User login
14-Year-Old Boy With Mild Antecedent Neck Pain in Setting of Acute Trauma: A Rare Case of Benign Fibrous Histiocytoma of the Spine
Benign fibrous histiocytoma (BFH) is a rare, well-recognized, primary skeletal tumor accounting for approximately 1% of all benign bone tumors. Spinal involvement is exceedingly rare with only 11 cases reported in the literature.1,2 We present a case of BFH located in the cervical spine of a pediatric patient that was successfully treated with curretage through an anterior surgical approach, along with a review of the literature and appropriate management concerning BFH of the spine.
Case Report
A 14-year-old boy was tackled while playing football and noticed immediate neck pain and subjective paresthesia in the upper extremities. Examination revealed a nontender spine (cervical, thoracic, lumbar) and normal strength and range of motion in all extremities. Sensation was diffusely intact, long tract signs were absent, and gait was normal. On questioning, the patient endorsed mild antecedent neck pain but denied prior history of any trauma. Neck pain did not radiate and was slightly worsened by activity but was mostly intermittent and random. As the neck pain was very mild and was not interfering with daily activities, the patient had not sought care before presenting to the emergency department. He had no pertinent past medical or surgical history.
The patient presented with a computed tomography (CT) scan of his head and cervical spine and a magnetic resonance imaging (MRI) scan of the cervical spine. A magnetic resonance angiography (MRA) scan of the neck was ordered after his arrival.
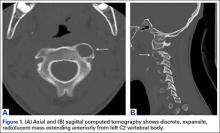
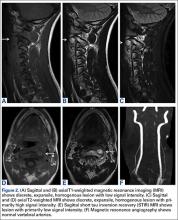
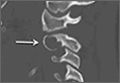
Axial and sagittal CT (Figures 1A, 1B) showed a 1×1.2-cm discrete, expansile, lytic, radiolucent mass extending anterior from the left C2 vertebral body. The mass appeared to abut the left vertebral artery foramen. The cortical bone surrounding the lesion was thin but uniform. Sagittal and axial T1-weighted MRI (Figures 2A, 2B) showed the discrete, expansile, homogenous lesion with the same intensity as normal bone marrow. Sagittal and axial T2-weighted MRI (Figures 2C, 2D) showed a discrete, expansile, homogenous lesion with primarily high signal intensity. Sagittal short tau inversion recovery (STIR) MRI (Figure 2E) again showed the lesion with primarily low intensity. Given the close proximity of the lesion to the vertebral foramen, MRA was ordered; it showed the lesion was not interfering with the vertebral artery (Figure 2F).
The tumor’s location, in the left anterior aspect of the C2 vertebral body, was not conducive to percutaneous biopsy for establishing tissue diagnosis, so the decision was made to surgically excise the lesion. A left-sided anterior incision was made 2 fingerbreadths inferior to the jaw line in a neck crease. A head and neck surgeon assisted with dissection. Dissection was carried down through the skin, subcutaneous tissue, and platysma on to the anterior part of the spine medial to the carotid sheath. Superior thyroid nerve and vessels and superior laryngeal nerve were identified and preserved. Fluoroscopy confirmed correct location at C2. The tumor was easily visualized, and the outer shell broke easily with palpation. Gentle curettage was necessary when removing the tumor off the vertebral artery. A portion of the specimen was sent during surgery for frozen section, which showed infrequent mitotic figures and no other findings concerning for malignancy. No instability was created after curettage and excision of the tumor, so no grafting or instrumentation was necessary.
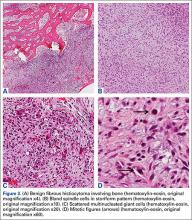
Grossly, the tumor was pale tan and firm. Histologic examination with hematoxylin-eosin staining revealed a bland spindle-cell neoplasm that focally involved bone. A storiform pattern was present. The cells had scant cytoplasm and oval to elongate nuclei with tapered ends. Significant nuclear pleomorphism was not seen. The stroma was loose, with focal myxoid change. Benign multinucleated giant cells were present. Mitotic activity was infrequent (Figures 3A–3D). Two attending pathologists reviewed the case material and the frozen and formalin-fixed specimens independently and concurred with the diagnosis of BFH. In addition, the case was reviewed at the surgical pathology consensus conference; the reviewers agreed on BFH, and additional studies were deemed unnecessary.
Given the patient’s complete clinical picture, the differential diagnosis included nonossifying fibroma (NOF), eosinophilic granuloma (EG), BFH, fibrous dysplasia, giant cell tumor (GCT), aneurysmal bone cyst (ABC), and osteoblastoma (OB).
Discussion
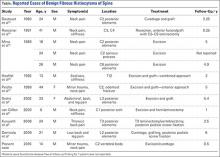
BFH is an extremely rare bone lesion, accounting for only 1% of all surgically managed bone tumors; not counting the present case, only 11 spine cases have been reported in the literature.1,2 BFH of the spine traditionally causes nonspecific, poorly localized pain. The Table lists the reported cases of spinal BFH and their presenting symptoms, location, and treatment. BFH usually occurs in young adults, but the age range is 5 to 75 years.2-4 Mean age of the 12 patients with spinal BFH in the literature (including ours) is 25 years.1 In addition, spinal BFH appears to have no predilection for sex.
