User login
What Is Your Diagnosis? Mycosis Fungoides
The Diagnosis: Mycosis Fungoides
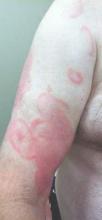
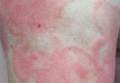
Physical examination revealed erythematous polycyclic and arcuate plaques with fine overlying scale on the right arm and shoulder (Figure 1). Mild wrinkling and telangiectasias were noted on the skin surrounding the lesions. Laboratory tests showed normal values for antinuclear antibodies, anti–Sjögren syndrome–related antigen A, and anti–Sjögren syndrome–related antigen B.
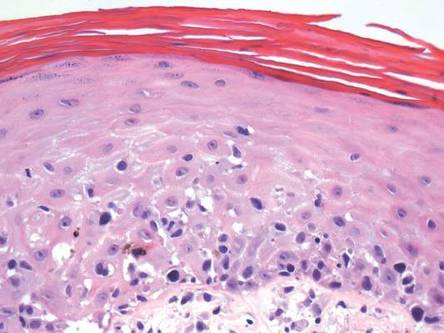
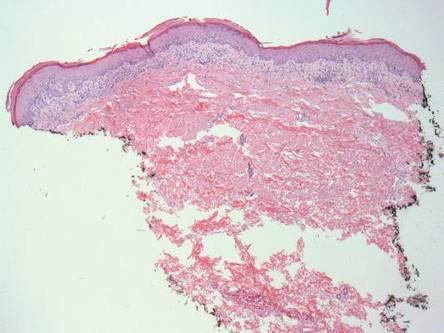
A skin biopsy of a plaque on the right upper arm showed enlarged pleomorphic lymphocytes arranged along the basal layer and in focal collections within the epidermis (Figure 2). Within the dermis were wiry bundles of collagen, a sparse superficial and patchy infiltrate of lymphocytes, and scattered large mononuclear cells (Figure 3). Immunoperoxidase staining revealed large intraepidermal lymphocytes positive for CD4 (Figure 4A) and CD5. Notably, these lymphocytes also stained positive for CD30 (Figure 4B). Staining for CD8, CD1a, CD56, and anaplastic lymphoma kinase was negative, with aberrant loss of CD3. The morphology and pattern of immunoreactivity supported the diagnosis of mycosis fungoides (MF).
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma.1 Its progression is classified in 3 stages: (1) early (patch) stage, (2) plaque stage, and (3) tumor stage. Conclusive diagnosis of early stage MF often is difficult due to its clinical features that are similar to more common benign dermatoses (eg, atopic dermatitis, psoriasis, lichen planus), leading to shortcomings in determining prognosis and selecting an appropriate treatment regimen. With this diagnositic difficulty in mind, guidelines have been created to aid in the diagnosis of early stage MF.2
Clinical features consistent with early stage MF include multiple erythematous, well-demarcated lesions with varying shapes that typically are greater than 5 cm in diameter.2 Lesions usually are flat or thinly elevated and may exhibit slight scaling. As was noted in our patient, poikiloderma of the surrounding skin is fairly specific for early stage MF, as it is not a feature associated with common clinical mimics of MF (eg, atopic dermatitis, psoriasis, lichen planus). The distribution of skin lesions in non–sun-exposed areas is common. The eruption is persistent, though it may wax and wane in severity.2
 |  | |
|
|
Histopathologic examination is necessary to confirm a diagnosis of MF. Typically, early stage MF is marked by enlarged T lymphocytes within the epidermis as well as the papillary and superficial reticular dermis. Cerebriform nuclei are a key finding in the diagnosis of MF. Lymphocytes frequently are arranged linearly along the basal layer of the epidermis. Within the epidermis, clusters of atypical lymphocytes (Pautrier microabscesses) without spongiosis are uncommon but are a characteristic finding of MF if present.1 Papillary dermal fibrosis also may be evident.2
