User login
Ultrasound Measurement to Estimate CVP
Severe sepsis and septic shock account for more than 750,000 hospital admissions and 215,000 deaths per year.1 Early fluid resuscitation is the cornerstone of treatment, and early goal‐directed therapy (EGDT), which includes a target central venous pressure (CVP) of 8 to 12 mm Hg, has been shown to improve outcomes, including mortality and length of stay.2 This goal allows appropriate initial resuscitation and may decrease the risk of excess fluid administration, which is related to adverse outcomes in critically ill patients.3 However, nonintensivists may not start early aggressive fluid resuscitation because of inability to accurately assess intravascular volume, concerns for inadvertent volume overload, or the difficulty of recognizing insidious illness. Assessment of volume status, primarily from inspection of the internal jugular vein to estimate CVP, is difficult to perform by clinical examination alone, especially if CVP is very low.4 Inspection of the external jugular vein is perhaps easier than inspecting the internal jugular vein and appears to accurately estimate CVP,5 but it does not allow the degree of precision necessary for EGDT. Echocardiography can estimate CVP based on respirophasic variation or collapsibility index, but this technique requires expensive equipment and sonographic expertise. The current gold standard technique for measuring CVP requires an invasive central venous catheter, which can delay timely resuscitation and is associated with complications.6
An alternative technique to guide resuscitation efforts should be accurate, safe, rapid, and easy to perform at the bedside, while providing real‐time measurement results. We hypothesized that CVP can be accurately assessed using noninvasive ultrasound imaging of the internal jugular vein, since jugular venous pressure is essentially equal to CVP.7 Specifically, our study estimated the diagnostic accuracy of ultrasound measurement of the aspect ratio (height/width) of the internal jugular vein compared with the invasively measured CVP target for EGDT. We expected that a lower aspect ratio would correlate with a lower CVP and a higher aspect ratio would correlate with a higher CVP.
Methods
Volunteers were enrolled at Saint Mary's Hospital (Mayo Clinic) in Rochester, MN, from January to March 2006, and patients were enrolled at Saint Mary's Hospital and at Abbott Northwestern Hospital (Allina Hospitals and Clinics) in Minneapolis, MN, from May 2006 to October 2007. The study was approved by the Institutional Review Boards of Mayo Clinic and Allina and had 2 phases. The first phase comprised ultrasound measurements of internal jugular vein aspect ratio and determination of intraobserver and interobserver agreement in healthy volunteers. The second phase involved measurement of internal jugular vein aspect ratio and invasive CVP in a convenience sample of 44 spontaneously breathing patients admitted to medical intensive care units: 9 patients at Saint Marys Hospital and 35 patients at Abbott Northwestern Hospital. Patients were enrolled only when study members were on duty in the intensive care unit and able to perform study measurements. As a result, a high proportion of patients who may have been eligible were not asked to participate.
Each volunteer was deemed euvolemic on the basis of normal orthostatic measurements and normal oral intake with no vomiting or diarrhea in the previous 5 days. Measurements of 19 volunteers were made by 1 author (A.S.K.), with subsequent measurements of 15 of the volunteers made by another author (O.G.) to determine interobserver variability; 4 participants did not undergo a second measurement because of scheduling conflicts.
Inclusion and exclusion criteria for the critically ill patients are provided in Table 1. Recruitment was based on presenting symptoms and test results that led the intensive care unit physicians to decide to place a CVP monitor. All the enrolled patients had invasive CVP measurement performed approximately 30 to 40 minutes after ultrasound measurement of the internal jugular vein; this delay was the time required to place the central line and obtain the measurement. All patients who were invited to participate in the study were included. No patients were excluded on the basis of the exclusion criteria or because of inability to place a central line. No complications related to central line placement occurred.
| Inclusion criteria |
| 1. Aged 18 years or older |
| 2. Admission to the intensive care unit |
| 3. Spontaneously breathing (not intubated/ventilated) |
| 4. Planned insertion of a central venous pressure monitor for therapy |
| Exclusion criteria |
| 1. Known cervical spine injuries or fusion |
| 2. Nonremovable cervical collars |
| 3. Surgical dressings that would prevent visualization of the internal jugular vein |
| 4. Inability of the patient to be properly positioned |
| 5. A code situation |
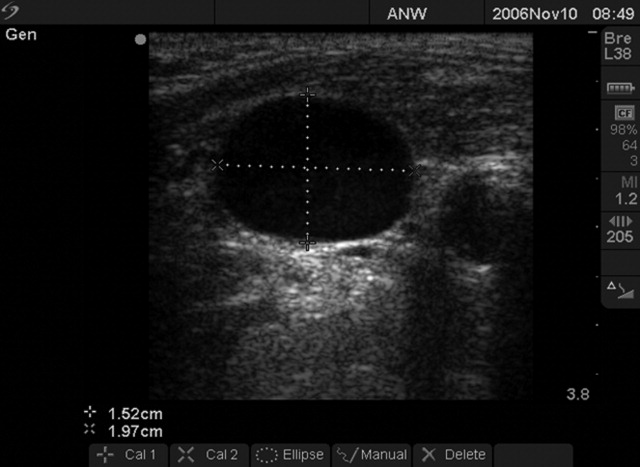
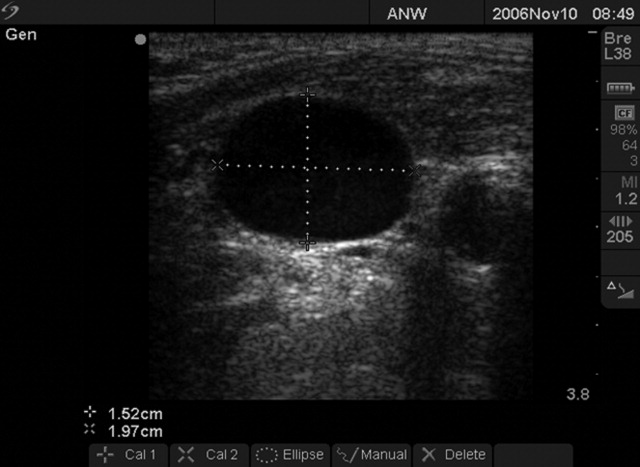
We followed a prescribed measurement technique (Table 2) to determine the internal jugular vein aspect ratio in all volunteers and patients. Measurements of the volunteers were made with a Site‐Rite 3 Ultrasound System (Bard Access Systems, Inc., Salt Lake City, UT) using a 9.0‐MHz transducer. Measurements of the critically ill patients were made with a SonoSite MicroMaxx ultrasound system (SonoSite, Inc., Bothell, WA) using a 10.5‐MHz transducer. Study team physicians initially were blinded to actual measured CVP. Internal jugular vein aspect ratio and CVP were measured at tidal volume end‐expiration for all patients. One measurement was obtained for each patient, with measurements being made by 1 of 4 physicians (2 intensivists, 1 critical care fellow, and 1 chief medicine resident). With no specific ultrasound training and with only minimal practice, the physicians could obtain the optimal aspect ratio within a few seconds (Figure 1).
| 1. Position the patient supine (0) with head and legs flat, ensuring overall comfort. A small pillow can be used to help keep head, neck, and trunk aligned |
| 2. Have the patient rotate his or her head slightly to the side (<30) to expose the internal jugular vein |
| 3. Place the transducer transversely on the patient's neck over the expected location of the internal jugular vein. The transducer should be perpendicular to the patient's neck |
| 4. Apply slight pressure to the transducer to locate the internal jugular vein on the view screen. Use the minimum pressure necessary to obtain a good quality ultrasound image |
| 5. Once the internal jugular vein is found, adjust the position of the transducer over the vein to obtain the most circular cross‐sectional image |
| 6. Have the patient breathe normally, then ask him or her to briefly stop breathing at normal (tidal volume) end‐expiration |
| 7. Store the best end‐expiration image (in which the internal jugular vein appears most circular) and have the patient resume normal breathing |
| 8. Measure the height and width of the internal jugular vein using the built‐in cursor function or a ruler |
This was an exploratory prospective study, and all methods of data collection were designed before patient enrollment. However, the ultrasound‐derived aspect ratio of 0.83 (which defined a CVP of 8 mm Hg) was determined post hoc to maximize sensitivity and specificity and was based on the aspect ratio of the euvolemic volunteers and the inflection point of the CVP vs aspect ratio curve for the critically ill patients.
