Article

Ocular Chemical Burns in the Dermatology Office: A Practical Approach to Managing Safety Precautions
- Author:
- Deborah J. Moon, MD
- Shawna Langley, MD
Dermatologists should be cognizant of potential hazards to the eyes during facial procedures and always take proper precautions to decrease the...
Article
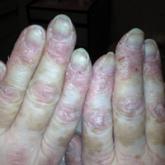
Amyopathic Dermatomyositis With Plantar Keratoderma Responding to Methotrexate Therapy
- Author:
- Travis J. Morrell, MD, MPH
- William Soren Mortensen, MD
- Shawna Langley, MD
Amyopathic dermatomyositis (ADM) represents a substantial subset of dermatomyositis (DM). Patients with this symptom of the disorder may present...
