Article
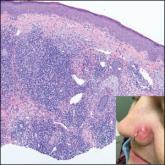
Erythematous Papule on the Nasal Ala
- Author:
- Rohit Gupta, MD
- Abdul Hafeez Diwan, MD, PhD
- Vicky Ren, MD
A 35-year-old woman presented with a slowly growing, smooth, erythematous papule of 2 months’ duration on the left nasal ala surrounding a...
Article
Asthma: Newer Tx options mean more targeted therapy
- Author:
- Parth M. Rali, MD
- Nana Yaa Baffour-Awuah, MD
- Rohit Gupta, MD
- Grishma Rali, MD
- Mayur Rali, MD, FAAFP
It’s an exciting era of asthma management, with the introduction of several novel modalities, including biological therapy and bronchial...
