User login
In reply: Stiff, numb hands
In Reply: Generally, it is preferable to measure the ionized calcium directly, particularly if there is uncertainty about whether the corrected serum calcium is reflective of the ionized calcium, if the patient’s symptoms are atypical, or if a reliable laboratory is available to measure ionized calcium.
Direct measurement of the ionized calcium concentration could be favored compared with measuring the corrected calcium in patients with symptoms of hypocalcemia in the setting of a normal total calcium concentration. Symptomatic hypocalcemia with normal total calcium but low ionized calcium can occasionally occur in patients with acute respiratory alkalosis due to increased binding of calcium to albumin. Thus, respiratory alkalosis may cause an acute decrease in ionized calcium. Furthermore, the ionized fraction can change without an alteration in the total serum calcium concentration, as with hyperparathyroidism, which increases the ionized calcium at the expense of that bound to albumin, and hyperphosphatemia, which increases the fraction bound to inorganic anions, decreasing ionized calcium. In patients who have chronic kidney disease and a low serum bicarbonate or a low serum albumin, or both, measuring the ionized calcium is preferable to measuring the total calcium in order to diagnose hypocalcemia or hypercalcemia.
The patient was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption, and she was followed by her primary care doctor. All the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since. We agree regarding the importance of checking on the ionized calcium to confirm the hypocalcemia and normalization after treatment as stated above. Ionized calcium was never checked during the hospital stay or during the follow-up after the discharge.
In Reply: Generally, it is preferable to measure the ionized calcium directly, particularly if there is uncertainty about whether the corrected serum calcium is reflective of the ionized calcium, if the patient’s symptoms are atypical, or if a reliable laboratory is available to measure ionized calcium.
Direct measurement of the ionized calcium concentration could be favored compared with measuring the corrected calcium in patients with symptoms of hypocalcemia in the setting of a normal total calcium concentration. Symptomatic hypocalcemia with normal total calcium but low ionized calcium can occasionally occur in patients with acute respiratory alkalosis due to increased binding of calcium to albumin. Thus, respiratory alkalosis may cause an acute decrease in ionized calcium. Furthermore, the ionized fraction can change without an alteration in the total serum calcium concentration, as with hyperparathyroidism, which increases the ionized calcium at the expense of that bound to albumin, and hyperphosphatemia, which increases the fraction bound to inorganic anions, decreasing ionized calcium. In patients who have chronic kidney disease and a low serum bicarbonate or a low serum albumin, or both, measuring the ionized calcium is preferable to measuring the total calcium in order to diagnose hypocalcemia or hypercalcemia.
The patient was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption, and she was followed by her primary care doctor. All the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since. We agree regarding the importance of checking on the ionized calcium to confirm the hypocalcemia and normalization after treatment as stated above. Ionized calcium was never checked during the hospital stay or during the follow-up after the discharge.
In Reply: Generally, it is preferable to measure the ionized calcium directly, particularly if there is uncertainty about whether the corrected serum calcium is reflective of the ionized calcium, if the patient’s symptoms are atypical, or if a reliable laboratory is available to measure ionized calcium.
Direct measurement of the ionized calcium concentration could be favored compared with measuring the corrected calcium in patients with symptoms of hypocalcemia in the setting of a normal total calcium concentration. Symptomatic hypocalcemia with normal total calcium but low ionized calcium can occasionally occur in patients with acute respiratory alkalosis due to increased binding of calcium to albumin. Thus, respiratory alkalosis may cause an acute decrease in ionized calcium. Furthermore, the ionized fraction can change without an alteration in the total serum calcium concentration, as with hyperparathyroidism, which increases the ionized calcium at the expense of that bound to albumin, and hyperphosphatemia, which increases the fraction bound to inorganic anions, decreasing ionized calcium. In patients who have chronic kidney disease and a low serum bicarbonate or a low serum albumin, or both, measuring the ionized calcium is preferable to measuring the total calcium in order to diagnose hypocalcemia or hypercalcemia.
The patient was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption, and she was followed by her primary care doctor. All the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since. We agree regarding the importance of checking on the ionized calcium to confirm the hypocalcemia and normalization after treatment as stated above. Ionized calcium was never checked during the hospital stay or during the follow-up after the discharge.
Stiff, numb hands
A 45-year-old woman with no chronic medical problems presented to the emergency room with a 1-day history of cramps and paresthesias in both hands and feet, mainly involving the fingers and toes. She said that after an argument with her daughter she began feeling anxious, and this was accompanied by shortness of breath and palpitations as well as generalized weakness, fatigue, and body aches. She also reported nausea and repeated vomiting but no abdominal pain, distention or change in bowel movements. She had had no loss of consciousness, confusion, incontinence, headache, dizziness, diplopia, or facial paresthesia.
She is a cigarette smoker, is alcohol-dependent, but does not use illicit drugs and is not on any medications.
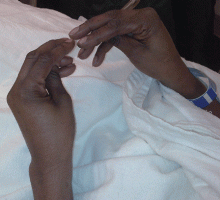
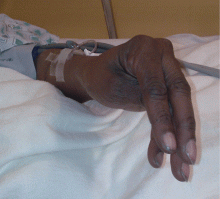
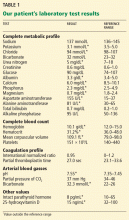
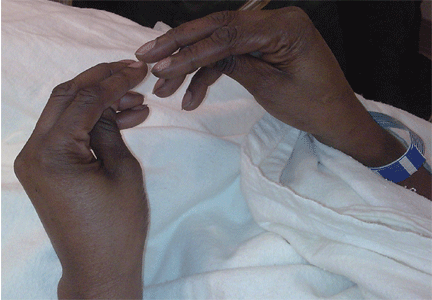
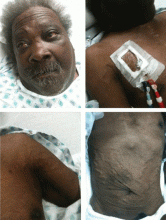
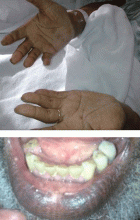
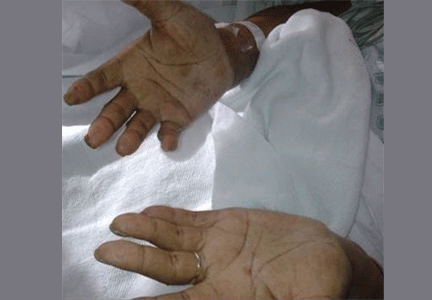
Examination revealed a temperature of 37.1°C (98.8°F), blood pressure 150/75 mm Hg, heart rate 105 bpm, respiratory rate 24 breaths per minute, and oxygen saturation 97% on room air. She appeared very fatigued, thin, and in mild distress due to her cramps. Her mucous membranes were dry, but she had no orthostatic changes. She had noticeable carpopedal spasms (Figure 1), reproducible by inflating a blood-pressure cuff placed on her arm (Trousseau sign) (Figure 2). Also noted was the Chvostek sign—contraction of the ipsilateral facial muscles when the facial nerve is tapped just in front of the ear. The rest of the systemic evaluation was normal. Laboratory investigations were as listed in Table 1. Electrocardiography showed a prolonged QTc interval (0.5 sec). The chest radiograph was normal.
HYPERVENTILATION AND TETANY
The presumptive diagnosis was latent tetany caused by an electrolyte derangement, in this case a combination of hypocalcemia, hypomagnesemia, and hypokalemia as the result of alcohol abuse, repeated vomiting, and hyperventilation brought on by a severe attack of anxiety.
Tetany results from increased excitability of nerves and muscles, leading to painful muscle cramps.1,2 Typical symptoms include circumoral and distal paresthesias, stiffness, clumsiness, myalgia, carpopedal spasm, laryngospasm, bronchospasm, and generalized seizure. The Chvostek and Trousseau signs help to confirm the diagnosis of tetany.3,4
The differential diagnosis of carpopedal spasm includes other conditions of involuntary muscle contraction, such as myotonic disorders; myokymia from Isaac syndrome (writhing movements of the muscles under the skin visualized by continuous “rippling” movements of the muscle); stiff-man syndrome (an autoimmune-antiglutamic acid decarboxylase antibody-associated muscle rigidity that waxes and wanes with concurrent spasms); and snake envenomation.
In addition, our patient’s symptoms were probably brought on by hyperventilation. In general, patients with hyperventilation syndrome are young females who display various manifestations of underlying anxiety and can develop tetany even after a brief episode of hyperventilation. At the time of presentation, our patient was found to have mixed respiratory and metabolic alkalosis. The anxiety-induced hyperventilation likely contributed to the respiratory alkalosis. She had no other symptoms or signs to suggest an acute organic respiratory illness such as pulmonary embolism, pneumothorax, or infection. Vomiting likely caused the metabolic alkalosis and hypokalemia.
Tetany is usually triggered by acute hypocalcemia and is uncommon unless the serum ionized calcium concentration falls below 4.3 mg/dL (1.1 mmol/L), which usually corresponds to a serum total calcium concentration of 7.0 to 7.5 mg/dL (1.8 to 1.9 mmol/L). Patients with a gradual onset of hypocalcemia tend to have fewer symptoms.3,4
Although alkalosis alone can cause tetany, it also enhances tetany by reducing the level of ionized calcium in the serum. Alkalemia causes hypocalcemia by an intravascular chelative mechanism in which the decrease in concentration of hydrogen ions leaves the negatively charged binding sites on albumin available to bind ionized calcium.3
The same happens to the magnesium, a cation with the same size and valence. Significant hypomagnesemia is common in tetanic patients with hyperventilation attacks and may, by itself or in combination with hypocalcemia, cause tetany.2,5,6 Hypokalemia can develop in patients with hypomagnesemia or metabolic alkalosis and may lead to tetany.6,7 Furthermore, our patient was dependent on alcohol, and this is known to cause hypomagnesemia from the excessive urinary excretion of magnesium. This defect of alcohol-induced tubular dysfunction is reversible within 4 weeks of abstinence. Magnesium depletion can cause hypocalcemia by producing resistance to parathyroid hormone or by decreasing its secretion, and this occurs in severe hypomagnesemia, ie, when the serum magnesium concentration falls below 1.0 mg/dL (0.4 mmol/L).
IDENTIFY AND TREAT THE UNDERLYING CAUSE
The management of tetany consists of identifying and treating the underlying cause. Infusion of calcium or magnesium is effective as acute therapy for tetany, and, if appropriate, vitamin D supplementation should also be provided.3,4,7 However, if associated hyperventilation syndrome is present, patients benefit from reassurance and treatment for underlying psychological stress. The traditional treatment of rebreathing into a paper bag is no longer recommended because of the potential risk of hypoxia. Sedatives and antidepressants should be reserved for patients who have not responded to conservative treatment.
Our patient was given an explanation of the condition together with breathing exercises. She received lorazepam and was immediately treated with intravenous hydration, along with intravenous infusion of magnesium, calcium, and potassium. These interventions led to an immediate resolution of her symptoms.
Her low level of intact parathyroid hormone may also have been caused by hypomagnesemia. She was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption and to see us or her primary care doctor should the symptoms recur. As expected, all the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 2008; 35:215–237.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Smets YF, Bokani N, de Meijer PH, Meinders AE. Tetany due to excessive use of alcohol: a possible magnesium deficiency [in Dutch]. Ned Tijdschr Geneeskd 2004; 148:641–644.
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–2652.
A 45-year-old woman with no chronic medical problems presented to the emergency room with a 1-day history of cramps and paresthesias in both hands and feet, mainly involving the fingers and toes. She said that after an argument with her daughter she began feeling anxious, and this was accompanied by shortness of breath and palpitations as well as generalized weakness, fatigue, and body aches. She also reported nausea and repeated vomiting but no abdominal pain, distention or change in bowel movements. She had had no loss of consciousness, confusion, incontinence, headache, dizziness, diplopia, or facial paresthesia.
She is a cigarette smoker, is alcohol-dependent, but does not use illicit drugs and is not on any medications.
Examination revealed a temperature of 37.1°C (98.8°F), blood pressure 150/75 mm Hg, heart rate 105 bpm, respiratory rate 24 breaths per minute, and oxygen saturation 97% on room air. She appeared very fatigued, thin, and in mild distress due to her cramps. Her mucous membranes were dry, but she had no orthostatic changes. She had noticeable carpopedal spasms (Figure 1), reproducible by inflating a blood-pressure cuff placed on her arm (Trousseau sign) (Figure 2). Also noted was the Chvostek sign—contraction of the ipsilateral facial muscles when the facial nerve is tapped just in front of the ear. The rest of the systemic evaluation was normal. Laboratory investigations were as listed in Table 1. Electrocardiography showed a prolonged QTc interval (0.5 sec). The chest radiograph was normal.
HYPERVENTILATION AND TETANY
The presumptive diagnosis was latent tetany caused by an electrolyte derangement, in this case a combination of hypocalcemia, hypomagnesemia, and hypokalemia as the result of alcohol abuse, repeated vomiting, and hyperventilation brought on by a severe attack of anxiety.
Tetany results from increased excitability of nerves and muscles, leading to painful muscle cramps.1,2 Typical symptoms include circumoral and distal paresthesias, stiffness, clumsiness, myalgia, carpopedal spasm, laryngospasm, bronchospasm, and generalized seizure. The Chvostek and Trousseau signs help to confirm the diagnosis of tetany.3,4
The differential diagnosis of carpopedal spasm includes other conditions of involuntary muscle contraction, such as myotonic disorders; myokymia from Isaac syndrome (writhing movements of the muscles under the skin visualized by continuous “rippling” movements of the muscle); stiff-man syndrome (an autoimmune-antiglutamic acid decarboxylase antibody-associated muscle rigidity that waxes and wanes with concurrent spasms); and snake envenomation.
In addition, our patient’s symptoms were probably brought on by hyperventilation. In general, patients with hyperventilation syndrome are young females who display various manifestations of underlying anxiety and can develop tetany even after a brief episode of hyperventilation. At the time of presentation, our patient was found to have mixed respiratory and metabolic alkalosis. The anxiety-induced hyperventilation likely contributed to the respiratory alkalosis. She had no other symptoms or signs to suggest an acute organic respiratory illness such as pulmonary embolism, pneumothorax, or infection. Vomiting likely caused the metabolic alkalosis and hypokalemia.
Tetany is usually triggered by acute hypocalcemia and is uncommon unless the serum ionized calcium concentration falls below 4.3 mg/dL (1.1 mmol/L), which usually corresponds to a serum total calcium concentration of 7.0 to 7.5 mg/dL (1.8 to 1.9 mmol/L). Patients with a gradual onset of hypocalcemia tend to have fewer symptoms.3,4
Although alkalosis alone can cause tetany, it also enhances tetany by reducing the level of ionized calcium in the serum. Alkalemia causes hypocalcemia by an intravascular chelative mechanism in which the decrease in concentration of hydrogen ions leaves the negatively charged binding sites on albumin available to bind ionized calcium.3
The same happens to the magnesium, a cation with the same size and valence. Significant hypomagnesemia is common in tetanic patients with hyperventilation attacks and may, by itself or in combination with hypocalcemia, cause tetany.2,5,6 Hypokalemia can develop in patients with hypomagnesemia or metabolic alkalosis and may lead to tetany.6,7 Furthermore, our patient was dependent on alcohol, and this is known to cause hypomagnesemia from the excessive urinary excretion of magnesium. This defect of alcohol-induced tubular dysfunction is reversible within 4 weeks of abstinence. Magnesium depletion can cause hypocalcemia by producing resistance to parathyroid hormone or by decreasing its secretion, and this occurs in severe hypomagnesemia, ie, when the serum magnesium concentration falls below 1.0 mg/dL (0.4 mmol/L).
IDENTIFY AND TREAT THE UNDERLYING CAUSE
The management of tetany consists of identifying and treating the underlying cause. Infusion of calcium or magnesium is effective as acute therapy for tetany, and, if appropriate, vitamin D supplementation should also be provided.3,4,7 However, if associated hyperventilation syndrome is present, patients benefit from reassurance and treatment for underlying psychological stress. The traditional treatment of rebreathing into a paper bag is no longer recommended because of the potential risk of hypoxia. Sedatives and antidepressants should be reserved for patients who have not responded to conservative treatment.
Our patient was given an explanation of the condition together with breathing exercises. She received lorazepam and was immediately treated with intravenous hydration, along with intravenous infusion of magnesium, calcium, and potassium. These interventions led to an immediate resolution of her symptoms.
Her low level of intact parathyroid hormone may also have been caused by hypomagnesemia. She was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption and to see us or her primary care doctor should the symptoms recur. As expected, all the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since.
A 45-year-old woman with no chronic medical problems presented to the emergency room with a 1-day history of cramps and paresthesias in both hands and feet, mainly involving the fingers and toes. She said that after an argument with her daughter she began feeling anxious, and this was accompanied by shortness of breath and palpitations as well as generalized weakness, fatigue, and body aches. She also reported nausea and repeated vomiting but no abdominal pain, distention or change in bowel movements. She had had no loss of consciousness, confusion, incontinence, headache, dizziness, diplopia, or facial paresthesia.
She is a cigarette smoker, is alcohol-dependent, but does not use illicit drugs and is not on any medications.
Examination revealed a temperature of 37.1°C (98.8°F), blood pressure 150/75 mm Hg, heart rate 105 bpm, respiratory rate 24 breaths per minute, and oxygen saturation 97% on room air. She appeared very fatigued, thin, and in mild distress due to her cramps. Her mucous membranes were dry, but she had no orthostatic changes. She had noticeable carpopedal spasms (Figure 1), reproducible by inflating a blood-pressure cuff placed on her arm (Trousseau sign) (Figure 2). Also noted was the Chvostek sign—contraction of the ipsilateral facial muscles when the facial nerve is tapped just in front of the ear. The rest of the systemic evaluation was normal. Laboratory investigations were as listed in Table 1. Electrocardiography showed a prolonged QTc interval (0.5 sec). The chest radiograph was normal.
HYPERVENTILATION AND TETANY
The presumptive diagnosis was latent tetany caused by an electrolyte derangement, in this case a combination of hypocalcemia, hypomagnesemia, and hypokalemia as the result of alcohol abuse, repeated vomiting, and hyperventilation brought on by a severe attack of anxiety.
Tetany results from increased excitability of nerves and muscles, leading to painful muscle cramps.1,2 Typical symptoms include circumoral and distal paresthesias, stiffness, clumsiness, myalgia, carpopedal spasm, laryngospasm, bronchospasm, and generalized seizure. The Chvostek and Trousseau signs help to confirm the diagnosis of tetany.3,4
The differential diagnosis of carpopedal spasm includes other conditions of involuntary muscle contraction, such as myotonic disorders; myokymia from Isaac syndrome (writhing movements of the muscles under the skin visualized by continuous “rippling” movements of the muscle); stiff-man syndrome (an autoimmune-antiglutamic acid decarboxylase antibody-associated muscle rigidity that waxes and wanes with concurrent spasms); and snake envenomation.
In addition, our patient’s symptoms were probably brought on by hyperventilation. In general, patients with hyperventilation syndrome are young females who display various manifestations of underlying anxiety and can develop tetany even after a brief episode of hyperventilation. At the time of presentation, our patient was found to have mixed respiratory and metabolic alkalosis. The anxiety-induced hyperventilation likely contributed to the respiratory alkalosis. She had no other symptoms or signs to suggest an acute organic respiratory illness such as pulmonary embolism, pneumothorax, or infection. Vomiting likely caused the metabolic alkalosis and hypokalemia.
Tetany is usually triggered by acute hypocalcemia and is uncommon unless the serum ionized calcium concentration falls below 4.3 mg/dL (1.1 mmol/L), which usually corresponds to a serum total calcium concentration of 7.0 to 7.5 mg/dL (1.8 to 1.9 mmol/L). Patients with a gradual onset of hypocalcemia tend to have fewer symptoms.3,4
Although alkalosis alone can cause tetany, it also enhances tetany by reducing the level of ionized calcium in the serum. Alkalemia causes hypocalcemia by an intravascular chelative mechanism in which the decrease in concentration of hydrogen ions leaves the negatively charged binding sites on albumin available to bind ionized calcium.3
The same happens to the magnesium, a cation with the same size and valence. Significant hypomagnesemia is common in tetanic patients with hyperventilation attacks and may, by itself or in combination with hypocalcemia, cause tetany.2,5,6 Hypokalemia can develop in patients with hypomagnesemia or metabolic alkalosis and may lead to tetany.6,7 Furthermore, our patient was dependent on alcohol, and this is known to cause hypomagnesemia from the excessive urinary excretion of magnesium. This defect of alcohol-induced tubular dysfunction is reversible within 4 weeks of abstinence. Magnesium depletion can cause hypocalcemia by producing resistance to parathyroid hormone or by decreasing its secretion, and this occurs in severe hypomagnesemia, ie, when the serum magnesium concentration falls below 1.0 mg/dL (0.4 mmol/L).
IDENTIFY AND TREAT THE UNDERLYING CAUSE
The management of tetany consists of identifying and treating the underlying cause. Infusion of calcium or magnesium is effective as acute therapy for tetany, and, if appropriate, vitamin D supplementation should also be provided.3,4,7 However, if associated hyperventilation syndrome is present, patients benefit from reassurance and treatment for underlying psychological stress. The traditional treatment of rebreathing into a paper bag is no longer recommended because of the potential risk of hypoxia. Sedatives and antidepressants should be reserved for patients who have not responded to conservative treatment.
Our patient was given an explanation of the condition together with breathing exercises. She received lorazepam and was immediately treated with intravenous hydration, along with intravenous infusion of magnesium, calcium, and potassium. These interventions led to an immediate resolution of her symptoms.
Her low level of intact parathyroid hormone may also have been caused by hypomagnesemia. She was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption and to see us or her primary care doctor should the symptoms recur. As expected, all the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 2008; 35:215–237.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Smets YF, Bokani N, de Meijer PH, Meinders AE. Tetany due to excessive use of alcohol: a possible magnesium deficiency [in Dutch]. Ned Tijdschr Geneeskd 2004; 148:641–644.
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–2652.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 2008; 35:215–237.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Smets YF, Bokani N, de Meijer PH, Meinders AE. Tetany due to excessive use of alcohol: a possible magnesium deficiency [in Dutch]. Ned Tijdschr Geneeskd 2004; 148:641–644.
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–2652.
A 47-year-old man with chest and neck pain
A 47-year-old man presented with acute shortness of breath and chest and neck pain, which began after he heard popping sounds while boarding a bus. The pain was right-sided, sharp, worse with deep breathing, and associated with a sensation of fullness over the right chest.
His medical conditions included controlled hypertension, gastroesophageal reflux disease, and chronic obstructive pulmonary disease (COPD). The COPD was managed with an albuterol inhaler only. He had a 50-pack-year history of smoking, and he drank alcohol occasionally.
On arrival, he was in mild respiratory distress, but his vital signs were stable. We could hear wheezing on both sides of his chest and feel subcutaneous crepitation on both sides of his chest and neck, the latter more on the right side. The rest of the examination was unremarkable.
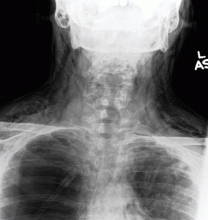
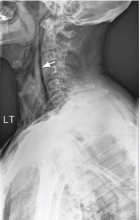
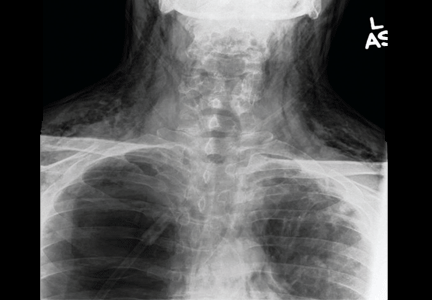
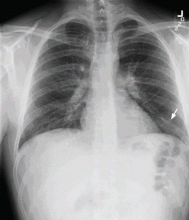
Results of a complete blood cell count and metabolic panel were within normal limits. Because of the above findings, nasopharyngeal radiogragraphy was ordered (Figures 1 and 2).
