User login
The Burden of Guardianship: A Matched Cohort Study
A central tenet of modern medicine is that patients must provide fully informed consent to receive or refuse medical care offered by their clinical teams.1–4 If a patient is unable to make and communicate a choice or clearly indicate an understanding of the information presented, then he or she is considered to lack the capacity to make medical decisions and the medical team must seek consent from the patient’s surrogate decision-maker.2-7 Every U.S. state recognizes a patient’s healthcare proxy (HCP) and a court-appointed guardian as a legally recognized surrogate.8,9 Most of the states also have statutes or regulations establishing a hierarchy of legally recognized surrogate decision-makers in the absence of a HCP or a court-appointed guardian, such as spouses, adult children, parents, siblings, and grandparents.8,10 In states that do not have such a statute, hospitals develop their own institutional policies for surrogate decision-making.
However, there are important limitations on the authority of these surrogate decision-makers.10 For instance, patients may not have a family member or a friend to serve as a surrogate decision-maker, often family members cannot override a patient’s objection, even when that patient lacks decision-making capacity, and certain decisions require a guardian or a HCP.8-10 In these circumstances, the hospital must petition a court to appoint a guardian as a legally recognized surrogate decision-maker. This can be an involved family member, if one exists, or an independent, typically volunteer, guardian.11 The process of guardian appointment is complex7,11 and can range from a few days to more than a month, largely dependent on court dates and finding a volunteer guardian. Much of the process occurs during the patient’s hospital stay. This prolongation of hospitalization would be expected to increase health care costs and iatrogenic complications,12–14 but data quantifying these for patients requiring guardianship are lacking. The goal of this study was to describe the characteristics of patients who undergo the process of guardianship and measure the associated burdens. These burdens include the financial costs to the medical system, the prolonged length of stay beyond medical necessity, and the costs to the patient in the form of hospital-acquired complications. Investigating the burden of guardianship is an important first step in uncovering opportunities to improve the process. We hypothesized that patients requiring guardianship would have lengths of stay and healthcare costs that were at least as large as those for patients whose conditions required similar durations of hospitalization prior to medical clearance, in part due to iatrogenic complications that would accrue while awaiting guardian appointment.
METHODS
Setting
We conducted a retrospective matched cohort study of adult inpatients at Beth Israel Deaconess Medical Center (BIDMC), a 651-bed academic, tertiary care facility in Boston, MA. The study was approved by the BIDMC Institutional Review Board as a nonhuman subject research consistent with hospital operations.
Population
For this matched cohort study, we identified case patients as those hospitalized for any reason for whom guardianship proceedings were initiated and obtained; only the first hospitalization during which the guardianship was pursued was used. Cases were identified by obtaining the data of all patients for whom the BIDMC general counsel completed the process of guardianship between October 2014 and September 2015. At BIDMC, all the guardianship proceedings are referred to the general counsel.
To determine the postclearance experience for referred patients compared with that for other patients with similar lengths of stay up to those of the referred patients’ point of clearance, we identified up to three matched controls for each case (Supplemental Figure 1). Medical clearance was defined as the date when the patient was medically stable to be discharged from the hospital, and it was determined in an iterative manner. We identified controls as hospitalized patients admitted for any cause and matched to the cases requiring guardianship on discharging service and length of stay prior to clearance. Specifically, we identified patients on the same service as the case whose length of stay was at least as long as the length of stay of the case patient until medical clearance, as defined below. We then determined the total and the excess length of stay, defined as the duration beyond clearance for each case referred for guardianship; for controls, the ‘excess’ length of stay was the number of hospitalized days beyond the corresponding time that a matched case had been provided clearance. To account for seasonal influences and the training level of house officers, we selected the three controls whose discharge date was closest (before or after) to the discharge date of their matched case.
From legal team files, we identified 61 patients hospitalized at BIDMC for whom new guardianship was pursued to completion. Of these 61 patients, 10 could not be matched to an appropriate control and were included in descriptive analyses but not in comparisons with controls.
Covariates and Outcomes
We collected the details regarding age, gender, primary language, highest level of education, marital status, insurance status, race, date of admission, date of discharge, discharge disposition, principal diagnosis, case mix index (CMI), and discharging service from our administrative and billing data. Outcomes of interest included length of stay and total hospital charges that were collected from the same databases. We used hospital charges, rather than payments, to ensure uniformity across payers.
Chart Review
Unique to cases, a team of two medical residents (JP, RP) and a hospitalist (DR) determined the date of medical clearance and hospital-associated complications by a chart review. The date of medical clearance was then used to calculate excess length of stay, ie, the duration of stay beyond the date of medical clearance, by subtracting the time to medical clearance from the total inpatient length of stay.
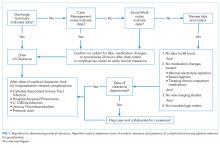
We developed a novel algorithm to determine the date of medical clearance consistently (Figure 1). We first determined whether the discharge summary indicated a clear date of medical readiness for discharge. If the discharge summary was unclear, then a case management or a social work note was used. The date of medical clearance determined by the case management or the social work note was then confirmed with clinical data. The date was confirmed if there were no significant laboratory orders and major medication changes or procedures for 24 h from the date identified. If notes were also inconclusive, then the medical clearance was determined by a review of provider order entry. Medical readiness for discharge was then defined as the first day when there were no laboratory orders for 48 h and no significant medication changes, imaging studies, or microbiologic orders.
Hospital-acquired complications were determined to be related to the guardianship process if they occurred after the date of medical stability but prior to discharge. We did not investigate hospital-acquired complications among controls. Hospital-acquired complications were defined as follows:
- Catheter-associated urinary tract infection (CAUTI): active Foley catheter order and positive urine culture that resulted in antibiotic administration.
- Hospital-acquired pneumonia (HAP): chest X-ray or computed tomography (CT) scan showing a consolidation that resulted in antibiotic administration.
- Venous thromboembolism (VTE): positive venous ultrasound or CT angiography of the chest for deep venous thrombosis (DVT) or pulmonary embolism (PE).
- Decubitus ulcer: new wound care consultation for sacral decubitus ulceration.
- Clostridium difficile (C. diff) infection: positive stool polymerase chain reaction that resulted in antibiotic administration.
The algorithm for identifying the date of clearance and the presence of complications was piloted independently by three investigators (RP, JP, DR) using a single chart review and was redesigned until a consensus was obtained. The same three investigators then independently reviewed three additional charts, including all notes, laboratory results, imaging results, and orders, with complete agreement for both date of clearance and presence of complications. Two investigators (RP, JP) then individually reviewed the remaining 57 charts. Of these, 10 were selected a priori for review by both investigators for interrater reliability, with a mean difference of 0.5 days in the estimated time to clearance and complete concordance in complications. In addition, a third investigator (DR) independently reread 5 of the 57 reviewed charts, with complete concordance in both time to clearance and presence of complications with the original readings.
Statistical Analysis
SAS 9.3 was used for all analyses (SAS Institute Inc., Cary, NC, USA).
We first examined the demographic and clinical characteristics of all 61 patients who underwent guardianship proceedings. Second, we described the primary outcomes of interest–length of stay, costs, and likelihood of complications–in this series of patients with associated 95% confidence intervals.
Third, we examined the associations between guardianship and length of stay and healthcare costs using generalized estimating equations with clustering by matched set and compound symmetry. For length of stay, we specifically assessed excess length of stay (the matching variable) to avoid immortal time bias; we also examined the total length of stay. For all regression analyses, we adjusted for the following covariates: age, gender, education, marital status, race/ethnicity, CMI, insurance status, discharging service, and principal diagnosis. To maximize normality of residuals, costs were log-transformed; length of stay beyond clearance was log-transformed after addition of 1. For both outcomes, we back-transformed the regression coefficients and presented percent change between case and control patients. All reported tests are two-sided.
RESULTS
A total of 61 guardianship cases and 118 controls were included in the analysis.
General Characteristics
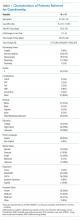
The characteristics of all cases prior to matching are included in Table 1. The department of internal medicine discharged the largest proportion of cases, followed by neurosurgery and neurology departments. More than 65% of cases were insured by Medicare or Medicaid. Three-quarters of cases were discharged from the hospital to another medical facility, with about half discharged to a skilled nursing facility (SNF) or a rehabilitation center and one-quarter to a long-term acute care hospital (LTACH).
The median length of stay for patients requiring guardianship was 28 (range, 23-36) days, and the median total charges were $171,083 ($106,897-$245,281), with a total cost approximating $10.9 million for these patients. Regarding hospital-acquired complications, 10 (16%; 95% confidence interval, 8%–28%) unique cases suffered from a complication, with HAP being the most frequently (n = 5) occurring complication.
Comparison with Matched Controls
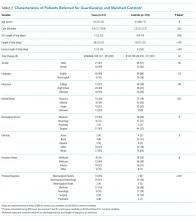
No statistically significant differences were observed between cases and controls in terms of age, primary language, highest level of education, ethnicity, insurance status, or discharging service as shown in Table 2; discharging service was a matched variable and comparable by design. However, cases tended to be less likely to be married and had a higher CMI.
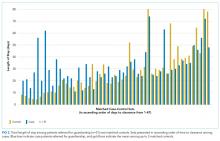
When compared with control patients in terms of similar services who stayed for at least as long as their duration to clearance, the cases had significantly longer lengths of stay compared to those of controls (29 total days compared to 18 days, P < .001; Figure 2). In addition, cases incurred significantly higher median total charges ($168,666) compared to those of controls ($104,190; P = .02).
After accounting for potential confounders, including age, gender, language, education, marital status, discharging service, ethnicity, insurance status, CMI, and principal diagnosis, guardianship was associated with 58% higher excess length of stay (P = .04, 95% CI [2%-145%]). Furthermore, guardianship was associated with 23% higher total charges (P = .02, 95% CI [4%-46%]) and 37% longer total length of stay (P = .002, 95% CI [12%-67%]).
DISCUSSION
To our knowledge, this is among the first studies to investigate healthcare costs and harm to the patient in the form of hospital-associated complications as a result of guardianship proceedings. Other studies15,16 have also demonstrated excessive length of stay attributed to nonclinical factors such as guardianship, though they did not quantify the excess stay or compare guardianship cases with a matched control. One study17 demonstrated total charges of $150,000 per patient requiring guardianship, which are similar to our results. However, Chen et al. also observed an average of 27.8 medically unnecessary days, which are 16 more days than those in our study sample. This may reflect the difference in how excess days were determined, namely, statistical process control analysis in the previous study compared with a manual chart review in our study. To our knowledge, no other study has compared guardianship cases with matched controls to compare their experiences to patients with similarly prolonged stays prior to clearance.
After matching by service and the length of stay until medical clearance in each guardianship case, the subsequent length of stay was higher among cases than among controls, even after adjustment for differences in CMI and diagnosis. This suggests that the process of obtaining guardianship results in a particularly prolonged length of stay, which is presumably attributable to factors other than medical complexity or ongoing illness.
It is probable that at least two interrelated mechanisms are responsible for the particularly high costs and the long stay of patients who require guardianship. First, the process of obtaining guardianship is itself protracted in several cases, necessitating long-term admissions well beyond the point of medical stability. Second, our results suggest that longer hospital stays are apt to grow further in a feed-forward cycle due to hospital-acquired complications that develop after the date of medical clearance. Indeed, in our series, 16% of patients sustained a complication that is readily attributable to hospital care after their date of clearance, and these types of complications are likely to lengthen the stay even further.
We compared cases referred for guardianship to control patients on the same services, at similar time points, whose length of stay was at least as long as the point of medical clearance as their corresponding case patient. Because cases were hospitalized with active medical needs to at least the point of clearance, we anticipated that costs might well be lower among cases, who had no medical necessity for hospitalization at the point of clearance, compared with controls who remained hospitalized presumably for active medical needs. Counter to this hypothesis, and accounting for potentially confounding variables, undergoing a guardianship proceeding was associated with nearly 25% higher costs of patient care. This may ultimately represent a substantial burden on the healthcare system. For example, in just 1 year in our hospital, the total hospital charges reached almost $11 million for the 61 patients who underwent guardianship proceedings. Considering that 65% of the patients requiring guardianship had Medicaid or Medicare coverage, there are significant financial implications for the hospital systems and to the public.
Limitations of our study relate to its retrospective nature at a single center. Investigating guardianship cases at a single center and with a small sample size of 61 patients limits generalizability. Nevertheless, we still had enough power to detect significant differences compared with matched controls, and this study remains the largest investigation into the cost associated with guardianship to date and the only study comparing guardianship cases with matched controls. Furthermore, we did not complete chart reviews of controls, which limits direct comparisons of complications and precluded our matching on variables that required detailed review.
The retrospective design may include confounders unaccounted for in our statistical design, though we attempted to match cases with controls to account for some of these potential differences and included a broad set of covariates that included measures of comorbidity and diagnosis. To this point, we included only CMI and principal diagnosis as the measures of severity, and adjustment for CMI, which includes features of the index hospitalization itself, may represent overadjustment. However, this type of overadjustment would tend to bias toward the null hypothesis.
Investigators only completed chart reviews for cases, which limits our ability to contrast the rate of hospital-associated complications for cases with that of controls. However, the rates of CAUTI and HAP complications among our cases were notably higher than national inpatient estimates, ie, 5% and 8% compared to 0.2%18 and 0.5%-1%,19 respectively. Furthermore, we demonstrated higher total costs and total lengths of stay among guardianship patients, analyses for which the attributed date of clearance for controls was not required, and the rate of complications among the case patients was sizable despite their being formally medically cleared. In other words, regardless of whether a complication rate of 16% is “typical” for inpatients hospitalized for these durations, this suggests that persistent hospitalization after clearance does not carry a benign prognosis.
In addition, to estimate healthcare costs, we relied on total hospital charges, which are readily available and reflect, at least in part, payer costs but do not reflect true costs to the medical center. Nonetheless, charges approximately reflect costs–with some variation across cost centers–and hence provide a useful metric for comparing cases and controls. To provide context, for academic medical centers such as ours, costs are typically about half of charges.
Finally, each state has different statutes for surrogate decision-making. The results of this study reflect the Massachusetts’ experience, with no public guardianship program or hierarchy statute. That being said, while this presumably causes the need for more guardianships in Massachusetts, the mechanisms for guardianship are broadly similar nationwide and are likely to result in excessive length of stay and cost similar to those in our population, as demonstrated in studies from other states.7,15–17
Implications
At a time where medical systems are searching for opportunities to reduce the length of stay, prevent unnecessary hospitalization, and improve the quality of care, reevaluating the guardianship process is ripe with opportunity. In this single academic center, the process of guardianship was associated with 58% excess length of stay and 23% higher total hospital charges. Furthermore, one in six patients requiring guardianship suffered from hospital-associated complications.
This matched cohort study adds quantitative data demonstrating substantial burdens to the healthcare system as a result of the guardianship process and can be used as an impetus for hospital administration and legal systems to expedite the process. Potential improvements include increasing HCP form completions (which would eliminate the need to pursue guardianship for most of such patients), identifying patients who lack a legally recognized surrogate decision-maker earlier in their hospital stay (ideally upon admission), and providing resources to assist clinical teams in the completion of affidavits necessary to support the appointment of a guardian, so that paperwork can be filed with courts sooner. Further research that provides more generalizable prospective data could potentially improve the guardianship process and reduce its burden on hospitals and patients even further.
Acknowledgments
The authors express their tremendous thanks to Gail Piatkowski for her invaluable assistance in collecting administrative and billing data.
Disclosures
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article
1. O’Neill O. Autonomy and Trust in Bioethics. Cambridge: Cambridge University Press; 2002. PubMed
2. Beauchamp T, Childress J. Principles of Biomedical Ethics. 7th ed. New York: Oxford University Press; 2013.
3. McMurray RJ, Clarke OW, Barrasso JA, et al. Decisions near the end of life. J Am Med Assoc. 1992;267(16):2229-2233.
4. American Medical Association. AMA Principles of Medical Ethics: Chapter 2 - Opinions on Consent, Communication and Decision Making.; 2016.
