User login
Patterns and Appropriateness of Thrombophilia Testing in an Academic Medical Center
Thrombophilia is a prothrombotic state, either acquired or inherited, leading to a thrombotic predisposition.1 The most common heritable thrombophilias include factor V Leiden (FVL) and prothrombin G20210A. The most common acquired thrombophilia is the presence of phospholipid antibodies.1 Thrombotic risk varies with thrombophilia type. For example, deficiencies of antithrombin, protein C and protein S, and the presence of phospholipid antibodies, confer higher risk than FVL and prothrombin G20210A.2-5 Other thrombophilias (eg, methylenetetrahydrofolate reductase mutation, increased factor VIII activity) are relatively uncommon and/or their impact on thrombosis risk appears to be either minimal or unknown.1-6 There is little clinical evidence that testing for thrombophilia impacts subsequent thrombosis prevention.5,7,8 Multiple clinical guidelines and medical societies recommend against the routine and indiscriminate use of thrombophilia testing.8-13 In general, thrombophilia testing should be considered only if the result would lead to changes in anticoagulant initiation, intensity, and/or duration, or might inform interventions to prevent thrombosis in asymptomatic family members.8-13 However, thrombophilia testing rarely changes the acute management of a thrombotic event and may have harmful effects on patients and their family members because positive results may unnecessarily increase anxiety and negative results may provide false reassurance.6,14-18 The cost-effectiveness of thrombophilia testing is unknown. Economic models have sought to quantify cost-effectiveness, but conclusions from these studies are limited.7
The utility of thrombophilia testing in emergency department (ED) and inpatient settings is further limited because patients are often treated and discharged before thrombophilia test results are available. Additionally, in these settings, multiple factors increase the risk of false-positive or false-negative results (eg, acute thrombosis, acute illness, pregnancy, and anticoagulant therapy).19,20 The purpose of this study was to systematically assess thrombophilia testing patterns in the ED and hospitalized patients at an academic medical center and to quantify the proportion of tests associated with minimal clinical utility. We hypothesize that the majority of thrombophilia tests completed in the inpatient setting are associated with minimal clinical utility.
METHODS
Setting and Patients
This study was conducted at University of Utah Health Care (UUHC) University Hospital, a 488-bed academic medical center with a level I trauma center, primary stroke center, and 50-bed ED. Laboratory services for UUHC, including thrombophilia testing, are provided by a national reference laboratory, Associated Regional and University Pathologists Laboratories. This study included patients ≥18 years of age who received thrombophilia testing (Supplementary Table 1) during an ED visit or inpatient admission at University Hospital between July 1, 2014 and December 31, 2014. There were no exclusion criteria. An institutional electronic data repository was used to identify patients matching inclusion criteria. All study activities were reviewed and approved by the UUHC Institutional Review Board with a waiver of informed consent.
Outcomes
An electronic database query was used to identify patients, collect patient demographic information, and collect test characteristics. Each patient’s electronic medical record was manually reviewed to collect all other outcomes. Indication for thrombophilia testing was identified by manual review of provider notes. Thrombophilia tests occurring in situations associated with minimal clinical utility were defined as tests meeting at least one of the following criteria: patient discharged before test results were available for review; test type not recommended by published guidelines or by UUHC Thrombosis Service physicians for thrombophilia testing (Supplementary Table 2); test performed in situations associated with decreased accuracy; test was a duplicate test as a result of different thrombophilia panels containing identical tests; and test followed a provoked venous thromboembolism (VTE). Testing in situations associated with decreased accuracy are summarized in Supplementary Table 3 and included at least one of the following at the time of the test: anticoagulant therapy, acute thrombosis, pregnant or <8 weeks postpartum, and receiving estrogen-containing medications. Only test types known to be affected by the respective situation were included. Testing following a provoked VTE was defined as testing prompted by an acute thrombosis and performed within 3 months following major surgery (defined administratively as any surgery performed in an operating room), during pregnancy, <8 weeks postpartum, or while on estrogen-containing medications. Thrombophilia testing during anticoagulant therapy was defined as testing within 4 half-lives of anticoagulant administration based on medication administration records. Anticoagulant therapy changes were identified by comparing prior-to-admission and discharge medication lists.
Data Analysis
Patient and laboratory characteristics were summarized using descriptive statistics, including mean and standard deviation (SD) for continuous variables and proportions for categorical variables. Data analysis was performed using Excel (Version 2013, Microsoft Corporation. Redmond, Washington).
RESULTS
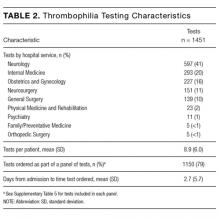
During the 6-month study period, 163 patients received at least 1 thrombophilia test during an ED visit or inpatient admission. Patient characteristics are summarized in Table 1. Tested patients were most commonly inpatients (96%) and female (71%). A total of 1451 thrombophilia tests were performed with a mean (± SD) of 8.9 ± 6.0 tests per patient. Testing characteristics are summarized in Table 2. Of the 39 different test types performed, the most commonly ordered were cardiolipin IgG and IgM antibodies (9% each), lupus anticoagulant (9%), and β2-glycoprotein 1 IgG and IgM antibodies (8% each). When combined with testing for phosphatidyl antibodies, antiphospholipid tests accounted for 70% of all tests. Overall, 134 (9%) test results were positive. The mean time for results to become available was 2.2 ± 2.5 days. The frequency of test types with corresponding positivity rates and mean time for results to become available are summarized in Supplementary Table 4.
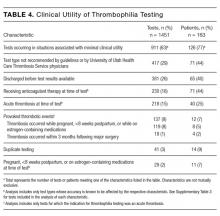
The indications for thrombophilia testing are summarized in Table 3. Ischemic stroke was the most common indication for testing (50% of tests; 35% of patients), followed by VTE (21% of tests; 21% of patients), and pregnancy-related conditions (eg, preeclampsia, intrauterine fetal demise; 15% of tests; 25% of patients). Overall, 911 tests (63%) occurred in situations associated with minimal clinical utility, with 126 patients (77%) receiving at least one of these tests (Table 4).
Anticoagulant therapy was changed in 43 patients (26%) in the following ways: initiated in 35 patients (21%), transitioned to a different anticoagulant in 6 patients (4%), and discontinued in 2 patients (1%). Of the 35 patients initiating anticoagulant therapy, 29 had documented thrombosis (24 had VTE, 4 had cerebral venous sinus thrombosis [CVST], and 1 had basilar artery thrombosis). Overall, 2 instances were identified in which initiation of anticoagulant therapy at discharge was in response to thrombophilia test results. In the first instance, warfarin without a parenteral anticoagulant bridge was initiated for a 54-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG antibodies, lupus anticoagulant, and protein S deficiency. In the second instance, warfarin with an enoxaparin bridge was initiated for a 26-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG and IgM antibodies, cardiolipin IgG antibodies, lupus anticoagulant, protein C deficiency, and antithrombin deficiency. Of the 163 patients receiving thrombophilia testing, only 2 patients (1%) had clear documentation of being offered genetic consultation.
DISCUSSION
In this retrospective analysis, 1451 thrombophilia tests were performed in 163 patients over 6 months. Tested patients were relatively young, which is likely explained by the number of patients tested for pregnancy-related conditions and the fact that a stroke or VTE in younger patients more frequently prompted providers to suspect thrombophilia. Nearly three-fourths of patients were female, which is likely due to testing for pregnancy-related conditions and possibly diagnostic suspicion bias given the comparative predilection of antiphospholipid syndrome for women. The patient characteristics in our study are consistent with other studies evaluating thrombophilia testing.21,22
Thrombophilia testing was most frequently prompted by stroke, VTE, and pregnancy-related conditions. Only 26% of patients had acute thrombosis identified during the admission, primarily because of the high proportion of tests for cryptogenic strokes and pregnancy-related conditions. Thrombophilia testing is recommended in patients who have had a stroke when the stroke is considered to be cryptogenic after a standard stroke evaluation.23 Thrombophilia testing in pregnancy-related conditions is controversial but is often considered in situations such as stillbirths with severe placental pathology and/or significant growth restriction, or in mothers with a personal or family history of thrombosis.24 The proportion of testing for pregnancy-related conditions may be greater than at other institutions because UUHC Maternal Fetal Medicine is a referral center for women with conditions associated with hypercoagulability. Anticoagulant therapy was initiated in 21% of patients, but specifically in response to thrombophilia testing in only 2 instances; in most cases, anticoagulant therapy was initiated regardless of thrombophilia test results.
The results of this study confirm our hypothesis because the majority of thrombophilia tests occurred in situations associated with minimal clinical utility. Testing in these situations was not isolated to specific patients or medical services because 77% of tested patients received at least 1 test associated with minimal clinical utility. Our study took a conservative approach in defining scenarios associated with minimal clinical utility because other situations can also affect testing accuracy (eg, hepatic disease, nephrotic syndrome) but were not included in our analysis of this outcome.
The results of this study highlight opportunities to improve thrombophilia testing practices at our institution and may be generalizable to institutions with similar testing patterns. Because multiple medical services order thrombophilia tests, strategies to improve testing practices are still being determined. The results of this study can serve as a baseline for comparison after strategies are implemented. The most common situation associated with minimal clinical utility was the use of test types not generally recommended by guidelines or UUHC Thrombosis Service physicians for thrombophilia testing (eg, β2-glycoprotein 1 IgA antibodies, phosphatidyl antibodies). We intend to require a hematology or thrombosis specialty consult prior to ordering these tests. This intervention alone could potentially decrease unnecessary testing by a third. Another consideration is to require a specialty consult prior to any inpatient thrombophilia testing. This strategy has been found to decrease inappropriate testing at other institutions.21 We also intend to streamline available thrombophilia testing panels because a poorly designed panel could lead to ordering of multiple tests associated with minimal clinical utility. At least 12 different thrombophilia panels are currently available in our computerized physician order entry system (see Supplementary Table 5). We hypothesize that current panel designs contribute to providers inadvertently ordering unintended or duplicate tests and that reducing the number of available panels and clearly delineating what tests are contained in each panel is likely to reduce unnecessary testing. Other strategies being considered include using electronic clinical decision support tools, implementing strict ordering criteria for all inpatient testing, and establishing a thrombosis stewardship program.
Our study was unique in at least 2 ways. First, previous studies describing thrombophilia testing have described testing patterns for patients with specific indications (eg, VTE), whereas our study described all thrombophilia tests regardless of indication. This allows for testing pattern comparisons across indications and medical services, increasing the generalizability of our results. Second, this study quantifies tests occurring in situations associated with a practical definition of minimal clinical utility.
Our study has several limitations: (1) Many variables were reliant on provider notes and other documentation, which allows for potential misclassification of variables. (2) It was not always possible to determine the ultimate utility of each test in clinical management decisions, and our study did not investigate the impact of thrombophilia testing on duration of anticoagulant therapy. Additionally, select situations could benefit from testing regardless if anticoagulant therapy is altered (eg, informing contraceptive choices). (3) Testing performed following a provoked acute thrombosis was defined as testing within 3 months following administratively defined major surgery. This definition could have included some minor procedures that do not substantially increase VTE risk, resulting in underestimated clinical utility. (4) The UUHC University Hospital serves as a referral hospital for a large geographical area, and investigators did not have access to outpatient records for a large proportion of discharged patients. As a result, frequency of repeat testing could not be assessed, possibly resulting in overestimated clinical utility. (5) In categorizing indications for testing, testing for CVST was subcategorized under testing for ischemic stroke based on presenting symptoms rather than on underlying pathophysiology. The rationale for this categorization is that patients with CVST were often tested based on presenting symptoms. Additionally, tests for CVST were ordered by the neurology service, which also ordered tests for all other ischemic stroke indications. (6) The purpose of our study was to investigate the subset of the hospital’s patient population that received thrombophilia testing, and patients were identified by tests received and not by diagnosis codes. As a result, we are unable to provide the proportion of total patients treated at the hospital for specific conditions who were tested (eg, the proportion of stroke patients that received thrombophilia testing). (7) Current practice guidelines do not recommend testing for phosphatidyl antibodies, even when traditional antiphospholipid testing is negative.25-27 Although expert panels continue to explore associations between phosphatidyl antibodies and pregnancy morbidity and thrombotic events, the low level of evidence is insufficient to guide clinical management.28 Therefore, we categorized all phosphatidyl testing as associated with minimal clinical utility.
CONCLUSIONS
In a large academic medical center, the majority of tests occurred in situations associated with minimal clinical utility. Strategies to improve thrombophilia testing practices are needed in order to minimize potentially inappropriate testing, provide more cost-effective care, and promote value-driven outcomes.
Disclosure
S.W. received financial support for this submitted work via a Bristol-Myers-Squibb grant. G.F. received financial support from Portola Pharmaceuticals for consulting and lectures that were not related to this submitted work.
1. Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109(4):369-384. PubMed
2. Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med. 1995;332(14):912-917. PubMed
3. Koster T, Rosendaal FR, de Ronde H, Briët E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet. 1993;342(8886-8887):1503-1506. PubMed
4. Margaglione M, Brancaccio V, Giuliani N, et al. Increased risk for venous thrombosis in carriers of the prothrombin G-->A20210 gene variant. Ann Intern Med. 1998;129(2):89-93. PubMed
5. De Stefano V, Martinelli I, Mannucci PM, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med. 1999;341:801-806. PubMed
6. Dickey TL. Can thrombophilia testing help to prevent recurrent VTE? Part 2. JAAPA. 2002;15(12):23-24, 27-29. PubMed
7. Simpson EL, Stevenson MD, Rawdin A, Papaioannou D. Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis. Health Technol Assess. 2009;13(2):iii, ix-x, 1-91. PubMed
8. National Institute for Health and Clinical Excellence. Venous thromboembolic disease: the management of venous thromboembolic diseases and the role of thrombophilia testing. NICE clinical guideline 144. https://www.nice.org.uk/guidance/cg144. Accessed on June 30, 2017.
9. Evalution of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67-76.
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S-494S. PubMed
11. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
12. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Hematology Am Soc Hematol Educ Program. 2013;2013:9-14. PubMed
13. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
14. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361. PubMed
15. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1-7. PubMed
16. Miles JS, Miletich JP, Goldhaber SZ, Hennekens CH, Ridker PM. G20210A mutation in the prothrombin gene and the risk of recurrent venous thromboembolism. J Am Coll Cardiol. 2001;37(1):215-218. PubMed
17. Eichinger S, Weltermann A, Mannhalter C, et al. The risk of recurrent venous thromboembolism in heterozygous carriers of factor V Leiden and a first spontaneous venous thromboembolism. Arch Intern Med. 2002;162(20):2357-2360. PubMed
18. Mazzolai L, Duchosal MA. Hereditary thrombophilia and venous thromboembolism: critical evaluation of the clinical implications of screening. Eur J Vasc Endovasc Surg. 2007;34(4):483-488. PubMed
19. Merriman L, Greaves M. Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699-704. PubMed
20. Favaloro EJ, McDonald D, Lippi G. Laboratory investigation of thrombophilia: the good, the bad, and the ugly. Semin Thromb Hemost. 2009;35(7):695-710. PubMed
21. Shen YM, Tsai J, Taiwo E, et al. Analysis of thrombophilia test ordering practices at an academic center: a proposal for appropriate testing to reduce harm and cost. PLoS One. 2016;11(5):e0155326. PubMed
22. Meyer MR, Witt DM, Delate T, et al. Thrombophilia testing patterns amongst patients with acute venous thromboembolism. Thromb Res. 2015;136(6):1160-1164. PubMed
23. Saver JL. Clinical practice: cryptogenic stroke. N Engl J Med. 2016;374(21):2065-2074. PubMed
24. ACOG practice bulletin no. 102: management of stillbirth. Obstet Gynecol. 2009;113(3):748-761. PubMed
25. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295-306. PubMed
26. Keeling D, Mackie I, Moore GW, Greer IA, Greaves M, British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157(1):47-58. PubMed
27. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice bulletin no. 132: antiphospholipid syndrome. Obstet Gynecol. 2012;120(6):1514-1521. PubMed
28. Bertolaccini ML, Amengual O, Andreoli L, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev. 2014;13(9):917-930. PubMed
Thrombophilia is a prothrombotic state, either acquired or inherited, leading to a thrombotic predisposition.1 The most common heritable thrombophilias include factor V Leiden (FVL) and prothrombin G20210A. The most common acquired thrombophilia is the presence of phospholipid antibodies.1 Thrombotic risk varies with thrombophilia type. For example, deficiencies of antithrombin, protein C and protein S, and the presence of phospholipid antibodies, confer higher risk than FVL and prothrombin G20210A.2-5 Other thrombophilias (eg, methylenetetrahydrofolate reductase mutation, increased factor VIII activity) are relatively uncommon and/or their impact on thrombosis risk appears to be either minimal or unknown.1-6 There is little clinical evidence that testing for thrombophilia impacts subsequent thrombosis prevention.5,7,8 Multiple clinical guidelines and medical societies recommend against the routine and indiscriminate use of thrombophilia testing.8-13 In general, thrombophilia testing should be considered only if the result would lead to changes in anticoagulant initiation, intensity, and/or duration, or might inform interventions to prevent thrombosis in asymptomatic family members.8-13 However, thrombophilia testing rarely changes the acute management of a thrombotic event and may have harmful effects on patients and their family members because positive results may unnecessarily increase anxiety and negative results may provide false reassurance.6,14-18 The cost-effectiveness of thrombophilia testing is unknown. Economic models have sought to quantify cost-effectiveness, but conclusions from these studies are limited.7
The utility of thrombophilia testing in emergency department (ED) and inpatient settings is further limited because patients are often treated and discharged before thrombophilia test results are available. Additionally, in these settings, multiple factors increase the risk of false-positive or false-negative results (eg, acute thrombosis, acute illness, pregnancy, and anticoagulant therapy).19,20 The purpose of this study was to systematically assess thrombophilia testing patterns in the ED and hospitalized patients at an academic medical center and to quantify the proportion of tests associated with minimal clinical utility. We hypothesize that the majority of thrombophilia tests completed in the inpatient setting are associated with minimal clinical utility.
METHODS
Setting and Patients
This study was conducted at University of Utah Health Care (UUHC) University Hospital, a 488-bed academic medical center with a level I trauma center, primary stroke center, and 50-bed ED. Laboratory services for UUHC, including thrombophilia testing, are provided by a national reference laboratory, Associated Regional and University Pathologists Laboratories. This study included patients ≥18 years of age who received thrombophilia testing (Supplementary Table 1) during an ED visit or inpatient admission at University Hospital between July 1, 2014 and December 31, 2014. There were no exclusion criteria. An institutional electronic data repository was used to identify patients matching inclusion criteria. All study activities were reviewed and approved by the UUHC Institutional Review Board with a waiver of informed consent.
Outcomes
An electronic database query was used to identify patients, collect patient demographic information, and collect test characteristics. Each patient’s electronic medical record was manually reviewed to collect all other outcomes. Indication for thrombophilia testing was identified by manual review of provider notes. Thrombophilia tests occurring in situations associated with minimal clinical utility were defined as tests meeting at least one of the following criteria: patient discharged before test results were available for review; test type not recommended by published guidelines or by UUHC Thrombosis Service physicians for thrombophilia testing (Supplementary Table 2); test performed in situations associated with decreased accuracy; test was a duplicate test as a result of different thrombophilia panels containing identical tests; and test followed a provoked venous thromboembolism (VTE). Testing in situations associated with decreased accuracy are summarized in Supplementary Table 3 and included at least one of the following at the time of the test: anticoagulant therapy, acute thrombosis, pregnant or <8 weeks postpartum, and receiving estrogen-containing medications. Only test types known to be affected by the respective situation were included. Testing following a provoked VTE was defined as testing prompted by an acute thrombosis and performed within 3 months following major surgery (defined administratively as any surgery performed in an operating room), during pregnancy, <8 weeks postpartum, or while on estrogen-containing medications. Thrombophilia testing during anticoagulant therapy was defined as testing within 4 half-lives of anticoagulant administration based on medication administration records. Anticoagulant therapy changes were identified by comparing prior-to-admission and discharge medication lists.
Data Analysis
Patient and laboratory characteristics were summarized using descriptive statistics, including mean and standard deviation (SD) for continuous variables and proportions for categorical variables. Data analysis was performed using Excel (Version 2013, Microsoft Corporation. Redmond, Washington).
RESULTS
During the 6-month study period, 163 patients received at least 1 thrombophilia test during an ED visit or inpatient admission. Patient characteristics are summarized in Table 1. Tested patients were most commonly inpatients (96%) and female (71%). A total of 1451 thrombophilia tests were performed with a mean (± SD) of 8.9 ± 6.0 tests per patient. Testing characteristics are summarized in Table 2. Of the 39 different test types performed, the most commonly ordered were cardiolipin IgG and IgM antibodies (9% each), lupus anticoagulant (9%), and β2-glycoprotein 1 IgG and IgM antibodies (8% each). When combined with testing for phosphatidyl antibodies, antiphospholipid tests accounted for 70% of all tests. Overall, 134 (9%) test results were positive. The mean time for results to become available was 2.2 ± 2.5 days. The frequency of test types with corresponding positivity rates and mean time for results to become available are summarized in Supplementary Table 4.
The indications for thrombophilia testing are summarized in Table 3. Ischemic stroke was the most common indication for testing (50% of tests; 35% of patients), followed by VTE (21% of tests; 21% of patients), and pregnancy-related conditions (eg, preeclampsia, intrauterine fetal demise; 15% of tests; 25% of patients). Overall, 911 tests (63%) occurred in situations associated with minimal clinical utility, with 126 patients (77%) receiving at least one of these tests (Table 4).
Anticoagulant therapy was changed in 43 patients (26%) in the following ways: initiated in 35 patients (21%), transitioned to a different anticoagulant in 6 patients (4%), and discontinued in 2 patients (1%). Of the 35 patients initiating anticoagulant therapy, 29 had documented thrombosis (24 had VTE, 4 had cerebral venous sinus thrombosis [CVST], and 1 had basilar artery thrombosis). Overall, 2 instances were identified in which initiation of anticoagulant therapy at discharge was in response to thrombophilia test results. In the first instance, warfarin without a parenteral anticoagulant bridge was initiated for a 54-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG antibodies, lupus anticoagulant, and protein S deficiency. In the second instance, warfarin with an enoxaparin bridge was initiated for a 26-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG and IgM antibodies, cardiolipin IgG antibodies, lupus anticoagulant, protein C deficiency, and antithrombin deficiency. Of the 163 patients receiving thrombophilia testing, only 2 patients (1%) had clear documentation of being offered genetic consultation.
DISCUSSION
In this retrospective analysis, 1451 thrombophilia tests were performed in 163 patients over 6 months. Tested patients were relatively young, which is likely explained by the number of patients tested for pregnancy-related conditions and the fact that a stroke or VTE in younger patients more frequently prompted providers to suspect thrombophilia. Nearly three-fourths of patients were female, which is likely due to testing for pregnancy-related conditions and possibly diagnostic suspicion bias given the comparative predilection of antiphospholipid syndrome for women. The patient characteristics in our study are consistent with other studies evaluating thrombophilia testing.21,22
Thrombophilia testing was most frequently prompted by stroke, VTE, and pregnancy-related conditions. Only 26% of patients had acute thrombosis identified during the admission, primarily because of the high proportion of tests for cryptogenic strokes and pregnancy-related conditions. Thrombophilia testing is recommended in patients who have had a stroke when the stroke is considered to be cryptogenic after a standard stroke evaluation.23 Thrombophilia testing in pregnancy-related conditions is controversial but is often considered in situations such as stillbirths with severe placental pathology and/or significant growth restriction, or in mothers with a personal or family history of thrombosis.24 The proportion of testing for pregnancy-related conditions may be greater than at other institutions because UUHC Maternal Fetal Medicine is a referral center for women with conditions associated with hypercoagulability. Anticoagulant therapy was initiated in 21% of patients, but specifically in response to thrombophilia testing in only 2 instances; in most cases, anticoagulant therapy was initiated regardless of thrombophilia test results.
The results of this study confirm our hypothesis because the majority of thrombophilia tests occurred in situations associated with minimal clinical utility. Testing in these situations was not isolated to specific patients or medical services because 77% of tested patients received at least 1 test associated with minimal clinical utility. Our study took a conservative approach in defining scenarios associated with minimal clinical utility because other situations can also affect testing accuracy (eg, hepatic disease, nephrotic syndrome) but were not included in our analysis of this outcome.
The results of this study highlight opportunities to improve thrombophilia testing practices at our institution and may be generalizable to institutions with similar testing patterns. Because multiple medical services order thrombophilia tests, strategies to improve testing practices are still being determined. The results of this study can serve as a baseline for comparison after strategies are implemented. The most common situation associated with minimal clinical utility was the use of test types not generally recommended by guidelines or UUHC Thrombosis Service physicians for thrombophilia testing (eg, β2-glycoprotein 1 IgA antibodies, phosphatidyl antibodies). We intend to require a hematology or thrombosis specialty consult prior to ordering these tests. This intervention alone could potentially decrease unnecessary testing by a third. Another consideration is to require a specialty consult prior to any inpatient thrombophilia testing. This strategy has been found to decrease inappropriate testing at other institutions.21 We also intend to streamline available thrombophilia testing panels because a poorly designed panel could lead to ordering of multiple tests associated with minimal clinical utility. At least 12 different thrombophilia panels are currently available in our computerized physician order entry system (see Supplementary Table 5). We hypothesize that current panel designs contribute to providers inadvertently ordering unintended or duplicate tests and that reducing the number of available panels and clearly delineating what tests are contained in each panel is likely to reduce unnecessary testing. Other strategies being considered include using electronic clinical decision support tools, implementing strict ordering criteria for all inpatient testing, and establishing a thrombosis stewardship program.
