User login
Opioid-Induced Androgen Deficiency in Veterans With Chronic Nonmalignant Pain
According to the CDC, the medical use of opioid painkillers has increased at least 10-fold during the past 20 years, “because of a movement toward more aggressive management of pain.”1 Although opioid therapy is generally considered effective for the treatment of pain, long-term use (both orally and intrathecally) is associated with adverse effects (AEs) such as constipation, fatigue, nausea, sleep disturbances, depression, sexual dysfunction, and hypogonadism.2,3Opioid-induced androgen deficiency (OPIAD), as defined by Smith and Elliot, is a clinical syndrome characterized by inappropriately low concentrations of gonadotropins (specifically, follicle-stimulating hormone [FSH] and luteinizing hormone [LH]), which leads to inadequate production of sex hormones, including estradiol and testosterone.4
Related: Testosterone Replacement Therapy: Playing Catch-up With Patients
The mechanism behind this phenomenon is initiated by either endogenous or exogenous opioids acting on opioid receptors in the hypothalamus, which causes a decrease in the release of gonadotropin- releasing hormone (GnRH). This decrease in GnRH causes a reduction in the release of LH and FSH from the pituitary gland as well as testosterone or estradiol from the gonads.4,5 Various guidelines report different cutoffs for the lower limit of normal total testosterone: The Endocrine Society recommends 300 ng/dL, the American Association of Clinical Endocrinologists suggests 200 ng/dL, and various other organizations suggest 230 ng/dL.6-8 Hypotestosteronism can result in patients presenting with a broad spectrum of clinical symptoms, including reduced libido, erectile dysfunction (ED), fatigue, hot flashes, depression, anemia, decreased muscle mass, weight gain, and osteopenia or osteoporosis.4 Women with low testosterone levels can experience irregular menstrual periods, oligomenorrhea, or amenorrhea.9 Opioid-induced androgen deficiency often goes unrecognized and untreated. The reported prevalence of opioid-induced hypogonadism ranges from 21% to 86%.4,9 Given the growing number of patients on chronic opioid therapy, OPIAD warrants further investigation to identify the prevalence in the veteran population to appropriately monitor and manage this deficiency.
The objective of this retrospective review was to identify the presence of secondary hypogonadism in chronic opioid users among a cohort of veterans receiving chronic opioids for nonmalignant pain. In addition to identifying the presence of secondary hypogonadism, the relationship between testosterone concentrations and total daily morphine equivalent doses (MEDs) was reviewed. These data along with the new information recently published on testosterone replacement therapy (TRT) and cardiovascular (CV) risk were then used to evaluate current practices at the West Palm Beach VAMC for OPIAD monitoring and management and to modify and update the local Criteria for Use (CFU) for TRT.
Methods
Patient data from the West Palm Beach VAMC in Florida from January 2013 to December 2013 were reviewed to identify patients who had a total testosterone (TT) level measured. All patient appointments for evaluation and treatment by the clinical pharmacy specialist in pain management were reviewed for data collection. This retrospective review was approved by the scientific advisory committee as part of the facility’s ongoing performance improvement efforts as defined by VHA Handbook 1058.05 and did not require written patient consent.10
Several distinct TT level data were collected. The descriptive data included patient age; gender; type of treated pain; testosterone level(s) drawn, including TT level before opioid therapy, TT level before/during/after TRT, and current total testosterone level; total daily MED of opioid therapy; duration of chronic opioid therapy; symptoms of exhibited hypogonadism; TRT formulation, dose, and duration; TRT prescriber; symptom change (if any); and laboratory tests ordered for TRT monitoring (lipid profile, liver profile, complete blood count, LH/FSH, and prostate specific antigen [PSA] panel).5,11,12
Related: Combination Treatment Relieves Opioid-Induced Constipation
Daily MED of opioid therapy was calculated using the VA/DoD opioid conversion table for patients on oxycodone, hydromorphone, or hydrocodone.13 For those on the fentanyl patch or methadone, conversion factors of 1:2 (fentanyl [µg/h]:morphine [mg/d]) and 1:3 (methadone:morphine) were used to convert to the MED.14 For patients on the buprenorphine patch, the package insert was used to convert to the corresponding MED.15 Combination therapies used the applicable conversions to calculate the total daily MED.
Once the data were collected, descriptive statistics were used to analyze the data. In addition, 4 graphs were generated to review potential relationships. The correlation coefficient was calculated using the Alcula Online Statistics Calculator (http://www.alcula.com; Correlation Coefficient Calculator).
Results
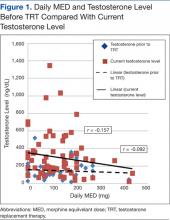
A total of 316 unique veteran patients were seen by the clinical pharmacy specialist in pain management from January 1, 2013, through December 31, 2013. Of these, 73 patients (23.1%) had at least 1 TT level drawn in 2013. Three patients with testosterone levels drawn (4.1%) were excluded from the data analysis for the following reasons: 1 patient did not have testosterone levels on file before receiving testosterone replacement from a non-VA source, 1 patient received opioids from a non-VA source (MED and duration of opioid therapy could not be calculated), and 1 patient inconsistently received opioids and MED used at the time of testosterone level draw. Per the local TRT CFU, a TT level > 350 ng/dL does not require treatment, whereas levels < 230 ng/dL (with symptoms) may require TRT, and < 200 ng/dL should be treated as hypogonadal (interpretation based on local laboratory’s reference range for TT).16 Of the 70 patients included in the analysis, 34 (48.6%) had a TT level < 230 ng/dL and would be considered eligible for TRT if they presented with symptoms of low testosterone. Of these 34 patients with a low testosterone level, 28 (40%) were being treated or had been treated with TRT (Figure 1).
The average age of the male patients with a testosterone level drawn was 58.3 years, which was not significantly different from the calculated median age of 60 years. No female patients had a testosterone level drawn. On average, the TT level was normal before starting opioids (reference range per local laboratory: 175-781 ng/dL). Once opioids were initiated, patients were treated for an average duration of 52.5 months (calculated through December 2013) with an average daily dose of 126.8 MED (Table). Fifty of the 70 patients (71.4%) with testosterone levels drawn in 2013 received TRT. The most common symptoms reported by patients related to low testosterone included ED, decreased libido, depression, chronic fatigue, generalized weakness, and hot flashes or night sweats.
The average TT level prior to TRT was 145.3, and the average testosterone level after initiation of or during treatment with TRT was 292.4, which is within the normal TT level range. Most patients receiving TRT were treated with testosterone cypionate injections, and this was also the formulation used for the longest periods, likely due to the local CFU. In addition to testosterone cypionate injections, patients were also treated with testosterone enanthate injections, testosterone patches, and testosterone gel.
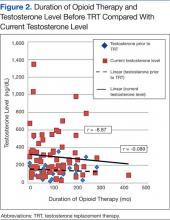
Figure 1 compares current testosterone level and testosterone level before TRT with total daily MEDs. Figure 2 compares current testosterone level and testosterone level before TRT with length of opioid therapy. The 2 figures use data from all patients included in the analysis and indicate a potential inverse relationship between the total daily MED and duration of therapy with the testosterone level, although none of the calculated correlation coefficients indicate that a strong relationship was present.
