmonary Embolism Prevention (PEP) Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355:1295–1302.
- Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126 suppl 3:234S–264S.
- Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2004; ( 1):CD004659.
- Coomarasamy A, Honest H, Papaioannou S, Gee H, Khan KS. Aspirin for prevention of preeclampsia in women with historical risk factors: a systematic review. Obstet Gynecol 2003; 101:1319–1332.
- Caritis SN, Sibai BM, Hauth J, et al, and the National Institute of Child Health and Human Development Network of Maternal Fetal Medicine Units. Low-dose aspirin to prevent preeclampsia in women at high risk. N Engl J Med 1998; 338:701–705.
- Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ 1997; 314:253–257.
- Kozer E, Nikfar S, Costei A, Boskovic R, Nulman I, Koren G. Aspirin consumption during the first trimester of pregnancy and congenital anomalies: a meta-analysis. Am J Obstet Gynecol 2002; 187:1623–1630.
- Sebastian C, Scherlag M, Kugelmass A, Schechter E. Primary stent implantation for acute myocardial infarction during pregnancy: use of abciximab, ticlopidine, and aspirin. Cathet Cardiovasc Diagn 1998; 45:275–249.
- Wilson AM, Boyle AJ, Fox P. Management of ischaemic heart disease in women of child-bearing age. Intern Med J 2004; 34:694–697.
- Klinzing P, Markert UR, Liesaus K, Peiker G. Case report: successful pregnancy and delivery after myocardial infarction and essential thrombocythemia treated with clopidogrel. Clin Exp Obstet Gynecol 2001; 28:215–216.
- Danhof M, de Boer A, Magnani HN, Stiekema JC. Pharmacokinetic considerations on Orgaran (Org 10172) therapy. Haemostasis 1992; 22:73–84.
- Tardy-Poncet B, Tardy B, Reynaud J, et al. Efficacy and safety of danaparoid sodium (ORG 10172) in critically ill patients with heparin-associated thrombocytopenia. Chest 1999; 115:1616–1620.
- Lagrange F, Vergnes C, Brun JL, et al. Absence of placental transfer of pentasaccharide (fondaparinux, Arixtra) in the dually perfused human cotyledon in vitro. Thromb Haemost 2002; 87:831–835.
- Dempfle CE. Minor transplacental passge of fondapinux in vivo. N Engl J Med 2004; 350:1914.
- Magnani HN. Heparin-induced thrombocytopenia (HIT): an overview of 230 patients treated with orgaran (Org 10172). Thromb Haemost 1993; 70:554–561.
- Lindhoff-Last E, Kreutzenbeck HJ, Magnani HN. Treatment of 51 pregnancies with danaparoid because of heparin intolerance. Thromb Haemost 2005; 93:63–69.
- Greinacher A, Eckhardt T, Mussmann J, Mueller-Eckhardt C. Pregnancy complicated by heparin associated thrombocytopenia: management by a prospectively in vitro selected heparinoid (Org 10172). Thromb Res 1993; 71:123–126.
- Schindewolf M, Mosch G, Bauersachs RM, Lindhoff-Last E. Safe anticoagulation with danaparoid in pregnancy and lactation. Thromb Haemost 2004; 92:211.
- Harenberg J. Treatment of a woman with lupus and thromboembolism and cutaneous intolerance to heparins using fondaparinux during pregnancy. Thromb Res 2007; 119:385–388.
- Wijesiriwardana A, Lees DA, Lush C. Fondaparinux as anticoagulant in a pregnant woman with heparin allergy. Blood Coagul Fibrinolysis 2006; 17:147–149.
- Mazzolai L, Hohlfeld P, Spertini F, Hayoz D, Schapira M, Duchosal MA. Fondaparinux is a safe alternative in case of heparin intolerance during pregnancy. Blood 2006; 108:1569–1570.
- Hawkins D, Evans J. Minimizing the risk of heparin-induced osteoporosis during pregnancy. Expert Opin Drug Saf 2005; 4:583–590.
- Brill-Edwards P, Ginsberg JS, Gent M, et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of clot in this pregnancy study group. N Engl J Med 2000; 343:1439–1444.
- Martinelli I, Legnani C, Bucciarelli P, Grandone E, De Stefano V, Mannucci PM. Risk of pregnancy-related venous thrombosis in carriers of severe inherited thrombophilia. Thromb Haemost 2001; 86:800–803.
- De Stefano V, Martinelli I, Rossi E, Battaglioli T, Za T, Mannucci PM, Leone G. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol 2006; 135:386–391.
- Barbour LA, Smith JM, Marlar RA. Heparin levels to guide thromboembolism prophylaxis during pregnancy. Am J Obstet Gynecol 1995; 173:1869–1873.
- Ensom MH, Stephenson MD. Pharmacokinetics of low molecular weight heparin and unfractionated heparin in pregnancy. J Soc Gynecol Investig 2004; 11:377–383.
- Crowther MA, Berry LR, Monagle PT, Chan AK. Mechanisms responsible for the failure of protamine to inactivate low-molecular-weight heparin. Br J Haematol 2002; 116:178–186.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Thomas LA, Summers RR, Cardwell MS. Use of Greenfield filters in pregnant women at risk for pulmonary embolism. South Med J 1997; 90:215–217.
- Maclean PS, Tait RC. Hereditary and acquired antithrombin deficiency: epidemiology, pathogenesis and treatment options. Drugs 2007; 67:1429–1440.
- Information from LactMed: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT, LactMed Record Number: 279. Accessed 11/26/2008.
- Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000; 342:374–380.
- Hirsch DR, Mikkola KM, Marks PW, et al. Pulmonary embolism and deep venous thrombosis during pregnancy or oral contraceptive use: prevalence of factor V Leiden. Am Heart J 1996; 131:1145–1148.
- Dizon-Townson DS, Nelson LM, Jang H, Varner MW, Ward K. The incidence of the factor V Leiden mutation in an obstetric population and its relationship to deep vein thrombosis. Am J Obstet Gynecol 1997; 176:883–886.
- McColl MD, Ramsay JE, Tait RC, et al. Risk factors for pregnancy associated venous thromboembolism. Thromb Haemost 1997; 78:1183–1188.
- Kupferminc MJ, Fait G, Many A, Gordon D, Eldor A, Lessing JB. Severe preeclampsia and high frequency of genetic thrombophilic mutations. Obstet Gynecol 2000; 96:45–49.
- Kupferminc MJ, Eldor A, Steinman N, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med 1999; 340:9–13.
- Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol 2006; 132:171–196.
- Brenner B, Hoffman R, Blumenfeld Z, Weiner Z, Younis JS. Gestational outcome in thrombophilic women with recurrent pregnancy loss treated by enoxaparin. Thromb Haemost 2000; 83:693–697.
- Carp H, Dolitzky M, Inbal A. Thromboprophylaxis improves the live birth rate in women with consecutive recurrent miscarriages and hereditary thrombophilia. J Thromb Haemost 2003; 1:433–438.
- Gris JC, Mercier E, Quere I, et al. Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood 2004; 103:3695–3699.
- Salazar E, Izaguirre R, Verdejo J, Mutchinick O. Failure of adjusted doses of subcutaneous heparin to prevent thromboembolic phenomena in pregnant patients with mechanical cardiac valve prostheses. J Am Coll Cardiol 1996; 27:1698–1703.
- Iturbe-Alessio I, Fonseca MC, Mutchinik O, Santos MA, Zajarias A, Salazar E. Risks of anticoagulant therapy in pregnant women with artificial heart valves. N Engl J Med 1986; 315:1390–1393.
- Rowan JA, McCowan LM, Raudkivi PJ, North RA. Enoxaparin treatment in women with mechanical heart valves during pregnancy. Am J Obstet Gynecol 2001; 185:633–637.
- Oran B, Lee-Parritz A, Ansell J. Low molecular weight heparin for the prophylaxis of thromboembolism in women with prosthetic mechanical heart valves during pregnancy. Thromb Haemost 2004; 92:747–751.
Anticoagulation is essential in a wide variety of conditions in women of child-bearing age. Some, such as venous thromboembolism, occur more often during pregnancy. Others, such as recurrent fetal loss in the setting of antiphospholipid antibodies, are specific to pregnancy.
While anticoagulants are useful in many circumstances, their use during pregnancy increases the risk of hemorrhage and other adverse effects on the mother and the fetus. Treatment with anticoagulants during pregnancy must therefore be carefully considered, with judicious selection of the agent, and with reflection on the physiologic changes of pregnancy to ensure appropriate dosing. In this article, we review these issues.
WHY IS THROMBOTIC RISK HIGHER DURING PREGNANCY?
Venous thromboembolism is among the leading causes of maternal death in developed countries.1–3 Modern care has dramatically reduced the risk of maternal death from hemorrhage, infection, and hypertension, but rates of morbidity and death from thrombosis have remained stable or increased in recent years.4
- Much higher levels of fibrinogen and factors VII, VIII, IX, and X
- Lower levels of protein S and increased resistance to activated protein C
- Impaired fibrinolysis, due to inhibitors derived from the placenta.
Acquired antithrombin deficiency may also occur in high-proteinuric states such as nephrotic syndrome or preeclampsia, further increasing thrombotic risk. Pooling of venous blood, caused by progesterone-mediated venous dilation and compounded by compression of the inferior vena cava by the uterus in later pregnancy, also increases thrombotic risk. And endothelial disruption of the pelvic vessels may occur during delivery, particularly during cesarean section.
Additional factors that increase thrombotic risk include immobilization, such as bed rest for pregnancy complications; surgery, including cesarean section; ovarian hyperstimulation during gonadotropin use for in vitro fertilization; trauma; malignancy; and hereditary or acquired hypercoagulable states.6 These hypercoagulable states include deficiencies of antithrombin or the intrinsic anticoagulant proteins C or S; resistance to activated protein C, usually due to the factor V Leiden mutation; the PT20210A mutation of the prothrombin gene; hyperhomocystinemia due to mutation of the methyltetrahydrofolate reductase (MTHFR) gene; and the sustained presence of antiphospholipid antibodies, including lupus anticoagulant antibodies, sometimes also with moderately high titers of anticardiolipin or beta-2-glycoprotein I antibodies.
Other conditions that increase thrombotic risk include hyperemesis gravidarum, obesity, inflammatory bowel disease, infection, smoking, and indwelling intravenous catheters.6 Given the multitude of risk factors, pregnant women have a risk of thrombotic complications three to five times higher than nonpregnant women.7
HEPARIN USE DURING PREGNANCY
Low-molecular-weight heparins (LMWHs)8 and unfractionated heparin bind to anti-thrombin and thus change the shape of the antithrombin molecule, dramatically increasing its interaction with the clotting factors Xa and prothrombin (factor II). The enhanced clearance of these procoagulant proteins leads to the anticoagulant effect. Unfractionated heparin has roughly equivalent interaction with factors Xa and II and prolongs the activated partial thromboplastin time (aPTT), which is therefore used to monitor the intensity of anticoagulation.
LMWHs, on the other hand, interact relatively little with factor II and do not predictably prolong the aPTT. Monitoring their effect is therefore more difficult and requires direct measurement of anti-factor-Xa activity. This test is widely available, but it is time-consuming (it takes several hours and results may not be available within 24 hours if the test is requested “after hours”), and therefore it is of limited use in the acute clinical setting. While weight-based dosing of LMWHs is reliable and safe in nonpregnant patients, it has not yet been validated for pregnant women.
Unfractionated heparin has been used for decades for many indications during pregnancy. It is a large molecule, so it does not cross the placenta and thus, in contrast to the coumarin derivatives, does not cause teratogenesis or toxic fetal effects. Its main limitations in pregnancy are its inconvenient dosing (at least twice daily when given subcutaneously) and its potential maternal adverse effects (mainly osteoporosis and heparin-induced thrombocytopenia).
Over the last 10 years LMWHs have become the preferred anticoagulants for treating and preventing thromboembolism in all patients. They are equivalent or superior to unfractionated heparin in efficacy and safety in the initial treatment of acute deep venous thrombosis9,10 and pulmonary embolism11,12 outside of pregnancy. While comparative data are much less robust in pregnant patients, several series have confirmed the safety and efficacy of LMWHs in pregnancy.13–15 LMWHs do not cross the placenta15–17 and thus have a fetal safety profile equivalent to that of unfractionated heparin.
Pregnancy alters metabolism of LMWHs
The physiologic changes of pregnancy alter the metabolism of LMWH, resulting in lower peak levels and a higher rate of clearance,18,19 and so a pregnant woman may need higher doses or more frequent dosing.
Recent evidence suggests that thromboprophylaxis can be done with lower, fixed, once-daily doses of LMWH throughout pregnancy,20 although some clinicians still prefer twice-daily dosing (particularly during the latter half of pregnancy).
