User login
The re-emerging role of therapeutic neuromodulation
Discuss this article at http://currentpsychiatry.blogspot.com/2010/11/therapeutic-neuromodulation.html#comments
The brain is an electrochemical organ, and its activity can be modulated for therapeutic purposes by electrical, pharmacologic, or combined approaches. In general, neuromodulation induces electrical current in peripheral or central nervous tissue, which is accomplished by various techniques, including:
- electroconvulsive therapy (ECT)
- vagus nerve stimulation (VNS)
- transcranial magnetic stimulation (TMS)
- deep brain stimulation (DBS).
It is thought that therapeutic benefit occurs by regulating functional disturbances in relevant distributed neural circuits.1 Depending on the stimulation method, the frequencies chosen may excite or inhibit different or the same areas of the brain in varying patterns. Unlike medication, neuromodulation impacts the brain episodically, which may mitigate adaptation to the therapy’s beneficial effects and avoid systemic adverse effects.
Neuromodulation techniques are categorized based on their risk level as invasive or noninvasive and seizurogenic or nonseizurogenic (Table 1). Although these and other approaches are being considered for various neuropsychiatric disorders (Table 2), the most common application is for severe, treatment-resistant depression. Therefore, this article focuses on FDA-approved neuromodulation treatments for depression, with limited discussion of other indications.
Table 1
Therapeutic neuromodulation: Categorization based on risk
| Noninvasive, nonseizurogenic TMS, tDCS, CES | ||
| Noninvasive, seizurogenic ECT, MST, FEAST | ||
| Invasive, nonseizurogenic VNS, DBS, EpCS | ||
| CES: cranial electrotherapy stimulation; DBS: deep brain stimulation; ECT: electroconvulsive therapy; EpCS: epidural prefrontal cortical stimulation; FEAST: focal electrically administered seizure therapy; MST: magnetic seizure therapy; tDCS: transcranial direct current stimulation; TMS: transcranial magnetic stimulation; VNS: vagus nerve stimulation | ||
Table 2
Approved and investigational indications of neuromodulation
| Approach | Description | Clinical application |
|---|---|---|
| CES | Uses small pulses of electrical current delivered across the head focused on the hypothalamic region with electrodes usually placed on the ear at the mastoid near the face | Depression Anxiety Sleep disorders |
| DBS | ‘Functional neurosurgical’ procedure that uses electrical current to directly modulate specific areas of the CNS | Depression OCD* Parkinson’s disease* Dystonia* |
| ECT | Short-term electrical stimulation sufficient to induce a seizure | Depression* Schizophrenia Mania |
| EpCS | Uses implantable stimulating paddles that do not come in contact with the brain and target the anterior frontal poles and the lateral prefrontal cortex | Depression Pain |
| FEAST | An alternate form of ECT that involves passage of electrical current unidirectionally from a small anode to a larger cathode electrode | Depression |
| MST | Intense, high-frequency magnetic pulses sufficient to induce a seizure | Depression |
| tDCS | Sustained, low-intensity constant current flow usually passing from anode to cathode electrodes placed on the scalp | Depression |
| TMS | Use of intense high- or low-frequency magnetic pulses to produce neuronal excitation or inhibition | Depression* PTSD OCD Schizophrenia Substance use disorders Tinnitus |
| VNS | Use of intermittent mild electrical pulses to the left vagus nerve, whose afferent fibers impact structures such as the locus ceruleus and the raphe nucleus | Epilepsy* Depression* |
| *FDA-approved indications CES: cranial electrotherapy stimulation; DBS: deep brain stimulation; ECT: electroconvulsive therapy; EpCS: epidural prefrontal cortical stimulation; FEAST: focal electrically administered seizure therapy; MST: magnetic seizure therapy; OCD: obsessive-compulsive disorder; PTSD: posttraumatic stress disorder; tDCS: transcranial direct current stimulation; TMS: transcranial magnetic stimulation; VNS: vagus nerve stimulation | ||
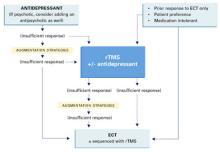
ECT: Oldest and most effective
ECT has remained the most effective therapeutic neuromodulation technique for more than 7 decades. It is indicated primarily for severe depressive episodes (eg, psychotic, melancholic), particularly in older patients.
ECT delivers electrical current to the CNS that is sufficient to produce a seizure. Under modified conditions, a typical course of 6 to 12 sessions can resolve severe depressive episodes and may also benefit other disorders, such as bipolar mania and acute psychosis. Although ECT is potentially life-saving, its use was markedly curtailed with the advent of effective antidepressants in the 1950s. Multiple factors impede its use, including:
- access and expertise are limited in many areas
- cognition is at least temporarily adversely affected
- relapse rates after acute benefit are high
- cost
- public perception often is negative.
Studies are addressing several of these concerns. For example, the National Institute of Mental Health-sponsored Consortium on Research with ECT (CORE) group is considering how to more effectively maintain acute benefits of ECT. They compared the potential merits of maintenance ECT with maintenance pharmacotherapy (nortriptyline plus lithium) over 6 months. Although the 2 strategies had comparable results, retention rates were <50% and about one-third relapsed in both groups.2,3 Potential alternative strategies include a more frequent ECT maintenance schedule and/or combining maintenance ECT with medication(s).
Magnetic seizure therapy (MST) and focal electrically administered seizure therapy (FEAST) are attempts to produce similar efficacy and less cognitive disruption compared with ECT.4,5 Work also continues on electrode placement (eg, bifrontal) and alteration of waveform characteristics (eg, ultra-brief) to maintain or enhance efficacy while minimizing adverse effects.6,7
Stimulating the vagus nerve
VNS was introduced for treating refractory epilepsy in 1997. In 2005, it became the first FDA-approved implantable device for managing chronic or recurrent treatment-resistant depression.
The vagus nerve is the principal parasympathetic, efferent tract regulating heart rate, intestinal motility, and gastric acid secretion. Information about pain, hunger, and satiety is conveyed by these fibers to the median raphe nucleus and locus coeruleus, brain regions with significant serotonergic and noradrenergic innervation. These neurotransmitters also are believed to play a pivotal role in major depression.
With VNS, a pacemaker-like pulse generator is surgically implanted subcutaneously in the patient’s upper left chest. Wires extend from this device to the left vagus nerve (80% of whose fibers are afferent) located in the neck, to which the pulse generator sends electrical signals every few seconds (Table 3). The right vagus nerve is not used because it provides parasympathetic innervation to the heart. A clinician adjusts stimulation parameters using a computer and a noninvasive handheld device. Common adverse effects include voice alteration or hoarseness, cough, and shortness of breath, which occur during active stimulation because of the proximity of the electrodes to the laryngeal and pharyngeal branches of the vagus nerve. These effects may improve by adjusting stimulation intensity. The device permits a wide range of duty cycles, but preclinical animal studies indicate that >50% activation periods may damage the vagus nerve. If patients become too uncomfortable, they may deactivate the device with a magnet held over the implantation area.
Two open-label studies evaluated VNS to treat major depression. The first involved 10 weeks of stimulation in 59 subjects with chronic or recurrent, nonpsychotic, unipolar or bipolar depression who failed at least 2 adequate antidepressant trials in the current episode.8 Stable doses of concomitant antidepressants or mood stabilizers were allowed. After 3 months, 18 (31%) patients responded within an average of 45.5 days, and nearly 15% achieved remission. Response was defined as 50% reduction in baseline Hamilton Depression Rating Scale-28 (HDRS-28) score; remission was defined as HDRS-28 score ≤10. Further, clinical response did not differ between unipolar and bipolar depression patients.
In the second trial, 74 patients with treatment-resistant depression received fixed dose antidepressants and VNS for 3 months, followed by 9 months of flexibly dosed VNS and antidepressants.9 At 3 months, response (≥50% reduction in HDRS-28 score) and remission (HDRS-28 score <10) rates were 37% and 17%, respectively, and increased to 53% and 33% at 1 year.
A sham-controlled trial of VNS in 235 depressed patients used similar inclusion and exclusion criteria as in the open-label study by Sackeim et al.8,10 Two weeks after device implantation, patients were randomized to active treatment (stimulator turned on) or sham control (stimulator left off). At 3 months, the primary outcome measure—response rate based on HDRS-24 score—did not differ significantly between the active and control groups (15% vs 10%, respectively). There was, however, a significantly greater improvement in Inventory of Depressive Symptomatology-Self Report Scale scores with active VNS vs sham VNS.
Patients on sham treatment then were switched to active treatment and both groups were followed for 12 additional months, at which time response and remission rates nearly doubled for both groups.11 In a post-hoc analysis, the same investigators found significant improvement with VNS compared with a naturalistic, matched control group with similar treatment-resistant depression.12 The FDA considered this adequate to support efficacy and approved the device for chronic or recurrent treatment-resistant depression in an episode not responsive to at least 4 adequate treatment trials with pharmacotherapy or ECT. Perhaps because post-hoc analyses typically are not sufficient to gain FDA approval, most insurance companies do not reimburse for VNS treatment of depression, and VNS is not frequently used for refractory depression.
Table 3
Vagus nerve stimulation treatment parameters
| Parameter | Units | Range | Median value at 12 months in pivotal study |
|---|---|---|---|
| Output current | Milliamps (mA) | 0 to 3.5 | 1 |
| Signal frequency | Hertz (Hz) | 1.30 | 20 |
| Pulse width | Microseconds (µsec) | 130 to 1,000 | 500 |
| Duty cycle: ON time* | Seconds | 7 to 60 | 30 |
| Duty cycle: OFF time* | Minutes | 0.2 to 180 | 5 |
| *Stimulation cycle is 24 hours per day Source: Epilepsy patient’s manual for vagus nerve stimulation with the VNS Therapy™ system. Houston, TX: Cyberonics, Inc.; 2002, 2004. Depression physician’s manual. Houston, TX: Cyberonics, Inc.; 2005 | |||
A newer option: TMS
TMS is the most recently FDA-approved therapeutic neuromodulation technique for treating depression. In October 2008, a TMS device became available for patients failing to respond to 1 adequate antidepressant trial during the current episode.
TMS delivers intense, intermittent magnetic pulses produced by an electrical charge into a ferromagnetic coil. The pulse intensity is similar to that produced by MRI. The coil usually is placed on the scalp over the left dorsolateral prefrontal cortex (DLPFC) and pulses are delivered in a rapid, repetitive train, causing neuronal depolarization in a small area of the adjacent cerebral cortex, as well as distal effects in other relevant neural circuits (Table 4). TMS typically is administered on an outpatient basis. A standard treatment course for depression consists of 5 treatment sessions per week for 4 to 8 weeks, depending on symptom severity and how quickly patients respond.
TMS initially was examined in several small, open-label studies that looked at various treatment parameters and stimulation sites. Several sham-controlled studies generally found TMS efficacious and further refined treatment administration. Its role in treating depression—and possibly other psychiatric disorders—has been supported by 2 recent meta-analyses.13,14
O’Reardon et al15 conducted the largest double-blind trial of active vs sham TMS (N=301) for moderately treatment-resistant major depression. This study began with a 4- to 6-week, blinded, randomized phase followed by 6 weeks of open-label TMS for initial nonresponders. The third phase reintroduced TMS over 6 months as needed to augment maintenance antidepressants. This trial utilized the most aggressive treatment parameters to date (ie, 10 Hz; 75 4-second trains; 26-second inter-train interval; 120% motor threshold) delivering 3,000 pulses per treatment over an average of 24 sessions. Compared with the sham procedure, patients who received active TMS showed significantly higher response rates on the Montgomery-Åsberg Depression Rating Scale (MADRS) at weeks 4 and 6. Similar results were found for the 17- and 24-item HDRS. At 6 weeks, remission rate—defined as a MADRS score <10—was significantly higher in the active treatment group (14%) compared with the sham procedure (6%). A post-hoc analysis found that the most robust benefit occurred in patients with only 1 failed adequate antidepressant trial (effect size=0.83).16 This administration protocol was well tolerated, with no deaths or seizures and a low rate of discontinuation because of adverse events (5%).17 The most common adverse effects were application site pain or discomfort and headaches.
Recently, the second largest (N=190) sham-controlled trial of TMS for treatment-resistant major depression was published.18 This National Institute of Mental Health-sponsored, multiphase study included an initial 2-week, treatment-free period; 3 weeks of daily treatments over the left DLPFC using the same device and parameters as in the O’Reardon study; and an additional 3 weeks of treatment in patients who were improving. Those not responding to initial treatment were crossed over to open-label active TMS. This study advanced TMS development by:
- using a novel somatosensory system that produced similar sensations with sham and active TMS
- assessing the success of maintaining the blind
- establishing a rigorous clinical rating system
- utilizing MRI-guided adjustment of coil placement in a subset of patients.
The authors concluded that active TMS was significantly better than sham treatment in achieving remission (14% vs 5%). In addition, the raters, treaters, and patients were effectively blinded to the treatment condition. MRI-assisted coil placement found that in 33% of the sample, site placement determined by standardized assessment was over the premotor cortex rather than the prefrontal cortex, so the coil was moved 1 additional cm anteriorly in these patients. Similar to those observed by O’Reardon et al, adverse effects of active TMS were generally mild to moderate, did not differ by treatment condition, and led to a low discontinuation rate (5.5%).
Table 4
Treatment parameters of transcranial magnetic stimulation
| Parameter | Comment |
|---|---|
| Motor threshold | Lowest intensity over primary motor cortex to produce contraction of the first dorsal interosseous or abductor pollicis brevis muscle; visual or electromyographically monitored |
| Stimulus coil location | Most common: Left dorsolateral prefrontal cortex (DLPFC) Less common: Right DLPFC, vertex |
| Stimulus pulse(s) or train | |
| Intensity | 80% to 120% of MT |
| Frequency | ≤1 to 20 Hz |
| Duration | ≤1 millisecond |
| Interpulse interval | 50 to 100 milliseconds |
| Stimulus train duration | 3 to 6 seconds |
| Inter-train interval | 20 to 60 seconds |
| Source: Janicak PG, Krasuski J, Beedle D, et al. Transcranial magnetic stimulation for neuropsychiatric disorders. Psychiatr Times. 1999;16:56-63 | |
Deep brain stimulation
DBS is a “functional neurosurgical” procedure that delivers electrical current directly to specific areas within the brain.19 Its mechanism of action remains uncertain; depolarization blockade, synaptic inhibition, and “neural jamming” are leading hypotheses. In contrast to conventional ablative surgeries, DBS is reversible and adjustable. Implantation involves positioning pacemaker-like battery devices subcutaneously in the left and right upper chest. Electrodes attached to wires are run subcutaneously behind the ears and, with stereotactic guidance, placed through burr holes in the skull into specific CNS areas implicated in the pathophysiology of conditions such as Parkinson’s disease, refractory depression, and severe obsessive-compulsive disorder (OCD).
Antidepressant effects. The FDA recently approved DBS under its humanitarian device exemption program for intractable, severe, disabling OCD based on promising results from open and blind trials that stimulated areas such as the internal capsule and adjacent ventral striatum.20-22 These studies reported that DBS of the caudate nucleus for OCD and subthalamic nucleus for Parkinson’s disease also produced antidepressant effects. Subsequently, trials targeting the subgenual region (Brodmann’s area 25), the ventral capsule/ventral striatum, and nucleus accumbens demonstrated antidepressant effects.23-27 Pending the results of ongoing pilot trials, large, multi-center studies using different devices and target areas are being planned to clarify the role of DBS for patients with severe, disabling, refractory depression.
Adverse effects of DBS can be:
- surgical-related (eg, seizure, bleeding, infection)
- device-related (eg, lead breakage, malfunction)
- stimulation-related (eg, paresthesia, dysarthria, memory disruption, cognitive changes, psychiatric symptoms).
The most serious risk is intracranial bleeding, which occurs in 2% to 3% of patients. Clearly, the risk-benefit ratio must be carefully considered.
Cost and reimbursement
Cost of treatment and potential for third-party reimbursement are important considerations for any risk-benefit analysis. Many patients who seek neuromodulation treatments will not have insurance or other coverage entitlements.28-30 Further, newer treatments are not routinely covered by insurance; however, individual case coverage may be allowed and some device manufacturers have programs to assist providers and patients obtain coverage.28-30 Even ECT, which has long been a covered treatment for major depression, is still considered investigational for other disorders. Thus, it is important to pre-certify with the patient’s health insurance provider before initiating treatment.
Coverage, however, is not the only consideration when weighing cost effectiveness. Economic studies can assist with clinical and ethical decisions relating to treatment choice.31 These studies, however, need to be critically evaluated (eg, what costs were included in the analysis). Although direct costs are easier to evaluate, indirect costs—such as the patient’s ability to continue to work while receiving the treatment, caretaker availability during treatment, and whether treatment is an inpatient or outpatient procedure—are more difficult to evaluate and should be discussed with the patient. Because these specialized options have the potential to further benefit patients with depression and other neuropsychiatric disorders, it is essential to balance the pressures of cost containment with the need for more effective and better tolerated treatments.32-34
Related Resource
- Brunoni AR, Teng CT, Correa C, et al. Neuromodulation approaches for the treatment of major depression: challenges and recommendations from a working group meeting. Arq Neuropsiquiatr. 2010;68(3):433-451.
Drug Brand Names
- Lithium • Eskalith, Lithobid
- Nortriptyline • Aventyl, Pamelor
1. Janicak PG, Pavuluri M, Marder S. Principles and practice of psychopharmacotherapy. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 323-359. In press.
2. Kellner CH, Knapp RG, Petrides G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression. A multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE). Arch Gen Psychiatry. 2006;63:1337-1344.
3. Rasmussen KG, Mueller M, Rummans TA, et al. Is baseline medication resistance associated with potential for relapse after successful remission of a depressive episode with ECT? Data from the Consortium for Research on Electroconvulsive Therapy (CORE). J Clin Psychiatry. 2009;70(2):232-237.
4. Spellman T, McClintock SM, Terrace H, et al. Differential effects of high-dose magnetic seizure therapy and electroconvulsive shock on cognitive function. Biol Psychiatry. 2008;63:1163-1170.
5. Spellman T, Peterchev AV, Lisanby SH. Focal electrically administered seizure therapy: a novel form of ECT illustrates the roles of current directionality, polarity, and electrode configuration in seizure induction. Neuropsychopharmacology. 2009;34(8):2002-2010.
6. Kellner CH, Knapp R, Husain MM, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry. 2010;196:226-234.
7. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimulat. 2008;1:71-83.
8. Sackeim HA, Rush JA, George MS, et al. Vagus nerve stimulation (VNSTM) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713-728.
9. Schlaepfer TE, Frick C, Zobel A, et al. Vagus nerve stimulation for depression: efficacy and safety in a European study. Psychol Med. 2008;38(5):651-661.
10. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized controlled acute phase trial. Biol Psychiatry. 2005;58:347-354.
11. Rush AJ, Sackeim HA, Marangell LB, et al. Effects of 12 months of vagus nerve stimulation in treatment resistant depression: a naturalistic study. Biol Psychiatry. 2005;58(5):355-363.
12. George MS, Rush AJ, Marangell LB, et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry. 2005;58:364-373.
13. Schutter DJ. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dordolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychol Med. 2009;39:65-75.
14. Slotema CW, Blom JD, Hoek HW, et al. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71(7):873-884.
15. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208-1216.
16. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522-534.
17. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
18. George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507-516.
19. Pilitsis JG, Bakay RAE. Deep brain stimulation for psychiatric disorders. Psychopharm Rev. 2007;42(9):67-74.
20. Greenberg BD, Gabriels LA, Malone DA, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15(1):64-79.
21. Mallet L, Plolsan M, Jaafari N, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121-2134.
22. Goodman WK, Foote KD, Greenberg BD, et al. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. 2010;67:535-542.
23. Mayberg HS, Lozano AM, McNeely HE, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
24. Lozano AM, Mayberg HS, Giacobbe P, et al. Subcallosal cingulated gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461-467.
25. McNeely HE, Mayberg HS, Lozano AM, et al. Neuropsychological impact of Cg25 deep brain stimulation for treatment-resistant depression: preliminary results over 12 months. J Nerv Ment Dis. 2008;196(5):405-410.
26. Malone DA, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
27. Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2):368-377.
28. Health insurance coverage NeuroStar TMS Therapy® Web site. Available at: http://www.neurostartms.com/TMSHealthInsurance/Health-Insurance-Coverage.aspx. Accessed June 2, 2010.
29. VNS insurance information Vagus nerve stimulation therapy for treatment-resistant depression Web site. Available at: http://www.vnstherapy.com/depression/insuranceinformation/coverage.asp. Accessed June 2, 2010.
30. Insurance coverage—DBS therapy for OCD Available at: http://www.medtronic.com/your-health/obsessive-compulsive-disorder-ocd/getting-therapy/insurance-coverage/index.htm. Accessed June 2, 2010.
31. Simpson KN, Welch MJ, Kozel FA, et al. Cost-effectiveness of transcranial magnetic stimulation in the treatment of major depression: a health economics analysis. Adv Ther. 2009;26(3):346-368.
32. Rado J, Dowd SM, Janicak PG. The emerging role of transcranial magnetic stimulation (TMS) for treatment of psychiatric disorders. Dir Psychiatry. 2008;28(25):215-331.
33. Dougherty DD, Rauch SL. Somatic therapies for treatment-resistant depression: new neurotherapeutic interventions. Psychiatr Clin N Am. 2007;30:31-37.
34. Olfson M, Marcus S, Sackeim HA, et al. Use of ECT for the inpatient treatment of recurrent major depression. Am J Psychiatry. 1998;155:22-29.
Discuss this article at http://currentpsychiatry.blogspot.com/2010/11/therapeutic-neuromodulation.html#comments
The brain is an electrochemical organ, and its activity can be modulated for therapeutic purposes by electrical, pharmacologic, or combined approaches. In general, neuromodulation induces electrical current in peripheral or central nervous tissue, which is accomplished by various techniques, including:
- electroconvulsive therapy (ECT)
- vagus nerve stimulation (VNS)
- transcranial magnetic stimulation (TMS)
- deep brain stimulation (DBS).
It is thought that therapeutic benefit occurs by regulating functional disturbances in relevant distributed neural circuits.1 Depending on the stimulation method, the frequencies chosen may excite or inhibit different or the same areas of the brain in varying patterns. Unlike medication, neuromodulation impacts the brain episodically, which may mitigate adaptation to the therapy’s beneficial effects and avoid systemic adverse effects.
Neuromodulation techniques are categorized based on their risk level as invasive or noninvasive and seizurogenic or nonseizurogenic (Table 1). Although these and other approaches are being considered for various neuropsychiatric disorders (Table 2), the most common application is for severe, treatment-resistant depression. Therefore, this article focuses on FDA-approved neuromodulation treatments for depression, with limited discussion of other indications.
Table 1
Therapeutic neuromodulation: Categorization based on risk
| Noninvasive, nonseizurogenic TMS, tDCS, CES | ||
| Noninvasive, seizurogenic ECT, MST, FEAST | ||
| Invasive, nonseizurogenic VNS, DBS, EpCS | ||
| CES: cranial electrotherapy stimulation; DBS: deep brain stimulation; ECT: electroconvulsive therapy; EpCS: epidural prefrontal cortical stimulation; FEAST: focal electrically administered seizure therapy; MST: magnetic seizure therapy; tDCS: transcranial direct current stimulation; TMS: transcranial magnetic stimulation; VNS: vagus nerve stimulation | ||
Table 2
Approved and investigational indications of neuromodulation
| Approach | Description | Clinical application |
|---|---|---|
| CES | Uses small pulses of electrical current delivered across the head focused on the hypothalamic region with electrodes usually placed on the ear at the mastoid near the face | Depression Anxiety Sleep disorders |
| DBS | ‘Functional neurosurgical’ procedure that uses electrical current to directly modulate specific areas of the CNS | Depression OCD* Parkinson’s disease* Dystonia* |
| ECT | Short-term electrical stimulation sufficient to induce a seizure | Depression* Schizophrenia Mania |
| EpCS | Uses implantable stimulating paddles that do not come in contact with the brain and target the anterior frontal poles and the lateral prefrontal cortex | Depression Pain |
| FEAST | An alternate form of ECT that involves passage of electrical current unidirectionally from a small anode to a larger cathode electrode | Depression |
| MST | Intense, high-frequency magnetic pulses sufficient to induce a seizure | Depression |
| tDCS | Sustained, low-intensity constant current flow usually passing from anode to cathode electrodes placed on the scalp | Depression |
| TMS | Use of intense high- or low-frequency magnetic pulses to produce neuronal excitation or inhibition | Depression* PTSD OCD Schizophrenia Substance use disorders Tinnitus |
| VNS | Use of intermittent mild electrical pulses to the left vagus nerve, whose afferent fibers impact structures such as the locus ceruleus and the raphe nucleus | Epilepsy* Depression* |
| *FDA-approved indications CES: cranial electrotherapy stimulation; DBS: deep brain stimulation; ECT: electroconvulsive therapy; EpCS: epidural prefrontal cortical stimulation; FEAST: focal electrically administered seizure therapy; MST: magnetic seizure therapy; OCD: obsessive-compulsive disorder; PTSD: posttraumatic stress disorder; tDCS: transcranial direct current stimulation; TMS: transcranial magnetic stimulation; VNS: vagus nerve stimulation | ||
ECT: Oldest and most effective
ECT has remained the most effective therapeutic neuromodulation technique for more than 7 decades. It is indicated primarily for severe depressive episodes (eg, psychotic, melancholic), particularly in older patients.
ECT delivers electrical current to the CNS that is sufficient to produce a seizure. Under modified conditions, a typical course of 6 to 12 sessions can resolve severe depressive episodes and may also benefit other disorders, such as bipolar mania and acute psychosis. Although ECT is potentially life-saving, its use was markedly curtailed with the advent of effective antidepressants in the 1950s. Multiple factors impede its use, including:
- access and expertise are limited in many areas
- cognition is at least temporarily adversely affected
- relapse rates after acute benefit are high
- cost
- public perception often is negative.
Studies are addressing several of these concerns. For example, the National Institute of Mental Health-sponsored Consortium on Research with ECT (CORE) group is considering how to more effectively maintain acute benefits of ECT. They compared the potential merits of maintenance ECT with maintenance pharmacotherapy (nortriptyline plus lithium) over 6 months. Although the 2 strategies had comparable results, retention rates were <50% and about one-third relapsed in both groups.2,3 Potential alternative strategies include a more frequent ECT maintenance schedule and/or combining maintenance ECT with medication(s).
Magnetic seizure therapy (MST) and focal electrically administered seizure therapy (FEAST) are attempts to produce similar efficacy and less cognitive disruption compared with ECT.4,5 Work also continues on electrode placement (eg, bifrontal) and alteration of waveform characteristics (eg, ultra-brief) to maintain or enhance efficacy while minimizing adverse effects.6,7
Stimulating the vagus nerve
VNS was introduced for treating refractory epilepsy in 1997. In 2005, it became the first FDA-approved implantable device for managing chronic or recurrent treatment-resistant depression.
The vagus nerve is the principal parasympathetic, efferent tract regulating heart rate, intestinal motility, and gastric acid secretion. Information about pain, hunger, and satiety is conveyed by these fibers to the median raphe nucleus and locus coeruleus, brain regions with significant serotonergic and noradrenergic innervation. These neurotransmitters also are believed to play a pivotal role in major depression.
With VNS, a pacemaker-like pulse generator is surgically implanted subcutaneously in the patient’s upper left chest. Wires extend from this device to the left vagus nerve (80% of whose fibers are afferent) located in the neck, to which the pulse generator sends electrical signals every few seconds (Table 3). The right vagus nerve is not used because it provides parasympathetic innervation to the heart. A clinician adjusts stimulation parameters using a computer and a noninvasive handheld device. Common adverse effects include voice alteration or hoarseness, cough, and shortness of breath, which occur during active stimulation because of the proximity of the electrodes to the laryngeal and pharyngeal branches of the vagus nerve. These effects may improve by adjusting stimulation intensity. The device permits a wide range of duty cycles, but preclinical animal studies indicate that >50% activation periods may damage the vagus nerve. If patients become too uncomfortable, they may deactivate the device with a magnet held over the implantation area.
