User login
Early look: Localized cryolysis reduced sebum by 40%
PHOENIX – One cycle of selective transepidermal cooling on the backs of 11 men with healthy, normal skin produced a transient 40% reduction in sebum production by sebaceous glands in a controlled pilot study.
Sebum production fell significantly by 1 and 2 weeks after treatment, compared with production prior to treatment, but by week 4, was not significantly different than at baseline, Dr. H. Ray Jalian and his associates reported at the annual meeting of the American Society for Laser Medicine and Surgery. Sebum production did not change significantly on untreated patches of skin that served as controls.
The results give hope that selective cryolysis of sebaceous glands might be a noninvasive treatment for acne vulgaris, which should be explored in additional studies, said Dr. Jalian of the Wellman Center for Photomedicine, Massachusetts General Hospital, Boston.
The study won a top award at the meeting and piqued the interest of attendees. Dr. Jeffrey Dover, immediate past president of the ASLMS, explained the excitement over this small pilot study.
"If you extend that to the future, after they do more studies; more cold for longer [time periods], or maybe more applications may produce prolonged sebaceous gland inactivity," he noted. "And maybe [achieve] permanent sebaceous gland inactivity, which theoretically could be a cure for acne – there could be Accutane-like results with an applicator that’s cold, with no side effects," said Dr. Dover, a dermatologist in Chestnut Hill, Mass. "Now, that would be an advance for dermatology," he said in an interview.
Sebumeter measurements at 2 weeks (the primary outcome measure) showed a 40% reduction in sebum production for two temperature settings, compared with baseline.
The study involved the off-label use of a handheld cooling applicator that has been approved for body sculpting to destroy fat in some body areas. Lipid-rich tissue is susceptible to cold injury, and the researchers hoped to target the lipid content of the sebaceous glands.
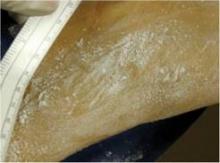
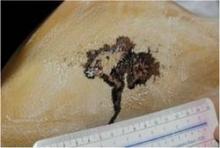
Participants were randomized to treatment on select areas of their backs at temperatures of –10° C or –15° C, and they received either a single 20-minute cooling cycle or two 10-minute cooling cycles with rewarming in between. Some skin areas on the scapulas served as control sites, and each subject had two treatment sites and one control site. Sebum measurements and standardized clinical photos were taken just before treatment and on follow-up visits at 3 days (72 hours) and at 1, 2, and 4 weeks.
Among secondary outcomes, a smaller but significant sebum reduction occurred at 1 week, but sebum production did not differ significantly at 72 hours or at 4 weeks, compared with baseline. Transient pain reported during treatment (average on a Visual Analog Scale score of approximately 3 on a 10-point scale) disappeared quickly post treatment and at follow-up visits. "It actually was a very-well-tolerated procedure," Dr. Jalian said.
Transient post-treatment erythema, dysesthesia, and edema also cleared quickly, though one subject had erythema lasting approximately 72 hours, according to blinded evaluators.
"Perhaps the most interesting part of our study was the histology," Dr. Jalian noted. Biopsies from a subset of participants showed histologic evidence of sebaceous gland damage after treatment that persisted at weeks 1 and 2.
There was no clinical or histologic evidence of damage to tissue outside of the treated areas.
"Selective cryolysis of sebaceous glands is achievable through noninvasive cooling," said Dr. Jalian. Future studies may examine whether repeated treatments could maintain the reduction in sebum output beyond 2 weeks, he added.
Previous studies have shown that a 30%-50% reduction in sebum is needed to produce a 50% improvement in acne (Br. J. Dermatol. 2010;163:683-8).
The human pilot study followed preclinical experiments on the ears of mice, sheep, and pigs that showed histologic damage in sebaceous glands after treatment, Dr. Jalian said.
The study was funded by Zeltiq Aesthetics, which makes the handheld cooling device. Dr. Jalian reported no other relevant disclosures.
On Twitter @sherryboschert
PHOENIX – One cycle of selective transepidermal cooling on the backs of 11 men with healthy, normal skin produced a transient 40% reduction in sebum production by sebaceous glands in a controlled pilot study.
Sebum production fell significantly by 1 and 2 weeks after treatment, compared with production prior to treatment, but by week 4, was not significantly different than at baseline, Dr. H. Ray Jalian and his associates reported at the annual meeting of the American Society for Laser Medicine and Surgery. Sebum production did not change significantly on untreated patches of skin that served as controls.
The results give hope that selective cryolysis of sebaceous glands might be a noninvasive treatment for acne vulgaris, which should be explored in additional studies, said Dr. Jalian of the Wellman Center for Photomedicine, Massachusetts General Hospital, Boston.
The study won a top award at the meeting and piqued the interest of attendees. Dr. Jeffrey Dover, immediate past president of the ASLMS, explained the excitement over this small pilot study.
"If you extend that to the future, after they do more studies; more cold for longer [time periods], or maybe more applications may produce prolonged sebaceous gland inactivity," he noted. "And maybe [achieve] permanent sebaceous gland inactivity, which theoretically could be a cure for acne – there could be Accutane-like results with an applicator that’s cold, with no side effects," said Dr. Dover, a dermatologist in Chestnut Hill, Mass. "Now, that would be an advance for dermatology," he said in an interview.
Sebumeter measurements at 2 weeks (the primary outcome measure) showed a 40% reduction in sebum production for two temperature settings, compared with baseline.
The study involved the off-label use of a handheld cooling applicator that has been approved for body sculpting to destroy fat in some body areas. Lipid-rich tissue is susceptible to cold injury, and the researchers hoped to target the lipid content of the sebaceous glands.
Participants were randomized to treatment on select areas of their backs at temperatures of –10° C or –15° C, and they received either a single 20-minute cooling cycle or two 10-minute cooling cycles with rewarming in between. Some skin areas on the scapulas served as control sites, and each subject had two treatment sites and one control site. Sebum measurements and standardized clinical photos were taken just before treatment and on follow-up visits at 3 days (72 hours) and at 1, 2, and 4 weeks.
Among secondary outcomes, a smaller but significant sebum reduction occurred at 1 week, but sebum production did not differ significantly at 72 hours or at 4 weeks, compared with baseline. Transient pain reported during treatment (average on a Visual Analog Scale score of approximately 3 on a 10-point scale) disappeared quickly post treatment and at follow-up visits. "It actually was a very-well-tolerated procedure," Dr. Jalian said.
Transient post-treatment erythema, dysesthesia, and edema also cleared quickly, though one subject had erythema lasting approximately 72 hours, according to blinded evaluators.
"Perhaps the most interesting part of our study was the histology," Dr. Jalian noted. Biopsies from a subset of participants showed histologic evidence of sebaceous gland damage after treatment that persisted at weeks 1 and 2.
There was no clinical or histologic evidence of damage to tissue outside of the treated areas.
"Selective cryolysis of sebaceous glands is achievable through noninvasive cooling," said Dr. Jalian. Future studies may examine whether repeated treatments could maintain the reduction in sebum output beyond 2 weeks, he added.
Previous studies have shown that a 30%-50% reduction in sebum is needed to produce a 50% improvement in acne (Br. J. Dermatol. 2010;163:683-8).
The human pilot study followed preclinical experiments on the ears of mice, sheep, and pigs that showed histologic damage in sebaceous glands after treatment, Dr. Jalian said.
The study was funded by Zeltiq Aesthetics, which makes the handheld cooling device. Dr. Jalian reported no other relevant disclosures.
On Twitter @sherryboschert
PHOENIX – One cycle of selective transepidermal cooling on the backs of 11 men with healthy, normal skin produced a transient 40% reduction in sebum production by sebaceous glands in a controlled pilot study.
Sebum production fell significantly by 1 and 2 weeks after treatment, compared with production prior to treatment, but by week 4, was not significantly different than at baseline, Dr. H. Ray Jalian and his associates reported at the annual meeting of the American Society for Laser Medicine and Surgery. Sebum production did not change significantly on untreated patches of skin that served as controls.
The results give hope that selective cryolysis of sebaceous glands might be a noninvasive treatment for acne vulgaris, which should be explored in additional studies, said Dr. Jalian of the Wellman Center for Photomedicine, Massachusetts General Hospital, Boston.
The study won a top award at the meeting and piqued the interest of attendees. Dr. Jeffrey Dover, immediate past president of the ASLMS, explained the excitement over this small pilot study.
"If you extend that to the future, after they do more studies; more cold for longer [time periods], or maybe more applications may produce prolonged sebaceous gland inactivity," he noted. "And maybe [achieve] permanent sebaceous gland inactivity, which theoretically could be a cure for acne – there could be Accutane-like results with an applicator that’s cold, with no side effects," said Dr. Dover, a dermatologist in Chestnut Hill, Mass. "Now, that would be an advance for dermatology," he said in an interview.
Sebumeter measurements at 2 weeks (the primary outcome measure) showed a 40% reduction in sebum production for two temperature settings, compared with baseline.
The study involved the off-label use of a handheld cooling applicator that has been approved for body sculpting to destroy fat in some body areas. Lipid-rich tissue is susceptible to cold injury, and the researchers hoped to target the lipid content of the sebaceous glands.
Participants were randomized to treatment on select areas of their backs at temperatures of –10° C or –15° C, and they received either a single 20-minute cooling cycle or two 10-minute cooling cycles with rewarming in between. Some skin areas on the scapulas served as control sites, and each subject had two treatment sites and one control site. Sebum measurements and standardized clinical photos were taken just before treatment and on follow-up visits at 3 days (72 hours) and at 1, 2, and 4 weeks.
Among secondary outcomes, a smaller but significant sebum reduction occurred at 1 week, but sebum production did not differ significantly at 72 hours or at 4 weeks, compared with baseline. Transient pain reported during treatment (average on a Visual Analog Scale score of approximately 3 on a 10-point scale) disappeared quickly post treatment and at follow-up visits. "It actually was a very-well-tolerated procedure," Dr. Jalian said.
Transient post-treatment erythema, dysesthesia, and edema also cleared quickly, though one subject had erythema lasting approximately 72 hours, according to blinded evaluators.
"Perhaps the most interesting part of our study was the histology," Dr. Jalian noted. Biopsies from a subset of participants showed histologic evidence of sebaceous gland damage after treatment that persisted at weeks 1 and 2.
There was no clinical or histologic evidence of damage to tissue outside of the treated areas.
"Selective cryolysis of sebaceous glands is achievable through noninvasive cooling," said Dr. Jalian. Future studies may examine whether repeated treatments could maintain the reduction in sebum output beyond 2 weeks, he added.
Previous studies have shown that a 30%-50% reduction in sebum is needed to produce a 50% improvement in acne (Br. J. Dermatol. 2010;163:683-8).
The human pilot study followed preclinical experiments on the ears of mice, sheep, and pigs that showed histologic damage in sebaceous glands after treatment, Dr. Jalian said.
The study was funded by Zeltiq Aesthetics, which makes the handheld cooling device. Dr. Jalian reported no other relevant disclosures.
On Twitter @sherryboschert
AT LASER 2014
Key clinical point: Cryolysis of the sebaceous glands might provide a noninvasive option for acne treatment.
Major finding: Sebum excretion was 40% lower 2 weeks after transepidermal cryolysis treatment of sebaceous glands, compared with baseline.
Data source: A prospective, controlled pilot study of 11 healthy men who were treated on selected areas on their backs.
