User login
Erythema and Induration on the Right Ear and Maxilla
Lepromatous Leprosy
Lepromatous leprosy (LL) is a chronic, cutaneous, granulomatous infection caused by Mycobacterium leprae or the newly discovered Mycobacterium lepromatosis, both acid-fast, intracellular, bacillus bacterium.1 Although decreasing in prevalence due to effective treatment with antimicrobials, LL continues to be endemic in warm tropical or subtropical areas in Southeast Asia, sub-Saharan Africa, the Indian subcontinent, and South America.1 The mode of transmission of infection is not well established.
The cutaneous manifestation of leprosy was previously classified based on the cell-mediated immune response of the patient, as described by Ridley and Jopling,2 ranging from tuberculoid leprosy (TT) to LL. In this spectrum of leprosy are the borderline lesions including borderline tuberculoid, borderline, and borderline lepromatous.2,3 Although this classification is popular, in 2012 the World Health Organization implemented a new 2-category classification system to standardize treatment regimens: paucibacillary (2–5 lesions or 1 nerve involvement) and multibacillary (>5 lesions or multiple nerve involvement).4
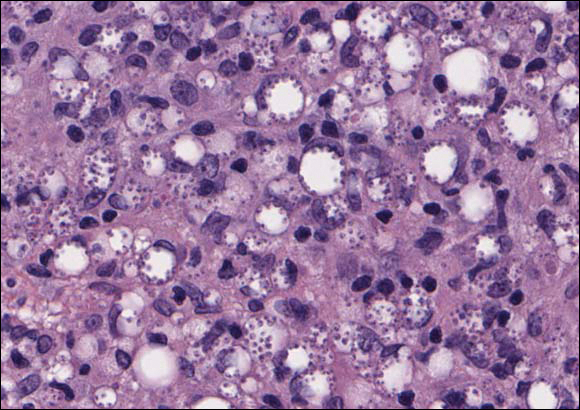
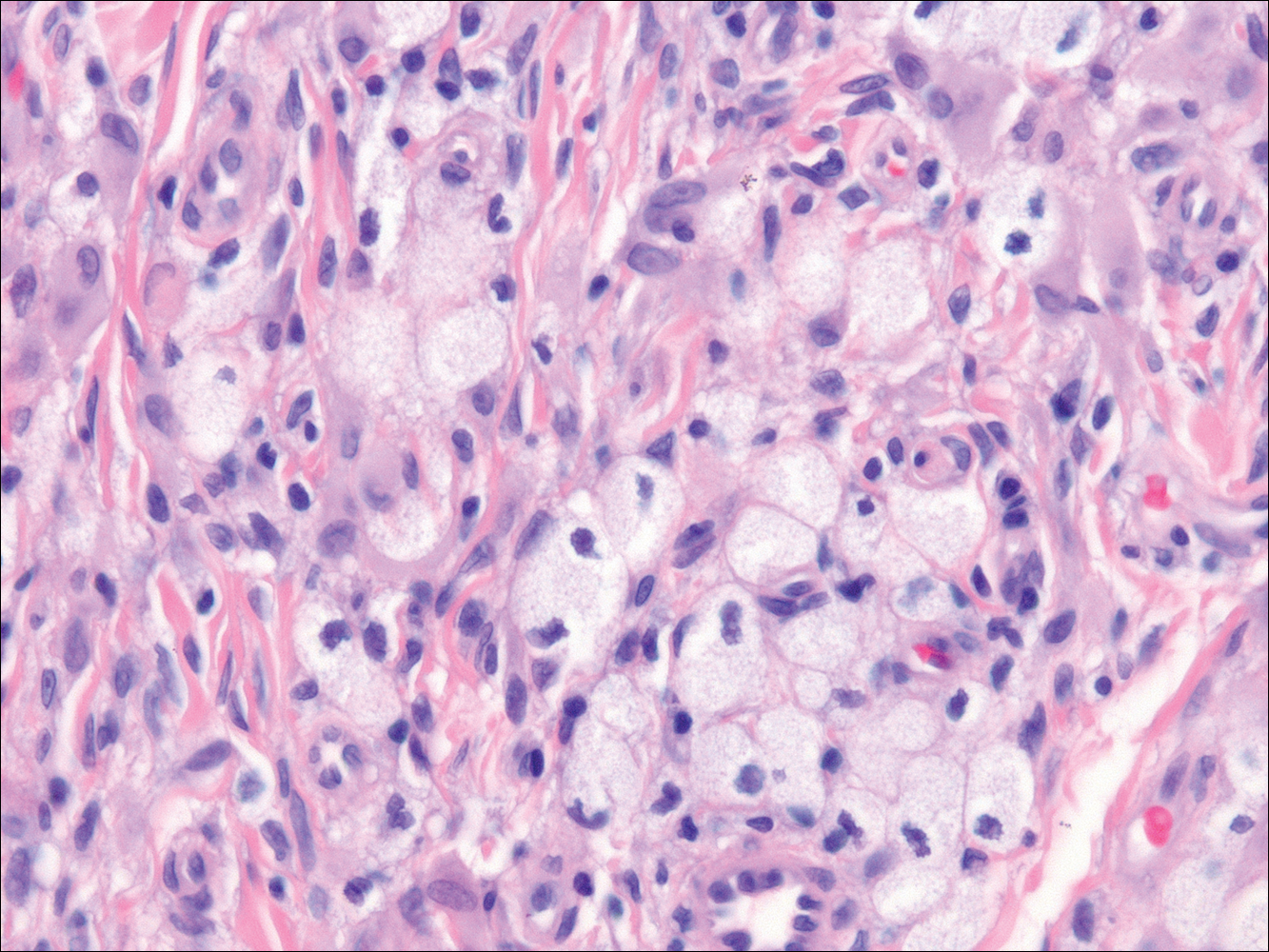