Statistical Analysis
Groups were compared using the 2 test for differences in proportions and the Wilcoxon rank sum test for continuous data. P < 0.05 was considered statistically significant. Bland‐Altman plots were used to describe the bias and variability of the aspect ratio within and between observers.8 This technique compares 2 methods of measurement to determine agreement and repeatability by plotting the mean of the differences (which should be zero) and the upper and lower limits of agreement (1.96 standard deviations [SDs] of those differences above and below the mean). Results were calculated using the available data; there was no adjustment for missing data. Analyses were performed using SPLUS and SAS/STAT software (SAS Institute, Inc., Cary, NC).
Results
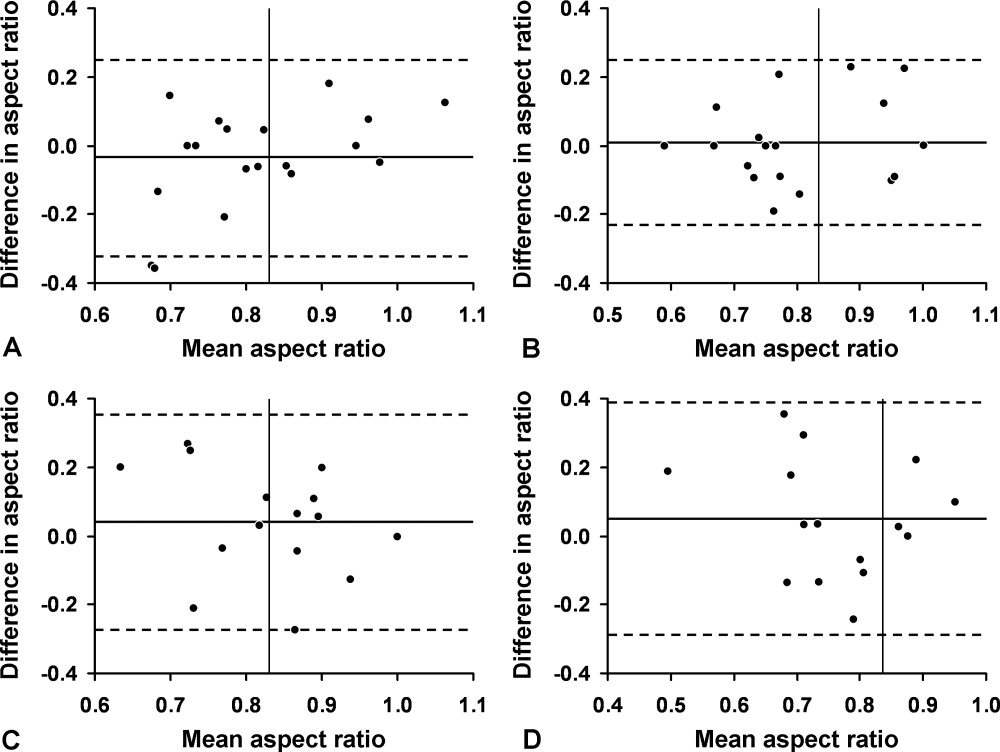
We first evaluated 19 white volunteers: 12 women and 7 men. Mean (SD) age was 42 (11) years and mean body mass index was 26.6 (4.5) kg/m2. Mean arterial pressure was 89 (13) mm Hg and mean heart rate was 71 (15) beats/minute. Mean aspect ratio of the right and left internal jugular vein for all volunteers was 0.82 (0.07). There was no difference in aspect ratio between the right (0.83 [0.10]) and left (0.81 [0.13]) vein (P > 0.10). Also, no difference in the aspect ratio was seen between men (0.81 [0.08]) and women (0.83 [0.07]) (P = 0.77). Bland‐Altman analysis indicated moderate intraobserver and interobserver agreement for the aspect ratio measurements (Figure 2).
We then compared the aspect ratio measured using ultrasound and CVP measured with an invasive monitor for 44 spontaneously breathing critically ill patients (22 women and 22 men; 38 were white). Mean (SD) age was 66 (14) years and mean body mass index was 28.8 (9.1) kg/m2. Mean arterial pressure (n = 36) was 67 (12) mm Hg and mean heart rate (n = 34) was 92 (22) beats/minute. Systemic inflammatory response syndrome (SIRS) criteria were present in 23 of 40 patients; complete data were unavailable for the other 4 patients. Of these 40 patients, 20 had sepsis, 15 had severe sepsis, and 5 had septic shock. The most common diagnoses were gastrointestinal tract bleeding in 6 patients and congestive heart failure in 4 patients. Acute Physiology and Chronic Health Evaluation (APACHE III) score, available for 8 of the 9 patients at Saint Marys Hospital, was 63 (10).
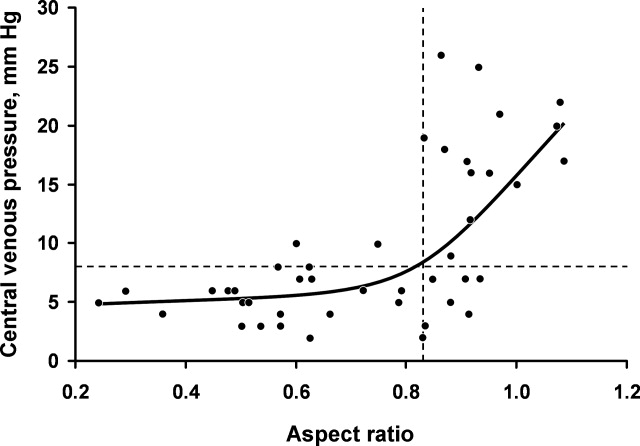
Figure 3 shows measured aspect ratios vs. invasively measured CVP for the critically ill patients. The curvilinear result is consistent with venous and right ventricular compliance ( volume/ pressure) characteristics. Note that the inflection point (beginning of the increased slope) of the curve corresponds to a CVP of about 8 mm Hg. Furthermore, the aspect ratio (0.8) at this point is the same as that seen in the euvolemic volunteers. These findings suggest that, in spontaneously breathing patients, a CVP of about 8 mm Hg and an aspect ratio of about 0.8 each defines the beginning of the plateau on the cardiac Frank‐Starling curve.