Disclosures: The study was funded by Zeltiq Aesthetics, which makes the handheld cooling device used in the study. Dr. Jalian reported no other relevant disclosures.
Three laser/light devices lead injury reports
PHOENIX – Radiofrequency devices, diode lasers, and intense pulsed light devices had the most reports of suspected injuries from 1991 through 2013, based on data from a Food and Drug Administration database.
During that period, 1,212 medical device reports were submitted for suspected injuries from laser or light device use or malfunction to the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database, as well as another 45 that were excluded from the study because of insufficient data. The FDA requires device users and manufacturers to submit the reports and collects voluntary reports from patients. Each report may contain more than one adverse event.
Blisters or burns were the most common problems reported to the MAUDE database for each of the top three devices, Dr. Anne Marie Tremaine and her associates reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Radiofrequency devices were the subject of 270 reports that included 294 adverse events. These reports included 119 burns or blisters; 85 incidents of fat loss, depression, or divot; 34 scars; 25 incidents of dyschromia (both hyper- and hypopigmentation); and 12 cases of sagging skin. In addition, a few reports noted burns in the area of the return pad, paresthesia or nerve palsy, dry eye or "floaters," and cracked dental crowns.
Insufficient coupling fluid and tearing or breakdown in the device tip membrane topped the reasons given for problems related to radiofrequency treatment, reported Dr. Tremaine of Massachusetts General Hospital’s Wellman Center for Photomedicine, Boston. Complications also were ascribed to tumescent anesthesia, intradermal lidocaine injections, and general anesthesia.
The most common treatment modality associated with adverse events was laser hair removal using diode lasers or intense pulsed light. The 252 reports on diode lasers included 297 burns or blisters, 42 incidents of dyschromia, 11 scars, and 8 cases of swelling. The 158 reports on adverse events from intense pulsed light included 126 burns or blisters; 24 incidents of dyschromia; 20 scars; 13 patients with bruising; 12 incidents of fat loss, depression, or divot; and smaller numbers of eye injury or delayed wound healing.
Many of the complications in the reports were attributed to improper settings or improperly maintained device tips.
"Physicians should be aware of the adverse events and malfunctions of the devices used in dermatology," Dr. Tremaine said. The MAUDE database "will help uncover otherwise unreported events seen in routine practice among all types of practitioners" to supplement data from initial studies and reports in the medical literature. This information should help physicians better inform patients about the potential risks of treatment, she said.
Dr. Tremaine and her associates searched the MAUDE database using terms including names of device manufacturers from a comprehensive list, specific product names, and the wavelengths and technology of the devices used in dermatology.
However, because adverse events are underreported to MAUDE, which provides only passive surveillance, the incidence or prevalence of problems with laser or light devices could not be discerned from this database, the investigators noted.
Dr. Tremaine reported having no disclosures.
On Twitter @sherryboschert
PHOENIX – Radiofrequency devices, diode lasers, and intense pulsed light devices had the most reports of suspected injuries from 1991 through 2013, based on data from a Food and Drug Administration database.
During that period, 1,212 medical device reports were submitted for suspected injuries from laser or light device use or malfunction to the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database, as well as another 45 that were excluded from the study because of insufficient data. The FDA requires device users and manufacturers to submit the reports and collects voluntary reports from patients. Each report may contain more than one adverse event.
Blisters or burns were the most common problems reported to the MAUDE database for each of the top three devices, Dr. Anne Marie Tremaine and her associates reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Radiofrequency devices were the subject of 270 reports that included 294 adverse events. These reports included 119 burns or blisters; 85 incidents of fat loss, depression, or divot; 34 scars; 25 incidents of dyschromia (both hyper- and hypopigmentation); and 12 cases of sagging skin. In addition, a few reports noted burns in the area of the return pad, paresthesia or nerve palsy, dry eye or "floaters," and cracked dental crowns.
Insufficient coupling fluid and tearing or breakdown in the device tip membrane topped the reasons given for problems related to radiofrequency treatment, reported Dr. Tremaine of Massachusetts General Hospital’s Wellman Center for Photomedicine, Boston. Complications also were ascribed to tumescent anesthesia, intradermal lidocaine injections, and general anesthesia.
The most common treatment modality associated with adverse events was laser hair removal using diode lasers or intense pulsed light. The 252 reports on diode lasers included 297 burns or blisters, 42 incidents of dyschromia, 11 scars, and 8 cases of swelling. The 158 reports on adverse events from intense pulsed light included 126 burns or blisters; 24 incidents of dyschromia; 20 scars; 13 patients with bruising; 12 incidents of fat loss, depression, or divot; and smaller numbers of eye injury or delayed wound healing.
Many of the complications in the reports were attributed to improper settings or improperly maintained device tips.
"Physicians should be aware of the adverse events and malfunctions of the devices used in dermatology," Dr. Tremaine said. The MAUDE database "will help uncover otherwise unreported events seen in routine practice among all types of practitioners" to supplement data from initial studies and reports in the medical literature. This information should help physicians better inform patients about the potential risks of treatment, she said.
Dr. Tremaine and her associates searched the MAUDE database using terms including names of device manufacturers from a comprehensive list, specific product names, and the wavelengths and technology of the devices used in dermatology.
However, because adverse events are underreported to MAUDE, which provides only passive surveillance, the incidence or prevalence of problems with laser or light devices could not be discerned from this database, the investigators noted.
Dr. Tremaine reported having no disclosures.
On Twitter @sherryboschert
PHOENIX – Radiofrequency devices, diode lasers, and intense pulsed light devices had the most reports of suspected injuries from 1991 through 2013, based on data from a Food and Drug Administration database.
During that period, 1,212 medical device reports were submitted for suspected injuries from laser or light device use or malfunction to the FDA’s MAUDE (Manufacturer and User Facility Device Experience) database, as well as another 45 that were excluded from the study because of insufficient data. The FDA requires device users and manufacturers to submit the reports and collects voluntary reports from patients. Each report may contain more than one adverse event.
Blisters or burns were the most common problems reported to the MAUDE database for each of the top three devices, Dr. Anne Marie Tremaine and her associates reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Radiofrequency devices were the subject of 270 reports that included 294 adverse events. These reports included 119 burns or blisters; 85 incidents of fat loss, depression, or divot; 34 scars; 25 incidents of dyschromia (both hyper- and hypopigmentation); and 12 cases of sagging skin. In addition, a few reports noted burns in the area of the return pad, paresthesia or nerve palsy, dry eye or "floaters," and cracked dental crowns.
Insufficient coupling fluid and tearing or breakdown in the device tip membrane topped the reasons given for problems related to radiofrequency treatment, reported Dr. Tremaine of Massachusetts General Hospital’s Wellman Center for Photomedicine, Boston. Complications also were ascribed to tumescent anesthesia, intradermal lidocaine injections, and general anesthesia.
The most common treatment modality associated with adverse events was laser hair removal using diode lasers or intense pulsed light. The 252 reports on diode lasers included 297 burns or blisters, 42 incidents of dyschromia, 11 scars, and 8 cases of swelling. The 158 reports on adverse events from intense pulsed light included 126 burns or blisters; 24 incidents of dyschromia; 20 scars; 13 patients with bruising; 12 incidents of fat loss, depression, or divot; and smaller numbers of eye injury or delayed wound healing.
Many of the complications in the reports were attributed to improper settings or improperly maintained device tips.
"Physicians should be aware of the adverse events and malfunctions of the devices used in dermatology," Dr. Tremaine said. The MAUDE database "will help uncover otherwise unreported events seen in routine practice among all types of practitioners" to supplement data from initial studies and reports in the medical literature. This information should help physicians better inform patients about the potential risks of treatment, she said.
Dr. Tremaine and her associates searched the MAUDE database using terms including names of device manufacturers from a comprehensive list, specific product names, and the wavelengths and technology of the devices used in dermatology.
However, because adverse events are underreported to MAUDE, which provides only passive surveillance, the incidence or prevalence of problems with laser or light devices could not be discerned from this database, the investigators noted.
Dr. Tremaine reported having no disclosures.
On Twitter @sherryboschert
AT LASER 2014
Major finding: Radiofrequency devices had the highest number of adverse-event reports (270), followed by diode lasers (252) and intense pulsed light devices (158).
Data source: A study of 1,212 medical device reports for laser or light devices in the FDA’s MAUDE database from 1991 through 2013.
Disclosures: Dr. Tremaine reported having no disclosures.
Laser therapy improved severe hyperhidrosis
PHOENIX – Laser treatment of severe hyperhidrosis significantly reduced underarm sweating after 6 months in two separate but similar studies of 20 and 13 patients.
Patients with scores of 3 or 4 on the 4-point Hyperhidrosis Disease Severity Scale (HDSS) underwent a single treatment to acutely damage eccrine glands using a 1,440-nm Nd:YAG laser with a new 800-mcm side-firing fiber designed to deliver targeted energy. Patients received tumescent anesthesia in each axilla.
Sixteen of 20 patients in one study (78%) reported at least a 2-point improvement in HDSS scores at a 6-month follow-up visit, and 17 (83%) reported at least a 1-point improvement at 1 year, compared with baseline, Dr. Bruce Katz and his associates reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Average HDSS scores were 3.6 before treatment, 2.3 at 3 months, 1.7 at 6 months, and 2.2 at 1 year, said Dr. Katz, a clinical professor of dermatology at Mount Sinai Hospital, New York.
The side-firing fiber inserted through a 150-mm handpiece and set at 10-15 W delivered 1,200-1,500 J to each of four 5 cm2 × 5 cm2 areas in each armpit. Both eccrine and apocrine glands were reduced based on post-treatment histological studies. Side effects included edema, bruising, and numbness, but these conditions resolved in 1-2 weeks.
Before the study, 13 patients (65%) had an HDSS score of 4. Five patients had tried treatment with botulinum toxin type A before the study and found it effective, with results lasting an average of 6 months, but they had discontinued that therapy because of the high cost, Dr. Katz said.
In a second study of 13 patients, HDSS scores improved by a mean of 1.3 at the 6-month follow-up visit, Dr. Alina Fratila and her associates reported in a separate presentation at the meeting.
Gravimetric measurements (in milligrams per minute) showed a mean 78% reduction in sweating at 6 months, said Dr. Fratila of the Fountain of Youth Clinic in Bonn, Germany.
Physicians rated the improvement in sweat as 71%, and patients rated it as 55%. The laser in this study was set at 7.5 W, delivering an average of 6,000 J per axilla (or 1,500 J per 5 cm2 × 5 cm2 quadrant).
Ten patients had tried a prescription antiperspirant prior to participating in the study, but they stopped because they found it either irritating or ineffective. Four patients had tried botulinum toxin therapy but stopped because of the cost, Dr. Fratila said.
The laser treatment has a low risk of side effects and causes essentially no downtime, Dr. Fratila said. She added that she plans to follow the patients to assess results after 1 and 2 years.
An estimated 3% of the U.S. population has primary focal hyperhidrosis, comprising nearly 8 million people, Dr. Katz said.
Other treatment options for primary focal hyperhidrosis include topical over-the-counter antiperspirants, aluminum chloride 10%-35% topical antiperspirant, intradermal injections of botulinum toxin type A, and surgical resection of local sweat glands.
Dr. Katz reported financial associations with Cynosure, which makes the laser systems used in his study, and with Allergan, Alma, and other companies. Dr. Fratila reported having no disclosures.
On Twitter @sherryboschert
PHOENIX – Laser treatment of severe hyperhidrosis significantly reduced underarm sweating after 6 months in two separate but similar studies of 20 and 13 patients.