Pending more research on weight-based dosing of LMWH in pregnancy, anti-factor- Xa activity levels should be measured after treatment is started and every 1 to 3 months thereafter during pregnancy.21 Doses should be adjusted to keep the peak anti-Xa level (ie, 4 hours after the dose) at 0.5 to 1.2 U/mL.22
Heparin-induced thrombocytopenia
Type-2 heparin-induced thrombocytopenia is an uncommon but serious adverse effect of unfractionated heparin therapy (and, less commonly of LMWH), caused by heparin-dependent immunoglobulin G (IgG) antibodies that activate platelets via their Fc receptors, potentially precipitating life-threatening arterial or venous thrombosis.
In a trial in nonpregnant orthopedic patients,23 clinical heparin-induced thrombocytopenia occurred in 2.7% of patients receiving unfractionated heparin vs 0% of those receiving LMWH; heparin-dependent IgG was present in 7.8% vs 2.2%, respectively.
Fortunately, heparin-induced thrombocytopenia seems to be very rare in pregnancy: two recent prospective series evaluating prolonged LMWH use in pregnancy13,15 revealed no episodes of this disease. Nonetheless, it is reasonable to measure the platelet count once or twice weekly during the first few weeks of LMWH use and less often thereafter, unless symptoms of heparin-induced thrombocytopenia develop. In pregnant women with heparin-induced thrombocytopenia or heparin-related skin reactions, other anticoagulants must be considered24 (see discussion later).
Heparin-induced osteoporosis
Heparin-induced osteoporosis, a potential effect of prolonged heparin therapy, is of concern, given the prolonged duration and high doses of unfractionated heparin often needed to treat venous thromboembolism during pregnancy. Several studies found significant loss of bone mineral density in the proximal femur25 and lumbar spine26 during extended use of unfractionated heparin in pregnancy.
Fortunately, LMWH appears to be much safer with respect to bone loss. Three recent studies27–30 evaluated the use of LMWH for extended periods during pregnancy, and none found any greater loss of bone mineral density than that seen in normal pregnant controls. Giving supplemental calcium (1,000–1,500 mg/day) and vitamin D (400–1,000 IU/day) concomitantly with unfractionated heparin or LMWH in pregnancy is advisable to further reduce the risk.
Interrupt heparin to permit regional anesthesia
Heparin therapy should be temporarily stopped during the immediate peripartum interval to minimize the risk of hemorrhage and to permit regional anesthesia. Because of the theoretical risk of paraspinal hemorrhage in women receiving heparin who undergo epidural or spinal anesthesia, many anesthetists will not perform neuraxial regional anesthesia in women who have recently received heparin.
Since unfractionated heparin has a relatively short duration of action, the American Society of Regional Anesthesia states that subcutaneous unfractionated heparin prophylaxis is not a contraindication to neuraxial regional anesthesia.31 However, LMWHs should be stopped for at least 12 to 24 hours before regional anesthesia can be considered safe. This issue is discussed in more detail in the section on peripartum and postpartum management of anticoagulation, below.
In summary, LMWH during pregnancy offers a number of advantages over unfractionated heparin: equivalent efficacy, once- or twice-daily dosing, lower risk of heparin-induced thrombocytopenia and osteoporosis, and less-intensive monitoring. Unfractionated heparin can be offered to women who cannot afford LMWH (which costs four to five times more), and it may be used peripartum to reduce hemorrhagic risk and to permit regional anesthesia.
COUMARINS
Coumarins are the mainstay of anticoagulant therapy in most nonpregnant women beyond the immediate thrombotic period.
Warfarin (Coumadin) is the most widely used coumarin because it has a predictable onset and duration of action and excellent bioavailability.32 Others, such as acenocoumarol (Sintrom) and phenprocoumon (Marcoumar), are used more outside the United States but can be ordered or brought into the United States.
Coumarins interfere with vitamin K metabolism, inhibiting the generation of vitamin-K-dependent procoagulant proteins (factors II, VII, IX, and X) and thereby preventing clotting. They also inhibit the formation of the vitamin-K-dependent intrinsic anticoagulant proteins C and S.
Major bleeding is the most significant side effect of coumarin therapy, occurring at a rate of 4% to 6% over 3 months when the prothrombin time is maintained at an international normalized ratio (INR) of 2 to 3,33 and more often if the INR is higher.
Other issues with warfarin are the effect of variations in dietary vitamin K intake on anticoagulation and potential drug interactions that may alter the anticoagulant effect. Thus, the INR needs to be monitored closely.
Risks to the fetus and the mother
Unlike the heparins, coumarins freely cross the placenta and thus pose a risk of teratogenicity. A cluster of fetal malformations including “warfarin embryopathy” (nasal bone hypoplasia and chondrodysplasia punctata) can occur when the drug is used between 6 and 12 weeks of gestation. Warfarin embryopathy may be avoided by stopping warfarin prior to 6 weeks from the onset of the last menstrual period (ie, 6-week “menstrual age” or 4-week gestational age34).
Later in pregnancy, warfarin is associated with potential fetal bleeding complications leading to central nervous system abnormalities, increased rates of intrauterine fetal death, and pregnancy loss. In pregnant women with mechanical cardiac valve prostheses who received oral anticoagulants throughout pregnancy, the incidence of congenital anomalies was 6.4% to 10.2%.35 Fetal demise (spontaneous abortion, stillbirth, neonatal death) was also very common (29.7% to 33.6% of pregnancies) in coumarin-treated women.
Severe maternal hemorrhage may also occur in pregnant women on oral anticoagulants, particularly those who remain fully anticoagulated around the time of labor and delivery.
General caveats to warfarin in pregnancy
Because of the many maternal and fetal concerns, oral anticoagulant use in pregnancy is largely restricted to women with older-generation prosthetic heart valves in whom the very high maternal thrombotic risk may outweigh the risk of maternal and fetal side effects.
While there are limited data on warfarin use in pregnant women with antiphospholipid syndrome,36 warfarin use in such patients should be considered only for those at highest risk and with careful informed consent. These issues are discussed further below in the section on mechanical heart valve prostheses.
ANTIPLATELET DRUGS
Aspirin is an antiplatelet agent rather than an anticoagulant. Although considered inadequate for preventing venous thrombosis in high-risk groups when used alone, aspirin can moderately reduce the risk of deep venous thrombosis and pulmonary embolism in nonpregnant patients.37 It also has a well-accepted role in preventing arterial thrombotic events, ie, coronary artery disease and stroke.38
Low-dose aspirin (≤ 100 mg/day) has been extensively evaluated during pregnancy39–41 and has been shown to be safe and effective in reducing the risk of preeclampsia in high-risk women39 and in treating women with antiphospholipid antibodies and recurrent pregnancy loss42 (in conjunction with prophylactic doses of heparin). Although higher doses of aspirin and other nonsteroidal anti-inflammatory drugs can be toxic to the fetus, low doses have been shown to be safe throughout pregnancy.43
Dipyridamole (Persantine) has been studied extensively in pregnancy, and while it appears to be safe, it has not found a well-defined therapeutic role.
Other antiplatelet drugs have been only rarely used, and data on their safety and efficacy during pregnancy are limited to case reports, for example, on ticlopidine44 (Ticlid) and clopidogrel45,46 (Plavix) given during pregnancy in women with cardiac disease. These drugs do not appear to be major teratogens or to cause specific fetal harm. Their use may be reasonable in some high-risk situations, such as recurrent thrombotic stroke despite aspirin therapy. They may be used alone or with other anticoagulants in women with a coronary or other vascular stent if fetal safety is uncertain or if there is an increased risk of maternal bleeding.
NEWER ANTICOAGULANTS
Danaparoid
The heparinoid danaparoid (Orgaran) is an LMWH, a combination of heparan, dermatan, and chondroitin sulfate. Since it is derived from heparin, in theory it can cross-react with antiheparin antibodies, but this is generally not a problem. Danaparoid inhibits factor Xa, and monitoring is via measurement of anti-factor-Xa activity levels. It has been shown to be safe and effective in nonpregnant patients with heparin-induced thrombocytopenia.51
Although no controlled study has been published on danaparoid in pregnancy, at least 51 pregnancies in 49 patients treated with danaparoid have been reported.52 Thirty-two of the patients received danaparoid because of heparin-induced thrombocytopenia and 19 because of heparin-induced skin intolerance. These reports suggest that danaparoid does not cross the placenta53 and that it may be effective and safe during pregnancy.54 For this reason, it is probably the preferred anticoagulant in pregnant patients with heparin-induced thrombocytopenia or other serious reactions to heparin.
Unfortunately, danaparoid has two major disadvantages. First, it has a prolonged half-life and no effective reversing agent, which makes its use problematic close to the time of delivery. Second, and perhaps more relevant to this discussion, it is not readily available in the United States; it was removed from the market by its manufacturer in April 2002 for business reasons rather than because of concerns over toxicity. It is still available in Canada and Europe, and it can be obtained in special circumstances in the United States via the US Food and Drug Administration (FDA); this may be worthwhile in pregnant patients who require a nonurgent alternative to heparin.
Direct thrombin inhibitors
Lepirudin (Refludan), bivalirudin (Angiomax), and argatroban are direct thrombin inhibitors and exert their anticoagulant effect independently of antithrombin. They are given by continuous intravenous infusion, and they have a very short half-life.
Lepirudin and argatroban are typically monitored via the aPTT. Bivalirudin can be monitored with the activated clotting time, partial thromboplastin time, or INR, depending on the circumstances. None of these agents generates or cross-reacts with antibodies generated in heparin-induced thrombocytopenia. None has an antidote, but the short half-life usually obviates the need for one.
Unfortunately, pregnancy data are very sparse for all three of these new agents. Argatroban has a low molecular weight and likely crosses the placenta. Also, because these agents are given intravenously, they are not practical for long-term use in pregnancy.
Fondaparinux
Fondaparinux (Arixtra), a direct factor Xa inhibitor, binds to antithrombin, causing an irreversible conformational change that increases antithrombin’s ability to inactivate factor Xa (as do the heparins). It has no effect on factor IIa (thrombin) and does not predictably affect the aPTT. Its half-life is 17 hours, and no agent is known to reverse its anticoagulant effect, although some experts would recommend a trial of high-dose recombinant factor VIIa (Novo-Seven) in uncontrolled hemorrhage.
While not FDA-approved for treating heparin-induced thrombocytopenia, it has been used for this in some patients.55–58 Animal studies and in vitro human placental perfusion studies suggest that fondaparinux does not cross the placenta in significant amounts.49 Since danaparoid is not available in the United States, fondaparinux would likely be the first choice among the newer anticoagulants when treating heparin-induced thrombocytopenia in pregnancy.
INDICATIONS FOR ANTICOAGULANTS DURING PREGNANCY
Acute deep venous thrombosis and pulmonary embolism
Anticoagulant therapy should begin as full doses of either LMWH or intravenous unfractionated heparin. We prefer starting with LMWH, as it can be started rapidly with less need for nursing care (eg, no need to start and maintain an intravenous line and monitor the aPTT) and has excellent safety. If LMWH is selected, initial dosing should be based on the current weight (Table 2). Subsequent monitoring of the peak anti-factor-Xa activity levels (ie, 4 hours after the dose) is recommended, with the first level drawn in the first few days of treatment, and repeat levels every 1 to 3 months for the rest of treatment. As mentioned earlier, weight-based dosing has not been systematically evaluated in pregnancy.
If unfractionated heparin is the initial agent, it should be given as a bolus followed by a continuous infusion, ideally utilizing a weight-based nomogram to estimate required doses, with adjustment of the infusion rate to maintain the aPTT at 1.5 to 2.5 times the baseline value (obtained during pregnancy). After several days, the heparin may be switched to LMWH in therapeutic doses (Table 2).
Alternatively, in women approaching term or who cannot afford LMWH, anticoagulation may be continued as adjusted-dose subcutaneous unfractionated heparin, ie, two or three large daily doses of subcutaneous heparin to provide therapeutic levels of anticoagulation. The starting dose can be calculated as the total units of heparin required to maintain full anticoagulation intravenously over 24 hours, given as two or three divided doses (Table 2). The aPTT at the mid-dosing interval (eg, 6 hours after the subcutaneous dose during every-12-hour dosing) should be monitored and the dose adjusted to maintain the aPTT at 1.5 to 2.5 times the baseline value.
A therapeutic level of anticoagulation should be maintained for at least 3 months after an acute thrombotic event during pregnancy, though many physicians prefer to continue full anticoagulation for a total of 6 months. Beyond this interval, if the woman is still pregnant, the anticoagulation may be reduced in intensity, perhaps even to a prophylactic level for the duration of the pregnancy (see discussion below on prior venous thromboembolic events) (Table 2). Peripartum and postpartum anticoagulation are discussed further below.
