User login
Fingernail Photo-onycholysis After Aminolevulinic Acid–Photodynamic Therapy Under Blue Light for Treatment of Actinic Keratoses on the Face
To the Editor:
Topical photodynamic therapy (PDT) is one of several effective treatments of actinic keratoses (AKs). Photodynamic therapy involves selection of a lesion field, application of a photosensitizer drug, incubation for an explicit period of time, and illumination of the area from a light source corresponding to the absorption spectrum of the chosen drug.1 A photosensitizer drug used in PDT to target AK is aminolevulinic acid (ALA). Aminolevulinic acid converts disease tissue to photoactivatable porphyrins, especially protoporphyrin IX, which has its largest absorption peak (410 nm) in the blue spectrum, with smaller absorption peaks at 505, 540, 580, and 630 nm. Photodynamic therapy treatments historically have been carried out under red light (peak emissions, 630 nm) to improve tissue penetration, which is superior in efficacy when treating Bowen disease and basal cell carcinoma.1,2 Broadband blue light (peak emission, 417 nm) now is routinely used and has been proven effective in combination with ALA for the treatment of AK.3 It was approved by the US Food and Drug Administration for AKs in 1999.4
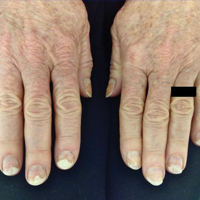
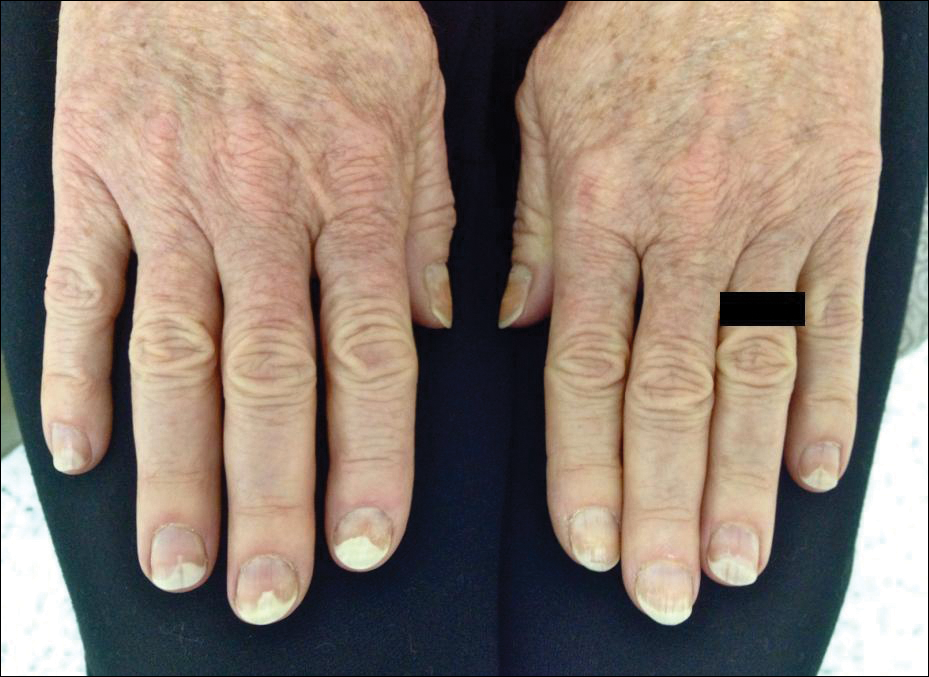
Photo-onycholysis is a photosensitivity reaction defined as separation of the nail plate from the nail bed. There are 4 different types of photo-onycholysis characterized by appearance and by the number of digits affected: Type I is denoted by the involvement of several fingers, with half-moon–shaped separations of the nail plate. Type II affects a single finger and corresponds to a brown, defined, circular notch opening distally. Type III, which involves several fingers, is defined as round yellow stains in the central portion of the nail that turn red after 5 to 10 days. Type IV has been associated with bullae under the nails.5 There have been cases of photo-onycholysis arising after exposure to UV light following ingestion of certain prescription drugs or spontaneously,6 and a single case following PDT to the hands with red light.5 We report a case of fingernail photo-onycholysis resulting from ALA-PDT for the treatment of perioral AK.