Skeletal BFH presents as a discrete, well-defined, osteolytic lesion with sharp borders and potentially a sclerotic rim.4-6 Cortical expansion and even cortical disruption with invasion into adjacent tissue have occurred in flat bones.7 Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in storiform pattern.6
BFH shares many of its radiologic and histologic characteristics and clinical symptoms with other benign bone lesions (the tumors listed above). Therefore, accurate diagnosis of BFH requires appropriate correlation of clinical, radiographic, and histologic data.2,3,8 Below is a comparison of BFH with related bone lesions.
Spinal BFH causes a nonspecific, poorly localized pain similar to that of EG, ABC, GCT, and OB.3,9 NOF and fibrous dysplasia generally do not cause pain, unless these lesions are discovered secondary to a pathologic fracture.8,10,11 Our patient had minor antecedent neck pain, which was brought to light by his football accident. ABC and OB are more locally aggressive than BFH and can cause neurologic symptoms by mass effect and spinal cord or nerve root compression.1,8 In this case and in the 6 other cases of BFH of the cervical spine, there were no neurologic changes.4,10
Of the tumors mentioned, NOF and EG almost always occur in children. However, NOF usually occurs in the metaphyseal region of long bones, and EG is usually accompanied by systemic symptoms, such as lymphadenopathy, hepatomegaly, and increased inflammatory markers.1,8 Fibrous dysplasia usually presents in childhood but does not become symptomatic until adulthood. GCTs and OB predominantly occur in adulthood.12,13 Our patient’s age and lack of other systemic symptoms supported the diagnosis of BFH.
Appearance on MRI is reported less with BFH than with other tumors, but heterogenous signal intensity similar to that of skeletal muscle on T1-weighted images and high signal intensity on T2-weighted images is typically reported.8,14 NOF and fibrous dysplasia do not disrupt the bony cortex unless a pathologic fracture has occurred.4 GCTs are more aggressive lytic lesions with more aggressive radiologic features. GCTs generally cause cortical expansion/attenuation, and lack a sclerotic rim. GCTs also have a heterogenous appearance on MRI and give a low to intermediate signal on both T1- and T2-weighted images.12,15 The appearance of EG is similar to that of BFH as an osteolytic lesion with a sclerotic rim, though EGs typically break through the cortex and acquire a “punched-out” look.1,8 ABC typically is described as an expansile osteolytic lesion with a “soap-bubble” appearance on radiographs; periosteal elevation and cortical attenuation can also be visualized. MRI shows the typical multilobular appearance of the lesion with fluid levels.13
OB appears as a radiolucent lesion, with or without calcifications, surrounded by a thin margin of reactive bone.14,16 A distinguishing characteristic of OB was thought to be intense radioisotope uptake on bone scintigraphy, but recently a bony BFH demonstrated intense uptake.17 OBs typically demonstrate nonspecific MRI results similar to those of BFH: low to intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images.13 In our patient’s case, the radiographic appearance and lack of specific radiographic findings consistent with the other tumors supported the diagnosis of BFH.
Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in a storiform pattern6 which was demonstrated in our patient’s case. In addition, significant nuclear pleomorphism, mitotic activity, and necrosis were absent—a difference between BFH and malignant fibrous histiocytoma.4,15 The microscopic characteristics of BFH readily differentiate it from OB, ABC, EG, and GCT, but not from NOF on microscopic appearance alone. Clinical and radiographic findings must be consistent, as mentioned.7,18
Complete surgical excision is the reported treatment for BFH. Prognosis after resection or curettage is usually good, and recurrences have been rare.1,2 Depending on the intraspinous location of BFH, stabilization after resection or curettage may be necessary to prevent residual instability. Three of the 11 reported cases of spinal BFH required stabilization by anterior fusion or posterior pedicle screw fixation after resection.1,2 The other 8 cases underwent excision alone or excision and grafting. All 11 patients were disease-free at a mean follow-up of 3.5 years.1 In nonspinal BFH, however, both local recurrence and lung metastasis have been reported.2,5,9,19 Clarke and colleagues9 reported local recurrences in 3 of 8 cases. These recurrences involved BFH in long bones of the leg, which had been treated with curettage and grafting. There has been no reliable report of a malignant change in BFH.2,9 The only case of lung metastasis, reported by Unni and Dahlin6 in their study of 10 cases, occurred 2 years after local recurrence in the distal femur.Our patient was doing well at most recent follow-up, 6 months after surgery. He had no pain and had returned to normal activities. Although there are no reported cases of spinal BFH recurrence, we will follow this patient with imaging on an annual basis. His case is of particular interest to orthopedic surgeons because they encounter benign bone lesions every day, and many of these lesions are in difficult anatomical locations. Knowing the characteristics, differential diagnoses, and appropriate diagnostic workups for benign bone lesions is important for optimal and timely patient care.