Our study was unique in at least 2 ways. First, previous studies describing thrombophilia testing have described testing patterns for patients with specific indications (eg, VTE), whereas our study described all thrombophilia tests regardless of indication. This allows for testing pattern comparisons across indications and medical services, increasing the generalizability of our results. Second, this study quantifies tests occurring in situations associated with a practical definition of minimal clinical utility.
Our study has several limitations: (1) Many variables were reliant on provider notes and other documentation, which allows for potential misclassification of variables. (2) It was not always possible to determine the ultimate utility of each test in clinical management decisions, and our study did not investigate the impact of thrombophilia testing on duration of anticoagulant therapy. Additionally, select situations could benefit from testing regardless if anticoagulant therapy is altered (eg, informing contraceptive choices). (3) Testing performed following a provoked acute thrombosis was defined as testing within 3 months following administratively defined major surgery. This definition could have included some minor procedures that do not substantially increase VTE risk, resulting in underestimated clinical utility. (4) The UUHC University Hospital serves as a referral hospital for a large geographical area, and investigators did not have access to outpatient records for a large proportion of discharged patients. As a result, frequency of repeat testing could not be assessed, possibly resulting in overestimated clinical utility. (5) In categorizing indications for testing, testing for CVST was subcategorized under testing for ischemic stroke based on presenting symptoms rather than on underlying pathophysiology. The rationale for this categorization is that patients with CVST were often tested based on presenting symptoms. Additionally, tests for CVST were ordered by the neurology service, which also ordered tests for all other ischemic stroke indications. (6) The purpose of our study was to investigate the subset of the hospital’s patient population that received thrombophilia testing, and patients were identified by tests received and not by diagnosis codes. As a result, we are unable to provide the proportion of total patients treated at the hospital for specific conditions who were tested (eg, the proportion of stroke patients that received thrombophilia testing). (7) Current practice guidelines do not recommend testing for phosphatidyl antibodies, even when traditional antiphospholipid testing is negative.25-27 Although expert panels continue to explore associations between phosphatidyl antibodies and pregnancy morbidity and thrombotic events, the low level of evidence is insufficient to guide clinical management.28 Therefore, we categorized all phosphatidyl testing as associated with minimal clinical utility.
CONCLUSIONS
In a large academic medical center, the majority of tests occurred in situations associated with minimal clinical utility. Strategies to improve thrombophilia testing practices are needed in order to minimize potentially inappropriate testing, provide more cost-effective care, and promote value-driven outcomes.
Disclosure
S.W. received financial support for this submitted work via a Bristol-Myers-Squibb grant. G.F. received financial support from Portola Pharmaceuticals for consulting and lectures that were not related to this submitted work.
Thrombophilia is a prothrombotic state, either acquired or inherited, leading to a thrombotic predisposition.1 The most common heritable thrombophilias include factor V Leiden (FVL) and prothrombin G20210A. The most common acquired thrombophilia is the presence of phospholipid antibodies.1 Thrombotic risk varies with thrombophilia type. For example, deficiencies of antithrombin, protein C and protein S, and the presence of phospholipid antibodies, confer higher risk than FVL and prothrombin G20210A.2-5 Other thrombophilias (eg, methylenetetrahydrofolate reductase mutation, increased factor VIII activity) are relatively uncommon and/or their impact on thrombosis risk appears to be either minimal or unknown.1-6 There is little clinical evidence that testing for thrombophilia impacts subsequent thrombosis prevention.5,7,8 Multiple clinical guidelines and medical societies recommend against the routine and indiscriminate use of thrombophilia testing.8-13 In general, thrombophilia testing should be considered only if the result would lead to changes in anticoagulant initiation, intensity, and/or duration, or might inform interventions to prevent thrombosis in asymptomatic family members.8-13 However, thrombophilia testing rarely changes the acute management of a thrombotic event and may have harmful effects on patients and their family members because positive results may unnecessarily increase anxiety and negative results may provide false reassurance.6,14-18 The cost-effectiveness of thrombophilia testing is unknown. Economic models have sought to quantify cost-effectiveness, but conclusions from these studies are limited.7
The utility of thrombophilia testing in emergency department (ED) and inpatient settings is further limited because patients are often treated and discharged before thrombophilia test results are available. Additionally, in these settings, multiple factors increase the risk of false-positive or false-negative results (eg, acute thrombosis, acute illness, pregnancy, and anticoagulant therapy).19,20 The purpose of this study was to systematically assess thrombophilia testing patterns in the ED and hospitalized patients at an academic medical center and to quantify the proportion of tests associated with minimal clinical utility. We hypothesize that the majority of thrombophilia tests completed in the inpatient setting are associated with minimal clinical utility.
METHODS
Setting and Patients
This study was conducted at University of Utah Health Care (UUHC) University Hospital, a 488-bed academic medical center with a level I trauma center, primary stroke center, and 50-bed ED. Laboratory services for UUHC, including thrombophilia testing, are provided by a national reference laboratory, Associated Regional and University Pathologists Laboratories. This study included patients ≥18 years of age who received thrombophilia testing (Supplementary Table 1) during an ED visit or inpatient admission at University Hospital between July 1, 2014 and December 31, 2014. There were no exclusion criteria. An institutional electronic data repository was used to identify patients matching inclusion criteria. All study activities were reviewed and approved by the UUHC Institutional Review Board with a waiver of informed consent.
Outcomes
An electronic database query was used to identify patients, collect patient demographic information, and collect test characteristics. Each patient’s electronic medical record was manually reviewed to collect all other outcomes. Indication for thrombophilia testing was identified by manual review of provider notes. Thrombophilia tests occurring in situations associated with minimal clinical utility were defined as tests meeting at least one of the following criteria: patient discharged before test results were available for review; test type not recommended by published guidelines or by UUHC Thrombosis Service physicians for thrombophilia testing (Supplementary Table 2); test performed in situations associated with decreased accuracy; test was a duplicate test as a result of different thrombophilia panels containing identical tests; and test followed a provoked venous thromboembolism (VTE). Testing in situations associated with decreased accuracy are summarized in Supplementary Table 3 and included at least one of the following at the time of the test: anticoagulant therapy, acute thrombosis, pregnant or <8 weeks postpartum, and receiving estrogen-containing medications. Only test types known to be affected by the respective situation were included. Testing following a provoked VTE was defined as testing prompted by an acute thrombosis and performed within 3 months following major surgery (defined administratively as any surgery performed in an operating room), during pregnancy, <8 weeks postpartum, or while on estrogen-containing medications. Thrombophilia testing during anticoagulant therapy was defined as testing within 4 half-lives of anticoagulant administration based on medication administration records. Anticoagulant therapy changes were identified by comparing prior-to-admission and discharge medication lists.
Data Analysis
Patient and laboratory characteristics were summarized using descriptive statistics, including mean and standard deviation (SD) for continuous variables and proportions for categorical variables. Data analysis was performed using Excel (Version 2013, Microsoft Corporation. Redmond, Washington).
RESULTS
During the 6-month study period, 163 patients received at least 1 thrombophilia test during an ED visit or inpatient admission. Patient characteristics are summarized in Table 1. Tested patients were most commonly inpatients (96%) and female (71%). A total of 1451 thrombophilia tests were performed with a mean (± SD) of 8.9 ± 6.0 tests per patient. Testing characteristics are summarized in Table 2. Of the 39 different test types performed, the most commonly ordered were cardiolipin IgG and IgM antibodies (9% each), lupus anticoagulant (9%), and β2-glycoprotein 1 IgG and IgM antibodies (8% each). When combined with testing for phosphatidyl antibodies, antiphospholipid tests accounted for 70% of all tests. Overall, 134 (9%) test results were positive. The mean time for results to become available was 2.2 ± 2.5 days. The frequency of test types with corresponding positivity rates and mean time for results to become available are summarized in Supplementary Table 4.
The indications for thrombophilia testing are summarized in Table 3. Ischemic stroke was the most common indication for testing (50% of tests; 35% of patients), followed by VTE (21% of tests; 21% of patients), and pregnancy-related conditions (eg, preeclampsia, intrauterine fetal demise; 15% of tests; 25% of patients). Overall, 911 tests (63%) occurred in situations associated with minimal clinical utility, with 126 patients (77%) receiving at least one of these tests (Table 4).
Anticoagulant therapy was changed in 43 patients (26%) in the following ways: initiated in 35 patients (21%), transitioned to a different anticoagulant in 6 patients (4%), and discontinued in 2 patients (1%). Of the 35 patients initiating anticoagulant therapy, 29 had documented thrombosis (24 had VTE, 4 had cerebral venous sinus thrombosis [CVST], and 1 had basilar artery thrombosis). Overall, 2 instances were identified in which initiation of anticoagulant therapy at discharge was in response to thrombophilia test results. In the first instance, warfarin without a parenteral anticoagulant bridge was initiated for a 54-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG antibodies, lupus anticoagulant, and protein S deficiency. In the second instance, warfarin with an enoxaparin bridge was initiated for a 26-year-old patient with a cryptogenic stroke who tested positive for β2-glycoprotein 1 IgG and IgM antibodies, cardiolipin IgG antibodies, lupus anticoagulant, protein C deficiency, and antithrombin deficiency. Of the 163 patients receiving thrombophilia testing, only 2 patients (1%) had clear documentation of being offered genetic consultation.
DISCUSSION
In this retrospective analysis, 1451 thrombophilia tests were performed in 163 patients over 6 months. Tested patients were relatively young, which is likely explained by the number of patients tested for pregnancy-related conditions and the fact that a stroke or VTE in younger patients more frequently prompted providers to suspect thrombophilia. Nearly three-fourths of patients were female, which is likely due to testing for pregnancy-related conditions and possibly diagnostic suspicion bias given the comparative predilection of antiphospholipid syndrome for women. The patient characteristics in our study are consistent with other studies evaluating thrombophilia testing.21,22
Thrombophilia testing was most frequently prompted by stroke, VTE, and pregnancy-related conditions. Only 26% of patients had acute thrombosis identified during the admission, primarily because of the high proportion of tests for cryptogenic strokes and pregnancy-related conditions. Thrombophilia testing is recommended in patients who have had a stroke when the stroke is considered to be cryptogenic after a standard stroke evaluation.23 Thrombophilia testing in pregnancy-related conditions is controversial but is often considered in situations such as stillbirths with severe placental pathology and/or significant growth restriction, or in mothers with a personal or family history of thrombosis.24 The proportion of testing for pregnancy-related conditions may be greater than at other institutions because UUHC Maternal Fetal Medicine is a referral center for women with conditions associated with hypercoagulability. Anticoagulant therapy was initiated in 21% of patients, but specifically in response to thrombophilia testing in only 2 instances; in most cases, anticoagulant therapy was initiated regardless of thrombophilia test results.
The results of this study confirm our hypothesis because the majority of thrombophilia tests occurred in situations associated with minimal clinical utility. Testing in these situations was not isolated to specific patients or medical services because 77% of tested patients received at least 1 test associated with minimal clinical utility. Our study took a conservative approach in defining scenarios associated with minimal clinical utility because other situations can also affect testing accuracy (eg, hepatic disease, nephrotic syndrome) but were not included in our analysis of this outcome.
The results of this study highlight opportunities to improve thrombophilia testing practices at our institution and may be generalizable to institutions with similar testing patterns. Because multiple medical services order thrombophilia tests, strategies to improve testing practices are still being determined. The results of this study can serve as a baseline for comparison after strategies are implemented. The most common situation associated with minimal clinical utility was the use of test types not generally recommended by guidelines or UUHC Thrombosis Service physicians for thrombophilia testing (eg, β2-glycoprotein 1 IgA antibodies, phosphatidyl antibodies). We intend to require a hematology or thrombosis specialty consult prior to ordering these tests. This intervention alone could potentially decrease unnecessary testing by a third. Another consideration is to require a specialty consult prior to any inpatient thrombophilia testing. This strategy has been found to decrease inappropriate testing at other institutions.21 We also intend to streamline available thrombophilia testing panels because a poorly designed panel could lead to ordering of multiple tests associated with minimal clinical utility. At least 12 different thrombophilia panels are currently available in our computerized physician order entry system (see Supplementary Table 5). We hypothesize that current panel designs contribute to providers inadvertently ordering unintended or duplicate tests and that reducing the number of available panels and clearly delineating what tests are contained in each panel is likely to reduce unnecessary testing. Other strategies being considered include using electronic clinical decision support tools, implementing strict ordering criteria for all inpatient testing, and establishing a thrombosis stewardship program.
