User login
Extended-release carbamazepine
Over 2 decades, carbamazepine has become a well-established, off-label alternative to lithium for treating acute mania. In December, the FDA approved an extended-release form of the anticonvulsant to treat type I bipolar disorder (Table 1).
This article addresses clinical use of extended-release capsules of carbamazepine (ERC-CBZ) and their safety, tolerability, and potential to interact with other medications.
Table 1
Extended-release capsules
of carbamazepine:
Fast facts
| Brand name: Equetro |
| Class: Anticonvulsant |
| FDA-approved indication: Bipolar mania |
| Manufacturer: Shire Pharmaceuticals Group |
| Dosing form: 100-, 200-, and 300-mg capsules |
| Recommended dosage: Manufacturer recommends starting at 400 mg/d in two divided doses.* Adjust dosage in 200-mg increments to achieve optimal response. Dosages >1,600 mg/d have not been studied. |
| *The author recommends starting at 200 mg/d using once-daily, nighttime dosing and titrating slowly based on tolerability and efficacy, continuing with nighttime dosing only. |
PHARMACOKINETICS
Because ERC-CBZ and carbamazepine yield similar molecules, the extended- and immediate-release forms have similar pharmacodynamic properties. There are notable pharmacokinetic differences, however.
With chronic use, carbamazepine autoinduces cytochrome P-450 (CYP) 3A4 hepatic enzymes. These enzymes rapidly break down carbamazepine to its active 10, 11-epoxide metabolite and the epoxide to the inactive diol. Because this sequence shortens carbamazepine’s half-life considerably—from 35 to 40 hours to 12 to 17 hours after 2 to 3 weeks of use—multiple daily dosing and minor dosage increases often are needed to maintain carbamazepine blood levels. Also, with immediate-release carbamazepine’s peak/trough variations, transient side effects such as ataxia, dizziness, or diplopia may emerge 2 to 3 hours after dosing once steady state is reached.
By contrast, ERC-CBZ should be more tolerable and easier to use because it smooths out these variations. Although studies of ERC-CBZ in mania have examined twice-daily dosing, using once-nightly dosing instead (starting at 200 mg) will harness carbamazepine’s sedative and other side effects to promote sleep onset, and lower levels throughout the day will further increase its tolerability.1 Patients who experience breakthrough afternoon or evening manic symptoms with once-nightly dosing can be returned to twice-daily dosing.
EFFICACY IN BIPOLAR DISORDER
ERC-CBZ has shown efficacy for treating bipolar disorder in three studies,2,3,4 including two large double-blind, placebo-controlled, multi-center trials that followed patients with type I bipolar disorder with current manic or mixed episodes.
In the first trial,2 204 patients received ERC-CBZ, 400 to 1,600 mg/d (mean±SD daily dosage 756.4±413.4 mg/d, mean plasma level 8.9 μg/mL), or placebo for 3 weeks. Young Mania Rating Scale (YMRS) scores decreased 50% in 41.5% of the treatment group and in 22.4% of the placebo group. Hamilton Rating Scale for Depression (HAM-D) scores decreased more in the ERC-CBZ group, but the difference was not clinically significant.
In a second trial of 239 patients,3 YMRS scores fell 50% across 3 weeks in 60.8% of those taking ERC-CBZ, 400 to 1,600 mg/d (mean±SD dosage 642±369.2 mg/d), compared with 28.7% of placebotreated patients. HAM-D total scores also improved significantly in ERC-CBZ-treated patients compared with the placebo group in a subanalysis of 188 intent-to-treat patients with a manic episode.
In a 6-month extension following 92 patients from two double-blind trials,4 mean total YMRS scores decreased among former placebo group patients switched to open-label ERC-CBZ, 200 to 1,600 mg/d (mean dosage 938 mg, mean serum level 6.6 μg/mL). Patients who had taken ERC-CBZ during the acute trial saw little change in YMRS scores during continued ERC-CBZ treatment, except in the second month. At end point, however, YMRS scores fell further for both treatment groups. HAM-D total scores differed little across 6 months, but 54 patients with mixed states maintained significant reductions.
Maintenance therapy. In many studies, carbamazepine has compared favorably with lithium for long-term bipolar maintenance in some patients. Consider patients who respond well to acute carbamazepine therapy for continuation treatment with ERC-CBZ. Patients who may be most likely to respond to carbamazepine therapy include those with:
- type II bipolar disorder
- substance abuse comorbidity
- mood-incongruent delusions
- no family history of bipolar illness among first-degree relatives.5
Contraindications (such as use during pregnancy or breast-feeding) are the same for extended- and immediate-release carbamazepine.
TOLERABILITY
Carbamazepine in any form can cause a range of common to rare side effects (Table 2), which have been reviewed elsewhere.4-6
Side effects of ERC-CBZ most commonly reported during the double-blind, placebo-controlled studies include dizziness, nausea, somnolence, headache, vomiting, dyspepsia, dry mouth, pruritus, and benign rash. Slower upward titration of single nighttime doses—instead of the twice-daily dosing used in these studies—could prevent most of these effects.
Only one patient in either study developed a serious side effect possibly related to ERC-CBZ (fever with rash); the rash resolved 6 days after the drug was stopped.
Total cholesterol in patients taking ERC-CBZ also rose 12% to 13% in the double-blind studies.2,3 Consider dietary and/or cholesterol-lowering medications in patients taking ERC-CBZ who are at high risk for cardiovascular events.
In the 6-month open-label study, headache, dizziness, and benign rash were most frequently reported. No serious adverse events related to the study drug were reported.
Table 2
Carbamazepine’s common to rare side effects
| Common | Infrequent |
| Ataxia | Hyponatremia (asymptomatic to symptomatic, reversed by demeclocycline or lithium) |
| Benign rash | Liver enzyme elevations |
| Benign white blood cell count suppression (reversed by lithium) | Tremor |
| Decreased thyroid hormones | Weight gain |
| Diplopia | Rare/serious |
| Dizziness | Agranulocytosis |
| Fatigue, sedation | Aplastic anemia |
| Increased cholesterol | Hyponatremia (symptomatic) |
| Nausea | Severe rash
|
| Spina bifida (following in utero exposure) |
INTERACTIONS WITH OTHER MEDICATIONS
Because of its potent induction of CYP 3A4 enzymes,6 carbamazepine in any form may substantially lower blood levels of several compounds metabolized principally by CYP 3A4 isoenzymes (Table 3), including typical antipsychotics such as haloperidol and the atypical antipsychotic aripiprazole. Even so, patients often improve with combination carbamazepine/haloperidol therapy despite lower haloperidol blood levels.
If a patient is taking oral contraceptives, inform her primary care physician or OB/GYN when prescribing carbamazepine. Because the anticonvulsant lowers circulating estrogen, a higher contraceptive dosage or alternate birth-control method should be considered to prevent unwanted pregnancy.
Most other drug-drug interactions have been well-delineated and can be avoided. Inform the patient and his or her primary care physician when giving carbamazepine concomitantly with any drug.
Numerous medications can also increase serum carbamazepine levels, causing problems in a patient already near his or her side-effect threshold (Table 4). Reduce the carbamazepine dosage to avoid these adverse effects.
Table 3
Carbamazepine decreases serum concentrations of these drugs
| Antipsychotics | Analgesics |
| Buprenorphine | Aripiprazole* |
| Methadone | Clozapine |
| Antimicrobials | Haloperidol* |
| Caspofungin | Olanzapine* |
| Doxycycline | Risperidone* |
| Anticoagulants | Thiothixene |
| Warfarin*† | Ziprasidone* |
| Anticonvulsants | Antivirals |
| Carbamazepine*† | Delavirdine |
| Lamotrigine*† | Protease inhibitors† |
| Oxcarbazepine | Anxiolytics/sedatives |
| Phenobarbital | Alprazolam* |
| Phenytoin | Steroids |
| Topiramate | Estrogen in hormonal contraceptives*† |
| Valproate*‡ | Mifepristone |
| Zonisamide | Prednisolone* |
| Antidepressants | Stimulants |
| Bupropion* | Methylphenidate* |
| Citalopram* | Modafinil* |
| Mirtazapine | Others |
| Tricyclics | Cisplatin |
| Doxorubicin | |
| Theophylline | |
| * Carbamazepine is often given with this medication. | |
| † Potentially serious interaction. | |
| ‡ Less-serious interaction likely with carbamazepine. | |
Table 4
These drugs increase serum carbamazepine and may cause toxicity
| Anticonvulsants | Macrolide antibiotics |
| Valproate (increases carbamazepine 10, 11-epoxide levels)*† | Clarithromycin*† |
| Antidepressants | Erythromycin*† |
| Fluoxetine*‡ | Flurithromycin*† |
| Fluvoxamine*‡ | Josamycin*† |
| Nefazodone*‡ | Ponsinomycin*† |
| Antimicrobials | Triacetyloleandromycin*† |
| Isoniazid† | Others |
| Quinupristin/dalfopristin | Acetazolamide |
| Calcium channel blockers | Cimetidine§ |
| Diltiazem*† | Danazol |
| Verapamil*† | d-Propoxyphene‡ |
| Hypolipidemics | Ketoconazole† |
| Gemfibrozil | Niacinamide |
| Nicotinamide | Omeprazole |
| Ritonavir† | |
| Ticlopidine | |
| * Carbamazepine is often given with this medication. | |
| † Potentially serious interaction. | |
| ‡ Less-serious interaction likely with carbamazepine. | |
| § Data on interactions with carbamazepine unclear. | |
CLINICAL IMPLICATIONS
Long-acting carbamazepine suitable for single nighttime dosing should facilitate adherence and reduce daytime side effects. Consider ERC-CBZ for patients not responding adequately to lithium or valproate, as individual response to any of these three drugs can vary greatly. Sideeffect tolerability (such as less weight gain with carbamazepine than with valproate) also could help guide drug choice. In patients with rapid cycling, carbamazepine plus lithium may be more effective than either drug alone.
New data suggest that carbamazepine offers acute antidepressant effects in some individuals and in long-term depression treatment.5 More research is needed to identify depressed patients most likely to respond to this agent.
For now, when using ERC-CBZ, we can draw from the larger experience with immediate-release carbamazepine to treat epilepsy, bipolar disorder, and related mood disorders. Once you master carbamazepine’s pharmacokinetic interactions with other commonly used agents, ERC-CBZ in slowly titrated, single nighttime dosages should simplify the compound’s administration and tolerability.
Related resources
- Carbamazepine (extended-release) Web site. www.equetro.com.
- Post RM, Speer AM, Obrocea GV, Leverich GS. Acute and prophylactic effects of anticonvulsants in bipolar depression. Clin Neurosci Res 2002;2:228-51.
- Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997;58:470-8.
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine (extended-release) • Equetro
- Carbamazepine (immediate-release) • Tegretol, others
- Haloperidol • Haldol
- Lithium • Eskalith, others
- Valproate • Depakote
Disclosure
Dr. Post reports no current financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Miller AD, Krauss GL, Hamzeh FM. Improved CNS tolerability following conversion from immediate- to extended-release carbamazepine. Acta Neurol Scand 2004;109:374-7.
2. Weisler RH, Kalali AH, Ketter TA. A multicenter, randomized, double-blind, placebo-controlled trial of extended-release carbamazepine capsules as monotherapy for bipolar disorder patients with manic or mixed episodes. J Clin Psychiatry 2004;65:478-84.
3. Weisler RH, Keck PE Jr, Swann AC, et al, for the SPD417 Study Group. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: A multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2005;66:323-30.
4. Ketter TA, Kalali AH, Weisler RH. A 6-month, multicenter, openlabel evaluation of beaded, extended-release carbamazepine capsule monotherapy in bipolar disorder patients with manic or mixed episodes. J Clin Psychiatry 2004;65:668-73.
5. Post RM, Frye MA. Carbamazepine. In: Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry (9th ed). New York: Lippincott Williams & Wilkins, 2005, in press.
6. Ketter TA, Wang PW, Post RM. Carbamazepine and oxcarbazepine. In: Schatzberg AF, Nemeroff CB (eds). Textbook of psychopharmacology. Washington, DC: American Psychiatric Press, 2004;581-606.
Over 2 decades, carbamazepine has become a well-established, off-label alternative to lithium for treating acute mania. In December, the FDA approved an extended-release form of the anticonvulsant to treat type I bipolar disorder (Table 1).
This article addresses clinical use of extended-release capsules of carbamazepine (ERC-CBZ) and their safety, tolerability, and potential to interact with other medications.
Table 1
Extended-release capsules
of carbamazepine:
Fast facts
| Brand name: Equetro |
| Class: Anticonvulsant |
| FDA-approved indication: Bipolar mania |
| Manufacturer: Shire Pharmaceuticals Group |
| Dosing form: 100-, 200-, and 300-mg capsules |
| Recommended dosage: Manufacturer recommends starting at 400 mg/d in two divided doses.* Adjust dosage in 200-mg increments to achieve optimal response. Dosages >1,600 mg/d have not been studied. |
| *The author recommends starting at 200 mg/d using once-daily, nighttime dosing and titrating slowly based on tolerability and efficacy, continuing with nighttime dosing only. |
PHARMACOKINETICS
Because ERC-CBZ and carbamazepine yield similar molecules, the extended- and immediate-release forms have similar pharmacodynamic properties. There are notable pharmacokinetic differences, however.
With chronic use, carbamazepine autoinduces cytochrome P-450 (CYP) 3A4 hepatic enzymes. These enzymes rapidly break down carbamazepine to its active 10, 11-epoxide metabolite and the epoxide to the inactive diol. Because this sequence shortens carbamazepine’s half-life considerably—from 35 to 40 hours to 12 to 17 hours after 2 to 3 weeks of use—multiple daily dosing and minor dosage increases often are needed to maintain carbamazepine blood levels. Also, with immediate-release carbamazepine’s peak/trough variations, transient side effects such as ataxia, dizziness, or diplopia may emerge 2 to 3 hours after dosing once steady state is reached.
By contrast, ERC-CBZ should be more tolerable and easier to use because it smooths out these variations. Although studies of ERC-CBZ in mania have examined twice-daily dosing, using once-nightly dosing instead (starting at 200 mg) will harness carbamazepine’s sedative and other side effects to promote sleep onset, and lower levels throughout the day will further increase its tolerability.1 Patients who experience breakthrough afternoon or evening manic symptoms with once-nightly dosing can be returned to twice-daily dosing.
EFFICACY IN BIPOLAR DISORDER
ERC-CBZ has shown efficacy for treating bipolar disorder in three studies,2,3,4 including two large double-blind, placebo-controlled, multi-center trials that followed patients with type I bipolar disorder with current manic or mixed episodes.
In the first trial,2 204 patients received ERC-CBZ, 400 to 1,600 mg/d (mean±SD daily dosage 756.4±413.4 mg/d, mean plasma level 8.9 μg/mL), or placebo for 3 weeks. Young Mania Rating Scale (YMRS) scores decreased 50% in 41.5% of the treatment group and in 22.4% of the placebo group. Hamilton Rating Scale for Depression (HAM-D) scores decreased more in the ERC-CBZ group, but the difference was not clinically significant.
In a second trial of 239 patients,3 YMRS scores fell 50% across 3 weeks in 60.8% of those taking ERC-CBZ, 400 to 1,600 mg/d (mean±SD dosage 642±369.2 mg/d), compared with 28.7% of placebotreated patients. HAM-D total scores also improved significantly in ERC-CBZ-treated patients compared with the placebo group in a subanalysis of 188 intent-to-treat patients with a manic episode.
In a 6-month extension following 92 patients from two double-blind trials,4 mean total YMRS scores decreased among former placebo group patients switched to open-label ERC-CBZ, 200 to 1,600 mg/d (mean dosage 938 mg, mean serum level 6.6 μg/mL). Patients who had taken ERC-CBZ during the acute trial saw little change in YMRS scores during continued ERC-CBZ treatment, except in the second month. At end point, however, YMRS scores fell further for both treatment groups. HAM-D total scores differed little across 6 months, but 54 patients with mixed states maintained significant reductions.
Maintenance therapy. In many studies, carbamazepine has compared favorably with lithium for long-term bipolar maintenance in some patients. Consider patients who respond well to acute carbamazepine therapy for continuation treatment with ERC-CBZ. Patients who may be most likely to respond to carbamazepine therapy include those with:
- type II bipolar disorder
- substance abuse comorbidity
- mood-incongruent delusions
- no family history of bipolar illness among first-degree relatives.5
Contraindications (such as use during pregnancy or breast-feeding) are the same for extended- and immediate-release carbamazepine.
TOLERABILITY
Carbamazepine in any form can cause a range of common to rare side effects (Table 2), which have been reviewed elsewhere.4-6
Side effects of ERC-CBZ most commonly reported during the double-blind, placebo-controlled studies include dizziness, nausea, somnolence, headache, vomiting, dyspepsia, dry mouth, pruritus, and benign rash. Slower upward titration of single nighttime doses—instead of the twice-daily dosing used in these studies—could prevent most of these effects.
Only one patient in either study developed a serious side effect possibly related to ERC-CBZ (fever with rash); the rash resolved 6 days after the drug was stopped.
Total cholesterol in patients taking ERC-CBZ also rose 12% to 13% in the double-blind studies.2,3 Consider dietary and/or cholesterol-lowering medications in patients taking ERC-CBZ who are at high risk for cardiovascular events.
In the 6-month open-label study, headache, dizziness, and benign rash were most frequently reported. No serious adverse events related to the study drug were reported.
Table 2
Carbamazepine’s common to rare side effects
| Common | Infrequent |
| Ataxia | Hyponatremia (asymptomatic to symptomatic, reversed by demeclocycline or lithium) |
| Benign rash | Liver enzyme elevations |
| Benign white blood cell count suppression (reversed by lithium) | Tremor |
| Decreased thyroid hormones | Weight gain |
| Diplopia | Rare/serious |
| Dizziness | Agranulocytosis |
| Fatigue, sedation | Aplastic anemia |
| Increased cholesterol | Hyponatremia (symptomatic) |
| Nausea | Severe rash
|
| Spina bifida (following in utero exposure) |
INTERACTIONS WITH OTHER MEDICATIONS
Because of its potent induction of CYP 3A4 enzymes,6 carbamazepine in any form may substantially lower blood levels of several compounds metabolized principally by CYP 3A4 isoenzymes (Table 3), including typical antipsychotics such as haloperidol and the atypical antipsychotic aripiprazole. Even so, patients often improve with combination carbamazepine/haloperidol therapy despite lower haloperidol blood levels.
If a patient is taking oral contraceptives, inform her primary care physician or OB/GYN when prescribing carbamazepine. Because the anticonvulsant lowers circulating estrogen, a higher contraceptive dosage or alternate birth-control method should be considered to prevent unwanted pregnancy.
Most other drug-drug interactions have been well-delineated and can be avoided. Inform the patient and his or her primary care physician when giving carbamazepine concomitantly with any drug.
Numerous medications can also increase serum carbamazepine levels, causing problems in a patient already near his or her side-effect threshold (Table 4). Reduce the carbamazepine dosage to avoid these adverse effects.
Table 3
Carbamazepine decreases serum concentrations of these drugs
| Antipsychotics | Analgesics |
| Buprenorphine | Aripiprazole* |
| Methadone | Clozapine |
| Antimicrobials | Haloperidol* |
| Caspofungin | Olanzapine* |
| Doxycycline | Risperidone* |
| Anticoagulants | Thiothixene |
| Warfarin*† | Ziprasidone* |
| Anticonvulsants | Antivirals |
| Carbamazepine*† | Delavirdine |
| Lamotrigine*† | Protease inhibitors† |
| Oxcarbazepine | Anxiolytics/sedatives |
| Phenobarbital | Alprazolam* |
| Phenytoin | Steroids |
| Topiramate | Estrogen in hormonal contraceptives*† |
| Valproate*‡ | Mifepristone |
| Zonisamide | Prednisolone* |
| Antidepressants | Stimulants |
| Bupropion* | Methylphenidate* |
| Citalopram* | Modafinil* |
| Mirtazapine | Others |
| Tricyclics | Cisplatin |
| Doxorubicin | |
| Theophylline | |
| * Carbamazepine is often given with this medication. | |
| † Potentially serious interaction. | |
| ‡ Less-serious interaction likely with carbamazepine. | |
Table 4
These drugs increase serum carbamazepine and may cause toxicity
| Anticonvulsants | Macrolide antibiotics |
| Valproate (increases carbamazepine 10, 11-epoxide levels)*† | Clarithromycin*† |
| Antidepressants | Erythromycin*† |
| Fluoxetine*‡ | Flurithromycin*† |
| Fluvoxamine*‡ | Josamycin*† |
| Nefazodone*‡ | Ponsinomycin*† |
| Antimicrobials | Triacetyloleandromycin*† |
| Isoniazid† | Others |
| Quinupristin/dalfopristin | Acetazolamide |
| Calcium channel blockers | Cimetidine§ |
| Diltiazem*† | Danazol |
| Verapamil*† | d-Propoxyphene‡ |
| Hypolipidemics | Ketoconazole† |
| Gemfibrozil | Niacinamide |
| Nicotinamide | Omeprazole |
| Ritonavir† | |
| Ticlopidine | |
| * Carbamazepine is often given with this medication. | |
| † Potentially serious interaction. | |
| ‡ Less-serious interaction likely with carbamazepine. | |
| § Data on interactions with carbamazepine unclear. | |
CLINICAL IMPLICATIONS
Long-acting carbamazepine suitable for single nighttime dosing should facilitate adherence and reduce daytime side effects. Consider ERC-CBZ for patients not responding adequately to lithium or valproate, as individual response to any of these three drugs can vary greatly. Sideeffect tolerability (such as less weight gain with carbamazepine than with valproate) also could help guide drug choice. In patients with rapid cycling, carbamazepine plus lithium may be more effective than either drug alone.
New data suggest that carbamazepine offers acute antidepressant effects in some individuals and in long-term depression treatment.5 More research is needed to identify depressed patients most likely to respond to this agent.
For now, when using ERC-CBZ, we can draw from the larger experience with immediate-release carbamazepine to treat epilepsy, bipolar disorder, and related mood disorders. Once you master carbamazepine’s pharmacokinetic interactions with other commonly used agents, ERC-CBZ in slowly titrated, single nighttime dosages should simplify the compound’s administration and tolerability.
Related resources
- Carbamazepine (extended-release) Web site. www.equetro.com.
- Post RM, Speer AM, Obrocea GV, Leverich GS. Acute and prophylactic effects of anticonvulsants in bipolar depression. Clin Neurosci Res 2002;2:228-51.
- Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997;58:470-8.
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine (extended-release) • Equetro
- Carbamazepine (immediate-release) • Tegretol, others
- Haloperidol • Haldol
- Lithium • Eskalith, others
- Valproate • Depakote
Disclosure
Dr. Post reports no current financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Over 2 decades, carbamazepine has become a well-established, off-label alternative to lithium for treating acute mania. In December, the FDA approved an extended-release form of the anticonvulsant to treat type I bipolar disorder (Table 1).
This article addresses clinical use of extended-release capsules of carbamazepine (ERC-CBZ) and their safety, tolerability, and potential to interact with other medications.
Table 1
Extended-release capsules
of carbamazepine:
Fast facts
| Brand name: Equetro |
| Class: Anticonvulsant |
| FDA-approved indication: Bipolar mania |
| Manufacturer: Shire Pharmaceuticals Group |
| Dosing form: 100-, 200-, and 300-mg capsules |
| Recommended dosage: Manufacturer recommends starting at 400 mg/d in two divided doses.* Adjust dosage in 200-mg increments to achieve optimal response. Dosages >1,600 mg/d have not been studied. |
| *The author recommends starting at 200 mg/d using once-daily, nighttime dosing and titrating slowly based on tolerability and efficacy, continuing with nighttime dosing only. |
PHARMACOKINETICS
Because ERC-CBZ and carbamazepine yield similar molecules, the extended- and immediate-release forms have similar pharmacodynamic properties. There are notable pharmacokinetic differences, however.
With chronic use, carbamazepine autoinduces cytochrome P-450 (CYP) 3A4 hepatic enzymes. These enzymes rapidly break down carbamazepine to its active 10, 11-epoxide metabolite and the epoxide to the inactive diol. Because this sequence shortens carbamazepine’s half-life considerably—from 35 to 40 hours to 12 to 17 hours after 2 to 3 weeks of use—multiple daily dosing and minor dosage increases often are needed to maintain carbamazepine blood levels. Also, with immediate-release carbamazepine’s peak/trough variations, transient side effects such as ataxia, dizziness, or diplopia may emerge 2 to 3 hours after dosing once steady state is reached.
By contrast, ERC-CBZ should be more tolerable and easier to use because it smooths out these variations. Although studies of ERC-CBZ in mania have examined twice-daily dosing, using once-nightly dosing instead (starting at 200 mg) will harness carbamazepine’s sedative and other side effects to promote sleep onset, and lower levels throughout the day will further increase its tolerability.1 Patients who experience breakthrough afternoon or evening manic symptoms with once-nightly dosing can be returned to twice-daily dosing.
EFFICACY IN BIPOLAR DISORDER
ERC-CBZ has shown efficacy for treating bipolar disorder in three studies,2,3,4 including two large double-blind, placebo-controlled, multi-center trials that followed patients with type I bipolar disorder with current manic or mixed episodes.
In the first trial,2 204 patients received ERC-CBZ, 400 to 1,600 mg/d (mean±SD daily dosage 756.4±413.4 mg/d, mean plasma level 8.9 μg/mL), or placebo for 3 weeks. Young Mania Rating Scale (YMRS) scores decreased 50% in 41.5% of the treatment group and in 22.4% of the placebo group. Hamilton Rating Scale for Depression (HAM-D) scores decreased more in the ERC-CBZ group, but the difference was not clinically significant.
In a second trial of 239 patients,3 YMRS scores fell 50% across 3 weeks in 60.8% of those taking ERC-CBZ, 400 to 1,600 mg/d (mean±SD dosage 642±369.2 mg/d), compared with 28.7% of placebotreated patients. HAM-D total scores also improved significantly in ERC-CBZ-treated patients compared with the placebo group in a subanalysis of 188 intent-to-treat patients with a manic episode.
In a 6-month extension following 92 patients from two double-blind trials,4 mean total YMRS scores decreased among former placebo group patients switched to open-label ERC-CBZ, 200 to 1,600 mg/d (mean dosage 938 mg, mean serum level 6.6 μg/mL). Patients who had taken ERC-CBZ during the acute trial saw little change in YMRS scores during continued ERC-CBZ treatment, except in the second month. At end point, however, YMRS scores fell further for both treatment groups. HAM-D total scores differed little across 6 months, but 54 patients with mixed states maintained significant reductions.
Maintenance therapy. In many studies, carbamazepine has compared favorably with lithium for long-term bipolar maintenance in some patients. Consider patients who respond well to acute carbamazepine therapy for continuation treatment with ERC-CBZ. Patients who may be most likely to respond to carbamazepine therapy include those with:
- type II bipolar disorder
- substance abuse comorbidity
- mood-incongruent delusions
- no family history of bipolar illness among first-degree relatives.5
Contraindications (such as use during pregnancy or breast-feeding) are the same for extended- and immediate-release carbamazepine.
TOLERABILITY
Carbamazepine in any form can cause a range of common to rare side effects (Table 2), which have been reviewed elsewhere.4-6
Side effects of ERC-CBZ most commonly reported during the double-blind, placebo-controlled studies include dizziness, nausea, somnolence, headache, vomiting, dyspepsia, dry mouth, pruritus, and benign rash. Slower upward titration of single nighttime doses—instead of the twice-daily dosing used in these studies—could prevent most of these effects.
Only one patient in either study developed a serious side effect possibly related to ERC-CBZ (fever with rash); the rash resolved 6 days after the drug was stopped.
Total cholesterol in patients taking ERC-CBZ also rose 12% to 13% in the double-blind studies.2,3 Consider dietary and/or cholesterol-lowering medications in patients taking ERC-CBZ who are at high risk for cardiovascular events.
In the 6-month open-label study, headache, dizziness, and benign rash were most frequently reported. No serious adverse events related to the study drug were reported.
Table 2
Carbamazepine’s common to rare side effects
| Common | Infrequent |
| Ataxia | Hyponatremia (asymptomatic to symptomatic, reversed by demeclocycline or lithium) |
| Benign rash | Liver enzyme elevations |
| Benign white blood cell count suppression (reversed by lithium) | Tremor |
| Decreased thyroid hormones | Weight gain |
| Diplopia | Rare/serious |
| Dizziness | Agranulocytosis |
| Fatigue, sedation | Aplastic anemia |
| Increased cholesterol | Hyponatremia (symptomatic) |
| Nausea | Severe rash
|
| Spina bifida (following in utero exposure) |
INTERACTIONS WITH OTHER MEDICATIONS
Because of its potent induction of CYP 3A4 enzymes,6 carbamazepine in any form may substantially lower blood levels of several compounds metabolized principally by CYP 3A4 isoenzymes (Table 3), including typical antipsychotics such as haloperidol and the atypical antipsychotic aripiprazole. Even so, patients often improve with combination carbamazepine/haloperidol therapy despite lower haloperidol blood levels.
If a patient is taking oral contraceptives, inform her primary care physician or OB/GYN when prescribing carbamazepine. Because the anticonvulsant lowers circulating estrogen, a higher contraceptive dosage or alternate birth-control method should be considered to prevent unwanted pregnancy.
Most other drug-drug interactions have been well-delineated and can be avoided. Inform the patient and his or her primary care physician when giving carbamazepine concomitantly with any drug.
Numerous medications can also increase serum carbamazepine levels, causing problems in a patient already near his or her side-effect threshold (Table 4). Reduce the carbamazepine dosage to avoid these adverse effects.
Table 3
Carbamazepine decreases serum concentrations of these drugs
| Antipsychotics | Analgesics |
| Buprenorphine | Aripiprazole* |
| Methadone | Clozapine |
| Antimicrobials | Haloperidol* |
| Caspofungin | Olanzapine* |
| Doxycycline | Risperidone* |
| Anticoagulants | Thiothixene |
| Warfarin*† | Ziprasidone* |
| Anticonvulsants | Antivirals |
| Carbamazepine*† | Delavirdine |
| Lamotrigine*† | Protease inhibitors† |
| Oxcarbazepine | Anxiolytics/sedatives |
| Phenobarbital | Alprazolam* |
| Phenytoin | Steroids |
| Topiramate | Estrogen in hormonal contraceptives*† |
| Valproate*‡ | Mifepristone |
| Zonisamide | Prednisolone* |
| Antidepressants | Stimulants |
| Bupropion* | Methylphenidate* |
| Citalopram* | Modafinil* |
| Mirtazapine | Others |
| Tricyclics | Cisplatin |
| Doxorubicin | |
| Theophylline | |
| * Carbamazepine is often given with this medication. | |
| † Potentially serious interaction. | |
| ‡ Less-serious interaction likely with carbamazepine. | |
Table 4
These drugs increase serum carbamazepine and may cause toxicity
| Anticonvulsants | Macrolide antibiotics |
| Valproate (increases carbamazepine 10, 11-epoxide levels)*† | Clarithromycin*† |
| Antidepressants | Erythromycin*† |
| Fluoxetine*‡ | Flurithromycin*† |
| Fluvoxamine*‡ | Josamycin*† |
| Nefazodone*‡ | Ponsinomycin*† |
| Antimicrobials | Triacetyloleandromycin*† |
| Isoniazid† | Others |
| Quinupristin/dalfopristin | Acetazolamide |
| Calcium channel blockers | Cimetidine§ |
| Diltiazem*† | Danazol |
| Verapamil*† | d-Propoxyphene‡ |
| Hypolipidemics | Ketoconazole† |
| Gemfibrozil | Niacinamide |
| Nicotinamide | Omeprazole |
| Ritonavir† | |
| Ticlopidine | |
| * Carbamazepine is often given with this medication. | |
| † Potentially serious interaction. | |
| ‡ Less-serious interaction likely with carbamazepine. | |
| § Data on interactions with carbamazepine unclear. | |
CLINICAL IMPLICATIONS
Long-acting carbamazepine suitable for single nighttime dosing should facilitate adherence and reduce daytime side effects. Consider ERC-CBZ for patients not responding adequately to lithium or valproate, as individual response to any of these three drugs can vary greatly. Sideeffect tolerability (such as less weight gain with carbamazepine than with valproate) also could help guide drug choice. In patients with rapid cycling, carbamazepine plus lithium may be more effective than either drug alone.
New data suggest that carbamazepine offers acute antidepressant effects in some individuals and in long-term depression treatment.5 More research is needed to identify depressed patients most likely to respond to this agent.
For now, when using ERC-CBZ, we can draw from the larger experience with immediate-release carbamazepine to treat epilepsy, bipolar disorder, and related mood disorders. Once you master carbamazepine’s pharmacokinetic interactions with other commonly used agents, ERC-CBZ in slowly titrated, single nighttime dosages should simplify the compound’s administration and tolerability.
Related resources
- Carbamazepine (extended-release) Web site. www.equetro.com.
- Post RM, Speer AM, Obrocea GV, Leverich GS. Acute and prophylactic effects of anticonvulsants in bipolar depression. Clin Neurosci Res 2002;2:228-51.
- Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997;58:470-8.
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine (extended-release) • Equetro
- Carbamazepine (immediate-release) • Tegretol, others
- Haloperidol • Haldol
- Lithium • Eskalith, others
- Valproate • Depakote
Disclosure
Dr. Post reports no current financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Miller AD, Krauss GL, Hamzeh FM. Improved CNS tolerability following conversion from immediate- to extended-release carbamazepine. Acta Neurol Scand 2004;109:374-7.
2. Weisler RH, Kalali AH, Ketter TA. A multicenter, randomized, double-blind, placebo-controlled trial of extended-release carbamazepine capsules as monotherapy for bipolar disorder patients with manic or mixed episodes. J Clin Psychiatry 2004;65:478-84.
3. Weisler RH, Keck PE Jr, Swann AC, et al, for the SPD417 Study Group. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: A multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2005;66:323-30.
4. Ketter TA, Kalali AH, Weisler RH. A 6-month, multicenter, openlabel evaluation of beaded, extended-release carbamazepine capsule monotherapy in bipolar disorder patients with manic or mixed episodes. J Clin Psychiatry 2004;65:668-73.
5. Post RM, Frye MA. Carbamazepine. In: Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry (9th ed). New York: Lippincott Williams & Wilkins, 2005, in press.
6. Ketter TA, Wang PW, Post RM. Carbamazepine and oxcarbazepine. In: Schatzberg AF, Nemeroff CB (eds). Textbook of psychopharmacology. Washington, DC: American Psychiatric Press, 2004;581-606.
1. Miller AD, Krauss GL, Hamzeh FM. Improved CNS tolerability following conversion from immediate- to extended-release carbamazepine. Acta Neurol Scand 2004;109:374-7.
2. Weisler RH, Kalali AH, Ketter TA. A multicenter, randomized, double-blind, placebo-controlled trial of extended-release carbamazepine capsules as monotherapy for bipolar disorder patients with manic or mixed episodes. J Clin Psychiatry 2004;65:478-84.
3. Weisler RH, Keck PE Jr, Swann AC, et al, for the SPD417 Study Group. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: A multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2005;66:323-30.
4. Ketter TA, Kalali AH, Weisler RH. A 6-month, multicenter, openlabel evaluation of beaded, extended-release carbamazepine capsule monotherapy in bipolar disorder patients with manic or mixed episodes. J Clin Psychiatry 2004;65:668-73.
5. Post RM, Frye MA. Carbamazepine. In: Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry (9th ed). New York: Lippincott Williams & Wilkins, 2005, in press.