Two open-label studies evaluated VNS to treat major depression. The first involved 10 weeks of stimulation in 59 subjects with chronic or recurrent, nonpsychotic, unipolar or bipolar depression who failed at least 2 adequate antidepressant trials in the current episode.8 Stable doses of concomitant antidepressants or mood stabilizers were allowed. After 3 months, 18 (31%) patients responded within an average of 45.5 days, and nearly 15% achieved remission. Response was defined as 50% reduction in baseline Hamilton Depression Rating Scale-28 (HDRS-28) score; remission was defined as HDRS-28 score ≤10. Further, clinical response did not differ between unipolar and bipolar depression patients.
In the second trial, 74 patients with treatment-resistant depression received fixed dose antidepressants and VNS for 3 months, followed by 9 months of flexibly dosed VNS and antidepressants.9 At 3 months, response (≥50% reduction in HDRS-28 score) and remission (HDRS-28 score <10) rates were 37% and 17%, respectively, and increased to 53% and 33% at 1 year.
A sham-controlled trial of VNS in 235 depressed patients used similar inclusion and exclusion criteria as in the open-label study by Sackeim et al.8,10 Two weeks after device implantation, patients were randomized to active treatment (stimulator turned on) or sham control (stimulator left off). At 3 months, the primary outcome measure—response rate based on HDRS-24 score—did not differ significantly between the active and control groups (15% vs 10%, respectively). There was, however, a significantly greater improvement in Inventory of Depressive Symptomatology-Self Report Scale scores with active VNS vs sham VNS.
Patients on sham treatment then were switched to active treatment and both groups were followed for 12 additional months, at which time response and remission rates nearly doubled for both groups.11 In a post-hoc analysis, the same investigators found significant improvement with VNS compared with a naturalistic, matched control group with similar treatment-resistant depression.12 The FDA considered this adequate to support efficacy and approved the device for chronic or recurrent treatment-resistant depression in an episode not responsive to at least 4 adequate treatment trials with pharmacotherapy or ECT. Perhaps because post-hoc analyses typically are not sufficient to gain FDA approval, most insurance companies do not reimburse for VNS treatment of depression, and VNS is not frequently used for refractory depression.
Table 3
Vagus nerve stimulation treatment parameters
| Parameter | Units | Range | Median value at 12 months in pivotal study |
|---|---|---|---|
| Output current | Milliamps (mA) | 0 to 3.5 | 1 |
| Signal frequency | Hertz (Hz) | 1.30 | 20 |
| Pulse width | Microseconds (µsec) | 130 to 1,000 | 500 |
| Duty cycle: ON time* | Seconds | 7 to 60 | 30 |
| Duty cycle: OFF time* | Minutes | 0.2 to 180 | 5 |
| *Stimulation cycle is 24 hours per day Source: Epilepsy patient’s manual for vagus nerve stimulation with the VNS Therapy™ system. Houston, TX: Cyberonics, Inc.; 2002, 2004. Depression physician’s manual. Houston, TX: Cyberonics, Inc.; 2005 | |||
A newer option: TMS
TMS is the most recently FDA-approved therapeutic neuromodulation technique for treating depression. In October 2008, a TMS device became available for patients failing to respond to 1 adequate antidepressant trial during the current episode.
TMS delivers intense, intermittent magnetic pulses produced by an electrical charge into a ferromagnetic coil. The pulse intensity is similar to that produced by MRI. The coil usually is placed on the scalp over the left dorsolateral prefrontal cortex (DLPFC) and pulses are delivered in a rapid, repetitive train, causing neuronal depolarization in a small area of the adjacent cerebral cortex, as well as distal effects in other relevant neural circuits (Table 4). TMS typically is administered on an outpatient basis. A standard treatment course for depression consists of 5 treatment sessions per week for 4 to 8 weeks, depending on symptom severity and how quickly patients respond.
TMS initially was examined in several small, open-label studies that looked at various treatment parameters and stimulation sites. Several sham-controlled studies generally found TMS efficacious and further refined treatment administration. Its role in treating depression—and possibly other psychiatric disorders—has been supported by 2 recent meta-analyses.13,14
O’Reardon et al15 conducted the largest double-blind trial of active vs sham TMS (N=301) for moderately treatment-resistant major depression. This study began with a 4- to 6-week, blinded, randomized phase followed by 6 weeks of open-label TMS for initial nonresponders. The third phase reintroduced TMS over 6 months as needed to augment maintenance antidepressants. This trial utilized the most aggressive treatment parameters to date (ie, 10 Hz; 75 4-second trains; 26-second inter-train interval; 120% motor threshold) delivering 3,000 pulses per treatment over an average of 24 sessions. Compared with the sham procedure, patients who received active TMS showed significantly higher response rates on the Montgomery-Åsberg Depression Rating Scale (MADRS) at weeks 4 and 6. Similar results were found for the 17- and 24-item HDRS. At 6 weeks, remission rate—defined as a MADRS score <10—was significantly higher in the active treatment group (14%) compared with the sham procedure (6%). A post-hoc analysis found that the most robust benefit occurred in patients with only 1 failed adequate antidepressant trial (effect size=0.83).16 This administration protocol was well tolerated, with no deaths or seizures and a low rate of discontinuation because of adverse events (5%).17 The most common adverse effects were application site pain or discomfort and headaches.
Recently, the second largest (N=190) sham-controlled trial of TMS for treatment-resistant major depression was published.18 This National Institute of Mental Health-sponsored, multiphase study included an initial 2-week, treatment-free period; 3 weeks of daily treatments over the left DLPFC using the same device and parameters as in the O’Reardon study; and an additional 3 weeks of treatment in patients who were improving. Those not responding to initial treatment were crossed over to open-label active TMS. This study advanced TMS development by:
- using a novel somatosensory system that produced similar sensations with sham and active TMS
- assessing the success of maintaining the blind
- establishing a rigorous clinical rating system
- utilizing MRI-guided adjustment of coil placement in a subset of patients.
The authors concluded that active TMS was significantly better than sham treatment in achieving remission (14% vs 5%). In addition, the raters, treaters, and patients were effectively blinded to the treatment condition. MRI-assisted coil placement found that in 33% of the sample, site placement determined by standardized assessment was over the premotor cortex rather than the prefrontal cortex, so the coil was moved 1 additional cm anteriorly in these patients. Similar to those observed by O’Reardon et al, adverse effects of active TMS were generally mild to moderate, did not differ by treatment condition, and led to a low discontinuation rate (5.5%).
Table 4
Treatment parameters of transcranial magnetic stimulation
| Parameter | Comment |
|---|---|
| Motor threshold | Lowest intensity over primary motor cortex to produce contraction of the first dorsal interosseous or abductor pollicis brevis muscle; visual or electromyographically monitored |
| Stimulus coil location | Most common: Left dorsolateral prefrontal cortex (DLPFC) Less common: Right DLPFC, vertex |
| Stimulus pulse(s) or train | |
| Intensity | 80% to 120% of MT |
| Frequency | ≤1 to 20 Hz |
| Duration | ≤1 millisecond |
| Interpulse interval | 50 to 100 milliseconds |
| Stimulus train duration | 3 to 6 seconds |
| Inter-train interval | 20 to 60 seconds |
| Source: Janicak PG, Krasuski J, Beedle D, et al. Transcranial magnetic stimulation for neuropsychiatric disorders. Psychiatr Times. 1999;16:56-63 | |
Deep brain stimulation
DBS is a “functional neurosurgical” procedure that delivers electrical current directly to specific areas within the brain.19 Its mechanism of action remains uncertain; depolarization blockade, synaptic inhibition, and “neural jamming” are leading hypotheses. In contrast to conventional ablative surgeries, DBS is reversible and adjustable. Implantation involves positioning pacemaker-like battery devices subcutaneously in the left and right upper chest. Electrodes attached to wires are run subcutaneously behind the ears and, with stereotactic guidance, placed through burr holes in the skull into specific CNS areas implicated in the pathophysiology of conditions such as Parkinson’s disease, refractory depression, and severe obsessive-compulsive disorder (OCD).
Antidepressant effects. The FDA recently approved DBS under its humanitarian device exemption program for intractable, severe, disabling OCD based on promising results from open and blind trials that stimulated areas such as the internal capsule and adjacent ventral striatum.20-22 These studies reported that DBS of the caudate nucleus for OCD and subthalamic nucleus for Parkinson’s disease also produced antidepressant effects. Subsequently, trials targeting the subgenual region (Brodmann’s area 25), the ventral capsule/ventral striatum, and nucleus accumbens demonstrated antidepressant effects.23-27 Pending the results of ongoing pilot trials, large, multi-center studies using different devices and target areas are being planned to clarify the role of DBS for patients with severe, disabling, refractory depression.
Adverse effects of DBS can be:
- surgical-related (eg, seizure, bleeding, infection)
- device-related (eg, lead breakage, malfunction)
- stimulation-related (eg, paresthesia, dysarthria, memory disruption, cognitive changes, psychiatric symptoms).
The most serious risk is intracranial bleeding, which occurs in 2% to 3% of patients. Clearly, the risk-benefit ratio must be carefully considered.
Cost and reimbursement
Cost of treatment and potential for third-party reimbursement are important considerations for any risk-benefit analysis. Many patients who seek neuromodulation treatments will not have insurance or other coverage entitlements.28-30 Further, newer treatments are not routinely covered by insurance; however, individual case coverage may be allowed and some device manufacturers have programs to assist providers and patients obtain coverage.28-30 Even ECT, which has long been a covered treatment for major depression, is still considered investigational for other disorders. Thus, it is important to pre-certify with the patient’s health insurance provider before initiating treatment.
Coverage, however, is not the only consideration when weighing cost effectiveness. Economic studies can assist with clinical and ethical decisions relating to treatment choice.31 These studies, however, need to be critically evaluated (eg, what costs were included in the analysis). Although direct costs are easier to evaluate, indirect costs—such as the patient’s ability to continue to work while receiving the treatment, caretaker availability during treatment, and whether treatment is an inpatient or outpatient procedure—are more difficult to evaluate and should be discussed with the patient. Because these specialized options have the potential to further benefit patients with depression and other neuropsychiatric disorders, it is essential to balance the pressures of cost containment with the need for more effective and better tolerated treatments.32-34
Related Resource
- Brunoni AR, Teng CT, Correa C, et al. Neuromodulation approaches for the treatment of major depression: challenges and recommendations from a working group meeting. Arq Neuropsiquiatr. 2010;68(3):433-451.
Drug Brand Names
- Lithium • Eskalith, Lithobid
- Nortriptyline • Aventyl, Pamelor
Discuss this article at http://currentpsychiatry.blogspot.com/2010/11/therapeutic-neuromodulation.html#comments
The brain is an electrochemical organ, and its activity can be modulated for therapeutic purposes by electrical, pharmacologic, or combined approaches. In general, neuromodulation induces electrical current in peripheral or central nervous tissue, which is accomplished by various techniques, including:
- electroconvulsive therapy (ECT)
- vagus nerve stimulation (VNS)
- transcranial magnetic stimulation (TMS)
- deep brain stimulation (DBS).
It is thought that therapeutic benefit occurs by regulating functional disturbances in relevant distributed neural circuits.1 Depending on the stimulation method, the frequencies chosen may excite or inhibit different or the same areas of the brain in varying patterns. Unlike medication, neuromodulation impacts the brain episodically, which may mitigate adaptation to the therapy’s beneficial effects and avoid systemic adverse effects.
Neuromodulation techniques are categorized based on their risk level as invasive or noninvasive and seizurogenic or nonseizurogenic (Table 1). Although these and other approaches are being considered for various neuropsychiatric disorders (Table 2), the most common application is for severe, treatment-resistant depression. Therefore, this article focuses on FDA-approved neuromodulation treatments for depression, with limited discussion of other indications.
Table 1
Therapeutic neuromodulation: Categorization based on risk
| Noninvasive, nonseizurogenic TMS, tDCS, CES | ||
| Noninvasive, seizurogenic ECT, MST, FEAST | ||
| Invasive, nonseizurogenic VNS, DBS, EpCS | ||
| CES: cranial electrotherapy stimulation; DBS: deep brain stimulation; ECT: electroconvulsive therapy; EpCS: epidural prefrontal cortical stimulation; FEAST: focal electrically administered seizure therapy; MST: magnetic seizure therapy; tDCS: transcranial direct current stimulation; TMS: transcranial magnetic stimulation; VNS: vagus nerve stimulation | ||
Table 2
Approved and investigational indications of neuromodulation
| Approach | Description | Clinical application |
|---|---|---|
| CES | Uses small pulses of electrical current delivered across the head focused on the hypothalamic region with electrodes usually placed on the ear at the mastoid near the face | Depression Anxiety Sleep disorders |
| DBS | ‘Functional neurosurgical’ procedure that uses electrical current to directly modulate specific areas of the CNS | Depression OCD* Parkinson’s disease* Dystonia* |
| ECT | Short-term electrical stimulation sufficient to induce a seizure | Depression* Schizophrenia Mania |
| EpCS | Uses implantable stimulating paddles that do not come in contact with the brain and target the anterior frontal poles and the lateral prefrontal cortex | Depression Pain |
| FEAST | An alternate form of ECT that involves passage of electrical current unidirectionally from a small anode to a larger cathode electrode | Depression |
| MST | Intense, high-frequency magnetic pulses sufficient to induce a seizure | Depression |
| tDCS | Sustained, low-intensity constant current flow usually passing from anode to cathode electrodes placed on the scalp | Depression |
| TMS | Use of intense high- or low-frequency magnetic pulses to produce neuronal excitation or inhibition | Depression* PTSD OCD Schizophrenia Substance use disorders Tinnitus |
| VNS | Use of intermittent mild electrical pulses to the left vagus nerve, whose afferent fibers impact structures such as the locus ceruleus and the raphe nucleus | Epilepsy* Depression* |
| *FDA-approved indications CES: cranial electrotherapy stimulation; DBS: deep brain stimulation; ECT: electroconvulsive therapy; EpCS: epidural prefrontal cortical stimulation; FEAST: focal electrically administered seizure therapy; MST: magnetic seizure therapy; OCD: obsessive-compulsive disorder; PTSD: posttraumatic stress disorder; tDCS: transcranial direct current stimulation; TMS: transcranial magnetic stimulation; VNS: vagus nerve stimulation | ||
ECT: Oldest and most effective
ECT has remained the most effective therapeutic neuromodulation technique for more than 7 decades. It is indicated primarily for severe depressive episodes (eg, psychotic, melancholic), particularly in older patients.
ECT delivers electrical current to the CNS that is sufficient to produce a seizure. Under modified conditions, a typical course of 6 to 12 sessions can resolve severe depressive episodes and may also benefit other disorders, such as bipolar mania and acute psychosis. Although ECT is potentially life-saving, its use was markedly curtailed with the advent of effective antidepressants in the 1950s. Multiple factors impede its use, including:
- access and expertise are limited in many areas
- cognition is at least temporarily adversely affected
- relapse rates after acute benefit are high
- cost
- public perception often is negative.
Studies are addressing several of these concerns. For example, the National Institute of Mental Health-sponsored Consortium on Research with ECT (CORE) group is considering how to more effectively maintain acute benefits of ECT. They compared the potential merits of maintenance ECT with maintenance pharmacotherapy (nortriptyline plus lithium) over 6 months. Although the 2 strategies had comparable results, retention rates were <50% and about one-third relapsed in both groups.2,3 Potential alternative strategies include a more frequent ECT maintenance schedule and/or combining maintenance ECT with medication(s).
Magnetic seizure therapy (MST) and focal electrically administered seizure therapy (FEAST) are attempts to produce similar efficacy and less cognitive disruption compared with ECT.4,5 Work also continues on electrode placement (eg, bifrontal) and alteration of waveform characteristics (eg, ultra-brief) to maintain or enhance efficacy while minimizing adverse effects.6,7
Stimulating the vagus nerve
VNS was introduced for treating refractory epilepsy in 1997. In 2005, it became the first FDA-approved implantable device for managing chronic or recurrent treatment-resistant depression.
The vagus nerve is the principal parasympathetic, efferent tract regulating heart rate, intestinal motility, and gastric acid secretion. Information about pain, hunger, and satiety is conveyed by these fibers to the median raphe nucleus and locus coeruleus, brain regions with significant serotonergic and noradrenergic innervation. These neurotransmitters also are believed to play a pivotal role in major depression.
With VNS, a pacemaker-like pulse generator is surgically implanted subcutaneously in the patient’s upper left chest. Wires extend from this device to the left vagus nerve (80% of whose fibers are afferent) located in the neck, to which the pulse generator sends electrical signals every few seconds (Table 3). The right vagus nerve is not used because it provides parasympathetic innervation to the heart. A clinician adjusts stimulation parameters using a computer and a noninvasive handheld device. Common adverse effects include voice alteration or hoarseness, cough, and shortness of breath, which occur during active stimulation because of the proximity of the electrodes to the laryngeal and pharyngeal branches of the vagus nerve. These effects may improve by adjusting stimulation intensity. The device permits a wide range of duty cycles, but preclinical animal studies indicate that >50% activation periods may damage the vagus nerve. If patients become too uncomfortable, they may deactivate the device with a magnet held over the implantation area.
Two open-label studies evaluated VNS to treat major depression. The first involved 10 weeks of stimulation in 59 subjects with chronic or recurrent, nonpsychotic, unipolar or bipolar depression who failed at least 2 adequate antidepressant trials in the current episode.8 Stable doses of concomitant antidepressants or mood stabilizers were allowed. After 3 months, 18 (31%) patients responded within an average of 45.5 days, and nearly 15% achieved remission. Response was defined as 50% reduction in baseline Hamilton Depression Rating Scale-28 (HDRS-28) score; remission was defined as HDRS-28 score ≤10. Further, clinical response did not differ between unipolar and bipolar depression patients.
In the second trial, 74 patients with treatment-resistant depression received fixed dose antidepressants and VNS for 3 months, followed by 9 months of flexibly dosed VNS and antidepressants.9 At 3 months, response (≥50% reduction in HDRS-28 score) and remission (HDRS-28 score <10) rates were 37% and 17%, respectively, and increased to 53% and 33% at 1 year.
A sham-controlled trial of VNS in 235 depressed patients used similar inclusion and exclusion criteria as in the open-label study by Sackeim et al.8,10 Two weeks after device implantation, patients were randomized to active treatment (stimulator turned on) or sham control (stimulator left off). At 3 months, the primary outcome measure—response rate based on HDRS-24 score—did not differ significantly between the active and control groups (15% vs 10%, respectively). There was, however, a significantly greater improvement in Inventory of Depressive Symptomatology-Self Report Scale scores with active VNS vs sham VNS.
Patients on sham treatment then were switched to active treatment and both groups were followed for 12 additional months, at which time response and remission rates nearly doubled for both groups.11 In a post-hoc analysis, the same investigators found significant improvement with VNS compared with a naturalistic, matched control group with similar treatment-resistant depression.12 The FDA considered this adequate to support efficacy and approved the device for chronic or recurrent treatment-resistant depression in an episode not responsive to at least 4 adequate treatment trials with pharmacotherapy or ECT. Perhaps because post-hoc analyses typically are not sufficient to gain FDA approval, most insurance companies do not reimburse for VNS treatment of depression, and VNS is not frequently used for refractory depression.
Table 3
Vagus nerve stimulation treatment parameters
| Parameter | Units | Range | Median value at 12 months in pivotal study |
|---|---|---|---|
| Output current | Milliamps (mA) | 0 to 3.5 | 1 |
| Signal frequency | Hertz (Hz) | 1.30 | 20 |
| Pulse width | Microseconds (µsec) | 130 to 1,000 | 500 |
| Duty cycle: ON time* | Seconds | 7 to 60 | 30 |
| Duty cycle: OFF time* | Minutes | 0.2 to 180 | 5 |
| *Stimulation cycle is 24 hours per day Source: Epilepsy patient’s manual for vagus nerve stimulation with the VNS Therapy™ system. Houston, TX: Cyberonics, Inc.; 2002, 2004. Depression physician’s manual. Houston, TX: Cyberonics, Inc.; 2005 | |||
A newer option: TMS
TMS is the most recently FDA-approved therapeutic neuromodulation technique for treating depression. In October 2008, a TMS device became available for patients failing to respond to 1 adequate antidepressant trial during the current episode.
TMS delivers intense, intermittent magnetic pulses produced by an electrical charge into a ferromagnetic coil. The pulse intensity is similar to that produced by MRI. The coil usually is placed on the scalp over the left dorsolateral prefrontal cortex (DLPFC) and pulses are delivered in a rapid, repetitive train, causing neuronal depolarization in a small area of the adjacent cerebral cortex, as well as distal effects in other relevant neural circuits (Table 4). TMS typically is administered on an outpatient basis. A standard treatment course for depression consists of 5 treatment sessions per week for 4 to 8 weeks, depending on symptom severity and how quickly patients respond.
TMS initially was examined in several small, open-label studies that looked at various treatment parameters and stimulation sites. Several sham-controlled studies generally found TMS efficacious and further refined treatment administration. Its role in treating depression—and possibly other psychiatric disorders—has been supported by 2 recent meta-analyses.13,14
O’Reardon et al15 conducted the largest double-blind trial of active vs sham TMS (N=301) for moderately treatment-resistant major depression. This study began with a 4- to 6-week, blinded, randomized phase followed by 6 weeks of open-label TMS for initial nonresponders. The third phase reintroduced TMS over 6 months as needed to augment maintenance antidepressants. This trial utilized the most aggressive treatment parameters to date (ie, 10 Hz; 75 4-second trains; 26-second inter-train interval; 120% motor threshold) delivering 3,000 pulses per treatment over an average of 24 sessions. Compared with the sham procedure, patients who received active TMS showed significantly higher response rates on the Montgomery-Åsberg Depression Rating Scale (MADRS) at weeks 4 and 6. Similar results were found for the 17- and 24-item HDRS. At 6 weeks, remission rate—defined as a MADRS score <10—was significantly higher in the active treatment group (14%) compared with the sham procedure (6%). A post-hoc analysis found that the most robust benefit occurred in patients with only 1 failed adequate antidepressant trial (effect size=0.83).16 This administration protocol was well tolerated, with no deaths or seizures and a low rate of discontinuation because of adverse events (5%).17 The most common adverse effects were application site pain or discomfort and headaches.
Recently, the second largest (N=190) sham-controlled trial of TMS for treatment-resistant major depression was published.18 This National Institute of Mental Health-sponsored, multiphase study included an initial 2-week, treatment-free period; 3 weeks of daily treatments over the left DLPFC using the same device and parameters as in the O’Reardon study; and an additional 3 weeks of treatment in patients who were improving. Those not responding to initial treatment were crossed over to open-label active TMS. This study advanced TMS development by:
- using a novel somatosensory system that produced similar sensations with sham and active TMS
- assessing the success of maintaining the blind
- establishing a rigorous clinical rating system
- utilizing MRI-guided adjustment of coil placement in a subset of patients.
The authors concluded that active TMS was significantly better than sham treatment in achieving remission (14% vs 5%). In addition, the raters, treaters, and patients were effectively blinded to the treatment condition. MRI-assisted coil placement found that in 33% of the sample, site placement determined by standardized assessment was over the premotor cortex rather than the prefrontal cortex, so the coil was moved 1 additional cm anteriorly in these patients. Similar to those observed by O’Reardon et al, adverse effects of active TMS were generally mild to moderate, did not differ by treatment condition, and led to a low discontinuation rate (5.5%).
Table 4
Treatment parameters of transcranial magnetic stimulation
| Parameter | Comment |
|---|---|
| Motor threshold | Lowest intensity over primary motor cortex to produce contraction of the first dorsal interosseous or abductor pollicis brevis muscle; visual or electromyographically monitored |
| Stimulus coil location | Most common: Left dorsolateral prefrontal cortex (DLPFC) Less common: Right DLPFC, vertex |
| Stimulus pulse(s) or train | |
| Intensity | 80% to 120% of MT |
| Frequency | ≤1 to 20 Hz |
| Duration | ≤1 millisecond |
| Interpulse interval | 50 to 100 milliseconds |
| Stimulus train duration | 3 to 6 seconds |
| Inter-train interval | 20 to 60 seconds |
| Source: Janicak PG, Krasuski J, Beedle D, et al. Transcranial magnetic stimulation for neuropsychiatric disorders. Psychiatr Times. 1999;16:56-63 | |
Deep brain stimulation
DBS is a “functional neurosurgical” procedure that delivers electrical current directly to specific areas within the brain.19 Its mechanism of action remains uncertain; depolarization blockade, synaptic inhibition, and “neural jamming” are leading hypotheses. In contrast to conventional ablative surgeries, DBS is reversible and adjustable. Implantation involves positioning pacemaker-like battery devices subcutaneously in the left and right upper chest. Electrodes attached to wires are run subcutaneously behind the ears and, with stereotactic guidance, placed through burr holes in the skull into specific CNS areas implicated in the pathophysiology of conditions such as Parkinson’s disease, refractory depression, and severe obsessive-compulsive disorder (OCD).
Antidepressant effects. The FDA recently approved DBS under its humanitarian device exemption program for intractable, severe, disabling OCD based on promising results from open and blind trials that stimulated areas such as the internal capsule and adjacent ventral striatum.20-22 These studies reported that DBS of the caudate nucleus for OCD and subthalamic nucleus for Parkinson’s disease also produced antidepressant effects. Subsequently, trials targeting the subgenual region (Brodmann’s area 25), the ventral capsule/ventral striatum, and nucleus accumbens demonstrated antidepressant effects.23-27 Pending the results of ongoing pilot trials, large, multi-center studies using different devices and target areas are being planned to clarify the role of DBS for patients with severe, disabling, refractory depression.
Adverse effects of DBS can be:
- surgical-related (eg, seizure, bleeding, infection)
- device-related (eg, lead breakage, malfunction)
- stimulation-related (eg, paresthesia, dysarthria, memory disruption, cognitive changes, psychiatric symptoms).
The most serious risk is intracranial bleeding, which occurs in 2% to 3% of patients. Clearly, the risk-benefit ratio must be carefully considered.
Cost and reimbursement
Cost of treatment and potential for third-party reimbursement are important considerations for any risk-benefit analysis. Many patients who seek neuromodulation treatments will not have insurance or other coverage entitlements.28-30 Further, newer treatments are not routinely covered by insurance; however, individual case coverage may be allowed and some device manufacturers have programs to assist providers and patients obtain coverage.28-30 Even ECT, which has long been a covered treatment for major depression, is still considered investigational for other disorders. Thus, it is important to pre-certify with the patient’s health insurance provider before initiating treatment.
Coverage, however, is not the only consideration when weighing cost effectiveness. Economic studies can assist with clinical and ethical decisions relating to treatment choice.31 These studies, however, need to be critically evaluated (eg, what costs were included in the analysis). Although direct costs are easier to evaluate, indirect costs—such as the patient’s ability to continue to work while receiving the treatment, caretaker availability during treatment, and whether treatment is an inpatient or outpatient procedure—are more difficult to evaluate and should be discussed with the patient. Because these specialized options have the potential to further benefit patients with depression and other neuropsychiatric disorders, it is essential to balance the pressures of cost containment with the need for more effective and better tolerated treatments.32-34
Related Resource
- Brunoni AR, Teng CT, Correa C, et al. Neuromodulation approaches for the treatment of major depression: challenges and recommendations from a working group meeting. Arq Neuropsiquiatr. 2010;68(3):433-451.
Drug Brand Names
- Lithium • Eskalith, Lithobid
- Nortriptyline • Aventyl, Pamelor
1. Janicak PG, Pavuluri M, Marder S. Principles and practice of psychopharmacotherapy. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 323-359. In press.
2. Kellner CH, Knapp RG, Petrides G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression. A multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE). Arch Gen Psychiatry. 2006;63:1337-1344.
