User login
Prolonged Stay Factors in Bronchiolitis
Prior studies have identified risk factors for increased severity of illness, readmission, or prolonged length of stay (LOS) in infants admitted with bronchiolitis.123 These risk factors include birth‐related factors (prematurity, birth within six months of respiratory syncytial virus [RSV] season, discharge from the neonatal intensive care unit during winter, multiple birth infant), environmental factors (day care attendance, school‐age siblings, smoke exposure), and underlying diseases (chronic lung disease and other pulmonary conditions, failure to thrive (FTT), congenital heart disease, immunologic disorders, and neuromuscular disease).123 Additional risk factors occurring during the bronchiolitis course that have been associated with prolonged hospital course are mechanical ventilation, intensive care unit (ICU) admission, hypoxia on admission, apnea, feeding problems, and duration of supplemental oxygen.2123
Having a reliable model to identify infants at high risk for prolonged LOS early in the course of an admission would be helpful, both for clinical care and for studies of interventions designed to reduce LOS. Prior attempts at developing a model have yielded mixed results. The Michigan Logistic Regression Model displayed excellent predictive ability with an area under the receiver‐operator curve (ROC) of 0.88 using variables including prematurity, FTT, pulmonary disease, other comorbid diseases, and early mechanical ventilation.21 However, when applied to another patient population it did not perform as well. The Rotterdam Model using the variables of weight and supplemental oxygen had an ROC of 0.65.24
Prior prediction models have focused more on birth‐ and disease‐related risk factors than on hospital course factors, particularly common clinical assessments including respiratory status and caloric intake. An additional limitation of prior models is some loss of ability to study the interaction between various predictor variables when using multivariate regression methods.
Our aims were: 1) to study the associations of various clinical markers identifiable during the first two days of the hospital admission with LOS; and 2) to develop a LOS prediction model, using both previously identified risk factors and more detailed clinical data from the first two days of the hospital admission.
MATERIALS AND METHODS
Study Population and Setting
We conducted a retrospective cohort study during a single bronchiolitis season to identify factors predictive of a prolonged length of stay.
Children's Hospital of Wisconsin (CHW) is a 242‐bed tertiary care academic center. The charts of all infants discharged from CHW who met the following criteria were reviewed:
Age <365 days;
Admitted between November 1, 2004 and April 15, 2005;
Bronchiolitis diagnosis using the International Classification of Diseases, 9th edition (ICD‐9) discharge codes 466.11 (RSV bronchiolitis) or 466.19 (bronchiolitis from other organisms);
Placement on the CHW bronchiolitis treatment protocol. Major elements of this protocol include:
respiratory therapists (RT) assessments three times daily providing a standard means of evaluating severity of illness throughout the admission;
pre‐ and post‐intervention assessments.
Infants in this protocol differ from those not on the protocol; their average LOS is one day shorter and their care is more closely aligned with practices established in the Child Health Accountability Initiative (CHAI)25 and the American Academy of Pediatrics Guidelines,26 including: emphasis on clinical diagnosis rather than using laboratory and radiologic testing; avoiding routine bronchodilator use; and decreasing continuous pulse oximetry use. Only patients placed on the bronchiolitis treatment protocol were studied because these infants have a consistent model of care proven to be effective at CHW and other institutions25, 27; 70% of infants admitted to CHW with bronchiolitis were placed on the protocol. Common reasons for not placing infants on the protocol include: 1) the diagnosis of bronchiolitis was initially unclear; 2) the infant had chronic respiratory problems; and 3) physician preference.
Infants with events occurring during the admission not related to bronchiolitis and impacting LOS were excluded. Infants admitted or transferred to the ICU were included if placed on the bronchiolitis protocol; however, few ICU patients were placed on the protocol, as its intent is mainly for the general units.
Data Collected
Five trained abstractors (two were study authors) abstracted the following information from patient records: 1) baseline patient characteristics; 2) initial evaluation: respiratory rate, oxygen saturation, supplemental oxygen use, presence of increased work of breathing, weight, height, Waterlow percentile (percent of ideal body weight);28 3) fluid and nutritional information on hospital days 15; 4) respiratory assessments and treatments on hospital days 15 (clinical respiratory scores, respiratory rates, oxygen saturation, use of supplemental oxygen, medications received; 5) laboratory and imaging results; and 6) diagnoses. Each hospital day was defined as 0600 to 0559 the following day.
Clinical Respiratory Scores
The Children's Hospital of Wisconsin Respiratory Score (CHWRS) is a marker of overall respiratory status (not yet validated.) It contains six variables scored 03 based on degree: breath sounds, dyspnea, retractions, respiratory rate, heart rate, and supplemental oxygen. Scores range from 0 to 18, with lower scores representing less respiratory distress.
Outcomes and Analysis
The primary outcome was LOS, defined as the number of hours from the time a subject arrived on the hospital unit to time of last nursing documentation at time of discharge. The average LOS at CHW of 2.5 days is comparable to the lower end of that reported in the literature (2.85 days21, 22, 29, 30). LOS was dichotomized as short or prolonged, with prolonged LOS defined as 108 hours. We chose this length as it represents the 80th percentile LOS at our institution. Most physicians caring for infants with bronchiolitis at CHW use discharge criteria aligned with those in the hospitalist group's bronchiolitis clinical practice guideline,31 the SOFFFAR criteria: Sno longer dependent on nasopharyngeal suctioning; Ooff oxygen, or to baseline oxygen requirement; Ffamily agreeable to discharge; F follow‐up plan in place; FFeeding well enough to maintain hydration; Aif albuterol responsive, requiring treatments no more frequently than every six hours, Rrespiratory status acceptable (not too tachypneic or in respiratory distress).
Univariate Analysis
We examined the association between selected variables and LOS group (short or prolonged). Three groups of variables were studied: 1) variables identifiable upon admission (Table 1); 2) variables identifiable on hospital days 1 and 2 (Table 2); and 3) variables identifiable later in the admission. The variables evaluated were all non‐normally distributed and, therefore, the MannWhitney test was used to examine differences between groups with continuous or categorical variables. Dichotomous variables were compared using chi‐square or Fisher's exact test. SPSS (Chicago, IL) was used for these analyses. Because of multiple comparisons, 90% power and an alpha of 0.01 were used.
| Variable | Median (IQR), N [% of subjects] | P Value | |
|---|---|---|---|
| Short (N = 225) | Long (N = 47) | ||
| |||
| Age (days) | 134 (63‐225.5) | 139 (63‐240) | 0.86 |
| Gestation (weeks) | 40 (37‐40) | 39 (35‐40) | 0.07 |
| Race | |||
| White | 108 [48] | 23 [49] | 0.91 |
| Other | 117 [52] | 24 [51] | |
| Gender | |||
| Male | 121 [54] | 25 [53] | 0.94 |
| Respiratory support at birth | 22 [10] | 12 [26] | 0.003* |
| Chronic respiratory disease | 21 [9] | 8 [17] | 0.12 |
| Respiratory rate on admission | 56 (44‐64) | 56 (46‐66) | 0.58 |
| Cardiac conditions | 4 [2] | 3 [6] | 0.10 |
| Waterlow percent | 100 (92‐109) {n =203} | 96 (88‐107) {n =46} | 0.16 |
| Days of cough prior to admission | 4 (2‐6) {n =202} | 4 (2‐5) {n = 40} | 0.78 |
| Days of congestion prior to admission | 3 (1‐5) {n =183} | 3 (1‐5) {n =35} | 0.98 |
| Days of fever prior to admission | 1 (0‐3) {n =206} | 1 (0‐2) {n =43} | 0.50 |
| Days of decreased oral intake prior to admission | 1 (0‐2) {n =181} | 1 (0‐1) {n =36} | 0.44 |
| Variable | Median (IQR) or N [%] | P | Median (IQR) or N [%] | P | ||
|---|---|---|---|---|---|---|
| Short | Long | Short | Long | |||
| Hospital Day 1 | Hospital Day 2 | |||||
| ||||||
| Hours of supplemental oxygen | 3 (0‐10) | 11 (5‐17) | <0.001* | 3 (0‐19) | 24 (17‐24) | <0.001* |
| Minimum supplemental oxygen use (liters) | 0 (0‐0.1) | 0.25 (0‐0.5) | <0.001* | 0 (0‐0) | 0.2 (0‐0.5) | <0.001* |
| Maximum supplemental oxygen use (liters) | 0.5 (0‐1) | 0.75 (0.5‐1.5) | <0.001* | 0.2 (0‐0.5) | 1 (0.5‐1.5) | <0.001* |
| Minimum oxygen saturation (percent) | 94 (92‐96) | 94 (92‐96) | 0.89 | 94 (92‐95) | 93 (91‐94) | 0.001* |
| Maximum oxygen saturation (percent) | 99 (98‐100) | 100 (99‐100) | 0.23 | 100 (98‐100) | 100 (99‐100) | 0.37 |
| Minimum respiratory rate | 36 (32‐46) | 36 (32‐46) | 0.92 | 34 (30‐40) | 36 (32‐41) | 0.11 |
| Maximum respiratory rate | 53 (45‐62) | 56 (48‐64) | 0.14 | 55 (48‐64) | 63 (52‐75) | <0.001* |
| Mean respiratory score | 4 (3‐5.5) | 5 (4‐6.7) | 0.008* | 3.4 (2.7‐4.5) | 4.8 (3.7‐7) | <0.001* |
| Change in respiratory score | 0 (0‐1) | 0 (1‐1.5) | 0.3 | 1 (0‐2) | 0 (‐2‐2) | 0.022 |
| Number of times nasopharyngeal suctioned | 1 (0‐2) | 2 (1‐3) | 0.012 | 1 (0‐3) | 4 (2‐5) | <0.001* |
| Calories consumed (Kcal/kg/day) | 53 (22‐82) | 54 (33‐79) | 0.801 | 66 (47‐90) | 54 (21‐72) | 0.001* |
| ICU (% of subjects) | 4 (1.8%) | 2 (4.3%) | 0.28 | 4 (1.8%) | 5 (10.6%) | 0.009* |
Recursive Partitioning Analysis
We chose recursive partitioning as the method for model creation instead of multivariate linear regression in order to: 1) study multiple possible variable interactions without having to create multiple interaction terms; and 2) generate an easy‐to‐use flow diagram to identify infants at risk for prolonged LOS without having to use a complex formula generated by multivariate regression. In recursive partitioning methodology, the statistical program selects the variable among the set of candidate variables that best separates the first parent node with all subjects into short and prolonged stay intermediate nodes. The process is repeated with additional variables selected that further separate the intermediate nodes into short and prolonged stay nodes, until finally a flow diagram is generated, resulting in terminal nodes of predicted short and prolonged stay subjects. Recursive partitioning was performed using Salford Systems' CART software San Diego, CA. The minimum number of cases required in parent/emntermediate nodes was 20, and in terminal nodes was 5. Eighty percent of cases were randomly selected for the learning tree, and 20% in the test tree for cross‐validation.