Our study was unique in at least 2 ways. First, previous studies describing thrombophilia testing have described testing patterns for patients with specific indications (eg, VTE), whereas our study described all thrombophilia tests regardless of indication. This allows for testing pattern comparisons across indications and medical services, increasing the generalizability of our results. Second, this study quantifies tests occurring in situations associated with a practical definition of minimal clinical utility.
Our study has several limitations: (1) Many variables were reliant on provider notes and other documentation, which allows for potential misclassification of variables. (2) It was not always possible to determine the ultimate utility of each test in clinical management decisions, and our study did not investigate the impact of thrombophilia testing on duration of anticoagulant therapy. Additionally, select situations could benefit from testing regardless if anticoagulant therapy is altered (eg, informing contraceptive choices). (3) Testing performed following a provoked acute thrombosis was defined as testing within 3 months following administratively defined major surgery. This definition could have included some minor procedures that do not substantially increase VTE risk, resulting in underestimated clinical utility. (4) The UUHC University Hospital serves as a referral hospital for a large geographical area, and investigators did not have access to outpatient records for a large proportion of discharged patients. As a result, frequency of repeat testing could not be assessed, possibly resulting in overestimated clinical utility. (5) In categorizing indications for testing, testing for CVST was subcategorized under testing for ischemic stroke based on presenting symptoms rather than on underlying pathophysiology. The rationale for this categorization is that patients with CVST were often tested based on presenting symptoms. Additionally, tests for CVST were ordered by the neurology service, which also ordered tests for all other ischemic stroke indications. (6) The purpose of our study was to investigate the subset of the hospital’s patient population that received thrombophilia testing, and patients were identified by tests received and not by diagnosis codes. As a result, we are unable to provide the proportion of total patients treated at the hospital for specific conditions who were tested (eg, the proportion of stroke patients that received thrombophilia testing). (7) Current practice guidelines do not recommend testing for phosphatidyl antibodies, even when traditional antiphospholipid testing is negative.25-27 Although expert panels continue to explore associations between phosphatidyl antibodies and pregnancy morbidity and thrombotic events, the low level of evidence is insufficient to guide clinical management.28 Therefore, we categorized all phosphatidyl testing as associated with minimal clinical utility.
CONCLUSIONS
In a large academic medical center, the majority of tests occurred in situations associated with minimal clinical utility. Strategies to improve thrombophilia testing practices are needed in order to minimize potentially inappropriate testing, provide more cost-effective care, and promote value-driven outcomes.
Disclosure
S.W. received financial support for this submitted work via a Bristol-Myers-Squibb grant. G.F. received financial support from Portola Pharmaceuticals for consulting and lectures that were not related to this submitted work.
1. Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109(4):369-384. PubMed
2. Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med. 1995;332(14):912-917. PubMed
3. Koster T, Rosendaal FR, de Ronde H, Briët E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet. 1993;342(8886-8887):1503-1506. PubMed
4. Margaglione M, Brancaccio V, Giuliani N, et al. Increased risk for venous thrombosis in carriers of the prothrombin G-->A20210 gene variant. Ann Intern Med. 1998;129(2):89-93. PubMed
5. De Stefano V, Martinelli I, Mannucci PM, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med. 1999;341:801-806. PubMed
6. Dickey TL. Can thrombophilia testing help to prevent recurrent VTE? Part 2. JAAPA. 2002;15(12):23-24, 27-29. PubMed
7. Simpson EL, Stevenson MD, Rawdin A, Papaioannou D. Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis. Health Technol Assess. 2009;13(2):iii, ix-x, 1-91. PubMed
8. National Institute for Health and Clinical Excellence. Venous thromboembolic disease: the management of venous thromboembolic diseases and the role of thrombophilia testing. NICE clinical guideline 144. https://www.nice.org.uk/guidance/cg144. Accessed on June 30, 2017.
9. Evalution of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67-76.
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S-494S. PubMed
11. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
12. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Hematology Am Soc Hematol Educ Program. 2013;2013:9-14. PubMed
13. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
14. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361. PubMed
15. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1-7. PubMed
16. Miles JS, Miletich JP, Goldhaber SZ, Hennekens CH, Ridker PM. G20210A mutation in the prothrombin gene and the risk of recurrent venous thromboembolism. J Am Coll Cardiol. 2001;37(1):215-218. PubMed
17. Eichinger S, Weltermann A, Mannhalter C, et al. The risk of recurrent venous thromboembolism in heterozygous carriers of factor V Leiden and a first spontaneous venous thromboembolism. Arch Intern Med. 2002;162(20):2357-2360. PubMed
18. Mazzolai L, Duchosal MA. Hereditary thrombophilia and venous thromboembolism: critical evaluation of the clinical implications of screening. Eur J Vasc Endovasc Surg. 2007;34(4):483-488. PubMed
19. Merriman L, Greaves M. Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699-704. PubMed
20. Favaloro EJ, McDonald D, Lippi G. Laboratory investigation of thrombophilia: the good, the bad, and the ugly. Semin Thromb Hemost. 2009;35(7):695-710. PubMed
21. Shen YM, Tsai J, Taiwo E, et al. Analysis of thrombophilia test ordering practices at an academic center: a proposal for appropriate testing to reduce harm and cost. PLoS One. 2016;11(5):e0155326. PubMed
22. Meyer MR, Witt DM, Delate T, et al. Thrombophilia testing patterns amongst patients with acute venous thromboembolism. Thromb Res. 2015;136(6):1160-1164. PubMed
23. Saver JL. Clinical practice: cryptogenic stroke. N Engl J Med. 2016;374(21):2065-2074. PubMed
24. ACOG practice bulletin no. 102: management of stillbirth. Obstet Gynecol. 2009;113(3):748-761. PubMed
25. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295-306. PubMed
26. Keeling D, Mackie I, Moore GW, Greer IA, Greaves M, British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157(1):47-58. PubMed
27. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice bulletin no. 132: antiphospholipid syndrome. Obstet Gynecol. 2012;120(6):1514-1521. PubMed
28. Bertolaccini ML, Amengual O, Andreoli L, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev. 2014;13(9):917-930. PubMed
1. Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109(4):369-384. PubMed
2. Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med. 1995;332(14):912-917. PubMed
3. Koster T, Rosendaal FR, de Ronde H, Briët E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet. 1993;342(8886-8887):1503-1506. PubMed
4. Margaglione M, Brancaccio V, Giuliani N, et al. Increased risk for venous thrombosis in carriers of the prothrombin G-->A20210 gene variant. Ann Intern Med. 1998;129(2):89-93. PubMed
5. De Stefano V, Martinelli I, Mannucci PM, et al. The risk of recurrent deep venous thrombosis among heterozygous carriers of both factor V Leiden and the G20210A prothrombin mutation. N Engl J Med. 1999;341:801-806. PubMed
6. Dickey TL. Can thrombophilia testing help to prevent recurrent VTE? Part 2. JAAPA. 2002;15(12):23-24, 27-29. PubMed
7. Simpson EL, Stevenson MD, Rawdin A, Papaioannou D. Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis. Health Technol Assess. 2009;13(2):iii, ix-x, 1-91. PubMed
8. National Institute for Health and Clinical Excellence. Venous thromboembolic disease: the management of venous thromboembolic diseases and the role of thrombophilia testing. NICE clinical guideline 144. https://www.nice.org.uk/guidance/cg144. Accessed on June 30, 2017.
9. Evalution of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67-76.
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S-494S. PubMed
11. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220. PubMed
12. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely® campaign: five hematologic tests and treatments to question. Hematology Am Soc Hematol Educ Program. 2013;2013:9-14. PubMed
13. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
14. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361. PubMed
15. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1-7. PubMed
16. Miles JS, Miletich JP, Goldhaber SZ, Hennekens CH, Ridker PM. G20210A mutation in the prothrombin gene and the risk of recurrent venous thromboembolism. J Am Coll Cardiol. 2001;37(1):215-218. PubMed
17. Eichinger S, Weltermann A, Mannhalter C, et al. The risk of recurrent venous thromboembolism in heterozygous carriers of factor V Leiden and a first spontaneous venous thromboembolism. Arch Intern Med. 2002;162(20):2357-2360. PubMed
18. Mazzolai L, Duchosal MA. Hereditary thrombophilia and venous thromboembolism: critical evaluation of the clinical implications of screening. Eur J Vasc Endovasc Surg. 2007;34(4):483-488. PubMed
19. Merriman L, Greaves M. Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699-704. PubMed
20. Favaloro EJ, McDonald D, Lippi G. Laboratory investigation of thrombophilia: the good, the bad, and the ugly. Semin Thromb Hemost. 2009;35(7):695-710. PubMed
21. Shen YM, Tsai J, Taiwo E, et al. Analysis of thrombophilia test ordering practices at an academic center: a proposal for appropriate testing to reduce harm and cost. PLoS One. 2016;11(5):e0155326. PubMed
22. Meyer MR, Witt DM, Delate T, et al. Thrombophilia testing patterns amongst patients with acute venous thromboembolism. Thromb Res. 2015;136(6):1160-1164. PubMed
23. Saver JL. Clinical practice: cryptogenic stroke. N Engl J Med. 2016;374(21):2065-2074. PubMed
24. ACOG practice bulletin no. 102: management of stillbirth. Obstet Gynecol. 2009;113(3):748-761. PubMed
25. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295-306. PubMed
26. Keeling D, Mackie I, Moore GW, Greer IA, Greaves M, British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157(1):47-58. PubMed
27. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice bulletin no. 132: antiphospholipid syndrome. Obstet Gynecol. 2012;120(6):1514-1521. PubMed
28. Bertolaccini ML, Amengual O, Andreoli L, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev. 2014;13(9):917-930. PubMed
© 2017 Society of Hospital Medicine
PICC and Venous Catheter Appropriateness
Vascular access devices (VADs), including peripherally inserted central venous catheters (PICCs) and traditional central venous catheters (CVCs), remain a cornerstone for the delivery of necessary therapy. VADs are used routinely to treat inpatients and increasingly outpatients too. PICCs possess characteristics that are often favorable in a variety of clinical settings when compared to traditional CVCs. However, a paucity of evidence regarding the indication, selection, application, duration, and risks associated with these devices exists. PICCs are often used in situations when peripheral venous catheters (PIVsincluding ultrasound‐guided peripheral intravenous catheters and midline catheters [midlines]) would meet patient needs and confer a lower risk of complications. An unmet need to define indications and promote utilization that conforms to optimal use currently exists. The purpose of this article was to highlight for hospitalists the methodology and subsequent key recommendations published recently[1] regarding appropriateness of PICCs as they pertain to other vascular access device use.
BACKGROUND
Greater utilization of PICCs to meet a variety of clinical needs has recently emerged in hospital‐based medicine.[2, 3] This phenomenon is likely a function of favorable characteristics when comparing PICCs with traditional CVCs. PICCs are often favored because of safety with insertion in the arm, compatibility with inpatient and outpatient therapies, ease of protocolization for insertion by vascular access nursing services, patient tolerability, and cost savings.[4, 5, 6, 7, 8] Yet limitations of PICCs exist and complications including malpositioning, dislodgement, and luminal occlusion[9, 10, 11] affect patient safety and outcomes. Most notably, PICCs are strongly associated with risk for thrombosis and infection, complications that are most frequent in hospitalized and critically ill patients.[12, 13, 14, 15, 16]
Vascular access devices and particularly PICCs pose a substantial risk for thrombosis.[16, 17, 18, 19, 20] PICCs represent the greatest risk factor for upper extremity deep vein thrombosis (DVT), and in one study, PICC‐associated DVT risk was double that with traditional CVCs.[17] Risk factors for the development of PICC‐associated DVT include ipsilateral paresis,[21] infection,[22] PICC diameter,[19, 20] and prolonged surgery (procedure duration >1 hour) with a PICC in place.[23] Recently, PICCs placed in the upper extremity have been described as a possible risk factor for lower extremity venous thrombosis as well.[24, 25]
Infection complicating CVCs is well described,[12, 15] and guidelines for the prevention of catheter‐associated blood stream infections exist.[26, 27] However, the magnitude of the risk of infection associated with PICCs compared with traditional CVCs remains uncertain. Some reports suggest a decrease risk for infection with the utilization of PICCs[28]; others suggest a similar risk.[29] Existing guidelines, however, do not recommend substituting PICCs for CVCs as a technique to reduce infection, especially in general medical patients.[30]
It is not surprising that variability in the clinical use of PICCs and inappropriate PICC utilization has been described[31, 32] given the heterogeneity of patients and clinical situations in which PICCs are used. Simple awareness of medical devices in place is central to optimizing care. Important to the hospitalist physician is a recent study that found that 1 in 5 physicians were unaware of a CVC being present in their patient.[33] Indeed, emphasis has been placed on optimizing the use of PICC lines nationally through the Choosing Wisely initiative.[34, 35]
A panel of experts was convened at the University of Michigan in an effort to further clarify the appropriate use of VADs. Panelists engaged in a RAND Corporation/University of California Los Angeles (RAND/UCLA) Appropriateness Methodology review[36] to provide guidance regarding VAD use. The RAND/UCLA methodology is a validated way to assess the appropriateness of medical and surgical resource utilization, and details of this methodology are published elsewhere.[1] In brief, each panelist was provided a series of clinical scenarios associated with the use of central venous catheters purposefully including areas of consensus, controversy, and ambiguity. Using a standardized method for rating appropriateness, whereby median ratings on opposite ends of a 1 to 9 scale were used to indicate preference of one device over another (for example 9 reflected appropriate and 13 reflected inappropriate), the methodology classified consensus results into three levels of appropriateness. These three levels are: appropriate when the panel median is between 7 and 9 and without disagreement, uncertain/neutral when the panel median is between 4 and 6 or disagreement exists regardless of the median, or inappropriate when the panel median is between 1 and 3 without disagreement.
