User login
Improving Anticoagulation Transitions
Anticoagulants are among the prescriptions with the highest risk of an adverse drug event (ADE) after discharge, and many of these events are preventable.[1, 2, 3] In recognition of the high risk for adverse events, the Institute for Healthcare Improvement Map details several key features of a safe anticoagulation management program, including the recommendation during the transition period that clinicians ensure proper lab monitoring and establish capacity for follow‐up testing.[4]
Despite the potential for harm, most hospitals do not have a structured process for the transmission of vital information related to warfarin management from the inpatient to the ambulatory setting. Our aim was to develop a concise report containing the essential information regarding the patient's anticoagulation regimen, the Safe Transitions Anticoagulation Report (STAR), and a process to ensure the report can be readily accessed and utilized by ambulatory clinicians.
METHODS
We assembled an interdisciplinary team to develop a new report and workflow to ensure that information on inpatient warfarin management was transmitted to outpatient providers in a reliable and structured manner. Explicit goals were to maximize use of the electronic medical record (EMR) to autopopulate aspects of the new report and create a process that worked seamlessly into the workflow. The final items were selected based on the risk of harm if not conveyed and feasibility of incorporation through the EMR:
- Warfarin doses: the 7 warfarin doses immediately prior to discharge
- International normalized ratio (INR) values: the 7 INR values immediately prior to discharge
- Bridging anticoagulation: the low‐molecular‐weight heparin (LMWH) prescribed as a bridging anticoagulant, if any
The STAR resides in both the discharge summary and the after visit summary (AVS) for patients discharged on warfarin. At our institution, the AVS contains a medication list, discharge instructions, and appointments, and is automatically produced through our EMR. Our institution utilizes the Epic EMR (Epic Systems Corp., Verona, WI) in the hospital, ambulatory clinics, and faculty practices.
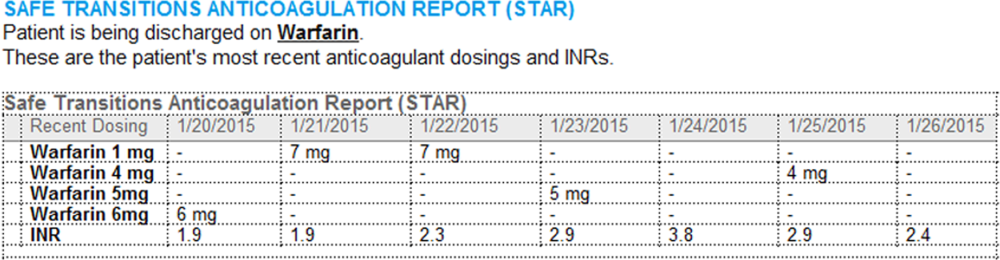
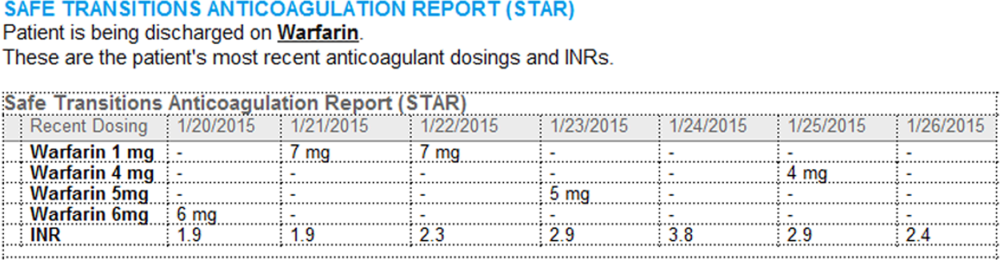
A process was developed where a structured table is automatically created (Figure 1). A field was added to our EMR's discharge summary template asking whether the patient is being discharged on warfarin. Answering yes produces a second question asking whether the patient is also being discharged on bridging anticoagulation with LMWH. The STAR is not inserted into the discharge summary if the clinician completing the discharge summary deletes the anticoagulation question. The STAR is automatically created for patients discharged on warfarin and inserted into the AVS by the EMR regardless of whether the discharge summary has been completed. Patients are instructed by their nurse to bring their AVS to their follow‐up appointments.