Sixteen variables were considered a priori as potentially important in affecting LOS and were candidates for inclusion. These included five baseline variables (age, gestation, Waterlow percentile, presence of chronic respiratory disease, and a marker for missing Waterlow percentile) and 11 variables from hospital day 2 (kcal/kg/day consumed, hours of supplemental oxygen, maximum supplemental oxygen use, maximum oxygen saturation, maximum respiratory rate, minimum supplemental oxygen use, minimum oxygen saturation, minimum respiratory rate, mean clinical respiratory score, change in respiratory score, and nasopharyngeal suctioning frequency). Hospital day 2 variables were chosen rather than hospital day 1, because hospital day 1 was only a partial day in the hospital for the majority of subjects. Several aspects of oxygenation were studied because oxygen need has been consistently found to be an important predictor of LOS. We sought to discover which particular aspect of oxygen need was most important. For comparison, recursive partitioning was performed on the variable sets taken from the Michigan (weight, congenital heart disease, failure to thrive, gestational age, chronic pulmonary diseases, and early mechanical ventilation)21 and Rotterdam (weight and need for supplemental oxygen)24 Models.
This study was approved by the CHW Institutional Review Board.
RESULTS
Three hundred forty‐seven infants were admitted during the 20042005 bronchiolitis season, with 273 placed in the bronchiolitis treatment protocol. The charts of these 273 patients were reviewed. One was excluded because of gastrostomy tube placement during the admission. Of the remaining 272 patients, 47 (17.3%) had a LOS 108 hours. The median LOS was 59 hours (range 10334 hours). Two patients had missing data for caloric intake on days 1 and 2; and 23 patients did not have height obtained, therefore their Waterlow classification could not be determined. Historical details concerning fever, congestion, cough, and diminished caloric intake preceding admission were variably reported, resulting in a smaller sample size for these baseline characteristics as described in Table 1.
Univariate Analysis
Baseline characteristics of infants having short and prolonged LOS are described in Table 1. Groups were statistically similar except that the long stay group contained a significantly larger proportion of infants requiring respiratory support at birth (defined as needing intubation, continuous positive airway pressure [CPAP], or oxygen).
Table 2 describes selected variables on hospital days 1 and 2 in infants having short and prolonged LOS. On hospital day 1, infants in the prolonged LOS group had a significantly greater need for supplemental oxygen, and mean respiratory score. On hospital day 2, infants in the prolonged LOS group had a significantly greater need for supplemental oxygen maximum respiratory rate, mean respiratory score, and number of times they were suctioned. They had a significantly lower minimum oxygen saturation and caloric intake. On hospital day 2, the prolonged LOS group had a greater proportion of subjects in the ICU, on CPAP, and on the ventilator.
We examined two characteristics identifiable after the second hospital day. There was a significant difference in: a) the median number of discharge diagnoses in the short LOS group (two) vs the prolonged LOS group (three) (P < 0.001); and b) the presence of apnea during the admission in the short LOS group (0.1%) vs the prolonged LOS group (9%) (P = 0.009).
Recursive Partitioning Model
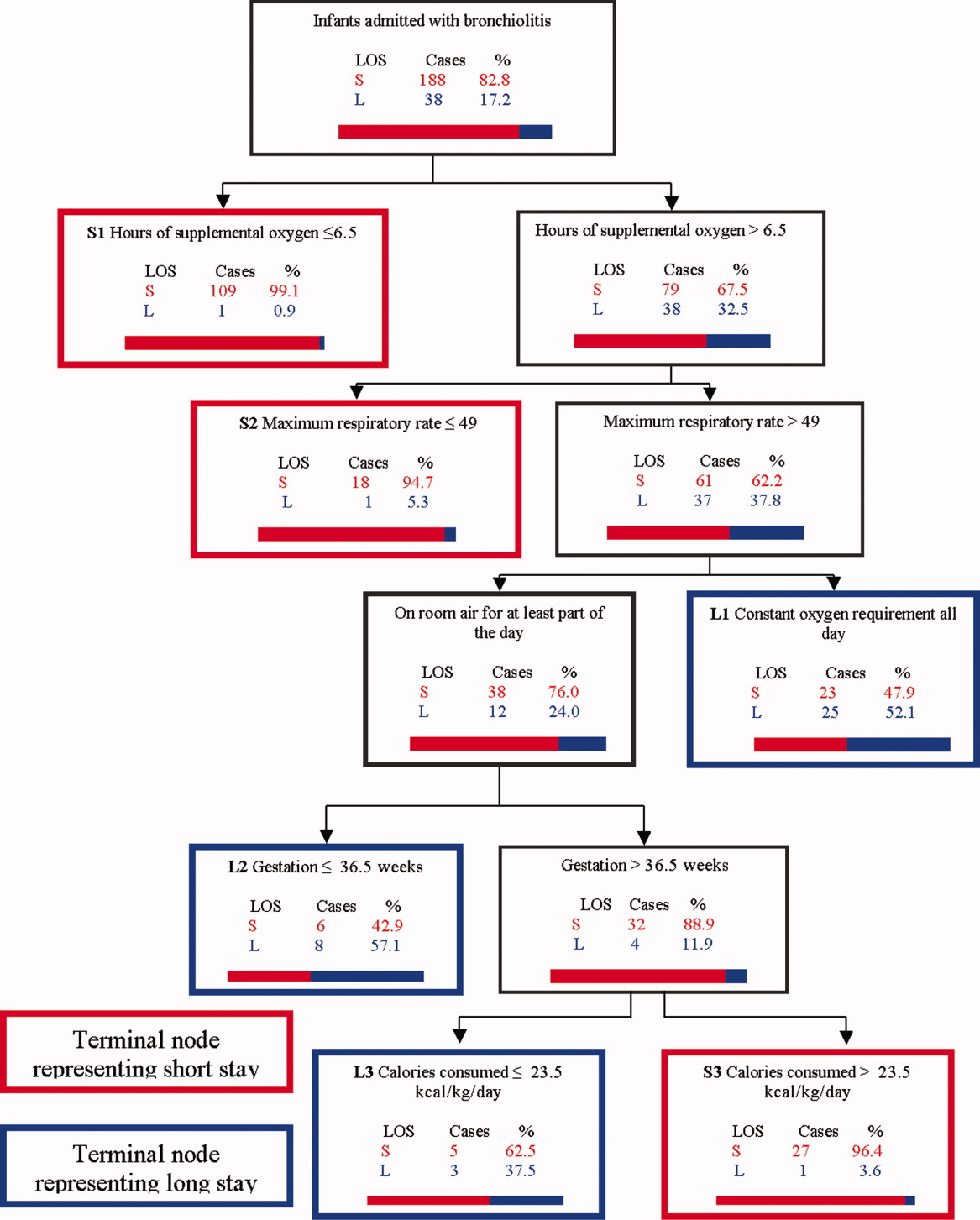
Figure 1 depicts the recursive partitioning model that best predicted LOS. Five variables were selected by the recursive partitioning model. Selected variables, in order of appearance (variable importance is related to order of appearance, ie, most important variable is first), were: hours of supplemental oxygen, maximum respiratory rate, minimum supplemental oxygen use, gestation, and kilocalories (kcal)/kilogram (kg)/day consumed. The characteristics of this model were: ROC 0.89 and 0.72 for the learning and test trees, respectively; sensitivity, 0.85; and specificity, 0.82
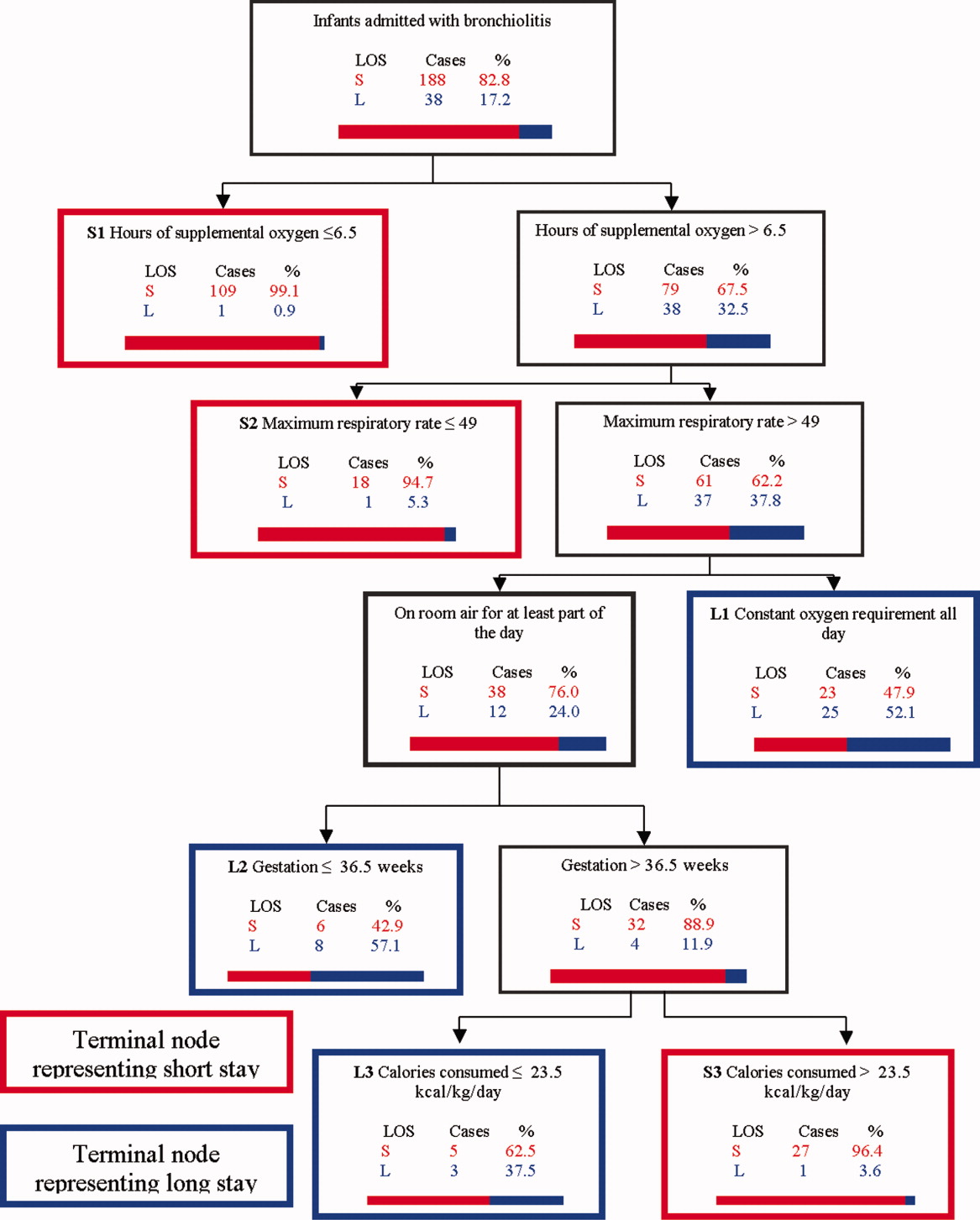
Infants predicted as having a short LOS had three distinct profiles labeled S1, S2, and S3 in Figure 1. The S1 group required 6.5 hours of oxygen. The S2 group required >6.5 hours of oxygen, but had a maximum respiratory rate 49. The S3 group required >6.5 hours of oxygen, had a maximum respiratory rate >49, but were >36.5 week gestation, and consumed >23.5 kcal/kg/day.