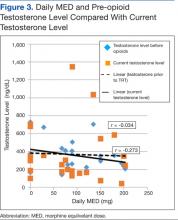
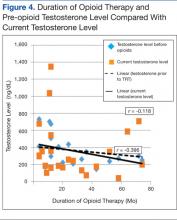
Figures 3 and 4 include data only for patients who had both a testosterone level collected before opioids (baseline testosterone level) and a current testosterone level. Figure 3 trends the data using total daily MED, and Figure 4 uses the duration of opioid therapy. The correlation for Figure 4 is slightly stronger; the strongest negative correlations were identified between total daily MED and testosterone level before opioid therapy (r = -0.273) and duration of opioid therapy and testosterone level prior to opioid therapy (r = -0.396). The trends indicate that most patients had a normal TT level before opioid treatment and that patients treated with higher MEDs and for longer durations of time were more likely to have lower total testosterone levels.
Discussion
Low testosterone levels can adversely affect patients’ quality of life (QOL) and add to patients’ medication burden with the initiation of TRT. Given new data analyzing the potential effects of TRT on CV event risk, the use of TRT should be carefully considered, as it may carry significant risks and may not be suitable for all patients.
In November 2013, a study was published regarding TRT and increased CV risk.17 This was a retrospective cohort study of men with low testosterone levels (< 300 ng/dL) who had undergone coronary angiography in the VA system between 2005 and 2011 (average age in testosterone group was 60.6 years). The results were significant for an absolute rate of events (all-cause mortality, myocardial infarction [MI], and ischemic stroke) of 19.9% in the no testosterone group and 25.7% in the TRT group, an absolute risk difference of 5.8% at 3 years after coronary angiography. Kaplan-Meier survival curves demonstrated that testosterone use was associated with increased risk of death, MI, and stroke. This result was unchanged when adjusted for the presence of coronary artery disease (CAD). In addition, no significant difference was found between the groups in terms of systolic blood pressure, low- density lipoprotein cholesterol level, or in the use of beta-blocker and statin medications. What is important to note is that in this cohort, 20% had a prior history of MI and heart failure, and more than 50% had confirmed obstructive CAD on angiography. In addition, as this was an observational study, confounding or bias may exist, and given the study population, generalizability may be limited to a veteran population.
Related: A Multidisciplinary Chronic Pain Management Clinic in an Indian Health Service Facility
Another retrospective cohort study assessed the risk of acute nonfatal MI following an initial TRT prescription in a large health care database (average age based on TRT prescription was 54.4 years).18 In men aged ≥ 65 years, a 2-fold increase in the risk of MI in the immediate 90 days after filling an initial TRT prescription declined to baseline after 91 to 180 days among those who did not refill their prescription. Younger men with a history of heart disease had a 2- to 3-fold increased risk of MI in the 90 days following initial TRT prescription. No excess risk was observed in the younger men without such a history. Again, this study has its limitations related to the retrospective design and use of a health care database as opposed to a randomized controlled trial.
In February 2014, a VA National Pharmacy Benefits Management (PBM) bulletin addressed 2 recent studies that had identified a possible risk of increased CV events in men receiving TRT. The bulletin noted that these studies had prompted the FDA to reassess the CV safety of TRT.19 The TRT CFU was updated by VISN 8 to ensure that the patients receive appropriate treatment and are monitored accordingly.
One of the major changes to the CFU was defining the reference ranges for TRT (interpretation based on a local laboratory’s reference range for total testosterone): serum TT < 200 ng/dL be “treated as hypogonadal, those with TT > 400 ng/dL be considered normal and those with TT 200-400 ng/dL be treated based on their clinical presentation if symptomatic; TT levels > 350 ng/dL do not require treatment, and levels below 230 ng/dL (with symptoms) may require testosterone replacement therapy.”16 Other important updates included revision of the exclusion criteria as well as highlighting special considerations related to TRT, including the use of free testosterone levels rather than TT levels in patients with suspected protein-binding issues, role in fertility treatments, limited use in patients on spironolactone therapy (due to spironolactone’s anti-androgen effects), and potential association with mood and behavior.16
As chronic opioid therapy is associated with OPIAD, the renewed interest in TRT and its potential AEs provides yet another reason to reconsider opioid therapy. This is especially valid when opioids are the potential cause of hypogonadism and the reaction is treating the AEs of opioids (as opposed to considering elimination of the causative agent) with a therapy that can potentially increase the risk for CV events so that opioids can be continued. Outside the potential CV risk with TRT, opioids carry the innate risk for substance abuse and addiction.
The Opioid Safety Initiative Requirements was released as a memorandum in April 2014 and is the VHA’s effort to “reduce harm from unsafe medications and/or excessive doses while adequately controlling pain in Veterans.”20 Although it does not discuss the risk of OPIAD, it does highlight the need to identify and mitigate high-risk patients as well as high-risk opioid regimens. All these factors, including the possibility of hypogonadism, should be considered before starting opioid therapy and at the time of opioid renewal, as it is known that opioid therapy is not without risks.
At the West Palm Beach VAMC, the primary care providers (PCPs) are responsible for the management of TRT, including the workup, renewal, and monitoring. The Chronic Nonmalignant Pain Management Clinic (CNMPMC) orders testosterone levels on patients who report symptoms of low testosterone, such as hot flashes, depression, and low energy level and refers them to their PCP as indicated. The authors believe that this is most appropriate for a number of reasons: (1) the CNMPMC is a consult service, and patients are not followed indefinitely; (2) patients should be fully evaluated for appropriateness of TRT (including assessment of CV risk) before starting therapy; and (3) the necessary monitoring parameters (laboratory testing, digital rectal exam, and osteoporosis screening) are not typically within the VA pain clinic provider’s scope of practice or expertise. A consideration for future practice would be to incorporate the use of a standardized questionnaire for OPIAD monitoring in patients receiving ≥ 100 mg of morphine daily (eg, the Aging Males’ Symptoms scale).21 It should, however, be at the forefront of the pain specialist’s and PCP’s minds that all patients on chronic opioid therapy or considering chronic opioid therapy should be counseled on the risk for OPIAD. If OPIAD is identified, the patient should be carefully considered for an opioid dose reduction as an initial management strategy.
Limitations
A limitation of this review is the lack of consistency or adequacy of serum testosterone sampling, noting that valid testosterone levels need to be drawn in the morning and not obtained during a time of acute illness. In addition, testosterone levels need to be drawn at an appropriate interval while on TRT (eg, at the midpoint between testosterone injections).16 Although the time of the sample collection is documented in the Computerized Patient Record System (CPRS), it is unknown whether the patient was acutely ill on the day of the sampling unless a progress note is entered, and it is difficult to determine whether the level timing was accurate based on the testosterone replacement formulation. Another limitation is that the average decline in serum testosterone levels with aging in men is 1% to 2% per year. A significant fraction of older men have levels below the lower limit of the normal range for healthy young men, so in older men it can be more difficult to determine whether low testosterone is related to chronic opioid use or to older age.5,16
As this was a retrospective review, additional limitations included the inability to measure subclinical OPIAD, and the data collection related to symptoms of hypogonadism was restricted by documentation in the CPRS progress notes. The lack of data for females does not contribute to the literature on OPIAD in women. Finally, as the total daily MED does not distinguish between short-acting and long-acting opioid therapy, no differences between the impacts of short-acting vs long- acting opioid therapy on risk for hypogonadism can be inferred. There is evidence to suggest that long-acting opioids are associated with a significantly higher risk for OPIAD compared with short-acting opioids, although the mechanism behind this is not well established.22,23
Conclusions
The average age of the patients on chronic opioid therapy with a testosterone level drawn in this cohort was 58.3 years, which is younger than originally anticipated. The median age of 60 years is not significantly different from the average age, indicating that outliers did not impact this calculation. On average, the TT level was normal before starting opioids. Once opioids were started, patients were treated for an average duration of 52.5 months with an average daily dose of 126.8 mg MED. In this veteran cohort, 48.6% of patients met the criteria for TRT based on TT level alone, which is within the reported prevalence range of opioid-induced hypogonadism already published.4,9 These results are in line with the original hypothesis that chronic opioid use can adversely impact testosterone levels and can have a poor effect on a patient’s QOL due to symptoms of low testosterone. In addition to TRT, possible and suggested (but not proven) treatment options for OPIAD include discontinuation of opioid therapy, opioid rotation, or conversion to buprenorphine.21 The approach used should account for multiple patient-specific factors and should be individualized.
