User login
Acral Necrosis After PD-L1 Immune Checkpoint Inhibitor Therapy
To the Editor:
A 67-year-old woman presented to the hospital with painful hands and feet. Two weeks prior, the patient experienced a few days of intermittent purple discoloration of the fingers, followed by black discoloration of the fingers, toes, and nose with notable pain. She reported no illness preceding the presenting symptoms, and there was no progression of symptoms in the days preceding presentation.
The patient had a history of smoking. She had a medical history of chronic obstructive pulmonary disease as well as recurrent non–small cell lung cancer that was treated most recently with a 1-year course of the programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor durvalumab (last treatment was 4 months prior to the current presentation).
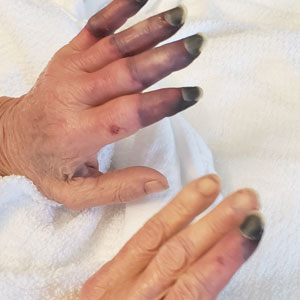
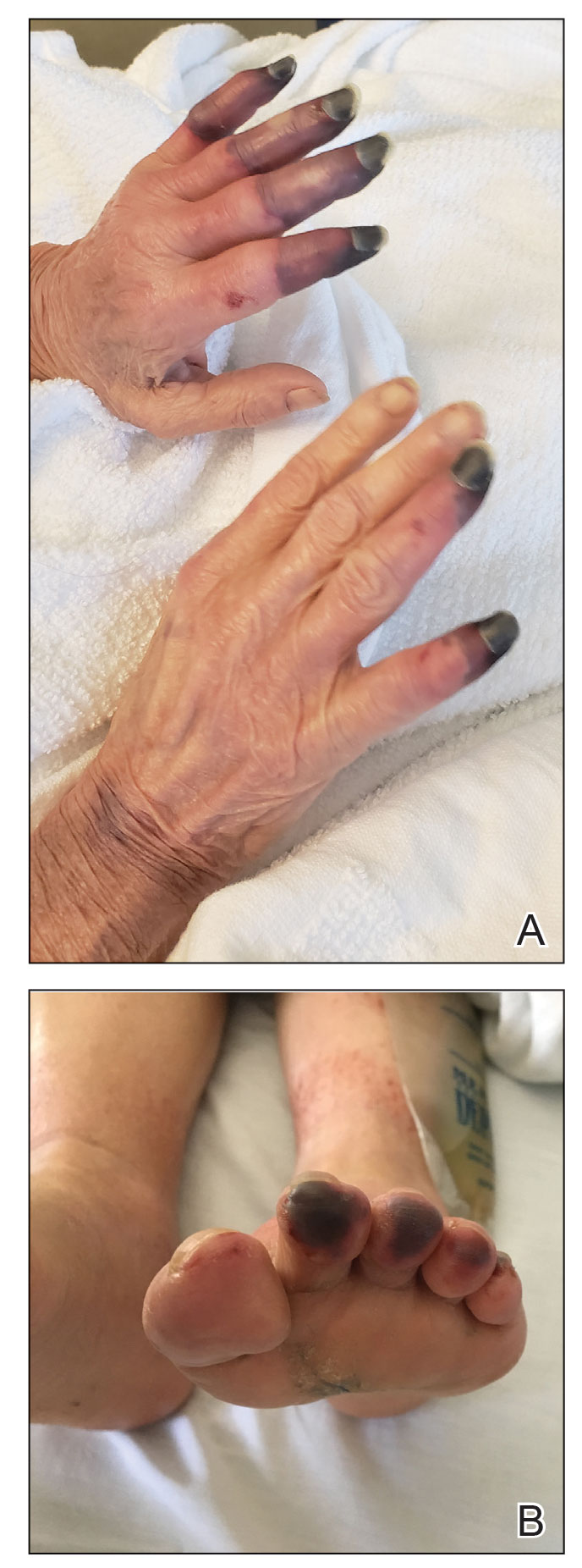
Physical examination revealed necrosis of the tips of the second, third, and fourth fingers of the left hand, as well as the tips of the third and fourth fingers of the right hand, progressing to purpura proximally on all involved fingers (Figure, A); scattered purpura and necrotic papules on the toe pads (Figure, B); and a 2- to 3-cm black plaque on the nasal tip. The patient was afebrile.