Q: What is the most likely cause of this presentation?
- Esophageal rupture
- Gas gangrene
- Asthma exacerbation
- Ruptured emphysematous bullae
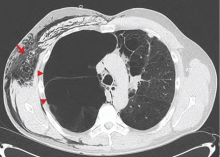
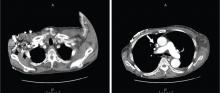
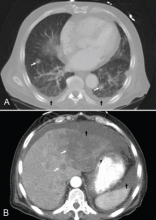
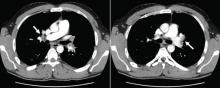
A: This patient had a history of COPD, which put him at risk of developing bullous emphysematous bullae that can rupture and cause subcutaneous emphysema. His nasopharyngeal radiograph (Figure1) showed bilateral extensive subcutaneous emphysema. His lateral nasopharyngeal radiograph (Figure 2) showed air-tracking within the mediastinum and into the retropharyngeal space (arrow). Computed tomography (Figure 3) showed extensive subcutaneous emphysema in the right lateral chest wall (arrow) and large bullae in the right upper lobe (arrow heads). As for the other possibilities:
Esophageal ruptures and tears are iatrogenic in most cases and usually occur after endoscopic procedures, but they can also occur in patients with intractable vomiting. Computed tomography often shows esophageal thickening, periesophageal fluid, mediastinal widening, and extraluminal air. However, in most cases, it is seen as pneumomediastinum and subcutaneous emphysema.1
Gas gangrene is a life-threatening soft-tissue and muscle infection caused by Clostridium perfringens in most cases.2 The pain is out of proportion to the findings on physical examination. Patients usually have toxic signs and symptoms such as fever and hypotension. Our patient was hemodynamically stable, with no changes in skin color.
Severe exacerbations of asthma can lead to alveolar rupture, pneumothorax, and subcutaneous emphysema, although this is a rare complication. Air can dissect along the bronchovascular sheaths into the neck and cause subcutaneous emphysema, or into the pleural space and cause pneumothorax. Our patient had no history of asthma and plainly had emphysematous bullae.3
SUBCUTANEOUS EMPHYSEMA
Subcutaneous emphysema is a collection of air within subcutaneous tissues. It usually presents as bloating of the skin around the neck and the chest wall. It is often seen in patients with pneumothorax.
The most common cause of subcutaneous emphysema is traumatic injury to the chest wall, such as from a motor vehicle accident or a stab wound,4 but it can also occur spontaneously in patients who have severe emphysema with large bullae. As the emphysema progresses, the bullae can easily rupture, and this can lead to pneumothorax, which can lead to subcutaneous emphysema. Primary spontaneous pneumothorax and subcutaneous emphysema can occur in people who have unrecognized lung disease and genetic disorders such as Marfan syndrome and Ehler-Danlos syndrome.5 Other causes include iatrogenic injury, Pneumocystis jirovecii pneumonia (common in patients with human immunodeficiency virus infection), and cystic fibrosis. Pneumothorax occurs in about 30% of cases of P jirovecii pneumonia,6 and in about 6% of patients with cystic fibrosis.7 Bronchocutaneous fistula is an extremely rare complication of lung cancer and can cause subcutaneous emphysema.8 Tuberculosis is another possible cause.9
Subcutaneous emphysema mainly presents with chest or neck pain and wheezing. In severe cases, air can track to the face, causing facial swelling and difficulty breathing due to compression of the larynx. Also, it can track down to the thighs, causing leg pain and swelling.10
On examination, subcutaneous emphysema can be detected by palpating the chest wall, which causes the air bubble to move and produce crackling sounds. Most cases of subcutaneous emphysema are diagnosed clinically. Chest radiography and computed tomography help identify the source of air leak. Ultrasonography is usually used in cases of blunt trauma to the chest as part of the Focal Assessment With Sonography for Trauma protocol.11
Subcutaneous emphysema can resolve spontaneously, requiring only pain management and supplemental oxygen.12 In severe cases, air collection can lead to what is called “massive subcutaneous emphysema,” which requires surgical drainage.
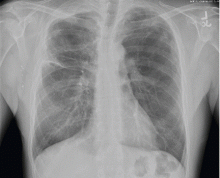
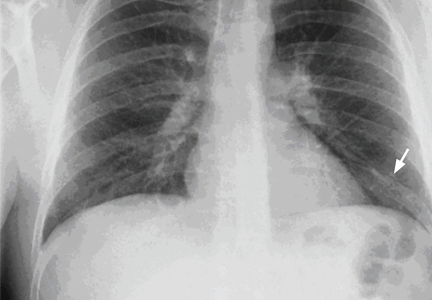
Our patient had large emphysematous bullae in the apical region of the right lung that ruptured and led to subcutaneous emphysema. After placement of a chest tube, he underwent right-sided thoracotomy with bullectomy. His postoperative course was uneventful, and he was discharged a few days later. Three weeks later, repeated chest radiography showed resolution of his subcutaneous emphysema (Figure 4).
- White CS, Templeton PA, Attar S. Esophageal perforation: CT findings. AJR Am J Roentgenol 1993; 160:767–770.
- Aggelidakis J, Lasithiotakis K, Topalidou A, Koutroumpas J, Kouvidis G, Katonis P. Limb salvage after gas gangrene: a case report and review of the literature. World J Emerg Surg 2011; 6:28.
- Romero KJ, Trujillo MH. Spontaneous pneumomediastinum and subcutaneous emphysema in asthma exacerbation: the Macklin effect. Heart Lung 2010; 39:444–447.
- Peart O. Subcutaneous emphysema. Radiol Technol 2006; 77:296.
- Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med 2006; 12:268–272.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest 2005; 128:720–728.
- Yalçinkaya S, Vural AH, Göncü MT, Özyazicioglu AF. Cavitary lung cancer presenting as subcutaneous emphysema on the contralateral side. Interact Cardiovasc Thorac Surg 2012; 14:338–339.
- Shamaei M, Tabarsi P, Pojhan S, et al. Tuberculosis-associated secondary pneumothorax: a retrospective study of 53 patients. Respir Care 2011; 56:298–302.
- Sherif HM, Ott DA. The use of subcutaneous drains to manage subcutaneous emphysema. Tex Heart Inst J 1999; 26:129–131.
- Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17:11–17.
- Mattox KL, Allen MK. Systematic approach to pneumothorax, haemothorax, pneumomediastinum and subcutaneous emphysema. Injury 1986; 17:309–312.
A 47-year-old man presented with acute shortness of breath and chest and neck pain, which began after he heard popping sounds while boarding a bus. The pain was right-sided, sharp, worse with deep breathing, and associated with a sensation of fullness over the right chest.
His medical conditions included controlled hypertension, gastroesophageal reflux disease, and chronic obstructive pulmonary disease (COPD). The COPD was managed with an albuterol inhaler only. He had a 50-pack-year history of smoking, and he drank alcohol occasionally.
On arrival, he was in mild respiratory distress, but his vital signs were stable. We could hear wheezing on both sides of his chest and feel subcutaneous crepitation on both sides of his chest and neck, the latter more on the right side. The rest of the examination was unremarkable.
Results of a complete blood cell count and metabolic panel were within normal limits. Because of the above findings, nasopharyngeal radiogragraphy was ordered (Figures 1 and 2).
Q: What is the most likely cause of this presentation?
- Esophageal rupture
- Gas gangrene
- Asthma exacerbation
- Ruptured emphysematous bullae
A: This patient had a history of COPD, which put him at risk of developing bullous emphysematous bullae that can rupture and cause subcutaneous emphysema. His nasopharyngeal radiograph (Figure1) showed bilateral extensive subcutaneous emphysema. His lateral nasopharyngeal radiograph (Figure 2) showed air-tracking within the mediastinum and into the retropharyngeal space (arrow). Computed tomography (Figure 3) showed extensive subcutaneous emphysema in the right lateral chest wall (arrow) and large bullae in the right upper lobe (arrow heads). As for the other possibilities:
Esophageal ruptures and tears are iatrogenic in most cases and usually occur after endoscopic procedures, but they can also occur in patients with intractable vomiting. Computed tomography often shows esophageal thickening, periesophageal fluid, mediastinal widening, and extraluminal air. However, in most cases, it is seen as pneumomediastinum and subcutaneous emphysema.1
Gas gangrene is a life-threatening soft-tissue and muscle infection caused by Clostridium perfringens in most cases.2 The pain is out of proportion to the findings on physical examination. Patients usually have toxic signs and symptoms such as fever and hypotension. Our patient was hemodynamically stable, with no changes in skin color.
Severe exacerbations of asthma can lead to alveolar rupture, pneumothorax, and subcutaneous emphysema, although this is a rare complication. Air can dissect along the bronchovascular sheaths into the neck and cause subcutaneous emphysema, or into the pleural space and cause pneumothorax. Our patient had no history of asthma and plainly had emphysematous bullae.3
SUBCUTANEOUS EMPHYSEMA
Subcutaneous emphysema is a collection of air within subcutaneous tissues. It usually presents as bloating of the skin around the neck and the chest wall. It is often seen in patients with pneumothorax.
The most common cause of subcutaneous emphysema is traumatic injury to the chest wall, such as from a motor vehicle accident or a stab wound,4 but it can also occur spontaneously in patients who have severe emphysema with large bullae. As the emphysema progresses, the bullae can easily rupture, and this can lead to pneumothorax, which can lead to subcutaneous emphysema. Primary spontaneous pneumothorax and subcutaneous emphysema can occur in people who have unrecognized lung disease and genetic disorders such as Marfan syndrome and Ehler-Danlos syndrome.5 Other causes include iatrogenic injury, Pneumocystis jirovecii pneumonia (common in patients with human immunodeficiency virus infection), and cystic fibrosis. Pneumothorax occurs in about 30% of cases of P jirovecii pneumonia,6 and in about 6% of patients with cystic fibrosis.7 Bronchocutaneous fistula is an extremely rare complication of lung cancer and can cause subcutaneous emphysema.8 Tuberculosis is another possible cause.9
Subcutaneous emphysema mainly presents with chest or neck pain and wheezing. In severe cases, air can track to the face, causing facial swelling and difficulty breathing due to compression of the larynx. Also, it can track down to the thighs, causing leg pain and swelling.10
On examination, subcutaneous emphysema can be detected by palpating the chest wall, which causes the air bubble to move and produce crackling sounds. Most cases of subcutaneous emphysema are diagnosed clinically. Chest radiography and computed tomography help identify the source of air leak. Ultrasonography is usually used in cases of blunt trauma to the chest as part of the Focal Assessment With Sonography for Trauma protocol.11
Subcutaneous emphysema can resolve spontaneously, requiring only pain management and supplemental oxygen.12 In severe cases, air collection can lead to what is called “massive subcutaneous emphysema,” which requires surgical drainage.
Our patient had large emphysematous bullae in the apical region of the right lung that ruptured and led to subcutaneous emphysema. After placement of a chest tube, he underwent right-sided thoracotomy with bullectomy. His postoperative course was uneventful, and he was discharged a few days later. Three weeks later, repeated chest radiography showed resolution of his subcutaneous emphysema (Figure 4).
A 47-year-old man presented with acute shortness of breath and chest and neck pain, which began after he heard popping sounds while boarding a bus. The pain was right-sided, sharp, worse with deep breathing, and associated with a sensation of fullness over the right chest.
His medical conditions included controlled hypertension, gastroesophageal reflux disease, and chronic obstructive pulmonary disease (COPD). The COPD was managed with an albuterol inhaler only. He had a 50-pack-year history of smoking, and he drank alcohol occasionally.
On arrival, he was in mild respiratory distress, but his vital signs were stable. We could hear wheezing on both sides of his chest and feel subcutaneous crepitation on both sides of his chest and neck, the latter more on the right side. The rest of the examination was unremarkable.
Results of a complete blood cell count and metabolic panel were within normal limits. Because of the above findings, nasopharyngeal radiogragraphy was ordered (Figures 1 and 2).
Q: What is the most likely cause of this presentation?
- Esophageal rupture
- Gas gangrene
- Asthma exacerbation
- Ruptured emphysematous bullae
A: This patient had a history of COPD, which put him at risk of developing bullous emphysematous bullae that can rupture and cause subcutaneous emphysema. His nasopharyngeal radiograph (Figure1) showed bilateral extensive subcutaneous emphysema. His lateral nasopharyngeal radiograph (Figure 2) showed air-tracking within the mediastinum and into the retropharyngeal space (arrow). Computed tomography (Figure 3) showed extensive subcutaneous emphysema in the right lateral chest wall (arrow) and large bullae in the right upper lobe (arrow heads). As for the other possibilities:
Esophageal ruptures and tears are iatrogenic in most cases and usually occur after endoscopic procedures, but they can also occur in patients with intractable vomiting. Computed tomography often shows esophageal thickening, periesophageal fluid, mediastinal widening, and extraluminal air. However, in most cases, it is seen as pneumomediastinum and subcutaneous emphysema.1
Gas gangrene is a life-threatening soft-tissue and muscle infection caused by Clostridium perfringens in most cases.2 The pain is out of proportion to the findings on physical examination. Patients usually have toxic signs and symptoms such as fever and hypotension. Our patient was hemodynamically stable, with no changes in skin color.
Severe exacerbations of asthma can lead to alveolar rupture, pneumothorax, and subcutaneous emphysema, although this is a rare complication. Air can dissect along the bronchovascular sheaths into the neck and cause subcutaneous emphysema, or into the pleural space and cause pneumothorax. Our patient had no history of asthma and plainly had emphysematous bullae.3
SUBCUTANEOUS EMPHYSEMA
Subcutaneous emphysema is a collection of air within subcutaneous tissues. It usually presents as bloating of the skin around the neck and the chest wall. It is often seen in patients with pneumothorax.
The most common cause of subcutaneous emphysema is traumatic injury to the chest wall, such as from a motor vehicle accident or a stab wound,4 but it can also occur spontaneously in patients who have severe emphysema with large bullae. As the emphysema progresses, the bullae can easily rupture, and this can lead to pneumothorax, which can lead to subcutaneous emphysema. Primary spontaneous pneumothorax and subcutaneous emphysema can occur in people who have unrecognized lung disease and genetic disorders such as Marfan syndrome and Ehler-Danlos syndrome.5 Other causes include iatrogenic injury, Pneumocystis jirovecii pneumonia (common in patients with human immunodeficiency virus infection), and cystic fibrosis. Pneumothorax occurs in about 30% of cases of P jirovecii pneumonia,6 and in about 6% of patients with cystic fibrosis.7 Bronchocutaneous fistula is an extremely rare complication of lung cancer and can cause subcutaneous emphysema.8 Tuberculosis is another possible cause.9
Subcutaneous emphysema mainly presents with chest or neck pain and wheezing. In severe cases, air can track to the face, causing facial swelling and difficulty breathing due to compression of the larynx. Also, it can track down to the thighs, causing leg pain and swelling.10
On examination, subcutaneous emphysema can be detected by palpating the chest wall, which causes the air bubble to move and produce crackling sounds. Most cases of subcutaneous emphysema are diagnosed clinically. Chest radiography and computed tomography help identify the source of air leak. Ultrasonography is usually used in cases of blunt trauma to the chest as part of the Focal Assessment With Sonography for Trauma protocol.11
Subcutaneous emphysema can resolve spontaneously, requiring only pain management and supplemental oxygen.12 In severe cases, air collection can lead to what is called “massive subcutaneous emphysema,” which requires surgical drainage.
Our patient had large emphysematous bullae in the apical region of the right lung that ruptured and led to subcutaneous emphysema. After placement of a chest tube, he underwent right-sided thoracotomy with bullectomy. His postoperative course was uneventful, and he was discharged a few days later. Three weeks later, repeated chest radiography showed resolution of his subcutaneous emphysema (Figure 4).
- White CS, Templeton PA, Attar S. Esophageal perforation: CT findings. AJR Am J Roentgenol 1993; 160:767–770.
- Aggelidakis J, Lasithiotakis K, Topalidou A, Koutroumpas J, Kouvidis G, Katonis P. Limb salvage after gas gangrene: a case report and review of the literature. World J Emerg Surg 2011; 6:28.
- Romero KJ, Trujillo MH. Spontaneous pneumomediastinum and subcutaneous emphysema in asthma exacerbation: the Macklin effect. Heart Lung 2010; 39:444–447.
- Peart O. Subcutaneous emphysema. Radiol Technol 2006; 77:296.
- Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med 2006; 12:268–272.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest 2005; 128:720–728.
- Yalçinkaya S, Vural AH, Göncü MT, Özyazicioglu AF. Cavitary lung cancer presenting as subcutaneous emphysema on the contralateral side. Interact Cardiovasc Thorac Surg 2012; 14:338–339.
- Shamaei M, Tabarsi P, Pojhan S, et al. Tuberculosis-associated secondary pneumothorax: a retrospective study of 53 patients. Respir Care 2011; 56:298–302.
- Sherif HM, Ott DA. The use of subcutaneous drains to manage subcutaneous emphysema. Tex Heart Inst J 1999; 26:129–131.
- Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17:11–17.
- Mattox KL, Allen MK. Systematic approach to pneumothorax, haemothorax, pneumomediastinum and subcutaneous emphysema. Injury 1986; 17:309–312.
- White CS, Templeton PA, Attar S. Esophageal perforation: CT findings. AJR Am J Roentgenol 1993; 160:767–770.
- Aggelidakis J, Lasithiotakis K, Topalidou A, Koutroumpas J, Kouvidis G, Katonis P. Limb salvage after gas gangrene: a case report and review of the literature. World J Emerg Surg 2011; 6:28.
- Romero KJ, Trujillo MH. Spontaneous pneumomediastinum and subcutaneous emphysema in asthma exacerbation: the Macklin effect. Heart Lung 2010; 39:444–447.
- Peart O. Subcutaneous emphysema. Radiol Technol 2006; 77:296.
- Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med 2006; 12:268–272.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest 2005; 128:720–728.
- Yalçinkaya S, Vural AH, Göncü MT, Özyazicioglu AF. Cavitary lung cancer presenting as subcutaneous emphysema on the contralateral side. Interact Cardiovasc Thorac Surg 2012; 14:338–339.
- Shamaei M, Tabarsi P, Pojhan S, et al. Tuberculosis-associated secondary pneumothorax: a retrospective study of 53 patients. Respir Care 2011; 56:298–302.
- Sherif HM, Ott DA. The use of subcutaneous drains to manage subcutaneous emphysema. Tex Heart Inst J 1999; 26:129–131.
- Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17:11–17.
- Mattox KL, Allen MK. Systematic approach to pneumothorax, haemothorax, pneumomediastinum and subcutaneous emphysema. Injury 1986; 17:309–312.
Superior vena cava syndrome
A 60-year-old man presented with progressive swelling of his face and neck, which had begun 2 weeks earlier. He denied any headache, lightheadedness, blurry vision, syncope, or change in his cognitive or memory function. A review of symptoms was unremarkable.
The patient had hypertension and end-stage renal disease, for which he was receiving hemodialysis via a catheter tunneled into his right internal jugular vein. He had undergone multiple unsuccessful attempts to create an arteriovenous fistula over the previous 2 years.
Doppler ultrasonography revealed chronic thrombosis and reverse flow in the right internal jugular vein and reverse flow in the right subclavian vein. These findings were consistent with central venous thrombosis and superior vena cava (SVC) syndrome.
Diagnosis: SVC syndrome secondary to intravascular thrombosis related to his central venous dialysis catheter.
SVC SYNDROME
The SVC is the major drainage vessel for venous blood from the head, neck, upper extremities, and upper thorax. Obstruction to its flow increases venous pressure, which results in interstitial edema and retrograde collateral flow.1
More than 80% of cases of SVC syndrome are caused by malignant lung tumors and lymphoma.
Nonmalignant causes include mediastinal fibrosis; vascular diseases (eg, aortic aneurysm, large-vessel vasculitis); infections such as histoplasmosis, tuberculosis, syphilis, and actinomycosis; benign mediastinal tumors such as teratoma, cystic hygroma, thymoma, and dermoid cyst; and thrombosis from central venous catheters, pacemaker leads, and guidewires.2–6 A recent report suggests that benign causes may now account for up to 40% of cases as a result of a rise in the use of indwelling central venous catheters and cardiac pacemakers during the past 2 decades, resulting in a higher incidence of SVC thrombosis.7
An obstructed SVC initiates collateral venous return to the heart from the upper half of the body through different pathways. The most important pathway is the azygos venous system, which includes the azygos vein. Occlusion of the SVC at the level of the azygos vein contributes to the appearance of collateral veins on the chest and abdominal walls, and venous blood flows via these collaterals into the inferior vena cava.1,8,9
Different presentations
The diagnosis of SVC syndrome is often made on clinical grounds alone, ie, the combination of the clinical presentation and, often, a thoracic malignancy or contributing factors such as a central catheter.1
With slowly progressive obstruction of the SVC, the most common presenting symptoms include swelling of the face, neck, and both arms. On the other hand, adequate collateral drainage may develop,1 and patients may have minimal symptoms.
However, a rapid onset of SVC syndrome in the absence of collateral circulation will cause a more dramatic and life-threatening presentation, often with neurologic and respiratory sequelae such as cerebral and laryngeal edema and respiratory embarrassment, which were not present in our patient’s case.1,10–15 These serious complications are rare and are considered an acute emergency. In these cases, special attention to airway, breathing, and circulation (the “ABCs”) is essential, and endovascular repairs and stenting or open surgical reconstruction and alternate approaches for renal replacement therapy should be considered.1,12,13,15
CT is diagnostic and provides accurate information about the location of the obstruction and about other critical surrounding structures such as the lungs, mediastinum, and adjacent blood vessels.1,7,10,11 Our patient’s CT scan confirmed a significant stenosis of the SVC due to thrombosis, with no compression coming from the lungs or mediastinal structures.
Thrombolytic therapy in acute cases
In cases of acute thrombosis (with symptom onset less than 2 days previously), thrombolytic therapy followed by anticoagulation is recommended and may both cause the symptoms to regress within several days and allow the central catheter to be kept in.16 However, thrombolytic therapy is less effective in chronic thrombosis (with onset of symptoms more than 10 days previously).16
Vascular or surgical intervention is often needed to treat SVC syndrome related to dialysis access.
Most experts recommend anticoagulation after thrombosis to prevent disease progression and recurrence, although the benefit of either short-term or long-term anticoagulation therapy for this syndrome is unclear.16
Recommended treatments for cancer-related SVC syndrome include chemotherapy and radiation to shrink the tumor that is causing the obstruction. Tissue diagnosis is often necessary to direct treatment decisions.1 However, percutaneous angioplasty and the use of intravenous stents are becoming increasingly common and are simple, safe, and effective in rapidly relieving SVC syndrome caused by malignant diseases.1 A bypass of the SVC may be indicated in some cases.1 Adjunctive therapies include diuretics, corticosteroids, thrombolytics, anticoagulation, and elevating the head of the patient’s bed.1
CASE CONTINUED
Our patient was started on heparin intravenously for 7 days and long-term oral anticoagulant therapy with warfarin (Coumadin) to continue as long as the catheter was in place, with a target international normalized ratio between 2 and 2.5. He required no other interventions, and his dialysis catheter remained functioning. He was monitored in the hospital for 2 weeks, during which his symptoms gradually improved, with noticeable resolution of his facial swelling.
He was discharged home to continue on an oral anticoagulant and was then followed to monitor for a reappearance of the symptoms (which would force the removal of the catheter), and to pursue possible percutaneous angioplasty, stenting, or surgical reconstruction of the SVC if needed.
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med 2007; 356:1862–1869.
- Parish JM, Marschke RF, Dines DE, Lee RE. Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc 1981; 56:407–413.
- Aurora R, Milite F, Vander Els NJ. Respiratory emergencies. Semin Oncol 2000; 27:256–269.
- Markman M. Diagnosis and management of superior vena cava syndrome. Cleve Clin J Med 1999; 66:59–61.