In LL, a cell-mediated immune response is not mounted against the infection in the patient. Clinically, the disease can manifest as macular and nodular erythematous cutaneous lesions with poorly defined borders that are preferentially located on the face, earlobes, and nasal mucosa. Chronic infections are associated with sensory loss. Histologically, the dermis is densely infiltrated by foamy macrophages (Virchow cells or lepra cells), which do not form granulomas (quiz image A). The infiltrate may have varying accompanying lymphocytes and plasma cells, which can extend deep into the subcutaneous adipose tissue. Between the dermal infiltrate and epidermis is an uninvolved band of superficial dermis called the Grenz zone. The epidermis is flattened and atrophic. Nerves often are surrounded by macrophages with degrees of hyalinization but rarely are swollen. On acid-fast staining (Wade-Fite or Ziehl-Neelsen), numerous acid-fast bacilli are present within dermal cells in densely packed, intracellular collections called globi (quiz image B).2,3,5
In TT, the robust immune response causes epithelioid granuloma formation, similar to cutaneous sarcoidosis, and few, if any, organisms can be found on special stains. The remaining borderline lesions have varying numbers of bacilli and varying amounts of granuloma formation.3,6,7 Many cases of TT resolve without specific treatment. For most leprous diseases, the World Health Organization currently recommends a regimen of dapsone, rifampin, and clofazimine combination treatments for 6 to 12 months depending on the type of leprosy.8
Cutaneous leishmaniasis should be included in the differential diagnosis for patients from LL endemic areas. Early lesions can have a histiocytic infiltration with associated mixed inflammation and prominent epidermal hyperplasia. These early lesions usually have parasitic organisms located within the periphery of the cytoplasm of macrophages (“marquee sign”) to help differentiate it from leprous diseases (Figure 1).9
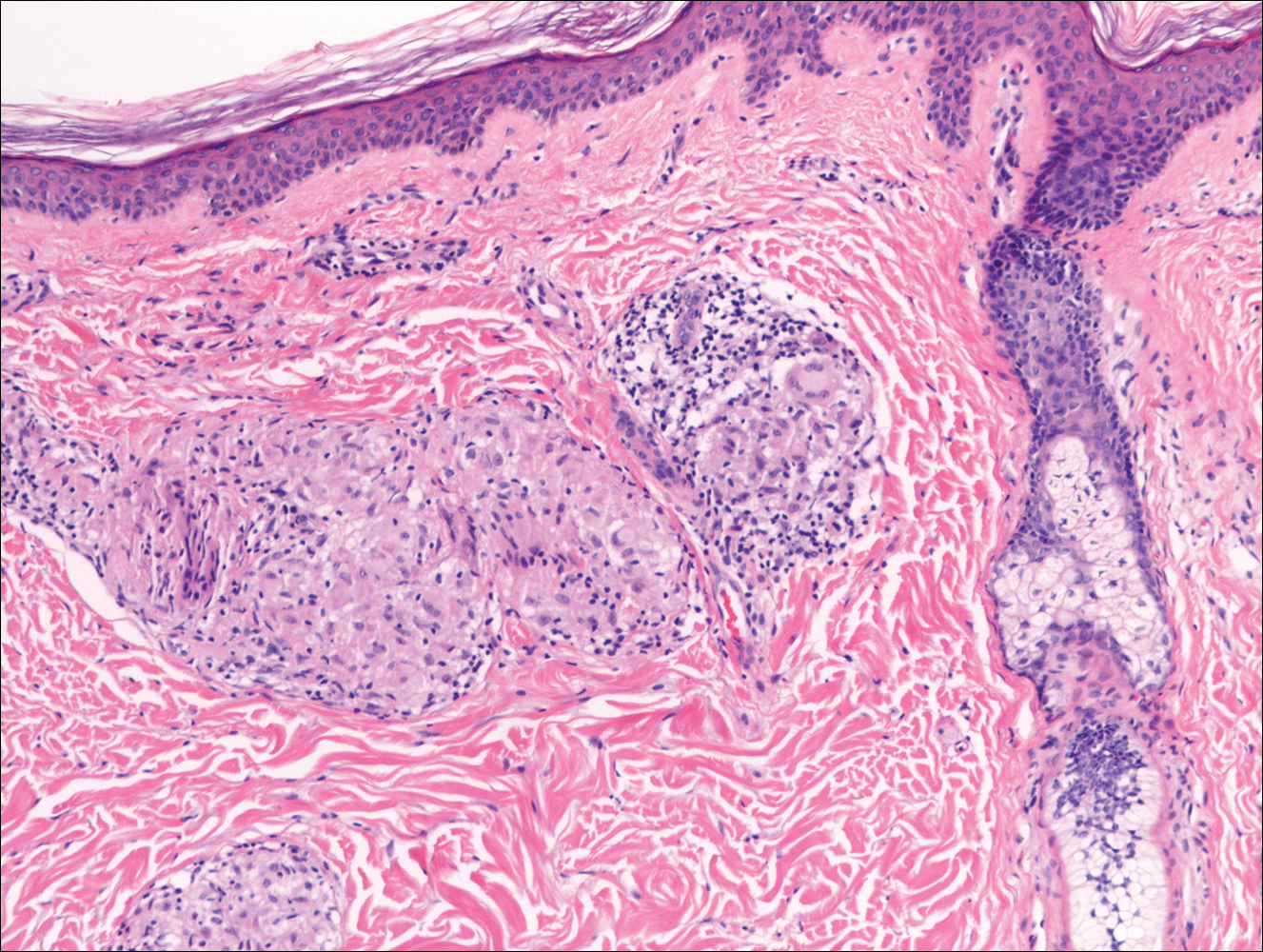
In nonendemic areas, leprous diseases often are mistaken for sarcoidosis, xanthomas, granular cell tumors, paraffinomas, or other histiocytic-rich lesions.10 Cutaneous sarcoidosis may be difficult to distinguish from TT, as both have noncaseating granulomas (Figure 2). Rare acid-fast bacilli may aid in the diagnosis, and sarcoid granulomas are not typically associated with cutaneous nerve involvement. New diagnostic tools such as polymerase chain reaction or genome sequencing can pick up rare organisms.
