Article
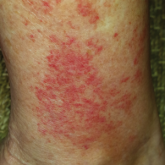
Exercise-Induced Vasculitis in a Patient With Negative Ultrasound Venous Reflux Study: A Mimic of Stasis Dermatitis
- Author:
- Swaminathan Sundaresan, MD
- Michael R. Migden, MD
- Sirunya Silapunt, MD
Exercise-induced vasculitis largely is documented in photographs or by history and may be misdiagnosed as stasis dermatitis due to its clinical...
Article
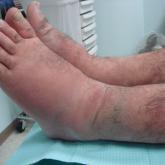
Severe Pretibial Myxedema Refractory to Systemic Immunosuppressants
- Author:
- Sirunya Silapunt, MD
- Abiara V. Agwu, MD
- Michael R. Migden, MD
Pretibial myxedema (PM) is a known manifestation of Graves disease that almost always occurs in the presence of Graves ophthalmopathy. The...
Article

Linear Terra Firma–Forme Dermatosis of the Midline Back
- Author:
- Benjamin D. Freemyer, BS
- Michael R. Migden, MD
- Sirunya Silapunt, MD
Terra firma–forme dermatosis (TFFD) is a benign and likely underdiagnosed disorder with relatively few reports in the literature. A 46-year-old...
Article

The Use of Sodium Sulfacetamide in Dermatology
- Author:
- Kristin Wolf, MD
- Sirunya Silapunt, MD
Sodium sulfacetamide is effective in the management of a variety of inflammatory facial dermatoses and often is used in combination with sulfur...