A 65-year-old woman was treated for AKs on the perioral region of the face with PDT. Aminolevulinic acid hydrochloride 20% was applied to the lips and allowed to incubate for 60 minutes. Her face was illuminated with 10 J/cm² of blue light (417 nm) for 16 minutes and 40 seconds. Sunscreen (sun protection factor 40) was applied to the area immediately after treatment, and the patient was thoroughly counseled to avoid sunlight for the next 48 hours and to use sun protection. Within 72 hours following treatment, the patient reported all 10 fingernails noticeably separated from the nail bed with minimal pain, corresponding to type I photo-onycholysis (Figure). The patient’s only medications were vitamin D (1000 mg once daily) and calcium supplements (1500 mg twice daily). Although the patient exercised strict UV light avoidance for the face, her hands were not protected when she went gardening directly after the treatment. At 5 weeks, the patient returned for her second ALA-PDT treatment of perioral AK and a fungal culture was taken of the left third fingernail, which returned negative results. Poly-ureaurethane nail lacquer 16% was prescribed and was used once daily to protect and strengthen the fingernails. The patient returned for follow-up in clinic after 13 weeks and photo-onycholysis was resolving. Photo-onycholysis is categorized as a phototoxic reaction often associated with drug intake, more specifically with the use of tetracyclines, psoralens, and fluoroquinolones; less commonly with oral contraceptives; or spontaneously.6 It usually is recognized as a crescent-shaped distal separation of the nail surrounded by pigment. The action spectrum is believed to include UVA and UVB, though the exact mechanisms have not been confirmed.5
Our case provides evidence for risks involving the development of photo-onycholysis following PDT. We have no reason to believe there was systemic absorption of ALA, as there were no visible vesicles on the arms or hands after the treatment. Negative fungal culture results excluded onychomycosis. It is our hypothesis that the patient touched her face with her fingernails during the 60-minute incubation time prior to ALA-PDT treatment under blue light, inadvertently collecting ALA under the fingernails. Once she exposed her hands to sunlight while gardening after treatment, the nails likely reacted with the ALA in response to the UV radiation, thus triggering photo-onycholysis.
This case represents a report of fingernail photo-onycholysis from ALA-PDT under blue light as well as a report following treatment of AK not located on the hands with PDT. Although the photo-onycholysis did resolve within a few months of treatment, our case demonstrates the importance of counseling patients more specifically about isolating the ALA treatment zone from nontreated areas on the body during incubation. Improper UV light protection following ALA-PDT is known to produce phototoxic reactions and our case supports this outcome.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Hauschild A. Photodynamic therapy for actinic keratoses: procedure matters? Br J Dermatol. 2012;166:3-5.
- Alexiades-Armenakas M. Laser-mediated photodynamic therapy. Clin Dermatol. 2006;24:16-25.
- Babilas P, Schreml S, Landthaler M, et al. Photodynamic therapy in dermatology: state-of-the-art. Photodermatol Photoimmunol Photomed. 2010;26:118-132.
- Hanneken S, Wessendorf U, Neumann NJ. Photodynamic onycholysis: first report of photo-onycholysis after photodynamic therapy. Clin Exp Dermatol. 2008;33:659-660.
- Baran R, Juhlin L. Photoonycholysis. Photodermatol Photoimmunol Photomed. 2002;18:202-207.
To the Editor:
Topical photodynamic therapy (PDT) is one of several effective treatments of actinic keratoses (AKs). Photodynamic therapy involves selection of a lesion field, application of a photosensitizer drug, incubation for an explicit period of time, and illumination of the area from a light source corresponding to the absorption spectrum of the chosen drug.1 A photosensitizer drug used in PDT to target AK is aminolevulinic acid (ALA). Aminolevulinic acid converts disease tissue to photoactivatable porphyrins, especially protoporphyrin IX, which has its largest absorption peak (410 nm) in the blue spectrum, with smaller absorption peaks at 505, 540, 580, and 630 nm. Photodynamic therapy treatments historically have been carried out under red light (peak emissions, 630 nm) to improve tissue penetration, which is superior in efficacy when treating Bowen disease and basal cell carcinoma.1,2 Broadband blue light (peak emission, 417 nm) now is routinely used and has been proven effective in combination with ALA for the treatment of AK.3 It was approved by the US Food and Drug Administration for AKs in 1999.4
Photo-onycholysis is a photosensitivity reaction defined as separation of the nail plate from the nail bed. There are 4 different types of photo-onycholysis characterized by appearance and by the number of digits affected: Type I is denoted by the involvement of several fingers, with half-moon–shaped separations of the nail plate. Type II affects a single finger and corresponds to a brown, defined, circular notch opening distally. Type III, which involves several fingers, is defined as round yellow stains in the central portion of the nail that turn red after 5 to 10 days. Type IV has been associated with bullae under the nails.5 There have been cases of photo-onycholysis arising after exposure to UV light following ingestion of certain prescription drugs or spontaneously,6 and a single case following PDT to the hands with red light.5 We report a case of fingernail photo-onycholysis resulting from ALA-PDT for the treatment of perioral AK.
