User login
Antireflux surgery in the proton pump inhibitor era
For most patients with gastroesophageal reflux disease (GERD), a proton pump inhibitor (PPI) is the first choice for treatment.1 But some patients have symptoms that persist despite PPI therapy, some desire surgery despite successful PPI therapy, and some have persistent extraesophageal symptoms or other complications of reflux. For these patients, surgery is an option.2
In this article, we review the management of GERD and clarify the indications for antireflux surgery based on evidence of safety and efficacy.
GERD DEFINED: SYMPTOMS OR COMPLICATIONS
Defining the role of antireflux surgery is difficult, given the variety of presentations and the absence of a gold standard for diagnosing GERD. Most adults experience several episodes of physiologic reflux daily without symptoms.3 But a broad array of symptoms have been attributed to GERD, including chest pain, cough, and sore throat, and some conditions caused by acid reflux (eg, Barrett esophagus) can be asymptomatic.4,5
HEARTBURN ISN’T ALWAYS GERD
Typical GERD presents with the classic symptoms of pyrosis (heartburn) or acid regurgitation, or both.
Although these symptoms are often thought to be specific for GERD, other causes of esophageal injury— eg, eosinophilic esophagitis, infection (Candida, cytomegalovirus, herpes simplex virus), pill-induced esophagitis, or radiation therapy—can produce similar symptoms. Other causes, including coronary artery disease, biliary colic, foregut malignancy, or peptic ulcer disease, should also be considered in patients with supposedly typical GERD. Life-threatening mimics of GERD, such as unstable angina, should be excluded if they are likely, before proceeding with evaluating for possible GERD. Therefore, the initial history and examination should focus on appropriate diagnosis, with careful delineation of symptom quality.
Alarm features for advanced pathology6–8 include involuntary weight loss, dysphagia, vomiting, evidence of gastrointestinal blood loss, anemia, chest pain, and an epigastric mass.7 Admittedly, these features are only mediocre for detecting or excluding gastric or esophageal cancer, with a sensitivity of 67% and a specificity 66%.9 Nevertheless, they should prompt an endoscopic examination. In patients who have alarm features but have not yet been treated for GERD, upper endoscopy can identify an abnormality in about 60% of patients.10–12
PPIs HAVE REPLACED ANTACIDS AND HISTAMINE-2 RECEPTOR ANTAGONISTS
When the symptoms suggest GERD and no alarm features are present, an initial trial of the following lifestyle changes is reasonable:
- Avoiding acidic or refluxogenic foods (coffee, alcohol, chocolate, peppermint, fatty foods, citrus foods)
- Avoiding certain medications (anticholinergics, estrogens, calcium-channel blockers, nitroglycerine, benzodiazepines)
- Losing weight
- Quitting smoking
- Raising the head of the bed
- Staying upright for 2 to 3 hours after meals.
For someone with mild symptoms, these changes pose minimal risk. Unfortunately, they are unlikely to provide adequate symptom control for most patients.13–17
Before PPIs were invented, drug therapy for GERD symptoms that did not resolve with lifestyle changes consisted of antacids and, later, histamine-2 receptor antagonists. When maximal therapy failed to control symptoms, fundoplication surgery was considered an appropriate next step.
PPIs substantially changed the management of GERD, suppressing acid secretion much better than histamine-2 receptor antagonists. Taken 30 minutes before breakfast, a single daily dose of a PPI normalizes esophageal acid exposure in 67% of patients.18 Adding a second dose 30 minutes before dinner raises the number to more than 90%.19
PPIs have consistently outperformed histamine-2 blockers in the healing of esophagitis and in improving heartburn symptoms and are now the first-line medical therapy for uncomplicated GERD.6,8,20–25
WHEN PPIs WORK, SURGERY OFFERS NO ADVANTAGE
The LOTUS trial (Long-Term Usage of Esomeprazole vs Surgery for Treatment of Chronic GERD) compared long-term drug therapy with surgery to maintain remission of symptoms in GERD.27 In this trial, 554 patients whose symptoms initially responded to the PPI esomeprazole (Nexium) were randomized to continue to receive esomeprazole (n = 266) or to undergo laparoscopic antireflux surgery (288 were randomly assigned, and 248 had the operation). Dose adjustment of the esomeprazole was allowed (20–40 mg/day). A total of 372 patients completed 5 years of follow-up (192 esomeprazole, 180 surgery).
Symptoms stayed in remission in 92% of the esomeprazole group and 85% of the surgery group (P = .048). However, the difference was no longer statistically significant after modeling the effects of study dropout. The rate of severe adverse events was similar in both groups: 24.1% with esomeprazole and 28.6% with surgery.
These findings indicate that if symptoms fully abate with medical therapy, surgery offers no advantage. In addition, patients who desire surgery in the hope of avoiding lifelong drug therapy should be made aware that drug therapy and reoperation are often necessary after surgery.28 In most cases, antireflux surgery is unnecessary for patients whose GERD fully responds to PPI therapy.
IF PPIs FAIL, FURTHER TESTING NEEDED
But many patients who take PPIs still have symptoms, even though these drugs suppress acid secretion and heal esophagitis. In fact, symptoms completely resolve in only about one-half of patients with erosive disease and one-third of those without erosive disease.21
Reasons for an incomplete symptomatic response to PPIs are various. Acid reflux can persist, but this accounts for only 10% of cases.29 About one-third of patients have persistent reflux that is weakly acidic, with a pH higher than 4.29. However, most patients with persistent typical GERD symptoms do not have significant, persistent reflux, or their symptoms are not related to reflux events. In these cases, an alternative cause of the refractory symptoms should be sought.
Further diagnostic testing is indicated when symptoms persist despite PPI therapy. Upper endoscopy will reveal an abnormality such as persistent erosive esophagitis, eosinophilic esophagitis, esophageal stricture, Barrett esophagus, or esophageal cancer in roughly 10% of patients in whom empiric therapy fails.10
Although patients with persistent symptoms have not been enrolled in many randomized controlled trials, a multivariate analysis showed that failure of medical therapy heralds a poor response to surgery.30 Data such as these have led most experts to discourage fundoplication for such patients now, unlike in the pre-PPI era.
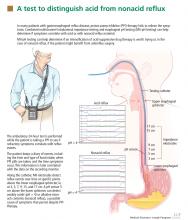
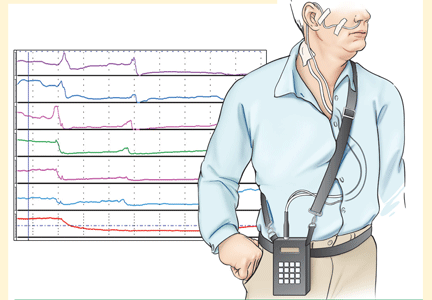
pH and intraluminal impedance testing
However, this recommendation against surgery is not a hard-and-fast rule.
In patients with esophageal regurgitation, most will not achieve adequate relief of symptoms with PPI therapy alone.34 The therapeutic gain of PPI therapy vs placebo averaged just 17% in seven randomized, controlled trials, more than 20% less than the response rate for heartburn.34 This is likely because of structural abnormalities such as reduced lower esophageal sphincter pressure, hiatal hernia, or delayed gastric emptying. Antireflux surgery can correct these structural abnormalities or prevent them from causing so much trouble; however, the presence of true regurgitation should first be confirmed by MII testing. If regurgitation is confirmed, antireflux surgery is warranted, particularly in patients with nocturnal symptoms who may be at high risk of aspiration. With careful patient selection, regurgitation symptoms improve in about 90% after surgery.2
In patients with heartburn, if esophageal acid exposure continues to be abnormal on MII-pH testing, then an escalation of therapy may improve symptoms, particularly if symptoms occur during reflux or if they partially responded to PPI therapy. Options in this scenario include alteration or intensification of acid-suppressive therapy, treatment with baclofen (Lioresal), and antireflux surgery.18,35,36 In randomized controlled trials of patients whose symptoms partially responded to PPIs, antireflux surgery has performed similarly to PPIs in terms of improving typical GERD symptoms, particularly regurgitation.27,37–41 Although this scenario is a reasonable indication for antireflux surgery, recommendations should be made with appropriate restraint since it is not easily reversible, some patients experience complications, and up to one-third will have no therapeutic benefit.30
Nonacid reflux. In some cases, MII-pH testing during PPI therapy will reveal reflux of weakly acidic (pH > 4) or alkaline stomach contents, often called “nonacid reflux.”29 Nonacid reflux is often present in patients with esophagitis that persists despite PPI therapy, indicating that even weakly acidic stomach contents can injure the mucosa.42 Since intensifying the acid-suppressive therapy is unlikely to improve these symptoms, antireflux surgery may have a role.
In one study,43 nonacid reflux was well controlled by laparoscopic Nissen fundoplication, although 15 (48%) of 31 patients had persistent symptoms of GERD after surgery. No patient had a strong symptom correlation with postoperative reflux events, suggesting an alternative cause of the persistent symptoms. Therefore, antireflux surgery for nonacid reflux should be limited exclusively to patients with strong symptom correlation, and even then it should be considered with restraint, given the limited evidence for benefit and the potential for harm.
If testing is negative. In studies investigating the diagnostic yield of MII-pH testing, more than 50% of patients who had refractory symptoms had a negative MII-pH test.29 In such situations, when the symptoms are strongly correlated with reflux events, the patient is said to have “esophageal hypersensitivity.” A few small studies have suggested that such patients may benefit from surgery, but these data have not been replicated in randomized controlled trials.32
When the testing is negative and there is no correlation between the patient’s symptoms and reflux events, the patient is unlikely to benefit from antireflux surgery. Care of these patients is beyond the scope of this review.
SURGERY RARELY IMPROVES COUGH, ASTHMA, OR LARYNGITIS
GERD has been implicated as a cause of chronic cough, asthma, and laryngitis, although each of these has many potential causes.44–46 Despite these associations, the evidence for therapeutic benefit from antireflux therapy is weak.
PPI therapy shows no benefit over placebo for chronic cough of uncertain etiology, but some benefit if GERD is objectively demonstrated.47 Laryngitis resolved in just 15% of patients on esomeprazole vs 16% of patients on placebo after excluding patients with moderate to severe heartburn.48
In a large randomized controlled trial in patients with asthma, there was no overall improvement in peak flow for the PPI group vs the placebo group, although significant improvement occurred in patients with heartburn and nocturnal respiratory symptoms.46
Potent antisecretory therapy seems to improve extraesophageal symptoms when typical GERD symptoms are also present, but it otherwise has shown little evidence of benefit.
The evidence for a benefit from antireflux surgery in patients with extraesophageal GERD syndromes is even more limited. Although one systematic review49 found that cough and other laryngeal symptoms improved in 60% to 100% of patients with objective evidence of GERD who underwent fundoplication, virtually all of the studies were uncontrolled case series.49
The lone randomized controlled trial in the systematic review compared Nissen fundoplication with ranitidine (Zantac) or antacids only for patients with asthma and GERD, and found no significant difference in peak expiratory flow among the three groups after 2 years. However, asthma symptom scores improved in 75% of the surgical group, 9% of the medical group, and 4% of the control group.50
In a study that was not included in the prior systematic review, patients with laryngopharyngeal reflux unresponsive to aggressive acid suppression who subsequently underwent fundoplication fared no better than those who did not.51
Thus, based on the available data, antireflux surgery is only rarely indicated for extraesophageal symptoms, especially in patients who have no typical GERD symptoms or in patients whose symptoms are refractory to medical therapy.
SURGERY FOR EROSIVE ESOPHAGITIS OR BARRETT ESOPHAGUS IF PPI FAILS
Lifelong antireflux therapy is indicated for patients with severe erosive esophagitis or Barrett esophagus. Erosive esophagitis recurs in more than 80% within 12 months of discontinuing antisecretory therapy.52 Both Barrett esophagus and esophageal adenocarcinoma are strongly associated with GERD, and nearly 10% of patients with chronic reflux have Barrett esophagus.53,54 It is suspected that suppressing reflux reduces the rate of progression of Barrett esophagus to esophageal adenocarcinoma, but this remains to be proven.
Perhaps the strongest indication for surgery in the PPI era is for patients who have persistent symptoms and severe erosive esophagitis (Los Angeles grade C or D) despite high-dose PPI therapy. If other causes of persistent esophagitis have been ruled out, fundoplication can induce healing and improve symptoms.55,56 In these cases, surgery is done to induce remission of the disease when maximal medical therapy has been truly unsuccessful.
Randomized controlled trials suggest that medical and surgical therapies are equally effective for preventing the recurrence of erosive esophagitis or the progression of Barrett esophagus. In a study of 225 patients, at 7 years of follow-up, esophagitis had recurred in 10.4% of patients on omeprazole vs 11.8% of those who had undergone antireflux surgery.40 Similarly, open Nissen fundoplication was no different from drug therapy (histamine-2 receptor antagonist or PPI) for progression of Barrett esophagus over a median of 5 years.57 A meta-analysis with nearly 5,000 person-years each in the medical and surgical groups also found no significant difference in rates of cancer progression.58
Notably, symptoms such as dysphagia, flatulence, and the inability to burp occurred significantly more often in the surgical groups in these studies.
In view of these data, antireflux surgery has no significant advantage over medical therapy for maintaining healing of erosive esophagitis or preventing progression of Barrett esophagus. Thus, it should be reserved for patients who do not desire lifelong drug therapy, provided they understand that there is no therapeutic advantage for their esophagitis or for Barrett esophagus.
SPECIFIC INDICATIONS FOR ANTIREFLUX SURGERY
Now that we have PPIs, several situations remain in which surgery for GERD is either indicated or worth considering.
Antireflux surgery is clearly indicated for:
- Patients with erosive esophagitis that does not heal with maximal drug therapy
- Patients with volume regurgitation, particularly if it occurs at night or if there is evidence of aspiration
- Patients who require lifelong treatment for reflux but who have had a serious adverse event related to PPI therapy, such as refractory Clostridium difficile infection.
Antireflux surgery is also worth considering in patients who for personal reasons wish to avoid long-term or lifelong drug therapy.
Patients should be informed, however, that antireflux surgery has not been shown to be better than medical therapy for maintaining remission of symptoms, for preventing progression of Barrett esophagus, or for maintaining healing of erosive esophagitis. Medical therapy is still the first option for these patients.
Surgery may also be considered in patients with persistent symptoms who have a partial response to medical therapy, who show persistent acidic or weakly acidic reflux on MII-pH testing, and whose symptoms have been correlated with reflux events. Although surgery is not sure to improve their symptoms, benefit is more likely in this patient population compared with those without these characteristics.
Extraesophageal GERD
In patients suspected of having extraesophageal GERD, surgery should be considered if typical GERD symptoms are present and improve with PPI therapy, if the extraesophageal syndrome partially responds to PPI therapy, and if MII-pH testing demonstrates a correlation between symptoms and reflux. Surgery may have a stronger indication in this setting if the patient has nocturnal reflux or extraesophageal symptoms.
When is surgery not an option?
In general, surgery should not be considered in patients who do not have a partial response to PPI therapy or who do not have a strong symptom-reflux correlation on MII-pH testing. In all cases of failed medical therapy without persistent severe erosive disease, the threshold for opting for surgery should be high, given the uncertain response of these patients to surgery.
Peristaltic dysfunction is a relative but not an absolute contraindication to surgery.59
RISKS, BENEFITS OF SURGERY FOR GERD
The patient’s preference for surgery over drug therapy should always be balanced against the risks of surgery, including both short-term and long-term adverse events, to allow the patient to make an adequately informed decision (Table 2).2,26
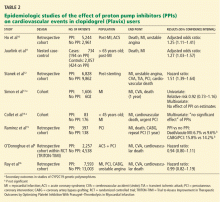
Adverse events associated with PPI therapy are rare and in many cases the association is debatable.26 Nonetheless, long-term PPI therapy has been most strongly associated with an increased risk of C difficile infection and other enteric infections, although the absolute risk of these events remains low.
Complication rates after antireflux surgery depend on the surgeon’s experience and technique. Death is exceedingly rare. In most high-volume centers, the need to convert from laparoscopic to open fundoplication occurs in fewer than 2.4% of patients.2
Potential perioperative complications include perforation (4%), wound infection (3%), and pneumothorax (2%).2
Antireflux surgery is also associated with a significant risk of dysphagia, bloating, an inability to burp, and excessive flatulence, all of which can markedly impair the quality of life.
A major consideration is that fundoplication is generally irreversible. Reoperation rates have been reported to range from 0% to 15%.2 Furthermore, up to 50% of patients still need medical therapy after surgery.60,61 Of note, only about 25% of patients on medical therapy after surgery will actually have an abnormal pH study.61
MORE STUDY NEEDED
Future studies directly comparing medical and surgical therapy for carefully selected patients with extraesophageal manifestations of GERD and refractory symptoms should help further delineate outcome in this difficult group of patients.
Under development are new drugs that may inhibit transient relaxation of the lower esophageal sphincter, as well as minimally invasive procedures, which may alter the indications for surgery in coming years.36
Acknowledgment: The research for this article was supported in part by a grant from the National Institutes of Health (T32 DK07634).
- Finks JF, Wei Y, Birkmeyer JD. The rise and fall of antireflux surgery in the United States. Surg Endosc 2006; 20:1698–1701.
- Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD; SAGES Guidelines Committee. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 2010; 24:2647–2669.
- Richter JE. Typical and atypical presentations of gastroesophageal reflux disease. The role of esophageal testing in diagnosis and management. Gastroenterol Clin North Am 1996; 25:75–102.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Dickman R, Kim JL, Camargo L, et al. Correlation of gastroesophageal reflux disease symptoms characteristics with long-segment Barrett’s esophagus. Dis Esophagus 2006; 19:360–365.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Armstrong D, Marshall JK, Chiba N, et al; Canadian Association of Gastroenterology GERD Consensus Group. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults - update 2004. Can J Gastroenterol 2005; 19:15–35.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.
- Vakil N, Moayyedi P, Fennerty MB, Talley NJ. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology 2006; 131:390–401.
- Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc 2010; 71:28–34.
- Dickman R, Mattek N, Holub J, Peters D, Fass R. Prevalence of upper gastrointestinal tract findings in patients with noncardiac chest pain versus those with gastroesophageal reflux disease (GERD)-related symptoms: results from a national endoscopic database. Am J Gastroenterol 2007; 102:1173–1179.
- Voutilainen M, Sipponen P, Mecklin JP, Juhola M, Färkkilä M. Gastroesophageal reflux disease: prevalence, clinical, endoscopic and histopathological findings in 1,128 consecutive patients referred for endoscopy due to dyspeptic and reflux symptoms. Digestion 2000; 61:6–13.
- Fraser-Moodie CA, Norton B, Gornall C, Magnago S, Weale AR, Holmes GK. Weight loss has an independent beneficial effect on symptoms of gastro-oesophageal reflux in patients who are overweight. Scand J Gastroenterol 1999; 34:337–340.
- Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA. Bodymass index and symptoms of gastroesophageal reflux in women. N Engl J Med 2006; 354:2340–2348.
- Kjellin A, Ramel S, Rössner S, Thor K. Gastroesophageal reflux in obese patients is not reduced by weight reduction. Scand J Gastroenterol 1996; 31:1047–1051.