5. Arnold RM, Kellum J. Moral justifications for surrogate decision making in the intensive care unit: Implications and limitations. Crit Care Med. 2003;31(Supplement):S347-S353. PubMed
6. Karp N, Wood E. Incapacitated and Alone: Healthcare Decision Making for Unbefriended Older People. Am Bar Assoc Hum Rights. 2003;31(2).
7. Bandy RJ, Helft PR, Bandy RW, Torke AM. Medical decision-making during the guardianship process for incapacitated, hospitalized adults: a descriptive cohort study. J Gen Intern Med. 2010;25(10):1003-1008. PubMed
8. Wynn S. Decisions by surrogates: an overview of surrogate consent laws in the United States. Bifocal. 2014;36(1):10-14.
9. Massachusetts General Laws. Chapter 201D: Health Care Proxies. https://malegislature.gov/Laws/GeneralLaws/PartII/TitleII/Chapter201D. Published 2017. Accessed March 31, 2017.
10. American Bar Association Commision on Law and Aging. Default Surrogate Consent Statutes. Am Bar Assoc. 2016:1-17.
11. Massachusetts General Laws. Chapter 190B: Massachusetts Probate Code. https://malegislature.gov/Laws/GeneralLaws/PartII/TitleII/Chapter190B. Published 2017. Accessed March 31, 2017.
12. Rosman M, Rachminov O, Segal O, Segal G. Prolonged patients’ in-hospital waiting period after discharge eligibility is associated with increased risk of infection, morbidity and mortality: a retrospective cohort analysis. BMC Health Serv Res. 2015;15:246. PubMed
13. Majeed MU, Williams DT, Pollock R, et al. Delay in discharge and its impact on unnecessary hospital bed occupancy. 2012. PubMed
14. Nobili A, Licata G, Salerno F, et al. Polypharmacy, length of hospital stay, and in-hospital mortality among elderly patients in internal medicine wards. The REPOSI study. Eur J Clin Pharmacol. 2011;67(5):507-519. PubMed
15. Chen JJ, Finn CT, Homa K, St Onge KP, Caller TA. Discharge delays for patients requiring in-hospital guardianship: A Cohort Analysis. J Healthc Qual. 2016;38(4):235-242. PubMed
16. Chen JJ, Kwon A, Stevens Y, Finn CT. Barriers beyond clinical control affecting timely hospital discharge for a patient requiring guardianship. Psychosomatics. 2015;56(2):206-209. PubMed
17. Chen JJ, Blanchard MA, Finn CT, et al. A clinical pathway for guardianship at dartmouth-hitchcock medical center. Jt Comm J Qual Patient Saf. 2014;40(9):389-397. PubMed
18. McEachern R, Campbell Jr GD. Hospital-Acquired Pneumonia: Epidemiology, Etiology, and Treatment. Infect Dis Clin North Am. 1998;12(3):761-779. PubMed
19. Zimlichman E, Henderson D, Tamir O, et al. Health care–associated infections. JAMA Intern Med. 2013;173(22):2039. PubMed
A central tenet of modern medicine is that patients must provide fully informed consent to receive or refuse medical care offered by their clinical teams.1–4 If a patient is unable to make and communicate a choice or clearly indicate an understanding of the information presented, then he or she is considered to lack the capacity to make medical decisions and the medical team must seek consent from the patient’s surrogate decision-maker.2-7 Every U.S. state recognizes a patient’s healthcare proxy (HCP) and a court-appointed guardian as a legally recognized surrogate.8,9 Most of the states also have statutes or regulations establishing a hierarchy of legally recognized surrogate decision-makers in the absence of a HCP or a court-appointed guardian, such as spouses, adult children, parents, siblings, and grandparents.8,10 In states that do not have such a statute, hospitals develop their own institutional policies for surrogate decision-making.
However, there are important limitations on the authority of these surrogate decision-makers.10 For instance, patients may not have a family member or a friend to serve as a surrogate decision-maker, often family members cannot override a patient’s objection, even when that patient lacks decision-making capacity, and certain decisions require a guardian or a HCP.8-10 In these circumstances, the hospital must petition a court to appoint a guardian as a legally recognized surrogate decision-maker. This can be an involved family member, if one exists, or an independent, typically volunteer, guardian.11 The process of guardian appointment is complex7,11 and can range from a few days to more than a month, largely dependent on court dates and finding a volunteer guardian. Much of the process occurs during the patient’s hospital stay. This prolongation of hospitalization would be expected to increase health care costs and iatrogenic complications,12–14 but data quantifying these for patients requiring guardianship are lacking. The goal of this study was to describe the characteristics of patients who undergo the process of guardianship and measure the associated burdens. These burdens include the financial costs to the medical system, the prolonged length of stay beyond medical necessity, and the costs to the patient in the form of hospital-acquired complications. Investigating the burden of guardianship is an important first step in uncovering opportunities to improve the process. We hypothesized that patients requiring guardianship would have lengths of stay and healthcare costs that were at least as large as those for patients whose conditions required similar durations of hospitalization prior to medical clearance, in part due to iatrogenic complications that would accrue while awaiting guardian appointment.
METHODS
Setting
We conducted a retrospective matched cohort study of adult inpatients at Beth Israel Deaconess Medical Center (BIDMC), a 651-bed academic, tertiary care facility in Boston, MA. The study was approved by the BIDMC Institutional Review Board as a nonhuman subject research consistent with hospital operations.
Population
For this matched cohort study, we identified case patients as those hospitalized for any reason for whom guardianship proceedings were initiated and obtained; only the first hospitalization during which the guardianship was pursued was used. Cases were identified by obtaining the data of all patients for whom the BIDMC general counsel completed the process of guardianship between October 2014 and September 2015. At BIDMC, all the guardianship proceedings are referred to the general counsel.
To determine the postclearance experience for referred patients compared with that for other patients with similar lengths of stay up to those of the referred patients’ point of clearance, we identified up to three matched controls for each case (Supplemental Figure 1). Medical clearance was defined as the date when the patient was medically stable to be discharged from the hospital, and it was determined in an iterative manner. We identified controls as hospitalized patients admitted for any cause and matched to the cases requiring guardianship on discharging service and length of stay prior to clearance. Specifically, we identified patients on the same service as the case whose length of stay was at least as long as the length of stay of the case patient until medical clearance, as defined below. We then determined the total and the excess length of stay, defined as the duration beyond clearance for each case referred for guardianship; for controls, the ‘excess’ length of stay was the number of hospitalized days beyond the corresponding time that a matched case had been provided clearance. To account for seasonal influences and the training level of house officers, we selected the three controls whose discharge date was closest (before or after) to the discharge date of their matched case.
From legal team files, we identified 61 patients hospitalized at BIDMC for whom new guardianship was pursued to completion. Of these 61 patients, 10 could not be matched to an appropriate control and were included in descriptive analyses but not in comparisons with controls.
Covariates and Outcomes
We collected the details regarding age, gender, primary language, highest level of education, marital status, insurance status, race, date of admission, date of discharge, discharge disposition, principal diagnosis, case mix index (CMI), and discharging service from our administrative and billing data. Outcomes of interest included length of stay and total hospital charges that were collected from the same databases. We used hospital charges, rather than payments, to ensure uniformity across payers.
Chart Review
Unique to cases, a team of two medical residents (JP, RP) and a hospitalist (DR) determined the date of medical clearance and hospital-associated complications by a chart review. The date of medical clearance was then used to calculate excess length of stay, ie, the duration of stay beyond the date of medical clearance, by subtracting the time to medical clearance from the total inpatient length of stay.
We developed a novel algorithm to determine the date of medical clearance consistently (Figure 1). We first determined whether the discharge summary indicated a clear date of medical readiness for discharge. If the discharge summary was unclear, then a case management or a social work note was used. The date of medical clearance determined by the case management or the social work note was then confirmed with clinical data. The date was confirmed if there were no significant laboratory orders and major medication changes or procedures for 24 h from the date identified. If notes were also inconclusive, then the medical clearance was determined by a review of provider order entry. Medical readiness for discharge was then defined as the first day when there were no laboratory orders for 48 h and no significant medication changes, imaging studies, or microbiologic orders.
Hospital-acquired complications were determined to be related to the guardianship process if they occurred after the date of medical stability but prior to discharge. We did not investigate hospital-acquired complications among controls. Hospital-acquired complications were defined as follows:
- Catheter-associated urinary tract infection (CAUTI): active Foley catheter order and positive urine culture that resulted in antibiotic administration.
- Hospital-acquired pneumonia (HAP): chest X-ray or computed tomography (CT) scan showing a consolidation that resulted in antibiotic administration.
- Venous thromboembolism (VTE): positive venous ultrasound or CT angiography of the chest for deep venous thrombosis (DVT) or pulmonary embolism (PE).
- Decubitus ulcer: new wound care consultation for sacral decubitus ulceration.
- Clostridium difficile (C. diff) infection: positive stool polymerase chain reaction that resulted in antibiotic administration.
The algorithm for identifying the date of clearance and the presence of complications was piloted independently by three investigators (RP, JP, DR) using a single chart review and was redesigned until a consensus was obtained. The same three investigators then independently reviewed three additional charts, including all notes, laboratory results, imaging results, and orders, with complete agreement for both date of clearance and presence of complications. Two investigators (RP, JP) then individually reviewed the remaining 57 charts. Of these, 10 were selected a priori for review by both investigators for interrater reliability, with a mean difference of 0.5 days in the estimated time to clearance and complete concordance in complications. In addition, a third investigator (DR) independently reread 5 of the 57 reviewed charts, with complete concordance in both time to clearance and presence of complications with the original readings.
Statistical Analysis
SAS 9.3 was used for all analyses (SAS Institute Inc., Cary, NC, USA).
We first examined the demographic and clinical characteristics of all 61 patients who underwent guardianship proceedings. Second, we described the primary outcomes of interest–length of stay, costs, and likelihood of complications–in this series of patients with associated 95% confidence intervals.
Third, we examined the associations between guardianship and length of stay and healthcare costs using generalized estimating equations with clustering by matched set and compound symmetry. For length of stay, we specifically assessed excess length of stay (the matching variable) to avoid immortal time bias; we also examined the total length of stay. For all regression analyses, we adjusted for the following covariates: age, gender, education, marital status, race/ethnicity, CMI, insurance status, discharging service, and principal diagnosis. To maximize normality of residuals, costs were log-transformed; length of stay beyond clearance was log-transformed after addition of 1. For both outcomes, we back-transformed the regression coefficients and presented percent change between case and control patients. All reported tests are two-sided.
RESULTS
A total of 61 guardianship cases and 118 controls were included in the analysis.
General Characteristics
The characteristics of all cases prior to matching are included in Table 1. The department of internal medicine discharged the largest proportion of cases, followed by neurosurgery and neurology departments. More than 65% of cases were insured by Medicare or Medicaid. Three-quarters of cases were discharged from the hospital to another medical facility, with about half discharged to a skilled nursing facility (SNF) or a rehabilitation center and one-quarter to a long-term acute care hospital (LTACH).
The median length of stay for patients requiring guardianship was 28 (range, 23-36) days, and the median total charges were $171,083 ($106,897-$245,281), with a total cost approximating $10.9 million for these patients. Regarding hospital-acquired complications, 10 (16%; 95% confidence interval, 8%–28%) unique cases suffered from a complication, with HAP being the most frequently (n = 5) occurring complication.
Comparison with Matched Controls
No statistically significant differences were observed between cases and controls in terms of age, primary language, highest level of education, ethnicity, insurance status, or discharging service as shown in Table 2; discharging service was a matched variable and comparable by design. However, cases tended to be less likely to be married and had a higher CMI.
When compared with control patients in terms of similar services who stayed for at least as long as their duration to clearance, the cases had significantly longer lengths of stay compared to those of controls (29 total days compared to 18 days, P < .001; Figure 2). In addition, cases incurred significantly higher median total charges ($168,666) compared to those of controls ($104,190; P = .02).
After accounting for potential confounders, including age, gender, language, education, marital status, discharging service, ethnicity, insurance status, CMI, and principal diagnosis, guardianship was associated with 58% higher excess length of stay (P = .04, 95% CI [2%-145%]). Furthermore, guardianship was associated with 23% higher total charges (P = .02, 95% CI [4%-46%]) and 37% longer total length of stay (P = .002, 95% CI [12%-67%]).
DISCUSSION
To our knowledge, this is among the first studies to investigate healthcare costs and harm to the patient in the form of hospital-associated complications as a result of guardianship proceedings. Other studies15,16 have also demonstrated excessive length of stay attributed to nonclinical factors such as guardianship, though they did not quantify the excess stay or compare guardianship cases with a matched control. One study17 demonstrated total charges of $150,000 per patient requiring guardianship, which are similar to our results. However, Chen et al. also observed an average of 27.8 medically unnecessary days, which are 16 more days than those in our study sample. This may reflect the difference in how excess days were determined, namely, statistical process control analysis in the previous study compared with a manual chart review in our study. To our knowledge, no other study has compared guardianship cases with matched controls to compare their experiences to patients with similarly prolonged stays prior to clearance.
After matching by service and the length of stay until medical clearance in each guardianship case, the subsequent length of stay was higher among cases than among controls, even after adjustment for differences in CMI and diagnosis. This suggests that the process of obtaining guardianship results in a particularly prolonged length of stay, which is presumably attributable to factors other than medical complexity or ongoing illness.
It is probable that at least two interrelated mechanisms are responsible for the particularly high costs and the long stay of patients who require guardianship. First, the process of obtaining guardianship is itself protracted in several cases, necessitating long-term admissions well beyond the point of medical stability. Second, our results suggest that longer hospital stays are apt to grow further in a feed-forward cycle due to hospital-acquired complications that develop after the date of medical clearance. Indeed, in our series, 16% of patients sustained a complication that is readily attributable to hospital care after their date of clearance, and these types of complications are likely to lengthen the stay even further.
We compared cases referred for guardianship to control patients on the same services, at similar time points, whose length of stay was at least as long as the point of medical clearance as their corresponding case patient. Because cases were hospitalized with active medical needs to at least the point of clearance, we anticipated that costs might well be lower among cases, who had no medical necessity for hospitalization at the point of clearance, compared with controls who remained hospitalized presumably for active medical needs. Counter to this hypothesis, and accounting for potentially confounding variables, undergoing a guardianship proceeding was associated with nearly 25% higher costs of patient care. This may ultimately represent a substantial burden on the healthcare system. For example, in just 1 year in our hospital, the total hospital charges reached almost $11 million for the 61 patients who underwent guardianship proceedings. Considering that 65% of the patients requiring guardianship had Medicaid or Medicare coverage, there are significant financial implications for the hospital systems and to the public.
Limitations of our study relate to its retrospective nature at a single center. Investigating guardianship cases at a single center and with a small sample size of 61 patients limits generalizability. Nevertheless, we still had enough power to detect significant differences compared with matched controls, and this study remains the largest investigation into the cost associated with guardianship to date and the only study comparing guardianship cases with matched controls. Furthermore, we did not complete chart reviews of controls, which limits direct comparisons of complications and precluded our matching on variables that required detailed review.
The retrospective design may include confounders unaccounted for in our statistical design, though we attempted to match cases with controls to account for some of these potential differences and included a broad set of covariates that included measures of comorbidity and diagnosis. To this point, we included only CMI and principal diagnosis as the measures of severity, and adjustment for CMI, which includes features of the index hospitalization itself, may represent overadjustment. However, this type of overadjustment would tend to bias toward the null hypothesis.
