Article
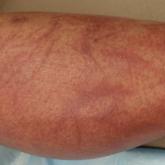
Flagellate Shiitake Mushroom Reaction With Histologic Features of Acute Generalized Exanthematous Pustulosis
- Author:
- Richard J. Browning, MD
- Ramin Fathi, MD
- Mahsa A. Smith, MD
- Theodore Alkousakis, MD
Ingestion of shiitake mushrooms and bleomycin is associated with flagellate dermatitis.
Article
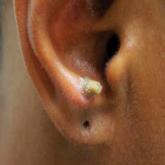
Cutaneous Sarcoidosis Presenting as a Cutaneous Horn
- Author:
- Richard J. Browning, MD
- Laura A. Greyling, MD
- Loretta S. Davis, MD
Biopsy of a cutaneous horn should be deep enough to capture the neoplastic or inflammatory process at the base of the lesion. Cutaneous...