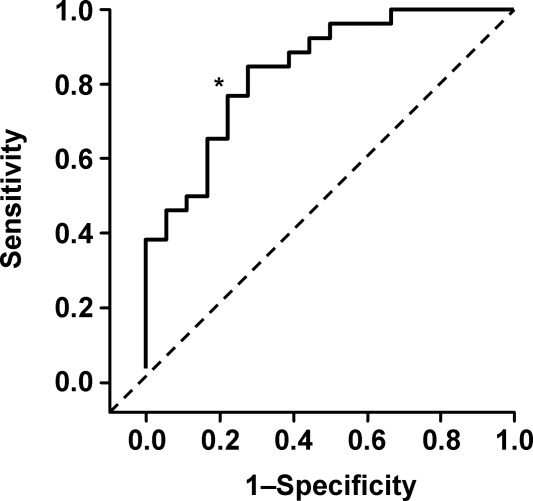
Ultrasound imaging of the internal jugular vein aspect ratio accurately estimated the CVP target of 8 mm Hg based on the area under the receiver operating characteristics curve of 0.84 (95% confidence interval [CI], 0.72‐0.96) (Figure 4). For an invasively measured CVP of less than 8 mm Hg, the likelihood ratio for a positive ultrasound test result (aspect ratio < 0.83) was 3.5 (95% CI, 1.4‐8.4) and for a negative test result (aspect ratio 0.83) was 0.30 (95% CI, 0.14‐0.62). Clinically, this means that patients with a measured aspect ratio of less than 0.83 require further fluid resuscitation, whereas patients with a measured aspect ratio of 0.83 or greater are less likely to benefit from fluid resuscitation.
Discussion
This study demonstrated that the EGDT CVP target of 8 to 12 mm Hg can be accurately estimated (referenced to invasive CVP monitoring) using noninvasive ultrasound measurement of the internal jugular vein in spontaneously breathing critically ill patients. The measurement process is simple to perform at the bedside and moderately reliable when performed by different observers; also, the results appear to be equivalent for both sides and for males or females. Images can be stored electronically for serial comparisons and for viewing by other caregivers. Because the aspect ratio is essentially constant over the length of the internal jugular vein, unlike diameter, measurements can be performed anywhere along the vein. Also, ultrasound imaging allows visualization of the internal jugular vein despite anatomic variation.9
Previous attempts at noninvasive hemodynamic monitoring using plethysmography, thoracic electrical bioimpedance, and external Doppler probes have shown these methods to be cumbersome or inaccurate.1013 Other investigators have used echocardiography14, 15 and handheld ultrasound16 to image the diameter of the inferior vena cava in order to assess intravascular volume status, but these techniques require expertise in sonographic imaging. An alternative technique is to measure peripheral venous pressure, which correlates with CVP.17 This method, however, requires technical expertise to zero the monitor and is not yet widely used for critically ill patients. A literature search found 1 letter to the editor suggesting that real‐time ultrasound imaging of the internal jugular vein could be used to qualitatively determine jugular venous pressure18 and 3 studies using ultrasound in conjunction with a pressure transducer or manometer to determine the pressure needed to collapse the vein (either the internal jugular or a peripheral vein), with subsequent correlation to CVP.1921 These latter techniques appear to be cumbersome and require custom equipment that is not readily available in most hospitals.
Any measurement of CVP, including our technique, assumes correlation with volume responsiveness as a surrogate for intravascular volume. However, CVP is governed by multiple physiologic and pathologic factors, including intravascular volume, vascular and ventricular compliance, ventricular function, tricuspid stenosis and regurgitation, cardiac tamponade, and atrioventricular dissociation.22, 23 Therefore, CVP alone may not be an accurate measure of volume responsiveness (intravascular volume). CVP may also have spontaneous variation similar to pulmonary capillary wedge pressure, which can be as high as 7 mm Hg in any given patient.24 Furthermore, invasive CVP monitors also have limitations, and the overall accuracy of the Philips system used at Saint Marys Hospital is 4% of the reading or 4 mm Hg, whichever is greater.25 Nonetheless, the EGDT algorithm that incorporates CVP measurement with a target of 8 to 12 mm Hg in spontaneously breathing patients and 12 mm Hg in mechanically ventilated patients has resulted in decreased mortality among patients with severe sepsis and is recommended by the Surviving Sepsis Campaign guidelines26 and the Institute for Healthcare Improvement.27
These study results are important because nonintensivists such as hospitalists and emergency department physicians can use this technique to provide rapid fluid resuscitation early in the course of severe sepsis and septic shock, when aggressive fluid resuscitation is most effective. Ultrasound imaging of the internal jugular vein is easy to perform without formal training, and the equipment is readily available in most hospitals. Future studies will evaluate outcomes in spontaneously breathing and ventilated patients to determine the accuracy of this measurement technique in volume‐depleted and volume‐overloaded states. If validated in different patient populations, ultrasound measurement of the internal jugular vein could substitute for the EGDT CVP target in critically ill patients and allow early aggressive fluid resuscitation before a central venous catheter is placed.
Limitations
This exploratory study enrolled a small convenience sample of primarily white patients. The convenience sample is potentially prone to selection bias since a majority of patients who may have been eligible were never asked to participate. Also, not all patients had sepsis syndrome; our intention was to measure CVP and aspect ratio for available critically ill patients. Accordingly, results may be different depending on severity of illness. In addition, some of the patients were transferred from outside medical centers or from emergency departments and therefore may have already been partly resuscitated. Another limitation is that the intraobserver and interobserver variability for the healthy volunteers showed only moderate agreement, possibly indicating limited repeatability, although these results could be due to the small sample size. Also, we did not determine intraobserver and interobserver variability for the critically ill patients; results may be different from those of the healthy volunteers. Furthermore, underlying conditions such as tricuspid stenosis or regurgitation and cardiac tamponade may affect measurement results, but we included all patients without formal assessment, since treatment was performed on an urgent/emergent basis as would happen in real clinical settings.
Acknowledgements
The authors dedicate this work to their patients with severe sepsis. They thank Lisa Kirkland, MD, and Murat Yilmaz, MD, for their assistance with this study. They also thank the Mayo Clinic Divisions of General Internal Medicine and Pulmonary and Critical Care Medicine for funding.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29(7):1303–1310.
- ,,, et al.Early Goal‐Directed Therapy Collaborative Group. Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345(19):1368–1377.
- ,.Fluid therapy in resuscitated sepsis: less is more.Chest.2008;133(1):252–263.
- ,.The rational clinical examination: does this patient have abnormal central venous pressure?JAMA.1996;275(8):630–634.
- ,,,,,.Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients.Arch Intern Med.2006;166(19):2132–2137.
- ,.Central venous catheterization.Crit Care Med.2007;35(5):1390–1396.
- .How to use central venous pressure measurements.Curr Opin Crit Care.2005;11(3):264–270.
- ,.Statistical methods for assessing agreement between two methods of clinical measurement.Lancet.1986;1(8476):307–310.
- ,.Anatomical variations of internal jugular vein location: impact on central venous access.Crit Care Med.1991;19(12):1516–1519.
- ,,.Noninvasive measurement of central venous pressure by neck inductive plethysmography.Chest.1991;100(2):371–375.
- ,,, et al.A new noninvasive method to determine central venous pressure.Resuscitation.2006;70(2):238–246.
- .Advances in critical care monitoring.Arch Surg.1997;132(7):734–739.
- ,,, et al.Continuous recording of pulmonary artery diastolic pressure and cardiac output using a novel ultrasound transducer.J Am Soc Echocardiogr.2002;15(11):1381–1386.
- ,,,,.Measurement of anterior‐posterior diameter of inferior vena cava by ultrasonography: a new non‐invasive method to assess acute changes in vascular filling state.Cardiovasc Res.1994;28(8):1269–1272.
- ,,,.Utility of the inferior vena cava diameter as a marker of dry weight in nonoliguric hemodialyzed patients.ASAIO J.2001;47(5):528–532.
- ,,, et al.Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic.Clin J Am Soc Nephrol.2006;1(4):749–753.
- ,,, et al.Comparison of peripheral and central venous pressures in critically ill patients.Anaesth Intensive Care.2003;31(1):34–39.
- .Determination of elevated jugular venous pressure by real‐time ultrasound.Ann Emerg Med.1999;34(1):115.
