User login
Clinical Characteristics and Outcomes of Non-ICU Hospitalization for COVID-19 in a Nonepicenter, Centrally Monitored Healthcare System
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is associated with a wide range of illness severity and community prevalence, with an estimated 20% to 30% of patients requiring hospitalization.1,2 Outcome studies of hospitalized patients to date have focused on epicenter healthcare systems operating at surge-level bed capacity in resource-limited settings with mortality exceeding 20% among patients with a discharge disposition3,4 and have had a publication bias toward those suffering critical illness.5-7 Generalizability of these results to nonepicenter hospital systems is unclear given potential differences in triage practices and resource availability according to disease prevalence, with nonepicenter systems that are operating below capacity potentially able to accommodate the needs of most, if not all patients, requiring inpatient level care. Clinical outcomes associated with non–critically ill patients in nonepicenter regions remain poorly characterized yet highly relevant because these will ultimately apply to most US and global healthcare environments.
Nonepicenter healthcare systems must anticipate disease requirements for noncritically ill patients hospitalized with COVID-19 in order to appropriately allocate resources, including monitoring services like continuous pulse oximetry and cardiac telemetry. Data regarding the incidence of in-hospital respiratory and cardiovascular complications, including arrhythmias, among non–intensive care unit (non-ICU) hospitalized patients with COVID-19 are limited, with little granularity in terms of associated variables.7-11 Further data are needed to guide prioritization of valuable non-ICU continuous monitoring resources to the highest-risk patients in order to minimize consumption of personal protective equipment, reduce healthcare worker exposure, and ensure adequate availability for the expansion of prepandemic services.
Projections indicate that COVID-19 incidence may persist in the coming months.11-13 As nonessential hospital operations simultaneously resume, planning for resource allocation for patients with COVID-19 must be incorporated into broader systems of care. Further data are needed to help hospitals anticipate resource needs during this transition, especially by most systems that are caring for COVID-19 patients in nonepicenter environments. Therefore, we conducted a retrospective study of a large, multihospital, nonepicenter health system equipped with centralized continuous monitoring services in order to describe the detailed clinical course, resource utilization, and risk factors for adverse events in patients with COVID-19 initially admitted to the non-ICU setting.
METHODS
Central Monitoring Unit
The central monitoring unit (CMU) provides standardized and continuous off-site secondary monitoring of cardiac telemetry and pulse oximetry for non-ICU patients within Cleveland Clinic hospitals (Ohio, Florida), with direct communication to bedside nursing and inpatient emergency response teams for clinically significant cardiac arrhythmias, respiratory events, and vital sign changes according to standardized indications, as previously reported.14 Clinical variables of interest, including electrocardiographic and vital sign data, are collected and periodically analyzed within a central registry for quality assurance, risk stratification, and resource allocation. The data registry carries Institutional Review Board approval for retrospective analysis and deidentified outcomes reporting with consent form waiver.
Study Design and Data Collection
All patients positive for SARS-CoV-2 infection by nasopharyngeal polymerase chain reaction assay (Applied Biosystems) admitted from the emergency department to a non-ICU bed at a CMU hospital on or after March 13, 2020, and subsequently discharged on or before May 1, 2020, were identified. Retrospective review of the electronic medical record was performed, with follow-up continued through hospital discharge. Data were collected on patient demographics, clinical characteristics including admission laboratories and chest x-ray findings (abnormal defined as presence of an infiltrate/opacity consistent with airspace disease), continuous monitoring utilization, respiratory support, medication treatment, ICU transfer, and final hospital disposition. In addition, prospective recordings of cardiac arrhythmias that prompted CMU notification of bedside nursing were reviewed.
The primary outcome was a composite of death, ICU transfer, or increased oxygen requirement defined as escalation from simple nasal cannula to either high-flow nasal cannula (HFNC), noninvasive ventilation (NIV) consisting of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), or mechanical ventilation. In accordance with published guidelines, patients were treated with supplemental oxygen to maintain peripheral oxygen saturation between 92% and 96%.15
Of note, based on the validated performance of high sensitivity troponin primarily for the diagnosis of acute myocardial infarction in patients presenting to the emergency department with chest pain, our system reserves its use for this context and prefers conventional (fourth generation) troponin T testing for inpatients. Therefore, conventional troponin T values are reported in this study.
Statistical Analyses
Continuous variables are expressed as mean ± standard deviation or median (interquartile range), and categorical variables are expressed as absolute numbers with percentages. Independent samples t and Mann-Whitney U tests were used to compare continuous variables, as appropriate, and chi-square testing was used to compare categorical variables. Clinical variables satisfying an a priori two-tailed threshold of P < .05 were retained for multivariable logistic regression analysis. Variables retaining P < .05 in multivariable modeling were considered statistically significant. Analyses were performed using SPSS software, Version 23 (SPSS Inc).
RESULTS
Baseline Characteristics
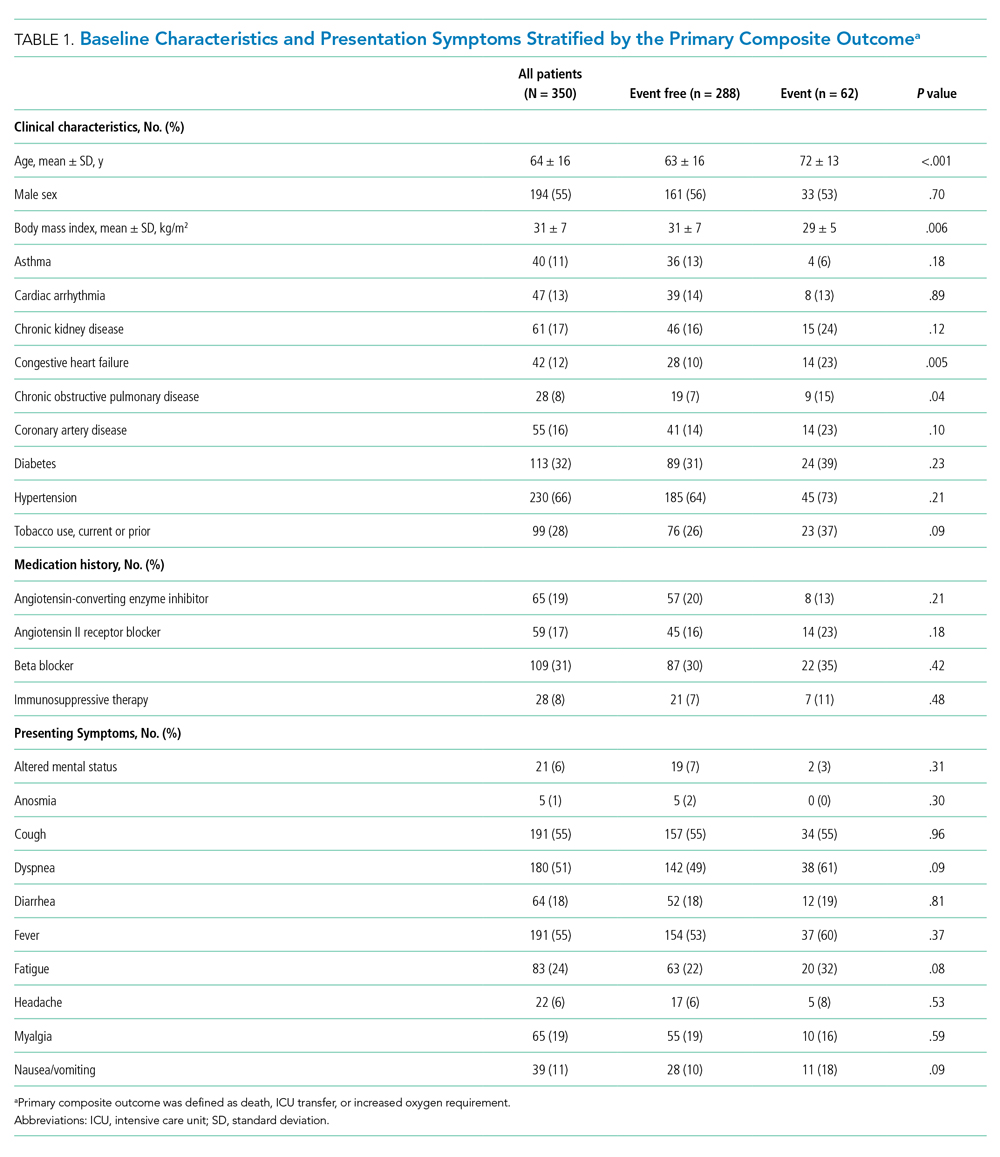
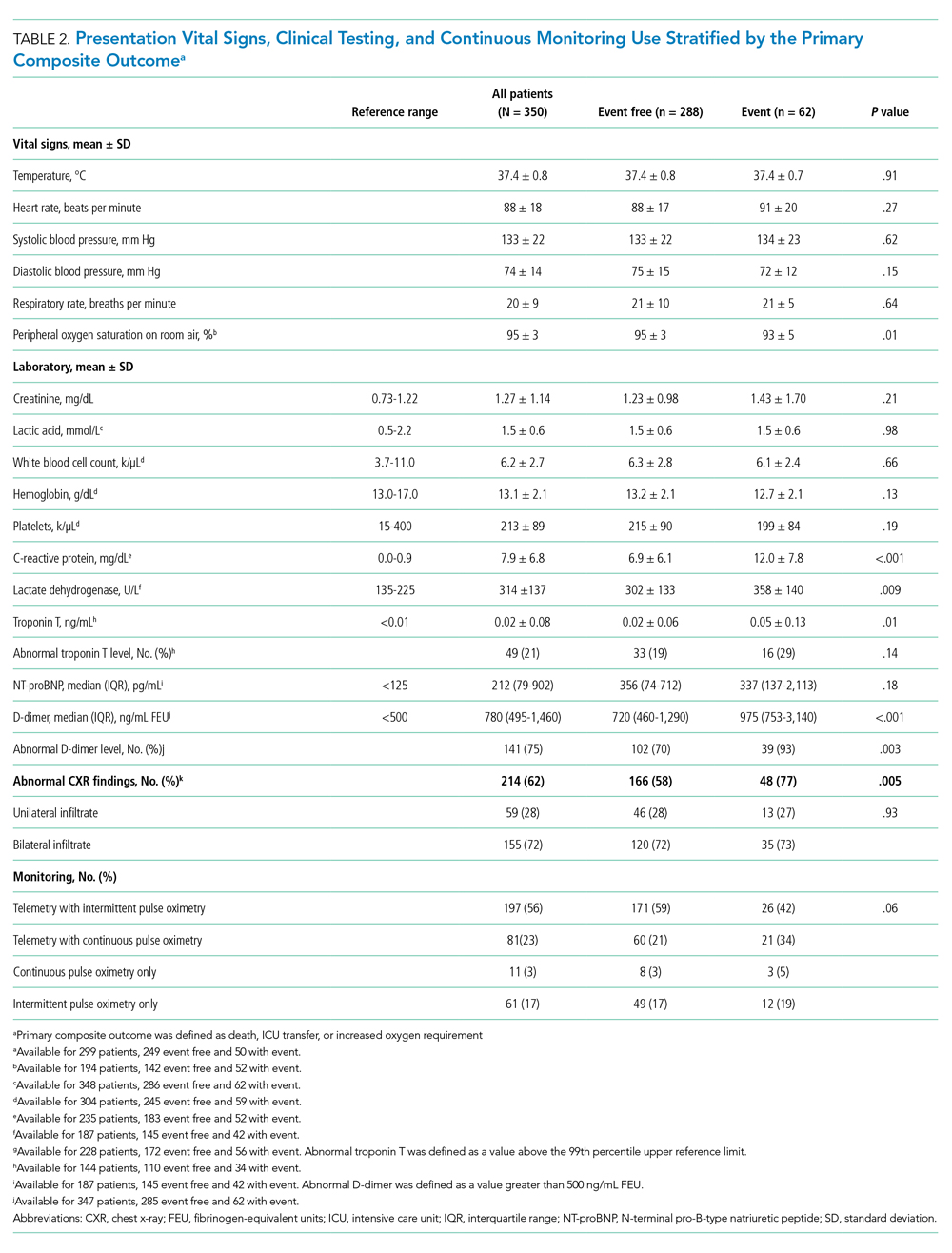
Between March 13, 2020, and May 1, 2020, a total of 350 patients admitted from the emergency department to a non-ICU inpatient bed had a final hospital disposition. Baseline characteristics, medication treatments, and continuous monitoring utilization are shown in Table 1 and Table 2. The average age was 64 ± 16 years, more than half of patients were male (n = 194; 55%), and most patients had at least one underlying comorbidity (n = 297; 85%), the most common being hypertension (n = 230; 66%), diabetes mellitus (n = 113; 32%), and current or prior tobacco use (n = 99; 28%). The presenting syndrome most frequently included subjective fever (n = 191; 55%), cough (n = 191; 55%), or dyspnea (n = 180; 51%).
Continuous Monitoring Use
Continuous monitoring was used in most patients (n = 289; 83%), including telemetry with intermittent pulse oximetry (n = 197; 56%), telemetry with continuous pulse oximetry (n = 81; 23%), or continuous pulse oximetry alone (n = 11; 3%). Among telemetry-monitored patients (n = 278; 79%), the most frequent indication was for a noncardiac disease state (n = 187; 67%), while indications for known cardiac arrhythmia (n = 74; 27%), heart failure (n = 10; 4%), or coronary artery disease (n = 7; 2%) were less common.
Oxygen Requirements and Cardiac Arrhythmias
The maximum level of respiratory support required by each patient is shown in Appendix Figure 1A. A total of 256 patients (73%) required 3 L/min or less of supplemental oxygen by nasal cannula, 45 (13%) required more than 3 L/min of supplemental oxygen by nasal cannula, 19 (5%) required HFNC, 8 (2%) required NIV, and 22 patients (6%) required mechanical ventilation. Among patients requiring HFNC or NIV, there were 13 (48%) who remained in a non-ICU bed, while the remaining 14 patients (52%) were transferred to the ICU.
Cardiac arrhythmias were detected in 39 (14%) of the 278 telemetry-monitored patients (Appendix Figure 1B). Clinical arrhythmias consisted of supraventricular tachycardia (SVT) in 17 patients (6%), nonsustained monomorphic ventricular tachycardia (VT) in 15 patients (5%), and a prolonged pause or severe bradyarrhythmia in 12 patients (4%). There were no cases of sustained monomorphic VT, polymorphic VT (including torsades de pointes), or ventricular fibrillation. All supraventricular tachycardias, nonsustained monomorphic VTs, and bradyarrhythmias/pauses were managed medically in the non-ICU setting, with the exception of one patient who was transferred to the ICU for a primary indication of atrial fibrillation with rapid ventricular response, which was treated with amiodarone. No patient with supraventricular tachycardia required emergent cardioversion, and no patient with a bradyarrhythmia or pause required temporary or permanent pacemaker implantation.
The detection of any arrhythmia was more common in patients with a history of cardiac arrhythmia (n = 18/41 vs 21/237; 44% vs 9%; P < .001), congestive heart failure (n = 11/36 vs 28/242; 31% vs 12%; P = .002), coronary artery disease (n = 12/49 vs 27/229; 24% vs 12%; P = .02), hypertension (n = 33/190 vs 6/88; 17% vs 7%; P = .02), and an abnormal admission troponin level (n = 13/40 vs 19/142; 33% vs 13%; P = .005). Notably, of the 39 patients with cardiac arrhythmias, 35 (90%) had either an abnormal admission troponin level or a history of cardiac arrhythmia, congestive heart failure, coronary artery disease, or hypertension. Of the 17 patients with SVT episodes, 13 (76%) had a known history of atrial fibrillation. Among patients who had a cardiac arrhythmia vs those who did not, there were no differences in levels of C-reactive protein (CRP; 7.3 ± 6.2 mg/dL vs. 7.8 ± 6.8 mg/dL, P = .63) or lactate dehydrogenase (LDH; 281 ± 89 U/L vs. 318 ± 142 U/L; P = .17). Approximately half of patients were treated with hydroxychloroquine (n = 185; 53%) or azithromycin (n = 182; 52%); 41% were treated with both (n = 142), with no observed association between any arrhythmia type and treatment with one or both medications (P > .05 for all comparisons).
Discharge Disposition and Adverse Outcomes
After an average length of stay of 6.1 ± 5.9 days, final hospital disposition included discharge to home (n = 278; 79%), discharge to subacute facility (n = 40; 11%), discharge to hospice (n = 8; 2%), death (n = 22, 6%), or release against medical advice (n = 2; 1%) (Figure). The primary composite outcome occurred in 62 patients (18%), including 22 deaths (6%), 48 ICU transfers (14%), and 49 patients with increased oxygen requirements (14%). Only two deaths occurred in the absence of an increased oxygen requirement or ICU transfer.

Increased oxygen requirement was the indication for ICU transfer in 37 of 48 patients (77%), with 22 patients (46%) requiring mechanical ventilation. Of the 48 patients requiring ICU transfer, 14 (29%) died, including 10 of the 22 patients (45%) treated with mechanical ventilation. Of the 302 patients who remained in the non-ICU setting, 8 (3%) died and 8 (3%) were discharged to hospice.
In univariable analyses, the primary composite outcome was more common among older patients (event vs event free, 72 ± 13 years vs 63 ± 16 years; P < .001); it was also more common in patients with congestive heart failure (n = 14/62 vs 28/288; 23% vs 10%; P = .005), chronic obstructive pulmonary disease (n = 9/62 vs 19/288; 15% vs 7%; P = .04), lower body mass index (29 ± 5 kg/m2 vs 31 ± 7 kg/m2; P = .006), lower peripheral oxygen saturation on room air (93% ± 5% vs 95% ± 3%; P = .005), higher CRP level (12.0 ± 7.8 mg/dL vs 6.9 ± 6.1 mg/dL; P < .001), higher LDH level (358 ± 140 U/L vs 302 ± 133 U/L; P = .009), higher troponin level (0.05 ± 0.13 ng/dL vs 0.02 ± 0.06 ng/dL; P = .01), abnormal D-dimer level (n = 39/42 vs 102/145; 93% vs 70%; P = .003), and abnormal chest x-ray findings (n = 48/62 vs 166/285; 77% vs 58%; P = .005) (Table 1 and Table 2). After multivariable adjustment, CRP level (odds ratio [OR], 1.09 per 1 mg/dL increase; 95% CI, 1.01-1.18; P = .04) and LDH level (OR, 1.006 per 1 U/L increase; 95% CI, 1.001-1.012; P = .03) remained significantly associated with the composite adverse outcome (Table 3). The rate of death, ICU transfer, or increased oxygen requirement was sixfold higher in patients with a CRP level in the fourth quartile (≥11.0 mg/dL) than it was among those in the first quartile (≤ 2.6 mg/dL) (P < .001 for trend), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L) than it was among those in the first quartile (≤ 232 U/L) (P = .001 for trend) (Appendix Figure 2). No patient with a CRP level in the reference range (≤ 0.9 mg/dL) experienced the composite adverse event, compared to three patients (n = 3/49, 6.1%) within the reference range for LDH level (≤ 225 U/L), all of whom had an elevated CRP.