Investigators only completed chart reviews for cases, which limits our ability to contrast the rate of hospital-associated complications for cases with that of controls. However, the rates of CAUTI and HAP complications among our cases were notably higher than national inpatient estimates, ie, 5% and 8% compared to 0.2%18 and 0.5%-1%,19 respectively. Furthermore, we demonstrated higher total costs and total lengths of stay among guardianship patients, analyses for which the attributed date of clearance for controls was not required, and the rate of complications among the case patients was sizable despite their being formally medically cleared. In other words, regardless of whether a complication rate of 16% is “typical” for inpatients hospitalized for these durations, this suggests that persistent hospitalization after clearance does not carry a benign prognosis.
In addition, to estimate healthcare costs, we relied on total hospital charges, which are readily available and reflect, at least in part, payer costs but do not reflect true costs to the medical center. Nonetheless, charges approximately reflect costs–with some variation across cost centers–and hence provide a useful metric for comparing cases and controls. To provide context, for academic medical centers such as ours, costs are typically about half of charges.
Finally, each state has different statutes for surrogate decision-making. The results of this study reflect the Massachusetts’ experience, with no public guardianship program or hierarchy statute. That being said, while this presumably causes the need for more guardianships in Massachusetts, the mechanisms for guardianship are broadly similar nationwide and are likely to result in excessive length of stay and cost similar to those in our population, as demonstrated in studies from other states.7,15–17
Implications
At a time where medical systems are searching for opportunities to reduce the length of stay, prevent unnecessary hospitalization, and improve the quality of care, reevaluating the guardianship process is ripe with opportunity. In this single academic center, the process of guardianship was associated with 58% excess length of stay and 23% higher total hospital charges. Furthermore, one in six patients requiring guardianship suffered from hospital-associated complications.
This matched cohort study adds quantitative data demonstrating substantial burdens to the healthcare system as a result of the guardianship process and can be used as an impetus for hospital administration and legal systems to expedite the process. Potential improvements include increasing HCP form completions (which would eliminate the need to pursue guardianship for most of such patients), identifying patients who lack a legally recognized surrogate decision-maker earlier in their hospital stay (ideally upon admission), and providing resources to assist clinical teams in the completion of affidavits necessary to support the appointment of a guardian, so that paperwork can be filed with courts sooner. Further research that provides more generalizable prospective data could potentially improve the guardianship process and reduce its burden on hospitals and patients even further.
Acknowledgments
The authors express their tremendous thanks to Gail Piatkowski for her invaluable assistance in collecting administrative and billing data.
Disclosures
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article
A central tenet of modern medicine is that patients must provide fully informed consent to receive or refuse medical care offered by their clinical teams.1–4 If a patient is unable to make and communicate a choice or clearly indicate an understanding of the information presented, then he or she is considered to lack the capacity to make medical decisions and the medical team must seek consent from the patient’s surrogate decision-maker.2-7 Every U.S. state recognizes a patient’s healthcare proxy (HCP) and a court-appointed guardian as a legally recognized surrogate.8,9 Most of the states also have statutes or regulations establishing a hierarchy of legally recognized surrogate decision-makers in the absence of a HCP or a court-appointed guardian, such as spouses, adult children, parents, siblings, and grandparents.8,10 In states that do not have such a statute, hospitals develop their own institutional policies for surrogate decision-making.
However, there are important limitations on the authority of these surrogate decision-makers.10 For instance, patients may not have a family member or a friend to serve as a surrogate decision-maker, often family members cannot override a patient’s objection, even when that patient lacks decision-making capacity, and certain decisions require a guardian or a HCP.8-10 In these circumstances, the hospital must petition a court to appoint a guardian as a legally recognized surrogate decision-maker. This can be an involved family member, if one exists, or an independent, typically volunteer, guardian.11 The process of guardian appointment is complex7,11 and can range from a few days to more than a month, largely dependent on court dates and finding a volunteer guardian. Much of the process occurs during the patient’s hospital stay. This prolongation of hospitalization would be expected to increase health care costs and iatrogenic complications,12–14 but data quantifying these for patients requiring guardianship are lacking. The goal of this study was to describe the characteristics of patients who undergo the process of guardianship and measure the associated burdens. These burdens include the financial costs to the medical system, the prolonged length of stay beyond medical necessity, and the costs to the patient in the form of hospital-acquired complications. Investigating the burden of guardianship is an important first step in uncovering opportunities to improve the process. We hypothesized that patients requiring guardianship would have lengths of stay and healthcare costs that were at least as large as those for patients whose conditions required similar durations of hospitalization prior to medical clearance, in part due to iatrogenic complications that would accrue while awaiting guardian appointment.
METHODS
Setting
We conducted a retrospective matched cohort study of adult inpatients at Beth Israel Deaconess Medical Center (BIDMC), a 651-bed academic, tertiary care facility in Boston, MA. The study was approved by the BIDMC Institutional Review Board as a nonhuman subject research consistent with hospital operations.
Population
For this matched cohort study, we identified case patients as those hospitalized for any reason for whom guardianship proceedings were initiated and obtained; only the first hospitalization during which the guardianship was pursued was used. Cases were identified by obtaining the data of all patients for whom the BIDMC general counsel completed the process of guardianship between October 2014 and September 2015. At BIDMC, all the guardianship proceedings are referred to the general counsel.
To determine the postclearance experience for referred patients compared with that for other patients with similar lengths of stay up to those of the referred patients’ point of clearance, we identified up to three matched controls for each case (Supplemental Figure 1). Medical clearance was defined as the date when the patient was medically stable to be discharged from the hospital, and it was determined in an iterative manner. We identified controls as hospitalized patients admitted for any cause and matched to the cases requiring guardianship on discharging service and length of stay prior to clearance. Specifically, we identified patients on the same service as the case whose length of stay was at least as long as the length of stay of the case patient until medical clearance, as defined below. We then determined the total and the excess length of stay, defined as the duration beyond clearance for each case referred for guardianship; for controls, the ‘excess’ length of stay was the number of hospitalized days beyond the corresponding time that a matched case had been provided clearance. To account for seasonal influences and the training level of house officers, we selected the three controls whose discharge date was closest (before or after) to the discharge date of their matched case.
From legal team files, we identified 61 patients hospitalized at BIDMC for whom new guardianship was pursued to completion. Of these 61 patients, 10 could not be matched to an appropriate control and were included in descriptive analyses but not in comparisons with controls.
Covariates and Outcomes
We collected the details regarding age, gender, primary language, highest level of education, marital status, insurance status, race, date of admission, date of discharge, discharge disposition, principal diagnosis, case mix index (CMI), and discharging service from our administrative and billing data. Outcomes of interest included length of stay and total hospital charges that were collected from the same databases. We used hospital charges, rather than payments, to ensure uniformity across payers.
Chart Review
Unique to cases, a team of two medical residents (JP, RP) and a hospitalist (DR) determined the date of medical clearance and hospital-associated complications by a chart review. The date of medical clearance was then used to calculate excess length of stay, ie, the duration of stay beyond the date of medical clearance, by subtracting the time to medical clearance from the total inpatient length of stay.
We developed a novel algorithm to determine the date of medical clearance consistently (Figure 1). We first determined whether the discharge summary indicated a clear date of medical readiness for discharge. If the discharge summary was unclear, then a case management or a social work note was used. The date of medical clearance determined by the case management or the social work note was then confirmed with clinical data. The date was confirmed if there were no significant laboratory orders and major medication changes or procedures for 24 h from the date identified. If notes were also inconclusive, then the medical clearance was determined by a review of provider order entry. Medical readiness for discharge was then defined as the first day when there were no laboratory orders for 48 h and no significant medication changes, imaging studies, or microbiologic orders.
Hospital-acquired complications were determined to be related to the guardianship process if they occurred after the date of medical stability but prior to discharge. We did not investigate hospital-acquired complications among controls. Hospital-acquired complications were defined as follows:
- Catheter-associated urinary tract infection (CAUTI): active Foley catheter order and positive urine culture that resulted in antibiotic administration.
- Hospital-acquired pneumonia (HAP): chest X-ray or computed tomography (CT) scan showing a consolidation that resulted in antibiotic administration.
- Venous thromboembolism (VTE): positive venous ultrasound or CT angiography of the chest for deep venous thrombosis (DVT) or pulmonary embolism (PE).
- Decubitus ulcer: new wound care consultation for sacral decubitus ulceration.
- Clostridium difficile (C. diff) infection: positive stool polymerase chain reaction that resulted in antibiotic administration.
The algorithm for identifying the date of clearance and the presence of complications was piloted independently by three investigators (RP, JP, DR) using a single chart review and was redesigned until a consensus was obtained. The same three investigators then independently reviewed three additional charts, including all notes, laboratory results, imaging results, and orders, with complete agreement for both date of clearance and presence of complications. Two investigators (RP, JP) then individually reviewed the remaining 57 charts. Of these, 10 were selected a priori for review by both investigators for interrater reliability, with a mean difference of 0.5 days in the estimated time to clearance and complete concordance in complications. In addition, a third investigator (DR) independently reread 5 of the 57 reviewed charts, with complete concordance in both time to clearance and presence of complications with the original readings.
Statistical Analysis
SAS 9.3 was used for all analyses (SAS Institute Inc., Cary, NC, USA).
We first examined the demographic and clinical characteristics of all 61 patients who underwent guardianship proceedings. Second, we described the primary outcomes of interest–length of stay, costs, and likelihood of complications–in this series of patients with associated 95% confidence intervals.
Third, we examined the associations between guardianship and length of stay and healthcare costs using generalized estimating equations with clustering by matched set and compound symmetry. For length of stay, we specifically assessed excess length of stay (the matching variable) to avoid immortal time bias; we also examined the total length of stay. For all regression analyses, we adjusted for the following covariates: age, gender, education, marital status, race/ethnicity, CMI, insurance status, discharging service, and principal diagnosis. To maximize normality of residuals, costs were log-transformed; length of stay beyond clearance was log-transformed after addition of 1. For both outcomes, we back-transformed the regression coefficients and presented percent change between case and control patients. All reported tests are two-sided.
RESULTS
A total of 61 guardianship cases and 118 controls were included in the analysis.
General Characteristics
The characteristics of all cases prior to matching are included in Table 1. The department of internal medicine discharged the largest proportion of cases, followed by neurosurgery and neurology departments. More than 65% of cases were insured by Medicare or Medicaid. Three-quarters of cases were discharged from the hospital to another medical facility, with about half discharged to a skilled nursing facility (SNF) or a rehabilitation center and one-quarter to a long-term acute care hospital (LTACH).
The median length of stay for patients requiring guardianship was 28 (range, 23-36) days, and the median total charges were $171,083 ($106,897-$245,281), with a total cost approximating $10.9 million for these patients. Regarding hospital-acquired complications, 10 (16%; 95% confidence interval, 8%–28%) unique cases suffered from a complication, with HAP being the most frequently (n = 5) occurring complication.
Comparison with Matched Controls
No statistically significant differences were observed between cases and controls in terms of age, primary language, highest level of education, ethnicity, insurance status, or discharging service as shown in Table 2; discharging service was a matched variable and comparable by design. However, cases tended to be less likely to be married and had a higher CMI.
When compared with control patients in terms of similar services who stayed for at least as long as their duration to clearance, the cases had significantly longer lengths of stay compared to those of controls (29 total days compared to 18 days, P < .001; Figure 2). In addition, cases incurred significantly higher median total charges ($168,666) compared to those of controls ($104,190; P = .02).
After accounting for potential confounders, including age, gender, language, education, marital status, discharging service, ethnicity, insurance status, CMI, and principal diagnosis, guardianship was associated with 58% higher excess length of stay (P = .04, 95% CI [2%-145%]). Furthermore, guardianship was associated with 23% higher total charges (P = .02, 95% CI [4%-46%]) and 37% longer total length of stay (P = .002, 95% CI [12%-67%]).
DISCUSSION
To our knowledge, this is among the first studies to investigate healthcare costs and harm to the patient in the form of hospital-associated complications as a result of guardianship proceedings. Other studies15,16 have also demonstrated excessive length of stay attributed to nonclinical factors such as guardianship, though they did not quantify the excess stay or compare guardianship cases with a matched control. One study17 demonstrated total charges of $150,000 per patient requiring guardianship, which are similar to our results. However, Chen et al. also observed an average of 27.8 medically unnecessary days, which are 16 more days than those in our study sample. This may reflect the difference in how excess days were determined, namely, statistical process control analysis in the previous study compared with a manual chart review in our study. To our knowledge, no other study has compared guardianship cases with matched controls to compare their experiences to patients with similarly prolonged stays prior to clearance.
After matching by service and the length of stay until medical clearance in each guardianship case, the subsequent length of stay was higher among cases than among controls, even after adjustment for differences in CMI and diagnosis. This suggests that the process of obtaining guardianship results in a particularly prolonged length of stay, which is presumably attributable to factors other than medical complexity or ongoing illness.
It is probable that at least two interrelated mechanisms are responsible for the particularly high costs and the long stay of patients who require guardianship. First, the process of obtaining guardianship is itself protracted in several cases, necessitating long-term admissions well beyond the point of medical stability. Second, our results suggest that longer hospital stays are apt to grow further in a feed-forward cycle due to hospital-acquired complications that develop after the date of medical clearance. Indeed, in our series, 16% of patients sustained a complication that is readily attributable to hospital care after their date of clearance, and these types of complications are likely to lengthen the stay even further.
We compared cases referred for guardianship to control patients on the same services, at similar time points, whose length of stay was at least as long as the point of medical clearance as their corresponding case patient. Because cases were hospitalized with active medical needs to at least the point of clearance, we anticipated that costs might well be lower among cases, who had no medical necessity for hospitalization at the point of clearance, compared with controls who remained hospitalized presumably for active medical needs. Counter to this hypothesis, and accounting for potentially confounding variables, undergoing a guardianship proceeding was associated with nearly 25% higher costs of patient care. This may ultimately represent a substantial burden on the healthcare system. For example, in just 1 year in our hospital, the total hospital charges reached almost $11 million for the 61 patients who underwent guardianship proceedings. Considering that 65% of the patients requiring guardianship had Medicaid or Medicare coverage, there are significant financial implications for the hospital systems and to the public.
Limitations of our study relate to its retrospective nature at a single center. Investigating guardianship cases at a single center and with a small sample size of 61 patients limits generalizability. Nevertheless, we still had enough power to detect significant differences compared with matched controls, and this study remains the largest investigation into the cost associated with guardianship to date and the only study comparing guardianship cases with matched controls. Furthermore, we did not complete chart reviews of controls, which limits direct comparisons of complications and precluded our matching on variables that required detailed review.
The retrospective design may include confounders unaccounted for in our statistical design, though we attempted to match cases with controls to account for some of these potential differences and included a broad set of covariates that included measures of comorbidity and diagnosis. To this point, we included only CMI and principal diagnosis as the measures of severity, and adjustment for CMI, which includes features of the index hospitalization itself, may represent overadjustment. However, this type of overadjustment would tend to bias toward the null hypothesis.
Investigators only completed chart reviews for cases, which limits our ability to contrast the rate of hospital-associated complications for cases with that of controls. However, the rates of CAUTI and HAP complications among our cases were notably higher than national inpatient estimates, ie, 5% and 8% compared to 0.2%18 and 0.5%-1%,19 respectively. Furthermore, we demonstrated higher total costs and total lengths of stay among guardianship patients, analyses for which the attributed date of clearance for controls was not required, and the rate of complications among the case patients was sizable despite their being formally medically cleared. In other words, regardless of whether a complication rate of 16% is “typical” for inpatients hospitalized for these durations, this suggests that persistent hospitalization after clearance does not carry a benign prognosis.
In addition, to estimate healthcare costs, we relied on total hospital charges, which are readily available and reflect, at least in part, payer costs but do not reflect true costs to the medical center. Nonetheless, charges approximately reflect costs–with some variation across cost centers–and hence provide a useful metric for comparing cases and controls. To provide context, for academic medical centers such as ours, costs are typically about half of charges.
