User login
Inferior Vena Cava Filter Placement in Patients with Venous Thromboembolism without Contraindication to Anticoagulation
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A nticoagulation is the cornerstone of acute venous thromboembolism (VTE) management. Nonetheless, the use of inferior vena cava (IVC) filters in addition to anticoagulation is increasing, with wide variation in practice patterns and a growing recognition of filter-related complications. Rigorous randomized controlled data demonstrating that IVC filters, particularly the increasingly commonly placed retrievable filters, provide a mortality benefit are sparse. Given our review of IVC filter use and the lack of evidence demonstrating that IVC filters provide a mortality benefit, we recommend using anticoagulation alone for stable medical service patients admitted with acute VTE. In nuanced cases, hospitalists should engage in multidisciplinary care to develop individualized treatment options.
CASE PRESENTATION
A 65-year-old woman with a history of diabetes mellitus, metastatic breast cancer, and peptic ulcer disease presents to the Emergency Department for the evaluation of right thigh swelling, chest pain, and dyspnea after a transcontinental flight. Physical examination is notable for a pulse of 114 beats per minute, blood pressure of 136/93 mm Hg, respiratory rate of 14 breaths per minute, oxygen saturation of 95% on room air, and swelling of the right thigh. Computerized tomography imaging demonstrates multiple bilateral pulmonary emboli. Emergency department physicians begin anticoagulation and inform you that they have ordered the placement of a retrievable inferior vena cava (IVC) filter.
BACKGROUND
Acute venous thromboembolism (VTE) accounts for more than 500,000 hospitalizations in the United States each year.1 Although the management of VTE centers around anticoagulation, the concurrent use of IVC filters has increased over the past several decades.2 Several observational studies have attempted to quantify IVC filter usage and have shown that overall filter placement has increased at an impressive rate. Within two decades, the number of patients undergoing IVC filter placement has increased nearly 25 times from 2,000 in 1979 to 49,000 in 1999.2 Recent Medicare data show that claims for IVC filter placement procedures have increased from 30,756 in 1999 to 65,041 in 2008.3 IVC filter placement rates are higher in the US than in other developed countries; one review projected that in 2012, the IVC filter placement rate in a given population in the US is 25 times higher than that in a similar population in Europe.4
The guidelines for IVC filter usage are largely based on expert opinion, and solid data regarding this intervention are lacking. This combination is problematic, especially because the practice is becoming commonplace, and filter-related complications are increasingly recognized. Additionally, the appropriateness of filter use varies among providers, as evidenced by a retrospective study in which three VTE experts reviewed medical records to determine the appropriateness of filter placement. They unanimously agreed that filter use was appropriate in 51% of the cases, unanimously agreed that filter use was inappropriate in 26% of the cases, and lacked consensus on the appropriateness of filter use in 23% of the cases.5 The striking lack of consensus among experts underscores the wide range of opinion regarding the appropriateness of IVC filter placement on a case-by-case basis. Moreover, evidence suggests that physician adherence to guidelines for appropriate IVC filter use is suboptimal. One single-center study showed that only 43.5% of filters placed by interventional radiology practitioners met the guidelines established by the American College of Chest Physicians (ACCP), with a slightly increased percentage of filter placement meeting guidelines if the requesting provider is an IM-trained physician.6
WHY YOU MIGHT THINK IVC FILTER PLACEMENT IS HELPFUL IN PATIENTS WITH VTE WITHOUT CONTRAINDICATION TO ANTICOAGULATION
In theory, the concept of IVC filters makes intuitive sense—filters block the ascent of any thrombus from the lower extremities to prevent the feared complication of a pulmonary embolism (PE). Unfortunately, rigorous data are limited, and consensus guidelines vary between different specialty organizations, further obfuscating the role of IVC filter placement in the management of VTE. For example, the ACCP recommends against the use of IVC filters in most patients with VTE receiving anticoagulation and does not list any prophylactic indications.7,8 Meanwhile, the Society of Interventional Radiology lists prophylactic indications for IVC filter placement in certain patient populations, such patients with a risk of VTE and a high risk of bleeding, and notes numerous relative indications for IVC filter placement.8 Notably, these differences in expert opinion likely influence practice patterns, as evidenced by the increase in IVC filter placement for relative indications.9,10
WHY IVC FILTERS PLACEMENT IN PATIENTS WITH VTE WHO CAN BE ANTICOAGULATED IS NOT HELPFUL
The Prevention du Risque d’Embolie Pulmonaire par Interruption Cave (PRECIP) trial is the most robust study supporting the 2016 ACCP recommendation against IVC filter use in patients that can receive anticoagulation.7,11 This study randomized 400 patients with deep vein thrombosis (DVT) at high risk for PE to anticoagulation with or without permanent filter placement to address VTE and mortality rates associated with IVC filter placement. The trial showed that the VTE burden shifts in the presence of IVC filters. At 2-year follow-up, the group with IVC filters had nonsignificantly fewer PEs than the control group and an increased incidence of DVT. Mortality rates did not differ between groups.11 At eight-year follow-up this shift in VTE burden is again seen given that the number of PEs in patients who received IVC filters decreased and the incidence of DVTs increased. Again, mortality did not differ between groups.12 A subsequent study randomized 399 patients with DVT and acute symptomatic PE with at least one additional marker of severity to anticoagulation with or without retrievable IVC filter placement and showed no difference in recurrent PE or mortality at 3 or 6 months.13 These results argue against placing retrievable filters in patients receiving anticoagulation.
The identification of associated adverse events further favor the judicious use of IVC filters. A retrospective review of the long-term complications of IVC filters based on imaging data showed a 14% fracture rate, 13% IVC thrombosis rate, and a 48% perforation rate.14 Multiple studies have shown that the associated complication rates of retrievable filters are higher than those of permanent filters; such an association is concerning given that retrievable filter usage exceeds permanent filter usage.14,15 The increase in retrievable filter usage is likely attributable to their attractive risk-benefit calculation. In theory, retrievable IVC filters should be perfect for patients who have conditions that increase VTE risk but create temporary contraindications, such as trauma or major surgery, to anticoagulation. However, anticoagulation is preferred over IVC filters in the long term because the complication rates of IVC filters increase with dwell time.16 Given the reports of adverse events and concern that IVC filters are not appropriately removed, the Food and Drug Administration recommends removing retrievable IVC filters once the risk of filters outweighs the benefits, which appears to be 29-54 days after implantation.17 However, successful retrieval rates are low, both because of the low rates of removal attempts and because of the interference of complications, such as embedded or thrombosed filters, with removal.10,18 As an example, in a retrospective review of all patients who received an IVC filter at an academic medical center over the period of 2003-2011, nearly 25% of patients were discharged on anticoagulation after IVC filter placement.10 This suggests that their contraindication to anticoagulation and need for IVC placement have passed by the time of discharge. Nevertheless, clinicians attempted filter retrieval in only 9.6% of these patients, representing a significant missed opportunity of treatment with anticoagulation rather than IVC filters.10
Factors such as filter plan documentation, hematology involvement, patient age ≤70 years, and establishment of dedicated IVC filter clinics are correlated with improved rates of filter removal; these correlations emphasize the importance of a clear follow-up plan in the timely removal of these devices.18,19
WHEN MIGHT IT BE HELPFUL TO PLACE IVC FILTERS IN PATIENTS WITH NO CONTRAINDICATION TO ANTICOAGULATION?
IVC filter placement is inappropriate in the vast majority of patients with VTE who can be anticoagulated. However the ACCP does acknowledge that a small subset of patients – specifically, those with severe or massive PE – may fall outside this guideline.7 Clinicians fear that these patients have low cardiopulmonary reserve and may experience hemodynamic collapse and death with another “hit” from a recurrent PE. This recommendation is consistent with the evidence that in unstable patients with PE, IVC filter placement is associated with decreased in-hospital mortality.20 Data remain limited for this situation, and the decision to place an IVC filter in anticoagulated but unstable patients is an individualized one.
WHAT YOU SHOULD DO INSTEAD: REFRAIN FROM IVC FILTER PLACEMENT AND TREAT WITH SYSTEMIC ANTICOAGULATION
In stable patients admitted to the medical service with VTE and who can be anticoagulated, there is little evidence that placement of an IVC filter will improve short- or long-term mortality. Hospitalists should anticoagulate these patients with a vitamin-K antagonist, heparin product, or novel oral anticoagulants.
RECOMMENDATIONS
- Anticoagulate hemodynamically stable patients who are admitted to the medical service with VTE and who do not have a contraindication to anticoagulation. Do not place a permanent or retrievable IVC filter.
- IVC filter placement may benefit unstable patients who may experience hemodynamic collapse with an increased PE burden. IVC filter placement should be discussed with a multidisciplinary team.
- When discharging a patient with an IVC filter, hospitalists should improve retrieval rates by scheduling subsequent removal. The discharge summary should contain information about the IVC filter, as well as clear instructions regarding the plan for removal. The instructions should include radiology follow-up information and the designation of responsible physicians in case of questions.
CONCLUSION
Although IVC filter use is increasing, the evidence does not support their use in hemodynamically stable patients who can be anticoagulated. The patient described in the initial case has no contraindication to systemic anticoagulation. Therefore, she should be started on anticoagulation, and an IVC filter should not be placed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors do not have any conflicts of interest to disclose
1. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations – United States, 2007-2009. MMWR. 2012;61:401-404. PubMed
2. Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164(14):1541-1545. doi: 10.1001/archinte.164.14.1541 PubMed
3. Duszak R Jr, Parker L, Levin DC, Rao VM. Placement and removal of inferior vena cava filters: national trends in the Medicare population. J Am Coll Radiol. 2011;8(7):483-489. doi: 10.1016/j.jacr.2010.12.021. PubMed
4. Wang SL, Llyod AJ. Clinical review: inferior vena cava filters in the age of patient-centered outcomes. Ann Med. 2013;45(7):474-481. doi: 10.3109/07853890.2013.832951. PubMed
5. Spencer FA, Bates SM, Goldberg RJ, et al. A population-based study of inferior vena cava filters in patients with acute venous thromboembolism. Arch Intern Med.2010;170(16):1456-1462. doi: 10.1001/archinternmed.2010.272. PubMed
6. Baadh AS, Zikria JF, Rivioli S, et al. Indications for inferior vena cava filter placement: do physicians comply with guidelines? J Vasc Interv Radiol. 2012;23(8):989-995. doi: 10.1016/j.jvir.2012.04.017. PubMed
7. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. doi: 10.1016/j.chest.2015.11.026. PubMed
8. Kaufman JA, Kinney TB, Streiff MB, et al. Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol. 2006;17(3):449-459. doi: 10.1097/01.rvi.0000203418.39769.0d. PubMed
9. Tao MJ, Montbriand JM, Eisenberg N, Sniderman KW, Roche-Nagle G. Temporary inferior vena cava filter indications, retrieval rates, and follow-up management at a multicenter tertiary care institution. J Vasc Surg. 2016;64(2):430-437. doi: 10.1016/j.jvs.2016.02.034. PubMed
10. Sarosiek S, Crowther M, Sloan JM. Indications, complications, and management of inferior vena cava filters. JAMA Intern Med.2013;173(7):513-517. doi: 10.1001/jamainternmed.2013.343. PubMed
11. Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena cava filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. N Engl J Med. 1998;338(7):409-415. doi: 10.1056/NEJM199802123380701. PubMed
12. PRECIP Study Group. Eight-year follow up of patients with permanent vena cava filters in the prevention of pulmonary embolism. Circulation. 2005;112(3):416-422. doi: 10.1161/CIRCULATIONAHA.104.512834. PubMed
13. Mismetti P, Laporte S, Pellerin O, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism. JAMA. 2015;313(16):1627-1635. doi: 10.1001/jama.2015.3780. PubMed
14. Wang SL, Siddiqui A, Rosenthal E. Long-term complications of inferior vena cava filters. J Vasc Surg Venous Lymphat Disord. 2017;5(1):33-41. doi: 10.1016/j.jvsv.2016.07.002. PubMed
15. Andreoli JM, Lewandowski RJ, Vogelzang RL, Ryu RK. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol. 2014;25(8):1181-1185. doi: 10.1016/j.jvir.2014.04.016. PubMed
16. Vijay K, Hughes JA, Burdette AS, et al. Fractured bard Recovery, G2, and G2 Express inferior vena cava filters: incidence, clinical consequences, and outcomes of removal attempts. J Vasc Interv Radiol. 2012;23(2):188-194. doi: 10.1016/j.jvir.2011.10.005. PubMed
17. Removing Retrievable Inferior Vena Cava Filters: FDA Safety Communication. FDA.gov. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm396377.htm. Published May 6, 2014. Accessed April 10, 2017.
