User login
The FREEDOM trial: In appropriate patients with diabetes and multivessel coronary artery disease, CABG beats PCI
Many patients with diabetes mellitus develop complex, accelerated, multifocal coronary artery disease. Moreover, if they undergo revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), their risk of morbidity and death afterward is higher than in those without diabetes.1,2
Over the last 2 decades, CABG and PCI have advanced significantly, as have antithrombotic therapy and drug therapies to modify cardiovascular risk factors such as hyperlipidemia, hypertension, and diabetes.
Several earlier studies showed CABG to be more beneficial than PCI in diabetic patients with multivessel coronary artery disease.3–5 However, the topic has been controversial, and a substantial proportion of these patients continue to undergo PCI rather than CABG.
There are two main reasons for the continued use of PCI in this population. First, PCI is evolving, with new adjuvant drugs and drugeluting stents. Many cardiologists believe that earlier trials, which did not use contemporary PCI techniques, are outdated and that current, state-of-the-art PCI may be equivalent to—if not superior to—CABG.
Second, PCI is often performed on an ad hoc basis immediately after diagnostic angiography, leaving little time for discussion with the patient about alternative treatments. In this scenario, patients are inclined to undergo PCI immediately, while they are already on the table in the catheterization suite, rather than CABG at a later date.6
In addition, although the current joint guide-lines of the American College of Cardiology and the American Heart Association state that CABG is preferable to PCI for patients with diabetes and multivessel coronary artery disease, they give it only a level IIa recommendation.7
The much-anticipated Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial8 was designed to settle the CABG-vs-PCI debate, thereby leading to a stronger guideline recommendation for the preferred revascularization strategy in this patient population.
WHY ARE DIABETIC PATIENTS DIFFERENT?
Diabetes mellitus is a major risk factor for premature and aggressive coronary artery disease. Several mechanisms have been proposed to explain this association.
Diabetic patients have higher concentrations of several inflammatory proteins than those without diabetes, including C-reactive protein, tumor necrosis factor, and platelet-derived soluble CD40 ligand. They also have higher levels of adhesion molecules such as vascular cell adhesion molecule-1 and intercellular adhesion molecule.9,10 In addition, when blood sugar levels are high, platelets express more glycoprotein IIb/IIIa receptors and are therefore more prone to aggregate.11
These prothrombotic and proinflammatory cytokines, in conjunction with endothelial dysfunction and metabolic disorders such as hyperglycemia, hyperlipidemia, obesity, insulin resistance, and oxidative stress, lead to accelerated atherosclerosis in patients with diabetes.12 Also, because diabetes is a systemic disease, the atherosclerotic process is diffuse, and many patients with diabetes have left main coronary artery lesions and diffuse multivessel coronary artery disease.13,14
Although the short-term outcomes of revascularization by any means are comparable in patients with and without diabetes, diabetic patients have lower long-term survival rates and higher rates of myocardial infarction and need for repeat procedures.15 Diabetic patients who undergo PCI have a high rate of stent thrombosis and restenosis.16,17 Similarly, those undergoing CABG have higher rates of postoperative infection and renal and neurologic complications.18,19
BEFORE THE FREEDOM TRIAL
The question of CABG vs PCI has plagued physicians ever since PCI came to the forefront in the 1980s. Before stents were widely used, PCI with balloon angioplasty was known to be comparable to CABG for single-vessel disease, but whether it was beneficial in patients with multivessel disease or left main disease was not entirely evident. Randomized clinical trials were launched to answer the question.
Studies of balloon angioplasty vs CABG
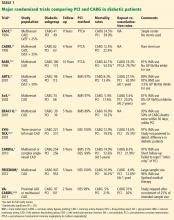
The BARI trial (Bypass Angioplasty Revascularization Investigation),5,20 published in 1996, compared PCI (using balloon angioplasty without a stent) and CABG in patients with multivessel coronary artery disease (Table 120–29).
Between 1988 and 1991, the trial randomly assigned 1,829 patients with multivessel disease to receive either PCI or CABG and compared their long-term outcomes. Although there was no difference in mortality rates between the two groups overall, the diabetic subgroup had a significantly better survival rate with CABG than with PCI, which was sustained over a follow-up period of 10 years.5
BARI had a significant clinical impact at the time and led to a clinical alert by the National Heart, Lung, and Blood Institute recommending CABG over PCI for patients with diabetes. However, not everyone accepted the results, because they were based on a small number of patients (n = 353) in a retrospectively determined subgroup. Further, the BARI trial was conducted before the advent of coronary stents, which were later shown to improve outcomes after PCI. Also, optimal medical therapy after revascularization was not specified in the protocol, which likely affected outcomes.
EAST (Emory Angioplasty Versus Surgery Trial)21 and CABRI (Coronary Angioplasty Versus Bypass Revascularization Investigation) 22 were similar randomized trials comparing angioplasty and CABG in patients with multivessel coronary artery disease. These showed better outcomes after CABG in patients with diabetes. However, lack of statistical significance because of small sample sizes limited their clinical impact.
Studies of PCI with bare-metal stents vs CABG
The ARTS trial (Arterial Revascularization Therapy Study) compared PCI (with bare-metal stents) and CABG in 1,205 patients with multivessel coronary artery disease.23 The mortality rate did not differ significantly between two treatment groups overall or in the diabetic subgroup. However, the repeat revascularization rate was higher with PCI than with CABG.
The SoS trial (Stenting or Surgery)24 had similar results.
The ERACI II trial (Argentine Randomized Study: Coronary Angioplasty With Stenting Versus Coronary Bypass Surgery in Multi-Vessel Disease)25 found no difference in mortality rates at 5 years with CABG vs PCI.
These trials were criticized, as none of them routinely used glycoprotein IIb/IIIa inhibitors with PCI, which by then had been shown to reduce mortality rates.30 However, these trials made it clear that restenosis requiring repeat revascularization was a major disadvantage of PCI with bare-metal stents compared with CABG in patients with diabetes. Drug-eluting stents, which significantly reduced the rates of in-stent restenosis and target-lesion revascularization, were expected to overcome this major disadvantage.
Studies of PCI with drug-eluting stents vs CABG
ARTS II was the first trial to compare PCI with drug-eluting stents vs CABG. This was a nonrandomized single-arm study of 607 patients (including 159 with diabetes) who were treated with drug-eluting stents; the outcomes were compared with the CABG group from the earlier ARTS trial.31
At 3 years, in the diabetic subgroup, the rates of death, myocardial infarction, and stroke were not significantly different between treatments, although a trend favored PCI. However, this comparison was limited by selection bias, as ARTS II was a nonrandomized trial in which operators chose patients for drug-eluting stents in an attempt to match already known outcomes from the CABG cohort of ARTS.
SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) was the first randomized trial comparing PCI with drug-eluting stents (in this trial, paclitaxel-eluting) vs CABG in patients with three-vessel or left main coronary artery disease.26,27 Subgroup analysis in patients with diabetes mellitus revealed a higher rate of major adverse cardiac and cerebrovascular events (death, myocardial infarction, stroke, or repeat revascularization) in the PCI group than in the CABG patients, largely driven by higher rates of repeat revascularization after PCI.32,33 SYNTAX was not designed to assess significant differences in rates of death.
The CARDIa trial (Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and multivessel coronary artery disease to PCI (about one-third with bare-metal stents and two-thirds with drug-eluting stents) or CABG. Rates of major adverse cardiac and cerebrovascular events were higher in the PCI group, again largely driven by higher rates of repeat revascularization.4 CARDIa was stopped early because of a lack of enrollment and could not provide sufficient evidence to endorse one strategy over the other.
VA-CARDS (Veteran Affairs Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and proximal left anterior descending artery or multivessel coronary artery disease to receive PCI with drug-eluting stents or CABG.28 Although the rate of death was lower with CABG than with PCI at 2 years, the trial was underpowered and was terminated at 25% of the initial intended patient enrollment. In addition, only 9% of diabetic patients screened were angiographically eligible for the study.29
Registry data. Analysis of a large data set from the National Cardiovascular Disease Registry and the Society of Thoracic Surgeons revealed a survival advantage of CABG over PCI for a follow-up period of 5 years.34 However, this was a nonrandomized study, so its conclusions were not definitive.
THE FREEDOM TRIAL
Given the limitations of the trials described above, the National Heart, Lung, and Blood Institute sponsored the FREEDOM trial—an appropriately powered, randomized comparison of PCI (with drug-eluting stents) and CABG (using arterial grafting) in patients with diabetes and multivessel coronary artery disease using contemporary techniques and concomitant optimal medical therapy.8
FREEDOM study design
The FREEDOM trial enrolled 1,900 patients with diabetes and angiographically confirmed multivessel coronary artery disease (83% with three-vessel disease) with stenosis of more than 70% in two or more major epicardial vessels involving at least two separate coronary-artery territories. The main exclusion criteria were severe left main coronary artery stenosis (≥ 50% stenosis), class III or IV congestive heart failure, and previous CABG or valve surgery. For CABG surgery, arterial revascularization was encouraged.
Dual antiplatelet therapy was recommended for at least 12 months in patients receiving a drug-eluting stent, and optimal medical management for diabetes, hypertension, and hyperlipidemia was strongly advocated.
Between April 2005 and April 2010, 32,966 patients were screened, of whom 3,309 were eligible for the trial and 1,900 consented and were randomized (953 to the PCI group and 947 to the CABG group). The patients were followed for a minimum of 2 years and had a median follow-up time of 3.8 years. Outcomes were measured with an intention-to-treat analysis.
Study results
Patients. The groups were comparable with regard to baseline demographics and cardiac risk factors.
The mean age was 63; 29% of the patients were women, and 83% had three-vessel coronary artery disease. The mean hemoglobin A1c was 7.8%, and the mean ejection fraction was 66%. The mean SYNTAX score, which defines the anatomic complexity of lesions, was 26 (≤ 22 is mild, 23–32 is intermediate, and ≥ 33 is high). The mean EURO score, which defines surgical risk, was 2.7 (a score ≥ 5 being associated with a lower rate of survival).
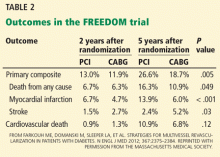
The primary composite outcome (death, nonfatal myocardial infarction, or nonfatal stroke) occurred less frequently in the CABG group than in the PCI group (Table 2). CABG was also associated with significantly lower rates of death from any cause and of myocardial infarction. Importantly, survival curves comparing the two groups diverged at 2-year follow-up. In contrast to other outcomes assessed, stroke occurred more often in the CABG group. The 5-year rates in the CABG group vs the PCI group were:
- Primary outcome—18.7% vs 26.6%, P = .005
- Death from any cause—10.9% vs 16.3%, P = .049
- Myocardial infarction—6% vs 13.9%, P < .0001
- Stroke—5.2% vs 2.4%, P = .03.
The secondary outcome (death, nonfatal myocardial infarction, nonfatal stroke, or repeat revascularization at 30 days or 12 months) had occurred significantly more often in the PCI group than in the CABG group at 1 year (16.8% vs 11.8%, P = .004), with most of the difference attributable to a higher repeat revascularization rate in the PCI group (12.6% vs 4.8%, P < .001).
Subgroup analysis. CABG was superior to PCI across all prespecified subgroups, covering the complexity of the coronary artery disease. Event rates with CABG vs PCI, by tertiles of the SYNTAX score:
- SYNTAX scores ≤ 22: 17.2% vs 23.2%
- SYNTAX scores 23–32: 17.7% vs 27.2%
- SYNTAX scores ≥ 33: 22.8% vs 30.6%.
Cost-effectiveness. Although up-front costs were higher with CABG, at $34,467 for the index hospitalization vs $25,845 for PCI (P < .001), when the in-trial results were extended to a lifetime horizon, CABG had an incremental cost-effectiveness ratio of $8,132 per quality-adjusted life-year gained vs PCI.35 Traditionally, therapies are considered costeffective if the incremental cost-effectiveness ratio is less than $50,000 per quality-adjusted life-year gained.
WHY MAY CABG BE SUPERIOR IN DIABETIC PATIENTS?
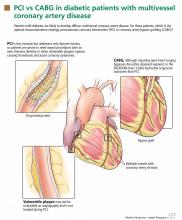
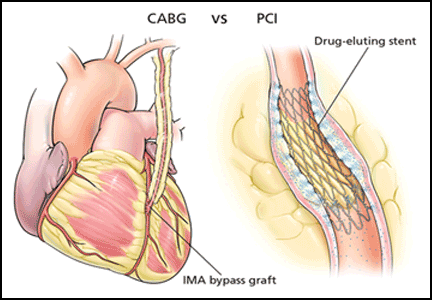
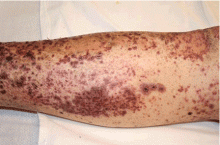
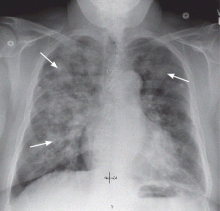
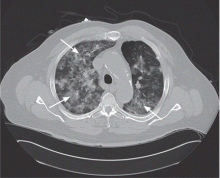
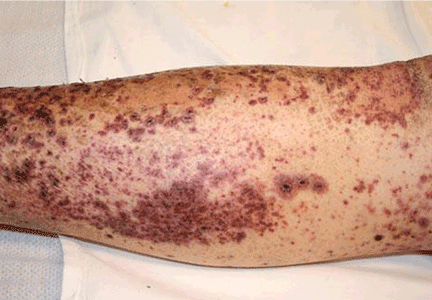
The major advantage of CABG over PCI is the ability to achieve complete revascularization. Diabetic patients with coronary artery disease tend to have diffuse, multifocal disease with several stenotic lesions in multiple coronary arteries. While stents only treat the focal area of most significant occlusion, CABG may bypass all proximal vulnerable plaques that could potentially develop into culprit lesions over time, truly bypassing the diseased segments (Figure 1).
In addition, heavy calcification may not allow optimal stenting in these patients.
Use of multiple stents increases the risk of restenosis, which could lead to a higher incidence of myocardial infarction and need for repeat revascularization. This was evident in the FREEDOM trial, in which the mean number of stents per patient was 4.2. Also, some lesions need to be left untreated because of the complexity involved.
The major improvement in outcomes after CABG has resulted from using arterial conduits such as the internal mammary artery rather than the saphenous vein.36 The patency rates of internal mammary artery grafts exceed 80% over 10 years.37 Internal mammary artery grafting was done in 94% of patients receiving CABG in the FREEDOM trial.
WHAT DOES THIS MEAN?
FREEDOM was a landmark trial that confirmed that CABG provides significant benefit compared with contemporary PCI with drug-eluting stents in patients with diabetes mellitus and multivessel coronary artery disease. It was a large multicenter trial that was adequately powered, unlike most of the earlier trials of this topic.
Unlike previous trials in which the benefit of CABG was driven by reduction in repeat revascularizations alone, FREEDOM showed lower incidence rates of all-cause mortality and myocardial infarction with CABG than with PCI. CABG was better regardless of SYNTAX score, number of diseased vessels, ejection fraction, race, or sex of the patient, indicating that it leads to superior outcomes across a wide spectrum of patients.
An argument that cardiologists often cite when recommending PCI is that it can save money due to lower length of index hospital stay and lower procedure costs of with PCI than with CABG. However, in FREEDOM, CABG also appeared to be highly cost-effective.
FREEDOM had limitations
While FREEDOM provided robust data proving the superiority of CABG, the study had several limitations.
Although there was an overall survival benefit with CABG compared with PCI, the difference in incidence of cardiovascular deaths (which accounted for 64% of all deaths) was not statistically significant.
The trial included only patients who were eligible for both PCI and CABG. Hence, the results may not be generalizable to all diabetic patients with multivessel coronary artery disease—indeed, only 10% of those screened were considered eligible for the trial. However, it is likely that several patients screened in the FREEDOM trial may not have been eligible for PCI or CABG at the time of screening, since the revascularization decision was made by a multidisciplinary team and a more appropriate decision (either CABG or PCI) was then made.
Other factors limiting the general applicability of the results were low numbers of female patients (28.6%), black patients (6.3%), patients with an ejection fraction of 40% or less (2.5%), and patients with a low SYNTAX score (35%).
There were several unexplained observations as well. The difference in events between the treatment groups was much higher in North America than in other regions. The number of coronary lesions in the CABG group was high (mean = 5.74), but the average numbers of grafts used was only 2.9, and data were not provided regarding use of sequential grafting. Similarly, an average of only 3.5 of the six stenotic lesions per patient in the PCI group were revascularized; whether this was the result of procedural limitations with PCI was not entirely clear.
In addition, while the investigators mention that an average patient received four stents, a surprising finding was that the mean total length of the stents used was only 26 mm. This appears too small, as the usual length of one drug-eluting stent is about 20 to 30 mm.
Since only high-volume centers with good outcome data were included in the trial, the results may lack validity for patients undergoing revascularization at low-volume community centers.