Based on the data, there is a trend toward lower testosterone levels in veterans treated with higher MED and for longer periods with chronic opioids. Given recent data that infer that TRT carries increased CV risk as well as the VHA’s Opioid Safety Initiative, it is imperative that providers closely evaluate the appropriateness of starting TRT and/or continuing chronic opioid therapy. All patients generally should have failed non- opioid management prior to opioid therapy for chronic nonmalignant pain, and this should be documented accordingly. It is also crucial to have the “opioid talk” with patients from time to time and discuss the risks vs benefits, the potential for addiction, overdose, dependence, tolerance, constipation, and OPIAD so patients can continue to be an active and informed participants in their care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Unintentional drug poisoning in the United States, 2010. Atlanta, GA: Centers for Disease Control and Prevention Website. http://www.cdc.gov /HomeandRecreationalSafety/pdf/poison-issue-brief .pdf. Published July 2010. Accessed August 28, 2015.
2. American Academy of Family Physicians. Using opioids in the management of chronic pain patients: challenges and future options. University of Kentucky Medical Center Website. http://www .mc.uky.edu/equip-4-pcps/documents/CRx%20Literature/Opioids%20for%20chronic%20pain.pdf. Published 2010. Accessed August 28, 2015.
3. Duarte RV, Raphael JH, Labib M, Southall JL, Ashford RL. Prevalence and influence of diagnostic criteria in the assessment of hypogonadism in intrathecal opioid therapy patients. Pain Physician. 2013;16(1):9-14.
4. Smith HS, Elliott JA. Opioid-induced androgen deficiency (OPIAD). Pain Physician. 2012;15(suppl 3):ES145-ES156.
5. De Maddalena C, Bellini M, Berra M, Meriggiola MC, Aloisi AM. Opioid-induced hypogonadism: why and how to treat it. Pain Physician. 2012;15(suppl 3):ES111-ES118.
6. Bhasin S, Cunningham GR, Hayes FJ, et al; VM Endocrine Society Task Force. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
7. Petak SM, Nankin HR, Spark RF, Swerdloff RS, Rodriguez-Rigau LJ; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients–2002 update. Endocr Pract. 2002;8(6):440-456.
8. Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl. 2009;30(1):1-9.
9. Reddy RG, Aung T, Karavitaki N, Wass JA. Opioid induced hypogonadism. BMJ. 2010;341:c4462.
10. U.S. Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1058.05: VHA operations activities that may constitute research. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications /ViewPublication.asp?pub_ID=2456. Published October 28, 2011. Accessed August 28, 2015.
11. AndroGel [package insert]. North Chicago, IL: AbbVie Inc; 2013.
12. Axiron [package insert]. Indianapolis, IL: Lilly USA, LLC; 2011.
13. U.S. Department of Veterans Affairs. Opioid therapy for chronic pain pocket guide. U.S. Department of Veterans Affairs. http://www.healthquality .va.gov/guidelines/pain/cot/opioidpocketguide23may2013v1.pdf. Published May 2013 Accessed August 28, 2015.
14. McPherson ML. Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing. Bethesda, MD: American Society of Health-System Pharmacists; 2009.
15. Butrans [package insert]. Stamford, CT: Purdue Pharma LP; 2014.
16. Testosterone Replacement Therapy Criteria for Use. VISN 8: VISN Pharmacist Executives, Veterans Health Administration, Department of Veterans Affairs; 2014. [Internal document.]
17. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
18. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805.
19. U.S. Department of Veterans Affairs. Testosterone products and cardiovascular safety. U.S. Department of Veterans Affairs Website. http://www.pbm .va.gov/PBM/vacenterformedicationsafety /nationalpbmbulletin/Testosterone_Products_and _Cardiovascular_Safety_NATIONAL_PBM _BULLETIN_02.pdf. Published February 7, 2014. Accessed August 28, 2015.
20. U.S. Department of Veterans Affairs Veterans Health Administration (VHA) Pharmacy Benefits Management Services (PBM), Medical Advisory Panel (MAP) and Center for Medication Safety (VA MEDSAFE). Memorandum: Opioid Safety Initiative Requirements. U.S. Department of Veterans Affairs Website. http://www.veterans.senate.gov/imo /media/doc/VA%20Testimony%20-%20April%2030%20SVAC%20Overmedication%20hearing.pdf. Published April 30, 2014. Accessed August 28, 2015.
21. Brennan MJ. The effect of opioid therapy on endocrine function. Am J Med. 2013;126(3)(suppl 1):S12-S18.
22. Rubinstein AL, Carpenter DM, Minkoff JR. Hypogonadism in men with chronic pain linked to the use of long-acting rather than short-acting opioids. Clin J Pain. 2013;29(10):840-845.
23. Rubinstein A, Carpenter DM. Elucidating risk factors for androgen deficiency associated with daily opioid use. Am J Med. 2014;127(12):1195-1201.
According to the CDC, the medical use of opioid painkillers has increased at least 10-fold during the past 20 years, “because of a movement toward more aggressive management of pain.”1 Although opioid therapy is generally considered effective for the treatment of pain, long-term use (both orally and intrathecally) is associated with adverse effects (AEs) such as constipation, fatigue, nausea, sleep disturbances, depression, sexual dysfunction, and hypogonadism.2,3Opioid-induced androgen deficiency (OPIAD), as defined by Smith and Elliot, is a clinical syndrome characterized by inappropriately low concentrations of gonadotropins (specifically, follicle-stimulating hormone [FSH] and luteinizing hormone [LH]), which leads to inadequate production of sex hormones, including estradiol and testosterone.4
Related: Testosterone Replacement Therapy: Playing Catch-up With Patients
The mechanism behind this phenomenon is initiated by either endogenous or exogenous opioids acting on opioid receptors in the hypothalamus, which causes a decrease in the release of gonadotropin- releasing hormone (GnRH). This decrease in GnRH causes a reduction in the release of LH and FSH from the pituitary gland as well as testosterone or estradiol from the gonads.4,5 Various guidelines report different cutoffs for the lower limit of normal total testosterone: The Endocrine Society recommends 300 ng/dL, the American Association of Clinical Endocrinologists suggests 200 ng/dL, and various other organizations suggest 230 ng/dL.6-8 Hypotestosteronism can result in patients presenting with a broad spectrum of clinical symptoms, including reduced libido, erectile dysfunction (ED), fatigue, hot flashes, depression, anemia, decreased muscle mass, weight gain, and osteopenia or osteoporosis.4 Women with low testosterone levels can experience irregular menstrual periods, oligomenorrhea, or amenorrhea.9 Opioid-induced androgen deficiency often goes unrecognized and untreated. The reported prevalence of opioid-induced hypogonadism ranges from 21% to 86%.4,9 Given the growing number of patients on chronic opioid therapy, OPIAD warrants further investigation to identify the prevalence in the veteran population to appropriately monitor and manage this deficiency.