A 65-year-old woman was treated for AKs on the perioral region of the face with PDT. Aminolevulinic acid hydrochloride 20% was applied to the lips and allowed to incubate for 60 minutes. Her face was illuminated with 10 J/cm² of blue light (417 nm) for 16 minutes and 40 seconds. Sunscreen (sun protection factor 40) was applied to the area immediately after treatment, and the patient was thoroughly counseled to avoid sunlight for the next 48 hours and to use sun protection. Within 72 hours following treatment, the patient reported all 10 fingernails noticeably separated from the nail bed with minimal pain, corresponding to type I photo-onycholysis (Figure). The patient’s only medications were vitamin D (1000 mg once daily) and calcium supplements (1500 mg twice daily). Although the patient exercised strict UV light avoidance for the face, her hands were not protected when she went gardening directly after the treatment. At 5 weeks, the patient returned for her second ALA-PDT treatment of perioral AK and a fungal culture was taken of the left third fingernail, which returned negative results. Poly-ureaurethane nail lacquer 16% was prescribed and was used once daily to protect and strengthen the fingernails. The patient returned for follow-up in clinic after 13 weeks and photo-onycholysis was resolving. Photo-onycholysis is categorized as a phototoxic reaction often associated with drug intake, more specifically with the use of tetracyclines, psoralens, and fluoroquinolones; less commonly with oral contraceptives; or spontaneously.6 It usually is recognized as a crescent-shaped distal separation of the nail surrounded by pigment. The action spectrum is believed to include UVA and UVB, though the exact mechanisms have not been confirmed.5

Our case provides evidence for risks involving the development of photo-onycholysis following PDT. We have no reason to believe there was systemic absorption of ALA, as there were no visible vesicles on the arms or hands after the treatment. Negative fungal culture results excluded onychomycosis. It is our hypothesis that the patient touched her face with her fingernails during the 60-minute incubation time prior to ALA-PDT treatment under blue light, inadvertently collecting ALA under the fingernails. Once she exposed her hands to sunlight while gardening after treatment, the nails likely reacted with the ALA in response to the UV radiation, thus triggering photo-onycholysis.
This case represents a report of fingernail photo-onycholysis from ALA-PDT under blue light as well as a report following treatment of AK not located on the hands with PDT. Although the photo-onycholysis did resolve within a few months of treatment, our case demonstrates the importance of counseling patients more specifically about isolating the ALA treatment zone from nontreated areas on the body during incubation. Improper UV light protection following ALA-PDT is known to produce phototoxic reactions and our case supports this outcome.
To the Editor:
Topical photodynamic therapy (PDT) is one of several effective treatments of actinic keratoses (AKs). Photodynamic therapy involves selection of a lesion field, application of a photosensitizer drug, incubation for an explicit period of time, and illumination of the area from a light source corresponding to the absorption spectrum of the chosen drug.1 A photosensitizer drug used in PDT to target AK is aminolevulinic acid (ALA). Aminolevulinic acid converts disease tissue to photoactivatable porphyrins, especially protoporphyrin IX, which has its largest absorption peak (410 nm) in the blue spectrum, with smaller absorption peaks at 505, 540, 580, and 630 nm. Photodynamic therapy treatments historically have been carried out under red light (peak emissions, 630 nm) to improve tissue penetration, which is superior in efficacy when treating Bowen disease and basal cell carcinoma.1,2 Broadband blue light (peak emission, 417 nm) now is routinely used and has been proven effective in combination with ALA for the treatment of AK.3 It was approved by the US Food and Drug Administration for AKs in 1999.4
Photo-onycholysis is a photosensitivity reaction defined as separation of the nail plate from the nail bed. There are 4 different types of photo-onycholysis characterized by appearance and by the number of digits affected: Type I is denoted by the involvement of several fingers, with half-moon–shaped separations of the nail plate. Type II affects a single finger and corresponds to a brown, defined, circular notch opening distally. Type III, which involves several fingers, is defined as round yellow stains in the central portion of the nail that turn red after 5 to 10 days. Type IV has been associated with bullae under the nails.5 There have been cases of photo-onycholysis arising after exposure to UV light following ingestion of certain prescription drugs or spontaneously,6 and a single case following PDT to the hands with red light.5 We report a case of fingernail photo-onycholysis resulting from ALA-PDT for the treatment of perioral AK.
