User login
When the dissociation curve shifts to the left
A 48-year-old woman presented to the emergency department after 2 days of nonproductive cough, chest discomfort, worsening shortness of breath, and subjective fever. She had a history of systemic sclerosis. She was currently taking prednisone 20 mg daily and aspirin 81 mg daily.
Physical examination revealed tachypnea (28 breaths per minute), and bronchial breath sounds in the left lower chest posteriorly.
The initial laboratory workup revealed:
- Hemoglobin 106 g/L (reference range 115–155)
- Mean corpuscular volume 84 fL (80–100)
- White blood cell count 29.4 × 109/L (3.70–11.0), with 85% neutrophils
- Platelet count 180 × 109/L (150–350)
- Lactate dehydrogenase 312 U/L (100–220).
Chest radiography showed opacification of the lower lobe of the left lung.
She was admitted to the hospital and started treatment with intravenous azithromycin and ceftriaxone for presumed community-acquired pneumonia, based on the clinical presentation and findings on chest radiography. Because of her immunosuppression (due to chronic prednisone therapy) and her high lactate dehydrogenase level, Pneumocystis jirovecii pneumonia was suspected, and because she had a history of allergy to trimethoprim-sulfamethoxazole and pentamidine, she was started on dapsone.
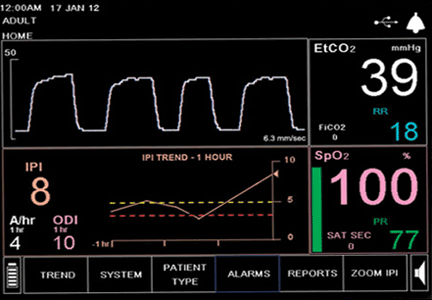
During the next 24 hours, she developed worsening dyspnea, hypoxia, and cyanosis. She was placed on an air-entrainment mask, with a fraction of inspired oxygen of 0.5. Pulse oximetry showed an oxygen saturation of 85%, but arterial blood gas analysis indicated an oxyhemoglobin concentration of 95%.
THE ‘SATURATION GAP’
1. Which is most likely to have caused the discrepancy between the oxyhemoglobin concentration and the oxygen saturation by pulse oximetry in this patient?
- Methemoglobinemia
- Carbon monoxide poisoning
- Inappropriate placement of the pulse oximeter probe
- Pulmonary embolism
Methemoglobinemia is the most likely cause of the discrepancy between the oxyhemoglobin levels and the oxygen saturation by pulse oximetry, a phenomenon also known as the “saturation gap.” Other common causes are cyanide poisoning and carbon monoxide poisoning.
Carbon monoxide poisoning, however, does not explain our patient’s cyanosis. On the contrary, carbon monoxide poisoning can actually cause the patient’s lips and mucous membranes to appear unnaturally bright pink. Also, carbon monoxide poisoning raises the blood concentration of carboxyhemoglobin (which has a high affinity for oxygen), and this usually causes pulse oximetry to read inappropriately high, whereas in our patient it read low.
Incorrect placement of the pulse oximeter probe can result in an inaccurate measurement of oxygen saturation. Visualization of the waveform on the plethysmograph or the signal quality index can be used to assess adequate placement of the pulse oximeter probe. However, inadequate probe placement does not explain our patient’s dyspnea and cyanosis.
Pulmonary embolism can lead to hypoxia as a result of ventilation-perfusion mismatch. However, pulmonary embolism leading to low oxygen saturation on pulse oximetry will also lead to concomitantly low oxyhemoglobin levels as measured by arterial blood gas analysis, and this was not seen in our patient.
BACK TO OUR PATIENT
Because there was a discrepancy between our patient’s pulse oximetry reading and oxyhemoglobin concentration by arterial blood gas measurement, her methemoglobin level was checked and was found to be 30%, thus confirming the diagnosis of methemoglobinemia.
WHAT IS METHEMOGLOBINEMIA, AND WHAT CAUSES IT?
Oxygen is normally bound to iron in its ferrous (Fe2+) form in hemoglobin to form oxyhemoglobin. Oxidative stress in the body can cause iron to change from the ferrous to the ferric (Fe3+) state, forming methemoglobin. Methemoglobin is normally present in the blood in low levels (< 1% of the total hemoglobin), and ferric iron is reduced and recycled back to the ferrous form by NADH-cytochrome b5 reductase, an enzyme present in red blood cells. This protective mechanism maintains methemoglobin levels within safe limits. But increased production can lead to accumulation of methemoglobin, resulting in dyspnea and hypoxia and the condition referred to as methemoglobinemia.1
Increased levels of methemoglobin relative to normal hemoglobin cause tissue hypoxia by several mechanisms. Methemoglobin cannot efficiently carry oxygen; instead, it binds to water or to a hydroxide ion depending on the pH of the environment.2 Therefore, the hemoglobin molecule does not carry its usual load of oxygen, and hypoxia results from the reduced delivery of oxygen to tissues. In addition, an increased concentration of methemoglobin causes a leftward shift in the oxygen-hemoglobin dissociation curve, representing an increased affinity to bound oxygen in the remaining heme groups. The tightly bound oxygen is not adequately released at the tissue level, thus causing cellular hypoxia.
Methemoglobinemia is most often caused by exposure to an oxidizing chemical or drug that increases production of methemoglobin. In rare cases, it is caused by a congenital deficiency of NADH-cytochrome b5 reductase.3
2. Which of the following drugs can cause methemoglobinemia?
- Acetaminophen
- Dapsone
- Benzocaine
- Primaquine
All four of these drugs are common culprits for causing acquired methemoglobinemia; others include chloroquine, nitroglycerin, and sulfonamides.4–6
The increased production of methemoglobin caused by these drugs overwhelms the protective effect of reducing enzymes and can lead to an accumulation of methemoglobin. However, because of variability in cellular metabolism, not every person who takes these drugs develops dangerous levels of methemoglobin.
Dapsone and benzocaine are the most commonly encountered drugs known to cause methemoglobinemia (Table 1). Dapsone is an anti-inflammatory and antimicrobial agent most commonly used for treating lepromatous leprosy and dermatitis herpetiformis. It is also often prescribed for prophylaxis and treatment of P jirovecii pneumonia in immunosuppressed individuals.7 Benzocaine is a local anesthetic and was commonly used before procedures such as oral or dental surgery, transesophageal echocardiography, and endoscopy.8–10 Even low doses of benzocaine can lead to high levels of methemoglobinemia. However, the availability of other, safer anesthetics now limits the use of benzocaine in major US centers. In addition, the topical anesthetic Emla (lidocaine plus prilocaine) has been recently reported as a cause of methemoglobinemia in infants and children.11,12
Also, potentially fatal methemoglobinemia has been reported in patients with a deficiency of G-6-phosphate dehydrogenase (G6PD) who received rasburicase, a recombinant version of urate oxidase enzyme used to prevent and treat tumor lysis syndrome.13,14
Lastly, methemoglobinemia has been reported in patients with inflammatory bowel disease treated with mesalamine.
Although this adverse reaction is rare, clinicians should be aware of it, since these agents are commonly used in everyday medical practice.15
RECOGNIZING THE DANGER SIGNS
The clinical manifestations of methemoglobinemia are directly proportional to the percentage of methemoglobin in red blood cells. Cyanosis generally becomes apparent at concentrations around 15%, at which point the patient may still have no symptoms. Anxiety, lightheadedness, tachycardia, and dizziness manifest at levels of 20% to 30%. Fatigue, confusion, dizziness, tachypnea, and worsening tachycardia occur at levels of 30% to 50%. Levels of 50% to 70% cause coma, seizures, arrhythmias, and acidosis, and levels over 70% are considered lethal.16
While these levels provide a general guideline of symptomatology in an otherwise healthy person, it is important to remember that patients with underlying conditions such as anemia, lung disease (both of which our patient had), sepsis, thalassemia, G6PD deficiency, and sickle cell disease can manifest symptoms at lower concentrations of methemoglobin.1,17
Most patients who develop clinically significant levels of methemoglobin do so within the first few hours of starting one of the culprit drugs.
DIAGNOSIS: METHEMOGLOBINEMIA AND THE SATURATION GAP
In patients with methemoglobinemia, pulse oximetry gives lower values than arterial blood gas oxygen measurements. Regular pulse oximetry works by measuring light absorbance at two distinct wavelengths (660 and 940 nm) to calculate the ratio of oxyhemoglobin to deoxyhemoglobin. Methemoglobin absorbs light at both these wavelengths, thus lowering the pulse oximetry values.1
In contrast, oxygen saturation of arterial blood gas (oxyhemoglobin) is calculated indirectly from the concentration of dissolved oxygen in the blood and does not include oxygen bound to hemoglobin. Therefore, the measured arterial oxygen saturation is often normal in patients with methemoglobinemia since it relies only on inspired oxygen content and is independent of the methemoglobin concentration.18
Oxygen supplementation can raise the level of oxyhemoglobin, which is a measure of dissolved oxygen, but the oxygen saturation as measured by pulse oximetry remains largely unchanged—ie, the saturation gap. A difference of more than 5% between the oxygen saturation by pulse oximetry and blood gas analysis is abnormal. Patients with clinically significant methemoglobinemia usually have a saturation gap greater than 10%.
Several other unique features should raise suspicion of methemoglobinemia. It should be considered in a patient presenting with cyanosis out of proportion to the oxygen saturation and in a patient with low oxygen saturation and a normal chest radiograph. Other clues include blood that is chocolate-colored on gross examination, rather than the dark red of deoxygenated blood.
Co-oximetry measures oxygen saturation using different wavelengths of light to distinguish between fractions of oxyhemoglobin, deoxyhemoglobin, and methemoglobin, but it is not widely available.
THE NEXT STEP
3. What is the next step in the management of our patient?
- Discontinue the dapsone
- Start methylene blue
- Start hyperbaric oxygen
- Give sodium thiosulfate
- Discontinue dapsone and start methylene blue
The next step in her management should be to stop the dapsone and start an infusion of methylene blue. Hyperbaric oxygen is used in treating carbon monoxide poisoning, and sodium thiosulfate is used in treating cyanide toxicity. They would not be appropriate in this patient’s care.
MANAGEMENT OF ACQUIRED METHEMOGLOBINEMIA
The first, most critical step in managing acquired methemoglobinemia is to immediately discontinue the suspected offending agent. In most patients without a concomitant condition such as anemia or lung disease and with a methemoglobin level below 20%, discontinuing the offending agent may suffice. Patients with a level of 20% or greater and patients with cardiac and pulmonary disease, who develop symptoms at lower concentrations of methemoglobin, require infusion of methylene blue.
Methylene blue is converted to its reduced form, leukomethylene blue, by NADPH-methemoglobin reductase. As it is oxidized, leukomethylene blue reduces methemoglobin to hemoglobin. A dose of 1 mg/kg intravenously is given at first. The response is usually dramatic, with a reduction in methemoglobin levels and improvement in symptoms often within 30 to 60 minutes. If levels remain high, the dose can be repeated 1 hour later.19
A caveat: methylene blue therapy should be avoided in patients with complete G6PD deficiency. Methylene blue works through the enzyme NADPH-methemoglobin reductase, and since patients with G6PD deficiency lack this enzyme, methylene blue is ineffective. In fact, since it cannot be reduced, excessive methylene blue can oxidize hemoglobin to methemoglobin, further exacerbating the condition. In patients with partial G6PD deficiency, methylene blue is still recommended as a first-line treatment, but at a lower initial dose (0.3–0.5 mg/kg). However, in patients with significant hemolysis, an exchange transfusion is the only treatment option.
CASE CONCLUDED
Since dapsone was identified as the likely cause of methemoglobinemia in our patient, it was immediately discontinued. Because she was symptomatic, 70 mg of methylene blue was given intravenously. Over the next 60 minutes, her clinical condition improved significantly. A repeat methemoglobin measurement was 3%.
She was discharged home the next day on oral antibiotics to complete treatment for community-acquired pneumonia.
TAKE-HOME POINTS
- Consider methemoglobinemia in a patient with unexplained cyanosis.
- Pulse oximetry gives lower values than arterial blood gas oxygen measurements in patients with methemoglobinemia, and pulse oximetry readings do not improve with supplemental oxygen.
- A saturation gap greater than 5% strongly suggests methemoglobinemia.
- The diagnosis of methemoglobinemia is confirmed by measuring the methemoglobin concentration.
- Most healthy patients develop symptoms at methemoglobin levels of 20%, but patients with comorbidities can develop symptoms at lower levels.
- A number of drugs can cause methemoglobinemia, even at therapeutic dosages.
- Treatment is generally indicated in patients who have symptoms or in healthy patients who have a methemoglobin level of 20% or greater.
- Identifying and promptly discontinuing the causative agent and initiating methylene blue infusion (1 mg/kg over 5 minutes) is the preferred treatment.
- Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth 2014; 28:1055–1059.
- Margulies DR, Manookian CM. Methemoglobinemia as a cause of respiratory failure. J Trauma 2002; 52:796–797.
- Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J 2011; 104:757–761.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Kanji HD, Mithani S, Boucher P, Dias VC, Yarema MC. Coma, metabolic acidosis, and methemoglobinemia in a patient with acetaminophen toxicity. J Popul Ther Clin Pharmacol 2013; 20:e207–e211.
- Kawasumi H, Tanaka E, Hoshi D, Kawaguchi Y, Yamanaka H. Methemoglobinemia induced by trimethoprim-sulfamethoxazole in a patient with systemic lupus erythematosus. Intern Med 2013; 52:1741–1743.
- Wieringa A, Bethlehem C, Hoogendoorn M, van der Maten J, van Roon EN. Very late recovery of dapsone-induced methemoglobinemia. Clin Toxicol (Phila) 2014; 52:80–81.
- Barclay JA, Ziemba SE, Ibrahim RB. Dapsone-induced methemoglobinemia: a primer for clinicians. Ann Pharmacother 2011; 45:1103–1115.
- Taleb M, Ashraf Z, Valavoor S, Tinkel J. Evaluation and management of acquired methemoglobinemia associated with topical benzocaine use. Am J Cardiovasc Drugs 2013; 13:325–330.
- Chowdhary S, Bukoye B, Bhansali AM, et al. Risk of topical anesthetic-induced methemoglobinemia: a 10-year retrospective case-control study. JAMA Intern Med 2013; 173:771–776.
- Larson A, Stidham T, Banerji S, Kaufman J. Seizures and methemoglobinemia in an infant after excessive EMLA application. Pediatr Emerg Care 2013; 29:377–379.
- Schmitt C, Matulic M, Kervégant M, et al. Methaemoglobinaemia in a child treated with Emla cream: circumstances and consequences of overdose [in French]. Ann Dermatol Venereol 2012; 139:824–827.