DISCUSSION
In this study of 350 patients initially admitted to a non-ICU hospital bed within a large, nonepicenter healthcare system, the primary outcome of death, ICU transfer, or increased oxygen requirement occurred in 18% of patients and was independently associated with higher admission CRP and LDH levels on multivariable analysis. Most patients (73%) required 3 L/min or less of supplemental oxygen, while 14% of patients required escalation to HFNC, NIV, or mechanical ventilation. Despite frequent telemetry use (79%), cardiac arrhythmias were uncommon (14%), including no life-threatening ventricular arrhythmias. Clinical deterioration requiring ICU transfer occurred in 14% of patients, most often for an indication of increased oxygen requirement (77%). In-hospital mortality was 6% for the entire cohort, 29% for patients requiring ICU transfer, and 3% for patients who remained in the non-ICU setting.
Nonepicenter, Non-ICU Mortality
This study offers an assessment of clinical outcomes in patients with COVID-19 hospitalized in a non-ICU, nonepicenter healthcare system operating below capacity. Although such systems account for most institutions caring for patients with COVID-19, this population has been underrepresented in the literature, which has focused on epicenter hospitals and critically ill patients.3-7 Existing epicenter estimates of in-hospital mortality for patients not requiring ICU-level care range from 6% in Northern California2 to at least 10% in New York, New York,3 and 11% in Wuhan, China.4 The corresponding non-ICU in-hospital mortality in our study was only 3%, supporting the vital role of social distancing in reducing COVID-19 mortality by facilitating care delivery in a non–resource limited hospital setting.
Oxygen Requirements and Cardiac Arrhythmias in Non-ICU Patients
Beyond nonepicenter mortality estimates, this study is the first to provide a detailed characterization of the clinical course and resource usage among patients with COVID-19 admitted to the non-ICU setting. Given the predicted persistence of SARS-CoV-2 spread,11-13 this information is crucial to healthcare systems that must anticipate resource requirements, such as respiratory support and continuous monitoring equipment, for the care of hospitalized patients with COVID-19. Such informed planning takes on even greater importance as prepandemic hospital services resume.
While most patients (73%) with COVID-19 admitted to a non-ICU bed required peak supplemental oxygen of 3 L/min or less, a relevant proportion (14%) developed a need for HFNC, NIV, or mechanical ventilation. Furthermore, among telemetry-monitored patients (79%), cardiac arrhythmias were uncommon (14%), and nearly all (90%) occurred in patients with either a positive troponin or known history of cardiac disease. There were no life-threatening ventricular arrhythmias associated with frequent use of hydroxychloroquine (53%) and azithromycin (52%).
These telemetry findings expand upon a smaller study of non-ICU patients receiving either hydroxychloroquine or azithromycin, in which no life-threatening ventricular tachyarrhythmias were detected.8 A separate study reported a 5.9% incidence of malignant ventricular tachyarrhythmias in hospitalized patients with COVID-19,10 but this study did not stratify arrhythmias by illness severity, and a high frequency of critical illness is suggested by the mechanical ventilation rate of 24%, thereby limiting comparison with our non-ICU telemetry findings.
CRP and LDH Levels as Predictors of Adverse Outcomes
This study supports the utility of obtaining CRP and LDH levels for risk stratification at the time of non-ICU hospital admission. In multivariable analysis, higher CRP and LDH levels were significantly associated with the composite adverse outcome. The adverse event rates was increased sixfold between patients with a CRP in the fourth quartile (≥ 11.0 mg/dL, 36%) and those in the first quartile (≤ 2.6 mg/dL, 5.3%), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L, 34%) compared with those in the first quartile (≤ 232 U/L, 7%).
These findings are consistent with prior studies that have associated elevated inflammatory markers with poor prognosis and death.7,9,16 In some cases, COVID-19 may manifest similar to a cytokine storm syndrome, which highlights the importance of inflammation-associated tissue injury and leads to widespread interest in the use of immunosuppressive medications.17,18 Several studies also have demonstrated an association between LDH level and severe illness,4,7,19 although this is the first to specifically demonstrate its association with clinical decompensation in the non-ICU hospitalized population. Given that SARS-CoV-2 can infect multiple organs,20,21 there is biological plausibility for the use of LDH levels as a nonspecific marker of tissue injury for early identification of more severe infection.
Notably, while elevated troponin levels have been strongly associated with the need for mechanical ventilation and with death, this has primarily been established using either high-sensitivity troponin assays at the time of admission22 or using peak conventional troponin levels during hospitalization.10 In this study, while abnormal conventional troponin levels at the time of non-ICU admission were not significantly associated with the primary outcome in multivariable analysis, absolute troponin values were significantly higher in univariable analysis. Incomplete troponin sampling and the lack of routine high-sensitivity troponin assay use may explain the lack of more robust troponin significance in this study.
Implications for Non-ICU Continuous Monitoring Resource Allocation
Prioritization of non-ICU continuous monitoring resources among patients with COVID-19 has numerous benefits, including reduced consumption of personal protective equipment, fewer healthcare worker exposures, and adequate availability of continuous monitoring for the expansion of prepandemic hospital services. While individualized clinical discretion is still required, the results of this study can be used as a guide for the allocation of continuous pulse oximetry and cardiac telemetry. Patients with a normal presenting CRP level and/or LDH level had a low incidence of clinical decompensation, which suggests that such patients could be monitored with intermittent rather than continuous pulse oximetry. Furthermore, cardiac telemetry could be reserved for patients with a history of cardiac comorbidities or abnormal troponin levels because such patients accounted for 90% of cardiac arrhythmias in this study.
Limitations
This study was limited to a single health system, and it lacks a direct comparison to nonhospitalized patients and those directly admitted to the ICU. Triage practices and thresholds for hospitalization may differ across institutions and regions, thereby limiting the generalizability of our study. Additional limitations include the lack of selected admission laboratories for all patients, as well as the lack of telemetry monitoring in all patients. However, any resulting selection bias may be more likely to attenuate the magnitude of observed effects given that additional testing and increased telemetry use may be expected in patients who are felt to be higher risk by routine clinical assessment.
CONCLUSION
In this study of non–critically ill patients hospitalized within a nonepicenter health system, the development of more severe illness or death was significantly associated with higher levels of CRP and LDH on admission. Clinical decompensation was driven largely by respiratory complications, while cardiac arrhythmias were rare. Overall, the non-ICU mortality rate was at least half of that reported in epicenter regions. Altogether, these findings provide valuable information for resource allocation planning while nonepicenter health systems continue caring for patients with COVID-19 as they also resume prepandemic operations.
1. Bialek S, Boundy E, Bowen V, et al; CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
2. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195-2198. https://doi.org/10.1001/jama.2020.7202
3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. Published online April 22, 2020. https://doi.org/10.1001/jama.2020.6775
4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
5. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612-1614. https://doi.org/10.1001/jama.2020.4326
6. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574-1581. https://doi.org/10.1001/jama.2020.5394
7. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
8. Chang D, Saleh M, Gabriels J, et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75(23):2992-2993. https://doi.org/10.1016/j.jacc.2020.04.032
9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. https://doi.org/10.1016/s0140-6736(20)30183-5
10. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):1-8. https://doi.org/10.1001/jamacardio.2020.1017
11. Centers for Disease Control and Prevention COVID-19 Forecasts. Accessed May 19, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/forecasting-us.html
12. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860-868. https://doi.org/10.1126/science.abb5793
13. Baker RE, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science. 2020;369(6501):315-319. https://doi.org/10.1126/science.abc2535
14. Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non-critically ill patients. JAMA. 2016;316(5):519-524. https://doi.org/10.1001/jama.2016.10258
15. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. https://doi.org/10.1097/ccm.0000000000004363
16. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
17. Mehta P, McAuley DF, Brown M, et al; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. https://doi.org/10.1016/s0140-6736(20)30628-0
18. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. Published online April 13, 2020. https://doi.org/10.1001/jama.2020.6019
19. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1-9. https://doi.org/10.1001/jamainternmed.2020.2033
20. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590-592. https://doi.org/10.1056/nejmc2011400
21. Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020;26(7):1077-1083. https://doi.org/10.1038/s41591-020-0912-6
22. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810. https://doi.org/10.1001/jamacardio.2020.0950
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is associated with a wide range of illness severity and community prevalence, with an estimated 20% to 30% of patients requiring hospitalization.1,2 Outcome studies of hospitalized patients to date have focused on epicenter healthcare systems operating at surge-level bed capacity in resource-limited settings with mortality exceeding 20% among patients with a discharge disposition3,4 and have had a publication bias toward those suffering critical illness.5-7 Generalizability of these results to nonepicenter hospital systems is unclear given potential differences in triage practices and resource availability according to disease prevalence, with nonepicenter systems that are operating below capacity potentially able to accommodate the needs of most, if not all patients, requiring inpatient level care. Clinical outcomes associated with non–critically ill patients in nonepicenter regions remain poorly characterized yet highly relevant because these will ultimately apply to most US and global healthcare environments.
Nonepicenter healthcare systems must anticipate disease requirements for noncritically ill patients hospitalized with COVID-19 in order to appropriately allocate resources, including monitoring services like continuous pulse oximetry and cardiac telemetry. Data regarding the incidence of in-hospital respiratory and cardiovascular complications, including arrhythmias, among non–intensive care unit (non-ICU) hospitalized patients with COVID-19 are limited, with little granularity in terms of associated variables.7-11 Further data are needed to guide prioritization of valuable non-ICU continuous monitoring resources to the highest-risk patients in order to minimize consumption of personal protective equipment, reduce healthcare worker exposure, and ensure adequate availability for the expansion of prepandemic services.
Projections indicate that COVID-19 incidence may persist in the coming months.11-13 As nonessential hospital operations simultaneously resume, planning for resource allocation for patients with COVID-19 must be incorporated into broader systems of care. Further data are needed to help hospitals anticipate resource needs during this transition, especially by most systems that are caring for COVID-19 patients in nonepicenter environments. Therefore, we conducted a retrospective study of a large, multihospital, nonepicenter health system equipped with centralized continuous monitoring services in order to describe the detailed clinical course, resource utilization, and risk factors for adverse events in patients with COVID-19 initially admitted to the non-ICU setting.
METHODS
Central Monitoring Unit
The central monitoring unit (CMU) provides standardized and continuous off-site secondary monitoring of cardiac telemetry and pulse oximetry for non-ICU patients within Cleveland Clinic hospitals (Ohio, Florida), with direct communication to bedside nursing and inpatient emergency response teams for clinically significant cardiac arrhythmias, respiratory events, and vital sign changes according to standardized indications, as previously reported.14 Clinical variables of interest, including electrocardiographic and vital sign data, are collected and periodically analyzed within a central registry for quality assurance, risk stratification, and resource allocation. The data registry carries Institutional Review Board approval for retrospective analysis and deidentified outcomes reporting with consent form waiver.
Study Design and Data Collection
All patients positive for SARS-CoV-2 infection by nasopharyngeal polymerase chain reaction assay (Applied Biosystems) admitted from the emergency department to a non-ICU bed at a CMU hospital on or after March 13, 2020, and subsequently discharged on or before May 1, 2020, were identified. Retrospective review of the electronic medical record was performed, with follow-up continued through hospital discharge. Data were collected on patient demographics, clinical characteristics including admission laboratories and chest x-ray findings (abnormal defined as presence of an infiltrate/opacity consistent with airspace disease), continuous monitoring utilization, respiratory support, medication treatment, ICU transfer, and final hospital disposition. In addition, prospective recordings of cardiac arrhythmias that prompted CMU notification of bedside nursing were reviewed.
The primary outcome was a composite of death, ICU transfer, or increased oxygen requirement defined as escalation from simple nasal cannula to either high-flow nasal cannula (HFNC), noninvasive ventilation (NIV) consisting of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), or mechanical ventilation. In accordance with published guidelines, patients were treated with supplemental oxygen to maintain peripheral oxygen saturation between 92% and 96%.15
Of note, based on the validated performance of high sensitivity troponin primarily for the diagnosis of acute myocardial infarction in patients presenting to the emergency department with chest pain, our system reserves its use for this context and prefers conventional (fourth generation) troponin T testing for inpatients. Therefore, conventional troponin T values are reported in this study.
Statistical Analyses
Continuous variables are expressed as mean ± standard deviation or median (interquartile range), and categorical variables are expressed as absolute numbers with percentages. Independent samples t and Mann-Whitney U tests were used to compare continuous variables, as appropriate, and chi-square testing was used to compare categorical variables. Clinical variables satisfying an a priori two-tailed threshold of P < .05 were retained for multivariable logistic regression analysis. Variables retaining P < .05 in multivariable modeling were considered statistically significant. Analyses were performed using SPSS software, Version 23 (SPSS Inc).
RESULTS
Baseline Characteristics
Between March 13, 2020, and May 1, 2020, a total of 350 patients admitted from the emergency department to a non-ICU inpatient bed had a final hospital disposition. Baseline characteristics, medication treatments, and continuous monitoring utilization are shown in Table 1 and Table 2. The average age was 64 ± 16 years, more than half of patients were male (n = 194; 55%), and most patients had at least one underlying comorbidity (n = 297; 85%), the most common being hypertension (n = 230; 66%), diabetes mellitus (n = 113; 32%), and current or prior tobacco use (n = 99; 28%). The presenting syndrome most frequently included subjective fever (n = 191; 55%), cough (n = 191; 55%), or dyspnea (n = 180; 51%).

Continuous Monitoring Use
Continuous monitoring was used in most patients (n = 289; 83%), including telemetry with intermittent pulse oximetry (n = 197; 56%), telemetry with continuous pulse oximetry (n = 81; 23%), or continuous pulse oximetry alone (n = 11; 3%). Among telemetry-monitored patients (n = 278; 79%), the most frequent indication was for a noncardiac disease state (n = 187; 67%), while indications for known cardiac arrhythmia (n = 74; 27%), heart failure (n = 10; 4%), or coronary artery disease (n = 7; 2%) were less common.

Oxygen Requirements and Cardiac Arrhythmias
The maximum level of respiratory support required by each patient is shown in Appendix Figure 1A. A total of 256 patients (73%) required 3 L/min or less of supplemental oxygen by nasal cannula, 45 (13%) required more than 3 L/min of supplemental oxygen by nasal cannula, 19 (5%) required HFNC, 8 (2%) required NIV, and 22 patients (6%) required mechanical ventilation. Among patients requiring HFNC or NIV, there were 13 (48%) who remained in a non-ICU bed, while the remaining 14 patients (52%) were transferred to the ICU.
Cardiac arrhythmias were detected in 39 (14%) of the 278 telemetry-monitored patients (Appendix Figure 1B). Clinical arrhythmias consisted of supraventricular tachycardia (SVT) in 17 patients (6%), nonsustained monomorphic ventricular tachycardia (VT) in 15 patients (5%), and a prolonged pause or severe bradyarrhythmia in 12 patients (4%). There were no cases of sustained monomorphic VT, polymorphic VT (including torsades de pointes), or ventricular fibrillation. All supraventricular tachycardias, nonsustained monomorphic VTs, and bradyarrhythmias/pauses were managed medically in the non-ICU setting, with the exception of one patient who was transferred to the ICU for a primary indication of atrial fibrillation with rapid ventricular response, which was treated with amiodarone. No patient with supraventricular tachycardia required emergent cardioversion, and no patient with a bradyarrhythmia or pause required temporary or permanent pacemaker implantation.
The detection of any arrhythmia was more common in patients with a history of cardiac arrhythmia (n = 18/41 vs 21/237; 44% vs 9%; P < .001), congestive heart failure (n = 11/36 vs 28/242; 31% vs 12%; P = .002), coronary artery disease (n = 12/49 vs 27/229; 24% vs 12%; P = .02), hypertension (n = 33/190 vs 6/88; 17% vs 7%; P = .02), and an abnormal admission troponin level (n = 13/40 vs 19/142; 33% vs 13%; P = .005). Notably, of the 39 patients with cardiac arrhythmias, 35 (90%) had either an abnormal admission troponin level or a history of cardiac arrhythmia, congestive heart failure, coronary artery disease, or hypertension. Of the 17 patients with SVT episodes, 13 (76%) had a known history of atrial fibrillation. Among patients who had a cardiac arrhythmia vs those who did not, there were no differences in levels of C-reactive protein (CRP; 7.3 ± 6.2 mg/dL vs. 7.8 ± 6.8 mg/dL, P = .63) or lactate dehydrogenase (LDH; 281 ± 89 U/L vs. 318 ± 142 U/L; P = .17). Approximately half of patients were treated with hydroxychloroquine (n = 185; 53%) or azithromycin (n = 182; 52%); 41% were treated with both (n = 142), with no observed association between any arrhythmia type and treatment with one or both medications (P > .05 for all comparisons).
Discharge Disposition and Adverse Outcomes
After an average length of stay of 6.1 ± 5.9 days, final hospital disposition included discharge to home (n = 278; 79%), discharge to subacute facility (n = 40; 11%), discharge to hospice (n = 8; 2%), death (n = 22, 6%), or release against medical advice (n = 2; 1%) (Figure). The primary composite outcome occurred in 62 patients (18%), including 22 deaths (6%), 48 ICU transfers (14%), and 49 patients with increased oxygen requirements (14%). Only two deaths occurred in the absence of an increased oxygen requirement or ICU transfer.

Increased oxygen requirement was the indication for ICU transfer in 37 of 48 patients (77%), with 22 patients (46%) requiring mechanical ventilation. Of the 48 patients requiring ICU transfer, 14 (29%) died, including 10 of the 22 patients (45%) treated with mechanical ventilation. Of the 302 patients who remained in the non-ICU setting, 8 (3%) died and 8 (3%) were discharged to hospice.
In univariable analyses, the primary composite outcome was more common among older patients (event vs event free, 72 ± 13 years vs 63 ± 16 years; P < .001); it was also more common in patients with congestive heart failure (n = 14/62 vs 28/288; 23% vs 10%; P = .005), chronic obstructive pulmonary disease (n = 9/62 vs 19/288; 15% vs 7%; P = .04), lower body mass index (29 ± 5 kg/m2 vs 31 ± 7 kg/m2; P = .006), lower peripheral oxygen saturation on room air (93% ± 5% vs 95% ± 3%; P = .005), higher CRP level (12.0 ± 7.8 mg/dL vs 6.9 ± 6.1 mg/dL; P < .001), higher LDH level (358 ± 140 U/L vs 302 ± 133 U/L; P = .009), higher troponin level (0.05 ± 0.13 ng/dL vs 0.02 ± 0.06 ng/dL; P = .01), abnormal D-dimer level (n = 39/42 vs 102/145; 93% vs 70%; P = .003), and abnormal chest x-ray findings (n = 48/62 vs 166/285; 77% vs 58%; P = .005) (Table 1 and Table 2). After multivariable adjustment, CRP level (odds ratio [OR], 1.09 per 1 mg/dL increase; 95% CI, 1.01-1.18; P = .04) and LDH level (OR, 1.006 per 1 U/L increase; 95% CI, 1.001-1.012; P = .03) remained significantly associated with the composite adverse outcome (Table 3). The rate of death, ICU transfer, or increased oxygen requirement was sixfold higher in patients with a CRP level in the fourth quartile (≥11.0 mg/dL) than it was among those in the first quartile (≤ 2.6 mg/dL) (P < .001 for trend), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L) than it was among those in the first quartile (≤ 232 U/L) (P = .001 for trend) (Appendix Figure 2). No patient with a CRP level in the reference range (≤ 0.9 mg/dL) experienced the composite adverse event, compared to three patients (n = 3/49, 6.1%) within the reference range for LDH level (≤ 225 U/L), all of whom had an elevated CRP.

DISCUSSION
In this study of 350 patients initially admitted to a non-ICU hospital bed within a large, nonepicenter healthcare system, the primary outcome of death, ICU transfer, or increased oxygen requirement occurred in 18% of patients and was independently associated with higher admission CRP and LDH levels on multivariable analysis. Most patients (73%) required 3 L/min or less of supplemental oxygen, while 14% of patients required escalation to HFNC, NIV, or mechanical ventilation. Despite frequent telemetry use (79%), cardiac arrhythmias were uncommon (14%), including no life-threatening ventricular arrhythmias. Clinical deterioration requiring ICU transfer occurred in 14% of patients, most often for an indication of increased oxygen requirement (77%). In-hospital mortality was 6% for the entire cohort, 29% for patients requiring ICU transfer, and 3% for patients who remained in the non-ICU setting.
Nonepicenter, Non-ICU Mortality
This study offers an assessment of clinical outcomes in patients with COVID-19 hospitalized in a non-ICU, nonepicenter healthcare system operating below capacity. Although such systems account for most institutions caring for patients with COVID-19, this population has been underrepresented in the literature, which has focused on epicenter hospitals and critically ill patients.3-7 Existing epicenter estimates of in-hospital mortality for patients not requiring ICU-level care range from 6% in Northern California2 to at least 10% in New York, New York,3 and 11% in Wuhan, China.4 The corresponding non-ICU in-hospital mortality in our study was only 3%, supporting the vital role of social distancing in reducing COVID-19 mortality by facilitating care delivery in a non–resource limited hospital setting.
Oxygen Requirements and Cardiac Arrhythmias in Non-ICU Patients
Beyond nonepicenter mortality estimates, this study is the first to provide a detailed characterization of the clinical course and resource usage among patients with COVID-19 admitted to the non-ICU setting. Given the predicted persistence of SARS-CoV-2 spread,11-13 this information is crucial to healthcare systems that must anticipate resource requirements, such as respiratory support and continuous monitoring equipment, for the care of hospitalized patients with COVID-19. Such informed planning takes on even greater importance as prepandemic hospital services resume.
While most patients (73%) with COVID-19 admitted to a non-ICU bed required peak supplemental oxygen of 3 L/min or less, a relevant proportion (14%) developed a need for HFNC, NIV, or mechanical ventilation. Furthermore, among telemetry-monitored patients (79%), cardiac arrhythmias were uncommon (14%), and nearly all (90%) occurred in patients with either a positive troponin or known history of cardiac disease. There were no life-threatening ventricular arrhythmias associated with frequent use of hydroxychloroquine (53%) and azithromycin (52%).
These telemetry findings expand upon a smaller study of non-ICU patients receiving either hydroxychloroquine or azithromycin, in which no life-threatening ventricular tachyarrhythmias were detected.8 A separate study reported a 5.9% incidence of malignant ventricular tachyarrhythmias in hospitalized patients with COVID-19,10 but this study did not stratify arrhythmias by illness severity, and a high frequency of critical illness is suggested by the mechanical ventilation rate of 24%, thereby limiting comparison with our non-ICU telemetry findings.
CRP and LDH Levels as Predictors of Adverse Outcomes
This study supports the utility of obtaining CRP and LDH levels for risk stratification at the time of non-ICU hospital admission. In multivariable analysis, higher CRP and LDH levels were significantly associated with the composite adverse outcome. The adverse event rates was increased sixfold between patients with a CRP in the fourth quartile (≥ 11.0 mg/dL, 36%) and those in the first quartile (≤ 2.6 mg/dL, 5.3%), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L, 34%) compared with those in the first quartile (≤ 232 U/L, 7%).
These findings are consistent with prior studies that have associated elevated inflammatory markers with poor prognosis and death.7,9,16 In some cases, COVID-19 may manifest similar to a cytokine storm syndrome, which highlights the importance of inflammation-associated tissue injury and leads to widespread interest in the use of immunosuppressive medications.17,18 Several studies also have demonstrated an association between LDH level and severe illness,4,7,19 although this is the first to specifically demonstrate its association with clinical decompensation in the non-ICU hospitalized population. Given that SARS-CoV-2 can infect multiple organs,20,21 there is biological plausibility for the use of LDH levels as a nonspecific marker of tissue injury for early identification of more severe infection.
Notably, while elevated troponin levels have been strongly associated with the need for mechanical ventilation and with death, this has primarily been established using either high-sensitivity troponin assays at the time of admission22 or using peak conventional troponin levels during hospitalization.10 In this study, while abnormal conventional troponin levels at the time of non-ICU admission were not significantly associated with the primary outcome in multivariable analysis, absolute troponin values were significantly higher in univariable analysis. Incomplete troponin sampling and the lack of routine high-sensitivity troponin assay use may explain the lack of more robust troponin significance in this study.
Implications for Non-ICU Continuous Monitoring Resource Allocation
Prioritization of non-ICU continuous monitoring resources among patients with COVID-19 has numerous benefits, including reduced consumption of personal protective equipment, fewer healthcare worker exposures, and adequate availability of continuous monitoring for the expansion of prepandemic hospital services. While individualized clinical discretion is still required, the results of this study can be used as a guide for the allocation of continuous pulse oximetry and cardiac telemetry. Patients with a normal presenting CRP level and/or LDH level had a low incidence of clinical decompensation, which suggests that such patients could be monitored with intermittent rather than continuous pulse oximetry. Furthermore, cardiac telemetry could be reserved for patients with a history of cardiac comorbidities or abnormal troponin levels because such patients accounted for 90% of cardiac arrhythmias in this study.
Limitations
This study was limited to a single health system, and it lacks a direct comparison to nonhospitalized patients and those directly admitted to the ICU. Triage practices and thresholds for hospitalization may differ across institutions and regions, thereby limiting the generalizability of our study. Additional limitations include the lack of selected admission laboratories for all patients, as well as the lack of telemetry monitoring in all patients. However, any resulting selection bias may be more likely to attenuate the magnitude of observed effects given that additional testing and increased telemetry use may be expected in patients who are felt to be higher risk by routine clinical assessment.
CONCLUSION
In this study of non–critically ill patients hospitalized within a nonepicenter health system, the development of more severe illness or death was significantly associated with higher levels of CRP and LDH on admission. Clinical decompensation was driven largely by respiratory complications, while cardiac arrhythmias were rare. Overall, the non-ICU mortality rate was at least half of that reported in epicenter regions. Altogether, these findings provide valuable information for resource allocation planning while nonepicenter health systems continue caring for patients with COVID-19 as they also resume prepandemic operations.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is associated with a wide range of illness severity and community prevalence, with an estimated 20% to 30% of patients requiring hospitalization.1,2 Outcome studies of hospitalized patients to date have focused on epicenter healthcare systems operating at surge-level bed capacity in resource-limited settings with mortality exceeding 20% among patients with a discharge disposition3,4 and have had a publication bias toward those suffering critical illness.5-7 Generalizability of these results to nonepicenter hospital systems is unclear given potential differences in triage practices and resource availability according to disease prevalence, with nonepicenter systems that are operating below capacity potentially able to accommodate the needs of most, if not all patients, requiring inpatient level care. Clinical outcomes associated with non–critically ill patients in nonepicenter regions remain poorly characterized yet highly relevant because these will ultimately apply to most US and global healthcare environments.
Nonepicenter healthcare systems must anticipate disease requirements for noncritically ill patients hospitalized with COVID-19 in order to appropriately allocate resources, including monitoring services like continuous pulse oximetry and cardiac telemetry. Data regarding the incidence of in-hospital respiratory and cardiovascular complications, including arrhythmias, among non–intensive care unit (non-ICU) hospitalized patients with COVID-19 are limited, with little granularity in terms of associated variables.7-11 Further data are needed to guide prioritization of valuable non-ICU continuous monitoring resources to the highest-risk patients in order to minimize consumption of personal protective equipment, reduce healthcare worker exposure, and ensure adequate availability for the expansion of prepandemic services.
Projections indicate that COVID-19 incidence may persist in the coming months.11-13 As nonessential hospital operations simultaneously resume, planning for resource allocation for patients with COVID-19 must be incorporated into broader systems of care. Further data are needed to help hospitals anticipate resource needs during this transition, especially by most systems that are caring for COVID-19 patients in nonepicenter environments. Therefore, we conducted a retrospective study of a large, multihospital, nonepicenter health system equipped with centralized continuous monitoring services in order to describe the detailed clinical course, resource utilization, and risk factors for adverse events in patients with COVID-19 initially admitted to the non-ICU setting.
METHODS
Central Monitoring Unit
The central monitoring unit (CMU) provides standardized and continuous off-site secondary monitoring of cardiac telemetry and pulse oximetry for non-ICU patients within Cleveland Clinic hospitals (Ohio, Florida), with direct communication to bedside nursing and inpatient emergency response teams for clinically significant cardiac arrhythmias, respiratory events, and vital sign changes according to standardized indications, as previously reported.14 Clinical variables of interest, including electrocardiographic and vital sign data, are collected and periodically analyzed within a central registry for quality assurance, risk stratification, and resource allocation. The data registry carries Institutional Review Board approval for retrospective analysis and deidentified outcomes reporting with consent form waiver.
Study Design and Data Collection
All patients positive for SARS-CoV-2 infection by nasopharyngeal polymerase chain reaction assay (Applied Biosystems) admitted from the emergency department to a non-ICU bed at a CMU hospital on or after March 13, 2020, and subsequently discharged on or before May 1, 2020, were identified. Retrospective review of the electronic medical record was performed, with follow-up continued through hospital discharge. Data were collected on patient demographics, clinical characteristics including admission laboratories and chest x-ray findings (abnormal defined as presence of an infiltrate/opacity consistent with airspace disease), continuous monitoring utilization, respiratory support, medication treatment, ICU transfer, and final hospital disposition. In addition, prospective recordings of cardiac arrhythmias that prompted CMU notification of bedside nursing were reviewed.
The primary outcome was a composite of death, ICU transfer, or increased oxygen requirement defined as escalation from simple nasal cannula to either high-flow nasal cannula (HFNC), noninvasive ventilation (NIV) consisting of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP), or mechanical ventilation. In accordance with published guidelines, patients were treated with supplemental oxygen to maintain peripheral oxygen saturation between 92% and 96%.15
Of note, based on the validated performance of high sensitivity troponin primarily for the diagnosis of acute myocardial infarction in patients presenting to the emergency department with chest pain, our system reserves its use for this context and prefers conventional (fourth generation) troponin T testing for inpatients. Therefore, conventional troponin T values are reported in this study.
Statistical Analyses
Continuous variables are expressed as mean ± standard deviation or median (interquartile range), and categorical variables are expressed as absolute numbers with percentages. Independent samples t and Mann-Whitney U tests were used to compare continuous variables, as appropriate, and chi-square testing was used to compare categorical variables. Clinical variables satisfying an a priori two-tailed threshold of P < .05 were retained for multivariable logistic regression analysis. Variables retaining P < .05 in multivariable modeling were considered statistically significant. Analyses were performed using SPSS software, Version 23 (SPSS Inc).
RESULTS
Baseline Characteristics
Between March 13, 2020, and May 1, 2020, a total of 350 patients admitted from the emergency department to a non-ICU inpatient bed had a final hospital disposition. Baseline characteristics, medication treatments, and continuous monitoring utilization are shown in Table 1 and Table 2. The average age was 64 ± 16 years, more than half of patients were male (n = 194; 55%), and most patients had at least one underlying comorbidity (n = 297; 85%), the most common being hypertension (n = 230; 66%), diabetes mellitus (n = 113; 32%), and current or prior tobacco use (n = 99; 28%). The presenting syndrome most frequently included subjective fever (n = 191; 55%), cough (n = 191; 55%), or dyspnea (n = 180; 51%).

