User login
Acral Necrosis After PD-L1 Immune Checkpoint Inhibitor Therapy
To the Editor:
A 67-year-old woman presented to the hospital with painful hands and feet. Two weeks prior, the patient experienced a few days of intermittent purple discoloration of the fingers, followed by black discoloration of the fingers, toes, and nose with notable pain. She reported no illness preceding the presenting symptoms, and there was no progression of symptoms in the days preceding presentation.
The patient had a history of smoking. She had a medical history of chronic obstructive pulmonary disease as well as recurrent non–small cell lung cancer that was treated most recently with a 1-year course of the programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor durvalumab (last treatment was 4 months prior to the current presentation).
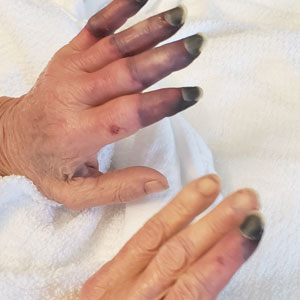
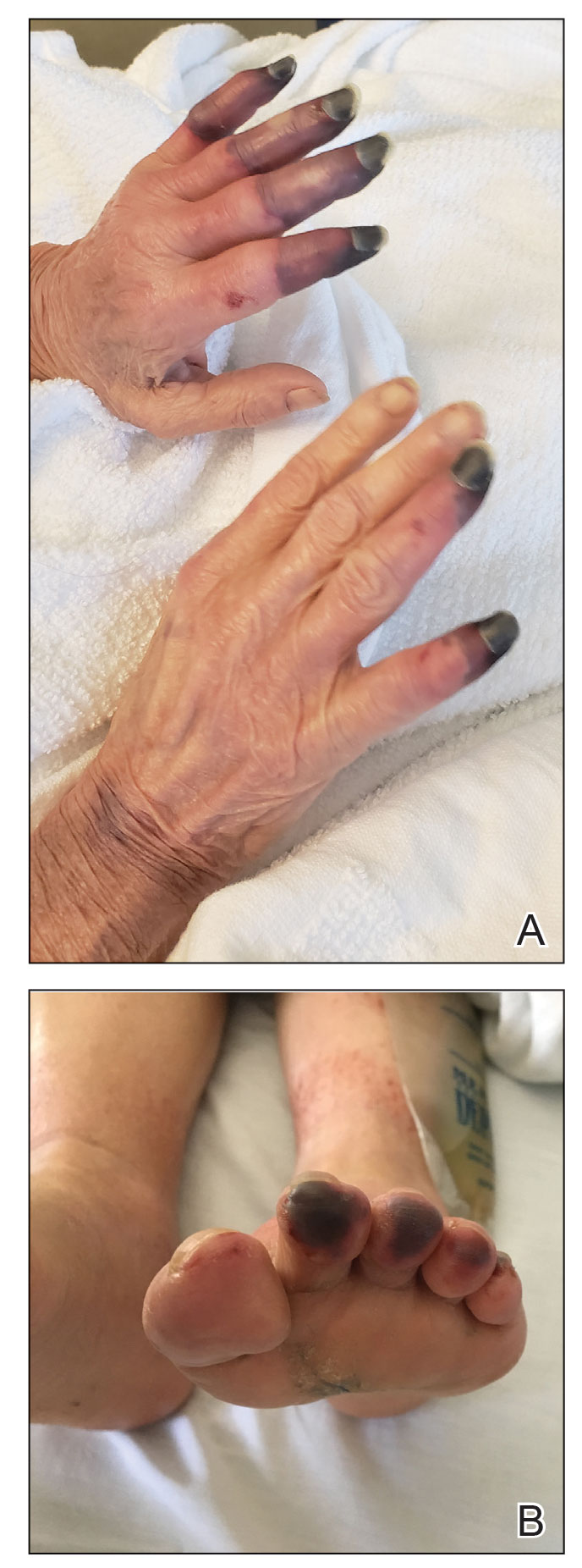
Physical examination revealed necrosis of the tips of the second, third, and fourth fingers of the left hand, as well as the tips of the third and fourth fingers of the right hand, progressing to purpura proximally on all involved fingers (Figure, A); scattered purpura and necrotic papules on the toe pads (Figure, B); and a 2- to 3-cm black plaque on the nasal tip. The patient was afebrile.
An embolic and vascular workup was performed. Transthoracic echocardiography was negative for thrombi, ankle brachial indices were within reference range, and computed tomography angiography revealed a few nonocclusive coronary plaques. Conventional angiography was not performed.
Laboratory testing revealed a mildly elevated level of cryofibrinogens (cryocrit, 2.5%); cold agglutinins (1:32); mild monoclonal κ IgG gammopathy (0.1 g/dL); and elevated inflammatory markers (C-reactive protein, 76 mg/L [reference range, 0–10 mg/L]; erythrocyte sedimentation rate, 38 mm/h [reference range, 0–20 mm/h]; fibrinogen, 571 mg/dL [reference range, 150–450 mg/dL]; and ferritin, 394 ng/mL [reference range, 10–180 ng/mL]). Additional laboratory studies were negative or within reference range, including tests of anti-RNA polymerase antibody, rheumatoid factor, antinuclear antibody, anticardiolipin antibody, anti-β2 glycoprotein antibody, antineutrophil cytoplasmic antibodies (myeloperoxidase and proteinase-3), cryoglobulins, and complement; human immunodeficiency virus and hepatitis B and C virus serologic studies; prothrombin time, partial thromboplastin time, and lupus anticoagulant; and a heparin-induced thrombocytopenia panel.
A skin biopsy adjacent to an area of necrosis on the finger showed thickened walls of dermal vessels, sparse leukocytoclastic debris, and evidence of recanalizing medium-sized vessels. Direct immunofluorescence studies were negative.
Based on the clinical history and histologic findings showing an absence of vasculitis, a diagnosis of acral necrosis associated with the PD-L1 immune checkpoint inhibitor durvalumab—a delayed immune-related event (DIRE)—was favored. The calcium channel blocker amlodipine was started at a dosage of 2.5 mg/d orally. Necrosis of the toes resolved over the course of 1 week; however, necrosis of the fingers remained unchanged. After 1 week of hospitalization, the patient was discharged at her request.
Acral necrosis following immune checkpoint inhibitor therapy has been reported as a rare and recalcitrant immune-related adverse event (AE).1-4 However, our patient’s symptoms occurred months after treatment was discontinued, which is consistent with a DIRE.5 The course of acral necrosis begins with acrocyanosis (a Raynaud disease–like phenomenon) of the fingers that progresses to necrosis. A history of Raynaud disease or other autoimmune disorder generally is absent.1 Our patient’s history indicated actively smoking at the time of presentation, similar to a case described by Khaddour et al.1 Similarly, in a case presented by Comont et al,3 the patient also had a history of smoking. In a recent study of acute vascular events associated with immune checkpoint inhibitors, 16 of 31 patients had a history of smoking.6
No definitive diagnostic laboratory or pathologic findings are associated with acral necrosis following immune checkpoint inhibitor therapy. Histopathologic analysis does not demonstrate vasculitis or other overt vascular pathology.2,3
The optimal treatment of immune checkpoint inhibitor–associated digital necrosis is unclear. Corticosteroids and discontinuation of the immune checkpoint inhibitor generally are employed,1-4 though treatment response has been variable. Other therapies such as calcium channel blockers (as in our case), sympathectomy,1 epoprostenol, botulinum injection, rituximab,2 and alprostadil4 have been attempted without clear effect.
We considered a diagnosis of paraneoplastic acral vascular syndrome in our patient, which was ruled out because the syndrome typically occurs in the setting of a worsening underlying malignancy7; our patient’s cancer was stable to improved. Thromboangiitis obliterans was ruled out by the absence of a characteristic thrombus on biopsy, the patient’s older age, and involvement of the nose.
We report an unusual case of acral necrosis occurring as a DIRE in response to administration of an immune checkpoint inhibitor. Further description is needed to clarify the diagnostic criteria for and treatment of this rare autoimmune phenomenon.
- Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019;19:449. doi:10.1186/s12885-019-5661-x
- Padda A, Schiopu E, Sovich J, et al. Ipilimumab induced digital vasculitis. J Immunother Cancer. 2018;6:12. doi:10.1186/s40425-018-0321-2
- Comont T, Sibaud V, Mourey L, et al. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer. 2018;6:120. doi:10.1186/s40425-018-0443-6
- Gambichler T, Strutzmann S, Tannapfel A, et al. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017;17:327. doi:10.1186/s12885-017-3313-6
- Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi:10.1186/s40425-019-0645-6
- Bar J, Markel G, Gottfried T, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122-131. doi:10.1016/j.ejca.2019.06.021
- Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. doi:10.1067/mjd.2002.120474
To the Editor:
A 67-year-old woman presented to the hospital with painful hands and feet. Two weeks prior, the patient experienced a few days of intermittent purple discoloration of the fingers, followed by black discoloration of the fingers, toes, and nose with notable pain. She reported no illness preceding the presenting symptoms, and there was no progression of symptoms in the days preceding presentation.
The patient had a history of smoking. She had a medical history of chronic obstructive pulmonary disease as well as recurrent non–small cell lung cancer that was treated most recently with a 1-year course of the programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor durvalumab (last treatment was 4 months prior to the current presentation).
Physical examination revealed necrosis of the tips of the second, third, and fourth fingers of the left hand, as well as the tips of the third and fourth fingers of the right hand, progressing to purpura proximally on all involved fingers (Figure, A); scattered purpura and necrotic papules on the toe pads (Figure, B); and a 2- to 3-cm black plaque on the nasal tip. The patient was afebrile.

