User login
Analysis of Hospital Resource Availability and COVID-19 Mortality Across the United States
The COVID-19 pandemic is a crisis of mismatch between resources and infection burden. There is extraordinary heterogeneity across time and geography in the pandemic impact, with hospitals in New York City initially inundated while hospitals in major urban areas of California were comparatively quiet. Efforts to “flatten the curve” are intended to improve outcomes by reducing health system overload.1 In the case of hospital-based care, health systems’ primary resources include emergency and critical care bed and staff capacity.
Prior work has documented wide variability in intensive care capacity across the United States and hypothesized that even moderate disease outbreaks could overwhelm hospital referral regions (HRRs).2,3 Various simulations of outbreaks suggested that thousands of deaths are potentially preventable depending on the health system’s response,4 although the degree to which resource limitations have contributed to mortality during this COVID-19 pandemic has yet to be explored. The objective of this analysis was to examine the association between hospital resources and COVID-19 deaths amongst HRRs in the United States in the period from March 1 to July 26, 2020.
METHODS
Data
This was an analysis of the American Hospital Association Annual Survey Database from 2017 and 2018, including hospital resource variables such as total hospital beds, hospitalists, intensive care beds, intensivists, emergency physicians, and nurses.5 The analysis was limited to general medical and surgical hospitals capable of providing acute care services, defined as those reporting at least 500 emergency department visits in 2018. Where data were missing on analysis variables (26.0% missing overall), the data were drawn from the 2017 survey results (reduced to 23.8% missing) from the same site as available, and the remainder were imputed with multivariate imputation by chained equations. An identical analysis without imputation was performed as a sensitivity analysis that showed a similar pattern of results. Total resources were tabulated amongst HRRs, and the hospital resources per COVID-19 case calculated. HRRs are a geographic category devised to represent regional health care markets, and each includes hospital sites performing major procedures.3 These were the focus of the analysis because they may represent a meaningful geographic division of hospital-based resources. COVID-19 case and death counts (as of July 26, 2020) were drawn from publicly available county-level data curated by the New York Times from state and local governments as well as health departments nationwide,6 separated by month (ie, March, April, May, June, and July). Data on New York City were available in aggregate (rather than separated by borough). Cases and deaths were therefore apportioned to the three HRRs involving New York City in proportion to that area’s population. To adjust for the lag between COVID-19 cases and deaths,7,8 we offset deaths 2 weeks into the future so that the April COVID-19 death count for a given HRR included deaths that occurred for 1 month beginning 2 weeks after the start of April, and so on.
Analysis
We estimated Poisson distribution regressions for the outcome of COVID-19 death count in each HRR and month with one model for each of our six hospital-based resource variables. The offset (exposure) variable was COVID-19 case count. To adjust for the possibility of varying effects of hospital resources on deaths by month (ie, in anticipation that health systems might evolve in response to the pandemic over time), each model includes terms for the interaction between hospital-based resource and an indicator variable for month, as well as a fifth term for month. Standard errors were adjusted for clustering within HRR. We report resultant incident rate ratios (IRRs) with 95% CIs, and we report these as statistically significant at the 5% level only after adjustment for multiple comparisons across our six hospital-resource variables using the conservative Bonferroni adjustment. The pseudo-R2 for each of these six models is also reported to summarize the amount of variation in deaths explained. For our model with ICU beds per COVID-19 case, we perform postestimation prediction of number of deaths by HRR, assuming the counterfactual in which HRRs with fewer than average ICU beds per COVID-19 case instead had the average observed number of ICU beds per COVID-19 case by HRR in April, which functioned as a measure of early excess deaths potentially related to resource limitations. The study was classified as exempt by the Institutional Review Board at the Yale School of Medicine, New Haven, Connecticut. Analyses were conducted in Stata 15 (StataCorp LLC) and R.