It remains to be seen if the benefits of CABG will be sustained over 10 years and longer, when saphenous vein grafts tend to fail and require repeat revascularization, commonly performed with PCI. Previous data suggest that the longer the follow-up, the better the results with CABG. However, long-term results (> 10 years) in studies comparing drugeluting stents and CABG are not available.
Despite limitations, FREEDOM may change clinical practice
Despite these limitations, the FREEDOM trial has the potential to change clinical practice and strengthen current recommendations for CABG in these patients.
The trial underscored the importance of a multidisciplinary heart team approach in managing patients with complex coronary artery disease, similar to that being used in patients with severe aortic stenosis since transcatheter aortic valve replacement became available.
It should also bring an end to the practice of ad hoc PCI, especially in patients with diabetes and multivessel coronary artery disease. It is now imperative that physicians discuss current evidence for therapeutic options with the patients and their families before performing diagnostic angiography rather than immediately afterward, to give the patients ample time to make an informed decision. This is important, as most patients are likely to choose PCI in the same setting over CABG unless there is extensive discussion about the risks and benefits of both strategies done in an unbiased manner before angiography.
The fear of open heart surgery, a longer hospital stay, and a higher risk of stroke with CABG may lead some patients to choose PCI instead. In addition, factors that may preclude CABG in otherwise-eligible patients include anatomic considerations (diffuse distal vessel disease, poor conduits), individual factors (frailty, poor renal function, poor pulmonary function, patient preference), and local expertise.
Nevertheless, the patient should be presented with current evidence, and discussions regarding the optimal procedure should be held with a heart team, which should include an interventional cardiologist, a cardiothoracic surgeon, and a noninvasive cardiologist to facilitate an unbiased decision.
Regardless of the strategy chosen, the importance of compliance with optimal medical therapy (statins, antiplatelet agents, diabetes treatment) should be continuously emphasized to the patient.
WHAT DOES THE FUTURE HOLD?
Despite unequivocal evidence that CABG is superior to PCI in eligible patients with diabetes mellitus in the current era, PCI technologies continue to evolve rapidly. Newer second-generation drug-eluting stents have shown lower rates of restenosis38,39 and may shorten the duration of post-PCI dual-antiplatelet therapy, a nuisance that has negatively affected outcomes with drug-eluting stents (because of problems of cost, poor compliance, and increased bleeding risk).
At the same time, CABG has also improved, with more extensive use of complete arterial conduits and use of an off-pump bypass technique that in theory poses a lower risk of stroke, although this has not yet been shown in a randomized trial.40
Alternative approaches are being investigated. One of them is a hybrid procedure in which minimally invasive off-pump arterial grafting is combined with drug-eluting stents, which may reduce the risk of stroke and speed postoperative recovery.
- Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2005; 293:1501–1508.
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008; 52:255–262.
- Mack MJ, Banning AP, Serruys PW, et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg 2011; 92:2140–2146.
- Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010; 55:432–440.
- The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007; 49:1600–1606.
- Hlatky MA. Compelling evidence for coronary-bypass surgery in patients with diabetes. N Engl J Med 2012; 367:2437–2438.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000; 102:2180–2184.
- Bluher M, Unger R, Rassoul F, et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or type II diabetes. Diabetologia 2002; 45:210–216.
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003; 108:1527–1532.
- Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003; 41:1071–1077.
- Waller BF, Palumbo PJ, Lie JT, Roberts WC. Status of the coronary arteries at necropsy in diabetes mellitus with onset after age 30 years. Analysis of 229 diabetic patients with and without clinical evidence of coronary heart disease and comparison to 183 control subjects. Am J Med 1980; 69:498–506.
- Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle-aged diabetic patients (the London Cohort of the WHO Multinational Study of Vascular Disease in Diabetics) I: causes and death rates. Diabetologia 1990; 33:538–541.
- Laskey WK, Selzer F, Vlachos HA, et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2002; 90:1062–1067.
- Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480.
- Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005; 111:143–149.
- Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetologica 1999; 36:77–84.
- Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100:642–647.
- The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med 1996; 335:217–225.
- King SB, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eightyear mortality in the Emory Angioplasty versus Surgery Trial (East). J Am Coll Cardiol 2000; 35:1116–1121.
- Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF; CABRI Investigators (Coronary Angioplasty versus Bypass Revascularization Investigation). Difference in the mortality of the CABRI diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J Cardiol 2001; 87:947–950.
- Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 2005; 46:575–581.
- Booth J, Clayton T, Pepper J, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation 2008; 118:381–388.
- Rodriguez AE, Baldi J, Fernandez Pereira C, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol 2005; 46:582–588.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013; 381:629–638.
- Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013; 61:808–816.
- Ellis SG. Coronary revascularization for patients with diabetes: updated data favor coronary artery bypass grafting. J Am Coll Cardiol 2013; 61:817–819.
- Bhatt DL, Marso SP, Lincoff AM, et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol 2000; 35:922–928.
- Serruys PW, Ong AT, Morice MC, et al. Arterial Revascularisation Therapies Study Part II - Sirolimus-eluting stents for the treatment of patients with multivessel de novo coronary artery lesions. EuroIntervention 2005; 1:147–156.
- Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013; 43:1006–1013.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366:1467–1476.
- Magnuson EA, Farkouh ME, Fuster V, et al; FREEDOM Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes and multivessel coronary artery disease: results from the FREEDOM trial. Circulation 2013; 127:820–831.
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986; 314:1–6.
- Tector AJ, Schmahl TM, Janson B, et al. The internal mammary artery graft. Its longevity after coronary bypass. JAMA 1981; 246:2181–2183.
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitax-eleluting stents in coronary artery disease. N Engl J Med 2010; 362:1663–1674.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010; 363:136–146.
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366:1489–1497.
Many patients with diabetes mellitus develop complex, accelerated, multifocal coronary artery disease. Moreover, if they undergo revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), their risk of morbidity and death afterward is higher than in those without diabetes.1,2
Over the last 2 decades, CABG and PCI have advanced significantly, as have antithrombotic therapy and drug therapies to modify cardiovascular risk factors such as hyperlipidemia, hypertension, and diabetes.
Several earlier studies showed CABG to be more beneficial than PCI in diabetic patients with multivessel coronary artery disease.3–5 However, the topic has been controversial, and a substantial proportion of these patients continue to undergo PCI rather than CABG.
There are two main reasons for the continued use of PCI in this population. First, PCI is evolving, with new adjuvant drugs and drugeluting stents. Many cardiologists believe that earlier trials, which did not use contemporary PCI techniques, are outdated and that current, state-of-the-art PCI may be equivalent to—if not superior to—CABG.
Second, PCI is often performed on an ad hoc basis immediately after diagnostic angiography, leaving little time for discussion with the patient about alternative treatments. In this scenario, patients are inclined to undergo PCI immediately, while they are already on the table in the catheterization suite, rather than CABG at a later date.6
In addition, although the current joint guide-lines of the American College of Cardiology and the American Heart Association state that CABG is preferable to PCI for patients with diabetes and multivessel coronary artery disease, they give it only a level IIa recommendation.7
The much-anticipated Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial8 was designed to settle the CABG-vs-PCI debate, thereby leading to a stronger guideline recommendation for the preferred revascularization strategy in this patient population.
WHY ARE DIABETIC PATIENTS DIFFERENT?
Diabetes mellitus is a major risk factor for premature and aggressive coronary artery disease. Several mechanisms have been proposed to explain this association.
Diabetic patients have higher concentrations of several inflammatory proteins than those without diabetes, including C-reactive protein, tumor necrosis factor, and platelet-derived soluble CD40 ligand. They also have higher levels of adhesion molecules such as vascular cell adhesion molecule-1 and intercellular adhesion molecule.9,10 In addition, when blood sugar levels are high, platelets express more glycoprotein IIb/IIIa receptors and are therefore more prone to aggregate.11
These prothrombotic and proinflammatory cytokines, in conjunction with endothelial dysfunction and metabolic disorders such as hyperglycemia, hyperlipidemia, obesity, insulin resistance, and oxidative stress, lead to accelerated atherosclerosis in patients with diabetes.12 Also, because diabetes is a systemic disease, the atherosclerotic process is diffuse, and many patients with diabetes have left main coronary artery lesions and diffuse multivessel coronary artery disease.13,14
Although the short-term outcomes of revascularization by any means are comparable in patients with and without diabetes, diabetic patients have lower long-term survival rates and higher rates of myocardial infarction and need for repeat procedures.15 Diabetic patients who undergo PCI have a high rate of stent thrombosis and restenosis.16,17 Similarly, those undergoing CABG have higher rates of postoperative infection and renal and neurologic complications.18,19
BEFORE THE FREEDOM TRIAL
The question of CABG vs PCI has plagued physicians ever since PCI came to the forefront in the 1980s. Before stents were widely used, PCI with balloon angioplasty was known to be comparable to CABG for single-vessel disease, but whether it was beneficial in patients with multivessel disease or left main disease was not entirely evident. Randomized clinical trials were launched to answer the question.
Studies of balloon angioplasty vs CABG
The BARI trial (Bypass Angioplasty Revascularization Investigation),5,20 published in 1996, compared PCI (using balloon angioplasty without a stent) and CABG in patients with multivessel coronary artery disease (Table 120–29).
Between 1988 and 1991, the trial randomly assigned 1,829 patients with multivessel disease to receive either PCI or CABG and compared their long-term outcomes. Although there was no difference in mortality rates between the two groups overall, the diabetic subgroup had a significantly better survival rate with CABG than with PCI, which was sustained over a follow-up period of 10 years.5
BARI had a significant clinical impact at the time and led to a clinical alert by the National Heart, Lung, and Blood Institute recommending CABG over PCI for patients with diabetes. However, not everyone accepted the results, because they were based on a small number of patients (n = 353) in a retrospectively determined subgroup. Further, the BARI trial was conducted before the advent of coronary stents, which were later shown to improve outcomes after PCI. Also, optimal medical therapy after revascularization was not specified in the protocol, which likely affected outcomes.
EAST (Emory Angioplasty Versus Surgery Trial)21 and CABRI (Coronary Angioplasty Versus Bypass Revascularization Investigation) 22 were similar randomized trials comparing angioplasty and CABG in patients with multivessel coronary artery disease. These showed better outcomes after CABG in patients with diabetes. However, lack of statistical significance because of small sample sizes limited their clinical impact.
Studies of PCI with bare-metal stents vs CABG
The ARTS trial (Arterial Revascularization Therapy Study) compared PCI (with bare-metal stents) and CABG in 1,205 patients with multivessel coronary artery disease.23 The mortality rate did not differ significantly between two treatment groups overall or in the diabetic subgroup. However, the repeat revascularization rate was higher with PCI than with CABG.
The SoS trial (Stenting or Surgery)24 had similar results.
The ERACI II trial (Argentine Randomized Study: Coronary Angioplasty With Stenting Versus Coronary Bypass Surgery in Multi-Vessel Disease)25 found no difference in mortality rates at 5 years with CABG vs PCI.
These trials were criticized, as none of them routinely used glycoprotein IIb/IIIa inhibitors with PCI, which by then had been shown to reduce mortality rates.30 However, these trials made it clear that restenosis requiring repeat revascularization was a major disadvantage of PCI with bare-metal stents compared with CABG in patients with diabetes. Drug-eluting stents, which significantly reduced the rates of in-stent restenosis and target-lesion revascularization, were expected to overcome this major disadvantage.
Studies of PCI with drug-eluting stents vs CABG
ARTS II was the first trial to compare PCI with drug-eluting stents vs CABG. This was a nonrandomized single-arm study of 607 patients (including 159 with diabetes) who were treated with drug-eluting stents; the outcomes were compared with the CABG group from the earlier ARTS trial.31
At 3 years, in the diabetic subgroup, the rates of death, myocardial infarction, and stroke were not significantly different between treatments, although a trend favored PCI. However, this comparison was limited by selection bias, as ARTS II was a nonrandomized trial in which operators chose patients for drug-eluting stents in an attempt to match already known outcomes from the CABG cohort of ARTS.
SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) was the first randomized trial comparing PCI with drug-eluting stents (in this trial, paclitaxel-eluting) vs CABG in patients with three-vessel or left main coronary artery disease.26,27 Subgroup analysis in patients with diabetes mellitus revealed a higher rate of major adverse cardiac and cerebrovascular events (death, myocardial infarction, stroke, or repeat revascularization) in the PCI group than in the CABG patients, largely driven by higher rates of repeat revascularization after PCI.32,33 SYNTAX was not designed to assess significant differences in rates of death.
The CARDIa trial (Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and multivessel coronary artery disease to PCI (about one-third with bare-metal stents and two-thirds with drug-eluting stents) or CABG. Rates of major adverse cardiac and cerebrovascular events were higher in the PCI group, again largely driven by higher rates of repeat revascularization.4 CARDIa was stopped early because of a lack of enrollment and could not provide sufficient evidence to endorse one strategy over the other.
VA-CARDS (Veteran Affairs Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and proximal left anterior descending artery or multivessel coronary artery disease to receive PCI with drug-eluting stents or CABG.28 Although the rate of death was lower with CABG than with PCI at 2 years, the trial was underpowered and was terminated at 25% of the initial intended patient enrollment. In addition, only 9% of diabetic patients screened were angiographically eligible for the study.29
Registry data. Analysis of a large data set from the National Cardiovascular Disease Registry and the Society of Thoracic Surgeons revealed a survival advantage of CABG over PCI for a follow-up period of 5 years.34 However, this was a nonrandomized study, so its conclusions were not definitive.
THE FREEDOM TRIAL
Given the limitations of the trials described above, the National Heart, Lung, and Blood Institute sponsored the FREEDOM trial—an appropriately powered, randomized comparison of PCI (with drug-eluting stents) and CABG (using arterial grafting) in patients with diabetes and multivessel coronary artery disease using contemporary techniques and concomitant optimal medical therapy.8
FREEDOM study design
The FREEDOM trial enrolled 1,900 patients with diabetes and angiographically confirmed multivessel coronary artery disease (83% with three-vessel disease) with stenosis of more than 70% in two or more major epicardial vessels involving at least two separate coronary-artery territories. The main exclusion criteria were severe left main coronary artery stenosis (≥ 50% stenosis), class III or IV congestive heart failure, and previous CABG or valve surgery. For CABG surgery, arterial revascularization was encouraged.
Dual antiplatelet therapy was recommended for at least 12 months in patients receiving a drug-eluting stent, and optimal medical management for diabetes, hypertension, and hyperlipidemia was strongly advocated.
Between April 2005 and April 2010, 32,966 patients were screened, of whom 3,309 were eligible for the trial and 1,900 consented and were randomized (953 to the PCI group and 947 to the CABG group). The patients were followed for a minimum of 2 years and had a median follow-up time of 3.8 years. Outcomes were measured with an intention-to-treat analysis.
Study results
Patients. The groups were comparable with regard to baseline demographics and cardiac risk factors.
The mean age was 63; 29% of the patients were women, and 83% had three-vessel coronary artery disease. The mean hemoglobin A1c was 7.8%, and the mean ejection fraction was 66%. The mean SYNTAX score, which defines the anatomic complexity of lesions, was 26 (≤ 22 is mild, 23–32 is intermediate, and ≥ 33 is high). The mean EURO score, which defines surgical risk, was 2.7 (a score ≥ 5 being associated with a lower rate of survival).
The primary composite outcome (death, nonfatal myocardial infarction, or nonfatal stroke) occurred less frequently in the CABG group than in the PCI group (Table 2). CABG was also associated with significantly lower rates of death from any cause and of myocardial infarction. Importantly, survival curves comparing the two groups diverged at 2-year follow-up. In contrast to other outcomes assessed, stroke occurred more often in the CABG group. The 5-year rates in the CABG group vs the PCI group were:
- Primary outcome—18.7% vs 26.6%, P = .005
- Death from any cause—10.9% vs 16.3%, P = .049
- Myocardial infarction—6% vs 13.9%, P < .0001
- Stroke—5.2% vs 2.4%, P = .03.
The secondary outcome (death, nonfatal myocardial infarction, nonfatal stroke, or repeat revascularization at 30 days or 12 months) had occurred significantly more often in the PCI group than in the CABG group at 1 year (16.8% vs 11.8%, P = .004), with most of the difference attributable to a higher repeat revascularization rate in the PCI group (12.6% vs 4.8%, P < .001).
Subgroup analysis. CABG was superior to PCI across all prespecified subgroups, covering the complexity of the coronary artery disease. Event rates with CABG vs PCI, by tertiles of the SYNTAX score:
- SYNTAX scores ≤ 22: 17.2% vs 23.2%
- SYNTAX scores 23–32: 17.7% vs 27.2%
- SYNTAX scores ≥ 33: 22.8% vs 30.6%.