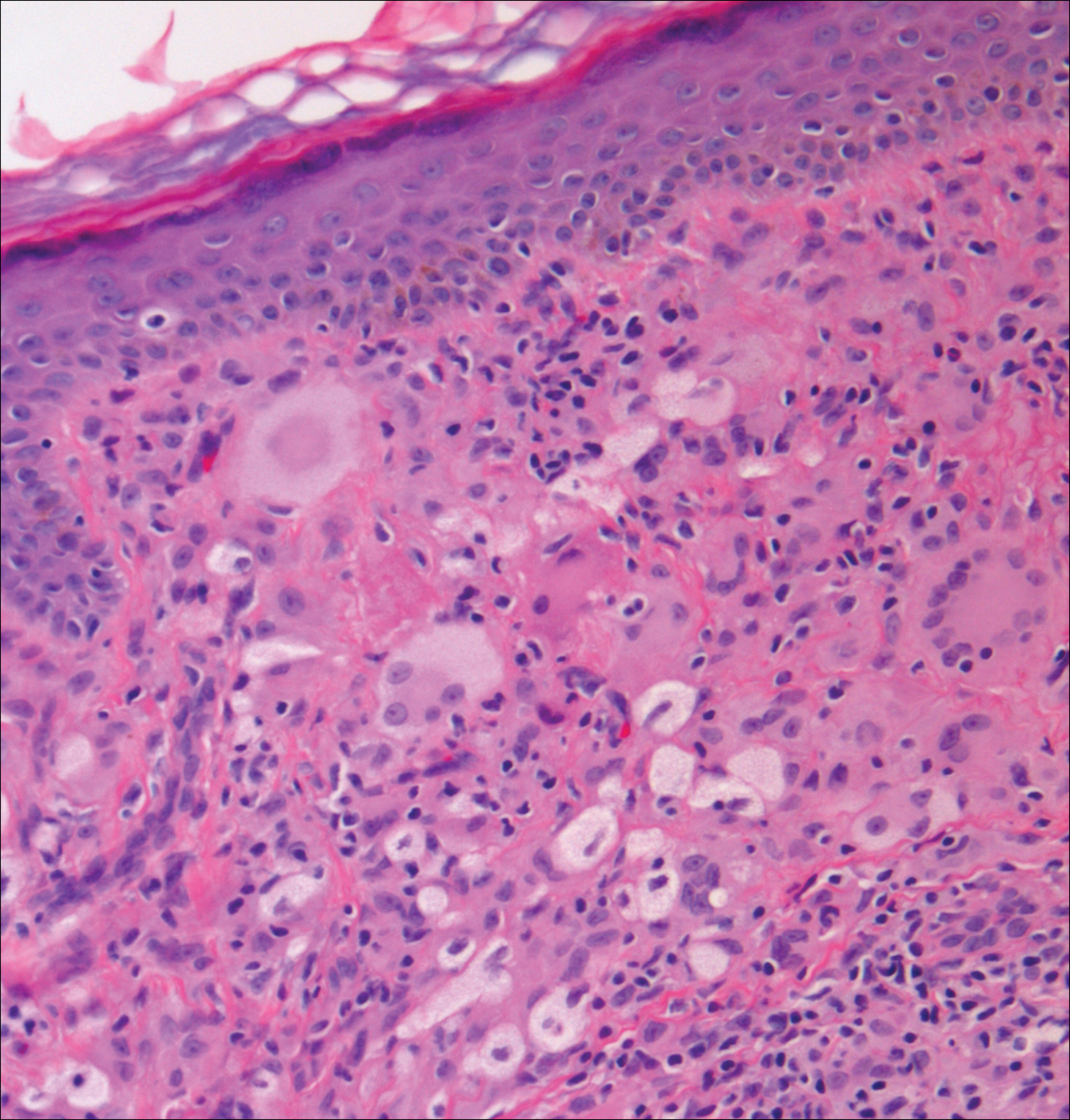
Xanthogranuolomas and xanthomas may histologically resemble LL with a dense dermal infiltrate of foamy histiocytes. No organisms are found in the infiltrate. Histologically, xanthogranulomas (juvenile or adult) will be a mixed infiltrate with foamy histiocytes; giant cell formation, especially Touton giant cells; lymphocytes; and granulocytes (Figure 3). Touton giant cells have a wreathlike formation of nuclei and an outer vacuolated cytoplasm. Xanthomas have sheets of large histiocytes with a foamy, lipid-filled interior and mild lymphocytic infiltrate (Figure 4).