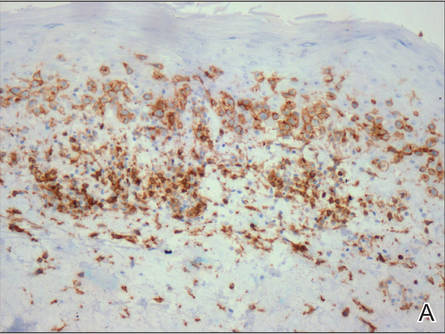 | 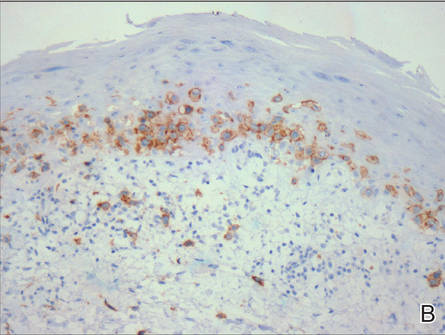 | |
Figure 4. Large intraepidermal lymphocytes were highlighted on CD4 (A) and CD30 immunostaining (B)(original magnification ×200 and ×200). | ||
Immunostaining typically reveals positivity for CD3 and CD4, as well as for lymphocyte antigens CD2 and CD5.1 CD30 positivity in early stage MF rarely has been reported in the literature.3,4 Such cases appear histologically similarly to CD30‒negative cases in other respects. One study showed that the presence of CD30-positive lymphocytes does not alter the clinical course of MF.3 Another study found that, while epidermal CD30-postive lymphocytes had no prognostic relevance, an increased percentage of dermal CD30-positive cells was linked to a higher stage at diagnosis and worse overall prognosis.5 Pathogenesis underlying CD30 positivity in early MF is unknown. It is important to note that CD30-positive cells commonly are seen in lymphomatoid papulosis and anaplastic large cell lymphoma, as well as a variety of nonneoplastic conditions.3,6,7
- Smoller BR. Mycosis fungoides: what do/do not we know? J Cutan Pathol. 2008;35(suppl 2):35-39.
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
- Wu H, Telang GH, Lessin SR, et al. Mycosis fungoides with CD30-positive cells in the epidermis. Am J Dermatopathol. 2000;22:212-216.
- Ohtani T, Kikuchi K, Koizumi H, et al. A case of CD30+ large-cell transformation in a patient with unilesional patch-stage mycosis fungoides. Int J Dermatol. 2009;48:623-626.
- Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33:1860-1868.
- Resnik KS, Kutzner H. Of lymphocytes and cutaneous epithelium: keratoacanthomatous hyperplasia in CD30+ lymphoproliferative disorders and CD30+ cells associated with keratoacanthoma. Am J Dermatopathol. 2010;32:314-315.
- Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J Cutan Pathol. 2006;33(suppl 1):58-70.
The Diagnosis: Mycosis Fungoides
Physical examination revealed erythematous polycyclic and arcuate plaques with fine overlying scale on the right arm and shoulder (Figure 1). Mild wrinkling and telangiectasias were noted on the skin surrounding the lesions. Laboratory tests showed normal values for antinuclear antibodies, anti–Sjögren syndrome–related antigen A, and anti–Sjögren syndrome–related antigen B.
A skin biopsy of a plaque on the right upper arm showed enlarged pleomorphic lymphocytes arranged along the basal layer and in focal collections within the epidermis (Figure 2). Within the dermis were wiry bundles of collagen, a sparse superficial and patchy infiltrate of lymphocytes, and scattered large mononuclear cells (Figure 3). Immunoperoxidase staining revealed large intraepidermal lymphocytes positive for CD4 (Figure 4A) and CD5. Notably, these lymphocytes also stained positive for CD30 (Figure 4B). Staining for CD8, CD1a, CD56, and anaplastic lymphoma kinase was negative, with aberrant loss of CD3. The morphology and pattern of immunoreactivity supported the diagnosis of mycosis fungoides (MF).