- Waring JP, Eastwood TF, Austin JM, Sanowski RA. The immediate effects of cessation of cigarette smoking on gastroesophageal reflux. Am J Gastroenterol 1989; 84:1076–1078.
- Pehl C, Waizenhoefer A, Wendl B, Schmidt T, Schepp W, Pfeiffer A. Effect of low and high fat meals on lower esophageal sphincter motility and gastroesophageal reflux in healthy subjects. Am J Gastroenterol 1999; 94:1192–1196.
- Bajbouj M, Becker V, Phillip V, Wilhelm D, Schmid RM, Meining A. High-dose esomeprazole for treatment of symptomatic refractory gastroesophageal reflux disease—a prospective pH-metry/impedance-controlled study. Digestion 2009; 80:112–118.
- Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol 2005; 100:283–289.
- Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev 2007;CD003244.
- Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol 2004; 2:656–664.
- Sabesin SM, Berlin RG, Humphries TJ, Bradstreet DC, Walton-Bowen KL, Zaidi S. Famotidine relieves symptoms of gastroesophageal reflux disease and heals erosions and ulcerations. Results of a multicenter, placebo-controlled, dose-ranging study. USA Merck Gastroesophageal Reflux Disease Study Group. Arch Intern Med 1991; 151:2394–2400.
- van Pinxteren B, Numans ME, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2004;CD002095.
- Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology 1997; 112:1798–1810.
- Venables TL, Newland RD, Patel AC, Hole J, Wilcock C, Turbitt ML. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams once daily, or ranitidine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastro-oesophageal reflux disease in general practice. Scand J Gastroenterol 1997; 32:965–973.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- Galmiche JP, Hatlebakk J, Attwood S, et al; LOTUS Trial Collaborators. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 2011; 305:1969–1977.
- Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: followup of a randomized controlled trial. JAMA 2001; 285:2331–2338.
- Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 2006; 55:1398–1402.
- Campos GM, Peters JH, DeMeester TR, et al. Multivariate analysis of factors predicting outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg 1999; 3:292–300.
- Becker V, Bajbouj M, Waller K, Schmid RM, Meining A. Clinical trial: persistent gastro-oesophageal reflux symptoms despite standard therapy with proton pump inhibitors - a follow-up study of intraluminal-impedance guided therapy. Aliment Pharmacol Ther 2007; 26:1355–1360.
- Mainie I, Tutuian R, Agrawal A, Adams D, Castell DO. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg 2006; 93:1483–1487.
- del Genio G, Tolone S, del Genio F, et al. Prospective assessment of patient selection for antireflux surgery by combined multichannel intraluminal impedance pH monitoring. J Gastrointest Surg 2008; 12:1491–1496.
- Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol 2011; 106:1419–1425.
- Koek GH, Sifrim D, Lerut T, Janssens J, Tack J. Effect of the GABA(B) agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut 2003; 52:1397–1402.
- Boeckxstaens GE. Reflux inhibitors: a new approach for GERD? Curr Opin Pharmacol 2008; 8:685–689.
- Anvari M, Allen C, Marshall J, et al. A randomized controlled trial of laparoscopic nissen fundoplication versus proton pump inhibitors for treatment of patients with chronic gastroesophageal reflux disease: One-year follow-up. Surg Innov 2006; 13:238–249.
- Mahon D, Rhodes M, Decadt B, et al. Randomized clinical trial of laparoscopic Nissen fundoplication compared with proton-pump inhibitors for treatment of chronic gastro-oesophageal reflux. Br J Surg 2005; 92:695–699.
- Mehta S, Bennett J, Mahon D, Rhodes M. Prospective trial of laparoscopic nissen fundoplication versus proton pump inhibitor therapy for gastroesophageal reflux disease: Seven-year follow-up. J Gastrointest Surg 2006; 10:1312–1316.
- Lundell L, Miettinen P, Myrvold HE, et al; Nordic GORD Study Group. Seven-year follow-up of a randomized clinical trial comparing proton-pump inhibition with surgical therapy for reflux oesophagitis. Br J Surg 2007; 94:198–203.
- Lundell L, Attwood S, Ell C, et al; LOTUS trial collaborators. Comparing laparoscopic antireflux surgery with esomeprazole in the management of patients with chronic gastro-oesophageal reflux disease: a 3-year interim analysis of the LOTUS trial. Gut 2008; 57:1207–1213.
- Frazzoni M, Conigliaro R, Melotti G. Weakly acidic refluxes have a major role in the pathogenesis of proton pump inhibitor-resistant reflux oesophagitis. Aliment Pharmacol Ther 2011; 33:601–606.
- Broeders JA, Bredenoord AJ, Hazebroek EJ, Broeders IA, Gooszen HG, Smout AJ. Effects of anti-reflux surgery on weakly acidic reflux and belching. Gut 2011; 60:435–441.
- American Gastroenterological Association medical position statement: guidelines on the use of esophageal pH recording. Gastroenterology 1996; 110:1981.
- el-Serag HB, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology 1997; 113:755–760.
- Kiljander TO, Laitinen JO. The prevalence of gastroesophageal reflux disease in adult asthmatics. Chest 2004; 126:1490–1494.
- Chang AB, Lasserson TJ, Kiljander TO, Connor FL, Gaffney JT, Garske LA. Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. BMJ 2006; 332:11–17.
- Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006; 116:254–260.
- Iqbal M, Batch AJ, Spychal RT, Cooper BT. Outcome of surgical fundoplication for extraesophageal (atypical) manifestations of gastroesophageal reflux disease in adults: a systematic review. J Laparoendosc Adv Surg Tech A 2008; 18:789–796.
- Sontag SJ, O’Connell S, Khandelwal S, et al. Asthmatics with gastroesophageal reflux: long term results of a randomized trial of medical and surgical antireflux therapies. Am J Gastroenterol 2003; 98:987–999.
- Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol 2006; 4:433–441.
- Johnson DA, Benjamin SB, Vakil NB, et al. Esomeprazole once daily for 6 months is effective therapy for maintaining healed erosive esophagitis and for controlling gastroesophageal reflux disease symptoms: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Gastroenterol 2001; 96:27–34.
- Winters C, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987; 92:118–124.
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61:226–231.
- Rosenthal R, Peterli R, Guenin MO, von Flüe M, Ackermann C. Laparoscopic antireflux surgery: long-term outcomes and quality of life. J Laparoendosc Adv Surg Tech A 2006; 16:557–561.
- Broeders JA, Draaisma WA, Bredenoord AJ, Smout AJ, Broeders IA, Gooszen HG. Long-term outcome of Nissen fundoplication in non-erosive and erosive gastro-oesophageal reflux disease. Br J Surg 2010; 97:845–352.
- Parrilla P, Martínez de Haro LF, Ortiz A, et al. Long-term results of a randomized prospective study comparing medical and surgical treatment of Barrett’s esophagus. Ann Surg 2003; 237:291–298.
- Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett’s esophagus? A meta-analysis. Am J Gastroenterol 2003; 98:2390–2394.
- Pandolfino JE, Kahrilas PJ; American Gastroenterological Association. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 2005; 128:209–224.
- Dominitz JA, Dire CA, Billingsley KG, Todd-Stenberg JA. Complications and antireflux medication use after antireflux surgery. Clin Gastroenterol Hepatol 2006; 4:299–305.
- Lord RV, Kaminski A, Oberg S, et al. Absence of gastroesophageal reflux disease in a majority of patients taking acid suppression medications after Nissen fundoplication. J Gastrointest Surg 2002; 6:3–9.
For most patients with gastroesophageal reflux disease (GERD), a proton pump inhibitor (PPI) is the first choice for treatment.1 But some patients have symptoms that persist despite PPI therapy, some desire surgery despite successful PPI therapy, and some have persistent extraesophageal symptoms or other complications of reflux. For these patients, surgery is an option.2
In this article, we review the management of GERD and clarify the indications for antireflux surgery based on evidence of safety and efficacy.
GERD DEFINED: SYMPTOMS OR COMPLICATIONS
Defining the role of antireflux surgery is difficult, given the variety of presentations and the absence of a gold standard for diagnosing GERD. Most adults experience several episodes of physiologic reflux daily without symptoms.3 But a broad array of symptoms have been attributed to GERD, including chest pain, cough, and sore throat, and some conditions caused by acid reflux (eg, Barrett esophagus) can be asymptomatic.4,5
HEARTBURN ISN’T ALWAYS GERD
Typical GERD presents with the classic symptoms of pyrosis (heartburn) or acid regurgitation, or both.
Although these symptoms are often thought to be specific for GERD, other causes of esophageal injury— eg, eosinophilic esophagitis, infection (Candida, cytomegalovirus, herpes simplex virus), pill-induced esophagitis, or radiation therapy—can produce similar symptoms. Other causes, including coronary artery disease, biliary colic, foregut malignancy, or peptic ulcer disease, should also be considered in patients with supposedly typical GERD. Life-threatening mimics of GERD, such as unstable angina, should be excluded if they are likely, before proceeding with evaluating for possible GERD. Therefore, the initial history and examination should focus on appropriate diagnosis, with careful delineation of symptom quality.
Alarm features for advanced pathology6–8 include involuntary weight loss, dysphagia, vomiting, evidence of gastrointestinal blood loss, anemia, chest pain, and an epigastric mass.7 Admittedly, these features are only mediocre for detecting or excluding gastric or esophageal cancer, with a sensitivity of 67% and a specificity 66%.9 Nevertheless, they should prompt an endoscopic examination. In patients who have alarm features but have not yet been treated for GERD, upper endoscopy can identify an abnormality in about 60% of patients.10–12
PPIs HAVE REPLACED ANTACIDS AND HISTAMINE-2 RECEPTOR ANTAGONISTS
When the symptoms suggest GERD and no alarm features are present, an initial trial of the following lifestyle changes is reasonable:
- Avoiding acidic or refluxogenic foods (coffee, alcohol, chocolate, peppermint, fatty foods, citrus foods)
- Avoiding certain medications (anticholinergics, estrogens, calcium-channel blockers, nitroglycerine, benzodiazepines)
- Losing weight
- Quitting smoking
- Raising the head of the bed
- Staying upright for 2 to 3 hours after meals.
For someone with mild symptoms, these changes pose minimal risk. Unfortunately, they are unlikely to provide adequate symptom control for most patients.13–17
Before PPIs were invented, drug therapy for GERD symptoms that did not resolve with lifestyle changes consisted of antacids and, later, histamine-2 receptor antagonists. When maximal therapy failed to control symptoms, fundoplication surgery was considered an appropriate next step.
PPIs substantially changed the management of GERD, suppressing acid secretion much better than histamine-2 receptor antagonists. Taken 30 minutes before breakfast, a single daily dose of a PPI normalizes esophageal acid exposure in 67% of patients.18 Adding a second dose 30 minutes before dinner raises the number to more than 90%.19
PPIs have consistently outperformed histamine-2 blockers in the healing of esophagitis and in improving heartburn symptoms and are now the first-line medical therapy for uncomplicated GERD.6,8,20–25
WHEN PPIs WORK, SURGERY OFFERS NO ADVANTAGE
The LOTUS trial (Long-Term Usage of Esomeprazole vs Surgery for Treatment of Chronic GERD) compared long-term drug therapy with surgery to maintain remission of symptoms in GERD.27 In this trial, 554 patients whose symptoms initially responded to the PPI esomeprazole (Nexium) were randomized to continue to receive esomeprazole (n = 266) or to undergo laparoscopic antireflux surgery (288 were randomly assigned, and 248 had the operation). Dose adjustment of the esomeprazole was allowed (20–40 mg/day). A total of 372 patients completed 5 years of follow-up (192 esomeprazole, 180 surgery).
Symptoms stayed in remission in 92% of the esomeprazole group and 85% of the surgery group (P = .048). However, the difference was no longer statistically significant after modeling the effects of study dropout. The rate of severe adverse events was similar in both groups: 24.1% with esomeprazole and 28.6% with surgery.
These findings indicate that if symptoms fully abate with medical therapy, surgery offers no advantage. In addition, patients who desire surgery in the hope of avoiding lifelong drug therapy should be made aware that drug therapy and reoperation are often necessary after surgery.28 In most cases, antireflux surgery is unnecessary for patients whose GERD fully responds to PPI therapy.
IF PPIs FAIL, FURTHER TESTING NEEDED
But many patients who take PPIs still have symptoms, even though these drugs suppress acid secretion and heal esophagitis. In fact, symptoms completely resolve in only about one-half of patients with erosive disease and one-third of those without erosive disease.21
Reasons for an incomplete symptomatic response to PPIs are various. Acid reflux can persist, but this accounts for only 10% of cases.29 About one-third of patients have persistent reflux that is weakly acidic, with a pH higher than 4.29. However, most patients with persistent typical GERD symptoms do not have significant, persistent reflux, or their symptoms are not related to reflux events. In these cases, an alternative cause of the refractory symptoms should be sought.
Further diagnostic testing is indicated when symptoms persist despite PPI therapy. Upper endoscopy will reveal an abnormality such as persistent erosive esophagitis, eosinophilic esophagitis, esophageal stricture, Barrett esophagus, or esophageal cancer in roughly 10% of patients in whom empiric therapy fails.10
Although patients with persistent symptoms have not been enrolled in many randomized controlled trials, a multivariate analysis showed that failure of medical therapy heralds a poor response to surgery.30 Data such as these have led most experts to discourage fundoplication for such patients now, unlike in the pre-PPI era.
pH and intraluminal impedance testing
However, this recommendation against surgery is not a hard-and-fast rule.
In patients with esophageal regurgitation, most will not achieve adequate relief of symptoms with PPI therapy alone.34 The therapeutic gain of PPI therapy vs placebo averaged just 17% in seven randomized, controlled trials, more than 20% less than the response rate for heartburn.34 This is likely because of structural abnormalities such as reduced lower esophageal sphincter pressure, hiatal hernia, or delayed gastric emptying. Antireflux surgery can correct these structural abnormalities or prevent them from causing so much trouble; however, the presence of true regurgitation should first be confirmed by MII testing. If regurgitation is confirmed, antireflux surgery is warranted, particularly in patients with nocturnal symptoms who may be at high risk of aspiration. With careful patient selection, regurgitation symptoms improve in about 90% after surgery.2
In patients with heartburn, if esophageal acid exposure continues to be abnormal on MII-pH testing, then an escalation of therapy may improve symptoms, particularly if symptoms occur during reflux or if they partially responded to PPI therapy. Options in this scenario include alteration or intensification of acid-suppressive therapy, treatment with baclofen (Lioresal), and antireflux surgery.18,35,36 In randomized controlled trials of patients whose symptoms partially responded to PPIs, antireflux surgery has performed similarly to PPIs in terms of improving typical GERD symptoms, particularly regurgitation.27,37–41 Although this scenario is a reasonable indication for antireflux surgery, recommendations should be made with appropriate restraint since it is not easily reversible, some patients experience complications, and up to one-third will have no therapeutic benefit.30
Nonacid reflux. In some cases, MII-pH testing during PPI therapy will reveal reflux of weakly acidic (pH > 4) or alkaline stomach contents, often called “nonacid reflux.”29 Nonacid reflux is often present in patients with esophagitis that persists despite PPI therapy, indicating that even weakly acidic stomach contents can injure the mucosa.42 Since intensifying the acid-suppressive therapy is unlikely to improve these symptoms, antireflux surgery may have a role.
In one study,43 nonacid reflux was well controlled by laparoscopic Nissen fundoplication, although 15 (48%) of 31 patients had persistent symptoms of GERD after surgery. No patient had a strong symptom correlation with postoperative reflux events, suggesting an alternative cause of the persistent symptoms. Therefore, antireflux surgery for nonacid reflux should be limited exclusively to patients with strong symptom correlation, and even then it should be considered with restraint, given the limited evidence for benefit and the potential for harm.
If testing is negative. In studies investigating the diagnostic yield of MII-pH testing, more than 50% of patients who had refractory symptoms had a negative MII-pH test.29 In such situations, when the symptoms are strongly correlated with reflux events, the patient is said to have “esophageal hypersensitivity.” A few small studies have suggested that such patients may benefit from surgery, but these data have not been replicated in randomized controlled trials.32
When the testing is negative and there is no correlation between the patient’s symptoms and reflux events, the patient is unlikely to benefit from antireflux surgery. Care of these patients is beyond the scope of this review.
SURGERY RARELY IMPROVES COUGH, ASTHMA, OR LARYNGITIS
GERD has been implicated as a cause of chronic cough, asthma, and laryngitis, although each of these has many potential causes.44–46 Despite these associations, the evidence for therapeutic benefit from antireflux therapy is weak.
PPI therapy shows no benefit over placebo for chronic cough of uncertain etiology, but some benefit if GERD is objectively demonstrated.47 Laryngitis resolved in just 15% of patients on esomeprazole vs 16% of patients on placebo after excluding patients with moderate to severe heartburn.48
In a large randomized controlled trial in patients with asthma, there was no overall improvement in peak flow for the PPI group vs the placebo group, although significant improvement occurred in patients with heartburn and nocturnal respiratory symptoms.46
Potent antisecretory therapy seems to improve extraesophageal symptoms when typical GERD symptoms are also present, but it otherwise has shown little evidence of benefit.
The evidence for a benefit from antireflux surgery in patients with extraesophageal GERD syndromes is even more limited. Although one systematic review49 found that cough and other laryngeal symptoms improved in 60% to 100% of patients with objective evidence of GERD who underwent fundoplication, virtually all of the studies were uncontrolled case series.49
The lone randomized controlled trial in the systematic review compared Nissen fundoplication with ranitidine (Zantac) or antacids only for patients with asthma and GERD, and found no significant difference in peak expiratory flow among the three groups after 2 years. However, asthma symptom scores improved in 75% of the surgical group, 9% of the medical group, and 4% of the control group.50
In a study that was not included in the prior systematic review, patients with laryngopharyngeal reflux unresponsive to aggressive acid suppression who subsequently underwent fundoplication fared no better than those who did not.51
Thus, based on the available data, antireflux surgery is only rarely indicated for extraesophageal symptoms, especially in patients who have no typical GERD symptoms or in patients whose symptoms are refractory to medical therapy.
SURGERY FOR EROSIVE ESOPHAGITIS OR BARRETT ESOPHAGUS IF PPI FAILS
Lifelong antireflux therapy is indicated for patients with severe erosive esophagitis or Barrett esophagus. Erosive esophagitis recurs in more than 80% within 12 months of discontinuing antisecretory therapy.52 Both Barrett esophagus and esophageal adenocarcinoma are strongly associated with GERD, and nearly 10% of patients with chronic reflux have Barrett esophagus.53,54 It is suspected that suppressing reflux reduces the rate of progression of Barrett esophagus to esophageal adenocarcinoma, but this remains to be proven.
Perhaps the strongest indication for surgery in the PPI era is for patients who have persistent symptoms and severe erosive esophagitis (Los Angeles grade C or D) despite high-dose PPI therapy. If other causes of persistent esophagitis have been ruled out, fundoplication can induce healing and improve symptoms.55,56 In these cases, surgery is done to induce remission of the disease when maximal medical therapy has been truly unsuccessful.