Infants predicted as having a long LOS had three distinct profiles labeled L1, L2, and L3 in Figure 1. The L1 group required >6.5 hours of supplemental oxygen, had a maximum respiratory rate >49, and required some level of oxygen support the entire day. The L2 group required >6.5 hours of supplemental oxygen, had a maximum respiratory rate >49, were on room air some portion of the day, but had a gestation 36.5 weeks. The L3 group required >6.5 hours of supplemental oxygen, had a maximum respiratory rate >49, were on room air for some portion of the day, had a gestation >36.5 weeks, but consumed 23.5 kcal/kg/day.
Table 3 compares the performance of our model (the Milwaukee Model), the Michigan Model, and the Rotterdam Model in predicting LOS group. Overall, the Milwaukee Model had the highest ROC 0.89/0.72 for the learning and test trees. All three models had good sensitivity (Milwaukee, 85%; Michigan, 85%), with the Rotterdam Model having the highest (98%). The Milwaukee Model also had good specificity (82%), while the Michigan and Rotterdam Models were less specific (46% and 44%).
| Model | Priors* | Sensitivity | Specificity | Learning Tree ROC | Test Tree ROC | |
|---|---|---|---|---|---|---|
| Long LOS | Short LOS | |||||
| ||||||
| Michigan | 0.5 | 0.5 | 0.85 | 0.46 | 0.69 | 0.56 |
| Rotterdam | 0.5 | 0.5 | 0.98 | 0.44 | 0.73 | 0.61 |
| Milwaukee | 0.5 | 0.5 | 0.85 | 0.82 | 0.89 | 0.72 |
DISCUSSION
We confirmed several previously recognized risk factors for prolonged LOS, including: apnea, at least part of the hospital stay in ICU, use of CPAP, mechanical ventilation, and prematurity. However, most patients admitted with bronchiolitis do not have these risk factors. The major contribution of this study is the evaluation of factors applicable to all patients admitted with bronchiolitis, and a more in‐depth analysis of clinical assessments performed on hospital days 1 and 2 than had been previously reported.
We did find strong associations between a number of clinical assessments and LOS. While some were apparent on day 1 of the admission, the number and degree of clinical differences between infants destined for a short vs prolonged stay were more apparent on hospital day 2. On this day, there were significant differences between the groups in the length and amount of oxygen received, oxygen saturation, maximum respiratory rate, respiratory scores, nasopharyngeal suctioning need, and caloric intake. Interestingly, it was noted the prolonged stay group had overall worsening or a lack of improvement in several clinical markers from day 1 to day 2, in areas where the short stay group showed improvements.
To our knowledge, the Milwaukee Model is the first bronchiolitis LOS prediction model to incorporate several clinical markers occurring early in the hospital stay. These clinical markers were found to be more effective predictors of LOS group in our study population than some of the traditional birth‐ and disease‐related risk factors previously reported. The model highlighted some important interactions among variables, and identified specific profiles of patients likely to have a short or prolonged LOS based on their day 2 clinical status.
The short LOS groups all shared one of the following three features: 1) low duration of oxygen use; 2) absence of tachypnea (tachypnea defined as a respiratory rate >60 in infants <2 months old and >50 for infants between 2 and 12 months old.3234); or 3) absence of severely diminished caloric intake. The prolonged LOS groups shared the common characteristics of higher duration of oxygen use and higher maximum respiratory rates. In addition to these two elements, each long stay group had either a constant oxygen requirement, prematurity (36.5 weeks), or very low caloric intake (<23.5 kcal/kg/day).
While all three models shared good sensitivity, the increased specificity of the Milwaukee Model limits the number of false positives (infants screening as destined for a prolonged LOS who actually will have a short LOS). For clinicians or researchers planning interventions for high‐risk infants, this greater specificity would reduce the number of infants who might unnecessarily receive those interventions. While there are limited proven therapies to hasten the recovery of patients with bronchiolits,35 many treatments and combinations of treatments are currently being used and studied. Nebulized hypertonic saline,36 airway secretion clearance modalities,37 and nutritional supplementation22, 38 are some examples of interventions that could be used and evaluated in infants screened as high risk for prolonged LOS. While it may have been better to identify short vs long stay immediately upon admission or after hospital day 1, it was the more clear separation between the short and long stay groups that occurred on day 2 that allowed us to develop an accurate predictive model. We believe that for infants destined to be in the hospital for at least three more days, a model based on hospital day 2 variables is worthwhile.
When evaluating the characteristics of the three models, it is important to note that they were initially studied in populations with some important differences. Only 11% of Milwaukee patients had chronic respiratory diseases, whereas the previously developed models were generated from a sample with a higher prevalence of chronic respiratory diseases (Michigan, 20%; Rotterdam, 23%). Only 3% of subjects needed placement in the ICU compared to higher rates in prior studies (Michigan, 15%; Rotterdam, 43.5%). In a population of patients with a lower prevalence of chronic respiratory diseases and need for ICU, early clinical markers may become more important in predicting LOS. While our model may generalize well in such a cohort of patients, it might not generalize as well to a cohort with a high prevalence of chronic lung disease and higher need for ICU. It is also important to note that the area under the ROC was lower in the test tree than the learning tree. This variation demonstrates the need for evaluating the performance of this model in additional populations.
This study has several limitations. First, it is a retrospective study of a single bronchiolitis season at a single institution. Second, the authors served as data abstractors and could have been biased, as they were not blinded. Third, four out of the five markers in our model are clinical markers that could vary based on clinical assessment skills and institutional practice. For example, oxygen use is dependent on the practice of nurses and respiratory therapists charged with regulating the oxygen delivery. However, the practice of initiating and weaning oxygen is fairly standardized at our institution. Fourth, environmental and social risk factors, such as day care attendance, school‐age siblings, and smoke exposure, can impact LOS but were not included in our model. Fifth, we do not have data on those infants not included in the bronchiolitis protocol. It is possible that they differed from those in protocol. Finally, six infants were either placed or transferred to the ICU on day 1, which may make them inherently different than the other infants in the model. However, four out of these six infants did go on to have a short stay, highlighting the fact that many other factors affect LOS.
We believe this model may be useful because the clinical markers it uses represent some of the key problems seen in bronchiolitis (poor oxygenation, tachypnea, and poor feeding). Careful assessment of these clinical markers can allow effective prediction of those infants likely to have a prolonged LOS. This early risk assessment could allow more effective targeting of interventions to help high‐risk infants.
CONCLUSIONS
There are important differences between infants with bronchiolitis having short and prolonged hospital stays, including several clinical markers identifiable on hospital day 2, such as the length and amount of oxygen received, minimum oxygen saturation, maximum respiratory rate, clinical respiratory scores, deep suctioning need, and caloric intake. The Milwaukee Model uses the number of hours of supplemental oxygen, respiratory rate, minimum supplemental oxygen use, gestation, and caloric intake to predict short or prolonged LOS. It performed well with a good ROC, sensitivity, and specificity in one population of infants with a low prevalence of chronic respiratory disease.
- ,.Clinical significance of respiratory syncytial virus.Postgrad Med.1964;35:460–465.
- ,,,,.Respiratory syncytial virus infection in young hospitalized children. Identification of risk patients and prevention of nosocomial spread by rapid diagnosis.Acta Paediatr Scand.1983;72(1):47–51.
- .Pathogenesis of bronchiolitis—epidemiologic considerations.Pediatr Res.1977;11(3 pt 2):239–243.
- ,,.Respiratory syncytial virus infection in children with bronchopulmonary dysplasia.Pediatrics.1988;82(2):199–203.
- ,,, et al.Respiratory syncytial viral infection in children with compromised immune function.N Engl J Med.1986;315(2):77–81.
- ,,,,.Risk of secondary bacterial infection in infants hospitalized with respiratory syncytial viral infection.J Pediatr.1988;113(2):266–271.
- ,,,,,.Respiratory syncytial viral infection in infants with congenital heart disease.N Engl J Med.1982;307(7):397–400.
- ,,, et al.Hospitalized children with respiratory syncytial virus infection and neuromuscular impairment face an increased risk of a complicated course.Pediatr Infect Dis J.2007;26(6):485–491.
- ,,.Rehospitalization for respiratory illness in infants of less than 32 weeks' gestation.Pediatrics.1991;88(3):527–532.
- ,,, et al.Respiratory syncytial virus (RSV) immune globulin intravenous therapy for RSV lower respiratory tract infection in infants and young children at high risk for severe RSV infections: Respiratory Syncytial Virus Immune Globulin Study Group.Pediatrics.1997;99(3):454–461.
- ,,,,.Risk factors for bronchiolitis‐associated deaths among infants in the United States.Pediatr Infect Dis J.2003;22(6):483–490.
- ,.Risk factors for severe respiratory syncytial virus infection in infants.Respir Med.2002;96(suppl B):S9–S14.
- ,,,,.Rehospitalization for respiratory syncytial virus among premature infants.Pediatrics.1999;104(4 pt 1):894–899.
- ,,,,.Risk of respiratory syncytial virus infection for infants from low‐income families in relationship to age, sex, ethnic group, and maternal antibody level.J Pediatr.1981;98(5):708–715.
- ,,,.Preterm twins and triplets. A high‐risk group for severe respiratory syncytial virus infection.Am J Dis Child.1993;147(3):303–306.
- ,,, et al.Rehospitalization because of respiratory syncytial virus infection in premature infants younger than 33 weeks of gestation: a prospective study. IRIS Study Group.Pediatr Infect Dis J.2000;19(7):592–597.
- ,.Parental smoking, presence of older siblings, and family history of asthma increase risk of bronchiolitis.Am J Dis Child.1986;140(8):806–812.
- ,,,,.Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis.J Pediatr.1988;113(5):826–830.
- ,,, et al.Variable morbidity of respiratory syncytial virus infection in patients with underlying lung disease: a review of the PICNIC RSV database. Pediatric Investigators Collaborative Network on Infections in Canada.Pediatr Infect Dis J.1999;18(10):866–869.
- ,,,,.Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid.J Pediatr.2000;137(6):865–870.
- ,.Severity of illness models for respiratory syncytial virus‐associated hospitalization.Am J Respir Crit Care Med.1999;159(4 pt 1):1234–1240.
- ,.Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis.Pediatrics.2008;121(3):470–475.
- ,,.Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection.J Pediatr.1995;126(2):212–219.
- ,,,.Prediction of duration of hospitalization in respiratory syncytial virus infection.Pediatr Pulmonol.2002;33(6):453–457.
- ,,,,,.Impact of a bronchiolitis guideline: a multisite demonstration project.Chest.2002;121(6):1789–1797.
- , , , , , , , , , Diagnosis and management of bronchiolitis.Pediatrics.2006;118(4):1774–1793.
- ,,, et al.Evaluation of an evidence‐based guideline for bronchiolitis.Pediatrics.1999;104(6):1334–1341.