18. Peterson EA, Yenson PR, Liu D, Lee AYY. Predictors of attempted inferior vena cava filters retrieval in a tertiary care centre. Thromb Res. 2014;134(2):300-304. doi: 10.1016/j.thromres.2014.05.029. PubMed
19. Minocha J, Idakoji I, Riaz A, et al. Improving inferior vena cava filter retrieval rates: impact of a dedicated inferior vena cava filter clinic. J Vasc Interv Radiol. 2010;21(12):1847-1851. doi: 10.1016/j.jvir.2010.09.003. PubMed
20. Stein PD, Matta F, Keyes DC, Willyerd GL. Impact of vena cava filters on in-hospital case fatality rate from pulmonary embolism. Am J Med. 2012;125(5):478-484. doi: 10.1016/j.amjmed.2011.05.025. PubMed
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A nticoagulation is the cornerstone of acute venous thromboembolism (VTE) management. Nonetheless, the use of inferior vena cava (IVC) filters in addition to anticoagulation is increasing, with wide variation in practice patterns and a growing recognition of filter-related complications. Rigorous randomized controlled data demonstrating that IVC filters, particularly the increasingly commonly placed retrievable filters, provide a mortality benefit are sparse. Given our review of IVC filter use and the lack of evidence demonstrating that IVC filters provide a mortality benefit, we recommend using anticoagulation alone for stable medical service patients admitted with acute VTE. In nuanced cases, hospitalists should engage in multidisciplinary care to develop individualized treatment options.
CASE PRESENTATION
A 65-year-old woman with a history of diabetes mellitus, metastatic breast cancer, and peptic ulcer disease presents to the Emergency Department for the evaluation of right thigh swelling, chest pain, and dyspnea after a transcontinental flight. Physical examination is notable for a pulse of 114 beats per minute, blood pressure of 136/93 mm Hg, respiratory rate of 14 breaths per minute, oxygen saturation of 95% on room air, and swelling of the right thigh. Computerized tomography imaging demonstrates multiple bilateral pulmonary emboli. Emergency department physicians begin anticoagulation and inform you that they have ordered the placement of a retrievable inferior vena cava (IVC) filter.
BACKGROUND
Acute venous thromboembolism (VTE) accounts for more than 500,000 hospitalizations in the United States each year.1 Although the management of VTE centers around anticoagulation, the concurrent use of IVC filters has increased over the past several decades.2 Several observational studies have attempted to quantify IVC filter usage and have shown that overall filter placement has increased at an impressive rate. Within two decades, the number of patients undergoing IVC filter placement has increased nearly 25 times from 2,000 in 1979 to 49,000 in 1999.2 Recent Medicare data show that claims for IVC filter placement procedures have increased from 30,756 in 1999 to 65,041 in 2008.3 IVC filter placement rates are higher in the US than in other developed countries; one review projected that in 2012, the IVC filter placement rate in a given population in the US is 25 times higher than that in a similar population in Europe.4
The guidelines for IVC filter usage are largely based on expert opinion, and solid data regarding this intervention are lacking. This combination is problematic, especially because the practice is becoming commonplace, and filter-related complications are increasingly recognized. Additionally, the appropriateness of filter use varies among providers, as evidenced by a retrospective study in which three VTE experts reviewed medical records to determine the appropriateness of filter placement. They unanimously agreed that filter use was appropriate in 51% of the cases, unanimously agreed that filter use was inappropriate in 26% of the cases, and lacked consensus on the appropriateness of filter use in 23% of the cases.5 The striking lack of consensus among experts underscores the wide range of opinion regarding the appropriateness of IVC filter placement on a case-by-case basis. Moreover, evidence suggests that physician adherence to guidelines for appropriate IVC filter use is suboptimal. One single-center study showed that only 43.5% of filters placed by interventional radiology practitioners met the guidelines established by the American College of Chest Physicians (ACCP), with a slightly increased percentage of filter placement meeting guidelines if the requesting provider is an IM-trained physician.6
WHY YOU MIGHT THINK IVC FILTER PLACEMENT IS HELPFUL IN PATIENTS WITH VTE WITHOUT CONTRAINDICATION TO ANTICOAGULATION
In theory, the concept of IVC filters makes intuitive sense—filters block the ascent of any thrombus from the lower extremities to prevent the feared complication of a pulmonary embolism (PE). Unfortunately, rigorous data are limited, and consensus guidelines vary between different specialty organizations, further obfuscating the role of IVC filter placement in the management of VTE. For example, the ACCP recommends against the use of IVC filters in most patients with VTE receiving anticoagulation and does not list any prophylactic indications.7,8 Meanwhile, the Society of Interventional Radiology lists prophylactic indications for IVC filter placement in certain patient populations, such patients with a risk of VTE and a high risk of bleeding, and notes numerous relative indications for IVC filter placement.8 Notably, these differences in expert opinion likely influence practice patterns, as evidenced by the increase in IVC filter placement for relative indications.9,10
WHY IVC FILTERS PLACEMENT IN PATIENTS WITH VTE WHO CAN BE ANTICOAGULATED IS NOT HELPFUL
The Prevention du Risque d’Embolie Pulmonaire par Interruption Cave (PRECIP) trial is the most robust study supporting the 2016 ACCP recommendation against IVC filter use in patients that can receive anticoagulation.7,11 This study randomized 400 patients with deep vein thrombosis (DVT) at high risk for PE to anticoagulation with or without permanent filter placement to address VTE and mortality rates associated with IVC filter placement. The trial showed that the VTE burden shifts in the presence of IVC filters. At 2-year follow-up, the group with IVC filters had nonsignificantly fewer PEs than the control group and an increased incidence of DVT. Mortality rates did not differ between groups.11 At eight-year follow-up this shift in VTE burden is again seen given that the number of PEs in patients who received IVC filters decreased and the incidence of DVTs increased. Again, mortality did not differ between groups.12 A subsequent study randomized 399 patients with DVT and acute symptomatic PE with at least one additional marker of severity to anticoagulation with or without retrievable IVC filter placement and showed no difference in recurrent PE or mortality at 3 or 6 months.13 These results argue against placing retrievable filters in patients receiving anticoagulation.
The identification of associated adverse events further favor the judicious use of IVC filters. A retrospective review of the long-term complications of IVC filters based on imaging data showed a 14% fracture rate, 13% IVC thrombosis rate, and a 48% perforation rate.14 Multiple studies have shown that the associated complication rates of retrievable filters are higher than those of permanent filters; such an association is concerning given that retrievable filter usage exceeds permanent filter usage.14,15 The increase in retrievable filter usage is likely attributable to their attractive risk-benefit calculation. In theory, retrievable IVC filters should be perfect for patients who have conditions that increase VTE risk but create temporary contraindications, such as trauma or major surgery, to anticoagulation. However, anticoagulation is preferred over IVC filters in the long term because the complication rates of IVC filters increase with dwell time.16 Given the reports of adverse events and concern that IVC filters are not appropriately removed, the Food and Drug Administration recommends removing retrievable IVC filters once the risk of filters outweighs the benefits, which appears to be 29-54 days after implantation.17 However, successful retrieval rates are low, both because of the low rates of removal attempts and because of the interference of complications, such as embedded or thrombosed filters, with removal.10,18 As an example, in a retrospective review of all patients who received an IVC filter at an academic medical center over the period of 2003-2011, nearly 25% of patients were discharged on anticoagulation after IVC filter placement.10 This suggests that their contraindication to anticoagulation and need for IVC placement have passed by the time of discharge. Nevertheless, clinicians attempted filter retrieval in only 9.6% of these patients, representing a significant missed opportunity of treatment with anticoagulation rather than IVC filters.10
Factors such as filter plan documentation, hematology involvement, patient age ≤70 years, and establishment of dedicated IVC filter clinics are correlated with improved rates of filter removal; these correlations emphasize the importance of a clear follow-up plan in the timely removal of these devices.18,19
WHEN MIGHT IT BE HELPFUL TO PLACE IVC FILTERS IN PATIENTS WITH NO CONTRAINDICATION TO ANTICOAGULATION?
IVC filter placement is inappropriate in the vast majority of patients with VTE who can be anticoagulated. However the ACCP does acknowledge that a small subset of patients – specifically, those with severe or massive PE – may fall outside this guideline.7 Clinicians fear that these patients have low cardiopulmonary reserve and may experience hemodynamic collapse and death with another “hit” from a recurrent PE. This recommendation is consistent with the evidence that in unstable patients with PE, IVC filter placement is associated with decreased in-hospital mortality.20 Data remain limited for this situation, and the decision to place an IVC filter in anticoagulated but unstable patients is an individualized one.
WHAT YOU SHOULD DO INSTEAD: REFRAIN FROM IVC FILTER PLACEMENT AND TREAT WITH SYSTEMIC ANTICOAGULATION
In stable patients admitted to the medical service with VTE and who can be anticoagulated, there is little evidence that placement of an IVC filter will improve short- or long-term mortality. Hospitalists should anticoagulate these patients with a vitamin-K antagonist, heparin product, or novel oral anticoagulants.
RECOMMENDATIONS
- Anticoagulate hemodynamically stable patients who are admitted to the medical service with VTE and who do not have a contraindication to anticoagulation. Do not place a permanent or retrievable IVC filter.
- IVC filter placement may benefit unstable patients who may experience hemodynamic collapse with an increased PE burden. IVC filter placement should be discussed with a multidisciplinary team.
- When discharging a patient with an IVC filter, hospitalists should improve retrieval rates by scheduling subsequent removal. The discharge summary should contain information about the IVC filter, as well as clear instructions regarding the plan for removal. The instructions should include radiology follow-up information and the designation of responsible physicians in case of questions.
CONCLUSION
Although IVC filter use is increasing, the evidence does not support their use in hemodynamically stable patients who can be anticoagulated. The patient described in the initial case has no contraindication to systemic anticoagulation. Therefore, she should be started on anticoagulation, and an IVC filter should not be placed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors do not have any conflicts of interest to disclose
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A nticoagulation is the cornerstone of acute venous thromboembolism (VTE) management. Nonetheless, the use of inferior vena cava (IVC) filters in addition to anticoagulation is increasing, with wide variation in practice patterns and a growing recognition of filter-related complications. Rigorous randomized controlled data demonstrating that IVC filters, particularly the increasingly commonly placed retrievable filters, provide a mortality benefit are sparse. Given our review of IVC filter use and the lack of evidence demonstrating that IVC filters provide a mortality benefit, we recommend using anticoagulation alone for stable medical service patients admitted with acute VTE. In nuanced cases, hospitalists should engage in multidisciplinary care to develop individualized treatment options.
CASE PRESENTATION
A 65-year-old woman with a history of diabetes mellitus, metastatic breast cancer, and peptic ulcer disease presents to the Emergency Department for the evaluation of right thigh swelling, chest pain, and dyspnea after a transcontinental flight. Physical examination is notable for a pulse of 114 beats per minute, blood pressure of 136/93 mm Hg, respiratory rate of 14 breaths per minute, oxygen saturation of 95% on room air, and swelling of the right thigh. Computerized tomography imaging demonstrates multiple bilateral pulmonary emboli. Emergency department physicians begin anticoagulation and inform you that they have ordered the placement of a retrievable inferior vena cava (IVC) filter.