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma.1 Its progression is classified in 3 stages: (1) early (patch) stage, (2) plaque stage, and (3) tumor stage. Conclusive diagnosis of early stage MF often is difficult due to its clinical features that are similar to more common benign dermatoses (eg, atopic dermatitis, psoriasis, lichen planus), leading to shortcomings in determining prognosis and selecting an appropriate treatment regimen. With this diagnositic difficulty in mind, guidelines have been created to aid in the diagnosis of early stage MF.2
Clinical features consistent with early stage MF include multiple erythematous, well-demarcated lesions with varying shapes that typically are greater than 5 cm in diameter.2 Lesions usually are flat or thinly elevated and may exhibit slight scaling. As was noted in our patient, poikiloderma of the surrounding skin is fairly specific for early stage MF, as it is not a feature associated with common clinical mimics of MF (eg, atopic dermatitis, psoriasis, lichen planus). The distribution of skin lesions in non–sun-exposed areas is common. The eruption is persistent, though it may wax and wane in severity.2
 |  | |
|
|
Histopathologic examination is necessary to confirm a diagnosis of MF. Typically, early stage MF is marked by enlarged T lymphocytes within the epidermis as well as the papillary and superficial reticular dermis. Cerebriform nuclei are a key finding in the diagnosis of MF. Lymphocytes frequently are arranged linearly along the basal layer of the epidermis. Within the epidermis, clusters of atypical lymphocytes (Pautrier microabscesses) without spongiosis are uncommon but are a characteristic finding of MF if present.1 Papillary dermal fibrosis also may be evident.2
 |  | |
Figure 4. Large intraepidermal lymphocytes were highlighted on CD4 (A) and CD30 immunostaining (B)(original magnification ×200 and ×200). | ||
Immunostaining typically reveals positivity for CD3 and CD4, as well as for lymphocyte antigens CD2 and CD5.1 CD30 positivity in early stage MF rarely has been reported in the literature.3,4 Such cases appear histologically similarly to CD30‒negative cases in other respects. One study showed that the presence of CD30-positive lymphocytes does not alter the clinical course of MF.3 Another study found that, while epidermal CD30-postive lymphocytes had no prognostic relevance, an increased percentage of dermal CD30-positive cells was linked to a higher stage at diagnosis and worse overall prognosis.5 Pathogenesis underlying CD30 positivity in early MF is unknown. It is important to note that CD30-positive cells commonly are seen in lymphomatoid papulosis and anaplastic large cell lymphoma, as well as a variety of nonneoplastic conditions.3,6,7
The Diagnosis: Mycosis Fungoides
Physical examination revealed erythematous polycyclic and arcuate plaques with fine overlying scale on the right arm and shoulder (Figure 1). Mild wrinkling and telangiectasias were noted on the skin surrounding the lesions. Laboratory tests showed normal values for antinuclear antibodies, anti–Sjögren syndrome–related antigen A, and anti–Sjögren syndrome–related antigen B.
A skin biopsy of a plaque on the right upper arm showed enlarged pleomorphic lymphocytes arranged along the basal layer and in focal collections within the epidermis (Figure 2). Within the dermis were wiry bundles of collagen, a sparse superficial and patchy infiltrate of lymphocytes, and scattered large mononuclear cells (Figure 3). Immunoperoxidase staining revealed large intraepidermal lymphocytes positive for CD4 (Figure 4A) and CD5. Notably, these lymphocytes also stained positive for CD30 (Figure 4B). Staining for CD8, CD1a, CD56, and anaplastic lymphoma kinase was negative, with aberrant loss of CD3. The morphology and pattern of immunoreactivity supported the diagnosis of mycosis fungoides (MF).
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma.1 Its progression is classified in 3 stages: (1) early (patch) stage, (2) plaque stage, and (3) tumor stage. Conclusive diagnosis of early stage MF often is difficult due to its clinical features that are similar to more common benign dermatoses (eg, atopic dermatitis, psoriasis, lichen planus), leading to shortcomings in determining prognosis and selecting an appropriate treatment regimen. With this diagnositic difficulty in mind, guidelines have been created to aid in the diagnosis of early stage MF.2
Clinical features consistent with early stage MF include multiple erythematous, well-demarcated lesions with varying shapes that typically are greater than 5 cm in diameter.2 Lesions usually are flat or thinly elevated and may exhibit slight scaling. As was noted in our patient, poikiloderma of the surrounding skin is fairly specific for early stage MF, as it is not a feature associated with common clinical mimics of MF (eg, atopic dermatitis, psoriasis, lichen planus). The distribution of skin lesions in non–sun-exposed areas is common. The eruption is persistent, though it may wax and wane in severity.2
 |  | |
|
|
Histopathologic examination is necessary to confirm a diagnosis of MF. Typically, early stage MF is marked by enlarged T lymphocytes within the epidermis as well as the papillary and superficial reticular dermis. Cerebriform nuclei are a key finding in the diagnosis of MF. Lymphocytes frequently are arranged linearly along the basal layer of the epidermis. Within the epidermis, clusters of atypical lymphocytes (Pautrier microabscesses) without spongiosis are uncommon but are a characteristic finding of MF if present.1 Papillary dermal fibrosis also may be evident.2
 |  | |
Figure 4. Large intraepidermal lymphocytes were highlighted on CD4 (A) and CD30 immunostaining (B)(original magnification ×200 and ×200). | ||
Immunostaining typically reveals positivity for CD3 and CD4, as well as for lymphocyte antigens CD2 and CD5.1 CD30 positivity in early stage MF rarely has been reported in the literature.3,4 Such cases appear histologically similarly to CD30‒negative cases in other respects. One study showed that the presence of CD30-positive lymphocytes does not alter the clinical course of MF.3 Another study found that, while epidermal CD30-postive lymphocytes had no prognostic relevance, an increased percentage of dermal CD30-positive cells was linked to a higher stage at diagnosis and worse overall prognosis.5 Pathogenesis underlying CD30 positivity in early MF is unknown. It is important to note that CD30-positive cells commonly are seen in lymphomatoid papulosis and anaplastic large cell lymphoma, as well as a variety of nonneoplastic conditions.3,6,7
- Smoller BR. Mycosis fungoides: what do/do not we know? J Cutan Pathol. 2008;35(suppl 2):35-39.
