User login
Using antipsychotics in patients with dementia
Three keys can help you safely treat dementia’s difficult behavioral and psychological symptoms:
- Differentiate medical from psychiatric causes of patients’ distress.
- Use antipsychotics and other drugs as adjuncts to psychosocial treatments.
- Start low and go slow when titrating dosages.
Although no treatment reverses the pathophysiology of progressive neurodegenerative disorders, managing agitation and other behaviors can alleviate patient suffering and reduce caregiver stress. Based on the evidence and our experience, this article describes a practical approach, including a treatment algorithm and evidence of atypical antipsychotics’ efficacy and side effects in this patient population.
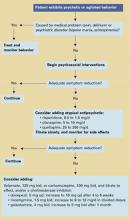
Algorithm Treating behavioral symptoms in patients with dementia
Dementia’s behavioral symptoms
An International Psychogeriatric Association consensus statement1 grouped dementia’s behavioral and psychological symptoms into two types:
- those usually assessed by interviewing patients and relatives—anxiety, depressed mood, hallucinations, and delusions
- those usually identified by observing patient behavior—aggression, screaming, restlessness, agitation, wandering, culturally inappropriate behaviors, sexual disinhibition, hoarding, cursing, and shadowing.
These behaviors in community-living patients are distressing to family members and increase the risk for caregiver burnout—the most common reason for placing older patients in long-term care. In the nursing home, dementia’s symptoms reduce patients’ quality of life; interfere with feeding, bathing, and dressing; and—when violent—may endanger staff and other patients.
Rule out a medical cause
Differential diagnosis. Behavioral symptoms in dementia tend to be unpredictable, which makes diagnosis and treatment challenging. The first step is to determine if a medical or psychiatric condition might account for the behavior. For instance:
- A patient with dementia may be agitated because of a distended bladder or arthritis but unable to communicate his or her pain in words.
- In mild dementia, a pre-existing psychiatric disorder such as schizophrenia might be causing a patient’s hallucinations or delusions.
- Pacing and restlessness may be drug side effects and might be controlled by reducing dosages or switching to less-activating agents.
Delirium is also a risk for older patients—especially those with degenerative neurologic disorders. Common triggers in older patients include acute illness such as a urinary tract infection or pneumonia, alcohol or benzodiazepine withdrawal, anticholinergic agents, medication changes, and dehydration.
Delirium is characterized by acute onset and fluctuating neuropsychiatric symptoms, including disturbed consciousness and changes in attention and cognition. Taking a careful history to learn the course of treatment and the patient’s baseline cognitive function can help you differentiate dementia from delirium. Family members, physicians, and nursing staff are valuable sources of this information.
Use antipsychotics as adjuncts
Psychosocial interventions. After medical causes have been ruled out, consensus guidelines2 recommend psychosocial interventions as first-line treatment of dementia’s behavioral symptoms (Algorithm). Suggested interventions for patients and caregivers are listed in Table 1.3
Antipsychotics. For patients who respond inadequately to psychosocial measures, the next step is to add an atypical antipsychotic. Because of side effects, conventional antipsychotics are not recommended for patients with dementia.
When prescribing atypicals, remember that older adults:
- are more sensitive to side effects than younger adults
- require lower starting and target dosages
- exhibit heterogeneity of response.
Older patients’ medical status can range from “fit” to “frail,” which influences individual response to medications. Generally, age-related changes in the way their bodies metabolize drugs account for older patients’ increased sensitivity to drug side effects (Box).4-11
Atypical antipsychotics and dosages that have been shown benefit for managing behavioral symptoms in older patients with dementia include:
- risperidone, 0.5 to 1.5 mg/d12
- olanzapine, 5 to 10 mg/d13
- quetiapine, 25 to 350 mg/d14 (Table 2).15,16
Start with low dosages, and titrate slowly. Increase once or twice a week until the lowest effective dosage is reached.
Augmenting agents. If antipsychotic monotherapy fails to achieve an adequate response or if side effects limit dosing, adjunctive agents may be added with caution. Augmenting agents that have shown benefit in some patients with dementia include:
- mood stabilizers such as divalproex17 or carbamazepine18
- cholinesterase inhibitors, such as donepezil, rivastigmine, or galantamine.19
Start divalproex at 125 mg bid or carbamazepine at 100 mg bid and titrate to effect. Concomitant carbamazepine will decrease blood levels of risperidone, olanzapine, and quetiapine because of hepatic enzyme induction.20
Start donepezil at 5 mg once daily and increase after 4 to 6 weeks to 10 mg qd. When using rivastigmine, start with 1.5 mg bid and titrate to 9 to 12 mg/d in divided doses. Start galantamine at 4 mg bid and increase after 1 month to 8 mg bid.
Table 1
Suggested psychosocial interventions for older patients with dementia
Communicate clearly
|
Minimize the impact of sensory deficits
|
Modify environment when necessary
|
Encourage consistent daily routines
|
Optimize social/physical stimulation
|
Encourage caregiver to:
|
Antipsychotic side effects
Atypical antipsychotics are more effective than conventional agents in treating negative symptoms and are associated with lower rates of extrapyramidal symptoms (EPS) and tardive dyskinesia (TD).21
Tardive dyskinesia. All antipsychotics can cause TD, although the risk is about 10 times greater with conventionals than atypicals. With conventionals, the annual cumulative TD incidence for young adults is 4 to 5%,22 and rates are much higher for middle-aged and older adults receiving chronic therapy:
- 29% after 1 year
- 50% after 2 years
- 63% after 3 years.23
In older patients, use atypical rather than conventional antipsychotics to minimize TD risk. Observe carefully; if TD symptoms occur, cautiously withdraw the antipsychotic and consider trying another agent.
Other risks. Atypical antipsychotics may cause sedation, orthostatic hypotension (with an increased risk for falls), increased serum prolactin, and weight gain (Table 2).
Weight gain from atypical antipsychotics has been associated with adverse effects on glucose metabolism and increased risk for type 2 diabetes.24 Some might argue that weight gain associated with olanzapine and other atypicals might benefit low-weight older patients. The frail elderly need to increase muscle mass, however, and the atypicals are associated with increases in fat mass.
Increased serum prolactin with risperidone theoretically could lead to loss of bone density, but evidence of this effect in older patients does not exist.
Start low, go slow
Clozapine may help control treatment-resistant psychosis in patients with schizophrenia and manage patients with severe TD.25 However, clozapine’s increased risk of agranulocytosis, neurologic side effects (seizures, sedation, confusion), and anticholinergic effects limit its use in older patients, particularly those with neurodegenerative disorders (Table 2).
Dosing. In rare cases when using clozapine in older patients, start with 6.25 to 12.5 mg/d. Increase by 6.25 to 12.5 mg once or twice a week to 50 to 100 mg/d.
Risperidone has been used to treat agitation in older patients with dementia in two small studies:
In a 9-week, open-label trial, 15 patients (mean age 78) with dementia were given risperidone, 0.5 to 3 mg/d. Agitation improved significantly, as measured by the Cohen-Mansfield Agitation Inventory (CMAI)—a 29-item questionnaire completed by caregivers.26 CMAI scores at study’s end averaged 49.5, compared with 70.5 at baseline.27
A 12-week, placebo-controlled, doubleblind study examined risperidone—0.5, 1, or 2 mg/d—in 625 institutionalized patients (mean age 83) with dementia and agitation. Ninety-six patients had Functional Assessment Staging Rating Scale scores of 6A, indicating moderate to severe dementia. In patients receiving risperidone, these behavioral measures were significantly reduced:
- Behavior Pathology in Alzheimer’s Disease Rating Scale (BEHAVE-AD) total scores, which measure behavior severity
- BEHAVE-AD psychosis subscale scores
- BEHAVE-AD aggressiveness scores
- CMAI verbal and aggression scores.
Adverse effects were reported at 82% for all three risperidone dosages and 85% for placebo. Side effects including somnolence, EPS, and peripheral edema were dose-related.12
Another trial compared risperidone or haloperidol, 0.5 to 4 mg/d, with placebo in treating 344 patients with behavioral symptoms of dementia. After 12 weeks of risperidone, mean dosage 1.1 mg/d:
- mean total BEHAVE-AD score decreased by 53%, compared with 37% in the placebo group
- CMAI score decreased by 32%, compared with 18% in the placebo group.
EPS were more severe with haloperidol than with risperidone or placebo.28
Risk of stroke. A small but significantly increased incidence of stroke and stroke-like events was recently reported in older patients with dementia when treated with risperidone. These events occurred in double-blind, placebocontrolled trials in patients (mean age 82) with Alzheimer’s, vascular, and mixed dementias.
Pharmacokinetic changes can influence concentrations of drugs in tissue compartments over time. Drug absorption declines with normal aging, but a clinically significant decrease in total absorption of psychotropics appears not to occur.13
In the liver, lipid-soluble psychotropics are metabolized into pharmacologically active or inactive metabolites. Some metabolic pathways, such as demethylation, may be influenced by age, leading to increased plasma concentrations of drugs and their metabolites.14,15 However, hydroxylation tends not to be affected by age.16
The ratio of body fat to water increases with aging,13 increasing the volume of distribution for lipid-soluble psychotropics. An age-related decrease in glomerular filtration accounts in part for increased accumulation of hydrophilic metabolites in some older patients.17,18
Pharmacodynamic changes with aging occur in neurotransmitter systems within cellular processing, such as at receptor or reuptake levels.19 These changes may exaggerate drug-drug interactions or affect complex neurotransmitter interactions.
The number of neurons in nigrostriatal pathways declines with age. Decreases are also seen in tyrosine hydroxylase activity, presynaptic dopamine D2 receptors, and dopamine levels—which may be particularly relevant to a discussion of antipsychotic medications.20
The net effect of these changes is the need to prescribe lower-than-usual starting and target dosages of many medications, including antipsychotics.
Most patients who experienced cerebrovascular events had one or more stroke risk factors, including diabetes, hypertension, atrial fibrillation, heart arrhythmia, atherosclerosis, or heart failure. They did not show a pattern of reduced blood pressure or orthostatic changes.12,29
Table 2
Antipsychotic side effects and dosages in older patients with dementia*
| Side effect | Clozapine (6.25 to 100 mg/d) | Risperidone (0.5 to 1.5 mg/d) | Olanzapine (5 to 10 mg/d) | Quetiapine (25 to 350 mg/d) |
|---|---|---|---|---|
| Orthostasis | ++++ | ++++ | +++ | ++ |
| Sedation | +++++ | ++ | +++ | ++ |
| Prolactin increase | 0 | +++ | + | 0 |
| Weight gain | ++++ | + | +++ | + |
| EPS | 0/+ | ++ | + | 0/+ |
| Tardive dyskinesia | 0 | + | + | ? |
| Anticholinergic effects | ++++ | + | + | 0 |
| Seizure risk | +++ | + | + | + |
| Hematologic effects | +++ | + | + | + |
| Source: Adapted from references 15 and 16. | ||||
| * Side-effect profiles and recommended dosages of ziprasidone and aripiprazole in older patients are not yet established. | ||||
| EPS: Extrapyramidal symptoms | ||||
| Key: | ||||
| 0 = none | ||||
| + = slight | ||||
| +++ = mild | ||||
| +++++ = marked | ||||
| 0/+ = none to slight | ||||
| ++ = very mild | ||||
| ++++ = moderate | ||||
Dosing. For older patients with dementia and psychosis, start risperidone at 0.25 to 0.5 mg/d and increase by no more than 0.25 to 0.5 mg once or twice per week. Do not exceed 3 mg/d. For agitation, a 1998 Expert Consensus Guideline Series panel2 recommended starting risperidone at 0.25 to 0.5 mg/d and increasing to an average of 0.5 to 1.5 mg/d.
Olanzapine. Two double-blind, placebo-controlled studies have examined olanzapine in treating agitation associated with dementia.
Saterlee et al30 compared olanzapine, mean 2.4 mg/d, with placebo in outpatients (mean age 79) with Alzheimer’s disease and psychosis. No significant differences were noted in hepatic transaminase levels, leukopenia, EPS, or orthostatic changes.
In a later study,13 nursing home patients (mean age 83) with Alzheimer’s disease, psychosis, and agitation were randomly assigned to receive olanzapine—5, 10, or 15 mg/d—or placebo. After 6 weeks, patients receiving olanzapine, 5 or 10 mg/d, showed significant improvement in Neuropsychiatric Inventory (NPI) total core scores. Olanzapine, 15 mg/d, was not significantly more effective than placebo.
Adverse events such as somnolence and abnormal gait occurred more often with olanzapine than placebo. The somnolence rate with olanzapine was 14% for 5 mg/d and 13% for 10 mg/d, compared with 3% for placebo. For abnormal gait, the rate with olanzapine was 11% for 5 mg/d and 7% for 10 mg/d, compared with 1% for placebo.
Dosing. Start olanzapine at 2.5 mg/d, and increase after 1 to 3 days to 5 mg/d. If symptoms are not adequately controlled, titrate by 2.5-mg increments to 10 mg/d.
Quetiapine. One open-label study14 examined using quetiapine in older patients with psychotic disorders. The study enrolled 184 patients (mean age 76) with Alzheimer’s disease, Parkinson’s disease, schizophrenia, vascular dementia, schizoaffective disorder, bipolar disorder, or major depression. Before the trial, patients were taking various conventional and atypical antipsychotics.
Brief Psychiatric Rating Scale (BPRS) and Clinical Global Impressions (CGI) scores improved significantly after 52 weeks of quetiapine, median 137.5 mg/d. BPRS scores improved 20% in 49% of patients who completed the study.
Less than one-half (48%) of enrolled patients completed the study. Reasons for withdrawal included lack of efficacy (19%), adverse events or illness (15%; adverse events alone, 11%), lost to follow-up (13%), protocol noncompliance (3%), or diminished need for treatment (2%).
EPS occurred in 13% of patients. Mean total scores on the Simpson-Angus Rating Scale for Extrapyramidal Side Effects decreased 1.8 points, indicating reduced parkinsonian symptoms.
