User login
The nuts and bolts of the pursuit to be ever better (Continuous Improvement, Part 2)
The U.S. health care system is renowned for cutting-edge technology and the best rescue care in the world. However, countless shortcomings of our current health care system are well chronicled: We spend twice as much on health care in the United States as do other developed countries, we fail to deliver basic quality of care half the time, we see more than 200,000 patients die annually from safety mishaps, and we waste more than $700 billion per year because of inefficiencies and unnecessary services.
These shortcomings are among some of the reasons for the evolution of the Affordable Care Act. Yet, meaningful transformation of our health care system will not come from legislation. Furthermore, change will not come easily, but will require learning and applying new skills in process improvement to redesign the way we deliver care, and in order to redesign care, those who actually deliver care must lead. The new knowledge and skills of how to redesign care are crucial, or as Henry Ford once stated, "If you always do what you have always done, you will always get what you have always gotten." In last month’s Systemness blog, I introduced process improvement. This month we turn toward gaining a basic understanding of what we mean.
[Read Part 1: What do making airbags and delivering health care have in common? (Continuous Improvement, Part 1)]
Principal No. 1 is that every single thing we do is a process, and process improvement is simply the use of a standard methodology to identify and eliminate waste. Waste can be classified by what author Jay Arthur calls the three D’s: defects, deviation, and delays (Lean Six Sigma for Hospitals, McGraw Hill, 2011.)
But the definition doesn’t stop there. Process improvement must attack waste that impacts the customer, in our case, the patient, the family, and the payer.
When you embark on a process improvement project, you must be able to answer the following questions affirmatively: Will fixing this process improve the access, quality, safety, experience, and/or cost of care? If you start from a position of "how will this help me, the provider?" you could be on the wrong track. We must allow the needs of the customer to drive our improvement.
It’s important to note what’s missing from the definition of process improvement: train and blame, memorization, promises of vigilance, education of patients, and altering patient behavior. These have been basic to patient care for more than a century, and of course they (sometimes to our detriment) have their prominent place in today’s health care delivery. However, process improvement should not depend on them. In the Checklist Manifesto, Atul Gawande makes the case that the complexity of health care delivery has outgrown these; physicians and the systems they operate within can depend on these no longer.
But which methodology is best? The answer to this question is not worth your worry. Three main process improvement methodologies dominate this discussion: lean, six sigma, and plan-do-check-act (PDCA). These methodologies overlap each other in many ways, and the unique aspects complement each other well. At our institution we have incorporated a blended approach using lean management and methodologies adapted to our local culture creating a generic six-phase process improvement framework: project definition, baseline analysis, investigation, improvement design, implementation, monitor. If we had simply adopted six sigma’s methodology (define, measure, analyze, improve, control), we would have been equally successful. Ditto if we had declared ourselves a PDCA institution.
The Bottom Line
For all of our technology, our education, our many bright minds, and for all the money poured into our industry, health care hasn’t met its potential. We have a lot of room to run. America generates $700 billion per year of health care waste. We have a $700 billion opportunity. Payers know this, and they are done overpaying. Reimbursements for physicians and hospitals are at their height. They are falling and will continue to fall.
Financial penalties for preventable errors (is there any other kind?), poor patient satisfaction, and nonstandard care are upon us. Gone are the days of craft-based medicine. The need to understand and apply process improvement is upon us – the golden age of systemness is here.
Dr. Pendleton is chief medical quality officer at University of Utah Health Care in Salt Lake City. He reports having no financial conflicts of interest. Find him on Twitter @MDBobP. Steven Johnson is a value engineer in the Value Engineering program at the University of Utah Health Care. He is a six sigma black belt certified by the American Society for Quality. Steve has been a lean six sigma practitioner for 18 years in aerospace, automotive, chemical, construction, and healthcare. Steve has a mechanical engineering degree, an MBA, and a graduate certificate in predictive analytics.
The U.S. health care system is renowned for cutting-edge technology and the best rescue care in the world. However, countless shortcomings of our current health care system are well chronicled: We spend twice as much on health care in the United States as do other developed countries, we fail to deliver basic quality of care half the time, we see more than 200,000 patients die annually from safety mishaps, and we waste more than $700 billion per year because of inefficiencies and unnecessary services.
These shortcomings are among some of the reasons for the evolution of the Affordable Care Act. Yet, meaningful transformation of our health care system will not come from legislation. Furthermore, change will not come easily, but will require learning and applying new skills in process improvement to redesign the way we deliver care, and in order to redesign care, those who actually deliver care must lead. The new knowledge and skills of how to redesign care are crucial, or as Henry Ford once stated, "If you always do what you have always done, you will always get what you have always gotten." In last month’s Systemness blog, I introduced process improvement. This month we turn toward gaining a basic understanding of what we mean.
[Read Part 1: What do making airbags and delivering health care have in common? (Continuous Improvement, Part 1)]
Principal No. 1 is that every single thing we do is a process, and process improvement is simply the use of a standard methodology to identify and eliminate waste. Waste can be classified by what author Jay Arthur calls the three D’s: defects, deviation, and delays (Lean Six Sigma for Hospitals, McGraw Hill, 2011.)
But the definition doesn’t stop there. Process improvement must attack waste that impacts the customer, in our case, the patient, the family, and the payer.
When you embark on a process improvement project, you must be able to answer the following questions affirmatively: Will fixing this process improve the access, quality, safety, experience, and/or cost of care? If you start from a position of "how will this help me, the provider?" you could be on the wrong track. We must allow the needs of the customer to drive our improvement.
It’s important to note what’s missing from the definition of process improvement: train and blame, memorization, promises of vigilance, education of patients, and altering patient behavior. These have been basic to patient care for more than a century, and of course they (sometimes to our detriment) have their prominent place in today’s health care delivery. However, process improvement should not depend on them. In the Checklist Manifesto, Atul Gawande makes the case that the complexity of health care delivery has outgrown these; physicians and the systems they operate within can depend on these no longer.
But which methodology is best? The answer to this question is not worth your worry. Three main process improvement methodologies dominate this discussion: lean, six sigma, and plan-do-check-act (PDCA). These methodologies overlap each other in many ways, and the unique aspects complement each other well. At our institution we have incorporated a blended approach using lean management and methodologies adapted to our local culture creating a generic six-phase process improvement framework: project definition, baseline analysis, investigation, improvement design, implementation, monitor. If we had simply adopted six sigma’s methodology (define, measure, analyze, improve, control), we would have been equally successful. Ditto if we had declared ourselves a PDCA institution.
The Bottom Line
For all of our technology, our education, our many bright minds, and for all the money poured into our industry, health care hasn’t met its potential. We have a lot of room to run. America generates $700 billion per year of health care waste. We have a $700 billion opportunity. Payers know this, and they are done overpaying. Reimbursements for physicians and hospitals are at their height. They are falling and will continue to fall.
Financial penalties for preventable errors (is there any other kind?), poor patient satisfaction, and nonstandard care are upon us. Gone are the days of craft-based medicine. The need to understand and apply process improvement is upon us – the golden age of systemness is here.
Dr. Pendleton is chief medical quality officer at University of Utah Health Care in Salt Lake City. He reports having no financial conflicts of interest. Find him on Twitter @MDBobP. Steven Johnson is a value engineer in the Value Engineering program at the University of Utah Health Care. He is a six sigma black belt certified by the American Society for Quality. Steve has been a lean six sigma practitioner for 18 years in aerospace, automotive, chemical, construction, and healthcare. Steve has a mechanical engineering degree, an MBA, and a graduate certificate in predictive analytics.
The U.S. health care system is renowned for cutting-edge technology and the best rescue care in the world. However, countless shortcomings of our current health care system are well chronicled: We spend twice as much on health care in the United States as do other developed countries, we fail to deliver basic quality of care half the time, we see more than 200,000 patients die annually from safety mishaps, and we waste more than $700 billion per year because of inefficiencies and unnecessary services.
These shortcomings are among some of the reasons for the evolution of the Affordable Care Act. Yet, meaningful transformation of our health care system will not come from legislation. Furthermore, change will not come easily, but will require learning and applying new skills in process improvement to redesign the way we deliver care, and in order to redesign care, those who actually deliver care must lead. The new knowledge and skills of how to redesign care are crucial, or as Henry Ford once stated, "If you always do what you have always done, you will always get what you have always gotten." In last month’s Systemness blog, I introduced process improvement. This month we turn toward gaining a basic understanding of what we mean.
[Read Part 1: What do making airbags and delivering health care have in common? (Continuous Improvement, Part 1)]
Principal No. 1 is that every single thing we do is a process, and process improvement is simply the use of a standard methodology to identify and eliminate waste. Waste can be classified by what author Jay Arthur calls the three D’s: defects, deviation, and delays (Lean Six Sigma for Hospitals, McGraw Hill, 2011.)
But the definition doesn’t stop there. Process improvement must attack waste that impacts the customer, in our case, the patient, the family, and the payer.
When you embark on a process improvement project, you must be able to answer the following questions affirmatively: Will fixing this process improve the access, quality, safety, experience, and/or cost of care? If you start from a position of "how will this help me, the provider?" you could be on the wrong track. We must allow the needs of the customer to drive our improvement.
It’s important to note what’s missing from the definition of process improvement: train and blame, memorization, promises of vigilance, education of patients, and altering patient behavior. These have been basic to patient care for more than a century, and of course they (sometimes to our detriment) have their prominent place in today’s health care delivery. However, process improvement should not depend on them. In the Checklist Manifesto, Atul Gawande makes the case that the complexity of health care delivery has outgrown these; physicians and the systems they operate within can depend on these no longer.
But which methodology is best? The answer to this question is not worth your worry. Three main process improvement methodologies dominate this discussion: lean, six sigma, and plan-do-check-act (PDCA). These methodologies overlap each other in many ways, and the unique aspects complement each other well. At our institution we have incorporated a blended approach using lean management and methodologies adapted to our local culture creating a generic six-phase process improvement framework: project definition, baseline analysis, investigation, improvement design, implementation, monitor. If we had simply adopted six sigma’s methodology (define, measure, analyze, improve, control), we would have been equally successful. Ditto if we had declared ourselves a PDCA institution.
The Bottom Line
For all of our technology, our education, our many bright minds, and for all the money poured into our industry, health care hasn’t met its potential. We have a lot of room to run. America generates $700 billion per year of health care waste. We have a $700 billion opportunity. Payers know this, and they are done overpaying. Reimbursements for physicians and hospitals are at their height. They are falling and will continue to fall.
Financial penalties for preventable errors (is there any other kind?), poor patient satisfaction, and nonstandard care are upon us. Gone are the days of craft-based medicine. The need to understand and apply process improvement is upon us – the golden age of systemness is here.
Dr. Pendleton is chief medical quality officer at University of Utah Health Care in Salt Lake City. He reports having no financial conflicts of interest. Find him on Twitter @MDBobP. Steven Johnson is a value engineer in the Value Engineering program at the University of Utah Health Care. He is a six sigma black belt certified by the American Society for Quality. Steve has been a lean six sigma practitioner for 18 years in aerospace, automotive, chemical, construction, and healthcare. Steve has a mechanical engineering degree, an MBA, and a graduate certificate in predictive analytics.
How safe is your hospital? Measuring patient safety is not so straightforward
Florence Nightingale once quipped, "The first duty of a hospital is that it should do the sick no harm." Yet the 1998 Institute of Medicine report, "To Err is Human," revealed that hospitalized patients all too frequently suffer the consequences of mishaps; some 44,000-98,000 patients every year die from medical mistakes.
Despite this troubling revelation and the subsequent flourishing field of patient safety (which can be defined as the prevention, avoidance, and amelioration of adverse outcomes originating from the processes of care delivery), little measurable progress has been made in patient safety in the United States during the past 15 years.
Certainly, success stories exist, such as the actualization that central line infection rates can be reduced to near zero with proper processes, procedures, and culture. Yet demonstration of broad-based impact of the patient safety field has been lacking and the cause is multifactorial: Patient safety is a relatively infant field, and suboptimal safety culture and team-based care, lack of knowledge and competency in safety science, and inadequate (or even perverse) incentives all likely play a role.
However, a more important contributing factor may be the inadequacy of routine safety measurement.
After all, measurement is the first step toward gaining the knowledge and control, which in turn leads to improvement. For many, safety measurement is simply defined by benchmarked metrics of harm occurrence (e.g. infection rates, falls, etc.). Assessing safety by these reactive measures, although informative, does not by itself tell us how dangerous it is now or will be in the future. The narrow view of defining safety based upon these lagging indicators –indicators that define safety just by those events that have already reached a patient – would be analogous to the nuclear power industry defining safety solely by the frequency of catastrophic events. To more meaningfully understand the safety in our hospitals, these rear-view metrics should be balanced with proactive measures of prevention and reliability, i.e., leading indicators. The essence of leading indicators (such as the results of a safety culture survey, information obtained during safety walk rounds, and system audits) is that they are proactive. They measure variables that are believed to be indicators or precursors of safety performance so that safety is achieved and maintained before harm actually occurs.
A proposed minimum safety measurement set for a hospital or care delivery unit is as follows:
Leading indicators of safety:
• Safety culture survey results.
• Safety walk rounds.
• Clinical care audits.
Lagging indicators of safety:
• Mortality.
• Hospital-acquired conditions.
• Incident reporting.
• Medication errors.
• Malpractice claims/patient complaints.
• Diagnostic errors.
• Appropriateness of care.
To truly know if our hospital (or clinic, or inpatient unit, or health care system) is safe, we need to be able to understand not only if safety has been demonstrated in the past, but also if it is being demonstrated in the present, and will be in the future; if care delivery is reliable; and if the system is learning from harm or errors in process.
Only through such a multiperspective measurement dashboard to shape culture and drive performance will health care truly achieve the performance that our patients deserve and that will allow us to uphold our moral and professional obligation of primum non nocere.
What safety measurements are you and your hospital using to achieve this goal?
Dr. Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City, and a member of the Hospitalist News advisory board.
Florence Nightingale once quipped, "The first duty of a hospital is that it should do the sick no harm." Yet the 1998 Institute of Medicine report, "To Err is Human," revealed that hospitalized patients all too frequently suffer the consequences of mishaps; some 44,000-98,000 patients every year die from medical mistakes.
Despite this troubling revelation and the subsequent flourishing field of patient safety (which can be defined as the prevention, avoidance, and amelioration of adverse outcomes originating from the processes of care delivery), little measurable progress has been made in patient safety in the United States during the past 15 years.
Certainly, success stories exist, such as the actualization that central line infection rates can be reduced to near zero with proper processes, procedures, and culture. Yet demonstration of broad-based impact of the patient safety field has been lacking and the cause is multifactorial: Patient safety is a relatively infant field, and suboptimal safety culture and team-based care, lack of knowledge and competency in safety science, and inadequate (or even perverse) incentives all likely play a role.
However, a more important contributing factor may be the inadequacy of routine safety measurement.