Randomized controlled trials suggest that medical and surgical therapies are equally effective for preventing the recurrence of erosive esophagitis or the progression of Barrett esophagus. In a study of 225 patients, at 7 years of follow-up, esophagitis had recurred in 10.4% of patients on omeprazole vs 11.8% of those who had undergone antireflux surgery.40 Similarly, open Nissen fundoplication was no different from drug therapy (histamine-2 receptor antagonist or PPI) for progression of Barrett esophagus over a median of 5 years.57 A meta-analysis with nearly 5,000 person-years each in the medical and surgical groups also found no significant difference in rates of cancer progression.58
Notably, symptoms such as dysphagia, flatulence, and the inability to burp occurred significantly more often in the surgical groups in these studies.
In view of these data, antireflux surgery has no significant advantage over medical therapy for maintaining healing of erosive esophagitis or preventing progression of Barrett esophagus. Thus, it should be reserved for patients who do not desire lifelong drug therapy, provided they understand that there is no therapeutic advantage for their esophagitis or for Barrett esophagus.
SPECIFIC INDICATIONS FOR ANTIREFLUX SURGERY
Now that we have PPIs, several situations remain in which surgery for GERD is either indicated or worth considering.
Antireflux surgery is clearly indicated for:
- Patients with erosive esophagitis that does not heal with maximal drug therapy
- Patients with volume regurgitation, particularly if it occurs at night or if there is evidence of aspiration
- Patients who require lifelong treatment for reflux but who have had a serious adverse event related to PPI therapy, such as refractory Clostridium difficile infection.
Antireflux surgery is also worth considering in patients who for personal reasons wish to avoid long-term or lifelong drug therapy.
Patients should be informed, however, that antireflux surgery has not been shown to be better than medical therapy for maintaining remission of symptoms, for preventing progression of Barrett esophagus, or for maintaining healing of erosive esophagitis. Medical therapy is still the first option for these patients.
Surgery may also be considered in patients with persistent symptoms who have a partial response to medical therapy, who show persistent acidic or weakly acidic reflux on MII-pH testing, and whose symptoms have been correlated with reflux events. Although surgery is not sure to improve their symptoms, benefit is more likely in this patient population compared with those without these characteristics.
Extraesophageal GERD
In patients suspected of having extraesophageal GERD, surgery should be considered if typical GERD symptoms are present and improve with PPI therapy, if the extraesophageal syndrome partially responds to PPI therapy, and if MII-pH testing demonstrates a correlation between symptoms and reflux. Surgery may have a stronger indication in this setting if the patient has nocturnal reflux or extraesophageal symptoms.
When is surgery not an option?
In general, surgery should not be considered in patients who do not have a partial response to PPI therapy or who do not have a strong symptom-reflux correlation on MII-pH testing. In all cases of failed medical therapy without persistent severe erosive disease, the threshold for opting for surgery should be high, given the uncertain response of these patients to surgery.
Peristaltic dysfunction is a relative but not an absolute contraindication to surgery.59
RISKS, BENEFITS OF SURGERY FOR GERD
The patient’s preference for surgery over drug therapy should always be balanced against the risks of surgery, including both short-term and long-term adverse events, to allow the patient to make an adequately informed decision (Table 2).2,26
Adverse events associated with PPI therapy are rare and in many cases the association is debatable.26 Nonetheless, long-term PPI therapy has been most strongly associated with an increased risk of C difficile infection and other enteric infections, although the absolute risk of these events remains low.
Complication rates after antireflux surgery depend on the surgeon’s experience and technique. Death is exceedingly rare. In most high-volume centers, the need to convert from laparoscopic to open fundoplication occurs in fewer than 2.4% of patients.2
Potential perioperative complications include perforation (4%), wound infection (3%), and pneumothorax (2%).2
Antireflux surgery is also associated with a significant risk of dysphagia, bloating, an inability to burp, and excessive flatulence, all of which can markedly impair the quality of life.
A major consideration is that fundoplication is generally irreversible. Reoperation rates have been reported to range from 0% to 15%.2 Furthermore, up to 50% of patients still need medical therapy after surgery.60,61 Of note, only about 25% of patients on medical therapy after surgery will actually have an abnormal pH study.61
MORE STUDY NEEDED
Future studies directly comparing medical and surgical therapy for carefully selected patients with extraesophageal manifestations of GERD and refractory symptoms should help further delineate outcome in this difficult group of patients.
Under development are new drugs that may inhibit transient relaxation of the lower esophageal sphincter, as well as minimally invasive procedures, which may alter the indications for surgery in coming years.36
Acknowledgment: The research for this article was supported in part by a grant from the National Institutes of Health (T32 DK07634).
For most patients with gastroesophageal reflux disease (GERD), a proton pump inhibitor (PPI) is the first choice for treatment.1 But some patients have symptoms that persist despite PPI therapy, some desire surgery despite successful PPI therapy, and some have persistent extraesophageal symptoms or other complications of reflux. For these patients, surgery is an option.2
In this article, we review the management of GERD and clarify the indications for antireflux surgery based on evidence of safety and efficacy.
GERD DEFINED: SYMPTOMS OR COMPLICATIONS
Defining the role of antireflux surgery is difficult, given the variety of presentations and the absence of a gold standard for diagnosing GERD. Most adults experience several episodes of physiologic reflux daily without symptoms.3 But a broad array of symptoms have been attributed to GERD, including chest pain, cough, and sore throat, and some conditions caused by acid reflux (eg, Barrett esophagus) can be asymptomatic.4,5
HEARTBURN ISN’T ALWAYS GERD
Typical GERD presents with the classic symptoms of pyrosis (heartburn) or acid regurgitation, or both.
Although these symptoms are often thought to be specific for GERD, other causes of esophageal injury— eg, eosinophilic esophagitis, infection (Candida, cytomegalovirus, herpes simplex virus), pill-induced esophagitis, or radiation therapy—can produce similar symptoms. Other causes, including coronary artery disease, biliary colic, foregut malignancy, or peptic ulcer disease, should also be considered in patients with supposedly typical GERD. Life-threatening mimics of GERD, such as unstable angina, should be excluded if they are likely, before proceeding with evaluating for possible GERD. Therefore, the initial history and examination should focus on appropriate diagnosis, with careful delineation of symptom quality.
Alarm features for advanced pathology6–8 include involuntary weight loss, dysphagia, vomiting, evidence of gastrointestinal blood loss, anemia, chest pain, and an epigastric mass.7 Admittedly, these features are only mediocre for detecting or excluding gastric or esophageal cancer, with a sensitivity of 67% and a specificity 66%.9 Nevertheless, they should prompt an endoscopic examination. In patients who have alarm features but have not yet been treated for GERD, upper endoscopy can identify an abnormality in about 60% of patients.10–12
PPIs HAVE REPLACED ANTACIDS AND HISTAMINE-2 RECEPTOR ANTAGONISTS
When the symptoms suggest GERD and no alarm features are present, an initial trial of the following lifestyle changes is reasonable:
- Avoiding acidic or refluxogenic foods (coffee, alcohol, chocolate, peppermint, fatty foods, citrus foods)
- Avoiding certain medications (anticholinergics, estrogens, calcium-channel blockers, nitroglycerine, benzodiazepines)
- Losing weight
- Quitting smoking
- Raising the head of the bed
- Staying upright for 2 to 3 hours after meals.
For someone with mild symptoms, these changes pose minimal risk. Unfortunately, they are unlikely to provide adequate symptom control for most patients.13–17
Before PPIs were invented, drug therapy for GERD symptoms that did not resolve with lifestyle changes consisted of antacids and, later, histamine-2 receptor antagonists. When maximal therapy failed to control symptoms, fundoplication surgery was considered an appropriate next step.
PPIs substantially changed the management of GERD, suppressing acid secretion much better than histamine-2 receptor antagonists. Taken 30 minutes before breakfast, a single daily dose of a PPI normalizes esophageal acid exposure in 67% of patients.18 Adding a second dose 30 minutes before dinner raises the number to more than 90%.19
PPIs have consistently outperformed histamine-2 blockers in the healing of esophagitis and in improving heartburn symptoms and are now the first-line medical therapy for uncomplicated GERD.6,8,20–25
WHEN PPIs WORK, SURGERY OFFERS NO ADVANTAGE
The LOTUS trial (Long-Term Usage of Esomeprazole vs Surgery for Treatment of Chronic GERD) compared long-term drug therapy with surgery to maintain remission of symptoms in GERD.27 In this trial, 554 patients whose symptoms initially responded to the PPI esomeprazole (Nexium) were randomized to continue to receive esomeprazole (n = 266) or to undergo laparoscopic antireflux surgery (288 were randomly assigned, and 248 had the operation). Dose adjustment of the esomeprazole was allowed (20–40 mg/day). A total of 372 patients completed 5 years of follow-up (192 esomeprazole, 180 surgery).
Symptoms stayed in remission in 92% of the esomeprazole group and 85% of the surgery group (P = .048). However, the difference was no longer statistically significant after modeling the effects of study dropout. The rate of severe adverse events was similar in both groups: 24.1% with esomeprazole and 28.6% with surgery.
These findings indicate that if symptoms fully abate with medical therapy, surgery offers no advantage. In addition, patients who desire surgery in the hope of avoiding lifelong drug therapy should be made aware that drug therapy and reoperation are often necessary after surgery.28 In most cases, antireflux surgery is unnecessary for patients whose GERD fully responds to PPI therapy.
IF PPIs FAIL, FURTHER TESTING NEEDED
But many patients who take PPIs still have symptoms, even though these drugs suppress acid secretion and heal esophagitis. In fact, symptoms completely resolve in only about one-half of patients with erosive disease and one-third of those without erosive disease.21
Reasons for an incomplete symptomatic response to PPIs are various. Acid reflux can persist, but this accounts for only 10% of cases.29 About one-third of patients have persistent reflux that is weakly acidic, with a pH higher than 4.29. However, most patients with persistent typical GERD symptoms do not have significant, persistent reflux, or their symptoms are not related to reflux events. In these cases, an alternative cause of the refractory symptoms should be sought.
Further diagnostic testing is indicated when symptoms persist despite PPI therapy. Upper endoscopy will reveal an abnormality such as persistent erosive esophagitis, eosinophilic esophagitis, esophageal stricture, Barrett esophagus, or esophageal cancer in roughly 10% of patients in whom empiric therapy fails.10
Although patients with persistent symptoms have not been enrolled in many randomized controlled trials, a multivariate analysis showed that failure of medical therapy heralds a poor response to surgery.30 Data such as these have led most experts to discourage fundoplication for such patients now, unlike in the pre-PPI era.
pH and intraluminal impedance testing
However, this recommendation against surgery is not a hard-and-fast rule.
In patients with esophageal regurgitation, most will not achieve adequate relief of symptoms with PPI therapy alone.34 The therapeutic gain of PPI therapy vs placebo averaged just 17% in seven randomized, controlled trials, more than 20% less than the response rate for heartburn.34 This is likely because of structural abnormalities such as reduced lower esophageal sphincter pressure, hiatal hernia, or delayed gastric emptying. Antireflux surgery can correct these structural abnormalities or prevent them from causing so much trouble; however, the presence of true regurgitation should first be confirmed by MII testing. If regurgitation is confirmed, antireflux surgery is warranted, particularly in patients with nocturnal symptoms who may be at high risk of aspiration. With careful patient selection, regurgitation symptoms improve in about 90% after surgery.2
In patients with heartburn, if esophageal acid exposure continues to be abnormal on MII-pH testing, then an escalation of therapy may improve symptoms, particularly if symptoms occur during reflux or if they partially responded to PPI therapy. Options in this scenario include alteration or intensification of acid-suppressive therapy, treatment with baclofen (Lioresal), and antireflux surgery.18,35,36 In randomized controlled trials of patients whose symptoms partially responded to PPIs, antireflux surgery has performed similarly to PPIs in terms of improving typical GERD symptoms, particularly regurgitation.27,37–41 Although this scenario is a reasonable indication for antireflux surgery, recommendations should be made with appropriate restraint since it is not easily reversible, some patients experience complications, and up to one-third will have no therapeutic benefit.30
Nonacid reflux. In some cases, MII-pH testing during PPI therapy will reveal reflux of weakly acidic (pH > 4) or alkaline stomach contents, often called “nonacid reflux.”29 Nonacid reflux is often present in patients with esophagitis that persists despite PPI therapy, indicating that even weakly acidic stomach contents can injure the mucosa.42 Since intensifying the acid-suppressive therapy is unlikely to improve these symptoms, antireflux surgery may have a role.
In one study,43 nonacid reflux was well controlled by laparoscopic Nissen fundoplication, although 15 (48%) of 31 patients had persistent symptoms of GERD after surgery. No patient had a strong symptom correlation with postoperative reflux events, suggesting an alternative cause of the persistent symptoms. Therefore, antireflux surgery for nonacid reflux should be limited exclusively to patients with strong symptom correlation, and even then it should be considered with restraint, given the limited evidence for benefit and the potential for harm.
If testing is negative. In studies investigating the diagnostic yield of MII-pH testing, more than 50% of patients who had refractory symptoms had a negative MII-pH test.29 In such situations, when the symptoms are strongly correlated with reflux events, the patient is said to have “esophageal hypersensitivity.” A few small studies have suggested that such patients may benefit from surgery, but these data have not been replicated in randomized controlled trials.32
When the testing is negative and there is no correlation between the patient’s symptoms and reflux events, the patient is unlikely to benefit from antireflux surgery. Care of these patients is beyond the scope of this review.
SURGERY RARELY IMPROVES COUGH, ASTHMA, OR LARYNGITIS
GERD has been implicated as a cause of chronic cough, asthma, and laryngitis, although each of these has many potential causes.44–46 Despite these associations, the evidence for therapeutic benefit from antireflux therapy is weak.
PPI therapy shows no benefit over placebo for chronic cough of uncertain etiology, but some benefit if GERD is objectively demonstrated.47 Laryngitis resolved in just 15% of patients on esomeprazole vs 16% of patients on placebo after excluding patients with moderate to severe heartburn.48
In a large randomized controlled trial in patients with asthma, there was no overall improvement in peak flow for the PPI group vs the placebo group, although significant improvement occurred in patients with heartburn and nocturnal respiratory symptoms.46
Potent antisecretory therapy seems to improve extraesophageal symptoms when typical GERD symptoms are also present, but it otherwise has shown little evidence of benefit.
The evidence for a benefit from antireflux surgery in patients with extraesophageal GERD syndromes is even more limited. Although one systematic review49 found that cough and other laryngeal symptoms improved in 60% to 100% of patients with objective evidence of GERD who underwent fundoplication, virtually all of the studies were uncontrolled case series.49
The lone randomized controlled trial in the systematic review compared Nissen fundoplication with ranitidine (Zantac) or antacids only for patients with asthma and GERD, and found no significant difference in peak expiratory flow among the three groups after 2 years. However, asthma symptom scores improved in 75% of the surgical group, 9% of the medical group, and 4% of the control group.50
In a study that was not included in the prior systematic review, patients with laryngopharyngeal reflux unresponsive to aggressive acid suppression who subsequently underwent fundoplication fared no better than those who did not.51
Thus, based on the available data, antireflux surgery is only rarely indicated for extraesophageal symptoms, especially in patients who have no typical GERD symptoms or in patients whose symptoms are refractory to medical therapy.
SURGERY FOR EROSIVE ESOPHAGITIS OR BARRETT ESOPHAGUS IF PPI FAILS
Lifelong antireflux therapy is indicated for patients with severe erosive esophagitis or Barrett esophagus. Erosive esophagitis recurs in more than 80% within 12 months of discontinuing antisecretory therapy.52 Both Barrett esophagus and esophageal adenocarcinoma are strongly associated with GERD, and nearly 10% of patients with chronic reflux have Barrett esophagus.53,54 It is suspected that suppressing reflux reduces the rate of progression of Barrett esophagus to esophageal adenocarcinoma, but this remains to be proven.
Perhaps the strongest indication for surgery in the PPI era is for patients who have persistent symptoms and severe erosive esophagitis (Los Angeles grade C or D) despite high-dose PPI therapy. If other causes of persistent esophagitis have been ruled out, fundoplication can induce healing and improve symptoms.55,56 In these cases, surgery is done to induce remission of the disease when maximal medical therapy has been truly unsuccessful.
Randomized controlled trials suggest that medical and surgical therapies are equally effective for preventing the recurrence of erosive esophagitis or the progression of Barrett esophagus. In a study of 225 patients, at 7 years of follow-up, esophagitis had recurred in 10.4% of patients on omeprazole vs 11.8% of those who had undergone antireflux surgery.40 Similarly, open Nissen fundoplication was no different from drug therapy (histamine-2 receptor antagonist or PPI) for progression of Barrett esophagus over a median of 5 years.57 A meta-analysis with nearly 5,000 person-years each in the medical and surgical groups also found no significant difference in rates of cancer progression.58
Notably, symptoms such as dysphagia, flatulence, and the inability to burp occurred significantly more often in the surgical groups in these studies.
In view of these data, antireflux surgery has no significant advantage over medical therapy for maintaining healing of erosive esophagitis or preventing progression of Barrett esophagus. Thus, it should be reserved for patients who do not desire lifelong drug therapy, provided they understand that there is no therapeutic advantage for their esophagitis or for Barrett esophagus.
SPECIFIC INDICATIONS FOR ANTIREFLUX SURGERY
Now that we have PPIs, several situations remain in which surgery for GERD is either indicated or worth considering.
Antireflux surgery is clearly indicated for:
- Patients with erosive esophagitis that does not heal with maximal drug therapy
- Patients with volume regurgitation, particularly if it occurs at night or if there is evidence of aspiration
- Patients who require lifelong treatment for reflux but who have had a serious adverse event related to PPI therapy, such as refractory Clostridium difficile infection.
Antireflux surgery is also worth considering in patients who for personal reasons wish to avoid long-term or lifelong drug therapy.
Patients should be informed, however, that antireflux surgery has not been shown to be better than medical therapy for maintaining remission of symptoms, for preventing progression of Barrett esophagus, or for maintaining healing of erosive esophagitis. Medical therapy is still the first option for these patients.
Surgery may also be considered in patients with persistent symptoms who have a partial response to medical therapy, who show persistent acidic or weakly acidic reflux on MII-pH testing, and whose symptoms have been correlated with reflux events. Although surgery is not sure to improve their symptoms, benefit is more likely in this patient population compared with those without these characteristics.
Extraesophageal GERD
In patients suspected of having extraesophageal GERD, surgery should be considered if typical GERD symptoms are present and improve with PPI therapy, if the extraesophageal syndrome partially responds to PPI therapy, and if MII-pH testing demonstrates a correlation between symptoms and reflux. Surgery may have a stronger indication in this setting if the patient has nocturnal reflux or extraesophageal symptoms.
When is surgery not an option?
In general, surgery should not be considered in patients who do not have a partial response to PPI therapy or who do not have a strong symptom-reflux correlation on MII-pH testing. In all cases of failed medical therapy without persistent severe erosive disease, the threshold for opting for surgery should be high, given the uncertain response of these patients to surgery.
Peristaltic dysfunction is a relative but not an absolute contraindication to surgery.59
RISKS, BENEFITS OF SURGERY FOR GERD
The patient’s preference for surgery over drug therapy should always be balanced against the risks of surgery, including both short-term and long-term adverse events, to allow the patient to make an adequately informed decision (Table 2).2,26
Adverse events associated with PPI therapy are rare and in many cases the association is debatable.26 Nonetheless, long-term PPI therapy has been most strongly associated with an increased risk of C difficile infection and other enteric infections, although the absolute risk of these events remains low.