An embolic and vascular workup was performed. Transthoracic echocardiography was negative for thrombi, ankle brachial indices were within reference range, and computed tomography angiography revealed a few nonocclusive coronary plaques. Conventional angiography was not performed.
Laboratory testing revealed a mildly elevated level of cryofibrinogens (cryocrit, 2.5%); cold agglutinins (1:32); mild monoclonal κ IgG gammopathy (0.1 g/dL); and elevated inflammatory markers (C-reactive protein, 76 mg/L [reference range, 0–10 mg/L]; erythrocyte sedimentation rate, 38 mm/h [reference range, 0–20 mm/h]; fibrinogen, 571 mg/dL [reference range, 150–450 mg/dL]; and ferritin, 394 ng/mL [reference range, 10–180 ng/mL]). Additional laboratory studies were negative or within reference range, including tests of anti-RNA polymerase antibody, rheumatoid factor, antinuclear antibody, anticardiolipin antibody, anti-β2 glycoprotein antibody, antineutrophil cytoplasmic antibodies (myeloperoxidase and proteinase-3), cryoglobulins, and complement; human immunodeficiency virus and hepatitis B and C virus serologic studies; prothrombin time, partial thromboplastin time, and lupus anticoagulant; and a heparin-induced thrombocytopenia panel.
A skin biopsy adjacent to an area of necrosis on the finger showed thickened walls of dermal vessels, sparse leukocytoclastic debris, and evidence of recanalizing medium-sized vessels. Direct immunofluorescence studies were negative.
Based on the clinical history and histologic findings showing an absence of vasculitis, a diagnosis of acral necrosis associated with the PD-L1 immune checkpoint inhibitor durvalumab—a delayed immune-related event (DIRE)—was favored. The calcium channel blocker amlodipine was started at a dosage of 2.5 mg/d orally. Necrosis of the toes resolved over the course of 1 week; however, necrosis of the fingers remained unchanged. After 1 week of hospitalization, the patient was discharged at her request.
Acral necrosis following immune checkpoint inhibitor therapy has been reported as a rare and recalcitrant immune-related adverse event (AE).1-4 However, our patient’s symptoms occurred months after treatment was discontinued, which is consistent with a DIRE.5 The course of acral necrosis begins with acrocyanosis (a Raynaud disease–like phenomenon) of the fingers that progresses to necrosis. A history of Raynaud disease or other autoimmune disorder generally is absent.1 Our patient’s history indicated actively smoking at the time of presentation, similar to a case described by Khaddour et al.1 Similarly, in a case presented by Comont et al,3 the patient also had a history of smoking. In a recent study of acute vascular events associated with immune checkpoint inhibitors, 16 of 31 patients had a history of smoking.6
No definitive diagnostic laboratory or pathologic findings are associated with acral necrosis following immune checkpoint inhibitor therapy. Histopathologic analysis does not demonstrate vasculitis or other overt vascular pathology.2,3
The optimal treatment of immune checkpoint inhibitor–associated digital necrosis is unclear. Corticosteroids and discontinuation of the immune checkpoint inhibitor generally are employed,1-4 though treatment response has been variable. Other therapies such as calcium channel blockers (as in our case), sympathectomy,1 epoprostenol, botulinum injection, rituximab,2 and alprostadil4 have been attempted without clear effect.
We considered a diagnosis of paraneoplastic acral vascular syndrome in our patient, which was ruled out because the syndrome typically occurs in the setting of a worsening underlying malignancy7; our patient’s cancer was stable to improved. Thromboangiitis obliterans was ruled out by the absence of a characteristic thrombus on biopsy, the patient’s older age, and involvement of the nose.
We report an unusual case of acral necrosis occurring as a DIRE in response to administration of an immune checkpoint inhibitor. Further description is needed to clarify the diagnostic criteria for and treatment of this rare autoimmune phenomenon.
To the Editor:
A 67-year-old woman presented to the hospital with painful hands and feet. Two weeks prior, the patient experienced a few days of intermittent purple discoloration of the fingers, followed by black discoloration of the fingers, toes, and nose with notable pain. She reported no illness preceding the presenting symptoms, and there was no progression of symptoms in the days preceding presentation.
The patient had a history of smoking. She had a medical history of chronic obstructive pulmonary disease as well as recurrent non–small cell lung cancer that was treated most recently with a 1-year course of the programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor durvalumab (last treatment was 4 months prior to the current presentation).
Physical examination revealed necrosis of the tips of the second, third, and fourth fingers of the left hand, as well as the tips of the third and fourth fingers of the right hand, progressing to purpura proximally on all involved fingers (Figure, A); scattered purpura and necrotic papules on the toe pads (Figure, B); and a 2- to 3-cm black plaque on the nasal tip. The patient was afebrile.

An embolic and vascular workup was performed. Transthoracic echocardiography was negative for thrombi, ankle brachial indices were within reference range, and computed tomography angiography revealed a few nonocclusive coronary plaques. Conventional angiography was not performed.
Laboratory testing revealed a mildly elevated level of cryofibrinogens (cryocrit, 2.5%); cold agglutinins (1:32); mild monoclonal κ IgG gammopathy (0.1 g/dL); and elevated inflammatory markers (C-reactive protein, 76 mg/L [reference range, 0–10 mg/L]; erythrocyte sedimentation rate, 38 mm/h [reference range, 0–20 mm/h]; fibrinogen, 571 mg/dL [reference range, 150–450 mg/dL]; and ferritin, 394 ng/mL [reference range, 10–180 ng/mL]). Additional laboratory studies were negative or within reference range, including tests of anti-RNA polymerase antibody, rheumatoid factor, antinuclear antibody, anticardiolipin antibody, anti-β2 glycoprotein antibody, antineutrophil cytoplasmic antibodies (myeloperoxidase and proteinase-3), cryoglobulins, and complement; human immunodeficiency virus and hepatitis B and C virus serologic studies; prothrombin time, partial thromboplastin time, and lupus anticoagulant; and a heparin-induced thrombocytopenia panel.
A skin biopsy adjacent to an area of necrosis on the finger showed thickened walls of dermal vessels, sparse leukocytoclastic debris, and evidence of recanalizing medium-sized vessels. Direct immunofluorescence studies were negative.
Based on the clinical history and histologic findings showing an absence of vasculitis, a diagnosis of acral necrosis associated with the PD-L1 immune checkpoint inhibitor durvalumab—a delayed immune-related event (DIRE)—was favored. The calcium channel blocker amlodipine was started at a dosage of 2.5 mg/d orally. Necrosis of the toes resolved over the course of 1 week; however, necrosis of the fingers remained unchanged. After 1 week of hospitalization, the patient was discharged at her request.
Acral necrosis following immune checkpoint inhibitor therapy has been reported as a rare and recalcitrant immune-related adverse event (AE).1-4 However, our patient’s symptoms occurred months after treatment was discontinued, which is consistent with a DIRE.5 The course of acral necrosis begins with acrocyanosis (a Raynaud disease–like phenomenon) of the fingers that progresses to necrosis. A history of Raynaud disease or other autoimmune disorder generally is absent.1 Our patient’s history indicated actively smoking at the time of presentation, similar to a case described by Khaddour et al.1 Similarly, in a case presented by Comont et al,3 the patient also had a history of smoking. In a recent study of acute vascular events associated with immune checkpoint inhibitors, 16 of 31 patients had a history of smoking.6
No definitive diagnostic laboratory or pathologic findings are associated with acral necrosis following immune checkpoint inhibitor therapy. Histopathologic analysis does not demonstrate vasculitis or other overt vascular pathology.2,3
The optimal treatment of immune checkpoint inhibitor–associated digital necrosis is unclear. Corticosteroids and discontinuation of the immune checkpoint inhibitor generally are employed,1-4 though treatment response has been variable. Other therapies such as calcium channel blockers (as in our case), sympathectomy,1 epoprostenol, botulinum injection, rituximab,2 and alprostadil4 have been attempted without clear effect.
We considered a diagnosis of paraneoplastic acral vascular syndrome in our patient, which was ruled out because the syndrome typically occurs in the setting of a worsening underlying malignancy7; our patient’s cancer was stable to improved. Thromboangiitis obliterans was ruled out by the absence of a characteristic thrombus on biopsy, the patient’s older age, and involvement of the nose.
We report an unusual case of acral necrosis occurring as a DIRE in response to administration of an immune checkpoint inhibitor. Further description is needed to clarify the diagnostic criteria for and treatment of this rare autoimmune phenomenon.
- Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019;19:449. doi:10.1186/s12885-019-5661-x
- Padda A, Schiopu E, Sovich J, et al. Ipilimumab induced digital vasculitis. J Immunother Cancer. 2018;6:12. doi:10.1186/s40425-018-0321-2
- Comont T, Sibaud V, Mourey L, et al. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer. 2018;6:120. doi:10.1186/s40425-018-0443-6
- Gambichler T, Strutzmann S, Tannapfel A, et al. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017;17:327. doi:10.1186/s12885-017-3313-6
- Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi:10.1186/s40425-019-0645-6
- Bar J, Markel G, Gottfried T, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122-131. doi:10.1016/j.ejca.2019.06.021
- Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. doi:10.1067/mjd.2002.120474
- Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019;19:449. doi:10.1186/s12885-019-5661-x
- Padda A, Schiopu E, Sovich J, et al. Ipilimumab induced digital vasculitis. J Immunother Cancer. 2018;6:12. doi:10.1186/s40425-018-0321-2
- Comont T, Sibaud V, Mourey L, et al. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer. 2018;6:120. doi:10.1186/s40425-018-0443-6
- Gambichler T, Strutzmann S, Tannapfel A, et al. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017;17:327. doi:10.1186/s12885-017-3313-6
- Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi:10.1186/s40425-019-0645-6
- Bar J, Markel G, Gottfried T, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122-131. doi:10.1016/j.ejca.2019.06.021
- Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. doi:10.1067/mjd.2002.120474
Practice Points
- Dermatologists should be aware of acral necrosis as a rare adverse event of treatment with an immune checkpoint inhibitor.