1. Demiralp B, Kose O, Oguz E, Sanal T, Ozcan A, Sehirlioglu A. Benign fibrous histiocytoma of the lumbar vertebrae. Skeletal Radiol. 2009;38(2):187-191.
2. Kuruvath S, O’Donovan DG, Aspoas AR, David KM. Benign fibrous histiocytoma of the thoracic spine: case report and review of the literature. J Neurosurg Spine. 2006;4(3):260-264.
3. Ceroni D, Dayer R, De Coulon G, Kaelin A. Benign fibrous histiocytoma of bone in a paediatric population: a report of 6 cases. Musculoskelet Surg. 2011;95(2):107-114.
4. Dorfman HD, Czerniak B. Bone Tumors. St. Louis, MO: Mosby; 1998.
5. Grohs JG, Nicolakis M, Kainberger F, Lang S, Kotz R. Benign fibrous histiocytoma of bone: a report of ten cases and review of literature. Wien Klin Wochenschr. 2002;114(1-2):56-63.
6. Unni KK, Dahlin DC. Dahlin’s Bone Tumors. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
7. Balasubramanian C, Rajaraman G, Singh CS, Baliga DK. Benign fibrous histiocytoma of the sacrum—diagnostic difficulties facing this rare bone tumor. Pediatr Neurosurg. 2005;41(5):253-257.
8. van Giffen NH, van Rhijn LW, van Ooij A, et al. Benign fibrous histiocytoma of the posterior arch of C1 in a 6-year old boy: a case report. Spine. 2003;28(18):E359-E363.
9. Clarke BE, Xipell JM, Thomas DP. Benign fibrous histiocytoma of bone. Am J Surg Pathol. 1985;9(11):806-815.
10. Peicha G, Siebert FJ, Bratschitsch G, Fankhauser F, Grechenig W. Pathologic odontoid fracture and benign fibrous histiocytoma of bone. Eur Spine J. 1999;8(2):161-163.
11. Unni KK, Inwards CY, Bridge JA, Kindblom LG, Wold LE. Tumors of the Bones and Joints (AFIP Atlas of Tumor Pathology Series IV). Annapolis Junction, MD: American Registry of Pathology Press; 2005.
12. Dee R. Principles of Orthopaedic Practice. 2nd ed. New York, NY: McGraw-Hill; 1997.
13. Murphey M, Andrews C, Flemming D, Temple HT, Smith WS, Smirniotopoulos JG. Primary tumors of the spine: radiologic–pathologic correlation. Radiographics. 1996;16(5):1131-1158.
14. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
15. Mirra JM, Picci P, Gold RH. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Vol 1. Philadelphia, PA: Lea & Febiger; 1989.
16. Theodorou DJ, Theodorou SJ, Sartoris DJ. An imaging overview of primary tumors of the spine: part 1. Benign tumors. Clin Imaging. 2008;32(3):196-203.
17. Li X, Meng Z, Li D, Tan J, Song X. Benign fibrous histiocytoma of a rib. Clin Nucl Med. 2014;39(9): 837-841.
18. Roessner A, Immenkamp M, Weidner A, Hobik HP, Grundmann E. Benign fibrous histiocytoma of bone. Light- and electron-microscopic observations. J Cancer Res Clin Oncol. 1981;101(2):191-202.
19. Destouet JM, Kyriakos M, Gilula LA. Fibrous histiocytoma (fibroxanthoma) of a cervical vertebra. A report with a review of the literature. Skeletal Radiol. 1980;5(4):241-246.
20. Hoeffel JC, Bomand-Ferrand F, Tachet F, Lascombes P, Czorny A, Bernard C. So-called benign fibrous histiocytoma: report of a case. J Pediatr Surg. 1992;27(5):672-674.
Benign fibrous histiocytoma (BFH) is a rare, well-recognized, primary skeletal tumor accounting for approximately 1% of all benign bone tumors. Spinal involvement is exceedingly rare with only 11 cases reported in the literature.1,2 We present a case of BFH located in the cervical spine of a pediatric patient that was successfully treated with curretage through an anterior surgical approach, along with a review of the literature and appropriate management concerning BFH of the spine.
Case Report
A 14-year-old boy was tackled while playing football and noticed immediate neck pain and subjective paresthesia in the upper extremities. Examination revealed a nontender spine (cervical, thoracic, lumbar) and normal strength and range of motion in all extremities. Sensation was diffusely intact, long tract signs were absent, and gait was normal. On questioning, the patient endorsed mild antecedent neck pain but denied prior history of any trauma. Neck pain did not radiate and was slightly worsened by activity but was mostly intermittent and random. As the neck pain was very mild and was not interfering with daily activities, the patient had not sought care before presenting to the emergency department. He had no pertinent past medical or surgical history.
The patient presented with a computed tomography (CT) scan of his head and cervical spine and a magnetic resonance imaging (MRI) scan of the cervical spine. A magnetic resonance angiography (MRA) scan of the neck was ordered after his arrival.
