User login
Deep T waves and chest pain
A 67-year-old man with a history of hypertension and hyperlipidemia presented to the emergency department after 3 hours of what he described as a burning sensation in his chest that woke him from sleep. He attributed it at first to a late-night meal and treated himself with some milk and yogurt, which seemed to relieve the symptoms. However, the pain recurred and was associated with difficulty breathing. At that point, he drove himself to the emergency department.
On arrival, his temperature was 36.5°C (97.7°F), blood pressure 134/67 mm Hg, heart rate 89 bpm, respirations 18/min, and oxygen saturation 98% on room air. His cardiovascular, lung, and neurologic examinations were normal. His cardiac enzyme levels (creatine kinase, creatine kinase MB fraction, and troponin T) were within normal limits.
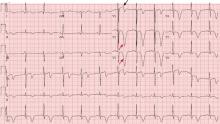
Figure 1 depicts his initial electrocardiogram. It showed deep, symmetric T-wave inversions in the precordial leads especially in V2 and V3, changes known as Wellens syndrome. The ST-T changes in lead V1 suggested a very proximal lesion in the left anterior descending artery (LAD), before the first septal perforator. Also, lateral and high lateral (V5 and V6) findings indicated stenoses of the branching diagonals and left circumflex myocardial territory. Furthermore, the inferior ST-T changes indicated that his LAD may have wrapped around the cardiac apex. All of these findings were prognostically significant.
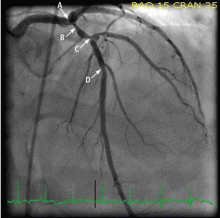
The patient was given aspirin and was started on intravenous unfractionated heparin and nitroglycerin. He was sent for urgent left-heart catheterization, which showed a 50% to 60% stenosis in the left main coronary artery, with involvement of the left circumflex artery proximally, in addition to a “tight” first-diagonal stenosis, a 90% stenosis in a large (> 3.0-mm) proximal segment of the LAD, an 80% stenosis in a large (> 3.0-mm) mid-LAD segment, and a 40% stenosis in a large (> 3.0-mm) second diagonal artery (Figure 2).
He was referred for cardiac surgery and underwent triple coronary artery bypass grafting: the left internal thoracic artery was grafted to the LAD, a reverse saphenous vein graft was performed to the diagonal artery, and a reverse saphenous vein graft was performed to the obtuse marginal artery.
A PRECURSOR TO INFARCTION
Wellens et al described specific precordial T-wave changes in patients with unstable angina who subsequently developed anterior wall myocardial infarction.1
The importance of Wellens syndrome is that it occurs in the pain-free interval when no other evidence of ischemia or angina may be present.1 Cardiac enzyme levels are typically normal or only minimally elevated; only 12% of patients with this syndrome have elevated cardiac biomarker levels.2
Given the extent of myocardial injury, urgent echocardiography can show a wall-motion abnormality even if cardiac enzyme levels are normal. This gives important insight into electrocardiographic changes and should prompt consideration of revascularization.
Even with extensive medical management, Wellens syndrome progresses to acute anterior wall ischemia. About 75% of patients with Wellens syndrome who receive medical management but do not undergo revascularization (eg, coronary artery bypass grafting, percutaneous coronary intervention) develop extensive anterior wall infarction within days.1,3 Despite negative cardiac biomarkers, Wellens syndrome is considered an acute coronary syndrome requiring urgent cardiac intervention.
- Movahed MR. Wellens’ syndrome or inverted U-waves? Clin Cardiol 2008; 31:133–134.
- de Zwaan C, Bär FW, Janssen JH, et al. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowing of the proximal LAD coronary artery. Am Heart J 1989; 117:657–665.
- de Zwaan C, Bär FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J 1982; 103:730–736.
A 67-year-old man with a history of hypertension and hyperlipidemia presented to the emergency department after 3 hours of what he described as a burning sensation in his chest that woke him from sleep. He attributed it at first to a late-night meal and treated himself with some milk and yogurt, which seemed to relieve the symptoms. However, the pain recurred and was associated with difficulty breathing. At that point, he drove himself to the emergency department.
On arrival, his temperature was 36.5°C (97.7°F), blood pressure 134/67 mm Hg, heart rate 89 bpm, respirations 18/min, and oxygen saturation 98% on room air. His cardiovascular, lung, and neurologic examinations were normal. His cardiac enzyme levels (creatine kinase, creatine kinase MB fraction, and troponin T) were within normal limits.