Patients with scores of 3 or 4 on the 4-point Hyperhidrosis Disease Severity Scale (HDSS) underwent a single treatment to acutely damage eccrine glands using a 1,440-nm Nd:YAG laser with a new 800-mcm side-firing fiber designed to deliver targeted energy. Patients received tumescent anesthesia in each axilla.
Sixteen of 20 patients in one study (78%) reported at least a 2-point improvement in HDSS scores at a 6-month follow-up visit, and 17 (83%) reported at least a 1-point improvement at 1 year, compared with baseline, Dr. Bruce Katz and his associates reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Average HDSS scores were 3.6 before treatment, 2.3 at 3 months, 1.7 at 6 months, and 2.2 at 1 year, said Dr. Katz, a clinical professor of dermatology at Mount Sinai Hospital, New York.
The side-firing fiber inserted through a 150-mm handpiece and set at 10-15 W delivered 1,200-1,500 J to each of four 5 cm2 × 5 cm2 areas in each armpit. Both eccrine and apocrine glands were reduced based on post-treatment histological studies. Side effects included edema, bruising, and numbness, but these conditions resolved in 1-2 weeks.
Before the study, 13 patients (65%) had an HDSS score of 4. Five patients had tried treatment with botulinum toxin type A before the study and found it effective, with results lasting an average of 6 months, but they had discontinued that therapy because of the high cost, Dr. Katz said.
In a second study of 13 patients, HDSS scores improved by a mean of 1.3 at the 6-month follow-up visit, Dr. Alina Fratila and her associates reported in a separate presentation at the meeting.
Gravimetric measurements (in milligrams per minute) showed a mean 78% reduction in sweating at 6 months, said Dr. Fratila of the Fountain of Youth Clinic in Bonn, Germany.
Physicians rated the improvement in sweat as 71%, and patients rated it as 55%. The laser in this study was set at 7.5 W, delivering an average of 6,000 J per axilla (or 1,500 J per 5 cm2 × 5 cm2 quadrant).
Ten patients had tried a prescription antiperspirant prior to participating in the study, but they stopped because they found it either irritating or ineffective. Four patients had tried botulinum toxin therapy but stopped because of the cost, Dr. Fratila said.
The laser treatment has a low risk of side effects and causes essentially no downtime, Dr. Fratila said. She added that she plans to follow the patients to assess results after 1 and 2 years.
An estimated 3% of the U.S. population has primary focal hyperhidrosis, comprising nearly 8 million people, Dr. Katz said.
Other treatment options for primary focal hyperhidrosis include topical over-the-counter antiperspirants, aluminum chloride 10%-35% topical antiperspirant, intradermal injections of botulinum toxin type A, and surgical resection of local sweat glands.
Dr. Katz reported financial associations with Cynosure, which makes the laser systems used in his study, and with Allergan, Alma, and other companies. Dr. Fratila reported having no disclosures.
On Twitter @sherryboschert
PHOENIX – Laser treatment of severe hyperhidrosis significantly reduced underarm sweating after 6 months in two separate but similar studies of 20 and 13 patients.
Patients with scores of 3 or 4 on the 4-point Hyperhidrosis Disease Severity Scale (HDSS) underwent a single treatment to acutely damage eccrine glands using a 1,440-nm Nd:YAG laser with a new 800-mcm side-firing fiber designed to deliver targeted energy. Patients received tumescent anesthesia in each axilla.
Sixteen of 20 patients in one study (78%) reported at least a 2-point improvement in HDSS scores at a 6-month follow-up visit, and 17 (83%) reported at least a 1-point improvement at 1 year, compared with baseline, Dr. Bruce Katz and his associates reported at the annual meeting of the American Society for Laser Medicine and Surgery.
Average HDSS scores were 3.6 before treatment, 2.3 at 3 months, 1.7 at 6 months, and 2.2 at 1 year, said Dr. Katz, a clinical professor of dermatology at Mount Sinai Hospital, New York.
The side-firing fiber inserted through a 150-mm handpiece and set at 10-15 W delivered 1,200-1,500 J to each of four 5 cm2 × 5 cm2 areas in each armpit. Both eccrine and apocrine glands were reduced based on post-treatment histological studies. Side effects included edema, bruising, and numbness, but these conditions resolved in 1-2 weeks.
Before the study, 13 patients (65%) had an HDSS score of 4. Five patients had tried treatment with botulinum toxin type A before the study and found it effective, with results lasting an average of 6 months, but they had discontinued that therapy because of the high cost, Dr. Katz said.
In a second study of 13 patients, HDSS scores improved by a mean of 1.3 at the 6-month follow-up visit, Dr. Alina Fratila and her associates reported in a separate presentation at the meeting.
Gravimetric measurements (in milligrams per minute) showed a mean 78% reduction in sweating at 6 months, said Dr. Fratila of the Fountain of Youth Clinic in Bonn, Germany.
Physicians rated the improvement in sweat as 71%, and patients rated it as 55%. The laser in this study was set at 7.5 W, delivering an average of 6,000 J per axilla (or 1,500 J per 5 cm2 × 5 cm2 quadrant).
Ten patients had tried a prescription antiperspirant prior to participating in the study, but they stopped because they found it either irritating or ineffective. Four patients had tried botulinum toxin therapy but stopped because of the cost, Dr. Fratila said.
The laser treatment has a low risk of side effects and causes essentially no downtime, Dr. Fratila said. She added that she plans to follow the patients to assess results after 1 and 2 years.
An estimated 3% of the U.S. population has primary focal hyperhidrosis, comprising nearly 8 million people, Dr. Katz said.
Other treatment options for primary focal hyperhidrosis include topical over-the-counter antiperspirants, aluminum chloride 10%-35% topical antiperspirant, intradermal injections of botulinum toxin type A, and surgical resection of local sweat glands.
Dr. Katz reported financial associations with Cynosure, which makes the laser systems used in his study, and with Allergan, Alma, and other companies. Dr. Fratila reported having no disclosures.
On Twitter @sherryboschert
AT LASER 2014
Key clinical point: Patients with severe hyperhidrosis who underwent laser treatment had minimal, short-term side effects and essentially no downtime.
Major finding: HDSS scores of underarm sweating 6 months after laser treatment improved by at least 2 points in one study and by a mean of 1.3 points in another study.
Data source: Separate single-center, uncontrolled trials of 20 and 13 patients, respectively, with severe primary focal hyperhidrosis.
Disclosures: Dr. Katz reported financial associations with Cynosure, which makes the laser systems used in the study, and with Allergan, Alma, and other companies. Dr. Fratila reported having no disclosures.
Evidence-Based Apps: Mental health apps promising but data limited
It seems the glass is half-full and half-empty when it comes to mental health apps. What data there are suggest that apps to treat or prevent anxiety, depression, stress, or substance abuse can work, but the paucity of good research makes it difficult to know which ones to recommend.
A recent randomized trial in 78 highly trait-anxious (but not diagnosed) adults found that a single session of using an experimental game app for attention-bias modification training (ABMT) significantly reduced acute stress responses and improved mood, compared with those of a control group (Clin. Psychol. Sci. 2014 March 6 [doi:10.1177/2167702614522228]). The work is preliminary but should not be taken for granted. A separate review of the literature on more than 3,000 mental health apps available to consumers found evidence for efficacy for only five apps in eight trials of less-than-desirable-quality that at least included a control group or pretest and posttest assessments (J. Med. Internet Res. 2013;15:e247).
The gamified ABMT app, which uses features such as animated characters, sound effects, and awarding of points to make it more of a game, recruited adult university students who scored high on anxiety assessments and randomized them to either a shorter training session on the app or a placebo app (25 minutes of game play with 20 minutes of rest breaks) or a longer training session on the app or a control (45 minutes of game play with brief breaks given as needed).
Validated assessments immediately before and after showed generally positive results, but with some surprises, reported Tracy A. Dennis, Ph.D., who is professor of psychology at Hunter College in New York City, and Laura J. O’Toole of the City University of New York.
ABMT seeks to reduce anxiety by reducing a person’s exaggerated attention to threats (also known as "threat bias"). For example, the app may show two faces – one menacing and the other neutral or pleasant – and use mechanisms such as game maneuvers or points to reinforce attention away from the threatening face (or "threat prompt"). Only the long ABMT session produced significantly less biased attention to threats in the app. Subjects in the long ABMT group also showed less difficulty disengaging from threat (compared with those in either the long control group or the short ABMT), which was associated with less negative mood and less nervous speech.
Unexpectedly, participants in the short ABMT session had greater difficulty disengaging from threat after the session, compared with controls, Dr. Dennis and Ms. O’Toole found. The short ABMT session, however, significantly reduced participants’ subjective reports of anxiety, compared with those by control groups or the long ABMT group.
Several previous studies of modifying ABMT for use on mobile devices or via the Internet reported mixed results and didn’t assess participants’ willingness to keep using the app, the investigators noted. They hope that turning it into a video game–like app may make it easy, inexpensive, and appealing for youths and young adults to use without stigma. Future studies should look at more sophisticated versions of the app used by clinically diagnosed populations in out-of-laboratory settings for different amounts of time, the investigators suggested.
In an earlier review of the literature on mental health apps, Tara Donker, Ph.D., and her associates reviewed 4,997 abstracts and excluded studies that targeted a medical disorder, that didn’t include outcomes data, or that had to be downloaded first to a computer before transfer to a mobile device. The remaining eight studies included 227 participants, reported Dr. Donker of the University of New South Wales, Sydney, Australia, and her associates.
Four trials focused on three apps assessing depression (Mobilyze!, mobiletype, and Get Happy Program), three trials focused on the Mobile Stress Management app that measured stress as a primary outcome, and one trial focused on the DBT Coach app for substance use. None of these appears to be commercially available in the United States.
The within-group effects sizes for the apps ranged from 0.29 to 2.28, and the between-group effects sizes ranged from 0.01 to 0.48 using validated mental health scales in intent-to-treat analyses. These are promising results suggesting that mobile health apps can help reduce depressive symptoms and "caseness," stress, anxiety, and substance use, but the overall mediocre quality of the studies means the findings need to be replicated, the investigators said.
Users gave the apps moderate to high ratings for helpfulness, usability, and satisfaction. Adherence rates (in the studies that reported them) were high, and higher than seen previously with Internet-based interventions. Technical problems such as battery failure, connectivity problems, or the app freezing were the main reason app use was interrupted.
Dr. Dennis and Dr. Donker reported having no financial disclosures. Dr. Dennis’s study was funded by the U.S. National Institute of Mental Health.
On Twitter @sherryboschert
It seems the glass is half-full and half-empty when it comes to mental health apps. What data there are suggest that apps to treat or prevent anxiety, depression, stress, or substance abuse can work, but the paucity of good research makes it difficult to know which ones to recommend.
A recent randomized trial in 78 highly trait-anxious (but not diagnosed) adults found that a single session of using an experimental game app for attention-bias modification training (ABMT) significantly reduced acute stress responses and improved mood, compared with those of a control group (Clin. Psychol. Sci. 2014 March 6 [doi:10.1177/2167702614522228]). The work is preliminary but should not be taken for granted. A separate review of the literature on more than 3,000 mental health apps available to consumers found evidence for efficacy for only five apps in eight trials of less-than-desirable-quality that at least included a control group or pretest and posttest assessments (J. Med. Internet Res. 2013;15:e247).