- Bucklin MH, Groth CM. Mortality following rasburicase-induced methemoglobinemia. Ann Pharmacother 2013; 47:1353–1358.
- Cheah CY, Lew TE, Seymour JF, Burbury K. Rasburicase causing severe oxidative hemolysis and methemoglobinemia in a patient with previously unrecognized glucose-6-phosphate dehydrogenase deficiency. Acta Haematol 2013; 130:254–259.
- Druez A, Rahier JF, Hébuterne X. Methaemoglobinaemia and renal failure following mesalazine for treatment of inflammatory bowel disease. J Crohns Colitis 2014; 8:900–901.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med 1999; 34:646–656.
- Groeper K, Katcher K, Tobias JD. Anesthetic management of a patient with methemoglobinemia. South Med J 2003; 96:504–509.
- Haymond S, Cariappa R, Eby CS, Scott MG. Laboratory assessment of oxygenation in methemoglobinemia. Clin Chem 2005; 51:434–444.
- Jang DH, Nelson LS, Hoffman RS. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol 2013; 9:242–249.
A 48-year-old woman presented to the emergency department after 2 days of nonproductive cough, chest discomfort, worsening shortness of breath, and subjective fever. She had a history of systemic sclerosis. She was currently taking prednisone 20 mg daily and aspirin 81 mg daily.
Physical examination revealed tachypnea (28 breaths per minute), and bronchial breath sounds in the left lower chest posteriorly.
The initial laboratory workup revealed:
- Hemoglobin 106 g/L (reference range 115–155)
- Mean corpuscular volume 84 fL (80–100)
- White blood cell count 29.4 × 109/L (3.70–11.0), with 85% neutrophils
- Platelet count 180 × 109/L (150–350)
- Lactate dehydrogenase 312 U/L (100–220).
Chest radiography showed opacification of the lower lobe of the left lung.
She was admitted to the hospital and started treatment with intravenous azithromycin and ceftriaxone for presumed community-acquired pneumonia, based on the clinical presentation and findings on chest radiography. Because of her immunosuppression (due to chronic prednisone therapy) and her high lactate dehydrogenase level, Pneumocystis jirovecii pneumonia was suspected, and because she had a history of allergy to trimethoprim-sulfamethoxazole and pentamidine, she was started on dapsone.
During the next 24 hours, she developed worsening dyspnea, hypoxia, and cyanosis. She was placed on an air-entrainment mask, with a fraction of inspired oxygen of 0.5. Pulse oximetry showed an oxygen saturation of 85%, but arterial blood gas analysis indicated an oxyhemoglobin concentration of 95%.
THE ‘SATURATION GAP’
1. Which is most likely to have caused the discrepancy between the oxyhemoglobin concentration and the oxygen saturation by pulse oximetry in this patient?
- Methemoglobinemia
- Carbon monoxide poisoning
- Inappropriate placement of the pulse oximeter probe
- Pulmonary embolism
Methemoglobinemia is the most likely cause of the discrepancy between the oxyhemoglobin levels and the oxygen saturation by pulse oximetry, a phenomenon also known as the “saturation gap.” Other common causes are cyanide poisoning and carbon monoxide poisoning.
Carbon monoxide poisoning, however, does not explain our patient’s cyanosis. On the contrary, carbon monoxide poisoning can actually cause the patient’s lips and mucous membranes to appear unnaturally bright pink. Also, carbon monoxide poisoning raises the blood concentration of carboxyhemoglobin (which has a high affinity for oxygen), and this usually causes pulse oximetry to read inappropriately high, whereas in our patient it read low.
Incorrect placement of the pulse oximeter probe can result in an inaccurate measurement of oxygen saturation. Visualization of the waveform on the plethysmograph or the signal quality index can be used to assess adequate placement of the pulse oximeter probe. However, inadequate probe placement does not explain our patient’s dyspnea and cyanosis.
Pulmonary embolism can lead to hypoxia as a result of ventilation-perfusion mismatch. However, pulmonary embolism leading to low oxygen saturation on pulse oximetry will also lead to concomitantly low oxyhemoglobin levels as measured by arterial blood gas analysis, and this was not seen in our patient.
BACK TO OUR PATIENT
Because there was a discrepancy between our patient’s pulse oximetry reading and oxyhemoglobin concentration by arterial blood gas measurement, her methemoglobin level was checked and was found to be 30%, thus confirming the diagnosis of methemoglobinemia.
WHAT IS METHEMOGLOBINEMIA, AND WHAT CAUSES IT?
Oxygen is normally bound to iron in its ferrous (Fe2+) form in hemoglobin to form oxyhemoglobin. Oxidative stress in the body can cause iron to change from the ferrous to the ferric (Fe3+) state, forming methemoglobin. Methemoglobin is normally present in the blood in low levels (< 1% of the total hemoglobin), and ferric iron is reduced and recycled back to the ferrous form by NADH-cytochrome b5 reductase, an enzyme present in red blood cells. This protective mechanism maintains methemoglobin levels within safe limits. But increased production can lead to accumulation of methemoglobin, resulting in dyspnea and hypoxia and the condition referred to as methemoglobinemia.1
Increased levels of methemoglobin relative to normal hemoglobin cause tissue hypoxia by several mechanisms. Methemoglobin cannot efficiently carry oxygen; instead, it binds to water or to a hydroxide ion depending on the pH of the environment.2 Therefore, the hemoglobin molecule does not carry its usual load of oxygen, and hypoxia results from the reduced delivery of oxygen to tissues. In addition, an increased concentration of methemoglobin causes a leftward shift in the oxygen-hemoglobin dissociation curve, representing an increased affinity to bound oxygen in the remaining heme groups. The tightly bound oxygen is not adequately released at the tissue level, thus causing cellular hypoxia.
Methemoglobinemia is most often caused by exposure to an oxidizing chemical or drug that increases production of methemoglobin. In rare cases, it is caused by a congenital deficiency of NADH-cytochrome b5 reductase.3
2. Which of the following drugs can cause methemoglobinemia?
- Acetaminophen
- Dapsone
- Benzocaine
- Primaquine
All four of these drugs are common culprits for causing acquired methemoglobinemia; others include chloroquine, nitroglycerin, and sulfonamides.4–6
The increased production of methemoglobin caused by these drugs overwhelms the protective effect of reducing enzymes and can lead to an accumulation of methemoglobin. However, because of variability in cellular metabolism, not every person who takes these drugs develops dangerous levels of methemoglobin.
Dapsone and benzocaine are the most commonly encountered drugs known to cause methemoglobinemia (Table 1). Dapsone is an anti-inflammatory and antimicrobial agent most commonly used for treating lepromatous leprosy and dermatitis herpetiformis. It is also often prescribed for prophylaxis and treatment of P jirovecii pneumonia in immunosuppressed individuals.7 Benzocaine is a local anesthetic and was commonly used before procedures such as oral or dental surgery, transesophageal echocardiography, and endoscopy.8–10 Even low doses of benzocaine can lead to high levels of methemoglobinemia. However, the availability of other, safer anesthetics now limits the use of benzocaine in major US centers. In addition, the topical anesthetic Emla (lidocaine plus prilocaine) has been recently reported as a cause of methemoglobinemia in infants and children.11,12
Also, potentially fatal methemoglobinemia has been reported in patients with a deficiency of G-6-phosphate dehydrogenase (G6PD) who received rasburicase, a recombinant version of urate oxidase enzyme used to prevent and treat tumor lysis syndrome.13,14
Lastly, methemoglobinemia has been reported in patients with inflammatory bowel disease treated with mesalamine.
Although this adverse reaction is rare, clinicians should be aware of it, since these agents are commonly used in everyday medical practice.15
RECOGNIZING THE DANGER SIGNS
The clinical manifestations of methemoglobinemia are directly proportional to the percentage of methemoglobin in red blood cells. Cyanosis generally becomes apparent at concentrations around 15%, at which point the patient may still have no symptoms. Anxiety, lightheadedness, tachycardia, and dizziness manifest at levels of 20% to 30%. Fatigue, confusion, dizziness, tachypnea, and worsening tachycardia occur at levels of 30% to 50%. Levels of 50% to 70% cause coma, seizures, arrhythmias, and acidosis, and levels over 70% are considered lethal.16
While these levels provide a general guideline of symptomatology in an otherwise healthy person, it is important to remember that patients with underlying conditions such as anemia, lung disease (both of which our patient had), sepsis, thalassemia, G6PD deficiency, and sickle cell disease can manifest symptoms at lower concentrations of methemoglobin.1,17
Most patients who develop clinically significant levels of methemoglobin do so within the first few hours of starting one of the culprit drugs.
DIAGNOSIS: METHEMOGLOBINEMIA AND THE SATURATION GAP
In patients with methemoglobinemia, pulse oximetry gives lower values than arterial blood gas oxygen measurements. Regular pulse oximetry works by measuring light absorbance at two distinct wavelengths (660 and 940 nm) to calculate the ratio of oxyhemoglobin to deoxyhemoglobin. Methemoglobin absorbs light at both these wavelengths, thus lowering the pulse oximetry values.1
In contrast, oxygen saturation of arterial blood gas (oxyhemoglobin) is calculated indirectly from the concentration of dissolved oxygen in the blood and does not include oxygen bound to hemoglobin. Therefore, the measured arterial oxygen saturation is often normal in patients with methemoglobinemia since it relies only on inspired oxygen content and is independent of the methemoglobin concentration.18
Oxygen supplementation can raise the level of oxyhemoglobin, which is a measure of dissolved oxygen, but the oxygen saturation as measured by pulse oximetry remains largely unchanged—ie, the saturation gap. A difference of more than 5% between the oxygen saturation by pulse oximetry and blood gas analysis is abnormal. Patients with clinically significant methemoglobinemia usually have a saturation gap greater than 10%.
Several other unique features should raise suspicion of methemoglobinemia. It should be considered in a patient presenting with cyanosis out of proportion to the oxygen saturation and in a patient with low oxygen saturation and a normal chest radiograph. Other clues include blood that is chocolate-colored on gross examination, rather than the dark red of deoxygenated blood.
Co-oximetry measures oxygen saturation using different wavelengths of light to distinguish between fractions of oxyhemoglobin, deoxyhemoglobin, and methemoglobin, but it is not widely available.
THE NEXT STEP
3. What is the next step in the management of our patient?
- Discontinue the dapsone
- Start methylene blue
- Start hyperbaric oxygen
- Give sodium thiosulfate
- Discontinue dapsone and start methylene blue
The next step in her management should be to stop the dapsone and start an infusion of methylene blue. Hyperbaric oxygen is used in treating carbon monoxide poisoning, and sodium thiosulfate is used in treating cyanide toxicity. They would not be appropriate in this patient’s care.
MANAGEMENT OF ACQUIRED METHEMOGLOBINEMIA
The first, most critical step in managing acquired methemoglobinemia is to immediately discontinue the suspected offending agent. In most patients without a concomitant condition such as anemia or lung disease and with a methemoglobin level below 20%, discontinuing the offending agent may suffice. Patients with a level of 20% or greater and patients with cardiac and pulmonary disease, who develop symptoms at lower concentrations of methemoglobin, require infusion of methylene blue.
Methylene blue is converted to its reduced form, leukomethylene blue, by NADPH-methemoglobin reductase. As it is oxidized, leukomethylene blue reduces methemoglobin to hemoglobin. A dose of 1 mg/kg intravenously is given at first. The response is usually dramatic, with a reduction in methemoglobin levels and improvement in symptoms often within 30 to 60 minutes. If levels remain high, the dose can be repeated 1 hour later.19
A caveat: methylene blue therapy should be avoided in patients with complete G6PD deficiency. Methylene blue works through the enzyme NADPH-methemoglobin reductase, and since patients with G6PD deficiency lack this enzyme, methylene blue is ineffective. In fact, since it cannot be reduced, excessive methylene blue can oxidize hemoglobin to methemoglobin, further exacerbating the condition. In patients with partial G6PD deficiency, methylene blue is still recommended as a first-line treatment, but at a lower initial dose (0.3–0.5 mg/kg). However, in patients with significant hemolysis, an exchange transfusion is the only treatment option.
CASE CONCLUDED
Since dapsone was identified as the likely cause of methemoglobinemia in our patient, it was immediately discontinued. Because she was symptomatic, 70 mg of methylene blue was given intravenously. Over the next 60 minutes, her clinical condition improved significantly. A repeat methemoglobin measurement was 3%.
She was discharged home the next day on oral antibiotics to complete treatment for community-acquired pneumonia.
TAKE-HOME POINTS
- Consider methemoglobinemia in a patient with unexplained cyanosis.
- Pulse oximetry gives lower values than arterial blood gas oxygen measurements in patients with methemoglobinemia, and pulse oximetry readings do not improve with supplemental oxygen.
- A saturation gap greater than 5% strongly suggests methemoglobinemia.
- The diagnosis of methemoglobinemia is confirmed by measuring the methemoglobin concentration.
- Most healthy patients develop symptoms at methemoglobin levels of 20%, but patients with comorbidities can develop symptoms at lower levels.
- A number of drugs can cause methemoglobinemia, even at therapeutic dosages.
- Treatment is generally indicated in patients who have symptoms or in healthy patients who have a methemoglobin level of 20% or greater.
- Identifying and promptly discontinuing the causative agent and initiating methylene blue infusion (1 mg/kg over 5 minutes) is the preferred treatment.
A 48-year-old woman presented to the emergency department after 2 days of nonproductive cough, chest discomfort, worsening shortness of breath, and subjective fever. She had a history of systemic sclerosis. She was currently taking prednisone 20 mg daily and aspirin 81 mg daily.
Physical examination revealed tachypnea (28 breaths per minute), and bronchial breath sounds in the left lower chest posteriorly.
The initial laboratory workup revealed:
- Hemoglobin 106 g/L (reference range 115–155)
- Mean corpuscular volume 84 fL (80–100)
- White blood cell count 29.4 × 109/L (3.70–11.0), with 85% neutrophils
- Platelet count 180 × 109/L (150–350)
- Lactate dehydrogenase 312 U/L (100–220).
Chest radiography showed opacification of the lower lobe of the left lung.
She was admitted to the hospital and started treatment with intravenous azithromycin and ceftriaxone for presumed community-acquired pneumonia, based on the clinical presentation and findings on chest radiography. Because of her immunosuppression (due to chronic prednisone therapy) and her high lactate dehydrogenase level, Pneumocystis jirovecii pneumonia was suspected, and because she had a history of allergy to trimethoprim-sulfamethoxazole and pentamidine, she was started on dapsone.