Cost-effectiveness. Although up-front costs were higher with CABG, at $34,467 for the index hospitalization vs $25,845 for PCI (P < .001), when the in-trial results were extended to a lifetime horizon, CABG had an incremental cost-effectiveness ratio of $8,132 per quality-adjusted life-year gained vs PCI.35 Traditionally, therapies are considered costeffective if the incremental cost-effectiveness ratio is less than $50,000 per quality-adjusted life-year gained.
WHY MAY CABG BE SUPERIOR IN DIABETIC PATIENTS?
The major advantage of CABG over PCI is the ability to achieve complete revascularization. Diabetic patients with coronary artery disease tend to have diffuse, multifocal disease with several stenotic lesions in multiple coronary arteries. While stents only treat the focal area of most significant occlusion, CABG may bypass all proximal vulnerable plaques that could potentially develop into culprit lesions over time, truly bypassing the diseased segments (Figure 1).
In addition, heavy calcification may not allow optimal stenting in these patients.
Use of multiple stents increases the risk of restenosis, which could lead to a higher incidence of myocardial infarction and need for repeat revascularization. This was evident in the FREEDOM trial, in which the mean number of stents per patient was 4.2. Also, some lesions need to be left untreated because of the complexity involved.
The major improvement in outcomes after CABG has resulted from using arterial conduits such as the internal mammary artery rather than the saphenous vein.36 The patency rates of internal mammary artery grafts exceed 80% over 10 years.37 Internal mammary artery grafting was done in 94% of patients receiving CABG in the FREEDOM trial.
WHAT DOES THIS MEAN?
FREEDOM was a landmark trial that confirmed that CABG provides significant benefit compared with contemporary PCI with drug-eluting stents in patients with diabetes mellitus and multivessel coronary artery disease. It was a large multicenter trial that was adequately powered, unlike most of the earlier trials of this topic.
Unlike previous trials in which the benefit of CABG was driven by reduction in repeat revascularizations alone, FREEDOM showed lower incidence rates of all-cause mortality and myocardial infarction with CABG than with PCI. CABG was better regardless of SYNTAX score, number of diseased vessels, ejection fraction, race, or sex of the patient, indicating that it leads to superior outcomes across a wide spectrum of patients.
An argument that cardiologists often cite when recommending PCI is that it can save money due to lower length of index hospital stay and lower procedure costs of with PCI than with CABG. However, in FREEDOM, CABG also appeared to be highly cost-effective.
FREEDOM had limitations
While FREEDOM provided robust data proving the superiority of CABG, the study had several limitations.
Although there was an overall survival benefit with CABG compared with PCI, the difference in incidence of cardiovascular deaths (which accounted for 64% of all deaths) was not statistically significant.
The trial included only patients who were eligible for both PCI and CABG. Hence, the results may not be generalizable to all diabetic patients with multivessel coronary artery disease—indeed, only 10% of those screened were considered eligible for the trial. However, it is likely that several patients screened in the FREEDOM trial may not have been eligible for PCI or CABG at the time of screening, since the revascularization decision was made by a multidisciplinary team and a more appropriate decision (either CABG or PCI) was then made.
Other factors limiting the general applicability of the results were low numbers of female patients (28.6%), black patients (6.3%), patients with an ejection fraction of 40% or less (2.5%), and patients with a low SYNTAX score (35%).
There were several unexplained observations as well. The difference in events between the treatment groups was much higher in North America than in other regions. The number of coronary lesions in the CABG group was high (mean = 5.74), but the average numbers of grafts used was only 2.9, and data were not provided regarding use of sequential grafting. Similarly, an average of only 3.5 of the six stenotic lesions per patient in the PCI group were revascularized; whether this was the result of procedural limitations with PCI was not entirely clear.
In addition, while the investigators mention that an average patient received four stents, a surprising finding was that the mean total length of the stents used was only 26 mm. This appears too small, as the usual length of one drug-eluting stent is about 20 to 30 mm.
Since only high-volume centers with good outcome data were included in the trial, the results may lack validity for patients undergoing revascularization at low-volume community centers.
It remains to be seen if the benefits of CABG will be sustained over 10 years and longer, when saphenous vein grafts tend to fail and require repeat revascularization, commonly performed with PCI. Previous data suggest that the longer the follow-up, the better the results with CABG. However, long-term results (> 10 years) in studies comparing drugeluting stents and CABG are not available.
Despite limitations, FREEDOM may change clinical practice
Despite these limitations, the FREEDOM trial has the potential to change clinical practice and strengthen current recommendations for CABG in these patients.
The trial underscored the importance of a multidisciplinary heart team approach in managing patients with complex coronary artery disease, similar to that being used in patients with severe aortic stenosis since transcatheter aortic valve replacement became available.
It should also bring an end to the practice of ad hoc PCI, especially in patients with diabetes and multivessel coronary artery disease. It is now imperative that physicians discuss current evidence for therapeutic options with the patients and their families before performing diagnostic angiography rather than immediately afterward, to give the patients ample time to make an informed decision. This is important, as most patients are likely to choose PCI in the same setting over CABG unless there is extensive discussion about the risks and benefits of both strategies done in an unbiased manner before angiography.
The fear of open heart surgery, a longer hospital stay, and a higher risk of stroke with CABG may lead some patients to choose PCI instead. In addition, factors that may preclude CABG in otherwise-eligible patients include anatomic considerations (diffuse distal vessel disease, poor conduits), individual factors (frailty, poor renal function, poor pulmonary function, patient preference), and local expertise.
Nevertheless, the patient should be presented with current evidence, and discussions regarding the optimal procedure should be held with a heart team, which should include an interventional cardiologist, a cardiothoracic surgeon, and a noninvasive cardiologist to facilitate an unbiased decision.
Regardless of the strategy chosen, the importance of compliance with optimal medical therapy (statins, antiplatelet agents, diabetes treatment) should be continuously emphasized to the patient.
WHAT DOES THE FUTURE HOLD?
Despite unequivocal evidence that CABG is superior to PCI in eligible patients with diabetes mellitus in the current era, PCI technologies continue to evolve rapidly. Newer second-generation drug-eluting stents have shown lower rates of restenosis38,39 and may shorten the duration of post-PCI dual-antiplatelet therapy, a nuisance that has negatively affected outcomes with drug-eluting stents (because of problems of cost, poor compliance, and increased bleeding risk).
At the same time, CABG has also improved, with more extensive use of complete arterial conduits and use of an off-pump bypass technique that in theory poses a lower risk of stroke, although this has not yet been shown in a randomized trial.40
Alternative approaches are being investigated. One of them is a hybrid procedure in which minimally invasive off-pump arterial grafting is combined with drug-eluting stents, which may reduce the risk of stroke and speed postoperative recovery.
Many patients with diabetes mellitus develop complex, accelerated, multifocal coronary artery disease. Moreover, if they undergo revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), their risk of morbidity and death afterward is higher than in those without diabetes.1,2
Over the last 2 decades, CABG and PCI have advanced significantly, as have antithrombotic therapy and drug therapies to modify cardiovascular risk factors such as hyperlipidemia, hypertension, and diabetes.
Several earlier studies showed CABG to be more beneficial than PCI in diabetic patients with multivessel coronary artery disease.3–5 However, the topic has been controversial, and a substantial proportion of these patients continue to undergo PCI rather than CABG.
There are two main reasons for the continued use of PCI in this population. First, PCI is evolving, with new adjuvant drugs and drugeluting stents. Many cardiologists believe that earlier trials, which did not use contemporary PCI techniques, are outdated and that current, state-of-the-art PCI may be equivalent to—if not superior to—CABG.
Second, PCI is often performed on an ad hoc basis immediately after diagnostic angiography, leaving little time for discussion with the patient about alternative treatments. In this scenario, patients are inclined to undergo PCI immediately, while they are already on the table in the catheterization suite, rather than CABG at a later date.6
In addition, although the current joint guide-lines of the American College of Cardiology and the American Heart Association state that CABG is preferable to PCI for patients with diabetes and multivessel coronary artery disease, they give it only a level IIa recommendation.7
The much-anticipated Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial8 was designed to settle the CABG-vs-PCI debate, thereby leading to a stronger guideline recommendation for the preferred revascularization strategy in this patient population.
WHY ARE DIABETIC PATIENTS DIFFERENT?
Diabetes mellitus is a major risk factor for premature and aggressive coronary artery disease. Several mechanisms have been proposed to explain this association.
Diabetic patients have higher concentrations of several inflammatory proteins than those without diabetes, including C-reactive protein, tumor necrosis factor, and platelet-derived soluble CD40 ligand. They also have higher levels of adhesion molecules such as vascular cell adhesion molecule-1 and intercellular adhesion molecule.9,10 In addition, when blood sugar levels are high, platelets express more glycoprotein IIb/IIIa receptors and are therefore more prone to aggregate.11
These prothrombotic and proinflammatory cytokines, in conjunction with endothelial dysfunction and metabolic disorders such as hyperglycemia, hyperlipidemia, obesity, insulin resistance, and oxidative stress, lead to accelerated atherosclerosis in patients with diabetes.12 Also, because diabetes is a systemic disease, the atherosclerotic process is diffuse, and many patients with diabetes have left main coronary artery lesions and diffuse multivessel coronary artery disease.13,14
Although the short-term outcomes of revascularization by any means are comparable in patients with and without diabetes, diabetic patients have lower long-term survival rates and higher rates of myocardial infarction and need for repeat procedures.15 Diabetic patients who undergo PCI have a high rate of stent thrombosis and restenosis.16,17 Similarly, those undergoing CABG have higher rates of postoperative infection and renal and neurologic complications.18,19
BEFORE THE FREEDOM TRIAL
The question of CABG vs PCI has plagued physicians ever since PCI came to the forefront in the 1980s. Before stents were widely used, PCI with balloon angioplasty was known to be comparable to CABG for single-vessel disease, but whether it was beneficial in patients with multivessel disease or left main disease was not entirely evident. Randomized clinical trials were launched to answer the question.
Studies of balloon angioplasty vs CABG
The BARI trial (Bypass Angioplasty Revascularization Investigation),5,20 published in 1996, compared PCI (using balloon angioplasty without a stent) and CABG in patients with multivessel coronary artery disease (Table 120–29).
Between 1988 and 1991, the trial randomly assigned 1,829 patients with multivessel disease to receive either PCI or CABG and compared their long-term outcomes. Although there was no difference in mortality rates between the two groups overall, the diabetic subgroup had a significantly better survival rate with CABG than with PCI, which was sustained over a follow-up period of 10 years.5
BARI had a significant clinical impact at the time and led to a clinical alert by the National Heart, Lung, and Blood Institute recommending CABG over PCI for patients with diabetes. However, not everyone accepted the results, because they were based on a small number of patients (n = 353) in a retrospectively determined subgroup. Further, the BARI trial was conducted before the advent of coronary stents, which were later shown to improve outcomes after PCI. Also, optimal medical therapy after revascularization was not specified in the protocol, which likely affected outcomes.
EAST (Emory Angioplasty Versus Surgery Trial)21 and CABRI (Coronary Angioplasty Versus Bypass Revascularization Investigation) 22 were similar randomized trials comparing angioplasty and CABG in patients with multivessel coronary artery disease. These showed better outcomes after CABG in patients with diabetes. However, lack of statistical significance because of small sample sizes limited their clinical impact.
Studies of PCI with bare-metal stents vs CABG
The ARTS trial (Arterial Revascularization Therapy Study) compared PCI (with bare-metal stents) and CABG in 1,205 patients with multivessel coronary artery disease.23 The mortality rate did not differ significantly between two treatment groups overall or in the diabetic subgroup. However, the repeat revascularization rate was higher with PCI than with CABG.
The SoS trial (Stenting or Surgery)24 had similar results.
The ERACI II trial (Argentine Randomized Study: Coronary Angioplasty With Stenting Versus Coronary Bypass Surgery in Multi-Vessel Disease)25 found no difference in mortality rates at 5 years with CABG vs PCI.
These trials were criticized, as none of them routinely used glycoprotein IIb/IIIa inhibitors with PCI, which by then had been shown to reduce mortality rates.30 However, these trials made it clear that restenosis requiring repeat revascularization was a major disadvantage of PCI with bare-metal stents compared with CABG in patients with diabetes. Drug-eluting stents, which significantly reduced the rates of in-stent restenosis and target-lesion revascularization, were expected to overcome this major disadvantage.
Studies of PCI with drug-eluting stents vs CABG
ARTS II was the first trial to compare PCI with drug-eluting stents vs CABG. This was a nonrandomized single-arm study of 607 patients (including 159 with diabetes) who were treated with drug-eluting stents; the outcomes were compared with the CABG group from the earlier ARTS trial.31
At 3 years, in the diabetic subgroup, the rates of death, myocardial infarction, and stroke were not significantly different between treatments, although a trend favored PCI. However, this comparison was limited by selection bias, as ARTS II was a nonrandomized trial in which operators chose patients for drug-eluting stents in an attempt to match already known outcomes from the CABG cohort of ARTS.
SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) was the first randomized trial comparing PCI with drug-eluting stents (in this trial, paclitaxel-eluting) vs CABG in patients with three-vessel or left main coronary artery disease.26,27 Subgroup analysis in patients with diabetes mellitus revealed a higher rate of major adverse cardiac and cerebrovascular events (death, myocardial infarction, stroke, or repeat revascularization) in the PCI group than in the CABG patients, largely driven by higher rates of repeat revascularization after PCI.32,33 SYNTAX was not designed to assess significant differences in rates of death.
The CARDIa trial (Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and multivessel coronary artery disease to PCI (about one-third with bare-metal stents and two-thirds with drug-eluting stents) or CABG. Rates of major adverse cardiac and cerebrovascular events were higher in the PCI group, again largely driven by higher rates of repeat revascularization.4 CARDIa was stopped early because of a lack of enrollment and could not provide sufficient evidence to endorse one strategy over the other.
VA-CARDS (Veteran Affairs Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and proximal left anterior descending artery or multivessel coronary artery disease to receive PCI with drug-eluting stents or CABG.28 Although the rate of death was lower with CABG than with PCI at 2 years, the trial was underpowered and was terminated at 25% of the initial intended patient enrollment. In addition, only 9% of diabetic patients screened were angiographically eligible for the study.29
Registry data. Analysis of a large data set from the National Cardiovascular Disease Registry and the Society of Thoracic Surgeons revealed a survival advantage of CABG over PCI for a follow-up period of 5 years.34 However, this was a nonrandomized study, so its conclusions were not definitive.
THE FREEDOM TRIAL
Given the limitations of the trials described above, the National Heart, Lung, and Blood Institute sponsored the FREEDOM trial—an appropriately powered, randomized comparison of PCI (with drug-eluting stents) and CABG (using arterial grafting) in patients with diabetes and multivessel coronary artery disease using contemporary techniques and concomitant optimal medical therapy.8
FREEDOM study design
The FREEDOM trial enrolled 1,900 patients with diabetes and angiographically confirmed multivessel coronary artery disease (83% with three-vessel disease) with stenosis of more than 70% in two or more major epicardial vessels involving at least two separate coronary-artery territories. The main exclusion criteria were severe left main coronary artery stenosis (≥ 50% stenosis), class III or IV congestive heart failure, and previous CABG or valve surgery. For CABG surgery, arterial revascularization was encouraged.
Dual antiplatelet therapy was recommended for at least 12 months in patients receiving a drug-eluting stent, and optimal medical management for diabetes, hypertension, and hyperlipidemia was strongly advocated.
Between April 2005 and April 2010, 32,966 patients were screened, of whom 3,309 were eligible for the trial and 1,900 consented and were randomized (953 to the PCI group and 947 to the CABG group). The patients were followed for a minimum of 2 years and had a median follow-up time of 3.8 years. Outcomes were measured with an intention-to-treat analysis.
Study results
Patients. The groups were comparable with regard to baseline demographics and cardiac risk factors.
The mean age was 63; 29% of the patients were women, and 83% had three-vessel coronary artery disease. The mean hemoglobin A1c was 7.8%, and the mean ejection fraction was 66%. The mean SYNTAX score, which defines the anatomic complexity of lesions, was 26 (≤ 22 is mild, 23–32 is intermediate, and ≥ 33 is high). The mean EURO score, which defines surgical risk, was 2.7 (a score ≥ 5 being associated with a lower rate of survival).
The primary composite outcome (death, nonfatal myocardial infarction, or nonfatal stroke) occurred less frequently in the CABG group than in the PCI group (Table 2). CABG was also associated with significantly lower rates of death from any cause and of myocardial infarction. Importantly, survival curves comparing the two groups diverged at 2-year follow-up. In contrast to other outcomes assessed, stroke occurred more often in the CABG group. The 5-year rates in the CABG group vs the PCI group were:
- Primary outcome—18.7% vs 26.6%, P = .005
- Death from any cause—10.9% vs 16.3%, P = .049
- Myocardial infarction—6% vs 13.9%, P < .0001
- Stroke—5.2% vs 2.4%, P = .03.