Complication rates after antireflux surgery depend on the surgeon’s experience and technique. Death is exceedingly rare. In most high-volume centers, the need to convert from laparoscopic to open fundoplication occurs in fewer than 2.4% of patients.2
Potential perioperative complications include perforation (4%), wound infection (3%), and pneumothorax (2%).2
Antireflux surgery is also associated with a significant risk of dysphagia, bloating, an inability to burp, and excessive flatulence, all of which can markedly impair the quality of life.
A major consideration is that fundoplication is generally irreversible. Reoperation rates have been reported to range from 0% to 15%.2 Furthermore, up to 50% of patients still need medical therapy after surgery.60,61 Of note, only about 25% of patients on medical therapy after surgery will actually have an abnormal pH study.61
MORE STUDY NEEDED
Future studies directly comparing medical and surgical therapy for carefully selected patients with extraesophageal manifestations of GERD and refractory symptoms should help further delineate outcome in this difficult group of patients.
Under development are new drugs that may inhibit transient relaxation of the lower esophageal sphincter, as well as minimally invasive procedures, which may alter the indications for surgery in coming years.36
Acknowledgment: The research for this article was supported in part by a grant from the National Institutes of Health (T32 DK07634).
- Finks JF, Wei Y, Birkmeyer JD. The rise and fall of antireflux surgery in the United States. Surg Endosc 2006; 20:1698–1701.
- Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD; SAGES Guidelines Committee. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 2010; 24:2647–2669.
- Richter JE. Typical and atypical presentations of gastroesophageal reflux disease. The role of esophageal testing in diagnosis and management. Gastroenterol Clin North Am 1996; 25:75–102.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Dickman R, Kim JL, Camargo L, et al. Correlation of gastroesophageal reflux disease symptoms characteristics with long-segment Barrett’s esophagus. Dis Esophagus 2006; 19:360–365.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Armstrong D, Marshall JK, Chiba N, et al; Canadian Association of Gastroenterology GERD Consensus Group. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults - update 2004. Can J Gastroenterol 2005; 19:15–35.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.
- Vakil N, Moayyedi P, Fennerty MB, Talley NJ. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology 2006; 131:390–401.
- Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc 2010; 71:28–34.
- Dickman R, Mattek N, Holub J, Peters D, Fass R. Prevalence of upper gastrointestinal tract findings in patients with noncardiac chest pain versus those with gastroesophageal reflux disease (GERD)-related symptoms: results from a national endoscopic database. Am J Gastroenterol 2007; 102:1173–1179.
- Voutilainen M, Sipponen P, Mecklin JP, Juhola M, Färkkilä M. Gastroesophageal reflux disease: prevalence, clinical, endoscopic and histopathological findings in 1,128 consecutive patients referred for endoscopy due to dyspeptic and reflux symptoms. Digestion 2000; 61:6–13.
- Fraser-Moodie CA, Norton B, Gornall C, Magnago S, Weale AR, Holmes GK. Weight loss has an independent beneficial effect on symptoms of gastro-oesophageal reflux in patients who are overweight. Scand J Gastroenterol 1999; 34:337–340.
- Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA. Bodymass index and symptoms of gastroesophageal reflux in women. N Engl J Med 2006; 354:2340–2348.
- Kjellin A, Ramel S, Rössner S, Thor K. Gastroesophageal reflux in obese patients is not reduced by weight reduction. Scand J Gastroenterol 1996; 31:1047–1051.
- Waring JP, Eastwood TF, Austin JM, Sanowski RA. The immediate effects of cessation of cigarette smoking on gastroesophageal reflux. Am J Gastroenterol 1989; 84:1076–1078.
- Pehl C, Waizenhoefer A, Wendl B, Schmidt T, Schepp W, Pfeiffer A. Effect of low and high fat meals on lower esophageal sphincter motility and gastroesophageal reflux in healthy subjects. Am J Gastroenterol 1999; 94:1192–1196.
- Bajbouj M, Becker V, Phillip V, Wilhelm D, Schmid RM, Meining A. High-dose esomeprazole for treatment of symptomatic refractory gastroesophageal reflux disease—a prospective pH-metry/impedance-controlled study. Digestion 2009; 80:112–118.
- Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol 2005; 100:283–289.
- Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev 2007;CD003244.
- Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol 2004; 2:656–664.
- Sabesin SM, Berlin RG, Humphries TJ, Bradstreet DC, Walton-Bowen KL, Zaidi S. Famotidine relieves symptoms of gastroesophageal reflux disease and heals erosions and ulcerations. Results of a multicenter, placebo-controlled, dose-ranging study. USA Merck Gastroesophageal Reflux Disease Study Group. Arch Intern Med 1991; 151:2394–2400.
- van Pinxteren B, Numans ME, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2004;CD002095.
- Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology 1997; 112:1798–1810.
- Venables TL, Newland RD, Patel AC, Hole J, Wilcock C, Turbitt ML. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams once daily, or ranitidine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastro-oesophageal reflux disease in general practice. Scand J Gastroenterol 1997; 32:965–973.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- Galmiche JP, Hatlebakk J, Attwood S, et al; LOTUS Trial Collaborators. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 2011; 305:1969–1977.
- Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: followup of a randomized controlled trial. JAMA 2001; 285:2331–2338.
- Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 2006; 55:1398–1402.
- Campos GM, Peters JH, DeMeester TR, et al. Multivariate analysis of factors predicting outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg 1999; 3:292–300.
- Becker V, Bajbouj M, Waller K, Schmid RM, Meining A. Clinical trial: persistent gastro-oesophageal reflux symptoms despite standard therapy with proton pump inhibitors - a follow-up study of intraluminal-impedance guided therapy. Aliment Pharmacol Ther 2007; 26:1355–1360.
- Mainie I, Tutuian R, Agrawal A, Adams D, Castell DO. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg 2006; 93:1483–1487.
- del Genio G, Tolone S, del Genio F, et al. Prospective assessment of patient selection for antireflux surgery by combined multichannel intraluminal impedance pH monitoring. J Gastrointest Surg 2008; 12:1491–1496.
- Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol 2011; 106:1419–1425.
- Koek GH, Sifrim D, Lerut T, Janssens J, Tack J. Effect of the GABA(B) agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut 2003; 52:1397–1402.
- Boeckxstaens GE. Reflux inhibitors: a new approach for GERD? Curr Opin Pharmacol 2008; 8:685–689.
- Anvari M, Allen C, Marshall J, et al. A randomized controlled trial of laparoscopic nissen fundoplication versus proton pump inhibitors for treatment of patients with chronic gastroesophageal reflux disease: One-year follow-up. Surg Innov 2006; 13:238–249.
- Mahon D, Rhodes M, Decadt B, et al. Randomized clinical trial of laparoscopic Nissen fundoplication compared with proton-pump inhibitors for treatment of chronic gastro-oesophageal reflux. Br J Surg 2005; 92:695–699.
- Mehta S, Bennett J, Mahon D, Rhodes M. Prospective trial of laparoscopic nissen fundoplication versus proton pump inhibitor therapy for gastroesophageal reflux disease: Seven-year follow-up. J Gastrointest Surg 2006; 10:1312–1316.
- Lundell L, Miettinen P, Myrvold HE, et al; Nordic GORD Study Group. Seven-year follow-up of a randomized clinical trial comparing proton-pump inhibition with surgical therapy for reflux oesophagitis. Br J Surg 2007; 94:198–203.
- Lundell L, Attwood S, Ell C, et al; LOTUS trial collaborators. Comparing laparoscopic antireflux surgery with esomeprazole in the management of patients with chronic gastro-oesophageal reflux disease: a 3-year interim analysis of the LOTUS trial. Gut 2008; 57:1207–1213.
- Frazzoni M, Conigliaro R, Melotti G. Weakly acidic refluxes have a major role in the pathogenesis of proton pump inhibitor-resistant reflux oesophagitis. Aliment Pharmacol Ther 2011; 33:601–606.
- Broeders JA, Bredenoord AJ, Hazebroek EJ, Broeders IA, Gooszen HG, Smout AJ. Effects of anti-reflux surgery on weakly acidic reflux and belching. Gut 2011; 60:435–441.
- American Gastroenterological Association medical position statement: guidelines on the use of esophageal pH recording. Gastroenterology 1996; 110:1981.
- el-Serag HB, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology 1997; 113:755–760.
- Kiljander TO, Laitinen JO. The prevalence of gastroesophageal reflux disease in adult asthmatics. Chest 2004; 126:1490–1494.
- Chang AB, Lasserson TJ, Kiljander TO, Connor FL, Gaffney JT, Garske LA. Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. BMJ 2006; 332:11–17.
- Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006; 116:254–260.
- Iqbal M, Batch AJ, Spychal RT, Cooper BT. Outcome of surgical fundoplication for extraesophageal (atypical) manifestations of gastroesophageal reflux disease in adults: a systematic review. J Laparoendosc Adv Surg Tech A 2008; 18:789–796.
- Sontag SJ, O’Connell S, Khandelwal S, et al. Asthmatics with gastroesophageal reflux: long term results of a randomized trial of medical and surgical antireflux therapies. Am J Gastroenterol 2003; 98:987–999.
- Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol 2006; 4:433–441.
- Johnson DA, Benjamin SB, Vakil NB, et al. Esomeprazole once daily for 6 months is effective therapy for maintaining healed erosive esophagitis and for controlling gastroesophageal reflux disease symptoms: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Gastroenterol 2001; 96:27–34.
- Winters C, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987; 92:118–124.
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61:226–231.
- Rosenthal R, Peterli R, Guenin MO, von Flüe M, Ackermann C. Laparoscopic antireflux surgery: long-term outcomes and quality of life. J Laparoendosc Adv Surg Tech A 2006; 16:557–561.
- Broeders JA, Draaisma WA, Bredenoord AJ, Smout AJ, Broeders IA, Gooszen HG. Long-term outcome of Nissen fundoplication in non-erosive and erosive gastro-oesophageal reflux disease. Br J Surg 2010; 97:845–352.
- Parrilla P, Martínez de Haro LF, Ortiz A, et al. Long-term results of a randomized prospective study comparing medical and surgical treatment of Barrett’s esophagus. Ann Surg 2003; 237:291–298.
- Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett’s esophagus? A meta-analysis. Am J Gastroenterol 2003; 98:2390–2394.
- Pandolfino JE, Kahrilas PJ; American Gastroenterological Association. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 2005; 128:209–224.
- Dominitz JA, Dire CA, Billingsley KG, Todd-Stenberg JA. Complications and antireflux medication use after antireflux surgery. Clin Gastroenterol Hepatol 2006; 4:299–305.
- Lord RV, Kaminski A, Oberg S, et al. Absence of gastroesophageal reflux disease in a majority of patients taking acid suppression medications after Nissen fundoplication. J Gastrointest Surg 2002; 6:3–9.
- Finks JF, Wei Y, Birkmeyer JD. The rise and fall of antireflux surgery in the United States. Surg Endosc 2006; 20:1698–1701.
- Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD; SAGES Guidelines Committee. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 2010; 24:2647–2669.
- Richter JE. Typical and atypical presentations of gastroesophageal reflux disease. The role of esophageal testing in diagnosis and management. Gastroenterol Clin North Am 1996; 25:75–102.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Dickman R, Kim JL, Camargo L, et al. Correlation of gastroesophageal reflux disease symptoms characteristics with long-segment Barrett’s esophagus. Dis Esophagus 2006; 19:360–365.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Armstrong D, Marshall JK, Chiba N, et al; Canadian Association of Gastroenterology GERD Consensus Group. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults - update 2004. Can J Gastroenterol 2005; 19:15–35.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.
- Vakil N, Moayyedi P, Fennerty MB, Talley NJ. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology 2006; 131:390–401.
- Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc 2010; 71:28–34.
- Dickman R, Mattek N, Holub J, Peters D, Fass R. Prevalence of upper gastrointestinal tract findings in patients with noncardiac chest pain versus those with gastroesophageal reflux disease (GERD)-related symptoms: results from a national endoscopic database. Am J Gastroenterol 2007; 102:1173–1179.
- Voutilainen M, Sipponen P, Mecklin JP, Juhola M, Färkkilä M. Gastroesophageal reflux disease: prevalence, clinical, endoscopic and histopathological findings in 1,128 consecutive patients referred for endoscopy due to dyspeptic and reflux symptoms. Digestion 2000; 61:6–13.
- Fraser-Moodie CA, Norton B, Gornall C, Magnago S, Weale AR, Holmes GK. Weight loss has an independent beneficial effect on symptoms of gastro-oesophageal reflux in patients who are overweight. Scand J Gastroenterol 1999; 34:337–340.
- Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA. Bodymass index and symptoms of gastroesophageal reflux in women. N Engl J Med 2006; 354:2340–2348.
- Kjellin A, Ramel S, Rössner S, Thor K. Gastroesophageal reflux in obese patients is not reduced by weight reduction. Scand J Gastroenterol 1996; 31:1047–1051.
- Waring JP, Eastwood TF, Austin JM, Sanowski RA. The immediate effects of cessation of cigarette smoking on gastroesophageal reflux. Am J Gastroenterol 1989; 84:1076–1078.
- Pehl C, Waizenhoefer A, Wendl B, Schmidt T, Schepp W, Pfeiffer A. Effect of low and high fat meals on lower esophageal sphincter motility and gastroesophageal reflux in healthy subjects. Am J Gastroenterol 1999; 94:1192–1196.
- Bajbouj M, Becker V, Phillip V, Wilhelm D, Schmid RM, Meining A. High-dose esomeprazole for treatment of symptomatic refractory gastroesophageal reflux disease—a prospective pH-metry/impedance-controlled study. Digestion 2009; 80:112–118.
- Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol 2005; 100:283–289.
- Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev 2007;CD003244.
- Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol 2004; 2:656–664.
- Sabesin SM, Berlin RG, Humphries TJ, Bradstreet DC, Walton-Bowen KL, Zaidi S. Famotidine relieves symptoms of gastroesophageal reflux disease and heals erosions and ulcerations. Results of a multicenter, placebo-controlled, dose-ranging study. USA Merck Gastroesophageal Reflux Disease Study Group. Arch Intern Med 1991; 151:2394–2400.
- van Pinxteren B, Numans ME, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2004;CD002095.
- Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology 1997; 112:1798–1810.
- Venables TL, Newland RD, Patel AC, Hole J, Wilcock C, Turbitt ML. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams once daily, or ranitidine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastro-oesophageal reflux disease in general practice. Scand J Gastroenterol 1997; 32:965–973.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- Galmiche JP, Hatlebakk J, Attwood S, et al; LOTUS Trial Collaborators. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 2011; 305:1969–1977.
- Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: followup of a randomized controlled trial. JAMA 2001; 285:2331–2338.
- Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 2006; 55:1398–1402.
- Campos GM, Peters JH, DeMeester TR, et al. Multivariate analysis of factors predicting outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg 1999; 3:292–300.
- Becker V, Bajbouj M, Waller K, Schmid RM, Meining A. Clinical trial: persistent gastro-oesophageal reflux symptoms despite standard therapy with proton pump inhibitors - a follow-up study of intraluminal-impedance guided therapy. Aliment Pharmacol Ther 2007; 26:1355–1360.
- Mainie I, Tutuian R, Agrawal A, Adams D, Castell DO. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg 2006; 93:1483–1487.
- del Genio G, Tolone S, del Genio F, et al. Prospective assessment of patient selection for antireflux surgery by combined multichannel intraluminal impedance pH monitoring. J Gastrointest Surg 2008; 12:1491–1496.
- Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol 2011; 106:1419–1425.
- Koek GH, Sifrim D, Lerut T, Janssens J, Tack J. Effect of the GABA(B) agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut 2003; 52:1397–1402.
- Boeckxstaens GE. Reflux inhibitors: a new approach for GERD? Curr Opin Pharmacol 2008; 8:685–689.
- Anvari M, Allen C, Marshall J, et al. A randomized controlled trial of laparoscopic nissen fundoplication versus proton pump inhibitors for treatment of patients with chronic gastroesophageal reflux disease: One-year follow-up. Surg Innov 2006; 13:238–249.
- Mahon D, Rhodes M, Decadt B, et al. Randomized clinical trial of laparoscopic Nissen fundoplication compared with proton-pump inhibitors for treatment of chronic gastro-oesophageal reflux. Br J Surg 2005; 92:695–699.
- Mehta S, Bennett J, Mahon D, Rhodes M. Prospective trial of laparoscopic nissen fundoplication versus proton pump inhibitor therapy for gastroesophageal reflux disease: Seven-year follow-up. J Gastrointest Surg 2006; 10:1312–1316.
- Lundell L, Miettinen P, Myrvold HE, et al; Nordic GORD Study Group. Seven-year follow-up of a randomized clinical trial comparing proton-pump inhibition with surgical therapy for reflux oesophagitis. Br J Surg 2007; 94:198–203.
- Lundell L, Attwood S, Ell C, et al; LOTUS trial collaborators. Comparing laparoscopic antireflux surgery with esomeprazole in the management of patients with chronic gastro-oesophageal reflux disease: a 3-year interim analysis of the LOTUS trial. Gut 2008; 57:1207–1213.
- Frazzoni M, Conigliaro R, Melotti G. Weakly acidic refluxes have a major role in the pathogenesis of proton pump inhibitor-resistant reflux oesophagitis. Aliment Pharmacol Ther 2011; 33:601–606.
- Broeders JA, Bredenoord AJ, Hazebroek EJ, Broeders IA, Gooszen HG, Smout AJ. Effects of anti-reflux surgery on weakly acidic reflux and belching. Gut 2011; 60:435–441.
- American Gastroenterological Association medical position statement: guidelines on the use of esophageal pH recording. Gastroenterology 1996; 110:1981.
- el-Serag HB, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology 1997; 113:755–760.
- Kiljander TO, Laitinen JO. The prevalence of gastroesophageal reflux disease in adult asthmatics. Chest 2004; 126:1490–1494.
- Chang AB, Lasserson TJ, Kiljander TO, Connor FL, Gaffney JT, Garske LA. Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. BMJ 2006; 332:11–17.
- Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006; 116:254–260.
- Iqbal M, Batch AJ, Spychal RT, Cooper BT. Outcome of surgical fundoplication for extraesophageal (atypical) manifestations of gastroesophageal reflux disease in adults: a systematic review. J Laparoendosc Adv Surg Tech A 2008; 18:789–796.
- Sontag SJ, O’Connell S, Khandelwal S, et al. Asthmatics with gastroesophageal reflux: long term results of a randomized trial of medical and surgical antireflux therapies. Am J Gastroenterol 2003; 98:987–999.
- Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol 2006; 4:433–441.
- Johnson DA, Benjamin SB, Vakil NB, et al. Esomeprazole once daily for 6 months is effective therapy for maintaining healed erosive esophagitis and for controlling gastroesophageal reflux disease symptoms: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Gastroenterol 2001; 96:27–34.
- Winters C, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology 1987; 92:118–124.
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005; 61:226–231.