After all, measurement is the first step toward gaining the knowledge and control, which in turn leads to improvement. For many, safety measurement is simply defined by benchmarked metrics of harm occurrence (e.g. infection rates, falls, etc.). Assessing safety by these reactive measures, although informative, does not by itself tell us how dangerous it is now or will be in the future. The narrow view of defining safety based upon these lagging indicators –indicators that define safety just by those events that have already reached a patient – would be analogous to the nuclear power industry defining safety solely by the frequency of catastrophic events. To more meaningfully understand the safety in our hospitals, these rear-view metrics should be balanced with proactive measures of prevention and reliability, i.e., leading indicators. The essence of leading indicators (such as the results of a safety culture survey, information obtained during safety walk rounds, and system audits) is that they are proactive. They measure variables that are believed to be indicators or precursors of safety performance so that safety is achieved and maintained before harm actually occurs.
A proposed minimum safety measurement set for a hospital or care delivery unit is as follows:
Leading indicators of safety:
• Safety culture survey results.
• Safety walk rounds.
• Clinical care audits.
Lagging indicators of safety:
• Mortality.
• Hospital-acquired conditions.
• Incident reporting.
• Medication errors.
• Malpractice claims/patient complaints.
• Diagnostic errors.
• Appropriateness of care.
To truly know if our hospital (or clinic, or inpatient unit, or health care system) is safe, we need to be able to understand not only if safety has been demonstrated in the past, but also if it is being demonstrated in the present, and will be in the future; if care delivery is reliable; and if the system is learning from harm or errors in process.
Only through such a multiperspective measurement dashboard to shape culture and drive performance will health care truly achieve the performance that our patients deserve and that will allow us to uphold our moral and professional obligation of primum non nocere.
What safety measurements are you and your hospital using to achieve this goal?
Dr. Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City, and a member of the Hospitalist News advisory board.
Florence Nightingale once quipped, "The first duty of a hospital is that it should do the sick no harm." Yet the 1998 Institute of Medicine report, "To Err is Human," revealed that hospitalized patients all too frequently suffer the consequences of mishaps; some 44,000-98,000 patients every year die from medical mistakes.
Despite this troubling revelation and the subsequent flourishing field of patient safety (which can be defined as the prevention, avoidance, and amelioration of adverse outcomes originating from the processes of care delivery), little measurable progress has been made in patient safety in the United States during the past 15 years.
Certainly, success stories exist, such as the actualization that central line infection rates can be reduced to near zero with proper processes, procedures, and culture. Yet demonstration of broad-based impact of the patient safety field has been lacking and the cause is multifactorial: Patient safety is a relatively infant field, and suboptimal safety culture and team-based care, lack of knowledge and competency in safety science, and inadequate (or even perverse) incentives all likely play a role.
However, a more important contributing factor may be the inadequacy of routine safety measurement.
After all, measurement is the first step toward gaining the knowledge and control, which in turn leads to improvement. For many, safety measurement is simply defined by benchmarked metrics of harm occurrence (e.g. infection rates, falls, etc.). Assessing safety by these reactive measures, although informative, does not by itself tell us how dangerous it is now or will be in the future. The narrow view of defining safety based upon these lagging indicators –indicators that define safety just by those events that have already reached a patient – would be analogous to the nuclear power industry defining safety solely by the frequency of catastrophic events. To more meaningfully understand the safety in our hospitals, these rear-view metrics should be balanced with proactive measures of prevention and reliability, i.e., leading indicators. The essence of leading indicators (such as the results of a safety culture survey, information obtained during safety walk rounds, and system audits) is that they are proactive. They measure variables that are believed to be indicators or precursors of safety performance so that safety is achieved and maintained before harm actually occurs.
A proposed minimum safety measurement set for a hospital or care delivery unit is as follows:
Leading indicators of safety:
• Safety culture survey results.
• Safety walk rounds.
• Clinical care audits.
Lagging indicators of safety:
• Mortality.
• Hospital-acquired conditions.
• Incident reporting.
• Medication errors.
• Malpractice claims/patient complaints.
• Diagnostic errors.
• Appropriateness of care.
To truly know if our hospital (or clinic, or inpatient unit, or health care system) is safe, we need to be able to understand not only if safety has been demonstrated in the past, but also if it is being demonstrated in the present, and will be in the future; if care delivery is reliable; and if the system is learning from harm or errors in process.
Only through such a multiperspective measurement dashboard to shape culture and drive performance will health care truly achieve the performance that our patients deserve and that will allow us to uphold our moral and professional obligation of primum non nocere.
What safety measurements are you and your hospital using to achieve this goal?
Dr. Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City, and a member of the Hospitalist News advisory board.
Continuing Medical Education Program in
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
Periprocedural Antithrombotic Management
The management of patients on long‐term antithrombotic therapy (vitamin K antagonists [VKA] or antiplatelet agents) who may require temporary disruption for an invasive procedure is challenging. Management is controversial due to methodologically limited prospective data and varied consensus opinions. Yet periprocedural anticoagulation management is a commonly encountered clinical problem. It is estimated that there are 2.5 million patients on long‐term VKA therapy in North America1 and 41% of the U.S. population over age 40 years is on antiplatelet therapy.2 Further, the need for temporary disruption of these therapies for an invasive procedure is frequent. As an example, in 1 European study, approximately 15% of patients on long‐term VKA required a major surgical procedure in 4 years of follow‐up.3 The role of the hospitalist physician in managing these patients is increasing as hospitalists care for an increasing number of surgical patients and provide periprocedural consultation both in and out of the hospital. Therefore, it is imperative for the hospitalist physician to be proficient in making thoughtful and individualized recommendations on the appropriate management of periprocedural anticoagulants, drawing from the available literature and evidence‐based practice guidelines. Importantly, the Society of Hospital Medicine has cited perioperative management as an important core competency.4
The hospitalist physician is likely to encounter numerous periprocedural scenarios, including the management of antiplatelet agents, identifying low bleeding risk procedures wherein interruption of anticoagulants is unnecessary, and recognizing patients with a low short‐term thromboembolic risk where anticoagulants can be disrupted without the need for heparin or low molecular weight heparin (LMWH) in the periprocedural period (defined as bridging therapy). Further, all other clinical scenarios require both a careful individualized assessment of the patient's risk of periprocedural bleeding and thromboembolism and a thoughtful discussion with all involved parties. This discussion may involve the person performing the procedure, the anesthesiologist, and the patient. The purpose of this work is to explore these relevant areas through a review of the literature with a particular focus on the recently published 2008 American College of Chest Physicians (ACCP) evidence‐based clinical practice guidelines.
We reviewed medical literature from 1990 through May 2008 with the following key words: bridging, anticoagulation, perioperative, antiplatelet, heparin, and low molecular weight heparin. Individual studies were then independently reviewed by the authors. Studies that were felt relevant to a hospitalist physician were retrieved and reviewed. If there was uncertainty regarding applicability to a hospitalist setting, a second author's opinion was rendered. Additionally, we reviewed 1 author's personal reference list of articles relating to periprocedural anticoagulation that has been compiled over the past 10 years. This list and the reference lists of retrieved articles were also reviewed. Data were summarized to answer 4 clinically relevant questions:
-
What is the optimal management of antiplatelet therapy in the periprocedural period?
-
Are there very low‐bleeding risk procedures that do not require interruption of oral anticoagulation?
-
Are there low thromboembolic risk populations who do not require periprocedural bridging?
-
How do you manage patients who must discontinue anticoagulants but are at an increased thrombotic risk?
Clinical Question #1: What Is the Optimal Management of Antiplatelet Therapy in the Periprocedural Period?
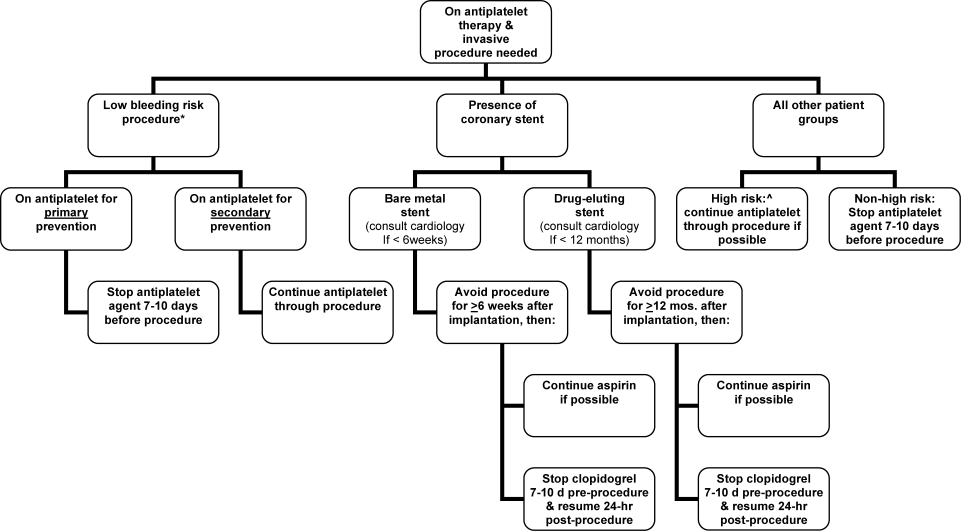
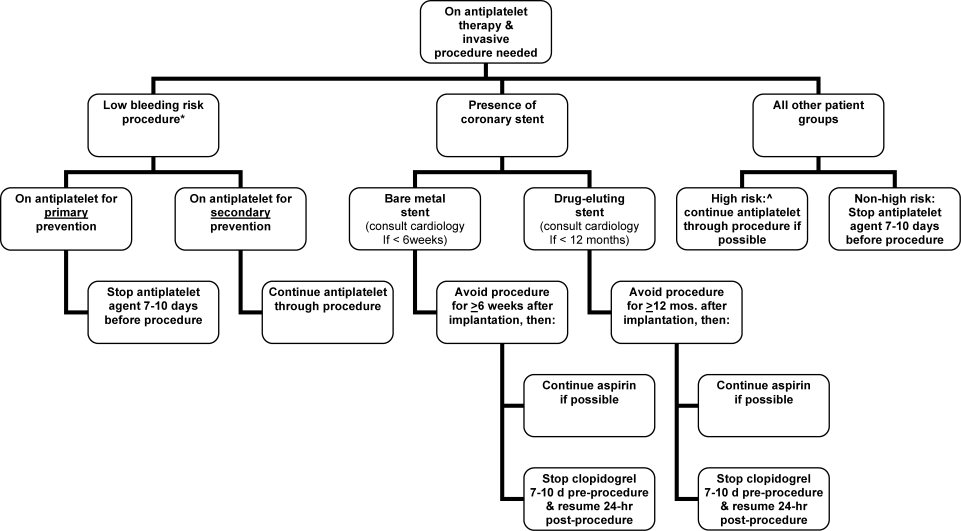
The optimal management of oral antiplatelet therapy in the periprocedural period is not well studied. Most reviews, expert recommendations, and consensus statements either do not comment on periprocedural antiplatelet management or recommend the routine discontinuation of therapy at least 7 days prior to surgery.3, 5, 6 However, as the 2008 ACCP guidelines highlight, the recommendation to routinely discontinue antiplatelet therapy 7 days prior to the procedure is an oversimplification.1 In the era of both bare metal cardiac stents and drug‐eluting stents, the optimal management of these patients requires that 2 primary questions be asked: (1) Is this a low‐bleeding risk procedure whereby antiplatelet therapy can be continued? (2) Does the patient have a coronary stent whereby the continuation of antiplatelet therapy or delay of the intervention is necessary?
In the context of ongoing aspirin therapy, certain procedures have a low risk of significant hemorrhagic complications. These low bleeding risk procedures include cataract surgery, cutaneous surgery, oral surgery, and endoscopic procedures, including those with mucosal biopsies.710 Patients undergoing these procedures may safely continue low dose aspirin therapy, especially if they have a high‐risk indication for their aspirin such as recent myocardial infarction, stroke, or the presence of a coronary stent.5, 710 Whether these procedures can be safely performed in the setting of a thienopyridine or combination antiplatelet therapy is uncertain.
In the past several decades, the management of obstructive coronary artery disease has undergone a major evolution. Placement of coronary stents has become commonplace, and there are now several million patients with drug‐eluting stents.11 The major complication of these devices is stent thrombosis, which results in death or myocardial infarction in up to 64% of patients.12 Fortunately, dual antiplatelet therapy (aspirin and a thienopyridine such as clopidogrel) markedly reduces this risk.13 Current guidelines recommend using combination antiplatelet therapy for at least 4 to 6 weeks and ideally up to 12 months after placement of a bare metal stent and at least 12 months after placement of either a sirolimus‐ or paclitaxel‐eluting stent.1, 14 During this period of dual antiplatelet therapy, the premature discontinuation of the thienopyridine may be catastrophic. To guide clinicians in managing these patients in the periprocedural period, recent consensus guidelines recommend the following:1, 12
-
In patients who are expected to need an invasive surgical procedure in the next 12 months, consideration should be given to avoiding drug‐eluting stents.
-
Elective procedures which have an increased risk of bleeding should be deferred for at least 6 weeks after bare metal stent implantation and 12 months after drug‐eluting stent implantation.
-
For patients undergoing a surgical procedure within 6 weeks of bare metal stent implantation and 12 months of drug‐eluting stent implantation, continuation of aspirin and clopidogrel is recommended. If bleeding risk prohibits this, then a cardiologist should be consulted.
-
In patients with a drug‐eluting stent who need to undergo a procedure whereby the thienopyridine needs to be discontinued, aspirin should be continued if at all possible, and the thienopyridine should be resumed as soon as possible after the procedure. It may be reasonable to consider a loading dose of clopidogrel, up to 600 mg, in this setting, although prospective supportive data is lacking.1
It is important to recognize that delayed stent thrombosis is now reported well beyond 1 year after drug‐eluting stent implantation, and that there may not be a diminution in risk after the initial 12 months.1517 Until additional data is available, it seems prudent, if possible, to at least continue aspirin in the periprocedural period in these patients. If bleeding concerns obviate this, then antiplatelet therapy should be discontinued and resumed as soon as possible.
For patients on chronic antiplatelet therapy who do not have a cardiac stent and who are not undergoing a low‐bleeding‐risk procedure, the risks and benefits of the continuation or discontinuation of antiplatelet therapy in the periprocedural period are uncertain as absolute risks in the periprocedural period have not been well studied. Relative risks/benefits, however, can be estimated from prior studies. Aspirin leads to an approximate 25% relative risk reduction in cardiac or thrombotic event rates compared to placebo.14, 18 Although important, the absolute benefit of 1 week of therapy (vs. no therapy during the periprocedural period) is estimated to be small. The small absolute benefit of continued aspirin therapy may be offset by an increase in significant bleeding events. Although, not well studied, continued aspirin increases significant bleeding by 50% with absolute event rates varying by type of procedure.8 In some procedures, such as intracranial surgery or transurethral prostatectomy, this bleeding risk is prohibitive. For others, the risk may be modest and the decision to continue vs. discontinue aspirin therapy may be at the discretion of the person performing the procedure. In general, for most patients who do not have a coronary stent and have not had a recent (past 3 months) myocardial infarction or stroke, discontinuation of antiplatelet therapy 7 to 10 days prior to the procedure seems prudent. The primary exceptions are patients who are undergoing percutaneous coronary intervention or coronary artery bypass grafting. For these procedures continuing aspirin is recommended.1 Figure 1 outlines a proposed management strategy based upon available evidence and guidelines.