A 65-year-old woman was treated for AKs on the perioral region of the face with PDT. Aminolevulinic acid hydrochloride 20% was applied to the lips and allowed to incubate for 60 minutes. Her face was illuminated with 10 J/cm² of blue light (417 nm) for 16 minutes and 40 seconds. Sunscreen (sun protection factor 40) was applied to the area immediately after treatment, and the patient was thoroughly counseled to avoid sunlight for the next 48 hours and to use sun protection. Within 72 hours following treatment, the patient reported all 10 fingernails noticeably separated from the nail bed with minimal pain, corresponding to type I photo-onycholysis (Figure). The patient’s only medications were vitamin D (1000 mg once daily) and calcium supplements (1500 mg twice daily). Although the patient exercised strict UV light avoidance for the face, her hands were not protected when she went gardening directly after the treatment. At 5 weeks, the patient returned for her second ALA-PDT treatment of perioral AK and a fungal culture was taken of the left third fingernail, which returned negative results. Poly-ureaurethane nail lacquer 16% was prescribed and was used once daily to protect and strengthen the fingernails. The patient returned for follow-up in clinic after 13 weeks and photo-onycholysis was resolving. Photo-onycholysis is categorized as a phototoxic reaction often associated with drug intake, more specifically with the use of tetracyclines, psoralens, and fluoroquinolones; less commonly with oral contraceptives; or spontaneously.6 It usually is recognized as a crescent-shaped distal separation of the nail surrounded by pigment. The action spectrum is believed to include UVA and UVB, though the exact mechanisms have not been confirmed.5

Our case provides evidence for risks involving the development of photo-onycholysis following PDT. We have no reason to believe there was systemic absorption of ALA, as there were no visible vesicles on the arms or hands after the treatment. Negative fungal culture results excluded onychomycosis. It is our hypothesis that the patient touched her face with her fingernails during the 60-minute incubation time prior to ALA-PDT treatment under blue light, inadvertently collecting ALA under the fingernails. Once she exposed her hands to sunlight while gardening after treatment, the nails likely reacted with the ALA in response to the UV radiation, thus triggering photo-onycholysis.
This case represents a report of fingernail photo-onycholysis from ALA-PDT under blue light as well as a report following treatment of AK not located on the hands with PDT. Although the photo-onycholysis did resolve within a few months of treatment, our case demonstrates the importance of counseling patients more specifically about isolating the ALA treatment zone from nontreated areas on the body during incubation. Improper UV light protection following ALA-PDT is known to produce phototoxic reactions and our case supports this outcome.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Hauschild A. Photodynamic therapy for actinic keratoses: procedure matters? Br J Dermatol. 2012;166:3-5.
- Alexiades-Armenakas M. Laser-mediated photodynamic therapy. Clin Dermatol. 2006;24:16-25.
- Babilas P, Schreml S, Landthaler M, et al. Photodynamic therapy in dermatology: state-of-the-art. Photodermatol Photoimmunol Photomed. 2010;26:118-132.
- Hanneken S, Wessendorf U, Neumann NJ. Photodynamic onycholysis: first report of photo-onycholysis after photodynamic therapy. Clin Exp Dermatol. 2008;33:659-660.
- Baran R, Juhlin L. Photoonycholysis. Photodermatol Photoimmunol Photomed. 2002;18:202-207.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Hauschild A. Photodynamic therapy for actinic keratoses: procedure matters? Br J Dermatol. 2012;166:3-5.
- Alexiades-Armenakas M. Laser-mediated photodynamic therapy. Clin Dermatol. 2006;24:16-25.