Dosing. Start quetiapine at 25 mg once at bedtime or bid; increase in 25-mg increments until the lowest effective dosage is achieved.
Ziprasidone. Little data exist on using ziprasidone in long-term care. In one recent study,31 ziprasidone (mean 100 mg/d) was given to 62 patients ages 64 to 92 with medical illnesses plus major depression, bipolar disorder, schizoaffective disorder, Alzheimer’s disease, or multi-infarct dementia. A retrospective chart review of 10 patients showed decreased agitation, as mean NPI scores declined from 76 to 33.
Sedation was the most common side effect. QTc findings, postural hypotension, and syncope rates did not change. Despite its limitations, this study suggests that ziprasidone is safe and effective in treating psychosis associated with dementia or other disorders.
Aripiprazole. As with ziprasidone, little data exist to guide the use of aripiprazole in older patients. In a randomized preliminary trial,32 192 noninstitutionalized patients with Alzheimer’s disease and psychosis were treated for 10 weeks with aripiprazole, mean 10 mg/d, or placebo.
At 8 and 10 weeks, BPRS psychosis subscale scores improved significantly in patients taking aripiprazole, compared with placebo. EPS and akathisia improved, and somnolence was the most common side effect. Although this study enrolled noninstitutionalized patients with dementia, the results suggest that aripiprazole may help treat long-term care residents with neurodegenerative disorders and behavioral disturbances.
Related resources
- Zaraa AS. Dementia update: Pharmacologic management of agitation and psychosis in older demented patients. Geriatrics 2003;58(10):48-53.
- Mills EJ, Chow TW. Randomized controlled trials in long-term care of residents with dementia: a systematic review. J Am Med Dir Assoc 2003;4(6):302-7.
- Alzheimer’s Association. Treating agitation. www.alz.org/PhysCare/Treating/agitation.htm
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Donepezil • Aricept
- Galantamine • Reminyl
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Rivastigmine • Exelon
- Valproate • Depakote
- Ziprasidone • Geodon
Disclosure
Dr. Kasckow receives research support from, is a consultant to, or is a speaker for Eli Lilly & Co., Forest Laboratories, Solvay Pharmaceuticals, AstraZeneca Pharmaceuticals, Organon, Janssen Pharmaceutica, and Pfizer Inc.
Dr. Mulchahey reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Mohamed receives research support form Forest Laboratories and is a speaker for Eli Lilly & Co.
1. Finkel S, Costa e Silva J, Cohen G, et al. Behavioral and psychological symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. Am J Geriatr Psychiatry 1998;6:97-100.
2. The Expert Consensus Panel for Agitation in Dementia. Treatment of agitation in older persons with dementia. Postgrad Med 1998;4(suppl):1-88.
3. Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review, summary, and critique. Am J Geriatr Psychiatry 2001;9(4):361-81.
4. Davidson J. Pharmacologic treatment. In: Busse E, Blazer D (eds). Textbook of geriatric psychiatry (2nd ed). Washington DC: American Psychiatric Publishing, 1996:359-79.
5. Nies A, Robinson DS, Friedman MJ, et al. Relationship between age and tricyclic antidepressant plasma levels. Am J Psychiatry 1977;134(7):790-3.
6. Greenblatt DJ, Shader RJ. Benzodiazepine kinetics in the elderly. In: Usdin E (ed). Clinical pharmacology in psychiatry. New York: Elsevier, 1981;174-81.
7. Pollock BG, Perel JM, Altieri LP, et al. Debrisoquine hydroxylation phenotyping in geriatric psychopharmacology. Psychopharmacol Bull. 1992;28(2):163-8.
8. Nelson JC, Atillasoy E, Mazure C, Jatlow PI. Hydroxydesipramine in the elderly. J Clin Psychopharmacol 1988;8(6):428-33.
9. Young RC, Alexopoulos GS, Shamoian CA, et al. Plasma 10-hydroxynortriptyline in elderly depressed patients. Clin Pharmacol Ther 1984;35(4):540-4.
10. Cantillon M, Molchan SE, Little J. Pharmacological and neuroendocrine probes in neuropsychiatric illness. In: Coffey CE, Cummings JL (eds). Textbook of geriatric neuropsychiatry. Washington, DC: American Psychiatric Publishing, 1994.
11. Young RC, Meyers BS. Psychopharmacology. In: Sadovoy J, Lazarus LW, Jarvik LF, Grossberg GT (eds). Comprehensive review of geriatric psychiatry. Washington DC: American Psychiatric Publishing, 1996;755-817.
12. Katz IR, Jeste DV, Mintzer JE, et al. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psychiatry 1999;60(2):107-15.
13. Street JS, Clark WS, Gannon KS, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU Study Group. Arch Gen Psychiatry 2000;57(10):968-76.
14. Tariot PN, Salzman C, Yeung PP, et al. Long-term use of quetiapine in elderly patients with psychotic disorders. Clin Ther 2000;22(9):1068-84.
15. Casey DE. The relationship of pharmacology to side effects. J Clin Psychiatry 1997;58(suppl):55-62.
16. Pickar D. Prospects for pharmacotherapy of schizophrenia. Lancet 1995;345:557-62.
17. Kasckow JW, McElroy SL, Cameron RL, et al. A pilot study on the use of divalproex sodium in the treatment of behavioral agitation in elderly patients with dementia: assessment with the BEHAVE-AD and CGI rating scales. Curr Ther Res 1997;58(12):981-9.
18. Tariot PN, Erb R, Podgorski CA, et al. Efficacy and tolerability of carbamazepine for agitation and aggression in dementia. Am J Psychiatry 1998;155(1):54-61.
19. Kasckow JW. Cognitive enhancers for dementia: do they work? Current Psychiatry 2002;1(3):22-8.
20. Lacy C, Armstrong L, Goldman M, Lance L. (eds) Lexicomp drug information handbook. Hudson, OH: Lexicomp, 2003-2004:1225-27, 1189-90, 1026-27.
21. Jeste DV, Lacro JP, Bailey A, et al. Lower incidence of tardive dyskinesia with risperidone compared with haloperidol in older patients. J Am Geriatr Soc 1999;47(6):716-19.
22. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988;45(9):789-96.
23. Jeste DV, Caligiuri MP, Paulsen JS, et al. Risk of tardive dyskinesia in older patients. A prospective longitudinal study of 266 outpatients. Arch Gen Psychiatry 1995;52(9):756-65.
24. Sernyak MJ, Leslie DL, Alarcon RD, et al. Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry 2002;159:561-6.
25. Chengappa KN, Baker RW, Kreinbrook SB, Adair D. Clozapine use in female geriatric patients with psychoses. JGeriatr Psychiatry Neurol 1995;8(1):12-15.
26. Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in the nursing home. J Gerontol 1989;44(3):M77-84.
27. Lavretsky H, Sultzer D. A structured trial of risperidone for the treatment of agitation in dementia. Am J Geriatr Psychiatry 1998;6(2):127-35.
28. De Deyn PP, Rabheru K, Rasmussen A, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology 1999;53(5):946-55.
29. Brodaty H, Ames D, Snowdon J, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry 2003;64(2):134-43.
30. Satterlee W, Reams SG, Burns PR, et al. A clinical update on olanzapine treatment in schizophrenia and in elderly Alzheimer’s disease patients (abstract). Psychopharmacol Bull 1995;31:534.-
31. Berkowitz A. Ziprasidone for elderly dementia: a case series (abstract). San Francisco, CA: American Psychiatric Association annual meeting, 2003.
32. De Deyn PP, Jeste D, Auby P, Carson W. Aripiprazole in dementia of the Alzheimer’s type (abstract). Honolulu, HI: American Association for Geriatric Psychiatry annual meeting, 2003.
Three keys can help you safely treat dementia’s difficult behavioral and psychological symptoms:
- Differentiate medical from psychiatric causes of patients’ distress.
- Use antipsychotics and other drugs as adjuncts to psychosocial treatments.
- Start low and go slow when titrating dosages.
Although no treatment reverses the pathophysiology of progressive neurodegenerative disorders, managing agitation and other behaviors can alleviate patient suffering and reduce caregiver stress. Based on the evidence and our experience, this article describes a practical approach, including a treatment algorithm and evidence of atypical antipsychotics’ efficacy and side effects in this patient population.
Algorithm Treating behavioral symptoms in patients with dementia
Dementia’s behavioral symptoms
An International Psychogeriatric Association consensus statement1 grouped dementia’s behavioral and psychological symptoms into two types:
- those usually assessed by interviewing patients and relatives—anxiety, depressed mood, hallucinations, and delusions
- those usually identified by observing patient behavior—aggression, screaming, restlessness, agitation, wandering, culturally inappropriate behaviors, sexual disinhibition, hoarding, cursing, and shadowing.
These behaviors in community-living patients are distressing to family members and increase the risk for caregiver burnout—the most common reason for placing older patients in long-term care. In the nursing home, dementia’s symptoms reduce patients’ quality of life; interfere with feeding, bathing, and dressing; and—when violent—may endanger staff and other patients.
Rule out a medical cause
Differential diagnosis. Behavioral symptoms in dementia tend to be unpredictable, which makes diagnosis and treatment challenging. The first step is to determine if a medical or psychiatric condition might account for the behavior. For instance:
- A patient with dementia may be agitated because of a distended bladder or arthritis but unable to communicate his or her pain in words.
- In mild dementia, a pre-existing psychiatric disorder such as schizophrenia might be causing a patient’s hallucinations or delusions.
- Pacing and restlessness may be drug side effects and might be controlled by reducing dosages or switching to less-activating agents.
Delirium is also a risk for older patients—especially those with degenerative neurologic disorders. Common triggers in older patients include acute illness such as a urinary tract infection or pneumonia, alcohol or benzodiazepine withdrawal, anticholinergic agents, medication changes, and dehydration.
Delirium is characterized by acute onset and fluctuating neuropsychiatric symptoms, including disturbed consciousness and changes in attention and cognition. Taking a careful history to learn the course of treatment and the patient’s baseline cognitive function can help you differentiate dementia from delirium. Family members, physicians, and nursing staff are valuable sources of this information.
Use antipsychotics as adjuncts
Psychosocial interventions. After medical causes have been ruled out, consensus guidelines2 recommend psychosocial interventions as first-line treatment of dementia’s behavioral symptoms (Algorithm). Suggested interventions for patients and caregivers are listed in Table 1.3
Antipsychotics. For patients who respond inadequately to psychosocial measures, the next step is to add an atypical antipsychotic. Because of side effects, conventional antipsychotics are not recommended for patients with dementia.
When prescribing atypicals, remember that older adults:
- are more sensitive to side effects than younger adults
- require lower starting and target dosages
- exhibit heterogeneity of response.
Older patients’ medical status can range from “fit” to “frail,” which influences individual response to medications. Generally, age-related changes in the way their bodies metabolize drugs account for older patients’ increased sensitivity to drug side effects (Box).4-11
Atypical antipsychotics and dosages that have been shown benefit for managing behavioral symptoms in older patients with dementia include:
- risperidone, 0.5 to 1.5 mg/d12
- olanzapine, 5 to 10 mg/d13
- quetiapine, 25 to 350 mg/d14 (Table 2).15,16
Start with low dosages, and titrate slowly. Increase once or twice a week until the lowest effective dosage is reached.
Augmenting agents. If antipsychotic monotherapy fails to achieve an adequate response or if side effects limit dosing, adjunctive agents may be added with caution. Augmenting agents that have shown benefit in some patients with dementia include:
- mood stabilizers such as divalproex17 or carbamazepine18
- cholinesterase inhibitors, such as donepezil, rivastigmine, or galantamine.19
Start divalproex at 125 mg bid or carbamazepine at 100 mg bid and titrate to effect. Concomitant carbamazepine will decrease blood levels of risperidone, olanzapine, and quetiapine because of hepatic enzyme induction.20
Start donepezil at 5 mg once daily and increase after 4 to 6 weeks to 10 mg qd. When using rivastigmine, start with 1.5 mg bid and titrate to 9 to 12 mg/d in divided doses. Start galantamine at 4 mg bid and increase after 1 month to 8 mg bid.
Table 1
Suggested psychosocial interventions for older patients with dementia
Communicate clearly
|
Minimize the impact of sensory deficits
|
Modify environment when necessary
|
Encourage consistent daily routines
|
Optimize social/physical stimulation
|
Encourage caregiver to:
|
Antipsychotic side effects
Atypical antipsychotics are more effective than conventional agents in treating negative symptoms and are associated with lower rates of extrapyramidal symptoms (EPS) and tardive dyskinesia (TD).21
Tardive dyskinesia. All antipsychotics can cause TD, although the risk is about 10 times greater with conventionals than atypicals. With conventionals, the annual cumulative TD incidence for young adults is 4 to 5%,22 and rates are much higher for middle-aged and older adults receiving chronic therapy:
- 29% after 1 year
- 50% after 2 years
- 63% after 3 years.23
In older patients, use atypical rather than conventional antipsychotics to minimize TD risk. Observe carefully; if TD symptoms occur, cautiously withdraw the antipsychotic and consider trying another agent.
Other risks. Atypical antipsychotics may cause sedation, orthostatic hypotension (with an increased risk for falls), increased serum prolactin, and weight gain (Table 2).
Weight gain from atypical antipsychotics has been associated with adverse effects on glucose metabolism and increased risk for type 2 diabetes.24 Some might argue that weight gain associated with olanzapine and other atypicals might benefit low-weight older patients. The frail elderly need to increase muscle mass, however, and the atypicals are associated with increases in fat mass.
Increased serum prolactin with risperidone theoretically could lead to loss of bone density, but evidence of this effect in older patients does not exist.
Start low, go slow
Clozapine may help control treatment-resistant psychosis in patients with schizophrenia and manage patients with severe TD.25 However, clozapine’s increased risk of agranulocytosis, neurologic side effects (seizures, sedation, confusion), and anticholinergic effects limit its use in older patients, particularly those with neurodegenerative disorders (Table 2).