Continuous Monitoring Use
Continuous monitoring was used in most patients (n = 289; 83%), including telemetry with intermittent pulse oximetry (n = 197; 56%), telemetry with continuous pulse oximetry (n = 81; 23%), or continuous pulse oximetry alone (n = 11; 3%). Among telemetry-monitored patients (n = 278; 79%), the most frequent indication was for a noncardiac disease state (n = 187; 67%), while indications for known cardiac arrhythmia (n = 74; 27%), heart failure (n = 10; 4%), or coronary artery disease (n = 7; 2%) were less common.

Oxygen Requirements and Cardiac Arrhythmias
The maximum level of respiratory support required by each patient is shown in Appendix Figure 1A. A total of 256 patients (73%) required 3 L/min or less of supplemental oxygen by nasal cannula, 45 (13%) required more than 3 L/min of supplemental oxygen by nasal cannula, 19 (5%) required HFNC, 8 (2%) required NIV, and 22 patients (6%) required mechanical ventilation. Among patients requiring HFNC or NIV, there were 13 (48%) who remained in a non-ICU bed, while the remaining 14 patients (52%) were transferred to the ICU.
Cardiac arrhythmias were detected in 39 (14%) of the 278 telemetry-monitored patients (Appendix Figure 1B). Clinical arrhythmias consisted of supraventricular tachycardia (SVT) in 17 patients (6%), nonsustained monomorphic ventricular tachycardia (VT) in 15 patients (5%), and a prolonged pause or severe bradyarrhythmia in 12 patients (4%). There were no cases of sustained monomorphic VT, polymorphic VT (including torsades de pointes), or ventricular fibrillation. All supraventricular tachycardias, nonsustained monomorphic VTs, and bradyarrhythmias/pauses were managed medically in the non-ICU setting, with the exception of one patient who was transferred to the ICU for a primary indication of atrial fibrillation with rapid ventricular response, which was treated with amiodarone. No patient with supraventricular tachycardia required emergent cardioversion, and no patient with a bradyarrhythmia or pause required temporary or permanent pacemaker implantation.
The detection of any arrhythmia was more common in patients with a history of cardiac arrhythmia (n = 18/41 vs 21/237; 44% vs 9%; P < .001), congestive heart failure (n = 11/36 vs 28/242; 31% vs 12%; P = .002), coronary artery disease (n = 12/49 vs 27/229; 24% vs 12%; P = .02), hypertension (n = 33/190 vs 6/88; 17% vs 7%; P = .02), and an abnormal admission troponin level (n = 13/40 vs 19/142; 33% vs 13%; P = .005). Notably, of the 39 patients with cardiac arrhythmias, 35 (90%) had either an abnormal admission troponin level or a history of cardiac arrhythmia, congestive heart failure, coronary artery disease, or hypertension. Of the 17 patients with SVT episodes, 13 (76%) had a known history of atrial fibrillation. Among patients who had a cardiac arrhythmia vs those who did not, there were no differences in levels of C-reactive protein (CRP; 7.3 ± 6.2 mg/dL vs. 7.8 ± 6.8 mg/dL, P = .63) or lactate dehydrogenase (LDH; 281 ± 89 U/L vs. 318 ± 142 U/L; P = .17). Approximately half of patients were treated with hydroxychloroquine (n = 185; 53%) or azithromycin (n = 182; 52%); 41% were treated with both (n = 142), with no observed association between any arrhythmia type and treatment with one or both medications (P > .05 for all comparisons).
Discharge Disposition and Adverse Outcomes
After an average length of stay of 6.1 ± 5.9 days, final hospital disposition included discharge to home (n = 278; 79%), discharge to subacute facility (n = 40; 11%), discharge to hospice (n = 8; 2%), death (n = 22, 6%), or release against medical advice (n = 2; 1%) (Figure). The primary composite outcome occurred in 62 patients (18%), including 22 deaths (6%), 48 ICU transfers (14%), and 49 patients with increased oxygen requirements (14%). Only two deaths occurred in the absence of an increased oxygen requirement or ICU transfer.

Increased oxygen requirement was the indication for ICU transfer in 37 of 48 patients (77%), with 22 patients (46%) requiring mechanical ventilation. Of the 48 patients requiring ICU transfer, 14 (29%) died, including 10 of the 22 patients (45%) treated with mechanical ventilation. Of the 302 patients who remained in the non-ICU setting, 8 (3%) died and 8 (3%) were discharged to hospice.
In univariable analyses, the primary composite outcome was more common among older patients (event vs event free, 72 ± 13 years vs 63 ± 16 years; P < .001); it was also more common in patients with congestive heart failure (n = 14/62 vs 28/288; 23% vs 10%; P = .005), chronic obstructive pulmonary disease (n = 9/62 vs 19/288; 15% vs 7%; P = .04), lower body mass index (29 ± 5 kg/m2 vs 31 ± 7 kg/m2; P = .006), lower peripheral oxygen saturation on room air (93% ± 5% vs 95% ± 3%; P = .005), higher CRP level (12.0 ± 7.8 mg/dL vs 6.9 ± 6.1 mg/dL; P < .001), higher LDH level (358 ± 140 U/L vs 302 ± 133 U/L; P = .009), higher troponin level (0.05 ± 0.13 ng/dL vs 0.02 ± 0.06 ng/dL; P = .01), abnormal D-dimer level (n = 39/42 vs 102/145; 93% vs 70%; P = .003), and abnormal chest x-ray findings (n = 48/62 vs 166/285; 77% vs 58%; P = .005) (Table 1 and Table 2). After multivariable adjustment, CRP level (odds ratio [OR], 1.09 per 1 mg/dL increase; 95% CI, 1.01-1.18; P = .04) and LDH level (OR, 1.006 per 1 U/L increase; 95% CI, 1.001-1.012; P = .03) remained significantly associated with the composite adverse outcome (Table 3). The rate of death, ICU transfer, or increased oxygen requirement was sixfold higher in patients with a CRP level in the fourth quartile (≥11.0 mg/dL) than it was among those in the first quartile (≤ 2.6 mg/dL) (P < .001 for trend), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L) than it was among those in the first quartile (≤ 232 U/L) (P = .001 for trend) (Appendix Figure 2). No patient with a CRP level in the reference range (≤ 0.9 mg/dL) experienced the composite adverse event, compared to three patients (n = 3/49, 6.1%) within the reference range for LDH level (≤ 225 U/L), all of whom had an elevated CRP.

DISCUSSION
In this study of 350 patients initially admitted to a non-ICU hospital bed within a large, nonepicenter healthcare system, the primary outcome of death, ICU transfer, or increased oxygen requirement occurred in 18% of patients and was independently associated with higher admission CRP and LDH levels on multivariable analysis. Most patients (73%) required 3 L/min or less of supplemental oxygen, while 14% of patients required escalation to HFNC, NIV, or mechanical ventilation. Despite frequent telemetry use (79%), cardiac arrhythmias were uncommon (14%), including no life-threatening ventricular arrhythmias. Clinical deterioration requiring ICU transfer occurred in 14% of patients, most often for an indication of increased oxygen requirement (77%). In-hospital mortality was 6% for the entire cohort, 29% for patients requiring ICU transfer, and 3% for patients who remained in the non-ICU setting.
Nonepicenter, Non-ICU Mortality
This study offers an assessment of clinical outcomes in patients with COVID-19 hospitalized in a non-ICU, nonepicenter healthcare system operating below capacity. Although such systems account for most institutions caring for patients with COVID-19, this population has been underrepresented in the literature, which has focused on epicenter hospitals and critically ill patients.3-7 Existing epicenter estimates of in-hospital mortality for patients not requiring ICU-level care range from 6% in Northern California2 to at least 10% in New York, New York,3 and 11% in Wuhan, China.4 The corresponding non-ICU in-hospital mortality in our study was only 3%, supporting the vital role of social distancing in reducing COVID-19 mortality by facilitating care delivery in a non–resource limited hospital setting.
Oxygen Requirements and Cardiac Arrhythmias in Non-ICU Patients
Beyond nonepicenter mortality estimates, this study is the first to provide a detailed characterization of the clinical course and resource usage among patients with COVID-19 admitted to the non-ICU setting. Given the predicted persistence of SARS-CoV-2 spread,11-13 this information is crucial to healthcare systems that must anticipate resource requirements, such as respiratory support and continuous monitoring equipment, for the care of hospitalized patients with COVID-19. Such informed planning takes on even greater importance as prepandemic hospital services resume.
While most patients (73%) with COVID-19 admitted to a non-ICU bed required peak supplemental oxygen of 3 L/min or less, a relevant proportion (14%) developed a need for HFNC, NIV, or mechanical ventilation. Furthermore, among telemetry-monitored patients (79%), cardiac arrhythmias were uncommon (14%), and nearly all (90%) occurred in patients with either a positive troponin or known history of cardiac disease. There were no life-threatening ventricular arrhythmias associated with frequent use of hydroxychloroquine (53%) and azithromycin (52%).
These telemetry findings expand upon a smaller study of non-ICU patients receiving either hydroxychloroquine or azithromycin, in which no life-threatening ventricular tachyarrhythmias were detected.8 A separate study reported a 5.9% incidence of malignant ventricular tachyarrhythmias in hospitalized patients with COVID-19,10 but this study did not stratify arrhythmias by illness severity, and a high frequency of critical illness is suggested by the mechanical ventilation rate of 24%, thereby limiting comparison with our non-ICU telemetry findings.
CRP and LDH Levels as Predictors of Adverse Outcomes
This study supports the utility of obtaining CRP and LDH levels for risk stratification at the time of non-ICU hospital admission. In multivariable analysis, higher CRP and LDH levels were significantly associated with the composite adverse outcome. The adverse event rates was increased sixfold between patients with a CRP in the fourth quartile (≥ 11.0 mg/dL, 36%) and those in the first quartile (≤ 2.6 mg/dL, 5.3%), and it was fivefold higher in patients with an LDH level in the fourth quartile (≥ 354 U/L, 34%) compared with those in the first quartile (≤ 232 U/L, 7%).
These findings are consistent with prior studies that have associated elevated inflammatory markers with poor prognosis and death.7,9,16 In some cases, COVID-19 may manifest similar to a cytokine storm syndrome, which highlights the importance of inflammation-associated tissue injury and leads to widespread interest in the use of immunosuppressive medications.17,18 Several studies also have demonstrated an association between LDH level and severe illness,4,7,19 although this is the first to specifically demonstrate its association with clinical decompensation in the non-ICU hospitalized population. Given that SARS-CoV-2 can infect multiple organs,20,21 there is biological plausibility for the use of LDH levels as a nonspecific marker of tissue injury for early identification of more severe infection.
Notably, while elevated troponin levels have been strongly associated with the need for mechanical ventilation and with death, this has primarily been established using either high-sensitivity troponin assays at the time of admission22 or using peak conventional troponin levels during hospitalization.10 In this study, while abnormal conventional troponin levels at the time of non-ICU admission were not significantly associated with the primary outcome in multivariable analysis, absolute troponin values were significantly higher in univariable analysis. Incomplete troponin sampling and the lack of routine high-sensitivity troponin assay use may explain the lack of more robust troponin significance in this study.
Implications for Non-ICU Continuous Monitoring Resource Allocation
Prioritization of non-ICU continuous monitoring resources among patients with COVID-19 has numerous benefits, including reduced consumption of personal protective equipment, fewer healthcare worker exposures, and adequate availability of continuous monitoring for the expansion of prepandemic hospital services. While individualized clinical discretion is still required, the results of this study can be used as a guide for the allocation of continuous pulse oximetry and cardiac telemetry. Patients with a normal presenting CRP level and/or LDH level had a low incidence of clinical decompensation, which suggests that such patients could be monitored with intermittent rather than continuous pulse oximetry. Furthermore, cardiac telemetry could be reserved for patients with a history of cardiac comorbidities or abnormal troponin levels because such patients accounted for 90% of cardiac arrhythmias in this study.
Limitations
This study was limited to a single health system, and it lacks a direct comparison to nonhospitalized patients and those directly admitted to the ICU. Triage practices and thresholds for hospitalization may differ across institutions and regions, thereby limiting the generalizability of our study. Additional limitations include the lack of selected admission laboratories for all patients, as well as the lack of telemetry monitoring in all patients. However, any resulting selection bias may be more likely to attenuate the magnitude of observed effects given that additional testing and increased telemetry use may be expected in patients who are felt to be higher risk by routine clinical assessment.
CONCLUSION
In this study of non–critically ill patients hospitalized within a nonepicenter health system, the development of more severe illness or death was significantly associated with higher levels of CRP and LDH on admission. Clinical decompensation was driven largely by respiratory complications, while cardiac arrhythmias were rare. Overall, the non-ICU mortality rate was at least half of that reported in epicenter regions. Altogether, these findings provide valuable information for resource allocation planning while nonepicenter health systems continue caring for patients with COVID-19 as they also resume prepandemic operations.
1. Bialek S, Boundy E, Bowen V, et al; CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
2. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195-2198. https://doi.org/10.1001/jama.2020.7202
3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. Published online April 22, 2020. https://doi.org/10.1001/jama.2020.6775
4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
5. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612-1614. https://doi.org/10.1001/jama.2020.4326
6. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574-1581. https://doi.org/10.1001/jama.2020.5394
7. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
8. Chang D, Saleh M, Gabriels J, et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75(23):2992-2993. https://doi.org/10.1016/j.jacc.2020.04.032
9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. https://doi.org/10.1016/s0140-6736(20)30183-5
10. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):1-8. https://doi.org/10.1001/jamacardio.2020.1017
11. Centers for Disease Control and Prevention COVID-19 Forecasts. Accessed May 19, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/forecasting-us.html
12. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860-868. https://doi.org/10.1126/science.abb5793
13. Baker RE, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science. 2020;369(6501):315-319. https://doi.org/10.1126/science.abc2535
14. Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non-critically ill patients. JAMA. 2016;316(5):519-524. https://doi.org/10.1001/jama.2016.10258
15. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. https://doi.org/10.1097/ccm.0000000000004363
16. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
17. Mehta P, McAuley DF, Brown M, et al; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. https://doi.org/10.1016/s0140-6736(20)30628-0
18. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. Published online April 13, 2020. https://doi.org/10.1001/jama.2020.6019
19. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1-9. https://doi.org/10.1001/jamainternmed.2020.2033
20. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590-592. https://doi.org/10.1056/nejmc2011400
21. Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020;26(7):1077-1083. https://doi.org/10.1038/s41591-020-0912-6
22. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810. https://doi.org/10.1001/jamacardio.2020.0950
1. Bialek S, Boundy E, Bowen V, et al; CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346. https://doi.org/10.15585/mmwr.mm6912e2
2. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195-2198. https://doi.org/10.1001/jama.2020.7202
3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. Published online April 22, 2020. https://doi.org/10.1001/jama.2020.6775
4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
5. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612-1614. https://doi.org/10.1001/jama.2020.4326
6. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574-1581. https://doi.org/10.1001/jama.2020.5394
7. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. https://doi.org/10.1001/jama.2020.1585
8. Chang D, Saleh M, Gabriels J, et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75(23):2992-2993. https://doi.org/10.1016/j.jacc.2020.04.032
9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. https://doi.org/10.1016/s0140-6736(20)30183-5
10. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):1-8. https://doi.org/10.1001/jamacardio.2020.1017
11. Centers for Disease Control and Prevention COVID-19 Forecasts. Accessed May 19, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/forecasting-us.html
12. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860-868. https://doi.org/10.1126/science.abb5793
13. Baker RE, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. Susceptible supply limits the role of climate in the early SARS-CoV-2 pandemic. Science. 2020;369(6501):315-319. https://doi.org/10.1126/science.abc2535
14. Cantillon DJ, Loy M, Burkle A, et al. Association between off-site central monitoring using standardized cardiac telemetry and clinical outcomes among non-critically ill patients. JAMA. 2016;316(5):519-524. https://doi.org/10.1001/jama.2016.10258
15. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440-e469. https://doi.org/10.1097/ccm.0000000000004363
16. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
17. Mehta P, McAuley DF, Brown M, et al; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034. https://doi.org/10.1016/s0140-6736(20)30628-0
18. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. Published online April 13, 2020. https://doi.org/10.1001/jama.2020.6019
19. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1-9. https://doi.org/10.1001/jamainternmed.2020.2033
20. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590-592. https://doi.org/10.1056/nejmc2011400
21. Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med. 2020;26(7):1077-1083. https://doi.org/10.1038/s41591-020-0912-6
22. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810. https://doi.org/10.1001/jamacardio.2020.0950
© 2021 Society of Hospital Medicine
Left ventricular thrombosis can still complicate acute myocardial infarction
A 62-year-old man with hypertension, type 2 diabetes mellitus, and hypercholesterolemia presented to the emergency department with substernal chest pain that started about 15 hours earlier while he was at rest watching television.
On examination, his pulse was 92 beats per minute and regular, his blood pressure was 160/88 mm Hg, and he had no evidence of jugular venous distention or pedal edema. Lung examination was positive for bibasilar crackles.
Electrocardiography revealed Q waves with ST elevation in leads I, aVL, V4, V5, and V6 with reciprocal ST depression in leads II, III, and aVF.
His troponin T level on presentation was markedly elevated.