RESULTS
Comprehensive results regarding appropriateness ratings are reported elsewhere.[1] Results especially key to hospital‐based practitioners are summarized below. Table 1 highlights common scenarios when PICC placement is considered appropriate and inappropriate.
|
| A. Appropriate indications for PICC use |
| Delivery of peripherally compatible infusates when the proposed duration is 6 or more days* |
| Delivery of nonperipherally compatible infusates (eg, irritants/vesicants) regardless of proposed duration of use |
| Delivery of cyclical or episodic chemotherapy that can be administered through a peripheral vein in patients with active cancer, provided the proposed duration of such treatment is 3 or more months |
| Invasive hemodynamic monitoring or necessary central venous access in a critically ill patient, provided the proposed duration is 15 or more days |
| Frequent phlebotomy (every 8 hours) in a hospitalized patient provided the proposed duration is 6 or more days |
| Intermittent infusions or infrequent phlebotomy in patients with poor/difficult peripheral venous access, provided that the proposed duration is 6 or more days |
| Intermittent infusions or infrequent phlebotomy in patients with poor/difficult peripheral venous access, provided that the proposed duration is 6 or more days |
| For infusions or palliative treatment during end‐of‐life care∥ |
| Delivery of peripherally compatible infusates for patients residing in skilled nursing facilities or transitioning from hospital to home, provided that the proposed duration is at least 15 or more days |
| B. Inappropriate indications for PICC use |
| Placement for any indication other than infusion of nonperipherally compatible infusates (eg, irritants/vesicants) when the proposed duration is 5 or fewer days |
| Placement in a patient with active cancer for cyclical chemotherapy that can be administered through a peripheral vein, when the proposed duration of treatment is 3 or fewer months and peripheral veins are available |
| Placement in a patient with stage 3b or greater chronic kidney disease (estimated glomerular filtration rate <44 mL/min) or in patients currently receiving renal replacement therapy via any modality |
| Insertion for nonfrequent phlebotomy if the proposed duration is 5 or fewer days |
| Patient or family request in a patient that is not actively dying/on hospice for comfort from daily lab draws |
| Medical or nursing provider request in the absence of other appropriate criteria for PICC use |
Appropriateness of PICCs in General Hospitalized Medical Patients
The appropriateness of PICCs when compared to other VADs among hospitalized medical patients can be broadly characterized based upon the planned infusate and the anticipated duration of use. PICCs were the preferred VAD when the anticipated duration of infusion was greater than 15 days or for any duration if the infusion was an irritant/vesicant (such as parenteral nutrition or chemotherapy). PICCs were considered appropriate if the proposed duration of use was 6 to 14 days, though preference for a midline or an ultrasound‐guided PIV was noted for this time‐frame. Tunneled catheters were considered appropriate only for the infusion of an irritant/vesicant when the anticipated duration was 15 days; similarly, implanted ports were rated as appropriate when an irritant/vesicant infusion was planned for 31 days. Both tunneled catheters and ports were rated as appropriate when episodic infusion over the duration of several months was necessary. Disagreement existed between the panelists regarding the appropriateness of PICC placement for the indication of frequent blood draws (3 phlebotomies per day) and among patients with difficult venous access, when phlebotomy would be needed for 5 days. In these cases an individualized patient‐centered approach was recommended. PICC placement was considered appropriate in these situations if venous access was required 6 days, but ultrasound‐guided and midline PIVs were again preferred to PICCs when the expected duration of use was <14 days.
Appropriateness of PICCs in Patients With Chronic Kidney Disease
The appropriateness of PICC use among patients with chronic kidney disease (CKD) takes into consideration disease stage as defined by the Kidney Disease: Improving Global Outcomes workgroup.[37] Although panelist recommendations did not differ for patients with stage 1 to 3a CKD (estimated GFR 45 mL/min) from those noted above, for patient's stage 3b or greater CKD, insertion of devices into an arm vein was rated as inappropriate (valuing the preservation of peripheral and central veins for possible hemodialysis/creation of arteriovenous fistulae and grafts). Among patients with stage 3b or greater CKD, PIV access in the dorsum of the hand was recommended for an expected duration of use 5 days. In consultation with a nephrologist, the use of a tunneled small‐bore central catheter (4 French or 5 French) inserted into the jugular vein was rated as appropriate in stage 3b or greater CKD patients requiring venous access for a longer duration.
Appropriateness of PICC Use in Patients with Cancer
The panelists' acknowledged the heterogeneity of thrombosis risk based on cancer type; recommendations reflect the assumption of cancer as a solid tumor. Vascular access choice among cancer patients is complicated by the cyclic nature of therapy frequently administered, the diversity of infusate (eg, nonirritant or nonvesicant versus irritant/vesicant), and uncertainties surrounding duration of therapy. To address this, the panelists chose a pragmatic approach considering the infusate (irritant/vesicant or not), and dichotomized treatment duration (3 months or not). Among cancer patients requiring nonvesicant/nonirritant chemotherapy for a duration 3 months, interval placement of PIVs was rated as appropriate, and disagreement existed among the panelists regarding the appropriateness of PICCs. If 3 months of chemotherapy was necessary, then PICCs or tunneled‐cuffed catheters were considered appropriate. Ports were rated as appropriate if the expected use was 6 months. Among cancer patients requiring vesicant/emrritant chemotherapy, PICCs and tunneled‐cuffed catheters were rated as appropriate for all time intervals, and ports were rated as neutral for 3‐ to 6‐month durations of infusion, and appropriate for durations greater than 6 months. When acceptable, PICCs were favored over tunneled‐cuffed catheters among cancer patients with coagulopathy (eg, severe thrombocytopenia, elevated international normalized ratios).
Appropriateness of PICCs in Patients With Critical Illness
Among critically ill patients, PIVs and midline catheters were rated as appropriate for infusion of 5 days, and 6 to 14 days, respectively, whereas PICCs were considered appropriate only when use 15 days was anticipated. Although both CVCs and PICCs were rated as appropriate among hemodynamically unstable patients in scenarios where invasive cardiovascular monitoring is necessary for durations of 14 days and 15 days, respectively, CVCs were favored over PICCs among patients who are hemodynamically unstable or requiring vasopressors.
Appropriateness of PICC Use In Special Populations
The existence of patients who require lifelong, often intermittent, intravenous access (eg, sickle cell anemia, short‐gut syndrome, cystic fibrosis) necessitates distinct recommendations for venous access. In this population, recommendations were categorized based on frequency of hospitalization. In patients that were hospitalized infrequently (<5 hospitalizations per year), use of midlines was preferred to PICCs when the hospitalization was expected to last 5 days; PICCs were rated as appropriate for a duration of use 15 days. However, in patients who require frequent hospitalization (6 hospitalizations annually), tunneled‐cuffed catheters were rated as appropriate and preferred over PICCs when the expected duration of use was 15 days per session.
For long‐term residents in skilled nursing facilities, PICCs were rated as appropriate for an expected duration of use 15 days, but uncertain for a duration of 6 to 14 days (when midlines were rated as appropriate). For venous access of 5 days, PIVs were rated as most appropriate.
How, When, by Whom, and Which PICCs Should Be Inserted
Societal recommendations[26] and guidelines[38] for routine placement and positioning of PICCs by dedicated nursing services exist.[39, 40] Panelists favored consultation with the specialists ordering vascular access devices (eg, infectious disease, nephrology, hematology, oncology) within the first few days of admission for optimal device selection and timing of insertion. For example, PICCs were rated as appropriate to be placed within 2 to 3 days of hospital admission for patients requiring longterm antimicrobial infusion (in the absence of bacteremia). Preferential PICC placement by interventional radiology was rated as appropriate if portable ultrasound did not identify a suitable target vein, the catheter fails to advance over the guidewire during a bedside attempt, or the patient requires sedation not appropriate for bedside placement. Interventional radiology insertion was also preferred in patients with bilateral mastectomy, altered chest anatomy, and for patients with permanent pacemakers or defibrillators if the contralateral arm is was not amenable for insertion. PICCs are generally placed at the bedside (with radiographic confirmation of catheter position, or with electrocardiography guidance when proficiency with this technique exists) or under direct visualization in the interventional radiology suite. As recommended elsewhere,[21, 26, 41] panelists rated the placement of the PICC catheter tip in the lower one‐third of the superior vena cava, at the cavoatrial junction, or in the right atrium as being appropriate. Nuanced recommendations surrounding PICC adjustment under varying circumstances can be found in the parent document.[1] Single‐lumen devices, which are associated with fewer complications, were rated as the appropriate default lumen of choice in the absence of a documented rationale for a multilumen PICC as a mechanism to decrease possible complications.[19, 20, 42] The insertion of multilumen PICCs for separating blood draws from infusions or ensuring a backup lumen is available was rated as inappropriate. Consistent with recent recommendations,[43, 44] normal saline rather than heparin was rated as appropriate to maintain catheter patency. The advancement of a migrated PICC was rated as inappropriate under all circumstances.
CONCLUSIONS
In‐hospital healthcare providers are routinely confronted with dilemmas surrounding choice of VAD. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative is a multidisciplinary effort to clarify decision‐making related to VAD use. The systematic literature review and RAND/UCLA appropriateness method applied by the MAGIC panelists identifies areas of broad consensus surrounding the use of PICCs in relation to other VADs, and highlights uncertainties regarding the best practice to guide clinical care. Appropriateness statements facilitate standardization for the use, care, and discontinuation of VADs. These recommendations may be important to healthcare quality officers and payers as they allow for measurement of, and adherence to, standardized practice. In an era of electronic medical records and embedded clinical decision support, these recommendations may facilitate a just‐in‐time resource for optimal VAD management, outcomes measurement, and patient follow‐up. In addition to directing clinical care, these recommendations may serve as a lattice for the formation of future randomized clinical trials to further clarify important areas of the uncertainty surrounding VAD use.
Disclosures: Drs. Woller and Stevens disclose financial support paid to their institution of employment (Intermountain Medical Center) for conducting clinical research (with no financial support paid to either investigator). Dr. Woller discloses serving as an expert panelist for the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative. The authors report no other conflicts of interest.
- , , , et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , , . Peripherally inserted central catheters may lower the incidence of catheter‐related blood stream infections in patients in surgical intensive care units. Surg Infect (Larchmt). 2011;12(4):279–282.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;35(1):34–42.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , . Facility wide benefits of radiology vascular access teams, part 2. Radiol Manage. 2010;32(3):39–43.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 3–4.
- , , , . Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncol. 2013;52(5):886–892.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , . Malposition of peripherally inserted central catheter: experience from 3,012 patients with cancer. Exp Ther Med. 2013;6(4):891–893.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , , et al. A randomised, controlled trial comparing the long‐term effects of peripherally inserted central catheter placement in chemotherapy patients using B‐mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94–103.
- , , , , . A retrospective study on the long‐term placement of peripherally inserted central catheters and the importance of nursing care and education. Cancer Nurs. 2011;34(1):E25–E30.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis. Chest. 2013;143(3):627–633.
- , , , , , . Risk factors associated with peripherally inserted central venous catheter‐related large vein thrombosis in neurological intensive care patients. Intensive Care Med. 2012;38(2):272–278.
- , , , , . Upper extremity venous thrombosis in patients with cancer with peripherally inserted central venous catheters: a retrospective analysis of risk factors. J Oncol Pract. 2013;9(1):e8–e12.
- , , , et al. 2008 Standards, Options and Recommendations (SOR) guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol. 2009;20(9):1459–1471.