BACKGROUND
Acute venous thromboembolism (VTE) accounts for more than 500,000 hospitalizations in the United States each year.1 Although the management of VTE centers around anticoagulation, the concurrent use of IVC filters has increased over the past several decades.2 Several observational studies have attempted to quantify IVC filter usage and have shown that overall filter placement has increased at an impressive rate. Within two decades, the number of patients undergoing IVC filter placement has increased nearly 25 times from 2,000 in 1979 to 49,000 in 1999.2 Recent Medicare data show that claims for IVC filter placement procedures have increased from 30,756 in 1999 to 65,041 in 2008.3 IVC filter placement rates are higher in the US than in other developed countries; one review projected that in 2012, the IVC filter placement rate in a given population in the US is 25 times higher than that in a similar population in Europe.4
The guidelines for IVC filter usage are largely based on expert opinion, and solid data regarding this intervention are lacking. This combination is problematic, especially because the practice is becoming commonplace, and filter-related complications are increasingly recognized. Additionally, the appropriateness of filter use varies among providers, as evidenced by a retrospective study in which three VTE experts reviewed medical records to determine the appropriateness of filter placement. They unanimously agreed that filter use was appropriate in 51% of the cases, unanimously agreed that filter use was inappropriate in 26% of the cases, and lacked consensus on the appropriateness of filter use in 23% of the cases.5 The striking lack of consensus among experts underscores the wide range of opinion regarding the appropriateness of IVC filter placement on a case-by-case basis. Moreover, evidence suggests that physician adherence to guidelines for appropriate IVC filter use is suboptimal. One single-center study showed that only 43.5% of filters placed by interventional radiology practitioners met the guidelines established by the American College of Chest Physicians (ACCP), with a slightly increased percentage of filter placement meeting guidelines if the requesting provider is an IM-trained physician.6
WHY YOU MIGHT THINK IVC FILTER PLACEMENT IS HELPFUL IN PATIENTS WITH VTE WITHOUT CONTRAINDICATION TO ANTICOAGULATION
In theory, the concept of IVC filters makes intuitive sense—filters block the ascent of any thrombus from the lower extremities to prevent the feared complication of a pulmonary embolism (PE). Unfortunately, rigorous data are limited, and consensus guidelines vary between different specialty organizations, further obfuscating the role of IVC filter placement in the management of VTE. For example, the ACCP recommends against the use of IVC filters in most patients with VTE receiving anticoagulation and does not list any prophylactic indications.7,8 Meanwhile, the Society of Interventional Radiology lists prophylactic indications for IVC filter placement in certain patient populations, such patients with a risk of VTE and a high risk of bleeding, and notes numerous relative indications for IVC filter placement.8 Notably, these differences in expert opinion likely influence practice patterns, as evidenced by the increase in IVC filter placement for relative indications.9,10
WHY IVC FILTERS PLACEMENT IN PATIENTS WITH VTE WHO CAN BE ANTICOAGULATED IS NOT HELPFUL
The Prevention du Risque d’Embolie Pulmonaire par Interruption Cave (PRECIP) trial is the most robust study supporting the 2016 ACCP recommendation against IVC filter use in patients that can receive anticoagulation.7,11 This study randomized 400 patients with deep vein thrombosis (DVT) at high risk for PE to anticoagulation with or without permanent filter placement to address VTE and mortality rates associated with IVC filter placement. The trial showed that the VTE burden shifts in the presence of IVC filters. At 2-year follow-up, the group with IVC filters had nonsignificantly fewer PEs than the control group and an increased incidence of DVT. Mortality rates did not differ between groups.11 At eight-year follow-up this shift in VTE burden is again seen given that the number of PEs in patients who received IVC filters decreased and the incidence of DVTs increased. Again, mortality did not differ between groups.12 A subsequent study randomized 399 patients with DVT and acute symptomatic PE with at least one additional marker of severity to anticoagulation with or without retrievable IVC filter placement and showed no difference in recurrent PE or mortality at 3 or 6 months.13 These results argue against placing retrievable filters in patients receiving anticoagulation.
The identification of associated adverse events further favor the judicious use of IVC filters. A retrospective review of the long-term complications of IVC filters based on imaging data showed a 14% fracture rate, 13% IVC thrombosis rate, and a 48% perforation rate.14 Multiple studies have shown that the associated complication rates of retrievable filters are higher than those of permanent filters; such an association is concerning given that retrievable filter usage exceeds permanent filter usage.14,15 The increase in retrievable filter usage is likely attributable to their attractive risk-benefit calculation. In theory, retrievable IVC filters should be perfect for patients who have conditions that increase VTE risk but create temporary contraindications, such as trauma or major surgery, to anticoagulation. However, anticoagulation is preferred over IVC filters in the long term because the complication rates of IVC filters increase with dwell time.16 Given the reports of adverse events and concern that IVC filters are not appropriately removed, the Food and Drug Administration recommends removing retrievable IVC filters once the risk of filters outweighs the benefits, which appears to be 29-54 days after implantation.17 However, successful retrieval rates are low, both because of the low rates of removal attempts and because of the interference of complications, such as embedded or thrombosed filters, with removal.10,18 As an example, in a retrospective review of all patients who received an IVC filter at an academic medical center over the period of 2003-2011, nearly 25% of patients were discharged on anticoagulation after IVC filter placement.10 This suggests that their contraindication to anticoagulation and need for IVC placement have passed by the time of discharge. Nevertheless, clinicians attempted filter retrieval in only 9.6% of these patients, representing a significant missed opportunity of treatment with anticoagulation rather than IVC filters.10
Factors such as filter plan documentation, hematology involvement, patient age ≤70 years, and establishment of dedicated IVC filter clinics are correlated with improved rates of filter removal; these correlations emphasize the importance of a clear follow-up plan in the timely removal of these devices.18,19
WHEN MIGHT IT BE HELPFUL TO PLACE IVC FILTERS IN PATIENTS WITH NO CONTRAINDICATION TO ANTICOAGULATION?
IVC filter placement is inappropriate in the vast majority of patients with VTE who can be anticoagulated. However the ACCP does acknowledge that a small subset of patients – specifically, those with severe or massive PE – may fall outside this guideline.7 Clinicians fear that these patients have low cardiopulmonary reserve and may experience hemodynamic collapse and death with another “hit” from a recurrent PE. This recommendation is consistent with the evidence that in unstable patients with PE, IVC filter placement is associated with decreased in-hospital mortality.20 Data remain limited for this situation, and the decision to place an IVC filter in anticoagulated but unstable patients is an individualized one.
WHAT YOU SHOULD DO INSTEAD: REFRAIN FROM IVC FILTER PLACEMENT AND TREAT WITH SYSTEMIC ANTICOAGULATION
In stable patients admitted to the medical service with VTE and who can be anticoagulated, there is little evidence that placement of an IVC filter will improve short- or long-term mortality. Hospitalists should anticoagulate these patients with a vitamin-K antagonist, heparin product, or novel oral anticoagulants.
RECOMMENDATIONS
- Anticoagulate hemodynamically stable patients who are admitted to the medical service with VTE and who do not have a contraindication to anticoagulation. Do not place a permanent or retrievable IVC filter.
- IVC filter placement may benefit unstable patients who may experience hemodynamic collapse with an increased PE burden. IVC filter placement should be discussed with a multidisciplinary team.
- When discharging a patient with an IVC filter, hospitalists should improve retrieval rates by scheduling subsequent removal. The discharge summary should contain information about the IVC filter, as well as clear instructions regarding the plan for removal. The instructions should include radiology follow-up information and the designation of responsible physicians in case of questions.
CONCLUSION
Although IVC filter use is increasing, the evidence does not support their use in hemodynamically stable patients who can be anticoagulated. The patient described in the initial case has no contraindication to systemic anticoagulation. Therefore, she should be started on anticoagulation, and an IVC filter should not be placed.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors do not have any conflicts of interest to disclose
1. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations – United States, 2007-2009. MMWR. 2012;61:401-404. PubMed
2. Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164(14):1541-1545. doi: 10.1001/archinte.164.14.1541 PubMed
3. Duszak R Jr, Parker L, Levin DC, Rao VM. Placement and removal of inferior vena cava filters: national trends in the Medicare population. J Am Coll Radiol. 2011;8(7):483-489. doi: 10.1016/j.jacr.2010.12.021. PubMed
4. Wang SL, Llyod AJ. Clinical review: inferior vena cava filters in the age of patient-centered outcomes. Ann Med. 2013;45(7):474-481. doi: 10.3109/07853890.2013.832951. PubMed
5. Spencer FA, Bates SM, Goldberg RJ, et al. A population-based study of inferior vena cava filters in patients with acute venous thromboembolism. Arch Intern Med.2010;170(16):1456-1462. doi: 10.1001/archinternmed.2010.272. PubMed
6. Baadh AS, Zikria JF, Rivioli S, et al. Indications for inferior vena cava filter placement: do physicians comply with guidelines? J Vasc Interv Radiol. 2012;23(8):989-995. doi: 10.1016/j.jvir.2012.04.017. PubMed
7. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. doi: 10.1016/j.chest.2015.11.026. PubMed
8. Kaufman JA, Kinney TB, Streiff MB, et al. Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol. 2006;17(3):449-459. doi: 10.1097/01.rvi.0000203418.39769.0d. PubMed
9. Tao MJ, Montbriand JM, Eisenberg N, Sniderman KW, Roche-Nagle G. Temporary inferior vena cava filter indications, retrieval rates, and follow-up management at a multicenter tertiary care institution. J Vasc Surg. 2016;64(2):430-437. doi: 10.1016/j.jvs.2016.02.034. PubMed
10. Sarosiek S, Crowther M, Sloan JM. Indications, complications, and management of inferior vena cava filters. JAMA Intern Med.2013;173(7):513-517. doi: 10.1001/jamainternmed.2013.343. PubMed
11. Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena cava filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. N Engl J Med. 1998;338(7):409-415. doi: 10.1056/NEJM199802123380701. PubMed
12. PRECIP Study Group. Eight-year follow up of patients with permanent vena cava filters in the prevention of pulmonary embolism. Circulation. 2005;112(3):416-422. doi: 10.1161/CIRCULATIONAHA.104.512834. PubMed
13. Mismetti P, Laporte S, Pellerin O, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism. JAMA. 2015;313(16):1627-1635. doi: 10.1001/jama.2015.3780. PubMed
14. Wang SL, Siddiqui A, Rosenthal E. Long-term complications of inferior vena cava filters. J Vasc Surg Venous Lymphat Disord. 2017;5(1):33-41. doi: 10.1016/j.jvsv.2016.07.002. PubMed
15. Andreoli JM, Lewandowski RJ, Vogelzang RL, Ryu RK. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol. 2014;25(8):1181-1185. doi: 10.1016/j.jvir.2014.04.016. PubMed
16. Vijay K, Hughes JA, Burdette AS, et al. Fractured bard Recovery, G2, and G2 Express inferior vena cava filters: incidence, clinical consequences, and outcomes of removal attempts. J Vasc Interv Radiol. 2012;23(2):188-194. doi: 10.1016/j.jvir.2011.10.005. PubMed
17. Removing Retrievable Inferior Vena Cava Filters: FDA Safety Communication. FDA.gov. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm396377.htm. Published May 6, 2014. Accessed April 10, 2017.
