User login
Cutaneous T-Cell Lymphoma in a Patient With Celiac Disease
To the Editor:
Mycosis fungoides (MF) is the most common form of a heterogeneous group of non-Hodgkin lymphomas known as cutaneous T-cell lymphomas. Celiac disease (CD) is associated with increased risk for development of enteropathy-associated T-cell lymphoma and other intraintestinal and extraintestinal non-Hodgkin lymphomas, but a firm association between CD and MF has not been established.1 The first and second cases of concomitant MF and CD were reported in 1985 and 2009 by Coulson and Sanderson2 and Moreira et al,3 respectively. Two other reports of celiac-associated dermatitis herpetiformis and MF exist.4,5 We report a patient with a unique constellation of MF, CD, and Sjögren syndrome (SS).
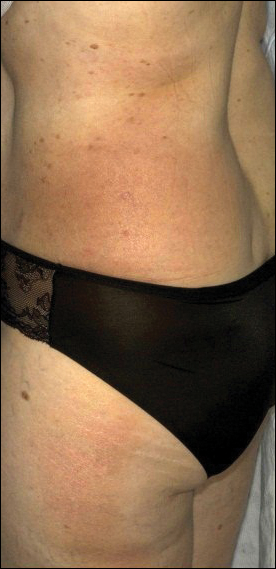
A 54-year-old woman presented with a worsening nonpruritic, slightly tender, eczematous patch on the back of 19 years’ duration. She had a history of SS diagnosed by salivary gland biopsy. She also had a diagnosis of CD confirmed with positive antigliadin IgA antibodies, with a dramatic improvement in symptoms on a gluten-free diet (GFD) after having abdominal pain and diarrhea for many years. She had no evidence of dermatitis herpetiformis. Recently, more red-brown areas of confluent light pink erythema without clear-cut borders had appeared on the axillae, trunk, and thigh (Figure). The patient also noted new lesions and more erythema of the patches when not adhering to a GFD. A biopsy specimen from the left side of the lateral trunk revealed a bandlike lymphocytic infiltrate with irregular nuclear contours displaying epidermotropism with a few Pautrier microabscesses. Immunohistochemistry showed strong CD3 and CD4 positivity with loss of CD7 and scattered CD8 staining. Peripheral blood flow cytometry showed no aberrant cell populations. The patient was diagnosed with MF stage IB and treated with topical corticosteroids and natural light with improvement.
It has been hypothesized that early MF is an autoimmune process caused by dysregulation of a lymphocytic reaction against chronic exogenous or endogenous antigens.4,5 The association of MF with CD supports the possibility of lymphocytic stimulation by a persistent antigen (ie, gluten) in the gastrointestinal tract. Porter et al4 suggested that in susceptible individuals, the resulting clonal T cells may migrate into the epidermis, causing MF. This theory also is supported by the finding that adherence to a GFD leads to decreased risk for malignancy and morbidity.6 In our patient, the chronic autoimmune stimulation in SS could be a factor in the pathogenesis of MF. Additionally, SS, CD, and MF are all strongly associated with increased incidence of specific but different HLA class II antigens. Mycosis fungoides is associated with HLA-DR5 and DQB1*03 alleles, CD with HLA-DQ2 and DQ8, and SS with HLA-DR15 and DR3. We do not know the HLA type of our patient, but she likely possessed multiple alleles, leading to the unique aggregation of diseases.
Furthermore, studies have shown that lymphocytes in CD patients display impaired regulatory T-cell function, causing increased incidence of autoimmune diseases and malignancy.7,8 By this theory, the occurrence of MF in patients is facilitated by the inability of CD lymphocytes to control the abnormal T-cell proliferation in the skin. Interestingly, the finding of SS in our patient supports the possibility of impaired regulatory T-cell function.
Although the occurrence of both MF and CD in our patient could be coincidental, the possibility of correlation must be considered as more cases are documented.
- Catassi C, Fabiani E, Corrao G, et al; Italian Working Group on Coeliac Disease and Non-Hodgkin’s-Lymphoma. Risk of non-Hodgkin Lymphoma in celiac disease. JAMA. 2002;287:1413-1419.
- Coulson IH, Sanderson KV. T-cell lymphoma presenting as tumour d’emblée mycosis fungoides associated with coeliac disease. J R Soc Med. 1985;78(suppl 11):23-24.
- Moreira AI, Menezes N, Varela P, et al. Primary cutaneous peripheral T cell lymphoma and celiac disease [in Portuguese]. Rev Assoc Med Bras. 2009;55:253-256.
- Porter WM, Dawe SA, Bunker CB. Dermatitis herpetiformis and cutaneous T-cell lymphoma. Clin Exp Dermatol. 2001;26:304-305.
- Sun G, Berthelot C, Duvic M. A second case of dermatitis herpetiformis and cutaneous T-cell lymphoma. Clin Exp Dermatol. 2008;33:506-507.
- Holmes GK, Prior P, Lane MR, et al. Malignancy in coeliac disease—effect of a gluten free diet. Gut. 1989;30:333-338.