The STAR project team utilized plan‐do‐study‐act cycles to test small changes and make revisions. The workflow was piloted on 2 medical/surgical units from January 2014 through March 2014, and revised based on feedback from clinicians and nursing staff. The STAR initiative was fully implemented across our institution in April 2014.
The study was evaluated by the institutional review board of the Icahn School of Medicine at Mount Sinai, and full review was waived.
Outcomes
Our primary outcomes were the timeliness of laboratory monitoring and quality of anticoagulation management for patients with an established relationship at 1 of the main outpatient practices in our system. Our institution has an anticoagulation clinic for patients followed at the general medicine clinic. We defined an established relationship as having been seen in the same practice on at least 2 occasions in the 12 months prior to admission. The primary outcomes were the percentage of patients who had an INR measurement done and the percentage who had a therapeutic INR value within 10 days after discharge. As the 10‐day period is arbitrary, we also assessed these outcomes for the 3‐, 7‐, and 30‐day periods after discharge. The therapeutic range was defined for all patients as an INR of 2.0 to 3.0, as this is the target range for the large majority of patients on warfarin in our system. Outcomes during the intervention period were compared to baseline values during the preintervention period. For patients with multiple admissions, the first admission during each period was included.
Ambulatory Physician Survey
We surveyed ambulatory care physicians at the main practices for our health system. The survey assessed how often the STAR was viewed and incorporated into decision making, whether the report improved workflow, and whether ambulatory providers perceived that the report increased patient safety. The survey was distributed at the 6‐month interval during the intervention phase. The survey was disseminated electronically on 3 occasions, and a paper version was distributed on 1 occasion to housestaff and general medicine faculty.
Statistical Analysis
Comparisons for categorical data were performed using the 2 test. P values were based on 2‐tailed tests of significance, and a value <0.05 was considered significant.
RESULTS
The STAR was embedded in the discharge summary for 1370 (78.6%) discharges during the intervention period. A total of 505 patients in the preintervention period and 292 patients in the intervention period were established patients at an affiliated practice and comprised the study population. Demographics and indications for warfarin for the preintervention and intervention groups are listed in the Table 1.
| Preintervention Group, N=505 | Intervention Group, N=292 | P Value | |
|---|---|---|---|
| |||
| Age, y | 66.7 | 68.0 | 0.29 |
| Male gender, n (%) | 236 (46.7) | 153 (52.4) | 0.12 |
| Discharged on bridging agent, n (%) | 90 (17.8) | 36 (12.3) | 0.04 |
| Average length of stay, d | 7.1 | 7.6 | 0.46 |
| Newly prescribed warfarin, n (%) | 147 (29.1) | 62 (21.2) | 0.01 |
| INR 2.03.0 range at discharge, n (%) | 187 (37.0) | 137 (46.9) | 0.02 |
| Warfarin indication, n (%)* | |||
| Venous thromboembolism | 93 (18.4) | 39 (13.4) | |
| Atrial fibrillation | 204 (40.3) | 127 (43.5) | |
| Mechanical heart valve | 19 (3.8) | 17 (6.5) | |
| Prevention of thromboembolism | 142 (28.1) | 94 (32.2) | |
| Intracardiac thrombosis | 3 (0.5) | 6 (2.1) | |
| Thrombophilia | 4 (0.8) | 5 (1.7) | |
| Other | 19 (3.8) | 12 (4.1) | |
| No indication | 32 (6.3) | 1 (3.8) | |
The frequency of INR testing within 10 days of discharge was similar for the preintervention and intervention periods (41.4% and 47.6%, respectively, P=0.09). Similarly, the likelihood of having at least 1 therapeutic INR value within 10 days of discharge was not statistically different for the groups (17.0% and 21.2%, P=0.14). The pattern was similar for the 3‐, 7‐, and 30‐day periods; a higher percentage of the intervention group had INR testing and attained a therapeutic INR value, though for no time period did this reach statistical significance. This pattern was also found when limiting the analysis to patients discharged home rather than to a facility, patients on warfarin, prior to admission, and patients started on warfarin during the hospitalization.