During the next 24 hours, she developed worsening dyspnea, hypoxia, and cyanosis. She was placed on an air-entrainment mask, with a fraction of inspired oxygen of 0.5. Pulse oximetry showed an oxygen saturation of 85%, but arterial blood gas analysis indicated an oxyhemoglobin concentration of 95%.
THE ‘SATURATION GAP’
1. Which is most likely to have caused the discrepancy between the oxyhemoglobin concentration and the oxygen saturation by pulse oximetry in this patient?
- Methemoglobinemia
- Carbon monoxide poisoning
- Inappropriate placement of the pulse oximeter probe
- Pulmonary embolism
Methemoglobinemia is the most likely cause of the discrepancy between the oxyhemoglobin levels and the oxygen saturation by pulse oximetry, a phenomenon also known as the “saturation gap.” Other common causes are cyanide poisoning and carbon monoxide poisoning.
Carbon monoxide poisoning, however, does not explain our patient’s cyanosis. On the contrary, carbon monoxide poisoning can actually cause the patient’s lips and mucous membranes to appear unnaturally bright pink. Also, carbon monoxide poisoning raises the blood concentration of carboxyhemoglobin (which has a high affinity for oxygen), and this usually causes pulse oximetry to read inappropriately high, whereas in our patient it read low.
Incorrect placement of the pulse oximeter probe can result in an inaccurate measurement of oxygen saturation. Visualization of the waveform on the plethysmograph or the signal quality index can be used to assess adequate placement of the pulse oximeter probe. However, inadequate probe placement does not explain our patient’s dyspnea and cyanosis.
Pulmonary embolism can lead to hypoxia as a result of ventilation-perfusion mismatch. However, pulmonary embolism leading to low oxygen saturation on pulse oximetry will also lead to concomitantly low oxyhemoglobin levels as measured by arterial blood gas analysis, and this was not seen in our patient.
BACK TO OUR PATIENT
Because there was a discrepancy between our patient’s pulse oximetry reading and oxyhemoglobin concentration by arterial blood gas measurement, her methemoglobin level was checked and was found to be 30%, thus confirming the diagnosis of methemoglobinemia.
WHAT IS METHEMOGLOBINEMIA, AND WHAT CAUSES IT?
Oxygen is normally bound to iron in its ferrous (Fe2+) form in hemoglobin to form oxyhemoglobin. Oxidative stress in the body can cause iron to change from the ferrous to the ferric (Fe3+) state, forming methemoglobin. Methemoglobin is normally present in the blood in low levels (< 1% of the total hemoglobin), and ferric iron is reduced and recycled back to the ferrous form by NADH-cytochrome b5 reductase, an enzyme present in red blood cells. This protective mechanism maintains methemoglobin levels within safe limits. But increased production can lead to accumulation of methemoglobin, resulting in dyspnea and hypoxia and the condition referred to as methemoglobinemia.1
Increased levels of methemoglobin relative to normal hemoglobin cause tissue hypoxia by several mechanisms. Methemoglobin cannot efficiently carry oxygen; instead, it binds to water or to a hydroxide ion depending on the pH of the environment.2 Therefore, the hemoglobin molecule does not carry its usual load of oxygen, and hypoxia results from the reduced delivery of oxygen to tissues. In addition, an increased concentration of methemoglobin causes a leftward shift in the oxygen-hemoglobin dissociation curve, representing an increased affinity to bound oxygen in the remaining heme groups. The tightly bound oxygen is not adequately released at the tissue level, thus causing cellular hypoxia.
Methemoglobinemia is most often caused by exposure to an oxidizing chemical or drug that increases production of methemoglobin. In rare cases, it is caused by a congenital deficiency of NADH-cytochrome b5 reductase.3
2. Which of the following drugs can cause methemoglobinemia?
- Acetaminophen
- Dapsone
- Benzocaine
- Primaquine
All four of these drugs are common culprits for causing acquired methemoglobinemia; others include chloroquine, nitroglycerin, and sulfonamides.4–6
The increased production of methemoglobin caused by these drugs overwhelms the protective effect of reducing enzymes and can lead to an accumulation of methemoglobin. However, because of variability in cellular metabolism, not every person who takes these drugs develops dangerous levels of methemoglobin.
Dapsone and benzocaine are the most commonly encountered drugs known to cause methemoglobinemia (Table 1). Dapsone is an anti-inflammatory and antimicrobial agent most commonly used for treating lepromatous leprosy and dermatitis herpetiformis. It is also often prescribed for prophylaxis and treatment of P jirovecii pneumonia in immunosuppressed individuals.7 Benzocaine is a local anesthetic and was commonly used before procedures such as oral or dental surgery, transesophageal echocardiography, and endoscopy.8–10 Even low doses of benzocaine can lead to high levels of methemoglobinemia. However, the availability of other, safer anesthetics now limits the use of benzocaine in major US centers. In addition, the topical anesthetic Emla (lidocaine plus prilocaine) has been recently reported as a cause of methemoglobinemia in infants and children.11,12
Also, potentially fatal methemoglobinemia has been reported in patients with a deficiency of G-6-phosphate dehydrogenase (G6PD) who received rasburicase, a recombinant version of urate oxidase enzyme used to prevent and treat tumor lysis syndrome.13,14
Lastly, methemoglobinemia has been reported in patients with inflammatory bowel disease treated with mesalamine.
Although this adverse reaction is rare, clinicians should be aware of it, since these agents are commonly used in everyday medical practice.15
RECOGNIZING THE DANGER SIGNS
The clinical manifestations of methemoglobinemia are directly proportional to the percentage of methemoglobin in red blood cells. Cyanosis generally becomes apparent at concentrations around 15%, at which point the patient may still have no symptoms. Anxiety, lightheadedness, tachycardia, and dizziness manifest at levels of 20% to 30%. Fatigue, confusion, dizziness, tachypnea, and worsening tachycardia occur at levels of 30% to 50%. Levels of 50% to 70% cause coma, seizures, arrhythmias, and acidosis, and levels over 70% are considered lethal.16
While these levels provide a general guideline of symptomatology in an otherwise healthy person, it is important to remember that patients with underlying conditions such as anemia, lung disease (both of which our patient had), sepsis, thalassemia, G6PD deficiency, and sickle cell disease can manifest symptoms at lower concentrations of methemoglobin.1,17
Most patients who develop clinically significant levels of methemoglobin do so within the first few hours of starting one of the culprit drugs.
DIAGNOSIS: METHEMOGLOBINEMIA AND THE SATURATION GAP
In patients with methemoglobinemia, pulse oximetry gives lower values than arterial blood gas oxygen measurements. Regular pulse oximetry works by measuring light absorbance at two distinct wavelengths (660 and 940 nm) to calculate the ratio of oxyhemoglobin to deoxyhemoglobin. Methemoglobin absorbs light at both these wavelengths, thus lowering the pulse oximetry values.1
In contrast, oxygen saturation of arterial blood gas (oxyhemoglobin) is calculated indirectly from the concentration of dissolved oxygen in the blood and does not include oxygen bound to hemoglobin. Therefore, the measured arterial oxygen saturation is often normal in patients with methemoglobinemia since it relies only on inspired oxygen content and is independent of the methemoglobin concentration.18
Oxygen supplementation can raise the level of oxyhemoglobin, which is a measure of dissolved oxygen, but the oxygen saturation as measured by pulse oximetry remains largely unchanged—ie, the saturation gap. A difference of more than 5% between the oxygen saturation by pulse oximetry and blood gas analysis is abnormal. Patients with clinically significant methemoglobinemia usually have a saturation gap greater than 10%.
Several other unique features should raise suspicion of methemoglobinemia. It should be considered in a patient presenting with cyanosis out of proportion to the oxygen saturation and in a patient with low oxygen saturation and a normal chest radiograph. Other clues include blood that is chocolate-colored on gross examination, rather than the dark red of deoxygenated blood.
Co-oximetry measures oxygen saturation using different wavelengths of light to distinguish between fractions of oxyhemoglobin, deoxyhemoglobin, and methemoglobin, but it is not widely available.
THE NEXT STEP
3. What is the next step in the management of our patient?
- Discontinue the dapsone
- Start methylene blue
- Start hyperbaric oxygen
- Give sodium thiosulfate
- Discontinue dapsone and start methylene blue
The next step in her management should be to stop the dapsone and start an infusion of methylene blue. Hyperbaric oxygen is used in treating carbon monoxide poisoning, and sodium thiosulfate is used in treating cyanide toxicity. They would not be appropriate in this patient’s care.
MANAGEMENT OF ACQUIRED METHEMOGLOBINEMIA
The first, most critical step in managing acquired methemoglobinemia is to immediately discontinue the suspected offending agent. In most patients without a concomitant condition such as anemia or lung disease and with a methemoglobin level below 20%, discontinuing the offending agent may suffice. Patients with a level of 20% or greater and patients with cardiac and pulmonary disease, who develop symptoms at lower concentrations of methemoglobin, require infusion of methylene blue.
Methylene blue is converted to its reduced form, leukomethylene blue, by NADPH-methemoglobin reductase. As it is oxidized, leukomethylene blue reduces methemoglobin to hemoglobin. A dose of 1 mg/kg intravenously is given at first. The response is usually dramatic, with a reduction in methemoglobin levels and improvement in symptoms often within 30 to 60 minutes. If levels remain high, the dose can be repeated 1 hour later.19
A caveat: methylene blue therapy should be avoided in patients with complete G6PD deficiency. Methylene blue works through the enzyme NADPH-methemoglobin reductase, and since patients with G6PD deficiency lack this enzyme, methylene blue is ineffective. In fact, since it cannot be reduced, excessive methylene blue can oxidize hemoglobin to methemoglobin, further exacerbating the condition. In patients with partial G6PD deficiency, methylene blue is still recommended as a first-line treatment, but at a lower initial dose (0.3–0.5 mg/kg). However, in patients with significant hemolysis, an exchange transfusion is the only treatment option.
CASE CONCLUDED
Since dapsone was identified as the likely cause of methemoglobinemia in our patient, it was immediately discontinued. Because she was symptomatic, 70 mg of methylene blue was given intravenously. Over the next 60 minutes, her clinical condition improved significantly. A repeat methemoglobin measurement was 3%.
She was discharged home the next day on oral antibiotics to complete treatment for community-acquired pneumonia.
TAKE-HOME POINTS
- Consider methemoglobinemia in a patient with unexplained cyanosis.
- Pulse oximetry gives lower values than arterial blood gas oxygen measurements in patients with methemoglobinemia, and pulse oximetry readings do not improve with supplemental oxygen.
- A saturation gap greater than 5% strongly suggests methemoglobinemia.
- The diagnosis of methemoglobinemia is confirmed by measuring the methemoglobin concentration.
- Most healthy patients develop symptoms at methemoglobin levels of 20%, but patients with comorbidities can develop symptoms at lower levels.
- A number of drugs can cause methemoglobinemia, even at therapeutic dosages.
- Treatment is generally indicated in patients who have symptoms or in healthy patients who have a methemoglobin level of 20% or greater.
- Identifying and promptly discontinuing the causative agent and initiating methylene blue infusion (1 mg/kg over 5 minutes) is the preferred treatment.
- Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth 2014; 28:1055–1059.
- Margulies DR, Manookian CM. Methemoglobinemia as a cause of respiratory failure. J Trauma 2002; 52:796–797.
- Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J 2011; 104:757–761.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Kanji HD, Mithani S, Boucher P, Dias VC, Yarema MC. Coma, metabolic acidosis, and methemoglobinemia in a patient with acetaminophen toxicity. J Popul Ther Clin Pharmacol 2013; 20:e207–e211.
- Kawasumi H, Tanaka E, Hoshi D, Kawaguchi Y, Yamanaka H. Methemoglobinemia induced by trimethoprim-sulfamethoxazole in a patient with systemic lupus erythematosus. Intern Med 2013; 52:1741–1743.
- Wieringa A, Bethlehem C, Hoogendoorn M, van der Maten J, van Roon EN. Very late recovery of dapsone-induced methemoglobinemia. Clin Toxicol (Phila) 2014; 52:80–81.
- Barclay JA, Ziemba SE, Ibrahim RB. Dapsone-induced methemoglobinemia: a primer for clinicians. Ann Pharmacother 2011; 45:1103–1115.
- Taleb M, Ashraf Z, Valavoor S, Tinkel J. Evaluation and management of acquired methemoglobinemia associated with topical benzocaine use. Am J Cardiovasc Drugs 2013; 13:325–330.
- Chowdhary S, Bukoye B, Bhansali AM, et al. Risk of topical anesthetic-induced methemoglobinemia: a 10-year retrospective case-control study. JAMA Intern Med 2013; 173:771–776.
- Larson A, Stidham T, Banerji S, Kaufman J. Seizures and methemoglobinemia in an infant after excessive EMLA application. Pediatr Emerg Care 2013; 29:377–379.
- Schmitt C, Matulic M, Kervégant M, et al. Methaemoglobinaemia in a child treated with Emla cream: circumstances and consequences of overdose [in French]. Ann Dermatol Venereol 2012; 139:824–827.
- Bucklin MH, Groth CM. Mortality following rasburicase-induced methemoglobinemia. Ann Pharmacother 2013; 47:1353–1358.
- Cheah CY, Lew TE, Seymour JF, Burbury K. Rasburicase causing severe oxidative hemolysis and methemoglobinemia in a patient with previously unrecognized glucose-6-phosphate dehydrogenase deficiency. Acta Haematol 2013; 130:254–259.
- Druez A, Rahier JF, Hébuterne X. Methaemoglobinaemia and renal failure following mesalazine for treatment of inflammatory bowel disease. J Crohns Colitis 2014; 8:900–901.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med 1999; 34:646–656.
- Groeper K, Katcher K, Tobias JD. Anesthetic management of a patient with methemoglobinemia. South Med J 2003; 96:504–509.
- Haymond S, Cariappa R, Eby CS, Scott MG. Laboratory assessment of oxygenation in methemoglobinemia. Clin Chem 2005; 51:434–444.
- Jang DH, Nelson LS, Hoffman RS. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol 2013; 9:242–249.
- Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth 2014; 28:1055–1059.
- Margulies DR, Manookian CM. Methemoglobinemia as a cause of respiratory failure. J Trauma 2002; 52:796–797.
- Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J 2011; 104:757–761.
- Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004; 83:265–273.
- Kanji HD, Mithani S, Boucher P, Dias VC, Yarema MC. Coma, metabolic acidosis, and methemoglobinemia in a patient with acetaminophen toxicity. J Popul Ther Clin Pharmacol 2013; 20:e207–e211.