Axial and sagittal CT (Figures 1A, 1B) showed a 1×1.2-cm discrete, expansile, lytic, radiolucent mass extending anterior from the left C2 vertebral body. The mass appeared to abut the left vertebral artery foramen. The cortical bone surrounding the lesion was thin but uniform. Sagittal and axial T1-weighted MRI (Figures 2A, 2B) showed the discrete, expansile, homogenous lesion with the same intensity as normal bone marrow. Sagittal and axial T2-weighted MRI (Figures 2C, 2D) showed a discrete, expansile, homogenous lesion with primarily high signal intensity. Sagittal short tau inversion recovery (STIR) MRI (Figure 2E) again showed the lesion with primarily low intensity. Given the close proximity of the lesion to the vertebral foramen, MRA was ordered; it showed the lesion was not interfering with the vertebral artery (Figure 2F).
The tumor’s location, in the left anterior aspect of the C2 vertebral body, was not conducive to percutaneous biopsy for establishing tissue diagnosis, so the decision was made to surgically excise the lesion. A left-sided anterior incision was made 2 fingerbreadths inferior to the jaw line in a neck crease. A head and neck surgeon assisted with dissection. Dissection was carried down through the skin, subcutaneous tissue, and platysma on to the anterior part of the spine medial to the carotid sheath. Superior thyroid nerve and vessels and superior laryngeal nerve were identified and preserved. Fluoroscopy confirmed correct location at C2. The tumor was easily visualized, and the outer shell broke easily with palpation. Gentle curettage was necessary when removing the tumor off the vertebral artery. A portion of the specimen was sent during surgery for frozen section, which showed infrequent mitotic figures and no other findings concerning for malignancy. No instability was created after curettage and excision of the tumor, so no grafting or instrumentation was necessary.
Grossly, the tumor was pale tan and firm. Histologic examination with hematoxylin-eosin staining revealed a bland spindle-cell neoplasm that focally involved bone. A storiform pattern was present. The cells had scant cytoplasm and oval to elongate nuclei with tapered ends. Significant nuclear pleomorphism was not seen. The stroma was loose, with focal myxoid change. Benign multinucleated giant cells were present. Mitotic activity was infrequent (Figures 3A–3D). Two attending pathologists reviewed the case material and the frozen and formalin-fixed specimens independently and concurred with the diagnosis of BFH. In addition, the case was reviewed at the surgical pathology consensus conference; the reviewers agreed on BFH, and additional studies were deemed unnecessary.
Given the patient’s complete clinical picture, the differential diagnosis included nonossifying fibroma (NOF), eosinophilic granuloma (EG), BFH, fibrous dysplasia, giant cell tumor (GCT), aneurysmal bone cyst (ABC), and osteoblastoma (OB).
Discussion
BFH is an extremely rare bone lesion, accounting for only 1% of all surgically managed bone tumors; not counting the present case, only 11 spine cases have been reported in the literature.1,2 BFH of the spine traditionally causes nonspecific, poorly localized pain. The Table lists the reported cases of spinal BFH and their presenting symptoms, location, and treatment. BFH usually occurs in young adults, but the age range is 5 to 75 years.2-4 Mean age of the 12 patients with spinal BFH in the literature (including ours) is 25 years.1 In addition, spinal BFH appears to have no predilection for sex.
Skeletal BFH presents as a discrete, well-defined, osteolytic lesion with sharp borders and potentially a sclerotic rim.4-6 Cortical expansion and even cortical disruption with invasion into adjacent tissue have occurred in flat bones.7 Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in storiform pattern.6
BFH shares many of its radiologic and histologic characteristics and clinical symptoms with other benign bone lesions (the tumors listed above). Therefore, accurate diagnosis of BFH requires appropriate correlation of clinical, radiographic, and histologic data.2,3,8 Below is a comparison of BFH with related bone lesions.
Spinal BFH causes a nonspecific, poorly localized pain similar to that of EG, ABC, GCT, and OB.3,9 NOF and fibrous dysplasia generally do not cause pain, unless these lesions are discovered secondary to a pathologic fracture.8,10,11 Our patient had minor antecedent neck pain, which was brought to light by his football accident. ABC and OB are more locally aggressive than BFH and can cause neurologic symptoms by mass effect and spinal cord or nerve root compression.1,8 In this case and in the 6 other cases of BFH of the cervical spine, there were no neurologic changes.4,10
Of the tumors mentioned, NOF and EG almost always occur in children. However, NOF usually occurs in the metaphyseal region of long bones, and EG is usually accompanied by systemic symptoms, such as lymphadenopathy, hepatomegaly, and increased inflammatory markers.1,8 Fibrous dysplasia usually presents in childhood but does not become symptomatic until adulthood. GCTs and OB predominantly occur in adulthood.12,13 Our patient’s age and lack of other systemic symptoms supported the diagnosis of BFH.