- , , , , . The association between picc use and venous thromboembolism in upper and lower extremities. Am J Med. 2015;128(9):986–993.e1.
- , , , et al. VTE Incidence and risk factors in patients with severe sepsis and septic shock. Chest. 2015;148(5):1224–1230.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S).
- , , , , , , et al. Healthcare Infection Control Practices Advisory Committee (HICPAC) (Appendix 1). Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter‐related Infections. Clin Infect Dis. 2011;52:1087–1099.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Clin Infect Dis. 2011;52(9):e162–e193.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- Choosing Wisely. American Society of Nephrology. Don't place peripherally inserted central catheters (PICC) in stage III‐V CKD patients without consulting nephrology. Available at: http://www.choosingwisely.org/clinician‐lists/american‐society‐nephrology‐peripherally‐inserted‐central‐catheters‐in‐stage‐iii‐iv‐ckd‐patients. Accessed November 3, 2015.
- Society of General Internal Medicine. Don't place, or leave in place, peripherally inserted central catheters for patient or provider convenience. Available at: http://www.choosingwisely.org/clinician‐lists/society‐general‐internal‐medicine‐peripherally‐inserted‐central‐catheters‐for‐patient‐provider‐convenience. Accessed November 3, 2015.
- , , , et al. The RAND/UCLA appropriateness method user's manual. Santa Monica, CA: RAND; 2001. Available at: http://www.rand.org/pubs/monograph_reports/MR1269.html.
- National Kidney Foundation/Kidney Disease Outcomes Quality Initiative. KDOQI 2012 clinical practice guidelines for chronic kidney disease. Kidney Inter. 2013;(suppl 3):1–150. Accessed November 3, 2015.
- , , , et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539–573.
- , , , , . Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enteral Nutr. 2005;29(5):374–379.
- , , , . Analysis of tip malposition and correction in peripherally inserted central catheters placed at bedside by a dedicated nursing team. J Vasc Interv Radiol. 2007;18(4):513–518.
- Food and Drug Administration Task Force. Precautions necessary with central venous catheters. FDA Drug Bull. 1989:15–16.
- , , , . Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864–868.
- , , , et al. Flushing the central venous catheter: is heparin necessary? J Vasc Access. 2014;15(4):241–248.
- , , , , , . Heparin versus 0.9% sodium chloride intermittent flushing for prevention of occlusion in central venous catheters in adults. Cochrane Database Syst Rev. 2014;10:CD008462.
Vascular access devices (VADs), including peripherally inserted central venous catheters (PICCs) and traditional central venous catheters (CVCs), remain a cornerstone for the delivery of necessary therapy. VADs are used routinely to treat inpatients and increasingly outpatients too. PICCs possess characteristics that are often favorable in a variety of clinical settings when compared to traditional CVCs. However, a paucity of evidence regarding the indication, selection, application, duration, and risks associated with these devices exists. PICCs are often used in situations when peripheral venous catheters (PIVsincluding ultrasound‐guided peripheral intravenous catheters and midline catheters [midlines]) would meet patient needs and confer a lower risk of complications. An unmet need to define indications and promote utilization that conforms to optimal use currently exists. The purpose of this article was to highlight for hospitalists the methodology and subsequent key recommendations published recently[1] regarding appropriateness of PICCs as they pertain to other vascular access device use.
BACKGROUND
Greater utilization of PICCs to meet a variety of clinical needs has recently emerged in hospital‐based medicine.[2, 3] This phenomenon is likely a function of favorable characteristics when comparing PICCs with traditional CVCs. PICCs are often favored because of safety with insertion in the arm, compatibility with inpatient and outpatient therapies, ease of protocolization for insertion by vascular access nursing services, patient tolerability, and cost savings.[4, 5, 6, 7, 8] Yet limitations of PICCs exist and complications including malpositioning, dislodgement, and luminal occlusion[9, 10, 11] affect patient safety and outcomes. Most notably, PICCs are strongly associated with risk for thrombosis and infection, complications that are most frequent in hospitalized and critically ill patients.[12, 13, 14, 15, 16]
Vascular access devices and particularly PICCs pose a substantial risk for thrombosis.[16, 17, 18, 19, 20] PICCs represent the greatest risk factor for upper extremity deep vein thrombosis (DVT), and in one study, PICC‐associated DVT risk was double that with traditional CVCs.[17] Risk factors for the development of PICC‐associated DVT include ipsilateral paresis,[21] infection,[22] PICC diameter,[19, 20] and prolonged surgery (procedure duration >1 hour) with a PICC in place.[23] Recently, PICCs placed in the upper extremity have been described as a possible risk factor for lower extremity venous thrombosis as well.[24, 25]
Infection complicating CVCs is well described,[12, 15] and guidelines for the prevention of catheter‐associated blood stream infections exist.[26, 27] However, the magnitude of the risk of infection associated with PICCs compared with traditional CVCs remains uncertain. Some reports suggest a decrease risk for infection with the utilization of PICCs[28]; others suggest a similar risk.[29] Existing guidelines, however, do not recommend substituting PICCs for CVCs as a technique to reduce infection, especially in general medical patients.[30]
It is not surprising that variability in the clinical use of PICCs and inappropriate PICC utilization has been described[31, 32] given the heterogeneity of patients and clinical situations in which PICCs are used. Simple awareness of medical devices in place is central to optimizing care. Important to the hospitalist physician is a recent study that found that 1 in 5 physicians were unaware of a CVC being present in their patient.[33] Indeed, emphasis has been placed on optimizing the use of PICC lines nationally through the Choosing Wisely initiative.[34, 35]
A panel of experts was convened at the University of Michigan in an effort to further clarify the appropriate use of VADs. Panelists engaged in a RAND Corporation/University of California Los Angeles (RAND/UCLA) Appropriateness Methodology review[36] to provide guidance regarding VAD use. The RAND/UCLA methodology is a validated way to assess the appropriateness of medical and surgical resource utilization, and details of this methodology are published elsewhere.[1] In brief, each panelist was provided a series of clinical scenarios associated with the use of central venous catheters purposefully including areas of consensus, controversy, and ambiguity. Using a standardized method for rating appropriateness, whereby median ratings on opposite ends of a 1 to 9 scale were used to indicate preference of one device over another (for example 9 reflected appropriate and 13 reflected inappropriate), the methodology classified consensus results into three levels of appropriateness. These three levels are: appropriate when the panel median is between 7 and 9 and without disagreement, uncertain/neutral when the panel median is between 4 and 6 or disagreement exists regardless of the median, or inappropriate when the panel median is between 1 and 3 without disagreement.
RESULTS
Comprehensive results regarding appropriateness ratings are reported elsewhere.[1] Results especially key to hospital‐based practitioners are summarized below. Table 1 highlights common scenarios when PICC placement is considered appropriate and inappropriate.
|
| A. Appropriate indications for PICC use |
| Delivery of peripherally compatible infusates when the proposed duration is 6 or more days* |
| Delivery of nonperipherally compatible infusates (eg, irritants/vesicants) regardless of proposed duration of use |
| Delivery of cyclical or episodic chemotherapy that can be administered through a peripheral vein in patients with active cancer, provided the proposed duration of such treatment is 3 or more months |
| Invasive hemodynamic monitoring or necessary central venous access in a critically ill patient, provided the proposed duration is 15 or more days |
| Frequent phlebotomy (every 8 hours) in a hospitalized patient provided the proposed duration is 6 or more days |
| Intermittent infusions or infrequent phlebotomy in patients with poor/difficult peripheral venous access, provided that the proposed duration is 6 or more days |
| Intermittent infusions or infrequent phlebotomy in patients with poor/difficult peripheral venous access, provided that the proposed duration is 6 or more days |
| For infusions or palliative treatment during end‐of‐life care∥ |
| Delivery of peripherally compatible infusates for patients residing in skilled nursing facilities or transitioning from hospital to home, provided that the proposed duration is at least 15 or more days |
| B. Inappropriate indications for PICC use |
| Placement for any indication other than infusion of nonperipherally compatible infusates (eg, irritants/vesicants) when the proposed duration is 5 or fewer days |
| Placement in a patient with active cancer for cyclical chemotherapy that can be administered through a peripheral vein, when the proposed duration of treatment is 3 or fewer months and peripheral veins are available |
| Placement in a patient with stage 3b or greater chronic kidney disease (estimated glomerular filtration rate <44 mL/min) or in patients currently receiving renal replacement therapy via any modality |
| Insertion for nonfrequent phlebotomy if the proposed duration is 5 or fewer days |
| Patient or family request in a patient that is not actively dying/on hospice for comfort from daily lab draws |
| Medical or nursing provider request in the absence of other appropriate criteria for PICC use |
Appropriateness of PICCs in General Hospitalized Medical Patients
The appropriateness of PICCs when compared to other VADs among hospitalized medical patients can be broadly characterized based upon the planned infusate and the anticipated duration of use. PICCs were the preferred VAD when the anticipated duration of infusion was greater than 15 days or for any duration if the infusion was an irritant/vesicant (such as parenteral nutrition or chemotherapy). PICCs were considered appropriate if the proposed duration of use was 6 to 14 days, though preference for a midline or an ultrasound‐guided PIV was noted for this time‐frame. Tunneled catheters were considered appropriate only for the infusion of an irritant/vesicant when the anticipated duration was 15 days; similarly, implanted ports were rated as appropriate when an irritant/vesicant infusion was planned for 31 days. Both tunneled catheters and ports were rated as appropriate when episodic infusion over the duration of several months was necessary. Disagreement existed between the panelists regarding the appropriateness of PICC placement for the indication of frequent blood draws (3 phlebotomies per day) and among patients with difficult venous access, when phlebotomy would be needed for 5 days. In these cases an individualized patient‐centered approach was recommended. PICC placement was considered appropriate in these situations if venous access was required 6 days, but ultrasound‐guided and midline PIVs were again preferred to PICCs when the expected duration of use was <14 days.
Appropriateness of PICCs in Patients With Chronic Kidney Disease
The appropriateness of PICC use among patients with chronic kidney disease (CKD) takes into consideration disease stage as defined by the Kidney Disease: Improving Global Outcomes workgroup.[37] Although panelist recommendations did not differ for patients with stage 1 to 3a CKD (estimated GFR 45 mL/min) from those noted above, for patient's stage 3b or greater CKD, insertion of devices into an arm vein was rated as inappropriate (valuing the preservation of peripheral and central veins for possible hemodialysis/creation of arteriovenous fistulae and grafts). Among patients with stage 3b or greater CKD, PIV access in the dorsum of the hand was recommended for an expected duration of use 5 days. In consultation with a nephrologist, the use of a tunneled small‐bore central catheter (4 French or 5 French) inserted into the jugular vein was rated as appropriate in stage 3b or greater CKD patients requiring venous access for a longer duration.
Appropriateness of PICC Use in Patients with Cancer
The panelists' acknowledged the heterogeneity of thrombosis risk based on cancer type; recommendations reflect the assumption of cancer as a solid tumor. Vascular access choice among cancer patients is complicated by the cyclic nature of therapy frequently administered, the diversity of infusate (eg, nonirritant or nonvesicant versus irritant/vesicant), and uncertainties surrounding duration of therapy. To address this, the panelists chose a pragmatic approach considering the infusate (irritant/vesicant or not), and dichotomized treatment duration (3 months or not). Among cancer patients requiring nonvesicant/nonirritant chemotherapy for a duration 3 months, interval placement of PIVs was rated as appropriate, and disagreement existed among the panelists regarding the appropriateness of PICCs. If 3 months of chemotherapy was necessary, then PICCs or tunneled‐cuffed catheters were considered appropriate. Ports were rated as appropriate if the expected use was 6 months. Among cancer patients requiring vesicant/emrritant chemotherapy, PICCs and tunneled‐cuffed catheters were rated as appropriate for all time intervals, and ports were rated as neutral for 3‐ to 6‐month durations of infusion, and appropriate for durations greater than 6 months. When acceptable, PICCs were favored over tunneled‐cuffed catheters among cancer patients with coagulopathy (eg, severe thrombocytopenia, elevated international normalized ratios).
Appropriateness of PICCs in Patients With Critical Illness
Among critically ill patients, PIVs and midline catheters were rated as appropriate for infusion of 5 days, and 6 to 14 days, respectively, whereas PICCs were considered appropriate only when use 15 days was anticipated. Although both CVCs and PICCs were rated as appropriate among hemodynamically unstable patients in scenarios where invasive cardiovascular monitoring is necessary for durations of 14 days and 15 days, respectively, CVCs were favored over PICCs among patients who are hemodynamically unstable or requiring vasopressors.