He underwent heart catheterization and was found to have 100% occlusion of the proximal left anterior descending artery. He underwent successful percutaneous coronary intervention with placement of a drug-eluting stent, and afterward had grade 3 flow on the Thrombolysis in Myocardial Infarction (TIMI) scale.
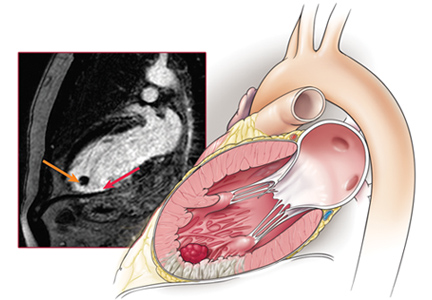
Echocardiography the next day revealed a mobile echo-dense mass in the left ventricular apex (Figure 1) and a left ventricular ejection fraction of 35%.
THE INCIDENCE OF LEFT VENTRICULAR THROMBOSIS IN ACUTE MI
1. What is the incidence of left ventricular thrombosis after acute myocardial infarction (MI), now that primary percutaneous coronary intervention is common?
- 0.1%
- 2%
- 20%
- 40%
Left ventricular thrombosis is a serious complication of acute MI that can cause systemic thromboembolism, including stroke.1 Before thrombolytic therapy was available, this complication occurred in 20% to 60% of patients with acute MI.2,3 But early reperfusion strategies, anticoagulation for the first 48 hours, and dual antiplatelet therapy have reduced the incidence of this complication significantly.
In the thrombolytic era, the incidence of left ventricular thrombosis was 5.1% in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) 3 study, which had 8,326 patients. A subset of patients who had an anterior MI had almost double the incidence (11.5%).3

The incidence has further declined with the advent of primary percutaneous coronary intervention, likely thanks to enhanced myocardial salvage, and now ranges from 2.5% to 15% (Table 1).4–11 The largest observational study, with 2,911 patients undergoing percutaneous coronary intervention, reported an incidence of 2.5% within 3 to 5 days of the MI.7 At our center, the incidence was found to be even lower, 1.8% in 1,700 patients presenting with ST-elevation MI undergoing primary percutaneous coronary intervention. Hence, of the answers to the question above, 2% would be closest.
Large infarct size with a low left ventricular ejection fraction (< 40%), anterior wall MI, hypertension, and delay in time from symptom onset to intervention were independent predictors of left ventricular thrombus formation in most studies.7,12 The risk is highest during the first 2 weeks after MI, and thrombosis almost never occurs more than 3 months after the index event.5,13–16
WHAT IS THE PATHOGENESIS OF LEFT VENTRICULAR THROMBOSIS?
A large transmural infarct results in loss of contractile function, which causes stagnation and pooling of blood adjacent to the infarcted ventricular segment. In addition, endocardial injury exposes tissue factor, which then initiates the coagulation cascade. To make matters worse, MI results in a hypercoagulable state through unclear mechanisms, which completes the Virchow triad for thrombus formation. Elevations of D-dimer, fibrinogen, anticardiolipin antibodies (IgM and IgG), and tissue factor have also been reported after acute MI.17
Thrombus formation begins with platelet aggregation at the site of endocardial damage, forming a platelet plug, followed by activation of clotting factors. These thrombi are referred to as “mural,” as they adhere to the chamber wall (endocardium). They are composed of fibrin and entrapped red and white blood cells (Figure 2).
The natural course of thrombus evolution is established but variable. A left ventricular thrombus may dislodge and embolize, resulting in stroke or other thromboembolic complications. Alternately, it can dissolve over time, aided by intrinsic fibrinolytic mechanisms. On other occasions, the thrombus may organize, a process characterized by ingrowth of smooth muscle cells, fibroblasts, and endothelium.
HOW IS LEFT VENTRICULAR THROMBOSIS DIAGNOSED?
2. What is the best imaging test for detecting a thrombus?
- Transesophageal echocardiography
- Transthoracic echocardiography
- Cardiac magnetic resonance imaging (MRI) without gadolinium contrast
- Cardiac MRI with gadolinium contrast
Evaluation of left ventricular function after acute MI carries a class I indication (ie, it should be performed).18
Echocardiography is commonly used, and it has a 60% sensitivity to detect a thrombus.19 In patients with poorer transthoracic echocardiographic windows, contrast can be used to better delineate the left ventricular cavity and show the thrombus. Transesophageal echocardiography is seldom useful, as the left ventricular apex is foreshortened and in the far field.
A left ventricular thrombus is confirmed if an echo-dense mass with well-demarcated margins distinct from the endocardium is seen throughout the cardiac cycle. It should be evident in at least two different views (apical and short-axis) and should be adjacent to a hypokinetic or akinetic left ventricular wall. False-positive findings can occur due to misidentified false tendons, papillary muscles, and trabeculae.
Cardiac MRI with late gadolinium enhancement is now the gold standard for diagnostic imaging, as it accurately characterizes the shape, size, and location of the thrombus (Figure 3). Gadolinium contrast increases the enhancement of the ventricular cavity, thus allowing easy detection of thrombus, which appears dark. Cardiac MRI with delayed enhancement has 88% to 91% sensitivity and 99% specificity to detect left ventricular thrombosis.20,21 However, compared with echocardiography, routine cardiac MRI is time-intensive, costly, and not routinely available. As a result, it should be performed only in patients with poor acoustic windows and a high clinical suspicion of left ventricular thrombosis.
Delayed-contrast cardiac computed tomography can be used to identify left ventricular thrombosis, using absence of contrast uptake. The need to use contrast is a disadvantage, but computed tomography can be an alternative in patients with contraindications to cardiac MRI.
WHAT COMPLICATIONS ARISE FROM LEFT VENTRICULAR THROMBOSIS?
The most feared complication of left ventricular thrombosis is thromboembolism. Cardioembolic stroke is generally severe, prone to early and long-term recurrence, and associated with a higher death rate than noncardioembolic ischemic stroke.22,23 Thrombi associated with thromboembolism are often acute and mobile rather than organized and immobile.24 They may embolize to the brain, spleen, kidneys, and bowel.25 In a meta-analysis of 11 studies, the pooled odds ratio for risk of embolization was 5.45 (95% confidence interval [CI] 3.02–9.83) with left ventricular thrombi vs without.26 Before systemic thrombolysis and antiplatelet therapy became available, stroke rates ranged from 1.5% to 10%.27–29
In a meta-analysis of 22 studies from 1978 to 2004, the incidence of ischemic stroke after MI during hospitalization was around 11.1 per 1,000 MIs.30 This study found that anterior MI was associated with a higher risk of stroke, but reported no difference in the incidence of stroke with percutaneous coronary intervention, systemic thrombolysis, or no reperfusion.
In a large prospective cohort study of 2,160 patients,31 259 (12%) had a stroke after MI. In multivariable analysis, age, diabetes, and previous stroke were predictors of stroke after MI. This study reported significantly fewer strokes in patients who underwent percutaneous coronary intervention than with other or no reperfusion therapies.31
ANTICOAGULATION TREATMENT
3. How would you treat a patient who has a drug-eluting stent in the left anterior descending artery and a new diagnosis of left ventricular thrombosis?
- Warfarin
- Aspirin and clopidogrel
- Aspirin, clopidogrel, and warfarin
- Aspirin and warfarin
The management of left ventricular thrombosis has been summarized in guidelines from the American College of Chest Physicians (ACCP) in 2012,32 and from the American College of Cardiology/American Heart Association in 2013,18 which recommend anticoagulation for at least 3 months, or indefinitely if bleeding risk is low, for all patients developing a left ventricular thrombus.
For patients with acute MI and left ventricular thrombosis, the ACCP guidelines recommend warfarin with a target international normalized ratio of 2.0 to 3.0 plus dual antiplatelet therapy (eg, aspirin plus clopidogrel) for 3 months, after which warfarin is discontinued but dual antiplatelet therapy is continued for up to 12 months.32
The European Society of Cardiology guidelines33 recommend 6 months of anticoagulation. However, if the patient is receiving dual antiplatelet therapy, they recommend repeated imaging of the left ventricle after 3 months of anticoagulation, which may allow for earlier discontinuation of anticoagulation if the thrombus has resolved and apical wall motion has recovered. Therefore, most experts recommend 3 months of anticoagulation when used in combination with dual antiplatelet therapy and repeating echocardiography at 3 months to safely discontinue anticoagulation. The best answer to the question posed here is aspirin, clopidogrel, and warfarin.
Decisions about antithrombotic therapy may also depend on stent type and the patient’s bleeding risk. With bare-metal stents, dual antiplatelet therapy along with anticoagulation should be used for 1 month, after which anticoagulation should be used with a single antiplatelet agent for another 2 months; after this, the anticoagulant can be discontinued and dual antiplatelet therapy can be resumed for a total of 12 months. Newer anticoagulants such as rivaroxaban, dabigatran, edoxaban, and apixaban may also have a role, but they have not yet been studied for this indication.
Surgical thrombectomy is rarely considered now, given the known efficacy of anticoagulants in dissolving the thrombus. It was done in the past for large, mobile, or protruding left ventricular thrombi, which have a higher potential for embolization.34 Currently, it can be done under very special circumstances, such as before placement of a left ventricular assist device or if the thrombus is large, to prevent embolism.35,36
BLEEDING COMPLICATIONS WITH TRIPLE ANTITHROMBOTIC THERAPY
After stent placement, almost all patients need to be on dual antiplatelet therapy for a specified duration depending on the type and generation of stent used. Such patients end up on “triple” antithrombotic therapy (two antiplatelet drugs plus an anticoagulant), which poses a high risk of bleeding.37 Consideration needs to be given to the risks of stroke, stent thrombosis, and major bleeding when selecting the antithrombotic regimen.38 Triple antithrombotic therapy has been associated with a risk of fatal and nonfatal bleeding of 4% to 16% when used for indications such as atrial fibrillation.39–41
Risks of triple antithrombotic therapy (aspirin 80–100 mg, clopidogrel 75 mg, and warfarin) were compared with those of clopidogrel plus warfarin in the What Is the Optimal Antiplatelet and Anticoagulant therapy in Patients With Oral Anticoagulation and Coronary Stenting Trial,37 which reported a significantly lower risk of major and minor bleeding with clopidogrel-plus-warfarin therapy than with triple antithrombotic therapy, 14.3% vs 31.7% (hazard ratio 0.40, 95% CI 0.28–0.58, P < .0001).
Additionally, the increased risk of major and minor bleeding associated with triple antithrombotic therapy has been confirmed in many observational studies; other studies found a trend toward lower risk with triple therapy, but this was not statistically significant (Table 2).38,40,42–55 A large multicenter European trial is being conducted to compare dual antiplatelet therapy vs triple antithrombotic therapy in patients with left ventricular thrombosis.
CASE FOLLOW-UP
Our patient was started on warfarin, clopidogrel 75 mg, and aspirin 75 mg at the time of discharge. He was continued on warfarin for 3 months, at which time a follow-up echocardiogram showed no thrombus in the left ventricle. Warfarin was discontinued, and he had no thromboembolic complications.
TAKE-HOME POINTS
Left ventricular thrombosis after an acute MI is very important to detect, as it can lead to serious complications through arterial embolism.
The incidence of left ventricular thrombosis has declined significantly with the use of percutaneous coronary intervention. However, it may still occur in a small number of patients with larger infarcts owing to delay in revascularization or proximal (left main or left anterior descending) occlusions with larger infarct size.
Echocardiography, which is routinely performed after acute MI to assess myocardial function, uncovers most left ventricular thrombi. In high-risk cases, MRI with late gadolinium enhancement can increase the diagnostic yield.
Anticoagulation with warfarin is recommended for at least 3 months. Post-MI patients undergoing stent implantation may need triple antithrombotic therapy, which, however, increases the bleeding risk significantly. Large randomized trials are needed to guide physicians in risk stratification of such patients.
- Lip GY, Piotrponikowski P, Andreotti F, et al; Heart Failure Association (EHFA) of the European Society of Cardiology (ESC) and the ESC Working Group on Thrombosis. Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost 2012; 108:1009–1022.
- Turpie AG, Robinson JG, Doyle DJ, et al. Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med 1989; 320:352–357.
- Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 1998; 81:822–827.
- Kalra A, Jang IK. Prevalence of early left ventricular thrombus after primary coronary intervention for acute myocardial infarction. J Thromb Thrombolysis 2000; 10:133–136.
- Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN. Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non-anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with or without coronary revascularization. Am J Cardiol 2004; 93:1529–1530.
- Rehan A, Kanwar M, Rosman H, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound 2006;4:20.
- Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 2008; 335:171–176.
- Osherov AB, Borovik-Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J 2009; 157:1074–1080.
- Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010; 106:1197–1200.
- Shacham Y, Leshem-Rubinow E, Ben Assa E, et al. Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am J Cardiol 2013; 112:57–60.
- Gianstefani S, Douiri A, Delithanasis I, et al. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 2014; 113:1111–1116.
- Shacham Y, Birati EY, Rogovski O, Cogan Y, Keren G, Roth A. Left ventricular thrombus formation and bleeding complications during continuous in-hospital anticoagulation for acute anterior myocardial infarction. Isr Med Assoc J 2012; 14:742–746.
- Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med 1981; 305:297–302.
- Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol 1989; 14:903–911.
- Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 1984; 100:789–794.
- Greaves SC, Zhi G, Lee RT, et al. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol 1997; 80:442–448.
- Solheim S, Seljeflot I, Lunde K, et al. Prothrombotic markers in patients with acute myocardial infarction and left ventricular thrombus formation treated with pci and dual antiplatelet therapy. Thromb J 2013; 11:1.
- O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–e425.
- Weinsaft JW, Kim HW, Crowley AL, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011; 4:702–712.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001; 12:171–180.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German Stroke Data Bank. Stroke 2001; 32:2559–2566.
- Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990; 15:790–800.
- Jordan RA, Miller RD, Edwards JE, Parker RL. Thrombo-embolism in acute and in healed myocardial infarction. I. Intracardiac mural thrombosis. Circulation 1952; 6:1–6.
- Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 1993; 22:1004–1009.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest 1980; 77:586–590.
- Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med 1983; 74:989–995.
- Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006; 119:354.e1–354.e9.
- Witt BJ, Brown RD Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005; 143:785–792.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e637S–e68S.
- Steg G, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33:2569–2619.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Kanemitsu S, Miyake Y, Okabe M. Surgical removal of a left ventricular thrombus associated with cardiac sarcoidosis. Interact Cardiovasc Thorac Surg 2008; 7:333–335.
- Engin C, Yagdi T, Balcioglu O, et al. Left ventricular assist device implantation in heart failure patients with a left ventricular thrombus. Transplant Proc 2013; 45:1017–1019.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv 2011; 4:522–534.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Karjalainen PP, Porela P, Ylitalo A, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28:726–732.
- Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009; 53:2019–2027.
- Azoulay L, Dell’Aniello S, Simon T, Renoux C, Suissa S. The concurrent use of antithrombotic therapies and the risk of bleeding in patients with atrial fibrillation. Thromb Haemost 2013; 109:431–439.
- Deshmukh A, Hilleman DE, Del Core M, Nair CK. Antithrombotic regimens in patients with indication for long-term anticoagulation undergoing coronary interventions-systematic analysis, review of literature, and implications on management. Am J Ther 2013; 20:654–663.
- Fosbol EL, Wang TY, Li S, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J 2013; 166:864–870.
- Gao F, Zhou YJ, Wang ZJ, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol 2011; 148:96–101.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Hermosillo AJ, Spinler SA. Aspirin, clopidogrel, and warfarin: is the combination appropriate and effective or inappropriate and too dangerous? Ann Pharmacother 2008; 42:790–805.
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am Coll Cardiol 2009; 54:95–109.
- Khurram Z, Chou E, Minutello R, et al. Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J Invasive Cardiol 2006; 18:162–164.
- Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J 2004; 147:463–467.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- DeEugenio D, Kolman L, DeCaro M, et al. Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 2007; 27:691–696.
- Ruiz-Nodar JM, Marin F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol 2008; 51:818–825.
- Sarafoff N, Ndrepepa G, Mehilli J, et al. Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med 2008; 264:472–480.
- Rossini R, Musumeci GF, Lettieri CF, et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol 2008; 102:1618–1623.
A 62-year-old man with hypertension, type 2 diabetes mellitus, and hypercholesterolemia presented to the emergency department with substernal chest pain that started about 15 hours earlier while he was at rest watching television.
On examination, his pulse was 92 beats per minute and regular, his blood pressure was 160/88 mm Hg, and he had no evidence of jugular venous distention or pedal edema. Lung examination was positive for bibasilar crackles.
Electrocardiography revealed Q waves with ST elevation in leads I, aVL, V4, V5, and V6 with reciprocal ST depression in leads II, III, and aVF.
His troponin T level on presentation was markedly elevated.

He underwent heart catheterization and was found to have 100% occlusion of the proximal left anterior descending artery. He underwent successful percutaneous coronary intervention with placement of a drug-eluting stent, and afterward had grade 3 flow on the Thrombolysis in Myocardial Infarction (TIMI) scale.
Echocardiography the next day revealed a mobile echo-dense mass in the left ventricular apex (Figure 1) and a left ventricular ejection fraction of 35%.
THE INCIDENCE OF LEFT VENTRICULAR THROMBOSIS IN ACUTE MI
1. What is the incidence of left ventricular thrombosis after acute myocardial infarction (MI), now that primary percutaneous coronary intervention is common?
- 0.1%
- 2%
- 20%
- 40%
Left ventricular thrombosis is a serious complication of acute MI that can cause systemic thromboembolism, including stroke.1 Before thrombolytic therapy was available, this complication occurred in 20% to 60% of patients with acute MI.2,3 But early reperfusion strategies, anticoagulation for the first 48 hours, and dual antiplatelet therapy have reduced the incidence of this complication significantly.
In the thrombolytic era, the incidence of left ventricular thrombosis was 5.1% in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) 3 study, which had 8,326 patients. A subset of patients who had an anterior MI had almost double the incidence (11.5%).3

The incidence has further declined with the advent of primary percutaneous coronary intervention, likely thanks to enhanced myocardial salvage, and now ranges from 2.5% to 15% (Table 1).4–11 The largest observational study, with 2,911 patients undergoing percutaneous coronary intervention, reported an incidence of 2.5% within 3 to 5 days of the MI.7 At our center, the incidence was found to be even lower, 1.8% in 1,700 patients presenting with ST-elevation MI undergoing primary percutaneous coronary intervention. Hence, of the answers to the question above, 2% would be closest.
Large infarct size with a low left ventricular ejection fraction (< 40%), anterior wall MI, hypertension, and delay in time from symptom onset to intervention were independent predictors of left ventricular thrombus formation in most studies.7,12 The risk is highest during the first 2 weeks after MI, and thrombosis almost never occurs more than 3 months after the index event.5,13–16
WHAT IS THE PATHOGENESIS OF LEFT VENTRICULAR THROMBOSIS?
A large transmural infarct results in loss of contractile function, which causes stagnation and pooling of blood adjacent to the infarcted ventricular segment. In addition, endocardial injury exposes tissue factor, which then initiates the coagulation cascade. To make matters worse, MI results in a hypercoagulable state through unclear mechanisms, which completes the Virchow triad for thrombus formation. Elevations of D-dimer, fibrinogen, anticardiolipin antibodies (IgM and IgG), and tissue factor have also been reported after acute MI.17
Thrombus formation begins with platelet aggregation at the site of endocardial damage, forming a platelet plug, followed by activation of clotting factors. These thrombi are referred to as “mural,” as they adhere to the chamber wall (endocardium). They are composed of fibrin and entrapped red and white blood cells (Figure 2).
The natural course of thrombus evolution is established but variable. A left ventricular thrombus may dislodge and embolize, resulting in stroke or other thromboembolic complications. Alternately, it can dissolve over time, aided by intrinsic fibrinolytic mechanisms. On other occasions, the thrombus may organize, a process characterized by ingrowth of smooth muscle cells, fibroblasts, and endothelium.
HOW IS LEFT VENTRICULAR THROMBOSIS DIAGNOSED?
2. What is the best imaging test for detecting a thrombus?
- Transesophageal echocardiography
- Transthoracic echocardiography
- Cardiac magnetic resonance imaging (MRI) without gadolinium contrast
- Cardiac MRI with gadolinium contrast
Evaluation of left ventricular function after acute MI carries a class I indication (ie, it should be performed).18
Echocardiography is commonly used, and it has a 60% sensitivity to detect a thrombus.19 In patients with poorer transthoracic echocardiographic windows, contrast can be used to better delineate the left ventricular cavity and show the thrombus. Transesophageal echocardiography is seldom useful, as the left ventricular apex is foreshortened and in the far field.
A left ventricular thrombus is confirmed if an echo-dense mass with well-demarcated margins distinct from the endocardium is seen throughout the cardiac cycle. It should be evident in at least two different views (apical and short-axis) and should be adjacent to a hypokinetic or akinetic left ventricular wall. False-positive findings can occur due to misidentified false tendons, papillary muscles, and trabeculae.
Cardiac MRI with late gadolinium enhancement is now the gold standard for diagnostic imaging, as it accurately characterizes the shape, size, and location of the thrombus (Figure 3). Gadolinium contrast increases the enhancement of the ventricular cavity, thus allowing easy detection of thrombus, which appears dark. Cardiac MRI with delayed enhancement has 88% to 91% sensitivity and 99% specificity to detect left ventricular thrombosis.20,21 However, compared with echocardiography, routine cardiac MRI is time-intensive, costly, and not routinely available. As a result, it should be performed only in patients with poor acoustic windows and a high clinical suspicion of left ventricular thrombosis.
Delayed-contrast cardiac computed tomography can be used to identify left ventricular thrombosis, using absence of contrast uptake. The need to use contrast is a disadvantage, but computed tomography can be an alternative in patients with contraindications to cardiac MRI.
WHAT COMPLICATIONS ARISE FROM LEFT VENTRICULAR THROMBOSIS?
The most feared complication of left ventricular thrombosis is thromboembolism. Cardioembolic stroke is generally severe, prone to early and long-term recurrence, and associated with a higher death rate than noncardioembolic ischemic stroke.22,23 Thrombi associated with thromboembolism are often acute and mobile rather than organized and immobile.24 They may embolize to the brain, spleen, kidneys, and bowel.25 In a meta-analysis of 11 studies, the pooled odds ratio for risk of embolization was 5.45 (95% confidence interval [CI] 3.02–9.83) with left ventricular thrombi vs without.26 Before systemic thrombolysis and antiplatelet therapy became available, stroke rates ranged from 1.5% to 10%.27–29
In a meta-analysis of 22 studies from 1978 to 2004, the incidence of ischemic stroke after MI during hospitalization was around 11.1 per 1,000 MIs.30 This study found that anterior MI was associated with a higher risk of stroke, but reported no difference in the incidence of stroke with percutaneous coronary intervention, systemic thrombolysis, or no reperfusion.
In a large prospective cohort study of 2,160 patients,31 259 (12%) had a stroke after MI. In multivariable analysis, age, diabetes, and previous stroke were predictors of stroke after MI. This study reported significantly fewer strokes in patients who underwent percutaneous coronary intervention than with other or no reperfusion therapies.31
ANTICOAGULATION TREATMENT
3. How would you treat a patient who has a drug-eluting stent in the left anterior descending artery and a new diagnosis of left ventricular thrombosis?
- Warfarin
- Aspirin and clopidogrel
- Aspirin, clopidogrel, and warfarin
- Aspirin and warfarin
The management of left ventricular thrombosis has been summarized in guidelines from the American College of Chest Physicians (ACCP) in 2012,32 and from the American College of Cardiology/American Heart Association in 2013,18 which recommend anticoagulation for at least 3 months, or indefinitely if bleeding risk is low, for all patients developing a left ventricular thrombus.
For patients with acute MI and left ventricular thrombosis, the ACCP guidelines recommend warfarin with a target international normalized ratio of 2.0 to 3.0 plus dual antiplatelet therapy (eg, aspirin plus clopidogrel) for 3 months, after which warfarin is discontinued but dual antiplatelet therapy is continued for up to 12 months.32
The European Society of Cardiology guidelines33 recommend 6 months of anticoagulation. However, if the patient is receiving dual antiplatelet therapy, they recommend repeated imaging of the left ventricle after 3 months of anticoagulation, which may allow for earlier discontinuation of anticoagulation if the thrombus has resolved and apical wall motion has recovered. Therefore, most experts recommend 3 months of anticoagulation when used in combination with dual antiplatelet therapy and repeating echocardiography at 3 months to safely discontinue anticoagulation. The best answer to the question posed here is aspirin, clopidogrel, and warfarin.
Decisions about antithrombotic therapy may also depend on stent type and the patient’s bleeding risk. With bare-metal stents, dual antiplatelet therapy along with anticoagulation should be used for 1 month, after which anticoagulation should be used with a single antiplatelet agent for another 2 months; after this, the anticoagulant can be discontinued and dual antiplatelet therapy can be resumed for a total of 12 months. Newer anticoagulants such as rivaroxaban, dabigatran, edoxaban, and apixaban may also have a role, but they have not yet been studied for this indication.
Surgical thrombectomy is rarely considered now, given the known efficacy of anticoagulants in dissolving the thrombus. It was done in the past for large, mobile, or protruding left ventricular thrombi, which have a higher potential for embolization.34 Currently, it can be done under very special circumstances, such as before placement of a left ventricular assist device or if the thrombus is large, to prevent embolism.35,36
BLEEDING COMPLICATIONS WITH TRIPLE ANTITHROMBOTIC THERAPY
After stent placement, almost all patients need to be on dual antiplatelet therapy for a specified duration depending on the type and generation of stent used. Such patients end up on “triple” antithrombotic therapy (two antiplatelet drugs plus an anticoagulant), which poses a high risk of bleeding.37 Consideration needs to be given to the risks of stroke, stent thrombosis, and major bleeding when selecting the antithrombotic regimen.38 Triple antithrombotic therapy has been associated with a risk of fatal and nonfatal bleeding of 4% to 16% when used for indications such as atrial fibrillation.39–41
Risks of triple antithrombotic therapy (aspirin 80–100 mg, clopidogrel 75 mg, and warfarin) were compared with those of clopidogrel plus warfarin in the What Is the Optimal Antiplatelet and Anticoagulant therapy in Patients With Oral Anticoagulation and Coronary Stenting Trial,37 which reported a significantly lower risk of major and minor bleeding with clopidogrel-plus-warfarin therapy than with triple antithrombotic therapy, 14.3% vs 31.7% (hazard ratio 0.40, 95% CI 0.28–0.58, P < .0001).
Additionally, the increased risk of major and minor bleeding associated with triple antithrombotic therapy has been confirmed in many observational studies; other studies found a trend toward lower risk with triple therapy, but this was not statistically significant (Table 2).38,40,42–55 A large multicenter European trial is being conducted to compare dual antiplatelet therapy vs triple antithrombotic therapy in patients with left ventricular thrombosis.
CASE FOLLOW-UP
Our patient was started on warfarin, clopidogrel 75 mg, and aspirin 75 mg at the time of discharge. He was continued on warfarin for 3 months, at which time a follow-up echocardiogram showed no thrombus in the left ventricle. Warfarin was discontinued, and he had no thromboembolic complications.
TAKE-HOME POINTS
Left ventricular thrombosis after an acute MI is very important to detect, as it can lead to serious complications through arterial embolism.
The incidence of left ventricular thrombosis has declined significantly with the use of percutaneous coronary intervention. However, it may still occur in a small number of patients with larger infarcts owing to delay in revascularization or proximal (left main or left anterior descending) occlusions with larger infarct size.
Echocardiography, which is routinely performed after acute MI to assess myocardial function, uncovers most left ventricular thrombi. In high-risk cases, MRI with late gadolinium enhancement can increase the diagnostic yield.
Anticoagulation with warfarin is recommended for at least 3 months. Post-MI patients undergoing stent implantation may need triple antithrombotic therapy, which, however, increases the bleeding risk significantly. Large randomized trials are needed to guide physicians in risk stratification of such patients.
A 62-year-old man with hypertension, type 2 diabetes mellitus, and hypercholesterolemia presented to the emergency department with substernal chest pain that started about 15 hours earlier while he was at rest watching television.
On examination, his pulse was 92 beats per minute and regular, his blood pressure was 160/88 mm Hg, and he had no evidence of jugular venous distention or pedal edema. Lung examination was positive for bibasilar crackles.
Electrocardiography revealed Q waves with ST elevation in leads I, aVL, V4, V5, and V6 with reciprocal ST depression in leads II, III, and aVF.
His troponin T level on presentation was markedly elevated.