Appearance on MRI is reported less with BFH than with other tumors, but heterogenous signal intensity similar to that of skeletal muscle on T1-weighted images and high signal intensity on T2-weighted images is typically reported.8,14 NOF and fibrous dysplasia do not disrupt the bony cortex unless a pathologic fracture has occurred.4 GCTs are more aggressive lytic lesions with more aggressive radiologic features. GCTs generally cause cortical expansion/attenuation, and lack a sclerotic rim. GCTs also have a heterogenous appearance on MRI and give a low to intermediate signal on both T1- and T2-weighted images.12,15 The appearance of EG is similar to that of BFH as an osteolytic lesion with a sclerotic rim, though EGs typically break through the cortex and acquire a “punched-out” look.1,8 ABC typically is described as an expansile osteolytic lesion with a “soap-bubble” appearance on radiographs; periosteal elevation and cortical attenuation can also be visualized. MRI shows the typical multilobular appearance of the lesion with fluid levels.13
OB appears as a radiolucent lesion, with or without calcifications, surrounded by a thin margin of reactive bone.14,16 A distinguishing characteristic of OB was thought to be intense radioisotope uptake on bone scintigraphy, but recently a bony BFH demonstrated intense uptake.17 OBs typically demonstrate nonspecific MRI results similar to those of BFH: low to intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images.13 In our patient’s case, the radiographic appearance and lack of specific radiographic findings consistent with the other tumors supported the diagnosis of BFH.
Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in a storiform pattern6 which was demonstrated in our patient’s case. In addition, significant nuclear pleomorphism, mitotic activity, and necrosis were absent—a difference between BFH and malignant fibrous histiocytoma.4,15 The microscopic characteristics of BFH readily differentiate it from OB, ABC, EG, and GCT, but not from NOF on microscopic appearance alone. Clinical and radiographic findings must be consistent, as mentioned.7,18
Complete surgical excision is the reported treatment for BFH. Prognosis after resection or curettage is usually good, and recurrences have been rare.1,2 Depending on the intraspinous location of BFH, stabilization after resection or curettage may be necessary to prevent residual instability. Three of the 11 reported cases of spinal BFH required stabilization by anterior fusion or posterior pedicle screw fixation after resection.1,2 The other 8 cases underwent excision alone or excision and grafting. All 11 patients were disease-free at a mean follow-up of 3.5 years.1 In nonspinal BFH, however, both local recurrence and lung metastasis have been reported.2,5,9,19 Clarke and colleagues9 reported local recurrences in 3 of 8 cases. These recurrences involved BFH in long bones of the leg, which had been treated with curettage and grafting. There has been no reliable report of a malignant change in BFH.2,9 The only case of lung metastasis, reported by Unni and Dahlin6 in their study of 10 cases, occurred 2 years after local recurrence in the distal femur.Our patient was doing well at most recent follow-up, 6 months after surgery. He had no pain and had returned to normal activities. Although there are no reported cases of spinal BFH recurrence, we will follow this patient with imaging on an annual basis. His case is of particular interest to orthopedic surgeons because they encounter benign bone lesions every day, and many of these lesions are in difficult anatomical locations. Knowing the characteristics, differential diagnoses, and appropriate diagnostic workups for benign bone lesions is important for optimal and timely patient care.
Benign fibrous histiocytoma (BFH) is a rare, well-recognized, primary skeletal tumor accounting for approximately 1% of all benign bone tumors. Spinal involvement is exceedingly rare with only 11 cases reported in the literature.1,2 We present a case of BFH located in the cervical spine of a pediatric patient that was successfully treated with curretage through an anterior surgical approach, along with a review of the literature and appropriate management concerning BFH of the spine.
Case Report
A 14-year-old boy was tackled while playing football and noticed immediate neck pain and subjective paresthesia in the upper extremities. Examination revealed a nontender spine (cervical, thoracic, lumbar) and normal strength and range of motion in all extremities. Sensation was diffusely intact, long tract signs were absent, and gait was normal. On questioning, the patient endorsed mild antecedent neck pain but denied prior history of any trauma. Neck pain did not radiate and was slightly worsened by activity but was mostly intermittent and random. As the neck pain was very mild and was not interfering with daily activities, the patient had not sought care before presenting to the emergency department. He had no pertinent past medical or surgical history.
The patient presented with a computed tomography (CT) scan of his head and cervical spine and a magnetic resonance imaging (MRI) scan of the cervical spine. A magnetic resonance angiography (MRA) scan of the neck was ordered after his arrival.
Axial and sagittal CT (Figures 1A, 1B) showed a 1×1.2-cm discrete, expansile, lytic, radiolucent mass extending anterior from the left C2 vertebral body. The mass appeared to abut the left vertebral artery foramen. The cortical bone surrounding the lesion was thin but uniform. Sagittal and axial T1-weighted MRI (Figures 2A, 2B) showed the discrete, expansile, homogenous lesion with the same intensity as normal bone marrow. Sagittal and axial T2-weighted MRI (Figures 2C, 2D) showed a discrete, expansile, homogenous lesion with primarily high signal intensity. Sagittal short tau inversion recovery (STIR) MRI (Figure 2E) again showed the lesion with primarily low intensity. Given the close proximity of the lesion to the vertebral foramen, MRA was ordered; it showed the lesion was not interfering with the vertebral artery (Figure 2F).