Finally, each state has different statutes for surrogate decision-making. The results of this study reflect the Massachusetts’ experience, with no public guardianship program or hierarchy statute. That being said, while this presumably causes the need for more guardianships in Massachusetts, the mechanisms for guardianship are broadly similar nationwide and are likely to result in excessive length of stay and cost similar to those in our population, as demonstrated in studies from other states.7,15–17
Implications
At a time where medical systems are searching for opportunities to reduce the length of stay, prevent unnecessary hospitalization, and improve the quality of care, reevaluating the guardianship process is ripe with opportunity. In this single academic center, the process of guardianship was associated with 58% excess length of stay and 23% higher total hospital charges. Furthermore, one in six patients requiring guardianship suffered from hospital-associated complications.
This matched cohort study adds quantitative data demonstrating substantial burdens to the healthcare system as a result of the guardianship process and can be used as an impetus for hospital administration and legal systems to expedite the process. Potential improvements include increasing HCP form completions (which would eliminate the need to pursue guardianship for most of such patients), identifying patients who lack a legally recognized surrogate decision-maker earlier in their hospital stay (ideally upon admission), and providing resources to assist clinical teams in the completion of affidavits necessary to support the appointment of a guardian, so that paperwork can be filed with courts sooner. Further research that provides more generalizable prospective data could potentially improve the guardianship process and reduce its burden on hospitals and patients even further.
Acknowledgments
The authors express their tremendous thanks to Gail Piatkowski for her invaluable assistance in collecting administrative and billing data.
Disclosures
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article
1. O’Neill O. Autonomy and Trust in Bioethics. Cambridge: Cambridge University Press; 2002. PubMed
2. Beauchamp T, Childress J. Principles of Biomedical Ethics. 7th ed. New York: Oxford University Press; 2013.
3. McMurray RJ, Clarke OW, Barrasso JA, et al. Decisions near the end of life. J Am Med Assoc. 1992;267(16):2229-2233.
4. American Medical Association. AMA Principles of Medical Ethics: Chapter 2 - Opinions on Consent, Communication and Decision Making.; 2016.
5. Arnold RM, Kellum J. Moral justifications for surrogate decision making in the intensive care unit: Implications and limitations. Crit Care Med. 2003;31(Supplement):S347-S353. PubMed
6. Karp N, Wood E. Incapacitated and Alone: Healthcare Decision Making for Unbefriended Older People. Am Bar Assoc Hum Rights. 2003;31(2).
7. Bandy RJ, Helft PR, Bandy RW, Torke AM. Medical decision-making during the guardianship process for incapacitated, hospitalized adults: a descriptive cohort study. J Gen Intern Med. 2010;25(10):1003-1008. PubMed
8. Wynn S. Decisions by surrogates: an overview of surrogate consent laws in the United States. Bifocal. 2014;36(1):10-14.
9. Massachusetts General Laws. Chapter 201D: Health Care Proxies. https://malegislature.gov/Laws/GeneralLaws/PartII/TitleII/Chapter201D. Published 2017. Accessed March 31, 2017.
10. American Bar Association Commision on Law and Aging. Default Surrogate Consent Statutes. Am Bar Assoc. 2016:1-17.
11. Massachusetts General Laws. Chapter 190B: Massachusetts Probate Code. https://malegislature.gov/Laws/GeneralLaws/PartII/TitleII/Chapter190B. Published 2017. Accessed March 31, 2017.
12. Rosman M, Rachminov O, Segal O, Segal G. Prolonged patients’ in-hospital waiting period after discharge eligibility is associated with increased risk of infection, morbidity and mortality: a retrospective cohort analysis. BMC Health Serv Res. 2015;15:246. PubMed
13. Majeed MU, Williams DT, Pollock R, et al. Delay in discharge and its impact on unnecessary hospital bed occupancy. 2012. PubMed
14. Nobili A, Licata G, Salerno F, et al. Polypharmacy, length of hospital stay, and in-hospital mortality among elderly patients in internal medicine wards. The REPOSI study. Eur J Clin Pharmacol. 2011;67(5):507-519. PubMed
15. Chen JJ, Finn CT, Homa K, St Onge KP, Caller TA. Discharge delays for patients requiring in-hospital guardianship: A Cohort Analysis. J Healthc Qual. 2016;38(4):235-242. PubMed
16. Chen JJ, Kwon A, Stevens Y, Finn CT. Barriers beyond clinical control affecting timely hospital discharge for a patient requiring guardianship. Psychosomatics. 2015;56(2):206-209. PubMed
17. Chen JJ, Blanchard MA, Finn CT, et al. A clinical pathway for guardianship at dartmouth-hitchcock medical center. Jt Comm J Qual Patient Saf. 2014;40(9):389-397. PubMed
18. McEachern R, Campbell Jr GD. Hospital-Acquired Pneumonia: Epidemiology, Etiology, and Treatment. Infect Dis Clin North Am. 1998;12(3):761-779. PubMed
19. Zimlichman E, Henderson D, Tamir O, et al. Health care–associated infections. JAMA Intern Med. 2013;173(22):2039. PubMed
1. O’Neill O. Autonomy and Trust in Bioethics. Cambridge: Cambridge University Press; 2002. PubMed
2. Beauchamp T, Childress J. Principles of Biomedical Ethics. 7th ed. New York: Oxford University Press; 2013.
3. McMurray RJ, Clarke OW, Barrasso JA, et al. Decisions near the end of life. J Am Med Assoc. 1992;267(16):2229-2233.
4. American Medical Association. AMA Principles of Medical Ethics: Chapter 2 - Opinions on Consent, Communication and Decision Making.; 2016.
5. Arnold RM, Kellum J. Moral justifications for surrogate decision making in the intensive care unit: Implications and limitations. Crit Care Med. 2003;31(Supplement):S347-S353. PubMed
6. Karp N, Wood E. Incapacitated and Alone: Healthcare Decision Making for Unbefriended Older People. Am Bar Assoc Hum Rights. 2003;31(2).
7. Bandy RJ, Helft PR, Bandy RW, Torke AM. Medical decision-making during the guardianship process for incapacitated, hospitalized adults: a descriptive cohort study. J Gen Intern Med. 2010;25(10):1003-1008. PubMed
8. Wynn S. Decisions by surrogates: an overview of surrogate consent laws in the United States. Bifocal. 2014;36(1):10-14.
9. Massachusetts General Laws. Chapter 201D: Health Care Proxies. https://malegislature.gov/Laws/GeneralLaws/PartII/TitleII/Chapter201D. Published 2017. Accessed March 31, 2017.
10. American Bar Association Commision on Law and Aging. Default Surrogate Consent Statutes. Am Bar Assoc. 2016:1-17.
11. Massachusetts General Laws. Chapter 190B: Massachusetts Probate Code. https://malegislature.gov/Laws/GeneralLaws/PartII/TitleII/Chapter190B. Published 2017. Accessed March 31, 2017.
12. Rosman M, Rachminov O, Segal O, Segal G. Prolonged patients’ in-hospital waiting period after discharge eligibility is associated with increased risk of infection, morbidity and mortality: a retrospective cohort analysis. BMC Health Serv Res. 2015;15:246. PubMed
13. Majeed MU, Williams DT, Pollock R, et al. Delay in discharge and its impact on unnecessary hospital bed occupancy. 2012. PubMed
14. Nobili A, Licata G, Salerno F, et al. Polypharmacy, length of hospital stay, and in-hospital mortality among elderly patients in internal medicine wards. The REPOSI study. Eur J Clin Pharmacol. 2011;67(5):507-519. PubMed
15. Chen JJ, Finn CT, Homa K, St Onge KP, Caller TA. Discharge delays for patients requiring in-hospital guardianship: A Cohort Analysis. J Healthc Qual. 2016;38(4):235-242. PubMed
16. Chen JJ, Kwon A, Stevens Y, Finn CT. Barriers beyond clinical control affecting timely hospital discharge for a patient requiring guardianship. Psychosomatics. 2015;56(2):206-209. PubMed
17. Chen JJ, Blanchard MA, Finn CT, et al. A clinical pathway for guardianship at dartmouth-hitchcock medical center. Jt Comm J Qual Patient Saf. 2014;40(9):389-397. PubMed
18. McEachern R, Campbell Jr GD. Hospital-Acquired Pneumonia: Epidemiology, Etiology, and Treatment. Infect Dis Clin North Am. 1998;12(3):761-779. PubMed
19. Zimlichman E, Henderson D, Tamir O, et al. Health care–associated infections. JAMA Intern Med. 2013;173(22):2039. PubMed
© 2018 Society of Hospital Medicine
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review
Bacterial bloodstream infections (BSIs) are a major cause of morbidity and mortality in the United States. Approximately 600,000 BSI cases occur annually, resulting in 85,000 deaths,1 at a cost exceeding $1 billion.2 Traditionally, BSIs have been managed with intravenous antimicrobials, which rapidly achieve therapeutic blood concentrations, and are viewed as more potent than oral alternatives. Indeed, for acutely ill patients with bacteremia and sepsis, timely intravenous antimicrobials are lifesaving.3
However, whether intravenous antimicrobials are essential for the entire treatment course in BSIs, particularly for uncomplicated episodes, is controversial. Patients that are clinically stable or have been stabilized after an initial septic presentation may be appropriate candidates for treatment with oral antimicrobials. There are costs and risks associated with extended courses of intravenous agents, such as the necessity for long-term intravenous catheters, which entail risks for procedural complications, secondary infections, and thrombosis. A prospective study of 192 peripherally inserted central catheter (PICC) episodes reported an overall complication rate of 30.2%, including central line-associated BSIs (CLABSI) or venous thrombosis.4 Other studies also identified high rates of thrombosis5 and PICC-related CLABSI, particularly in patients with malignancy, where sepsis-related complications approach 25%.6 Additionally, appropriate care of indwelling catheters requires significant financial and healthcare resources.
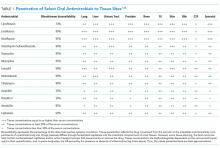
Oral antimicrobial therapy for bacterial BSIs offers several potential benefits. Direct economic and healthcare workforce savings are expected to be significant, and procedural and catheter-related complications would be eliminated.7 Moreover, oral therapy provides antimicrobial stewardship by reducing the use of broad-spectrum intravenous agents.8 Recent infectious disease “Choosing Wisely” initiatives recommend clinicians “prefer oral formulations of highly bioavailable antimicrobials whenever possible”,9 and this approach is supported by the Centers for Disease Control and Prevention antibiotic stewardship program.10 However, the expected savings and benefits of oral therapy would be lost should they be less effective and result in treatment failure or relapse of the primary BSI. Pathogen susceptibility, gastrointestinal absorption, oral bioavailability, patient tolerability, and adherence with therapy need to be carefully considered before choosing oral antimicrobials. Thus, oral antimicrobial therapy for BSI should be utilized in carefully selected circumstances.
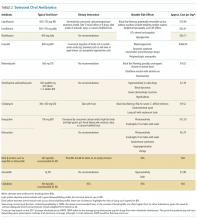
In this narrative review, we highlight areas where oral therapy is safe and effective in treating bloodstream infections, as well as offer guidance to clinicians managing patients experiencing BSI. Given the lack of robust clinical trials on this subject, the evidence for performing a systematic review was insufficient. Thus, the articles and recommendations cited in this review were selected based on the authors’ experiences to represent the best available evidence.
Infection Source Control
Pathogen and Antimicrobial Factors
Patient Factors
Evidence Regarding Bloodstream Infections due to Gram-Negative Rods
BSIs due to gram-negative rods (GNRs) are common and cause significant morbidity and mortality. GNRs represent a broad and diverse array of pathogens. We focus on the Enterobacteriaceae family and Pseudomonas aeruginosa, because they are frequently encountered in clinical practice.1
Gram-Negative Rods, Enterobacteriaceae Family
The Enterobacteriaceae family includes Escherichia coli, Klebsiella, Salmonella, Proteus, Enterobacter, Serratia, and Citrobacter species. The range of illnesses caused by Enterobacteriaceae is as diverse as the family, encompassing most body sites. Although most Enterobacteriaceae are intrinsically susceptible to antibiotics, there is potential for significant multi-drug resistance. Of particular recent concern has been the emergence of Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBL) and even carbapenem-resistant strains.14
However, Enterobacteriaceae species susceptible to oral antimicrobials are often suitable candidates for oral BSI therapy. Among 106 patients with GNR BSI treated with a highly bioavailable oral antibiotic (eg, levofloxacin), the treatment failure rate was only 2% (versus 14% when an antimicrobial with only moderate or low bioavailability was selected).15 Oral treatment of Enterobacteriaceae BSIs secondary to urinary tract infection has been best studied. A prospective randomized, controlled trial evaluated oral versus intravenous ciprofloxacin amongst 141 patients with severe pyelonephritis or complicated urinary tract infections, in which the rate of bacteremia was 38%.16 Notably, patients with obstruction or renal abscess were excluded from the trial. No significant differences in microbiological failure or unsatisfactory clinical responses were found between the IV and oral treatment groups. Additionally, a Cochrane review reported that oral antibiotic therapy is no less effective than intravenous therapy for severe UTI, although data on BSI frequency were not provided.17
Resistance to fluoroquinolones such as ciprofloxacin has been identified as a risk factor for GNR BSI oral treatment failure, highlighting the importance of confirming susceptibilities before committing to an oral treatment plan.18,19 Even ESBL Enterobacteriaceae can be considered for treatment with fluoroquinolones if susceptibilities allow.20
The ideal duration of therapy for GNR BSI is an area of active research. A recent retrospective trial showed no difference in all-cause mortality or recurrent BSI in GNR BSI treated for 8 versus 15 days.21 A recent meta-analysis suggested that 7 days of therapy was noninferior to a longer duration therapy (10–14 days) for pyelonephritis, in which a subset was bacteremic.22 However, another trial reported that short course therapy for GNR BSI (<7 days) is associated with higher risk of treatment failure.22 Further data are needed.
Gram-Negative Rods, Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common pathogen, intrinsically resistant to many antimicrobials, and readily develops antimicrobial resistance during therapy. Fluoroquinolones (such as ciprofloxacin, levofloxacin, and delafloxacin) are the only currently available oral agents with antipseudomonal activity. However, fluoroquinolones may not achieve blood concentrations appropriate for P. aeruginosa treatment at standard doses, while higher dose regimens may be associated with increased risk for undesirable side effects.24,25 Currently, given the minimal trial data comparing oral versus intravenous therapy for P. aeruginosa BSIs, and multiple studies indicating increased mortality when P. aeruginosa is treated inappropriately,26,27 we prefer a conservative approach and consider oral therapy a less-preferred option.
Evidence Regarding Bloodstream Infections due to Gram-Positive Cocci
The majority of bloodstream infections in the United States, and the resultant morbidity and mortality, are from gram-positive cocci (GPCs) such as Staphylococcus, Streptococcus, and Enterococcus species.1
Gram-Positive Cocci, Streptococcus pneumoniae
Of the approximately 900,000 annual cases of S. pneumoniae infection in the United States, approximately 40,000 are complicated by BSI, with 70% of those cases being secondary to pneumococcal pneumonia.28 In studies on patients with pneumococcal pneumonia, bacteremic cases generally fare worse than those without bacteremia.29,30 However, several trials demonstrated comparable outcomes in the setting of bacteremic pneumococcal pneumonia when switched early (within 3 days) from intravenous to oral antibiotics to complete a 7-day course.31,32 Before pneumococcal penicillin resistance became widespread, oral penicillin was shown to be effective, and remains an option for susceptible strains.33 It is worth noting, however, that other trials have shown a mortality benefit from treating bacteremic pneumococcal pneumonia initially with dual-therapy including a β-lactam and macrolide such as azithromycin. This observation highlights the importance of knowing the final susceptibility data prior to consolidating to monotherapy with an oral agent, and that macrolides may have beneficial anti-inflammatory effects, though further research is needed.34,35
Although the evidence for treating bacteremic pneumococcal pneumonia using a highly active and absorbable oral agent is fairly robust, S. pneumoniae BSI secondary to other sites of infection sites is less well studied and may require a more conservative approach.