Clinical Question #2: Are There Very‐Low‐Bleeding‐Risk Procedures That Do Not Require Interruption of Oral Anticoagulation?
Some procedures are associated with a low‐enough risk of bleeding that it is safe to proceed without interrupting VKA anticoagulation. This approach spares the risk and cost that occur with the holding of oral anticoagulants and institution of bridging therapy. When considering this strategy, it is important that the specialist performing the procedure is included in the discussion. Dental, dermatologic, and cataract procedures are common outpatient procedures that are associated with low bleeding risk. The relative safety of these procedures in patients who are anticoagulated is discussed thoroughly in the ACCP guidelines.1 Other low‐bleeding‐risk procedures for which a hospitalist may be consulted include certain endoscopic procedures, paracentesis, central venous catheter placement, and arthrocentesis.
The American Society for Gastrointestinal Endoscopy has published guidelines recommending that anticoagulation can be safely continued in patients undergoing the following endoscopic procedures with a low bleeding risk: esophagogastroduodenoscopy (EGD), flexible sigmoidoscopy, and colonoscopy, all with or without mucosal biopsy; enteroscopy, biliary/pancreatic stent placement, endoscopic ultrasound without biopsy, and endoscopic retrograde cholangiopancreatography (ERCP) without sphincterotomy.19 Conversely, high‐risk procedures for which interruption of anticoagulation is recommended include polypectomy, biliary sphincterotomy, variceal treatment, percutaneous endoscopic gastrostomy (PEG) placement, dilation of strictures, and endoscopic ultrasound‐guided fine‐needle aspiration.
Limited data suggest that paracentesis, central venous catheter placement, and arthrocentesis may be safe to perform in the setting of anticoagulation. For patients undergoing paracentesis there is little evidence in anticoagulated patients; however, it is probably safe to continue anticoagulation as studies have demonstrated the safety of this procedure in patients with significant thrombocytopenia and coagulopathy.20, 21 Limited data also supports that central venous catheter placement may be safely performed in the setting of abnormal coagulation tests, although some recommend avoiding the subclavian site due to the risk of hemothorax and the inability to apply adequate compression.2226 With regard to arthrocentesis, multiple authors have endorsed the idea that joint and soft‐tissue aspirations and injections present a low risk of serious bleeding even with anticoagulation.2729 This is supported by limited data.30, 31
Other procedures such as lumbar puncture, thoracentesis, and cardiac catheterization are somewhat more controversial in the anticoagulated patient. Anticoagulation should generally be interrupted for lumbar puncture,29, 32 as 1 study involving patients who were started on heparin immediately after the procedure had a 2% incidence of spinal hematoma and 6.7% major complication rate.33 With regard to thoracentesis, evidence is very limited, but experts generally accept that it may be safely performed in patients with mild coagulopathy.34, 35 One frequently‐cited study found no bleeding complications in 57 patients with mild elevation in prothrombin time, which correlated to an International Normalized Ratio of approximately 2.2 or less.36 A recent report also revealed no serious bleeding complications in 33 thoracenteses performed on patients receiving full anticoagulation with warfarin, heparin, and/or low molecular weight heparin.37
Therapeutic anticoagulation has traditionally been felt to be a relative contraindication to cardiac catheterization.38, 39 In spite of this, several observational studies have suggested it may be safely performed using a standard approach,40 using vascular closure devices,41 or using a radial artery approach instead of the more commonly used femoral site.4244 The small size of these observational reports, the diagnostic rather than therapeutic nature of most cases, the limited use of other antithrombotic and antiplatelet medications, and the experience required to use the transradial approach are all major limitations preventing widespread acceptance of cardiac catheterization in therapeutically anticoagulated patients.
In summary, there are numerous procedures that may be safely pursued in the setting of therapeutic anticoagulation. However, for most of these procedures the data is somewhat limited. As such, it is paramount for the hospitalist physician to recognize these clinical scenarios and to discuss management options with the patient and the person performing the procedure, if applicable.
Clinical Question #3: Are There LowThromboembolic‐Risk Populations Who Do Not Require Periprocedural Bridging?
Although it has previously been noted that there is a wide variation of opinion on when and how to perform periprocedural bridging, it is generally agreed that in the following conditions the risk of thrombosis is low enough that bridging with full dose heparin or LMWH is not necessary:1, 5, 4549
-
Atrial fibrillation without previous stroke or transient ischemic attack (TIA) and no more than 2 additional thrombotic risk factors on the CHADS2 scoring system (Table 1).
-
A single venous thromboembolic event that occurred greater than 12 months ago with no ongoing risk factors such as active malignancy, high risk thrombophilia, or the antiphospholipid antibody syndrome.
-
Bileaflet aortic valve without the presence of additional risk factors (ie, patients <75 years of age with the absence of atrial fibrillation, prior stroke or transient ischemic attack, hypertension, diabetes, or congestive heart failure).
| CHADS2 Score* | Annual Risk of Stroke (%) |
|---|---|
| |
| 0 | 1.9 |
| 1 | 2.8 |
| 2 | 4.0 |
| 3 | 5.9 |
| 4 | 8.5 |
| 5 | 12.5 |
| 6 | 18.2 |
Clinical Question #4: How Do You Manage Patients Who Must Discontinue Anticoagulants But Are at an Increased Thrombotic Risk?
When anticoagulation must be held and the patient does not have a very low thromboembolic risk, a decision of whether or not to use bridging anticoagulation must be made. The current ACCP guideline gives grade 1C and 2C recommendations (evidence from observational studies, case series, or controlled trials with serious flaws) regarding for whom and how to implement bridging.1 The grade C designation is due to a lack of high‐quality randomized clinical trials. As such, the clinician must carefully consider an individual patient's estimated thromboembolic risk, procedurally‐related bleeding risk, patient‐related bleeding risk factors, and the patient's values regarding concerns of thromboembolism or bleeding. In these situations it is also imperative that the person performing the procedure is involved in the risk‐to‐benefit discussion.
When evaluating an individual patient's risk of thromboembolism, clinicians sometimes estimate the perioperative risk by prorating the annual incidence of thromboembolic complications to the few days that anticoagulation is withheld.67 Making this extrapolation discounts the effect of a potential increase in thromboembolic risk induced by surgery. As an example, an average patient with atrial fibrillation who has a 5% predicted annual stroke rate would be estimated to have a stroke risk of 0.05% if they are not anticoagulated for 4 days. However, studies have shown that the actual rate of perioperative thromboembolism is approximately 1%.1 With these limitations and uncertainties in mind, and until there is better prospective outcomes data, we must consider relative risks in the context of absolute event rate estimates when deciding a perioperative anticoagulant management plan. The estimated annual incidence of thrombosis without anticoagulation for various indications and the current guideline recommendations are presented in Table 2.
| Practice Guideline Preferred Management Recommendations | ||||
|---|---|---|---|---|
| Indication for chronic anticoagulation | Estimated Annual Thrombotic Risk Without Anticoagulation | ACCP*1 | ACC/AHA45, 46 | British Haematologic Society70 |
| ||||
| Dual prosthetic or older‐generation valve | >10% | Bridge | Bridge | Bridge |
| VTE within 3 months or severe thrombophilias | Bridge | N/A | Bridge | |
| Pregnancy with prosthetic valve | Bridge | Bridge | N/A | |
| Bileaflet valve in the mitral position | Bridge | Bridge | Prophylaxis | |
| Valve with acute embolism <6 months | Bridge | N/A | Bridge | |
| A‐fib valvular or CHADS2 score 5‐6 | Bridge | Consider bridging | N/A | |
| Recurrent venous thromboembolism | 4‐10% | Bridge | N/A | N/A |
| VTE within 3‐12 months or active cancer | Bridge | N/A | Prophylaxis | |
| Bileaflet aortic valve with additional risk factors | Bridge | Bridge | Prophylaxis | |
| A‐fib CHADS2 score 3‐4 | Bridge | Consider bridging | N/A | |
| Bileaflet aortic valve without additional risk factors | <4% | Prophylaxis or no bridging | No bridging | Prophylaxis |
| VTE >12 months | Prophylaxis or no bridging | N/A | Prophylaxis | |
| A‐fib CHADS2 score 0‐2 and no previous CVA/TIA | Prophylaxis or no bridging | No bridging | N/A | |
In addition to thromboembolic risk, we must also consider the bleeding risk associated with the procedure/surgery. Importantly, therapeutic heparin started early in the postoperative period is associated with major bleeding event rates as high as 10% to 20%.1, 50 Once a major bleeding event occurs, this will often lead to an extended interruption of anticoagulant therapy, placing the patient at a more prolonged risk of an associated thromboembolic event. For this reason, the resumption of full‐dose anticoagulation with LMWH/heparin should be delayed for at least 48 hours in most patients undergoing a surgery or procedure associated with an increased risk of bleeding. Examples of these higher‐bleeding‐risk procedures include major thoracic surgery, intracranial or spinal surgery, major vascular surgery, major orthopedic surgery, urologic surgery involving the bladder or prostrate, major oncologic surgery, reconstructive plastic surgery, colonoscopy with associated polypectomy, renal or prostate biopsies, and placement of a cardiac pacemaker/defibrillator.1, 5157
Taken together, these uncertainties surrounding thromboembolic and bleeding risk estimates imply that there are multiple options for periprocedural management. Several studies, many of which included patients with mechanical heart valves, have shown similar safety and efficacy between LMWH and intravenous (IV) unfractionated heparin.5864 Table 3 summarizes these studies. The ACCP recommends bridging with LMWH over IV unfractionated heparin due to equal efficacy and cost savings with LMWH.1 When bridging is used, careful attention must be given to the timing and dose of anticoagulation in both the preoperative and postoperative periods. Table 4 lists dosing of commonly used LMWHs in North America. When using LMWHs in the preprocedural setting it is important to note that unacceptably high levels of anticoagulation remain present when a patient is given a full once‐daily LMWH dose the morning prior to the procedure or when a full‐dose, twice‐daily LMWH dose is given the evening prior to the procedure.65, 66 For this reason, the ACCP recommends administering the last preoperative dose 24 hours before surgery and if full‐dose once‐daily LMWH is used, the dose should be decreased by one‐half on the day before the surgery in order to ensure that no residual anticoagulant effect remains at the time of surgery.
| AuthorReference/Study Type | Number of Patients | Patient Population | Type of Procedure | Bridging Strategy | Major Bleeds | Minor Bleeds | TE Rate |
|---|---|---|---|---|---|---|---|
| |||||||
| Turpie and Douketis63/single arm cohort | 174 | 66% aortic valve; 34% mitral or dual prosthetic valve | Not specified | Enoxaparin 1 mg/kg twice daily | 2.3% | Not specified | None |
| Kovacs et al.61/single arm cohort | 224 | Prosthetic heart valves or a‐fib plus 1 major risk factor | 67 surgical; 157 nonsurgical | Preoperative bridging with dalteparin 200 IU/kg daily; dose reduced to 100 IU/kg on preoperative day 1; restarted at 100 IU/kg on POD 1; dose reduced to 5000 IU daily if high risk for bleeding | 6.7%; 8/15 occurred intraoperatively or <6 hours postoperatively; 2/15 occurred after 4 weeks | Not specified | 3.6%; 6/8 episodes occurred after warfarin held secondary to bleeding; 2/8 thrombotic episodes judged to be due to cardioembolism |
| Douketis et al.59/prospective registry | 650 | A‐fib 58%; mechanical heart valve 33% | 251 surgical; 399 nonsurgical | Dalteparin 100 IU/kg twice daily; held after high bleeding risk procedure and patients with poor hemostasis | 0.92% | 5.9% | 0.6% |
| Spyropolous et al.62/prospective registry; 14 centers in United States and Canada | 901 | UFH: 40% mechanical valves, 33% a‐fib; LMWH: 24% mechanical valve, 40% a‐fib | 394 surgical; 507 nonsurgical | LMWH mostly given twice daily 80%; UFH 20% | 5.5% UFH; 3.3% LMWH | 9.1% UFH; 12.0% LMWH | 2.4% UFH; 0.9% LMWH |
| Dunn et al.66/prospective cohort | 260 | A‐fib 68% or prior DVT 37% (excluding prosthetic heart valves) | 105 surgical; 145 nonsurgical | Enoxaparin 1.5 mg/kg daily | 3.5% overall; minor surgery/procedures 0.9%; major surgery 28% | 42% | 1.9%; 1/5 events occurred after bleeding led to withdrawal of AC |
| Omran et al.77/prospective registry | 779 | Various indications | Major and minor procedures | All patients bridged with enoxaparin; moderate TE risk 1 mg/kg daily; high TE risk 1 mg/kg twice daily | 0.5%; all in high‐risk group | 5.9% | 0 |
| Garcia et al.71/prospective, observational cohort of 101 sites in United States | 1024 patients with 1293 interruptions of AC | A‐fib 53%; VTE 14%; prosthetic valve 13% | Outpatient procedures only | At discretion of provider. Bridging performed in 8.3% of interruptions; 3% a‐fib, 10% VTE, and 29% mechanical valves | 0.6%; 4/6 patients with major bleed received bridging | 1.7%;10/17 patients with minor bleed received bridging | 0.7%; no events in patients who were bridged |
| Wysokinski et al.64/prospective cohort | 345 consecutive patients undergoing 386 procedures | 100% nonvalvular a‐fib | Major and minor surgeries/procedures | Individualized in AC clinic; 52% of patients bridged | 2.7%; no difference whether patient received bridging or not | 3.0%; 10/11 occurred in bridged patients | 1.1%; no difference in bridged vs. nonbridged patients |
| Low Molecular Weight Heparin | Subcutaneous Dose |
|---|---|
| |
| Dalteparin | |
| Low dose (prophylaxis dose) | 5,000 IU once daily |
| Full dose | 100 IU/kg twice daily or 200 IU/kg once daily |
| Enoxaparin | |
| Low dose (prophylaxis dose) | 30 mg twice daily or 40mg daily |
| Full dose | 1 mg/kg twice daily or 1.5 mg/kg once daily |
| Tinzaparin (full dose) | 175 IU/kg once daily |
In the postprocedural setting, timing and dose of anticoagulant is important, as major bleeding with the use of therapeutic anticoagulation can occur in up to 10% to 20% of cases. When restarting anticoagulation after the procedure, it is important to evaluate intraoperative hemostasis and to consider patient‐related factors that may further increase bleeding risk. These include advanced age, concomitant antiplatelet or nonsteroidal antiinflammatory medications, renal insufficiency, placement of spinal/epidural catheter, worsening liver disease, or the presence of other comorbid illnesses such as cancer.30, 67, 68 The ACCP recommends withholding full‐dose anticoagulation for at least 48 to 72 hours in patients who are felt to be at a high risk for postoperative bleeding.1 Figure 2 is a proposed management approach to the use of bridging anticoagulants that is consistent with the 2008 ACCP recommendations.

