User login
In reply: Stiff, numb hands
In Reply: Generally, it is preferable to measure the ionized calcium directly, particularly if there is uncertainty about whether the corrected serum calcium is reflective of the ionized calcium, if the patient’s symptoms are atypical, or if a reliable laboratory is available to measure ionized calcium.
Direct measurement of the ionized calcium concentration could be favored compared with measuring the corrected calcium in patients with symptoms of hypocalcemia in the setting of a normal total calcium concentration. Symptomatic hypocalcemia with normal total calcium but low ionized calcium can occasionally occur in patients with acute respiratory alkalosis due to increased binding of calcium to albumin. Thus, respiratory alkalosis may cause an acute decrease in ionized calcium. Furthermore, the ionized fraction can change without an alteration in the total serum calcium concentration, as with hyperparathyroidism, which increases the ionized calcium at the expense of that bound to albumin, and hyperphosphatemia, which increases the fraction bound to inorganic anions, decreasing ionized calcium. In patients who have chronic kidney disease and a low serum bicarbonate or a low serum albumin, or both, measuring the ionized calcium is preferable to measuring the total calcium in order to diagnose hypocalcemia or hypercalcemia.
The patient was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption, and she was followed by her primary care doctor. All the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since. We agree regarding the importance of checking on the ionized calcium to confirm the hypocalcemia and normalization after treatment as stated above. Ionized calcium was never checked during the hospital stay or during the follow-up after the discharge.
In Reply: Generally, it is preferable to measure the ionized calcium directly, particularly if there is uncertainty about whether the corrected serum calcium is reflective of the ionized calcium, if the patient’s symptoms are atypical, or if a reliable laboratory is available to measure ionized calcium.
Direct measurement of the ionized calcium concentration could be favored compared with measuring the corrected calcium in patients with symptoms of hypocalcemia in the setting of a normal total calcium concentration. Symptomatic hypocalcemia with normal total calcium but low ionized calcium can occasionally occur in patients with acute respiratory alkalosis due to increased binding of calcium to albumin. Thus, respiratory alkalosis may cause an acute decrease in ionized calcium. Furthermore, the ionized fraction can change without an alteration in the total serum calcium concentration, as with hyperparathyroidism, which increases the ionized calcium at the expense of that bound to albumin, and hyperphosphatemia, which increases the fraction bound to inorganic anions, decreasing ionized calcium. In patients who have chronic kidney disease and a low serum bicarbonate or a low serum albumin, or both, measuring the ionized calcium is preferable to measuring the total calcium in order to diagnose hypocalcemia or hypercalcemia.
The patient was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption, and she was followed by her primary care doctor. All the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since. We agree regarding the importance of checking on the ionized calcium to confirm the hypocalcemia and normalization after treatment as stated above. Ionized calcium was never checked during the hospital stay or during the follow-up after the discharge.
In Reply: Generally, it is preferable to measure the ionized calcium directly, particularly if there is uncertainty about whether the corrected serum calcium is reflective of the ionized calcium, if the patient’s symptoms are atypical, or if a reliable laboratory is available to measure ionized calcium.
Direct measurement of the ionized calcium concentration could be favored compared with measuring the corrected calcium in patients with symptoms of hypocalcemia in the setting of a normal total calcium concentration. Symptomatic hypocalcemia with normal total calcium but low ionized calcium can occasionally occur in patients with acute respiratory alkalosis due to increased binding of calcium to albumin. Thus, respiratory alkalosis may cause an acute decrease in ionized calcium. Furthermore, the ionized fraction can change without an alteration in the total serum calcium concentration, as with hyperparathyroidism, which increases the ionized calcium at the expense of that bound to albumin, and hyperphosphatemia, which increases the fraction bound to inorganic anions, decreasing ionized calcium. In patients who have chronic kidney disease and a low serum bicarbonate or a low serum albumin, or both, measuring the ionized calcium is preferable to measuring the total calcium in order to diagnose hypocalcemia or hypercalcemia.
The patient was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption, and she was followed by her primary care doctor. All the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since. We agree regarding the importance of checking on the ionized calcium to confirm the hypocalcemia and normalization after treatment as stated above. Ionized calcium was never checked during the hospital stay or during the follow-up after the discharge.