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
- Wu H, Telang GH, Lessin SR, et al. Mycosis fungoides with CD30-positive cells in the epidermis. Am J Dermatopathol. 2000;22:212-216.
- Ohtani T, Kikuchi K, Koizumi H, et al. A case of CD30+ large-cell transformation in a patient with unilesional patch-stage mycosis fungoides. Int J Dermatol. 2009;48:623-626.
- Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33:1860-1868.
- Resnik KS, Kutzner H. Of lymphocytes and cutaneous epithelium: keratoacanthomatous hyperplasia in CD30+ lymphoproliferative disorders and CD30+ cells associated with keratoacanthoma. Am J Dermatopathol. 2010;32:314-315.
- Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J Cutan Pathol. 2006;33(suppl 1):58-70.
- Smoller BR. Mycosis fungoides: what do/do not we know? J Cutan Pathol. 2008;35(suppl 2):35-39.
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
- Wu H, Telang GH, Lessin SR, et al. Mycosis fungoides with CD30-positive cells in the epidermis. Am J Dermatopathol. 2000;22:212-216.
- Ohtani T, Kikuchi K, Koizumi H, et al. A case of CD30+ large-cell transformation in a patient with unilesional patch-stage mycosis fungoides. Int J Dermatol. 2009;48:623-626.
- Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33:1860-1868.
- Resnik KS, Kutzner H. Of lymphocytes and cutaneous epithelium: keratoacanthomatous hyperplasia in CD30+ lymphoproliferative disorders and CD30+ cells associated with keratoacanthoma. Am J Dermatopathol. 2010;32:314-315.
- Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J Cutan Pathol. 2006;33(suppl 1):58-70.
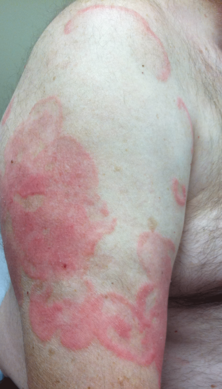
An otherwise healthy 62-year-old man presented for evaluation of multiple scaly erythematous plaques on the right upper arm and shoulder of 10 years’ duration. The patient reported a burning sensation but no exacerbation of the lesions upon sun exposure. He previously had been treated for a presumed clinical diagnosis of erythema annulare centrifugum but experienced only modest improvement with topical corticosteroids and tacrolimus ointment 0.1%. Previous trials of systemic antifungals also yielded minimal benefit.
Vegetative Sacral Plaque in a Patient With Human Immunodeficiency Virus
The Diagnosis: Herpes Simplex Vegetans
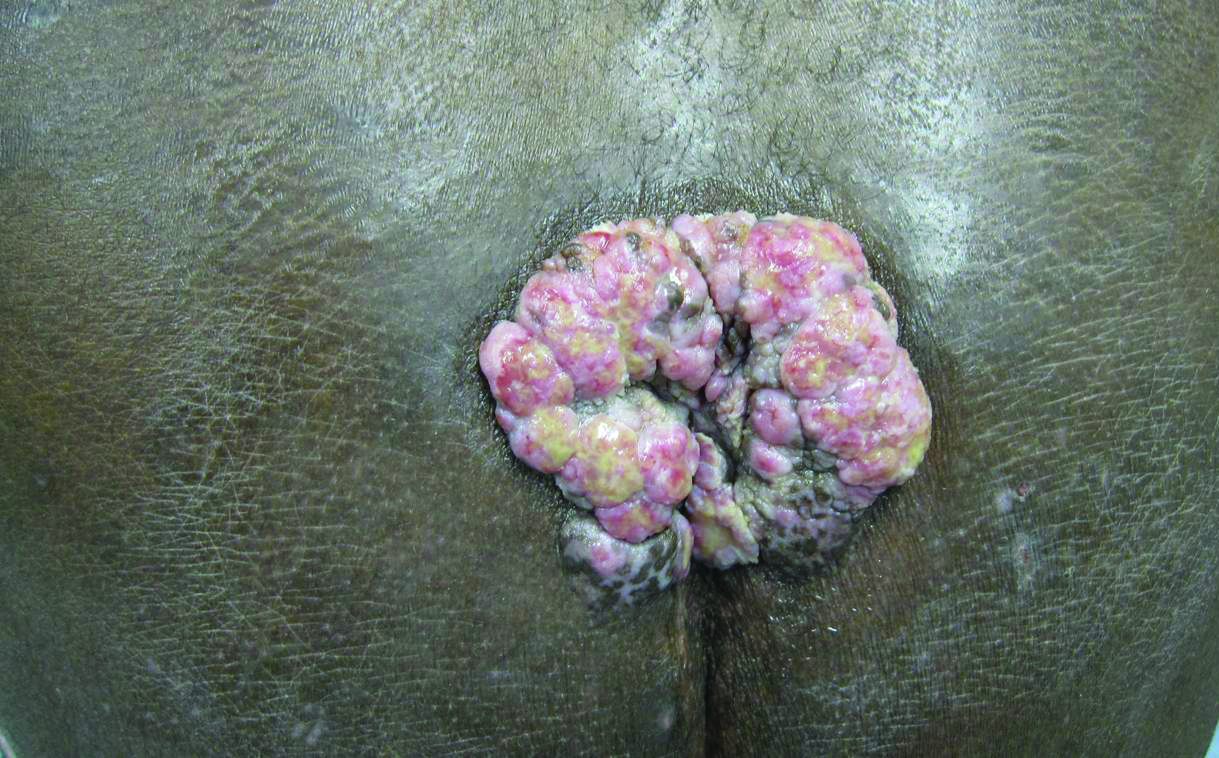
Histopathologic examination using hematoxylin and eosin stain demonstrated marked pseudoepitheliomatous hyperplasia with granulation tissue, ulceration, and abundant exudate joined by a dense mixed inflammatory cell infiltrate that included a myriad of eosinophils (Figure, A). At higher power (Figure, B), many single and multinucleate acantholytic keratinocytes showed ground-glass nuclei and peripheral margination of chromatin within zones of ulceration and crust. Viral culture and direct fluorescent antibody assay identified herpes simplex virus (HSV) type 2. Based on the clinical and histopathologic findings, the patient was diagnosed with herpes simplex vegetans. He was initially treated with oral acyclovir and then oral famciclovir but showed minimal improvement. Eventually, he was referred to surgery and the mass was totally excised with clear margins and no evidence of underlying malignancy.