The tumor’s location, in the left anterior aspect of the C2 vertebral body, was not conducive to percutaneous biopsy for establishing tissue diagnosis, so the decision was made to surgically excise the lesion. A left-sided anterior incision was made 2 fingerbreadths inferior to the jaw line in a neck crease. A head and neck surgeon assisted with dissection. Dissection was carried down through the skin, subcutaneous tissue, and platysma on to the anterior part of the spine medial to the carotid sheath. Superior thyroid nerve and vessels and superior laryngeal nerve were identified and preserved. Fluoroscopy confirmed correct location at C2. The tumor was easily visualized, and the outer shell broke easily with palpation. Gentle curettage was necessary when removing the tumor off the vertebral artery. A portion of the specimen was sent during surgery for frozen section, which showed infrequent mitotic figures and no other findings concerning for malignancy. No instability was created after curettage and excision of the tumor, so no grafting or instrumentation was necessary.
Grossly, the tumor was pale tan and firm. Histologic examination with hematoxylin-eosin staining revealed a bland spindle-cell neoplasm that focally involved bone. A storiform pattern was present. The cells had scant cytoplasm and oval to elongate nuclei with tapered ends. Significant nuclear pleomorphism was not seen. The stroma was loose, with focal myxoid change. Benign multinucleated giant cells were present. Mitotic activity was infrequent (Figures 3A–3D). Two attending pathologists reviewed the case material and the frozen and formalin-fixed specimens independently and concurred with the diagnosis of BFH. In addition, the case was reviewed at the surgical pathology consensus conference; the reviewers agreed on BFH, and additional studies were deemed unnecessary.
Given the patient’s complete clinical picture, the differential diagnosis included nonossifying fibroma (NOF), eosinophilic granuloma (EG), BFH, fibrous dysplasia, giant cell tumor (GCT), aneurysmal bone cyst (ABC), and osteoblastoma (OB).
Discussion
BFH is an extremely rare bone lesion, accounting for only 1% of all surgically managed bone tumors; not counting the present case, only 11 spine cases have been reported in the literature.1,2 BFH of the spine traditionally causes nonspecific, poorly localized pain. The Table lists the reported cases of spinal BFH and their presenting symptoms, location, and treatment. BFH usually occurs in young adults, but the age range is 5 to 75 years.2-4 Mean age of the 12 patients with spinal BFH in the literature (including ours) is 25 years.1 In addition, spinal BFH appears to have no predilection for sex.
Skeletal BFH presents as a discrete, well-defined, osteolytic lesion with sharp borders and potentially a sclerotic rim.4-6 Cortical expansion and even cortical disruption with invasion into adjacent tissue have occurred in flat bones.7 Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in storiform pattern.6
BFH shares many of its radiologic and histologic characteristics and clinical symptoms with other benign bone lesions (the tumors listed above). Therefore, accurate diagnosis of BFH requires appropriate correlation of clinical, radiographic, and histologic data.2,3,8 Below is a comparison of BFH with related bone lesions.
Spinal BFH causes a nonspecific, poorly localized pain similar to that of EG, ABC, GCT, and OB.3,9 NOF and fibrous dysplasia generally do not cause pain, unless these lesions are discovered secondary to a pathologic fracture.8,10,11 Our patient had minor antecedent neck pain, which was brought to light by his football accident. ABC and OB are more locally aggressive than BFH and can cause neurologic symptoms by mass effect and spinal cord or nerve root compression.1,8 In this case and in the 6 other cases of BFH of the cervical spine, there were no neurologic changes.4,10
Of the tumors mentioned, NOF and EG almost always occur in children. However, NOF usually occurs in the metaphyseal region of long bones, and EG is usually accompanied by systemic symptoms, such as lymphadenopathy, hepatomegaly, and increased inflammatory markers.1,8 Fibrous dysplasia usually presents in childhood but does not become symptomatic until adulthood. GCTs and OB predominantly occur in adulthood.12,13 Our patient’s age and lack of other systemic symptoms supported the diagnosis of BFH.
