Article

Does taking BP medicine at night (vs morning) result in fewer cardiovascular events?
- Author:
- Robert Martin, DO
- Rick Guthmann, MD, MPH
EVIDENCE-BASED ANSWER: Probably not. In patients who have hypertension, the timing of administration of antihypertensive medications does not...
Article
Are manual therapies effective at reducing chronic tension headache frequency in adults?
- Author:
- Denver Hager, DO
- Rick Guthmann, MD, MPH
EVIDENCE-BASED ANSWER: MAYBE. Among patients with chronic tension headaches, manual therapies may reduce headache frequency more than sham manual...
Article
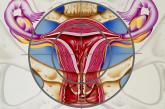
Which medications work best for menorrhagia?
- Author:
- Allison Lale, MD, MPH
- Elise Halajian, DO
- Rick Guthmann, MD, MPH
- Joan Nashelsky, MLS
EVIDENCE-BASED ANSWER: Four medications have been shown to reduce menstrual blood loss (MBL) significantly in placebo-controlled randomized...