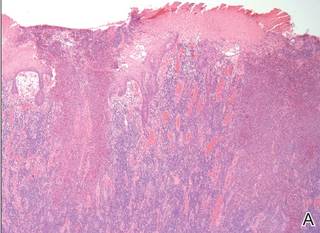  |
| Histopathology revealed marked pseudoepitheliomatous hyperplasia, ulceration, and a dense mixed inflammatory cell infiltrate (A)(H&E, original magnification ×20). Many multinucleate acantholytic keratinocytes with ground-glass nuclei and peripheral margination of chromatin were shown (B)(H&E, original magnification ×400). |
Herpes simplex virus is one of the most common sexually transmitted infections, with a notably increased incidence and prevalence among individuals with human immunodeficiency virus (HIV) infection.1 Although typical HSV manifestation in immunocompetent patients includes clustered vesicles and/or ulcerations, immunocompromised patients may have unusual presentations, such as persistent and extensive ulcerations or nodular hyperkeratotic lesions.2,3 Herpes vegetans, a term used to describe these atypical exophytic lesions, rarely has been reported in literature, but its presence should raise suspicion for possible underlying immunocompromise. The pathogenesis behind the hypertrophic nature of these lesions is not well understood, but it is postulated that the immune dysregulation from concomitant HIV and HSV infection plays a role.2 Overproduction of tumor necrosis factor and IL-6 by HIV-infected dermal dendritic cells causes an increase in antiapoptotic factors within the epidermis, resulting in enhanced keratinocyte proliferation and clinical hyperkeratosis.2,4
The differential diagnosis for herpes vegetans is somewhat broad, owing to the verrucous and often eroded appearance of the lesions. Biopsy and cultures can be obtained to differentiate from condyloma acuminatum, condyloma latum (secondary syphilis), pyoderma vegetans, pemphigus vegetans, granuloma inguinale, extraintestinal Crohn disease, deep fungal infections, cutaneous tuberculosis, and malignancy.2-4 Histopathology shows epithelial hyperplasia and ulceration with scattered multinucleate keratinocytes, usually at the periphery of the ulcer, and intranuclear inclusions typical of HSV. In addition, a dense dermal infiltrate of lymphocytes, histiocytes, plasma cells, and eosinophils is usually present beneath the base of the ulcer.2,4
Treatment options for herpes vegetans are limited due to the high prevalence of acyclovir-resistant (ACV-R) HSV-2 strains in HIV patients. Valacyclovir and penciclovir have been largely ineffective against ACV-R HSV due to their dependence on the same enzyme—thymidine kinase—involved in the mechanism of acyclovir resistance. Intravenous foscarnet and cidofovir have shown efficacy against ACV-R virus, but concerns of nephrotoxicity have limited their use over prolonged intervals.5 Castelo-Soccio et al6 reported promising results with intralesional cidofovir. This route of administration provides the advantage of increased bioavailability with reduced risk for nephrotoxicity.6 Finally, surgical resection may be considered for refractory lesions to circumvent the toxicity from systemically administered drugs.3
- Severson JL, Tyring SK. Relation between herpes simplex viruses and human immunodeficiency virus infections. Arch Dermatol. 1999;135:1393-1397.