- Kawasumi H, Tanaka E, Hoshi D, Kawaguchi Y, Yamanaka H. Methemoglobinemia induced by trimethoprim-sulfamethoxazole in a patient with systemic lupus erythematosus. Intern Med 2013; 52:1741–1743.
- Wieringa A, Bethlehem C, Hoogendoorn M, van der Maten J, van Roon EN. Very late recovery of dapsone-induced methemoglobinemia. Clin Toxicol (Phila) 2014; 52:80–81.
- Barclay JA, Ziemba SE, Ibrahim RB. Dapsone-induced methemoglobinemia: a primer for clinicians. Ann Pharmacother 2011; 45:1103–1115.
- Taleb M, Ashraf Z, Valavoor S, Tinkel J. Evaluation and management of acquired methemoglobinemia associated with topical benzocaine use. Am J Cardiovasc Drugs 2013; 13:325–330.
- Chowdhary S, Bukoye B, Bhansali AM, et al. Risk of topical anesthetic-induced methemoglobinemia: a 10-year retrospective case-control study. JAMA Intern Med 2013; 173:771–776.
- Larson A, Stidham T, Banerji S, Kaufman J. Seizures and methemoglobinemia in an infant after excessive EMLA application. Pediatr Emerg Care 2013; 29:377–379.
- Schmitt C, Matulic M, Kervégant M, et al. Methaemoglobinaemia in a child treated with Emla cream: circumstances and consequences of overdose [in French]. Ann Dermatol Venereol 2012; 139:824–827.
- Bucklin MH, Groth CM. Mortality following rasburicase-induced methemoglobinemia. Ann Pharmacother 2013; 47:1353–1358.
- Cheah CY, Lew TE, Seymour JF, Burbury K. Rasburicase causing severe oxidative hemolysis and methemoglobinemia in a patient with previously unrecognized glucose-6-phosphate dehydrogenase deficiency. Acta Haematol 2013; 130:254–259.
- Druez A, Rahier JF, Hébuterne X. Methaemoglobinaemia and renal failure following mesalazine for treatment of inflammatory bowel disease. J Crohns Colitis 2014; 8:900–901.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med 1999; 34:646–656.
- Groeper K, Katcher K, Tobias JD. Anesthetic management of a patient with methemoglobinemia. South Med J 2003; 96:504–509.
- Haymond S, Cariappa R, Eby CS, Scott MG. Laboratory assessment of oxygenation in methemoglobinemia. Clin Chem 2005; 51:434–444.
- Jang DH, Nelson LS, Hoffman RS. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol 2013; 9:242–249.
Why are we doing cardiovascular outcome trials in type 2 diabetes?
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
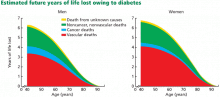
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
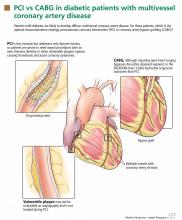
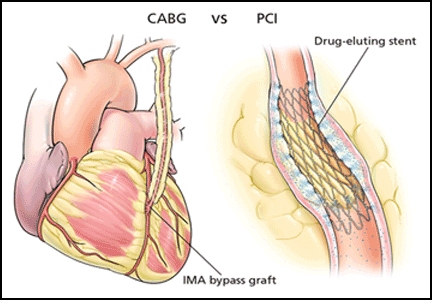
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
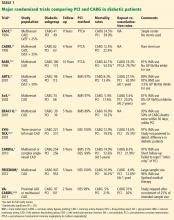
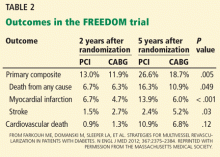
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
KEY POINTS
- The worldwide burden of type 2 diabetes is increasing dramatically as obesity rates increase, populations age, and people around the world adopt a Western diet.
- Diabetes increases the risk of atherosclerotic cardiovascular disease, which remains the leading cause of death in diabetic patients.
- Lowering blood glucose alone may not necessarily amount to reduction in adverse cardiovascular events.
- Clinical trials of new drugs for type 2 diabetes must prove cardiovascular safety in addition to glucose-lowering potential before the drugs gain final regulatory approval.
- Aggressive risk factor modification (smoking cessation, control of hypertension, and treatment of hyperlipidemia with statins) remains paramount in reducing cardiovascular risk in people with diabetes.
In reply: The FREEDOM trial
In Reply: We appreciate the comments of Dr. Saeed and colleagues. As stated in our article, given that the patients included in the FREEDOM trial represent a select group with diabetes and multivessel coronary artery disease, they may not represent all patients encountered in a real-world setting. We highlighted that only 10% of the patients screened were included for randomization, which limits the generalizability of the results. Also, the overall patient population may not be at high risk, as evidenced by low mean EuroSCORE and SYNTAX scores and by the low proportion of patients with ejection fractions less than 40%. However, patients with left main coronary artery disease (even without diabetes) have been shown to have better outcomes with coronary artery bypass grafting than with PCI, although a head-to-head trial in a diabetic subgroup is currently not available.1,2 In addition, it is important to realize that the FREEDOM trial deals with stable angina; therefore, the results may not extend to patients with acute coronary syndrome wherein primary PCI remains the most feasible option in most cases.
Diabetes mellitus is independently associated with complex, accelerated, and multifocal coronary artery disease. Therefore, outcomes after revascularization (with bypass grafting or PCI) are worse in diabetic patients than in those without diabetes. However, this association does not prove the superiority of PCI over bypass grafting.
As we stated in our paper, the FREEDOM trial did not clearly define the strategy for arterial grafts in patients undergoing bypass grafting. The mean number of coronary lesions in the bypass grafting group was high (mean = 5.74), but the average number of grafts used was only 2.9, and data were not provided on the use of sequential grafting and multiple arterial conduits. Lastly, it is true that the FREEDOM trial had relatively fewer patients (18.5%) that underwent off-pump bypass grafting surgery; however, this approach has never been shown to be superior in large randomized trials.3,4
In conclusion, no randomized trial should replace clinical judgment to define the targeted revascularization strategy for an individual patient. Rather, results from the FREEDOM trial should help support clinical decision-making in the context of the patient and the institution.
- Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009; 373:1190–1197.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Diegeler A, Börgermann J, Kappert U, et al. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. N Engl J Med 2013; 368:1189–1198.
- Lamy A, Devereaux PJ, Prabhakaran D, et al; CORONARY Investigators. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med 2013; 368:1179–1188.
In Reply: We appreciate the comments of Dr. Saeed and colleagues. As stated in our article, given that the patients included in the FREEDOM trial represent a select group with diabetes and multivessel coronary artery disease, they may not represent all patients encountered in a real-world setting. We highlighted that only 10% of the patients screened were included for randomization, which limits the generalizability of the results. Also, the overall patient population may not be at high risk, as evidenced by low mean EuroSCORE and SYNTAX scores and by the low proportion of patients with ejection fractions less than 40%. However, patients with left main coronary artery disease (even without diabetes) have been shown to have better outcomes with coronary artery bypass grafting than with PCI, although a head-to-head trial in a diabetic subgroup is currently not available.1,2 In addition, it is important to realize that the FREEDOM trial deals with stable angina; therefore, the results may not extend to patients with acute coronary syndrome wherein primary PCI remains the most feasible option in most cases.
Diabetes mellitus is independently associated with complex, accelerated, and multifocal coronary artery disease. Therefore, outcomes after revascularization (with bypass grafting or PCI) are worse in diabetic patients than in those without diabetes. However, this association does not prove the superiority of PCI over bypass grafting.
As we stated in our paper, the FREEDOM trial did not clearly define the strategy for arterial grafts in patients undergoing bypass grafting. The mean number of coronary lesions in the bypass grafting group was high (mean = 5.74), but the average number of grafts used was only 2.9, and data were not provided on the use of sequential grafting and multiple arterial conduits. Lastly, it is true that the FREEDOM trial had relatively fewer patients (18.5%) that underwent off-pump bypass grafting surgery; however, this approach has never been shown to be superior in large randomized trials.3,4
In conclusion, no randomized trial should replace clinical judgment to define the targeted revascularization strategy for an individual patient. Rather, results from the FREEDOM trial should help support clinical decision-making in the context of the patient and the institution.
In Reply: We appreciate the comments of Dr. Saeed and colleagues. As stated in our article, given that the patients included in the FREEDOM trial represent a select group with diabetes and multivessel coronary artery disease, they may not represent all patients encountered in a real-world setting. We highlighted that only 10% of the patients screened were included for randomization, which limits the generalizability of the results. Also, the overall patient population may not be at high risk, as evidenced by low mean EuroSCORE and SYNTAX scores and by the low proportion of patients with ejection fractions less than 40%. However, patients with left main coronary artery disease (even without diabetes) have been shown to have better outcomes with coronary artery bypass grafting than with PCI, although a head-to-head trial in a diabetic subgroup is currently not available.1,2 In addition, it is important to realize that the FREEDOM trial deals with stable angina; therefore, the results may not extend to patients with acute coronary syndrome wherein primary PCI remains the most feasible option in most cases.
Diabetes mellitus is independently associated with complex, accelerated, and multifocal coronary artery disease. Therefore, outcomes after revascularization (with bypass grafting or PCI) are worse in diabetic patients than in those without diabetes. However, this association does not prove the superiority of PCI over bypass grafting.
As we stated in our paper, the FREEDOM trial did not clearly define the strategy for arterial grafts in patients undergoing bypass grafting. The mean number of coronary lesions in the bypass grafting group was high (mean = 5.74), but the average number of grafts used was only 2.9, and data were not provided on the use of sequential grafting and multiple arterial conduits. Lastly, it is true that the FREEDOM trial had relatively fewer patients (18.5%) that underwent off-pump bypass grafting surgery; however, this approach has never been shown to be superior in large randomized trials.3,4
In conclusion, no randomized trial should replace clinical judgment to define the targeted revascularization strategy for an individual patient. Rather, results from the FREEDOM trial should help support clinical decision-making in the context of the patient and the institution.
- Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009; 373:1190–1197.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Diegeler A, Börgermann J, Kappert U, et al. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. N Engl J Med 2013; 368:1189–1198.
- Lamy A, Devereaux PJ, Prabhakaran D, et al; CORONARY Investigators. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med 2013; 368:1179–1188.
- Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009; 373:1190–1197.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Diegeler A, Börgermann J, Kappert U, et al. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. N Engl J Med 2013; 368:1189–1198.
- Lamy A, Devereaux PJ, Prabhakaran D, et al; CORONARY Investigators. Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med 2013; 368:1179–1188.
The FREEDOM trial: In appropriate patients with diabetes and multivessel coronary artery disease, CABG beats PCI
Many patients with diabetes mellitus develop complex, accelerated, multifocal coronary artery disease. Moreover, if they undergo revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), their risk of morbidity and death afterward is higher than in those without diabetes.1,2
Over the last 2 decades, CABG and PCI have advanced significantly, as have antithrombotic therapy and drug therapies to modify cardiovascular risk factors such as hyperlipidemia, hypertension, and diabetes.
Several earlier studies showed CABG to be more beneficial than PCI in diabetic patients with multivessel coronary artery disease.3–5 However, the topic has been controversial, and a substantial proportion of these patients continue to undergo PCI rather than CABG.
There are two main reasons for the continued use of PCI in this population. First, PCI is evolving, with new adjuvant drugs and drugeluting stents. Many cardiologists believe that earlier trials, which did not use contemporary PCI techniques, are outdated and that current, state-of-the-art PCI may be equivalent to—if not superior to—CABG.
Second, PCI is often performed on an ad hoc basis immediately after diagnostic angiography, leaving little time for discussion with the patient about alternative treatments. In this scenario, patients are inclined to undergo PCI immediately, while they are already on the table in the catheterization suite, rather than CABG at a later date.6
In addition, although the current joint guide-lines of the American College of Cardiology and the American Heart Association state that CABG is preferable to PCI for patients with diabetes and multivessel coronary artery disease, they give it only a level IIa recommendation.7
The much-anticipated Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial8 was designed to settle the CABG-vs-PCI debate, thereby leading to a stronger guideline recommendation for the preferred revascularization strategy in this patient population.
WHY ARE DIABETIC PATIENTS DIFFERENT?
Diabetes mellitus is a major risk factor for premature and aggressive coronary artery disease. Several mechanisms have been proposed to explain this association.
Diabetic patients have higher concentrations of several inflammatory proteins than those without diabetes, including C-reactive protein, tumor necrosis factor, and platelet-derived soluble CD40 ligand. They also have higher levels of adhesion molecules such as vascular cell adhesion molecule-1 and intercellular adhesion molecule.9,10 In addition, when blood sugar levels are high, platelets express more glycoprotein IIb/IIIa receptors and are therefore more prone to aggregate.11
These prothrombotic and proinflammatory cytokines, in conjunction with endothelial dysfunction and metabolic disorders such as hyperglycemia, hyperlipidemia, obesity, insulin resistance, and oxidative stress, lead to accelerated atherosclerosis in patients with diabetes.12 Also, because diabetes is a systemic disease, the atherosclerotic process is diffuse, and many patients with diabetes have left main coronary artery lesions and diffuse multivessel coronary artery disease.13,14
Although the short-term outcomes of revascularization by any means are comparable in patients with and without diabetes, diabetic patients have lower long-term survival rates and higher rates of myocardial infarction and need for repeat procedures.15 Diabetic patients who undergo PCI have a high rate of stent thrombosis and restenosis.16,17 Similarly, those undergoing CABG have higher rates of postoperative infection and renal and neurologic complications.18,19
BEFORE THE FREEDOM TRIAL
The question of CABG vs PCI has plagued physicians ever since PCI came to the forefront in the 1980s. Before stents were widely used, PCI with balloon angioplasty was known to be comparable to CABG for single-vessel disease, but whether it was beneficial in patients with multivessel disease or left main disease was not entirely evident. Randomized clinical trials were launched to answer the question.
Studies of balloon angioplasty vs CABG
The BARI trial (Bypass Angioplasty Revascularization Investigation),5,20 published in 1996, compared PCI (using balloon angioplasty without a stent) and CABG in patients with multivessel coronary artery disease (Table 120–29).
Between 1988 and 1991, the trial randomly assigned 1,829 patients with multivessel disease to receive either PCI or CABG and compared their long-term outcomes. Although there was no difference in mortality rates between the two groups overall, the diabetic subgroup had a significantly better survival rate with CABG than with PCI, which was sustained over a follow-up period of 10 years.5
BARI had a significant clinical impact at the time and led to a clinical alert by the National Heart, Lung, and Blood Institute recommending CABG over PCI for patients with diabetes. However, not everyone accepted the results, because they were based on a small number of patients (n = 353) in a retrospectively determined subgroup. Further, the BARI trial was conducted before the advent of coronary stents, which were later shown to improve outcomes after PCI. Also, optimal medical therapy after revascularization was not specified in the protocol, which likely affected outcomes.