The objective of this retrospective review was to identify the presence of secondary hypogonadism in chronic opioid users among a cohort of veterans receiving chronic opioids for nonmalignant pain. In addition to identifying the presence of secondary hypogonadism, the relationship between testosterone concentrations and total daily morphine equivalent doses (MEDs) was reviewed. These data along with the new information recently published on testosterone replacement therapy (TRT) and cardiovascular (CV) risk were then used to evaluate current practices at the West Palm Beach VAMC for OPIAD monitoring and management and to modify and update the local Criteria for Use (CFU) for TRT.
Methods
Patient data from the West Palm Beach VAMC in Florida from January 2013 to December 2013 were reviewed to identify patients who had a total testosterone (TT) level measured. All patient appointments for evaluation and treatment by the clinical pharmacy specialist in pain management were reviewed for data collection. This retrospective review was approved by the scientific advisory committee as part of the facility’s ongoing performance improvement efforts as defined by VHA Handbook 1058.05 and did not require written patient consent.10
Several distinct TT level data were collected. The descriptive data included patient age; gender; type of treated pain; testosterone level(s) drawn, including TT level before opioid therapy, TT level before/during/after TRT, and current total testosterone level; total daily MED of opioid therapy; duration of chronic opioid therapy; symptoms of exhibited hypogonadism; TRT formulation, dose, and duration; TRT prescriber; symptom change (if any); and laboratory tests ordered for TRT monitoring (lipid profile, liver profile, complete blood count, LH/FSH, and prostate specific antigen [PSA] panel).5,11,12
Related: Combination Treatment Relieves Opioid-Induced Constipation
Daily MED of opioid therapy was calculated using the VA/DoD opioid conversion table for patients on oxycodone, hydromorphone, or hydrocodone.13 For those on the fentanyl patch or methadone, conversion factors of 1:2 (fentanyl [µg/h]:morphine [mg/d]) and 1:3 (methadone:morphine) were used to convert to the MED.14 For patients on the buprenorphine patch, the package insert was used to convert to the corresponding MED.15 Combination therapies used the applicable conversions to calculate the total daily MED.
Once the data were collected, descriptive statistics were used to analyze the data. In addition, 4 graphs were generated to review potential relationships. The correlation coefficient was calculated using the Alcula Online Statistics Calculator (http://www.alcula.com; Correlation Coefficient Calculator).
Results
A total of 316 unique veteran patients were seen by the clinical pharmacy specialist in pain management from January 1, 2013, through December 31, 2013. Of these, 73 patients (23.1%) had at least 1 TT level drawn in 2013. Three patients with testosterone levels drawn (4.1%) were excluded from the data analysis for the following reasons: 1 patient did not have testosterone levels on file before receiving testosterone replacement from a non-VA source, 1 patient received opioids from a non-VA source (MED and duration of opioid therapy could not be calculated), and 1 patient inconsistently received opioids and MED used at the time of testosterone level draw. Per the local TRT CFU, a TT level > 350 ng/dL does not require treatment, whereas levels < 230 ng/dL (with symptoms) may require TRT, and < 200 ng/dL should be treated as hypogonadal (interpretation based on local laboratory’s reference range for TT).16 Of the 70 patients included in the analysis, 34 (48.6%) had a TT level < 230 ng/dL and would be considered eligible for TRT if they presented with symptoms of low testosterone. Of these 34 patients with a low testosterone level, 28 (40%) were being treated or had been treated with TRT (Figure 1).
The average age of the male patients with a testosterone level drawn was 58.3 years, which was not significantly different from the calculated median age of 60 years. No female patients had a testosterone level drawn. On average, the TT level was normal before starting opioids (reference range per local laboratory: 175-781 ng/dL). Once opioids were initiated, patients were treated for an average duration of 52.5 months (calculated through December 2013) with an average daily dose of 126.8 MED (Table). Fifty of the 70 patients (71.4%) with testosterone levels drawn in 2013 received TRT. The most common symptoms reported by patients related to low testosterone included ED, decreased libido, depression, chronic fatigue, generalized weakness, and hot flashes or night sweats.
The average TT level prior to TRT was 145.3, and the average testosterone level after initiation of or during treatment with TRT was 292.4, which is within the normal TT level range. Most patients receiving TRT were treated with testosterone cypionate injections, and this was also the formulation used for the longest periods, likely due to the local CFU. In addition to testosterone cypionate injections, patients were also treated with testosterone enanthate injections, testosterone patches, and testosterone gel.
Figure 1 compares current testosterone level and testosterone level before TRT with total daily MEDs. Figure 2 compares current testosterone level and testosterone level before TRT with length of opioid therapy. The 2 figures use data from all patients included in the analysis and indicate a potential inverse relationship between the total daily MED and duration of therapy with the testosterone level, although none of the calculated correlation coefficients indicate that a strong relationship was present.
Figures 3 and 4 include data only for patients who had both a testosterone level collected before opioids (baseline testosterone level) and a current testosterone level. Figure 3 trends the data using total daily MED, and Figure 4 uses the duration of opioid therapy. The correlation for Figure 4 is slightly stronger; the strongest negative correlations were identified between total daily MED and testosterone level before opioid therapy (r = -0.273) and duration of opioid therapy and testosterone level prior to opioid therapy (r = -0.396). The trends indicate that most patients had a normal TT level before opioid treatment and that patients treated with higher MEDs and for longer durations of time were more likely to have lower total testosterone levels.
Discussion
Low testosterone levels can adversely affect patients’ quality of life (QOL) and add to patients’ medication burden with the initiation of TRT. Given new data analyzing the potential effects of TRT on CV event risk, the use of TRT should be carefully considered, as it may carry significant risks and may not be suitable for all patients.
In November 2013, a study was published regarding TRT and increased CV risk.17 This was a retrospective cohort study of men with low testosterone levels (< 300 ng/dL) who had undergone coronary angiography in the VA system between 2005 and 2011 (average age in testosterone group was 60.6 years). The results were significant for an absolute rate of events (all-cause mortality, myocardial infarction [MI], and ischemic stroke) of 19.9% in the no testosterone group and 25.7% in the TRT group, an absolute risk difference of 5.8% at 3 years after coronary angiography. Kaplan-Meier survival curves demonstrated that testosterone use was associated with increased risk of death, MI, and stroke. This result was unchanged when adjusted for the presence of coronary artery disease (CAD). In addition, no significant difference was found between the groups in terms of systolic blood pressure, low- density lipoprotein cholesterol level, or in the use of beta-blocker and statin medications. What is important to note is that in this cohort, 20% had a prior history of MI and heart failure, and more than 50% had confirmed obstructive CAD on angiography. In addition, as this was an observational study, confounding or bias may exist, and given the study population, generalizability may be limited to a veteran population.
Related: A Multidisciplinary Chronic Pain Management Clinic in an Indian Health Service Facility
Another retrospective cohort study assessed the risk of acute nonfatal MI following an initial TRT prescription in a large health care database (average age based on TRT prescription was 54.4 years).18 In men aged ≥ 65 years, a 2-fold increase in the risk of MI in the immediate 90 days after filling an initial TRT prescription declined to baseline after 91 to 180 days among those who did not refill their prescription. Younger men with a history of heart disease had a 2- to 3-fold increased risk of MI in the 90 days following initial TRT prescription. No excess risk was observed in the younger men without such a history. Again, this study has its limitations related to the retrospective design and use of a health care database as opposed to a randomized controlled trial.