Appropriateness of PICC Use In Special Populations
The existence of patients who require lifelong, often intermittent, intravenous access (eg, sickle cell anemia, short‐gut syndrome, cystic fibrosis) necessitates distinct recommendations for venous access. In this population, recommendations were categorized based on frequency of hospitalization. In patients that were hospitalized infrequently (<5 hospitalizations per year), use of midlines was preferred to PICCs when the hospitalization was expected to last 5 days; PICCs were rated as appropriate for a duration of use 15 days. However, in patients who require frequent hospitalization (6 hospitalizations annually), tunneled‐cuffed catheters were rated as appropriate and preferred over PICCs when the expected duration of use was 15 days per session.
For long‐term residents in skilled nursing facilities, PICCs were rated as appropriate for an expected duration of use 15 days, but uncertain for a duration of 6 to 14 days (when midlines were rated as appropriate). For venous access of 5 days, PIVs were rated as most appropriate.
How, When, by Whom, and Which PICCs Should Be Inserted
Societal recommendations[26] and guidelines[38] for routine placement and positioning of PICCs by dedicated nursing services exist.[39, 40] Panelists favored consultation with the specialists ordering vascular access devices (eg, infectious disease, nephrology, hematology, oncology) within the first few days of admission for optimal device selection and timing of insertion. For example, PICCs were rated as appropriate to be placed within 2 to 3 days of hospital admission for patients requiring longterm antimicrobial infusion (in the absence of bacteremia). Preferential PICC placement by interventional radiology was rated as appropriate if portable ultrasound did not identify a suitable target vein, the catheter fails to advance over the guidewire during a bedside attempt, or the patient requires sedation not appropriate for bedside placement. Interventional radiology insertion was also preferred in patients with bilateral mastectomy, altered chest anatomy, and for patients with permanent pacemakers or defibrillators if the contralateral arm is was not amenable for insertion. PICCs are generally placed at the bedside (with radiographic confirmation of catheter position, or with electrocardiography guidance when proficiency with this technique exists) or under direct visualization in the interventional radiology suite. As recommended elsewhere,[21, 26, 41] panelists rated the placement of the PICC catheter tip in the lower one‐third of the superior vena cava, at the cavoatrial junction, or in the right atrium as being appropriate. Nuanced recommendations surrounding PICC adjustment under varying circumstances can be found in the parent document.[1] Single‐lumen devices, which are associated with fewer complications, were rated as the appropriate default lumen of choice in the absence of a documented rationale for a multilumen PICC as a mechanism to decrease possible complications.[19, 20, 42] The insertion of multilumen PICCs for separating blood draws from infusions or ensuring a backup lumen is available was rated as inappropriate. Consistent with recent recommendations,[43, 44] normal saline rather than heparin was rated as appropriate to maintain catheter patency. The advancement of a migrated PICC was rated as inappropriate under all circumstances.
CONCLUSIONS
In‐hospital healthcare providers are routinely confronted with dilemmas surrounding choice of VAD. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative is a multidisciplinary effort to clarify decision‐making related to VAD use. The systematic literature review and RAND/UCLA appropriateness method applied by the MAGIC panelists identifies areas of broad consensus surrounding the use of PICCs in relation to other VADs, and highlights uncertainties regarding the best practice to guide clinical care. Appropriateness statements facilitate standardization for the use, care, and discontinuation of VADs. These recommendations may be important to healthcare quality officers and payers as they allow for measurement of, and adherence to, standardized practice. In an era of electronic medical records and embedded clinical decision support, these recommendations may facilitate a just‐in‐time resource for optimal VAD management, outcomes measurement, and patient follow‐up. In addition to directing clinical care, these recommendations may serve as a lattice for the formation of future randomized clinical trials to further clarify important areas of the uncertainty surrounding VAD use.
Disclosures: Drs. Woller and Stevens disclose financial support paid to their institution of employment (Intermountain Medical Center) for conducting clinical research (with no financial support paid to either investigator). Dr. Woller discloses serving as an expert panelist for the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative. The authors report no other conflicts of interest.
Vascular access devices (VADs), including peripherally inserted central venous catheters (PICCs) and traditional central venous catheters (CVCs), remain a cornerstone for the delivery of necessary therapy. VADs are used routinely to treat inpatients and increasingly outpatients too. PICCs possess characteristics that are often favorable in a variety of clinical settings when compared to traditional CVCs. However, a paucity of evidence regarding the indication, selection, application, duration, and risks associated with these devices exists. PICCs are often used in situations when peripheral venous catheters (PIVsincluding ultrasound‐guided peripheral intravenous catheters and midline catheters [midlines]) would meet patient needs and confer a lower risk of complications. An unmet need to define indications and promote utilization that conforms to optimal use currently exists. The purpose of this article was to highlight for hospitalists the methodology and subsequent key recommendations published recently[1] regarding appropriateness of PICCs as they pertain to other vascular access device use.
BACKGROUND
Greater utilization of PICCs to meet a variety of clinical needs has recently emerged in hospital‐based medicine.[2, 3] This phenomenon is likely a function of favorable characteristics when comparing PICCs with traditional CVCs. PICCs are often favored because of safety with insertion in the arm, compatibility with inpatient and outpatient therapies, ease of protocolization for insertion by vascular access nursing services, patient tolerability, and cost savings.[4, 5, 6, 7, 8] Yet limitations of PICCs exist and complications including malpositioning, dislodgement, and luminal occlusion[9, 10, 11] affect patient safety and outcomes. Most notably, PICCs are strongly associated with risk for thrombosis and infection, complications that are most frequent in hospitalized and critically ill patients.[12, 13, 14, 15, 16]
Vascular access devices and particularly PICCs pose a substantial risk for thrombosis.[16, 17, 18, 19, 20] PICCs represent the greatest risk factor for upper extremity deep vein thrombosis (DVT), and in one study, PICC‐associated DVT risk was double that with traditional CVCs.[17] Risk factors for the development of PICC‐associated DVT include ipsilateral paresis,[21] infection,[22] PICC diameter,[19, 20] and prolonged surgery (procedure duration >1 hour) with a PICC in place.[23] Recently, PICCs placed in the upper extremity have been described as a possible risk factor for lower extremity venous thrombosis as well.[24, 25]
Infection complicating CVCs is well described,[12, 15] and guidelines for the prevention of catheter‐associated blood stream infections exist.[26, 27] However, the magnitude of the risk of infection associated with PICCs compared with traditional CVCs remains uncertain. Some reports suggest a decrease risk for infection with the utilization of PICCs[28]; others suggest a similar risk.[29] Existing guidelines, however, do not recommend substituting PICCs for CVCs as a technique to reduce infection, especially in general medical patients.[30]
It is not surprising that variability in the clinical use of PICCs and inappropriate PICC utilization has been described[31, 32] given the heterogeneity of patients and clinical situations in which PICCs are used. Simple awareness of medical devices in place is central to optimizing care. Important to the hospitalist physician is a recent study that found that 1 in 5 physicians were unaware of a CVC being present in their patient.[33] Indeed, emphasis has been placed on optimizing the use of PICC lines nationally through the Choosing Wisely initiative.[34, 35]
A panel of experts was convened at the University of Michigan in an effort to further clarify the appropriate use of VADs. Panelists engaged in a RAND Corporation/University of California Los Angeles (RAND/UCLA) Appropriateness Methodology review[36] to provide guidance regarding VAD use. The RAND/UCLA methodology is a validated way to assess the appropriateness of medical and surgical resource utilization, and details of this methodology are published elsewhere.[1] In brief, each panelist was provided a series of clinical scenarios associated with the use of central venous catheters purposefully including areas of consensus, controversy, and ambiguity. Using a standardized method for rating appropriateness, whereby median ratings on opposite ends of a 1 to 9 scale were used to indicate preference of one device over another (for example 9 reflected appropriate and 13 reflected inappropriate), the methodology classified consensus results into three levels of appropriateness. These three levels are: appropriate when the panel median is between 7 and 9 and without disagreement, uncertain/neutral when the panel median is between 4 and 6 or disagreement exists regardless of the median, or inappropriate when the panel median is between 1 and 3 without disagreement.
RESULTS
Comprehensive results regarding appropriateness ratings are reported elsewhere.[1] Results especially key to hospital‐based practitioners are summarized below. Table 1 highlights common scenarios when PICC placement is considered appropriate and inappropriate.
|
| A. Appropriate indications for PICC use |
| Delivery of peripherally compatible infusates when the proposed duration is 6 or more days* |
| Delivery of nonperipherally compatible infusates (eg, irritants/vesicants) regardless of proposed duration of use |
| Delivery of cyclical or episodic chemotherapy that can be administered through a peripheral vein in patients with active cancer, provided the proposed duration of such treatment is 3 or more months |
| Invasive hemodynamic monitoring or necessary central venous access in a critically ill patient, provided the proposed duration is 15 or more days |
| Frequent phlebotomy (every 8 hours) in a hospitalized patient provided the proposed duration is 6 or more days |
| Intermittent infusions or infrequent phlebotomy in patients with poor/difficult peripheral venous access, provided that the proposed duration is 6 or more days |
| Intermittent infusions or infrequent phlebotomy in patients with poor/difficult peripheral venous access, provided that the proposed duration is 6 or more days |
| For infusions or palliative treatment during end‐of‐life care∥ |
| Delivery of peripherally compatible infusates for patients residing in skilled nursing facilities or transitioning from hospital to home, provided that the proposed duration is at least 15 or more days |
| B. Inappropriate indications for PICC use |
| Placement for any indication other than infusion of nonperipherally compatible infusates (eg, irritants/vesicants) when the proposed duration is 5 or fewer days |
| Placement in a patient with active cancer for cyclical chemotherapy that can be administered through a peripheral vein, when the proposed duration of treatment is 3 or fewer months and peripheral veins are available |
| Placement in a patient with stage 3b or greater chronic kidney disease (estimated glomerular filtration rate <44 mL/min) or in patients currently receiving renal replacement therapy via any modality |
| Insertion for nonfrequent phlebotomy if the proposed duration is 5 or fewer days |
| Patient or family request in a patient that is not actively dying/on hospice for comfort from daily lab draws |
| Medical or nursing provider request in the absence of other appropriate criteria for PICC use |
Appropriateness of PICCs in General Hospitalized Medical Patients
The appropriateness of PICCs when compared to other VADs among hospitalized medical patients can be broadly characterized based upon the planned infusate and the anticipated duration of use. PICCs were the preferred VAD when the anticipated duration of infusion was greater than 15 days or for any duration if the infusion was an irritant/vesicant (such as parenteral nutrition or chemotherapy). PICCs were considered appropriate if the proposed duration of use was 6 to 14 days, though preference for a midline or an ultrasound‐guided PIV was noted for this time‐frame. Tunneled catheters were considered appropriate only for the infusion of an irritant/vesicant when the anticipated duration was 15 days; similarly, implanted ports were rated as appropriate when an irritant/vesicant infusion was planned for 31 days. Both tunneled catheters and ports were rated as appropriate when episodic infusion over the duration of several months was necessary. Disagreement existed between the panelists regarding the appropriateness of PICC placement for the indication of frequent blood draws (3 phlebotomies per day) and among patients with difficult venous access, when phlebotomy would be needed for 5 days. In these cases an individualized patient‐centered approach was recommended. PICC placement was considered appropriate in these situations if venous access was required 6 days, but ultrasound‐guided and midline PIVs were again preferred to PICCs when the expected duration of use was <14 days.
Appropriateness of PICCs in Patients With Chronic Kidney Disease
The appropriateness of PICC use among patients with chronic kidney disease (CKD) takes into consideration disease stage as defined by the Kidney Disease: Improving Global Outcomes workgroup.[37] Although panelist recommendations did not differ for patients with stage 1 to 3a CKD (estimated GFR 45 mL/min) from those noted above, for patient's stage 3b or greater CKD, insertion of devices into an arm vein was rated as inappropriate (valuing the preservation of peripheral and central veins for possible hemodialysis/creation of arteriovenous fistulae and grafts). Among patients with stage 3b or greater CKD, PIV access in the dorsum of the hand was recommended for an expected duration of use 5 days. In consultation with a nephrologist, the use of a tunneled small‐bore central catheter (4 French or 5 French) inserted into the jugular vein was rated as appropriate in stage 3b or greater CKD patients requiring venous access for a longer duration.
Appropriateness of PICC Use in Patients with Cancer
The panelists' acknowledged the heterogeneity of thrombosis risk based on cancer type; recommendations reflect the assumption of cancer as a solid tumor. Vascular access choice among cancer patients is complicated by the cyclic nature of therapy frequently administered, the diversity of infusate (eg, nonirritant or nonvesicant versus irritant/vesicant), and uncertainties surrounding duration of therapy. To address this, the panelists chose a pragmatic approach considering the infusate (irritant/vesicant or not), and dichotomized treatment duration (3 months or not). Among cancer patients requiring nonvesicant/nonirritant chemotherapy for a duration 3 months, interval placement of PIVs was rated as appropriate, and disagreement existed among the panelists regarding the appropriateness of PICCs. If 3 months of chemotherapy was necessary, then PICCs or tunneled‐cuffed catheters were considered appropriate. Ports were rated as appropriate if the expected use was 6 months. Among cancer patients requiring vesicant/emrritant chemotherapy, PICCs and tunneled‐cuffed catheters were rated as appropriate for all time intervals, and ports were rated as neutral for 3‐ to 6‐month durations of infusion, and appropriate for durations greater than 6 months. When acceptable, PICCs were favored over tunneled‐cuffed catheters among cancer patients with coagulopathy (eg, severe thrombocytopenia, elevated international normalized ratios).