- Granzotto M, dal Bo S, Quaglia S, et al. Regulatory T-cell function is impaired in celiac disease. Dig Dis Sci. 2009;54:1513-1519.
- Roychoudhuri R, Hirahara K, Mousavi K, et al. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis [published online June 2, 2013]. Nature. 2013;498:506-510.
To the Editor:
Mycosis fungoides (MF) is the most common form of a heterogeneous group of non-Hodgkin lymphomas known as cutaneous T-cell lymphomas. Celiac disease (CD) is associated with increased risk for development of enteropathy-associated T-cell lymphoma and other intraintestinal and extraintestinal non-Hodgkin lymphomas, but a firm association between CD and MF has not been established.1 The first and second cases of concomitant MF and CD were reported in 1985 and 2009 by Coulson and Sanderson2 and Moreira et al,3 respectively. Two other reports of celiac-associated dermatitis herpetiformis and MF exist.4,5 We report a patient with a unique constellation of MF, CD, and Sjögren syndrome (SS).
A 54-year-old woman presented with a worsening nonpruritic, slightly tender, eczematous patch on the back of 19 years’ duration. She had a history of SS diagnosed by salivary gland biopsy. She also had a diagnosis of CD confirmed with positive antigliadin IgA antibodies, with a dramatic improvement in symptoms on a gluten-free diet (GFD) after having abdominal pain and diarrhea for many years. She had no evidence of dermatitis herpetiformis. Recently, more red-brown areas of confluent light pink erythema without clear-cut borders had appeared on the axillae, trunk, and thigh (Figure). The patient also noted new lesions and more erythema of the patches when not adhering to a GFD. A biopsy specimen from the left side of the lateral trunk revealed a bandlike lymphocytic infiltrate with irregular nuclear contours displaying epidermotropism with a few Pautrier microabscesses. Immunohistochemistry showed strong CD3 and CD4 positivity with loss of CD7 and scattered CD8 staining. Peripheral blood flow cytometry showed no aberrant cell populations. The patient was diagnosed with MF stage IB and treated with topical corticosteroids and natural light with improvement.

It has been hypothesized that early MF is an autoimmune process caused by dysregulation of a lymphocytic reaction against chronic exogenous or endogenous antigens.4,5 The association of MF with CD supports the possibility of lymphocytic stimulation by a persistent antigen (ie, gluten) in the gastrointestinal tract. Porter et al4 suggested that in susceptible individuals, the resulting clonal T cells may migrate into the epidermis, causing MF. This theory also is supported by the finding that adherence to a GFD leads to decreased risk for malignancy and morbidity.6 In our patient, the chronic autoimmune stimulation in SS could be a factor in the pathogenesis of MF. Additionally, SS, CD, and MF are all strongly associated with increased incidence of specific but different HLA class II antigens. Mycosis fungoides is associated with HLA-DR5 and DQB1*03 alleles, CD with HLA-DQ2 and DQ8, and SS with HLA-DR15 and DR3. We do not know the HLA type of our patient, but she likely possessed multiple alleles, leading to the unique aggregation of diseases.
Furthermore, studies have shown that lymphocytes in CD patients display impaired regulatory T-cell function, causing increased incidence of autoimmune diseases and malignancy.7,8 By this theory, the occurrence of MF in patients is facilitated by the inability of CD lymphocytes to control the abnormal T-cell proliferation in the skin. Interestingly, the finding of SS in our patient supports the possibility of impaired regulatory T-cell function.
Although the occurrence of both MF and CD in our patient could be coincidental, the possibility of correlation must be considered as more cases are documented.
To the Editor:
Mycosis fungoides (MF) is the most common form of a heterogeneous group of non-Hodgkin lymphomas known as cutaneous T-cell lymphomas. Celiac disease (CD) is associated with increased risk for development of enteropathy-associated T-cell lymphoma and other intraintestinal and extraintestinal non-Hodgkin lymphomas, but a firm association between CD and MF has not been established.1 The first and second cases of concomitant MF and CD were reported in 1985 and 2009 by Coulson and Sanderson2 and Moreira et al,3 respectively. Two other reports of celiac-associated dermatitis herpetiformis and MF exist.4,5 We report a patient with a unique constellation of MF, CD, and Sjögren syndrome (SS).
A 54-year-old woman presented with a worsening nonpruritic, slightly tender, eczematous patch on the back of 19 years’ duration. She had a history of SS diagnosed by salivary gland biopsy. She also had a diagnosis of CD confirmed with positive antigliadin IgA antibodies, with a dramatic improvement in symptoms on a gluten-free diet (GFD) after having abdominal pain and diarrhea for many years. She had no evidence of dermatitis herpetiformis. Recently, more red-brown areas of confluent light pink erythema without clear-cut borders had appeared on the axillae, trunk, and thigh (Figure). The patient also noted new lesions and more erythema of the patches when not adhering to a GFD. A biopsy specimen from the left side of the lateral trunk revealed a bandlike lymphocytic infiltrate with irregular nuclear contours displaying epidermotropism with a few Pautrier microabscesses. Immunohistochemistry showed strong CD3 and CD4 positivity with loss of CD7 and scattered CD8 staining. Peripheral blood flow cytometry showed no aberrant cell populations. The patient was diagnosed with MF stage IB and treated with topical corticosteroids and natural light with improvement.