- .Classification and definition of protein‐calorie malnutrition.Br Med J.1972;3(5826):566–569.
- ,,,,.Inpatient care for uncomplicated bronchiolitis: comparison with Milliman and Robertson guidelines.Arch Pediatr Adolesc Med.2001;155(12):1323–1327.
- ,,,.Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations.Arch Pediatr Adolesc Med.2004;158(6):527–530.
- .Bronchiolitis clinical practice guideline.Children's Hospital of Wisconsin Intranet;2007. http://clinicalpractice.chw.org/display/displayFile.asp?docid=393185(2):319–336.
- ,.The rational clinical examination. Does this infant have pneumonia?JAMA.1998;279(4):308–313.
- ,.Focused ethnographic studies in the WHO Programme for the Control of Acute Respiratory Infections.Med Anthropol.1994;15(4):409–424.
- ,.Bronchiolitis: recent evidence on diagnosis and management.Pediatrics.125(2):342–349.
- ,,,.Nebulized hypertonic saline solution for acute bronchiolitis in infants.Cochrane Database Syst Rev.2008(4):CD006458.
- .Airway clearance applications in infants and children.Respir Care.2007;52(10):1382–1391.
- ,.Immunonutrition in critically ill patients: a systematic review and analysis of the literature.Intensive Care Med.2008;34(11):1980–1990.
Prior studies have identified risk factors for increased severity of illness, readmission, or prolonged length of stay (LOS) in infants admitted with bronchiolitis.123 These risk factors include birth‐related factors (prematurity, birth within six months of respiratory syncytial virus [RSV] season, discharge from the neonatal intensive care unit during winter, multiple birth infant), environmental factors (day care attendance, school‐age siblings, smoke exposure), and underlying diseases (chronic lung disease and other pulmonary conditions, failure to thrive (FTT), congenital heart disease, immunologic disorders, and neuromuscular disease).123 Additional risk factors occurring during the bronchiolitis course that have been associated with prolonged hospital course are mechanical ventilation, intensive care unit (ICU) admission, hypoxia on admission, apnea, feeding problems, and duration of supplemental oxygen.2123
Having a reliable model to identify infants at high risk for prolonged LOS early in the course of an admission would be helpful, both for clinical care and for studies of interventions designed to reduce LOS. Prior attempts at developing a model have yielded mixed results. The Michigan Logistic Regression Model displayed excellent predictive ability with an area under the receiver‐operator curve (ROC) of 0.88 using variables including prematurity, FTT, pulmonary disease, other comorbid diseases, and early mechanical ventilation.21 However, when applied to another patient population it did not perform as well. The Rotterdam Model using the variables of weight and supplemental oxygen had an ROC of 0.65.24
Prior prediction models have focused more on birth‐ and disease‐related risk factors than on hospital course factors, particularly common clinical assessments including respiratory status and caloric intake. An additional limitation of prior models is some loss of ability to study the interaction between various predictor variables when using multivariate regression methods.
Our aims were: 1) to study the associations of various clinical markers identifiable during the first two days of the hospital admission with LOS; and 2) to develop a LOS prediction model, using both previously identified risk factors and more detailed clinical data from the first two days of the hospital admission.
MATERIALS AND METHODS
Study Population and Setting
We conducted a retrospective cohort study during a single bronchiolitis season to identify factors predictive of a prolonged length of stay.
Children's Hospital of Wisconsin (CHW) is a 242‐bed tertiary care academic center. The charts of all infants discharged from CHW who met the following criteria were reviewed:
Age <365 days;
Admitted between November 1, 2004 and April 15, 2005;
Bronchiolitis diagnosis using the International Classification of Diseases, 9th edition (ICD‐9) discharge codes 466.11 (RSV bronchiolitis) or 466.19 (bronchiolitis from other organisms);
Placement on the CHW bronchiolitis treatment protocol. Major elements of this protocol include:
respiratory therapists (RT) assessments three times daily providing a standard means of evaluating severity of illness throughout the admission;
pre‐ and post‐intervention assessments.
Infants in this protocol differ from those not on the protocol; their average LOS is one day shorter and their care is more closely aligned with practices established in the Child Health Accountability Initiative (CHAI)25 and the American Academy of Pediatrics Guidelines,26 including: emphasis on clinical diagnosis rather than using laboratory and radiologic testing; avoiding routine bronchodilator use; and decreasing continuous pulse oximetry use. Only patients placed on the bronchiolitis treatment protocol were studied because these infants have a consistent model of care proven to be effective at CHW and other institutions25, 27; 70% of infants admitted to CHW with bronchiolitis were placed on the protocol. Common reasons for not placing infants on the protocol include: 1) the diagnosis of bronchiolitis was initially unclear; 2) the infant had chronic respiratory problems; and 3) physician preference.
Infants with events occurring during the admission not related to bronchiolitis and impacting LOS were excluded. Infants admitted or transferred to the ICU were included if placed on the bronchiolitis protocol; however, few ICU patients were placed on the protocol, as its intent is mainly for the general units.
Data Collected
Five trained abstractors (two were study authors) abstracted the following information from patient records: 1) baseline patient characteristics; 2) initial evaluation: respiratory rate, oxygen saturation, supplemental oxygen use, presence of increased work of breathing, weight, height, Waterlow percentile (percent of ideal body weight);28 3) fluid and nutritional information on hospital days 15; 4) respiratory assessments and treatments on hospital days 15 (clinical respiratory scores, respiratory rates, oxygen saturation, use of supplemental oxygen, medications received; 5) laboratory and imaging results; and 6) diagnoses. Each hospital day was defined as 0600 to 0559 the following day.
Clinical Respiratory Scores
The Children's Hospital of Wisconsin Respiratory Score (CHWRS) is a marker of overall respiratory status (not yet validated.) It contains six variables scored 03 based on degree: breath sounds, dyspnea, retractions, respiratory rate, heart rate, and supplemental oxygen. Scores range from 0 to 18, with lower scores representing less respiratory distress.
Outcomes and Analysis
The primary outcome was LOS, defined as the number of hours from the time a subject arrived on the hospital unit to time of last nursing documentation at time of discharge. The average LOS at CHW of 2.5 days is comparable to the lower end of that reported in the literature (2.85 days21, 22, 29, 30). LOS was dichotomized as short or prolonged, with prolonged LOS defined as 108 hours. We chose this length as it represents the 80th percentile LOS at our institution. Most physicians caring for infants with bronchiolitis at CHW use discharge criteria aligned with those in the hospitalist group's bronchiolitis clinical practice guideline,31 the SOFFFAR criteria: Sno longer dependent on nasopharyngeal suctioning; Ooff oxygen, or to baseline oxygen requirement; Ffamily agreeable to discharge; F follow‐up plan in place; FFeeding well enough to maintain hydration; Aif albuterol responsive, requiring treatments no more frequently than every six hours, Rrespiratory status acceptable (not too tachypneic or in respiratory distress).
Univariate Analysis
We examined the association between selected variables and LOS group (short or prolonged). Three groups of variables were studied: 1) variables identifiable upon admission (Table 1); 2) variables identifiable on hospital days 1 and 2 (Table 2); and 3) variables identifiable later in the admission. The variables evaluated were all non‐normally distributed and, therefore, the MannWhitney test was used to examine differences between groups with continuous or categorical variables. Dichotomous variables were compared using chi‐square or Fisher's exact test. SPSS (Chicago, IL) was used for these analyses. Because of multiple comparisons, 90% power and an alpha of 0.01 were used.
| Variable | Median (IQR), N [% of subjects] | P Value | |
|---|---|---|---|
| Short (N = 225) | Long (N = 47) | ||
| |||
| Age (days) | 134 (63‐225.5) | 139 (63‐240) | 0.86 |
| Gestation (weeks) | 40 (37‐40) | 39 (35‐40) | 0.07 |
| Race | |||
| White | 108 [48] | 23 [49] | 0.91 |
| Other | 117 [52] | 24 [51] | |
| Gender | |||
| Male | 121 [54] | 25 [53] | 0.94 |
| Respiratory support at birth | 22 [10] | 12 [26] | 0.003* |
| Chronic respiratory disease | 21 [9] | 8 [17] | 0.12 |
| Respiratory rate on admission | 56 (44‐64) | 56 (46‐66) | 0.58 |
| Cardiac conditions | 4 [2] | 3 [6] | 0.10 |
| Waterlow percent | 100 (92‐109) {n =203} | 96 (88‐107) {n =46} | 0.16 |
| Days of cough prior to admission | 4 (2‐6) {n =202} | 4 (2‐5) {n = 40} | 0.78 |
| Days of congestion prior to admission | 3 (1‐5) {n =183} | 3 (1‐5) {n =35} | 0.98 |
| Days of fever prior to admission | 1 (0‐3) {n =206} | 1 (0‐2) {n =43} | 0.50 |
| Days of decreased oral intake prior to admission | 1 (0‐2) {n =181} | 1 (0‐1) {n =36} | 0.44 |
| Variable | Median (IQR) or N [%] | P | Median (IQR) or N [%] | P | ||
|---|---|---|---|---|---|---|
| Short | Long | Short | Long | |||
| Hospital Day 1 | Hospital Day 2 | |||||
| ||||||
| Hours of supplemental oxygen | 3 (0‐10) | 11 (5‐17) | <0.001* | 3 (0‐19) | 24 (17‐24) | <0.001* |
| Minimum supplemental oxygen use (liters) | 0 (0‐0.1) | 0.25 (0‐0.5) | <0.001* | 0 (0‐0) | 0.2 (0‐0.5) | <0.001* |
| Maximum supplemental oxygen use (liters) | 0.5 (0‐1) | 0.75 (0.5‐1.5) | <0.001* | 0.2 (0‐0.5) | 1 (0.5‐1.5) | <0.001* |
| Minimum oxygen saturation (percent) | 94 (92‐96) | 94 (92‐96) | 0.89 | 94 (92‐95) | 93 (91‐94) | 0.001* |
| Maximum oxygen saturation (percent) | 99 (98‐100) | 100 (99‐100) | 0.23 | 100 (98‐100) | 100 (99‐100) | 0.37 |
| Minimum respiratory rate | 36 (32‐46) | 36 (32‐46) | 0.92 | 34 (30‐40) | 36 (32‐41) | 0.11 |
| Maximum respiratory rate | 53 (45‐62) | 56 (48‐64) | 0.14 | 55 (48‐64) | 63 (52‐75) | <0.001* |
| Mean respiratory score | 4 (3‐5.5) | 5 (4‐6.7) | 0.008* | 3.4 (2.7‐4.5) | 4.8 (3.7‐7) | <0.001* |
| Change in respiratory score | 0 (0‐1) | 0 (1‐1.5) | 0.3 | 1 (0‐2) | 0 (‐2‐2) | 0.022 |
| Number of times nasopharyngeal suctioned | 1 (0‐2) | 2 (1‐3) | 0.012 | 1 (0‐3) | 4 (2‐5) | <0.001* |
| Calories consumed (Kcal/kg/day) | 53 (22‐82) | 54 (33‐79) | 0.801 | 66 (47‐90) | 54 (21‐72) | 0.001* |
| ICU (% of subjects) | 4 (1.8%) | 2 (4.3%) | 0.28 | 4 (1.8%) | 5 (10.6%) | 0.009* |
Recursive Partitioning Analysis
We chose recursive partitioning as the method for model creation instead of multivariate linear regression in order to: 1) study multiple possible variable interactions without having to create multiple interaction terms; and 2) generate an easy‐to‐use flow diagram to identify infants at risk for prolonged LOS without having to use a complex formula generated by multivariate regression. In recursive partitioning methodology, the statistical program selects the variable among the set of candidate variables that best separates the first parent node with all subjects into short and prolonged stay intermediate nodes. The process is repeated with additional variables selected that further separate the intermediate nodes into short and prolonged stay nodes, until finally a flow diagram is generated, resulting in terminal nodes of predicted short and prolonged stay subjects. Recursive partitioning was performed using Salford Systems' CART software San Diego, CA. The minimum number of cases required in parent/emntermediate nodes was 20, and in terminal nodes was 5. Eighty percent of cases were randomly selected for the learning tree, and 20% in the test tree for cross‐validation.