- Babilas P, Schreml S, Landthaler M, et al. Photodynamic therapy in dermatology: state-of-the-art. Photodermatol Photoimmunol Photomed. 2010;26:118-132.
- Hanneken S, Wessendorf U, Neumann NJ. Photodynamic onycholysis: first report of photo-onycholysis after photodynamic therapy. Clin Exp Dermatol. 2008;33:659-660.
- Baran R, Juhlin L. Photoonycholysis. Photodermatol Photoimmunol Photomed. 2002;18:202-207.
Practice Points
- Photodynamic therapy with aminolevulinic acid (ALA) is an effective treatment of actinic keratoses but can produce unexpected side effects in locations distant from initial therapy sites.
- It is important to counsel patients prior to initiating photodynamic therapy with ALA about isolating the ALA treatment zone from nontreated areas on the body during incubation.
What Is Your Diagnosis? Mycosis Fungoides
The Diagnosis: Mycosis Fungoides
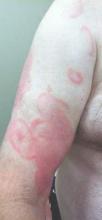
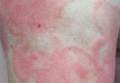
Physical examination revealed erythematous polycyclic and arcuate plaques with fine overlying scale on the right arm and shoulder (Figure 1). Mild wrinkling and telangiectasias were noted on the skin surrounding the lesions. Laboratory tests showed normal values for antinuclear antibodies, anti–Sjögren syndrome–related antigen A, and anti–Sjögren syndrome–related antigen B.
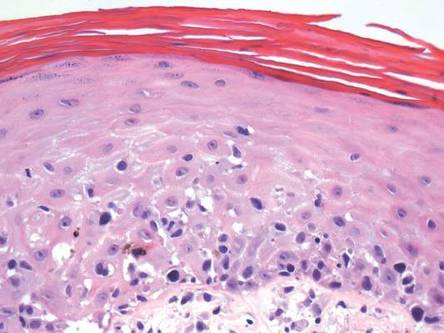
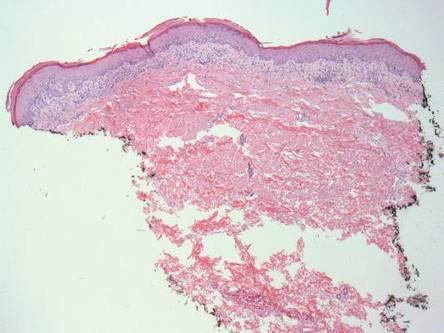
A skin biopsy of a plaque on the right upper arm showed enlarged pleomorphic lymphocytes arranged along the basal layer and in focal collections within the epidermis (Figure 2). Within the dermis were wiry bundles of collagen, a sparse superficial and patchy infiltrate of lymphocytes, and scattered large mononuclear cells (Figure 3). Immunoperoxidase staining revealed large intraepidermal lymphocytes positive for CD4 (Figure 4A) and CD5. Notably, these lymphocytes also stained positive for CD30 (Figure 4B). Staining for CD8, CD1a, CD56, and anaplastic lymphoma kinase was negative, with aberrant loss of CD3. The morphology and pattern of immunoreactivity supported the diagnosis of mycosis fungoides (MF).