- Delayed immune-related events are sequelae of immune checkpoint inhibitors that can occur months after treatment is discontinued.
Inequity, Bias, Racism, and Physician Burnout: Staying Connected to Purpose and Identity as an Antidote
“Where are you really from?”
When I tell patients I am from Casper, Wyoming—wh ere I have lived the majority of my life—it’smet with disbelief. The subtext: YOU can’t be from THERE.
I didn’t used to think much of comments like this, but as I have continued to hear them, I find myself feeling tired—tired of explaining myself, tired of being treated differently than my colleagues, and tired of justifying myself. My experiences as a woman of color sadly are not uncommon in medicine.
Sara Martinez-Garcia, BA
Racial bias and racism are steeped in the culture of medicine—from the medical school admissions process1,2 to the medical training itself.3 More than half of medical students who identify as underrepresented in medicine (UIM) experience microaggressions.4 Experiencing racism and sexism in the learning environment can lead to burnout, and microaggressions promote feelings of self-doubt and isolation. Medical students who experience microaggressions are more likely to report feelings of burnout and impaired learning.4 These experiences can leave one feeling as if “You do not belong” and “You are unworthy of being in this position.”
Addressing physician burnout already is complex, and addressing burnout caused by inequity, bias, and racism is even more so. In an ideal world, we would eliminate inequity, bias, and racism in medicine through institutional and individual actions. There has been movement to do so. For example, the Accreditation Council for Graduate Medical Education (ACGME), which oversees standards for US resident and fellow training, launched ACGME Equity Matters (https://www.acgme.org/what-we-do/diversity-equity-and-inclusion/ACGME-Equity-Matters/), an initiative aimed to improve diversity, equity, and antiracism practices within graduate medical eduation. However, we know that education alone isn’t enough to fix this monumental problem. Traditional diversity training as we have known it has never been demonstrated to contribute to lasting changes in behavior; it takes much more extensive and complex interventions to meaningfully reduce bias.5 In the meantime, we need action. As a medical community, we need to be better about not turning the other way when we see these things happening in our classrooms and in our hospitals. As individuals, we must self-reflect on the role that we each play in contributing to or combatting injustices and seek out bystander training to empower us to speak out against acts of bias such as sexism or racism. Whether it is supporting a fellow colleague or speaking out against an inappropriate interaction, we can all do our part. A very brief list of actions and resources to support our UIM students and colleagues are listed in the Table; those interested in more in-depth resources are encouraged to explore the Association of American Medical Colleges Diversity and Inclusion Toolkit (https://www.aamc.org/professional-development/affinity-groups/cfas/diversity-inclusion-toolkit/resources).

We can’t change the culture of medicine quickly or even in our lifetime. In the meantime, those who are UIM will continue to experience these events that erode our well-being. They will continue to need support. Discussing mental health has long been stigmatized, and physicians are no exception. Many physicians are hesitant to discuss mental health issues out of fear of judgement and perceived or even real repercussions on their careers.10 However, times are changing and evolving with the current generation of medical students. It’s no secret that medicine is stressful. Most medical schools provide free counseling services, which lowers the barrier for discussions of mental health from the beginning. Making talk about mental health just as normal as talking about other aspects of health takes away the fear that “something is wrong with me” if someone seeks out counseling and mental health services. Faculty should actively check in and maintain open lines of communication, which can be invaluable for UIM students and their training experience. Creating an environment where trainees can be real and honest about the struggles they face in and out of the classroom can make everyone feel like they are not alone.
Addressing burnout in medicine is going to require an all-hands-on-deck approach. At an institutional level, there is a lot of room for improvement—improving systems for physicians so they are able to operate at their highest level (eg, addressing the burdens of prior authorizations and the electronic medical record), setting reasonable expectations around productivity, and creating work structures that respect work-life balance.11 But what can we do for ourselves? We believe that one of the most important ways to protect ourselves from burnout is to remember why. As a medical student, there is enormous pressure—pressure to learn an enormous volume of information, pass examinations, get involved in extracurricular activities, make connections, and seek research opportunities, while also cooking healthy food, grocery shopping, maintaining relationships with loved ones, and generally taking care of oneself. At times it can feel as if our lives outside of medical school are not important enough or valuable enough to make time for, but the pieces of our identity outside of medicine are what shape us into who we are today and are the roots of our purpose in medicine. Sometimes you can feel the most motivated, valued, and supported when you make time to have dinner with friends, call a family member, or simply spend time alone in the outdoors. Who you are and how you got to this point in your life are your identity. Reminding yourself of that can help when experiencing microaggressions or when that voice tries to tell you that you are not worthy. As you progress further in your career, maintaining that relationship with who you are outside of medicine can be your armor against burnout.
- Capers Q IV, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369.
- Lucey CR, Saguil A. The consequences of structural racism on MCAT scores and medical school admissions: the past is prologue. Acad Med. 2020;95:351-356.
- Nguemeni Tiako MJ, South EC, Ray V. Medical schools as racialized organizations: a primer. Ann Intern Med. 2021;174:1143-1144.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2021;113:310-314.
- Dobbin F, Kalev A. Why doesn’t diversity training work? the challenge for industry and academia. Anthropology Now. 2018;10:48-55.
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Int J Womens Dermatol. 2020;6:57-60.
- Hackworth JM, Kotagal M, Bignall ONR, et al. Microaggressions: privileged observers’ duty to act and what they can do [published online December 1, 2021]. Pediatrics. doi:10.1542/peds.2021-052758.
- Wheeler DJ, Zapata J, Davis D, et al. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2019;41:1112-1117.
- Scott K. Just Work: How to Root Out Bias, Prejudice, and Bullying to Build a Kick-Ass Culture of Inclusivity. St. Martin’s Press; 2021.
- Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289:3161-3166.
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
“Where are you really from?”
When I tell patients I am from Casper, Wyoming—wh ere I have lived the majority of my life—it’smet with disbelief. The subtext: YOU can’t be from THERE.
I didn’t used to think much of comments like this, but as I have continued to hear them, I find myself feeling tired—tired of explaining myself, tired of being treated differently than my colleagues, and tired of justifying myself. My experiences as a woman of color sadly are not uncommon in medicine.
Sara Martinez-Garcia, BA
Racial bias and racism are steeped in the culture of medicine—from the medical school admissions process1,2 to the medical training itself.3 More than half of medical students who identify as underrepresented in medicine (UIM) experience microaggressions.4 Experiencing racism and sexism in the learning environment can lead to burnout, and microaggressions promote feelings of self-doubt and isolation. Medical students who experience microaggressions are more likely to report feelings of burnout and impaired learning.4 These experiences can leave one feeling as if “You do not belong” and “You are unworthy of being in this position.”
Addressing physician burnout already is complex, and addressing burnout caused by inequity, bias, and racism is even more so. In an ideal world, we would eliminate inequity, bias, and racism in medicine through institutional and individual actions. There has been movement to do so. For example, the Accreditation Council for Graduate Medical Education (ACGME), which oversees standards for US resident and fellow training, launched ACGME Equity Matters (https://www.acgme.org/what-we-do/diversity-equity-and-inclusion/ACGME-Equity-Matters/), an initiative aimed to improve diversity, equity, and antiracism practices within graduate medical eduation. However, we know that education alone isn’t enough to fix this monumental problem. Traditional diversity training as we have known it has never been demonstrated to contribute to lasting changes in behavior; it takes much more extensive and complex interventions to meaningfully reduce bias.5 In the meantime, we need action. As a medical community, we need to be better about not turning the other way when we see these things happening in our classrooms and in our hospitals. As individuals, we must self-reflect on the role that we each play in contributing to or combatting injustices and seek out bystander training to empower us to speak out against acts of bias such as sexism or racism. Whether it is supporting a fellow colleague or speaking out against an inappropriate interaction, we can all do our part. A very brief list of actions and resources to support our UIM students and colleagues are listed in the Table; those interested in more in-depth resources are encouraged to explore the Association of American Medical Colleges Diversity and Inclusion Toolkit (https://www.aamc.org/professional-development/affinity-groups/cfas/diversity-inclusion-toolkit/resources).

We can’t change the culture of medicine quickly or even in our lifetime. In the meantime, those who are UIM will continue to experience these events that erode our well-being. They will continue to need support. Discussing mental health has long been stigmatized, and physicians are no exception. Many physicians are hesitant to discuss mental health issues out of fear of judgement and perceived or even real repercussions on their careers.10 However, times are changing and evolving with the current generation of medical students. It’s no secret that medicine is stressful. Most medical schools provide free counseling services, which lowers the barrier for discussions of mental health from the beginning. Making talk about mental health just as normal as talking about other aspects of health takes away the fear that “something is wrong with me” if someone seeks out counseling and mental health services. Faculty should actively check in and maintain open lines of communication, which can be invaluable for UIM students and their training experience. Creating an environment where trainees can be real and honest about the struggles they face in and out of the classroom can make everyone feel like they are not alone.