The gamified ABMT app, which uses features such as animated characters, sound effects, and awarding of points to make it more of a game, recruited adult university students who scored high on anxiety assessments and randomized them to either a shorter training session on the app or a placebo app (25 minutes of game play with 20 minutes of rest breaks) or a longer training session on the app or a control (45 minutes of game play with brief breaks given as needed).
Validated assessments immediately before and after showed generally positive results, but with some surprises, reported Tracy A. Dennis, Ph.D., who is professor of psychology at Hunter College in New York City, and Laura J. O’Toole of the City University of New York.
ABMT seeks to reduce anxiety by reducing a person’s exaggerated attention to threats (also known as "threat bias"). For example, the app may show two faces – one menacing and the other neutral or pleasant – and use mechanisms such as game maneuvers or points to reinforce attention away from the threatening face (or "threat prompt"). Only the long ABMT session produced significantly less biased attention to threats in the app. Subjects in the long ABMT group also showed less difficulty disengaging from threat (compared with those in either the long control group or the short ABMT), which was associated with less negative mood and less nervous speech.
Unexpectedly, participants in the short ABMT session had greater difficulty disengaging from threat after the session, compared with controls, Dr. Dennis and Ms. O’Toole found. The short ABMT session, however, significantly reduced participants’ subjective reports of anxiety, compared with those by control groups or the long ABMT group.
Several previous studies of modifying ABMT for use on mobile devices or via the Internet reported mixed results and didn’t assess participants’ willingness to keep using the app, the investigators noted. They hope that turning it into a video game–like app may make it easy, inexpensive, and appealing for youths and young adults to use without stigma. Future studies should look at more sophisticated versions of the app used by clinically diagnosed populations in out-of-laboratory settings for different amounts of time, the investigators suggested.
In an earlier review of the literature on mental health apps, Tara Donker, Ph.D., and her associates reviewed 4,997 abstracts and excluded studies that targeted a medical disorder, that didn’t include outcomes data, or that had to be downloaded first to a computer before transfer to a mobile device. The remaining eight studies included 227 participants, reported Dr. Donker of the University of New South Wales, Sydney, Australia, and her associates.
Four trials focused on three apps assessing depression (Mobilyze!, mobiletype, and Get Happy Program), three trials focused on the Mobile Stress Management app that measured stress as a primary outcome, and one trial focused on the DBT Coach app for substance use. None of these appears to be commercially available in the United States.
The within-group effects sizes for the apps ranged from 0.29 to 2.28, and the between-group effects sizes ranged from 0.01 to 0.48 using validated mental health scales in intent-to-treat analyses. These are promising results suggesting that mobile health apps can help reduce depressive symptoms and "caseness," stress, anxiety, and substance use, but the overall mediocre quality of the studies means the findings need to be replicated, the investigators said.
Users gave the apps moderate to high ratings for helpfulness, usability, and satisfaction. Adherence rates (in the studies that reported them) were high, and higher than seen previously with Internet-based interventions. Technical problems such as battery failure, connectivity problems, or the app freezing were the main reason app use was interrupted.
Dr. Dennis and Dr. Donker reported having no financial disclosures. Dr. Dennis’s study was funded by the U.S. National Institute of Mental Health.
On Twitter @sherryboschert
It seems the glass is half-full and half-empty when it comes to mental health apps. What data there are suggest that apps to treat or prevent anxiety, depression, stress, or substance abuse can work, but the paucity of good research makes it difficult to know which ones to recommend.
A recent randomized trial in 78 highly trait-anxious (but not diagnosed) adults found that a single session of using an experimental game app for attention-bias modification training (ABMT) significantly reduced acute stress responses and improved mood, compared with those of a control group (Clin. Psychol. Sci. 2014 March 6 [doi:10.1177/2167702614522228]). The work is preliminary but should not be taken for granted. A separate review of the literature on more than 3,000 mental health apps available to consumers found evidence for efficacy for only five apps in eight trials of less-than-desirable-quality that at least included a control group or pretest and posttest assessments (J. Med. Internet Res. 2013;15:e247).
The gamified ABMT app, which uses features such as animated characters, sound effects, and awarding of points to make it more of a game, recruited adult university students who scored high on anxiety assessments and randomized them to either a shorter training session on the app or a placebo app (25 minutes of game play with 20 minutes of rest breaks) or a longer training session on the app or a control (45 minutes of game play with brief breaks given as needed).
Validated assessments immediately before and after showed generally positive results, but with some surprises, reported Tracy A. Dennis, Ph.D., who is professor of psychology at Hunter College in New York City, and Laura J. O’Toole of the City University of New York.
ABMT seeks to reduce anxiety by reducing a person’s exaggerated attention to threats (also known as "threat bias"). For example, the app may show two faces – one menacing and the other neutral or pleasant – and use mechanisms such as game maneuvers or points to reinforce attention away from the threatening face (or "threat prompt"). Only the long ABMT session produced significantly less biased attention to threats in the app. Subjects in the long ABMT group also showed less difficulty disengaging from threat (compared with those in either the long control group or the short ABMT), which was associated with less negative mood and less nervous speech.
Unexpectedly, participants in the short ABMT session had greater difficulty disengaging from threat after the session, compared with controls, Dr. Dennis and Ms. O’Toole found. The short ABMT session, however, significantly reduced participants’ subjective reports of anxiety, compared with those by control groups or the long ABMT group.
Several previous studies of modifying ABMT for use on mobile devices or via the Internet reported mixed results and didn’t assess participants’ willingness to keep using the app, the investigators noted. They hope that turning it into a video game–like app may make it easy, inexpensive, and appealing for youths and young adults to use without stigma. Future studies should look at more sophisticated versions of the app used by clinically diagnosed populations in out-of-laboratory settings for different amounts of time, the investigators suggested.
In an earlier review of the literature on mental health apps, Tara Donker, Ph.D., and her associates reviewed 4,997 abstracts and excluded studies that targeted a medical disorder, that didn’t include outcomes data, or that had to be downloaded first to a computer before transfer to a mobile device. The remaining eight studies included 227 participants, reported Dr. Donker of the University of New South Wales, Sydney, Australia, and her associates.
Four trials focused on three apps assessing depression (Mobilyze!, mobiletype, and Get Happy Program), three trials focused on the Mobile Stress Management app that measured stress as a primary outcome, and one trial focused on the DBT Coach app for substance use. None of these appears to be commercially available in the United States.
The within-group effects sizes for the apps ranged from 0.29 to 2.28, and the between-group effects sizes ranged from 0.01 to 0.48 using validated mental health scales in intent-to-treat analyses. These are promising results suggesting that mobile health apps can help reduce depressive symptoms and "caseness," stress, anxiety, and substance use, but the overall mediocre quality of the studies means the findings need to be replicated, the investigators said.
Users gave the apps moderate to high ratings for helpfulness, usability, and satisfaction. Adherence rates (in the studies that reported them) were high, and higher than seen previously with Internet-based interventions. Technical problems such as battery failure, connectivity problems, or the app freezing were the main reason app use was interrupted.
Dr. Dennis and Dr. Donker reported having no financial disclosures. Dr. Dennis’s study was funded by the U.S. National Institute of Mental Health.
On Twitter @sherryboschert
VIDEO: Laser's novel effect improves restrictive burn scars
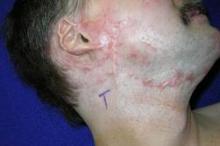
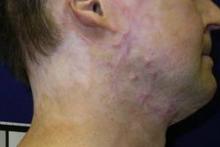
PHOENIX – New techniques using very-low-density, very-high-energy ablative fractional carbon dioxide laser are helping thousands of patients – mostly soldiers – with restrictive scars from bombs or burns.
It’s a treatment to which the far larger number of civilian burn patients should have access, but few do, explained Dr. Nathan S. Uebelhoer, who received an award and a standing ovation at the annual meeting of the American Society for Laser Medicine and Surgery for his work in this area.
In an interview, Dr. Uebelhoer of Aroostook Medical Center, Presque Isle, Maine, describes the techniques used to achieve such promising results with restrictive scars, and he discusses what might be necessary to make the treatment more widely available.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
PHOENIX – New techniques using very-low-density, very-high-energy ablative fractional carbon dioxide laser are helping thousands of patients – mostly soldiers – with restrictive scars from bombs or burns.
It’s a treatment to which the far larger number of civilian burn patients should have access, but few do, explained Dr. Nathan S. Uebelhoer, who received an award and a standing ovation at the annual meeting of the American Society for Laser Medicine and Surgery for his work in this area.
In an interview, Dr. Uebelhoer of Aroostook Medical Center, Presque Isle, Maine, describes the techniques used to achieve such promising results with restrictive scars, and he discusses what might be necessary to make the treatment more widely available.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
PHOENIX – New techniques using very-low-density, very-high-energy ablative fractional carbon dioxide laser are helping thousands of patients – mostly soldiers – with restrictive scars from bombs or burns.
It’s a treatment to which the far larger number of civilian burn patients should have access, but few do, explained Dr. Nathan S. Uebelhoer, who received an award and a standing ovation at the annual meeting of the American Society for Laser Medicine and Surgery for his work in this area.
In an interview, Dr. Uebelhoer of Aroostook Medical Center, Presque Isle, Maine, describes the techniques used to achieve such promising results with restrictive scars, and he discusses what might be necessary to make the treatment more widely available.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
AT LASER 2014
Evidence-Based Apps: Skin-patch device influencing ambulatory cardiac monitoring
An easy-to-use skin-patch device detected 96 arrhythmia events during 2 weeks of use, compared with 61 events detected using 24-hour Holter monitoring, in a study of 146 patients referred for evaluation of cardiac arrhythmias.
Patients referred to the cardiac investigations laboratory at Scripps Green Hospital, La Jolla, Calif., between April and July 2012 were fitted simultaneously with a 24-hour Holter monitor and a Zio Patch, which is a lightweight, single-use electrocardiographic (ECG) device that was attached over the left pectoral region of the patient’s chest by an adhesive patch. Patients wore the patch for a median of 11 days, but it can be worn for as long as 14 days.
Among 102 physicians surveyed in the study, 90% said they thought that the data from the patch provided a definitive diagnosis, compared with 64% who thought the Holter data allowed a definitive diagnosis, Dr. Paddy M. Barrett and his associates reported (Am. J. Med. 2014;127:95.e11-95.e17).
Patients showed a clear preference for the water-resistant patch, saying that it affected just 10% of their activities of daily living, compared with 76% of activities affected by the Holter monitor. The patch was comfortable to wear, according to 94%, and 52% said the Holter monitor was comfortable. Asked to choose between the two, 81% of patients said that they preferred the patch.
Researchers applied the patch, but, outside of the study, it sometimes is mailed directly to a patient for self-application. Patients can press a button on it to indicate when they feel symptoms. At the end of the monitoring period, the patch is mailed to an iRhythm facility for data analysis, from which a digital report is sent to the ordering physician. The Zio Patch is approved by the Food and Drug Administration.
During the first 24 hours when both devices were worn in the study, the Holter monitor detected 11 arrhythmic events not detected by the patch, but these and more were found during the extended use of the patch. A review of those 11 events led to an adjustment in the device’s algorithm and extra training for the staff who review the data and produce the reports for clinicians, according to Dr. Barrett, who led the study while he was a fellow at Scripps Translational Science Institute, La Jolla, and now is a cardiology fellow at St. Vincent’s University College Hospital, Dublin.
Six arrhythmias were included in the definition of arrhythmic events: supraventricular tachycardia; atrial fibrillation/flutter; a pause greater than 3 seconds; atrioventricular block; ventricular tachycardia; or polymorphic ventricular tachycardia/ventricular fibrillation.