The secondary outcome (death, nonfatal myocardial infarction, nonfatal stroke, or repeat revascularization at 30 days or 12 months) had occurred significantly more often in the PCI group than in the CABG group at 1 year (16.8% vs 11.8%, P = .004), with most of the difference attributable to a higher repeat revascularization rate in the PCI group (12.6% vs 4.8%, P < .001).
Subgroup analysis. CABG was superior to PCI across all prespecified subgroups, covering the complexity of the coronary artery disease. Event rates with CABG vs PCI, by tertiles of the SYNTAX score:
- SYNTAX scores ≤ 22: 17.2% vs 23.2%
- SYNTAX scores 23–32: 17.7% vs 27.2%
- SYNTAX scores ≥ 33: 22.8% vs 30.6%.
Cost-effectiveness. Although up-front costs were higher with CABG, at $34,467 for the index hospitalization vs $25,845 for PCI (P < .001), when the in-trial results were extended to a lifetime horizon, CABG had an incremental cost-effectiveness ratio of $8,132 per quality-adjusted life-year gained vs PCI.35 Traditionally, therapies are considered costeffective if the incremental cost-effectiveness ratio is less than $50,000 per quality-adjusted life-year gained.
WHY MAY CABG BE SUPERIOR IN DIABETIC PATIENTS?
The major advantage of CABG over PCI is the ability to achieve complete revascularization. Diabetic patients with coronary artery disease tend to have diffuse, multifocal disease with several stenotic lesions in multiple coronary arteries. While stents only treat the focal area of most significant occlusion, CABG may bypass all proximal vulnerable plaques that could potentially develop into culprit lesions over time, truly bypassing the diseased segments (Figure 1).
In addition, heavy calcification may not allow optimal stenting in these patients.
Use of multiple stents increases the risk of restenosis, which could lead to a higher incidence of myocardial infarction and need for repeat revascularization. This was evident in the FREEDOM trial, in which the mean number of stents per patient was 4.2. Also, some lesions need to be left untreated because of the complexity involved.
The major improvement in outcomes after CABG has resulted from using arterial conduits such as the internal mammary artery rather than the saphenous vein.36 The patency rates of internal mammary artery grafts exceed 80% over 10 years.37 Internal mammary artery grafting was done in 94% of patients receiving CABG in the FREEDOM trial.
WHAT DOES THIS MEAN?
FREEDOM was a landmark trial that confirmed that CABG provides significant benefit compared with contemporary PCI with drug-eluting stents in patients with diabetes mellitus and multivessel coronary artery disease. It was a large multicenter trial that was adequately powered, unlike most of the earlier trials of this topic.
Unlike previous trials in which the benefit of CABG was driven by reduction in repeat revascularizations alone, FREEDOM showed lower incidence rates of all-cause mortality and myocardial infarction with CABG than with PCI. CABG was better regardless of SYNTAX score, number of diseased vessels, ejection fraction, race, or sex of the patient, indicating that it leads to superior outcomes across a wide spectrum of patients.
An argument that cardiologists often cite when recommending PCI is that it can save money due to lower length of index hospital stay and lower procedure costs of with PCI than with CABG. However, in FREEDOM, CABG also appeared to be highly cost-effective.
FREEDOM had limitations
While FREEDOM provided robust data proving the superiority of CABG, the study had several limitations.
Although there was an overall survival benefit with CABG compared with PCI, the difference in incidence of cardiovascular deaths (which accounted for 64% of all deaths) was not statistically significant.
The trial included only patients who were eligible for both PCI and CABG. Hence, the results may not be generalizable to all diabetic patients with multivessel coronary artery disease—indeed, only 10% of those screened were considered eligible for the trial. However, it is likely that several patients screened in the FREEDOM trial may not have been eligible for PCI or CABG at the time of screening, since the revascularization decision was made by a multidisciplinary team and a more appropriate decision (either CABG or PCI) was then made.
Other factors limiting the general applicability of the results were low numbers of female patients (28.6%), black patients (6.3%), patients with an ejection fraction of 40% or less (2.5%), and patients with a low SYNTAX score (35%).
There were several unexplained observations as well. The difference in events between the treatment groups was much higher in North America than in other regions. The number of coronary lesions in the CABG group was high (mean = 5.74), but the average numbers of grafts used was only 2.9, and data were not provided regarding use of sequential grafting. Similarly, an average of only 3.5 of the six stenotic lesions per patient in the PCI group were revascularized; whether this was the result of procedural limitations with PCI was not entirely clear.
In addition, while the investigators mention that an average patient received four stents, a surprising finding was that the mean total length of the stents used was only 26 mm. This appears too small, as the usual length of one drug-eluting stent is about 20 to 30 mm.
Since only high-volume centers with good outcome data were included in the trial, the results may lack validity for patients undergoing revascularization at low-volume community centers.
It remains to be seen if the benefits of CABG will be sustained over 10 years and longer, when saphenous vein grafts tend to fail and require repeat revascularization, commonly performed with PCI. Previous data suggest that the longer the follow-up, the better the results with CABG. However, long-term results (> 10 years) in studies comparing drugeluting stents and CABG are not available.
Despite limitations, FREEDOM may change clinical practice
Despite these limitations, the FREEDOM trial has the potential to change clinical practice and strengthen current recommendations for CABG in these patients.
The trial underscored the importance of a multidisciplinary heart team approach in managing patients with complex coronary artery disease, similar to that being used in patients with severe aortic stenosis since transcatheter aortic valve replacement became available.
It should also bring an end to the practice of ad hoc PCI, especially in patients with diabetes and multivessel coronary artery disease. It is now imperative that physicians discuss current evidence for therapeutic options with the patients and their families before performing diagnostic angiography rather than immediately afterward, to give the patients ample time to make an informed decision. This is important, as most patients are likely to choose PCI in the same setting over CABG unless there is extensive discussion about the risks and benefits of both strategies done in an unbiased manner before angiography.
The fear of open heart surgery, a longer hospital stay, and a higher risk of stroke with CABG may lead some patients to choose PCI instead. In addition, factors that may preclude CABG in otherwise-eligible patients include anatomic considerations (diffuse distal vessel disease, poor conduits), individual factors (frailty, poor renal function, poor pulmonary function, patient preference), and local expertise.
Nevertheless, the patient should be presented with current evidence, and discussions regarding the optimal procedure should be held with a heart team, which should include an interventional cardiologist, a cardiothoracic surgeon, and a noninvasive cardiologist to facilitate an unbiased decision.
Regardless of the strategy chosen, the importance of compliance with optimal medical therapy (statins, antiplatelet agents, diabetes treatment) should be continuously emphasized to the patient.
WHAT DOES THE FUTURE HOLD?
Despite unequivocal evidence that CABG is superior to PCI in eligible patients with diabetes mellitus in the current era, PCI technologies continue to evolve rapidly. Newer second-generation drug-eluting stents have shown lower rates of restenosis38,39 and may shorten the duration of post-PCI dual-antiplatelet therapy, a nuisance that has negatively affected outcomes with drug-eluting stents (because of problems of cost, poor compliance, and increased bleeding risk).
At the same time, CABG has also improved, with more extensive use of complete arterial conduits and use of an off-pump bypass technique that in theory poses a lower risk of stroke, although this has not yet been shown in a randomized trial.40
Alternative approaches are being investigated. One of them is a hybrid procedure in which minimally invasive off-pump arterial grafting is combined with drug-eluting stents, which may reduce the risk of stroke and speed postoperative recovery.
- Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2005; 293:1501–1508.
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008; 52:255–262.
- Mack MJ, Banning AP, Serruys PW, et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg 2011; 92:2140–2146.
- Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010; 55:432–440.
- The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007; 49:1600–1606.
- Hlatky MA. Compelling evidence for coronary-bypass surgery in patients with diabetes. N Engl J Med 2012; 367:2437–2438.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000; 102:2180–2184.
- Bluher M, Unger R, Rassoul F, et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or type II diabetes. Diabetologia 2002; 45:210–216.
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003; 108:1527–1532.
- Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003; 41:1071–1077.
- Waller BF, Palumbo PJ, Lie JT, Roberts WC. Status of the coronary arteries at necropsy in diabetes mellitus with onset after age 30 years. Analysis of 229 diabetic patients with and without clinical evidence of coronary heart disease and comparison to 183 control subjects. Am J Med 1980; 69:498–506.
- Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle-aged diabetic patients (the London Cohort of the WHO Multinational Study of Vascular Disease in Diabetics) I: causes and death rates. Diabetologia 1990; 33:538–541.
- Laskey WK, Selzer F, Vlachos HA, et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2002; 90:1062–1067.
- Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480.
- Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005; 111:143–149.
- Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetologica 1999; 36:77–84.
- Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100:642–647.
- The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med 1996; 335:217–225.
- King SB, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eightyear mortality in the Emory Angioplasty versus Surgery Trial (East). J Am Coll Cardiol 2000; 35:1116–1121.
- Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF; CABRI Investigators (Coronary Angioplasty versus Bypass Revascularization Investigation). Difference in the mortality of the CABRI diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J Cardiol 2001; 87:947–950.
- Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 2005; 46:575–581.
- Booth J, Clayton T, Pepper J, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation 2008; 118:381–388.
- Rodriguez AE, Baldi J, Fernandez Pereira C, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol 2005; 46:582–588.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013; 381:629–638.
- Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013; 61:808–816.
- Ellis SG. Coronary revascularization for patients with diabetes: updated data favor coronary artery bypass grafting. J Am Coll Cardiol 2013; 61:817–819.
- Bhatt DL, Marso SP, Lincoff AM, et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol 2000; 35:922–928.
- Serruys PW, Ong AT, Morice MC, et al. Arterial Revascularisation Therapies Study Part II - Sirolimus-eluting stents for the treatment of patients with multivessel de novo coronary artery lesions. EuroIntervention 2005; 1:147–156.
- Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013; 43:1006–1013.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366:1467–1476.
- Magnuson EA, Farkouh ME, Fuster V, et al; FREEDOM Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes and multivessel coronary artery disease: results from the FREEDOM trial. Circulation 2013; 127:820–831.
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986; 314:1–6.
- Tector AJ, Schmahl TM, Janson B, et al. The internal mammary artery graft. Its longevity after coronary bypass. JAMA 1981; 246:2181–2183.
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitax-eleluting stents in coronary artery disease. N Engl J Med 2010; 362:1663–1674.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010; 363:136–146.
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366:1489–1497.
- Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2005; 293:1501–1508.
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008; 52:255–262.
- Mack MJ, Banning AP, Serruys PW, et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg 2011; 92:2140–2146.
- Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010; 55:432–440.
- The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007; 49:1600–1606.
- Hlatky MA. Compelling evidence for coronary-bypass surgery in patients with diabetes. N Engl J Med 2012; 367:2437–2438.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000; 102:2180–2184.
- Bluher M, Unger R, Rassoul F, et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or type II diabetes. Diabetologia 2002; 45:210–216.
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003; 108:1527–1532.
- Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003; 41:1071–1077.
- Waller BF, Palumbo PJ, Lie JT, Roberts WC. Status of the coronary arteries at necropsy in diabetes mellitus with onset after age 30 years. Analysis of 229 diabetic patients with and without clinical evidence of coronary heart disease and comparison to 183 control subjects. Am J Med 1980; 69:498–506.
- Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle-aged diabetic patients (the London Cohort of the WHO Multinational Study of Vascular Disease in Diabetics) I: causes and death rates. Diabetologia 1990; 33:538–541.
- Laskey WK, Selzer F, Vlachos HA, et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2002; 90:1062–1067.
- Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480.
- Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005; 111:143–149.
- Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetologica 1999; 36:77–84.
- Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100:642–647.
- The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med 1996; 335:217–225.
- King SB, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eightyear mortality in the Emory Angioplasty versus Surgery Trial (East). J Am Coll Cardiol 2000; 35:1116–1121.
- Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF; CABRI Investigators (Coronary Angioplasty versus Bypass Revascularization Investigation). Difference in the mortality of the CABRI diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J Cardiol 2001; 87:947–950.
- Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 2005; 46:575–581.
- Booth J, Clayton T, Pepper J, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation 2008; 118:381–388.
- Rodriguez AE, Baldi J, Fernandez Pereira C, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol 2005; 46:582–588.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013; 381:629–638.
- Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013; 61:808–816.
- Ellis SG. Coronary revascularization for patients with diabetes: updated data favor coronary artery bypass grafting. J Am Coll Cardiol 2013; 61:817–819.
- Bhatt DL, Marso SP, Lincoff AM, et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol 2000; 35:922–928.
- Serruys PW, Ong AT, Morice MC, et al. Arterial Revascularisation Therapies Study Part II - Sirolimus-eluting stents for the treatment of patients with multivessel de novo coronary artery lesions. EuroIntervention 2005; 1:147–156.
- Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013; 43:1006–1013.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366:1467–1476.
- Magnuson EA, Farkouh ME, Fuster V, et al; FREEDOM Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes and multivessel coronary artery disease: results from the FREEDOM trial. Circulation 2013; 127:820–831.
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986; 314:1–6.
- Tector AJ, Schmahl TM, Janson B, et al. The internal mammary artery graft. Its longevity after coronary bypass. JAMA 1981; 246:2181–2183.
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitax-eleluting stents in coronary artery disease. N Engl J Med 2010; 362:1663–1674.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010; 363:136–146.
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366:1489–1497.
KEY POINTS
- Patients with diabetes have a higher prevalence of multivessel coronary artery disease and often have complex, diffuse lesions.
- Bypass surgery is the preferred method of revascularization in appropriately selected patients with diabetes and multivessel coronary artery disease.
- In the FREEDOM trial, only about 10% of the screened patients were eligible for the study, limiting its generalizability; however, this is comparable to exclusion rates in previous large randomized trials.
- When choosing a revascularization method, the physician team needs to discuss the options with the patient before performing diagnostic angiography. The team should include a cardiac surgeon and a cardiologist.
A 72-year-old man with a purpuric rash
A 72-year-old man whose medical history includes diabetes mellitus, hypertension, coronary artery disease, aortic valve replacement, atrial fibrillation, and chronic obstructive pulmonary disease was in his usual state of health until 2 weeks ago, when he developed a purpuric rash on his legs. His physician started him on prednisone 40 mg daily for the rash; however, 1 week later he presented to a hospital emergency room when his family found him confused and diaphoretic.
In the emergency room, he was found to be hypoglycemic, with a serum glucose level of 40 mg/dL, which was promptly treated. His mental status improved partially. In the hospital, the rash worsened and progressed upwards to his trunk and upper extremities. He was transferred to our institution for further workup and management.
A review of systems reveals occasional epistaxis in the summer, recent fatigue, cough, and shortness of breath on exertion. His medications at the time of transfer include warfarin (Coumadin), amlodipine (Norvasc), insulin, ipratropium and albuterol (Combivent) inhalers, and prednisone 40 mg daily. He has not undergone surgery recently.
PHYSICAL EXAMINATION
He is alert and oriented to person but not to time and place.
Vital signs. Oral temperature 101.1°F (38.4°C), pulse rate 108, blood pressure 108/79 mm/Hg, respiratory rate 22, oxygen saturation 93% by pulse oximetry on room air, weight 94 kg (207 lb).
Head, eyes, ears, nose, and throat. No pallor or icterus, pupils are equally reactive, nasal mucosa not inflamed or ulcerated, mucous membranes moist, no sinus tenderness.
Neck. No jugular venous distention and no cervical lymphadenopathy.
Cardiovascular. Tachycardia, irregularly irregular rhythm, prosthetic valve sounds, no murmurs, rubs, or gallops.
Respiratory. Bibasal crackles (right side more than the left). No wheezing.
Abdomen. Soft, nontender, nondistended, no palpable organomegaly, bowel sounds normal.
Extremities. No edema, good peripheral pulses.
Neurologic. No focal deficits noted.
Lymphatic. No enlarged lymph nodes.
Musculoskeletal. Traumatic right second distal interphalangeal amputation. Otherwise, no joint abnormality or restriction of movement.
Initial laboratory values:
- White blood cell count 15.78 × 109/L (normal 4.5–11.0)
- Absolute neutrophil count 13.3 × 109/L (4.0–11.0)
- Hemoglobin 13.3 g/dL (13.5–17.5)
- Platelet count 133 × 109/L (150–400)
- International normalized ratio (INR) 1.8
- Sodium 136 mmol/L (135–146)
- Potassium 4.6 mmol/L (3.5–5.0)
- Blood urea nitrogen 31 mg/dL (10–25)
- Creatinine 1.6 mg/dL (0.70–1.40)
- Glucose 62 mg/dL (65–100)
- Bicarbonate 23 mmol/L (23–32)
- Albumin 2.5 g/dL (3.5–5.0)
- Total protein 4.6 g/dL (6.0–8.4)
- Bilirubin 1.2 mg/dL (0.0–1.5)
- Aspartate aminotransferase 41 U/L (7–40)
- Alanine aminotransferase 74 U/L (5–50)
- Alkaline phosphatase 55 U/L (40–150)
- C-reactive protein 9.9 mg/dL (0.0–1.0).