- Rosenthal R, Peterli R, Guenin MO, von Flüe M, Ackermann C. Laparoscopic antireflux surgery: long-term outcomes and quality of life. J Laparoendosc Adv Surg Tech A 2006; 16:557–561.
- Broeders JA, Draaisma WA, Bredenoord AJ, Smout AJ, Broeders IA, Gooszen HG. Long-term outcome of Nissen fundoplication in non-erosive and erosive gastro-oesophageal reflux disease. Br J Surg 2010; 97:845–352.
- Parrilla P, Martínez de Haro LF, Ortiz A, et al. Long-term results of a randomized prospective study comparing medical and surgical treatment of Barrett’s esophagus. Ann Surg 2003; 237:291–298.
- Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett’s esophagus? A meta-analysis. Am J Gastroenterol 2003; 98:2390–2394.
- Pandolfino JE, Kahrilas PJ; American Gastroenterological Association. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 2005; 128:209–224.
- Dominitz JA, Dire CA, Billingsley KG, Todd-Stenberg JA. Complications and antireflux medication use after antireflux surgery. Clin Gastroenterol Hepatol 2006; 4:299–305.
- Lord RV, Kaminski A, Oberg S, et al. Absence of gastroesophageal reflux disease in a majority of patients taking acid suppression medications after Nissen fundoplication. J Gastrointest Surg 2002; 6:3–9.
KEY POINTS
- If a PPI in twice-daily doses fails to relieve GERD symptoms, a pH study combined with multichannel intraluminal impedance testing can help in deciding whether to try surgery.
- Antireflux surgery can be considered for erosive esophagitis that does not resolve with drug therapy, for volume regurgitation (particularly if it occurs at night or if there is a risk of aspiration), and for patients who need lifelong treatment for reflux but have had a serious adverse event related to PPI therapy.
- Studies are needed to directly compare medical and surgical therapy in patients with extraesophageal manifestations of GERD and refractory symptoms, a difficult group of patients.
- Drugs that inhibit transient relaxation of the lower esophageal sphincter are under investigation, as are minimally invasive procedures to manipulate the physical barrier to reflux.
In reply: Coadministration of clopidogrel and proton pump inhibitors
In Reply: I thank Dr. Keller for his interest in my review on the side effects and drug interactions of proton pump inhibitors (PPIs).1 In particular, the concern about the potentially increased risk of a cardiovascular event in patients taking a PPI while on clopidogrel is a matter of active research. Since the prevention of death, myocardial infarction, or stroke is the desired outcome in patients receiving antiplatelet therapy, any reduction in the antiplatelet effect of clopidogrel could put patients at increased risk. Because of the enormous number of patients on both PPIs and clopidogrel, investigators are studying the effect of PPIs on clopidogrel to determine the true significance in day-to-day practice. We should expect that the data will continue to evolve in the coming years as more research is done on this important interaction.
The FDA Web site that Dr. Keller brings up2 was posted a few months after the submission of my manuscript. But even with the FDA’s cautionary words, it is important to realize that the risk that purportedly exists with the interaction of omeprazole and clopidogrel and the suggestion for the alternative use of pantoprazole are both based on pharmacokinetic, pharmacodynamic, and epidemiologic studies, not on clinical outcome data.
As much as we would like to rely on such studies, pharmacokinetic and pharmacodynamic studies do not address clinical outcomes, and observational studies cannot account for every confounder, because patients in these studies are not randomly assigned to the intervention, which is the rationale behind the necessity for a prospective trial. The Clopidogrel and the Optimization of Gastrointestinal Events (COGENT) study,3 a prospective randomized controlled trial with 3,761 analyzed patients, found no differences in adjudicated cardiovascular outcomes between groups who received a clopidogrel plus omeprazole vs clopidogrel alone.3 Although the COGENT study ended prematurely because of bankruptcy of the funding source, these outcomes represent the only randomized prospective data that can be found to date on PubMed. With such large numbers of patients in each group (1,876 and 1,885, respectively) and no differences in outcomes, it stands to reason that only a study with massive sample sizes would be able to detect a statistically significant difference. Differences between clopidogrel-treated patients taking and not taking omeprazole are likely be found in a well-designed prospective trial; however, it would be virtually impossible to find differences among PPIs.
To make matters even less convincing that therapy should be altered, the Working Group on High On-treatment Platelet Reactivity stated in their recent consensus paper that there are “limited data to support that alteration of therapy based on platelet function measurements actually improves outcomes.” 4 Additionally, a recent multisociety Expert Consensus Document discussing the concomitant use of PPIs and thienopyridine drugs to reduce gastrointestinal complications further supports this argument.5 Therefore, it is difficult to justify a marked increase in cost of the PPI selected (pantoprazole costs nearly seven times more per dose than omeprazole, according to one Web site6) for a benefit that is supported only by theoretical and observational data, not by outcome data.
As Dr. Keller also mentions, Aggrenox can be used for secondary stroke prophylaxis, but a discussion about a therapeutic exchange between clopidogrel and other antiplatelet agents was beyond the scope of my review. A recently published joint guideline of the American Heart Association and the American Stroke Association guideline should be consulted for further information.7
Other gastroprotective therapies are available. However, misoprostol (as mentioned) is associated with significant gastrointestinal side effects and must be taken four times a day. H2-receptor antagonists are not considered to be as effective as PPIs.8,9
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
- Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010; 56:919–33.
- Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines:a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol 2010; 105:2533–2549.
- HealthWarehouse. www.healthwarehouse.com. Accessed March 23, 2011.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents.Circulation 2008; 118:1894–1909.
- Lanza FL, Chan FK, Quigley EM, et al. Guidelines forprevention of NSAID-related ulcer complications. Am JGastroenterol 2009; 104:728–738.
In Reply: I thank Dr. Keller for his interest in my review on the side effects and drug interactions of proton pump inhibitors (PPIs).1 In particular, the concern about the potentially increased risk of a cardiovascular event in patients taking a PPI while on clopidogrel is a matter of active research. Since the prevention of death, myocardial infarction, or stroke is the desired outcome in patients receiving antiplatelet therapy, any reduction in the antiplatelet effect of clopidogrel could put patients at increased risk. Because of the enormous number of patients on both PPIs and clopidogrel, investigators are studying the effect of PPIs on clopidogrel to determine the true significance in day-to-day practice. We should expect that the data will continue to evolve in the coming years as more research is done on this important interaction.
The FDA Web site that Dr. Keller brings up2 was posted a few months after the submission of my manuscript. But even with the FDA’s cautionary words, it is important to realize that the risk that purportedly exists with the interaction of omeprazole and clopidogrel and the suggestion for the alternative use of pantoprazole are both based on pharmacokinetic, pharmacodynamic, and epidemiologic studies, not on clinical outcome data.
As much as we would like to rely on such studies, pharmacokinetic and pharmacodynamic studies do not address clinical outcomes, and observational studies cannot account for every confounder, because patients in these studies are not randomly assigned to the intervention, which is the rationale behind the necessity for a prospective trial. The Clopidogrel and the Optimization of Gastrointestinal Events (COGENT) study,3 a prospective randomized controlled trial with 3,761 analyzed patients, found no differences in adjudicated cardiovascular outcomes between groups who received a clopidogrel plus omeprazole vs clopidogrel alone.3 Although the COGENT study ended prematurely because of bankruptcy of the funding source, these outcomes represent the only randomized prospective data that can be found to date on PubMed. With such large numbers of patients in each group (1,876 and 1,885, respectively) and no differences in outcomes, it stands to reason that only a study with massive sample sizes would be able to detect a statistically significant difference. Differences between clopidogrel-treated patients taking and not taking omeprazole are likely be found in a well-designed prospective trial; however, it would be virtually impossible to find differences among PPIs.
To make matters even less convincing that therapy should be altered, the Working Group on High On-treatment Platelet Reactivity stated in their recent consensus paper that there are “limited data to support that alteration of therapy based on platelet function measurements actually improves outcomes.” 4 Additionally, a recent multisociety Expert Consensus Document discussing the concomitant use of PPIs and thienopyridine drugs to reduce gastrointestinal complications further supports this argument.5 Therefore, it is difficult to justify a marked increase in cost of the PPI selected (pantoprazole costs nearly seven times more per dose than omeprazole, according to one Web site6) for a benefit that is supported only by theoretical and observational data, not by outcome data.
As Dr. Keller also mentions, Aggrenox can be used for secondary stroke prophylaxis, but a discussion about a therapeutic exchange between clopidogrel and other antiplatelet agents was beyond the scope of my review. A recently published joint guideline of the American Heart Association and the American Stroke Association guideline should be consulted for further information.7
Other gastroprotective therapies are available. However, misoprostol (as mentioned) is associated with significant gastrointestinal side effects and must be taken four times a day. H2-receptor antagonists are not considered to be as effective as PPIs.8,9
In Reply: I thank Dr. Keller for his interest in my review on the side effects and drug interactions of proton pump inhibitors (PPIs).1 In particular, the concern about the potentially increased risk of a cardiovascular event in patients taking a PPI while on clopidogrel is a matter of active research. Since the prevention of death, myocardial infarction, or stroke is the desired outcome in patients receiving antiplatelet therapy, any reduction in the antiplatelet effect of clopidogrel could put patients at increased risk. Because of the enormous number of patients on both PPIs and clopidogrel, investigators are studying the effect of PPIs on clopidogrel to determine the true significance in day-to-day practice. We should expect that the data will continue to evolve in the coming years as more research is done on this important interaction.
The FDA Web site that Dr. Keller brings up2 was posted a few months after the submission of my manuscript. But even with the FDA’s cautionary words, it is important to realize that the risk that purportedly exists with the interaction of omeprazole and clopidogrel and the suggestion for the alternative use of pantoprazole are both based on pharmacokinetic, pharmacodynamic, and epidemiologic studies, not on clinical outcome data.
As much as we would like to rely on such studies, pharmacokinetic and pharmacodynamic studies do not address clinical outcomes, and observational studies cannot account for every confounder, because patients in these studies are not randomly assigned to the intervention, which is the rationale behind the necessity for a prospective trial. The Clopidogrel and the Optimization of Gastrointestinal Events (COGENT) study,3 a prospective randomized controlled trial with 3,761 analyzed patients, found no differences in adjudicated cardiovascular outcomes between groups who received a clopidogrel plus omeprazole vs clopidogrel alone.3 Although the COGENT study ended prematurely because of bankruptcy of the funding source, these outcomes represent the only randomized prospective data that can be found to date on PubMed. With such large numbers of patients in each group (1,876 and 1,885, respectively) and no differences in outcomes, it stands to reason that only a study with massive sample sizes would be able to detect a statistically significant difference. Differences between clopidogrel-treated patients taking and not taking omeprazole are likely be found in a well-designed prospective trial; however, it would be virtually impossible to find differences among PPIs.
To make matters even less convincing that therapy should be altered, the Working Group on High On-treatment Platelet Reactivity stated in their recent consensus paper that there are “limited data to support that alteration of therapy based on platelet function measurements actually improves outcomes.” 4 Additionally, a recent multisociety Expert Consensus Document discussing the concomitant use of PPIs and thienopyridine drugs to reduce gastrointestinal complications further supports this argument.5 Therefore, it is difficult to justify a marked increase in cost of the PPI selected (pantoprazole costs nearly seven times more per dose than omeprazole, according to one Web site6) for a benefit that is supported only by theoretical and observational data, not by outcome data.
As Dr. Keller also mentions, Aggrenox can be used for secondary stroke prophylaxis, but a discussion about a therapeutic exchange between clopidogrel and other antiplatelet agents was beyond the scope of my review. A recently published joint guideline of the American Heart Association and the American Stroke Association guideline should be consulted for further information.7
Other gastroprotective therapies are available. However, misoprostol (as mentioned) is associated with significant gastrointestinal side effects and must be taken four times a day. H2-receptor antagonists are not considered to be as effective as PPIs.8,9
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
- Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010; 56:919–33.
- Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines:a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol 2010; 105:2533–2549.
- HealthWarehouse. www.healthwarehouse.com. Accessed March 23, 2011.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents.Circulation 2008; 118:1894–1909.
- Lanza FL, Chan FK, Quigley EM, et al. Guidelines forprevention of NSAID-related ulcer complications. Am JGastroenterol 2009; 104:728–738.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
- Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010; 56:919–33.
- Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines:a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol 2010; 105:2533–2549.
- HealthWarehouse. www.healthwarehouse.com. Accessed March 23, 2011.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents.Circulation 2008; 118:1894–1909.
- Lanza FL, Chan FK, Quigley EM, et al. Guidelines forprevention of NSAID-related ulcer complications. Am JGastroenterol 2009; 104:728–738.
Proton pump inhibitor side effects and drug interactions: Much ado about nothing?
The development and introduction of the first proton pump inhibitor (PPI), omeprazole (Prilosec), for the management of acid-peptic disorders marks one of the great success stories in gastroenterology. Until the latter part of the 20th century, complications of acid-peptic disease were among the most common problems faced in gastroenterology. Severe peptic strictures were once a highly prevalent cause of dysphagia, and operations for peptic ulcer disease were routinely learned by surgical trainees.
The success of these drugs, with sales total-ling $13.6 billion worldwide in 2009,1 is not just a result of their potency and effectiveness in improving symptoms and complications of acid-peptic disease. Their safety among pharmacologic agents has been unparalleled. When the drugs were first introduced, their use was limited to short courses out of concern that gastric carcinoids could develop, but decades of use have not shown this issue to be of clinical relevance. Serious, acute adverse effects are also exceedingly uncommon.
However, recent reports have questioned the long-term safety of PPIs. Furthermore, these drugs are too often used in patients who have no valid indication for them,2,3 exposing these patients to unnecessary risks.
The goals of this review are to analyze the recent literature about the risks of PPIs and to provide a rational approach for managing patients on PPI therapy in light of these concerns.
DO PPIs REDUCE THE EFFECT OF CLOPIDOGREL?
Clopidogrel (Plavix) is a potent antiplatelet agent commonly used in patients with atherosclerotic cardiac or cerebrovascular disease, sometimes in combination with aspirin. Because of the risk of significant gastrointestinal bleeding, a 2008 multisociety task force recommended prescribing a PPI when both clopidogrel and aspirin are used as dual antiplatelet therapy.4
Studies of clopidogrel plus PPIs: Discrepant results
To date, only one prospective randomized controlled trial has specifically investigated the effect of PPIs on cardiovascular outcomes in patients using clopidogrel. In this trial, patients on dual antiplatelet therapy with clopidogrel and aspirin were randomized to receive either omeprazole 20 mg or placebo. Analysis of the data revealed no significant increase in the composite end point of cardiovascular events (hazard ratio [HR] 0.99, 95% confidence interval [CI] 0.68–1.44, P = .96), but a statistically significant decrease in composite gastrointestinal events (HR 0.34, 95% CI 0.18–0.63, P < .001).17
Unfortunately, this trial had to be terminated before the prespecified sample size and duration of follow-up were reached because the study sponsor declared bankruptcy.
One additional recent retrospective cohort study16 likewise found no significant risk of serious cardiovascular disease related to PPI use in clopidogrel users. It also found that the adjusted incidence of hospitalization for upper gastrointestinal bleeding was 50% lower in patients who used PPIs than in those who did not (HR 0.50, 95% CI 0.39–0.65).
Do factors other than PPIs account for the higher risk in some of the studies?
The discrepant results of these studies suggest that the higher risk of cardiovascular events may be due, either completely or in part, to a factor other than the pharmacologic interaction of PPIs and clopidogrel. It is difficult to infer causality from the available data. In situations in which no randomized controlled trials exist, one looks to observational (case-control or cohort) studies to try to obtain the best estimate of the actual risk. With PPIs and clopidogrel, a randomized controlled trial was performed but terminated before patient enrollment was complete.
The increased risk found in some of these studies may be real, may be due to chance, or may even represent an increased risk from PPIs alone (although data do not support this possibility).18 However, the major concern in observational studies is the inability to account for unmeasured confounders, a problem virtually eliminated by randomization strategies in prospective studies.
In the studies that found a higher risk with the combination of omeprazole plus clopidogrel, the principal concern is confounding by indication, in which distortions of the risk estimates arise from an imbalance in prognostic factors between compared treatment groups that remains unmeasured.19 Stated another way, physicians who believed some patients to be “sicker” or to have a higher risk of serious events may have treated them with a PPI on the basis of factors that remained unaccounted for in the epidemiologic investigation.
This possibility has been reinforced by findings from a nonrandomized subgroup analysis of a randomized controlled trial in which patients who had been receiving a PPI had a higher rate of cardiovascular events whether they received clopidogrel or placebo.20
FDA alert: Avoid using omeprazole or esomeprazole with clopidogrel
Nonetheless, on November 17, 2009, the US Food and Drug Administration (FDA) issued an alert to health care professionals and the public about the potential interaction between clopidogrel and omeprazole.21 In this alert, the FDA stated that the use of omeprazole or esomeprazole (Nexium) with clopidogrel should be avoided.
An algorithm to use when considering clopidogrel plus a PPI
Physicians are now left in a bind between the minimal, if any, pooled risk seen in the available data and the FDA recommendation. What is the best action to take?
First, assess the need for dual antiplatelet therapy. If dual antiplatelet therapy (clopidogrel plus aspirin) is required, then a PPI is warranted for gastric protection because the risk of life-threatening bleeding outweighs any increased risk of cardiovascular events.4
If antiplatelet monotherapy (clopidogrel alone) is required, then assess the reason for antisecretory therapy.
For complicated disease, such as gastroesophageal reflux disease with Barrett esophagus or peptic strictures, PPI therapy is warranted to prevent progression or recurrence of complications. If the antisecretory therapy is being provided for noncomplicated symptomatic disorders such as nonerosive gastroesophageal reflux disease or dyspepsia, then one should try to “step down” the therapy by lowering the PPI dose as much as possible while still controlling symptoms to the patient’s tolerance, then possibly stepping further by substituting a histamine-2-receptor antagonist, an antacid, or “on-demand” use of PPIs.22,23
However, if the rationale for antisecretory therapy is simply for gastrointestinal protection, then further risk stratification for gastro intestinal bleeding should be undertaken.4 For patients with a high risk of future gastrointestinal bleeding, such as those with prior episodes of bleeding or concurrent use of nonsteroidal anti-inflammatory drugs, antisecretory therapy is still recommended. Therefore, if a patient is on monotherapy with clopidogrel, has no complicated or symptomatic gastrointestinal disorder, and does not have a high risk of gastrointestinal bleeding, then therapy with a PPI should be reconsidered.
There are no strong data to indicate that one particular PPI should be used or avoided if one of the above criteria indicates the concurrent need for clopidogrel and a PPI. In their health alert about the potential interaction, the FDA did not issue the same warning for PPIs other than omeprazole and esomeprazole, but fell short of recommending a change to another PPI because of a lack of data to support or refute a similar interaction.
Because the half-lives of clopidogrel and PPIs are short, separating their administration could in theory decrease or eliminate the risk of competitive inhibition. The PPI could be given in the morning before breakfast and the clopidogrel could be given at night, or the clopidogrel could be given at lunchtime and the PPI before dinner. Although the FDA does not believe this strategy will reduce this interaction,21 one expert in the field has suggested it.18
DO PPIs CAUSE OSTEOPOROSIS, FRACTURES?