Addressing burnout in medicine is going to require an all-hands-on-deck approach. At an institutional level, there is a lot of room for improvement—improving systems for physicians so they are able to operate at their highest level (eg, addressing the burdens of prior authorizations and the electronic medical record), setting reasonable expectations around productivity, and creating work structures that respect work-life balance.11 But what can we do for ourselves? We believe that one of the most important ways to protect ourselves from burnout is to remember why. As a medical student, there is enormous pressure—pressure to learn an enormous volume of information, pass examinations, get involved in extracurricular activities, make connections, and seek research opportunities, while also cooking healthy food, grocery shopping, maintaining relationships with loved ones, and generally taking care of oneself. At times it can feel as if our lives outside of medical school are not important enough or valuable enough to make time for, but the pieces of our identity outside of medicine are what shape us into who we are today and are the roots of our purpose in medicine. Sometimes you can feel the most motivated, valued, and supported when you make time to have dinner with friends, call a family member, or simply spend time alone in the outdoors. Who you are and how you got to this point in your life are your identity. Reminding yourself of that can help when experiencing microaggressions or when that voice tries to tell you that you are not worthy. As you progress further in your career, maintaining that relationship with who you are outside of medicine can be your armor against burnout.
“Where are you really from?”
When I tell patients I am from Casper, Wyoming—wh ere I have lived the majority of my life—it’smet with disbelief. The subtext: YOU can’t be from THERE.
I didn’t used to think much of comments like this, but as I have continued to hear them, I find myself feeling tired—tired of explaining myself, tired of being treated differently than my colleagues, and tired of justifying myself. My experiences as a woman of color sadly are not uncommon in medicine.
Sara Martinez-Garcia, BA
Racial bias and racism are steeped in the culture of medicine—from the medical school admissions process1,2 to the medical training itself.3 More than half of medical students who identify as underrepresented in medicine (UIM) experience microaggressions.4 Experiencing racism and sexism in the learning environment can lead to burnout, and microaggressions promote feelings of self-doubt and isolation. Medical students who experience microaggressions are more likely to report feelings of burnout and impaired learning.4 These experiences can leave one feeling as if “You do not belong” and “You are unworthy of being in this position.”
Addressing physician burnout already is complex, and addressing burnout caused by inequity, bias, and racism is even more so. In an ideal world, we would eliminate inequity, bias, and racism in medicine through institutional and individual actions. There has been movement to do so. For example, the Accreditation Council for Graduate Medical Education (ACGME), which oversees standards for US resident and fellow training, launched ACGME Equity Matters (https://www.acgme.org/what-we-do/diversity-equity-and-inclusion/ACGME-Equity-Matters/), an initiative aimed to improve diversity, equity, and antiracism practices within graduate medical eduation. However, we know that education alone isn’t enough to fix this monumental problem. Traditional diversity training as we have known it has never been demonstrated to contribute to lasting changes in behavior; it takes much more extensive and complex interventions to meaningfully reduce bias.5 In the meantime, we need action. As a medical community, we need to be better about not turning the other way when we see these things happening in our classrooms and in our hospitals. As individuals, we must self-reflect on the role that we each play in contributing to or combatting injustices and seek out bystander training to empower us to speak out against acts of bias such as sexism or racism. Whether it is supporting a fellow colleague or speaking out against an inappropriate interaction, we can all do our part. A very brief list of actions and resources to support our UIM students and colleagues are listed in the Table; those interested in more in-depth resources are encouraged to explore the Association of American Medical Colleges Diversity and Inclusion Toolkit (https://www.aamc.org/professional-development/affinity-groups/cfas/diversity-inclusion-toolkit/resources).

We can’t change the culture of medicine quickly or even in our lifetime. In the meantime, those who are UIM will continue to experience these events that erode our well-being. They will continue to need support. Discussing mental health has long been stigmatized, and physicians are no exception. Many physicians are hesitant to discuss mental health issues out of fear of judgement and perceived or even real repercussions on their careers.10 However, times are changing and evolving with the current generation of medical students. It’s no secret that medicine is stressful. Most medical schools provide free counseling services, which lowers the barrier for discussions of mental health from the beginning. Making talk about mental health just as normal as talking about other aspects of health takes away the fear that “something is wrong with me” if someone seeks out counseling and mental health services. Faculty should actively check in and maintain open lines of communication, which can be invaluable for UIM students and their training experience. Creating an environment where trainees can be real and honest about the struggles they face in and out of the classroom can make everyone feel like they are not alone.
Addressing burnout in medicine is going to require an all-hands-on-deck approach. At an institutional level, there is a lot of room for improvement—improving systems for physicians so they are able to operate at their highest level (eg, addressing the burdens of prior authorizations and the electronic medical record), setting reasonable expectations around productivity, and creating work structures that respect work-life balance.11 But what can we do for ourselves? We believe that one of the most important ways to protect ourselves from burnout is to remember why. As a medical student, there is enormous pressure—pressure to learn an enormous volume of information, pass examinations, get involved in extracurricular activities, make connections, and seek research opportunities, while also cooking healthy food, grocery shopping, maintaining relationships with loved ones, and generally taking care of oneself. At times it can feel as if our lives outside of medical school are not important enough or valuable enough to make time for, but the pieces of our identity outside of medicine are what shape us into who we are today and are the roots of our purpose in medicine. Sometimes you can feel the most motivated, valued, and supported when you make time to have dinner with friends, call a family member, or simply spend time alone in the outdoors. Who you are and how you got to this point in your life are your identity. Reminding yourself of that can help when experiencing microaggressions or when that voice tries to tell you that you are not worthy. As you progress further in your career, maintaining that relationship with who you are outside of medicine can be your armor against burnout.
- Capers Q IV, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369.
- Lucey CR, Saguil A. The consequences of structural racism on MCAT scores and medical school admissions: the past is prologue. Acad Med. 2020;95:351-356.
- Nguemeni Tiako MJ, South EC, Ray V. Medical schools as racialized organizations: a primer. Ann Intern Med. 2021;174:1143-1144.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2021;113:310-314.
- Dobbin F, Kalev A. Why doesn’t diversity training work? the challenge for industry and academia. Anthropology Now. 2018;10:48-55.
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Int J Womens Dermatol. 2020;6:57-60.
- Hackworth JM, Kotagal M, Bignall ONR, et al. Microaggressions: privileged observers’ duty to act and what they can do [published online December 1, 2021]. Pediatrics. doi:10.1542/peds.2021-052758.
- Wheeler DJ, Zapata J, Davis D, et al. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2019;41:1112-1117.
- Scott K. Just Work: How to Root Out Bias, Prejudice, and Bullying to Build a Kick-Ass Culture of Inclusivity. St. Martin’s Press; 2021.
- Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289:3161-3166.
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
- Capers Q IV, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369.
- Lucey CR, Saguil A. The consequences of structural racism on MCAT scores and medical school admissions: the past is prologue. Acad Med. 2020;95:351-356.
- Nguemeni Tiako MJ, South EC, Ray V. Medical schools as racialized organizations: a primer. Ann Intern Med. 2021;174:1143-1144.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2021;113:310-314.
- Dobbin F, Kalev A. Why doesn’t diversity training work? the challenge for industry and academia. Anthropology Now. 2018;10:48-55.
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Int J Womens Dermatol. 2020;6:57-60.
- Hackworth JM, Kotagal M, Bignall ONR, et al. Microaggressions: privileged observers’ duty to act and what they can do [published online December 1, 2021]. Pediatrics. doi:10.1542/peds.2021-052758.
- Wheeler DJ, Zapata J, Davis D, et al. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2019;41:1112-1117.
- Scott K. Just Work: How to Root Out Bias, Prejudice, and Bullying to Build a Kick-Ass Culture of Inclusivity. St. Martin’s Press; 2021.
- Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289:3161-3166.
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
Racial Limitations of Fitzpatrick Skin Type
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
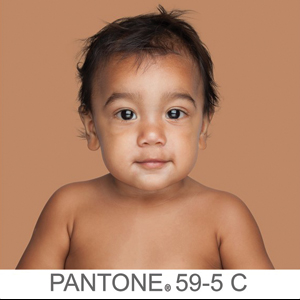
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.
© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
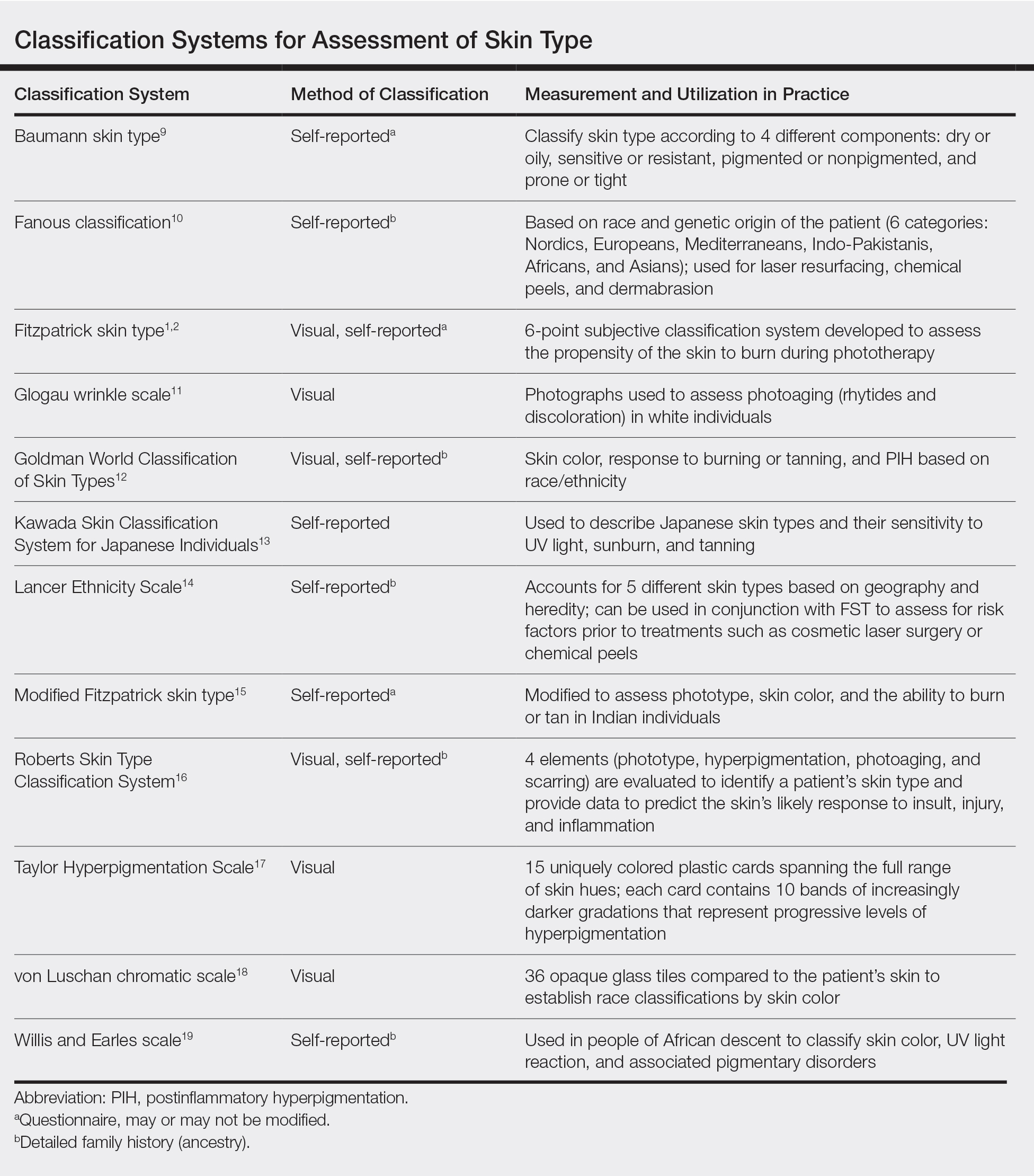
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20
Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.
© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20
Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.
© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20
Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
Practice Points
- Medical providers should be cognizant of conflating race and ethnicity with Fitzpatrick skin type (FST).
- Misuse of FST may occur more frequently among physicians who do not identify as having skin of color.
- Although alternative skin type classification systems have been proposed, more clinically relevant methods for describing skin of color need to be developed.
Barriers and Job Satisfaction Among Dermatology Hospitalists
Consultative dermatologists, or dermatology hospitalists (DHs), perform a critical role in the care of inpatients with skin disease, providing efficient diagnosis and management of patients with complex skin conditions as well as education of patients and trainees in the hospital setting.1 In 2013, 27% of the US population was seen by a physician for a skin disease.2 In 2014, there were nearly 650,000 hospital admissions principally for skin disease.3 Input by dermatologists facilitates accurate diagnosis and management of inpatients with skin disease,4 including a substantial number of cutaneous malignancies diagnosed in the inpatient setting.5 Several studies have highlighted the generally low level of diagnostic concordance between referring services and dermatology consultants,4,6 with dermatology consultants frequently noting diagnoses not considered by referring services,7 reinforcing the importance of having access to dermatologists in the hospital setting.
The care of skin disease in the inpatient setting has become increasingly complex. The Society for Dermatology Hospitalists (SDH) was created in 2009 to address this complexity, with the goal to “strive to develop the highest standards of clinical care of hospitalized patients with skin disease.”8 A recent survey found that 50% of DHs spend between 41 to 52 weeks per year on service.9 Despite this degree of commitment, there are considerable barriers that prevent the majority of dermatologists from efficiently providing inpatient consultative care. The inpatient and outpatient provision of dermatology care varies greatly, including the variety of ethical situations encountered and the diversity of skin conditions treated.10-12 Additionally, the transition between inpatient and outpatient care can be challenging for providers.13
The goal of this study was to evaluate the overall job satisfaction of DHs and further describe potential barriers to inpatient dermatology consultations.
Methods
An anonymous 31-question electronic survey was sent via email to all current members of the SDH from November 20 to December 10, 2018. The study was reviewed and determined to be exempt from federal human subjects regulations by the University of Washington Human Subjects Division (Seattle, Washington)(STUDY00005832).
Results
At the time of survey distribution, the SDH had 145 members, including attending-level dermatologists and resident members. Thirty-seven self-identified DHs (46% [17/37] women; 54% [20/37] men) completed the survey. The majority of respondents were junior faculty, with 46% (17/37) assistant professors, 5% (2/37) acting instructors, 32% (12/37) associate professors, and 16% (6/37) professors. All regions of the United States were represented.
Time Dedicated to Providing Inpatient Dermatology Consultations
The majority of those surveyed were satisfied or very satisfied (68% [25/37]) with the amount of time allotted for inpatient dermatology consultations, while 14% (5/37) were unsatisfied or very unsatisfied. Of those surveyed, 46% (17/37) reported that 21% to 50% of their time is dedicated to inpatient dermatology consultations. The majority (57% [21/37]) reported that their outpatient clinic efforts are reduced when providing dermatology inpatient consultations.
Regarding travel to the inpatient practice site, 60% (22/37) rated their travel time/effort as very easy, with 38% (14/37) reporting that the sites at which they provide inpatient dermatology consultations and their main outpatient clinics are the same physical location; 38% (14/37) reported travel times of less than 15 minutes between clinical practice sites.
Eighty-nine percent (33/37) of respondents said they are able to spend more time teaching trainees when providing inpatient dermatology consultations compared to their time spent in clinic. Similarly, 70% (26/37) said they are able to spend more time learning about patients and their conditions when providing inpatient dermatology consultations. Respondents also reported additional time expenditures because of inpatient dermatology consultations, primarily additional teaching requirements (49% [18/37]), additional electronic medical record training (35% [13/37]), and credentialing requirements (24% [9/37]).
Infrastructure for Providing Inpatient Dermatology Consultations
For many respondents (30% [11/37]), only 2 faculty dermatologists regularly provide inpatient dermatology consultations at their institutions. Four respondents reported having at least 5 faculty dermatologists who regularly provide inpatient dermatology consultations; excluding these, the average number of DHs was 2.42 faculty per institution.
Most respondents (57% [21/37]) reported their institutions support inpatient dermatology services by providing salary support for residents to cover services. Other methods of support included dedicated office spaces (30% [11/37]), free hospital parking while providing inpatient consultations (24% [9/37]), and administrative support (11% [4/37]).
Consultation Composition
Respondents indicated that requests for DH consultations most often come from medical services, including medical intensive care, internal medicine, and family medicine (95% [35/37]); the emergency department (95% [35/37]); surgical services (92% [34/37]); and hematology/oncology (89% [33/37]). Fewer DHs reported receiving consultation requests from pediatrics (70% [26/37]).
Many respondents (49% [18/37]) reported consulting for patients with skin disorders that they considered to be life-threatening or potentially life-threatening either very frequently (daily) or frequently (several times weekly), with only 16% (6/37) responding that they see such patients about once per month.
Compensation for Inpatient Dermatology Consultation
The most commonly reported compensation models for DHs were fixed salary plus productivity or performance incentives and fixed salary only models (49% [18/37] and 32% [12/37], respectively), with relative value unit (RVU) models and other models less frequently reported (16% [6/37] and 3% [1/37], respectively). Only 46% (17/37) of respondents were satisfied or very satisfied with their institutions’ compensation models; the remainder (54% [20/37]) were either neutral, unsatisfied, or very unsatisfied regarding their institutions’ compensation models. Overall compensation satisfaction was higher, with 60% (22/37) of DHs reporting they were satisfied or very satisfied with their salaries and 41% (15/37) reporting they were either neutral or not satisfied. The majority (60% [2/37]) of respondents felt that fixed salary plus productivity or performance incentives models would be the ideal compensation model for DHs.
Of the DHs whose compensations models were RVU based (6/37 [16%]), 67% (4/6) said they receive incentive pay upon meeting their RVU targets. No respondents reported that they were expected to generate an equivalent number of RVUs when performing inpatient consultations as compared to an outpatient session. Only 32% (12/37) of respondents reported keeping the revenue/RVUs generated by inpatient dermatology consultations; most (57% [21/37]) noted that their dermatology divisions/departments keep the revenue/RVUs, followed by university hospitals (27% [10/37]), schools of medicine (11% [4/37]), and departments of medicine (3% [1/37]). The remainder of respondents (22% [8/37]) were unsure who keeps the revenue/RVUs generated by inpatient dermatology consultations.
Most respondents (70% [26/37]) reported that the revenue (or RVU equivalent) generated by inpatient dermatology consultations does not fully support their salary for the time spent as consultants. Rather, these DHs noted sources of additional financial support, primarily the DHs themselves (69% [18/26]), followed by dermatology divisions/departments (50% [13/26]), departments of medicine (23% [6/26]), university hospitals (23% [6/26]), and schools of medicine (12% [3/26]).
Job Fulfillment Among DHs
Most respondents said they choose to provide inpatient dermatology consultations due to their interest in complex medical dermatology and their desire to work with other medical teams and specialties (92% [34/37] and 76% [28/37], respectively). Seventy percent (26/37) said they choose to provide inpatient consultations to be able to teach medical students and residents as well as to take advantage of the added opportunities to practice in a variety of settings beyond their outpatient clinics (57% [21/37]). Only 3% (1/37) of respondents reported that they provide inpatient dermatology consultations because they are “required to do so.”
Most DHs (84% [31/37]) said they feel their institutions as well as their dermatology divisions/departments value having access to inpatient dermatology services, though some did not feel this way (16% [6/37] neutral or strongly disagree). Nearly all respondents (97% [36/37]) felt they provide a critical service when performing inpatient dermatology consultations. All respondents (100%) said they found providing inpatient dermatology consultations fulfilling, and 65% (24/37) said they prefer providing inpatient dermatology consultations to spending time in clinic. Of the DHs who were surveyed, 68% (25/37) said they were satisfied with the balance of outpatient and inpatient services in their clinical practice and 30% (11/37) said they were not.