6. Ketter TA, Wang PW, Post RM. Carbamazepine and oxcarbazepine. In: Schatzberg AF, Nemeroff CB (eds). Textbook of psychopharmacology. Washington, DC: American Psychiatric Press, 2004;581-606.
Bipolar depression dilemma: Continue antidepressants after remission—or not?
Psychiatrists in the trenches are not alone in being unsure how to treat breakthrough bipolar depression. No panelist or other expert attending a recent American College of Neuropsychopharmacology symposium could definitively recommend:
- when to add an antidepressant to mood-stabilizer therapy
- whether to discontinue antidepressants after bipolar depression is stabilized.
Until controlled trials address these issues, we must use limited evidence to treat patients with bipolar depression. To help you meet this challenge, this article offers provisional suggestions based on recent published and unpublished reports.
Evidence for continuing
No published, randomized studies have examined how long to continue antidepressants after bipolar depression has stabilized. Until recently, conventional wisdom has been to discontinue antidepressants as soon as possible because of worries about antidepressant-induced mania. Then two case-controlled studies—one retrospective and one prospective—challenged that assumption.
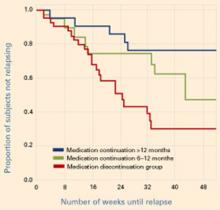
Figure Reduced relapse rates in patients whose antidepressants were continued
In a prospective case-controlled study, patients with bipolar disorder who continued antidepressant treatment for 6 to 12 months or >12 months after depressive episode remission had lower relapse rates than those who discontinued antidepressants within 6 months.
Source: Reprinted with permission from reference 2. Copyright 2003. American Psychiatric AssociationUsing similar methodologies, Altshuler et al1,2 examined bipolar patients who remained in remission for 6 weeks on mood stabilizers plus adjunctive antidepressants. Those who continued the antidepressants were less likely to relapse into depression (without an increase in manic episodes) than those whose antidepressants were discontinued (Figure).
Relapse rates in the first study1 were 35% at 1 year in those who continued antidepressants and 68% in those who did not. In the second study,2 36% and 70% of patients, respectively, had relapsed at 1 year. In the latter study, those who continued to take antidepressants for at least 6 months—instead of at least 12 months—had intermediate depressive relapse rates (53% for 6 months vs. 24% for 12 months).
When interpreting these data, keep several caveats in mind. For one, patients were not randomly assigned to continue or discontinue antidepressants but were designated by patient and clinician choice. When comparing the two patient groups, however, the authors found no inherent differences in illness characteristics that might have biased the results.
More importantly, few patients initially treated with antidepressant augmentation responded well and remained in remission. In the prospective study, 549 of 1,078 patients in the Stanley Foundation Bipolar Network received an antidepressant for breakthrough depressive symptoms.2 Only 189 remained on antidepressants for 2 months, and only 84 (15%) of the original population remained in remission for 2 months.
Summary. A small subgroup of patients appears to respond well to antidepressants and sustains this response for 6 to 8 weeks. For this subset, continuing antidepressant therapy would appear to be an appropriate strategy, based on:
- a significantly reduced risk for depressive relapse
- no increased risk of switching to mania, which is the usual reason to terminate antidepressants early.
Subsequent evidence
One needs to assess these observations in light of an interim analysis reported halfway through a small, open, randomized study. Ghaemi et al3 found no significant difference in outcomes—regardless of rapid-cycling status—among 14 bipolar patients who remained on antidepressants and 19 who discontinued across an average 60 and 50 weeks, respectively.
Table
Evidence on continuing antidepressants in breakthrough bipolar depression
| Study | Design | Results |
|---|---|---|
| Altshuler et al 1 | Retrospective, case-control, 39 patients (1 year) | Relapse rates: 35% in patients who continued antidepressants after mood stabilization and and 68% in those who did not; study included no rapid-cyclers |
| Altshuler et al 2 | Prospective, case control 84 patients (1 year) | Relapse rates: 36% in patients who continued antidepressants after mood stabilization and 70% in those who did not; study included no rapid cyclers |
| Ghaemi et al 3 (unpublished) | Open, randomized 33 patients (approx. 1 yr) | Relapse rate: 50% within 20 weeks, whether or not patients continued antidepressants; one-third of patients were rapid cyclers |
A survival analysis initially suggested some benefit for continuing antidepressants, but this disappeared when the authors adjusted for potential confounding factors—which was not done in the Altshuler et al studies. Patients receiving short-term or long-term antidepressants had a similar number of depressive episodes per year (1.00 vs. 0.75 episodes/year, respectively). For some reason, nonrapid-cycling patients showed greater depressive morbidity than those with rapid cycling.
Rapid cyclers comprised 30% of patients who continued antidepressants and 37% of those who discontinued. This may explain the 50% relapse rate within 20 weeks in both groups. By comparison, the Altshuler et al studies included no rapid cyclers.1,2 More details on this unpublished data are expected.
Are antidepressants the answer?
The high relapse rate in patients taking mood stabilizers—with or without an antidepressant—and the Ghaemi et al data3 suggest that we need alternate antidepressant approaches to bipolar depression. Potential regimens—anticonvulsants, atypical antipsychotics, and other agents—merit further study. So far the evidence is mixed, and the most effective approaches are not well-delineated.
Anticonvulsants. Sachs et al4 reported that adding lamotrigine or lamotrigine plus an antidepressant to mood stabilizer therapy did not appear more effective in treating bipolar depression than simply maintaining the mood stabilizers.
Similarly, a small randomized, double-blind study in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) was stopped early because of low total response. Adjunctive lamotrigine (24%) and inositol (17%) appeared more effective than risperidone (5%) for refractory bipolar depression.
Antipsychotics. In an 8-week randomized trial by Tohen et al,5 833 patients with moderate to severe bipolar I depression were treated with olanzapine, olanzapine plus fluoxetine, or placebo. Core depression symptoms improved significantly more with olanzapine alone or with fluoxetine compared with placebo, as measured by mean changes in the Montgomery-Asberg Depression Rating Scale.
In a 6-month open trial, 192 patients whose bipolar I depression remitted with any of the three treatments6 continued olanzapine and then, if needed after the first week, the olanzapine/fluoxetine combination (OFC). Nearly two-thirds of patients (62%) remained without a depressive recurrence, and 94% remained without a manic recurrence while taking the OFC. These unpublished data indicate that the OFC provided greater prophylactic antimanic than antidepressant effects.
Calabrese et al7 recently presented unpublished data comparing the effects of quetiapine monotherapy, 300 or 600 mg/d, with placebo in patients with bipolar depression. Beginning in the first week of treatment, both quetiapine dosages produced significantly greater antidepressant, antianxiety, and anti-insomnia effects than placebo (P < 0.001). Remission rates with both dosages were >50%.
Summary. These first controlled studies of atypical antipsychotics in bipolar depression suggest that this drug class may have antidepressant as well as their demonstrated antimanic effects.
Recommendations—for now
Controlled clinical trials have not been conducted, and the evidence on using adjunctive antidepressants in bipolar depression is ambiguous.8-11 As a result, it is unclear when or how long to use antidepressants, and many of the inferences and suggestions in this paper remain highly provisional.
Initiating antidepressants. My personal treatment guidelines—and those of many other clinicians—are to use the unimodal antidepressants to augment mood stabilizers in bipolar patients experiencing breakthrough depression, as long as they have not had:
- ultra-rapid cycling (>4 episodes/week)
- antidepressant-induced cycle acceleration
- or multiple episodes of antidepressant-induced mania, despite co-treatment with mood stabilizers.
If any of these variables is present, I would instead add another mood stabilizer or an atypical antipsychotic.
Continuing antidepressants. If the patient remains stable for 2 months after the antidepressant is added—with no depression or manic occurrence—I would continue the antidepressant indefinitely, based on the Altshuler et al data.12
Mood charting.I also recommend that clinicians help patients develop an individual method for mood charting, such as that used in the National Institute of Mental Health Life Chart Method (NIMH-LCM)12,13 or the STEP-BD program.14 Goals of the 5-year STEP-BD are to:
- determine the most effective treatments and relapse prevention strategies for bipolar disorder
- evaluate the psychotropic benefit of anticonvulsants, atypical antipsychotics, cholinesterase inhibitors, and neurotransmitter precursors
- determine the benefit of psychotropic combinations.
Mood charts can provide a retrospective and prospective overview of a patient’s illness course and response to medications. Compared with patient recall, mood charts help clinicians evaluate more precisely the risk of switching and the risks and benefits of starting, continuing, or discontinuing antidepressants. Mood charts may be your most effective tool for managing a patient’s bipolar depression and achieving and maintaining long-term remission.
Summary. In the absence of consensus or guidelines for treating bipolar depression, I suggest:
- a conservative approach—ie, no changes in medication—when the patient remains well on a given regimen
- an aggressive—if not radical—series of treatments and revisions when the illness course remains problematic.
Many medication classes and adjunctive strategies are available for treating bipolar illness.15-18 I recommend that you continue to systematically explore adjunctive treatments and combinations until the patient improves or—even better—attains remission. Good response can be achieved—even in treatment-refractory patients ill for long periods or with recurrent bipolar depression—although complex combination therapy is often required.
Related resources
- National Institute of Mental Health-Life Chart Method. Retrospective and prospective mood-tracking charts for patients with bipolar disorder. www.bipolarnews.org
- Systematic Treatment Enhancement Program for Bipolar Depression (STEP-BD). National Institute of Mental Health. www.stepbd.org/research/
Drug brand names
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosure
Dr. Post is a consultant to Abbott Laboratories, Astra-Zeneca Pharmaceuticals, Bristol-Myers Squibb Co., Elan Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., Shire Pharmaceuticals, and UCB Pharma.
1. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-16.
2. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003;160:1252-62.
3. Ghaemi S, El-Mallakh R, Baldassano CF, et al. Antidepressant treatment in bipolar depression: Long-term outcome (abstract). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
4. Sachs GS. Mood stabilizers alone vs.mood stabilizers plus antidepressant in bipolar depression (symposium). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
5. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60:1079-88.
6. Tohen M, Vieta E, Ketter TA, et al. Acute and long-term efficacy of olanzapine and olanzapine/fluoxetine combination for bipolar depression (abstract). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
7. Calabrese JR, Macfadden W, McCoy R, et al. Double-blind, placebo-controlled study of quetiapine in bipolar depression (abstract). New York: American Psychiatric Association annual meeting, 2004.
8. Post RM, Denicoff KD, Leverich GS, et al. Presentations of depression in bipolar illness. Clin Neurosci Res 2002a;2:142-57.
9. Post RM, Leverich GS, Nolen WA, et al. A re-evaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2003a;5:396-406.
10. Post RM, Speer AM, Leverich GS. Bipolar illness: Which critical treatment issues need studying? Clinical Approaches in Bipolar Disorders 2003b;2:24-30.
11. Goodwin GM. Bipolar disorder: is psychiatry’s Cinderella starting to get out a little more? Acta Neuropsychiatry 2000;12:105.-
12. Leverich GS, Post RM. Life charting the course of bipolar disorder. Current Review of Mood and Anxiety Disorders 1996;1:48-61.
13. Leverich GS, Post RM. Life charting of affective disorders. CNS Spectrums 1998;3:21-37.
14. Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry 2003;53:1028-42.
15. Post RM, Speer AM, Obrocea GV, Leverich GS. Acute and prophylactic effects of anticonvulsants in bipolar depression. Clin Neurosci Res 2002;2:228-51.
16. Post RM, Speer AM. A brief history of anticonvulsant use in affective disorders. In: Trimble MR, Schmitz B (eds). Seizures, affective disorders and anticonvulsant drugs Surrey, UK: Clarius Press 2002;53:81.-
17. Post RM, Speer AM, Leverich GS. Complex combination therapy: the evolution toward rational polypharmacy in lithium-resistant bipolar illness, In: Akiskal H, Tohen M (eds). 50 years: the psychopharmacology of bipolar illness London: John Wiley & Sons Ltd. 2004 (in press).
18. Post RM, Altshuler LL. Mood disorders: treatment of bipolar disorders. Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry (8th ed) New York: Lippincott Williams & Wilkins, 2004 (in press).
Psychiatrists in the trenches are not alone in being unsure how to treat breakthrough bipolar depression. No panelist or other expert attending a recent American College of Neuropsychopharmacology symposium could definitively recommend:
- when to add an antidepressant to mood-stabilizer therapy
- whether to discontinue antidepressants after bipolar depression is stabilized.
Until controlled trials address these issues, we must use limited evidence to treat patients with bipolar depression. To help you meet this challenge, this article offers provisional suggestions based on recent published and unpublished reports.
Evidence for continuing
No published, randomized studies have examined how long to continue antidepressants after bipolar depression has stabilized. Until recently, conventional wisdom has been to discontinue antidepressants as soon as possible because of worries about antidepressant-induced mania. Then two case-controlled studies—one retrospective and one prospective—challenged that assumption.
Figure Reduced relapse rates in patients whose antidepressants were continued
In a prospective case-controlled study, patients with bipolar disorder who continued antidepressant treatment for 6 to 12 months or >12 months after depressive episode remission had lower relapse rates than those who discontinued antidepressants within 6 months.
Source: Reprinted with permission from reference 2. Copyright 2003. American Psychiatric AssociationUsing similar methodologies, Altshuler et al1,2 examined bipolar patients who remained in remission for 6 weeks on mood stabilizers plus adjunctive antidepressants. Those who continued the antidepressants were less likely to relapse into depression (without an increase in manic episodes) than those whose antidepressants were discontinued (Figure).
Relapse rates in the first study1 were 35% at 1 year in those who continued antidepressants and 68% in those who did not. In the second study,2 36% and 70% of patients, respectively, had relapsed at 1 year. In the latter study, those who continued to take antidepressants for at least 6 months—instead of at least 12 months—had intermediate depressive relapse rates (53% for 6 months vs. 24% for 12 months).
When interpreting these data, keep several caveats in mind. For one, patients were not randomly assigned to continue or discontinue antidepressants but were designated by patient and clinician choice. When comparing the two patient groups, however, the authors found no inherent differences in illness characteristics that might have biased the results.
More importantly, few patients initially treated with antidepressant augmentation responded well and remained in remission. In the prospective study, 549 of 1,078 patients in the Stanley Foundation Bipolar Network received an antidepressant for breakthrough depressive symptoms.2 Only 189 remained on antidepressants for 2 months, and only 84 (15%) of the original population remained in remission for 2 months.
Summary. A small subgroup of patients appears to respond well to antidepressants and sustains this response for 6 to 8 weeks. For this subset, continuing antidepressant therapy would appear to be an appropriate strategy, based on:
- a significantly reduced risk for depressive relapse
- no increased risk of switching to mania, which is the usual reason to terminate antidepressants early.
Subsequent evidence
One needs to assess these observations in light of an interim analysis reported halfway through a small, open, randomized study. Ghaemi et al3 found no significant difference in outcomes—regardless of rapid-cycling status—among 14 bipolar patients who remained on antidepressants and 19 who discontinued across an average 60 and 50 weeks, respectively.
Table
Evidence on continuing antidepressants in breakthrough bipolar depression
| Study | Design | Results |
|---|---|---|
| Altshuler et al 1 | Retrospective, case-control, 39 patients (1 year) | Relapse rates: 35% in patients who continued antidepressants after mood stabilization and and 68% in those who did not; study included no rapid-cyclers |
| Altshuler et al 2 | Prospective, case control 84 patients (1 year) | Relapse rates: 36% in patients who continued antidepressants after mood stabilization and 70% in those who did not; study included no rapid cyclers |
| Ghaemi et al 3 (unpublished) | Open, randomized 33 patients (approx. 1 yr) | Relapse rate: 50% within 20 weeks, whether or not patients continued antidepressants; one-third of patients were rapid cyclers |
A survival analysis initially suggested some benefit for continuing antidepressants, but this disappeared when the authors adjusted for potential confounding factors—which was not done in the Altshuler et al studies. Patients receiving short-term or long-term antidepressants had a similar number of depressive episodes per year (1.00 vs. 0.75 episodes/year, respectively). For some reason, nonrapid-cycling patients showed greater depressive morbidity than those with rapid cycling.
Rapid cyclers comprised 30% of patients who continued antidepressants and 37% of those who discontinued. This may explain the 50% relapse rate within 20 weeks in both groups. By comparison, the Altshuler et al studies included no rapid cyclers.1,2 More details on this unpublished data are expected.
Are antidepressants the answer?
The high relapse rate in patients taking mood stabilizers—with or without an antidepressant—and the Ghaemi et al data3 suggest that we need alternate antidepressant approaches to bipolar depression. Potential regimens—anticonvulsants, atypical antipsychotics, and other agents—merit further study. So far the evidence is mixed, and the most effective approaches are not well-delineated.
Anticonvulsants. Sachs et al4 reported that adding lamotrigine or lamotrigine plus an antidepressant to mood stabilizer therapy did not appear more effective in treating bipolar depression than simply maintaining the mood stabilizers.
Similarly, a small randomized, double-blind study in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) was stopped early because of low total response. Adjunctive lamotrigine (24%) and inositol (17%) appeared more effective than risperidone (5%) for refractory bipolar depression.
Antipsychotics. In an 8-week randomized trial by Tohen et al,5 833 patients with moderate to severe bipolar I depression were treated with olanzapine, olanzapine plus fluoxetine, or placebo. Core depression symptoms improved significantly more with olanzapine alone or with fluoxetine compared with placebo, as measured by mean changes in the Montgomery-Asberg Depression Rating Scale.
In a 6-month open trial, 192 patients whose bipolar I depression remitted with any of the three treatments6 continued olanzapine and then, if needed after the first week, the olanzapine/fluoxetine combination (OFC). Nearly two-thirds of patients (62%) remained without a depressive recurrence, and 94% remained without a manic recurrence while taking the OFC. These unpublished data indicate that the OFC provided greater prophylactic antimanic than antidepressant effects.
Calabrese et al7 recently presented unpublished data comparing the effects of quetiapine monotherapy, 300 or 600 mg/d, with placebo in patients with bipolar depression. Beginning in the first week of treatment, both quetiapine dosages produced significantly greater antidepressant, antianxiety, and anti-insomnia effects than placebo (P < 0.001). Remission rates with both dosages were >50%.
Summary. These first controlled studies of atypical antipsychotics in bipolar depression suggest that this drug class may have antidepressant as well as their demonstrated antimanic effects.
Recommendations—for now
Controlled clinical trials have not been conducted, and the evidence on using adjunctive antidepressants in bipolar depression is ambiguous.8-11 As a result, it is unclear when or how long to use antidepressants, and many of the inferences and suggestions in this paper remain highly provisional.
Initiating antidepressants. My personal treatment guidelines—and those of many other clinicians—are to use the unimodal antidepressants to augment mood stabilizers in bipolar patients experiencing breakthrough depression, as long as they have not had:
- ultra-rapid cycling (>4 episodes/week)
- antidepressant-induced cycle acceleration
- or multiple episodes of antidepressant-induced mania, despite co-treatment with mood stabilizers.
If any of these variables is present, I would instead add another mood stabilizer or an atypical antipsychotic.
Continuing antidepressants. If the patient remains stable for 2 months after the antidepressant is added—with no depression or manic occurrence—I would continue the antidepressant indefinitely, based on the Altshuler et al data.12
Mood charting.I also recommend that clinicians help patients develop an individual method for mood charting, such as that used in the National Institute of Mental Health Life Chart Method (NIMH-LCM)12,13 or the STEP-BD program.14 Goals of the 5-year STEP-BD are to:
- determine the most effective treatments and relapse prevention strategies for bipolar disorder
- evaluate the psychotropic benefit of anticonvulsants, atypical antipsychotics, cholinesterase inhibitors, and neurotransmitter precursors
- determine the benefit of psychotropic combinations.
Mood charts can provide a retrospective and prospective overview of a patient’s illness course and response to medications. Compared with patient recall, mood charts help clinicians evaluate more precisely the risk of switching and the risks and benefits of starting, continuing, or discontinuing antidepressants. Mood charts may be your most effective tool for managing a patient’s bipolar depression and achieving and maintaining long-term remission.
Summary. In the absence of consensus or guidelines for treating bipolar depression, I suggest:
- a conservative approach—ie, no changes in medication—when the patient remains well on a given regimen
- an aggressive—if not radical—series of treatments and revisions when the illness course remains problematic.
Many medication classes and adjunctive strategies are available for treating bipolar illness.15-18 I recommend that you continue to systematically explore adjunctive treatments and combinations until the patient improves or—even better—attains remission. Good response can be achieved—even in treatment-refractory patients ill for long periods or with recurrent bipolar depression—although complex combination therapy is often required.
Related resources
- National Institute of Mental Health-Life Chart Method. Retrospective and prospective mood-tracking charts for patients with bipolar disorder. www.bipolarnews.org
- Systematic Treatment Enhancement Program for Bipolar Depression (STEP-BD). National Institute of Mental Health. www.stepbd.org/research/
Drug brand names
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosure
Dr. Post is a consultant to Abbott Laboratories, Astra-Zeneca Pharmaceuticals, Bristol-Myers Squibb Co., Elan Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., Shire Pharmaceuticals, and UCB Pharma.
Psychiatrists in the trenches are not alone in being unsure how to treat breakthrough bipolar depression. No panelist or other expert attending a recent American College of Neuropsychopharmacology symposium could definitively recommend:
- when to add an antidepressant to mood-stabilizer therapy
- whether to discontinue antidepressants after bipolar depression is stabilized.
Until controlled trials address these issues, we must use limited evidence to treat patients with bipolar depression. To help you meet this challenge, this article offers provisional suggestions based on recent published and unpublished reports.
Evidence for continuing
No published, randomized studies have examined how long to continue antidepressants after bipolar depression has stabilized. Until recently, conventional wisdom has been to discontinue antidepressants as soon as possible because of worries about antidepressant-induced mania. Then two case-controlled studies—one retrospective and one prospective—challenged that assumption.
Figure Reduced relapse rates in patients whose antidepressants were continued
In a prospective case-controlled study, patients with bipolar disorder who continued antidepressant treatment for 6 to 12 months or >12 months after depressive episode remission had lower relapse rates than those who discontinued antidepressants within 6 months.
Source: Reprinted with permission from reference 2. Copyright 2003. American Psychiatric AssociationUsing similar methodologies, Altshuler et al1,2 examined bipolar patients who remained in remission for 6 weeks on mood stabilizers plus adjunctive antidepressants. Those who continued the antidepressants were less likely to relapse into depression (without an increase in manic episodes) than those whose antidepressants were discontinued (Figure).
Relapse rates in the first study1 were 35% at 1 year in those who continued antidepressants and 68% in those who did not. In the second study,2 36% and 70% of patients, respectively, had relapsed at 1 year. In the latter study, those who continued to take antidepressants for at least 6 months—instead of at least 12 months—had intermediate depressive relapse rates (53% for 6 months vs. 24% for 12 months).
When interpreting these data, keep several caveats in mind. For one, patients were not randomly assigned to continue or discontinue antidepressants but were designated by patient and clinician choice. When comparing the two patient groups, however, the authors found no inherent differences in illness characteristics that might have biased the results.
More importantly, few patients initially treated with antidepressant augmentation responded well and remained in remission. In the prospective study, 549 of 1,078 patients in the Stanley Foundation Bipolar Network received an antidepressant for breakthrough depressive symptoms.2 Only 189 remained on antidepressants for 2 months, and only 84 (15%) of the original population remained in remission for 2 months.
Summary. A small subgroup of patients appears to respond well to antidepressants and sustains this response for 6 to 8 weeks. For this subset, continuing antidepressant therapy would appear to be an appropriate strategy, based on:
- a significantly reduced risk for depressive relapse
- no increased risk of switching to mania, which is the usual reason to terminate antidepressants early.
Subsequent evidence
One needs to assess these observations in light of an interim analysis reported halfway through a small, open, randomized study. Ghaemi et al3 found no significant difference in outcomes—regardless of rapid-cycling status—among 14 bipolar patients who remained on antidepressants and 19 who discontinued across an average 60 and 50 weeks, respectively.
Table
Evidence on continuing antidepressants in breakthrough bipolar depression
| Study | Design | Results |
|---|---|---|
| Altshuler et al 1 | Retrospective, case-control, 39 patients (1 year) | Relapse rates: 35% in patients who continued antidepressants after mood stabilization and and 68% in those who did not; study included no rapid-cyclers |
| Altshuler et al 2 | Prospective, case control 84 patients (1 year) | Relapse rates: 36% in patients who continued antidepressants after mood stabilization and 70% in those who did not; study included no rapid cyclers |
| Ghaemi et al 3 (unpublished) | Open, randomized 33 patients (approx. 1 yr) | Relapse rate: 50% within 20 weeks, whether or not patients continued antidepressants; one-third of patients were rapid cyclers |
A survival analysis initially suggested some benefit for continuing antidepressants, but this disappeared when the authors adjusted for potential confounding factors—which was not done in the Altshuler et al studies. Patients receiving short-term or long-term antidepressants had a similar number of depressive episodes per year (1.00 vs. 0.75 episodes/year, respectively). For some reason, nonrapid-cycling patients showed greater depressive morbidity than those with rapid cycling.
Rapid cyclers comprised 30% of patients who continued antidepressants and 37% of those who discontinued. This may explain the 50% relapse rate within 20 weeks in both groups. By comparison, the Altshuler et al studies included no rapid cyclers.1,2 More details on this unpublished data are expected.
Are antidepressants the answer?
The high relapse rate in patients taking mood stabilizers—with or without an antidepressant—and the Ghaemi et al data3 suggest that we need alternate antidepressant approaches to bipolar depression. Potential regimens—anticonvulsants, atypical antipsychotics, and other agents—merit further study. So far the evidence is mixed, and the most effective approaches are not well-delineated.
Anticonvulsants. Sachs et al4 reported that adding lamotrigine or lamotrigine plus an antidepressant to mood stabilizer therapy did not appear more effective in treating bipolar depression than simply maintaining the mood stabilizers.
Similarly, a small randomized, double-blind study in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) was stopped early because of low total response. Adjunctive lamotrigine (24%) and inositol (17%) appeared more effective than risperidone (5%) for refractory bipolar depression.
Antipsychotics. In an 8-week randomized trial by Tohen et al,5 833 patients with moderate to severe bipolar I depression were treated with olanzapine, olanzapine plus fluoxetine, or placebo. Core depression symptoms improved significantly more with olanzapine alone or with fluoxetine compared with placebo, as measured by mean changes in the Montgomery-Asberg Depression Rating Scale.
In a 6-month open trial, 192 patients whose bipolar I depression remitted with any of the three treatments6 continued olanzapine and then, if needed after the first week, the olanzapine/fluoxetine combination (OFC). Nearly two-thirds of patients (62%) remained without a depressive recurrence, and 94% remained without a manic recurrence while taking the OFC. These unpublished data indicate that the OFC provided greater prophylactic antimanic than antidepressant effects.
Calabrese et al7 recently presented unpublished data comparing the effects of quetiapine monotherapy, 300 or 600 mg/d, with placebo in patients with bipolar depression. Beginning in the first week of treatment, both quetiapine dosages produced significantly greater antidepressant, antianxiety, and anti-insomnia effects than placebo (P < 0.001). Remission rates with both dosages were >50%.
Summary. These first controlled studies of atypical antipsychotics in bipolar depression suggest that this drug class may have antidepressant as well as their demonstrated antimanic effects.
Recommendations—for now
Controlled clinical trials have not been conducted, and the evidence on using adjunctive antidepressants in bipolar depression is ambiguous.8-11 As a result, it is unclear when or how long to use antidepressants, and many of the inferences and suggestions in this paper remain highly provisional.
Initiating antidepressants. My personal treatment guidelines—and those of many other clinicians—are to use the unimodal antidepressants to augment mood stabilizers in bipolar patients experiencing breakthrough depression, as long as they have not had:
- ultra-rapid cycling (>4 episodes/week)
- antidepressant-induced cycle acceleration
- or multiple episodes of antidepressant-induced mania, despite co-treatment with mood stabilizers.
If any of these variables is present, I would instead add another mood stabilizer or an atypical antipsychotic.
Continuing antidepressants. If the patient remains stable for 2 months after the antidepressant is added—with no depression or manic occurrence—I would continue the antidepressant indefinitely, based on the Altshuler et al data.12
Mood charting.I also recommend that clinicians help patients develop an individual method for mood charting, such as that used in the National Institute of Mental Health Life Chart Method (NIMH-LCM)12,13 or the STEP-BD program.14 Goals of the 5-year STEP-BD are to:
- determine the most effective treatments and relapse prevention strategies for bipolar disorder
- evaluate the psychotropic benefit of anticonvulsants, atypical antipsychotics, cholinesterase inhibitors, and neurotransmitter precursors
- determine the benefit of psychotropic combinations.
Mood charts can provide a retrospective and prospective overview of a patient’s illness course and response to medications. Compared with patient recall, mood charts help clinicians evaluate more precisely the risk of switching and the risks and benefits of starting, continuing, or discontinuing antidepressants. Mood charts may be your most effective tool for managing a patient’s bipolar depression and achieving and maintaining long-term remission.
Summary. In the absence of consensus or guidelines for treating bipolar depression, I suggest:
- a conservative approach—ie, no changes in medication—when the patient remains well on a given regimen
- an aggressive—if not radical—series of treatments and revisions when the illness course remains problematic.
Many medication classes and adjunctive strategies are available for treating bipolar illness.15-18 I recommend that you continue to systematically explore adjunctive treatments and combinations until the patient improves or—even better—attains remission. Good response can be achieved—even in treatment-refractory patients ill for long periods or with recurrent bipolar depression—although complex combination therapy is often required.
Related resources
- National Institute of Mental Health-Life Chart Method. Retrospective and prospective mood-tracking charts for patients with bipolar disorder. www.bipolarnews.org
- Systematic Treatment Enhancement Program for Bipolar Depression (STEP-BD). National Institute of Mental Health. www.stepbd.org/research/
Drug brand names
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Olanzapine/fluoxetine • Symbyax
- Quetiapine • Seroquel
- Risperidone • Risperdal
Disclosure
Dr. Post is a consultant to Abbott Laboratories, Astra-Zeneca Pharmaceuticals, Bristol-Myers Squibb Co., Elan Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., Shire Pharmaceuticals, and UCB Pharma.
1. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-16.
2. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003;160:1252-62.
3. Ghaemi S, El-Mallakh R, Baldassano CF, et al. Antidepressant treatment in bipolar depression: Long-term outcome (abstract). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
4. Sachs GS. Mood stabilizers alone vs.mood stabilizers plus antidepressant in bipolar depression (symposium). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
5. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60:1079-88.
6. Tohen M, Vieta E, Ketter TA, et al. Acute and long-term efficacy of olanzapine and olanzapine/fluoxetine combination for bipolar depression (abstract). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
7. Calabrese JR, Macfadden W, McCoy R, et al. Double-blind, placebo-controlled study of quetiapine in bipolar depression (abstract). New York: American Psychiatric Association annual meeting, 2004.
8. Post RM, Denicoff KD, Leverich GS, et al. Presentations of depression in bipolar illness. Clin Neurosci Res 2002a;2:142-57.
9. Post RM, Leverich GS, Nolen WA, et al. A re-evaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2003a;5:396-406.
10. Post RM, Speer AM, Leverich GS. Bipolar illness: Which critical treatment issues need studying? Clinical Approaches in Bipolar Disorders 2003b;2:24-30.
11. Goodwin GM. Bipolar disorder: is psychiatry’s Cinderella starting to get out a little more? Acta Neuropsychiatry 2000;12:105.-
12. Leverich GS, Post RM. Life charting the course of bipolar disorder. Current Review of Mood and Anxiety Disorders 1996;1:48-61.
13. Leverich GS, Post RM. Life charting of affective disorders. CNS Spectrums 1998;3:21-37.
14. Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry 2003;53:1028-42.
15. Post RM, Speer AM, Obrocea GV, Leverich GS. Acute and prophylactic effects of anticonvulsants in bipolar depression. Clin Neurosci Res 2002;2:228-51.
16. Post RM, Speer AM. A brief history of anticonvulsant use in affective disorders. In: Trimble MR, Schmitz B (eds). Seizures, affective disorders and anticonvulsant drugs Surrey, UK: Clarius Press 2002;53:81.-
17. Post RM, Speer AM, Leverich GS. Complex combination therapy: the evolution toward rational polypharmacy in lithium-resistant bipolar illness, In: Akiskal H, Tohen M (eds). 50 years: the psychopharmacology of bipolar illness London: John Wiley & Sons Ltd. 2004 (in press).
18. Post RM, Altshuler LL. Mood disorders: treatment of bipolar disorders. Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry (8th ed) New York: Lippincott Williams & Wilkins, 2004 (in press).
1. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-16.
2. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003;160:1252-62.
3. Ghaemi S, El-Mallakh R, Baldassano CF, et al. Antidepressant treatment in bipolar depression: Long-term outcome (abstract). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
4. Sachs GS. Mood stabilizers alone vs.mood stabilizers plus antidepressant in bipolar depression (symposium). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
5. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60:1079-88.
6. Tohen M, Vieta E, Ketter TA, et al. Acute and long-term efficacy of olanzapine and olanzapine/fluoxetine combination for bipolar depression (abstract). San Juan, PR: American College of Neuropsychopharmacology annual meeting, 2003.
7. Calabrese JR, Macfadden W, McCoy R, et al. Double-blind, placebo-controlled study of quetiapine in bipolar depression (abstract). New York: American Psychiatric Association annual meeting, 2004.
8. Post RM, Denicoff KD, Leverich GS, et al. Presentations of depression in bipolar illness. Clin Neurosci Res 2002a;2:142-57.
9. Post RM, Leverich GS, Nolen WA, et al. A re-evaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2003a;5:396-406.
10. Post RM, Speer AM, Leverich GS. Bipolar illness: Which critical treatment issues need studying? Clinical Approaches in Bipolar Disorders 2003b;2:24-30.
11. Goodwin GM. Bipolar disorder: is psychiatry’s Cinderella starting to get out a little more? Acta Neuropsychiatry 2000;12:105.-
12. Leverich GS, Post RM. Life charting the course of bipolar disorder. Current Review of Mood and Anxiety Disorders 1996;1:48-61.
13. Leverich GS, Post RM. Life charting of affective disorders. CNS Spectrums 1998;3:21-37.
14. Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry 2003;53:1028-42.
15. Post RM, Speer AM, Obrocea GV, Leverich GS. Acute and prophylactic effects of anticonvulsants in bipolar depression. Clin Neurosci Res 2002;2:228-51.
16. Post RM, Speer AM. A brief history of anticonvulsant use in affective disorders. In: Trimble MR, Schmitz B (eds). Seizures, affective disorders and anticonvulsant drugs Surrey, UK: Clarius Press 2002;53:81.-
17. Post RM, Speer AM, Leverich GS. Complex combination therapy: the evolution toward rational polypharmacy in lithium-resistant bipolar illness, In: Akiskal H, Tohen M (eds). 50 years: the psychopharmacology of bipolar illness London: John Wiley & Sons Ltd. 2004 (in press).
18. Post RM, Altshuler LL. Mood disorders: treatment of bipolar disorders. Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry (8th ed) New York: Lippincott Williams & Wilkins, 2004 (in press).
From the Stanley Foundation Bipolar Network: Efficacy of adjunctive therapies for treatment-resistant patients
When choosing psychopharmacologic agents for patients with bipolar disorder, the clinician lacks guidance from controlled clinical trials at virtually every therapeutic step. To help remedy this problem, we in the Stanley Foundation Bipolar Network (SFBN) evaluated compounds with potentially important therapeutic profiles. We began by documenting the demographics and illness characteristics of patients with bipolar disorder. Then we assessed some 16 treatments in naturalistic or formal studies, some of which are complete and others ongoing.