- ,,,.Ultrasound‐guided noninvasive measurement of a patient's central venous pressure.Conf Proc IEEE Eng Med Biol Soc.2006;1:3843–3849.
- ,,, et al.Noninvasive central venous pressure measurement by controlled compression sonography at the forearm.J Am Coll Cardiol.2007;50(16):1584–1589.
- ,,,,,.Estimation of central venous pressure by ultrasound.Resuscitation.2005;64(2):193–199.
- ,,, et al.Determination of total effective vascular compliance in patients with sepsis syndrome.Am J Respir Crit Care Med.1998;157(1):50–56.
- ,,.Central venous pressure: uses and limitations. In: Pinsky MR, Payen D, eds.Functional Hemodynamic Monitoring.Berlin, Germany:Springer‐Verlag Berlin Heidelberg;2006:101.
- ,.Normal fluctuations in pulmonary artery and pulmonary capillary wedge pressures in acutely ill patients.Heart Lung.1982;11(5):393–398.
- Philips M3012A Data Sheet.Hemodynamic extension to the multi‐measurement server.Amsterdam:Koninklijke Philips Electronics N.V.;2003.
- ,,, et al.Surviving Sepsis Campaign Management Guidelines Committee. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32(3):858–873. [Erratua: Crit Care Med. 2004;32(6):1448. Correction of dosage error in text. Crit Care Med. 2004;32(10):2169–2170.]
- Institute for Healthcare Improvement.Sepsis.Cambridge, MA:Institute for Healthcare Improvement. Available at:http://www.ihi.org/IHI/Topics/CriticalCare/Sepsis. Accessed March 2009.
Severe sepsis and septic shock account for more than 750,000 hospital admissions and 215,000 deaths per year.1 Early fluid resuscitation is the cornerstone of treatment, and early goal‐directed therapy (EGDT), which includes a target central venous pressure (CVP) of 8 to 12 mm Hg, has been shown to improve outcomes, including mortality and length of stay.2 This goal allows appropriate initial resuscitation and may decrease the risk of excess fluid administration, which is related to adverse outcomes in critically ill patients.3 However, nonintensivists may not start early aggressive fluid resuscitation because of inability to accurately assess intravascular volume, concerns for inadvertent volume overload, or the difficulty of recognizing insidious illness. Assessment of volume status, primarily from inspection of the internal jugular vein to estimate CVP, is difficult to perform by clinical examination alone, especially if CVP is very low.4 Inspection of the external jugular vein is perhaps easier than inspecting the internal jugular vein and appears to accurately estimate CVP,5 but it does not allow the degree of precision necessary for EGDT. Echocardiography can estimate CVP based on respirophasic variation or collapsibility index, but this technique requires expensive equipment and sonographic expertise. The current gold standard technique for measuring CVP requires an invasive central venous catheter, which can delay timely resuscitation and is associated with complications.6
An alternative technique to guide resuscitation efforts should be accurate, safe, rapid, and easy to perform at the bedside, while providing real‐time measurement results. We hypothesized that CVP can be accurately assessed using noninvasive ultrasound imaging of the internal jugular vein, since jugular venous pressure is essentially equal to CVP.7 Specifically, our study estimated the diagnostic accuracy of ultrasound measurement of the aspect ratio (height/width) of the internal jugular vein compared with the invasively measured CVP target for EGDT. We expected that a lower aspect ratio would correlate with a lower CVP and a higher aspect ratio would correlate with a higher CVP.
Methods
Volunteers were enrolled at Saint Mary's Hospital (Mayo Clinic) in Rochester, MN, from January to March 2006, and patients were enrolled at Saint Mary's Hospital and at Abbott Northwestern Hospital (Allina Hospitals and Clinics) in Minneapolis, MN, from May 2006 to October 2007. The study was approved by the Institutional Review Boards of Mayo Clinic and Allina and had 2 phases. The first phase comprised ultrasound measurements of internal jugular vein aspect ratio and determination of intraobserver and interobserver agreement in healthy volunteers. The second phase involved measurement of internal jugular vein aspect ratio and invasive CVP in a convenience sample of 44 spontaneously breathing patients admitted to medical intensive care units: 9 patients at Saint Marys Hospital and 35 patients at Abbott Northwestern Hospital. Patients were enrolled only when study members were on duty in the intensive care unit and able to perform study measurements. As a result, a high proportion of patients who may have been eligible were not asked to participate.
Each volunteer was deemed euvolemic on the basis of normal orthostatic measurements and normal oral intake with no vomiting or diarrhea in the previous 5 days. Measurements of 19 volunteers were made by 1 author (A.S.K.), with subsequent measurements of 15 of the volunteers made by another author (O.G.) to determine interobserver variability; 4 participants did not undergo a second measurement because of scheduling conflicts.
Inclusion and exclusion criteria for the critically ill patients are provided in Table 1. Recruitment was based on presenting symptoms and test results that led the intensive care unit physicians to decide to place a CVP monitor. All the enrolled patients had invasive CVP measurement performed approximately 30 to 40 minutes after ultrasound measurement of the internal jugular vein; this delay was the time required to place the central line and obtain the measurement. All patients who were invited to participate in the study were included. No patients were excluded on the basis of the exclusion criteria or because of inability to place a central line. No complications related to central line placement occurred.
| Inclusion criteria |
| 1. Aged 18 years or older |
| 2. Admission to the intensive care unit |
| 3. Spontaneously breathing (not intubated/ventilated) |
| 4. Planned insertion of a central venous pressure monitor for therapy |
| Exclusion criteria |
| 1. Known cervical spine injuries or fusion |
| 2. Nonremovable cervical collars |
| 3. Surgical dressings that would prevent visualization of the internal jugular vein |
| 4. Inability of the patient to be properly positioned |
| 5. A code situation |
We followed a prescribed measurement technique (Table 2) to determine the internal jugular vein aspect ratio in all volunteers and patients. Measurements of the volunteers were made with a Site‐Rite 3 Ultrasound System (Bard Access Systems, Inc., Salt Lake City, UT) using a 9.0‐MHz transducer. Measurements of the critically ill patients were made with a SonoSite MicroMaxx ultrasound system (SonoSite, Inc., Bothell, WA) using a 10.5‐MHz transducer. Study team physicians initially were blinded to actual measured CVP. Internal jugular vein aspect ratio and CVP were measured at tidal volume end‐expiration for all patients. One measurement was obtained for each patient, with measurements being made by 1 of 4 physicians (2 intensivists, 1 critical care fellow, and 1 chief medicine resident). With no specific ultrasound training and with only minimal practice, the physicians could obtain the optimal aspect ratio within a few seconds (Figure 1).

| 1. Position the patient supine (0) with head and legs flat, ensuring overall comfort. A small pillow can be used to help keep head, neck, and trunk aligned |
| 2. Have the patient rotate his or her head slightly to the side (<30) to expose the internal jugular vein |
| 3. Place the transducer transversely on the patient's neck over the expected location of the internal jugular vein. The transducer should be perpendicular to the patient's neck |
| 4. Apply slight pressure to the transducer to locate the internal jugular vein on the view screen. Use the minimum pressure necessary to obtain a good quality ultrasound image |
| 5. Once the internal jugular vein is found, adjust the position of the transducer over the vein to obtain the most circular cross‐sectional image |
| 6. Have the patient breathe normally, then ask him or her to briefly stop breathing at normal (tidal volume) end‐expiration |
| 7. Store the best end‐expiration image (in which the internal jugular vein appears most circular) and have the patient resume normal breathing |
| 8. Measure the height and width of the internal jugular vein using the built‐in cursor function or a ruler |
This was an exploratory prospective study, and all methods of data collection were designed before patient enrollment. However, the ultrasound‐derived aspect ratio of 0.83 (which defined a CVP of 8 mm Hg) was determined post hoc to maximize sensitivity and specificity and was based on the aspect ratio of the euvolemic volunteers and the inflection point of the CVP vs aspect ratio curve for the critically ill patients.