Appearance on MRI is reported less with BFH than with other tumors, but heterogenous signal intensity similar to that of skeletal muscle on T1-weighted images and high signal intensity on T2-weighted images is typically reported.8,14 NOF and fibrous dysplasia do not disrupt the bony cortex unless a pathologic fracture has occurred.4 GCTs are more aggressive lytic lesions with more aggressive radiologic features. GCTs generally cause cortical expansion/attenuation, and lack a sclerotic rim. GCTs also have a heterogenous appearance on MRI and give a low to intermediate signal on both T1- and T2-weighted images.12,15 The appearance of EG is similar to that of BFH as an osteolytic lesion with a sclerotic rim, though EGs typically break through the cortex and acquire a “punched-out” look.1,8 ABC typically is described as an expansile osteolytic lesion with a “soap-bubble” appearance on radiographs; periosteal elevation and cortical attenuation can also be visualized. MRI shows the typical multilobular appearance of the lesion with fluid levels.13
OB appears as a radiolucent lesion, with or without calcifications, surrounded by a thin margin of reactive bone.14,16 A distinguishing characteristic of OB was thought to be intense radioisotope uptake on bone scintigraphy, but recently a bony BFH demonstrated intense uptake.17 OBs typically demonstrate nonspecific MRI results similar to those of BFH: low to intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images.13 In our patient’s case, the radiographic appearance and lack of specific radiographic findings consistent with the other tumors supported the diagnosis of BFH.
Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in a storiform pattern6 which was demonstrated in our patient’s case. In addition, significant nuclear pleomorphism, mitotic activity, and necrosis were absent—a difference between BFH and malignant fibrous histiocytoma.4,15 The microscopic characteristics of BFH readily differentiate it from OB, ABC, EG, and GCT, but not from NOF on microscopic appearance alone. Clinical and radiographic findings must be consistent, as mentioned.7,18
Complete surgical excision is the reported treatment for BFH. Prognosis after resection or curettage is usually good, and recurrences have been rare.1,2 Depending on the intraspinous location of BFH, stabilization after resection or curettage may be necessary to prevent residual instability. Three of the 11 reported cases of spinal BFH required stabilization by anterior fusion or posterior pedicle screw fixation after resection.1,2 The other 8 cases underwent excision alone or excision and grafting. All 11 patients were disease-free at a mean follow-up of 3.5 years.1 In nonspinal BFH, however, both local recurrence and lung metastasis have been reported.2,5,9,19 Clarke and colleagues9 reported local recurrences in 3 of 8 cases. These recurrences involved BFH in long bones of the leg, which had been treated with curettage and grafting. There has been no reliable report of a malignant change in BFH.2,9 The only case of lung metastasis, reported by Unni and Dahlin6 in their study of 10 cases, occurred 2 years after local recurrence in the distal femur.Our patient was doing well at most recent follow-up, 6 months after surgery. He had no pain and had returned to normal activities. Although there are no reported cases of spinal BFH recurrence, we will follow this patient with imaging on an annual basis. His case is of particular interest to orthopedic surgeons because they encounter benign bone lesions every day, and many of these lesions are in difficult anatomical locations. Knowing the characteristics, differential diagnoses, and appropriate diagnostic workups for benign bone lesions is important for optimal and timely patient care.
1. Demiralp B, Kose O, Oguz E, Sanal T, Ozcan A, Sehirlioglu A. Benign fibrous histiocytoma of the lumbar vertebrae. Skeletal Radiol. 2009;38(2):187-191.
2. Kuruvath S, O’Donovan DG, Aspoas AR, David KM. Benign fibrous histiocytoma of the thoracic spine: case report and review of the literature. J Neurosurg Spine. 2006;4(3):260-264.
3. Ceroni D, Dayer R, De Coulon G, Kaelin A. Benign fibrous histiocytoma of bone in a paediatric population: a report of 6 cases. Musculoskelet Surg. 2011;95(2):107-114.
4. Dorfman HD, Czerniak B. Bone Tumors. St. Louis, MO: Mosby; 1998.
5. Grohs JG, Nicolakis M, Kainberger F, Lang S, Kotz R. Benign fibrous histiocytoma of bone: a report of ten cases and review of literature. Wien Klin Wochenschr. 2002;114(1-2):56-63.
6. Unni KK, Dahlin DC. Dahlin’s Bone Tumors. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
7. Balasubramanian C, Rajaraman G, Singh CS, Baliga DK. Benign fibrous histiocytoma of the sacrum—diagnostic difficulties facing this rare bone tumor. Pediatr Neurosurg. 2005;41(5):253-257.
8. van Giffen NH, van Rhijn LW, van Ooij A, et al. Benign fibrous histiocytoma of the posterior arch of C1 in a 6-year old boy: a case report. Spine. 2003;28(18):E359-E363.
9. Clarke BE, Xipell JM, Thomas DP. Benign fibrous histiocytoma of bone. Am J Surg Pathol. 1985;9(11):806-815.
10. Peicha G, Siebert FJ, Bratschitsch G, Fankhauser F, Grechenig W. Pathologic odontoid fracture and benign fibrous histiocytoma of bone. Eur Spine J. 1999;8(2):161-163.
11. Unni KK, Inwards CY, Bridge JA, Kindblom LG, Wold LE. Tumors of the Bones and Joints (AFIP Atlas of Tumor Pathology Series IV). Annapolis Junction, MD: American Registry of Pathology Press; 2005.
12. Dee R. Principles of Orthopaedic Practice. 2nd ed. New York, NY: McGraw-Hill; 1997.
13. Murphey M, Andrews C, Flemming D, Temple HT, Smith WS, Smirniotopoulos JG. Primary tumors of the spine: radiologic–pathologic correlation. Radiographics. 1996;16(5):1131-1158.
14. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
15. Mirra JM, Picci P, Gold RH. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Vol 1. Philadelphia, PA: Lea & Febiger; 1989.
16. Theodorou DJ, Theodorou SJ, Sartoris DJ. An imaging overview of primary tumors of the spine: part 1. Benign tumors. Clin Imaging. 2008;32(3):196-203.
17. Li X, Meng Z, Li D, Tan J, Song X. Benign fibrous histiocytoma of a rib. Clin Nucl Med. 2014;39(9): 837-841.
18. Roessner A, Immenkamp M, Weidner A, Hobik HP, Grundmann E. Benign fibrous histiocytoma of bone. Light- and electron-microscopic observations. J Cancer Res Clin Oncol. 1981;101(2):191-202.
19. Destouet JM, Kyriakos M, Gilula LA. Fibrous histiocytoma (fibroxanthoma) of a cervical vertebra. A report with a review of the literature. Skeletal Radiol. 1980;5(4):241-246.
20. Hoeffel JC, Bomand-Ferrand F, Tachet F, Lascombes P, Czorny A, Bernard C. So-called benign fibrous histiocytoma: report of a case. J Pediatr Surg. 1992;27(5):672-674.
1. Demiralp B, Kose O, Oguz E, Sanal T, Ozcan A, Sehirlioglu A. Benign fibrous histiocytoma of the lumbar vertebrae. Skeletal Radiol. 2009;38(2):187-191.
2. Kuruvath S, O’Donovan DG, Aspoas AR, David KM. Benign fibrous histiocytoma of the thoracic spine: case report and review of the literature. J Neurosurg Spine. 2006;4(3):260-264.
3. Ceroni D, Dayer R, De Coulon G, Kaelin A. Benign fibrous histiocytoma of bone in a paediatric population: a report of 6 cases. Musculoskelet Surg. 2011;95(2):107-114.
4. Dorfman HD, Czerniak B. Bone Tumors. St. Louis, MO: Mosby; 1998.
5. Grohs JG, Nicolakis M, Kainberger F, Lang S, Kotz R. Benign fibrous histiocytoma of bone: a report of ten cases and review of literature. Wien Klin Wochenschr. 2002;114(1-2):56-63.
6. Unni KK, Dahlin DC. Dahlin’s Bone Tumors. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
7. Balasubramanian C, Rajaraman G, Singh CS, Baliga DK. Benign fibrous histiocytoma of the sacrum—diagnostic difficulties facing this rare bone tumor. Pediatr Neurosurg. 2005;41(5):253-257.
8. van Giffen NH, van Rhijn LW, van Ooij A, et al. Benign fibrous histiocytoma of the posterior arch of C1 in a 6-year old boy: a case report. Spine. 2003;28(18):E359-E363.
9. Clarke BE, Xipell JM, Thomas DP. Benign fibrous histiocytoma of bone. Am J Surg Pathol. 1985;9(11):806-815.
10. Peicha G, Siebert FJ, Bratschitsch G, Fankhauser F, Grechenig W. Pathologic odontoid fracture and benign fibrous histiocytoma of bone. Eur Spine J. 1999;8(2):161-163.
11. Unni KK, Inwards CY, Bridge JA, Kindblom LG, Wold LE. Tumors of the Bones and Joints (AFIP Atlas of Tumor Pathology Series IV). Annapolis Junction, MD: American Registry of Pathology Press; 2005.
12. Dee R. Principles of Orthopaedic Practice. 2nd ed. New York, NY: McGraw-Hill; 1997.
13. Murphey M, Andrews C, Flemming D, Temple HT, Smith WS, Smirniotopoulos JG. Primary tumors of the spine: radiologic–pathologic correlation. Radiographics. 1996;16(5):1131-1158.
14. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
15. Mirra JM, Picci P, Gold RH. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Vol 1. Philadelphia, PA: Lea & Febiger; 1989.
16. Theodorou DJ, Theodorou SJ, Sartoris DJ. An imaging overview of primary tumors of the spine: part 1. Benign tumors. Clin Imaging. 2008;32(3):196-203.
17. Li X, Meng Z, Li D, Tan J, Song X. Benign fibrous histiocytoma of a rib. Clin Nucl Med. 2014;39(9): 837-841.
18. Roessner A, Immenkamp M, Weidner A, Hobik HP, Grundmann E. Benign fibrous histiocytoma of bone. Light- and electron-microscopic observations. J Cancer Res Clin Oncol. 1981;101(2):191-202.
19. Destouet JM, Kyriakos M, Gilula LA. Fibrous histiocytoma (fibroxanthoma) of a cervical vertebra. A report with a review of the literature. Skeletal Radiol. 1980;5(4):241-246.
20. Hoeffel JC, Bomand-Ferrand F, Tachet F, Lascombes P, Czorny A, Bernard C. So-called benign fibrous histiocytoma: report of a case. J Pediatr Surg. 1992;27(5):672-674.