He underwent heart catheterization and was found to have 100% occlusion of the proximal left anterior descending artery. He underwent successful percutaneous coronary intervention with placement of a drug-eluting stent, and afterward had grade 3 flow on the Thrombolysis in Myocardial Infarction (TIMI) scale.
Echocardiography the next day revealed a mobile echo-dense mass in the left ventricular apex (Figure 1) and a left ventricular ejection fraction of 35%.
THE INCIDENCE OF LEFT VENTRICULAR THROMBOSIS IN ACUTE MI
1. What is the incidence of left ventricular thrombosis after acute myocardial infarction (MI), now that primary percutaneous coronary intervention is common?
- 0.1%
- 2%
- 20%
- 40%
Left ventricular thrombosis is a serious complication of acute MI that can cause systemic thromboembolism, including stroke.1 Before thrombolytic therapy was available, this complication occurred in 20% to 60% of patients with acute MI.2,3 But early reperfusion strategies, anticoagulation for the first 48 hours, and dual antiplatelet therapy have reduced the incidence of this complication significantly.
In the thrombolytic era, the incidence of left ventricular thrombosis was 5.1% in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) 3 study, which had 8,326 patients. A subset of patients who had an anterior MI had almost double the incidence (11.5%).3

The incidence has further declined with the advent of primary percutaneous coronary intervention, likely thanks to enhanced myocardial salvage, and now ranges from 2.5% to 15% (Table 1).4–11 The largest observational study, with 2,911 patients undergoing percutaneous coronary intervention, reported an incidence of 2.5% within 3 to 5 days of the MI.7 At our center, the incidence was found to be even lower, 1.8% in 1,700 patients presenting with ST-elevation MI undergoing primary percutaneous coronary intervention. Hence, of the answers to the question above, 2% would be closest.
Large infarct size with a low left ventricular ejection fraction (< 40%), anterior wall MI, hypertension, and delay in time from symptom onset to intervention were independent predictors of left ventricular thrombus formation in most studies.7,12 The risk is highest during the first 2 weeks after MI, and thrombosis almost never occurs more than 3 months after the index event.5,13–16
WHAT IS THE PATHOGENESIS OF LEFT VENTRICULAR THROMBOSIS?
A large transmural infarct results in loss of contractile function, which causes stagnation and pooling of blood adjacent to the infarcted ventricular segment. In addition, endocardial injury exposes tissue factor, which then initiates the coagulation cascade. To make matters worse, MI results in a hypercoagulable state through unclear mechanisms, which completes the Virchow triad for thrombus formation. Elevations of D-dimer, fibrinogen, anticardiolipin antibodies (IgM and IgG), and tissue factor have also been reported after acute MI.17
Thrombus formation begins with platelet aggregation at the site of endocardial damage, forming a platelet plug, followed by activation of clotting factors. These thrombi are referred to as “mural,” as they adhere to the chamber wall (endocardium). They are composed of fibrin and entrapped red and white blood cells (Figure 2).
The natural course of thrombus evolution is established but variable. A left ventricular thrombus may dislodge and embolize, resulting in stroke or other thromboembolic complications. Alternately, it can dissolve over time, aided by intrinsic fibrinolytic mechanisms. On other occasions, the thrombus may organize, a process characterized by ingrowth of smooth muscle cells, fibroblasts, and endothelium.
HOW IS LEFT VENTRICULAR THROMBOSIS DIAGNOSED?
2. What is the best imaging test for detecting a thrombus?
- Transesophageal echocardiography
- Transthoracic echocardiography
- Cardiac magnetic resonance imaging (MRI) without gadolinium contrast
- Cardiac MRI with gadolinium contrast
Evaluation of left ventricular function after acute MI carries a class I indication (ie, it should be performed).18
Echocardiography is commonly used, and it has a 60% sensitivity to detect a thrombus.19 In patients with poorer transthoracic echocardiographic windows, contrast can be used to better delineate the left ventricular cavity and show the thrombus. Transesophageal echocardiography is seldom useful, as the left ventricular apex is foreshortened and in the far field.
A left ventricular thrombus is confirmed if an echo-dense mass with well-demarcated margins distinct from the endocardium is seen throughout the cardiac cycle. It should be evident in at least two different views (apical and short-axis) and should be adjacent to a hypokinetic or akinetic left ventricular wall. False-positive findings can occur due to misidentified false tendons, papillary muscles, and trabeculae.
Cardiac MRI with late gadolinium enhancement is now the gold standard for diagnostic imaging, as it accurately characterizes the shape, size, and location of the thrombus (Figure 3). Gadolinium contrast increases the enhancement of the ventricular cavity, thus allowing easy detection of thrombus, which appears dark. Cardiac MRI with delayed enhancement has 88% to 91% sensitivity and 99% specificity to detect left ventricular thrombosis.20,21 However, compared with echocardiography, routine cardiac MRI is time-intensive, costly, and not routinely available. As a result, it should be performed only in patients with poor acoustic windows and a high clinical suspicion of left ventricular thrombosis.
Delayed-contrast cardiac computed tomography can be used to identify left ventricular thrombosis, using absence of contrast uptake. The need to use contrast is a disadvantage, but computed tomography can be an alternative in patients with contraindications to cardiac MRI.
WHAT COMPLICATIONS ARISE FROM LEFT VENTRICULAR THROMBOSIS?
The most feared complication of left ventricular thrombosis is thromboembolism. Cardioembolic stroke is generally severe, prone to early and long-term recurrence, and associated with a higher death rate than noncardioembolic ischemic stroke.22,23 Thrombi associated with thromboembolism are often acute and mobile rather than organized and immobile.24 They may embolize to the brain, spleen, kidneys, and bowel.25 In a meta-analysis of 11 studies, the pooled odds ratio for risk of embolization was 5.45 (95% confidence interval [CI] 3.02–9.83) with left ventricular thrombi vs without.26 Before systemic thrombolysis and antiplatelet therapy became available, stroke rates ranged from 1.5% to 10%.27–29
In a meta-analysis of 22 studies from 1978 to 2004, the incidence of ischemic stroke after MI during hospitalization was around 11.1 per 1,000 MIs.30 This study found that anterior MI was associated with a higher risk of stroke, but reported no difference in the incidence of stroke with percutaneous coronary intervention, systemic thrombolysis, or no reperfusion.
In a large prospective cohort study of 2,160 patients,31 259 (12%) had a stroke after MI. In multivariable analysis, age, diabetes, and previous stroke were predictors of stroke after MI. This study reported significantly fewer strokes in patients who underwent percutaneous coronary intervention than with other or no reperfusion therapies.31
ANTICOAGULATION TREATMENT
3. How would you treat a patient who has a drug-eluting stent in the left anterior descending artery and a new diagnosis of left ventricular thrombosis?
- Warfarin
- Aspirin and clopidogrel
- Aspirin, clopidogrel, and warfarin
- Aspirin and warfarin
The management of left ventricular thrombosis has been summarized in guidelines from the American College of Chest Physicians (ACCP) in 2012,32 and from the American College of Cardiology/American Heart Association in 2013,18 which recommend anticoagulation for at least 3 months, or indefinitely if bleeding risk is low, for all patients developing a left ventricular thrombus.
For patients with acute MI and left ventricular thrombosis, the ACCP guidelines recommend warfarin with a target international normalized ratio of 2.0 to 3.0 plus dual antiplatelet therapy (eg, aspirin plus clopidogrel) for 3 months, after which warfarin is discontinued but dual antiplatelet therapy is continued for up to 12 months.32
The European Society of Cardiology guidelines33 recommend 6 months of anticoagulation. However, if the patient is receiving dual antiplatelet therapy, they recommend repeated imaging of the left ventricle after 3 months of anticoagulation, which may allow for earlier discontinuation of anticoagulation if the thrombus has resolved and apical wall motion has recovered. Therefore, most experts recommend 3 months of anticoagulation when used in combination with dual antiplatelet therapy and repeating echocardiography at 3 months to safely discontinue anticoagulation. The best answer to the question posed here is aspirin, clopidogrel, and warfarin.
Decisions about antithrombotic therapy may also depend on stent type and the patient’s bleeding risk. With bare-metal stents, dual antiplatelet therapy along with anticoagulation should be used for 1 month, after which anticoagulation should be used with a single antiplatelet agent for another 2 months; after this, the anticoagulant can be discontinued and dual antiplatelet therapy can be resumed for a total of 12 months. Newer anticoagulants such as rivaroxaban, dabigatran, edoxaban, and apixaban may also have a role, but they have not yet been studied for this indication.
Surgical thrombectomy is rarely considered now, given the known efficacy of anticoagulants in dissolving the thrombus. It was done in the past for large, mobile, or protruding left ventricular thrombi, which have a higher potential for embolization.34 Currently, it can be done under very special circumstances, such as before placement of a left ventricular assist device or if the thrombus is large, to prevent embolism.35,36
BLEEDING COMPLICATIONS WITH TRIPLE ANTITHROMBOTIC THERAPY
After stent placement, almost all patients need to be on dual antiplatelet therapy for a specified duration depending on the type and generation of stent used. Such patients end up on “triple” antithrombotic therapy (two antiplatelet drugs plus an anticoagulant), which poses a high risk of bleeding.37 Consideration needs to be given to the risks of stroke, stent thrombosis, and major bleeding when selecting the antithrombotic regimen.38 Triple antithrombotic therapy has been associated with a risk of fatal and nonfatal bleeding of 4% to 16% when used for indications such as atrial fibrillation.39–41
Risks of triple antithrombotic therapy (aspirin 80–100 mg, clopidogrel 75 mg, and warfarin) were compared with those of clopidogrel plus warfarin in the What Is the Optimal Antiplatelet and Anticoagulant therapy in Patients With Oral Anticoagulation and Coronary Stenting Trial,37 which reported a significantly lower risk of major and minor bleeding with clopidogrel-plus-warfarin therapy than with triple antithrombotic therapy, 14.3% vs 31.7% (hazard ratio 0.40, 95% CI 0.28–0.58, P < .0001).
Additionally, the increased risk of major and minor bleeding associated with triple antithrombotic therapy has been confirmed in many observational studies; other studies found a trend toward lower risk with triple therapy, but this was not statistically significant (Table 2).38,40,42–55 A large multicenter European trial is being conducted to compare dual antiplatelet therapy vs triple antithrombotic therapy in patients with left ventricular thrombosis.
CASE FOLLOW-UP
Our patient was started on warfarin, clopidogrel 75 mg, and aspirin 75 mg at the time of discharge. He was continued on warfarin for 3 months, at which time a follow-up echocardiogram showed no thrombus in the left ventricle. Warfarin was discontinued, and he had no thromboembolic complications.
TAKE-HOME POINTS
Left ventricular thrombosis after an acute MI is very important to detect, as it can lead to serious complications through arterial embolism.
The incidence of left ventricular thrombosis has declined significantly with the use of percutaneous coronary intervention. However, it may still occur in a small number of patients with larger infarcts owing to delay in revascularization or proximal (left main or left anterior descending) occlusions with larger infarct size.
Echocardiography, which is routinely performed after acute MI to assess myocardial function, uncovers most left ventricular thrombi. In high-risk cases, MRI with late gadolinium enhancement can increase the diagnostic yield.
Anticoagulation with warfarin is recommended for at least 3 months. Post-MI patients undergoing stent implantation may need triple antithrombotic therapy, which, however, increases the bleeding risk significantly. Large randomized trials are needed to guide physicians in risk stratification of such patients.
- Lip GY, Piotrponikowski P, Andreotti F, et al; Heart Failure Association (EHFA) of the European Society of Cardiology (ESC) and the ESC Working Group on Thrombosis. Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost 2012; 108:1009–1022.
- Turpie AG, Robinson JG, Doyle DJ, et al. Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med 1989; 320:352–357.
- Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 1998; 81:822–827.
- Kalra A, Jang IK. Prevalence of early left ventricular thrombus after primary coronary intervention for acute myocardial infarction. J Thromb Thrombolysis 2000; 10:133–136.
- Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN. Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non-anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with or without coronary revascularization. Am J Cardiol 2004; 93:1529–1530.
- Rehan A, Kanwar M, Rosman H, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound 2006;4:20.
- Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 2008; 335:171–176.
- Osherov AB, Borovik-Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J 2009; 157:1074–1080.
- Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010; 106:1197–1200.
- Shacham Y, Leshem-Rubinow E, Ben Assa E, et al. Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am J Cardiol 2013; 112:57–60.
- Gianstefani S, Douiri A, Delithanasis I, et al. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 2014; 113:1111–1116.
- Shacham Y, Birati EY, Rogovski O, Cogan Y, Keren G, Roth A. Left ventricular thrombus formation and bleeding complications during continuous in-hospital anticoagulation for acute anterior myocardial infarction. Isr Med Assoc J 2012; 14:742–746.
- Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med 1981; 305:297–302.
- Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol 1989; 14:903–911.
- Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 1984; 100:789–794.
- Greaves SC, Zhi G, Lee RT, et al. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol 1997; 80:442–448.
- Solheim S, Seljeflot I, Lunde K, et al. Prothrombotic markers in patients with acute myocardial infarction and left ventricular thrombus formation treated with pci and dual antiplatelet therapy. Thromb J 2013; 11:1.
- O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–e425.
- Weinsaft JW, Kim HW, Crowley AL, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011; 4:702–712.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001; 12:171–180.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German Stroke Data Bank. Stroke 2001; 32:2559–2566.
- Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990; 15:790–800.
- Jordan RA, Miller RD, Edwards JE, Parker RL. Thrombo-embolism in acute and in healed myocardial infarction. I. Intracardiac mural thrombosis. Circulation 1952; 6:1–6.
- Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 1993; 22:1004–1009.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest 1980; 77:586–590.
- Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med 1983; 74:989–995.
- Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006; 119:354.e1–354.e9.
- Witt BJ, Brown RD Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005; 143:785–792.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e637S–e68S.
- Steg G, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33:2569–2619.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Kanemitsu S, Miyake Y, Okabe M. Surgical removal of a left ventricular thrombus associated with cardiac sarcoidosis. Interact Cardiovasc Thorac Surg 2008; 7:333–335.
- Engin C, Yagdi T, Balcioglu O, et al. Left ventricular assist device implantation in heart failure patients with a left ventricular thrombus. Transplant Proc 2013; 45:1017–1019.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv 2011; 4:522–534.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Karjalainen PP, Porela P, Ylitalo A, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28:726–732.
- Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009; 53:2019–2027.
- Azoulay L, Dell’Aniello S, Simon T, Renoux C, Suissa S. The concurrent use of antithrombotic therapies and the risk of bleeding in patients with atrial fibrillation. Thromb Haemost 2013; 109:431–439.
- Deshmukh A, Hilleman DE, Del Core M, Nair CK. Antithrombotic regimens in patients with indication for long-term anticoagulation undergoing coronary interventions-systematic analysis, review of literature, and implications on management. Am J Ther 2013; 20:654–663.
- Fosbol EL, Wang TY, Li S, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J 2013; 166:864–870.
- Gao F, Zhou YJ, Wang ZJ, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol 2011; 148:96–101.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Hermosillo AJ, Spinler SA. Aspirin, clopidogrel, and warfarin: is the combination appropriate and effective or inappropriate and too dangerous? Ann Pharmacother 2008; 42:790–805.
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am Coll Cardiol 2009; 54:95–109.
- Khurram Z, Chou E, Minutello R, et al. Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J Invasive Cardiol 2006; 18:162–164.
- Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J 2004; 147:463–467.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- DeEugenio D, Kolman L, DeCaro M, et al. Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 2007; 27:691–696.
- Ruiz-Nodar JM, Marin F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol 2008; 51:818–825.
- Sarafoff N, Ndrepepa G, Mehilli J, et al. Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med 2008; 264:472–480.
- Rossini R, Musumeci GF, Lettieri CF, et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol 2008; 102:1618–1623.
- Lip GY, Piotrponikowski P, Andreotti F, et al; Heart Failure Association (EHFA) of the European Society of Cardiology (ESC) and the ESC Working Group on Thrombosis. Thromboembolism and antithrombotic therapy for heart failure in sinus rhythm: an executive summary of a joint consensus document from the ESC Heart Failure Association and the ESC Working Group on Thrombosis. Thromb Haemost 2012; 108:1009–1022.
- Turpie AG, Robinson JG, Doyle DJ, et al. Comparison of high-dose with low-dose subcutaneous heparin to prevent left ventricular mural thrombosis in patients with acute transmural anterior myocardial infarction. N Engl J Med 1989; 320:352–357.
- Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am J Cardiol 1998; 81:822–827.
- Kalra A, Jang IK. Prevalence of early left ventricular thrombus after primary coronary intervention for acute myocardial infarction. J Thromb Thrombolysis 2000; 10:133–136.
- Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN. Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non-anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with or without coronary revascularization. Am J Cardiol 2004; 93:1529–1530.
- Rehan A, Kanwar M, Rosman H, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound 2006;4:20.
- Zielinska M, Kaczmarek K, Tylkowski M. Predictors of left ventricular thrombus formation in acute myocardial infarction treated with successful primary angioplasty with stenting. Am J Med Sci 2008; 335:171–176.
- Osherov AB, Borovik-Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J 2009; 157:1074–1080.
- Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010; 106:1197–1200.
- Shacham Y, Leshem-Rubinow E, Ben Assa E, et al. Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am J Cardiol 2013; 112:57–60.
- Gianstefani S, Douiri A, Delithanasis I, et al. Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol 2014; 113:1111–1116.
- Shacham Y, Birati EY, Rogovski O, Cogan Y, Keren G, Roth A. Left ventricular thrombus formation and bleeding complications during continuous in-hospital anticoagulation for acute anterior myocardial infarction. Isr Med Assoc J 2012; 14:742–746.
- Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med 1981; 305:297–302.
- Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol 1989; 14:903–911.
- Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med 1984; 100:789–794.
- Greaves SC, Zhi G, Lee RT, et al. Incidence and natural history of left ventricular thrombus following anterior wall acute myocardial infarction. Am J Cardiol 1997; 80:442–448.
- Solheim S, Seljeflot I, Lunde K, et al. Prothrombotic markers in patients with acute myocardial infarction and left ventricular thrombus formation treated with pci and dual antiplatelet therapy. Thromb J 2013; 11:1.
- O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127:e362–e425.
- Weinsaft JW, Kim HW, Crowley AL, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011; 4:702–712.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001; 12:171–180.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German Stroke Data Bank. Stroke 2001; 32:2559–2566.
- Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol 1990; 15:790–800.
- Jordan RA, Miller RD, Edwards JE, Parker RL. Thrombo-embolism in acute and in healed myocardial infarction. I. Intracardiac mural thrombosis. Circulation 1952; 6:1–6.
- Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol 1993; 22:1004–1009.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest 1980; 77:586–590.
- Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am J Med 1983; 74:989–995.
- Witt BJ, Ballman KV, Brown RD Jr, Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006; 119:354.e1–354.e9.
- Witt BJ, Brown RD Jr, Jacobsen SJ, Weston SA, Yawn BP, Roger VL. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005; 143:785–792.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):e637S–e68S.
- Steg G, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33:2569–2619.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Kanemitsu S, Miyake Y, Okabe M. Surgical removal of a left ventricular thrombus associated with cardiac sarcoidosis. Interact Cardiovasc Thorac Surg 2008; 7:333–335.
- Engin C, Yagdi T, Balcioglu O, et al. Left ventricular assist device implantation in heart failure patients with a left ventricular thrombus. Transplant Proc 2013; 45:1017–1019.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- Faxon DP, Eikelboom JW, Berger PB, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: a North American perspective: executive summary. Circ Cardiovasc Interv 2011; 4:522–534.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Karjalainen PP, Porela P, Ylitalo A, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28:726–732.
- Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009; 53:2019–2027.
- Azoulay L, Dell’Aniello S, Simon T, Renoux C, Suissa S. The concurrent use of antithrombotic therapies and the risk of bleeding in patients with atrial fibrillation. Thromb Haemost 2013; 109:431–439.
- Deshmukh A, Hilleman DE, Del Core M, Nair CK. Antithrombotic regimens in patients with indication for long-term anticoagulation undergoing coronary interventions-systematic analysis, review of literature, and implications on management. Am J Ther 2013; 20:654–663.
- Fosbol EL, Wang TY, Li S, et al. Warfarin use among older atrial fibrillation patients with non-ST-segment elevation myocardial infarction managed with coronary stenting and dual antiplatelet therapy. Am Heart J 2013; 166:864–870.
- Gao F, Zhou YJ, Wang ZJ, et al. Meta-analysis of the combination of warfarin and dual antiplatelet therapy after coronary stenting in patients with indications for chronic oral anticoagulation. Int J Cardiol 2011; 148:96–101.
- Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170:1433–1441.
- Hermosillo AJ, Spinler SA. Aspirin, clopidogrel, and warfarin: is the combination appropriate and effective or inappropriate and too dangerous? Ann Pharmacother 2008; 42:790–805.
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am Coll Cardiol 2009; 54:95–109.
- Khurram Z, Chou E, Minutello R, et al. Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J Invasive Cardiol 2006; 18:162–164.
- Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patients with an indication for anticoagulation. Am Heart J 2004; 147:463–467.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- DeEugenio D, Kolman L, DeCaro M, et al. Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 2007; 27:691–696.
- Ruiz-Nodar JM, Marin F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol 2008; 51:818–825.
- Sarafoff N, Ndrepepa G, Mehilli J, et al. Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J Intern Med 2008; 264:472–480.
- Rossini R, Musumeci GF, Lettieri CF, et al. Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol 2008; 102:1618–1623.
Troponin elevation after noncardiac surgery: Significance and management