In this second installment, we report our current information about the efficacy of second-generation antidepressants, atypical antipsychotics, and anticonvulsant agents.
Mood stabilizers
As mood stabilizers, the anticonvulsants carbamazepine and valproate are well-accepted alternatives or adjuncts to lithium carbonate, which remains a mainstay treatment for acute episodes and long-term prophylaxis.1
Valproate is approved by the FDA for treatment of acute mania but not for long-term prevention. Open studies in rapid-cycling, treatment-refractory patients demonstrate substantial improvement for both manic and depressive phases with valproate. In the most recent controlled study, valproate did not prevent manic episodes to a statistically significant degree, but it did show greater antidepressant effects than placebo or lithium.2
Carbamazepine has been widely studied in acute mania and compared with lithium for long-term prophylaxis. Most studies show nonsignificant differences between carbamazepine and lithium in preventing manic and depressive episodes, although several studies indicate less-robust antimanic effects of carbamazepine,3 particularly for patients with classic euphoric mania without psychosis, bipolar II, or associated substance abuse.4
In atypical patients, carbamazepine appeared to outperform lithium, suggesting clinical differences in these agents that have different mechanisms of action across neurotransmitter and peptide systems.
Low response rates Most randomized controlled trials and open adjunct studies suggest that 50% or more patients respond to lithium, valproate, or carbamazepine. Outcomes may be far less positive in clinical practice, however. Despite the use of mood stabilizers and a variety of antidepressants, benzodiazepines, and neuroleptics, our patients experienced substantial residual manic and depressive symptoms and functional impairment.
Similarly, clinician ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM) indicated a high rate of breakthrough episodes during carefully monitored and aggressive psychopharmacologic treatment.5 Two-thirds of the first 258 bipolar outpatients that we followed and rated daily for 1 year continued to be substantially impaired. One-quarter of them were ill for more than 75% of the year, despite using an average 4.1 psychopharmacologic agents per patient, including mood stabilizers, antidepressants, antipsychotics, and other agents.
Acute and long-term combination therapy appears more effective than monotherapy, particularly for treatment-refractory rapid cyclers.3 Even so, many patients do not respond to combination therapy. For example, in a 1-year prophylaxis study, 25% or fewer patients were rated “much improved” to “very much improved” on the Clinical Global Impressions (CGI) scale with lithium or carbamazepine as the baseline mood stabilizer and with antidepressants, neuroleptics, and benzodiazepines used as needed. In the third year of combination therapy, the overall response rate, including rapid cyclers, was roughly 50%.3
A low rate of response is apparent for lithium and valproate. While treating rapidly cycling patients, Calabrese and associates found that less than one-quarter of them responded well enough to the two-drug combination that they could be enrolled in a randomized double-blind comparison of each drug in monotherapy. Of those who were eligible, almost all relapsed with either lithium or valproate monotherapy and again required combination therapy.6
Table 1
RATE OF SWITCHING (%) INTO MANIA IN DEPRESSED BIPOLAR PATIENTS DURING ANTIDEPRESSANT TREATMENT*
| Acute treatment (10 wks) | Continuation treatment (52 wks)‡ | |||||
|---|---|---|---|---|---|---|
| Type of switch | Open (n=27) | Blind (n=100) | Total (n=127) | Open (n=12) | Blind (n=55) | Total (n=67) |
| Brief hypomania | 3.7% | 6% | 5.5% | – | 3.6% | 3% |
| Recurrent brief hypomania | 11 | 12 | 11.8 | 8.3% | 18.2 | 16.4 |
| Hypomania | 11 | 13 | 12.6 | 8.3 | 23.6 | 20.9 |
| Mania | 14.8 | 12 | 12.6 | 33.3 | 14.5 | 17.9 |
| Total | 25.8 | 25 | 25.2 | 41.6 | 38.1 | 38.8 |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | ||||||
| ‡ Most patients (80%) dropped out of this phase because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. | ||||||
| Source: Post RM et al. Bipolar Disord 2001;3:259-65, and unpublished data. | ||||||
Taken together, these studies indicate that even with our most effective and most studied agents, many patients respond inadequately. On the basis of this clinical reality, we began to explore additional options that might enhance clinical effectiveness.
Atypical antipsychotics
We assessed the addition of the atypical antipsychotic olanzapine in patients with treatment-refractory bipolar disorder7 and saw improvement in 57% of subjects with manic, mixed, and depressive components. These data foreshadowed more recent controlled findings of olanzapine’s usefulness in depression and mania.8,9 Our preliminary open clinical trial experience allowed for more controlled studies and subsequent approval and wider clinical use of olanzapine in bipolar illness.
Our initial assessment of other atypical antipsychotics found that quetiapine was associated with significantly fewer depressive symptoms on the Inventory of Depressive Symptomatology (IDS) when used as part of the regular treatment regimen. This improvement, which was seen in the first month, was maintained over 4 months of treatment, whereas no significant improvement in depression severity was seen with the use of risperidone or clozapine.10
It would be highly preliminary to conclude from these uncontrolled data that quetiapine had greater effects on bipolar depressive symptoms than did risperidone or clozapine. We cannot rule out the possibility that patients with different degrees of treatment resistance were enrolled and treated with the different concomitant drugs. However, patients did not differ at baseline in severity of their depression.
In this initial look at the atypicals, the severity of depressive symptoms on the IDS was much greater than that of mania, as measured by the Young Mania Rating Scale (YMRS). Thus, the precise patterns of illness being treated and the impact of these antipsychotics on depressive cyclicity and duration await closer examination. We intend to look at the relative efficacy and side-effect profiles of each of the atypical antipsychotics as used in clinical practice.
Second-generation antidepressants
Bupropion, sertraline, and venlafaxine have different mechanisms of action but appear to be of value as adjuncts to bipolar disorder treatment. When we used them as adjuncts to mood stabilizers in a prospective study, we found a 40 to 50% acute response rate and some switching into hypomania and mania (Table 1).11 Many switches appeared to involve only isolated brief bursts of hypomania or recurrent brief hypomanias, but more sustained episodes of hypomania (7 days) could be more clinically problematic. Only 13% of patients treated acutely with these antidepressants had more full-blown manic symptoms (i.e., those associated with moderate or greater dysfunction on the NIMH-LCM).
During the next year, when apparent acute antidepressant responders were offered continuation treatment, 18% switched into mania. However, most patients (about 80%) dropped out because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. The 127 patients who were randomized received 175 acute antidepressant trials. Only 9% were associated with a switch into mania with moderate dysfunction, whereas another 9% showed periods of hypomania lasting longer than 1 week.12
Table 2
RELAPSE INTO DEPRESSION IN BIPOLAR PATIENTS AFTER 1 YEAR WITH OR WITHOUT ADJUNCTIVE ANTIDEPRESSANT TREATMENT*
| Rate of relapse | |
|---|---|
| Continued on antidepressants | |
| Study #1 (n=19) | 35% |
| Study #2 (n=41) | 41% |
| Discontinued antidepressants | |
| Study #1 (n=25) | 68% |
| Study #2 (n=43) | 71% |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | |
| Study #1: Altshuler et al. J Clin Psychiatry 2001;62:612-6. | |
| Study #2: Altshuler et al. (submitted for publication 2002). | |
We have entered 176 patients into this double-blind, randomized comparison of bupropion, sertraline, and venlafaxine, with another 27 randomized openly. The study will attempt to examine any possible between-drug differences. Even without the blind being broken, it appears that these antidepressants are associated with a somewhat lower-than-expected rate of switching into mania with dysfunction, but a direct comparison with a placebo is not available.
In two other series, patients who remained well for 2 months on antidepressants plus mood stabilizers and then continued on their antidepressants had a much lower incidence of depressive relapses over the following year than those who discontinued their antidepressants (Table 2).13,14 Surprisingly, continuing the antidepressant was not associated with an increased rate of switching into mania.
Prospective randomized, controlled studies are required to confirm these findings. Even so, our studies appear to challenge conventional wisdom for the first time. Stopping antidepressant treatment in bipolar patients as soon as possible for fear of inducing a manic episode may not be the optimal recommendation in the admittedly small subgroup (about 15%) of patients who have responded well to an antidepressant for at least 2 months.
Table 3
SIX STUDIES COMPLETED BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number studied | Response rate |
|---|---|---|---|---|
| Antidepressants bupropion | Yes | Double-blind | 173 | ~ 47% |
| vs. sertraline vs. venlafaxine (Post et al, 2001) | Yes | Open | 27 | |
| Total = 200 | ||||
| Olanzapine (McElroy et al, 1998) | No | Open | 14 | ~ 57% |
| Lamotrigine (Suppes et al, 1999) | No | Open | 17 | ~ 65% |
| Gabapentin (Altshuler et al, 1999) | No | Open | 28 | 78% (14/18) for mania or hypomania 100% (n = 5) for depression 20% (1/5) for cycling |
| Topiramate (McElroy et al, 2000) | No | Open | 54 | 63% for mania or cycling 27% for acute depression (weight loss observed) |
| Tiagabine (Suppes et al, 2002) | No | Open | 17 | 18% (3/17) (3 possible seizures) |
Second-generation anticonvulsants
Based on our patients’ substantial residual symptoms, we explored a series of newly approved anticonvulsants for potential use as mood stabilizers.
Lamotrigine Consistent with other open and double-blind, placebo-controlled studies, lamotrigine showed promise as an adjunctive treatment, with improvement in depressive symptoms and cycling.15 One such study showed acute antidepressant effects of lamotrigine monotherapy compared with a placebo at dosages of 50 or 200 mg/d.16
Another double-blind, randomized, placebo-controlled NIMH study (partially funded by the Stanley Foundation) compared lamotrigine with gabapentin and a placebo in patients for whom lithium, carbamazepine, and valproate had failed.17 Most of these treatment-refractory patients received all three agents through three 6-week randomizations. Lamotrigine was much more effective in depression and overall illness than either a placebo or gabapentin. Men with bipolar compared with unipolar illness and patients with fewer unsuccessful clinical trials and hospitalizations for depression showed the greatest response.18
In two recent industry-sponsored controlled trials of long-term prophylaxis (following a recent manic or depressive episode), lamotrigine was significantly more effective than a placebo (Bowden et al and Calabrese et al, unpublished data). Lamotrigine was more effective than lithium in preventing depressive episodes, whereas lithium was more effective than lamotrigine for manic episodes, although lamotrigine was significantly better than the placebo.
These data suggest that lamotrigine may differ from valproate, carbamazepine, and lithium in being a more effective antidepressant than antimanic agent.
Topiramate Adjunctive use of topiramate in rapid-cycling and treatment-resistant patients has been examined in open studies, but controlled studies have not yet been published. In our open-label case series observations, 19 of 30 (63%) patients taking topiramate for mania or cycling were “much” or “very much” improved after 10 weeks, whereas only 3 of 11 (27%) patients taking topiramate for acute depression were so improved.19
Table 4
STUDIES IN PROGRESS BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number enrolled* | Notes |
|---|---|---|---|---|
| Topiramate vs. sibutramine | Yes | Open | 42 | Relative weight loss and mood stabilization |
| Omega-3 fatty acids (6 grams EPA vs. placebo) (for depression cycling) (Keck et al) | Yes Yes | Double-blind Double-blind | 62 60 | Data from both studies being analyzed |
| Acamprosate (for alcohol craving) (Grunze et al) | No | Open | 23 | Goal: 60 patients |
| Levetiracetam (Post et al) | No | Open | 13 | Goal: 30 patients |
| Zonisamide (McElroy et al) | No | Open | 45 | Goal: 60 patients European sites only |
| Tranylcypromine vs. lamotrigine (for refractory depression) (Nolen et al) | Yes | Open | 17 | Single-site study |
| Quetiapine (McElroy et al) | No | Open | 48 | Apparent superior antidepressant effects compared with risperidone or clozapine |
| Risperidone (Grunze et al) | No | Open | 37 | No significant effect on IDS‡ depression ratings |
| Clozapine (Suppes et al) | No | Open | 19 | No significant effect on IDS depression ratings |
| Modafinil vs. placebo (Frye et al) | Yes | Double-blind | Started 4/02 | |
| * Enrollment as of April 2002 n = 696 total patients in tables 3 and 4 | ||||
| ‡ IDS = Inventory of Depressive Symptomatology | ||||
An industry-sponsored double-blind, randomized trial of topiramate in acute mania looked promising in the first 67 patients, but not as encouraging in subsequent subjects. A positive drug effect re-emerged if one eliminated patients whose manic episodes were precipitated by antidepressants.
The acute antidepressant effects of topiramate did not appear promising in our study. On the other hand, a single-blind, randomized study of topiramate versus bupropion as adjunctive therapy for breakthrough bipolar depression suggested excellent acute response (approximately 56%) to both agents.20 These data leave the potential antidepressant effects of topiramate undetermined.
In patients being treated with topiramate, we found significant weight loss (average –0.7 kg at 4 weeks, –1.6 kg at 10 weeks, –4.7 kg at 6 months, and –6.2 kg at 1 year).19 Average weight loss was 4.5±6.7 kg at the final evaluation (body mass index [BMI] change of –6.3% and –5% at 1 year and last evaluation, respectively).
We and others have thus seen substantial weight loss in one-third to one-half of patients taking topiramate for bipolar disorder.21 This weight loss is maintained over relatively long periods, which differentiates topiramate from other weight-loss regimens. Topiramate’s ability to decrease weight—even while it is in the early stages of evaluation as a mood stabilizer—is of considerable interest, given the substantial problem of overweight in the bipolar population and the fact that many drug therapies used in the illness are associated with weight gain.
We are proceeding with a randomized open comparison of the relative weight loss and psychotropic efficacy of topiramate versus the antiobesity agent sibutramine. Sibutramine has been reported to have antidepressant properties and is FDA-approved for weight loss.
Gabapentin and tiagabine Our group and many others have reported substantial overall clinical improvement when gabapentin was used as adjunctive therapy in an open study of patients with inadequate response to previous treatments.22 As monotherapy, however, gabapentin was not more effective than a placebo17 and was less effective than lamotrigine. Similarly, gabapentin was not an effective acute antimanic agent when compared with a placebo,23 although other double-blind, controlled studies have indicated positive effects in anxiety disorders24 and social phobias.25 Thus, the gabapentin literature appears to be divergent—i.e., positive studies support its adjunctive use in bipolar patients, but controlled studies suggest that it is not directly antimanic or mood-stabilizing.
Gabapentin’s effect on syndromes that are highly comorbid with bipolar illness could account for its apparent success as open adjunctive therapy. For example, in addition to its antianxiety effects, gabapentin shows positive effects in alcohol withdrawal, pain syndromes, sleep disorders (including restless leg syndrome), and obsessive-compulsive disorder. Also, combining gabapentin with other psychotropics with other mechanisms of action may have a more robust therapeutic effect than monotherapy.
Our preliminary observations with the selective GABA-reuptake inhibitor tiagabine were not promising. In an open study, only 3 of the first 17 SFBN patients with treatment-resistant affective disorders showed a partial response (moderate improvement on the Clinical Global Impressions Scale for Bipolar Illness [CGI-BP]) to adjunctive tiagabine treatment when the dosage was gradually increased. Serious side effects, including two patients with possible seizures, were noted.26 Similar results were seen when tiagabine doses were rapidly increased in eight patients with acute mania; none responded, and one had a major motor seizure.27
Thus, our initial observations led us to discontinue use of tiagabine and suggest any further clinical consideration of this agent for treatment-resistant affective disorders would require great care. This perspective contrasts with several previous positive case reports. It is unknown whether selected patients or those treated with lower dosages would be more responsive to this agent.
Like valproate, gabapentin and tiagabine increase brain GABA, but their apparent lack of antimanic efficacy strongly suggests that raising brain GABA alone does little to counter mania. These data further support the viewpoint already offered that all anticonvulsants are not alike and that anticonvulsants as a class are not necessarily mood stabilizers.
Zonisamide and levetiracetam Open studies of these two other anticonvulsants are near completion. Each has an interesting mechanistic profile, good tolerability, and the (potentially positive) side effect of inducing some weight loss, based on studies of neurologic patients.
Omega-3 fatty acids Based on positive reports of antidepressant effects of omega-3 fatty acids,28-30 we recently recruited more than 120 patients with bipolar disorder for two double-blind, randomized, controlled trials. Those patients with either an acute depression or a cyclic course will receive 6 grams of omega-3 fatty acid (the eicosapentaenoate form) or a placebo for 4 months of double-blind treatment, followed by an 8-month open, active continuation phase.
If results are positive, omega-3 fatty acids would represent the much-needed addition of a well-tolerated dietary agent to the therapeutic armamentarium directed at bipolar illness.
Conclusion
The Stanley Foundation Bipolar Network has begun to evaluate the usefulness of numerous older and new compounds for patients with bipolar disorder, including second-generation antidepressants, anticonvulsants, atypical antipsychotic agents, and omega-3 fatty acids. Based on initial observations from 16 treatment studies (Table 3), we have drawn these conclusions:
- Second-generation antidepressants such as bupropion, sertraline, and venlafaxine appear to have a lower-than-expected switch rate into mania, compared with the use of the older tricyclic antidepressants.
- If the addition of a second-generation antidepressant results in good antidepressant response for 2 months and no switches into mania, one should consider continuing the antidepressant in this small minority of patients, rather than stopping it as soon as possible as previously recommended.
- Adjunctive use of lamotrigine appears to be of value, particularly for acute depression and prophylaxis, for many patients who have not responded to other treatments.
- While our group and others have reported improvement with adjunctive gabapentin in bipolar patients in uncontrolled case series, its primary antimanic or mood-stabilizing utility (as well as that of tiagabine) does not appear promising.
- Topiramate’s ability to induce substantial weight loss may emerge as clinically therapeutic for patients with problematic weight gain related to other psychotropic drugs, independent of its ultimate utility as a possible mood stabilizer.
We are preparing to analyze other open case series assessing acute and long-term treatment outcomes as well as randomized, double-blind clinical trials. Even though the funding of this unique collaborative network is ending, we look forward to more effective treatment of bipolar disorder in the future with continued development of new psychotropic agents and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and the VA Cooperative studies.31
Related resources
- National Alliance for the Mentally Ill (NAMI). www.nami.org
- National Depressive and Manic-Depressive Association (NDMDA). www.ndmda.org
Drug brand names
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sibutramine • Meridia
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproate • Depakote
- Venlafaxine • Effexor
- Zonisamide • Zonegran
Acknowledgement
The authors are particularly indebted to the generosity of The Stanley Foundation in making funds available for this series of studies and several about to be completed.
Disclosure
Financial disclosure statements have been provided by all authors and are available upon request at current.psychiatry@dowdenhealth.com.
1. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press, 1990.
2. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
3. Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997a;58:470-8.
4. Greil W, Kleindienst N. The comparative prophylactic efficacy of lithium and carbamazepine in patients with bipolar I disorder. Int Clin Psychopharmacol 1999;14:277-81.
5. Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for one year with daily prospective ratings on the NIMH-LCM (submitted for publication, 2002).
6. Calabrese JR, Rapport DJ. Mood stabilizers and the evolution of maintenance study designs in bipolar I disorder. J Clin Psychiatry 1999;60(suppl 5):5-13.
7. McElroy SL, Frye M, Denicoff K, et al. Olanzapine in treatment-resistant bipolar disorder. J Affect Disord 1998;49:119-22.
8. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
9. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
10. Keck PE, Jr. New antiepileptics in the treatment of bipolar disorder. Syllabus and Proceedings Summary of the 2001 American Psychiatric Association Meeting, New Orleans, May 5-10, 2001. Abstract 34E, 93.
11. Post RM, Altshuler LL, Frye MA, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001;3:259-65.
12. Post RM, Leverich GS, Nolen WA, et al. A reevaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2002 (in press).
13. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-6.
14. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute remission from bipolar depression on rates of depressive relapse at one-year follow-up (submitted for publication 2002).
15. Suppes T, Brown ES, McElroy SL, et al. Lamotrigine for the treatment of bipolar disorder: a clinical case series. J Affective Disord 1999;53:95-8.
16. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60:79-88.
17. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14.
18. Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biolog Psychiatry 2002;51:253-60.
19. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biolog Psychiatry 2000;47:1025-33.
20. McIntyre RS, Mancini DA, McCann SM, et al. Topiramiate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 2002;4:207-13.
21. Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults. Epilepsia 2000;41(suppl 1):S66-S71.
22. Altshuler LL, Keck PE, Jr, McElroy SL, et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1999;1:61-5.
23. Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord 2000;2:249-55.
24. Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000;20:467-71.
25. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999;19:341-8.
26. Suppes T, Chisholm K, Dhvule D, et al. Tiagabine in bipolar disorder: adverse events in an open case series (submitted for publication, 2002).
27. Grunze H, Erfurth A, Marcuse A, et al. Tiagabine appears not to be efficacious in the treatment of acute mania. J Clin Psychiatry 1999;60:759-62.
28. Stoll AL, Severus WE, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56:407-12.
29. Horrobin DF, Peet M. A dose-ranging study of ethyl-eicosapentaenoate in treatment-unresponsive depression. Biolog Psychiatry 2001;49:37S.-
30. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-9.
31. Bauer MS, Williford WO, Dawson EE, et al. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: Reducing the efficacy-effectiveness gap in bipolar disorder. J Affect Disord 2001;67:61-78.
When choosing psychopharmacologic agents for patients with bipolar disorder, the clinician lacks guidance from controlled clinical trials at virtually every therapeutic step. To help remedy this problem, we in the Stanley Foundation Bipolar Network (SFBN) evaluated compounds with potentially important therapeutic profiles. We began by documenting the demographics and illness characteristics of patients with bipolar disorder. Then we assessed some 16 treatments in naturalistic or formal studies, some of which are complete and others ongoing.
In this second installment, we report our current information about the efficacy of second-generation antidepressants, atypical antipsychotics, and anticonvulsant agents.
Mood stabilizers
As mood stabilizers, the anticonvulsants carbamazepine and valproate are well-accepted alternatives or adjuncts to lithium carbonate, which remains a mainstay treatment for acute episodes and long-term prophylaxis.1
Valproate is approved by the FDA for treatment of acute mania but not for long-term prevention. Open studies in rapid-cycling, treatment-refractory patients demonstrate substantial improvement for both manic and depressive phases with valproate. In the most recent controlled study, valproate did not prevent manic episodes to a statistically significant degree, but it did show greater antidepressant effects than placebo or lithium.2
Carbamazepine has been widely studied in acute mania and compared with lithium for long-term prophylaxis. Most studies show nonsignificant differences between carbamazepine and lithium in preventing manic and depressive episodes, although several studies indicate less-robust antimanic effects of carbamazepine,3 particularly for patients with classic euphoric mania without psychosis, bipolar II, or associated substance abuse.4
In atypical patients, carbamazepine appeared to outperform lithium, suggesting clinical differences in these agents that have different mechanisms of action across neurotransmitter and peptide systems.
Low response rates Most randomized controlled trials and open adjunct studies suggest that 50% or more patients respond to lithium, valproate, or carbamazepine. Outcomes may be far less positive in clinical practice, however. Despite the use of mood stabilizers and a variety of antidepressants, benzodiazepines, and neuroleptics, our patients experienced substantial residual manic and depressive symptoms and functional impairment.
Similarly, clinician ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM) indicated a high rate of breakthrough episodes during carefully monitored and aggressive psychopharmacologic treatment.5 Two-thirds of the first 258 bipolar outpatients that we followed and rated daily for 1 year continued to be substantially impaired. One-quarter of them were ill for more than 75% of the year, despite using an average 4.1 psychopharmacologic agents per patient, including mood stabilizers, antidepressants, antipsychotics, and other agents.
Acute and long-term combination therapy appears more effective than monotherapy, particularly for treatment-refractory rapid cyclers.3 Even so, many patients do not respond to combination therapy. For example, in a 1-year prophylaxis study, 25% or fewer patients were rated “much improved” to “very much improved” on the Clinical Global Impressions (CGI) scale with lithium or carbamazepine as the baseline mood stabilizer and with antidepressants, neuroleptics, and benzodiazepines used as needed. In the third year of combination therapy, the overall response rate, including rapid cyclers, was roughly 50%.3
A low rate of response is apparent for lithium and valproate. While treating rapidly cycling patients, Calabrese and associates found that less than one-quarter of them responded well enough to the two-drug combination that they could be enrolled in a randomized double-blind comparison of each drug in monotherapy. Of those who were eligible, almost all relapsed with either lithium or valproate monotherapy and again required combination therapy.6
Table 1
RATE OF SWITCHING (%) INTO MANIA IN DEPRESSED BIPOLAR PATIENTS DURING ANTIDEPRESSANT TREATMENT*
| Acute treatment (10 wks) | Continuation treatment (52 wks)‡ | |||||
|---|---|---|---|---|---|---|
| Type of switch | Open (n=27) | Blind (n=100) | Total (n=127) | Open (n=12) | Blind (n=55) | Total (n=67) |
| Brief hypomania | 3.7% | 6% | 5.5% | – | 3.6% | 3% |
| Recurrent brief hypomania | 11 | 12 | 11.8 | 8.3% | 18.2 | 16.4 |
| Hypomania | 11 | 13 | 12.6 | 8.3 | 23.6 | 20.9 |
| Mania | 14.8 | 12 | 12.6 | 33.3 | 14.5 | 17.9 |
| Total | 25.8 | 25 | 25.2 | 41.6 | 38.1 | 38.8 |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | ||||||
| ‡ Most patients (80%) dropped out of this phase because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. | ||||||
| Source: Post RM et al. Bipolar Disord 2001;3:259-65, and unpublished data. | ||||||
Taken together, these studies indicate that even with our most effective and most studied agents, many patients respond inadequately. On the basis of this clinical reality, we began to explore additional options that might enhance clinical effectiveness.
Atypical antipsychotics
We assessed the addition of the atypical antipsychotic olanzapine in patients with treatment-refractory bipolar disorder7 and saw improvement in 57% of subjects with manic, mixed, and depressive components. These data foreshadowed more recent controlled findings of olanzapine’s usefulness in depression and mania.8,9 Our preliminary open clinical trial experience allowed for more controlled studies and subsequent approval and wider clinical use of olanzapine in bipolar illness.
Our initial assessment of other atypical antipsychotics found that quetiapine was associated with significantly fewer depressive symptoms on the Inventory of Depressive Symptomatology (IDS) when used as part of the regular treatment regimen. This improvement, which was seen in the first month, was maintained over 4 months of treatment, whereas no significant improvement in depression severity was seen with the use of risperidone or clozapine.10
It would be highly preliminary to conclude from these uncontrolled data that quetiapine had greater effects on bipolar depressive symptoms than did risperidone or clozapine. We cannot rule out the possibility that patients with different degrees of treatment resistance were enrolled and treated with the different concomitant drugs. However, patients did not differ at baseline in severity of their depression.
In this initial look at the atypicals, the severity of depressive symptoms on the IDS was much greater than that of mania, as measured by the Young Mania Rating Scale (YMRS). Thus, the precise patterns of illness being treated and the impact of these antipsychotics on depressive cyclicity and duration await closer examination. We intend to look at the relative efficacy and side-effect profiles of each of the atypical antipsychotics as used in clinical practice.
Second-generation antidepressants
Bupropion, sertraline, and venlafaxine have different mechanisms of action but appear to be of value as adjuncts to bipolar disorder treatment. When we used them as adjuncts to mood stabilizers in a prospective study, we found a 40 to 50% acute response rate and some switching into hypomania and mania (Table 1).11 Many switches appeared to involve only isolated brief bursts of hypomania or recurrent brief hypomanias, but more sustained episodes of hypomania (7 days) could be more clinically problematic. Only 13% of patients treated acutely with these antidepressants had more full-blown manic symptoms (i.e., those associated with moderate or greater dysfunction on the NIMH-LCM).
During the next year, when apparent acute antidepressant responders were offered continuation treatment, 18% switched into mania. However, most patients (about 80%) dropped out because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. The 127 patients who were randomized received 175 acute antidepressant trials. Only 9% were associated with a switch into mania with moderate dysfunction, whereas another 9% showed periods of hypomania lasting longer than 1 week.12
Table 2
RELAPSE INTO DEPRESSION IN BIPOLAR PATIENTS AFTER 1 YEAR WITH OR WITHOUT ADJUNCTIVE ANTIDEPRESSANT TREATMENT*
| Rate of relapse | |
|---|---|
| Continued on antidepressants | |
| Study #1 (n=19) | 35% |
| Study #2 (n=41) | 41% |
| Discontinued antidepressants | |
| Study #1 (n=25) | 68% |
| Study #2 (n=43) | 71% |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | |
| Study #1: Altshuler et al. J Clin Psychiatry 2001;62:612-6. | |
| Study #2: Altshuler et al. (submitted for publication 2002). | |
We have entered 176 patients into this double-blind, randomized comparison of bupropion, sertraline, and venlafaxine, with another 27 randomized openly. The study will attempt to examine any possible between-drug differences. Even without the blind being broken, it appears that these antidepressants are associated with a somewhat lower-than-expected rate of switching into mania with dysfunction, but a direct comparison with a placebo is not available.
In two other series, patients who remained well for 2 months on antidepressants plus mood stabilizers and then continued on their antidepressants had a much lower incidence of depressive relapses over the following year than those who discontinued their antidepressants (Table 2).13,14 Surprisingly, continuing the antidepressant was not associated with an increased rate of switching into mania.
Prospective randomized, controlled studies are required to confirm these findings. Even so, our studies appear to challenge conventional wisdom for the first time. Stopping antidepressant treatment in bipolar patients as soon as possible for fear of inducing a manic episode may not be the optimal recommendation in the admittedly small subgroup (about 15%) of patients who have responded well to an antidepressant for at least 2 months.
Table 3
SIX STUDIES COMPLETED BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number studied | Response rate |
|---|---|---|---|---|
| Antidepressants bupropion | Yes | Double-blind | 173 | ~ 47% |
| vs. sertraline vs. venlafaxine (Post et al, 2001) | Yes | Open | 27 | |
| Total = 200 | ||||
| Olanzapine (McElroy et al, 1998) | No | Open | 14 | ~ 57% |
| Lamotrigine (Suppes et al, 1999) | No | Open | 17 | ~ 65% |
| Gabapentin (Altshuler et al, 1999) | No | Open | 28 | 78% (14/18) for mania or hypomania 100% (n = 5) for depression 20% (1/5) for cycling |
| Topiramate (McElroy et al, 2000) | No | Open | 54 | 63% for mania or cycling 27% for acute depression (weight loss observed) |
| Tiagabine (Suppes et al, 2002) | No | Open | 17 | 18% (3/17) (3 possible seizures) |
Second-generation anticonvulsants
Based on our patients’ substantial residual symptoms, we explored a series of newly approved anticonvulsants for potential use as mood stabilizers.
Lamotrigine Consistent with other open and double-blind, placebo-controlled studies, lamotrigine showed promise as an adjunctive treatment, with improvement in depressive symptoms and cycling.15 One such study showed acute antidepressant effects of lamotrigine monotherapy compared with a placebo at dosages of 50 or 200 mg/d.16
Another double-blind, randomized, placebo-controlled NIMH study (partially funded by the Stanley Foundation) compared lamotrigine with gabapentin and a placebo in patients for whom lithium, carbamazepine, and valproate had failed.17 Most of these treatment-refractory patients received all three agents through three 6-week randomizations. Lamotrigine was much more effective in depression and overall illness than either a placebo or gabapentin. Men with bipolar compared with unipolar illness and patients with fewer unsuccessful clinical trials and hospitalizations for depression showed the greatest response.18
In two recent industry-sponsored controlled trials of long-term prophylaxis (following a recent manic or depressive episode), lamotrigine was significantly more effective than a placebo (Bowden et al and Calabrese et al, unpublished data). Lamotrigine was more effective than lithium in preventing depressive episodes, whereas lithium was more effective than lamotrigine for manic episodes, although lamotrigine was significantly better than the placebo.
These data suggest that lamotrigine may differ from valproate, carbamazepine, and lithium in being a more effective antidepressant than antimanic agent.
Topiramate Adjunctive use of topiramate in rapid-cycling and treatment-resistant patients has been examined in open studies, but controlled studies have not yet been published. In our open-label case series observations, 19 of 30 (63%) patients taking topiramate for mania or cycling were “much” or “very much” improved after 10 weeks, whereas only 3 of 11 (27%) patients taking topiramate for acute depression were so improved.19
Table 4
STUDIES IN PROGRESS BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number enrolled* | Notes |
|---|---|---|---|---|
| Topiramate vs. sibutramine | Yes | Open | 42 | Relative weight loss and mood stabilization |
| Omega-3 fatty acids (6 grams EPA vs. placebo) (for depression cycling) (Keck et al) | Yes Yes | Double-blind Double-blind | 62 60 | Data from both studies being analyzed |
| Acamprosate (for alcohol craving) (Grunze et al) | No | Open | 23 | Goal: 60 patients |
| Levetiracetam (Post et al) | No | Open | 13 | Goal: 30 patients |
| Zonisamide (McElroy et al) | No | Open | 45 | Goal: 60 patients European sites only |
| Tranylcypromine vs. lamotrigine (for refractory depression) (Nolen et al) | Yes | Open | 17 | Single-site study |
| Quetiapine (McElroy et al) | No | Open | 48 | Apparent superior antidepressant effects compared with risperidone or clozapine |
| Risperidone (Grunze et al) | No | Open | 37 | No significant effect on IDS‡ depression ratings |
| Clozapine (Suppes et al) | No | Open | 19 | No significant effect on IDS depression ratings |
| Modafinil vs. placebo (Frye et al) | Yes | Double-blind | Started 4/02 | |
| * Enrollment as of April 2002 n = 696 total patients in tables 3 and 4 | ||||
| ‡ IDS = Inventory of Depressive Symptomatology | ||||
An industry-sponsored double-blind, randomized trial of topiramate in acute mania looked promising in the first 67 patients, but not as encouraging in subsequent subjects. A positive drug effect re-emerged if one eliminated patients whose manic episodes were precipitated by antidepressants.
The acute antidepressant effects of topiramate did not appear promising in our study. On the other hand, a single-blind, randomized study of topiramate versus bupropion as adjunctive therapy for breakthrough bipolar depression suggested excellent acute response (approximately 56%) to both agents.20 These data leave the potential antidepressant effects of topiramate undetermined.
In patients being treated with topiramate, we found significant weight loss (average –0.7 kg at 4 weeks, –1.6 kg at 10 weeks, –4.7 kg at 6 months, and –6.2 kg at 1 year).19 Average weight loss was 4.5±6.7 kg at the final evaluation (body mass index [BMI] change of –6.3% and –5% at 1 year and last evaluation, respectively).
We and others have thus seen substantial weight loss in one-third to one-half of patients taking topiramate for bipolar disorder.21 This weight loss is maintained over relatively long periods, which differentiates topiramate from other weight-loss regimens. Topiramate’s ability to decrease weight—even while it is in the early stages of evaluation as a mood stabilizer—is of considerable interest, given the substantial problem of overweight in the bipolar population and the fact that many drug therapies used in the illness are associated with weight gain.
We are proceeding with a randomized open comparison of the relative weight loss and psychotropic efficacy of topiramate versus the antiobesity agent sibutramine. Sibutramine has been reported to have antidepressant properties and is FDA-approved for weight loss.
Gabapentin and tiagabine Our group and many others have reported substantial overall clinical improvement when gabapentin was used as adjunctive therapy in an open study of patients with inadequate response to previous treatments.22 As monotherapy, however, gabapentin was not more effective than a placebo17 and was less effective than lamotrigine. Similarly, gabapentin was not an effective acute antimanic agent when compared with a placebo,23 although other double-blind, controlled studies have indicated positive effects in anxiety disorders24 and social phobias.25 Thus, the gabapentin literature appears to be divergent—i.e., positive studies support its adjunctive use in bipolar patients, but controlled studies suggest that it is not directly antimanic or mood-stabilizing.