- Patel AB, Rosen T. Herpes vegetans as a sign of HIV infection. Dermatol Online J. 2008;14:6.
- Chung VQ, Parker DC, Parker SR. Surgical excision for vegetative herpes simplex virus infection. Dermatol Surg. 2007;33:1374-1379.
- Beasley KL, Cooley GE, Kao GF, et al. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5, pt 2):860-863.
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126.
The Diagnosis: Herpes Simplex Vegetans
Histopathologic examination using hematoxylin and eosin stain demonstrated marked pseudoepitheliomatous hyperplasia with granulation tissue, ulceration, and abundant exudate joined by a dense mixed inflammatory cell infiltrate that included a myriad of eosinophils (Figure, A). At higher power (Figure, B), many single and multinucleate acantholytic keratinocytes showed ground-glass nuclei and peripheral margination of chromatin within zones of ulceration and crust. Viral culture and direct fluorescent antibody assay identified herpes simplex virus (HSV) type 2. Based on the clinical and histopathologic findings, the patient was diagnosed with herpes simplex vegetans. He was initially treated with oral acyclovir and then oral famciclovir but showed minimal improvement. Eventually, he was referred to surgery and the mass was totally excised with clear margins and no evidence of underlying malignancy.
  |
| Histopathology revealed marked pseudoepitheliomatous hyperplasia, ulceration, and a dense mixed inflammatory cell infiltrate (A)(H&E, original magnification ×20). Many multinucleate acantholytic keratinocytes with ground-glass nuclei and peripheral margination of chromatin were shown (B)(H&E, original magnification ×400). |
Herpes simplex virus is one of the most common sexually transmitted infections, with a notably increased incidence and prevalence among individuals with human immunodeficiency virus (HIV) infection.1 Although typical HSV manifestation in immunocompetent patients includes clustered vesicles and/or ulcerations, immunocompromised patients may have unusual presentations, such as persistent and extensive ulcerations or nodular hyperkeratotic lesions.2,3 Herpes vegetans, a term used to describe these atypical exophytic lesions, rarely has been reported in literature, but its presence should raise suspicion for possible underlying immunocompromise. The pathogenesis behind the hypertrophic nature of these lesions is not well understood, but it is postulated that the immune dysregulation from concomitant HIV and HSV infection plays a role.2 Overproduction of tumor necrosis factor and IL-6 by HIV-infected dermal dendritic cells causes an increase in antiapoptotic factors within the epidermis, resulting in enhanced keratinocyte proliferation and clinical hyperkeratosis.2,4
The differential diagnosis for herpes vegetans is somewhat broad, owing to the verrucous and often eroded appearance of the lesions. Biopsy and cultures can be obtained to differentiate from condyloma acuminatum, condyloma latum (secondary syphilis), pyoderma vegetans, pemphigus vegetans, granuloma inguinale, extraintestinal Crohn disease, deep fungal infections, cutaneous tuberculosis, and malignancy.2-4 Histopathology shows epithelial hyperplasia and ulceration with scattered multinucleate keratinocytes, usually at the periphery of the ulcer, and intranuclear inclusions typical of HSV. In addition, a dense dermal infiltrate of lymphocytes, histiocytes, plasma cells, and eosinophils is usually present beneath the base of the ulcer.2,4
Treatment options for herpes vegetans are limited due to the high prevalence of acyclovir-resistant (ACV-R) HSV-2 strains in HIV patients. Valacyclovir and penciclovir have been largely ineffective against ACV-R HSV due to their dependence on the same enzyme—thymidine kinase—involved in the mechanism of acyclovir resistance. Intravenous foscarnet and cidofovir have shown efficacy against ACV-R virus, but concerns of nephrotoxicity have limited their use over prolonged intervals.5 Castelo-Soccio et al6 reported promising results with intralesional cidofovir. This route of administration provides the advantage of increased bioavailability with reduced risk for nephrotoxicity.6 Finally, surgical resection may be considered for refractory lesions to circumvent the toxicity from systemically administered drugs.3
The Diagnosis: Herpes Simplex Vegetans
Histopathologic examination using hematoxylin and eosin stain demonstrated marked pseudoepitheliomatous hyperplasia with granulation tissue, ulceration, and abundant exudate joined by a dense mixed inflammatory cell infiltrate that included a myriad of eosinophils (Figure, A). At higher power (Figure, B), many single and multinucleate acantholytic keratinocytes showed ground-glass nuclei and peripheral margination of chromatin within zones of ulceration and crust. Viral culture and direct fluorescent antibody assay identified herpes simplex virus (HSV) type 2. Based on the clinical and histopathologic findings, the patient was diagnosed with herpes simplex vegetans. He was initially treated with oral acyclovir and then oral famciclovir but showed minimal improvement. Eventually, he was referred to surgery and the mass was totally excised with clear margins and no evidence of underlying malignancy.