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma.1 Its progression is classified in 3 stages: (1) early (patch) stage, (2) plaque stage, and (3) tumor stage. Conclusive diagnosis of early stage MF often is difficult due to its clinical features that are similar to more common benign dermatoses (eg, atopic dermatitis, psoriasis, lichen planus), leading to shortcomings in determining prognosis and selecting an appropriate treatment regimen. With this diagnositic difficulty in mind, guidelines have been created to aid in the diagnosis of early stage MF.2
Clinical features consistent with early stage MF include multiple erythematous, well-demarcated lesions with varying shapes that typically are greater than 5 cm in diameter.2 Lesions usually are flat or thinly elevated and may exhibit slight scaling. As was noted in our patient, poikiloderma of the surrounding skin is fairly specific for early stage MF, as it is not a feature associated with common clinical mimics of MF (eg, atopic dermatitis, psoriasis, lichen planus). The distribution of skin lesions in non–sun-exposed areas is common. The eruption is persistent, though it may wax and wane in severity.2
 |  | |
|
|
Histopathologic examination is necessary to confirm a diagnosis of MF. Typically, early stage MF is marked by enlarged T lymphocytes within the epidermis as well as the papillary and superficial reticular dermis. Cerebriform nuclei are a key finding in the diagnosis of MF. Lymphocytes frequently are arranged linearly along the basal layer of the epidermis. Within the epidermis, clusters of atypical lymphocytes (Pautrier microabscesses) without spongiosis are uncommon but are a characteristic finding of MF if present.1 Papillary dermal fibrosis also may be evident.2
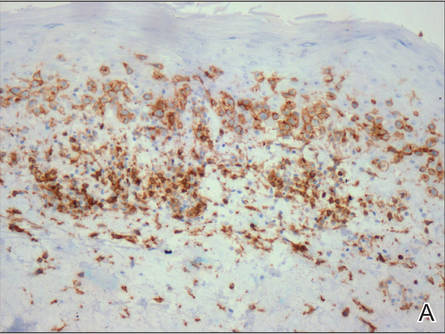 | 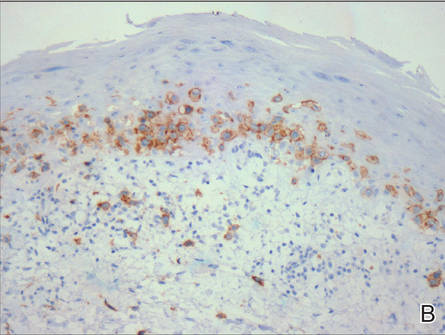 | |
Figure 4. Large intraepidermal lymphocytes were highlighted on CD4 (A) and CD30 immunostaining (B)(original magnification ×200 and ×200). | ||
Immunostaining typically reveals positivity for CD3 and CD4, as well as for lymphocyte antigens CD2 and CD5.1 CD30 positivity in early stage MF rarely has been reported in the literature.3,4 Such cases appear histologically similarly to CD30‒negative cases in other respects. One study showed that the presence of CD30-positive lymphocytes does not alter the clinical course of MF.3 Another study found that, while epidermal CD30-postive lymphocytes had no prognostic relevance, an increased percentage of dermal CD30-positive cells was linked to a higher stage at diagnosis and worse overall prognosis.5 Pathogenesis underlying CD30 positivity in early MF is unknown. It is important to note that CD30-positive cells commonly are seen in lymphomatoid papulosis and anaplastic large cell lymphoma, as well as a variety of nonneoplastic conditions.3,6,7
- Smoller BR. Mycosis fungoides: what do/do not we know? J Cutan Pathol. 2008;35(suppl 2):35-39.
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
- Wu H, Telang GH, Lessin SR, et al. Mycosis fungoides with CD30-positive cells in the epidermis. Am J Dermatopathol. 2000;22:212-216.
- Ohtani T, Kikuchi K, Koizumi H, et al. A case of CD30+ large-cell transformation in a patient with unilesional patch-stage mycosis fungoides. Int J Dermatol. 2009;48:623-626.
- Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33:1860-1868.
- Resnik KS, Kutzner H. Of lymphocytes and cutaneous epithelium: keratoacanthomatous hyperplasia in CD30+ lymphoproliferative disorders and CD30+ cells associated with keratoacanthoma. Am J Dermatopathol. 2010;32:314-315.
- Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J Cutan Pathol. 2006;33(suppl 1):58-70.
The Diagnosis: Mycosis Fungoides
Physical examination revealed erythematous polycyclic and arcuate plaques with fine overlying scale on the right arm and shoulder (Figure 1). Mild wrinkling and telangiectasias were noted on the skin surrounding the lesions. Laboratory tests showed normal values for antinuclear antibodies, anti–Sjögren syndrome–related antigen A, and anti–Sjögren syndrome–related antigen B.
A skin biopsy of a plaque on the right upper arm showed enlarged pleomorphic lymphocytes arranged along the basal layer and in focal collections within the epidermis (Figure 2). Within the dermis were wiry bundles of collagen, a sparse superficial and patchy infiltrate of lymphocytes, and scattered large mononuclear cells (Figure 3). Immunoperoxidase staining revealed large intraepidermal lymphocytes positive for CD4 (Figure 4A) and CD5. Notably, these lymphocytes also stained positive for CD30 (Figure 4B). Staining for CD8, CD1a, CD56, and anaplastic lymphoma kinase was negative, with aberrant loss of CD3. The morphology and pattern of immunoreactivity supported the diagnosis of mycosis fungoides (MF).