A total of 87of 207 (42.0%) clinicians responded to the survey. Of respondents, 75% reported that they had seen 1 patient who had been discharged on warfarin since the STAR initiative had begun, 58% of whom reported having viewed the STAR. Most respondents who viewed the STAR found it to be helpful or very helpful in guiding warfarin management (67%), improving their workflow and efficiency (58%), and improving patient safety (77%). Approximately one‐third of respondents who had viewed the STAR (34%) reported that they selected a different dose than they would have chosen had the STAR not been available.
DISCUSSION
We developed a concise report that is seamlessly created and inserted into the discharge summary. Though the STAR was perceived as improving patient safety by ambulatory care providers, there was no impact on attaining a therapeutic INR after discharge. There are several possible explanations for a lack of benefit. Most notably, our intervention was comprised of a stand‐alone EMR‐based tool and focused on 1 component of the transitions process. Given the complexity of healthcare delivery and anticoagulant management, it is likely that broader interventions are required to improve clinical outcomes over the transition period. Potential targets of multifaceted approaches may include improving access to care, providing greater access to anticoagulation clinics, enhancing patient education, and promoting direct physician‐physician communication. Bundled interventions will likely need to include involvement of an interdisciplinary team, such as pharmacist involvement in the medication reconciliation process.
The transition period from the hospital to the outpatient setting has the potential to jeopardize patient safety if vital information is not reliably transmitted across venues.[5, 6, 7, 8] Forster and colleagues noted an 11% incidence of ADEs in the posthospitalization period, of which 60% were either preventable or ameliorable.[3] To decrease the risk to patient safety during the transitions period, the Transitions of Care Consensus policy statement by the Society of Hospital Medicine and other medical organizations called for incorporation of standard data transfer forms (templates and transmission protocols).[9] Despite the high risk and preventable nature of many of the events, few specific tools have been developed. As part of broader initiatives to improve the transitions process, the STAR may have the potential to be a means for health systems to improve the quality of the transition of care for patients on anticoagulants.
Our study has several limitations. First, it was performed at a single health system. It is unknown whether the EMR‐based report could be similarly employed at other systems. Second, our study was unable to assess clinical endpoints. Given the lack of effect on attaining a therapeutic INR, it is unlikely that downstream outcomes, such as thromboembolism, were impacted. Lastly, we were unable to examine whether our intervention improved the care of patients whose outpatient provider was external to our system.
The STAR is a concise tool developed to provide essential anticoagulant‐related information to ambulatory providers. Though the report was perceived as improving patient safety, our finding of a lack of impact on attaining a therapeutic INR after discharge suggests that the tool would need to be a component of a broader multifaceted intervention to impact clinical outcomes.
Disclosures
This project was funded by a grant from the Cardinal Health E3 Foundation. The authors report no conflicts of interest.
- , , , Medication errors: experience of the United States Pharmacopeia (USP) MEDMARX reporting system. J Clin Pharm. 2003;43:760–767.
- , , , , The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167.
- , , , , Adverse drug events occurring following hospital discharge. J Gen Int Med. 2005;204:317–323.
- IHI Improvement Map. Available at: http://app.ihi.org/imap/tool/#Process=54aa289b‐16fd‐4a64‐8329‐3941dfc565d1. Accessed February 20 2015.
- , , , Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323.
- , , , , , Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831–841.
- Transitions of care in patients receiving oral anticoagulants: general principles, procedures, and impact of new oral anticoagulants. J Cardiovasc Nurs. 2013;28:54–65.
- Care transitions in anticoagulation management for patients with atrial fibrillation: an emphasis on safety. Oschner J. 2013;13:419–427.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4:364–370.