Figure 1 depicts his initial electrocardiogram. It showed deep, symmetric T-wave inversions in the precordial leads especially in V2 and V3, changes known as Wellens syndrome. The ST-T changes in lead V1 suggested a very proximal lesion in the left anterior descending artery (LAD), before the first septal perforator. Also, lateral and high lateral (V5 and V6) findings indicated stenoses of the branching diagonals and left circumflex myocardial territory. Furthermore, the inferior ST-T changes indicated that his LAD may have wrapped around the cardiac apex. All of these findings were prognostically significant.
The patient was given aspirin and was started on intravenous unfractionated heparin and nitroglycerin. He was sent for urgent left-heart catheterization, which showed a 50% to 60% stenosis in the left main coronary artery, with involvement of the left circumflex artery proximally, in addition to a “tight” first-diagonal stenosis, a 90% stenosis in a large (> 3.0-mm) proximal segment of the LAD, an 80% stenosis in a large (> 3.0-mm) mid-LAD segment, and a 40% stenosis in a large (> 3.0-mm) second diagonal artery (Figure 2).
He was referred for cardiac surgery and underwent triple coronary artery bypass grafting: the left internal thoracic artery was grafted to the LAD, a reverse saphenous vein graft was performed to the diagonal artery, and a reverse saphenous vein graft was performed to the obtuse marginal artery.
A PRECURSOR TO INFARCTION
Wellens et al described specific precordial T-wave changes in patients with unstable angina who subsequently developed anterior wall myocardial infarction.1
The importance of Wellens syndrome is that it occurs in the pain-free interval when no other evidence of ischemia or angina may be present.1 Cardiac enzyme levels are typically normal or only minimally elevated; only 12% of patients with this syndrome have elevated cardiac biomarker levels.2
Given the extent of myocardial injury, urgent echocardiography can show a wall-motion abnormality even if cardiac enzyme levels are normal. This gives important insight into electrocardiographic changes and should prompt consideration of revascularization.
Even with extensive medical management, Wellens syndrome progresses to acute anterior wall ischemia. About 75% of patients with Wellens syndrome who receive medical management but do not undergo revascularization (eg, coronary artery bypass grafting, percutaneous coronary intervention) develop extensive anterior wall infarction within days.1,3 Despite negative cardiac biomarkers, Wellens syndrome is considered an acute coronary syndrome requiring urgent cardiac intervention.
A 67-year-old man with a history of hypertension and hyperlipidemia presented to the emergency department after 3 hours of what he described as a burning sensation in his chest that woke him from sleep. He attributed it at first to a late-night meal and treated himself with some milk and yogurt, which seemed to relieve the symptoms. However, the pain recurred and was associated with difficulty breathing. At that point, he drove himself to the emergency department.
On arrival, his temperature was 36.5°C (97.7°F), blood pressure 134/67 mm Hg, heart rate 89 bpm, respirations 18/min, and oxygen saturation 98% on room air. His cardiovascular, lung, and neurologic examinations were normal. His cardiac enzyme levels (creatine kinase, creatine kinase MB fraction, and troponin T) were within normal limits.
Figure 1 depicts his initial electrocardiogram. It showed deep, symmetric T-wave inversions in the precordial leads especially in V2 and V3, changes known as Wellens syndrome. The ST-T changes in lead V1 suggested a very proximal lesion in the left anterior descending artery (LAD), before the first septal perforator. Also, lateral and high lateral (V5 and V6) findings indicated stenoses of the branching diagonals and left circumflex myocardial territory. Furthermore, the inferior ST-T changes indicated that his LAD may have wrapped around the cardiac apex. All of these findings were prognostically significant.
The patient was given aspirin and was started on intravenous unfractionated heparin and nitroglycerin. He was sent for urgent left-heart catheterization, which showed a 50% to 60% stenosis in the left main coronary artery, with involvement of the left circumflex artery proximally, in addition to a “tight” first-diagonal stenosis, a 90% stenosis in a large (> 3.0-mm) proximal segment of the LAD, an 80% stenosis in a large (> 3.0-mm) mid-LAD segment, and a 40% stenosis in a large (> 3.0-mm) second diagonal artery (Figure 2).
He was referred for cardiac surgery and underwent triple coronary artery bypass grafting: the left internal thoracic artery was grafted to the LAD, a reverse saphenous vein graft was performed to the diagonal artery, and a reverse saphenous vein graft was performed to the obtuse marginal artery.