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma.1 Its progression is classified in 3 stages: (1) early (patch) stage, (2) plaque stage, and (3) tumor stage. Conclusive diagnosis of early stage MF often is difficult due to its clinical features that are similar to more common benign dermatoses (eg, atopic dermatitis, psoriasis, lichen planus), leading to shortcomings in determining prognosis and selecting an appropriate treatment regimen. With this diagnositic difficulty in mind, guidelines have been created to aid in the diagnosis of early stage MF.2
Clinical features consistent with early stage MF include multiple erythematous, well-demarcated lesions with varying shapes that typically are greater than 5 cm in diameter.2 Lesions usually are flat or thinly elevated and may exhibit slight scaling. As was noted in our patient, poikiloderma of the surrounding skin is fairly specific for early stage MF, as it is not a feature associated with common clinical mimics of MF (eg, atopic dermatitis, psoriasis, lichen planus). The distribution of skin lesions in non–sun-exposed areas is common. The eruption is persistent, though it may wax and wane in severity.2
 |  | |
|
|
Histopathologic examination is necessary to confirm a diagnosis of MF. Typically, early stage MF is marked by enlarged T lymphocytes within the epidermis as well as the papillary and superficial reticular dermis. Cerebriform nuclei are a key finding in the diagnosis of MF. Lymphocytes frequently are arranged linearly along the basal layer of the epidermis. Within the epidermis, clusters of atypical lymphocytes (Pautrier microabscesses) without spongiosis are uncommon but are a characteristic finding of MF if present.1 Papillary dermal fibrosis also may be evident.2
 |  | |
Figure 4. Large intraepidermal lymphocytes were highlighted on CD4 (A) and CD30 immunostaining (B)(original magnification ×200 and ×200). | ||
Immunostaining typically reveals positivity for CD3 and CD4, as well as for lymphocyte antigens CD2 and CD5.1 CD30 positivity in early stage MF rarely has been reported in the literature.3,4 Such cases appear histologically similarly to CD30‒negative cases in other respects. One study showed that the presence of CD30-positive lymphocytes does not alter the clinical course of MF.3 Another study found that, while epidermal CD30-postive lymphocytes had no prognostic relevance, an increased percentage of dermal CD30-positive cells was linked to a higher stage at diagnosis and worse overall prognosis.5 Pathogenesis underlying CD30 positivity in early MF is unknown. It is important to note that CD30-positive cells commonly are seen in lymphomatoid papulosis and anaplastic large cell lymphoma, as well as a variety of nonneoplastic conditions.3,6,7
The Diagnosis: Mycosis Fungoides
Physical examination revealed erythematous polycyclic and arcuate plaques with fine overlying scale on the right arm and shoulder (Figure 1). Mild wrinkling and telangiectasias were noted on the skin surrounding the lesions. Laboratory tests showed normal values for antinuclear antibodies, anti–Sjögren syndrome–related antigen A, and anti–Sjögren syndrome–related antigen B.
A skin biopsy of a plaque on the right upper arm showed enlarged pleomorphic lymphocytes arranged along the basal layer and in focal collections within the epidermis (Figure 2). Within the dermis were wiry bundles of collagen, a sparse superficial and patchy infiltrate of lymphocytes, and scattered large mononuclear cells (Figure 3). Immunoperoxidase staining revealed large intraepidermal lymphocytes positive for CD4 (Figure 4A) and CD5. Notably, these lymphocytes also stained positive for CD30 (Figure 4B). Staining for CD8, CD1a, CD56, and anaplastic lymphoma kinase was negative, with aberrant loss of CD3. The morphology and pattern of immunoreactivity supported the diagnosis of mycosis fungoides (MF).