- Khanna S, Sniderman K, Simons M, Besley M, Uldall R. Superior vena cava stenosis associated with hemodialysis catheters. Am J Kidney Dis 1993; 21:278–281.
- Bertrand M, Presant CA, Klein L, Scott E. Iatrogenic superior vena cava syndrome. A new entity. Cancer 1984; 54:376–378.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006; 85:37–42.
- Plekker D, Ellis T, Irusen EM, Bolliger CT, Diacon AH. Clinical and radiological grading of superior vena cava obstruction. Respiration 2008; 76:69–75.
- Sheth S, Ebert MD, Fishman EK. Superior vena cava obstruction evaluation with MDCT. AJR Am J Roentgenol 2010; 194:W336–W346.
- DeMichele A, Glick J. Cancer-related emergencies. In:Lenhard R, Osteen R, Gansler T, eds. Clinical Oncology. Atlanta, GA: American Cancer Society; 2001:733–764.
- Chen JC, Bongard F, Klein SR. A contemporary perspective on superior vena cava syndrome. Am J Surg 1990; 160:207–211.
- Sheikh MA, Fernandez BB, Gray BH, Graham LM, Carman TL. Endovascular stenting of nonmalignant superior vena cava syndrome. Catheter Cardiovasc Interv 2005; 65:405–411.
- Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008; 6:1262–1266.
- Greenberg S, Kosinski R, Daniels J. Treatment of superior vena cava thrombosis with recombinant tissue type plasminogen activator. Chest 1991; 99:1298–1301.
- Molhem A, Sabry A, Bawadekji H, Al Saran K. Superior vena cava syndrome in hemodialysis patient. Saudi J Kidney Dis Transpl 2011; 22:381–386.
- Akoglu H, Yilmaz R, Peynircioglu B, et al. A rare complication of hemodialysis catheters: superior vena cava syndrome. Hemodial Int 2007; 11:385–391.
A 60-year-old man presented with progressive swelling of his face and neck, which had begun 2 weeks earlier. He denied any headache, lightheadedness, blurry vision, syncope, or change in his cognitive or memory function. A review of symptoms was unremarkable.
The patient had hypertension and end-stage renal disease, for which he was receiving hemodialysis via a catheter tunneled into his right internal jugular vein. He had undergone multiple unsuccessful attempts to create an arteriovenous fistula over the previous 2 years.
Doppler ultrasonography revealed chronic thrombosis and reverse flow in the right internal jugular vein and reverse flow in the right subclavian vein. These findings were consistent with central venous thrombosis and superior vena cava (SVC) syndrome.
Diagnosis: SVC syndrome secondary to intravascular thrombosis related to his central venous dialysis catheter.
SVC SYNDROME
The SVC is the major drainage vessel for venous blood from the head, neck, upper extremities, and upper thorax. Obstruction to its flow increases venous pressure, which results in interstitial edema and retrograde collateral flow.1
More than 80% of cases of SVC syndrome are caused by malignant lung tumors and lymphoma.
Nonmalignant causes include mediastinal fibrosis; vascular diseases (eg, aortic aneurysm, large-vessel vasculitis); infections such as histoplasmosis, tuberculosis, syphilis, and actinomycosis; benign mediastinal tumors such as teratoma, cystic hygroma, thymoma, and dermoid cyst; and thrombosis from central venous catheters, pacemaker leads, and guidewires.2–6 A recent report suggests that benign causes may now account for up to 40% of cases as a result of a rise in the use of indwelling central venous catheters and cardiac pacemakers during the past 2 decades, resulting in a higher incidence of SVC thrombosis.7
An obstructed SVC initiates collateral venous return to the heart from the upper half of the body through different pathways. The most important pathway is the azygos venous system, which includes the azygos vein. Occlusion of the SVC at the level of the azygos vein contributes to the appearance of collateral veins on the chest and abdominal walls, and venous blood flows via these collaterals into the inferior vena cava.1,8,9
Different presentations
The diagnosis of SVC syndrome is often made on clinical grounds alone, ie, the combination of the clinical presentation and, often, a thoracic malignancy or contributing factors such as a central catheter.1
With slowly progressive obstruction of the SVC, the most common presenting symptoms include swelling of the face, neck, and both arms. On the other hand, adequate collateral drainage may develop,1 and patients may have minimal symptoms.
However, a rapid onset of SVC syndrome in the absence of collateral circulation will cause a more dramatic and life-threatening presentation, often with neurologic and respiratory sequelae such as cerebral and laryngeal edema and respiratory embarrassment, which were not present in our patient’s case.1,10–15 These serious complications are rare and are considered an acute emergency. In these cases, special attention to airway, breathing, and circulation (the “ABCs”) is essential, and endovascular repairs and stenting or open surgical reconstruction and alternate approaches for renal replacement therapy should be considered.1,12,13,15
CT is diagnostic and provides accurate information about the location of the obstruction and about other critical surrounding structures such as the lungs, mediastinum, and adjacent blood vessels.1,7,10,11 Our patient’s CT scan confirmed a significant stenosis of the SVC due to thrombosis, with no compression coming from the lungs or mediastinal structures.
Thrombolytic therapy in acute cases
In cases of acute thrombosis (with symptom onset less than 2 days previously), thrombolytic therapy followed by anticoagulation is recommended and may both cause the symptoms to regress within several days and allow the central catheter to be kept in.16 However, thrombolytic therapy is less effective in chronic thrombosis (with onset of symptoms more than 10 days previously).16
Vascular or surgical intervention is often needed to treat SVC syndrome related to dialysis access.
Most experts recommend anticoagulation after thrombosis to prevent disease progression and recurrence, although the benefit of either short-term or long-term anticoagulation therapy for this syndrome is unclear.16
Recommended treatments for cancer-related SVC syndrome include chemotherapy and radiation to shrink the tumor that is causing the obstruction. Tissue diagnosis is often necessary to direct treatment decisions.1 However, percutaneous angioplasty and the use of intravenous stents are becoming increasingly common and are simple, safe, and effective in rapidly relieving SVC syndrome caused by malignant diseases.1 A bypass of the SVC may be indicated in some cases.1 Adjunctive therapies include diuretics, corticosteroids, thrombolytics, anticoagulation, and elevating the head of the patient’s bed.1
CASE CONTINUED
Our patient was started on heparin intravenously for 7 days and long-term oral anticoagulant therapy with warfarin (Coumadin) to continue as long as the catheter was in place, with a target international normalized ratio between 2 and 2.5. He required no other interventions, and his dialysis catheter remained functioning. He was monitored in the hospital for 2 weeks, during which his symptoms gradually improved, with noticeable resolution of his facial swelling.
He was discharged home to continue on an oral anticoagulant and was then followed to monitor for a reappearance of the symptoms (which would force the removal of the catheter), and to pursue possible percutaneous angioplasty, stenting, or surgical reconstruction of the SVC if needed.
A 60-year-old man presented with progressive swelling of his face and neck, which had begun 2 weeks earlier. He denied any headache, lightheadedness, blurry vision, syncope, or change in his cognitive or memory function. A review of symptoms was unremarkable.
The patient had hypertension and end-stage renal disease, for which he was receiving hemodialysis via a catheter tunneled into his right internal jugular vein. He had undergone multiple unsuccessful attempts to create an arteriovenous fistula over the previous 2 years.
Doppler ultrasonography revealed chronic thrombosis and reverse flow in the right internal jugular vein and reverse flow in the right subclavian vein. These findings were consistent with central venous thrombosis and superior vena cava (SVC) syndrome.
Diagnosis: SVC syndrome secondary to intravascular thrombosis related to his central venous dialysis catheter.
SVC SYNDROME
The SVC is the major drainage vessel for venous blood from the head, neck, upper extremities, and upper thorax. Obstruction to its flow increases venous pressure, which results in interstitial edema and retrograde collateral flow.1
More than 80% of cases of SVC syndrome are caused by malignant lung tumors and lymphoma.
Nonmalignant causes include mediastinal fibrosis; vascular diseases (eg, aortic aneurysm, large-vessel vasculitis); infections such as histoplasmosis, tuberculosis, syphilis, and actinomycosis; benign mediastinal tumors such as teratoma, cystic hygroma, thymoma, and dermoid cyst; and thrombosis from central venous catheters, pacemaker leads, and guidewires.2–6 A recent report suggests that benign causes may now account for up to 40% of cases as a result of a rise in the use of indwelling central venous catheters and cardiac pacemakers during the past 2 decades, resulting in a higher incidence of SVC thrombosis.7
An obstructed SVC initiates collateral venous return to the heart from the upper half of the body through different pathways. The most important pathway is the azygos venous system, which includes the azygos vein. Occlusion of the SVC at the level of the azygos vein contributes to the appearance of collateral veins on the chest and abdominal walls, and venous blood flows via these collaterals into the inferior vena cava.1,8,9
Different presentations
The diagnosis of SVC syndrome is often made on clinical grounds alone, ie, the combination of the clinical presentation and, often, a thoracic malignancy or contributing factors such as a central catheter.1
With slowly progressive obstruction of the SVC, the most common presenting symptoms include swelling of the face, neck, and both arms. On the other hand, adequate collateral drainage may develop,1 and patients may have minimal symptoms.
However, a rapid onset of SVC syndrome in the absence of collateral circulation will cause a more dramatic and life-threatening presentation, often with neurologic and respiratory sequelae such as cerebral and laryngeal edema and respiratory embarrassment, which were not present in our patient’s case.1,10–15 These serious complications are rare and are considered an acute emergency. In these cases, special attention to airway, breathing, and circulation (the “ABCs”) is essential, and endovascular repairs and stenting or open surgical reconstruction and alternate approaches for renal replacement therapy should be considered.1,12,13,15
CT is diagnostic and provides accurate information about the location of the obstruction and about other critical surrounding structures such as the lungs, mediastinum, and adjacent blood vessels.1,7,10,11 Our patient’s CT scan confirmed a significant stenosis of the SVC due to thrombosis, with no compression coming from the lungs or mediastinal structures.
Thrombolytic therapy in acute cases
In cases of acute thrombosis (with symptom onset less than 2 days previously), thrombolytic therapy followed by anticoagulation is recommended and may both cause the symptoms to regress within several days and allow the central catheter to be kept in.16 However, thrombolytic therapy is less effective in chronic thrombosis (with onset of symptoms more than 10 days previously).16
Vascular or surgical intervention is often needed to treat SVC syndrome related to dialysis access.
Most experts recommend anticoagulation after thrombosis to prevent disease progression and recurrence, although the benefit of either short-term or long-term anticoagulation therapy for this syndrome is unclear.16
Recommended treatments for cancer-related SVC syndrome include chemotherapy and radiation to shrink the tumor that is causing the obstruction. Tissue diagnosis is often necessary to direct treatment decisions.1 However, percutaneous angioplasty and the use of intravenous stents are becoming increasingly common and are simple, safe, and effective in rapidly relieving SVC syndrome caused by malignant diseases.1 A bypass of the SVC may be indicated in some cases.1 Adjunctive therapies include diuretics, corticosteroids, thrombolytics, anticoagulation, and elevating the head of the patient’s bed.1
CASE CONTINUED
Our patient was started on heparin intravenously for 7 days and long-term oral anticoagulant therapy with warfarin (Coumadin) to continue as long as the catheter was in place, with a target international normalized ratio between 2 and 2.5. He required no other interventions, and his dialysis catheter remained functioning. He was monitored in the hospital for 2 weeks, during which his symptoms gradually improved, with noticeable resolution of his facial swelling.
He was discharged home to continue on an oral anticoagulant and was then followed to monitor for a reappearance of the symptoms (which would force the removal of the catheter), and to pursue possible percutaneous angioplasty, stenting, or surgical reconstruction of the SVC if needed.
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med 2007; 356:1862–1869.
- Parish JM, Marschke RF, Dines DE, Lee RE. Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc 1981; 56:407–413.
- Aurora R, Milite F, Vander Els NJ. Respiratory emergencies. Semin Oncol 2000; 27:256–269.
- Markman M. Diagnosis and management of superior vena cava syndrome. Cleve Clin J Med 1999; 66:59–61.
- Khanna S, Sniderman K, Simons M, Besley M, Uldall R. Superior vena cava stenosis associated with hemodialysis catheters. Am J Kidney Dis 1993; 21:278–281.
- Bertrand M, Presant CA, Klein L, Scott E. Iatrogenic superior vena cava syndrome. A new entity. Cancer 1984; 54:376–378.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006; 85:37–42.
- Plekker D, Ellis T, Irusen EM, Bolliger CT, Diacon AH. Clinical and radiological grading of superior vena cava obstruction. Respiration 2008; 76:69–75.
- Sheth S, Ebert MD, Fishman EK. Superior vena cava obstruction evaluation with MDCT. AJR Am J Roentgenol 2010; 194:W336–W346.
- DeMichele A, Glick J. Cancer-related emergencies. In:Lenhard R, Osteen R, Gansler T, eds. Clinical Oncology. Atlanta, GA: American Cancer Society; 2001:733–764.
- Chen JC, Bongard F, Klein SR. A contemporary perspective on superior vena cava syndrome. Am J Surg 1990; 160:207–211.
- Sheikh MA, Fernandez BB, Gray BH, Graham LM, Carman TL. Endovascular stenting of nonmalignant superior vena cava syndrome. Catheter Cardiovasc Interv 2005; 65:405–411.
- Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008; 6:1262–1266.
- Greenberg S, Kosinski R, Daniels J. Treatment of superior vena cava thrombosis with recombinant tissue type plasminogen activator. Chest 1991; 99:1298–1301.
- Molhem A, Sabry A, Bawadekji H, Al Saran K. Superior vena cava syndrome in hemodialysis patient. Saudi J Kidney Dis Transpl 2011; 22:381–386.
- Akoglu H, Yilmaz R, Peynircioglu B, et al. A rare complication of hemodialysis catheters: superior vena cava syndrome. Hemodial Int 2007; 11:385–391.
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med 2007; 356:1862–1869.
- Parish JM, Marschke RF, Dines DE, Lee RE. Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc 1981; 56:407–413.
- Aurora R, Milite F, Vander Els NJ. Respiratory emergencies. Semin Oncol 2000; 27:256–269.
- Markman M. Diagnosis and management of superior vena cava syndrome. Cleve Clin J Med 1999; 66:59–61.
- Khanna S, Sniderman K, Simons M, Besley M, Uldall R. Superior vena cava stenosis associated with hemodialysis catheters. Am J Kidney Dis 1993; 21:278–281.
- Bertrand M, Presant CA, Klein L, Scott E. Iatrogenic superior vena cava syndrome. A new entity. Cancer 1984; 54:376–378.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006; 85:37–42.
- Plekker D, Ellis T, Irusen EM, Bolliger CT, Diacon AH. Clinical and radiological grading of superior vena cava obstruction. Respiration 2008; 76:69–75.
- Sheth S, Ebert MD, Fishman EK. Superior vena cava obstruction evaluation with MDCT. AJR Am J Roentgenol 2010; 194:W336–W346.
- DeMichele A, Glick J. Cancer-related emergencies. In:Lenhard R, Osteen R, Gansler T, eds. Clinical Oncology. Atlanta, GA: American Cancer Society; 2001:733–764.
- Chen JC, Bongard F, Klein SR. A contemporary perspective on superior vena cava syndrome. Am J Surg 1990; 160:207–211.
- Sheikh MA, Fernandez BB, Gray BH, Graham LM, Carman TL. Endovascular stenting of nonmalignant superior vena cava syndrome. Catheter Cardiovasc Interv 2005; 65:405–411.
- Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008; 6:1262–1266.
- Greenberg S, Kosinski R, Daniels J. Treatment of superior vena cava thrombosis with recombinant tissue type plasminogen activator. Chest 1991; 99:1298–1301.
- Molhem A, Sabry A, Bawadekji H, Al Saran K. Superior vena cava syndrome in hemodialysis patient. Saudi J Kidney Dis Transpl 2011; 22:381–386.
- Akoglu H, Yilmaz R, Peynircioglu B, et al. A rare complication of hemodialysis catheters: superior vena cava syndrome. Hemodial Int 2007; 11:385–391.
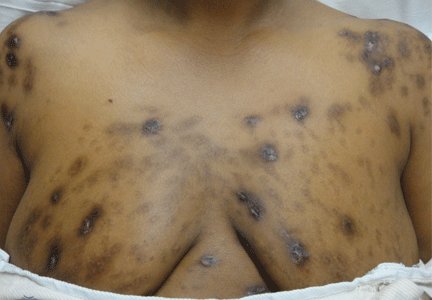
Hyperpigmentation and hypotension
A 65-year-old man presents with a 2-month history of generalized weakness, dizziness, and blurred vision. His symptoms began gradually and have been progressing over the last few weeks, so that they now affect his ability to perform normal daily activities.
He has lost 20 lb and has become anorectic. He has no fever, night sweats, headache, cough, hemoptysis, or dyspnea. He has no history of abdominal pain, changes in bowel habits, nausea, vomiting, or urinary symptoms. He was admitted 6 weeks ago for the same symptoms; he was treated for hypotension and received intravenous (IV) fluids and electrolyte supplements for dehydration.
He has a history of hypertension, stroke, vascular dementia, and atrial fibrillation. He is taking warfarin (Coumadin), extended-release diltiazem (Cardizem), simvastatin (Zocor), and donepezil (Aricept). He underwent right hemicolectomy 5 years ago for a large tubular adenoma with high-grade dysplasia in the cecum.
Initial laboratory values are as follows:
- White blood cell count 7.4 × 109/L (reference range 4.5–11.0), with a normal differential
- Mild anemia, with a hemoglobin of 116 g/L (140–175)
- Activated partial thromboplastin time 59.9 sec (23.0–32.4)
- Serum sodium 135 mmol/L (136–142)
- Serum potassium 4.6 mmol/L (3.5–5.0)
- Aspartate aminotransferase 58 U/L (10–30)
- Alanine aminotransferase 16 U/L (10–40)
- Alkaline phosphatase 328 U/L (30–120)
- Urea, creatinine, and corrected calcium are normal.
Electrocardiography shows atrial fibrillation with low-voltage QRS complexes. Chest radiography is normal. A stool test is negative for occult blood. A workup for sepsis is negative.
Q: Which is the appropriate test at this point to determine the cause of the hypotension?
- Serum parathyroid-hormone-related protein
- Baseline serum cortisol, plasma adrenocorticotropic hormone (ACTH) levels, and an ACTH stimulation test with cosyntropin (Cortrosyn)
- Serum thyrotropin level
- Aspiration biopsy of subcutaneous fat with Congo red and immunostaining
- Late-night salivary cortisol
A: The correct next step is to measure baseline serum cortisol, to test ACTH levels, and to order an ACTH stimulation test with cosyntropin.
Primary adrenocortical insufficiency should be considered in patients with metastatic malignancy who present with peripheral vascular collapse, particularly when it is associated with cutaneous hyperpigmentation, chronic malaise, fatigue, weakness, anorexia, weight loss, hypoglycemia, and electrolyte disturbances such as hyponatremia and hyperkalemia.
Checking the baseline serum cortisol and ACTH levels and cosyntropin stimulation testing are vital steps in making an early diagnosis of primary adrenocortical insufficiency. Inappropriately low serum cortisol is highly suggestive of primary adrenal insufficiency, especially if accompanied by simultaneous elevation of the plasma ACTH level. The result of the ACTH stimulation test with cosyntropin is often confirmatory.
Measuring the serum parathyroid-hormone-related protein level is not indicated, since the patient has a normal corrected calcium. Patients with ectopic Cushing syndrome may present with weight loss due to underlying malignancy, but the presence of hypotension and a lack of hypokalemia makes such a diagnosis unlikely, and, therefore, measurement of late-night salivary cortisol is not the best answer. Amyloidosis, hypothyroidism, or hyperthyroidism are unlikely to have this patient’s presentation.
RESULTS OF FURTHER EVALUATION
Our patient’s ACTH serum level was elevated, and an ACTH stimulation test with cosyntropin confirmed the diagnosis of primary adrenal insufficiency.
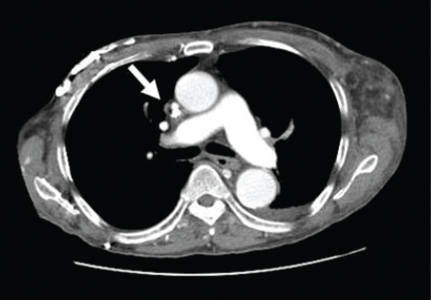
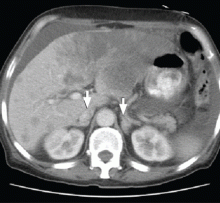
CT of the abdomen failed to demonstrate primary tumors, but both adrenal glands were enlarged, likely from metastasis (Figure 4). His hypotension responded to treatment with hydrocortisone and fludrocortisone, and his symptoms resolved. No further testing or therapy was directed to the primary occult malignancy, as it was considered advanced. The prognosis was discussed with the patient, and he deferred any further management and was discharged to hospice care. He died a few months later.
PRIMARY ADRENOCORTICAL INSUFFICIENCY
Primary adrenocortical insufficiency is an uncommon disorder caused by destruction or dysfunction of the adrenal cortices. It is characterized by chronic deficiency of cortisol, aldosterone, and adrenal androgens. In the United States, nearly 6 million people are considered to have undiagnosed adrenal insufficiency, which is clinically significant only during times of physiologic stress.1
Primary adrenocortical insufficiency affects men and women equally. However, the idiopathic autoimmune form of adrenal insufficiency (Addison disease) is two to three times more common in women than in men.
If the condition is undiagnosed or ineffectively treated, the risk of significant morbidity and death is high. Symptoms and signs are nonspecific, and the onset is insidious.
Almost all patients with primary adrenal insufficiency have malaise, fatigue, anorexia, and weight loss. Vomiting, abdominal pain, and fever are more common during an adrenal crisis, when a patient with subclinical disease is subjected to major stress. Postural dizziness or syncope is a common result of volume depletion and hypotension.2–4 It is commonly accompanied by hyponatremia and hyperkalemia.
Hyperpigmentation is the most characteristic physical finding and is caused by an ACTH-mediated increase in melanin content in the skin.2,4,5 The resulting brown hyperpigmentation is most obvious in areas exposed to sunlight (face, neck, backs of hands), and in areas exposed to chronic friction or pressure, such as the elbows, knees, knuckles, waist, and shoulders (brassiere straps).4 Pigmentation is also prominent in the palmar creases, areolae, axillae, perineum, surgical scars, and umbilicus. Other patterns of hyperpigmentation are patchy pigmentation on the inner surface of lips, the buccal mucosa, under the tongue, and on the hard palate.3,5 The hyperpigmentation begins to fade within several days and largely disappears after a few months of adequate glucocorticoid therapy.4
In the United States, 80% of cases of primary adrenocortical insufficiency are caused by autoimmune adrenal destruction. The remainder are caused by infectious diseases (eg, tuberculosis, fungal infection, cytomegalovirus infection, and Mycobacterium aviumintracellulare infection in the context of human immunodeficiency virus infection), by infiltration of the adrenal glands by metastatic cancer, by adrenal hemorrhage, or by drugs such as ketoconazole, fluconazole (Diflucan), metyrapone (Metopirone), mitotane (Lysodren), and etomidate (Amidate).4,6
Adrenal metastatic disease
Infiltration of the adrenal glands by metastatic cancer is not uncommon, probably because of their rich sinusoidal blood supply, and the adrenals are the fourth most common site of metastasis. Common primary tumors are lung, breast, melanoma, gastric, esophageal, and colorectal cancers, while metastasis due to an undetermined primary tumor is the least common.7
Clinically evident adrenal insufficiency produced by metastatic carcinoma is uncommon because most of the adrenal cortex must be destroyed before hypofunction becomes evident.7–9
Malignancy rarely presents first as adrenal insufficiency caused by metastatic infiltration.10
Hormonal therapy may significantly improve symptoms and quality of life in patients with metastatic adrenal insufficiency.8,11
DIAGNOSIS AND MANAGEMENT
Once primary adrenal insufficiency is suspected, prompt diagnosis and treatment are essential. A low plasma cortisol level (< 3 μg/dL) at 8 am is highly suggestive of adrenal insufficiency if exposure to exogenous glucocorticoids has been excluded (including oral, inhaled, and injected),12,13 especially if accompanied by simultaneous elevation of the plasma ACTH level (usually > 200 pg/mL). An 8 am cortisol concentration above 15 μg/dL makes adrenal insufficiency highly unlikely, but levels between 3 and 15 μg/dL are nondiagnostic and need to be further evaluated by an ACTH stimulation test with cosyntropin.4,7
Imaging in primary adrenal insufficiency may be considered when the condition is not clearly autoimmune.14 Abdominal CT is the ideal imaging test for detecting abnormal adrenal glands. CT shows small, noncalcified adrenals in autoimmune Addison disease. It demonstrates enlarged adrenals in about 85% of cases caused by metastatic or granulomatous disease; and calcification is noted in cases of tuberculous adrenal disease.4
Management involves treating the underlying cause and starting hormone replacement therapy. Hormonal therapy consists of corticosteroids and mineralocorticoids; hydrocortisone is the drug of choice and is usually given with fludrocortisone acetate, which has a potent sodium-retaining effect. In the presence of a stressor (fever, surgery, severe illness), the dose of hydrocortisone should be doubled (> 50 mg hydrocortisone per day) for at least 3 to 5 days.2,4
- Erichsen MM, Løvås K, Fougner KJ, et al. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur J Endocrinol 2009; 160:233–237.