PRIOR VENOUS THROMBOEMBOLIC EVENT
While all pregnant women are at higher risk of venous thrombosis, the overall incidence of thromboembolism is only about one event per 1,000 pregnancies. Routine thromboprophylaxis in all pregnant women is therefore not justified. However, women who have previously had a venous thromboembolic event are at a substantially higher risk of recurrent thrombosis and should be considered for thromboprophylaxis in all subsequent high-risk situations, including pregnancy.
For women on indefinite therapeutic anticoagulation (ie, because of recurrent thrombosis), full therapeutic anticoagulation with LMWH or adjusted-dose unfractionated heparin should be maintained throughout pregnancy, as described above.
Which other women should receive prophylactic anticoagulation is a topic of ongoing debate and controversy.
How great is the risk of recurrent thromboembolism?
A small observational study59 examined the risk of recurrent venous thromboembolism during subsequent pregnancies in women with a prior thrombotic event. Anticoagulation was withheld during the antepartum period and restarted briefly after delivery. Among the 125 women enrolled, recurrent venous thromboembolism occurred in 4.8%, with half of the events occurring during the antepartum period. Among those with underlying thrombophilia, the rate of recurrent venous thromboembolism was 13% (95% confidence interval [CI] 1.7%–40.5%) to 20% (95% CI 2.5%–56.5%), and those with a prior idiopathic clot without thrombophilia had an event rate of 7.7% (95% CI 0.01%–25.1%). The subgroup with a prior reversible risk factor (at the time of their initial venous thromboembolic event) and without detectable thrombophilia had no recurrent events.
This study suggests that women with prior venous thromboembolism and thrombophilia or a prior idiopathic thrombotic event are at a substantial risk of recurrent thrombotic events during pregnancy. And other data confirm the high risk of recurrent venous thromboembolism in thrombophilic pregnant women.60 These women should all be offered active antepartum and postpartum thromboprophylaxis with LMWH or unfractionated heparin (Tables 2 and 4). Women without thrombophilia but with a history of venous thromboembolism related to pregnancy or oral contraceptive use also have a substantial risk of recurrent venous thrombosis and should be offered antepartum and postpartum thromboprophylaxis.61 In contrast, women with a prior “secondary” clot, no thrombophilia, and no additional current risk factors (Table 1) appear to be at low risk of recurrent venous thromboembolism.
The risks should be discussed with these women, with an option for close clinical surveillance during pregnancy (Table 4), but with a low threshold to investigate any worrisome symptoms. Such women may also elect to take LMWH or unfractionated heparin during pregnancy.
Which heparin to use?
Prophylactic anticoagulation during pregnancy can be with either LMWH or unfractionated heparin. For most women this involves “prophylactic” dosing with the goal of maintaining a mid-interval anti-factor-Xa activity level of approximately 0.05 to 0.2 U/mL. Thromboprophylaxis with LMWH can be with lower, fixed, once-daily doses throughout pregnancy20 (Table 2), although some clinicians still prefer twice-daily dosing. The heparin should be started as soon as pregnancy is confirmed, as the pregnancy-associated increase in thrombotic risk begins by the middle of the first trimester.
To maintain effective prophylactic levels, the dose of unfractionated heparin should be increased sequentially over the trimesters62,63: approximately 5,000 units subcutaneously twice daily in the first trimester, then 7,500 units twice daily in the second trimester, and 10,000 units twice daily in the third trimester for a woman of average size.
When to add low-dose aspirin
Women with antiphospholipid antibodies, particularly those with prior recurrent pregnancy loss or fetal demise, should receive aspirin 81 mg/day in addition to heparin.39 The aspirin may be started prior to conception or when pregnancy is confirmed.
Other measures
Women on anticoagulant therapy who are at risk of recurrent venous thromboembolism should be encouraged to wear elastic compression stockings. Intermittent pneumatic compression of the legs via automated devices may be considered for women hospitalized for any reason or on bedrest.
Whichever measures are used, a high index of suspicion and a low threshold for investigating for recurrent thrombosis should be maintained throughout pregnancy and the puerperium.
PERIPARTUM AND POSTPARTUM MANAGEMENT OF ANTICOAGULATION
Heparin therapy must be interrupted temporarily during the immediate peripartum interval to minimize the risk of hemorrhage and to allow for the option of regional anesthesia. As mentioned earlier, because of the theoretical risk of paraspinal hemorrhage in women receiving heparin who undergo epidural or spinal anesthesia, the American Society of Regional Anesthesia guidelines advise waiting to insert the needle at least 10 to 12 hours after the last prophylactic dose of LMWH, and at least 24 hours after the last therapeutic dose.31
The guidelines state that neuraxial anesthesia is not contraindicated in patients on prophylactic unfractionated heparin.31
To facilitate use of regional anesthesia in these women, therefore, options include:
- Electively stopping LMWH 24 hours before planned induction of labor
- Electively stopping prophylactic-dose LMWH or unfractionated heparin at about 38 weeks of gestation, to await spontaneous labor, or
- Switching therapeutic or prophylactic LMWH to unfractionated heparin at about 36 weeks of gestation, with instructions to discontinue the injections in the earliest stages of spontaneous labor. This aims to shorten the heparin-free period required before neuraxial anesthesia while minimizing maternal thrombotic risk.
Additional advantages to using unfractionated heparin peripartum include the option of obtaining a rapid aPTT measurement to confirm the absence of a significant ongoing heparin effect prior to regional anesthesia or delivery, and the ability to completely reverse the heparin effect with protamine sulfate if major bleeding occurs. LMWHs are only partially reversible.64
Interrupting anticoagulation after an initial thrombotic event
If therapeutic anticoagulation must be interrupted for labor within 1 month of the initial thrombotic event, the risk of recurrent thrombotic complications is high65; these women must be observed very carefully and may benefit from intravenous heparin before and after delivery. They may even merit placement of a temporary vena cava filter (particularly if less than 2 weeks have elapsed since the venous thromboembolic event and in women with a large deep venous clot burden), a procedure that has been used safely but little studied in pregnant women.66
Fluoroscopic guidance may be needed for filter placement. This exposes the fetus to radiation, but the low-level exposure at this late gestational age is unlikely to pose a significant risk. The filter may be removed within 1 to 2 weeks postpartum, assuming there are no ongoing contraindications to anticoagulation.
In the rare woman with antithrombin deficiency and a recent or prior thrombotic event, giving antithrombin concentrate during the peripartum (heparin-free) interval has been described and may be considered under the guidance of a hematologist.67
Ongoing anticoagulation is essential postpartum, as the puerperium is the period of highest day-to-day risk of thromboembolic events: about one-third of pregnancy-associated events occur during these 6 to 12 weeks.2 Heparin should be resumed 6 to 12 hours after delivery, once hemostasis is confirmed.
Options for women requiring ongoing therapeutic anticoagulation include intravenous heparin started without a bolus, to minimize bleeding risk, with aPTT measured 12 hours later, or an initial prophylactic dose of LMWH 6 to 12 hours postpartum, with therapeutic dosing resumed on postpartum day 1. If prophylactic dosing is desired, unfractionated heparin or LMWH may be given subcutaneously starting at about 6 hours postpartum.
Warfarin in the puerperium
Women may subsequently be maintained on either LMWH or unfractionated heparin, or switched to an oral anticoagulant such as warfarin. Although warfarin may appear in minute amounts in breast milk, it has not been associated with adverse events in newborns and is considered compatible with breastfeeding.68 Heparin should be continued during the initial days of warfarin therapy, until the INR is at a therapeutic level for 24 hours. Some physicians prefer to delay warfarin for several days, giving LMWH alone in the immediate postpartum period, to allow wound-healing and to reduce bleeding risk.
Postpartum, anticoagulation should be continued for at least 6 to 12 weeks, at which point the physiologic changes in the coagulation system related to pregnancy will have returned to normal.
THROMBOPHILIA WITHOUT A PREVIOUS THROMBOEMBOLIC EVENT
Over the last 5 to 10 years, practitioners have been seeing many more young women with genetic or acquired thrombophilias who have never had a venous thromboembolic event. Physicians must advise these women about their risk of thromboembolic events during pregnancy and about the appropriateness of anticoagulant use.
Thrombophilias are often detected in women who develop venous thrombosis during pregnancy,69–71 but they are also very common in the general population (around 15%). While women with thrombophilia are at above-average risk of venous thromboembolism during pregnancy, the magnitude of risk in an individual patient is often difficult to estimate.
Data suggest that some types of thrombophilia confer greater thrombotic risk than others. McColl et al72 derived risk estimates for a primary event in women with several of the disorders: 0.23% in women heterozygous for the factor V Leiden mutation, 0.88% in women with protein C deficiency, and 2.4% to 35.7% in women with antithrombin deficiency. A case-control study70 found that all thrombophilic states were more common in women with pregnancy-associated venous thromboembolism than in healthy pregnant controls, except those with the MTHFR mutation and protein S deficiency. The estimated risk during pregnancy was 0.03% in women with no defect, 0.1% in women with protein C deficiency, 0.25% in women with the factor V Leiden mutation, 0.4% in those with antithrombin deficiency, 0.5% in those with the prothrombin gene mutation, and 4.6% in those with both factor V Leiden and prothrombin gene mutations.
Routine anticoagulation not advised in pregnant thrombophilic women
Because the risk of a primary venous thromboembolic event is less than 1% for most thrombophilic women, routine anticoagulant therapy does not seem prudent for this indication. Given the low absolute risk of venous thromboembolism, the cost and potential side effects of anticoagulant use are difficult to justify.
The women who seem at higher risk and in whom anticoagulation should be considered include those with antithrombin deficiency; those with high-titer anticardiolipin antibodies or a lupus anticoagulant antibody (treat with heparin and low-dose aspirin); those with combined thrombophilic defects or who are homozygotes for the factor V Leiden or prothrombin gene mutations; and those with multiple other current risk factors for venous thromboembolism (Table 1).
Since anticoagulants for primary prevention of adverse pregnancy outcomes in thrombophilic women have not yet been shown to have a definitive benefit, they are not recommended for this purpose.
ADVERSE PREGNANCY OUTCOMES IN WOMEN WITH THROMBOPHILIAS
Women with antiphospholipid antibodies and a previous poor obstetric outcome are clearly at increased risk of recurrent adverse pregnancy outcomes such as recurrent spontaneous abortion, unexplained fetal death, placental insufficiency, and early or severe preeclampsia. In such women who have both antiphospholipid antibodies and a history of venous thromboembolism or adverse pregnancy outcome, treatment during subsequent pregnancy with low-dose aspirin and prophylactic-dose LMWH or unfractionated heparin improves pregnancy outcomes.36–42 Women with antiphospholipid antibodies without previous thrombosis or pregnancy complications may also be at increased risk, but it is unclear whether thromboprophylaxis improves their outcomes.
Recent epidemiologic data reveal that women with other thrombophilic conditions also are at increased risk of early, severe preeclampsia73 as well as other pregnancy complications, including recurrent pregnancy loss, placental abruption, fetal growth restriction, and stillbirth.74 A recent meta-analysis75 looked at individual thrombophilias and found that factor V Leiden and prothrombin gene mutations were associated with recurrent fetal loss, stillbirth, and preeclampsia; that protein S deficiency was associated with recurrent fetal loss and stillbirth; that antiphospholipid antibodies were associated with recurrent pregnancy loss, preeclampsia, and intrauterine growth restriction; that the MTHFR mutation (homozygous) was associated with preeclampsia; and that protein C and antithrombin deficiencies were not significantly associated with adverse pregnancy outcomes. Data were scant for some of the rarer thrombophilias.75
Several recent small studies76–78 suggest that anticoagulants may improve pregnancy outcomes in women with genetic thrombophilias and recurrent pregnancy loss. These findings have not yet been confirmed in high-quality clinical trials, but such trials are under way. It is still unclear whether anticoagulants also reduce the risk of other adverse pregnancy outcomes associated with thrombophilias.
The current American College of Chest Physicians guidelines recommend testing of women with adverse pregnancy outcomes (recurrent pregnancy loss, prior severe or recurrent preeclampsia, abruptions, or otherwise unexplained intrauterine death) for congenital thrombophilias and antiphospholipid antibodies, and offering treatment to such women, if thrombophilic, with low-dose aspirin plus prophylactic heparin (unfractionated or LMWH).22 The authors of the guidelines admit that the evidence for this recommendation is weak, but they argue that the heparin will also serve as thromboprophylaxis in this high-risk group. Hopefully, the randomized clinical trials currently under way will provide clearer guidance regarding the most appropriate therapy in this difficult clinical situation.