3. Rasmussen KG, Mueller M, Rummans TA, et al. Is baseline medication resistance associated with potential for relapse after successful remission of a depressive episode with ECT? Data from the Consortium for Research on Electroconvulsive Therapy (CORE). J Clin Psychiatry. 2009;70(2):232-237.
4. Spellman T, McClintock SM, Terrace H, et al. Differential effects of high-dose magnetic seizure therapy and electroconvulsive shock on cognitive function. Biol Psychiatry. 2008;63:1163-1170.
5. Spellman T, Peterchev AV, Lisanby SH. Focal electrically administered seizure therapy: a novel form of ECT illustrates the roles of current directionality, polarity, and electrode configuration in seizure induction. Neuropsychopharmacology. 2009;34(8):2002-2010.
6. Kellner CH, Knapp R, Husain MM, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry. 2010;196:226-234.
7. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimulat. 2008;1:71-83.
8. Sackeim HA, Rush JA, George MS, et al. Vagus nerve stimulation (VNSTM) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713-728.
9. Schlaepfer TE, Frick C, Zobel A, et al. Vagus nerve stimulation for depression: efficacy and safety in a European study. Psychol Med. 2008;38(5):651-661.
10. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized controlled acute phase trial. Biol Psychiatry. 2005;58:347-354.
11. Rush AJ, Sackeim HA, Marangell LB, et al. Effects of 12 months of vagus nerve stimulation in treatment resistant depression: a naturalistic study. Biol Psychiatry. 2005;58(5):355-363.
12. George MS, Rush AJ, Marangell LB, et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry. 2005;58:364-373.
13. Schutter DJ. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dordolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychol Med. 2009;39:65-75.
14. Slotema CW, Blom JD, Hoek HW, et al. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71(7):873-884.
15. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208-1216.
16. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522-534.
17. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
18. George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507-516.
19. Pilitsis JG, Bakay RAE. Deep brain stimulation for psychiatric disorders. Psychopharm Rev. 2007;42(9):67-74.
20. Greenberg BD, Gabriels LA, Malone DA, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15(1):64-79.
21. Mallet L, Plolsan M, Jaafari N, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121-2134.
22. Goodman WK, Foote KD, Greenberg BD, et al. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. 2010;67:535-542.
23. Mayberg HS, Lozano AM, McNeely HE, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
24. Lozano AM, Mayberg HS, Giacobbe P, et al. Subcallosal cingulated gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461-467.
25. McNeely HE, Mayberg HS, Lozano AM, et al. Neuropsychological impact of Cg25 deep brain stimulation for treatment-resistant depression: preliminary results over 12 months. J Nerv Ment Dis. 2008;196(5):405-410.
26. Malone DA, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
27. Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2):368-377.
28. Health insurance coverage NeuroStar TMS Therapy® Web site. Available at: http://www.neurostartms.com/TMSHealthInsurance/Health-Insurance-Coverage.aspx. Accessed June 2, 2010.
29. VNS insurance information Vagus nerve stimulation therapy for treatment-resistant depression Web site. Available at: http://www.vnstherapy.com/depression/insuranceinformation/coverage.asp. Accessed June 2, 2010.
30. Insurance coverage—DBS therapy for OCD Available at: http://www.medtronic.com/your-health/obsessive-compulsive-disorder-ocd/getting-therapy/insurance-coverage/index.htm. Accessed June 2, 2010.
31. Simpson KN, Welch MJ, Kozel FA, et al. Cost-effectiveness of transcranial magnetic stimulation in the treatment of major depression: a health economics analysis. Adv Ther. 2009;26(3):346-368.
32. Rado J, Dowd SM, Janicak PG. The emerging role of transcranial magnetic stimulation (TMS) for treatment of psychiatric disorders. Dir Psychiatry. 2008;28(25):215-331.
33. Dougherty DD, Rauch SL. Somatic therapies for treatment-resistant depression: new neurotherapeutic interventions. Psychiatr Clin N Am. 2007;30:31-37.
34. Olfson M, Marcus S, Sackeim HA, et al. Use of ECT for the inpatient treatment of recurrent major depression. Am J Psychiatry. 1998;155:22-29.
1. Janicak PG, Pavuluri M, Marder S. Principles and practice of psychopharmacotherapy. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 323-359. In press.
2. Kellner CH, Knapp RG, Petrides G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression. A multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE). Arch Gen Psychiatry. 2006;63:1337-1344.
3. Rasmussen KG, Mueller M, Rummans TA, et al. Is baseline medication resistance associated with potential for relapse after successful remission of a depressive episode with ECT? Data from the Consortium for Research on Electroconvulsive Therapy (CORE). J Clin Psychiatry. 2009;70(2):232-237.
4. Spellman T, McClintock SM, Terrace H, et al. Differential effects of high-dose magnetic seizure therapy and electroconvulsive shock on cognitive function. Biol Psychiatry. 2008;63:1163-1170.
5. Spellman T, Peterchev AV, Lisanby SH. Focal electrically administered seizure therapy: a novel form of ECT illustrates the roles of current directionality, polarity, and electrode configuration in seizure induction. Neuropsychopharmacology. 2009;34(8):2002-2010.
6. Kellner CH, Knapp R, Husain MM, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry. 2010;196:226-234.
7. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimulat. 2008;1:71-83.
8. Sackeim HA, Rush JA, George MS, et al. Vagus nerve stimulation (VNSTM) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713-728.
9. Schlaepfer TE, Frick C, Zobel A, et al. Vagus nerve stimulation for depression: efficacy and safety in a European study. Psychol Med. 2008;38(5):651-661.
10. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized controlled acute phase trial. Biol Psychiatry. 2005;58:347-354.
11. Rush AJ, Sackeim HA, Marangell LB, et al. Effects of 12 months of vagus nerve stimulation in treatment resistant depression: a naturalistic study. Biol Psychiatry. 2005;58(5):355-363.
12. George MS, Rush AJ, Marangell LB, et al. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatry. 2005;58:364-373.
13. Schutter DJ. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dordolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychol Med. 2009;39:65-75.
14. Slotema CW, Blom JD, Hoek HW, et al. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71(7):873-884.
15. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208-1216.
16. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522-534.
17. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
18. George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507-516.
19. Pilitsis JG, Bakay RAE. Deep brain stimulation for psychiatric disorders. Psychopharm Rev. 2007;42(9):67-74.
20. Greenberg BD, Gabriels LA, Malone DA, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15(1):64-79.
21. Mallet L, Plolsan M, Jaafari N, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121-2134.
22. Goodman WK, Foote KD, Greenberg BD, et al. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. 2010;67:535-542.
23. Mayberg HS, Lozano AM, McNeely HE, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651-660.
24. Lozano AM, Mayberg HS, Giacobbe P, et al. Subcallosal cingulated gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461-467.
25. McNeely HE, Mayberg HS, Lozano AM, et al. Neuropsychological impact of Cg25 deep brain stimulation for treatment-resistant depression: preliminary results over 12 months. J Nerv Ment Dis. 2008;196(5):405-410.
26. Malone DA, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
27. Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2):368-377.
28. Health insurance coverage NeuroStar TMS Therapy® Web site. Available at: http://www.neurostartms.com/TMSHealthInsurance/Health-Insurance-Coverage.aspx. Accessed June 2, 2010.
29. VNS insurance information Vagus nerve stimulation therapy for treatment-resistant depression Web site. Available at: http://www.vnstherapy.com/depression/insuranceinformation/coverage.asp. Accessed June 2, 2010.
30. Insurance coverage—DBS therapy for OCD Available at: http://www.medtronic.com/your-health/obsessive-compulsive-disorder-ocd/getting-therapy/insurance-coverage/index.htm. Accessed June 2, 2010.
31. Simpson KN, Welch MJ, Kozel FA, et al. Cost-effectiveness of transcranial magnetic stimulation in the treatment of major depression: a health economics analysis. Adv Ther. 2009;26(3):346-368.
32. Rado J, Dowd SM, Janicak PG. The emerging role of transcranial magnetic stimulation (TMS) for treatment of psychiatric disorders. Dir Psychiatry. 2008;28(25):215-331.
33. Dougherty DD, Rauch SL. Somatic therapies for treatment-resistant depression: new neurotherapeutic interventions. Psychiatr Clin N Am. 2007;30:31-37.
34. Olfson M, Marcus S, Sackeim HA, et al. Use of ECT for the inpatient treatment of recurrent major depression. Am J Psychiatry. 1998;155:22-29.
Transcranial magnetic stimulation for depression
Only 28% to 33% of patients with major depression experience remission after their first antidepressant treatment, according to results of the Sequenced Treatment Alternative to Relieve Depression (STAR*D) trial.1 Therapeutic options include switching to an alternate antidepressant, augmentation with a second antidepressant, psychotherapy, mood stabilizers, or second-generation antipsychotics.
In October 2008, the FDA approved a new option: transcranial magnetic stimulation (NeuroStar TMS Therapy), a neuro-modulation approach indicated for patients with major depressive disorder (MDD) who failed 1 adequate antidepressant trial in the current episode (Table 1).
Table 1
Transcranial magnetic stimulation: Fast facts
| Brand name: NeuroStar TMS Therapy |
| Class: Class II medical device |
| Indication: Treatment of major depressive disorder in adults who failed to achieve satisfactory improvement from 1 prior antidepressant medication at or above the minimal effective dose and duration in the current depressive episode |
| Approval date: October 7, 2008 |
| Availability: Limited number of treatment centers; see www.NeuroStarTMS.com |
| Manufacturer: Neuronetics, Inc. |
| Recommended dose: 75 10-Hz, 4-second trains; 26-second intertrain interval; administered over the left dorsolateral prefrontal cortex; 5 days a week, up to 6 weeks |
How it works
TMS delivers intense intermittent magnetic pulses produced by an electrical charge into a ferromagnetic coil. The intensity of the pulse is similar to that of MRI (1.5 to 2 tesla); however, in MRI the magnetic field is constantly on, whereas in TMS the field is exceptionally brief (milliseconds).
For depression treatment, the coil is usually placed on the scalp over the left dorsolateral prefrontal cortex (DLPFC). Pulses are delivered in a rapid, repetitive train, causing neuronal depolarization in a small area of the cerebral cortex and distal effects in other neurocircuits.
For depression, standard outpatient treatment consists of 5 daily sessions per week for up to 6 weeks. Each session takes approximately 40 minutes, and patients typically return to normal daily activities without difficulty. Initially, NeuroStar TMS will be available in a limited number of treatment centers (see Related Resource).
Intensity of treatment is individualized by adjusting parameters that affect delivery of the magnetic pulses. Motor threshold (MT) is the level of stimulation required to produce movement in a contralateral target muscle, such as the abductor pollicis brevis that causes contraction of the thumb. Once this level is determined, pulses are administered at an intensity relative to the MT (such as 120%). Single TMS pulses are used to find the relevant area of the motor cortex, whereas repetitive pulses are applied over the left DLPFC for therapy.
Frequency of stimulation is measured in cycles per second or hertz (Hz). Stimulation train is the duration during which pulses are administered, and the intertrain interval (ITI) is the time between stimulation trains. Other parameters include site of stimulation and number of treatments per day, week, and course. Recommended treatment levels appear in (Table 2).
Table 2
TMS depression treatment parameters
| Parameter | Definition | Recommended treatment level | |
|---|---|---|---|
| Motor threshold | Level of stimulation required to produce contractions in the contralateral target muscle (abductor pollicis brevis, which causes contraction of the thumb) | 120% | |
| Frequency of stimulation | Measured in cycles per second or hertz (Hz) | 10 Hz | |
| Stimulation train | Duration of the stimulation | 4 seconds | |
| Intertrain interval | Time between stimulation trains | 26 seconds | |
| Site of stimulation | Where in the brain the stimulation will occur | Left dorsolateral prefrontal cortex | |
| Number of treatments | How many times the patient receives stimulation/treatment | 5 days per week for up to 6 weeks | |
| Total stimulation time | Number of stimulations given in a session | 3,000 stimulations per session | |
| TMS: transcranial magnetic stimulation | |||
Efficacy
George et al2 first reported TMS for depression in 1995. Initial small, open-label studies examined a variety of treatment intensities, durations, and stimulation sites. Several sham-controlled studies further refined treatment parameters. These studies generally found TMS efficacious, but questioned the robustness of the clinical effect.
To better assess the antidepressant effect of TMS, studies employed larger samples and more aggressive treatment parameters. Avery et al3 randomized 68 patients to 15 sessions of active or sham TMS over the left DLPFC. Each treatment consisted of 32 10-Hz, 5-second trains at 110% MT with a 25-second ITI. At 1 and 2 weeks after treatment, 31% of subjects in the active treatment group showed a significant decrease in symptoms—defined as ≥50% reduction in Hamilton Depression Rating Scale (HDRS) score—versus 6% in the sham group. In addition, 20% of subjects in the active TMS group achieved remission (defined as HDRS score
The largest trial of TMS monotherapy (N=301) for moderately treatment-resistant major depression was completed in 2007.4 This 3-phase study began with a 4- to 6-week, randomized, double-blind activeversus-sham TMS procedure, followed by 6 weeks of open-label TMS in initial nonresponders. The third phase reintroduced TMS over 6 months as needed to augment maintenance antidepressant medication.
This trial used the most aggressive treatment parameters to date: 75 10-Hz, 4-second trains at 120% MT with a 26-second ITI, delivering 3,000 pulses per treatment over an average of 26 sessions. To maintain an adequate blind, the study utilized sham and active coils with similar appearances, placement, and acoustic properties. The sham coil had an embedded aluminum shield, which limited the magnetic energy reaching the cortex to ≤10% of the active coil. Although there was no assessment of the adequacy of the blind in this trial:
- subjects were naive to TMS in the sham-controlled phase
- TMS operators did not assess efficacy
- TMS operators and subjects did not discuss the treatment experience with the efficacy raters.
Compared with those who received the sham procedure, subjects who received active TMS showed significantly better response rates on the Montgomery-Åsberg Depression Rating Scale (MADRS) at weeks 4 and 6. Similar results were found for the 17- and 24-item HDRS. At 6 weeks, the remission rate (defined as a MADRS score
A post-hoc analysis found that the greatest benefit occurred in patients who had only 1 failed adequate antidepressant trial (effect size=0.83).5
TMS vs ECT. Dowd et al6 summarized 8 published trials that compared TMS with electroconvulsive therapy (ECT) for severe depression:
- 5 reported equivalent efficacy
- 1 found unilateral ECT (UL-ECT) and bilateral ECT (BL-ECT) superior to TMS
- 1 reported UL-ECT superior to TMS
- 1 found UL-ECT plus medication superior to TMS monotherapy in patients with psychosis but comparable in efficacy to TMS in the absence of psychosis.
These results need to be interpreted with caution because of the studies’ diverse designs, nonblinded assessments, and small sample sizes.
Tolerability and safety
The most frequently reported adverse effects of TMS are headache and pain at the site of stimulation. Seizures had been reported in early trials, but the extremely low occurrence has been much lower since Wasserman7 published consensus guidelines on the safe use of TMS in 1996.
Janicak et al8 examined safety data from the 3-phase trial mentioned above, which included >10,000 cumulative treatment sessions. TMS was well-tolerated, with a low discontinuation rate associated with adverse effects: 4.5% in the active treatment group versus 3.4% in the sham TMS procedure group. No deaths, seizures, or cases of treatment-emergent mania occurred. The most commonly reported adverse effects were transient headache and discomfort at the stimulation site. Most patients acclimated to these effects in the first week. No changes were seen in cognitive functioning or auditory thresholds.
As in previous studies, TMS was safely combined with antidepressants in the third phase of this trial; however, patients at risk for seizure or on medications that could lower the seizure threshold were excluded. Thus, risk of seizure may be increased under these conditions. TMS is contraindicated for patients with implanted metallic devices or nonremovable objects in or around the head, except for dental hardware or braces.
- For availability information, contact the manufacturer, Neuronetics, at (877) 6000-7555 or www.NeuroStarTMS.com.
Disclosures
Drs. Dowd, Rado, and Janicak receive research support from and are consultants to Neuronetics, Inc.
Dr. Welch receives research support from Neuronetics, Inc.
1. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163(1):28-40.
2. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995;6(14):1853-6.
3. Avery DH, Holtzheimer PE, III, Fawaz W, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry 2006;59:187-94.
4. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multi-site randomized controlled trial. Biol Psychiatry 2007;62:1208-16.
5. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology Epub 2008 Aug 13.
6. Dowd SM, Janicak PG. Transcranial magnetic stimulation for major depression: part II. Psychopharm Review 2007;42(1):1-8.
7. Wasserman EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol 1998;108(1):1-16.
8. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction. J Clin Psychiatry 2008;69:222-33.
Only 28% to 33% of patients with major depression experience remission after their first antidepressant treatment, according to results of the Sequenced Treatment Alternative to Relieve Depression (STAR*D) trial.1 Therapeutic options include switching to an alternate antidepressant, augmentation with a second antidepressant, psychotherapy, mood stabilizers, or second-generation antipsychotics.
In October 2008, the FDA approved a new option: transcranial magnetic stimulation (NeuroStar TMS Therapy), a neuro-modulation approach indicated for patients with major depressive disorder (MDD) who failed 1 adequate antidepressant trial in the current episode (Table 1).
Table 1
Transcranial magnetic stimulation: Fast facts
| Brand name: NeuroStar TMS Therapy |
| Class: Class II medical device |
| Indication: Treatment of major depressive disorder in adults who failed to achieve satisfactory improvement from 1 prior antidepressant medication at or above the minimal effective dose and duration in the current depressive episode |
| Approval date: October 7, 2008 |
| Availability: Limited number of treatment centers; see www.NeuroStarTMS.com |
| Manufacturer: Neuronetics, Inc. |
| Recommended dose: 75 10-Hz, 4-second trains; 26-second intertrain interval; administered over the left dorsolateral prefrontal cortex; 5 days a week, up to 6 weeks |
How it works
TMS delivers intense intermittent magnetic pulses produced by an electrical charge into a ferromagnetic coil. The intensity of the pulse is similar to that of MRI (1.5 to 2 tesla); however, in MRI the magnetic field is constantly on, whereas in TMS the field is exceptionally brief (milliseconds).
For depression treatment, the coil is usually placed on the scalp over the left dorsolateral prefrontal cortex (DLPFC). Pulses are delivered in a rapid, repetitive train, causing neuronal depolarization in a small area of the cerebral cortex and distal effects in other neurocircuits.
For depression, standard outpatient treatment consists of 5 daily sessions per week for up to 6 weeks. Each session takes approximately 40 minutes, and patients typically return to normal daily activities without difficulty. Initially, NeuroStar TMS will be available in a limited number of treatment centers (see Related Resource).
Intensity of treatment is individualized by adjusting parameters that affect delivery of the magnetic pulses. Motor threshold (MT) is the level of stimulation required to produce movement in a contralateral target muscle, such as the abductor pollicis brevis that causes contraction of the thumb. Once this level is determined, pulses are administered at an intensity relative to the MT (such as 120%). Single TMS pulses are used to find the relevant area of the motor cortex, whereas repetitive pulses are applied over the left DLPFC for therapy.
Frequency of stimulation is measured in cycles per second or hertz (Hz). Stimulation train is the duration during which pulses are administered, and the intertrain interval (ITI) is the time between stimulation trains. Other parameters include site of stimulation and number of treatments per day, week, and course. Recommended treatment levels appear in (Table 2).
Table 2
TMS depression treatment parameters
| Parameter | Definition | Recommended treatment level | |
|---|---|---|---|
| Motor threshold | Level of stimulation required to produce contractions in the contralateral target muscle (abductor pollicis brevis, which causes contraction of the thumb) | 120% | |
| Frequency of stimulation | Measured in cycles per second or hertz (Hz) | 10 Hz | |
| Stimulation train | Duration of the stimulation | 4 seconds | |
| Intertrain interval | Time between stimulation trains | 26 seconds | |
| Site of stimulation | Where in the brain the stimulation will occur | Left dorsolateral prefrontal cortex | |
| Number of treatments | How many times the patient receives stimulation/treatment | 5 days per week for up to 6 weeks | |
| Total stimulation time | Number of stimulations given in a session | 3,000 stimulations per session | |
| TMS: transcranial magnetic stimulation | |||
Efficacy
George et al2 first reported TMS for depression in 1995. Initial small, open-label studies examined a variety of treatment intensities, durations, and stimulation sites. Several sham-controlled studies further refined treatment parameters. These studies generally found TMS efficacious, but questioned the robustness of the clinical effect.
To better assess the antidepressant effect of TMS, studies employed larger samples and more aggressive treatment parameters. Avery et al3 randomized 68 patients to 15 sessions of active or sham TMS over the left DLPFC. Each treatment consisted of 32 10-Hz, 5-second trains at 110% MT with a 25-second ITI. At 1 and 2 weeks after treatment, 31% of subjects in the active treatment group showed a significant decrease in symptoms—defined as ≥50% reduction in Hamilton Depression Rating Scale (HDRS) score—versus 6% in the sham group. In addition, 20% of subjects in the active TMS group achieved remission (defined as HDRS score
The largest trial of TMS monotherapy (N=301) for moderately treatment-resistant major depression was completed in 2007.4 This 3-phase study began with a 4- to 6-week, randomized, double-blind activeversus-sham TMS procedure, followed by 6 weeks of open-label TMS in initial nonresponders. The third phase reintroduced TMS over 6 months as needed to augment maintenance antidepressant medication.
This trial used the most aggressive treatment parameters to date: 75 10-Hz, 4-second trains at 120% MT with a 26-second ITI, delivering 3,000 pulses per treatment over an average of 26 sessions. To maintain an adequate blind, the study utilized sham and active coils with similar appearances, placement, and acoustic properties. The sham coil had an embedded aluminum shield, which limited the magnetic energy reaching the cortex to ≤10% of the active coil. Although there was no assessment of the adequacy of the blind in this trial:
- subjects were naive to TMS in the sham-controlled phase
- TMS operators did not assess efficacy
- TMS operators and subjects did not discuss the treatment experience with the efficacy raters.
Compared with those who received the sham procedure, subjects who received active TMS showed significantly better response rates on the Montgomery-Åsberg Depression Rating Scale (MADRS) at weeks 4 and 6. Similar results were found for the 17- and 24-item HDRS. At 6 weeks, the remission rate (defined as a MADRS score
A post-hoc analysis found that the greatest benefit occurred in patients who had only 1 failed adequate antidepressant trial (effect size=0.83).5
TMS vs ECT. Dowd et al6 summarized 8 published trials that compared TMS with electroconvulsive therapy (ECT) for severe depression:
- 5 reported equivalent efficacy
- 1 found unilateral ECT (UL-ECT) and bilateral ECT (BL-ECT) superior to TMS
- 1 reported UL-ECT superior to TMS
- 1 found UL-ECT plus medication superior to TMS monotherapy in patients with psychosis but comparable in efficacy to TMS in the absence of psychosis.
These results need to be interpreted with caution because of the studies’ diverse designs, nonblinded assessments, and small sample sizes.
Tolerability and safety
The most frequently reported adverse effects of TMS are headache and pain at the site of stimulation. Seizures had been reported in early trials, but the extremely low occurrence has been much lower since Wasserman7 published consensus guidelines on the safe use of TMS in 1996.
Janicak et al8 examined safety data from the 3-phase trial mentioned above, which included >10,000 cumulative treatment sessions. TMS was well-tolerated, with a low discontinuation rate associated with adverse effects: 4.5% in the active treatment group versus 3.4% in the sham TMS procedure group. No deaths, seizures, or cases of treatment-emergent mania occurred. The most commonly reported adverse effects were transient headache and discomfort at the stimulation site. Most patients acclimated to these effects in the first week. No changes were seen in cognitive functioning or auditory thresholds.
As in previous studies, TMS was safely combined with antidepressants in the third phase of this trial; however, patients at risk for seizure or on medications that could lower the seizure threshold were excluded. Thus, risk of seizure may be increased under these conditions. TMS is contraindicated for patients with implanted metallic devices or nonremovable objects in or around the head, except for dental hardware or braces.
- For availability information, contact the manufacturer, Neuronetics, at (877) 6000-7555 or www.NeuroStarTMS.com.
Disclosures
Drs. Dowd, Rado, and Janicak receive research support from and are consultants to Neuronetics, Inc.
Dr. Welch receives research support from Neuronetics, Inc.
Only 28% to 33% of patients with major depression experience remission after their first antidepressant treatment, according to results of the Sequenced Treatment Alternative to Relieve Depression (STAR*D) trial.1 Therapeutic options include switching to an alternate antidepressant, augmentation with a second antidepressant, psychotherapy, mood stabilizers, or second-generation antipsychotics.
In October 2008, the FDA approved a new option: transcranial magnetic stimulation (NeuroStar TMS Therapy), a neuro-modulation approach indicated for patients with major depressive disorder (MDD) who failed 1 adequate antidepressant trial in the current episode (Table 1).
Table 1
Transcranial magnetic stimulation: Fast facts
| Brand name: NeuroStar TMS Therapy |
| Class: Class II medical device |
| Indication: Treatment of major depressive disorder in adults who failed to achieve satisfactory improvement from 1 prior antidepressant medication at or above the minimal effective dose and duration in the current depressive episode |
| Approval date: October 7, 2008 |
| Availability: Limited number of treatment centers; see www.NeuroStarTMS.com |
| Manufacturer: Neuronetics, Inc. |
| Recommended dose: 75 10-Hz, 4-second trains; 26-second intertrain interval; administered over the left dorsolateral prefrontal cortex; 5 days a week, up to 6 weeks |
How it works
TMS delivers intense intermittent magnetic pulses produced by an electrical charge into a ferromagnetic coil. The intensity of the pulse is similar to that of MRI (1.5 to 2 tesla); however, in MRI the magnetic field is constantly on, whereas in TMS the field is exceptionally brief (milliseconds).
For depression treatment, the coil is usually placed on the scalp over the left dorsolateral prefrontal cortex (DLPFC). Pulses are delivered in a rapid, repetitive train, causing neuronal depolarization in a small area of the cerebral cortex and distal effects in other neurocircuits.
For depression, standard outpatient treatment consists of 5 daily sessions per week for up to 6 weeks. Each session takes approximately 40 minutes, and patients typically return to normal daily activities without difficulty. Initially, NeuroStar TMS will be available in a limited number of treatment centers (see Related Resource).
Intensity of treatment is individualized by adjusting parameters that affect delivery of the magnetic pulses. Motor threshold (MT) is the level of stimulation required to produce movement in a contralateral target muscle, such as the abductor pollicis brevis that causes contraction of the thumb. Once this level is determined, pulses are administered at an intensity relative to the MT (such as 120%). Single TMS pulses are used to find the relevant area of the motor cortex, whereas repetitive pulses are applied over the left DLPFC for therapy.
Frequency of stimulation is measured in cycles per second or hertz (Hz). Stimulation train is the duration during which pulses are administered, and the intertrain interval (ITI) is the time between stimulation trains. Other parameters include site of stimulation and number of treatments per day, week, and course. Recommended treatment levels appear in (Table 2).
Table 2
TMS depression treatment parameters
| Parameter | Definition | Recommended treatment level | |
|---|---|---|---|
| Motor threshold | Level of stimulation required to produce contractions in the contralateral target muscle (abductor pollicis brevis, which causes contraction of the thumb) | 120% | |
| Frequency of stimulation | Measured in cycles per second or hertz (Hz) | 10 Hz | |
| Stimulation train | Duration of the stimulation | 4 seconds | |
| Intertrain interval | Time between stimulation trains | 26 seconds | |
| Site of stimulation | Where in the brain the stimulation will occur | Left dorsolateral prefrontal cortex | |
| Number of treatments | How many times the patient receives stimulation/treatment | 5 days per week for up to 6 weeks | |
| Total stimulation time | Number of stimulations given in a session | 3,000 stimulations per session | |
| TMS: transcranial magnetic stimulation | |||
Efficacy
George et al2 first reported TMS for depression in 1995. Initial small, open-label studies examined a variety of treatment intensities, durations, and stimulation sites. Several sham-controlled studies further refined treatment parameters. These studies generally found TMS efficacious, but questioned the robustness of the clinical effect.