18. Peterson EA, Yenson PR, Liu D, Lee AYY. Predictors of attempted inferior vena cava filters retrieval in a tertiary care centre. Thromb Res. 2014;134(2):300-304. doi: 10.1016/j.thromres.2014.05.029. PubMed
19. Minocha J, Idakoji I, Riaz A, et al. Improving inferior vena cava filter retrieval rates: impact of a dedicated inferior vena cava filter clinic. J Vasc Interv Radiol. 2010;21(12):1847-1851. doi: 10.1016/j.jvir.2010.09.003. PubMed
20. Stein PD, Matta F, Keyes DC, Willyerd GL. Impact of vena cava filters on in-hospital case fatality rate from pulmonary embolism. Am J Med. 2012;125(5):478-484. doi: 10.1016/j.amjmed.2011.05.025. PubMed
1. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations – United States, 2007-2009. MMWR. 2012;61:401-404. PubMed
2. Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164(14):1541-1545. doi: 10.1001/archinte.164.14.1541 PubMed
3. Duszak R Jr, Parker L, Levin DC, Rao VM. Placement and removal of inferior vena cava filters: national trends in the Medicare population. J Am Coll Radiol. 2011;8(7):483-489. doi: 10.1016/j.jacr.2010.12.021. PubMed
4. Wang SL, Llyod AJ. Clinical review: inferior vena cava filters in the age of patient-centered outcomes. Ann Med. 2013;45(7):474-481. doi: 10.3109/07853890.2013.832951. PubMed
5. Spencer FA, Bates SM, Goldberg RJ, et al. A population-based study of inferior vena cava filters in patients with acute venous thromboembolism. Arch Intern Med.2010;170(16):1456-1462. doi: 10.1001/archinternmed.2010.272. PubMed
6. Baadh AS, Zikria JF, Rivioli S, et al. Indications for inferior vena cava filter placement: do physicians comply with guidelines? J Vasc Interv Radiol. 2012;23(8):989-995. doi: 10.1016/j.jvir.2012.04.017. PubMed
7. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. doi: 10.1016/j.chest.2015.11.026. PubMed
8. Kaufman JA, Kinney TB, Streiff MB, et al. Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol. 2006;17(3):449-459. doi: 10.1097/01.rvi.0000203418.39769.0d. PubMed
9. Tao MJ, Montbriand JM, Eisenberg N, Sniderman KW, Roche-Nagle G. Temporary inferior vena cava filter indications, retrieval rates, and follow-up management at a multicenter tertiary care institution. J Vasc Surg. 2016;64(2):430-437. doi: 10.1016/j.jvs.2016.02.034. PubMed
10. Sarosiek S, Crowther M, Sloan JM. Indications, complications, and management of inferior vena cava filters. JAMA Intern Med.2013;173(7):513-517. doi: 10.1001/jamainternmed.2013.343. PubMed
11. Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena cava filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. N Engl J Med. 1998;338(7):409-415. doi: 10.1056/NEJM199802123380701. PubMed
12. PRECIP Study Group. Eight-year follow up of patients with permanent vena cava filters in the prevention of pulmonary embolism. Circulation. 2005;112(3):416-422. doi: 10.1161/CIRCULATIONAHA.104.512834. PubMed
13. Mismetti P, Laporte S, Pellerin O, et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism. JAMA. 2015;313(16):1627-1635. doi: 10.1001/jama.2015.3780. PubMed
14. Wang SL, Siddiqui A, Rosenthal E. Long-term complications of inferior vena cava filters. J Vasc Surg Venous Lymphat Disord. 2017;5(1):33-41. doi: 10.1016/j.jvsv.2016.07.002. PubMed
15. Andreoli JM, Lewandowski RJ, Vogelzang RL, Ryu RK. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol. 2014;25(8):1181-1185. doi: 10.1016/j.jvir.2014.04.016. PubMed
16. Vijay K, Hughes JA, Burdette AS, et al. Fractured bard Recovery, G2, and G2 Express inferior vena cava filters: incidence, clinical consequences, and outcomes of removal attempts. J Vasc Interv Radiol. 2012;23(2):188-194. doi: 10.1016/j.jvir.2011.10.005. PubMed
17. Removing Retrievable Inferior Vena Cava Filters: FDA Safety Communication. FDA.gov. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm396377.htm. Published May 6, 2014. Accessed April 10, 2017.
18. Peterson EA, Yenson PR, Liu D, Lee AYY. Predictors of attempted inferior vena cava filters retrieval in a tertiary care centre. Thromb Res. 2014;134(2):300-304. doi: 10.1016/j.thromres.2014.05.029. PubMed
19. Minocha J, Idakoji I, Riaz A, et al. Improving inferior vena cava filter retrieval rates: impact of a dedicated inferior vena cava filter clinic. J Vasc Interv Radiol. 2010;21(12):1847-1851. doi: 10.1016/j.jvir.2010.09.003. PubMed
20. Stein PD, Matta F, Keyes DC, Willyerd GL. Impact of vena cava filters on in-hospital case fatality rate from pulmonary embolism. Am J Med. 2012;125(5):478-484. doi: 10.1016/j.amjmed.2011.05.025. PubMed
© 2018 Society of Hospital Medicine
The Burden of Guardianship: A Matched Cohort Study
A central tenet of modern medicine is that patients must provide fully informed consent to receive or refuse medical care offered by their clinical teams.1–4 If a patient is unable to make and communicate a choice or clearly indicate an understanding of the information presented, then he or she is considered to lack the capacity to make medical decisions and the medical team must seek consent from the patient’s surrogate decision-maker.2-7 Every U.S. state recognizes a patient’s healthcare proxy (HCP) and a court-appointed guardian as a legally recognized surrogate.8,9 Most of the states also have statutes or regulations establishing a hierarchy of legally recognized surrogate decision-makers in the absence of a HCP or a court-appointed guardian, such as spouses, adult children, parents, siblings, and grandparents.8,10 In states that do not have such a statute, hospitals develop their own institutional policies for surrogate decision-making.
However, there are important limitations on the authority of these surrogate decision-makers.10 For instance, patients may not have a family member or a friend to serve as a surrogate decision-maker, often family members cannot override a patient’s objection, even when that patient lacks decision-making capacity, and certain decisions require a guardian or a HCP.8-10 In these circumstances, the hospital must petition a court to appoint a guardian as a legally recognized surrogate decision-maker. This can be an involved family member, if one exists, or an independent, typically volunteer, guardian.11 The process of guardian appointment is complex7,11 and can range from a few days to more than a month, largely dependent on court dates and finding a volunteer guardian. Much of the process occurs during the patient’s hospital stay. This prolongation of hospitalization would be expected to increase health care costs and iatrogenic complications,12–14 but data quantifying these for patients requiring guardianship are lacking. The goal of this study was to describe the characteristics of patients who undergo the process of guardianship and measure the associated burdens. These burdens include the financial costs to the medical system, the prolonged length of stay beyond medical necessity, and the costs to the patient in the form of hospital-acquired complications. Investigating the burden of guardianship is an important first step in uncovering opportunities to improve the process. We hypothesized that patients requiring guardianship would have lengths of stay and healthcare costs that were at least as large as those for patients whose conditions required similar durations of hospitalization prior to medical clearance, in part due to iatrogenic complications that would accrue while awaiting guardian appointment.
METHODS
Setting
We conducted a retrospective matched cohort study of adult inpatients at Beth Israel Deaconess Medical Center (BIDMC), a 651-bed academic, tertiary care facility in Boston, MA. The study was approved by the BIDMC Institutional Review Board as a nonhuman subject research consistent with hospital operations.
Population
For this matched cohort study, we identified case patients as those hospitalized for any reason for whom guardianship proceedings were initiated and obtained; only the first hospitalization during which the guardianship was pursued was used. Cases were identified by obtaining the data of all patients for whom the BIDMC general counsel completed the process of guardianship between October 2014 and September 2015. At BIDMC, all the guardianship proceedings are referred to the general counsel.
To determine the postclearance experience for referred patients compared with that for other patients with similar lengths of stay up to those of the referred patients’ point of clearance, we identified up to three matched controls for each case (Supplemental Figure 1). Medical clearance was defined as the date when the patient was medically stable to be discharged from the hospital, and it was determined in an iterative manner. We identified controls as hospitalized patients admitted for any cause and matched to the cases requiring guardianship on discharging service and length of stay prior to clearance. Specifically, we identified patients on the same service as the case whose length of stay was at least as long as the length of stay of the case patient until medical clearance, as defined below. We then determined the total and the excess length of stay, defined as the duration beyond clearance for each case referred for guardianship; for controls, the ‘excess’ length of stay was the number of hospitalized days beyond the corresponding time that a matched case had been provided clearance. To account for seasonal influences and the training level of house officers, we selected the three controls whose discharge date was closest (before or after) to the discharge date of their matched case.
From legal team files, we identified 61 patients hospitalized at BIDMC for whom new guardianship was pursued to completion. Of these 61 patients, 10 could not be matched to an appropriate control and were included in descriptive analyses but not in comparisons with controls.
Covariates and Outcomes
We collected the details regarding age, gender, primary language, highest level of education, marital status, insurance status, race, date of admission, date of discharge, discharge disposition, principal diagnosis, case mix index (CMI), and discharging service from our administrative and billing data. Outcomes of interest included length of stay and total hospital charges that were collected from the same databases. We used hospital charges, rather than payments, to ensure uniformity across payers.
Chart Review
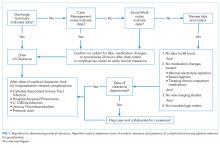
Unique to cases, a team of two medical residents (JP, RP) and a hospitalist (DR) determined the date of medical clearance and hospital-associated complications by a chart review. The date of medical clearance was then used to calculate excess length of stay, ie, the duration of stay beyond the date of medical clearance, by subtracting the time to medical clearance from the total inpatient length of stay.
We developed a novel algorithm to determine the date of medical clearance consistently (Figure 1). We first determined whether the discharge summary indicated a clear date of medical readiness for discharge. If the discharge summary was unclear, then a case management or a social work note was used. The date of medical clearance determined by the case management or the social work note was then confirmed with clinical data. The date was confirmed if there were no significant laboratory orders and major medication changes or procedures for 24 h from the date identified. If notes were also inconclusive, then the medical clearance was determined by a review of provider order entry. Medical readiness for discharge was then defined as the first day when there were no laboratory orders for 48 h and no significant medication changes, imaging studies, or microbiologic orders.
Hospital-acquired complications were determined to be related to the guardianship process if they occurred after the date of medical stability but prior to discharge. We did not investigate hospital-acquired complications among controls. Hospital-acquired complications were defined as follows:
- Catheter-associated urinary tract infection (CAUTI): active Foley catheter order and positive urine culture that resulted in antibiotic administration.
- Hospital-acquired pneumonia (HAP): chest X-ray or computed tomography (CT) scan showing a consolidation that resulted in antibiotic administration.
- Venous thromboembolism (VTE): positive venous ultrasound or CT angiography of the chest for deep venous thrombosis (DVT) or pulmonary embolism (PE).
- Decubitus ulcer: new wound care consultation for sacral decubitus ulceration.
- Clostridium difficile (C. diff) infection: positive stool polymerase chain reaction that resulted in antibiotic administration.
The algorithm for identifying the date of clearance and the presence of complications was piloted independently by three investigators (RP, JP, DR) using a single chart review and was redesigned until a consensus was obtained. The same three investigators then independently reviewed three additional charts, including all notes, laboratory results, imaging results, and orders, with complete agreement for both date of clearance and presence of complications. Two investigators (RP, JP) then individually reviewed the remaining 57 charts. Of these, 10 were selected a priori for review by both investigators for interrater reliability, with a mean difference of 0.5 days in the estimated time to clearance and complete concordance in complications. In addition, a third investigator (DR) independently reread 5 of the 57 reviewed charts, with complete concordance in both time to clearance and presence of complications with the original readings.
Statistical Analysis
SAS 9.3 was used for all analyses (SAS Institute Inc., Cary, NC, USA).
We first examined the demographic and clinical characteristics of all 61 patients who underwent guardianship proceedings. Second, we described the primary outcomes of interest–length of stay, costs, and likelihood of complications–in this series of patients with associated 95% confidence intervals.
Third, we examined the associations between guardianship and length of stay and healthcare costs using generalized estimating equations with clustering by matched set and compound symmetry. For length of stay, we specifically assessed excess length of stay (the matching variable) to avoid immortal time bias; we also examined the total length of stay. For all regression analyses, we adjusted for the following covariates: age, gender, education, marital status, race/ethnicity, CMI, insurance status, discharging service, and principal diagnosis. To maximize normality of residuals, costs were log-transformed; length of stay beyond clearance was log-transformed after addition of 1. For both outcomes, we back-transformed the regression coefficients and presented percent change between case and control patients. All reported tests are two-sided.
RESULTS
A total of 61 guardianship cases and 118 controls were included in the analysis.