A PRECURSOR TO INFARCTION
Wellens et al described specific precordial T-wave changes in patients with unstable angina who subsequently developed anterior wall myocardial infarction.1
The importance of Wellens syndrome is that it occurs in the pain-free interval when no other evidence of ischemia or angina may be present.1 Cardiac enzyme levels are typically normal or only minimally elevated; only 12% of patients with this syndrome have elevated cardiac biomarker levels.2
Given the extent of myocardial injury, urgent echocardiography can show a wall-motion abnormality even if cardiac enzyme levels are normal. This gives important insight into electrocardiographic changes and should prompt consideration of revascularization.
Even with extensive medical management, Wellens syndrome progresses to acute anterior wall ischemia. About 75% of patients with Wellens syndrome who receive medical management but do not undergo revascularization (eg, coronary artery bypass grafting, percutaneous coronary intervention) develop extensive anterior wall infarction within days.1,3 Despite negative cardiac biomarkers, Wellens syndrome is considered an acute coronary syndrome requiring urgent cardiac intervention.
- Movahed MR. Wellens’ syndrome or inverted U-waves? Clin Cardiol 2008; 31:133–134.
- de Zwaan C, Bär FW, Janssen JH, et al. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowing of the proximal LAD coronary artery. Am Heart J 1989; 117:657–665.
- de Zwaan C, Bär FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J 1982; 103:730–736.
- Movahed MR. Wellens’ syndrome or inverted U-waves? Clin Cardiol 2008; 31:133–134.
- de Zwaan C, Bär FW, Janssen JH, et al. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowing of the proximal LAD coronary artery. Am Heart J 1989; 117:657–665.
- de Zwaan C, Bär FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J 1982; 103:730–736.
A 47-year-old man with chest and neck pain
A 47-year-old man presented with acute shortness of breath and chest and neck pain, which began after he heard popping sounds while boarding a bus. The pain was right-sided, sharp, worse with deep breathing, and associated with a sensation of fullness over the right chest.
His medical conditions included controlled hypertension, gastroesophageal reflux disease, and chronic obstructive pulmonary disease (COPD). The COPD was managed with an albuterol inhaler only. He had a 50-pack-year history of smoking, and he drank alcohol occasionally.
On arrival, he was in mild respiratory distress, but his vital signs were stable. We could hear wheezing on both sides of his chest and feel subcutaneous crepitation on both sides of his chest and neck, the latter more on the right side. The rest of the examination was unremarkable.
Results of a complete blood cell count and metabolic panel were within normal limits. Because of the above findings, nasopharyngeal radiogragraphy was ordered (Figures 1 and 2).
Q: What is the most likely cause of this presentation?
- Esophageal rupture
- Gas gangrene
- Asthma exacerbation
- Ruptured emphysematous bullae
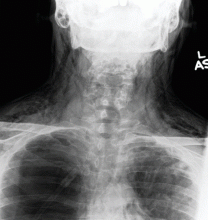
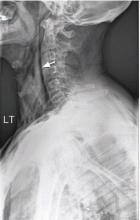
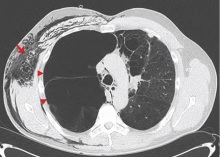
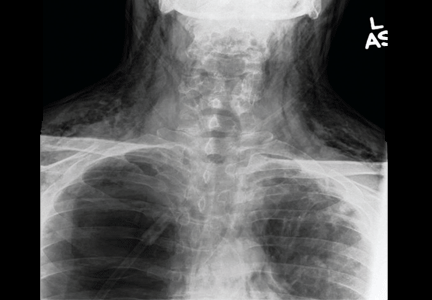
A: This patient had a history of COPD, which put him at risk of developing bullous emphysematous bullae that can rupture and cause subcutaneous emphysema. His nasopharyngeal radiograph (Figure1) showed bilateral extensive subcutaneous emphysema. His lateral nasopharyngeal radiograph (Figure 2) showed air-tracking within the mediastinum and into the retropharyngeal space (arrow). Computed tomography (Figure 3) showed extensive subcutaneous emphysema in the right lateral chest wall (arrow) and large bullae in the right upper lobe (arrow heads). As for the other possibilities:
Esophageal ruptures and tears are iatrogenic in most cases and usually occur after endoscopic procedures, but they can also occur in patients with intractable vomiting. Computed tomography often shows esophageal thickening, periesophageal fluid, mediastinal widening, and extraluminal air. However, in most cases, it is seen as pneumomediastinum and subcutaneous emphysema.1
Gas gangrene is a life-threatening soft-tissue and muscle infection caused by Clostridium perfringens in most cases.2 The pain is out of proportion to the findings on physical examination. Patients usually have toxic signs and symptoms such as fever and hypotension. Our patient was hemodynamically stable, with no changes in skin color.