Other studies
Electrocardiography shows atrial fibrillation and left ventricular hypertrophy, but no acute changes.
Computed tomography (CT) of the head shows no evidence of hemorrhage or infarction.
Blood cultures are sent at the time of hospital admission.
WHICH TEST IS NEXT?
1. Which is the most appropriate next step for this patient?
- Urinalysis
- CT of the chest
- Echocardiography
- Skin biopsy
The rash in Figure 1 is palpable purpura, which strongly suggests small-vessel cutaneous vasculitis, a condition that can occur in a broad range of settings. An underlying cause is identified in over 70% of cases. Cutaneous vasculitis may herald a primary small-vessel systemic vasculitis such as Wegener granulomatosis, microscopic polyangiitis, or Henoch-Schönlein purpura. It can also be secondary to a spectrum of underlying triggers or diseases that include medications, infections, malignancies such as lymphoproliferative disorders, cryoglobulinemia secondary to hepatitis C viral infection, and connective tissue diseases such as rheumatoid arthritis and systemic lupus erythematosus.
Infective endocarditis is associated with a secondary form of vasculitis and is a strong possibility in this patient, who has a prosthetic aortic valve, fever, and a high white blood cell count.
Thrombocytopenia should also prompt an assessment for any drugs the patient is taking that affect platelet function. However, thrombocytopenia typically results in nonpalpable purpura.
Idiopathic isolated cutaneous vasculitis, in which no underlying cause for the cutaneous vasculitis can be identified, is the diagnosis in less than 30% of cases.
A vasculitic disease process can involve multiple sites, which may be asymptomatic on presentation. Identifying these sites is important, not only to establish the diagnosis, but also to detect potentially life-threatening complications early.
Thus, in this patient, urinalysis should be done promptly to check for active sediment consisting of red cell casts, which would suggest renal involvement (glomerulonephritis). Also, a rising blood pressure and creatinine would point to renal involvement and warrant more aggressive initial therapy.
Chest radiography should be done to rule out pulmonary infiltrates, septic emboli, nodules, or cavities that could represent vasculitic or infectious involvement of the lungs. CT of the chest may be needed to further characterize abnormalities on chest radiography.
Echocardiography should certainly be pursued as part of the workup for endocarditis, but urinalysis is of the utmost importance in this patient at this point.
More diagnostic information is needed before considering skin biopsy.
Clues from the urinalysis and chest radiography
Our patient’s sedimentation rate is 24 mm/hour. The urinalysis is strongly positive for blood and a moderate amount of protein but negative for leukocyte esterase and nitrite. The urine sediment contains numerous red blood cell casts and 6 to 10 white blood cells per high-power field.
Pending return of blood cultures, ceftriaxone (Rocephin) and azithromycin (Zithromax) are started to treat possible community-acquired pneumonia. Vancomycin (Vancocin) is empirically added, given the possibility of prosthetic valve endocarditis. Gram stain on blood cultures shows gram-positive cocci.
Ground-glass attenuation implies focal or diffuse opacification of the lung that does not obscure the vascular structures, is not associated with air bronchograms, and appears as a “veil” across the lung parenchyma.1 It can be seen in acute diseases such as infection (including pneumonia from atypical bacteria, viruses, acid-fast bacilli, and Pneumocystis jiroveci), pulmonary hemorrhage of any cause, acute viral, eosinophilic, and interstitial pneumonias, and hypersensitivity pneumonitis. It can also be seen in chronic disease states such as interstitial lung disease, bronchoalveolar carcinoma, alveolar proteinosis, and sarcoidosis.
WHICH TEST WOULD NOT HELP?
2. Which of the following tests is least likely to help in the diagnostic evaluation at this point?
- Transthoracic echocardiography
- Transesophageal echocardiography
- Bronchoscopy
- Cystoscopy
In this case, the least likely to help is cystoscopy.
This patient’s vasculitic-appearing rash, ground-glass pulmonary infiltrates, and impaired renal function with red cell casts suggest a pulmonary-renal syndrome, and with this constellation of features, a systemic vasculitis is very likely. Therefore, the focus of the evaluation should be on any evidence to support a diagnosis of vasculitis, as well as other possible causes.
In a patient with diabetes, an artificial heart valve, and fever, the possibility of infection, especially endocarditis, remains high. Transthoracic echocardiography is warranted, and if it is negative for vegetations, transesophageal echocardiography would be a reasonable next option.
Bronchoscopy is warranted to determine if the infiltrates represent pulmonary hemorrhage, which can be seen in certain types of vasculitic and systemic disorders.
The finding of red cell casts in the urine indicates glomerulonephritis, and therefore the kidneys are the likely source of the urinary red blood cells, making cystoscopy of no utility in this current, acute setting.
Case continued: His condition worsens
Transthoracic echocardiography reveals a well-seated mechanical prosthetic aortic valve, trivial aortic regurgitation, a peak gradient of 23 mm Hg and a mean gradient of 12 mm Hg (normal values for his prosthetic valve), and no valvular vegetations. Transesophageal echocardiography confirms the absence of vegetations.
His oxygen requirement increases, and analysis of arterial blood gases reveals a pH of 7.37, Pco2 49 mm Hg (normal range 35–45), Po2 102 mm Hg (normal 80–100), and bicarbonate 28 mmol/L while breathing 100% supplemental oxygen by a nonrebreather face mask. He is taken to the medical intensive care unit for intubation and mechanical ventilation. Bronchoscopy performed while he is intubated confirms diffuse alveolar hemorrhage. Pulse intravenous methylprednisolone (Solu-Medrol) therapy is started.
DIFFUSE ALVEOLAR HEMORRHAGE
3. Which of the following is not true about diffuse alveolar hemorrhage?
- Its onset is usually abrupt or of short duration
- It is always associated with hemoptysis
- Radiography most commonly shows new patchy or diffuse alveolar opacities
- Pulmonary function testing shows increased diffusing capacity of the lung for carbon monoxide (Dlco)
Hemoptysis is absent at presentation in as many as 33% of patients.
The onset of diffuse alveolar hemorrhage is usually abrupt or of short duration, with initial symptoms of cough, fever, and dyspnea. Some patients, such as ours, can present with severe acute respiratory distress syndrome requiring mechanical ventilation. Radiography most often shows new patchy alveolar opacities, and CT may reveal a ground-glass appearance. On pulmonary function testing, the Dlco is high, owing to the hemoglobin within the alveoli.
ACUTE GLOMERULONEPHRITIS PLUS PULMONARY HEMORRHAGE EQUALS…?
4. Which disease could have manifestations consistent with acute glomerulonephritis and pulmonary hemorrhage?
- Antiglomerular basement membrane disease
- Wegener granulomatosis
- Microscopic polyangiitis
- Systemic lupus erythematosus
All of these are possible.
The combined presentation of acute glomerulonephritis and pulmonary hemorrhage (also called pulmonary-renal syndrome) is usually seen in antiglomerular basement membrane disease (Goodpasture syndrome) and small-vessel systemic vasculitides such as Wegener granulomatosis and microscopic polyangiitis.2,3 It can also be seen in patients with systemic lupus erythematosus.
Antiglomerular basement membrane disease
In antiglomerular basement membrane disease, circulating antibodies are directed towards an antigen intrinsic to the glomerular basement membrane, typically leading to acute glomerulonephritis associated with crescent formation. It may present as acute renal failure in which urinalysis shows proteinuria with sediment characterized by red cell casts. Pulmonary involvement, usually alveolar hemorrhage, is present in approximately 60% to 70% of cases.
The diagnosis requires demonstration of antiglomerular basement membrane antibodies in either the serum or the kidney. Renal biopsy is usually recommended because the accuracy of serum assays is variable.
A key histologic feature of the renal lesion in antiglomerular basement membrane disease is crescentic glomerulonephritis in which immunofluorescence microscopy demonstrates the virtually pathognomonic finding of linear deposition of immunoglobulin G along the glomerular capillaries.
The treatment of choice for antiglomerular basement membrane disease is plasmapheresis and immunosuppression with a combination of glucocorticoids and cyclophosphamide (Cytoxan). If the disease is high on the differential diagnosis, empiric plasmapheresis should be started while waiting for diagnostic studies, because the prognosis of untreated glomerulonephritis is poor.
Wegener granulomatosis
Wegener granulomatosis is a systemic vasculitis of the medium and small arteries, arterioles, and venules that classically involves the upper and lower respiratory tracts and the kidneys. Patients may present with persistent rhinorrhea and epistaxis, cough with chest radiographs showing nodules, fixed infiltrates, or cavities, and abnormal urinary sediment with microscopic hematuria with or without red cell casts.
From 75% to 90% of patients with active Wegener granulomatosis are positive for antineutrophil cytoplasmic antibody (ANCA). In 60% to 80% of cases, ANCA is directed against proteinase 3 (PR3), which produces a cytoplasmic standing pattern by immunofluorescence (cANCA), while 5% to 20% have ANCA directed against myeloper-oxidase, which produces a perinuclear staining pattern (pANCA). A small number of patients with Wegener granulomatosis are ANCA-negative.
The diagnosis is usually confirmed by tissue biopsy at the site of active disease, which shows necrotizing vasculitis with granulomatous inflammation. The renal lesion is typically that of a focal, segmental, necrotizing glomerulonephritis that has few to no immune complexes (pauci-immune glomerulonephritis).
The treatment of severe disease involves a combination of cyclophosphamide and glucocorticoids initially to achieve remission followed by maintenance therapy with methotrexate or azathioprine (Imuran).
Microscopic polyangiitis
Microscopic polyangiitis is a systemic vasculitis of the capillaries, venules, and arterioles, with little or no immune complex deposition. Nearly all patients have renal involvement, and 10% to 30% have lung involvement. In those with lung involvement, diffuse alveolar hemorrhage is the most common manifestation.
On histopathologic study, microscopic polyangiitis differs from Wegener granulomatosis in that it does not have granuloma formation. However, the renal lesion is that of a pauci-immune glomerulonephritis and is identical to that seen in Wegener granulomatosis. From 70% to 85% of patients with microscopic polyangiitis are ANCA-positive, and most of these have pANCA.
The management of active severe microscopic polyangiitis is identical to that of Wegener granulomatosis.
Systemic lupus erythematosus
Systemic lupus erythematosus is an autoimmune disease characterized by tissue-binding autoantibody and immune-complex-mediated organ damage. It can involve multiple organ systems, and the diagnosis is based on characteristic clinical features and autoantibodies. The sensitivity of antinuclear antibody for lupus is close to 100%, which makes it a good screening tool. Antibodies to dsDNA and Smith antigen have high specificity for lupus.
About 75% of patients have renal involvement at some point in their disease course. The different types of renal disease in systemic lupus are usually differentiated with a renal biopsy, with immune-complex-mediated glomerular diseases being the most common.
The most common pulmonary manifestation is pleuritis with or without pleural effusion. Life-threatening pulmonary manifestations include pulmonary hemorrhage and interstitial inflammation leading to fibrosis.
Lupus has great clinical variability and the treatment approach is based on the organ manifestations, disease activity, and severity.
CASE CONTINUED: ARRIVING AT THE DIAGNOSIS
We start our patient on cyclophosphamide 175 mg daily in view of possible Wegener granulomatosis.
Even though purpura is extremely rare in primary antiglomerular basement membrane disease, this patient has life-threatening pulmonary hemorrhage, a complication seen in over 50% of these patients. Therefore, plasmapheresis is started empirically.
On the second day of cyclophosphamide treatment, tests for ANCA, glomerular basement membrane antibody, and antinuclear antibody are reported as negative, and complement levels are normal. Bronchoalveolar lavage shows no infection. Follow-up blood cultures are negative.
To summarize the findings so far, this patient has a purpuric skin rash, active urine sediment with red cell casts indicating glomerulonephritis, acute renal failure, and severe pulmonary hemorrhage requiring mechanical ventilation. Although one set of blood cultures showed gram-positive cocci, no source of infection, particularly endocarditis, could be identified.
Antiglomerular basement membrane disease would still be high on the list of suspected diagnoses, given his diffuse alveolar hemorrhage. As mentioned earlier, renal biopsy is imperative to making a diagnosis, because serologic tests have variable accuracy. And making the correct diagnosis has therapeutic implications.
Renal biopsy is performed and shows immune-complex mesangiopathic glomerulonephritis with positive immunofluorescent staining in the mesangium for IgA. Only one glomerulus shows fibrinoid necrosis.
Skin biopsy results obtained earlier showed positive direct immunofluorescence for IgA. Both renal and skin biopsies suggested Henoch-Schönlein purpura.
IgA deposition in the kidney and skin has been associated with liver cirrhosis, celiac disease, and infections with agents such as human immunodeficiency virus, cytomegalovirus, Haemophilus parainfluenzae, and Staphylococcus aureus. In a Japanese study,4 renal biopsy specimens from 116 patients with IgA nephropathy and from 122 patients with other types of kidney disease were examined for the presence of S aureus antigen in the glomeruli. Although antigen was not detected in non-IgA disease, 68% of specimens from patients with IgA nephropathy had S aureus cell envelope antigen together with IgA antibody in the glomeruli. However, no single antigen has been consistently identified, so it seems more probable that the development of IgA deposition in kidneys is a consequence of aberrant IgA immune response rather than the antigen itself.
HENOCH-SCHÖNLEIN PURPURA
Henoch-Schönlein purpura is a systemic vasculitis with a prominent cutaneous component. It is characterized by the tissue deposition of IgA-containing immune complexes. It is predominantly a disease of children but it can be seen in adults. A UK study found the prevalence to be 20 per 100,000 children, with the highest prevalence between ages 4 and 7 (70 per 100,000).5
The four cardinal clinical features of Henoch-Schönlein purpura are purpuric rash, abdominal pain, arthralgia, and renal involvement. Almost all patients have purpuric rash at some point in their disease course. Arthralgia with or without arthritis is typically migratory, oligoarticular, and nondeforming, usually affecting the large joints of the lower extremities; involvement of the upper extremities is less common.
Skin biopsy typically shows leukocytoclastic vasculitis in postcapillary venules with IgA deposition, and these findings are pathognomonic of Henoch-Schönlein purpura.
Gastrointestinal involvement can range from mild symptoms such as nausea, vomiting, abdominal pain, and paralytic ileus to severe disease such as gastrointestinal hemorrhage, bowel infarction, bowel perforation, and intussusception.
Renal involvement is common and is important, as it can in rare cases progress to end-stage renal disease. The urinalysis usually shows mild proteinuria with active sediment with red cell casts. Most patients have relatively mild disease, characterized by asymptomatic hematuria with a normal or slightly elevated creatinine. However, severe involvement may occur, with nephrotic syndrome, hypertension, and acute renal failure.
Different presentation in adults vs children
Adults with Henoch-Schönlein purpura only rarely present with bowel intussusception, whereas some studies have found that adults are more likely than children to develop significant renal involvement, including end-stage renal disease.6,7
There is a general but not absolute correlation between the severity of clinical manifestations and the findings on renal biopsy. A poor prognosis (significant proteinuria, hypertension, renal insufficiency, or end-stage renal disease) is associated with crescent formation involving more than 50% of the glomeruli.8
Our current understanding of the longterm outcome of the renal disease in Henoch-Schönlein purpura is primarily derived from studies in children. In one study, complete recovery occurred in 94% of children and 89% of adults.7 A long-term study of 250 adults with Henoch-Schönlein purpura and renal involvement of sufficient severity to require biopsy reported that, at a median follow up of 15 years, 11% had become dialysis-dependent and 13% had severe renal failure (creatinine clearance < 30 mL/min).6 Recurrence is common, occurring in approximately one-third of patients, more likely in those with nephritis.8
The diagnosis of Henoch-Schönlein purpura is typically made on the basis of key clinical features. In patients such as ours who have an atypical presentation, biopsy of affected skin and renal biopsy can be essential in the diagnosis. Diffuse alveolar hemorrhage is exceedingly rare in Henoch-Schönlein purpura but can be seen, as in our patient.9,10 In this setting, the findings of IgA deposits in skin and renal biopsy specimens, together with the absence of other clinical, serologic, or histologic features of other more-common potential causes, secured the diagnosis in this patient.
Henoch-Schönlein purpura is usually self-limited and requires no specific therapy. Evidence suggests that glucocorticoids enhance the rate of resolution of the arthritis and abdominal pain but do not appear to prevent recurrent disease or lessen the likelihood of progression of renal disease.8 Patients with severe renal involvement with renal function impairment may benefit from pulse intravenous corticosteroid therapy (methylprednisolone 250–1,000 mg per day for 3 days), followed by oral steroids for 3 months.11
In anecdotal reports, renal function improved in 19 of 21 children with Henoch-Schönlein purpura and severe crescentic nephritis.12 Studies have evaluated cyclophosphamide13 and plasmapheresis,14 but their role remains uncertain. Renal transplantation is an option in patients who progress to end-stage renal disease.