Dosing. In rare cases when using clozapine in older patients, start with 6.25 to 12.5 mg/d. Increase by 6.25 to 12.5 mg once or twice a week to 50 to 100 mg/d.
Risperidone has been used to treat agitation in older patients with dementia in two small studies:
In a 9-week, open-label trial, 15 patients (mean age 78) with dementia were given risperidone, 0.5 to 3 mg/d. Agitation improved significantly, as measured by the Cohen-Mansfield Agitation Inventory (CMAI)—a 29-item questionnaire completed by caregivers.26 CMAI scores at study’s end averaged 49.5, compared with 70.5 at baseline.27
A 12-week, placebo-controlled, doubleblind study examined risperidone—0.5, 1, or 2 mg/d—in 625 institutionalized patients (mean age 83) with dementia and agitation. Ninety-six patients had Functional Assessment Staging Rating Scale scores of 6A, indicating moderate to severe dementia. In patients receiving risperidone, these behavioral measures were significantly reduced:
- Behavior Pathology in Alzheimer’s Disease Rating Scale (BEHAVE-AD) total scores, which measure behavior severity
- BEHAVE-AD psychosis subscale scores
- BEHAVE-AD aggressiveness scores
- CMAI verbal and aggression scores.
Adverse effects were reported at 82% for all three risperidone dosages and 85% for placebo. Side effects including somnolence, EPS, and peripheral edema were dose-related.12
Another trial compared risperidone or haloperidol, 0.5 to 4 mg/d, with placebo in treating 344 patients with behavioral symptoms of dementia. After 12 weeks of risperidone, mean dosage 1.1 mg/d:
- mean total BEHAVE-AD score decreased by 53%, compared with 37% in the placebo group
- CMAI score decreased by 32%, compared with 18% in the placebo group.
EPS were more severe with haloperidol than with risperidone or placebo.28
Risk of stroke. A small but significantly increased incidence of stroke and stroke-like events was recently reported in older patients with dementia when treated with risperidone. These events occurred in double-blind, placebocontrolled trials in patients (mean age 82) with Alzheimer’s, vascular, and mixed dementias.
Pharmacokinetic changes can influence concentrations of drugs in tissue compartments over time. Drug absorption declines with normal aging, but a clinically significant decrease in total absorption of psychotropics appears not to occur.13
In the liver, lipid-soluble psychotropics are metabolized into pharmacologically active or inactive metabolites. Some metabolic pathways, such as demethylation, may be influenced by age, leading to increased plasma concentrations of drugs and their metabolites.14,15 However, hydroxylation tends not to be affected by age.16
The ratio of body fat to water increases with aging,13 increasing the volume of distribution for lipid-soluble psychotropics. An age-related decrease in glomerular filtration accounts in part for increased accumulation of hydrophilic metabolites in some older patients.17,18
Pharmacodynamic changes with aging occur in neurotransmitter systems within cellular processing, such as at receptor or reuptake levels.19 These changes may exaggerate drug-drug interactions or affect complex neurotransmitter interactions.
The number of neurons in nigrostriatal pathways declines with age. Decreases are also seen in tyrosine hydroxylase activity, presynaptic dopamine D2 receptors, and dopamine levels—which may be particularly relevant to a discussion of antipsychotic medications.20
The net effect of these changes is the need to prescribe lower-than-usual starting and target dosages of many medications, including antipsychotics.
Most patients who experienced cerebrovascular events had one or more stroke risk factors, including diabetes, hypertension, atrial fibrillation, heart arrhythmia, atherosclerosis, or heart failure. They did not show a pattern of reduced blood pressure or orthostatic changes.12,29
Table 2
Antipsychotic side effects and dosages in older patients with dementia*
| Side effect | Clozapine (6.25 to 100 mg/d) | Risperidone (0.5 to 1.5 mg/d) | Olanzapine (5 to 10 mg/d) | Quetiapine (25 to 350 mg/d) |
|---|---|---|---|---|
| Orthostasis | ++++ | ++++ | +++ | ++ |
| Sedation | +++++ | ++ | +++ | ++ |
| Prolactin increase | 0 | +++ | + | 0 |
| Weight gain | ++++ | + | +++ | + |
| EPS | 0/+ | ++ | + | 0/+ |
| Tardive dyskinesia | 0 | + | + | ? |
| Anticholinergic effects | ++++ | + | + | 0 |
| Seizure risk | +++ | + | + | + |
| Hematologic effects | +++ | + | + | + |
| Source: Adapted from references 15 and 16. | ||||
| * Side-effect profiles and recommended dosages of ziprasidone and aripiprazole in older patients are not yet established. | ||||
| EPS: Extrapyramidal symptoms | ||||
| Key: | ||||
| 0 = none | ||||
| + = slight | ||||
| +++ = mild | ||||
| +++++ = marked | ||||
| 0/+ = none to slight | ||||
| ++ = very mild | ||||
| ++++ = moderate | ||||
Dosing. For older patients with dementia and psychosis, start risperidone at 0.25 to 0.5 mg/d and increase by no more than 0.25 to 0.5 mg once or twice per week. Do not exceed 3 mg/d. For agitation, a 1998 Expert Consensus Guideline Series panel2 recommended starting risperidone at 0.25 to 0.5 mg/d and increasing to an average of 0.5 to 1.5 mg/d.
Olanzapine. Two double-blind, placebo-controlled studies have examined olanzapine in treating agitation associated with dementia.
Saterlee et al30 compared olanzapine, mean 2.4 mg/d, with placebo in outpatients (mean age 79) with Alzheimer’s disease and psychosis. No significant differences were noted in hepatic transaminase levels, leukopenia, EPS, or orthostatic changes.
In a later study,13 nursing home patients (mean age 83) with Alzheimer’s disease, psychosis, and agitation were randomly assigned to receive olanzapine—5, 10, or 15 mg/d—or placebo. After 6 weeks, patients receiving olanzapine, 5 or 10 mg/d, showed significant improvement in Neuropsychiatric Inventory (NPI) total core scores. Olanzapine, 15 mg/d, was not significantly more effective than placebo.
Adverse events such as somnolence and abnormal gait occurred more often with olanzapine than placebo. The somnolence rate with olanzapine was 14% for 5 mg/d and 13% for 10 mg/d, compared with 3% for placebo. For abnormal gait, the rate with olanzapine was 11% for 5 mg/d and 7% for 10 mg/d, compared with 1% for placebo.
Dosing. Start olanzapine at 2.5 mg/d, and increase after 1 to 3 days to 5 mg/d. If symptoms are not adequately controlled, titrate by 2.5-mg increments to 10 mg/d.
Quetiapine. One open-label study14 examined using quetiapine in older patients with psychotic disorders. The study enrolled 184 patients (mean age 76) with Alzheimer’s disease, Parkinson’s disease, schizophrenia, vascular dementia, schizoaffective disorder, bipolar disorder, or major depression. Before the trial, patients were taking various conventional and atypical antipsychotics.
Brief Psychiatric Rating Scale (BPRS) and Clinical Global Impressions (CGI) scores improved significantly after 52 weeks of quetiapine, median 137.5 mg/d. BPRS scores improved 20% in 49% of patients who completed the study.
Less than one-half (48%) of enrolled patients completed the study. Reasons for withdrawal included lack of efficacy (19%), adverse events or illness (15%; adverse events alone, 11%), lost to follow-up (13%), protocol noncompliance (3%), or diminished need for treatment (2%).
EPS occurred in 13% of patients. Mean total scores on the Simpson-Angus Rating Scale for Extrapyramidal Side Effects decreased 1.8 points, indicating reduced parkinsonian symptoms.
Dosing. Start quetiapine at 25 mg once at bedtime or bid; increase in 25-mg increments until the lowest effective dosage is achieved.
Ziprasidone. Little data exist on using ziprasidone in long-term care. In one recent study,31 ziprasidone (mean 100 mg/d) was given to 62 patients ages 64 to 92 with medical illnesses plus major depression, bipolar disorder, schizoaffective disorder, Alzheimer’s disease, or multi-infarct dementia. A retrospective chart review of 10 patients showed decreased agitation, as mean NPI scores declined from 76 to 33.
Sedation was the most common side effect. QTc findings, postural hypotension, and syncope rates did not change. Despite its limitations, this study suggests that ziprasidone is safe and effective in treating psychosis associated with dementia or other disorders.
Aripiprazole. As with ziprasidone, little data exist to guide the use of aripiprazole in older patients. In a randomized preliminary trial,32 192 noninstitutionalized patients with Alzheimer’s disease and psychosis were treated for 10 weeks with aripiprazole, mean 10 mg/d, or placebo.
At 8 and 10 weeks, BPRS psychosis subscale scores improved significantly in patients taking aripiprazole, compared with placebo. EPS and akathisia improved, and somnolence was the most common side effect. Although this study enrolled noninstitutionalized patients with dementia, the results suggest that aripiprazole may help treat long-term care residents with neurodegenerative disorders and behavioral disturbances.
Related resources
- Zaraa AS. Dementia update: Pharmacologic management of agitation and psychosis in older demented patients. Geriatrics 2003;58(10):48-53.
- Mills EJ, Chow TW. Randomized controlled trials in long-term care of residents with dementia: a systematic review. J Am Med Dir Assoc 2003;4(6):302-7.
- Alzheimer’s Association. Treating agitation. www.alz.org/PhysCare/Treating/agitation.htm
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Donepezil • Aricept
- Galantamine • Reminyl
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Rivastigmine • Exelon
- Valproate • Depakote
- Ziprasidone • Geodon
Disclosure
Dr. Kasckow receives research support from, is a consultant to, or is a speaker for Eli Lilly & Co., Forest Laboratories, Solvay Pharmaceuticals, AstraZeneca Pharmaceuticals, Organon, Janssen Pharmaceutica, and Pfizer Inc.
Dr. Mulchahey reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Mohamed receives research support form Forest Laboratories and is a speaker for Eli Lilly & Co.
Three keys can help you safely treat dementia’s difficult behavioral and psychological symptoms:
- Differentiate medical from psychiatric causes of patients’ distress.
- Use antipsychotics and other drugs as adjuncts to psychosocial treatments.
- Start low and go slow when titrating dosages.
Although no treatment reverses the pathophysiology of progressive neurodegenerative disorders, managing agitation and other behaviors can alleviate patient suffering and reduce caregiver stress. Based on the evidence and our experience, this article describes a practical approach, including a treatment algorithm and evidence of atypical antipsychotics’ efficacy and side effects in this patient population.
Algorithm Treating behavioral symptoms in patients with dementia
Dementia’s behavioral symptoms
An International Psychogeriatric Association consensus statement1 grouped dementia’s behavioral and psychological symptoms into two types:
- those usually assessed by interviewing patients and relatives—anxiety, depressed mood, hallucinations, and delusions
- those usually identified by observing patient behavior—aggression, screaming, restlessness, agitation, wandering, culturally inappropriate behaviors, sexual disinhibition, hoarding, cursing, and shadowing.
These behaviors in community-living patients are distressing to family members and increase the risk for caregiver burnout—the most common reason for placing older patients in long-term care. In the nursing home, dementia’s symptoms reduce patients’ quality of life; interfere with feeding, bathing, and dressing; and—when violent—may endanger staff and other patients.
Rule out a medical cause
Differential diagnosis. Behavioral symptoms in dementia tend to be unpredictable, which makes diagnosis and treatment challenging. The first step is to determine if a medical or psychiatric condition might account for the behavior. For instance:
- A patient with dementia may be agitated because of a distended bladder or arthritis but unable to communicate his or her pain in words.
- In mild dementia, a pre-existing psychiatric disorder such as schizophrenia might be causing a patient’s hallucinations or delusions.
- Pacing and restlessness may be drug side effects and might be controlled by reducing dosages or switching to less-activating agents.
Delirium is also a risk for older patients—especially those with degenerative neurologic disorders. Common triggers in older patients include acute illness such as a urinary tract infection or pneumonia, alcohol or benzodiazepine withdrawal, anticholinergic agents, medication changes, and dehydration.
Delirium is characterized by acute onset and fluctuating neuropsychiatric symptoms, including disturbed consciousness and changes in attention and cognition. Taking a careful history to learn the course of treatment and the patient’s baseline cognitive function can help you differentiate dementia from delirium. Family members, physicians, and nursing staff are valuable sources of this information.
Use antipsychotics as adjuncts
Psychosocial interventions. After medical causes have been ruled out, consensus guidelines2 recommend psychosocial interventions as first-line treatment of dementia’s behavioral symptoms (Algorithm). Suggested interventions for patients and caregivers are listed in Table 1.3
Antipsychotics. For patients who respond inadequately to psychosocial measures, the next step is to add an atypical antipsychotic. Because of side effects, conventional antipsychotics are not recommended for patients with dementia.
When prescribing atypicals, remember that older adults:
- are more sensitive to side effects than younger adults
- require lower starting and target dosages
- exhibit heterogeneity of response.
Older patients’ medical status can range from “fit” to “frail,” which influences individual response to medications. Generally, age-related changes in the way their bodies metabolize drugs account for older patients’ increased sensitivity to drug side effects (Box).4-11
Atypical antipsychotics and dosages that have been shown benefit for managing behavioral symptoms in older patients with dementia include:
- risperidone, 0.5 to 1.5 mg/d12
- olanzapine, 5 to 10 mg/d13
- quetiapine, 25 to 350 mg/d14 (Table 2).15,16
Start with low dosages, and titrate slowly. Increase once or twice a week until the lowest effective dosage is reached.
Augmenting agents. If antipsychotic monotherapy fails to achieve an adequate response or if side effects limit dosing, adjunctive agents may be added with caution. Augmenting agents that have shown benefit in some patients with dementia include:
- mood stabilizers such as divalproex17 or carbamazepine18
- cholinesterase inhibitors, such as donepezil, rivastigmine, or galantamine.19
Start divalproex at 125 mg bid or carbamazepine at 100 mg bid and titrate to effect. Concomitant carbamazepine will decrease blood levels of risperidone, olanzapine, and quetiapine because of hepatic enzyme induction.20
Start donepezil at 5 mg once daily and increase after 4 to 6 weeks to 10 mg qd. When using rivastigmine, start with 1.5 mg bid and titrate to 9 to 12 mg/d in divided doses. Start galantamine at 4 mg bid and increase after 1 month to 8 mg bid.