In February 2014, a VA National Pharmacy Benefits Management (PBM) bulletin addressed 2 recent studies that had identified a possible risk of increased CV events in men receiving TRT. The bulletin noted that these studies had prompted the FDA to reassess the CV safety of TRT.19 The TRT CFU was updated by VISN 8 to ensure that the patients receive appropriate treatment and are monitored accordingly.
One of the major changes to the CFU was defining the reference ranges for TRT (interpretation based on a local laboratory’s reference range for total testosterone): serum TT < 200 ng/dL be “treated as hypogonadal, those with TT > 400 ng/dL be considered normal and those with TT 200-400 ng/dL be treated based on their clinical presentation if symptomatic; TT levels > 350 ng/dL do not require treatment, and levels below 230 ng/dL (with symptoms) may require testosterone replacement therapy.”16 Other important updates included revision of the exclusion criteria as well as highlighting special considerations related to TRT, including the use of free testosterone levels rather than TT levels in patients with suspected protein-binding issues, role in fertility treatments, limited use in patients on spironolactone therapy (due to spironolactone’s anti-androgen effects), and potential association with mood and behavior.16
As chronic opioid therapy is associated with OPIAD, the renewed interest in TRT and its potential AEs provides yet another reason to reconsider opioid therapy. This is especially valid when opioids are the potential cause of hypogonadism and the reaction is treating the AEs of opioids (as opposed to considering elimination of the causative agent) with a therapy that can potentially increase the risk for CV events so that opioids can be continued. Outside the potential CV risk with TRT, opioids carry the innate risk for substance abuse and addiction.
The Opioid Safety Initiative Requirements was released as a memorandum in April 2014 and is the VHA’s effort to “reduce harm from unsafe medications and/or excessive doses while adequately controlling pain in Veterans.”20 Although it does not discuss the risk of OPIAD, it does highlight the need to identify and mitigate high-risk patients as well as high-risk opioid regimens. All these factors, including the possibility of hypogonadism, should be considered before starting opioid therapy and at the time of opioid renewal, as it is known that opioid therapy is not without risks.
At the West Palm Beach VAMC, the primary care providers (PCPs) are responsible for the management of TRT, including the workup, renewal, and monitoring. The Chronic Nonmalignant Pain Management Clinic (CNMPMC) orders testosterone levels on patients who report symptoms of low testosterone, such as hot flashes, depression, and low energy level and refers them to their PCP as indicated. The authors believe that this is most appropriate for a number of reasons: (1) the CNMPMC is a consult service, and patients are not followed indefinitely; (2) patients should be fully evaluated for appropriateness of TRT (including assessment of CV risk) before starting therapy; and (3) the necessary monitoring parameters (laboratory testing, digital rectal exam, and osteoporosis screening) are not typically within the VA pain clinic provider’s scope of practice or expertise. A consideration for future practice would be to incorporate the use of a standardized questionnaire for OPIAD monitoring in patients receiving ≥ 100 mg of morphine daily (eg, the Aging Males’ Symptoms scale).21 It should, however, be at the forefront of the pain specialist’s and PCP’s minds that all patients on chronic opioid therapy or considering chronic opioid therapy should be counseled on the risk for OPIAD. If OPIAD is identified, the patient should be carefully considered for an opioid dose reduction as an initial management strategy.
Limitations
A limitation of this review is the lack of consistency or adequacy of serum testosterone sampling, noting that valid testosterone levels need to be drawn in the morning and not obtained during a time of acute illness. In addition, testosterone levels need to be drawn at an appropriate interval while on TRT (eg, at the midpoint between testosterone injections).16 Although the time of the sample collection is documented in the Computerized Patient Record System (CPRS), it is unknown whether the patient was acutely ill on the day of the sampling unless a progress note is entered, and it is difficult to determine whether the level timing was accurate based on the testosterone replacement formulation. Another limitation is that the average decline in serum testosterone levels with aging in men is 1% to 2% per year. A significant fraction of older men have levels below the lower limit of the normal range for healthy young men, so in older men it can be more difficult to determine whether low testosterone is related to chronic opioid use or to older age.5,16
As this was a retrospective review, additional limitations included the inability to measure subclinical OPIAD, and the data collection related to symptoms of hypogonadism was restricted by documentation in the CPRS progress notes. The lack of data for females does not contribute to the literature on OPIAD in women. Finally, as the total daily MED does not distinguish between short-acting and long-acting opioid therapy, no differences between the impacts of short-acting vs long- acting opioid therapy on risk for hypogonadism can be inferred. There is evidence to suggest that long-acting opioids are associated with a significantly higher risk for OPIAD compared with short-acting opioids, although the mechanism behind this is not well established.22,23
Conclusions
The average age of the patients on chronic opioid therapy with a testosterone level drawn in this cohort was 58.3 years, which is younger than originally anticipated. The median age of 60 years is not significantly different from the average age, indicating that outliers did not impact this calculation. On average, the TT level was normal before starting opioids. Once opioids were started, patients were treated for an average duration of 52.5 months with an average daily dose of 126.8 mg MED. In this veteran cohort, 48.6% of patients met the criteria for TRT based on TT level alone, which is within the reported prevalence range of opioid-induced hypogonadism already published.4,9 These results are in line with the original hypothesis that chronic opioid use can adversely impact testosterone levels and can have a poor effect on a patient’s QOL due to symptoms of low testosterone. In addition to TRT, possible and suggested (but not proven) treatment options for OPIAD include discontinuation of opioid therapy, opioid rotation, or conversion to buprenorphine.21 The approach used should account for multiple patient-specific factors and should be individualized.
Based on the data, there is a trend toward lower testosterone levels in veterans treated with higher MED and for longer periods with chronic opioids. Given recent data that infer that TRT carries increased CV risk as well as the VHA’s Opioid Safety Initiative, it is imperative that providers closely evaluate the appropriateness of starting TRT and/or continuing chronic opioid therapy. All patients generally should have failed non- opioid management prior to opioid therapy for chronic nonmalignant pain, and this should be documented accordingly. It is also crucial to have the “opioid talk” with patients from time to time and discuss the risks vs benefits, the potential for addiction, overdose, dependence, tolerance, constipation, and OPIAD so patients can continue to be an active and informed participants in their care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
According to the CDC, the medical use of opioid painkillers has increased at least 10-fold during the past 20 years, “because of a movement toward more aggressive management of pain.”1 Although opioid therapy is generally considered effective for the treatment of pain, long-term use (both orally and intrathecally) is associated with adverse effects (AEs) such as constipation, fatigue, nausea, sleep disturbances, depression, sexual dysfunction, and hypogonadism.2,3Opioid-induced androgen deficiency (OPIAD), as defined by Smith and Elliot, is a clinical syndrome characterized by inappropriately low concentrations of gonadotropins (specifically, follicle-stimulating hormone [FSH] and luteinizing hormone [LH]), which leads to inadequate production of sex hormones, including estradiol and testosterone.4
Related: Testosterone Replacement Therapy: Playing Catch-up With Patients
The mechanism behind this phenomenon is initiated by either endogenous or exogenous opioids acting on opioid receptors in the hypothalamus, which causes a decrease in the release of gonadotropin- releasing hormone (GnRH). This decrease in GnRH causes a reduction in the release of LH and FSH from the pituitary gland as well as testosterone or estradiol from the gonads.4,5 Various guidelines report different cutoffs for the lower limit of normal total testosterone: The Endocrine Society recommends 300 ng/dL, the American Association of Clinical Endocrinologists suggests 200 ng/dL, and various other organizations suggest 230 ng/dL.6-8 Hypotestosteronism can result in patients presenting with a broad spectrum of clinical symptoms, including reduced libido, erectile dysfunction (ED), fatigue, hot flashes, depression, anemia, decreased muscle mass, weight gain, and osteopenia or osteoporosis.4 Women with low testosterone levels can experience irregular menstrual periods, oligomenorrhea, or amenorrhea.9 Opioid-induced androgen deficiency often goes unrecognized and untreated. The reported prevalence of opioid-induced hypogonadism ranges from 21% to 86%.4,9 Given the growing number of patients on chronic opioid therapy, OPIAD warrants further investigation to identify the prevalence in the veteran population to appropriately monitor and manage this deficiency.
