Article

Scarring alopecia in a woman with psoriasis
- Author:
- Lance Nussbaum, DO
- Matthew Morrissey, MD
- Simon Ritchie, MD
Was this patient’s plaque psoriasis causing her progressive hair loss—or was it something else?
Article
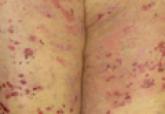
Bullous Pemphigoid Treated With Intravenous Immunoglobulin
- Author:
- Papapit Tuchinda, MD
- Simon Ritchie, MD
- Anthony A. Gaspari, MD
Bullous pemphigoid (BP) is a blistering autoimmune disease that primarily affects elderly patients who commonly present with comorbidities. Side...