RESULTS
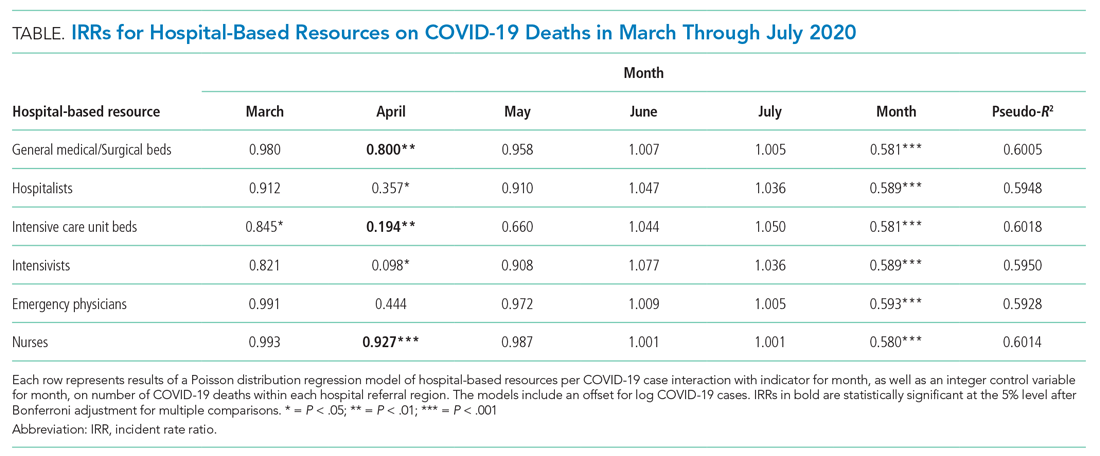
A total of 4,453 hospitals across 306 HRRs were included and linked to 2,827 county-level COVID-19 case and death counts in each of 5 months (March through July 2020). The median HRR in our analysis included 14 hospitals, with a maximum of 76 hospitals (Los Angeles, California) and a minimum of 1 (Longview, Texas). Among HRRs, 206 (67.3%) had experienced caseloads exceeding 20 per 10,000 population, while 85 (27.8%) had experienced greater than 100 per 10,000 population in the peak month during the study period. The Table depicts results of each of six Poisson distribution regression models, with the finding that greater number of ICU beds (IRR, 0.194; 95% CI, 0.076-0.491), general medical/surgical beds (IRR, 0.800; 95% CI, 0.696-0.920), and nurses (IRR, 0.927; 95% CI, 0.888-0.967) per COVID-19 case in April were statistically significantly associated with reduced deaths.
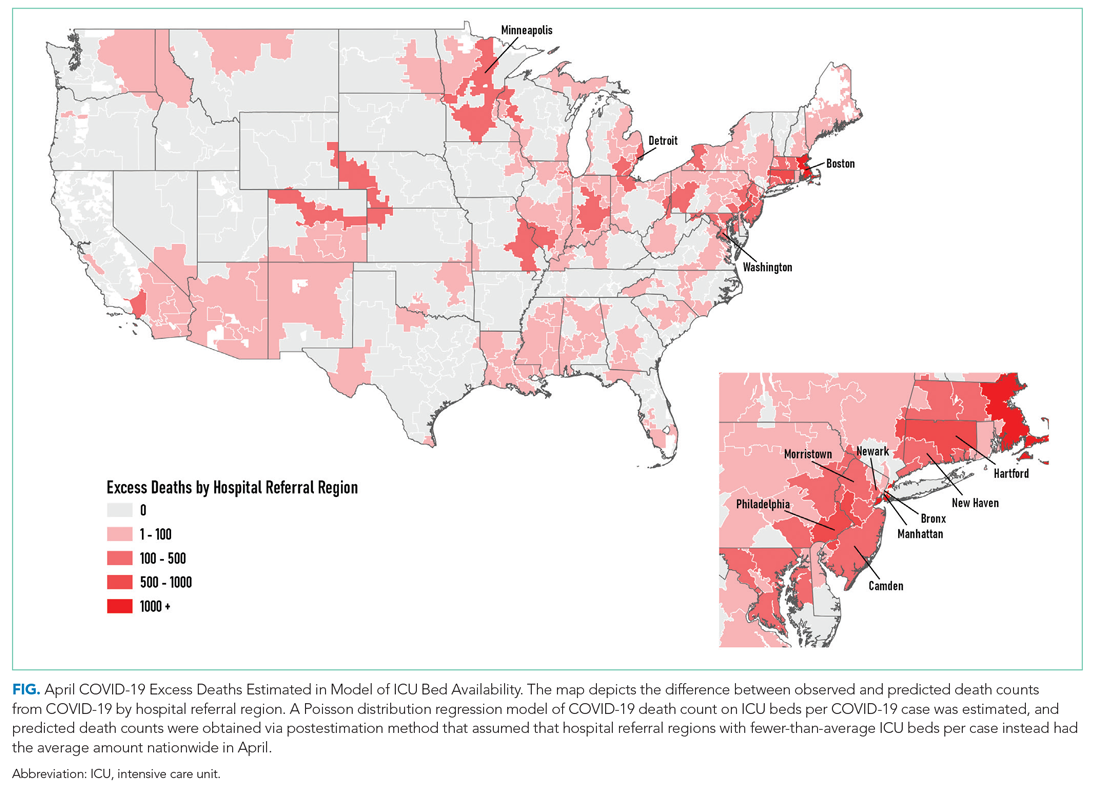
The model including ICU beds per COVID-19 case had the largest pseudo-R2 at 0.6018, which suggests that ICU bed availability explains the most variation in death count among hospital resource variables analyzed. The incident rate ratio in this model implies that, for an entire additional ICU bed for each COVID-19 case (a one-unit increase in that variable), there is an associated one-fifth decrease in incidence rate (IRR, 0.194) of death in April. The mean value among HRRs in April was 0.25 ICU beds per case (one ICU bed for every four COVID-19 cases), but it was as low as 0.01 to 0.005 in hard-hit areas (one ICU bed for every 100 to 200 COVID-19 cases). The early excess deaths observed in April were not observed in later months. The magnitude of this effect can be summarized as follows: If the 152 HRRs in April with fewer than the mean number of ICU beds per COVID-19 case were to instead have the mean number (one ICU bed for every four COVID-19 cases), our model estimates that there would have been 15,571 fewer deaths that month. The HRRs with the largest number of early excess deaths were Manhattan in New York City (1,466), Bronx in New York City (1,315), Boston, Massachusetts (1,293), Philadelphia, Pennsylvania (955), Hartford, Connecticut (682), Detroit, Michigan (499), and Camden, New Jersey (484). The Figure depicts HRRs in the United States with early excess deaths by this measure in April.