In a widely publicized paper published in 2006, Yang and colleagues25 reported the results of a large nested case-control study in the United Kingdom. The risk of hip fracture was significantly greater in patients who had been using PPIs for at least 1 year than in those who had not. The risk appeared to increase with longer use and higher doses of PPIs.
A similar risk of hip fracture was seen in a larger Danish case-control study published the same year.26 This study also found an increased odds ratio for PPI use in patients with spine fractures as well as in patients with any type of fracture. Interestingly, this study found a lower risk of fracture in patients using a histamine-2-receptor antagonist instead of a PPI.
Targownik et al27 found that the risk of hip fracture was not significantly higher until after 5 years of PPI exposure, with an even stronger risk after 7 years.
However, the data on both association and causal relationship are not uniform.
The Women’s Health Initiative,30 with more than 1 million person-years of followup, found no association between PPI use and hip fracture, but a modest association between PPI use and spine, arm, and wrist fractures, as well as total fractures.
A study in the United Kingdom found that patients without any major risk factors for hip fracture (defined by a risk ratio > 2) accounted for only 25% of cases but 53% of controls. When only these two average-risk groups were compared, the risk of hip fracture was similar in cases and controls.31
Corley et al32 also found that the risk of fracture associated with PPI use was only significant in the presence of another risk factor. These findings suggest that residual confounding may be to blame, at least in part, for the estimates of increased risk in the prior studies.
Another way to interpret these data is that PPIs increase the risk in patients at high risk to begin with, but not in those at average risk. This is an example of interaction (or effect modification) in which the risk is unequally distributed across groups with different characteristics.
A recently published study refutes the theory that impaired calcium absorption is responsible for the increase in fractures.33 In this study, investigators queried the Manitoba Bone Mineral Density Database to determine the relationship between antisecretory therapy with PPIs and osteoporosis or loss of bone mineral density—and they found none. This study may support the theory that residual confounding is the reason for the finding of an increased risk, but it also leaves open the possibility that PPIs induce other changes in bone microstructure that could increase the risk of fracture.
FDA labeling: Possible risk of fracture with PPIs
Based on the data so far, it appears possible that there is a small, albeit statistically significant, association between PPI use and fracture risk. The association is indeed biologically plausible, but it remains to be seen if this association is clinically significant, as the risk is relatively low. Even though the studies had methodologic limitations, on May 25, 2010, the FDA announced a change in the required labeling information for PPIs to indicate a possible risk of fracture with these drugs.34
Reassess the need for chronic PPI therapy
Although patients may worry that they will develop osteoporosis and fractures if they take PPIs, the data do not support a strong risk. Nevertheless, when faced with a patient on chronic PPI therapy, especially with a high dose, providers should use the opportunity to reassess the indication for the PPI to decide if chronic therapy is required, in a matter similar to the algorithm provided for PPI-clopidogrel cotherapy (FIGURE 2). Providers should educate patients about the data, and limit new and recurring PPI prescriptions to patients who require a PPI for appropriate indications, at the lowest dose, and for the shortest time possible.
DO PPIs INCREASE THE RISK OF PNEUMONIA?
Several recent studies have also raised concern about an association between PPI use and pneumonia.
Normally, the stomach remains free of bacteria (except for Helicobacter pylori) because its acidic milieu destroys nearly all bacteria swallowed. If the stomach becomes less acidic, it loses this protective mechanism, and ingested organisms can survive and proliferate.35 In theory, when gastroesophageal reflux occurs, these bacteria could be carried up to the hypopharynx where microaspiration into the lower airways could lead to pneumonia, especially in patients with compromised oropharyngeal protective reflexes (eg, patients on mechanical ventilation).
This possible association came to the attention of the general medical community when a Dutch study,36 in which 5,551 cases of community-acquired pneumonia developed in 364,683 people, found that the incidence of pneumonia was about 4.5 times higher in people exposed to acid-suppressive drugs (both PPIs and histamine-2-receptor antagonists) than in unexposed individuals. Patients who developed pneumonia also had higher odds of significant comorbid conditions, including heart failure and chronic obstructive pulmonary disease. The authors calculated that about one case of pneumonia per 226 patients treated with a PPI would be attributable to the PPI. A major limitation of this study, however, was that only 18% of the patients diagnosed with pneumonia actually had radiologic or microbiologic confirmation of pneumonia.
Other studies later examined the relationship between PPIs and community-acquired pneumonia,37–41 and most have revealed a modestly higher risk of community-acquired pneumonia in patients exposed to PPIs.
This risk was confirmed in a recent metaanalysis, which found a higher risk of community-acquired pneumonia with PPI use (odds ratio 1.36, 95% CI 1.12–1.65).42 However, the authors refrained from drawing definitive conclusions from these data because of significant heterogeneity between the studies. One study37 found that recent onset of use (within 7 days) had a much stronger association with community-acquired pneumonia than longer-term use, which is contradictory to a causal association, since longer-term use should lead to more cases of pneumonia.
Another study investigated the association between acid-suppressive drugs and hospital-acquired pneumonia in nonventilated patients.43 In a 4-year period, there were 63,878 admissions in 42,093 unique patients. Acid-suppressive drugs were prescribed in 32,922 admissions (52%); the drugs included PPIs in 83% of these. Hospital-acquired pneumonia occurred in 2,219 admissions (3.5%), with a higher incidence in patients exposed to acid-suppressive drugs than in the unexposed group (4.6% vs 2.0%). The adjusted odds ratio for pneumonia was 1.3 (95% CI 1.1–1.4) in the exposed group. Subgroup analysis revealed that the association remained significant for PPIs but not for histamine-2-receptor antagonists.
Adequate studies of mechanically ventilated patients in the current era of intravenous PPI use are lacking. Older studies in this group of patients may not be generalizable to current practice because of the reduction in gastric volume with intravenous PPIs that may offset the theoretical risk of aspiration.35
Although the data supporting the association are not exceedingly strong, the relationship is biologically plausible. If there is a risk, it seems to be greatest in the sickest patients, who can least afford to develop pneumonia. Therefore, prudent prescribing should be the rule for both inpatients and outpatients, especially in patients with comorbidities, in whom pneumonia could have serious consequences.
PPIs AND ENTERIC INFECTIONS
Traditionally, gastric acid was not believed to be important in protecting against Clostridium difficile infection because acid-resistant spores were presumed to be the principal vector of transmission.44 Recently, this thought has been challenged, as several studies have found a higher risk of C difficile infection in PPI users. In theory, PPIs may increase the risk of C difficile infection by increasing the ability of the spore to convert to the vegetative form and to survive intraluminally.
A recent meta-analysis of 11 papers, including nearly 127,000 patients, found a significant relationship between PPI use and C difficile infection, with an odds ratio of 2.05 (95% CI 1.47–2.85).45 Further supporting the hypothesis of a direct causative association, a recent study found a significant dose-response, with more aggressive acid-suppression associated with higher odds ratios.46 In view of this association, patients using PPIs who develop diarrhea should be evaluated for C difficile, perhaps even in the absence of other risk factors.
Other enteric infections have been found to be associated with PPIs.44,45 Small intestinal bacterial overgrowth, a condition that is associated with bloating, diarrhea, and malabsorption, has recently been associated with PPI use, although the significance of the association is uncertain.47
Based on a change in the intestinal flora, recent reports have additionally implied that there is a relationship between PPI use and the development of spontaneous bacterial peritonitis in hospitalized cirrhotic patients with ascites. One study found a strong association (odds ratio 4.3, 95% CI 1.3–11.7) between PPIs and spontaneous bacterial pneumonitis,48 whereas another study found no significant association (odds ratio 1.0, 95% CI 0.4–2.6).49
Both studies were small case-control studies of hospitalized patients. No firm conclusion can be drawn about the relevance of this association from these investigations at this point.
PPIs AND ACUTE INTERSTITIAL NEPHRITIS
Several case reports have implicated PPIs as a cause of acute interstitial nephritis.
A systematic review from 2007 found 64 cases documented in the literature, 12 of which were considered certainly associated, and 9 of which were probably associated.50 Initial symptoms were nonspecific and included nausea, malaise, and fever. With such extensive use worldwide as the denominator, the authors concluded that acute interstitial nephritis was a rare, idiosyncratic occurrence related to PPI use, but did not find enough evidence to support a causative relationship. Despite the rarity of the syndrome, they recommended maintaining a high level of clinical suspicion to detect acute interstitial nephritis early in its course, especially soon after the initiation of PPI therapy.
POSSIBLE ASSOCIATIONS WITH IRON AND B12 DEFICIENCIES
Long-term PPI therapy has been thought to be associated with micronutrient deficiencies, especially of iron and vitamin B12. Hydrochloric acid in the stomach assists in the dissociation of iron salts from food and the reduction of ferric iron to the more soluble ferrous iron.51 Gastric acid also facilitates the release of vitamin B12 bound to proteins within ingested foodstuffs to permit binding to R-proteins for eventual absorption in the terminal ileum.51,52
Despite the biologic plausibility of these deficiencies, there is currently little evidence to support a clinically relevant association to recommend a change in current practice.
NO THERAPY IS COMPLETELY WITHOUT RISK
Although concerns have been raised about the long-term safety of PPIs, the preponderance of the evidence does not strongly support the apprehensions publicized over the last few years. When translating these studies into the routine management of patients, it is important to recall some very basic tenets of good patient care.
No therapy is completely without risk—whether pharmacologic, surgical, or psychological, and no matter how benign or straightforward. Consequently, no drug, procedure, or treatment plan should be ordered without a valid indication. Even with an indication, the risk-benefit ratio of the therapy prescribed should always be considered. If the indication for the PPI is weak or uncertain, then even a slight risk tips the balance away from the drug, and the drug should be discontinued.
When seeing patients in long-term care, the indication and necessity for all drugs, including PPIs, should be reviewed. The algorithm proposed in Figure 2 can be adapted for virtually any of the possible associations.
Consider the indication for the PPI. Was the PPI started during a hospitalization and then routinely continued after discharge? This is one situation in which the use of a PPI could potentially be discontinued.2
For complicated acid-peptic disease, dose reduction or cessation of PPI therapy may not be possible.
If the PPI was prescribed only for symptom relief, as in cases of dyspepsia or nonerosive gastroesophageal reflux disease, reduce the dose of PPI to as low as possible to maintain symptom control. Should chronic therapy still be required, no specific monitoring is recommended, apart from routine monitoring that takes place in the course of patient care.
Lastly, because of the media attention that several of these concerns have garnered, patients may still harbor significant concerns about PPIs, even their short-term use. In such cases, the prescriber should take the opportunity to communicate the reason for the decision to prescribe the PPI, as well as the best available data about the risks PPIs may pose. None of these outcomes is very common in the absence of PPIs, with the possible exception of recurrent cardiovascular events, and the risks provided in all of these studies are relative to the baseline risk. Even if the risk of a particular outcome doubles with long-term PPI use, twice a small risk remains a small risk.
- Gatyas G. IMS Health reports U.S. prescription sales grew 5.1 percent in 2009, to $300.3 Billion. IMS Health. http://www.imshealth.com/portal/site/imshealth/menuitem.a46c6d4df3db4b3d88f611019418c22a/?vgnextoid=d690a27e9d5b7210VgnVCM100000ed152ca2RCRD&vgnextfmt=default. Accessed 10/7/2010.
- Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005; 21:1203–1209.
- Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol 2006; 101:2200–2205.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118:1894–1909.
- Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol 2004; 95:2–8.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008; 51:256–260.
- Small DS, Farid NA, Payne CD, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Sibbing D, Morath T, Stegherr J, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost 2009; 101:714–719.
- O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009; 180:713–718.
- Stanek EJ, Aubert RE, Flockhart DA, et al. A national study of the effect of individual proton pump inhibitors on cardiovascular outcomes in patients treated with clopidogrel following coronary stenting: the Clopidogrel Medco Outcomes Study. Program and abstracts of the 32nd Annual SCAI Scientific Sessions May 6, 2009; Las Vegas, Nevada.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Ramirez JF, Selzer F, Chakaprani R, et al. Proton pump inhibitor and clopidogrel combination is not associated with adverse clinical outcomes after PCI: the NHLBI dynamic registry (abstract). J Am Coll Cardiol 2009; 53(suppl 1):A27.
- Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med 2010; 152:337–345.
- Bhatt DL, Cryer B, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol 2010; 105:34–41.
- Walker AM. Confounding by indication. Epidemiology 1996; 7:335–336.
- Dunn SP, Macaulay TE, Brennan DM, et al. Baseline proton pump inhibitor use is associated with increased cardiovascular events with and without the use of clopidogrel in the CREDO trial (abstract). Circulation 2008; 118:S815.
- US Food and Drug Administration. Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). U.S. Department of Health and Human Services, 11/17/2009. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm. Accessed 9/23/2010.
- Inadomi JM, Jamal R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology 2001; 121:1095–1100.
- Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol 2003; 98:1940–1944.
- O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 2005; 118:778–781.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 2006; 79:76–83.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008; 179:319–326.
- Roux C, Briot K, Gossec L, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int 2009; 84:13–19.
- Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int 2008; 83:251–259.
- Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med 2010; 170:765–771.
- Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 2008; 28:951–959.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 2010; 138:896–904.
- US Food and Drug Administration. FDA Drug Safety Communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. U.S. Department of Health and Human Services, 5/25/2010. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed 12/7/2010.
- Vakil N. Acid inhibition and infections outside the gastrointestinal tract. Am J Gastroenterol 2009; 104(suppl 2):S17–S20.
- Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004; 292:1955–1960.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008; 149:391–398.
- Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community-acquired pneumonia and the use of statins, ACE inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf 2009; 18:269–275.
- Rodríguez LA, Ruigómez A, Wallander MA, Johansson S. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology 2009; 20:800–806.
- Eurich DT, Sadowski CA, Simpson SH, Marrie TJ, Majumdar SR. Recurrent community-acquired pneumonia in patients starting acid-suppressing drugs. Am J Med 2010; 123:47–53.
- Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther 2010; 31:1165–1177.
- Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA 2009; 301:2120–2128.
- Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol 2009; 104(suppl 2):S10–S16.
- Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007; 102:2047–2056.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2010; 8:504–508.
- Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol 2009; 104:1130–1134.
- Campbell MS, Obstein K, Reddy KR, Yang YX. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci 2008; 53:394–398.
- Sierra F, Suarez M, Rey M, Vela MF. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther 2007; 26:545–553.
- McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol 2009; 104(suppl 2):S5–S9.
- Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med 2009; 122:896–903.
The development and introduction of the first proton pump inhibitor (PPI), omeprazole (Prilosec), for the management of acid-peptic disorders marks one of the great success stories in gastroenterology. Until the latter part of the 20th century, complications of acid-peptic disease were among the most common problems faced in gastroenterology. Severe peptic strictures were once a highly prevalent cause of dysphagia, and operations for peptic ulcer disease were routinely learned by surgical trainees.
The success of these drugs, with sales total-ling $13.6 billion worldwide in 2009,1 is not just a result of their potency and effectiveness in improving symptoms and complications of acid-peptic disease. Their safety among pharmacologic agents has been unparalleled. When the drugs were first introduced, their use was limited to short courses out of concern that gastric carcinoids could develop, but decades of use have not shown this issue to be of clinical relevance. Serious, acute adverse effects are also exceedingly uncommon.
However, recent reports have questioned the long-term safety of PPIs. Furthermore, these drugs are too often used in patients who have no valid indication for them,2,3 exposing these patients to unnecessary risks.
The goals of this review are to analyze the recent literature about the risks of PPIs and to provide a rational approach for managing patients on PPI therapy in light of these concerns.
DO PPIs REDUCE THE EFFECT OF CLOPIDOGREL?
Clopidogrel (Plavix) is a potent antiplatelet agent commonly used in patients with atherosclerotic cardiac or cerebrovascular disease, sometimes in combination with aspirin. Because of the risk of significant gastrointestinal bleeding, a 2008 multisociety task force recommended prescribing a PPI when both clopidogrel and aspirin are used as dual antiplatelet therapy.4
Studies of clopidogrel plus PPIs: Discrepant results
To date, only one prospective randomized controlled trial has specifically investigated the effect of PPIs on cardiovascular outcomes in patients using clopidogrel. In this trial, patients on dual antiplatelet therapy with clopidogrel and aspirin were randomized to receive either omeprazole 20 mg or placebo. Analysis of the data revealed no significant increase in the composite end point of cardiovascular events (hazard ratio [HR] 0.99, 95% confidence interval [CI] 0.68–1.44, P = .96), but a statistically significant decrease in composite gastrointestinal events (HR 0.34, 95% CI 0.18–0.63, P < .001).17
Unfortunately, this trial had to be terminated before the prespecified sample size and duration of follow-up were reached because the study sponsor declared bankruptcy.
One additional recent retrospective cohort study16 likewise found no significant risk of serious cardiovascular disease related to PPI use in clopidogrel users. It also found that the adjusted incidence of hospitalization for upper gastrointestinal bleeding was 50% lower in patients who used PPIs than in those who did not (HR 0.50, 95% CI 0.39–0.65).
Do factors other than PPIs account for the higher risk in some of the studies?
The discrepant results of these studies suggest that the higher risk of cardiovascular events may be due, either completely or in part, to a factor other than the pharmacologic interaction of PPIs and clopidogrel. It is difficult to infer causality from the available data. In situations in which no randomized controlled trials exist, one looks to observational (case-control or cohort) studies to try to obtain the best estimate of the actual risk. With PPIs and clopidogrel, a randomized controlled trial was performed but terminated before patient enrollment was complete.
The increased risk found in some of these studies may be real, may be due to chance, or may even represent an increased risk from PPIs alone (although data do not support this possibility).18 However, the major concern in observational studies is the inability to account for unmeasured confounders, a problem virtually eliminated by randomization strategies in prospective studies.
In the studies that found a higher risk with the combination of omeprazole plus clopidogrel, the principal concern is confounding by indication, in which distortions of the risk estimates arise from an imbalance in prognostic factors between compared treatment groups that remains unmeasured.19 Stated another way, physicians who believed some patients to be “sicker” or to have a higher risk of serious events may have treated them with a PPI on the basis of factors that remained unaccounted for in the epidemiologic investigation.
This possibility has been reinforced by findings from a nonrandomized subgroup analysis of a randomized controlled trial in which patients who had been receiving a PPI had a higher rate of cardiovascular events whether they received clopidogrel or placebo.20
FDA alert: Avoid using omeprazole or esomeprazole with clopidogrel
Nonetheless, on November 17, 2009, the US Food and Drug Administration (FDA) issued an alert to health care professionals and the public about the potential interaction between clopidogrel and omeprazole.21 In this alert, the FDA stated that the use of omeprazole or esomeprazole (Nexium) with clopidogrel should be avoided.
An algorithm to use when considering clopidogrel plus a PPI
Physicians are now left in a bind between the minimal, if any, pooled risk seen in the available data and the FDA recommendation. What is the best action to take?
First, assess the need for dual antiplatelet therapy. If dual antiplatelet therapy (clopidogrel plus aspirin) is required, then a PPI is warranted for gastric protection because the risk of life-threatening bleeding outweighs any increased risk of cardiovascular events.4
If antiplatelet monotherapy (clopidogrel alone) is required, then assess the reason for antisecretory therapy.