To better assess the antidepressant effect of TMS, studies employed larger samples and more aggressive treatment parameters. Avery et al3 randomized 68 patients to 15 sessions of active or sham TMS over the left DLPFC. Each treatment consisted of 32 10-Hz, 5-second trains at 110% MT with a 25-second ITI. At 1 and 2 weeks after treatment, 31% of subjects in the active treatment group showed a significant decrease in symptoms—defined as ≥50% reduction in Hamilton Depression Rating Scale (HDRS) score—versus 6% in the sham group. In addition, 20% of subjects in the active TMS group achieved remission (defined as HDRS score
The largest trial of TMS monotherapy (N=301) for moderately treatment-resistant major depression was completed in 2007.4 This 3-phase study began with a 4- to 6-week, randomized, double-blind activeversus-sham TMS procedure, followed by 6 weeks of open-label TMS in initial nonresponders. The third phase reintroduced TMS over 6 months as needed to augment maintenance antidepressant medication.
This trial used the most aggressive treatment parameters to date: 75 10-Hz, 4-second trains at 120% MT with a 26-second ITI, delivering 3,000 pulses per treatment over an average of 26 sessions. To maintain an adequate blind, the study utilized sham and active coils with similar appearances, placement, and acoustic properties. The sham coil had an embedded aluminum shield, which limited the magnetic energy reaching the cortex to ≤10% of the active coil. Although there was no assessment of the adequacy of the blind in this trial:
- subjects were naive to TMS in the sham-controlled phase
- TMS operators did not assess efficacy
- TMS operators and subjects did not discuss the treatment experience with the efficacy raters.
Compared with those who received the sham procedure, subjects who received active TMS showed significantly better response rates on the Montgomery-Åsberg Depression Rating Scale (MADRS) at weeks 4 and 6. Similar results were found for the 17- and 24-item HDRS. At 6 weeks, the remission rate (defined as a MADRS score
A post-hoc analysis found that the greatest benefit occurred in patients who had only 1 failed adequate antidepressant trial (effect size=0.83).5
TMS vs ECT. Dowd et al6 summarized 8 published trials that compared TMS with electroconvulsive therapy (ECT) for severe depression:
- 5 reported equivalent efficacy
- 1 found unilateral ECT (UL-ECT) and bilateral ECT (BL-ECT) superior to TMS
- 1 reported UL-ECT superior to TMS
- 1 found UL-ECT plus medication superior to TMS monotherapy in patients with psychosis but comparable in efficacy to TMS in the absence of psychosis.
These results need to be interpreted with caution because of the studies’ diverse designs, nonblinded assessments, and small sample sizes.
Tolerability and safety
The most frequently reported adverse effects of TMS are headache and pain at the site of stimulation. Seizures had been reported in early trials, but the extremely low occurrence has been much lower since Wasserman7 published consensus guidelines on the safe use of TMS in 1996.
Janicak et al8 examined safety data from the 3-phase trial mentioned above, which included >10,000 cumulative treatment sessions. TMS was well-tolerated, with a low discontinuation rate associated with adverse effects: 4.5% in the active treatment group versus 3.4% in the sham TMS procedure group. No deaths, seizures, or cases of treatment-emergent mania occurred. The most commonly reported adverse effects were transient headache and discomfort at the stimulation site. Most patients acclimated to these effects in the first week. No changes were seen in cognitive functioning or auditory thresholds.
As in previous studies, TMS was safely combined with antidepressants in the third phase of this trial; however, patients at risk for seizure or on medications that could lower the seizure threshold were excluded. Thus, risk of seizure may be increased under these conditions. TMS is contraindicated for patients with implanted metallic devices or nonremovable objects in or around the head, except for dental hardware or braces.
- For availability information, contact the manufacturer, Neuronetics, at (877) 6000-7555 or www.NeuroStarTMS.com.
Disclosures
Drs. Dowd, Rado, and Janicak receive research support from and are consultants to Neuronetics, Inc.
Dr. Welch receives research support from Neuronetics, Inc.
1. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163(1):28-40.
2. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995;6(14):1853-6.
3. Avery DH, Holtzheimer PE, III, Fawaz W, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry 2006;59:187-94.
4. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multi-site randomized controlled trial. Biol Psychiatry 2007;62:1208-16.
5. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology Epub 2008 Aug 13.
6. Dowd SM, Janicak PG. Transcranial magnetic stimulation for major depression: part II. Psychopharm Review 2007;42(1):1-8.
7. Wasserman EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol 1998;108(1):1-16.
8. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction. J Clin Psychiatry 2008;69:222-33.
1. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163(1):28-40.
2. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995;6(14):1853-6.
3. Avery DH, Holtzheimer PE, III, Fawaz W, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry 2006;59:187-94.
4. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multi-site randomized controlled trial. Biol Psychiatry 2007;62:1208-16.
5. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology Epub 2008 Aug 13.
6. Dowd SM, Janicak PG. Transcranial magnetic stimulation for major depression: part II. Psychopharm Review 2007;42(1):1-8.
7. Wasserman EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol 1998;108(1):1-16.
8. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction. J Clin Psychiatry 2008;69:222-33.
Paliperidone ER: Reformulated antipsychotic for schizophrenia Tx
In the 9 months since paliperidone extended-release was FDA-approved for schizophrenia, the 3 acute pivotal trials supporting its approval have been published.1-3 They join a handful of post hoc analyses of this second-generation antipsychotic (SGA) in schizophrenia subgroups, including patients over age 65, recently diagnosed patients, and those with predominant negative symptoms.
This article discusses the evidence and paliperidone ER’s probable clinical benefits and adverse effects, with focus on its:
- pharmacodynamics and pharmacokinetics
- potential efficacy in schizophrenia and for specific patients and symptoms
- safety and tolerability.
How does paliperidone ER work?
Paliperidone ER was approved for schizophrenia treatment in December 2006 based on three 6-week, randomized, placebo-controlled trials. Paliperidone ER is the active metabolite of risperidone (9-OH risperidone) delivered in a once-daily, time-released formulation (Table 1).
Pharmacodynamics. Similar to risperidone, paliperidone ER has high binding affinity for dopamine (D2) and serotonin (5-HT2A) receptors, with additional affinity for histaminic (H1) and adrenergic receptors (alpha1 and alpha2) but not for muscarinic-cholinergic receptors.
Pharmacokinetics. After oral administration, the medication is widely and rapidly distributed. The drug’s terminal half-life is about 23 hours, and steady-state concentration is reached in 4 to 5 days.4,5
Thus paliperidone ER—when compared with risperidone and other antipsychotics that are metabolized primarily in the liver—is less likely to be involved in hepatic drug-drug or drug-disease interactions. However, some drugs that can induce CYP-450 enzymes—such as carbamazepine—may affect paliperidone’s metabolism.7
Paliperidone has an osmotic controlled-release oral delivery system (OROS®) for steady medication delivery across 24 hours8 (Table 2).1-3 The tablet consists of an osmotically active tri-layer core surrounded by a semipermeable membrane. When the tablet is swallowed, the membrane controls the rate of water reaching the tablet core, which determines the rate of drug delivery.6 The result is less variation between peak and trough drug concentrations, compared with immediate-release formulations.
Table 1
How paliperidone ER compares with risperidone
| Characteristic | Paliperidone ER | Risperidone |
|---|---|---|
| Formulation | OROS extended-release | Immediate release |
| Active moiety | 9-OH risperidone | Risperidone plus 9-OH risperidone |
| Metabolism | Primarily renal | Primarily hepatic |
| Drug interactions | Minimal | Primarily through cytochrome P-450 enzyme 2D6 |
| Dosing | Start at target dose | Titrate to target dose |
| OROS: osmotic controlled-release oral delivery system | ||
Paliperidone ER’s clinical characteristics
| Second-generation antipsychotic approved for schizophrenia |
| 9-OH active metabolite of risperidone |
| Osmotic controlled-release system provides steady-state drug delivery over 24 hours |
| Terminal half-life (time for 50% of drug to be eliminated from the body) ~23 hours |
| Available in 3-mg, 6-mg, and 9-mg tablets; recommended starting dose is 6 mg/d, and labeled dose range is 3 to 12 mg/d |
| Excreted primarily by the kidney; maximum recommended dose for patients with oderate to severe renal impairment is 3 mg/d |
| Source: References 1-3 |
Clinical use
Paliperidone ER offers potential therapeutic benefits in treating schizophrenia patients, although not without the risk of adverse events such as extrapyramidal symptoms (EPS) (Table 3).1-3
Patient selection. Because of its slow-release formulation and relatively stable plasma concentrations, paliperidone ER might be useful for patients who are highly sensitive to antipsychotics’ side effects. In particular, paliperidone ER might be ideal for patients who respond to but may not tolerate risperidone.
Paliperidone ER appears to be safe in patients with liver disease. Its primary renal excretion should minimize the risk of hepatic-related drug interactions in patients taking multiple medications.
Dosage and titration. For treating schizophrenia, the suggested starting dose of paliperidone ER is 6 mg/d taken in the morning. In the 3 pivotal trials, 6 mg was the lowest dose to show broad efficacy with minimal adverse events.9
For many patients, the 6-mg starting dose will be the therapeutic dose. When needed, the dose may be increased in 3-mg increments every 1 to 2 weeks to a maximum 12 mg/d (a 15-mg dose was used in clinical trials, but the adverse effects out-weighed the benefits). Lower maximum doses are recommended for patients with renal impairment:
- 6 mg/d for those with creatinine clearance ≥50 to
- 3 mg/d for those with creatinine clearance 10 to 10
Safety and tolerability. Pooled data from the 3 trials indicate that adverse events (AEs) occurred during treatment in 66% to 77% of patients receiving paliperidone ER vs 66% in placebo groups. The most common AEs were headache (11% to 18%), insomnia (4% to 12%), and anxiety (6% to 9%).9
EPS. Risk of EPS-related AEs (such as akathisia and parkinsonian symptoms) with 3-mg and 6-mg paliperidone ER doses (13% and 10%, respectively) was similar to placebo (11%) but increased with the 9-mg, 12-mg, and 15-mg doses (25%, 26%, and 24%, respectively). Should EPS occur, reduce the paliperidone ER dose or consider adding antiparkinsonian medications.
Lab values. No clinically relevant changes were noted in blood glucose, insulin, or lipids.12 Similar to risperidone, paliperidone ER elevated prolactin levels.
Weight gain with paliperidone ER is dose-dependent; in the clinical trials, mean body weight change for all doses was ≤1.9 kg, which is similar to the weight gain seen with risperidone and in the moderate range compared with other SGAs. When using paliperidone ER, follow the American Diabetes Association/American Psychiatric Association guidelines13 for monitoring weight gain and metabolic parameters with antipsychotics. Also monitor patients for clinical symptoms of hyperprolactinemia, and—if intolerable—adjust the dose or switch to another SGA.
Tachycardia. Advise patients that they may experience a rapid heart rate while taking paliperidone ER. In clinical trials, tachycardia occurred in ≤14% of patients—twice the rate with placebo—but did not contribute to more serious cardiac rhythm disturbances or to discontinuation. Incidence of prolonged corrected QT interval (QTc) was 3% to 5% in the paliperidone ER group vs 3% in the placebo group.
Patient education. Because of paliperidone ER’s pharmacokinetic properties, counsel patients to:
- take 1 tablet each day in the morning
- not chew, split, or crush the tablets but swallow whole to preserve the controlled-release delivery.
Table 3
Paliperidone ER’s potential benefits and risks in clinical practice
| Potential benefits | Details |
|---|---|
| Efficacy | Data support acute (6 weeks) and chronic (up to 24 weeks) improvement in schizophrenia symptoms, patient function, and quality of life |
| Pharmacokinetics | Primarily renal excretion decreases risk of hepatic drug-drug or drug-disease interactions |
| Long-acting formulation | Once-daily dosing simplifies treatment and may improve adherence |
| EPS | Risk similar to placebo at 3-mg and 6-mg doses, but increased at higher doses |
| Weight gain | Similar to risperidone |
| Hyperprolactinemia | Similar to risperidone |
| Tachycardia | Occurred in up to 14% of patients in clinical trials (twice the rate of placebo [7%]) |
| QTc prolongation | Increase up to 12 msec on average, with no patients exceeding 500 msec and no clinically adverse events during trials; use paliperidone with caution in patients with arrhythmias or cardiovascular disease or who are taking other medication that can prolong the QT interval |
| EPS: extrapyramidal symptoms | |
| Source: References 1-3 | |
Efficacy trials in schizophrenia
Three 6-week trials1-3 examined paliperidone ER’s efficacy in a total of 1,692 patients with chronic schizophrenia who were hospitalized ≥14 days with acute exacerbations. The trials were double-blind, randomized, fixed-dose, parallel-group, and placebo- and active-controlled (compared with olanzapine, 10 mg/d). Patients showed no significant differences in demographic or baseline characteristics or in the use of rescue medications.
The primary outcome measure was mean change in Positive and Negative Syndrome Scale (PANSS) total score, which quantifies positive, negative, and global psychopathologic symptom severity. Secondary outcome measures included:
- PANSS Marder factor scores14 (derived from PANSS items that reflect positive and negative symptoms, anxiety and depression, hostility, and thought disorganization).
- Clinical Global Impressions-Severity (CGI-S) score, which measures overall illness severity.15
- Personal and Social Performance (PSP) scores, which rate socially useful activities, relationships, self-care, and disturbing and aggressive behaviors; improvement by 1 category (10 points) reflects a clinically meaningful change.16,17
A total of 43% of patients completed the study—34% taking placebo; 46% taking paliperidone ER, 6 mg; 48% taking paliperidone ER, 12 mg; and 45% taking olanzapine. Demographic and baseline characteristics of the 432 patients who received ≥1 dose were similar across all groups. Approximately 75% of patients in each group used rescue medications—primarily lorazepam—for agitation, restlessness, or insomnia for a mean of 8 days.
Patients taking either paliperidone ER dose showed statistically significant greater improvement in PANSS total score compared with those taking placebo (6 mg, P = 0.006; 12 mg, P
Clinical response rates were similar with the 6-mg and 12-mg paliperidone ER doses—50% and 51%, respectively—and greater than with placebo (34%). The higher response rates with paliperidone ER were statistically significant compared with placebo (6 mg, P
Discontinuation rates for lack of efficacy were lower with paliperidone ER (6 mg, 23%; 12 mg, 14%) than with placebo (35%). A substantially lower percentage of patients taking this agent remained classified as “marked/severe/extremely severe” on the CGI-S score from baseline to endpoint, compared with the placebo group;
- 6 mg paliperidone ER, 58% to 26%
- 12 mg paliperidone ER, 64% to 21%
- placebo, 60% to 45%.
The second study2 included U.S. and international sites and compared 3 fixed doses of paliperidone ER (6-, 9-, and 12-mg) with placebo. Among the 630 patients enrolled, 66% completed the study. Patients were randomly assigned to 6 mg, 9 mg, or 12 mg of paliperidone ER; 10 mg of olanzapine; or placebo. The number of patients who dropped out because of adverse events was comparable across the groups.
Patient groups assigned to paliperidone ER showed significant improvement when compared with placebo (P 30% reduction in PANSS total score from baseline to endpoint included:
- 6 mg paliperidone ER, 56%
- 9 mg paliperidone ER, 51%
- 12 mg paliperidone ER, 61%
- placebo, 30%.
- 6 mg paliperidone ER, 63% at baseline to 22% at endpoint
- 9 mg paliperidone ER, 58% to 23%
- 12 mg paliperidone ER, 64% to 16%
- placebo, 60% to 51%.
The third study3 was a multicenter international trial that compared 3 fixed doses of paliperidone ER (3, 9, and 15 mg) with placebo. Among the 618 randomized patients, 365 (59%) completed the study: 70 of 127 (55%) on 3-mg paliperidone ER, 78 of 125 (62%) on 9-mg paliperidone ER, 82 of 115 (71%) on 15-mg paliperidone ER, and 47 of 123 (38%) on placebo.
- 3 mg paliperidone ER, 40%
- 9 mg paliperidone ER, 46%
- 15 mg paliperidone ER, 53%
- placebo, 18% (P ≤0.005).
- 3 mg paliperidone ER, 54% to 32%
- 9 mg paliperidone ER, 52% to 23%
- 15 mg paliperidone ER, 57% to 17%
- placebo, 56% to 50%.
Additional trial evidence
Schizophrenia subpopulations. Post hoc analyses of data reported from the 3 pivotal trials suggest that paliperidone ER may be useful for specific groups of schizophrenia patients, including those who are recently diagnosed, age >65, or severely ill or have predominant negative symptoms or sleep problems (Table 4).18-23
Efficacy in delaying recurrence. Paliperidone ER’s efficacy in delaying symptom recurrence was examined in a randomized, double-blind, placebo-controlled study of 207 patients who had been stabilized on open-label, flexible-dosed paliperidone ER.24 Time to first recurrence of schizophrenia symptoms was the primary efficacy measure. Starting dose was 9 mg/d (flexible dose range 3 to 15 mg/d).
The study was halted at a planned interim analysis because time-to-recurrence was significantly longer for patients receiving paliperidone ER compared with placebo (P
Final analysis of the 179 patients who completed the study confirmed the interim findings. Ongoing treatment maintained improvement in patients’ acute symptoms, functioning, and quality-of-life measures.
Table 4
Studies of paliperidone ER in schizophrenia subpopulations
| Patient population | Study design | Findings |
|---|---|---|
| Recently diagnosed | 413 patients diagnosed within 5 years of study entry compared with 893 patients who had been ill ≥5 years*18,19 | Tolerability was similar, but recently diagnosed patients were more likely to experience movement disorders and somnolence |
| Age ≥65 years | 114 schizophrenia patients age ≥65 given paliperidone ER, 3 to 12 mg/d, or placebo in 6-week, double-blind, randomized, placebo-controlled trial20 | Rates of cardiovascular, cerebrovascular, neuromotor, and metabolic changes similar to placebo, except for tachycardia (16% with paliperidone vs 0% with placebo) |
| Severely ill | 217 patients with marked to severe symptoms (baseline total PANSS score ≥105)*21 | Patients treated with paliperidone showed significantly greater improvement vs placebo in mean total PANSS score (–26.7 vs –5.7) and other measures |
| Substantial negative symptoms | 299 patients with predominant negative symptoms from 3 acute efficacy trials*22 | Patients treated with paliperidone showed significant improvements vs placebo on primary and secondary measures of negative symptoms |
| Sleep problems | 36 patients age 18 to 45 diagnosed with schizophrenia and schizophrenia-related insomnia*23 | In stable patients, paliperidone improved sleep architecture, continuity, and patient-rated sleep quality without producing or worsening daytime sleepiness |
| * Studies marked with asterisks represent post hoc analyses of data from the 3 clinical trials on which the FDA based its approval of paliperidone ER. | ||
| PANSS: Positive and Negative Syndrome Scale | ||
- Paliperidone extended release. Prescribing information. www.invega.com.
- Johnson & Johnson. U.S. District Court upholds Risperdal® (risperidone) patent (press release). October 16, 2006. www.jnj.com/news/jnj_news/20061016_094453.htm.
- Carbamazepine • Tegretol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Paliperidone ER • Invega
- Risperidone • Risperdal
Dr. Rado and Dr. Dowd receive research support from Neuronetics, sanofi-aventis, Janssen Pharmaceutica, and Solvay.
Dr. Janicak receives research support from Astra-Zeneca, Bristol-Myers Squibb, Janssen Pharmaceutica, Neuronetics, Solvay, and sanofi-aventis. He is a consultant to Astra-Zeneca, Bristol-Myers Squibb, Janssen Pharmaceutica, Neuronetics, and Solvay, and a speaker for Abbott Laboratories, Astra-Zeneca, Bristol-Myers Squibb, Janssen Pharmaceutica, and Pfizer.
1. Marder S, Kramer M, Ford L, et al. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled study. Biol Psychiatry 2007; Jun 27; Epub ahead of print.
2. Kane J, Canas F, Kramer M, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr Res 2007;90(1-3):147-61.
3. Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and early response of paliperidone extended-release tablets (paliperidone ER): results of a 6-week, randomized, placebo-controlled study. Schizophr Res 2007;93(1-3):117-30.
4. Rossenu SAC, Rusch S, Janssens L, et al. Extended release formulation of paliperidone shows dose proportional pharmacokinetics. Presented at: Annual Meeting of the American Association of Pharmaceutical Scientists; October 29, 2006; San Antonio, TX.
5. Vermeir M, Boom S, Naessens I, et al. Absorption, metabolism, and excretion of a single oral dose of 14C-paliperidone 1 mg in healthy subjects. Eur Neuropsychopharmacol 2005;15(suppl):S648-9.
6. Conley R, Gupta SK, Sathyan G. Clinical spectrum of the osmotic-controlled release oral delivery system (OROS), an advanced oral delivery form. Curr Med Res Opin 2006;22(10):1879-92.
7. Spina E, Avenoso A, Facciola G, et al. Plasma concentrations of risperidone and 9-hydroxyrisperidone: effect of comedication with carbamazepine or valproate. Ther Drug Monit 2000;22(4):481-5.
8. Paliperidone extended release. Prescribing information. Available at: http://www.invega.com. Accessed August 8, 2007.
9. Meltzer H, Kramer M, Gassmann-Mayer C, et al. Efficacy and tolerability of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 6-week placebo controlled studies. Int J Neuropsychopharmacol 2006;9(suppl 1):S225.-
10. Thyssen A, Cleton A, Osselae NV, et al. Effects of renal impairment on the pharmacokinetic profile of paliperidone extended-release tablets. Clin Pharmacol Ther 2007. In press.
11. Thyssen A, Crauwels H, Cleton A, et al. Effects of hepatic impairment on the pharmacokinetics of paliperidone immediate-release. Presented at: 46th Annual Meeting of the New Clinical Drug Evaluation Unit (NCDEU); June 12-15, 2006; Boca Raton, FL.
12. Meyer J, Kramer M, Lane R, et al. Metabolic outcomes in patients with schizophrenia treated with oral paliperidone extended release tablets: pooled analysis of three 6 week placebo-controlled studies. Int J Neuropsychopharmacol 2006;9(suppl 1):S282.-
13. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. J Clin Psychiatry 2004;65:267-72.
14. Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry 1997;58:538-46.
15. Guy W. Clinical Global Impressions Scale. Early clinical drug evaluation unit (ECDEU) assessment manual for psychopharmacology. Rockville, MD: National Institute of Mental Health, Department of Health, Education, and Welfare; 1976:218-22. ADM publication 76-338.
16. Morosini PL, Magliano L, Brambilla L, et al. Development, reliability and acceptability of a new version of the DSMIV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand 2000;101:323-9.
17. Patrick D, Adriaenssen I, Morosini P, Rothman M. Reliability, validity and sensitivity to change of the Personal and Social Performance scale in patients with acute schizophrenia. Int J Neuropsychopharmacol 2006;9(suppl 1):S287-8.
18. Kostic D, Bossie C, Turkoz I, et al. Paliperidone extended-release tablets in patients recently diagnosed with schizophrenia. Int J Neuropsychopharmacol 2006;9(suppl 1):S161.-
19. Kostic D, Bossie C, Turkoz I, et al. Paliperidone extended-release tablets in patients recently diagnosed with schizophrenia. Presented at: Congress of the Collegium Internationale Neruo-Psychopharmacologicum (CINP); July 9-13, 2006; Chicago, IL.
20. Tzimos A, Kramer M, Ford L, et al. A 6-week placebo-controlled study of the safety and tolerability of flexible doses of oral paliperidone extended release tablets in the treatment of schizophrenia in elderly patients. Int J Neuropsychopharmacol 2006;9(suppl 1):S155.-
21. Canuso C, Youssef E, Dirks B, et al. Paliperidone extended-release in severely-ill patients with schizophrenia. Presented at: 58th Annual Institute on Psychiatric Services; October 5-8, 2006; New York, NY.
22. Dirks B, Eerdekens M, Turkoz I, et al. Efficacy of paliperidone extended-release tablets in patients with schizophrenia and predominant negative symptoms. Int J Neuropsychopharmacol 2006;9(suppl 1):S162.-
23. Luthringer R, Staner L, Noel N, et al. Sleep assessments in patients with schizophrenia following treatment with paliperidone extended-release tablets. Eur Neuropsychopharmacol 2006;16(suppl 4):S224.-
24. Kramer M, Simpson G, Maciulis V, et al. Paliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomized double-blind, placebo-controlled study [published correction appears in J Clin Psychopharmacol. 2007;27(3):258]. J Clin Psychopharmacol 2007;27(1):6-14.
In the 9 months since paliperidone extended-release was FDA-approved for schizophrenia, the 3 acute pivotal trials supporting its approval have been published.1-3 They join a handful of post hoc analyses of this second-generation antipsychotic (SGA) in schizophrenia subgroups, including patients over age 65, recently diagnosed patients, and those with predominant negative symptoms.
This article discusses the evidence and paliperidone ER’s probable clinical benefits and adverse effects, with focus on its:
- pharmacodynamics and pharmacokinetics
- potential efficacy in schizophrenia and for specific patients and symptoms
- safety and tolerability.
How does paliperidone ER work?
Paliperidone ER was approved for schizophrenia treatment in December 2006 based on three 6-week, randomized, placebo-controlled trials. Paliperidone ER is the active metabolite of risperidone (9-OH risperidone) delivered in a once-daily, time-released formulation (Table 1).
Pharmacodynamics. Similar to risperidone, paliperidone ER has high binding affinity for dopamine (D2) and serotonin (5-HT2A) receptors, with additional affinity for histaminic (H1) and adrenergic receptors (alpha1 and alpha2) but not for muscarinic-cholinergic receptors.
Pharmacokinetics. After oral administration, the medication is widely and rapidly distributed. The drug’s terminal half-life is about 23 hours, and steady-state concentration is reached in 4 to 5 days.4,5
Thus paliperidone ER—when compared with risperidone and other antipsychotics that are metabolized primarily in the liver—is less likely to be involved in hepatic drug-drug or drug-disease interactions. However, some drugs that can induce CYP-450 enzymes—such as carbamazepine—may affect paliperidone’s metabolism.7
Paliperidone has an osmotic controlled-release oral delivery system (OROS®) for steady medication delivery across 24 hours8 (Table 2).1-3 The tablet consists of an osmotically active tri-layer core surrounded by a semipermeable membrane. When the tablet is swallowed, the membrane controls the rate of water reaching the tablet core, which determines the rate of drug delivery.6 The result is less variation between peak and trough drug concentrations, compared with immediate-release formulations.
Table 1
How paliperidone ER compares with risperidone
| Characteristic | Paliperidone ER | Risperidone |
|---|---|---|
| Formulation | OROS extended-release | Immediate release |
| Active moiety | 9-OH risperidone | Risperidone plus 9-OH risperidone |
| Metabolism | Primarily renal | Primarily hepatic |
| Drug interactions | Minimal | Primarily through cytochrome P-450 enzyme 2D6 |
| Dosing | Start at target dose | Titrate to target dose |
| OROS: osmotic controlled-release oral delivery system | ||
Paliperidone ER’s clinical characteristics
| Second-generation antipsychotic approved for schizophrenia |
| 9-OH active metabolite of risperidone |
| Osmotic controlled-release system provides steady-state drug delivery over 24 hours |
| Terminal half-life (time for 50% of drug to be eliminated from the body) ~23 hours |
| Available in 3-mg, 6-mg, and 9-mg tablets; recommended starting dose is 6 mg/d, and labeled dose range is 3 to 12 mg/d |
| Excreted primarily by the kidney; maximum recommended dose for patients with oderate to severe renal impairment is 3 mg/d |
| Source: References 1-3 |
Clinical use
Paliperidone ER offers potential therapeutic benefits in treating schizophrenia patients, although not without the risk of adverse events such as extrapyramidal symptoms (EPS) (Table 3).1-3
Patient selection. Because of its slow-release formulation and relatively stable plasma concentrations, paliperidone ER might be useful for patients who are highly sensitive to antipsychotics’ side effects. In particular, paliperidone ER might be ideal for patients who respond to but may not tolerate risperidone.