EAST (Emory Angioplasty Versus Surgery Trial)21 and CABRI (Coronary Angioplasty Versus Bypass Revascularization Investigation) 22 were similar randomized trials comparing angioplasty and CABG in patients with multivessel coronary artery disease. These showed better outcomes after CABG in patients with diabetes. However, lack of statistical significance because of small sample sizes limited their clinical impact.
Studies of PCI with bare-metal stents vs CABG
The ARTS trial (Arterial Revascularization Therapy Study) compared PCI (with bare-metal stents) and CABG in 1,205 patients with multivessel coronary artery disease.23 The mortality rate did not differ significantly between two treatment groups overall or in the diabetic subgroup. However, the repeat revascularization rate was higher with PCI than with CABG.
The SoS trial (Stenting or Surgery)24 had similar results.
The ERACI II trial (Argentine Randomized Study: Coronary Angioplasty With Stenting Versus Coronary Bypass Surgery in Multi-Vessel Disease)25 found no difference in mortality rates at 5 years with CABG vs PCI.
These trials were criticized, as none of them routinely used glycoprotein IIb/IIIa inhibitors with PCI, which by then had been shown to reduce mortality rates.30 However, these trials made it clear that restenosis requiring repeat revascularization was a major disadvantage of PCI with bare-metal stents compared with CABG in patients with diabetes. Drug-eluting stents, which significantly reduced the rates of in-stent restenosis and target-lesion revascularization, were expected to overcome this major disadvantage.
Studies of PCI with drug-eluting stents vs CABG
ARTS II was the first trial to compare PCI with drug-eluting stents vs CABG. This was a nonrandomized single-arm study of 607 patients (including 159 with diabetes) who were treated with drug-eluting stents; the outcomes were compared with the CABG group from the earlier ARTS trial.31
At 3 years, in the diabetic subgroup, the rates of death, myocardial infarction, and stroke were not significantly different between treatments, although a trend favored PCI. However, this comparison was limited by selection bias, as ARTS II was a nonrandomized trial in which operators chose patients for drug-eluting stents in an attempt to match already known outcomes from the CABG cohort of ARTS.
SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) was the first randomized trial comparing PCI with drug-eluting stents (in this trial, paclitaxel-eluting) vs CABG in patients with three-vessel or left main coronary artery disease.26,27 Subgroup analysis in patients with diabetes mellitus revealed a higher rate of major adverse cardiac and cerebrovascular events (death, myocardial infarction, stroke, or repeat revascularization) in the PCI group than in the CABG patients, largely driven by higher rates of repeat revascularization after PCI.32,33 SYNTAX was not designed to assess significant differences in rates of death.
The CARDIa trial (Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and multivessel coronary artery disease to PCI (about one-third with bare-metal stents and two-thirds with drug-eluting stents) or CABG. Rates of major adverse cardiac and cerebrovascular events were higher in the PCI group, again largely driven by higher rates of repeat revascularization.4 CARDIa was stopped early because of a lack of enrollment and could not provide sufficient evidence to endorse one strategy over the other.
VA-CARDS (Veteran Affairs Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and proximal left anterior descending artery or multivessel coronary artery disease to receive PCI with drug-eluting stents or CABG.28 Although the rate of death was lower with CABG than with PCI at 2 years, the trial was underpowered and was terminated at 25% of the initial intended patient enrollment. In addition, only 9% of diabetic patients screened were angiographically eligible for the study.29
Registry data. Analysis of a large data set from the National Cardiovascular Disease Registry and the Society of Thoracic Surgeons revealed a survival advantage of CABG over PCI for a follow-up period of 5 years.34 However, this was a nonrandomized study, so its conclusions were not definitive.
THE FREEDOM TRIAL
Given the limitations of the trials described above, the National Heart, Lung, and Blood Institute sponsored the FREEDOM trial—an appropriately powered, randomized comparison of PCI (with drug-eluting stents) and CABG (using arterial grafting) in patients with diabetes and multivessel coronary artery disease using contemporary techniques and concomitant optimal medical therapy.8
FREEDOM study design
The FREEDOM trial enrolled 1,900 patients with diabetes and angiographically confirmed multivessel coronary artery disease (83% with three-vessel disease) with stenosis of more than 70% in two or more major epicardial vessels involving at least two separate coronary-artery territories. The main exclusion criteria were severe left main coronary artery stenosis (≥ 50% stenosis), class III or IV congestive heart failure, and previous CABG or valve surgery. For CABG surgery, arterial revascularization was encouraged.
Dual antiplatelet therapy was recommended for at least 12 months in patients receiving a drug-eluting stent, and optimal medical management for diabetes, hypertension, and hyperlipidemia was strongly advocated.
Between April 2005 and April 2010, 32,966 patients were screened, of whom 3,309 were eligible for the trial and 1,900 consented and were randomized (953 to the PCI group and 947 to the CABG group). The patients were followed for a minimum of 2 years and had a median follow-up time of 3.8 years. Outcomes were measured with an intention-to-treat analysis.
Study results
Patients. The groups were comparable with regard to baseline demographics and cardiac risk factors.
The mean age was 63; 29% of the patients were women, and 83% had three-vessel coronary artery disease. The mean hemoglobin A1c was 7.8%, and the mean ejection fraction was 66%. The mean SYNTAX score, which defines the anatomic complexity of lesions, was 26 (≤ 22 is mild, 23–32 is intermediate, and ≥ 33 is high). The mean EURO score, which defines surgical risk, was 2.7 (a score ≥ 5 being associated with a lower rate of survival).
The primary composite outcome (death, nonfatal myocardial infarction, or nonfatal stroke) occurred less frequently in the CABG group than in the PCI group (Table 2). CABG was also associated with significantly lower rates of death from any cause and of myocardial infarction. Importantly, survival curves comparing the two groups diverged at 2-year follow-up. In contrast to other outcomes assessed, stroke occurred more often in the CABG group. The 5-year rates in the CABG group vs the PCI group were:
- Primary outcome—18.7% vs 26.6%, P = .005
- Death from any cause—10.9% vs 16.3%, P = .049
- Myocardial infarction—6% vs 13.9%, P < .0001
- Stroke—5.2% vs 2.4%, P = .03.
The secondary outcome (death, nonfatal myocardial infarction, nonfatal stroke, or repeat revascularization at 30 days or 12 months) had occurred significantly more often in the PCI group than in the CABG group at 1 year (16.8% vs 11.8%, P = .004), with most of the difference attributable to a higher repeat revascularization rate in the PCI group (12.6% vs 4.8%, P < .001).
Subgroup analysis. CABG was superior to PCI across all prespecified subgroups, covering the complexity of the coronary artery disease. Event rates with CABG vs PCI, by tertiles of the SYNTAX score:
- SYNTAX scores ≤ 22: 17.2% vs 23.2%
- SYNTAX scores 23–32: 17.7% vs 27.2%
- SYNTAX scores ≥ 33: 22.8% vs 30.6%.
Cost-effectiveness. Although up-front costs were higher with CABG, at $34,467 for the index hospitalization vs $25,845 for PCI (P < .001), when the in-trial results were extended to a lifetime horizon, CABG had an incremental cost-effectiveness ratio of $8,132 per quality-adjusted life-year gained vs PCI.35 Traditionally, therapies are considered costeffective if the incremental cost-effectiveness ratio is less than $50,000 per quality-adjusted life-year gained.
WHY MAY CABG BE SUPERIOR IN DIABETIC PATIENTS?
The major advantage of CABG over PCI is the ability to achieve complete revascularization. Diabetic patients with coronary artery disease tend to have diffuse, multifocal disease with several stenotic lesions in multiple coronary arteries. While stents only treat the focal area of most significant occlusion, CABG may bypass all proximal vulnerable plaques that could potentially develop into culprit lesions over time, truly bypassing the diseased segments (Figure 1).
In addition, heavy calcification may not allow optimal stenting in these patients.
Use of multiple stents increases the risk of restenosis, which could lead to a higher incidence of myocardial infarction and need for repeat revascularization. This was evident in the FREEDOM trial, in which the mean number of stents per patient was 4.2. Also, some lesions need to be left untreated because of the complexity involved.
The major improvement in outcomes after CABG has resulted from using arterial conduits such as the internal mammary artery rather than the saphenous vein.36 The patency rates of internal mammary artery grafts exceed 80% over 10 years.37 Internal mammary artery grafting was done in 94% of patients receiving CABG in the FREEDOM trial.
WHAT DOES THIS MEAN?
FREEDOM was a landmark trial that confirmed that CABG provides significant benefit compared with contemporary PCI with drug-eluting stents in patients with diabetes mellitus and multivessel coronary artery disease. It was a large multicenter trial that was adequately powered, unlike most of the earlier trials of this topic.
Unlike previous trials in which the benefit of CABG was driven by reduction in repeat revascularizations alone, FREEDOM showed lower incidence rates of all-cause mortality and myocardial infarction with CABG than with PCI. CABG was better regardless of SYNTAX score, number of diseased vessels, ejection fraction, race, or sex of the patient, indicating that it leads to superior outcomes across a wide spectrum of patients.
An argument that cardiologists often cite when recommending PCI is that it can save money due to lower length of index hospital stay and lower procedure costs of with PCI than with CABG. However, in FREEDOM, CABG also appeared to be highly cost-effective.
FREEDOM had limitations
While FREEDOM provided robust data proving the superiority of CABG, the study had several limitations.
Although there was an overall survival benefit with CABG compared with PCI, the difference in incidence of cardiovascular deaths (which accounted for 64% of all deaths) was not statistically significant.
The trial included only patients who were eligible for both PCI and CABG. Hence, the results may not be generalizable to all diabetic patients with multivessel coronary artery disease—indeed, only 10% of those screened were considered eligible for the trial. However, it is likely that several patients screened in the FREEDOM trial may not have been eligible for PCI or CABG at the time of screening, since the revascularization decision was made by a multidisciplinary team and a more appropriate decision (either CABG or PCI) was then made.
Other factors limiting the general applicability of the results were low numbers of female patients (28.6%), black patients (6.3%), patients with an ejection fraction of 40% or less (2.5%), and patients with a low SYNTAX score (35%).
There were several unexplained observations as well. The difference in events between the treatment groups was much higher in North America than in other regions. The number of coronary lesions in the CABG group was high (mean = 5.74), but the average numbers of grafts used was only 2.9, and data were not provided regarding use of sequential grafting. Similarly, an average of only 3.5 of the six stenotic lesions per patient in the PCI group were revascularized; whether this was the result of procedural limitations with PCI was not entirely clear.
In addition, while the investigators mention that an average patient received four stents, a surprising finding was that the mean total length of the stents used was only 26 mm. This appears too small, as the usual length of one drug-eluting stent is about 20 to 30 mm.
Since only high-volume centers with good outcome data were included in the trial, the results may lack validity for patients undergoing revascularization at low-volume community centers.
It remains to be seen if the benefits of CABG will be sustained over 10 years and longer, when saphenous vein grafts tend to fail and require repeat revascularization, commonly performed with PCI. Previous data suggest that the longer the follow-up, the better the results with CABG. However, long-term results (> 10 years) in studies comparing drugeluting stents and CABG are not available.
Despite limitations, FREEDOM may change clinical practice
Despite these limitations, the FREEDOM trial has the potential to change clinical practice and strengthen current recommendations for CABG in these patients.
The trial underscored the importance of a multidisciplinary heart team approach in managing patients with complex coronary artery disease, similar to that being used in patients with severe aortic stenosis since transcatheter aortic valve replacement became available.
It should also bring an end to the practice of ad hoc PCI, especially in patients with diabetes and multivessel coronary artery disease. It is now imperative that physicians discuss current evidence for therapeutic options with the patients and their families before performing diagnostic angiography rather than immediately afterward, to give the patients ample time to make an informed decision. This is important, as most patients are likely to choose PCI in the same setting over CABG unless there is extensive discussion about the risks and benefits of both strategies done in an unbiased manner before angiography.
The fear of open heart surgery, a longer hospital stay, and a higher risk of stroke with CABG may lead some patients to choose PCI instead. In addition, factors that may preclude CABG in otherwise-eligible patients include anatomic considerations (diffuse distal vessel disease, poor conduits), individual factors (frailty, poor renal function, poor pulmonary function, patient preference), and local expertise.
Nevertheless, the patient should be presented with current evidence, and discussions regarding the optimal procedure should be held with a heart team, which should include an interventional cardiologist, a cardiothoracic surgeon, and a noninvasive cardiologist to facilitate an unbiased decision.
Regardless of the strategy chosen, the importance of compliance with optimal medical therapy (statins, antiplatelet agents, diabetes treatment) should be continuously emphasized to the patient.
WHAT DOES THE FUTURE HOLD?
Despite unequivocal evidence that CABG is superior to PCI in eligible patients with diabetes mellitus in the current era, PCI technologies continue to evolve rapidly. Newer second-generation drug-eluting stents have shown lower rates of restenosis38,39 and may shorten the duration of post-PCI dual-antiplatelet therapy, a nuisance that has negatively affected outcomes with drug-eluting stents (because of problems of cost, poor compliance, and increased bleeding risk).
At the same time, CABG has also improved, with more extensive use of complete arterial conduits and use of an off-pump bypass technique that in theory poses a lower risk of stroke, although this has not yet been shown in a randomized trial.40
Alternative approaches are being investigated. One of them is a hybrid procedure in which minimally invasive off-pump arterial grafting is combined with drug-eluting stents, which may reduce the risk of stroke and speed postoperative recovery.
- Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2005; 293:1501–1508.
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008; 52:255–262.
- Mack MJ, Banning AP, Serruys PW, et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg 2011; 92:2140–2146.
- Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010; 55:432–440.
- The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007; 49:1600–1606.
- Hlatky MA. Compelling evidence for coronary-bypass surgery in patients with diabetes. N Engl J Med 2012; 367:2437–2438.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000; 102:2180–2184.
- Bluher M, Unger R, Rassoul F, et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or type II diabetes. Diabetologia 2002; 45:210–216.
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003; 108:1527–1532.
- Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003; 41:1071–1077.
- Waller BF, Palumbo PJ, Lie JT, Roberts WC. Status of the coronary arteries at necropsy in diabetes mellitus with onset after age 30 years. Analysis of 229 diabetic patients with and without clinical evidence of coronary heart disease and comparison to 183 control subjects. Am J Med 1980; 69:498–506.
- Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle-aged diabetic patients (the London Cohort of the WHO Multinational Study of Vascular Disease in Diabetics) I: causes and death rates. Diabetologia 1990; 33:538–541.