Gram-Positive Cocci, β-hemolytic Streptococcus species
β-Hemolytic Streptococci include groups A to H, of which groups A (S. pyogenes) and B (S. agalactiae) are the most commonly implicated in BSIs.36 Group A Streptococcus (GAS) is classically associated with streptococcal pharyngitis and Group B Streptococcus (GBS) is associated with postpartum endometritis and neonatal meningitis, though both are virulent organisms with a potential to cause invasive infection throughout the body and in all age-groups. Up to 14% of GAS and 41% GBS BSIs have no clear source;37,38 given these are skin pathogens, such scenarios likely represent invasion via microabrasion. As β-hemolytic streptococcal BSI is often observed in the context of necrotizing skin and soft tissue infections, surgical source control is particularly important.39 GAS remains exquisitely susceptible to penicillin, and intravenous penicillin remains the mainstay for invasive disease; GBS has higher penicillin resistance rates than GAS.40 Clindamycin should be added when there is concern for severe disease or toxic shock.41 Unfortunately, oral penicillin is poorly bioavailable (approximately 50%), and there has been recent concern regarding inducible clindamycin resistance in GAS.42 Thus, oral penicillin V and/or clindamycin is a potentially risky strategy, with no clinical trials supporting this approach; however, they may be reasonable options in selected patients with source control and stable hemodynamics. Amoxicillin has high bioavailability (85%) and may be effective; however, there is lack of supporting data. Highly bioavailable agents such as levofloxacin and linezolid have GAS and GBS activity43 and might be expected to produce satisfactory outcomes. Because no clinical trials have compared these agents with intravenous therapy for BSI, caution is advised. Although bacteriostatic against Staphylococcus, linezolid is bactericidal against Streptococcus.44 Fluoroquinolone resistance amongst β-hemolytic Streptococcus is rare (approximately 0.5%) but does occur.45
Gram-Positive Cocci, Staphylococcus Species
Staphylococcus species include S. aureus (including methicillin susceptible and resistant strains: MSSA and MRSA, respectively) and coagulase-negative species, which include organisms such as S. epidermidis. S. aureus is the most common cause of BSI mortality in the United States,1 with mortality rates estimated at 20%–40% per episode.46 Infectious disease consultation has been associated with decreased mortality and is recommended.47 The guidelines of the Infectious Diseases Society of America for the treatment of MRSA recommend the use of parenteral agents for BSI.48 It is important to consider if a patient with S. aureus BSI has infective endocarditis.
Oral antibiotic therapy for S. aureus BSI is not currently standard practice. Although trimethoprim-sulfamethoxazole (TMP-SMX) has favorable pharmacokinetics and case series of using it successfully for BSI exist,49 TMP-SMX showed inferior outcomes in a randomized trial comparing oral TMP-SMX with intravenous vancomycin in a series of 101 S. aureus infections.50 This observation has been replicated.51 Data on doxycycline or clindamycin for S. aureus BSI are limited, and IDSA guidelines advise against their use in this setting because they are predominantly bacteriostatic.48 Linezolid has favorable pharmacokinetics, with approximately 100% bioavailability, and S. aureus resistance to linezolid is rare.52 Several randomized trials have compared oral linezolid with intravenous vancomycin for S. aureus BSI; for instance, Stevens et al. randomized 460 patients with S. aureus infection (of whom 18% had BSI) to linezolid versus vancomycin and observed similar clinical cure rates.53 A pooled analysis showed oral linezolid was noninferior to vancomycin specifically for S. aureus BSI.54 However, long-term use is often limited by hematologic toxicity, peripheral or optic neuropathy (which can be permanent), and induced serotonin syndrome. Additionally, linezolid is bacteriostatic, not bactericidal against S. aureus. Using oral linezolid as a first-line option for S. aureus BSI would not be recommended; however, it may be used as a second-line treatment option in selected cases. Tedizolid has similar pharmacokinetics and spectrum of activity with fewer side effects; however, clinical data on its use for S. aureus BSI are lacking.55 Fluoroquinolones such as levofloxacin and the newer agent delafloxacin have activity against S. aureus, including MRSA, but on-treatment emergence of fluoroquinolone resistance is a concern, and data on delafloxacin for BSI are lacking.56,57 Older literature suggested the combination of ciprofloxacin and rifampin was effective against right-sided S. aureus endocarditis,58 and other oral fluoroquinolone-rifamycin combinations have also been found to be effective59 However, this approach is currently not a standard therapy, nor is it widely used. Decisions on the duration of therapy for S. aureus BSI should be made in conjunction with an infectious diseases specialist; 14 days is currently regarded as a minimum.47,48
Published data regarding oral treatment of coagulase-negative Staphylococcus (CoNS) BSI are limited. Most CoNS bacteremia and up to 80% Staphylococcus epidermidis bacteremia represent blood culture contamination, though true infection from CoNS is not uncommon, particularly in patients with indwelling catheters.60 An exception is the CoNS species Staphylococcus lugdunensis, which is more virulent, and bacteremia with this organism usually warrants antibiotics. Oral antimicrobial therapy is currently not a standard treatment practice for CoNS BSI that is felt to represent true infection; however, linezolid has been successfully used in case series.61
Gram-Positive Cocci, Enterococcus
E. faecium and E. faecalis are commonly implicated in BSI.1 Similar to S. aureus, infective endocarditis must be ruled out when treating enterococcus BSI; a scoring system has been proposed to assist in deciding if such patients require echocardiography.62 Intravenous ampicillin is a preferred, highly effective agent for enterococci treatment when the organism is susceptible.44 However, oral ampicillin has poor bioavailability (50%), and data for its use in BSI are lacking. For susceptible strains, amoxicillin has comparable efficacy for enterococci and enhanced bioavailability (85%); high dose oral amoxicillin could be considered, but there is minimal clinical trial data to support this approach. Fluoroquinolones exhibit only modest activity against enterococci and would be an inferior choice for BSI.63 Although often sensitive to oral tetracyclines, data on their use in enterococcal BSI are insufficient. Nitrofurantoin can be used for susceptible enterococcal urinary tract infection; however, it does not achieve high blood concentrations and should not be used for BSI.
There is significant data comparing oral linezolid with intravenous daptomycin for vancomycin-resistant enterococci (VRE) BSI. In a systematic review including 10 trials using 30-day all-cause mortality as the primary outcome, patients treated with daptomycin demonstrated higher odds of death (OR 1.61, 95% CI 1.08–2.40) compared with those treated with linezolid.64 However, more recent data suggested that higher daptomycin doses than those used in these earlier trials resulted in improved VRE BSI outcomes.65 A subsequent study reported that VRE BSI treatment with linezolid is associated with significantly higher treatment failure and mortality compared with daptomycin therapy.66 Further research is needed, but should the side-effect profile of linezolid be tolerable, it remains an effective option for oral treatment of enterococcal BSIs.
Evidence Regarding Anaerobic Bacterial Blood Stream Infection
Anaerobic bacteria include Bacteroides, Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Veillonella, and Clostridium. Anaerobes account for approximately 4% of bacterial BSIs, and are often seen in the context of polymicrobial infection.67 Given that anaerobes are difficult to recover, and that antimicrobial resistance testing is more labor intensive, antibiotic therapy choices are often made empirically.67 Unfortunately, antibiotic resistance amongst anaerobes is increasing.68 However, metronidazole remains highly active against a majority of anaerobes, with only a handful of treatment failures reported,69 and has a highly favorable pharmacokinetic profile for oral treatment. Oral metronidazole remains an effective choice for many anaerobic BSIs. Considering the polymicrobial nature of many anaerobic infections, source control is important, and concomitant GNR infection must be ruled out before using metronidazole monotherapy.
Clindamycin has significant anaerobic activity, but Bacteroides resistance has increased significantly in recent years, as high as 26%-44%.70 Amoxicillin-clavulanate has good anaerobic coverage, but bioavailability of clavulanate is limited (50%), making it inferior for BSI. Bioavailability is also limited for cephalosporins with anaerobic activity, such as cefuroxime. Moxifloxacin is a fluoroquinolone with some anaerobic coverage and a good oral pharmacokinetic profile, but Bacteroides resistance can be as high as 50%, making it a risky empiric choice.68
Conclusions
Bacterial BSIs are common and result in significant morbidity and mortality, with high associated healthcare costs. Although BSIs are traditionally treated with intravenous antimicrobials, many BSIs can be safely and effectively cured using oral antibiotics. When appropriately selected, oral antibiotics offer lower costs, fewer side effects, promote antimicrobial stewardship, and are easier for patients. Ultimately, the decision to use oral versus intravenous antibiotics must consider the characteristics of the pathogen, patient, and drug.
Disclosures
None of the authors report any conflicts of interest.
1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501-509. PubMed
2. Kilgore M, Brossette S. Cost of bloodstream infections. Am J Infect Control. 2008;36(10):S172.e1-3. PubMed
3. Youkee D, Hulme W, Roberts T, Daniels R, Nutbeam T, Keep J. Time Matters: Antibiotic Timing in Sepsis and Septic Shock. Crit Care Med. 2016;44(10):e1016-1017. PubMed
4. Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;28;6:18. PubMed
5. Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, Waybill PN. Venous Thrombosis Associated with the Placement of Peripherally Inserted Central Catheters. J Vasc Interv Radiol. 2000;11(10):1309-1314. PubMed
6. Cheong K, Perry D, Karapetis C, Koczwara B. High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004;34(5):234-238. PubMed
7. Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006;90(6):1197-1222. PubMed
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014;5(2):83-87. PubMed
9. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
10. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
11. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
12. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393-407. PubMed
13. Cua YM, Kripalani S. Medication Use in the Transition from Hospital to Home. Ann Acad Med Singapore. 2008;37(2):136. PubMed
14. Paterson DL. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am J Med. 2006; 119(6):S20-28.
15. Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. PubMed
16. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M Oral vs Intravenous Ciprofloxacin in the Initial Empirical Management of Severe Pyelonephritis or Complicated Urinary Tract Infections: A Prospective Randomized Clinical Trial. Arch Intern Med. 1999;159(1):53-58. PubMed
17. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003237. PubMed
18. Brigmon MM, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Impact of fluoroquinolone resistance in Gram-negative bloodstream infections on healthcare utilization. Clin Microbiol Infect. 2015;21(9):843-849. PubMed
19. Ortega M, Marco F, Soriano A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63(3):568-574. PubMed
20. Lo CL, Lee CC, Li CW, et al. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2017;50(3):355-361. PubMed
21. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the Outcomes of Adults With Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score–Matched Cohort. Clin Infect Dis. 2017; cix767. doi.org/10.1093/cid/cix767 PubMed
22. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection-- 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183-2191. PubMed
23. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 2017;45(5):613-620. PubMed
24. Zelenitsky S, Ariano R, Harding G, Forrest A. Evaluating Ciprofloxacin Dosing for Pseudomonas aeruginosa Infection by Using Clinical Outcome-Based Monte Carlo Simulations. Antimicrob Agents Chemother. 2005;49(10):4009-4014. PubMed
25. Cazaubon Y, Bourguignon L, Goutelle S, Martin O, Maire P, Ducher M. Are ciprofloxacin dosage regimens adequate for antimicrobial efficacy and prevention of resistance? Pseudomonas aeruginosa bloodstream infection in elderly patients as a simulation case study. Fundam Clin Pharmacol. 2015;29(6):615-624. PubMed
26. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob Agents Chemother. 2005;49(4):1306-1311. PubMed
27. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of Combination Antimicrobial Therapy for Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756-2764. PubMed
28. The Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Emerging Infections Program Network Streptococcus pneumoniae, 2013. https://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Published November, 2014. Accessed September 26, 2017.
29. Brandenburg JA, Marrie TJ, Coley CM, et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000;15(9):638-646. PubMed
30. Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000;79(4):210-221. PubMed
31. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001; 161(6):848-850. PubMed
32. Oosterheert JJ, Bonten MJM, Schneider MME, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333(7580):1193. PubMed
33. Austrian R, Winston AL. The efficacy of penicillin V (phenoxymethyl-penicillin) in the treatment of mild and of moderately severe pneumococcal pneumonia. Am J Med Sci. 1956;232(6):624-628. PubMed
34. Waterer GW, Somes GW, Wunderink RG. Monotherapy May Be Suboptimal for Severe Bacteremic Pneumococcal Pneumonia. Arch Intern Med. 2001; 161(15):1837-1842. PubMed
35. Baddour LM, Yu VL, Klugman KP, et al. International Pneumococcal Study Group. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440-444. PubMed
36. Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic streptococcus group g: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112(8):622-626. PubMed
37. Davies HD, McGeer A, Schwartz B, Green, et al; Ontario Group A Streptococcal Study Group. Invasive Group A Streptococcal Infections in Ontario, Canada. N Engl J Med. 1996;335(8):547-554. PubMed
38. Farley MM, Harvey C, Stull T, et al. A Population-Based Assessment of Invasive Disease Due to Group B Streptococcus in Nonpregnant Adults. N Engl J Med. 1993;328(25):1807-1811. PubMed
39. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478-486. PubMed
40. Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo JJ. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994;38(9):2183-2186. PubMed
41. Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096-1100. PubMed
42. Chen I, Kaufisi P, Erdem G. Emergence of erythromycin- and clindamycin-resistant Streptococcus pyogenes emm 90 strains in Hawaii. J Clin Microbiol. 2011;49(1):439-441. PubMed
43. Biedenbach DJ, Jones RN. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25(1):47–51. PubMed
44. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT. Sanford Guide To Antimicrobial Therapy 2017. Dallas, TX. Antimicrobial Theapy, Inc, 2017.
45. Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn Microbiol Infect Dis. 2006;55(2):119-127. PubMed
46. Shurland S, Zhan M, Bradham DD, Roghmann M-C. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):2739. PubMed
47. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56(4):527-535. PubMed
48. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55. PubMed
49. Adra M, Lawrence KR. Trimethoprim/Sulfamethoxazole for Treatment of Severe Staphylococcus aureus Infections. Ann Pharmacother. 2004;38(2):338-341. PubMed
50. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117(5):390-398. PubMed
51. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ. 2015;350:h2219. PubMed
52. Sánchez García M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260-2264. PubMed
53. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481-1490. PubMed
54. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923-929. PubMed
55. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health-Syst Pharm. 2014;71(8):621-633. PubMed
56. Gade ND, Qazi MS. Fluoroquinolone Therapy in Staphylococcus aureus Infections: Where Do We Stand? J Lab Physicians. 2013;5(2):109-112. PubMed
57. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71(3):821-829. PubMed
58. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071-1073. PubMed
59. Schrenzel J, Harbarth S, Schockmel G, et al. A Randomized Clinical Trial to Compare Fleroxacin-Rifampicin with Flucloxacillin or Vancomycin for the Treatment of Staphylococcal Infection. Clin Infect Dis. 2004;39(9):1285-1292. PubMed
60. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. PubMed
61. Antony SJ, Diaz-Vasquez E, Stratton C. Clinical experience with linezolid in the treatment of resistant gram-positive infections. J Natl Med Assoc. 2001;93(10):386-391. PubMed
62. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis. 2015;60(4):528-535. PubMed
63. Martínez-Martínez L, Joyanes P, Pascual A, Terrero E, Perea EJ. Activity of eight fluoroquinolones against enterococci. Clin Microbiol Infect. 1997;3(4):497-499. PubMed
64. Balli EP, Venetis CA, Miyakis S. Systematic Review and Meta-Analysis of Linezolid versus Daptomycin for Treatment of Vancomycin-Resistant Enterococcal Bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-739. PubMed
70. Snydman DR, Jacobus NV, McDermott LA, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007;51(5):1649-1655. PubMed
69. Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis. 2010;50 Suppl 1:S26-33. PubMed
68. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2012;56(3):1247-1252. PubMed
67. Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26(6):1413-1417. PubMed
66. Britt NS, Potter EM, Patel N, Steed ME. Comparison of the Effectiveness and Safety of Linezolid and Daptomycin in Vancomycin-Resistant Enterococcal Bloodstream Infection: A National Cohort Study of Veterans Affairs Patients. Clin Infect Dis. 2015;61(6):871-878. PubMed
65. Chuang YC, Lin HY, Chen PY, et al. Effect of Daptomycin Dose on the Outcome of Vancomycin-Resistant, Daptomycin-Susceptible Enterococcus faecium Bacteremia. Clin Infect Dis. 2017;64(8):1026-1034. PubMed
Bacterial bloodstream infections (BSIs) are a major cause of morbidity and mortality in the United States. Approximately 600,000 BSI cases occur annually, resulting in 85,000 deaths,1 at a cost exceeding $1 billion.2 Traditionally, BSIs have been managed with intravenous antimicrobials, which rapidly achieve therapeutic blood concentrations, and are viewed as more potent than oral alternatives. Indeed, for acutely ill patients with bacteremia and sepsis, timely intravenous antimicrobials are lifesaving.3
However, whether intravenous antimicrobials are essential for the entire treatment course in BSIs, particularly for uncomplicated episodes, is controversial. Patients that are clinically stable or have been stabilized after an initial septic presentation may be appropriate candidates for treatment with oral antimicrobials. There are costs and risks associated with extended courses of intravenous agents, such as the necessity for long-term intravenous catheters, which entail risks for procedural complications, secondary infections, and thrombosis. A prospective study of 192 peripherally inserted central catheter (PICC) episodes reported an overall complication rate of 30.2%, including central line-associated BSIs (CLABSI) or venous thrombosis.4 Other studies also identified high rates of thrombosis5 and PICC-related CLABSI, particularly in patients with malignancy, where sepsis-related complications approach 25%.6 Additionally, appropriate care of indwelling catheters requires significant financial and healthcare resources.