In an interview, Dr. Barrett expressed particular interest in the device’s ability to detect asymptomatic atrial fibrillation that commonly is missed by 24-hour Holter monitoring. "The detection of more [events] allows risk stratification and implementation of treatment strategies," he said. The investigators have no plans to conduct a long-term study to see if the incremental diagnoses obtained by the patch ultimately improve health outcomes.
Dr. Gregory Engel, a cardiologist and electrophysiologist at Silicon Valley Cardiology who is not associated with the study or with the device maker, said the medical literature suggests that finding and treating "silent" atrial fibrillation will reduce mortality and morbidity. He uses the Zio Patch system in his 10-person private group practice at three offices in northern California and believes the patch will replace most, but not all, Holter monitor use.
"We own our Holter, so if I just need 24 hours of monitoring" in a patient whose arrhythmia already is known, "I might as well use the product I’ve already paid for," he said in an interview. "From a pure information and diagnostic perspective," however, the Zio Patch is "far superior to the Holter."
The Zio also would not be his first choice for cases in which he wants monitoring that provides instant feedback. Mobile cardiac telemetry or other instant-feedback systems may be appropriate for some patients.
In a separate retrospective study of data from 1,171 Zio Patch monitoring reports on patients who’d had a stroke or transient ischemic attack (TIA), the device detected atrial fibrillation in nearly 5% and supraventricular tachycardia in 51% of records, Dr. Christie E. Tung and her associates reported in a poster presentation at the International Stroke Conference in San Diego.
Patients wore the patch for a mean of 11 days. In the 4.2% of reports showing paroxysmal atrial fibrillation, the first episode did not occur until after 48 hours of monitoring in 14% of wearers, suggesting that these would have been missed by conventional 24-hour or 48-hour Holter monitoring, said Dr. Tung of Stanford (Calif.) University. That’s important because detection of atrial fibrillation in patients with stroke or TIA changes the recommended antithrombotic regimen from antiplatelet medications to oral anticoagulants, the investigators explained.
The list price of the Zio device and service is $595, according to a spokeswoman for iRhythm Technologies, which markets the Zio. Dr. Barrett and Dr. Engel said the cost is roughly comparable to that of 24-hour Holter monitoring and less expensive than other forms of extended ECG monitoring. Most insurers will cover the Zio Patch, Dr. Engel said. Aetna, one of the largest U.S. insurance companies, began covering the Zio Patch for long-term ECG monitoring in January 2014.
iRhythm Technologies helped fund the studies. Dr. Barrett, Dr. Tung, and their associates reported having no financial disclosures.
On Twitter @sherryboschert
An easy-to-use skin-patch device detected 96 arrhythmia events during 2 weeks of use, compared with 61 events detected using 24-hour Holter monitoring, in a study of 146 patients referred for evaluation of cardiac arrhythmias.
Patients referred to the cardiac investigations laboratory at Scripps Green Hospital, La Jolla, Calif., between April and July 2012 were fitted simultaneously with a 24-hour Holter monitor and a Zio Patch, which is a lightweight, single-use electrocardiographic (ECG) device that was attached over the left pectoral region of the patient’s chest by an adhesive patch. Patients wore the patch for a median of 11 days, but it can be worn for as long as 14 days.
Among 102 physicians surveyed in the study, 90% said they thought that the data from the patch provided a definitive diagnosis, compared with 64% who thought the Holter data allowed a definitive diagnosis, Dr. Paddy M. Barrett and his associates reported (Am. J. Med. 2014;127:95.e11-95.e17).
Patients showed a clear preference for the water-resistant patch, saying that it affected just 10% of their activities of daily living, compared with 76% of activities affected by the Holter monitor. The patch was comfortable to wear, according to 94%, and 52% said the Holter monitor was comfortable. Asked to choose between the two, 81% of patients said that they preferred the patch.
Researchers applied the patch, but, outside of the study, it sometimes is mailed directly to a patient for self-application. Patients can press a button on it to indicate when they feel symptoms. At the end of the monitoring period, the patch is mailed to an iRhythm facility for data analysis, from which a digital report is sent to the ordering physician. The Zio Patch is approved by the Food and Drug Administration.
During the first 24 hours when both devices were worn in the study, the Holter monitor detected 11 arrhythmic events not detected by the patch, but these and more were found during the extended use of the patch. A review of those 11 events led to an adjustment in the device’s algorithm and extra training for the staff who review the data and produce the reports for clinicians, according to Dr. Barrett, who led the study while he was a fellow at Scripps Translational Science Institute, La Jolla, and now is a cardiology fellow at St. Vincent’s University College Hospital, Dublin.
Six arrhythmias were included in the definition of arrhythmic events: supraventricular tachycardia; atrial fibrillation/flutter; a pause greater than 3 seconds; atrioventricular block; ventricular tachycardia; or polymorphic ventricular tachycardia/ventricular fibrillation.
In an interview, Dr. Barrett expressed particular interest in the device’s ability to detect asymptomatic atrial fibrillation that commonly is missed by 24-hour Holter monitoring. "The detection of more [events] allows risk stratification and implementation of treatment strategies," he said. The investigators have no plans to conduct a long-term study to see if the incremental diagnoses obtained by the patch ultimately improve health outcomes.
Dr. Gregory Engel, a cardiologist and electrophysiologist at Silicon Valley Cardiology who is not associated with the study or with the device maker, said the medical literature suggests that finding and treating "silent" atrial fibrillation will reduce mortality and morbidity. He uses the Zio Patch system in his 10-person private group practice at three offices in northern California and believes the patch will replace most, but not all, Holter monitor use.
"We own our Holter, so if I just need 24 hours of monitoring" in a patient whose arrhythmia already is known, "I might as well use the product I’ve already paid for," he said in an interview. "From a pure information and diagnostic perspective," however, the Zio Patch is "far superior to the Holter."
The Zio also would not be his first choice for cases in which he wants monitoring that provides instant feedback. Mobile cardiac telemetry or other instant-feedback systems may be appropriate for some patients.
In a separate retrospective study of data from 1,171 Zio Patch monitoring reports on patients who’d had a stroke or transient ischemic attack (TIA), the device detected atrial fibrillation in nearly 5% and supraventricular tachycardia in 51% of records, Dr. Christie E. Tung and her associates reported in a poster presentation at the International Stroke Conference in San Diego.
Patients wore the patch for a mean of 11 days. In the 4.2% of reports showing paroxysmal atrial fibrillation, the first episode did not occur until after 48 hours of monitoring in 14% of wearers, suggesting that these would have been missed by conventional 24-hour or 48-hour Holter monitoring, said Dr. Tung of Stanford (Calif.) University. That’s important because detection of atrial fibrillation in patients with stroke or TIA changes the recommended antithrombotic regimen from antiplatelet medications to oral anticoagulants, the investigators explained.
The list price of the Zio device and service is $595, according to a spokeswoman for iRhythm Technologies, which markets the Zio. Dr. Barrett and Dr. Engel said the cost is roughly comparable to that of 24-hour Holter monitoring and less expensive than other forms of extended ECG monitoring. Most insurers will cover the Zio Patch, Dr. Engel said. Aetna, one of the largest U.S. insurance companies, began covering the Zio Patch for long-term ECG monitoring in January 2014.
iRhythm Technologies helped fund the studies. Dr. Barrett, Dr. Tung, and their associates reported having no financial disclosures.
On Twitter @sherryboschert
An easy-to-use skin-patch device detected 96 arrhythmia events during 2 weeks of use, compared with 61 events detected using 24-hour Holter monitoring, in a study of 146 patients referred for evaluation of cardiac arrhythmias.
Patients referred to the cardiac investigations laboratory at Scripps Green Hospital, La Jolla, Calif., between April and July 2012 were fitted simultaneously with a 24-hour Holter monitor and a Zio Patch, which is a lightweight, single-use electrocardiographic (ECG) device that was attached over the left pectoral region of the patient’s chest by an adhesive patch. Patients wore the patch for a median of 11 days, but it can be worn for as long as 14 days.
Among 102 physicians surveyed in the study, 90% said they thought that the data from the patch provided a definitive diagnosis, compared with 64% who thought the Holter data allowed a definitive diagnosis, Dr. Paddy M. Barrett and his associates reported (Am. J. Med. 2014;127:95.e11-95.e17).
Patients showed a clear preference for the water-resistant patch, saying that it affected just 10% of their activities of daily living, compared with 76% of activities affected by the Holter monitor. The patch was comfortable to wear, according to 94%, and 52% said the Holter monitor was comfortable. Asked to choose between the two, 81% of patients said that they preferred the patch.
Researchers applied the patch, but, outside of the study, it sometimes is mailed directly to a patient for self-application. Patients can press a button on it to indicate when they feel symptoms. At the end of the monitoring period, the patch is mailed to an iRhythm facility for data analysis, from which a digital report is sent to the ordering physician. The Zio Patch is approved by the Food and Drug Administration.
During the first 24 hours when both devices were worn in the study, the Holter monitor detected 11 arrhythmic events not detected by the patch, but these and more were found during the extended use of the patch. A review of those 11 events led to an adjustment in the device’s algorithm and extra training for the staff who review the data and produce the reports for clinicians, according to Dr. Barrett, who led the study while he was a fellow at Scripps Translational Science Institute, La Jolla, and now is a cardiology fellow at St. Vincent’s University College Hospital, Dublin.
Six arrhythmias were included in the definition of arrhythmic events: supraventricular tachycardia; atrial fibrillation/flutter; a pause greater than 3 seconds; atrioventricular block; ventricular tachycardia; or polymorphic ventricular tachycardia/ventricular fibrillation.
In an interview, Dr. Barrett expressed particular interest in the device’s ability to detect asymptomatic atrial fibrillation that commonly is missed by 24-hour Holter monitoring. "The detection of more [events] allows risk stratification and implementation of treatment strategies," he said. The investigators have no plans to conduct a long-term study to see if the incremental diagnoses obtained by the patch ultimately improve health outcomes.
Dr. Gregory Engel, a cardiologist and electrophysiologist at Silicon Valley Cardiology who is not associated with the study or with the device maker, said the medical literature suggests that finding and treating "silent" atrial fibrillation will reduce mortality and morbidity. He uses the Zio Patch system in his 10-person private group practice at three offices in northern California and believes the patch will replace most, but not all, Holter monitor use.
"We own our Holter, so if I just need 24 hours of monitoring" in a patient whose arrhythmia already is known, "I might as well use the product I’ve already paid for," he said in an interview. "From a pure information and diagnostic perspective," however, the Zio Patch is "far superior to the Holter."
The Zio also would not be his first choice for cases in which he wants monitoring that provides instant feedback. Mobile cardiac telemetry or other instant-feedback systems may be appropriate for some patients.
In a separate retrospective study of data from 1,171 Zio Patch monitoring reports on patients who’d had a stroke or transient ischemic attack (TIA), the device detected atrial fibrillation in nearly 5% and supraventricular tachycardia in 51% of records, Dr. Christie E. Tung and her associates reported in a poster presentation at the International Stroke Conference in San Diego.
Patients wore the patch for a mean of 11 days. In the 4.2% of reports showing paroxysmal atrial fibrillation, the first episode did not occur until after 48 hours of monitoring in 14% of wearers, suggesting that these would have been missed by conventional 24-hour or 48-hour Holter monitoring, said Dr. Tung of Stanford (Calif.) University. That’s important because detection of atrial fibrillation in patients with stroke or TIA changes the recommended antithrombotic regimen from antiplatelet medications to oral anticoagulants, the investigators explained.