Table 1
Suggested psychosocial interventions for older patients with dementia
Communicate clearly
|
Minimize the impact of sensory deficits
|
Modify environment when necessary
|
Encourage consistent daily routines
|
Optimize social/physical stimulation
|
Encourage caregiver to:
|
Antipsychotic side effects
Atypical antipsychotics are more effective than conventional agents in treating negative symptoms and are associated with lower rates of extrapyramidal symptoms (EPS) and tardive dyskinesia (TD).21
Tardive dyskinesia. All antipsychotics can cause TD, although the risk is about 10 times greater with conventionals than atypicals. With conventionals, the annual cumulative TD incidence for young adults is 4 to 5%,22 and rates are much higher for middle-aged and older adults receiving chronic therapy:
- 29% after 1 year
- 50% after 2 years
- 63% after 3 years.23
In older patients, use atypical rather than conventional antipsychotics to minimize TD risk. Observe carefully; if TD symptoms occur, cautiously withdraw the antipsychotic and consider trying another agent.
Other risks. Atypical antipsychotics may cause sedation, orthostatic hypotension (with an increased risk for falls), increased serum prolactin, and weight gain (Table 2).
Weight gain from atypical antipsychotics has been associated with adverse effects on glucose metabolism and increased risk for type 2 diabetes.24 Some might argue that weight gain associated with olanzapine and other atypicals might benefit low-weight older patients. The frail elderly need to increase muscle mass, however, and the atypicals are associated with increases in fat mass.
Increased serum prolactin with risperidone theoretically could lead to loss of bone density, but evidence of this effect in older patients does not exist.
Start low, go slow
Clozapine may help control treatment-resistant psychosis in patients with schizophrenia and manage patients with severe TD.25 However, clozapine’s increased risk of agranulocytosis, neurologic side effects (seizures, sedation, confusion), and anticholinergic effects limit its use in older patients, particularly those with neurodegenerative disorders (Table 2).
Dosing. In rare cases when using clozapine in older patients, start with 6.25 to 12.5 mg/d. Increase by 6.25 to 12.5 mg once or twice a week to 50 to 100 mg/d.
Risperidone has been used to treat agitation in older patients with dementia in two small studies:
In a 9-week, open-label trial, 15 patients (mean age 78) with dementia were given risperidone, 0.5 to 3 mg/d. Agitation improved significantly, as measured by the Cohen-Mansfield Agitation Inventory (CMAI)—a 29-item questionnaire completed by caregivers.26 CMAI scores at study’s end averaged 49.5, compared with 70.5 at baseline.27
A 12-week, placebo-controlled, doubleblind study examined risperidone—0.5, 1, or 2 mg/d—in 625 institutionalized patients (mean age 83) with dementia and agitation. Ninety-six patients had Functional Assessment Staging Rating Scale scores of 6A, indicating moderate to severe dementia. In patients receiving risperidone, these behavioral measures were significantly reduced:
- Behavior Pathology in Alzheimer’s Disease Rating Scale (BEHAVE-AD) total scores, which measure behavior severity
- BEHAVE-AD psychosis subscale scores
- BEHAVE-AD aggressiveness scores
- CMAI verbal and aggression scores.
Adverse effects were reported at 82% for all three risperidone dosages and 85% for placebo. Side effects including somnolence, EPS, and peripheral edema were dose-related.12
Another trial compared risperidone or haloperidol, 0.5 to 4 mg/d, with placebo in treating 344 patients with behavioral symptoms of dementia. After 12 weeks of risperidone, mean dosage 1.1 mg/d:
- mean total BEHAVE-AD score decreased by 53%, compared with 37% in the placebo group
- CMAI score decreased by 32%, compared with 18% in the placebo group.
EPS were more severe with haloperidol than with risperidone or placebo.28
Risk of stroke. A small but significantly increased incidence of stroke and stroke-like events was recently reported in older patients with dementia when treated with risperidone. These events occurred in double-blind, placebocontrolled trials in patients (mean age 82) with Alzheimer’s, vascular, and mixed dementias.
Pharmacokinetic changes can influence concentrations of drugs in tissue compartments over time. Drug absorption declines with normal aging, but a clinically significant decrease in total absorption of psychotropics appears not to occur.13
In the liver, lipid-soluble psychotropics are metabolized into pharmacologically active or inactive metabolites. Some metabolic pathways, such as demethylation, may be influenced by age, leading to increased plasma concentrations of drugs and their metabolites.14,15 However, hydroxylation tends not to be affected by age.16
The ratio of body fat to water increases with aging,13 increasing the volume of distribution for lipid-soluble psychotropics. An age-related decrease in glomerular filtration accounts in part for increased accumulation of hydrophilic metabolites in some older patients.17,18
Pharmacodynamic changes with aging occur in neurotransmitter systems within cellular processing, such as at receptor or reuptake levels.19 These changes may exaggerate drug-drug interactions or affect complex neurotransmitter interactions.
The number of neurons in nigrostriatal pathways declines with age. Decreases are also seen in tyrosine hydroxylase activity, presynaptic dopamine D2 receptors, and dopamine levels—which may be particularly relevant to a discussion of antipsychotic medications.20
The net effect of these changes is the need to prescribe lower-than-usual starting and target dosages of many medications, including antipsychotics.
Most patients who experienced cerebrovascular events had one or more stroke risk factors, including diabetes, hypertension, atrial fibrillation, heart arrhythmia, atherosclerosis, or heart failure. They did not show a pattern of reduced blood pressure or orthostatic changes.12,29
Table 2
Antipsychotic side effects and dosages in older patients with dementia*
| Side effect | Clozapine (6.25 to 100 mg/d) | Risperidone (0.5 to 1.5 mg/d) | Olanzapine (5 to 10 mg/d) | Quetiapine (25 to 350 mg/d) |
|---|---|---|---|---|
| Orthostasis | ++++ | ++++ | +++ | ++ |
| Sedation | +++++ | ++ | +++ | ++ |
| Prolactin increase | 0 | +++ | + | 0 |
| Weight gain | ++++ | + | +++ | + |
| EPS | 0/+ | ++ | + | 0/+ |
| Tardive dyskinesia | 0 | + | + | ? |
| Anticholinergic effects | ++++ | + | + | 0 |
| Seizure risk | +++ | + | + | + |
| Hematologic effects | +++ | + | + | + |
| Source: Adapted from references 15 and 16. | ||||
| * Side-effect profiles and recommended dosages of ziprasidone and aripiprazole in older patients are not yet established. | ||||
| EPS: Extrapyramidal symptoms | ||||
| Key: | ||||
| 0 = none | ||||
| + = slight | ||||
| +++ = mild | ||||
| +++++ = marked | ||||
| 0/+ = none to slight | ||||
| ++ = very mild | ||||
| ++++ = moderate | ||||
Dosing. For older patients with dementia and psychosis, start risperidone at 0.25 to 0.5 mg/d and increase by no more than 0.25 to 0.5 mg once or twice per week. Do not exceed 3 mg/d. For agitation, a 1998 Expert Consensus Guideline Series panel2 recommended starting risperidone at 0.25 to 0.5 mg/d and increasing to an average of 0.5 to 1.5 mg/d.
Olanzapine. Two double-blind, placebo-controlled studies have examined olanzapine in treating agitation associated with dementia.
Saterlee et al30 compared olanzapine, mean 2.4 mg/d, with placebo in outpatients (mean age 79) with Alzheimer’s disease and psychosis. No significant differences were noted in hepatic transaminase levels, leukopenia, EPS, or orthostatic changes.
In a later study,13 nursing home patients (mean age 83) with Alzheimer’s disease, psychosis, and agitation were randomly assigned to receive olanzapine—5, 10, or 15 mg/d—or placebo. After 6 weeks, patients receiving olanzapine, 5 or 10 mg/d, showed significant improvement in Neuropsychiatric Inventory (NPI) total core scores. Olanzapine, 15 mg/d, was not significantly more effective than placebo.
Adverse events such as somnolence and abnormal gait occurred more often with olanzapine than placebo. The somnolence rate with olanzapine was 14% for 5 mg/d and 13% for 10 mg/d, compared with 3% for placebo. For abnormal gait, the rate with olanzapine was 11% for 5 mg/d and 7% for 10 mg/d, compared with 1% for placebo.
Dosing. Start olanzapine at 2.5 mg/d, and increase after 1 to 3 days to 5 mg/d. If symptoms are not adequately controlled, titrate by 2.5-mg increments to 10 mg/d.
Quetiapine. One open-label study14 examined using quetiapine in older patients with psychotic disorders. The study enrolled 184 patients (mean age 76) with Alzheimer’s disease, Parkinson’s disease, schizophrenia, vascular dementia, schizoaffective disorder, bipolar disorder, or major depression. Before the trial, patients were taking various conventional and atypical antipsychotics.
Brief Psychiatric Rating Scale (BPRS) and Clinical Global Impressions (CGI) scores improved significantly after 52 weeks of quetiapine, median 137.5 mg/d. BPRS scores improved 20% in 49% of patients who completed the study.
Less than one-half (48%) of enrolled patients completed the study. Reasons for withdrawal included lack of efficacy (19%), adverse events or illness (15%; adverse events alone, 11%), lost to follow-up (13%), protocol noncompliance (3%), or diminished need for treatment (2%).
EPS occurred in 13% of patients. Mean total scores on the Simpson-Angus Rating Scale for Extrapyramidal Side Effects decreased 1.8 points, indicating reduced parkinsonian symptoms.
Dosing. Start quetiapine at 25 mg once at bedtime or bid; increase in 25-mg increments until the lowest effective dosage is achieved.
Ziprasidone. Little data exist on using ziprasidone in long-term care. In one recent study,31 ziprasidone (mean 100 mg/d) was given to 62 patients ages 64 to 92 with medical illnesses plus major depression, bipolar disorder, schizoaffective disorder, Alzheimer’s disease, or multi-infarct dementia. A retrospective chart review of 10 patients showed decreased agitation, as mean NPI scores declined from 76 to 33.
Sedation was the most common side effect. QTc findings, postural hypotension, and syncope rates did not change. Despite its limitations, this study suggests that ziprasidone is safe and effective in treating psychosis associated with dementia or other disorders.
Aripiprazole. As with ziprasidone, little data exist to guide the use of aripiprazole in older patients. In a randomized preliminary trial,32 192 noninstitutionalized patients with Alzheimer’s disease and psychosis were treated for 10 weeks with aripiprazole, mean 10 mg/d, or placebo.
At 8 and 10 weeks, BPRS psychosis subscale scores improved significantly in patients taking aripiprazole, compared with placebo. EPS and akathisia improved, and somnolence was the most common side effect. Although this study enrolled noninstitutionalized patients with dementia, the results suggest that aripiprazole may help treat long-term care residents with neurodegenerative disorders and behavioral disturbances.
Related resources
- Zaraa AS. Dementia update: Pharmacologic management of agitation and psychosis in older demented patients. Geriatrics 2003;58(10):48-53.
- Mills EJ, Chow TW. Randomized controlled trials in long-term care of residents with dementia: a systematic review. J Am Med Dir Assoc 2003;4(6):302-7.
- Alzheimer’s Association. Treating agitation. www.alz.org/PhysCare/Treating/agitation.htm
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Donepezil • Aricept
- Galantamine • Reminyl
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Rivastigmine • Exelon
- Valproate • Depakote
- Ziprasidone • Geodon
Disclosure
Dr. Kasckow receives research support from, is a consultant to, or is a speaker for Eli Lilly & Co., Forest Laboratories, Solvay Pharmaceuticals, AstraZeneca Pharmaceuticals, Organon, Janssen Pharmaceutica, and Pfizer Inc.
Dr. Mulchahey reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Mohamed receives research support form Forest Laboratories and is a speaker for Eli Lilly & Co.
1. Finkel S, Costa e Silva J, Cohen G, et al. Behavioral and psychological symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. Am J Geriatr Psychiatry 1998;6:97-100.
2. The Expert Consensus Panel for Agitation in Dementia. Treatment of agitation in older persons with dementia. Postgrad Med 1998;4(suppl):1-88.
3. Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review, summary, and critique. Am J Geriatr Psychiatry 2001;9(4):361-81.
4. Davidson J. Pharmacologic treatment. In: Busse E, Blazer D (eds). Textbook of geriatric psychiatry (2nd ed). Washington DC: American Psychiatric Publishing, 1996:359-79.
5. Nies A, Robinson DS, Friedman MJ, et al. Relationship between age and tricyclic antidepressant plasma levels. Am J Psychiatry 1977;134(7):790-3.
6. Greenblatt DJ, Shader RJ. Benzodiazepine kinetics in the elderly. In: Usdin E (ed). Clinical pharmacology in psychiatry. New York: Elsevier, 1981;174-81.
7. Pollock BG, Perel JM, Altieri LP, et al. Debrisoquine hydroxylation phenotyping in geriatric psychopharmacology. Psychopharmacol Bull. 1992;28(2):163-8.
8. Nelson JC, Atillasoy E, Mazure C, Jatlow PI. Hydroxydesipramine in the elderly. J Clin Psychopharmacol 1988;8(6):428-33.
9. Young RC, Alexopoulos GS, Shamoian CA, et al. Plasma 10-hydroxynortriptyline in elderly depressed patients. Clin Pharmacol Ther 1984;35(4):540-4.
10. Cantillon M, Molchan SE, Little J. Pharmacological and neuroendocrine probes in neuropsychiatric illness. In: Coffey CE, Cummings JL (eds). Textbook of geriatric neuropsychiatry. Washington, DC: American Psychiatric Publishing, 1994.
11. Young RC, Meyers BS. Psychopharmacology. In: Sadovoy J, Lazarus LW, Jarvik LF, Grossberg GT (eds). Comprehensive review of geriatric psychiatry. Washington DC: American Psychiatric Publishing, 1996;755-817.