MECHANICAL HEART VALVES
Internists may occasionally encounter a woman with a mechanical heart valve prosthesis who is either pregnant or is planning a pregnancy and therefore needs advice regarding optimal anticoagulant management. This should generally be undertaken in a multi-disciplinary fashion, with input from cardiology, hematology, and maternal-fetal medicine. The substantial maternal and fetal risks and the lack of definitive data on which to base treatment decisions make it a treacherous and stressful undertaking. Nonetheless, all internists should have a basic understanding of the complex issues regarding this management.
Outside of pregnancy, oral anticoagulants are the mainstay of therapy for patients with mechanical heart valves. Unfortunately, as discussed above, the use of these agents during pregnancy carries a risk of teratogenicity and toxic fetal effects and increases the risk of pregnancy loss and maternal hemorrhage. Heparins have been used in this setting for many years, but data on their efficacy and safety are very limited, and there are numerous reports of catastrophic maternal thrombotic complications.79,80
A systematic review of anticoagulation in pregnant women with prosthetic heart valves34 found very limited data on heparin use throughout pregnancy. Women maintained on warfarin vs heparin between pregnancy weeks 6 and 12 had higher rates of congenital anomalies (6.4% with warfarin vs 3.4% with heparin) and total fetal wastage (33.6% vs 26.5%). The warfarin group had fewer maternal thromboembolic complications (3.9% vs 9.2%), however, and a slightly lower rate of maternal death (1.8% vs 4.2%). Most of the women had higher-risk older-generation valves in the mitral position.
Recent data on LMWH consist mainly of case reports and case series,81 with a likely bias to publication of worse outcomes. Controlled trials in this area will be difficult to conduct. Still, aggressive anticoagulation with LMWH or unfractionated heparin, with close monitoring of the intensity of anticoagulation, may be safe and effective for pregnant women with newer-generation mechanical heart valves.82 A recent consensus statement22 suggested several regimens for pregnant women with mechanical heart valves:
- Twice-daily LMWH throughout pregnancy, with the dose adjusted either by weight, or to keep the 4-hour postinjection anti-factor-Xa activity level around 1.0 to 1.2 U/mL
- Aggressive adjusted-dose unfractionated heparin throughout pregnancy, given subcutaneously every 12 hours and adjusted to keep the mid-interval aPTT at least twice the control value or to attain a mid-interval anti-factor-Xa activity level of 0.35 to 0.70 U/mL
- Unfractionated heparin or LMWH (as above) until gestation week 13, then warfarin until the middle of the third trimester, and then heparin again.22
The authors also recommended adding low-dose aspirin (75–162 mg/day) in high-risk women.22
These options all seem reasonable, given our current knowledge, though warfarin use during pregnancy should be restricted to very-high-risk situations, such as women with older-generation mitral prostheses. LM-WHs may become the preferred therapy for this indication once further controlled data regarding their efficacy and safety become available.
Anticoagulation is essential in a wide variety of conditions in women of child-bearing age. Some, such as venous thromboembolism, occur more often during pregnancy. Others, such as recurrent fetal loss in the setting of antiphospholipid antibodies, are specific to pregnancy.
While anticoagulants are useful in many circumstances, their use during pregnancy increases the risk of hemorrhage and other adverse effects on the mother and the fetus. Treatment with anticoagulants during pregnancy must therefore be carefully considered, with judicious selection of the agent, and with reflection on the physiologic changes of pregnancy to ensure appropriate dosing. In this article, we review these issues.
WHY IS THROMBOTIC RISK HIGHER DURING PREGNANCY?
Venous thromboembolism is among the leading causes of maternal death in developed countries.1–3 Modern care has dramatically reduced the risk of maternal death from hemorrhage, infection, and hypertension, but rates of morbidity and death from thrombosis have remained stable or increased in recent years.4
- Much higher levels of fibrinogen and factors VII, VIII, IX, and X
- Lower levels of protein S and increased resistance to activated protein C
- Impaired fibrinolysis, due to inhibitors derived from the placenta.
Acquired antithrombin deficiency may also occur in high-proteinuric states such as nephrotic syndrome or preeclampsia, further increasing thrombotic risk. Pooling of venous blood, caused by progesterone-mediated venous dilation and compounded by compression of the inferior vena cava by the uterus in later pregnancy, also increases thrombotic risk. And endothelial disruption of the pelvic vessels may occur during delivery, particularly during cesarean section.
Additional factors that increase thrombotic risk include immobilization, such as bed rest for pregnancy complications; surgery, including cesarean section; ovarian hyperstimulation during gonadotropin use for in vitro fertilization; trauma; malignancy; and hereditary or acquired hypercoagulable states.6 These hypercoagulable states include deficiencies of antithrombin or the intrinsic anticoagulant proteins C or S; resistance to activated protein C, usually due to the factor V Leiden mutation; the PT20210A mutation of the prothrombin gene; hyperhomocystinemia due to mutation of the methyltetrahydrofolate reductase (MTHFR) gene; and the sustained presence of antiphospholipid antibodies, including lupus anticoagulant antibodies, sometimes also with moderately high titers of anticardiolipin or beta-2-glycoprotein I antibodies.
Other conditions that increase thrombotic risk include hyperemesis gravidarum, obesity, inflammatory bowel disease, infection, smoking, and indwelling intravenous catheters.6 Given the multitude of risk factors, pregnant women have a risk of thrombotic complications three to five times higher than nonpregnant women.7
HEPARIN USE DURING PREGNANCY
Low-molecular-weight heparins (LMWHs)8 and unfractionated heparin bind to anti-thrombin and thus change the shape of the antithrombin molecule, dramatically increasing its interaction with the clotting factors Xa and prothrombin (factor II). The enhanced clearance of these procoagulant proteins leads to the anticoagulant effect. Unfractionated heparin has roughly equivalent interaction with factors Xa and II and prolongs the activated partial thromboplastin time (aPTT), which is therefore used to monitor the intensity of anticoagulation.
LMWHs, on the other hand, interact relatively little with factor II and do not predictably prolong the aPTT. Monitoring their effect is therefore more difficult and requires direct measurement of anti-factor-Xa activity. This test is widely available, but it is time-consuming (it takes several hours and results may not be available within 24 hours if the test is requested “after hours”), and therefore it is of limited use in the acute clinical setting. While weight-based dosing of LMWHs is reliable and safe in nonpregnant patients, it has not yet been validated for pregnant women.
Unfractionated heparin has been used for decades for many indications during pregnancy. It is a large molecule, so it does not cross the placenta and thus, in contrast to the coumarin derivatives, does not cause teratogenesis or toxic fetal effects. Its main limitations in pregnancy are its inconvenient dosing (at least twice daily when given subcutaneously) and its potential maternal adverse effects (mainly osteoporosis and heparin-induced thrombocytopenia).
Over the last 10 years LMWHs have become the preferred anticoagulants for treating and preventing thromboembolism in all patients. They are equivalent or superior to unfractionated heparin in efficacy and safety in the initial treatment of acute deep venous thrombosis9,10 and pulmonary embolism11,12 outside of pregnancy. While comparative data are much less robust in pregnant patients, several series have confirmed the safety and efficacy of LMWHs in pregnancy.13–15 LMWHs do not cross the placenta15–17 and thus have a fetal safety profile equivalent to that of unfractionated heparin.
Pregnancy alters metabolism of LMWHs
The physiologic changes of pregnancy alter the metabolism of LMWH, resulting in lower peak levels and a higher rate of clearance,18,19 and so a pregnant woman may need higher doses or more frequent dosing.
Recent evidence suggests that thromboprophylaxis can be done with lower, fixed, once-daily doses of LMWH throughout pregnancy,20 although some clinicians still prefer twice-daily dosing (particularly during the latter half of pregnancy).
Pending more research on weight-based dosing of LMWH in pregnancy, anti-factor- Xa activity levels should be measured after treatment is started and every 1 to 3 months thereafter during pregnancy.21 Doses should be adjusted to keep the peak anti-Xa level (ie, 4 hours after the dose) at 0.5 to 1.2 U/mL.22
Heparin-induced thrombocytopenia
Type-2 heparin-induced thrombocytopenia is an uncommon but serious adverse effect of unfractionated heparin therapy (and, less commonly of LMWH), caused by heparin-dependent immunoglobulin G (IgG) antibodies that activate platelets via their Fc receptors, potentially precipitating life-threatening arterial or venous thrombosis.
In a trial in nonpregnant orthopedic patients,23 clinical heparin-induced thrombocytopenia occurred in 2.7% of patients receiving unfractionated heparin vs 0% of those receiving LMWH; heparin-dependent IgG was present in 7.8% vs 2.2%, respectively.
Fortunately, heparin-induced thrombocytopenia seems to be very rare in pregnancy: two recent prospective series evaluating prolonged LMWH use in pregnancy13,15 revealed no episodes of this disease. Nonetheless, it is reasonable to measure the platelet count once or twice weekly during the first few weeks of LMWH use and less often thereafter, unless symptoms of heparin-induced thrombocytopenia develop. In pregnant women with heparin-induced thrombocytopenia or heparin-related skin reactions, other anticoagulants must be considered24 (see discussion later).
Heparin-induced osteoporosis
Heparin-induced osteoporosis, a potential effect of prolonged heparin therapy, is of concern, given the prolonged duration and high doses of unfractionated heparin often needed to treat venous thromboembolism during pregnancy. Several studies found significant loss of bone mineral density in the proximal femur25 and lumbar spine26 during extended use of unfractionated heparin in pregnancy.
Fortunately, LMWH appears to be much safer with respect to bone loss. Three recent studies27–30 evaluated the use of LMWH for extended periods during pregnancy, and none found any greater loss of bone mineral density than that seen in normal pregnant controls. Giving supplemental calcium (1,000–1,500 mg/day) and vitamin D (400–1,000 IU/day) concomitantly with unfractionated heparin or LMWH in pregnancy is advisable to further reduce the risk.
Interrupt heparin to permit regional anesthesia
Heparin therapy should be temporarily stopped during the immediate peripartum interval to minimize the risk of hemorrhage and to permit regional anesthesia. Because of the theoretical risk of paraspinal hemorrhage in women receiving heparin who undergo epidural or spinal anesthesia, many anesthetists will not perform neuraxial regional anesthesia in women who have recently received heparin.
Since unfractionated heparin has a relatively short duration of action, the American Society of Regional Anesthesia states that subcutaneous unfractionated heparin prophylaxis is not a contraindication to neuraxial regional anesthesia.31 However, LMWHs should be stopped for at least 12 to 24 hours before regional anesthesia can be considered safe. This issue is discussed in more detail in the section on peripartum and postpartum management of anticoagulation, below.
In summary, LMWH during pregnancy offers a number of advantages over unfractionated heparin: equivalent efficacy, once- or twice-daily dosing, lower risk of heparin-induced thrombocytopenia and osteoporosis, and less-intensive monitoring. Unfractionated heparin can be offered to women who cannot afford LMWH (which costs four to five times more), and it may be used peripartum to reduce hemorrhagic risk and to permit regional anesthesia.
COUMARINS
Coumarins are the mainstay of anticoagulant therapy in most nonpregnant women beyond the immediate thrombotic period.
Warfarin (Coumadin) is the most widely used coumarin because it has a predictable onset and duration of action and excellent bioavailability.32 Others, such as acenocoumarol (Sintrom) and phenprocoumon (Marcoumar), are used more outside the United States but can be ordered or brought into the United States.
Coumarins interfere with vitamin K metabolism, inhibiting the generation of vitamin-K-dependent procoagulant proteins (factors II, VII, IX, and X) and thereby preventing clotting. They also inhibit the formation of the vitamin-K-dependent intrinsic anticoagulant proteins C and S.
Major bleeding is the most significant side effect of coumarin therapy, occurring at a rate of 4% to 6% over 3 months when the prothrombin time is maintained at an international normalized ratio (INR) of 2 to 3,33 and more often if the INR is higher.
Other issues with warfarin are the effect of variations in dietary vitamin K intake on anticoagulation and potential drug interactions that may alter the anticoagulant effect. Thus, the INR needs to be monitored closely.
Risks to the fetus and the mother
Unlike the heparins, coumarins freely cross the placenta and thus pose a risk of teratogenicity. A cluster of fetal malformations including “warfarin embryopathy” (nasal bone hypoplasia and chondrodysplasia punctata) can occur when the drug is used between 6 and 12 weeks of gestation. Warfarin embryopathy may be avoided by stopping warfarin prior to 6 weeks from the onset of the last menstrual period (ie, 6-week “menstrual age” or 4-week gestational age34).