Paliperidone ER appears to be safe in patients with liver disease. Its primary renal excretion should minimize the risk of hepatic-related drug interactions in patients taking multiple medications.
Dosage and titration. For treating schizophrenia, the suggested starting dose of paliperidone ER is 6 mg/d taken in the morning. In the 3 pivotal trials, 6 mg was the lowest dose to show broad efficacy with minimal adverse events.9
For many patients, the 6-mg starting dose will be the therapeutic dose. When needed, the dose may be increased in 3-mg increments every 1 to 2 weeks to a maximum 12 mg/d (a 15-mg dose was used in clinical trials, but the adverse effects out-weighed the benefits). Lower maximum doses are recommended for patients with renal impairment:
- 6 mg/d for those with creatinine clearance ≥50 to
- 3 mg/d for those with creatinine clearance 10 to 10
Safety and tolerability. Pooled data from the 3 trials indicate that adverse events (AEs) occurred during treatment in 66% to 77% of patients receiving paliperidone ER vs 66% in placebo groups. The most common AEs were headache (11% to 18%), insomnia (4% to 12%), and anxiety (6% to 9%).9
EPS. Risk of EPS-related AEs (such as akathisia and parkinsonian symptoms) with 3-mg and 6-mg paliperidone ER doses (13% and 10%, respectively) was similar to placebo (11%) but increased with the 9-mg, 12-mg, and 15-mg doses (25%, 26%, and 24%, respectively). Should EPS occur, reduce the paliperidone ER dose or consider adding antiparkinsonian medications.
Lab values. No clinically relevant changes were noted in blood glucose, insulin, or lipids.12 Similar to risperidone, paliperidone ER elevated prolactin levels.
Weight gain with paliperidone ER is dose-dependent; in the clinical trials, mean body weight change for all doses was ≤1.9 kg, which is similar to the weight gain seen with risperidone and in the moderate range compared with other SGAs. When using paliperidone ER, follow the American Diabetes Association/American Psychiatric Association guidelines13 for monitoring weight gain and metabolic parameters with antipsychotics. Also monitor patients for clinical symptoms of hyperprolactinemia, and—if intolerable—adjust the dose or switch to another SGA.
Tachycardia. Advise patients that they may experience a rapid heart rate while taking paliperidone ER. In clinical trials, tachycardia occurred in ≤14% of patients—twice the rate with placebo—but did not contribute to more serious cardiac rhythm disturbances or to discontinuation. Incidence of prolonged corrected QT interval (QTc) was 3% to 5% in the paliperidone ER group vs 3% in the placebo group.
Patient education. Because of paliperidone ER’s pharmacokinetic properties, counsel patients to:
- take 1 tablet each day in the morning
- not chew, split, or crush the tablets but swallow whole to preserve the controlled-release delivery.
Table 3
Paliperidone ER’s potential benefits and risks in clinical practice
| Potential benefits | Details |
|---|---|
| Efficacy | Data support acute (6 weeks) and chronic (up to 24 weeks) improvement in schizophrenia symptoms, patient function, and quality of life |
| Pharmacokinetics | Primarily renal excretion decreases risk of hepatic drug-drug or drug-disease interactions |
| Long-acting formulation | Once-daily dosing simplifies treatment and may improve adherence |
| EPS | Risk similar to placebo at 3-mg and 6-mg doses, but increased at higher doses |
| Weight gain | Similar to risperidone |
| Hyperprolactinemia | Similar to risperidone |
| Tachycardia | Occurred in up to 14% of patients in clinical trials (twice the rate of placebo [7%]) |
| QTc prolongation | Increase up to 12 msec on average, with no patients exceeding 500 msec and no clinically adverse events during trials; use paliperidone with caution in patients with arrhythmias or cardiovascular disease or who are taking other medication that can prolong the QT interval |
| EPS: extrapyramidal symptoms | |
| Source: References 1-3 | |
Efficacy trials in schizophrenia
Three 6-week trials1-3 examined paliperidone ER’s efficacy in a total of 1,692 patients with chronic schizophrenia who were hospitalized ≥14 days with acute exacerbations. The trials were double-blind, randomized, fixed-dose, parallel-group, and placebo- and active-controlled (compared with olanzapine, 10 mg/d). Patients showed no significant differences in demographic or baseline characteristics or in the use of rescue medications.
The primary outcome measure was mean change in Positive and Negative Syndrome Scale (PANSS) total score, which quantifies positive, negative, and global psychopathologic symptom severity. Secondary outcome measures included:
- PANSS Marder factor scores14 (derived from PANSS items that reflect positive and negative symptoms, anxiety and depression, hostility, and thought disorganization).
- Clinical Global Impressions-Severity (CGI-S) score, which measures overall illness severity.15
- Personal and Social Performance (PSP) scores, which rate socially useful activities, relationships, self-care, and disturbing and aggressive behaviors; improvement by 1 category (10 points) reflects a clinically meaningful change.16,17
A total of 43% of patients completed the study—34% taking placebo; 46% taking paliperidone ER, 6 mg; 48% taking paliperidone ER, 12 mg; and 45% taking olanzapine. Demographic and baseline characteristics of the 432 patients who received ≥1 dose were similar across all groups. Approximately 75% of patients in each group used rescue medications—primarily lorazepam—for agitation, restlessness, or insomnia for a mean of 8 days.
Patients taking either paliperidone ER dose showed statistically significant greater improvement in PANSS total score compared with those taking placebo (6 mg, P = 0.006; 12 mg, P
Clinical response rates were similar with the 6-mg and 12-mg paliperidone ER doses—50% and 51%, respectively—and greater than with placebo (34%). The higher response rates with paliperidone ER were statistically significant compared with placebo (6 mg, P
Discontinuation rates for lack of efficacy were lower with paliperidone ER (6 mg, 23%; 12 mg, 14%) than with placebo (35%). A substantially lower percentage of patients taking this agent remained classified as “marked/severe/extremely severe” on the CGI-S score from baseline to endpoint, compared with the placebo group;
- 6 mg paliperidone ER, 58% to 26%
- 12 mg paliperidone ER, 64% to 21%
- placebo, 60% to 45%.
The second study2 included U.S. and international sites and compared 3 fixed doses of paliperidone ER (6-, 9-, and 12-mg) with placebo. Among the 630 patients enrolled, 66% completed the study. Patients were randomly assigned to 6 mg, 9 mg, or 12 mg of paliperidone ER; 10 mg of olanzapine; or placebo. The number of patients who dropped out because of adverse events was comparable across the groups.
Patient groups assigned to paliperidone ER showed significant improvement when compared with placebo (P 30% reduction in PANSS total score from baseline to endpoint included:
- 6 mg paliperidone ER, 56%
- 9 mg paliperidone ER, 51%
- 12 mg paliperidone ER, 61%
- placebo, 30%.
- 6 mg paliperidone ER, 63% at baseline to 22% at endpoint
- 9 mg paliperidone ER, 58% to 23%
- 12 mg paliperidone ER, 64% to 16%
- placebo, 60% to 51%.
The third study3 was a multicenter international trial that compared 3 fixed doses of paliperidone ER (3, 9, and 15 mg) with placebo. Among the 618 randomized patients, 365 (59%) completed the study: 70 of 127 (55%) on 3-mg paliperidone ER, 78 of 125 (62%) on 9-mg paliperidone ER, 82 of 115 (71%) on 15-mg paliperidone ER, and 47 of 123 (38%) on placebo.
- 3 mg paliperidone ER, 40%
- 9 mg paliperidone ER, 46%
- 15 mg paliperidone ER, 53%
- placebo, 18% (P ≤0.005).
- 3 mg paliperidone ER, 54% to 32%
- 9 mg paliperidone ER, 52% to 23%
- 15 mg paliperidone ER, 57% to 17%
- placebo, 56% to 50%.
Additional trial evidence
Schizophrenia subpopulations. Post hoc analyses of data reported from the 3 pivotal trials suggest that paliperidone ER may be useful for specific groups of schizophrenia patients, including those who are recently diagnosed, age >65, or severely ill or have predominant negative symptoms or sleep problems (Table 4).18-23
Efficacy in delaying recurrence. Paliperidone ER’s efficacy in delaying symptom recurrence was examined in a randomized, double-blind, placebo-controlled study of 207 patients who had been stabilized on open-label, flexible-dosed paliperidone ER.24 Time to first recurrence of schizophrenia symptoms was the primary efficacy measure. Starting dose was 9 mg/d (flexible dose range 3 to 15 mg/d).
The study was halted at a planned interim analysis because time-to-recurrence was significantly longer for patients receiving paliperidone ER compared with placebo (P
Final analysis of the 179 patients who completed the study confirmed the interim findings. Ongoing treatment maintained improvement in patients’ acute symptoms, functioning, and quality-of-life measures.
Table 4
Studies of paliperidone ER in schizophrenia subpopulations
| Patient population | Study design | Findings |
|---|---|---|
| Recently diagnosed | 413 patients diagnosed within 5 years of study entry compared with 893 patients who had been ill ≥5 years*18,19 | Tolerability was similar, but recently diagnosed patients were more likely to experience movement disorders and somnolence |
| Age ≥65 years | 114 schizophrenia patients age ≥65 given paliperidone ER, 3 to 12 mg/d, or placebo in 6-week, double-blind, randomized, placebo-controlled trial20 | Rates of cardiovascular, cerebrovascular, neuromotor, and metabolic changes similar to placebo, except for tachycardia (16% with paliperidone vs 0% with placebo) |
| Severely ill | 217 patients with marked to severe symptoms (baseline total PANSS score ≥105)*21 | Patients treated with paliperidone showed significantly greater improvement vs placebo in mean total PANSS score (–26.7 vs –5.7) and other measures |
| Substantial negative symptoms | 299 patients with predominant negative symptoms from 3 acute efficacy trials*22 | Patients treated with paliperidone showed significant improvements vs placebo on primary and secondary measures of negative symptoms |
| Sleep problems | 36 patients age 18 to 45 diagnosed with schizophrenia and schizophrenia-related insomnia*23 | In stable patients, paliperidone improved sleep architecture, continuity, and patient-rated sleep quality without producing or worsening daytime sleepiness |
| * Studies marked with asterisks represent post hoc analyses of data from the 3 clinical trials on which the FDA based its approval of paliperidone ER. | ||
| PANSS: Positive and Negative Syndrome Scale | ||
- Paliperidone extended release. Prescribing information. www.invega.com.
- Johnson & Johnson. U.S. District Court upholds Risperdal® (risperidone) patent (press release). October 16, 2006. www.jnj.com/news/jnj_news/20061016_094453.htm.
- Carbamazepine • Tegretol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Paliperidone ER • Invega
- Risperidone • Risperdal
Dr. Rado and Dr. Dowd receive research support from Neuronetics, sanofi-aventis, Janssen Pharmaceutica, and Solvay.
Dr. Janicak receives research support from Astra-Zeneca, Bristol-Myers Squibb, Janssen Pharmaceutica, Neuronetics, Solvay, and sanofi-aventis. He is a consultant to Astra-Zeneca, Bristol-Myers Squibb, Janssen Pharmaceutica, Neuronetics, and Solvay, and a speaker for Abbott Laboratories, Astra-Zeneca, Bristol-Myers Squibb, Janssen Pharmaceutica, and Pfizer.
In the 9 months since paliperidone extended-release was FDA-approved for schizophrenia, the 3 acute pivotal trials supporting its approval have been published.1-3 They join a handful of post hoc analyses of this second-generation antipsychotic (SGA) in schizophrenia subgroups, including patients over age 65, recently diagnosed patients, and those with predominant negative symptoms.
This article discusses the evidence and paliperidone ER’s probable clinical benefits and adverse effects, with focus on its:
- pharmacodynamics and pharmacokinetics
- potential efficacy in schizophrenia and for specific patients and symptoms
- safety and tolerability.
How does paliperidone ER work?
Paliperidone ER was approved for schizophrenia treatment in December 2006 based on three 6-week, randomized, placebo-controlled trials. Paliperidone ER is the active metabolite of risperidone (9-OH risperidone) delivered in a once-daily, time-released formulation (Table 1).
Pharmacodynamics. Similar to risperidone, paliperidone ER has high binding affinity for dopamine (D2) and serotonin (5-HT2A) receptors, with additional affinity for histaminic (H1) and adrenergic receptors (alpha1 and alpha2) but not for muscarinic-cholinergic receptors.
Pharmacokinetics. After oral administration, the medication is widely and rapidly distributed. The drug’s terminal half-life is about 23 hours, and steady-state concentration is reached in 4 to 5 days.4,5
Thus paliperidone ER—when compared with risperidone and other antipsychotics that are metabolized primarily in the liver—is less likely to be involved in hepatic drug-drug or drug-disease interactions. However, some drugs that can induce CYP-450 enzymes—such as carbamazepine—may affect paliperidone’s metabolism.7
Paliperidone has an osmotic controlled-release oral delivery system (OROS®) for steady medication delivery across 24 hours8 (Table 2).1-3 The tablet consists of an osmotically active tri-layer core surrounded by a semipermeable membrane. When the tablet is swallowed, the membrane controls the rate of water reaching the tablet core, which determines the rate of drug delivery.6 The result is less variation between peak and trough drug concentrations, compared with immediate-release formulations.
Table 1
How paliperidone ER compares with risperidone
| Characteristic | Paliperidone ER | Risperidone |
|---|---|---|
| Formulation | OROS extended-release | Immediate release |
| Active moiety | 9-OH risperidone | Risperidone plus 9-OH risperidone |
| Metabolism | Primarily renal | Primarily hepatic |
| Drug interactions | Minimal | Primarily through cytochrome P-450 enzyme 2D6 |
| Dosing | Start at target dose | Titrate to target dose |
| OROS: osmotic controlled-release oral delivery system | ||
Paliperidone ER’s clinical characteristics
| Second-generation antipsychotic approved for schizophrenia |
| 9-OH active metabolite of risperidone |
| Osmotic controlled-release system provides steady-state drug delivery over 24 hours |
| Terminal half-life (time for 50% of drug to be eliminated from the body) ~23 hours |
| Available in 3-mg, 6-mg, and 9-mg tablets; recommended starting dose is 6 mg/d, and labeled dose range is 3 to 12 mg/d |
| Excreted primarily by the kidney; maximum recommended dose for patients with oderate to severe renal impairment is 3 mg/d |
| Source: References 1-3 |
Clinical use
Paliperidone ER offers potential therapeutic benefits in treating schizophrenia patients, although not without the risk of adverse events such as extrapyramidal symptoms (EPS) (Table 3).1-3
Patient selection. Because of its slow-release formulation and relatively stable plasma concentrations, paliperidone ER might be useful for patients who are highly sensitive to antipsychotics’ side effects. In particular, paliperidone ER might be ideal for patients who respond to but may not tolerate risperidone.
Paliperidone ER appears to be safe in patients with liver disease. Its primary renal excretion should minimize the risk of hepatic-related drug interactions in patients taking multiple medications.
Dosage and titration. For treating schizophrenia, the suggested starting dose of paliperidone ER is 6 mg/d taken in the morning. In the 3 pivotal trials, 6 mg was the lowest dose to show broad efficacy with minimal adverse events.9
For many patients, the 6-mg starting dose will be the therapeutic dose. When needed, the dose may be increased in 3-mg increments every 1 to 2 weeks to a maximum 12 mg/d (a 15-mg dose was used in clinical trials, but the adverse effects out-weighed the benefits). Lower maximum doses are recommended for patients with renal impairment:
- 6 mg/d for those with creatinine clearance ≥50 to
- 3 mg/d for those with creatinine clearance 10 to 10
Safety and tolerability. Pooled data from the 3 trials indicate that adverse events (AEs) occurred during treatment in 66% to 77% of patients receiving paliperidone ER vs 66% in placebo groups. The most common AEs were headache (11% to 18%), insomnia (4% to 12%), and anxiety (6% to 9%).9
EPS. Risk of EPS-related AEs (such as akathisia and parkinsonian symptoms) with 3-mg and 6-mg paliperidone ER doses (13% and 10%, respectively) was similar to placebo (11%) but increased with the 9-mg, 12-mg, and 15-mg doses (25%, 26%, and 24%, respectively). Should EPS occur, reduce the paliperidone ER dose or consider adding antiparkinsonian medications.
Lab values. No clinically relevant changes were noted in blood glucose, insulin, or lipids.12 Similar to risperidone, paliperidone ER elevated prolactin levels.
Weight gain with paliperidone ER is dose-dependent; in the clinical trials, mean body weight change for all doses was ≤1.9 kg, which is similar to the weight gain seen with risperidone and in the moderate range compared with other SGAs. When using paliperidone ER, follow the American Diabetes Association/American Psychiatric Association guidelines13 for monitoring weight gain and metabolic parameters with antipsychotics. Also monitor patients for clinical symptoms of hyperprolactinemia, and—if intolerable—adjust the dose or switch to another SGA.
Tachycardia. Advise patients that they may experience a rapid heart rate while taking paliperidone ER. In clinical trials, tachycardia occurred in ≤14% of patients—twice the rate with placebo—but did not contribute to more serious cardiac rhythm disturbances or to discontinuation. Incidence of prolonged corrected QT interval (QTc) was 3% to 5% in the paliperidone ER group vs 3% in the placebo group.
Patient education. Because of paliperidone ER’s pharmacokinetic properties, counsel patients to:
- take 1 tablet each day in the morning
- not chew, split, or crush the tablets but swallow whole to preserve the controlled-release delivery.
Table 3
Paliperidone ER’s potential benefits and risks in clinical practice
| Potential benefits | Details |
|---|---|
| Efficacy | Data support acute (6 weeks) and chronic (up to 24 weeks) improvement in schizophrenia symptoms, patient function, and quality of life |
| Pharmacokinetics | Primarily renal excretion decreases risk of hepatic drug-drug or drug-disease interactions |
| Long-acting formulation | Once-daily dosing simplifies treatment and may improve adherence |
| EPS | Risk similar to placebo at 3-mg and 6-mg doses, but increased at higher doses |
| Weight gain | Similar to risperidone |
| Hyperprolactinemia | Similar to risperidone |
| Tachycardia | Occurred in up to 14% of patients in clinical trials (twice the rate of placebo [7%]) |
| QTc prolongation | Increase up to 12 msec on average, with no patients exceeding 500 msec and no clinically adverse events during trials; use paliperidone with caution in patients with arrhythmias or cardiovascular disease or who are taking other medication that can prolong the QT interval |
| EPS: extrapyramidal symptoms | |
| Source: References 1-3 | |
Efficacy trials in schizophrenia
Three 6-week trials1-3 examined paliperidone ER’s efficacy in a total of 1,692 patients with chronic schizophrenia who were hospitalized ≥14 days with acute exacerbations. The trials were double-blind, randomized, fixed-dose, parallel-group, and placebo- and active-controlled (compared with olanzapine, 10 mg/d). Patients showed no significant differences in demographic or baseline characteristics or in the use of rescue medications.
The primary outcome measure was mean change in Positive and Negative Syndrome Scale (PANSS) total score, which quantifies positive, negative, and global psychopathologic symptom severity. Secondary outcome measures included:
- PANSS Marder factor scores14 (derived from PANSS items that reflect positive and negative symptoms, anxiety and depression, hostility, and thought disorganization).
- Clinical Global Impressions-Severity (CGI-S) score, which measures overall illness severity.15
- Personal and Social Performance (PSP) scores, which rate socially useful activities, relationships, self-care, and disturbing and aggressive behaviors; improvement by 1 category (10 points) reflects a clinically meaningful change.16,17
A total of 43% of patients completed the study—34% taking placebo; 46% taking paliperidone ER, 6 mg; 48% taking paliperidone ER, 12 mg; and 45% taking olanzapine. Demographic and baseline characteristics of the 432 patients who received ≥1 dose were similar across all groups. Approximately 75% of patients in each group used rescue medications—primarily lorazepam—for agitation, restlessness, or insomnia for a mean of 8 days.
Patients taking either paliperidone ER dose showed statistically significant greater improvement in PANSS total score compared with those taking placebo (6 mg, P = 0.006; 12 mg, P
Clinical response rates were similar with the 6-mg and 12-mg paliperidone ER doses—50% and 51%, respectively—and greater than with placebo (34%). The higher response rates with paliperidone ER were statistically significant compared with placebo (6 mg, P
Discontinuation rates for lack of efficacy were lower with paliperidone ER (6 mg, 23%; 12 mg, 14%) than with placebo (35%). A substantially lower percentage of patients taking this agent remained classified as “marked/severe/extremely severe” on the CGI-S score from baseline to endpoint, compared with the placebo group;
- 6 mg paliperidone ER, 58% to 26%
- 12 mg paliperidone ER, 64% to 21%
- placebo, 60% to 45%.
The second study2 included U.S. and international sites and compared 3 fixed doses of paliperidone ER (6-, 9-, and 12-mg) with placebo. Among the 630 patients enrolled, 66% completed the study. Patients were randomly assigned to 6 mg, 9 mg, or 12 mg of paliperidone ER; 10 mg of olanzapine; or placebo. The number of patients who dropped out because of adverse events was comparable across the groups.
Patient groups assigned to paliperidone ER showed significant improvement when compared with placebo (P 30% reduction in PANSS total score from baseline to endpoint included:
- 6 mg paliperidone ER, 56%
- 9 mg paliperidone ER, 51%
- 12 mg paliperidone ER, 61%
- placebo, 30%.
- 6 mg paliperidone ER, 63% at baseline to 22% at endpoint
- 9 mg paliperidone ER, 58% to 23%
- 12 mg paliperidone ER, 64% to 16%
- placebo, 60% to 51%.
The third study3 was a multicenter international trial that compared 3 fixed doses of paliperidone ER (3, 9, and 15 mg) with placebo. Among the 618 randomized patients, 365 (59%) completed the study: 70 of 127 (55%) on 3-mg paliperidone ER, 78 of 125 (62%) on 9-mg paliperidone ER, 82 of 115 (71%) on 15-mg paliperidone ER, and 47 of 123 (38%) on placebo.
- 3 mg paliperidone ER, 40%
- 9 mg paliperidone ER, 46%
- 15 mg paliperidone ER, 53%
- placebo, 18% (P ≤0.005).
- 3 mg paliperidone ER, 54% to 32%
- 9 mg paliperidone ER, 52% to 23%
- 15 mg paliperidone ER, 57% to 17%
- placebo, 56% to 50%.
Additional trial evidence
Schizophrenia subpopulations. Post hoc analyses of data reported from the 3 pivotal trials suggest that paliperidone ER may be useful for specific groups of schizophrenia patients, including those who are recently diagnosed, age >65, or severely ill or have predominant negative symptoms or sleep problems (Table 4).18-23
Efficacy in delaying recurrence. Paliperidone ER’s efficacy in delaying symptom recurrence was examined in a randomized, double-blind, placebo-controlled study of 207 patients who had been stabilized on open-label, flexible-dosed paliperidone ER.24 Time to first recurrence of schizophrenia symptoms was the primary efficacy measure. Starting dose was 9 mg/d (flexible dose range 3 to 15 mg/d).
The study was halted at a planned interim analysis because time-to-recurrence was significantly longer for patients receiving paliperidone ER compared with placebo (P
Final analysis of the 179 patients who completed the study confirmed the interim findings. Ongoing treatment maintained improvement in patients’ acute symptoms, functioning, and quality-of-life measures.
Table 4
Studies of paliperidone ER in schizophrenia subpopulations
| Patient population | Study design | Findings |
|---|---|---|
| Recently diagnosed | 413 patients diagnosed within 5 years of study entry compared with 893 patients who had been ill ≥5 years*18,19 | Tolerability was similar, but recently diagnosed patients were more likely to experience movement disorders and somnolence |
| Age ≥65 years | 114 schizophrenia patients age ≥65 given paliperidone ER, 3 to 12 mg/d, or placebo in 6-week, double-blind, randomized, placebo-controlled trial20 | Rates of cardiovascular, cerebrovascular, neuromotor, and metabolic changes similar to placebo, except for tachycardia (16% with paliperidone vs 0% with placebo) |
| Severely ill | 217 patients with marked to severe symptoms (baseline total PANSS score ≥105)*21 | Patients treated with paliperidone showed significantly greater improvement vs placebo in mean total PANSS score (–26.7 vs –5.7) and other measures |
| Substantial negative symptoms | 299 patients with predominant negative symptoms from 3 acute efficacy trials*22 | Patients treated with paliperidone showed significant improvements vs placebo on primary and secondary measures of negative symptoms |
| Sleep problems | 36 patients age 18 to 45 diagnosed with schizophrenia and schizophrenia-related insomnia*23 | In stable patients, paliperidone improved sleep architecture, continuity, and patient-rated sleep quality without producing or worsening daytime sleepiness |
| * Studies marked with asterisks represent post hoc analyses of data from the 3 clinical trials on which the FDA based its approval of paliperidone ER. | ||
| PANSS: Positive and Negative Syndrome Scale | ||
- Paliperidone extended release. Prescribing information. www.invega.com.
- Johnson & Johnson. U.S. District Court upholds Risperdal® (risperidone) patent (press release). October 16, 2006. www.jnj.com/news/jnj_news/20061016_094453.htm.
- Carbamazepine • Tegretol
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Paliperidone ER • Invega
- Risperidone • Risperdal
Dr. Rado and Dr. Dowd receive research support from Neuronetics, sanofi-aventis, Janssen Pharmaceutica, and Solvay.
Dr. Janicak receives research support from Astra-Zeneca, Bristol-Myers Squibb, Janssen Pharmaceutica, Neuronetics, Solvay, and sanofi-aventis. He is a consultant to Astra-Zeneca, Bristol-Myers Squibb, Janssen Pharmaceutica, Neuronetics, and Solvay, and a speaker for Abbott Laboratories, Astra-Zeneca, Bristol-Myers Squibb, Janssen Pharmaceutica, and Pfizer.
1. Marder S, Kramer M, Ford L, et al. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled study. Biol Psychiatry 2007; Jun 27; Epub ahead of print.
2. Kane J, Canas F, Kramer M, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr Res 2007;90(1-3):147-61.
3. Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and early response of paliperidone extended-release tablets (paliperidone ER): results of a 6-week, randomized, placebo-controlled study. Schizophr Res 2007;93(1-3):117-30.
4. Rossenu SAC, Rusch S, Janssens L, et al. Extended release formulation of paliperidone shows dose proportional pharmacokinetics. Presented at: Annual Meeting of the American Association of Pharmaceutical Scientists; October 29, 2006; San Antonio, TX.
5. Vermeir M, Boom S, Naessens I, et al. Absorption, metabolism, and excretion of a single oral dose of 14C-paliperidone 1 mg in healthy subjects. Eur Neuropsychopharmacol 2005;15(suppl):S648-9.
6. Conley R, Gupta SK, Sathyan G. Clinical spectrum of the osmotic-controlled release oral delivery system (OROS), an advanced oral delivery form. Curr Med Res Opin 2006;22(10):1879-92.
7. Spina E, Avenoso A, Facciola G, et al. Plasma concentrations of risperidone and 9-hydroxyrisperidone: effect of comedication with carbamazepine or valproate. Ther Drug Monit 2000;22(4):481-5.
8. Paliperidone extended release. Prescribing information. Available at: http://www.invega.com. Accessed August 8, 2007.
9. Meltzer H, Kramer M, Gassmann-Mayer C, et al. Efficacy and tolerability of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 6-week placebo controlled studies. Int J Neuropsychopharmacol 2006;9(suppl 1):S225.-
10. Thyssen A, Cleton A, Osselae NV, et al. Effects of renal impairment on the pharmacokinetic profile of paliperidone extended-release tablets. Clin Pharmacol Ther 2007. In press.
11. Thyssen A, Crauwels H, Cleton A, et al. Effects of hepatic impairment on the pharmacokinetics of paliperidone immediate-release. Presented at: 46th Annual Meeting of the New Clinical Drug Evaluation Unit (NCDEU); June 12-15, 2006; Boca Raton, FL.