OUR CASE CONTINUED
In our patient, plasmapheresis was discontinued. As the pulmonary hemorrhage had developed during treatment with prednisone, we decided to continue cyclophosphamide, given the life-threatening nature of his disease. His pulmonary status improved and he was extubated.
During his initial hospital stay, he was taking heparin for anticoagulation therapy. However, given the life-threatening diffuse alveolar hemorrhage, heparin was stopped during the course of his stay in the intensive care unit. Once he was stable and was transferred out of the intensive care unit, heparin was resumed, and his anticoagulation therapy was bridged to warfarin just before discharge. He was eventually discharged on a tapering dose of oral prednisone and cyclophosphamide for 3 months, after which he was switched to azathioprine for maintenance therapy. He was doing well 6 months later, with a serum creatinine level of 1.6 mg/dL, no red cell casts in the urine, and no rash.
TAKE-HOME POINT
In any case of suspected vasculitis that presents with skin disease, it is essential to look for other sites with potentially life-threatening involvement. Henoch-Schönlein purpura is a systemic vasculitis with a prominent cutaneous component. It is not always benign and can be associated with serious complications such as renal failure, gastrointestinal events, and, very rarely, diffuse alveolar hemorrhage.
- Collins J, Stern EJ. Ground-glass opacity at CT: the ABCs. AJR Am J Roentgenol 1997; 169:355–367.
- Boyce NW, Holdsworth SR. Pulmonary manifestations of the clinical syndrome of acute glomerulonephritis and lung hemorrhage. Am J Kidney Dis 1986; 8:31–36.
- Gallagher H, Kwan JT, Jayne DR. Pulmonary renal syndrome: a 4-year, single-center experience. Am J Kidney Dis 2002; 39:42–47.
- Koyama A, Sharmin S, Sakurai H, et al. Staphylococcus aureus cell envelope antigen is a new candidate for the induction of IgA nephropathy. Kidney Int 2004; 66:121–132.
- Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002; 360:1197–1202.
- Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch-Schönlein purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002; 13:1271–1278.
- Blanco R, Martinez-Taboada VM, Rodriguez-Valverde V, Garcia-Fuentes M, Gonzalez-Gay MA. Henoch-Schönlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis Rheum 1997; 40:859–864.
- Saulsbury FT. Henoch-Schönlein purpura in children. Report of 100 patients and review of the literature. Medicine (Baltimore) 1999; 78:395–409.
- Nadrous HF, Yu AC, Specks U, Ryu JH. Pulmonary involvement in Henoch-Schönlein purpura. Mayo Clin Proc 2004; 79:1151–1157.
- Vats KR, Vats A, Kim Y, Dassenko D, Sinaiko AR. Henoch-Schönlein purpura and pulmonary hemorrhage: a report and literature review. Pediatr Nephrol 1999; 13:530–534.
- Niaudet P, Habib R. Methylprednisolone pulse therapy in the treatment of severe forms of Schönlein-Henoch purpura nephritis. Pediatr Nephrol 1998; 12:238–243.
- Bergstein J, Leiser J, Andreoli SP. Response of crescentic Henoch-Schöenlein purpura nephritis to corticosteroid and azathioprine therapy. Clin Nephrol 1998; 49:9–14.
- Tarshish P, Bernstein J, Edelmann CM. Henoch-Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol 2004; 19:51–56.
- Hattori M, Ito K, Konomoto T, Kawaguchi H, Yoshioka T, Khono M. Plasmapheresis as the sole therapy for rapidly progressive Henoch-Schönlein purpura nephritis in children. Am J Kidney Dis 1999; 33:427–433.
A 72-year-old man whose medical history includes diabetes mellitus, hypertension, coronary artery disease, aortic valve replacement, atrial fibrillation, and chronic obstructive pulmonary disease was in his usual state of health until 2 weeks ago, when he developed a purpuric rash on his legs. His physician started him on prednisone 40 mg daily for the rash; however, 1 week later he presented to a hospital emergency room when his family found him confused and diaphoretic.
In the emergency room, he was found to be hypoglycemic, with a serum glucose level of 40 mg/dL, which was promptly treated. His mental status improved partially. In the hospital, the rash worsened and progressed upwards to his trunk and upper extremities. He was transferred to our institution for further workup and management.
A review of systems reveals occasional epistaxis in the summer, recent fatigue, cough, and shortness of breath on exertion. His medications at the time of transfer include warfarin (Coumadin), amlodipine (Norvasc), insulin, ipratropium and albuterol (Combivent) inhalers, and prednisone 40 mg daily. He has not undergone surgery recently.
PHYSICAL EXAMINATION
He is alert and oriented to person but not to time and place.
Vital signs. Oral temperature 101.1°F (38.4°C), pulse rate 108, blood pressure 108/79 mm/Hg, respiratory rate 22, oxygen saturation 93% by pulse oximetry on room air, weight 94 kg (207 lb).
Head, eyes, ears, nose, and throat. No pallor or icterus, pupils are equally reactive, nasal mucosa not inflamed or ulcerated, mucous membranes moist, no sinus tenderness.
Neck. No jugular venous distention and no cervical lymphadenopathy.
Cardiovascular. Tachycardia, irregularly irregular rhythm, prosthetic valve sounds, no murmurs, rubs, or gallops.
Respiratory. Bibasal crackles (right side more than the left). No wheezing.
Abdomen. Soft, nontender, nondistended, no palpable organomegaly, bowel sounds normal.
Extremities. No edema, good peripheral pulses.
Neurologic. No focal deficits noted.
Lymphatic. No enlarged lymph nodes.
Musculoskeletal. Traumatic right second distal interphalangeal amputation. Otherwise, no joint abnormality or restriction of movement.
Initial laboratory values:
- White blood cell count 15.78 × 109/L (normal 4.5–11.0)
- Absolute neutrophil count 13.3 × 109/L (4.0–11.0)
- Hemoglobin 13.3 g/dL (13.5–17.5)
- Platelet count 133 × 109/L (150–400)
- International normalized ratio (INR) 1.8
- Sodium 136 mmol/L (135–146)
- Potassium 4.6 mmol/L (3.5–5.0)
- Blood urea nitrogen 31 mg/dL (10–25)
- Creatinine 1.6 mg/dL (0.70–1.40)
- Glucose 62 mg/dL (65–100)
- Bicarbonate 23 mmol/L (23–32)
- Albumin 2.5 g/dL (3.5–5.0)
- Total protein 4.6 g/dL (6.0–8.4)
- Bilirubin 1.2 mg/dL (0.0–1.5)
- Aspartate aminotransferase 41 U/L (7–40)
- Alanine aminotransferase 74 U/L (5–50)
- Alkaline phosphatase 55 U/L (40–150)
- C-reactive protein 9.9 mg/dL (0.0–1.0).
Other studies
Electrocardiography shows atrial fibrillation and left ventricular hypertrophy, but no acute changes.
Computed tomography (CT) of the head shows no evidence of hemorrhage or infarction.
Blood cultures are sent at the time of hospital admission.
WHICH TEST IS NEXT?
1. Which is the most appropriate next step for this patient?
- Urinalysis
- CT of the chest
- Echocardiography
- Skin biopsy
The rash in Figure 1 is palpable purpura, which strongly suggests small-vessel cutaneous vasculitis, a condition that can occur in a broad range of settings. An underlying cause is identified in over 70% of cases. Cutaneous vasculitis may herald a primary small-vessel systemic vasculitis such as Wegener granulomatosis, microscopic polyangiitis, or Henoch-Schönlein purpura. It can also be secondary to a spectrum of underlying triggers or diseases that include medications, infections, malignancies such as lymphoproliferative disorders, cryoglobulinemia secondary to hepatitis C viral infection, and connective tissue diseases such as rheumatoid arthritis and systemic lupus erythematosus.
Infective endocarditis is associated with a secondary form of vasculitis and is a strong possibility in this patient, who has a prosthetic aortic valve, fever, and a high white blood cell count.
Thrombocytopenia should also prompt an assessment for any drugs the patient is taking that affect platelet function. However, thrombocytopenia typically results in nonpalpable purpura.
Idiopathic isolated cutaneous vasculitis, in which no underlying cause for the cutaneous vasculitis can be identified, is the diagnosis in less than 30% of cases.
A vasculitic disease process can involve multiple sites, which may be asymptomatic on presentation. Identifying these sites is important, not only to establish the diagnosis, but also to detect potentially life-threatening complications early.
Thus, in this patient, urinalysis should be done promptly to check for active sediment consisting of red cell casts, which would suggest renal involvement (glomerulonephritis). Also, a rising blood pressure and creatinine would point to renal involvement and warrant more aggressive initial therapy.
Chest radiography should be done to rule out pulmonary infiltrates, septic emboli, nodules, or cavities that could represent vasculitic or infectious involvement of the lungs. CT of the chest may be needed to further characterize abnormalities on chest radiography.
Echocardiography should certainly be pursued as part of the workup for endocarditis, but urinalysis is of the utmost importance in this patient at this point.
More diagnostic information is needed before considering skin biopsy.
Clues from the urinalysis and chest radiography
Our patient’s sedimentation rate is 24 mm/hour. The urinalysis is strongly positive for blood and a moderate amount of protein but negative for leukocyte esterase and nitrite. The urine sediment contains numerous red blood cell casts and 6 to 10 white blood cells per high-power field.
Pending return of blood cultures, ceftriaxone (Rocephin) and azithromycin (Zithromax) are started to treat possible community-acquired pneumonia. Vancomycin (Vancocin) is empirically added, given the possibility of prosthetic valve endocarditis. Gram stain on blood cultures shows gram-positive cocci.
Ground-glass attenuation implies focal or diffuse opacification of the lung that does not obscure the vascular structures, is not associated with air bronchograms, and appears as a “veil” across the lung parenchyma.1 It can be seen in acute diseases such as infection (including pneumonia from atypical bacteria, viruses, acid-fast bacilli, and Pneumocystis jiroveci), pulmonary hemorrhage of any cause, acute viral, eosinophilic, and interstitial pneumonias, and hypersensitivity pneumonitis. It can also be seen in chronic disease states such as interstitial lung disease, bronchoalveolar carcinoma, alveolar proteinosis, and sarcoidosis.
WHICH TEST WOULD NOT HELP?
2. Which of the following tests is least likely to help in the diagnostic evaluation at this point?
- Transthoracic echocardiography
- Transesophageal echocardiography
- Bronchoscopy
- Cystoscopy
In this case, the least likely to help is cystoscopy.
This patient’s vasculitic-appearing rash, ground-glass pulmonary infiltrates, and impaired renal function with red cell casts suggest a pulmonary-renal syndrome, and with this constellation of features, a systemic vasculitis is very likely. Therefore, the focus of the evaluation should be on any evidence to support a diagnosis of vasculitis, as well as other possible causes.
In a patient with diabetes, an artificial heart valve, and fever, the possibility of infection, especially endocarditis, remains high. Transthoracic echocardiography is warranted, and if it is negative for vegetations, transesophageal echocardiography would be a reasonable next option.
Bronchoscopy is warranted to determine if the infiltrates represent pulmonary hemorrhage, which can be seen in certain types of vasculitic and systemic disorders.
The finding of red cell casts in the urine indicates glomerulonephritis, and therefore the kidneys are the likely source of the urinary red blood cells, making cystoscopy of no utility in this current, acute setting.
Case continued: His condition worsens
Transthoracic echocardiography reveals a well-seated mechanical prosthetic aortic valve, trivial aortic regurgitation, a peak gradient of 23 mm Hg and a mean gradient of 12 mm Hg (normal values for his prosthetic valve), and no valvular vegetations. Transesophageal echocardiography confirms the absence of vegetations.
His oxygen requirement increases, and analysis of arterial blood gases reveals a pH of 7.37, Pco2 49 mm Hg (normal range 35–45), Po2 102 mm Hg (normal 80–100), and bicarbonate 28 mmol/L while breathing 100% supplemental oxygen by a nonrebreather face mask. He is taken to the medical intensive care unit for intubation and mechanical ventilation. Bronchoscopy performed while he is intubated confirms diffuse alveolar hemorrhage. Pulse intravenous methylprednisolone (Solu-Medrol) therapy is started.
DIFFUSE ALVEOLAR HEMORRHAGE
3. Which of the following is not true about diffuse alveolar hemorrhage?
- Its onset is usually abrupt or of short duration
- It is always associated with hemoptysis
- Radiography most commonly shows new patchy or diffuse alveolar opacities
- Pulmonary function testing shows increased diffusing capacity of the lung for carbon monoxide (Dlco)
Hemoptysis is absent at presentation in as many as 33% of patients.
The onset of diffuse alveolar hemorrhage is usually abrupt or of short duration, with initial symptoms of cough, fever, and dyspnea. Some patients, such as ours, can present with severe acute respiratory distress syndrome requiring mechanical ventilation. Radiography most often shows new patchy alveolar opacities, and CT may reveal a ground-glass appearance. On pulmonary function testing, the Dlco is high, owing to the hemoglobin within the alveoli.
ACUTE GLOMERULONEPHRITIS PLUS PULMONARY HEMORRHAGE EQUALS…?
4. Which disease could have manifestations consistent with acute glomerulonephritis and pulmonary hemorrhage?
- Antiglomerular basement membrane disease
- Wegener granulomatosis
- Microscopic polyangiitis
- Systemic lupus erythematosus
All of these are possible.
The combined presentation of acute glomerulonephritis and pulmonary hemorrhage (also called pulmonary-renal syndrome) is usually seen in antiglomerular basement membrane disease (Goodpasture syndrome) and small-vessel systemic vasculitides such as Wegener granulomatosis and microscopic polyangiitis.2,3 It can also be seen in patients with systemic lupus erythematosus.
Antiglomerular basement membrane disease
In antiglomerular basement membrane disease, circulating antibodies are directed towards an antigen intrinsic to the glomerular basement membrane, typically leading to acute glomerulonephritis associated with crescent formation. It may present as acute renal failure in which urinalysis shows proteinuria with sediment characterized by red cell casts. Pulmonary involvement, usually alveolar hemorrhage, is present in approximately 60% to 70% of cases.
The diagnosis requires demonstration of antiglomerular basement membrane antibodies in either the serum or the kidney. Renal biopsy is usually recommended because the accuracy of serum assays is variable.
A key histologic feature of the renal lesion in antiglomerular basement membrane disease is crescentic glomerulonephritis in which immunofluorescence microscopy demonstrates the virtually pathognomonic finding of linear deposition of immunoglobulin G along the glomerular capillaries.
The treatment of choice for antiglomerular basement membrane disease is plasmapheresis and immunosuppression with a combination of glucocorticoids and cyclophosphamide (Cytoxan). If the disease is high on the differential diagnosis, empiric plasmapheresis should be started while waiting for diagnostic studies, because the prognosis of untreated glomerulonephritis is poor.
Wegener granulomatosis
Wegener granulomatosis is a systemic vasculitis of the medium and small arteries, arterioles, and venules that classically involves the upper and lower respiratory tracts and the kidneys. Patients may present with persistent rhinorrhea and epistaxis, cough with chest radiographs showing nodules, fixed infiltrates, or cavities, and abnormal urinary sediment with microscopic hematuria with or without red cell casts.
From 75% to 90% of patients with active Wegener granulomatosis are positive for antineutrophil cytoplasmic antibody (ANCA). In 60% to 80% of cases, ANCA is directed against proteinase 3 (PR3), which produces a cytoplasmic standing pattern by immunofluorescence (cANCA), while 5% to 20% have ANCA directed against myeloper-oxidase, which produces a perinuclear staining pattern (pANCA). A small number of patients with Wegener granulomatosis are ANCA-negative.
The diagnosis is usually confirmed by tissue biopsy at the site of active disease, which shows necrotizing vasculitis with granulomatous inflammation. The renal lesion is typically that of a focal, segmental, necrotizing glomerulonephritis that has few to no immune complexes (pauci-immune glomerulonephritis).
The treatment of severe disease involves a combination of cyclophosphamide and glucocorticoids initially to achieve remission followed by maintenance therapy with methotrexate or azathioprine (Imuran).
Microscopic polyangiitis
Microscopic polyangiitis is a systemic vasculitis of the capillaries, venules, and arterioles, with little or no immune complex deposition. Nearly all patients have renal involvement, and 10% to 30% have lung involvement. In those with lung involvement, diffuse alveolar hemorrhage is the most common manifestation.
On histopathologic study, microscopic polyangiitis differs from Wegener granulomatosis in that it does not have granuloma formation. However, the renal lesion is that of a pauci-immune glomerulonephritis and is identical to that seen in Wegener granulomatosis. From 70% to 85% of patients with microscopic polyangiitis are ANCA-positive, and most of these have pANCA.
The management of active severe microscopic polyangiitis is identical to that of Wegener granulomatosis.