The list price of the Zio device and service is $595, according to a spokeswoman for iRhythm Technologies, which markets the Zio. Dr. Barrett and Dr. Engel said the cost is roughly comparable to that of 24-hour Holter monitoring and less expensive than other forms of extended ECG monitoring. Most insurers will cover the Zio Patch, Dr. Engel said. Aetna, one of the largest U.S. insurance companies, began covering the Zio Patch for long-term ECG monitoring in January 2014.
iRhythm Technologies helped fund the studies. Dr. Barrett, Dr. Tung, and their associates reported having no financial disclosures.
On Twitter @sherryboschert
CO2 laser works best for hypertrophic scars
PHOENIX – A fractional carbon dioxide laser at 10,600 nm was the only one of three laser treatments for hypertrophic scars that produced significant overall improvements in a comparison of data on 141 scars in 66 patients.
Less successful were treatments using a vascular KTP laser at 532 nm or a 1,550-nm nonablative fractional erbium glass laser.
"The only one that showed significant improvement compared with the control scar, adjusted for the baseline scar, was the fractional CO2 laser," Dr. Sigrid Blome-Eberwein said at the annual meeting of the American Society for Laser Medicine and Surgery.
The data came from three prospective controlled studies at one institution. The patients were burn survivors who had at least two scars of similar appearance and physiologic function in the same body area that were at least 6 months from wound healing. Patients underwent a series of at least three treatments at 4-week intervals on one or more scars, with a similar scar left untreated as the control scar.
Subjective assessments of the scars showed statistically significant improvements in scores on the Vancouver Scar Scale with all three treatments compared with control scars, but fewer changes were seen in objective measurements of scar qualities. "Measurements of these improvements are really difficult to tackle. We tried to add some objective measurement instruments to our evaluations," reported Dr. Blome-Eberwein of Lehigh Valley Regional Burn Center, Allentown, Pa.
None of the treatments produced significant changes in elasticity as measured by the Cutometer device. "It’s really a complete mix of results in this value," she said.
The fractional erbium laser produced one statistically significant improvement, compared with control scars, in thickness as measured by high-resolution ultrasound used to assess scar thickness (so that patients could avoid biopsies). The KTP laser also produced one statistically significant improvement, compared with control scars: improved sensation as assessed by Semmes Weinstein monofilaments.
The CO2 laser, however, produced greater improvements in scar thickness and sensation, as well as significant improvements in erythema and pigment (both measured by spectrometry), compared with control scars. Measurements of pain and pruritus did not differ significantly between treated and control scars in any of the studies; pain levels tended to improve in both the treated and control areas and pruritus tended to remain steady.
The KTP laser caused blisters in some patients. The fractional CO2 and fractional erbium lasers produced minimal damage. Increased power was more likely to produce collateral damage and blisters, Dr. Blome-Eberwein said.
Any results were persistent and additive. Because penetration of these lasers is limited, multiple treatments are needed for thick scars, she noted.
Based on these findings and her experience at the burn center, Dr. Blome-Eberwein recommended treating scars that seem to turn hypertrophic early (within 4 weeks of wound healing) and frequently (every 3 weeks) with a nonablative fractional erbium glass laser or pulsed dye laser. The erbium glass laser does seem to prevent "some degree of hypertrophy early on," she said. Either of these early treatments may decrease hypertrophic activity and prevent hypertrophy with no dermal damage.
Dr. Blome-Eberwein said she treats hypertrophic scars 3-12 months after healing with fractional CO2 laser. Treating scar pigment remains a challenge, she added.
"In the future, there will be handheld devices" to treat scars at home "because a lot of people are affected by this problem," she said.
Most of the scars in the studies were from burns, with a few from trauma.
Dr. Blome-Eberwein reported having no financial disclosures.
On Twitter @sherryboschert
PHOENIX – A fractional carbon dioxide laser at 10,600 nm was the only one of three laser treatments for hypertrophic scars that produced significant overall improvements in a comparison of data on 141 scars in 66 patients.
Less successful were treatments using a vascular KTP laser at 532 nm or a 1,550-nm nonablative fractional erbium glass laser.
"The only one that showed significant improvement compared with the control scar, adjusted for the baseline scar, was the fractional CO2 laser," Dr. Sigrid Blome-Eberwein said at the annual meeting of the American Society for Laser Medicine and Surgery.
The data came from three prospective controlled studies at one institution. The patients were burn survivors who had at least two scars of similar appearance and physiologic function in the same body area that were at least 6 months from wound healing. Patients underwent a series of at least three treatments at 4-week intervals on one or more scars, with a similar scar left untreated as the control scar.
Subjective assessments of the scars showed statistically significant improvements in scores on the Vancouver Scar Scale with all three treatments compared with control scars, but fewer changes were seen in objective measurements of scar qualities. "Measurements of these improvements are really difficult to tackle. We tried to add some objective measurement instruments to our evaluations," reported Dr. Blome-Eberwein of Lehigh Valley Regional Burn Center, Allentown, Pa.
None of the treatments produced significant changes in elasticity as measured by the Cutometer device. "It’s really a complete mix of results in this value," she said.
The fractional erbium laser produced one statistically significant improvement, compared with control scars, in thickness as measured by high-resolution ultrasound used to assess scar thickness (so that patients could avoid biopsies). The KTP laser also produced one statistically significant improvement, compared with control scars: improved sensation as assessed by Semmes Weinstein monofilaments.
The CO2 laser, however, produced greater improvements in scar thickness and sensation, as well as significant improvements in erythema and pigment (both measured by spectrometry), compared with control scars. Measurements of pain and pruritus did not differ significantly between treated and control scars in any of the studies; pain levels tended to improve in both the treated and control areas and pruritus tended to remain steady.
The KTP laser caused blisters in some patients. The fractional CO2 and fractional erbium lasers produced minimal damage. Increased power was more likely to produce collateral damage and blisters, Dr. Blome-Eberwein said.
Any results were persistent and additive. Because penetration of these lasers is limited, multiple treatments are needed for thick scars, she noted.
Based on these findings and her experience at the burn center, Dr. Blome-Eberwein recommended treating scars that seem to turn hypertrophic early (within 4 weeks of wound healing) and frequently (every 3 weeks) with a nonablative fractional erbium glass laser or pulsed dye laser. The erbium glass laser does seem to prevent "some degree of hypertrophy early on," she said. Either of these early treatments may decrease hypertrophic activity and prevent hypertrophy with no dermal damage.
Dr. Blome-Eberwein said she treats hypertrophic scars 3-12 months after healing with fractional CO2 laser. Treating scar pigment remains a challenge, she added.
"In the future, there will be handheld devices" to treat scars at home "because a lot of people are affected by this problem," she said.
Most of the scars in the studies were from burns, with a few from trauma.
Dr. Blome-Eberwein reported having no financial disclosures.
On Twitter @sherryboschert
PHOENIX – A fractional carbon dioxide laser at 10,600 nm was the only one of three laser treatments for hypertrophic scars that produced significant overall improvements in a comparison of data on 141 scars in 66 patients.
Less successful were treatments using a vascular KTP laser at 532 nm or a 1,550-nm nonablative fractional erbium glass laser.
"The only one that showed significant improvement compared with the control scar, adjusted for the baseline scar, was the fractional CO2 laser," Dr. Sigrid Blome-Eberwein said at the annual meeting of the American Society for Laser Medicine and Surgery.
The data came from three prospective controlled studies at one institution. The patients were burn survivors who had at least two scars of similar appearance and physiologic function in the same body area that were at least 6 months from wound healing. Patients underwent a series of at least three treatments at 4-week intervals on one or more scars, with a similar scar left untreated as the control scar.
Subjective assessments of the scars showed statistically significant improvements in scores on the Vancouver Scar Scale with all three treatments compared with control scars, but fewer changes were seen in objective measurements of scar qualities. "Measurements of these improvements are really difficult to tackle. We tried to add some objective measurement instruments to our evaluations," reported Dr. Blome-Eberwein of Lehigh Valley Regional Burn Center, Allentown, Pa.
None of the treatments produced significant changes in elasticity as measured by the Cutometer device. "It’s really a complete mix of results in this value," she said.
The fractional erbium laser produced one statistically significant improvement, compared with control scars, in thickness as measured by high-resolution ultrasound used to assess scar thickness (so that patients could avoid biopsies). The KTP laser also produced one statistically significant improvement, compared with control scars: improved sensation as assessed by Semmes Weinstein monofilaments.
The CO2 laser, however, produced greater improvements in scar thickness and sensation, as well as significant improvements in erythema and pigment (both measured by spectrometry), compared with control scars. Measurements of pain and pruritus did not differ significantly between treated and control scars in any of the studies; pain levels tended to improve in both the treated and control areas and pruritus tended to remain steady.
The KTP laser caused blisters in some patients. The fractional CO2 and fractional erbium lasers produced minimal damage. Increased power was more likely to produce collateral damage and blisters, Dr. Blome-Eberwein said.
Any results were persistent and additive. Because penetration of these lasers is limited, multiple treatments are needed for thick scars, she noted.
Based on these findings and her experience at the burn center, Dr. Blome-Eberwein recommended treating scars that seem to turn hypertrophic early (within 4 weeks of wound healing) and frequently (every 3 weeks) with a nonablative fractional erbium glass laser or pulsed dye laser. The erbium glass laser does seem to prevent "some degree of hypertrophy early on," she said. Either of these early treatments may decrease hypertrophic activity and prevent hypertrophy with no dermal damage.
Dr. Blome-Eberwein said she treats hypertrophic scars 3-12 months after healing with fractional CO2 laser. Treating scar pigment remains a challenge, she added.
"In the future, there will be handheld devices" to treat scars at home "because a lot of people are affected by this problem," she said.
Most of the scars in the studies were from burns, with a few from trauma.
Dr. Blome-Eberwein reported having no financial disclosures.
On Twitter @sherryboschert
AT LASER 2014
Major finding: The fractional CO2 laser significantly improved scar thickness, sensation, erythema, and pigment, compared with untreated scars. The KTP laser improved sensation and the erbium glass laser improved thickness in treated scars, compared with control scars.
Data source: An analysis of data from three prospective controlled studies of laser treatments in 66 patients with 141 scars.
Disclosures: Dr. Blome-Eberwein reported having no financial disclosures.
Evidence-Based Apps: Is it possible to diagnose epileptic seizure digitally?
A new app seems to help nonneurologist health care workers diagnose seizures as epileptic or nonepileptic, findings from a small study to be presented at the American Academy of Neurology meeting suggest.
The epilepsy app, which will be called Epilepsy Diagnosis when it becomes available, was created by neurologist Victor Patterson of Belfast, Northern Ireland, and built by a contracted company. He asked 67 patients at an epilepsy clinic in Nepal (51 of whom had epileptic seizures) 26 questions about their seizures and incorporated into the app the 11 most helpful questions and answers for predicting an epileptic seizure.
Nonphysician health care workers and a few physicians-in-training tested the app on 132 patients attending epilepsy clinics in Nepal and India. They either used the app before a formal diagnosis was made or were blinded to the patient’s clinical diagnosis.
The app was informative in 87% of cases (115 patients), and app results ultimately agreed with a physician’s diagnosis of epileptic or nonepileptic seizure in 97% of those cases (112 patients), according to Dr. Patterson. In addition to developing the app, he has been a visiting neurologist or teleneurologist for nonprofit international aid organizations or for hospitals in Nepal and Sudan, he said in an interview.