Statistical Analysis
Groups were compared using the 2 test for differences in proportions and the Wilcoxon rank sum test for continuous data. P < 0.05 was considered statistically significant. Bland‐Altman plots were used to describe the bias and variability of the aspect ratio within and between observers.8 This technique compares 2 methods of measurement to determine agreement and repeatability by plotting the mean of the differences (which should be zero) and the upper and lower limits of agreement (1.96 standard deviations [SDs] of those differences above and below the mean). Results were calculated using the available data; there was no adjustment for missing data. Analyses were performed using SPLUS and SAS/STAT software (SAS Institute, Inc., Cary, NC).
Results
We first evaluated 19 white volunteers: 12 women and 7 men. Mean (SD) age was 42 (11) years and mean body mass index was 26.6 (4.5) kg/m2. Mean arterial pressure was 89 (13) mm Hg and mean heart rate was 71 (15) beats/minute. Mean aspect ratio of the right and left internal jugular vein for all volunteers was 0.82 (0.07). There was no difference in aspect ratio between the right (0.83 [0.10]) and left (0.81 [0.13]) vein (P > 0.10). Also, no difference in the aspect ratio was seen between men (0.81 [0.08]) and women (0.83 [0.07]) (P = 0.77). Bland‐Altman analysis indicated moderate intraobserver and interobserver agreement for the aspect ratio measurements (Figure 2).

We then compared the aspect ratio measured using ultrasound and CVP measured with an invasive monitor for 44 spontaneously breathing critically ill patients (22 women and 22 men; 38 were white). Mean (SD) age was 66 (14) years and mean body mass index was 28.8 (9.1) kg/m2. Mean arterial pressure (n = 36) was 67 (12) mm Hg and mean heart rate (n = 34) was 92 (22) beats/minute. Systemic inflammatory response syndrome (SIRS) criteria were present in 23 of 40 patients; complete data were unavailable for the other 4 patients. Of these 40 patients, 20 had sepsis, 15 had severe sepsis, and 5 had septic shock. The most common diagnoses were gastrointestinal tract bleeding in 6 patients and congestive heart failure in 4 patients. Acute Physiology and Chronic Health Evaluation (APACHE III) score, available for 8 of the 9 patients at Saint Marys Hospital, was 63 (10).
Figure 3 shows measured aspect ratios vs. invasively measured CVP for the critically ill patients. The curvilinear result is consistent with venous and right ventricular compliance ( volume/ pressure) characteristics. Note that the inflection point (beginning of the increased slope) of the curve corresponds to a CVP of about 8 mm Hg. Furthermore, the aspect ratio (0.8) at this point is the same as that seen in the euvolemic volunteers. These findings suggest that, in spontaneously breathing patients, a CVP of about 8 mm Hg and an aspect ratio of about 0.8 each defines the beginning of the plateau on the cardiac Frank‐Starling curve.

Ultrasound imaging of the internal jugular vein aspect ratio accurately estimated the CVP target of 8 mm Hg based on the area under the receiver operating characteristics curve of 0.84 (95% confidence interval [CI], 0.72‐0.96) (Figure 4). For an invasively measured CVP of less than 8 mm Hg, the likelihood ratio for a positive ultrasound test result (aspect ratio < 0.83) was 3.5 (95% CI, 1.4‐8.4) and for a negative test result (aspect ratio 0.83) was 0.30 (95% CI, 0.14‐0.62). Clinically, this means that patients with a measured aspect ratio of less than 0.83 require further fluid resuscitation, whereas patients with a measured aspect ratio of 0.83 or greater are less likely to benefit from fluid resuscitation.

Discussion
This study demonstrated that the EGDT CVP target of 8 to 12 mm Hg can be accurately estimated (referenced to invasive CVP monitoring) using noninvasive ultrasound measurement of the internal jugular vein in spontaneously breathing critically ill patients. The measurement process is simple to perform at the bedside and moderately reliable when performed by different observers; also, the results appear to be equivalent for both sides and for males or females. Images can be stored electronically for serial comparisons and for viewing by other caregivers. Because the aspect ratio is essentially constant over the length of the internal jugular vein, unlike diameter, measurements can be performed anywhere along the vein. Also, ultrasound imaging allows visualization of the internal jugular vein despite anatomic variation.9
Previous attempts at noninvasive hemodynamic monitoring using plethysmography, thoracic electrical bioimpedance, and external Doppler probes have shown these methods to be cumbersome or inaccurate.1013 Other investigators have used echocardiography14, 15 and handheld ultrasound16 to image the diameter of the inferior vena cava in order to assess intravascular volume status, but these techniques require expertise in sonographic imaging. An alternative technique is to measure peripheral venous pressure, which correlates with CVP.17 This method, however, requires technical expertise to zero the monitor and is not yet widely used for critically ill patients. A literature search found 1 letter to the editor suggesting that real‐time ultrasound imaging of the internal jugular vein could be used to qualitatively determine jugular venous pressure18 and 3 studies using ultrasound in conjunction with a pressure transducer or manometer to determine the pressure needed to collapse the vein (either the internal jugular or a peripheral vein), with subsequent correlation to CVP.1921 These latter techniques appear to be cumbersome and require custom equipment that is not readily available in most hospitals.
Any measurement of CVP, including our technique, assumes correlation with volume responsiveness as a surrogate for intravascular volume. However, CVP is governed by multiple physiologic and pathologic factors, including intravascular volume, vascular and ventricular compliance, ventricular function, tricuspid stenosis and regurgitation, cardiac tamponade, and atrioventricular dissociation.22, 23 Therefore, CVP alone may not be an accurate measure of volume responsiveness (intravascular volume). CVP may also have spontaneous variation similar to pulmonary capillary wedge pressure, which can be as high as 7 mm Hg in any given patient.24 Furthermore, invasive CVP monitors also have limitations, and the overall accuracy of the Philips system used at Saint Marys Hospital is 4% of the reading or 4 mm Hg, whichever is greater.25 Nonetheless, the EGDT algorithm that incorporates CVP measurement with a target of 8 to 12 mm Hg in spontaneously breathing patients and 12 mm Hg in mechanically ventilated patients has resulted in decreased mortality among patients with severe sepsis and is recommended by the Surviving Sepsis Campaign guidelines26 and the Institute for Healthcare Improvement.27
These study results are important because nonintensivists such as hospitalists and emergency department physicians can use this technique to provide rapid fluid resuscitation early in the course of severe sepsis and septic shock, when aggressive fluid resuscitation is most effective. Ultrasound imaging of the internal jugular vein is easy to perform without formal training, and the equipment is readily available in most hospitals. Future studies will evaluate outcomes in spontaneously breathing and ventilated patients to determine the accuracy of this measurement technique in volume‐depleted and volume‐overloaded states. If validated in different patient populations, ultrasound measurement of the internal jugular vein could substitute for the EGDT CVP target in critically ill patients and allow early aggressive fluid resuscitation before a central venous catheter is placed.