Sixteen variables were considered a priori as potentially important in affecting LOS and were candidates for inclusion. These included five baseline variables (age, gestation, Waterlow percentile, presence of chronic respiratory disease, and a marker for missing Waterlow percentile) and 11 variables from hospital day 2 (kcal/kg/day consumed, hours of supplemental oxygen, maximum supplemental oxygen use, maximum oxygen saturation, maximum respiratory rate, minimum supplemental oxygen use, minimum oxygen saturation, minimum respiratory rate, mean clinical respiratory score, change in respiratory score, and nasopharyngeal suctioning frequency). Hospital day 2 variables were chosen rather than hospital day 1, because hospital day 1 was only a partial day in the hospital for the majority of subjects. Several aspects of oxygenation were studied because oxygen need has been consistently found to be an important predictor of LOS. We sought to discover which particular aspect of oxygen need was most important. For comparison, recursive partitioning was performed on the variable sets taken from the Michigan (weight, congenital heart disease, failure to thrive, gestational age, chronic pulmonary diseases, and early mechanical ventilation)21 and Rotterdam (weight and need for supplemental oxygen)24 Models.
This study was approved by the CHW Institutional Review Board.
RESULTS
Three hundred forty‐seven infants were admitted during the 20042005 bronchiolitis season, with 273 placed in the bronchiolitis treatment protocol. The charts of these 273 patients were reviewed. One was excluded because of gastrostomy tube placement during the admission. Of the remaining 272 patients, 47 (17.3%) had a LOS 108 hours. The median LOS was 59 hours (range 10334 hours). Two patients had missing data for caloric intake on days 1 and 2; and 23 patients did not have height obtained, therefore their Waterlow classification could not be determined. Historical details concerning fever, congestion, cough, and diminished caloric intake preceding admission were variably reported, resulting in a smaller sample size for these baseline characteristics as described in Table 1.
Univariate Analysis
Baseline characteristics of infants having short and prolonged LOS are described in Table 1. Groups were statistically similar except that the long stay group contained a significantly larger proportion of infants requiring respiratory support at birth (defined as needing intubation, continuous positive airway pressure [CPAP], or oxygen).
Table 2 describes selected variables on hospital days 1 and 2 in infants having short and prolonged LOS. On hospital day 1, infants in the prolonged LOS group had a significantly greater need for supplemental oxygen, and mean respiratory score. On hospital day 2, infants in the prolonged LOS group had a significantly greater need for supplemental oxygen maximum respiratory rate, mean respiratory score, and number of times they were suctioned. They had a significantly lower minimum oxygen saturation and caloric intake. On hospital day 2, the prolonged LOS group had a greater proportion of subjects in the ICU, on CPAP, and on the ventilator.
We examined two characteristics identifiable after the second hospital day. There was a significant difference in: a) the median number of discharge diagnoses in the short LOS group (two) vs the prolonged LOS group (three) (P < 0.001); and b) the presence of apnea during the admission in the short LOS group (0.1%) vs the prolonged LOS group (9%) (P = 0.009).
Recursive Partitioning Model
Figure 1 depicts the recursive partitioning model that best predicted LOS. Five variables were selected by the recursive partitioning model. Selected variables, in order of appearance (variable importance is related to order of appearance, ie, most important variable is first), were: hours of supplemental oxygen, maximum respiratory rate, minimum supplemental oxygen use, gestation, and kilocalories (kcal)/kilogram (kg)/day consumed. The characteristics of this model were: ROC 0.89 and 0.72 for the learning and test trees, respectively; sensitivity, 0.85; and specificity, 0.82

Infants predicted as having a short LOS had three distinct profiles labeled S1, S2, and S3 in Figure 1. The S1 group required 6.5 hours of oxygen. The S2 group required >6.5 hours of oxygen, but had a maximum respiratory rate 49. The S3 group required >6.5 hours of oxygen, had a maximum respiratory rate >49, but were >36.5 week gestation, and consumed >23.5 kcal/kg/day.
Infants predicted as having a long LOS had three distinct profiles labeled L1, L2, and L3 in Figure 1. The L1 group required >6.5 hours of supplemental oxygen, had a maximum respiratory rate >49, and required some level of oxygen support the entire day. The L2 group required >6.5 hours of supplemental oxygen, had a maximum respiratory rate >49, were on room air some portion of the day, but had a gestation 36.5 weeks. The L3 group required >6.5 hours of supplemental oxygen, had a maximum respiratory rate >49, were on room air for some portion of the day, had a gestation >36.5 weeks, but consumed 23.5 kcal/kg/day.
Table 3 compares the performance of our model (the Milwaukee Model), the Michigan Model, and the Rotterdam Model in predicting LOS group. Overall, the Milwaukee Model had the highest ROC 0.89/0.72 for the learning and test trees. All three models had good sensitivity (Milwaukee, 85%; Michigan, 85%), with the Rotterdam Model having the highest (98%). The Milwaukee Model also had good specificity (82%), while the Michigan and Rotterdam Models were less specific (46% and 44%).
| Model | Priors* | Sensitivity | Specificity | Learning Tree ROC | Test Tree ROC | |
|---|---|---|---|---|---|---|
| Long LOS | Short LOS | |||||
| ||||||
| Michigan | 0.5 | 0.5 | 0.85 | 0.46 | 0.69 | 0.56 |
| Rotterdam | 0.5 | 0.5 | 0.98 | 0.44 | 0.73 | 0.61 |
| Milwaukee | 0.5 | 0.5 | 0.85 | 0.82 | 0.89 | 0.72 |
DISCUSSION
We confirmed several previously recognized risk factors for prolonged LOS, including: apnea, at least part of the hospital stay in ICU, use of CPAP, mechanical ventilation, and prematurity. However, most patients admitted with bronchiolitis do not have these risk factors. The major contribution of this study is the evaluation of factors applicable to all patients admitted with bronchiolitis, and a more in‐depth analysis of clinical assessments performed on hospital days 1 and 2 than had been previously reported.
We did find strong associations between a number of clinical assessments and LOS. While some were apparent on day 1 of the admission, the number and degree of clinical differences between infants destined for a short vs prolonged stay were more apparent on hospital day 2. On this day, there were significant differences between the groups in the length and amount of oxygen received, oxygen saturation, maximum respiratory rate, respiratory scores, nasopharyngeal suctioning need, and caloric intake. Interestingly, it was noted the prolonged stay group had overall worsening or a lack of improvement in several clinical markers from day 1 to day 2, in areas where the short stay group showed improvements.
To our knowledge, the Milwaukee Model is the first bronchiolitis LOS prediction model to incorporate several clinical markers occurring early in the hospital stay. These clinical markers were found to be more effective predictors of LOS group in our study population than some of the traditional birth‐ and disease‐related risk factors previously reported. The model highlighted some important interactions among variables, and identified specific profiles of patients likely to have a short or prolonged LOS based on their day 2 clinical status.
The short LOS groups all shared one of the following three features: 1) low duration of oxygen use; 2) absence of tachypnea (tachypnea defined as a respiratory rate >60 in infants <2 months old and >50 for infants between 2 and 12 months old.3234); or 3) absence of severely diminished caloric intake. The prolonged LOS groups shared the common characteristics of higher duration of oxygen use and higher maximum respiratory rates. In addition to these two elements, each long stay group had either a constant oxygen requirement, prematurity (36.5 weeks), or very low caloric intake (<23.5 kcal/kg/day).
While all three models shared good sensitivity, the increased specificity of the Milwaukee Model limits the number of false positives (infants screening as destined for a prolonged LOS who actually will have a short LOS). For clinicians or researchers planning interventions for high‐risk infants, this greater specificity would reduce the number of infants who might unnecessarily receive those interventions. While there are limited proven therapies to hasten the recovery of patients with bronchiolits,35 many treatments and combinations of treatments are currently being used and studied. Nebulized hypertonic saline,36 airway secretion clearance modalities,37 and nutritional supplementation22, 38 are some examples of interventions that could be used and evaluated in infants screened as high risk for prolonged LOS. While it may have been better to identify short vs long stay immediately upon admission or after hospital day 1, it was the more clear separation between the short and long stay groups that occurred on day 2 that allowed us to develop an accurate predictive model. We believe that for infants destined to be in the hospital for at least three more days, a model based on hospital day 2 variables is worthwhile.
When evaluating the characteristics of the three models, it is important to note that they were initially studied in populations with some important differences. Only 11% of Milwaukee patients had chronic respiratory diseases, whereas the previously developed models were generated from a sample with a higher prevalence of chronic respiratory diseases (Michigan, 20%; Rotterdam, 23%). Only 3% of subjects needed placement in the ICU compared to higher rates in prior studies (Michigan, 15%; Rotterdam, 43.5%). In a population of patients with a lower prevalence of chronic respiratory diseases and need for ICU, early clinical markers may become more important in predicting LOS. While our model may generalize well in such a cohort of patients, it might not generalize as well to a cohort with a high prevalence of chronic lung disease and higher need for ICU. It is also important to note that the area under the ROC was lower in the test tree than the learning tree. This variation demonstrates the need for evaluating the performance of this model in additional populations.
This study has several limitations. First, it is a retrospective study of a single bronchiolitis season at a single institution. Second, the authors served as data abstractors and could have been biased, as they were not blinded. Third, four out of the five markers in our model are clinical markers that could vary based on clinical assessment skills and institutional practice. For example, oxygen use is dependent on the practice of nurses and respiratory therapists charged with regulating the oxygen delivery. However, the practice of initiating and weaning oxygen is fairly standardized at our institution. Fourth, environmental and social risk factors, such as day care attendance, school‐age siblings, and smoke exposure, can impact LOS but were not included in our model. Fifth, we do not have data on those infants not included in the bronchiolitis protocol. It is possible that they differed from those in protocol. Finally, six infants were either placed or transferred to the ICU on day 1, which may make them inherently different than the other infants in the model. However, four out of these six infants did go on to have a short stay, highlighting the fact that many other factors affect LOS.