Anticoagulants are among the prescriptions with the highest risk of an adverse drug event (ADE) after discharge, and many of these events are preventable.[1, 2, 3] In recognition of the high risk for adverse events, the Institute for Healthcare Improvement Map details several key features of a safe anticoagulation management program, including the recommendation during the transition period that clinicians ensure proper lab monitoring and establish capacity for follow‐up testing.[4]
Despite the potential for harm, most hospitals do not have a structured process for the transmission of vital information related to warfarin management from the inpatient to the ambulatory setting. Our aim was to develop a concise report containing the essential information regarding the patient's anticoagulation regimen, the Safe Transitions Anticoagulation Report (STAR), and a process to ensure the report can be readily accessed and utilized by ambulatory clinicians.
METHODS
We assembled an interdisciplinary team to develop a new report and workflow to ensure that information on inpatient warfarin management was transmitted to outpatient providers in a reliable and structured manner. Explicit goals were to maximize use of the electronic medical record (EMR) to autopopulate aspects of the new report and create a process that worked seamlessly into the workflow. The final items were selected based on the risk of harm if not conveyed and feasibility of incorporation through the EMR:
- Warfarin doses: the 7 warfarin doses immediately prior to discharge
- International normalized ratio (INR) values: the 7 INR values immediately prior to discharge
- Bridging anticoagulation: the low‐molecular‐weight heparin (LMWH) prescribed as a bridging anticoagulant, if any
The STAR resides in both the discharge summary and the after visit summary (AVS) for patients discharged on warfarin. At our institution, the AVS contains a medication list, discharge instructions, and appointments, and is automatically produced through our EMR. Our institution utilizes the Epic EMR (Epic Systems Corp., Verona, WI) in the hospital, ambulatory clinics, and faculty practices.
A process was developed where a structured table is automatically created (Figure 1). A field was added to our EMR's discharge summary template asking whether the patient is being discharged on warfarin. Answering yes produces a second question asking whether the patient is also being discharged on bridging anticoagulation with LMWH. The STAR is not inserted into the discharge summary if the clinician completing the discharge summary deletes the anticoagulation question. The STAR is automatically created for patients discharged on warfarin and inserted into the AVS by the EMR regardless of whether the discharge summary has been completed. Patients are instructed by their nurse to bring their AVS to their follow‐up appointments.

The STAR project team utilized plan‐do‐study‐act cycles to test small changes and make revisions. The workflow was piloted on 2 medical/surgical units from January 2014 through March 2014, and revised based on feedback from clinicians and nursing staff. The STAR initiative was fully implemented across our institution in April 2014.
The study was evaluated by the institutional review board of the Icahn School of Medicine at Mount Sinai, and full review was waived.
Outcomes
Our primary outcomes were the timeliness of laboratory monitoring and quality of anticoagulation management for patients with an established relationship at 1 of the main outpatient practices in our system. Our institution has an anticoagulation clinic for patients followed at the general medicine clinic. We defined an established relationship as having been seen in the same practice on at least 2 occasions in the 12 months prior to admission. The primary outcomes were the percentage of patients who had an INR measurement done and the percentage who had a therapeutic INR value within 10 days after discharge. As the 10‐day period is arbitrary, we also assessed these outcomes for the 3‐, 7‐, and 30‐day periods after discharge. The therapeutic range was defined for all patients as an INR of 2.0 to 3.0, as this is the target range for the large majority of patients on warfarin in our system. Outcomes during the intervention period were compared to baseline values during the preintervention period. For patients with multiple admissions, the first admission during each period was included.
Ambulatory Physician Survey
We surveyed ambulatory care physicians at the main practices for our health system. The survey assessed how often the STAR was viewed and incorporated into decision making, whether the report improved workflow, and whether ambulatory providers perceived that the report increased patient safety. The survey was distributed at the 6‐month interval during the intervention phase. The survey was disseminated electronically on 3 occasions, and a paper version was distributed on 1 occasion to housestaff and general medicine faculty.
Statistical Analysis
Comparisons for categorical data were performed using the 2 test. P values were based on 2‐tailed tests of significance, and a value <0.05 was considered significant.