CONCLUSION
The evaluation and management of patients on long‐term antiplatelet or VKA therapy who require an invasive procedure or surgery is a common, complicated, and controversial area. Importantly, it is an area in which the hospitalist physician must be adept. Although there remain many unanswered clinical questions, an evolving literature base and recent practice guidelines can help guide management decisions.
- ,,, et al.The Perioperative Management of Antithrombotic Therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6_suppl):299S–339S.
- ,,,,.Aspirin use among adults aged 40 and older in the United States: results of a national survey.Am J Prev Med.2007;32(5):403–407.
- ,.Oral anticoagulation in surgical procedures: risks and recommendations.Br J Haematol.2003;123(4):676–682.
- Perioperative medicine.J Hosp Med.2006;1(supp 1):30–31.
- .Perioperative anticoagulation management in patients who are receiving oral anticoagulant therapy: a practical guide for clinicians.Thromb Res.2002;108(1):3–13.
- ,,,.Periprocedural bridging therapy in patients receiving chronic oral anticoagulation therapy.Curr Med Res Opin.2006;22(6):1109–1122.
- ,,,,.Effect of aspirin intake on bleeding during cataract surgery.J Cataract Refract Surg.1998;24(9):1243–1246.
- ,,,.Low‐dose aspirin for secondary cardiovascular prevention–cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation—review and meta‐analysis.J Intern Med.2005;257(5):399–414.
- ,,.Aspirin does not increase bleeding complications after transbronchial biopsy.Chest.2002;122(4):1461–1464.
- ,.The management of patients on anticoagulants prior to cutaneous surgery: case report of a thromboembolic complication, review of the literature, and evidence‐based recommendations.Plast Reconstr Surg.2006;118(5):110e–117e.
- .Unanswered questions—drug‐eluting stents and the risk of late thrombosis.N Engl J Med.2007;356(10):981–984.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115(6):813–818.
- ,,, et al.The Primary and Secondary Prevention of Coronary Artery Disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6_suppl):776S–814S.
- ,,, et al.2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee.Circulation.2008;117(2):261–295.
- ,,,,,.Late thrombosis of drug‐eluting stents: a meta‐analysis of randomized clinical trials.Am J Med.2006;119(12):1056–1061.
- ,,, et al.Early and late coronary stent thrombosis of sirolimus‐eluting and paclitaxel‐eluting stents in routine clinical practice: data from a large two‐institutional cohort study.Lancet.2007;369(9562):667–678.
- ,,, et al.Clopidogrel use and long‐term clinical outcomes after drug‐eluting stent implantation.JAMA.2007;297(2):159–168.
- Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.Antiplatelet Trialists' Collaboration.Br Med J (Clin Res Ed).1994;308(6921):81–106.
- ,,, et al.Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures.Gastrointest Endosc.2002;55(7):775–779.
- ,.Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease.Aliment Pharmacol Ther.2005;21(5):525–529.
- ,,, et al.Performance standards for therapeutic abdominal paracentesis.Hepatology.2004;40(2):484–488.
- ,.Percutaneous cannulation of the internal jugular vein in patients with coagulopathies: an experience based on 1,000 attempts.Anesthesiology.1982;56(4):321–323.
- ,,,,,.Central venous catheterization in patients with coagulopathy.Arch Surg.1992;127(3):273–275.
- ,,.Central venous catheter placement in patients with disorders of hemostasis.Chest.1996;110(1):185–188.
- ,,.US‐guided placement of central vein catheters in patients with disorders of hemostasis.Eur J Radiol.2008;65(2):253–256.
- ,.Central venous cannulation in patients with liver disease and coagulopathy—a prospective audit.Intensive Care Med.1999;25(5):481–485.
- ,.Perioperative management of patients receiving oral anticoagulants: a systematic review.Arch Intern Med.2003;163(8):901–908.
- ,,, et al.Kelley's Textbook of Rheumatology.7th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,.Clinical Procedures in Emergency Medicine.4th ed.Philadelphia, PA:W.B. Saunders;2004.
- ,.A prospective study of the safety of joint and soft tissue aspirations and injections in patients taking warfarin sodium.Arthritis Rheum.1998;41(4):736–739.
- ,,, et al.[Frequency of the bleeding risk in patients receiving warfarin submitted to arthrocentesis of the knee].Reumatismo.2003;55(3):159–163.
- .Textbook of Critical Care.5th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,.Complications of lumbar puncture followed by anticoagulation.Stroke.1981;12(6):879–881.
- ,,,.Mason, Murray and Nadel's Textbook of Respiratory Medicine.4th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,,.Videos in clinical medicine. Thoracentesis.N Engl J Med.2006;355(15):e16.
- ,.Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities.Transfusion.1991;31(2):164–171.
- .Risk of bleeding during thoracentesis in anticoagulated patients.Chest.2006;130(4):141S–d‐2.
- ,,, et al.Guidelines for prevention of thromboembolic events in valvular heart disease. Study Group of the Working Group on Valvular Heart Disease of the European Society of Cardiology.Eur Heart J.1995;16(10):1320–1330.
- ,.Coronary angiography and intravascular ultrasonography. In: Braunwald E, Zipes DP, Libby P, eds.Heart Disease: A Textbook of Cardiovascular Medicine.6th ed.Philadelphia, PA:WB Saunders;2001:387–421.
- ,,, et al.Effectiveness of manual pressure hemostasis following transfemoral coronary angiography in patients on therapeutic warfarin anticoagulation.Am J Cardiol.2006;97(4):485–488.
- ,,,,,.Elective coronary angiography and percutaneous coronary intervention during uninterrupted warfarin therapy.Catheter Cardiovasc Interv.2003;60(2):180–184.
- ,,,.Coronary angiography in the fully anticoagulated patient: the transradial route is successful and safe.Catheter Cardiovasc Interv.2003;58(1):8–10.
- ,,,,.Percutaneous left and right heart catheterization in fully anticoagulated patients utilizing the radial artery and forearm vein: a two‐center experience.J Interv Cardiol.2006;19(3):258–263.
- ,,, et al.[Safety of diagnostic transradial catheterization in patients undergoing long‐term anticoagulation with coumarin derivatives].Rev Esp Cardiol.2007;60(9):988–991. [Spanish]
- ,,,,,,,,,,,,,2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Valvular Heart Disease).J Am Coll Cardiol.2008;52:e1–142.
- ,,, et al.ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in Collaboration With the North American Society of Pacing and Electrophysiology.J Am Coll Cardiol.2001;38(4):1231–1266.
- .Periprocedural thromboprophylaxis in patients receiving chronic anticoagulation therapy.Am Heart J.2004;147(1):3–15.
- ,.Management of anticoagulation before and after elective surgery.N Engl J Med.1997;336(21):1506–1511.
- ,,,,,.Modern management of prosthetic valve anticoagulation.Mayo Clin Proc.1998;73(7):665–680.
- ,.Anticoagulant‐related bleeding: clinical epidemiology, prediction, and prevention.Am J Med.1993;95(3):315–328.
- ,,,,,.Reconstruction of musculoskeletal defects following oncologic resection in 76 patients.Ann Plast Surg.2006;57(2):190–194.
- ,,,,.Biopsy of the prostate guided by transrectal ultrasound: relation between warfarin use and incidence of bleeding complications.Clin Radiol.2005;60(4):459–463; discussion 457‐458.
- ,.Anticoagulation in neurosurgical patients.Neurosurgery.1999;45(4):838–847; discussion 847‐848.
- ,,,,.Post‐operative blood loss after transurethral prostatectomy is dependent on in situ fibrinolysis.Br J Urol.1997;80(6):889–893.
- ,,.Complications of heparin therapy after total joint arthroplasty.J Bone Joint Surg.1989;71(8):1130–1134.
- ,,,,,.Postpolypectomy lower GI bleeding: descriptive analysis.Gastrointest Endosc.2000;51(6):690–696.
- ,,, et al.Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy.Chest.2004;126(4):1177–1186.
- ,,.[Perioperative bridging with enoxaparin. results of the prospective BRAVE registry with 779 patients].Med Klin (Munich).2007;102(10):809–815. [German]
- ,,.Low molecular weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen.Arch Intern Med.2004;164(12):1319–1326.
- ,,.Bridging therapy in patients on long‐term oral anticoagulants who require surgery: the Prospective Peri‐operative Enoxaparin Cohort Trial (PROSPECT).J Thromb Haemost.2007;5(11):2211–2218.
- ,,, et al.Single‐arm study of bridging therapy with low molecular weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin.Circulation.2004;110(12):1658–1663.
- ,,,.Costs and clinical outcomes associated with low molecular weight heparin vs unfractionated heparin for perioperative bridging in patients receiving long‐term oral anticoagulant therapy.Chest.2004;125(5):1642–1650.
- ,.Enoxaparin is effective and safe as bridging anticoagulation in patients with a mechanical prosthetic heart valve who require temporary interruption of warfarin because of surgery or an invasive procedure. [Abstract #703]. ASH Annual Meeting Abstracts.Blood.2004;104:202A.
- ,,, et al.Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation.Mayo Clin Proc.2008;83(6):639–645.
- ,,,.Bridging anticoagulation with low molecular weight heparin after interruption of warfarin therapy is associated with a residual anticoagulant effect prior to surgery.Thromb Haemost.2005;94(3):528–531.
- ,,, et al.Brief communication: preoperative anticoagulant activity after bridging low molecular weight heparin for temporary interruption of warfarin.Ann Intern Med.2007;146(3):184–187.
- National Institute for Clinical Excellence. Atrial fibrillation: national clinical guideline for management in primary and secondary care.2006. Available at: http://www.nice.org.uk/nicemedia/pdf/cg036fullguideline. pdf. Accessed May 2009.
- ,,, et al.Clinical outcomes with unfractionated heparin or low molecular weight heparin as bridging therapy in patients on long‐term oral anticoagulants: the REGIMEN registry.J Thromb Haemost.2006;4(6):1246–1252.
- ,,,,,.Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation.JAMA.2001;285(22):2864–2870.
- ,,.Guidelines on oral anticoagulation (warfarin). 3rd ed. 2005 update.Br J Haematol.2006;132(3):277–285.
- ,,, et al.Risk of thromboembolism with short‐term interruption of warfarin therapy.Arch Intern Med.2008;168(1):63–69.
The management of patients on long‐term antithrombotic therapy (vitamin K antagonists [VKA] or antiplatelet agents) who may require temporary disruption for an invasive procedure is challenging. Management is controversial due to methodologically limited prospective data and varied consensus opinions. Yet periprocedural anticoagulation management is a commonly encountered clinical problem. It is estimated that there are 2.5 million patients on long‐term VKA therapy in North America1 and 41% of the U.S. population over age 40 years is on antiplatelet therapy.2 Further, the need for temporary disruption of these therapies for an invasive procedure is frequent. As an example, in 1 European study, approximately 15% of patients on long‐term VKA required a major surgical procedure in 4 years of follow‐up.3 The role of the hospitalist physician in managing these patients is increasing as hospitalists care for an increasing number of surgical patients and provide periprocedural consultation both in and out of the hospital. Therefore, it is imperative for the hospitalist physician to be proficient in making thoughtful and individualized recommendations on the appropriate management of periprocedural anticoagulants, drawing from the available literature and evidence‐based practice guidelines. Importantly, the Society of Hospital Medicine has cited perioperative management as an important core competency.4
The hospitalist physician is likely to encounter numerous periprocedural scenarios, including the management of antiplatelet agents, identifying low bleeding risk procedures wherein interruption of anticoagulants is unnecessary, and recognizing patients with a low short‐term thromboembolic risk where anticoagulants can be disrupted without the need for heparin or low molecular weight heparin (LMWH) in the periprocedural period (defined as bridging therapy). Further, all other clinical scenarios require both a careful individualized assessment of the patient's risk of periprocedural bleeding and thromboembolism and a thoughtful discussion with all involved parties. This discussion may involve the person performing the procedure, the anesthesiologist, and the patient. The purpose of this work is to explore these relevant areas through a review of the literature with a particular focus on the recently published 2008 American College of Chest Physicians (ACCP) evidence‐based clinical practice guidelines.
We reviewed medical literature from 1990 through May 2008 with the following key words: bridging, anticoagulation, perioperative, antiplatelet, heparin, and low molecular weight heparin. Individual studies were then independently reviewed by the authors. Studies that were felt relevant to a hospitalist physician were retrieved and reviewed. If there was uncertainty regarding applicability to a hospitalist setting, a second author's opinion was rendered. Additionally, we reviewed 1 author's personal reference list of articles relating to periprocedural anticoagulation that has been compiled over the past 10 years. This list and the reference lists of retrieved articles were also reviewed. Data were summarized to answer 4 clinically relevant questions:
-
What is the optimal management of antiplatelet therapy in the periprocedural period?
-
Are there very low‐bleeding risk procedures that do not require interruption of oral anticoagulation?
-
Are there low thromboembolic risk populations who do not require periprocedural bridging?
-
How do you manage patients who must discontinue anticoagulants but are at an increased thrombotic risk?
Clinical Question #1: What Is the Optimal Management of Antiplatelet Therapy in the Periprocedural Period?
The optimal management of oral antiplatelet therapy in the periprocedural period is not well studied. Most reviews, expert recommendations, and consensus statements either do not comment on periprocedural antiplatelet management or recommend the routine discontinuation of therapy at least 7 days prior to surgery.3, 5, 6 However, as the 2008 ACCP guidelines highlight, the recommendation to routinely discontinue antiplatelet therapy 7 days prior to the procedure is an oversimplification.1 In the era of both bare metal cardiac stents and drug‐eluting stents, the optimal management of these patients requires that 2 primary questions be asked: (1) Is this a low‐bleeding risk procedure whereby antiplatelet therapy can be continued? (2) Does the patient have a coronary stent whereby the continuation of antiplatelet therapy or delay of the intervention is necessary?
In the context of ongoing aspirin therapy, certain procedures have a low risk of significant hemorrhagic complications. These low bleeding risk procedures include cataract surgery, cutaneous surgery, oral surgery, and endoscopic procedures, including those with mucosal biopsies.710 Patients undergoing these procedures may safely continue low dose aspirin therapy, especially if they have a high‐risk indication for their aspirin such as recent myocardial infarction, stroke, or the presence of a coronary stent.5, 710 Whether these procedures can be safely performed in the setting of a thienopyridine or combination antiplatelet therapy is uncertain.
In the past several decades, the management of obstructive coronary artery disease has undergone a major evolution. Placement of coronary stents has become commonplace, and there are now several million patients with drug‐eluting stents.11 The major complication of these devices is stent thrombosis, which results in death or myocardial infarction in up to 64% of patients.12 Fortunately, dual antiplatelet therapy (aspirin and a thienopyridine such as clopidogrel) markedly reduces this risk.13 Current guidelines recommend using combination antiplatelet therapy for at least 4 to 6 weeks and ideally up to 12 months after placement of a bare metal stent and at least 12 months after placement of either a sirolimus‐ or paclitaxel‐eluting stent.1, 14 During this period of dual antiplatelet therapy, the premature discontinuation of the thienopyridine may be catastrophic. To guide clinicians in managing these patients in the periprocedural period, recent consensus guidelines recommend the following:1, 12
-
In patients who are expected to need an invasive surgical procedure in the next 12 months, consideration should be given to avoiding drug‐eluting stents.