We believe this model may be useful because the clinical markers it uses represent some of the key problems seen in bronchiolitis (poor oxygenation, tachypnea, and poor feeding). Careful assessment of these clinical markers can allow effective prediction of those infants likely to have a prolonged LOS. This early risk assessment could allow more effective targeting of interventions to help high‐risk infants.
CONCLUSIONS
There are important differences between infants with bronchiolitis having short and prolonged hospital stays, including several clinical markers identifiable on hospital day 2, such as the length and amount of oxygen received, minimum oxygen saturation, maximum respiratory rate, clinical respiratory scores, deep suctioning need, and caloric intake. The Milwaukee Model uses the number of hours of supplemental oxygen, respiratory rate, minimum supplemental oxygen use, gestation, and caloric intake to predict short or prolonged LOS. It performed well with a good ROC, sensitivity, and specificity in one population of infants with a low prevalence of chronic respiratory disease.
Prior studies have identified risk factors for increased severity of illness, readmission, or prolonged length of stay (LOS) in infants admitted with bronchiolitis.123 These risk factors include birth‐related factors (prematurity, birth within six months of respiratory syncytial virus [RSV] season, discharge from the neonatal intensive care unit during winter, multiple birth infant), environmental factors (day care attendance, school‐age siblings, smoke exposure), and underlying diseases (chronic lung disease and other pulmonary conditions, failure to thrive (FTT), congenital heart disease, immunologic disorders, and neuromuscular disease).123 Additional risk factors occurring during the bronchiolitis course that have been associated with prolonged hospital course are mechanical ventilation, intensive care unit (ICU) admission, hypoxia on admission, apnea, feeding problems, and duration of supplemental oxygen.2123
Having a reliable model to identify infants at high risk for prolonged LOS early in the course of an admission would be helpful, both for clinical care and for studies of interventions designed to reduce LOS. Prior attempts at developing a model have yielded mixed results. The Michigan Logistic Regression Model displayed excellent predictive ability with an area under the receiver‐operator curve (ROC) of 0.88 using variables including prematurity, FTT, pulmonary disease, other comorbid diseases, and early mechanical ventilation.21 However, when applied to another patient population it did not perform as well. The Rotterdam Model using the variables of weight and supplemental oxygen had an ROC of 0.65.24
Prior prediction models have focused more on birth‐ and disease‐related risk factors than on hospital course factors, particularly common clinical assessments including respiratory status and caloric intake. An additional limitation of prior models is some loss of ability to study the interaction between various predictor variables when using multivariate regression methods.
Our aims were: 1) to study the associations of various clinical markers identifiable during the first two days of the hospital admission with LOS; and 2) to develop a LOS prediction model, using both previously identified risk factors and more detailed clinical data from the first two days of the hospital admission.
MATERIALS AND METHODS
Study Population and Setting
We conducted a retrospective cohort study during a single bronchiolitis season to identify factors predictive of a prolonged length of stay.
Children's Hospital of Wisconsin (CHW) is a 242‐bed tertiary care academic center. The charts of all infants discharged from CHW who met the following criteria were reviewed:
Age <365 days;
Admitted between November 1, 2004 and April 15, 2005;
Bronchiolitis diagnosis using the International Classification of Diseases, 9th edition (ICD‐9) discharge codes 466.11 (RSV bronchiolitis) or 466.19 (bronchiolitis from other organisms);
Placement on the CHW bronchiolitis treatment protocol. Major elements of this protocol include:
respiratory therapists (RT) assessments three times daily providing a standard means of evaluating severity of illness throughout the admission;
pre‐ and post‐intervention assessments.
Infants in this protocol differ from those not on the protocol; their average LOS is one day shorter and their care is more closely aligned with practices established in the Child Health Accountability Initiative (CHAI)25 and the American Academy of Pediatrics Guidelines,26 including: emphasis on clinical diagnosis rather than using laboratory and radiologic testing; avoiding routine bronchodilator use; and decreasing continuous pulse oximetry use. Only patients placed on the bronchiolitis treatment protocol were studied because these infants have a consistent model of care proven to be effective at CHW and other institutions25, 27; 70% of infants admitted to CHW with bronchiolitis were placed on the protocol. Common reasons for not placing infants on the protocol include: 1) the diagnosis of bronchiolitis was initially unclear; 2) the infant had chronic respiratory problems; and 3) physician preference.
Infants with events occurring during the admission not related to bronchiolitis and impacting LOS were excluded. Infants admitted or transferred to the ICU were included if placed on the bronchiolitis protocol; however, few ICU patients were placed on the protocol, as its intent is mainly for the general units.
Data Collected
Five trained abstractors (two were study authors) abstracted the following information from patient records: 1) baseline patient characteristics; 2) initial evaluation: respiratory rate, oxygen saturation, supplemental oxygen use, presence of increased work of breathing, weight, height, Waterlow percentile (percent of ideal body weight);28 3) fluid and nutritional information on hospital days 15; 4) respiratory assessments and treatments on hospital days 15 (clinical respiratory scores, respiratory rates, oxygen saturation, use of supplemental oxygen, medications received; 5) laboratory and imaging results; and 6) diagnoses. Each hospital day was defined as 0600 to 0559 the following day.
Clinical Respiratory Scores
The Children's Hospital of Wisconsin Respiratory Score (CHWRS) is a marker of overall respiratory status (not yet validated.) It contains six variables scored 03 based on degree: breath sounds, dyspnea, retractions, respiratory rate, heart rate, and supplemental oxygen. Scores range from 0 to 18, with lower scores representing less respiratory distress.
Outcomes and Analysis
The primary outcome was LOS, defined as the number of hours from the time a subject arrived on the hospital unit to time of last nursing documentation at time of discharge. The average LOS at CHW of 2.5 days is comparable to the lower end of that reported in the literature (2.85 days21, 22, 29, 30). LOS was dichotomized as short or prolonged, with prolonged LOS defined as 108 hours. We chose this length as it represents the 80th percentile LOS at our institution. Most physicians caring for infants with bronchiolitis at CHW use discharge criteria aligned with those in the hospitalist group's bronchiolitis clinical practice guideline,31 the SOFFFAR criteria: Sno longer dependent on nasopharyngeal suctioning; Ooff oxygen, or to baseline oxygen requirement; Ffamily agreeable to discharge; F follow‐up plan in place; FFeeding well enough to maintain hydration; Aif albuterol responsive, requiring treatments no more frequently than every six hours, Rrespiratory status acceptable (not too tachypneic or in respiratory distress).
Univariate Analysis
We examined the association between selected variables and LOS group (short or prolonged). Three groups of variables were studied: 1) variables identifiable upon admission (Table 1); 2) variables identifiable on hospital days 1 and 2 (Table 2); and 3) variables identifiable later in the admission. The variables evaluated were all non‐normally distributed and, therefore, the MannWhitney test was used to examine differences between groups with continuous or categorical variables. Dichotomous variables were compared using chi‐square or Fisher's exact test. SPSS (Chicago, IL) was used for these analyses. Because of multiple comparisons, 90% power and an alpha of 0.01 were used.
| Variable | Median (IQR), N [% of subjects] | P Value | |
|---|---|---|---|
| Short (N = 225) | Long (N = 47) | ||
| |||
| Age (days) | 134 (63‐225.5) | 139 (63‐240) | 0.86 |
| Gestation (weeks) | 40 (37‐40) | 39 (35‐40) | 0.07 |
| Race | |||
| White | 108 [48] | 23 [49] | 0.91 |
| Other | 117 [52] | 24 [51] | |
| Gender | |||
| Male | 121 [54] | 25 [53] | 0.94 |
| Respiratory support at birth | 22 [10] | 12 [26] | 0.003* |
| Chronic respiratory disease | 21 [9] | 8 [17] | 0.12 |
| Respiratory rate on admission | 56 (44‐64) | 56 (46‐66) | 0.58 |
| Cardiac conditions | 4 [2] | 3 [6] | 0.10 |
| Waterlow percent | 100 (92‐109) {n =203} | 96 (88‐107) {n =46} | 0.16 |
| Days of cough prior to admission | 4 (2‐6) {n =202} | 4 (2‐5) {n = 40} | 0.78 |
| Days of congestion prior to admission | 3 (1‐5) {n =183} | 3 (1‐5) {n =35} | 0.98 |
| Days of fever prior to admission | 1 (0‐3) {n =206} | 1 (0‐2) {n =43} | 0.50 |
| Days of decreased oral intake prior to admission | 1 (0‐2) {n =181} | 1 (0‐1) {n =36} | 0.44 |
| Variable | Median (IQR) or N [%] | P | Median (IQR) or N [%] | P | ||
|---|---|---|---|---|---|---|
| Short | Long | Short | Long | |||
| Hospital Day 1 | Hospital Day 2 | |||||
| ||||||
| Hours of supplemental oxygen | 3 (0‐10) | 11 (5‐17) | <0.001* | 3 (0‐19) | 24 (17‐24) | <0.001* |
| Minimum supplemental oxygen use (liters) | 0 (0‐0.1) | 0.25 (0‐0.5) | <0.001* | 0 (0‐0) | 0.2 (0‐0.5) | <0.001* |
| Maximum supplemental oxygen use (liters) | 0.5 (0‐1) | 0.75 (0.5‐1.5) | <0.001* | 0.2 (0‐0.5) | 1 (0.5‐1.5) | <0.001* |
| Minimum oxygen saturation (percent) | 94 (92‐96) | 94 (92‐96) | 0.89 | 94 (92‐95) | 93 (91‐94) | 0.001* |
| Maximum oxygen saturation (percent) | 99 (98‐100) | 100 (99‐100) | 0.23 | 100 (98‐100) | 100 (99‐100) | 0.37 |
| Minimum respiratory rate | 36 (32‐46) | 36 (32‐46) | 0.92 | 34 (30‐40) | 36 (32‐41) | 0.11 |
| Maximum respiratory rate | 53 (45‐62) | 56 (48‐64) | 0.14 | 55 (48‐64) | 63 (52‐75) | <0.001* |
| Mean respiratory score | 4 (3‐5.5) | 5 (4‐6.7) | 0.008* | 3.4 (2.7‐4.5) | 4.8 (3.7‐7) | <0.001* |
| Change in respiratory score | 0 (0‐1) | 0 (1‐1.5) | 0.3 | 1 (0‐2) | 0 (‐2‐2) | 0.022 |
| Number of times nasopharyngeal suctioned | 1 (0‐2) | 2 (1‐3) | 0.012 | 1 (0‐3) | 4 (2‐5) | <0.001* |
| Calories consumed (Kcal/kg/day) | 53 (22‐82) | 54 (33‐79) | 0.801 | 66 (47‐90) | 54 (21‐72) | 0.001* |
| ICU (% of subjects) | 4 (1.8%) | 2 (4.3%) | 0.28 | 4 (1.8%) | 5 (10.6%) | 0.009* |
Recursive Partitioning Analysis
We chose recursive partitioning as the method for model creation instead of multivariate linear regression in order to: 1) study multiple possible variable interactions without having to create multiple interaction terms; and 2) generate an easy‐to‐use flow diagram to identify infants at risk for prolonged LOS without having to use a complex formula generated by multivariate regression. In recursive partitioning methodology, the statistical program selects the variable among the set of candidate variables that best separates the first parent node with all subjects into short and prolonged stay intermediate nodes. The process is repeated with additional variables selected that further separate the intermediate nodes into short and prolonged stay nodes, until finally a flow diagram is generated, resulting in terminal nodes of predicted short and prolonged stay subjects. Recursive partitioning was performed using Salford Systems' CART software San Diego, CA. The minimum number of cases required in parent/emntermediate nodes was 20, and in terminal nodes was 5. Eighty percent of cases were randomly selected for the learning tree, and 20% in the test tree for cross‐validation.