Limitations
This exploratory study enrolled a small convenience sample of primarily white patients. The convenience sample is potentially prone to selection bias since a majority of patients who may have been eligible were never asked to participate. Also, not all patients had sepsis syndrome; our intention was to measure CVP and aspect ratio for available critically ill patients. Accordingly, results may be different depending on severity of illness. In addition, some of the patients were transferred from outside medical centers or from emergency departments and therefore may have already been partly resuscitated. Another limitation is that the intraobserver and interobserver variability for the healthy volunteers showed only moderate agreement, possibly indicating limited repeatability, although these results could be due to the small sample size. Also, we did not determine intraobserver and interobserver variability for the critically ill patients; results may be different from those of the healthy volunteers. Furthermore, underlying conditions such as tricuspid stenosis or regurgitation and cardiac tamponade may affect measurement results, but we included all patients without formal assessment, since treatment was performed on an urgent/emergent basis as would happen in real clinical settings.
Acknowledgements
The authors dedicate this work to their patients with severe sepsis. They thank Lisa Kirkland, MD, and Murat Yilmaz, MD, for their assistance with this study. They also thank the Mayo Clinic Divisions of General Internal Medicine and Pulmonary and Critical Care Medicine for funding.
Severe sepsis and septic shock account for more than 750,000 hospital admissions and 215,000 deaths per year.1 Early fluid resuscitation is the cornerstone of treatment, and early goal‐directed therapy (EGDT), which includes a target central venous pressure (CVP) of 8 to 12 mm Hg, has been shown to improve outcomes, including mortality and length of stay.2 This goal allows appropriate initial resuscitation and may decrease the risk of excess fluid administration, which is related to adverse outcomes in critically ill patients.3 However, nonintensivists may not start early aggressive fluid resuscitation because of inability to accurately assess intravascular volume, concerns for inadvertent volume overload, or the difficulty of recognizing insidious illness. Assessment of volume status, primarily from inspection of the internal jugular vein to estimate CVP, is difficult to perform by clinical examination alone, especially if CVP is very low.4 Inspection of the external jugular vein is perhaps easier than inspecting the internal jugular vein and appears to accurately estimate CVP,5 but it does not allow the degree of precision necessary for EGDT. Echocardiography can estimate CVP based on respirophasic variation or collapsibility index, but this technique requires expensive equipment and sonographic expertise. The current gold standard technique for measuring CVP requires an invasive central venous catheter, which can delay timely resuscitation and is associated with complications.6
An alternative technique to guide resuscitation efforts should be accurate, safe, rapid, and easy to perform at the bedside, while providing real‐time measurement results. We hypothesized that CVP can be accurately assessed using noninvasive ultrasound imaging of the internal jugular vein, since jugular venous pressure is essentially equal to CVP.7 Specifically, our study estimated the diagnostic accuracy of ultrasound measurement of the aspect ratio (height/width) of the internal jugular vein compared with the invasively measured CVP target for EGDT. We expected that a lower aspect ratio would correlate with a lower CVP and a higher aspect ratio would correlate with a higher CVP.
Methods
Volunteers were enrolled at Saint Mary's Hospital (Mayo Clinic) in Rochester, MN, from January to March 2006, and patients were enrolled at Saint Mary's Hospital and at Abbott Northwestern Hospital (Allina Hospitals and Clinics) in Minneapolis, MN, from May 2006 to October 2007. The study was approved by the Institutional Review Boards of Mayo Clinic and Allina and had 2 phases. The first phase comprised ultrasound measurements of internal jugular vein aspect ratio and determination of intraobserver and interobserver agreement in healthy volunteers. The second phase involved measurement of internal jugular vein aspect ratio and invasive CVP in a convenience sample of 44 spontaneously breathing patients admitted to medical intensive care units: 9 patients at Saint Marys Hospital and 35 patients at Abbott Northwestern Hospital. Patients were enrolled only when study members were on duty in the intensive care unit and able to perform study measurements. As a result, a high proportion of patients who may have been eligible were not asked to participate.
Each volunteer was deemed euvolemic on the basis of normal orthostatic measurements and normal oral intake with no vomiting or diarrhea in the previous 5 days. Measurements of 19 volunteers were made by 1 author (A.S.K.), with subsequent measurements of 15 of the volunteers made by another author (O.G.) to determine interobserver variability; 4 participants did not undergo a second measurement because of scheduling conflicts.
Inclusion and exclusion criteria for the critically ill patients are provided in Table 1. Recruitment was based on presenting symptoms and test results that led the intensive care unit physicians to decide to place a CVP monitor. All the enrolled patients had invasive CVP measurement performed approximately 30 to 40 minutes after ultrasound measurement of the internal jugular vein; this delay was the time required to place the central line and obtain the measurement. All patients who were invited to participate in the study were included. No patients were excluded on the basis of the exclusion criteria or because of inability to place a central line. No complications related to central line placement occurred.
| Inclusion criteria |
| 1. Aged 18 years or older |
| 2. Admission to the intensive care unit |
| 3. Spontaneously breathing (not intubated/ventilated) |
| 4. Planned insertion of a central venous pressure monitor for therapy |
| Exclusion criteria |
| 1. Known cervical spine injuries or fusion |
| 2. Nonremovable cervical collars |
| 3. Surgical dressings that would prevent visualization of the internal jugular vein |
| 4. Inability of the patient to be properly positioned |
| 5. A code situation |
We followed a prescribed measurement technique (Table 2) to determine the internal jugular vein aspect ratio in all volunteers and patients. Measurements of the volunteers were made with a Site‐Rite 3 Ultrasound System (Bard Access Systems, Inc., Salt Lake City, UT) using a 9.0‐MHz transducer. Measurements of the critically ill patients were made with a SonoSite MicroMaxx ultrasound system (SonoSite, Inc., Bothell, WA) using a 10.5‐MHz transducer. Study team physicians initially were blinded to actual measured CVP. Internal jugular vein aspect ratio and CVP were measured at tidal volume end‐expiration for all patients. One measurement was obtained for each patient, with measurements being made by 1 of 4 physicians (2 intensivists, 1 critical care fellow, and 1 chief medicine resident). With no specific ultrasound training and with only minimal practice, the physicians could obtain the optimal aspect ratio within a few seconds (Figure 1).

| 1. Position the patient supine (0) with head and legs flat, ensuring overall comfort. A small pillow can be used to help keep head, neck, and trunk aligned |
| 2. Have the patient rotate his or her head slightly to the side (<30) to expose the internal jugular vein |
| 3. Place the transducer transversely on the patient's neck over the expected location of the internal jugular vein. The transducer should be perpendicular to the patient's neck |
| 4. Apply slight pressure to the transducer to locate the internal jugular vein on the view screen. Use the minimum pressure necessary to obtain a good quality ultrasound image |
| 5. Once the internal jugular vein is found, adjust the position of the transducer over the vein to obtain the most circular cross‐sectional image |
| 6. Have the patient breathe normally, then ask him or her to briefly stop breathing at normal (tidal volume) end‐expiration |
| 7. Store the best end‐expiration image (in which the internal jugular vein appears most circular) and have the patient resume normal breathing |
| 8. Measure the height and width of the internal jugular vein using the built‐in cursor function or a ruler |
This was an exploratory prospective study, and all methods of data collection were designed before patient enrollment. However, the ultrasound‐derived aspect ratio of 0.83 (which defined a CVP of 8 mm Hg) was determined post hoc to maximize sensitivity and specificity and was based on the aspect ratio of the euvolemic volunteers and the inflection point of the CVP vs aspect ratio curve for the critically ill patients.
Statistical Analysis
Groups were compared using the 2 test for differences in proportions and the Wilcoxon rank sum test for continuous data. P < 0.05 was considered statistically significant. Bland‐Altman plots were used to describe the bias and variability of the aspect ratio within and between observers.8 This technique compares 2 methods of measurement to determine agreement and repeatability by plotting the mean of the differences (which should be zero) and the upper and lower limits of agreement (1.96 standard deviations [SDs] of those differences above and below the mean). Results were calculated using the available data; there was no adjustment for missing data. Analyses were performed using SPLUS and SAS/STAT software (SAS Institute, Inc., Cary, NC).