- Oelkers W. Adrenal insufficiency. N Engl J Med 1996; 335:1206–1212.
- Redman BG, Pazdur R, Zingas AP, Loredo R. Prospective evaluation of adrenal insufficiency in patients with adrenal metastasis. Cancer 1987; 60:103–107.
- Berger M., Hypofunction of the adrenal cortex in infancy. Manit Med Rev 1949; 29:132.
- Stulberg DL, Clark N, Tovey D. Common hyperpigmentation disorders in adults: Part I. Diagnostic approach, café au lait macules, diffuse hyperpigmentation, sun exposure, and phototoxic reactions. Am Fam Physician 2003; 68:1955–1960.
- Zelissen PM, Bast EJ, Croughs RJ. Associated autoimmunity in Addison’s disease. J Autoimmun 1995; 8:121–130.
- Lutz A, Stojkovic M, Schmidt M, Arlt W, Allolio B, Reincke M. Adrenocortical function in patients with macrometastases of the adrenal gland. Eur J Endocrinol 2000; 143:91–97.
- Kung AW, Pun KK, Lam K, Wang C, Leung CY. Addisonian crisis as presenting feature in malignancies. Cancer 1990; 65:177–179.
- Cedermark BJ, Sjöberg HE. The clinical significance of metastases to the adrenal glands. Surg Gynecol Obstet 1981; 152:607–610.
- Rosenthal FD, Davies MK, Burden AC. Malignant disease presenting as Addison’s disease. Br Med J 1978; 1:1591–1592.
- Seidenwurm DJ, Elmer EB, Kaplan LM, Williams EK, Morris DG, Hoffman AR. Metastases to the adrenal glands and the development of Addison’s disease. Cancer 1984; 54:552–557.
- Santiago AH, Ratzan S. Acute adrenal crisis in an asthmatic child treated with inhaled fluticasone proprionate. Int J Pediatr Endocrinol 2010; 2010. pii:749239.
- Holme J, Tomlinson JW, Stockley RA, Stewart PM, Barlow N, Sullivan AL. Adrenal suppression in bronchiectasis and the impact of inhaled corticosteroids. Eur Respir J 2008; 32:1047–1052.
- Mohammad K, Sadikot RT. Adrenal insufficiency as a presenting manifestation of nonsmall cell lung cancer. South Med J 2009; 102:665–667.
A 65-year-old man presents with a 2-month history of generalized weakness, dizziness, and blurred vision. His symptoms began gradually and have been progressing over the last few weeks, so that they now affect his ability to perform normal daily activities.
He has lost 20 lb and has become anorectic. He has no fever, night sweats, headache, cough, hemoptysis, or dyspnea. He has no history of abdominal pain, changes in bowel habits, nausea, vomiting, or urinary symptoms. He was admitted 6 weeks ago for the same symptoms; he was treated for hypotension and received intravenous (IV) fluids and electrolyte supplements for dehydration.
He has a history of hypertension, stroke, vascular dementia, and atrial fibrillation. He is taking warfarin (Coumadin), extended-release diltiazem (Cardizem), simvastatin (Zocor), and donepezil (Aricept). He underwent right hemicolectomy 5 years ago for a large tubular adenoma with high-grade dysplasia in the cecum.
Initial laboratory values are as follows:
- White blood cell count 7.4 × 109/L (reference range 4.5–11.0), with a normal differential
- Mild anemia, with a hemoglobin of 116 g/L (140–175)
- Activated partial thromboplastin time 59.9 sec (23.0–32.4)
- Serum sodium 135 mmol/L (136–142)
- Serum potassium 4.6 mmol/L (3.5–5.0)
- Aspartate aminotransferase 58 U/L (10–30)
- Alanine aminotransferase 16 U/L (10–40)
- Alkaline phosphatase 328 U/L (30–120)
- Urea, creatinine, and corrected calcium are normal.
Electrocardiography shows atrial fibrillation with low-voltage QRS complexes. Chest radiography is normal. A stool test is negative for occult blood. A workup for sepsis is negative.
Q: Which is the appropriate test at this point to determine the cause of the hypotension?
- Serum parathyroid-hormone-related protein
- Baseline serum cortisol, plasma adrenocorticotropic hormone (ACTH) levels, and an ACTH stimulation test with cosyntropin (Cortrosyn)
- Serum thyrotropin level
- Aspiration biopsy of subcutaneous fat with Congo red and immunostaining
- Late-night salivary cortisol
A: The correct next step is to measure baseline serum cortisol, to test ACTH levels, and to order an ACTH stimulation test with cosyntropin.
Primary adrenocortical insufficiency should be considered in patients with metastatic malignancy who present with peripheral vascular collapse, particularly when it is associated with cutaneous hyperpigmentation, chronic malaise, fatigue, weakness, anorexia, weight loss, hypoglycemia, and electrolyte disturbances such as hyponatremia and hyperkalemia.
Checking the baseline serum cortisol and ACTH levels and cosyntropin stimulation testing are vital steps in making an early diagnosis of primary adrenocortical insufficiency. Inappropriately low serum cortisol is highly suggestive of primary adrenal insufficiency, especially if accompanied by simultaneous elevation of the plasma ACTH level. The result of the ACTH stimulation test with cosyntropin is often confirmatory.
Measuring the serum parathyroid-hormone-related protein level is not indicated, since the patient has a normal corrected calcium. Patients with ectopic Cushing syndrome may present with weight loss due to underlying malignancy, but the presence of hypotension and a lack of hypokalemia makes such a diagnosis unlikely, and, therefore, measurement of late-night salivary cortisol is not the best answer. Amyloidosis, hypothyroidism, or hyperthyroidism are unlikely to have this patient’s presentation.
RESULTS OF FURTHER EVALUATION
Our patient’s ACTH serum level was elevated, and an ACTH stimulation test with cosyntropin confirmed the diagnosis of primary adrenal insufficiency.
CT of the abdomen failed to demonstrate primary tumors, but both adrenal glands were enlarged, likely from metastasis (Figure 4). His hypotension responded to treatment with hydrocortisone and fludrocortisone, and his symptoms resolved. No further testing or therapy was directed to the primary occult malignancy, as it was considered advanced. The prognosis was discussed with the patient, and he deferred any further management and was discharged to hospice care. He died a few months later.
PRIMARY ADRENOCORTICAL INSUFFICIENCY
Primary adrenocortical insufficiency is an uncommon disorder caused by destruction or dysfunction of the adrenal cortices. It is characterized by chronic deficiency of cortisol, aldosterone, and adrenal androgens. In the United States, nearly 6 million people are considered to have undiagnosed adrenal insufficiency, which is clinically significant only during times of physiologic stress.1
Primary adrenocortical insufficiency affects men and women equally. However, the idiopathic autoimmune form of adrenal insufficiency (Addison disease) is two to three times more common in women than in men.
If the condition is undiagnosed or ineffectively treated, the risk of significant morbidity and death is high. Symptoms and signs are nonspecific, and the onset is insidious.
Almost all patients with primary adrenal insufficiency have malaise, fatigue, anorexia, and weight loss. Vomiting, abdominal pain, and fever are more common during an adrenal crisis, when a patient with subclinical disease is subjected to major stress. Postural dizziness or syncope is a common result of volume depletion and hypotension.2–4 It is commonly accompanied by hyponatremia and hyperkalemia.
Hyperpigmentation is the most characteristic physical finding and is caused by an ACTH-mediated increase in melanin content in the skin.2,4,5 The resulting brown hyperpigmentation is most obvious in areas exposed to sunlight (face, neck, backs of hands), and in areas exposed to chronic friction or pressure, such as the elbows, knees, knuckles, waist, and shoulders (brassiere straps).4 Pigmentation is also prominent in the palmar creases, areolae, axillae, perineum, surgical scars, and umbilicus. Other patterns of hyperpigmentation are patchy pigmentation on the inner surface of lips, the buccal mucosa, under the tongue, and on the hard palate.3,5 The hyperpigmentation begins to fade within several days and largely disappears after a few months of adequate glucocorticoid therapy.4
In the United States, 80% of cases of primary adrenocortical insufficiency are caused by autoimmune adrenal destruction. The remainder are caused by infectious diseases (eg, tuberculosis, fungal infection, cytomegalovirus infection, and Mycobacterium aviumintracellulare infection in the context of human immunodeficiency virus infection), by infiltration of the adrenal glands by metastatic cancer, by adrenal hemorrhage, or by drugs such as ketoconazole, fluconazole (Diflucan), metyrapone (Metopirone), mitotane (Lysodren), and etomidate (Amidate).4,6
Adrenal metastatic disease
Infiltration of the adrenal glands by metastatic cancer is not uncommon, probably because of their rich sinusoidal blood supply, and the adrenals are the fourth most common site of metastasis. Common primary tumors are lung, breast, melanoma, gastric, esophageal, and colorectal cancers, while metastasis due to an undetermined primary tumor is the least common.7
Clinically evident adrenal insufficiency produced by metastatic carcinoma is uncommon because most of the adrenal cortex must be destroyed before hypofunction becomes evident.7–9
Malignancy rarely presents first as adrenal insufficiency caused by metastatic infiltration.10
Hormonal therapy may significantly improve symptoms and quality of life in patients with metastatic adrenal insufficiency.8,11
DIAGNOSIS AND MANAGEMENT
Once primary adrenal insufficiency is suspected, prompt diagnosis and treatment are essential. A low plasma cortisol level (< 3 μg/dL) at 8 am is highly suggestive of adrenal insufficiency if exposure to exogenous glucocorticoids has been excluded (including oral, inhaled, and injected),12,13 especially if accompanied by simultaneous elevation of the plasma ACTH level (usually > 200 pg/mL). An 8 am cortisol concentration above 15 μg/dL makes adrenal insufficiency highly unlikely, but levels between 3 and 15 μg/dL are nondiagnostic and need to be further evaluated by an ACTH stimulation test with cosyntropin.4,7
Imaging in primary adrenal insufficiency may be considered when the condition is not clearly autoimmune.14 Abdominal CT is the ideal imaging test for detecting abnormal adrenal glands. CT shows small, noncalcified adrenals in autoimmune Addison disease. It demonstrates enlarged adrenals in about 85% of cases caused by metastatic or granulomatous disease; and calcification is noted in cases of tuberculous adrenal disease.4
Management involves treating the underlying cause and starting hormone replacement therapy. Hormonal therapy consists of corticosteroids and mineralocorticoids; hydrocortisone is the drug of choice and is usually given with fludrocortisone acetate, which has a potent sodium-retaining effect. In the presence of a stressor (fever, surgery, severe illness), the dose of hydrocortisone should be doubled (> 50 mg hydrocortisone per day) for at least 3 to 5 days.2,4
A 65-year-old man presents with a 2-month history of generalized weakness, dizziness, and blurred vision. His symptoms began gradually and have been progressing over the last few weeks, so that they now affect his ability to perform normal daily activities.
He has lost 20 lb and has become anorectic. He has no fever, night sweats, headache, cough, hemoptysis, or dyspnea. He has no history of abdominal pain, changes in bowel habits, nausea, vomiting, or urinary symptoms. He was admitted 6 weeks ago for the same symptoms; he was treated for hypotension and received intravenous (IV) fluids and electrolyte supplements for dehydration.
He has a history of hypertension, stroke, vascular dementia, and atrial fibrillation. He is taking warfarin (Coumadin), extended-release diltiazem (Cardizem), simvastatin (Zocor), and donepezil (Aricept). He underwent right hemicolectomy 5 years ago for a large tubular adenoma with high-grade dysplasia in the cecum.
Initial laboratory values are as follows:
- White blood cell count 7.4 × 109/L (reference range 4.5–11.0), with a normal differential
- Mild anemia, with a hemoglobin of 116 g/L (140–175)
- Activated partial thromboplastin time 59.9 sec (23.0–32.4)
- Serum sodium 135 mmol/L (136–142)
- Serum potassium 4.6 mmol/L (3.5–5.0)
- Aspartate aminotransferase 58 U/L (10–30)
- Alanine aminotransferase 16 U/L (10–40)
- Alkaline phosphatase 328 U/L (30–120)
- Urea, creatinine, and corrected calcium are normal.
Electrocardiography shows atrial fibrillation with low-voltage QRS complexes. Chest radiography is normal. A stool test is negative for occult blood. A workup for sepsis is negative.
Q: Which is the appropriate test at this point to determine the cause of the hypotension?
- Serum parathyroid-hormone-related protein
- Baseline serum cortisol, plasma adrenocorticotropic hormone (ACTH) levels, and an ACTH stimulation test with cosyntropin (Cortrosyn)
- Serum thyrotropin level
- Aspiration biopsy of subcutaneous fat with Congo red and immunostaining
- Late-night salivary cortisol
A: The correct next step is to measure baseline serum cortisol, to test ACTH levels, and to order an ACTH stimulation test with cosyntropin.
Primary adrenocortical insufficiency should be considered in patients with metastatic malignancy who present with peripheral vascular collapse, particularly when it is associated with cutaneous hyperpigmentation, chronic malaise, fatigue, weakness, anorexia, weight loss, hypoglycemia, and electrolyte disturbances such as hyponatremia and hyperkalemia.
Checking the baseline serum cortisol and ACTH levels and cosyntropin stimulation testing are vital steps in making an early diagnosis of primary adrenocortical insufficiency. Inappropriately low serum cortisol is highly suggestive of primary adrenal insufficiency, especially if accompanied by simultaneous elevation of the plasma ACTH level. The result of the ACTH stimulation test with cosyntropin is often confirmatory.
Measuring the serum parathyroid-hormone-related protein level is not indicated, since the patient has a normal corrected calcium. Patients with ectopic Cushing syndrome may present with weight loss due to underlying malignancy, but the presence of hypotension and a lack of hypokalemia makes such a diagnosis unlikely, and, therefore, measurement of late-night salivary cortisol is not the best answer. Amyloidosis, hypothyroidism, or hyperthyroidism are unlikely to have this patient’s presentation.
RESULTS OF FURTHER EVALUATION
Our patient’s ACTH serum level was elevated, and an ACTH stimulation test with cosyntropin confirmed the diagnosis of primary adrenal insufficiency.
CT of the abdomen failed to demonstrate primary tumors, but both adrenal glands were enlarged, likely from metastasis (Figure 4). His hypotension responded to treatment with hydrocortisone and fludrocortisone, and his symptoms resolved. No further testing or therapy was directed to the primary occult malignancy, as it was considered advanced. The prognosis was discussed with the patient, and he deferred any further management and was discharged to hospice care. He died a few months later.
PRIMARY ADRENOCORTICAL INSUFFICIENCY
Primary adrenocortical insufficiency is an uncommon disorder caused by destruction or dysfunction of the adrenal cortices. It is characterized by chronic deficiency of cortisol, aldosterone, and adrenal androgens. In the United States, nearly 6 million people are considered to have undiagnosed adrenal insufficiency, which is clinically significant only during times of physiologic stress.1
Primary adrenocortical insufficiency affects men and women equally. However, the idiopathic autoimmune form of adrenal insufficiency (Addison disease) is two to three times more common in women than in men.
If the condition is undiagnosed or ineffectively treated, the risk of significant morbidity and death is high. Symptoms and signs are nonspecific, and the onset is insidious.
Almost all patients with primary adrenal insufficiency have malaise, fatigue, anorexia, and weight loss. Vomiting, abdominal pain, and fever are more common during an adrenal crisis, when a patient with subclinical disease is subjected to major stress. Postural dizziness or syncope is a common result of volume depletion and hypotension.2–4 It is commonly accompanied by hyponatremia and hyperkalemia.
Hyperpigmentation is the most characteristic physical finding and is caused by an ACTH-mediated increase in melanin content in the skin.2,4,5 The resulting brown hyperpigmentation is most obvious in areas exposed to sunlight (face, neck, backs of hands), and in areas exposed to chronic friction or pressure, such as the elbows, knees, knuckles, waist, and shoulders (brassiere straps).4 Pigmentation is also prominent in the palmar creases, areolae, axillae, perineum, surgical scars, and umbilicus. Other patterns of hyperpigmentation are patchy pigmentation on the inner surface of lips, the buccal mucosa, under the tongue, and on the hard palate.3,5 The hyperpigmentation begins to fade within several days and largely disappears after a few months of adequate glucocorticoid therapy.4
In the United States, 80% of cases of primary adrenocortical insufficiency are caused by autoimmune adrenal destruction. The remainder are caused by infectious diseases (eg, tuberculosis, fungal infection, cytomegalovirus infection, and Mycobacterium aviumintracellulare infection in the context of human immunodeficiency virus infection), by infiltration of the adrenal glands by metastatic cancer, by adrenal hemorrhage, or by drugs such as ketoconazole, fluconazole (Diflucan), metyrapone (Metopirone), mitotane (Lysodren), and etomidate (Amidate).4,6
Adrenal metastatic disease
Infiltration of the adrenal glands by metastatic cancer is not uncommon, probably because of their rich sinusoidal blood supply, and the adrenals are the fourth most common site of metastasis. Common primary tumors are lung, breast, melanoma, gastric, esophageal, and colorectal cancers, while metastasis due to an undetermined primary tumor is the least common.7
Clinically evident adrenal insufficiency produced by metastatic carcinoma is uncommon because most of the adrenal cortex must be destroyed before hypofunction becomes evident.7–9
Malignancy rarely presents first as adrenal insufficiency caused by metastatic infiltration.10
Hormonal therapy may significantly improve symptoms and quality of life in patients with metastatic adrenal insufficiency.8,11
DIAGNOSIS AND MANAGEMENT
Once primary adrenal insufficiency is suspected, prompt diagnosis and treatment are essential. A low plasma cortisol level (< 3 μg/dL) at 8 am is highly suggestive of adrenal insufficiency if exposure to exogenous glucocorticoids has been excluded (including oral, inhaled, and injected),12,13 especially if accompanied by simultaneous elevation of the plasma ACTH level (usually > 200 pg/mL). An 8 am cortisol concentration above 15 μg/dL makes adrenal insufficiency highly unlikely, but levels between 3 and 15 μg/dL are nondiagnostic and need to be further evaluated by an ACTH stimulation test with cosyntropin.4,7
Imaging in primary adrenal insufficiency may be considered when the condition is not clearly autoimmune.14 Abdominal CT is the ideal imaging test for detecting abnormal adrenal glands. CT shows small, noncalcified adrenals in autoimmune Addison disease. It demonstrates enlarged adrenals in about 85% of cases caused by metastatic or granulomatous disease; and calcification is noted in cases of tuberculous adrenal disease.4
Management involves treating the underlying cause and starting hormone replacement therapy. Hormonal therapy consists of corticosteroids and mineralocorticoids; hydrocortisone is the drug of choice and is usually given with fludrocortisone acetate, which has a potent sodium-retaining effect. In the presence of a stressor (fever, surgery, severe illness), the dose of hydrocortisone should be doubled (> 50 mg hydrocortisone per day) for at least 3 to 5 days.2,4
- Erichsen MM, Løvås K, Fougner KJ, et al. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur J Endocrinol 2009; 160:233–237.
- Oelkers W. Adrenal insufficiency. N Engl J Med 1996; 335:1206–1212.
- Redman BG, Pazdur R, Zingas AP, Loredo R. Prospective evaluation of adrenal insufficiency in patients with adrenal metastasis. Cancer 1987; 60:103–107.
- Berger M., Hypofunction of the adrenal cortex in infancy. Manit Med Rev 1949; 29:132.
- Stulberg DL, Clark N, Tovey D. Common hyperpigmentation disorders in adults: Part I. Diagnostic approach, café au lait macules, diffuse hyperpigmentation, sun exposure, and phototoxic reactions. Am Fam Physician 2003; 68:1955–1960.
- Zelissen PM, Bast EJ, Croughs RJ. Associated autoimmunity in Addison’s disease. J Autoimmun 1995; 8:121–130.
- Lutz A, Stojkovic M, Schmidt M, Arlt W, Allolio B, Reincke M. Adrenocortical function in patients with macrometastases of the adrenal gland. Eur J Endocrinol 2000; 143:91–97.
- Kung AW, Pun KK, Lam K, Wang C, Leung CY. Addisonian crisis as presenting feature in malignancies. Cancer 1990; 65:177–179.
- Cedermark BJ, Sjöberg HE. The clinical significance of metastases to the adrenal glands. Surg Gynecol Obstet 1981; 152:607–610.
- Rosenthal FD, Davies MK, Burden AC. Malignant disease presenting as Addison’s disease. Br Med J 1978; 1:1591–1592.
- Seidenwurm DJ, Elmer EB, Kaplan LM, Williams EK, Morris DG, Hoffman AR. Metastases to the adrenal glands and the development of Addison’s disease. Cancer 1984; 54:552–557.
- Santiago AH, Ratzan S. Acute adrenal crisis in an asthmatic child treated with inhaled fluticasone proprionate. Int J Pediatr Endocrinol 2010; 2010. pii:749239.
- Holme J, Tomlinson JW, Stockley RA, Stewart PM, Barlow N, Sullivan AL. Adrenal suppression in bronchiectasis and the impact of inhaled corticosteroids. Eur Respir J 2008; 32:1047–1052.
- Mohammad K, Sadikot RT. Adrenal insufficiency as a presenting manifestation of nonsmall cell lung cancer. South Med J 2009; 102:665–667.
- Erichsen MM, Løvås K, Fougner KJ, et al. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur J Endocrinol 2009; 160:233–237.
- Oelkers W. Adrenal insufficiency. N Engl J Med 1996; 335:1206–1212.
- Redman BG, Pazdur R, Zingas AP, Loredo R. Prospective evaluation of adrenal insufficiency in patients with adrenal metastasis. Cancer 1987; 60:103–107.
- Berger M., Hypofunction of the adrenal cortex in infancy. Manit Med Rev 1949; 29:132.
- Stulberg DL, Clark N, Tovey D. Common hyperpigmentation disorders in adults: Part I. Diagnostic approach, café au lait macules, diffuse hyperpigmentation, sun exposure, and phototoxic reactions. Am Fam Physician 2003; 68:1955–1960.
- Zelissen PM, Bast EJ, Croughs RJ. Associated autoimmunity in Addison’s disease. J Autoimmun 1995; 8:121–130.
- Lutz A, Stojkovic M, Schmidt M, Arlt W, Allolio B, Reincke M. Adrenocortical function in patients with macrometastases of the adrenal gland. Eur J Endocrinol 2000; 143:91–97.