Oral antimicrobial therapy for bacterial BSIs offers several potential benefits. Direct economic and healthcare workforce savings are expected to be significant, and procedural and catheter-related complications would be eliminated.7 Moreover, oral therapy provides antimicrobial stewardship by reducing the use of broad-spectrum intravenous agents.8 Recent infectious disease “Choosing Wisely” initiatives recommend clinicians “prefer oral formulations of highly bioavailable antimicrobials whenever possible”,9 and this approach is supported by the Centers for Disease Control and Prevention antibiotic stewardship program.10 However, the expected savings and benefits of oral therapy would be lost should they be less effective and result in treatment failure or relapse of the primary BSI. Pathogen susceptibility, gastrointestinal absorption, oral bioavailability, patient tolerability, and adherence with therapy need to be carefully considered before choosing oral antimicrobials. Thus, oral antimicrobial therapy for BSI should be utilized in carefully selected circumstances.
In this narrative review, we highlight areas where oral therapy is safe and effective in treating bloodstream infections, as well as offer guidance to clinicians managing patients experiencing BSI. Given the lack of robust clinical trials on this subject, the evidence for performing a systematic review was insufficient. Thus, the articles and recommendations cited in this review were selected based on the authors’ experiences to represent the best available evidence.
Infection Source Control
Pathogen and Antimicrobial Factors
Patient Factors
Evidence Regarding Bloodstream Infections due to Gram-Negative Rods
BSIs due to gram-negative rods (GNRs) are common and cause significant morbidity and mortality. GNRs represent a broad and diverse array of pathogens. We focus on the Enterobacteriaceae family and Pseudomonas aeruginosa, because they are frequently encountered in clinical practice.1
Gram-Negative Rods, Enterobacteriaceae Family
The Enterobacteriaceae family includes Escherichia coli, Klebsiella, Salmonella, Proteus, Enterobacter, Serratia, and Citrobacter species. The range of illnesses caused by Enterobacteriaceae is as diverse as the family, encompassing most body sites. Although most Enterobacteriaceae are intrinsically susceptible to antibiotics, there is potential for significant multi-drug resistance. Of particular recent concern has been the emergence of Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBL) and even carbapenem-resistant strains.14
However, Enterobacteriaceae species susceptible to oral antimicrobials are often suitable candidates for oral BSI therapy. Among 106 patients with GNR BSI treated with a highly bioavailable oral antibiotic (eg, levofloxacin), the treatment failure rate was only 2% (versus 14% when an antimicrobial with only moderate or low bioavailability was selected).15 Oral treatment of Enterobacteriaceae BSIs secondary to urinary tract infection has been best studied. A prospective randomized, controlled trial evaluated oral versus intravenous ciprofloxacin amongst 141 patients with severe pyelonephritis or complicated urinary tract infections, in which the rate of bacteremia was 38%.16 Notably, patients with obstruction or renal abscess were excluded from the trial. No significant differences in microbiological failure or unsatisfactory clinical responses were found between the IV and oral treatment groups. Additionally, a Cochrane review reported that oral antibiotic therapy is no less effective than intravenous therapy for severe UTI, although data on BSI frequency were not provided.17
Resistance to fluoroquinolones such as ciprofloxacin has been identified as a risk factor for GNR BSI oral treatment failure, highlighting the importance of confirming susceptibilities before committing to an oral treatment plan.18,19 Even ESBL Enterobacteriaceae can be considered for treatment with fluoroquinolones if susceptibilities allow.20
The ideal duration of therapy for GNR BSI is an area of active research. A recent retrospective trial showed no difference in all-cause mortality or recurrent BSI in GNR BSI treated for 8 versus 15 days.21 A recent meta-analysis suggested that 7 days of therapy was noninferior to a longer duration therapy (10–14 days) for pyelonephritis, in which a subset was bacteremic.22 However, another trial reported that short course therapy for GNR BSI (<7 days) is associated with higher risk of treatment failure.22 Further data are needed.
Gram-Negative Rods, Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common pathogen, intrinsically resistant to many antimicrobials, and readily develops antimicrobial resistance during therapy. Fluoroquinolones (such as ciprofloxacin, levofloxacin, and delafloxacin) are the only currently available oral agents with antipseudomonal activity. However, fluoroquinolones may not achieve blood concentrations appropriate for P. aeruginosa treatment at standard doses, while higher dose regimens may be associated with increased risk for undesirable side effects.24,25 Currently, given the minimal trial data comparing oral versus intravenous therapy for P. aeruginosa BSIs, and multiple studies indicating increased mortality when P. aeruginosa is treated inappropriately,26,27 we prefer a conservative approach and consider oral therapy a less-preferred option.
Evidence Regarding Bloodstream Infections due to Gram-Positive Cocci
The majority of bloodstream infections in the United States, and the resultant morbidity and mortality, are from gram-positive cocci (GPCs) such as Staphylococcus, Streptococcus, and Enterococcus species.1
Gram-Positive Cocci, Streptococcus pneumoniae
Of the approximately 900,000 annual cases of S. pneumoniae infection in the United States, approximately 40,000 are complicated by BSI, with 70% of those cases being secondary to pneumococcal pneumonia.28 In studies on patients with pneumococcal pneumonia, bacteremic cases generally fare worse than those without bacteremia.29,30 However, several trials demonstrated comparable outcomes in the setting of bacteremic pneumococcal pneumonia when switched early (within 3 days) from intravenous to oral antibiotics to complete a 7-day course.31,32 Before pneumococcal penicillin resistance became widespread, oral penicillin was shown to be effective, and remains an option for susceptible strains.33 It is worth noting, however, that other trials have shown a mortality benefit from treating bacteremic pneumococcal pneumonia initially with dual-therapy including a β-lactam and macrolide such as azithromycin. This observation highlights the importance of knowing the final susceptibility data prior to consolidating to monotherapy with an oral agent, and that macrolides may have beneficial anti-inflammatory effects, though further research is needed.34,35
Although the evidence for treating bacteremic pneumococcal pneumonia using a highly active and absorbable oral agent is fairly robust, S. pneumoniae BSI secondary to other sites of infection sites is less well studied and may require a more conservative approach.
Gram-Positive Cocci, β-hemolytic Streptococcus species
β-Hemolytic Streptococci include groups A to H, of which groups A (S. pyogenes) and B (S. agalactiae) are the most commonly implicated in BSIs.36 Group A Streptococcus (GAS) is classically associated with streptococcal pharyngitis and Group B Streptococcus (GBS) is associated with postpartum endometritis and neonatal meningitis, though both are virulent organisms with a potential to cause invasive infection throughout the body and in all age-groups. Up to 14% of GAS and 41% GBS BSIs have no clear source;37,38 given these are skin pathogens, such scenarios likely represent invasion via microabrasion. As β-hemolytic streptococcal BSI is often observed in the context of necrotizing skin and soft tissue infections, surgical source control is particularly important.39 GAS remains exquisitely susceptible to penicillin, and intravenous penicillin remains the mainstay for invasive disease; GBS has higher penicillin resistance rates than GAS.40 Clindamycin should be added when there is concern for severe disease or toxic shock.41 Unfortunately, oral penicillin is poorly bioavailable (approximately 50%), and there has been recent concern regarding inducible clindamycin resistance in GAS.42 Thus, oral penicillin V and/or clindamycin is a potentially risky strategy, with no clinical trials supporting this approach; however, they may be reasonable options in selected patients with source control and stable hemodynamics. Amoxicillin has high bioavailability (85%) and may be effective; however, there is lack of supporting data. Highly bioavailable agents such as levofloxacin and linezolid have GAS and GBS activity43 and might be expected to produce satisfactory outcomes. Because no clinical trials have compared these agents with intravenous therapy for BSI, caution is advised. Although bacteriostatic against Staphylococcus, linezolid is bactericidal against Streptococcus.44 Fluoroquinolone resistance amongst β-hemolytic Streptococcus is rare (approximately 0.5%) but does occur.45
Gram-Positive Cocci, Staphylococcus Species
Staphylococcus species include S. aureus (including methicillin susceptible and resistant strains: MSSA and MRSA, respectively) and coagulase-negative species, which include organisms such as S. epidermidis. S. aureus is the most common cause of BSI mortality in the United States,1 with mortality rates estimated at 20%–40% per episode.46 Infectious disease consultation has been associated with decreased mortality and is recommended.47 The guidelines of the Infectious Diseases Society of America for the treatment of MRSA recommend the use of parenteral agents for BSI.48 It is important to consider if a patient with S. aureus BSI has infective endocarditis.
Oral antibiotic therapy for S. aureus BSI is not currently standard practice. Although trimethoprim-sulfamethoxazole (TMP-SMX) has favorable pharmacokinetics and case series of using it successfully for BSI exist,49 TMP-SMX showed inferior outcomes in a randomized trial comparing oral TMP-SMX with intravenous vancomycin in a series of 101 S. aureus infections.50 This observation has been replicated.51 Data on doxycycline or clindamycin for S. aureus BSI are limited, and IDSA guidelines advise against their use in this setting because they are predominantly bacteriostatic.48 Linezolid has favorable pharmacokinetics, with approximately 100% bioavailability, and S. aureus resistance to linezolid is rare.52 Several randomized trials have compared oral linezolid with intravenous vancomycin for S. aureus BSI; for instance, Stevens et al. randomized 460 patients with S. aureus infection (of whom 18% had BSI) to linezolid versus vancomycin and observed similar clinical cure rates.53 A pooled analysis showed oral linezolid was noninferior to vancomycin specifically for S. aureus BSI.54 However, long-term use is often limited by hematologic toxicity, peripheral or optic neuropathy (which can be permanent), and induced serotonin syndrome. Additionally, linezolid is bacteriostatic, not bactericidal against S. aureus. Using oral linezolid as a first-line option for S. aureus BSI would not be recommended; however, it may be used as a second-line treatment option in selected cases. Tedizolid has similar pharmacokinetics and spectrum of activity with fewer side effects; however, clinical data on its use for S. aureus BSI are lacking.55 Fluoroquinolones such as levofloxacin and the newer agent delafloxacin have activity against S. aureus, including MRSA, but on-treatment emergence of fluoroquinolone resistance is a concern, and data on delafloxacin for BSI are lacking.56,57 Older literature suggested the combination of ciprofloxacin and rifampin was effective against right-sided S. aureus endocarditis,58 and other oral fluoroquinolone-rifamycin combinations have also been found to be effective59 However, this approach is currently not a standard therapy, nor is it widely used. Decisions on the duration of therapy for S. aureus BSI should be made in conjunction with an infectious diseases specialist; 14 days is currently regarded as a minimum.47,48
Published data regarding oral treatment of coagulase-negative Staphylococcus (CoNS) BSI are limited. Most CoNS bacteremia and up to 80% Staphylococcus epidermidis bacteremia represent blood culture contamination, though true infection from CoNS is not uncommon, particularly in patients with indwelling catheters.60 An exception is the CoNS species Staphylococcus lugdunensis, which is more virulent, and bacteremia with this organism usually warrants antibiotics. Oral antimicrobial therapy is currently not a standard treatment practice for CoNS BSI that is felt to represent true infection; however, linezolid has been successfully used in case series.61
Gram-Positive Cocci, Enterococcus
E. faecium and E. faecalis are commonly implicated in BSI.1 Similar to S. aureus, infective endocarditis must be ruled out when treating enterococcus BSI; a scoring system has been proposed to assist in deciding if such patients require echocardiography.62 Intravenous ampicillin is a preferred, highly effective agent for enterococci treatment when the organism is susceptible.44 However, oral ampicillin has poor bioavailability (50%), and data for its use in BSI are lacking. For susceptible strains, amoxicillin has comparable efficacy for enterococci and enhanced bioavailability (85%); high dose oral amoxicillin could be considered, but there is minimal clinical trial data to support this approach. Fluoroquinolones exhibit only modest activity against enterococci and would be an inferior choice for BSI.63 Although often sensitive to oral tetracyclines, data on their use in enterococcal BSI are insufficient. Nitrofurantoin can be used for susceptible enterococcal urinary tract infection; however, it does not achieve high blood concentrations and should not be used for BSI.
There is significant data comparing oral linezolid with intravenous daptomycin for vancomycin-resistant enterococci (VRE) BSI. In a systematic review including 10 trials using 30-day all-cause mortality as the primary outcome, patients treated with daptomycin demonstrated higher odds of death (OR 1.61, 95% CI 1.08–2.40) compared with those treated with linezolid.64 However, more recent data suggested that higher daptomycin doses than those used in these earlier trials resulted in improved VRE BSI outcomes.65 A subsequent study reported that VRE BSI treatment with linezolid is associated with significantly higher treatment failure and mortality compared with daptomycin therapy.66 Further research is needed, but should the side-effect profile of linezolid be tolerable, it remains an effective option for oral treatment of enterococcal BSIs.
Evidence Regarding Anaerobic Bacterial Blood Stream Infection
Anaerobic bacteria include Bacteroides, Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Veillonella, and Clostridium. Anaerobes account for approximately 4% of bacterial BSIs, and are often seen in the context of polymicrobial infection.67 Given that anaerobes are difficult to recover, and that antimicrobial resistance testing is more labor intensive, antibiotic therapy choices are often made empirically.67 Unfortunately, antibiotic resistance amongst anaerobes is increasing.68 However, metronidazole remains highly active against a majority of anaerobes, with only a handful of treatment failures reported,69 and has a highly favorable pharmacokinetic profile for oral treatment. Oral metronidazole remains an effective choice for many anaerobic BSIs. Considering the polymicrobial nature of many anaerobic infections, source control is important, and concomitant GNR infection must be ruled out before using metronidazole monotherapy.
Clindamycin has significant anaerobic activity, but Bacteroides resistance has increased significantly in recent years, as high as 26%-44%.70 Amoxicillin-clavulanate has good anaerobic coverage, but bioavailability of clavulanate is limited (50%), making it inferior for BSI. Bioavailability is also limited for cephalosporins with anaerobic activity, such as cefuroxime. Moxifloxacin is a fluoroquinolone with some anaerobic coverage and a good oral pharmacokinetic profile, but Bacteroides resistance can be as high as 50%, making it a risky empiric choice.68
Conclusions
Bacterial BSIs are common and result in significant morbidity and mortality, with high associated healthcare costs. Although BSIs are traditionally treated with intravenous antimicrobials, many BSIs can be safely and effectively cured using oral antibiotics. When appropriately selected, oral antibiotics offer lower costs, fewer side effects, promote antimicrobial stewardship, and are easier for patients. Ultimately, the decision to use oral versus intravenous antibiotics must consider the characteristics of the pathogen, patient, and drug.