An embolic and vascular workup was performed. Transthoracic echocardiography was negative for thrombi, ankle brachial indices were within reference range, and computed tomography angiography revealed a few nonocclusive coronary plaques. Conventional angiography was not performed.
Laboratory testing revealed a mildly elevated level of cryofibrinogens (cryocrit, 2.5%); cold agglutinins (1:32); mild monoclonal κ IgG gammopathy (0.1 g/dL); and elevated inflammatory markers (C-reactive protein, 76 mg/L [reference range, 0–10 mg/L]; erythrocyte sedimentation rate, 38 mm/h [reference range, 0–20 mm/h]; fibrinogen, 571 mg/dL [reference range, 150–450 mg/dL]; and ferritin, 394 ng/mL [reference range, 10–180 ng/mL]). Additional laboratory studies were negative or within reference range, including tests of anti-RNA polymerase antibody, rheumatoid factor, antinuclear antibody, anticardiolipin antibody, anti-β2 glycoprotein antibody, antineutrophil cytoplasmic antibodies (myeloperoxidase and proteinase-3), cryoglobulins, and complement; human immunodeficiency virus and hepatitis B and C virus serologic studies; prothrombin time, partial thromboplastin time, and lupus anticoagulant; and a heparin-induced thrombocytopenia panel.
A skin biopsy adjacent to an area of necrosis on the finger showed thickened walls of dermal vessels, sparse leukocytoclastic debris, and evidence of recanalizing medium-sized vessels. Direct immunofluorescence studies were negative.
Based on the clinical history and histologic findings showing an absence of vasculitis, a diagnosis of acral necrosis associated with the PD-L1 immune checkpoint inhibitor durvalumab—a delayed immune-related event (DIRE)—was favored. The calcium channel blocker amlodipine was started at a dosage of 2.5 mg/d orally. Necrosis of the toes resolved over the course of 1 week; however, necrosis of the fingers remained unchanged. After 1 week of hospitalization, the patient was discharged at her request.
Acral necrosis following immune checkpoint inhibitor therapy has been reported as a rare and recalcitrant immune-related adverse event (AE).1-4 However, our patient’s symptoms occurred months after treatment was discontinued, which is consistent with a DIRE.5 The course of acral necrosis begins with acrocyanosis (a Raynaud disease–like phenomenon) of the fingers that progresses to necrosis. A history of Raynaud disease or other autoimmune disorder generally is absent.1 Our patient’s history indicated actively smoking at the time of presentation, similar to a case described by Khaddour et al.1 Similarly, in a case presented by Comont et al,3 the patient also had a history of smoking. In a recent study of acute vascular events associated with immune checkpoint inhibitors, 16 of 31 patients had a history of smoking.6
No definitive diagnostic laboratory or pathologic findings are associated with acral necrosis following immune checkpoint inhibitor therapy. Histopathologic analysis does not demonstrate vasculitis or other overt vascular pathology.2,3
The optimal treatment of immune checkpoint inhibitor–associated digital necrosis is unclear. Corticosteroids and discontinuation of the immune checkpoint inhibitor generally are employed,1-4 though treatment response has been variable. Other therapies such as calcium channel blockers (as in our case), sympathectomy,1 epoprostenol, botulinum injection, rituximab,2 and alprostadil4 have been attempted without clear effect.
We considered a diagnosis of paraneoplastic acral vascular syndrome in our patient, which was ruled out because the syndrome typically occurs in the setting of a worsening underlying malignancy7; our patient’s cancer was stable to improved. Thromboangiitis obliterans was ruled out by the absence of a characteristic thrombus on biopsy, the patient’s older age, and involvement of the nose.
We report an unusual case of acral necrosis occurring as a DIRE in response to administration of an immune checkpoint inhibitor. Further description is needed to clarify the diagnostic criteria for and treatment of this rare autoimmune phenomenon.
- Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019;19:449. doi:10.1186/s12885-019-5661-x
- Padda A, Schiopu E, Sovich J, et al. Ipilimumab induced digital vasculitis. J Immunother Cancer. 2018;6:12. doi:10.1186/s40425-018-0321-2
- Comont T, Sibaud V, Mourey L, et al. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer. 2018;6:120. doi:10.1186/s40425-018-0443-6
- Gambichler T, Strutzmann S, Tannapfel A, et al. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017;17:327. doi:10.1186/s12885-017-3313-6
- Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi:10.1186/s40425-019-0645-6
- Bar J, Markel G, Gottfried T, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122-131. doi:10.1016/j.ejca.2019.06.021
- Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. doi:10.1067/mjd.2002.120474
To the Editor:
A 67-year-old woman presented to the hospital with painful hands and feet. Two weeks prior, the patient experienced a few days of intermittent purple discoloration of the fingers, followed by black discoloration of the fingers, toes, and nose with notable pain. She reported no illness preceding the presenting symptoms, and there was no progression of symptoms in the days preceding presentation.
The patient had a history of smoking. She had a medical history of chronic obstructive pulmonary disease as well as recurrent non–small cell lung cancer that was treated most recently with a 1-year course of the programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor durvalumab (last treatment was 4 months prior to the current presentation).
Physical examination revealed necrosis of the tips of the second, third, and fourth fingers of the left hand, as well as the tips of the third and fourth fingers of the right hand, progressing to purpura proximally on all involved fingers (Figure, A); scattered purpura and necrotic papules on the toe pads (Figure, B); and a 2- to 3-cm black plaque on the nasal tip. The patient was afebrile.

An embolic and vascular workup was performed. Transthoracic echocardiography was negative for thrombi, ankle brachial indices were within reference range, and computed tomography angiography revealed a few nonocclusive coronary plaques. Conventional angiography was not performed.
Laboratory testing revealed a mildly elevated level of cryofibrinogens (cryocrit, 2.5%); cold agglutinins (1:32); mild monoclonal κ IgG gammopathy (0.1 g/dL); and elevated inflammatory markers (C-reactive protein, 76 mg/L [reference range, 0–10 mg/L]; erythrocyte sedimentation rate, 38 mm/h [reference range, 0–20 mm/h]; fibrinogen, 571 mg/dL [reference range, 150–450 mg/dL]; and ferritin, 394 ng/mL [reference range, 10–180 ng/mL]). Additional laboratory studies were negative or within reference range, including tests of anti-RNA polymerase antibody, rheumatoid factor, antinuclear antibody, anticardiolipin antibody, anti-β2 glycoprotein antibody, antineutrophil cytoplasmic antibodies (myeloperoxidase and proteinase-3), cryoglobulins, and complement; human immunodeficiency virus and hepatitis B and C virus serologic studies; prothrombin time, partial thromboplastin time, and lupus anticoagulant; and a heparin-induced thrombocytopenia panel.
A skin biopsy adjacent to an area of necrosis on the finger showed thickened walls of dermal vessels, sparse leukocytoclastic debris, and evidence of recanalizing medium-sized vessels. Direct immunofluorescence studies were negative.
Based on the clinical history and histologic findings showing an absence of vasculitis, a diagnosis of acral necrosis associated with the PD-L1 immune checkpoint inhibitor durvalumab—a delayed immune-related event (DIRE)—was favored. The calcium channel blocker amlodipine was started at a dosage of 2.5 mg/d orally. Necrosis of the toes resolved over the course of 1 week; however, necrosis of the fingers remained unchanged. After 1 week of hospitalization, the patient was discharged at her request.