General Characteristics
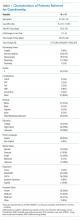
The characteristics of all cases prior to matching are included in Table 1. The department of internal medicine discharged the largest proportion of cases, followed by neurosurgery and neurology departments. More than 65% of cases were insured by Medicare or Medicaid. Three-quarters of cases were discharged from the hospital to another medical facility, with about half discharged to a skilled nursing facility (SNF) or a rehabilitation center and one-quarter to a long-term acute care hospital (LTACH).
The median length of stay for patients requiring guardianship was 28 (range, 23-36) days, and the median total charges were $171,083 ($106,897-$245,281), with a total cost approximating $10.9 million for these patients. Regarding hospital-acquired complications, 10 (16%; 95% confidence interval, 8%–28%) unique cases suffered from a complication, with HAP being the most frequently (n = 5) occurring complication.
Comparison with Matched Controls
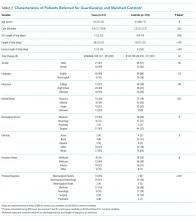
No statistically significant differences were observed between cases and controls in terms of age, primary language, highest level of education, ethnicity, insurance status, or discharging service as shown in Table 2; discharging service was a matched variable and comparable by design. However, cases tended to be less likely to be married and had a higher CMI.
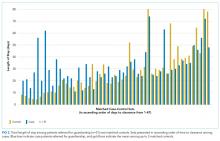
When compared with control patients in terms of similar services who stayed for at least as long as their duration to clearance, the cases had significantly longer lengths of stay compared to those of controls (29 total days compared to 18 days, P < .001; Figure 2). In addition, cases incurred significantly higher median total charges ($168,666) compared to those of controls ($104,190; P = .02).
After accounting for potential confounders, including age, gender, language, education, marital status, discharging service, ethnicity, insurance status, CMI, and principal diagnosis, guardianship was associated with 58% higher excess length of stay (P = .04, 95% CI [2%-145%]). Furthermore, guardianship was associated with 23% higher total charges (P = .02, 95% CI [4%-46%]) and 37% longer total length of stay (P = .002, 95% CI [12%-67%]).
DISCUSSION
To our knowledge, this is among the first studies to investigate healthcare costs and harm to the patient in the form of hospital-associated complications as a result of guardianship proceedings. Other studies15,16 have also demonstrated excessive length of stay attributed to nonclinical factors such as guardianship, though they did not quantify the excess stay or compare guardianship cases with a matched control. One study17 demonstrated total charges of $150,000 per patient requiring guardianship, which are similar to our results. However, Chen et al. also observed an average of 27.8 medically unnecessary days, which are 16 more days than those in our study sample. This may reflect the difference in how excess days were determined, namely, statistical process control analysis in the previous study compared with a manual chart review in our study. To our knowledge, no other study has compared guardianship cases with matched controls to compare their experiences to patients with similarly prolonged stays prior to clearance.
After matching by service and the length of stay until medical clearance in each guardianship case, the subsequent length of stay was higher among cases than among controls, even after adjustment for differences in CMI and diagnosis. This suggests that the process of obtaining guardianship results in a particularly prolonged length of stay, which is presumably attributable to factors other than medical complexity or ongoing illness.
It is probable that at least two interrelated mechanisms are responsible for the particularly high costs and the long stay of patients who require guardianship. First, the process of obtaining guardianship is itself protracted in several cases, necessitating long-term admissions well beyond the point of medical stability. Second, our results suggest that longer hospital stays are apt to grow further in a feed-forward cycle due to hospital-acquired complications that develop after the date of medical clearance. Indeed, in our series, 16% of patients sustained a complication that is readily attributable to hospital care after their date of clearance, and these types of complications are likely to lengthen the stay even further.
We compared cases referred for guardianship to control patients on the same services, at similar time points, whose length of stay was at least as long as the point of medical clearance as their corresponding case patient. Because cases were hospitalized with active medical needs to at least the point of clearance, we anticipated that costs might well be lower among cases, who had no medical necessity for hospitalization at the point of clearance, compared with controls who remained hospitalized presumably for active medical needs. Counter to this hypothesis, and accounting for potentially confounding variables, undergoing a guardianship proceeding was associated with nearly 25% higher costs of patient care. This may ultimately represent a substantial burden on the healthcare system. For example, in just 1 year in our hospital, the total hospital charges reached almost $11 million for the 61 patients who underwent guardianship proceedings. Considering that 65% of the patients requiring guardianship had Medicaid or Medicare coverage, there are significant financial implications for the hospital systems and to the public.
Limitations of our study relate to its retrospective nature at a single center. Investigating guardianship cases at a single center and with a small sample size of 61 patients limits generalizability. Nevertheless, we still had enough power to detect significant differences compared with matched controls, and this study remains the largest investigation into the cost associated with guardianship to date and the only study comparing guardianship cases with matched controls. Furthermore, we did not complete chart reviews of controls, which limits direct comparisons of complications and precluded our matching on variables that required detailed review.
The retrospective design may include confounders unaccounted for in our statistical design, though we attempted to match cases with controls to account for some of these potential differences and included a broad set of covariates that included measures of comorbidity and diagnosis. To this point, we included only CMI and principal diagnosis as the measures of severity, and adjustment for CMI, which includes features of the index hospitalization itself, may represent overadjustment. However, this type of overadjustment would tend to bias toward the null hypothesis.
Investigators only completed chart reviews for cases, which limits our ability to contrast the rate of hospital-associated complications for cases with that of controls. However, the rates of CAUTI and HAP complications among our cases were notably higher than national inpatient estimates, ie, 5% and 8% compared to 0.2%18 and 0.5%-1%,19 respectively. Furthermore, we demonstrated higher total costs and total lengths of stay among guardianship patients, analyses for which the attributed date of clearance for controls was not required, and the rate of complications among the case patients was sizable despite their being formally medically cleared. In other words, regardless of whether a complication rate of 16% is “typical” for inpatients hospitalized for these durations, this suggests that persistent hospitalization after clearance does not carry a benign prognosis.
In addition, to estimate healthcare costs, we relied on total hospital charges, which are readily available and reflect, at least in part, payer costs but do not reflect true costs to the medical center. Nonetheless, charges approximately reflect costs–with some variation across cost centers–and hence provide a useful metric for comparing cases and controls. To provide context, for academic medical centers such as ours, costs are typically about half of charges.
Finally, each state has different statutes for surrogate decision-making. The results of this study reflect the Massachusetts’ experience, with no public guardianship program or hierarchy statute. That being said, while this presumably causes the need for more guardianships in Massachusetts, the mechanisms for guardianship are broadly similar nationwide and are likely to result in excessive length of stay and cost similar to those in our population, as demonstrated in studies from other states.7,15–17
Implications
At a time where medical systems are searching for opportunities to reduce the length of stay, prevent unnecessary hospitalization, and improve the quality of care, reevaluating the guardianship process is ripe with opportunity. In this single academic center, the process of guardianship was associated with 58% excess length of stay and 23% higher total hospital charges. Furthermore, one in six patients requiring guardianship suffered from hospital-associated complications.
This matched cohort study adds quantitative data demonstrating substantial burdens to the healthcare system as a result of the guardianship process and can be used as an impetus for hospital administration and legal systems to expedite the process. Potential improvements include increasing HCP form completions (which would eliminate the need to pursue guardianship for most of such patients), identifying patients who lack a legally recognized surrogate decision-maker earlier in their hospital stay (ideally upon admission), and providing resources to assist clinical teams in the completion of affidavits necessary to support the appointment of a guardian, so that paperwork can be filed with courts sooner. Further research that provides more generalizable prospective data could potentially improve the guardianship process and reduce its burden on hospitals and patients even further.
Acknowledgments
The authors express their tremendous thanks to Gail Piatkowski for her invaluable assistance in collecting administrative and billing data.
Disclosures
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article
1. O’Neill O. Autonomy and Trust in Bioethics. Cambridge: Cambridge University Press; 2002. PubMed
2. Beauchamp T, Childress J. Principles of Biomedical Ethics. 7th ed. New York: Oxford University Press; 2013.
3. McMurray RJ, Clarke OW, Barrasso JA, et al. Decisions near the end of life. J Am Med Assoc. 1992;267(16):2229-2233.
4. American Medical Association. AMA Principles of Medical Ethics: Chapter 2 - Opinions on Consent, Communication and Decision Making.; 2016.
5. Arnold RM, Kellum J. Moral justifications for surrogate decision making in the intensive care unit: Implications and limitations. Crit Care Med. 2003;31(Supplement):S347-S353. PubMed
6. Karp N, Wood E. Incapacitated and Alone: Healthcare Decision Making for Unbefriended Older People. Am Bar Assoc Hum Rights. 2003;31(2).
7. Bandy RJ, Helft PR, Bandy RW, Torke AM. Medical decision-making during the guardianship process for incapacitated, hospitalized adults: a descriptive cohort study. J Gen Intern Med. 2010;25(10):1003-1008. PubMed
8. Wynn S. Decisions by surrogates: an overview of surrogate consent laws in the United States. Bifocal. 2014;36(1):10-14.
9. Massachusetts General Laws. Chapter 201D: Health Care Proxies. https://malegislature.gov/Laws/GeneralLaws/PartII/TitleII/Chapter201D. Published 2017. Accessed March 31, 2017.
10. American Bar Association Commision on Law and Aging. Default Surrogate Consent Statutes. Am Bar Assoc. 2016:1-17.
11. Massachusetts General Laws. Chapter 190B: Massachusetts Probate Code. https://malegislature.gov/Laws/GeneralLaws/PartII/TitleII/Chapter190B. Published 2017. Accessed March 31, 2017.
12. Rosman M, Rachminov O, Segal O, Segal G. Prolonged patients’ in-hospital waiting period after discharge eligibility is associated with increased risk of infection, morbidity and mortality: a retrospective cohort analysis. BMC Health Serv Res. 2015;15:246. PubMed
13. Majeed MU, Williams DT, Pollock R, et al. Delay in discharge and its impact on unnecessary hospital bed occupancy. 2012. PubMed
14. Nobili A, Licata G, Salerno F, et al. Polypharmacy, length of hospital stay, and in-hospital mortality among elderly patients in internal medicine wards. The REPOSI study. Eur J Clin Pharmacol. 2011;67(5):507-519. PubMed
15. Chen JJ, Finn CT, Homa K, St Onge KP, Caller TA. Discharge delays for patients requiring in-hospital guardianship: A Cohort Analysis. J Healthc Qual. 2016;38(4):235-242. PubMed
16. Chen JJ, Kwon A, Stevens Y, Finn CT. Barriers beyond clinical control affecting timely hospital discharge for a patient requiring guardianship. Psychosomatics. 2015;56(2):206-209. PubMed
17. Chen JJ, Blanchard MA, Finn CT, et al. A clinical pathway for guardianship at dartmouth-hitchcock medical center. Jt Comm J Qual Patient Saf. 2014;40(9):389-397. PubMed
18. McEachern R, Campbell Jr GD. Hospital-Acquired Pneumonia: Epidemiology, Etiology, and Treatment. Infect Dis Clin North Am. 1998;12(3):761-779. PubMed
19. Zimlichman E, Henderson D, Tamir O, et al. Health care–associated infections. JAMA Intern Med. 2013;173(22):2039. PubMed
A central tenet of modern medicine is that patients must provide fully informed consent to receive or refuse medical care offered by their clinical teams.1–4 If a patient is unable to make and communicate a choice or clearly indicate an understanding of the information presented, then he or she is considered to lack the capacity to make medical decisions and the medical team must seek consent from the patient’s surrogate decision-maker.2-7 Every U.S. state recognizes a patient’s healthcare proxy (HCP) and a court-appointed guardian as a legally recognized surrogate.8,9 Most of the states also have statutes or regulations establishing a hierarchy of legally recognized surrogate decision-makers in the absence of a HCP or a court-appointed guardian, such as spouses, adult children, parents, siblings, and grandparents.8,10 In states that do not have such a statute, hospitals develop their own institutional policies for surrogate decision-making.