Using the app, health care providers were able to separate epileptic from nonepileptic seizures with "near-complete reliability," according to the abstract for his upcoming poster presentation on May 1 at the American Academy of Neurology annual meeting in Philadelphia. "Nondoctors were able to use it with minimal training."
Helping health care workers diagnose epileptic seizures could "save precious medical time," he said. "The diagnosis of episodes of altered consciousness as epileptic seizures is key to the management of epilepsy."
Neurologists typically distinguish an epileptic seizure from a nonepileptic one by taking a careful history of the attack both from the patient and an eyewitness and then use their medical knowledge to decide whether the episode is epileptic or not, Dr. Patterson said in an interview. The app uses what he considered to be the 11 most helpful questions in diagnosing Nepalese patients to take a history from the patient and an eyewitness. The app is programmed to analyze the answers and give a probability of the episode being epileptic.
"It’s the same process, with the app substituting for the neurologist," he said.
Primary care physicians, nurses, and other nonneurologist health care workers in more developed countries (like the United States) might be able to use the app in areas where neurologists are in short supply, but Dr. Patterson cautioned that the app was programmed based on the population in Nepal, which may be different from the U.S. population. For example, the rate of convulsive seizures or other conditions may differ between the two countries.
"I recommend physicians in different parts of the world to test this to see if it works for them," he said.
Health workers in resource-poor areas of the world will be able to get the app for free but a fee will be charged to physicians. Any profits will go toward more research into methods for "closing the epilepsy treatment gap," he added.
Dr. Patterson reported having no financial disclosures other than those disclosed above.
On Twitter @sherryboschert
A new app seems to help nonneurologist health care workers diagnose seizures as epileptic or nonepileptic, findings from a small study to be presented at the American Academy of Neurology meeting suggest.
The epilepsy app, which will be called Epilepsy Diagnosis when it becomes available, was created by neurologist Victor Patterson of Belfast, Northern Ireland, and built by a contracted company. He asked 67 patients at an epilepsy clinic in Nepal (51 of whom had epileptic seizures) 26 questions about their seizures and incorporated into the app the 11 most helpful questions and answers for predicting an epileptic seizure.
Nonphysician health care workers and a few physicians-in-training tested the app on 132 patients attending epilepsy clinics in Nepal and India. They either used the app before a formal diagnosis was made or were blinded to the patient’s clinical diagnosis.
The app was informative in 87% of cases (115 patients), and app results ultimately agreed with a physician’s diagnosis of epileptic or nonepileptic seizure in 97% of those cases (112 patients), according to Dr. Patterson. In addition to developing the app, he has been a visiting neurologist or teleneurologist for nonprofit international aid organizations or for hospitals in Nepal and Sudan, he said in an interview.
Using the app, health care providers were able to separate epileptic from nonepileptic seizures with "near-complete reliability," according to the abstract for his upcoming poster presentation on May 1 at the American Academy of Neurology annual meeting in Philadelphia. "Nondoctors were able to use it with minimal training."
Helping health care workers diagnose epileptic seizures could "save precious medical time," he said. "The diagnosis of episodes of altered consciousness as epileptic seizures is key to the management of epilepsy."
Neurologists typically distinguish an epileptic seizure from a nonepileptic one by taking a careful history of the attack both from the patient and an eyewitness and then use their medical knowledge to decide whether the episode is epileptic or not, Dr. Patterson said in an interview. The app uses what he considered to be the 11 most helpful questions in diagnosing Nepalese patients to take a history from the patient and an eyewitness. The app is programmed to analyze the answers and give a probability of the episode being epileptic.
"It’s the same process, with the app substituting for the neurologist," he said.
Primary care physicians, nurses, and other nonneurologist health care workers in more developed countries (like the United States) might be able to use the app in areas where neurologists are in short supply, but Dr. Patterson cautioned that the app was programmed based on the population in Nepal, which may be different from the U.S. population. For example, the rate of convulsive seizures or other conditions may differ between the two countries.
"I recommend physicians in different parts of the world to test this to see if it works for them," he said.
Health workers in resource-poor areas of the world will be able to get the app for free but a fee will be charged to physicians. Any profits will go toward more research into methods for "closing the epilepsy treatment gap," he added.
Dr. Patterson reported having no financial disclosures other than those disclosed above.
On Twitter @sherryboschert
A new app seems to help nonneurologist health care workers diagnose seizures as epileptic or nonepileptic, findings from a small study to be presented at the American Academy of Neurology meeting suggest.
The epilepsy app, which will be called Epilepsy Diagnosis when it becomes available, was created by neurologist Victor Patterson of Belfast, Northern Ireland, and built by a contracted company. He asked 67 patients at an epilepsy clinic in Nepal (51 of whom had epileptic seizures) 26 questions about their seizures and incorporated into the app the 11 most helpful questions and answers for predicting an epileptic seizure.
Nonphysician health care workers and a few physicians-in-training tested the app on 132 patients attending epilepsy clinics in Nepal and India. They either used the app before a formal diagnosis was made or were blinded to the patient’s clinical diagnosis.
The app was informative in 87% of cases (115 patients), and app results ultimately agreed with a physician’s diagnosis of epileptic or nonepileptic seizure in 97% of those cases (112 patients), according to Dr. Patterson. In addition to developing the app, he has been a visiting neurologist or teleneurologist for nonprofit international aid organizations or for hospitals in Nepal and Sudan, he said in an interview.
Using the app, health care providers were able to separate epileptic from nonepileptic seizures with "near-complete reliability," according to the abstract for his upcoming poster presentation on May 1 at the American Academy of Neurology annual meeting in Philadelphia. "Nondoctors were able to use it with minimal training."
Helping health care workers diagnose epileptic seizures could "save precious medical time," he said. "The diagnosis of episodes of altered consciousness as epileptic seizures is key to the management of epilepsy."
Neurologists typically distinguish an epileptic seizure from a nonepileptic one by taking a careful history of the attack both from the patient and an eyewitness and then use their medical knowledge to decide whether the episode is epileptic or not, Dr. Patterson said in an interview. The app uses what he considered to be the 11 most helpful questions in diagnosing Nepalese patients to take a history from the patient and an eyewitness. The app is programmed to analyze the answers and give a probability of the episode being epileptic.
"It’s the same process, with the app substituting for the neurologist," he said.
Primary care physicians, nurses, and other nonneurologist health care workers in more developed countries (like the United States) might be able to use the app in areas where neurologists are in short supply, but Dr. Patterson cautioned that the app was programmed based on the population in Nepal, which may be different from the U.S. population. For example, the rate of convulsive seizures or other conditions may differ between the two countries.
"I recommend physicians in different parts of the world to test this to see if it works for them," he said.
Health workers in resource-poor areas of the world will be able to get the app for free but a fee will be charged to physicians. Any profits will go toward more research into methods for "closing the epilepsy treatment gap," he added.
Dr. Patterson reported having no financial disclosures other than those disclosed above.
On Twitter @sherryboschert
Where’s the evidence that medical apps are clinically useful?
The idea that smartphones, tablets, apps, and mobile technology might improve medical care certainly is appealing, but is there any evidence that these are useful clinical tools and not just glamorous new toys?
Welcome to our newest column – Evidence-Based Apps – in which we’ll track the growing field of mobile-device medicine for you and deliver the data that you need to decide whether an app might complement your clinical practice.
First, here’s the definition we’ll use: A medical app, or application, is software running on a phone, computer, some other electronic device, or the Internet that’s intended to improve health outcomes or act as a medical device. This may include products that you download to your phone or tablet, text-messaging interventions aimed at motivating patients, or small electronic devices and software that could replace larger, older, costlier medical technology.
Does that smartphone app for mental health decrease depression or anxiety? Can a smart skin patch replace Holter monitors? Will texts or other electronic reminders improve patient adherence to medication regimens? And will any of those ultimately improve patient health in the long term, increase convenience, or reduce medical costs? The developers of these devices would like you to think so, but here’s our mantra for this column: Show me the data!
A review of 1,500 health apps that cost between 69 cents and $999 found that 331 claimed to treat or cure medical problems, but all were disputed by medical experts, according to the New England Center for Investigative Reporting. Twelve of these apps relied on cell-phone light for "treatment," two said that the phone’s vibrations were therapeutic, and a whopping 43% claimed that cell-phone sounds were treatments.
Mobile health products (also called mHealth) are a booming field, and we’re now starting to see studies of apps that report clinical outcomes. The Food and Drug Administration regulates more than 100 apps that it sees as stand-ins for previously approved medical devices, and hospitals have put their own brands on more than 200 apps so far.
At least 9% of all U.S. adults have at least one health app on a smartphone, according to the Pew Research Center’s "Mobile Health 2012" report. Most popular are health apps to track and promote exercise, healthful diet, and weight loss.
In 2013, 95 million Americans accessed health information or tools through mobile phones, 27% more than in 2012, according to a report by Manhattan Research. Patients with the following diseases were most likely to be doing this, Manhattan Research said: cystic fibrosis, growth hormone deficiency, acne, ADD or ADHD, hepatitis C virus infection, migraine, Crohn’s disease, chronic kidney disease, generalized anxiety disorder, and bipolar disorder.
Makers of digital health products have lagged in conducting studies to verify the efficacy of these products, but it’s a problem that’s common to medicine, Forbes.com contributor David Shaywitz noted in a recent post. An estimated 40% of current medical practices aren’t backed by evidence from good clinical trials.
This column will offer reports on which of the more than 40,000 health and medical apps on the marketplace have some clinical trial evidence behind them. If there are any particular apps or topics you’d like us to cover, we’d love to hear from you!
On Twitter @sherryboschert
The idea that smartphones, tablets, apps, and mobile technology might improve medical care certainly is appealing, but is there any evidence that these are useful clinical tools and not just glamorous new toys?
Welcome to our newest column – Evidence-Based Apps – in which we’ll track the growing field of mobile-device medicine for you and deliver the data that you need to decide whether an app might complement your clinical practice.
First, here’s the definition we’ll use: A medical app, or application, is software running on a phone, computer, some other electronic device, or the Internet that’s intended to improve health outcomes or act as a medical device. This may include products that you download to your phone or tablet, text-messaging interventions aimed at motivating patients, or small electronic devices and software that could replace larger, older, costlier medical technology.
Does that smartphone app for mental health decrease depression or anxiety? Can a smart skin patch replace Holter monitors? Will texts or other electronic reminders improve patient adherence to medication regimens? And will any of those ultimately improve patient health in the long term, increase convenience, or reduce medical costs? The developers of these devices would like you to think so, but here’s our mantra for this column: Show me the data!
A review of 1,500 health apps that cost between 69 cents and $999 found that 331 claimed to treat or cure medical problems, but all were disputed by medical experts, according to the New England Center for Investigative Reporting. Twelve of these apps relied on cell-phone light for "treatment," two said that the phone’s vibrations were therapeutic, and a whopping 43% claimed that cell-phone sounds were treatments.
Mobile health products (also called mHealth) are a booming field, and we’re now starting to see studies of apps that report clinical outcomes. The Food and Drug Administration regulates more than 100 apps that it sees as stand-ins for previously approved medical devices, and hospitals have put their own brands on more than 200 apps so far.
At least 9% of all U.S. adults have at least one health app on a smartphone, according to the Pew Research Center’s "Mobile Health 2012" report. Most popular are health apps to track and promote exercise, healthful diet, and weight loss.