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma.1 Its progression is classified in 3 stages: (1) early (patch) stage, (2) plaque stage, and (3) tumor stage. Conclusive diagnosis of early stage MF often is difficult due to its clinical features that are similar to more common benign dermatoses (eg, atopic dermatitis, psoriasis, lichen planus), leading to shortcomings in determining prognosis and selecting an appropriate treatment regimen. With this diagnositic difficulty in mind, guidelines have been created to aid in the diagnosis of early stage MF.2
Clinical features consistent with early stage MF include multiple erythematous, well-demarcated lesions with varying shapes that typically are greater than 5 cm in diameter.2 Lesions usually are flat or thinly elevated and may exhibit slight scaling. As was noted in our patient, poikiloderma of the surrounding skin is fairly specific for early stage MF, as it is not a feature associated with common clinical mimics of MF (eg, atopic dermatitis, psoriasis, lichen planus). The distribution of skin lesions in non–sun-exposed areas is common. The eruption is persistent, though it may wax and wane in severity.2
 |  | |
|
|
Histopathologic examination is necessary to confirm a diagnosis of MF. Typically, early stage MF is marked by enlarged T lymphocytes within the epidermis as well as the papillary and superficial reticular dermis. Cerebriform nuclei are a key finding in the diagnosis of MF. Lymphocytes frequently are arranged linearly along the basal layer of the epidermis. Within the epidermis, clusters of atypical lymphocytes (Pautrier microabscesses) without spongiosis are uncommon but are a characteristic finding of MF if present.1 Papillary dermal fibrosis also may be evident.2
 |  | |
Figure 4. Large intraepidermal lymphocytes were highlighted on CD4 (A) and CD30 immunostaining (B)(original magnification ×200 and ×200). | ||
Immunostaining typically reveals positivity for CD3 and CD4, as well as for lymphocyte antigens CD2 and CD5.1 CD30 positivity in early stage MF rarely has been reported in the literature.3,4 Such cases appear histologically similarly to CD30‒negative cases in other respects. One study showed that the presence of CD30-positive lymphocytes does not alter the clinical course of MF.3 Another study found that, while epidermal CD30-postive lymphocytes had no prognostic relevance, an increased percentage of dermal CD30-positive cells was linked to a higher stage at diagnosis and worse overall prognosis.5 Pathogenesis underlying CD30 positivity in early MF is unknown. It is important to note that CD30-positive cells commonly are seen in lymphomatoid papulosis and anaplastic large cell lymphoma, as well as a variety of nonneoplastic conditions.3,6,7
- Smoller BR. Mycosis fungoides: what do/do not we know? J Cutan Pathol. 2008;35(suppl 2):35-39.
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
- Wu H, Telang GH, Lessin SR, et al. Mycosis fungoides with CD30-positive cells in the epidermis. Am J Dermatopathol. 2000;22:212-216.
- Ohtani T, Kikuchi K, Koizumi H, et al. A case of CD30+ large-cell transformation in a patient with unilesional patch-stage mycosis fungoides. Int J Dermatol. 2009;48:623-626.
- Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33:1860-1868.
- Resnik KS, Kutzner H. Of lymphocytes and cutaneous epithelium: keratoacanthomatous hyperplasia in CD30+ lymphoproliferative disorders and CD30+ cells associated with keratoacanthoma. Am J Dermatopathol. 2010;32:314-315.
- Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J Cutan Pathol. 2006;33(suppl 1):58-70.
- Smoller BR. Mycosis fungoides: what do/do not we know? J Cutan Pathol. 2008;35(suppl 2):35-39.
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
- Wu H, Telang GH, Lessin SR, et al. Mycosis fungoides with CD30-positive cells in the epidermis. Am J Dermatopathol. 2000;22:212-216.
- Ohtani T, Kikuchi K, Koizumi H, et al. A case of CD30+ large-cell transformation in a patient with unilesional patch-stage mycosis fungoides. Int J Dermatol. 2009;48:623-626.
- Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33:1860-1868.
- Resnik KS, Kutzner H. Of lymphocytes and cutaneous epithelium: keratoacanthomatous hyperplasia in CD30+ lymphoproliferative disorders and CD30+ cells associated with keratoacanthoma. Am J Dermatopathol. 2010;32:314-315.
- Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J Cutan Pathol. 2006;33(suppl 1):58-70.
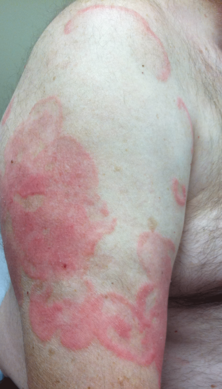
An otherwise healthy 62-year-old man presented for evaluation of multiple scaly erythematous plaques on the right upper arm and shoulder of 10 years’ duration. The patient reported a burning sensation but no exacerbation of the lesions upon sun exposure. He previously had been treated for a presumed clinical diagnosis of erythema annulare centrifugum but experienced only modest improvement with topical corticosteroids and tacrolimus ointment 0.1%. Previous trials of systemic antifungals also yielded minimal benefit.