- Han XY, Seo YH, Sizer KC, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130:856-864.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. a five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255-273.
- Ridley DS. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51:451-465.
- World Health Organization. WHO Expert Committee on Leprosy. World Health Organ Tech Rep Ser. 2012;968:1-61.
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- Crowson AN, Magro C, Mihm M Jr. Treponemal diseases. In: Elder DE, Elenitsas R, Johnson Jr BL, et al. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:540-579.
- Dacso MM, Jacobson RR, Scollard DM, et al. Evaluation of multi-drug therapy for leprosy in the United States using daily rifampin. South Med J. 2011;104:689-694.
- Handler MZ, Patel PA, Kapila R, et al. Cutaneous and mucocutaneous leishmaniasis: differential diagnosis, diagnosis, histopathology, and management. J Am Acad Dermatol. 2015;73:911-926.
- Massone C, Nunzi E, Cerroni L. Histopathologic diagnosis of leprosy in a nonendemic area. Am J Dermatopathol. 2010;32:417-419.
Lepromatous Leprosy
Lepromatous leprosy (LL) is a chronic, cutaneous, granulomatous infection caused by Mycobacterium leprae or the newly discovered Mycobacterium lepromatosis, both acid-fast, intracellular, bacillus bacterium.1 Although decreasing in prevalence due to effective treatment with antimicrobials, LL continues to be endemic in warm tropical or subtropical areas in Southeast Asia, sub-Saharan Africa, the Indian subcontinent, and South America.1 The mode of transmission of infection is not well established.
The cutaneous manifestation of leprosy was previously classified based on the cell-mediated immune response of the patient, as described by Ridley and Jopling,2 ranging from tuberculoid leprosy (TT) to LL. In this spectrum of leprosy are the borderline lesions including borderline tuberculoid, borderline, and borderline lepromatous.2,3 Although this classification is popular, in 2012 the World Health Organization implemented a new 2-category classification system to standardize treatment regimens: paucibacillary (2–5 lesions or 1 nerve involvement) and multibacillary (>5 lesions or multiple nerve involvement).4
In LL, a cell-mediated immune response is not mounted against the infection in the patient. Clinically, the disease can manifest as macular and nodular erythematous cutaneous lesions with poorly defined borders that are preferentially located on the face, earlobes, and nasal mucosa. Chronic infections are associated with sensory loss. Histologically, the dermis is densely infiltrated by foamy macrophages (Virchow cells or lepra cells), which do not form granulomas (quiz image A). The infiltrate may have varying accompanying lymphocytes and plasma cells, which can extend deep into the subcutaneous adipose tissue. Between the dermal infiltrate and epidermis is an uninvolved band of superficial dermis called the Grenz zone. The epidermis is flattened and atrophic. Nerves often are surrounded by macrophages with degrees of hyalinization but rarely are swollen. On acid-fast staining (Wade-Fite or Ziehl-Neelsen), numerous acid-fast bacilli are present within dermal cells in densely packed, intracellular collections called globi (quiz image B).2,3,5
In TT, the robust immune response causes epithelioid granuloma formation, similar to cutaneous sarcoidosis, and few, if any, organisms can be found on special stains. The remaining borderline lesions have varying numbers of bacilli and varying amounts of granuloma formation.3,6,7 Many cases of TT resolve without specific treatment. For most leprous diseases, the World Health Organization currently recommends a regimen of dapsone, rifampin, and clofazimine combination treatments for 6 to 12 months depending on the type of leprosy.8
Cutaneous leishmaniasis should be included in the differential diagnosis for patients from LL endemic areas. Early lesions can have a histiocytic infiltration with associated mixed inflammation and prominent epidermal hyperplasia. These early lesions usually have parasitic organisms located within the periphery of the cytoplasm of macrophages (“marquee sign”) to help differentiate it from leprous diseases (Figure 1).9

In nonendemic areas, leprous diseases often are mistaken for sarcoidosis, xanthomas, granular cell tumors, paraffinomas, or other histiocytic-rich lesions.10 Cutaneous sarcoidosis may be difficult to distinguish from TT, as both have noncaseating granulomas (Figure 2). Rare acid-fast bacilli may aid in the diagnosis, and sarcoid granulomas are not typically associated with cutaneous nerve involvement. New diagnostic tools such as polymerase chain reaction or genome sequencing can pick up rare organisms.