Disclosures
None of the authors report any conflicts of interest.
Bacterial bloodstream infections (BSIs) are a major cause of morbidity and mortality in the United States. Approximately 600,000 BSI cases occur annually, resulting in 85,000 deaths,1 at a cost exceeding $1 billion.2 Traditionally, BSIs have been managed with intravenous antimicrobials, which rapidly achieve therapeutic blood concentrations, and are viewed as more potent than oral alternatives. Indeed, for acutely ill patients with bacteremia and sepsis, timely intravenous antimicrobials are lifesaving.3
However, whether intravenous antimicrobials are essential for the entire treatment course in BSIs, particularly for uncomplicated episodes, is controversial. Patients that are clinically stable or have been stabilized after an initial septic presentation may be appropriate candidates for treatment with oral antimicrobials. There are costs and risks associated with extended courses of intravenous agents, such as the necessity for long-term intravenous catheters, which entail risks for procedural complications, secondary infections, and thrombosis. A prospective study of 192 peripherally inserted central catheter (PICC) episodes reported an overall complication rate of 30.2%, including central line-associated BSIs (CLABSI) or venous thrombosis.4 Other studies also identified high rates of thrombosis5 and PICC-related CLABSI, particularly in patients with malignancy, where sepsis-related complications approach 25%.6 Additionally, appropriate care of indwelling catheters requires significant financial and healthcare resources.
Oral antimicrobial therapy for bacterial BSIs offers several potential benefits. Direct economic and healthcare workforce savings are expected to be significant, and procedural and catheter-related complications would be eliminated.7 Moreover, oral therapy provides antimicrobial stewardship by reducing the use of broad-spectrum intravenous agents.8 Recent infectious disease “Choosing Wisely” initiatives recommend clinicians “prefer oral formulations of highly bioavailable antimicrobials whenever possible”,9 and this approach is supported by the Centers for Disease Control and Prevention antibiotic stewardship program.10 However, the expected savings and benefits of oral therapy would be lost should they be less effective and result in treatment failure or relapse of the primary BSI. Pathogen susceptibility, gastrointestinal absorption, oral bioavailability, patient tolerability, and adherence with therapy need to be carefully considered before choosing oral antimicrobials. Thus, oral antimicrobial therapy for BSI should be utilized in carefully selected circumstances.
In this narrative review, we highlight areas where oral therapy is safe and effective in treating bloodstream infections, as well as offer guidance to clinicians managing patients experiencing BSI. Given the lack of robust clinical trials on this subject, the evidence for performing a systematic review was insufficient. Thus, the articles and recommendations cited in this review were selected based on the authors’ experiences to represent the best available evidence.
Infection Source Control
Pathogen and Antimicrobial Factors
Patient Factors
Evidence Regarding Bloodstream Infections due to Gram-Negative Rods
BSIs due to gram-negative rods (GNRs) are common and cause significant morbidity and mortality. GNRs represent a broad and diverse array of pathogens. We focus on the Enterobacteriaceae family and Pseudomonas aeruginosa, because they are frequently encountered in clinical practice.1
Gram-Negative Rods, Enterobacteriaceae Family
The Enterobacteriaceae family includes Escherichia coli, Klebsiella, Salmonella, Proteus, Enterobacter, Serratia, and Citrobacter species. The range of illnesses caused by Enterobacteriaceae is as diverse as the family, encompassing most body sites. Although most Enterobacteriaceae are intrinsically susceptible to antibiotics, there is potential for significant multi-drug resistance. Of particular recent concern has been the emergence of Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBL) and even carbapenem-resistant strains.14
However, Enterobacteriaceae species susceptible to oral antimicrobials are often suitable candidates for oral BSI therapy. Among 106 patients with GNR BSI treated with a highly bioavailable oral antibiotic (eg, levofloxacin), the treatment failure rate was only 2% (versus 14% when an antimicrobial with only moderate or low bioavailability was selected).15 Oral treatment of Enterobacteriaceae BSIs secondary to urinary tract infection has been best studied. A prospective randomized, controlled trial evaluated oral versus intravenous ciprofloxacin amongst 141 patients with severe pyelonephritis or complicated urinary tract infections, in which the rate of bacteremia was 38%.16 Notably, patients with obstruction or renal abscess were excluded from the trial. No significant differences in microbiological failure or unsatisfactory clinical responses were found between the IV and oral treatment groups. Additionally, a Cochrane review reported that oral antibiotic therapy is no less effective than intravenous therapy for severe UTI, although data on BSI frequency were not provided.17
Resistance to fluoroquinolones such as ciprofloxacin has been identified as a risk factor for GNR BSI oral treatment failure, highlighting the importance of confirming susceptibilities before committing to an oral treatment plan.18,19 Even ESBL Enterobacteriaceae can be considered for treatment with fluoroquinolones if susceptibilities allow.20
The ideal duration of therapy for GNR BSI is an area of active research. A recent retrospective trial showed no difference in all-cause mortality or recurrent BSI in GNR BSI treated for 8 versus 15 days.21 A recent meta-analysis suggested that 7 days of therapy was noninferior to a longer duration therapy (10–14 days) for pyelonephritis, in which a subset was bacteremic.22 However, another trial reported that short course therapy for GNR BSI (<7 days) is associated with higher risk of treatment failure.22 Further data are needed.
Gram-Negative Rods, Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common pathogen, intrinsically resistant to many antimicrobials, and readily develops antimicrobial resistance during therapy. Fluoroquinolones (such as ciprofloxacin, levofloxacin, and delafloxacin) are the only currently available oral agents with antipseudomonal activity. However, fluoroquinolones may not achieve blood concentrations appropriate for P. aeruginosa treatment at standard doses, while higher dose regimens may be associated with increased risk for undesirable side effects.24,25 Currently, given the minimal trial data comparing oral versus intravenous therapy for P. aeruginosa BSIs, and multiple studies indicating increased mortality when P. aeruginosa is treated inappropriately,26,27 we prefer a conservative approach and consider oral therapy a less-preferred option.
Evidence Regarding Bloodstream Infections due to Gram-Positive Cocci
The majority of bloodstream infections in the United States, and the resultant morbidity and mortality, are from gram-positive cocci (GPCs) such as Staphylococcus, Streptococcus, and Enterococcus species.1
Gram-Positive Cocci, Streptococcus pneumoniae
Of the approximately 900,000 annual cases of S. pneumoniae infection in the United States, approximately 40,000 are complicated by BSI, with 70% of those cases being secondary to pneumococcal pneumonia.28 In studies on patients with pneumococcal pneumonia, bacteremic cases generally fare worse than those without bacteremia.29,30 However, several trials demonstrated comparable outcomes in the setting of bacteremic pneumococcal pneumonia when switched early (within 3 days) from intravenous to oral antibiotics to complete a 7-day course.31,32 Before pneumococcal penicillin resistance became widespread, oral penicillin was shown to be effective, and remains an option for susceptible strains.33 It is worth noting, however, that other trials have shown a mortality benefit from treating bacteremic pneumococcal pneumonia initially with dual-therapy including a β-lactam and macrolide such as azithromycin. This observation highlights the importance of knowing the final susceptibility data prior to consolidating to monotherapy with an oral agent, and that macrolides may have beneficial anti-inflammatory effects, though further research is needed.34,35
Although the evidence for treating bacteremic pneumococcal pneumonia using a highly active and absorbable oral agent is fairly robust, S. pneumoniae BSI secondary to other sites of infection sites is less well studied and may require a more conservative approach.
Gram-Positive Cocci, β-hemolytic Streptococcus species
β-Hemolytic Streptococci include groups A to H, of which groups A (S. pyogenes) and B (S. agalactiae) are the most commonly implicated in BSIs.36 Group A Streptococcus (GAS) is classically associated with streptococcal pharyngitis and Group B Streptococcus (GBS) is associated with postpartum endometritis and neonatal meningitis, though both are virulent organisms with a potential to cause invasive infection throughout the body and in all age-groups. Up to 14% of GAS and 41% GBS BSIs have no clear source;37,38 given these are skin pathogens, such scenarios likely represent invasion via microabrasion. As β-hemolytic streptococcal BSI is often observed in the context of necrotizing skin and soft tissue infections, surgical source control is particularly important.39 GAS remains exquisitely susceptible to penicillin, and intravenous penicillin remains the mainstay for invasive disease; GBS has higher penicillin resistance rates than GAS.40 Clindamycin should be added when there is concern for severe disease or toxic shock.41 Unfortunately, oral penicillin is poorly bioavailable (approximately 50%), and there has been recent concern regarding inducible clindamycin resistance in GAS.42 Thus, oral penicillin V and/or clindamycin is a potentially risky strategy, with no clinical trials supporting this approach; however, they may be reasonable options in selected patients with source control and stable hemodynamics. Amoxicillin has high bioavailability (85%) and may be effective; however, there is lack of supporting data. Highly bioavailable agents such as levofloxacin and linezolid have GAS and GBS activity43 and might be expected to produce satisfactory outcomes. Because no clinical trials have compared these agents with intravenous therapy for BSI, caution is advised. Although bacteriostatic against Staphylococcus, linezolid is bactericidal against Streptococcus.44 Fluoroquinolone resistance amongst β-hemolytic Streptococcus is rare (approximately 0.5%) but does occur.45
Gram-Positive Cocci, Staphylococcus Species
Staphylococcus species include S. aureus (including methicillin susceptible and resistant strains: MSSA and MRSA, respectively) and coagulase-negative species, which include organisms such as S. epidermidis. S. aureus is the most common cause of BSI mortality in the United States,1 with mortality rates estimated at 20%–40% per episode.46 Infectious disease consultation has been associated with decreased mortality and is recommended.47 The guidelines of the Infectious Diseases Society of America for the treatment of MRSA recommend the use of parenteral agents for BSI.48 It is important to consider if a patient with S. aureus BSI has infective endocarditis.
Oral antibiotic therapy for S. aureus BSI is not currently standard practice. Although trimethoprim-sulfamethoxazole (TMP-SMX) has favorable pharmacokinetics and case series of using it successfully for BSI exist,49 TMP-SMX showed inferior outcomes in a randomized trial comparing oral TMP-SMX with intravenous vancomycin in a series of 101 S. aureus infections.50 This observation has been replicated.51 Data on doxycycline or clindamycin for S. aureus BSI are limited, and IDSA guidelines advise against their use in this setting because they are predominantly bacteriostatic.48 Linezolid has favorable pharmacokinetics, with approximately 100% bioavailability, and S. aureus resistance to linezolid is rare.52 Several randomized trials have compared oral linezolid with intravenous vancomycin for S. aureus BSI; for instance, Stevens et al. randomized 460 patients with S. aureus infection (of whom 18% had BSI) to linezolid versus vancomycin and observed similar clinical cure rates.53 A pooled analysis showed oral linezolid was noninferior to vancomycin specifically for S. aureus BSI.54 However, long-term use is often limited by hematologic toxicity, peripheral or optic neuropathy (which can be permanent), and induced serotonin syndrome. Additionally, linezolid is bacteriostatic, not bactericidal against S. aureus. Using oral linezolid as a first-line option for S. aureus BSI would not be recommended; however, it may be used as a second-line treatment option in selected cases. Tedizolid has similar pharmacokinetics and spectrum of activity with fewer side effects; however, clinical data on its use for S. aureus BSI are lacking.55 Fluoroquinolones such as levofloxacin and the newer agent delafloxacin have activity against S. aureus, including MRSA, but on-treatment emergence of fluoroquinolone resistance is a concern, and data on delafloxacin for BSI are lacking.56,57 Older literature suggested the combination of ciprofloxacin and rifampin was effective against right-sided S. aureus endocarditis,58 and other oral fluoroquinolone-rifamycin combinations have also been found to be effective59 However, this approach is currently not a standard therapy, nor is it widely used. Decisions on the duration of therapy for S. aureus BSI should be made in conjunction with an infectious diseases specialist; 14 days is currently regarded as a minimum.47,48
Published data regarding oral treatment of coagulase-negative Staphylococcus (CoNS) BSI are limited. Most CoNS bacteremia and up to 80% Staphylococcus epidermidis bacteremia represent blood culture contamination, though true infection from CoNS is not uncommon, particularly in patients with indwelling catheters.60 An exception is the CoNS species Staphylococcus lugdunensis, which is more virulent, and bacteremia with this organism usually warrants antibiotics. Oral antimicrobial therapy is currently not a standard treatment practice for CoNS BSI that is felt to represent true infection; however, linezolid has been successfully used in case series.61
Gram-Positive Cocci, Enterococcus
E. faecium and E. faecalis are commonly implicated in BSI.1 Similar to S. aureus, infective endocarditis must be ruled out when treating enterococcus BSI; a scoring system has been proposed to assist in deciding if such patients require echocardiography.62 Intravenous ampicillin is a preferred, highly effective agent for enterococci treatment when the organism is susceptible.44 However, oral ampicillin has poor bioavailability (50%), and data for its use in BSI are lacking. For susceptible strains, amoxicillin has comparable efficacy for enterococci and enhanced bioavailability (85%); high dose oral amoxicillin could be considered, but there is minimal clinical trial data to support this approach. Fluoroquinolones exhibit only modest activity against enterococci and would be an inferior choice for BSI.63 Although often sensitive to oral tetracyclines, data on their use in enterococcal BSI are insufficient. Nitrofurantoin can be used for susceptible enterococcal urinary tract infection; however, it does not achieve high blood concentrations and should not be used for BSI.
There is significant data comparing oral linezolid with intravenous daptomycin for vancomycin-resistant enterococci (VRE) BSI. In a systematic review including 10 trials using 30-day all-cause mortality as the primary outcome, patients treated with daptomycin demonstrated higher odds of death (OR 1.61, 95% CI 1.08–2.40) compared with those treated with linezolid.64 However, more recent data suggested that higher daptomycin doses than those used in these earlier trials resulted in improved VRE BSI outcomes.65 A subsequent study reported that VRE BSI treatment with linezolid is associated with significantly higher treatment failure and mortality compared with daptomycin therapy.66 Further research is needed, but should the side-effect profile of linezolid be tolerable, it remains an effective option for oral treatment of enterococcal BSIs.
Evidence Regarding Anaerobic Bacterial Blood Stream Infection
Anaerobic bacteria include Bacteroides, Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Veillonella, and Clostridium. Anaerobes account for approximately 4% of bacterial BSIs, and are often seen in the context of polymicrobial infection.67 Given that anaerobes are difficult to recover, and that antimicrobial resistance testing is more labor intensive, antibiotic therapy choices are often made empirically.67 Unfortunately, antibiotic resistance amongst anaerobes is increasing.68 However, metronidazole remains highly active against a majority of anaerobes, with only a handful of treatment failures reported,69 and has a highly favorable pharmacokinetic profile for oral treatment. Oral metronidazole remains an effective choice for many anaerobic BSIs. Considering the polymicrobial nature of many anaerobic infections, source control is important, and concomitant GNR infection must be ruled out before using metronidazole monotherapy.
Clindamycin has significant anaerobic activity, but Bacteroides resistance has increased significantly in recent years, as high as 26%-44%.70 Amoxicillin-clavulanate has good anaerobic coverage, but bioavailability of clavulanate is limited (50%), making it inferior for BSI. Bioavailability is also limited for cephalosporins with anaerobic activity, such as cefuroxime. Moxifloxacin is a fluoroquinolone with some anaerobic coverage and a good oral pharmacokinetic profile, but Bacteroides resistance can be as high as 50%, making it a risky empiric choice.68
Conclusions
Bacterial BSIs are common and result in significant morbidity and mortality, with high associated healthcare costs. Although BSIs are traditionally treated with intravenous antimicrobials, many BSIs can be safely and effectively cured using oral antibiotics. When appropriately selected, oral antibiotics offer lower costs, fewer side effects, promote antimicrobial stewardship, and are easier for patients. Ultimately, the decision to use oral versus intravenous antibiotics must consider the characteristics of the pathogen, patient, and drug.