DISCUSSION
We found significant associations between availability of hospital-based resources and COVID-19 deaths in the month of April 2020. This observation was consistent across measures of both hospital bed and staff capacity but not statistically significant in all cases. This provides empiric evidence in support of a preprint simulation publication by Branas et al showing the potential for thousands of excess deaths related to lack of available resources.4 Interestingly, the relationship between hospital-based resources per COVID-19 case and death count is not seen in May, June, or July. This may be because hospitals and health systems were rapidly adapting to pandemic demands9 by shifting resources or reorganizing existing infrastructure to free up beds and personnel.
Our findings underscore the importance of analyses that address heterogeneity in health system response over time and across different geographic areas. That the relationship is not seen after the early pandemic period, when hospitals and health systems were most overwhelmed, suggests that health systems and communities were able to adapt. Importantly, this work does not address the likely complex relationships among hospital resources and outcomes (for example, the benefit of ICU bed availability is likely limited when there are insufficient intensivists and nurses). These complexities should be a focus of future work. Furthermore, hospital resource flexibility, community efforts to slow transmission, and improvements in testing availability and the management of COVID-19 among hospitalized patients may all play a role in attenuating the relationship between baseline resource limitations and outcomes for patients with COVID-19.
These results merit further granular studies to examine specific hospital resources and observed variation in outcomes. Prior literature has linked inpatient capacity—variously defined as high census, acuity, turnover, or delayed admission—to outcomes including mortality among patients with stroke, among those with acute coronary syndrome, and among those requiring intensive care.10 Literature from Italy’s experience shows there was large variation in the case fatality rate among regions of Northern Italy and argues this was partially due to hospital resource limitations.11 Future work can also address whether just-in-time resource mobilization, such as temporary ICU expansion, physician cross-staffing, telemedicine, and dedicated units for COVID-19 patients, attenuated the impact of potential hospital resource scarcity on outcomes.
The present analysis is limited by the quality of the data. There is likely variation of available COVID-19 testing by HRR. It may be that areas with larger outbreaks early on generally tested a smaller, sicker proportion of population-level cases than did those with smaller outbreaks. This effect may be reversed if larger HRRs in urban areas have health systems and public health departments more inclined toward or capable of doing more testing. Furthermore, deaths related to COVID-19 are likely related to community-based factors, including nonhealthcare resources and underlying population characteristics, that likely correlate with the availability of hospital-based resources within HRRs. Some have called into question whether, a priori, we should expect hospital-based capacity to be an important driver of mortality at all,12 arguing that, when critical care capacity is exceeded, resources may be efficiently reallocated away from patients who are least likely to benefit. Because we used the American Hospital Association data, this snapshot of hospital resources is not limited to critical care capacity because there could be alternative explanations for situations in which mortality for both COVID-19 and non–COVID-19 patients may be lower and hospital resources are better matched with demand. For example, patients may seek care earlier in their disease course (whether COVID-19 or otherwise)13 if their local emergency department is not thought to be overwhelmed with case volume.
CONCLUSION
We find that COVID-19 deaths vary among HRRs. The availability of several hospital-based resources is associated with death rates and supports early efforts across the United States to “flatten the curve” to prevent hospital overload. Continued surveillance of this relationship is essential to guide policymakers and hospitals seeking to balance the still limited supply of resources with the demands of caring for both infectious and noninfectious patients in the coming months of this outbreak and in future pandemics.
Acknowledgment
The authors gratefully acknowledge the help of Carolyn Lusch, AICP, in generating depictions of results in Geographic Information Systems.