Stiff, numb hands
A 45-year-old woman with no chronic medical problems presented to the emergency room with a 1-day history of cramps and paresthesias in both hands and feet, mainly involving the fingers and toes. She said that after an argument with her daughter she began feeling anxious, and this was accompanied by shortness of breath and palpitations as well as generalized weakness, fatigue, and body aches. She also reported nausea and repeated vomiting but no abdominal pain, distention or change in bowel movements. She had had no loss of consciousness, confusion, incontinence, headache, dizziness, diplopia, or facial paresthesia.
She is a cigarette smoker, is alcohol-dependent, but does not use illicit drugs and is not on any medications.
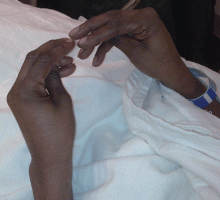
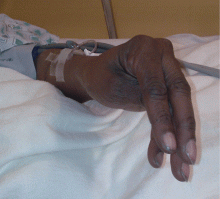
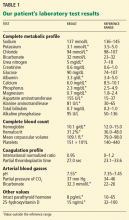
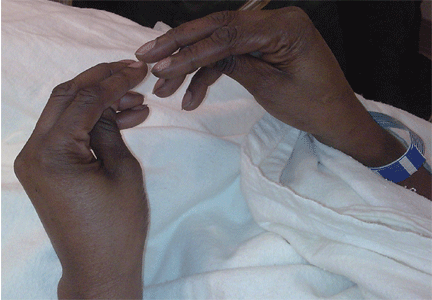
Examination revealed a temperature of 37.1°C (98.8°F), blood pressure 150/75 mm Hg, heart rate 105 bpm, respiratory rate 24 breaths per minute, and oxygen saturation 97% on room air. She appeared very fatigued, thin, and in mild distress due to her cramps. Her mucous membranes were dry, but she had no orthostatic changes. She had noticeable carpopedal spasms (Figure 1), reproducible by inflating a blood-pressure cuff placed on her arm (Trousseau sign) (Figure 2). Also noted was the Chvostek sign—contraction of the ipsilateral facial muscles when the facial nerve is tapped just in front of the ear. The rest of the systemic evaluation was normal. Laboratory investigations were as listed in Table 1. Electrocardiography showed a prolonged QTc interval (0.5 sec). The chest radiograph was normal.
HYPERVENTILATION AND TETANY
The presumptive diagnosis was latent tetany caused by an electrolyte derangement, in this case a combination of hypocalcemia, hypomagnesemia, and hypokalemia as the result of alcohol abuse, repeated vomiting, and hyperventilation brought on by a severe attack of anxiety.
Tetany results from increased excitability of nerves and muscles, leading to painful muscle cramps.1,2 Typical symptoms include circumoral and distal paresthesias, stiffness, clumsiness, myalgia, carpopedal spasm, laryngospasm, bronchospasm, and generalized seizure. The Chvostek and Trousseau signs help to confirm the diagnosis of tetany.3,4
The differential diagnosis of carpopedal spasm includes other conditions of involuntary muscle contraction, such as myotonic disorders; myokymia from Isaac syndrome (writhing movements of the muscles under the skin visualized by continuous “rippling” movements of the muscle); stiff-man syndrome (an autoimmune-antiglutamic acid decarboxylase antibody-associated muscle rigidity that waxes and wanes with concurrent spasms); and snake envenomation.
In addition, our patient’s symptoms were probably brought on by hyperventilation. In general, patients with hyperventilation syndrome are young females who display various manifestations of underlying anxiety and can develop tetany even after a brief episode of hyperventilation. At the time of presentation, our patient was found to have mixed respiratory and metabolic alkalosis. The anxiety-induced hyperventilation likely contributed to the respiratory alkalosis. She had no other symptoms or signs to suggest an acute organic respiratory illness such as pulmonary embolism, pneumothorax, or infection. Vomiting likely caused the metabolic alkalosis and hypokalemia.
Tetany is usually triggered by acute hypocalcemia and is uncommon unless the serum ionized calcium concentration falls below 4.3 mg/dL (1.1 mmol/L), which usually corresponds to a serum total calcium concentration of 7.0 to 7.5 mg/dL (1.8 to 1.9 mmol/L). Patients with a gradual onset of hypocalcemia tend to have fewer symptoms.3,4
Although alkalosis alone can cause tetany, it also enhances tetany by reducing the level of ionized calcium in the serum. Alkalemia causes hypocalcemia by an intravascular chelative mechanism in which the decrease in concentration of hydrogen ions leaves the negatively charged binding sites on albumin available to bind ionized calcium.3
The same happens to the magnesium, a cation with the same size and valence. Significant hypomagnesemia is common in tetanic patients with hyperventilation attacks and may, by itself or in combination with hypocalcemia, cause tetany.2,5,6 Hypokalemia can develop in patients with hypomagnesemia or metabolic alkalosis and may lead to tetany.6,7 Furthermore, our patient was dependent on alcohol, and this is known to cause hypomagnesemia from the excessive urinary excretion of magnesium. This defect of alcohol-induced tubular dysfunction is reversible within 4 weeks of abstinence. Magnesium depletion can cause hypocalcemia by producing resistance to parathyroid hormone or by decreasing its secretion, and this occurs in severe hypomagnesemia, ie, when the serum magnesium concentration falls below 1.0 mg/dL (0.4 mmol/L).