Gabapentin’s effect on syndromes that are highly comorbid with bipolar illness could account for its apparent success as open adjunctive therapy. For example, in addition to its antianxiety effects, gabapentin shows positive effects in alcohol withdrawal, pain syndromes, sleep disorders (including restless leg syndrome), and obsessive-compulsive disorder. Also, combining gabapentin with other psychotropics with other mechanisms of action may have a more robust therapeutic effect than monotherapy.
Our preliminary observations with the selective GABA-reuptake inhibitor tiagabine were not promising. In an open study, only 3 of the first 17 SFBN patients with treatment-resistant affective disorders showed a partial response (moderate improvement on the Clinical Global Impressions Scale for Bipolar Illness [CGI-BP]) to adjunctive tiagabine treatment when the dosage was gradually increased. Serious side effects, including two patients with possible seizures, were noted.26 Similar results were seen when tiagabine doses were rapidly increased in eight patients with acute mania; none responded, and one had a major motor seizure.27
Thus, our initial observations led us to discontinue use of tiagabine and suggest any further clinical consideration of this agent for treatment-resistant affective disorders would require great care. This perspective contrasts with several previous positive case reports. It is unknown whether selected patients or those treated with lower dosages would be more responsive to this agent.
Like valproate, gabapentin and tiagabine increase brain GABA, but their apparent lack of antimanic efficacy strongly suggests that raising brain GABA alone does little to counter mania. These data further support the viewpoint already offered that all anticonvulsants are not alike and that anticonvulsants as a class are not necessarily mood stabilizers.
Zonisamide and levetiracetam Open studies of these two other anticonvulsants are near completion. Each has an interesting mechanistic profile, good tolerability, and the (potentially positive) side effect of inducing some weight loss, based on studies of neurologic patients.
Omega-3 fatty acids Based on positive reports of antidepressant effects of omega-3 fatty acids,28-30 we recently recruited more than 120 patients with bipolar disorder for two double-blind, randomized, controlled trials. Those patients with either an acute depression or a cyclic course will receive 6 grams of omega-3 fatty acid (the eicosapentaenoate form) or a placebo for 4 months of double-blind treatment, followed by an 8-month open, active continuation phase.
If results are positive, omega-3 fatty acids would represent the much-needed addition of a well-tolerated dietary agent to the therapeutic armamentarium directed at bipolar illness.
Conclusion
The Stanley Foundation Bipolar Network has begun to evaluate the usefulness of numerous older and new compounds for patients with bipolar disorder, including second-generation antidepressants, anticonvulsants, atypical antipsychotic agents, and omega-3 fatty acids. Based on initial observations from 16 treatment studies (Table 3), we have drawn these conclusions:
- Second-generation antidepressants such as bupropion, sertraline, and venlafaxine appear to have a lower-than-expected switch rate into mania, compared with the use of the older tricyclic antidepressants.
- If the addition of a second-generation antidepressant results in good antidepressant response for 2 months and no switches into mania, one should consider continuing the antidepressant in this small minority of patients, rather than stopping it as soon as possible as previously recommended.
- Adjunctive use of lamotrigine appears to be of value, particularly for acute depression and prophylaxis, for many patients who have not responded to other treatments.
- While our group and others have reported improvement with adjunctive gabapentin in bipolar patients in uncontrolled case series, its primary antimanic or mood-stabilizing utility (as well as that of tiagabine) does not appear promising.
- Topiramate’s ability to induce substantial weight loss may emerge as clinically therapeutic for patients with problematic weight gain related to other psychotropic drugs, independent of its ultimate utility as a possible mood stabilizer.
We are preparing to analyze other open case series assessing acute and long-term treatment outcomes as well as randomized, double-blind clinical trials. Even though the funding of this unique collaborative network is ending, we look forward to more effective treatment of bipolar disorder in the future with continued development of new psychotropic agents and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and the VA Cooperative studies.31
Related resources
- National Alliance for the Mentally Ill (NAMI). www.nami.org
- National Depressive and Manic-Depressive Association (NDMDA). www.ndmda.org
Drug brand names
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sibutramine • Meridia
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproate • Depakote
- Venlafaxine • Effexor
- Zonisamide • Zonegran
Acknowledgement
The authors are particularly indebted to the generosity of The Stanley Foundation in making funds available for this series of studies and several about to be completed.
Disclosure
Financial disclosure statements have been provided by all authors and are available upon request at current.psychiatry@dowdenhealth.com.
When choosing psychopharmacologic agents for patients with bipolar disorder, the clinician lacks guidance from controlled clinical trials at virtually every therapeutic step. To help remedy this problem, we in the Stanley Foundation Bipolar Network (SFBN) evaluated compounds with potentially important therapeutic profiles. We began by documenting the demographics and illness characteristics of patients with bipolar disorder. Then we assessed some 16 treatments in naturalistic or formal studies, some of which are complete and others ongoing.
In this second installment, we report our current information about the efficacy of second-generation antidepressants, atypical antipsychotics, and anticonvulsant agents.
Mood stabilizers
As mood stabilizers, the anticonvulsants carbamazepine and valproate are well-accepted alternatives or adjuncts to lithium carbonate, which remains a mainstay treatment for acute episodes and long-term prophylaxis.1
Valproate is approved by the FDA for treatment of acute mania but not for long-term prevention. Open studies in rapid-cycling, treatment-refractory patients demonstrate substantial improvement for both manic and depressive phases with valproate. In the most recent controlled study, valproate did not prevent manic episodes to a statistically significant degree, but it did show greater antidepressant effects than placebo or lithium.2
Carbamazepine has been widely studied in acute mania and compared with lithium for long-term prophylaxis. Most studies show nonsignificant differences between carbamazepine and lithium in preventing manic and depressive episodes, although several studies indicate less-robust antimanic effects of carbamazepine,3 particularly for patients with classic euphoric mania without psychosis, bipolar II, or associated substance abuse.4
In atypical patients, carbamazepine appeared to outperform lithium, suggesting clinical differences in these agents that have different mechanisms of action across neurotransmitter and peptide systems.
Low response rates Most randomized controlled trials and open adjunct studies suggest that 50% or more patients respond to lithium, valproate, or carbamazepine. Outcomes may be far less positive in clinical practice, however. Despite the use of mood stabilizers and a variety of antidepressants, benzodiazepines, and neuroleptics, our patients experienced substantial residual manic and depressive symptoms and functional impairment.
Similarly, clinician ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM) indicated a high rate of breakthrough episodes during carefully monitored and aggressive psychopharmacologic treatment.5 Two-thirds of the first 258 bipolar outpatients that we followed and rated daily for 1 year continued to be substantially impaired. One-quarter of them were ill for more than 75% of the year, despite using an average 4.1 psychopharmacologic agents per patient, including mood stabilizers, antidepressants, antipsychotics, and other agents.
Acute and long-term combination therapy appears more effective than monotherapy, particularly for treatment-refractory rapid cyclers.3 Even so, many patients do not respond to combination therapy. For example, in a 1-year prophylaxis study, 25% or fewer patients were rated “much improved” to “very much improved” on the Clinical Global Impressions (CGI) scale with lithium or carbamazepine as the baseline mood stabilizer and with antidepressants, neuroleptics, and benzodiazepines used as needed. In the third year of combination therapy, the overall response rate, including rapid cyclers, was roughly 50%.3
A low rate of response is apparent for lithium and valproate. While treating rapidly cycling patients, Calabrese and associates found that less than one-quarter of them responded well enough to the two-drug combination that they could be enrolled in a randomized double-blind comparison of each drug in monotherapy. Of those who were eligible, almost all relapsed with either lithium or valproate monotherapy and again required combination therapy.6
Table 1
RATE OF SWITCHING (%) INTO MANIA IN DEPRESSED BIPOLAR PATIENTS DURING ANTIDEPRESSANT TREATMENT*
| Acute treatment (10 wks) | Continuation treatment (52 wks)‡ | |||||
|---|---|---|---|---|---|---|
| Type of switch | Open (n=27) | Blind (n=100) | Total (n=127) | Open (n=12) | Blind (n=55) | Total (n=67) |
| Brief hypomania | 3.7% | 6% | 5.5% | – | 3.6% | 3% |
| Recurrent brief hypomania | 11 | 12 | 11.8 | 8.3% | 18.2 | 16.4 |
| Hypomania | 11 | 13 | 12.6 | 8.3 | 23.6 | 20.9 |
| Mania | 14.8 | 12 | 12.6 | 33.3 | 14.5 | 17.9 |
| Total | 25.8 | 25 | 25.2 | 41.6 | 38.1 | 38.8 |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | ||||||
| ‡ Most patients (80%) dropped out of this phase because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. | ||||||
| Source: Post RM et al. Bipolar Disord 2001;3:259-65, and unpublished data. | ||||||
Taken together, these studies indicate that even with our most effective and most studied agents, many patients respond inadequately. On the basis of this clinical reality, we began to explore additional options that might enhance clinical effectiveness.
Atypical antipsychotics
We assessed the addition of the atypical antipsychotic olanzapine in patients with treatment-refractory bipolar disorder7 and saw improvement in 57% of subjects with manic, mixed, and depressive components. These data foreshadowed more recent controlled findings of olanzapine’s usefulness in depression and mania.8,9 Our preliminary open clinical trial experience allowed for more controlled studies and subsequent approval and wider clinical use of olanzapine in bipolar illness.
Our initial assessment of other atypical antipsychotics found that quetiapine was associated with significantly fewer depressive symptoms on the Inventory of Depressive Symptomatology (IDS) when used as part of the regular treatment regimen. This improvement, which was seen in the first month, was maintained over 4 months of treatment, whereas no significant improvement in depression severity was seen with the use of risperidone or clozapine.10
It would be highly preliminary to conclude from these uncontrolled data that quetiapine had greater effects on bipolar depressive symptoms than did risperidone or clozapine. We cannot rule out the possibility that patients with different degrees of treatment resistance were enrolled and treated with the different concomitant drugs. However, patients did not differ at baseline in severity of their depression.
In this initial look at the atypicals, the severity of depressive symptoms on the IDS was much greater than that of mania, as measured by the Young Mania Rating Scale (YMRS). Thus, the precise patterns of illness being treated and the impact of these antipsychotics on depressive cyclicity and duration await closer examination. We intend to look at the relative efficacy and side-effect profiles of each of the atypical antipsychotics as used in clinical practice.
Second-generation antidepressants
Bupropion, sertraline, and venlafaxine have different mechanisms of action but appear to be of value as adjuncts to bipolar disorder treatment. When we used them as adjuncts to mood stabilizers in a prospective study, we found a 40 to 50% acute response rate and some switching into hypomania and mania (Table 1).11 Many switches appeared to involve only isolated brief bursts of hypomania or recurrent brief hypomanias, but more sustained episodes of hypomania (7 days) could be more clinically problematic. Only 13% of patients treated acutely with these antidepressants had more full-blown manic symptoms (i.e., those associated with moderate or greater dysfunction on the NIMH-LCM).
During the next year, when apparent acute antidepressant responders were offered continuation treatment, 18% switched into mania. However, most patients (about 80%) dropped out because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. The 127 patients who were randomized received 175 acute antidepressant trials. Only 9% were associated with a switch into mania with moderate dysfunction, whereas another 9% showed periods of hypomania lasting longer than 1 week.12
Table 2
RELAPSE INTO DEPRESSION IN BIPOLAR PATIENTS AFTER 1 YEAR WITH OR WITHOUT ADJUNCTIVE ANTIDEPRESSANT TREATMENT*
| Rate of relapse | |
|---|---|
| Continued on antidepressants | |
| Study #1 (n=19) | 35% |
| Study #2 (n=41) | 41% |
| Discontinued antidepressants | |
| Study #1 (n=25) | 68% |
| Study #2 (n=43) | 71% |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | |
| Study #1: Altshuler et al. J Clin Psychiatry 2001;62:612-6. | |
| Study #2: Altshuler et al. (submitted for publication 2002). | |
We have entered 176 patients into this double-blind, randomized comparison of bupropion, sertraline, and venlafaxine, with another 27 randomized openly. The study will attempt to examine any possible between-drug differences. Even without the blind being broken, it appears that these antidepressants are associated with a somewhat lower-than-expected rate of switching into mania with dysfunction, but a direct comparison with a placebo is not available.
In two other series, patients who remained well for 2 months on antidepressants plus mood stabilizers and then continued on their antidepressants had a much lower incidence of depressive relapses over the following year than those who discontinued their antidepressants (Table 2).13,14 Surprisingly, continuing the antidepressant was not associated with an increased rate of switching into mania.
Prospective randomized, controlled studies are required to confirm these findings. Even so, our studies appear to challenge conventional wisdom for the first time. Stopping antidepressant treatment in bipolar patients as soon as possible for fear of inducing a manic episode may not be the optimal recommendation in the admittedly small subgroup (about 15%) of patients who have responded well to an antidepressant for at least 2 months.
Table 3
SIX STUDIES COMPLETED BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number studied | Response rate |
|---|---|---|---|---|
| Antidepressants bupropion | Yes | Double-blind | 173 | ~ 47% |
| vs. sertraline vs. venlafaxine (Post et al, 2001) | Yes | Open | 27 | |
| Total = 200 | ||||
| Olanzapine (McElroy et al, 1998) | No | Open | 14 | ~ 57% |
| Lamotrigine (Suppes et al, 1999) | No | Open | 17 | ~ 65% |
| Gabapentin (Altshuler et al, 1999) | No | Open | 28 | 78% (14/18) for mania or hypomania 100% (n = 5) for depression 20% (1/5) for cycling |
| Topiramate (McElroy et al, 2000) | No | Open | 54 | 63% for mania or cycling 27% for acute depression (weight loss observed) |
| Tiagabine (Suppes et al, 2002) | No | Open | 17 | 18% (3/17) (3 possible seizures) |
Second-generation anticonvulsants
Based on our patients’ substantial residual symptoms, we explored a series of newly approved anticonvulsants for potential use as mood stabilizers.
Lamotrigine Consistent with other open and double-blind, placebo-controlled studies, lamotrigine showed promise as an adjunctive treatment, with improvement in depressive symptoms and cycling.15 One such study showed acute antidepressant effects of lamotrigine monotherapy compared with a placebo at dosages of 50 or 200 mg/d.16
Another double-blind, randomized, placebo-controlled NIMH study (partially funded by the Stanley Foundation) compared lamotrigine with gabapentin and a placebo in patients for whom lithium, carbamazepine, and valproate had failed.17 Most of these treatment-refractory patients received all three agents through three 6-week randomizations. Lamotrigine was much more effective in depression and overall illness than either a placebo or gabapentin. Men with bipolar compared with unipolar illness and patients with fewer unsuccessful clinical trials and hospitalizations for depression showed the greatest response.18
In two recent industry-sponsored controlled trials of long-term prophylaxis (following a recent manic or depressive episode), lamotrigine was significantly more effective than a placebo (Bowden et al and Calabrese et al, unpublished data). Lamotrigine was more effective than lithium in preventing depressive episodes, whereas lithium was more effective than lamotrigine for manic episodes, although lamotrigine was significantly better than the placebo.
These data suggest that lamotrigine may differ from valproate, carbamazepine, and lithium in being a more effective antidepressant than antimanic agent.
Topiramate Adjunctive use of topiramate in rapid-cycling and treatment-resistant patients has been examined in open studies, but controlled studies have not yet been published. In our open-label case series observations, 19 of 30 (63%) patients taking topiramate for mania or cycling were “much” or “very much” improved after 10 weeks, whereas only 3 of 11 (27%) patients taking topiramate for acute depression were so improved.19
Table 4
STUDIES IN PROGRESS BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number enrolled* | Notes |
|---|---|---|---|---|
| Topiramate vs. sibutramine | Yes | Open | 42 | Relative weight loss and mood stabilization |
| Omega-3 fatty acids (6 grams EPA vs. placebo) (for depression cycling) (Keck et al) | Yes Yes | Double-blind Double-blind | 62 60 | Data from both studies being analyzed |
| Acamprosate (for alcohol craving) (Grunze et al) | No | Open | 23 | Goal: 60 patients |
| Levetiracetam (Post et al) | No | Open | 13 | Goal: 30 patients |
| Zonisamide (McElroy et al) | No | Open | 45 | Goal: 60 patients European sites only |
| Tranylcypromine vs. lamotrigine (for refractory depression) (Nolen et al) | Yes | Open | 17 | Single-site study |
| Quetiapine (McElroy et al) | No | Open | 48 | Apparent superior antidepressant effects compared with risperidone or clozapine |
| Risperidone (Grunze et al) | No | Open | 37 | No significant effect on IDS‡ depression ratings |
| Clozapine (Suppes et al) | No | Open | 19 | No significant effect on IDS depression ratings |
| Modafinil vs. placebo (Frye et al) | Yes | Double-blind | Started 4/02 | |
| * Enrollment as of April 2002 n = 696 total patients in tables 3 and 4 | ||||
| ‡ IDS = Inventory of Depressive Symptomatology | ||||
An industry-sponsored double-blind, randomized trial of topiramate in acute mania looked promising in the first 67 patients, but not as encouraging in subsequent subjects. A positive drug effect re-emerged if one eliminated patients whose manic episodes were precipitated by antidepressants.
The acute antidepressant effects of topiramate did not appear promising in our study. On the other hand, a single-blind, randomized study of topiramate versus bupropion as adjunctive therapy for breakthrough bipolar depression suggested excellent acute response (approximately 56%) to both agents.20 These data leave the potential antidepressant effects of topiramate undetermined.
In patients being treated with topiramate, we found significant weight loss (average –0.7 kg at 4 weeks, –1.6 kg at 10 weeks, –4.7 kg at 6 months, and –6.2 kg at 1 year).19 Average weight loss was 4.5±6.7 kg at the final evaluation (body mass index [BMI] change of –6.3% and –5% at 1 year and last evaluation, respectively).
We and others have thus seen substantial weight loss in one-third to one-half of patients taking topiramate for bipolar disorder.21 This weight loss is maintained over relatively long periods, which differentiates topiramate from other weight-loss regimens. Topiramate’s ability to decrease weight—even while it is in the early stages of evaluation as a mood stabilizer—is of considerable interest, given the substantial problem of overweight in the bipolar population and the fact that many drug therapies used in the illness are associated with weight gain.
We are proceeding with a randomized open comparison of the relative weight loss and psychotropic efficacy of topiramate versus the antiobesity agent sibutramine. Sibutramine has been reported to have antidepressant properties and is FDA-approved for weight loss.
Gabapentin and tiagabine Our group and many others have reported substantial overall clinical improvement when gabapentin was used as adjunctive therapy in an open study of patients with inadequate response to previous treatments.22 As monotherapy, however, gabapentin was not more effective than a placebo17 and was less effective than lamotrigine. Similarly, gabapentin was not an effective acute antimanic agent when compared with a placebo,23 although other double-blind, controlled studies have indicated positive effects in anxiety disorders24 and social phobias.25 Thus, the gabapentin literature appears to be divergent—i.e., positive studies support its adjunctive use in bipolar patients, but controlled studies suggest that it is not directly antimanic or mood-stabilizing.
Gabapentin’s effect on syndromes that are highly comorbid with bipolar illness could account for its apparent success as open adjunctive therapy. For example, in addition to its antianxiety effects, gabapentin shows positive effects in alcohol withdrawal, pain syndromes, sleep disorders (including restless leg syndrome), and obsessive-compulsive disorder. Also, combining gabapentin with other psychotropics with other mechanisms of action may have a more robust therapeutic effect than monotherapy.
Our preliminary observations with the selective GABA-reuptake inhibitor tiagabine were not promising. In an open study, only 3 of the first 17 SFBN patients with treatment-resistant affective disorders showed a partial response (moderate improvement on the Clinical Global Impressions Scale for Bipolar Illness [CGI-BP]) to adjunctive tiagabine treatment when the dosage was gradually increased. Serious side effects, including two patients with possible seizures, were noted.26 Similar results were seen when tiagabine doses were rapidly increased in eight patients with acute mania; none responded, and one had a major motor seizure.27
Thus, our initial observations led us to discontinue use of tiagabine and suggest any further clinical consideration of this agent for treatment-resistant affective disorders would require great care. This perspective contrasts with several previous positive case reports. It is unknown whether selected patients or those treated with lower dosages would be more responsive to this agent.
Like valproate, gabapentin and tiagabine increase brain GABA, but their apparent lack of antimanic efficacy strongly suggests that raising brain GABA alone does little to counter mania. These data further support the viewpoint already offered that all anticonvulsants are not alike and that anticonvulsants as a class are not necessarily mood stabilizers.
Zonisamide and levetiracetam Open studies of these two other anticonvulsants are near completion. Each has an interesting mechanistic profile, good tolerability, and the (potentially positive) side effect of inducing some weight loss, based on studies of neurologic patients.
Omega-3 fatty acids Based on positive reports of antidepressant effects of omega-3 fatty acids,28-30 we recently recruited more than 120 patients with bipolar disorder for two double-blind, randomized, controlled trials. Those patients with either an acute depression or a cyclic course will receive 6 grams of omega-3 fatty acid (the eicosapentaenoate form) or a placebo for 4 months of double-blind treatment, followed by an 8-month open, active continuation phase.
If results are positive, omega-3 fatty acids would represent the much-needed addition of a well-tolerated dietary agent to the therapeutic armamentarium directed at bipolar illness.
Conclusion
The Stanley Foundation Bipolar Network has begun to evaluate the usefulness of numerous older and new compounds for patients with bipolar disorder, including second-generation antidepressants, anticonvulsants, atypical antipsychotic agents, and omega-3 fatty acids. Based on initial observations from 16 treatment studies (Table 3), we have drawn these conclusions:
- Second-generation antidepressants such as bupropion, sertraline, and venlafaxine appear to have a lower-than-expected switch rate into mania, compared with the use of the older tricyclic antidepressants.
- If the addition of a second-generation antidepressant results in good antidepressant response for 2 months and no switches into mania, one should consider continuing the antidepressant in this small minority of patients, rather than stopping it as soon as possible as previously recommended.
- Adjunctive use of lamotrigine appears to be of value, particularly for acute depression and prophylaxis, for many patients who have not responded to other treatments.
- While our group and others have reported improvement with adjunctive gabapentin in bipolar patients in uncontrolled case series, its primary antimanic or mood-stabilizing utility (as well as that of tiagabine) does not appear promising.
- Topiramate’s ability to induce substantial weight loss may emerge as clinically therapeutic for patients with problematic weight gain related to other psychotropic drugs, independent of its ultimate utility as a possible mood stabilizer.
We are preparing to analyze other open case series assessing acute and long-term treatment outcomes as well as randomized, double-blind clinical trials. Even though the funding of this unique collaborative network is ending, we look forward to more effective treatment of bipolar disorder in the future with continued development of new psychotropic agents and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and the VA Cooperative studies.31
Related resources
- National Alliance for the Mentally Ill (NAMI). www.nami.org
- National Depressive and Manic-Depressive Association (NDMDA). www.ndmda.org
Drug brand names
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sibutramine • Meridia
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproate • Depakote
- Venlafaxine • Effexor
- Zonisamide • Zonegran
Acknowledgement
The authors are particularly indebted to the generosity of The Stanley Foundation in making funds available for this series of studies and several about to be completed.
Disclosure
Financial disclosure statements have been provided by all authors and are available upon request at current.psychiatry@dowdenhealth.com.
1. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press, 1990.
2. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
3. Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997a;58:470-8.
4. Greil W, Kleindienst N. The comparative prophylactic efficacy of lithium and carbamazepine in patients with bipolar I disorder. Int Clin Psychopharmacol 1999;14:277-81.
5. Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for one year with daily prospective ratings on the NIMH-LCM (submitted for publication, 2002).
6. Calabrese JR, Rapport DJ. Mood stabilizers and the evolution of maintenance study designs in bipolar I disorder. J Clin Psychiatry 1999;60(suppl 5):5-13.
7. McElroy SL, Frye M, Denicoff K, et al. Olanzapine in treatment-resistant bipolar disorder. J Affect Disord 1998;49:119-22.
8. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
9. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
10. Keck PE, Jr. New antiepileptics in the treatment of bipolar disorder. Syllabus and Proceedings Summary of the 2001 American Psychiatric Association Meeting, New Orleans, May 5-10, 2001. Abstract 34E, 93.
11. Post RM, Altshuler LL, Frye MA, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001;3:259-65.
12. Post RM, Leverich GS, Nolen WA, et al. A reevaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2002 (in press).
13. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-6.
14. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute remission from bipolar depression on rates of depressive relapse at one-year follow-up (submitted for publication 2002).
15. Suppes T, Brown ES, McElroy SL, et al. Lamotrigine for the treatment of bipolar disorder: a clinical case series. J Affective Disord 1999;53:95-8.
16. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60:79-88.
17. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14.
18. Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biolog Psychiatry 2002;51:253-60.
19. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biolog Psychiatry 2000;47:1025-33.
20. McIntyre RS, Mancini DA, McCann SM, et al. Topiramiate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 2002;4:207-13.
21. Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults. Epilepsia 2000;41(suppl 1):S66-S71.
22. Altshuler LL, Keck PE, Jr, McElroy SL, et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1999;1:61-5.
23. Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord 2000;2:249-55.
24. Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000;20:467-71.
25. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999;19:341-8.
26. Suppes T, Chisholm K, Dhvule D, et al. Tiagabine in bipolar disorder: adverse events in an open case series (submitted for publication, 2002).
27. Grunze H, Erfurth A, Marcuse A, et al. Tiagabine appears not to be efficacious in the treatment of acute mania. J Clin Psychiatry 1999;60:759-62.
28. Stoll AL, Severus WE, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56:407-12.
29. Horrobin DF, Peet M. A dose-ranging study of ethyl-eicosapentaenoate in treatment-unresponsive depression. Biolog Psychiatry 2001;49:37S.-
30. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-9.
31. Bauer MS, Williford WO, Dawson EE, et al. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: Reducing the efficacy-effectiveness gap in bipolar disorder. J Affect Disord 2001;67:61-78.
1. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press, 1990.
2. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
3. Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997a;58:470-8.
4. Greil W, Kleindienst N. The comparative prophylactic efficacy of lithium and carbamazepine in patients with bipolar I disorder. Int Clin Psychopharmacol 1999;14:277-81.
5. Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for one year with daily prospective ratings on the NIMH-LCM (submitted for publication, 2002).
6. Calabrese JR, Rapport DJ. Mood stabilizers and the evolution of maintenance study designs in bipolar I disorder. J Clin Psychiatry 1999;60(suppl 5):5-13.
7. McElroy SL, Frye M, Denicoff K, et al. Olanzapine in treatment-resistant bipolar disorder. J Affect Disord 1998;49:119-22.
8. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
9. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
10. Keck PE, Jr. New antiepileptics in the treatment of bipolar disorder. Syllabus and Proceedings Summary of the 2001 American Psychiatric Association Meeting, New Orleans, May 5-10, 2001. Abstract 34E, 93.
11. Post RM, Altshuler LL, Frye MA, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001;3:259-65.
12. Post RM, Leverich GS, Nolen WA, et al. A reevaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2002 (in press).
13. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-6.
14. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute remission from bipolar depression on rates of depressive relapse at one-year follow-up (submitted for publication 2002).
15. Suppes T, Brown ES, McElroy SL, et al. Lamotrigine for the treatment of bipolar disorder: a clinical case series. J Affective Disord 1999;53:95-8.
16. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60:79-88.
17. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14.
18. Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biolog Psychiatry 2002;51:253-60.
19. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biolog Psychiatry 2000;47:1025-33.
20. McIntyre RS, Mancini DA, McCann SM, et al. Topiramiate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 2002;4:207-13.
21. Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults. Epilepsia 2000;41(suppl 1):S66-S71.
22. Altshuler LL, Keck PE, Jr, McElroy SL, et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1999;1:61-5.
23. Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord 2000;2:249-55.
24. Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000;20:467-71.
25. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999;19:341-8.
26. Suppes T, Chisholm K, Dhvule D, et al. Tiagabine in bipolar disorder: adverse events in an open case series (submitted for publication, 2002).
27. Grunze H, Erfurth A, Marcuse A, et al. Tiagabine appears not to be efficacious in the treatment of acute mania. J Clin Psychiatry 1999;60:759-62.
28. Stoll AL, Severus WE, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56:407-12.
29. Horrobin DF, Peet M. A dose-ranging study of ethyl-eicosapentaenoate in treatment-unresponsive depression. Biolog Psychiatry 2001;49:37S.-
30. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-9.
31. Bauer MS, Williford WO, Dawson EE, et al. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: Reducing the efficacy-effectiveness gap in bipolar disorder. J Affect Disord 2001;67:61-78.
From the Stanley Foundation Bipolar Network: Efficacy of adjunctive therapies for treatment-resistant patients
When choosing psychopharmacologic agents for patients with bipolar disorder, the clinician lacks guidance from controlled clinical trials at virtually every therapeutic step. To help remedy this problem, we in the Stanley Foundation Bipolar Network (SFBN) evaluated compounds with potentially important therapeutic profiles. We began by documenting the demographics and illness characteristics of patients with bipolar disorder. Then we assessed some 16 treatments in naturalistic or formal studies, some of which are complete and others ongoing.
In this second installment, we report our current information about the efficacy of second-generation antidepressants, atypical antipsychotics, and anticonvulsant agents.
Mood stabilizers
As mood stabilizers, the anticonvulsants carbamazepine and valproate are well-accepted alternatives or adjuncts to lithium carbonate, which remains a mainstay treatment for acute episodes and long-term prophylaxis.1
Valproate is approved by the FDA for treatment of acute mania but not for long-term prevention. Open studies in rapid-cycling, treatment-refractory patients demonstrate substantial improvement for both manic and depressive phases with valproate. In the most recent controlled study, valproate did not prevent manic episodes to a statistically significant degree, but it did show greater antidepressant effects than placebo or lithium.2
Carbamazepine has been widely studied in acute mania and compared with lithium for long-term prophylaxis. Most studies show nonsignificant differences between carbamazepine and lithium in preventing manic and depressive episodes, although several studies indicate less-robust antimanic effects of carbamazepine,3 particularly for patients with classic euphoric mania without psychosis, bipolar II, or associated substance abuse.4
In atypical patients, carbamazepine appeared to outperform lithium, suggesting clinical differences in these agents that have different mechanisms of action across neurotransmitter and peptide systems.
Low response rates Most randomized controlled trials and open adjunct studies suggest that 50% or more patients respond to lithium, valproate, or carbamazepine. Outcomes may be far less positive in clinical practice, however. Despite the use of mood stabilizers and a variety of antidepressants, benzodiazepines, and neuroleptics, our patients experienced substantial residual manic and depressive symptoms and functional impairment.
Similarly, clinician ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM) indicated a high rate of breakthrough episodes during carefully monitored and aggressive psychopharmacologic treatment.5 Two-thirds of the first 258 bipolar outpatients that we followed and rated daily for 1 year continued to be substantially impaired. One-quarter of them were ill for more than 75% of the year, despite using an average 4.1 psychopharmacologic agents per patient, including mood stabilizers, antidepressants, antipsychotics, and other agents.
Acute and long-term combination therapy appears more effective than monotherapy, particularly for treatment-refractory rapid cyclers.3 Even so, many patients do not respond to combination therapy. For example, in a 1-year prophylaxis study, 25% or fewer patients were rated “much improved” to “very much improved” on the Clinical Global Impressions (CGI) scale with lithium or carbamazepine as the baseline mood stabilizer and with antidepressants, neuroleptics, and benzodiazepines used as needed. In the third year of combination therapy, the overall response rate, including rapid cyclers, was roughly 50%.3
A low rate of response is apparent for lithium and valproate. While treating rapidly cycling patients, Calabrese and associates found that less than one-quarter of them responded well enough to the two-drug combination that they could be enrolled in a randomized double-blind comparison of each drug in monotherapy. Of those who were eligible, almost all relapsed with either lithium or valproate monotherapy and again required combination therapy.6
Table 1
RATE OF SWITCHING (%) INTO MANIA IN DEPRESSED BIPOLAR PATIENTS DURING ANTIDEPRESSANT TREATMENT*
| Acute treatment (10 wks) | Continuation treatment (52 wks)‡ | |||||
|---|---|---|---|---|---|---|
| Type of switch | Open (n=27) | Blind (n=100) | Total (n=127) | Open (n=12) | Blind (n=55) | Total (n=67) |
| Brief hypomania | 3.7% | 6% | 5.5% | – | 3.6% | 3% |
| Recurrent brief hypomania | 11 | 12 | 11.8 | 8.3% | 18.2 | 16.4 |
| Hypomania | 11 | 13 | 12.6 | 8.3 | 23.6 | 20.9 |
| Mania | 14.8 | 12 | 12.6 | 33.3 | 14.5 | 17.9 |
| Total | 25.8 | 25 | 25.2 | 41.6 | 38.1 | 38.8 |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | ||||||
| ‡ Most patients (80%) dropped out of this phase because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. | ||||||
| Source: Post RM et al. Bipolar Disord 2001;3:259-65, and unpublished data. | ||||||
Taken together, these studies indicate that even with our most effective and most studied agents, many patients respond inadequately. On the basis of this clinical reality, we began to explore additional options that might enhance clinical effectiveness.
Atypical antipsychotics
We assessed the addition of the atypical antipsychotic olanzapine in patients with treatment-refractory bipolar disorder7 and saw improvement in 57% of subjects with manic, mixed, and depressive components. These data foreshadowed more recent controlled findings of olanzapine’s usefulness in depression and mania.8,9 Our preliminary open clinical trial experience allowed for more controlled studies and subsequent approval and wider clinical use of olanzapine in bipolar illness.
Our initial assessment of other atypical antipsychotics found that quetiapine was associated with significantly fewer depressive symptoms on the Inventory of Depressive Symptomatology (IDS) when used as part of the regular treatment regimen. This improvement, which was seen in the first month, was maintained over 4 months of treatment, whereas no significant improvement in depression severity was seen with the use of risperidone or clozapine.10
It would be highly preliminary to conclude from these uncontrolled data that quetiapine had greater effects on bipolar depressive symptoms than did risperidone or clozapine. We cannot rule out the possibility that patients with different degrees of treatment resistance were enrolled and treated with the different concomitant drugs. However, patients did not differ at baseline in severity of their depression.
In this initial look at the atypicals, the severity of depressive symptoms on the IDS was much greater than that of mania, as measured by the Young Mania Rating Scale (YMRS). Thus, the precise patterns of illness being treated and the impact of these antipsychotics on depressive cyclicity and duration await closer examination. We intend to look at the relative efficacy and side-effect profiles of each of the atypical antipsychotics as used in clinical practice.
Second-generation antidepressants
Bupropion, sertraline, and venlafaxine have different mechanisms of action but appear to be of value as adjuncts to bipolar disorder treatment. When we used them as adjuncts to mood stabilizers in a prospective study, we found a 40 to 50% acute response rate and some switching into hypomania and mania (Table 1).11 Many switches appeared to involve only isolated brief bursts of hypomania or recurrent brief hypomanias, but more sustained episodes of hypomania (7 days) could be more clinically problematic. Only 13% of patients treated acutely with these antidepressants had more full-blown manic symptoms (i.e., those associated with moderate or greater dysfunction on the NIMH-LCM).