Systemic lupus erythematosus
Systemic lupus erythematosus is an autoimmune disease characterized by tissue-binding autoantibody and immune-complex-mediated organ damage. It can involve multiple organ systems, and the diagnosis is based on characteristic clinical features and autoantibodies. The sensitivity of antinuclear antibody for lupus is close to 100%, which makes it a good screening tool. Antibodies to dsDNA and Smith antigen have high specificity for lupus.
About 75% of patients have renal involvement at some point in their disease course. The different types of renal disease in systemic lupus are usually differentiated with a renal biopsy, with immune-complex-mediated glomerular diseases being the most common.
The most common pulmonary manifestation is pleuritis with or without pleural effusion. Life-threatening pulmonary manifestations include pulmonary hemorrhage and interstitial inflammation leading to fibrosis.
Lupus has great clinical variability and the treatment approach is based on the organ manifestations, disease activity, and severity.
CASE CONTINUED: ARRIVING AT THE DIAGNOSIS
We start our patient on cyclophosphamide 175 mg daily in view of possible Wegener granulomatosis.
Even though purpura is extremely rare in primary antiglomerular basement membrane disease, this patient has life-threatening pulmonary hemorrhage, a complication seen in over 50% of these patients. Therefore, plasmapheresis is started empirically.
On the second day of cyclophosphamide treatment, tests for ANCA, glomerular basement membrane antibody, and antinuclear antibody are reported as negative, and complement levels are normal. Bronchoalveolar lavage shows no infection. Follow-up blood cultures are negative.
To summarize the findings so far, this patient has a purpuric skin rash, active urine sediment with red cell casts indicating glomerulonephritis, acute renal failure, and severe pulmonary hemorrhage requiring mechanical ventilation. Although one set of blood cultures showed gram-positive cocci, no source of infection, particularly endocarditis, could be identified.
Antiglomerular basement membrane disease would still be high on the list of suspected diagnoses, given his diffuse alveolar hemorrhage. As mentioned earlier, renal biopsy is imperative to making a diagnosis, because serologic tests have variable accuracy. And making the correct diagnosis has therapeutic implications.
Renal biopsy is performed and shows immune-complex mesangiopathic glomerulonephritis with positive immunofluorescent staining in the mesangium for IgA. Only one glomerulus shows fibrinoid necrosis.
Skin biopsy results obtained earlier showed positive direct immunofluorescence for IgA. Both renal and skin biopsies suggested Henoch-Schönlein purpura.
IgA deposition in the kidney and skin has been associated with liver cirrhosis, celiac disease, and infections with agents such as human immunodeficiency virus, cytomegalovirus, Haemophilus parainfluenzae, and Staphylococcus aureus. In a Japanese study,4 renal biopsy specimens from 116 patients with IgA nephropathy and from 122 patients with other types of kidney disease were examined for the presence of S aureus antigen in the glomeruli. Although antigen was not detected in non-IgA disease, 68% of specimens from patients with IgA nephropathy had S aureus cell envelope antigen together with IgA antibody in the glomeruli. However, no single antigen has been consistently identified, so it seems more probable that the development of IgA deposition in kidneys is a consequence of aberrant IgA immune response rather than the antigen itself.
HENOCH-SCHÖNLEIN PURPURA
Henoch-Schönlein purpura is a systemic vasculitis with a prominent cutaneous component. It is characterized by the tissue deposition of IgA-containing immune complexes. It is predominantly a disease of children but it can be seen in adults. A UK study found the prevalence to be 20 per 100,000 children, with the highest prevalence between ages 4 and 7 (70 per 100,000).5
The four cardinal clinical features of Henoch-Schönlein purpura are purpuric rash, abdominal pain, arthralgia, and renal involvement. Almost all patients have purpuric rash at some point in their disease course. Arthralgia with or without arthritis is typically migratory, oligoarticular, and nondeforming, usually affecting the large joints of the lower extremities; involvement of the upper extremities is less common.
Skin biopsy typically shows leukocytoclastic vasculitis in postcapillary venules with IgA deposition, and these findings are pathognomonic of Henoch-Schönlein purpura.
Gastrointestinal involvement can range from mild symptoms such as nausea, vomiting, abdominal pain, and paralytic ileus to severe disease such as gastrointestinal hemorrhage, bowel infarction, bowel perforation, and intussusception.
Renal involvement is common and is important, as it can in rare cases progress to end-stage renal disease. The urinalysis usually shows mild proteinuria with active sediment with red cell casts. Most patients have relatively mild disease, characterized by asymptomatic hematuria with a normal or slightly elevated creatinine. However, severe involvement may occur, with nephrotic syndrome, hypertension, and acute renal failure.
Different presentation in adults vs children
Adults with Henoch-Schönlein purpura only rarely present with bowel intussusception, whereas some studies have found that adults are more likely than children to develop significant renal involvement, including end-stage renal disease.6,7
There is a general but not absolute correlation between the severity of clinical manifestations and the findings on renal biopsy. A poor prognosis (significant proteinuria, hypertension, renal insufficiency, or end-stage renal disease) is associated with crescent formation involving more than 50% of the glomeruli.8
Our current understanding of the longterm outcome of the renal disease in Henoch-Schönlein purpura is primarily derived from studies in children. In one study, complete recovery occurred in 94% of children and 89% of adults.7 A long-term study of 250 adults with Henoch-Schönlein purpura and renal involvement of sufficient severity to require biopsy reported that, at a median follow up of 15 years, 11% had become dialysis-dependent and 13% had severe renal failure (creatinine clearance < 30 mL/min).6 Recurrence is common, occurring in approximately one-third of patients, more likely in those with nephritis.8
The diagnosis of Henoch-Schönlein purpura is typically made on the basis of key clinical features. In patients such as ours who have an atypical presentation, biopsy of affected skin and renal biopsy can be essential in the diagnosis. Diffuse alveolar hemorrhage is exceedingly rare in Henoch-Schönlein purpura but can be seen, as in our patient.9,10 In this setting, the findings of IgA deposits in skin and renal biopsy specimens, together with the absence of other clinical, serologic, or histologic features of other more-common potential causes, secured the diagnosis in this patient.
Henoch-Schönlein purpura is usually self-limited and requires no specific therapy. Evidence suggests that glucocorticoids enhance the rate of resolution of the arthritis and abdominal pain but do not appear to prevent recurrent disease or lessen the likelihood of progression of renal disease.8 Patients with severe renal involvement with renal function impairment may benefit from pulse intravenous corticosteroid therapy (methylprednisolone 250–1,000 mg per day for 3 days), followed by oral steroids for 3 months.11
In anecdotal reports, renal function improved in 19 of 21 children with Henoch-Schönlein purpura and severe crescentic nephritis.12 Studies have evaluated cyclophosphamide13 and plasmapheresis,14 but their role remains uncertain. Renal transplantation is an option in patients who progress to end-stage renal disease.
OUR CASE CONTINUED
In our patient, plasmapheresis was discontinued. As the pulmonary hemorrhage had developed during treatment with prednisone, we decided to continue cyclophosphamide, given the life-threatening nature of his disease. His pulmonary status improved and he was extubated.
During his initial hospital stay, he was taking heparin for anticoagulation therapy. However, given the life-threatening diffuse alveolar hemorrhage, heparin was stopped during the course of his stay in the intensive care unit. Once he was stable and was transferred out of the intensive care unit, heparin was resumed, and his anticoagulation therapy was bridged to warfarin just before discharge. He was eventually discharged on a tapering dose of oral prednisone and cyclophosphamide for 3 months, after which he was switched to azathioprine for maintenance therapy. He was doing well 6 months later, with a serum creatinine level of 1.6 mg/dL, no red cell casts in the urine, and no rash.
TAKE-HOME POINT
In any case of suspected vasculitis that presents with skin disease, it is essential to look for other sites with potentially life-threatening involvement. Henoch-Schönlein purpura is a systemic vasculitis with a prominent cutaneous component. It is not always benign and can be associated with serious complications such as renal failure, gastrointestinal events, and, very rarely, diffuse alveolar hemorrhage.
A 72-year-old man whose medical history includes diabetes mellitus, hypertension, coronary artery disease, aortic valve replacement, atrial fibrillation, and chronic obstructive pulmonary disease was in his usual state of health until 2 weeks ago, when he developed a purpuric rash on his legs. His physician started him on prednisone 40 mg daily for the rash; however, 1 week later he presented to a hospital emergency room when his family found him confused and diaphoretic.
In the emergency room, he was found to be hypoglycemic, with a serum glucose level of 40 mg/dL, which was promptly treated. His mental status improved partially. In the hospital, the rash worsened and progressed upwards to his trunk and upper extremities. He was transferred to our institution for further workup and management.
A review of systems reveals occasional epistaxis in the summer, recent fatigue, cough, and shortness of breath on exertion. His medications at the time of transfer include warfarin (Coumadin), amlodipine (Norvasc), insulin, ipratropium and albuterol (Combivent) inhalers, and prednisone 40 mg daily. He has not undergone surgery recently.
PHYSICAL EXAMINATION
He is alert and oriented to person but not to time and place.
Vital signs. Oral temperature 101.1°F (38.4°C), pulse rate 108, blood pressure 108/79 mm/Hg, respiratory rate 22, oxygen saturation 93% by pulse oximetry on room air, weight 94 kg (207 lb).
Head, eyes, ears, nose, and throat. No pallor or icterus, pupils are equally reactive, nasal mucosa not inflamed or ulcerated, mucous membranes moist, no sinus tenderness.
Neck. No jugular venous distention and no cervical lymphadenopathy.
Cardiovascular. Tachycardia, irregularly irregular rhythm, prosthetic valve sounds, no murmurs, rubs, or gallops.
Respiratory. Bibasal crackles (right side more than the left). No wheezing.
Abdomen. Soft, nontender, nondistended, no palpable organomegaly, bowel sounds normal.
Extremities. No edema, good peripheral pulses.
Neurologic. No focal deficits noted.
Lymphatic. No enlarged lymph nodes.
Musculoskeletal. Traumatic right second distal interphalangeal amputation. Otherwise, no joint abnormality or restriction of movement.
Initial laboratory values:
- White blood cell count 15.78 × 109/L (normal 4.5–11.0)
- Absolute neutrophil count 13.3 × 109/L (4.0–11.0)
- Hemoglobin 13.3 g/dL (13.5–17.5)
- Platelet count 133 × 109/L (150–400)
- International normalized ratio (INR) 1.8
- Sodium 136 mmol/L (135–146)
- Potassium 4.6 mmol/L (3.5–5.0)
- Blood urea nitrogen 31 mg/dL (10–25)
- Creatinine 1.6 mg/dL (0.70–1.40)
- Glucose 62 mg/dL (65–100)
- Bicarbonate 23 mmol/L (23–32)
- Albumin 2.5 g/dL (3.5–5.0)
- Total protein 4.6 g/dL (6.0–8.4)
- Bilirubin 1.2 mg/dL (0.0–1.5)
- Aspartate aminotransferase 41 U/L (7–40)
- Alanine aminotransferase 74 U/L (5–50)
- Alkaline phosphatase 55 U/L (40–150)
- C-reactive protein 9.9 mg/dL (0.0–1.0).
Other studies
Electrocardiography shows atrial fibrillation and left ventricular hypertrophy, but no acute changes.
Computed tomography (CT) of the head shows no evidence of hemorrhage or infarction.
Blood cultures are sent at the time of hospital admission.
WHICH TEST IS NEXT?
1. Which is the most appropriate next step for this patient?
- Urinalysis
- CT of the chest
- Echocardiography
- Skin biopsy
The rash in Figure 1 is palpable purpura, which strongly suggests small-vessel cutaneous vasculitis, a condition that can occur in a broad range of settings. An underlying cause is identified in over 70% of cases. Cutaneous vasculitis may herald a primary small-vessel systemic vasculitis such as Wegener granulomatosis, microscopic polyangiitis, or Henoch-Schönlein purpura. It can also be secondary to a spectrum of underlying triggers or diseases that include medications, infections, malignancies such as lymphoproliferative disorders, cryoglobulinemia secondary to hepatitis C viral infection, and connective tissue diseases such as rheumatoid arthritis and systemic lupus erythematosus.
Infective endocarditis is associated with a secondary form of vasculitis and is a strong possibility in this patient, who has a prosthetic aortic valve, fever, and a high white blood cell count.
Thrombocytopenia should also prompt an assessment for any drugs the patient is taking that affect platelet function. However, thrombocytopenia typically results in nonpalpable purpura.
Idiopathic isolated cutaneous vasculitis, in which no underlying cause for the cutaneous vasculitis can be identified, is the diagnosis in less than 30% of cases.
A vasculitic disease process can involve multiple sites, which may be asymptomatic on presentation. Identifying these sites is important, not only to establish the diagnosis, but also to detect potentially life-threatening complications early.
Thus, in this patient, urinalysis should be done promptly to check for active sediment consisting of red cell casts, which would suggest renal involvement (glomerulonephritis). Also, a rising blood pressure and creatinine would point to renal involvement and warrant more aggressive initial therapy.
Chest radiography should be done to rule out pulmonary infiltrates, septic emboli, nodules, or cavities that could represent vasculitic or infectious involvement of the lungs. CT of the chest may be needed to further characterize abnormalities on chest radiography.
Echocardiography should certainly be pursued as part of the workup for endocarditis, but urinalysis is of the utmost importance in this patient at this point.
More diagnostic information is needed before considering skin biopsy.
Clues from the urinalysis and chest radiography
Our patient’s sedimentation rate is 24 mm/hour. The urinalysis is strongly positive for blood and a moderate amount of protein but negative for leukocyte esterase and nitrite. The urine sediment contains numerous red blood cell casts and 6 to 10 white blood cells per high-power field.
Pending return of blood cultures, ceftriaxone (Rocephin) and azithromycin (Zithromax) are started to treat possible community-acquired pneumonia. Vancomycin (Vancocin) is empirically added, given the possibility of prosthetic valve endocarditis. Gram stain on blood cultures shows gram-positive cocci.
Ground-glass attenuation implies focal or diffuse opacification of the lung that does not obscure the vascular structures, is not associated with air bronchograms, and appears as a “veil” across the lung parenchyma.1 It can be seen in acute diseases such as infection (including pneumonia from atypical bacteria, viruses, acid-fast bacilli, and Pneumocystis jiroveci), pulmonary hemorrhage of any cause, acute viral, eosinophilic, and interstitial pneumonias, and hypersensitivity pneumonitis. It can also be seen in chronic disease states such as interstitial lung disease, bronchoalveolar carcinoma, alveolar proteinosis, and sarcoidosis.
WHICH TEST WOULD NOT HELP?
2. Which of the following tests is least likely to help in the diagnostic evaluation at this point?
- Transthoracic echocardiography
- Transesophageal echocardiography
- Bronchoscopy
- Cystoscopy
In this case, the least likely to help is cystoscopy.
This patient’s vasculitic-appearing rash, ground-glass pulmonary infiltrates, and impaired renal function with red cell casts suggest a pulmonary-renal syndrome, and with this constellation of features, a systemic vasculitis is very likely. Therefore, the focus of the evaluation should be on any evidence to support a diagnosis of vasculitis, as well as other possible causes.
In a patient with diabetes, an artificial heart valve, and fever, the possibility of infection, especially endocarditis, remains high. Transthoracic echocardiography is warranted, and if it is negative for vegetations, transesophageal echocardiography would be a reasonable next option.
Bronchoscopy is warranted to determine if the infiltrates represent pulmonary hemorrhage, which can be seen in certain types of vasculitic and systemic disorders.
The finding of red cell casts in the urine indicates glomerulonephritis, and therefore the kidneys are the likely source of the urinary red blood cells, making cystoscopy of no utility in this current, acute setting.
Case continued: His condition worsens
Transthoracic echocardiography reveals a well-seated mechanical prosthetic aortic valve, trivial aortic regurgitation, a peak gradient of 23 mm Hg and a mean gradient of 12 mm Hg (normal values for his prosthetic valve), and no valvular vegetations. Transesophageal echocardiography confirms the absence of vegetations.
His oxygen requirement increases, and analysis of arterial blood gases reveals a pH of 7.37, Pco2 49 mm Hg (normal range 35–45), Po2 102 mm Hg (normal 80–100), and bicarbonate 28 mmol/L while breathing 100% supplemental oxygen by a nonrebreather face mask. He is taken to the medical intensive care unit for intubation and mechanical ventilation. Bronchoscopy performed while he is intubated confirms diffuse alveolar hemorrhage. Pulse intravenous methylprednisolone (Solu-Medrol) therapy is started.
DIFFUSE ALVEOLAR HEMORRHAGE
3. Which of the following is not true about diffuse alveolar hemorrhage?
- Its onset is usually abrupt or of short duration
- It is always associated with hemoptysis
- Radiography most commonly shows new patchy or diffuse alveolar opacities
- Pulmonary function testing shows increased diffusing capacity of the lung for carbon monoxide (Dlco)
Hemoptysis is absent at presentation in as many as 33% of patients.