More than 200 million patients undergo noncardiac surgery each year, and the volume is increasing.1 Cardiovascular complications are a major cause of morbidity and mortality in the perioperative period.
Before the advent of modern cardiac biomarkers, an estimated 2% to 3% of all patients undergoing noncardiac surgery had a major adverse cardiac event.2 However, more recent studies suggest that 5% to 25% of patients have troponin elevations after noncardiac surgery, depending on the patient population,3–6 and many are asymptomatic, suggesting that many patients are sustaining undetected myocardial injury. Those who suffer a myocardial infarction or myocardial injury have elevated morbidity and mortality rates, not only perioperatively, but also at 30 days and even at up to 1 year.3–5,7–11
Yet there are almost no data on how best to manage these patients; the available guidelines, therefore, do not provide sufficient recommendations for clinical practice.
To address the lack of guidelines, we examine the incidence and proposed mechanisms of myocardial injury after noncardiac surgery, suggest an approach to identifying patients at risk, recommend treatment strategies, and consider future directions.
CARDIAC BIOMARKERS
When cardiac cellular injury from ischemia, direct trauma, or other cause disrupts the cell membrane, intracellular contents enter the extracellular space, including the blood stream. If the myocyte damage is extensive enough, biochemical assays can detect these substances.
Troponin, creatine kinase, myoglobin, and lactate dehydrogenase are common biomarkers of necrosis that, when detected in the plasma, may indicate cardiac injury. Each can be detected at varying times after cardiac injury (Figure 1).12
Cardiac troponins I and T
Of the biomarkers, cardiac troponin I and cardiac troponin T are now the most widely used and are the most specific for myocyte injury.
Troponins are proteins that regulate the calcium-induced interaction between myosin and actin that results in muscle contraction. Troponin is a complex consisting of three subunits: troponin C, troponin I, and troponin T. The cardiac troponin I and T isoforms are distinct from those found in skeletal muscle, making them specific for myocyte injury, and they are currently the recommended markers for diagnosing acute myocardial infarction.13
The troponin immunoassays currently available are not standardized among laboratories and point-of-care methods, and thus, levels cannot be compared across testing centers.14 Each assay has unique performance characteristics, but guidelines recommend using the 99th percentile value from a normal reference population for a given assay to define whether myocardial injury is present.13
Troponin elevation has prognostic value in patients presenting with acute coronary syndromes,15–18 and the degree of elevation correlates with infarct size.19–21
Controversy exists as to whether troponin and other biomarkers are released only after myocardial necrosis or after reversible injury as well. Using newer, highly sensitive assays, troponin elevations have been detected after short periods of ischemia during stress testing22,23 and in patients with stable angina,24 suggesting that reversible cardiac stress and injury can lead to troponin release. This mechanism may play an important role during the myocardial injury that can occur in patients undergoing noncardiac surgery.
MYOCARDIAL INFARCTION vs MYOCARDIAL INJURY
In 2000, the Joint Task Force of the European Society of Cardiology, American College of Cardiology Foundation, American Heart Association, and World Heart Federation revised the criteria for the diagnosis of myocardial infarction created by the World Health Organization in 1979. The definition was revised again in 2007 and once more in 2012 to create the third universal definition of myocardial infarction.
Acute myocardial infarction
Acute myocardial infarction is defined as evidence of myocardial necrosis in a setting of myocardial ischemia, not related to causes such as trauma or pulmonary embolism, with a rise or a fall (or a rise and a fall) of cardiac biomarkers (at least one value being above the 99th percentile in the reference population) and any of the following:
- Symptoms of ischemia
- New ST-segment changes or new left bundle branch block
- Pathologic Q waves
- Imaging evidence of new loss of viable myocardium or new regional wall-motion abnormality
- Intracoronary thrombus by angiography or autopsy.13
Myocardial injury after noncardiac surgery
Studies10,11 have shown that many patients undergoing noncardiac surgery have evidence of cardiac biomarker release but do not meet the universal definition of myocardial infarction.
The Perioperative Ischemic Evaluation (POISE) trial10 reported that 415 (5%) of its patients met the definition of myocardial infarction, of whom only about 35% had symptoms of ischemia. Another 697 patients (8.3%) had isolated elevations in biomarkers without meeting the definition of myocardial infarction.
The VISION study11 (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation) prospectively screened more than 15,000 patients in several countries for troponin elevation during the first 3 postoperative days and for ischemic symptoms and features. Of the patients screened, approximately 1,200 (8%) had troponin elevations, with fewer than half fulfilling the criteria for myocardial infarction.
In another study, van Waes et al6 prospectively screened 2,232 patients ages 60 and older undergoing intermediate- to high-risk noncardiac surgery. Troponin levels were elevated in 19% of the patients, but only 10 of these patients met the universal definition of myocardial infarction.
In all of these studies, patients with isolated elevation in myocardial biomarkers had worse short-term and long-term outcomes than those without. These observations led to a proposed definition of “myocardial injury after noncardiac surgery” that is broader than that of myocardial infarction and requires only elevation of cardiac biomarkers judged to be due to myocardial ischemia (ie, not from another obvious cause such as pulmonary embolism or myocarditis).3
FIVE TYPES OF MYOCARDIAL INFARCTION
The Joint Task Force13 categorizes myocardial infarction into five distinct types:
- Type 1—due to plaque rupture
- Type 2—due to imbalance between oxygen supply and demand
- Type 3—sudden cardiac death
- Type 4a—associated with percutaneous coronary intervention
- Type 4b—associated with stent thrombosis
- Type 5—associated with coronary artery bypass surgery.
Types 1 and 2 have both been implicated in perioperative myocardial infarction and injury. Patient characteristics and the physiologic response to surgical and anesthetic stressors likely contribute to the development of myocardial infarction and injury after noncardiac surgery.
Plaque rupture as a cause of postoperative myocardial infarction
The mechanism of type 1 myocardial infarction—plaque rupture or erosion leading to thrombosis and infarction—plays a significant role in most cases of acute coronary syndromes. Its role in perioperative and postoperative myocardial infarction or injury, however, is less clear.
In an autopsy study of 26 patients who died of myocardial infarction after noncardiac surgery, plaque rupture was evident in 12 (46%).25 A prospective angiographic study of 120 patients with acute coronary syndromes after noncardiac surgery found that nearly 50% had evidence of plaque rupture.26
Higher levels of catecholamines, cortisol,27,28 platelet reactivity,29 procoagulant factors,30 and coronary artery shear stress31 are all present in the postoperative period and may contribute to an increased propensity for plaque rupture or erosion. Whether plaque rupture is present in patients who have isolated troponin elevation but do not meet the criteria for myocardial infarction has not been investigated.
Oxygen supply-demand imbalance during and after surgery
Oxygen supply-demand imbalance (the mechanism in type 2 myocardial infarction) leading to myocyte stress, ischemia, and subsequent infarction is likely common in the perioperative and postoperative periods. As previously discussed, this imbalance may be present with or without symptoms.
Oxygen demand may increase in this period as a result of tachycardia32 caused by bleeding, pain, and catecholamines or increased wall stress from hypertension due to vasoconstriction or pain.33 Oxygen supply can be decreased secondary to tachycardia, anemia,34 hypotension, hypoxemia, hypercarbia, intravascular fluid shifts (bleeding or volume overload), or coronary vasoconstriction.33,35
These mechanisms of myocardial injury, infarction, or both can occur with or without underlying significant obstructive coronary artery disease. However, severe coronary artery disease is more common in those who have had a perioperative myocardial infarction.36
POSTOPERATIVE TROPONIN ELEVATION CARRIES A WORSE PROGNOSIS
Patients who suffer a myocardial infarction after noncardiac surgery have worse short- and long-term outcomes than their counterparts.4,5,7, 8,10,33 In the POISE trial,10 the 30-day mortality rate was 11.6% in those who had had a perioperative myocardial infarction, compared with 2.2% in those who did not (P < .001). The patients who had had a myocardial infarction were also more likely to have nonfatal cardiac arrest, coronary revascularization, and congestive heart failure.
Myocardial injury not fulfilling the criteria for myocardial infarction after noncardiac surgery is also associated with worse short-term and long-term outcomes.3,6,10,11,37,38 POISE patients with isolated elevations in cardiac biomarkers had a higher 30-day risk of coronary revascularization and nonfatal arrest.10 In the VISION trial, an elevation in troponin was the strongest predictor of death within 30 days after noncardiac surgery. This analysis also showed that the higher the peak troponin value, the greater the risk of death and the shorter the median time until death.11
A meta-analysis of 14 studies in 3,139 patients found that elevated troponin after noncardiac surgery was an independent predictor of death within 1 year (odds ratio [OR] 6.7, 95% confidence interval [CI] 4.1–10.9) and beyond 1 year (OR 1.8, 95% CI 1.4–2.3).37
SHOULD SCREENING BE ROUTINE AFTER NONCARDIAC SURGERY?
Since patients suffering myocardial infarction or injury after noncardiac surgery have a worse prognosis, many experts advocate routinely screening all high-risk patients and those undergoing moderate- to high-risk surgery. Many tools exist to determine which patients undergoing noncardiac surgery are at high risk of cardiac complications.
The revised Goldman Cardiac Risk Index is commonly used and well validated. Variables in this index that predict major cardiac complications are:
- High-risk surgery (vascular surgery, orthopedic surgery, and intraperitoneal or intrathoracic surgery)
- History of ischemic heart disease
- History of congestive heart failure
- History of cerebrovascular disease
- Diabetes requiring insulin therapy
- Chronic kidney disease with a creatinine > 2.0 mg/dL.
The more of these variables that are present, the higher the risk of perioperative cardiac events2,4:
- No risk factors: 0.4% risk (95% CI 0.1–0.8)
- One risk factor: 1.0% risk (95% CI 0.5–1.4)
- Two risk factors: 2.4% risk (95% CI 1.3–3.5)
- Three or more risk factors: 5.4% risk (95% CI 2.7–7.9).
Current guidelines from the American College of Cardiology and the American Heart Association give a class I recommendation (the highest) for measuring troponin levels after noncardiac surgery in patients who have symptoms or signs suggesting myocardial ischemia. They give a class IIb recommendation (usefulness is less well established) for screening those at high risk but without symptoms or signs of ischemia, despite the previously cited evidence that patients with troponin elevation are at increased risk. The IIb recommendation is due to a lack of validated treatment strategies to modify and attenuate the recognized risk with troponin elevation in this setting.39
LITTLE EVIDENCE TO GUIDE TREATMENT
In current practice, internists and cardiologists are often asked to consult on patients with troponin elevations noted after noncardiac surgery. Although published and ongoing studies examine strategies to prevent cardiovascular events during noncardiac surgery, we lack data on managing the cases of myocardial infarction and injury that actually occur after noncardiac surgery.
When managing a patient who has a troponin elevation after surgery, many clinical factors must be weighed, including hemodynamic and clinical stability and risk of bleeding. Confronted with ST-segment elevation myocardial infarction or high-risk non–ST-segment elevation myocardial infarction, most clinicians would favor an early invasive reperfusion strategy in accordance with guidelines on managing acute coronary syndrome. Fibrinolytic drugs for ST-segment elevation myocardial infarction are likely to be contraindicated in the postoperative period because they pose an unacceptable risk of bleeding.
Guideline-directed medical therapies for those suffering perioperative myocardial infarction may lower the risk of future cardiovascular events, as suggested by a retrospective study of 66 patients diagnosed with perioperative myocardial infarction after vascular surgery.40 Those in whom medical therapy for coronary artery disease was not intensified—defined as adding or increasing the dose of antiplatelet agent, statin, beta-blocker, or angiotensin-converting enzyme inhibitor—had higher rates of cardiovascular events at 12 months (hazard ratio [HR] 2.80, 95% CI 1.05–24.2).40
In those with asymptomatic myocardial infarction or isolated elevation in cardiac biomarkers, no treatment strategies have been assessed prospectively or in randomized trials. However, statins and aspirin have been suggested as providing some benefit. In a substudy of the POISE trial, the use of aspirin was associated with a 46% reduction in the 30-day mortality rate in those suffering a perioperative myocardial infarction, and statins were associated with a 76% reduction.10 In a single-center retrospective analysis of 337 patients undergoing moderate- to high-risk vascular surgery, statin therapy was associated with a lower 1-year mortality rate (OR 0.63, 95% CI 0.40–0.98).38
We propose a treatment algorithm for patients identified as having cardiovascular events after noncardiac surgery (Figure 2), based on current evidence and guidelines. Ultimately, treatment decisions should be tailored to the individual patient. Discussion of the risks and benefits of therapeutic options should include the patient and surgeon.
Ongoing and future trials
Ongoing and future trials are aimed at addressing definitive treatment strategies in this patient population.
The MANAGE trial (Management of Myocardial Injury After Non-cardiac Surgery Trial) is randomizing patients suffering myocardial injury after noncardiac surgery to receive either dabigatran and omeprazole or placebo to assess the efficacy of these agents in preventing major adverse cardiac events and the safety of anticoagulation (ClinicalTrials.gov Identifier: NCT01661101).
The INTREPID trial (Study of Ticagrelor Versus Aspirin Treatment in Patients With Myocardial Injury Post Major Non-Cardiac Surgery) will assess the efficacy and safety of ticagrelor treatment compared with aspirin in a similar population (ClinicalTrial.gov Identifier: NCT02291419). The trial will enroll approximately 1,000 patients identified as having a postoperative troponin elevation more than two times the upper limit of normal of the assay during the index hospitalization (Figure 3). Enrollment was to have begun in mid-2015.
- Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372:139–144.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173:627–634.
- McFalls EO, Ward HB, Moritz TE, et al. Predictors and outcomes of a perioperative myocardial infarction following elective vascular surgery in patients with documented coronary artery disease: results of the CARP trial. Eur Heart J 2008; 29:394–401.
- van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation 2013; 127:2264–2271.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc 2009; 84:917–938.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012; 126:2020–2035.
- Apple FS, Quist HE, Doyle PJ, Otto AP, Murakami MM. Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clin Chem 2003; 49:1331–1336.
- Ottani F, Galvani M, Nicolini FA, et al. Elevated cardiac troponin levels predict the risk of adverse outcome in patients with acute coronary syndromes. Am Heart J 2000; 140:917–927.
- Ohman EM, Armstrong PW, White HD, et al. Risk stratification with a point-of-care cardiac troponin T test in acute myocardial infarction. GUSTO III investigators. Global Use of Strategies to Open Occluded Coronary Arteries. Am J Cardiol 1999; 84:1281–1286.
- deFilippi CR, Tocchi M, Parmar RJ, et al. Cardiac troponin T in chest pain unit patients without ischemic electrocardiographic changes: angiographic correlates and long-term clinical outcomes. J Am Coll Cardiol 2000; 35:1827–1834.
- Heidenreich PA, Alloggiamento T, Melsop K, McDonald KM, Go AS, Hlatky MA. The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol 2001; 38:478–485.
- Steen H, Giannitsis E, Futterer S, Merten C, Juenger C, Katus HA. Cardiac troponin T at 96 hours after acute myocardial infarction correlates with infarct size and cardiac function. J Am Coll Cardiol 2006; 48:2192–2194.
- Licka M, Zimmermann R, Zehelein J, Dengler TJ, Katus HA, Kubler W. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart 2002; 87:520–524.
- Vasile VC, Babuin L, Giannitsis E, Katus HA, Jaffe AS. Relationship of MRI-determined infarct size and cTnI measurements in patients with ST-elevation myocardial infarction. Clin Chem 2008; 54:617–619.
- Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J 2009; 30:162–169.
- Siriwardena M, Campbell V, Richards AM, Pemberton CJ. Cardiac biomarker responses to dobutamine stress echocardiography in healthy volunteers and patients with coronary artery disease. Clin Chem 2012; 58:1492–1494.
- Turer AT, Addo TA, Martin JL, et al. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol 2011; 57:2398–2405.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- Gualandro DM, Campos CA, Calderaro D, et al. Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: frequent and dangerous. Atherosclerosis 2012; 222:191–195.
- Sametz W, Metzler H, Gries M, et al. Perioperative catecholamine changes in cardiac risk patients. Eur J Clin Invest 1999; 29:582–587.
- Frank SM, Higgins MS, Breslow MJ, et al. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia. A randomized clinical trial. Anesthesiology 1995; 82:83–93.
- Rosenfeld BA, Faraday N, Campbell D, et al. Perioperative platelet reactivity and the effects of clonidine. Anesthesiology 1993; 79:255–261.
- Lison S, Weiss G, Spannagl M, Heindl B. Postoperative changes in procoagulant factors after major surgery. Blood Coagul Fibrinolysis 2011; 22:190–196.
- Fukumoto Y, Hiro T, Fujii T, et al. Localized elevation of shear stress is related to coronary plaque rupture: a 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J Am Coll Cardiol 2008; 51:645–650.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114:I-344–I-349.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Nelson AH, Fleisher LA, Rosenbaum SH. Relationship between postoperative anemia and cardiac morbidity in high-risk vascular patients in the intensive care unit. Crit Care Med 1993; 21:860–866.
- Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation 2009; 119:2936–2944.
- Ellis SG, Hertzer NR, Young JR, Brener S. Angiographic correlates of cardiac death and myocardial infarction complicating major nonthoracic vascular surgery. Am J Cardiol 1996; 77:1126–1128.
- Levy M, Heels-Ansdell D, Hiralal R, et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta-analysis. Anesthesiology 2011; 114:796–806.
- Garcia S, Marston N, Sandoval Y, et al. Prognostic value of 12-lead electrocardiogram and peak troponin I level after vascular surgery. J Vasc Surg 2013; 57:166–172.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Foucrier A, Rodseth R, Aissaoui M, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
More than 200 million patients undergo noncardiac surgery each year, and the volume is increasing.1 Cardiovascular complications are a major cause of morbidity and mortality in the perioperative period.
Before the advent of modern cardiac biomarkers, an estimated 2% to 3% of all patients undergoing noncardiac surgery had a major adverse cardiac event.2 However, more recent studies suggest that 5% to 25% of patients have troponin elevations after noncardiac surgery, depending on the patient population,3–6 and many are asymptomatic, suggesting that many patients are sustaining undetected myocardial injury. Those who suffer a myocardial infarction or myocardial injury have elevated morbidity and mortality rates, not only perioperatively, but also at 30 days and even at up to 1 year.3–5,7–11
Yet there are almost no data on how best to manage these patients; the available guidelines, therefore, do not provide sufficient recommendations for clinical practice.
To address the lack of guidelines, we examine the incidence and proposed mechanisms of myocardial injury after noncardiac surgery, suggest an approach to identifying patients at risk, recommend treatment strategies, and consider future directions.
CARDIAC BIOMARKERS
When cardiac cellular injury from ischemia, direct trauma, or other cause disrupts the cell membrane, intracellular contents enter the extracellular space, including the blood stream. If the myocyte damage is extensive enough, biochemical assays can detect these substances.
Troponin, creatine kinase, myoglobin, and lactate dehydrogenase are common biomarkers of necrosis that, when detected in the plasma, may indicate cardiac injury. Each can be detected at varying times after cardiac injury (Figure 1).12
Cardiac troponins I and T
Of the biomarkers, cardiac troponin I and cardiac troponin T are now the most widely used and are the most specific for myocyte injury.
Troponins are proteins that regulate the calcium-induced interaction between myosin and actin that results in muscle contraction. Troponin is a complex consisting of three subunits: troponin C, troponin I, and troponin T. The cardiac troponin I and T isoforms are distinct from those found in skeletal muscle, making them specific for myocyte injury, and they are currently the recommended markers for diagnosing acute myocardial infarction.13
The troponin immunoassays currently available are not standardized among laboratories and point-of-care methods, and thus, levels cannot be compared across testing centers.14 Each assay has unique performance characteristics, but guidelines recommend using the 99th percentile value from a normal reference population for a given assay to define whether myocardial injury is present.13
Troponin elevation has prognostic value in patients presenting with acute coronary syndromes,15–18 and the degree of elevation correlates with infarct size.19–21
Controversy exists as to whether troponin and other biomarkers are released only after myocardial necrosis or after reversible injury as well. Using newer, highly sensitive assays, troponin elevations have been detected after short periods of ischemia during stress testing22,23 and in patients with stable angina,24 suggesting that reversible cardiac stress and injury can lead to troponin release. This mechanism may play an important role during the myocardial injury that can occur in patients undergoing noncardiac surgery.
MYOCARDIAL INFARCTION vs MYOCARDIAL INJURY
In 2000, the Joint Task Force of the European Society of Cardiology, American College of Cardiology Foundation, American Heart Association, and World Heart Federation revised the criteria for the diagnosis of myocardial infarction created by the World Health Organization in 1979. The definition was revised again in 2007 and once more in 2012 to create the third universal definition of myocardial infarction.
Acute myocardial infarction
Acute myocardial infarction is defined as evidence of myocardial necrosis in a setting of myocardial ischemia, not related to causes such as trauma or pulmonary embolism, with a rise or a fall (or a rise and a fall) of cardiac biomarkers (at least one value being above the 99th percentile in the reference population) and any of the following:
- Symptoms of ischemia
- New ST-segment changes or new left bundle branch block
- Pathologic Q waves
- Imaging evidence of new loss of viable myocardium or new regional wall-motion abnormality
- Intracoronary thrombus by angiography or autopsy.13
Myocardial injury after noncardiac surgery
Studies10,11 have shown that many patients undergoing noncardiac surgery have evidence of cardiac biomarker release but do not meet the universal definition of myocardial infarction.
The Perioperative Ischemic Evaluation (POISE) trial10 reported that 415 (5%) of its patients met the definition of myocardial infarction, of whom only about 35% had symptoms of ischemia. Another 697 patients (8.3%) had isolated elevations in biomarkers without meeting the definition of myocardial infarction.
The VISION study11 (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation) prospectively screened more than 15,000 patients in several countries for troponin elevation during the first 3 postoperative days and for ischemic symptoms and features. Of the patients screened, approximately 1,200 (8%) had troponin elevations, with fewer than half fulfilling the criteria for myocardial infarction.