Sixteen variables were considered a priori as potentially important in affecting LOS and were candidates for inclusion. These included five baseline variables (age, gestation, Waterlow percentile, presence of chronic respiratory disease, and a marker for missing Waterlow percentile) and 11 variables from hospital day 2 (kcal/kg/day consumed, hours of supplemental oxygen, maximum supplemental oxygen use, maximum oxygen saturation, maximum respiratory rate, minimum supplemental oxygen use, minimum oxygen saturation, minimum respiratory rate, mean clinical respiratory score, change in respiratory score, and nasopharyngeal suctioning frequency). Hospital day 2 variables were chosen rather than hospital day 1, because hospital day 1 was only a partial day in the hospital for the majority of subjects. Several aspects of oxygenation were studied because oxygen need has been consistently found to be an important predictor of LOS. We sought to discover which particular aspect of oxygen need was most important. For comparison, recursive partitioning was performed on the variable sets taken from the Michigan (weight, congenital heart disease, failure to thrive, gestational age, chronic pulmonary diseases, and early mechanical ventilation)21 and Rotterdam (weight and need for supplemental oxygen)24 Models.
This study was approved by the CHW Institutional Review Board.
RESULTS
Three hundred forty‐seven infants were admitted during the 20042005 bronchiolitis season, with 273 placed in the bronchiolitis treatment protocol. The charts of these 273 patients were reviewed. One was excluded because of gastrostomy tube placement during the admission. Of the remaining 272 patients, 47 (17.3%) had a LOS 108 hours. The median LOS was 59 hours (range 10334 hours). Two patients had missing data for caloric intake on days 1 and 2; and 23 patients did not have height obtained, therefore their Waterlow classification could not be determined. Historical details concerning fever, congestion, cough, and diminished caloric intake preceding admission were variably reported, resulting in a smaller sample size for these baseline characteristics as described in Table 1.
Univariate Analysis
Baseline characteristics of infants having short and prolonged LOS are described in Table 1. Groups were statistically similar except that the long stay group contained a significantly larger proportion of infants requiring respiratory support at birth (defined as needing intubation, continuous positive airway pressure [CPAP], or oxygen).
Table 2 describes selected variables on hospital days 1 and 2 in infants having short and prolonged LOS. On hospital day 1, infants in the prolonged LOS group had a significantly greater need for supplemental oxygen, and mean respiratory score. On hospital day 2, infants in the prolonged LOS group had a significantly greater need for supplemental oxygen maximum respiratory rate, mean respiratory score, and number of times they were suctioned. They had a significantly lower minimum oxygen saturation and caloric intake. On hospital day 2, the prolonged LOS group had a greater proportion of subjects in the ICU, on CPAP, and on the ventilator.
We examined two characteristics identifiable after the second hospital day. There was a significant difference in: a) the median number of discharge diagnoses in the short LOS group (two) vs the prolonged LOS group (three) (P < 0.001); and b) the presence of apnea during the admission in the short LOS group (0.1%) vs the prolonged LOS group (9%) (P = 0.009).
Recursive Partitioning Model
Figure 1 depicts the recursive partitioning model that best predicted LOS. Five variables were selected by the recursive partitioning model. Selected variables, in order of appearance (variable importance is related to order of appearance, ie, most important variable is first), were: hours of supplemental oxygen, maximum respiratory rate, minimum supplemental oxygen use, gestation, and kilocalories (kcal)/kilogram (kg)/day consumed. The characteristics of this model were: ROC 0.89 and 0.72 for the learning and test trees, respectively; sensitivity, 0.85; and specificity, 0.82

Infants predicted as having a short LOS had three distinct profiles labeled S1, S2, and S3 in Figure 1. The S1 group required 6.5 hours of oxygen. The S2 group required >6.5 hours of oxygen, but had a maximum respiratory rate 49. The S3 group required >6.5 hours of oxygen, had a maximum respiratory rate >49, but were >36.5 week gestation, and consumed >23.5 kcal/kg/day.
Infants predicted as having a long LOS had three distinct profiles labeled L1, L2, and L3 in Figure 1. The L1 group required >6.5 hours of supplemental oxygen, had a maximum respiratory rate >49, and required some level of oxygen support the entire day. The L2 group required >6.5 hours of supplemental oxygen, had a maximum respiratory rate >49, were on room air some portion of the day, but had a gestation 36.5 weeks. The L3 group required >6.5 hours of supplemental oxygen, had a maximum respiratory rate >49, were on room air for some portion of the day, had a gestation >36.5 weeks, but consumed 23.5 kcal/kg/day.
Table 3 compares the performance of our model (the Milwaukee Model), the Michigan Model, and the Rotterdam Model in predicting LOS group. Overall, the Milwaukee Model had the highest ROC 0.89/0.72 for the learning and test trees. All three models had good sensitivity (Milwaukee, 85%; Michigan, 85%), with the Rotterdam Model having the highest (98%). The Milwaukee Model also had good specificity (82%), while the Michigan and Rotterdam Models were less specific (46% and 44%).
| Model | Priors* | Sensitivity | Specificity | Learning Tree ROC | Test Tree ROC | |
|---|---|---|---|---|---|---|
| Long LOS | Short LOS | |||||
| ||||||
| Michigan | 0.5 | 0.5 | 0.85 | 0.46 | 0.69 | 0.56 |
| Rotterdam | 0.5 | 0.5 | 0.98 | 0.44 | 0.73 | 0.61 |
| Milwaukee | 0.5 | 0.5 | 0.85 | 0.82 | 0.89 | 0.72 |
DISCUSSION
We confirmed several previously recognized risk factors for prolonged LOS, including: apnea, at least part of the hospital stay in ICU, use of CPAP, mechanical ventilation, and prematurity. However, most patients admitted with bronchiolitis do not have these risk factors. The major contribution of this study is the evaluation of factors applicable to all patients admitted with bronchiolitis, and a more in‐depth analysis of clinical assessments performed on hospital days 1 and 2 than had been previously reported.
We did find strong associations between a number of clinical assessments and LOS. While some were apparent on day 1 of the admission, the number and degree of clinical differences between infants destined for a short vs prolonged stay were more apparent on hospital day 2. On this day, there were significant differences between the groups in the length and amount of oxygen received, oxygen saturation, maximum respiratory rate, respiratory scores, nasopharyngeal suctioning need, and caloric intake. Interestingly, it was noted the prolonged stay group had overall worsening or a lack of improvement in several clinical markers from day 1 to day 2, in areas where the short stay group showed improvements.
To our knowledge, the Milwaukee Model is the first bronchiolitis LOS prediction model to incorporate several clinical markers occurring early in the hospital stay. These clinical markers were found to be more effective predictors of LOS group in our study population than some of the traditional birth‐ and disease‐related risk factors previously reported. The model highlighted some important interactions among variables, and identified specific profiles of patients likely to have a short or prolonged LOS based on their day 2 clinical status.
The short LOS groups all shared one of the following three features: 1) low duration of oxygen use; 2) absence of tachypnea (tachypnea defined as a respiratory rate >60 in infants <2 months old and >50 for infants between 2 and 12 months old.3234); or 3) absence of severely diminished caloric intake. The prolonged LOS groups shared the common characteristics of higher duration of oxygen use and higher maximum respiratory rates. In addition to these two elements, each long stay group had either a constant oxygen requirement, prematurity (36.5 weeks), or very low caloric intake (<23.5 kcal/kg/day).
While all three models shared good sensitivity, the increased specificity of the Milwaukee Model limits the number of false positives (infants screening as destined for a prolonged LOS who actually will have a short LOS). For clinicians or researchers planning interventions for high‐risk infants, this greater specificity would reduce the number of infants who might unnecessarily receive those interventions. While there are limited proven therapies to hasten the recovery of patients with bronchiolits,35 many treatments and combinations of treatments are currently being used and studied. Nebulized hypertonic saline,36 airway secretion clearance modalities,37 and nutritional supplementation22, 38 are some examples of interventions that could be used and evaluated in infants screened as high risk for prolonged LOS. While it may have been better to identify short vs long stay immediately upon admission or after hospital day 1, it was the more clear separation between the short and long stay groups that occurred on day 2 that allowed us to develop an accurate predictive model. We believe that for infants destined to be in the hospital for at least three more days, a model based on hospital day 2 variables is worthwhile.
When evaluating the characteristics of the three models, it is important to note that they were initially studied in populations with some important differences. Only 11% of Milwaukee patients had chronic respiratory diseases, whereas the previously developed models were generated from a sample with a higher prevalence of chronic respiratory diseases (Michigan, 20%; Rotterdam, 23%). Only 3% of subjects needed placement in the ICU compared to higher rates in prior studies (Michigan, 15%; Rotterdam, 43.5%). In a population of patients with a lower prevalence of chronic respiratory diseases and need for ICU, early clinical markers may become more important in predicting LOS. While our model may generalize well in such a cohort of patients, it might not generalize as well to a cohort with a high prevalence of chronic lung disease and higher need for ICU. It is also important to note that the area under the ROC was lower in the test tree than the learning tree. This variation demonstrates the need for evaluating the performance of this model in additional populations.
This study has several limitations. First, it is a retrospective study of a single bronchiolitis season at a single institution. Second, the authors served as data abstractors and could have been biased, as they were not blinded. Third, four out of the five markers in our model are clinical markers that could vary based on clinical assessment skills and institutional practice. For example, oxygen use is dependent on the practice of nurses and respiratory therapists charged with regulating the oxygen delivery. However, the practice of initiating and weaning oxygen is fairly standardized at our institution. Fourth, environmental and social risk factors, such as day care attendance, school‐age siblings, and smoke exposure, can impact LOS but were not included in our model. Fifth, we do not have data on those infants not included in the bronchiolitis protocol. It is possible that they differed from those in protocol. Finally, six infants were either placed or transferred to the ICU on day 1, which may make them inherently different than the other infants in the model. However, four out of these six infants did go on to have a short stay, highlighting the fact that many other factors affect LOS.
We believe this model may be useful because the clinical markers it uses represent some of the key problems seen in bronchiolitis (poor oxygenation, tachypnea, and poor feeding). Careful assessment of these clinical markers can allow effective prediction of those infants likely to have a prolonged LOS. This early risk assessment could allow more effective targeting of interventions to help high‐risk infants.