Xanthogranuolomas and xanthomas may histologically resemble LL with a dense dermal infiltrate of foamy histiocytes. No organisms are found in the infiltrate. Histologically, xanthogranulomas (juvenile or adult) will be a mixed infiltrate with foamy histiocytes; giant cell formation, especially Touton giant cells; lymphocytes; and granulocytes (Figure 3). Touton giant cells have a wreathlike formation of nuclei and an outer vacuolated cytoplasm. Xanthomas have sheets of large histiocytes with a foamy, lipid-filled interior and mild lymphocytic infiltrate (Figure 4).


Lepromatous Leprosy
Lepromatous leprosy (LL) is a chronic, cutaneous, granulomatous infection caused by Mycobacterium leprae or the newly discovered Mycobacterium lepromatosis, both acid-fast, intracellular, bacillus bacterium.1 Although decreasing in prevalence due to effective treatment with antimicrobials, LL continues to be endemic in warm tropical or subtropical areas in Southeast Asia, sub-Saharan Africa, the Indian subcontinent, and South America.1 The mode of transmission of infection is not well established.
The cutaneous manifestation of leprosy was previously classified based on the cell-mediated immune response of the patient, as described by Ridley and Jopling,2 ranging from tuberculoid leprosy (TT) to LL. In this spectrum of leprosy are the borderline lesions including borderline tuberculoid, borderline, and borderline lepromatous.2,3 Although this classification is popular, in 2012 the World Health Organization implemented a new 2-category classification system to standardize treatment regimens: paucibacillary (2–5 lesions or 1 nerve involvement) and multibacillary (>5 lesions or multiple nerve involvement).4
In LL, a cell-mediated immune response is not mounted against the infection in the patient. Clinically, the disease can manifest as macular and nodular erythematous cutaneous lesions with poorly defined borders that are preferentially located on the face, earlobes, and nasal mucosa. Chronic infections are associated with sensory loss. Histologically, the dermis is densely infiltrated by foamy macrophages (Virchow cells or lepra cells), which do not form granulomas (quiz image A). The infiltrate may have varying accompanying lymphocytes and plasma cells, which can extend deep into the subcutaneous adipose tissue. Between the dermal infiltrate and epidermis is an uninvolved band of superficial dermis called the Grenz zone. The epidermis is flattened and atrophic. Nerves often are surrounded by macrophages with degrees of hyalinization but rarely are swollen. On acid-fast staining (Wade-Fite or Ziehl-Neelsen), numerous acid-fast bacilli are present within dermal cells in densely packed, intracellular collections called globi (quiz image B).2,3,5
In TT, the robust immune response causes epithelioid granuloma formation, similar to cutaneous sarcoidosis, and few, if any, organisms can be found on special stains. The remaining borderline lesions have varying numbers of bacilli and varying amounts of granuloma formation.3,6,7 Many cases of TT resolve without specific treatment. For most leprous diseases, the World Health Organization currently recommends a regimen of dapsone, rifampin, and clofazimine combination treatments for 6 to 12 months depending on the type of leprosy.8
Cutaneous leishmaniasis should be included in the differential diagnosis for patients from LL endemic areas. Early lesions can have a histiocytic infiltration with associated mixed inflammation and prominent epidermal hyperplasia. These early lesions usually have parasitic organisms located within the periphery of the cytoplasm of macrophages (“marquee sign”) to help differentiate it from leprous diseases (Figure 1).9

In nonendemic areas, leprous diseases often are mistaken for sarcoidosis, xanthomas, granular cell tumors, paraffinomas, or other histiocytic-rich lesions.10 Cutaneous sarcoidosis may be difficult to distinguish from TT, as both have noncaseating granulomas (Figure 2). Rare acid-fast bacilli may aid in the diagnosis, and sarcoid granulomas are not typically associated with cutaneous nerve involvement. New diagnostic tools such as polymerase chain reaction or genome sequencing can pick up rare organisms.