The objective of this retrospective review was to identify the presence of secondary hypogonadism in chronic opioid users among a cohort of veterans receiving chronic opioids for nonmalignant pain. In addition to identifying the presence of secondary hypogonadism, the relationship between testosterone concentrations and total daily morphine equivalent doses (MEDs) was reviewed. These data along with the new information recently published on testosterone replacement therapy (TRT) and cardiovascular (CV) risk were then used to evaluate current practices at the West Palm Beach VAMC for OPIAD monitoring and management and to modify and update the local Criteria for Use (CFU) for TRT.
Methods
Patient data from the West Palm Beach VAMC in Florida from January 2013 to December 2013 were reviewed to identify patients who had a total testosterone (TT) level measured. All patient appointments for evaluation and treatment by the clinical pharmacy specialist in pain management were reviewed for data collection. This retrospective review was approved by the scientific advisory committee as part of the facility’s ongoing performance improvement efforts as defined by VHA Handbook 1058.05 and did not require written patient consent.10
Several distinct TT level data were collected. The descriptive data included patient age; gender; type of treated pain; testosterone level(s) drawn, including TT level before opioid therapy, TT level before/during/after TRT, and current total testosterone level; total daily MED of opioid therapy; duration of chronic opioid therapy; symptoms of exhibited hypogonadism; TRT formulation, dose, and duration; TRT prescriber; symptom change (if any); and laboratory tests ordered for TRT monitoring (lipid profile, liver profile, complete blood count, LH/FSH, and prostate specific antigen [PSA] panel).5,11,12
Related: Combination Treatment Relieves Opioid-Induced Constipation
Daily MED of opioid therapy was calculated using the VA/DoD opioid conversion table for patients on oxycodone, hydromorphone, or hydrocodone.13 For those on the fentanyl patch or methadone, conversion factors of 1:2 (fentanyl [µg/h]:morphine [mg/d]) and 1:3 (methadone:morphine) were used to convert to the MED.14 For patients on the buprenorphine patch, the package insert was used to convert to the corresponding MED.15 Combination therapies used the applicable conversions to calculate the total daily MED.
Once the data were collected, descriptive statistics were used to analyze the data. In addition, 4 graphs were generated to review potential relationships. The correlation coefficient was calculated using the Alcula Online Statistics Calculator (http://www.alcula.com; Correlation Coefficient Calculator).
Results
A total of 316 unique veteran patients were seen by the clinical pharmacy specialist in pain management from January 1, 2013, through December 31, 2013. Of these, 73 patients (23.1%) had at least 1 TT level drawn in 2013. Three patients with testosterone levels drawn (4.1%) were excluded from the data analysis for the following reasons: 1 patient did not have testosterone levels on file before receiving testosterone replacement from a non-VA source, 1 patient received opioids from a non-VA source (MED and duration of opioid therapy could not be calculated), and 1 patient inconsistently received opioids and MED used at the time of testosterone level draw. Per the local TRT CFU, a TT level > 350 ng/dL does not require treatment, whereas levels < 230 ng/dL (with symptoms) may require TRT, and < 200 ng/dL should be treated as hypogonadal (interpretation based on local laboratory’s reference range for TT).16 Of the 70 patients included in the analysis, 34 (48.6%) had a TT level < 230 ng/dL and would be considered eligible for TRT if they presented with symptoms of low testosterone. Of these 34 patients with a low testosterone level, 28 (40%) were being treated or had been treated with TRT (Figure 1).
The average age of the male patients with a testosterone level drawn was 58.3 years, which was not significantly different from the calculated median age of 60 years. No female patients had a testosterone level drawn. On average, the TT level was normal before starting opioids (reference range per local laboratory: 175-781 ng/dL). Once opioids were initiated, patients were treated for an average duration of 52.5 months (calculated through December 2013) with an average daily dose of 126.8 MED (Table). Fifty of the 70 patients (71.4%) with testosterone levels drawn in 2013 received TRT. The most common symptoms reported by patients related to low testosterone included ED, decreased libido, depression, chronic fatigue, generalized weakness, and hot flashes or night sweats.
The average TT level prior to TRT was 145.3, and the average testosterone level after initiation of or during treatment with TRT was 292.4, which is within the normal TT level range. Most patients receiving TRT were treated with testosterone cypionate injections, and this was also the formulation used for the longest periods, likely due to the local CFU. In addition to testosterone cypionate injections, patients were also treated with testosterone enanthate injections, testosterone patches, and testosterone gel.
Figure 1 compares current testosterone level and testosterone level before TRT with total daily MEDs. Figure 2 compares current testosterone level and testosterone level before TRT with length of opioid therapy. The 2 figures use data from all patients included in the analysis and indicate a potential inverse relationship between the total daily MED and duration of therapy with the testosterone level, although none of the calculated correlation coefficients indicate that a strong relationship was present.
Figures 3 and 4 include data only for patients who had both a testosterone level collected before opioids (baseline testosterone level) and a current testosterone level. Figure 3 trends the data using total daily MED, and Figure 4 uses the duration of opioid therapy. The correlation for Figure 4 is slightly stronger; the strongest negative correlations were identified between total daily MED and testosterone level before opioid therapy (r = -0.273) and duration of opioid therapy and testosterone level prior to opioid therapy (r = -0.396). The trends indicate that most patients had a normal TT level before opioid treatment and that patients treated with higher MEDs and for longer durations of time were more likely to have lower total testosterone levels.
Discussion
Low testosterone levels can adversely affect patients’ quality of life (QOL) and add to patients’ medication burden with the initiation of TRT. Given new data analyzing the potential effects of TRT on CV event risk, the use of TRT should be carefully considered, as it may carry significant risks and may not be suitable for all patients.
In November 2013, a study was published regarding TRT and increased CV risk.17 This was a retrospective cohort study of men with low testosterone levels (< 300 ng/dL) who had undergone coronary angiography in the VA system between 2005 and 2011 (average age in testosterone group was 60.6 years). The results were significant for an absolute rate of events (all-cause mortality, myocardial infarction [MI], and ischemic stroke) of 19.9% in the no testosterone group and 25.7% in the TRT group, an absolute risk difference of 5.8% at 3 years after coronary angiography. Kaplan-Meier survival curves demonstrated that testosterone use was associated with increased risk of death, MI, and stroke. This result was unchanged when adjusted for the presence of coronary artery disease (CAD). In addition, no significant difference was found between the groups in terms of systolic blood pressure, low- density lipoprotein cholesterol level, or in the use of beta-blocker and statin medications. What is important to note is that in this cohort, 20% had a prior history of MI and heart failure, and more than 50% had confirmed obstructive CAD on angiography. In addition, as this was an observational study, confounding or bias may exist, and given the study population, generalizability may be limited to a veteran population.