For complicated disease, such as gastroesophageal reflux disease with Barrett esophagus or peptic strictures, PPI therapy is warranted to prevent progression or recurrence of complications. If the antisecretory therapy is being provided for noncomplicated symptomatic disorders such as nonerosive gastroesophageal reflux disease or dyspepsia, then one should try to “step down” the therapy by lowering the PPI dose as much as possible while still controlling symptoms to the patient’s tolerance, then possibly stepping further by substituting a histamine-2-receptor antagonist, an antacid, or “on-demand” use of PPIs.22,23
However, if the rationale for antisecretory therapy is simply for gastrointestinal protection, then further risk stratification for gastro intestinal bleeding should be undertaken.4 For patients with a high risk of future gastrointestinal bleeding, such as those with prior episodes of bleeding or concurrent use of nonsteroidal anti-inflammatory drugs, antisecretory therapy is still recommended. Therefore, if a patient is on monotherapy with clopidogrel, has no complicated or symptomatic gastrointestinal disorder, and does not have a high risk of gastrointestinal bleeding, then therapy with a PPI should be reconsidered.
There are no strong data to indicate that one particular PPI should be used or avoided if one of the above criteria indicates the concurrent need for clopidogrel and a PPI. In their health alert about the potential interaction, the FDA did not issue the same warning for PPIs other than omeprazole and esomeprazole, but fell short of recommending a change to another PPI because of a lack of data to support or refute a similar interaction.
Because the half-lives of clopidogrel and PPIs are short, separating their administration could in theory decrease or eliminate the risk of competitive inhibition. The PPI could be given in the morning before breakfast and the clopidogrel could be given at night, or the clopidogrel could be given at lunchtime and the PPI before dinner. Although the FDA does not believe this strategy will reduce this interaction,21 one expert in the field has suggested it.18
DO PPIs CAUSE OSTEOPOROSIS, FRACTURES?
In a widely publicized paper published in 2006, Yang and colleagues25 reported the results of a large nested case-control study in the United Kingdom. The risk of hip fracture was significantly greater in patients who had been using PPIs for at least 1 year than in those who had not. The risk appeared to increase with longer use and higher doses of PPIs.
A similar risk of hip fracture was seen in a larger Danish case-control study published the same year.26 This study also found an increased odds ratio for PPI use in patients with spine fractures as well as in patients with any type of fracture. Interestingly, this study found a lower risk of fracture in patients using a histamine-2-receptor antagonist instead of a PPI.
Targownik et al27 found that the risk of hip fracture was not significantly higher until after 5 years of PPI exposure, with an even stronger risk after 7 years.
However, the data on both association and causal relationship are not uniform.
The Women’s Health Initiative,30 with more than 1 million person-years of followup, found no association between PPI use and hip fracture, but a modest association between PPI use and spine, arm, and wrist fractures, as well as total fractures.
A study in the United Kingdom found that patients without any major risk factors for hip fracture (defined by a risk ratio > 2) accounted for only 25% of cases but 53% of controls. When only these two average-risk groups were compared, the risk of hip fracture was similar in cases and controls.31
Corley et al32 also found that the risk of fracture associated with PPI use was only significant in the presence of another risk factor. These findings suggest that residual confounding may be to blame, at least in part, for the estimates of increased risk in the prior studies.
Another way to interpret these data is that PPIs increase the risk in patients at high risk to begin with, but not in those at average risk. This is an example of interaction (or effect modification) in which the risk is unequally distributed across groups with different characteristics.
A recently published study refutes the theory that impaired calcium absorption is responsible for the increase in fractures.33 In this study, investigators queried the Manitoba Bone Mineral Density Database to determine the relationship between antisecretory therapy with PPIs and osteoporosis or loss of bone mineral density—and they found none. This study may support the theory that residual confounding is the reason for the finding of an increased risk, but it also leaves open the possibility that PPIs induce other changes in bone microstructure that could increase the risk of fracture.
FDA labeling: Possible risk of fracture with PPIs
Based on the data so far, it appears possible that there is a small, albeit statistically significant, association between PPI use and fracture risk. The association is indeed biologically plausible, but it remains to be seen if this association is clinically significant, as the risk is relatively low. Even though the studies had methodologic limitations, on May 25, 2010, the FDA announced a change in the required labeling information for PPIs to indicate a possible risk of fracture with these drugs.34
Reassess the need for chronic PPI therapy
Although patients may worry that they will develop osteoporosis and fractures if they take PPIs, the data do not support a strong risk. Nevertheless, when faced with a patient on chronic PPI therapy, especially with a high dose, providers should use the opportunity to reassess the indication for the PPI to decide if chronic therapy is required, in a matter similar to the algorithm provided for PPI-clopidogrel cotherapy (FIGURE 2). Providers should educate patients about the data, and limit new and recurring PPI prescriptions to patients who require a PPI for appropriate indications, at the lowest dose, and for the shortest time possible.
DO PPIs INCREASE THE RISK OF PNEUMONIA?
Several recent studies have also raised concern about an association between PPI use and pneumonia.
Normally, the stomach remains free of bacteria (except for Helicobacter pylori) because its acidic milieu destroys nearly all bacteria swallowed. If the stomach becomes less acidic, it loses this protective mechanism, and ingested organisms can survive and proliferate.35 In theory, when gastroesophageal reflux occurs, these bacteria could be carried up to the hypopharynx where microaspiration into the lower airways could lead to pneumonia, especially in patients with compromised oropharyngeal protective reflexes (eg, patients on mechanical ventilation).
This possible association came to the attention of the general medical community when a Dutch study,36 in which 5,551 cases of community-acquired pneumonia developed in 364,683 people, found that the incidence of pneumonia was about 4.5 times higher in people exposed to acid-suppressive drugs (both PPIs and histamine-2-receptor antagonists) than in unexposed individuals. Patients who developed pneumonia also had higher odds of significant comorbid conditions, including heart failure and chronic obstructive pulmonary disease. The authors calculated that about one case of pneumonia per 226 patients treated with a PPI would be attributable to the PPI. A major limitation of this study, however, was that only 18% of the patients diagnosed with pneumonia actually had radiologic or microbiologic confirmation of pneumonia.
Other studies later examined the relationship between PPIs and community-acquired pneumonia,37–41 and most have revealed a modestly higher risk of community-acquired pneumonia in patients exposed to PPIs.
This risk was confirmed in a recent metaanalysis, which found a higher risk of community-acquired pneumonia with PPI use (odds ratio 1.36, 95% CI 1.12–1.65).42 However, the authors refrained from drawing definitive conclusions from these data because of significant heterogeneity between the studies. One study37 found that recent onset of use (within 7 days) had a much stronger association with community-acquired pneumonia than longer-term use, which is contradictory to a causal association, since longer-term use should lead to more cases of pneumonia.
Another study investigated the association between acid-suppressive drugs and hospital-acquired pneumonia in nonventilated patients.43 In a 4-year period, there were 63,878 admissions in 42,093 unique patients. Acid-suppressive drugs were prescribed in 32,922 admissions (52%); the drugs included PPIs in 83% of these. Hospital-acquired pneumonia occurred in 2,219 admissions (3.5%), with a higher incidence in patients exposed to acid-suppressive drugs than in the unexposed group (4.6% vs 2.0%). The adjusted odds ratio for pneumonia was 1.3 (95% CI 1.1–1.4) in the exposed group. Subgroup analysis revealed that the association remained significant for PPIs but not for histamine-2-receptor antagonists.
Adequate studies of mechanically ventilated patients in the current era of intravenous PPI use are lacking. Older studies in this group of patients may not be generalizable to current practice because of the reduction in gastric volume with intravenous PPIs that may offset the theoretical risk of aspiration.35
Although the data supporting the association are not exceedingly strong, the relationship is biologically plausible. If there is a risk, it seems to be greatest in the sickest patients, who can least afford to develop pneumonia. Therefore, prudent prescribing should be the rule for both inpatients and outpatients, especially in patients with comorbidities, in whom pneumonia could have serious consequences.
PPIs AND ENTERIC INFECTIONS
Traditionally, gastric acid was not believed to be important in protecting against Clostridium difficile infection because acid-resistant spores were presumed to be the principal vector of transmission.44 Recently, this thought has been challenged, as several studies have found a higher risk of C difficile infection in PPI users. In theory, PPIs may increase the risk of C difficile infection by increasing the ability of the spore to convert to the vegetative form and to survive intraluminally.
A recent meta-analysis of 11 papers, including nearly 127,000 patients, found a significant relationship between PPI use and C difficile infection, with an odds ratio of 2.05 (95% CI 1.47–2.85).45 Further supporting the hypothesis of a direct causative association, a recent study found a significant dose-response, with more aggressive acid-suppression associated with higher odds ratios.46 In view of this association, patients using PPIs who develop diarrhea should be evaluated for C difficile, perhaps even in the absence of other risk factors.
Other enteric infections have been found to be associated with PPIs.44,45 Small intestinal bacterial overgrowth, a condition that is associated with bloating, diarrhea, and malabsorption, has recently been associated with PPI use, although the significance of the association is uncertain.47
Based on a change in the intestinal flora, recent reports have additionally implied that there is a relationship between PPI use and the development of spontaneous bacterial peritonitis in hospitalized cirrhotic patients with ascites. One study found a strong association (odds ratio 4.3, 95% CI 1.3–11.7) between PPIs and spontaneous bacterial pneumonitis,48 whereas another study found no significant association (odds ratio 1.0, 95% CI 0.4–2.6).49
Both studies were small case-control studies of hospitalized patients. No firm conclusion can be drawn about the relevance of this association from these investigations at this point.
PPIs AND ACUTE INTERSTITIAL NEPHRITIS
Several case reports have implicated PPIs as a cause of acute interstitial nephritis.
A systematic review from 2007 found 64 cases documented in the literature, 12 of which were considered certainly associated, and 9 of which were probably associated.50 Initial symptoms were nonspecific and included nausea, malaise, and fever. With such extensive use worldwide as the denominator, the authors concluded that acute interstitial nephritis was a rare, idiosyncratic occurrence related to PPI use, but did not find enough evidence to support a causative relationship. Despite the rarity of the syndrome, they recommended maintaining a high level of clinical suspicion to detect acute interstitial nephritis early in its course, especially soon after the initiation of PPI therapy.
POSSIBLE ASSOCIATIONS WITH IRON AND B12 DEFICIENCIES
Long-term PPI therapy has been thought to be associated with micronutrient deficiencies, especially of iron and vitamin B12. Hydrochloric acid in the stomach assists in the dissociation of iron salts from food and the reduction of ferric iron to the more soluble ferrous iron.51 Gastric acid also facilitates the release of vitamin B12 bound to proteins within ingested foodstuffs to permit binding to R-proteins for eventual absorption in the terminal ileum.51,52
Despite the biologic plausibility of these deficiencies, there is currently little evidence to support a clinically relevant association to recommend a change in current practice.
NO THERAPY IS COMPLETELY WITHOUT RISK
Although concerns have been raised about the long-term safety of PPIs, the preponderance of the evidence does not strongly support the apprehensions publicized over the last few years. When translating these studies into the routine management of patients, it is important to recall some very basic tenets of good patient care.
No therapy is completely without risk—whether pharmacologic, surgical, or psychological, and no matter how benign or straightforward. Consequently, no drug, procedure, or treatment plan should be ordered without a valid indication. Even with an indication, the risk-benefit ratio of the therapy prescribed should always be considered. If the indication for the PPI is weak or uncertain, then even a slight risk tips the balance away from the drug, and the drug should be discontinued.
When seeing patients in long-term care, the indication and necessity for all drugs, including PPIs, should be reviewed. The algorithm proposed in Figure 2 can be adapted for virtually any of the possible associations.
Consider the indication for the PPI. Was the PPI started during a hospitalization and then routinely continued after discharge? This is one situation in which the use of a PPI could potentially be discontinued.2
For complicated acid-peptic disease, dose reduction or cessation of PPI therapy may not be possible.
If the PPI was prescribed only for symptom relief, as in cases of dyspepsia or nonerosive gastroesophageal reflux disease, reduce the dose of PPI to as low as possible to maintain symptom control. Should chronic therapy still be required, no specific monitoring is recommended, apart from routine monitoring that takes place in the course of patient care.
Lastly, because of the media attention that several of these concerns have garnered, patients may still harbor significant concerns about PPIs, even their short-term use. In such cases, the prescriber should take the opportunity to communicate the reason for the decision to prescribe the PPI, as well as the best available data about the risks PPIs may pose. None of these outcomes is very common in the absence of PPIs, with the possible exception of recurrent cardiovascular events, and the risks provided in all of these studies are relative to the baseline risk. Even if the risk of a particular outcome doubles with long-term PPI use, twice a small risk remains a small risk.
The development and introduction of the first proton pump inhibitor (PPI), omeprazole (Prilosec), for the management of acid-peptic disorders marks one of the great success stories in gastroenterology. Until the latter part of the 20th century, complications of acid-peptic disease were among the most common problems faced in gastroenterology. Severe peptic strictures were once a highly prevalent cause of dysphagia, and operations for peptic ulcer disease were routinely learned by surgical trainees.
The success of these drugs, with sales total-ling $13.6 billion worldwide in 2009,1 is not just a result of their potency and effectiveness in improving symptoms and complications of acid-peptic disease. Their safety among pharmacologic agents has been unparalleled. When the drugs were first introduced, their use was limited to short courses out of concern that gastric carcinoids could develop, but decades of use have not shown this issue to be of clinical relevance. Serious, acute adverse effects are also exceedingly uncommon.
However, recent reports have questioned the long-term safety of PPIs. Furthermore, these drugs are too often used in patients who have no valid indication for them,2,3 exposing these patients to unnecessary risks.
The goals of this review are to analyze the recent literature about the risks of PPIs and to provide a rational approach for managing patients on PPI therapy in light of these concerns.
DO PPIs REDUCE THE EFFECT OF CLOPIDOGREL?
Clopidogrel (Plavix) is a potent antiplatelet agent commonly used in patients with atherosclerotic cardiac or cerebrovascular disease, sometimes in combination with aspirin. Because of the risk of significant gastrointestinal bleeding, a 2008 multisociety task force recommended prescribing a PPI when both clopidogrel and aspirin are used as dual antiplatelet therapy.4
Studies of clopidogrel plus PPIs: Discrepant results
To date, only one prospective randomized controlled trial has specifically investigated the effect of PPIs on cardiovascular outcomes in patients using clopidogrel. In this trial, patients on dual antiplatelet therapy with clopidogrel and aspirin were randomized to receive either omeprazole 20 mg or placebo. Analysis of the data revealed no significant increase in the composite end point of cardiovascular events (hazard ratio [HR] 0.99, 95% confidence interval [CI] 0.68–1.44, P = .96), but a statistically significant decrease in composite gastrointestinal events (HR 0.34, 95% CI 0.18–0.63, P < .001).17
Unfortunately, this trial had to be terminated before the prespecified sample size and duration of follow-up were reached because the study sponsor declared bankruptcy.
One additional recent retrospective cohort study16 likewise found no significant risk of serious cardiovascular disease related to PPI use in clopidogrel users. It also found that the adjusted incidence of hospitalization for upper gastrointestinal bleeding was 50% lower in patients who used PPIs than in those who did not (HR 0.50, 95% CI 0.39–0.65).
Do factors other than PPIs account for the higher risk in some of the studies?
The discrepant results of these studies suggest that the higher risk of cardiovascular events may be due, either completely or in part, to a factor other than the pharmacologic interaction of PPIs and clopidogrel. It is difficult to infer causality from the available data. In situations in which no randomized controlled trials exist, one looks to observational (case-control or cohort) studies to try to obtain the best estimate of the actual risk. With PPIs and clopidogrel, a randomized controlled trial was performed but terminated before patient enrollment was complete.
The increased risk found in some of these studies may be real, may be due to chance, or may even represent an increased risk from PPIs alone (although data do not support this possibility).18 However, the major concern in observational studies is the inability to account for unmeasured confounders, a problem virtually eliminated by randomization strategies in prospective studies.
In the studies that found a higher risk with the combination of omeprazole plus clopidogrel, the principal concern is confounding by indication, in which distortions of the risk estimates arise from an imbalance in prognostic factors between compared treatment groups that remains unmeasured.19 Stated another way, physicians who believed some patients to be “sicker” or to have a higher risk of serious events may have treated them with a PPI on the basis of factors that remained unaccounted for in the epidemiologic investigation.
This possibility has been reinforced by findings from a nonrandomized subgroup analysis of a randomized controlled trial in which patients who had been receiving a PPI had a higher rate of cardiovascular events whether they received clopidogrel or placebo.20
FDA alert: Avoid using omeprazole or esomeprazole with clopidogrel
Nonetheless, on November 17, 2009, the US Food and Drug Administration (FDA) issued an alert to health care professionals and the public about the potential interaction between clopidogrel and omeprazole.21 In this alert, the FDA stated that the use of omeprazole or esomeprazole (Nexium) with clopidogrel should be avoided.
An algorithm to use when considering clopidogrel plus a PPI
Physicians are now left in a bind between the minimal, if any, pooled risk seen in the available data and the FDA recommendation. What is the best action to take?
First, assess the need for dual antiplatelet therapy. If dual antiplatelet therapy (clopidogrel plus aspirin) is required, then a PPI is warranted for gastric protection because the risk of life-threatening bleeding outweighs any increased risk of cardiovascular events.4
If antiplatelet monotherapy (clopidogrel alone) is required, then assess the reason for antisecretory therapy.
For complicated disease, such as gastroesophageal reflux disease with Barrett esophagus or peptic strictures, PPI therapy is warranted to prevent progression or recurrence of complications. If the antisecretory therapy is being provided for noncomplicated symptomatic disorders such as nonerosive gastroesophageal reflux disease or dyspepsia, then one should try to “step down” the therapy by lowering the PPI dose as much as possible while still controlling symptoms to the patient’s tolerance, then possibly stepping further by substituting a histamine-2-receptor antagonist, an antacid, or “on-demand” use of PPIs.22,23
However, if the rationale for antisecretory therapy is simply for gastrointestinal protection, then further risk stratification for gastro intestinal bleeding should be undertaken.4 For patients with a high risk of future gastrointestinal bleeding, such as those with prior episodes of bleeding or concurrent use of nonsteroidal anti-inflammatory drugs, antisecretory therapy is still recommended. Therefore, if a patient is on monotherapy with clopidogrel, has no complicated or symptomatic gastrointestinal disorder, and does not have a high risk of gastrointestinal bleeding, then therapy with a PPI should be reconsidered.
There are no strong data to indicate that one particular PPI should be used or avoided if one of the above criteria indicates the concurrent need for clopidogrel and a PPI. In their health alert about the potential interaction, the FDA did not issue the same warning for PPIs other than omeprazole and esomeprazole, but fell short of recommending a change to another PPI because of a lack of data to support or refute a similar interaction.
Because the half-lives of clopidogrel and PPIs are short, separating their administration could in theory decrease or eliminate the risk of competitive inhibition. The PPI could be given in the morning before breakfast and the clopidogrel could be given at night, or the clopidogrel could be given at lunchtime and the PPI before dinner. Although the FDA does not believe this strategy will reduce this interaction,21 one expert in the field has suggested it.18
DO PPIs CAUSE OSTEOPOROSIS, FRACTURES?