However, there are important limitations on the authority of these surrogate decision-makers.10 For instance, patients may not have a family member or a friend to serve as a surrogate decision-maker, often family members cannot override a patient’s objection, even when that patient lacks decision-making capacity, and certain decisions require a guardian or a HCP.8-10 In these circumstances, the hospital must petition a court to appoint a guardian as a legally recognized surrogate decision-maker. This can be an involved family member, if one exists, or an independent, typically volunteer, guardian.11 The process of guardian appointment is complex7,11 and can range from a few days to more than a month, largely dependent on court dates and finding a volunteer guardian. Much of the process occurs during the patient’s hospital stay. This prolongation of hospitalization would be expected to increase health care costs and iatrogenic complications,12–14 but data quantifying these for patients requiring guardianship are lacking. The goal of this study was to describe the characteristics of patients who undergo the process of guardianship and measure the associated burdens. These burdens include the financial costs to the medical system, the prolonged length of stay beyond medical necessity, and the costs to the patient in the form of hospital-acquired complications. Investigating the burden of guardianship is an important first step in uncovering opportunities to improve the process. We hypothesized that patients requiring guardianship would have lengths of stay and healthcare costs that were at least as large as those for patients whose conditions required similar durations of hospitalization prior to medical clearance, in part due to iatrogenic complications that would accrue while awaiting guardian appointment.
METHODS
Setting
We conducted a retrospective matched cohort study of adult inpatients at Beth Israel Deaconess Medical Center (BIDMC), a 651-bed academic, tertiary care facility in Boston, MA. The study was approved by the BIDMC Institutional Review Board as a nonhuman subject research consistent with hospital operations.
Population
For this matched cohort study, we identified case patients as those hospitalized for any reason for whom guardianship proceedings were initiated and obtained; only the first hospitalization during which the guardianship was pursued was used. Cases were identified by obtaining the data of all patients for whom the BIDMC general counsel completed the process of guardianship between October 2014 and September 2015. At BIDMC, all the guardianship proceedings are referred to the general counsel.
To determine the postclearance experience for referred patients compared with that for other patients with similar lengths of stay up to those of the referred patients’ point of clearance, we identified up to three matched controls for each case (Supplemental Figure 1). Medical clearance was defined as the date when the patient was medically stable to be discharged from the hospital, and it was determined in an iterative manner. We identified controls as hospitalized patients admitted for any cause and matched to the cases requiring guardianship on discharging service and length of stay prior to clearance. Specifically, we identified patients on the same service as the case whose length of stay was at least as long as the length of stay of the case patient until medical clearance, as defined below. We then determined the total and the excess length of stay, defined as the duration beyond clearance for each case referred for guardianship; for controls, the ‘excess’ length of stay was the number of hospitalized days beyond the corresponding time that a matched case had been provided clearance. To account for seasonal influences and the training level of house officers, we selected the three controls whose discharge date was closest (before or after) to the discharge date of their matched case.
From legal team files, we identified 61 patients hospitalized at BIDMC for whom new guardianship was pursued to completion. Of these 61 patients, 10 could not be matched to an appropriate control and were included in descriptive analyses but not in comparisons with controls.
Covariates and Outcomes
We collected the details regarding age, gender, primary language, highest level of education, marital status, insurance status, race, date of admission, date of discharge, discharge disposition, principal diagnosis, case mix index (CMI), and discharging service from our administrative and billing data. Outcomes of interest included length of stay and total hospital charges that were collected from the same databases. We used hospital charges, rather than payments, to ensure uniformity across payers.
Chart Review
Unique to cases, a team of two medical residents (JP, RP) and a hospitalist (DR) determined the date of medical clearance and hospital-associated complications by a chart review. The date of medical clearance was then used to calculate excess length of stay, ie, the duration of stay beyond the date of medical clearance, by subtracting the time to medical clearance from the total inpatient length of stay.
We developed a novel algorithm to determine the date of medical clearance consistently (Figure 1). We first determined whether the discharge summary indicated a clear date of medical readiness for discharge. If the discharge summary was unclear, then a case management or a social work note was used. The date of medical clearance determined by the case management or the social work note was then confirmed with clinical data. The date was confirmed if there were no significant laboratory orders and major medication changes or procedures for 24 h from the date identified. If notes were also inconclusive, then the medical clearance was determined by a review of provider order entry. Medical readiness for discharge was then defined as the first day when there were no laboratory orders for 48 h and no significant medication changes, imaging studies, or microbiologic orders.
Hospital-acquired complications were determined to be related to the guardianship process if they occurred after the date of medical stability but prior to discharge. We did not investigate hospital-acquired complications among controls. Hospital-acquired complications were defined as follows:
- Catheter-associated urinary tract infection (CAUTI): active Foley catheter order and positive urine culture that resulted in antibiotic administration.
- Hospital-acquired pneumonia (HAP): chest X-ray or computed tomography (CT) scan showing a consolidation that resulted in antibiotic administration.
- Venous thromboembolism (VTE): positive venous ultrasound or CT angiography of the chest for deep venous thrombosis (DVT) or pulmonary embolism (PE).
- Decubitus ulcer: new wound care consultation for sacral decubitus ulceration.
- Clostridium difficile (C. diff) infection: positive stool polymerase chain reaction that resulted in antibiotic administration.
The algorithm for identifying the date of clearance and the presence of complications was piloted independently by three investigators (RP, JP, DR) using a single chart review and was redesigned until a consensus was obtained. The same three investigators then independently reviewed three additional charts, including all notes, laboratory results, imaging results, and orders, with complete agreement for both date of clearance and presence of complications. Two investigators (RP, JP) then individually reviewed the remaining 57 charts. Of these, 10 were selected a priori for review by both investigators for interrater reliability, with a mean difference of 0.5 days in the estimated time to clearance and complete concordance in complications. In addition, a third investigator (DR) independently reread 5 of the 57 reviewed charts, with complete concordance in both time to clearance and presence of complications with the original readings.
Statistical Analysis
SAS 9.3 was used for all analyses (SAS Institute Inc., Cary, NC, USA).
We first examined the demographic and clinical characteristics of all 61 patients who underwent guardianship proceedings. Second, we described the primary outcomes of interest–length of stay, costs, and likelihood of complications–in this series of patients with associated 95% confidence intervals.
Third, we examined the associations between guardianship and length of stay and healthcare costs using generalized estimating equations with clustering by matched set and compound symmetry. For length of stay, we specifically assessed excess length of stay (the matching variable) to avoid immortal time bias; we also examined the total length of stay. For all regression analyses, we adjusted for the following covariates: age, gender, education, marital status, race/ethnicity, CMI, insurance status, discharging service, and principal diagnosis. To maximize normality of residuals, costs were log-transformed; length of stay beyond clearance was log-transformed after addition of 1. For both outcomes, we back-transformed the regression coefficients and presented percent change between case and control patients. All reported tests are two-sided.
RESULTS
A total of 61 guardianship cases and 118 controls were included in the analysis.
General Characteristics
The characteristics of all cases prior to matching are included in Table 1. The department of internal medicine discharged the largest proportion of cases, followed by neurosurgery and neurology departments. More than 65% of cases were insured by Medicare or Medicaid. Three-quarters of cases were discharged from the hospital to another medical facility, with about half discharged to a skilled nursing facility (SNF) or a rehabilitation center and one-quarter to a long-term acute care hospital (LTACH).
The median length of stay for patients requiring guardianship was 28 (range, 23-36) days, and the median total charges were $171,083 ($106,897-$245,281), with a total cost approximating $10.9 million for these patients. Regarding hospital-acquired complications, 10 (16%; 95% confidence interval, 8%–28%) unique cases suffered from a complication, with HAP being the most frequently (n = 5) occurring complication.
Comparison with Matched Controls
No statistically significant differences were observed between cases and controls in terms of age, primary language, highest level of education, ethnicity, insurance status, or discharging service as shown in Table 2; discharging service was a matched variable and comparable by design. However, cases tended to be less likely to be married and had a higher CMI.
When compared with control patients in terms of similar services who stayed for at least as long as their duration to clearance, the cases had significantly longer lengths of stay compared to those of controls (29 total days compared to 18 days, P < .001; Figure 2). In addition, cases incurred significantly higher median total charges ($168,666) compared to those of controls ($104,190; P = .02).
After accounting for potential confounders, including age, gender, language, education, marital status, discharging service, ethnicity, insurance status, CMI, and principal diagnosis, guardianship was associated with 58% higher excess length of stay (P = .04, 95% CI [2%-145%]). Furthermore, guardianship was associated with 23% higher total charges (P = .02, 95% CI [4%-46%]) and 37% longer total length of stay (P = .002, 95% CI [12%-67%]).
DISCUSSION
To our knowledge, this is among the first studies to investigate healthcare costs and harm to the patient in the form of hospital-associated complications as a result of guardianship proceedings. Other studies15,16 have also demonstrated excessive length of stay attributed to nonclinical factors such as guardianship, though they did not quantify the excess stay or compare guardianship cases with a matched control. One study17 demonstrated total charges of $150,000 per patient requiring guardianship, which are similar to our results. However, Chen et al. also observed an average of 27.8 medically unnecessary days, which are 16 more days than those in our study sample. This may reflect the difference in how excess days were determined, namely, statistical process control analysis in the previous study compared with a manual chart review in our study. To our knowledge, no other study has compared guardianship cases with matched controls to compare their experiences to patients with similarly prolonged stays prior to clearance.
After matching by service and the length of stay until medical clearance in each guardianship case, the subsequent length of stay was higher among cases than among controls, even after adjustment for differences in CMI and diagnosis. This suggests that the process of obtaining guardianship results in a particularly prolonged length of stay, which is presumably attributable to factors other than medical complexity or ongoing illness.
It is probable that at least two interrelated mechanisms are responsible for the particularly high costs and the long stay of patients who require guardianship. First, the process of obtaining guardianship is itself protracted in several cases, necessitating long-term admissions well beyond the point of medical stability. Second, our results suggest that longer hospital stays are apt to grow further in a feed-forward cycle due to hospital-acquired complications that develop after the date of medical clearance. Indeed, in our series, 16% of patients sustained a complication that is readily attributable to hospital care after their date of clearance, and these types of complications are likely to lengthen the stay even further.
We compared cases referred for guardianship to control patients on the same services, at similar time points, whose length of stay was at least as long as the point of medical clearance as their corresponding case patient. Because cases were hospitalized with active medical needs to at least the point of clearance, we anticipated that costs might well be lower among cases, who had no medical necessity for hospitalization at the point of clearance, compared with controls who remained hospitalized presumably for active medical needs. Counter to this hypothesis, and accounting for potentially confounding variables, undergoing a guardianship proceeding was associated with nearly 25% higher costs of patient care. This may ultimately represent a substantial burden on the healthcare system. For example, in just 1 year in our hospital, the total hospital charges reached almost $11 million for the 61 patients who underwent guardianship proceedings. Considering that 65% of the patients requiring guardianship had Medicaid or Medicare coverage, there are significant financial implications for the hospital systems and to the public.
Limitations of our study relate to its retrospective nature at a single center. Investigating guardianship cases at a single center and with a small sample size of 61 patients limits generalizability. Nevertheless, we still had enough power to detect significant differences compared with matched controls, and this study remains the largest investigation into the cost associated with guardianship to date and the only study comparing guardianship cases with matched controls. Furthermore, we did not complete chart reviews of controls, which limits direct comparisons of complications and precluded our matching on variables that required detailed review.
The retrospective design may include confounders unaccounted for in our statistical design, though we attempted to match cases with controls to account for some of these potential differences and included a broad set of covariates that included measures of comorbidity and diagnosis. To this point, we included only CMI and principal diagnosis as the measures of severity, and adjustment for CMI, which includes features of the index hospitalization itself, may represent overadjustment. However, this type of overadjustment would tend to bias toward the null hypothesis.
Investigators only completed chart reviews for cases, which limits our ability to contrast the rate of hospital-associated complications for cases with that of controls. However, the rates of CAUTI and HAP complications among our cases were notably higher than national inpatient estimates, ie, 5% and 8% compared to 0.2%18 and 0.5%-1%,19 respectively. Furthermore, we demonstrated higher total costs and total lengths of stay among guardianship patients, analyses for which the attributed date of clearance for controls was not required, and the rate of complications among the case patients was sizable despite their being formally medically cleared. In other words, regardless of whether a complication rate of 16% is “typical” for inpatients hospitalized for these durations, this suggests that persistent hospitalization after clearance does not carry a benign prognosis.