-
Elective procedures which have an increased risk of bleeding should be deferred for at least 6 weeks after bare metal stent implantation and 12 months after drug‐eluting stent implantation.
-
For patients undergoing a surgical procedure within 6 weeks of bare metal stent implantation and 12 months of drug‐eluting stent implantation, continuation of aspirin and clopidogrel is recommended. If bleeding risk prohibits this, then a cardiologist should be consulted.
-
In patients with a drug‐eluting stent who need to undergo a procedure whereby the thienopyridine needs to be discontinued, aspirin should be continued if at all possible, and the thienopyridine should be resumed as soon as possible after the procedure. It may be reasonable to consider a loading dose of clopidogrel, up to 600 mg, in this setting, although prospective supportive data is lacking.1
It is important to recognize that delayed stent thrombosis is now reported well beyond 1 year after drug‐eluting stent implantation, and that there may not be a diminution in risk after the initial 12 months.1517 Until additional data is available, it seems prudent, if possible, to at least continue aspirin in the periprocedural period in these patients. If bleeding concerns obviate this, then antiplatelet therapy should be discontinued and resumed as soon as possible.
For patients on chronic antiplatelet therapy who do not have a cardiac stent and who are not undergoing a low‐bleeding‐risk procedure, the risks and benefits of the continuation or discontinuation of antiplatelet therapy in the periprocedural period are uncertain as absolute risks in the periprocedural period have not been well studied. Relative risks/benefits, however, can be estimated from prior studies. Aspirin leads to an approximate 25% relative risk reduction in cardiac or thrombotic event rates compared to placebo.14, 18 Although important, the absolute benefit of 1 week of therapy (vs. no therapy during the periprocedural period) is estimated to be small. The small absolute benefit of continued aspirin therapy may be offset by an increase in significant bleeding events. Although, not well studied, continued aspirin increases significant bleeding by 50% with absolute event rates varying by type of procedure.8 In some procedures, such as intracranial surgery or transurethral prostatectomy, this bleeding risk is prohibitive. For others, the risk may be modest and the decision to continue vs. discontinue aspirin therapy may be at the discretion of the person performing the procedure. In general, for most patients who do not have a coronary stent and have not had a recent (past 3 months) myocardial infarction or stroke, discontinuation of antiplatelet therapy 7 to 10 days prior to the procedure seems prudent. The primary exceptions are patients who are undergoing percutaneous coronary intervention or coronary artery bypass grafting. For these procedures continuing aspirin is recommended.1 Figure 1 outlines a proposed management strategy based upon available evidence and guidelines.

Clinical Question #2: Are There Very‐Low‐Bleeding‐Risk Procedures That Do Not Require Interruption of Oral Anticoagulation?
Some procedures are associated with a low‐enough risk of bleeding that it is safe to proceed without interrupting VKA anticoagulation. This approach spares the risk and cost that occur with the holding of oral anticoagulants and institution of bridging therapy. When considering this strategy, it is important that the specialist performing the procedure is included in the discussion. Dental, dermatologic, and cataract procedures are common outpatient procedures that are associated with low bleeding risk. The relative safety of these procedures in patients who are anticoagulated is discussed thoroughly in the ACCP guidelines.1 Other low‐bleeding‐risk procedures for which a hospitalist may be consulted include certain endoscopic procedures, paracentesis, central venous catheter placement, and arthrocentesis.
The American Society for Gastrointestinal Endoscopy has published guidelines recommending that anticoagulation can be safely continued in patients undergoing the following endoscopic procedures with a low bleeding risk: esophagogastroduodenoscopy (EGD), flexible sigmoidoscopy, and colonoscopy, all with or without mucosal biopsy; enteroscopy, biliary/pancreatic stent placement, endoscopic ultrasound without biopsy, and endoscopic retrograde cholangiopancreatography (ERCP) without sphincterotomy.19 Conversely, high‐risk procedures for which interruption of anticoagulation is recommended include polypectomy, biliary sphincterotomy, variceal treatment, percutaneous endoscopic gastrostomy (PEG) placement, dilation of strictures, and endoscopic ultrasound‐guided fine‐needle aspiration.
Limited data suggest that paracentesis, central venous catheter placement, and arthrocentesis may be safe to perform in the setting of anticoagulation. For patients undergoing paracentesis there is little evidence in anticoagulated patients; however, it is probably safe to continue anticoagulation as studies have demonstrated the safety of this procedure in patients with significant thrombocytopenia and coagulopathy.20, 21 Limited data also supports that central venous catheter placement may be safely performed in the setting of abnormal coagulation tests, although some recommend avoiding the subclavian site due to the risk of hemothorax and the inability to apply adequate compression.2226 With regard to arthrocentesis, multiple authors have endorsed the idea that joint and soft‐tissue aspirations and injections present a low risk of serious bleeding even with anticoagulation.2729 This is supported by limited data.30, 31
Other procedures such as lumbar puncture, thoracentesis, and cardiac catheterization are somewhat more controversial in the anticoagulated patient. Anticoagulation should generally be interrupted for lumbar puncture,29, 32 as 1 study involving patients who were started on heparin immediately after the procedure had a 2% incidence of spinal hematoma and 6.7% major complication rate.33 With regard to thoracentesis, evidence is very limited, but experts generally accept that it may be safely performed in patients with mild coagulopathy.34, 35 One frequently‐cited study found no bleeding complications in 57 patients with mild elevation in prothrombin time, which correlated to an International Normalized Ratio of approximately 2.2 or less.36 A recent report also revealed no serious bleeding complications in 33 thoracenteses performed on patients receiving full anticoagulation with warfarin, heparin, and/or low molecular weight heparin.37
Therapeutic anticoagulation has traditionally been felt to be a relative contraindication to cardiac catheterization.38, 39 In spite of this, several observational studies have suggested it may be safely performed using a standard approach,40 using vascular closure devices,41 or using a radial artery approach instead of the more commonly used femoral site.4244 The small size of these observational reports, the diagnostic rather than therapeutic nature of most cases, the limited use of other antithrombotic and antiplatelet medications, and the experience required to use the transradial approach are all major limitations preventing widespread acceptance of cardiac catheterization in therapeutically anticoagulated patients.
In summary, there are numerous procedures that may be safely pursued in the setting of therapeutic anticoagulation. However, for most of these procedures the data is somewhat limited. As such, it is paramount for the hospitalist physician to recognize these clinical scenarios and to discuss management options with the patient and the person performing the procedure, if applicable.
Clinical Question #3: Are There LowThromboembolic‐Risk Populations Who Do Not Require Periprocedural Bridging?
Although it has previously been noted that there is a wide variation of opinion on when and how to perform periprocedural bridging, it is generally agreed that in the following conditions the risk of thrombosis is low enough that bridging with full dose heparin or LMWH is not necessary:1, 5, 4549
-
Atrial fibrillation without previous stroke or transient ischemic attack (TIA) and no more than 2 additional thrombotic risk factors on the CHADS2 scoring system (Table 1).
-
A single venous thromboembolic event that occurred greater than 12 months ago with no ongoing risk factors such as active malignancy, high risk thrombophilia, or the antiphospholipid antibody syndrome.
-
Bileaflet aortic valve without the presence of additional risk factors (ie, patients <75 years of age with the absence of atrial fibrillation, prior stroke or transient ischemic attack, hypertension, diabetes, or congestive heart failure).
| CHADS2 Score* | Annual Risk of Stroke (%) |
|---|---|
| |
| 0 | 1.9 |
| 1 | 2.8 |
| 2 | 4.0 |
| 3 | 5.9 |
| 4 | 8.5 |
| 5 | 12.5 |
| 6 | 18.2 |
Clinical Question #4: How Do You Manage Patients Who Must Discontinue Anticoagulants But Are at an Increased Thrombotic Risk?
When anticoagulation must be held and the patient does not have a very low thromboembolic risk, a decision of whether or not to use bridging anticoagulation must be made. The current ACCP guideline gives grade 1C and 2C recommendations (evidence from observational studies, case series, or controlled trials with serious flaws) regarding for whom and how to implement bridging.1 The grade C designation is due to a lack of high‐quality randomized clinical trials. As such, the clinician must carefully consider an individual patient's estimated thromboembolic risk, procedurally‐related bleeding risk, patient‐related bleeding risk factors, and the patient's values regarding concerns of thromboembolism or bleeding. In these situations it is also imperative that the person performing the procedure is involved in the risk‐to‐benefit discussion.
When evaluating an individual patient's risk of thromboembolism, clinicians sometimes estimate the perioperative risk by prorating the annual incidence of thromboembolic complications to the few days that anticoagulation is withheld.67 Making this extrapolation discounts the effect of a potential increase in thromboembolic risk induced by surgery. As an example, an average patient with atrial fibrillation who has a 5% predicted annual stroke rate would be estimated to have a stroke risk of 0.05% if they are not anticoagulated for 4 days. However, studies have shown that the actual rate of perioperative thromboembolism is approximately 1%.1 With these limitations and uncertainties in mind, and until there is better prospective outcomes data, we must consider relative risks in the context of absolute event rate estimates when deciding a perioperative anticoagulant management plan. The estimated annual incidence of thrombosis without anticoagulation for various indications and the current guideline recommendations are presented in Table 2.
| Practice Guideline Preferred Management Recommendations | ||||
|---|---|---|---|---|
| Indication for chronic anticoagulation | Estimated Annual Thrombotic Risk Without Anticoagulation | ACCP*1 | ACC/AHA45, 46 | British Haematologic Society70 |
| ||||
| Dual prosthetic or older‐generation valve | >10% | Bridge | Bridge | Bridge |
| VTE within 3 months or severe thrombophilias | Bridge | N/A | Bridge | |
| Pregnancy with prosthetic valve | Bridge | Bridge | N/A | |
| Bileaflet valve in the mitral position | Bridge | Bridge | Prophylaxis | |
| Valve with acute embolism <6 months | Bridge | N/A | Bridge | |
| A‐fib valvular or CHADS2 score 5‐6 | Bridge | Consider bridging | N/A | |
| Recurrent venous thromboembolism | 4‐10% | Bridge | N/A | N/A |
| VTE within 3‐12 months or active cancer | Bridge | N/A | Prophylaxis | |
| Bileaflet aortic valve with additional risk factors | Bridge | Bridge | Prophylaxis | |
| A‐fib CHADS2 score 3‐4 | Bridge | Consider bridging | N/A | |
| Bileaflet aortic valve without additional risk factors | <4% | Prophylaxis or no bridging | No bridging | Prophylaxis |
| VTE >12 months | Prophylaxis or no bridging | N/A | Prophylaxis | |
| A‐fib CHADS2 score 0‐2 and no previous CVA/TIA | Prophylaxis or no bridging | No bridging | N/A | |
In addition to thromboembolic risk, we must also consider the bleeding risk associated with the procedure/surgery. Importantly, therapeutic heparin started early in the postoperative period is associated with major bleeding event rates as high as 10% to 20%.1, 50 Once a major bleeding event occurs, this will often lead to an extended interruption of anticoagulant therapy, placing the patient at a more prolonged risk of an associated thromboembolic event. For this reason, the resumption of full‐dose anticoagulation with LMWH/heparin should be delayed for at least 48 hours in most patients undergoing a surgery or procedure associated with an increased risk of bleeding. Examples of these higher‐bleeding‐risk procedures include major thoracic surgery, intracranial or spinal surgery, major vascular surgery, major orthopedic surgery, urologic surgery involving the bladder or prostrate, major oncologic surgery, reconstructive plastic surgery, colonoscopy with associated polypectomy, renal or prostate biopsies, and placement of a cardiac pacemaker/defibrillator.1, 5157
Taken together, these uncertainties surrounding thromboembolic and bleeding risk estimates imply that there are multiple options for periprocedural management. Several studies, many of which included patients with mechanical heart valves, have shown similar safety and efficacy between LMWH and intravenous (IV) unfractionated heparin.5864 Table 3 summarizes these studies. The ACCP recommends bridging with LMWH over IV unfractionated heparin due to equal efficacy and cost savings with LMWH.1 When bridging is used, careful attention must be given to the timing and dose of anticoagulation in both the preoperative and postoperative periods. Table 4 lists dosing of commonly used LMWHs in North America. When using LMWHs in the preprocedural setting it is important to note that unacceptably high levels of anticoagulation remain present when a patient is given a full once‐daily LMWH dose the morning prior to the procedure or when a full‐dose, twice‐daily LMWH dose is given the evening prior to the procedure.65, 66 For this reason, the ACCP recommends administering the last preoperative dose 24 hours before surgery and if full‐dose once‐daily LMWH is used, the dose should be decreased by one‐half on the day before the surgery in order to ensure that no residual anticoagulant effect remains at the time of surgery.
| AuthorReference/Study Type | Number of Patients | Patient Population | Type of Procedure | Bridging Strategy | Major Bleeds | Minor Bleeds | TE Rate |
|---|---|---|---|---|---|---|---|
| |||||||
| Turpie and Douketis63/single arm cohort | 174 | 66% aortic valve; 34% mitral or dual prosthetic valve | Not specified | Enoxaparin 1 mg/kg twice daily | 2.3% | Not specified | None |
| Kovacs et al.61/single arm cohort | 224 | Prosthetic heart valves or a‐fib plus 1 major risk factor | 67 surgical; 157 nonsurgical | Preoperative bridging with dalteparin 200 IU/kg daily; dose reduced to 100 IU/kg on preoperative day 1; restarted at 100 IU/kg on POD 1; dose reduced to 5000 IU daily if high risk for bleeding | 6.7%; 8/15 occurred intraoperatively or <6 hours postoperatively; 2/15 occurred after 4 weeks | Not specified | 3.6%; 6/8 episodes occurred after warfarin held secondary to bleeding; 2/8 thrombotic episodes judged to be due to cardioembolism |
| Douketis et al.59/prospective registry | 650 | A‐fib 58%; mechanical heart valve 33% | 251 surgical; 399 nonsurgical | Dalteparin 100 IU/kg twice daily; held after high bleeding risk procedure and patients with poor hemostasis | 0.92% | 5.9% | 0.6% |
| Spyropolous et al.62/prospective registry; 14 centers in United States and Canada | 901 | UFH: 40% mechanical valves, 33% a‐fib; LMWH: 24% mechanical valve, 40% a‐fib | 394 surgical; 507 nonsurgical | LMWH mostly given twice daily 80%; UFH 20% | 5.5% UFH; 3.3% LMWH | 9.1% UFH; 12.0% LMWH | 2.4% UFH; 0.9% LMWH |
| Dunn et al.66/prospective cohort | 260 | A‐fib 68% or prior DVT 37% (excluding prosthetic heart valves) | 105 surgical; 145 nonsurgical | Enoxaparin 1.5 mg/kg daily | 3.5% overall; minor surgery/procedures 0.9%; major surgery 28% | 42% | 1.9%; 1/5 events occurred after bleeding led to withdrawal of AC |
| Omran et al.77/prospective registry | 779 | Various indications | Major and minor procedures | All patients bridged with enoxaparin; moderate TE risk 1 mg/kg daily; high TE risk 1 mg/kg twice daily | 0.5%; all in high‐risk group | 5.9% | 0 |
| Garcia et al.71/prospective, observational cohort of 101 sites in United States | 1024 patients with 1293 interruptions of AC | A‐fib 53%; VTE 14%; prosthetic valve 13% | Outpatient procedures only | At discretion of provider. Bridging performed in 8.3% of interruptions; 3% a‐fib, 10% VTE, and 29% mechanical valves | 0.6%; 4/6 patients with major bleed received bridging | 1.7%;10/17 patients with minor bleed received bridging | 0.7%; no events in patients who were bridged |
| Wysokinski et al.64/prospective cohort | 345 consecutive patients undergoing 386 procedures | 100% nonvalvular a‐fib | Major and minor surgeries/procedures | Individualized in AC clinic; 52% of patients bridged | 2.7%; no difference whether patient received bridging or not | 3.0%; 10/11 occurred in bridged patients | 1.1%; no difference in bridged vs. nonbridged patients |
| Low Molecular Weight Heparin | Subcutaneous Dose |
|---|---|
| |
| Dalteparin | |
| Low dose (prophylaxis dose) | 5,000 IU once daily |
| Full dose | 100 IU/kg twice daily or 200 IU/kg once daily |
| Enoxaparin | |
| Low dose (prophylaxis dose) | 30 mg twice daily or 40mg daily |
| Full dose | 1 mg/kg twice daily or 1.5 mg/kg once daily |
| Tinzaparin (full dose) | 175 IU/kg once daily |
In the postprocedural setting, timing and dose of anticoagulant is important, as major bleeding with the use of therapeutic anticoagulation can occur in up to 10% to 20% of cases. When restarting anticoagulation after the procedure, it is important to evaluate intraoperative hemostasis and to consider patient‐related factors that may further increase bleeding risk. These include advanced age, concomitant antiplatelet or nonsteroidal antiinflammatory medications, renal insufficiency, placement of spinal/epidural catheter, worsening liver disease, or the presence of other comorbid illnesses such as cancer.30, 67, 68 The ACCP recommends withholding full‐dose anticoagulation for at least 48 to 72 hours in patients who are felt to be at a high risk for postoperative bleeding.1 Figure 2 is a proposed management approach to the use of bridging anticoagulants that is consistent with the 2008 ACCP recommendations.