Later in pregnancy, warfarin is associated with potential fetal bleeding complications leading to central nervous system abnormalities, increased rates of intrauterine fetal death, and pregnancy loss. In pregnant women with mechanical cardiac valve prostheses who received oral anticoagulants throughout pregnancy, the incidence of congenital anomalies was 6.4% to 10.2%.35 Fetal demise (spontaneous abortion, stillbirth, neonatal death) was also very common (29.7% to 33.6% of pregnancies) in coumarin-treated women.
Severe maternal hemorrhage may also occur in pregnant women on oral anticoagulants, particularly those who remain fully anticoagulated around the time of labor and delivery.
General caveats to warfarin in pregnancy
Because of the many maternal and fetal concerns, oral anticoagulant use in pregnancy is largely restricted to women with older-generation prosthetic heart valves in whom the very high maternal thrombotic risk may outweigh the risk of maternal and fetal side effects.
While there are limited data on warfarin use in pregnant women with antiphospholipid syndrome,36 warfarin use in such patients should be considered only for those at highest risk and with careful informed consent. These issues are discussed further below in the section on mechanical heart valve prostheses.
ANTIPLATELET DRUGS
Aspirin is an antiplatelet agent rather than an anticoagulant. Although considered inadequate for preventing venous thrombosis in high-risk groups when used alone, aspirin can moderately reduce the risk of deep venous thrombosis and pulmonary embolism in nonpregnant patients.37 It also has a well-accepted role in preventing arterial thrombotic events, ie, coronary artery disease and stroke.38
Low-dose aspirin (≤ 100 mg/day) has been extensively evaluated during pregnancy39–41 and has been shown to be safe and effective in reducing the risk of preeclampsia in high-risk women39 and in treating women with antiphospholipid antibodies and recurrent pregnancy loss42 (in conjunction with prophylactic doses of heparin). Although higher doses of aspirin and other nonsteroidal anti-inflammatory drugs can be toxic to the fetus, low doses have been shown to be safe throughout pregnancy.43
Dipyridamole (Persantine) has been studied extensively in pregnancy, and while it appears to be safe, it has not found a well-defined therapeutic role.
Other antiplatelet drugs have been only rarely used, and data on their safety and efficacy during pregnancy are limited to case reports, for example, on ticlopidine44 (Ticlid) and clopidogrel45,46 (Plavix) given during pregnancy in women with cardiac disease. These drugs do not appear to be major teratogens or to cause specific fetal harm. Their use may be reasonable in some high-risk situations, such as recurrent thrombotic stroke despite aspirin therapy. They may be used alone or with other anticoagulants in women with a coronary or other vascular stent if fetal safety is uncertain or if there is an increased risk of maternal bleeding.
NEWER ANTICOAGULANTS
Danaparoid
The heparinoid danaparoid (Orgaran) is an LMWH, a combination of heparan, dermatan, and chondroitin sulfate. Since it is derived from heparin, in theory it can cross-react with antiheparin antibodies, but this is generally not a problem. Danaparoid inhibits factor Xa, and monitoring is via measurement of anti-factor-Xa activity levels. It has been shown to be safe and effective in nonpregnant patients with heparin-induced thrombocytopenia.51
Although no controlled study has been published on danaparoid in pregnancy, at least 51 pregnancies in 49 patients treated with danaparoid have been reported.52 Thirty-two of the patients received danaparoid because of heparin-induced thrombocytopenia and 19 because of heparin-induced skin intolerance. These reports suggest that danaparoid does not cross the placenta53 and that it may be effective and safe during pregnancy.54 For this reason, it is probably the preferred anticoagulant in pregnant patients with heparin-induced thrombocytopenia or other serious reactions to heparin.
Unfortunately, danaparoid has two major disadvantages. First, it has a prolonged half-life and no effective reversing agent, which makes its use problematic close to the time of delivery. Second, and perhaps more relevant to this discussion, it is not readily available in the United States; it was removed from the market by its manufacturer in April 2002 for business reasons rather than because of concerns over toxicity. It is still available in Canada and Europe, and it can be obtained in special circumstances in the United States via the US Food and Drug Administration (FDA); this may be worthwhile in pregnant patients who require a nonurgent alternative to heparin.
Direct thrombin inhibitors
Lepirudin (Refludan), bivalirudin (Angiomax), and argatroban are direct thrombin inhibitors and exert their anticoagulant effect independently of antithrombin. They are given by continuous intravenous infusion, and they have a very short half-life.
Lepirudin and argatroban are typically monitored via the aPTT. Bivalirudin can be monitored with the activated clotting time, partial thromboplastin time, or INR, depending on the circumstances. None of these agents generates or cross-reacts with antibodies generated in heparin-induced thrombocytopenia. None has an antidote, but the short half-life usually obviates the need for one.
Unfortunately, pregnancy data are very sparse for all three of these new agents. Argatroban has a low molecular weight and likely crosses the placenta. Also, because these agents are given intravenously, they are not practical for long-term use in pregnancy.
Fondaparinux
Fondaparinux (Arixtra), a direct factor Xa inhibitor, binds to antithrombin, causing an irreversible conformational change that increases antithrombin’s ability to inactivate factor Xa (as do the heparins). It has no effect on factor IIa (thrombin) and does not predictably affect the aPTT. Its half-life is 17 hours, and no agent is known to reverse its anticoagulant effect, although some experts would recommend a trial of high-dose recombinant factor VIIa (Novo-Seven) in uncontrolled hemorrhage.
While not FDA-approved for treating heparin-induced thrombocytopenia, it has been used for this in some patients.55–58 Animal studies and in vitro human placental perfusion studies suggest that fondaparinux does not cross the placenta in significant amounts.49 Since danaparoid is not available in the United States, fondaparinux would likely be the first choice among the newer anticoagulants when treating heparin-induced thrombocytopenia in pregnancy.
INDICATIONS FOR ANTICOAGULANTS DURING PREGNANCY
Acute deep venous thrombosis and pulmonary embolism
Anticoagulant therapy should begin as full doses of either LMWH or intravenous unfractionated heparin. We prefer starting with LMWH, as it can be started rapidly with less need for nursing care (eg, no need to start and maintain an intravenous line and monitor the aPTT) and has excellent safety. If LMWH is selected, initial dosing should be based on the current weight (Table 2). Subsequent monitoring of the peak anti-factor-Xa activity levels (ie, 4 hours after the dose) is recommended, with the first level drawn in the first few days of treatment, and repeat levels every 1 to 3 months for the rest of treatment. As mentioned earlier, weight-based dosing has not been systematically evaluated in pregnancy.
If unfractionated heparin is the initial agent, it should be given as a bolus followed by a continuous infusion, ideally utilizing a weight-based nomogram to estimate required doses, with adjustment of the infusion rate to maintain the aPTT at 1.5 to 2.5 times the baseline value (obtained during pregnancy). After several days, the heparin may be switched to LMWH in therapeutic doses (Table 2).
Alternatively, in women approaching term or who cannot afford LMWH, anticoagulation may be continued as adjusted-dose subcutaneous unfractionated heparin, ie, two or three large daily doses of subcutaneous heparin to provide therapeutic levels of anticoagulation. The starting dose can be calculated as the total units of heparin required to maintain full anticoagulation intravenously over 24 hours, given as two or three divided doses (Table 2). The aPTT at the mid-dosing interval (eg, 6 hours after the subcutaneous dose during every-12-hour dosing) should be monitored and the dose adjusted to maintain the aPTT at 1.5 to 2.5 times the baseline value.
A therapeutic level of anticoagulation should be maintained for at least 3 months after an acute thrombotic event during pregnancy, though many physicians prefer to continue full anticoagulation for a total of 6 months. Beyond this interval, if the woman is still pregnant, the anticoagulation may be reduced in intensity, perhaps even to a prophylactic level for the duration of the pregnancy (see discussion below on prior venous thromboembolic events) (Table 2). Peripartum and postpartum anticoagulation are discussed further below.
PRIOR VENOUS THROMBOEMBOLIC EVENT
While all pregnant women are at higher risk of venous thrombosis, the overall incidence of thromboembolism is only about one event per 1,000 pregnancies. Routine thromboprophylaxis in all pregnant women is therefore not justified. However, women who have previously had a venous thromboembolic event are at a substantially higher risk of recurrent thrombosis and should be considered for thromboprophylaxis in all subsequent high-risk situations, including pregnancy.
For women on indefinite therapeutic anticoagulation (ie, because of recurrent thrombosis), full therapeutic anticoagulation with LMWH or adjusted-dose unfractionated heparin should be maintained throughout pregnancy, as described above.
Which other women should receive prophylactic anticoagulation is a topic of ongoing debate and controversy.
How great is the risk of recurrent thromboembolism?
A small observational study59 examined the risk of recurrent venous thromboembolism during subsequent pregnancies in women with a prior thrombotic event. Anticoagulation was withheld during the antepartum period and restarted briefly after delivery. Among the 125 women enrolled, recurrent venous thromboembolism occurred in 4.8%, with half of the events occurring during the antepartum period. Among those with underlying thrombophilia, the rate of recurrent venous thromboembolism was 13% (95% confidence interval [CI] 1.7%–40.5%) to 20% (95% CI 2.5%–56.5%), and those with a prior idiopathic clot without thrombophilia had an event rate of 7.7% (95% CI 0.01%–25.1%). The subgroup with a prior reversible risk factor (at the time of their initial venous thromboembolic event) and without detectable thrombophilia had no recurrent events.
This study suggests that women with prior venous thromboembolism and thrombophilia or a prior idiopathic thrombotic event are at a substantial risk of recurrent thrombotic events during pregnancy. And other data confirm the high risk of recurrent venous thromboembolism in thrombophilic pregnant women.60 These women should all be offered active antepartum and postpartum thromboprophylaxis with LMWH or unfractionated heparin (Tables 2 and 4). Women without thrombophilia but with a history of venous thromboembolism related to pregnancy or oral contraceptive use also have a substantial risk of recurrent venous thrombosis and should be offered antepartum and postpartum thromboprophylaxis.61 In contrast, women with a prior “secondary” clot, no thrombophilia, and no additional current risk factors (Table 1) appear to be at low risk of recurrent venous thromboembolism.
The risks should be discussed with these women, with an option for close clinical surveillance during pregnancy (Table 4), but with a low threshold to investigate any worrisome symptoms. Such women may also elect to take LMWH or unfractionated heparin during pregnancy.
Which heparin to use?
Prophylactic anticoagulation during pregnancy can be with either LMWH or unfractionated heparin. For most women this involves “prophylactic” dosing with the goal of maintaining a mid-interval anti-factor-Xa activity level of approximately 0.05 to 0.2 U/mL. Thromboprophylaxis with LMWH can be with lower, fixed, once-daily doses throughout pregnancy20 (Table 2), although some clinicians still prefer twice-daily dosing. The heparin should be started as soon as pregnancy is confirmed, as the pregnancy-associated increase in thrombotic risk begins by the middle of the first trimester.
To maintain effective prophylactic levels, the dose of unfractionated heparin should be increased sequentially over the trimesters62,63: approximately 5,000 units subcutaneously twice daily in the first trimester, then 7,500 units twice daily in the second trimester, and 10,000 units twice daily in the third trimester for a woman of average size.
When to add low-dose aspirin
Women with antiphospholipid antibodies, particularly those with prior recurrent pregnancy loss or fetal demise, should receive aspirin 81 mg/day in addition to heparin.39 The aspirin may be started prior to conception or when pregnancy is confirmed.
Other measures
Women on anticoagulant therapy who are at risk of recurrent venous thromboembolism should be encouraged to wear elastic compression stockings. Intermittent pneumatic compression of the legs via automated devices may be considered for women hospitalized for any reason or on bedrest.
Whichever measures are used, a high index of suspicion and a low threshold for investigating for recurrent thrombosis should be maintained throughout pregnancy and the puerperium.
PERIPARTUM AND POSTPARTUM MANAGEMENT OF ANTICOAGULATION
Heparin therapy must be interrupted temporarily during the immediate peripartum interval to minimize the risk of hemorrhage and to allow for the option of regional anesthesia. As mentioned earlier, because of the theoretical risk of paraspinal hemorrhage in women receiving heparin who undergo epidural or spinal anesthesia, the American Society of Regional Anesthesia guidelines advise waiting to insert the needle at least 10 to 12 hours after the last prophylactic dose of LMWH, and at least 24 hours after the last therapeutic dose.31
The guidelines state that neuraxial anesthesia is not contraindicated in patients on prophylactic unfractionated heparin.31
To facilitate use of regional anesthesia in these women, therefore, options include:
- Electively stopping LMWH 24 hours before planned induction of labor
- Electively stopping prophylactic-dose LMWH or unfractionated heparin at about 38 weeks of gestation, to await spontaneous labor, or
- Switching therapeutic or prophylactic LMWH to unfractionated heparin at about 36 weeks of gestation, with instructions to discontinue the injections in the earliest stages of spontaneous labor. This aims to shorten the heparin-free period required before neuraxial anesthesia while minimizing maternal thrombotic risk.