- Laskey WK, Selzer F, Vlachos HA, et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2002; 90:1062–1067.
- Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480.
- Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005; 111:143–149.
- Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetologica 1999; 36:77–84.
- Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100:642–647.
- The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med 1996; 335:217–225.
- King SB, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eightyear mortality in the Emory Angioplasty versus Surgery Trial (East). J Am Coll Cardiol 2000; 35:1116–1121.
- Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF; CABRI Investigators (Coronary Angioplasty versus Bypass Revascularization Investigation). Difference in the mortality of the CABRI diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J Cardiol 2001; 87:947–950.
- Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 2005; 46:575–581.
- Booth J, Clayton T, Pepper J, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation 2008; 118:381–388.
- Rodriguez AE, Baldi J, Fernandez Pereira C, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol 2005; 46:582–588.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013; 381:629–638.
- Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013; 61:808–816.
- Ellis SG. Coronary revascularization for patients with diabetes: updated data favor coronary artery bypass grafting. J Am Coll Cardiol 2013; 61:817–819.
- Bhatt DL, Marso SP, Lincoff AM, et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol 2000; 35:922–928.
- Serruys PW, Ong AT, Morice MC, et al. Arterial Revascularisation Therapies Study Part II - Sirolimus-eluting stents for the treatment of patients with multivessel de novo coronary artery lesions. EuroIntervention 2005; 1:147–156.
- Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013; 43:1006–1013.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366:1467–1476.
- Magnuson EA, Farkouh ME, Fuster V, et al; FREEDOM Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes and multivessel coronary artery disease: results from the FREEDOM trial. Circulation 2013; 127:820–831.
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986; 314:1–6.
- Tector AJ, Schmahl TM, Janson B, et al. The internal mammary artery graft. Its longevity after coronary bypass. JAMA 1981; 246:2181–2183.
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitax-eleluting stents in coronary artery disease. N Engl J Med 2010; 362:1663–1674.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010; 363:136–146.
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366:1489–1497.
Many patients with diabetes mellitus develop complex, accelerated, multifocal coronary artery disease. Moreover, if they undergo revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), their risk of morbidity and death afterward is higher than in those without diabetes.1,2
Over the last 2 decades, CABG and PCI have advanced significantly, as have antithrombotic therapy and drug therapies to modify cardiovascular risk factors such as hyperlipidemia, hypertension, and diabetes.
Several earlier studies showed CABG to be more beneficial than PCI in diabetic patients with multivessel coronary artery disease.3–5 However, the topic has been controversial, and a substantial proportion of these patients continue to undergo PCI rather than CABG.
There are two main reasons for the continued use of PCI in this population. First, PCI is evolving, with new adjuvant drugs and drugeluting stents. Many cardiologists believe that earlier trials, which did not use contemporary PCI techniques, are outdated and that current, state-of-the-art PCI may be equivalent to—if not superior to—CABG.
Second, PCI is often performed on an ad hoc basis immediately after diagnostic angiography, leaving little time for discussion with the patient about alternative treatments. In this scenario, patients are inclined to undergo PCI immediately, while they are already on the table in the catheterization suite, rather than CABG at a later date.6
In addition, although the current joint guide-lines of the American College of Cardiology and the American Heart Association state that CABG is preferable to PCI for patients with diabetes and multivessel coronary artery disease, they give it only a level IIa recommendation.7
The much-anticipated Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial8 was designed to settle the CABG-vs-PCI debate, thereby leading to a stronger guideline recommendation for the preferred revascularization strategy in this patient population.
WHY ARE DIABETIC PATIENTS DIFFERENT?
Diabetes mellitus is a major risk factor for premature and aggressive coronary artery disease. Several mechanisms have been proposed to explain this association.
Diabetic patients have higher concentrations of several inflammatory proteins than those without diabetes, including C-reactive protein, tumor necrosis factor, and platelet-derived soluble CD40 ligand. They also have higher levels of adhesion molecules such as vascular cell adhesion molecule-1 and intercellular adhesion molecule.9,10 In addition, when blood sugar levels are high, platelets express more glycoprotein IIb/IIIa receptors and are therefore more prone to aggregate.11
These prothrombotic and proinflammatory cytokines, in conjunction with endothelial dysfunction and metabolic disorders such as hyperglycemia, hyperlipidemia, obesity, insulin resistance, and oxidative stress, lead to accelerated atherosclerosis in patients with diabetes.12 Also, because diabetes is a systemic disease, the atherosclerotic process is diffuse, and many patients with diabetes have left main coronary artery lesions and diffuse multivessel coronary artery disease.13,14
Although the short-term outcomes of revascularization by any means are comparable in patients with and without diabetes, diabetic patients have lower long-term survival rates and higher rates of myocardial infarction and need for repeat procedures.15 Diabetic patients who undergo PCI have a high rate of stent thrombosis and restenosis.16,17 Similarly, those undergoing CABG have higher rates of postoperative infection and renal and neurologic complications.18,19
BEFORE THE FREEDOM TRIAL
The question of CABG vs PCI has plagued physicians ever since PCI came to the forefront in the 1980s. Before stents were widely used, PCI with balloon angioplasty was known to be comparable to CABG for single-vessel disease, but whether it was beneficial in patients with multivessel disease or left main disease was not entirely evident. Randomized clinical trials were launched to answer the question.
Studies of balloon angioplasty vs CABG
The BARI trial (Bypass Angioplasty Revascularization Investigation),5,20 published in 1996, compared PCI (using balloon angioplasty without a stent) and CABG in patients with multivessel coronary artery disease (Table 120–29).
Between 1988 and 1991, the trial randomly assigned 1,829 patients with multivessel disease to receive either PCI or CABG and compared their long-term outcomes. Although there was no difference in mortality rates between the two groups overall, the diabetic subgroup had a significantly better survival rate with CABG than with PCI, which was sustained over a follow-up period of 10 years.5
BARI had a significant clinical impact at the time and led to a clinical alert by the National Heart, Lung, and Blood Institute recommending CABG over PCI for patients with diabetes. However, not everyone accepted the results, because they were based on a small number of patients (n = 353) in a retrospectively determined subgroup. Further, the BARI trial was conducted before the advent of coronary stents, which were later shown to improve outcomes after PCI. Also, optimal medical therapy after revascularization was not specified in the protocol, which likely affected outcomes.
EAST (Emory Angioplasty Versus Surgery Trial)21 and CABRI (Coronary Angioplasty Versus Bypass Revascularization Investigation) 22 were similar randomized trials comparing angioplasty and CABG in patients with multivessel coronary artery disease. These showed better outcomes after CABG in patients with diabetes. However, lack of statistical significance because of small sample sizes limited their clinical impact.
Studies of PCI with bare-metal stents vs CABG
The ARTS trial (Arterial Revascularization Therapy Study) compared PCI (with bare-metal stents) and CABG in 1,205 patients with multivessel coronary artery disease.23 The mortality rate did not differ significantly between two treatment groups overall or in the diabetic subgroup. However, the repeat revascularization rate was higher with PCI than with CABG.
The SoS trial (Stenting or Surgery)24 had similar results.
The ERACI II trial (Argentine Randomized Study: Coronary Angioplasty With Stenting Versus Coronary Bypass Surgery in Multi-Vessel Disease)25 found no difference in mortality rates at 5 years with CABG vs PCI.
These trials were criticized, as none of them routinely used glycoprotein IIb/IIIa inhibitors with PCI, which by then had been shown to reduce mortality rates.30 However, these trials made it clear that restenosis requiring repeat revascularization was a major disadvantage of PCI with bare-metal stents compared with CABG in patients with diabetes. Drug-eluting stents, which significantly reduced the rates of in-stent restenosis and target-lesion revascularization, were expected to overcome this major disadvantage.
Studies of PCI with drug-eluting stents vs CABG
ARTS II was the first trial to compare PCI with drug-eluting stents vs CABG. This was a nonrandomized single-arm study of 607 patients (including 159 with diabetes) who were treated with drug-eluting stents; the outcomes were compared with the CABG group from the earlier ARTS trial.31
At 3 years, in the diabetic subgroup, the rates of death, myocardial infarction, and stroke were not significantly different between treatments, although a trend favored PCI. However, this comparison was limited by selection bias, as ARTS II was a nonrandomized trial in which operators chose patients for drug-eluting stents in an attempt to match already known outcomes from the CABG cohort of ARTS.
SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) was the first randomized trial comparing PCI with drug-eluting stents (in this trial, paclitaxel-eluting) vs CABG in patients with three-vessel or left main coronary artery disease.26,27 Subgroup analysis in patients with diabetes mellitus revealed a higher rate of major adverse cardiac and cerebrovascular events (death, myocardial infarction, stroke, or repeat revascularization) in the PCI group than in the CABG patients, largely driven by higher rates of repeat revascularization after PCI.32,33 SYNTAX was not designed to assess significant differences in rates of death.
The CARDIa trial (Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and multivessel coronary artery disease to PCI (about one-third with bare-metal stents and two-thirds with drug-eluting stents) or CABG. Rates of major adverse cardiac and cerebrovascular events were higher in the PCI group, again largely driven by higher rates of repeat revascularization.4 CARDIa was stopped early because of a lack of enrollment and could not provide sufficient evidence to endorse one strategy over the other.
VA-CARDS (Veteran Affairs Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and proximal left anterior descending artery or multivessel coronary artery disease to receive PCI with drug-eluting stents or CABG.28 Although the rate of death was lower with CABG than with PCI at 2 years, the trial was underpowered and was terminated at 25% of the initial intended patient enrollment. In addition, only 9% of diabetic patients screened were angiographically eligible for the study.29
Registry data. Analysis of a large data set from the National Cardiovascular Disease Registry and the Society of Thoracic Surgeons revealed a survival advantage of CABG over PCI for a follow-up period of 5 years.34 However, this was a nonrandomized study, so its conclusions were not definitive.
THE FREEDOM TRIAL
Given the limitations of the trials described above, the National Heart, Lung, and Blood Institute sponsored the FREEDOM trial—an appropriately powered, randomized comparison of PCI (with drug-eluting stents) and CABG (using arterial grafting) in patients with diabetes and multivessel coronary artery disease using contemporary techniques and concomitant optimal medical therapy.8
FREEDOM study design
The FREEDOM trial enrolled 1,900 patients with diabetes and angiographically confirmed multivessel coronary artery disease (83% with three-vessel disease) with stenosis of more than 70% in two or more major epicardial vessels involving at least two separate coronary-artery territories. The main exclusion criteria were severe left main coronary artery stenosis (≥ 50% stenosis), class III or IV congestive heart failure, and previous CABG or valve surgery. For CABG surgery, arterial revascularization was encouraged.
Dual antiplatelet therapy was recommended for at least 12 months in patients receiving a drug-eluting stent, and optimal medical management for diabetes, hypertension, and hyperlipidemia was strongly advocated.
Between April 2005 and April 2010, 32,966 patients were screened, of whom 3,309 were eligible for the trial and 1,900 consented and were randomized (953 to the PCI group and 947 to the CABG group). The patients were followed for a minimum of 2 years and had a median follow-up time of 3.8 years. Outcomes were measured with an intention-to-treat analysis.
Study results
Patients. The groups were comparable with regard to baseline demographics and cardiac risk factors.
The mean age was 63; 29% of the patients were women, and 83% had three-vessel coronary artery disease. The mean hemoglobin A1c was 7.8%, and the mean ejection fraction was 66%. The mean SYNTAX score, which defines the anatomic complexity of lesions, was 26 (≤ 22 is mild, 23–32 is intermediate, and ≥ 33 is high). The mean EURO score, which defines surgical risk, was 2.7 (a score ≥ 5 being associated with a lower rate of survival).
The primary composite outcome (death, nonfatal myocardial infarction, or nonfatal stroke) occurred less frequently in the CABG group than in the PCI group (Table 2). CABG was also associated with significantly lower rates of death from any cause and of myocardial infarction. Importantly, survival curves comparing the two groups diverged at 2-year follow-up. In contrast to other outcomes assessed, stroke occurred more often in the CABG group. The 5-year rates in the CABG group vs the PCI group were:
- Primary outcome—18.7% vs 26.6%, P = .005
- Death from any cause—10.9% vs 16.3%, P = .049
- Myocardial infarction—6% vs 13.9%, P < .0001
- Stroke—5.2% vs 2.4%, P = .03.
The secondary outcome (death, nonfatal myocardial infarction, nonfatal stroke, or repeat revascularization at 30 days or 12 months) had occurred significantly more often in the PCI group than in the CABG group at 1 year (16.8% vs 11.8%, P = .004), with most of the difference attributable to a higher repeat revascularization rate in the PCI group (12.6% vs 4.8%, P < .001).
Subgroup analysis. CABG was superior to PCI across all prespecified subgroups, covering the complexity of the coronary artery disease. Event rates with CABG vs PCI, by tertiles of the SYNTAX score:
- SYNTAX scores ≤ 22: 17.2% vs 23.2%
- SYNTAX scores 23–32: 17.7% vs 27.2%
- SYNTAX scores ≥ 33: 22.8% vs 30.6%.
Cost-effectiveness. Although up-front costs were higher with CABG, at $34,467 for the index hospitalization vs $25,845 for PCI (P < .001), when the in-trial results were extended to a lifetime horizon, CABG had an incremental cost-effectiveness ratio of $8,132 per quality-adjusted life-year gained vs PCI.35 Traditionally, therapies are considered costeffective if the incremental cost-effectiveness ratio is less than $50,000 per quality-adjusted life-year gained.
WHY MAY CABG BE SUPERIOR IN DIABETIC PATIENTS?
The major advantage of CABG over PCI is the ability to achieve complete revascularization. Diabetic patients with coronary artery disease tend to have diffuse, multifocal disease with several stenotic lesions in multiple coronary arteries. While stents only treat the focal area of most significant occlusion, CABG may bypass all proximal vulnerable plaques that could potentially develop into culprit lesions over time, truly bypassing the diseased segments (Figure 1).
In addition, heavy calcification may not allow optimal stenting in these patients.
Use of multiple stents increases the risk of restenosis, which could lead to a higher incidence of myocardial infarction and need for repeat revascularization. This was evident in the FREEDOM trial, in which the mean number of stents per patient was 4.2. Also, some lesions need to be left untreated because of the complexity involved.
The major improvement in outcomes after CABG has resulted from using arterial conduits such as the internal mammary artery rather than the saphenous vein.36 The patency rates of internal mammary artery grafts exceed 80% over 10 years.37 Internal mammary artery grafting was done in 94% of patients receiving CABG in the FREEDOM trial.
WHAT DOES THIS MEAN?