The onset of diffuse alveolar hemorrhage is usually abrupt or of short duration, with initial symptoms of cough, fever, and dyspnea. Some patients, such as ours, can present with severe acute respiratory distress syndrome requiring mechanical ventilation. Radiography most often shows new patchy alveolar opacities, and CT may reveal a ground-glass appearance. On pulmonary function testing, the Dlco is high, owing to the hemoglobin within the alveoli.
ACUTE GLOMERULONEPHRITIS PLUS PULMONARY HEMORRHAGE EQUALS…?
4. Which disease could have manifestations consistent with acute glomerulonephritis and pulmonary hemorrhage?
- Antiglomerular basement membrane disease
- Wegener granulomatosis
- Microscopic polyangiitis
- Systemic lupus erythematosus
All of these are possible.
The combined presentation of acute glomerulonephritis and pulmonary hemorrhage (also called pulmonary-renal syndrome) is usually seen in antiglomerular basement membrane disease (Goodpasture syndrome) and small-vessel systemic vasculitides such as Wegener granulomatosis and microscopic polyangiitis.2,3 It can also be seen in patients with systemic lupus erythematosus.
Antiglomerular basement membrane disease
In antiglomerular basement membrane disease, circulating antibodies are directed towards an antigen intrinsic to the glomerular basement membrane, typically leading to acute glomerulonephritis associated with crescent formation. It may present as acute renal failure in which urinalysis shows proteinuria with sediment characterized by red cell casts. Pulmonary involvement, usually alveolar hemorrhage, is present in approximately 60% to 70% of cases.
The diagnosis requires demonstration of antiglomerular basement membrane antibodies in either the serum or the kidney. Renal biopsy is usually recommended because the accuracy of serum assays is variable.
A key histologic feature of the renal lesion in antiglomerular basement membrane disease is crescentic glomerulonephritis in which immunofluorescence microscopy demonstrates the virtually pathognomonic finding of linear deposition of immunoglobulin G along the glomerular capillaries.
The treatment of choice for antiglomerular basement membrane disease is plasmapheresis and immunosuppression with a combination of glucocorticoids and cyclophosphamide (Cytoxan). If the disease is high on the differential diagnosis, empiric plasmapheresis should be started while waiting for diagnostic studies, because the prognosis of untreated glomerulonephritis is poor.
Wegener granulomatosis
Wegener granulomatosis is a systemic vasculitis of the medium and small arteries, arterioles, and venules that classically involves the upper and lower respiratory tracts and the kidneys. Patients may present with persistent rhinorrhea and epistaxis, cough with chest radiographs showing nodules, fixed infiltrates, or cavities, and abnormal urinary sediment with microscopic hematuria with or without red cell casts.
From 75% to 90% of patients with active Wegener granulomatosis are positive for antineutrophil cytoplasmic antibody (ANCA). In 60% to 80% of cases, ANCA is directed against proteinase 3 (PR3), which produces a cytoplasmic standing pattern by immunofluorescence (cANCA), while 5% to 20% have ANCA directed against myeloper-oxidase, which produces a perinuclear staining pattern (pANCA). A small number of patients with Wegener granulomatosis are ANCA-negative.
The diagnosis is usually confirmed by tissue biopsy at the site of active disease, which shows necrotizing vasculitis with granulomatous inflammation. The renal lesion is typically that of a focal, segmental, necrotizing glomerulonephritis that has few to no immune complexes (pauci-immune glomerulonephritis).
The treatment of severe disease involves a combination of cyclophosphamide and glucocorticoids initially to achieve remission followed by maintenance therapy with methotrexate or azathioprine (Imuran).
Microscopic polyangiitis
Microscopic polyangiitis is a systemic vasculitis of the capillaries, venules, and arterioles, with little or no immune complex deposition. Nearly all patients have renal involvement, and 10% to 30% have lung involvement. In those with lung involvement, diffuse alveolar hemorrhage is the most common manifestation.
On histopathologic study, microscopic polyangiitis differs from Wegener granulomatosis in that it does not have granuloma formation. However, the renal lesion is that of a pauci-immune glomerulonephritis and is identical to that seen in Wegener granulomatosis. From 70% to 85% of patients with microscopic polyangiitis are ANCA-positive, and most of these have pANCA.
The management of active severe microscopic polyangiitis is identical to that of Wegener granulomatosis.
Systemic lupus erythematosus
Systemic lupus erythematosus is an autoimmune disease characterized by tissue-binding autoantibody and immune-complex-mediated organ damage. It can involve multiple organ systems, and the diagnosis is based on characteristic clinical features and autoantibodies. The sensitivity of antinuclear antibody for lupus is close to 100%, which makes it a good screening tool. Antibodies to dsDNA and Smith antigen have high specificity for lupus.
About 75% of patients have renal involvement at some point in their disease course. The different types of renal disease in systemic lupus are usually differentiated with a renal biopsy, with immune-complex-mediated glomerular diseases being the most common.
The most common pulmonary manifestation is pleuritis with or without pleural effusion. Life-threatening pulmonary manifestations include pulmonary hemorrhage and interstitial inflammation leading to fibrosis.
Lupus has great clinical variability and the treatment approach is based on the organ manifestations, disease activity, and severity.
CASE CONTINUED: ARRIVING AT THE DIAGNOSIS
We start our patient on cyclophosphamide 175 mg daily in view of possible Wegener granulomatosis.
Even though purpura is extremely rare in primary antiglomerular basement membrane disease, this patient has life-threatening pulmonary hemorrhage, a complication seen in over 50% of these patients. Therefore, plasmapheresis is started empirically.
On the second day of cyclophosphamide treatment, tests for ANCA, glomerular basement membrane antibody, and antinuclear antibody are reported as negative, and complement levels are normal. Bronchoalveolar lavage shows no infection. Follow-up blood cultures are negative.
To summarize the findings so far, this patient has a purpuric skin rash, active urine sediment with red cell casts indicating glomerulonephritis, acute renal failure, and severe pulmonary hemorrhage requiring mechanical ventilation. Although one set of blood cultures showed gram-positive cocci, no source of infection, particularly endocarditis, could be identified.
Antiglomerular basement membrane disease would still be high on the list of suspected diagnoses, given his diffuse alveolar hemorrhage. As mentioned earlier, renal biopsy is imperative to making a diagnosis, because serologic tests have variable accuracy. And making the correct diagnosis has therapeutic implications.
Renal biopsy is performed and shows immune-complex mesangiopathic glomerulonephritis with positive immunofluorescent staining in the mesangium for IgA. Only one glomerulus shows fibrinoid necrosis.
Skin biopsy results obtained earlier showed positive direct immunofluorescence for IgA. Both renal and skin biopsies suggested Henoch-Schönlein purpura.
IgA deposition in the kidney and skin has been associated with liver cirrhosis, celiac disease, and infections with agents such as human immunodeficiency virus, cytomegalovirus, Haemophilus parainfluenzae, and Staphylococcus aureus. In a Japanese study,4 renal biopsy specimens from 116 patients with IgA nephropathy and from 122 patients with other types of kidney disease were examined for the presence of S aureus antigen in the glomeruli. Although antigen was not detected in non-IgA disease, 68% of specimens from patients with IgA nephropathy had S aureus cell envelope antigen together with IgA antibody in the glomeruli. However, no single antigen has been consistently identified, so it seems more probable that the development of IgA deposition in kidneys is a consequence of aberrant IgA immune response rather than the antigen itself.
HENOCH-SCHÖNLEIN PURPURA
Henoch-Schönlein purpura is a systemic vasculitis with a prominent cutaneous component. It is characterized by the tissue deposition of IgA-containing immune complexes. It is predominantly a disease of children but it can be seen in adults. A UK study found the prevalence to be 20 per 100,000 children, with the highest prevalence between ages 4 and 7 (70 per 100,000).5
The four cardinal clinical features of Henoch-Schönlein purpura are purpuric rash, abdominal pain, arthralgia, and renal involvement. Almost all patients have purpuric rash at some point in their disease course. Arthralgia with or without arthritis is typically migratory, oligoarticular, and nondeforming, usually affecting the large joints of the lower extremities; involvement of the upper extremities is less common.
Skin biopsy typically shows leukocytoclastic vasculitis in postcapillary venules with IgA deposition, and these findings are pathognomonic of Henoch-Schönlein purpura.
Gastrointestinal involvement can range from mild symptoms such as nausea, vomiting, abdominal pain, and paralytic ileus to severe disease such as gastrointestinal hemorrhage, bowel infarction, bowel perforation, and intussusception.
Renal involvement is common and is important, as it can in rare cases progress to end-stage renal disease. The urinalysis usually shows mild proteinuria with active sediment with red cell casts. Most patients have relatively mild disease, characterized by asymptomatic hematuria with a normal or slightly elevated creatinine. However, severe involvement may occur, with nephrotic syndrome, hypertension, and acute renal failure.
Different presentation in adults vs children
Adults with Henoch-Schönlein purpura only rarely present with bowel intussusception, whereas some studies have found that adults are more likely than children to develop significant renal involvement, including end-stage renal disease.6,7
There is a general but not absolute correlation between the severity of clinical manifestations and the findings on renal biopsy. A poor prognosis (significant proteinuria, hypertension, renal insufficiency, or end-stage renal disease) is associated with crescent formation involving more than 50% of the glomeruli.8
Our current understanding of the longterm outcome of the renal disease in Henoch-Schönlein purpura is primarily derived from studies in children. In one study, complete recovery occurred in 94% of children and 89% of adults.7 A long-term study of 250 adults with Henoch-Schönlein purpura and renal involvement of sufficient severity to require biopsy reported that, at a median follow up of 15 years, 11% had become dialysis-dependent and 13% had severe renal failure (creatinine clearance < 30 mL/min).6 Recurrence is common, occurring in approximately one-third of patients, more likely in those with nephritis.8
The diagnosis of Henoch-Schönlein purpura is typically made on the basis of key clinical features. In patients such as ours who have an atypical presentation, biopsy of affected skin and renal biopsy can be essential in the diagnosis. Diffuse alveolar hemorrhage is exceedingly rare in Henoch-Schönlein purpura but can be seen, as in our patient.9,10 In this setting, the findings of IgA deposits in skin and renal biopsy specimens, together with the absence of other clinical, serologic, or histologic features of other more-common potential causes, secured the diagnosis in this patient.
Henoch-Schönlein purpura is usually self-limited and requires no specific therapy. Evidence suggests that glucocorticoids enhance the rate of resolution of the arthritis and abdominal pain but do not appear to prevent recurrent disease or lessen the likelihood of progression of renal disease.8 Patients with severe renal involvement with renal function impairment may benefit from pulse intravenous corticosteroid therapy (methylprednisolone 250–1,000 mg per day for 3 days), followed by oral steroids for 3 months.11
In anecdotal reports, renal function improved in 19 of 21 children with Henoch-Schönlein purpura and severe crescentic nephritis.12 Studies have evaluated cyclophosphamide13 and plasmapheresis,14 but their role remains uncertain. Renal transplantation is an option in patients who progress to end-stage renal disease.
OUR CASE CONTINUED
In our patient, plasmapheresis was discontinued. As the pulmonary hemorrhage had developed during treatment with prednisone, we decided to continue cyclophosphamide, given the life-threatening nature of his disease. His pulmonary status improved and he was extubated.
During his initial hospital stay, he was taking heparin for anticoagulation therapy. However, given the life-threatening diffuse alveolar hemorrhage, heparin was stopped during the course of his stay in the intensive care unit. Once he was stable and was transferred out of the intensive care unit, heparin was resumed, and his anticoagulation therapy was bridged to warfarin just before discharge. He was eventually discharged on a tapering dose of oral prednisone and cyclophosphamide for 3 months, after which he was switched to azathioprine for maintenance therapy. He was doing well 6 months later, with a serum creatinine level of 1.6 mg/dL, no red cell casts in the urine, and no rash.
TAKE-HOME POINT
In any case of suspected vasculitis that presents with skin disease, it is essential to look for other sites with potentially life-threatening involvement. Henoch-Schönlein purpura is a systemic vasculitis with a prominent cutaneous component. It is not always benign and can be associated with serious complications such as renal failure, gastrointestinal events, and, very rarely, diffuse alveolar hemorrhage.
- Collins J, Stern EJ. Ground-glass opacity at CT: the ABCs. AJR Am J Roentgenol 1997; 169:355–367.
- Boyce NW, Holdsworth SR. Pulmonary manifestations of the clinical syndrome of acute glomerulonephritis and lung hemorrhage. Am J Kidney Dis 1986; 8:31–36.
- Gallagher H, Kwan JT, Jayne DR. Pulmonary renal syndrome: a 4-year, single-center experience. Am J Kidney Dis 2002; 39:42–47.
- Koyama A, Sharmin S, Sakurai H, et al. Staphylococcus aureus cell envelope antigen is a new candidate for the induction of IgA nephropathy. Kidney Int 2004; 66:121–132.
- Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002; 360:1197–1202.
- Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch-Schönlein purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002; 13:1271–1278.
- Blanco R, Martinez-Taboada VM, Rodriguez-Valverde V, Garcia-Fuentes M, Gonzalez-Gay MA. Henoch-Schönlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis Rheum 1997; 40:859–864.
- Saulsbury FT. Henoch-Schönlein purpura in children. Report of 100 patients and review of the literature. Medicine (Baltimore) 1999; 78:395–409.
- Nadrous HF, Yu AC, Specks U, Ryu JH. Pulmonary involvement in Henoch-Schönlein purpura. Mayo Clin Proc 2004; 79:1151–1157.
- Vats KR, Vats A, Kim Y, Dassenko D, Sinaiko AR. Henoch-Schönlein purpura and pulmonary hemorrhage: a report and literature review. Pediatr Nephrol 1999; 13:530–534.
- Niaudet P, Habib R. Methylprednisolone pulse therapy in the treatment of severe forms of Schönlein-Henoch purpura nephritis. Pediatr Nephrol 1998; 12:238–243.
- Bergstein J, Leiser J, Andreoli SP. Response of crescentic Henoch-Schöenlein purpura nephritis to corticosteroid and azathioprine therapy. Clin Nephrol 1998; 49:9–14.
- Tarshish P, Bernstein J, Edelmann CM. Henoch-Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol 2004; 19:51–56.
- Hattori M, Ito K, Konomoto T, Kawaguchi H, Yoshioka T, Khono M. Plasmapheresis as the sole therapy for rapidly progressive Henoch-Schönlein purpura nephritis in children. Am J Kidney Dis 1999; 33:427–433.
- Collins J, Stern EJ. Ground-glass opacity at CT: the ABCs. AJR Am J Roentgenol 1997; 169:355–367.
- Boyce NW, Holdsworth SR. Pulmonary manifestations of the clinical syndrome of acute glomerulonephritis and lung hemorrhage. Am J Kidney Dis 1986; 8:31–36.
- Gallagher H, Kwan JT, Jayne DR. Pulmonary renal syndrome: a 4-year, single-center experience. Am J Kidney Dis 2002; 39:42–47.
- Koyama A, Sharmin S, Sakurai H, et al. Staphylococcus aureus cell envelope antigen is a new candidate for the induction of IgA nephropathy. Kidney Int 2004; 66:121–132.
- Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002; 360:1197–1202.
- Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch-Schönlein purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002; 13:1271–1278.
- Blanco R, Martinez-Taboada VM, Rodriguez-Valverde V, Garcia-Fuentes M, Gonzalez-Gay MA. Henoch-Schönlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis Rheum 1997; 40:859–864.
- Saulsbury FT. Henoch-Schönlein purpura in children. Report of 100 patients and review of the literature. Medicine (Baltimore) 1999; 78:395–409.
- Nadrous HF, Yu AC, Specks U, Ryu JH. Pulmonary involvement in Henoch-Schönlein purpura. Mayo Clin Proc 2004; 79:1151–1157.
- Vats KR, Vats A, Kim Y, Dassenko D, Sinaiko AR. Henoch-Schönlein purpura and pulmonary hemorrhage: a report and literature review. Pediatr Nephrol 1999; 13:530–534.
- Niaudet P, Habib R. Methylprednisolone pulse therapy in the treatment of severe forms of Schönlein-Henoch purpura nephritis. Pediatr Nephrol 1998; 12:238–243.
- Bergstein J, Leiser J, Andreoli SP. Response of crescentic Henoch-Schöenlein purpura nephritis to corticosteroid and azathioprine therapy. Clin Nephrol 1998; 49:9–14.
- Tarshish P, Bernstein J, Edelmann CM. Henoch-Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol 2004; 19:51–56.
- Hattori M, Ito K, Konomoto T, Kawaguchi H, Yoshioka T, Khono M. Plasmapheresis as the sole therapy for rapidly progressive Henoch-Schönlein purpura nephritis in children. Am J Kidney Dis 1999; 33:427–433.