CONCLUSION
The evaluation and management of patients on long‐term antiplatelet or VKA therapy who require an invasive procedure or surgery is a common, complicated, and controversial area. Importantly, it is an area in which the hospitalist physician must be adept. Although there remain many unanswered clinical questions, an evolving literature base and recent practice guidelines can help guide management decisions.
The management of patients on long‐term antithrombotic therapy (vitamin K antagonists [VKA] or antiplatelet agents) who may require temporary disruption for an invasive procedure is challenging. Management is controversial due to methodologically limited prospective data and varied consensus opinions. Yet periprocedural anticoagulation management is a commonly encountered clinical problem. It is estimated that there are 2.5 million patients on long‐term VKA therapy in North America1 and 41% of the U.S. population over age 40 years is on antiplatelet therapy.2 Further, the need for temporary disruption of these therapies for an invasive procedure is frequent. As an example, in 1 European study, approximately 15% of patients on long‐term VKA required a major surgical procedure in 4 years of follow‐up.3 The role of the hospitalist physician in managing these patients is increasing as hospitalists care for an increasing number of surgical patients and provide periprocedural consultation both in and out of the hospital. Therefore, it is imperative for the hospitalist physician to be proficient in making thoughtful and individualized recommendations on the appropriate management of periprocedural anticoagulants, drawing from the available literature and evidence‐based practice guidelines. Importantly, the Society of Hospital Medicine has cited perioperative management as an important core competency.4
The hospitalist physician is likely to encounter numerous periprocedural scenarios, including the management of antiplatelet agents, identifying low bleeding risk procedures wherein interruption of anticoagulants is unnecessary, and recognizing patients with a low short‐term thromboembolic risk where anticoagulants can be disrupted without the need for heparin or low molecular weight heparin (LMWH) in the periprocedural period (defined as bridging therapy). Further, all other clinical scenarios require both a careful individualized assessment of the patient's risk of periprocedural bleeding and thromboembolism and a thoughtful discussion with all involved parties. This discussion may involve the person performing the procedure, the anesthesiologist, and the patient. The purpose of this work is to explore these relevant areas through a review of the literature with a particular focus on the recently published 2008 American College of Chest Physicians (ACCP) evidence‐based clinical practice guidelines.
We reviewed medical literature from 1990 through May 2008 with the following key words: bridging, anticoagulation, perioperative, antiplatelet, heparin, and low molecular weight heparin. Individual studies were then independently reviewed by the authors. Studies that were felt relevant to a hospitalist physician were retrieved and reviewed. If there was uncertainty regarding applicability to a hospitalist setting, a second author's opinion was rendered. Additionally, we reviewed 1 author's personal reference list of articles relating to periprocedural anticoagulation that has been compiled over the past 10 years. This list and the reference lists of retrieved articles were also reviewed. Data were summarized to answer 4 clinically relevant questions:
-
What is the optimal management of antiplatelet therapy in the periprocedural period?
-
Are there very low‐bleeding risk procedures that do not require interruption of oral anticoagulation?
-
Are there low thromboembolic risk populations who do not require periprocedural bridging?
-
How do you manage patients who must discontinue anticoagulants but are at an increased thrombotic risk?
Clinical Question #1: What Is the Optimal Management of Antiplatelet Therapy in the Periprocedural Period?
The optimal management of oral antiplatelet therapy in the periprocedural period is not well studied. Most reviews, expert recommendations, and consensus statements either do not comment on periprocedural antiplatelet management or recommend the routine discontinuation of therapy at least 7 days prior to surgery.3, 5, 6 However, as the 2008 ACCP guidelines highlight, the recommendation to routinely discontinue antiplatelet therapy 7 days prior to the procedure is an oversimplification.1 In the era of both bare metal cardiac stents and drug‐eluting stents, the optimal management of these patients requires that 2 primary questions be asked: (1) Is this a low‐bleeding risk procedure whereby antiplatelet therapy can be continued? (2) Does the patient have a coronary stent whereby the continuation of antiplatelet therapy or delay of the intervention is necessary?
In the context of ongoing aspirin therapy, certain procedures have a low risk of significant hemorrhagic complications. These low bleeding risk procedures include cataract surgery, cutaneous surgery, oral surgery, and endoscopic procedures, including those with mucosal biopsies.710 Patients undergoing these procedures may safely continue low dose aspirin therapy, especially if they have a high‐risk indication for their aspirin such as recent myocardial infarction, stroke, or the presence of a coronary stent.5, 710 Whether these procedures can be safely performed in the setting of a thienopyridine or combination antiplatelet therapy is uncertain.
In the past several decades, the management of obstructive coronary artery disease has undergone a major evolution. Placement of coronary stents has become commonplace, and there are now several million patients with drug‐eluting stents.11 The major complication of these devices is stent thrombosis, which results in death or myocardial infarction in up to 64% of patients.12 Fortunately, dual antiplatelet therapy (aspirin and a thienopyridine such as clopidogrel) markedly reduces this risk.13 Current guidelines recommend using combination antiplatelet therapy for at least 4 to 6 weeks and ideally up to 12 months after placement of a bare metal stent and at least 12 months after placement of either a sirolimus‐ or paclitaxel‐eluting stent.1, 14 During this period of dual antiplatelet therapy, the premature discontinuation of the thienopyridine may be catastrophic. To guide clinicians in managing these patients in the periprocedural period, recent consensus guidelines recommend the following:1, 12
-
In patients who are expected to need an invasive surgical procedure in the next 12 months, consideration should be given to avoiding drug‐eluting stents.
-
Elective procedures which have an increased risk of bleeding should be deferred for at least 6 weeks after bare metal stent implantation and 12 months after drug‐eluting stent implantation.
-
For patients undergoing a surgical procedure within 6 weeks of bare metal stent implantation and 12 months of drug‐eluting stent implantation, continuation of aspirin and clopidogrel is recommended. If bleeding risk prohibits this, then a cardiologist should be consulted.
-
In patients with a drug‐eluting stent who need to undergo a procedure whereby the thienopyridine needs to be discontinued, aspirin should be continued if at all possible, and the thienopyridine should be resumed as soon as possible after the procedure. It may be reasonable to consider a loading dose of clopidogrel, up to 600 mg, in this setting, although prospective supportive data is lacking.1
It is important to recognize that delayed stent thrombosis is now reported well beyond 1 year after drug‐eluting stent implantation, and that there may not be a diminution in risk after the initial 12 months.1517 Until additional data is available, it seems prudent, if possible, to at least continue aspirin in the periprocedural period in these patients. If bleeding concerns obviate this, then antiplatelet therapy should be discontinued and resumed as soon as possible.
For patients on chronic antiplatelet therapy who do not have a cardiac stent and who are not undergoing a low‐bleeding‐risk procedure, the risks and benefits of the continuation or discontinuation of antiplatelet therapy in the periprocedural period are uncertain as absolute risks in the periprocedural period have not been well studied. Relative risks/benefits, however, can be estimated from prior studies. Aspirin leads to an approximate 25% relative risk reduction in cardiac or thrombotic event rates compared to placebo.14, 18 Although important, the absolute benefit of 1 week of therapy (vs. no therapy during the periprocedural period) is estimated to be small. The small absolute benefit of continued aspirin therapy may be offset by an increase in significant bleeding events. Although, not well studied, continued aspirin increases significant bleeding by 50% with absolute event rates varying by type of procedure.8 In some procedures, such as intracranial surgery or transurethral prostatectomy, this bleeding risk is prohibitive. For others, the risk may be modest and the decision to continue vs. discontinue aspirin therapy may be at the discretion of the person performing the procedure. In general, for most patients who do not have a coronary stent and have not had a recent (past 3 months) myocardial infarction or stroke, discontinuation of antiplatelet therapy 7 to 10 days prior to the procedure seems prudent. The primary exceptions are patients who are undergoing percutaneous coronary intervention or coronary artery bypass grafting. For these procedures continuing aspirin is recommended.1 Figure 1 outlines a proposed management strategy based upon available evidence and guidelines.

Clinical Question #2: Are There Very‐Low‐Bleeding‐Risk Procedures That Do Not Require Interruption of Oral Anticoagulation?
Some procedures are associated with a low‐enough risk of bleeding that it is safe to proceed without interrupting VKA anticoagulation. This approach spares the risk and cost that occur with the holding of oral anticoagulants and institution of bridging therapy. When considering this strategy, it is important that the specialist performing the procedure is included in the discussion. Dental, dermatologic, and cataract procedures are common outpatient procedures that are associated with low bleeding risk. The relative safety of these procedures in patients who are anticoagulated is discussed thoroughly in the ACCP guidelines.1 Other low‐bleeding‐risk procedures for which a hospitalist may be consulted include certain endoscopic procedures, paracentesis, central venous catheter placement, and arthrocentesis.
The American Society for Gastrointestinal Endoscopy has published guidelines recommending that anticoagulation can be safely continued in patients undergoing the following endoscopic procedures with a low bleeding risk: esophagogastroduodenoscopy (EGD), flexible sigmoidoscopy, and colonoscopy, all with or without mucosal biopsy; enteroscopy, biliary/pancreatic stent placement, endoscopic ultrasound without biopsy, and endoscopic retrograde cholangiopancreatography (ERCP) without sphincterotomy.19 Conversely, high‐risk procedures for which interruption of anticoagulation is recommended include polypectomy, biliary sphincterotomy, variceal treatment, percutaneous endoscopic gastrostomy (PEG) placement, dilation of strictures, and endoscopic ultrasound‐guided fine‐needle aspiration.
Limited data suggest that paracentesis, central venous catheter placement, and arthrocentesis may be safe to perform in the setting of anticoagulation. For patients undergoing paracentesis there is little evidence in anticoagulated patients; however, it is probably safe to continue anticoagulation as studies have demonstrated the safety of this procedure in patients with significant thrombocytopenia and coagulopathy.20, 21 Limited data also supports that central venous catheter placement may be safely performed in the setting of abnormal coagulation tests, although some recommend avoiding the subclavian site due to the risk of hemothorax and the inability to apply adequate compression.2226 With regard to arthrocentesis, multiple authors have endorsed the idea that joint and soft‐tissue aspirations and injections present a low risk of serious bleeding even with anticoagulation.2729 This is supported by limited data.30, 31
Other procedures such as lumbar puncture, thoracentesis, and cardiac catheterization are somewhat more controversial in the anticoagulated patient. Anticoagulation should generally be interrupted for lumbar puncture,29, 32 as 1 study involving patients who were started on heparin immediately after the procedure had a 2% incidence of spinal hematoma and 6.7% major complication rate.33 With regard to thoracentesis, evidence is very limited, but experts generally accept that it may be safely performed in patients with mild coagulopathy.34, 35 One frequently‐cited study found no bleeding complications in 57 patients with mild elevation in prothrombin time, which correlated to an International Normalized Ratio of approximately 2.2 or less.36 A recent report also revealed no serious bleeding complications in 33 thoracenteses performed on patients receiving full anticoagulation with warfarin, heparin, and/or low molecular weight heparin.37
Therapeutic anticoagulation has traditionally been felt to be a relative contraindication to cardiac catheterization.38, 39 In spite of this, several observational studies have suggested it may be safely performed using a standard approach,40 using vascular closure devices,41 or using a radial artery approach instead of the more commonly used femoral site.4244 The small size of these observational reports, the diagnostic rather than therapeutic nature of most cases, the limited use of other antithrombotic and antiplatelet medications, and the experience required to use the transradial approach are all major limitations preventing widespread acceptance of cardiac catheterization in therapeutically anticoagulated patients.
In summary, there are numerous procedures that may be safely pursued in the setting of therapeutic anticoagulation. However, for most of these procedures the data is somewhat limited. As such, it is paramount for the hospitalist physician to recognize these clinical scenarios and to discuss management options with the patient and the person performing the procedure, if applicable.
Clinical Question #3: Are There LowThromboembolic‐Risk Populations Who Do Not Require Periprocedural Bridging?
Although it has previously been noted that there is a wide variation of opinion on when and how to perform periprocedural bridging, it is generally agreed that in the following conditions the risk of thrombosis is low enough that bridging with full dose heparin or LMWH is not necessary:1, 5, 4549
-
Atrial fibrillation without previous stroke or transient ischemic attack (TIA) and no more than 2 additional thrombotic risk factors on the CHADS2 scoring system (Table 1).
-
A single venous thromboembolic event that occurred greater than 12 months ago with no ongoing risk factors such as active malignancy, high risk thrombophilia, or the antiphospholipid antibody syndrome.
-
Bileaflet aortic valve without the presence of additional risk factors (ie, patients <75 years of age with the absence of atrial fibrillation, prior stroke or transient ischemic attack, hypertension, diabetes, or congestive heart failure).
| CHADS2 Score* | Annual Risk of Stroke (%) |
|---|---|
| |
| 0 | 1.9 |
| 1 | 2.8 |
| 2 | 4.0 |
| 3 | 5.9 |
| 4 | 8.5 |
| 5 | 12.5 |
| 6 | 18.2 |
Clinical Question #4: How Do You Manage Patients Who Must Discontinue Anticoagulants But Are at an Increased Thrombotic Risk?