  |
| Histopathology revealed marked pseudoepitheliomatous hyperplasia, ulceration, and a dense mixed inflammatory cell infiltrate (A)(H&E, original magnification ×20). Many multinucleate acantholytic keratinocytes with ground-glass nuclei and peripheral margination of chromatin were shown (B)(H&E, original magnification ×400). |
Herpes simplex virus is one of the most common sexually transmitted infections, with a notably increased incidence and prevalence among individuals with human immunodeficiency virus (HIV) infection.1 Although typical HSV manifestation in immunocompetent patients includes clustered vesicles and/or ulcerations, immunocompromised patients may have unusual presentations, such as persistent and extensive ulcerations or nodular hyperkeratotic lesions.2,3 Herpes vegetans, a term used to describe these atypical exophytic lesions, rarely has been reported in literature, but its presence should raise suspicion for possible underlying immunocompromise. The pathogenesis behind the hypertrophic nature of these lesions is not well understood, but it is postulated that the immune dysregulation from concomitant HIV and HSV infection plays a role.2 Overproduction of tumor necrosis factor and IL-6 by HIV-infected dermal dendritic cells causes an increase in antiapoptotic factors within the epidermis, resulting in enhanced keratinocyte proliferation and clinical hyperkeratosis.2,4
The differential diagnosis for herpes vegetans is somewhat broad, owing to the verrucous and often eroded appearance of the lesions. Biopsy and cultures can be obtained to differentiate from condyloma acuminatum, condyloma latum (secondary syphilis), pyoderma vegetans, pemphigus vegetans, granuloma inguinale, extraintestinal Crohn disease, deep fungal infections, cutaneous tuberculosis, and malignancy.2-4 Histopathology shows epithelial hyperplasia and ulceration with scattered multinucleate keratinocytes, usually at the periphery of the ulcer, and intranuclear inclusions typical of HSV. In addition, a dense dermal infiltrate of lymphocytes, histiocytes, plasma cells, and eosinophils is usually present beneath the base of the ulcer.2,4
Treatment options for herpes vegetans are limited due to the high prevalence of acyclovir-resistant (ACV-R) HSV-2 strains in HIV patients. Valacyclovir and penciclovir have been largely ineffective against ACV-R HSV due to their dependence on the same enzyme—thymidine kinase—involved in the mechanism of acyclovir resistance. Intravenous foscarnet and cidofovir have shown efficacy against ACV-R virus, but concerns of nephrotoxicity have limited their use over prolonged intervals.5 Castelo-Soccio et al6 reported promising results with intralesional cidofovir. This route of administration provides the advantage of increased bioavailability with reduced risk for nephrotoxicity.6 Finally, surgical resection may be considered for refractory lesions to circumvent the toxicity from systemically administered drugs.3
- Severson JL, Tyring SK. Relation between herpes simplex viruses and human immunodeficiency virus infections. Arch Dermatol. 1999;135:1393-1397.
- Patel AB, Rosen T. Herpes vegetans as a sign of HIV infection. Dermatol Online J. 2008;14:6.
- Chung VQ, Parker DC, Parker SR. Surgical excision for vegetative herpes simplex virus infection. Dermatol Surg. 2007;33:1374-1379.
- Beasley KL, Cooley GE, Kao GF, et al. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5, pt 2):860-863.