1. Phua J, Weng L, Ling L, et al; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506-517. https://doi.org/10.1016/s2213-2600(20)30161-2
2. Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371-1372. https://doi.org/10.1001/jama.2010.394
3. General FAQ. Dartmouth Atlas Project. 2020. Accessed July 8, 2020. https://www.dartmouthatlas.org/faq/
4. Branas CC, Rundle A, Pei S, et al. Flattening the curve before it flattens us: hospital critical care capacity limits and mortality from novel coronavirus (SARS-CoV2) cases in US counties. medRxiv. Preprint posted online April 6, 2020. https://doi.org/10.1101/2020.04.01.20049759
5. American Hospital Association Annual Survey Database. American Hospital Association. 2018. Accessed July 8, 2020. https://www.ahadata.com/aha-annual-survey-database
6. An Ongoing Repository of Data on Coronavirus Cases and Deaths in the U.S. New York Times. 2020. Accessed July 8, 2020. https://github.com/nytimes/covid-19-data
7. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. https://doi.org/10.1016/s1473-3099(20)30195-x
8. Rosakis P, Marketou ME. Rethinking case fatality ratios for COVID-19 from a data-driven viewpoint. J Infect. 2020;81(2);e162-e164. https://doi.org/10.1016/j.jinf.2020.06.010
9. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
10. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guide JM. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
11. Volpato S, Landi F, Incalzi RA. A frail health care system for an old population: lesson form [sic] the COVID-19 outbreak in Italy. J Gerontol Series A. 2020;75(9):e126-e127. https://doi.org/10.1093/gerona/glaa087
12. Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447-455. https://doi.org/10.7326/0003-4819-159-7-201310010-00004
13. Moroni F, Gramegna M, Agello S, et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2(10):1620-1624. https://doi.org/10.1016/j.jaccas.2020.04.010
The COVID-19 pandemic is a crisis of mismatch between resources and infection burden. There is extraordinary heterogeneity across time and geography in the pandemic impact, with hospitals in New York City initially inundated while hospitals in major urban areas of California were comparatively quiet. Efforts to “flatten the curve” are intended to improve outcomes by reducing health system overload.1 In the case of hospital-based care, health systems’ primary resources include emergency and critical care bed and staff capacity.
Prior work has documented wide variability in intensive care capacity across the United States and hypothesized that even moderate disease outbreaks could overwhelm hospital referral regions (HRRs).2,3 Various simulations of outbreaks suggested that thousands of deaths are potentially preventable depending on the health system’s response,4 although the degree to which resource limitations have contributed to mortality during this COVID-19 pandemic has yet to be explored. The objective of this analysis was to examine the association between hospital resources and COVID-19 deaths amongst HRRs in the United States in the period from March 1 to July 26, 2020.
METHODS
Data
This was an analysis of the American Hospital Association Annual Survey Database from 2017 and 2018, including hospital resource variables such as total hospital beds, hospitalists, intensive care beds, intensivists, emergency physicians, and nurses.5 The analysis was limited to general medical and surgical hospitals capable of providing acute care services, defined as those reporting at least 500 emergency department visits in 2018. Where data were missing on analysis variables (26.0% missing overall), the data were drawn from the 2017 survey results (reduced to 23.8% missing) from the same site as available, and the remainder were imputed with multivariate imputation by chained equations. An identical analysis without imputation was performed as a sensitivity analysis that showed a similar pattern of results. Total resources were tabulated amongst HRRs, and the hospital resources per COVID-19 case calculated. HRRs are a geographic category devised to represent regional health care markets, and each includes hospital sites performing major procedures.3 These were the focus of the analysis because they may represent a meaningful geographic division of hospital-based resources. COVID-19 case and death counts (as of July 26, 2020) were drawn from publicly available county-level data curated by the New York Times from state and local governments as well as health departments nationwide,6 separated by month (ie, March, April, May, June, and July). Data on New York City were available in aggregate (rather than separated by borough). Cases and deaths were therefore apportioned to the three HRRs involving New York City in proportion to that area’s population. To adjust for the lag between COVID-19 cases and deaths,7,8 we offset deaths 2 weeks into the future so that the April COVID-19 death count for a given HRR included deaths that occurred for 1 month beginning 2 weeks after the start of April, and so on.
Analysis
We estimated Poisson distribution regressions for the outcome of COVID-19 death count in each HRR and month with one model for each of our six hospital-based resource variables. The offset (exposure) variable was COVID-19 case count. To adjust for the possibility of varying effects of hospital resources on deaths by month (ie, in anticipation that health systems might evolve in response to the pandemic over time), each model includes terms for the interaction between hospital-based resource and an indicator variable for month, as well as a fifth term for month. Standard errors were adjusted for clustering within HRR. We report resultant incident rate ratios (IRRs) with 95% CIs, and we report these as statistically significant at the 5% level only after adjustment for multiple comparisons across our six hospital-resource variables using the conservative Bonferroni adjustment. The pseudo-R2 for each of these six models is also reported to summarize the amount of variation in deaths explained. For our model with ICU beds per COVID-19 case, we perform postestimation prediction of number of deaths by HRR, assuming the counterfactual in which HRRs with fewer than average ICU beds per COVID-19 case instead had the average observed number of ICU beds per COVID-19 case by HRR in April, which functioned as a measure of early excess deaths potentially related to resource limitations. The study was classified as exempt by the Institutional Review Board at the Yale School of Medicine, New Haven, Connecticut. Analyses were conducted in Stata 15 (StataCorp LLC) and R.