Additional advantages to using unfractionated heparin peripartum include the option of obtaining a rapid aPTT measurement to confirm the absence of a significant ongoing heparin effect prior to regional anesthesia or delivery, and the ability to completely reverse the heparin effect with protamine sulfate if major bleeding occurs. LMWHs are only partially reversible.64
Interrupting anticoagulation after an initial thrombotic event
If therapeutic anticoagulation must be interrupted for labor within 1 month of the initial thrombotic event, the risk of recurrent thrombotic complications is high65; these women must be observed very carefully and may benefit from intravenous heparin before and after delivery. They may even merit placement of a temporary vena cava filter (particularly if less than 2 weeks have elapsed since the venous thromboembolic event and in women with a large deep venous clot burden), a procedure that has been used safely but little studied in pregnant women.66
Fluoroscopic guidance may be needed for filter placement. This exposes the fetus to radiation, but the low-level exposure at this late gestational age is unlikely to pose a significant risk. The filter may be removed within 1 to 2 weeks postpartum, assuming there are no ongoing contraindications to anticoagulation.
In the rare woman with antithrombin deficiency and a recent or prior thrombotic event, giving antithrombin concentrate during the peripartum (heparin-free) interval has been described and may be considered under the guidance of a hematologist.67
Ongoing anticoagulation is essential postpartum, as the puerperium is the period of highest day-to-day risk of thromboembolic events: about one-third of pregnancy-associated events occur during these 6 to 12 weeks.2 Heparin should be resumed 6 to 12 hours after delivery, once hemostasis is confirmed.
Options for women requiring ongoing therapeutic anticoagulation include intravenous heparin started without a bolus, to minimize bleeding risk, with aPTT measured 12 hours later, or an initial prophylactic dose of LMWH 6 to 12 hours postpartum, with therapeutic dosing resumed on postpartum day 1. If prophylactic dosing is desired, unfractionated heparin or LMWH may be given subcutaneously starting at about 6 hours postpartum.
Warfarin in the puerperium
Women may subsequently be maintained on either LMWH or unfractionated heparin, or switched to an oral anticoagulant such as warfarin. Although warfarin may appear in minute amounts in breast milk, it has not been associated with adverse events in newborns and is considered compatible with breastfeeding.68 Heparin should be continued during the initial days of warfarin therapy, until the INR is at a therapeutic level for 24 hours. Some physicians prefer to delay warfarin for several days, giving LMWH alone in the immediate postpartum period, to allow wound-healing and to reduce bleeding risk.
Postpartum, anticoagulation should be continued for at least 6 to 12 weeks, at which point the physiologic changes in the coagulation system related to pregnancy will have returned to normal.
THROMBOPHILIA WITHOUT A PREVIOUS THROMBOEMBOLIC EVENT
Over the last 5 to 10 years, practitioners have been seeing many more young women with genetic or acquired thrombophilias who have never had a venous thromboembolic event. Physicians must advise these women about their risk of thromboembolic events during pregnancy and about the appropriateness of anticoagulant use.
Thrombophilias are often detected in women who develop venous thrombosis during pregnancy,69–71 but they are also very common in the general population (around 15%). While women with thrombophilia are at above-average risk of venous thromboembolism during pregnancy, the magnitude of risk in an individual patient is often difficult to estimate.
Data suggest that some types of thrombophilia confer greater thrombotic risk than others. McColl et al72 derived risk estimates for a primary event in women with several of the disorders: 0.23% in women heterozygous for the factor V Leiden mutation, 0.88% in women with protein C deficiency, and 2.4% to 35.7% in women with antithrombin deficiency. A case-control study70 found that all thrombophilic states were more common in women with pregnancy-associated venous thromboembolism than in healthy pregnant controls, except those with the MTHFR mutation and protein S deficiency. The estimated risk during pregnancy was 0.03% in women with no defect, 0.1% in women with protein C deficiency, 0.25% in women with the factor V Leiden mutation, 0.4% in those with antithrombin deficiency, 0.5% in those with the prothrombin gene mutation, and 4.6% in those with both factor V Leiden and prothrombin gene mutations.
Routine anticoagulation not advised in pregnant thrombophilic women
Because the risk of a primary venous thromboembolic event is less than 1% for most thrombophilic women, routine anticoagulant therapy does not seem prudent for this indication. Given the low absolute risk of venous thromboembolism, the cost and potential side effects of anticoagulant use are difficult to justify.
The women who seem at higher risk and in whom anticoagulation should be considered include those with antithrombin deficiency; those with high-titer anticardiolipin antibodies or a lupus anticoagulant antibody (treat with heparin and low-dose aspirin); those with combined thrombophilic defects or who are homozygotes for the factor V Leiden or prothrombin gene mutations; and those with multiple other current risk factors for venous thromboembolism (Table 1).
Since anticoagulants for primary prevention of adverse pregnancy outcomes in thrombophilic women have not yet been shown to have a definitive benefit, they are not recommended for this purpose.
ADVERSE PREGNANCY OUTCOMES IN WOMEN WITH THROMBOPHILIAS
Women with antiphospholipid antibodies and a previous poor obstetric outcome are clearly at increased risk of recurrent adverse pregnancy outcomes such as recurrent spontaneous abortion, unexplained fetal death, placental insufficiency, and early or severe preeclampsia. In such women who have both antiphospholipid antibodies and a history of venous thromboembolism or adverse pregnancy outcome, treatment during subsequent pregnancy with low-dose aspirin and prophylactic-dose LMWH or unfractionated heparin improves pregnancy outcomes.36–42 Women with antiphospholipid antibodies without previous thrombosis or pregnancy complications may also be at increased risk, but it is unclear whether thromboprophylaxis improves their outcomes.
Recent epidemiologic data reveal that women with other thrombophilic conditions also are at increased risk of early, severe preeclampsia73 as well as other pregnancy complications, including recurrent pregnancy loss, placental abruption, fetal growth restriction, and stillbirth.74 A recent meta-analysis75 looked at individual thrombophilias and found that factor V Leiden and prothrombin gene mutations were associated with recurrent fetal loss, stillbirth, and preeclampsia; that protein S deficiency was associated with recurrent fetal loss and stillbirth; that antiphospholipid antibodies were associated with recurrent pregnancy loss, preeclampsia, and intrauterine growth restriction; that the MTHFR mutation (homozygous) was associated with preeclampsia; and that protein C and antithrombin deficiencies were not significantly associated with adverse pregnancy outcomes. Data were scant for some of the rarer thrombophilias.75
Several recent small studies76–78 suggest that anticoagulants may improve pregnancy outcomes in women with genetic thrombophilias and recurrent pregnancy loss. These findings have not yet been confirmed in high-quality clinical trials, but such trials are under way. It is still unclear whether anticoagulants also reduce the risk of other adverse pregnancy outcomes associated with thrombophilias.
The current American College of Chest Physicians guidelines recommend testing of women with adverse pregnancy outcomes (recurrent pregnancy loss, prior severe or recurrent preeclampsia, abruptions, or otherwise unexplained intrauterine death) for congenital thrombophilias and antiphospholipid antibodies, and offering treatment to such women, if thrombophilic, with low-dose aspirin plus prophylactic heparin (unfractionated or LMWH).22 The authors of the guidelines admit that the evidence for this recommendation is weak, but they argue that the heparin will also serve as thromboprophylaxis in this high-risk group. Hopefully, the randomized clinical trials currently under way will provide clearer guidance regarding the most appropriate therapy in this difficult clinical situation.
MECHANICAL HEART VALVES
Internists may occasionally encounter a woman with a mechanical heart valve prosthesis who is either pregnant or is planning a pregnancy and therefore needs advice regarding optimal anticoagulant management. This should generally be undertaken in a multi-disciplinary fashion, with input from cardiology, hematology, and maternal-fetal medicine. The substantial maternal and fetal risks and the lack of definitive data on which to base treatment decisions make it a treacherous and stressful undertaking. Nonetheless, all internists should have a basic understanding of the complex issues regarding this management.
Outside of pregnancy, oral anticoagulants are the mainstay of therapy for patients with mechanical heart valves. Unfortunately, as discussed above, the use of these agents during pregnancy carries a risk of teratogenicity and toxic fetal effects and increases the risk of pregnancy loss and maternal hemorrhage. Heparins have been used in this setting for many years, but data on their efficacy and safety are very limited, and there are numerous reports of catastrophic maternal thrombotic complications.79,80
A systematic review of anticoagulation in pregnant women with prosthetic heart valves34 found very limited data on heparin use throughout pregnancy. Women maintained on warfarin vs heparin between pregnancy weeks 6 and 12 had higher rates of congenital anomalies (6.4% with warfarin vs 3.4% with heparin) and total fetal wastage (33.6% vs 26.5%). The warfarin group had fewer maternal thromboembolic complications (3.9% vs 9.2%), however, and a slightly lower rate of maternal death (1.8% vs 4.2%). Most of the women had higher-risk older-generation valves in the mitral position.
Recent data on LMWH consist mainly of case reports and case series,81 with a likely bias to publication of worse outcomes. Controlled trials in this area will be difficult to conduct. Still, aggressive anticoagulation with LMWH or unfractionated heparin, with close monitoring of the intensity of anticoagulation, may be safe and effective for pregnant women with newer-generation mechanical heart valves.82 A recent consensus statement22 suggested several regimens for pregnant women with mechanical heart valves:
- Twice-daily LMWH throughout pregnancy, with the dose adjusted either by weight, or to keep the 4-hour postinjection anti-factor-Xa activity level around 1.0 to 1.2 U/mL
- Aggressive adjusted-dose unfractionated heparin throughout pregnancy, given subcutaneously every 12 hours and adjusted to keep the mid-interval aPTT at least twice the control value or to attain a mid-interval anti-factor-Xa activity level of 0.35 to 0.70 U/mL
- Unfractionated heparin or LMWH (as above) until gestation week 13, then warfarin until the middle of the third trimester, and then heparin again.22
The authors also recommended adding low-dose aspirin (75–162 mg/day) in high-risk women.22
These options all seem reasonable, given our current knowledge, though warfarin use during pregnancy should be restricted to very-high-risk situations, such as women with older-generation mitral prostheses. LM-WHs may become the preferred therapy for this indication once further controlled data regarding their efficacy and safety become available.
- Chang J, Elam-Evans LD, Berg CJ, et al. Pregnancy-related mortality surveillance-United States, 1991–1999. MMWR Surveill Summ 2003; 52:1–8.
- Lewis G, Drife JO, Clutton-Brock T, et al. Why Mothers Die, 2000–2002. The Sixth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG Press, 2004.
- Health Canada. Special Report on Maternal Mortality and Severe Morbidity in Canada—Enhanced Surveillance: The Path to Prevention. Ottawa: Minister of Public Works and Government Services Canada, 2004. www.phac-aspc.gc.ca/rhs-ssg/srmm-rsmm/page1-eng.php. Accessed 11/26/2008.
- Stein PD, Hull RD, Kayali F, et al. Venous thromboembolism in pregnancy: 21-year trends. Am J Med 2004; 117:121–125.
- Greer IA. Thrombosis in pregnancy: maternal and fetal issues. Lancet 1999; 353:1258–1265.
- Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet 1999; 353:1167–1173.
- Gherman RB, Goodwin TM, Leung B, et al. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol 1999; 94:730–734.
- Weitz JI. Low-molecular-weight heparins. N Engl J Med 1997; 337:688–698.
- Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996; 334:677–681.
- Koopman MM, Prandoni P, Piovella F, et al. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. The Tasman Study Group. N Engl J Med 1996; 334:682–687.
- Simonneau G, Sors H, Charbonnier B, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. The THESEE Study Group. Tinzaparine ou Heparine Standard: Evaluations dans l’Embolie Pulmonaire. N Engl J Med 1997; 337:663–669.
- Hull RD, Raskob GE, Brant RF, et al. Low-molecular-weight heparin vs heparin in the treatment of patients with pulmonary embolism. American-Canadian Thrombosis Study Group. Arch Intern Med 2000; 160:229–236.
- Sanson BJ, Lensing AW, Prins MH, et al. Safety of low-molecular-weight heparin in pregnancy: a systematic review. Thromb Haemost 1999; 81:668–672.
- Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood 2005; 106:401–407.
- Melissari E, Parker CJ, Wilson NV, et al. Use of low molecular weight heparin in pregnancy. Thromb Haemost 1992; 68:652–656.
- Forestier F, Daffos F, Capella-Pavlovsky M. Low molecular weight heparin (PK 10169) does not cross the placenta during the second trimester of pregnancy study by direct fetal blood sampling under ultrasound. Thromb Res 1984; 34:557–560.