12. Katz IR, Jeste DV, Mintzer JE, et al. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psychiatry 1999;60(2):107-15.
13. Street JS, Clark WS, Gannon KS, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU Study Group. Arch Gen Psychiatry 2000;57(10):968-76.
14. Tariot PN, Salzman C, Yeung PP, et al. Long-term use of quetiapine in elderly patients with psychotic disorders. Clin Ther 2000;22(9):1068-84.
15. Casey DE. The relationship of pharmacology to side effects. J Clin Psychiatry 1997;58(suppl):55-62.
16. Pickar D. Prospects for pharmacotherapy of schizophrenia. Lancet 1995;345:557-62.
17. Kasckow JW, McElroy SL, Cameron RL, et al. A pilot study on the use of divalproex sodium in the treatment of behavioral agitation in elderly patients with dementia: assessment with the BEHAVE-AD and CGI rating scales. Curr Ther Res 1997;58(12):981-9.
18. Tariot PN, Erb R, Podgorski CA, et al. Efficacy and tolerability of carbamazepine for agitation and aggression in dementia. Am J Psychiatry 1998;155(1):54-61.
19. Kasckow JW. Cognitive enhancers for dementia: do they work? Current Psychiatry 2002;1(3):22-8.
20. Lacy C, Armstrong L, Goldman M, Lance L. (eds) Lexicomp drug information handbook. Hudson, OH: Lexicomp, 2003-2004:1225-27, 1189-90, 1026-27.
21. Jeste DV, Lacro JP, Bailey A, et al. Lower incidence of tardive dyskinesia with risperidone compared with haloperidol in older patients. J Am Geriatr Soc 1999;47(6):716-19.
22. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988;45(9):789-96.
23. Jeste DV, Caligiuri MP, Paulsen JS, et al. Risk of tardive dyskinesia in older patients. A prospective longitudinal study of 266 outpatients. Arch Gen Psychiatry 1995;52(9):756-65.
24. Sernyak MJ, Leslie DL, Alarcon RD, et al. Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry 2002;159:561-6.
25. Chengappa KN, Baker RW, Kreinbrook SB, Adair D. Clozapine use in female geriatric patients with psychoses. JGeriatr Psychiatry Neurol 1995;8(1):12-15.
26. Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in the nursing home. J Gerontol 1989;44(3):M77-84.
27. Lavretsky H, Sultzer D. A structured trial of risperidone for the treatment of agitation in dementia. Am J Geriatr Psychiatry 1998;6(2):127-35.
28. De Deyn PP, Rabheru K, Rasmussen A, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology 1999;53(5):946-55.
29. Brodaty H, Ames D, Snowdon J, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry 2003;64(2):134-43.
30. Satterlee W, Reams SG, Burns PR, et al. A clinical update on olanzapine treatment in schizophrenia and in elderly Alzheimer’s disease patients (abstract). Psychopharmacol Bull 1995;31:534.-
31. Berkowitz A. Ziprasidone for elderly dementia: a case series (abstract). San Francisco, CA: American Psychiatric Association annual meeting, 2003.
32. De Deyn PP, Jeste D, Auby P, Carson W. Aripiprazole in dementia of the Alzheimer’s type (abstract). Honolulu, HI: American Association for Geriatric Psychiatry annual meeting, 2003.
1. Finkel S, Costa e Silva J, Cohen G, et al. Behavioral and psychological symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. Am J Geriatr Psychiatry 1998;6:97-100.
2. The Expert Consensus Panel for Agitation in Dementia. Treatment of agitation in older persons with dementia. Postgrad Med 1998;4(suppl):1-88.
3. Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review, summary, and critique. Am J Geriatr Psychiatry 2001;9(4):361-81.
4. Davidson J. Pharmacologic treatment. In: Busse E, Blazer D (eds). Textbook of geriatric psychiatry (2nd ed). Washington DC: American Psychiatric Publishing, 1996:359-79.
5. Nies A, Robinson DS, Friedman MJ, et al. Relationship between age and tricyclic antidepressant plasma levels. Am J Psychiatry 1977;134(7):790-3.
6. Greenblatt DJ, Shader RJ. Benzodiazepine kinetics in the elderly. In: Usdin E (ed). Clinical pharmacology in psychiatry. New York: Elsevier, 1981;174-81.
7. Pollock BG, Perel JM, Altieri LP, et al. Debrisoquine hydroxylation phenotyping in geriatric psychopharmacology. Psychopharmacol Bull. 1992;28(2):163-8.
8. Nelson JC, Atillasoy E, Mazure C, Jatlow PI. Hydroxydesipramine in the elderly. J Clin Psychopharmacol 1988;8(6):428-33.
9. Young RC, Alexopoulos GS, Shamoian CA, et al. Plasma 10-hydroxynortriptyline in elderly depressed patients. Clin Pharmacol Ther 1984;35(4):540-4.
10. Cantillon M, Molchan SE, Little J. Pharmacological and neuroendocrine probes in neuropsychiatric illness. In: Coffey CE, Cummings JL (eds). Textbook of geriatric neuropsychiatry. Washington, DC: American Psychiatric Publishing, 1994.
11. Young RC, Meyers BS. Psychopharmacology. In: Sadovoy J, Lazarus LW, Jarvik LF, Grossberg GT (eds). Comprehensive review of geriatric psychiatry. Washington DC: American Psychiatric Publishing, 1996;755-817.
12. Katz IR, Jeste DV, Mintzer JE, et al. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psychiatry 1999;60(2):107-15.
13. Street JS, Clark WS, Gannon KS, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU Study Group. Arch Gen Psychiatry 2000;57(10):968-76.
14. Tariot PN, Salzman C, Yeung PP, et al. Long-term use of quetiapine in elderly patients with psychotic disorders. Clin Ther 2000;22(9):1068-84.
15. Casey DE. The relationship of pharmacology to side effects. J Clin Psychiatry 1997;58(suppl):55-62.
16. Pickar D. Prospects for pharmacotherapy of schizophrenia. Lancet 1995;345:557-62.
17. Kasckow JW, McElroy SL, Cameron RL, et al. A pilot study on the use of divalproex sodium in the treatment of behavioral agitation in elderly patients with dementia: assessment with the BEHAVE-AD and CGI rating scales. Curr Ther Res 1997;58(12):981-9.
18. Tariot PN, Erb R, Podgorski CA, et al. Efficacy and tolerability of carbamazepine for agitation and aggression in dementia. Am J Psychiatry 1998;155(1):54-61.
19. Kasckow JW. Cognitive enhancers for dementia: do they work? Current Psychiatry 2002;1(3):22-8.
20. Lacy C, Armstrong L, Goldman M, Lance L. (eds) Lexicomp drug information handbook. Hudson, OH: Lexicomp, 2003-2004:1225-27, 1189-90, 1026-27.
21. Jeste DV, Lacro JP, Bailey A, et al. Lower incidence of tardive dyskinesia with risperidone compared with haloperidol in older patients. J Am Geriatr Soc 1999;47(6):716-19.
22. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988;45(9):789-96.
23. Jeste DV, Caligiuri MP, Paulsen JS, et al. Risk of tardive dyskinesia in older patients. A prospective longitudinal study of 266 outpatients. Arch Gen Psychiatry 1995;52(9):756-65.
24. Sernyak MJ, Leslie DL, Alarcon RD, et al. Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry 2002;159:561-6.
25. Chengappa KN, Baker RW, Kreinbrook SB, Adair D. Clozapine use in female geriatric patients with psychoses. JGeriatr Psychiatry Neurol 1995;8(1):12-15.
26. Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in the nursing home. J Gerontol 1989;44(3):M77-84.
27. Lavretsky H, Sultzer D. A structured trial of risperidone for the treatment of agitation in dementia. Am J Geriatr Psychiatry 1998;6(2):127-35.
28. De Deyn PP, Rabheru K, Rasmussen A, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology 1999;53(5):946-55.
29. Brodaty H, Ames D, Snowdon J, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry 2003;64(2):134-43.
30. Satterlee W, Reams SG, Burns PR, et al. A clinical update on olanzapine treatment in schizophrenia and in elderly Alzheimer’s disease patients (abstract). Psychopharmacol Bull 1995;31:534.-
31. Berkowitz A. Ziprasidone for elderly dementia: a case series (abstract). San Francisco, CA: American Psychiatric Association annual meeting, 2003.
32. De Deyn PP, Jeste D, Auby P, Carson W. Aripiprazole in dementia of the Alzheimer’s type (abstract). Honolulu, HI: American Association for Geriatric Psychiatry annual meeting, 2003.
Preventing late-life suicide: 6 steps to detect the warning signs
CASE REPORT: I have a gun
Mr. V, age 77, appears depressed and anxious during his appointment at a mental hygiene clinic. He reports insomnia, concentration trouble, and anhedonia. He tells the psychiatrist he keeps a loaded gun at home and is not sure he can control his suicidal impulses.
The patient is Caucasian and has a history of heart failure, pulmonary disease, and type 2 diabetes. His wife died 18 months ago. He lives alone, but his sister lives nearby. He recently received a right hip replacement, which required 3 months of rehabilitation in a nursing home to recover from surgical complications. He still has trouble walking.
As in Mr. V’s case, treating older patients referred for psychiatric care often involves evaluating suicide risk. His age, race, gender, depressed mood, recent bereavement, and medical ailments place him in the population at highest risk of suicide (Box, Table 1).1-8
Approximately 20% of all suicides in the United States are committed by persons age 65 or older,1 who account for 13% of the total population. The suicide rate among persons older than 75 is three times higher than it is for the young.2 Older Caucasian men have the highest per-capita rate of completed suicide, compared with any other group of Americans.3
Psychiatric disorders. The rates of Axis I disorders among older persons who commit suicide fall within the average range for all age groups (70 to 90%). However, the types of disorders seen in the older population differ from those of younger suicides (Table 1).4-8
Affective illness has been termed “the predominant psychopathology associated with suicide in later life.”4 Among older persons who commit suicide, three-fourths (76%) have diagnosable mood disorders4 and nearly two-thirds (63%) have depression.6 Contributing risk factors include alcoholism and substance abuse,4,6,7 Axis II disorders, and dementia.6
Losses and medical illness. In later life, bereavement, loss of independence, or financial reversals may lead to depression. Older persons who take their own lives also tend to have greater physical health burdens and more functional disabilities than those who do not commit suicide.6,8
This article describes an age-based psychiatric workup of the suicidal older patient, including factors to consider when screening for depressive symptoms, prescribing drug therapy, and determining the need for hospitalization.
AGE-BASED CLINICAL WORKUP
For older patients who report suicidal ideation, an age-appropriate workup—using clinical interviews and screening instruments—is essential. The clinical interview can build rapport and gather information about the patient’s suicidal plan or intent. Based on our experience, we recommend the following 6-step screening interview, summarized in Table 2.
- Ask about a specific plan. Does the patient have the means readily available to carry out this plan? What is the timeline (imminent versus vaguely futuristic)? Does the patient report having control over this plan?
- Gather a suicide history. Has the patient attempted suicide before? By what means? Is there a family history of suicide? If yes, by what means did this family member commit suicide, and how was the patient affected?
- Assess social status. How isolated is the patient? Have there been recent changes in his or her social circle, such as loss of a spouse? Can the patient identify at least one person who would be negatively affected by the suicide?
- Assess medical health. Does the patient suffer from chronic pain? Does the patient have a recently diagnosed medical condition? Has a longstanding medical condition become more debilitating? Does the patient report feeling hopeless about impending medical difficulties? Has he or she been keeping regularly scheduled medical appointments with outpatient clinicians?
- Assess mental health. Does the patient meet DSM-IV criteria for depression or schizophrenia, which are associated with high suicide risk? Does he or she report being hopeless or helpless? Is the suicidal ideation ego dystonic?
- Ascertain clinical signs of suicidal intent. Has the patient:
Table 1
Suicide risk with mental and physical illness, by patient age
| Risk factors | Young (21 to 34 yrs) | Middle-aged (35 to 54 yrs) | Young-old (55 to 74 yrs) | Elderly (77+ yrs) |
|---|---|---|---|---|
| Psychiatric disorders | • | • | • | • |
| Mood disorders | ○ | • | • | |
| Alcohol abuse | ○ | • | ○ | |
| Primary psychoses | • | ○ | ||
| Personality disorders | ○ | |||
| Physical ailments | • | • | ||
| • Significant risk factor ○ Potential risk factor | ||||
| Source: Compiled from information in references 4-8. | ||||
CASE REPORT continued: Some telling signs
Mr. V’s laboratory screening reveals slightly elevated serum glucose and mild anemia. An ECG reveals a type I heart block, but all other lab results are unremarkable. His sister reports he recently gave away his dog, which he and his wife had owned for many years. He has also mentioned a desire to revise his will when speaking to other family members. Hospital records indicate he has missed numerous medical appointments over the past 4 months.
SCREENING INSTRUMENTS
Psychological assessments can often buttress the clinical interview findings. Several measurements are well-suited for detecting suicidal risk and concomitant depression (Table 3).
Beck SSI-C. The Beck Scale for Suicide Ideation – Current (SSI-C) assesses a patient’s preparation and motivation to commit suicide.9 This short (19-item) self-report measure asks patients to rate their wish to die, desire to attempt suicide, duration (and frequency) of suicidal thoughts, sense of control over suicide, and deterrents they face. The SSI-C helps to measure or monitor suicidality and is reliable and valid for psychiatric outpatients.9
BDI-II. The Beck Depression Inventory—recently revised in a second edition (BDI-II)10—can be useful because depression is one of the strongest risk factors for elder suicide. The 21-item BDI-II—a psychometrically sound, self-report instrument—asks about general symptoms of depression and gauges their severity. It can be applied to diverse patient populations and ages11 and is appropriate for older patients who are also being treated medically.