- Kung AW, Pun KK, Lam K, Wang C, Leung CY. Addisonian crisis as presenting feature in malignancies. Cancer 1990; 65:177–179.
- Cedermark BJ, Sjöberg HE. The clinical significance of metastases to the adrenal glands. Surg Gynecol Obstet 1981; 152:607–610.
- Rosenthal FD, Davies MK, Burden AC. Malignant disease presenting as Addison’s disease. Br Med J 1978; 1:1591–1592.
- Seidenwurm DJ, Elmer EB, Kaplan LM, Williams EK, Morris DG, Hoffman AR. Metastases to the adrenal glands and the development of Addison’s disease. Cancer 1984; 54:552–557.
- Santiago AH, Ratzan S. Acute adrenal crisis in an asthmatic child treated with inhaled fluticasone proprionate. Int J Pediatr Endocrinol 2010; 2010. pii:749239.
- Holme J, Tomlinson JW, Stockley RA, Stewart PM, Barlow N, Sullivan AL. Adrenal suppression in bronchiectasis and the impact of inhaled corticosteroids. Eur Respir J 2008; 32:1047–1052.
- Mohammad K, Sadikot RT. Adrenal insufficiency as a presenting manifestation of nonsmall cell lung cancer. South Med J 2009; 102:665–667.
Factor V Leiden: How great is the risk of venous thromboembolism?
A 29-year-old white man with no chronic medical problems presents to the emergency department with shortness of breath, left-sided pleuritic chest pain, cough, and hemoptysis. These symptoms began abruptly 1 day ago and have persisted. He also has mild pain and swelling in both calves. He denies having any fever, night sweats, or chills. On further questioning, he reports having taken a long, nonstop driving trip that lasted 8 hours 1 week ago.
His medical history is negative, and he specifically reports no history of deep venous thrombosis or pulmonary embolism. He underwent appendectomy 10 years ago but has had no other operations. He does not take any medications. His family history is noncontributory and is negative for venous thromboembolism. He smokes and uses alcohol occasionally but not illicit drugs.
Examination. He appears to be in considerable distress because of his chest pain. His temperature is 100.4°F (38.0°C), blood pressure 125/70 mm Hg, heart rate 125 beats per minute, respiratory rate 26 breaths per minute, oxygen saturation 92% on room air, and body mass index 19 kg/m2.
Chest examination reveals diminished vesicular breathing in the left base, which is normal to percussion without added sounds. Both calves are swollen and tender to palpation without skin discoloration. The rest of his examination is normal.
Laboratory values:
- White blood cell count 9.3 × 109/L (reference range 4.5–11.0)
- Hemoglobin 15.9 g/dL (14.0–17.5)
- Platelets 205 × 109/L (150–350)
- Sodium 140 mEq/L (136–142)
- Potassium 3.9 mEq/L (3.5–5.0)
- Chloride 108 mEq/L (96–106)
- Bicarbonate 23 mEq/L (21–28)
- Blood urea nitrogen 14 mg/dL (8–23)
- Creatinine 0.9 mg/dL (0.6–1.2)
- Glucose 95 mg/dL (70–110)
- International normalized ratio (INR) 0.90 (0.00–1.2)
- Partial thromboplastin time 27.5 seconds (24.6–31.8)
- Creatine phosphokinase 205 U/L (39–308)
- Troponin T < 0.015 ng/mL (0.01–0.045).
Pulmonary embolism is diagnosed
Factor V Leiden is diagnosed, and the patient recovers with treatment
Anticoagulation is started in the emergency department.
Given this patient’s young age and clot burden, a hypercoagulable state is suspected. Thrombophilia screening is performed, with tests for the factor V Leiden mutation, the prothrombin G20210A mutation, and antiphospholipid and lupus anticoagulant antibodies. The rest of the thrombophilia panel, including antithrombin III, factor VIII, protein C, and protein S, is deferred because the levels of these substances would be expected to change during the acute thrombosis.
The direct test for factor V Leiden mutation is positive for the heterozygous type. The test for the prothrombin G20210A mutation is negative, and his antiphospholipid antibody levels, including the lupus anticoagulant titer, are within normal limits.
The patient is kept on a standard regimen of unfractionated heparin, overlapped with warfarin (Coumadin) until his INR is 2.0 to 3.0 on 2 consecutive days. His hospital course is uneventful and his condition gradually improves.
He is discharged home to continue on oral anticoagulation for 6 months with a target INR of 2.0 to 3.0. Two weeks after completing his anticoagulation therapy, his levels of antithrombin III, factor VIII, protein C, and protein S are all within normal limits.
FACTOR V LEIDEN IS COMMON
Factor V Leiden is the most common inherited thrombophilia, with a prevalence of 3% to 7% in the general US population,1 approximately 5% in whites, 2.2% in Hispanics, and 1.2% in blacks.2 Its prevalence in patients with venous thromboembolism, however, is 50%.1,3 The annual incidence of venous thromboembolism in patients with factor V Leiden is 0.5%.4,5
MORE COAGULATION, LESS ANTICOAGULATION
Factor V has a critical position in both the coagulant and anticoagulant pathways. Factor V Leiden results in a hypercoagulable state by both increasing coagulation and decreasing anticoagulation.
This mutation causes factor V to be resistant to being cleaved and inactivated by activated protein C, a condition known as APC resistance. As a result, more factor Va is available within the prothrombinase complex, increasing coagulation by increased generation of thrombin.6–8
Furthermore, a cofactor formed by cleavage of factor V at position 506 is thought to support activated protein C in degrading factor VIIIa (in the tenase complex), along with protein S. People with factor V Leiden lack this cleavage product and thus have less anticoagulant activity from activated protein C. The increased coagulation and decreased anticoagulation appear to contribute equally to the hypercoagulable state in factor V Leiden-associated APC resistance.9–11
Heterozygosity for the factor V Leiden mutation accounts for 90% to 95% of cases of APC resistance. A much smaller number of people are homozygous for it.1
People who are homozygous for factor V Leiden are at higher risk of venous thromboembolism than those who are heterozygous for it, since the latter group’s blood contains both factor V Leiden and normal factor V. The normal factor V allows anticoagulation via the second pathway of inactivation of factor VIIIa by activated protein C, giving some protection against thrombosis. In people who are homozygous for factor V Leiden, the lack of normal factor V acting as an anticoagulant protein results in a higher thrombotic risk.9–11
Other factor V mutations may also cause APC resistance
Although factor V Leiden is the only genetic defect for which a causal relationship with APC resistance has been clearly determined, other, rarer hereditary factor V mutations or polymorphisms have been described, such as factor V Cambridge (Arg306Thr)12 and factor V Hong Kong (Arg306Gly).13 These mutations may result in APC resistance, but their clinical association with thrombosis is less clear.14 Factor V Liverpool (Ile359Thr) is associated with a higher risk of thrombosis, apparently because of reduced APC-mediated inactivation of factor Va and because it is a poor cofactor with activated protein C for the inactivation of factor VIIIa.15
An R2 haplotype has also been described in association with APC resistance.16,17 The phenomenon may be due to a reduction in activated protein C cofactor activity.9 However, not all studies have been convincing regarding the role of this haplotype in clinical disease.18 Coinheritance of this haplotype with factor V Leiden may increase the risk of venous thromboembolism above that associated with factor V Leiden alone.19
Although factor V Leiden is the most common cause of inherited APC resistance, other changes in hemostasis cause acquired APC resistance and may contribute to the thrombotic tendency in these patients.20–22 The most common causes of acquired APC resistance include elevated factor VIII levels,23–25 pregnancy,26–28 use of oral contraceptives,29,30 and antiphospholipid antibodies.31
USUALLY MANIFESTS AS DEEP VEIN THROMBOSIS
Factor V Leiden usually manifests as deep vein thrombosis with or without pulmonary embolism, but thrombosis in unusual locations also occurs.32
The risk of a first episode of venous thromboembolism is two to five times higher with heterozygous factor V Leiden. However, even though the relative risk is high, the absolute risk is low. Furthermore, despite the higher risk of venous thrombosis, there is no evidence that heterozygosity for factor V Leiden increases the overall mortality rate.4,33–36
In people with homozygous factor V Leiden or with combined inherited thrombophilias, the risk of venous thromboembolism is increased to a greater degree: it is 20 to 50 times higher.7,8,37–39 However, whether the risk of death is higher is not clear.
VENOUS THROMBOEMBOLISM IS MULTIFACTORIAL
The pathogenesis of venous thromboembolism is multifactorial and involves an interaction between inherited and acquired factors. Very often, people with factor V Leiden have additional risk factors that contribute to the development of venous clots, and it is very unusual for them to have thrombosis in the absence of these additional factors.
These factors include older age, surgery, obesity, prolonged travel, immobility, hospitalization, oral contraceptive use, hormonal replacement therapy, pregnancy, and malignancy. They increase the risk of venous thrombosis in normal individuals as well, but more so in people with factor V Leiden.40–43
Testing for other known causes of thrombophilia may also be pursued. These include elevated homocysteine levels, the factor II (prothrombin) G20210A mutation, anticardiolipin antibody, lupus anticoagulant, and deficiencies of antithrombin III, protein C, and protein S.
Factor V Leiden by itself does not appear to increase the risk of arterial thrombosis, ie, heart attack and stroke.33,38,44–46
Family history: A risk indicator for venous thrombosis
Family history is an important indicator of risk for a first venous thromboembolic event, regardless of other risk factors identified. The risk of a first event is two to three times higher in people with a family history of thrombosis in a first-degree relative. The risk is four times higher when multiple family members are affected, at least one of them before age 50.47
In people with genetic thrombophilia, the risk of thrombosis (especially unprovoked thrombosis at a young age) is also higher in those with a strong family history than in those without a family history. In those with factor V Leiden, the risk of venous thromboembolism is three to four times higher if there is a positive family history. The risk is five times higher in carriers of factor V Leiden with a family history of venous thromboembolism before age 50, and 13 times higher in those with more than one affected family member.47
Possible shared environmental factors or coinheritance of other unidentified genetic factors may also contribute to the higher susceptibility in thrombosis-prone families.
TESTING FOR APC RESISTANCE AND FACTOR V LEIDEN
The factor V Leiden mutation can be detected directly by genetic testing of peripheral blood mononuclear cells. This method is relatively time-consuming and expensive, however.
At present, the most cost-effective approach is to test first for APC resistance using a second-generation coagulation assay—the modified APC sensitivity test. In this clot-based method, the patient’s sample is prediluted with factor V-deficient plasma to eliminate the effect of lupus anticoagulants and factor deficiencies that could prolong the baseline clotting time, and heparin is inactivated by polybrene. Then either an augmented partial-thromboplastin-time-based assay or a tissue-factor-dependent factor V assay is performed.
This test is nearly 100% sensitive and specific for factor V Leiden, in contrast to the first-generation, or classic, APC sensitivity test, which lacked specificity and sensitivity for it.9–11,48–60 This modification also permits testing of patients receiving anticoagulants or who have abnormal augmented partial thromboplastin times due to coagulation factor deficiencies.
A positive result on the modified APC sensitivity test should be confirmed by a direct genetic test for the factor V Leiden mutation. An APC resistance assay is unnecessary if a direct genetic test is used initially.
HOW LONG TO GIVE ANTICOAGULATION AFTER VENOUS THROMBOEMBOLISM?
Patients who have had an episode of venous thromboembolism have to be treated with anticoagulants.
In general, the initial management of venous thromboembolism in patients with heritable thrombophilias is no different from that in any other patient with a clot. Anticoagulants such as warfarin are given at a target INR of 2.5 (range 2.0–3.0).32 The duration of treatment is based on the risk factors that resulted in the thrombotic event.
After a first event, some authorities recommend anticoagulant therapy for 6 months.32 A shorter period (3 months) is recommended if there is a transient risk factor (eg, surgery, oral contraceptive use, travel, pregnancy, the puerperium) and the thrombosis is confined to distal veins (eg, the calf veins).32
Factor V Leiden does not necessarily increase the risk of recurrent events in patients who have a transient risk factor. Therefore, people who are heterozygous for this mutation do not usually need to be treated lifelong with anticoagulants if they have had only one episode of deep vein thrombosis or pulmonary embolism, given the risk of bleeding associated with anticoagulation, unless they have additional risk factors.
Conditions in which indefinite anticoagulation may be required after careful consideration of the risks and benefits are:
- Life-threatening events such as near-fatal pulmonary embolism
- Cerebral or visceral vein thrombosis
- Recurrent thrombotic events
- Additional persistent risk factors (eg, active malignant neoplasm, extremity paresis, and antiphospholipid antibodies)
- Combined thrombophilias (eg, combined heterozygosity for factor V Leiden and the prothrombin G20210A mutation)
- Homozygosity for factor V Leiden.32,46,48
Factor V Leiden by itself or combined with other thrombophilic abnormalities is not associated with a higher risk of recurrent venous thromboembolism during warfarin therapy (a possible exception is the combination of factor V Leiden plus antiphospholipid antibodies).32,34 Furthermore, current evidence suggests that no thrombophilic defect is a clinically important risk factor for recurrent venous thromboembolism after anticoagulant therapy is stopped. All these facts indicate that clinical factors are probably more important than laboratory abnormalities in determining the duration of anticoagulation therapy.32,35,36,61–63
PRIMARY PROPHYLAXIS IN PATIENTS WITH FACTOR V LEIDEN
Factor V Leiden is only one of many risk factors for deep vein thrombosis or pulmonary embolism. If carriers of factor V Leiden have never had a blood clot, then they are not routinely treated with an anticoagulant. Rather, they should be counseled about reducing or eliminating other factors that may add to their risk of developing a clot in the future.
Usually, the effect of risk factors is additive: the more risk factors present, the higher the risk.46,50 Sometimes, however, the effect of multiple risk factors is more than additive.
Some risk factors, such as genetics or age, are not alterable, but many can be controlled by medications or lifestyle modifications. Therefore, general measures and precautions are recommended to minimize the risk of thrombosis. For example:
Losing weight (if the patient is overweight) is an important intervention for risk reduction, since obesity is probably the most common modifiable risk factor for developing blood clots.
Avoiding long periods of immobility is recommended. For example, if the patient is taking a long car ride (more than 2 hours), then stopping every few hours and walking around for a few minutes is a good way to keep the blood circulating. If the patient has a desk job, getting up and walking around the office periodically is advised. On long airplane trips, a walk in the aisle every so often and preventing dehydration by drinking plenty of fluids and avoiding alcohol are recommended.
Wearing elastic stockings with a graduated elastic pressure may prevent deep venous thrombosis from developing on long flights.63–65
Staying active and getting regular exercise through such activities as walking, bicycling, or swimming are helpful.
Avoiding smoking is critical.50,63
Thromboprophylaxis is recommended for most acutely ill hospitalized patients, especially those confined to bed with additional risk factors. Guidelines for prophylaxis are based on an individualized risk assessment and not on thrombophilia status. Prophylactic anticoagulation is routinely recommended for patients undergoing major high-risk surgery, such as an orthopedic, urologic, gynecologic, or bariatric procedure. Any excess thrombotic risk conferred by thrombophilia is likely small compared with the risk of surgery, and recommendations on the duration and intensity of thromboprophylaxis are not based on thrombophilic status.46,48
Education. Pain, swelling, redness of a limb, unexplained shortness of breath, and chest pain are the most common symptoms of deep vein thrombosis and pulmonary embolism.46,50 It is crucial to teach patients with factor V Leiden to recognize these symptoms and to seek early medical attention in case they experience any of them.
SCREENING FAMILY MEMBERS FOR THE FACTOR V LEIDEN MUTATION
Factor V Leiden by itself is a relatively mild thrombophilic defect that does not cause thrombosis in all carriers, and there is no evidence that early diagnosis reduces rates of morbidity or mortality. Therefore, routine screening of all asymptomatic relatives of affected patients with venous thrombosis is not recommended. Rather, the decision to screen should be made on an individual basis.50,66
Screening may be beneficial in selected cases, especially when patients have a strong family history of recurrent venous thrombosis at a young age (younger than 50 years) and the family member has additional risk factors for venous thromboembolism such as oral contraception or is planning for pregnancy.32,48,49,66
- Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet 1995; 346:1133–1134.
- Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA 1997; 277:1305–1307.
- Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 1995; 85:1504–1508.
- Stolz E, Kemkes-Matthes B, Pötzsch B, et al. Screening for thrombophilic risk factors among 25 German patients with cerebral venous thrombosis. Acta Neurol Scand 2000; 102:31–36.
- Langlois NJ, Wells PS. Risk of venous thromboembolism in relatives of symptomatic probands with thrombophilia: a systematic review. Thromb Haemost 2003; 90:17–26.
- Juul K, Tybjaerg-Hansen A, Mortensen J, Lange P, Vestbo J, Nordestgaard BG. Factor V leiden homozygosity, dyspnea, and reduced pulmonary function. Arch Intern Med 2005; 165:2032–2036.
- Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994; 369:64–67.
- Dahlbäck B. New molecular insights into the genetics of thrombophilia. Resistance to activated protein C caused by Arg506 to Gln mutation in factor V as a pathogenic risk factor for venous thrombosis. Thromb Haemost 1995; 74:139–148.
- Castoldi E, Brugge JM, Nicolaes GA, Girelli D, Tans G, Rosing J. Impaired APC cofactor activity of factor V plays a major role in the APC resistance associated with the factor V Leiden (R506Q) and R2 (H1299R) mutations. Blood 2004; 103:4173–4179.
- Dahlback B. Anticoagulant factor V and thrombosis risk (editorial). Blood 2004; 103:3995.
- Simioni P, Castoldi E, Lunghi B, Tormene D, Rosing J, Bernardi F. An underestimated combination of opposites resulting in enhanced thrombotic tendency. Blood 2005; 106:2363–2365.
- Williamson D, Brown K, Luddington R, Baglin C, Baglin T. Factor V Cambridge: a new mutation (Arg306-->Thr) associated with resistance to activated protein C. Blood 1998; 91:1140–1144.
- Chan WP, Lee CK, Kwong YL, Lam CK, Liang R. A novel mutation of Arg306 of factor V gene in Hong Kong Chinese. Blood 1998; 91:1135–1139.
- Liang R, Lee CK, Wat MS, Kwong YL, Lam CK, Liu HW. Clinical significance of Arg306 mutations of factor V gene. Blood 1998; 92:2599–2600.
- Steen M, Norstrøm EA, Tholander AL, et al. Functional characterization of factor V-Ile359Thr: a novel mutation associated with thrombosis. Blood 2004; 103:3381–3387.
- Bernardi F, Faioni EM, Castoldi E, et al. A factor V genetic component differing from factor V R506Q contributes to the activated protein C resistance phenotype. Blood 1997; 90:1552–1557.
- Lunghi B, Castoldi E, Mingozzi F, Bernardi F. A new factor V gene polymorphism (His 1254 Arg) present in subjects of African origin mimics the R2 polymorphism (His 1299 Arg). Blood 1998; 91:364–365.
- Luddington R, Jackson A, Pannerselvam S, Brown K, Baglin T. The factor V R2 allele: risk of venous thromboembolism, factor V levels and resistance to activated protein C. Thromb Haemost 2000; 83:204–208.
- Faioni EM, Franchi F, Bucciarelli P, et al. Coinheritance of the HR2 haplotype in the factor V gene confers an increased risk of venous thromboembolism to carriers of factor V R506Q (factor V Leiden). Blood 1999; 94:3062–3066.
- Clark P, Walker ID. The phenomenon known as acquired activated protein C resistance. Br J Haematol 2001; 115:767–773.
- Tosetto A, Simioni M, Madeo D, Rodeghiero F. Intraindividual consistency of the activated protein C resistance phenotype. Br J Haematol 2004; 126:405–409.
- de Visser MC, Rosendaal FR, Bertina RM. A reduced sensitivity for activated protein C in the absence of factor V Leiden increases the risk of venous thrombosis. Blood 1999; 93:1271–1276.
- Kraaijenhagen RA, in’t Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost 2000; 83:5–9.
- Kamphuisen PW, Eikenboom JC, Bertina RM. Elevated factor VIII levels and the risk of thrombosis. Arterioscler Thromb Vasc Biol 2001; 21:731–738.
- Koster T, Blann AD, Briët E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995; 345:152–155.
- Clark P, Brennand J, Conkie JA, McCall F, Greer IA, Walker ID. Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost 1998; 79:1166–1170.
- Cumming AM, Tait RC, Fildes S, Yoong A, Keeney S, Hay CR. Development of resistance to activated protein C during pregnancy. Br J Haematol 1995; 90:725–727.
- Mathonnet F, de Mazancourt P, Bastenaire B, et al. Activated protein C sensitivity ratio in pregnant women at delivery. Br J Haematol 1996; 92:244–246.
- Post MS, Rosing J, Van Der Mooren MJ, et al; Ageing Women’ and the Institute for Cardiovascular Research-Vrije Universiteit (ICaRVU). Increased resistance to activated protein C after short-term oral hormone replacement therapy in healthy post-menopausal women. Br J Haematol 2002; 119:1017–1023.
- Olivieri O, Friso S, Manzato F, et al. Resistance to activated protein C in healthy women taking oral contraceptives. Br J Haematol 1995; 91:465–470.
- Bokarewa MI, Blombäck M, Egberg N, Rosén S. A new variant of interaction between phospholipid antibodies and the protein C system. Blood Coagul Fibrinolysis 1994; 5:37–41.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- van Stralen KJ, Doggen CJ, Bezemer ID, Pomp ER, Lisman T, Rosendaal FR. Mechanisms of the factor V Leiden paradox. Arterioscler Thromb Vasc Biol 2008; 28:1872–1877.
- Agaoglu N, Mustafa NA, Turkyilmaz S. Prothrombotic disorders in patients with mesenteric vein thrombosis. J Invest Surg 2003; 16:299–304.
- El-Karaksy H, El-Koofy N, El-Hawary M, et al. Prevalence of factor V Leiden mutation and other hereditary thrombophilic factors in Egyptian children with portal vein thrombosis: results of a single-center case-control study. Ann Hematol 2004; 83:712–715.
- Heijmans BT, Westendorp RG, Knook DL, Kluft C, Slagboom PE. The risk of mortality and the factor V Leiden mutation in a population-based cohort. Thromb Haemost 1998; 80:607–609.
- Turkstra F, Karemaker R, Kuijer PM, Prins MH, Büller HR. Is the prevalence of the factor V Leiden mutation in patients with pulmonary embolism and deep vein thrombosis really different? Thromb Haemost 1999; 81:345–348.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Manten B, Westendorp RG, Koster T, Reitsma PH, Rosendaal FR. Risk factor profiles in patients with different clinical manifestations of venous thromboembolism: a focus on the factor V Leiden mutation. Thromb Haemost 1996; 76:510–513.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, Büller HR, Vandenbroucke JP. Enhancement by factor V Leiden mutation of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet 1995; 346:1593–1596.
- Murphy PT. Factor V Leiden and venous thromboembolism. Ann Intern Med 2004; 141:483–484.
- Nizankowska-Mogilnicka E, Adamek L, Grzanka P, et al. Genetic polymorphisms associated with acute pulmonary embolism and deep venous thrombosis. Eur Respir J 2003; 21:25–30.
- Arsov T, Miladinova D, Spiroski M. Factor V Leiden is associated with higher risk of deep venous thrombosis of large blood vessels. Croat Med J 2006; 47:433–439.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood 2000; 96:3329–3333.
- Ornstein DL, Cushman M. Cardiology patient page. Factor V Leiden. Circulation 2003; 107:e94–e97.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Press RD, Bauer KA, Kujovich JL, Heit JA. Clinical utility of factor V leiden (R506Q) testing for the diagnosis and management of thromboembolic disorders. Arch Pathol Lab Med 2002; 126:1304–1318.
- Gadelha T, Roldán V, Lecumberri R, et al; RIETE Investigators. Clinical characteristics of patients with factor V Leiden or prothrombin G20210A and a first episode of venous thromboembolism. Findings from the RIETE Registry. Thromb Res 2010; 126:283–286.