During the next year, when apparent acute antidepressant responders were offered continuation treatment, 18% switched into mania. However, most patients (about 80%) dropped out because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. The 127 patients who were randomized received 175 acute antidepressant trials. Only 9% were associated with a switch into mania with moderate dysfunction, whereas another 9% showed periods of hypomania lasting longer than 1 week.12
Table 2
RELAPSE INTO DEPRESSION IN BIPOLAR PATIENTS AFTER 1 YEAR WITH OR WITHOUT ADJUNCTIVE ANTIDEPRESSANT TREATMENT*
| Rate of relapse | |
|---|---|
| Continued on antidepressants | |
| Study #1 (n=19) | 35% |
| Study #2 (n=41) | 41% |
| Discontinued antidepressants | |
| Study #1 (n=25) | 68% |
| Study #2 (n=43) | 71% |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | |
| Study #1: Altshuler et al. J Clin Psychiatry 2001;62:612-6. | |
| Study #2: Altshuler et al. (submitted for publication 2002). | |
We have entered 176 patients into this double-blind, randomized comparison of bupropion, sertraline, and venlafaxine, with another 27 randomized openly. The study will attempt to examine any possible between-drug differences. Even without the blind being broken, it appears that these antidepressants are associated with a somewhat lower-than-expected rate of switching into mania with dysfunction, but a direct comparison with a placebo is not available.
In two other series, patients who remained well for 2 months on antidepressants plus mood stabilizers and then continued on their antidepressants had a much lower incidence of depressive relapses over the following year than those who discontinued their antidepressants (Table 2).13,14 Surprisingly, continuing the antidepressant was not associated with an increased rate of switching into mania.
Prospective randomized, controlled studies are required to confirm these findings. Even so, our studies appear to challenge conventional wisdom for the first time. Stopping antidepressant treatment in bipolar patients as soon as possible for fear of inducing a manic episode may not be the optimal recommendation in the admittedly small subgroup (about 15%) of patients who have responded well to an antidepressant for at least 2 months.
Table 3
SIX STUDIES COMPLETED BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number studied | Response rate |
|---|---|---|---|---|
| Antidepressants bupropion | Yes | Double-blind | 173 | ~ 47% |
| vs. sertraline vs. venlafaxine (Post et al, 2001) | Yes | Open | 27 | |
| Total = 200 | ||||
| Olanzapine (McElroy et al, 1998) | No | Open | 14 | ~ 57% |
| Lamotrigine (Suppes et al, 1999) | No | Open | 17 | ~ 65% |
| Gabapentin (Altshuler et al, 1999) | No | Open | 28 | 78% (14/18) for mania or hypomania 100% (n = 5) for depression 20% (1/5) for cycling |
| Topiramate (McElroy et al, 2000) | No | Open | 54 | 63% for mania or cycling 27% for acute depression (weight loss observed) |
| Tiagabine (Suppes et al, 2002) | No | Open | 17 | 18% (3/17) (3 possible seizures) |
Second-generation anticonvulsants
Based on our patients’ substantial residual symptoms, we explored a series of newly approved anticonvulsants for potential use as mood stabilizers.
Lamotrigine Consistent with other open and double-blind, placebo-controlled studies, lamotrigine showed promise as an adjunctive treatment, with improvement in depressive symptoms and cycling.15 One such study showed acute antidepressant effects of lamotrigine monotherapy compared with a placebo at dosages of 50 or 200 mg/d.16
Another double-blind, randomized, placebo-controlled NIMH study (partially funded by the Stanley Foundation) compared lamotrigine with gabapentin and a placebo in patients for whom lithium, carbamazepine, and valproate had failed.17 Most of these treatment-refractory patients received all three agents through three 6-week randomizations. Lamotrigine was much more effective in depression and overall illness than either a placebo or gabapentin. Men with bipolar compared with unipolar illness and patients with fewer unsuccessful clinical trials and hospitalizations for depression showed the greatest response.18
In two recent industry-sponsored controlled trials of long-term prophylaxis (following a recent manic or depressive episode), lamotrigine was significantly more effective than a placebo (Bowden et al and Calabrese et al, unpublished data). Lamotrigine was more effective than lithium in preventing depressive episodes, whereas lithium was more effective than lamotrigine for manic episodes, although lamotrigine was significantly better than the placebo.
These data suggest that lamotrigine may differ from valproate, carbamazepine, and lithium in being a more effective antidepressant than antimanic agent.
Topiramate Adjunctive use of topiramate in rapid-cycling and treatment-resistant patients has been examined in open studies, but controlled studies have not yet been published. In our open-label case series observations, 19 of 30 (63%) patients taking topiramate for mania or cycling were “much” or “very much” improved after 10 weeks, whereas only 3 of 11 (27%) patients taking topiramate for acute depression were so improved.19
Table 4
STUDIES IN PROGRESS BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number enrolled* | Notes |
|---|---|---|---|---|
| Topiramate vs. sibutramine | Yes | Open | 42 | Relative weight loss and mood stabilization |
| Omega-3 fatty acids (6 grams EPA vs. placebo) (for depression cycling) (Keck et al) | Yes Yes | Double-blind Double-blind | 62 60 | Data from both studies being analyzed |
| Acamprosate (for alcohol craving) (Grunze et al) | No | Open | 23 | Goal: 60 patients |
| Levetiracetam (Post et al) | No | Open | 13 | Goal: 30 patients |
| Zonisamide (McElroy et al) | No | Open | 45 | Goal: 60 patients European sites only |
| Tranylcypromine vs. lamotrigine (for refractory depression) (Nolen et al) | Yes | Open | 17 | Single-site study |
| Quetiapine (McElroy et al) | No | Open | 48 | Apparent superior antidepressant effects compared with risperidone or clozapine |
| Risperidone (Grunze et al) | No | Open | 37 | No significant effect on IDS‡ depression ratings |
| Clozapine (Suppes et al) | No | Open | 19 | No significant effect on IDS depression ratings |
| Modafinil vs. placebo (Frye et al) | Yes | Double-blind | Started 4/02 | |
| * Enrollment as of April 2002 n = 696 total patients in tables 3 and 4 | ||||
| ‡ IDS = Inventory of Depressive Symptomatology | ||||
An industry-sponsored double-blind, randomized trial of topiramate in acute mania looked promising in the first 67 patients, but not as encouraging in subsequent subjects. A positive drug effect re-emerged if one eliminated patients whose manic episodes were precipitated by antidepressants.
The acute antidepressant effects of topiramate did not appear promising in our study. On the other hand, a single-blind, randomized study of topiramate versus bupropion as adjunctive therapy for breakthrough bipolar depression suggested excellent acute response (approximately 56%) to both agents.20 These data leave the potential antidepressant effects of topiramate undetermined.
In patients being treated with topiramate, we found significant weight loss (average –0.7 kg at 4 weeks, –1.6 kg at 10 weeks, –4.7 kg at 6 months, and –6.2 kg at 1 year).19 Average weight loss was 4.5±6.7 kg at the final evaluation (body mass index [BMI] change of –6.3% and –5% at 1 year and last evaluation, respectively).
We and others have thus seen substantial weight loss in one-third to one-half of patients taking topiramate for bipolar disorder.21 This weight loss is maintained over relatively long periods, which differentiates topiramate from other weight-loss regimens. Topiramate’s ability to decrease weight—even while it is in the early stages of evaluation as a mood stabilizer—is of considerable interest, given the substantial problem of overweight in the bipolar population and the fact that many drug therapies used in the illness are associated with weight gain.
We are proceeding with a randomized open comparison of the relative weight loss and psychotropic efficacy of topiramate versus the antiobesity agent sibutramine. Sibutramine has been reported to have antidepressant properties and is FDA-approved for weight loss.
Gabapentin and tiagabine Our group and many others have reported substantial overall clinical improvement when gabapentin was used as adjunctive therapy in an open study of patients with inadequate response to previous treatments.22 As monotherapy, however, gabapentin was not more effective than a placebo17 and was less effective than lamotrigine. Similarly, gabapentin was not an effective acute antimanic agent when compared with a placebo,23 although other double-blind, controlled studies have indicated positive effects in anxiety disorders24 and social phobias.25 Thus, the gabapentin literature appears to be divergent—i.e., positive studies support its adjunctive use in bipolar patients, but controlled studies suggest that it is not directly antimanic or mood-stabilizing.
Gabapentin’s effect on syndromes that are highly comorbid with bipolar illness could account for its apparent success as open adjunctive therapy. For example, in addition to its antianxiety effects, gabapentin shows positive effects in alcohol withdrawal, pain syndromes, sleep disorders (including restless leg syndrome), and obsessive-compulsive disorder. Also, combining gabapentin with other psychotropics with other mechanisms of action may have a more robust therapeutic effect than monotherapy.
Our preliminary observations with the selective GABA-reuptake inhibitor tiagabine were not promising. In an open study, only 3 of the first 17 SFBN patients with treatment-resistant affective disorders showed a partial response (moderate improvement on the Clinical Global Impressions Scale for Bipolar Illness [CGI-BP]) to adjunctive tiagabine treatment when the dosage was gradually increased. Serious side effects, including two patients with possible seizures, were noted.26 Similar results were seen when tiagabine doses were rapidly increased in eight patients with acute mania; none responded, and one had a major motor seizure.27
Thus, our initial observations led us to discontinue use of tiagabine and suggest any further clinical consideration of this agent for treatment-resistant affective disorders would require great care. This perspective contrasts with several previous positive case reports. It is unknown whether selected patients or those treated with lower dosages would be more responsive to this agent.
Like valproate, gabapentin and tiagabine increase brain GABA, but their apparent lack of antimanic efficacy strongly suggests that raising brain GABA alone does little to counter mania. These data further support the viewpoint already offered that all anticonvulsants are not alike and that anticonvulsants as a class are not necessarily mood stabilizers.
Zonisamide and levetiracetam Open studies of these two other anticonvulsants are near completion. Each has an interesting mechanistic profile, good tolerability, and the (potentially positive) side effect of inducing some weight loss, based on studies of neurologic patients.
Omega-3 fatty acids Based on positive reports of antidepressant effects of omega-3 fatty acids,28-30 we recently recruited more than 120 patients with bipolar disorder for two double-blind, randomized, controlled trials. Those patients with either an acute depression or a cyclic course will receive 6 grams of omega-3 fatty acid (the eicosapentaenoate form) or a placebo for 4 months of double-blind treatment, followed by an 8-month open, active continuation phase.
If results are positive, omega-3 fatty acids would represent the much-needed addition of a well-tolerated dietary agent to the therapeutic armamentarium directed at bipolar illness.
Conclusion
The Stanley Foundation Bipolar Network has begun to evaluate the usefulness of numerous older and new compounds for patients with bipolar disorder, including second-generation antidepressants, anticonvulsants, atypical antipsychotic agents, and omega-3 fatty acids. Based on initial observations from 16 treatment studies (Table 3), we have drawn these conclusions:
- Second-generation antidepressants such as bupropion, sertraline, and venlafaxine appear to have a lower-than-expected switch rate into mania, compared with the use of the older tricyclic antidepressants.
- If the addition of a second-generation antidepressant results in good antidepressant response for 2 months and no switches into mania, one should consider continuing the antidepressant in this small minority of patients, rather than stopping it as soon as possible as previously recommended.
- Adjunctive use of lamotrigine appears to be of value, particularly for acute depression and prophylaxis, for many patients who have not responded to other treatments.
- While our group and others have reported improvement with adjunctive gabapentin in bipolar patients in uncontrolled case series, its primary antimanic or mood-stabilizing utility (as well as that of tiagabine) does not appear promising.
- Topiramate’s ability to induce substantial weight loss may emerge as clinically therapeutic for patients with problematic weight gain related to other psychotropic drugs, independent of its ultimate utility as a possible mood stabilizer.
We are preparing to analyze other open case series assessing acute and long-term treatment outcomes as well as randomized, double-blind clinical trials. Even though the funding of this unique collaborative network is ending, we look forward to more effective treatment of bipolar disorder in the future with continued development of new psychotropic agents and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and the VA Cooperative studies.31
Related resources
- National Alliance for the Mentally Ill (NAMI). www.nami.org
- National Depressive and Manic-Depressive Association (NDMDA). www.ndmda.org
Drug brand names
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sibutramine • Meridia
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproate • Depakote
- Venlafaxine • Effexor
- Zonisamide • Zonegran
Acknowledgement
The authors are particularly indebted to the generosity of The Stanley Foundation in making funds available for this series of studies and several about to be completed.
Disclosure
Financial disclosure statements have been provided by all authors and are available upon request at current.psychiatry@dowdenhealth.com.
1. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press, 1990.
2. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
3. Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997a;58:470-8.
4. Greil W, Kleindienst N. The comparative prophylactic efficacy of lithium and carbamazepine in patients with bipolar I disorder. Int Clin Psychopharmacol 1999;14:277-81.
5. Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for one year with daily prospective ratings on the NIMH-LCM (submitted for publication, 2002).
6. Calabrese JR, Rapport DJ. Mood stabilizers and the evolution of maintenance study designs in bipolar I disorder. J Clin Psychiatry 1999;60(suppl 5):5-13.
7. McElroy SL, Frye M, Denicoff K, et al. Olanzapine in treatment-resistant bipolar disorder. J Affect Disord 1998;49:119-22.
8. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
9. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
10. Keck PE, Jr. New antiepileptics in the treatment of bipolar disorder. Syllabus and Proceedings Summary of the 2001 American Psychiatric Association Meeting, New Orleans, May 5-10, 2001. Abstract 34E, 93.
11. Post RM, Altshuler LL, Frye MA, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001;3:259-65.
12. Post RM, Leverich GS, Nolen WA, et al. A reevaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2002 (in press).
13. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-6.
14. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute remission from bipolar depression on rates of depressive relapse at one-year follow-up (submitted for publication 2002).
15. Suppes T, Brown ES, McElroy SL, et al. Lamotrigine for the treatment of bipolar disorder: a clinical case series. J Affective Disord 1999;53:95-8.
16. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60:79-88.
17. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14.
18. Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biolog Psychiatry 2002;51:253-60.
19. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biolog Psychiatry 2000;47:1025-33.
20. McIntyre RS, Mancini DA, McCann SM, et al. Topiramiate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 2002;4:207-13.
21. Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults. Epilepsia 2000;41(suppl 1):S66-S71.
22. Altshuler LL, Keck PE, Jr, McElroy SL, et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1999;1:61-5.
23. Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord 2000;2:249-55.
24. Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000;20:467-71.
25. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999;19:341-8.
26. Suppes T, Chisholm K, Dhvule D, et al. Tiagabine in bipolar disorder: adverse events in an open case series (submitted for publication, 2002).
27. Grunze H, Erfurth A, Marcuse A, et al. Tiagabine appears not to be efficacious in the treatment of acute mania. J Clin Psychiatry 1999;60:759-62.
28. Stoll AL, Severus WE, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56:407-12.
29. Horrobin DF, Peet M. A dose-ranging study of ethyl-eicosapentaenoate in treatment-unresponsive depression. Biolog Psychiatry 2001;49:37S.-
30. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-9.
31. Bauer MS, Williford WO, Dawson EE, et al. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: Reducing the efficacy-effectiveness gap in bipolar disorder. J Affect Disord 2001;67:61-78.
When choosing psychopharmacologic agents for patients with bipolar disorder, the clinician lacks guidance from controlled clinical trials at virtually every therapeutic step. To help remedy this problem, we in the Stanley Foundation Bipolar Network (SFBN) evaluated compounds with potentially important therapeutic profiles. We began by documenting the demographics and illness characteristics of patients with bipolar disorder. Then we assessed some 16 treatments in naturalistic or formal studies, some of which are complete and others ongoing.
In this second installment, we report our current information about the efficacy of second-generation antidepressants, atypical antipsychotics, and anticonvulsant agents.
Mood stabilizers
As mood stabilizers, the anticonvulsants carbamazepine and valproate are well-accepted alternatives or adjuncts to lithium carbonate, which remains a mainstay treatment for acute episodes and long-term prophylaxis.1
Valproate is approved by the FDA for treatment of acute mania but not for long-term prevention. Open studies in rapid-cycling, treatment-refractory patients demonstrate substantial improvement for both manic and depressive phases with valproate. In the most recent controlled study, valproate did not prevent manic episodes to a statistically significant degree, but it did show greater antidepressant effects than placebo or lithium.2
Carbamazepine has been widely studied in acute mania and compared with lithium for long-term prophylaxis. Most studies show nonsignificant differences between carbamazepine and lithium in preventing manic and depressive episodes, although several studies indicate less-robust antimanic effects of carbamazepine,3 particularly for patients with classic euphoric mania without psychosis, bipolar II, or associated substance abuse.4
In atypical patients, carbamazepine appeared to outperform lithium, suggesting clinical differences in these agents that have different mechanisms of action across neurotransmitter and peptide systems.
Low response rates Most randomized controlled trials and open adjunct studies suggest that 50% or more patients respond to lithium, valproate, or carbamazepine. Outcomes may be far less positive in clinical practice, however. Despite the use of mood stabilizers and a variety of antidepressants, benzodiazepines, and neuroleptics, our patients experienced substantial residual manic and depressive symptoms and functional impairment.
Similarly, clinician ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM) indicated a high rate of breakthrough episodes during carefully monitored and aggressive psychopharmacologic treatment.5 Two-thirds of the first 258 bipolar outpatients that we followed and rated daily for 1 year continued to be substantially impaired. One-quarter of them were ill for more than 75% of the year, despite using an average 4.1 psychopharmacologic agents per patient, including mood stabilizers, antidepressants, antipsychotics, and other agents.
Acute and long-term combination therapy appears more effective than monotherapy, particularly for treatment-refractory rapid cyclers.3 Even so, many patients do not respond to combination therapy. For example, in a 1-year prophylaxis study, 25% or fewer patients were rated “much improved” to “very much improved” on the Clinical Global Impressions (CGI) scale with lithium or carbamazepine as the baseline mood stabilizer and with antidepressants, neuroleptics, and benzodiazepines used as needed. In the third year of combination therapy, the overall response rate, including rapid cyclers, was roughly 50%.3
A low rate of response is apparent for lithium and valproate. While treating rapidly cycling patients, Calabrese and associates found that less than one-quarter of them responded well enough to the two-drug combination that they could be enrolled in a randomized double-blind comparison of each drug in monotherapy. Of those who were eligible, almost all relapsed with either lithium or valproate monotherapy and again required combination therapy.6
Table 1
RATE OF SWITCHING (%) INTO MANIA IN DEPRESSED BIPOLAR PATIENTS DURING ANTIDEPRESSANT TREATMENT*
| Acute treatment (10 wks) | Continuation treatment (52 wks)‡ | |||||
|---|---|---|---|---|---|---|
| Type of switch | Open (n=27) | Blind (n=100) | Total (n=127) | Open (n=12) | Blind (n=55) | Total (n=67) |
| Brief hypomania | 3.7% | 6% | 5.5% | – | 3.6% | 3% |
| Recurrent brief hypomania | 11 | 12 | 11.8 | 8.3% | 18.2 | 16.4 |
| Hypomania | 11 | 13 | 12.6 | 8.3 | 23.6 | 20.9 |
| Mania | 14.8 | 12 | 12.6 | 33.3 | 14.5 | 17.9 |
| Total | 25.8 | 25 | 25.2 | 41.6 | 38.1 | 38.8 |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | ||||||
| ‡ Most patients (80%) dropped out of this phase because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. | ||||||
| Source: Post RM et al. Bipolar Disord 2001;3:259-65, and unpublished data. | ||||||
Taken together, these studies indicate that even with our most effective and most studied agents, many patients respond inadequately. On the basis of this clinical reality, we began to explore additional options that might enhance clinical effectiveness.
Atypical antipsychotics
We assessed the addition of the atypical antipsychotic olanzapine in patients with treatment-refractory bipolar disorder7 and saw improvement in 57% of subjects with manic, mixed, and depressive components. These data foreshadowed more recent controlled findings of olanzapine’s usefulness in depression and mania.8,9 Our preliminary open clinical trial experience allowed for more controlled studies and subsequent approval and wider clinical use of olanzapine in bipolar illness.
Our initial assessment of other atypical antipsychotics found that quetiapine was associated with significantly fewer depressive symptoms on the Inventory of Depressive Symptomatology (IDS) when used as part of the regular treatment regimen. This improvement, which was seen in the first month, was maintained over 4 months of treatment, whereas no significant improvement in depression severity was seen with the use of risperidone or clozapine.10
It would be highly preliminary to conclude from these uncontrolled data that quetiapine had greater effects on bipolar depressive symptoms than did risperidone or clozapine. We cannot rule out the possibility that patients with different degrees of treatment resistance were enrolled and treated with the different concomitant drugs. However, patients did not differ at baseline in severity of their depression.
In this initial look at the atypicals, the severity of depressive symptoms on the IDS was much greater than that of mania, as measured by the Young Mania Rating Scale (YMRS). Thus, the precise patterns of illness being treated and the impact of these antipsychotics on depressive cyclicity and duration await closer examination. We intend to look at the relative efficacy and side-effect profiles of each of the atypical antipsychotics as used in clinical practice.
Second-generation antidepressants
Bupropion, sertraline, and venlafaxine have different mechanisms of action but appear to be of value as adjuncts to bipolar disorder treatment. When we used them as adjuncts to mood stabilizers in a prospective study, we found a 40 to 50% acute response rate and some switching into hypomania and mania (Table 1).11 Many switches appeared to involve only isolated brief bursts of hypomania or recurrent brief hypomanias, but more sustained episodes of hypomania (7 days) could be more clinically problematic. Only 13% of patients treated acutely with these antidepressants had more full-blown manic symptoms (i.e., those associated with moderate or greater dysfunction on the NIMH-LCM).
During the next year, when apparent acute antidepressant responders were offered continuation treatment, 18% switched into mania. However, most patients (about 80%) dropped out because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. The 127 patients who were randomized received 175 acute antidepressant trials. Only 9% were associated with a switch into mania with moderate dysfunction, whereas another 9% showed periods of hypomania lasting longer than 1 week.12
Table 2
RELAPSE INTO DEPRESSION IN BIPOLAR PATIENTS AFTER 1 YEAR WITH OR WITHOUT ADJUNCTIVE ANTIDEPRESSANT TREATMENT*
| Rate of relapse | |
|---|---|
| Continued on antidepressants | |
| Study #1 (n=19) | 35% |
| Study #2 (n=41) | 41% |
| Discontinued antidepressants | |
| Study #1 (n=25) | 68% |
| Study #2 (n=43) | 71% |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | |
| Study #1: Altshuler et al. J Clin Psychiatry 2001;62:612-6. | |
| Study #2: Altshuler et al. (submitted for publication 2002). | |
We have entered 176 patients into this double-blind, randomized comparison of bupropion, sertraline, and venlafaxine, with another 27 randomized openly. The study will attempt to examine any possible between-drug differences. Even without the blind being broken, it appears that these antidepressants are associated with a somewhat lower-than-expected rate of switching into mania with dysfunction, but a direct comparison with a placebo is not available.
In two other series, patients who remained well for 2 months on antidepressants plus mood stabilizers and then continued on their antidepressants had a much lower incidence of depressive relapses over the following year than those who discontinued their antidepressants (Table 2).13,14 Surprisingly, continuing the antidepressant was not associated with an increased rate of switching into mania.
Prospective randomized, controlled studies are required to confirm these findings. Even so, our studies appear to challenge conventional wisdom for the first time. Stopping antidepressant treatment in bipolar patients as soon as possible for fear of inducing a manic episode may not be the optimal recommendation in the admittedly small subgroup (about 15%) of patients who have responded well to an antidepressant for at least 2 months.
Table 3
SIX STUDIES COMPLETED BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number studied | Response rate |
|---|---|---|---|---|
| Antidepressants bupropion | Yes | Double-blind | 173 | ~ 47% |
| vs. sertraline vs. venlafaxine (Post et al, 2001) | Yes | Open | 27 | |
| Total = 200 | ||||
| Olanzapine (McElroy et al, 1998) | No | Open | 14 | ~ 57% |
| Lamotrigine (Suppes et al, 1999) | No | Open | 17 | ~ 65% |
| Gabapentin (Altshuler et al, 1999) | No | Open | 28 | 78% (14/18) for mania or hypomania 100% (n = 5) for depression 20% (1/5) for cycling |
| Topiramate (McElroy et al, 2000) | No | Open | 54 | 63% for mania or cycling 27% for acute depression (weight loss observed) |
| Tiagabine (Suppes et al, 2002) | No | Open | 17 | 18% (3/17) (3 possible seizures) |
Second-generation anticonvulsants
Based on our patients’ substantial residual symptoms, we explored a series of newly approved anticonvulsants for potential use as mood stabilizers.
Lamotrigine Consistent with other open and double-blind, placebo-controlled studies, lamotrigine showed promise as an adjunctive treatment, with improvement in depressive symptoms and cycling.15 One such study showed acute antidepressant effects of lamotrigine monotherapy compared with a placebo at dosages of 50 or 200 mg/d.16
Another double-blind, randomized, placebo-controlled NIMH study (partially funded by the Stanley Foundation) compared lamotrigine with gabapentin and a placebo in patients for whom lithium, carbamazepine, and valproate had failed.17 Most of these treatment-refractory patients received all three agents through three 6-week randomizations. Lamotrigine was much more effective in depression and overall illness than either a placebo or gabapentin. Men with bipolar compared with unipolar illness and patients with fewer unsuccessful clinical trials and hospitalizations for depression showed the greatest response.18
In two recent industry-sponsored controlled trials of long-term prophylaxis (following a recent manic or depressive episode), lamotrigine was significantly more effective than a placebo (Bowden et al and Calabrese et al, unpublished data). Lamotrigine was more effective than lithium in preventing depressive episodes, whereas lithium was more effective than lamotrigine for manic episodes, although lamotrigine was significantly better than the placebo.
These data suggest that lamotrigine may differ from valproate, carbamazepine, and lithium in being a more effective antidepressant than antimanic agent.
Topiramate Adjunctive use of topiramate in rapid-cycling and treatment-resistant patients has been examined in open studies, but controlled studies have not yet been published. In our open-label case series observations, 19 of 30 (63%) patients taking topiramate for mania or cycling were “much” or “very much” improved after 10 weeks, whereas only 3 of 11 (27%) patients taking topiramate for acute depression were so improved.19
Table 4
STUDIES IN PROGRESS BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number enrolled* | Notes |
|---|---|---|---|---|
| Topiramate vs. sibutramine | Yes | Open | 42 | Relative weight loss and mood stabilization |
| Omega-3 fatty acids (6 grams EPA vs. placebo) (for depression cycling) (Keck et al) | Yes Yes | Double-blind Double-blind | 62 60 | Data from both studies being analyzed |
| Acamprosate (for alcohol craving) (Grunze et al) | No | Open | 23 | Goal: 60 patients |
| Levetiracetam (Post et al) | No | Open | 13 | Goal: 30 patients |
| Zonisamide (McElroy et al) | No | Open | 45 | Goal: 60 patients European sites only |
| Tranylcypromine vs. lamotrigine (for refractory depression) (Nolen et al) | Yes | Open | 17 | Single-site study |
| Quetiapine (McElroy et al) | No | Open | 48 | Apparent superior antidepressant effects compared with risperidone or clozapine |
| Risperidone (Grunze et al) | No | Open | 37 | No significant effect on IDS‡ depression ratings |
| Clozapine (Suppes et al) | No | Open | 19 | No significant effect on IDS depression ratings |
| Modafinil vs. placebo (Frye et al) | Yes | Double-blind | Started 4/02 | |
| * Enrollment as of April 2002 n = 696 total patients in tables 3 and 4 | ||||
| ‡ IDS = Inventory of Depressive Symptomatology | ||||
An industry-sponsored double-blind, randomized trial of topiramate in acute mania looked promising in the first 67 patients, but not as encouraging in subsequent subjects. A positive drug effect re-emerged if one eliminated patients whose manic episodes were precipitated by antidepressants.
The acute antidepressant effects of topiramate did not appear promising in our study. On the other hand, a single-blind, randomized study of topiramate versus bupropion as adjunctive therapy for breakthrough bipolar depression suggested excellent acute response (approximately 56%) to both agents.20 These data leave the potential antidepressant effects of topiramate undetermined.
In patients being treated with topiramate, we found significant weight loss (average –0.7 kg at 4 weeks, –1.6 kg at 10 weeks, –4.7 kg at 6 months, and –6.2 kg at 1 year).19 Average weight loss was 4.5±6.7 kg at the final evaluation (body mass index [BMI] change of –6.3% and –5% at 1 year and last evaluation, respectively).
We and others have thus seen substantial weight loss in one-third to one-half of patients taking topiramate for bipolar disorder.21 This weight loss is maintained over relatively long periods, which differentiates topiramate from other weight-loss regimens. Topiramate’s ability to decrease weight—even while it is in the early stages of evaluation as a mood stabilizer—is of considerable interest, given the substantial problem of overweight in the bipolar population and the fact that many drug therapies used in the illness are associated with weight gain.
We are proceeding with a randomized open comparison of the relative weight loss and psychotropic efficacy of topiramate versus the antiobesity agent sibutramine. Sibutramine has been reported to have antidepressant properties and is FDA-approved for weight loss.
Gabapentin and tiagabine Our group and many others have reported substantial overall clinical improvement when gabapentin was used as adjunctive therapy in an open study of patients with inadequate response to previous treatments.22 As monotherapy, however, gabapentin was not more effective than a placebo17 and was less effective than lamotrigine. Similarly, gabapentin was not an effective acute antimanic agent when compared with a placebo,23 although other double-blind, controlled studies have indicated positive effects in anxiety disorders24 and social phobias.25 Thus, the gabapentin literature appears to be divergent—i.e., positive studies support its adjunctive use in bipolar patients, but controlled studies suggest that it is not directly antimanic or mood-stabilizing.
Gabapentin’s effect on syndromes that are highly comorbid with bipolar illness could account for its apparent success as open adjunctive therapy. For example, in addition to its antianxiety effects, gabapentin shows positive effects in alcohol withdrawal, pain syndromes, sleep disorders (including restless leg syndrome), and obsessive-compulsive disorder. Also, combining gabapentin with other psychotropics with other mechanisms of action may have a more robust therapeutic effect than monotherapy.
Our preliminary observations with the selective GABA-reuptake inhibitor tiagabine were not promising. In an open study, only 3 of the first 17 SFBN patients with treatment-resistant affective disorders showed a partial response (moderate improvement on the Clinical Global Impressions Scale for Bipolar Illness [CGI-BP]) to adjunctive tiagabine treatment when the dosage was gradually increased. Serious side effects, including two patients with possible seizures, were noted.26 Similar results were seen when tiagabine doses were rapidly increased in eight patients with acute mania; none responded, and one had a major motor seizure.27
Thus, our initial observations led us to discontinue use of tiagabine and suggest any further clinical consideration of this agent for treatment-resistant affective disorders would require great care. This perspective contrasts with several previous positive case reports. It is unknown whether selected patients or those treated with lower dosages would be more responsive to this agent.
Like valproate, gabapentin and tiagabine increase brain GABA, but their apparent lack of antimanic efficacy strongly suggests that raising brain GABA alone does little to counter mania. These data further support the viewpoint already offered that all anticonvulsants are not alike and that anticonvulsants as a class are not necessarily mood stabilizers.
Zonisamide and levetiracetam Open studies of these two other anticonvulsants are near completion. Each has an interesting mechanistic profile, good tolerability, and the (potentially positive) side effect of inducing some weight loss, based on studies of neurologic patients.
Omega-3 fatty acids Based on positive reports of antidepressant effects of omega-3 fatty acids,28-30 we recently recruited more than 120 patients with bipolar disorder for two double-blind, randomized, controlled trials. Those patients with either an acute depression or a cyclic course will receive 6 grams of omega-3 fatty acid (the eicosapentaenoate form) or a placebo for 4 months of double-blind treatment, followed by an 8-month open, active continuation phase.
If results are positive, omega-3 fatty acids would represent the much-needed addition of a well-tolerated dietary agent to the therapeutic armamentarium directed at bipolar illness.
Conclusion
The Stanley Foundation Bipolar Network has begun to evaluate the usefulness of numerous older and new compounds for patients with bipolar disorder, including second-generation antidepressants, anticonvulsants, atypical antipsychotic agents, and omega-3 fatty acids. Based on initial observations from 16 treatment studies (Table 3), we have drawn these conclusions:
- Second-generation antidepressants such as bupropion, sertraline, and venlafaxine appear to have a lower-than-expected switch rate into mania, compared with the use of the older tricyclic antidepressants.
- If the addition of a second-generation antidepressant results in good antidepressant response for 2 months and no switches into mania, one should consider continuing the antidepressant in this small minority of patients, rather than stopping it as soon as possible as previously recommended.
- Adjunctive use of lamotrigine appears to be of value, particularly for acute depression and prophylaxis, for many patients who have not responded to other treatments.
- While our group and others have reported improvement with adjunctive gabapentin in bipolar patients in uncontrolled case series, its primary antimanic or mood-stabilizing utility (as well as that of tiagabine) does not appear promising.
- Topiramate’s ability to induce substantial weight loss may emerge as clinically therapeutic for patients with problematic weight gain related to other psychotropic drugs, independent of its ultimate utility as a possible mood stabilizer.
We are preparing to analyze other open case series assessing acute and long-term treatment outcomes as well as randomized, double-blind clinical trials. Even though the funding of this unique collaborative network is ending, we look forward to more effective treatment of bipolar disorder in the future with continued development of new psychotropic agents and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and the VA Cooperative studies.31
Related resources
- National Alliance for the Mentally Ill (NAMI). www.nami.org
- National Depressive and Manic-Depressive Association (NDMDA). www.ndmda.org
Drug brand names
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sibutramine • Meridia
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproate • Depakote
- Venlafaxine • Effexor
- Zonisamide • Zonegran
Acknowledgement
The authors are particularly indebted to the generosity of The Stanley Foundation in making funds available for this series of studies and several about to be completed.
Disclosure
Financial disclosure statements have been provided by all authors and are available upon request at current.psychiatry@dowdenhealth.com.
When choosing psychopharmacologic agents for patients with bipolar disorder, the clinician lacks guidance from controlled clinical trials at virtually every therapeutic step. To help remedy this problem, we in the Stanley Foundation Bipolar Network (SFBN) evaluated compounds with potentially important therapeutic profiles. We began by documenting the demographics and illness characteristics of patients with bipolar disorder. Then we assessed some 16 treatments in naturalistic or formal studies, some of which are complete and others ongoing.
In this second installment, we report our current information about the efficacy of second-generation antidepressants, atypical antipsychotics, and anticonvulsant agents.
Mood stabilizers
As mood stabilizers, the anticonvulsants carbamazepine and valproate are well-accepted alternatives or adjuncts to lithium carbonate, which remains a mainstay treatment for acute episodes and long-term prophylaxis.1
Valproate is approved by the FDA for treatment of acute mania but not for long-term prevention. Open studies in rapid-cycling, treatment-refractory patients demonstrate substantial improvement for both manic and depressive phases with valproate. In the most recent controlled study, valproate did not prevent manic episodes to a statistically significant degree, but it did show greater antidepressant effects than placebo or lithium.2
Carbamazepine has been widely studied in acute mania and compared with lithium for long-term prophylaxis. Most studies show nonsignificant differences between carbamazepine and lithium in preventing manic and depressive episodes, although several studies indicate less-robust antimanic effects of carbamazepine,3 particularly for patients with classic euphoric mania without psychosis, bipolar II, or associated substance abuse.4
In atypical patients, carbamazepine appeared to outperform lithium, suggesting clinical differences in these agents that have different mechanisms of action across neurotransmitter and peptide systems.
Low response rates Most randomized controlled trials and open adjunct studies suggest that 50% or more patients respond to lithium, valproate, or carbamazepine. Outcomes may be far less positive in clinical practice, however. Despite the use of mood stabilizers and a variety of antidepressants, benzodiazepines, and neuroleptics, our patients experienced substantial residual manic and depressive symptoms and functional impairment.