In addition, to estimate healthcare costs, we relied on total hospital charges, which are readily available and reflect, at least in part, payer costs but do not reflect true costs to the medical center. Nonetheless, charges approximately reflect costs–with some variation across cost centers–and hence provide a useful metric for comparing cases and controls. To provide context, for academic medical centers such as ours, costs are typically about half of charges.
Finally, each state has different statutes for surrogate decision-making. The results of this study reflect the Massachusetts’ experience, with no public guardianship program or hierarchy statute. That being said, while this presumably causes the need for more guardianships in Massachusetts, the mechanisms for guardianship are broadly similar nationwide and are likely to result in excessive length of stay and cost similar to those in our population, as demonstrated in studies from other states.7,15–17
Implications
At a time where medical systems are searching for opportunities to reduce the length of stay, prevent unnecessary hospitalization, and improve the quality of care, reevaluating the guardianship process is ripe with opportunity. In this single academic center, the process of guardianship was associated with 58% excess length of stay and 23% higher total hospital charges. Furthermore, one in six patients requiring guardianship suffered from hospital-associated complications.
This matched cohort study adds quantitative data demonstrating substantial burdens to the healthcare system as a result of the guardianship process and can be used as an impetus for hospital administration and legal systems to expedite the process. Potential improvements include increasing HCP form completions (which would eliminate the need to pursue guardianship for most of such patients), identifying patients who lack a legally recognized surrogate decision-maker earlier in their hospital stay (ideally upon admission), and providing resources to assist clinical teams in the completion of affidavits necessary to support the appointment of a guardian, so that paperwork can be filed with courts sooner. Further research that provides more generalizable prospective data could potentially improve the guardianship process and reduce its burden on hospitals and patients even further.
Acknowledgments
The authors express their tremendous thanks to Gail Piatkowski for her invaluable assistance in collecting administrative and billing data.
Disclosures
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article
A central tenet of modern medicine is that patients must provide fully informed consent to receive or refuse medical care offered by their clinical teams.1–4 If a patient is unable to make and communicate a choice or clearly indicate an understanding of the information presented, then he or she is considered to lack the capacity to make medical decisions and the medical team must seek consent from the patient’s surrogate decision-maker.2-7 Every U.S. state recognizes a patient’s healthcare proxy (HCP) and a court-appointed guardian as a legally recognized surrogate.8,9 Most of the states also have statutes or regulations establishing a hierarchy of legally recognized surrogate decision-makers in the absence of a HCP or a court-appointed guardian, such as spouses, adult children, parents, siblings, and grandparents.8,10 In states that do not have such a statute, hospitals develop their own institutional policies for surrogate decision-making.
However, there are important limitations on the authority of these surrogate decision-makers.10 For instance, patients may not have a family member or a friend to serve as a surrogate decision-maker, often family members cannot override a patient’s objection, even when that patient lacks decision-making capacity, and certain decisions require a guardian or a HCP.8-10 In these circumstances, the hospital must petition a court to appoint a guardian as a legally recognized surrogate decision-maker. This can be an involved family member, if one exists, or an independent, typically volunteer, guardian.11 The process of guardian appointment is complex7,11 and can range from a few days to more than a month, largely dependent on court dates and finding a volunteer guardian. Much of the process occurs during the patient’s hospital stay. This prolongation of hospitalization would be expected to increase health care costs and iatrogenic complications,12–14 but data quantifying these for patients requiring guardianship are lacking. The goal of this study was to describe the characteristics of patients who undergo the process of guardianship and measure the associated burdens. These burdens include the financial costs to the medical system, the prolonged length of stay beyond medical necessity, and the costs to the patient in the form of hospital-acquired complications. Investigating the burden of guardianship is an important first step in uncovering opportunities to improve the process. We hypothesized that patients requiring guardianship would have lengths of stay and healthcare costs that were at least as large as those for patients whose conditions required similar durations of hospitalization prior to medical clearance, in part due to iatrogenic complications that would accrue while awaiting guardian appointment.
METHODS
Setting
We conducted a retrospective matched cohort study of adult inpatients at Beth Israel Deaconess Medical Center (BIDMC), a 651-bed academic, tertiary care facility in Boston, MA. The study was approved by the BIDMC Institutional Review Board as a nonhuman subject research consistent with hospital operations.
Population
For this matched cohort study, we identified case patients as those hospitalized for any reason for whom guardianship proceedings were initiated and obtained; only the first hospitalization during which the guardianship was pursued was used. Cases were identified by obtaining the data of all patients for whom the BIDMC general counsel completed the process of guardianship between October 2014 and September 2015. At BIDMC, all the guardianship proceedings are referred to the general counsel.
To determine the postclearance experience for referred patients compared with that for other patients with similar lengths of stay up to those of the referred patients’ point of clearance, we identified up to three matched controls for each case (Supplemental Figure 1). Medical clearance was defined as the date when the patient was medically stable to be discharged from the hospital, and it was determined in an iterative manner. We identified controls as hospitalized patients admitted for any cause and matched to the cases requiring guardianship on discharging service and length of stay prior to clearance. Specifically, we identified patients on the same service as the case whose length of stay was at least as long as the length of stay of the case patient until medical clearance, as defined below. We then determined the total and the excess length of stay, defined as the duration beyond clearance for each case referred for guardianship; for controls, the ‘excess’ length of stay was the number of hospitalized days beyond the corresponding time that a matched case had been provided clearance. To account for seasonal influences and the training level of house officers, we selected the three controls whose discharge date was closest (before or after) to the discharge date of their matched case.
From legal team files, we identified 61 patients hospitalized at BIDMC for whom new guardianship was pursued to completion. Of these 61 patients, 10 could not be matched to an appropriate control and were included in descriptive analyses but not in comparisons with controls.
Covariates and Outcomes
We collected the details regarding age, gender, primary language, highest level of education, marital status, insurance status, race, date of admission, date of discharge, discharge disposition, principal diagnosis, case mix index (CMI), and discharging service from our administrative and billing data. Outcomes of interest included length of stay and total hospital charges that were collected from the same databases. We used hospital charges, rather than payments, to ensure uniformity across payers.
Chart Review
Unique to cases, a team of two medical residents (JP, RP) and a hospitalist (DR) determined the date of medical clearance and hospital-associated complications by a chart review. The date of medical clearance was then used to calculate excess length of stay, ie, the duration of stay beyond the date of medical clearance, by subtracting the time to medical clearance from the total inpatient length of stay.
We developed a novel algorithm to determine the date of medical clearance consistently (Figure 1). We first determined whether the discharge summary indicated a clear date of medical readiness for discharge. If the discharge summary was unclear, then a case management or a social work note was used. The date of medical clearance determined by the case management or the social work note was then confirmed with clinical data. The date was confirmed if there were no significant laboratory orders and major medication changes or procedures for 24 h from the date identified. If notes were also inconclusive, then the medical clearance was determined by a review of provider order entry. Medical readiness for discharge was then defined as the first day when there were no laboratory orders for 48 h and no significant medication changes, imaging studies, or microbiologic orders.
Hospital-acquired complications were determined to be related to the guardianship process if they occurred after the date of medical stability but prior to discharge. We did not investigate hospital-acquired complications among controls. Hospital-acquired complications were defined as follows:
- Catheter-associated urinary tract infection (CAUTI): active Foley catheter order and positive urine culture that resulted in antibiotic administration.
- Hospital-acquired pneumonia (HAP): chest X-ray or computed tomography (CT) scan showing a consolidation that resulted in antibiotic administration.
- Venous thromboembolism (VTE): positive venous ultrasound or CT angiography of the chest for deep venous thrombosis (DVT) or pulmonary embolism (PE).
- Decubitus ulcer: new wound care consultation for sacral decubitus ulceration.
- Clostridium difficile (C. diff) infection: positive stool polymerase chain reaction that resulted in antibiotic administration.
The algorithm for identifying the date of clearance and the presence of complications was piloted independently by three investigators (RP, JP, DR) using a single chart review and was redesigned until a consensus was obtained. The same three investigators then independently reviewed three additional charts, including all notes, laboratory results, imaging results, and orders, with complete agreement for both date of clearance and presence of complications. Two investigators (RP, JP) then individually reviewed the remaining 57 charts. Of these, 10 were selected a priori for review by both investigators for interrater reliability, with a mean difference of 0.5 days in the estimated time to clearance and complete concordance in complications. In addition, a third investigator (DR) independently reread 5 of the 57 reviewed charts, with complete concordance in both time to clearance and presence of complications with the original readings.
Statistical Analysis
SAS 9.3 was used for all analyses (SAS Institute Inc., Cary, NC, USA).
We first examined the demographic and clinical characteristics of all 61 patients who underwent guardianship proceedings. Second, we described the primary outcomes of interest–length of stay, costs, and likelihood of complications–in this series of patients with associated 95% confidence intervals.
Third, we examined the associations between guardianship and length of stay and healthcare costs using generalized estimating equations with clustering by matched set and compound symmetry. For length of stay, we specifically assessed excess length of stay (the matching variable) to avoid immortal time bias; we also examined the total length of stay. For all regression analyses, we adjusted for the following covariates: age, gender, education, marital status, race/ethnicity, CMI, insurance status, discharging service, and principal diagnosis. To maximize normality of residuals, costs were log-transformed; length of stay beyond clearance was log-transformed after addition of 1. For both outcomes, we back-transformed the regression coefficients and presented percent change between case and control patients. All reported tests are two-sided.
RESULTS
A total of 61 guardianship cases and 118 controls were included in the analysis.
General Characteristics
The characteristics of all cases prior to matching are included in Table 1. The department of internal medicine discharged the largest proportion of cases, followed by neurosurgery and neurology departments. More than 65% of cases were insured by Medicare or Medicaid. Three-quarters of cases were discharged from the hospital to another medical facility, with about half discharged to a skilled nursing facility (SNF) or a rehabilitation center and one-quarter to a long-term acute care hospital (LTACH).
The median length of stay for patients requiring guardianship was 28 (range, 23-36) days, and the median total charges were $171,083 ($106,897-$245,281), with a total cost approximating $10.9 million for these patients. Regarding hospital-acquired complications, 10 (16%; 95% confidence interval, 8%–28%) unique cases suffered from a complication, with HAP being the most frequently (n = 5) occurring complication.
Comparison with Matched Controls
No statistically significant differences were observed between cases and controls in terms of age, primary language, highest level of education, ethnicity, insurance status, or discharging service as shown in Table 2; discharging service was a matched variable and comparable by design. However, cases tended to be less likely to be married and had a higher CMI.
When compared with control patients in terms of similar services who stayed for at least as long as their duration to clearance, the cases had significantly longer lengths of stay compared to those of controls (29 total days compared to 18 days, P < .001; Figure 2). In addition, cases incurred significantly higher median total charges ($168,666) compared to those of controls ($104,190; P = .02).
After accounting for potential confounders, including age, gender, language, education, marital status, discharging service, ethnicity, insurance status, CMI, and principal diagnosis, guardianship was associated with 58% higher excess length of stay (P = .04, 95% CI [2%-145%]). Furthermore, guardianship was associated with 23% higher total charges (P = .02, 95% CI [4%-46%]) and 37% longer total length of stay (P = .002, 95% CI [12%-67%]).
DISCUSSION
To our knowledge, this is among the first studies to investigate healthcare costs and harm to the patient in the form of hospital-associated complications as a result of guardianship proceedings. Other studies15,16 have also demonstrated excessive length of stay attributed to nonclinical factors such as guardianship, though they did not quantify the excess stay or compare guardianship cases with a matched control. One study17 demonstrated total charges of $150,000 per patient requiring guardianship, which are similar to our results. However, Chen et al. also observed an average of 27.8 medically unnecessary days, which are 16 more days than those in our study sample. This may reflect the difference in how excess days were determined, namely, statistical process control analysis in the previous study compared with a manual chart review in our study. To our knowledge, no other study has compared guardianship cases with matched controls to compare their experiences to patients with similarly prolonged stays prior to clearance.