- Severinsen MT, Overvad K, Johnsen SP, et al. Genetic susceptibility, smoking, obesity and risk of venous thromboembolism. Br J Haematol 2010; 149:273–279.
- Kujovich JL. Factor V Leiden thrombophilia. Genet Med 2011; 13:1–16.
- Lijfering WM, Brouwer JL, Veeger NJ, et al. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006; 166:729–736.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Strobl FJ, Hoffman S, Huber S, Williams EC, Voelkerding KV. Activated protein C resistance assay performance: improvement by sample dilution with factor V-deficient plasma. Arch Pathol Lab Med 1998; 122:430–433.
- Legnani C, Palareti G, Biagi R, et al. Activated protein C resistance: a comparison between two clotting assays and their relationship to the presence of the factor V Leiden mutation. Br J Haematol 1996; 93:694–699.
- Gouault-Heilmann M, Leroy-Matheron C. Factor V Leiden-dependent APC resistance: improved sensitivity and specificity of the APC resistance test by plasma dilution in factor V-depleted plasma. Thromb Res 1996; 82:281–283.
- Svensson PJ, Zöller B, Dahlbäck B. Evaluation of original and modified APC-resistance tests in unselected outpatients with clinically suspected thrombosis and in healthy controls. Thromb Haemost 1997; 77:332–335.
- Tripodi A, Negri B, Bertina RM, Mannucci PM. Screening for the FV:Q506 mutation—evaluation of thirteen plasma-based methods for their diagnostic efficacy in comparison with DNA analysis. Thromb Haemost 1997; 77:436–439.
- Wåhlander K, Larson G, Lindahl TL, et al. Factor V Leiden (G1691A) and prothrombin gene G20210A mutations as potential risk factors for venous thromboembolism after total hip or total knee replacement surgery. Thromb Haemost 2002; 87:580–585.
- Joseph JE, Low J, Courtenay B, Neil MJ, McGrath M, Ma D. A single-centre prospective study of clinical and haemostatic risk factors for venous thromboembolism following lower limb arthroplasty. Br J Haematol 2005; 129:87–92.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):381S–453S.
- Brenner B. Prophylaxis for travel-related thrombosis? Yes. J Thromb Haemost 2004; 2:2089–2091.
- Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med 2011; 6:113–116.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
A 29-year-old white man with no chronic medical problems presents to the emergency department with shortness of breath, left-sided pleuritic chest pain, cough, and hemoptysis. These symptoms began abruptly 1 day ago and have persisted. He also has mild pain and swelling in both calves. He denies having any fever, night sweats, or chills. On further questioning, he reports having taken a long, nonstop driving trip that lasted 8 hours 1 week ago.
His medical history is negative, and he specifically reports no history of deep venous thrombosis or pulmonary embolism. He underwent appendectomy 10 years ago but has had no other operations. He does not take any medications. His family history is noncontributory and is negative for venous thromboembolism. He smokes and uses alcohol occasionally but not illicit drugs.
Examination. He appears to be in considerable distress because of his chest pain. His temperature is 100.4°F (38.0°C), blood pressure 125/70 mm Hg, heart rate 125 beats per minute, respiratory rate 26 breaths per minute, oxygen saturation 92% on room air, and body mass index 19 kg/m2.
Chest examination reveals diminished vesicular breathing in the left base, which is normal to percussion without added sounds. Both calves are swollen and tender to palpation without skin discoloration. The rest of his examination is normal.
Laboratory values:
- White blood cell count 9.3 × 109/L (reference range 4.5–11.0)
- Hemoglobin 15.9 g/dL (14.0–17.5)
- Platelets 205 × 109/L (150–350)
- Sodium 140 mEq/L (136–142)
- Potassium 3.9 mEq/L (3.5–5.0)
- Chloride 108 mEq/L (96–106)
- Bicarbonate 23 mEq/L (21–28)
- Blood urea nitrogen 14 mg/dL (8–23)
- Creatinine 0.9 mg/dL (0.6–1.2)
- Glucose 95 mg/dL (70–110)
- International normalized ratio (INR) 0.90 (0.00–1.2)
- Partial thromboplastin time 27.5 seconds (24.6–31.8)
- Creatine phosphokinase 205 U/L (39–308)
- Troponin T < 0.015 ng/mL (0.01–0.045).
Pulmonary embolism is diagnosed
Factor V Leiden is diagnosed, and the patient recovers with treatment
Anticoagulation is started in the emergency department.
Given this patient’s young age and clot burden, a hypercoagulable state is suspected. Thrombophilia screening is performed, with tests for the factor V Leiden mutation, the prothrombin G20210A mutation, and antiphospholipid and lupus anticoagulant antibodies. The rest of the thrombophilia panel, including antithrombin III, factor VIII, protein C, and protein S, is deferred because the levels of these substances would be expected to change during the acute thrombosis.
The direct test for factor V Leiden mutation is positive for the heterozygous type. The test for the prothrombin G20210A mutation is negative, and his antiphospholipid antibody levels, including the lupus anticoagulant titer, are within normal limits.
The patient is kept on a standard regimen of unfractionated heparin, overlapped with warfarin (Coumadin) until his INR is 2.0 to 3.0 on 2 consecutive days. His hospital course is uneventful and his condition gradually improves.
He is discharged home to continue on oral anticoagulation for 6 months with a target INR of 2.0 to 3.0. Two weeks after completing his anticoagulation therapy, his levels of antithrombin III, factor VIII, protein C, and protein S are all within normal limits.
FACTOR V LEIDEN IS COMMON
Factor V Leiden is the most common inherited thrombophilia, with a prevalence of 3% to 7% in the general US population,1 approximately 5% in whites, 2.2% in Hispanics, and 1.2% in blacks.2 Its prevalence in patients with venous thromboembolism, however, is 50%.1,3 The annual incidence of venous thromboembolism in patients with factor V Leiden is 0.5%.4,5
MORE COAGULATION, LESS ANTICOAGULATION
Factor V has a critical position in both the coagulant and anticoagulant pathways. Factor V Leiden results in a hypercoagulable state by both increasing coagulation and decreasing anticoagulation.
This mutation causes factor V to be resistant to being cleaved and inactivated by activated protein C, a condition known as APC resistance. As a result, more factor Va is available within the prothrombinase complex, increasing coagulation by increased generation of thrombin.6–8
Furthermore, a cofactor formed by cleavage of factor V at position 506 is thought to support activated protein C in degrading factor VIIIa (in the tenase complex), along with protein S. People with factor V Leiden lack this cleavage product and thus have less anticoagulant activity from activated protein C. The increased coagulation and decreased anticoagulation appear to contribute equally to the hypercoagulable state in factor V Leiden-associated APC resistance.9–11
Heterozygosity for the factor V Leiden mutation accounts for 90% to 95% of cases of APC resistance. A much smaller number of people are homozygous for it.1
People who are homozygous for factor V Leiden are at higher risk of venous thromboembolism than those who are heterozygous for it, since the latter group’s blood contains both factor V Leiden and normal factor V. The normal factor V allows anticoagulation via the second pathway of inactivation of factor VIIIa by activated protein C, giving some protection against thrombosis. In people who are homozygous for factor V Leiden, the lack of normal factor V acting as an anticoagulant protein results in a higher thrombotic risk.9–11
Other factor V mutations may also cause APC resistance
Although factor V Leiden is the only genetic defect for which a causal relationship with APC resistance has been clearly determined, other, rarer hereditary factor V mutations or polymorphisms have been described, such as factor V Cambridge (Arg306Thr)12 and factor V Hong Kong (Arg306Gly).13 These mutations may result in APC resistance, but their clinical association with thrombosis is less clear.14 Factor V Liverpool (Ile359Thr) is associated with a higher risk of thrombosis, apparently because of reduced APC-mediated inactivation of factor Va and because it is a poor cofactor with activated protein C for the inactivation of factor VIIIa.15
An R2 haplotype has also been described in association with APC resistance.16,17 The phenomenon may be due to a reduction in activated protein C cofactor activity.9 However, not all studies have been convincing regarding the role of this haplotype in clinical disease.18 Coinheritance of this haplotype with factor V Leiden may increase the risk of venous thromboembolism above that associated with factor V Leiden alone.19
Although factor V Leiden is the most common cause of inherited APC resistance, other changes in hemostasis cause acquired APC resistance and may contribute to the thrombotic tendency in these patients.20–22 The most common causes of acquired APC resistance include elevated factor VIII levels,23–25 pregnancy,26–28 use of oral contraceptives,29,30 and antiphospholipid antibodies.31
USUALLY MANIFESTS AS DEEP VEIN THROMBOSIS
Factor V Leiden usually manifests as deep vein thrombosis with or without pulmonary embolism, but thrombosis in unusual locations also occurs.32
The risk of a first episode of venous thromboembolism is two to five times higher with heterozygous factor V Leiden. However, even though the relative risk is high, the absolute risk is low. Furthermore, despite the higher risk of venous thrombosis, there is no evidence that heterozygosity for factor V Leiden increases the overall mortality rate.4,33–36
In people with homozygous factor V Leiden or with combined inherited thrombophilias, the risk of venous thromboembolism is increased to a greater degree: it is 20 to 50 times higher.7,8,37–39 However, whether the risk of death is higher is not clear.
VENOUS THROMBOEMBOLISM IS MULTIFACTORIAL
The pathogenesis of venous thromboembolism is multifactorial and involves an interaction between inherited and acquired factors. Very often, people with factor V Leiden have additional risk factors that contribute to the development of venous clots, and it is very unusual for them to have thrombosis in the absence of these additional factors.
These factors include older age, surgery, obesity, prolonged travel, immobility, hospitalization, oral contraceptive use, hormonal replacement therapy, pregnancy, and malignancy. They increase the risk of venous thrombosis in normal individuals as well, but more so in people with factor V Leiden.40–43
Testing for other known causes of thrombophilia may also be pursued. These include elevated homocysteine levels, the factor II (prothrombin) G20210A mutation, anticardiolipin antibody, lupus anticoagulant, and deficiencies of antithrombin III, protein C, and protein S.
Factor V Leiden by itself does not appear to increase the risk of arterial thrombosis, ie, heart attack and stroke.33,38,44–46
Family history: A risk indicator for venous thrombosis
Family history is an important indicator of risk for a first venous thromboembolic event, regardless of other risk factors identified. The risk of a first event is two to three times higher in people with a family history of thrombosis in a first-degree relative. The risk is four times higher when multiple family members are affected, at least one of them before age 50.47
In people with genetic thrombophilia, the risk of thrombosis (especially unprovoked thrombosis at a young age) is also higher in those with a strong family history than in those without a family history. In those with factor V Leiden, the risk of venous thromboembolism is three to four times higher if there is a positive family history. The risk is five times higher in carriers of factor V Leiden with a family history of venous thromboembolism before age 50, and 13 times higher in those with more than one affected family member.47
Possible shared environmental factors or coinheritance of other unidentified genetic factors may also contribute to the higher susceptibility in thrombosis-prone families.
TESTING FOR APC RESISTANCE AND FACTOR V LEIDEN
The factor V Leiden mutation can be detected directly by genetic testing of peripheral blood mononuclear cells. This method is relatively time-consuming and expensive, however.
At present, the most cost-effective approach is to test first for APC resistance using a second-generation coagulation assay—the modified APC sensitivity test. In this clot-based method, the patient’s sample is prediluted with factor V-deficient plasma to eliminate the effect of lupus anticoagulants and factor deficiencies that could prolong the baseline clotting time, and heparin is inactivated by polybrene. Then either an augmented partial-thromboplastin-time-based assay or a tissue-factor-dependent factor V assay is performed.
This test is nearly 100% sensitive and specific for factor V Leiden, in contrast to the first-generation, or classic, APC sensitivity test, which lacked specificity and sensitivity for it.9–11,48–60 This modification also permits testing of patients receiving anticoagulants or who have abnormal augmented partial thromboplastin times due to coagulation factor deficiencies.
A positive result on the modified APC sensitivity test should be confirmed by a direct genetic test for the factor V Leiden mutation. An APC resistance assay is unnecessary if a direct genetic test is used initially.
HOW LONG TO GIVE ANTICOAGULATION AFTER VENOUS THROMBOEMBOLISM?
Patients who have had an episode of venous thromboembolism have to be treated with anticoagulants.
In general, the initial management of venous thromboembolism in patients with heritable thrombophilias is no different from that in any other patient with a clot. Anticoagulants such as warfarin are given at a target INR of 2.5 (range 2.0–3.0).32 The duration of treatment is based on the risk factors that resulted in the thrombotic event.
After a first event, some authorities recommend anticoagulant therapy for 6 months.32 A shorter period (3 months) is recommended if there is a transient risk factor (eg, surgery, oral contraceptive use, travel, pregnancy, the puerperium) and the thrombosis is confined to distal veins (eg, the calf veins).32
Factor V Leiden does not necessarily increase the risk of recurrent events in patients who have a transient risk factor. Therefore, people who are heterozygous for this mutation do not usually need to be treated lifelong with anticoagulants if they have had only one episode of deep vein thrombosis or pulmonary embolism, given the risk of bleeding associated with anticoagulation, unless they have additional risk factors.
Conditions in which indefinite anticoagulation may be required after careful consideration of the risks and benefits are:
- Life-threatening events such as near-fatal pulmonary embolism
- Cerebral or visceral vein thrombosis
- Recurrent thrombotic events
- Additional persistent risk factors (eg, active malignant neoplasm, extremity paresis, and antiphospholipid antibodies)
- Combined thrombophilias (eg, combined heterozygosity for factor V Leiden and the prothrombin G20210A mutation)
- Homozygosity for factor V Leiden.32,46,48
Factor V Leiden by itself or combined with other thrombophilic abnormalities is not associated with a higher risk of recurrent venous thromboembolism during warfarin therapy (a possible exception is the combination of factor V Leiden plus antiphospholipid antibodies).32,34 Furthermore, current evidence suggests that no thrombophilic defect is a clinically important risk factor for recurrent venous thromboembolism after anticoagulant therapy is stopped. All these facts indicate that clinical factors are probably more important than laboratory abnormalities in determining the duration of anticoagulation therapy.32,35,36,61–63
PRIMARY PROPHYLAXIS IN PATIENTS WITH FACTOR V LEIDEN
Factor V Leiden is only one of many risk factors for deep vein thrombosis or pulmonary embolism. If carriers of factor V Leiden have never had a blood clot, then they are not routinely treated with an anticoagulant. Rather, they should be counseled about reducing or eliminating other factors that may add to their risk of developing a clot in the future.
Usually, the effect of risk factors is additive: the more risk factors present, the higher the risk.46,50 Sometimes, however, the effect of multiple risk factors is more than additive.
Some risk factors, such as genetics or age, are not alterable, but many can be controlled by medications or lifestyle modifications. Therefore, general measures and precautions are recommended to minimize the risk of thrombosis. For example:
Losing weight (if the patient is overweight) is an important intervention for risk reduction, since obesity is probably the most common modifiable risk factor for developing blood clots.
Avoiding long periods of immobility is recommended. For example, if the patient is taking a long car ride (more than 2 hours), then stopping every few hours and walking around for a few minutes is a good way to keep the blood circulating. If the patient has a desk job, getting up and walking around the office periodically is advised. On long airplane trips, a walk in the aisle every so often and preventing dehydration by drinking plenty of fluids and avoiding alcohol are recommended.
Wearing elastic stockings with a graduated elastic pressure may prevent deep venous thrombosis from developing on long flights.63–65
Staying active and getting regular exercise through such activities as walking, bicycling, or swimming are helpful.
Avoiding smoking is critical.50,63
Thromboprophylaxis is recommended for most acutely ill hospitalized patients, especially those confined to bed with additional risk factors. Guidelines for prophylaxis are based on an individualized risk assessment and not on thrombophilia status. Prophylactic anticoagulation is routinely recommended for patients undergoing major high-risk surgery, such as an orthopedic, urologic, gynecologic, or bariatric procedure. Any excess thrombotic risk conferred by thrombophilia is likely small compared with the risk of surgery, and recommendations on the duration and intensity of thromboprophylaxis are not based on thrombophilic status.46,48
Education. Pain, swelling, redness of a limb, unexplained shortness of breath, and chest pain are the most common symptoms of deep vein thrombosis and pulmonary embolism.46,50 It is crucial to teach patients with factor V Leiden to recognize these symptoms and to seek early medical attention in case they experience any of them.
SCREENING FAMILY MEMBERS FOR THE FACTOR V LEIDEN MUTATION
Factor V Leiden by itself is a relatively mild thrombophilic defect that does not cause thrombosis in all carriers, and there is no evidence that early diagnosis reduces rates of morbidity or mortality. Therefore, routine screening of all asymptomatic relatives of affected patients with venous thrombosis is not recommended. Rather, the decision to screen should be made on an individual basis.50,66
Screening may be beneficial in selected cases, especially when patients have a strong family history of recurrent venous thrombosis at a young age (younger than 50 years) and the family member has additional risk factors for venous thromboembolism such as oral contraception or is planning for pregnancy.32,48,49,66
A 29-year-old white man with no chronic medical problems presents to the emergency department with shortness of breath, left-sided pleuritic chest pain, cough, and hemoptysis. These symptoms began abruptly 1 day ago and have persisted. He also has mild pain and swelling in both calves. He denies having any fever, night sweats, or chills. On further questioning, he reports having taken a long, nonstop driving trip that lasted 8 hours 1 week ago.
His medical history is negative, and he specifically reports no history of deep venous thrombosis or pulmonary embolism. He underwent appendectomy 10 years ago but has had no other operations. He does not take any medications. His family history is noncontributory and is negative for venous thromboembolism. He smokes and uses alcohol occasionally but not illicit drugs.
Examination. He appears to be in considerable distress because of his chest pain. His temperature is 100.4°F (38.0°C), blood pressure 125/70 mm Hg, heart rate 125 beats per minute, respiratory rate 26 breaths per minute, oxygen saturation 92% on room air, and body mass index 19 kg/m2.
Chest examination reveals diminished vesicular breathing in the left base, which is normal to percussion without added sounds. Both calves are swollen and tender to palpation without skin discoloration. The rest of his examination is normal.
Laboratory values:
- White blood cell count 9.3 × 109/L (reference range 4.5–11.0)
- Hemoglobin 15.9 g/dL (14.0–17.5)
- Platelets 205 × 109/L (150–350)
- Sodium 140 mEq/L (136–142)
- Potassium 3.9 mEq/L (3.5–5.0)
- Chloride 108 mEq/L (96–106)
- Bicarbonate 23 mEq/L (21–28)
- Blood urea nitrogen 14 mg/dL (8–23)
- Creatinine 0.9 mg/dL (0.6–1.2)
- Glucose 95 mg/dL (70–110)
- International normalized ratio (INR) 0.90 (0.00–1.2)
- Partial thromboplastin time 27.5 seconds (24.6–31.8)
- Creatine phosphokinase 205 U/L (39–308)
- Troponin T < 0.015 ng/mL (0.01–0.045).
Pulmonary embolism is diagnosed
Factor V Leiden is diagnosed, and the patient recovers with treatment
Anticoagulation is started in the emergency department.
Given this patient’s young age and clot burden, a hypercoagulable state is suspected. Thrombophilia screening is performed, with tests for the factor V Leiden mutation, the prothrombin G20210A mutation, and antiphospholipid and lupus anticoagulant antibodies. The rest of the thrombophilia panel, including antithrombin III, factor VIII, protein C, and protein S, is deferred because the levels of these substances would be expected to change during the acute thrombosis.
The direct test for factor V Leiden mutation is positive for the heterozygous type. The test for the prothrombin G20210A mutation is negative, and his antiphospholipid antibody levels, including the lupus anticoagulant titer, are within normal limits.
The patient is kept on a standard regimen of unfractionated heparin, overlapped with warfarin (Coumadin) until his INR is 2.0 to 3.0 on 2 consecutive days. His hospital course is uneventful and his condition gradually improves.
He is discharged home to continue on oral anticoagulation for 6 months with a target INR of 2.0 to 3.0. Two weeks after completing his anticoagulation therapy, his levels of antithrombin III, factor VIII, protein C, and protein S are all within normal limits.
FACTOR V LEIDEN IS COMMON
Factor V Leiden is the most common inherited thrombophilia, with a prevalence of 3% to 7% in the general US population,1 approximately 5% in whites, 2.2% in Hispanics, and 1.2% in blacks.2 Its prevalence in patients with venous thromboembolism, however, is 50%.1,3 The annual incidence of venous thromboembolism in patients with factor V Leiden is 0.5%.4,5
MORE COAGULATION, LESS ANTICOAGULATION
Factor V has a critical position in both the coagulant and anticoagulant pathways. Factor V Leiden results in a hypercoagulable state by both increasing coagulation and decreasing anticoagulation.
This mutation causes factor V to be resistant to being cleaved and inactivated by activated protein C, a condition known as APC resistance. As a result, more factor Va is available within the prothrombinase complex, increasing coagulation by increased generation of thrombin.6–8
Furthermore, a cofactor formed by cleavage of factor V at position 506 is thought to support activated protein C in degrading factor VIIIa (in the tenase complex), along with protein S. People with factor V Leiden lack this cleavage product and thus have less anticoagulant activity from activated protein C. The increased coagulation and decreased anticoagulation appear to contribute equally to the hypercoagulable state in factor V Leiden-associated APC resistance.9–11
Heterozygosity for the factor V Leiden mutation accounts for 90% to 95% of cases of APC resistance. A much smaller number of people are homozygous for it.1
People who are homozygous for factor V Leiden are at higher risk of venous thromboembolism than those who are heterozygous for it, since the latter group’s blood contains both factor V Leiden and normal factor V. The normal factor V allows anticoagulation via the second pathway of inactivation of factor VIIIa by activated protein C, giving some protection against thrombosis. In people who are homozygous for factor V Leiden, the lack of normal factor V acting as an anticoagulant protein results in a higher thrombotic risk.9–11
Other factor V mutations may also cause APC resistance
Although factor V Leiden is the only genetic defect for which a causal relationship with APC resistance has been clearly determined, other, rarer hereditary factor V mutations or polymorphisms have been described, such as factor V Cambridge (Arg306Thr)12 and factor V Hong Kong (Arg306Gly).13 These mutations may result in APC resistance, but their clinical association with thrombosis is less clear.14 Factor V Liverpool (Ile359Thr) is associated with a higher risk of thrombosis, apparently because of reduced APC-mediated inactivation of factor Va and because it is a poor cofactor with activated protein C for the inactivation of factor VIIIa.15
An R2 haplotype has also been described in association with APC resistance.16,17 The phenomenon may be due to a reduction in activated protein C cofactor activity.9 However, not all studies have been convincing regarding the role of this haplotype in clinical disease.18 Coinheritance of this haplotype with factor V Leiden may increase the risk of venous thromboembolism above that associated with factor V Leiden alone.19
Although factor V Leiden is the most common cause of inherited APC resistance, other changes in hemostasis cause acquired APC resistance and may contribute to the thrombotic tendency in these patients.20–22 The most common causes of acquired APC resistance include elevated factor VIII levels,23–25 pregnancy,26–28 use of oral contraceptives,29,30 and antiphospholipid antibodies.31
USUALLY MANIFESTS AS DEEP VEIN THROMBOSIS
Factor V Leiden usually manifests as deep vein thrombosis with or without pulmonary embolism, but thrombosis in unusual locations also occurs.32
The risk of a first episode of venous thromboembolism is two to five times higher with heterozygous factor V Leiden. However, even though the relative risk is high, the absolute risk is low. Furthermore, despite the higher risk of venous thrombosis, there is no evidence that heterozygosity for factor V Leiden increases the overall mortality rate.4,33–36
In people with homozygous factor V Leiden or with combined inherited thrombophilias, the risk of venous thromboembolism is increased to a greater degree: it is 20 to 50 times higher.7,8,37–39 However, whether the risk of death is higher is not clear.