Comment
Factors such as patient care, hospital infrastructure, and procedural support have all been cited by DHs as crucial aspects of their contributions to the care of hospitalized pa
Dermatology is primarily an outpatient specialty, and our study highlighted several important challenges for providers performing inpatient dermatology consultations. A major issue is time expenditures, including additional teaching requirements, additional electronic medical record training, and credentialing requirements. Travel time to inpatient hospital sites does not appear to be one of these hindering factors; nearly 60% of respondents rated their travel time/effort as very easy, with approximately 75% of respondents’ consultation locations being either at the same physical location as their main outpatient clinic or less than 15 minutes away. Maintaining easy travel between outpatient and inpatient settings is important to the success of the DH.
Our data suggest that compensation of DHs is a potential limitation to providing inpatient dermatology care. Our survey reinforced that providers who do inpatient dermatology consultations generally do not generate the revenue necessary to cover these efforts. More than 40% of DH respondents said they either feel neutral about or unsatisfied with their overall salary, and more than half said they feel similarly regarding their institutions’ compensation models. Most respondents said that a fixed salary model plus productivity or performance incentives is the ideal compensation model for those providing inpatient dermatology consultations, though only half said they actually are compensated according to this model. This discrepancy highlights the disconnect between the current accepted compensation models and the DH’s ideal model and provides direction for dermatology chairs and division heads as to what compensation model is preferable to support the success of DHs at their institutions.
Despite the barriers and compensation constraints we identified, DHs report high job satisfaction, which we hypothesize could combat burnout. In our study, all DHs surveyed say they find providing inpatient dermatology consultations fulfilling, and most were satisfied with the amount of time allotted for consultations. Some of the possible reasons why DHs may find their work fulfilling include increased time for teaching trainees and learning about patients and their conditions while consulting, as well as a preference for providing inpatient dermatology consultations to spending time in clinic. Most DHs said they choose to provide inpatient dermatology consultation rather than do so as a requirement, primarily due to their interest in complex medical dermatology and their desire to work with other medical teams/specialties; thankfully, only a small percentage said they provide these consultations because they are required to do so.
This study was conducted to analyze job satisfaction among DHs who provided inpatient dermatology consultations and determine common barriers and obstacles to their job satisfaction. Limitations to our study included the small sample size and the possibly limited representation of the intended population, as only the members of the SDH were surveyed, potentially excluding providers who regularly perform inpatient dermatology consultations but are not members of the SDH. Further limitations included recall bias and the qualitative nature of the survey instrument.
Final Thoughts
There was near-unanimous agreement among the DHs we surveyed regarding the importance of the role they play in their divisions/departments, but there are clear barriers to provision of inpatient dermatology consultation, specifically relating to extraneous time expenditures and compensation. Despite these barriers, the majority of respondents said they are very satisfied with the role they play in the inpatient setting and feel that their contributions are valued by the institutions where they work. Protecting these benefits of providing dermatology hospital consultations will be critical for maintaining this high job satisfaction and balancing out the barriers to providing these consultations. Protecting the time required to provide consultations is paramount so DHs continue to gain fulfillment from teaching trainees, caring for complex patients, and maintaining their place as valuable colleagues in the hospital setting.
Acknowledgment
The authors thank the members of the SDH for their participation in this survey.
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2.
- Arnold JD, Yoon SJ, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2018;80:425-432.
- Mancusi S, Festa Neto C. Inpatient dermatological consultations in a university hospital. Clinics (Sao Paulo). 2010;65:851-855.
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:e116-e118.
- Pereira AR, Porro AM, Seque CA, et al. Inpatient dermatology consultations in renal transplant recipients. Actas Dermosifiliogr. 2018;109:900-907.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Hansra NK, Shinkai K, Fox LP. Ethical issues in inpatient consultative dermatology. Clin Dermatol. 2012;30:496-500.
- El-Azhary R, Weenig RH, Gibson LE. The dermatology hospitalist: creating value by rapid clinical pathologic correlation in a patient-centered care model. Int J Dermatol. 2012;51:1461-1466.
- Ahronowitz I, Fox LP. Herpes zoster in hospitalized adults: practice gaps, new evidence, and remaining questions. J Am Acad Dermatol. 2018;78:223-230.e3.
- Rosenbach M. The logistics of an inpatient dermatology service. Semin Cutan Med Surg. 2017;36:3-8.
- Ackerman L, Kessler M. The efficient, effective community hospital inpatient dermatology consult. Semin Cutan Med Surg. 2017;36:9-11.
Consultative dermatologists, or dermatology hospitalists (DHs), perform a critical role in the care of inpatients with skin disease, providing efficient diagnosis and management of patients with complex skin conditions as well as education of patients and trainees in the hospital setting.1 In 2013, 27% of the US population was seen by a physician for a skin disease.2 In 2014, there were nearly 650,000 hospital admissions principally for skin disease.3 Input by dermatologists facilitates accurate diagnosis and management of inpatients with skin disease,4 including a substantial number of cutaneous malignancies diagnosed in the inpatient setting.5 Several studies have highlighted the generally low level of diagnostic concordance between referring services and dermatology consultants,4,6 with dermatology consultants frequently noting diagnoses not considered by referring services,7 reinforcing the importance of having access to dermatologists in the hospital setting.
The care of skin disease in the inpatient setting has become increasingly complex. The Society for Dermatology Hospitalists (SDH) was created in 2009 to address this complexity, with the goal to “strive to develop the highest standards of clinical care of hospitalized patients with skin disease.”8 A recent survey found that 50% of DHs spend between 41 to 52 weeks per year on service.9 Despite this degree of commitment, there are considerable barriers that prevent the majority of dermatologists from efficiently providing inpatient consultative care. The inpatient and outpatient provision of dermatology care varies greatly, including the variety of ethical situations encountered and the diversity of skin conditions treated.10-12 Additionally, the transition between inpatient and outpatient care can be challenging for providers.13
The goal of this study was to evaluate the overall job satisfaction of DHs and further describe potential barriers to inpatient dermatology consultations.
Methods
An anonymous 31-question electronic survey was sent via email to all current members of the SDH from November 20 to December 10, 2018. The study was reviewed and determined to be exempt from federal human subjects regulations by the University of Washington Human Subjects Division (Seattle, Washington)(STUDY00005832).
Results
At the time of survey distribution, the SDH had 145 members, including attending-level dermatologists and resident members. Thirty-seven self-identified DHs (46% [17/37] women; 54% [20/37] men) completed the survey. The majority of respondents were junior faculty, with 46% (17/37) assistant professors, 5% (2/37) acting instructors, 32% (12/37) associate professors, and 16% (6/37) professors. All regions of the United States were represented.
Time Dedicated to Providing Inpatient Dermatology Consultations
The majority of those surveyed were satisfied or very satisfied (68% [25/37]) with the amount of time allotted for inpatient dermatology consultations, while 14% (5/37) were unsatisfied or very unsatisfied. Of those surveyed, 46% (17/37) reported that 21% to 50% of their time is dedicated to inpatient dermatology consultations. The majority (57% [21/37]) reported that their outpatient clinic efforts are reduced when providing dermatology inpatient consultations.
Regarding travel to the inpatient practice site, 60% (22/37) rated their travel time/effort as very easy, with 38% (14/37) reporting that the sites at which they provide inpatient dermatology consultations and their main outpatient clinics are the same physical location; 38% (14/37) reported travel times of less than 15 minutes between clinical practice sites.
Eighty-nine percent (33/37) of respondents said they are able to spend more time teaching trainees when providing inpatient dermatology consultations compared to their time spent in clinic. Similarly, 70% (26/37) said they are able to spend more time learning about patients and their conditions when providing inpatient dermatology consultations. Respondents also reported additional time expenditures because of inpatient dermatology consultations, primarily additional teaching requirements (49% [18/37]), additional electronic medical record training (35% [13/37]), and credentialing requirements (24% [9/37]).
Infrastructure for Providing Inpatient Dermatology Consultations
For many respondents (30% [11/37]), only 2 faculty dermatologists regularly provide inpatient dermatology consultations at their institutions. Four respondents reported having at least 5 faculty dermatologists who regularly provide inpatient dermatology consultations; excluding these, the average number of DHs was 2.42 faculty per institution.
Most respondents (57% [21/37]) reported their institutions support inpatient dermatology services by providing salary support for residents to cover services. Other methods of support included dedicated office spaces (30% [11/37]), free hospital parking while providing inpatient consultations (24% [9/37]), and administrative support (11% [4/37]).
Consultation Composition
Respondents indicated that requests for DH consultations most often come from medical services, including medical intensive care, internal medicine, and family medicine (95% [35/37]); the emergency department (95% [35/37]); surgical services (92% [34/37]); and hematology/oncology (89% [33/37]). Fewer DHs reported receiving consultation requests from pediatrics (70% [26/37]).
Many respondents (49% [18/37]) reported consulting for patients with skin disorders that they considered to be life-threatening or potentially life-threatening either very frequently (daily) or frequently (several times weekly), with only 16% (6/37) responding that they see such patients about once per month.
Compensation for Inpatient Dermatology Consultation
The most commonly reported compensation models for DHs were fixed salary plus productivity or performance incentives and fixed salary only models (49% [18/37] and 32% [12/37], respectively), with relative value unit (RVU) models and other models less frequently reported (16% [6/37] and 3% [1/37], respectively). Only 46% (17/37) of respondents were satisfied or very satisfied with their institutions’ compensation models; the remainder (54% [20/37]) were either neutral, unsatisfied, or very unsatisfied regarding their institutions’ compensation models. Overall compensation satisfaction was higher, with 60% (22/37) of DHs reporting they were satisfied or very satisfied with their salaries and 41% (15/37) reporting they were either neutral or not satisfied. The majority (60% [2/37]) of respondents felt that fixed salary plus productivity or performance incentives models would be the ideal compensation model for DHs.