12. Meyer J, Kramer M, Lane R, et al. Metabolic outcomes in patients with schizophrenia treated with oral paliperidone extended release tablets: pooled analysis of three 6 week placebo-controlled studies. Int J Neuropsychopharmacol 2006;9(suppl 1):S282.-
13. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. J Clin Psychiatry 2004;65:267-72.
14. Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry 1997;58:538-46.
15. Guy W. Clinical Global Impressions Scale. Early clinical drug evaluation unit (ECDEU) assessment manual for psychopharmacology. Rockville, MD: National Institute of Mental Health, Department of Health, Education, and Welfare; 1976:218-22. ADM publication 76-338.
16. Morosini PL, Magliano L, Brambilla L, et al. Development, reliability and acceptability of a new version of the DSMIV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand 2000;101:323-9.
17. Patrick D, Adriaenssen I, Morosini P, Rothman M. Reliability, validity and sensitivity to change of the Personal and Social Performance scale in patients with acute schizophrenia. Int J Neuropsychopharmacol 2006;9(suppl 1):S287-8.
18. Kostic D, Bossie C, Turkoz I, et al. Paliperidone extended-release tablets in patients recently diagnosed with schizophrenia. Int J Neuropsychopharmacol 2006;9(suppl 1):S161.-
19. Kostic D, Bossie C, Turkoz I, et al. Paliperidone extended-release tablets in patients recently diagnosed with schizophrenia. Presented at: Congress of the Collegium Internationale Neruo-Psychopharmacologicum (CINP); July 9-13, 2006; Chicago, IL.
20. Tzimos A, Kramer M, Ford L, et al. A 6-week placebo-controlled study of the safety and tolerability of flexible doses of oral paliperidone extended release tablets in the treatment of schizophrenia in elderly patients. Int J Neuropsychopharmacol 2006;9(suppl 1):S155.-
21. Canuso C, Youssef E, Dirks B, et al. Paliperidone extended-release in severely-ill patients with schizophrenia. Presented at: 58th Annual Institute on Psychiatric Services; October 5-8, 2006; New York, NY.
22. Dirks B, Eerdekens M, Turkoz I, et al. Efficacy of paliperidone extended-release tablets in patients with schizophrenia and predominant negative symptoms. Int J Neuropsychopharmacol 2006;9(suppl 1):S162.-
23. Luthringer R, Staner L, Noel N, et al. Sleep assessments in patients with schizophrenia following treatment with paliperidone extended-release tablets. Eur Neuropsychopharmacol 2006;16(suppl 4):S224.-
24. Kramer M, Simpson G, Maciulis V, et al. Paliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomized double-blind, placebo-controlled study [published correction appears in J Clin Psychopharmacol. 2007;27(3):258]. J Clin Psychopharmacol 2007;27(1):6-14.
1. Marder S, Kramer M, Ford L, et al. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled study. Biol Psychiatry 2007; Jun 27; Epub ahead of print.
2. Kane J, Canas F, Kramer M, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr Res 2007;90(1-3):147-61.
3. Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and early response of paliperidone extended-release tablets (paliperidone ER): results of a 6-week, randomized, placebo-controlled study. Schizophr Res 2007;93(1-3):117-30.
4. Rossenu SAC, Rusch S, Janssens L, et al. Extended release formulation of paliperidone shows dose proportional pharmacokinetics. Presented at: Annual Meeting of the American Association of Pharmaceutical Scientists; October 29, 2006; San Antonio, TX.
5. Vermeir M, Boom S, Naessens I, et al. Absorption, metabolism, and excretion of a single oral dose of 14C-paliperidone 1 mg in healthy subjects. Eur Neuropsychopharmacol 2005;15(suppl):S648-9.
6. Conley R, Gupta SK, Sathyan G. Clinical spectrum of the osmotic-controlled release oral delivery system (OROS), an advanced oral delivery form. Curr Med Res Opin 2006;22(10):1879-92.
7. Spina E, Avenoso A, Facciola G, et al. Plasma concentrations of risperidone and 9-hydroxyrisperidone: effect of comedication with carbamazepine or valproate. Ther Drug Monit 2000;22(4):481-5.
8. Paliperidone extended release. Prescribing information. Available at: http://www.invega.com. Accessed August 8, 2007.
9. Meltzer H, Kramer M, Gassmann-Mayer C, et al. Efficacy and tolerability of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 6-week placebo controlled studies. Int J Neuropsychopharmacol 2006;9(suppl 1):S225.-
10. Thyssen A, Cleton A, Osselae NV, et al. Effects of renal impairment on the pharmacokinetic profile of paliperidone extended-release tablets. Clin Pharmacol Ther 2007. In press.
11. Thyssen A, Crauwels H, Cleton A, et al. Effects of hepatic impairment on the pharmacokinetics of paliperidone immediate-release. Presented at: 46th Annual Meeting of the New Clinical Drug Evaluation Unit (NCDEU); June 12-15, 2006; Boca Raton, FL.
12. Meyer J, Kramer M, Lane R, et al. Metabolic outcomes in patients with schizophrenia treated with oral paliperidone extended release tablets: pooled analysis of three 6 week placebo-controlled studies. Int J Neuropsychopharmacol 2006;9(suppl 1):S282.-
13. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. J Clin Psychiatry 2004;65:267-72.
14. Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry 1997;58:538-46.
15. Guy W. Clinical Global Impressions Scale. Early clinical drug evaluation unit (ECDEU) assessment manual for psychopharmacology. Rockville, MD: National Institute of Mental Health, Department of Health, Education, and Welfare; 1976:218-22. ADM publication 76-338.
16. Morosini PL, Magliano L, Brambilla L, et al. Development, reliability and acceptability of a new version of the DSMIV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand 2000;101:323-9.
17. Patrick D, Adriaenssen I, Morosini P, Rothman M. Reliability, validity and sensitivity to change of the Personal and Social Performance scale in patients with acute schizophrenia. Int J Neuropsychopharmacol 2006;9(suppl 1):S287-8.
18. Kostic D, Bossie C, Turkoz I, et al. Paliperidone extended-release tablets in patients recently diagnosed with schizophrenia. Int J Neuropsychopharmacol 2006;9(suppl 1):S161.-
19. Kostic D, Bossie C, Turkoz I, et al. Paliperidone extended-release tablets in patients recently diagnosed with schizophrenia. Presented at: Congress of the Collegium Internationale Neruo-Psychopharmacologicum (CINP); July 9-13, 2006; Chicago, IL.
20. Tzimos A, Kramer M, Ford L, et al. A 6-week placebo-controlled study of the safety and tolerability of flexible doses of oral paliperidone extended release tablets in the treatment of schizophrenia in elderly patients. Int J Neuropsychopharmacol 2006;9(suppl 1):S155.-
21. Canuso C, Youssef E, Dirks B, et al. Paliperidone extended-release in severely-ill patients with schizophrenia. Presented at: 58th Annual Institute on Psychiatric Services; October 5-8, 2006; New York, NY.
22. Dirks B, Eerdekens M, Turkoz I, et al. Efficacy of paliperidone extended-release tablets in patients with schizophrenia and predominant negative symptoms. Int J Neuropsychopharmacol 2006;9(suppl 1):S162.-
23. Luthringer R, Staner L, Noel N, et al. Sleep assessments in patients with schizophrenia following treatment with paliperidone extended-release tablets. Eur Neuropsychopharmacol 2006;16(suppl 4):S224.-
24. Kramer M, Simpson G, Maciulis V, et al. Paliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomized double-blind, placebo-controlled study [published correction appears in J Clin Psychopharmacol. 2007;27(3):258]. J Clin Psychopharmacol 2007;27(1):6-14.
Exercise for depression: It really does help—here’s how to get patients moving
Ms. H, age 26, is being evaluated for moderate to severe depressive symptoms, including oversleeping and overeating. She has had difficulty adhering to medication in the past and is ambivalent about taking antidepressants. She takes a passive approach to managing her depression, preferring to “wait for it to pass.”
Her psychiatrist prescribes fluoxetine, 20 mg in the morning, and recommends that Ms. H change her coping strategies from napping and snacking to increased physical activity. She encourages Ms. H to think about what activities interest her and to set exercise goals.
Ms. H says she has considered buying exercise equipment (an elliptical machine) and increasing her walking outside. She sets a goal to walk 20 minutes most days and to spend 10 to 15 minutes using the elliptical machine while watching television.
Physical activity’s mental health benefits are less well-known than its well-documented medical benefits—reduced risk of heart disease, hypertension, and diabetes; weight control; bone mass preservation; better sleep, and improved cholesterol levels.1 By encouraging exercise, you can improve patients’ mood, well-being, and quality of life, independent of medication and psychotherapy. In this article, we:
- explore the relationship between physical activity and mental health
- compare exercise with medication and psychotherapies for easing depression
- discuss counseling strategies shown to be effective in helping sedentary patients become more physically active.
Table 1
Why physical activity may improve mental health
|
| Psychological theories Physical activity:
|
| Source: References 10 and 11 |
Mental benefits of exercise
Adults who exercise regularly report lower levels of depressive and anxiety disorders than the overall U.S. population.2 As a therapeutic intervention, exercise has been studied primarily in depressed individuals, although some data also support its efficacy in:
- reducing anxiety symptoms in panic disorder3
- reducing disruptive behavior in developmentally disabled patients4
- alleviating chronic fatigue symptoms5
- improving body esteem in patients with body image disturbance6
- increasing function in chronic pain7
- reducing urges to smoke and improving smoking abstinence among nicotine-dependent individuals.8
Why exercise helps. Mechanisms that would explain exercise’s positive effect on mood are not well understood.9 Physiologic and psychological hypotheses have been suggested (Table 1),10,11 and researchers are attempting to elucidate them by using animal models.13
Case report: Feeling more energetic
At follow-up 6 weeks later, Ms. H. reported a substantial reduction in depressive symptoms. She noted increased energy, improved sleep, decreased overeating, higher self-esteem, and greater confidence in her ability to manage her depression.
Exercising also helped structure her day. She noticed that on days she did not exercise she was more likely to take a nap, miss her medication, or feel pessimistic about her depression.
Exercise as an antidepressant
Exercise vs psychotherapy. Exercise has been shown to be more effective at reducing depressive symptoms than no treatment, occupational therapy, cognitive therapy, health seminars, routine care, or meditation. Interventions used in these meta-analyses ranged from nonaerobic exercise training several times a week to 1 hour of supervised running 4 times a week.12 Literature reviews also have concluded that exercise training compares favorably with individual or group psychotherapy and with cognitive therapy for treating depression.7
Exercise vs medication. Exercise training has also been compared with drug therapy in treating depression.
In a randomized, controlled trial, 156 men and women over age 50 with major depression received exercise training, sertraline, or exercise plus sertraline. Subjects in the exercise groups completed 40 minutes of aerobic exercise (biking or brisk walking/ jogging) 3 times a week. Subjects treated with sertraline received 50 to 200 mg/d, depending on response.
After 16 weeks, all three groups were significantly improved, with no clinically or statistically significant differences in depressive symptoms, as measured with the Hamilton Rating Scale for Depression (HRSD) and Beck Depression Inventory.13
In a follow-up study 6 months later,14 the exercise group had significantly lower rates of relapse (defined as HRSD scores >15 and meeting diagnostic criteria) than did the medication group. Combining exercise with medication did not provide an added benefit in preventing relapse.
Exercise as monotherapy. Some studies have investigated using exercise instead of medication and psychotherapy. Many of these trials, however, were limited by methodologic weaknesses such as nonrandomized samples or lack of appropriate control groups.12
To address the need for higher-quality evidence, the Depression Outcomes Study of Exercise (DOSE) is investigating the dose-continued from page 12 response effects of exercise as monotherapy for major depressive disorder (MDD).5 The 12-week trial included 80 men and women ages 20 to 45 diagnosed with mild-to-moderate MDD using the Structured Clinical Interview for Depression. They were randomly assigned to one of five supervised exercise regimens:
- 7.0 kcal/kg/week in 3 days/week
- 7.0 kcal/kg/week in 5 days/week
- 17.5 kcal/kg/week in 3 days/week
- 17.5 kcal/kg/week in 5 days/week
- 3 days/week of stretching and flexibility exercises for 15 to 20 min/session.
Table 2
How much physical activity is recommended for adults?
For physical and mental health
|
For weight loss and management
|
Depressive symptoms were measured with the HRSD and Inventory of Depressive Symptoms (clinician and self-report). Other outcome measures included cardiorespiratory fitness, self-efficacy, and quality of life. Results are being prepared for publication and will likely help clarify the role of physical activity in treating patients with MDD.
Table 3
Why patients don’t exercise: Common barriers they perceive
Practical limitations
| |
Medical limitations
| |
Psychological limitations
| |
| Source: References 15 and 16 | |
How much exercise is therapeutic?
In the absence of physical activity guidelines specific to mental health, we suggest using standard public health guidelines (Table 2):
- 30 minutes or more of moderate-intensity physical activity (brisk walking, swimming, dancing, cycling) most days of the week (recommended by the Centers for Disease Control and Prevention and American College of Sports Medicine)1
- 60 minutes of moderate-intensity physical activity daily for weight loss and maintenance (recommended by the Institute of Medicine).16
A recent study investigated the effects of exercise duration and intensity on weight loss in overweight, sedentary women. These researchers recommended setting the initial intervention target at 150 minutes or more of moderate-intensity exercise per week and progressing to 60 minutes per day as appropriate.16
Increasing the number of steps taken per day, as measured by a pedometer, also can be beneficial. Encourage patients to obtain a baseline measure of daily steps and to gradually increase toward a moderate goal of 10,000 steps per day.17
Case report: Accentuating the positive
On follow-up, Ms. H was quick to report the many barriers to exercise she had experienced and the times she did not meet her goal. Rather than dwell on shortcomings, the psychiatrist redirected her to examine the many positive actions she had taken to manage her depression.
As she considered how to overcome barriers to exercise, she reported increased confidence that she could stick with her medication and exercise regimen. She continues to exercise regularly and adheres to her fluoxetine. Her depressive symptoms remain well-controlled.
Overcoming barriers to exercise
Patient obstacles. Many patients acknowledge that regular exercise makes them feel physically and emotionally healthier but have difficulty exercising long term. Less than one-half of those who start an exercise program stick with it beyond 6 months.18 Drop-out reasons include injuries, lack of time, and low motivation (Table 3).19,20
Depressive symptoms—fatigue, loss of interest, low self-esteem, feelings of helplessness, and psychomotor retardation—make exercise adherence even more difficult.
Physician obstacles. The U.S. Preventive Services Task Force recommends that physicians advise all patients to increase physical activity, but the national rate of physician counseling about exercise is low. In a population-based survey of more than 9,000 patients, 34% said their physicians counseled them about exercise at their most recent visit within the past year.21
Physician-reported barriers to exercise counseling include:
- competing demands for limited clinical time
- perceived ineffectiveness of advice to exercise
- lack of training and knowledge about exercise counseling and prescription.22,23
Patients are more likely to become active and continue exercising when their physicians help them set achievable goals.
Project PACE. Physicians can overcome barriers to counseling patients about exercise. Those who participated in Project PACE (Physician-based Assessment and Counseling for Exercise)24 said they felt more confident that they could counsel patients about physical activity in 1 to 5 minutes.
In a controlled study of 212 sedentary adults, patients who received PACE counseling from their physicians significantly increased their minutes of weekly walking compared with a control group. Also, 52% of patients who received PACE counseling adopted some physical activity, compared with 12% of controls.25
Though modest initial goals are not sufficient for achieving the full benefits of exercise, success with a small goal is a powerful motivator. Rather than giving up, patients feel encouraged and are more likely to set a subsequent, more ambitious goal.
Recommendations. To help patients start exercising, determine how motivated and ready they are. Start by asking them to describe their current activities. Ask if they were ever more active and what they liked about it. Did they experience any benefits? Establish which of increased activity’s benefits—improved sleep, reduced depression, increased energy—would most benefit the patient, based on his or her symptoms.
Discuss barriers to physical activity and encourage problem-solving to overcome them and incorporate physical activity into their lives. Encourage patients to seek support from family, friends, coworkers, and exercise groups.
Help them set realistic, achievable goals. Even a modest 10 minutes of activity has been shown to enhance mood,26 and a 10-minute brisk walk is one-third of the day’s public health guideline. Suggest that patients choose a variety of activities they enjoy.
During follow-up visits, reinforce any progress toward change. When patients’ exercise efforts fall short, explain that the process of becoming more active often includes setbacks. Advise them to seek support and to consider adopting more-achievable goals.
Related resources
- Getting started. Resources on nutrition and physical activity from the National Center for Chronic Disease Prevention and Health Promotion. http://www.cdc.gov/nccdphp/dnpa/physical/starting/index.htm
- Marcus B, Forsyth L. Motivating people to be physically active. Champaign, IL: Human Kinetics, 2002.
Drug brand names
- Fluoxetine • Prozac
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995;273(5):402-7.
2. Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med 2003;36:698-703.
3. Broocks A, Bandelow B, Pekrun G, et al. Comparison of aerobic exercise, clomipramine and placebo in the treatment of panic disorder. Am J Psychiatry 1998;155:603-9.
4. Gabler-Halle D, Halle JW, Chung YB. The effects of aerobic exercise on psychological and behavioral variables of individuals with developmental disabilities. A critical review. Res Dev Disabil 1993;14:359-86.
5. Powell P, Bentall RP, Nye FJ, Edwards RH. Patient education to encourage graded exercise in chronic fatigue syndrome. Br J Psychiatry 2004;184:142-6.
6. Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psychooncology 2003;12(2):118-26.
7. Tkachuk GA, Martin GL. Exercise therapy for patients with psychiatric disorders: research and clinical implications. Prof Psychol Res Pract 1999;30:275-82
8. Ussher MH, Taylor AH, West R, McEwen A. Does exercise aid smoking cessation? A systematic review. Addiction 2000;95(2):199-208.
9. Van Hoomissen JD, Chambliss HO, Holmes PV, Dishman RK. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain Res 2003;974:228-235.
10. Plante TG, Rodin J. Physical fitness and enhanced psychological health. Curr Psychol Res Rev 1990;9:3-24.
11. Weyerer A, Kupfer B. Physical exercise and psychological health. Sports Med 1994;17(2):108-16.
12. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomized controlled trials. Br Med J 2001;322:1-8.
13. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older adults with major depression. Arch Intern Med 1999;159:2349-56.
14. Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000;62:633-8.
15. Dunn AL, Trivedi MH, Kampert JB, et al. The DOSE study: a clinical trial to examine efficacy and dose response of exercise as treatment for depression. Control Clin Trials 2002;23:584-603.
16. Jakicic JM, Marcus BH, Gallagher KI, et al. Effect of exercise duration and intensity on weight loss in overweight, sedentary women. JAMA 2003;290:1323-30.
17. Tudor-Locke C, Bassett DR, Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 2004;34(1):1-8.
18. Dishman RK. Compliance/adherence in health-related exercise. Health Psychol 1982;1:237-67.
19. Sallis JF, Hovell MF. Determinants of exercise behavior. Exerc Sport Sci Rev 1990;18:307-30.
20. Heesch KC, Brown DR, Blanton CJ. Perceived barriers to exercise and stage of exercise adoption in older women of different racial/ethnic groups. Women Health 2000;30(4):61-76.
21. Wee CC, McCarthy EP, Davis RB, Phillips RS. Physician counseling about exercise. JAMA 1999;282(16):1583-8.
22. Kennedy MF, Meeuwisse WH. Exercise counseling by family physicians in Canada. Prev Med 2003 Sep;37(3):226-32.
23. Reed BD, Jensen JD, Gorenflo DW. Physicians and exercise promotion. Am J Prev Med 1991;7:410-15.
24. Long BJ, Calfas KJ, Wooten W, et al. A multisite field test of the acceptability of physical activity counseling in primary care: project PACE. Am J Prev Med 1996;12(2):73-81.
25. Calfas KJ, Long BJ, Sallis JF, et al. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med 1996;25(3):225-33.
26. Hansen CJ, Stevens LC, Coast JR. Exercise duration and mood state: how much is enough to feel better? Health Psychol 2001;20(4):267-75.
Ms. H, age 26, is being evaluated for moderate to severe depressive symptoms, including oversleeping and overeating. She has had difficulty adhering to medication in the past and is ambivalent about taking antidepressants. She takes a passive approach to managing her depression, preferring to “wait for it to pass.”
Her psychiatrist prescribes fluoxetine, 20 mg in the morning, and recommends that Ms. H change her coping strategies from napping and snacking to increased physical activity. She encourages Ms. H to think about what activities interest her and to set exercise goals.
Ms. H says she has considered buying exercise equipment (an elliptical machine) and increasing her walking outside. She sets a goal to walk 20 minutes most days and to spend 10 to 15 minutes using the elliptical machine while watching television.
Physical activity’s mental health benefits are less well-known than its well-documented medical benefits—reduced risk of heart disease, hypertension, and diabetes; weight control; bone mass preservation; better sleep, and improved cholesterol levels.1 By encouraging exercise, you can improve patients’ mood, well-being, and quality of life, independent of medication and psychotherapy. In this article, we:
- explore the relationship between physical activity and mental health
- compare exercise with medication and psychotherapies for easing depression
- discuss counseling strategies shown to be effective in helping sedentary patients become more physically active.
Table 1
Why physical activity may improve mental health
|
| Psychological theories Physical activity:
|
| Source: References 10 and 11 |
Mental benefits of exercise
Adults who exercise regularly report lower levels of depressive and anxiety disorders than the overall U.S. population.2 As a therapeutic intervention, exercise has been studied primarily in depressed individuals, although some data also support its efficacy in:
- reducing anxiety symptoms in panic disorder3
- reducing disruptive behavior in developmentally disabled patients4
- alleviating chronic fatigue symptoms5
- improving body esteem in patients with body image disturbance6
- increasing function in chronic pain7
- reducing urges to smoke and improving smoking abstinence among nicotine-dependent individuals.8
Why exercise helps. Mechanisms that would explain exercise’s positive effect on mood are not well understood.9 Physiologic and psychological hypotheses have been suggested (Table 1),10,11 and researchers are attempting to elucidate them by using animal models.13
Case report: Feeling more energetic
At follow-up 6 weeks later, Ms. H. reported a substantial reduction in depressive symptoms. She noted increased energy, improved sleep, decreased overeating, higher self-esteem, and greater confidence in her ability to manage her depression.
Exercising also helped structure her day. She noticed that on days she did not exercise she was more likely to take a nap, miss her medication, or feel pessimistic about her depression.
Exercise as an antidepressant
Exercise vs psychotherapy. Exercise has been shown to be more effective at reducing depressive symptoms than no treatment, occupational therapy, cognitive therapy, health seminars, routine care, or meditation. Interventions used in these meta-analyses ranged from nonaerobic exercise training several times a week to 1 hour of supervised running 4 times a week.12 Literature reviews also have concluded that exercise training compares favorably with individual or group psychotherapy and with cognitive therapy for treating depression.7
Exercise vs medication. Exercise training has also been compared with drug therapy in treating depression.
In a randomized, controlled trial, 156 men and women over age 50 with major depression received exercise training, sertraline, or exercise plus sertraline. Subjects in the exercise groups completed 40 minutes of aerobic exercise (biking or brisk walking/ jogging) 3 times a week. Subjects treated with sertraline received 50 to 200 mg/d, depending on response.
After 16 weeks, all three groups were significantly improved, with no clinically or statistically significant differences in depressive symptoms, as measured with the Hamilton Rating Scale for Depression (HRSD) and Beck Depression Inventory.13
In a follow-up study 6 months later,14 the exercise group had significantly lower rates of relapse (defined as HRSD scores >15 and meeting diagnostic criteria) than did the medication group. Combining exercise with medication did not provide an added benefit in preventing relapse.
Exercise as monotherapy. Some studies have investigated using exercise instead of medication and psychotherapy. Many of these trials, however, were limited by methodologic weaknesses such as nonrandomized samples or lack of appropriate control groups.12
To address the need for higher-quality evidence, the Depression Outcomes Study of Exercise (DOSE) is investigating the dose-continued from page 12 response effects of exercise as monotherapy for major depressive disorder (MDD).5 The 12-week trial included 80 men and women ages 20 to 45 diagnosed with mild-to-moderate MDD using the Structured Clinical Interview for Depression. They were randomly assigned to one of five supervised exercise regimens:
- 7.0 kcal/kg/week in 3 days/week
- 7.0 kcal/kg/week in 5 days/week
- 17.5 kcal/kg/week in 3 days/week
- 17.5 kcal/kg/week in 5 days/week
- 3 days/week of stretching and flexibility exercises for 15 to 20 min/session.
Table 2
How much physical activity is recommended for adults?
For physical and mental health
|
For weight loss and management
|
Depressive symptoms were measured with the HRSD and Inventory of Depressive Symptoms (clinician and self-report). Other outcome measures included cardiorespiratory fitness, self-efficacy, and quality of life. Results are being prepared for publication and will likely help clarify the role of physical activity in treating patients with MDD.
Table 3
Why patients don’t exercise: Common barriers they perceive
Practical limitations
| |
Medical limitations
| |
Psychological limitations
| |
| Source: References 15 and 16 | |
How much exercise is therapeutic?
In the absence of physical activity guidelines specific to mental health, we suggest using standard public health guidelines (Table 2):
- 30 minutes or more of moderate-intensity physical activity (brisk walking, swimming, dancing, cycling) most days of the week (recommended by the Centers for Disease Control and Prevention and American College of Sports Medicine)1
- 60 minutes of moderate-intensity physical activity daily for weight loss and maintenance (recommended by the Institute of Medicine).16
A recent study investigated the effects of exercise duration and intensity on weight loss in overweight, sedentary women. These researchers recommended setting the initial intervention target at 150 minutes or more of moderate-intensity exercise per week and progressing to 60 minutes per day as appropriate.16
Increasing the number of steps taken per day, as measured by a pedometer, also can be beneficial. Encourage patients to obtain a baseline measure of daily steps and to gradually increase toward a moderate goal of 10,000 steps per day.17
Case report: Accentuating the positive
On follow-up, Ms. H was quick to report the many barriers to exercise she had experienced and the times she did not meet her goal. Rather than dwell on shortcomings, the psychiatrist redirected her to examine the many positive actions she had taken to manage her depression.
As she considered how to overcome barriers to exercise, she reported increased confidence that she could stick with her medication and exercise regimen. She continues to exercise regularly and adheres to her fluoxetine. Her depressive symptoms remain well-controlled.
Overcoming barriers to exercise
Patient obstacles. Many patients acknowledge that regular exercise makes them feel physically and emotionally healthier but have difficulty exercising long term. Less than one-half of those who start an exercise program stick with it beyond 6 months.18 Drop-out reasons include injuries, lack of time, and low motivation (Table 3).19,20
Depressive symptoms—fatigue, loss of interest, low self-esteem, feelings of helplessness, and psychomotor retardation—make exercise adherence even more difficult.
Physician obstacles. The U.S. Preventive Services Task Force recommends that physicians advise all patients to increase physical activity, but the national rate of physician counseling about exercise is low. In a population-based survey of more than 9,000 patients, 34% said their physicians counseled them about exercise at their most recent visit within the past year.21
Physician-reported barriers to exercise counseling include:
- competing demands for limited clinical time
- perceived ineffectiveness of advice to exercise
- lack of training and knowledge about exercise counseling and prescription.22,23
Patients are more likely to become active and continue exercising when their physicians help them set achievable goals.
Project PACE. Physicians can overcome barriers to counseling patients about exercise. Those who participated in Project PACE (Physician-based Assessment and Counseling for Exercise)24 said they felt more confident that they could counsel patients about physical activity in 1 to 5 minutes.
In a controlled study of 212 sedentary adults, patients who received PACE counseling from their physicians significantly increased their minutes of weekly walking compared with a control group. Also, 52% of patients who received PACE counseling adopted some physical activity, compared with 12% of controls.25
Though modest initial goals are not sufficient for achieving the full benefits of exercise, success with a small goal is a powerful motivator. Rather than giving up, patients feel encouraged and are more likely to set a subsequent, more ambitious goal.