RESULTS
The STAR was embedded in the discharge summary for 1370 (78.6%) discharges during the intervention period. A total of 505 patients in the preintervention period and 292 patients in the intervention period were established patients at an affiliated practice and comprised the study population. Demographics and indications for warfarin for the preintervention and intervention groups are listed in the Table 1.
| Preintervention Group, N=505 | Intervention Group, N=292 | P Value | |
|---|---|---|---|
| |||
| Age, y | 66.7 | 68.0 | 0.29 |
| Male gender, n (%) | 236 (46.7) | 153 (52.4) | 0.12 |
| Discharged on bridging agent, n (%) | 90 (17.8) | 36 (12.3) | 0.04 |
| Average length of stay, d | 7.1 | 7.6 | 0.46 |
| Newly prescribed warfarin, n (%) | 147 (29.1) | 62 (21.2) | 0.01 |
| INR 2.03.0 range at discharge, n (%) | 187 (37.0) | 137 (46.9) | 0.02 |
| Warfarin indication, n (%)* | |||
| Venous thromboembolism | 93 (18.4) | 39 (13.4) | |
| Atrial fibrillation | 204 (40.3) | 127 (43.5) | |
| Mechanical heart valve | 19 (3.8) | 17 (6.5) | |
| Prevention of thromboembolism | 142 (28.1) | 94 (32.2) | |
| Intracardiac thrombosis | 3 (0.5) | 6 (2.1) | |
| Thrombophilia | 4 (0.8) | 5 (1.7) | |
| Other | 19 (3.8) | 12 (4.1) | |
| No indication | 32 (6.3) | 1 (3.8) | |
The frequency of INR testing within 10 days of discharge was similar for the preintervention and intervention periods (41.4% and 47.6%, respectively, P=0.09). Similarly, the likelihood of having at least 1 therapeutic INR value within 10 days of discharge was not statistically different for the groups (17.0% and 21.2%, P=0.14). The pattern was similar for the 3‐, 7‐, and 30‐day periods; a higher percentage of the intervention group had INR testing and attained a therapeutic INR value, though for no time period did this reach statistical significance. This pattern was also found when limiting the analysis to patients discharged home rather than to a facility, patients on warfarin, prior to admission, and patients started on warfarin during the hospitalization.
A total of 87of 207 (42.0%) clinicians responded to the survey. Of respondents, 75% reported that they had seen 1 patient who had been discharged on warfarin since the STAR initiative had begun, 58% of whom reported having viewed the STAR. Most respondents who viewed the STAR found it to be helpful or very helpful in guiding warfarin management (67%), improving their workflow and efficiency (58%), and improving patient safety (77%). Approximately one‐third of respondents who had viewed the STAR (34%) reported that they selected a different dose than they would have chosen had the STAR not been available.
DISCUSSION
We developed a concise report that is seamlessly created and inserted into the discharge summary. Though the STAR was perceived as improving patient safety by ambulatory care providers, there was no impact on attaining a therapeutic INR after discharge. There are several possible explanations for a lack of benefit. Most notably, our intervention was comprised of a stand‐alone EMR‐based tool and focused on 1 component of the transitions process. Given the complexity of healthcare delivery and anticoagulant management, it is likely that broader interventions are required to improve clinical outcomes over the transition period. Potential targets of multifaceted approaches may include improving access to care, providing greater access to anticoagulation clinics, enhancing patient education, and promoting direct physician‐physician communication. Bundled interventions will likely need to include involvement of an interdisciplinary team, such as pharmacist involvement in the medication reconciliation process.
The transition period from the hospital to the outpatient setting has the potential to jeopardize patient safety if vital information is not reliably transmitted across venues.[5, 6, 7, 8] Forster and colleagues noted an 11% incidence of ADEs in the posthospitalization period, of which 60% were either preventable or ameliorable.[3] To decrease the risk to patient safety during the transitions period, the Transitions of Care Consensus policy statement by the Society of Hospital Medicine and other medical organizations called for incorporation of standard data transfer forms (templates and transmission protocols).[9] Despite the high risk and preventable nature of many of the events, few specific tools have been developed. As part of broader initiatives to improve the transitions process, the STAR may have the potential to be a means for health systems to improve the quality of the transition of care for patients on anticoagulants.