- Forestier F, Daffos F, Rainaut M, Toulemonde F. Low molecular weight heparin (CY 216) does not cross the placenta during the third trimester of pregnancy. Thromb Haemost 1987; 57:234.
- Barbour LA, Oja JL, Schultz LK. A prospective trial that demonstrates that dalteparin requirements increase in pregnancy to maintain therapeutic levels of anticoagulation. Am J Obstet Gynecol 2004; 191:1024–1029.
- Smith MP, Norris LA, Steer PJ, Savidge GF, Bonnar J. Tinzaparin sodium for thrombosis treatment and prevention during pregnancy. Am J Obstet Gynecol 2004; 190:495–501.
- Ellison J, Walker ID, Greer IA. Antenatal use of enoxaparin for prevention and treatment of thromboembolism in pregnancy. BJOG 2000; 107:1116–1121.
- Sarig G, Brenner B. Monitoring of low molecular weight heparin (LMWH) in pregnancy. Thromb Res 2005; 115 suppl 1:84–86.
- Bates SM, Greer IA, Hirsh J, Ginsberg JS. Use of antithrombotic agents during pregnancy: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004; 126 suppl 3:627S–644S.
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995; 332:1330–1335.
- Hassell K. The management of patients with heparin-induced thrombocytopenia who require anticoagulant therapy. Chest 2005; 127 suppl 2:1S–8S.
- Barbour LA, Kick SD, Steiner JF, et al. A prospective study of heparin-induced osteoporosis in pregnancy using bone densitometry. Am J Obst Gynecol 1994; 170:862–869.
- Douketis JD, Ginsberg JS, Burrows RF, Duku EK, Webber CE, Brill-Edwards P. The effects of long-term heparin therapy during pregnancy on bone density. A prospective matched cohort study. Thromb Haemost 1996; 75:254–257.
- Pettila V, Leinonen P, Markkola A, Hiilesmaa V, Kaaja R. Postpartum bone mineral density in women treated for thromboprophylaxis with unfractionated heparin or LMW heparin. Thromb Haemost 2002; 87:182–186.
- Carlin AJ, Farquharson RG, Quenby SM, Topping J, Fraser WD. Prospective observational study of bone mineral density during pregnancy: low molecular weight heparin versus control. Hum Reprod 2004; 19:1211–1214.
- Casele HL, Laifer SA. Prospective evaluation of bone density in pregnant women receiving the low molecular weight heparin enoxaparin sodium. J Matern Fetal Med 2000; 9:122–125.
- Casele H, Haney EI, James A, Rosene-Montella K, Carson M. Bone density changes in women who receive thromboprophylaxis in pregnancy. Am J Obstet Gynecol 2006; 195:1109–1113.
- Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med 2003; 28:172–197.
- Hirsh J, Dalen JE, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 2001; 119 suppl 1:8S–21S.
- Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004; 126 suppl 3:287S–310S.
- Holmes LB. Teratogen-induced limb defects. Am J Med Genet 2002; 112:297–303.
- Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med 2000; 160:191–196.
- Pauzner R, Dulitzki M, Langevitz P, Livneh A, Kenett R, Many A. Low molecular weight heparin and warfarin in the treatment of patients with antiphospholipid syndrome during pregnancy. Thromb Haemost 2001; 86:1379–1384.
- Pulmonary Embolism Prevention (PEP) Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355:1295–1302.
- Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126 suppl 3:234S–264S.
- Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2004; ( 1):CD004659.
- Coomarasamy A, Honest H, Papaioannou S, Gee H, Khan KS. Aspirin for prevention of preeclampsia in women with historical risk factors: a systematic review. Obstet Gynecol 2003; 101:1319–1332.
- Caritis SN, Sibai BM, Hauth J, et al, and the National Institute of Child Health and Human Development Network of Maternal Fetal Medicine Units. Low-dose aspirin to prevent preeclampsia in women at high risk. N Engl J Med 1998; 338:701–705.
- Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ 1997; 314:253–257.
- Kozer E, Nikfar S, Costei A, Boskovic R, Nulman I, Koren G. Aspirin consumption during the first trimester of pregnancy and congenital anomalies: a meta-analysis. Am J Obstet Gynecol 2002; 187:1623–1630.
- Sebastian C, Scherlag M, Kugelmass A, Schechter E. Primary stent implantation for acute myocardial infarction during pregnancy: use of abciximab, ticlopidine, and aspirin. Cathet Cardiovasc Diagn 1998; 45:275–249.
- Wilson AM, Boyle AJ, Fox P. Management of ischaemic heart disease in women of child-bearing age. Intern Med J 2004; 34:694–697.
- Klinzing P, Markert UR, Liesaus K, Peiker G. Case report: successful pregnancy and delivery after myocardial infarction and essential thrombocythemia treated with clopidogrel. Clin Exp Obstet Gynecol 2001; 28:215–216.
- Danhof M, de Boer A, Magnani HN, Stiekema JC. Pharmacokinetic considerations on Orgaran (Org 10172) therapy. Haemostasis 1992; 22:73–84.
- Tardy-Poncet B, Tardy B, Reynaud J, et al. Efficacy and safety of danaparoid sodium (ORG 10172) in critically ill patients with heparin-associated thrombocytopenia. Chest 1999; 115:1616–1620.
- Lagrange F, Vergnes C, Brun JL, et al. Absence of placental transfer of pentasaccharide (fondaparinux, Arixtra) in the dually perfused human cotyledon in vitro. Thromb Haemost 2002; 87:831–835.
- Dempfle CE. Minor transplacental passge of fondapinux in vivo. N Engl J Med 2004; 350:1914.
- Magnani HN. Heparin-induced thrombocytopenia (HIT): an overview of 230 patients treated with orgaran (Org 10172). Thromb Haemost 1993; 70:554–561.
- Lindhoff-Last E, Kreutzenbeck HJ, Magnani HN. Treatment of 51 pregnancies with danaparoid because of heparin intolerance. Thromb Haemost 2005; 93:63–69.
- Greinacher A, Eckhardt T, Mussmann J, Mueller-Eckhardt C. Pregnancy complicated by heparin associated thrombocytopenia: management by a prospectively in vitro selected heparinoid (Org 10172). Thromb Res 1993; 71:123–126.
- Schindewolf M, Mosch G, Bauersachs RM, Lindhoff-Last E. Safe anticoagulation with danaparoid in pregnancy and lactation. Thromb Haemost 2004; 92:211.
- Harenberg J. Treatment of a woman with lupus and thromboembolism and cutaneous intolerance to heparins using fondaparinux during pregnancy. Thromb Res 2007; 119:385–388.
- Wijesiriwardana A, Lees DA, Lush C. Fondaparinux as anticoagulant in a pregnant woman with heparin allergy. Blood Coagul Fibrinolysis 2006; 17:147–149.
- Mazzolai L, Hohlfeld P, Spertini F, Hayoz D, Schapira M, Duchosal MA. Fondaparinux is a safe alternative in case of heparin intolerance during pregnancy. Blood 2006; 108:1569–1570.
- Hawkins D, Evans J. Minimizing the risk of heparin-induced osteoporosis during pregnancy. Expert Opin Drug Saf 2005; 4:583–590.
- Brill-Edwards P, Ginsberg JS, Gent M, et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of clot in this pregnancy study group. N Engl J Med 2000; 343:1439–1444.
- Martinelli I, Legnani C, Bucciarelli P, Grandone E, De Stefano V, Mannucci PM. Risk of pregnancy-related venous thrombosis in carriers of severe inherited thrombophilia. Thromb Haemost 2001; 86:800–803.
- De Stefano V, Martinelli I, Rossi E, Battaglioli T, Za T, Mannucci PM, Leone G. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol 2006; 135:386–391.
- Barbour LA, Smith JM, Marlar RA. Heparin levels to guide thromboembolism prophylaxis during pregnancy. Am J Obstet Gynecol 1995; 173:1869–1873.
- Ensom MH, Stephenson MD. Pharmacokinetics of low molecular weight heparin and unfractionated heparin in pregnancy. J Soc Gynecol Investig 2004; 11:377–383.
- Crowther MA, Berry LR, Monagle PT, Chan AK. Mechanisms responsible for the failure of protamine to inactivate low-molecular-weight heparin. Br J Haematol 2002; 116:178–186.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Thomas LA, Summers RR, Cardwell MS. Use of Greenfield filters in pregnant women at risk for pulmonary embolism. South Med J 1997; 90:215–217.
- Maclean PS, Tait RC. Hereditary and acquired antithrombin deficiency: epidemiology, pathogenesis and treatment options. Drugs 2007; 67:1429–1440.
- Information from LactMed: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT, LactMed Record Number: 279. Accessed 11/26/2008.
- Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000; 342:374–380.
- Hirsch DR, Mikkola KM, Marks PW, et al. Pulmonary embolism and deep venous thrombosis during pregnancy or oral contraceptive use: prevalence of factor V Leiden. Am Heart J 1996; 131:1145–1148.
- Dizon-Townson DS, Nelson LM, Jang H, Varner MW, Ward K. The incidence of the factor V Leiden mutation in an obstetric population and its relationship to deep vein thrombosis. Am J Obstet Gynecol 1997; 176:883–886.
- McColl MD, Ramsay JE, Tait RC, et al. Risk factors for pregnancy associated venous thromboembolism. Thromb Haemost 1997; 78:1183–1188.
- Kupferminc MJ, Fait G, Many A, Gordon D, Eldor A, Lessing JB. Severe preeclampsia and high frequency of genetic thrombophilic mutations. Obstet Gynecol 2000; 96:45–49.
- Kupferminc MJ, Eldor A, Steinman N, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med 1999; 340:9–13.
- Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol 2006; 132:171–196.
- Brenner B, Hoffman R, Blumenfeld Z, Weiner Z, Younis JS. Gestational outcome in thrombophilic women with recurrent pregnancy loss treated by enoxaparin. Thromb Haemost 2000; 83:693–697.
- Carp H, Dolitzky M, Inbal A. Thromboprophylaxis improves the live birth rate in women with consecutive recurrent miscarriages and hereditary thrombophilia. J Thromb Haemost 2003; 1:433–438.
- Gris JC, Mercier E, Quere I, et al. Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood 2004; 103:3695–3699.
- Salazar E, Izaguirre R, Verdejo J, Mutchinick O. Failure of adjusted doses of subcutaneous heparin to prevent thromboembolic phenomena in pregnant patients with mechanical cardiac valve prostheses. J Am Coll Cardiol 1996; 27:1698–1703.
- Iturbe-Alessio I, Fonseca MC, Mutchinik O, Santos MA, Zajarias A, Salazar E. Risks of anticoagulant therapy in pregnant women with artificial heart valves. N Engl J Med 1986; 315:1390–1393.
- Rowan JA, McCowan LM, Raudkivi PJ, North RA. Enoxaparin treatment in women with mechanical heart valves during pregnancy. Am J Obstet Gynecol 2001; 185:633–637.
- Oran B, Lee-Parritz A, Ansell J. Low molecular weight heparin for the prophylaxis of thromboembolism in women with prosthetic mechanical heart valves during pregnancy. Thromb Haemost 2004; 92:747–751.
- Chang J, Elam-Evans LD, Berg CJ, et al. Pregnancy-related mortality surveillance-United States, 1991–1999. MMWR Surveill Summ 2003; 52:1–8.
- Lewis G, Drife JO, Clutton-Brock T, et al. Why Mothers Die, 2000–2002. The Sixth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London: RCOG Press, 2004.
- Health Canada. Special Report on Maternal Mortality and Severe Morbidity in Canada—Enhanced Surveillance: The Path to Prevention. Ottawa: Minister of Public Works and Government Services Canada, 2004. www.phac-aspc.gc.ca/rhs-ssg/srmm-rsmm/page1-eng.php. Accessed 11/26/2008.
- Stein PD, Hull RD, Kayali F, et al. Venous thromboembolism in pregnancy: 21-year trends. Am J Med 2004; 117:121–125.
- Greer IA. Thrombosis in pregnancy: maternal and fetal issues. Lancet 1999; 353:1258–1265.
- Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet 1999; 353:1167–1173.
- Gherman RB, Goodwin TM, Leung B, et al. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol 1999; 94:730–734.
- Weitz JI. Low-molecular-weight heparins. N Engl J Med 1997; 337:688–698.
- Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996; 334:677–681.
- Koopman MM, Prandoni P, Piovella F, et al. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. The Tasman Study Group. N Engl J Med 1996; 334:682–687.
- Simonneau G, Sors H, Charbonnier B, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. The THESEE Study Group. Tinzaparine ou Heparine Standard: Evaluations dans l’Embolie Pulmonaire. N Engl J Med 1997; 337:663–669.