FREEDOM was a landmark trial that confirmed that CABG provides significant benefit compared with contemporary PCI with drug-eluting stents in patients with diabetes mellitus and multivessel coronary artery disease. It was a large multicenter trial that was adequately powered, unlike most of the earlier trials of this topic.
Unlike previous trials in which the benefit of CABG was driven by reduction in repeat revascularizations alone, FREEDOM showed lower incidence rates of all-cause mortality and myocardial infarction with CABG than with PCI. CABG was better regardless of SYNTAX score, number of diseased vessels, ejection fraction, race, or sex of the patient, indicating that it leads to superior outcomes across a wide spectrum of patients.
An argument that cardiologists often cite when recommending PCI is that it can save money due to lower length of index hospital stay and lower procedure costs of with PCI than with CABG. However, in FREEDOM, CABG also appeared to be highly cost-effective.
FREEDOM had limitations
While FREEDOM provided robust data proving the superiority of CABG, the study had several limitations.
Although there was an overall survival benefit with CABG compared with PCI, the difference in incidence of cardiovascular deaths (which accounted for 64% of all deaths) was not statistically significant.
The trial included only patients who were eligible for both PCI and CABG. Hence, the results may not be generalizable to all diabetic patients with multivessel coronary artery disease—indeed, only 10% of those screened were considered eligible for the trial. However, it is likely that several patients screened in the FREEDOM trial may not have been eligible for PCI or CABG at the time of screening, since the revascularization decision was made by a multidisciplinary team and a more appropriate decision (either CABG or PCI) was then made.
Other factors limiting the general applicability of the results were low numbers of female patients (28.6%), black patients (6.3%), patients with an ejection fraction of 40% or less (2.5%), and patients with a low SYNTAX score (35%).
There were several unexplained observations as well. The difference in events between the treatment groups was much higher in North America than in other regions. The number of coronary lesions in the CABG group was high (mean = 5.74), but the average numbers of grafts used was only 2.9, and data were not provided regarding use of sequential grafting. Similarly, an average of only 3.5 of the six stenotic lesions per patient in the PCI group were revascularized; whether this was the result of procedural limitations with PCI was not entirely clear.
In addition, while the investigators mention that an average patient received four stents, a surprising finding was that the mean total length of the stents used was only 26 mm. This appears too small, as the usual length of one drug-eluting stent is about 20 to 30 mm.
Since only high-volume centers with good outcome data were included in the trial, the results may lack validity for patients undergoing revascularization at low-volume community centers.
It remains to be seen if the benefits of CABG will be sustained over 10 years and longer, when saphenous vein grafts tend to fail and require repeat revascularization, commonly performed with PCI. Previous data suggest that the longer the follow-up, the better the results with CABG. However, long-term results (> 10 years) in studies comparing drugeluting stents and CABG are not available.
Despite limitations, FREEDOM may change clinical practice
Despite these limitations, the FREEDOM trial has the potential to change clinical practice and strengthen current recommendations for CABG in these patients.
The trial underscored the importance of a multidisciplinary heart team approach in managing patients with complex coronary artery disease, similar to that being used in patients with severe aortic stenosis since transcatheter aortic valve replacement became available.
It should also bring an end to the practice of ad hoc PCI, especially in patients with diabetes and multivessel coronary artery disease. It is now imperative that physicians discuss current evidence for therapeutic options with the patients and their families before performing diagnostic angiography rather than immediately afterward, to give the patients ample time to make an informed decision. This is important, as most patients are likely to choose PCI in the same setting over CABG unless there is extensive discussion about the risks and benefits of both strategies done in an unbiased manner before angiography.
The fear of open heart surgery, a longer hospital stay, and a higher risk of stroke with CABG may lead some patients to choose PCI instead. In addition, factors that may preclude CABG in otherwise-eligible patients include anatomic considerations (diffuse distal vessel disease, poor conduits), individual factors (frailty, poor renal function, poor pulmonary function, patient preference), and local expertise.
Nevertheless, the patient should be presented with current evidence, and discussions regarding the optimal procedure should be held with a heart team, which should include an interventional cardiologist, a cardiothoracic surgeon, and a noninvasive cardiologist to facilitate an unbiased decision.
Regardless of the strategy chosen, the importance of compliance with optimal medical therapy (statins, antiplatelet agents, diabetes treatment) should be continuously emphasized to the patient.
WHAT DOES THE FUTURE HOLD?
Despite unequivocal evidence that CABG is superior to PCI in eligible patients with diabetes mellitus in the current era, PCI technologies continue to evolve rapidly. Newer second-generation drug-eluting stents have shown lower rates of restenosis38,39 and may shorten the duration of post-PCI dual-antiplatelet therapy, a nuisance that has negatively affected outcomes with drug-eluting stents (because of problems of cost, poor compliance, and increased bleeding risk).
At the same time, CABG has also improved, with more extensive use of complete arterial conduits and use of an off-pump bypass technique that in theory poses a lower risk of stroke, although this has not yet been shown in a randomized trial.40
Alternative approaches are being investigated. One of them is a hybrid procedure in which minimally invasive off-pump arterial grafting is combined with drug-eluting stents, which may reduce the risk of stroke and speed postoperative recovery.
Many patients with diabetes mellitus develop complex, accelerated, multifocal coronary artery disease. Moreover, if they undergo revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), their risk of morbidity and death afterward is higher than in those without diabetes.1,2
Over the last 2 decades, CABG and PCI have advanced significantly, as have antithrombotic therapy and drug therapies to modify cardiovascular risk factors such as hyperlipidemia, hypertension, and diabetes.
Several earlier studies showed CABG to be more beneficial than PCI in diabetic patients with multivessel coronary artery disease.3–5 However, the topic has been controversial, and a substantial proportion of these patients continue to undergo PCI rather than CABG.
There are two main reasons for the continued use of PCI in this population. First, PCI is evolving, with new adjuvant drugs and drugeluting stents. Many cardiologists believe that earlier trials, which did not use contemporary PCI techniques, are outdated and that current, state-of-the-art PCI may be equivalent to—if not superior to—CABG.
Second, PCI is often performed on an ad hoc basis immediately after diagnostic angiography, leaving little time for discussion with the patient about alternative treatments. In this scenario, patients are inclined to undergo PCI immediately, while they are already on the table in the catheterization suite, rather than CABG at a later date.6
In addition, although the current joint guide-lines of the American College of Cardiology and the American Heart Association state that CABG is preferable to PCI for patients with diabetes and multivessel coronary artery disease, they give it only a level IIa recommendation.7
The much-anticipated Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial8 was designed to settle the CABG-vs-PCI debate, thereby leading to a stronger guideline recommendation for the preferred revascularization strategy in this patient population.
WHY ARE DIABETIC PATIENTS DIFFERENT?
Diabetes mellitus is a major risk factor for premature and aggressive coronary artery disease. Several mechanisms have been proposed to explain this association.
Diabetic patients have higher concentrations of several inflammatory proteins than those without diabetes, including C-reactive protein, tumor necrosis factor, and platelet-derived soluble CD40 ligand. They also have higher levels of adhesion molecules such as vascular cell adhesion molecule-1 and intercellular adhesion molecule.9,10 In addition, when blood sugar levels are high, platelets express more glycoprotein IIb/IIIa receptors and are therefore more prone to aggregate.11
These prothrombotic and proinflammatory cytokines, in conjunction with endothelial dysfunction and metabolic disorders such as hyperglycemia, hyperlipidemia, obesity, insulin resistance, and oxidative stress, lead to accelerated atherosclerosis in patients with diabetes.12 Also, because diabetes is a systemic disease, the atherosclerotic process is diffuse, and many patients with diabetes have left main coronary artery lesions and diffuse multivessel coronary artery disease.13,14
Although the short-term outcomes of revascularization by any means are comparable in patients with and without diabetes, diabetic patients have lower long-term survival rates and higher rates of myocardial infarction and need for repeat procedures.15 Diabetic patients who undergo PCI have a high rate of stent thrombosis and restenosis.16,17 Similarly, those undergoing CABG have higher rates of postoperative infection and renal and neurologic complications.18,19
BEFORE THE FREEDOM TRIAL
The question of CABG vs PCI has plagued physicians ever since PCI came to the forefront in the 1980s. Before stents were widely used, PCI with balloon angioplasty was known to be comparable to CABG for single-vessel disease, but whether it was beneficial in patients with multivessel disease or left main disease was not entirely evident. Randomized clinical trials were launched to answer the question.
Studies of balloon angioplasty vs CABG
The BARI trial (Bypass Angioplasty Revascularization Investigation),5,20 published in 1996, compared PCI (using balloon angioplasty without a stent) and CABG in patients with multivessel coronary artery disease (Table 120–29).
Between 1988 and 1991, the trial randomly assigned 1,829 patients with multivessel disease to receive either PCI or CABG and compared their long-term outcomes. Although there was no difference in mortality rates between the two groups overall, the diabetic subgroup had a significantly better survival rate with CABG than with PCI, which was sustained over a follow-up period of 10 years.5
BARI had a significant clinical impact at the time and led to a clinical alert by the National Heart, Lung, and Blood Institute recommending CABG over PCI for patients with diabetes. However, not everyone accepted the results, because they were based on a small number of patients (n = 353) in a retrospectively determined subgroup. Further, the BARI trial was conducted before the advent of coronary stents, which were later shown to improve outcomes after PCI. Also, optimal medical therapy after revascularization was not specified in the protocol, which likely affected outcomes.
EAST (Emory Angioplasty Versus Surgery Trial)21 and CABRI (Coronary Angioplasty Versus Bypass Revascularization Investigation) 22 were similar randomized trials comparing angioplasty and CABG in patients with multivessel coronary artery disease. These showed better outcomes after CABG in patients with diabetes. However, lack of statistical significance because of small sample sizes limited their clinical impact.
Studies of PCI with bare-metal stents vs CABG
The ARTS trial (Arterial Revascularization Therapy Study) compared PCI (with bare-metal stents) and CABG in 1,205 patients with multivessel coronary artery disease.23 The mortality rate did not differ significantly between two treatment groups overall or in the diabetic subgroup. However, the repeat revascularization rate was higher with PCI than with CABG.
The SoS trial (Stenting or Surgery)24 had similar results.
The ERACI II trial (Argentine Randomized Study: Coronary Angioplasty With Stenting Versus Coronary Bypass Surgery in Multi-Vessel Disease)25 found no difference in mortality rates at 5 years with CABG vs PCI.
These trials were criticized, as none of them routinely used glycoprotein IIb/IIIa inhibitors with PCI, which by then had been shown to reduce mortality rates.30 However, these trials made it clear that restenosis requiring repeat revascularization was a major disadvantage of PCI with bare-metal stents compared with CABG in patients with diabetes. Drug-eluting stents, which significantly reduced the rates of in-stent restenosis and target-lesion revascularization, were expected to overcome this major disadvantage.
Studies of PCI with drug-eluting stents vs CABG
ARTS II was the first trial to compare PCI with drug-eluting stents vs CABG. This was a nonrandomized single-arm study of 607 patients (including 159 with diabetes) who were treated with drug-eluting stents; the outcomes were compared with the CABG group from the earlier ARTS trial.31
At 3 years, in the diabetic subgroup, the rates of death, myocardial infarction, and stroke were not significantly different between treatments, although a trend favored PCI. However, this comparison was limited by selection bias, as ARTS II was a nonrandomized trial in which operators chose patients for drug-eluting stents in an attempt to match already known outcomes from the CABG cohort of ARTS.
SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) was the first randomized trial comparing PCI with drug-eluting stents (in this trial, paclitaxel-eluting) vs CABG in patients with three-vessel or left main coronary artery disease.26,27 Subgroup analysis in patients with diabetes mellitus revealed a higher rate of major adverse cardiac and cerebrovascular events (death, myocardial infarction, stroke, or repeat revascularization) in the PCI group than in the CABG patients, largely driven by higher rates of repeat revascularization after PCI.32,33 SYNTAX was not designed to assess significant differences in rates of death.
The CARDIa trial (Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and multivessel coronary artery disease to PCI (about one-third with bare-metal stents and two-thirds with drug-eluting stents) or CABG. Rates of major adverse cardiac and cerebrovascular events were higher in the PCI group, again largely driven by higher rates of repeat revascularization.4 CARDIa was stopped early because of a lack of enrollment and could not provide sufficient evidence to endorse one strategy over the other.
VA-CARDS (Veteran Affairs Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and proximal left anterior descending artery or multivessel coronary artery disease to receive PCI with drug-eluting stents or CABG.28 Although the rate of death was lower with CABG than with PCI at 2 years, the trial was underpowered and was terminated at 25% of the initial intended patient enrollment. In addition, only 9% of diabetic patients screened were angiographically eligible for the study.29
Registry data. Analysis of a large data set from the National Cardiovascular Disease Registry and the Society of Thoracic Surgeons revealed a survival advantage of CABG over PCI for a follow-up period of 5 years.34 However, this was a nonrandomized study, so its conclusions were not definitive.
THE FREEDOM TRIAL
Given the limitations of the trials described above, the National Heart, Lung, and Blood Institute sponsored the FREEDOM trial—an appropriately powered, randomized comparison of PCI (with drug-eluting stents) and CABG (using arterial grafting) in patients with diabetes and multivessel coronary artery disease using contemporary techniques and concomitant optimal medical therapy.8
FREEDOM study design
The FREEDOM trial enrolled 1,900 patients with diabetes and angiographically confirmed multivessel coronary artery disease (83% with three-vessel disease) with stenosis of more than 70% in two or more major epicardial vessels involving at least two separate coronary-artery territories. The main exclusion criteria were severe left main coronary artery stenosis (≥ 50% stenosis), class III or IV congestive heart failure, and previous CABG or valve surgery. For CABG surgery, arterial revascularization was encouraged.
Dual antiplatelet therapy was recommended for at least 12 months in patients receiving a drug-eluting stent, and optimal medical management for diabetes, hypertension, and hyperlipidemia was strongly advocated.
Between April 2005 and April 2010, 32,966 patients were screened, of whom 3,309 were eligible for the trial and 1,900 consented and were randomized (953 to the PCI group and 947 to the CABG group). The patients were followed for a minimum of 2 years and had a median follow-up time of 3.8 years. Outcomes were measured with an intention-to-treat analysis.
Study results
Patients. The groups were comparable with regard to baseline demographics and cardiac risk factors.
The mean age was 63; 29% of the patients were women, and 83% had three-vessel coronary artery disease. The mean hemoglobin A1c was 7.8%, and the mean ejection fraction was 66%. The mean SYNTAX score, which defines the anatomic complexity of lesions, was 26 (≤ 22 is mild, 23–32 is intermediate, and ≥ 33 is high). The mean EURO score, which defines surgical risk, was 2.7 (a score ≥ 5 being associated with a lower rate of survival).