In 2013, 95 million Americans accessed health information or tools through mobile phones, 27% more than in 2012, according to a report by Manhattan Research. Patients with the following diseases were most likely to be doing this, Manhattan Research said: cystic fibrosis, growth hormone deficiency, acne, ADD or ADHD, hepatitis C virus infection, migraine, Crohn’s disease, chronic kidney disease, generalized anxiety disorder, and bipolar disorder.
Makers of digital health products have lagged in conducting studies to verify the efficacy of these products, but it’s a problem that’s common to medicine, Forbes.com contributor David Shaywitz noted in a recent post. An estimated 40% of current medical practices aren’t backed by evidence from good clinical trials.
This column will offer reports on which of the more than 40,000 health and medical apps on the marketplace have some clinical trial evidence behind them. If there are any particular apps or topics you’d like us to cover, we’d love to hear from you!
On Twitter @sherryboschert
The idea that smartphones, tablets, apps, and mobile technology might improve medical care certainly is appealing, but is there any evidence that these are useful clinical tools and not just glamorous new toys?
Welcome to our newest column – Evidence-Based Apps – in which we’ll track the growing field of mobile-device medicine for you and deliver the data that you need to decide whether an app might complement your clinical practice.
First, here’s the definition we’ll use: A medical app, or application, is software running on a phone, computer, some other electronic device, or the Internet that’s intended to improve health outcomes or act as a medical device. This may include products that you download to your phone or tablet, text-messaging interventions aimed at motivating patients, or small electronic devices and software that could replace larger, older, costlier medical technology.
Does that smartphone app for mental health decrease depression or anxiety? Can a smart skin patch replace Holter monitors? Will texts or other electronic reminders improve patient adherence to medication regimens? And will any of those ultimately improve patient health in the long term, increase convenience, or reduce medical costs? The developers of these devices would like you to think so, but here’s our mantra for this column: Show me the data!
A review of 1,500 health apps that cost between 69 cents and $999 found that 331 claimed to treat or cure medical problems, but all were disputed by medical experts, according to the New England Center for Investigative Reporting. Twelve of these apps relied on cell-phone light for "treatment," two said that the phone’s vibrations were therapeutic, and a whopping 43% claimed that cell-phone sounds were treatments.
Mobile health products (also called mHealth) are a booming field, and we’re now starting to see studies of apps that report clinical outcomes. The Food and Drug Administration regulates more than 100 apps that it sees as stand-ins for previously approved medical devices, and hospitals have put their own brands on more than 200 apps so far.
At least 9% of all U.S. adults have at least one health app on a smartphone, according to the Pew Research Center’s "Mobile Health 2012" report. Most popular are health apps to track and promote exercise, healthful diet, and weight loss.
In 2013, 95 million Americans accessed health information or tools through mobile phones, 27% more than in 2012, according to a report by Manhattan Research. Patients with the following diseases were most likely to be doing this, Manhattan Research said: cystic fibrosis, growth hormone deficiency, acne, ADD or ADHD, hepatitis C virus infection, migraine, Crohn’s disease, chronic kidney disease, generalized anxiety disorder, and bipolar disorder.
Makers of digital health products have lagged in conducting studies to verify the efficacy of these products, but it’s a problem that’s common to medicine, Forbes.com contributor David Shaywitz noted in a recent post. An estimated 40% of current medical practices aren’t backed by evidence from good clinical trials.
This column will offer reports on which of the more than 40,000 health and medical apps on the marketplace have some clinical trial evidence behind them. If there are any particular apps or topics you’d like us to cover, we’d love to hear from you!
On Twitter @sherryboschert
Minority Correctly Use EpiPen or Metered Dose Inhaler
SAN DIEGO – Only 12% of 91 patients in Texas allergy/immunology clinics knew how to use an epinephrine autoinjector correctly, and only 7% of 41 patients could demonstrate correct use of their metered dose inhaler with a spacer, a small prospective study showed.
That’s not good enough, Dr. Rana S. Bonds said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. Her team has begun studying interventions to improve correct use of those devices and "will be sharing that data in the near future," she said.
They asked patients to demonstrate the use of an EpiPen or a metered dose inhaler (MDI) and spacer and scored the patients’ adherence to the EpiPen manufacturer’s instructions or published standards for MDI/spacers.
For the EpiPen, 25% of patients missed one of the five steps for correct use, 18% missed two steps, and 19% missed three steps. "It was fairly alarming" to find that 31% got four steps wrong and 8% missed all five steps, said Dr. Bonds of the University of Texas Medical Branch, Galveston. (Percentages total more than 100% because they were rounded.)
The most common mistake with the EpiPen involved the final step. "Once they deployed the epinephrine injection, they didn’t hold it down long enough. They were bouncing it off the thigh or whatever body part they thought they should inject it into," she said.
For the MDI/spacer, 16% of patients performed 1 of 11 steps for use incorrectly, 16% missed 2 steps, 21% missed 3 steps, and 18% missed 4 steps. Another 11% missed 5 steps, 5% missed 6 steps, 11% missed 7 steps, and 3% got all 11 steps wrong, Dr. Bonds and her associates reported. The most common mistake was failing to exhale before triggering the inhaler for inhalation.
The study recruited patients from the university’s main allergy/immunology clinic and its satellite clinics. Trainers are available at each clinic, and patients are supposed to see them before leaving with one of the devices, but the findings raise the question of whether health care providers at the clinics consistently make sure that happens, she said.
Younger patients, males, and patients with a medical background were more likely to show that they could use the EpiPen correctly. Being African American or less educated was associated with a greater likelihood of incorrect use. Correct usage rates differed significantly between some of the clinic sites. Factors that didn’t correlate with correct or incorrect use of the EpiPen included whether a family member also used the device, being prescribed the EpiPen more or less than 1 year ago, and whether patients had ever used the EpiPen (most hadn’t).
The number of patients in the MDI/spacer group was too small to permit risk factors to be analyzed, Dr. Bonds said.
Previously published studies have reported that 22% of food-allergic adolescents could demonstrate correct use of epinephrine and that rates of incorrect inhaler use ranged from 50% to 94%, she said. Other studies have shown that incorrect use reduces the treatment’s clinical efficacy, and that repeated instruction increases the likelihood of correct use.
Dr. Bonds reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN DIEGO – Only 12% of 91 patients in Texas allergy/immunology clinics knew how to use an epinephrine autoinjector correctly, and only 7% of 41 patients could demonstrate correct use of their metered dose inhaler with a spacer, a small prospective study showed.
That’s not good enough, Dr. Rana S. Bonds said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. Her team has begun studying interventions to improve correct use of those devices and "will be sharing that data in the near future," she said.
They asked patients to demonstrate the use of an EpiPen or a metered dose inhaler (MDI) and spacer and scored the patients’ adherence to the EpiPen manufacturer’s instructions or published standards for MDI/spacers.
For the EpiPen, 25% of patients missed one of the five steps for correct use, 18% missed two steps, and 19% missed three steps. "It was fairly alarming" to find that 31% got four steps wrong and 8% missed all five steps, said Dr. Bonds of the University of Texas Medical Branch, Galveston. (Percentages total more than 100% because they were rounded.)
The most common mistake with the EpiPen involved the final step. "Once they deployed the epinephrine injection, they didn’t hold it down long enough. They were bouncing it off the thigh or whatever body part they thought they should inject it into," she said.
For the MDI/spacer, 16% of patients performed 1 of 11 steps for use incorrectly, 16% missed 2 steps, 21% missed 3 steps, and 18% missed 4 steps. Another 11% missed 5 steps, 5% missed 6 steps, 11% missed 7 steps, and 3% got all 11 steps wrong, Dr. Bonds and her associates reported. The most common mistake was failing to exhale before triggering the inhaler for inhalation.
The study recruited patients from the university’s main allergy/immunology clinic and its satellite clinics. Trainers are available at each clinic, and patients are supposed to see them before leaving with one of the devices, but the findings raise the question of whether health care providers at the clinics consistently make sure that happens, she said.
Younger patients, males, and patients with a medical background were more likely to show that they could use the EpiPen correctly. Being African American or less educated was associated with a greater likelihood of incorrect use. Correct usage rates differed significantly between some of the clinic sites. Factors that didn’t correlate with correct or incorrect use of the EpiPen included whether a family member also used the device, being prescribed the EpiPen more or less than 1 year ago, and whether patients had ever used the EpiPen (most hadn’t).
The number of patients in the MDI/spacer group was too small to permit risk factors to be analyzed, Dr. Bonds said.
Previously published studies have reported that 22% of food-allergic adolescents could demonstrate correct use of epinephrine and that rates of incorrect inhaler use ranged from 50% to 94%, she said. Other studies have shown that incorrect use reduces the treatment’s clinical efficacy, and that repeated instruction increases the likelihood of correct use.
Dr. Bonds reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN DIEGO – Only 12% of 91 patients in Texas allergy/immunology clinics knew how to use an epinephrine autoinjector correctly, and only 7% of 41 patients could demonstrate correct use of their metered dose inhaler with a spacer, a small prospective study showed.
That’s not good enough, Dr. Rana S. Bonds said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. Her team has begun studying interventions to improve correct use of those devices and "will be sharing that data in the near future," she said.
They asked patients to demonstrate the use of an EpiPen or a metered dose inhaler (MDI) and spacer and scored the patients’ adherence to the EpiPen manufacturer’s instructions or published standards for MDI/spacers.
For the EpiPen, 25% of patients missed one of the five steps for correct use, 18% missed two steps, and 19% missed three steps. "It was fairly alarming" to find that 31% got four steps wrong and 8% missed all five steps, said Dr. Bonds of the University of Texas Medical Branch, Galveston. (Percentages total more than 100% because they were rounded.)
The most common mistake with the EpiPen involved the final step. "Once they deployed the epinephrine injection, they didn’t hold it down long enough. They were bouncing it off the thigh or whatever body part they thought they should inject it into," she said.
For the MDI/spacer, 16% of patients performed 1 of 11 steps for use incorrectly, 16% missed 2 steps, 21% missed 3 steps, and 18% missed 4 steps. Another 11% missed 5 steps, 5% missed 6 steps, 11% missed 7 steps, and 3% got all 11 steps wrong, Dr. Bonds and her associates reported. The most common mistake was failing to exhale before triggering the inhaler for inhalation.
The study recruited patients from the university’s main allergy/immunology clinic and its satellite clinics. Trainers are available at each clinic, and patients are supposed to see them before leaving with one of the devices, but the findings raise the question of whether health care providers at the clinics consistently make sure that happens, she said.
Younger patients, males, and patients with a medical background were more likely to show that they could use the EpiPen correctly. Being African American or less educated was associated with a greater likelihood of incorrect use. Correct usage rates differed significantly between some of the clinic sites. Factors that didn’t correlate with correct or incorrect use of the EpiPen included whether a family member also used the device, being prescribed the EpiPen more or less than 1 year ago, and whether patients had ever used the EpiPen (most hadn’t).
The number of patients in the MDI/spacer group was too small to permit risk factors to be analyzed, Dr. Bonds said.
Previously published studies have reported that 22% of food-allergic adolescents could demonstrate correct use of epinephrine and that rates of incorrect inhaler use ranged from 50% to 94%, she said. Other studies have shown that incorrect use reduces the treatment’s clinical efficacy, and that repeated instruction increases the likelihood of correct use.
Dr. Bonds reported having no relevant financial disclosures.
On Twitter @sherryboschert