Results
We first evaluated 19 white volunteers: 12 women and 7 men. Mean (SD) age was 42 (11) years and mean body mass index was 26.6 (4.5) kg/m2. Mean arterial pressure was 89 (13) mm Hg and mean heart rate was 71 (15) beats/minute. Mean aspect ratio of the right and left internal jugular vein for all volunteers was 0.82 (0.07). There was no difference in aspect ratio between the right (0.83 [0.10]) and left (0.81 [0.13]) vein (P > 0.10). Also, no difference in the aspect ratio was seen between men (0.81 [0.08]) and women (0.83 [0.07]) (P = 0.77). Bland‐Altman analysis indicated moderate intraobserver and interobserver agreement for the aspect ratio measurements (Figure 2).

We then compared the aspect ratio measured using ultrasound and CVP measured with an invasive monitor for 44 spontaneously breathing critically ill patients (22 women and 22 men; 38 were white). Mean (SD) age was 66 (14) years and mean body mass index was 28.8 (9.1) kg/m2. Mean arterial pressure (n = 36) was 67 (12) mm Hg and mean heart rate (n = 34) was 92 (22) beats/minute. Systemic inflammatory response syndrome (SIRS) criteria were present in 23 of 40 patients; complete data were unavailable for the other 4 patients. Of these 40 patients, 20 had sepsis, 15 had severe sepsis, and 5 had septic shock. The most common diagnoses were gastrointestinal tract bleeding in 6 patients and congestive heart failure in 4 patients. Acute Physiology and Chronic Health Evaluation (APACHE III) score, available for 8 of the 9 patients at Saint Marys Hospital, was 63 (10).
Figure 3 shows measured aspect ratios vs. invasively measured CVP for the critically ill patients. The curvilinear result is consistent with venous and right ventricular compliance ( volume/ pressure) characteristics. Note that the inflection point (beginning of the increased slope) of the curve corresponds to a CVP of about 8 mm Hg. Furthermore, the aspect ratio (0.8) at this point is the same as that seen in the euvolemic volunteers. These findings suggest that, in spontaneously breathing patients, a CVP of about 8 mm Hg and an aspect ratio of about 0.8 each defines the beginning of the plateau on the cardiac Frank‐Starling curve.

Ultrasound imaging of the internal jugular vein aspect ratio accurately estimated the CVP target of 8 mm Hg based on the area under the receiver operating characteristics curve of 0.84 (95% confidence interval [CI], 0.72‐0.96) (Figure 4). For an invasively measured CVP of less than 8 mm Hg, the likelihood ratio for a positive ultrasound test result (aspect ratio < 0.83) was 3.5 (95% CI, 1.4‐8.4) and for a negative test result (aspect ratio 0.83) was 0.30 (95% CI, 0.14‐0.62). Clinically, this means that patients with a measured aspect ratio of less than 0.83 require further fluid resuscitation, whereas patients with a measured aspect ratio of 0.83 or greater are less likely to benefit from fluid resuscitation.

Discussion
This study demonstrated that the EGDT CVP target of 8 to 12 mm Hg can be accurately estimated (referenced to invasive CVP monitoring) using noninvasive ultrasound measurement of the internal jugular vein in spontaneously breathing critically ill patients. The measurement process is simple to perform at the bedside and moderately reliable when performed by different observers; also, the results appear to be equivalent for both sides and for males or females. Images can be stored electronically for serial comparisons and for viewing by other caregivers. Because the aspect ratio is essentially constant over the length of the internal jugular vein, unlike diameter, measurements can be performed anywhere along the vein. Also, ultrasound imaging allows visualization of the internal jugular vein despite anatomic variation.9
Previous attempts at noninvasive hemodynamic monitoring using plethysmography, thoracic electrical bioimpedance, and external Doppler probes have shown these methods to be cumbersome or inaccurate.1013 Other investigators have used echocardiography14, 15 and handheld ultrasound16 to image the diameter of the inferior vena cava in order to assess intravascular volume status, but these techniques require expertise in sonographic imaging. An alternative technique is to measure peripheral venous pressure, which correlates with CVP.17 This method, however, requires technical expertise to zero the monitor and is not yet widely used for critically ill patients. A literature search found 1 letter to the editor suggesting that real‐time ultrasound imaging of the internal jugular vein could be used to qualitatively determine jugular venous pressure18 and 3 studies using ultrasound in conjunction with a pressure transducer or manometer to determine the pressure needed to collapse the vein (either the internal jugular or a peripheral vein), with subsequent correlation to CVP.1921 These latter techniques appear to be cumbersome and require custom equipment that is not readily available in most hospitals.
Any measurement of CVP, including our technique, assumes correlation with volume responsiveness as a surrogate for intravascular volume. However, CVP is governed by multiple physiologic and pathologic factors, including intravascular volume, vascular and ventricular compliance, ventricular function, tricuspid stenosis and regurgitation, cardiac tamponade, and atrioventricular dissociation.22, 23 Therefore, CVP alone may not be an accurate measure of volume responsiveness (intravascular volume). CVP may also have spontaneous variation similar to pulmonary capillary wedge pressure, which can be as high as 7 mm Hg in any given patient.24 Furthermore, invasive CVP monitors also have limitations, and the overall accuracy of the Philips system used at Saint Marys Hospital is 4% of the reading or 4 mm Hg, whichever is greater.25 Nonetheless, the EGDT algorithm that incorporates CVP measurement with a target of 8 to 12 mm Hg in spontaneously breathing patients and 12 mm Hg in mechanically ventilated patients has resulted in decreased mortality among patients with severe sepsis and is recommended by the Surviving Sepsis Campaign guidelines26 and the Institute for Healthcare Improvement.27
These study results are important because nonintensivists such as hospitalists and emergency department physicians can use this technique to provide rapid fluid resuscitation early in the course of severe sepsis and septic shock, when aggressive fluid resuscitation is most effective. Ultrasound imaging of the internal jugular vein is easy to perform without formal training, and the equipment is readily available in most hospitals. Future studies will evaluate outcomes in spontaneously breathing and ventilated patients to determine the accuracy of this measurement technique in volume‐depleted and volume‐overloaded states. If validated in different patient populations, ultrasound measurement of the internal jugular vein could substitute for the EGDT CVP target in critically ill patients and allow early aggressive fluid resuscitation before a central venous catheter is placed.
Limitations
This exploratory study enrolled a small convenience sample of primarily white patients. The convenience sample is potentially prone to selection bias since a majority of patients who may have been eligible were never asked to participate. Also, not all patients had sepsis syndrome; our intention was to measure CVP and aspect ratio for available critically ill patients. Accordingly, results may be different depending on severity of illness. In addition, some of the patients were transferred from outside medical centers or from emergency departments and therefore may have already been partly resuscitated. Another limitation is that the intraobserver and interobserver variability for the healthy volunteers showed only moderate agreement, possibly indicating limited repeatability, although these results could be due to the small sample size. Also, we did not determine intraobserver and interobserver variability for the critically ill patients; results may be different from those of the healthy volunteers. Furthermore, underlying conditions such as tricuspid stenosis or regurgitation and cardiac tamponade may affect measurement results, but we included all patients without formal assessment, since treatment was performed on an urgent/emergent basis as would happen in real clinical settings.