Of the DHs whose compensations models were RVU based (6/37 [16%]), 67% (4/6) said they receive incentive pay upon meeting their RVU targets. No respondents reported that they were expected to generate an equivalent number of RVUs when performing inpatient consultations as compared to an outpatient session. Only 32% (12/37) of respondents reported keeping the revenue/RVUs generated by inpatient dermatology consultations; most (57% [21/37]) noted that their dermatology divisions/departments keep the revenue/RVUs, followed by university hospitals (27% [10/37]), schools of medicine (11% [4/37]), and departments of medicine (3% [1/37]). The remainder of respondents (22% [8/37]) were unsure who keeps the revenue/RVUs generated by inpatient dermatology consultations.
Most respondents (70% [26/37]) reported that the revenue (or RVU equivalent) generated by inpatient dermatology consultations does not fully support their salary for the time spent as consultants. Rather, these DHs noted sources of additional financial support, primarily the DHs themselves (69% [18/26]), followed by dermatology divisions/departments (50% [13/26]), departments of medicine (23% [6/26]), university hospitals (23% [6/26]), and schools of medicine (12% [3/26]).
Job Fulfillment Among DHs
Most respondents said they choose to provide inpatient dermatology consultations due to their interest in complex medical dermatology and their desire to work with other medical teams and specialties (92% [34/37] and 76% [28/37], respectively). Seventy percent (26/37) said they choose to provide inpatient consultations to be able to teach medical students and residents as well as to take advantage of the added opportunities to practice in a variety of settings beyond their outpatient clinics (57% [21/37]). Only 3% (1/37) of respondents reported that they provide inpatient dermatology consultations because they are “required to do so.”
Most DHs (84% [31/37]) said they feel their institutions as well as their dermatology divisions/departments value having access to inpatient dermatology services, though some did not feel this way (16% [6/37] neutral or strongly disagree). Nearly all respondents (97% [36/37]) felt they provide a critical service when performing inpatient dermatology consultations. All respondents (100%) said they found providing inpatient dermatology consultations fulfilling, and 65% (24/37) said they prefer providing inpatient dermatology consultations to spending time in clinic. Of the DHs who were surveyed, 68% (25/37) said they were satisfied with the balance of outpatient and inpatient services in their clinical practice and 30% (11/37) said they were not.
Comment
Factors such as patient care, hospital infrastructure, and procedural support have all been cited by DHs as crucial aspects of their contributions to the care of hospitalized pa
Dermatology is primarily an outpatient specialty, and our study highlighted several important challenges for providers performing inpatient dermatology consultations. A major issue is time expenditures, including additional teaching requirements, additional electronic medical record training, and credentialing requirements. Travel time to inpatient hospital sites does not appear to be one of these hindering factors; nearly 60% of respondents rated their travel time/effort as very easy, with approximately 75% of respondents’ consultation locations being either at the same physical location as their main outpatient clinic or less than 15 minutes away. Maintaining easy travel between outpatient and inpatient settings is important to the success of the DH.
Our data suggest that compensation of DHs is a potential limitation to providing inpatient dermatology care. Our survey reinforced that providers who do inpatient dermatology consultations generally do not generate the revenue necessary to cover these efforts. More than 40% of DH respondents said they either feel neutral about or unsatisfied with their overall salary, and more than half said they feel similarly regarding their institutions’ compensation models. Most respondents said that a fixed salary model plus productivity or performance incentives is the ideal compensation model for those providing inpatient dermatology consultations, though only half said they actually are compensated according to this model. This discrepancy highlights the disconnect between the current accepted compensation models and the DH’s ideal model and provides direction for dermatology chairs and division heads as to what compensation model is preferable to support the success of DHs at their institutions.
Despite the barriers and compensation constraints we identified, DHs report high job satisfaction, which we hypothesize could combat burnout. In our study, all DHs surveyed say they find providing inpatient dermatology consultations fulfilling, and most were satisfied with the amount of time allotted for consultations. Some of the possible reasons why DHs may find their work fulfilling include increased time for teaching trainees and learning about patients and their conditions while consulting, as well as a preference for providing inpatient dermatology consultations to spending time in clinic. Most DHs said they choose to provide inpatient dermatology consultation rather than do so as a requirement, primarily due to their interest in complex medical dermatology and their desire to work with other medical teams/specialties; thankfully, only a small percentage said they provide these consultations because they are required to do so.
This study was conducted to analyze job satisfaction among DHs who provided inpatient dermatology consultations and determine common barriers and obstacles to their job satisfaction. Limitations to our study included the small sample size and the possibly limited representation of the intended population, as only the members of the SDH were surveyed, potentially excluding providers who regularly perform inpatient dermatology consultations but are not members of the SDH. Further limitations included recall bias and the qualitative nature of the survey instrument.
Final Thoughts
There was near-unanimous agreement among the DHs we surveyed regarding the importance of the role they play in their divisions/departments, but there are clear barriers to provision of inpatient dermatology consultation, specifically relating to extraneous time expenditures and compensation. Despite these barriers, the majority of respondents said they are very satisfied with the role they play in the inpatient setting and feel that their contributions are valued by the institutions where they work. Protecting these benefits of providing dermatology hospital consultations will be critical for maintaining this high job satisfaction and balancing out the barriers to providing these consultations. Protecting the time required to provide consultations is paramount so DHs continue to gain fulfillment from teaching trainees, caring for complex patients, and maintaining their place as valuable colleagues in the hospital setting.
Acknowledgment
The authors thank the members of the SDH for their participation in this survey.
Consultative dermatologists, or dermatology hospitalists (DHs), perform a critical role in the care of inpatients with skin disease, providing efficient diagnosis and management of patients with complex skin conditions as well as education of patients and trainees in the hospital setting.1 In 2013, 27% of the US population was seen by a physician for a skin disease.2 In 2014, there were nearly 650,000 hospital admissions principally for skin disease.3 Input by dermatologists facilitates accurate diagnosis and management of inpatients with skin disease,4 including a substantial number of cutaneous malignancies diagnosed in the inpatient setting.5 Several studies have highlighted the generally low level of diagnostic concordance between referring services and dermatology consultants,4,6 with dermatology consultants frequently noting diagnoses not considered by referring services,7 reinforcing the importance of having access to dermatologists in the hospital setting.
The care of skin disease in the inpatient setting has become increasingly complex. The Society for Dermatology Hospitalists (SDH) was created in 2009 to address this complexity, with the goal to “strive to develop the highest standards of clinical care of hospitalized patients with skin disease.”8 A recent survey found that 50% of DHs spend between 41 to 52 weeks per year on service.9 Despite this degree of commitment, there are considerable barriers that prevent the majority of dermatologists from efficiently providing inpatient consultative care. The inpatient and outpatient provision of dermatology care varies greatly, including the variety of ethical situations encountered and the diversity of skin conditions treated.10-12 Additionally, the transition between inpatient and outpatient care can be challenging for providers.13
The goal of this study was to evaluate the overall job satisfaction of DHs and further describe potential barriers to inpatient dermatology consultations.
Methods
An anonymous 31-question electronic survey was sent via email to all current members of the SDH from November 20 to December 10, 2018. The study was reviewed and determined to be exempt from federal human subjects regulations by the University of Washington Human Subjects Division (Seattle, Washington)(STUDY00005832).
Results
At the time of survey distribution, the SDH had 145 members, including attending-level dermatologists and resident members. Thirty-seven self-identified DHs (46% [17/37] women; 54% [20/37] men) completed the survey. The majority of respondents were junior faculty, with 46% (17/37) assistant professors, 5% (2/37) acting instructors, 32% (12/37) associate professors, and 16% (6/37) professors. All regions of the United States were represented.
Time Dedicated to Providing Inpatient Dermatology Consultations
The majority of those surveyed were satisfied or very satisfied (68% [25/37]) with the amount of time allotted for inpatient dermatology consultations, while 14% (5/37) were unsatisfied or very unsatisfied. Of those surveyed, 46% (17/37) reported that 21% to 50% of their time is dedicated to inpatient dermatology consultations. The majority (57% [21/37]) reported that their outpatient clinic efforts are reduced when providing dermatology inpatient consultations.
Regarding travel to the inpatient practice site, 60% (22/37) rated their travel time/effort as very easy, with 38% (14/37) reporting that the sites at which they provide inpatient dermatology consultations and their main outpatient clinics are the same physical location; 38% (14/37) reported travel times of less than 15 minutes between clinical practice sites.
Eighty-nine percent (33/37) of respondents said they are able to spend more time teaching trainees when providing inpatient dermatology consultations compared to their time spent in clinic. Similarly, 70% (26/37) said they are able to spend more time learning about patients and their conditions when providing inpatient dermatology consultations. Respondents also reported additional time expenditures because of inpatient dermatology consultations, primarily additional teaching requirements (49% [18/37]), additional electronic medical record training (35% [13/37]), and credentialing requirements (24% [9/37]).
Infrastructure for Providing Inpatient Dermatology Consultations
For many respondents (30% [11/37]), only 2 faculty dermatologists regularly provide inpatient dermatology consultations at their institutions. Four respondents reported having at least 5 faculty dermatologists who regularly provide inpatient dermatology consultations; excluding these, the average number of DHs was 2.42 faculty per institution.
Most respondents (57% [21/37]) reported their institutions support inpatient dermatology services by providing salary support for residents to cover services. Other methods of support included dedicated office spaces (30% [11/37]), free hospital parking while providing inpatient consultations (24% [9/37]), and administrative support (11% [4/37]).