Disclosures
None of the authors report any conflicts of interest.
1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501-509. PubMed
2. Kilgore M, Brossette S. Cost of bloodstream infections. Am J Infect Control. 2008;36(10):S172.e1-3. PubMed
3. Youkee D, Hulme W, Roberts T, Daniels R, Nutbeam T, Keep J. Time Matters: Antibiotic Timing in Sepsis and Septic Shock. Crit Care Med. 2016;44(10):e1016-1017. PubMed
4. Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;28;6:18. PubMed
5. Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, Waybill PN. Venous Thrombosis Associated with the Placement of Peripherally Inserted Central Catheters. J Vasc Interv Radiol. 2000;11(10):1309-1314. PubMed
6. Cheong K, Perry D, Karapetis C, Koczwara B. High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004;34(5):234-238. PubMed
7. Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006;90(6):1197-1222. PubMed
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014;5(2):83-87. PubMed
9. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
10. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
11. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
12. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393-407. PubMed
13. Cua YM, Kripalani S. Medication Use in the Transition from Hospital to Home. Ann Acad Med Singapore. 2008;37(2):136. PubMed
14. Paterson DL. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am J Med. 2006; 119(6):S20-28.
15. Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. PubMed
16. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M Oral vs Intravenous Ciprofloxacin in the Initial Empirical Management of Severe Pyelonephritis or Complicated Urinary Tract Infections: A Prospective Randomized Clinical Trial. Arch Intern Med. 1999;159(1):53-58. PubMed
17. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003237. PubMed
18. Brigmon MM, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Impact of fluoroquinolone resistance in Gram-negative bloodstream infections on healthcare utilization. Clin Microbiol Infect. 2015;21(9):843-849. PubMed
19. Ortega M, Marco F, Soriano A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63(3):568-574. PubMed
20. Lo CL, Lee CC, Li CW, et al. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2017;50(3):355-361. PubMed
21. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the Outcomes of Adults With Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score–Matched Cohort. Clin Infect Dis. 2017; cix767. doi.org/10.1093/cid/cix767 PubMed
22. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection-- 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183-2191. PubMed
23. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 2017;45(5):613-620. PubMed
24. Zelenitsky S, Ariano R, Harding G, Forrest A. Evaluating Ciprofloxacin Dosing for Pseudomonas aeruginosa Infection by Using Clinical Outcome-Based Monte Carlo Simulations. Antimicrob Agents Chemother. 2005;49(10):4009-4014. PubMed
25. Cazaubon Y, Bourguignon L, Goutelle S, Martin O, Maire P, Ducher M. Are ciprofloxacin dosage regimens adequate for antimicrobial efficacy and prevention of resistance? Pseudomonas aeruginosa bloodstream infection in elderly patients as a simulation case study. Fundam Clin Pharmacol. 2015;29(6):615-624. PubMed
26. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob Agents Chemother. 2005;49(4):1306-1311. PubMed
27. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of Combination Antimicrobial Therapy for Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756-2764. PubMed
28. The Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Emerging Infections Program Network Streptococcus pneumoniae, 2013. https://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Published November, 2014. Accessed September 26, 2017.
29. Brandenburg JA, Marrie TJ, Coley CM, et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000;15(9):638-646. PubMed
30. Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000;79(4):210-221. PubMed
31. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001; 161(6):848-850. PubMed
32. Oosterheert JJ, Bonten MJM, Schneider MME, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333(7580):1193. PubMed
33. Austrian R, Winston AL. The efficacy of penicillin V (phenoxymethyl-penicillin) in the treatment of mild and of moderately severe pneumococcal pneumonia. Am J Med Sci. 1956;232(6):624-628. PubMed
34. Waterer GW, Somes GW, Wunderink RG. Monotherapy May Be Suboptimal for Severe Bacteremic Pneumococcal Pneumonia. Arch Intern Med. 2001; 161(15):1837-1842. PubMed
35. Baddour LM, Yu VL, Klugman KP, et al. International Pneumococcal Study Group. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440-444. PubMed
36. Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic streptococcus group g: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112(8):622-626. PubMed
37. Davies HD, McGeer A, Schwartz B, Green, et al; Ontario Group A Streptococcal Study Group. Invasive Group A Streptococcal Infections in Ontario, Canada. N Engl J Med. 1996;335(8):547-554. PubMed
38. Farley MM, Harvey C, Stull T, et al. A Population-Based Assessment of Invasive Disease Due to Group B Streptococcus in Nonpregnant Adults. N Engl J Med. 1993;328(25):1807-1811. PubMed
39. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478-486. PubMed
40. Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo JJ. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994;38(9):2183-2186. PubMed
41. Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096-1100. PubMed
42. Chen I, Kaufisi P, Erdem G. Emergence of erythromycin- and clindamycin-resistant Streptococcus pyogenes emm 90 strains in Hawaii. J Clin Microbiol. 2011;49(1):439-441. PubMed
43. Biedenbach DJ, Jones RN. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25(1):47–51. PubMed
44. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT. Sanford Guide To Antimicrobial Therapy 2017. Dallas, TX. Antimicrobial Theapy, Inc, 2017.
45. Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn Microbiol Infect Dis. 2006;55(2):119-127. PubMed
46. Shurland S, Zhan M, Bradham DD, Roghmann M-C. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):2739. PubMed
47. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56(4):527-535. PubMed
48. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55. PubMed
49. Adra M, Lawrence KR. Trimethoprim/Sulfamethoxazole for Treatment of Severe Staphylococcus aureus Infections. Ann Pharmacother. 2004;38(2):338-341. PubMed
50. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117(5):390-398. PubMed
51. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ. 2015;350:h2219. PubMed
52. Sánchez García M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260-2264. PubMed
53. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481-1490. PubMed
54. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923-929. PubMed
55. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health-Syst Pharm. 2014;71(8):621-633. PubMed
56. Gade ND, Qazi MS. Fluoroquinolone Therapy in Staphylococcus aureus Infections: Where Do We Stand? J Lab Physicians. 2013;5(2):109-112. PubMed
57. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71(3):821-829. PubMed
58. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071-1073. PubMed
59. Schrenzel J, Harbarth S, Schockmel G, et al. A Randomized Clinical Trial to Compare Fleroxacin-Rifampicin with Flucloxacillin or Vancomycin for the Treatment of Staphylococcal Infection. Clin Infect Dis. 2004;39(9):1285-1292. PubMed
60. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. PubMed
61. Antony SJ, Diaz-Vasquez E, Stratton C. Clinical experience with linezolid in the treatment of resistant gram-positive infections. J Natl Med Assoc. 2001;93(10):386-391. PubMed
62. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis. 2015;60(4):528-535. PubMed
63. Martínez-Martínez L, Joyanes P, Pascual A, Terrero E, Perea EJ. Activity of eight fluoroquinolones against enterococci. Clin Microbiol Infect. 1997;3(4):497-499. PubMed
64. Balli EP, Venetis CA, Miyakis S. Systematic Review and Meta-Analysis of Linezolid versus Daptomycin for Treatment of Vancomycin-Resistant Enterococcal Bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-739. PubMed
70. Snydman DR, Jacobus NV, McDermott LA, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007;51(5):1649-1655. PubMed
69. Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis. 2010;50 Suppl 1:S26-33. PubMed
68. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2012;56(3):1247-1252. PubMed
67. Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26(6):1413-1417. PubMed
66. Britt NS, Potter EM, Patel N, Steed ME. Comparison of the Effectiveness and Safety of Linezolid and Daptomycin in Vancomycin-Resistant Enterococcal Bloodstream Infection: A National Cohort Study of Veterans Affairs Patients. Clin Infect Dis. 2015;61(6):871-878. PubMed
65. Chuang YC, Lin HY, Chen PY, et al. Effect of Daptomycin Dose on the Outcome of Vancomycin-Resistant, Daptomycin-Susceptible Enterococcus faecium Bacteremia. Clin Infect Dis. 2017;64(8):1026-1034. PubMed
1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501-509. PubMed
2. Kilgore M, Brossette S. Cost of bloodstream infections. Am J Infect Control. 2008;36(10):S172.e1-3. PubMed
3. Youkee D, Hulme W, Roberts T, Daniels R, Nutbeam T, Keep J. Time Matters: Antibiotic Timing in Sepsis and Septic Shock. Crit Care Med. 2016;44(10):e1016-1017. PubMed
4. Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;28;6:18. PubMed
5. Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, Waybill PN. Venous Thrombosis Associated with the Placement of Peripherally Inserted Central Catheters. J Vasc Interv Radiol. 2000;11(10):1309-1314. PubMed
6. Cheong K, Perry D, Karapetis C, Koczwara B. High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004;34(5):234-238. PubMed
7. Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006;90(6):1197-1222. PubMed
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014;5(2):83-87. PubMed
9. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
10. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
11. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
12. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393-407. PubMed
13. Cua YM, Kripalani S. Medication Use in the Transition from Hospital to Home. Ann Acad Med Singapore. 2008;37(2):136. PubMed
14. Paterson DL. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am J Med. 2006; 119(6):S20-28.
15. Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. PubMed
16. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M Oral vs Intravenous Ciprofloxacin in the Initial Empirical Management of Severe Pyelonephritis or Complicated Urinary Tract Infections: A Prospective Randomized Clinical Trial. Arch Intern Med. 1999;159(1):53-58. PubMed
17. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003237. PubMed
18. Brigmon MM, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Impact of fluoroquinolone resistance in Gram-negative bloodstream infections on healthcare utilization. Clin Microbiol Infect. 2015;21(9):843-849. PubMed
19. Ortega M, Marco F, Soriano A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63(3):568-574. PubMed
20. Lo CL, Lee CC, Li CW, et al. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2017;50(3):355-361. PubMed
21. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the Outcomes of Adults With Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score–Matched Cohort. Clin Infect Dis. 2017; cix767. doi.org/10.1093/cid/cix767 PubMed
22. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection-- 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183-2191. PubMed
23. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 2017;45(5):613-620. PubMed
24. Zelenitsky S, Ariano R, Harding G, Forrest A. Evaluating Ciprofloxacin Dosing for Pseudomonas aeruginosa Infection by Using Clinical Outcome-Based Monte Carlo Simulations. Antimicrob Agents Chemother. 2005;49(10):4009-4014. PubMed
25. Cazaubon Y, Bourguignon L, Goutelle S, Martin O, Maire P, Ducher M. Are ciprofloxacin dosage regimens adequate for antimicrobial efficacy and prevention of resistance? Pseudomonas aeruginosa bloodstream infection in elderly patients as a simulation case study. Fundam Clin Pharmacol. 2015;29(6):615-624. PubMed
26. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob Agents Chemother. 2005;49(4):1306-1311. PubMed
27. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of Combination Antimicrobial Therapy for Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756-2764. PubMed
28. The Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Emerging Infections Program Network Streptococcus pneumoniae, 2013. https://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Published November, 2014. Accessed September 26, 2017.
29. Brandenburg JA, Marrie TJ, Coley CM, et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000;15(9):638-646. PubMed
30. Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000;79(4):210-221. PubMed
31. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001; 161(6):848-850. PubMed
32. Oosterheert JJ, Bonten MJM, Schneider MME, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333(7580):1193. PubMed
33. Austrian R, Winston AL. The efficacy of penicillin V (phenoxymethyl-penicillin) in the treatment of mild and of moderately severe pneumococcal pneumonia. Am J Med Sci. 1956;232(6):624-628. PubMed
34. Waterer GW, Somes GW, Wunderink RG. Monotherapy May Be Suboptimal for Severe Bacteremic Pneumococcal Pneumonia. Arch Intern Med. 2001; 161(15):1837-1842. PubMed
35. Baddour LM, Yu VL, Klugman KP, et al. International Pneumococcal Study Group. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440-444. PubMed
36. Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic streptococcus group g: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112(8):622-626. PubMed
37. Davies HD, McGeer A, Schwartz B, Green, et al; Ontario Group A Streptococcal Study Group. Invasive Group A Streptococcal Infections in Ontario, Canada. N Engl J Med. 1996;335(8):547-554. PubMed
38. Farley MM, Harvey C, Stull T, et al. A Population-Based Assessment of Invasive Disease Due to Group B Streptococcus in Nonpregnant Adults. N Engl J Med. 1993;328(25):1807-1811. PubMed
39. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478-486. PubMed
40. Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo JJ. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994;38(9):2183-2186. PubMed
41. Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096-1100. PubMed
42. Chen I, Kaufisi P, Erdem G. Emergence of erythromycin- and clindamycin-resistant Streptococcus pyogenes emm 90 strains in Hawaii. J Clin Microbiol. 2011;49(1):439-441. PubMed
43. Biedenbach DJ, Jones RN. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25(1):47–51. PubMed
44. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT. Sanford Guide To Antimicrobial Therapy 2017. Dallas, TX. Antimicrobial Theapy, Inc, 2017.
45. Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn Microbiol Infect Dis. 2006;55(2):119-127. PubMed
46. Shurland S, Zhan M, Bradham DD, Roghmann M-C. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):2739. PubMed
47. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56(4):527-535. PubMed
48. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55. PubMed
49. Adra M, Lawrence KR. Trimethoprim/Sulfamethoxazole for Treatment of Severe Staphylococcus aureus Infections. Ann Pharmacother. 2004;38(2):338-341. PubMed
50. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117(5):390-398. PubMed
51. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ. 2015;350:h2219. PubMed
52. Sánchez García M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260-2264. PubMed
53. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481-1490. PubMed
54. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923-929. PubMed
55. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health-Syst Pharm. 2014;71(8):621-633. PubMed
56. Gade ND, Qazi MS. Fluoroquinolone Therapy in Staphylococcus aureus Infections: Where Do We Stand? J Lab Physicians. 2013;5(2):109-112. PubMed
57. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71(3):821-829. PubMed
58. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071-1073. PubMed
59. Schrenzel J, Harbarth S, Schockmel G, et al. A Randomized Clinical Trial to Compare Fleroxacin-Rifampicin with Flucloxacillin or Vancomycin for the Treatment of Staphylococcal Infection. Clin Infect Dis. 2004;39(9):1285-1292. PubMed
60. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. PubMed
61. Antony SJ, Diaz-Vasquez E, Stratton C. Clinical experience with linezolid in the treatment of resistant gram-positive infections. J Natl Med Assoc. 2001;93(10):386-391. PubMed
62. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis. 2015;60(4):528-535. PubMed
63. Martínez-Martínez L, Joyanes P, Pascual A, Terrero E, Perea EJ. Activity of eight fluoroquinolones against enterococci. Clin Microbiol Infect. 1997;3(4):497-499. PubMed
64. Balli EP, Venetis CA, Miyakis S. Systematic Review and Meta-Analysis of Linezolid versus Daptomycin for Treatment of Vancomycin-Resistant Enterococcal Bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-739. PubMed
70. Snydman DR, Jacobus NV, McDermott LA, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007;51(5):1649-1655. PubMed
69. Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis. 2010;50 Suppl 1:S26-33. PubMed
68. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2012;56(3):1247-1252. PubMed
67. Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26(6):1413-1417. PubMed
66. Britt NS, Potter EM, Patel N, Steed ME. Comparison of the Effectiveness and Safety of Linezolid and Daptomycin in Vancomycin-Resistant Enterococcal Bloodstream Infection: A National Cohort Study of Veterans Affairs Patients. Clin Infect Dis. 2015;61(6):871-878. PubMed
65. Chuang YC, Lin HY, Chen PY, et al. Effect of Daptomycin Dose on the Outcome of Vancomycin-Resistant, Daptomycin-Susceptible Enterococcus faecium Bacteremia. Clin Infect Dis. 2017;64(8):1026-1034. PubMed
© 2018 Society of Hospital Medicine