Recommendations. To help patients start exercising, determine how motivated and ready they are. Start by asking them to describe their current activities. Ask if they were ever more active and what they liked about it. Did they experience any benefits? Establish which of increased activity’s benefits—improved sleep, reduced depression, increased energy—would most benefit the patient, based on his or her symptoms.
Discuss barriers to physical activity and encourage problem-solving to overcome them and incorporate physical activity into their lives. Encourage patients to seek support from family, friends, coworkers, and exercise groups.
Help them set realistic, achievable goals. Even a modest 10 minutes of activity has been shown to enhance mood,26 and a 10-minute brisk walk is one-third of the day’s public health guideline. Suggest that patients choose a variety of activities they enjoy.
During follow-up visits, reinforce any progress toward change. When patients’ exercise efforts fall short, explain that the process of becoming more active often includes setbacks. Advise them to seek support and to consider adopting more-achievable goals.
Related resources
- Getting started. Resources on nutrition and physical activity from the National Center for Chronic Disease Prevention and Health Promotion. http://www.cdc.gov/nccdphp/dnpa/physical/starting/index.htm
- Marcus B, Forsyth L. Motivating people to be physically active. Champaign, IL: Human Kinetics, 2002.
Drug brand names
- Fluoxetine • Prozac
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. H, age 26, is being evaluated for moderate to severe depressive symptoms, including oversleeping and overeating. She has had difficulty adhering to medication in the past and is ambivalent about taking antidepressants. She takes a passive approach to managing her depression, preferring to “wait for it to pass.”
Her psychiatrist prescribes fluoxetine, 20 mg in the morning, and recommends that Ms. H change her coping strategies from napping and snacking to increased physical activity. She encourages Ms. H to think about what activities interest her and to set exercise goals.
Ms. H says she has considered buying exercise equipment (an elliptical machine) and increasing her walking outside. She sets a goal to walk 20 minutes most days and to spend 10 to 15 minutes using the elliptical machine while watching television.
Physical activity’s mental health benefits are less well-known than its well-documented medical benefits—reduced risk of heart disease, hypertension, and diabetes; weight control; bone mass preservation; better sleep, and improved cholesterol levels.1 By encouraging exercise, you can improve patients’ mood, well-being, and quality of life, independent of medication and psychotherapy. In this article, we:
- explore the relationship between physical activity and mental health
- compare exercise with medication and psychotherapies for easing depression
- discuss counseling strategies shown to be effective in helping sedentary patients become more physically active.
Table 1
Why physical activity may improve mental health
|
| Psychological theories Physical activity:
|
| Source: References 10 and 11 |
Mental benefits of exercise
Adults who exercise regularly report lower levels of depressive and anxiety disorders than the overall U.S. population.2 As a therapeutic intervention, exercise has been studied primarily in depressed individuals, although some data also support its efficacy in:
- reducing anxiety symptoms in panic disorder3
- reducing disruptive behavior in developmentally disabled patients4
- alleviating chronic fatigue symptoms5
- improving body esteem in patients with body image disturbance6
- increasing function in chronic pain7
- reducing urges to smoke and improving smoking abstinence among nicotine-dependent individuals.8
Why exercise helps. Mechanisms that would explain exercise’s positive effect on mood are not well understood.9 Physiologic and psychological hypotheses have been suggested (Table 1),10,11 and researchers are attempting to elucidate them by using animal models.13
Case report: Feeling more energetic
At follow-up 6 weeks later, Ms. H. reported a substantial reduction in depressive symptoms. She noted increased energy, improved sleep, decreased overeating, higher self-esteem, and greater confidence in her ability to manage her depression.
Exercising also helped structure her day. She noticed that on days she did not exercise she was more likely to take a nap, miss her medication, or feel pessimistic about her depression.
Exercise as an antidepressant
Exercise vs psychotherapy. Exercise has been shown to be more effective at reducing depressive symptoms than no treatment, occupational therapy, cognitive therapy, health seminars, routine care, or meditation. Interventions used in these meta-analyses ranged from nonaerobic exercise training several times a week to 1 hour of supervised running 4 times a week.12 Literature reviews also have concluded that exercise training compares favorably with individual or group psychotherapy and with cognitive therapy for treating depression.7
Exercise vs medication. Exercise training has also been compared with drug therapy in treating depression.
In a randomized, controlled trial, 156 men and women over age 50 with major depression received exercise training, sertraline, or exercise plus sertraline. Subjects in the exercise groups completed 40 minutes of aerobic exercise (biking or brisk walking/ jogging) 3 times a week. Subjects treated with sertraline received 50 to 200 mg/d, depending on response.
After 16 weeks, all three groups were significantly improved, with no clinically or statistically significant differences in depressive symptoms, as measured with the Hamilton Rating Scale for Depression (HRSD) and Beck Depression Inventory.13
In a follow-up study 6 months later,14 the exercise group had significantly lower rates of relapse (defined as HRSD scores >15 and meeting diagnostic criteria) than did the medication group. Combining exercise with medication did not provide an added benefit in preventing relapse.
Exercise as monotherapy. Some studies have investigated using exercise instead of medication and psychotherapy. Many of these trials, however, were limited by methodologic weaknesses such as nonrandomized samples or lack of appropriate control groups.12
To address the need for higher-quality evidence, the Depression Outcomes Study of Exercise (DOSE) is investigating the dose-continued from page 12 response effects of exercise as monotherapy for major depressive disorder (MDD).5 The 12-week trial included 80 men and women ages 20 to 45 diagnosed with mild-to-moderate MDD using the Structured Clinical Interview for Depression. They were randomly assigned to one of five supervised exercise regimens:
- 7.0 kcal/kg/week in 3 days/week
- 7.0 kcal/kg/week in 5 days/week
- 17.5 kcal/kg/week in 3 days/week
- 17.5 kcal/kg/week in 5 days/week
- 3 days/week of stretching and flexibility exercises for 15 to 20 min/session.
Table 2
How much physical activity is recommended for adults?
For physical and mental health
|
For weight loss and management
|
Depressive symptoms were measured with the HRSD and Inventory of Depressive Symptoms (clinician and self-report). Other outcome measures included cardiorespiratory fitness, self-efficacy, and quality of life. Results are being prepared for publication and will likely help clarify the role of physical activity in treating patients with MDD.
Table 3
Why patients don’t exercise: Common barriers they perceive
Practical limitations
| |
Medical limitations
| |
Psychological limitations
| |
| Source: References 15 and 16 | |
How much exercise is therapeutic?
In the absence of physical activity guidelines specific to mental health, we suggest using standard public health guidelines (Table 2):
- 30 minutes or more of moderate-intensity physical activity (brisk walking, swimming, dancing, cycling) most days of the week (recommended by the Centers for Disease Control and Prevention and American College of Sports Medicine)1
- 60 minutes of moderate-intensity physical activity daily for weight loss and maintenance (recommended by the Institute of Medicine).16
A recent study investigated the effects of exercise duration and intensity on weight loss in overweight, sedentary women. These researchers recommended setting the initial intervention target at 150 minutes or more of moderate-intensity exercise per week and progressing to 60 minutes per day as appropriate.16
Increasing the number of steps taken per day, as measured by a pedometer, also can be beneficial. Encourage patients to obtain a baseline measure of daily steps and to gradually increase toward a moderate goal of 10,000 steps per day.17
Case report: Accentuating the positive
On follow-up, Ms. H was quick to report the many barriers to exercise she had experienced and the times she did not meet her goal. Rather than dwell on shortcomings, the psychiatrist redirected her to examine the many positive actions she had taken to manage her depression.
As she considered how to overcome barriers to exercise, she reported increased confidence that she could stick with her medication and exercise regimen. She continues to exercise regularly and adheres to her fluoxetine. Her depressive symptoms remain well-controlled.
Overcoming barriers to exercise
Patient obstacles. Many patients acknowledge that regular exercise makes them feel physically and emotionally healthier but have difficulty exercising long term. Less than one-half of those who start an exercise program stick with it beyond 6 months.18 Drop-out reasons include injuries, lack of time, and low motivation (Table 3).19,20
Depressive symptoms—fatigue, loss of interest, low self-esteem, feelings of helplessness, and psychomotor retardation—make exercise adherence even more difficult.
Physician obstacles. The U.S. Preventive Services Task Force recommends that physicians advise all patients to increase physical activity, but the national rate of physician counseling about exercise is low. In a population-based survey of more than 9,000 patients, 34% said their physicians counseled them about exercise at their most recent visit within the past year.21
Physician-reported barriers to exercise counseling include:
- competing demands for limited clinical time
- perceived ineffectiveness of advice to exercise
- lack of training and knowledge about exercise counseling and prescription.22,23
Patients are more likely to become active and continue exercising when their physicians help them set achievable goals.
Project PACE. Physicians can overcome barriers to counseling patients about exercise. Those who participated in Project PACE (Physician-based Assessment and Counseling for Exercise)24 said they felt more confident that they could counsel patients about physical activity in 1 to 5 minutes.
In a controlled study of 212 sedentary adults, patients who received PACE counseling from their physicians significantly increased their minutes of weekly walking compared with a control group. Also, 52% of patients who received PACE counseling adopted some physical activity, compared with 12% of controls.25
Though modest initial goals are not sufficient for achieving the full benefits of exercise, success with a small goal is a powerful motivator. Rather than giving up, patients feel encouraged and are more likely to set a subsequent, more ambitious goal.
Recommendations. To help patients start exercising, determine how motivated and ready they are. Start by asking them to describe their current activities. Ask if they were ever more active and what they liked about it. Did they experience any benefits? Establish which of increased activity’s benefits—improved sleep, reduced depression, increased energy—would most benefit the patient, based on his or her symptoms.
Discuss barriers to physical activity and encourage problem-solving to overcome them and incorporate physical activity into their lives. Encourage patients to seek support from family, friends, coworkers, and exercise groups.
Help them set realistic, achievable goals. Even a modest 10 minutes of activity has been shown to enhance mood,26 and a 10-minute brisk walk is one-third of the day’s public health guideline. Suggest that patients choose a variety of activities they enjoy.
During follow-up visits, reinforce any progress toward change. When patients’ exercise efforts fall short, explain that the process of becoming more active often includes setbacks. Advise them to seek support and to consider adopting more-achievable goals.
Related resources
- Getting started. Resources on nutrition and physical activity from the National Center for Chronic Disease Prevention and Health Promotion. http://www.cdc.gov/nccdphp/dnpa/physical/starting/index.htm
- Marcus B, Forsyth L. Motivating people to be physically active. Champaign, IL: Human Kinetics, 2002.
Drug brand names
- Fluoxetine • Prozac
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995;273(5):402-7.
2. Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med 2003;36:698-703.
3. Broocks A, Bandelow B, Pekrun G, et al. Comparison of aerobic exercise, clomipramine and placebo in the treatment of panic disorder. Am J Psychiatry 1998;155:603-9.
4. Gabler-Halle D, Halle JW, Chung YB. The effects of aerobic exercise on psychological and behavioral variables of individuals with developmental disabilities. A critical review. Res Dev Disabil 1993;14:359-86.
5. Powell P, Bentall RP, Nye FJ, Edwards RH. Patient education to encourage graded exercise in chronic fatigue syndrome. Br J Psychiatry 2004;184:142-6.
6. Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psychooncology 2003;12(2):118-26.
7. Tkachuk GA, Martin GL. Exercise therapy for patients with psychiatric disorders: research and clinical implications. Prof Psychol Res Pract 1999;30:275-82
8. Ussher MH, Taylor AH, West R, McEwen A. Does exercise aid smoking cessation? A systematic review. Addiction 2000;95(2):199-208.
9. Van Hoomissen JD, Chambliss HO, Holmes PV, Dishman RK. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain Res 2003;974:228-235.
10. Plante TG, Rodin J. Physical fitness and enhanced psychological health. Curr Psychol Res Rev 1990;9:3-24.
11. Weyerer A, Kupfer B. Physical exercise and psychological health. Sports Med 1994;17(2):108-16.
12. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomized controlled trials. Br Med J 2001;322:1-8.
13. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older adults with major depression. Arch Intern Med 1999;159:2349-56.
14. Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000;62:633-8.
15. Dunn AL, Trivedi MH, Kampert JB, et al. The DOSE study: a clinical trial to examine efficacy and dose response of exercise as treatment for depression. Control Clin Trials 2002;23:584-603.
16. Jakicic JM, Marcus BH, Gallagher KI, et al. Effect of exercise duration and intensity on weight loss in overweight, sedentary women. JAMA 2003;290:1323-30.
17. Tudor-Locke C, Bassett DR, Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 2004;34(1):1-8.
18. Dishman RK. Compliance/adherence in health-related exercise. Health Psychol 1982;1:237-67.
19. Sallis JF, Hovell MF. Determinants of exercise behavior. Exerc Sport Sci Rev 1990;18:307-30.
20. Heesch KC, Brown DR, Blanton CJ. Perceived barriers to exercise and stage of exercise adoption in older women of different racial/ethnic groups. Women Health 2000;30(4):61-76.
21. Wee CC, McCarthy EP, Davis RB, Phillips RS. Physician counseling about exercise. JAMA 1999;282(16):1583-8.
22. Kennedy MF, Meeuwisse WH. Exercise counseling by family physicians in Canada. Prev Med 2003 Sep;37(3):226-32.
23. Reed BD, Jensen JD, Gorenflo DW. Physicians and exercise promotion. Am J Prev Med 1991;7:410-15.
24. Long BJ, Calfas KJ, Wooten W, et al. A multisite field test of the acceptability of physical activity counseling in primary care: project PACE. Am J Prev Med 1996;12(2):73-81.
25. Calfas KJ, Long BJ, Sallis JF, et al. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med 1996;25(3):225-33.
26. Hansen CJ, Stevens LC, Coast JR. Exercise duration and mood state: how much is enough to feel better? Health Psychol 2001;20(4):267-75.
1. Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995;273(5):402-7.
2. Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med 2003;36:698-703.
3. Broocks A, Bandelow B, Pekrun G, et al. Comparison of aerobic exercise, clomipramine and placebo in the treatment of panic disorder. Am J Psychiatry 1998;155:603-9.
4. Gabler-Halle D, Halle JW, Chung YB. The effects of aerobic exercise on psychological and behavioral variables of individuals with developmental disabilities. A critical review. Res Dev Disabil 1993;14:359-86.
5. Powell P, Bentall RP, Nye FJ, Edwards RH. Patient education to encourage graded exercise in chronic fatigue syndrome. Br J Psychiatry 2004;184:142-6.
6. Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psychooncology 2003;12(2):118-26.
7. Tkachuk GA, Martin GL. Exercise therapy for patients with psychiatric disorders: research and clinical implications. Prof Psychol Res Pract 1999;30:275-82
8. Ussher MH, Taylor AH, West R, McEwen A. Does exercise aid smoking cessation? A systematic review. Addiction 2000;95(2):199-208.
9. Van Hoomissen JD, Chambliss HO, Holmes PV, Dishman RK. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain Res 2003;974:228-235.
10. Plante TG, Rodin J. Physical fitness and enhanced psychological health. Curr Psychol Res Rev 1990;9:3-24.
11. Weyerer A, Kupfer B. Physical exercise and psychological health. Sports Med 1994;17(2):108-16.
12. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomized controlled trials. Br Med J 2001;322:1-8.
13. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older adults with major depression. Arch Intern Med 1999;159:2349-56.
14. Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000;62:633-8.
15. Dunn AL, Trivedi MH, Kampert JB, et al. The DOSE study: a clinical trial to examine efficacy and dose response of exercise as treatment for depression. Control Clin Trials 2002;23:584-603.
16. Jakicic JM, Marcus BH, Gallagher KI, et al. Effect of exercise duration and intensity on weight loss in overweight, sedentary women. JAMA 2003;290:1323-30.
17. Tudor-Locke C, Bassett DR, Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 2004;34(1):1-8.
18. Dishman RK. Compliance/adherence in health-related exercise. Health Psychol 1982;1:237-67.
19. Sallis JF, Hovell MF. Determinants of exercise behavior. Exerc Sport Sci Rev 1990;18:307-30.
20. Heesch KC, Brown DR, Blanton CJ. Perceived barriers to exercise and stage of exercise adoption in older women of different racial/ethnic groups. Women Health 2000;30(4):61-76.
21. Wee CC, McCarthy EP, Davis RB, Phillips RS. Physician counseling about exercise. JAMA 1999;282(16):1583-8.
22. Kennedy MF, Meeuwisse WH. Exercise counseling by family physicians in Canada. Prev Med 2003 Sep;37(3):226-32.
23. Reed BD, Jensen JD, Gorenflo DW. Physicians and exercise promotion. Am J Prev Med 1991;7:410-15.
24. Long BJ, Calfas KJ, Wooten W, et al. A multisite field test of the acceptability of physical activity counseling in primary care: project PACE. Am J Prev Med 1996;12(2):73-81.
25. Calfas KJ, Long BJ, Sallis JF, et al. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med 1996;25(3):225-33.
26. Hansen CJ, Stevens LC, Coast JR. Exercise duration and mood state: how much is enough to feel better? Health Psychol 2001;20(4):267-75.
Therapy-resistant major depression The attraction of magnetism: How effective—and safe—is rTMS?
Using magnets to improve health is sometimes hawked in dubious classified ads and “infomercials.” However, a legitimate use of magnetism—repetitive transcranial magnetic stimulation (rTMS)—is showing promise in treating severe depression (Box) 1-4 and other psychiatric disorders.
Patients or their families are likely to ask psychiatrists about rTMS as more becomes known about this investigational technology. Drawing from our experience and the evidence, we offer an update on whether rTMS may be an alternative for treating depression and address issues that must be resolved before it could be used in clinical practice.
WHAT IS RTMS?
rTMS consists of a series of magnetic pulses produced by a stimulator, which can be adjusted for:
- coil type and placement
- stimulation site, intensity, frequency, and number
- amount of time between stimulations
- treatment duration.
In 1985, Barker and colleagues developed single-pulse transcranial magnetic stimulation to examine motor cortex function.1 The single-pulse mechanism they discovered was subsequently adapted to deliver repetitive pulses and is referred to as repetitive transcranial magnetic stimulation (rTMS).
How rTMS works. Transcranial magnetic stimulation uses an electromagnetic coil applied to the head to produce an intense, localized, fluctuating magnetic field that passes unimpeded into a small area of the brain, inducing an electrical current. This results in neuronal depolarization in a localized area under the coil, and possibly distal effects as well.2 During the neurophysiological studies, it was discovered that subjects also experienced a change in mood.
Antidepressant effects. Similar physiologic effects induced by rTMS, electroconvulsive therapy (ECT), and antidepressants on the endocrine system, sleep parameters, and biochemical measures suggest antidepressant properties.3 In 1993, the first published study examining rTMS in psychiatric patients reported reduced depressive symptoms in two subjects.4 Since then, several clinical trials have examined rTMS’ antidepressive effects. In 2001, Canada’s Health Ministry approved rTMS for treating major depression. In the United States, rTMS remains investigational and is FDA-approved only for clinical trials.
Coil type and placement. Initial studies involved stimulation—typically low-frequency—over the vertex, but most subsequent rTMS trials in depression have stimulated the left dorsolateral prefrontal cortex. Neuroimaging studies have shown prefrontal functioning abnormalities in depressed subjects, and it is hypothesized that stimulating this area (plus possible distal effects) may produce an antidepressant effect.5
Various configurations have been used, but circular and figure-eight-shaped coils are most common. These flat coils are made of tightly wound ferromagnetic material such as copper, enclosed in a heavy plastic cover. With the figure-eight coil, the intersection of the two loops produces the strongest magnetic field.
Stimulation site. Stimulation intensity depends on the individual’s motor threshold, and the site can be determined visually or electrophysiologically.
- With the visual method, the motor threshold over the left primary motor cortex site for the first dorsal interosseous muscle (FDI) or the abductor pollius brevis (APB) is determined by iteration. This involves placing the coil at a progression of sites and increasing stimulation intensity until reliable (in 5 of 10 stimulations) contractions are seen in the right FDI or APB.
- Similarly, the electrophysiologic method uses 5 of 10 motorevoked potentials of 50 microvolts to locate the site.
The only small trial that compared visual and electrophysiologic site determination showed similar results with both methods.6 The most common stimulation site is the left dorsolateral prefrontal cortex, 5 cm anterior and parasagittal to the FDI or APB motor cortex. Alternately, frameless stereotactic systems or the international 10-20 proportional system used in EEG labs have been recommended to target sites more accurately.
Stimulus intensity. Each individual’s motor threshold determines stimulus intensity. Using functional MRI studies, researchers from the Medical University of South Carolina concluded that higher stimulation intensity relative to the motor threshold may have a more robust effect, as the magnetic field declines with distance from the coil.7 However, intensities >120% of the motor threshold are generally avoided because of possible increased seizure risk.9
Frequency of stimulation. Most researchers apply frequencies of 1 to 20 Hz over the left dorsolateral prefrontal cortex, but also use lower frequencies (<1 Hz) over the right dorsolateral prefrontal cortex. Using higher frequencies in major depression is attractive in theory because of:
- the reported association of decreased regional cerebral blood flow with hypometabolism in the left dorsolateral prefrontal cortex
- higher-frequency stimulation’s ability to produce temporary excitation and neuronal depolarization.
Number of stimulations. The number of stimulations is determined by frequency (Hz) and stimulation train duration (for example, 10 Hz for 5 seconds equals 50 stimulations). A typical treatment session incorporates 10 to 30 stimulation trains several seconds apart (the inter-train interval). Thus, a typical session delivers 1,000 to 1,200 stimulations. In studies of unmedicated depressed patients, the total number of stimulations has varied from 8,000 to 32,000 per treatment course.
Duration between two stimulation trains. Chen et al have demonstrated that shorter (<1 second) inter-train intervals increase seizure risk with higher frequencies (such as 20 Hz) and intensities (>100% of motor threshold) of stimulation.9 Based on their studies with healthy volunteers, they recommended several “safe” ranges (such as 5 seconds at 110% of motor threshold). Most trials use 30- to 60-second inter-train intervals.
Most treatments continue 2 to 4 weeks, Monday through Friday, although more frequent treatments are being studied.
EFFICACY FOR DEPRESSION
Most studies of rTMS in depression have compared real rTMS to a sham control or electroconvulsive therapy (ECT).
In earlier studies, the sham procedure typically involved tilting the coil away from the skull. This method has been questioned, however, because of evidence of neuronal depolarization.10
More recent sham coils mimic the real coils’ sound and sensation, without magnetic stimulation.
Despite these methodologic problems and some mixed results, depressed patients receiving rTMS show more favorable results than those receiving sham rTMS.11,12 Several meta-analyses have attempted to quantify rTMS’ efficacy for depression:
- Holtzheimer et al concluded that rTMS was statistically superior to sham rTMS, but the clinical significance of these findings was modest in a population of mostly outpatients with less-severe depression.13
- Burt et al found a statistically strong antidepressant effect, but its magnitude varied and few of the studies yielded a substantial clinical response or remission. The team also noted that rTMS’ long-term efficacy or adverse effects are unknown.14
- Kozel et al concluded that left prefrontal rTMS rendered a significant antidepressant effect with measurable clinical improvement.15
- Gershon et al16 supported an antidepressant effect for rTMS when compared with sham rTMS or ECT.
Ongoing rTMS research includes subjects with many types of mild to severe psychiatric illnesses, including major depression, obsessive-compulsive disorder, and psychosis. Typically, patients referred for experimental approaches have not responded to or tolerated available treatments. Exclusion criteria used by most rTMS studies are listed in the Table.
Table
Medical conditions that preclude use of rTMS
| Serious medical conditions History of seizures Increased intracranial pressure Serious head trauma |
| Myocardial infarction within the past 6 months |
| Pregnancy or childbearing potential (unless reliable contraception is being used) |
| Intracranial metallic implants |
| Pacemakers or other implanted devices |
rTMS vs. ECT. Four randomized, controlled trials have compared rTMS with ECT for treating severely ill, often medication-resistant patients.17-20 Although their methodologies differed, all four studies concluded that rTMS and ECT offer similar efficacy, except that rTMS may be less effective for treating psychotic depression.
One study found ECT more effective than rTMS for psychotic depression, although the patients who received ECT were also treated with antipsychotics and/or antidepressants.17 Our study,19 which did not use these agents, has not corroborated this observation. Preliminary data also indicate comparable relapse rates following acute ECT and rTMS when subjects are followed on maintenance medication.21
ADVERSE EFFECTS
The potential adverse effects of new treatments must always be considered. Thus far, rTMS appears to produce minimal, relatively benign complications, including:
- mild discomfort at the stimulation site
- localized muscle twitching during stimulation
- mild post-treatment headaches—believed caused by muscle contractions—which usually respond to aspirin or acetaminophen
- treatment stimulation-related seizures (rarely).8
The rTMS device makes a loud clicking noise, and subjects wear protective ear plugs during treatment.
Patient experience. The first rTMS session—during which the patient’s motor threshold is determined—can last up to 45 minutes. Subsequent sessions are usually 15 to 20 minutes. Patients are typically apprehensive before the first session but become more relaxed with experience and tolerate the treatments easily.
During the procedure, many patients describe a tapping sensation on the forehead, and some experience slight muscle twitching around the eye or corner of the mouth. As the coil warms, the skin it touches sometimes flushes pink, although this does not seem to bother our patients. They can return to their daily routines immediately after a session.
rTMS for major depression. In our experience, rTMS may help patients with major depression. For example, one patient diagnosed with a major depressive episode with psychotic features was referred to our study comparing rTMS with ECT.19 Her depression had lasted several months, with partial response to ECT treatments. She signed informed consent and was randomly assigned to receive rTMS treatment.
At study admission, the patient’s Hamilton Depression Rating Scale (HDRS) score was 48, indicating moderate to severe depression. Following 10 rTMS sessions, her HDRS score had dropped to 2, with remission of symptoms. No follow-up results were documented.
Cognitive effects. Whereas mood disorders are associated with medication-independent neuropsychological deficits, most studies have found no adverse cognitive effects with rTMS.22 Indeed, some of our rTMS patients have improved in certain cognitive tests, although this may be explained by test-retest effects or better attention and concentration associated with mood improvement.
Figure Potential roles for rTMS in treating major depression
Solid lines represent current standards of practice. Dotted lines represent hypothetical roles for rTMS.
Source: Adapted and reprinted with permission from Dowd et al. Is repetitive transcranial magnetic stimulation an alternative to ECTfor the treatment of depression? Contemp Psychiatry 2002;1:1-10.
POTENTIAL ROLE FOR rTMS
Today’s standard treatment of major depressive episodes begins with an antidepressant (plus an antipsychotic, if necessary) and proceeds to augmentation strategies if response is insufficient. rTMS may one day become an augmentation or monotherapy option for patients who do not respond sufficiently to standard treatments (Figure).
ECT treatment may be initiated if a patient has had a prior good response to ECT, is intolerant to medication, or prefers ECT. In that case, rTMS may be used as an alternate initial treatment or with ECT. Thus, rTMS may be used:
- to augment antidepressants
- as an alternative to antidepressants or ECT
- or sequentially with ECT.
Before that can happen, however, optimal treatment parameters need to be clarified by larger, well-designed, controlled studies comparing rTMS to a valid sham treatment, antidepressants, and ECT.
Related resources
- International Society for Transcranial Stimulation. www.ists.unibe.ch/
- Repetitive Transcranial Magnetic Stimulation Research Clinic at Yale-New Haven Psychiatric Hospital.
Disclosure
The authors report that they have no proprietary interest in the technology discussed in this article.
1. Barker A, Jalinous R, Freeston I. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985;1:1106-7.
2. Lisanby SH, Datto CJ, Szuba MP. ECT and rTMS: past, present, and future. Depress Anxiety 2000;12:115-17.
3. Post A, Keck PE, Jr. Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do we know about the neurobiological mechanisms? J Psychiatr Res 2001;35:193-215.
4. Holfich G, Kasper S, Hufnagel A, et al. Application of transcranial magnetic stimulation in treatment of drug resistant major depression—a report of two cases. Human Psychopharmacol 1993;8:361-5.