Related: A Multidisciplinary Chronic Pain Management Clinic in an Indian Health Service Facility
Another retrospective cohort study assessed the risk of acute nonfatal MI following an initial TRT prescription in a large health care database (average age based on TRT prescription was 54.4 years).18 In men aged ≥ 65 years, a 2-fold increase in the risk of MI in the immediate 90 days after filling an initial TRT prescription declined to baseline after 91 to 180 days among those who did not refill their prescription. Younger men with a history of heart disease had a 2- to 3-fold increased risk of MI in the 90 days following initial TRT prescription. No excess risk was observed in the younger men without such a history. Again, this study has its limitations related to the retrospective design and use of a health care database as opposed to a randomized controlled trial.
In February 2014, a VA National Pharmacy Benefits Management (PBM) bulletin addressed 2 recent studies that had identified a possible risk of increased CV events in men receiving TRT. The bulletin noted that these studies had prompted the FDA to reassess the CV safety of TRT.19 The TRT CFU was updated by VISN 8 to ensure that the patients receive appropriate treatment and are monitored accordingly.
One of the major changes to the CFU was defining the reference ranges for TRT (interpretation based on a local laboratory’s reference range for total testosterone): serum TT < 200 ng/dL be “treated as hypogonadal, those with TT > 400 ng/dL be considered normal and those with TT 200-400 ng/dL be treated based on their clinical presentation if symptomatic; TT levels > 350 ng/dL do not require treatment, and levels below 230 ng/dL (with symptoms) may require testosterone replacement therapy.”16 Other important updates included revision of the exclusion criteria as well as highlighting special considerations related to TRT, including the use of free testosterone levels rather than TT levels in patients with suspected protein-binding issues, role in fertility treatments, limited use in patients on spironolactone therapy (due to spironolactone’s anti-androgen effects), and potential association with mood and behavior.16
As chronic opioid therapy is associated with OPIAD, the renewed interest in TRT and its potential AEs provides yet another reason to reconsider opioid therapy. This is especially valid when opioids are the potential cause of hypogonadism and the reaction is treating the AEs of opioids (as opposed to considering elimination of the causative agent) with a therapy that can potentially increase the risk for CV events so that opioids can be continued. Outside the potential CV risk with TRT, opioids carry the innate risk for substance abuse and addiction.
The Opioid Safety Initiative Requirements was released as a memorandum in April 2014 and is the VHA’s effort to “reduce harm from unsafe medications and/or excessive doses while adequately controlling pain in Veterans.”20 Although it does not discuss the risk of OPIAD, it does highlight the need to identify and mitigate high-risk patients as well as high-risk opioid regimens. All these factors, including the possibility of hypogonadism, should be considered before starting opioid therapy and at the time of opioid renewal, as it is known that opioid therapy is not without risks.
At the West Palm Beach VAMC, the primary care providers (PCPs) are responsible for the management of TRT, including the workup, renewal, and monitoring. The Chronic Nonmalignant Pain Management Clinic (CNMPMC) orders testosterone levels on patients who report symptoms of low testosterone, such as hot flashes, depression, and low energy level and refers them to their PCP as indicated. The authors believe that this is most appropriate for a number of reasons: (1) the CNMPMC is a consult service, and patients are not followed indefinitely; (2) patients should be fully evaluated for appropriateness of TRT (including assessment of CV risk) before starting therapy; and (3) the necessary monitoring parameters (laboratory testing, digital rectal exam, and osteoporosis screening) are not typically within the VA pain clinic provider’s scope of practice or expertise. A consideration for future practice would be to incorporate the use of a standardized questionnaire for OPIAD monitoring in patients receiving ≥ 100 mg of morphine daily (eg, the Aging Males’ Symptoms scale).21 It should, however, be at the forefront of the pain specialist’s and PCP’s minds that all patients on chronic opioid therapy or considering chronic opioid therapy should be counseled on the risk for OPIAD. If OPIAD is identified, the patient should be carefully considered for an opioid dose reduction as an initial management strategy.
Limitations
A limitation of this review is the lack of consistency or adequacy of serum testosterone sampling, noting that valid testosterone levels need to be drawn in the morning and not obtained during a time of acute illness. In addition, testosterone levels need to be drawn at an appropriate interval while on TRT (eg, at the midpoint between testosterone injections).16 Although the time of the sample collection is documented in the Computerized Patient Record System (CPRS), it is unknown whether the patient was acutely ill on the day of the sampling unless a progress note is entered, and it is difficult to determine whether the level timing was accurate based on the testosterone replacement formulation. Another limitation is that the average decline in serum testosterone levels with aging in men is 1% to 2% per year. A significant fraction of older men have levels below the lower limit of the normal range for healthy young men, so in older men it can be more difficult to determine whether low testosterone is related to chronic opioid use or to older age.5,16
As this was a retrospective review, additional limitations included the inability to measure subclinical OPIAD, and the data collection related to symptoms of hypogonadism was restricted by documentation in the CPRS progress notes. The lack of data for females does not contribute to the literature on OPIAD in women. Finally, as the total daily MED does not distinguish between short-acting and long-acting opioid therapy, no differences between the impacts of short-acting vs long- acting opioid therapy on risk for hypogonadism can be inferred. There is evidence to suggest that long-acting opioids are associated with a significantly higher risk for OPIAD compared with short-acting opioids, although the mechanism behind this is not well established.22,23
Conclusions
The average age of the patients on chronic opioid therapy with a testosterone level drawn in this cohort was 58.3 years, which is younger than originally anticipated. The median age of 60 years is not significantly different from the average age, indicating that outliers did not impact this calculation. On average, the TT level was normal before starting opioids. Once opioids were started, patients were treated for an average duration of 52.5 months with an average daily dose of 126.8 mg MED. In this veteran cohort, 48.6% of patients met the criteria for TRT based on TT level alone, which is within the reported prevalence range of opioid-induced hypogonadism already published.4,9 These results are in line with the original hypothesis that chronic opioid use can adversely impact testosterone levels and can have a poor effect on a patient’s QOL due to symptoms of low testosterone. In addition to TRT, possible and suggested (but not proven) treatment options for OPIAD include discontinuation of opioid therapy, opioid rotation, or conversion to buprenorphine.21 The approach used should account for multiple patient-specific factors and should be individualized.
Based on the data, there is a trend toward lower testosterone levels in veterans treated with higher MED and for longer periods with chronic opioids. Given recent data that infer that TRT carries increased CV risk as well as the VHA’s Opioid Safety Initiative, it is imperative that providers closely evaluate the appropriateness of starting TRT and/or continuing chronic opioid therapy. All patients generally should have failed non- opioid management prior to opioid therapy for chronic nonmalignant pain, and this should be documented accordingly. It is also crucial to have the “opioid talk” with patients from time to time and discuss the risks vs benefits, the potential for addiction, overdose, dependence, tolerance, constipation, and OPIAD so patients can continue to be an active and informed participants in their care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Unintentional drug poisoning in the United States, 2010. Atlanta, GA: Centers for Disease Control and Prevention Website. http://www.cdc.gov /HomeandRecreationalSafety/pdf/poison-issue-brief .pdf. Published July 2010. Accessed August 28, 2015.
2. American Academy of Family Physicians. Using opioids in the management of chronic pain patients: challenges and future options. University of Kentucky Medical Center Website. http://www .mc.uky.edu/equip-4-pcps/documents/CRx%20Literature/Opioids%20for%20chronic%20pain.pdf. Published 2010. Accessed August 28, 2015.
3. Duarte RV, Raphael JH, Labib M, Southall JL, Ashford RL. Prevalence and influence of diagnostic criteria in the assessment of hypogonadism in intrathecal opioid therapy patients. Pain Physician. 2013;16(1):9-14.