Beck Hopelessness Scale. Hopelessness has been recognized as a possible harbinger of suicide.12 One study showed that depression became a clinically meaningful suicide predictor only when accompanied by hopelessness.13
A score of 10 or more on the Beck Hopelessness Scale identified 91% of patients in one study who eventually committed suicide. The hopelessness patients expressed on this scale more strongly differentiated between those who did or did not commit suicide than did their scores on the BDI or SSI-C.14
Table 2
6-step clinical interview with an older suicidal patient
|
|
| Source: Adapted from the Cincinnati Veterans Affairs Medical Center general psychological suicide assessment |
HRSD-R. The revised Hamilton Rating Scale for Depression (HRSD-R) documents patients’ levels of mood disturbance and suicidality. One item in this 21-item, clinician-administered instrument specifically asks about the patient’s level of suicidality in the past week. The scale has well-documented reliability and validity and is appropriate for psychiatric populations.15
CASE REPORT continued: Alarming findings
Along with the clinical interview, Mr. V. is screened with the Beck Hopelessness Scale and Beck Depression Inventory-II. These instruments are chosen because they are easy to administer, and patients can readily comprehend the questions—even when under duress. Mr. V’s results reveal moderate depression and severe hopelessness.
INPATIENT VS. OUTPATIENT CARE
Older patients are often referred to a psychiatrist because of vague suicidal ideation, but they may also present in an acute crisis—with immediate plans for suicide and readily accessible means. The first concern for their safety is to ensure they are not left alone.
Patient interview. First, listen empathetically and ask detailed questions, especially ones that remind patients of their daily connections and responsibilities. For instance, ask, “Do you have children who would be affected by your decision?” Address patients’ immediate needs, such as hunger, thirst, or pain.16 Work on building a therapeutic alliance before asking questions that may appear trivial to agitated patients (such as tasks assessing cognitive abilities).
Avoid arguing with patients, and refrain from offering advice or sermonizing. Allow them to describe their emotions, and communicate that you understand their concerns. Discuss how they can expect to receive treatment to ease their discomfort. Inform them that mental health specialists can treat them and monitor their progress.
Hospitalization. Begin discussing treatment options and broach the notion of hospital admission if necessary. One way to foster an alliance is to frame inpatient care as a way of helping them recover from their crisis in a safe environment.
To ensure patient safety, it is best to err on the side of admission. Admitting the suicidal patient not only guarantees strict supervision but also allows time for necessary psychological assessment. Hospitalization may also allow family members to remove any weapons or hazardous conditions from the patient’s home.
Including the family in problem-solving is especially important when managing older suicidal patients. For patients who are isolated from family or friends, recovery may depend on improving their support network.
Table 3
Comparing screening instruments for suicide risk
| Measure | Description | Time (minutes) |
|---|---|---|
| Beck Depression Inventory (BDI) | 21-item, self-administered; identifies depressive symptoms in past week | 10 |
| Beck Hopelessness Scale (BHS) | 20-item, self-administered; measures hopelessness, fatalism, and pessimism in past week | 5 |
| Beck Scale for Suicide Ideation-Current (SSI-C) | 19-item, self-administered; gauges suicidal intention | 10 |
| Hamilton Rating Scale for Depression-revised (HRSD-R) | 21-item, clinician-administered; rates depressive symptoms in past week | 25 |
Outpatient care. Not all acutely suicidal older patients require hospital admission. They may be safely managed as outpatients if they:
- have strong social support
- are not isolated
- have no access to firearms or other dangerous weapons.
Safety can be enhanced by having family members take responsibility for the senior’s well-being and by asking the patient to contract for safety. A safety contract may include:
- verbal confirmation—and ideally a written statement—that the patient will not commit suicide within a specified period
- a list of people the patient will contact when feeling suicidal
- steps being taken to monitor the patient’s welfare.
Finally, schedule follow-up appointments soon after discharge to certify patients are being closely monitored. To encourage outpatient medication adherence, build strong alliances with family members and ask patients to bring in their pill bottles during follow-up appointments.
CASE REPORT continued: Observation begins
The staff is clearly concerned about Mr. V’s suicide risk and requests that he voluntarily admit himself to the VA hospital. This decision is based on his level of isolation, the lethality of his suicide plan, access to a weapon, and the depression and hopelessness revealed by his screening tests. He reluctantly agrees and is admitted to the inpatient psychiatric unit for observation and treatment by a geriatric internist and a geriatric psychiatrist.
DRUG THERAPY FOR SUICIDALITY
For patients with mild depressive symptoms, psychotherapy may be sufficient to manage depression associated with suicidality. However, those with moderate-to-severe depression require both drug treatment and psychotherapy.
Drug selection depends upon the underlying psychiatric illness. If the older patient is experiencing a depressive disorder, a selective serotonin reuptake inhibitor (SSRI) or another antidepressant could serve as first-line treatment (Table 4). These medications are safe for suicidal patients because they are not fatal in overdose.
Administration. Because age-related changes in pharmacokinetics and spharmacodynamics can slow medication clearance, reduced dosages usually achieve a therapeutic effect and minimize the risk of side effects in geriatric patients.
Antidepressants commonly used for older patients are shown in Table 4. Excepting citalopram and escitalopram, these dosages are lower than usual. We start healthy older patients on one-half the usual dosage and those who are medically ill or have neurodegenerative disorders on one-third to one-fourth the usual dosage. We also titrate more slowly to reduce the risk of side effects.
Table 4
Antidepressants commonly used to treat geriatric depression
| Medication | Recommended dosage (mg/d) |
|---|---|
| SSRIs | |
| Citalopram | 20 to 40 |
| Escitalopram | 10 to 20 |
| Fluoxetine | 10 to 40 |
| Paroxetine | 10 to 40 |
| Sertraline | 25 to 150 |
| Others | |
| Bupropion | 100 to 400 |
| Mirtazapine | 15 to 45 |
| Venlafaxine | 75 to 225 |
As in younger patients, the most common side effects of SSRIs in older patients include GI difficulties, overactivation, and sexual dysfunction. Paroxetine’s potential for anticholinergic effects may be a concern for some older patients.
Drug-drug interactions are of great concern when treating older patients, who take an average of six to nine medications per day.17 Compared with other SSRIs, fluoxetine and paroxetine, are more likely to inhibit cytochrome P-450 enzymes 2D6 and 3A4. They could thus increase blood levels of drugs taken concomitantly that are substrates of 2D6 or 3A4.
Antidepressant side effects can sometimes be used to advantage. For example, mirtazapine’s sedating property at lower dosages could help older patients with insomnia.
CASE REPORT concluded: Finding support
Mr. V is started on an SSRI antidepressant. He also receives supportive and milieu therapy and coping skills training. During his hospitalization, Mr. V contracts for safety and allows his sister to remove the handgun from his home.
Upon discharge, Mr. V is referred to a day treatment program that operates 3 to 5 days a week and offers case management, group therapy, and individual psychotherapy. The program helps him meet other older patients and allows him to discuss his life’s accomplishments and losses with others his age. His sister is an integral part of the program, and he maintains close contact with her.
Mr. V reports vague and occasional suicidal ideation, with no specific plan or intent. He and his sister note that his medical condition improved soon after his psychiatric condition stabilized.
Related resources
- Surgeon General’s Call to Action to Prevent Suicide. www.mental-health.org/suicideprevention/calltoaction.pdf
- Centers for Disease Control and Prevention. National Center for Injury Prevention and Control.
Drug brand names
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
Drs. Montross reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Mohamed is a speaker and consultant to Forest Laboratories and Eli Lilly and Co.
Dr. Kasckow receives research support from, is a consultant to, and is a speaker for Forest Laboratories, Eli Lilly and Co., Pfizer Inc., and Janssen Pharmaceutica.
Dr. Zisook is a consultant to GlaxoSmithKline and a speaker for GlaxoSmithKline, Wyeth Pharmaceuticals, Pfizer Inc., Forest Laboratories, and Bristol-Myers Squibb Co.
1. Centers for Disease Control and Prevention. Surveillance for Injuries and Violence Among Older Adults (CDC Surveillance Summary, December 17, 1999, Chap. 3:27-50). www.cdc.gov/mmwr/PDF/SS/SS4808.pdf
2. Kaplan HI, Sadock BJ. Synopsis of psychiatry (6th ed). Baltimore: Williams & Wilkins, 1991.
3. Lyon DE, Chase LS, Farrell SP. Using an interview guide to assess suicidal ideation. Nurse Practitioner 2002;27:26-31.
4. Conwell Y, Lyness JM, Duberstein P, et al. Completed suicide among older patients in primary care practices: A controlled study. J Am Geriatr Soc 2000;48:23-9.
5. Conwell Y, Duberstein PR, Cox C, et al. Relationships of age and Axis I diagnoses in victims of completed suicide: A psychological autopsy study. Am J Psychiatry 1996;153:1001-8.
6. Harwood D, Hawton K, Hope T, Jacoby R. Psychiatric disorder and personality factors associated with suicide in older people: A descriptive and case-control study. Int J Geriatr Psychiatry 2001;16:155-65.
7. Henriksson MM, Marttunen MJ, Isometsa ET, et al. Mental disorders in elderly suicide. Int Psychogeriatr 1995;7:275-86.
8. Conwell Y, Duberstein PR, Caine ED. Risk factors for suicide in later life. Biol Psychiatry 2002;52:193-204.
9. Beck AT, Brown GK, Steer RA. Psychometric characteristics of the Scale for Suicide Ideation with psychiatric outpatients. Behav Res Ther 1997;35:1039-46.
10. Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, TX: The Psychological Corporation, 1987.
11. Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years later. Clin Psychol Rev 1988;8:77-100.
12. Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: The hopelessness scale. J Consult Clin Psychol 1974;42:861-5.
13. Drake RE, Cotton PG. Depression, hopelessness and suicide in chronic schizophrenia. Br J Psychiatry 1986;148:554-9.
14. Beck AT, Steer RA, Kovacs M, Garrison B. Hopelessness and eventual suicide: A 10-year perspective study of patients hospitalized with suicidal ideation. Am J Psychiatry 1985;142:559-63.
15. Riskind JH, Beck AT, Brown G, Steer RA. Taking the measure of anxiety and depression: Validity of the reconstructed Hamilton Scales. J Nerv Ment Dis 1987;175:474-9.
16. Lamberg L. Psychiatric emergencies call for comprehensive assessment and treatment. JAMA 2002;288:686-7.
17. Sadavoy J, Lazarus LW, Jarvik LF. Grossberg GT (eds). Comprehensive review of geriatric psychiatry (2nd ed). Washington, DC: American Psychiatric Press, 1996.
CASE REPORT: I have a gun
Mr. V, age 77, appears depressed and anxious during his appointment at a mental hygiene clinic. He reports insomnia, concentration trouble, and anhedonia. He tells the psychiatrist he keeps a loaded gun at home and is not sure he can control his suicidal impulses.
The patient is Caucasian and has a history of heart failure, pulmonary disease, and type 2 diabetes. His wife died 18 months ago. He lives alone, but his sister lives nearby. He recently received a right hip replacement, which required 3 months of rehabilitation in a nursing home to recover from surgical complications. He still has trouble walking.
As in Mr. V’s case, treating older patients referred for psychiatric care often involves evaluating suicide risk. His age, race, gender, depressed mood, recent bereavement, and medical ailments place him in the population at highest risk of suicide (Box, Table 1).1-8
Approximately 20% of all suicides in the United States are committed by persons age 65 or older,1 who account for 13% of the total population. The suicide rate among persons older than 75 is three times higher than it is for the young.2 Older Caucasian men have the highest per-capita rate of completed suicide, compared with any other group of Americans.3
Psychiatric disorders. The rates of Axis I disorders among older persons who commit suicide fall within the average range for all age groups (70 to 90%). However, the types of disorders seen in the older population differ from those of younger suicides (Table 1).4-8
Affective illness has been termed “the predominant psychopathology associated with suicide in later life.”4 Among older persons who commit suicide, three-fourths (76%) have diagnosable mood disorders4 and nearly two-thirds (63%) have depression.6 Contributing risk factors include alcoholism and substance abuse,4,6,7 Axis II disorders, and dementia.6
Losses and medical illness. In later life, bereavement, loss of independence, or financial reversals may lead to depression. Older persons who take their own lives also tend to have greater physical health burdens and more functional disabilities than those who do not commit suicide.6,8
This article describes an age-based psychiatric workup of the suicidal older patient, including factors to consider when screening for depressive symptoms, prescribing drug therapy, and determining the need for hospitalization.
AGE-BASED CLINICAL WORKUP
For older patients who report suicidal ideation, an age-appropriate workup—using clinical interviews and screening instruments—is essential. The clinical interview can build rapport and gather information about the patient’s suicidal plan or intent. Based on our experience, we recommend the following 6-step screening interview, summarized in Table 2.
- Ask about a specific plan. Does the patient have the means readily available to carry out this plan? What is the timeline (imminent versus vaguely futuristic)? Does the patient report having control over this plan?
- Gather a suicide history. Has the patient attempted suicide before? By what means? Is there a family history of suicide? If yes, by what means did this family member commit suicide, and how was the patient affected?
- Assess social status. How isolated is the patient? Have there been recent changes in his or her social circle, such as loss of a spouse? Can the patient identify at least one person who would be negatively affected by the suicide?
- Assess medical health. Does the patient suffer from chronic pain? Does the patient have a recently diagnosed medical condition? Has a longstanding medical condition become more debilitating? Does the patient report feeling hopeless about impending medical difficulties? Has he or she been keeping regularly scheduled medical appointments with outpatient clinicians?
- Assess mental health. Does the patient meet DSM-IV criteria for depression or schizophrenia, which are associated with high suicide risk? Does he or she report being hopeless or helpless? Is the suicidal ideation ego dystonic?