When anticoagulation must be held and the patient does not have a very low thromboembolic risk, a decision of whether or not to use bridging anticoagulation must be made. The current ACCP guideline gives grade 1C and 2C recommendations (evidence from observational studies, case series, or controlled trials with serious flaws) regarding for whom and how to implement bridging.1 The grade C designation is due to a lack of high‐quality randomized clinical trials. As such, the clinician must carefully consider an individual patient's estimated thromboembolic risk, procedurally‐related bleeding risk, patient‐related bleeding risk factors, and the patient's values regarding concerns of thromboembolism or bleeding. In these situations it is also imperative that the person performing the procedure is involved in the risk‐to‐benefit discussion.
When evaluating an individual patient's risk of thromboembolism, clinicians sometimes estimate the perioperative risk by prorating the annual incidence of thromboembolic complications to the few days that anticoagulation is withheld.67 Making this extrapolation discounts the effect of a potential increase in thromboembolic risk induced by surgery. As an example, an average patient with atrial fibrillation who has a 5% predicted annual stroke rate would be estimated to have a stroke risk of 0.05% if they are not anticoagulated for 4 days. However, studies have shown that the actual rate of perioperative thromboembolism is approximately 1%.1 With these limitations and uncertainties in mind, and until there is better prospective outcomes data, we must consider relative risks in the context of absolute event rate estimates when deciding a perioperative anticoagulant management plan. The estimated annual incidence of thrombosis without anticoagulation for various indications and the current guideline recommendations are presented in Table 2.
| Practice Guideline Preferred Management Recommendations | ||||
|---|---|---|---|---|
| Indication for chronic anticoagulation | Estimated Annual Thrombotic Risk Without Anticoagulation | ACCP*1 | ACC/AHA45, 46 | British Haematologic Society70 |
| ||||
| Dual prosthetic or older‐generation valve | >10% | Bridge | Bridge | Bridge |
| VTE within 3 months or severe thrombophilias | Bridge | N/A | Bridge | |
| Pregnancy with prosthetic valve | Bridge | Bridge | N/A | |
| Bileaflet valve in the mitral position | Bridge | Bridge | Prophylaxis | |
| Valve with acute embolism <6 months | Bridge | N/A | Bridge | |
| A‐fib valvular or CHADS2 score 5‐6 | Bridge | Consider bridging | N/A | |
| Recurrent venous thromboembolism | 4‐10% | Bridge | N/A | N/A |
| VTE within 3‐12 months or active cancer | Bridge | N/A | Prophylaxis | |
| Bileaflet aortic valve with additional risk factors | Bridge | Bridge | Prophylaxis | |
| A‐fib CHADS2 score 3‐4 | Bridge | Consider bridging | N/A | |
| Bileaflet aortic valve without additional risk factors | <4% | Prophylaxis or no bridging | No bridging | Prophylaxis |
| VTE >12 months | Prophylaxis or no bridging | N/A | Prophylaxis | |
| A‐fib CHADS2 score 0‐2 and no previous CVA/TIA | Prophylaxis or no bridging | No bridging | N/A | |
In addition to thromboembolic risk, we must also consider the bleeding risk associated with the procedure/surgery. Importantly, therapeutic heparin started early in the postoperative period is associated with major bleeding event rates as high as 10% to 20%.1, 50 Once a major bleeding event occurs, this will often lead to an extended interruption of anticoagulant therapy, placing the patient at a more prolonged risk of an associated thromboembolic event. For this reason, the resumption of full‐dose anticoagulation with LMWH/heparin should be delayed for at least 48 hours in most patients undergoing a surgery or procedure associated with an increased risk of bleeding. Examples of these higher‐bleeding‐risk procedures include major thoracic surgery, intracranial or spinal surgery, major vascular surgery, major orthopedic surgery, urologic surgery involving the bladder or prostrate, major oncologic surgery, reconstructive plastic surgery, colonoscopy with associated polypectomy, renal or prostate biopsies, and placement of a cardiac pacemaker/defibrillator.1, 5157
Taken together, these uncertainties surrounding thromboembolic and bleeding risk estimates imply that there are multiple options for periprocedural management. Several studies, many of which included patients with mechanical heart valves, have shown similar safety and efficacy between LMWH and intravenous (IV) unfractionated heparin.5864 Table 3 summarizes these studies. The ACCP recommends bridging with LMWH over IV unfractionated heparin due to equal efficacy and cost savings with LMWH.1 When bridging is used, careful attention must be given to the timing and dose of anticoagulation in both the preoperative and postoperative periods. Table 4 lists dosing of commonly used LMWHs in North America. When using LMWHs in the preprocedural setting it is important to note that unacceptably high levels of anticoagulation remain present when a patient is given a full once‐daily LMWH dose the morning prior to the procedure or when a full‐dose, twice‐daily LMWH dose is given the evening prior to the procedure.65, 66 For this reason, the ACCP recommends administering the last preoperative dose 24 hours before surgery and if full‐dose once‐daily LMWH is used, the dose should be decreased by one‐half on the day before the surgery in order to ensure that no residual anticoagulant effect remains at the time of surgery.
| AuthorReference/Study Type | Number of Patients | Patient Population | Type of Procedure | Bridging Strategy | Major Bleeds | Minor Bleeds | TE Rate |
|---|---|---|---|---|---|---|---|
| |||||||
| Turpie and Douketis63/single arm cohort | 174 | 66% aortic valve; 34% mitral or dual prosthetic valve | Not specified | Enoxaparin 1 mg/kg twice daily | 2.3% | Not specified | None |
| Kovacs et al.61/single arm cohort | 224 | Prosthetic heart valves or a‐fib plus 1 major risk factor | 67 surgical; 157 nonsurgical | Preoperative bridging with dalteparin 200 IU/kg daily; dose reduced to 100 IU/kg on preoperative day 1; restarted at 100 IU/kg on POD 1; dose reduced to 5000 IU daily if high risk for bleeding | 6.7%; 8/15 occurred intraoperatively or <6 hours postoperatively; 2/15 occurred after 4 weeks | Not specified | 3.6%; 6/8 episodes occurred after warfarin held secondary to bleeding; 2/8 thrombotic episodes judged to be due to cardioembolism |
| Douketis et al.59/prospective registry | 650 | A‐fib 58%; mechanical heart valve 33% | 251 surgical; 399 nonsurgical | Dalteparin 100 IU/kg twice daily; held after high bleeding risk procedure and patients with poor hemostasis | 0.92% | 5.9% | 0.6% |
| Spyropolous et al.62/prospective registry; 14 centers in United States and Canada | 901 | UFH: 40% mechanical valves, 33% a‐fib; LMWH: 24% mechanical valve, 40% a‐fib | 394 surgical; 507 nonsurgical | LMWH mostly given twice daily 80%; UFH 20% | 5.5% UFH; 3.3% LMWH | 9.1% UFH; 12.0% LMWH | 2.4% UFH; 0.9% LMWH |
| Dunn et al.66/prospective cohort | 260 | A‐fib 68% or prior DVT 37% (excluding prosthetic heart valves) | 105 surgical; 145 nonsurgical | Enoxaparin 1.5 mg/kg daily | 3.5% overall; minor surgery/procedures 0.9%; major surgery 28% | 42% | 1.9%; 1/5 events occurred after bleeding led to withdrawal of AC |
| Omran et al.77/prospective registry | 779 | Various indications | Major and minor procedures | All patients bridged with enoxaparin; moderate TE risk 1 mg/kg daily; high TE risk 1 mg/kg twice daily | 0.5%; all in high‐risk group | 5.9% | 0 |
| Garcia et al.71/prospective, observational cohort of 101 sites in United States | 1024 patients with 1293 interruptions of AC | A‐fib 53%; VTE 14%; prosthetic valve 13% | Outpatient procedures only | At discretion of provider. Bridging performed in 8.3% of interruptions; 3% a‐fib, 10% VTE, and 29% mechanical valves | 0.6%; 4/6 patients with major bleed received bridging | 1.7%;10/17 patients with minor bleed received bridging | 0.7%; no events in patients who were bridged |
| Wysokinski et al.64/prospective cohort | 345 consecutive patients undergoing 386 procedures | 100% nonvalvular a‐fib | Major and minor surgeries/procedures | Individualized in AC clinic; 52% of patients bridged | 2.7%; no difference whether patient received bridging or not | 3.0%; 10/11 occurred in bridged patients | 1.1%; no difference in bridged vs. nonbridged patients |
| Low Molecular Weight Heparin | Subcutaneous Dose |
|---|---|
| |
| Dalteparin | |
| Low dose (prophylaxis dose) | 5,000 IU once daily |
| Full dose | 100 IU/kg twice daily or 200 IU/kg once daily |
| Enoxaparin | |
| Low dose (prophylaxis dose) | 30 mg twice daily or 40mg daily |
| Full dose | 1 mg/kg twice daily or 1.5 mg/kg once daily |
| Tinzaparin (full dose) | 175 IU/kg once daily |
In the postprocedural setting, timing and dose of anticoagulant is important, as major bleeding with the use of therapeutic anticoagulation can occur in up to 10% to 20% of cases. When restarting anticoagulation after the procedure, it is important to evaluate intraoperative hemostasis and to consider patient‐related factors that may further increase bleeding risk. These include advanced age, concomitant antiplatelet or nonsteroidal antiinflammatory medications, renal insufficiency, placement of spinal/epidural catheter, worsening liver disease, or the presence of other comorbid illnesses such as cancer.30, 67, 68 The ACCP recommends withholding full‐dose anticoagulation for at least 48 to 72 hours in patients who are felt to be at a high risk for postoperative bleeding.1 Figure 2 is a proposed management approach to the use of bridging anticoagulants that is consistent with the 2008 ACCP recommendations.

CONCLUSION
The evaluation and management of patients on long‐term antiplatelet or VKA therapy who require an invasive procedure or surgery is a common, complicated, and controversial area. Importantly, it is an area in which the hospitalist physician must be adept. Although there remain many unanswered clinical questions, an evolving literature base and recent practice guidelines can help guide management decisions.
- ,,, et al.The Perioperative Management of Antithrombotic Therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6_suppl):299S–339S.
- ,,,,.Aspirin use among adults aged 40 and older in the United States: results of a national survey.Am J Prev Med.2007;32(5):403–407.
- ,.Oral anticoagulation in surgical procedures: risks and recommendations.Br J Haematol.2003;123(4):676–682.
- Perioperative medicine.J Hosp Med.2006;1(supp 1):30–31.
- .Perioperative anticoagulation management in patients who are receiving oral anticoagulant therapy: a practical guide for clinicians.Thromb Res.2002;108(1):3–13.
- ,,,.Periprocedural bridging therapy in patients receiving chronic oral anticoagulation therapy.Curr Med Res Opin.2006;22(6):1109–1122.
- ,,,,.Effect of aspirin intake on bleeding during cataract surgery.J Cataract Refract Surg.1998;24(9):1243–1246.
- ,,,.Low‐dose aspirin for secondary cardiovascular prevention–cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation—review and meta‐analysis.J Intern Med.2005;257(5):399–414.
- ,,.Aspirin does not increase bleeding complications after transbronchial biopsy.Chest.2002;122(4):1461–1464.
- ,.The management of patients on anticoagulants prior to cutaneous surgery: case report of a thromboembolic complication, review of the literature, and evidence‐based recommendations.Plast Reconstr Surg.2006;118(5):110e–117e.
- .Unanswered questions—drug‐eluting stents and the risk of late thrombosis.N Engl J Med.2007;356(10):981–984.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115(6):813–818.
- ,,, et al.The Primary and Secondary Prevention of Coronary Artery Disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6_suppl):776S–814S.
- ,,, et al.2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee.Circulation.2008;117(2):261–295.
- ,,,,,.Late thrombosis of drug‐eluting stents: a meta‐analysis of randomized clinical trials.Am J Med.2006;119(12):1056–1061.
- ,,, et al.Early and late coronary stent thrombosis of sirolimus‐eluting and paclitaxel‐eluting stents in routine clinical practice: data from a large two‐institutional cohort study.Lancet.2007;369(9562):667–678.
- ,,, et al.Clopidogrel use and long‐term clinical outcomes after drug‐eluting stent implantation.JAMA.2007;297(2):159–168.
- Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.Antiplatelet Trialists' Collaboration.Br Med J (Clin Res Ed).1994;308(6921):81–106.
- ,,, et al.Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures.Gastrointest Endosc.2002;55(7):775–779.
- ,.Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease.Aliment Pharmacol Ther.2005;21(5):525–529.
- ,,, et al.Performance standards for therapeutic abdominal paracentesis.Hepatology.2004;40(2):484–488.
- ,.Percutaneous cannulation of the internal jugular vein in patients with coagulopathies: an experience based on 1,000 attempts.Anesthesiology.1982;56(4):321–323.
- ,,,,,.Central venous catheterization in patients with coagulopathy.Arch Surg.1992;127(3):273–275.
- ,,.Central venous catheter placement in patients with disorders of hemostasis.Chest.1996;110(1):185–188.
- ,,.US‐guided placement of central vein catheters in patients with disorders of hemostasis.Eur J Radiol.2008;65(2):253–256.
- ,.Central venous cannulation in patients with liver disease and coagulopathy—a prospective audit.Intensive Care Med.1999;25(5):481–485.
- ,.Perioperative management of patients receiving oral anticoagulants: a systematic review.Arch Intern Med.2003;163(8):901–908.
- ,,, et al.Kelley's Textbook of Rheumatology.7th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,.Clinical Procedures in Emergency Medicine.4th ed.Philadelphia, PA:W.B. Saunders;2004.
- ,.A prospective study of the safety of joint and soft tissue aspirations and injections in patients taking warfarin sodium.Arthritis Rheum.1998;41(4):736–739.
- ,,, et al.[Frequency of the bleeding risk in patients receiving warfarin submitted to arthrocentesis of the knee].Reumatismo.2003;55(3):159–163.
- .Textbook of Critical Care.5th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,.Complications of lumbar puncture followed by anticoagulation.Stroke.1981;12(6):879–881.
- ,,,.Mason, Murray and Nadel's Textbook of Respiratory Medicine.4th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,,.Videos in clinical medicine. Thoracentesis.N Engl J Med.2006;355(15):e16.
- ,.Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities.Transfusion.1991;31(2):164–171.
- .Risk of bleeding during thoracentesis in anticoagulated patients.Chest.2006;130(4):141S–d‐2.
- ,,, et al.Guidelines for prevention of thromboembolic events in valvular heart disease. Study Group of the Working Group on Valvular Heart Disease of the European Society of Cardiology.Eur Heart J.1995;16(10):1320–1330.
- ,.Coronary angiography and intravascular ultrasonography. In: Braunwald E, Zipes DP, Libby P, eds.Heart Disease: A Textbook of Cardiovascular Medicine.6th ed.Philadelphia, PA:WB Saunders;2001:387–421.