Xanthogranuolomas and xanthomas may histologically resemble LL with a dense dermal infiltrate of foamy histiocytes. No organisms are found in the infiltrate. Histologically, xanthogranulomas (juvenile or adult) will be a mixed infiltrate with foamy histiocytes; giant cell formation, especially Touton giant cells; lymphocytes; and granulocytes (Figure 3). Touton giant cells have a wreathlike formation of nuclei and an outer vacuolated cytoplasm. Xanthomas have sheets of large histiocytes with a foamy, lipid-filled interior and mild lymphocytic infiltrate (Figure 4).


- Han XY, Seo YH, Sizer KC, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130:856-864.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. a five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255-273.
- Ridley DS. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51:451-465.
- World Health Organization. WHO Expert Committee on Leprosy. World Health Organ Tech Rep Ser. 2012;968:1-61.
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- Crowson AN, Magro C, Mihm M Jr. Treponemal diseases. In: Elder DE, Elenitsas R, Johnson Jr BL, et al. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:540-579.
- Dacso MM, Jacobson RR, Scollard DM, et al. Evaluation of multi-drug therapy for leprosy in the United States using daily rifampin. South Med J. 2011;104:689-694.
- Handler MZ, Patel PA, Kapila R, et al. Cutaneous and mucocutaneous leishmaniasis: differential diagnosis, diagnosis, histopathology, and management. J Am Acad Dermatol. 2015;73:911-926.
- Massone C, Nunzi E, Cerroni L. Histopathologic diagnosis of leprosy in a nonendemic area. Am J Dermatopathol. 2010;32:417-419.
- Han XY, Seo YH, Sizer KC, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130:856-864.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. a five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255-273.
- Ridley DS. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51:451-465.
- World Health Organization. WHO Expert Committee on Leprosy. World Health Organ Tech Rep Ser. 2012;968:1-61.
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- Crowson AN, Magro C, Mihm M Jr. Treponemal diseases. In: Elder DE, Elenitsas R, Johnson Jr BL, et al. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:540-579.
- Dacso MM, Jacobson RR, Scollard DM, et al. Evaluation of multi-drug therapy for leprosy in the United States using daily rifampin. South Med J. 2011;104:689-694.
- Handler MZ, Patel PA, Kapila R, et al. Cutaneous and mucocutaneous leishmaniasis: differential diagnosis, diagnosis, histopathology, and management. J Am Acad Dermatol. 2015;73:911-926.
- Massone C, Nunzi E, Cerroni L. Histopathologic diagnosis of leprosy in a nonendemic area. Am J Dermatopathol. 2010;32:417-419.
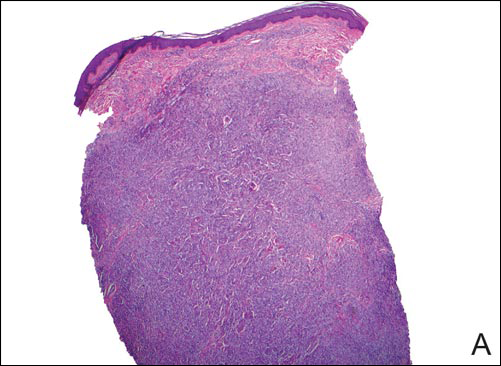
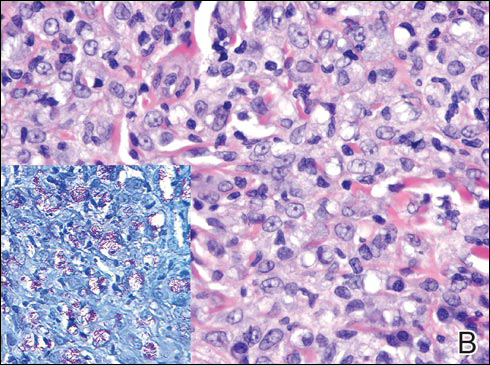
A 43-year-old man from Ghana presented with erythema and induration on the skin of the right maxillary region and right ear of several weeks’ duration.
The best diagnosis is:
a. cutaneous leishmaniasis
b. lepromatous leprosy
c. sarcoidosis
d. xanthogranuloma
e. xanthoma