Similarly, clinician ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM) indicated a high rate of breakthrough episodes during carefully monitored and aggressive psychopharmacologic treatment.5 Two-thirds of the first 258 bipolar outpatients that we followed and rated daily for 1 year continued to be substantially impaired. One-quarter of them were ill for more than 75% of the year, despite using an average 4.1 psychopharmacologic agents per patient, including mood stabilizers, antidepressants, antipsychotics, and other agents.
Acute and long-term combination therapy appears more effective than monotherapy, particularly for treatment-refractory rapid cyclers.3 Even so, many patients do not respond to combination therapy. For example, in a 1-year prophylaxis study, 25% or fewer patients were rated “much improved” to “very much improved” on the Clinical Global Impressions (CGI) scale with lithium or carbamazepine as the baseline mood stabilizer and with antidepressants, neuroleptics, and benzodiazepines used as needed. In the third year of combination therapy, the overall response rate, including rapid cyclers, was roughly 50%.3
A low rate of response is apparent for lithium and valproate. While treating rapidly cycling patients, Calabrese and associates found that less than one-quarter of them responded well enough to the two-drug combination that they could be enrolled in a randomized double-blind comparison of each drug in monotherapy. Of those who were eligible, almost all relapsed with either lithium or valproate monotherapy and again required combination therapy.6
Table 1
RATE OF SWITCHING (%) INTO MANIA IN DEPRESSED BIPOLAR PATIENTS DURING ANTIDEPRESSANT TREATMENT*
| Acute treatment (10 wks) | Continuation treatment (52 wks)‡ | |||||
|---|---|---|---|---|---|---|
| Type of switch | Open (n=27) | Blind (n=100) | Total (n=127) | Open (n=12) | Blind (n=55) | Total (n=67) |
| Brief hypomania | 3.7% | 6% | 5.5% | – | 3.6% | 3% |
| Recurrent brief hypomania | 11 | 12 | 11.8 | 8.3% | 18.2 | 16.4 |
| Hypomania | 11 | 13 | 12.6 | 8.3 | 23.6 | 20.9 |
| Mania | 14.8 | 12 | 12.6 | 33.3 | 14.5 | 17.9 |
| Total | 25.8 | 25 | 25.2 | 41.6 | 38.1 | 38.8 |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | ||||||
| ‡ Most patients (80%) dropped out of this phase because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. | ||||||
| Source: Post RM et al. Bipolar Disord 2001;3:259-65, and unpublished data. | ||||||
Taken together, these studies indicate that even with our most effective and most studied agents, many patients respond inadequately. On the basis of this clinical reality, we began to explore additional options that might enhance clinical effectiveness.
Atypical antipsychotics
We assessed the addition of the atypical antipsychotic olanzapine in patients with treatment-refractory bipolar disorder7 and saw improvement in 57% of subjects with manic, mixed, and depressive components. These data foreshadowed more recent controlled findings of olanzapine’s usefulness in depression and mania.8,9 Our preliminary open clinical trial experience allowed for more controlled studies and subsequent approval and wider clinical use of olanzapine in bipolar illness.
Our initial assessment of other atypical antipsychotics found that quetiapine was associated with significantly fewer depressive symptoms on the Inventory of Depressive Symptomatology (IDS) when used as part of the regular treatment regimen. This improvement, which was seen in the first month, was maintained over 4 months of treatment, whereas no significant improvement in depression severity was seen with the use of risperidone or clozapine.10
It would be highly preliminary to conclude from these uncontrolled data that quetiapine had greater effects on bipolar depressive symptoms than did risperidone or clozapine. We cannot rule out the possibility that patients with different degrees of treatment resistance were enrolled and treated with the different concomitant drugs. However, patients did not differ at baseline in severity of their depression.
In this initial look at the atypicals, the severity of depressive symptoms on the IDS was much greater than that of mania, as measured by the Young Mania Rating Scale (YMRS). Thus, the precise patterns of illness being treated and the impact of these antipsychotics on depressive cyclicity and duration await closer examination. We intend to look at the relative efficacy and side-effect profiles of each of the atypical antipsychotics as used in clinical practice.
Second-generation antidepressants
Bupropion, sertraline, and venlafaxine have different mechanisms of action but appear to be of value as adjuncts to bipolar disorder treatment. When we used them as adjuncts to mood stabilizers in a prospective study, we found a 40 to 50% acute response rate and some switching into hypomania and mania (Table 1).11 Many switches appeared to involve only isolated brief bursts of hypomania or recurrent brief hypomanias, but more sustained episodes of hypomania (7 days) could be more clinically problematic. Only 13% of patients treated acutely with these antidepressants had more full-blown manic symptoms (i.e., those associated with moderate or greater dysfunction on the NIMH-LCM).
During the next year, when apparent acute antidepressant responders were offered continuation treatment, 18% switched into mania. However, most patients (about 80%) dropped out because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. The 127 patients who were randomized received 175 acute antidepressant trials. Only 9% were associated with a switch into mania with moderate dysfunction, whereas another 9% showed periods of hypomania lasting longer than 1 week.12
Table 2
RELAPSE INTO DEPRESSION IN BIPOLAR PATIENTS AFTER 1 YEAR WITH OR WITHOUT ADJUNCTIVE ANTIDEPRESSANT TREATMENT*
| Rate of relapse | |
|---|---|
| Continued on antidepressants | |
| Study #1 (n=19) | 35% |
| Study #2 (n=41) | 41% |
| Discontinued antidepressants | |
| Study #1 (n=25) | 68% |
| Study #2 (n=43) | 71% |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | |
| Study #1: Altshuler et al. J Clin Psychiatry 2001;62:612-6. | |
| Study #2: Altshuler et al. (submitted for publication 2002). | |
We have entered 176 patients into this double-blind, randomized comparison of bupropion, sertraline, and venlafaxine, with another 27 randomized openly. The study will attempt to examine any possible between-drug differences. Even without the blind being broken, it appears that these antidepressants are associated with a somewhat lower-than-expected rate of switching into mania with dysfunction, but a direct comparison with a placebo is not available.
In two other series, patients who remained well for 2 months on antidepressants plus mood stabilizers and then continued on their antidepressants had a much lower incidence of depressive relapses over the following year than those who discontinued their antidepressants (Table 2).13,14 Surprisingly, continuing the antidepressant was not associated with an increased rate of switching into mania.
Prospective randomized, controlled studies are required to confirm these findings. Even so, our studies appear to challenge conventional wisdom for the first time. Stopping antidepressant treatment in bipolar patients as soon as possible for fear of inducing a manic episode may not be the optimal recommendation in the admittedly small subgroup (about 15%) of patients who have responded well to an antidepressant for at least 2 months.
Table 3
SIX STUDIES COMPLETED BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number studied | Response rate |
|---|---|---|---|---|
| Antidepressants bupropion | Yes | Double-blind | 173 | ~ 47% |
| vs. sertraline vs. venlafaxine (Post et al, 2001) | Yes | Open | 27 | |
| Total = 200 | ||||
| Olanzapine (McElroy et al, 1998) | No | Open | 14 | ~ 57% |
| Lamotrigine (Suppes et al, 1999) | No | Open | 17 | ~ 65% |
| Gabapentin (Altshuler et al, 1999) | No | Open | 28 | 78% (14/18) for mania or hypomania 100% (n = 5) for depression 20% (1/5) for cycling |
| Topiramate (McElroy et al, 2000) | No | Open | 54 | 63% for mania or cycling 27% for acute depression (weight loss observed) |
| Tiagabine (Suppes et al, 2002) | No | Open | 17 | 18% (3/17) (3 possible seizures) |
Second-generation anticonvulsants
Based on our patients’ substantial residual symptoms, we explored a series of newly approved anticonvulsants for potential use as mood stabilizers.
Lamotrigine Consistent with other open and double-blind, placebo-controlled studies, lamotrigine showed promise as an adjunctive treatment, with improvement in depressive symptoms and cycling.15 One such study showed acute antidepressant effects of lamotrigine monotherapy compared with a placebo at dosages of 50 or 200 mg/d.16
Another double-blind, randomized, placebo-controlled NIMH study (partially funded by the Stanley Foundation) compared lamotrigine with gabapentin and a placebo in patients for whom lithium, carbamazepine, and valproate had failed.17 Most of these treatment-refractory patients received all three agents through three 6-week randomizations. Lamotrigine was much more effective in depression and overall illness than either a placebo or gabapentin. Men with bipolar compared with unipolar illness and patients with fewer unsuccessful clinical trials and hospitalizations for depression showed the greatest response.18
In two recent industry-sponsored controlled trials of long-term prophylaxis (following a recent manic or depressive episode), lamotrigine was significantly more effective than a placebo (Bowden et al and Calabrese et al, unpublished data). Lamotrigine was more effective than lithium in preventing depressive episodes, whereas lithium was more effective than lamotrigine for manic episodes, although lamotrigine was significantly better than the placebo.
These data suggest that lamotrigine may differ from valproate, carbamazepine, and lithium in being a more effective antidepressant than antimanic agent.
Topiramate Adjunctive use of topiramate in rapid-cycling and treatment-resistant patients has been examined in open studies, but controlled studies have not yet been published. In our open-label case series observations, 19 of 30 (63%) patients taking topiramate for mania or cycling were “much” or “very much” improved after 10 weeks, whereas only 3 of 11 (27%) patients taking topiramate for acute depression were so improved.19
Table 4
STUDIES IN PROGRESS BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number enrolled* | Notes |
|---|---|---|---|---|
| Topiramate vs. sibutramine | Yes | Open | 42 | Relative weight loss and mood stabilization |
| Omega-3 fatty acids (6 grams EPA vs. placebo) (for depression cycling) (Keck et al) | Yes Yes | Double-blind Double-blind | 62 60 | Data from both studies being analyzed |
| Acamprosate (for alcohol craving) (Grunze et al) | No | Open | 23 | Goal: 60 patients |
| Levetiracetam (Post et al) | No | Open | 13 | Goal: 30 patients |
| Zonisamide (McElroy et al) | No | Open | 45 | Goal: 60 patients European sites only |
| Tranylcypromine vs. lamotrigine (for refractory depression) (Nolen et al) | Yes | Open | 17 | Single-site study |
| Quetiapine (McElroy et al) | No | Open | 48 | Apparent superior antidepressant effects compared with risperidone or clozapine |
| Risperidone (Grunze et al) | No | Open | 37 | No significant effect on IDS‡ depression ratings |
| Clozapine (Suppes et al) | No | Open | 19 | No significant effect on IDS depression ratings |
| Modafinil vs. placebo (Frye et al) | Yes | Double-blind | Started 4/02 | |
| * Enrollment as of April 2002 n = 696 total patients in tables 3 and 4 | ||||
| ‡ IDS = Inventory of Depressive Symptomatology | ||||
An industry-sponsored double-blind, randomized trial of topiramate in acute mania looked promising in the first 67 patients, but not as encouraging in subsequent subjects. A positive drug effect re-emerged if one eliminated patients whose manic episodes were precipitated by antidepressants.
The acute antidepressant effects of topiramate did not appear promising in our study. On the other hand, a single-blind, randomized study of topiramate versus bupropion as adjunctive therapy for breakthrough bipolar depression suggested excellent acute response (approximately 56%) to both agents.20 These data leave the potential antidepressant effects of topiramate undetermined.
In patients being treated with topiramate, we found significant weight loss (average –0.7 kg at 4 weeks, –1.6 kg at 10 weeks, –4.7 kg at 6 months, and –6.2 kg at 1 year).19 Average weight loss was 4.5±6.7 kg at the final evaluation (body mass index [BMI] change of –6.3% and –5% at 1 year and last evaluation, respectively).
We and others have thus seen substantial weight loss in one-third to one-half of patients taking topiramate for bipolar disorder.21 This weight loss is maintained over relatively long periods, which differentiates topiramate from other weight-loss regimens. Topiramate’s ability to decrease weight—even while it is in the early stages of evaluation as a mood stabilizer—is of considerable interest, given the substantial problem of overweight in the bipolar population and the fact that many drug therapies used in the illness are associated with weight gain.
We are proceeding with a randomized open comparison of the relative weight loss and psychotropic efficacy of topiramate versus the antiobesity agent sibutramine. Sibutramine has been reported to have antidepressant properties and is FDA-approved for weight loss.
Gabapentin and tiagabine Our group and many others have reported substantial overall clinical improvement when gabapentin was used as adjunctive therapy in an open study of patients with inadequate response to previous treatments.22 As monotherapy, however, gabapentin was not more effective than a placebo17 and was less effective than lamotrigine. Similarly, gabapentin was not an effective acute antimanic agent when compared with a placebo,23 although other double-blind, controlled studies have indicated positive effects in anxiety disorders24 and social phobias.25 Thus, the gabapentin literature appears to be divergent—i.e., positive studies support its adjunctive use in bipolar patients, but controlled studies suggest that it is not directly antimanic or mood-stabilizing.
Gabapentin’s effect on syndromes that are highly comorbid with bipolar illness could account for its apparent success as open adjunctive therapy. For example, in addition to its antianxiety effects, gabapentin shows positive effects in alcohol withdrawal, pain syndromes, sleep disorders (including restless leg syndrome), and obsessive-compulsive disorder. Also, combining gabapentin with other psychotropics with other mechanisms of action may have a more robust therapeutic effect than monotherapy.
Our preliminary observations with the selective GABA-reuptake inhibitor tiagabine were not promising. In an open study, only 3 of the first 17 SFBN patients with treatment-resistant affective disorders showed a partial response (moderate improvement on the Clinical Global Impressions Scale for Bipolar Illness [CGI-BP]) to adjunctive tiagabine treatment when the dosage was gradually increased. Serious side effects, including two patients with possible seizures, were noted.26 Similar results were seen when tiagabine doses were rapidly increased in eight patients with acute mania; none responded, and one had a major motor seizure.27
Thus, our initial observations led us to discontinue use of tiagabine and suggest any further clinical consideration of this agent for treatment-resistant affective disorders would require great care. This perspective contrasts with several previous positive case reports. It is unknown whether selected patients or those treated with lower dosages would be more responsive to this agent.
Like valproate, gabapentin and tiagabine increase brain GABA, but their apparent lack of antimanic efficacy strongly suggests that raising brain GABA alone does little to counter mania. These data further support the viewpoint already offered that all anticonvulsants are not alike and that anticonvulsants as a class are not necessarily mood stabilizers.
Zonisamide and levetiracetam Open studies of these two other anticonvulsants are near completion. Each has an interesting mechanistic profile, good tolerability, and the (potentially positive) side effect of inducing some weight loss, based on studies of neurologic patients.
Omega-3 fatty acids Based on positive reports of antidepressant effects of omega-3 fatty acids,28-30 we recently recruited more than 120 patients with bipolar disorder for two double-blind, randomized, controlled trials. Those patients with either an acute depression or a cyclic course will receive 6 grams of omega-3 fatty acid (the eicosapentaenoate form) or a placebo for 4 months of double-blind treatment, followed by an 8-month open, active continuation phase.
If results are positive, omega-3 fatty acids would represent the much-needed addition of a well-tolerated dietary agent to the therapeutic armamentarium directed at bipolar illness.
Conclusion
The Stanley Foundation Bipolar Network has begun to evaluate the usefulness of numerous older and new compounds for patients with bipolar disorder, including second-generation antidepressants, anticonvulsants, atypical antipsychotic agents, and omega-3 fatty acids. Based on initial observations from 16 treatment studies (Table 3), we have drawn these conclusions:
- Second-generation antidepressants such as bupropion, sertraline, and venlafaxine appear to have a lower-than-expected switch rate into mania, compared with the use of the older tricyclic antidepressants.
- If the addition of a second-generation antidepressant results in good antidepressant response for 2 months and no switches into mania, one should consider continuing the antidepressant in this small minority of patients, rather than stopping it as soon as possible as previously recommended.
- Adjunctive use of lamotrigine appears to be of value, particularly for acute depression and prophylaxis, for many patients who have not responded to other treatments.
- While our group and others have reported improvement with adjunctive gabapentin in bipolar patients in uncontrolled case series, its primary antimanic or mood-stabilizing utility (as well as that of tiagabine) does not appear promising.
- Topiramate’s ability to induce substantial weight loss may emerge as clinically therapeutic for patients with problematic weight gain related to other psychotropic drugs, independent of its ultimate utility as a possible mood stabilizer.
We are preparing to analyze other open case series assessing acute and long-term treatment outcomes as well as randomized, double-blind clinical trials. Even though the funding of this unique collaborative network is ending, we look forward to more effective treatment of bipolar disorder in the future with continued development of new psychotropic agents and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and the VA Cooperative studies.31
Related resources
- National Alliance for the Mentally Ill (NAMI). www.nami.org
- National Depressive and Manic-Depressive Association (NDMDA). www.ndmda.org
Drug brand names
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sibutramine • Meridia
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproate • Depakote
- Venlafaxine • Effexor
- Zonisamide • Zonegran
Acknowledgement
The authors are particularly indebted to the generosity of The Stanley Foundation in making funds available for this series of studies and several about to be completed.
Disclosure
Financial disclosure statements have been provided by all authors and are available upon request at current.psychiatry@dowdenhealth.com.
1. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press, 1990.
2. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
3. Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997a;58:470-8.
4. Greil W, Kleindienst N. The comparative prophylactic efficacy of lithium and carbamazepine in patients with bipolar I disorder. Int Clin Psychopharmacol 1999;14:277-81.
5. Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for one year with daily prospective ratings on the NIMH-LCM (submitted for publication, 2002).
6. Calabrese JR, Rapport DJ. Mood stabilizers and the evolution of maintenance study designs in bipolar I disorder. J Clin Psychiatry 1999;60(suppl 5):5-13.
7. McElroy SL, Frye M, Denicoff K, et al. Olanzapine in treatment-resistant bipolar disorder. J Affect Disord 1998;49:119-22.
8. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
9. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
10. Keck PE, Jr. New antiepileptics in the treatment of bipolar disorder. Syllabus and Proceedings Summary of the 2001 American Psychiatric Association Meeting, New Orleans, May 5-10, 2001. Abstract 34E, 93.
11. Post RM, Altshuler LL, Frye MA, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001;3:259-65.
12. Post RM, Leverich GS, Nolen WA, et al. A reevaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2002 (in press).
13. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-6.
14. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute remission from bipolar depression on rates of depressive relapse at one-year follow-up (submitted for publication 2002).
15. Suppes T, Brown ES, McElroy SL, et al. Lamotrigine for the treatment of bipolar disorder: a clinical case series. J Affective Disord 1999;53:95-8.
16. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60:79-88.
17. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14.
18. Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biolog Psychiatry 2002;51:253-60.
19. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biolog Psychiatry 2000;47:1025-33.
20. McIntyre RS, Mancini DA, McCann SM, et al. Topiramiate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 2002;4:207-13.
21. Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults. Epilepsia 2000;41(suppl 1):S66-S71.
22. Altshuler LL, Keck PE, Jr, McElroy SL, et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1999;1:61-5.
23. Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord 2000;2:249-55.
24. Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000;20:467-71.
25. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999;19:341-8.
26. Suppes T, Chisholm K, Dhvule D, et al. Tiagabine in bipolar disorder: adverse events in an open case series (submitted for publication, 2002).
27. Grunze H, Erfurth A, Marcuse A, et al. Tiagabine appears not to be efficacious in the treatment of acute mania. J Clin Psychiatry 1999;60:759-62.
28. Stoll AL, Severus WE, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56:407-12.
29. Horrobin DF, Peet M. A dose-ranging study of ethyl-eicosapentaenoate in treatment-unresponsive depression. Biolog Psychiatry 2001;49:37S.-
30. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-9.
31. Bauer MS, Williford WO, Dawson EE, et al. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: Reducing the efficacy-effectiveness gap in bipolar disorder. J Affect Disord 2001;67:61-78.
1. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press, 1990.
2. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
3. Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997a;58:470-8.
4. Greil W, Kleindienst N. The comparative prophylactic efficacy of lithium and carbamazepine in patients with bipolar I disorder. Int Clin Psychopharmacol 1999;14:277-81.
5. Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for one year with daily prospective ratings on the NIMH-LCM (submitted for publication, 2002).
6. Calabrese JR, Rapport DJ. Mood stabilizers and the evolution of maintenance study designs in bipolar I disorder. J Clin Psychiatry 1999;60(suppl 5):5-13.
7. McElroy SL, Frye M, Denicoff K, et al. Olanzapine in treatment-resistant bipolar disorder. J Affect Disord 1998;49:119-22.
8. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
9. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
10. Keck PE, Jr. New antiepileptics in the treatment of bipolar disorder. Syllabus and Proceedings Summary of the 2001 American Psychiatric Association Meeting, New Orleans, May 5-10, 2001. Abstract 34E, 93.
11. Post RM, Altshuler LL, Frye MA, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001;3:259-65.
12. Post RM, Leverich GS, Nolen WA, et al. A reevaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2002 (in press).
13. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-6.
14. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute remission from bipolar depression on rates of depressive relapse at one-year follow-up (submitted for publication 2002).
15. Suppes T, Brown ES, McElroy SL, et al. Lamotrigine for the treatment of bipolar disorder: a clinical case series. J Affective Disord 1999;53:95-8.
16. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60:79-88.
17. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14.
18. Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biolog Psychiatry 2002;51:253-60.
19. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biolog Psychiatry 2000;47:1025-33.
20. McIntyre RS, Mancini DA, McCann SM, et al. Topiramiate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 2002;4:207-13.
21. Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults. Epilepsia 2000;41(suppl 1):S66-S71.
22. Altshuler LL, Keck PE, Jr, McElroy SL, et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1999;1:61-5.
23. Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord 2000;2:249-55.
24. Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000;20:467-71.
25. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999;19:341-8.
26. Suppes T, Chisholm K, Dhvule D, et al. Tiagabine in bipolar disorder: adverse events in an open case series (submitted for publication, 2002).
27. Grunze H, Erfurth A, Marcuse A, et al. Tiagabine appears not to be efficacious in the treatment of acute mania. J Clin Psychiatry 1999;60:759-62.
28. Stoll AL, Severus WE, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56:407-12.
29. Horrobin DF, Peet M. A dose-ranging study of ethyl-eicosapentaenoate in treatment-unresponsive depression. Biolog Psychiatry 2001;49:37S.-
30. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-9.
31. Bauer MS, Williford WO, Dawson EE, et al. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: Reducing the efficacy-effectiveness gap in bipolar disorder. J Affect Disord 2001;67:61-78.
From the Stanley Foundation Bipolar Network: New findings on suicide attempts, substance abuse, obesity, and more
Even with psychiatry’s most effective and best studied agents, many patients with bipolar disorder are inadequately treated and respond insufficiently to available psychopharmacologic agents. To address this problem, the Stanley Foundation Bipolar Network (SFBN) was created to explore acute and long-term response to treatment of bipolar disorder.
For the past 6 years, our group (Box) has followed an international cohort of patients with bipolar disorder and offered them opportunities to participate in clinical trials at seven field centers. We have made considerable progress as we focused on the longitudinal course of bipolar illness and evaluated various combinations of psychopharmacologic therapies. The SFBN also has explored factors that increase the risk of suicide, quantified the incidence of anxiety disorder and substance abuse, and looked at other variables that may affect treatment outcome.
This report for the readers of Current Psychiatry provides a brief state-of-the-art overview of what we have learned about the course and management of bipolar disorder. It brings together and summarizes the findings from studies published, in press, or under review. Our goal is to update current findings, which may help you provide optimum care for these at-risk patients.
Since Robert Prien’s pioneering studies in the 1970s, the National Institute of Mental Health (NIMH) had funded little work on prophylaxis of bipolar disorders or acute bipolar depression. Following two NIMH conferences on bipolar illness in 1989 and 1994, the Stanley Foundation Bipolar Network (SFBN) was established to help overcome these research deficiencies, with funding provided by the Theodore and Vada Stanley Foundation.
Patients were recruited into the network from the general community and with the use of newspaper and newsletter advertisements. In contrast to many other clinical populations, few who wished to enter the network were excluded. It was clear, however, that filling out demographic, clinical, and rating forms would require considerable effort from each patient. All patients gave written informed consent to participate in the naturalistic part of the study, and were reconsented for any open or blind randomized clinical trial.
Patients provided daily self-ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM), a unique longitudinal instrument. Clinicians then completed their own separate LCMs, as more severely ill patients often could not complete their self-ratings, and patients often underestimated the severity and functional disability associated with the hypomanic and manic phases of bipolar illness. With the use of the NIMH-LCM, network investigators were able, for the first time, to assess the details of mood fluctuations associated with bipolar illness and to more precisely delineate the qualitative and quantitative aspects of response to psychopharmacologic interventions.
The SFBN includes seven research field centers in the United States, the Netherlands, and Germany. The leaders of those centers collaborated with Robert M. Post, MD, to prepare this report of the network findings. The collaborators are:
| Gabriele S. Leverich, MSW, and Kirk D. Denicoff, MD Biological Psychiatry Branch National Institute of Mental Health Bethesda, MD | Paul E. Keck, Jr., MD, and Susan L. McElroy, MD Biological Psychiatry Program University of Cincinnati College of Medicine Cincinnati, OH |
| Lori L. Altshuler, MD, and Mark A. Frye, MD UCLA Mood Disorders Research Program VA Medical Center Los Angeles, CA | Willem A. Nolen, MD, and Ralph Kupka, MD Altrecht Institute for Mental Health Care University Medical Center Utrecht, The Netherlands |
| John Rush, MD, and Trisha Suppes, MD Department of Psychiatry Southwest Medical Center University of Texas, Dallas | Joerg Walden, MD Department of Psychiatry University of Freiburg Freiburg, Germany |
| Keith Kramlinger, MD Mayo Clinic, Rochester, MN (Administrative center) | Heinz Grunze, MD Psychiatrische Klinik der LMU Munich, Germany |
In part one, we discuss the demographics of our cohort of 676 community-based bipolar patients, including risk factors for suicide attempts, frequency of comorbid psychiatric diagnoses, problems of overweight and obesity, and preliminary findings about thyroiditis.
In part two, we describe our clinical trials assessing mood stabilizers and adjunctive agents such as atypical antipsychotics, second-generation antidepressants, and anticonvulsants. We also will address a soon-to-be analyzed study of omega-3 fatty acids.
Table 1
CHARACTERISTICS OF THE 676 BIPOLAR OUTPATIENTS
| Mean age | 41 | |
| % Female | 43 | |
| Diagnosis (%) | ||
| Bipolar I | 79 | |
| Bipolar II | 16 | |
| Bipolar NOS | 4 | |
| Marital status (%) | ||
| Married/cohabitating | 44 | |
| Divorced/separated | 22 | |
| Income (%) | ||
| <$20,000 | 37 | |
| $20,000-40,000 | 22 | |
| $40,000-100,000 | 32 | |
| >$100,000 | 9 | |
| Education (completed, %) | ||
| High school | 34 | |
| Jr. college/technical | 13 | |
| College, 4-year | 27 | |
| Graduate degree | 16 | |
| Positive family history (1st-degree relatives, %) | ||
| Bipolar disorder | 56 | |
| Unipolar disorder | 64 | |
| Schizophrenia | 24 | |
| Drug dependence | 44 | |
| Other psychiatric disorder | 52 | |
| Alcohol abuse/dependence | 52 | |
| Suicide (complete or serious attempt) | 38 | |
| Environmental adversity (%): | Child/adolescent | Adult |
| Physical abuse | 29 | 8 |
| Sexual abuse | 30 | 8 |
| Illness course and treatment (age in yrs) | ||
| Age of onset (symptoms) | 19 | |
| Early onset (age <14) | 34 | |
| Age at first treatment | 29 | |
| Age at first hospitalization | 30 | |
| Cycling pattern (n = 643, %) | ||
| Rapid (or faster cycling) | 47 | |
| Ultrarapid (or faster cycling) | 30 | |
| Rapid-cycling only | 17 | |
| Ultrarapid cycling only | 10 | |
| Ultradian cycling | 20 | |
Study population
The SFBN recruited outpatients with bipolar disorders I, II, or not otherwise specified (NOS). We did not exclude patients with comorbid illnesses, except those with active substance abuse disorder requiring treatment in another setting.1 Patients were recruited almost exclusively from the community and rarely from inpatient hospitalization. The average age was 41, and 43% were college graduates. Most were employed but indicated that their illness limited their employment level or ability to function at work (Table 1).
One striking finding was a decadelong lag between the onset of symptoms reaching thresholds for diagnosis and the onset of first treatment.2 This long delay could have adverse consequences for illness outcome and represents a major public health target. Age of onset appeared to have both genetic/familial and environmental determinants.3 Subjects with a history of both extreme early life adversities (i.e., physical or sexual abuse) and a positive family history of mood disorder had the earliest onset of bipolar illness. Those who had neither of these factors had late-onset illness.
Suicide attempts
Prior to network entry, 25 to 50% of our study population had made a serious suicide attempt (defined as requiring medical attention and/or hospitalization), pointing to bipolar disorder’s potential lethality.
History of abuse A history of physical or sexual abuse in childhood or adolescence was a prominent risk factor for attempted suicide.4 With either type of abuse there was an approximate 15% increase in incidence of suicide attempts over the baseline rate of about 25%. More than 55% of patients with a history of both physical and sexual abuse had made a serious suicide attempt. The frequency of physical and sexual abuse was also positively related to increased incidence of suicide attempts. Taken together, these data suggest a “stressor dose-response” relationship between adverse childhood or adolescent experiences and an increased incidence of suicide attempts.
Table 2
FACTORS THAT APPEAR TO INCREASE SUICIDE RISK IN BIPOLAR DISORDER*
| Course-of-illness characteristics |
| Early onset |
| More time ill |
| More time depressed |
| More-severe depression |
| Social and medical support |
| Loss of significant other |
| Lack of a confidant |
| Single motherhood |
| Decreased access to health care |
| Lack of medical insurance |
| Demographics |
| Less education |
| Lower income |
| Being single |
| Comorbidities |
| Axis I |
| Eating disorders |
| Anxiety disorders |
| Axis II |
| Cluster A, B, C |
| Medical |
| Traumatic life events |
| Sexual abuse |
| Physical abuse |
| Genetics |
| Family history of suicide and drug abuse |
| * Identified in the SFBN cohort |
Comorbidities Other factors that appear to be associated with bipolar disorder and increased risk of attempted suicide include anxiety and eating disorders, early onset of symptoms, at least four previous hospitalizations for depression, and medical comorbidity (Table 2). Limited access to psychiatric and medical services and inadequate health insurance also were correlated with having made a serious suicide attempt. Thus, lack of medical care might exacerbate demoralization and increase the risk of suicide.
Family history of suicide or serious suicide attempts and drug abuse were associated with an increase in suicide attempts, but family history of unipolar or bipolar illness were not. These data are consistent with other studies suggesting that vulnerability to suicide may have a separate genetic component from that of mood disorders per se.
Severity of illness Increased severity of bipolar illness also was associated with prior psychosocial stressors3 and serious suicide attempts. We confirmed self-reported history, using daily mood charting, the clinician-rated Inventory of Depressive Symptomatology (IDS-C), and the Young Mania Rating Scale (YMRS) to rate increase in percentage of time ill, percentage of time depressed, and severity of depression.
Clinical implications We are uncertain how directly these data regarding suicide attempts relate to completed suicide. However, patients with bipolar disorder are among those at highest risk for completed suicide, and a prior serious attempt is often a precursor to completed suicide.5,6
Factors that appear particularly related to increased risk of suicide in patients with bipolar disorder are family history of suicide, history of early psychosocial stressors, comorbid personality disorders, substance abuse, and a relatively severe course of bipolar illness. Patients with these risk factors need support for two types of psychosocial stressors:
- poor access to health care
- stressors related to concurrent events (e.g., occurring with new affective episodes).
It may also be important for clinicians to consider the prophylactic use of lithium because of its demonstrated ability to reduce mortality in bipolar illness.7 Additionally, we need data on the efficacy of new drugs in preventing affective episodes and reducing suicidal ideation and acts.
Common comorbidities
We found considerable axis I lifetime comorbidities in the SFBN cohort (Table 3), including a 42% incidence of anxiety disorder and a similarly high rate of substance abuse. In addition to bipolar disorder, patients averaged approximately two lifetime comorbid diagnoses:
- 35% had none
- 65% had one or more
- 42% had two or more
- 24% had three or more.
Table 3
PSYCHIATRIC COMORBIDITY IN BIPOLAR OUTPATIENTS (%)*.
| Any substance abuse disorder | 42% |
| Alcohol | 33 |
| Marijuana | 16 |
| Stimulant | 9 |
| Sedative | 8 |
| Opiate | 7 |
| Hallucinogens | 6 |
| Any anxiety disorder | 42% |
| Panic disorder | 20 |
| Social phobia | 16 |
| Simple phobia | 10 |
| OCD | 9 |
| PTSD | 7 |
| GAD | 3 |
| Other | 3 |
| Any eating disorder | 11% |
| Bulimia nervosa | 8 |
| Anorexia nervosa | 4 |
| * Lifetime comorbid axis I disorders based on Structured Clinical Interview for DSM-IV (SCID) interviews with 288 SFBN patients. | |
Men with bipolar disorder had a higher absolute prevalence of alcoholism, but the relative risk compared with the general population was much higher in women (odds ratio 7.35) compared with men (odds ratio 2.77). In women, alcohol use and abuse were related to history of social phobia, greater number of depressive episodes prior to network entry, and history of abusing more than one substance. In men, alcohol comorbidity was related to history of alcohol abuse in first-degree relatives and history of early physical abuse.
These data suggest that female patients, in particular, were self-medicating their affective episodes and comorbid anxiety disorders with alcohol. They suggest the importance of asking patients directly about alcohol use to identify the need for primary and secondary prevention. In an adolescent or young adult, affective illness—and bipolar disorder in particular—should be considered a major risk factor for subsequent problems with alcohol and drug abuse. When taking a careful history, the treating physician should include questions regarding any substance abuse.
Overweight and obesity
One-half of the women and two-thirds of the men in the SFBN were overweight at network entry.8 Although this rate of overweight sounds alarmingly high, it was not significantly different from rates in the general U.S. population. A greater percentage of women than men in the network had morbid obesity, however.
In our cohort, being overweight or obese was associated with an increased incidence of cardiovascular disease, diabetes, arthritis, hypertension, hypothyroidism, and cancer. The degree of obesity also correlated significantly with past use of the numerous psychotropic drugs that have been associated with weight gain. Also, as might be expected, increased weight was associated with physical inactivity.
Many of the psychotropic drugs routinely used to treat bipolar disorder are associated with weight gain. Among these are the mood stabilizers lithium and valproate and the atypical antipsychotics, with the exception of ziprasidone (and, to follow, aripiprazole). Lithium’s liability to cause weight gain may be counterbalanced by its antisuicide effects and its ability to reverse excess medical mortality associated with affective illness. Thus, lithium should continue to play a major therapeutic role in bipolar disorder.
It may be more effective and easier to prevent weight gain than to attempt to reduce weight that has been gained. Recommending exercise and other weight-reduction measures may be helpful, as may co-administering drugs associated with weight loss, such as topiramate.9
Thyroiditis
Antithyroid antibodies were detected in 28% of network patients, compared with rates of 3% in the general population and 18% in psychiatric controls.10 The increased rate was associated with the need to augment thyroid function in 17% of network patients, but it was not related to age, mood state, rapid cycling status, or lithium treatment.
These data indicate that bipolar patients are prone to autoimmune thyroid changes. Further analysis is required to ascertain whether this finding has therapeutic implications, especially related to adjunctive T3 and T4 treatment.11
1. Leverich GS, Nolen WA, Rush AJ, et al. The Stanley Foundation Bipolar Treatment Outcome Network. I. Longitudinal methodology. J Affect Disord 2001;67:33-44.