5. George MS, Nahas Z, Speer AM, et al. Transcranial magnetic stimulation—a new method for investigating the neuroanatomy of depression. In: Ebert D, Ebmeier K (eds). New models for depression. New York: Karger, 1998;94-122.
6. Pridmore A, Americo Fernandes Filho J, Nahas Z, et al. Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. J ECT 1998;14(1):25-7.
7. Kozel FA, Nahas Z, deBrux C, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci 2000;13:376-84.
8. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, 1996. Electroencephalogr Clin Neurophysiol 1998;108:1-16.
9. Chen R, Gerloff C, Classen J, et al. Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol 1997;105:415-21.
10. Loo CK, Taylor JL, Gandevia SC, et al. Transcranial magnetic stimulation in controlled treatment studies: Are some “sham” forms active? Biol Psychiatry. 2000;47:325-31.
11. George MS, Nahas Z, Molloy M, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry 2000;48:962-70.
12. Berman RM, Narasimhan M, Sanacora G, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry 2000;47:332-7.
13. Holtzheimer PE, Russo J, Avery D. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull 2001;35:149-69.
14. Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta-analysis. Int J Neuropsychopharmacol 2002;5:73-103.
15. Kozel FE, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract 2002;8:270-5.
16. Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am JPsychiatry 2003;160(5):835-45.
17. Grunhaus L, Dannon PN, Schreiber S, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry 2000;47:314-24.
18. Pridmore S, Bruno R, Turnier-Shea Y, et al. Comparison of unlimited numbers of rapid transcranial magnetic stimulation and ECT treatment sessions in major depression episodes. Int J Neuropsychopharmacol 2000;3:129-34.
19. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biol Psychiatry 2002;51:659-67
20. Grunhaus L, Schreiber S, Dolberg OT, et al. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry 2003;53:324-31.
21. Dannon PH, Dolberg OT, Schreiber S, Grunhaus L. Three and six month outcome following courses of either ECT or rTMS in a population of severely depressed individuals—preliminary report. Biol Psychiatry 2002;15:687-90.
22. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiology (in press).
Using magnets to improve health is sometimes hawked in dubious classified ads and “infomercials.” However, a legitimate use of magnetism—repetitive transcranial magnetic stimulation (rTMS)—is showing promise in treating severe depression (Box) 1-4 and other psychiatric disorders.
Patients or their families are likely to ask psychiatrists about rTMS as more becomes known about this investigational technology. Drawing from our experience and the evidence, we offer an update on whether rTMS may be an alternative for treating depression and address issues that must be resolved before it could be used in clinical practice.
WHAT IS RTMS?
rTMS consists of a series of magnetic pulses produced by a stimulator, which can be adjusted for:
- coil type and placement
- stimulation site, intensity, frequency, and number
- amount of time between stimulations
- treatment duration.
In 1985, Barker and colleagues developed single-pulse transcranial magnetic stimulation to examine motor cortex function.1 The single-pulse mechanism they discovered was subsequently adapted to deliver repetitive pulses and is referred to as repetitive transcranial magnetic stimulation (rTMS).
How rTMS works. Transcranial magnetic stimulation uses an electromagnetic coil applied to the head to produce an intense, localized, fluctuating magnetic field that passes unimpeded into a small area of the brain, inducing an electrical current. This results in neuronal depolarization in a localized area under the coil, and possibly distal effects as well.2 During the neurophysiological studies, it was discovered that subjects also experienced a change in mood.
Antidepressant effects. Similar physiologic effects induced by rTMS, electroconvulsive therapy (ECT), and antidepressants on the endocrine system, sleep parameters, and biochemical measures suggest antidepressant properties.3 In 1993, the first published study examining rTMS in psychiatric patients reported reduced depressive symptoms in two subjects.4 Since then, several clinical trials have examined rTMS’ antidepressive effects. In 2001, Canada’s Health Ministry approved rTMS for treating major depression. In the United States, rTMS remains investigational and is FDA-approved only for clinical trials.
Coil type and placement. Initial studies involved stimulation—typically low-frequency—over the vertex, but most subsequent rTMS trials in depression have stimulated the left dorsolateral prefrontal cortex. Neuroimaging studies have shown prefrontal functioning abnormalities in depressed subjects, and it is hypothesized that stimulating this area (plus possible distal effects) may produce an antidepressant effect.5
Various configurations have been used, but circular and figure-eight-shaped coils are most common. These flat coils are made of tightly wound ferromagnetic material such as copper, enclosed in a heavy plastic cover. With the figure-eight coil, the intersection of the two loops produces the strongest magnetic field.
Stimulation site. Stimulation intensity depends on the individual’s motor threshold, and the site can be determined visually or electrophysiologically.
- With the visual method, the motor threshold over the left primary motor cortex site for the first dorsal interosseous muscle (FDI) or the abductor pollius brevis (APB) is determined by iteration. This involves placing the coil at a progression of sites and increasing stimulation intensity until reliable (in 5 of 10 stimulations) contractions are seen in the right FDI or APB.
- Similarly, the electrophysiologic method uses 5 of 10 motorevoked potentials of 50 microvolts to locate the site.
The only small trial that compared visual and electrophysiologic site determination showed similar results with both methods.6 The most common stimulation site is the left dorsolateral prefrontal cortex, 5 cm anterior and parasagittal to the FDI or APB motor cortex. Alternately, frameless stereotactic systems or the international 10-20 proportional system used in EEG labs have been recommended to target sites more accurately.
Stimulus intensity. Each individual’s motor threshold determines stimulus intensity. Using functional MRI studies, researchers from the Medical University of South Carolina concluded that higher stimulation intensity relative to the motor threshold may have a more robust effect, as the magnetic field declines with distance from the coil.7 However, intensities >120% of the motor threshold are generally avoided because of possible increased seizure risk.9
Frequency of stimulation. Most researchers apply frequencies of 1 to 20 Hz over the left dorsolateral prefrontal cortex, but also use lower frequencies (<1 Hz) over the right dorsolateral prefrontal cortex. Using higher frequencies in major depression is attractive in theory because of:
- the reported association of decreased regional cerebral blood flow with hypometabolism in the left dorsolateral prefrontal cortex
- higher-frequency stimulation’s ability to produce temporary excitation and neuronal depolarization.
Number of stimulations. The number of stimulations is determined by frequency (Hz) and stimulation train duration (for example, 10 Hz for 5 seconds equals 50 stimulations). A typical treatment session incorporates 10 to 30 stimulation trains several seconds apart (the inter-train interval). Thus, a typical session delivers 1,000 to 1,200 stimulations. In studies of unmedicated depressed patients, the total number of stimulations has varied from 8,000 to 32,000 per treatment course.
Duration between two stimulation trains. Chen et al have demonstrated that shorter (<1 second) inter-train intervals increase seizure risk with higher frequencies (such as 20 Hz) and intensities (>100% of motor threshold) of stimulation.9 Based on their studies with healthy volunteers, they recommended several “safe” ranges (such as 5 seconds at 110% of motor threshold). Most trials use 30- to 60-second inter-train intervals.
Most treatments continue 2 to 4 weeks, Monday through Friday, although more frequent treatments are being studied.
EFFICACY FOR DEPRESSION
Most studies of rTMS in depression have compared real rTMS to a sham control or electroconvulsive therapy (ECT).
In earlier studies, the sham procedure typically involved tilting the coil away from the skull. This method has been questioned, however, because of evidence of neuronal depolarization.10
More recent sham coils mimic the real coils’ sound and sensation, without magnetic stimulation.
Despite these methodologic problems and some mixed results, depressed patients receiving rTMS show more favorable results than those receiving sham rTMS.11,12 Several meta-analyses have attempted to quantify rTMS’ efficacy for depression:
- Holtzheimer et al concluded that rTMS was statistically superior to sham rTMS, but the clinical significance of these findings was modest in a population of mostly outpatients with less-severe depression.13
- Burt et al found a statistically strong antidepressant effect, but its magnitude varied and few of the studies yielded a substantial clinical response or remission. The team also noted that rTMS’ long-term efficacy or adverse effects are unknown.14
- Kozel et al concluded that left prefrontal rTMS rendered a significant antidepressant effect with measurable clinical improvement.15
- Gershon et al16 supported an antidepressant effect for rTMS when compared with sham rTMS or ECT.
Ongoing rTMS research includes subjects with many types of mild to severe psychiatric illnesses, including major depression, obsessive-compulsive disorder, and psychosis. Typically, patients referred for experimental approaches have not responded to or tolerated available treatments. Exclusion criteria used by most rTMS studies are listed in the Table.
Table
Medical conditions that preclude use of rTMS
| Serious medical conditions History of seizures Increased intracranial pressure Serious head trauma |
| Myocardial infarction within the past 6 months |
| Pregnancy or childbearing potential (unless reliable contraception is being used) |
| Intracranial metallic implants |
| Pacemakers or other implanted devices |
rTMS vs. ECT. Four randomized, controlled trials have compared rTMS with ECT for treating severely ill, often medication-resistant patients.17-20 Although their methodologies differed, all four studies concluded that rTMS and ECT offer similar efficacy, except that rTMS may be less effective for treating psychotic depression.
One study found ECT more effective than rTMS for psychotic depression, although the patients who received ECT were also treated with antipsychotics and/or antidepressants.17 Our study,19 which did not use these agents, has not corroborated this observation. Preliminary data also indicate comparable relapse rates following acute ECT and rTMS when subjects are followed on maintenance medication.21
ADVERSE EFFECTS
The potential adverse effects of new treatments must always be considered. Thus far, rTMS appears to produce minimal, relatively benign complications, including:
- mild discomfort at the stimulation site
- localized muscle twitching during stimulation
- mild post-treatment headaches—believed caused by muscle contractions—which usually respond to aspirin or acetaminophen
- treatment stimulation-related seizures (rarely).8
The rTMS device makes a loud clicking noise, and subjects wear protective ear plugs during treatment.
Patient experience. The first rTMS session—during which the patient’s motor threshold is determined—can last up to 45 minutes. Subsequent sessions are usually 15 to 20 minutes. Patients are typically apprehensive before the first session but become more relaxed with experience and tolerate the treatments easily.
During the procedure, many patients describe a tapping sensation on the forehead, and some experience slight muscle twitching around the eye or corner of the mouth. As the coil warms, the skin it touches sometimes flushes pink, although this does not seem to bother our patients. They can return to their daily routines immediately after a session.
rTMS for major depression. In our experience, rTMS may help patients with major depression. For example, one patient diagnosed with a major depressive episode with psychotic features was referred to our study comparing rTMS with ECT.19 Her depression had lasted several months, with partial response to ECT treatments. She signed informed consent and was randomly assigned to receive rTMS treatment.
At study admission, the patient’s Hamilton Depression Rating Scale (HDRS) score was 48, indicating moderate to severe depression. Following 10 rTMS sessions, her HDRS score had dropped to 2, with remission of symptoms. No follow-up results were documented.
Cognitive effects. Whereas mood disorders are associated with medication-independent neuropsychological deficits, most studies have found no adverse cognitive effects with rTMS.22 Indeed, some of our rTMS patients have improved in certain cognitive tests, although this may be explained by test-retest effects or better attention and concentration associated with mood improvement.
Figure Potential roles for rTMS in treating major depression
Solid lines represent current standards of practice. Dotted lines represent hypothetical roles for rTMS.
Source: Adapted and reprinted with permission from Dowd et al. Is repetitive transcranial magnetic stimulation an alternative to ECTfor the treatment of depression? Contemp Psychiatry 2002;1:1-10.
POTENTIAL ROLE FOR rTMS
Today’s standard treatment of major depressive episodes begins with an antidepressant (plus an antipsychotic, if necessary) and proceeds to augmentation strategies if response is insufficient. rTMS may one day become an augmentation or monotherapy option for patients who do not respond sufficiently to standard treatments (Figure).
ECT treatment may be initiated if a patient has had a prior good response to ECT, is intolerant to medication, or prefers ECT. In that case, rTMS may be used as an alternate initial treatment or with ECT. Thus, rTMS may be used:
- to augment antidepressants
- as an alternative to antidepressants or ECT
- or sequentially with ECT.
Before that can happen, however, optimal treatment parameters need to be clarified by larger, well-designed, controlled studies comparing rTMS to a valid sham treatment, antidepressants, and ECT.
Related resources
- International Society for Transcranial Stimulation. www.ists.unibe.ch/
- Repetitive Transcranial Magnetic Stimulation Research Clinic at Yale-New Haven Psychiatric Hospital.
Disclosure
The authors report that they have no proprietary interest in the technology discussed in this article.
Using magnets to improve health is sometimes hawked in dubious classified ads and “infomercials.” However, a legitimate use of magnetism—repetitive transcranial magnetic stimulation (rTMS)—is showing promise in treating severe depression (Box) 1-4 and other psychiatric disorders.
Patients or their families are likely to ask psychiatrists about rTMS as more becomes known about this investigational technology. Drawing from our experience and the evidence, we offer an update on whether rTMS may be an alternative for treating depression and address issues that must be resolved before it could be used in clinical practice.
WHAT IS RTMS?
rTMS consists of a series of magnetic pulses produced by a stimulator, which can be adjusted for:
- coil type and placement
- stimulation site, intensity, frequency, and number
- amount of time between stimulations
- treatment duration.
In 1985, Barker and colleagues developed single-pulse transcranial magnetic stimulation to examine motor cortex function.1 The single-pulse mechanism they discovered was subsequently adapted to deliver repetitive pulses and is referred to as repetitive transcranial magnetic stimulation (rTMS).
How rTMS works. Transcranial magnetic stimulation uses an electromagnetic coil applied to the head to produce an intense, localized, fluctuating magnetic field that passes unimpeded into a small area of the brain, inducing an electrical current. This results in neuronal depolarization in a localized area under the coil, and possibly distal effects as well.2 During the neurophysiological studies, it was discovered that subjects also experienced a change in mood.
Antidepressant effects. Similar physiologic effects induced by rTMS, electroconvulsive therapy (ECT), and antidepressants on the endocrine system, sleep parameters, and biochemical measures suggest antidepressant properties.3 In 1993, the first published study examining rTMS in psychiatric patients reported reduced depressive symptoms in two subjects.4 Since then, several clinical trials have examined rTMS’ antidepressive effects. In 2001, Canada’s Health Ministry approved rTMS for treating major depression. In the United States, rTMS remains investigational and is FDA-approved only for clinical trials.
Coil type and placement. Initial studies involved stimulation—typically low-frequency—over the vertex, but most subsequent rTMS trials in depression have stimulated the left dorsolateral prefrontal cortex. Neuroimaging studies have shown prefrontal functioning abnormalities in depressed subjects, and it is hypothesized that stimulating this area (plus possible distal effects) may produce an antidepressant effect.5
Various configurations have been used, but circular and figure-eight-shaped coils are most common. These flat coils are made of tightly wound ferromagnetic material such as copper, enclosed in a heavy plastic cover. With the figure-eight coil, the intersection of the two loops produces the strongest magnetic field.
Stimulation site. Stimulation intensity depends on the individual’s motor threshold, and the site can be determined visually or electrophysiologically.
- With the visual method, the motor threshold over the left primary motor cortex site for the first dorsal interosseous muscle (FDI) or the abductor pollius brevis (APB) is determined by iteration. This involves placing the coil at a progression of sites and increasing stimulation intensity until reliable (in 5 of 10 stimulations) contractions are seen in the right FDI or APB.
- Similarly, the electrophysiologic method uses 5 of 10 motorevoked potentials of 50 microvolts to locate the site.
The only small trial that compared visual and electrophysiologic site determination showed similar results with both methods.6 The most common stimulation site is the left dorsolateral prefrontal cortex, 5 cm anterior and parasagittal to the FDI or APB motor cortex. Alternately, frameless stereotactic systems or the international 10-20 proportional system used in EEG labs have been recommended to target sites more accurately.
Stimulus intensity. Each individual’s motor threshold determines stimulus intensity. Using functional MRI studies, researchers from the Medical University of South Carolina concluded that higher stimulation intensity relative to the motor threshold may have a more robust effect, as the magnetic field declines with distance from the coil.7 However, intensities >120% of the motor threshold are generally avoided because of possible increased seizure risk.9
Frequency of stimulation. Most researchers apply frequencies of 1 to 20 Hz over the left dorsolateral prefrontal cortex, but also use lower frequencies (<1 Hz) over the right dorsolateral prefrontal cortex. Using higher frequencies in major depression is attractive in theory because of:
- the reported association of decreased regional cerebral blood flow with hypometabolism in the left dorsolateral prefrontal cortex
- higher-frequency stimulation’s ability to produce temporary excitation and neuronal depolarization.
Number of stimulations. The number of stimulations is determined by frequency (Hz) and stimulation train duration (for example, 10 Hz for 5 seconds equals 50 stimulations). A typical treatment session incorporates 10 to 30 stimulation trains several seconds apart (the inter-train interval). Thus, a typical session delivers 1,000 to 1,200 stimulations. In studies of unmedicated depressed patients, the total number of stimulations has varied from 8,000 to 32,000 per treatment course.
Duration between two stimulation trains. Chen et al have demonstrated that shorter (<1 second) inter-train intervals increase seizure risk with higher frequencies (such as 20 Hz) and intensities (>100% of motor threshold) of stimulation.9 Based on their studies with healthy volunteers, they recommended several “safe” ranges (such as 5 seconds at 110% of motor threshold). Most trials use 30- to 60-second inter-train intervals.
Most treatments continue 2 to 4 weeks, Monday through Friday, although more frequent treatments are being studied.
EFFICACY FOR DEPRESSION
Most studies of rTMS in depression have compared real rTMS to a sham control or electroconvulsive therapy (ECT).
In earlier studies, the sham procedure typically involved tilting the coil away from the skull. This method has been questioned, however, because of evidence of neuronal depolarization.10
More recent sham coils mimic the real coils’ sound and sensation, without magnetic stimulation.
Despite these methodologic problems and some mixed results, depressed patients receiving rTMS show more favorable results than those receiving sham rTMS.11,12 Several meta-analyses have attempted to quantify rTMS’ efficacy for depression:
- Holtzheimer et al concluded that rTMS was statistically superior to sham rTMS, but the clinical significance of these findings was modest in a population of mostly outpatients with less-severe depression.13
- Burt et al found a statistically strong antidepressant effect, but its magnitude varied and few of the studies yielded a substantial clinical response or remission. The team also noted that rTMS’ long-term efficacy or adverse effects are unknown.14
- Kozel et al concluded that left prefrontal rTMS rendered a significant antidepressant effect with measurable clinical improvement.15
- Gershon et al16 supported an antidepressant effect for rTMS when compared with sham rTMS or ECT.
Ongoing rTMS research includes subjects with many types of mild to severe psychiatric illnesses, including major depression, obsessive-compulsive disorder, and psychosis. Typically, patients referred for experimental approaches have not responded to or tolerated available treatments. Exclusion criteria used by most rTMS studies are listed in the Table.
Table
Medical conditions that preclude use of rTMS
| Serious medical conditions History of seizures Increased intracranial pressure Serious head trauma |
| Myocardial infarction within the past 6 months |
| Pregnancy or childbearing potential (unless reliable contraception is being used) |
| Intracranial metallic implants |
| Pacemakers or other implanted devices |
rTMS vs. ECT. Four randomized, controlled trials have compared rTMS with ECT for treating severely ill, often medication-resistant patients.17-20 Although their methodologies differed, all four studies concluded that rTMS and ECT offer similar efficacy, except that rTMS may be less effective for treating psychotic depression.
One study found ECT more effective than rTMS for psychotic depression, although the patients who received ECT were also treated with antipsychotics and/or antidepressants.17 Our study,19 which did not use these agents, has not corroborated this observation. Preliminary data also indicate comparable relapse rates following acute ECT and rTMS when subjects are followed on maintenance medication.21
ADVERSE EFFECTS
The potential adverse effects of new treatments must always be considered. Thus far, rTMS appears to produce minimal, relatively benign complications, including:
- mild discomfort at the stimulation site
- localized muscle twitching during stimulation
- mild post-treatment headaches—believed caused by muscle contractions—which usually respond to aspirin or acetaminophen
- treatment stimulation-related seizures (rarely).8
The rTMS device makes a loud clicking noise, and subjects wear protective ear plugs during treatment.
Patient experience. The first rTMS session—during which the patient’s motor threshold is determined—can last up to 45 minutes. Subsequent sessions are usually 15 to 20 minutes. Patients are typically apprehensive before the first session but become more relaxed with experience and tolerate the treatments easily.
During the procedure, many patients describe a tapping sensation on the forehead, and some experience slight muscle twitching around the eye or corner of the mouth. As the coil warms, the skin it touches sometimes flushes pink, although this does not seem to bother our patients. They can return to their daily routines immediately after a session.
rTMS for major depression. In our experience, rTMS may help patients with major depression. For example, one patient diagnosed with a major depressive episode with psychotic features was referred to our study comparing rTMS with ECT.19 Her depression had lasted several months, with partial response to ECT treatments. She signed informed consent and was randomly assigned to receive rTMS treatment.
At study admission, the patient’s Hamilton Depression Rating Scale (HDRS) score was 48, indicating moderate to severe depression. Following 10 rTMS sessions, her HDRS score had dropped to 2, with remission of symptoms. No follow-up results were documented.
Cognitive effects. Whereas mood disorders are associated with medication-independent neuropsychological deficits, most studies have found no adverse cognitive effects with rTMS.22 Indeed, some of our rTMS patients have improved in certain cognitive tests, although this may be explained by test-retest effects or better attention and concentration associated with mood improvement.
Figure Potential roles for rTMS in treating major depression
Solid lines represent current standards of practice. Dotted lines represent hypothetical roles for rTMS.
Source: Adapted and reprinted with permission from Dowd et al. Is repetitive transcranial magnetic stimulation an alternative to ECTfor the treatment of depression? Contemp Psychiatry 2002;1:1-10.
POTENTIAL ROLE FOR rTMS
Today’s standard treatment of major depressive episodes begins with an antidepressant (plus an antipsychotic, if necessary) and proceeds to augmentation strategies if response is insufficient. rTMS may one day become an augmentation or monotherapy option for patients who do not respond sufficiently to standard treatments (Figure).
ECT treatment may be initiated if a patient has had a prior good response to ECT, is intolerant to medication, or prefers ECT. In that case, rTMS may be used as an alternate initial treatment or with ECT. Thus, rTMS may be used:
- to augment antidepressants
- as an alternative to antidepressants or ECT
- or sequentially with ECT.
Before that can happen, however, optimal treatment parameters need to be clarified by larger, well-designed, controlled studies comparing rTMS to a valid sham treatment, antidepressants, and ECT.
Related resources
- International Society for Transcranial Stimulation. www.ists.unibe.ch/
- Repetitive Transcranial Magnetic Stimulation Research Clinic at Yale-New Haven Psychiatric Hospital.
Disclosure
The authors report that they have no proprietary interest in the technology discussed in this article.
1. Barker A, Jalinous R, Freeston I. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985;1:1106-7.
2. Lisanby SH, Datto CJ, Szuba MP. ECT and rTMS: past, present, and future. Depress Anxiety 2000;12:115-17.
3. Post A, Keck PE, Jr. Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do we know about the neurobiological mechanisms? J Psychiatr Res 2001;35:193-215.
4. Holfich G, Kasper S, Hufnagel A, et al. Application of transcranial magnetic stimulation in treatment of drug resistant major depression—a report of two cases. Human Psychopharmacol 1993;8:361-5.
5. George MS, Nahas Z, Speer AM, et al. Transcranial magnetic stimulation—a new method for investigating the neuroanatomy of depression. In: Ebert D, Ebmeier K (eds). New models for depression. New York: Karger, 1998;94-122.
6. Pridmore A, Americo Fernandes Filho J, Nahas Z, et al. Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. J ECT 1998;14(1):25-7.
7. Kozel FA, Nahas Z, deBrux C, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci 2000;13:376-84.
8. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, 1996. Electroencephalogr Clin Neurophysiol 1998;108:1-16.
9. Chen R, Gerloff C, Classen J, et al. Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol 1997;105:415-21.
10. Loo CK, Taylor JL, Gandevia SC, et al. Transcranial magnetic stimulation in controlled treatment studies: Are some “sham” forms active? Biol Psychiatry. 2000;47:325-31.
11. George MS, Nahas Z, Molloy M, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry 2000;48:962-70.
12. Berman RM, Narasimhan M, Sanacora G, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry 2000;47:332-7.
13. Holtzheimer PE, Russo J, Avery D. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull 2001;35:149-69.
14. Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta-analysis. Int J Neuropsychopharmacol 2002;5:73-103.
15. Kozel FE, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract 2002;8:270-5.
16. Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am JPsychiatry 2003;160(5):835-45.
17. Grunhaus L, Dannon PN, Schreiber S, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry 2000;47:314-24.
18. Pridmore S, Bruno R, Turnier-Shea Y, et al. Comparison of unlimited numbers of rapid transcranial magnetic stimulation and ECT treatment sessions in major depression episodes. Int J Neuropsychopharmacol 2000;3:129-34.
19. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biol Psychiatry 2002;51:659-67
20. Grunhaus L, Schreiber S, Dolberg OT, et al. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry 2003;53:324-31.
21. Dannon PH, Dolberg OT, Schreiber S, Grunhaus L. Three and six month outcome following courses of either ECT or rTMS in a population of severely depressed individuals—preliminary report. Biol Psychiatry 2002;15:687-90.
22. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiology (in press).
1. Barker A, Jalinous R, Freeston I. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985;1:1106-7.
2. Lisanby SH, Datto CJ, Szuba MP. ECT and rTMS: past, present, and future. Depress Anxiety 2000;12:115-17.
3. Post A, Keck PE, Jr. Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do we know about the neurobiological mechanisms? J Psychiatr Res 2001;35:193-215.
4. Holfich G, Kasper S, Hufnagel A, et al. Application of transcranial magnetic stimulation in treatment of drug resistant major depression—a report of two cases. Human Psychopharmacol 1993;8:361-5.
5. George MS, Nahas Z, Speer AM, et al. Transcranial magnetic stimulation—a new method for investigating the neuroanatomy of depression. In: Ebert D, Ebmeier K (eds). New models for depression. New York: Karger, 1998;94-122.
6. Pridmore A, Americo Fernandes Filho J, Nahas Z, et al. Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. J ECT 1998;14(1):25-7.
7. Kozel FA, Nahas Z, deBrux C, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci 2000;13:376-84.
8. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, 1996. Electroencephalogr Clin Neurophysiol 1998;108:1-16.
9. Chen R, Gerloff C, Classen J, et al. Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol 1997;105:415-21.
10. Loo CK, Taylor JL, Gandevia SC, et al. Transcranial magnetic stimulation in controlled treatment studies: Are some “sham” forms active? Biol Psychiatry. 2000;47:325-31.
11. George MS, Nahas Z, Molloy M, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry 2000;48:962-70.
12. Berman RM, Narasimhan M, Sanacora G, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry 2000;47:332-7.
13. Holtzheimer PE, Russo J, Avery D. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacol Bull 2001;35:149-69.
14. Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta-analysis. Int J Neuropsychopharmacol 2002;5:73-103.
15. Kozel FE, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract 2002;8:270-5.
16. Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am JPsychiatry 2003;160(5):835-45.
17. Grunhaus L, Dannon PN, Schreiber S, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry 2000;47:314-24.
18. Pridmore S, Bruno R, Turnier-Shea Y, et al. Comparison of unlimited numbers of rapid transcranial magnetic stimulation and ECT treatment sessions in major depression episodes. Int J Neuropsychopharmacol 2000;3:129-34.
19. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biol Psychiatry 2002;51:659-67
20. Grunhaus L, Schreiber S, Dolberg OT, et al. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry 2003;53:324-31.
21. Dannon PH, Dolberg OT, Schreiber S, Grunhaus L. Three and six month outcome following courses of either ECT or rTMS in a population of severely depressed individuals—preliminary report. Biol Psychiatry 2002;15:687-90.
22. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiology (in press).