Consultation Composition
Respondents indicated that requests for DH consultations most often come from medical services, including medical intensive care, internal medicine, and family medicine (95% [35/37]); the emergency department (95% [35/37]); surgical services (92% [34/37]); and hematology/oncology (89% [33/37]). Fewer DHs reported receiving consultation requests from pediatrics (70% [26/37]).
Many respondents (49% [18/37]) reported consulting for patients with skin disorders that they considered to be life-threatening or potentially life-threatening either very frequently (daily) or frequently (several times weekly), with only 16% (6/37) responding that they see such patients about once per month.
Compensation for Inpatient Dermatology Consultation
The most commonly reported compensation models for DHs were fixed salary plus productivity or performance incentives and fixed salary only models (49% [18/37] and 32% [12/37], respectively), with relative value unit (RVU) models and other models less frequently reported (16% [6/37] and 3% [1/37], respectively). Only 46% (17/37) of respondents were satisfied or very satisfied with their institutions’ compensation models; the remainder (54% [20/37]) were either neutral, unsatisfied, or very unsatisfied regarding their institutions’ compensation models. Overall compensation satisfaction was higher, with 60% (22/37) of DHs reporting they were satisfied or very satisfied with their salaries and 41% (15/37) reporting they were either neutral or not satisfied. The majority (60% [2/37]) of respondents felt that fixed salary plus productivity or performance incentives models would be the ideal compensation model for DHs.
Of the DHs whose compensations models were RVU based (6/37 [16%]), 67% (4/6) said they receive incentive pay upon meeting their RVU targets. No respondents reported that they were expected to generate an equivalent number of RVUs when performing inpatient consultations as compared to an outpatient session. Only 32% (12/37) of respondents reported keeping the revenue/RVUs generated by inpatient dermatology consultations; most (57% [21/37]) noted that their dermatology divisions/departments keep the revenue/RVUs, followed by university hospitals (27% [10/37]), schools of medicine (11% [4/37]), and departments of medicine (3% [1/37]). The remainder of respondents (22% [8/37]) were unsure who keeps the revenue/RVUs generated by inpatient dermatology consultations.
Most respondents (70% [26/37]) reported that the revenue (or RVU equivalent) generated by inpatient dermatology consultations does not fully support their salary for the time spent as consultants. Rather, these DHs noted sources of additional financial support, primarily the DHs themselves (69% [18/26]), followed by dermatology divisions/departments (50% [13/26]), departments of medicine (23% [6/26]), university hospitals (23% [6/26]), and schools of medicine (12% [3/26]).
Job Fulfillment Among DHs
Most respondents said they choose to provide inpatient dermatology consultations due to their interest in complex medical dermatology and their desire to work with other medical teams and specialties (92% [34/37] and 76% [28/37], respectively). Seventy percent (26/37) said they choose to provide inpatient consultations to be able to teach medical students and residents as well as to take advantage of the added opportunities to practice in a variety of settings beyond their outpatient clinics (57% [21/37]). Only 3% (1/37) of respondents reported that they provide inpatient dermatology consultations because they are “required to do so.”
Most DHs (84% [31/37]) said they feel their institutions as well as their dermatology divisions/departments value having access to inpatient dermatology services, though some did not feel this way (16% [6/37] neutral or strongly disagree). Nearly all respondents (97% [36/37]) felt they provide a critical service when performing inpatient dermatology consultations. All respondents (100%) said they found providing inpatient dermatology consultations fulfilling, and 65% (24/37) said they prefer providing inpatient dermatology consultations to spending time in clinic. Of the DHs who were surveyed, 68% (25/37) said they were satisfied with the balance of outpatient and inpatient services in their clinical practice and 30% (11/37) said they were not.
Comment
Factors such as patient care, hospital infrastructure, and procedural support have all been cited by DHs as crucial aspects of their contributions to the care of hospitalized pa
Dermatology is primarily an outpatient specialty, and our study highlighted several important challenges for providers performing inpatient dermatology consultations. A major issue is time expenditures, including additional teaching requirements, additional electronic medical record training, and credentialing requirements. Travel time to inpatient hospital sites does not appear to be one of these hindering factors; nearly 60% of respondents rated their travel time/effort as very easy, with approximately 75% of respondents’ consultation locations being either at the same physical location as their main outpatient clinic or less than 15 minutes away. Maintaining easy travel between outpatient and inpatient settings is important to the success of the DH.
Our data suggest that compensation of DHs is a potential limitation to providing inpatient dermatology care. Our survey reinforced that providers who do inpatient dermatology consultations generally do not generate the revenue necessary to cover these efforts. More than 40% of DH respondents said they either feel neutral about or unsatisfied with their overall salary, and more than half said they feel similarly regarding their institutions’ compensation models. Most respondents said that a fixed salary model plus productivity or performance incentives is the ideal compensation model for those providing inpatient dermatology consultations, though only half said they actually are compensated according to this model. This discrepancy highlights the disconnect between the current accepted compensation models and the DH’s ideal model and provides direction for dermatology chairs and division heads as to what compensation model is preferable to support the success of DHs at their institutions.
Despite the barriers and compensation constraints we identified, DHs report high job satisfaction, which we hypothesize could combat burnout. In our study, all DHs surveyed say they find providing inpatient dermatology consultations fulfilling, and most were satisfied with the amount of time allotted for consultations. Some of the possible reasons why DHs may find their work fulfilling include increased time for teaching trainees and learning about patients and their conditions while consulting, as well as a preference for providing inpatient dermatology consultations to spending time in clinic. Most DHs said they choose to provide inpatient dermatology consultation rather than do so as a requirement, primarily due to their interest in complex medical dermatology and their desire to work with other medical teams/specialties; thankfully, only a small percentage said they provide these consultations because they are required to do so.
This study was conducted to analyze job satisfaction among DHs who provided inpatient dermatology consultations and determine common barriers and obstacles to their job satisfaction. Limitations to our study included the small sample size and the possibly limited representation of the intended population, as only the members of the SDH were surveyed, potentially excluding providers who regularly perform inpatient dermatology consultations but are not members of the SDH. Further limitations included recall bias and the qualitative nature of the survey instrument.
Final Thoughts
There was near-unanimous agreement among the DHs we surveyed regarding the importance of the role they play in their divisions/departments, but there are clear barriers to provision of inpatient dermatology consultation, specifically relating to extraneous time expenditures and compensation. Despite these barriers, the majority of respondents said they are very satisfied with the role they play in the inpatient setting and feel that their contributions are valued by the institutions where they work. Protecting these benefits of providing dermatology hospital consultations will be critical for maintaining this high job satisfaction and balancing out the barriers to providing these consultations. Protecting the time required to provide consultations is paramount so DHs continue to gain fulfillment from teaching trainees, caring for complex patients, and maintaining their place as valuable colleagues in the hospital setting.
Acknowledgment
The authors thank the members of the SDH for their participation in this survey.
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2.
- Arnold JD, Yoon SJ, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2018;80:425-432.
- Mancusi S, Festa Neto C. Inpatient dermatological consultations in a university hospital. Clinics (Sao Paulo). 2010;65:851-855.
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:e116-e118.
- Pereira AR, Porro AM, Seque CA, et al. Inpatient dermatology consultations in renal transplant recipients. Actas Dermosifiliogr. 2018;109:900-907.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Hansra NK, Shinkai K, Fox LP. Ethical issues in inpatient consultative dermatology. Clin Dermatol. 2012;30:496-500.
- El-Azhary R, Weenig RH, Gibson LE. The dermatology hospitalist: creating value by rapid clinical pathologic correlation in a patient-centered care model. Int J Dermatol. 2012;51:1461-1466.
- Ahronowitz I, Fox LP. Herpes zoster in hospitalized adults: practice gaps, new evidence, and remaining questions. J Am Acad Dermatol. 2018;78:223-230.e3.
- Rosenbach M. The logistics of an inpatient dermatology service. Semin Cutan Med Surg. 2017;36:3-8.
- Ackerman L, Kessler M. The efficient, effective community hospital inpatient dermatology consult. Semin Cutan Med Surg. 2017;36:9-11.
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2.
- Arnold JD, Yoon SJ, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2018;80:425-432.
- Mancusi S, Festa Neto C. Inpatient dermatological consultations in a university hospital. Clinics (Sao Paulo). 2010;65:851-855.
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:e116-e118.
- Pereira AR, Porro AM, Seque CA, et al. Inpatient dermatology consultations in renal transplant recipients. Actas Dermosifiliogr. 2018;109:900-907.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Hansra NK, Shinkai K, Fox LP. Ethical issues in inpatient consultative dermatology. Clin Dermatol. 2012;30:496-500.
- El-Azhary R, Weenig RH, Gibson LE. The dermatology hospitalist: creating value by rapid clinical pathologic correlation in a patient-centered care model. Int J Dermatol. 2012;51:1461-1466.
- Ahronowitz I, Fox LP. Herpes zoster in hospitalized adults: practice gaps, new evidence, and remaining questions. J Am Acad Dermatol. 2018;78:223-230.e3.
- Rosenbach M. The logistics of an inpatient dermatology service. Semin Cutan Med Surg. 2017;36:3-8.
- Ackerman L, Kessler M. The efficient, effective community hospital inpatient dermatology consult. Semin Cutan Med Surg. 2017;36:9-11.
Practice Points
• Dermatology hospitalists play a critical role in the specialized care of hospitalized patients with
skin conditions.
• Dermatology hospitalists have high job satisfaction, with opportunities to teach trainees and practice complex medical dermatology.
• Most dermatology hospitalists do not generate sufficient revenue providing inpatient dermatology consultations to fully support their salary for the time spent as consultants; alternate payment models are needed to maintain dermatology’s presence in the hospital.