The primary composite outcome (death, nonfatal myocardial infarction, or nonfatal stroke) occurred less frequently in the CABG group than in the PCI group (Table 2). CABG was also associated with significantly lower rates of death from any cause and of myocardial infarction. Importantly, survival curves comparing the two groups diverged at 2-year follow-up. In contrast to other outcomes assessed, stroke occurred more often in the CABG group. The 5-year rates in the CABG group vs the PCI group were:
- Primary outcome—18.7% vs 26.6%, P = .005
- Death from any cause—10.9% vs 16.3%, P = .049
- Myocardial infarction—6% vs 13.9%, P < .0001
- Stroke—5.2% vs 2.4%, P = .03.
The secondary outcome (death, nonfatal myocardial infarction, nonfatal stroke, or repeat revascularization at 30 days or 12 months) had occurred significantly more often in the PCI group than in the CABG group at 1 year (16.8% vs 11.8%, P = .004), with most of the difference attributable to a higher repeat revascularization rate in the PCI group (12.6% vs 4.8%, P < .001).
Subgroup analysis. CABG was superior to PCI across all prespecified subgroups, covering the complexity of the coronary artery disease. Event rates with CABG vs PCI, by tertiles of the SYNTAX score:
- SYNTAX scores ≤ 22: 17.2% vs 23.2%
- SYNTAX scores 23–32: 17.7% vs 27.2%
- SYNTAX scores ≥ 33: 22.8% vs 30.6%.
Cost-effectiveness. Although up-front costs were higher with CABG, at $34,467 for the index hospitalization vs $25,845 for PCI (P < .001), when the in-trial results were extended to a lifetime horizon, CABG had an incremental cost-effectiveness ratio of $8,132 per quality-adjusted life-year gained vs PCI.35 Traditionally, therapies are considered costeffective if the incremental cost-effectiveness ratio is less than $50,000 per quality-adjusted life-year gained.
WHY MAY CABG BE SUPERIOR IN DIABETIC PATIENTS?
The major advantage of CABG over PCI is the ability to achieve complete revascularization. Diabetic patients with coronary artery disease tend to have diffuse, multifocal disease with several stenotic lesions in multiple coronary arteries. While stents only treat the focal area of most significant occlusion, CABG may bypass all proximal vulnerable plaques that could potentially develop into culprit lesions over time, truly bypassing the diseased segments (Figure 1).
In addition, heavy calcification may not allow optimal stenting in these patients.
Use of multiple stents increases the risk of restenosis, which could lead to a higher incidence of myocardial infarction and need for repeat revascularization. This was evident in the FREEDOM trial, in which the mean number of stents per patient was 4.2. Also, some lesions need to be left untreated because of the complexity involved.
The major improvement in outcomes after CABG has resulted from using arterial conduits such as the internal mammary artery rather than the saphenous vein.36 The patency rates of internal mammary artery grafts exceed 80% over 10 years.37 Internal mammary artery grafting was done in 94% of patients receiving CABG in the FREEDOM trial.
WHAT DOES THIS MEAN?
FREEDOM was a landmark trial that confirmed that CABG provides significant benefit compared with contemporary PCI with drug-eluting stents in patients with diabetes mellitus and multivessel coronary artery disease. It was a large multicenter trial that was adequately powered, unlike most of the earlier trials of this topic.
Unlike previous trials in which the benefit of CABG was driven by reduction in repeat revascularizations alone, FREEDOM showed lower incidence rates of all-cause mortality and myocardial infarction with CABG than with PCI. CABG was better regardless of SYNTAX score, number of diseased vessels, ejection fraction, race, or sex of the patient, indicating that it leads to superior outcomes across a wide spectrum of patients.
An argument that cardiologists often cite when recommending PCI is that it can save money due to lower length of index hospital stay and lower procedure costs of with PCI than with CABG. However, in FREEDOM, CABG also appeared to be highly cost-effective.
FREEDOM had limitations
While FREEDOM provided robust data proving the superiority of CABG, the study had several limitations.
Although there was an overall survival benefit with CABG compared with PCI, the difference in incidence of cardiovascular deaths (which accounted for 64% of all deaths) was not statistically significant.
The trial included only patients who were eligible for both PCI and CABG. Hence, the results may not be generalizable to all diabetic patients with multivessel coronary artery disease—indeed, only 10% of those screened were considered eligible for the trial. However, it is likely that several patients screened in the FREEDOM trial may not have been eligible for PCI or CABG at the time of screening, since the revascularization decision was made by a multidisciplinary team and a more appropriate decision (either CABG or PCI) was then made.
Other factors limiting the general applicability of the results were low numbers of female patients (28.6%), black patients (6.3%), patients with an ejection fraction of 40% or less (2.5%), and patients with a low SYNTAX score (35%).
There were several unexplained observations as well. The difference in events between the treatment groups was much higher in North America than in other regions. The number of coronary lesions in the CABG group was high (mean = 5.74), but the average numbers of grafts used was only 2.9, and data were not provided regarding use of sequential grafting. Similarly, an average of only 3.5 of the six stenotic lesions per patient in the PCI group were revascularized; whether this was the result of procedural limitations with PCI was not entirely clear.
In addition, while the investigators mention that an average patient received four stents, a surprising finding was that the mean total length of the stents used was only 26 mm. This appears too small, as the usual length of one drug-eluting stent is about 20 to 30 mm.
Since only high-volume centers with good outcome data were included in the trial, the results may lack validity for patients undergoing revascularization at low-volume community centers.
It remains to be seen if the benefits of CABG will be sustained over 10 years and longer, when saphenous vein grafts tend to fail and require repeat revascularization, commonly performed with PCI. Previous data suggest that the longer the follow-up, the better the results with CABG. However, long-term results (> 10 years) in studies comparing drugeluting stents and CABG are not available.
Despite limitations, FREEDOM may change clinical practice
Despite these limitations, the FREEDOM trial has the potential to change clinical practice and strengthen current recommendations for CABG in these patients.
The trial underscored the importance of a multidisciplinary heart team approach in managing patients with complex coronary artery disease, similar to that being used in patients with severe aortic stenosis since transcatheter aortic valve replacement became available.
It should also bring an end to the practice of ad hoc PCI, especially in patients with diabetes and multivessel coronary artery disease. It is now imperative that physicians discuss current evidence for therapeutic options with the patients and their families before performing diagnostic angiography rather than immediately afterward, to give the patients ample time to make an informed decision. This is important, as most patients are likely to choose PCI in the same setting over CABG unless there is extensive discussion about the risks and benefits of both strategies done in an unbiased manner before angiography.
The fear of open heart surgery, a longer hospital stay, and a higher risk of stroke with CABG may lead some patients to choose PCI instead. In addition, factors that may preclude CABG in otherwise-eligible patients include anatomic considerations (diffuse distal vessel disease, poor conduits), individual factors (frailty, poor renal function, poor pulmonary function, patient preference), and local expertise.
Nevertheless, the patient should be presented with current evidence, and discussions regarding the optimal procedure should be held with a heart team, which should include an interventional cardiologist, a cardiothoracic surgeon, and a noninvasive cardiologist to facilitate an unbiased decision.
Regardless of the strategy chosen, the importance of compliance with optimal medical therapy (statins, antiplatelet agents, diabetes treatment) should be continuously emphasized to the patient.
WHAT DOES THE FUTURE HOLD?
Despite unequivocal evidence that CABG is superior to PCI in eligible patients with diabetes mellitus in the current era, PCI technologies continue to evolve rapidly. Newer second-generation drug-eluting stents have shown lower rates of restenosis38,39 and may shorten the duration of post-PCI dual-antiplatelet therapy, a nuisance that has negatively affected outcomes with drug-eluting stents (because of problems of cost, poor compliance, and increased bleeding risk).
At the same time, CABG has also improved, with more extensive use of complete arterial conduits and use of an off-pump bypass technique that in theory poses a lower risk of stroke, although this has not yet been shown in a randomized trial.40
Alternative approaches are being investigated. One of them is a hybrid procedure in which minimally invasive off-pump arterial grafting is combined with drug-eluting stents, which may reduce the risk of stroke and speed postoperative recovery.
- Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2005; 293:1501–1508.
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008; 52:255–262.
- Mack MJ, Banning AP, Serruys PW, et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg 2011; 92:2140–2146.
- Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010; 55:432–440.
- The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007; 49:1600–1606.
- Hlatky MA. Compelling evidence for coronary-bypass surgery in patients with diabetes. N Engl J Med 2012; 367:2437–2438.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000; 102:2180–2184.
- Bluher M, Unger R, Rassoul F, et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or type II diabetes. Diabetologia 2002; 45:210–216.
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003; 108:1527–1532.
- Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003; 41:1071–1077.
- Waller BF, Palumbo PJ, Lie JT, Roberts WC. Status of the coronary arteries at necropsy in diabetes mellitus with onset after age 30 years. Analysis of 229 diabetic patients with and without clinical evidence of coronary heart disease and comparison to 183 control subjects. Am J Med 1980; 69:498–506.
- Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle-aged diabetic patients (the London Cohort of the WHO Multinational Study of Vascular Disease in Diabetics) I: causes and death rates. Diabetologia 1990; 33:538–541.
- Laskey WK, Selzer F, Vlachos HA, et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2002; 90:1062–1067.
- Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480.
- Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005; 111:143–149.
- Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetologica 1999; 36:77–84.
- Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100:642–647.
- The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med 1996; 335:217–225.
- King SB, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eightyear mortality in the Emory Angioplasty versus Surgery Trial (East). J Am Coll Cardiol 2000; 35:1116–1121.
- Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF; CABRI Investigators (Coronary Angioplasty versus Bypass Revascularization Investigation). Difference in the mortality of the CABRI diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J Cardiol 2001; 87:947–950.
- Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 2005; 46:575–581.
- Booth J, Clayton T, Pepper J, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation 2008; 118:381–388.
- Rodriguez AE, Baldi J, Fernandez Pereira C, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol 2005; 46:582–588.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013; 381:629–638.
- Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013; 61:808–816.
- Ellis SG. Coronary revascularization for patients with diabetes: updated data favor coronary artery bypass grafting. J Am Coll Cardiol 2013; 61:817–819.
- Bhatt DL, Marso SP, Lincoff AM, et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol 2000; 35:922–928.
- Serruys PW, Ong AT, Morice MC, et al. Arterial Revascularisation Therapies Study Part II - Sirolimus-eluting stents for the treatment of patients with multivessel de novo coronary artery lesions. EuroIntervention 2005; 1:147–156.
- Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013; 43:1006–1013.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366:1467–1476.
- Magnuson EA, Farkouh ME, Fuster V, et al; FREEDOM Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes and multivessel coronary artery disease: results from the FREEDOM trial. Circulation 2013; 127:820–831.
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986; 314:1–6.
- Tector AJ, Schmahl TM, Janson B, et al. The internal mammary artery graft. Its longevity after coronary bypass. JAMA 1981; 246:2181–2183.
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitax-eleluting stents in coronary artery disease. N Engl J Med 2010; 362:1663–1674.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010; 363:136–146.
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366:1489–1497.
- Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2005; 293:1501–1508.
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008; 52:255–262.
- Mack MJ, Banning AP, Serruys PW, et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg 2011; 92:2140–2146.
- Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010; 55:432–440.
- The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007; 49:1600–1606.
- Hlatky MA. Compelling evidence for coronary-bypass surgery in patients with diabetes. N Engl J Med 2012; 367:2437–2438.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000; 102:2180–2184.
- Bluher M, Unger R, Rassoul F, et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or type II diabetes. Diabetologia 2002; 45:210–216.
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003; 108:1527–1532.
- Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003; 41:1071–1077.
- Waller BF, Palumbo PJ, Lie JT, Roberts WC. Status of the coronary arteries at necropsy in diabetes mellitus with onset after age 30 years. Analysis of 229 diabetic patients with and without clinical evidence of coronary heart disease and comparison to 183 control subjects. Am J Med 1980; 69:498–506.
- Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle-aged diabetic patients (the London Cohort of the WHO Multinational Study of Vascular Disease in Diabetics) I: causes and death rates. Diabetologia 1990; 33:538–541.
- Laskey WK, Selzer F, Vlachos HA, et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2002; 90:1062–1067.
- Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480.
- Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005; 111:143–149.
- Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetologica 1999; 36:77–84.
- Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100:642–647.
- The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med 1996; 335:217–225.
- King SB, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eightyear mortality in the Emory Angioplasty versus Surgery Trial (East). J Am Coll Cardiol 2000; 35:1116–1121.
- Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF; CABRI Investigators (Coronary Angioplasty versus Bypass Revascularization Investigation). Difference in the mortality of the CABRI diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J Cardiol 2001; 87:947–950.
- Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 2005; 46:575–581.
- Booth J, Clayton T, Pepper J, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation 2008; 118:381–388.
- Rodriguez AE, Baldi J, Fernandez Pereira C, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol 2005; 46:582–588.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013; 381:629–638.
- Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013; 61:808–816.
- Ellis SG. Coronary revascularization for patients with diabetes: updated data favor coronary artery bypass grafting. J Am Coll Cardiol 2013; 61:817–819.
- Bhatt DL, Marso SP, Lincoff AM, et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol 2000; 35:922–928.
- Serruys PW, Ong AT, Morice MC, et al. Arterial Revascularisation Therapies Study Part II - Sirolimus-eluting stents for the treatment of patients with multivessel de novo coronary artery lesions. EuroIntervention 2005; 1:147–156.
- Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013; 43:1006–1013.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366:1467–1476.
- Magnuson EA, Farkouh ME, Fuster V, et al; FREEDOM Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes and multivessel coronary artery disease: results from the FREEDOM trial. Circulation 2013; 127:820–831.
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986; 314:1–6.
- Tector AJ, Schmahl TM, Janson B, et al. The internal mammary artery graft. Its longevity after coronary bypass. JAMA 1981; 246:2181–2183.
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitax-eleluting stents in coronary artery disease. N Engl J Med 2010; 362:1663–1674.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010; 363:136–146.
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366:1489–1497.
KEY POINTS
- Patients with diabetes have a higher prevalence of multivessel coronary artery disease and often have complex, diffuse lesions.
- Bypass surgery is the preferred method of revascularization in appropriately selected patients with diabetes and multivessel coronary artery disease.
- In the FREEDOM trial, only about 10% of the screened patients were eligible for the study, limiting its generalizability; however, this is comparable to exclusion rates in previous large randomized trials.
- When choosing a revascularization method, the physician team needs to discuss the options with the patient before performing diagnostic angiography. The team should include a cardiac surgeon and a cardiologist.