Acknowledgements
The authors dedicate this work to their patients with severe sepsis. They thank Lisa Kirkland, MD, and Murat Yilmaz, MD, for their assistance with this study. They also thank the Mayo Clinic Divisions of General Internal Medicine and Pulmonary and Critical Care Medicine for funding.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29(7):1303–1310.
- ,,, et al.Early Goal‐Directed Therapy Collaborative Group. Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345(19):1368–1377.
- ,.Fluid therapy in resuscitated sepsis: less is more.Chest.2008;133(1):252–263.
- ,.The rational clinical examination: does this patient have abnormal central venous pressure?JAMA.1996;275(8):630–634.
- ,,,,,.Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients.Arch Intern Med.2006;166(19):2132–2137.
- ,.Central venous catheterization.Crit Care Med.2007;35(5):1390–1396.
- .How to use central venous pressure measurements.Curr Opin Crit Care.2005;11(3):264–270.
- ,.Statistical methods for assessing agreement between two methods of clinical measurement.Lancet.1986;1(8476):307–310.
- ,.Anatomical variations of internal jugular vein location: impact on central venous access.Crit Care Med.1991;19(12):1516–1519.
- ,,.Noninvasive measurement of central venous pressure by neck inductive plethysmography.Chest.1991;100(2):371–375.
- ,,, et al.A new noninvasive method to determine central venous pressure.Resuscitation.2006;70(2):238–246.
- .Advances in critical care monitoring.Arch Surg.1997;132(7):734–739.
- ,,, et al.Continuous recording of pulmonary artery diastolic pressure and cardiac output using a novel ultrasound transducer.J Am Soc Echocardiogr.2002;15(11):1381–1386.
- ,,,,.Measurement of anterior‐posterior diameter of inferior vena cava by ultrasonography: a new non‐invasive method to assess acute changes in vascular filling state.Cardiovasc Res.1994;28(8):1269–1272.
- ,,,.Utility of the inferior vena cava diameter as a marker of dry weight in nonoliguric hemodialyzed patients.ASAIO J.2001;47(5):528–532.
- ,,, et al.Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic.Clin J Am Soc Nephrol.2006;1(4):749–753.
- ,,, et al.Comparison of peripheral and central venous pressures in critically ill patients.Anaesth Intensive Care.2003;31(1):34–39.
- .Determination of elevated jugular venous pressure by real‐time ultrasound.Ann Emerg Med.1999;34(1):115.
- ,,,.Ultrasound‐guided noninvasive measurement of a patient's central venous pressure.Conf Proc IEEE Eng Med Biol Soc.2006;1:3843–3849.
- ,,, et al.Noninvasive central venous pressure measurement by controlled compression sonography at the forearm.J Am Coll Cardiol.2007;50(16):1584–1589.
- ,,,,,.Estimation of central venous pressure by ultrasound.Resuscitation.2005;64(2):193–199.
- ,,, et al.Determination of total effective vascular compliance in patients with sepsis syndrome.Am J Respir Crit Care Med.1998;157(1):50–56.
- ,,.Central venous pressure: uses and limitations. In: Pinsky MR, Payen D, eds.Functional Hemodynamic Monitoring.Berlin, Germany:Springer‐Verlag Berlin Heidelberg;2006:101.
- ,.Normal fluctuations in pulmonary artery and pulmonary capillary wedge pressures in acutely ill patients.Heart Lung.1982;11(5):393–398.
- Philips M3012A Data Sheet.Hemodynamic extension to the multi‐measurement server.Amsterdam:Koninklijke Philips Electronics N.V.;2003.
- ,,, et al.Surviving Sepsis Campaign Management Guidelines Committee. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32(3):858–873. [Erratua: Crit Care Med. 2004;32(6):1448. Correction of dosage error in text. Crit Care Med. 2004;32(10):2169–2170.]
- Institute for Healthcare Improvement.Sepsis.Cambridge, MA:Institute for Healthcare Improvement. Available at:http://www.ihi.org/IHI/Topics/CriticalCare/Sepsis. Accessed March 2009.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29(7):1303–1310.
- ,,, et al.Early Goal‐Directed Therapy Collaborative Group. Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345(19):1368–1377.
- ,.Fluid therapy in resuscitated sepsis: less is more.Chest.2008;133(1):252–263.
- ,.The rational clinical examination: does this patient have abnormal central venous pressure?JAMA.1996;275(8):630–634.
- ,,,,,.Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients.Arch Intern Med.2006;166(19):2132–2137.
- ,.Central venous catheterization.Crit Care Med.2007;35(5):1390–1396.
- .How to use central venous pressure measurements.Curr Opin Crit Care.2005;11(3):264–270.
- ,.Statistical methods for assessing agreement between two methods of clinical measurement.Lancet.1986;1(8476):307–310.
- ,.Anatomical variations of internal jugular vein location: impact on central venous access.Crit Care Med.1991;19(12):1516–1519.
- ,,.Noninvasive measurement of central venous pressure by neck inductive plethysmography.Chest.1991;100(2):371–375.
- ,,, et al.A new noninvasive method to determine central venous pressure.Resuscitation.2006;70(2):238–246.
- .Advances in critical care monitoring.Arch Surg.1997;132(7):734–739.
- ,,, et al.Continuous recording of pulmonary artery diastolic pressure and cardiac output using a novel ultrasound transducer.J Am Soc Echocardiogr.2002;15(11):1381–1386.
- ,,,,.Measurement of anterior‐posterior diameter of inferior vena cava by ultrasonography: a new non‐invasive method to assess acute changes in vascular filling state.Cardiovasc Res.1994;28(8):1269–1272.
- ,,,.Utility of the inferior vena cava diameter as a marker of dry weight in nonoliguric hemodialyzed patients.ASAIO J.2001;47(5):528–532.
- ,,, et al.Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic.Clin J Am Soc Nephrol.2006;1(4):749–753.
- ,,, et al.Comparison of peripheral and central venous pressures in critically ill patients.Anaesth Intensive Care.2003;31(1):34–39.
- .Determination of elevated jugular venous pressure by real‐time ultrasound.Ann Emerg Med.1999;34(1):115.
- ,,,.Ultrasound‐guided noninvasive measurement of a patient's central venous pressure.Conf Proc IEEE Eng Med Biol Soc.2006;1:3843–3849.
- ,,, et al.Noninvasive central venous pressure measurement by controlled compression sonography at the forearm.J Am Coll Cardiol.2007;50(16):1584–1589.
- ,,,,,.Estimation of central venous pressure by ultrasound.Resuscitation.2005;64(2):193–199.
- ,,, et al.Determination of total effective vascular compliance in patients with sepsis syndrome.Am J Respir Crit Care Med.1998;157(1):50–56.
- ,,.Central venous pressure: uses and limitations. In: Pinsky MR, Payen D, eds.Functional Hemodynamic Monitoring.Berlin, Germany:Springer‐Verlag Berlin Heidelberg;2006:101.
- ,.Normal fluctuations in pulmonary artery and pulmonary capillary wedge pressures in acutely ill patients.Heart Lung.1982;11(5):393–398.
- Philips M3012A Data Sheet.Hemodynamic extension to the multi‐measurement server.Amsterdam:Koninklijke Philips Electronics N.V.;2003.
- ,,, et al.Surviving Sepsis Campaign Management Guidelines Committee. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32(3):858–873. [Erratua: Crit Care Med. 2004;32(6):1448. Correction of dosage error in text. Crit Care Med. 2004;32(10):2169–2170.]
- Institute for Healthcare Improvement.Sepsis.Cambridge, MA:Institute for Healthcare Improvement. Available at:http://www.ihi.org/IHI/Topics/CriticalCare/Sepsis. Accessed March 2009.
Copyright © 2009 Society of Hospital Medicine