In another study, van Waes et al6 prospectively screened 2,232 patients ages 60 and older undergoing intermediate- to high-risk noncardiac surgery. Troponin levels were elevated in 19% of the patients, but only 10 of these patients met the universal definition of myocardial infarction.
In all of these studies, patients with isolated elevation in myocardial biomarkers had worse short-term and long-term outcomes than those without. These observations led to a proposed definition of “myocardial injury after noncardiac surgery” that is broader than that of myocardial infarction and requires only elevation of cardiac biomarkers judged to be due to myocardial ischemia (ie, not from another obvious cause such as pulmonary embolism or myocarditis).3
FIVE TYPES OF MYOCARDIAL INFARCTION
The Joint Task Force13 categorizes myocardial infarction into five distinct types:
- Type 1—due to plaque rupture
- Type 2—due to imbalance between oxygen supply and demand
- Type 3—sudden cardiac death
- Type 4a—associated with percutaneous coronary intervention
- Type 4b—associated with stent thrombosis
- Type 5—associated with coronary artery bypass surgery.
Types 1 and 2 have both been implicated in perioperative myocardial infarction and injury. Patient characteristics and the physiologic response to surgical and anesthetic stressors likely contribute to the development of myocardial infarction and injury after noncardiac surgery.
Plaque rupture as a cause of postoperative myocardial infarction
The mechanism of type 1 myocardial infarction—plaque rupture or erosion leading to thrombosis and infarction—plays a significant role in most cases of acute coronary syndromes. Its role in perioperative and postoperative myocardial infarction or injury, however, is less clear.
In an autopsy study of 26 patients who died of myocardial infarction after noncardiac surgery, plaque rupture was evident in 12 (46%).25 A prospective angiographic study of 120 patients with acute coronary syndromes after noncardiac surgery found that nearly 50% had evidence of plaque rupture.26
Higher levels of catecholamines, cortisol,27,28 platelet reactivity,29 procoagulant factors,30 and coronary artery shear stress31 are all present in the postoperative period and may contribute to an increased propensity for plaque rupture or erosion. Whether plaque rupture is present in patients who have isolated troponin elevation but do not meet the criteria for myocardial infarction has not been investigated.
Oxygen supply-demand imbalance during and after surgery
Oxygen supply-demand imbalance (the mechanism in type 2 myocardial infarction) leading to myocyte stress, ischemia, and subsequent infarction is likely common in the perioperative and postoperative periods. As previously discussed, this imbalance may be present with or without symptoms.
Oxygen demand may increase in this period as a result of tachycardia32 caused by bleeding, pain, and catecholamines or increased wall stress from hypertension due to vasoconstriction or pain.33 Oxygen supply can be decreased secondary to tachycardia, anemia,34 hypotension, hypoxemia, hypercarbia, intravascular fluid shifts (bleeding or volume overload), or coronary vasoconstriction.33,35
These mechanisms of myocardial injury, infarction, or both can occur with or without underlying significant obstructive coronary artery disease. However, severe coronary artery disease is more common in those who have had a perioperative myocardial infarction.36
POSTOPERATIVE TROPONIN ELEVATION CARRIES A WORSE PROGNOSIS
Patients who suffer a myocardial infarction after noncardiac surgery have worse short- and long-term outcomes than their counterparts.4,5,7, 8,10,33 In the POISE trial,10 the 30-day mortality rate was 11.6% in those who had had a perioperative myocardial infarction, compared with 2.2% in those who did not (P < .001). The patients who had had a myocardial infarction were also more likely to have nonfatal cardiac arrest, coronary revascularization, and congestive heart failure.
Myocardial injury not fulfilling the criteria for myocardial infarction after noncardiac surgery is also associated with worse short-term and long-term outcomes.3,6,10,11,37,38 POISE patients with isolated elevations in cardiac biomarkers had a higher 30-day risk of coronary revascularization and nonfatal arrest.10 In the VISION trial, an elevation in troponin was the strongest predictor of death within 30 days after noncardiac surgery. This analysis also showed that the higher the peak troponin value, the greater the risk of death and the shorter the median time until death.11
A meta-analysis of 14 studies in 3,139 patients found that elevated troponin after noncardiac surgery was an independent predictor of death within 1 year (odds ratio [OR] 6.7, 95% confidence interval [CI] 4.1–10.9) and beyond 1 year (OR 1.8, 95% CI 1.4–2.3).37
SHOULD SCREENING BE ROUTINE AFTER NONCARDIAC SURGERY?
Since patients suffering myocardial infarction or injury after noncardiac surgery have a worse prognosis, many experts advocate routinely screening all high-risk patients and those undergoing moderate- to high-risk surgery. Many tools exist to determine which patients undergoing noncardiac surgery are at high risk of cardiac complications.
The revised Goldman Cardiac Risk Index is commonly used and well validated. Variables in this index that predict major cardiac complications are:
- High-risk surgery (vascular surgery, orthopedic surgery, and intraperitoneal or intrathoracic surgery)
- History of ischemic heart disease
- History of congestive heart failure
- History of cerebrovascular disease
- Diabetes requiring insulin therapy
- Chronic kidney disease with a creatinine > 2.0 mg/dL.
The more of these variables that are present, the higher the risk of perioperative cardiac events2,4:
- No risk factors: 0.4% risk (95% CI 0.1–0.8)
- One risk factor: 1.0% risk (95% CI 0.5–1.4)
- Two risk factors: 2.4% risk (95% CI 1.3–3.5)
- Three or more risk factors: 5.4% risk (95% CI 2.7–7.9).
Current guidelines from the American College of Cardiology and the American Heart Association give a class I recommendation (the highest) for measuring troponin levels after noncardiac surgery in patients who have symptoms or signs suggesting myocardial ischemia. They give a class IIb recommendation (usefulness is less well established) for screening those at high risk but without symptoms or signs of ischemia, despite the previously cited evidence that patients with troponin elevation are at increased risk. The IIb recommendation is due to a lack of validated treatment strategies to modify and attenuate the recognized risk with troponin elevation in this setting.39
LITTLE EVIDENCE TO GUIDE TREATMENT
In current practice, internists and cardiologists are often asked to consult on patients with troponin elevations noted after noncardiac surgery. Although published and ongoing studies examine strategies to prevent cardiovascular events during noncardiac surgery, we lack data on managing the cases of myocardial infarction and injury that actually occur after noncardiac surgery.
When managing a patient who has a troponin elevation after surgery, many clinical factors must be weighed, including hemodynamic and clinical stability and risk of bleeding. Confronted with ST-segment elevation myocardial infarction or high-risk non–ST-segment elevation myocardial infarction, most clinicians would favor an early invasive reperfusion strategy in accordance with guidelines on managing acute coronary syndrome. Fibrinolytic drugs for ST-segment elevation myocardial infarction are likely to be contraindicated in the postoperative period because they pose an unacceptable risk of bleeding.
Guideline-directed medical therapies for those suffering perioperative myocardial infarction may lower the risk of future cardiovascular events, as suggested by a retrospective study of 66 patients diagnosed with perioperative myocardial infarction after vascular surgery.40 Those in whom medical therapy for coronary artery disease was not intensified—defined as adding or increasing the dose of antiplatelet agent, statin, beta-blocker, or angiotensin-converting enzyme inhibitor—had higher rates of cardiovascular events at 12 months (hazard ratio [HR] 2.80, 95% CI 1.05–24.2).40
In those with asymptomatic myocardial infarction or isolated elevation in cardiac biomarkers, no treatment strategies have been assessed prospectively or in randomized trials. However, statins and aspirin have been suggested as providing some benefit. In a substudy of the POISE trial, the use of aspirin was associated with a 46% reduction in the 30-day mortality rate in those suffering a perioperative myocardial infarction, and statins were associated with a 76% reduction.10 In a single-center retrospective analysis of 337 patients undergoing moderate- to high-risk vascular surgery, statin therapy was associated with a lower 1-year mortality rate (OR 0.63, 95% CI 0.40–0.98).38
We propose a treatment algorithm for patients identified as having cardiovascular events after noncardiac surgery (Figure 2), based on current evidence and guidelines. Ultimately, treatment decisions should be tailored to the individual patient. Discussion of the risks and benefits of therapeutic options should include the patient and surgeon.
Ongoing and future trials
Ongoing and future trials are aimed at addressing definitive treatment strategies in this patient population.
The MANAGE trial (Management of Myocardial Injury After Non-cardiac Surgery Trial) is randomizing patients suffering myocardial injury after noncardiac surgery to receive either dabigatran and omeprazole or placebo to assess the efficacy of these agents in preventing major adverse cardiac events and the safety of anticoagulation (ClinicalTrials.gov Identifier: NCT01661101).
The INTREPID trial (Study of Ticagrelor Versus Aspirin Treatment in Patients With Myocardial Injury Post Major Non-Cardiac Surgery) will assess the efficacy and safety of ticagrelor treatment compared with aspirin in a similar population (ClinicalTrial.gov Identifier: NCT02291419). The trial will enroll approximately 1,000 patients identified as having a postoperative troponin elevation more than two times the upper limit of normal of the assay during the index hospitalization (Figure 3). Enrollment was to have begun in mid-2015.
More than 200 million patients undergo noncardiac surgery each year, and the volume is increasing.1 Cardiovascular complications are a major cause of morbidity and mortality in the perioperative period.
Before the advent of modern cardiac biomarkers, an estimated 2% to 3% of all patients undergoing noncardiac surgery had a major adverse cardiac event.2 However, more recent studies suggest that 5% to 25% of patients have troponin elevations after noncardiac surgery, depending on the patient population,3–6 and many are asymptomatic, suggesting that many patients are sustaining undetected myocardial injury. Those who suffer a myocardial infarction or myocardial injury have elevated morbidity and mortality rates, not only perioperatively, but also at 30 days and even at up to 1 year.3–5,7–11
Yet there are almost no data on how best to manage these patients; the available guidelines, therefore, do not provide sufficient recommendations for clinical practice.
To address the lack of guidelines, we examine the incidence and proposed mechanisms of myocardial injury after noncardiac surgery, suggest an approach to identifying patients at risk, recommend treatment strategies, and consider future directions.
CARDIAC BIOMARKERS
When cardiac cellular injury from ischemia, direct trauma, or other cause disrupts the cell membrane, intracellular contents enter the extracellular space, including the blood stream. If the myocyte damage is extensive enough, biochemical assays can detect these substances.
Troponin, creatine kinase, myoglobin, and lactate dehydrogenase are common biomarkers of necrosis that, when detected in the plasma, may indicate cardiac injury. Each can be detected at varying times after cardiac injury (Figure 1).12
Cardiac troponins I and T
Of the biomarkers, cardiac troponin I and cardiac troponin T are now the most widely used and are the most specific for myocyte injury.
Troponins are proteins that regulate the calcium-induced interaction between myosin and actin that results in muscle contraction. Troponin is a complex consisting of three subunits: troponin C, troponin I, and troponin T. The cardiac troponin I and T isoforms are distinct from those found in skeletal muscle, making them specific for myocyte injury, and they are currently the recommended markers for diagnosing acute myocardial infarction.13
The troponin immunoassays currently available are not standardized among laboratories and point-of-care methods, and thus, levels cannot be compared across testing centers.14 Each assay has unique performance characteristics, but guidelines recommend using the 99th percentile value from a normal reference population for a given assay to define whether myocardial injury is present.13
Troponin elevation has prognostic value in patients presenting with acute coronary syndromes,15–18 and the degree of elevation correlates with infarct size.19–21
Controversy exists as to whether troponin and other biomarkers are released only after myocardial necrosis or after reversible injury as well. Using newer, highly sensitive assays, troponin elevations have been detected after short periods of ischemia during stress testing22,23 and in patients with stable angina,24 suggesting that reversible cardiac stress and injury can lead to troponin release. This mechanism may play an important role during the myocardial injury that can occur in patients undergoing noncardiac surgery.
MYOCARDIAL INFARCTION vs MYOCARDIAL INJURY
In 2000, the Joint Task Force of the European Society of Cardiology, American College of Cardiology Foundation, American Heart Association, and World Heart Federation revised the criteria for the diagnosis of myocardial infarction created by the World Health Organization in 1979. The definition was revised again in 2007 and once more in 2012 to create the third universal definition of myocardial infarction.
Acute myocardial infarction
Acute myocardial infarction is defined as evidence of myocardial necrosis in a setting of myocardial ischemia, not related to causes such as trauma or pulmonary embolism, with a rise or a fall (or a rise and a fall) of cardiac biomarkers (at least one value being above the 99th percentile in the reference population) and any of the following:
- Symptoms of ischemia
- New ST-segment changes or new left bundle branch block
- Pathologic Q waves
- Imaging evidence of new loss of viable myocardium or new regional wall-motion abnormality
- Intracoronary thrombus by angiography or autopsy.13
Myocardial injury after noncardiac surgery
Studies10,11 have shown that many patients undergoing noncardiac surgery have evidence of cardiac biomarker release but do not meet the universal definition of myocardial infarction.
The Perioperative Ischemic Evaluation (POISE) trial10 reported that 415 (5%) of its patients met the definition of myocardial infarction, of whom only about 35% had symptoms of ischemia. Another 697 patients (8.3%) had isolated elevations in biomarkers without meeting the definition of myocardial infarction.
The VISION study11 (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation) prospectively screened more than 15,000 patients in several countries for troponin elevation during the first 3 postoperative days and for ischemic symptoms and features. Of the patients screened, approximately 1,200 (8%) had troponin elevations, with fewer than half fulfilling the criteria for myocardial infarction.
In another study, van Waes et al6 prospectively screened 2,232 patients ages 60 and older undergoing intermediate- to high-risk noncardiac surgery. Troponin levels were elevated in 19% of the patients, but only 10 of these patients met the universal definition of myocardial infarction.
In all of these studies, patients with isolated elevation in myocardial biomarkers had worse short-term and long-term outcomes than those without. These observations led to a proposed definition of “myocardial injury after noncardiac surgery” that is broader than that of myocardial infarction and requires only elevation of cardiac biomarkers judged to be due to myocardial ischemia (ie, not from another obvious cause such as pulmonary embolism or myocarditis).3
FIVE TYPES OF MYOCARDIAL INFARCTION
The Joint Task Force13 categorizes myocardial infarction into five distinct types:
- Type 1—due to plaque rupture
- Type 2—due to imbalance between oxygen supply and demand
- Type 3—sudden cardiac death
- Type 4a—associated with percutaneous coronary intervention
- Type 4b—associated with stent thrombosis
- Type 5—associated with coronary artery bypass surgery.
Types 1 and 2 have both been implicated in perioperative myocardial infarction and injury. Patient characteristics and the physiologic response to surgical and anesthetic stressors likely contribute to the development of myocardial infarction and injury after noncardiac surgery.
Plaque rupture as a cause of postoperative myocardial infarction
The mechanism of type 1 myocardial infarction—plaque rupture or erosion leading to thrombosis and infarction—plays a significant role in most cases of acute coronary syndromes. Its role in perioperative and postoperative myocardial infarction or injury, however, is less clear.
In an autopsy study of 26 patients who died of myocardial infarction after noncardiac surgery, plaque rupture was evident in 12 (46%).25 A prospective angiographic study of 120 patients with acute coronary syndromes after noncardiac surgery found that nearly 50% had evidence of plaque rupture.26
Higher levels of catecholamines, cortisol,27,28 platelet reactivity,29 procoagulant factors,30 and coronary artery shear stress31 are all present in the postoperative period and may contribute to an increased propensity for plaque rupture or erosion. Whether plaque rupture is present in patients who have isolated troponin elevation but do not meet the criteria for myocardial infarction has not been investigated.
Oxygen supply-demand imbalance during and after surgery
Oxygen supply-demand imbalance (the mechanism in type 2 myocardial infarction) leading to myocyte stress, ischemia, and subsequent infarction is likely common in the perioperative and postoperative periods. As previously discussed, this imbalance may be present with or without symptoms.
Oxygen demand may increase in this period as a result of tachycardia32 caused by bleeding, pain, and catecholamines or increased wall stress from hypertension due to vasoconstriction or pain.33 Oxygen supply can be decreased secondary to tachycardia, anemia,34 hypotension, hypoxemia, hypercarbia, intravascular fluid shifts (bleeding or volume overload), or coronary vasoconstriction.33,35
These mechanisms of myocardial injury, infarction, or both can occur with or without underlying significant obstructive coronary artery disease. However, severe coronary artery disease is more common in those who have had a perioperative myocardial infarction.36
POSTOPERATIVE TROPONIN ELEVATION CARRIES A WORSE PROGNOSIS
Patients who suffer a myocardial infarction after noncardiac surgery have worse short- and long-term outcomes than their counterparts.4,5,7, 8,10,33 In the POISE trial,10 the 30-day mortality rate was 11.6% in those who had had a perioperative myocardial infarction, compared with 2.2% in those who did not (P < .001). The patients who had had a myocardial infarction were also more likely to have nonfatal cardiac arrest, coronary revascularization, and congestive heart failure.
Myocardial injury not fulfilling the criteria for myocardial infarction after noncardiac surgery is also associated with worse short-term and long-term outcomes.3,6,10,11,37,38 POISE patients with isolated elevations in cardiac biomarkers had a higher 30-day risk of coronary revascularization and nonfatal arrest.10 In the VISION trial, an elevation in troponin was the strongest predictor of death within 30 days after noncardiac surgery. This analysis also showed that the higher the peak troponin value, the greater the risk of death and the shorter the median time until death.11
A meta-analysis of 14 studies in 3,139 patients found that elevated troponin after noncardiac surgery was an independent predictor of death within 1 year (odds ratio [OR] 6.7, 95% confidence interval [CI] 4.1–10.9) and beyond 1 year (OR 1.8, 95% CI 1.4–2.3).37
SHOULD SCREENING BE ROUTINE AFTER NONCARDIAC SURGERY?
Since patients suffering myocardial infarction or injury after noncardiac surgery have a worse prognosis, many experts advocate routinely screening all high-risk patients and those undergoing moderate- to high-risk surgery. Many tools exist to determine which patients undergoing noncardiac surgery are at high risk of cardiac complications.
The revised Goldman Cardiac Risk Index is commonly used and well validated. Variables in this index that predict major cardiac complications are:
- High-risk surgery (vascular surgery, orthopedic surgery, and intraperitoneal or intrathoracic surgery)
- History of ischemic heart disease
- History of congestive heart failure
- History of cerebrovascular disease
- Diabetes requiring insulin therapy
- Chronic kidney disease with a creatinine > 2.0 mg/dL.
The more of these variables that are present, the higher the risk of perioperative cardiac events2,4:
- No risk factors: 0.4% risk (95% CI 0.1–0.8)
- One risk factor: 1.0% risk (95% CI 0.5–1.4)
- Two risk factors: 2.4% risk (95% CI 1.3–3.5)
- Three or more risk factors: 5.4% risk (95% CI 2.7–7.9).
Current guidelines from the American College of Cardiology and the American Heart Association give a class I recommendation (the highest) for measuring troponin levels after noncardiac surgery in patients who have symptoms or signs suggesting myocardial ischemia. They give a class IIb recommendation (usefulness is less well established) for screening those at high risk but without symptoms or signs of ischemia, despite the previously cited evidence that patients with troponin elevation are at increased risk. The IIb recommendation is due to a lack of validated treatment strategies to modify and attenuate the recognized risk with troponin elevation in this setting.39
LITTLE EVIDENCE TO GUIDE TREATMENT
In current practice, internists and cardiologists are often asked to consult on patients with troponin elevations noted after noncardiac surgery. Although published and ongoing studies examine strategies to prevent cardiovascular events during noncardiac surgery, we lack data on managing the cases of myocardial infarction and injury that actually occur after noncardiac surgery.
When managing a patient who has a troponin elevation after surgery, many clinical factors must be weighed, including hemodynamic and clinical stability and risk of bleeding. Confronted with ST-segment elevation myocardial infarction or high-risk non–ST-segment elevation myocardial infarction, most clinicians would favor an early invasive reperfusion strategy in accordance with guidelines on managing acute coronary syndrome. Fibrinolytic drugs for ST-segment elevation myocardial infarction are likely to be contraindicated in the postoperative period because they pose an unacceptable risk of bleeding.
Guideline-directed medical therapies for those suffering perioperative myocardial infarction may lower the risk of future cardiovascular events, as suggested by a retrospective study of 66 patients diagnosed with perioperative myocardial infarction after vascular surgery.40 Those in whom medical therapy for coronary artery disease was not intensified—defined as adding or increasing the dose of antiplatelet agent, statin, beta-blocker, or angiotensin-converting enzyme inhibitor—had higher rates of cardiovascular events at 12 months (hazard ratio [HR] 2.80, 95% CI 1.05–24.2).40
In those with asymptomatic myocardial infarction or isolated elevation in cardiac biomarkers, no treatment strategies have been assessed prospectively or in randomized trials. However, statins and aspirin have been suggested as providing some benefit. In a substudy of the POISE trial, the use of aspirin was associated with a 46% reduction in the 30-day mortality rate in those suffering a perioperative myocardial infarction, and statins were associated with a 76% reduction.10 In a single-center retrospective analysis of 337 patients undergoing moderate- to high-risk vascular surgery, statin therapy was associated with a lower 1-year mortality rate (OR 0.63, 95% CI 0.40–0.98).38
We propose a treatment algorithm for patients identified as having cardiovascular events after noncardiac surgery (Figure 2), based on current evidence and guidelines. Ultimately, treatment decisions should be tailored to the individual patient. Discussion of the risks and benefits of therapeutic options should include the patient and surgeon.
Ongoing and future trials
Ongoing and future trials are aimed at addressing definitive treatment strategies in this patient population.
The MANAGE trial (Management of Myocardial Injury After Non-cardiac Surgery Trial) is randomizing patients suffering myocardial injury after noncardiac surgery to receive either dabigatran and omeprazole or placebo to assess the efficacy of these agents in preventing major adverse cardiac events and the safety of anticoagulation (ClinicalTrials.gov Identifier: NCT01661101).