VENOUS THROMBOEMBOLISM IS MULTIFACTORIAL
The pathogenesis of venous thromboembolism is multifactorial and involves an interaction between inherited and acquired factors. Very often, people with factor V Leiden have additional risk factors that contribute to the development of venous clots, and it is very unusual for them to have thrombosis in the absence of these additional factors.
These factors include older age, surgery, obesity, prolonged travel, immobility, hospitalization, oral contraceptive use, hormonal replacement therapy, pregnancy, and malignancy. They increase the risk of venous thrombosis in normal individuals as well, but more so in people with factor V Leiden.40–43
Testing for other known causes of thrombophilia may also be pursued. These include elevated homocysteine levels, the factor II (prothrombin) G20210A mutation, anticardiolipin antibody, lupus anticoagulant, and deficiencies of antithrombin III, protein C, and protein S.
Factor V Leiden by itself does not appear to increase the risk of arterial thrombosis, ie, heart attack and stroke.33,38,44–46
Family history: A risk indicator for venous thrombosis
Family history is an important indicator of risk for a first venous thromboembolic event, regardless of other risk factors identified. The risk of a first event is two to three times higher in people with a family history of thrombosis in a first-degree relative. The risk is four times higher when multiple family members are affected, at least one of them before age 50.47
In people with genetic thrombophilia, the risk of thrombosis (especially unprovoked thrombosis at a young age) is also higher in those with a strong family history than in those without a family history. In those with factor V Leiden, the risk of venous thromboembolism is three to four times higher if there is a positive family history. The risk is five times higher in carriers of factor V Leiden with a family history of venous thromboembolism before age 50, and 13 times higher in those with more than one affected family member.47
Possible shared environmental factors or coinheritance of other unidentified genetic factors may also contribute to the higher susceptibility in thrombosis-prone families.
TESTING FOR APC RESISTANCE AND FACTOR V LEIDEN
The factor V Leiden mutation can be detected directly by genetic testing of peripheral blood mononuclear cells. This method is relatively time-consuming and expensive, however.
At present, the most cost-effective approach is to test first for APC resistance using a second-generation coagulation assay—the modified APC sensitivity test. In this clot-based method, the patient’s sample is prediluted with factor V-deficient plasma to eliminate the effect of lupus anticoagulants and factor deficiencies that could prolong the baseline clotting time, and heparin is inactivated by polybrene. Then either an augmented partial-thromboplastin-time-based assay or a tissue-factor-dependent factor V assay is performed.
This test is nearly 100% sensitive and specific for factor V Leiden, in contrast to the first-generation, or classic, APC sensitivity test, which lacked specificity and sensitivity for it.9–11,48–60 This modification also permits testing of patients receiving anticoagulants or who have abnormal augmented partial thromboplastin times due to coagulation factor deficiencies.
A positive result on the modified APC sensitivity test should be confirmed by a direct genetic test for the factor V Leiden mutation. An APC resistance assay is unnecessary if a direct genetic test is used initially.
HOW LONG TO GIVE ANTICOAGULATION AFTER VENOUS THROMBOEMBOLISM?
Patients who have had an episode of venous thromboembolism have to be treated with anticoagulants.
In general, the initial management of venous thromboembolism in patients with heritable thrombophilias is no different from that in any other patient with a clot. Anticoagulants such as warfarin are given at a target INR of 2.5 (range 2.0–3.0).32 The duration of treatment is based on the risk factors that resulted in the thrombotic event.
After a first event, some authorities recommend anticoagulant therapy for 6 months.32 A shorter period (3 months) is recommended if there is a transient risk factor (eg, surgery, oral contraceptive use, travel, pregnancy, the puerperium) and the thrombosis is confined to distal veins (eg, the calf veins).32
Factor V Leiden does not necessarily increase the risk of recurrent events in patients who have a transient risk factor. Therefore, people who are heterozygous for this mutation do not usually need to be treated lifelong with anticoagulants if they have had only one episode of deep vein thrombosis or pulmonary embolism, given the risk of bleeding associated with anticoagulation, unless they have additional risk factors.
Conditions in which indefinite anticoagulation may be required after careful consideration of the risks and benefits are:
- Life-threatening events such as near-fatal pulmonary embolism
- Cerebral or visceral vein thrombosis
- Recurrent thrombotic events
- Additional persistent risk factors (eg, active malignant neoplasm, extremity paresis, and antiphospholipid antibodies)
- Combined thrombophilias (eg, combined heterozygosity for factor V Leiden and the prothrombin G20210A mutation)
- Homozygosity for factor V Leiden.32,46,48
Factor V Leiden by itself or combined with other thrombophilic abnormalities is not associated with a higher risk of recurrent venous thromboembolism during warfarin therapy (a possible exception is the combination of factor V Leiden plus antiphospholipid antibodies).32,34 Furthermore, current evidence suggests that no thrombophilic defect is a clinically important risk factor for recurrent venous thromboembolism after anticoagulant therapy is stopped. All these facts indicate that clinical factors are probably more important than laboratory abnormalities in determining the duration of anticoagulation therapy.32,35,36,61–63
PRIMARY PROPHYLAXIS IN PATIENTS WITH FACTOR V LEIDEN
Factor V Leiden is only one of many risk factors for deep vein thrombosis or pulmonary embolism. If carriers of factor V Leiden have never had a blood clot, then they are not routinely treated with an anticoagulant. Rather, they should be counseled about reducing or eliminating other factors that may add to their risk of developing a clot in the future.
Usually, the effect of risk factors is additive: the more risk factors present, the higher the risk.46,50 Sometimes, however, the effect of multiple risk factors is more than additive.
Some risk factors, such as genetics or age, are not alterable, but many can be controlled by medications or lifestyle modifications. Therefore, general measures and precautions are recommended to minimize the risk of thrombosis. For example:
Losing weight (if the patient is overweight) is an important intervention for risk reduction, since obesity is probably the most common modifiable risk factor for developing blood clots.
Avoiding long periods of immobility is recommended. For example, if the patient is taking a long car ride (more than 2 hours), then stopping every few hours and walking around for a few minutes is a good way to keep the blood circulating. If the patient has a desk job, getting up and walking around the office periodically is advised. On long airplane trips, a walk in the aisle every so often and preventing dehydration by drinking plenty of fluids and avoiding alcohol are recommended.
Wearing elastic stockings with a graduated elastic pressure may prevent deep venous thrombosis from developing on long flights.63–65
Staying active and getting regular exercise through such activities as walking, bicycling, or swimming are helpful.
Avoiding smoking is critical.50,63
Thromboprophylaxis is recommended for most acutely ill hospitalized patients, especially those confined to bed with additional risk factors. Guidelines for prophylaxis are based on an individualized risk assessment and not on thrombophilia status. Prophylactic anticoagulation is routinely recommended for patients undergoing major high-risk surgery, such as an orthopedic, urologic, gynecologic, or bariatric procedure. Any excess thrombotic risk conferred by thrombophilia is likely small compared with the risk of surgery, and recommendations on the duration and intensity of thromboprophylaxis are not based on thrombophilic status.46,48
Education. Pain, swelling, redness of a limb, unexplained shortness of breath, and chest pain are the most common symptoms of deep vein thrombosis and pulmonary embolism.46,50 It is crucial to teach patients with factor V Leiden to recognize these symptoms and to seek early medical attention in case they experience any of them.
SCREENING FAMILY MEMBERS FOR THE FACTOR V LEIDEN MUTATION
Factor V Leiden by itself is a relatively mild thrombophilic defect that does not cause thrombosis in all carriers, and there is no evidence that early diagnosis reduces rates of morbidity or mortality. Therefore, routine screening of all asymptomatic relatives of affected patients with venous thrombosis is not recommended. Rather, the decision to screen should be made on an individual basis.50,66
Screening may be beneficial in selected cases, especially when patients have a strong family history of recurrent venous thrombosis at a young age (younger than 50 years) and the family member has additional risk factors for venous thromboembolism such as oral contraception or is planning for pregnancy.32,48,49,66
- Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet 1995; 346:1133–1134.
- Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA 1997; 277:1305–1307.
- Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 1995; 85:1504–1508.
- Stolz E, Kemkes-Matthes B, Pötzsch B, et al. Screening for thrombophilic risk factors among 25 German patients with cerebral venous thrombosis. Acta Neurol Scand 2000; 102:31–36.
- Langlois NJ, Wells PS. Risk of venous thromboembolism in relatives of symptomatic probands with thrombophilia: a systematic review. Thromb Haemost 2003; 90:17–26.
- Juul K, Tybjaerg-Hansen A, Mortensen J, Lange P, Vestbo J, Nordestgaard BG. Factor V leiden homozygosity, dyspnea, and reduced pulmonary function. Arch Intern Med 2005; 165:2032–2036.
- Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994; 369:64–67.
- Dahlbäck B. New molecular insights into the genetics of thrombophilia. Resistance to activated protein C caused by Arg506 to Gln mutation in factor V as a pathogenic risk factor for venous thrombosis. Thromb Haemost 1995; 74:139–148.
- Castoldi E, Brugge JM, Nicolaes GA, Girelli D, Tans G, Rosing J. Impaired APC cofactor activity of factor V plays a major role in the APC resistance associated with the factor V Leiden (R506Q) and R2 (H1299R) mutations. Blood 2004; 103:4173–4179.
- Dahlback B. Anticoagulant factor V and thrombosis risk (editorial). Blood 2004; 103:3995.
- Simioni P, Castoldi E, Lunghi B, Tormene D, Rosing J, Bernardi F. An underestimated combination of opposites resulting in enhanced thrombotic tendency. Blood 2005; 106:2363–2365.
- Williamson D, Brown K, Luddington R, Baglin C, Baglin T. Factor V Cambridge: a new mutation (Arg306-->Thr) associated with resistance to activated protein C. Blood 1998; 91:1140–1144.
- Chan WP, Lee CK, Kwong YL, Lam CK, Liang R. A novel mutation of Arg306 of factor V gene in Hong Kong Chinese. Blood 1998; 91:1135–1139.
- Liang R, Lee CK, Wat MS, Kwong YL, Lam CK, Liu HW. Clinical significance of Arg306 mutations of factor V gene. Blood 1998; 92:2599–2600.
- Steen M, Norstrøm EA, Tholander AL, et al. Functional characterization of factor V-Ile359Thr: a novel mutation associated with thrombosis. Blood 2004; 103:3381–3387.
- Bernardi F, Faioni EM, Castoldi E, et al. A factor V genetic component differing from factor V R506Q contributes to the activated protein C resistance phenotype. Blood 1997; 90:1552–1557.
- Lunghi B, Castoldi E, Mingozzi F, Bernardi F. A new factor V gene polymorphism (His 1254 Arg) present in subjects of African origin mimics the R2 polymorphism (His 1299 Arg). Blood 1998; 91:364–365.
- Luddington R, Jackson A, Pannerselvam S, Brown K, Baglin T. The factor V R2 allele: risk of venous thromboembolism, factor V levels and resistance to activated protein C. Thromb Haemost 2000; 83:204–208.
- Faioni EM, Franchi F, Bucciarelli P, et al. Coinheritance of the HR2 haplotype in the factor V gene confers an increased risk of venous thromboembolism to carriers of factor V R506Q (factor V Leiden). Blood 1999; 94:3062–3066.
- Clark P, Walker ID. The phenomenon known as acquired activated protein C resistance. Br J Haematol 2001; 115:767–773.
- Tosetto A, Simioni M, Madeo D, Rodeghiero F. Intraindividual consistency of the activated protein C resistance phenotype. Br J Haematol 2004; 126:405–409.
- de Visser MC, Rosendaal FR, Bertina RM. A reduced sensitivity for activated protein C in the absence of factor V Leiden increases the risk of venous thrombosis. Blood 1999; 93:1271–1276.
- Kraaijenhagen RA, in’t Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost 2000; 83:5–9.
- Kamphuisen PW, Eikenboom JC, Bertina RM. Elevated factor VIII levels and the risk of thrombosis. Arterioscler Thromb Vasc Biol 2001; 21:731–738.
- Koster T, Blann AD, Briët E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995; 345:152–155.
- Clark P, Brennand J, Conkie JA, McCall F, Greer IA, Walker ID. Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost 1998; 79:1166–1170.
- Cumming AM, Tait RC, Fildes S, Yoong A, Keeney S, Hay CR. Development of resistance to activated protein C during pregnancy. Br J Haematol 1995; 90:725–727.
- Mathonnet F, de Mazancourt P, Bastenaire B, et al. Activated protein C sensitivity ratio in pregnant women at delivery. Br J Haematol 1996; 92:244–246.
- Post MS, Rosing J, Van Der Mooren MJ, et al; Ageing Women’ and the Institute for Cardiovascular Research-Vrije Universiteit (ICaRVU). Increased resistance to activated protein C after short-term oral hormone replacement therapy in healthy post-menopausal women. Br J Haematol 2002; 119:1017–1023.
- Olivieri O, Friso S, Manzato F, et al. Resistance to activated protein C in healthy women taking oral contraceptives. Br J Haematol 1995; 91:465–470.
- Bokarewa MI, Blombäck M, Egberg N, Rosén S. A new variant of interaction between phospholipid antibodies and the protein C system. Blood Coagul Fibrinolysis 1994; 5:37–41.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- van Stralen KJ, Doggen CJ, Bezemer ID, Pomp ER, Lisman T, Rosendaal FR. Mechanisms of the factor V Leiden paradox. Arterioscler Thromb Vasc Biol 2008; 28:1872–1877.
- Agaoglu N, Mustafa NA, Turkyilmaz S. Prothrombotic disorders in patients with mesenteric vein thrombosis. J Invest Surg 2003; 16:299–304.
- El-Karaksy H, El-Koofy N, El-Hawary M, et al. Prevalence of factor V Leiden mutation and other hereditary thrombophilic factors in Egyptian children with portal vein thrombosis: results of a single-center case-control study. Ann Hematol 2004; 83:712–715.
- Heijmans BT, Westendorp RG, Knook DL, Kluft C, Slagboom PE. The risk of mortality and the factor V Leiden mutation in a population-based cohort. Thromb Haemost 1998; 80:607–609.
- Turkstra F, Karemaker R, Kuijer PM, Prins MH, Büller HR. Is the prevalence of the factor V Leiden mutation in patients with pulmonary embolism and deep vein thrombosis really different? Thromb Haemost 1999; 81:345–348.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Manten B, Westendorp RG, Koster T, Reitsma PH, Rosendaal FR. Risk factor profiles in patients with different clinical manifestations of venous thromboembolism: a focus on the factor V Leiden mutation. Thromb Haemost 1996; 76:510–513.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, Büller HR, Vandenbroucke JP. Enhancement by factor V Leiden mutation of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet 1995; 346:1593–1596.
- Murphy PT. Factor V Leiden and venous thromboembolism. Ann Intern Med 2004; 141:483–484.
- Nizankowska-Mogilnicka E, Adamek L, Grzanka P, et al. Genetic polymorphisms associated with acute pulmonary embolism and deep venous thrombosis. Eur Respir J 2003; 21:25–30.
- Arsov T, Miladinova D, Spiroski M. Factor V Leiden is associated with higher risk of deep venous thrombosis of large blood vessels. Croat Med J 2006; 47:433–439.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood 2000; 96:3329–3333.
- Ornstein DL, Cushman M. Cardiology patient page. Factor V Leiden. Circulation 2003; 107:e94–e97.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Press RD, Bauer KA, Kujovich JL, Heit JA. Clinical utility of factor V leiden (R506Q) testing for the diagnosis and management of thromboembolic disorders. Arch Pathol Lab Med 2002; 126:1304–1318.
- Gadelha T, Roldán V, Lecumberri R, et al; RIETE Investigators. Clinical characteristics of patients with factor V Leiden or prothrombin G20210A and a first episode of venous thromboembolism. Findings from the RIETE Registry. Thromb Res 2010; 126:283–286.
- Severinsen MT, Overvad K, Johnsen SP, et al. Genetic susceptibility, smoking, obesity and risk of venous thromboembolism. Br J Haematol 2010; 149:273–279.
- Kujovich JL. Factor V Leiden thrombophilia. Genet Med 2011; 13:1–16.
- Lijfering WM, Brouwer JL, Veeger NJ, et al. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006; 166:729–736.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Strobl FJ, Hoffman S, Huber S, Williams EC, Voelkerding KV. Activated protein C resistance assay performance: improvement by sample dilution with factor V-deficient plasma. Arch Pathol Lab Med 1998; 122:430–433.
- Legnani C, Palareti G, Biagi R, et al. Activated protein C resistance: a comparison between two clotting assays and their relationship to the presence of the factor V Leiden mutation. Br J Haematol 1996; 93:694–699.
- Gouault-Heilmann M, Leroy-Matheron C. Factor V Leiden-dependent APC resistance: improved sensitivity and specificity of the APC resistance test by plasma dilution in factor V-depleted plasma. Thromb Res 1996; 82:281–283.
- Svensson PJ, Zöller B, Dahlbäck B. Evaluation of original and modified APC-resistance tests in unselected outpatients with clinically suspected thrombosis and in healthy controls. Thromb Haemost 1997; 77:332–335.
- Tripodi A, Negri B, Bertina RM, Mannucci PM. Screening for the FV:Q506 mutation—evaluation of thirteen plasma-based methods for their diagnostic efficacy in comparison with DNA analysis. Thromb Haemost 1997; 77:436–439.
- Wåhlander K, Larson G, Lindahl TL, et al. Factor V Leiden (G1691A) and prothrombin gene G20210A mutations as potential risk factors for venous thromboembolism after total hip or total knee replacement surgery. Thromb Haemost 2002; 87:580–585.
- Joseph JE, Low J, Courtenay B, Neil MJ, McGrath M, Ma D. A single-centre prospective study of clinical and haemostatic risk factors for venous thromboembolism following lower limb arthroplasty. Br J Haematol 2005; 129:87–92.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):381S–453S.
- Brenner B. Prophylaxis for travel-related thrombosis? Yes. J Thromb Haemost 2004; 2:2089–2091.
- Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med 2011; 6:113–116.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
- Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet 1995; 346:1133–1134.
- Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA 1997; 277:1305–1307.
- Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 1995; 85:1504–1508.
- Stolz E, Kemkes-Matthes B, Pötzsch B, et al. Screening for thrombophilic risk factors among 25 German patients with cerebral venous thrombosis. Acta Neurol Scand 2000; 102:31–36.
- Langlois NJ, Wells PS. Risk of venous thromboembolism in relatives of symptomatic probands with thrombophilia: a systematic review. Thromb Haemost 2003; 90:17–26.
- Juul K, Tybjaerg-Hansen A, Mortensen J, Lange P, Vestbo J, Nordestgaard BG. Factor V leiden homozygosity, dyspnea, and reduced pulmonary function. Arch Intern Med 2005; 165:2032–2036.
- Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994; 369:64–67.
- Dahlbäck B. New molecular insights into the genetics of thrombophilia. Resistance to activated protein C caused by Arg506 to Gln mutation in factor V as a pathogenic risk factor for venous thrombosis. Thromb Haemost 1995; 74:139–148.
- Castoldi E, Brugge JM, Nicolaes GA, Girelli D, Tans G, Rosing J. Impaired APC cofactor activity of factor V plays a major role in the APC resistance associated with the factor V Leiden (R506Q) and R2 (H1299R) mutations. Blood 2004; 103:4173–4179.
- Dahlback B. Anticoagulant factor V and thrombosis risk (editorial). Blood 2004; 103:3995.
- Simioni P, Castoldi E, Lunghi B, Tormene D, Rosing J, Bernardi F. An underestimated combination of opposites resulting in enhanced thrombotic tendency. Blood 2005; 106:2363–2365.
- Williamson D, Brown K, Luddington R, Baglin C, Baglin T. Factor V Cambridge: a new mutation (Arg306-->Thr) associated with resistance to activated protein C. Blood 1998; 91:1140–1144.
- Chan WP, Lee CK, Kwong YL, Lam CK, Liang R. A novel mutation of Arg306 of factor V gene in Hong Kong Chinese. Blood 1998; 91:1135–1139.
- Liang R, Lee CK, Wat MS, Kwong YL, Lam CK, Liu HW. Clinical significance of Arg306 mutations of factor V gene. Blood 1998; 92:2599–2600.
- Steen M, Norstrøm EA, Tholander AL, et al. Functional characterization of factor V-Ile359Thr: a novel mutation associated with thrombosis. Blood 2004; 103:3381–3387.
- Bernardi F, Faioni EM, Castoldi E, et al. A factor V genetic component differing from factor V R506Q contributes to the activated protein C resistance phenotype. Blood 1997; 90:1552–1557.
- Lunghi B, Castoldi E, Mingozzi F, Bernardi F. A new factor V gene polymorphism (His 1254 Arg) present in subjects of African origin mimics the R2 polymorphism (His 1299 Arg). Blood 1998; 91:364–365.
- Luddington R, Jackson A, Pannerselvam S, Brown K, Baglin T. The factor V R2 allele: risk of venous thromboembolism, factor V levels and resistance to activated protein C. Thromb Haemost 2000; 83:204–208.
- Faioni EM, Franchi F, Bucciarelli P, et al. Coinheritance of the HR2 haplotype in the factor V gene confers an increased risk of venous thromboembolism to carriers of factor V R506Q (factor V Leiden). Blood 1999; 94:3062–3066.
- Clark P, Walker ID. The phenomenon known as acquired activated protein C resistance. Br J Haematol 2001; 115:767–773.
- Tosetto A, Simioni M, Madeo D, Rodeghiero F. Intraindividual consistency of the activated protein C resistance phenotype. Br J Haematol 2004; 126:405–409.
- de Visser MC, Rosendaal FR, Bertina RM. A reduced sensitivity for activated protein C in the absence of factor V Leiden increases the risk of venous thrombosis. Blood 1999; 93:1271–1276.
- Kraaijenhagen RA, in’t Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost 2000; 83:5–9.
- Kamphuisen PW, Eikenboom JC, Bertina RM. Elevated factor VIII levels and the risk of thrombosis. Arterioscler Thromb Vasc Biol 2001; 21:731–738.
- Koster T, Blann AD, Briët E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995; 345:152–155.
- Clark P, Brennand J, Conkie JA, McCall F, Greer IA, Walker ID. Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost 1998; 79:1166–1170.
- Cumming AM, Tait RC, Fildes S, Yoong A, Keeney S, Hay CR. Development of resistance to activated protein C during pregnancy. Br J Haematol 1995; 90:725–727.
- Mathonnet F, de Mazancourt P, Bastenaire B, et al. Activated protein C sensitivity ratio in pregnant women at delivery. Br J Haematol 1996; 92:244–246.
- Post MS, Rosing J, Van Der Mooren MJ, et al; Ageing Women’ and the Institute for Cardiovascular Research-Vrije Universiteit (ICaRVU). Increased resistance to activated protein C after short-term oral hormone replacement therapy in healthy post-menopausal women. Br J Haematol 2002; 119:1017–1023.
- Olivieri O, Friso S, Manzato F, et al. Resistance to activated protein C in healthy women taking oral contraceptives. Br J Haematol 1995; 91:465–470.
- Bokarewa MI, Blombäck M, Egberg N, Rosén S. A new variant of interaction between phospholipid antibodies and the protein C system. Blood Coagul Fibrinolysis 1994; 5:37–41.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- van Stralen KJ, Doggen CJ, Bezemer ID, Pomp ER, Lisman T, Rosendaal FR. Mechanisms of the factor V Leiden paradox. Arterioscler Thromb Vasc Biol 2008; 28:1872–1877.
- Agaoglu N, Mustafa NA, Turkyilmaz S. Prothrombotic disorders in patients with mesenteric vein thrombosis. J Invest Surg 2003; 16:299–304.
- El-Karaksy H, El-Koofy N, El-Hawary M, et al. Prevalence of factor V Leiden mutation and other hereditary thrombophilic factors in Egyptian children with portal vein thrombosis: results of a single-center case-control study. Ann Hematol 2004; 83:712–715.