In a widely publicized paper published in 2006, Yang and colleagues25 reported the results of a large nested case-control study in the United Kingdom. The risk of hip fracture was significantly greater in patients who had been using PPIs for at least 1 year than in those who had not. The risk appeared to increase with longer use and higher doses of PPIs.
A similar risk of hip fracture was seen in a larger Danish case-control study published the same year.26 This study also found an increased odds ratio for PPI use in patients with spine fractures as well as in patients with any type of fracture. Interestingly, this study found a lower risk of fracture in patients using a histamine-2-receptor antagonist instead of a PPI.
Targownik et al27 found that the risk of hip fracture was not significantly higher until after 5 years of PPI exposure, with an even stronger risk after 7 years.
However, the data on both association and causal relationship are not uniform.
The Women’s Health Initiative,30 with more than 1 million person-years of followup, found no association between PPI use and hip fracture, but a modest association between PPI use and spine, arm, and wrist fractures, as well as total fractures.
A study in the United Kingdom found that patients without any major risk factors for hip fracture (defined by a risk ratio > 2) accounted for only 25% of cases but 53% of controls. When only these two average-risk groups were compared, the risk of hip fracture was similar in cases and controls.31
Corley et al32 also found that the risk of fracture associated with PPI use was only significant in the presence of another risk factor. These findings suggest that residual confounding may be to blame, at least in part, for the estimates of increased risk in the prior studies.
Another way to interpret these data is that PPIs increase the risk in patients at high risk to begin with, but not in those at average risk. This is an example of interaction (or effect modification) in which the risk is unequally distributed across groups with different characteristics.
A recently published study refutes the theory that impaired calcium absorption is responsible for the increase in fractures.33 In this study, investigators queried the Manitoba Bone Mineral Density Database to determine the relationship between antisecretory therapy with PPIs and osteoporosis or loss of bone mineral density—and they found none. This study may support the theory that residual confounding is the reason for the finding of an increased risk, but it also leaves open the possibility that PPIs induce other changes in bone microstructure that could increase the risk of fracture.
FDA labeling: Possible risk of fracture with PPIs
Based on the data so far, it appears possible that there is a small, albeit statistically significant, association between PPI use and fracture risk. The association is indeed biologically plausible, but it remains to be seen if this association is clinically significant, as the risk is relatively low. Even though the studies had methodologic limitations, on May 25, 2010, the FDA announced a change in the required labeling information for PPIs to indicate a possible risk of fracture with these drugs.34
Reassess the need for chronic PPI therapy
Although patients may worry that they will develop osteoporosis and fractures if they take PPIs, the data do not support a strong risk. Nevertheless, when faced with a patient on chronic PPI therapy, especially with a high dose, providers should use the opportunity to reassess the indication for the PPI to decide if chronic therapy is required, in a matter similar to the algorithm provided for PPI-clopidogrel cotherapy (FIGURE 2). Providers should educate patients about the data, and limit new and recurring PPI prescriptions to patients who require a PPI for appropriate indications, at the lowest dose, and for the shortest time possible.
DO PPIs INCREASE THE RISK OF PNEUMONIA?
Several recent studies have also raised concern about an association between PPI use and pneumonia.
Normally, the stomach remains free of bacteria (except for Helicobacter pylori) because its acidic milieu destroys nearly all bacteria swallowed. If the stomach becomes less acidic, it loses this protective mechanism, and ingested organisms can survive and proliferate.35 In theory, when gastroesophageal reflux occurs, these bacteria could be carried up to the hypopharynx where microaspiration into the lower airways could lead to pneumonia, especially in patients with compromised oropharyngeal protective reflexes (eg, patients on mechanical ventilation).
This possible association came to the attention of the general medical community when a Dutch study,36 in which 5,551 cases of community-acquired pneumonia developed in 364,683 people, found that the incidence of pneumonia was about 4.5 times higher in people exposed to acid-suppressive drugs (both PPIs and histamine-2-receptor antagonists) than in unexposed individuals. Patients who developed pneumonia also had higher odds of significant comorbid conditions, including heart failure and chronic obstructive pulmonary disease. The authors calculated that about one case of pneumonia per 226 patients treated with a PPI would be attributable to the PPI. A major limitation of this study, however, was that only 18% of the patients diagnosed with pneumonia actually had radiologic or microbiologic confirmation of pneumonia.
Other studies later examined the relationship between PPIs and community-acquired pneumonia,37–41 and most have revealed a modestly higher risk of community-acquired pneumonia in patients exposed to PPIs.
This risk was confirmed in a recent metaanalysis, which found a higher risk of community-acquired pneumonia with PPI use (odds ratio 1.36, 95% CI 1.12–1.65).42 However, the authors refrained from drawing definitive conclusions from these data because of significant heterogeneity between the studies. One study37 found that recent onset of use (within 7 days) had a much stronger association with community-acquired pneumonia than longer-term use, which is contradictory to a causal association, since longer-term use should lead to more cases of pneumonia.
Another study investigated the association between acid-suppressive drugs and hospital-acquired pneumonia in nonventilated patients.43 In a 4-year period, there were 63,878 admissions in 42,093 unique patients. Acid-suppressive drugs were prescribed in 32,922 admissions (52%); the drugs included PPIs in 83% of these. Hospital-acquired pneumonia occurred in 2,219 admissions (3.5%), with a higher incidence in patients exposed to acid-suppressive drugs than in the unexposed group (4.6% vs 2.0%). The adjusted odds ratio for pneumonia was 1.3 (95% CI 1.1–1.4) in the exposed group. Subgroup analysis revealed that the association remained significant for PPIs but not for histamine-2-receptor antagonists.
Adequate studies of mechanically ventilated patients in the current era of intravenous PPI use are lacking. Older studies in this group of patients may not be generalizable to current practice because of the reduction in gastric volume with intravenous PPIs that may offset the theoretical risk of aspiration.35
Although the data supporting the association are not exceedingly strong, the relationship is biologically plausible. If there is a risk, it seems to be greatest in the sickest patients, who can least afford to develop pneumonia. Therefore, prudent prescribing should be the rule for both inpatients and outpatients, especially in patients with comorbidities, in whom pneumonia could have serious consequences.
PPIs AND ENTERIC INFECTIONS
Traditionally, gastric acid was not believed to be important in protecting against Clostridium difficile infection because acid-resistant spores were presumed to be the principal vector of transmission.44 Recently, this thought has been challenged, as several studies have found a higher risk of C difficile infection in PPI users. In theory, PPIs may increase the risk of C difficile infection by increasing the ability of the spore to convert to the vegetative form and to survive intraluminally.
A recent meta-analysis of 11 papers, including nearly 127,000 patients, found a significant relationship between PPI use and C difficile infection, with an odds ratio of 2.05 (95% CI 1.47–2.85).45 Further supporting the hypothesis of a direct causative association, a recent study found a significant dose-response, with more aggressive acid-suppression associated with higher odds ratios.46 In view of this association, patients using PPIs who develop diarrhea should be evaluated for C difficile, perhaps even in the absence of other risk factors.
Other enteric infections have been found to be associated with PPIs.44,45 Small intestinal bacterial overgrowth, a condition that is associated with bloating, diarrhea, and malabsorption, has recently been associated with PPI use, although the significance of the association is uncertain.47
Based on a change in the intestinal flora, recent reports have additionally implied that there is a relationship between PPI use and the development of spontaneous bacterial peritonitis in hospitalized cirrhotic patients with ascites. One study found a strong association (odds ratio 4.3, 95% CI 1.3–11.7) between PPIs and spontaneous bacterial pneumonitis,48 whereas another study found no significant association (odds ratio 1.0, 95% CI 0.4–2.6).49
Both studies were small case-control studies of hospitalized patients. No firm conclusion can be drawn about the relevance of this association from these investigations at this point.
PPIs AND ACUTE INTERSTITIAL NEPHRITIS
Several case reports have implicated PPIs as a cause of acute interstitial nephritis.
A systematic review from 2007 found 64 cases documented in the literature, 12 of which were considered certainly associated, and 9 of which were probably associated.50 Initial symptoms were nonspecific and included nausea, malaise, and fever. With such extensive use worldwide as the denominator, the authors concluded that acute interstitial nephritis was a rare, idiosyncratic occurrence related to PPI use, but did not find enough evidence to support a causative relationship. Despite the rarity of the syndrome, they recommended maintaining a high level of clinical suspicion to detect acute interstitial nephritis early in its course, especially soon after the initiation of PPI therapy.
POSSIBLE ASSOCIATIONS WITH IRON AND B12 DEFICIENCIES
Long-term PPI therapy has been thought to be associated with micronutrient deficiencies, especially of iron and vitamin B12. Hydrochloric acid in the stomach assists in the dissociation of iron salts from food and the reduction of ferric iron to the more soluble ferrous iron.51 Gastric acid also facilitates the release of vitamin B12 bound to proteins within ingested foodstuffs to permit binding to R-proteins for eventual absorption in the terminal ileum.51,52
Despite the biologic plausibility of these deficiencies, there is currently little evidence to support a clinically relevant association to recommend a change in current practice.
NO THERAPY IS COMPLETELY WITHOUT RISK
Although concerns have been raised about the long-term safety of PPIs, the preponderance of the evidence does not strongly support the apprehensions publicized over the last few years. When translating these studies into the routine management of patients, it is important to recall some very basic tenets of good patient care.
No therapy is completely without risk—whether pharmacologic, surgical, or psychological, and no matter how benign or straightforward. Consequently, no drug, procedure, or treatment plan should be ordered without a valid indication. Even with an indication, the risk-benefit ratio of the therapy prescribed should always be considered. If the indication for the PPI is weak or uncertain, then even a slight risk tips the balance away from the drug, and the drug should be discontinued.
When seeing patients in long-term care, the indication and necessity for all drugs, including PPIs, should be reviewed. The algorithm proposed in Figure 2 can be adapted for virtually any of the possible associations.
Consider the indication for the PPI. Was the PPI started during a hospitalization and then routinely continued after discharge? This is one situation in which the use of a PPI could potentially be discontinued.2
For complicated acid-peptic disease, dose reduction or cessation of PPI therapy may not be possible.
If the PPI was prescribed only for symptom relief, as in cases of dyspepsia or nonerosive gastroesophageal reflux disease, reduce the dose of PPI to as low as possible to maintain symptom control. Should chronic therapy still be required, no specific monitoring is recommended, apart from routine monitoring that takes place in the course of patient care.
Lastly, because of the media attention that several of these concerns have garnered, patients may still harbor significant concerns about PPIs, even their short-term use. In such cases, the prescriber should take the opportunity to communicate the reason for the decision to prescribe the PPI, as well as the best available data about the risks PPIs may pose. None of these outcomes is very common in the absence of PPIs, with the possible exception of recurrent cardiovascular events, and the risks provided in all of these studies are relative to the baseline risk. Even if the risk of a particular outcome doubles with long-term PPI use, twice a small risk remains a small risk.
- Gatyas G. IMS Health reports U.S. prescription sales grew 5.1 percent in 2009, to $300.3 Billion. IMS Health. http://www.imshealth.com/portal/site/imshealth/menuitem.a46c6d4df3db4b3d88f611019418c22a/?vgnextoid=d690a27e9d5b7210VgnVCM100000ed152ca2RCRD&vgnextfmt=default. Accessed 10/7/2010.
- Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005; 21:1203–1209.
- Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol 2006; 101:2200–2205.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118:1894–1909.
- Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol 2004; 95:2–8.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008; 51:256–260.
- Small DS, Farid NA, Payne CD, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Sibbing D, Morath T, Stegherr J, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost 2009; 101:714–719.
- O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009; 180:713–718.
- Stanek EJ, Aubert RE, Flockhart DA, et al. A national study of the effect of individual proton pump inhibitors on cardiovascular outcomes in patients treated with clopidogrel following coronary stenting: the Clopidogrel Medco Outcomes Study. Program and abstracts of the 32nd Annual SCAI Scientific Sessions May 6, 2009; Las Vegas, Nevada.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Ramirez JF, Selzer F, Chakaprani R, et al. Proton pump inhibitor and clopidogrel combination is not associated with adverse clinical outcomes after PCI: the NHLBI dynamic registry (abstract). J Am Coll Cardiol 2009; 53(suppl 1):A27.
- Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med 2010; 152:337–345.
- Bhatt DL, Cryer B, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol 2010; 105:34–41.
- Walker AM. Confounding by indication. Epidemiology 1996; 7:335–336.
- Dunn SP, Macaulay TE, Brennan DM, et al. Baseline proton pump inhibitor use is associated with increased cardiovascular events with and without the use of clopidogrel in the CREDO trial (abstract). Circulation 2008; 118:S815.
- US Food and Drug Administration. Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). U.S. Department of Health and Human Services, 11/17/2009. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm. Accessed 9/23/2010.
- Inadomi JM, Jamal R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology 2001; 121:1095–1100.
- Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol 2003; 98:1940–1944.
- O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 2005; 118:778–781.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 2006; 79:76–83.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008; 179:319–326.
- Roux C, Briot K, Gossec L, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int 2009; 84:13–19.
- Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int 2008; 83:251–259.
- Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med 2010; 170:765–771.
- Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 2008; 28:951–959.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 2010; 138:896–904.
- US Food and Drug Administration. FDA Drug Safety Communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. U.S. Department of Health and Human Services, 5/25/2010. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed 12/7/2010.
- Vakil N. Acid inhibition and infections outside the gastrointestinal tract. Am J Gastroenterol 2009; 104(suppl 2):S17–S20.
- Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004; 292:1955–1960.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008; 149:391–398.
- Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community-acquired pneumonia and the use of statins, ACE inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf 2009; 18:269–275.
- Rodríguez LA, Ruigómez A, Wallander MA, Johansson S. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology 2009; 20:800–806.
- Eurich DT, Sadowski CA, Simpson SH, Marrie TJ, Majumdar SR. Recurrent community-acquired pneumonia in patients starting acid-suppressing drugs. Am J Med 2010; 123:47–53.
- Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther 2010; 31:1165–1177.
- Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA 2009; 301:2120–2128.
- Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol 2009; 104(suppl 2):S10–S16.
- Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007; 102:2047–2056.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2010; 8:504–508.
- Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol 2009; 104:1130–1134.
- Campbell MS, Obstein K, Reddy KR, Yang YX. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci 2008; 53:394–398.
- Sierra F, Suarez M, Rey M, Vela MF. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther 2007; 26:545–553.
- McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol 2009; 104(suppl 2):S5–S9.
- Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med 2009; 122:896–903.
- Gatyas G. IMS Health reports U.S. prescription sales grew 5.1 percent in 2009, to $300.3 Billion. IMS Health. http://www.imshealth.com/portal/site/imshealth/menuitem.a46c6d4df3db4b3d88f611019418c22a/?vgnextoid=d690a27e9d5b7210VgnVCM100000ed152ca2RCRD&vgnextfmt=default. Accessed 10/7/2010.
- Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther 2005; 21:1203–1209.
- Heidelbaugh JJ, Inadomi JM. Magnitude and economic impact of inappropriate use of stress ulcer prophylaxis in non-ICU hospitalized patients. Am J Gastroenterol 2006; 101:2200–2205.
- Bhatt DL, Scheiman J, Abraham NS, et al; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118:1894–1909.
- Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol 2004; 95:2–8.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 2008; 51:256–260.
- Small DS, Farid NA, Payne CD, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol 2008; 48:475–484.
- Sibbing D, Morath T, Stegherr J, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost 2009; 101:714–719.
- O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009; 374:989–997.
- Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2009; 301:937–944.
- Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 2009; 180:713–718.
- Stanek EJ, Aubert RE, Flockhart DA, et al. A national study of the effect of individual proton pump inhibitors on cardiovascular outcomes in patients treated with clopidogrel following coronary stenting: the Clopidogrel Medco Outcomes Study. Program and abstracts of the 32nd Annual SCAI Scientific Sessions May 6, 2009; Las Vegas, Nevada.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Ramirez JF, Selzer F, Chakaprani R, et al. Proton pump inhibitor and clopidogrel combination is not associated with adverse clinical outcomes after PCI: the NHLBI dynamic registry (abstract). J Am Coll Cardiol 2009; 53(suppl 1):A27.
- Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med 2010; 152:337–345.
- Bhatt DL, Cryer B, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol 2010; 105:34–41.
- Walker AM. Confounding by indication. Epidemiology 1996; 7:335–336.
- Dunn SP, Macaulay TE, Brennan DM, et al. Baseline proton pump inhibitor use is associated with increased cardiovascular events with and without the use of clopidogrel in the CREDO trial (abstract). Circulation 2008; 118:S815.
- US Food and Drug Administration. Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). U.S. Department of Health and Human Services, 11/17/2009. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm. Accessed 9/23/2010.
- Inadomi JM, Jamal R, Murata GH, et al. Step-down management of gastroesophageal reflux disease. Gastroenterology 2001; 121:1095–1100.
- Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step-down from multiple- to single-dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol 2003; 98:1940–1944.
- O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 2005; 118:778–781.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int 2006; 79:76–83.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008; 179:319–326.
- Roux C, Briot K, Gossec L, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int 2009; 84:13–19.
- Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int 2008; 83:251–259.
- Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med 2010; 170:765–771.
- Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy 2008; 28:951–959.
- Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010; 139:93–101.
- Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology 2010; 138:896–904.
- US Food and Drug Administration. FDA Drug Safety Communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. U.S. Department of Health and Human Services, 5/25/2010. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm. Accessed 12/7/2010.
- Vakil N. Acid inhibition and infections outside the gastrointestinal tract. Am J Gastroenterol 2009; 104(suppl 2):S17–S20.
- Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004; 292:1955–1960.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008; 149:391–398.
- Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community-acquired pneumonia and the use of statins, ACE inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf 2009; 18:269–275.
- Rodríguez LA, Ruigómez A, Wallander MA, Johansson S. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology 2009; 20:800–806.
- Eurich DT, Sadowski CA, Simpson SH, Marrie TJ, Majumdar SR. Recurrent community-acquired pneumonia in patients starting acid-suppressing drugs. Am J Med 2010; 123:47–53.
- Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther 2010; 31:1165–1177.
- Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA 2009; 301:2120–2128.
- Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol 2009; 104(suppl 2):S10–S16.
- Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007; 102:2047–2056.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2010; 8:504–508.
- Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol 2009; 104:1130–1134.
- Campbell MS, Obstein K, Reddy KR, Yang YX. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci 2008; 53:394–398.
- Sierra F, Suarez M, Rey M, Vela MF. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther 2007; 26:545–553.
- McColl KE. Effect of proton pump inhibitors on vitamins and iron. Am J Gastroenterol 2009; 104(suppl 2):S5–S9.
- Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med 2009; 122:896–903.
KEY POINTS
- The US Food and Drug Administration has issued alerts that PPIs may increase the rate of osteoporosis-related fractures and may decrease the effectiveness of clopidogrel (Plavix) for preventing serious cardiovascular events.
- Other concerns include increased rates of pneumonia, Clostridium difficile infection, and other infections.
- A prudent approach to managing these concerns in day-to-day practice is required: PPIs, like any other drugs, should be prescribed only if indicated.