4. Smith HS, Elliott JA. Opioid-induced androgen deficiency (OPIAD). Pain Physician. 2012;15(suppl 3):ES145-ES156.
5. De Maddalena C, Bellini M, Berra M, Meriggiola MC, Aloisi AM. Opioid-induced hypogonadism: why and how to treat it. Pain Physician. 2012;15(suppl 3):ES111-ES118.
6. Bhasin S, Cunningham GR, Hayes FJ, et al; VM Endocrine Society Task Force. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
7. Petak SM, Nankin HR, Spark RF, Swerdloff RS, Rodriguez-Rigau LJ; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients–2002 update. Endocr Pract. 2002;8(6):440-456.
8. Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl. 2009;30(1):1-9.
9. Reddy RG, Aung T, Karavitaki N, Wass JA. Opioid induced hypogonadism. BMJ. 2010;341:c4462.
10. U.S. Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1058.05: VHA operations activities that may constitute research. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications /ViewPublication.asp?pub_ID=2456. Published October 28, 2011. Accessed August 28, 2015.
11. AndroGel [package insert]. North Chicago, IL: AbbVie Inc; 2013.
12. Axiron [package insert]. Indianapolis, IL: Lilly USA, LLC; 2011.
13. U.S. Department of Veterans Affairs. Opioid therapy for chronic pain pocket guide. U.S. Department of Veterans Affairs. http://www.healthquality .va.gov/guidelines/pain/cot/opioidpocketguide23may2013v1.pdf. Published May 2013 Accessed August 28, 2015.
14. McPherson ML. Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing. Bethesda, MD: American Society of Health-System Pharmacists; 2009.
15. Butrans [package insert]. Stamford, CT: Purdue Pharma LP; 2014.
16. Testosterone Replacement Therapy Criteria for Use. VISN 8: VISN Pharmacist Executives, Veterans Health Administration, Department of Veterans Affairs; 2014. [Internal document.]
17. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
18. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805.
19. U.S. Department of Veterans Affairs. Testosterone products and cardiovascular safety. U.S. Department of Veterans Affairs Website. http://www.pbm .va.gov/PBM/vacenterformedicationsafety /nationalpbmbulletin/Testosterone_Products_and _Cardiovascular_Safety_NATIONAL_PBM _BULLETIN_02.pdf. Published February 7, 2014. Accessed August 28, 2015.
20. U.S. Department of Veterans Affairs Veterans Health Administration (VHA) Pharmacy Benefits Management Services (PBM), Medical Advisory Panel (MAP) and Center for Medication Safety (VA MEDSAFE). Memorandum: Opioid Safety Initiative Requirements. U.S. Department of Veterans Affairs Website. http://www.veterans.senate.gov/imo /media/doc/VA%20Testimony%20-%20April%2030%20SVAC%20Overmedication%20hearing.pdf. Published April 30, 2014. Accessed August 28, 2015.
21. Brennan MJ. The effect of opioid therapy on endocrine function. Am J Med. 2013;126(3)(suppl 1):S12-S18.
22. Rubinstein AL, Carpenter DM, Minkoff JR. Hypogonadism in men with chronic pain linked to the use of long-acting rather than short-acting opioids. Clin J Pain. 2013;29(10):840-845.
23. Rubinstein A, Carpenter DM. Elucidating risk factors for androgen deficiency associated with daily opioid use. Am J Med. 2014;127(12):1195-1201.
1. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Unintentional drug poisoning in the United States, 2010. Atlanta, GA: Centers for Disease Control and Prevention Website. http://www.cdc.gov /HomeandRecreationalSafety/pdf/poison-issue-brief .pdf. Published July 2010. Accessed August 28, 2015.
2. American Academy of Family Physicians. Using opioids in the management of chronic pain patients: challenges and future options. University of Kentucky Medical Center Website. http://www .mc.uky.edu/equip-4-pcps/documents/CRx%20Literature/Opioids%20for%20chronic%20pain.pdf. Published 2010. Accessed August 28, 2015.
3. Duarte RV, Raphael JH, Labib M, Southall JL, Ashford RL. Prevalence and influence of diagnostic criteria in the assessment of hypogonadism in intrathecal opioid therapy patients. Pain Physician. 2013;16(1):9-14.
4. Smith HS, Elliott JA. Opioid-induced androgen deficiency (OPIAD). Pain Physician. 2012;15(suppl 3):ES145-ES156.
5. De Maddalena C, Bellini M, Berra M, Meriggiola MC, Aloisi AM. Opioid-induced hypogonadism: why and how to treat it. Pain Physician. 2012;15(suppl 3):ES111-ES118.
6. Bhasin S, Cunningham GR, Hayes FJ, et al; VM Endocrine Society Task Force. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
7. Petak SM, Nankin HR, Spark RF, Swerdloff RS, Rodriguez-Rigau LJ; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients–2002 update. Endocr Pract. 2002;8(6):440-456.
8. Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl. 2009;30(1):1-9.
9. Reddy RG, Aung T, Karavitaki N, Wass JA. Opioid induced hypogonadism. BMJ. 2010;341:c4462.
10. U.S. Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1058.05: VHA operations activities that may constitute research. U.S. Department of Veterans Affairs Website. http://www.va.gov/vhapublications /ViewPublication.asp?pub_ID=2456. Published October 28, 2011. Accessed August 28, 2015.
11. AndroGel [package insert]. North Chicago, IL: AbbVie Inc; 2013.
12. Axiron [package insert]. Indianapolis, IL: Lilly USA, LLC; 2011.
13. U.S. Department of Veterans Affairs. Opioid therapy for chronic pain pocket guide. U.S. Department of Veterans Affairs. http://www.healthquality .va.gov/guidelines/pain/cot/opioidpocketguide23may2013v1.pdf. Published May 2013 Accessed August 28, 2015.
14. McPherson ML. Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing. Bethesda, MD: American Society of Health-System Pharmacists; 2009.
15. Butrans [package insert]. Stamford, CT: Purdue Pharma LP; 2014.
16. Testosterone Replacement Therapy Criteria for Use. VISN 8: VISN Pharmacist Executives, Veterans Health Administration, Department of Veterans Affairs; 2014. [Internal document.]
17. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
18. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805.
19. U.S. Department of Veterans Affairs. Testosterone products and cardiovascular safety. U.S. Department of Veterans Affairs Website. http://www.pbm .va.gov/PBM/vacenterformedicationsafety /nationalpbmbulletin/Testosterone_Products_and _Cardiovascular_Safety_NATIONAL_PBM _BULLETIN_02.pdf. Published February 7, 2014. Accessed August 28, 2015.
20. U.S. Department of Veterans Affairs Veterans Health Administration (VHA) Pharmacy Benefits Management Services (PBM), Medical Advisory Panel (MAP) and Center for Medication Safety (VA MEDSAFE). Memorandum: Opioid Safety Initiative Requirements. U.S. Department of Veterans Affairs Website. http://www.veterans.senate.gov/imo /media/doc/VA%20Testimony%20-%20April%2030%20SVAC%20Overmedication%20hearing.pdf. Published April 30, 2014. Accessed August 28, 2015.
21. Brennan MJ. The effect of opioid therapy on endocrine function. Am J Med. 2013;126(3)(suppl 1):S12-S18.
22. Rubinstein AL, Carpenter DM, Minkoff JR. Hypogonadism in men with chronic pain linked to the use of long-acting rather than short-acting opioids. Clin J Pain. 2013;29(10):840-845.
23. Rubinstein A, Carpenter DM. Elucidating risk factors for androgen deficiency associated with daily opioid use. Am J Med. 2014;127(12):1195-1201.