- Hull RD, Raskob GE, Brant RF, et al. Low-molecular-weight heparin vs heparin in the treatment of patients with pulmonary embolism. American-Canadian Thrombosis Study Group. Arch Intern Med 2000; 160:229–236.
- Sanson BJ, Lensing AW, Prins MH, et al. Safety of low-molecular-weight heparin in pregnancy: a systematic review. Thromb Haemost 1999; 81:668–672.
- Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood 2005; 106:401–407.
- Melissari E, Parker CJ, Wilson NV, et al. Use of low molecular weight heparin in pregnancy. Thromb Haemost 1992; 68:652–656.
- Forestier F, Daffos F, Capella-Pavlovsky M. Low molecular weight heparin (PK 10169) does not cross the placenta during the second trimester of pregnancy study by direct fetal blood sampling under ultrasound. Thromb Res 1984; 34:557–560.
- Forestier F, Daffos F, Rainaut M, Toulemonde F. Low molecular weight heparin (CY 216) does not cross the placenta during the third trimester of pregnancy. Thromb Haemost 1987; 57:234.
- Barbour LA, Oja JL, Schultz LK. A prospective trial that demonstrates that dalteparin requirements increase in pregnancy to maintain therapeutic levels of anticoagulation. Am J Obstet Gynecol 2004; 191:1024–1029.
- Smith MP, Norris LA, Steer PJ, Savidge GF, Bonnar J. Tinzaparin sodium for thrombosis treatment and prevention during pregnancy. Am J Obstet Gynecol 2004; 190:495–501.
- Ellison J, Walker ID, Greer IA. Antenatal use of enoxaparin for prevention and treatment of thromboembolism in pregnancy. BJOG 2000; 107:1116–1121.
- Sarig G, Brenner B. Monitoring of low molecular weight heparin (LMWH) in pregnancy. Thromb Res 2005; 115 suppl 1:84–86.
- Bates SM, Greer IA, Hirsh J, Ginsberg JS. Use of antithrombotic agents during pregnancy: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004; 126 suppl 3:627S–644S.
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995; 332:1330–1335.
- Hassell K. The management of patients with heparin-induced thrombocytopenia who require anticoagulant therapy. Chest 2005; 127 suppl 2:1S–8S.
- Barbour LA, Kick SD, Steiner JF, et al. A prospective study of heparin-induced osteoporosis in pregnancy using bone densitometry. Am J Obst Gynecol 1994; 170:862–869.
- Douketis JD, Ginsberg JS, Burrows RF, Duku EK, Webber CE, Brill-Edwards P. The effects of long-term heparin therapy during pregnancy on bone density. A prospective matched cohort study. Thromb Haemost 1996; 75:254–257.
- Pettila V, Leinonen P, Markkola A, Hiilesmaa V, Kaaja R. Postpartum bone mineral density in women treated for thromboprophylaxis with unfractionated heparin or LMW heparin. Thromb Haemost 2002; 87:182–186.
- Carlin AJ, Farquharson RG, Quenby SM, Topping J, Fraser WD. Prospective observational study of bone mineral density during pregnancy: low molecular weight heparin versus control. Hum Reprod 2004; 19:1211–1214.
- Casele HL, Laifer SA. Prospective evaluation of bone density in pregnant women receiving the low molecular weight heparin enoxaparin sodium. J Matern Fetal Med 2000; 9:122–125.
- Casele H, Haney EI, James A, Rosene-Montella K, Carson M. Bone density changes in women who receive thromboprophylaxis in pregnancy. Am J Obstet Gynecol 2006; 195:1109–1113.
- Horlocker TT, Wedel DJ, Benzon H, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med 2003; 28:172–197.
- Hirsh J, Dalen JE, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 2001; 119 suppl 1:8S–21S.
- Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004; 126 suppl 3:287S–310S.
- Holmes LB. Teratogen-induced limb defects. Am J Med Genet 2002; 112:297–303.
- Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med 2000; 160:191–196.
- Pauzner R, Dulitzki M, Langevitz P, Livneh A, Kenett R, Many A. Low molecular weight heparin and warfarin in the treatment of patients with antiphospholipid syndrome during pregnancy. Thromb Haemost 2001; 86:1379–1384.
- Pulmonary Embolism Prevention (PEP) Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355:1295–1302.
- Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126 suppl 3:234S–264S.
- Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2004; ( 1):CD004659.
- Coomarasamy A, Honest H, Papaioannou S, Gee H, Khan KS. Aspirin for prevention of preeclampsia in women with historical risk factors: a systematic review. Obstet Gynecol 2003; 101:1319–1332.
- Caritis SN, Sibai BM, Hauth J, et al, and the National Institute of Child Health and Human Development Network of Maternal Fetal Medicine Units. Low-dose aspirin to prevent preeclampsia in women at high risk. N Engl J Med 1998; 338:701–705.
- Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ 1997; 314:253–257.
- Kozer E, Nikfar S, Costei A, Boskovic R, Nulman I, Koren G. Aspirin consumption during the first trimester of pregnancy and congenital anomalies: a meta-analysis. Am J Obstet Gynecol 2002; 187:1623–1630.
- Sebastian C, Scherlag M, Kugelmass A, Schechter E. Primary stent implantation for acute myocardial infarction during pregnancy: use of abciximab, ticlopidine, and aspirin. Cathet Cardiovasc Diagn 1998; 45:275–249.
- Wilson AM, Boyle AJ, Fox P. Management of ischaemic heart disease in women of child-bearing age. Intern Med J 2004; 34:694–697.
- Klinzing P, Markert UR, Liesaus K, Peiker G. Case report: successful pregnancy and delivery after myocardial infarction and essential thrombocythemia treated with clopidogrel. Clin Exp Obstet Gynecol 2001; 28:215–216.
- Danhof M, de Boer A, Magnani HN, Stiekema JC. Pharmacokinetic considerations on Orgaran (Org 10172) therapy. Haemostasis 1992; 22:73–84.
- Tardy-Poncet B, Tardy B, Reynaud J, et al. Efficacy and safety of danaparoid sodium (ORG 10172) in critically ill patients with heparin-associated thrombocytopenia. Chest 1999; 115:1616–1620.
- Lagrange F, Vergnes C, Brun JL, et al. Absence of placental transfer of pentasaccharide (fondaparinux, Arixtra) in the dually perfused human cotyledon in vitro. Thromb Haemost 2002; 87:831–835.
- Dempfle CE. Minor transplacental passge of fondapinux in vivo. N Engl J Med 2004; 350:1914.
- Magnani HN. Heparin-induced thrombocytopenia (HIT): an overview of 230 patients treated with orgaran (Org 10172). Thromb Haemost 1993; 70:554–561.
- Lindhoff-Last E, Kreutzenbeck HJ, Magnani HN. Treatment of 51 pregnancies with danaparoid because of heparin intolerance. Thromb Haemost 2005; 93:63–69.
- Greinacher A, Eckhardt T, Mussmann J, Mueller-Eckhardt C. Pregnancy complicated by heparin associated thrombocytopenia: management by a prospectively in vitro selected heparinoid (Org 10172). Thromb Res 1993; 71:123–126.
- Schindewolf M, Mosch G, Bauersachs RM, Lindhoff-Last E. Safe anticoagulation with danaparoid in pregnancy and lactation. Thromb Haemost 2004; 92:211.
- Harenberg J. Treatment of a woman with lupus and thromboembolism and cutaneous intolerance to heparins using fondaparinux during pregnancy. Thromb Res 2007; 119:385–388.
- Wijesiriwardana A, Lees DA, Lush C. Fondaparinux as anticoagulant in a pregnant woman with heparin allergy. Blood Coagul Fibrinolysis 2006; 17:147–149.
- Mazzolai L, Hohlfeld P, Spertini F, Hayoz D, Schapira M, Duchosal MA. Fondaparinux is a safe alternative in case of heparin intolerance during pregnancy. Blood 2006; 108:1569–1570.
- Hawkins D, Evans J. Minimizing the risk of heparin-induced osteoporosis during pregnancy. Expert Opin Drug Saf 2005; 4:583–590.
- Brill-Edwards P, Ginsberg JS, Gent M, et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of clot in this pregnancy study group. N Engl J Med 2000; 343:1439–1444.
- Martinelli I, Legnani C, Bucciarelli P, Grandone E, De Stefano V, Mannucci PM. Risk of pregnancy-related venous thrombosis in carriers of severe inherited thrombophilia. Thromb Haemost 2001; 86:800–803.
- De Stefano V, Martinelli I, Rossi E, Battaglioli T, Za T, Mannucci PM, Leone G. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol 2006; 135:386–391.
- Barbour LA, Smith JM, Marlar RA. Heparin levels to guide thromboembolism prophylaxis during pregnancy. Am J Obstet Gynecol 1995; 173:1869–1873.
- Ensom MH, Stephenson MD. Pharmacokinetics of low molecular weight heparin and unfractionated heparin in pregnancy. J Soc Gynecol Investig 2004; 11:377–383.
- Crowther MA, Berry LR, Monagle PT, Chan AK. Mechanisms responsible for the failure of protamine to inactivate low-molecular-weight heparin. Br J Haematol 2002; 116:178–186.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Thomas LA, Summers RR, Cardwell MS. Use of Greenfield filters in pregnant women at risk for pulmonary embolism. South Med J 1997; 90:215–217.
- Maclean PS, Tait RC. Hereditary and acquired antithrombin deficiency: epidemiology, pathogenesis and treatment options. Drugs 2007; 67:1429–1440.
- Information from LactMed: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT, LactMed Record Number: 279. Accessed 11/26/2008.
- Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000; 342:374–380.
- Hirsch DR, Mikkola KM, Marks PW, et al. Pulmonary embolism and deep venous thrombosis during pregnancy or oral contraceptive use: prevalence of factor V Leiden. Am Heart J 1996; 131:1145–1148.
- Dizon-Townson DS, Nelson LM, Jang H, Varner MW, Ward K. The incidence of the factor V Leiden mutation in an obstetric population and its relationship to deep vein thrombosis. Am J Obstet Gynecol 1997; 176:883–886.
- McColl MD, Ramsay JE, Tait RC, et al. Risk factors for pregnancy associated venous thromboembolism. Thromb Haemost 1997; 78:1183–1188.
- Kupferminc MJ, Fait G, Many A, Gordon D, Eldor A, Lessing JB. Severe preeclampsia and high frequency of genetic thrombophilic mutations. Obstet Gynecol 2000; 96:45–49.
- Kupferminc MJ, Eldor A, Steinman N, et al. Increased frequency of genetic thrombophilia in women with complications of pregnancy. N Engl J Med 1999; 340:9–13.
- Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol 2006; 132:171–196.
- Brenner B, Hoffman R, Blumenfeld Z, Weiner Z, Younis JS. Gestational outcome in thrombophilic women with recurrent pregnancy loss treated by enoxaparin. Thromb Haemost 2000; 83:693–697.
- Carp H, Dolitzky M, Inbal A. Thromboprophylaxis improves the live birth rate in women with consecutive recurrent miscarriages and hereditary thrombophilia. J Thromb Haemost 2003; 1:433–438.
- Gris JC, Mercier E, Quere I, et al. Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood 2004; 103:3695–3699.
- Salazar E, Izaguirre R, Verdejo J, Mutchinick O. Failure of adjusted doses of subcutaneous heparin to prevent thromboembolic phenomena in pregnant patients with mechanical cardiac valve prostheses. J Am Coll Cardiol 1996; 27:1698–1703.
- Iturbe-Alessio I, Fonseca MC, Mutchinik O, Santos MA, Zajarias A, Salazar E. Risks of anticoagulant therapy in pregnant women with artificial heart valves. N Engl J Med 1986; 315:1390–1393.
- Rowan JA, McCowan LM, Raudkivi PJ, North RA. Enoxaparin treatment in women with mechanical heart valves during pregnancy. Am J Obstet Gynecol 2001; 185:633–637.
- Oran B, Lee-Parritz A, Ansell J. Low molecular weight heparin for the prophylaxis of thromboembolism in women with prosthetic mechanical heart valves during pregnancy. Thromb Haemost 2004; 92:747–751.
KEY POINTS
- Pregnancy is a hypercoagulable state. Thrombotic risk in an individual pregnancy depends on many maternal and situational factors.
- When indicated, careful anticoagulation can proceed with minimal risk to the mother and fetus.
- Heparins, especially LMWHs, are the main anticoagulants used in pregnancy. Dosing depends on the clinical indications and on the agent selected.
- If anticoagulation is absolutely necessary and LMWH is contraindicated, a newer, alternative anticoagulant should be considered.
- Warfarin should not be used in pregnancy in any but the highest-risk situations.