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126.
- Severson JL, Tyring SK. Relation between herpes simplex viruses and human immunodeficiency virus infections. Arch Dermatol. 1999;135:1393-1397.
- Patel AB, Rosen T. Herpes vegetans as a sign of HIV infection. Dermatol Online J. 2008;14:6.
- Chung VQ, Parker DC, Parker SR. Surgical excision for vegetative herpes simplex virus infection. Dermatol Surg. 2007;33:1374-1379.
- Beasley KL, Cooley GE, Kao GF, et al. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J Am Acad Dermatol. 1997;37(5, pt 2):860-863.
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320.
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126.
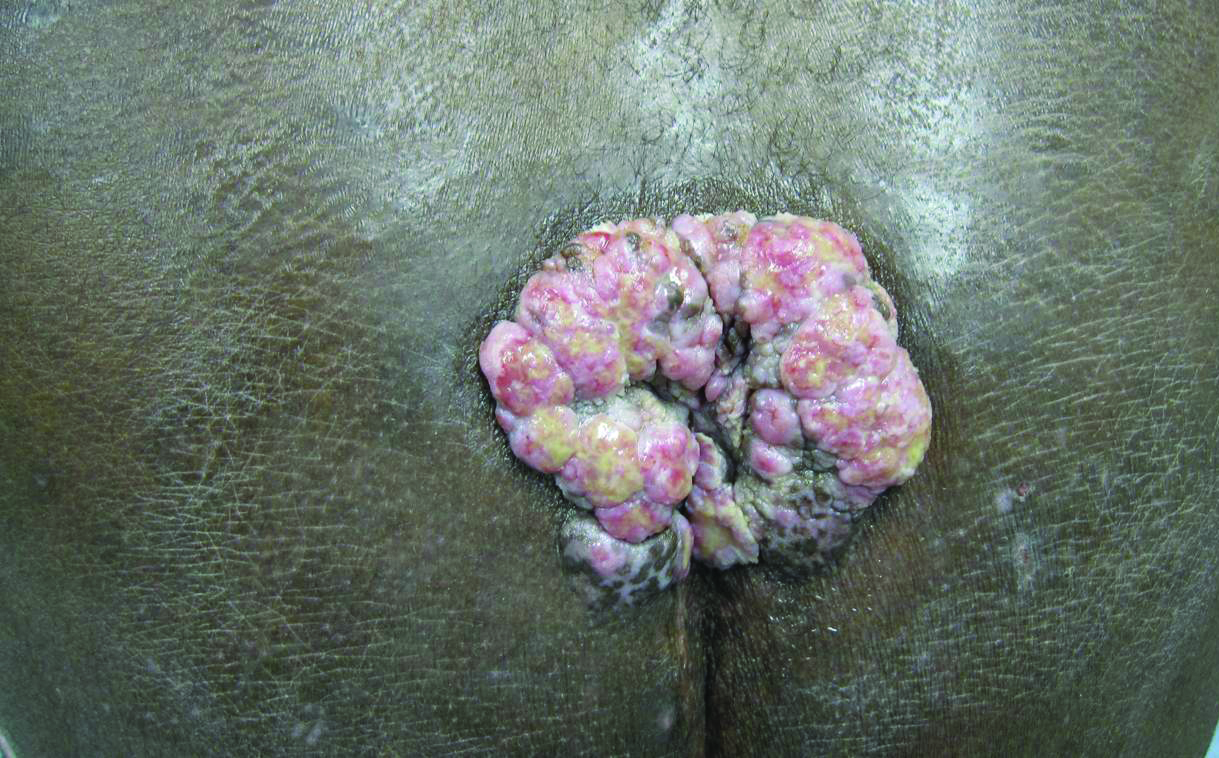
A 53-year-old man presented to our clinic with a sacral mass that had progressively enlarged over 2 years. The patient reported occasional oozing from the mass as well as pain when laying flat but denied fever or other symptoms. His medical history was remarkable for human immunodeficiency virus infection with variable adherence to a highly active antiretroviral therapy regimen. At the time of presentation, the patient had a CD4 lymphocyte count of 78 cells/mm3 (reference range, 500–1400 cells/mm3) and a viral load of 290 copies/mL (reference range, 0 copies/mL). Physical examination revealed a 10-cm discrete, moist and pink, exophytic plaque on the sacrum with superficial erosions. The plaque was nontender and without associated lymphadenopathy. The skin and mucous membranes were otherwise clear. A cutaneous biopsy specimen was obtained from the tumor and sent for histopathologic analysis.