RESULTS
A total of 4,453 hospitals across 306 HRRs were included and linked to 2,827 county-level COVID-19 case and death counts in each of 5 months (March through July 2020). The median HRR in our analysis included 14 hospitals, with a maximum of 76 hospitals (Los Angeles, California) and a minimum of 1 (Longview, Texas). Among HRRs, 206 (67.3%) had experienced caseloads exceeding 20 per 10,000 population, while 85 (27.8%) had experienced greater than 100 per 10,000 population in the peak month during the study period. The Table depicts results of each of six Poisson distribution regression models, with the finding that greater number of ICU beds (IRR, 0.194; 95% CI, 0.076-0.491), general medical/surgical beds (IRR, 0.800; 95% CI, 0.696-0.920), and nurses (IRR, 0.927; 95% CI, 0.888-0.967) per COVID-19 case in April were statistically significantly associated with reduced deaths.

The model including ICU beds per COVID-19 case had the largest pseudo-R2 at 0.6018, which suggests that ICU bed availability explains the most variation in death count among hospital resource variables analyzed. The incident rate ratio in this model implies that, for an entire additional ICU bed for each COVID-19 case (a one-unit increase in that variable), there is an associated one-fifth decrease in incidence rate (IRR, 0.194) of death in April. The mean value among HRRs in April was 0.25 ICU beds per case (one ICU bed for every four COVID-19 cases), but it was as low as 0.01 to 0.005 in hard-hit areas (one ICU bed for every 100 to 200 COVID-19 cases). The early excess deaths observed in April were not observed in later months. The magnitude of this effect can be summarized as follows: If the 152 HRRs in April with fewer than the mean number of ICU beds per COVID-19 case were to instead have the mean number (one ICU bed for every four COVID-19 cases), our model estimates that there would have been 15,571 fewer deaths that month. The HRRs with the largest number of early excess deaths were Manhattan in New York City (1,466), Bronx in New York City (1,315), Boston, Massachusetts (1,293), Philadelphia, Pennsylvania (955), Hartford, Connecticut (682), Detroit, Michigan (499), and Camden, New Jersey (484). The Figure depicts HRRs in the United States with early excess deaths by this measure in April.

DISCUSSION
We found significant associations between availability of hospital-based resources and COVID-19 deaths in the month of April 2020. This observation was consistent across measures of both hospital bed and staff capacity but not statistically significant in all cases. This provides empiric evidence in support of a preprint simulation publication by Branas et al showing the potential for thousands of excess deaths related to lack of available resources.4 Interestingly, the relationship between hospital-based resources per COVID-19 case and death count is not seen in May, June, or July. This may be because hospitals and health systems were rapidly adapting to pandemic demands9 by shifting resources or reorganizing existing infrastructure to free up beds and personnel.
Our findings underscore the importance of analyses that address heterogeneity in health system response over time and across different geographic areas. That the relationship is not seen after the early pandemic period, when hospitals and health systems were most overwhelmed, suggests that health systems and communities were able to adapt. Importantly, this work does not address the likely complex relationships among hospital resources and outcomes (for example, the benefit of ICU bed availability is likely limited when there are insufficient intensivists and nurses). These complexities should be a focus of future work. Furthermore, hospital resource flexibility, community efforts to slow transmission, and improvements in testing availability and the management of COVID-19 among hospitalized patients may all play a role in attenuating the relationship between baseline resource limitations and outcomes for patients with COVID-19.
These results merit further granular studies to examine specific hospital resources and observed variation in outcomes. Prior literature has linked inpatient capacity—variously defined as high census, acuity, turnover, or delayed admission—to outcomes including mortality among patients with stroke, among those with acute coronary syndrome, and among those requiring intensive care.10 Literature from Italy’s experience shows there was large variation in the case fatality rate among regions of Northern Italy and argues this was partially due to hospital resource limitations.11 Future work can also address whether just-in-time resource mobilization, such as temporary ICU expansion, physician cross-staffing, telemedicine, and dedicated units for COVID-19 patients, attenuated the impact of potential hospital resource scarcity on outcomes.