IDENTIFY AND TREAT THE UNDERLYING CAUSE
The management of tetany consists of identifying and treating the underlying cause. Infusion of calcium or magnesium is effective as acute therapy for tetany, and, if appropriate, vitamin D supplementation should also be provided.3,4,7 However, if associated hyperventilation syndrome is present, patients benefit from reassurance and treatment for underlying psychological stress. The traditional treatment of rebreathing into a paper bag is no longer recommended because of the potential risk of hypoxia. Sedatives and antidepressants should be reserved for patients who have not responded to conservative treatment.
Our patient was given an explanation of the condition together with breathing exercises. She received lorazepam and was immediately treated with intravenous hydration, along with intravenous infusion of magnesium, calcium, and potassium. These interventions led to an immediate resolution of her symptoms.
Her low level of intact parathyroid hormone may also have been caused by hypomagnesemia. She was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption and to see us or her primary care doctor should the symptoms recur. As expected, all the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 2008; 35:215–237.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Smets YF, Bokani N, de Meijer PH, Meinders AE. Tetany due to excessive use of alcohol: a possible magnesium deficiency [in Dutch]. Ned Tijdschr Geneeskd 2004; 148:641–644.
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–2652.
A 45-year-old woman with no chronic medical problems presented to the emergency room with a 1-day history of cramps and paresthesias in both hands and feet, mainly involving the fingers and toes. She said that after an argument with her daughter she began feeling anxious, and this was accompanied by shortness of breath and palpitations as well as generalized weakness, fatigue, and body aches. She also reported nausea and repeated vomiting but no abdominal pain, distention or change in bowel movements. She had had no loss of consciousness, confusion, incontinence, headache, dizziness, diplopia, or facial paresthesia.
She is a cigarette smoker, is alcohol-dependent, but does not use illicit drugs and is not on any medications.
Examination revealed a temperature of 37.1°C (98.8°F), blood pressure 150/75 mm Hg, heart rate 105 bpm, respiratory rate 24 breaths per minute, and oxygen saturation 97% on room air. She appeared very fatigued, thin, and in mild distress due to her cramps. Her mucous membranes were dry, but she had no orthostatic changes. She had noticeable carpopedal spasms (Figure 1), reproducible by inflating a blood-pressure cuff placed on her arm (Trousseau sign) (Figure 2). Also noted was the Chvostek sign—contraction of the ipsilateral facial muscles when the facial nerve is tapped just in front of the ear. The rest of the systemic evaluation was normal. Laboratory investigations were as listed in Table 1. Electrocardiography showed a prolonged QTc interval (0.5 sec). The chest radiograph was normal.
HYPERVENTILATION AND TETANY
The presumptive diagnosis was latent tetany caused by an electrolyte derangement, in this case a combination of hypocalcemia, hypomagnesemia, and hypokalemia as the result of alcohol abuse, repeated vomiting, and hyperventilation brought on by a severe attack of anxiety.
Tetany results from increased excitability of nerves and muscles, leading to painful muscle cramps.1,2 Typical symptoms include circumoral and distal paresthesias, stiffness, clumsiness, myalgia, carpopedal spasm, laryngospasm, bronchospasm, and generalized seizure. The Chvostek and Trousseau signs help to confirm the diagnosis of tetany.3,4
The differential diagnosis of carpopedal spasm includes other conditions of involuntary muscle contraction, such as myotonic disorders; myokymia from Isaac syndrome (writhing movements of the muscles under the skin visualized by continuous “rippling” movements of the muscle); stiff-man syndrome (an autoimmune-antiglutamic acid decarboxylase antibody-associated muscle rigidity that waxes and wanes with concurrent spasms); and snake envenomation.