After matching by service and the length of stay until medical clearance in each guardianship case, the subsequent length of stay was higher among cases than among controls, even after adjustment for differences in CMI and diagnosis. This suggests that the process of obtaining guardianship results in a particularly prolonged length of stay, which is presumably attributable to factors other than medical complexity or ongoing illness.
It is probable that at least two interrelated mechanisms are responsible for the particularly high costs and the long stay of patients who require guardianship. First, the process of obtaining guardianship is itself protracted in several cases, necessitating long-term admissions well beyond the point of medical stability. Second, our results suggest that longer hospital stays are apt to grow further in a feed-forward cycle due to hospital-acquired complications that develop after the date of medical clearance. Indeed, in our series, 16% of patients sustained a complication that is readily attributable to hospital care after their date of clearance, and these types of complications are likely to lengthen the stay even further.
We compared cases referred for guardianship to control patients on the same services, at similar time points, whose length of stay was at least as long as the point of medical clearance as their corresponding case patient. Because cases were hospitalized with active medical needs to at least the point of clearance, we anticipated that costs might well be lower among cases, who had no medical necessity for hospitalization at the point of clearance, compared with controls who remained hospitalized presumably for active medical needs. Counter to this hypothesis, and accounting for potentially confounding variables, undergoing a guardianship proceeding was associated with nearly 25% higher costs of patient care. This may ultimately represent a substantial burden on the healthcare system. For example, in just 1 year in our hospital, the total hospital charges reached almost $11 million for the 61 patients who underwent guardianship proceedings. Considering that 65% of the patients requiring guardianship had Medicaid or Medicare coverage, there are significant financial implications for the hospital systems and to the public.
Limitations of our study relate to its retrospective nature at a single center. Investigating guardianship cases at a single center and with a small sample size of 61 patients limits generalizability. Nevertheless, we still had enough power to detect significant differences compared with matched controls, and this study remains the largest investigation into the cost associated with guardianship to date and the only study comparing guardianship cases with matched controls. Furthermore, we did not complete chart reviews of controls, which limits direct comparisons of complications and precluded our matching on variables that required detailed review.
The retrospective design may include confounders unaccounted for in our statistical design, though we attempted to match cases with controls to account for some of these potential differences and included a broad set of covariates that included measures of comorbidity and diagnosis. To this point, we included only CMI and principal diagnosis as the measures of severity, and adjustment for CMI, which includes features of the index hospitalization itself, may represent overadjustment. However, this type of overadjustment would tend to bias toward the null hypothesis.
Investigators only completed chart reviews for cases, which limits our ability to contrast the rate of hospital-associated complications for cases with that of controls. However, the rates of CAUTI and HAP complications among our cases were notably higher than national inpatient estimates, ie, 5% and 8% compared to 0.2%18 and 0.5%-1%,19 respectively. Furthermore, we demonstrated higher total costs and total lengths of stay among guardianship patients, analyses for which the attributed date of clearance for controls was not required, and the rate of complications among the case patients was sizable despite their being formally medically cleared. In other words, regardless of whether a complication rate of 16% is “typical” for inpatients hospitalized for these durations, this suggests that persistent hospitalization after clearance does not carry a benign prognosis.
In addition, to estimate healthcare costs, we relied on total hospital charges, which are readily available and reflect, at least in part, payer costs but do not reflect true costs to the medical center. Nonetheless, charges approximately reflect costs–with some variation across cost centers–and hence provide a useful metric for comparing cases and controls. To provide context, for academic medical centers such as ours, costs are typically about half of charges.
Finally, each state has different statutes for surrogate decision-making. The results of this study reflect the Massachusetts’ experience, with no public guardianship program or hierarchy statute. That being said, while this presumably causes the need for more guardianships in Massachusetts, the mechanisms for guardianship are broadly similar nationwide and are likely to result in excessive length of stay and cost similar to those in our population, as demonstrated in studies from other states.7,15–17
Implications
At a time where medical systems are searching for opportunities to reduce the length of stay, prevent unnecessary hospitalization, and improve the quality of care, reevaluating the guardianship process is ripe with opportunity. In this single academic center, the process of guardianship was associated with 58% excess length of stay and 23% higher total hospital charges. Furthermore, one in six patients requiring guardianship suffered from hospital-associated complications.
This matched cohort study adds quantitative data demonstrating substantial burdens to the healthcare system as a result of the guardianship process and can be used as an impetus for hospital administration and legal systems to expedite the process. Potential improvements include increasing HCP form completions (which would eliminate the need to pursue guardianship for most of such patients), identifying patients who lack a legally recognized surrogate decision-maker earlier in their hospital stay (ideally upon admission), and providing resources to assist clinical teams in the completion of affidavits necessary to support the appointment of a guardian, so that paperwork can be filed with courts sooner. Further research that provides more generalizable prospective data could potentially improve the guardianship process and reduce its burden on hospitals and patients even further.
Acknowledgments
The authors express their tremendous thanks to Gail Piatkowski for her invaluable assistance in collecting administrative and billing data.
Disclosures
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article
1. O’Neill O. Autonomy and Trust in Bioethics. Cambridge: Cambridge University Press; 2002. PubMed
2. Beauchamp T, Childress J. Principles of Biomedical Ethics. 7th ed. New York: Oxford University Press; 2013.
3. McMurray RJ, Clarke OW, Barrasso JA, et al. Decisions near the end of life. J Am Med Assoc. 1992;267(16):2229-2233.
4. American Medical Association. AMA Principles of Medical Ethics: Chapter 2 - Opinions on Consent, Communication and Decision Making.; 2016.
5. Arnold RM, Kellum J. Moral justifications for surrogate decision making in the intensive care unit: Implications and limitations. Crit Care Med. 2003;31(Supplement):S347-S353. PubMed
6. Karp N, Wood E. Incapacitated and Alone: Healthcare Decision Making for Unbefriended Older People. Am Bar Assoc Hum Rights. 2003;31(2).
7. Bandy RJ, Helft PR, Bandy RW, Torke AM. Medical decision-making during the guardianship process for incapacitated, hospitalized adults: a descriptive cohort study. J Gen Intern Med. 2010;25(10):1003-1008. PubMed
8. Wynn S. Decisions by surrogates: an overview of surrogate consent laws in the United States. Bifocal. 2014;36(1):10-14.
9. Massachusetts General Laws. Chapter 201D: Health Care Proxies. https://malegislature.gov/Laws/GeneralLaws/PartII/TitleII/Chapter201D. Published 2017. Accessed March 31, 2017.
10. American Bar Association Commision on Law and Aging. Default Surrogate Consent Statutes. Am Bar Assoc. 2016:1-17.
11. Massachusetts General Laws. Chapter 190B: Massachusetts Probate Code. https://malegislature.gov/Laws/GeneralLaws/PartII/TitleII/Chapter190B. Published 2017. Accessed March 31, 2017.
12. Rosman M, Rachminov O, Segal O, Segal G. Prolonged patients’ in-hospital waiting period after discharge eligibility is associated with increased risk of infection, morbidity and mortality: a retrospective cohort analysis. BMC Health Serv Res. 2015;15:246. PubMed
13. Majeed MU, Williams DT, Pollock R, et al. Delay in discharge and its impact on unnecessary hospital bed occupancy. 2012. PubMed
14. Nobili A, Licata G, Salerno F, et al. Polypharmacy, length of hospital stay, and in-hospital mortality among elderly patients in internal medicine wards. The REPOSI study. Eur J Clin Pharmacol. 2011;67(5):507-519. PubMed
15. Chen JJ, Finn CT, Homa K, St Onge KP, Caller TA. Discharge delays for patients requiring in-hospital guardianship: A Cohort Analysis. J Healthc Qual. 2016;38(4):235-242. PubMed
16. Chen JJ, Kwon A, Stevens Y, Finn CT. Barriers beyond clinical control affecting timely hospital discharge for a patient requiring guardianship. Psychosomatics. 2015;56(2):206-209. PubMed
17. Chen JJ, Blanchard MA, Finn CT, et al. A clinical pathway for guardianship at dartmouth-hitchcock medical center. Jt Comm J Qual Patient Saf. 2014;40(9):389-397. PubMed
18. McEachern R, Campbell Jr GD. Hospital-Acquired Pneumonia: Epidemiology, Etiology, and Treatment. Infect Dis Clin North Am. 1998;12(3):761-779. PubMed
19. Zimlichman E, Henderson D, Tamir O, et al. Health care–associated infections. JAMA Intern Med. 2013;173(22):2039. PubMed
1. O’Neill O. Autonomy and Trust in Bioethics. Cambridge: Cambridge University Press; 2002. PubMed
2. Beauchamp T, Childress J. Principles of Biomedical Ethics. 7th ed. New York: Oxford University Press; 2013.
3. McMurray RJ, Clarke OW, Barrasso JA, et al. Decisions near the end of life. J Am Med Assoc. 1992;267(16):2229-2233.
4. American Medical Association. AMA Principles of Medical Ethics: Chapter 2 - Opinions on Consent, Communication and Decision Making.; 2016.
5. Arnold RM, Kellum J. Moral justifications for surrogate decision making in the intensive care unit: Implications and limitations. Crit Care Med. 2003;31(Supplement):S347-S353. PubMed
6. Karp N, Wood E. Incapacitated and Alone: Healthcare Decision Making for Unbefriended Older People. Am Bar Assoc Hum Rights. 2003;31(2).
7. Bandy RJ, Helft PR, Bandy RW, Torke AM. Medical decision-making during the guardianship process for incapacitated, hospitalized adults: a descriptive cohort study. J Gen Intern Med. 2010;25(10):1003-1008. PubMed
8. Wynn S. Decisions by surrogates: an overview of surrogate consent laws in the United States. Bifocal. 2014;36(1):10-14.
9. Massachusetts General Laws. Chapter 201D: Health Care Proxies. https://malegislature.gov/Laws/GeneralLaws/PartII/TitleII/Chapter201D. Published 2017. Accessed March 31, 2017.
10. American Bar Association Commision on Law and Aging. Default Surrogate Consent Statutes. Am Bar Assoc. 2016:1-17.
11. Massachusetts General Laws. Chapter 190B: Massachusetts Probate Code. https://malegislature.gov/Laws/GeneralLaws/PartII/TitleII/Chapter190B. Published 2017. Accessed March 31, 2017.
12. Rosman M, Rachminov O, Segal O, Segal G. Prolonged patients’ in-hospital waiting period after discharge eligibility is associated with increased risk of infection, morbidity and mortality: a retrospective cohort analysis. BMC Health Serv Res. 2015;15:246. PubMed
13. Majeed MU, Williams DT, Pollock R, et al. Delay in discharge and its impact on unnecessary hospital bed occupancy. 2012. PubMed
14. Nobili A, Licata G, Salerno F, et al. Polypharmacy, length of hospital stay, and in-hospital mortality among elderly patients in internal medicine wards. The REPOSI study. Eur J Clin Pharmacol. 2011;67(5):507-519. PubMed
15. Chen JJ, Finn CT, Homa K, St Onge KP, Caller TA. Discharge delays for patients requiring in-hospital guardianship: A Cohort Analysis. J Healthc Qual. 2016;38(4):235-242. PubMed
16. Chen JJ, Kwon A, Stevens Y, Finn CT. Barriers beyond clinical control affecting timely hospital discharge for a patient requiring guardianship. Psychosomatics. 2015;56(2):206-209. PubMed
17. Chen JJ, Blanchard MA, Finn CT, et al. A clinical pathway for guardianship at dartmouth-hitchcock medical center. Jt Comm J Qual Patient Saf. 2014;40(9):389-397. PubMed
18. McEachern R, Campbell Jr GD. Hospital-Acquired Pneumonia: Epidemiology, Etiology, and Treatment. Infect Dis Clin North Am. 1998;12(3):761-779. PubMed
19. Zimlichman E, Henderson D, Tamir O, et al. Health care–associated infections. JAMA Intern Med. 2013;173(22):2039. PubMed
© 2018 Society of Hospital Medicine