Appropriateness of PICCs in Patients With Critical Illness
Among critically ill patients, PIVs and midline catheters were rated as appropriate for infusion of 5 days, and 6 to 14 days, respectively, whereas PICCs were considered appropriate only when use 15 days was anticipated. Although both CVCs and PICCs were rated as appropriate among hemodynamically unstable patients in scenarios where invasive cardiovascular monitoring is necessary for durations of 14 days and 15 days, respectively, CVCs were favored over PICCs among patients who are hemodynamically unstable or requiring vasopressors.
Appropriateness of PICC Use In Special Populations
The existence of patients who require lifelong, often intermittent, intravenous access (eg, sickle cell anemia, short‐gut syndrome, cystic fibrosis) necessitates distinct recommendations for venous access. In this population, recommendations were categorized based on frequency of hospitalization. In patients that were hospitalized infrequently (<5 hospitalizations per year), use of midlines was preferred to PICCs when the hospitalization was expected to last 5 days; PICCs were rated as appropriate for a duration of use 15 days. However, in patients who require frequent hospitalization (6 hospitalizations annually), tunneled‐cuffed catheters were rated as appropriate and preferred over PICCs when the expected duration of use was 15 days per session.
For long‐term residents in skilled nursing facilities, PICCs were rated as appropriate for an expected duration of use 15 days, but uncertain for a duration of 6 to 14 days (when midlines were rated as appropriate). For venous access of 5 days, PIVs were rated as most appropriate.
How, When, by Whom, and Which PICCs Should Be Inserted
Societal recommendations[26] and guidelines[38] for routine placement and positioning of PICCs by dedicated nursing services exist.[39, 40] Panelists favored consultation with the specialists ordering vascular access devices (eg, infectious disease, nephrology, hematology, oncology) within the first few days of admission for optimal device selection and timing of insertion. For example, PICCs were rated as appropriate to be placed within 2 to 3 days of hospital admission for patients requiring longterm antimicrobial infusion (in the absence of bacteremia). Preferential PICC placement by interventional radiology was rated as appropriate if portable ultrasound did not identify a suitable target vein, the catheter fails to advance over the guidewire during a bedside attempt, or the patient requires sedation not appropriate for bedside placement. Interventional radiology insertion was also preferred in patients with bilateral mastectomy, altered chest anatomy, and for patients with permanent pacemakers or defibrillators if the contralateral arm is was not amenable for insertion. PICCs are generally placed at the bedside (with radiographic confirmation of catheter position, or with electrocardiography guidance when proficiency with this technique exists) or under direct visualization in the interventional radiology suite. As recommended elsewhere,[21, 26, 41] panelists rated the placement of the PICC catheter tip in the lower one‐third of the superior vena cava, at the cavoatrial junction, or in the right atrium as being appropriate. Nuanced recommendations surrounding PICC adjustment under varying circumstances can be found in the parent document.[1] Single‐lumen devices, which are associated with fewer complications, were rated as the appropriate default lumen of choice in the absence of a documented rationale for a multilumen PICC as a mechanism to decrease possible complications.[19, 20, 42] The insertion of multilumen PICCs for separating blood draws from infusions or ensuring a backup lumen is available was rated as inappropriate. Consistent with recent recommendations,[43, 44] normal saline rather than heparin was rated as appropriate to maintain catheter patency. The advancement of a migrated PICC was rated as inappropriate under all circumstances.
CONCLUSIONS
In‐hospital healthcare providers are routinely confronted with dilemmas surrounding choice of VAD. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative is a multidisciplinary effort to clarify decision‐making related to VAD use. The systematic literature review and RAND/UCLA appropriateness method applied by the MAGIC panelists identifies areas of broad consensus surrounding the use of PICCs in relation to other VADs, and highlights uncertainties regarding the best practice to guide clinical care. Appropriateness statements facilitate standardization for the use, care, and discontinuation of VADs. These recommendations may be important to healthcare quality officers and payers as they allow for measurement of, and adherence to, standardized practice. In an era of electronic medical records and embedded clinical decision support, these recommendations may facilitate a just‐in‐time resource for optimal VAD management, outcomes measurement, and patient follow‐up. In addition to directing clinical care, these recommendations may serve as a lattice for the formation of future randomized clinical trials to further clarify important areas of the uncertainty surrounding VAD use.
Disclosures: Drs. Woller and Stevens disclose financial support paid to their institution of employment (Intermountain Medical Center) for conducting clinical research (with no financial support paid to either investigator). Dr. Woller discloses serving as an expert panelist for the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative. The authors report no other conflicts of interest.
- , , , et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , , . Peripherally inserted central catheters may lower the incidence of catheter‐related blood stream infections in patients in surgical intensive care units. Surg Infect (Larchmt). 2011;12(4):279–282.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;35(1):34–42.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , . Facility wide benefits of radiology vascular access teams, part 2. Radiol Manage. 2010;32(3):39–43.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 3–4.
- , , , . Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncol. 2013;52(5):886–892.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , . Malposition of peripherally inserted central catheter: experience from 3,012 patients with cancer. Exp Ther Med. 2013;6(4):891–893.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , , et al. A randomised, controlled trial comparing the long‐term effects of peripherally inserted central catheter placement in chemotherapy patients using B‐mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94–103.
- , , , , . A retrospective study on the long‐term placement of peripherally inserted central catheters and the importance of nursing care and education. Cancer Nurs. 2011;34(1):E25–E30.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis. Chest. 2013;143(3):627–633.
- , , , , , . Risk factors associated with peripherally inserted central venous catheter‐related large vein thrombosis in neurological intensive care patients. Intensive Care Med. 2012;38(2):272–278.
- , , , , . Upper extremity venous thrombosis in patients with cancer with peripherally inserted central venous catheters: a retrospective analysis of risk factors. J Oncol Pract. 2013;9(1):e8–e12.
- , , , et al. 2008 Standards, Options and Recommendations (SOR) guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol. 2009;20(9):1459–1471.
- , , , , . The association between picc use and venous thromboembolism in upper and lower extremities. Am J Med. 2015;128(9):986–993.e1.
- , , , et al. VTE Incidence and risk factors in patients with severe sepsis and septic shock. Chest. 2015;148(5):1224–1230.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S).
- , , , , , , et al. Healthcare Infection Control Practices Advisory Committee (HICPAC) (Appendix 1). Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter‐related Infections. Clin Infect Dis. 2011;52:1087–1099.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Clin Infect Dis. 2011;52(9):e162–e193.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- Choosing Wisely. American Society of Nephrology. Don't place peripherally inserted central catheters (PICC) in stage III‐V CKD patients without consulting nephrology. Available at: http://www.choosingwisely.org/clinician‐lists/american‐society‐nephrology‐peripherally‐inserted‐central‐catheters‐in‐stage‐iii‐iv‐ckd‐patients. Accessed November 3, 2015.
- Society of General Internal Medicine. Don't place, or leave in place, peripherally inserted central catheters for patient or provider convenience. Available at: http://www.choosingwisely.org/clinician‐lists/society‐general‐internal‐medicine‐peripherally‐inserted‐central‐catheters‐for‐patient‐provider‐convenience. Accessed November 3, 2015.
- , , , et al. The RAND/UCLA appropriateness method user's manual. Santa Monica, CA: RAND; 2001. Available at: http://www.rand.org/pubs/monograph_reports/MR1269.html.
- National Kidney Foundation/Kidney Disease Outcomes Quality Initiative. KDOQI 2012 clinical practice guidelines for chronic kidney disease. Kidney Inter. 2013;(suppl 3):1–150. Accessed November 3, 2015.
- , , , et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539–573.
- , , , , . Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enteral Nutr. 2005;29(5):374–379.
- , , , . Analysis of tip malposition and correction in peripherally inserted central catheters placed at bedside by a dedicated nursing team. J Vasc Interv Radiol. 2007;18(4):513–518.
- Food and Drug Administration Task Force. Precautions necessary with central venous catheters. FDA Drug Bull. 1989:15–16.
- , , , . Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864–868.
- , , , et al. Flushing the central venous catheter: is heparin necessary? J Vasc Access. 2014;15(4):241–248.
- , , , , , . Heparin versus 0.9% sodium chloride intermittent flushing for prevention of occlusion in central venous catheters in adults. Cochrane Database Syst Rev. 2014;10:CD008462.
- , , , et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , , . Peripherally inserted central catheters may lower the incidence of catheter‐related blood stream infections in patients in surgical intensive care units. Surg Infect (Larchmt). 2011;12(4):279–282.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;35(1):34–42.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , . Facility wide benefits of radiology vascular access teams, part 2. Radiol Manage. 2010;32(3):39–43.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 3–4.
- , , , . Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncol. 2013;52(5):886–892.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , . Malposition of peripherally inserted central catheter: experience from 3,012 patients with cancer. Exp Ther Med. 2013;6(4):891–893.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , , et al. A randomised, controlled trial comparing the long‐term effects of peripherally inserted central catheter placement in chemotherapy patients using B‐mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94–103.
- , , , , . A retrospective study on the long‐term placement of peripherally inserted central catheters and the importance of nursing care and education. Cancer Nurs. 2011;34(1):E25–E30.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , . Upper extremity deep vein thrombosis: a community‐based perspective. Am J Med. 2007;120(8):678–684.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis. Chest. 2013;143(3):627–633.
- , , , , , . Risk factors associated with peripherally inserted central venous catheter‐related large vein thrombosis in neurological intensive care patients. Intensive Care Med. 2012;38(2):272–278.
- , , , , . Upper extremity venous thrombosis in patients with cancer with peripherally inserted central venous catheters: a retrospective analysis of risk factors. J Oncol Pract. 2013;9(1):e8–e12.
- , , , et al. 2008 Standards, Options and Recommendations (SOR) guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol. 2009;20(9):1459–1471.
- , , , , . The association between picc use and venous thromboembolism in upper and lower extremities. Am J Med. 2015;128(9):986–993.e1.
- , , , et al. VTE Incidence and risk factors in patients with severe sepsis and septic shock. Chest. 2015;148(5):1224–1230.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S).
- , , , , , , et al. Healthcare Infection Control Practices Advisory Committee (HICPAC) (Appendix 1). Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter‐related Infections. Clin Infect Dis. 2011;52:1087–1099.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Clin Infect Dis. 2011;52(9):e162–e193.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- Choosing Wisely. American Society of Nephrology. Don't place peripherally inserted central catheters (PICC) in stage III‐V CKD patients without consulting nephrology. Available at: http://www.choosingwisely.org/clinician‐lists/american‐society‐nephrology‐peripherally‐inserted‐central‐catheters‐in‐stage‐iii‐iv‐ckd‐patients. Accessed November 3, 2015.
- Society of General Internal Medicine. Don't place, or leave in place, peripherally inserted central catheters for patient or provider convenience. Available at: http://www.choosingwisely.org/clinician‐lists/society‐general‐internal‐medicine‐peripherally‐inserted‐central‐catheters‐for‐patient‐provider‐convenience. Accessed November 3, 2015.
- , , , et al. The RAND/UCLA appropriateness method user's manual. Santa Monica, CA: RAND; 2001. Available at: http://www.rand.org/pubs/monograph_reports/MR1269.html.
- National Kidney Foundation/Kidney Disease Outcomes Quality Initiative. KDOQI 2012 clinical practice guidelines for chronic kidney disease. Kidney Inter. 2013;(suppl 3):1–150. Accessed November 3, 2015.
- , , , et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539–573.
- , , , , . Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enteral Nutr. 2005;29(5):374–379.
- , , , . Analysis of tip malposition and correction in peripherally inserted central catheters placed at bedside by a dedicated nursing team. J Vasc Interv Radiol. 2007;18(4):513–518.
- Food and Drug Administration Task Force. Precautions necessary with central venous catheters. FDA Drug Bull. 1989:15–16.
- , , , . Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864–868.
- , , , et al. Flushing the central venous catheter: is heparin necessary? J Vasc Access. 2014;15(4):241–248.
- , , , , , . Heparin versus 0.9% sodium chloride intermittent flushing for prevention of occlusion in central venous catheters in adults. Cochrane Database Syst Rev. 2014;10:CD008462.