In addition, our patient’s symptoms were probably brought on by hyperventilation. In general, patients with hyperventilation syndrome are young females who display various manifestations of underlying anxiety and can develop tetany even after a brief episode of hyperventilation. At the time of presentation, our patient was found to have mixed respiratory and metabolic alkalosis. The anxiety-induced hyperventilation likely contributed to the respiratory alkalosis. She had no other symptoms or signs to suggest an acute organic respiratory illness such as pulmonary embolism, pneumothorax, or infection. Vomiting likely caused the metabolic alkalosis and hypokalemia.
Tetany is usually triggered by acute hypocalcemia and is uncommon unless the serum ionized calcium concentration falls below 4.3 mg/dL (1.1 mmol/L), which usually corresponds to a serum total calcium concentration of 7.0 to 7.5 mg/dL (1.8 to 1.9 mmol/L). Patients with a gradual onset of hypocalcemia tend to have fewer symptoms.3,4
Although alkalosis alone can cause tetany, it also enhances tetany by reducing the level of ionized calcium in the serum. Alkalemia causes hypocalcemia by an intravascular chelative mechanism in which the decrease in concentration of hydrogen ions leaves the negatively charged binding sites on albumin available to bind ionized calcium.3
The same happens to the magnesium, a cation with the same size and valence. Significant hypomagnesemia is common in tetanic patients with hyperventilation attacks and may, by itself or in combination with hypocalcemia, cause tetany.2,5,6 Hypokalemia can develop in patients with hypomagnesemia or metabolic alkalosis and may lead to tetany.6,7 Furthermore, our patient was dependent on alcohol, and this is known to cause hypomagnesemia from the excessive urinary excretion of magnesium. This defect of alcohol-induced tubular dysfunction is reversible within 4 weeks of abstinence. Magnesium depletion can cause hypocalcemia by producing resistance to parathyroid hormone or by decreasing its secretion, and this occurs in severe hypomagnesemia, ie, when the serum magnesium concentration falls below 1.0 mg/dL (0.4 mmol/L).
IDENTIFY AND TREAT THE UNDERLYING CAUSE
The management of tetany consists of identifying and treating the underlying cause. Infusion of calcium or magnesium is effective as acute therapy for tetany, and, if appropriate, vitamin D supplementation should also be provided.3,4,7 However, if associated hyperventilation syndrome is present, patients benefit from reassurance and treatment for underlying psychological stress. The traditional treatment of rebreathing into a paper bag is no longer recommended because of the potential risk of hypoxia. Sedatives and antidepressants should be reserved for patients who have not responded to conservative treatment.
Our patient was given an explanation of the condition together with breathing exercises. She received lorazepam and was immediately treated with intravenous hydration, along with intravenous infusion of magnesium, calcium, and potassium. These interventions led to an immediate resolution of her symptoms.
Her low level of intact parathyroid hormone may also have been caused by hypomagnesemia. She was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption and to see us or her primary care doctor should the symptoms recur. As expected, all the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since.
A 45-year-old woman with no chronic medical problems presented to the emergency room with a 1-day history of cramps and paresthesias in both hands and feet, mainly involving the fingers and toes. She said that after an argument with her daughter she began feeling anxious, and this was accompanied by shortness of breath and palpitations as well as generalized weakness, fatigue, and body aches. She also reported nausea and repeated vomiting but no abdominal pain, distention or change in bowel movements. She had had no loss of consciousness, confusion, incontinence, headache, dizziness, diplopia, or facial paresthesia.
She is a cigarette smoker, is alcohol-dependent, but does not use illicit drugs and is not on any medications.
Examination revealed a temperature of 37.1°C (98.8°F), blood pressure 150/75 mm Hg, heart rate 105 bpm, respiratory rate 24 breaths per minute, and oxygen saturation 97% on room air. She appeared very fatigued, thin, and in mild distress due to her cramps. Her mucous membranes were dry, but she had no orthostatic changes. She had noticeable carpopedal spasms (Figure 1), reproducible by inflating a blood-pressure cuff placed on her arm (Trousseau sign) (Figure 2). Also noted was the Chvostek sign—contraction of the ipsilateral facial muscles when the facial nerve is tapped just in front of the ear. The rest of the systemic evaluation was normal. Laboratory investigations were as listed in Table 1. Electrocardiography showed a prolonged QTc interval (0.5 sec). The chest radiograph was normal.