Our study has several limitations. First, it was performed at a single health system. It is unknown whether the EMR‐based report could be similarly employed at other systems. Second, our study was unable to assess clinical endpoints. Given the lack of effect on attaining a therapeutic INR, it is unlikely that downstream outcomes, such as thromboembolism, were impacted. Lastly, we were unable to examine whether our intervention improved the care of patients whose outpatient provider was external to our system.
The STAR is a concise tool developed to provide essential anticoagulant‐related information to ambulatory providers. Though the report was perceived as improving patient safety, our finding of a lack of impact on attaining a therapeutic INR after discharge suggests that the tool would need to be a component of a broader multifaceted intervention to impact clinical outcomes.
Disclosures
This project was funded by a grant from the Cardinal Health E3 Foundation. The authors report no conflicts of interest.
Anticoagulants are among the prescriptions with the highest risk of an adverse drug event (ADE) after discharge, and many of these events are preventable.[1, 2, 3] In recognition of the high risk for adverse events, the Institute for Healthcare Improvement Map details several key features of a safe anticoagulation management program, including the recommendation during the transition period that clinicians ensure proper lab monitoring and establish capacity for follow‐up testing.[4]
Despite the potential for harm, most hospitals do not have a structured process for the transmission of vital information related to warfarin management from the inpatient to the ambulatory setting. Our aim was to develop a concise report containing the essential information regarding the patient's anticoagulation regimen, the Safe Transitions Anticoagulation Report (STAR), and a process to ensure the report can be readily accessed and utilized by ambulatory clinicians.
METHODS
We assembled an interdisciplinary team to develop a new report and workflow to ensure that information on inpatient warfarin management was transmitted to outpatient providers in a reliable and structured manner. Explicit goals were to maximize use of the electronic medical record (EMR) to autopopulate aspects of the new report and create a process that worked seamlessly into the workflow. The final items were selected based on the risk of harm if not conveyed and feasibility of incorporation through the EMR:
- Warfarin doses: the 7 warfarin doses immediately prior to discharge
- International normalized ratio (INR) values: the 7 INR values immediately prior to discharge
- Bridging anticoagulation: the low‐molecular‐weight heparin (LMWH) prescribed as a bridging anticoagulant, if any
The STAR resides in both the discharge summary and the after visit summary (AVS) for patients discharged on warfarin. At our institution, the AVS contains a medication list, discharge instructions, and appointments, and is automatically produced through our EMR. Our institution utilizes the Epic EMR (Epic Systems Corp., Verona, WI) in the hospital, ambulatory clinics, and faculty practices.
A process was developed where a structured table is automatically created (Figure 1). A field was added to our EMR's discharge summary template asking whether the patient is being discharged on warfarin. Answering yes produces a second question asking whether the patient is also being discharged on bridging anticoagulation with LMWH. The STAR is not inserted into the discharge summary if the clinician completing the discharge summary deletes the anticoagulation question. The STAR is automatically created for patients discharged on warfarin and inserted into the AVS by the EMR regardless of whether the discharge summary has been completed. Patients are instructed by their nurse to bring their AVS to their follow‐up appointments.

The STAR project team utilized plan‐do‐study‐act cycles to test small changes and make revisions. The workflow was piloted on 2 medical/surgical units from January 2014 through March 2014, and revised based on feedback from clinicians and nursing staff. The STAR initiative was fully implemented across our institution in April 2014.
The study was evaluated by the institutional review board of the Icahn School of Medicine at Mount Sinai, and full review was waived.
