Article
Functional heartburn: An underrecognized cause of PPI-refractory symptoms
- Author:
- Scott Gabbard, MD
- Sonya Vijayvargiya
Functional heartburn is the most common cause of failure of proton pump inhibitor therapy.
Article

A man with progressive dysphagia
- Author:
- Adam Jacob Kichler, DO
- Scott Gabbard, MD
Difficulty swallowing can be caused by problems in the oropharynx or in the esophagus.
Article
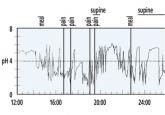
GERD: Diagnosing and treating the burn
- Author:
- Mohammed Alzubaidi, MD
- Scott Gabbard, MD
If symptoms do not respond to a proton pump inhibitor or are atypical, testing may be needed.