Severe exacerbations of asthma can lead to alveolar rupture, pneumothorax, and subcutaneous emphysema, although this is a rare complication. Air can dissect along the bronchovascular sheaths into the neck and cause subcutaneous emphysema, or into the pleural space and cause pneumothorax. Our patient had no history of asthma and plainly had emphysematous bullae.3
SUBCUTANEOUS EMPHYSEMA
Subcutaneous emphysema is a collection of air within subcutaneous tissues. It usually presents as bloating of the skin around the neck and the chest wall. It is often seen in patients with pneumothorax.
The most common cause of subcutaneous emphysema is traumatic injury to the chest wall, such as from a motor vehicle accident or a stab wound,4 but it can also occur spontaneously in patients who have severe emphysema with large bullae. As the emphysema progresses, the bullae can easily rupture, and this can lead to pneumothorax, which can lead to subcutaneous emphysema. Primary spontaneous pneumothorax and subcutaneous emphysema can occur in people who have unrecognized lung disease and genetic disorders such as Marfan syndrome and Ehler-Danlos syndrome.5 Other causes include iatrogenic injury, Pneumocystis jirovecii pneumonia (common in patients with human immunodeficiency virus infection), and cystic fibrosis. Pneumothorax occurs in about 30% of cases of P jirovecii pneumonia,6 and in about 6% of patients with cystic fibrosis.7 Bronchocutaneous fistula is an extremely rare complication of lung cancer and can cause subcutaneous emphysema.8 Tuberculosis is another possible cause.9
Subcutaneous emphysema mainly presents with chest or neck pain and wheezing. In severe cases, air can track to the face, causing facial swelling and difficulty breathing due to compression of the larynx. Also, it can track down to the thighs, causing leg pain and swelling.10
On examination, subcutaneous emphysema can be detected by palpating the chest wall, which causes the air bubble to move and produce crackling sounds. Most cases of subcutaneous emphysema are diagnosed clinically. Chest radiography and computed tomography help identify the source of air leak. Ultrasonography is usually used in cases of blunt trauma to the chest as part of the Focal Assessment With Sonography for Trauma protocol.11
Subcutaneous emphysema can resolve spontaneously, requiring only pain management and supplemental oxygen.12 In severe cases, air collection can lead to what is called “massive subcutaneous emphysema,” which requires surgical drainage.
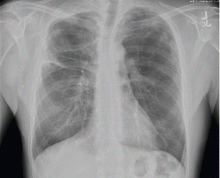
Our patient had large emphysematous bullae in the apical region of the right lung that ruptured and led to subcutaneous emphysema. After placement of a chest tube, he underwent right-sided thoracotomy with bullectomy. His postoperative course was uneventful, and he was discharged a few days later. Three weeks later, repeated chest radiography showed resolution of his subcutaneous emphysema (Figure 4).
- White CS, Templeton PA, Attar S. Esophageal perforation: CT findings. AJR Am J Roentgenol 1993; 160:767–770.
- Aggelidakis J, Lasithiotakis K, Topalidou A, Koutroumpas J, Kouvidis G, Katonis P. Limb salvage after gas gangrene: a case report and review of the literature. World J Emerg Surg 2011; 6:28.
- Romero KJ, Trujillo MH. Spontaneous pneumomediastinum and subcutaneous emphysema in asthma exacerbation: the Macklin effect. Heart Lung 2010; 39:444–447.
- Peart O. Subcutaneous emphysema. Radiol Technol 2006; 77:296.
- Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med 2006; 12:268–272.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest 2005; 128:720–728.
- Yalçinkaya S, Vural AH, Göncü MT, Özyazicioglu AF. Cavitary lung cancer presenting as subcutaneous emphysema on the contralateral side. Interact Cardiovasc Thorac Surg 2012; 14:338–339.
- Shamaei M, Tabarsi P, Pojhan S, et al. Tuberculosis-associated secondary pneumothorax: a retrospective study of 53 patients. Respir Care 2011; 56:298–302.