- ,,, et al.Effectiveness of manual pressure hemostasis following transfemoral coronary angiography in patients on therapeutic warfarin anticoagulation.Am J Cardiol.2006;97(4):485–488.
- ,,,,,.Elective coronary angiography and percutaneous coronary intervention during uninterrupted warfarin therapy.Catheter Cardiovasc Interv.2003;60(2):180–184.
- ,,,.Coronary angiography in the fully anticoagulated patient: the transradial route is successful and safe.Catheter Cardiovasc Interv.2003;58(1):8–10.
- ,,,,.Percutaneous left and right heart catheterization in fully anticoagulated patients utilizing the radial artery and forearm vein: a two‐center experience.J Interv Cardiol.2006;19(3):258–263.
- ,,, et al.[Safety of diagnostic transradial catheterization in patients undergoing long‐term anticoagulation with coumarin derivatives].Rev Esp Cardiol.2007;60(9):988–991. [Spanish]
- ,,,,,,,,,,,,,2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Valvular Heart Disease).J Am Coll Cardiol.2008;52:e1–142.
- ,,, et al.ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in Collaboration With the North American Society of Pacing and Electrophysiology.J Am Coll Cardiol.2001;38(4):1231–1266.
- .Periprocedural thromboprophylaxis in patients receiving chronic anticoagulation therapy.Am Heart J.2004;147(1):3–15.
- ,.Management of anticoagulation before and after elective surgery.N Engl J Med.1997;336(21):1506–1511.
- ,,,,,.Modern management of prosthetic valve anticoagulation.Mayo Clin Proc.1998;73(7):665–680.
- ,.Anticoagulant‐related bleeding: clinical epidemiology, prediction, and prevention.Am J Med.1993;95(3):315–328.
- ,,,,,.Reconstruction of musculoskeletal defects following oncologic resection in 76 patients.Ann Plast Surg.2006;57(2):190–194.
- ,,,,.Biopsy of the prostate guided by transrectal ultrasound: relation between warfarin use and incidence of bleeding complications.Clin Radiol.2005;60(4):459–463; discussion 457‐458.
- ,.Anticoagulation in neurosurgical patients.Neurosurgery.1999;45(4):838–847; discussion 847‐848.
- ,,,,.Post‐operative blood loss after transurethral prostatectomy is dependent on in situ fibrinolysis.Br J Urol.1997;80(6):889–893.
- ,,.Complications of heparin therapy after total joint arthroplasty.J Bone Joint Surg.1989;71(8):1130–1134.
- ,,,,,.Postpolypectomy lower GI bleeding: descriptive analysis.Gastrointest Endosc.2000;51(6):690–696.
- ,,, et al.Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy.Chest.2004;126(4):1177–1186.
- ,,.[Perioperative bridging with enoxaparin. results of the prospective BRAVE registry with 779 patients].Med Klin (Munich).2007;102(10):809–815. [German]
- ,,.Low molecular weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen.Arch Intern Med.2004;164(12):1319–1326.
- ,,.Bridging therapy in patients on long‐term oral anticoagulants who require surgery: the Prospective Peri‐operative Enoxaparin Cohort Trial (PROSPECT).J Thromb Haemost.2007;5(11):2211–2218.
- ,,, et al.Single‐arm study of bridging therapy with low molecular weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin.Circulation.2004;110(12):1658–1663.
- ,,,.Costs and clinical outcomes associated with low molecular weight heparin vs unfractionated heparin for perioperative bridging in patients receiving long‐term oral anticoagulant therapy.Chest.2004;125(5):1642–1650.
- ,.Enoxaparin is effective and safe as bridging anticoagulation in patients with a mechanical prosthetic heart valve who require temporary interruption of warfarin because of surgery or an invasive procedure. [Abstract #703]. ASH Annual Meeting Abstracts.Blood.2004;104:202A.
- ,,, et al.Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation.Mayo Clin Proc.2008;83(6):639–645.
- ,,,.Bridging anticoagulation with low molecular weight heparin after interruption of warfarin therapy is associated with a residual anticoagulant effect prior to surgery.Thromb Haemost.2005;94(3):528–531.
- ,,, et al.Brief communication: preoperative anticoagulant activity after bridging low molecular weight heparin for temporary interruption of warfarin.Ann Intern Med.2007;146(3):184–187.
- National Institute for Clinical Excellence. Atrial fibrillation: national clinical guideline for management in primary and secondary care.2006. Available at: http://www.nice.org.uk/nicemedia/pdf/cg036fullguideline. pdf. Accessed May 2009.
- ,,, et al.Clinical outcomes with unfractionated heparin or low molecular weight heparin as bridging therapy in patients on long‐term oral anticoagulants: the REGIMEN registry.J Thromb Haemost.2006;4(6):1246–1252.
- ,,,,,.Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation.JAMA.2001;285(22):2864–2870.
- ,,.Guidelines on oral anticoagulation (warfarin). 3rd ed. 2005 update.Br J Haematol.2006;132(3):277–285.
- ,,, et al.Risk of thromboembolism with short‐term interruption of warfarin therapy.Arch Intern Med.2008;168(1):63–69.
- ,,, et al.The Perioperative Management of Antithrombotic Therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6_suppl):299S–339S.
- ,,,,.Aspirin use among adults aged 40 and older in the United States: results of a national survey.Am J Prev Med.2007;32(5):403–407.
- ,.Oral anticoagulation in surgical procedures: risks and recommendations.Br J Haematol.2003;123(4):676–682.
- Perioperative medicine.J Hosp Med.2006;1(supp 1):30–31.
- .Perioperative anticoagulation management in patients who are receiving oral anticoagulant therapy: a practical guide for clinicians.Thromb Res.2002;108(1):3–13.
- ,,,.Periprocedural bridging therapy in patients receiving chronic oral anticoagulation therapy.Curr Med Res Opin.2006;22(6):1109–1122.
- ,,,,.Effect of aspirin intake on bleeding during cataract surgery.J Cataract Refract Surg.1998;24(9):1243–1246.
- ,,,.Low‐dose aspirin for secondary cardiovascular prevention–cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation—review and meta‐analysis.J Intern Med.2005;257(5):399–414.
- ,,.Aspirin does not increase bleeding complications after transbronchial biopsy.Chest.2002;122(4):1461–1464.
- ,.The management of patients on anticoagulants prior to cutaneous surgery: case report of a thromboembolic complication, review of the literature, and evidence‐based recommendations.Plast Reconstr Surg.2006;118(5):110e–117e.
- .Unanswered questions—drug‐eluting stents and the risk of late thrombosis.N Engl J Med.2007;356(10):981–984.
- ,,, et al.Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians.Circulation.2007;115(6):813–818.
- ,,, et al.The Primary and Secondary Prevention of Coronary Artery Disease: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133(6_suppl):776S–814S.
- ,,, et al.2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee.Circulation.2008;117(2):261–295.
- ,,,,,.Late thrombosis of drug‐eluting stents: a meta‐analysis of randomized clinical trials.Am J Med.2006;119(12):1056–1061.
- ,,, et al.Early and late coronary stent thrombosis of sirolimus‐eluting and paclitaxel‐eluting stents in routine clinical practice: data from a large two‐institutional cohort study.Lancet.2007;369(9562):667–678.
- ,,, et al.Clopidogrel use and long‐term clinical outcomes after drug‐eluting stent implantation.JAMA.2007;297(2):159–168.
- Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients.Antiplatelet Trialists' Collaboration.Br Med J (Clin Res Ed).1994;308(6921):81–106.
- ,,, et al.Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures.Gastrointest Endosc.2002;55(7):775–779.
- ,.Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease.Aliment Pharmacol Ther.2005;21(5):525–529.
- ,,, et al.Performance standards for therapeutic abdominal paracentesis.Hepatology.2004;40(2):484–488.
- ,.Percutaneous cannulation of the internal jugular vein in patients with coagulopathies: an experience based on 1,000 attempts.Anesthesiology.1982;56(4):321–323.
- ,,,,,.Central venous catheterization in patients with coagulopathy.Arch Surg.1992;127(3):273–275.
- ,,.Central venous catheter placement in patients with disorders of hemostasis.Chest.1996;110(1):185–188.
- ,,.US‐guided placement of central vein catheters in patients with disorders of hemostasis.Eur J Radiol.2008;65(2):253–256.
- ,.Central venous cannulation in patients with liver disease and coagulopathy—a prospective audit.Intensive Care Med.1999;25(5):481–485.
- ,.Perioperative management of patients receiving oral anticoagulants: a systematic review.Arch Intern Med.2003;163(8):901–908.
- ,,, et al.Kelley's Textbook of Rheumatology.7th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,.Clinical Procedures in Emergency Medicine.4th ed.Philadelphia, PA:W.B. Saunders;2004.
- ,.A prospective study of the safety of joint and soft tissue aspirations and injections in patients taking warfarin sodium.Arthritis Rheum.1998;41(4):736–739.
- ,,, et al.[Frequency of the bleeding risk in patients receiving warfarin submitted to arthrocentesis of the knee].Reumatismo.2003;55(3):159–163.
- .Textbook of Critical Care.5th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,.Complications of lumbar puncture followed by anticoagulation.Stroke.1981;12(6):879–881.
- ,,,.Mason, Murray and Nadel's Textbook of Respiratory Medicine.4th ed.Philadelphia, PA:Elsevier Saunders;2005.
- ,,.Videos in clinical medicine. Thoracentesis.N Engl J Med.2006;355(15):e16.
- ,.Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities.Transfusion.1991;31(2):164–171.
- .Risk of bleeding during thoracentesis in anticoagulated patients.Chest.2006;130(4):141S–d‐2.
- ,,, et al.Guidelines for prevention of thromboembolic events in valvular heart disease. Study Group of the Working Group on Valvular Heart Disease of the European Society of Cardiology.Eur Heart J.1995;16(10):1320–1330.
- ,.Coronary angiography and intravascular ultrasonography. In: Braunwald E, Zipes DP, Libby P, eds.Heart Disease: A Textbook of Cardiovascular Medicine.6th ed.Philadelphia, PA:WB Saunders;2001:387–421.
- ,,, et al.Effectiveness of manual pressure hemostasis following transfemoral coronary angiography in patients on therapeutic warfarin anticoagulation.Am J Cardiol.2006;97(4):485–488.
- ,,,,,.Elective coronary angiography and percutaneous coronary intervention during uninterrupted warfarin therapy.Catheter Cardiovasc Interv.2003;60(2):180–184.
- ,,,.Coronary angiography in the fully anticoagulated patient: the transradial route is successful and safe.Catheter Cardiovasc Interv.2003;58(1):8–10.
- ,,,,.Percutaneous left and right heart catheterization in fully anticoagulated patients utilizing the radial artery and forearm vein: a two‐center experience.J Interv Cardiol.2006;19(3):258–263.
- ,,, et al.[Safety of diagnostic transradial catheterization in patients undergoing long‐term anticoagulation with coumarin derivatives].Rev Esp Cardiol.2007;60(9):988–991. [Spanish]
- ,,,,,,,,,,,,,2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Valvular Heart Disease).J Am Coll Cardiol.2008;52:e1–142.
- ,,, et al.ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in Collaboration With the North American Society of Pacing and Electrophysiology.J Am Coll Cardiol.2001;38(4):1231–1266.
- .Periprocedural thromboprophylaxis in patients receiving chronic anticoagulation therapy.Am Heart J.2004;147(1):3–15.
- ,.Management of anticoagulation before and after elective surgery.N Engl J Med.1997;336(21):1506–1511.
- ,,,,,.Modern management of prosthetic valve anticoagulation.Mayo Clin Proc.1998;73(7):665–680.
- ,.Anticoagulant‐related bleeding: clinical epidemiology, prediction, and prevention.Am J Med.1993;95(3):315–328.
- ,,,,,.Reconstruction of musculoskeletal defects following oncologic resection in 76 patients.Ann Plast Surg.2006;57(2):190–194.
- ,,,,.Biopsy of the prostate guided by transrectal ultrasound: relation between warfarin use and incidence of bleeding complications.Clin Radiol.2005;60(4):459–463; discussion 457‐458.
- ,.Anticoagulation in neurosurgical patients.Neurosurgery.1999;45(4):838–847; discussion 847‐848.
- ,,,,.Post‐operative blood loss after transurethral prostatectomy is dependent on in situ fibrinolysis.Br J Urol.1997;80(6):889–893.
- ,,.Complications of heparin therapy after total joint arthroplasty.J Bone Joint Surg.1989;71(8):1130–1134.
- ,,,,,.Postpolypectomy lower GI bleeding: descriptive analysis.Gastrointest Endosc.2000;51(6):690–696.
- ,,, et al.Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy.Chest.2004;126(4):1177–1186.
- ,,.[Perioperative bridging with enoxaparin. results of the prospective BRAVE registry with 779 patients].Med Klin (Munich).2007;102(10):809–815. [German]
- ,,.Low molecular weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen.Arch Intern Med.2004;164(12):1319–1326.
- ,,.Bridging therapy in patients on long‐term oral anticoagulants who require surgery: the Prospective Peri‐operative Enoxaparin Cohort Trial (PROSPECT).J Thromb Haemost.2007;5(11):2211–2218.
- ,,, et al.Single‐arm study of bridging therapy with low molecular weight heparin for patients at risk of arterial embolism who require temporary interruption of warfarin.Circulation.2004;110(12):1658–1663.
- ,,,.Costs and clinical outcomes associated with low molecular weight heparin vs unfractionated heparin for perioperative bridging in patients receiving long‐term oral anticoagulant therapy.Chest.2004;125(5):1642–1650.
- ,.Enoxaparin is effective and safe as bridging anticoagulation in patients with a mechanical prosthetic heart valve who require temporary interruption of warfarin because of surgery or an invasive procedure. [Abstract #703]. ASH Annual Meeting Abstracts.Blood.2004;104:202A.
- ,,, et al.Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation.Mayo Clin Proc.2008;83(6):639–645.
- ,,,.Bridging anticoagulation with low molecular weight heparin after interruption of warfarin therapy is associated with a residual anticoagulant effect prior to surgery.Thromb Haemost.2005;94(3):528–531.
- ,,, et al.Brief communication: preoperative anticoagulant activity after bridging low molecular weight heparin for temporary interruption of warfarin.Ann Intern Med.2007;146(3):184–187.
- National Institute for Clinical Excellence. Atrial fibrillation: national clinical guideline for management in primary and secondary care.2006. Available at: http://www.nice.org.uk/nicemedia/pdf/cg036fullguideline. pdf. Accessed May 2009.
- ,,, et al.Clinical outcomes with unfractionated heparin or low molecular weight heparin as bridging therapy in patients on long‐term oral anticoagulants: the REGIMEN registry.J Thromb Haemost.2006;4(6):1246–1252.
- ,,,,,.Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation.JAMA.2001;285(22):2864–2870.
- ,,.Guidelines on oral anticoagulation (warfarin). 3rd ed. 2005 update.Br J Haematol.2006;132(3):277–285.
- ,,, et al.Risk of thromboembolism with short‐term interruption of warfarin therapy.Arch Intern Med.2008;168(1):63–69.