- Heijmans BT, Westendorp RG, Knook DL, Kluft C, Slagboom PE. The risk of mortality and the factor V Leiden mutation in a population-based cohort. Thromb Haemost 1998; 80:607–609.
- Turkstra F, Karemaker R, Kuijer PM, Prins MH, Büller HR. Is the prevalence of the factor V Leiden mutation in patients with pulmonary embolism and deep vein thrombosis really different? Thromb Haemost 1999; 81:345–348.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Manten B, Westendorp RG, Koster T, Reitsma PH, Rosendaal FR. Risk factor profiles in patients with different clinical manifestations of venous thromboembolism: a focus on the factor V Leiden mutation. Thromb Haemost 1996; 76:510–513.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, Büller HR, Vandenbroucke JP. Enhancement by factor V Leiden mutation of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet 1995; 346:1593–1596.
- Murphy PT. Factor V Leiden and venous thromboembolism. Ann Intern Med 2004; 141:483–484.
- Nizankowska-Mogilnicka E, Adamek L, Grzanka P, et al. Genetic polymorphisms associated with acute pulmonary embolism and deep venous thrombosis. Eur Respir J 2003; 21:25–30.
- Arsov T, Miladinova D, Spiroski M. Factor V Leiden is associated with higher risk of deep venous thrombosis of large blood vessels. Croat Med J 2006; 47:433–439.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood 2000; 96:3329–3333.
- Ornstein DL, Cushman M. Cardiology patient page. Factor V Leiden. Circulation 2003; 107:e94–e97.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Press RD, Bauer KA, Kujovich JL, Heit JA. Clinical utility of factor V leiden (R506Q) testing for the diagnosis and management of thromboembolic disorders. Arch Pathol Lab Med 2002; 126:1304–1318.
- Gadelha T, Roldán V, Lecumberri R, et al; RIETE Investigators. Clinical characteristics of patients with factor V Leiden or prothrombin G20210A and a first episode of venous thromboembolism. Findings from the RIETE Registry. Thromb Res 2010; 126:283–286.
- Severinsen MT, Overvad K, Johnsen SP, et al. Genetic susceptibility, smoking, obesity and risk of venous thromboembolism. Br J Haematol 2010; 149:273–279.
- Kujovich JL. Factor V Leiden thrombophilia. Genet Med 2011; 13:1–16.
- Lijfering WM, Brouwer JL, Veeger NJ, et al. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006; 166:729–736.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Strobl FJ, Hoffman S, Huber S, Williams EC, Voelkerding KV. Activated protein C resistance assay performance: improvement by sample dilution with factor V-deficient plasma. Arch Pathol Lab Med 1998; 122:430–433.
- Legnani C, Palareti G, Biagi R, et al. Activated protein C resistance: a comparison between two clotting assays and their relationship to the presence of the factor V Leiden mutation. Br J Haematol 1996; 93:694–699.
- Gouault-Heilmann M, Leroy-Matheron C. Factor V Leiden-dependent APC resistance: improved sensitivity and specificity of the APC resistance test by plasma dilution in factor V-depleted plasma. Thromb Res 1996; 82:281–283.
- Svensson PJ, Zöller B, Dahlbäck B. Evaluation of original and modified APC-resistance tests in unselected outpatients with clinically suspected thrombosis and in healthy controls. Thromb Haemost 1997; 77:332–335.
- Tripodi A, Negri B, Bertina RM, Mannucci PM. Screening for the FV:Q506 mutation—evaluation of thirteen plasma-based methods for their diagnostic efficacy in comparison with DNA analysis. Thromb Haemost 1997; 77:436–439.
- Wåhlander K, Larson G, Lindahl TL, et al. Factor V Leiden (G1691A) and prothrombin gene G20210A mutations as potential risk factors for venous thromboembolism after total hip or total knee replacement surgery. Thromb Haemost 2002; 87:580–585.
- Joseph JE, Low J, Courtenay B, Neil MJ, McGrath M, Ma D. A single-centre prospective study of clinical and haemostatic risk factors for venous thromboembolism following lower limb arthroplasty. Br J Haematol 2005; 129:87–92.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):381S–453S.
- Brenner B. Prophylaxis for travel-related thrombosis? Yes. J Thromb Haemost 2004; 2:2089–2091.
- Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med 2011; 6:113–116.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
KEY POINTS
- The pathogenesis of venous thromboembolism is complex and multifactorial, often reflecting the interplay between environmental, clinical, and genetic factors.
- Factor V Leiden increases the risk of venous thromboembolism but by itself does not appear to increase the risk of arterial thrombosis.
- Often, people with factor V Leiden may have additional risk factors that increase the rate of venous clots, such as older age, surgery, obesity, immobility, prolonged travel, hospitalization, oral contraceptive use, hormonal replacement therapy, pregnancy, and malignancy.
- General measures and precautions are needed to minimize the risk of venous thromboembolism in people with the factor V Leiden mutation, especially when modifiable factors are present, such as obesity and long periods of immobilization.
A 40-year-old woman with excoriated skin lesions
She denies any history of itching, insect bites, exposure to new medications or jewelry, allergies, recent change in medications, travel, or intravenous drug abuse.
Q: Which is the most likely diagnosis?
- Allergic contact dermatitis
- Xerosis
- Dermatotillomania
- Folliculitis
- Infestation (scabies)
A: Dermatotillomania, ie, pathologic skin picking, is the correct diagnosis. On further questioning, the patient reveals that the wounds have been self-inflicted over many years, starting in her adolescence. The wounds are located only in areas she can reach. She admits that social and emotional stressors had made the condition significantly worse and that lately she had lost control of her skin-picking. She denies nail-biting, trichotillomania, or obsessive-compulsive behavior.
As for the other possible diagnoses:
Allergic contact dermatitis occurs when contact with a particular substance elicits a hypersensitivity reaction. This reaction is of the delayed type (type IV). The affected individual can develop skin erythema and swelling with vesicles that are intensely pruritic at the contact site. The erythema may become evident hours after exposure, or not until weeks later, which can make the diagnosis difficult at times.
Our patient’s lesions were not pruritic, and she denied recent exposure to allergens.
Xerosis. Xerotic (dry) skin is usually rough, with fine scales and fissures. Xerosis can affect people of all ages and is often more intense during the winter. It affects mainly the arms, legs, and hands. Patients note pruritus, which can be treated with liberal use of emollients and tepid water baths.
Our patient’s lesions were scarred, hyperpigmented, and nonpruritic.
Folliculitis is a superficial infection of the hair follicle that presents as an erythematous pustule on the extremities, buttocks, or scalp. The pustule can be tender to palpation and can progress to an abscess that requires incision and drainage and intravenous antibiotics. A moist environment and poor hygiene are predisposing factors. Staphylococcus aureus is the culprit in most cases.
Our patient’s lesions were on the chest and upper back, where hair follicles were sparse or absent, and there was no erythema or tenderness.
Scabies is a skin infestation with Sarcoptes scabiei mites, which burrow in the skin and cause intense pruritus, especially at night. Scabies usually affects the sides and webs of the fingers and skin folds. Sexual contact is a common way of transmission; however, transmission can also occur by sharing beds and towels.
Patients with dermatotillomania lack intense pruritus, and skin-picking occurs during the day, while the patient is awake.
SELF-INFLICTED WOUNDS
Pathologic skin-picking, neurotic excoriation, excoriated acne, or dermatotillomania results from scratching, picking, gouging, or squeezing of one’s skin via teeth, fingernails, tweezers, or other objects.1–3 Lesions are usually found on skin that the patient can easily reach, such as the face, chest, upper and lower extremities, and upper back.4
The prevalence of pathologic skin-picking is estimated at 2% in dermatology patients.5 The overall prevalence of psychiatric disorders in all dermatology outpatients is estimated at 30% to 40%. Women outnumber men with this disorder.6
Dermatotillomania is thought to be on the spectrum of obsessive-compulsive disorder, in which patients exhibit impulses and compulsions.5 It starts in childhood or early adulthood, with an average lifetime duration of 21 years.7 It is usually associated with anxiety, depression, obsessive-compulsive traits, eating disorders, body dysmorphic disorders, or hypochondriasis. Psychosocial stress is the main trigger. Patients report feelings of tension and stress before picking and relief while picking; there is no suicidal ideation.8
Treatments are both pharmacologic and behavioral.9 Cognitive behavioral therapy and habit reversal therapy have each been successful when used alone.8 In addition, several case reports10 and double-blind studies11,12 have shown that treatment with a selective serotonin-reuptake inhibitor (SSRI) can reduce pathologic skin-picking.13,14 However, SSRIs have also been reported to induce or aggravate this behavior in patients with underlying mild skin-picking and a family history of skin-picking.15 Thus, it is pertinent to extract a detailed history from the patient before prescribing an SSRI.
We referred our patient for behavioral therapy and prescribed fluoxetine (Prozac) 20 mg daily. She showed improvement in symptoms in 4 weeks and has since stopped skin-picking completely.
- Arnold LM. Phenomenology and therapeutic options for dermatotillomania. Expert Rev Neurother 2002; 2:725–730.
- Bohne A, Keuthen N, Wilhelm S. Pathologic hairpulling, skin picking, and nail biting. Ann Clin Psychiatry 2005; 17:227–232.
- Gattu S, Rashid RM, Khachemoune A. Self-induced skin lesions: a review of dermatitis artefacta. Cutis 2009; 84:247–251.
- Keuthen NJ, Deckersbach T, Wilhelm S, et al. Repetitive skin-picking in a student population and comparison with a sample of self-injurious skin-pickers. Psychosomatics 2000; 41:210–215.
- Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs 2001; 15:351–359.
- Wilhelm S, Keuthen NJ, Deckersbach T, et al. Self-injurious skin picking: clinical characteristics and comorbidity. J Clin Psychiatry 1999; 60:454–459.
- Gupta MA, Gupta AK, Haberman HF. Neurotic excoriations: a review and some new perspectives. Compr Psychiatry 1986; 27:381–386.
- Rosenbaum MS, Ayllon T. The behavioral treatment of neurodermatitis through habit-reversal. Behav Res Ther 1981; 19:313–318.
- Deckersbach T, Wilhelm S, Keuthen N. Self-injurious skin picking: clinical characteristics, assessment methods, and treatment modalities. Brief Treatment and Crisis Intervention 2003; 3:249–260.
- Sharma H. Psychogenic excoriation responding to fluoxetine: a case report. J Indian Med Assoc 2008; 106:245,262.
- Bloch MR, Elliott M, Thompson H, Koran LM. Fluoxetine in pathologic skin-picking: open-label and double-blind results. Psychosomatics 2001; 42:314–319.
- Simeon D, Stein DJ, Gross S, Islam N, Schmeidler J, Hollander E. A double-blind trial of fluoxetine in pathologic skin picking. J Clin Psychiatry 1997; 58:341–347.
- Gupta MA, Gupta AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol 2001; 15:512–518.
- Keuthen NJ, Jameson M, Loh R, Deckersbach T, Wilhelm S, Dougherty DD. Open-label escitalopram treatment for pathological skin picking. Int Clin Psychopharmacol 2007; 22:268–274.
- Denys D, van Megen HJ, Westenberg HG. Emerging skin-picking behaviour after serotonin reuptake inhibitor-treatment in patients with obsessive-compulsive disorder: possible mechanisms and implications for clinical care. J Psychopharmacol 2003; 17:127–129.
She denies any history of itching, insect bites, exposure to new medications or jewelry, allergies, recent change in medications, travel, or intravenous drug abuse.
Q: Which is the most likely diagnosis?
- Allergic contact dermatitis
- Xerosis
- Dermatotillomania
- Folliculitis
- Infestation (scabies)
A: Dermatotillomania, ie, pathologic skin picking, is the correct diagnosis. On further questioning, the patient reveals that the wounds have been self-inflicted over many years, starting in her adolescence. The wounds are located only in areas she can reach. She admits that social and emotional stressors had made the condition significantly worse and that lately she had lost control of her skin-picking. She denies nail-biting, trichotillomania, or obsessive-compulsive behavior.
As for the other possible diagnoses:
Allergic contact dermatitis occurs when contact with a particular substance elicits a hypersensitivity reaction. This reaction is of the delayed type (type IV). The affected individual can develop skin erythema and swelling with vesicles that are intensely pruritic at the contact site. The erythema may become evident hours after exposure, or not until weeks later, which can make the diagnosis difficult at times.
Our patient’s lesions were not pruritic, and she denied recent exposure to allergens.
Xerosis. Xerotic (dry) skin is usually rough, with fine scales and fissures. Xerosis can affect people of all ages and is often more intense during the winter. It affects mainly the arms, legs, and hands. Patients note pruritus, which can be treated with liberal use of emollients and tepid water baths.
Our patient’s lesions were scarred, hyperpigmented, and nonpruritic.
Folliculitis is a superficial infection of the hair follicle that presents as an erythematous pustule on the extremities, buttocks, or scalp. The pustule can be tender to palpation and can progress to an abscess that requires incision and drainage and intravenous antibiotics. A moist environment and poor hygiene are predisposing factors. Staphylococcus aureus is the culprit in most cases.
Our patient’s lesions were on the chest and upper back, where hair follicles were sparse or absent, and there was no erythema or tenderness.
Scabies is a skin infestation with Sarcoptes scabiei mites, which burrow in the skin and cause intense pruritus, especially at night. Scabies usually affects the sides and webs of the fingers and skin folds. Sexual contact is a common way of transmission; however, transmission can also occur by sharing beds and towels.
Patients with dermatotillomania lack intense pruritus, and skin-picking occurs during the day, while the patient is awake.
SELF-INFLICTED WOUNDS
Pathologic skin-picking, neurotic excoriation, excoriated acne, or dermatotillomania results from scratching, picking, gouging, or squeezing of one’s skin via teeth, fingernails, tweezers, or other objects.1–3 Lesions are usually found on skin that the patient can easily reach, such as the face, chest, upper and lower extremities, and upper back.4
The prevalence of pathologic skin-picking is estimated at 2% in dermatology patients.5 The overall prevalence of psychiatric disorders in all dermatology outpatients is estimated at 30% to 40%. Women outnumber men with this disorder.6
Dermatotillomania is thought to be on the spectrum of obsessive-compulsive disorder, in which patients exhibit impulses and compulsions.5 It starts in childhood or early adulthood, with an average lifetime duration of 21 years.7 It is usually associated with anxiety, depression, obsessive-compulsive traits, eating disorders, body dysmorphic disorders, or hypochondriasis. Psychosocial stress is the main trigger. Patients report feelings of tension and stress before picking and relief while picking; there is no suicidal ideation.8
Treatments are both pharmacologic and behavioral.9 Cognitive behavioral therapy and habit reversal therapy have each been successful when used alone.8 In addition, several case reports10 and double-blind studies11,12 have shown that treatment with a selective serotonin-reuptake inhibitor (SSRI) can reduce pathologic skin-picking.13,14 However, SSRIs have also been reported to induce or aggravate this behavior in patients with underlying mild skin-picking and a family history of skin-picking.15 Thus, it is pertinent to extract a detailed history from the patient before prescribing an SSRI.
We referred our patient for behavioral therapy and prescribed fluoxetine (Prozac) 20 mg daily. She showed improvement in symptoms in 4 weeks and has since stopped skin-picking completely.
She denies any history of itching, insect bites, exposure to new medications or jewelry, allergies, recent change in medications, travel, or intravenous drug abuse.
Q: Which is the most likely diagnosis?
- Allergic contact dermatitis
- Xerosis
- Dermatotillomania
- Folliculitis
- Infestation (scabies)
A: Dermatotillomania, ie, pathologic skin picking, is the correct diagnosis. On further questioning, the patient reveals that the wounds have been self-inflicted over many years, starting in her adolescence. The wounds are located only in areas she can reach. She admits that social and emotional stressors had made the condition significantly worse and that lately she had lost control of her skin-picking. She denies nail-biting, trichotillomania, or obsessive-compulsive behavior.
As for the other possible diagnoses:
Allergic contact dermatitis occurs when contact with a particular substance elicits a hypersensitivity reaction. This reaction is of the delayed type (type IV). The affected individual can develop skin erythema and swelling with vesicles that are intensely pruritic at the contact site. The erythema may become evident hours after exposure, or not until weeks later, which can make the diagnosis difficult at times.
Our patient’s lesions were not pruritic, and she denied recent exposure to allergens.
Xerosis. Xerotic (dry) skin is usually rough, with fine scales and fissures. Xerosis can affect people of all ages and is often more intense during the winter. It affects mainly the arms, legs, and hands. Patients note pruritus, which can be treated with liberal use of emollients and tepid water baths.
Our patient’s lesions were scarred, hyperpigmented, and nonpruritic.
Folliculitis is a superficial infection of the hair follicle that presents as an erythematous pustule on the extremities, buttocks, or scalp. The pustule can be tender to palpation and can progress to an abscess that requires incision and drainage and intravenous antibiotics. A moist environment and poor hygiene are predisposing factors. Staphylococcus aureus is the culprit in most cases.
Our patient’s lesions were on the chest and upper back, where hair follicles were sparse or absent, and there was no erythema or tenderness.
Scabies is a skin infestation with Sarcoptes scabiei mites, which burrow in the skin and cause intense pruritus, especially at night. Scabies usually affects the sides and webs of the fingers and skin folds. Sexual contact is a common way of transmission; however, transmission can also occur by sharing beds and towels.
Patients with dermatotillomania lack intense pruritus, and skin-picking occurs during the day, while the patient is awake.
SELF-INFLICTED WOUNDS
Pathologic skin-picking, neurotic excoriation, excoriated acne, or dermatotillomania results from scratching, picking, gouging, or squeezing of one’s skin via teeth, fingernails, tweezers, or other objects.1–3 Lesions are usually found on skin that the patient can easily reach, such as the face, chest, upper and lower extremities, and upper back.4
The prevalence of pathologic skin-picking is estimated at 2% in dermatology patients.5 The overall prevalence of psychiatric disorders in all dermatology outpatients is estimated at 30% to 40%. Women outnumber men with this disorder.6
Dermatotillomania is thought to be on the spectrum of obsessive-compulsive disorder, in which patients exhibit impulses and compulsions.5 It starts in childhood or early adulthood, with an average lifetime duration of 21 years.7 It is usually associated with anxiety, depression, obsessive-compulsive traits, eating disorders, body dysmorphic disorders, or hypochondriasis. Psychosocial stress is the main trigger. Patients report feelings of tension and stress before picking and relief while picking; there is no suicidal ideation.8
Treatments are both pharmacologic and behavioral.9 Cognitive behavioral therapy and habit reversal therapy have each been successful when used alone.8 In addition, several case reports10 and double-blind studies11,12 have shown that treatment with a selective serotonin-reuptake inhibitor (SSRI) can reduce pathologic skin-picking.13,14 However, SSRIs have also been reported to induce or aggravate this behavior in patients with underlying mild skin-picking and a family history of skin-picking.15 Thus, it is pertinent to extract a detailed history from the patient before prescribing an SSRI.
We referred our patient for behavioral therapy and prescribed fluoxetine (Prozac) 20 mg daily. She showed improvement in symptoms in 4 weeks and has since stopped skin-picking completely.
- Arnold LM. Phenomenology and therapeutic options for dermatotillomania. Expert Rev Neurother 2002; 2:725–730.
- Bohne A, Keuthen N, Wilhelm S. Pathologic hairpulling, skin picking, and nail biting. Ann Clin Psychiatry 2005; 17:227–232.
- Gattu S, Rashid RM, Khachemoune A. Self-induced skin lesions: a review of dermatitis artefacta. Cutis 2009; 84:247–251.
- Keuthen NJ, Deckersbach T, Wilhelm S, et al. Repetitive skin-picking in a student population and comparison with a sample of self-injurious skin-pickers. Psychosomatics 2000; 41:210–215.
- Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs 2001; 15:351–359.
- Wilhelm S, Keuthen NJ, Deckersbach T, et al. Self-injurious skin picking: clinical characteristics and comorbidity. J Clin Psychiatry 1999; 60:454–459.
- Gupta MA, Gupta AK, Haberman HF. Neurotic excoriations: a review and some new perspectives. Compr Psychiatry 1986; 27:381–386.
- Rosenbaum MS, Ayllon T. The behavioral treatment of neurodermatitis through habit-reversal. Behav Res Ther 1981; 19:313–318.
- Deckersbach T, Wilhelm S, Keuthen N. Self-injurious skin picking: clinical characteristics, assessment methods, and treatment modalities. Brief Treatment and Crisis Intervention 2003; 3:249–260.
- Sharma H. Psychogenic excoriation responding to fluoxetine: a case report. J Indian Med Assoc 2008; 106:245,262.
- Bloch MR, Elliott M, Thompson H, Koran LM. Fluoxetine in pathologic skin-picking: open-label and double-blind results. Psychosomatics 2001; 42:314–319.
- Simeon D, Stein DJ, Gross S, Islam N, Schmeidler J, Hollander E. A double-blind trial of fluoxetine in pathologic skin picking. J Clin Psychiatry 1997; 58:341–347.
- Gupta MA, Gupta AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol 2001; 15:512–518.
- Keuthen NJ, Jameson M, Loh R, Deckersbach T, Wilhelm S, Dougherty DD. Open-label escitalopram treatment for pathological skin picking. Int Clin Psychopharmacol 2007; 22:268–274.
- Denys D, van Megen HJ, Westenberg HG. Emerging skin-picking behaviour after serotonin reuptake inhibitor-treatment in patients with obsessive-compulsive disorder: possible mechanisms and implications for clinical care. J Psychopharmacol 2003; 17:127–129.
- Arnold LM. Phenomenology and therapeutic options for dermatotillomania. Expert Rev Neurother 2002; 2:725–730.
- Bohne A, Keuthen N, Wilhelm S. Pathologic hairpulling, skin picking, and nail biting. Ann Clin Psychiatry 2005; 17:227–232.
- Gattu S, Rashid RM, Khachemoune A. Self-induced skin lesions: a review of dermatitis artefacta. Cutis 2009; 84:247–251.
- Keuthen NJ, Deckersbach T, Wilhelm S, et al. Repetitive skin-picking in a student population and comparison with a sample of self-injurious skin-pickers. Psychosomatics 2000; 41:210–215.
- Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs 2001; 15:351–359.
- Wilhelm S, Keuthen NJ, Deckersbach T, et al. Self-injurious skin picking: clinical characteristics and comorbidity. J Clin Psychiatry 1999; 60:454–459.
- Gupta MA, Gupta AK, Haberman HF. Neurotic excoriations: a review and some new perspectives. Compr Psychiatry 1986; 27:381–386.
- Rosenbaum MS, Ayllon T. The behavioral treatment of neurodermatitis through habit-reversal. Behav Res Ther 1981; 19:313–318.
- Deckersbach T, Wilhelm S, Keuthen N. Self-injurious skin picking: clinical characteristics, assessment methods, and treatment modalities. Brief Treatment and Crisis Intervention 2003; 3:249–260.
- Sharma H. Psychogenic excoriation responding to fluoxetine: a case report. J Indian Med Assoc 2008; 106:245,262.
- Bloch MR, Elliott M, Thompson H, Koran LM. Fluoxetine in pathologic skin-picking: open-label and double-blind results. Psychosomatics 2001; 42:314–319.
- Simeon D, Stein DJ, Gross S, Islam N, Schmeidler J, Hollander E. A double-blind trial of fluoxetine in pathologic skin picking. J Clin Psychiatry 1997; 58:341–347.
- Gupta MA, Gupta AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol 2001; 15:512–518.
- Keuthen NJ, Jameson M, Loh R, Deckersbach T, Wilhelm S, Dougherty DD. Open-label escitalopram treatment for pathological skin picking. Int Clin Psychopharmacol 2007; 22:268–274.
- Denys D, van Megen HJ, Westenberg HG. Emerging skin-picking behaviour after serotonin reuptake inhibitor-treatment in patients with obsessive-compulsive disorder: possible mechanisms and implications for clinical care. J Psychopharmacol 2003; 17:127–129.