- Ascertain clinical signs of suicidal intent. Has the patient:
Table 1
Suicide risk with mental and physical illness, by patient age
| Risk factors | Young (21 to 34 yrs) | Middle-aged (35 to 54 yrs) | Young-old (55 to 74 yrs) | Elderly (77+ yrs) |
|---|---|---|---|---|
| Psychiatric disorders | • | • | • | • |
| Mood disorders | ○ | • | • | |
| Alcohol abuse | ○ | • | ○ | |
| Primary psychoses | • | ○ | ||
| Personality disorders | ○ | |||
| Physical ailments | • | • | ||
| • Significant risk factor ○ Potential risk factor | ||||
| Source: Compiled from information in references 4-8. | ||||
CASE REPORT continued: Some telling signs
Mr. V’s laboratory screening reveals slightly elevated serum glucose and mild anemia. An ECG reveals a type I heart block, but all other lab results are unremarkable. His sister reports he recently gave away his dog, which he and his wife had owned for many years. He has also mentioned a desire to revise his will when speaking to other family members. Hospital records indicate he has missed numerous medical appointments over the past 4 months.
SCREENING INSTRUMENTS
Psychological assessments can often buttress the clinical interview findings. Several measurements are well-suited for detecting suicidal risk and concomitant depression (Table 3).
Beck SSI-C. The Beck Scale for Suicide Ideation – Current (SSI-C) assesses a patient’s preparation and motivation to commit suicide.9 This short (19-item) self-report measure asks patients to rate their wish to die, desire to attempt suicide, duration (and frequency) of suicidal thoughts, sense of control over suicide, and deterrents they face. The SSI-C helps to measure or monitor suicidality and is reliable and valid for psychiatric outpatients.9
BDI-II. The Beck Depression Inventory—recently revised in a second edition (BDI-II)10—can be useful because depression is one of the strongest risk factors for elder suicide. The 21-item BDI-II—a psychometrically sound, self-report instrument—asks about general symptoms of depression and gauges their severity. It can be applied to diverse patient populations and ages11 and is appropriate for older patients who are also being treated medically.
Beck Hopelessness Scale. Hopelessness has been recognized as a possible harbinger of suicide.12 One study showed that depression became a clinically meaningful suicide predictor only when accompanied by hopelessness.13
A score of 10 or more on the Beck Hopelessness Scale identified 91% of patients in one study who eventually committed suicide. The hopelessness patients expressed on this scale more strongly differentiated between those who did or did not commit suicide than did their scores on the BDI or SSI-C.14
Table 2
6-step clinical interview with an older suicidal patient
|
|
| Source: Adapted from the Cincinnati Veterans Affairs Medical Center general psychological suicide assessment |
HRSD-R. The revised Hamilton Rating Scale for Depression (HRSD-R) documents patients’ levels of mood disturbance and suicidality. One item in this 21-item, clinician-administered instrument specifically asks about the patient’s level of suicidality in the past week. The scale has well-documented reliability and validity and is appropriate for psychiatric populations.15
CASE REPORT continued: Alarming findings
Along with the clinical interview, Mr. V. is screened with the Beck Hopelessness Scale and Beck Depression Inventory-II. These instruments are chosen because they are easy to administer, and patients can readily comprehend the questions—even when under duress. Mr. V’s results reveal moderate depression and severe hopelessness.
INPATIENT VS. OUTPATIENT CARE
Older patients are often referred to a psychiatrist because of vague suicidal ideation, but they may also present in an acute crisis—with immediate plans for suicide and readily accessible means. The first concern for their safety is to ensure they are not left alone.
Patient interview. First, listen empathetically and ask detailed questions, especially ones that remind patients of their daily connections and responsibilities. For instance, ask, “Do you have children who would be affected by your decision?” Address patients’ immediate needs, such as hunger, thirst, or pain.16 Work on building a therapeutic alliance before asking questions that may appear trivial to agitated patients (such as tasks assessing cognitive abilities).
Avoid arguing with patients, and refrain from offering advice or sermonizing. Allow them to describe their emotions, and communicate that you understand their concerns. Discuss how they can expect to receive treatment to ease their discomfort. Inform them that mental health specialists can treat them and monitor their progress.
Hospitalization. Begin discussing treatment options and broach the notion of hospital admission if necessary. One way to foster an alliance is to frame inpatient care as a way of helping them recover from their crisis in a safe environment.
To ensure patient safety, it is best to err on the side of admission. Admitting the suicidal patient not only guarantees strict supervision but also allows time for necessary psychological assessment. Hospitalization may also allow family members to remove any weapons or hazardous conditions from the patient’s home.
Including the family in problem-solving is especially important when managing older suicidal patients. For patients who are isolated from family or friends, recovery may depend on improving their support network.
Table 3
Comparing screening instruments for suicide risk
| Measure | Description | Time (minutes) |
|---|---|---|
| Beck Depression Inventory (BDI) | 21-item, self-administered; identifies depressive symptoms in past week | 10 |
| Beck Hopelessness Scale (BHS) | 20-item, self-administered; measures hopelessness, fatalism, and pessimism in past week | 5 |
| Beck Scale for Suicide Ideation-Current (SSI-C) | 19-item, self-administered; gauges suicidal intention | 10 |
| Hamilton Rating Scale for Depression-revised (HRSD-R) | 21-item, clinician-administered; rates depressive symptoms in past week | 25 |
Outpatient care. Not all acutely suicidal older patients require hospital admission. They may be safely managed as outpatients if they:
- have strong social support
- are not isolated
- have no access to firearms or other dangerous weapons.
Safety can be enhanced by having family members take responsibility for the senior’s well-being and by asking the patient to contract for safety. A safety contract may include:
- verbal confirmation—and ideally a written statement—that the patient will not commit suicide within a specified period
- a list of people the patient will contact when feeling suicidal
- steps being taken to monitor the patient’s welfare.
Finally, schedule follow-up appointments soon after discharge to certify patients are being closely monitored. To encourage outpatient medication adherence, build strong alliances with family members and ask patients to bring in their pill bottles during follow-up appointments.
CASE REPORT continued: Observation begins
The staff is clearly concerned about Mr. V’s suicide risk and requests that he voluntarily admit himself to the VA hospital. This decision is based on his level of isolation, the lethality of his suicide plan, access to a weapon, and the depression and hopelessness revealed by his screening tests. He reluctantly agrees and is admitted to the inpatient psychiatric unit for observation and treatment by a geriatric internist and a geriatric psychiatrist.
DRUG THERAPY FOR SUICIDALITY
For patients with mild depressive symptoms, psychotherapy may be sufficient to manage depression associated with suicidality. However, those with moderate-to-severe depression require both drug treatment and psychotherapy.
Drug selection depends upon the underlying psychiatric illness. If the older patient is experiencing a depressive disorder, a selective serotonin reuptake inhibitor (SSRI) or another antidepressant could serve as first-line treatment (Table 4). These medications are safe for suicidal patients because they are not fatal in overdose.
Administration. Because age-related changes in pharmacokinetics and spharmacodynamics can slow medication clearance, reduced dosages usually achieve a therapeutic effect and minimize the risk of side effects in geriatric patients.
Antidepressants commonly used for older patients are shown in Table 4. Excepting citalopram and escitalopram, these dosages are lower than usual. We start healthy older patients on one-half the usual dosage and those who are medically ill or have neurodegenerative disorders on one-third to one-fourth the usual dosage. We also titrate more slowly to reduce the risk of side effects.
Table 4
Antidepressants commonly used to treat geriatric depression
| Medication | Recommended dosage (mg/d) |
|---|---|
| SSRIs | |
| Citalopram | 20 to 40 |
| Escitalopram | 10 to 20 |
| Fluoxetine | 10 to 40 |
| Paroxetine | 10 to 40 |
| Sertraline | 25 to 150 |
| Others | |
| Bupropion | 100 to 400 |
| Mirtazapine | 15 to 45 |
| Venlafaxine | 75 to 225 |
As in younger patients, the most common side effects of SSRIs in older patients include GI difficulties, overactivation, and sexual dysfunction. Paroxetine’s potential for anticholinergic effects may be a concern for some older patients.
Drug-drug interactions are of great concern when treating older patients, who take an average of six to nine medications per day.17 Compared with other SSRIs, fluoxetine and paroxetine, are more likely to inhibit cytochrome P-450 enzymes 2D6 and 3A4. They could thus increase blood levels of drugs taken concomitantly that are substrates of 2D6 or 3A4.
Antidepressant side effects can sometimes be used to advantage. For example, mirtazapine’s sedating property at lower dosages could help older patients with insomnia.
CASE REPORT concluded: Finding support
Mr. V is started on an SSRI antidepressant. He also receives supportive and milieu therapy and coping skills training. During his hospitalization, Mr. V contracts for safety and allows his sister to remove the handgun from his home.
Upon discharge, Mr. V is referred to a day treatment program that operates 3 to 5 days a week and offers case management, group therapy, and individual psychotherapy. The program helps him meet other older patients and allows him to discuss his life’s accomplishments and losses with others his age. His sister is an integral part of the program, and he maintains close contact with her.
Mr. V reports vague and occasional suicidal ideation, with no specific plan or intent. He and his sister note that his medical condition improved soon after his psychiatric condition stabilized.
Related resources
- Surgeon General’s Call to Action to Prevent Suicide. www.mental-health.org/suicideprevention/calltoaction.pdf
- Centers for Disease Control and Prevention. National Center for Injury Prevention and Control.
Drug brand names
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
Drs. Montross reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Mohamed is a speaker and consultant to Forest Laboratories and Eli Lilly and Co.
Dr. Kasckow receives research support from, is a consultant to, and is a speaker for Forest Laboratories, Eli Lilly and Co., Pfizer Inc., and Janssen Pharmaceutica.
Dr. Zisook is a consultant to GlaxoSmithKline and a speaker for GlaxoSmithKline, Wyeth Pharmaceuticals, Pfizer Inc., Forest Laboratories, and Bristol-Myers Squibb Co.
CASE REPORT: I have a gun
Mr. V, age 77, appears depressed and anxious during his appointment at a mental hygiene clinic. He reports insomnia, concentration trouble, and anhedonia. He tells the psychiatrist he keeps a loaded gun at home and is not sure he can control his suicidal impulses.
The patient is Caucasian and has a history of heart failure, pulmonary disease, and type 2 diabetes. His wife died 18 months ago. He lives alone, but his sister lives nearby. He recently received a right hip replacement, which required 3 months of rehabilitation in a nursing home to recover from surgical complications. He still has trouble walking.
As in Mr. V’s case, treating older patients referred for psychiatric care often involves evaluating suicide risk. His age, race, gender, depressed mood, recent bereavement, and medical ailments place him in the population at highest risk of suicide (Box, Table 1).1-8
Approximately 20% of all suicides in the United States are committed by persons age 65 or older,1 who account for 13% of the total population. The suicide rate among persons older than 75 is three times higher than it is for the young.2 Older Caucasian men have the highest per-capita rate of completed suicide, compared with any other group of Americans.3
Psychiatric disorders. The rates of Axis I disorders among older persons who commit suicide fall within the average range for all age groups (70 to 90%). However, the types of disorders seen in the older population differ from those of younger suicides (Table 1).4-8
Affective illness has been termed “the predominant psychopathology associated with suicide in later life.”4 Among older persons who commit suicide, three-fourths (76%) have diagnosable mood disorders4 and nearly two-thirds (63%) have depression.6 Contributing risk factors include alcoholism and substance abuse,4,6,7 Axis II disorders, and dementia.6
Losses and medical illness. In later life, bereavement, loss of independence, or financial reversals may lead to depression. Older persons who take their own lives also tend to have greater physical health burdens and more functional disabilities than those who do not commit suicide.6,8
This article describes an age-based psychiatric workup of the suicidal older patient, including factors to consider when screening for depressive symptoms, prescribing drug therapy, and determining the need for hospitalization.
AGE-BASED CLINICAL WORKUP
For older patients who report suicidal ideation, an age-appropriate workup—using clinical interviews and screening instruments—is essential. The clinical interview can build rapport and gather information about the patient’s suicidal plan or intent. Based on our experience, we recommend the following 6-step screening interview, summarized in Table 2.
- Ask about a specific plan. Does the patient have the means readily available to carry out this plan? What is the timeline (imminent versus vaguely futuristic)? Does the patient report having control over this plan?
- Gather a suicide history. Has the patient attempted suicide before? By what means? Is there a family history of suicide? If yes, by what means did this family member commit suicide, and how was the patient affected?
- Assess social status. How isolated is the patient? Have there been recent changes in his or her social circle, such as loss of a spouse? Can the patient identify at least one person who would be negatively affected by the suicide?
- Assess medical health. Does the patient suffer from chronic pain? Does the patient have a recently diagnosed medical condition? Has a longstanding medical condition become more debilitating? Does the patient report feeling hopeless about impending medical difficulties? Has he or she been keeping regularly scheduled medical appointments with outpatient clinicians?
- Assess mental health. Does the patient meet DSM-IV criteria for depression or schizophrenia, which are associated with high suicide risk? Does he or she report being hopeless or helpless? Is the suicidal ideation ego dystonic?
- Ascertain clinical signs of suicidal intent. Has the patient:
Table 1
Suicide risk with mental and physical illness, by patient age
| Risk factors | Young (21 to 34 yrs) | Middle-aged (35 to 54 yrs) | Young-old (55 to 74 yrs) | Elderly (77+ yrs) |
|---|---|---|---|---|
| Psychiatric disorders | • | • | • | • |
| Mood disorders | ○ | • | • | |
| Alcohol abuse | ○ | • | ○ | |
| Primary psychoses | • | ○ | ||
| Personality disorders | ○ | |||
| Physical ailments | • | • | ||
| • Significant risk factor ○ Potential risk factor | ||||
| Source: Compiled from information in references 4-8. | ||||
CASE REPORT continued: Some telling signs
Mr. V’s laboratory screening reveals slightly elevated serum glucose and mild anemia. An ECG reveals a type I heart block, but all other lab results are unremarkable. His sister reports he recently gave away his dog, which he and his wife had owned for many years. He has also mentioned a desire to revise his will when speaking to other family members. Hospital records indicate he has missed numerous medical appointments over the past 4 months.
SCREENING INSTRUMENTS
Psychological assessments can often buttress the clinical interview findings. Several measurements are well-suited for detecting suicidal risk and concomitant depression (Table 3).