The INTREPID trial (Study of Ticagrelor Versus Aspirin Treatment in Patients With Myocardial Injury Post Major Non-Cardiac Surgery) will assess the efficacy and safety of ticagrelor treatment compared with aspirin in a similar population (ClinicalTrial.gov Identifier: NCT02291419). The trial will enroll approximately 1,000 patients identified as having a postoperative troponin elevation more than two times the upper limit of normal of the assay during the index hospitalization (Figure 3). Enrollment was to have begun in mid-2015.
- Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372:139–144.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173:627–634.
- McFalls EO, Ward HB, Moritz TE, et al. Predictors and outcomes of a perioperative myocardial infarction following elective vascular surgery in patients with documented coronary artery disease: results of the CARP trial. Eur Heart J 2008; 29:394–401.
- van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation 2013; 127:2264–2271.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc 2009; 84:917–938.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012; 126:2020–2035.
- Apple FS, Quist HE, Doyle PJ, Otto AP, Murakami MM. Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clin Chem 2003; 49:1331–1336.
- Ottani F, Galvani M, Nicolini FA, et al. Elevated cardiac troponin levels predict the risk of adverse outcome in patients with acute coronary syndromes. Am Heart J 2000; 140:917–927.
- Ohman EM, Armstrong PW, White HD, et al. Risk stratification with a point-of-care cardiac troponin T test in acute myocardial infarction. GUSTO III investigators. Global Use of Strategies to Open Occluded Coronary Arteries. Am J Cardiol 1999; 84:1281–1286.
- deFilippi CR, Tocchi M, Parmar RJ, et al. Cardiac troponin T in chest pain unit patients without ischemic electrocardiographic changes: angiographic correlates and long-term clinical outcomes. J Am Coll Cardiol 2000; 35:1827–1834.
- Heidenreich PA, Alloggiamento T, Melsop K, McDonald KM, Go AS, Hlatky MA. The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol 2001; 38:478–485.
- Steen H, Giannitsis E, Futterer S, Merten C, Juenger C, Katus HA. Cardiac troponin T at 96 hours after acute myocardial infarction correlates with infarct size and cardiac function. J Am Coll Cardiol 2006; 48:2192–2194.
- Licka M, Zimmermann R, Zehelein J, Dengler TJ, Katus HA, Kubler W. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart 2002; 87:520–524.
- Vasile VC, Babuin L, Giannitsis E, Katus HA, Jaffe AS. Relationship of MRI-determined infarct size and cTnI measurements in patients with ST-elevation myocardial infarction. Clin Chem 2008; 54:617–619.
- Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J 2009; 30:162–169.
- Siriwardena M, Campbell V, Richards AM, Pemberton CJ. Cardiac biomarker responses to dobutamine stress echocardiography in healthy volunteers and patients with coronary artery disease. Clin Chem 2012; 58:1492–1494.
- Turer AT, Addo TA, Martin JL, et al. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol 2011; 57:2398–2405.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- Gualandro DM, Campos CA, Calderaro D, et al. Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: frequent and dangerous. Atherosclerosis 2012; 222:191–195.
- Sametz W, Metzler H, Gries M, et al. Perioperative catecholamine changes in cardiac risk patients. Eur J Clin Invest 1999; 29:582–587.
- Frank SM, Higgins MS, Breslow MJ, et al. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia. A randomized clinical trial. Anesthesiology 1995; 82:83–93.
- Rosenfeld BA, Faraday N, Campbell D, et al. Perioperative platelet reactivity and the effects of clonidine. Anesthesiology 1993; 79:255–261.
- Lison S, Weiss G, Spannagl M, Heindl B. Postoperative changes in procoagulant factors after major surgery. Blood Coagul Fibrinolysis 2011; 22:190–196.
- Fukumoto Y, Hiro T, Fujii T, et al. Localized elevation of shear stress is related to coronary plaque rupture: a 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J Am Coll Cardiol 2008; 51:645–650.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114:I-344–I-349.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Nelson AH, Fleisher LA, Rosenbaum SH. Relationship between postoperative anemia and cardiac morbidity in high-risk vascular patients in the intensive care unit. Crit Care Med 1993; 21:860–866.
- Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation 2009; 119:2936–2944.
- Ellis SG, Hertzer NR, Young JR, Brener S. Angiographic correlates of cardiac death and myocardial infarction complicating major nonthoracic vascular surgery. Am J Cardiol 1996; 77:1126–1128.
- Levy M, Heels-Ansdell D, Hiralal R, et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta-analysis. Anesthesiology 2011; 114:796–806.
- Garcia S, Marston N, Sandoval Y, et al. Prognostic value of 12-lead electrocardiogram and peak troponin I level after vascular surgery. J Vasc Surg 2013; 57:166–172.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Foucrier A, Rodseth R, Aissaoui M, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
- Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372:139–144.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120:564–578.
- Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 2005; 173:627–634.
- McFalls EO, Ward HB, Moritz TE, et al. Predictors and outcomes of a perioperative myocardial infarction following elective vascular surgery in patients with documented coronary artery disease: results of the CARP trial. Eur Heart J 2008; 29:394–401.
- van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation 2013; 127:2264–2271.
- Badner NH, Knill RL, Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery. Anesthesiology 1998; 88:572–578.
- Kim LJ, Martinez EA, Faraday N, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation 2002; 106:2366–2371.
- Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol 2003; 42:1547–1554.
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011; 154:523–528.
- Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc 2009; 84:917–938.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012; 126:2020–2035.
- Apple FS, Quist HE, Doyle PJ, Otto AP, Murakami MM. Plasma 99th percentile reference limits for cardiac troponin and creatine kinase MB mass for use with European Society of Cardiology/American College of Cardiology consensus recommendations. Clin Chem 2003; 49:1331–1336.
- Ottani F, Galvani M, Nicolini FA, et al. Elevated cardiac troponin levels predict the risk of adverse outcome in patients with acute coronary syndromes. Am Heart J 2000; 140:917–927.
- Ohman EM, Armstrong PW, White HD, et al. Risk stratification with a point-of-care cardiac troponin T test in acute myocardial infarction. GUSTO III investigators. Global Use of Strategies to Open Occluded Coronary Arteries. Am J Cardiol 1999; 84:1281–1286.
- deFilippi CR, Tocchi M, Parmar RJ, et al. Cardiac troponin T in chest pain unit patients without ischemic electrocardiographic changes: angiographic correlates and long-term clinical outcomes. J Am Coll Cardiol 2000; 35:1827–1834.
- Heidenreich PA, Alloggiamento T, Melsop K, McDonald KM, Go AS, Hlatky MA. The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol 2001; 38:478–485.
- Steen H, Giannitsis E, Futterer S, Merten C, Juenger C, Katus HA. Cardiac troponin T at 96 hours after acute myocardial infarction correlates with infarct size and cardiac function. J Am Coll Cardiol 2006; 48:2192–2194.
- Licka M, Zimmermann R, Zehelein J, Dengler TJ, Katus HA, Kubler W. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart 2002; 87:520–524.
- Vasile VC, Babuin L, Giannitsis E, Katus HA, Jaffe AS. Relationship of MRI-determined infarct size and cTnI measurements in patients with ST-elevation myocardial infarction. Clin Chem 2008; 54:617–619.
- Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J 2009; 30:162–169.
- Siriwardena M, Campbell V, Richards AM, Pemberton CJ. Cardiac biomarker responses to dobutamine stress echocardiography in healthy volunteers and patients with coronary artery disease. Clin Chem 2012; 58:1492–1494.
- Turer AT, Addo TA, Martin JL, et al. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol 2011; 57:2398–2405.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- Gualandro DM, Campos CA, Calderaro D, et al. Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: frequent and dangerous. Atherosclerosis 2012; 222:191–195.
- Sametz W, Metzler H, Gries M, et al. Perioperative catecholamine changes in cardiac risk patients. Eur J Clin Invest 1999; 29:582–587.
- Frank SM, Higgins MS, Breslow MJ, et al. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia. A randomized clinical trial. Anesthesiology 1995; 82:83–93.
- Rosenfeld BA, Faraday N, Campbell D, et al. Perioperative platelet reactivity and the effects of clonidine. Anesthesiology 1993; 79:255–261.
- Lison S, Weiss G, Spannagl M, Heindl B. Postoperative changes in procoagulant factors after major surgery. Blood Coagul Fibrinolysis 2011; 22:190–196.
- Fukumoto Y, Hiro T, Fujii T, et al. Localized elevation of shear stress is related to coronary plaque rupture: a 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J Am Coll Cardiol 2008; 51:645–650.
- Feringa HH, Bax JJ, Boersma E, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006; 114:I-344–I-349.
- Landesberg G. The pathophysiology of perioperative myocardial infarction: facts and perspectives. J Cardiothorac Vasc Anesth 2003; 17:90–100.
- Nelson AH, Fleisher LA, Rosenbaum SH. Relationship between postoperative anemia and cardiac morbidity in high-risk vascular patients in the intensive care unit. Crit Care Med 1993; 21:860–866.
- Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation 2009; 119:2936–2944.
- Ellis SG, Hertzer NR, Young JR, Brener S. Angiographic correlates of cardiac death and myocardial infarction complicating major nonthoracic vascular surgery. Am J Cardiol 1996; 77:1126–1128.
- Levy M, Heels-Ansdell D, Hiralal R, et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta-analysis. Anesthesiology 2011; 114:796–806.
- Garcia S, Marston N, Sandoval Y, et al. Prognostic value of 12-lead electrocardiogram and peak troponin I level after vascular surgery. J Vasc Surg 2013; 57:166–172.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Foucrier A, Rodseth R, Aissaoui M, et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg 2014; 119:1053–1063.
KEY POINTS
- Cardiovascular events are a major cause of morbidity and mortality in patients undergoing noncardiac surgery and occur frequently, especially in high-risk patients.
- Myocardial injury or infarction after noncardiac surgery heightens the short- and long-term risk of mortality and major adverse cardiac events.
- The dominant mechanism of myocardial injury after noncardiac surgery remains uncertain.
- In the absence of therapies proven to affect the outcome, the benefit of screening to identify these patients remains uncertain.
- Clinical trials are under way to help clinicians provide optimal care to this at-risk population.
Why are we doing cardiovascular outcome trials in type 2 diabetes?
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11
STRATEGIES TO REDUCE CARDIOVASCULAR RISK IN DIABETES
While the incidence of diabetes mellitus has risen alarmingly, the incidence of cardiovascular complications of diabetes has declined over the years. Lowering blood glucose has not been the critical factor mediating this risk reduction. In addition to smoking cessation, proven measures that clearly reduce long-term cardiovascular risk in diabetes are blood pressure control and reduction in low-density lipoprotein cholesterol with statins.
Lower the blood pressure to less than 140 mm Hg
ADVANCE.12 In the ADVANCE trial, in addition to being randomized to usual vs intensive glucose-lowering therapy, participants were also simultaneously randomized to receive either placebo or the combination of an angiotensin-converting enzyme inhibitor and a diuretic (ie, perindopril and indapamide). Blood pressure was reduced by a mean of 5.6 mm Hg systolic and 2.2 mm Hg diastolic in the active-treatment group. This moderate reduction in blood pressure was associated with an 18% relative risk reduction in death from cardiovascular disease and a 14% relative risk reduction in death from any cause.
The ACCORD trial13 lowered systolic blood pressure even more in the intensive-treatment group, with a target systolic blood pressure of less than 120 mm Hg compared with less than 140 mm Hg in the control group. Intensive therapy did not prove to significantly reduce the risk of major cardiovascular events and was associated with a significantly higher rate of serious adverse events.
Therefore, while antihypertensive therapy lowered the mortality rate in diabetic patients, lowering blood pressure beyond conventional blood pressure targets did not decrease the risk more. The latest hypertension treatment guidelines (from the eighth Joint National Committee) emphasize a blood pressure goal of 140/90 mm Hg or less in adults with diabetes.14
Prescribe a statin regardless of the baseline lipid level
The Collaborative Atorvastatin Diabetes Study (CARDS) randomized nearly 3,000 patients with type 2 diabetes mellitus and no history of cardiovascular disease to either atorvastatin 10 mg or placebo regardless of cholesterol status. The trial was terminated 2 years early because a prespecified efficacy end point had already been met: the treatment group experienced a markedly lower incidence of major cardiovascular events, including stroke.15
A large meta-analysis of randomized trials of statins noted a 9% reduction in all-cause mortality (relative risk [RR] 0.91, 99% confidence interval 0.82–1.01; P = .02) per mmol/L reduction in low-density lipoprotein cholesterol in patients with diabetes mellitus.16 Use of statins also led to significant reductions in rates of major coronary events (RR 0.78), coronary revascularization (RR 0.75), and stroke (RR 0.79).
The latest American College of Cardiology/American Heart Association guidelines endorse moderate-intensity or high-intensity statin treatment in patients with diabetes who are over age 40.17
Encourage smoking cessation
Smoking increases the lifetime risk of developing type 2 diabetes.18 It is also associated with premature development of microvascular and macrovascular complications of diabetes,19 and it leads to increased mortality risk in people with diabetes mellitus in a dose-dependent manner.20 Therefore, smoking cessation remains paramount in reducing cardiovascular risk, and patients should be encouraged to quit as soon as possible.
Role of antiplatelet agents
Use of antiplatelet drugs such as aspirin for primary prevention of cardiovascular disease in patients with diabetes is controversial. Initial studies showed a potential reduction in the incidence of myocardial infarction in men and stroke in women with diabetes with low-dose aspirin.21,22 Subsequent randomized trials and meta-analyses, however, yielded contrasting results, showing no benefit in cardiovascular risk reduction and potential risk of bleeding and other gastrointestinal adverse effects.23,24
The US Food and Drug Administration (FDA) has not approved aspirin for primary prevention of cardiovascular disease in people who have no history of cardiovascular disease. In contrast, the American Heart Association and the American Diabetes Association endorse low-dose aspirin (75–162 mg/day) for adults with diabetes and no history of vascular disease who are at increased cardiovascular risk (estimated 10-year risk of events > 10%) and who are not at increased risk of bleeding.
In the absence of a clear consensus and given the lack of randomized data, the role of aspirin in patients with diabetes remains controversial.
WHAT IS THE ROLE OF STRESS TESTING IN ASYMPTOMATIC DIABETIC PATIENTS?
People with diabetes also have a high incidence of silent (asymptomatic) ischemia that potentially leads to worse outcomes.25 Whether screening for silent ischemia improves outcomes in these patients is debatable.
The Detection of Anemia in Asymptomatic Diabetics (DIAD) trial randomized more than 1,000 asymptomatic diabetic participants to either screening for coronary artery disease with stress testing or no screening.26 Over a 5-year follow-up, there was no significant difference in the incidence of myocardial infarction and death from cardiac causes.
The guidelines remain divided on this clinical conundrum. While the American Diabetes Association recommends against routine screening for coronary artery disease in asymptomatic patients with diabetes, the American College of Cardiology/American Heart Association guidelines recommend screening with radionuclide imaging in patients with diabetes and a high risk of coronary artery disease.27
ROLE OF REVASCULARIZATION IN DIABETIC PATIENTS WITH STABLE CORONARY ARTERY DISEASE
Patients with coronary artery disease and diabetes fare worse than those without diabetes, despite revascularization by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).28
The choice of CABG or PCI as the preferred revascularization strategy was recently studied in the Future Revascularization Evaluation in Patients With DM: Optimal Management of Multivessel Disease (FREEDOM) trial.29 This study randomized 1,900 patients with diabetes and multivessel coronary artery disease to revascularization with PCI or CABG. After 5 years, there was a significantly lower rate of death and myocardial infarction with CABG than with PCI.
The role of revascularization in patients with diabetes and stable coronary artery disease has also been questioned. The Bypass Angioplasty Revascularization Investigation 2 DM (BARI-2D) randomized 2,368 patients with diabetes and stable coronary artery disease to undergo revascularization (PCI or CABG) or to receive intensive medical therapy alone.30 At 5 years, there was no significant difference in the rates of death and major cardiovascular events between patients undergoing revascularization and those undergoing medical therapy alone. Subgroup analysis revealed a potential benefit with CABG over medical therapy in patients with more extensive coronary artery disease.31
CAN DIABETES THERAPY CAUSE HARM?
New diabetes drugs must show no cardiovascular harm
Several drugs that were approved purely on the basis of their potential to reduce blood glucose were reevaluated for impact on adverse cardiovascular outcomes.
Muraglitazar is a peroxisome proliferator-activated receptor agonist that was shown in phase 2 and 3 studies to dramatically lower triglyceride levels in a dose-dependent fashion while raising high-density lipoprotein levels and being neutral to low-density lipoprotein levels. It also lowers blood glucose. The FDA Advisory Committee voted to approve its use for type 2 diabetes based on phase 2 trial data. But soon after, a meta-analysis revealed that the drug was associated with more than twice the incidence of cardiovascular complications and death than standard therapy.32 Further development of this drug subsequently ceased.
A similar meta-analysis was performed on rosiglitazone, a drug that has been available since 1997 and had been used by millions of patients. Rosiglitazone was also found to be associated with a significantly increased risk of cardiovascular death, as well as death from all causes.33
In light of these findings, the FDA in 2008 issued new guidelines to the diabetes drug development industry. Henceforth, new diabetes drugs must not only lower blood glucose, they must also be shown in a large clinical trial not to increase cardiovascular risk.
Current trials will provide critical information
Numerous trials are now under way to assess cardiovascular outcomes with promising new diabetes drugs. Tens of thousands of patients are involved in trials testing dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 agonists, sodium-glucose-linked transporter-2 agents, and a GPR40 agonist. Because of the change in guidelines, results over the next decade should reveal much more about the impact of lowering blood glucose on heart disease than we learned in the previous century.
Two apparently neutral but clinically relevant trials recently examined cardiovascular outcomes associated with diabetes drugs.
EXAMINE.34 The Examination of Cardiovascular Outcomes Study of Alogliptin Versus Standard of Care study randomized 5,380 patients with type 2 diabetes and a recent acute coronary syndrome event (acute myocardial infarction or unstable angina requiring hospitalization) to receive either alogliptin (a DPP-4 inhibitor) or placebo in addition to existing standard diabetes and cardiovascular therapy. Patients were followed for up to 40 months (median 18 months). Hemoglobin A1c levels were significantly lower with alogliptin than with placebo, but the time to the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was not significantly different between the two groups.
SAVOR.35 The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with DM (SAVOR–TIMI 53) trial randomized more than 16,000 patients with established cardiovascular disease or multiple risk factors to either the DPP-4 inhibitor saxagliptin or placebo. The patients’ physicians were permitted to adjust all other medications, including standard diabetes medications. The median treatment period was just over 2 years. Similar to EXAMINE, this study found no difference between the two groups in the primary end point of cardiovascular death, myocardial infarction, or ischemic stroke, even though glycemic control was better in the saxagliptin group.
Thus, both drugs were shown not to increase cardiovascular risk, an FDA criterion for drug marketing and approval.
CONTROL MODIFIABLE RISK FACTORS
There has been an alarming rise in the incidence of diabetes and obesity throughout the world. Cardiovascular disease remains the leading cause of death in patients with diabetes. While elevated blood glucose in diabetic patients leads to increased cardiovascular risk, efforts to reduce blood glucose to euglycemic levels may not lead to a reduction in this risk and may even cause harm.
Success in cardiovascular risk reduction in addition to glucose-lowering remains the holy grail in the development of new diabetes drugs. But in the meantime, aggressive control of other modifiable risk factors such as hypertension, smoking, and hyperlipidemia remains critical to reducing cardiovascular risk in diabetic patients.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
- Centers for Disease Control and Prevention. National diabetes statistics report. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2014.
- International Diabetes Federation. IDF Diabetes Atlas, 6th edition. Brussels: International Diabetes Federation, 2013.
- Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375:2215–2222.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011; 364:829–841.
- Hess K, Marx N, Lehrke M. Cardiovascular disease and diabetes: the vulnerable patient. Eur Heart J Suppl 2012; 14(suppl B):B4–B13.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379.
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee. JAMA 2014; 311:507–520.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–696.
- Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371:117–125.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 ACC/AHA cholesterol guideline. Ann Intern Med 2014; 160:339–343.
- Benjamin RM. A report of the Surgeon General. How tobacco smoke causes disease...what it means to you. www.cdc.gov/tobacco/data_statistics/sgr/2010/consumer_booklet/pdfs/consumer.pdf. Accessed September 30, 2014.
- Haire-Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999; 22:1887–1898.
- Chaturvedi N, Stevens L, Fuller JH. Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 1997; 20:1266–1272.
- ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. JAMA 1992; 268:1292–1300.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- Simpson SH, Gamble JM, Mereu L, Chambers T. Effect of aspirin dose on mortality and cardiovascular events in people with diabetes: a meta-analysis. J Gen Intern Med 2011; 26:1336–1344.
- Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 1999; 22:1396–1400.
- Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009; 301:1547–1555.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2010; 56:e50–e103.
- Roffi M, Angiolillo DJ, Kappetein AP. Current concepts on coronary revascularization in diabetic patients. Eur Heart J 2011; 32:2748–2757.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009; 120:2529–2540.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294:2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326.
KEY POINTS
- The worldwide burden of type 2 diabetes is increasing dramatically as obesity rates increase, populations age, and people around the world adopt a Western diet.
- Diabetes increases the risk of atherosclerotic cardiovascular disease, which remains the leading cause of death in diabetic patients.
- Lowering blood glucose alone may not necessarily amount to reduction in adverse cardiovascular events.
- Clinical trials of new drugs for type 2 diabetes must prove cardiovascular safety in addition to glucose-lowering potential before the drugs gain final regulatory approval.
- Aggressive risk factor modification (smoking cessation, control of hypertension, and treatment of hyperlipidemia with statins) remains paramount in reducing cardiovascular risk in people with diabetes.