Acral necrosis following immune checkpoint inhibitor therapy has been reported as a rare and recalcitrant immune-related adverse event (AE).1-4 However, our patient’s symptoms occurred months after treatment was discontinued, which is consistent with a DIRE.5 The course of acral necrosis begins with acrocyanosis (a Raynaud disease–like phenomenon) of the fingers that progresses to necrosis. A history of Raynaud disease or other autoimmune disorder generally is absent.1 Our patient’s history indicated actively smoking at the time of presentation, similar to a case described by Khaddour et al.1 Similarly, in a case presented by Comont et al,3 the patient also had a history of smoking. In a recent study of acute vascular events associated with immune checkpoint inhibitors, 16 of 31 patients had a history of smoking.6
No definitive diagnostic laboratory or pathologic findings are associated with acral necrosis following immune checkpoint inhibitor therapy. Histopathologic analysis does not demonstrate vasculitis or other overt vascular pathology.2,3
The optimal treatment of immune checkpoint inhibitor–associated digital necrosis is unclear. Corticosteroids and discontinuation of the immune checkpoint inhibitor generally are employed,1-4 though treatment response has been variable. Other therapies such as calcium channel blockers (as in our case), sympathectomy,1 epoprostenol, botulinum injection, rituximab,2 and alprostadil4 have been attempted without clear effect.
We considered a diagnosis of paraneoplastic acral vascular syndrome in our patient, which was ruled out because the syndrome typically occurs in the setting of a worsening underlying malignancy7; our patient’s cancer was stable to improved. Thromboangiitis obliterans was ruled out by the absence of a characteristic thrombus on biopsy, the patient’s older age, and involvement of the nose.
We report an unusual case of acral necrosis occurring as a DIRE in response to administration of an immune checkpoint inhibitor. Further description is needed to clarify the diagnostic criteria for and treatment of this rare autoimmune phenomenon.
To the Editor:
A 67-year-old woman presented to the hospital with painful hands and feet. Two weeks prior, the patient experienced a few days of intermittent purple discoloration of the fingers, followed by black discoloration of the fingers, toes, and nose with notable pain. She reported no illness preceding the presenting symptoms, and there was no progression of symptoms in the days preceding presentation.
The patient had a history of smoking. She had a medical history of chronic obstructive pulmonary disease as well as recurrent non–small cell lung cancer that was treated most recently with a 1-year course of the programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor durvalumab (last treatment was 4 months prior to the current presentation).
Physical examination revealed necrosis of the tips of the second, third, and fourth fingers of the left hand, as well as the tips of the third and fourth fingers of the right hand, progressing to purpura proximally on all involved fingers (Figure, A); scattered purpura and necrotic papules on the toe pads (Figure, B); and a 2- to 3-cm black plaque on the nasal tip. The patient was afebrile.

An embolic and vascular workup was performed. Transthoracic echocardiography was negative for thrombi, ankle brachial indices were within reference range, and computed tomography angiography revealed a few nonocclusive coronary plaques. Conventional angiography was not performed.
Laboratory testing revealed a mildly elevated level of cryofibrinogens (cryocrit, 2.5%); cold agglutinins (1:32); mild monoclonal κ IgG gammopathy (0.1 g/dL); and elevated inflammatory markers (C-reactive protein, 76 mg/L [reference range, 0–10 mg/L]; erythrocyte sedimentation rate, 38 mm/h [reference range, 0–20 mm/h]; fibrinogen, 571 mg/dL [reference range, 150–450 mg/dL]; and ferritin, 394 ng/mL [reference range, 10–180 ng/mL]). Additional laboratory studies were negative or within reference range, including tests of anti-RNA polymerase antibody, rheumatoid factor, antinuclear antibody, anticardiolipin antibody, anti-β2 glycoprotein antibody, antineutrophil cytoplasmic antibodies (myeloperoxidase and proteinase-3), cryoglobulins, and complement; human immunodeficiency virus and hepatitis B and C virus serologic studies; prothrombin time, partial thromboplastin time, and lupus anticoagulant; and a heparin-induced thrombocytopenia panel.
A skin biopsy adjacent to an area of necrosis on the finger showed thickened walls of dermal vessels, sparse leukocytoclastic debris, and evidence of recanalizing medium-sized vessels. Direct immunofluorescence studies were negative.