The present analysis is limited by the quality of the data. There is likely variation of available COVID-19 testing by HRR. It may be that areas with larger outbreaks early on generally tested a smaller, sicker proportion of population-level cases than did those with smaller outbreaks. This effect may be reversed if larger HRRs in urban areas have health systems and public health departments more inclined toward or capable of doing more testing. Furthermore, deaths related to COVID-19 are likely related to community-based factors, including nonhealthcare resources and underlying population characteristics, that likely correlate with the availability of hospital-based resources within HRRs. Some have called into question whether, a priori, we should expect hospital-based capacity to be an important driver of mortality at all,12 arguing that, when critical care capacity is exceeded, resources may be efficiently reallocated away from patients who are least likely to benefit. Because we used the American Hospital Association data, this snapshot of hospital resources is not limited to critical care capacity because there could be alternative explanations for situations in which mortality for both COVID-19 and non–COVID-19 patients may be lower and hospital resources are better matched with demand. For example, patients may seek care earlier in their disease course (whether COVID-19 or otherwise)13 if their local emergency department is not thought to be overwhelmed with case volume.
CONCLUSION
We find that COVID-19 deaths vary among HRRs. The availability of several hospital-based resources is associated with death rates and supports early efforts across the United States to “flatten the curve” to prevent hospital overload. Continued surveillance of this relationship is essential to guide policymakers and hospitals seeking to balance the still limited supply of resources with the demands of caring for both infectious and noninfectious patients in the coming months of this outbreak and in future pandemics.
Acknowledgment
The authors gratefully acknowledge the help of Carolyn Lusch, AICP, in generating depictions of results in Geographic Information Systems.
The COVID-19 pandemic is a crisis of mismatch between resources and infection burden. There is extraordinary heterogeneity across time and geography in the pandemic impact, with hospitals in New York City initially inundated while hospitals in major urban areas of California were comparatively quiet. Efforts to “flatten the curve” are intended to improve outcomes by reducing health system overload.1 In the case of hospital-based care, health systems’ primary resources include emergency and critical care bed and staff capacity.
Prior work has documented wide variability in intensive care capacity across the United States and hypothesized that even moderate disease outbreaks could overwhelm hospital referral regions (HRRs).2,3 Various simulations of outbreaks suggested that thousands of deaths are potentially preventable depending on the health system’s response,4 although the degree to which resource limitations have contributed to mortality during this COVID-19 pandemic has yet to be explored. The objective of this analysis was to examine the association between hospital resources and COVID-19 deaths amongst HRRs in the United States in the period from March 1 to July 26, 2020.
METHODS
Data
This was an analysis of the American Hospital Association Annual Survey Database from 2017 and 2018, including hospital resource variables such as total hospital beds, hospitalists, intensive care beds, intensivists, emergency physicians, and nurses.5 The analysis was limited to general medical and surgical hospitals capable of providing acute care services, defined as those reporting at least 500 emergency department visits in 2018. Where data were missing on analysis variables (26.0% missing overall), the data were drawn from the 2017 survey results (reduced to 23.8% missing) from the same site as available, and the remainder were imputed with multivariate imputation by chained equations. An identical analysis without imputation was performed as a sensitivity analysis that showed a similar pattern of results. Total resources were tabulated amongst HRRs, and the hospital resources per COVID-19 case calculated. HRRs are a geographic category devised to represent regional health care markets, and each includes hospital sites performing major procedures.3 These were the focus of the analysis because they may represent a meaningful geographic division of hospital-based resources. COVID-19 case and death counts (as of July 26, 2020) were drawn from publicly available county-level data curated by the New York Times from state and local governments as well as health departments nationwide,6 separated by month (ie, March, April, May, June, and July). Data on New York City were available in aggregate (rather than separated by borough). Cases and deaths were therefore apportioned to the three HRRs involving New York City in proportion to that area’s population. To adjust for the lag between COVID-19 cases and deaths,7,8 we offset deaths 2 weeks into the future so that the April COVID-19 death count for a given HRR included deaths that occurred for 1 month beginning 2 weeks after the start of April, and so on.
Analysis
We estimated Poisson distribution regressions for the outcome of COVID-19 death count in each HRR and month with one model for each of our six hospital-based resource variables. The offset (exposure) variable was COVID-19 case count. To adjust for the possibility of varying effects of hospital resources on deaths by month (ie, in anticipation that health systems might evolve in response to the pandemic over time), each model includes terms for the interaction between hospital-based resource and an indicator variable for month, as well as a fifth term for month. Standard errors were adjusted for clustering within HRR. We report resultant incident rate ratios (IRRs) with 95% CIs, and we report these as statistically significant at the 5% level only after adjustment for multiple comparisons across our six hospital-resource variables using the conservative Bonferroni adjustment. The pseudo-R2 for each of these six models is also reported to summarize the amount of variation in deaths explained. For our model with ICU beds per COVID-19 case, we perform postestimation prediction of number of deaths by HRR, assuming the counterfactual in which HRRs with fewer than average ICU beds per COVID-19 case instead had the average observed number of ICU beds per COVID-19 case by HRR in April, which functioned as a measure of early excess deaths potentially related to resource limitations. The study was classified as exempt by the Institutional Review Board at the Yale School of Medicine, New Haven, Connecticut. Analyses were conducted in Stata 15 (StataCorp LLC) and R.