2. Suppes T, Leverich GS, Keck PE, et al. The Stanley Foundation Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disord 2001b;67:45-59.
3. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological Psychiatry 2002;51:288-97.
4. Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. Manuscript submitted for publication, 2002.
5. Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39:896-9.
6. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch.Gen.Psychiatry 1999;56:617-26.
7. Ahrens B, Muller-Oerlinghausen B, Schou M, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord 1995;33:67-75.
8. McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry 2002;63:207-13.
9. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biological Psychiatry 2000;47:1025-33.
10. Kupka RW, Nolen WA, Post RM, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biological Psychiatry 2002;51:305-11.
11. Bauer M, Priebe S, Berghofer A, et al. Subjective response to and tolerability of long-term supraphysiological doses of levothyroxine in refractory mood disorders. J Affect Disord 2001;64:35-42.
Even with psychiatry’s most effective and best studied agents, many patients with bipolar disorder are inadequately treated and respond insufficiently to available psychopharmacologic agents. To address this problem, the Stanley Foundation Bipolar Network (SFBN) was created to explore acute and long-term response to treatment of bipolar disorder.
For the past 6 years, our group (Box) has followed an international cohort of patients with bipolar disorder and offered them opportunities to participate in clinical trials at seven field centers. We have made considerable progress as we focused on the longitudinal course of bipolar illness and evaluated various combinations of psychopharmacologic therapies. The SFBN also has explored factors that increase the risk of suicide, quantified the incidence of anxiety disorder and substance abuse, and looked at other variables that may affect treatment outcome.
This report for the readers of Current Psychiatry provides a brief state-of-the-art overview of what we have learned about the course and management of bipolar disorder. It brings together and summarizes the findings from studies published, in press, or under review. Our goal is to update current findings, which may help you provide optimum care for these at-risk patients.
Since Robert Prien’s pioneering studies in the 1970s, the National Institute of Mental Health (NIMH) had funded little work on prophylaxis of bipolar disorders or acute bipolar depression. Following two NIMH conferences on bipolar illness in 1989 and 1994, the Stanley Foundation Bipolar Network (SFBN) was established to help overcome these research deficiencies, with funding provided by the Theodore and Vada Stanley Foundation.
Patients were recruited into the network from the general community and with the use of newspaper and newsletter advertisements. In contrast to many other clinical populations, few who wished to enter the network were excluded. It was clear, however, that filling out demographic, clinical, and rating forms would require considerable effort from each patient. All patients gave written informed consent to participate in the naturalistic part of the study, and were reconsented for any open or blind randomized clinical trial.
Patients provided daily self-ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM), a unique longitudinal instrument. Clinicians then completed their own separate LCMs, as more severely ill patients often could not complete their self-ratings, and patients often underestimated the severity and functional disability associated with the hypomanic and manic phases of bipolar illness. With the use of the NIMH-LCM, network investigators were able, for the first time, to assess the details of mood fluctuations associated with bipolar illness and to more precisely delineate the qualitative and quantitative aspects of response to psychopharmacologic interventions.
The SFBN includes seven research field centers in the United States, the Netherlands, and Germany. The leaders of those centers collaborated with Robert M. Post, MD, to prepare this report of the network findings. The collaborators are:
| Gabriele S. Leverich, MSW, and Kirk D. Denicoff, MD Biological Psychiatry Branch National Institute of Mental Health Bethesda, MD | Paul E. Keck, Jr., MD, and Susan L. McElroy, MD Biological Psychiatry Program University of Cincinnati College of Medicine Cincinnati, OH |
| Lori L. Altshuler, MD, and Mark A. Frye, MD UCLA Mood Disorders Research Program VA Medical Center Los Angeles, CA | Willem A. Nolen, MD, and Ralph Kupka, MD Altrecht Institute for Mental Health Care University Medical Center Utrecht, The Netherlands |
| John Rush, MD, and Trisha Suppes, MD Department of Psychiatry Southwest Medical Center University of Texas, Dallas | Joerg Walden, MD Department of Psychiatry University of Freiburg Freiburg, Germany |
| Keith Kramlinger, MD Mayo Clinic, Rochester, MN (Administrative center) | Heinz Grunze, MD Psychiatrische Klinik der LMU Munich, Germany |
In part one, we discuss the demographics of our cohort of 676 community-based bipolar patients, including risk factors for suicide attempts, frequency of comorbid psychiatric diagnoses, problems of overweight and obesity, and preliminary findings about thyroiditis.
In part two, we describe our clinical trials assessing mood stabilizers and adjunctive agents such as atypical antipsychotics, second-generation antidepressants, and anticonvulsants. We also will address a soon-to-be analyzed study of omega-3 fatty acids.
Table 1
CHARACTERISTICS OF THE 676 BIPOLAR OUTPATIENTS
| Mean age | 41 | |
| % Female | 43 | |
| Diagnosis (%) | ||
| Bipolar I | 79 | |
| Bipolar II | 16 | |
| Bipolar NOS | 4 | |
| Marital status (%) | ||
| Married/cohabitating | 44 | |
| Divorced/separated | 22 | |
| Income (%) | ||
| <$20,000 | 37 | |
| $20,000-40,000 | 22 | |
| $40,000-100,000 | 32 | |
| >$100,000 | 9 | |
| Education (completed, %) | ||
| High school | 34 | |
| Jr. college/technical | 13 | |
| College, 4-year | 27 | |
| Graduate degree | 16 | |
| Positive family history (1st-degree relatives, %) | ||
| Bipolar disorder | 56 | |
| Unipolar disorder | 64 | |
| Schizophrenia | 24 | |
| Drug dependence | 44 | |
| Other psychiatric disorder | 52 | |
| Alcohol abuse/dependence | 52 | |
| Suicide (complete or serious attempt) | 38 | |
| Environmental adversity (%): | Child/adolescent | Adult |
| Physical abuse | 29 | 8 |
| Sexual abuse | 30 | 8 |
| Illness course and treatment (age in yrs) | ||
| Age of onset (symptoms) | 19 | |
| Early onset (age <14) | 34 | |
| Age at first treatment | 29 | |
| Age at first hospitalization | 30 | |
| Cycling pattern (n = 643, %) | ||
| Rapid (or faster cycling) | 47 | |
| Ultrarapid (or faster cycling) | 30 | |
| Rapid-cycling only | 17 | |
| Ultrarapid cycling only | 10 | |
| Ultradian cycling | 20 | |
Study population
The SFBN recruited outpatients with bipolar disorders I, II, or not otherwise specified (NOS). We did not exclude patients with comorbid illnesses, except those with active substance abuse disorder requiring treatment in another setting.1 Patients were recruited almost exclusively from the community and rarely from inpatient hospitalization. The average age was 41, and 43% were college graduates. Most were employed but indicated that their illness limited their employment level or ability to function at work (Table 1).
One striking finding was a decadelong lag between the onset of symptoms reaching thresholds for diagnosis and the onset of first treatment.2 This long delay could have adverse consequences for illness outcome and represents a major public health target. Age of onset appeared to have both genetic/familial and environmental determinants.3 Subjects with a history of both extreme early life adversities (i.e., physical or sexual abuse) and a positive family history of mood disorder had the earliest onset of bipolar illness. Those who had neither of these factors had late-onset illness.
Suicide attempts
Prior to network entry, 25 to 50% of our study population had made a serious suicide attempt (defined as requiring medical attention and/or hospitalization), pointing to bipolar disorder’s potential lethality.
History of abuse A history of physical or sexual abuse in childhood or adolescence was a prominent risk factor for attempted suicide.4 With either type of abuse there was an approximate 15% increase in incidence of suicide attempts over the baseline rate of about 25%. More than 55% of patients with a history of both physical and sexual abuse had made a serious suicide attempt. The frequency of physical and sexual abuse was also positively related to increased incidence of suicide attempts. Taken together, these data suggest a “stressor dose-response” relationship between adverse childhood or adolescent experiences and an increased incidence of suicide attempts.
Table 2
FACTORS THAT APPEAR TO INCREASE SUICIDE RISK IN BIPOLAR DISORDER*
| Course-of-illness characteristics |
| Early onset |
| More time ill |
| More time depressed |
| More-severe depression |
| Social and medical support |
| Loss of significant other |
| Lack of a confidant |
| Single motherhood |
| Decreased access to health care |
| Lack of medical insurance |
| Demographics |
| Less education |
| Lower income |
| Being single |
| Comorbidities |
| Axis I |
| Eating disorders |
| Anxiety disorders |
| Axis II |
| Cluster A, B, C |
| Medical |
| Traumatic life events |
| Sexual abuse |
| Physical abuse |
| Genetics |
| Family history of suicide and drug abuse |
| * Identified in the SFBN cohort |
Comorbidities Other factors that appear to be associated with bipolar disorder and increased risk of attempted suicide include anxiety and eating disorders, early onset of symptoms, at least four previous hospitalizations for depression, and medical comorbidity (Table 2). Limited access to psychiatric and medical services and inadequate health insurance also were correlated with having made a serious suicide attempt. Thus, lack of medical care might exacerbate demoralization and increase the risk of suicide.
Family history of suicide or serious suicide attempts and drug abuse were associated with an increase in suicide attempts, but family history of unipolar or bipolar illness were not. These data are consistent with other studies suggesting that vulnerability to suicide may have a separate genetic component from that of mood disorders per se.
Severity of illness Increased severity of bipolar illness also was associated with prior psychosocial stressors3 and serious suicide attempts. We confirmed self-reported history, using daily mood charting, the clinician-rated Inventory of Depressive Symptomatology (IDS-C), and the Young Mania Rating Scale (YMRS) to rate increase in percentage of time ill, percentage of time depressed, and severity of depression.
Clinical implications We are uncertain how directly these data regarding suicide attempts relate to completed suicide. However, patients with bipolar disorder are among those at highest risk for completed suicide, and a prior serious attempt is often a precursor to completed suicide.5,6
Factors that appear particularly related to increased risk of suicide in patients with bipolar disorder are family history of suicide, history of early psychosocial stressors, comorbid personality disorders, substance abuse, and a relatively severe course of bipolar illness. Patients with these risk factors need support for two types of psychosocial stressors:
- poor access to health care
- stressors related to concurrent events (e.g., occurring with new affective episodes).
It may also be important for clinicians to consider the prophylactic use of lithium because of its demonstrated ability to reduce mortality in bipolar illness.7 Additionally, we need data on the efficacy of new drugs in preventing affective episodes and reducing suicidal ideation and acts.
Common comorbidities
We found considerable axis I lifetime comorbidities in the SFBN cohort (Table 3), including a 42% incidence of anxiety disorder and a similarly high rate of substance abuse. In addition to bipolar disorder, patients averaged approximately two lifetime comorbid diagnoses:
- 35% had none
- 65% had one or more
- 42% had two or more
- 24% had three or more.
Table 3
PSYCHIATRIC COMORBIDITY IN BIPOLAR OUTPATIENTS (%)*.
| Any substance abuse disorder | 42% |
| Alcohol | 33 |
| Marijuana | 16 |
| Stimulant | 9 |
| Sedative | 8 |
| Opiate | 7 |
| Hallucinogens | 6 |
| Any anxiety disorder | 42% |
| Panic disorder | 20 |
| Social phobia | 16 |
| Simple phobia | 10 |
| OCD | 9 |
| PTSD | 7 |
| GAD | 3 |
| Other | 3 |
| Any eating disorder | 11% |
| Bulimia nervosa | 8 |
| Anorexia nervosa | 4 |
| * Lifetime comorbid axis I disorders based on Structured Clinical Interview for DSM-IV (SCID) interviews with 288 SFBN patients. | |
Men with bipolar disorder had a higher absolute prevalence of alcoholism, but the relative risk compared with the general population was much higher in women (odds ratio 7.35) compared with men (odds ratio 2.77). In women, alcohol use and abuse were related to history of social phobia, greater number of depressive episodes prior to network entry, and history of abusing more than one substance. In men, alcohol comorbidity was related to history of alcohol abuse in first-degree relatives and history of early physical abuse.
These data suggest that female patients, in particular, were self-medicating their affective episodes and comorbid anxiety disorders with alcohol. They suggest the importance of asking patients directly about alcohol use to identify the need for primary and secondary prevention. In an adolescent or young adult, affective illness—and bipolar disorder in particular—should be considered a major risk factor for subsequent problems with alcohol and drug abuse. When taking a careful history, the treating physician should include questions regarding any substance abuse.
Overweight and obesity
One-half of the women and two-thirds of the men in the SFBN were overweight at network entry.8 Although this rate of overweight sounds alarmingly high, it was not significantly different from rates in the general U.S. population. A greater percentage of women than men in the network had morbid obesity, however.
In our cohort, being overweight or obese was associated with an increased incidence of cardiovascular disease, diabetes, arthritis, hypertension, hypothyroidism, and cancer. The degree of obesity also correlated significantly with past use of the numerous psychotropic drugs that have been associated with weight gain. Also, as might be expected, increased weight was associated with physical inactivity.
Many of the psychotropic drugs routinely used to treat bipolar disorder are associated with weight gain. Among these are the mood stabilizers lithium and valproate and the atypical antipsychotics, with the exception of ziprasidone (and, to follow, aripiprazole). Lithium’s liability to cause weight gain may be counterbalanced by its antisuicide effects and its ability to reverse excess medical mortality associated with affective illness. Thus, lithium should continue to play a major therapeutic role in bipolar disorder.
It may be more effective and easier to prevent weight gain than to attempt to reduce weight that has been gained. Recommending exercise and other weight-reduction measures may be helpful, as may co-administering drugs associated with weight loss, such as topiramate.9
Thyroiditis
Antithyroid antibodies were detected in 28% of network patients, compared with rates of 3% in the general population and 18% in psychiatric controls.10 The increased rate was associated with the need to augment thyroid function in 17% of network patients, but it was not related to age, mood state, rapid cycling status, or lithium treatment.
These data indicate that bipolar patients are prone to autoimmune thyroid changes. Further analysis is required to ascertain whether this finding has therapeutic implications, especially related to adjunctive T3 and T4 treatment.11
Even with psychiatry’s most effective and best studied agents, many patients with bipolar disorder are inadequately treated and respond insufficiently to available psychopharmacologic agents. To address this problem, the Stanley Foundation Bipolar Network (SFBN) was created to explore acute and long-term response to treatment of bipolar disorder.
For the past 6 years, our group (Box) has followed an international cohort of patients with bipolar disorder and offered them opportunities to participate in clinical trials at seven field centers. We have made considerable progress as we focused on the longitudinal course of bipolar illness and evaluated various combinations of psychopharmacologic therapies. The SFBN also has explored factors that increase the risk of suicide, quantified the incidence of anxiety disorder and substance abuse, and looked at other variables that may affect treatment outcome.
This report for the readers of Current Psychiatry provides a brief state-of-the-art overview of what we have learned about the course and management of bipolar disorder. It brings together and summarizes the findings from studies published, in press, or under review. Our goal is to update current findings, which may help you provide optimum care for these at-risk patients.
Since Robert Prien’s pioneering studies in the 1970s, the National Institute of Mental Health (NIMH) had funded little work on prophylaxis of bipolar disorders or acute bipolar depression. Following two NIMH conferences on bipolar illness in 1989 and 1994, the Stanley Foundation Bipolar Network (SFBN) was established to help overcome these research deficiencies, with funding provided by the Theodore and Vada Stanley Foundation.
Patients were recruited into the network from the general community and with the use of newspaper and newsletter advertisements. In contrast to many other clinical populations, few who wished to enter the network were excluded. It was clear, however, that filling out demographic, clinical, and rating forms would require considerable effort from each patient. All patients gave written informed consent to participate in the naturalistic part of the study, and were reconsented for any open or blind randomized clinical trial.
Patients provided daily self-ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM), a unique longitudinal instrument. Clinicians then completed their own separate LCMs, as more severely ill patients often could not complete their self-ratings, and patients often underestimated the severity and functional disability associated with the hypomanic and manic phases of bipolar illness. With the use of the NIMH-LCM, network investigators were able, for the first time, to assess the details of mood fluctuations associated with bipolar illness and to more precisely delineate the qualitative and quantitative aspects of response to psychopharmacologic interventions.
The SFBN includes seven research field centers in the United States, the Netherlands, and Germany. The leaders of those centers collaborated with Robert M. Post, MD, to prepare this report of the network findings. The collaborators are:
| Gabriele S. Leverich, MSW, and Kirk D. Denicoff, MD Biological Psychiatry Branch National Institute of Mental Health Bethesda, MD | Paul E. Keck, Jr., MD, and Susan L. McElroy, MD Biological Psychiatry Program University of Cincinnati College of Medicine Cincinnati, OH |
| Lori L. Altshuler, MD, and Mark A. Frye, MD UCLA Mood Disorders Research Program VA Medical Center Los Angeles, CA | Willem A. Nolen, MD, and Ralph Kupka, MD Altrecht Institute for Mental Health Care University Medical Center Utrecht, The Netherlands |
| John Rush, MD, and Trisha Suppes, MD Department of Psychiatry Southwest Medical Center University of Texas, Dallas | Joerg Walden, MD Department of Psychiatry University of Freiburg Freiburg, Germany |
| Keith Kramlinger, MD Mayo Clinic, Rochester, MN (Administrative center) | Heinz Grunze, MD Psychiatrische Klinik der LMU Munich, Germany |
In part one, we discuss the demographics of our cohort of 676 community-based bipolar patients, including risk factors for suicide attempts, frequency of comorbid psychiatric diagnoses, problems of overweight and obesity, and preliminary findings about thyroiditis.
In part two, we describe our clinical trials assessing mood stabilizers and adjunctive agents such as atypical antipsychotics, second-generation antidepressants, and anticonvulsants. We also will address a soon-to-be analyzed study of omega-3 fatty acids.
Table 1
CHARACTERISTICS OF THE 676 BIPOLAR OUTPATIENTS
| Mean age | 41 | |
| % Female | 43 | |
| Diagnosis (%) | ||
| Bipolar I | 79 | |
| Bipolar II | 16 | |
| Bipolar NOS | 4 | |
| Marital status (%) | ||
| Married/cohabitating | 44 | |
| Divorced/separated | 22 | |
| Income (%) | ||
| <$20,000 | 37 | |
| $20,000-40,000 | 22 | |
| $40,000-100,000 | 32 | |
| >$100,000 | 9 | |
| Education (completed, %) | ||
| High school | 34 | |
| Jr. college/technical | 13 | |
| College, 4-year | 27 | |
| Graduate degree | 16 | |
| Positive family history (1st-degree relatives, %) | ||
| Bipolar disorder | 56 | |
| Unipolar disorder | 64 | |
| Schizophrenia | 24 | |
| Drug dependence | 44 | |
| Other psychiatric disorder | 52 | |
| Alcohol abuse/dependence | 52 | |
| Suicide (complete or serious attempt) | 38 | |
| Environmental adversity (%): | Child/adolescent | Adult |
| Physical abuse | 29 | 8 |
| Sexual abuse | 30 | 8 |
| Illness course and treatment (age in yrs) | ||
| Age of onset (symptoms) | 19 | |
| Early onset (age <14) | 34 | |
| Age at first treatment | 29 | |
| Age at first hospitalization | 30 | |
| Cycling pattern (n = 643, %) | ||
| Rapid (or faster cycling) | 47 | |
| Ultrarapid (or faster cycling) | 30 | |
| Rapid-cycling only | 17 | |
| Ultrarapid cycling only | 10 | |
| Ultradian cycling | 20 | |
Study population
The SFBN recruited outpatients with bipolar disorders I, II, or not otherwise specified (NOS). We did not exclude patients with comorbid illnesses, except those with active substance abuse disorder requiring treatment in another setting.1 Patients were recruited almost exclusively from the community and rarely from inpatient hospitalization. The average age was 41, and 43% were college graduates. Most were employed but indicated that their illness limited their employment level or ability to function at work (Table 1).
One striking finding was a decadelong lag between the onset of symptoms reaching thresholds for diagnosis and the onset of first treatment.2 This long delay could have adverse consequences for illness outcome and represents a major public health target. Age of onset appeared to have both genetic/familial and environmental determinants.3 Subjects with a history of both extreme early life adversities (i.e., physical or sexual abuse) and a positive family history of mood disorder had the earliest onset of bipolar illness. Those who had neither of these factors had late-onset illness.
Suicide attempts
Prior to network entry, 25 to 50% of our study population had made a serious suicide attempt (defined as requiring medical attention and/or hospitalization), pointing to bipolar disorder’s potential lethality.
History of abuse A history of physical or sexual abuse in childhood or adolescence was a prominent risk factor for attempted suicide.4 With either type of abuse there was an approximate 15% increase in incidence of suicide attempts over the baseline rate of about 25%. More than 55% of patients with a history of both physical and sexual abuse had made a serious suicide attempt. The frequency of physical and sexual abuse was also positively related to increased incidence of suicide attempts. Taken together, these data suggest a “stressor dose-response” relationship between adverse childhood or adolescent experiences and an increased incidence of suicide attempts.
Table 2
FACTORS THAT APPEAR TO INCREASE SUICIDE RISK IN BIPOLAR DISORDER*
| Course-of-illness characteristics |
| Early onset |
| More time ill |
| More time depressed |
| More-severe depression |
| Social and medical support |
| Loss of significant other |
| Lack of a confidant |
| Single motherhood |
| Decreased access to health care |
| Lack of medical insurance |
| Demographics |
| Less education |
| Lower income |
| Being single |
| Comorbidities |
| Axis I |
| Eating disorders |
| Anxiety disorders |
| Axis II |
| Cluster A, B, C |
| Medical |
| Traumatic life events |
| Sexual abuse |
| Physical abuse |
| Genetics |
| Family history of suicide and drug abuse |
| * Identified in the SFBN cohort |
Comorbidities Other factors that appear to be associated with bipolar disorder and increased risk of attempted suicide include anxiety and eating disorders, early onset of symptoms, at least four previous hospitalizations for depression, and medical comorbidity (Table 2). Limited access to psychiatric and medical services and inadequate health insurance also were correlated with having made a serious suicide attempt. Thus, lack of medical care might exacerbate demoralization and increase the risk of suicide.
Family history of suicide or serious suicide attempts and drug abuse were associated with an increase in suicide attempts, but family history of unipolar or bipolar illness were not. These data are consistent with other studies suggesting that vulnerability to suicide may have a separate genetic component from that of mood disorders per se.
Severity of illness Increased severity of bipolar illness also was associated with prior psychosocial stressors3 and serious suicide attempts. We confirmed self-reported history, using daily mood charting, the clinician-rated Inventory of Depressive Symptomatology (IDS-C), and the Young Mania Rating Scale (YMRS) to rate increase in percentage of time ill, percentage of time depressed, and severity of depression.
Clinical implications We are uncertain how directly these data regarding suicide attempts relate to completed suicide. However, patients with bipolar disorder are among those at highest risk for completed suicide, and a prior serious attempt is often a precursor to completed suicide.5,6
Factors that appear particularly related to increased risk of suicide in patients with bipolar disorder are family history of suicide, history of early psychosocial stressors, comorbid personality disorders, substance abuse, and a relatively severe course of bipolar illness. Patients with these risk factors need support for two types of psychosocial stressors:
- poor access to health care
- stressors related to concurrent events (e.g., occurring with new affective episodes).
It may also be important for clinicians to consider the prophylactic use of lithium because of its demonstrated ability to reduce mortality in bipolar illness.7 Additionally, we need data on the efficacy of new drugs in preventing affective episodes and reducing suicidal ideation and acts.
Common comorbidities
We found considerable axis I lifetime comorbidities in the SFBN cohort (Table 3), including a 42% incidence of anxiety disorder and a similarly high rate of substance abuse. In addition to bipolar disorder, patients averaged approximately two lifetime comorbid diagnoses:
- 35% had none
- 65% had one or more
- 42% had two or more
- 24% had three or more.
Table 3
PSYCHIATRIC COMORBIDITY IN BIPOLAR OUTPATIENTS (%)*.
| Any substance abuse disorder | 42% |
| Alcohol | 33 |
| Marijuana | 16 |
| Stimulant | 9 |
| Sedative | 8 |
| Opiate | 7 |
| Hallucinogens | 6 |
| Any anxiety disorder | 42% |
| Panic disorder | 20 |
| Social phobia | 16 |
| Simple phobia | 10 |
| OCD | 9 |
| PTSD | 7 |
| GAD | 3 |
| Other | 3 |
| Any eating disorder | 11% |
| Bulimia nervosa | 8 |
| Anorexia nervosa | 4 |
| * Lifetime comorbid axis I disorders based on Structured Clinical Interview for DSM-IV (SCID) interviews with 288 SFBN patients. | |
Men with bipolar disorder had a higher absolute prevalence of alcoholism, but the relative risk compared with the general population was much higher in women (odds ratio 7.35) compared with men (odds ratio 2.77). In women, alcohol use and abuse were related to history of social phobia, greater number of depressive episodes prior to network entry, and history of abusing more than one substance. In men, alcohol comorbidity was related to history of alcohol abuse in first-degree relatives and history of early physical abuse.
These data suggest that female patients, in particular, were self-medicating their affective episodes and comorbid anxiety disorders with alcohol. They suggest the importance of asking patients directly about alcohol use to identify the need for primary and secondary prevention. In an adolescent or young adult, affective illness—and bipolar disorder in particular—should be considered a major risk factor for subsequent problems with alcohol and drug abuse. When taking a careful history, the treating physician should include questions regarding any substance abuse.
Overweight and obesity
One-half of the women and two-thirds of the men in the SFBN were overweight at network entry.8 Although this rate of overweight sounds alarmingly high, it was not significantly different from rates in the general U.S. population. A greater percentage of women than men in the network had morbid obesity, however.
In our cohort, being overweight or obese was associated with an increased incidence of cardiovascular disease, diabetes, arthritis, hypertension, hypothyroidism, and cancer. The degree of obesity also correlated significantly with past use of the numerous psychotropic drugs that have been associated with weight gain. Also, as might be expected, increased weight was associated with physical inactivity.
Many of the psychotropic drugs routinely used to treat bipolar disorder are associated with weight gain. Among these are the mood stabilizers lithium and valproate and the atypical antipsychotics, with the exception of ziprasidone (and, to follow, aripiprazole). Lithium’s liability to cause weight gain may be counterbalanced by its antisuicide effects and its ability to reverse excess medical mortality associated with affective illness. Thus, lithium should continue to play a major therapeutic role in bipolar disorder.
It may be more effective and easier to prevent weight gain than to attempt to reduce weight that has been gained. Recommending exercise and other weight-reduction measures may be helpful, as may co-administering drugs associated with weight loss, such as topiramate.9
Thyroiditis
Antithyroid antibodies were detected in 28% of network patients, compared with rates of 3% in the general population and 18% in psychiatric controls.10 The increased rate was associated with the need to augment thyroid function in 17% of network patients, but it was not related to age, mood state, rapid cycling status, or lithium treatment.
These data indicate that bipolar patients are prone to autoimmune thyroid changes. Further analysis is required to ascertain whether this finding has therapeutic implications, especially related to adjunctive T3 and T4 treatment.11
1. Leverich GS, Nolen WA, Rush AJ, et al. The Stanley Foundation Bipolar Treatment Outcome Network. I. Longitudinal methodology. J Affect Disord 2001;67:33-44.
2. Suppes T, Leverich GS, Keck PE, et al. The Stanley Foundation Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disord 2001b;67:45-59.
3. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological Psychiatry 2002;51:288-97.
4. Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. Manuscript submitted for publication, 2002.
5. Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39:896-9.
6. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch.Gen.Psychiatry 1999;56:617-26.
7. Ahrens B, Muller-Oerlinghausen B, Schou M, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord 1995;33:67-75.
8. McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry 2002;63:207-13.
9. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biological Psychiatry 2000;47:1025-33.
10. Kupka RW, Nolen WA, Post RM, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biological Psychiatry 2002;51:305-11.
11. Bauer M, Priebe S, Berghofer A, et al. Subjective response to and tolerability of long-term supraphysiological doses of levothyroxine in refractory mood disorders. J Affect Disord 2001;64:35-42.
1. Leverich GS, Nolen WA, Rush AJ, et al. The Stanley Foundation Bipolar Treatment Outcome Network. I. Longitudinal methodology. J Affect Disord 2001;67:33-44.
2. Suppes T, Leverich GS, Keck PE, et al. The Stanley Foundation Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disord 2001b;67:45-59.
3. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological Psychiatry 2002;51:288-97.
4. Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. Manuscript submitted for publication, 2002.
5. Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39:896-9.
6. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch.Gen.Psychiatry 1999;56:617-26.
7. Ahrens B, Muller-Oerlinghausen B, Schou M, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord 1995;33:67-75.
8. McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry 2002;63:207-13.
9. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biological Psychiatry 2000;47:1025-33.
10. Kupka RW, Nolen WA, Post RM, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biological Psychiatry 2002;51:305-11.
11. Bauer M, Priebe S, Berghofer A, et al. Subjective response to and tolerability of long-term supraphysiological doses of levothyroxine in refractory mood disorders. J Affect Disord 2001;64:35-42.
From the Stanley Foundation Bipolar Network: New findings on suicide attempts, substance abuse, obesity, and more
Even with psychiatry’s most effective and best studied agents, many patients with bipolar disorder are inadequately treated and respond insufficiently to available psychopharmacologic agents. To address this problem, the Stanley Foundation Bipolar Network (SFBN) was created to explore acute and long-term response to treatment of bipolar disorder.
For the past 6 years, our group (Box) has followed an international cohort of patients with bipolar disorder and offered them opportunities to participate in clinical trials at seven field centers. We have made considerable progress as we focused on the longitudinal course of bipolar illness and evaluated various combinations of psychopharmacologic therapies. The SFBN also has explored factors that increase the risk of suicide, quantified the incidence of anxiety disorder and substance abuse, and looked at other variables that may affect treatment outcome.
This report for the readers of Current Psychiatry provides a brief state-of-the-art overview of what we have learned about the course and management of bipolar disorder. It brings together and summarizes the findings from studies published, in press, or under review. Our goal is to update current findings, which may help you provide optimum care for these at-risk patients.
Since Robert Prien’s pioneering studies in the 1970s, the National Institute of Mental Health (NIMH) had funded little work on prophylaxis of bipolar disorders or acute bipolar depression. Following two NIMH conferences on bipolar illness in 1989 and 1994, the Stanley Foundation Bipolar Network (SFBN) was established to help overcome these research deficiencies, with funding provided by the Theodore and Vada Stanley Foundation.
Patients were recruited into the network from the general community and with the use of newspaper and newsletter advertisements. In contrast to many other clinical populations, few who wished to enter the network were excluded. It was clear, however, that filling out demographic, clinical, and rating forms would require considerable effort from each patient. All patients gave written informed consent to participate in the naturalistic part of the study, and were reconsented for any open or blind randomized clinical trial.
Patients provided daily self-ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM), a unique longitudinal instrument. Clinicians then completed their own separate LCMs, as more severely ill patients often could not complete their self-ratings, and patients often underestimated the severity and functional disability associated with the hypomanic and manic phases of bipolar illness. With the use of the NIMH-LCM, network investigators were able, for the first time, to assess the details of mood fluctuations associated with bipolar illness and to more precisely delineate the qualitative and quantitative aspects of response to psychopharmacologic interventions.
The SFBN includes seven research field centers in the United States, the Netherlands, and Germany. The leaders of those centers collaborated with Robert M. Post, MD, to prepare this report of the network findings. The collaborators are:
| Gabriele S. Leverich, MSW, and Kirk D. Denicoff, MD Biological Psychiatry Branch National Institute of Mental Health Bethesda, MD | Paul E. Keck, Jr., MD, and Susan L. McElroy, MD Biological Psychiatry Program University of Cincinnati College of Medicine Cincinnati, OH |
| Lori L. Altshuler, MD, and Mark A. Frye, MD UCLA Mood Disorders Research Program VA Medical Center Los Angeles, CA | Willem A. Nolen, MD, and Ralph Kupka, MD Altrecht Institute for Mental Health Care University Medical Center Utrecht, The Netherlands |
| John Rush, MD, and Trisha Suppes, MD Department of Psychiatry Southwest Medical Center University of Texas, Dallas | Joerg Walden, MD Department of Psychiatry University of Freiburg Freiburg, Germany |
| Keith Kramlinger, MD Mayo Clinic, Rochester, MN (Administrative center) | Heinz Grunze, MD Psychiatrische Klinik der LMU Munich, Germany |
In part one, we discuss the demographics of our cohort of 676 community-based bipolar patients, including risk factors for suicide attempts, frequency of comorbid psychiatric diagnoses, problems of overweight and obesity, and preliminary findings about thyroiditis.
In part two, we describe our clinical trials assessing mood stabilizers and adjunctive agents such as atypical antipsychotics, second-generation antidepressants, and anticonvulsants. We also will address a soon-to-be analyzed study of omega-3 fatty acids.
Table 1
CHARACTERISTICS OF THE 676 BIPOLAR OUTPATIENTS
| Mean age | 41 | |
| % Female | 43 | |
| Diagnosis (%) | ||
| Bipolar I | 79 | |
| Bipolar II | 16 | |
| Bipolar NOS | 4 | |
| Marital status (%) | ||
| Married/cohabitating | 44 | |
| Divorced/separated | 22 | |
| Income (%) | ||
| <$20,000 | 37 | |
| $20,000-40,000 | 22 | |
| $40,000-100,000 | 32 | |
| >$100,000 | 9 | |
| Education (completed, %) | ||
| High school | 34 | |
| Jr. college/technical | 13 | |
| College, 4-year | 27 | |
| Graduate degree | 16 | |
| Positive family history (1st-degree relatives, %) | ||
| Bipolar disorder | 56 | |
| Unipolar disorder | 64 | |
| Schizophrenia | 24 | |
| Drug dependence | 44 | |
| Other psychiatric disorder | 52 | |
| Alcohol abuse/dependence | 52 | |
| Suicide (complete or serious attempt) | 38 | |
| Environmental adversity (%): | Child/adolescent | Adult |
| Physical abuse | 29 | 8 |
| Sexual abuse | 30 | 8 |
| Illness course and treatment (age in yrs) | ||
| Age of onset (symptoms) | 19 | |
| Early onset (age <14) | 34 | |
| Age at first treatment | 29 | |
| Age at first hospitalization | 30 | |
| Cycling pattern (n = 643, %) | ||
| Rapid (or faster cycling) | 47 | |
| Ultrarapid (or faster cycling) | 30 | |
| Rapid-cycling only | 17 | |
| Ultrarapid cycling only | 10 | |
| Ultradian cycling | 20 | |
Study population
The SFBN recruited outpatients with bipolar disorders I, II, or not otherwise specified (NOS). We did not exclude patients with comorbid illnesses, except those with active substance abuse disorder requiring treatment in another setting.1 Patients were recruited almost exclusively from the community and rarely from inpatient hospitalization. The average age was 41, and 43% were college graduates. Most were employed but indicated that their illness limited their employment level or ability to function at work (Table 1).
One striking finding was a decadelong lag between the onset of symptoms reaching thresholds for diagnosis and the onset of first treatment.2 This long delay could have adverse consequences for illness outcome and represents a major public health target. Age of onset appeared to have both genetic/familial and environmental determinants.3 Subjects with a history of both extreme early life adversities (i.e., physical or sexual abuse) and a positive family history of mood disorder had the earliest onset of bipolar illness. Those who had neither of these factors had late-onset illness.
Suicide attempts
Prior to network entry, 25 to 50% of our study population had made a serious suicide attempt (defined as requiring medical attention and/or hospitalization), pointing to bipolar disorder’s potential lethality.