Outcomes
Our primary outcomes were the timeliness of laboratory monitoring and quality of anticoagulation management for patients with an established relationship at 1 of the main outpatient practices in our system. Our institution has an anticoagulation clinic for patients followed at the general medicine clinic. We defined an established relationship as having been seen in the same practice on at least 2 occasions in the 12 months prior to admission. The primary outcomes were the percentage of patients who had an INR measurement done and the percentage who had a therapeutic INR value within 10 days after discharge. As the 10‐day period is arbitrary, we also assessed these outcomes for the 3‐, 7‐, and 30‐day periods after discharge. The therapeutic range was defined for all patients as an INR of 2.0 to 3.0, as this is the target range for the large majority of patients on warfarin in our system. Outcomes during the intervention period were compared to baseline values during the preintervention period. For patients with multiple admissions, the first admission during each period was included.
Ambulatory Physician Survey
We surveyed ambulatory care physicians at the main practices for our health system. The survey assessed how often the STAR was viewed and incorporated into decision making, whether the report improved workflow, and whether ambulatory providers perceived that the report increased patient safety. The survey was distributed at the 6‐month interval during the intervention phase. The survey was disseminated electronically on 3 occasions, and a paper version was distributed on 1 occasion to housestaff and general medicine faculty.
Statistical Analysis
Comparisons for categorical data were performed using the 2 test. P values were based on 2‐tailed tests of significance, and a value <0.05 was considered significant.
RESULTS
The STAR was embedded in the discharge summary for 1370 (78.6%) discharges during the intervention period. A total of 505 patients in the preintervention period and 292 patients in the intervention period were established patients at an affiliated practice and comprised the study population. Demographics and indications for warfarin for the preintervention and intervention groups are listed in the Table 1.
| Preintervention Group, N=505 | Intervention Group, N=292 | P Value | |
|---|---|---|---|
| |||
| Age, y | 66.7 | 68.0 | 0.29 |
| Male gender, n (%) | 236 (46.7) | 153 (52.4) | 0.12 |
| Discharged on bridging agent, n (%) | 90 (17.8) | 36 (12.3) | 0.04 |
| Average length of stay, d | 7.1 | 7.6 | 0.46 |
| Newly prescribed warfarin, n (%) | 147 (29.1) | 62 (21.2) | 0.01 |
| INR 2.03.0 range at discharge, n (%) | 187 (37.0) | 137 (46.9) | 0.02 |
| Warfarin indication, n (%)* | |||
| Venous thromboembolism | 93 (18.4) | 39 (13.4) | |
| Atrial fibrillation | 204 (40.3) | 127 (43.5) | |
| Mechanical heart valve | 19 (3.8) | 17 (6.5) | |
| Prevention of thromboembolism | 142 (28.1) | 94 (32.2) | |
| Intracardiac thrombosis | 3 (0.5) | 6 (2.1) | |
| Thrombophilia | 4 (0.8) | 5 (1.7) | |
| Other | 19 (3.8) | 12 (4.1) | |
| No indication | 32 (6.3) | 1 (3.8) | |
The frequency of INR testing within 10 days of discharge was similar for the preintervention and intervention periods (41.4% and 47.6%, respectively, P=0.09). Similarly, the likelihood of having at least 1 therapeutic INR value within 10 days of discharge was not statistically different for the groups (17.0% and 21.2%, P=0.14). The pattern was similar for the 3‐, 7‐, and 30‐day periods; a higher percentage of the intervention group had INR testing and attained a therapeutic INR value, though for no time period did this reach statistical significance. This pattern was also found when limiting the analysis to patients discharged home rather than to a facility, patients on warfarin, prior to admission, and patients started on warfarin during the hospitalization.
A total of 87of 207 (42.0%) clinicians responded to the survey. Of respondents, 75% reported that they had seen 1 patient who had been discharged on warfarin since the STAR initiative had begun, 58% of whom reported having viewed the STAR. Most respondents who viewed the STAR found it to be helpful or very helpful in guiding warfarin management (67%), improving their workflow and efficiency (58%), and improving patient safety (77%). Approximately one‐third of respondents who had viewed the STAR (34%) reported that they selected a different dose than they would have chosen had the STAR not been available.