RESULTS
A total of 4,453 hospitals across 306 HRRs were included and linked to 2,827 county-level COVID-19 case and death counts in each of 5 months (March through July 2020). The median HRR in our analysis included 14 hospitals, with a maximum of 76 hospitals (Los Angeles, California) and a minimum of 1 (Longview, Texas). Among HRRs, 206 (67.3%) had experienced caseloads exceeding 20 per 10,000 population, while 85 (27.8%) had experienced greater than 100 per 10,000 population in the peak month during the study period. The Table depicts results of each of six Poisson distribution regression models, with the finding that greater number of ICU beds (IRR, 0.194; 95% CI, 0.076-0.491), general medical/surgical beds (IRR, 0.800; 95% CI, 0.696-0.920), and nurses (IRR, 0.927; 95% CI, 0.888-0.967) per COVID-19 case in April were statistically significantly associated with reduced deaths.

The model including ICU beds per COVID-19 case had the largest pseudo-R2 at 0.6018, which suggests that ICU bed availability explains the most variation in death count among hospital resource variables analyzed. The incident rate ratio in this model implies that, for an entire additional ICU bed for each COVID-19 case (a one-unit increase in that variable), there is an associated one-fifth decrease in incidence rate (IRR, 0.194) of death in April. The mean value among HRRs in April was 0.25 ICU beds per case (one ICU bed for every four COVID-19 cases), but it was as low as 0.01 to 0.005 in hard-hit areas (one ICU bed for every 100 to 200 COVID-19 cases). The early excess deaths observed in April were not observed in later months. The magnitude of this effect can be summarized as follows: If the 152 HRRs in April with fewer than the mean number of ICU beds per COVID-19 case were to instead have the mean number (one ICU bed for every four COVID-19 cases), our model estimates that there would have been 15,571 fewer deaths that month. The HRRs with the largest number of early excess deaths were Manhattan in New York City (1,466), Bronx in New York City (1,315), Boston, Massachusetts (1,293), Philadelphia, Pennsylvania (955), Hartford, Connecticut (682), Detroit, Michigan (499), and Camden, New Jersey (484). The Figure depicts HRRs in the United States with early excess deaths by this measure in April.

DISCUSSION
We found significant associations between availability of hospital-based resources and COVID-19 deaths in the month of April 2020. This observation was consistent across measures of both hospital bed and staff capacity but not statistically significant in all cases. This provides empiric evidence in support of a preprint simulation publication by Branas et al showing the potential for thousands of excess deaths related to lack of available resources.4 Interestingly, the relationship between hospital-based resources per COVID-19 case and death count is not seen in May, June, or July. This may be because hospitals and health systems were rapidly adapting to pandemic demands9 by shifting resources or reorganizing existing infrastructure to free up beds and personnel.
Our findings underscore the importance of analyses that address heterogeneity in health system response over time and across different geographic areas. That the relationship is not seen after the early pandemic period, when hospitals and health systems were most overwhelmed, suggests that health systems and communities were able to adapt. Importantly, this work does not address the likely complex relationships among hospital resources and outcomes (for example, the benefit of ICU bed availability is likely limited when there are insufficient intensivists and nurses). These complexities should be a focus of future work. Furthermore, hospital resource flexibility, community efforts to slow transmission, and improvements in testing availability and the management of COVID-19 among hospitalized patients may all play a role in attenuating the relationship between baseline resource limitations and outcomes for patients with COVID-19.
These results merit further granular studies to examine specific hospital resources and observed variation in outcomes. Prior literature has linked inpatient capacity—variously defined as high census, acuity, turnover, or delayed admission—to outcomes including mortality among patients with stroke, among those with acute coronary syndrome, and among those requiring intensive care.10 Literature from Italy’s experience shows there was large variation in the case fatality rate among regions of Northern Italy and argues this was partially due to hospital resource limitations.11 Future work can also address whether just-in-time resource mobilization, such as temporary ICU expansion, physician cross-staffing, telemedicine, and dedicated units for COVID-19 patients, attenuated the impact of potential hospital resource scarcity on outcomes.
The present analysis is limited by the quality of the data. There is likely variation of available COVID-19 testing by HRR. It may be that areas with larger outbreaks early on generally tested a smaller, sicker proportion of population-level cases than did those with smaller outbreaks. This effect may be reversed if larger HRRs in urban areas have health systems and public health departments more inclined toward or capable of doing more testing. Furthermore, deaths related to COVID-19 are likely related to community-based factors, including nonhealthcare resources and underlying population characteristics, that likely correlate with the availability of hospital-based resources within HRRs. Some have called into question whether, a priori, we should expect hospital-based capacity to be an important driver of mortality at all,12 arguing that, when critical care capacity is exceeded, resources may be efficiently reallocated away from patients who are least likely to benefit. Because we used the American Hospital Association data, this snapshot of hospital resources is not limited to critical care capacity because there could be alternative explanations for situations in which mortality for both COVID-19 and non–COVID-19 patients may be lower and hospital resources are better matched with demand. For example, patients may seek care earlier in their disease course (whether COVID-19 or otherwise)13 if their local emergency department is not thought to be overwhelmed with case volume.