It has been hypothesized that early MF is an autoimmune process caused by dysregulation of a lymphocytic reaction against chronic exogenous or endogenous antigens.4,5 The association of MF with CD supports the possibility of lymphocytic stimulation by a persistent antigen (ie, gluten) in the gastrointestinal tract. Porter et al4 suggested that in susceptible individuals, the resulting clonal T cells may migrate into the epidermis, causing MF. This theory also is supported by the finding that adherence to a GFD leads to decreased risk for malignancy and morbidity.6 In our patient, the chronic autoimmune stimulation in SS could be a factor in the pathogenesis of MF. Additionally, SS, CD, and MF are all strongly associated with increased incidence of specific but different HLA class II antigens. Mycosis fungoides is associated with HLA-DR5 and DQB1*03 alleles, CD with HLA-DQ2 and DQ8, and SS with HLA-DR15 and DR3. We do not know the HLA type of our patient, but she likely possessed multiple alleles, leading to the unique aggregation of diseases.
Furthermore, studies have shown that lymphocytes in CD patients display impaired regulatory T-cell function, causing increased incidence of autoimmune diseases and malignancy.7,8 By this theory, the occurrence of MF in patients is facilitated by the inability of CD lymphocytes to control the abnormal T-cell proliferation in the skin. Interestingly, the finding of SS in our patient supports the possibility of impaired regulatory T-cell function.
Although the occurrence of both MF and CD in our patient could be coincidental, the possibility of correlation must be considered as more cases are documented.
- Catassi C, Fabiani E, Corrao G, et al; Italian Working Group on Coeliac Disease and Non-Hodgkin’s-Lymphoma. Risk of non-Hodgkin Lymphoma in celiac disease. JAMA. 2002;287:1413-1419.
- Coulson IH, Sanderson KV. T-cell lymphoma presenting as tumour d’emblée mycosis fungoides associated with coeliac disease. J R Soc Med. 1985;78(suppl 11):23-24.
- Moreira AI, Menezes N, Varela P, et al. Primary cutaneous peripheral T cell lymphoma and celiac disease [in Portuguese]. Rev Assoc Med Bras. 2009;55:253-256.
- Porter WM, Dawe SA, Bunker CB. Dermatitis herpetiformis and cutaneous T-cell lymphoma. Clin Exp Dermatol. 2001;26:304-305.
- Sun G, Berthelot C, Duvic M. A second case of dermatitis herpetiformis and cutaneous T-cell lymphoma. Clin Exp Dermatol. 2008;33:506-507.
- Holmes GK, Prior P, Lane MR, et al. Malignancy in coeliac disease—effect of a gluten free diet. Gut. 1989;30:333-338.
- Granzotto M, dal Bo S, Quaglia S, et al. Regulatory T-cell function is impaired in celiac disease. Dig Dis Sci. 2009;54:1513-1519.
- Roychoudhuri R, Hirahara K, Mousavi K, et al. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis [published online June 2, 2013]. Nature. 2013;498:506-510.
- Catassi C, Fabiani E, Corrao G, et al; Italian Working Group on Coeliac Disease and Non-Hodgkin’s-Lymphoma. Risk of non-Hodgkin Lymphoma in celiac disease. JAMA. 2002;287:1413-1419.
- Coulson IH, Sanderson KV. T-cell lymphoma presenting as tumour d’emblée mycosis fungoides associated with coeliac disease. J R Soc Med. 1985;78(suppl 11):23-24.
- Moreira AI, Menezes N, Varela P, et al. Primary cutaneous peripheral T cell lymphoma and celiac disease [in Portuguese]. Rev Assoc Med Bras. 2009;55:253-256.
- Porter WM, Dawe SA, Bunker CB. Dermatitis herpetiformis and cutaneous T-cell lymphoma. Clin Exp Dermatol. 2001;26:304-305.
- Sun G, Berthelot C, Duvic M. A second case of dermatitis herpetiformis and cutaneous T-cell lymphoma. Clin Exp Dermatol. 2008;33:506-507.
- Holmes GK, Prior P, Lane MR, et al. Malignancy in coeliac disease—effect of a gluten free diet. Gut. 1989;30:333-338.
- Granzotto M, dal Bo S, Quaglia S, et al. Regulatory T-cell function is impaired in celiac disease. Dig Dis Sci. 2009;54:1513-1519.
- Roychoudhuri R, Hirahara K, Mousavi K, et al. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis [published online June 2, 2013]. Nature. 2013;498:506-510.
Practice Points
- Mycosis fungoides, the most common type of cutaneous T-cell lymphoma, is an entity for which the pathogenesis is largely unknown.
- Our case and other cases of celiac disease and mycosis fungoides seem to support the immunologic hypothesis of lymphocytic stimulation by a persistent antigen.