DISCUSSION
We developed a concise report that is seamlessly created and inserted into the discharge summary. Though the STAR was perceived as improving patient safety by ambulatory care providers, there was no impact on attaining a therapeutic INR after discharge. There are several possible explanations for a lack of benefit. Most notably, our intervention was comprised of a stand‐alone EMR‐based tool and focused on 1 component of the transitions process. Given the complexity of healthcare delivery and anticoagulant management, it is likely that broader interventions are required to improve clinical outcomes over the transition period. Potential targets of multifaceted approaches may include improving access to care, providing greater access to anticoagulation clinics, enhancing patient education, and promoting direct physician‐physician communication. Bundled interventions will likely need to include involvement of an interdisciplinary team, such as pharmacist involvement in the medication reconciliation process.
The transition period from the hospital to the outpatient setting has the potential to jeopardize patient safety if vital information is not reliably transmitted across venues.[5, 6, 7, 8] Forster and colleagues noted an 11% incidence of ADEs in the posthospitalization period, of which 60% were either preventable or ameliorable.[3] To decrease the risk to patient safety during the transitions period, the Transitions of Care Consensus policy statement by the Society of Hospital Medicine and other medical organizations called for incorporation of standard data transfer forms (templates and transmission protocols).[9] Despite the high risk and preventable nature of many of the events, few specific tools have been developed. As part of broader initiatives to improve the transitions process, the STAR may have the potential to be a means for health systems to improve the quality of the transition of care for patients on anticoagulants.
Our study has several limitations. First, it was performed at a single health system. It is unknown whether the EMR‐based report could be similarly employed at other systems. Second, our study was unable to assess clinical endpoints. Given the lack of effect on attaining a therapeutic INR, it is unlikely that downstream outcomes, such as thromboembolism, were impacted. Lastly, we were unable to examine whether our intervention improved the care of patients whose outpatient provider was external to our system.
The STAR is a concise tool developed to provide essential anticoagulant‐related information to ambulatory providers. Though the report was perceived as improving patient safety, our finding of a lack of impact on attaining a therapeutic INR after discharge suggests that the tool would need to be a component of a broader multifaceted intervention to impact clinical outcomes.
Disclosures
This project was funded by a grant from the Cardinal Health E3 Foundation. The authors report no conflicts of interest.
- , , , Medication errors: experience of the United States Pharmacopeia (USP) MEDMARX reporting system. J Clin Pharm. 2003;43:760–767.
- , , , , The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167.
- , , , , Adverse drug events occurring following hospital discharge. J Gen Int Med. 2005;204:317–323.
- IHI Improvement Map. Available at: http://app.ihi.org/imap/tool/#Process=54aa289b‐16fd‐4a64‐8329‐3941dfc565d1. Accessed February 20 2015.
- , , , Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323.
- , , , , , Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831–841.
- Transitions of care in patients receiving oral anticoagulants: general principles, procedures, and impact of new oral anticoagulants. J Cardiovasc Nurs. 2013;28:54–65.
- Care transitions in anticoagulation management for patients with atrial fibrillation: an emphasis on safety. Oschner J. 2013;13:419–427.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4:364–370.
- , , , Medication errors: experience of the United States Pharmacopeia (USP) MEDMARX reporting system. J Clin Pharm. 2003;43:760–767.
- , , , , The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167.
- , , , , Adverse drug events occurring following hospital discharge. J Gen Int Med. 2005;204:317–323.
- IHI Improvement Map. Available at: http://app.ihi.org/imap/tool/#Process=54aa289b‐16fd‐4a64‐8329‐3941dfc565d1. Accessed February 20 2015.
- , , , Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323.
- , , , , , Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831–841.
- Transitions of care in patients receiving oral anticoagulants: general principles, procedures, and impact of new oral anticoagulants. J Cardiovasc Nurs. 2013;28:54–65.
- Care transitions in anticoagulation management for patients with atrial fibrillation: an emphasis on safety. Oschner J. 2013;13:419–427.
- , , , et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College Of Emergency Physicians, and Society for Academic Emergency Medicine. J Hosp Med. 2009;4:364–370.