CONCLUSION
We find that COVID-19 deaths vary among HRRs. The availability of several hospital-based resources is associated with death rates and supports early efforts across the United States to “flatten the curve” to prevent hospital overload. Continued surveillance of this relationship is essential to guide policymakers and hospitals seeking to balance the still limited supply of resources with the demands of caring for both infectious and noninfectious patients in the coming months of this outbreak and in future pandemics.
Acknowledgment
The authors gratefully acknowledge the help of Carolyn Lusch, AICP, in generating depictions of results in Geographic Information Systems.
1. Phua J, Weng L, Ling L, et al; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506-517. https://doi.org/10.1016/s2213-2600(20)30161-2
2. Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371-1372. https://doi.org/10.1001/jama.2010.394
3. General FAQ. Dartmouth Atlas Project. 2020. Accessed July 8, 2020. https://www.dartmouthatlas.org/faq/
4. Branas CC, Rundle A, Pei S, et al. Flattening the curve before it flattens us: hospital critical care capacity limits and mortality from novel coronavirus (SARS-CoV2) cases in US counties. medRxiv. Preprint posted online April 6, 2020. https://doi.org/10.1101/2020.04.01.20049759
5. American Hospital Association Annual Survey Database. American Hospital Association. 2018. Accessed July 8, 2020. https://www.ahadata.com/aha-annual-survey-database
6. An Ongoing Repository of Data on Coronavirus Cases and Deaths in the U.S. New York Times. 2020. Accessed July 8, 2020. https://github.com/nytimes/covid-19-data
7. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. https://doi.org/10.1016/s1473-3099(20)30195-x
8. Rosakis P, Marketou ME. Rethinking case fatality ratios for COVID-19 from a data-driven viewpoint. J Infect. 2020;81(2);e162-e164. https://doi.org/10.1016/j.jinf.2020.06.010
9. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
10. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guide JM. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
11. Volpato S, Landi F, Incalzi RA. A frail health care system for an old population: lesson form [sic] the COVID-19 outbreak in Italy. J Gerontol Series A. 2020;75(9):e126-e127. https://doi.org/10.1093/gerona/glaa087
12. Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447-455. https://doi.org/10.7326/0003-4819-159-7-201310010-00004
13. Moroni F, Gramegna M, Agello S, et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2(10):1620-1624. https://doi.org/10.1016/j.jaccas.2020.04.010
1. Phua J, Weng L, Ling L, et al; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506-517. https://doi.org/10.1016/s2213-2600(20)30161-2
2. Carr BG, Addyson DK, Kahn JM. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371-1372. https://doi.org/10.1001/jama.2010.394
3. General FAQ. Dartmouth Atlas Project. 2020. Accessed July 8, 2020. https://www.dartmouthatlas.org/faq/
4. Branas CC, Rundle A, Pei S, et al. Flattening the curve before it flattens us: hospital critical care capacity limits and mortality from novel coronavirus (SARS-CoV2) cases in US counties. medRxiv. Preprint posted online April 6, 2020. https://doi.org/10.1101/2020.04.01.20049759
5. American Hospital Association Annual Survey Database. American Hospital Association. 2018. Accessed July 8, 2020. https://www.ahadata.com/aha-annual-survey-database
6. An Ongoing Repository of Data on Coronavirus Cases and Deaths in the U.S. New York Times. 2020. Accessed July 8, 2020. https://github.com/nytimes/covid-19-data
7. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. https://doi.org/10.1016/s1473-3099(20)30195-x
8. Rosakis P, Marketou ME. Rethinking case fatality ratios for COVID-19 from a data-driven viewpoint. J Infect. 2020;81(2);e162-e164. https://doi.org/10.1016/j.jinf.2020.06.010
9. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
10. Eriksson CO, Stoner RC, Eden KB, Newgard CD, Guide JM. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. https://doi.org/10.1007/s11606-016-3936-3
11. Volpato S, Landi F, Incalzi RA. A frail health care system for an old population: lesson form [sic] the COVID-19 outbreak in Italy. J Gerontol Series A. 2020;75(9):e126-e127. https://doi.org/10.1093/gerona/glaa087
12. Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med. 2013;159(7):447-455. https://doi.org/10.7326/0003-4819-159-7-201310010-00004
13. Moroni F, Gramegna M, Agello S, et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2(10):1620-1624. https://doi.org/10.1016/j.jaccas.2020.04.010
© 2021 Society of Hospital Medicine