CONCLUSIONS
There are important differences between infants with bronchiolitis having short and prolonged hospital stays, including several clinical markers identifiable on hospital day 2, such as the length and amount of oxygen received, minimum oxygen saturation, maximum respiratory rate, clinical respiratory scores, deep suctioning need, and caloric intake. The Milwaukee Model uses the number of hours of supplemental oxygen, respiratory rate, minimum supplemental oxygen use, gestation, and caloric intake to predict short or prolonged LOS. It performed well with a good ROC, sensitivity, and specificity in one population of infants with a low prevalence of chronic respiratory disease.
- ,.Clinical significance of respiratory syncytial virus.Postgrad Med.1964;35:460–465.
- ,,,,.Respiratory syncytial virus infection in young hospitalized children. Identification of risk patients and prevention of nosocomial spread by rapid diagnosis.Acta Paediatr Scand.1983;72(1):47–51.
- .Pathogenesis of bronchiolitis—epidemiologic considerations.Pediatr Res.1977;11(3 pt 2):239–243.
- ,,.Respiratory syncytial virus infection in children with bronchopulmonary dysplasia.Pediatrics.1988;82(2):199–203.
- ,,, et al.Respiratory syncytial viral infection in children with compromised immune function.N Engl J Med.1986;315(2):77–81.
- ,,,,.Risk of secondary bacterial infection in infants hospitalized with respiratory syncytial viral infection.J Pediatr.1988;113(2):266–271.
- ,,,,,.Respiratory syncytial viral infection in infants with congenital heart disease.N Engl J Med.1982;307(7):397–400.
- ,,, et al.Hospitalized children with respiratory syncytial virus infection and neuromuscular impairment face an increased risk of a complicated course.Pediatr Infect Dis J.2007;26(6):485–491.
- ,,.Rehospitalization for respiratory illness in infants of less than 32 weeks' gestation.Pediatrics.1991;88(3):527–532.
- ,,, et al.Respiratory syncytial virus (RSV) immune globulin intravenous therapy for RSV lower respiratory tract infection in infants and young children at high risk for severe RSV infections: Respiratory Syncytial Virus Immune Globulin Study Group.Pediatrics.1997;99(3):454–461.
- ,,,,.Risk factors for bronchiolitis‐associated deaths among infants in the United States.Pediatr Infect Dis J.2003;22(6):483–490.
- ,.Risk factors for severe respiratory syncytial virus infection in infants.Respir Med.2002;96(suppl B):S9–S14.
- ,,,,.Rehospitalization for respiratory syncytial virus among premature infants.Pediatrics.1999;104(4 pt 1):894–899.
- ,,,,.Risk of respiratory syncytial virus infection for infants from low‐income families in relationship to age, sex, ethnic group, and maternal antibody level.J Pediatr.1981;98(5):708–715.
- ,,,.Preterm twins and triplets. A high‐risk group for severe respiratory syncytial virus infection.Am J Dis Child.1993;147(3):303–306.
- ,,, et al.Rehospitalization because of respiratory syncytial virus infection in premature infants younger than 33 weeks of gestation: a prospective study. IRIS Study Group.Pediatr Infect Dis J.2000;19(7):592–597.
- ,.Parental smoking, presence of older siblings, and family history of asthma increase risk of bronchiolitis.Am J Dis Child.1986;140(8):806–812.
- ,,,,.Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis.J Pediatr.1988;113(5):826–830.
- ,,, et al.Variable morbidity of respiratory syncytial virus infection in patients with underlying lung disease: a review of the PICNIC RSV database. Pediatric Investigators Collaborative Network on Infections in Canada.Pediatr Infect Dis J.1999;18(10):866–869.
- ,,,,.Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid.J Pediatr.2000;137(6):865–870.
- ,.Severity of illness models for respiratory syncytial virus‐associated hospitalization.Am J Respir Crit Care Med.1999;159(4 pt 1):1234–1240.
- ,.Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis.Pediatrics.2008;121(3):470–475.
- ,,.Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection.J Pediatr.1995;126(2):212–219.
- ,,,.Prediction of duration of hospitalization in respiratory syncytial virus infection.Pediatr Pulmonol.2002;33(6):453–457.
- ,,,,,.Impact of a bronchiolitis guideline: a multisite demonstration project.Chest.2002;121(6):1789–1797.
- , , , , , , , , , Diagnosis and management of bronchiolitis.Pediatrics.2006;118(4):1774–1793.
- ,,, et al.Evaluation of an evidence‐based guideline for bronchiolitis.Pediatrics.1999;104(6):1334–1341.
- .Classification and definition of protein‐calorie malnutrition.Br Med J.1972;3(5826):566–569.
- ,,,,.Inpatient care for uncomplicated bronchiolitis: comparison with Milliman and Robertson guidelines.Arch Pediatr Adolesc Med.2001;155(12):1323–1327.
- ,,,.Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations.Arch Pediatr Adolesc Med.2004;158(6):527–530.
- .Bronchiolitis clinical practice guideline.Children's Hospital of Wisconsin Intranet;2007. http://clinicalpractice.chw.org/display/displayFile.asp?docid=393185(2):319–336.
- ,.The rational clinical examination. Does this infant have pneumonia?JAMA.1998;279(4):308–313.
- ,.Focused ethnographic studies in the WHO Programme for the Control of Acute Respiratory Infections.Med Anthropol.1994;15(4):409–424.
- ,.Bronchiolitis: recent evidence on diagnosis and management.Pediatrics.125(2):342–349.
- ,,,.Nebulized hypertonic saline solution for acute bronchiolitis in infants.Cochrane Database Syst Rev.2008(4):CD006458.
- .Airway clearance applications in infants and children.Respir Care.2007;52(10):1382–1391.
- ,.Immunonutrition in critically ill patients: a systematic review and analysis of the literature.Intensive Care Med.2008;34(11):1980–1990.
- ,.Clinical significance of respiratory syncytial virus.Postgrad Med.1964;35:460–465.
- ,,,,.Respiratory syncytial virus infection in young hospitalized children. Identification of risk patients and prevention of nosocomial spread by rapid diagnosis.Acta Paediatr Scand.1983;72(1):47–51.
- .Pathogenesis of bronchiolitis—epidemiologic considerations.Pediatr Res.1977;11(3 pt 2):239–243.
- ,,.Respiratory syncytial virus infection in children with bronchopulmonary dysplasia.Pediatrics.1988;82(2):199–203.
- ,,, et al.Respiratory syncytial viral infection in children with compromised immune function.N Engl J Med.1986;315(2):77–81.
- ,,,,.Risk of secondary bacterial infection in infants hospitalized with respiratory syncytial viral infection.J Pediatr.1988;113(2):266–271.
- ,,,,,.Respiratory syncytial viral infection in infants with congenital heart disease.N Engl J Med.1982;307(7):397–400.
- ,,, et al.Hospitalized children with respiratory syncytial virus infection and neuromuscular impairment face an increased risk of a complicated course.Pediatr Infect Dis J.2007;26(6):485–491.
- ,,.Rehospitalization for respiratory illness in infants of less than 32 weeks' gestation.Pediatrics.1991;88(3):527–532.
- ,,, et al.Respiratory syncytial virus (RSV) immune globulin intravenous therapy for RSV lower respiratory tract infection in infants and young children at high risk for severe RSV infections: Respiratory Syncytial Virus Immune Globulin Study Group.Pediatrics.1997;99(3):454–461.
- ,,,,.Risk factors for bronchiolitis‐associated deaths among infants in the United States.Pediatr Infect Dis J.2003;22(6):483–490.
- ,.Risk factors for severe respiratory syncytial virus infection in infants.Respir Med.2002;96(suppl B):S9–S14.
- ,,,,.Rehospitalization for respiratory syncytial virus among premature infants.Pediatrics.1999;104(4 pt 1):894–899.
- ,,,,.Risk of respiratory syncytial virus infection for infants from low‐income families in relationship to age, sex, ethnic group, and maternal antibody level.J Pediatr.1981;98(5):708–715.
- ,,,.Preterm twins and triplets. A high‐risk group for severe respiratory syncytial virus infection.Am J Dis Child.1993;147(3):303–306.
- ,,, et al.Rehospitalization because of respiratory syncytial virus infection in premature infants younger than 33 weeks of gestation: a prospective study. IRIS Study Group.Pediatr Infect Dis J.2000;19(7):592–597.
- ,.Parental smoking, presence of older siblings, and family history of asthma increase risk of bronchiolitis.Am J Dis Child.1986;140(8):806–812.
- ,,,,.Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis.J Pediatr.1988;113(5):826–830.
- ,,, et al.Variable morbidity of respiratory syncytial virus infection in patients with underlying lung disease: a review of the PICNIC RSV database. Pediatric Investigators Collaborative Network on Infections in Canada.Pediatr Infect Dis J.1999;18(10):866–869.
- ,,,,.Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid.J Pediatr.2000;137(6):865–870.
- ,.Severity of illness models for respiratory syncytial virus‐associated hospitalization.Am J Respir Crit Care Med.1999;159(4 pt 1):1234–1240.
- ,.Effect of oxygen supplementation on length of stay for infants hospitalized with acute viral bronchiolitis.Pediatrics.2008;121(3):470–475.
- ,,.Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection.J Pediatr.1995;126(2):212–219.
- ,,,.Prediction of duration of hospitalization in respiratory syncytial virus infection.Pediatr Pulmonol.2002;33(6):453–457.
- ,,,,,.Impact of a bronchiolitis guideline: a multisite demonstration project.Chest.2002;121(6):1789–1797.
- , , , , , , , , , Diagnosis and management of bronchiolitis.Pediatrics.2006;118(4):1774–1793.
- ,,, et al.Evaluation of an evidence‐based guideline for bronchiolitis.Pediatrics.1999;104(6):1334–1341.
- .Classification and definition of protein‐calorie malnutrition.Br Med J.1972;3(5826):566–569.
- ,,,,.Inpatient care for uncomplicated bronchiolitis: comparison with Milliman and Robertson guidelines.Arch Pediatr Adolesc Med.2001;155(12):1323–1327.
- ,,,.Impact of pulse oximetry and oxygen therapy on length of stay in bronchiolitis hospitalizations.Arch Pediatr Adolesc Med.2004;158(6):527–530.
- .Bronchiolitis clinical practice guideline.Children's Hospital of Wisconsin Intranet;2007. http://clinicalpractice.chw.org/display/displayFile.asp?docid=393185(2):319–336.
- ,.The rational clinical examination. Does this infant have pneumonia?JAMA.1998;279(4):308–313.
- ,.Focused ethnographic studies in the WHO Programme for the Control of Acute Respiratory Infections.Med Anthropol.1994;15(4):409–424.
- ,.Bronchiolitis: recent evidence on diagnosis and management.Pediatrics.125(2):342–349.
- ,,,.Nebulized hypertonic saline solution for acute bronchiolitis in infants.Cochrane Database Syst Rev.2008(4):CD006458.
- .Airway clearance applications in infants and children.Respir Care.2007;52(10):1382–1391.
- ,.Immunonutrition in critically ill patients: a systematic review and analysis of the literature.Intensive Care Med.2008;34(11):1980–1990.
Copyright © 2011 Society of Hospital Medicine