- Sherif HM, Ott DA. The use of subcutaneous drains to manage subcutaneous emphysema. Tex Heart Inst J 1999; 26:129–131.
- Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17:11–17.
- Mattox KL, Allen MK. Systematic approach to pneumothorax, haemothorax, pneumomediastinum and subcutaneous emphysema. Injury 1986; 17:309–312.
A 47-year-old man presented with acute shortness of breath and chest and neck pain, which began after he heard popping sounds while boarding a bus. The pain was right-sided, sharp, worse with deep breathing, and associated with a sensation of fullness over the right chest.
His medical conditions included controlled hypertension, gastroesophageal reflux disease, and chronic obstructive pulmonary disease (COPD). The COPD was managed with an albuterol inhaler only. He had a 50-pack-year history of smoking, and he drank alcohol occasionally.
On arrival, he was in mild respiratory distress, but his vital signs were stable. We could hear wheezing on both sides of his chest and feel subcutaneous crepitation on both sides of his chest and neck, the latter more on the right side. The rest of the examination was unremarkable.
Results of a complete blood cell count and metabolic panel were within normal limits. Because of the above findings, nasopharyngeal radiogragraphy was ordered (Figures 1 and 2).
Q: What is the most likely cause of this presentation?
- Esophageal rupture
- Gas gangrene
- Asthma exacerbation
- Ruptured emphysematous bullae
A: This patient had a history of COPD, which put him at risk of developing bullous emphysematous bullae that can rupture and cause subcutaneous emphysema. His nasopharyngeal radiograph (Figure1) showed bilateral extensive subcutaneous emphysema. His lateral nasopharyngeal radiograph (Figure 2) showed air-tracking within the mediastinum and into the retropharyngeal space (arrow). Computed tomography (Figure 3) showed extensive subcutaneous emphysema in the right lateral chest wall (arrow) and large bullae in the right upper lobe (arrow heads). As for the other possibilities:
Esophageal ruptures and tears are iatrogenic in most cases and usually occur after endoscopic procedures, but they can also occur in patients with intractable vomiting. Computed tomography often shows esophageal thickening, periesophageal fluid, mediastinal widening, and extraluminal air. However, in most cases, it is seen as pneumomediastinum and subcutaneous emphysema.1
Gas gangrene is a life-threatening soft-tissue and muscle infection caused by Clostridium perfringens in most cases.2 The pain is out of proportion to the findings on physical examination. Patients usually have toxic signs and symptoms such as fever and hypotension. Our patient was hemodynamically stable, with no changes in skin color.
Severe exacerbations of asthma can lead to alveolar rupture, pneumothorax, and subcutaneous emphysema, although this is a rare complication. Air can dissect along the bronchovascular sheaths into the neck and cause subcutaneous emphysema, or into the pleural space and cause pneumothorax. Our patient had no history of asthma and plainly had emphysematous bullae.3
SUBCUTANEOUS EMPHYSEMA
Subcutaneous emphysema is a collection of air within subcutaneous tissues. It usually presents as bloating of the skin around the neck and the chest wall. It is often seen in patients with pneumothorax.
The most common cause of subcutaneous emphysema is traumatic injury to the chest wall, such as from a motor vehicle accident or a stab wound,4 but it can also occur spontaneously in patients who have severe emphysema with large bullae. As the emphysema progresses, the bullae can easily rupture, and this can lead to pneumothorax, which can lead to subcutaneous emphysema. Primary spontaneous pneumothorax and subcutaneous emphysema can occur in people who have unrecognized lung disease and genetic disorders such as Marfan syndrome and Ehler-Danlos syndrome.5 Other causes include iatrogenic injury, Pneumocystis jirovecii pneumonia (common in patients with human immunodeficiency virus infection), and cystic fibrosis. Pneumothorax occurs in about 30% of cases of P jirovecii pneumonia,6 and in about 6% of patients with cystic fibrosis.7 Bronchocutaneous fistula is an extremely rare complication of lung cancer and can cause subcutaneous emphysema.8 Tuberculosis is another possible cause.9
Subcutaneous emphysema mainly presents with chest or neck pain and wheezing. In severe cases, air can track to the face, causing facial swelling and difficulty breathing due to compression of the larynx. Also, it can track down to the thighs, causing leg pain and swelling.10
On examination, subcutaneous emphysema can be detected by palpating the chest wall, which causes the air bubble to move and produce crackling sounds. Most cases of subcutaneous emphysema are diagnosed clinically. Chest radiography and computed tomography help identify the source of air leak. Ultrasonography is usually used in cases of blunt trauma to the chest as part of the Focal Assessment With Sonography for Trauma protocol.11
Subcutaneous emphysema can resolve spontaneously, requiring only pain management and supplemental oxygen.12 In severe cases, air collection can lead to what is called “massive subcutaneous emphysema,” which requires surgical drainage.