Beck SSI-C. The Beck Scale for Suicide Ideation – Current (SSI-C) assesses a patient’s preparation and motivation to commit suicide.9 This short (19-item) self-report measure asks patients to rate their wish to die, desire to attempt suicide, duration (and frequency) of suicidal thoughts, sense of control over suicide, and deterrents they face. The SSI-C helps to measure or monitor suicidality and is reliable and valid for psychiatric outpatients.9
BDI-II. The Beck Depression Inventory—recently revised in a second edition (BDI-II)10—can be useful because depression is one of the strongest risk factors for elder suicide. The 21-item BDI-II—a psychometrically sound, self-report instrument—asks about general symptoms of depression and gauges their severity. It can be applied to diverse patient populations and ages11 and is appropriate for older patients who are also being treated medically.
Beck Hopelessness Scale. Hopelessness has been recognized as a possible harbinger of suicide.12 One study showed that depression became a clinically meaningful suicide predictor only when accompanied by hopelessness.13
A score of 10 or more on the Beck Hopelessness Scale identified 91% of patients in one study who eventually committed suicide. The hopelessness patients expressed on this scale more strongly differentiated between those who did or did not commit suicide than did their scores on the BDI or SSI-C.14
Table 2
6-step clinical interview with an older suicidal patient
|
|
| Source: Adapted from the Cincinnati Veterans Affairs Medical Center general psychological suicide assessment |
HRSD-R. The revised Hamilton Rating Scale for Depression (HRSD-R) documents patients’ levels of mood disturbance and suicidality. One item in this 21-item, clinician-administered instrument specifically asks about the patient’s level of suicidality in the past week. The scale has well-documented reliability and validity and is appropriate for psychiatric populations.15
CASE REPORT continued: Alarming findings
Along with the clinical interview, Mr. V. is screened with the Beck Hopelessness Scale and Beck Depression Inventory-II. These instruments are chosen because they are easy to administer, and patients can readily comprehend the questions—even when under duress. Mr. V’s results reveal moderate depression and severe hopelessness.
INPATIENT VS. OUTPATIENT CARE
Older patients are often referred to a psychiatrist because of vague suicidal ideation, but they may also present in an acute crisis—with immediate plans for suicide and readily accessible means. The first concern for their safety is to ensure they are not left alone.
Patient interview. First, listen empathetically and ask detailed questions, especially ones that remind patients of their daily connections and responsibilities. For instance, ask, “Do you have children who would be affected by your decision?” Address patients’ immediate needs, such as hunger, thirst, or pain.16 Work on building a therapeutic alliance before asking questions that may appear trivial to agitated patients (such as tasks assessing cognitive abilities).
Avoid arguing with patients, and refrain from offering advice or sermonizing. Allow them to describe their emotions, and communicate that you understand their concerns. Discuss how they can expect to receive treatment to ease their discomfort. Inform them that mental health specialists can treat them and monitor their progress.
Hospitalization. Begin discussing treatment options and broach the notion of hospital admission if necessary. One way to foster an alliance is to frame inpatient care as a way of helping them recover from their crisis in a safe environment.
To ensure patient safety, it is best to err on the side of admission. Admitting the suicidal patient not only guarantees strict supervision but also allows time for necessary psychological assessment. Hospitalization may also allow family members to remove any weapons or hazardous conditions from the patient’s home.
Including the family in problem-solving is especially important when managing older suicidal patients. For patients who are isolated from family or friends, recovery may depend on improving their support network.
Table 3
Comparing screening instruments for suicide risk
| Measure | Description | Time (minutes) |
|---|---|---|
| Beck Depression Inventory (BDI) | 21-item, self-administered; identifies depressive symptoms in past week | 10 |
| Beck Hopelessness Scale (BHS) | 20-item, self-administered; measures hopelessness, fatalism, and pessimism in past week | 5 |
| Beck Scale for Suicide Ideation-Current (SSI-C) | 19-item, self-administered; gauges suicidal intention | 10 |
| Hamilton Rating Scale for Depression-revised (HRSD-R) | 21-item, clinician-administered; rates depressive symptoms in past week | 25 |
Outpatient care. Not all acutely suicidal older patients require hospital admission. They may be safely managed as outpatients if they:
- have strong social support
- are not isolated
- have no access to firearms or other dangerous weapons.
Safety can be enhanced by having family members take responsibility for the senior’s well-being and by asking the patient to contract for safety. A safety contract may include:
- verbal confirmation—and ideally a written statement—that the patient will not commit suicide within a specified period
- a list of people the patient will contact when feeling suicidal
- steps being taken to monitor the patient’s welfare.
Finally, schedule follow-up appointments soon after discharge to certify patients are being closely monitored. To encourage outpatient medication adherence, build strong alliances with family members and ask patients to bring in their pill bottles during follow-up appointments.
CASE REPORT continued: Observation begins
The staff is clearly concerned about Mr. V’s suicide risk and requests that he voluntarily admit himself to the VA hospital. This decision is based on his level of isolation, the lethality of his suicide plan, access to a weapon, and the depression and hopelessness revealed by his screening tests. He reluctantly agrees and is admitted to the inpatient psychiatric unit for observation and treatment by a geriatric internist and a geriatric psychiatrist.
DRUG THERAPY FOR SUICIDALITY
For patients with mild depressive symptoms, psychotherapy may be sufficient to manage depression associated with suicidality. However, those with moderate-to-severe depression require both drug treatment and psychotherapy.
Drug selection depends upon the underlying psychiatric illness. If the older patient is experiencing a depressive disorder, a selective serotonin reuptake inhibitor (SSRI) or another antidepressant could serve as first-line treatment (Table 4). These medications are safe for suicidal patients because they are not fatal in overdose.
Administration. Because age-related changes in pharmacokinetics and spharmacodynamics can slow medication clearance, reduced dosages usually achieve a therapeutic effect and minimize the risk of side effects in geriatric patients.
Antidepressants commonly used for older patients are shown in Table 4. Excepting citalopram and escitalopram, these dosages are lower than usual. We start healthy older patients on one-half the usual dosage and those who are medically ill or have neurodegenerative disorders on one-third to one-fourth the usual dosage. We also titrate more slowly to reduce the risk of side effects.
Table 4
Antidepressants commonly used to treat geriatric depression
| Medication | Recommended dosage (mg/d) |
|---|---|
| SSRIs | |
| Citalopram | 20 to 40 |
| Escitalopram | 10 to 20 |
| Fluoxetine | 10 to 40 |
| Paroxetine | 10 to 40 |
| Sertraline | 25 to 150 |
| Others | |
| Bupropion | 100 to 400 |
| Mirtazapine | 15 to 45 |
| Venlafaxine | 75 to 225 |
As in younger patients, the most common side effects of SSRIs in older patients include GI difficulties, overactivation, and sexual dysfunction. Paroxetine’s potential for anticholinergic effects may be a concern for some older patients.
Drug-drug interactions are of great concern when treating older patients, who take an average of six to nine medications per day.17 Compared with other SSRIs, fluoxetine and paroxetine, are more likely to inhibit cytochrome P-450 enzymes 2D6 and 3A4. They could thus increase blood levels of drugs taken concomitantly that are substrates of 2D6 or 3A4.
Antidepressant side effects can sometimes be used to advantage. For example, mirtazapine’s sedating property at lower dosages could help older patients with insomnia.
CASE REPORT concluded: Finding support
Mr. V is started on an SSRI antidepressant. He also receives supportive and milieu therapy and coping skills training. During his hospitalization, Mr. V contracts for safety and allows his sister to remove the handgun from his home.
Upon discharge, Mr. V is referred to a day treatment program that operates 3 to 5 days a week and offers case management, group therapy, and individual psychotherapy. The program helps him meet other older patients and allows him to discuss his life’s accomplishments and losses with others his age. His sister is an integral part of the program, and he maintains close contact with her.
Mr. V reports vague and occasional suicidal ideation, with no specific plan or intent. He and his sister note that his medical condition improved soon after his psychiatric condition stabilized.
Related resources
- Surgeon General’s Call to Action to Prevent Suicide. www.mental-health.org/suicideprevention/calltoaction.pdf
- Centers for Disease Control and Prevention. National Center for Injury Prevention and Control.
Drug brand names
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosure
Drs. Montross reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Mohamed is a speaker and consultant to Forest Laboratories and Eli Lilly and Co.
Dr. Kasckow receives research support from, is a consultant to, and is a speaker for Forest Laboratories, Eli Lilly and Co., Pfizer Inc., and Janssen Pharmaceutica.
Dr. Zisook is a consultant to GlaxoSmithKline and a speaker for GlaxoSmithKline, Wyeth Pharmaceuticals, Pfizer Inc., Forest Laboratories, and Bristol-Myers Squibb Co.
1. Centers for Disease Control and Prevention. Surveillance for Injuries and Violence Among Older Adults (CDC Surveillance Summary, December 17, 1999, Chap. 3:27-50). www.cdc.gov/mmwr/PDF/SS/SS4808.pdf
2. Kaplan HI, Sadock BJ. Synopsis of psychiatry (6th ed). Baltimore: Williams & Wilkins, 1991.
3. Lyon DE, Chase LS, Farrell SP. Using an interview guide to assess suicidal ideation. Nurse Practitioner 2002;27:26-31.
4. Conwell Y, Lyness JM, Duberstein P, et al. Completed suicide among older patients in primary care practices: A controlled study. J Am Geriatr Soc 2000;48:23-9.
5. Conwell Y, Duberstein PR, Cox C, et al. Relationships of age and Axis I diagnoses in victims of completed suicide: A psychological autopsy study. Am J Psychiatry 1996;153:1001-8.
6. Harwood D, Hawton K, Hope T, Jacoby R. Psychiatric disorder and personality factors associated with suicide in older people: A descriptive and case-control study. Int J Geriatr Psychiatry 2001;16:155-65.
7. Henriksson MM, Marttunen MJ, Isometsa ET, et al. Mental disorders in elderly suicide. Int Psychogeriatr 1995;7:275-86.
8. Conwell Y, Duberstein PR, Caine ED. Risk factors for suicide in later life. Biol Psychiatry 2002;52:193-204.
9. Beck AT, Brown GK, Steer RA. Psychometric characteristics of the Scale for Suicide Ideation with psychiatric outpatients. Behav Res Ther 1997;35:1039-46.
10. Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, TX: The Psychological Corporation, 1987.
11. Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years later. Clin Psychol Rev 1988;8:77-100.
12. Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: The hopelessness scale. J Consult Clin Psychol 1974;42:861-5.
13. Drake RE, Cotton PG. Depression, hopelessness and suicide in chronic schizophrenia. Br J Psychiatry 1986;148:554-9.
14. Beck AT, Steer RA, Kovacs M, Garrison B. Hopelessness and eventual suicide: A 10-year perspective study of patients hospitalized with suicidal ideation. Am J Psychiatry 1985;142:559-63.
15. Riskind JH, Beck AT, Brown G, Steer RA. Taking the measure of anxiety and depression: Validity of the reconstructed Hamilton Scales. J Nerv Ment Dis 1987;175:474-9.
16. Lamberg L. Psychiatric emergencies call for comprehensive assessment and treatment. JAMA 2002;288:686-7.
17. Sadavoy J, Lazarus LW, Jarvik LF. Grossberg GT (eds). Comprehensive review of geriatric psychiatry (2nd ed). Washington, DC: American Psychiatric Press, 1996.
1. Centers for Disease Control and Prevention. Surveillance for Injuries and Violence Among Older Adults (CDC Surveillance Summary, December 17, 1999, Chap. 3:27-50). www.cdc.gov/mmwr/PDF/SS/SS4808.pdf
2. Kaplan HI, Sadock BJ. Synopsis of psychiatry (6th ed). Baltimore: Williams & Wilkins, 1991.
3. Lyon DE, Chase LS, Farrell SP. Using an interview guide to assess suicidal ideation. Nurse Practitioner 2002;27:26-31.
4. Conwell Y, Lyness JM, Duberstein P, et al. Completed suicide among older patients in primary care practices: A controlled study. J Am Geriatr Soc 2000;48:23-9.
5. Conwell Y, Duberstein PR, Cox C, et al. Relationships of age and Axis I diagnoses in victims of completed suicide: A psychological autopsy study. Am J Psychiatry 1996;153:1001-8.
6. Harwood D, Hawton K, Hope T, Jacoby R. Psychiatric disorder and personality factors associated with suicide in older people: A descriptive and case-control study. Int J Geriatr Psychiatry 2001;16:155-65.
7. Henriksson MM, Marttunen MJ, Isometsa ET, et al. Mental disorders in elderly suicide. Int Psychogeriatr 1995;7:275-86.
8. Conwell Y, Duberstein PR, Caine ED. Risk factors for suicide in later life. Biol Psychiatry 2002;52:193-204.
9. Beck AT, Brown GK, Steer RA. Psychometric characteristics of the Scale for Suicide Ideation with psychiatric outpatients. Behav Res Ther 1997;35:1039-46.
10. Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, TX: The Psychological Corporation, 1987.
11. Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years later. Clin Psychol Rev 1988;8:77-100.
12. Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: The hopelessness scale. J Consult Clin Psychol 1974;42:861-5.
13. Drake RE, Cotton PG. Depression, hopelessness and suicide in chronic schizophrenia. Br J Psychiatry 1986;148:554-9.
14. Beck AT, Steer RA, Kovacs M, Garrison B. Hopelessness and eventual suicide: A 10-year perspective study of patients hospitalized with suicidal ideation. Am J Psychiatry 1985;142:559-63.
15. Riskind JH, Beck AT, Brown G, Steer RA. Taking the measure of anxiety and depression: Validity of the reconstructed Hamilton Scales. J Nerv Ment Dis 1987;175:474-9.
16. Lamberg L. Psychiatric emergencies call for comprehensive assessment and treatment. JAMA 2002;288:686-7.
17. Sadavoy J, Lazarus LW, Jarvik LF. Grossberg GT (eds). Comprehensive review of geriatric psychiatry (2nd ed). Washington, DC: American Psychiatric Press, 1996.