HYPERVENTILATION AND TETANY
The presumptive diagnosis was latent tetany caused by an electrolyte derangement, in this case a combination of hypocalcemia, hypomagnesemia, and hypokalemia as the result of alcohol abuse, repeated vomiting, and hyperventilation brought on by a severe attack of anxiety.
Tetany results from increased excitability of nerves and muscles, leading to painful muscle cramps.1,2 Typical symptoms include circumoral and distal paresthesias, stiffness, clumsiness, myalgia, carpopedal spasm, laryngospasm, bronchospasm, and generalized seizure. The Chvostek and Trousseau signs help to confirm the diagnosis of tetany.3,4
The differential diagnosis of carpopedal spasm includes other conditions of involuntary muscle contraction, such as myotonic disorders; myokymia from Isaac syndrome (writhing movements of the muscles under the skin visualized by continuous “rippling” movements of the muscle); stiff-man syndrome (an autoimmune-antiglutamic acid decarboxylase antibody-associated muscle rigidity that waxes and wanes with concurrent spasms); and snake envenomation.
In addition, our patient’s symptoms were probably brought on by hyperventilation. In general, patients with hyperventilation syndrome are young females who display various manifestations of underlying anxiety and can develop tetany even after a brief episode of hyperventilation. At the time of presentation, our patient was found to have mixed respiratory and metabolic alkalosis. The anxiety-induced hyperventilation likely contributed to the respiratory alkalosis. She had no other symptoms or signs to suggest an acute organic respiratory illness such as pulmonary embolism, pneumothorax, or infection. Vomiting likely caused the metabolic alkalosis and hypokalemia.
Tetany is usually triggered by acute hypocalcemia and is uncommon unless the serum ionized calcium concentration falls below 4.3 mg/dL (1.1 mmol/L), which usually corresponds to a serum total calcium concentration of 7.0 to 7.5 mg/dL (1.8 to 1.9 mmol/L). Patients with a gradual onset of hypocalcemia tend to have fewer symptoms.3,4
Although alkalosis alone can cause tetany, it also enhances tetany by reducing the level of ionized calcium in the serum. Alkalemia causes hypocalcemia by an intravascular chelative mechanism in which the decrease in concentration of hydrogen ions leaves the negatively charged binding sites on albumin available to bind ionized calcium.3
The same happens to the magnesium, a cation with the same size and valence. Significant hypomagnesemia is common in tetanic patients with hyperventilation attacks and may, by itself or in combination with hypocalcemia, cause tetany.2,5,6 Hypokalemia can develop in patients with hypomagnesemia or metabolic alkalosis and may lead to tetany.6,7 Furthermore, our patient was dependent on alcohol, and this is known to cause hypomagnesemia from the excessive urinary excretion of magnesium. This defect of alcohol-induced tubular dysfunction is reversible within 4 weeks of abstinence. Magnesium depletion can cause hypocalcemia by producing resistance to parathyroid hormone or by decreasing its secretion, and this occurs in severe hypomagnesemia, ie, when the serum magnesium concentration falls below 1.0 mg/dL (0.4 mmol/L).
IDENTIFY AND TREAT THE UNDERLYING CAUSE
The management of tetany consists of identifying and treating the underlying cause. Infusion of calcium or magnesium is effective as acute therapy for tetany, and, if appropriate, vitamin D supplementation should also be provided.3,4,7 However, if associated hyperventilation syndrome is present, patients benefit from reassurance and treatment for underlying psychological stress. The traditional treatment of rebreathing into a paper bag is no longer recommended because of the potential risk of hypoxia. Sedatives and antidepressants should be reserved for patients who have not responded to conservative treatment.
Our patient was given an explanation of the condition together with breathing exercises. She received lorazepam and was immediately treated with intravenous hydration, along with intravenous infusion of magnesium, calcium, and potassium. These interventions led to an immediate resolution of her symptoms.
Her low level of intact parathyroid hormone may also have been caused by hypomagnesemia. She was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption and to see us or her primary care doctor should the symptoms recur. As expected, all the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 2008; 35:215–237.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Smets YF, Bokani N, de Meijer PH, Meinders AE. Tetany due to excessive use of alcohol: a possible magnesium deficiency [in Dutch]. Ned Tijdschr Geneeskd 2004; 148:641–644.
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–2652.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 2008; 35:215–237.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Smets YF, Bokani N, de Meijer PH, Meinders AE. Tetany due to excessive use of alcohol: a possible magnesium deficiency [in Dutch]. Ned Tijdschr Geneeskd 2004; 148:641–644.
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–2652.