Our patient had large emphysematous bullae in the apical region of the right lung that ruptured and led to subcutaneous emphysema. After placement of a chest tube, he underwent right-sided thoracotomy with bullectomy. His postoperative course was uneventful, and he was discharged a few days later. Three weeks later, repeated chest radiography showed resolution of his subcutaneous emphysema (Figure 4).
A 47-year-old man presented with acute shortness of breath and chest and neck pain, which began after he heard popping sounds while boarding a bus. The pain was right-sided, sharp, worse with deep breathing, and associated with a sensation of fullness over the right chest.
His medical conditions included controlled hypertension, gastroesophageal reflux disease, and chronic obstructive pulmonary disease (COPD). The COPD was managed with an albuterol inhaler only. He had a 50-pack-year history of smoking, and he drank alcohol occasionally.
On arrival, he was in mild respiratory distress, but his vital signs were stable. We could hear wheezing on both sides of his chest and feel subcutaneous crepitation on both sides of his chest and neck, the latter more on the right side. The rest of the examination was unremarkable.
Results of a complete blood cell count and metabolic panel were within normal limits. Because of the above findings, nasopharyngeal radiogragraphy was ordered (Figures 1 and 2).
Q: What is the most likely cause of this presentation?
- Esophageal rupture
- Gas gangrene
- Asthma exacerbation
- Ruptured emphysematous bullae
A: This patient had a history of COPD, which put him at risk of developing bullous emphysematous bullae that can rupture and cause subcutaneous emphysema. His nasopharyngeal radiograph (Figure1) showed bilateral extensive subcutaneous emphysema. His lateral nasopharyngeal radiograph (Figure 2) showed air-tracking within the mediastinum and into the retropharyngeal space (arrow). Computed tomography (Figure 3) showed extensive subcutaneous emphysema in the right lateral chest wall (arrow) and large bullae in the right upper lobe (arrow heads). As for the other possibilities:
Esophageal ruptures and tears are iatrogenic in most cases and usually occur after endoscopic procedures, but they can also occur in patients with intractable vomiting. Computed tomography often shows esophageal thickening, periesophageal fluid, mediastinal widening, and extraluminal air. However, in most cases, it is seen as pneumomediastinum and subcutaneous emphysema.1
Gas gangrene is a life-threatening soft-tissue and muscle infection caused by Clostridium perfringens in most cases.2 The pain is out of proportion to the findings on physical examination. Patients usually have toxic signs and symptoms such as fever and hypotension. Our patient was hemodynamically stable, with no changes in skin color.
Severe exacerbations of asthma can lead to alveolar rupture, pneumothorax, and subcutaneous emphysema, although this is a rare complication. Air can dissect along the bronchovascular sheaths into the neck and cause subcutaneous emphysema, or into the pleural space and cause pneumothorax. Our patient had no history of asthma and plainly had emphysematous bullae.3
SUBCUTANEOUS EMPHYSEMA
Subcutaneous emphysema is a collection of air within subcutaneous tissues. It usually presents as bloating of the skin around the neck and the chest wall. It is often seen in patients with pneumothorax.