History of abuse A history of physical or sexual abuse in childhood or adolescence was a prominent risk factor for attempted suicide.4 With either type of abuse there was an approximate 15% increase in incidence of suicide attempts over the baseline rate of about 25%. More than 55% of patients with a history of both physical and sexual abuse had made a serious suicide attempt. The frequency of physical and sexual abuse was also positively related to increased incidence of suicide attempts. Taken together, these data suggest a “stressor dose-response” relationship between adverse childhood or adolescent experiences and an increased incidence of suicide attempts.
Table 2
FACTORS THAT APPEAR TO INCREASE SUICIDE RISK IN BIPOLAR DISORDER*
| Course-of-illness characteristics |
| Early onset |
| More time ill |
| More time depressed |
| More-severe depression |
| Social and medical support |
| Loss of significant other |
| Lack of a confidant |
| Single motherhood |
| Decreased access to health care |
| Lack of medical insurance |
| Demographics |
| Less education |
| Lower income |
| Being single |
| Comorbidities |
| Axis I |
| Eating disorders |
| Anxiety disorders |
| Axis II |
| Cluster A, B, C |
| Medical |
| Traumatic life events |
| Sexual abuse |
| Physical abuse |
| Genetics |
| Family history of suicide and drug abuse |
| * Identified in the SFBN cohort |
Comorbidities Other factors that appear to be associated with bipolar disorder and increased risk of attempted suicide include anxiety and eating disorders, early onset of symptoms, at least four previous hospitalizations for depression, and medical comorbidity (Table 2). Limited access to psychiatric and medical services and inadequate health insurance also were correlated with having made a serious suicide attempt. Thus, lack of medical care might exacerbate demoralization and increase the risk of suicide.
Family history of suicide or serious suicide attempts and drug abuse were associated with an increase in suicide attempts, but family history of unipolar or bipolar illness were not. These data are consistent with other studies suggesting that vulnerability to suicide may have a separate genetic component from that of mood disorders per se.
Severity of illness Increased severity of bipolar illness also was associated with prior psychosocial stressors3 and serious suicide attempts. We confirmed self-reported history, using daily mood charting, the clinician-rated Inventory of Depressive Symptomatology (IDS-C), and the Young Mania Rating Scale (YMRS) to rate increase in percentage of time ill, percentage of time depressed, and severity of depression.
Clinical implications We are uncertain how directly these data regarding suicide attempts relate to completed suicide. However, patients with bipolar disorder are among those at highest risk for completed suicide, and a prior serious attempt is often a precursor to completed suicide.5,6
Factors that appear particularly related to increased risk of suicide in patients with bipolar disorder are family history of suicide, history of early psychosocial stressors, comorbid personality disorders, substance abuse, and a relatively severe course of bipolar illness. Patients with these risk factors need support for two types of psychosocial stressors:
- poor access to health care
- stressors related to concurrent events (e.g., occurring with new affective episodes).
It may also be important for clinicians to consider the prophylactic use of lithium because of its demonstrated ability to reduce mortality in bipolar illness.7 Additionally, we need data on the efficacy of new drugs in preventing affective episodes and reducing suicidal ideation and acts.
Common comorbidities
We found considerable axis I lifetime comorbidities in the SFBN cohort (Table 3), including a 42% incidence of anxiety disorder and a similarly high rate of substance abuse. In addition to bipolar disorder, patients averaged approximately two lifetime comorbid diagnoses:
- 35% had none
- 65% had one or more
- 42% had two or more
- 24% had three or more.
Table 3
PSYCHIATRIC COMORBIDITY IN BIPOLAR OUTPATIENTS (%)*.
| Any substance abuse disorder | 42% |
| Alcohol | 33 |
| Marijuana | 16 |
| Stimulant | 9 |
| Sedative | 8 |
| Opiate | 7 |
| Hallucinogens | 6 |
| Any anxiety disorder | 42% |
| Panic disorder | 20 |
| Social phobia | 16 |
| Simple phobia | 10 |
| OCD | 9 |
| PTSD | 7 |
| GAD | 3 |
| Other | 3 |
| Any eating disorder | 11% |
| Bulimia nervosa | 8 |
| Anorexia nervosa | 4 |
| * Lifetime comorbid axis I disorders based on Structured Clinical Interview for DSM-IV (SCID) interviews with 288 SFBN patients. | |
Men with bipolar disorder had a higher absolute prevalence of alcoholism, but the relative risk compared with the general population was much higher in women (odds ratio 7.35) compared with men (odds ratio 2.77). In women, alcohol use and abuse were related to history of social phobia, greater number of depressive episodes prior to network entry, and history of abusing more than one substance. In men, alcohol comorbidity was related to history of alcohol abuse in first-degree relatives and history of early physical abuse.
These data suggest that female patients, in particular, were self-medicating their affective episodes and comorbid anxiety disorders with alcohol. They suggest the importance of asking patients directly about alcohol use to identify the need for primary and secondary prevention. In an adolescent or young adult, affective illness—and bipolar disorder in particular—should be considered a major risk factor for subsequent problems with alcohol and drug abuse. When taking a careful history, the treating physician should include questions regarding any substance abuse.
Overweight and obesity
One-half of the women and two-thirds of the men in the SFBN were overweight at network entry.8 Although this rate of overweight sounds alarmingly high, it was not significantly different from rates in the general U.S. population. A greater percentage of women than men in the network had morbid obesity, however.
In our cohort, being overweight or obese was associated with an increased incidence of cardiovascular disease, diabetes, arthritis, hypertension, hypothyroidism, and cancer. The degree of obesity also correlated significantly with past use of the numerous psychotropic drugs that have been associated with weight gain. Also, as might be expected, increased weight was associated with physical inactivity.
Many of the psychotropic drugs routinely used to treat bipolar disorder are associated with weight gain. Among these are the mood stabilizers lithium and valproate and the atypical antipsychotics, with the exception of ziprasidone (and, to follow, aripiprazole). Lithium’s liability to cause weight gain may be counterbalanced by its antisuicide effects and its ability to reverse excess medical mortality associated with affective illness. Thus, lithium should continue to play a major therapeutic role in bipolar disorder.
It may be more effective and easier to prevent weight gain than to attempt to reduce weight that has been gained. Recommending exercise and other weight-reduction measures may be helpful, as may co-administering drugs associated with weight loss, such as topiramate.9
Thyroiditis
Antithyroid antibodies were detected in 28% of network patients, compared with rates of 3% in the general population and 18% in psychiatric controls.10 The increased rate was associated with the need to augment thyroid function in 17% of network patients, but it was not related to age, mood state, rapid cycling status, or lithium treatment.
These data indicate that bipolar patients are prone to autoimmune thyroid changes. Further analysis is required to ascertain whether this finding has therapeutic implications, especially related to adjunctive T3 and T4 treatment.11
1. Leverich GS, Nolen WA, Rush AJ, et al. The Stanley Foundation Bipolar Treatment Outcome Network. I. Longitudinal methodology. J Affect Disord 2001;67:33-44.
2. Suppes T, Leverich GS, Keck PE, et al. The Stanley Foundation Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disord 2001b;67:45-59.
3. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological Psychiatry 2002;51:288-97.
4. Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. Manuscript submitted for publication, 2002.
5. Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39:896-9.
6. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch.Gen.Psychiatry 1999;56:617-26.
7. Ahrens B, Muller-Oerlinghausen B, Schou M, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord 1995;33:67-75.
8. McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry 2002;63:207-13.
9. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biological Psychiatry 2000;47:1025-33.
10. Kupka RW, Nolen WA, Post RM, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biological Psychiatry 2002;51:305-11.
11. Bauer M, Priebe S, Berghofer A, et al. Subjective response to and tolerability of long-term supraphysiological doses of levothyroxine in refractory mood disorders. J Affect Disord 2001;64:35-42.
Even with psychiatry’s most effective and best studied agents, many patients with bipolar disorder are inadequately treated and respond insufficiently to available psychopharmacologic agents. To address this problem, the Stanley Foundation Bipolar Network (SFBN) was created to explore acute and long-term response to treatment of bipolar disorder.
For the past 6 years, our group (Box) has followed an international cohort of patients with bipolar disorder and offered them opportunities to participate in clinical trials at seven field centers. We have made considerable progress as we focused on the longitudinal course of bipolar illness and evaluated various combinations of psychopharmacologic therapies. The SFBN also has explored factors that increase the risk of suicide, quantified the incidence of anxiety disorder and substance abuse, and looked at other variables that may affect treatment outcome.
This report for the readers of Current Psychiatry provides a brief state-of-the-art overview of what we have learned about the course and management of bipolar disorder. It brings together and summarizes the findings from studies published, in press, or under review. Our goal is to update current findings, which may help you provide optimum care for these at-risk patients.
Since Robert Prien’s pioneering studies in the 1970s, the National Institute of Mental Health (NIMH) had funded little work on prophylaxis of bipolar disorders or acute bipolar depression. Following two NIMH conferences on bipolar illness in 1989 and 1994, the Stanley Foundation Bipolar Network (SFBN) was established to help overcome these research deficiencies, with funding provided by the Theodore and Vada Stanley Foundation.
Patients were recruited into the network from the general community and with the use of newspaper and newsletter advertisements. In contrast to many other clinical populations, few who wished to enter the network were excluded. It was clear, however, that filling out demographic, clinical, and rating forms would require considerable effort from each patient. All patients gave written informed consent to participate in the naturalistic part of the study, and were reconsented for any open or blind randomized clinical trial.
Patients provided daily self-ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM), a unique longitudinal instrument. Clinicians then completed their own separate LCMs, as more severely ill patients often could not complete their self-ratings, and patients often underestimated the severity and functional disability associated with the hypomanic and manic phases of bipolar illness. With the use of the NIMH-LCM, network investigators were able, for the first time, to assess the details of mood fluctuations associated with bipolar illness and to more precisely delineate the qualitative and quantitative aspects of response to psychopharmacologic interventions.
The SFBN includes seven research field centers in the United States, the Netherlands, and Germany. The leaders of those centers collaborated with Robert M. Post, MD, to prepare this report of the network findings. The collaborators are:
| Gabriele S. Leverich, MSW, and Kirk D. Denicoff, MD Biological Psychiatry Branch National Institute of Mental Health Bethesda, MD | Paul E. Keck, Jr., MD, and Susan L. McElroy, MD Biological Psychiatry Program University of Cincinnati College of Medicine Cincinnati, OH |
| Lori L. Altshuler, MD, and Mark A. Frye, MD UCLA Mood Disorders Research Program VA Medical Center Los Angeles, CA | Willem A. Nolen, MD, and Ralph Kupka, MD Altrecht Institute for Mental Health Care University Medical Center Utrecht, The Netherlands |
| John Rush, MD, and Trisha Suppes, MD Department of Psychiatry Southwest Medical Center University of Texas, Dallas | Joerg Walden, MD Department of Psychiatry University of Freiburg Freiburg, Germany |
| Keith Kramlinger, MD Mayo Clinic, Rochester, MN (Administrative center) | Heinz Grunze, MD Psychiatrische Klinik der LMU Munich, Germany |
In part one, we discuss the demographics of our cohort of 676 community-based bipolar patients, including risk factors for suicide attempts, frequency of comorbid psychiatric diagnoses, problems of overweight and obesity, and preliminary findings about thyroiditis.
In part two, we describe our clinical trials assessing mood stabilizers and adjunctive agents such as atypical antipsychotics, second-generation antidepressants, and anticonvulsants. We also will address a soon-to-be analyzed study of omega-3 fatty acids.
Table 1
CHARACTERISTICS OF THE 676 BIPOLAR OUTPATIENTS
| Mean age | 41 | |
| % Female | 43 | |
| Diagnosis (%) | ||
| Bipolar I | 79 | |
| Bipolar II | 16 | |
| Bipolar NOS | 4 | |
| Marital status (%) | ||
| Married/cohabitating | 44 | |
| Divorced/separated | 22 | |
| Income (%) | ||
| <$20,000 | 37 | |
| $20,000-40,000 | 22 | |
| $40,000-100,000 | 32 | |
| >$100,000 | 9 | |
| Education (completed, %) | ||
| High school | 34 | |
| Jr. college/technical | 13 | |
| College, 4-year | 27 | |
| Graduate degree | 16 | |
| Positive family history (1st-degree relatives, %) | ||
| Bipolar disorder | 56 | |
| Unipolar disorder | 64 | |
| Schizophrenia | 24 | |
| Drug dependence | 44 | |
| Other psychiatric disorder | 52 | |
| Alcohol abuse/dependence | 52 | |
| Suicide (complete or serious attempt) | 38 | |
| Environmental adversity (%): | Child/adolescent | Adult |
| Physical abuse | 29 | 8 |
| Sexual abuse | 30 | 8 |
| Illness course and treatment (age in yrs) | ||
| Age of onset (symptoms) | 19 | |
| Early onset (age <14) | 34 | |
| Age at first treatment | 29 | |
| Age at first hospitalization | 30 | |
| Cycling pattern (n = 643, %) | ||
| Rapid (or faster cycling) | 47 | |
| Ultrarapid (or faster cycling) | 30 | |
| Rapid-cycling only | 17 | |
| Ultrarapid cycling only | 10 | |
| Ultradian cycling | 20 | |
Study population
The SFBN recruited outpatients with bipolar disorders I, II, or not otherwise specified (NOS). We did not exclude patients with comorbid illnesses, except those with active substance abuse disorder requiring treatment in another setting.1 Patients were recruited almost exclusively from the community and rarely from inpatient hospitalization. The average age was 41, and 43% were college graduates. Most were employed but indicated that their illness limited their employment level or ability to function at work (Table 1).
One striking finding was a decadelong lag between the onset of symptoms reaching thresholds for diagnosis and the onset of first treatment.2 This long delay could have adverse consequences for illness outcome and represents a major public health target. Age of onset appeared to have both genetic/familial and environmental determinants.3 Subjects with a history of both extreme early life adversities (i.e., physical or sexual abuse) and a positive family history of mood disorder had the earliest onset of bipolar illness. Those who had neither of these factors had late-onset illness.
Suicide attempts
Prior to network entry, 25 to 50% of our study population had made a serious suicide attempt (defined as requiring medical attention and/or hospitalization), pointing to bipolar disorder’s potential lethality.
History of abuse A history of physical or sexual abuse in childhood or adolescence was a prominent risk factor for attempted suicide.4 With either type of abuse there was an approximate 15% increase in incidence of suicide attempts over the baseline rate of about 25%. More than 55% of patients with a history of both physical and sexual abuse had made a serious suicide attempt. The frequency of physical and sexual abuse was also positively related to increased incidence of suicide attempts. Taken together, these data suggest a “stressor dose-response” relationship between adverse childhood or adolescent experiences and an increased incidence of suicide attempts.
Table 2
FACTORS THAT APPEAR TO INCREASE SUICIDE RISK IN BIPOLAR DISORDER*
| Course-of-illness characteristics |
| Early onset |
| More time ill |
| More time depressed |
| More-severe depression |
| Social and medical support |
| Loss of significant other |
| Lack of a confidant |
| Single motherhood |
| Decreased access to health care |
| Lack of medical insurance |
| Demographics |
| Less education |
| Lower income |
| Being single |
| Comorbidities |
| Axis I |
| Eating disorders |
| Anxiety disorders |
| Axis II |
| Cluster A, B, C |
| Medical |
| Traumatic life events |
| Sexual abuse |
| Physical abuse |
| Genetics |
| Family history of suicide and drug abuse |
| * Identified in the SFBN cohort |
Comorbidities Other factors that appear to be associated with bipolar disorder and increased risk of attempted suicide include anxiety and eating disorders, early onset of symptoms, at least four previous hospitalizations for depression, and medical comorbidity (Table 2). Limited access to psychiatric and medical services and inadequate health insurance also were correlated with having made a serious suicide attempt. Thus, lack of medical care might exacerbate demoralization and increase the risk of suicide.
Family history of suicide or serious suicide attempts and drug abuse were associated with an increase in suicide attempts, but family history of unipolar or bipolar illness were not. These data are consistent with other studies suggesting that vulnerability to suicide may have a separate genetic component from that of mood disorders per se.
Severity of illness Increased severity of bipolar illness also was associated with prior psychosocial stressors3 and serious suicide attempts. We confirmed self-reported history, using daily mood charting, the clinician-rated Inventory of Depressive Symptomatology (IDS-C), and the Young Mania Rating Scale (YMRS) to rate increase in percentage of time ill, percentage of time depressed, and severity of depression.
Clinical implications We are uncertain how directly these data regarding suicide attempts relate to completed suicide. However, patients with bipolar disorder are among those at highest risk for completed suicide, and a prior serious attempt is often a precursor to completed suicide.5,6
Factors that appear particularly related to increased risk of suicide in patients with bipolar disorder are family history of suicide, history of early psychosocial stressors, comorbid personality disorders, substance abuse, and a relatively severe course of bipolar illness. Patients with these risk factors need support for two types of psychosocial stressors:
- poor access to health care
- stressors related to concurrent events (e.g., occurring with new affective episodes).
It may also be important for clinicians to consider the prophylactic use of lithium because of its demonstrated ability to reduce mortality in bipolar illness.7 Additionally, we need data on the efficacy of new drugs in preventing affective episodes and reducing suicidal ideation and acts.
Common comorbidities
We found considerable axis I lifetime comorbidities in the SFBN cohort (Table 3), including a 42% incidence of anxiety disorder and a similarly high rate of substance abuse. In addition to bipolar disorder, patients averaged approximately two lifetime comorbid diagnoses:
- 35% had none
- 65% had one or more
- 42% had two or more
- 24% had three or more.
Table 3
PSYCHIATRIC COMORBIDITY IN BIPOLAR OUTPATIENTS (%)*.
| Any substance abuse disorder | 42% |
| Alcohol | 33 |
| Marijuana | 16 |
| Stimulant | 9 |
| Sedative | 8 |
| Opiate | 7 |
| Hallucinogens | 6 |
| Any anxiety disorder | 42% |
| Panic disorder | 20 |
| Social phobia | 16 |
| Simple phobia | 10 |
| OCD | 9 |
| PTSD | 7 |
| GAD | 3 |
| Other | 3 |
| Any eating disorder | 11% |
| Bulimia nervosa | 8 |
| Anorexia nervosa | 4 |
| * Lifetime comorbid axis I disorders based on Structured Clinical Interview for DSM-IV (SCID) interviews with 288 SFBN patients. | |
Men with bipolar disorder had a higher absolute prevalence of alcoholism, but the relative risk compared with the general population was much higher in women (odds ratio 7.35) compared with men (odds ratio 2.77). In women, alcohol use and abuse were related to history of social phobia, greater number of depressive episodes prior to network entry, and history of abusing more than one substance. In men, alcohol comorbidity was related to history of alcohol abuse in first-degree relatives and history of early physical abuse.
These data suggest that female patients, in particular, were self-medicating their affective episodes and comorbid anxiety disorders with alcohol. They suggest the importance of asking patients directly about alcohol use to identify the need for primary and secondary prevention. In an adolescent or young adult, affective illness—and bipolar disorder in particular—should be considered a major risk factor for subsequent problems with alcohol and drug abuse. When taking a careful history, the treating physician should include questions regarding any substance abuse.
Overweight and obesity
One-half of the women and two-thirds of the men in the SFBN were overweight at network entry.8 Although this rate of overweight sounds alarmingly high, it was not significantly different from rates in the general U.S. population. A greater percentage of women than men in the network had morbid obesity, however.
In our cohort, being overweight or obese was associated with an increased incidence of cardiovascular disease, diabetes, arthritis, hypertension, hypothyroidism, and cancer. The degree of obesity also correlated significantly with past use of the numerous psychotropic drugs that have been associated with weight gain. Also, as might be expected, increased weight was associated with physical inactivity.
Many of the psychotropic drugs routinely used to treat bipolar disorder are associated with weight gain. Among these are the mood stabilizers lithium and valproate and the atypical antipsychotics, with the exception of ziprasidone (and, to follow, aripiprazole). Lithium’s liability to cause weight gain may be counterbalanced by its antisuicide effects and its ability to reverse excess medical mortality associated with affective illness. Thus, lithium should continue to play a major therapeutic role in bipolar disorder.
It may be more effective and easier to prevent weight gain than to attempt to reduce weight that has been gained. Recommending exercise and other weight-reduction measures may be helpful, as may co-administering drugs associated with weight loss, such as topiramate.9
Thyroiditis
Antithyroid antibodies were detected in 28% of network patients, compared with rates of 3% in the general population and 18% in psychiatric controls.10 The increased rate was associated with the need to augment thyroid function in 17% of network patients, but it was not related to age, mood state, rapid cycling status, or lithium treatment.
These data indicate that bipolar patients are prone to autoimmune thyroid changes. Further analysis is required to ascertain whether this finding has therapeutic implications, especially related to adjunctive T3 and T4 treatment.11
Even with psychiatry’s most effective and best studied agents, many patients with bipolar disorder are inadequately treated and respond insufficiently to available psychopharmacologic agents. To address this problem, the Stanley Foundation Bipolar Network (SFBN) was created to explore acute and long-term response to treatment of bipolar disorder.
For the past 6 years, our group (Box) has followed an international cohort of patients with bipolar disorder and offered them opportunities to participate in clinical trials at seven field centers. We have made considerable progress as we focused on the longitudinal course of bipolar illness and evaluated various combinations of psychopharmacologic therapies. The SFBN also has explored factors that increase the risk of suicide, quantified the incidence of anxiety disorder and substance abuse, and looked at other variables that may affect treatment outcome.
This report for the readers of Current Psychiatry provides a brief state-of-the-art overview of what we have learned about the course and management of bipolar disorder. It brings together and summarizes the findings from studies published, in press, or under review. Our goal is to update current findings, which may help you provide optimum care for these at-risk patients.
Since Robert Prien’s pioneering studies in the 1970s, the National Institute of Mental Health (NIMH) had funded little work on prophylaxis of bipolar disorders or acute bipolar depression. Following two NIMH conferences on bipolar illness in 1989 and 1994, the Stanley Foundation Bipolar Network (SFBN) was established to help overcome these research deficiencies, with funding provided by the Theodore and Vada Stanley Foundation.
Patients were recruited into the network from the general community and with the use of newspaper and newsletter advertisements. In contrast to many other clinical populations, few who wished to enter the network were excluded. It was clear, however, that filling out demographic, clinical, and rating forms would require considerable effort from each patient. All patients gave written informed consent to participate in the naturalistic part of the study, and were reconsented for any open or blind randomized clinical trial.
Patients provided daily self-ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM), a unique longitudinal instrument. Clinicians then completed their own separate LCMs, as more severely ill patients often could not complete their self-ratings, and patients often underestimated the severity and functional disability associated with the hypomanic and manic phases of bipolar illness. With the use of the NIMH-LCM, network investigators were able, for the first time, to assess the details of mood fluctuations associated with bipolar illness and to more precisely delineate the qualitative and quantitative aspects of response to psychopharmacologic interventions.
The SFBN includes seven research field centers in the United States, the Netherlands, and Germany. The leaders of those centers collaborated with Robert M. Post, MD, to prepare this report of the network findings. The collaborators are:
| Gabriele S. Leverich, MSW, and Kirk D. Denicoff, MD Biological Psychiatry Branch National Institute of Mental Health Bethesda, MD | Paul E. Keck, Jr., MD, and Susan L. McElroy, MD Biological Psychiatry Program University of Cincinnati College of Medicine Cincinnati, OH |
| Lori L. Altshuler, MD, and Mark A. Frye, MD UCLA Mood Disorders Research Program VA Medical Center Los Angeles, CA | Willem A. Nolen, MD, and Ralph Kupka, MD Altrecht Institute for Mental Health Care University Medical Center Utrecht, The Netherlands |
| John Rush, MD, and Trisha Suppes, MD Department of Psychiatry Southwest Medical Center University of Texas, Dallas | Joerg Walden, MD Department of Psychiatry University of Freiburg Freiburg, Germany |
| Keith Kramlinger, MD Mayo Clinic, Rochester, MN (Administrative center) | Heinz Grunze, MD Psychiatrische Klinik der LMU Munich, Germany |
In part one, we discuss the demographics of our cohort of 676 community-based bipolar patients, including risk factors for suicide attempts, frequency of comorbid psychiatric diagnoses, problems of overweight and obesity, and preliminary findings about thyroiditis.
In part two, we describe our clinical trials assessing mood stabilizers and adjunctive agents such as atypical antipsychotics, second-generation antidepressants, and anticonvulsants. We also will address a soon-to-be analyzed study of omega-3 fatty acids.
Table 1
CHARACTERISTICS OF THE 676 BIPOLAR OUTPATIENTS
| Mean age | 41 | |
| % Female | 43 | |
| Diagnosis (%) | ||
| Bipolar I | 79 | |
| Bipolar II | 16 | |
| Bipolar NOS | 4 | |
| Marital status (%) | ||
| Married/cohabitating | 44 | |
| Divorced/separated | 22 | |
| Income (%) | ||
| <$20,000 | 37 | |
| $20,000-40,000 | 22 | |
| $40,000-100,000 | 32 | |
| >$100,000 | 9 | |
| Education (completed, %) | ||
| High school | 34 | |
| Jr. college/technical | 13 | |
| College, 4-year | 27 | |
| Graduate degree | 16 | |
| Positive family history (1st-degree relatives, %) | ||
| Bipolar disorder | 56 | |
| Unipolar disorder | 64 | |
| Schizophrenia | 24 | |
| Drug dependence | 44 | |
| Other psychiatric disorder | 52 | |
| Alcohol abuse/dependence | 52 | |
| Suicide (complete or serious attempt) | 38 | |
| Environmental adversity (%): | Child/adolescent | Adult |
| Physical abuse | 29 | 8 |
| Sexual abuse | 30 | 8 |
| Illness course and treatment (age in yrs) | ||
| Age of onset (symptoms) | 19 | |
| Early onset (age <14) | 34 | |
| Age at first treatment | 29 | |
| Age at first hospitalization | 30 | |
| Cycling pattern (n = 643, %) | ||
| Rapid (or faster cycling) | 47 | |
| Ultrarapid (or faster cycling) | 30 | |
| Rapid-cycling only | 17 | |
| Ultrarapid cycling only | 10 | |
| Ultradian cycling | 20 | |
Study population
The SFBN recruited outpatients with bipolar disorders I, II, or not otherwise specified (NOS). We did not exclude patients with comorbid illnesses, except those with active substance abuse disorder requiring treatment in another setting.1 Patients were recruited almost exclusively from the community and rarely from inpatient hospitalization. The average age was 41, and 43% were college graduates. Most were employed but indicated that their illness limited their employment level or ability to function at work (Table 1).
One striking finding was a decadelong lag between the onset of symptoms reaching thresholds for diagnosis and the onset of first treatment.2 This long delay could have adverse consequences for illness outcome and represents a major public health target. Age of onset appeared to have both genetic/familial and environmental determinants.3 Subjects with a history of both extreme early life adversities (i.e., physical or sexual abuse) and a positive family history of mood disorder had the earliest onset of bipolar illness. Those who had neither of these factors had late-onset illness.
Suicide attempts
Prior to network entry, 25 to 50% of our study population had made a serious suicide attempt (defined as requiring medical attention and/or hospitalization), pointing to bipolar disorder’s potential lethality.
History of abuse A history of physical or sexual abuse in childhood or adolescence was a prominent risk factor for attempted suicide.4 With either type of abuse there was an approximate 15% increase in incidence of suicide attempts over the baseline rate of about 25%. More than 55% of patients with a history of both physical and sexual abuse had made a serious suicide attempt. The frequency of physical and sexual abuse was also positively related to increased incidence of suicide attempts. Taken together, these data suggest a “stressor dose-response” relationship between adverse childhood or adolescent experiences and an increased incidence of suicide attempts.
Table 2
FACTORS THAT APPEAR TO INCREASE SUICIDE RISK IN BIPOLAR DISORDER*
| Course-of-illness characteristics |
| Early onset |
| More time ill |
| More time depressed |
| More-severe depression |
| Social and medical support |
| Loss of significant other |
| Lack of a confidant |
| Single motherhood |
| Decreased access to health care |
| Lack of medical insurance |
| Demographics |
| Less education |
| Lower income |
| Being single |
| Comorbidities |
| Axis I |
| Eating disorders |
| Anxiety disorders |
| Axis II |
| Cluster A, B, C |
| Medical |
| Traumatic life events |
| Sexual abuse |
| Physical abuse |
| Genetics |
| Family history of suicide and drug abuse |
| * Identified in the SFBN cohort |
Comorbidities Other factors that appear to be associated with bipolar disorder and increased risk of attempted suicide include anxiety and eating disorders, early onset of symptoms, at least four previous hospitalizations for depression, and medical comorbidity (Table 2). Limited access to psychiatric and medical services and inadequate health insurance also were correlated with having made a serious suicide attempt. Thus, lack of medical care might exacerbate demoralization and increase the risk of suicide.
Family history of suicide or serious suicide attempts and drug abuse were associated with an increase in suicide attempts, but family history of unipolar or bipolar illness were not. These data are consistent with other studies suggesting that vulnerability to suicide may have a separate genetic component from that of mood disorders per se.
Severity of illness Increased severity of bipolar illness also was associated with prior psychosocial stressors3 and serious suicide attempts. We confirmed self-reported history, using daily mood charting, the clinician-rated Inventory of Depressive Symptomatology (IDS-C), and the Young Mania Rating Scale (YMRS) to rate increase in percentage of time ill, percentage of time depressed, and severity of depression.
Clinical implications We are uncertain how directly these data regarding suicide attempts relate to completed suicide. However, patients with bipolar disorder are among those at highest risk for completed suicide, and a prior serious attempt is often a precursor to completed suicide.5,6
Factors that appear particularly related to increased risk of suicide in patients with bipolar disorder are family history of suicide, history of early psychosocial stressors, comorbid personality disorders, substance abuse, and a relatively severe course of bipolar illness. Patients with these risk factors need support for two types of psychosocial stressors:
- poor access to health care
- stressors related to concurrent events (e.g., occurring with new affective episodes).
It may also be important for clinicians to consider the prophylactic use of lithium because of its demonstrated ability to reduce mortality in bipolar illness.7 Additionally, we need data on the efficacy of new drugs in preventing affective episodes and reducing suicidal ideation and acts.
Common comorbidities
We found considerable axis I lifetime comorbidities in the SFBN cohort (Table 3), including a 42% incidence of anxiety disorder and a similarly high rate of substance abuse. In addition to bipolar disorder, patients averaged approximately two lifetime comorbid diagnoses:
- 35% had none
- 65% had one or more
- 42% had two or more
- 24% had three or more.
Table 3
PSYCHIATRIC COMORBIDITY IN BIPOLAR OUTPATIENTS (%)*.
| Any substance abuse disorder | 42% |
| Alcohol | 33 |
| Marijuana | 16 |
| Stimulant | 9 |
| Sedative | 8 |
| Opiate | 7 |
| Hallucinogens | 6 |
| Any anxiety disorder | 42% |
| Panic disorder | 20 |
| Social phobia | 16 |
| Simple phobia | 10 |
| OCD | 9 |
| PTSD | 7 |
| GAD | 3 |
| Other | 3 |
| Any eating disorder | 11% |
| Bulimia nervosa | 8 |
| Anorexia nervosa | 4 |
| * Lifetime comorbid axis I disorders based on Structured Clinical Interview for DSM-IV (SCID) interviews with 288 SFBN patients. | |
Men with bipolar disorder had a higher absolute prevalence of alcoholism, but the relative risk compared with the general population was much higher in women (odds ratio 7.35) compared with men (odds ratio 2.77). In women, alcohol use and abuse were related to history of social phobia, greater number of depressive episodes prior to network entry, and history of abusing more than one substance. In men, alcohol comorbidity was related to history of alcohol abuse in first-degree relatives and history of early physical abuse.
These data suggest that female patients, in particular, were self-medicating their affective episodes and comorbid anxiety disorders with alcohol. They suggest the importance of asking patients directly about alcohol use to identify the need for primary and secondary prevention. In an adolescent or young adult, affective illness—and bipolar disorder in particular—should be considered a major risk factor for subsequent problems with alcohol and drug abuse. When taking a careful history, the treating physician should include questions regarding any substance abuse.
Overweight and obesity
One-half of the women and two-thirds of the men in the SFBN were overweight at network entry.8 Although this rate of overweight sounds alarmingly high, it was not significantly different from rates in the general U.S. population. A greater percentage of women than men in the network had morbid obesity, however.
In our cohort, being overweight or obese was associated with an increased incidence of cardiovascular disease, diabetes, arthritis, hypertension, hypothyroidism, and cancer. The degree of obesity also correlated significantly with past use of the numerous psychotropic drugs that have been associated with weight gain. Also, as might be expected, increased weight was associated with physical inactivity.
Many of the psychotropic drugs routinely used to treat bipolar disorder are associated with weight gain. Among these are the mood stabilizers lithium and valproate and the atypical antipsychotics, with the exception of ziprasidone (and, to follow, aripiprazole). Lithium’s liability to cause weight gain may be counterbalanced by its antisuicide effects and its ability to reverse excess medical mortality associated with affective illness. Thus, lithium should continue to play a major therapeutic role in bipolar disorder.
It may be more effective and easier to prevent weight gain than to attempt to reduce weight that has been gained. Recommending exercise and other weight-reduction measures may be helpful, as may co-administering drugs associated with weight loss, such as topiramate.9
Thyroiditis
Antithyroid antibodies were detected in 28% of network patients, compared with rates of 3% in the general population and 18% in psychiatric controls.10 The increased rate was associated with the need to augment thyroid function in 17% of network patients, but it was not related to age, mood state, rapid cycling status, or lithium treatment.
These data indicate that bipolar patients are prone to autoimmune thyroid changes. Further analysis is required to ascertain whether this finding has therapeutic implications, especially related to adjunctive T3 and T4 treatment.11
1. Leverich GS, Nolen WA, Rush AJ, et al. The Stanley Foundation Bipolar Treatment Outcome Network. I. Longitudinal methodology. J Affect Disord 2001;67:33-44.
2. Suppes T, Leverich GS, Keck PE, et al. The Stanley Foundation Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disord 2001b;67:45-59.
3. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological Psychiatry 2002;51:288-97.
4. Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. Manuscript submitted for publication, 2002.
5. Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39:896-9.
6. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch.Gen.Psychiatry 1999;56:617-26.
7. Ahrens B, Muller-Oerlinghausen B, Schou M, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord 1995;33:67-75.
8. McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry 2002;63:207-13.
9. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biological Psychiatry 2000;47:1025-33.
10. Kupka RW, Nolen WA, Post RM, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biological Psychiatry 2002;51:305-11.
11. Bauer M, Priebe S, Berghofer A, et al. Subjective response to and tolerability of long-term supraphysiological doses of levothyroxine in refractory mood disorders. J Affect Disord 2001;64:35-42.
1. Leverich GS, Nolen WA, Rush AJ, et al. The Stanley Foundation Bipolar Treatment Outcome Network. I. Longitudinal methodology. J Affect Disord 2001;67:33-44.
2. Suppes T, Leverich GS, Keck PE, et al. The Stanley Foundation Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disord 2001b;67:45-59.
3. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological Psychiatry 2002;51:288-97.
4. Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. Manuscript submitted for publication, 2002.
5. Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39:896-9.
6. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch.Gen.Psychiatry 1999;56:617-26.
7. Ahrens B, Muller-Oerlinghausen B, Schou M, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord 1995;33:67-75.
8. McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry 2002;63:207-13.
9. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biological Psychiatry 2000;47:1025-33.
10. Kupka RW, Nolen WA, Post RM, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biological Psychiatry 2002;51:305-11.
11. Bauer M, Priebe S, Berghofer A, et al. Subjective response to and tolerability of long-term supraphysiological doses of levothyroxine in refractory mood disorders. J Affect Disord 2001;64:35-42.