Based on the clinical history and histologic findings showing an absence of vasculitis, a diagnosis of acral necrosis associated with the PD-L1 immune checkpoint inhibitor durvalumab—a delayed immune-related event (DIRE)—was favored. The calcium channel blocker amlodipine was started at a dosage of 2.5 mg/d orally. Necrosis of the toes resolved over the course of 1 week; however, necrosis of the fingers remained unchanged. After 1 week of hospitalization, the patient was discharged at her request.
Acral necrosis following immune checkpoint inhibitor therapy has been reported as a rare and recalcitrant immune-related adverse event (AE).1-4 However, our patient’s symptoms occurred months after treatment was discontinued, which is consistent with a DIRE.5 The course of acral necrosis begins with acrocyanosis (a Raynaud disease–like phenomenon) of the fingers that progresses to necrosis. A history of Raynaud disease or other autoimmune disorder generally is absent.1 Our patient’s history indicated actively smoking at the time of presentation, similar to a case described by Khaddour et al.1 Similarly, in a case presented by Comont et al,3 the patient also had a history of smoking. In a recent study of acute vascular events associated with immune checkpoint inhibitors, 16 of 31 patients had a history of smoking.6
No definitive diagnostic laboratory or pathologic findings are associated with acral necrosis following immune checkpoint inhibitor therapy. Histopathologic analysis does not demonstrate vasculitis or other overt vascular pathology.2,3
The optimal treatment of immune checkpoint inhibitor–associated digital necrosis is unclear. Corticosteroids and discontinuation of the immune checkpoint inhibitor generally are employed,1-4 though treatment response has been variable. Other therapies such as calcium channel blockers (as in our case), sympathectomy,1 epoprostenol, botulinum injection, rituximab,2 and alprostadil4 have been attempted without clear effect.
We considered a diagnosis of paraneoplastic acral vascular syndrome in our patient, which was ruled out because the syndrome typically occurs in the setting of a worsening underlying malignancy7; our patient’s cancer was stable to improved. Thromboangiitis obliterans was ruled out by the absence of a characteristic thrombus on biopsy, the patient’s older age, and involvement of the nose.
We report an unusual case of acral necrosis occurring as a DIRE in response to administration of an immune checkpoint inhibitor. Further description is needed to clarify the diagnostic criteria for and treatment of this rare autoimmune phenomenon.
- Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019;19:449. doi:10.1186/s12885-019-5661-x
- Padda A, Schiopu E, Sovich J, et al. Ipilimumab induced digital vasculitis. J Immunother Cancer. 2018;6:12. doi:10.1186/s40425-018-0321-2
- Comont T, Sibaud V, Mourey L, et al. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer. 2018;6:120. doi:10.1186/s40425-018-0443-6
- Gambichler T, Strutzmann S, Tannapfel A, et al. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017;17:327. doi:10.1186/s12885-017-3313-6
- Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi:10.1186/s40425-019-0645-6
- Bar J, Markel G, Gottfried T, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122-131. doi:10.1016/j.ejca.2019.06.021
- Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. doi:10.1067/mjd.2002.120474
- Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019;19:449. doi:10.1186/s12885-019-5661-x
- Padda A, Schiopu E, Sovich J, et al. Ipilimumab induced digital vasculitis. J Immunother Cancer. 2018;6:12. doi:10.1186/s40425-018-0321-2
- Comont T, Sibaud V, Mourey L, et al. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer. 2018;6:120. doi:10.1186/s40425-018-0443-6
- Gambichler T, Strutzmann S, Tannapfel A, et al. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017;17:327. doi:10.1186/s12885-017-3313-6
- Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi:10.1186/s40425-019-0645-6
- Bar J, Markel G, Gottfried T, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122-131. doi:10.1016/j.ejca.2019.06.021
- Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. doi:10.1067/mjd.2002.120474
Practice Points
- Dermatologists should be aware of acral necrosis as a rare adverse event of treatment with an immune checkpoint inhibitor.
- Delayed immune-related events are sequelae of immune checkpoint inhibitors that can occur months after treatment is discontinued.