The most common cause of subcutaneous emphysema is traumatic injury to the chest wall, such as from a motor vehicle accident or a stab wound,4 but it can also occur spontaneously in patients who have severe emphysema with large bullae. As the emphysema progresses, the bullae can easily rupture, and this can lead to pneumothorax, which can lead to subcutaneous emphysema. Primary spontaneous pneumothorax and subcutaneous emphysema can occur in people who have unrecognized lung disease and genetic disorders such as Marfan syndrome and Ehler-Danlos syndrome.5 Other causes include iatrogenic injury, Pneumocystis jirovecii pneumonia (common in patients with human immunodeficiency virus infection), and cystic fibrosis. Pneumothorax occurs in about 30% of cases of P jirovecii pneumonia,6 and in about 6% of patients with cystic fibrosis.7 Bronchocutaneous fistula is an extremely rare complication of lung cancer and can cause subcutaneous emphysema.8 Tuberculosis is another possible cause.9
Subcutaneous emphysema mainly presents with chest or neck pain and wheezing. In severe cases, air can track to the face, causing facial swelling and difficulty breathing due to compression of the larynx. Also, it can track down to the thighs, causing leg pain and swelling.10
On examination, subcutaneous emphysema can be detected by palpating the chest wall, which causes the air bubble to move and produce crackling sounds. Most cases of subcutaneous emphysema are diagnosed clinically. Chest radiography and computed tomography help identify the source of air leak. Ultrasonography is usually used in cases of blunt trauma to the chest as part of the Focal Assessment With Sonography for Trauma protocol.11
Subcutaneous emphysema can resolve spontaneously, requiring only pain management and supplemental oxygen.12 In severe cases, air collection can lead to what is called “massive subcutaneous emphysema,” which requires surgical drainage.
Our patient had large emphysematous bullae in the apical region of the right lung that ruptured and led to subcutaneous emphysema. After placement of a chest tube, he underwent right-sided thoracotomy with bullectomy. His postoperative course was uneventful, and he was discharged a few days later. Three weeks later, repeated chest radiography showed resolution of his subcutaneous emphysema (Figure 4).
- White CS, Templeton PA, Attar S. Esophageal perforation: CT findings. AJR Am J Roentgenol 1993; 160:767–770.
- Aggelidakis J, Lasithiotakis K, Topalidou A, Koutroumpas J, Kouvidis G, Katonis P. Limb salvage after gas gangrene: a case report and review of the literature. World J Emerg Surg 2011; 6:28.
- Romero KJ, Trujillo MH. Spontaneous pneumomediastinum and subcutaneous emphysema in asthma exacerbation: the Macklin effect. Heart Lung 2010; 39:444–447.
- Peart O. Subcutaneous emphysema. Radiol Technol 2006; 77:296.
- Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med 2006; 12:268–272.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest 2005; 128:720–728.
- Yalçinkaya S, Vural AH, Göncü MT, Özyazicioglu AF. Cavitary lung cancer presenting as subcutaneous emphysema on the contralateral side. Interact Cardiovasc Thorac Surg 2012; 14:338–339.
- Shamaei M, Tabarsi P, Pojhan S, et al. Tuberculosis-associated secondary pneumothorax: a retrospective study of 53 patients. Respir Care 2011; 56:298–302.
- Sherif HM, Ott DA. The use of subcutaneous drains to manage subcutaneous emphysema. Tex Heart Inst J 1999; 26:129–131.
- Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17:11–17.
- Mattox KL, Allen MK. Systematic approach to pneumothorax, haemothorax, pneumomediastinum and subcutaneous emphysema. Injury 1986; 17:309–312.
- White CS, Templeton PA, Attar S. Esophageal perforation: CT findings. AJR Am J Roentgenol 1993; 160:767–770.
- Aggelidakis J, Lasithiotakis K, Topalidou A, Koutroumpas J, Kouvidis G, Katonis P. Limb salvage after gas gangrene: a case report and review of the literature. World J Emerg Surg 2011; 6:28.
- Romero KJ, Trujillo MH. Spontaneous pneumomediastinum and subcutaneous emphysema in asthma exacerbation: the Macklin effect. Heart Lung 2010; 39:444–447.
- Peart O. Subcutaneous emphysema. Radiol Technol 2006; 77:296.
- Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med 2006; 12:268–272.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Flume PA, Strange C, Ye X, Ebeling M, Hulsey T, Clark LL. Pneumothorax in cystic fibrosis. Chest 2005; 128:720–728.
- Yalçinkaya S, Vural AH, Göncü MT, Özyazicioglu AF. Cavitary lung cancer presenting as subcutaneous emphysema on the contralateral side. Interact Cardiovasc Thorac Surg 2012; 14:338–339.
- Shamaei M, Tabarsi P, Pojhan S, et al. Tuberculosis-associated secondary pneumothorax: a retrospective study of 53 patients. Respir Care 2011; 56:298–302.
- Sherif HM, Ott DA. The use of subcutaneous drains to manage subcutaneous emphysema. Tex Heart Inst J 1999; 26:129–131.
- Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17:11–17.
- Mattox KL, Allen MK. Systematic approach to pneumothorax, haemothorax, pneumomediastinum and subcutaneous emphysema. Injury 1986; 17:309–312.